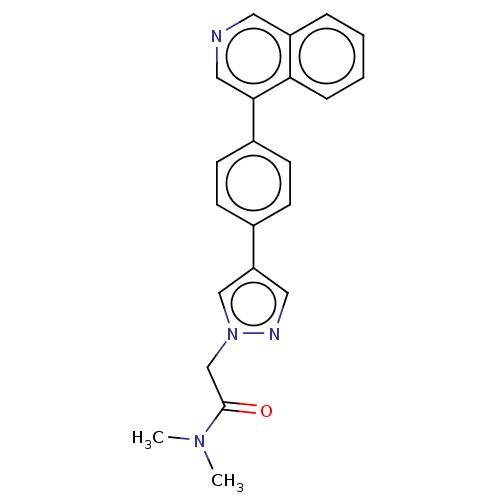
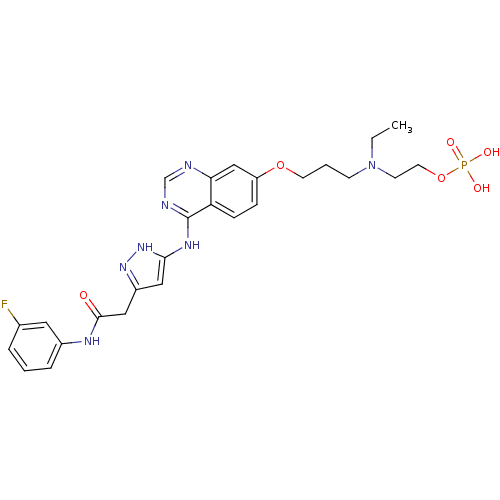
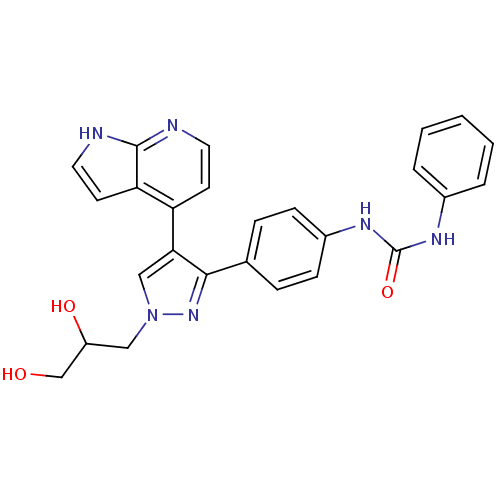
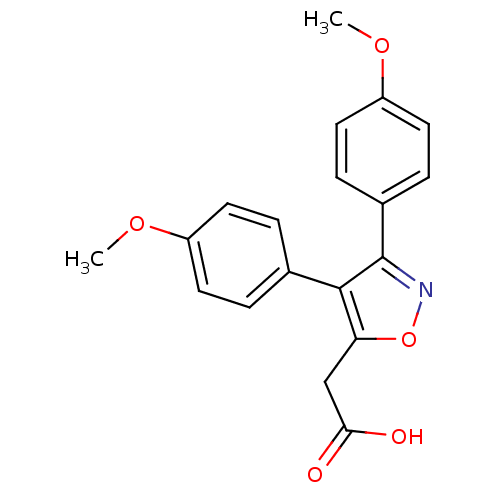
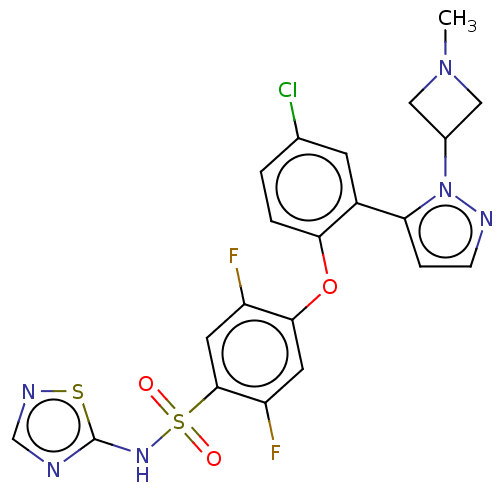
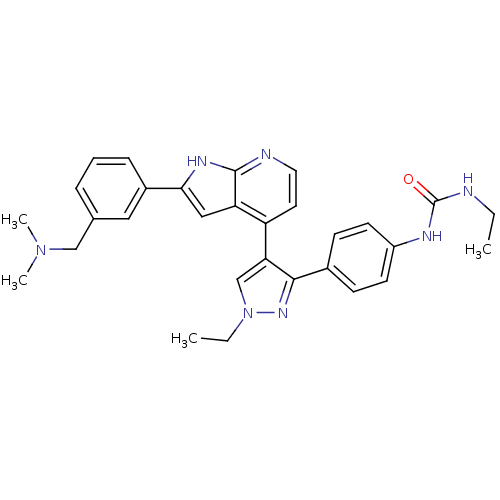
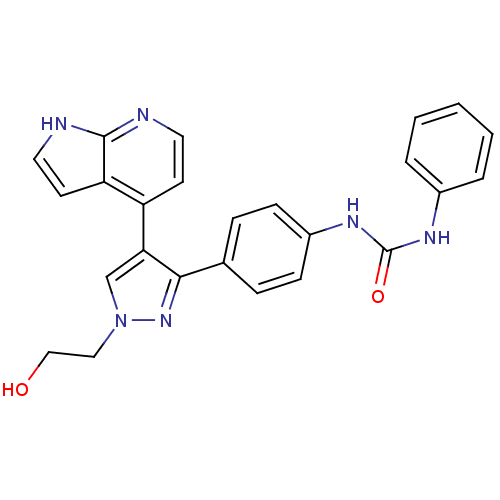
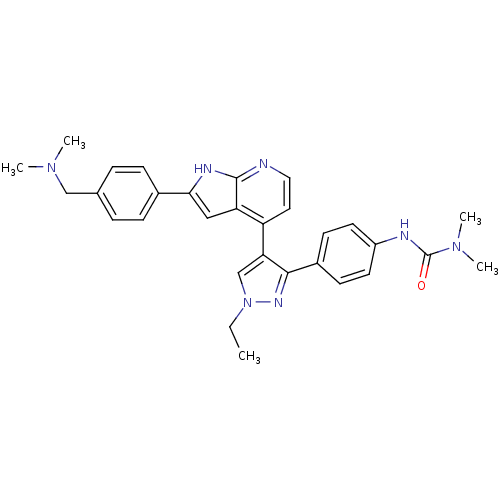
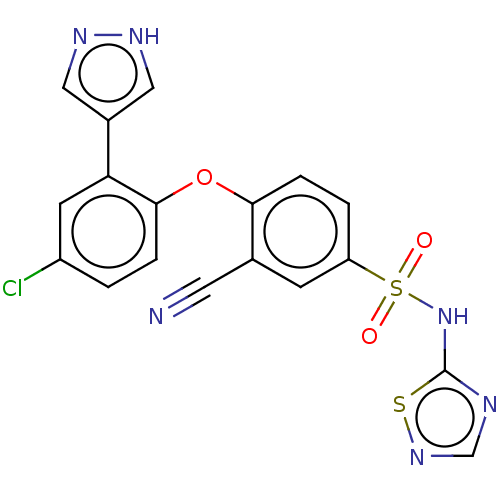
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
Affinity DataKi: 0.380nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Competitive inhibition of human Aurora B ATP binding site by rapid dilution methodMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
Affinity DataKi: 0.600nMAssay Description:Inhibition of Aurora AMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
Affinity DataKi: 1.5nMAssay Description:Competitive inhibition of human Aurora C ATP binding siteMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
Affinity DataKi: 1.80nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
Affinity DataKi: 4.60nMAssay Description:Competitive inhibition of Aurora C ATP binding siteMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
Affinity DataKi: 490nMAssay Description:Competitive inhibition of human Aurora A ATP binding siteMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Banyu Pharmaceutical
Curated by PDSP Ki Database
Banyu Pharmaceutical
Curated by PDSP Ki Database
Affinity DataKi: 2.00E+3nMAssay Description:Noncompetitive inhibition of P300 (unknown origin) using acetyl CoA as substrate after 15 mins by double reciprocal plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 9.40E+3nMAssay Description:Noncompetitive inhibition of P300 (unknown origin) using biotinylated H3 as substrate after 15 mins by double reciprocal plot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of CDK8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of CDK8/CyclinC (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Okayama University Graduate School Of Medicine
Curated by ChEMBL
Okayama University Graduate School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of Vitronectin binding to GPIIb/IIIIa Vitronectin receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho...More data for this Ligand-Target Pair

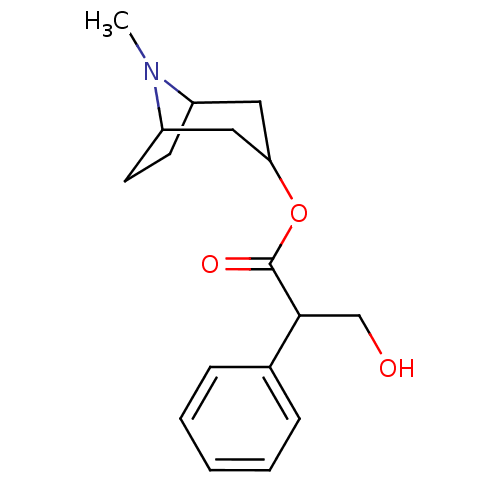
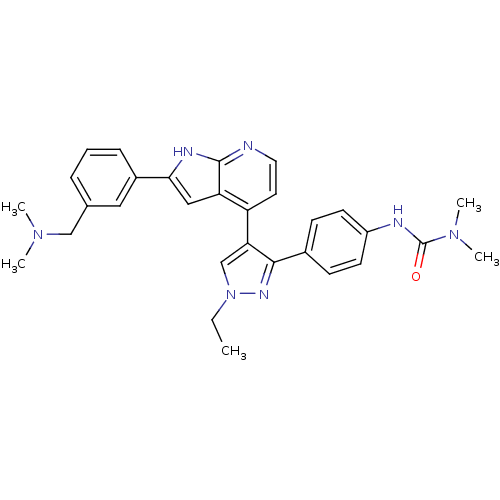
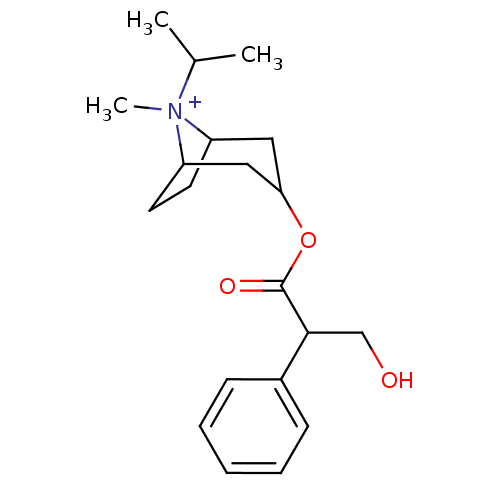
 3D Structure (crystal)
3D Structure (crystal)