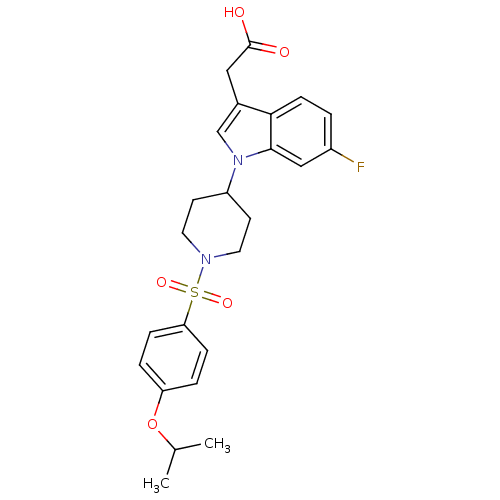
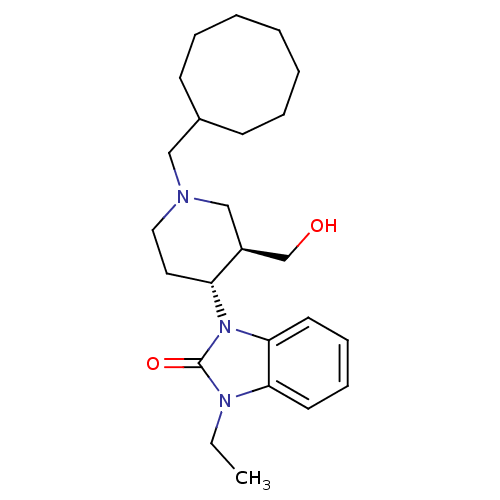
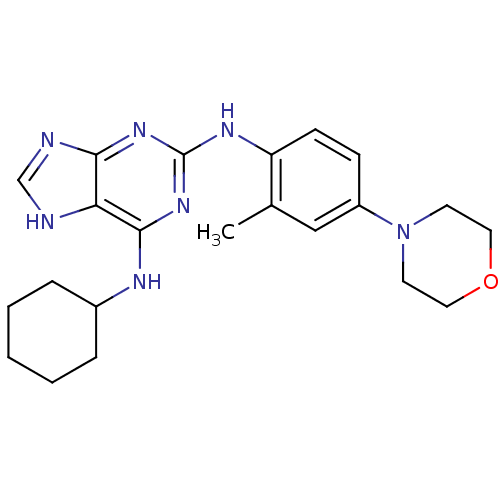
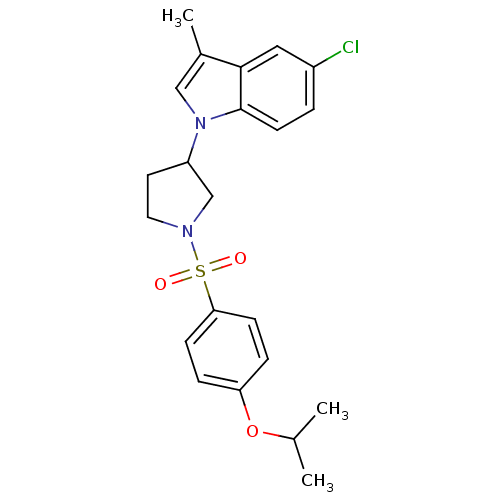
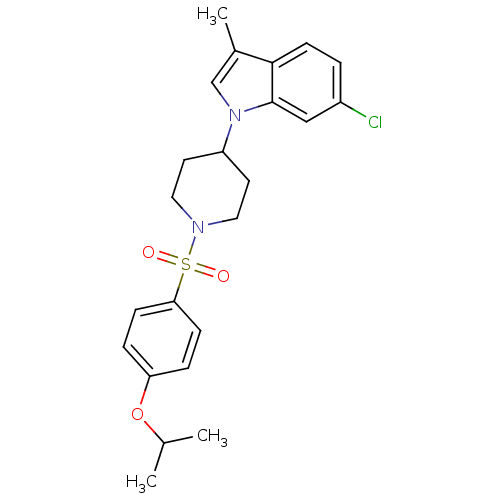
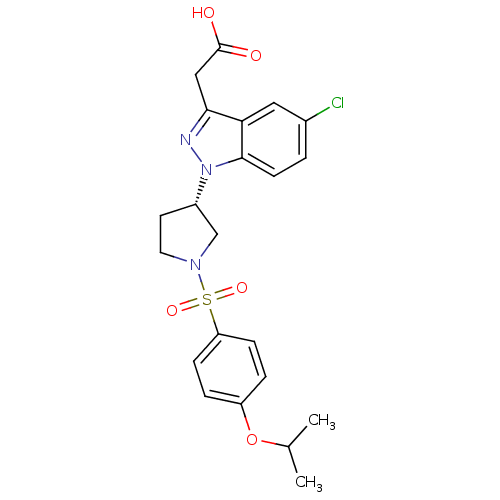
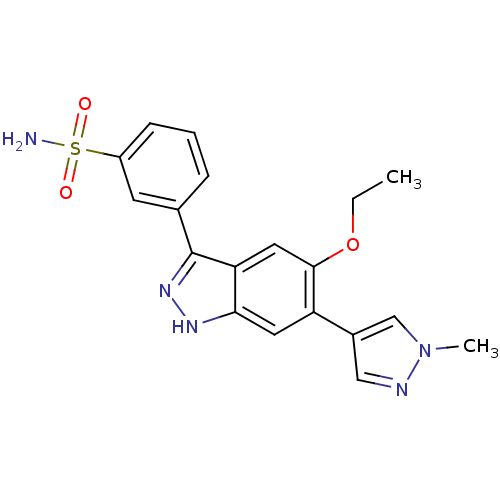
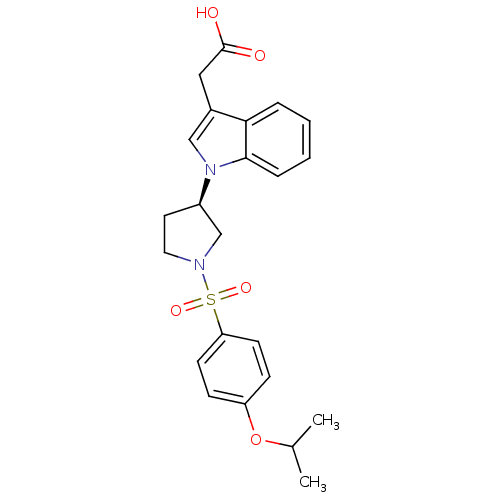
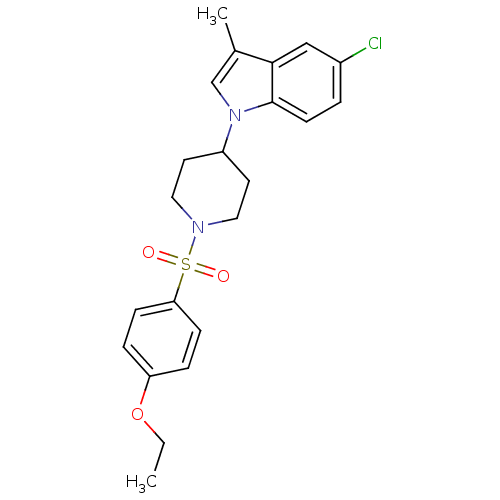
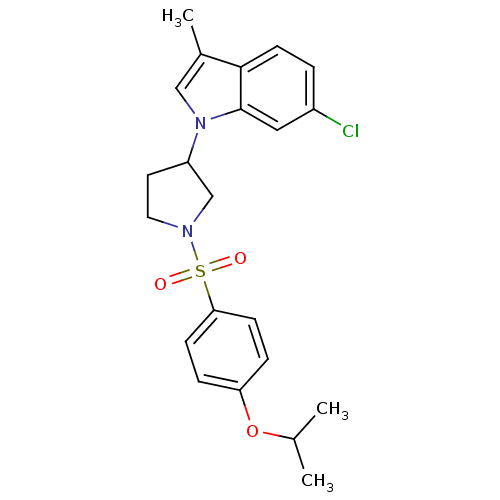
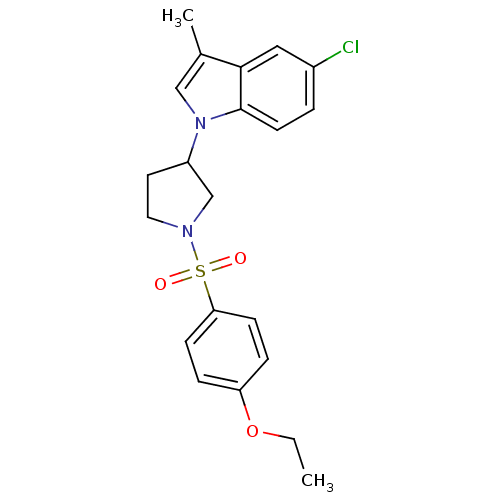
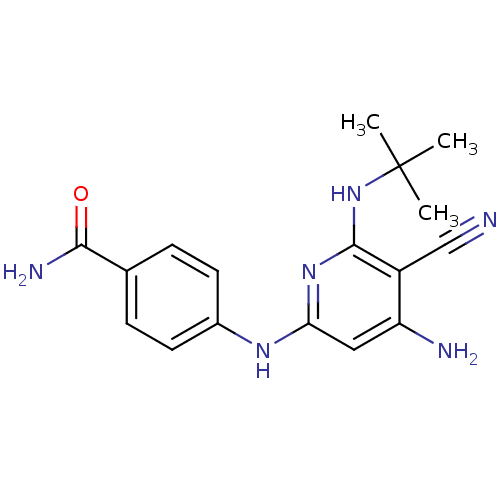
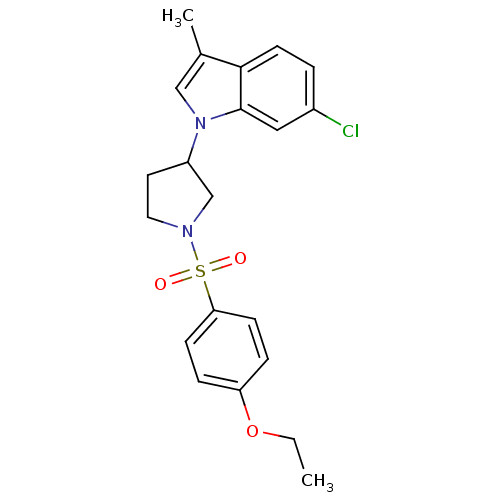
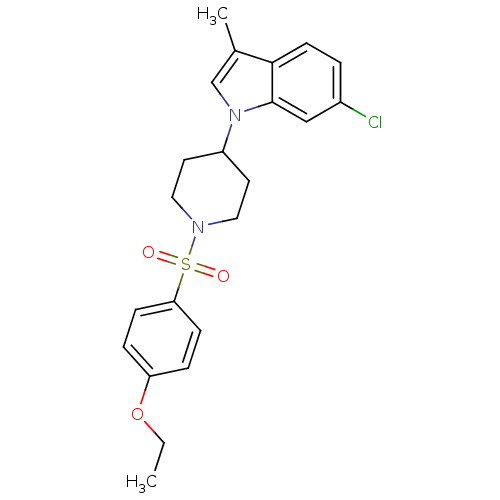
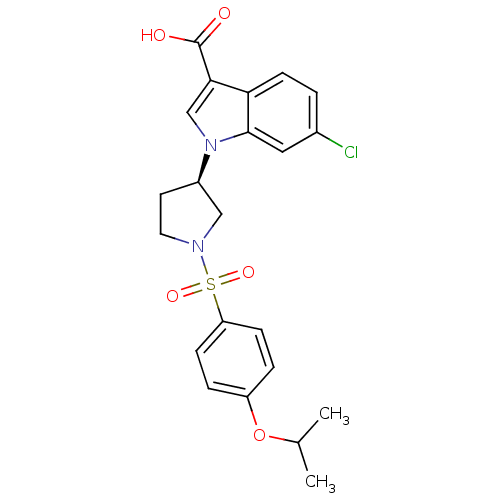
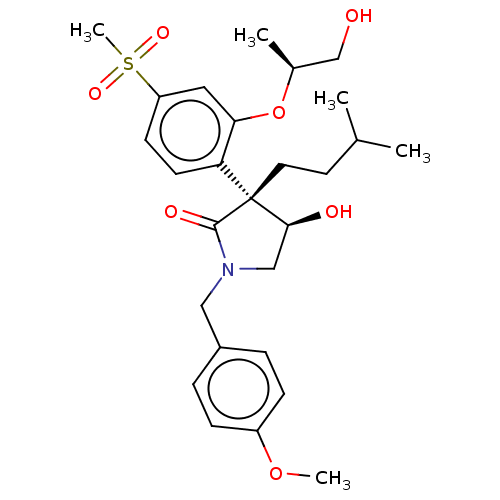
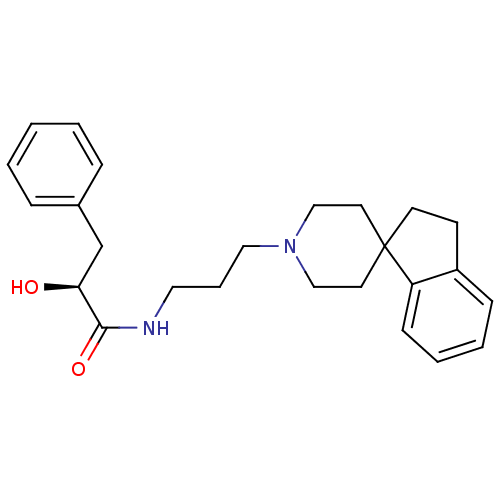
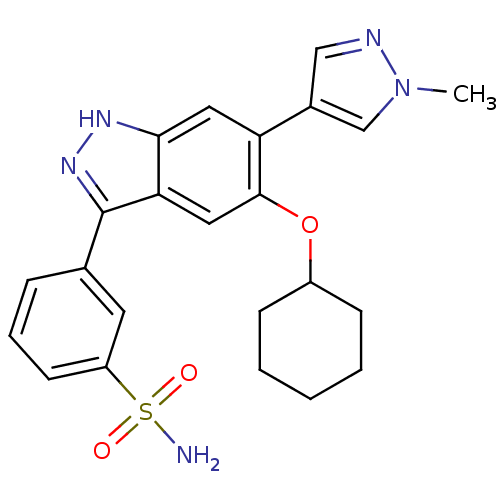
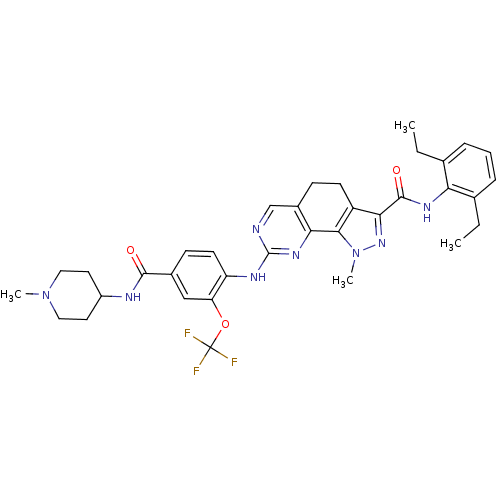
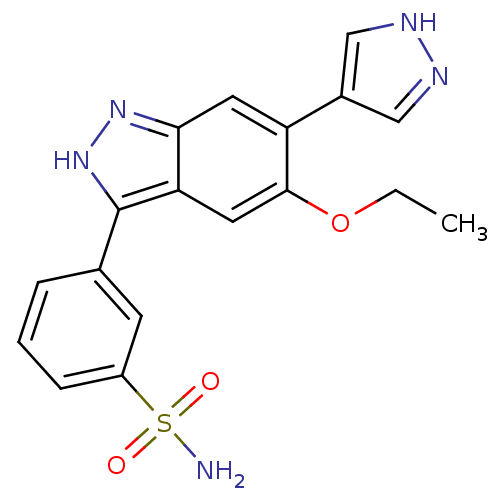
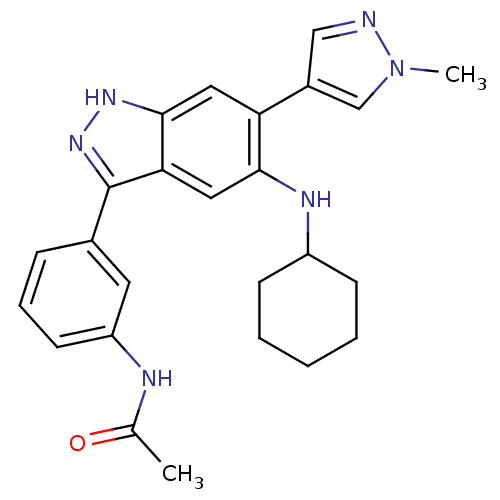
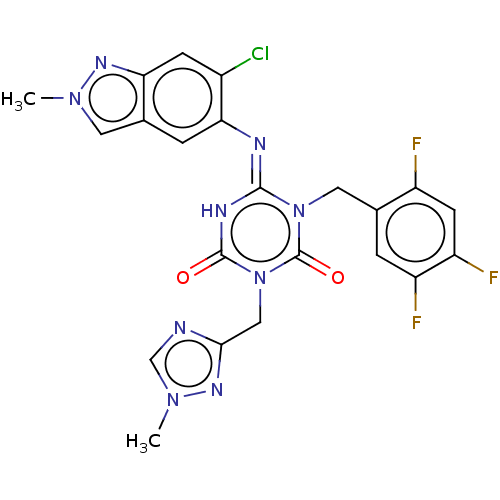
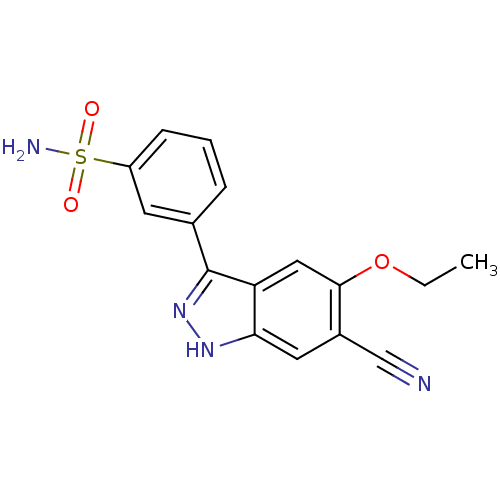
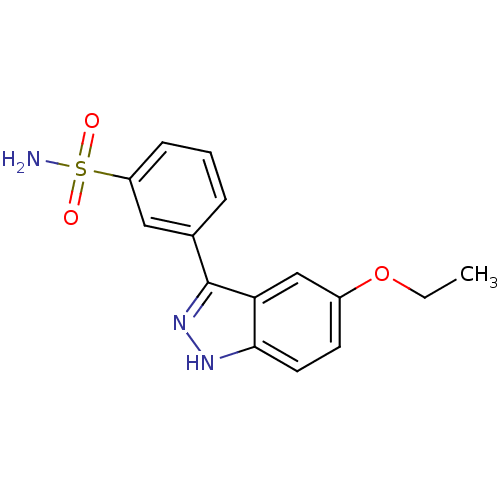
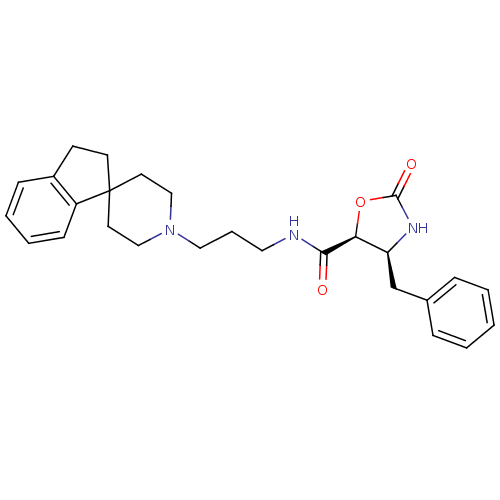
Report error Found 286 with Last Name = 'tachibana' and Initial = 'y'
Affinity DataKi: 1.40nM IC50: 1.60nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataKi: 4.30nM IC50: 2.90nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataKi: 5.40nM IC50: 1.20nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nM IC50: 2.20nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataKi: 7.70nM IC50: 4.70nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataKi: 22nM IC50: 4.10nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.120nMAssay Description:Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.270nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of N-terminal 6His-tagged full length human recombinant NNMT (1 to 264 residues) expressed in Escherichia coli BL21 (DE3) cells using S-ad...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human MPS1 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of JNK1-mediated ATF2 phosphorylation after 1 hr by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMAssay Description:Inhibition of Mps1-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:A prepared WP was homogenated and a membrane fraction was collected with high-speed centrifugation. A compound of the present invention was added to ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.10nMAssay Description:Inhibition of wild type HIV1 protease expressed in Escherichia coli BL21 DE3 assessed as cleavage of fluorogenic substrate using EDANS-RESGIFLETSKR-D...More data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of MPS1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMAssay Description:Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of N-terminal 10-His-tagged SARS-CoV-2 3CL protease (1 to 306 residues) expressed in Escherichia coli BL21(DE3) using Dabcyl-KTSAVLQSGFRKM...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The 3CL protease inhibition assay was conducted in 384-well plates (Corning 3702). The substance solution (10 mM dimethyl sulfoxide [DMSO] solution) ...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of N-terminal 6His-tagged full length human recombinant NNMT (1 to 264 residues) expressed in Escherichia coli BL21 (DE3) cells using S-ad...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of FLAG-tagged MPS1 phosphorylation in human RERF-LC-AI Tet-off cells after 3 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
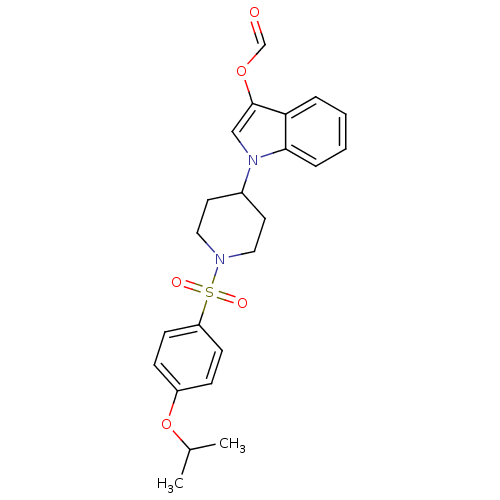
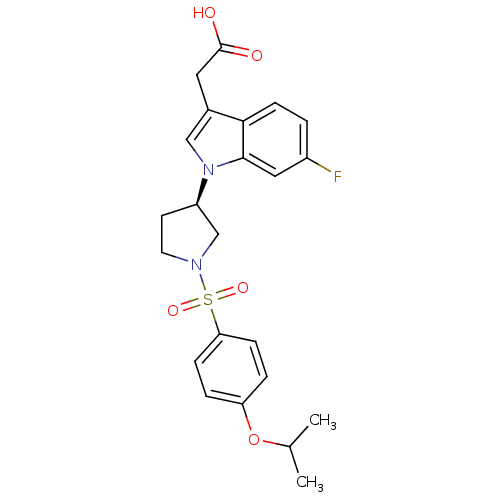
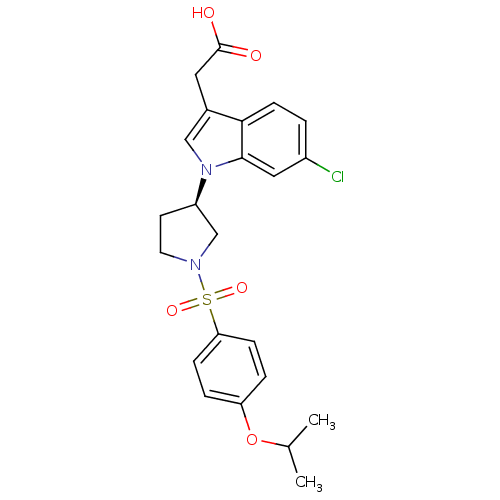
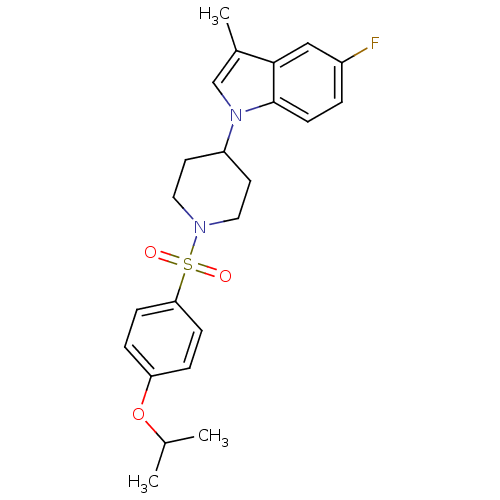
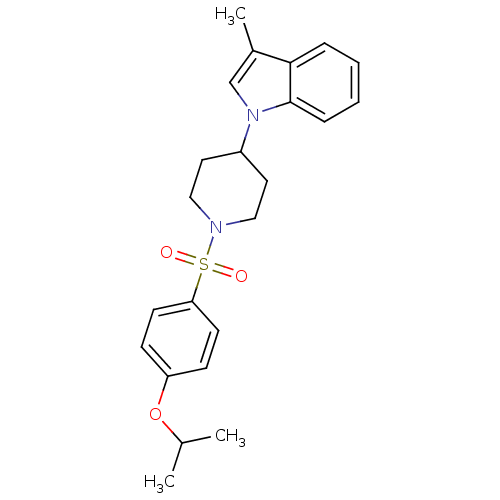
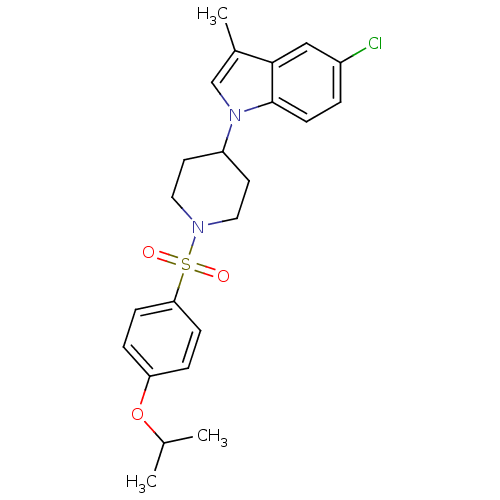
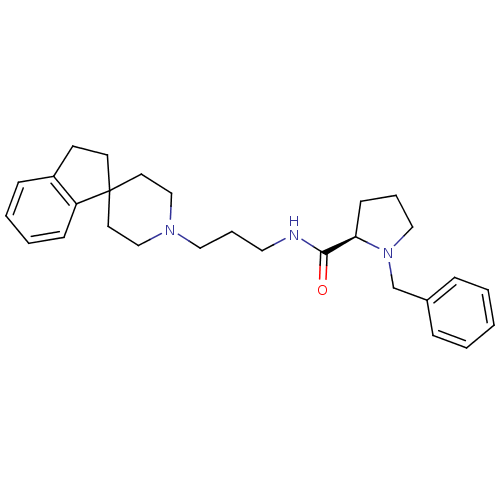
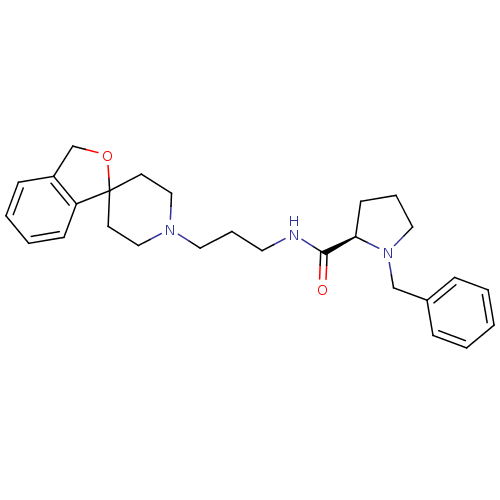
 3D Structure (crystal)
3D Structure (crystal)