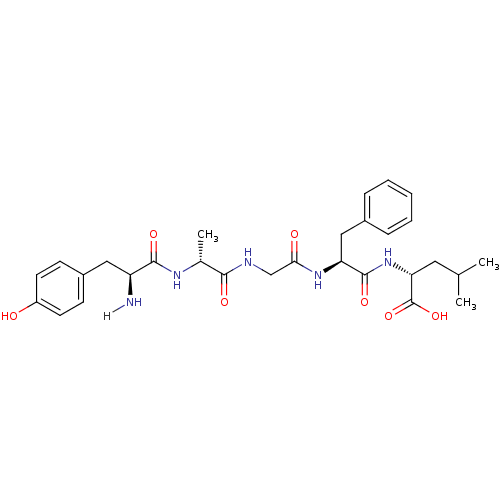
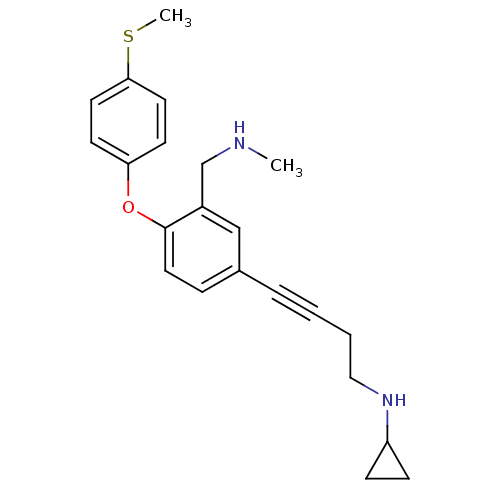
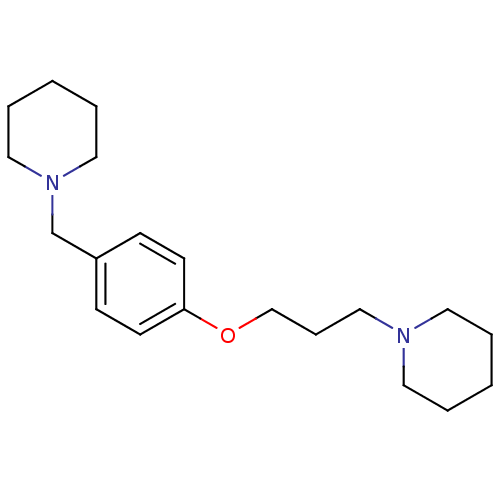
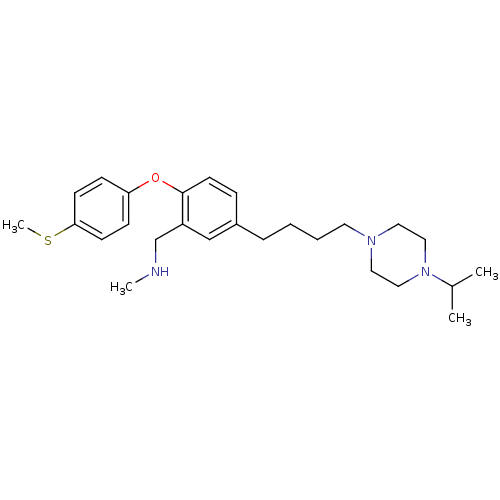
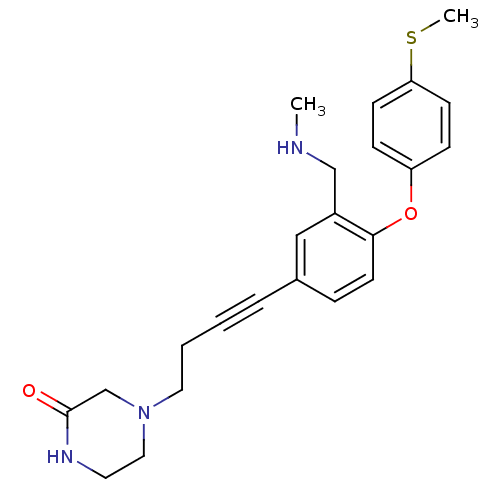
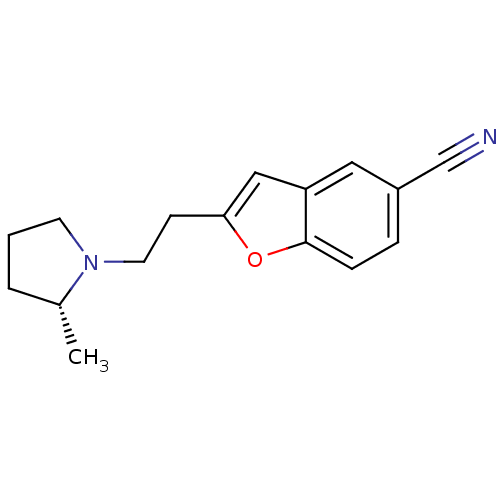
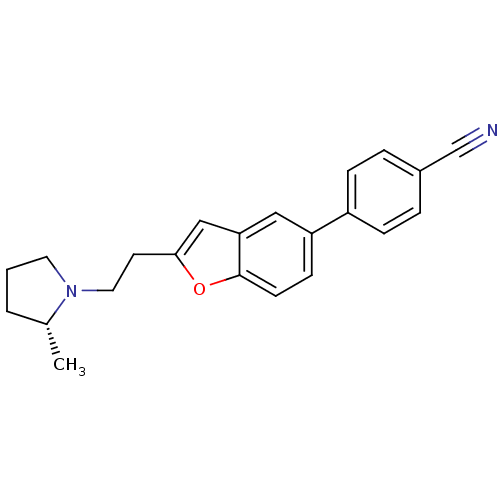
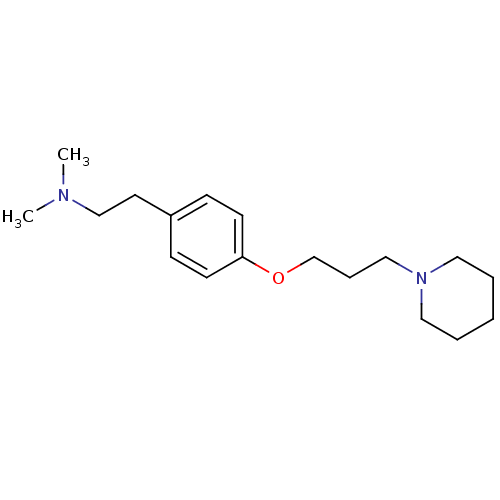
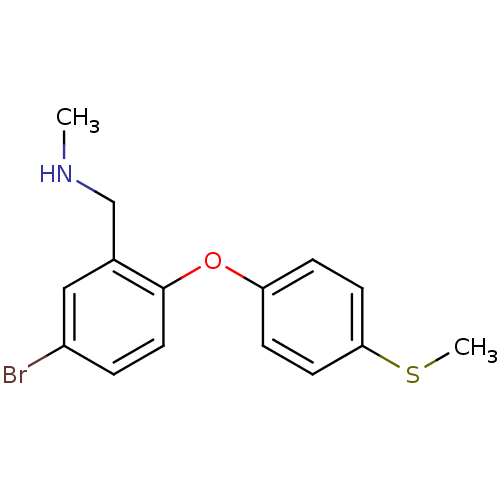
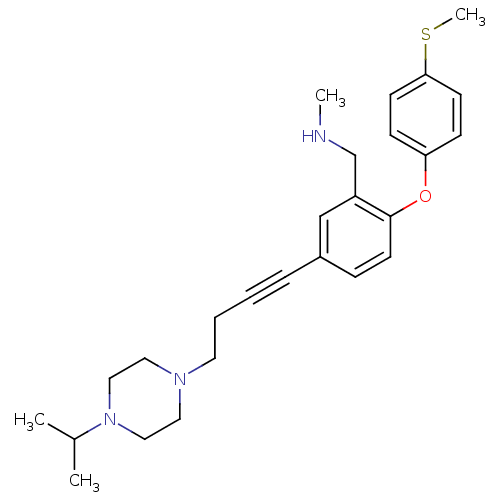
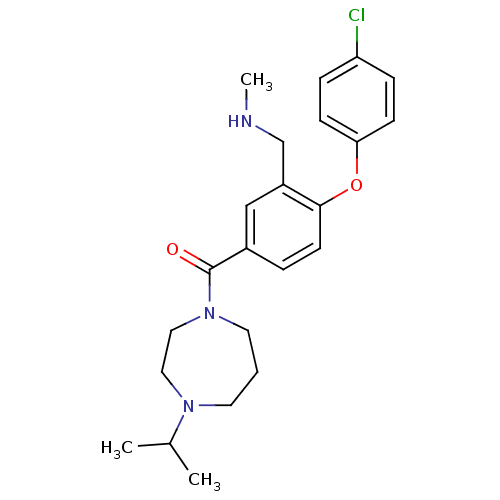
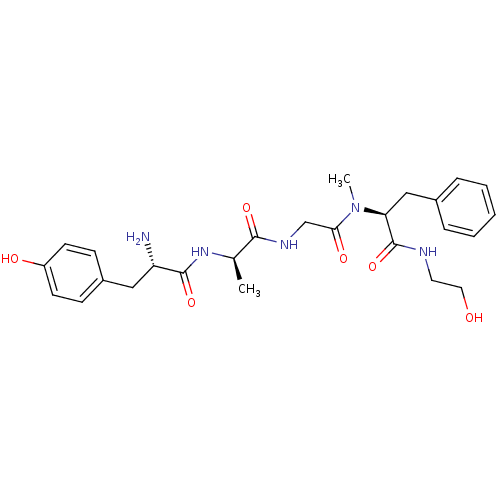
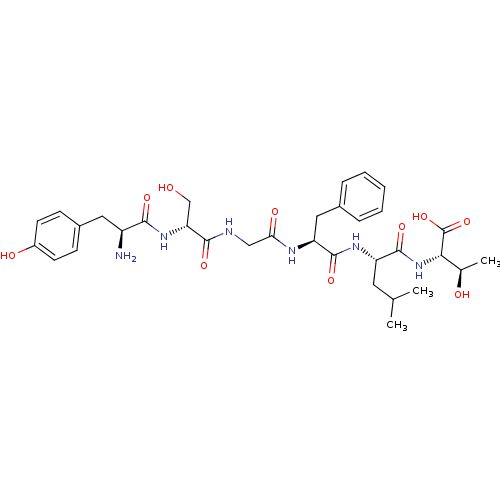
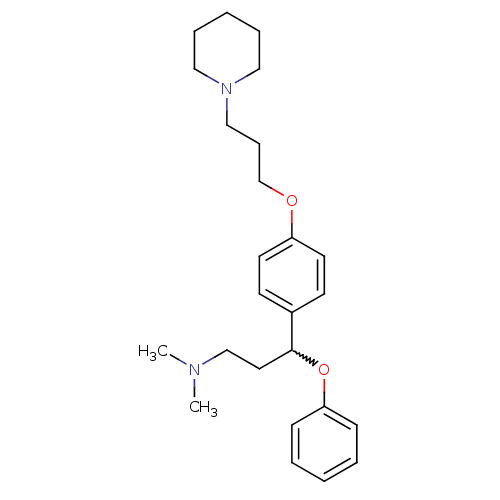
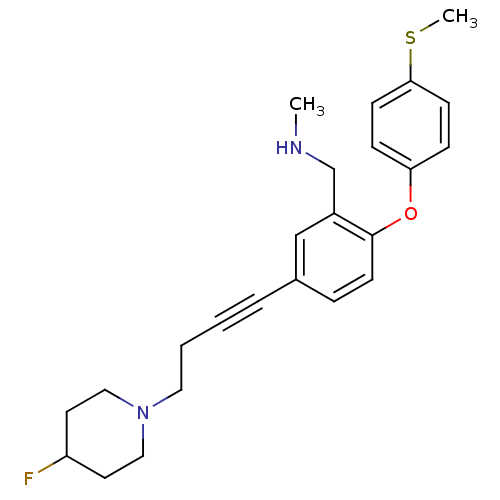
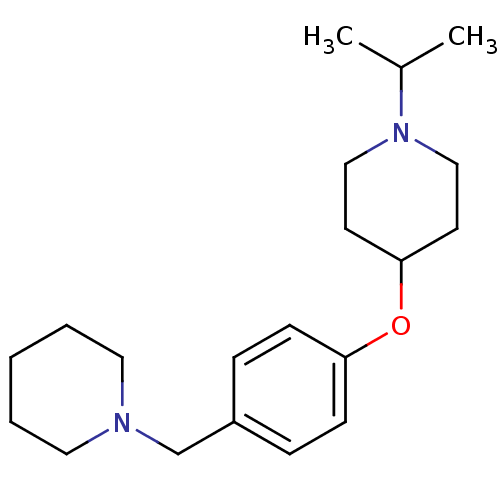
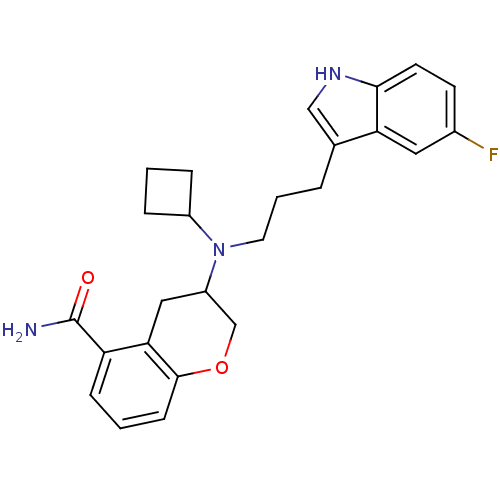
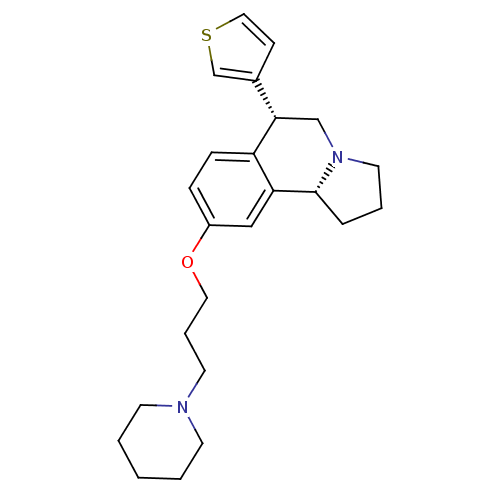
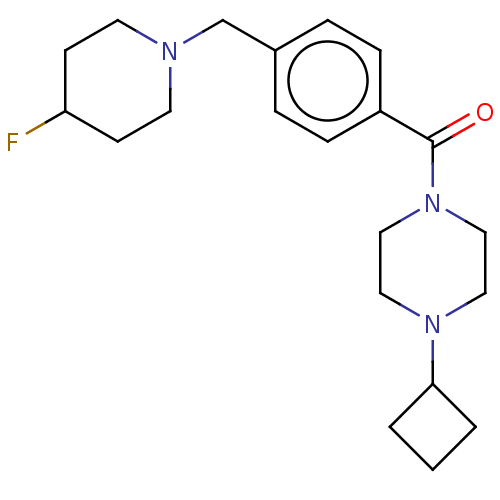
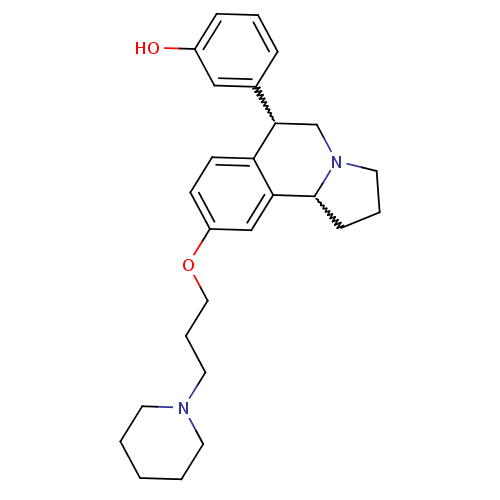
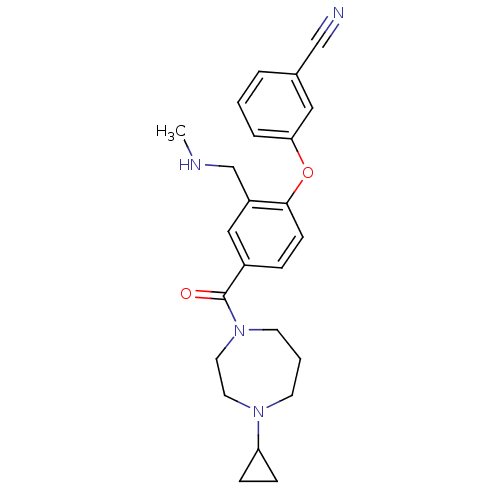
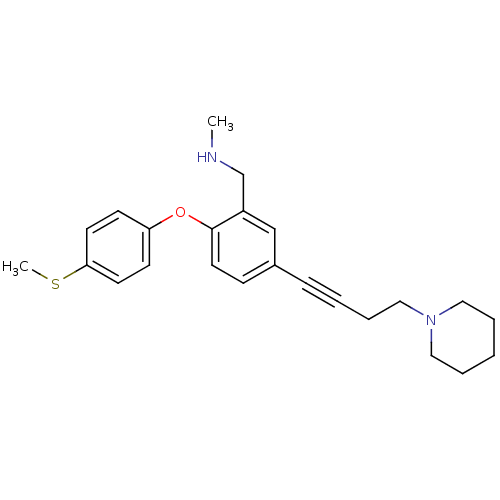
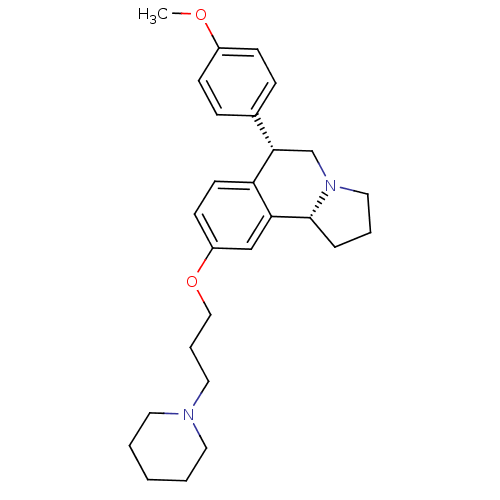
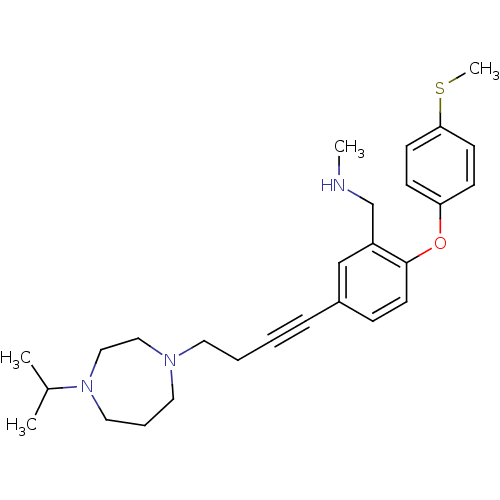
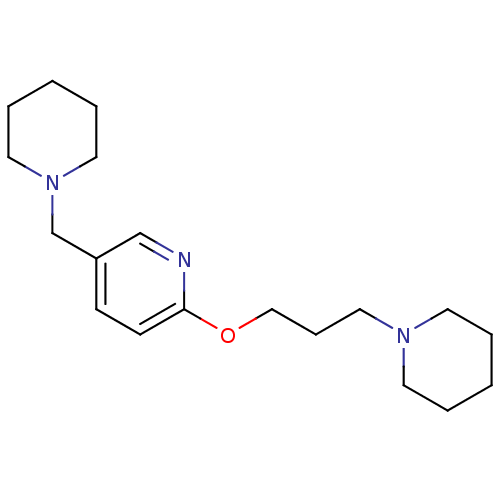
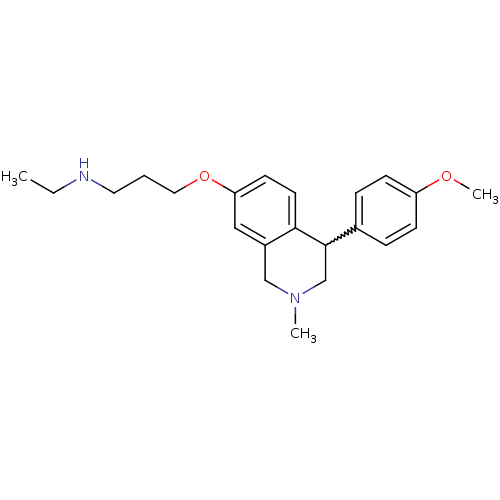
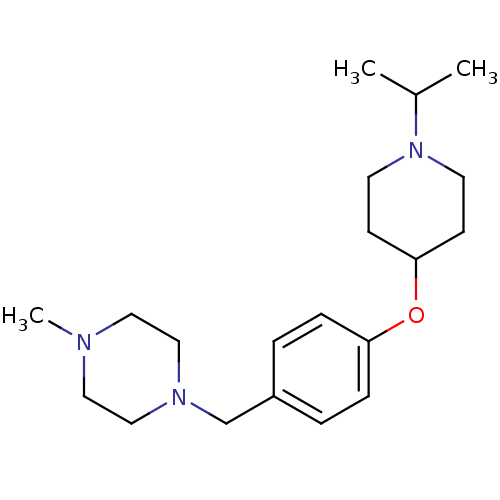
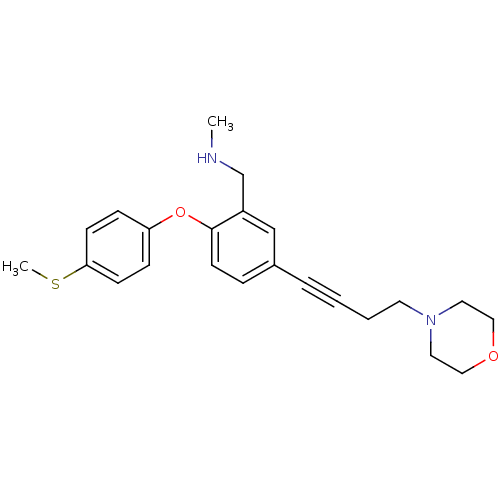
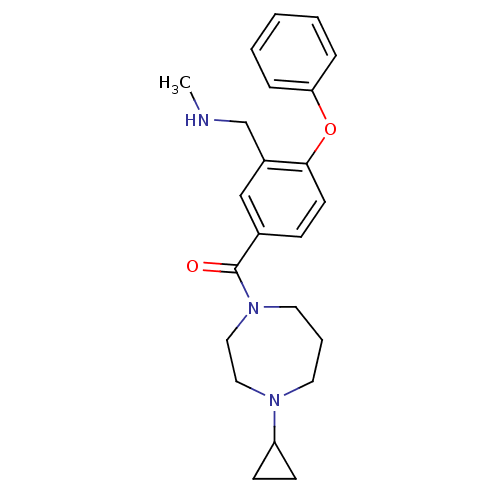
TargetDelta-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
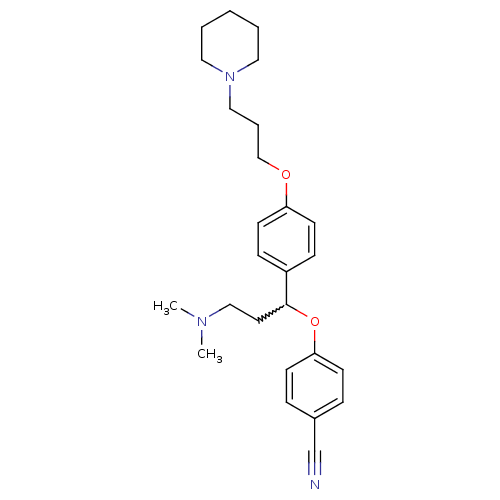
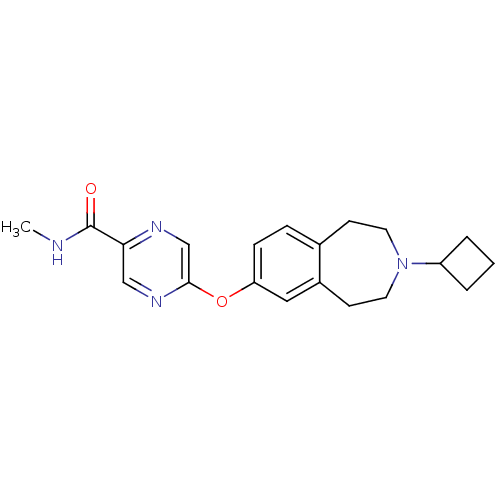
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Binding affinity at human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.210nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
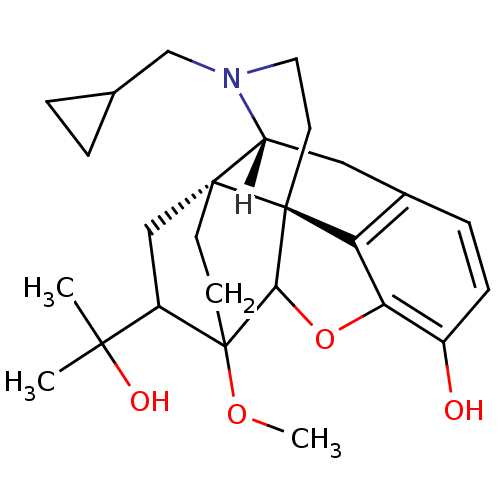
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.25nMAssay Description:Displacement of [3H]paroxetine from 5HT transporter in rat cortical membraneMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
TargetMu-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
TargetKappa-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.447nMAssay Description:Mean binding affinity for human H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.450nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
TargetDelta-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
TargetDelta-type opioid receptor(Homo sapiens (Human))
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Binding affinity at human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.631nMAssay Description:Mean binding affinity for human H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 minsMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity at human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity at human histamine H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Binding affinity at human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.720nMAssay Description:Binding affinity at human histamine H3More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 0.730nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.741nMAssay Description:Mean binding affinity for human H3 receptorMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Binding affinity at human histamine H3 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human histamine H3 receptorMore data for this Ligand-Target Pair