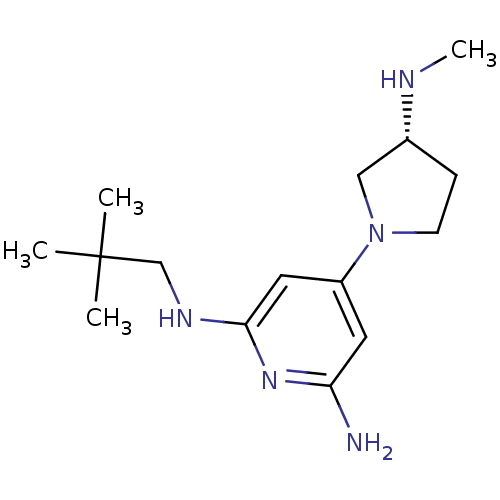
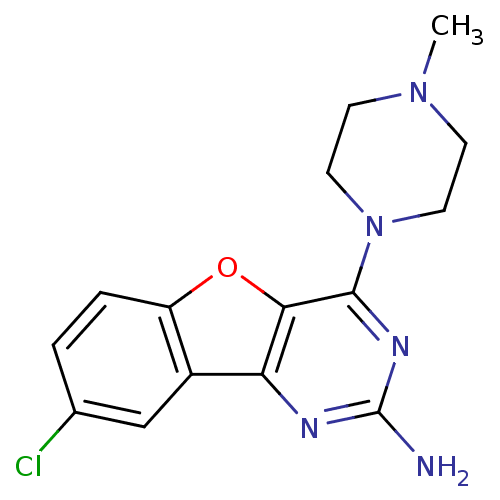
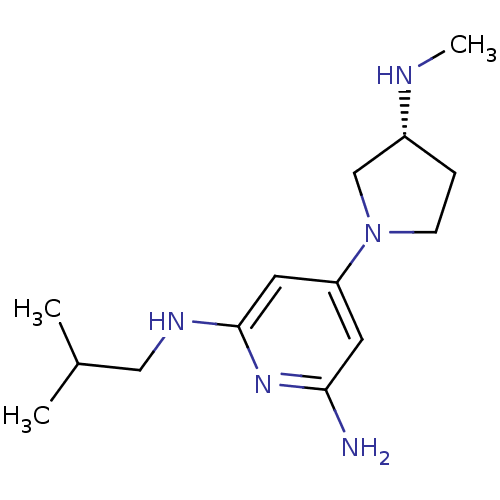
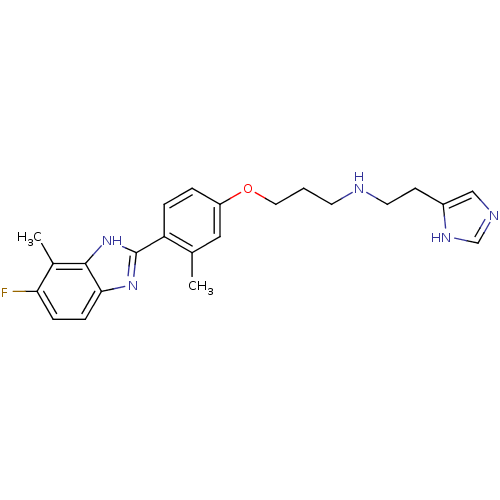
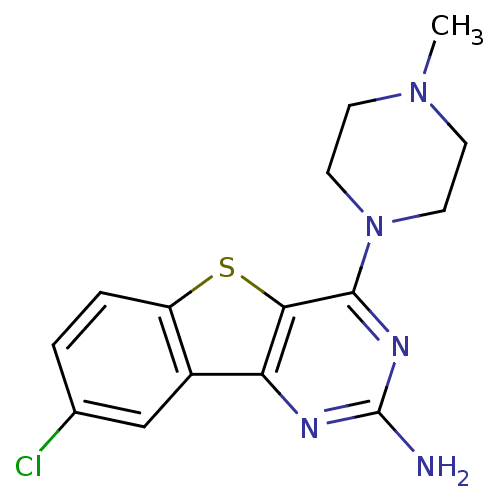
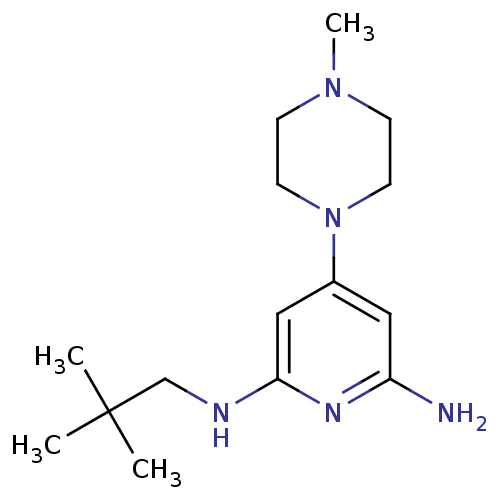
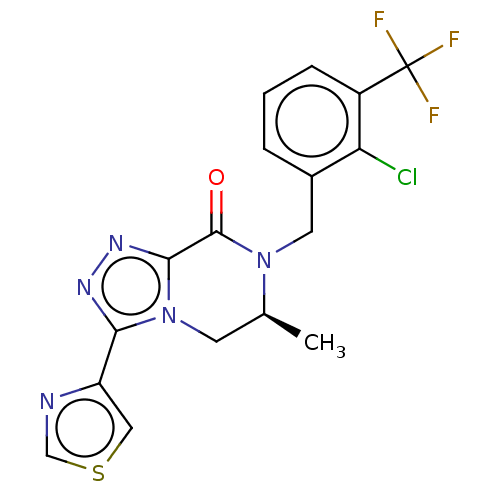
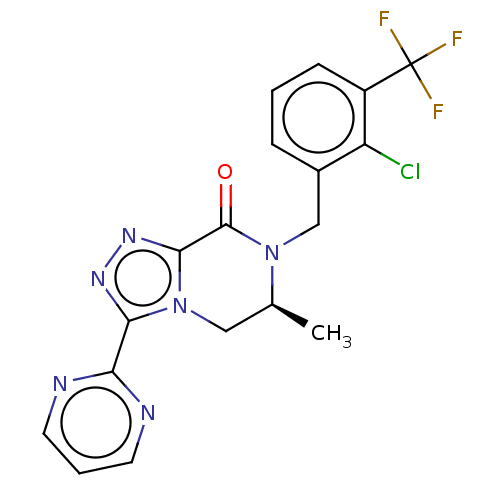
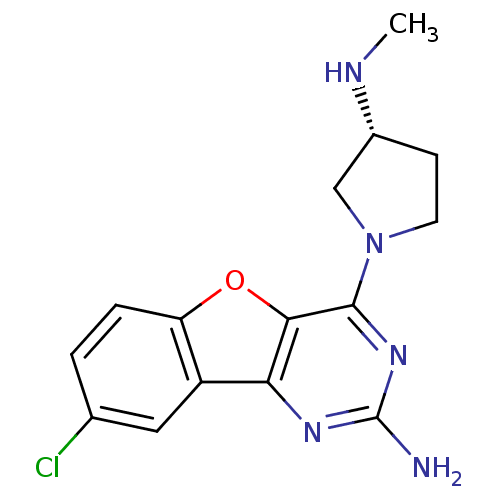
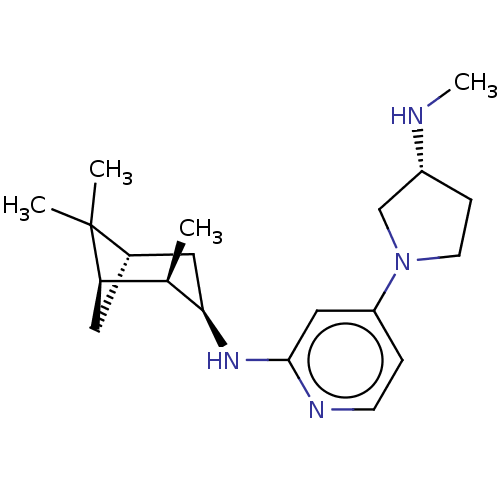
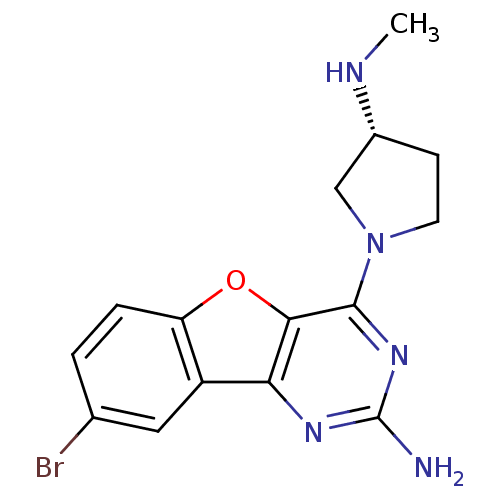
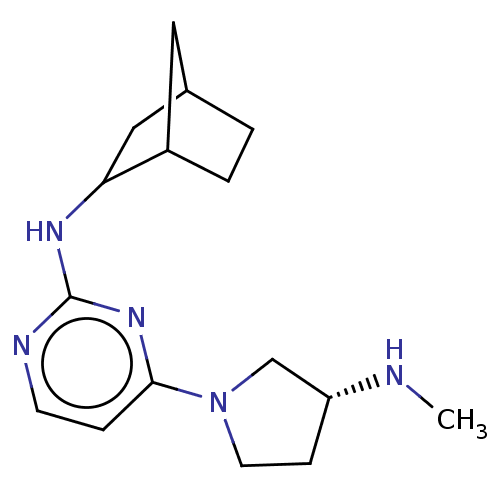
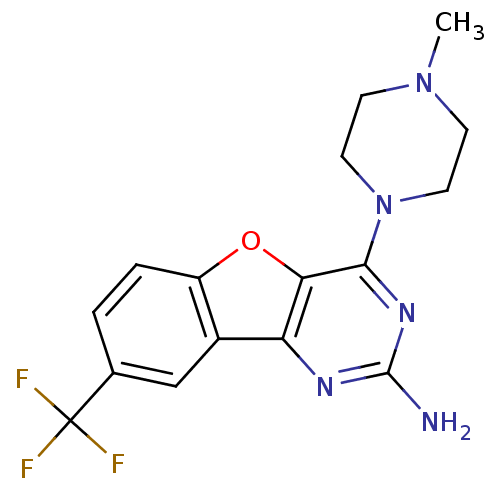
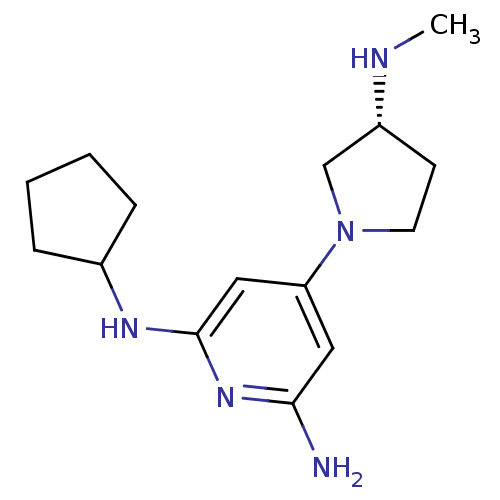
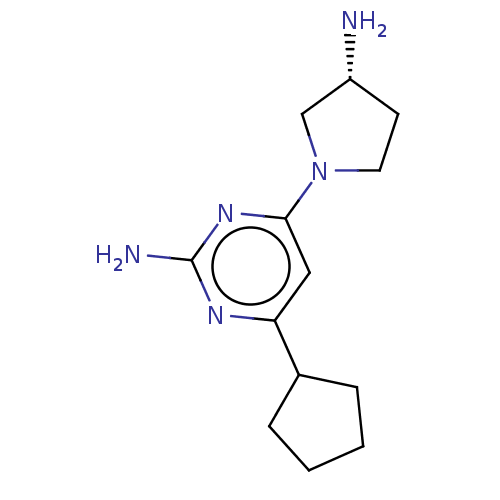
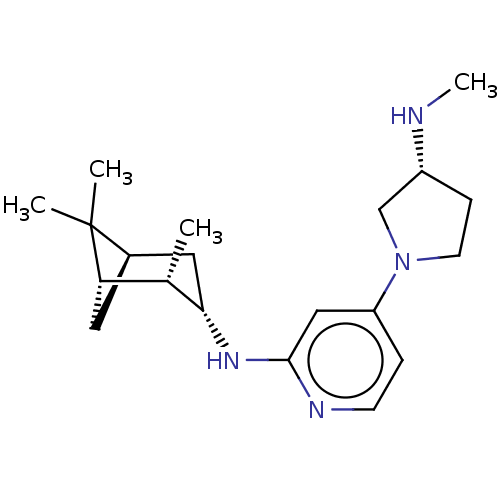
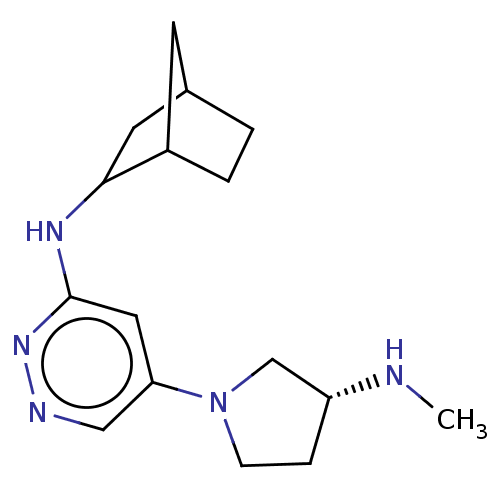
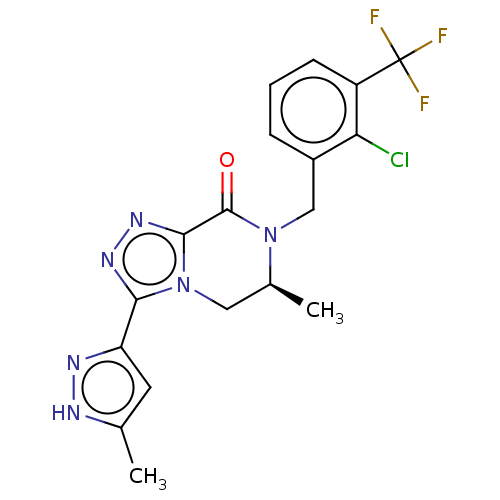
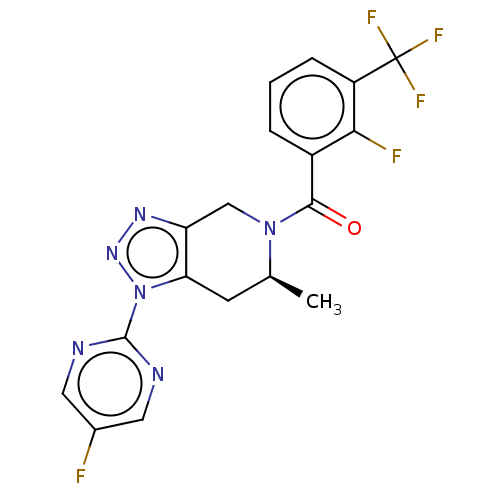
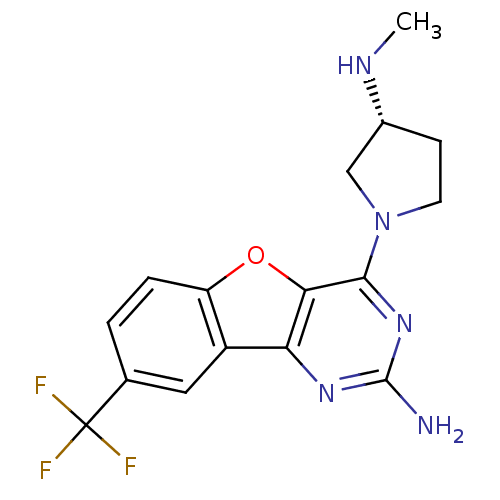
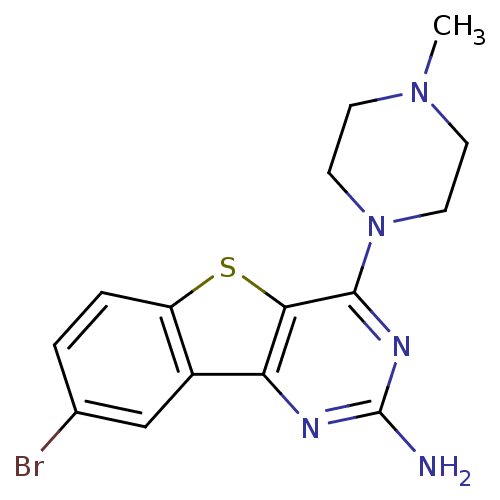
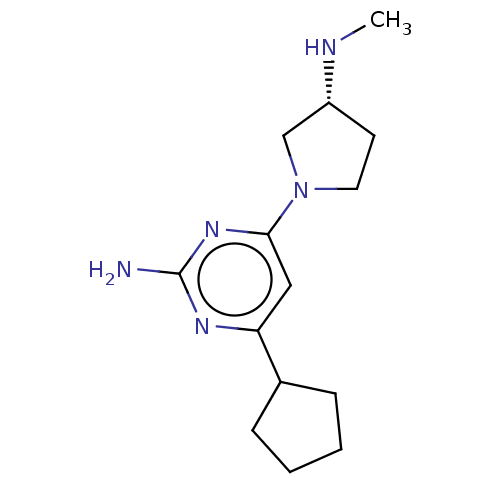
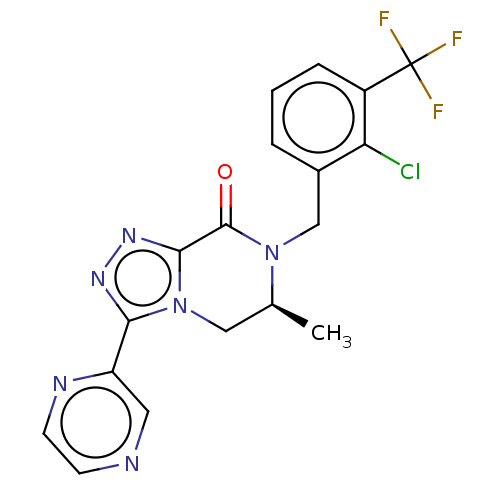
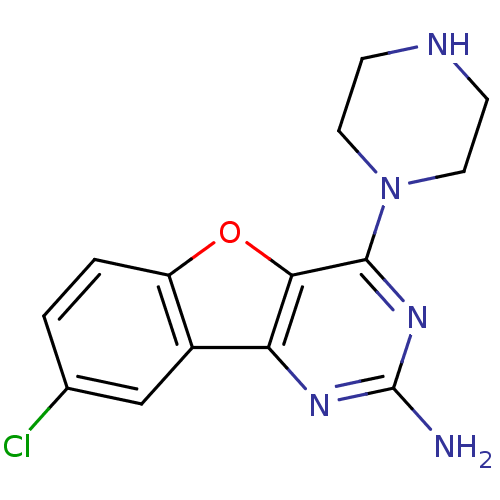
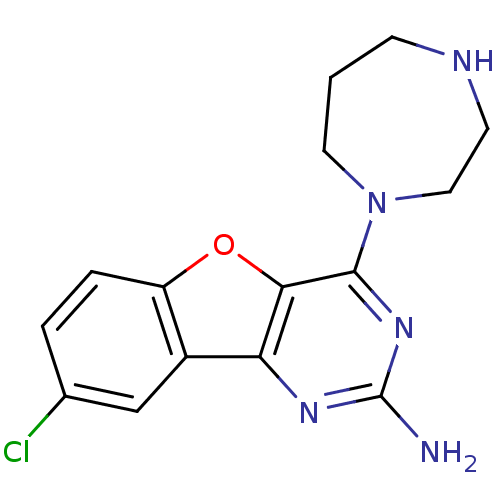
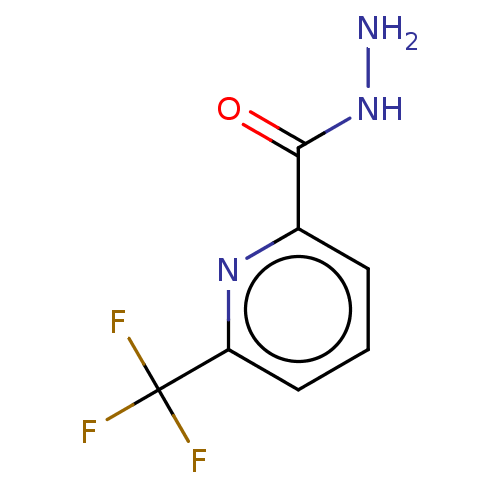
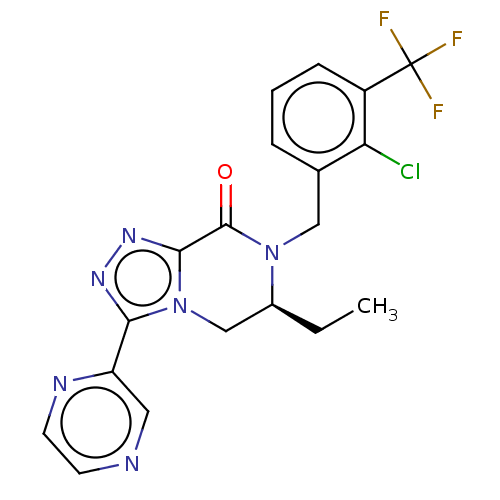
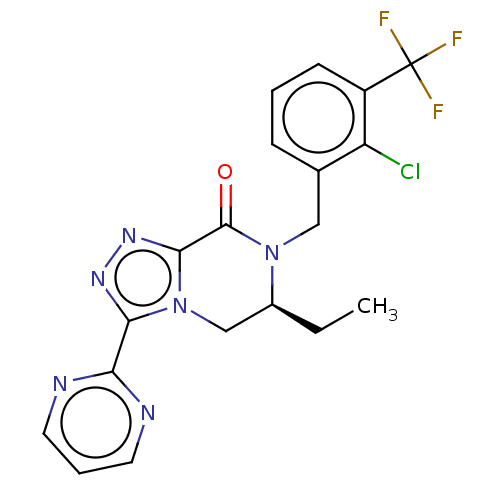
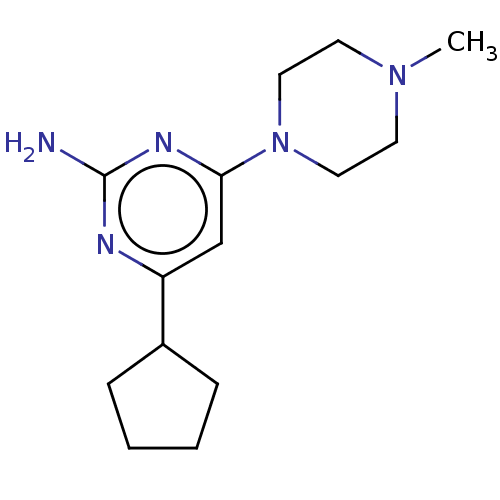
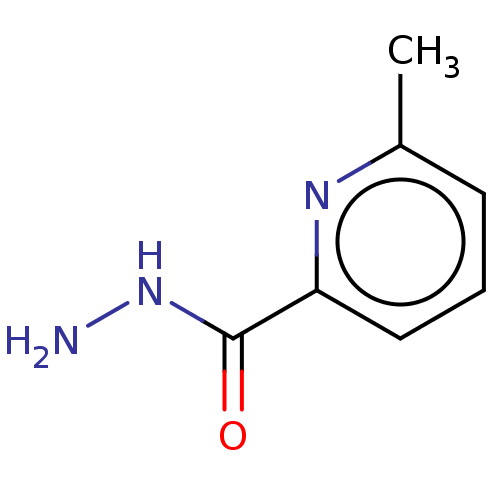
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
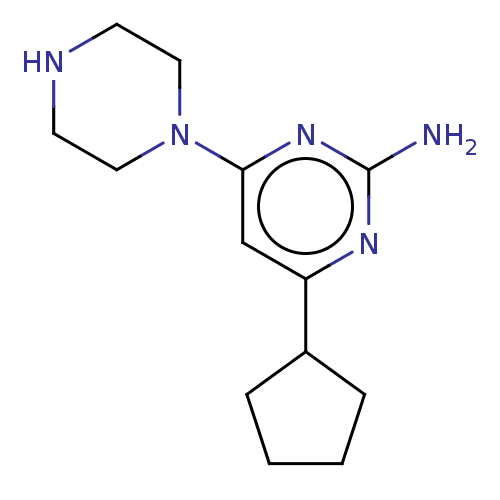
Affinity DataKi: 0.0400nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
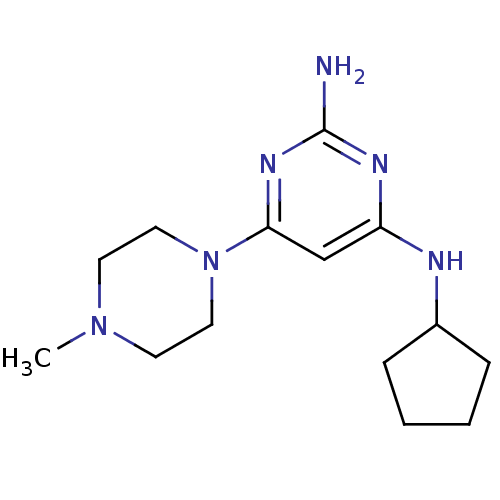
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
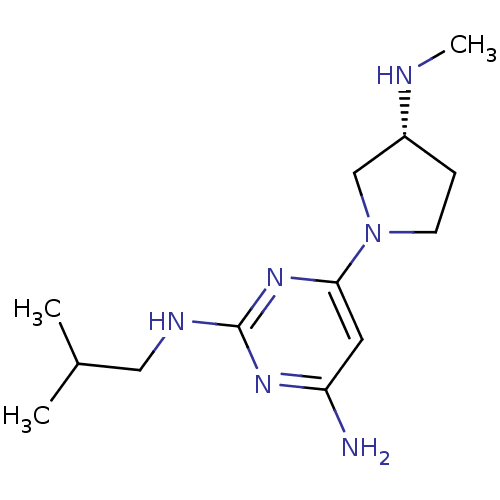
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
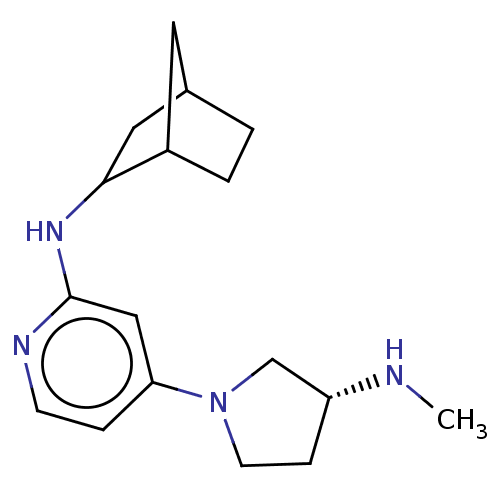
Affinity DataKi: 0.210nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.920nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]histamine from human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Human or rat P2X7-1321 N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Radioligand binding: human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparation...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @ −80° C. On the day of the experiment, cell membrane preparations were made according...More data for this Ligand-Target Pair
TargetHistamine H4 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:human or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according ...More data for this Ligand-Target Pair