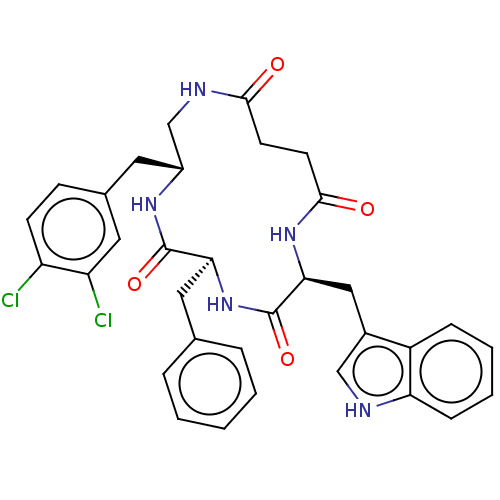
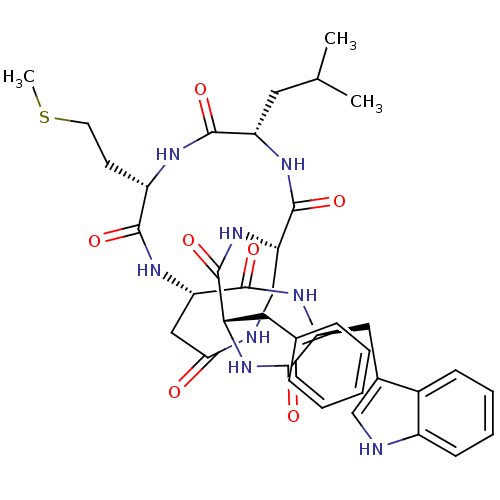
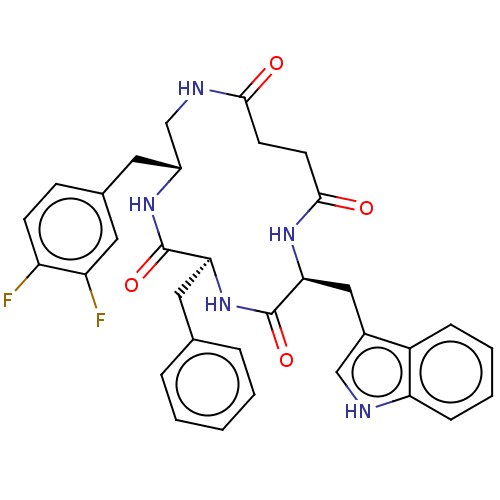
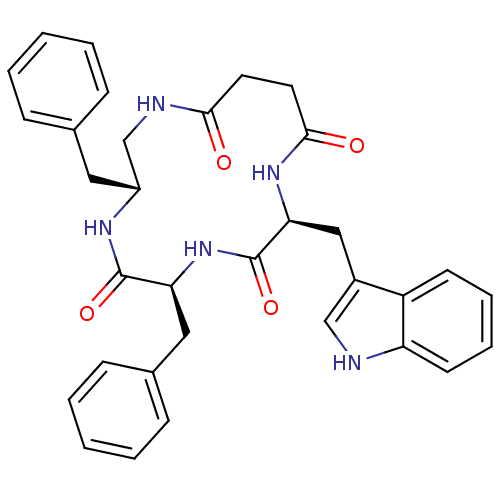
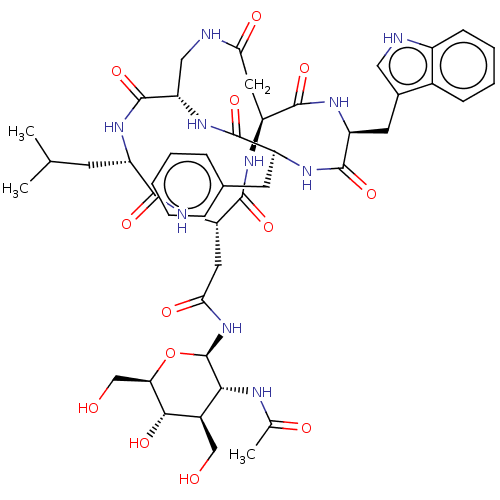
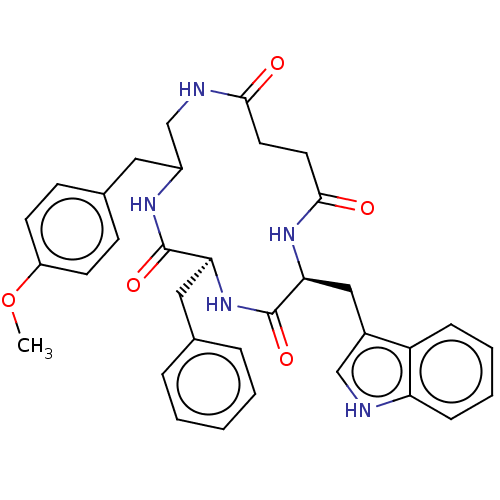
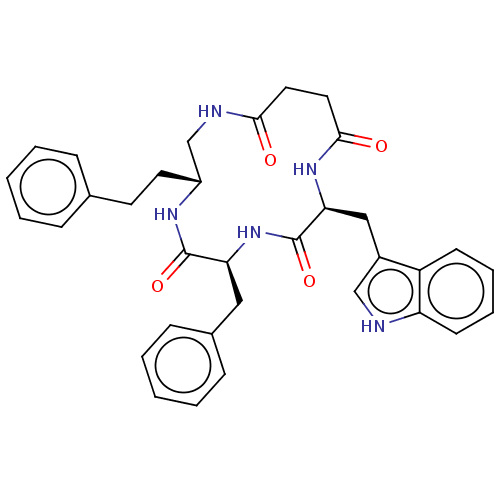
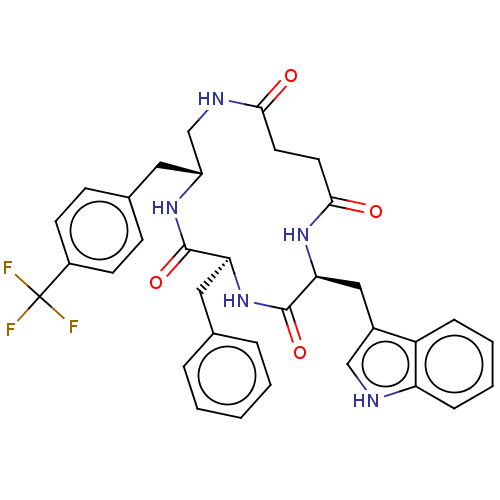
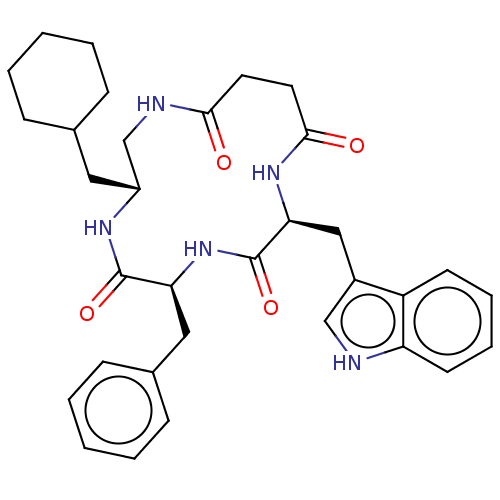
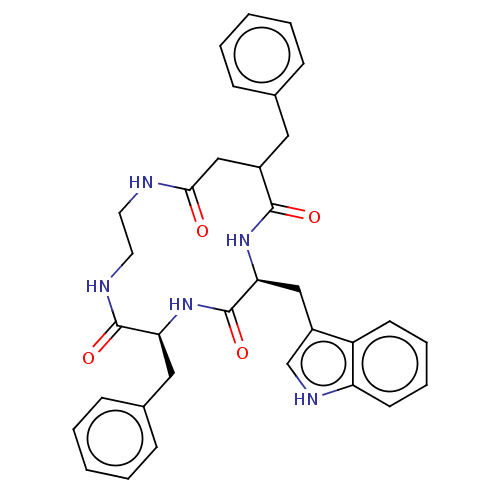
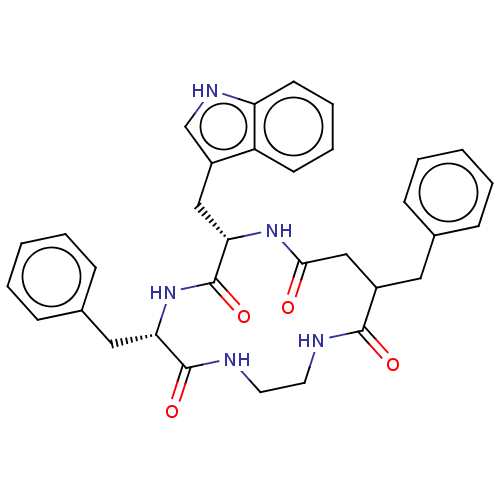
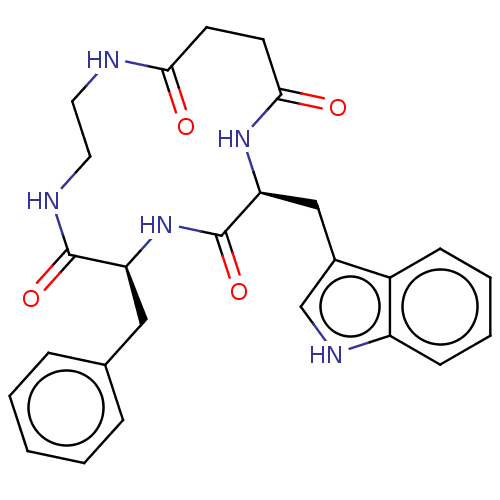
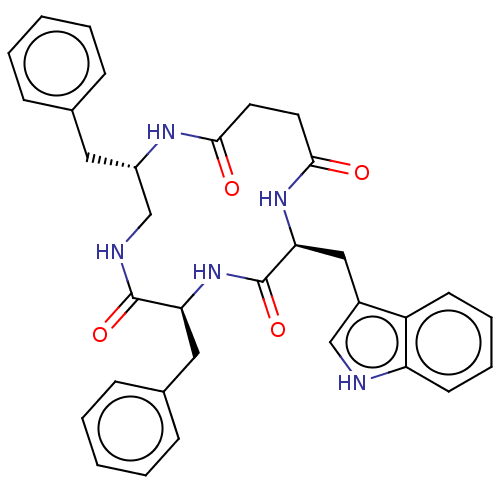
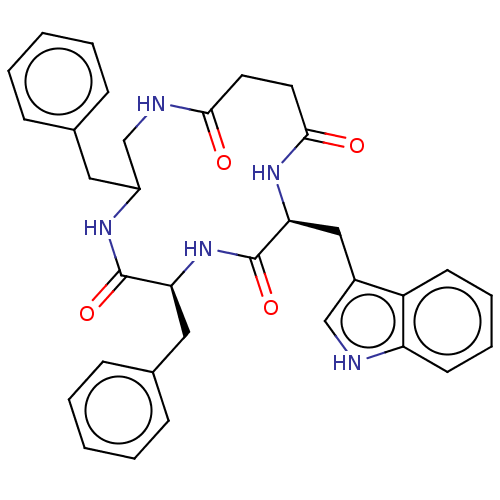
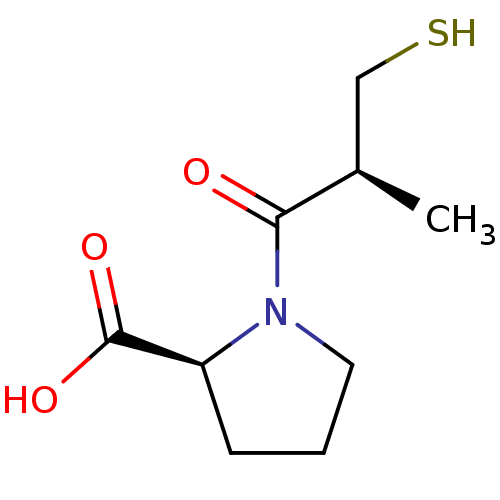
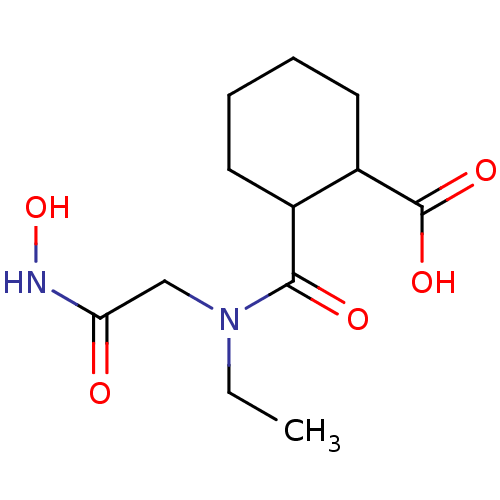
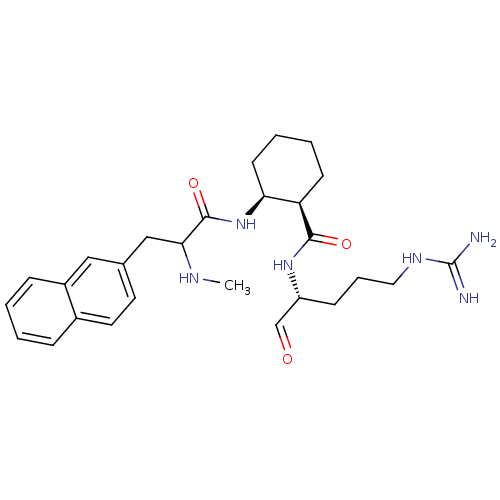
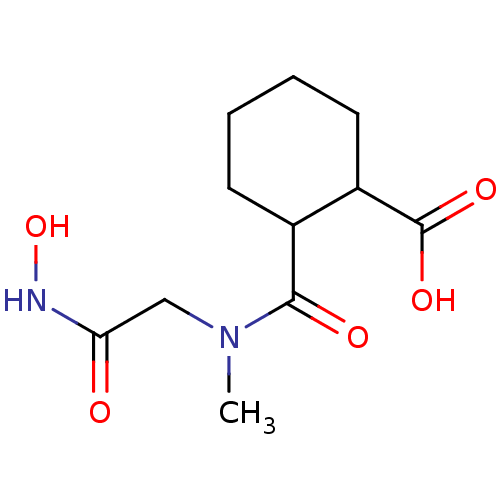
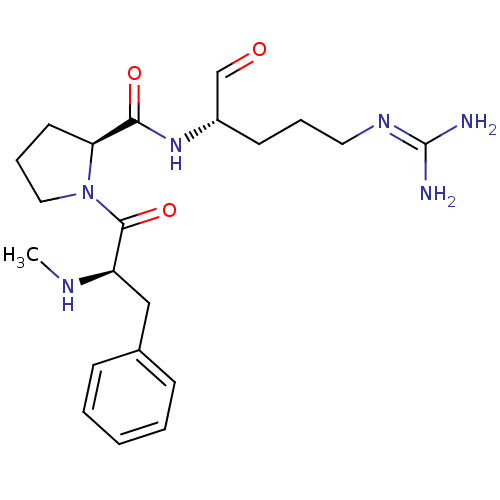
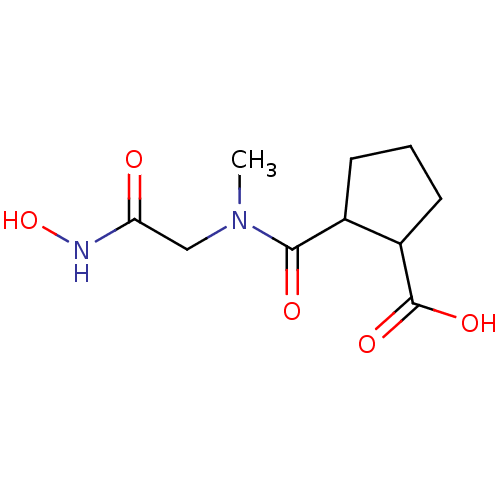
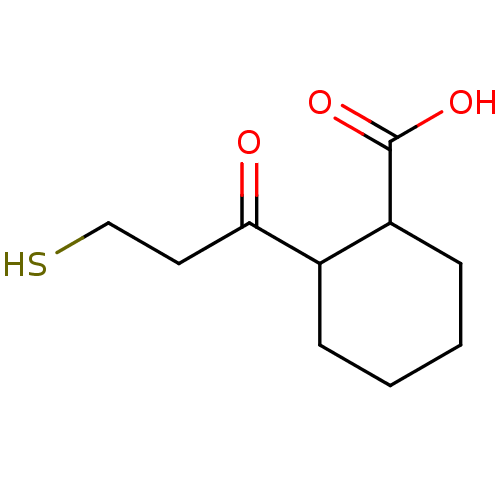
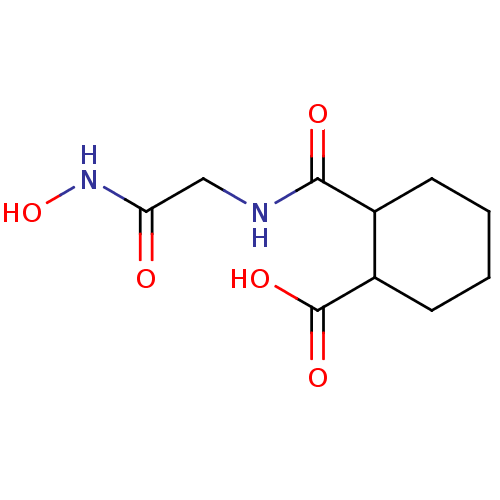
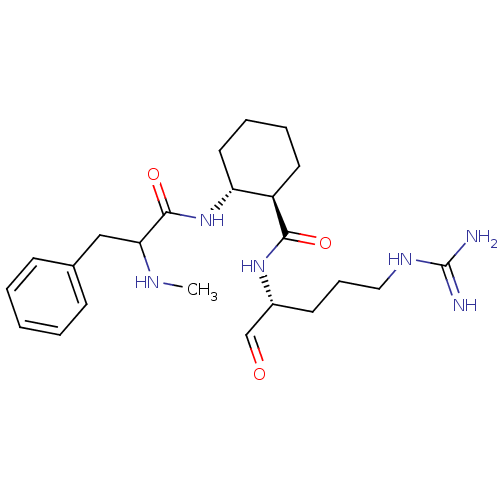
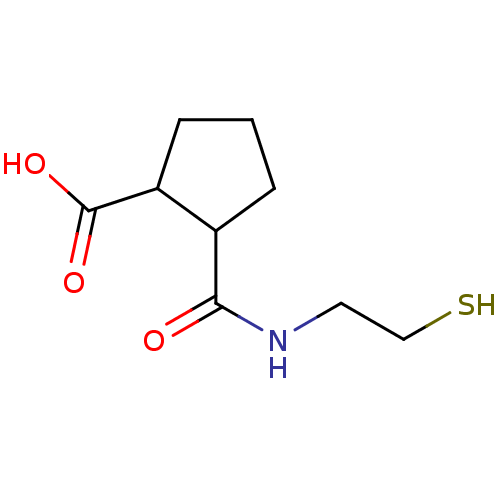
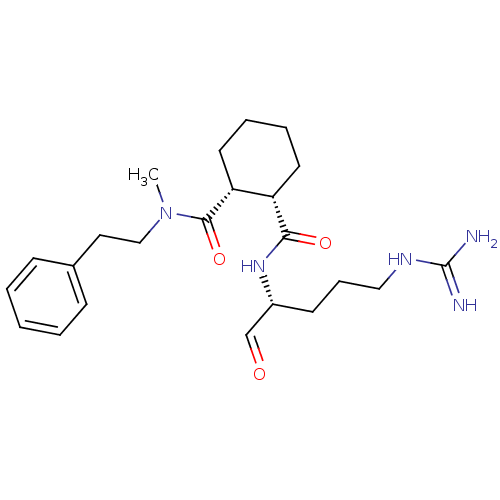
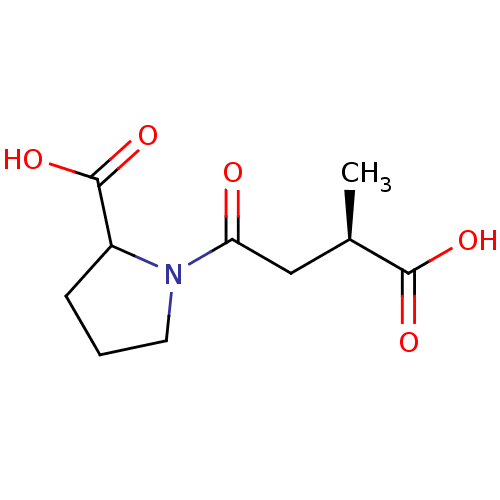
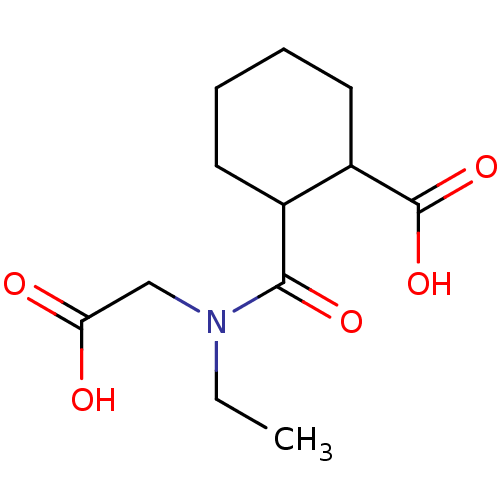
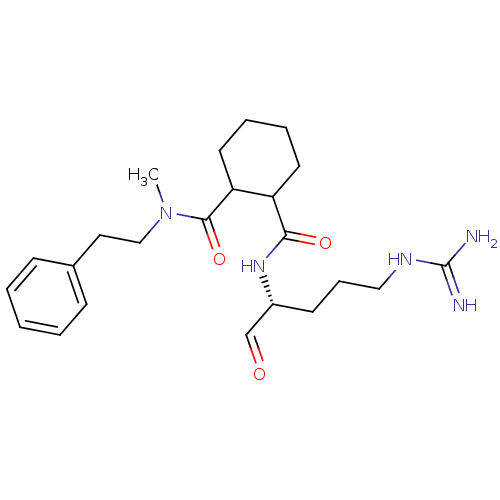
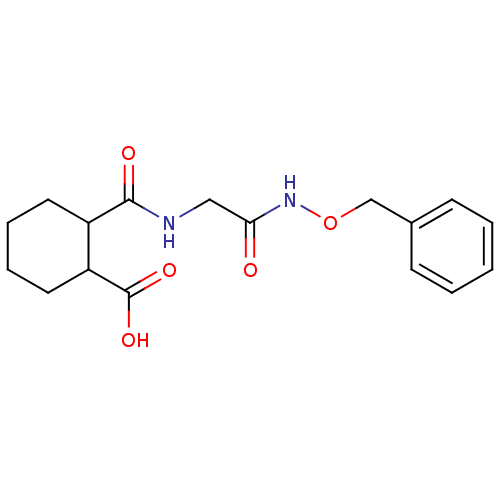
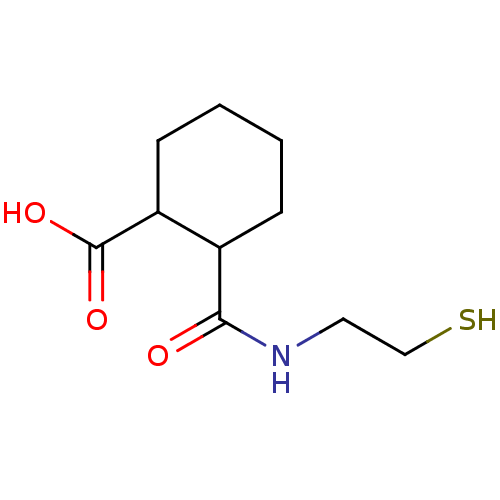
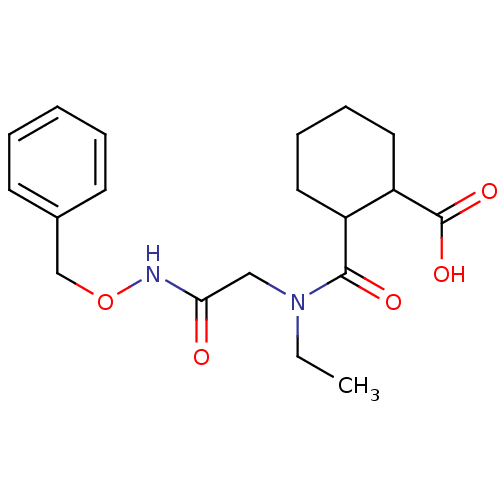
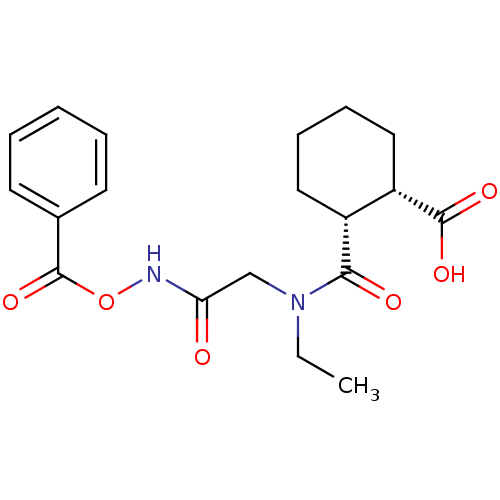
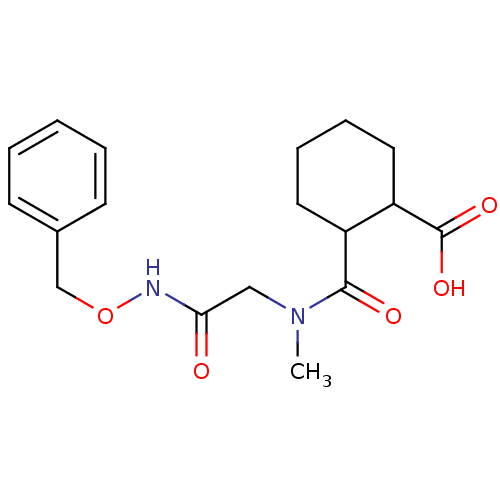
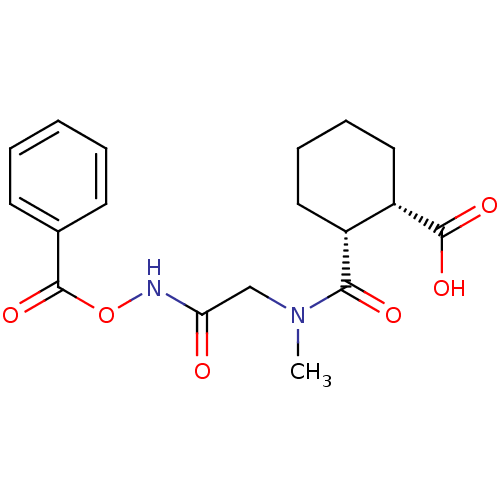
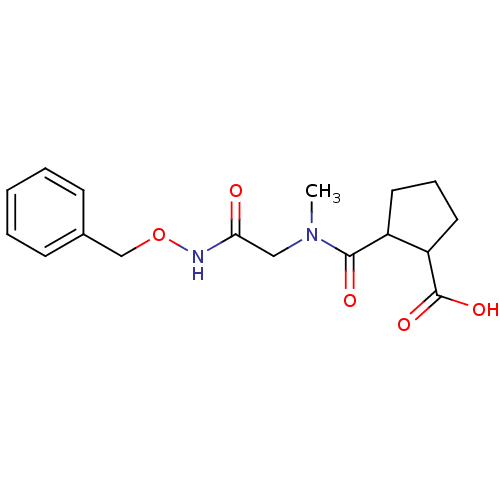
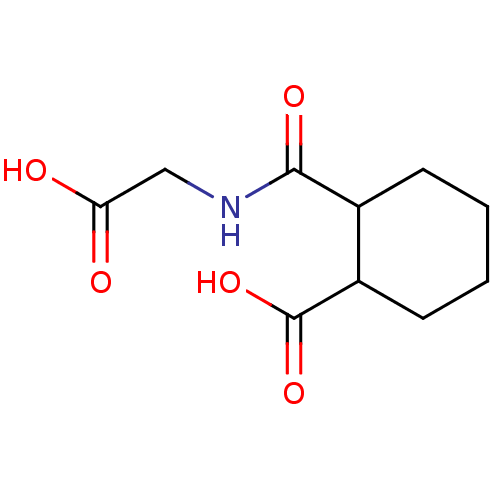
Affinity DataKi: 0.100nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 0.631nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 316nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 631nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 794nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+3nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+3nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+3nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+3nMAssay Description:Binding affinity towards Tachykinin receptor 2More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym THMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym THMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym THMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym THMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+4nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+4nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+4nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+4nMAssay Description:Compound was evaluated for the inhibition of thrombin using the synthetic substrate chromozym THMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+5nMAssay Description:In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrateMore data for this Ligand-Target Pair