Report error Found 5 Enz. Inhib. hit(s) with all data for entry = 50012703
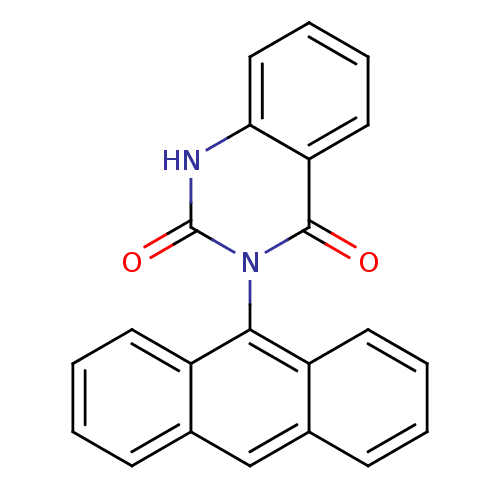
Affinity DataIC50: 2.90E+3nMAssay Description:Puromycin sensitive aminopeptidase inhibitory activity by the use of L-Ala AMC with MOLT-4More data for this Ligand-Target Pair
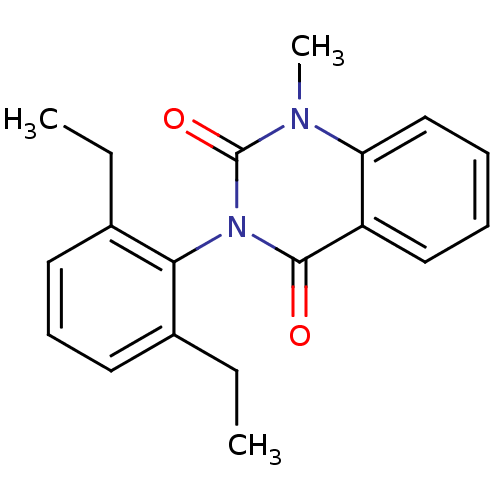
Affinity DataIC50: 3.40E+3nMAssay Description:Puromycin sensitive aminopeptidase inhibitory activity by the use of L-Ala AMC with MOLT-4More data for this Ligand-Target Pair
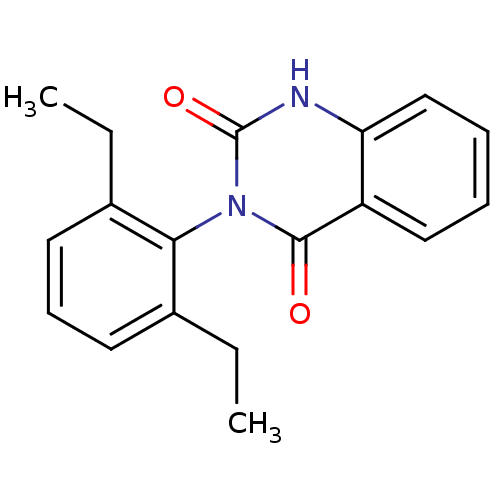
Affinity DataIC50: 3.80E+3nMAssay Description:Puromycin sensitive aminopeptidase inhibitory activity by the use of L-Ala AMC with MOLT-4More data for this Ligand-Target Pair
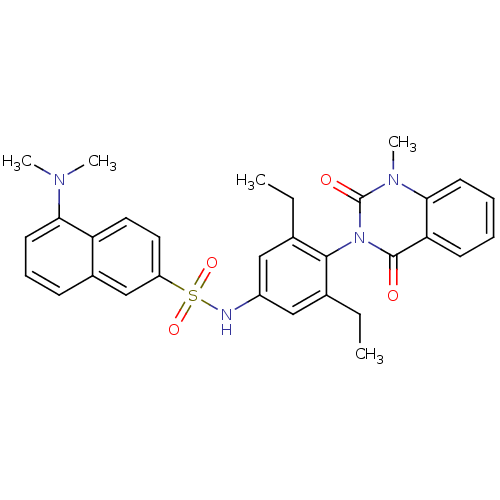
Affinity DataIC50: 4.60E+3nMAssay Description:Puromycin sensitive aminopeptidase inhibitory activity by the use of L-Ala AMC with MOLT-4More data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:Puromycin sensitive aminopeptidase inhibitory activity by the use of L-Ala AMC with MOLT-4More data for this Ligand-Target Pair