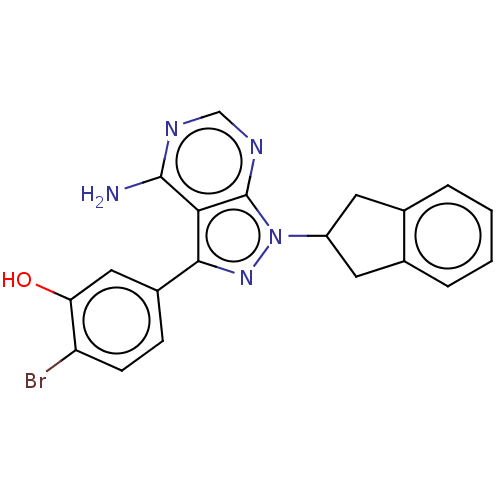
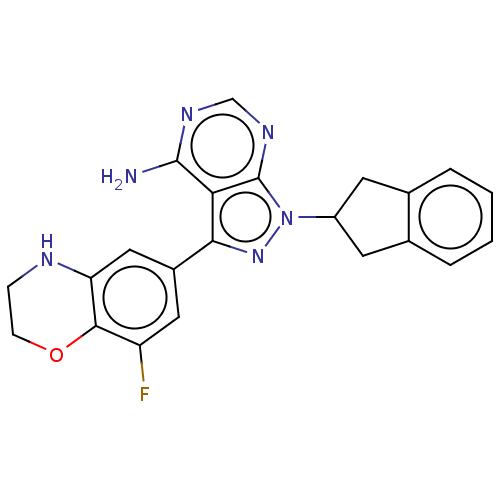
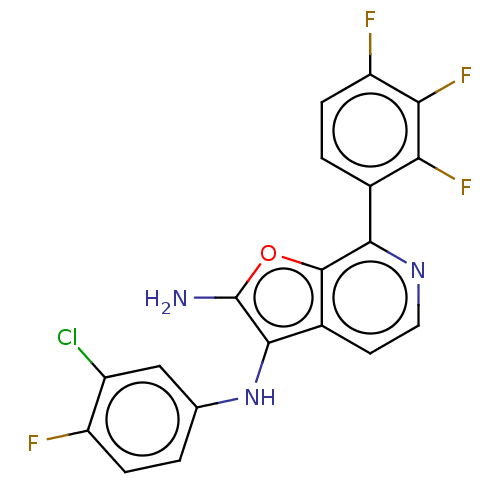
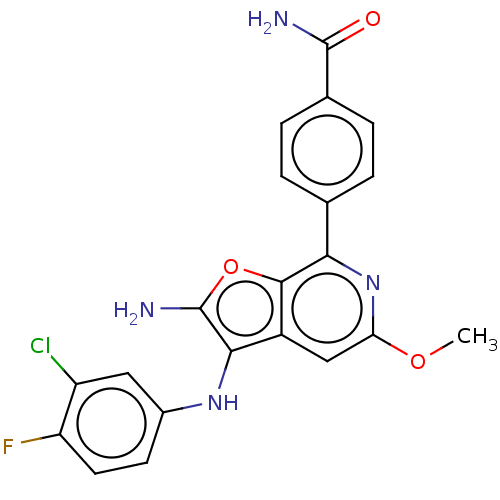
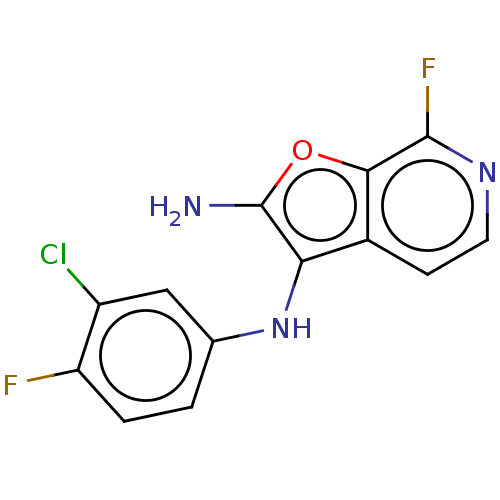
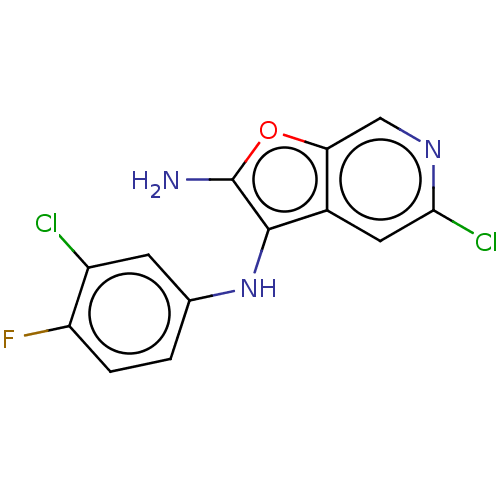
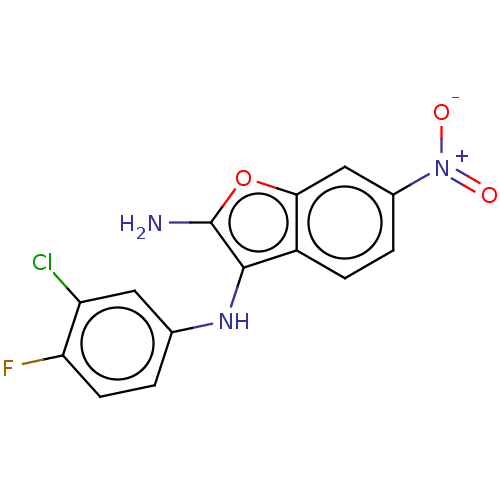
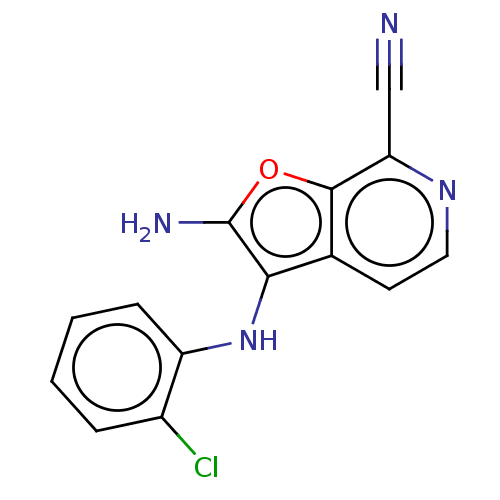
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: <0.5nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
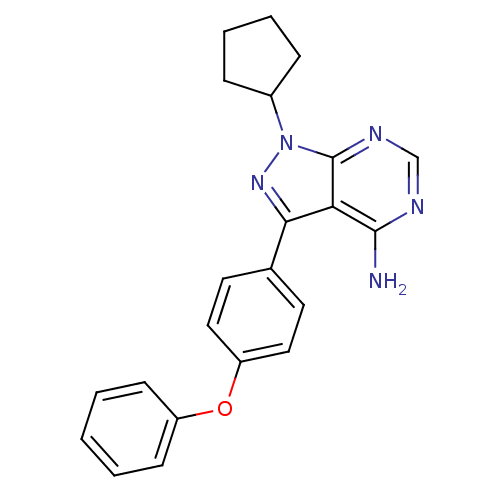
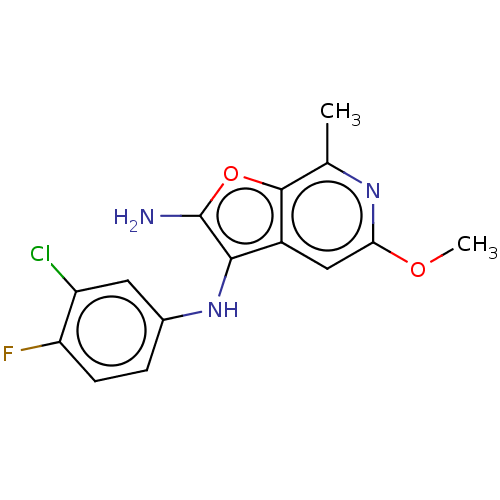
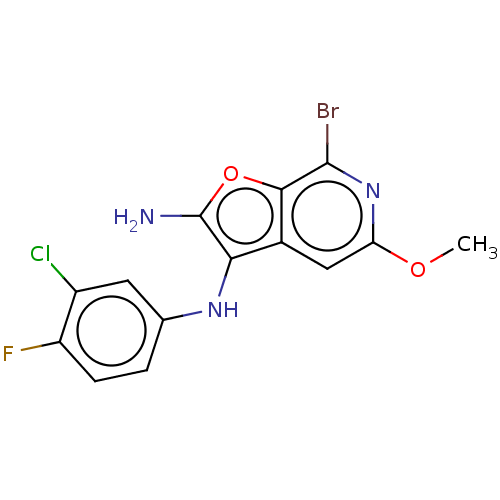
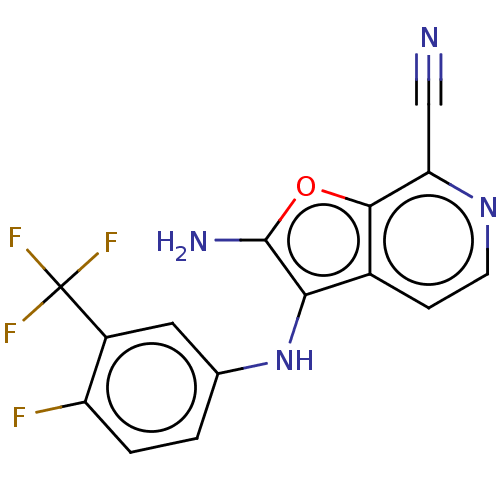
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of PI3Kdelta (unknown origin) in presence of [gamma-32P]ATP by phosphorimaging assayMore data for this Ligand-Target Pair
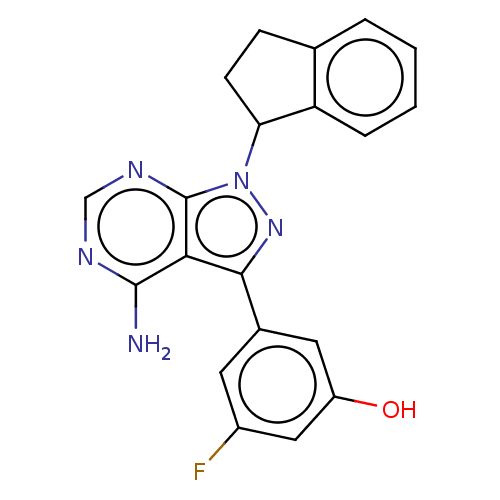
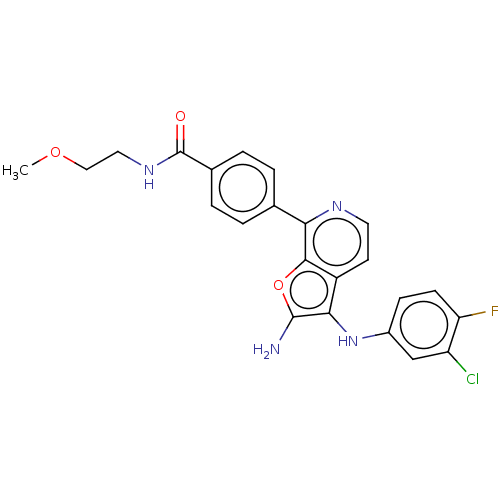
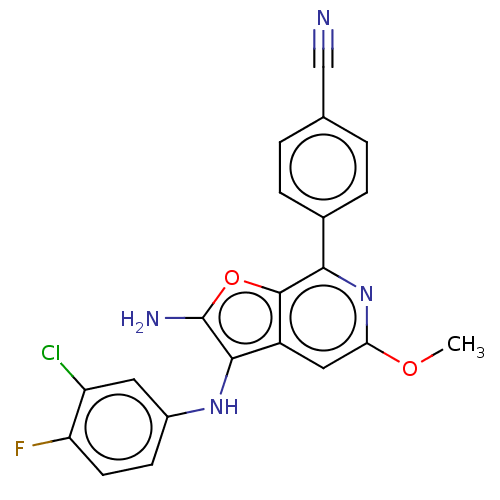
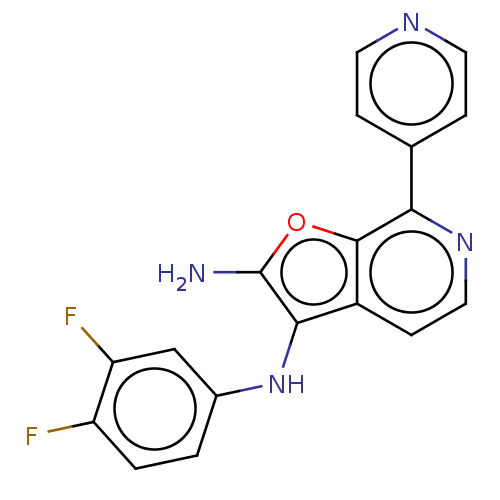
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
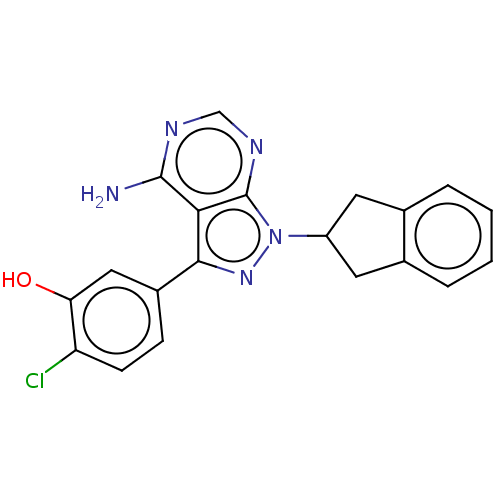
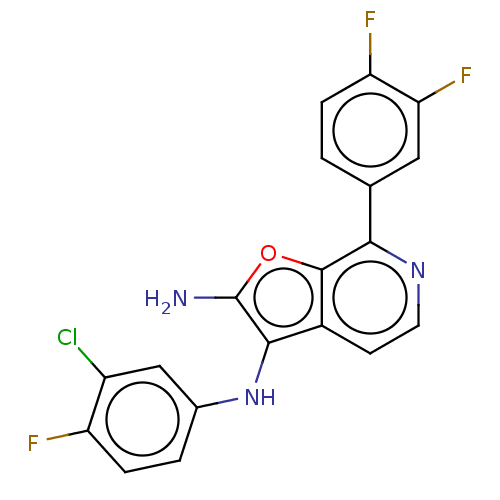
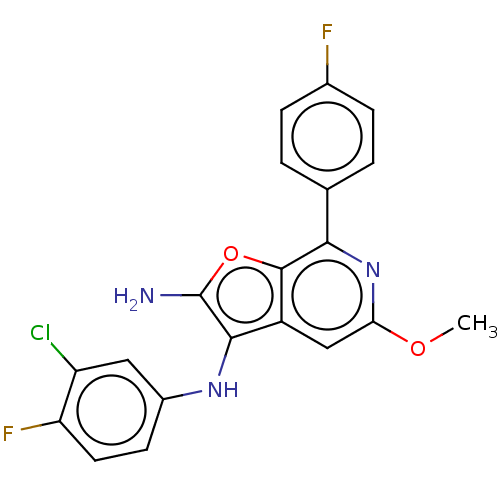
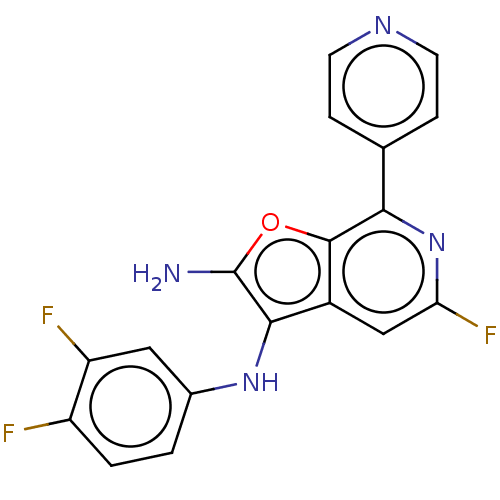
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:Inhibition of BTK (unknown origin) by FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of PI3Kgamma (unknown origin) in presence of [gamma-32P]ATP by phosphorimaging assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 61nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 84nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 86nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 104nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 142nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 172nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 176nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 176nMAssay Description:Inhibition of human BTK using KVEKIGEGTYGVVYK as substrate after 20 mins by [gamma-33P]ATP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair
Affinity DataIC50: <200nMAssay Description:Human indoleamine 2,3-dioxygenasel (hIDO1) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formyl...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)