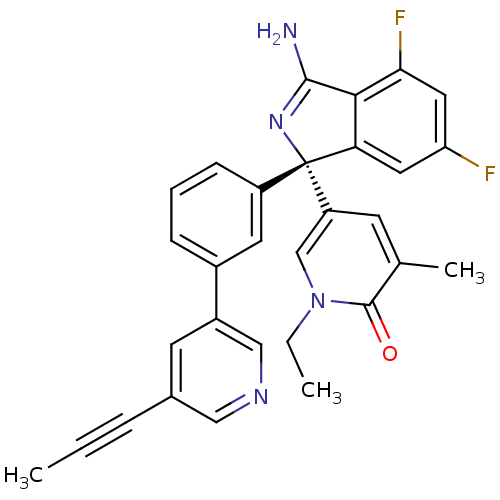
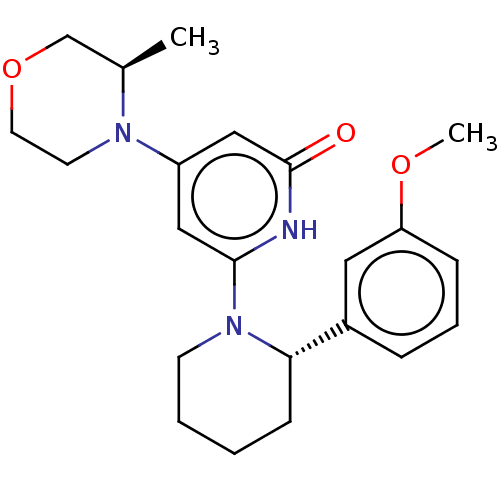
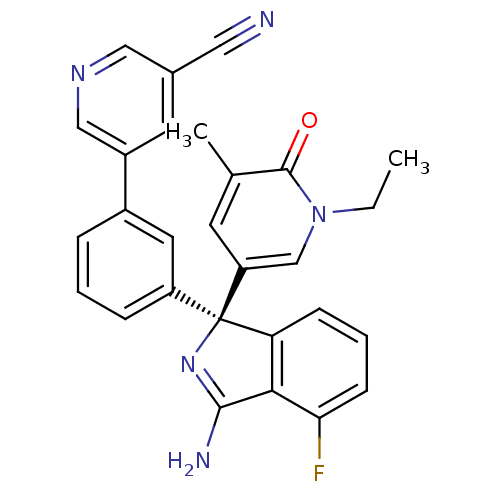
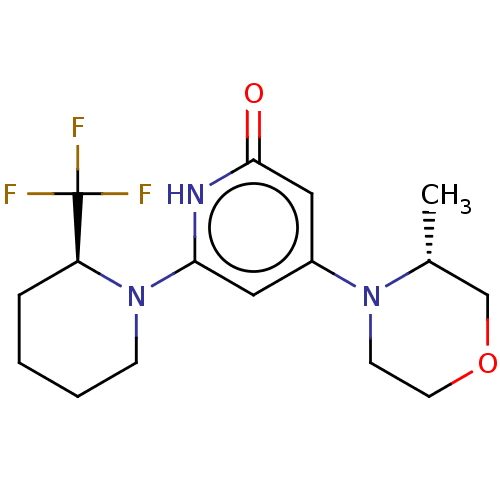
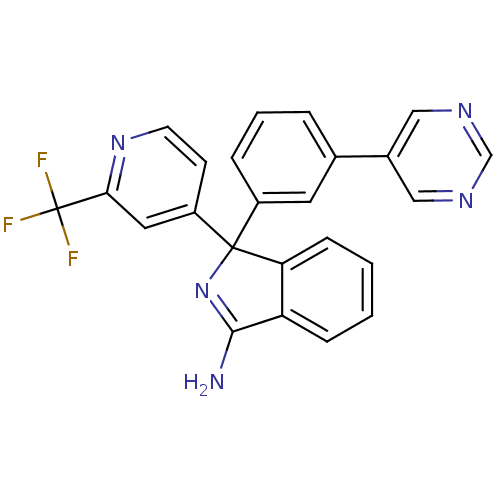
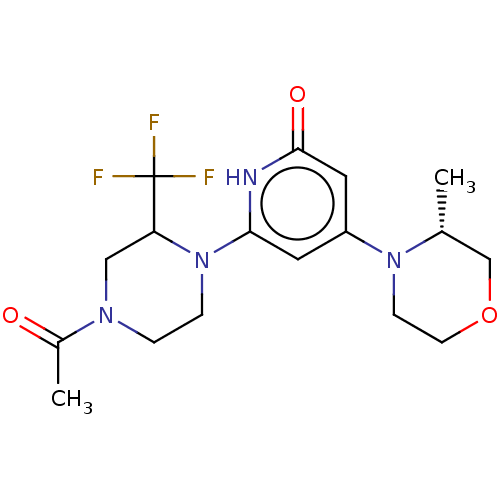
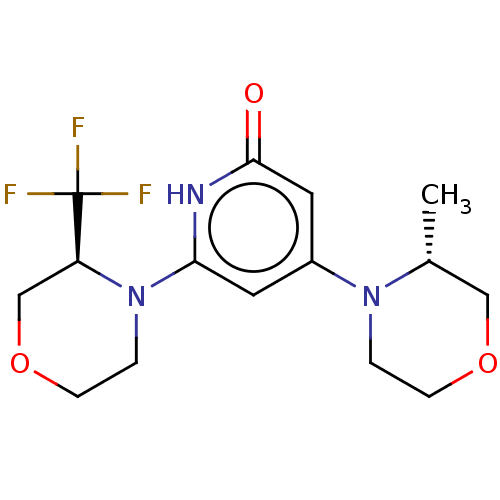
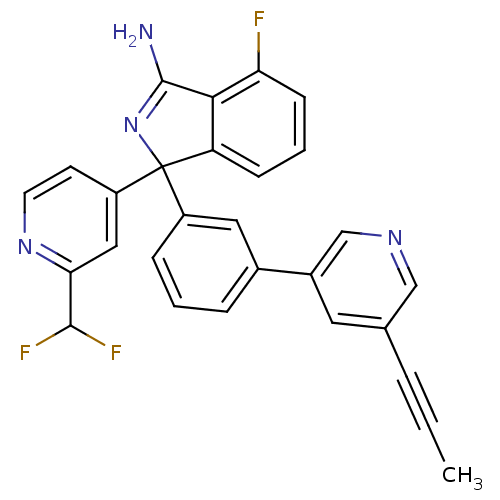
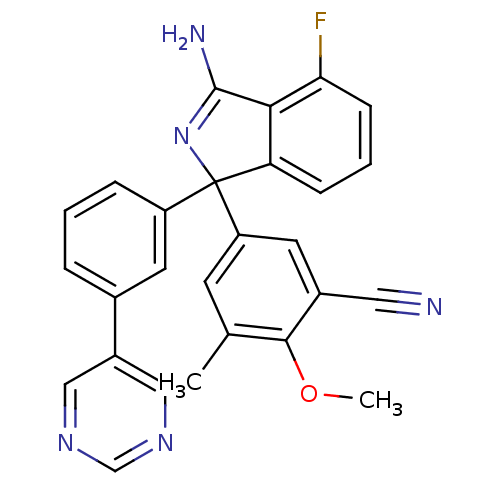
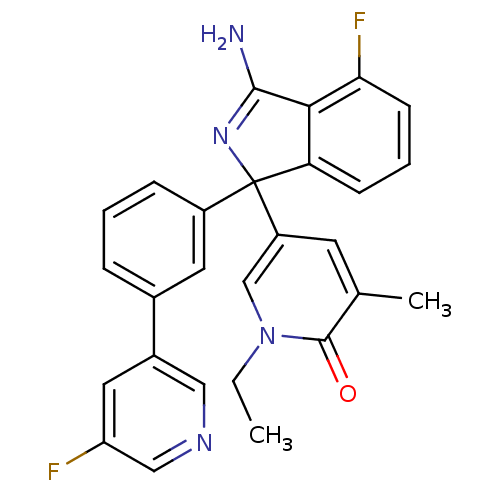
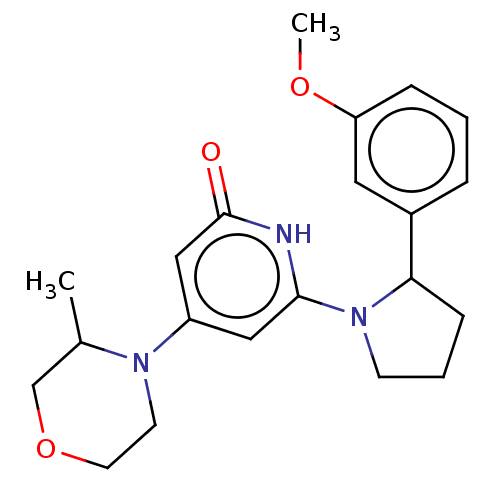
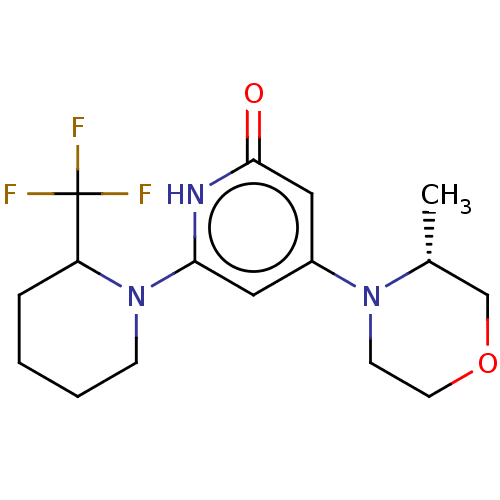
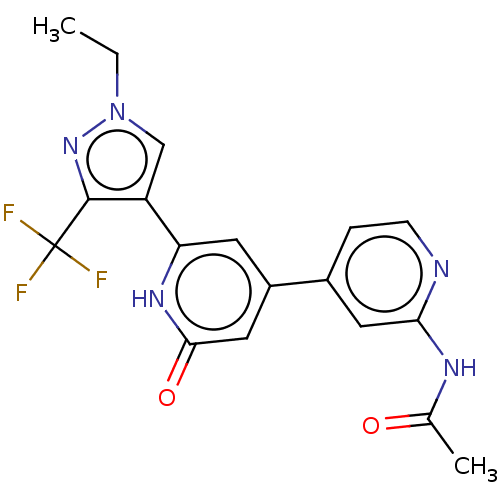
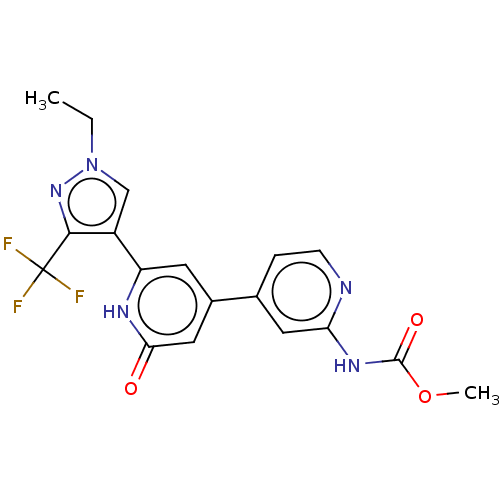
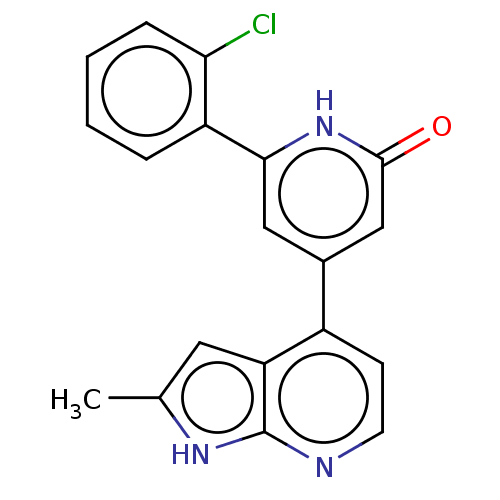
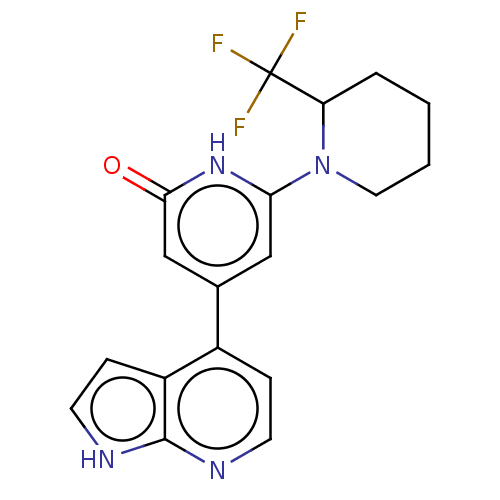
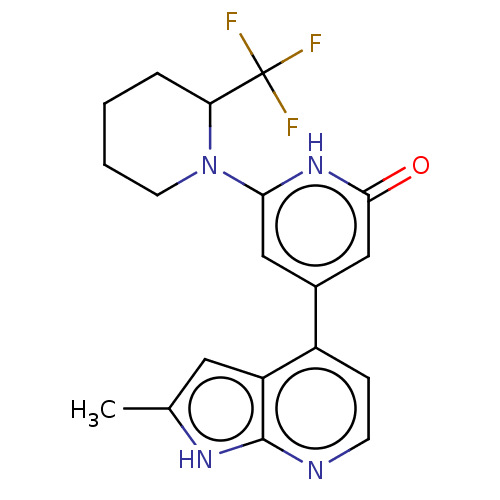
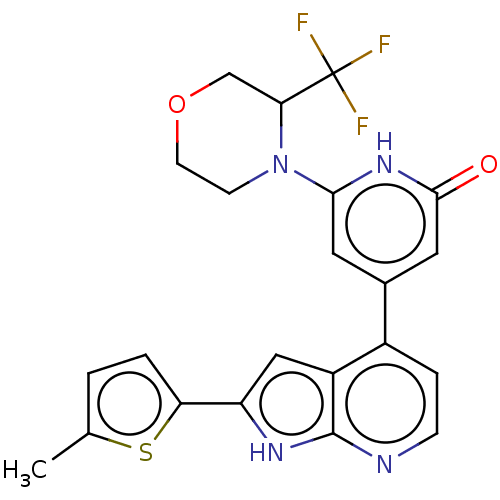
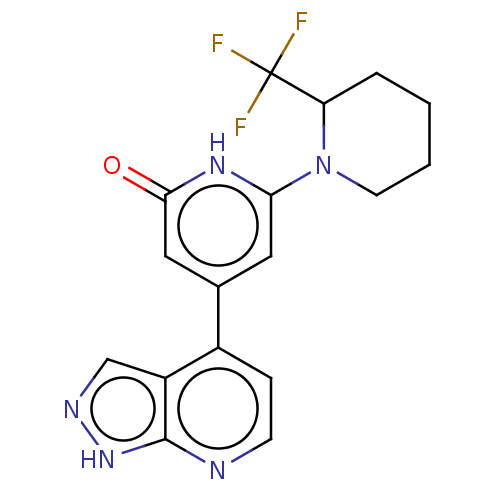
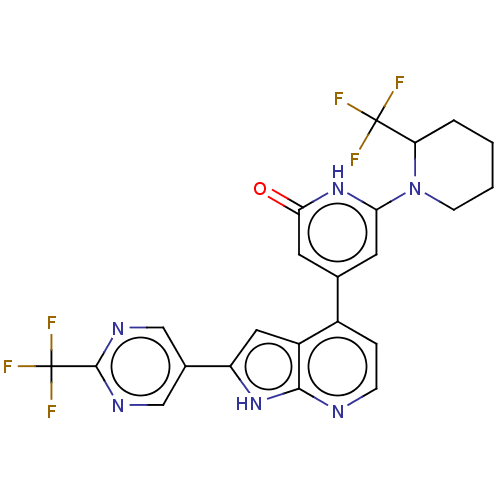
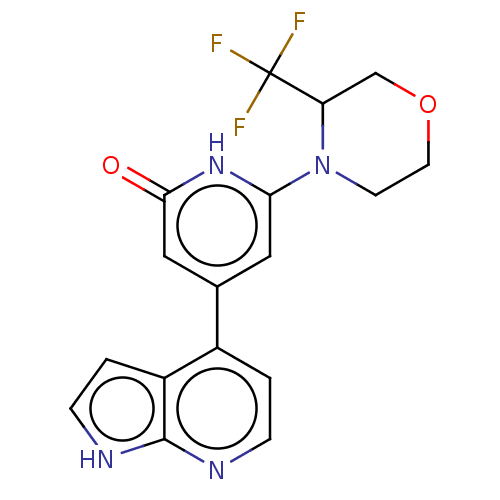
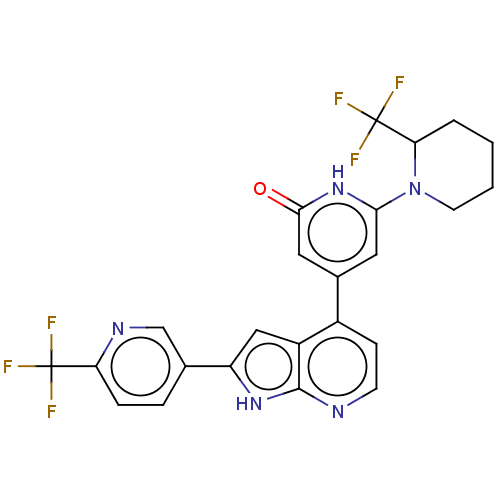
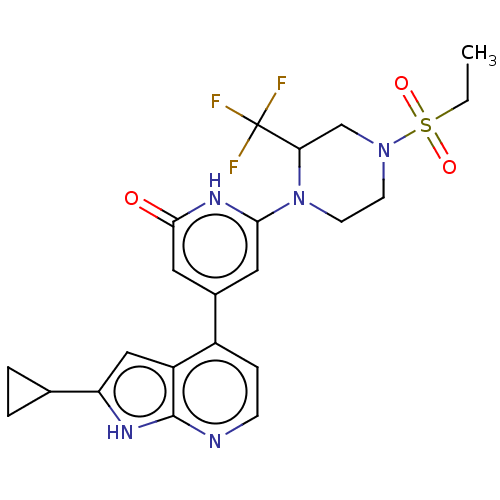
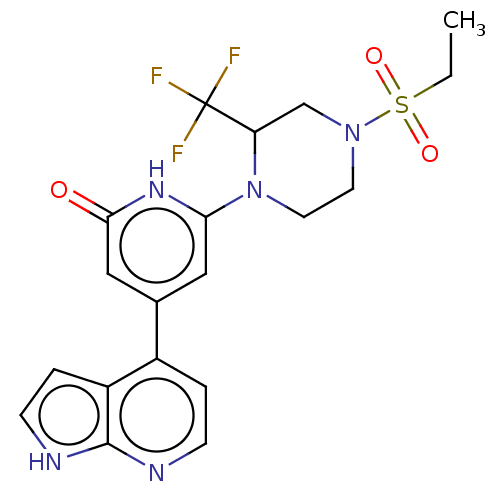
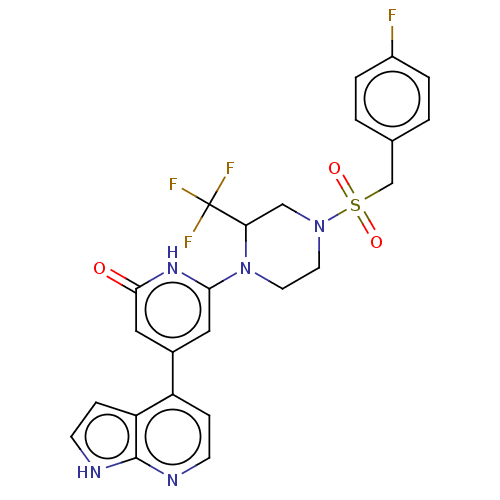
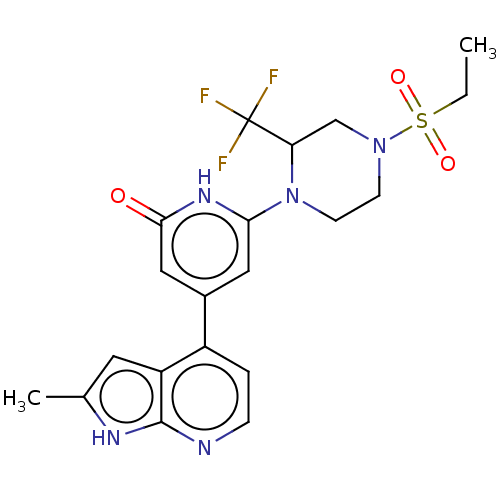
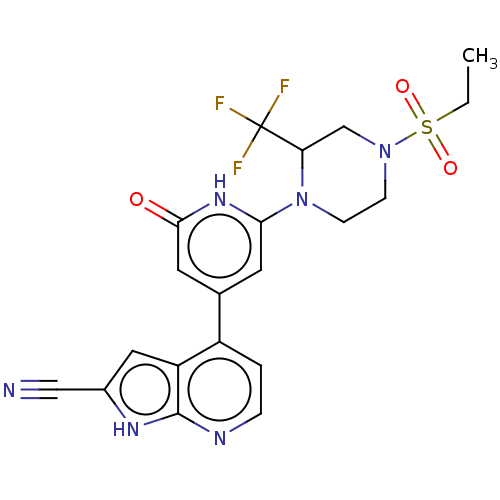
Affinity DataKi: 7.5nMAssay Description:Inhibition of human recombinant BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
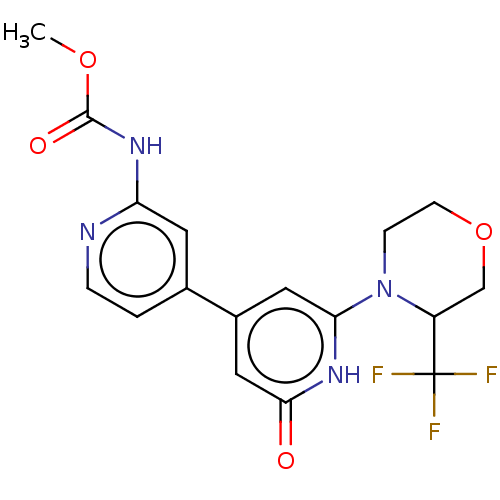
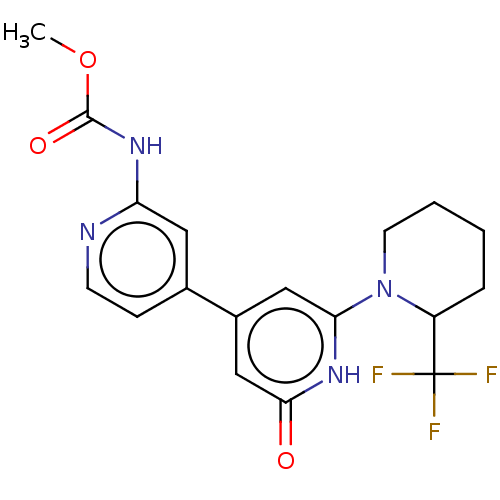
Affinity DataKi: 78nMAssay Description:Inhibition of human recombinant BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
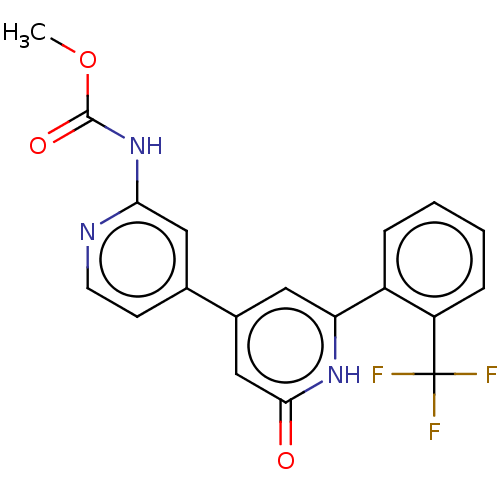
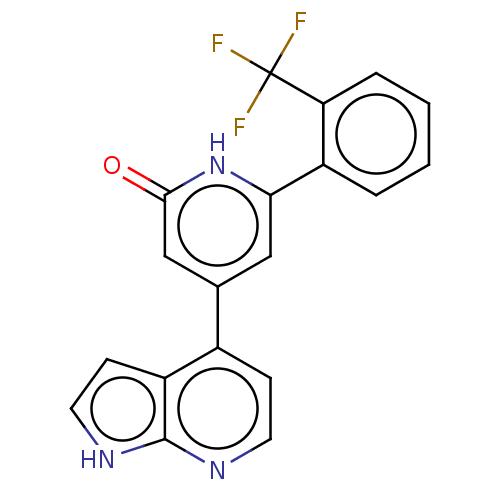
Affinity DataKi: 370nMAssay Description:Inhibition of human recombinant BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
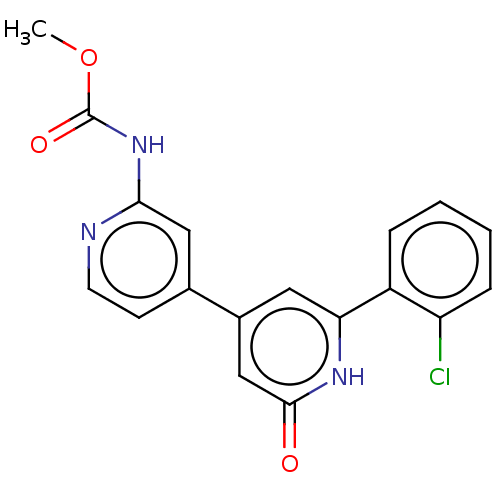
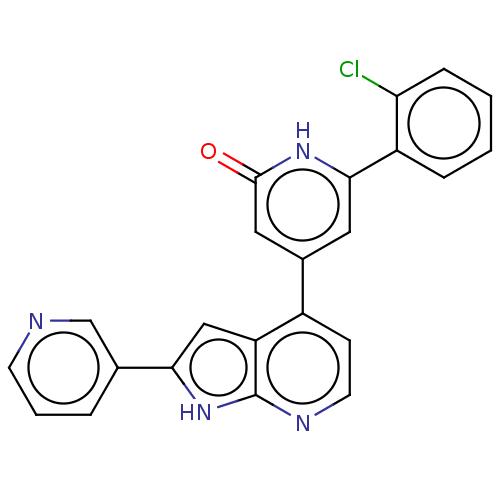
Affinity DataKi: 740nMAssay Description:Inhibition of human recombinant BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataKi: 770nMAssay Description:Inhibition of human recombinant BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Inhibition of human recombinant BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of human recombinant BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: 1nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: 2nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human recombinant BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of human recombinant BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human recombinant BACE1 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of BACE1-mediated amyloid beta 40 release in C57/BL6 mouse primary cortical neurons after overnight incubation by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: 3nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: 3nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of BACE1-mediated amyloid beta 40 release in C57/BL6 mouse primary cortical neurons after overnight incubation by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells assessed as inhibition of sAPPbeta release after 16 hrs by immunoassayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: 4nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: 4nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase catalytic subunit type 3(Homo sapiens (Human))
Sprint Bioscience
US Patent
Sprint Bioscience
US Patent
Affinity DataIC50: <5nMAssay Description:Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ...More data for this Ligand-Target Pair


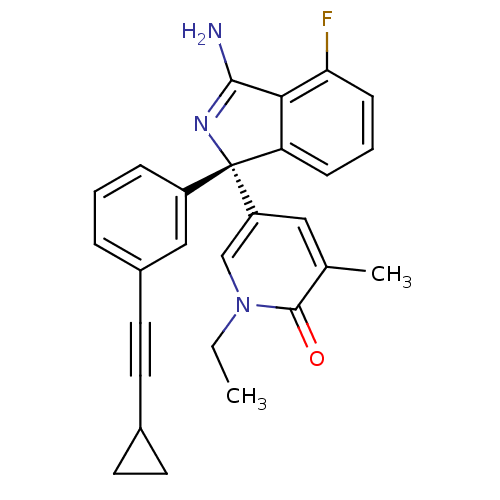
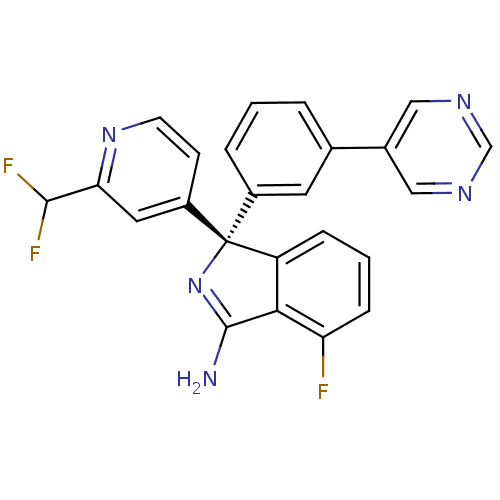
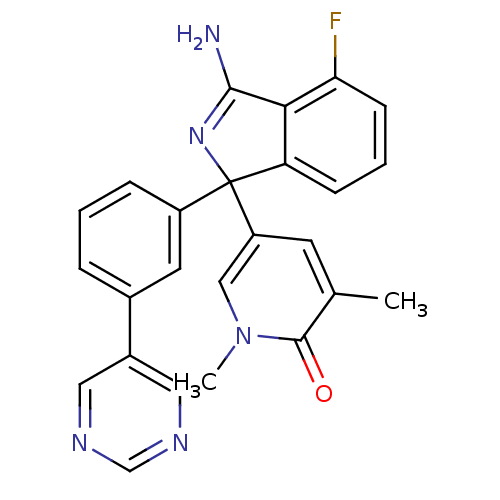
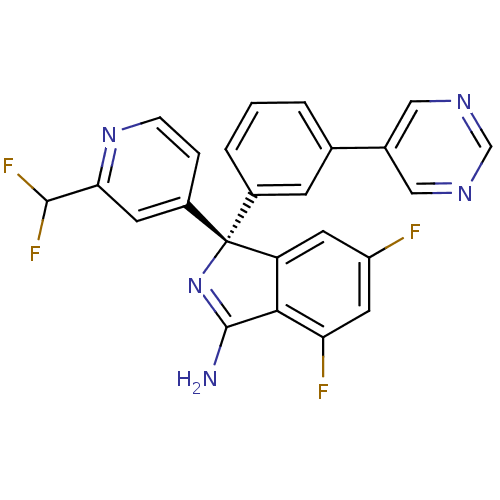
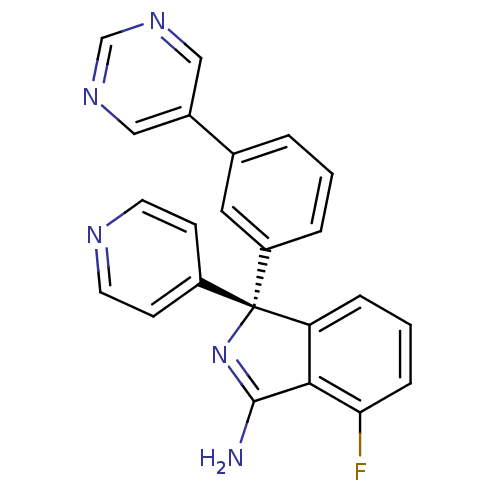
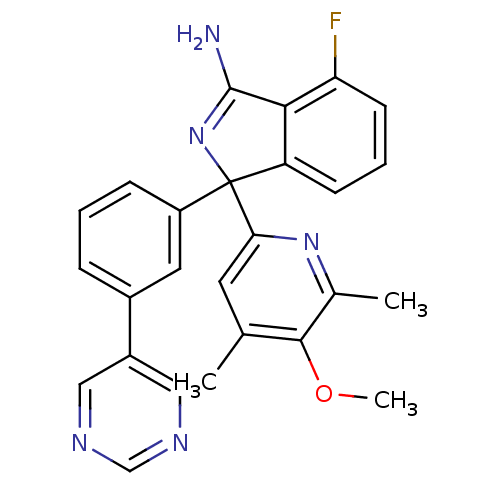
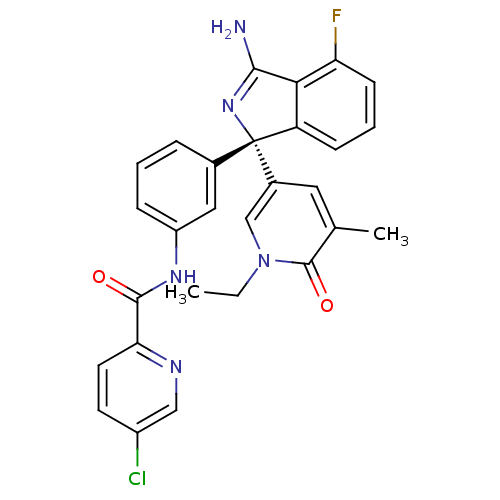
 3D Structure (crystal)
3D Structure (crystal)