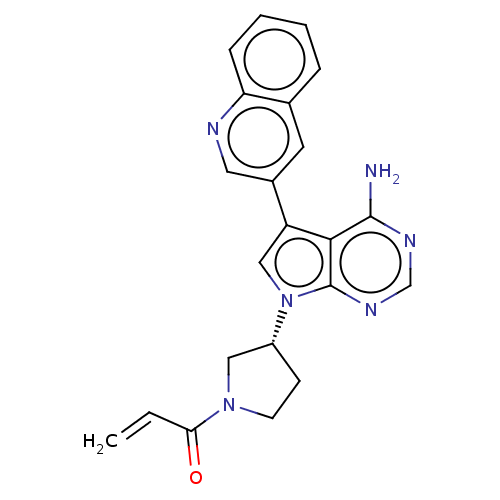
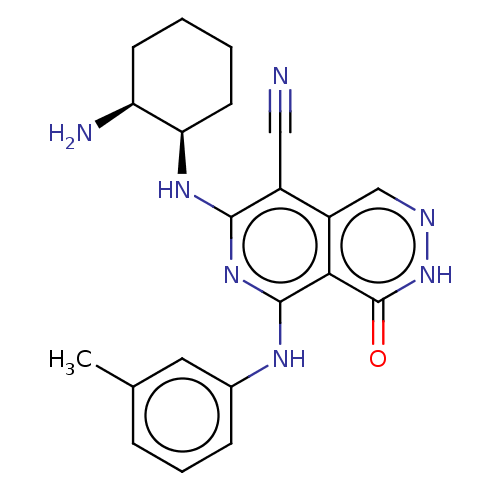
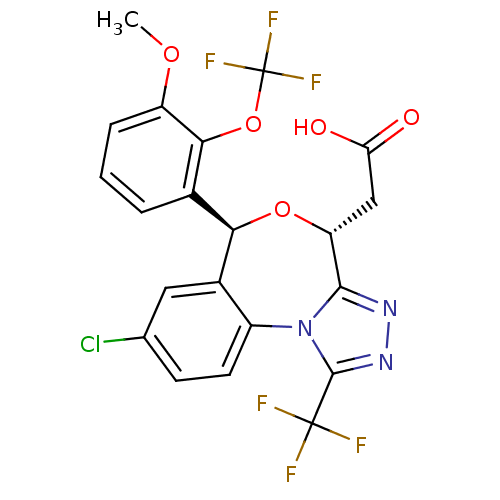
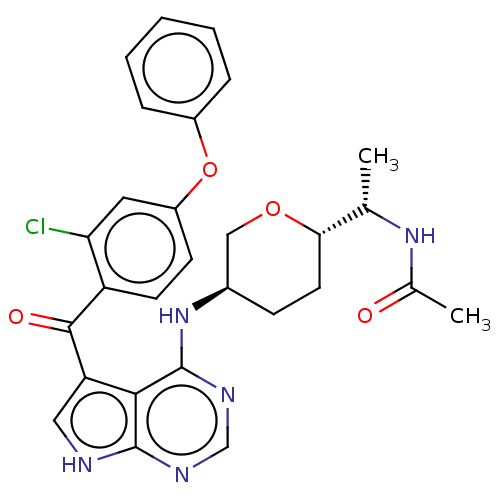
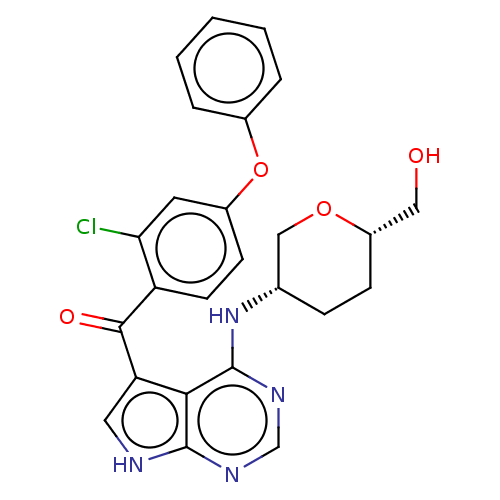
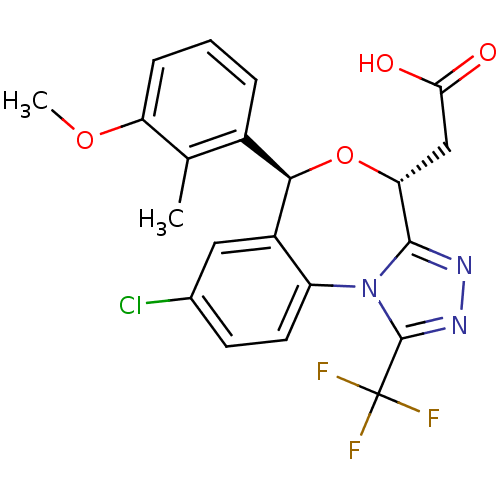
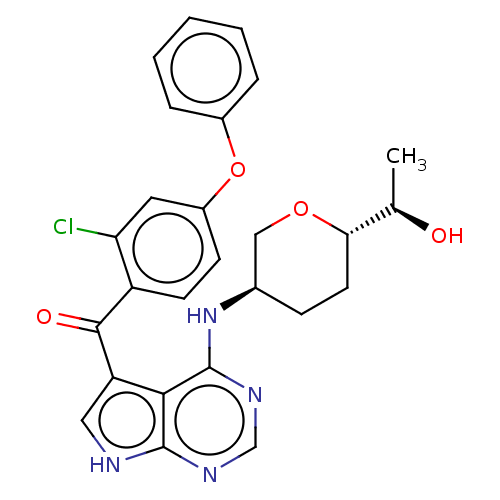
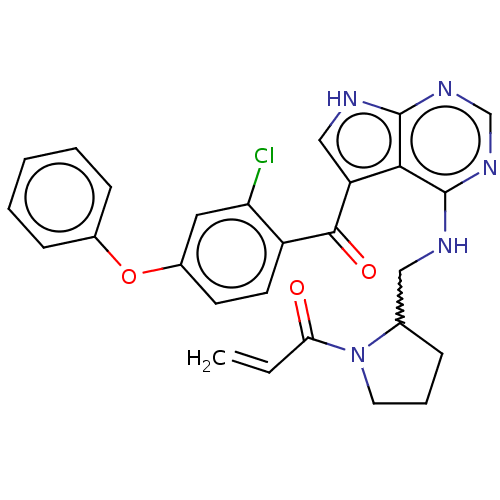
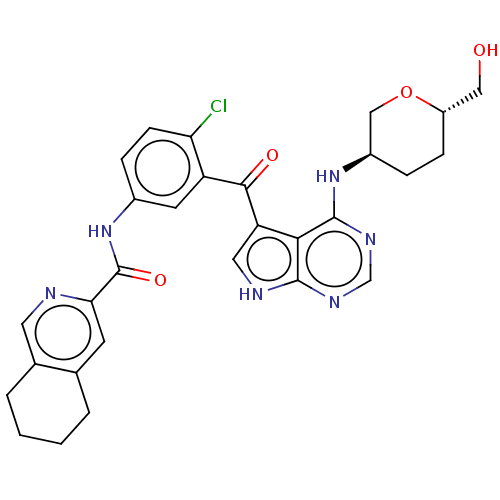
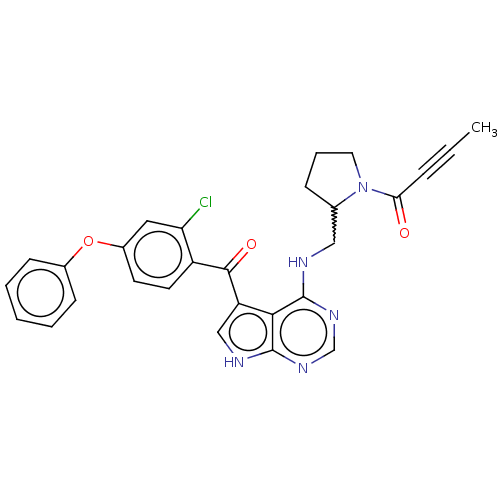
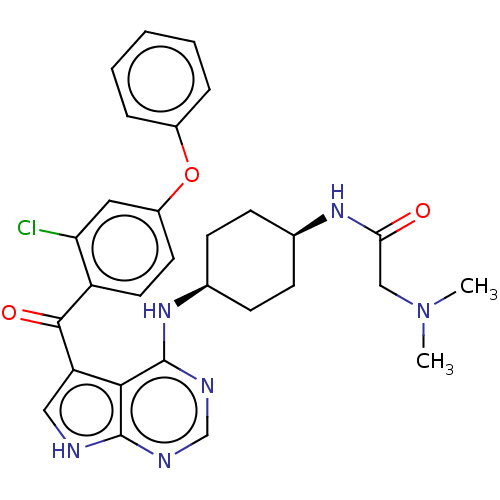
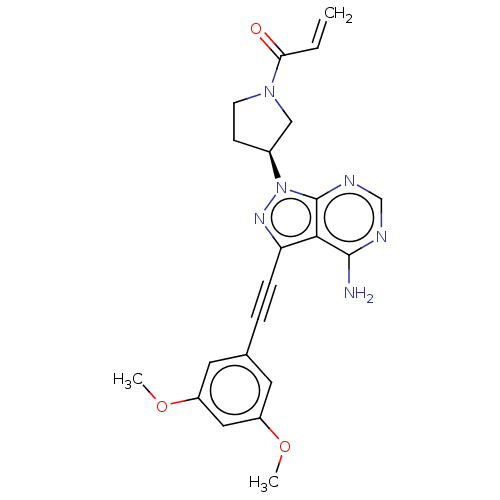
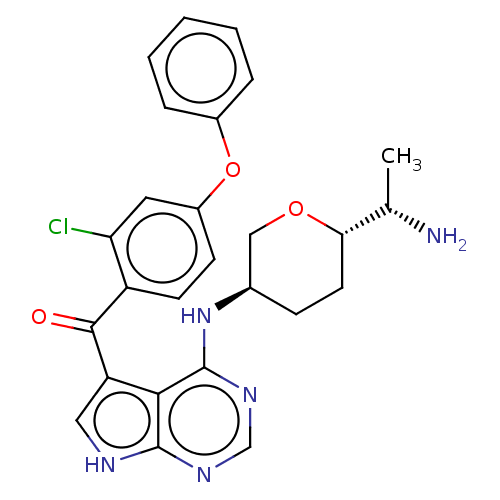
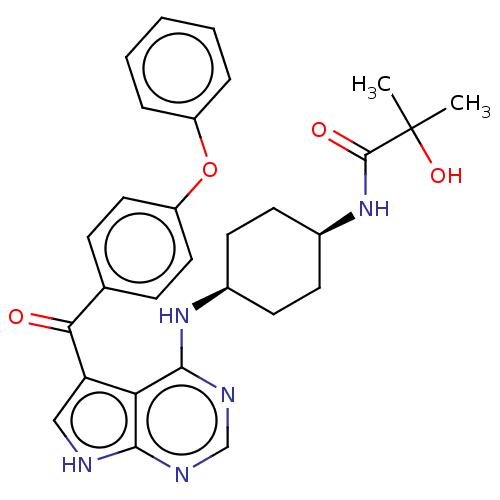
Affinity DataKi: 0.470nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.40nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 58nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 280nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMAssay Description:Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human EGFR del19/T790M using biotinEEPLYWSFPAKKK-NH2 as substrate incubated for 120 mins by TR-FRET assayMore data for this Ligand-Target Pair
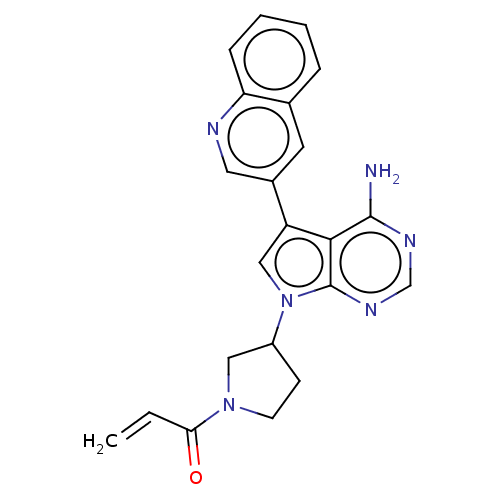
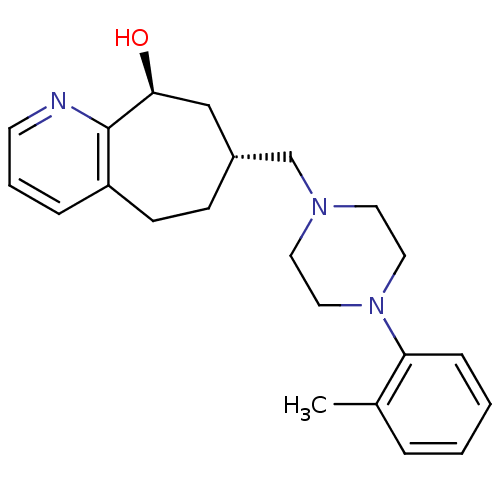
Ligand Info
Affinity DataIC50: 0.490nMAssay Description:Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide...More data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMAssay Description:Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMAssay Description:Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea...More data for this Ligand-Target Pair
Affinity DataIC50: 0.790nMAssay Description:Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.930nMAssay Description:Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea...More data for this Ligand-Target Pair
Affinity DataIC50: 0.980nMAssay Description:Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki...More data for this Ligand-Target Pair
Affinity DataIC50: 0.980nMAssay Description:Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki...More data for this Ligand-Target Pair
Affinity DataIC50: 0.980nMAssay Description:Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki...More data for this Ligand-Target Pair
Affinity DataIC50: 0.980nMAssay Description:The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant cytoplasmic domain FGFR2 (8 to 134 residues) incubated for 120 mins by mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.07nMAssay Description:Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of recombinant human EGFR del19/T790M using biotinEEPLYWSFPAKKK-NH2 as substrate incubated for 120 mins by TR-FRET assayMore data for this Ligand-Target Pair
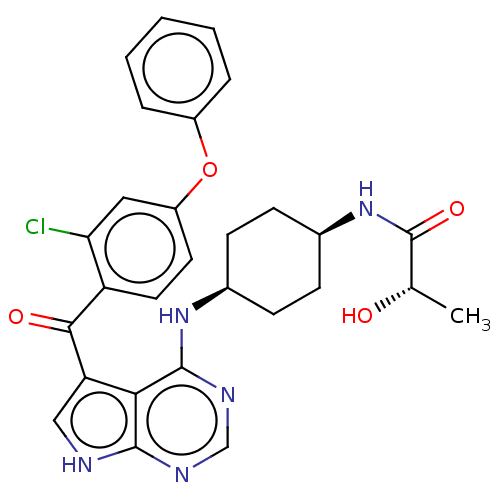
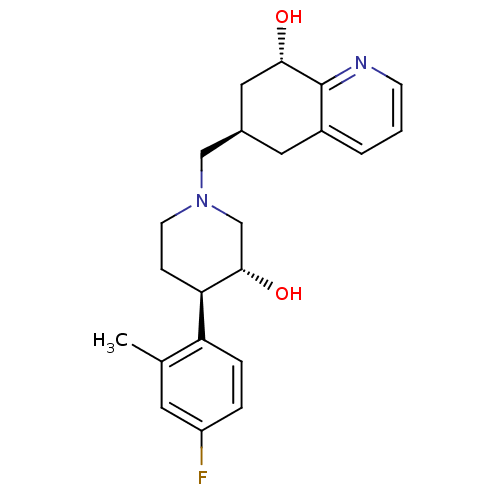
Ligand Info
Affinity DataIC50: 1.20nMAssay Description:The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a...More data for this Ligand-Target Pair

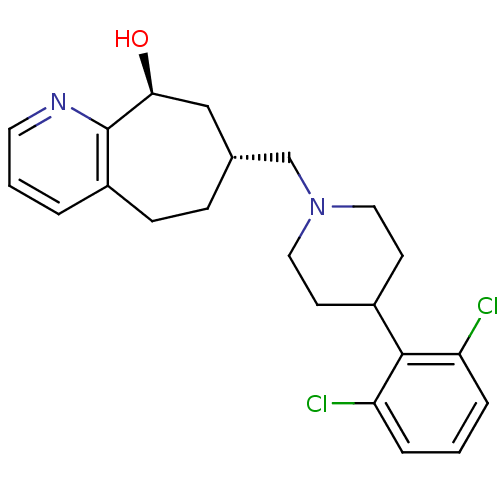
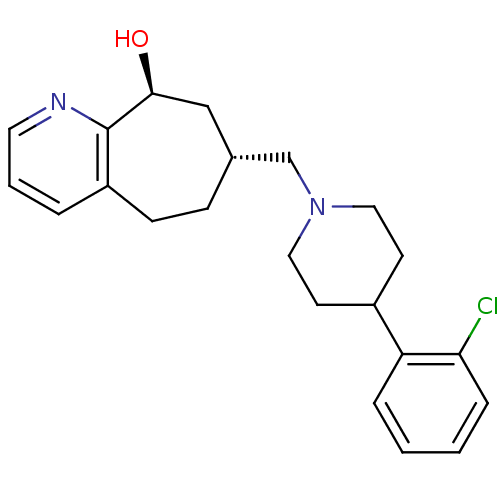
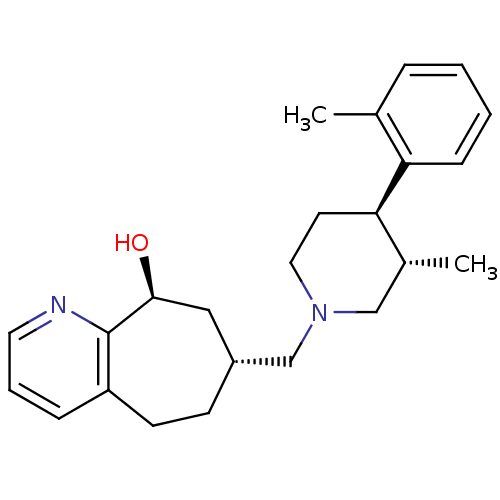
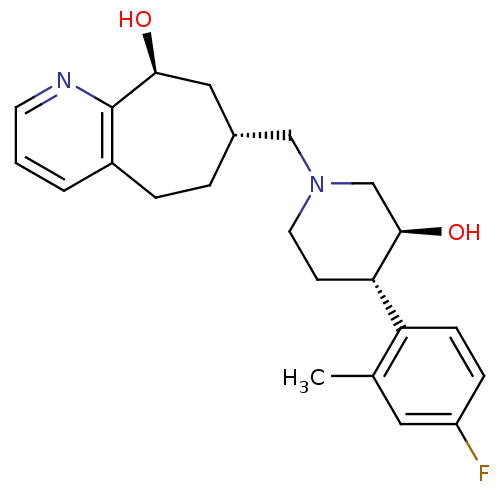
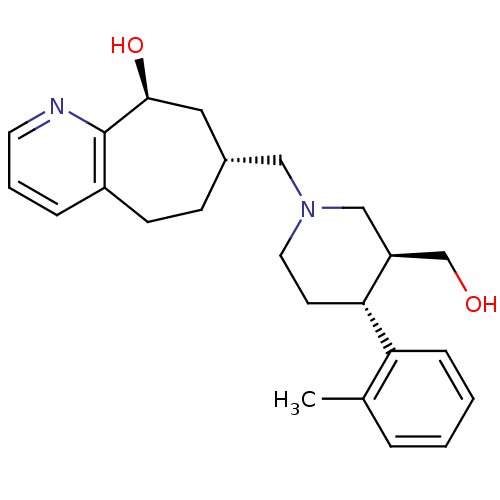

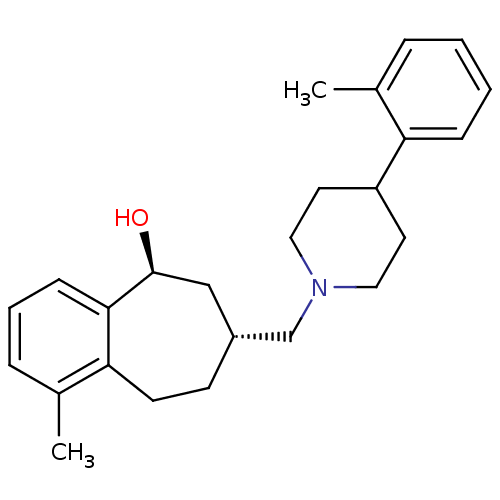
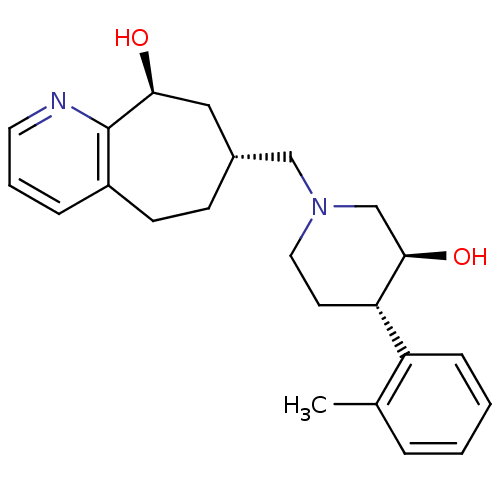
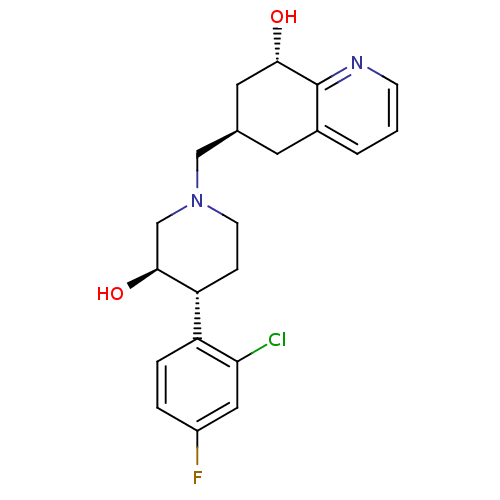
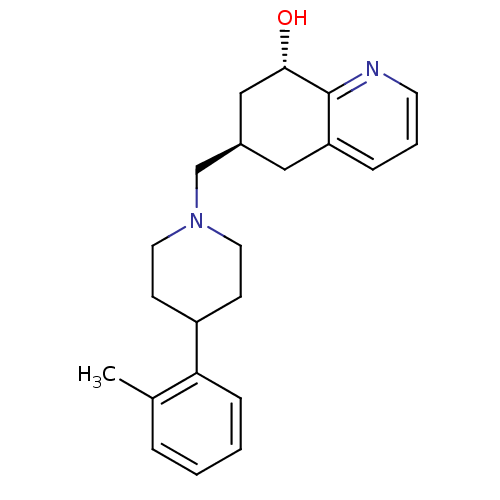
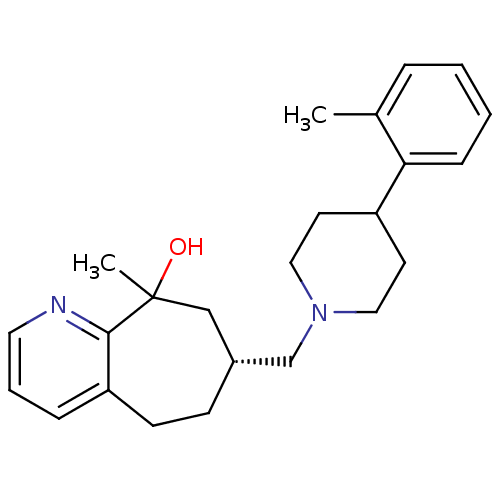
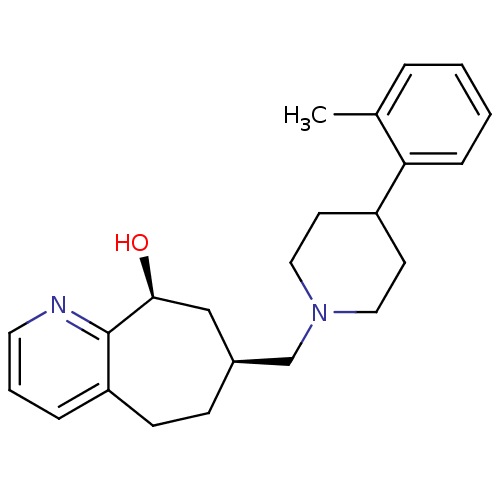
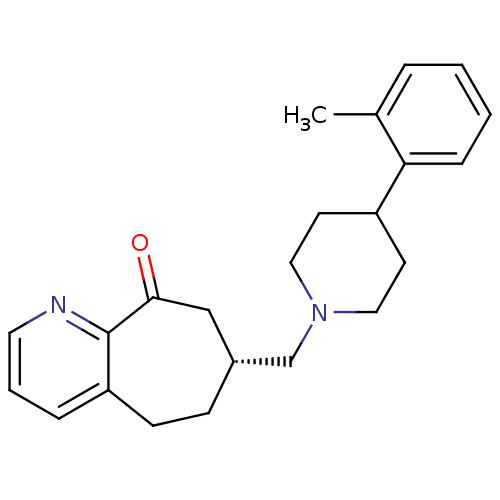
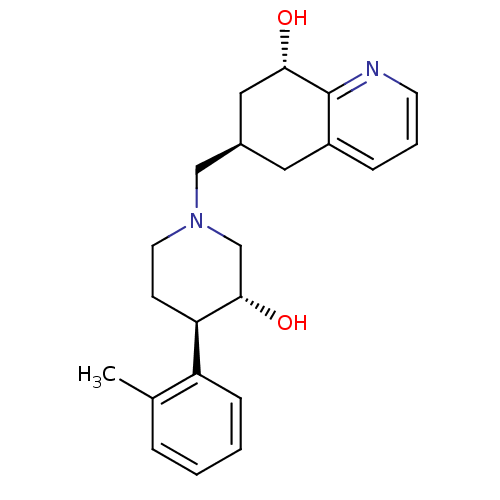
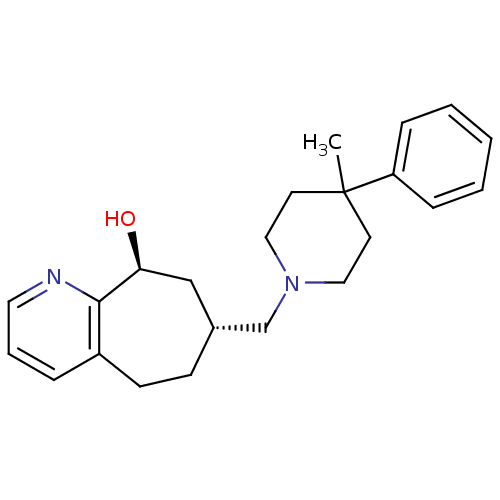
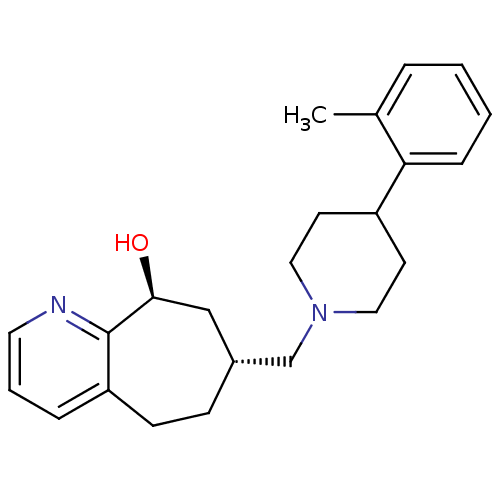

 3D Structure (crystal)
3D Structure (crystal)