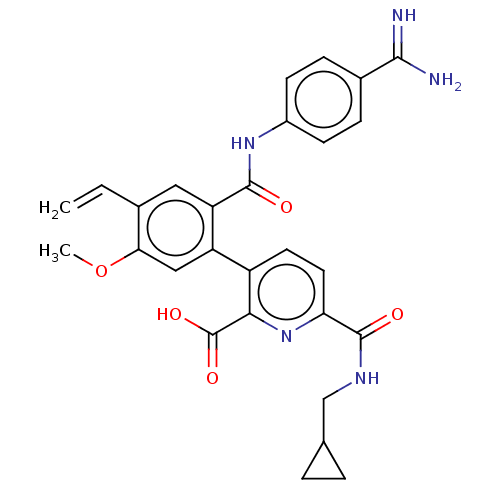
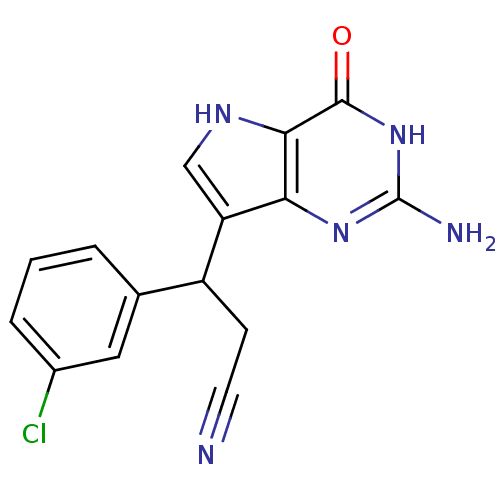
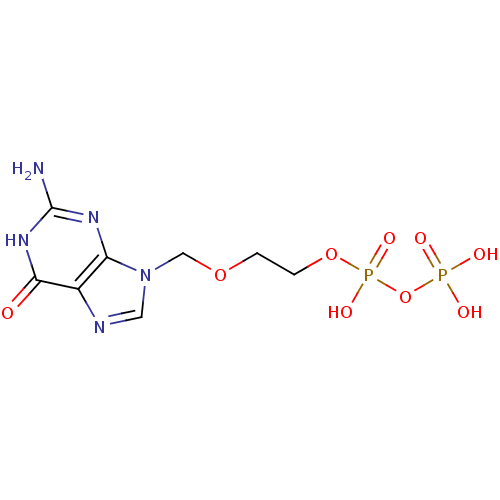
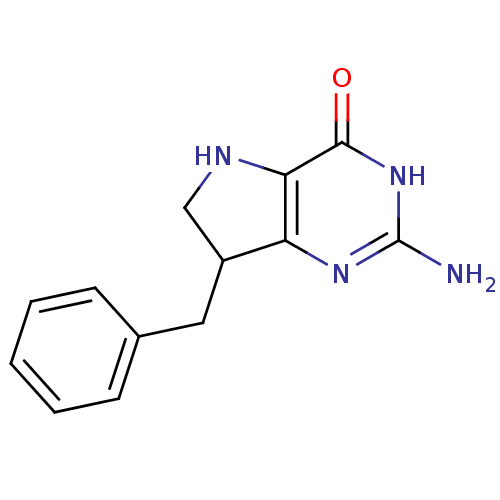
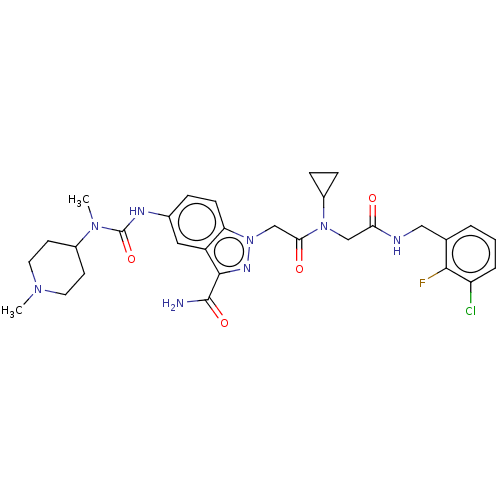
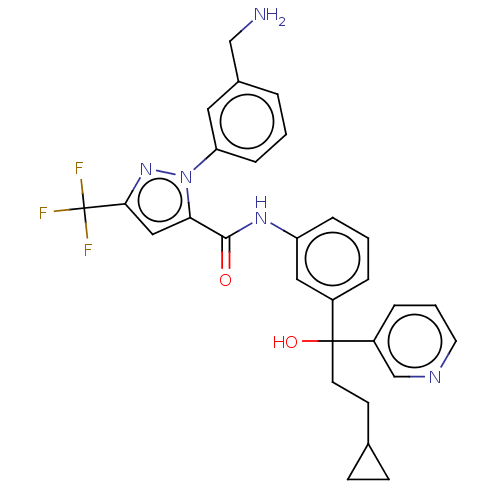
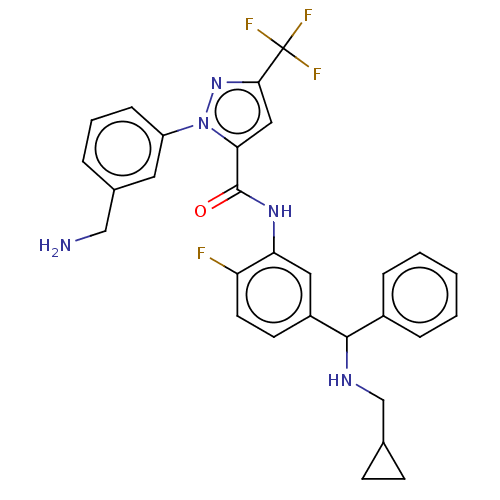
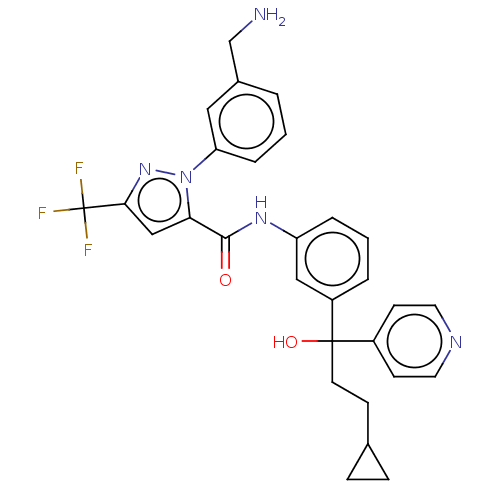
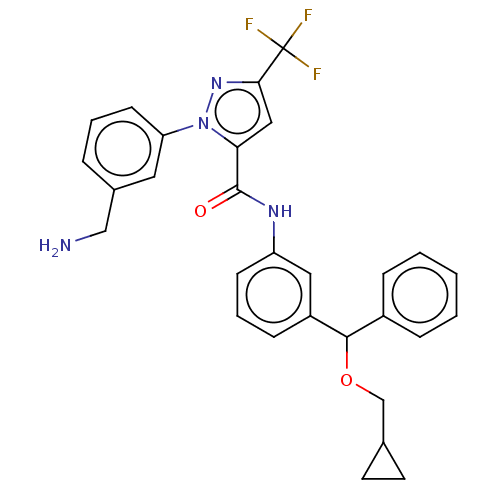
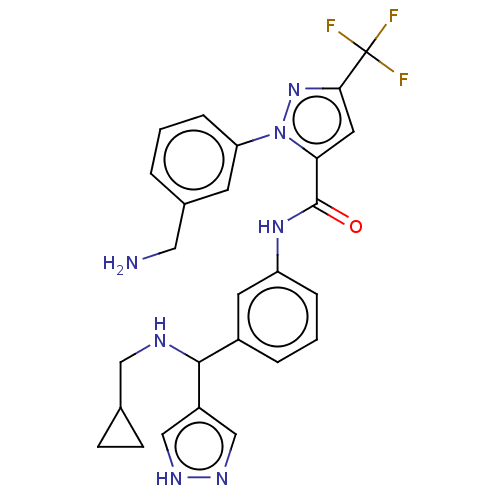
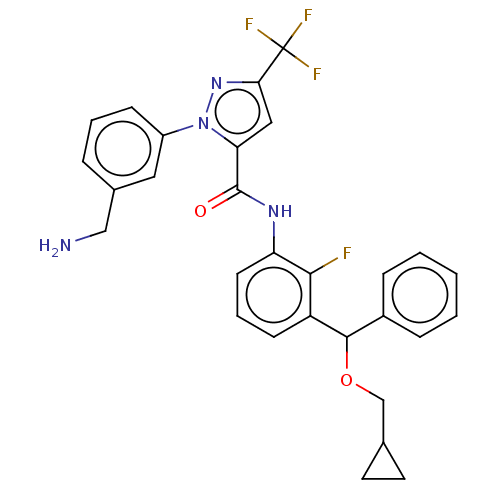
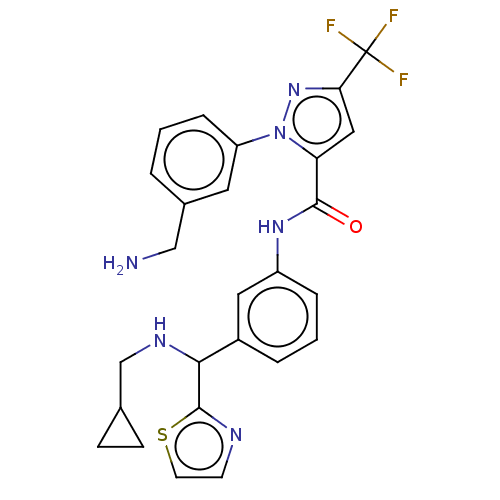
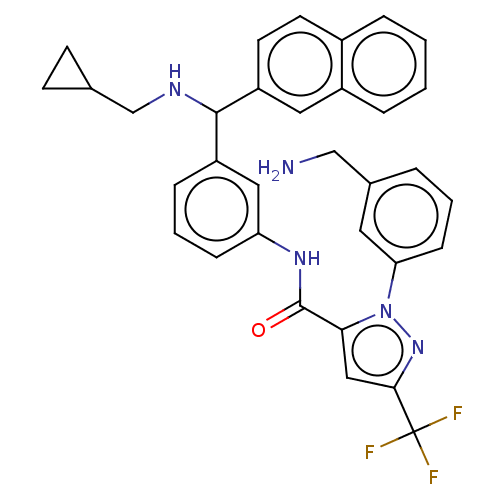
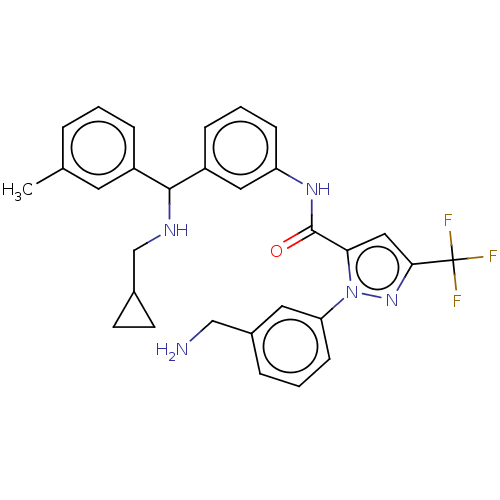
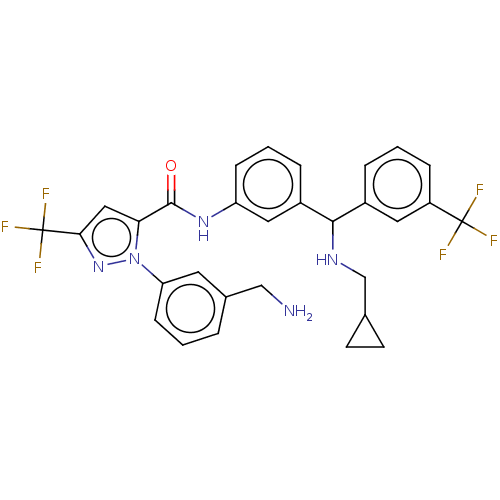
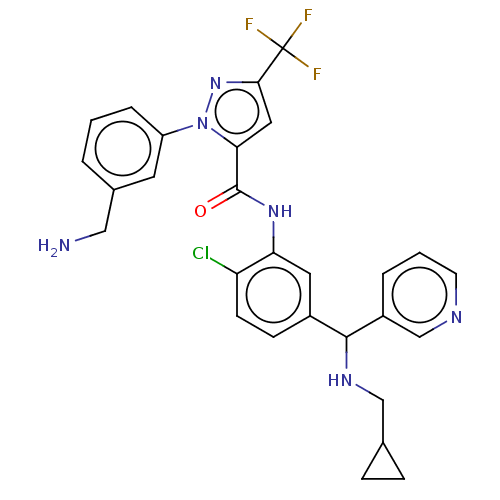
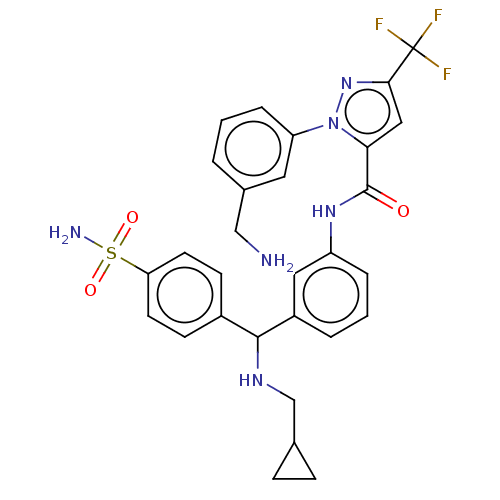
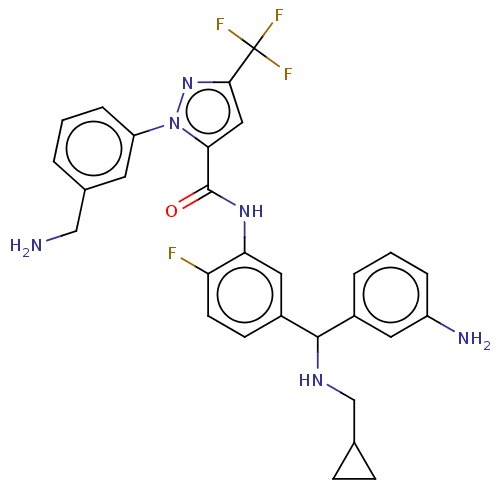
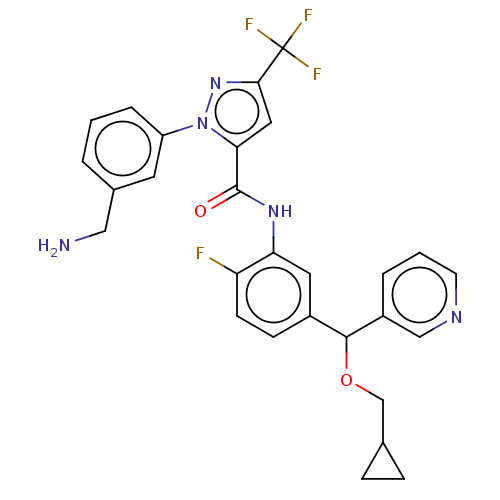
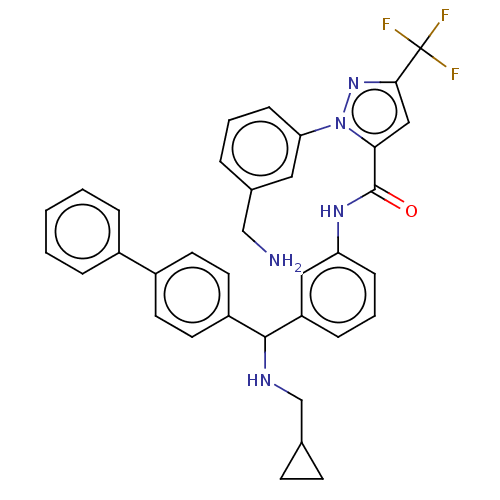
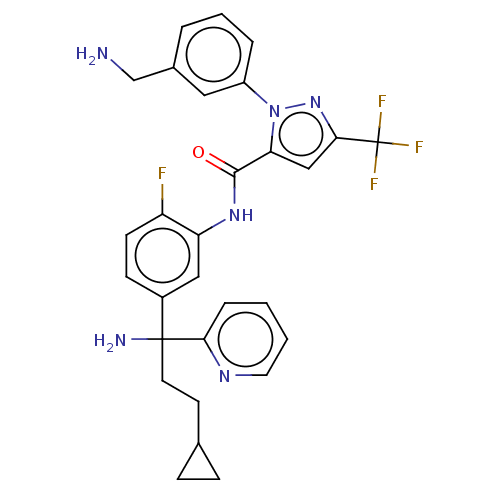
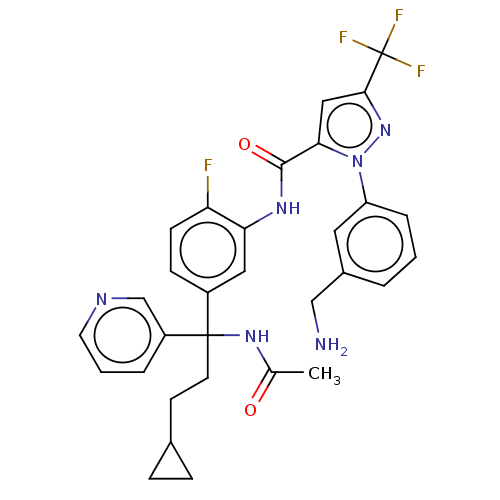
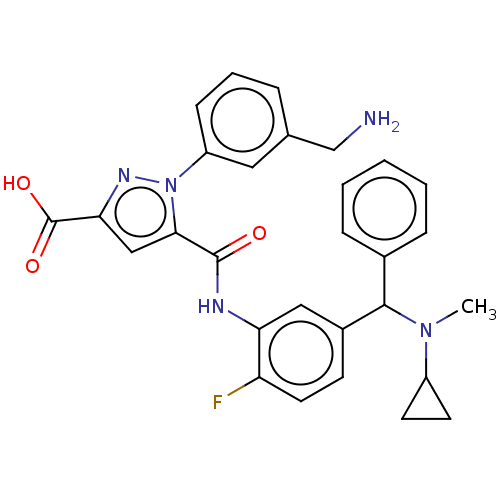
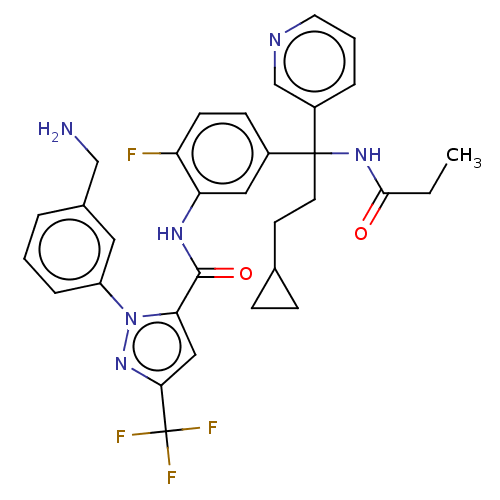
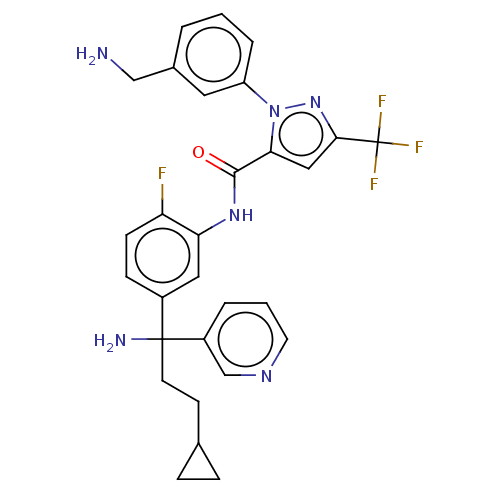
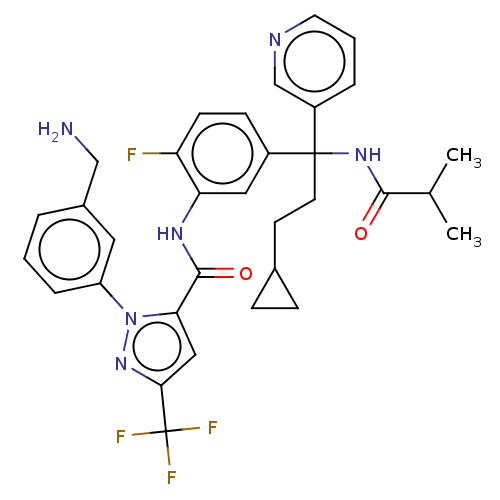
Affinity DataKi: 0.260nMAssay Description:Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m...More data for this Ligand-Target Pair
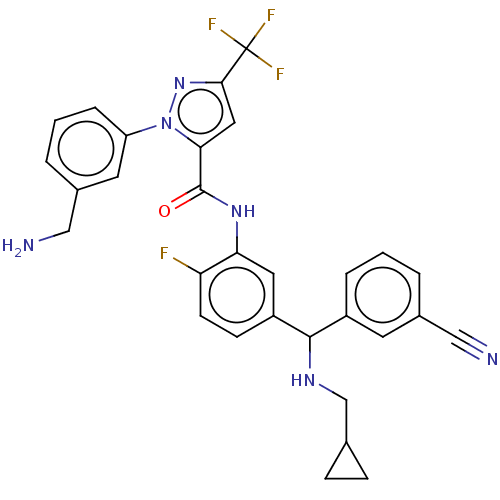
Affinity DataKi: 0.440nMAssay Description:Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m...More data for this Ligand-Target Pair
Target5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase(Escherichia coli (strain K12))
Biocryst Pharmaceuticals
Curated by ChEMBL
Biocryst Pharmaceuticals
Curated by ChEMBL
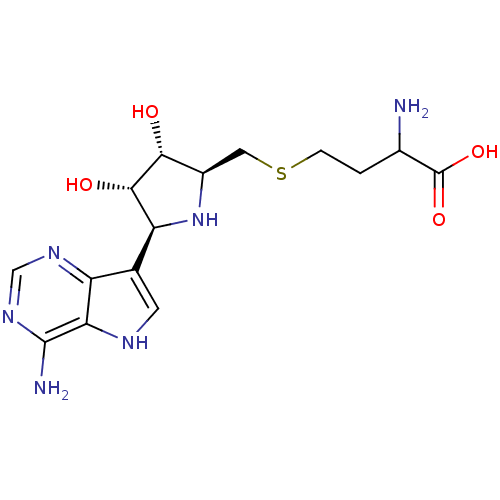
Affinity DataKi: 1.70nMAssay Description:Inhibition of Escherichia coli MTANMore data for this Ligand-Target Pair
TargetNeuraminidase(Influenza B virus (B/Lee/40))
University Of Alabama At Birmingham
Curated by ChEMBL
University Of Alabama At Birmingham
Curated by ChEMBL
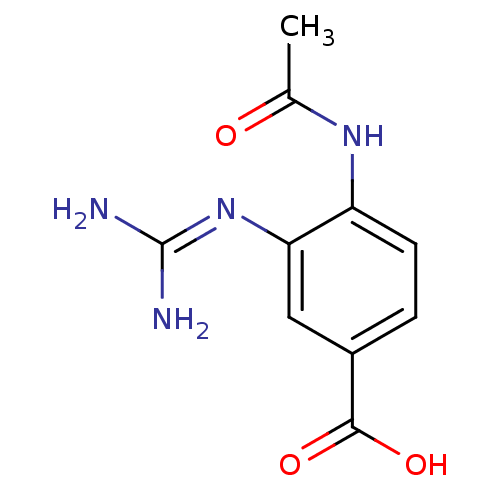
Affinity DataKi: 2.5nMAssay Description:In vitro inhibitory activity of the compound against B/Lee/40 Influenza B Neuraminidase.More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity against Purine nucleoside Phosphorylase from calf spleenMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Biocryst Pharmaceuticals
Curated by ChEMBL
Biocryst Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8.70nMAssay Description:Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4More data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Biocryst Pharmaceuticals
Curated by ChEMBL
Biocryst Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8.70nMAssay Description:Binding affinity against hydrophobic pocket of Purine nucleoside PhosphorylaseMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Biocryst Pharmaceuticals
Curated by ChEMBL
Biocryst Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 8.70nMAssay Description:Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibition of purine nucleoside phosphorylase against the enzyme from calf spleen in 1 mM phosphateMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from calf spleen in 1 mM phosphateMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Inhibition of human complement factor D using Z-L-Lys-SBzl hydrochloride as substrate preincubated for 15 mins followed by substrate addition and mea...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair
Affinity DataKi: 25.1nMAssay Description:The effect of compounds of the invention on human plasma kallikrein activity was determined using the chromogenic substrates (DiaPharma Group, Inc., ...More data for this Ligand-Target Pair