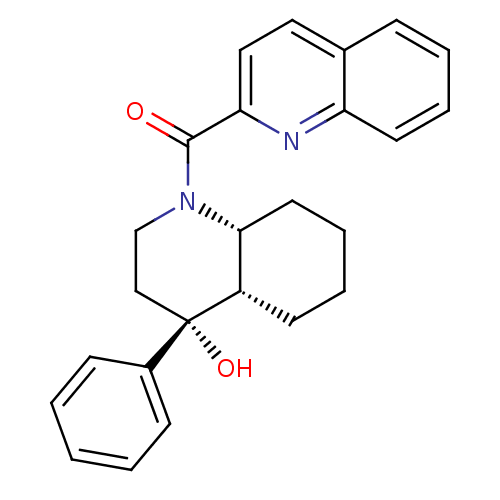
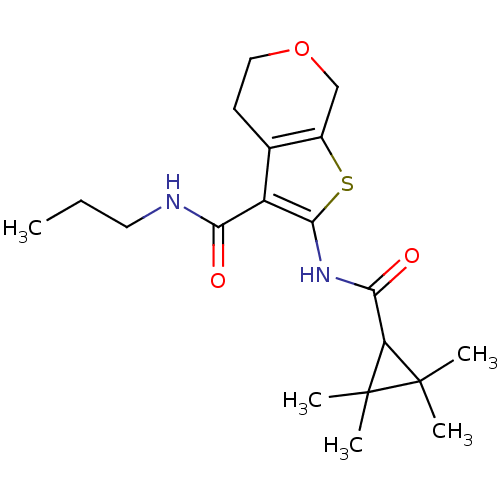
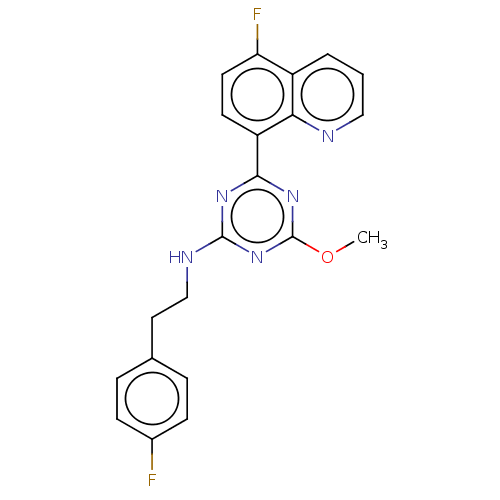
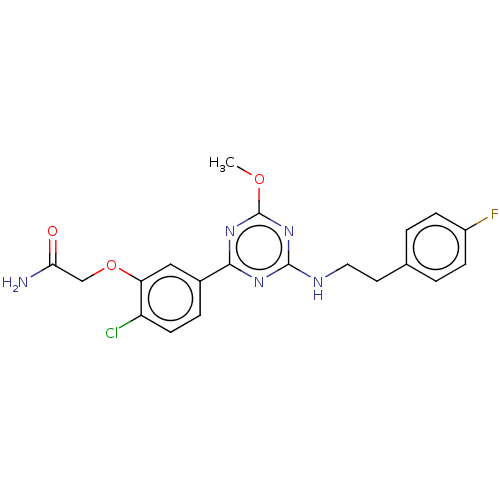
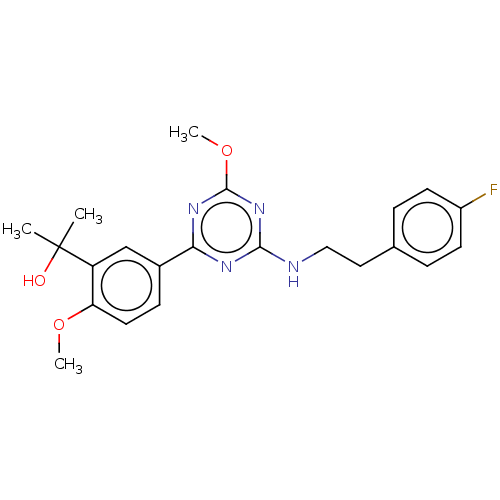
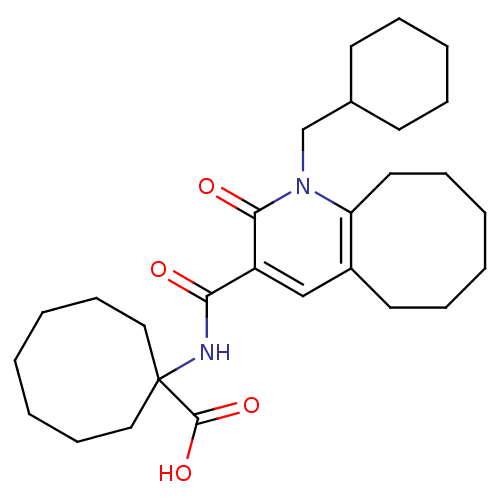
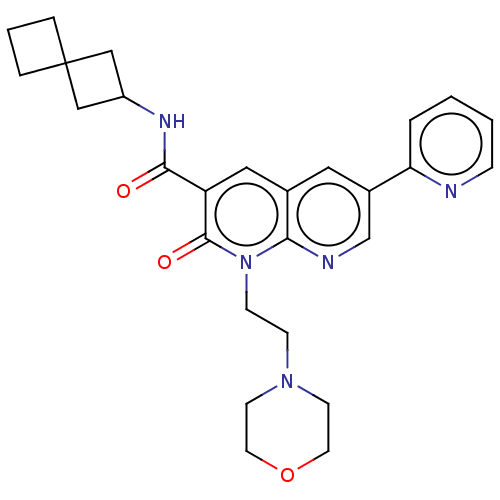
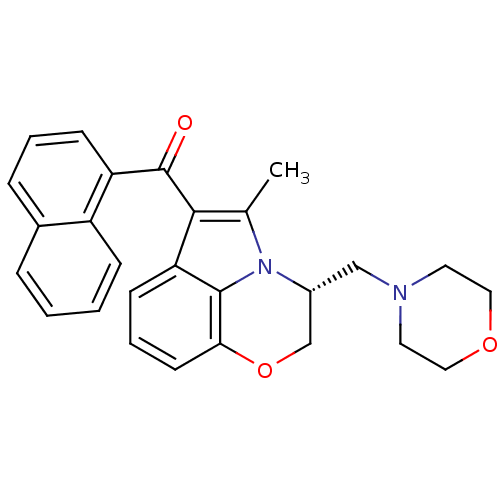
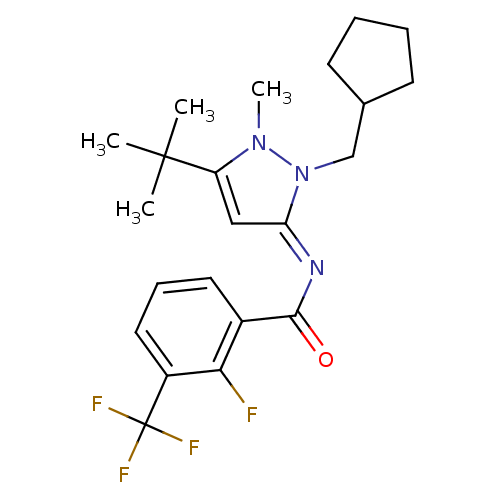
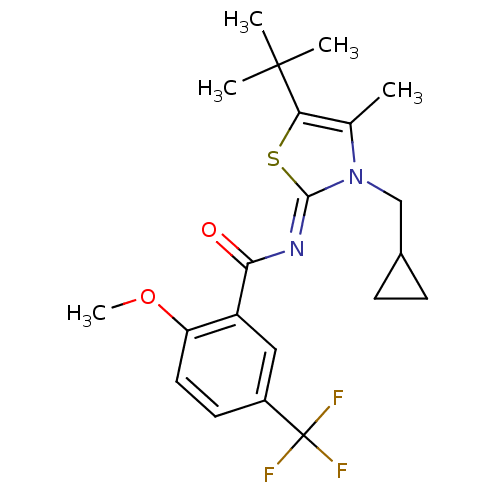
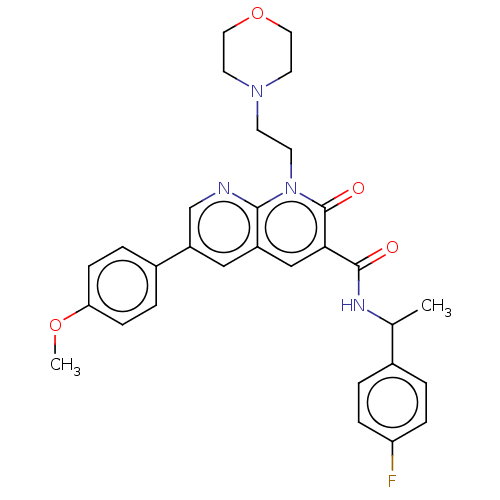
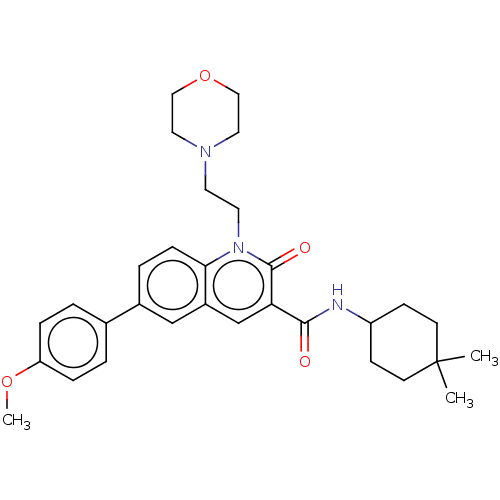
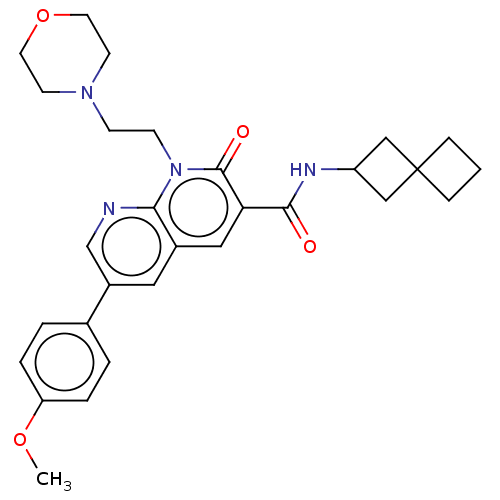
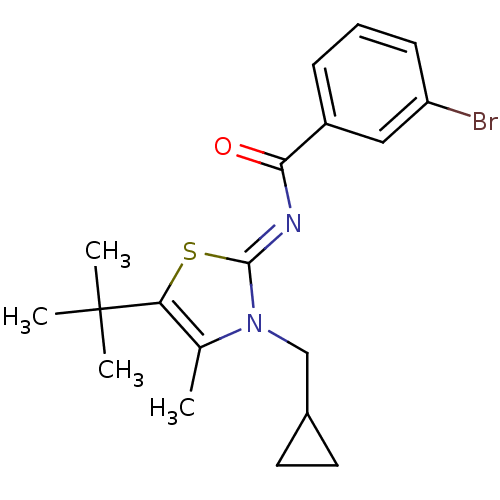
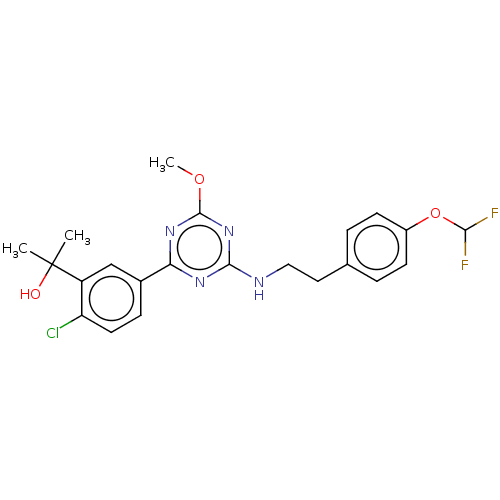
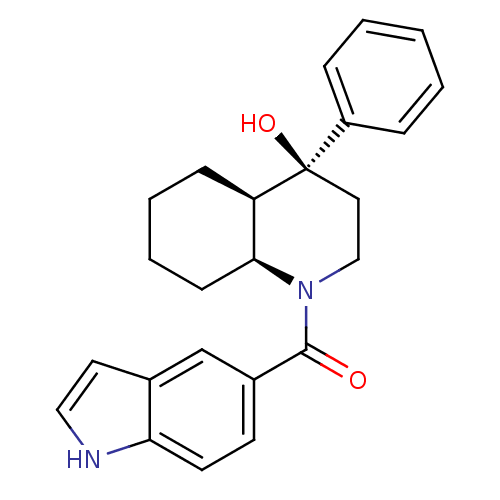
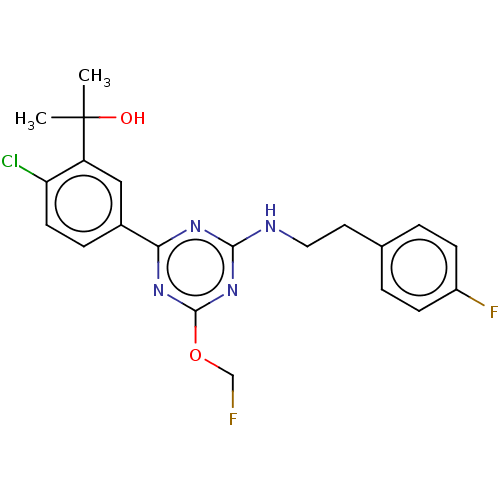
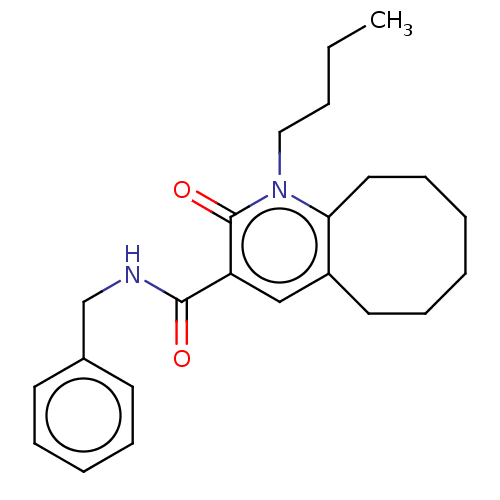
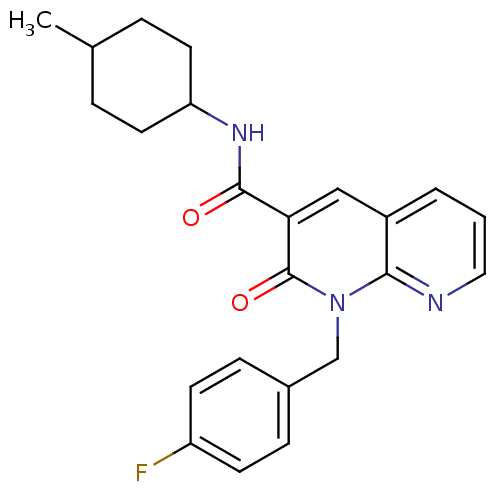
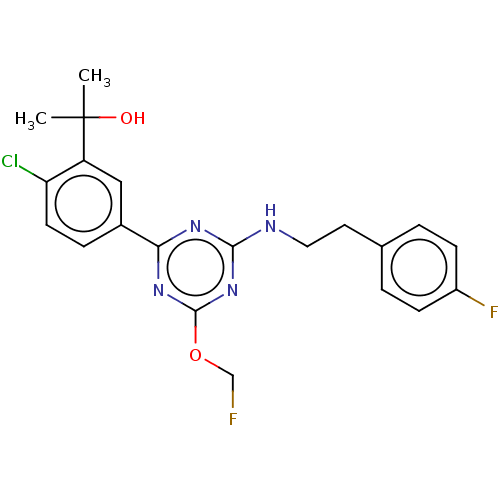
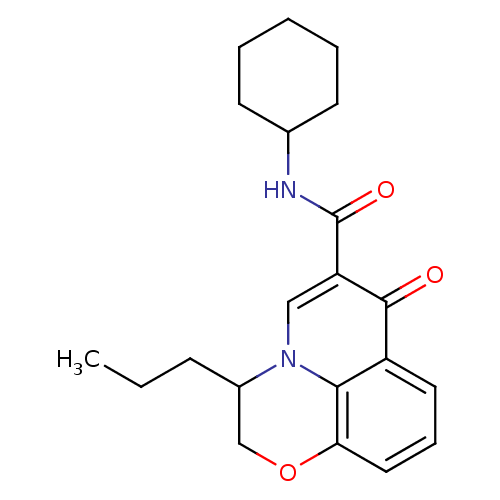
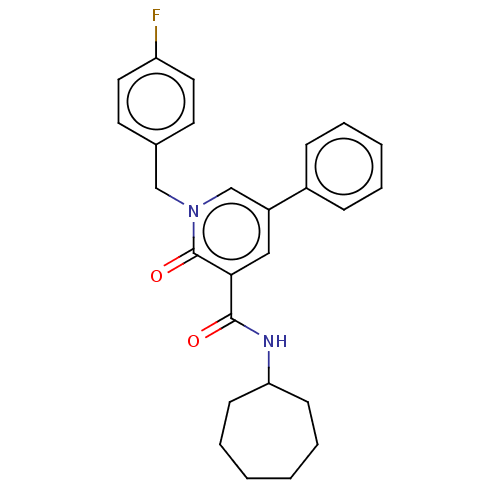
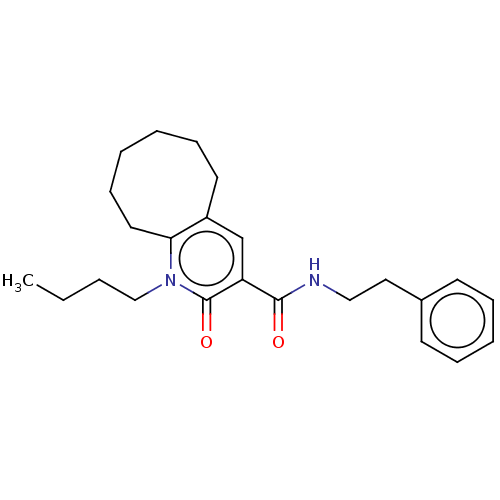
Affinity DataIC50: 0.140nMAssay Description:Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo...More data for this Ligand-Target Pair
Affinity DataIC50: <0.200nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Agonist activity at human recombinant CB2 receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 20 min...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP levelMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28...More data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMAssay Description:Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMAssay Description:Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo...More data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo...More data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo...More data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Binding affinity to human CB2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl...More data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo...More data for this Ligand-Target Pair
Affinity DataIC50: 0.630nMAssay Description:Inhibition of [35S]GTP-gamma-S binding to human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP levelMore data for this Ligand-Target Pair
Affinity DataIC50: 0.720nMAssay Description:HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE...More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Binding affinity to CB2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.790nMAssay Description:Inhibition of [35S]GTP-gamma-S binding to human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl...More data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMAssay Description:Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMAssay Description:Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Agonist activity at human recombinant CB2 receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 20 min...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release after 45 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Agonist activity at human CB2 in CHO cells assessed as inhibition of cAMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [18F]-LU13 from human CB2 receptor transfected in CHO cells membrane homogenate incubated for 90 mins by competitive radioligand bind...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Binding affinity to human CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of CB2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.53nMAssay Description:Agonist activity at human recombinant CB2 receptor expressed in CHO cell membranes assessed as inhibition of forskolin-induced cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inverse agonist activity at human CB2 receptor expressed in CHO membranes by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP productionMore data for this Ligand-Target Pair
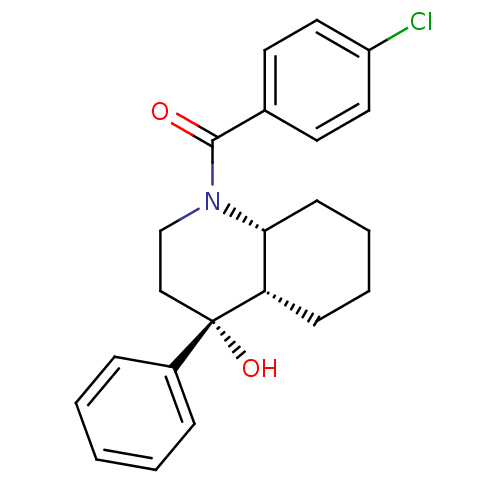
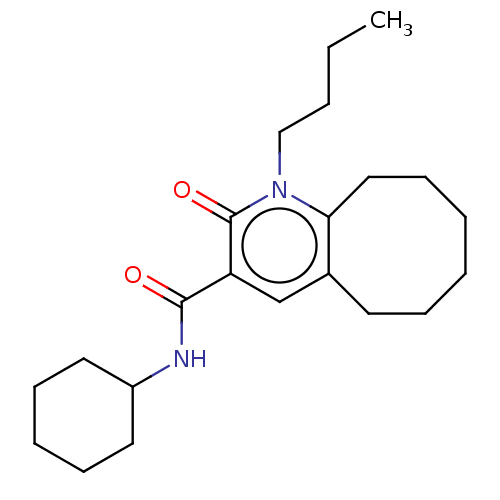
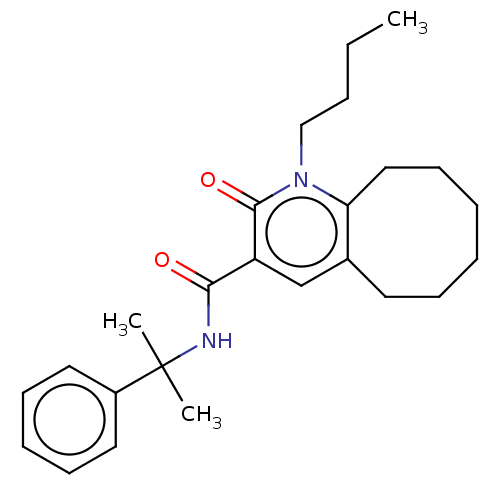
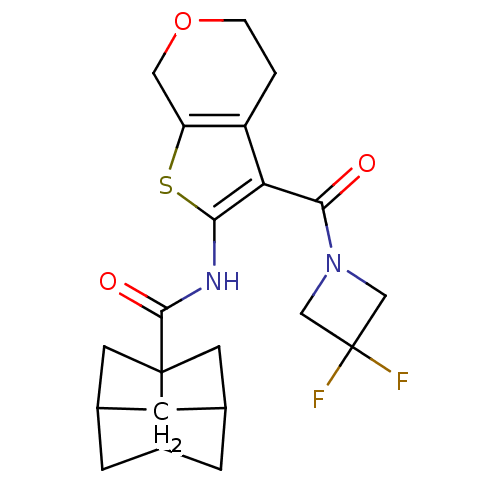
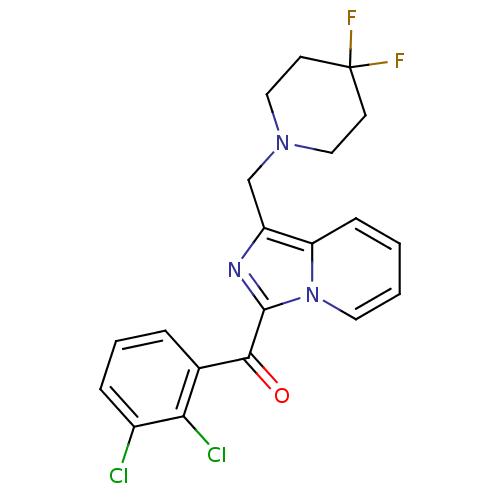
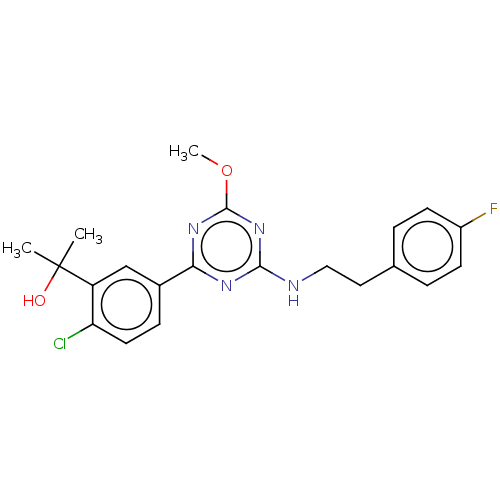
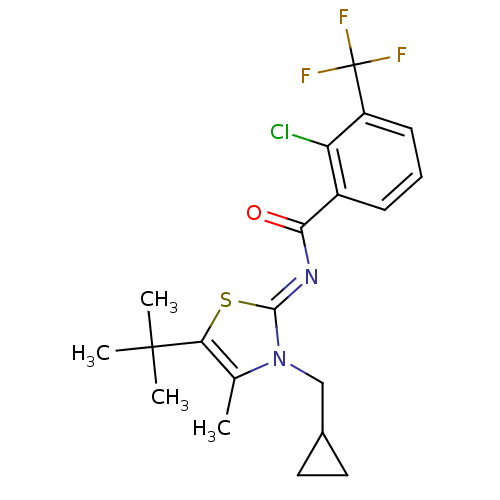
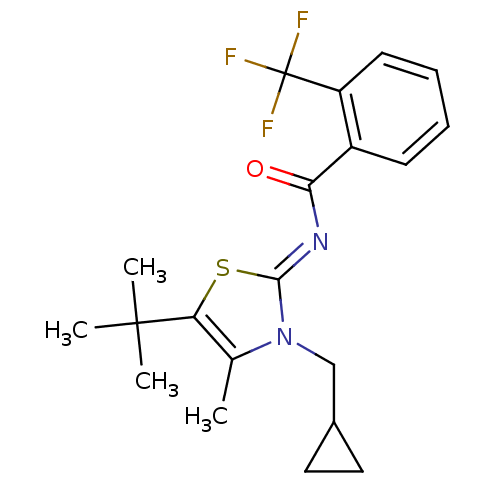
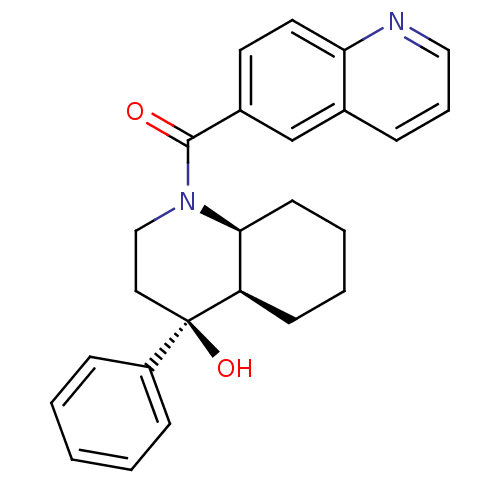
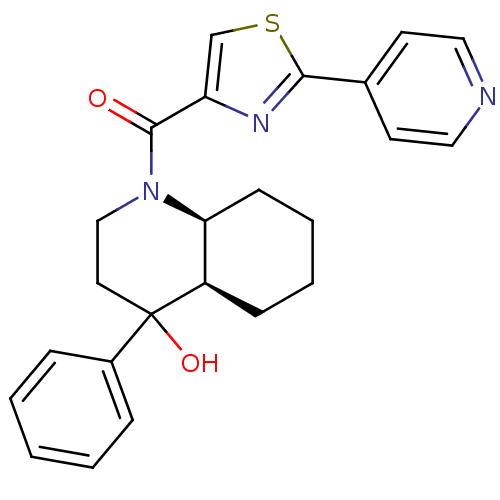
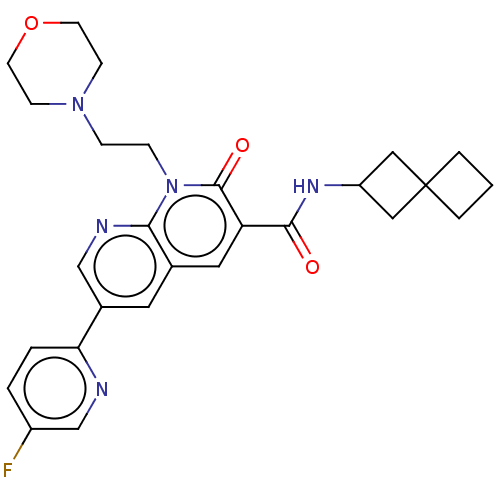
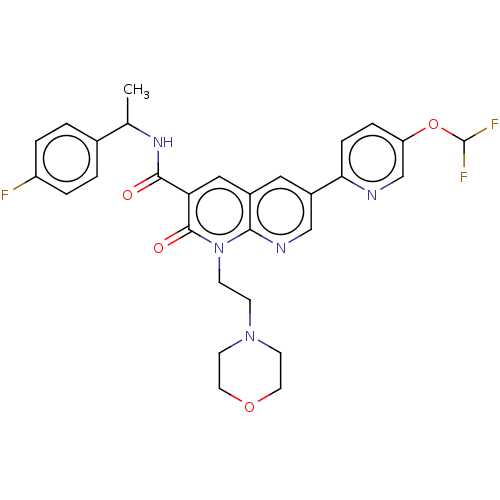
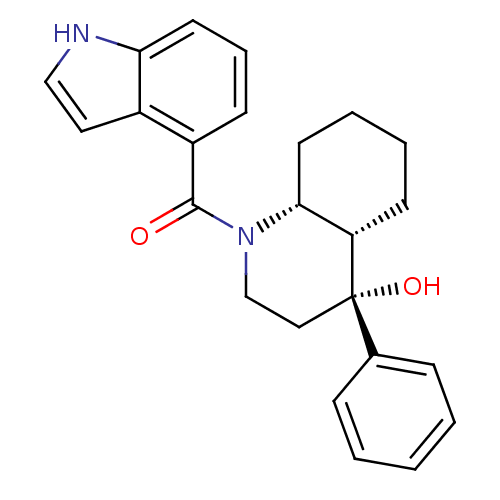
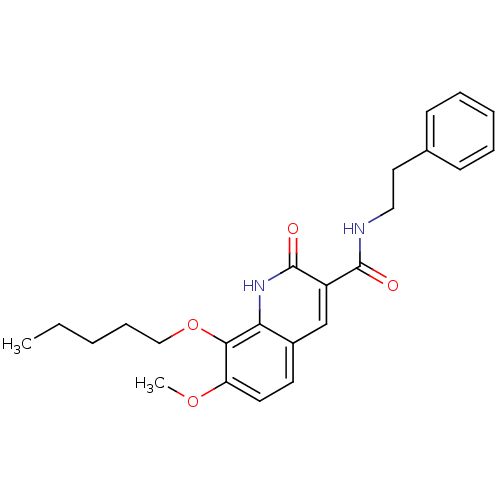
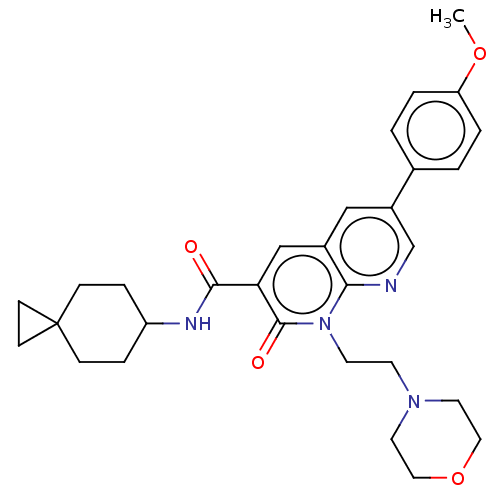
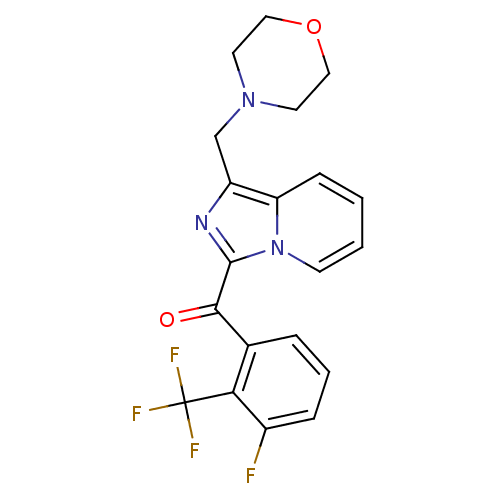
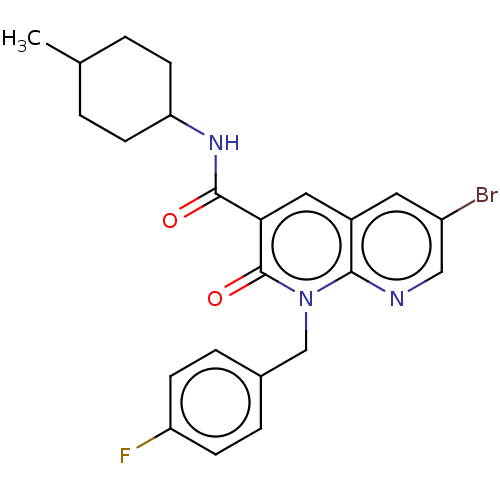
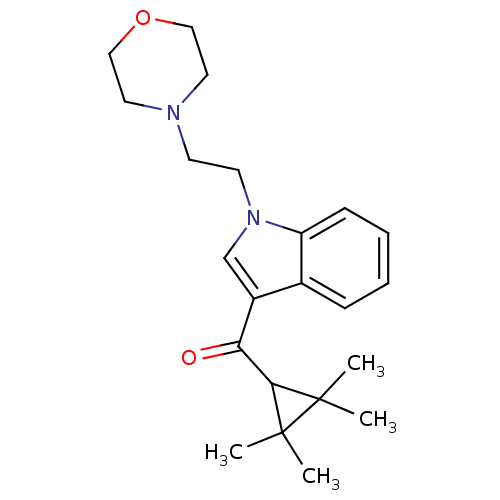
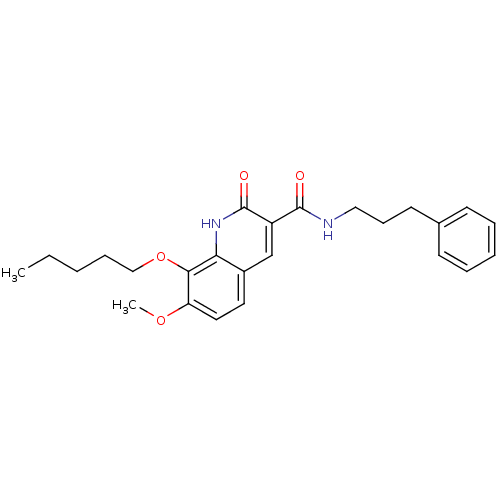

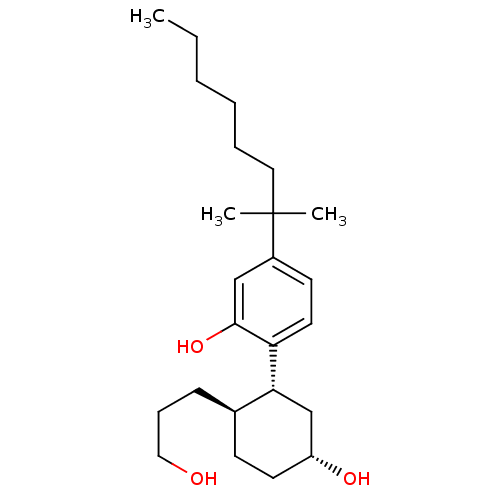
 3D Structure (crystal)
3D Structure (crystal)