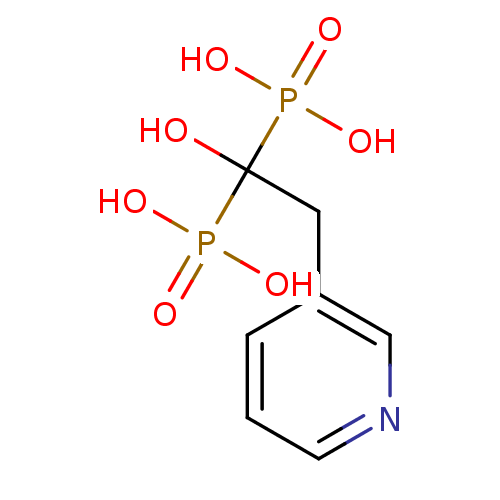
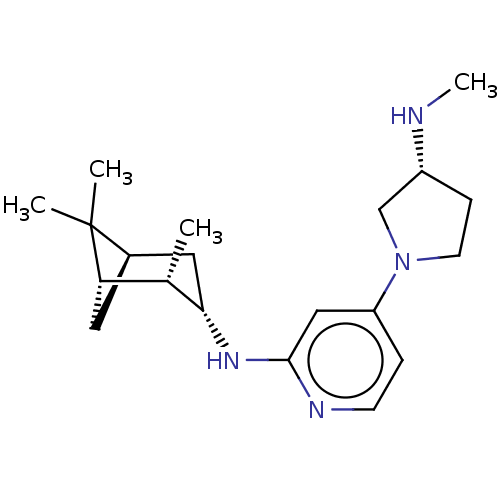
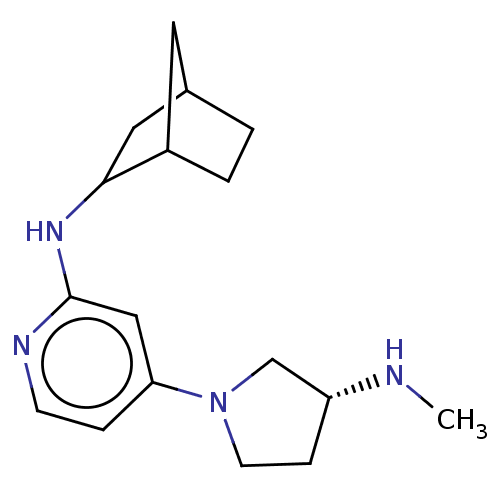
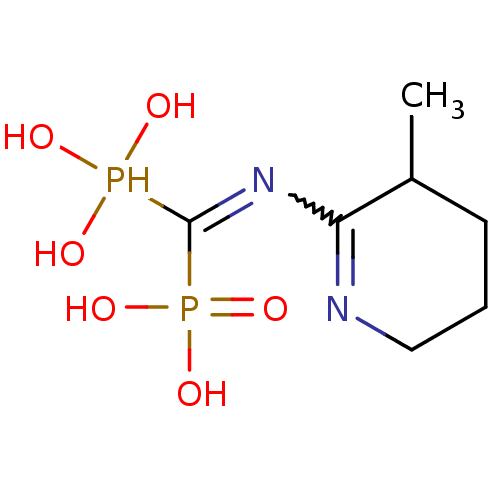
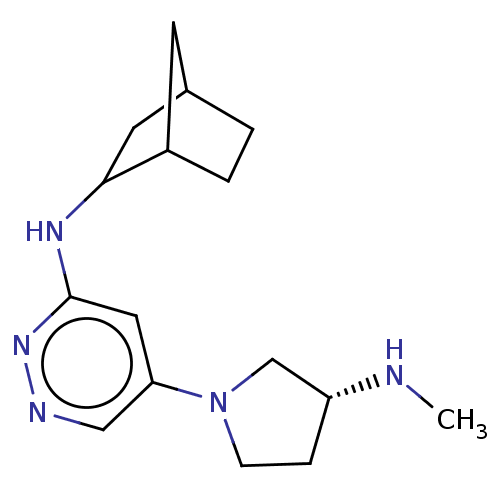
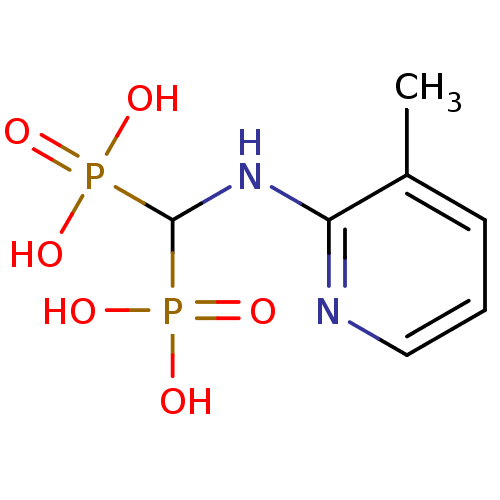
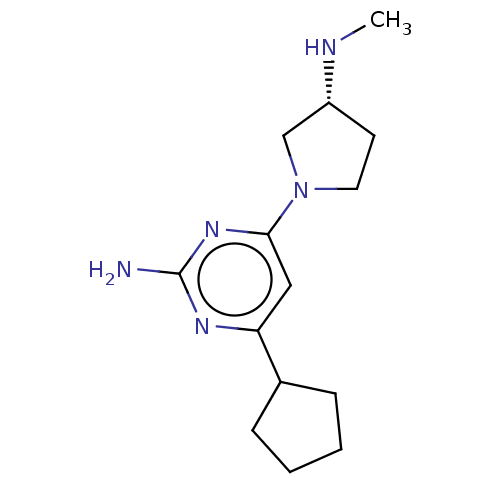
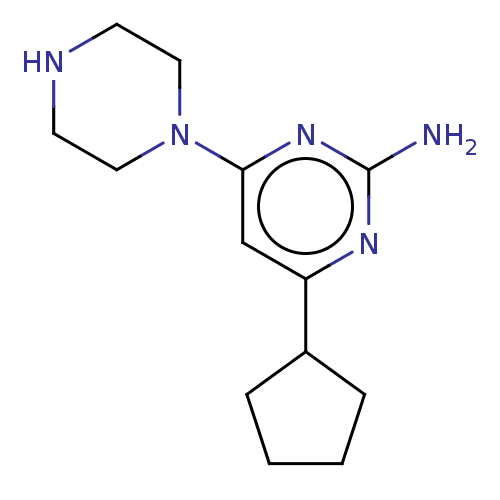
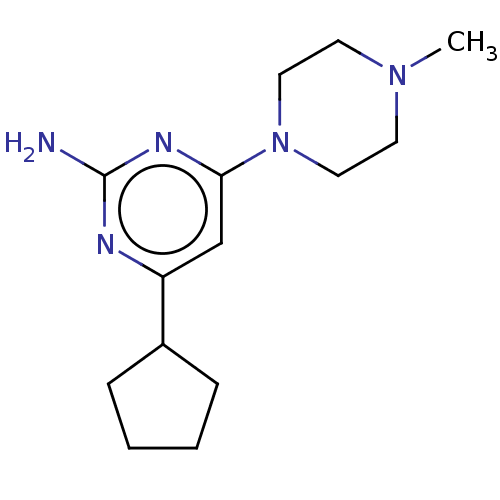
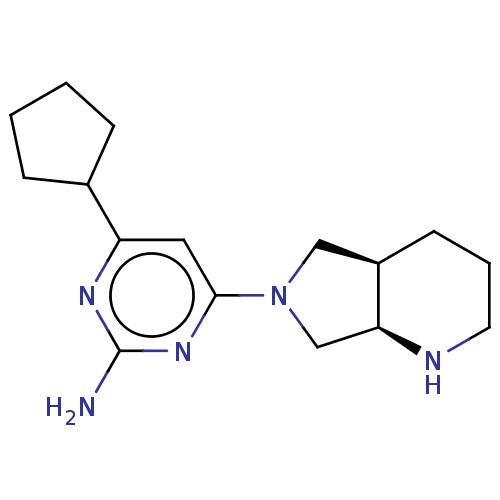
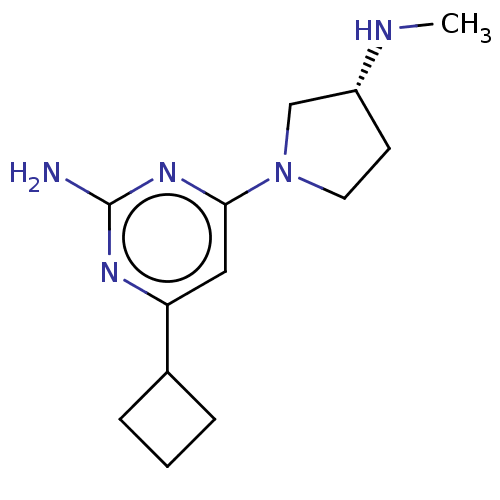
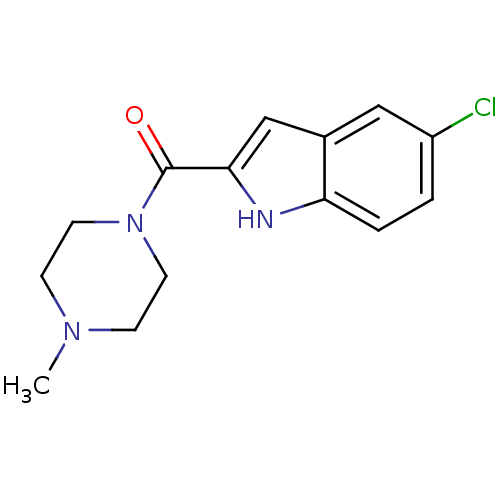
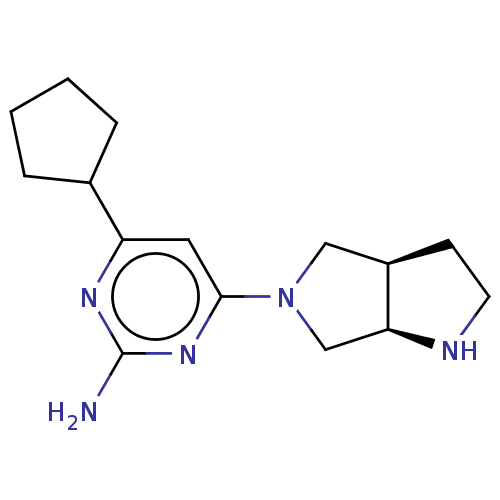
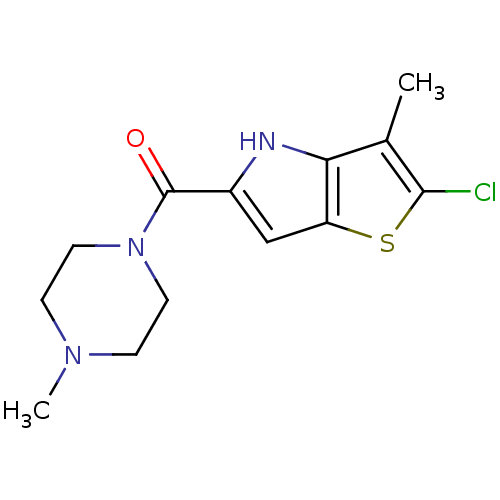
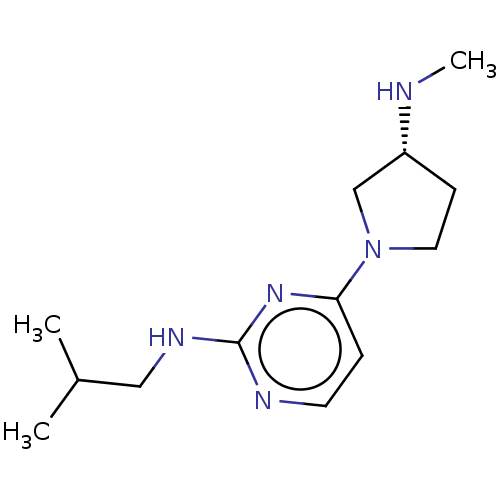
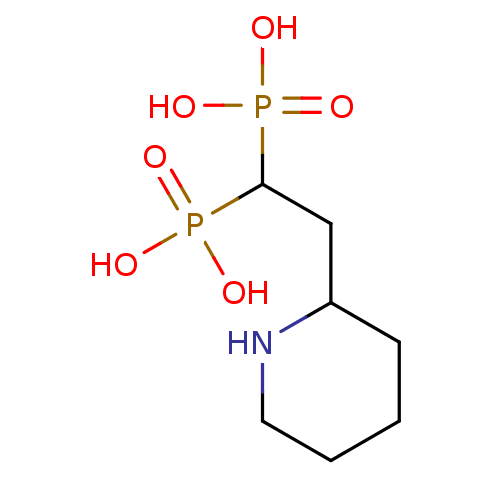
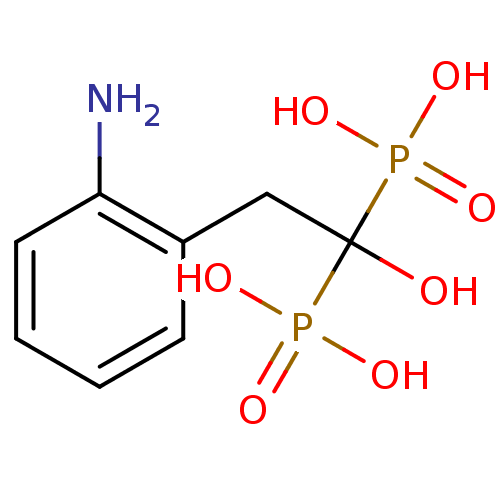
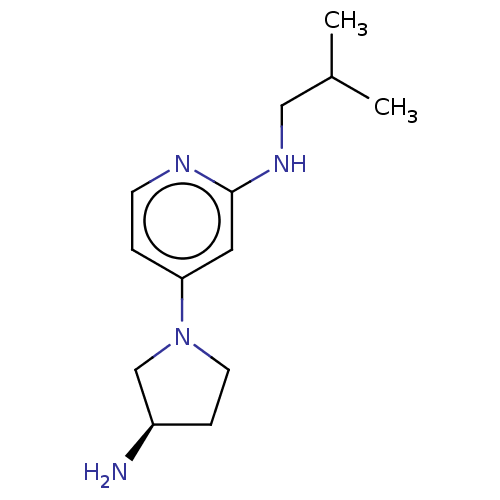
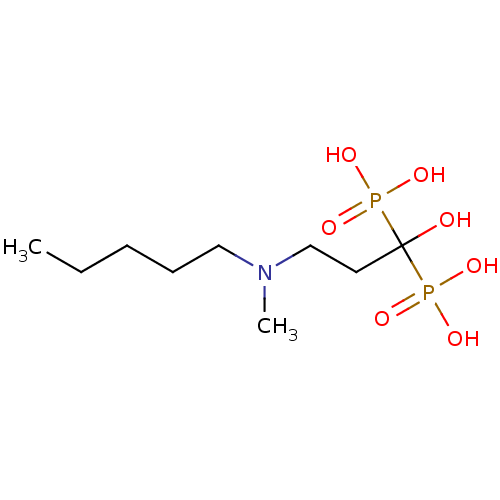
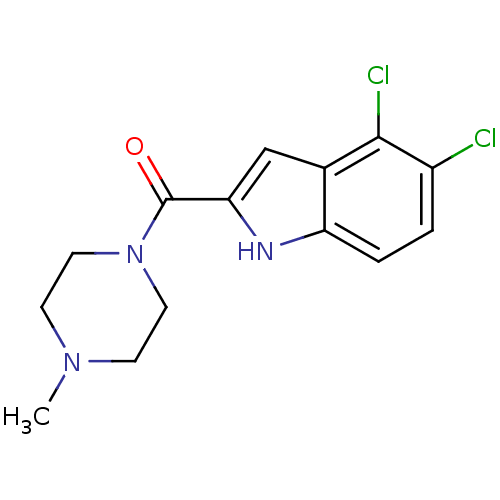
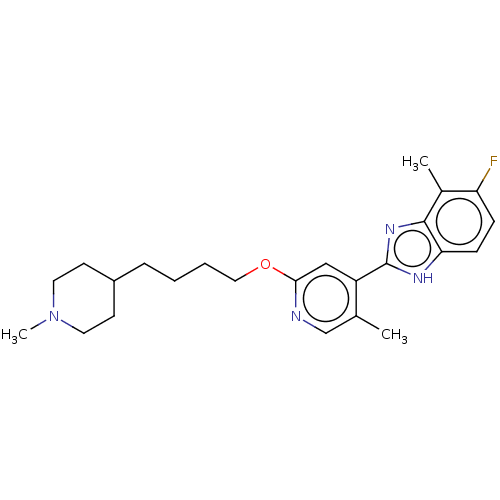
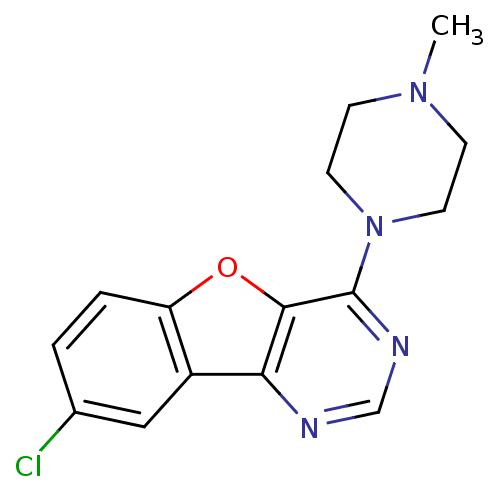
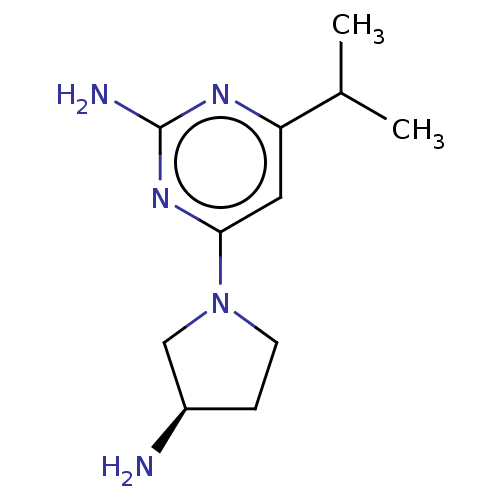
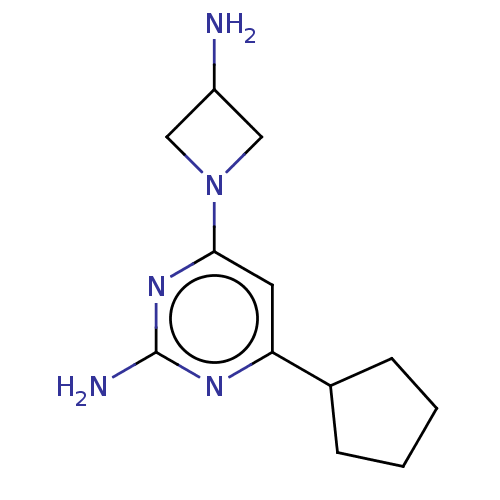
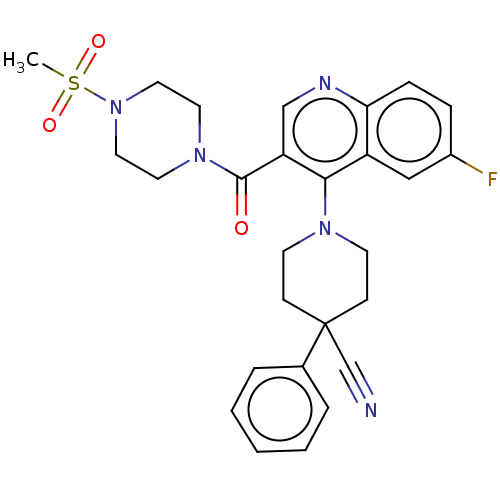
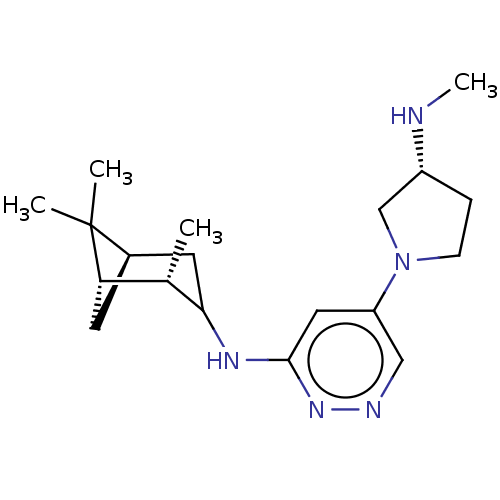
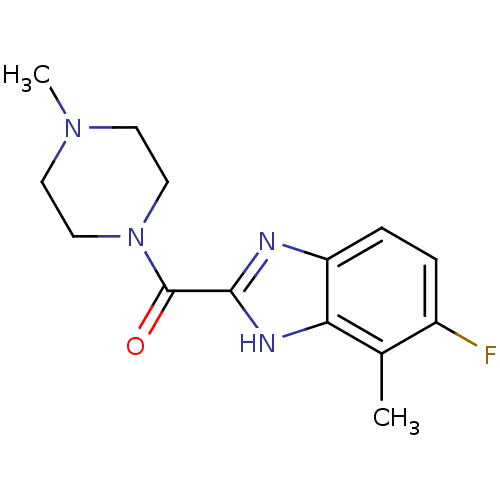
Affinity DataKi: 0.0500nM ΔG°: -61.2kJ/mole IC50: 30.1nMpH: 7.7 T: 2°CAssay Description:Enzymatic assay using CpNPPPS was assayed using Reed and Rilling method with some modification. More data for this Ligand-Target Pair
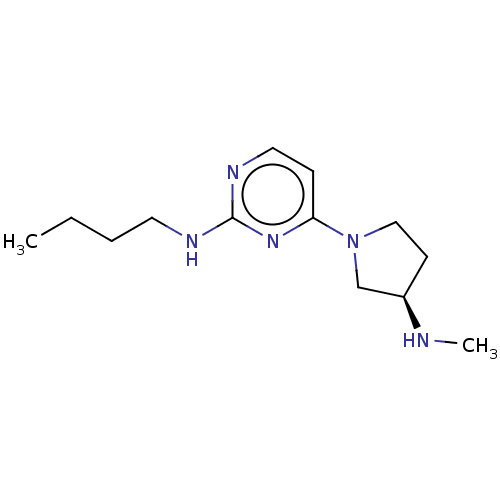
Affinity DataKi: 0.0700nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 0.360nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
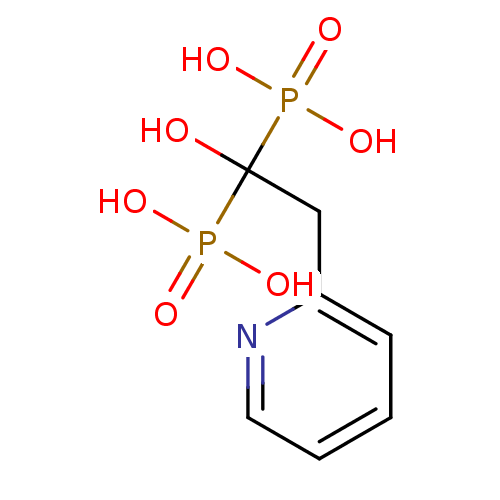
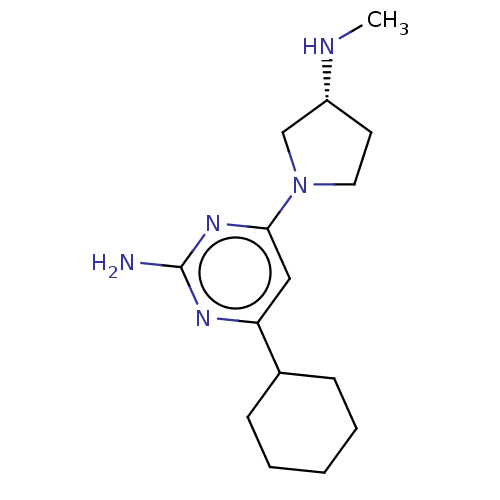
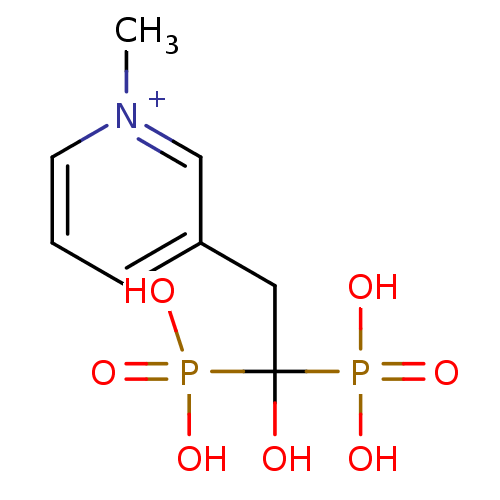
Affinity DataKi: 0.380nM ΔG°: -55.9kJ/mole IC50: 45.7nMpH: 7.7 T: 2°CAssay Description:Enzymatic assay using CpNPPPS was assayed using Reed and Rilling method with some modification. More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 0.740nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
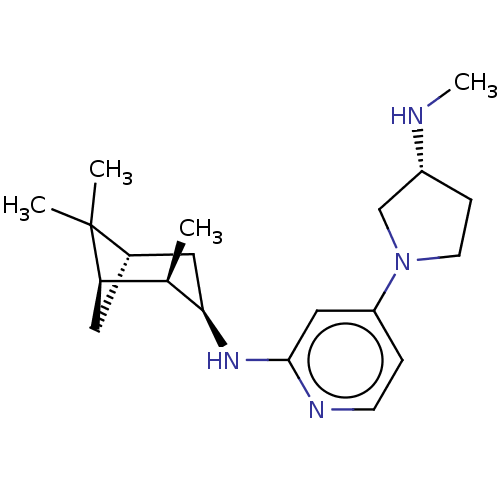
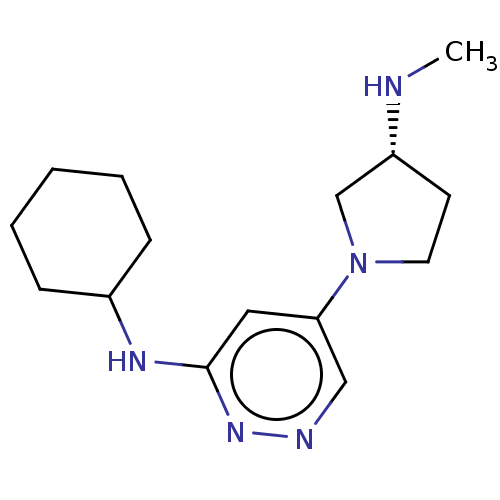
Affinity DataKi: 0.920nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 1.09nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
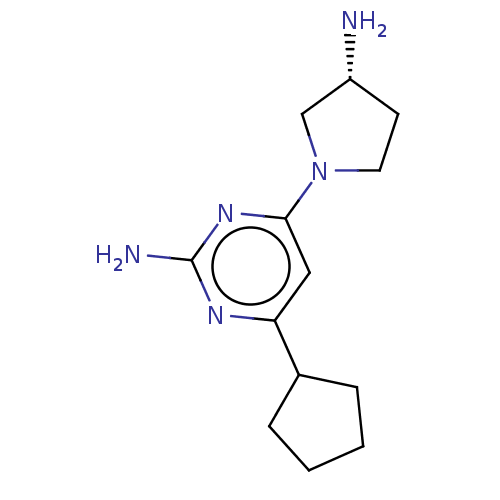
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
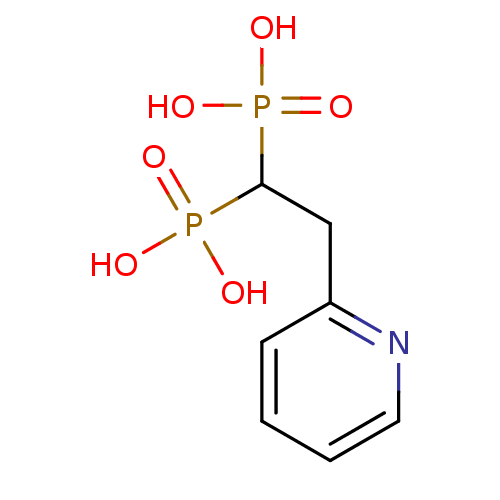
Affinity DataKi: 1.60nM ΔG°: -52.2kJ/mole IC50: 52.7nMpH: 7.7 T: 2°CAssay Description:Enzymatic assay using CpNPPPS was assayed using Reed and Rilling method with some modification. More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nM ΔG°: -49.0kJ/molepH: 7.5 T: 2°CAssay Description:The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff.More data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.08nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 3.28nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.10nM ΔG°: -47.9kJ/molepH: 7.5 T: 2°CAssay Description:The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff.More data for this Ligand-Target Pair
Affinity DataKi: 4.45nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nM ΔG°: -47.6kJ/molepH: 7.5 T: 2°CAssay Description:The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff.More data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.30nMAssay Description:Displacement of [3H]histamine from mouse histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysisMore data for this Ligand-Target Pair
TargetAldehyde dehydrogenase 1A1(Homo sapiens (Human))
National Center For Advancing Translational Sciences
Curated by ChEMBL
National Center For Advancing Translational Sciences
Curated by ChEMBL
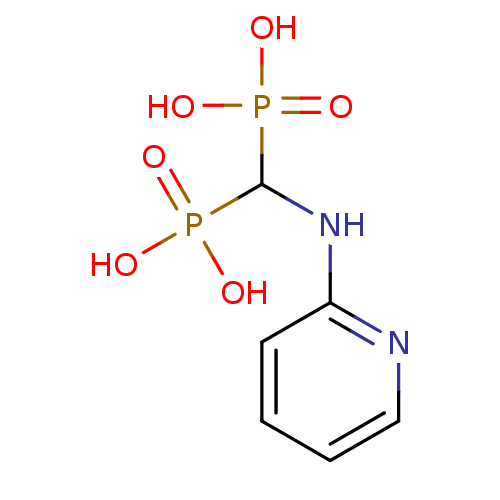
Affinity DataKi: 5.5nMAssay Description:Inhibition of human ALDH1A1 using propionaldehyde as substrate and varied concentration of NAD+ as cofactor preincubated for 15 mins followed by subs...More data for this Ligand-Target Pair
Affinity DataKi: 5.90nMAssay Description:Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cellsMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cellsMore data for this Ligand-Target Pair

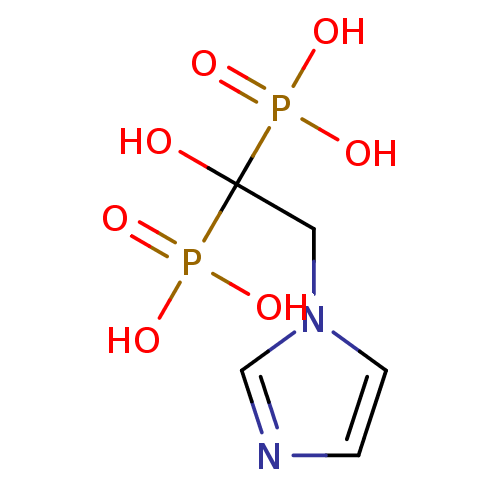
 3D Structure (crystal)
3D Structure (crystal)