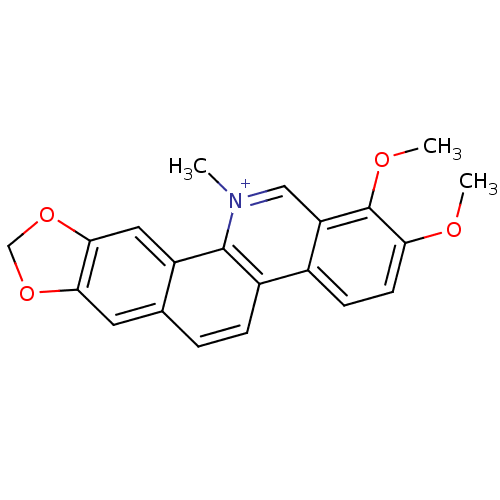
BDBM25524 17,18-dimethoxy-21-methyl-5,7-dioxa-21-azapentacyclo[11.8.0.0^{2,10}.0^{4,8}.0^{14,19}]henicosa-1(13),2,4(8),9,11,14(19),15,17,20-nonaen-21-ium::CHEMBL13045::CHEMBL258893::Chelerytherine::Toddalin::chelerythrine
SMILES C[n+]1cc2c(ccc(c2OC)OC)c3c1c4cc5c(cc4cc3)OCO5
InChI Key InChIKey=LLEJIEBFSOEYIV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 26 hits for monomerid = 25524
Found 26 hits for monomerid = 25524
Affinity DataIC50: 24nMAssay Description:Inhibition of PKCepsilonMore data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Competitive inhibition of recombinant human MAO-A using kynuramine as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Competitive inhibition of human AChE using acetyl thiocholine iodide as substrate by Lineweaver-Burk double-reciprocal analysisMore data for this Ligand-Target Pair
Affinity DataKi: 480nMAssay Description:Competitive inhibition of electric eel AChE using acetyl thiocholine iodide as substrate by Lineweaver-Burk double-reciprocal analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Inhibition of recombinant human MAO-A using kynuramine as substrate incubated for 20 mins by spectrophotometric methodMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
University Clinic Hospital of Valencia
Curated by ChEMBL
University Clinic Hospital of Valencia
Curated by ChEMBL
Affinity DataIC50: 566nMAssay Description:Agonist activity at PPARgamma (unknown origin)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
University Clinic Hospital of Valencia
Curated by ChEMBL
University Clinic Hospital of Valencia
Curated by ChEMBL
Affinity DataIC50: 566nMAssay Description:Binding affinity to human 6xHis-tagged PPARgamma ligand binding domain (206 to 477 residues) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 920nMAssay Description:Uncompetitive inhibition of electric eel AChE using acetyl thiocholine iodide as substrate by Lineweaver-Burk double-reciprocal analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.12E+3nMAssay Description:Uncompetitive inhibition of human AChE using acetyl thiocholine iodide as substrate by Lineweaver-Burk double-reciprocal analysisMore data for this Ligand-Target Pair
TargetSarcoplasmic/endoplasmic reticulum calcium ATPase 1(Rabbit)
University of Chemistry and Technology Prague
Curated by ChEMBL
University of Chemistry and Technology Prague
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of rabbit skeletal muscle microsomes SERCA1a incubated for 10 mins by enzyme-coupled methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+3nMAssay Description:Inhibition of AChE (unknown origin) preincubated for 30 mins followed by substrate addition acetylthiocholineiodide measured after 40 mins by Ellman'...More data for this Ligand-Target Pair
Affinity DataIC50: 1.54E+3nMAssay Description:Inhibition of human AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.78E+3nMAssay Description:Inhibition of electric eel AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.33E+3nMAssay Description:Inhibition of horse BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.58E+3nMAssay Description:Inhibition of BuChE (unknown origin) preincubated for 30 mins followed by substrate addition acetylthiocholineiodide measured after 40 mins by Ellman...More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of human BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant human MAO-B using benzylamine as substrate incubated for 30 mins by spectrophotometric methodMore data for this Ligand-Target Pair
TargetSarcoplasmic/endoplasmic reticulum calcium ATPase 1(Human)
University of Chemistry and Technology Prague
Curated by ChEMBL
University of Chemistry and Technology Prague
Curated by ChEMBL
Affinity DataIC50: 2.70E+4nMAssay Description:Inhibition of SERCA1a (unknown origin) incubated for 10 mins by enzyme-coupled methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Cdc42 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Rac1 GTPase activity assessed as incorporation of BODIPY-GTP after 40 mins by nucleotide binding competition assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:In the fluorescence polarization assay, the Bcl-XL protein is incubated with a fluorescein-tagged Bak-BH3 peptide. The Bcl-XL:Bak-BH3 peptide complex...More data for this Ligand-Target Pair
Affinity DataKi: 1.06E+5nMAssay Description:Displacement of Flu-Bak peptide from recombinant antiapoptotic Bcl-w protein by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.71E+5nMAssay Description:Displacement of Flu-Bak peptide from recombinant antiapoptotic Bcl2 protein by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus type 1)
University of Illinois
Curated by ChEMBL
University of Illinois
Curated by ChEMBL
Affinity DataIC50: 5.74E+5nMAssay Description:Inhibition of HIV1 RTMore data for this Ligand-Target Pair
Affinity DataIC50: 1.28E+6nMAssay Description:Inhibition of recombinant human DOPA decarboxylase assessed as inhibition of dopamine production after 30 mins by HPLC methodMore data for this Ligand-Target Pair