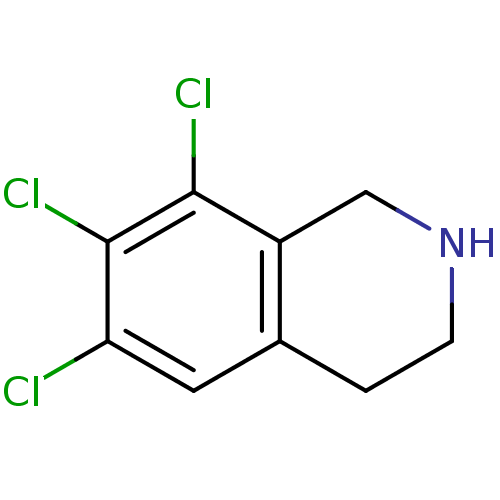
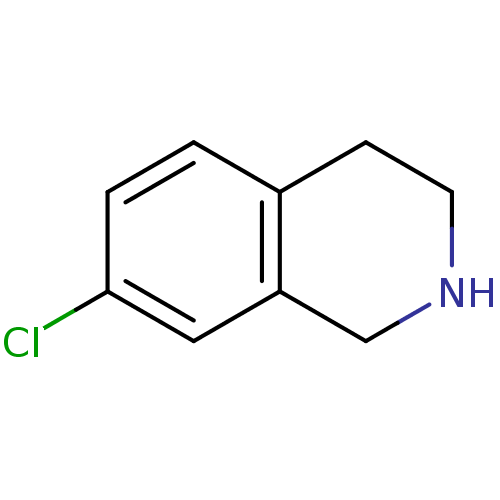
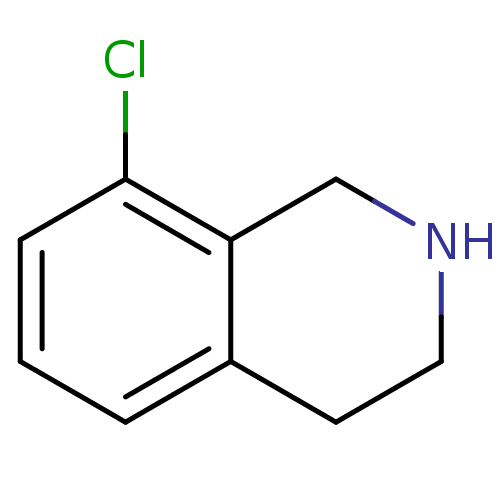
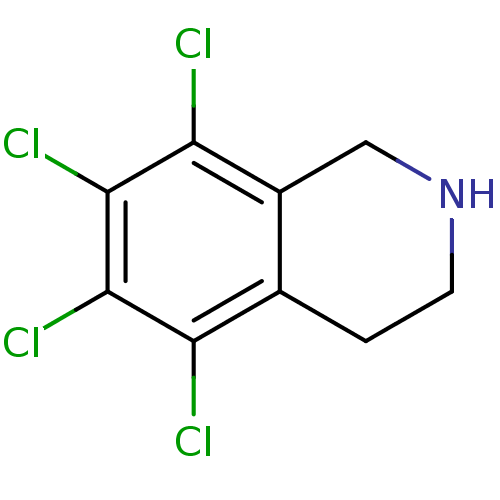
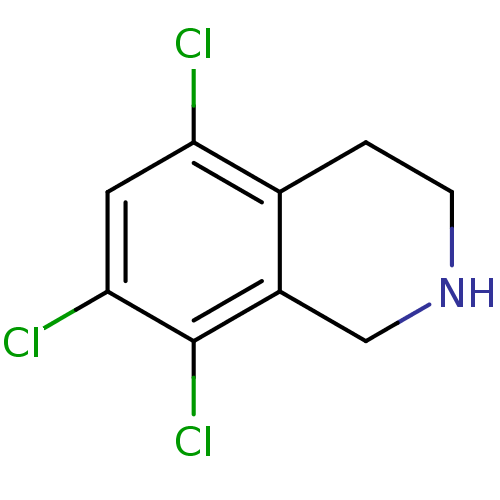
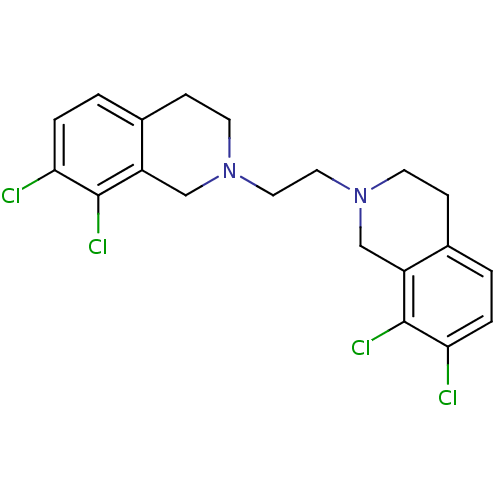
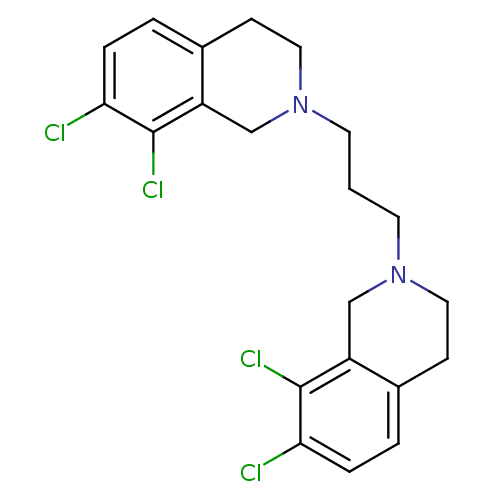
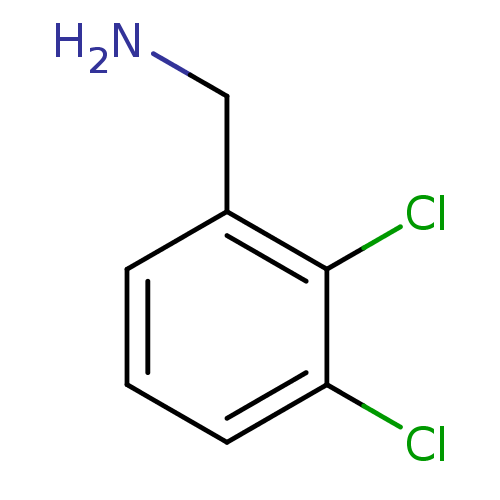
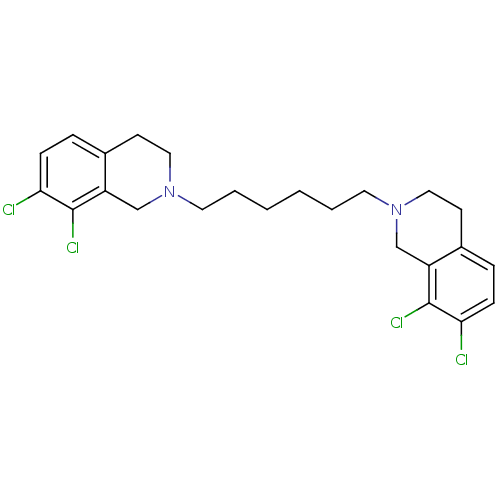
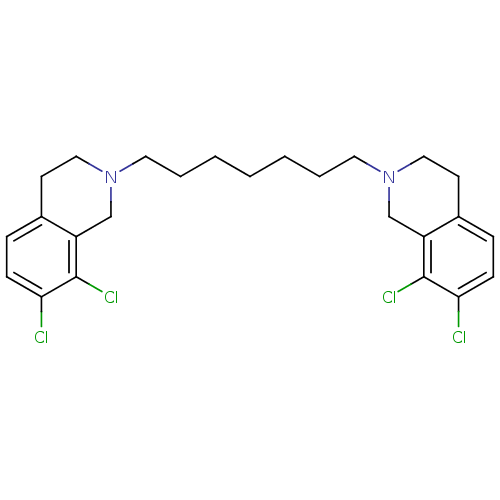
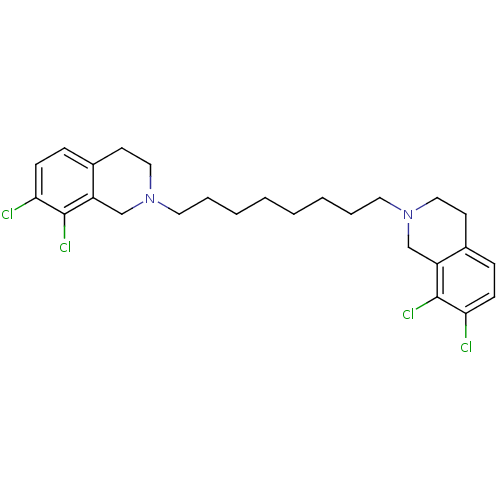
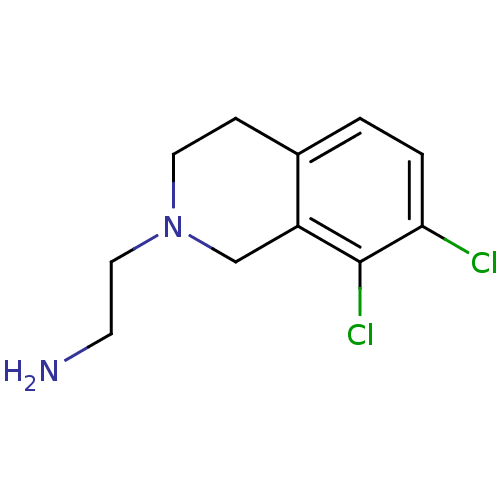
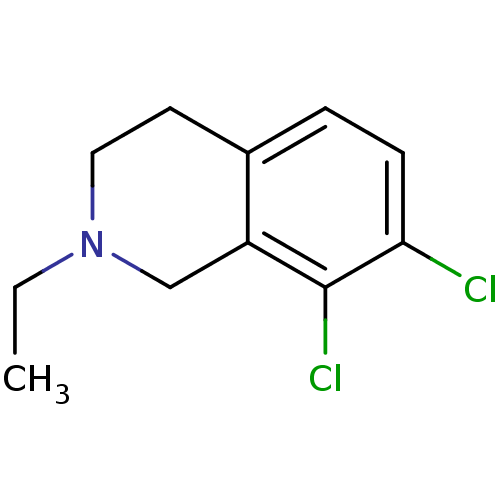
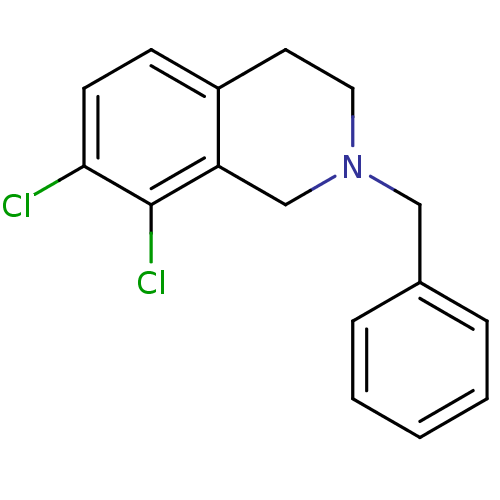
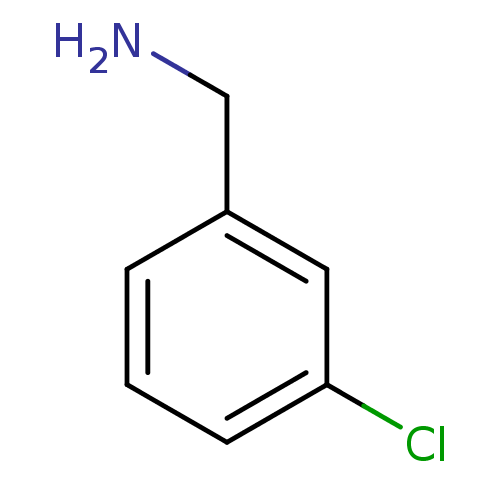
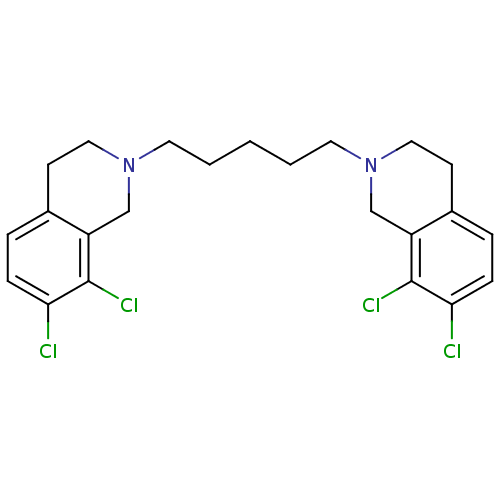
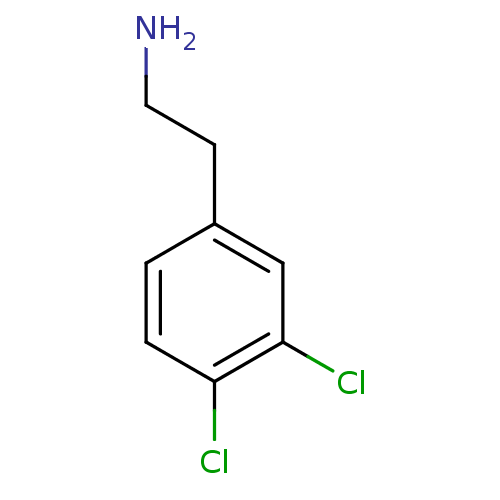
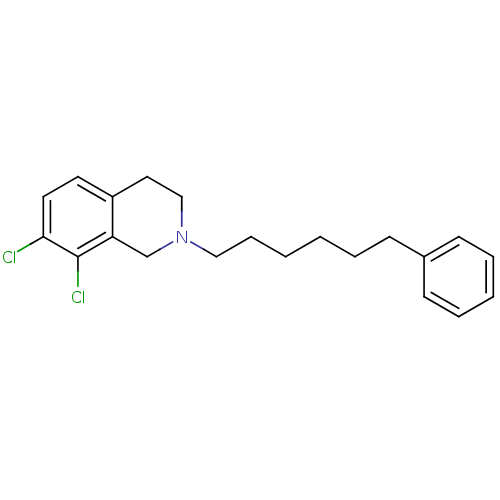
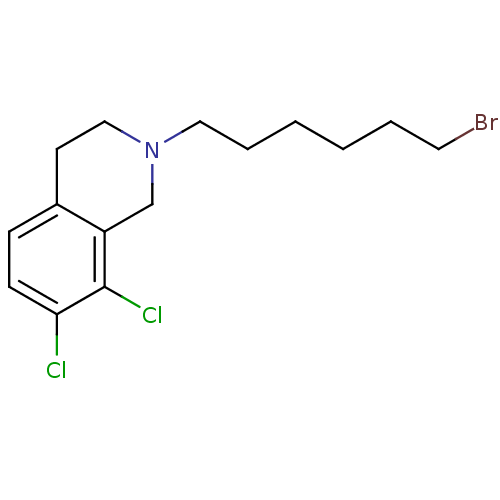
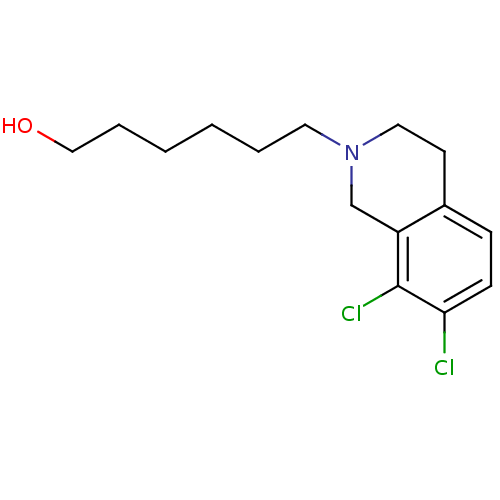
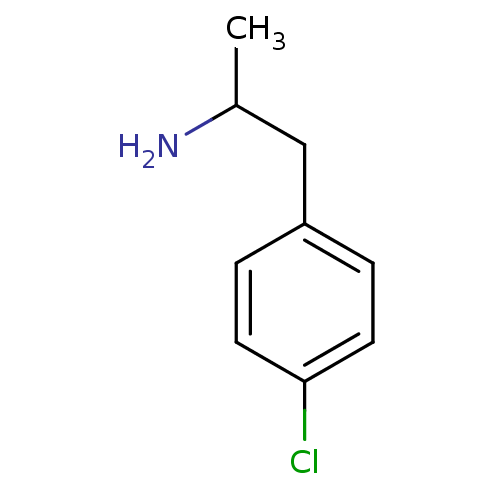
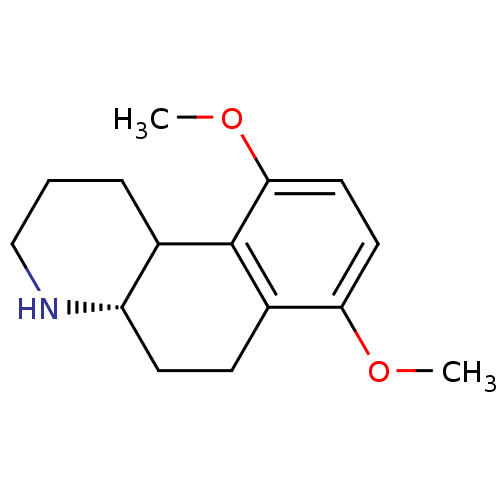
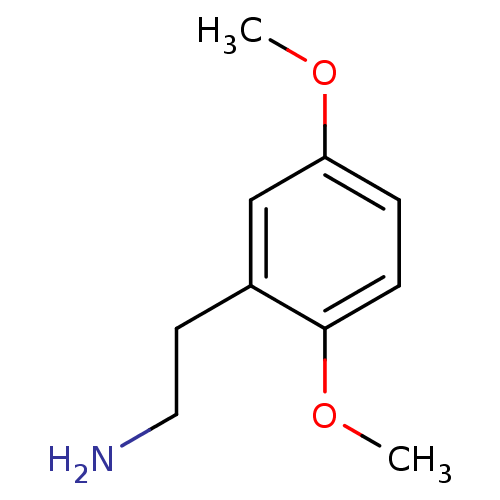
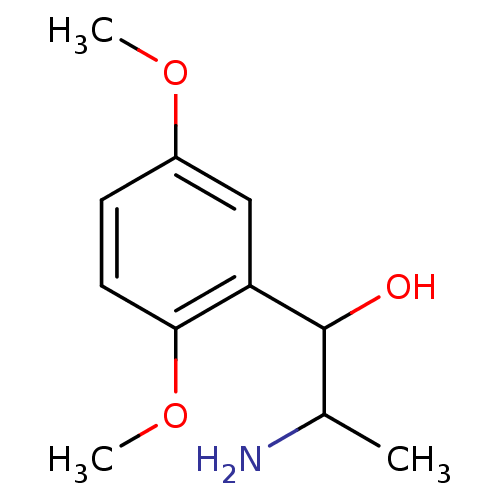
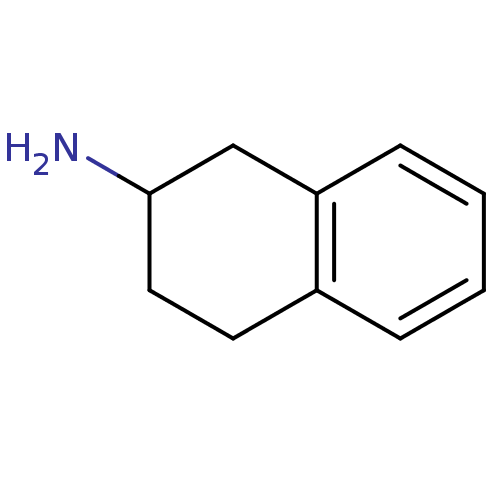TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity for phenylethanolamine N-methyl-transferase was determined.More data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potencyMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Bos taurus (bovine))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Binding affinity for phenylethanolamine N-methyl-transferase was determined.More data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potencyMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 52nMAssay Description:Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potencyMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 59nMAssay Description:Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potencyMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potencyMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potencyMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potencyMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 9.00E+3nMAssay Description:Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:In vitro inhibitory activity against human phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.00E+4nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferaseMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.00E+5nMAssay Description:Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
Affinity DataEC50: 170nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 3.00E+4nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 120nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 3.00E+4nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 200nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 5.30E+3nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 1.10E+4nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 3.00E+4nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 720nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair
Affinity DataEC50: 340nMAssay Description:Alpha-1 adrenergic receptor agonist activity in rabbit ear arteryMore data for this Ligand-Target Pair

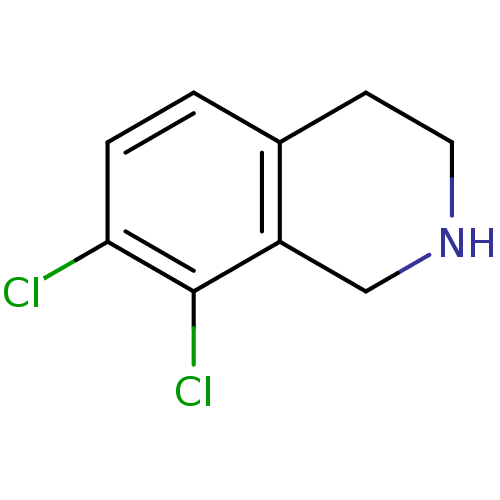
 3D Structure (crystal)
3D Structure (crystal)