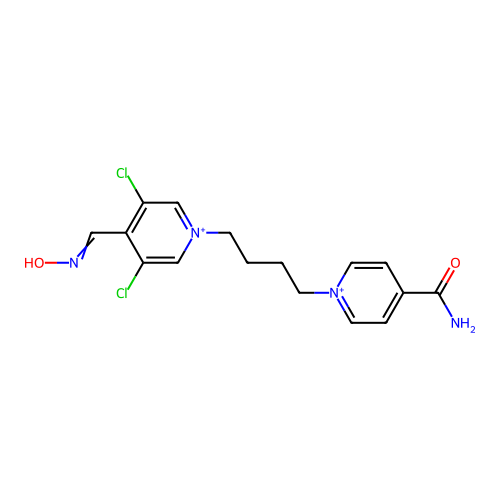
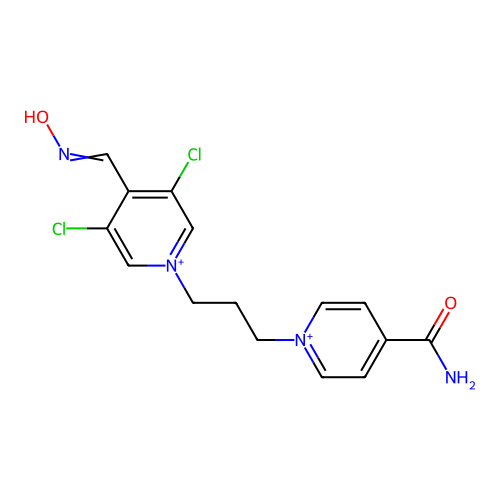
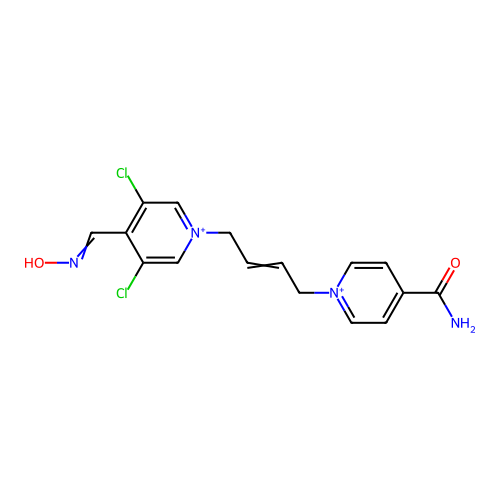
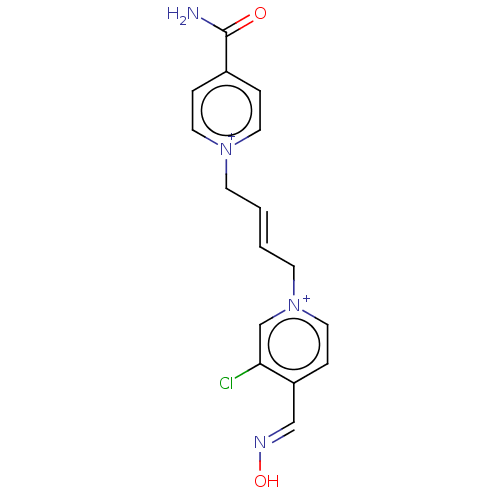
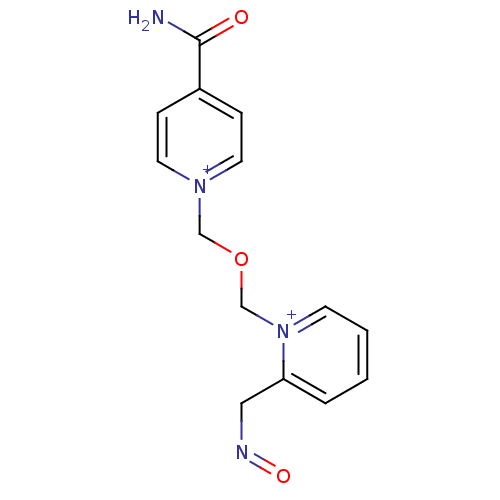
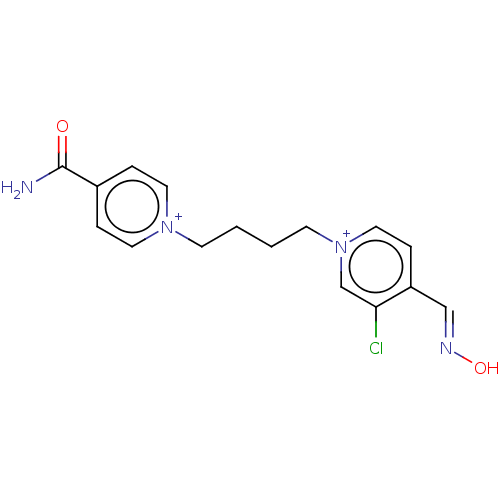
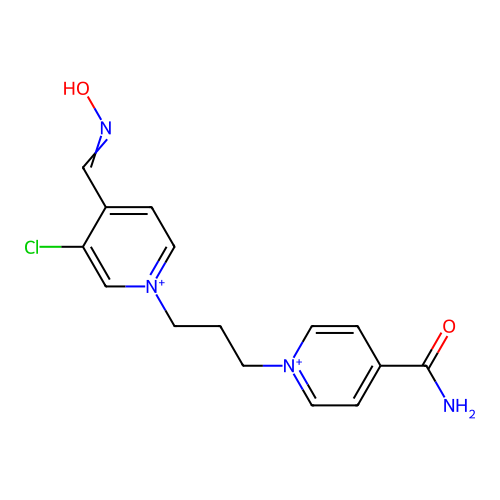
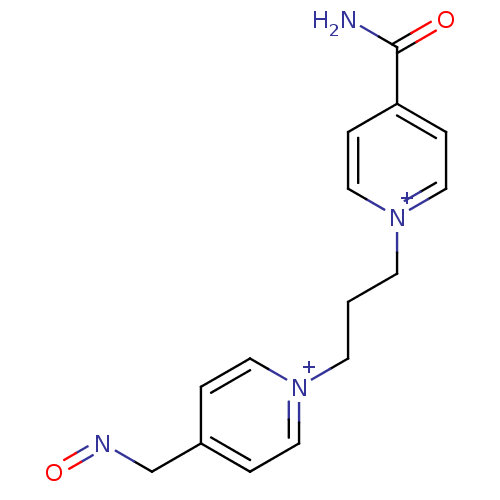
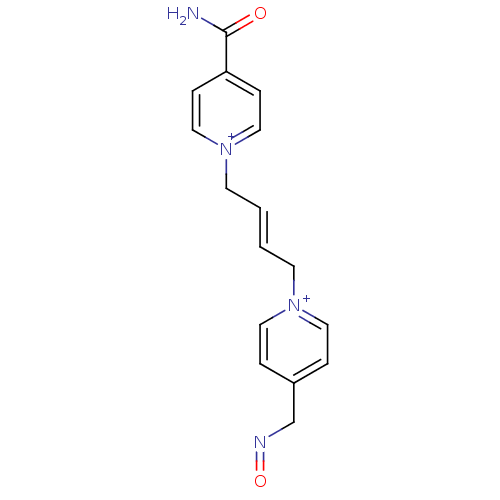
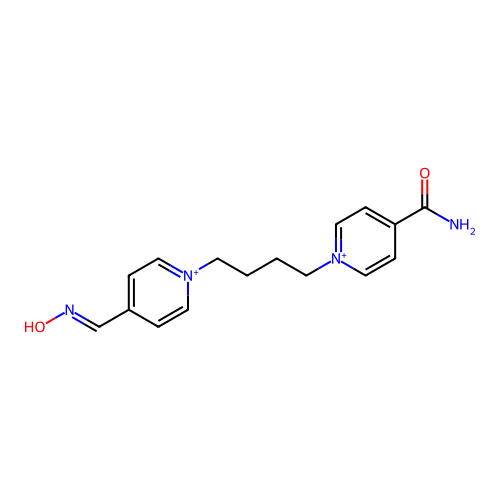
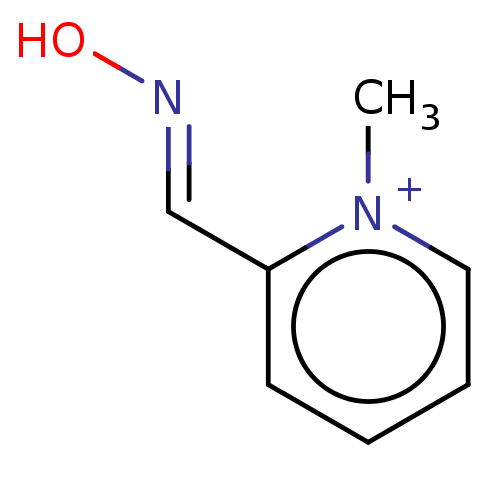
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.20E+4nMAssay Description:Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 2.10E+4nMAssay Description:Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 2.40E+4nMAssay Description:Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 2.50E+4nMAssay Description:Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 3.60E+4nMAssay Description:Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 4.20E+4nMAssay Description:Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 7.30E+4nMAssay Description:Reversible inhibition of human erythrocytic AChE using acetylthiocholine iodide as substrate measured up to 2 mins by spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 9.00E+4nMAssay Description:Reversible inhibition of human erythrocytic AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.10E+5nMAssay Description:Reversible inhibition of human erythrocytic AChE using acetylthiocholine iodide as substrate measured up to 2 mins by spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.40E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.80E+5nMAssay Description:Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.87E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 1.95E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 2.15E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 2.65E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 2.73E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 2.94E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 3.90E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Institute For Medical Research And Occupational Health
Curated by ChEMBL
Affinity DataKi: 9.10E+5nMAssay Description:Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric methodMore data for this Ligand-Target Pair