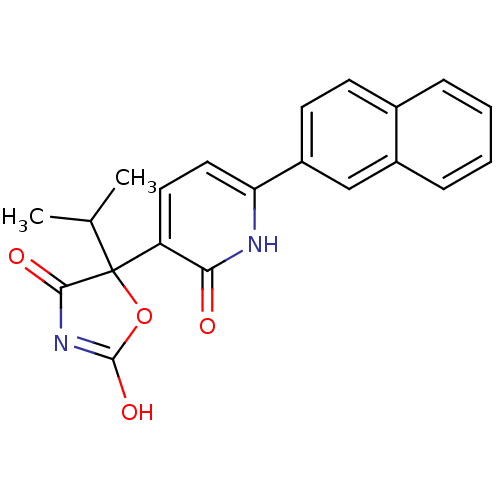
BDBM50384443 CHEMBL1770317
SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1
InChI Key InChIKey=HXVAXOYPIPUCRY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 50384443
Found 19 hits for monomerid = 50384443
Affinity DataKi: 5.01nMAssay Description:Binding affinity to human EP3More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [3H]-PGE2 from human EP3 expressed in Chem-1 cell membranes incubated for 2 hrs by TopCount scintillation plate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati...More data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Antagonist activity at human EP3 expressed in CHO cells assessed as suppression of sulprostone-induced inhibition of forskolin induced cAMP productio...More data for this Ligand-Target Pair
Affinity DataKi: >5.01E+4nMAssay Description:Antagonist activity at EP1 receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Binding affinity at human DP receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Antagonist activity against human EP2 receptor expressed in CHO-K1 cells assessed as inhibition of cAMP production after 4 hrs by FRET signal in LANC...More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Antagonist activity at FP receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 48 hrs by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Binding affinity at human EP4 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX2 in rat whole blood assessed as decreased PGE2 productionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX1 in rat whole blood assessed as decreased PGE2 productionMore data for this Ligand-Target Pair