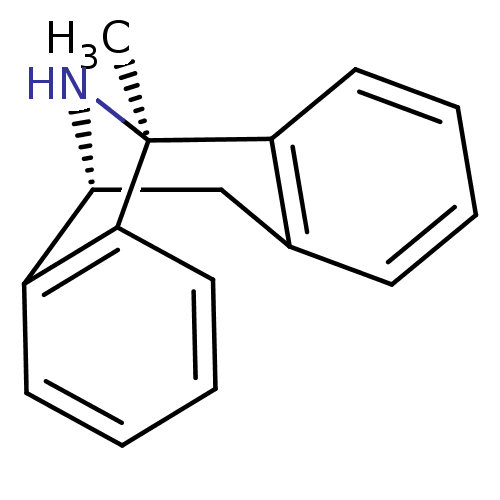Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glutamate receptor ionotropic, NMDA 2A
Ligand
BDBM50344263
Substrate
n/a
Meas. Tech.
ChEMBL_748237 (CHEMBL1781200)
IC50
29±n/a nM
Citation
 Brown, DG; Maier, DL; Sylvester, MA; Hoerter, TN; Menhaji-Klotz, E; Lasota, CC; Hirata, LT; Wilkins, DE; Scott, CW; Trivedi, S; Chen, T; McCarthy, DJ; Maciag, CM; Sutton, EJ; Cumberledge, J; Mathisen, D; Roberts, J; Gupta, A; Liu, F; Elmore, CS; Alhambra, C; Krumrine, JR; Wang, X; Ciaccio, PJ; Wood, MW; Campbell, JB; Johansson, MJ; Xia, J; Wen, X; Jiang, J; Wang, X; Peng, Z; Hu, T; Wang, J 2,6-Disubstituted pyrazines and related analogs as NR2B site antagonists of the NMDA receptor with anti-depressant activity. Bioorg Med Chem Lett 21:3399-403 (2011) [PubMed] Article
Brown, DG; Maier, DL; Sylvester, MA; Hoerter, TN; Menhaji-Klotz, E; Lasota, CC; Hirata, LT; Wilkins, DE; Scott, CW; Trivedi, S; Chen, T; McCarthy, DJ; Maciag, CM; Sutton, EJ; Cumberledge, J; Mathisen, D; Roberts, J; Gupta, A; Liu, F; Elmore, CS; Alhambra, C; Krumrine, JR; Wang, X; Ciaccio, PJ; Wood, MW; Campbell, JB; Johansson, MJ; Xia, J; Wen, X; Jiang, J; Wang, X; Peng, Z; Hu, T; Wang, J 2,6-Disubstituted pyrazines and related analogs as NR2B site antagonists of the NMDA receptor with anti-depressant activity. Bioorg Med Chem Lett 21:3399-403 (2011) [PubMed] Article More Info.:
Target
Name:
Glutamate receptor ionotropic, NMDA 2A
Synonyms:
GRIN2A | GluN2A | Glutamate [NMDA] receptor | Glutamate [NMDA] receptor subunit epsilon 1 | Glutamate [NMDA] receptor subunit epsilon-1 | N-methyl D-aspartate receptor subtype 2A | NMDA receptor subtype 2A protein (NR2A) | NMDAR2A | NMDE1_HUMAN | NR2A | hNR2A
Type:
n/a
Mol. Mass.:
165293.76
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
1464
Sequence:
MGRVGYWTLLVLPALLVWRGPAPSAAAEKGPPALNIAVMLGHSHDVTERELRTLWGPEQAAGLPLDVNVVALLMNRTDPKSLITHVCDLMSGARIHGLVFGDDTDQEAVAQMLDFISSHTFVPILGIHGGASMIMADKDPTSTFFQFGASIQQQATVMLKIMQDYDWHVFSLVTTIFPGYREFISFVKTTVDNSFVGWDMQNVITLDTSFEDAKTQVQLKKIHSSVILLYCSKDEAVLILSEARSLGLTGYDFFWIVPSLVSGNTELIPKEFPSGLISVSYDDWDYSLEARVRDGIGILTTAASSMLEKFSYIPEAKASCYGQMERPEVPMHTLHPFMVNVTWDGKDLSFTEEGYQVHPRLVVIVLNKDREWEKVGKWENHTLSLRHAVWPRYKSFSDCEPDDNHLSIVTLEEAPFVIVEDIDPLTETCVRNTVPCRKFVKINNSTNEGMNVKKCCKGFCIDILKKLSRTVKFTYDLYLVTNGKHGKKVNNVWNGMIGEVVYQRAVMAVGSLTINEERSEVVDFSVPFVETGISVMVSRSNGTVSPSAFLEPFSASVWVMMFVMLLIVSAIAVFVFEYFSPVGYNRNLAKGKAPHGPSFTIGKAIWLLWGLVFNNSVPVQNPKGTTSKIMVSVWAFFAVIFLASYTANLAAFMIQEEFVDQVTGLSDKKFQRPHDYSPPFRFGTVPNGSTERNIRNNYPYMHQYMTKFNQKGVEDALVSLKTGKLDAFIYDAAVLNYKAGRDEGCKLVTIGSGYIFATTGYGIALQKGSPWKRQIDLALLQFVGDGEMEELETLWLTGICHNEKNEVMSSQLDIDNMAGVFYMLAAAMALSLITFIWEHLFYWKLRFCFTGVCSDRPGLLFSISRGIYSCIHGVHIEEKKKSPDFNLTGSQSNMLKLLRSAKNISSMSNMNSSRMDSPKRAADFIQRGSLIMDMVSDKGNLMYSDNRSFQGKESIFGDNMNELQTFVANRQKDNLNNYVFQGQHPLTLNESNPNTVEVAVSTESKANSRPRQLWKKSVDSIRQDSLSQNPVSQRDEATAENRTHSLKSPRYLPEEMAHSDISETSNRATCHREPDNSKNHKTKDNFKRSVASKYPKDCSEVERTYLKTKSSSPRDKIYTIDGEKEPGFHLDPPQFVENVTLPENVDFPDPYQDPSENFRKGDSTLPMNRNPLHNEEGLSNNDQYKLYSKHFTLKDKGSPHSETSERYRQNSTHCRSCLSNMPTYSGHFTMRSPFKCDACLRMGNLYDIDEDQMLQETGNPATGEQVYQQDWAQNNALQLQKNKLRISRQHSYDNIVDKPRELDLSRPSRSISLKDRERLLEGNFYGSLFSVPSSKLSGKKSSLFPQGLEDSKRSKSLLPDHTSDNPFLHSHRDDQRLVIGRCPSDPYKHSLPSQAVNDSYLRSSLRSTASYCSRDSRGHNDVYISEHVMPYAANKNNMYSTPRVLNSCSNRRVYKKMPSIESDV
Inhibitor
Name:
BDBM50344263
Synonyms:
(+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene | (+)-MK-801 | (+)MK-801 | (+/-) MK-8011-methyl-(9R,1R)-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene | (+/-)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene | (+/-)-MK801 | (-)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene | (-)-MK801 | (1S,9R)-1-methyl-16-azatetracyclo[7.6.1.0^{2,7}.0^{10,15}]hexadeca-2(7),3,5,10(15),11,13-hexaene | (1S,9R)-1-methyl-16-azatetracyclo[7.6.1.0^{2,7}.0^{10,15}]hexadeca-2,4,6,10(15),11,13-hexaene | (5S,10R)-(+)-5-methyl-10,11-dihydro-5Hdibenzo[a,d]cyclohepten-5,10-imine | (5S,10S)-(+)-5-methyl-10,11-dihydro-5Hdibenzo[a,d]cyclohepten-5,10-imine | (Dizocilpine)1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene | (MK-801)1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene | 1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene | 1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10(15),11,13-hexaene(MK-801) | 1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2(7),3,5,10,12,14-hexaene | 1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]hexadeca-2,4,6,10(15),11,13-hexaene | 10,11-Dihydro-5-methyl-5H-dibenzo[a,d]cyclohepten-5,10-imine.(MK-801) | CHEMBL284237 | MK-801 | MK-801 (Dizocilpine) | dizocilpine
Type:
Small organic molecule
Emp. Form.:
C16H15N
Mol. Mass.:
221.297
SMILES:
C[C@]12N[C@H](Cc3ccccc13)c1ccccc21 |r|