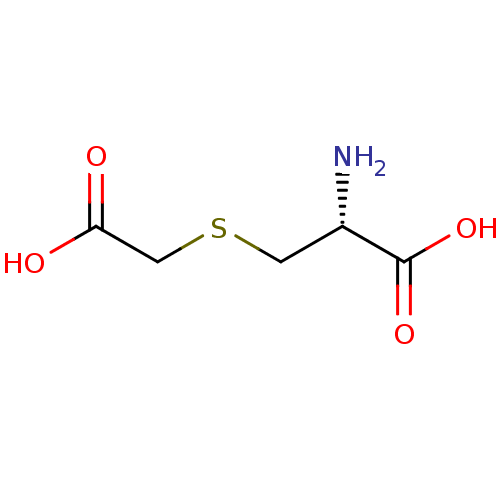
 carbocysteine (R)-S-(carboxymethyl)cysteine (2R)-2-amino-3-[(carboxymethyl)sulfanyl]propanoic acidS-(carboxymethyl)-L-cysteine BDBM50213735 CHEMBL396416 S-(carboxymethyl)-(R)-cysteine S-carboxymethyl-L-cysteine (L)-2-Amino-3-(carboxymethylthio)propionic acid L-3-((carboxymethyl)thio)alanine
carbocysteine (R)-S-(carboxymethyl)cysteine (2R)-2-amino-3-[(carboxymethyl)sulfanyl]propanoic acidS-(carboxymethyl)-L-cysteine BDBM50213735 CHEMBL396416 S-(carboxymethyl)-(R)-cysteine S-carboxymethyl-L-cysteine (L)-2-Amino-3-(carboxymethylthio)propionic acid L-3-((carboxymethyl)thio)alanine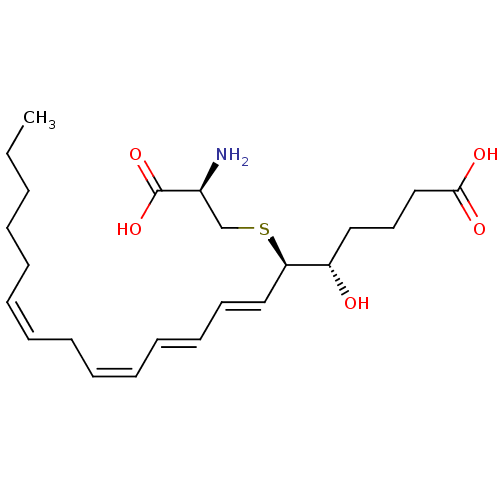 leukotriene E4 (5S-(5R*,6S*(S*),7E,9E,11Z,14Z))-6-((2-Amino-2-carboxyethyl)thio)-5-hydroxy-7,9,11,14-eicosatetraenoic acid BDBM50297387 LTE4 CHEMBL509456 5S-hydroxy,6R-(S-cysteinyl),7E,9E,11Z,14Z-eicosatetraenoic acid (5S,6R,7E,9E,11Z,14Z)-6-(cystein-S-yl)-5-hydroxyicosa-7,9,11,14-tetraenoic acidS-{(1R,2E,4E,6Z,9Z)-1-[(1S)-4-carboxy-1-hydroxybutyl]pentadeca-2,4,6,9-tetraen-1-yl}-L-cysteine
leukotriene E4 (5S-(5R*,6S*(S*),7E,9E,11Z,14Z))-6-((2-Amino-2-carboxyethyl)thio)-5-hydroxy-7,9,11,14-eicosatetraenoic acid BDBM50297387 LTE4 CHEMBL509456 5S-hydroxy,6R-(S-cysteinyl),7E,9E,11Z,14Z-eicosatetraenoic acid (5S,6R,7E,9E,11Z,14Z)-6-(cystein-S-yl)-5-hydroxyicosa-7,9,11,14-tetraenoic acidS-{(1R,2E,4E,6Z,9Z)-1-[(1S)-4-carboxy-1-hydroxybutyl]pentadeca-2,4,6,9-tetraen-1-yl}-L-cysteine
- Macchiarulo, A; Gioiello, A; Thomas, C; Pols, TW; Nuti, R; Ferrari, C; Giacchè, N; De Franco, F; Pruzanski, M; Auwerx, J; Schoonjans, K; Pellicciari, R Probing the Binding Site of Bile Acids in TGR5. ACS Med Chem Lett 4: 1158-62 (2013)
- Mostarda, S; Passeri, D; Carotti, A; Cerra, B; Colliva, C; Benicchi, T; Macchiarulo, A; Pellicciari, R; Gioiello, A Synthesis, physicochemical properties, and biological activity of bile acids 3-glucuronides: Novel insights into bile acid signalling and detoxification. Eur J Med Chem 144: 349-358 (2018)
- Wang, H; Cao, R; Ke, CF; Liu, Y; Wada, T; Inoue, Y Diastereomeric molecular recognition and binding behavior of bile acids by L/D-tryptophan-modified beta-cyclodextrins. J Org Chem 70: 8703-11 (2005)
- Luxenburger, A; Harris, LD; Ure, EM; Jiao, W; Woolhouse, AD; Cameron, SA; Weymouth-Wilson, A; Furneaux, RH; Pitman, JL; Hinkley, SFR The discovery of 12β-methyl-17-epi-18-nor-bile acids as potent and selective TGR5 agonists. Eur J Med Chem 250: (2023)
- Pellicciari, R; Sato, H; Gioiello, A; Costantino, G; Macchiarulo, A; Sadeghpour, BM; Giorgi, G; Schoonjans, K; Auwerx, J Nongenomic actions of bile acids. Synthesis and preliminary characterization of 23- and 6,23-alkyl-substituted bile acid derivatives as selective modulators for the G-protein coupled receptor TGR5. J Med Chem 50: 4265-8 (2007)
- Akita, H; Suzuki, H; Ito, K; Kinoshita, S; Sato, N; Takikawa, H; Sugiyama, Y Characterization of bile acid transport mediated by multidrug resistance associated protein 2 and bile salt export pump. Biochim Biophys Acta 1511: 7-16 (2001)
- Rais, R; Acharya, C; Tririya, G; Mackerell, AD; Polli, JE Molecular switch controlling the binding of anionic bile acid conjugates to human apical sodium-dependent bile acid transporter. J Med Chem 53: 4749-60 (2010)
- Gioiello, A; Macchiarulo, A; Carotti, A; Filipponi, P; Costantino, G; Rizzo, G; Adorini, L; Pellicciari, R Extending SAR of bile acids as FXR ligands: discovery of 23-N-(carbocinnamyloxy)-3a,7a-dihydroxy-6a-ethyl-24-nor-5β-cholan-23-amine. Bioorg Med Chem 19: 2650-8 (2011)
- Funk, C; Pantze, M; Jehle, L; Ponelle, C; Scheuermann, G; Lazendic, M; Gasser, R Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and trogl Toxicology 167: 83-98 (2001)
- Hallén, S; Björquist, A; Ostlund-Lindqvist, AM; Sachs, G Identification of a region of the ileal-type sodium/bile acid cotransporter interacting with a competitive bile acid transport inhibitor. Biochemistry 41: 14916-14924 (2002)
- Eckhardt, M; Frattini, S; Langkopf, E; Wagner, H Indanyloxydihydrobenzofuranylacetic acids US Patent US9597310 (2017)
- Tu, N; Link, JT; Sorensen, BK; Emery, M; Grynfarb, M; Goos-Nilsson, A; Nguyen, B Bile acid conjugates of a nonsteroidal glucocorticoid receptor modulator. Bioorg Med Chem Lett 14: 4179-83 (2004)
- Yu, DD; Sousa, KM; Mattern, DL; Wagner, J; Fu, X; Vaidehi, N; Forman, BM; Huang, W Stereoselective synthesis, biological evaluation, and modeling of novel bile acid-derived G-protein coupled bile acid receptor 1 (GP-BAR1, TGR5) agonists. Bioorg Med Chem 23: 1613-28 (2015)
- Firooznia, F; Lin, T; Mertz, E; Sidduri, A; So, S; Tilley, JW Piperidinyl naphthylacetic acids US Patent US8691993 (2014)
- Firooznia, F; Lin, T; Mertz, E; So, S; Sidduri, A; Tilley, JW Substituted naphthylacetic acids US Patent US9000044 (2015)
- Nakhi, A; Wong, HL; Weldy, M; Khoruts, A; Sadowsky, MJ; Dosa, PI Structural modifications that increase gut restriction of bile acid derivatives. RSC Med Chem 12: 394-405 (2021)
- Swierczek, K; Pandey, AS; Peters, JW; Hengge, AC A comparison of phosphonothioic acids with phosphonic acids as phosphatase inhibitors. J Med Chem 46: 3703-8 (2003)
- Iwata, F; Sato, S; Mukai, T; Yamada, S; Takeo, J; Abe, A; Okita, T; Kawahara, H Lorneic acids, trialkyl-substituted aromatic acids from a marine-derived actinomycete. J Nat Prod 72: 2046-8 (2009)
- Cheng, K; Khurana, S; Chen, Y; Kennedy, RH; Zimniak, P; Raufman, JP Lithocholylcholine, a bile acid/acetylcholine hybrid, is a muscarinic receptor antagonist. J Pharmacol Exp Ther 303: 29-35 (2002)
- Richards, SJ; von Geldern, TW; Jacobson, P; Wilcox, D; Nguyen, P; Ohman, L; Osterlund, M; Gelius, B; Grynfarb, M; Goos-Nilsson, A; Wang, J; Fung, S; Kalmanovich, M Synthesis and activity of novel bile-acid conjugated glucocorticoid receptor antagonists. Bioorg Med Chem Lett 16: 6086-90 (2006)
- Sepe, V; Renga, B; Festa, C; D'Amore, C; Masullo, D; Cipriani, S; Di Leva, FS; Monti, MC; Novellino, E; Limongelli, V; Zampella, A; Fiorucci, S Modification on ursodeoxycholic acid (UDCA) scaffold. discovery of bile acid derivatives as selective agonists of cell-surface G-protein coupled bile acid receptor 1 (GP-BAR1). J Med Chem 57: 7687-701 (2014)
- Zhang, Z; Ruddraraju, KV N-aryl oxamic acids US Patent US11192850 (2021)
- Hazra, B; Pore, V; Dey, S; Datta, S; Darokar, M; Saikia, D; Khanuja, SP; Thakur, A Bile acid amides derived from chiral amino alcohols: novel antimicrobials and antifungals. Bioorg Med Chem Lett 14: 773-7 (2004)
- Or, YS; Wang, G; Shen, R; Xing, X Bile acid analogs as FXR/TGR5 agonists and methods of use thereof US Patent US10266560 (2019)
- Wang, G; Or, YS; Shen, R; Long, J; Dai, P; Xing, X; He, J Bile acid derivatives as FXR/TGR5 agonists and methods of use thereof US Patent US10208081 (2019)
- Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors. Preparation of potent sulfonamides inhibitors incorporating bile acid tails. Bioorg Med Chem Lett 12: 1551-7 (2002)
- Xu, Y Recent Progress on Bile Acid Receptor Modulators for Treatment of Metabolic Diseases. J Med Chem 59: 6553-79 (2016)
- Ellingboe, J; Alessi, T; Millen, J; Sredy, J; King, A; Prusiewicz, C; Guzzo, F; VanEngen, D; Bagli, J (Pyrimidinyloxy)acetic acids and pyrimidineacetic acids as a novel class of aldose reductase inhibitors. J Med Chem 33: 2892-9 (1990)
- Hirano, M; Maeda, K; Hayashi, H; Kusuhara, H; Sugiyama, Y Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J Pharmacol Exp Ther 314: 876-82 (2005)
- Liu, Y; Zhang, L; Yan, H; Wang, Z; Sun, G; Song, X; Zhou, Z; Peng, B; Yan, L; Wu, Q; Li, W; Qi, X Design of Dimeric Bile Acid Derivatives as Potent and Selective Human NTCP Inhibitors. J Med Chem 64: 5973-6007 (2021)
- Tremont, SJ; Lee, LF; Huang, HC; Keller, BT; Banerjee, SC; Both, SR; Carpenter, AJ; Wang, CC; Garland, DJ; Huang, W; Jones, C; Koeller, KJ; Kolodziej, SA; Li, J; Manning, RE; Mahoney, MW; Miller, RE; Mischke, DA; Rath, NP; Fletcher, T; Reinhard, EJ; Tollefson, MB; Vernier, WF; Wagner, GM; Rapp, SR; Beaudry, J; Glenn, K; Regina, K; Schuh, JR; Smith, ME; Trivedi, JS; Reitz, DB Discovery of potent, nonsystemic apical sodium-codependent bile acid transporter inhibitors (Part 1). J Med Chem 48: 5837-52 (2005)
- Huang, HC; Tremont, SJ; Lee, LF; Keller, BT; Carpenter, AJ; Wang, CC; Banerjee, SC; Both, SR; Fletcher, T; Garland, DJ; Huang, W; Jones, C; Koeller, KJ; Kolodziej, SA; Li, J; Manning, RE; Mahoney, MW; Miller, RE; Mischke, DA; Rath, NP; Reinhard, EJ; Tollefson, MB; Vernier, WF; Wagner, GM; Rapp, SR; Beaudry, J; Glenn, K; Regina, K; Schuh, JR; Smith, ME; Trivedi, JS; Reitz, DB Discovery of potent, nonsystemic apical sodium-codependent bile acid transporter inhibitors (Part 2). J Med Chem 48: 5853-68 (2005)
- Liu, Y; Yang, YW; Cao, R; Song, SH; Zhang, HY; Wang, LH Thermodynamic Origin of Molecular Selective Binding of Bile Salts by Aminated β-Cyclodextrins J Phys Chem B 107: 14130 (2003)
- Wess, G; Kramer, W; Han, XB; Bock, K; Enhsen, A; Glombik, H; Baringhaus, KH; Böger, G; Urmann, M; Hoffmann, A Synthesis and biological activity of bile acid-derived HMG-CoA reductase inhibitors. The role of 21-methyl in recognition of HMG-CoA reductase and the ileal bile acid transport system. J Med Chem 37: 3240-6 (1994)
- Kinder, DH; Katzenellenbogen, JA Acylamino boronic acids and difluoroborane analogues of amino acids: potent inhibitors of chymotrypsin and elastase. J Med Chem 28: 1917-25 (1986)
- Ho, W; Tutwiler, GF; Cottrell, SC; Morgans, DJ; Tarhan, O; Mohrbacher, RJ Alkylglycidic acids: potential new hypoglycemic agents. J Med Chem 29: 2184-90 (1986)
- Luithle, J; Bob, F; Erb, C; Schnizler, K; Flessner, T; Kampen, MV; Methfessel, C Amides of acetic and propionic acids US Patent US9000008 (2015)
- Kolasa, T; Gunn, DE; Bhatia, P; Woods, KW; Gane, T; Stewart, AO; Bouska, JB; Harris, RR; Hulkower, KI; Malo, PE; Bell, RL; Carter, GW; Brooks, CD Heteroarylmethoxyphenylalkoxyiminoalkylcarboxylic acids as leukotriene biosynthesis inhibitors. J Med Chem 43: 690-705 (2000)
- Cha, J; Munoz, M; Reilly, M; Cooper, N; Leftheris, K; Morgans, Jr., DJ; Hom, T; Zheng, Y Substituted amino acids as integrin inhibitors US Patent US11180494 (2021)
- Wright, SW; Pinto, DJ; Sherk, SR; Green, AM; Magolda, RL Vinylogous hydroxamic acids: 5-lipoxygenase inhibitors Bioorg Med Chem Lett 2: 1079-1084 (1992)
- Wang, D; Girard, TJ; Kasten, TP; LaChance, RM; Miller-Wideman, MA; Durley, RC Inhibitory activity of unsaturated fatty acids and anacardic acids toward soluble tissue factor-factor VIIa complex. J Nat Prod 61: 1352-5 (1999)
- Masuda, M; I'izuka, Y; Yamazaki, M; Nishigaki, R; Kato, Y; Ni'inuma, K; Suzuki, H; Sugiyama, Y Methotrexate is excreted into the bile by canalicular multispecific organic anion transporter in rats. Cancer Res 57: 3506-10 (1997)
- Bhat, L; Jandeleit, B; Dias, TM; Moors, TL; Gallop, MA Synthesis and biological evaluation of novel steroidal pyrazoles as substrates for bile acid transporters. Bioorg Med Chem Lett 15: 85-7 (2004)
- Byrne, JA; Strautnieks, SS; Mieli-Vergani, G; Higgins, CF; Linton, KJ; Thompson, RJ The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology 123: 1649-58 (2002)
- Skinner, PJ; Cherrier, MC; Webb, PJ; Sage, CR; Dang, HT; Pride, CC; Chen, R; Tamura, SY; Richman, JG; Connolly, DT; Semple, G 3-Nitro-4-amino benzoic acids and 6-amino nicotinic acids are highly selective agonists of GPR109b. Bioorg Med Chem Lett 17: 6619-22 (2007)
- Tollefson, MB; Kolodziej, SA; Fletcher, TR; Vernier, WF; Beaudry, JA; Keller, BT; Reitz, DB A novel class of apical sodium co-dependent bile acid transporter inhibitors: the 1,2-benzothiazepines. Bioorg Med Chem Lett 13: 3727-30 (2003)
- Or, YS; Xing, X; Wang, G; Shen, R; He, J Benzoic acid derivatives of bile acid as FXR/TGR5 agonists and methods of use thereof US Patent US10323060 (2019)
- Costantino, G; Macchiarulo, A; Entrena-Guadix, A; Camaioni, E; Pellicciari, R Binding mode of 6ECDCA, a potent bile acid agonist of the farnesoid X receptor (FXR). Bioorg Med Chem Lett 13: 1865-8 (2003)
- Craddock, AL; Love, MW; Daniel, RW; Kirby, LC; Walters, HC; Wong, MH; Dawson, PA Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol 274: 157-69 (1998)
- Menard, PR; Suh, JT; Jones, H; Loev, B; Neiss, ES; Wilde, J; Schwab, A; Mann, WS Angiotensin converting enzyme inhibitors. (Mercaptoaroyl)amino acids. J Med Chem 28: 328-32 (1985)
- Eckhardt, M; Wagner, H; Peters, S Benzylaminopyridylcyclopropanecarboxylic acids, pharmaceutical compositions and uses thereof US Patent US10919859 (2021)
- Taouai, M; Chakroun, K; Sommer, R; Michaud, G; Giacalone, D; Ben Maaouia, MA; Vallin-Butruille, A; Mathiron, D; Abidi, R; Darbre, T; Cragg, PJ; Mullié, C; Reymond, JL; O'Toole, GA; Benazza, M Glycocluster Tetrahydroxamic Acids Exhibiting Unprecedented Inhibition of J Med Chem 62: 7722-7738 (2019)
- Eckhardt, M; Wagner, H; Peters, S Indanylaminopyrazinylcyclopropanecarboxylic acids, pharmaceutical compositions and uses thereof US Patent US10253004 (2019)
- Eckhardt, M; Wagner, H; Peters, S Indanylaminopyridylcyclopropanecarboxylic acids, pharmaceutical compositions and uses thereof US Patent US10125101 (2018)
- Hu, X; Zhu, J; Srivathsan, S; Pei, D Peptidyl hydroxamic acids as methionine aminopeptidase inhibitors. Bioorg Med Chem Lett 14: 77-9 (2003)
- Mawer, IM; Kulagowski, JJ; Leeson, PD; Grimwood, S; Marshall, GR Tetramic acids as novel glycine site antagonists Bioorg Med Chem Lett 5: 2643-2648 (1995)
- Burstein, SH The cannabinoid acids, analogs and endogenous counterparts. Bioorg Med Chem 22: 2830-43 (2014)
- Porcelloni, M; D'Andrea, P; Altamura, M; Catalioto, RM; Giuliani, S; Meini, S; Fattori, D Cinnamic acids and mono-substituted benzoic acids as useful capping groups for the preparation of hNK2 receptor antagonists. Bioorg Med Chem Lett 18: 4705-7 (2008)
- Chen, T; Reich, NW; Bell, N; Finn, PD; Rodriguez, D; Kohler, J; Kozuka, K; He, L; Spencer, AG; Charmot, D; Navre, M; Carreras, CW; Koo-McCoy, S; Tabora, J; Caldwell, JS; Jacobs, JW; Lewis, JG Design of Gut-Restricted Thiazolidine Agonists of G Protein-Coupled Bile Acid Receptor 1 (GPBAR1, TGR5). J Med Chem 61: 7589-7613 (2018)
- D'Amore, C; Di Leva, FS; Sepe, V; Renga, B; Del Gaudio, C; D'Auria, MV; Zampella, A; Fiorucci, S; Limongelli, V Design, synthesis, and biological evaluation of potent dual agonists of nuclear and membrane bile acid receptors. J Med Chem 57: 937-54 (2014)
- Kim, RB; Leake, B; Cvetkovic, M; Roden, MM; Nadeau, J; Walubo, A; Wilkinson, GR Modulation by drugs of human hepatic sodium-dependent bile acid transporter (sodium taurocholate cotransporting polypeptide) activity. J Pharmacol Exp Ther 291: 1204-9 (1999)
- Son, S; Chae, SY; Kim, CW; Choi, YG; Jung, SY; Lee, S; Lee, KC Preparation and structural, biochemical, and pharmaceutical characterizations of bile acid-modified long-acting exendin-4 derivatives. J Med Chem 52: 6889-96 (2009)
- Herbert, MR; Siegel, DL; Staszewski, L; Cayanan, C; Banerjee, U; Dhamija, S; Anderson, J; Fan, A; Wang, L; Rix, P; Shiau, AK; Rao, TS; Noble, SA; Heyman, RA; Bischoff, E; Guha, M; Kabakibi, A; Pinkerton, AB Synthesis and SAR of 2-aryl-3-aminomethylquinolines as agonists of the bile acid receptor TGR5. Bioorg Med Chem Lett 20: 5718-21 (2010)
- Klopfenstein, SR; Evdokimov, AG; Colson, AO; Fairweather, NT; Neuman, JJ; Maier, MB; Gray, JL; Gerwe, GS; Stake, GE; Howard, BW; Farmer, JA; Pokross, ME; Downs, TR; Kasibhatla, B; Peters, KG 1,2,3,4-Tetrahydroisoquinolinyl sulfamic acids as phosphatase PTP1B inhibitors. Bioorg Med Chem Lett 16: 1574-8 (2006)
- Stanley, NJ; Pedersen, DS; Nielsen, B; Kvist, T; Mathiesen, JM; Bräuner-Osborne, H; Taylor, DK; Abell, AD 1,2,3-triazolyl amino acids as AMPA receptor ligands. Bioorg Med Chem Lett 20: 7512-5 (2010)
- Hoshina, Y; Ikegami, S; Okuyama, A; Fukui, H; Inoguchi, K; Maruyama, T; Fujimoto, K; Matsumura, Y; Aoyama, A; Harada, T; Tanaka, H; Nakamura, T 2,3-Diphenylpropionic acids as potent VLA-4 antagonists. Bioorg Med Chem Lett 15: 217-20 (2004)
- Schulz, JM; Lanovoi, HT; Ames, AM; McKegg, PC; Patrone, JD Concise Modular Synthesis of Thalassotalic Acids A-C. J Nat Prod 82: 1045-1048 (2019)
- Gao, X; Wang, J; Liu, J; Guiadeen, D; Krikorian, A; Boga, SB; Alhassan, AB; Selyutin, O; Yu, W; Yu, Y; Anand, R; Liu, S; Yang, C; Wu, H; Cai, J; Cooper, A; Zhu, H; Maloney, K; Gao, YD; Fischmann, TO; Presland, J; Mansueto, M; Xu, Z; Leccese, E; Zhang-Hoover, J; Knemeyer, I; Garlisi, CG; Bays, N; Stivers, P; Brandish, PE; Hicks, A; Kim, R; Kozlowski, JA Discovery of novel BTK inhibitors with carboxylic acids. Bioorg Med Chem Lett 27: 1471-1477 (2017)
- Stanton, MG; Hubbs, J; Sloman, D; Hamblett, C; Andrade, P; Angagaw, M; Bi, G; Black, RM; Crispino, J; Cruz, JC; Fan, E; Farris, G; Hughes, BL; Kenific, CM; Middleton, RE; Nikov, G; Sajonz, P; Shah, S; Shomer, N; Szewczak, AA; Tanga, F; Tudge, MT; Shearman, M; Munoz, B Fluorinated piperidine acetic acids as gamma-secretase modulators. Bioorg Med Chem Lett 20: 755-8 (2010)
- Hwang, N; Sun, L; Noe, D; Lam, PYS; Zhou, T; Block, TM; Du, Y Hepatoselective Dihydroquinolizinone Bis-acids for HBsAg mRNA Degradation. ACS Med Chem Lett 12: 1130-1136 (2021)
- Atigadda, VR; Brouillette, WJ; Duarte, F; Babu, YS; Bantia, S; Chand, P; Chu, N; Montgomery, JA; Walsh, DA; Sudbeck, E; Finley, J; Air, GM; Luo, M; Laver, GW Hydrophobic benzoic acids as inhibitors of influenza neuraminidase. Bioorg Med Chem 7: 2487-97 (1999)
- Jiao, G; Johnson, AT; O''Malley, S; Kim, SJ Hydroxamic acids comprising pyrazole moiety and uses thereof US Patent US11046652 (2021)
- Wickens, P; Zhang, C; Ma, X; Zhao, Q; Amatruda, J; Bullock, W; Burns, M; Cantin, LD; Chuang, CY; Claus, T; Dai, M; Dela Cruz, F; Dickson, D; Ehrgott, FJ; Fan, D; Heald, S; Hentemann, M; Iwuagwu, CI; Johnson, JS; Kumarasinghe, E; Ladner, D; Lavoie, R; Liang, S; Livingston, JN; Lowe, D; Magnuson, S; Mannelly, G; Mugge, I; Ogutu, H; Pleasic-Williams, S; Schoenleber, RW; Shapiro, J; Shelekhin, T; Sweet, L; Town, C; Tsutsumi, M Indanylacetic acids as PPAR-delta activator insulin sensitizers. Bioorg Med Chem Lett 17: 4369-73 (2007)
- Simeth, NA; Bause, M; Dobmeier, M; Kling, RC; Lachmann, D; Hübner, H; Einsiedel, J; Gmeiner, P; König, B NTS2-selective neurotensin mimetics with tetrahydrofuran amino acids. Bioorg Med Chem 25: 350-359 (2017)
- Dufresne, C; Jones, ET; Omstead, MN; Bergstrom, JD; Wilson, KE Novel Zaragozic Acids from Leptodontidium elatius J Nat Prod 59: 52-54 (1996)
- Altman, MD; Childers, ML; Jewell, JP; Lesburg, CA; Siu, T Oxo-tetrahydro-isoquinoline carboxylic acids as STING inhibitors US Patent US11311528 (2022)
- Stranix, BR; Wu, JJ; Milot, G; Beaulieu, F; Bouchard, JE; Gouveia, K; Forte, A; Garde, S; Wang, Z; Mouscadet, JF; Delelis, O; Xiao, Y Pyridoxine hydroxamic acids as novel HIV-integrase inhibitors. Bioorg Med Chem Lett 26: 1233-6 (2016)
- Lowe, DB; Bifulco, N; Bullock, WH; Claus, T; Coish, P; Dai, M; Dela Cruz, FE; Dickson, D; Fan, D; Hoover-Litty, H; Li, T; Ma, X; Mannelly, G; Monahan, MK; Muegge, I; O'Connor, S; Rodriguez, M; Shelekhin, T; Stolle, A; Sweet, L; Wang, M; Wang, Y; Zhang, C; Zhang, HJ; Zhang, M; Zhao, K; Zhao, Q; Zhu, J; Zhu, L; Tsutsumi, M Substituted indanylacetic acids as PPAR-alpha-gamma activators. Bioorg Med Chem Lett 16: 297-301 (2005)
- Kumar, S; Kayastha, AM Inhibition studies of soybean (Glycine max) urease with heavy metals, sodium salts of mineral acids, boric acid, and boronic acids. J Enzyme Inhib Med Chem 25: 646-52 (2010)
- Musser, JH; Kreft, AF; Bender, RH; Kubrak, DM; Grimes, D; Carlson, RP; Hand, JM; Chang, J N-[(arylmethoxy)phenyl] carboxylic acids, hydroxamic acids, tetrazoles, and sulfonyl carboxamides. Potent orally active leukotriene D4 antagonists of novel structure. J Med Chem 33: 240-5 (1990)
- Tollefson, MB; Vernier, WF; Huang, HC; Chen, FP; Reinhard, EJ; Beaudry, J; Keller, BT; Reitz, DB A novel class of apical sodium co-dependent bile acid transporter inhibitors: the 2,3-disubstituted-4-phenylquinolines. Bioorg Med Chem Lett 10: 277-9 (2000)
- Baker, SR; Bleakman, D; Ezquerra, J; Ballyk, BA; Deverill, M; Ho, K; Kamboj, RK; Collado, I; Domínguez, C; Escribano, A; Mateo, AI; Pedregal, C; Rubio, A 4-Alkylidenyl glutamic acids, potent and selective GluR5 agonists. Bioorg Med Chem Lett 10: 1807-10 (2000)
- Rossi, C; Porcelloni, M; D'Andrea, P; Fincham, CI; Ettorre, A; Mauro, S; Squarcia, A; Bigioni, M; Parlani, M; Nardelli, F; Binaschi, M; Maggi, CA; Fattori, D Alkyl piperidine and piperazine hydroxamic acids as HDAC inhibitors. Bioorg Med Chem Lett 21: 2305-8 (2011)
- Morera, E; Di Marzo, V; Monti, L; Allarà, M; Schiano Moriello, A; Nalli, M; Ortar, G; De Petrocellis, L Arylboronic acids as dual-action FAAH and TRPV1 ligands. Bioorg Med Chem Lett 26: 1401-5 (2016)
- Baxter, AD; Bhogal, R; Bird, J; Keily, JF; Manallack, DT; Montana, JG; Owen, DA; Pitt, WR; Watson, RJ; Wills, RE Arylsulphonyl hydroxamic acids: potent and selective matrix metalloproteinase inhibitors. Bioorg Med Chem Lett 11: 1465-8 (2001)
- Plewe, MB; Butler, SL; Dress, KR; Hu, Q; Johnson, TW; Kuehler, JE; Kuki, A; Lam, H; Liu, W; Nowlin, D; Peng, Q; Rahavendran, SV; Tanis, SP; Tran, KT; Wang, H; Yang, A; Zhang, J Azaindole hydroxamic acids are potent HIV-1 integrase inhibitors. J Med Chem 52: 7211-9 (2009)
- Thompson, SA; Andrews, PR; Hanzlik, RP Carboxyl-modified amino acids and peptides as protease inhibitors. J Med Chem 29: 104-11 (1986)
- Brenneman, JB; Ginn, JD; Hopkins, TD; Lowe, MD; Sarko, CR; Westbrook, JA; Yu, M; Zhang, Z Heterocyclic carboxylic acids as activators of soluble guanylate cyclase US Patent US9353090 (2016)
- Eckhardt, M; Wagner, H; Peters, S Indanylaminoazadihydrobenzofuranylacetic acids, pharmaceutical compositions for the treatment of diabetes US Patent US10550127 (2020)
- Vang, T; Xie, Y; Liu, WH; Vidovic, D; Liu, Y; Wu, S; Smith, DH; Rinderspacher, A; Chung, C; Gong, G; Mustelin, T; Landry, DW; Rickert, RC; Schürer, SC; Deng, SX; Tautz, L Inhibition of lymphoid tyrosine phosphatase by benzofuran salicylic acids. J Med Chem 54: 562-71 (2011)
- McGeary, RP; Vella, P; Mak, JY; Guddat, LW; Schenk, G Inhibition of purple acid phosphatase with alpha-alkoxynaphthylmethylphosphonic acids. Bioorg Med Chem Lett 19: 163-6 (2008)
- Wong, CG; Meyer, RB Inhibitors of inosinic acid dehydrogenase. 2-Substituted inosinic acids. J Med Chem 27: 429-32 (1984)
- Narlawar, R; Pérez Revuelta, BI; Baumann, K; Schubenel, R; Haass, C; Steiner, H; Schmidt, B N-Substituted carbazolyloxyacetic acids modulate Alzheimer associated gamma-secretase. Bioorg Med Chem Lett 17: 176-82 (2007)
- Maltas, PJ; Watson, S; Langg{hacek over (a)}rd, M; David, L N-substituted-5-substituted phthalamic acids as sortilin inhibitors US Patent US9682967 (2017)
- Malamas, MS; Millen, J Quinazolineacetic acids and related analogues as aldose reductase inhibitors. J Med Chem 34: 1492-503 (1991)
- Lloyd, J; Ryono, DE; Bird, JE; Buote, J; Delaney, CL; Dejneka, T; Dickinson, KE; Moreland, S; Normandin, DE; Skwish, S; Spitzmiller, ER; Waldron, TL Quinoline-4-carboxylic acids as angiotensin II receptor antagonists Bioorg Med Chem Lett 4: 195-200 (1994)
- Klein, M; Schadt, O; Haselmayer, P; Busch, M Substituted boronic acids and boronate esters as immunoproteasome inhibitors US Patent US10294246 (2019)
- Grzywa, R; Winiarski, L; Psurski, M; Rudnicka, A; Wietrzyk, J; Gajda, T; Oleksyszyn, J Synthesis and biological activity of diisothiocyanate-derived mercapturic acids. Bioorg Med Chem Lett 26: 667-71 (2016)
- Bonafoux, D; Abibi, A; Bettencourt, B; Burchat, A; Ericsson, A; Harris, CM; Kebede, T; Morytko, M; McPherson, M; Wallace, G; Wu, X Thienopyrrole acetic acids as antagonists of the CRTH2 receptor. Bioorg Med Chem Lett 21: 1861-4 (2011)
- Han, LQ; Yuan, X; Wu, XY; Li, RD; Xu, B; Cheng, Q; Liu, ZM; Zhou, TY; An, HY; Wang, X; Cheng, TM; Ge, ZM; Cui, JR; Li, RT Urea-containing peptide boronic acids as potent proteasome inhibitors. Eur J Med Chem 125: 925-939 (2017)
- Han, F; Ning, M; Cao, H; Ye, Y; Feng, Y; Leng, Y; Shen, J Design of G-protein-coupled bile acid receptor 1 (GPBAR1, TGR5) soft drugs with reduced gallbladder-filling effects. Eur J Med Chem 203: (2020)
- Li, J; Liu, M; Li, Y; Sun, DD; Shu, Z; Tan, Q; Guo, S; Xie, R; Gao, L; Ru, H; Zang, Y; Liu, H; Li, J; Zhou, Y Discovery and Optimization of Non-bile Acid FXR Agonists as Preclinical Candidates for the Treatment of Nonalcoholic Steatohepatitis. J Med Chem 63: 12748-12772 (2020)
- Stieger, B; Fattinger, K; Madon, J; Kullak-Ublick, GA; Meier, PJ Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology 118: 422-30 (2000)
- Morgan, RE; Trauner, M; van Staden, CJ; Lee, PH; Ramachandran, B; Eschenberg, M; Afshari, CA; Qualls, CW; Lightfoot-Dunn, R; Hamadeh, HK Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci 118: 485-500 (2010)
- Zelcer, N; Reid, G; Wielinga, P; Kuil, A; van der Heijden, I; Schuetz, JD; Borst, P Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4). Biochem J 371: 361-7 (2003)
- Xiao, H; Li, P; Li, X; He, H; Wang, J; Guo, F; Zhang, J; Wei, L; Zhang, H; Shi, Y; Hou, L; Shen, L; Chen, Z; Du, C; Fu, S; Zhang, P; Hao, F; Wang, P; Xu, D; Liang, W; Tian, X; Zhang, A; Cheng, X; Yang, L; Wang, X; Zhang, X; Li, J; Chen, S Synthesis and Biological Evaluation of a Series of Bile Acid Derivatives as FXR Agonists for Treatment of NASH. ACS Med Chem Lett 8: 1246-1251 (2017)
- Fattinger, K; Funk, C; Pantze, M; Weber, C; Reichen, J; Stieger, B; Meier, PJ The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 69: 223-31 (2001)
- Klutchko, S; Hoefle, ML; Smith, RD; Essenburg, AD; Parker, RB; Nemeth, VL; Ryan, MJ; Dugan, DH; Kaplan, HR Synthesis and angiotensin-converting enzyme inhibitory activity of 3-(Mercaptomethyl)-2-oxo-1-pyrrolidineacetic acids and 3-(Mercaptomethyl)-2-oxo-1-piperidineacetic acids. J Med Chem 24: 104-9 (1981)
- Sato, H; Macchiarulo, A; Thomas, C; Gioiello, A; Une, M; Hofmann, AF; Saladin, R; Schoonjans, K; Pellicciari, R; Auwerx, J Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem 51: 1831-41 (2008)
- Andrés, M; Bravo, M; Buil, MA; Calbet, M; Castro, J; Domènech, T; Eichhorn, P; Ferrer, M; Gómez, E; Lehner, MD; Moreno, I; Roberts, RS; Sevilla, S 2-(1H-Pyrazol-4-yl)acetic acids as CRTh2 antagonists. Bioorg Med Chem Lett 23: 3349-53 (2013)
- Carballeira, NM; Cartagena, M; Sanabria, D; Tasdemir, D; Prada, CF; Reguera, RM; Balaña-Fouce, R 2-Alkynoic fatty acids inhibit topoisomerase IB from Leishmania donovani. Bioorg Med Chem Lett 22: 6185-9 (2012)
- Golebiowski, A; Paul Beckett, R; Van Zandt, M; Ji, MK; Whitehouse, D; Ryder, TR; Jagdmann, E; Andreoli, M; Mazur, A; Padmanilayam, M; Cousido-Siah, A; Mitschler, A; Ruiz, FX; Podjarny, A; Schroeter, H 2-Substituted-2-amino-6-boronohexanoic acids as arginase inhibitors. Bioorg Med Chem Lett 23: 2027-30 (2013)
- Walsh, SP; Severino, A; Zhou, C; He, J; Liang, GB; Tan, CP; Cao, J; Eiermann, GJ; Xu, L; Salituro, G; Howard, AD; Mills, SG; Yang, L 3-Substituted 3-(4-aryloxyaryl)-propanoic acids as GPR40 agonists. Bioorg Med Chem Lett 21: 3390-4 (2011)
- Jackson, WP; Islip, PJ; Kneen, G; Pugh, A; Wates, PJ Acetohydroxamic acids as potent, selective, orally active 5-lipoxygenase inhibitors. J Med Chem 31: 499-500 (1988)
- Gruenfeld, N; Stanton, JL; Yuan, AM; Ebetino, FH; Browne, LJ; Gude, C; Huebner, CF Angiotensin converting enzyme inhibitors: 1-glutarylindoline-2-carboxylic acids derivatives. J Med Chem 26: 1277-82 (1983)
- Leban, J; Kralik, M; Mies, J; Baumgartner, R; Gassen, M; Tasler, S Biphenyl-4-ylcarbamoyl thiophene carboxylic acids as potent DHODH inhibitors. Bioorg Med Chem Lett 16: 267-70 (2006)
- Buldenko, VM; Trush, VV; Kobzar, OL; Drapailo, AB; Kalchenko, VI; Vovk, AI Calixarene-based phosphinic acids as inhibitors of protein tyrosine phosphatases. Bioorg Med Chem Lett 29: 797-801 (2019)
- Misra, RN; Botti, CM; Haslanger, MF; Engebrecht, JR; Mahoney, EM; Ciosek, CP Cyclic aryl hydroxamic acids: synthesis and inhibition of 5-lipoxygenase Bioorg Med Chem Lett 1: 295-298 (1991)
- Tessier, P; Smil, DV; Wahhab, A; Leit, S; Rahil, J; Li, Z; Déziel, R; Besterman, JM Diphenylmethylene hydroxamic acids as selective class IIa histone deacetylase inhibitors. Bioorg Med Chem Lett 19: 5684-8 (2009)
- Nomura, M; Yumoto, K; Shinozaki, T; Isogai, S; Takano, Y; Murakami, K Discovery of cyclic amine-substituted benzoic acids as PPARa agonists. Bioorg Med Chem Lett 22: 334-8 (2011)
- Zhang, H; Barr, KJ; Lapointe, BT; Gunaydin, H; Liu, K; Trotter, BW Heteroaryl substituted benzoic acids as RORgammaT inhibitors and uses thereof US Patent US10584121 (2020)
- Van Cleemput, M; Cattoor, K; De Bosscher, K; Haegeman, G; De Keukeleire, D; Heyerick, A Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J Nat Prod 72: 1220-30 (2009)
- Iqbal, J; Saeed, A; Raza, R; Matin, A; Hameed, A; Furtmann, N; Lecka, J; Sévigny, J; Bajorath, J Identification of sulfonic acids as efficient ecto-5'-nucleotidase inhibitors. Eur J Med Chem 70: 685-91 (2013)
- Dai, Y; Guo, Y; Guo, J; Pease, LJ; Li, J; Marcotte, PA; Glaser, KB; Tapang, P; Albert, DH; Richardson, PL; Davidsen, SK; Michaelides, MR Indole amide hydroxamic acids as potent inhibitors of histone deacetylases. Bioorg Med Chem Lett 13: 1897-901 (2003)
- Goulet, JL; Kinneary, JF; Durette, PL; Stein, RL; Harrison, RK; Izquierdo-Martin, M; Kuo, DW; Lin, TY; Hagmann, WK Inhibition of stromelysin-1 (MMP-3) by peptidyl phosphinic acids Bioorg Med Chem Lett 4: 1221-1224 (1994)
- Bobkova, EV; Liu, WH; Colayco, S; Rascon, J; Vasile, S; Gasior, C; Critton, DA; Chan, X; Dahl, R; Su, Y; Sergienko, E; Chung, TD; Mustelin, T; Page, R; Tautz, L Inhibition of the Hematopoietic Protein Tyrosine Phosphatase by Phenoxyacetic Acids. ACS Med Chem Lett 2: 113-118 (2011)
- Martin, R; Gold, M; Jones, J Inhibition of the RTEM-1 β-lactamase by boronic acids Bioorg Med Chem Lett 4: 1229-1234 (1994)
- Zhao, H; Liu, G; Xin, Z; Serby, MD; Pei, Z; Szczepankiewicz, BG; Hajduk, PJ; Abad-Zapatero, C; Hutchins, CW; Lubben, TH; Ballaron, SJ; Haasch, DL; Kaszubska, W; Rondinone, CM; Trevillyan, JM; Jirousek, MR Isoxazole carboxylic acids as protein tyrosine phosphatase 1B (PTP1B) inhibitors. Bioorg Med Chem Lett 14: 5543-6 (2004)
- Cowart, M; Kowaluk, EA; Daanen, JF; Kohlhaas, KL; Alexander, KM; Wagenaar, FL; Kerwin, JF Nitroaromatic amino acids as inhibitors of neuronal nitric oxide synthase. J Med Chem 41: 2636-42 (1998)
- Varghese, S; Senanayake, T; Murray-Stewart, T; Doering, K; Fraser, A; Casero, RA; Woster, PM Polyaminohydroxamic acids and polyaminobenzamides as isoform selective histone deacetylase inhibitors. J Med Chem 51: 2447-56 (2008)
- Adams, J; Behnke, M; Chen, S; Cruickshank, AA; Dick, LR; Grenier, L; Klunder, JM; Ma, YT; Plamondon, L; Stein, RL Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett 8: 333-8 (1999)
- Venkatraman, S; Wu, W; Prongay, A; Girijavallabhan, V; George Njoroge, F Potent inhibitors of HCV-NS3 protease derived from boronic acids. Bioorg Med Chem Lett 19: 180-3 (2009)
- Repine, JT; Kaltenbronn, JS; Doherty, AM; Hamby, JM; Himmelsbach, RJ; Kornberg, BE; Taylor, MD; Lunney, EA; Humblet, C; Rapundalo, ST Renin inhibitors containing alpha-heteroatom amino acids as P2 residues. J Med Chem 35: 1032-42 (1992)
- Curtin, ML; Garland, RB; Heyman, HR; Frey, RR; Michaelides, MR; Li, J; Pease, LJ; Glaser, KB; Marcotte, PA; Davidsen, SK Succinimide hydroxamic acids as potent inhibitors of histone deacetylase (HDAC). Bioorg Med Chem Lett 12: 2919-23 (2002)
- Conti, P; De Amici, M; Joppolo Di Ventimiglia, S; Stensbøl, TB; Madsen, U; Bräuner-Osborne, H; Russo, E; De Sarro, G; Bruno, G; De Micheli, C Synthesis and anticonvulsant activity of novel bicyclic acidic amino acids. J Med Chem 46: 3102-8 (2003)
- Larbig, G; Schmidt, B Synthesis of tetramic and tetronic acids as beta-secretase inhibitors. J Comb Chem 8: 480-90 (2006)
- Kozikowski, A; Shen, S; Bergman, J; Gaisina, I Tetrahydroquinoline substituted hydroxamic acids as selective histone deacetylase 6 inhibitors US Patent US10456394 (2019)
- Lee, JS; Yang, MY; Yeo, H; Kim, J; Lee, HS; Ahn, JS Uncarinic acids: phospholipase Cgamma1 inhibitors from hooks of Uncaria rhynchophylla. Bioorg Med Chem Lett 9: 1429-32 (1999)
- Magnin, DR; Biller, SA; Chen, Y; Dickson, JK; Fryszman, OM; Lawrence, RM; Logan, JV; Sieber-McMaster, ES; Sulsky, RB; Traeger, SC; Hsieh, DC; Lan, SJ; Rinehart, JK; Harrity, TW; Jolibois, KG; Kunselman, LK; Rich, LC; Slusarchyk, DA; Ciosek, CP alpha-Phosphonosulfonic acids: potent and selective inhibitors of squalene synthase. J Med Chem 39: 657-60 (1996)
- Wang, Y; Jia, S; Tseng, B; Drewe, J; Cai, SX Dipeptidyl aspartyl fluoromethylketones as potent caspase inhibitors: peptidomimetic replacement of the P(2) amino acid by 2-aminoaryl acids and other non-natural amino acids. Bioorg Med Chem Lett 17: 6178-82 (2007)
- Kurata, H; Suzuki, S; Ohhata, Y; Ikeda, T; Hasegawa, T; Kitayama, K; Inaba, T; Kono, K; Kohama, T A novel class of apical sodium-dependent bile acid transporter inhibitors: the amphiphilic 4-oxo-1-phenyl-1,4-dihydroquinoline derivatives. Bioorg Med Chem Lett 14: 1183-6 (2004)
- Wu, Y; Aquino, CJ; Cowan, DJ; Anderson, DL; Ambroso, JL; Bishop, MJ; Boros, EE; Chen, L; Cunningham, A; Dobbins, RL; Feldman, PL; Harston, LT; Kaldor, IW; Klein, R; Liang, X; McIntyre, MS; Merrill, CL; Patterson, KM; Prescott, JS; Ray, JS; Roller, SG; Yao, X; Young, A; Yuen, J; Collins, JL Discovery of a highly potent, nonabsorbable apical sodium-dependent bile acid transporter inhibitor (GSK2330672) for treatment of type 2 diabetes. J Med Chem 56: 5094-114 (2013)
- Dawson, S; Stahl, S; Paul, N; Barber, J; Kenna, JG In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 40: 130-8 (2011)
- Fiorillo, B; Sepe, V; Conflitti, P; Roselli, R; Biagioli, M; Marchianò, S; De Luca, P; Baronissi, G; Rapacciuolo, P; Cassiano, C; Catalanotti, B; Zampella, A; Limongelli, V; Fiorucci, S Structural Basis for Developing Multitarget Compounds Acting on Cysteinyl Leukotriene Receptor 1 and G-Protein-Coupled Bile Acid Receptor 1. J Med Chem 64: 16512-16529 (2021)
- Gelmi, ML; Caputo, F; Clerici, F; Pellegrino, S; Giannaccini, G; Betti, L; Fabbrini, L; Schmid, L; Palego, L; Lucacchini, A 1-Aminocyclopentane-1,2,4-tricarboxylic acids screening on glutamatergic and serotonergic systems. Bioorg Med Chem 15: 7581-9 (2007)
- van Veldhoven, JP; Liu, R; Thee, SA; Wouters, Y; Verhoork, SJ; Mooiman, C; Louvel, J; IJzerman, AP Affinity and kinetics study of anthranilic acids as HCA2 receptor agonists. Bioorg Med Chem 23: 4013-25 (2015)
- Belvedere, S; Witter, DJ; Yan, J; Secrist, JP; Richon, V; Miller, TA Aminosuberoyl hydroxamic acids (ASHAs): a potent new class of HDAC inhibitors. Bioorg Med Chem Lett 17: 3969-71 (2007)
- Cerelli, MJ; Curtis, DL; Dunn, JP; Nelson, PH; Peak, TM; Waterbury, LD Antiinflammatory and aldose reductase inhibitory activity of some tricyclic arylacetic acids. J Med Chem 29: 2347-51 (1986)
- Zajdel, P; Subra, G; Bojarski, AJ; Duszynska, B; Pawlowski, M; Martinez, J Arylpiperazines with N-acylated amino acids as 5-HT1A receptor ligands. Bioorg Med Chem Lett 16: 3406-10 (2006)
- Hammer, K; Jönsson, M; Krüger, L Bicyclic hydroxamic acids useful as inhibitors of mammalian histone deacetylase activity US Patent US10654814 (2020)
- Bioisoteres for carboxylic acids: From ionized isosteres to novel unionized replacements.
- Ringbom, T; Huss, U; Stenholm, A; Flock, S; Skattebøl, L; Perera, P; Bohlin, L Cox-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod 64: 745-9 (2001)
- Liu, J; Han, J; Izawa, K; Sato, T; White, S; Meanwell, NA; Soloshonok, VA Cyclic tailor-made amino acids in the design of modern pharmaceuticals. Eur J Med Chem 208: (2020)
- Shen, W; Garvey, DS; Cohen, J; Stein, H; Rosenberg, SH Cyclopentanedi- and tricarboxylic acids as squalene synthase inhibitors: syntheses and evaluation. Bioorg Med Chem Lett 8: 891-6 (1999)
- Krennhrubec, K; Marshall, BL; Hedglin, M; Verdin, E; Ulrich, SM Design and evaluation of 'Linkerless' hydroxamic acids as selective HDAC8 inhibitors. Bioorg Med Chem Lett 17: 2874-8 (2007)
- Hack, S; Wörlein, B; Höfner, G; Pabel, J; Wanner, KT Development of imidazole alkanoic acids as mGAT3 selective GABA uptake inhibitors. Eur J Med Chem 46: 1483-98 (2011)
- Wang, W; Devasthale, P; Farrelly, D; Gu, L; Harrity, T; Cap, M; Chu, C; Kunselman, L; Morgan, N; Ponticiello, R; Zebo, R; Zhang, L; Locke, K; Lippy, J; O'Malley, K; Hosagrahara, V; Zhang, L; Kadiyala, P; Chang, C; Muckelbauer, J; Doweyko, AM; Zahler, R; Ryono, D; Hariharan, N; Cheng, PT Discovery of azetidinone acids as conformationally-constrained dual PPARalpha/gamma agonists. Bioorg Med Chem Lett 18: 1939-44 (2008)
- Lee, S; Wang, SW; Yu, CL; Tai, HC; Yen, JY; Tuan, YL; Wang, HH; Liu, YT; Chen, SS; Lee, HY Effect of phenylurea hydroxamic acids on histone deacetylase and VEGFR-2. Bioorg Med Chem 50: (2021)
- Cadoni, R; Pala, N; Lomelino, C; Mahon, BP; McKenna, R; Dallocchio, R; Dessì, A; Carcelli, M; Rogolino, D; Sanna, V; Rassu, M; Iaccarino, C; Vullo, D; Supuran, CT; Sechi, M Exploring Heteroaryl-pyrazole Carboxylic Acids as Human Carbonic Anhydrase XII Inhibitors. ACS Med Chem Lett 8: 941-946 (2017)
- Shobha, SV; Ramadoss, CS; Ravindranath, B Inhibition of Soybean Lipoxygenase-1 by Anacardic Acids, Cardols, and Cardanols J Nat Prod 57: 1755-1757 (1994)
- Taddei, M; Cini, E; Giannotti, L; Giannini, G; Battistuzzi, G; Vignola, D; Vesci, L; Cabri, W Lactam based 7-amino suberoylamide hydroxamic acids as potent HDAC inhibitors. Bioorg Med Chem Lett 24: 61-4 (2014)
- Soltane, R; Alhadrami, HA; Alasiri, A; Jannet, HB; Chouaib, K; Chrouda, A; Mostafa, A; Pashameah, RA Maslinic and oleanolic acids derivatives for treating SARS-CoV-2 infection US Patent US11266632 (2022)
- St-Georges, C; Désilets, A; Béliveau, F; Ghinet, M; Dion, SP; Colombo, É; Boudreault, PL; Najmanovich, RJ; Leduc, R; Marsault, É Modulating the selectivity of matriptase-2 inhibitors with unnatural amino acids. Eur J Med Chem 129: 110-123 (2017)
- Barlaam, B; Bird, TG; Lambert-Van Der Brempt, C; Campbell, D; Foster, SJ; Maciewicz, R New alpha-substituted succinate-based hydroxamic acids as TNFalpha convertase inhibitors. J Med Chem 42: 4890-908 (1999)
- Behrends, M; Wagner, S; Kopka, K; Schober, O; Schäfers, M; Kumbhar, S; Waller, M; Haufe, G New matrix metalloproteinase inhibitors based on¿-fluorinateda-aminocarboxylic anda-aminohydroxamic acids. Bioorg Med Chem 23: 3809-18 (2015)
- Powell, DA; Black, WC; Bleasby, K; Chan, CC; Deschenes, D; Gagnon, M; Gordon, R; Guay, J; Guiral, S; Hafey, MJ; Huang, Z; Isabel, E; Leblanc, Y; Styhler, A; Xu, LJ; Zhang, L; Oballa, RM Nicotinic acids: liver-targeted SCD inhibitors with preclinical anti-diabetic efficacy. Bioorg Med Chem Lett 21: 7281-6 (2011)
- Buckle, DR; Cantello, BC; Cawthorne, MA; Coyle, PJ; Dean, DK; Faller, A; Haigh, D; Hindley, RM; Jefcott, LJ; Lister, CA; Pinto, IL; Rami, HK; Smith, DG; Smith, SA Non thiazolidinedione antihyperglycaemic agents. 1: α-Heteroatom substituted β-phenylpropanoic acids Bioorg Med Chem Lett 6: 2121-2126 (1996)
- Jankowska, A; Satała, G; Kołaczkowski, M; Bucki, A; Głuch-Lutwin, M; Świerczek, A; Pociecha, K; Partyka, A; Jastrzębska-Więsek, M; Lubelska, A; Latacz, G; Gawalska, A; Bojarski, AJ; Wyska, E; Chłoń-Rzepa, G Novel anilide and benzylamide derivatives of arylpiperazinylalkanoic acids as 5-HT Eur J Med Chem 201: (2020)
- Adams, AD; Hu, Z; von Langen, D; Dadiz, A; Elbrecht, A; MacNaul, KL; Berger, JP; Zhou, G; Doebber, TW; Meurer, R; Forrest, MJ; Moller, DE; Jones, AB O-arylmandelic acids as highly selective human PPAR alpha/gamma agonists. Bioorg Med Chem Lett 13: 3185-90 (2003)
- Seiple, LA; Cardellina, JH; Akee, R; Stivers, JT Potent inhibition of human apurinic/apyrimidinic endonuclease 1 by arylstibonic acids. Mol Pharmacol 73: 669-77 (2008)
- Schmidt, D; Smenton, A; Raghavan, S; Carballo-Jane, E; Lubell, S; Ciecko, T; Holt, TG; Wolff, M; Taggart, A; Wilsie, L; Krsmanovic, M; Ren, N; Blom, D; Cheng, K; McCann, PE; Gerard Waters, M; Tata, J; Colletti, S Pyrazole acids as niacin receptor agonists for the treatment of dyslipidemia. Bioorg Med Chem Lett 19: 4768-72 (2009)
- Jeng, AY; Chou, M; Parker, DT Sulfonamide-based hydroxamic acids as potent inhibitors of mouse macrophage metalloelastase. Bioorg Med Chem Lett 8: 897-902 (1999)
- Markowska, A; Bruzgo, M; Surazynski, A; Midura-Nowaczek, K Tripeptides with non-code amino acids as potential serine proteases inhibitors. J Enzyme Inhib Med Chem 28: 639-43 (2013)
- Loesche, A; Wiemann, J; Al Halabi, Z; Karasch, J; Sippl, W; Csuk, R Unexpected AChE inhibitory activity of (2E)α,β-unsaturated fatty acids. Bioorg Med Chem Lett 28: 3315-3319 (2018)
- Aleo, MD; Luo, Y; Swiss, R; Bonin, PD; Potter, DM; Will, Y Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology 60: 1015-22 (2014)
- Dhanoa, DS; Bagley, SW; Chang, RS; Lotti, VJ; Chen, TB; Kivlighn, SD; Zingaro, GJ; Siegl, PK; Chakravarty, PK; Patchett, AA (Dipropylphenoxy)phenylacetic acids: a new generation of nonpeptide angiotensin II receptor antagonists. J Med Chem 36: 3738-42 (1994)
- Ojha, R; Huang, HL; HuangFu, WC; Wu, YW; Nepali, K; Lai, MJ; Su, CJ; Sung, TY; Chen, YL; Pan, SL; Liou, JP 1-Aroylindoline-hydroxamic acids as anticancer agents, inhibitors of HSP90 and HDAC. Eur J Med Chem 150: 667-677 (2018)
- Berthelot, P; Vaccher, C; Flouquet, N; Debaert, M; Luyckx, M; Brunet, C 3-Thienyl- and 3-furylaminobutyric acids. Synthesis and binding GABAB receptor studies. J Med Chem 34: 2557-60 (1991)
- Shinozuka, T; Shimada, K; Matsui, S; Yamane, T; Ama, M; Fukuda, T; Taki, M; Naito, S 4-Aminophenoxyacetic acids as a novel class of reversible cathepsin K inhibitors. Bioorg Med Chem Lett 16: 1502-5 (2006)
- Varghese, S; Gupta, D; Baran, T; Jiemjit, A; Gore, SD; Casero, RA; Woster, PM Alkyl-substituted polyaminohydroxamic acids: a novel class of targeted histone deacetylase inhibitors. J Med Chem 48: 6350-65 (2005)
- Buzzoni, V; Blazquez, J; Ferrari, S; Calò, S; Venturelli, A; Costi, MP Aza-boronic acids as non-beta-lactam inhibitors of AmpC-beta-lactamase. Bioorg Med Chem Lett 14: 3979-83 (2004)
- Lin, L; Shen, Q; Chen, GR; Xie, J Beta-C-glycosiduronic acids and beta-C-glycosyl compounds: new PTP1B inhibitors. Bioorg Med Chem Lett 18: 6348-51 (2008)
- He, Y; Zeng, LF; Yu, ZH; He, R; Liu, S; Zhang, ZY Bicyclic benzofuran and indole-based salicylic acids as protein tyrosine phosphatase inhibitors. Bioorg Med Chem 20: 1940-6 (2012)
- Trush, VV; Cherenok, SO; Tanchuk, VY; Kukhar, VP; Kalchenko, VI; Vovk, AI Calix[4]arene methylenebisphosphonic acids as inhibitors of protein tyrosine phosphatase 1B. Bioorg Med Chem Lett 23: 5619-23 (2013)
- Tilley, JW; Danho, W; Lovey, K; Wagner, R; Swistok, J; Makofske, R; Michalewsky, J; Triscari, J; Nelson, D; Weatherford, S Carboxylic acids and tetrazoles as isosteric replacements for sulfate in cholecystokinin analogues. J Med Chem 34: 1125-36 (1991)
- Wang, Z; Chen, L; Bayly, SF; Torrence, PF Convergent synthesis of ribonuclease L-active 2',5'-oligoadenylate-peptide nucleic acids. Bioorg Med Chem Lett 10: 1357-60 (2000)
- Pingali, H; Jain, M; Shah, S; Zaware, P; Makadia, P; Pola, S; Thube, B; Patel, D; Patil, P; Priyadarshini, P; Suthar, D; Shah, M; Giri, S; Patel, P Design and synthesis of novel bis-oximinoalkanoic acids as potent PPARalpha agonists. Bioorg Med Chem Lett 20: 1156-61 (2010)
- Asteian, A; Blayo, AL; He, Y; Koenig, M; Shin, Y; Kuruvilla, DS; Corzo, CA; Cameron, MD; Lin, L; Ruiz, C; Khan, S; Kumar, N; Busby, S; Marciano, DP; Garcia-Ordonez, RD; Griffin, PR; Kamenecka, TM Design, Synthesis, and Biological Evaluation of Indole Biphenylcarboxylic Acids as PPARγ Antagonists. ACS Med Chem Lett 6: 998-1003 (2015)
- Takano, R; Yoshida, M; Inoue, M; Honda, T; Nakashima, R; Matsumoto, K; Yano, T; Ogata, T; Watanabe, N; Toda, N Discovery of 3-aryl-3-ethoxypropanoic acids as orally active GPR40 agonists. Bioorg Med Chem Lett 24: 2949-53 (2014)
- Chen, YT; Seto, CT Divalent and trivalent alpha-ketocarboxylic acids as inhibitors of protein tyrosine phosphatases. J Med Chem 45: 3946-52 (2002)
- Grimstrup, M; Receveur, JM; Rist, Ø; Frimurer, TM; Nielsen, PA; Mathiesen, JM; Högberg, T Exploration of SAR features by modifications of thiazoleacetic acids as CRTH2 antagonists. Bioorg Med Chem Lett 20: 1638-41 (2010)
- Marciano, D; Ben-Baruch, G; Marom, M; Egozi, Y; Haklai, R; Kloog, Y Farnesyl derivatives of rigid carboxylic acids-inhibitors of ras-dependent cell growth. J Med Chem 38: 1267-72 (1995)
- Liu, D; Kong, G; Chen, QC; Wang, G; Li, J; Xu, Y; lin, T; Tian, Y; Zhang, X; Yao, X; Feng, G; Lu, Z; Chen, H Fatty acids as natural specific inhibitors of the proto-oncogenic protein Shp2. Bioorg Med Chem Lett 21: 6833-7 (2011)
- Skinner, PJ; Cherrier, MC; Webb, PJ; Shin, YJ; Gharbaoui, T; Lindstrom, A; Hong, V; Tamura, SY; Dang, HT; Pride, CC; Chen, R; Richman, JG; Connolly, DT; Semple, G Fluorinated pyrazole acids are agonists of the high affinity niacin receptor GPR109a. Bioorg Med Chem Lett 17: 5620-3 (2007)
- Dang, Q; Brown, BS; Liu, Y; Rydzewski, RM; Robinson, ED; van Poelje, PD; Reddy, MR; Erion, MD Fructose-1,6-bisphosphatase inhibitors. 1. Purine phosphonic acids as novel AMP mimics. J Med Chem 52: 2880-98 (2009)
- McCoull, W; Bailey, A; Barton, P; Birch, AM; Brown, AJ; Butler, HS; Boyd, S; Butlin, RJ; Chappell, B; Clarkson, P; Collins, S; Davies, RM; Ertan, A; Hammond, CD; Holmes, JL; Lenaghan, C; Midha, A; Morentin-Gutierrez, P; Moore, JE; Raubo, P; Robb, G Indazole-6-phenylcyclopropylcarboxylic Acids as Selective GPR120 Agonists with in Vivo Efficacy. J Med Chem 60: 3187-3197 (2017)
- Zur, AA; Chien, HC; Augustyn, E; Flint, A; Heeren, N; Finke, K; Hernandez, C; Hansen, L; Miller, S; Lin, L; Giacomini, KM; Colas, C; Schlessinger, A; Thomas, AA LAT1 activity of carboxylic acid bioisosteres: Evaluation of hydroxamic acids as substrates. Bioorg Med Chem Lett 26: 5000-5006 (2016)
- Schneider, HJ Ligand binding to nucleic acids and proteins: Does selectivity increase with strength? Eur J Med Chem 43: 2307-15 (2008)
- Leonard, K; Pan, W; Anaclerio, B; Gushue, JM; Guo, Z; DesJarlais, RL; Chaikin, MA; Lattanze, J; Crysler, C; Manthey, CL; Tomczuk, BE; Marugan, JJ Non-peptidic alpha(v)beta3 antagonists containing indol-1-yl propionic acids. Bioorg Med Chem Lett 15: 2679-84 (2005)
- Kubo, K; Kohara, Y; Imamiya, E; Sugiura, Y; Inada, Y; Furukawa, Y; Nishikawa, K; Naka, T Nonpeptide angiotensin II receptor antagonists. Synthesis and biological activity of benzimidazolecarboxylic acids. J Med Chem 36: 2182-95 (1993)
- Evans, KA; Shearer, BG; Wisnoski, DD; Shi, D; Sparks, SM; Sternbach, DD; Winegar, DA; Billin, AN; Britt, C; Way, JM; Epperly, AH; Leesnitzer, LM; Merrihew, RV; Xu, RX; Lambert, MH; Jin, J Phenoxyacetic acids as PPARd partial agonists: synthesis, optimization, and in vivo efficacy. Bioorg Med Chem Lett 21: 2345-50 (2011)
- De Corte, BL; Kinney, WA; Liu, L; Ghosh, S; Brunner, L; Hoekstra, WJ; Santulli, RJ; Tuman, RW; Baker, J; Burns, C; Proost, JC; Tounge, BA; Damiano, BP; Maryanoff, BE; Johnson, DL; Galemmo, RA Piperidine-containing beta-arylpropionic acids as potent antagonists of alphavbeta3/alphavbeta5 integrins. Bioorg Med Chem Lett 14: 5227-32 (2004)
- Marsault, E; Benakli, K; Beaubien, S; Saint-Louis, C; Déziel, R; Fraser, G Potent macrocyclic antagonists to the motilin receptor presenting novel unnatural amino acids. Bioorg Med Chem Lett 17: 4187-90 (2007)
- Munroe, JE; Shepherd, TA; Jungheim, LN; Hornback, WJ; Hatch, SD; Muesing, MA; Wiskerchen, M; Su, KS; Campanale, KM; Baxter, AJ; Colacino, JM Potent, orally bioavailable HIV-1 protease inhibitors containing noncoded D-amino acids Bioorg Med Chem Lett 5: 2897-902 (1995)
- Reiter, LA; Robinson, RP; McClure, KF; Jones, CS; Reese, MR; Mitchell, PG; Otterness, IG; Bliven, ML; Liras, J; Cortina, SR; Donahue, KM; Eskra, JD; Griffiths, RJ; Lame, ME; Lopez-Anaya, A; Martinelli, GJ; McGahee, SM; Yocum, SA; Lopresti-Morrow, LL; Tobiassen, LM; Vaughn-Bowser, ML Pyran-containing sulfonamide hydroxamic acids: potent MMP inhibitors that spare MMP-1. Bioorg Med Chem Lett 14: 3389-95 (2004)
- Kuduk, SD; Chang, RK; Di Marco, CN; Ray, WJ; Ma, L; Wittmann, M; Seager, MA; Koeplinger, KA; Thompson, CD; Hartman, GD; Bilodeau, MT Quinolizidinone carboxylic acids as CNS penetrant, selective m1 allosteric muscarinic receptor modulators. ACS Med Chem Lett 1: 263-267 (2010)
- LeMahieu, RA; Carson, M; Han, RJ; Nason, WC; O'Donnell, M; Brown, DL; Crowley, HJ; Welton, AF Substituted (aryloxy)alkanoic acids as antagonists of slow-reacting substance of anaphylaxis. J Med Chem 30: 173-8 (1987)
- Bräuer, N; Nagel, J; Irlbacher, H; Rotgeri, A; Schwede, W; Dahllöf, H; Koppitz, M; Peters, M; Godinho-Coelho, A Substituted pyridyl-cycloalkyl-carboxylic acids, compositions containing them and medical uses thereof US Patent US10172814 (2019)
- Zhang, YM; Fan, X; Xiang, B; Chakravarty, D; Scannevin, R; Burke, S; Karnachi, P; Rhodes, K; Jackson, P Synthesis and SAR of alpha-sulfonylcarboxylic acids as potent matrix metalloproteinase inhibitors. Bioorg Med Chem Lett 16: 3096-100 (2006)
- Pfister, JR; Wymann, WE; Mahoney, JM; Waterbury, LD Synthesis and aldose reductase inhibitory activity of 7-sulfamoylxanthone-2-carboxylic acids. J Med Chem 23: 1264-7 (1981)
- Cardillo, G; Gentilucci, L; Melchiorre, P; Spampinato, S Synthesis and binding activity of endomorphin-1 analogues containing beta-amino acids. Bioorg Med Chem Lett 10: 2755-8 (2000)
- Han, L; Wen, Y; Li, R; Xu, B; Ge, Z; Wang, X; Cheng, T; Cui, J; Li, R Synthesis and biological activity of peptide proline-boronic acids as proteasome inhibitors. Bioorg Med Chem 25: 4031-4044 (2017)
- Kaila, N; Janz, K; DeBernardo, S; Bedard, PW; Camphausen, RT; Tam, S; Tsao, DH; Keith, JC; Nickerson-Nutter, C; Shilling, A; Young-Sciame, R; Wang, Q Synthesis and biological evaluation of quinoline salicylic acids as P-selectin antagonists. J Med Chem 50: 21-39 (2007)
- Connolly, PJ; Wetter, SK; Murray, WV; Johnson, DL; McMahon, FJ; Farrell, FX; Tullai, J; Jolliffe, LK Synthesis and erythropoietin receptor binding affinities of N,N-disubstituted amino acids. Bioorg Med Chem Lett 10: 1995-9 (2001)
- Tóth, GK; Bakos, K; Penke, B; Pávó, I; Varga, C; Török, G; Péter, A; Fülöp, F Synthesis of oxytocin antagonists containing conformationally constrained amino acids in position 2. Bioorg Med Chem Lett 9: 667-72 (1999)
- Schobert, R; Schlenk, A Tetramic and tetronic acids: an update on new derivatives and biological aspects. Bioorg Med Chem 16: 4203-21 (2008)
- Holmes, IP; Gaines, S; Watson, SP; Lorthioir, O; Walker, A; Baddeley, SJ; Herbert, S; Egan, D; Convery, MA; Singh, OM; Gross, JW; Strelow, JM; Smith, RH; Amour, AJ; Brown, D; Martin, SL The identification of beta-hydroxy carboxylic acids as selective MMP-12 inhibitors. Bioorg Med Chem Lett 19: 5760-3 (2009)
- Jütten, P; Schumann, W; Härtl, A; Dahse, HM; Gräfe, U Thiosemicarbazones of formyl benzoic acids as novel potent inhibitors of estrone sulfatase. J Med Chem 50: 3661-6 (2007)
- Lasalle, M; Hoguet, V; Hennuyer, N; Leroux, F; Piveteau, C; Belloy, L; Lestavel, S; Vallez, E; Dorchies, E; Duplan, I; Sevin, E; Culot, M; Gosselet, F; Boulahjar, R; Herledan, A; Staels, B; Deprez, B; Tailleux, A; Charton, J Topical Intestinal Aminoimidazole Agonists of G-Protein-Coupled Bile Acid Receptor 1 Promote Glucagon Like Peptide-1 Secretion and Improve Glucose Tolerance. J Med Chem 60: 4185-4211 (2017)
- Tully, DC; Rucker, PV; Chianelli, D; Williams, J; Vidal, A; Alper, PB; Mutnick, D; Bursulaya, B; Schmeits, J; Wu, X; Bao, D; Zoll, J; Kim, Y; Groessl, T; McNamara, P; Seidel, HM; Molteni, V; Liu, B; Phimister, A; Joseph, SB; Laffitte, B Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH). J Med Chem 60: 9960-9973 (2017)
- Misawa, T; Hayashi, H; Makishima, M; Sugiyama, Y; Hashimoto, Y E297G mutated bile salt export pump (BSEP) function enhancers derived from GW4064: structural development study and separation from farnesoid X receptor-agonistic activity. Bioorg Med Chem Lett 22: 3962-6 (2012)
- Warner, DJ; Chen, H; Cantin, LD; Kenna, JG; Stahl, S; Walker, CL; Noeske, T Mitigating the inhibition of human bile salt export pump by drugs: opportunities provided by physicochemical property modulation, in silico modeling, and structural modification. Drug Metab Dispos 40: 2332-41 (2012)
- Elliott, JD; Lago, MA; Cousins, RD; Gao, A; Leber, JD; Erhard, KF; Nambi, P; Elshourbagy, NA; Kumar, C; Lee, JA 1,3-Diarylindan-2-carboxylic acids, potent and selective non-peptide endothelin receptor antagonists. J Med Chem 37: 1553-7 (1994)
- Weinstock, J; Keenan, RM; Samanen, J; Hempel, J; Finkelstein, JA; Franz, RG; Gaitanopoulos, DE; Girard, GR; Gleason, JG; Hill, DT 1-(carboxybenzyl)imidazole-5-acrylic acids: potent and selective angiotensin II receptor antagonists. J Med Chem 34: 1514-7 (1991)
- Valgeirsson, J; Nielsen, EØ; Peters, D; Varming, T; Mathiesen, C; Kristensen, AS; Madsen, U 2-arylureidobenzoic acids: selective noncompetitive antagonists for the homomeric kainate receptor subtype GluR5. J Med Chem 46: 5834-43 (2003)
- Islam, I; Bryant, J; May, K; Mohan, R; Yuan, S; Kent, L; Morser, J; Zhao, L; Vergona, R; White, K; Adler, M; Whitlow, M; Buckman, BO 3-Mercaptopropionic acids as efficacious inhibitors of activated thrombin activatable fibrinolysis inhibitor (TAFIa). Bioorg Med Chem Lett 17: 1349-54 (2007)
- Mak, LH; Knott, J; Scott, KA; Scott, C; Whyte, GF; Ye, Y; Mann, DJ; Ces, O; Stivers, J; Woscholski, R Arylstibonic acids are potent and isoform-selective inhibitors of Cdc25a and Cdc25b phosphatases. Bioorg Med Chem 20: 4371-6 (2012)
- Glenn, MP; Pattenden, LK; Reid, RC; Tyssen, DP; Tyndall, JD; Birch, CJ; Fairlie, DP Beta-strand mimicking macrocyclic amino acids: templates for protease inhibitors with antiviral activity. J Med Chem 45: 371-81 (2002)
- Watanabe, M; Hanashima, S; Mizushina, Y; Yoshida, H; Oshige, M; Sakaguchi, K; Sugawara, F Biotinylated lithocholic acids for affinity chromatography of mammalian DNA polymerases alpha and beta. Bioorg Med Chem Lett 12: 287-90 (2002)
- Patil, AD; Kokke, WC; Cochran, S; Francis, TA; Tomszek, T; Westley, JW Brominated polyacetylenic acids from the marine sponge Xestospongia muta: inhibitors of HIV protease. J Nat Prod 55: 1170-7 (1992)
- Abdel-Aziz, AA; El-Azab, AS; Ceruso, M; Supuran, CT Carbonic anhydrase inhibitory activity of sulfonamides and carboxylic acids incorporating cyclic imide scaffolds. Bioorg Med Chem Lett 24: 5185-9 (2014)
- Alcaro, S; Gaspar, A; Ortuso, F; Milhazes, N; Orallo, F; Uriarte, E; Yáñez, M; Borges, F Chromone-2- and -3-carboxylic acids inhibit differently monoamine oxidases A and B. Bioorg Med Chem Lett 20: 2709-12 (2010)
- Pikul, S; McDow Dunham, KL; Almstead, NG; De, B; Natchus, MG; Anastasio, MV; McPhail, SJ; Snider, CE; Taiwo, YO; Chen, L; Dunaway, CM; Gu, F; Mieling, GE Design and synthesis of phosphinamide-based hydroxamic acids as inhibitors of matrix metalloproteinases. J Med Chem 42: 87-94 (1999)
- Kuo, CH; Robichaud, AJ; Rew, DJ; Bergstrom, JD; Berger, GD Design and synthesis of squalene synthase inhibitors - acylic mimics of the zaragazic acids Bioorg Med Chem Lett 4: 1591-1594 (1994)
- Anandan, SK; Ward, JS; Brokx, RD; Denny, T; Bray, MR; Patel, DV; Xiao, XY Design and synthesis of thiazole-5-hydroxamic acids as novel histone deacetylase inhibitors. Bioorg Med Chem Lett 17: 5995-9 (2007)
- Zhang, R; Deangelis, A; Wang, A; Sieber-McMaster, E; Li, X; Russell, R; Pelton, P; Xu, J; Zhu, P; Zhou, L; Demarest, K; Murray, WV; Kuo, GH Discovery and SAR of para-alkylthiophenoxyacetic acids as potent and selective PPARdelta agonists. Bioorg Med Chem Lett 19: 1101-4 (2009)
- Ishii, A; Kotani, T; Nagaki, Y; Shibayama, Y; Toyomaki, Y; Okukado, N; Ienaga, K; Okamoto, K Highly selective aldose reductase inhibitors. 1. 3-(Arylalkyl)-2,4,5-trioxoimidazolidine-1-acetic acids. J Med Chem 39: 1924-7 (1996)
- Dvorak, CA; Liu, C; Shelton, J; Kuei, C; Sutton, SW; Lovenberg, TW; Carruthers, NI Identification of Hydroxybenzoic Acids as Selective Lactate Receptor (GPR81) Agonists with Antilipolytic Effects. ACS Med Chem Lett 3: 637-639 (2012)
- Kobzar, O; Shulha, Y; Buldenko, V; Cherenok, S; Silenko, O; Kalchenko, V; Vovk, A Inhibition of glutathione S-transferases by photoactive calix[4]arene α-ketophosphonic acids. Bioorg Med Chem Lett 77: (2022)
- Maresca, A; Vullo, D; Scozzafava, A; Manole, G; Supuran, CT Inhibition of the ß-class carbonic anhydrases from Mycobacterium tuberculosis with carboxylic acids. J Enzyme Inhib Med Chem 28: 392-6 (2013)
- Balunas, MJ; Su, B; Landini, S; Brueggemeier, RW; Kinghorn, AD Interference by naturally occurring fatty acids in a noncellular enzyme-based aromatase bioassay. J Nat Prod 69: 700-3 (2006)
- Stranix, BR; Lavallee, JF; Sevigny, G; Yelle, J; Perron, V; LeBerre, N; Herbart, D; Wu, JJ Lysine sulfonamides as novel HIV-protease inhibitors: Nepsilon-acyl aromatic alpha-amino acids. Bioorg Med Chem Lett 16: 3459-62 (2006)
- Breuning, A; Degel, B; Schulz, F; Büchold, C; Stempka, M; Machon, U; Heppner, S; Gelhaus, C; Leippe, M; Leyh, M; Kisker, C; Rath, J; Stich, A; Gut, J; Rosenthal, PJ; Schmuck, C; Schirmeister, T Michael acceptor based antiplasmodial and antitrypanosomal cysteine protease inhibitors with unusual amino acids. J Med Chem 53: 1951-63 (2010)
- Tolomelli, A; Baiula, M; Belvisi, L; Viola, A; Gentilucci, L; Troisi, S; Dattoli, SD; Spampinato, S; Civera, M; Juaristi, E; Escudero, M Modulation ofavß3- anda5ß1-integrin-mediated adhesion by dehydro-ß-amino acids containing peptidomimetics. Eur J Med Chem 66: 258-68 (2013)
- Kettle, JG; Faull, AW; Barker, AJ; Davies, DH; Stone, MA N-Benzylindole-2-carboxylic acids: potent functional antagonists of the CCR2b chemokine receptor. Bioorg Med Chem Lett 14: 405-8 (2003)
- Grzywa, R; Sokol, AM; Sienczyk, M; Radziszewicz, M; Kosciolek, B; Carty, MP; Oleksyszyn, J New aromatic monoesters of alpha-aminoaralkylphosphonic acids as inhibitors of aminopeptidase N/CD13. Bioorg Med Chem 18: 2930-6 (2010)
- Ferrer-Casal, M; Li, C; Galizzi, M; Stortz, CA; Szajnman, SH; Docampo, R; Moreno, SN; Rodriguez, JB New insights into molecular recognition of 1,1-bisphosphonic acids by farnesyl diphosphate synthase. Bioorg Med Chem 22: 398-405 (2013)
- Flynn, DL; Capiris, T; Cetenko, WJ; Connor, DT; Dyer, RD; Kostlan, CR; Nies, DE; Schrier, DJ; Sircar, JC Nonsteroidal antiinflammatory drug hydroxamic acids. Dual inhibitors of both cyclooxygenase and 5-lipoxygenase. J Med Chem 33: 2070-2 (1990)
- Liu, H; Wu, G; Yun, H Oxathiolane carboxylic acids and derivatives for the treatment and prophylaxis of virus infection US Patent US10183954 (2019)
- Schäfer, S; Saunders, L; Eliseeva, E; Velena, A; Jung, M; Schwienhorst, A; Strasser, A; Dickmanns, A; Ficner, R; Schlimme, S; Sippl, W; Verdin, E; Jung, M Phenylalanine-containing hydroxamic acids as selective inhibitors of class IIb histone deacetylases (HDACs). Bioorg Med Chem 16: 2011-33 (2008)
- Singh, R; Reed, AN; Chu, P; Scully, CC; Yau, MK; Suen, JY; Durek, T; Reid, RC; Fairlie, DP Potent complement C3a receptor agonists derived from oxazole amino acids: Structure-activity relationships. Bioorg Med Chem Lett 25: 5604-8 (2015)
- Keenan, RM; Weinstock, J; Finkelstein, JA; Franz, RG; Gaitanopoulos, DE; Girard, GR; Hill, DT; Morgan, TM; Samanen, JM; Peishoff, CE Potent nonpeptide angiotensin II receptor antagonists. 2. 1-(Carboxybenzyl)imidazole-5-acrylic acids. J Med Chem 36: 1880-92 (1993)
- Humphries, PS; Almaden, JV; Barnum, SJ; Carlson, TJ; Do, QQ; Fraser, JD; Hess, M; Kim, YH; Ogilvie, KM; Sun, S Pyridine-2-propanoic acids: Discovery of dual PPARalpha/gamma agonists as antidiabetic agents. Bioorg Med Chem Lett 16: 6116-9 (2006)
- Humphries, PS; Bailey, S; Almaden, JV; Barnum, SJ; Carlson, TJ; Christie, LC; Do, QQ; Fraser, JD; Hess, M; Kellum, J; Kim, YH; McClellan, GA; Ogilvie, KM; Simmons, BH; Skalitzky, D; Sun, S; Wilhite, D; Zehnder, LR Pyridine-3-propanoic acids: Discovery of dual PPARalpha/gamma agonists as antidiabetic agents. Bioorg Med Chem Lett 16: 6120-3 (2006)
- Vovk, AI; Mischenko, IM; Tanchuk, VY; Kachkovskii, GA; Sheiko, SY; Kolodyazhnyi, OI; Kukhar, VP Stereoselectivity of binding of alpha-(N-benzylamino)benzylphosphonic acids to prostatic acid phosphatase. Bioorg Med Chem Lett 18: 4620-3 (2008)
- Fatmawati, S; Shimizu, K; Kondo, R Structure-activity relationships of ganoderma acids from Ganoderma lucidum as aldose reductase inhibitors. Bioorg Med Chem Lett 21: 7295-7 (2011)
- Kuhn, B; Hilpert, H; Benz, J; Binggeli, A; Grether, U; Humm, R; Märki, HP; Meyer, M; Mohr, P Structure-based design of indole propionic acids as novel PPARalpha/gamma co-agonists. Bioorg Med Chem Lett 16: 4016-20 (2006)
- Hagmann, WK; Dorn, CP; Frankshun, RA; O'Grady, LA; Bailey, PJ; Rackham, A; Dougherty, HW Synthesis and antiinflammatory/analgesic activities of 11H-dibenzo[b, e,][1,4]dioxepinacetic acids. J Med Chem 29: 1436-41 (1986)
- Fichna, J; Lewellyn, K; Yan, F; Roth, BL; Zjawiony, JK Synthesis and biological evaluation of new salvinorin A analogues incorporating natural amino acids. Bioorg Med Chem Lett 21: 160-3 (2010)
- Bezuglov, V; Bobrov, M; Gretskaya, N; Gonchar, A; Zinchenko, G; Melck, D; Bisogno, T; Di Marzo, V; Kuklev, D; Rossi, JC; Vidal, JP; Durand, T Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorg Med Chem Lett 11: 447-9 (2001)
- Kubota, K; Kurebayashi, H; Miyachi, H; Tobe, M; Onishi, M; Isobe, Y Synthesis and structure-activity relationship of tricyclic carboxylic acids as novel anti-histamines. Bioorg Med Chem 19: 3005-21 (2011)
- Szermerski, M; Melesina, J; Wichapong, K; Löppenberg, M; Jose, J; Sippl, W; Holl, R Synthesis, biological evaluation and molecular docking studies of benzyloxyacetohydroxamic acids as LpxC inhibitors. Bioorg Med Chem 22: 1016-28 (2014)
- Hale, JJ; Doherty, G; Toth, L; Li, Z; Mills, SG; Hajdu, R; Ann Keohane, C; Rosenbach, M; Milligan, J; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Rosen, H; Mandala, S The discovery of 3-(N-alkyl)aminopropylphosphonic acids as potent S1P receptor agonists. Bioorg Med Chem Lett 14: 3495-9 (2004)
- Xiang, Y; Hirth, B; Asmussen, G; Biemann, HP; Bishop, KA; Good, A; Fitzgerald, M; Gladysheva, T; Jain, A; Jancsics, K; Liu, J; Metz, M; Papoulis, A; Skerlj, R; Stepp, JD; Wei, RR The discovery of novel benzofuran-2-carboxylic acids as potent Pim-1 inhibitors. Bioorg Med Chem Lett 21: 3050-6 (2011)
- Tumey, LN; Huck, B; Gleason, E; Wang, J; Silver, D; Brunden, K; Boozer, S; Rundlett, S; Sherf, B; Murphy, S; Bailey, A; Dent, T; Leventhal, C; Harrington, J; Bennani, YL The identification and optimization of 2,4-diketobutyric acids as flap endonuclease 1 inhibitors. Bioorg Med Chem Lett 14: 4915-8 (2004)
- Bhagwat, SS; Gude, C; Cohen, DS; Lee, W; Furness, P; Clarke, FH Thromboxane receptor antagonism combined with thromboxane synthase inhibition. 1. (+/-)-(3-pyridinylbicycloheptyl)alkanoic acids. J Med Chem 34: 1790-7 (1991)
- Casara, P; Ganzhorn, A; Philippo, C; Chanal, MC; Danzin, C Unsaturated thioacetic acids as novel mechanism-based inhibitors of peptidylglycine α-hydroxylating monooxygenase Bioorg Med Chem Lett 6: 393-396 (1996)
- Vaisburg, A; Bernstein, N; Frechette, S; Allan, M; Abou-Khalil, E; Leit, S; Moradei, O; Bouchain, G; Wang, J; Woo, SH; Fournel, M; Yan, PT; Trachy-Bourget, MC; Kalita, A; Beaulieu, C; Li, Z; MacLeod, AR; Besterman, JM; Delorme, D (2-amino-phenyl)-amides of omega-substituted alkanoic acids as new histone deacetylase inhibitors. Bioorg Med Chem Lett 14: 283-7 (2003)
- Koyama, H; Boueres, JK; Miller, DJ; Berger, JP; MacNaul, KL; Wang, PR; Ippolito, MC; Wright, SD; Agrawal, AK; Moller, DE; Sahoo, SP (2R)-2-methylchromane-2-carboxylic acids: discovery of selective PPARalpha agonists as hypolipidemic agents. Bioorg Med Chem Lett 15: 3347-51 (2005)
- Mederski, WW; Osswald, M; Dorsch, D; Christadler, M; Schmitges, CJ; Wilm, C 1,4-Diaryl-2-oxo-1,2-dihydro-quinoline-3-carboxylic acids as endothelin receptor antagonists Bioorg Med Chem Lett 7: 1883-1886 (1997)
- Chen, P; Horton, LB; Mikulski, RL; Deng, L; Sundriyal, S; Palzkill, T; Song, Y 2-Substituted 4,5-dihydrothiazole-4-carboxylic acids are novel inhibitors of metallo-ß-lactamases. Bioorg Med Chem Lett 22: 6229-32 (2012)
- Ilies, M; Di Costanzo, L; North, ML; Scott, JA; Christianson, DW 2-aminoimidazole amino acids as inhibitors of the binuclear manganese metalloenzyme human arginase I. J Med Chem 53: 4266-76 (2010)
- Cohen, N; Weber, G; Banner, BL; Lopresti, RJ; Schaer, B; Focella, A; Zenchoff, GB; Chiu, AM; Todaro, L; O'Donnell, M 3,4-Dihydro-2H-1-benzopyran-2-carboxylic acids and related compounds as leukotriene antagonists. J Med Chem 32: 1842-60 (1989)
- Zhi, Y; Gao, LX; Jin, Y; Tang, CL; Li, JY; Li, J; Long, YQ 4-Quinolone-3-carboxylic acids as cell-permeable inhibitors of protein tyrosine phosphatase 1B. Bioorg Med Chem 22: 3670-83 (2014)
- Nakane, M; Reid, JA; Han, WC; Das, J; Truc, VC; Haslanger, MF; Garber, D; Harris, DN; Hedberg, A; Ogletree, ML 7-Oxabicyclo[2.2.1]heptyl carboxylic acids as thromboxane A2 antagonists: aza omega-chain analogues. J Med Chem 33: 2465-76 (1990)
- Eldehna, WM; Nocentini, A; Elsayed, ZM; Al-Warhi, T; Aljaeed, N; Alotaibi, OJ; Al-Sanea, MM; Abdel-Aziz, HA; Supuran, CT Benzofuran-Based Carboxylic Acids as Carbonic Anhydrase Inhibitors and Antiproliferative Agents against Breast Cancer. ACS Med Chem Lett 11: 1022-1027 (2020)
- Milton, J; Slater, MJ; Bird, AJ; Spinks, D; Scott, G; Price, CE; Downing, S; Green, DV; Madar, S; Bethell, R; Stammers, DK Biaryl acids: novel non-nucleoside inhibitors of HIV reverse transcriptase types 1 and 2. Bioorg Med Chem Lett 8: 2623-8 (1999)
- Ilies, M; Di Costanzo, L; Dowling, DP; Thorn, KJ; Christianson, DW Binding ofa,a-disubstituted amino acids to arginase suggests new avenues for inhibitor design. J Med Chem 54: 5432-43 (2011)
- Szajnman, SH; Montalvetti, A; Wang, Y; Docampo, R; Rodriguez, JB Bisphosphonates derived from fatty acids are potent inhibitors of Trypanosoma cruzi farnesyl pyrophosphate synthase. Bioorg Med Chem Lett 13: 3231-5 (2003)
- Malovichko, OL; Petrus, AS; Krysko, AA; Kabanova, TA; Andronati, SA; Karaseva, TL; Kiriyak, AV Derivatives of 7-amino-1,2,3,4-tetrahydroisoquinoline and isophthalic acids as novel fibrinogen receptor antagonists. Bioorg Med Chem Lett 16: 5294-7 (2006)
- Mallik, B; Katragadda, M; Spruce, LA; Carafides, C; Tsokos, CG; Morikis, D; Lambris, JD Design and NMR characterization of active analogues of compstatin containing non-natural amino acids. J Med Chem 48: 274-86 (2005)
- Xue, P; Lu, HH; Zhu, YY; Ju, XL; Pannecouque, C; Zheng, XJ; Liu, GY; Zhang, XL; Gu, SX Design and synthesis of hybrids of diarylpyrimidines and diketo acids as HIV-1 inhibitors. Bioorg Med Chem Lett 27: 1640-1643 (2017)
- Matthews, JM; Chen, X; Cryan, E; Hlasta, DJ; Rybczynski, PJ; Strauss, K; Tang, Y; Xu, JZ; Yang, M; Zhou, L; Demarest, KT Design and synthesis of indane-ureido-thioisobutyric acids: A novel class of PPARalpha agonists. Bioorg Med Chem Lett 17: 6773-8 (2008)
- Sechi, M; Rizzi, G; Bacchi, A; Carcelli, M; Rogolino, D; Pala, N; Sanchez, TW; Taheri, L; Dayam, R; Neamati, N Design and synthesis of novel dihydroquinoline-3-carboxylic acids as HIV-1 integrase inhibitors. Bioorg Med Chem 17: 2925-35 (2009)
- Zhang, J; Han, M; Ma, X; Xu, L; Cao, J; Zhou, Y; Li, J; Liu, T; Hu, Y Design, Synthesis and Biological Evaluation of Peptidyl Epoxyketone Proteasome Inhibitors Composed of ??????-amino Acids. Chem Biol Drug Des 84: 497-504 (2014)
- Recher, M; Barboza, AP; Li, ZH; Galizzi, M; Ferrer-Casal, M; Szajnman, SH; Docampo, R; Moreno, SN; Rodriguez, JB Design, synthesis and biological evaluation of sulfur-containing 1,1-bisphosphonic acids as antiparasitic agents. Eur J Med Chem 60: 431-40 (2013)
- Levoin, N; Labeeuw, O; Krief, S; Calmels, T; Poupardin-Olivier, O; Berrebi-Bertrand, I; Lecomte, JM; Schwartz, JC; Capet, M Determination of the binding mode and interacting amino-acids for dibasic H3 receptor antagonists. Bioorg Med Chem 21: 4526-9 (2013)
- Xin, Z; Peng, H; Zhang, A; Talreja, T; Kumaravel, G; Xu, L; Rohde, E; Jung, MY; Shackett, MN; Kocisko, D; Chollate, S; Dunah, AW; Snodgrass-Belt, PA; Arnold, HM; Taveras, AG; Rhodes, KJ; Scannevin, RH Discovery of 4-aminomethylphenylacetic acids as¿-secretase modulators via a scaffold design approach. Bioorg Med Chem Lett 21: 7277-80 (2011)
- Minkkilä, A; Saario, SM; Käsnänen, H; Leppänen, J; Poso, A; Nevalainen, T Discovery of Boronic Acids as Novel and Potent Inhibitors of Fatty Acid Amide Hydrolase. J Med Chem 51: 7057-60 (2008)
- Crosignani, S; Jorand-Lebrun, C; Campbell, G; Prêtre, A; Grippi-Vallotton, T; Quattropani, A; Bouscary-Desforges, G; Bombrun, A; Missotten, M; Humbert, Y; Frémaux, C; Pâquet, M; El Harkani, K; Bradshaw, CG; Cleva, C; Abla, N; Daff, H; Schott, O; Pittet, PA; Arrighi, JF; Gaudet, M; Johnson, Z Discovery of a Novel Series of CRTH2 (DP2) Receptor Antagonists Devoid of Carboxylic Acids. ACS Med Chem Lett 2: 938-942 (2011)
- Duan, JJ; Chen, L; Lu, Z; Xue, CB; Liu, RQ; Covington, MB; Qian, M; Wasserman, ZR; Vaddi, K; Christ, DD; Trzaskos, JM; Newton, RC; Decicco, CP Discovery of beta-benzamido hydroxamic acids as potent, selective, and orally bioavailable TACE inhibitors. Bioorg Med Chem Lett 18: 241-6 (2008)
- Bihovsky, R; Levinson, BL; Loewi, RC; Erhardt, PW; Polokoff, MA Hydroxamic acids as potent inhibitors of endothelin-converting enzyme from human bronchiolar smooth muscle. J Med Chem 38: 2119-29 (1995)
- Jacobsen, P; Labouta, IM; Schaumburg, K; Falch, E; Krogsgaard-Larsen, P Hydroxy- and amino-substituted piperidinecarboxylic acids as gamma-aminobutyric acid agonists and uptake inhibitors. J Med Chem 25: 1157-62 (1982)
- Wang, H; Ning, R; Shen, Y; Chen, Z; Li, J; Zhang, R; Leng, Y; Zhao, W Lithocarpic Acids A-N, 3,4-seco-Cycloartane Derivatives from the Cupules of Lithocarpus polystachyus. J Nat Prod 77: 1910-20 (2014)
- Ning, RN; Wang, HM; Shen, Y; Chen, ZH; Zhang, RJ; Leng, Y; Zhao, WM Lithocarpic acids O-S, five homo-cycloartane derivatives from the cupules of Lithocarpus polystachyus. Bioorg Med Chem Lett 24: 5395-8 (2015)
- Shen, S; Doubleday, PF; Weerawarna, PM; Zhu, W; Kelleher, NL; Silverman, RB Mechanism-Based Design of 3-Amino-4-Halocyclopentenecarboxylic Acids as Inactivators of GABA Aminotransferase. ACS Med Chem Lett 11: 1949-1955 (2020)
- Sawa, M; Kiyoi, T; Kurokawa, K; Kumihara, H; Yamamoto, M; Miyasaka, T; Ito, Y; Hirayama, R; Inoue, T; Kirii, Y; Nishiwaki, E; Ohmoto, H; Maeda, Y; Ishibushi, E; Inoue, Y; Yoshino, K; Kondo, H New type of metalloproteinase inhibitor: design and synthesis of new phosphonamide-based hydroxamic acids. J Med Chem 45: 919-29 (2002)
- Buckle, DR; Cantello, BC; Cawthorne, MA; Coyle, PJ; Dean, DK; Faller, A; Haigh, D; Hindley, RM; Jefcott, LJ; Lister, CA; Pinto, IL; Rami, HK; Smith, DG; Smith, SA Non thiazolidinedione antihyperglycaemic agents. 2: α-Carbon substituted β-phenylpropanoic acids1 Bioorg Med Chem Lett 6: 2127-2130 (1996)
- Tucker, H; Thomas, DF Novel inhibitors of prolyl 4-hydroxylase. 2. 5-Amide substituted pyridine-2-carboxylic acids. J Med Chem 35: 804-7 (1992)
- Sauerberg, P; Pettersson, I; Jeppesen, L; Bury, PS; Mogensen, JP; Wassermann, K; Brand, CL; Sturis, J; Wöldike, HF; Fleckner, J; Andersen, AS; Mortensen, SB; Svensson, LA; Rasmussen, HB; Lehmann, SV; Polivka, Z; Sindelar, K; Panajotova, V; Ynddal, L; Wulff, EM Novel tricyclic-alpha-alkyloxyphenylpropionic acids: dual PPARalpha/gamma agonists with hypolipidemic and antidiabetic activity. J Med Chem 45: 789-804 (2002)
- Zhang, J; Didierlaurent, S; Fortin, M; Lefrançois, D; Uridat, E; Vevert, JP Potent nonpeptide endothelin antagonists: synthesis and structure-activity relationships of pyrazole-5-carboxylic acids. Bioorg Med Chem Lett 10: 2575-8 (2001)
- Jae, HS; Winn, M; Dixon, DB; Marsh, KC; Nguyen, B; Opgenorth, TJ; von Geldern, TW Pyrrolidine-3-carboxylic acids as endothelin antagonists. 2. Sulfonamide-based ETA/ETB mixed antagonists. J Med Chem 40: 3217-27 (1997)
- Le Diguarher, T; Chollet, AM; Bertrand, M; Hennig, P; Raimbaud, E; Sabatini, M; Guilbaud, N; Pierré, A; Tucker, GC; Casara, P Stereospecific synthesis of 5-substituted 2-bisarylthiocyclopentane carboxylic acids as specific matrix metalloproteinase inhibitors. J Med Chem 46: 3840-52 (2003)
- Smith, TP; Windsor, IW; Forest, KT; Raines, RT Stilbene Boronic Acids Form a Covalent Bond with Human Transthyretin and Inhibit Its Aggregation. J Med Chem 60: 7820-7834 (2017)
- Kato, Y; Hin, N; Maita, N; Thomas, AG; Kurosawa, S; Rojas, C; Yorita, K; Slusher, BS; Fukui, K; Tsukamoto, T Structural basis for potent inhibition of d-amino acid oxidase by thiophene carboxylic acids. Eur J Med Chem 159: 23-34 (2018)
- Luker, T; Bonnert, R; Brough, S; Cook, AR; Dickinson, MR; Dougall, I; Logan, C; Mohammed, RT; Paine, S; Sanganee, HJ; Sargent, C; Schmidt, JA; Teague, S; Thom, S Substituted indole-1-acetic acids as potent and selective CRTh2 antagonists-discovery of AZD1981. Bioorg Med Chem Lett 21: 6288-92 (2011)
- Piper, JR; McCaleb, GS; Montgomery, JA; Schmid, FA; Sirotnak, FM Syntheses and evaluation as antifolates of MTX analogues derived from 2, omega-diaminoalkanoic acids. J Med Chem 28: 1016-25 (1985)
- Synthesis and anti-liver fibrosis activity of imidazole and thiazole compounds containing amino acids.
- Inglis, SR; Zervosen, A; Woon, EC; Gerards, T; Teller, N; Fischer, DS; Luxen, A; Schofield, CJ Synthesis and evaluation of 3-(dihydroxyboryl)benzoic acids as D,D-carboxypeptidase R39 inhibitors. J Med Chem 52: 6097-106 (2009)
- Hasan, P; Pillalamarri, VK; Aneja, B; Irfan, M; Azam, M; Perwez, A; Maguire, R; Yadava, U; Kavanagh, K; Daniliuc, CG; Ahmad, MB; Rizvi, MMA; Rizwanul Haq, QM; Addlagatta, A; Abid, M Synthesis and mechanistic studies of diketo acids and their bioisosteres as potential antibacterial agents. Eur J Med Chem 163: 67-82 (2019)
- Beard, RL; Chandraratna, RA; Colon, DF; Gillett, SJ; Henry, E; Marler, DK; Song, T; Denys, L; Garst, ME; Arefieg, T Synthesis and structure-activity relationships of stilbene retinoid analogs substituted with heteroaromatic carboxylic acids. J Med Chem 38: 2820-9 (1995)
- Sitka, I; Allmendinger, L; Fülep, G; Höfner, G; Wanner, KT Synthesis of N-substituted acyclicß-amino acids and their investigation as GABA uptake inhibitors. Eur J Med Chem 65: 487-99 (2013)
- Kramer, JB; Boschelli, DH; Connor, DT; Kostlan, CR; Flynn, DL; Dyer, RD; Bornemeier, DA; Kennedy, JA; Wright, CD; Kuipers, PJ Synthesis of reversed hydroxamic acids of indomethacin: dual inhibitors of cyclooxygenase and 5-lipoxygenase Bioorg Med Chem Lett 2: 1655-1660 (1992)
- Smith, G; Mikkelsen, G; Eskildsen, J; Bundgaard, C The synthesis and SAR of 2-arylsulfanylphenyl-1-oxyalkylamino acids as GlyT-1 inhibitors. Bioorg Med Chem Lett 16: 3981-4 (2006)
- Ni, WW; Liu, Q; Ren, SZ; Li, WY; Yi, LL; Jing, H; Sheng, LX; Wan, Q; Zhong, PF; Fang, HL; Ouyang, H; Xiao, ZP; Zhu, HL The synthesis and evaluation of phenoxyacylhydroxamic acids as potential agents for Helicobacter pylori infections. Bioorg Med Chem 26: 4145-4152 (2018)
- Bhagwat, SS; Boswell, C; Gude, C; Contardo, N; Cohen, DS; Mathis, J; Dotson, R; Lee, W; Shetty, S Thromboxane receptor antagonism combined with thromboxane synthase inhibition. 6. 4-substituted 3-pyridinylalkanoic acids. Bioorg Med Chem Lett 2: 1619-1622 (1992)
- Bhagwat, SS; Roland, DM; Main, AJ; Gude, C; Grim, K; Goldstein, R; Cohen, DS; Dotson, R; Mathis, J; Lee, W Thromboxane receptor antagonism combined with thromboxane synthase inhibition. 7. pyridinylalkyl-substituted arylsulfonylamino arylalkanoic acids. Bioorg Med Chem Lett 2: 1623-1626 (1992)
- Kelley, JL; McLean, EW; Crouch, RC; Averett, DR; Tuttle, JV [[(Guaninylalkyl)phosphinico]methyl]phosphonic acids. Multisubstrate analogue inhibitors of human erythrocyte purine nucleoside phosphorylase. J Med Chem 38: 1005-14 (1995)
- Marinović, MA; Bekić, SS; Kugler, M; Brynda, J; Škerlová, J; Škorić, DĐ; Řezáčová, P; Petri, ET; Ćelić, AS X-ray structure of human aldo-keto reductase 1C3 in complex with a bile acid fused tetrazole inhibitor: experimental validation, molecular docking and structural analysis. RSC Med Chem 14: 341-355 (2023)
- Falke, H; Chaikuad, A; Becker, A; Loaëc, N; Lozach, O; Abu Jhaisha, S; Becker, W; Jones, PG; Preu, L; Baumann, K; Knapp, S; Meijer, L; Kunick, C 10-iodo-11H-indolo[3,2-c]quinoline-6-carboxylic acids are selective inhibitors of DYRK1A. J Med Chem 58: 3131-43 (2015)
- de Souza, AS; Pacheco, BDC; Pinheiro, S; Muri, EMF; Dias, LRS; Lima, CHS; Garrett, R; de Moraes, MBM; de Souza, BEG; Puzer, L 3-Acyltetramic acids as a novel class of inhibitors for human kallikreins 5 and 7. Bioorg Med Chem Lett 29: 1094-1098 (2019)
- Baran, JS; Laos, I; Langford, DD; Miller, JE; Jett, C; Taite, B; Rohrbacher, E 3-Alkyl-3-hydroxyglutaric acids: a new class of hypocholesterolemic HMG CoA reductase inhibitors. 1. J Med Chem 28: 597-601 (1985)
- Noe, MC; Snow, SL; Wolf-Gouveia, LA; Mitchell, PG; Lopresti-Morrow, L; Reeves, LM; Yocum, SA; Liras, JL; Vaughn, M 3-Hydroxy-4-arylsulfonyltetrahydropyranyl-3-hydroxamic acids are novel inhibitors of MMP-13 and aggrecanase. Bioorg Med Chem Lett 14: 4727-30 (2004)
- He, T; Edwards, TC; Xie, J; Aihara, H; Geraghty, RJ; Wang, Z 4,5-Dihydroxypyrimidine Methyl Carboxylates, Carboxylic Acids, and Carboxamides as Inhibitors of Human Cytomegalovirus pUL89 Endonuclease. J Med Chem 65: 5830-5849 (2022)
- Skinner, PJ; Webb, PJ; Sage, CR; Dang, TH; Pride, CC; Chen, R; Tamura, SY; Richman, JG; Connolly, DT; Semple, G 5-N,N-Disubstituted 5-aminopyrazole-3-carboxylic acids are highly potent agonists of GPR109b. Bioorg Med Chem Lett 19: 4207-9 (2009)
- Rossello, A; Nuti, E; Catalani, MP; Carelli, P; Orlandini, E; Rapposelli, S; Tuccinardi, T; Atkinson, SJ; Murphy, G; Balsamo, A A new development of matrix metalloproteinase inhibitors: twin hydroxamic acids as potent inhibitors of MMPs. Bioorg Med Chem Lett 15: 2311-4 (2005)
- Lavrador, K; Guillerm, D; Guillerm, G A new series of cyclic amino acids as inhibitors of S-adenosyl L-methionine synthetase. Bioorg Med Chem Lett 8: 1629-34 (1999)
- Liu, Q; Shi, WK; Ren, SZ; Ni, WW; Li, WY; Chen, HM; Liu, P; Yuan, J; He, XS; Liu, JJ; Cao, P; Yang, PZ; Xiao, ZP; Zhu, HL Arylamino containing hydroxamic acids as potent urease inhibitors for the treatment of Helicobacter pylori infection. Eur J Med Chem 156: 126-136 (2018)
- Holt, DA; Yamashita, DS; Konialian-Beck, AL; Luengo, JI; Abell, AD; Bergsma, DJ; Brandt, M; Levy, MA Benzophenone- and indolecarboxylic acids: potent type-2 specific inhibitors of human steroid 5 alpha-reductase. J Med Chem 38: 13-5 (1995)
- Sundén, H; Schäfer, A; Scheepstra, M; Leysen, S; Malo, M; Ma, JN; Burstein, ES; Ottmann, C; Brunsveld, L; Olsson, R Chiral Dihydrobenzofuran Acids Show Potent Retinoid X Receptor-Nuclear Receptor Related 1 Protein Dimer Activation. J Med Chem 59: 1232-8 (2016)
- Zhou, Y; Mowlazadeh Haghighi, S; Zoi, I; Sawyer, JR; Hruby, VJ; Cai, M Design of MC1R Selectiveγ-MSH Analogues with Canonical Amino Acids Leads to Potency and Pigmentation. J Med Chem 60: 9320-9329 (2017)
- Madak, JT; Cuthbertson, CR; Miyata, Y; Tamura, S; Petrunak, EM; Stuckey, JA; Han, Y; He, M; Sun, D; Showalter, HD; Neamati, N Design, Synthesis, and Biological Evaluation of 4-Quinoline Carboxylic Acids as Inhibitors of Dihydroorotate Dehydrogenase. J Med Chem 61: 5162-5186 (2018)
- Zhang, X; Cai, C; Winters, M; Wells, M; Wall, M; Lanter, J; Sui, Z; Ma, J; Novack, A; Nashashibi, I; Wang, Y; Yan, W; Suckow, A; Hua, H; Bell, A; Haug, P; Clapper, W; Jenkinson, C; Gunnet, J; Leonard, J; Murray, WV Design, synthesis and SAR of a novel series of heterocyclic phenylpropanoic acids as GPR120 agonists. Bioorg Med Chem Lett 27: 3272-3278 (2017)
- Eidam, O; Romagnoli, C; Caselli, E; Babaoglu, K; Pohlhaus, DT; Karpiak, J; Bonnet, R; Shoichet, BK; Prati, F Design, synthesis, crystal structures, and antimicrobial activity of sulfonamide boronic acids asß-lactamase inhibitors. J Med Chem 53: 7852-63 (2010)
- Beard, RL; Colon, DF; Klein, ES; Vorse, KA; Chandraratna, RA Differential RXR & RAR activity of stilbene retinoid analogs bearing thiazole and imidazole carboxylic acids Bioorg Med Chem Lett 5: 2729-2734 (1995)
- Liu, G; Szczepankiewicz, BG; Pei, Z; Janowick, DA; Xin, Z; Hajduk, PJ; Abad-Zapatero, C; Liang, H; Hutchins, CW; Fesik, SW; Ballaron, SJ; Stashko, MA; Lubben, T; Mika, AK; Zinker, BA; Trevillyan, JM; Jirousek, MR Discovery and structure-activity relationship of oxalylarylaminobenzoic acids as inhibitors of protein tyrosine phosphatase 1B. J Med Chem 46: 2093-103 (2003)
- Jiménez-Aligaga, K; Bermejo-Bescós, P; Martín-Aragón, S; Csákÿ, AG Discovery of alkenylboronic acids as neuroprotective agents affecting multiple biological targets involved in Alzheimer's disease. Bioorg Med Chem Lett 23: 426-9 (2012)
- Zhang, R; Wang, A; DeAngelis, A; Pelton, P; Xu, J; Zhu, P; Zhou, L; Demarest, K; Murray, WV; Kuo, GH Discovery of para-alkylthiophenoxyacetic acids as a novel series of potent and selective PPARdelta agonists. Bioorg Med Chem Lett 17: 3855-9 (2007)
- Mládková, J; Vanek, V; Budešínský, M; Elbert, T; Demianová, Z; Garrow, TA; Jirácek, J Double-headed sulfur-linked amino acids as first inhibitors for betaine-homocysteine S-methyltransferase 2. J Med Chem 55: 6822-31 (2012)
- Petrocchi, A; Koch, U; Matassa, VG; Pacini, B; Stillmock, KA; Summa, V From dihydroxypyrimidine carboxylic acids to carboxamide HIV-1 integrase inhibitors: SAR around the amide moiety. Bioorg Med Chem Lett 17: 350-3 (2007)
- Li, B; Li, L; Peng, Z; Liu, D; Si, L; Wang, J; Yuan, B; Huang, J; Proksch, P; Lin, W Harzianoic acids A and B, new natural scaffolds with inhibitory effects against hepatitis C virus. Bioorg Med Chem 27: 560-567 (2019)
- Bresciani, A; Ontoria, JM; Biancofiore, I; Cellucci, A; Ciammaichella, A; Di Marco, A; Ferrigno, F; Francone, A; Malancona, S; Monteagudo, E; Nizi, E; Pace, P; Ponzi, S; Rossetti, I; Veneziano, M; Summa, V; Harper, S Improved Selective Class I HDAC and Novel Selective HDAC3 Inhibitors: Beyond Hydroxamic Acids and Benzamides. ACS Med Chem Lett 10: 481-486 (2019)
- Pham, NB; Butler, MS; Hooper, JN; Moni, RW; Quinn, RJ Isolation of xestosterol esters of brominated acetylenic fatty acids from the marine sponge xestospongia testudinaria J Nat Prod 62: 1439-42 (1999)
- Wang, GT; Mantei, RA; Kawai, M; Tedrow, JS; Barnes, DM; Wang, J; Zhang, Q; Lou, P; Garcia, LA; Bouska, J; Yates, M; Park, C; Judge, RA; Lesniewski, R; Sheppard, GS; Bell, RL Lead optimization of methionine aminopeptidase-2 (MetAP2) inhibitors containing sulfonamides of 5,6-disubstituted anthranilic acids. Bioorg Med Chem Lett 17: 2817-22 (2007)
- Burdick, DJ; Paris, K; Weese, K; Stanley, M; Beresini, M; Clark, K; McDowell, RS; Marsters, JC; Gadek, TR N-Benzoyl amino acids as LFA-1/ICAM inhibitors 1: amino acid structure-activity relationship. Bioorg Med Chem Lett 13: 1015-8 (2003)
- Keresztes, A; Szucs, M; Borics, A; Kövér, KE; Forró, E; Fülöp, F; Tömböly, C; Péter, A; Páhi, A; Fábián, G; Murányi, M; Tóth, G New endomorphin analogues containing alicyclic beta-amino acids: influence on bioactive conformation and pharmacological profile. J Med Chem 51: 4270-9 (2008)
- Kumar, S; Namkung, W; Verkman, AS; Sharma, PK Novel 5-substituted benzyloxy-2-arylbenzofuran-3-carboxylic acids as calcium activated chloride channel inhibitors. Bioorg Med Chem 20: 4237-44 (2012)
- Salem, OI; Frotscher, M; Scherer, C; Neugebauer, A; Biemel, K; Streiber, M; Maas, R; Hartmann, RW Novel 5alpha-reductase inhibitors: synthesis, structure-activity studies, and pharmacokinetic profile of phenoxybenzoylphenyl acetic acids. J Med Chem 49: 748-59 (2006)
- Rist, Ø; Grimstrup, M; Receveur, JM; Frimurer, TM; Ulven, T; Kostenis, E; Högberg, T Novel selective thiazoleacetic acids as CRTH2 antagonists developed from in silico derived hits. Part 1. Bioorg Med Chem Lett 20: 1177-80 (2010)
- Grimstrup, M; Rist, Ø; Receveur, JM; Frimurer, TM; Ulven, T; Mathiesen, JM; Kostenis, E; Högberg, T Novel selective thiazoleacetic acids as CRTH2 antagonists developed from in silico derived hits. Part 2. Bioorg Med Chem Lett 20: 1181-5 (2010)
- Labaudinière, R; Hilboll, G; Leon-Lomeli, A; Terlain, B; Cavy, F; Parnham, M; Kuhl, P; Dereu, N Omega-[(omega-arylalkyl)thienyl]alkanoic acids: from specific LTA4 hydrolase inhibitors to LTB4 receptor antagonists. J Med Chem 35: 3170-9 (1992)
- Goyal, S; Banerjee, S; Mazumdar, S Oxygenation of monoenoic fatty acids by CYP175A1, an orphan cytochrome P450 from Thermus thermophilus HB27. Biochemistry 51: 7880-90 (2012)
- Meyners, C; Wolff, B; Kleinschek, A; Krämer, A; Meyer-Almes, FJ Perfluorinated hydroxamic acids are potent and selective inhibitors of HDAC-like enzymes from Pseudomonas aeruginosa. Bioorg Med Chem Lett 27: 1508-1512 (2017)
- Cherney, RJ; Decicco, CP; Nelson, DJ; Wang, L; Meyer, DT; Hardman, KD; Copeland, RA; Arner, EC Potent carboxylate inhibitors of stromelysin containing P2′ piperazic acids and P1′ biaryl moeities Bioorg Med Chem Lett 7: 1757-1762 (1997)
- Carrigan, CN; Esslinger, CS; Bartlett, RD; Bridges, RJ; Thompson, CM Quinoline-2,4-dicarboxylic acids: synthesis and evaluation as inhibitors of the glutamate vesicular transport system. Bioorg Med Chem Lett 9: 2607-12 (1999)
- Umemiya, H; Fukasawa, H; Ebisawa, M; Eyrolles, L; Kawachi, E; Eisenmann, G; Gronemeyer, H; Hashimoto, Y; Shudo, K; Kagechika, H Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the RXR-RAR heterodimers. J Med Chem 40: 4222-34 (1998)
- Dankwardt, SM; Billedeau, RJ; Lawley, LK; Abbot, SC; Martin, RL; Chan, CS; Van Wart, HE; Walker, KA Solid-phase synthesis of di- and tripeptidic hydroxamic acids as inhibitors of procollagen C-proteinase. Bioorg Med Chem Lett 10: 2513-6 (2001)
- Holt, DA; Levy, MA; Ladd, DL; Oh, HJ; Erb, JM; Heaslip, JI; Brandt, M; Metcalf, BW Steroidal A ring aryl carboxylic acids: a new class of steroid 5 alpha-reductase inhibitors. J Med Chem 33: 937-42 (1990)
- Oyallon, B; Brachet-Botineau, M; Logé, C; Bonnet, P; Souab, M; Robert, T; Ruchaud, S; Bach, S; Berthelot, P; Gouilleux, F; Viaud-Massuard, MC; Denevault-Sabourin, C Structure-based design of novel quinoxaline-2-carboxylic acids and analogues as Pim-1 inhibitors. Eur J Med Chem 154: 101-109 (2018)
- Afifi, AH; Kagiyama, I; El-Desoky, AH; Kato, H; Mangindaan, REP; de Voogd, NJ; Ammar, NM; Hifnawy, MS; Tsukamoto, S Sulawesins A-C, Furanosesterterpene Tetronic Acids That Inhibit USP7, from a Psammocinia sp. Marine Sponge. J Nat Prod 80: 2045-2050 (2017)
- Ohemeng, KA; Appollina, MA; Nguyen, VN; Schwender, CF; Singer, M; Steber, M; Ansell, J; Argentieri, D; Hageman, W Synthesis and 5-lipoxygenase inhibitory activities of some novel 2-substituted 5-benzofuran hydroxamic acids. J Med Chem 37: 3663-7 (1994)
- Vögerl, K; Ong, N; Senger, J; Herp, D; Schmidtkunz, K; Marek, M; Müller, M; Bartel, K; Shaik, TB; Porter, NJ; Robaa, D; Christianson, DW; Romier, C; Sippl, W; Jung, M; Bracher, F Synthesis and Biological Investigation of Phenothiazine-Based Benzhydroxamic Acids as Selective Histone Deacetylase 6 Inhibitors. J Med Chem 62: 1138-1166 (2019)
- Shearer, BG; Chao, EY; Uehling, DE; Deaton, DN; Cowan, C; Sherman, BW; Milliken, T; Faison, W; Brown, K; Adkison, KK; Lee, F Synthesis and evaluation of potent and selective beta3 adrenergic receptor agonists containing heterobiaryl carboxylic acids. Bioorg Med Chem Lett 17: 4670-7 (2007)
- Campbell, I; Collington, E; Finch, H; Hayes, R; Lumley, P; Mills, K; Pike, N; Robertson, G; Watts, I Synthesis and pharmacological evaluation of combined thromboxane receptor antagonist/synthase inhibitors: pyridine-containing sulphonamido acids Bioorg Med Chem Lett 1: 699-704 (1991)
- Lin, LS; Kopka, IE; Mumford, RA; Magriotis, PA; Lanza, T; Durette, PL; Kamenecka, T; Young, DN; de Laszlo, SE; McCauley, E; Riper, GV; Kidambi, U; Egger, LA; Tong, X; Lyons, K; Vincent, S; Stearns, R; Colletti, A; Teffera, Y; Fenyk-Melody, J; Schmidt, JA; MacCoss, M; Hagmann, WK The discovery of acylated beta-amino acids as potent and orally bioavailable VLA-4 antagonists. Bioorg Med Chem Lett 12: 611-4 (2002)
- Smith, G; Ruhland, T; Mikkelsen, G; Andersen, K; Christoffersen, CT; Alifrangis, LH; Mørk, A; Wren, SP; Harris, N; Wyman, BM; Brandt, G The synthesis and SAR of 2-arylsulfanyl-phenyl piperazinyl acetic acids as glyT-1 inhibitors. Bioorg Med Chem Lett 14: 4027-30 (2004)
- Castelli, R; Tognolini, M; Vacondio, F; Incerti, M; Pala, D; Callegari, D; Bertoni, S; Giorgio, C; Hassan-Mohamed, I; Zanotti, I; Bugatti, A; Rusnati, M; Festuccia, C; Rivara, S; Barocelli, E; Mor, M; Lodola, A ¿(5)-Cholenoyl-amino acids as selective and orally available antagonists of the Eph-ephrin system. Eur J Med Chem 103: 312-24 (2015)
- Festa, C; Renga, B; D'Amore, C; Sepe, V; Finamore, C; De Marino, S; Carino, A; Cipriani, S; Monti, MC; Zampella, A; Fiorucci, S Exploitation of cholane scaffold for the discovery of potent and selective farnesoid X receptor (FXR) and G-protein coupled bile acid receptor 1 (GP-BAR1) ligands. J Med Chem 57: 8477-95 (2014)
- Thompson, AM; Rewcastle, GW; Tercel, M; Dobrusin, EM; Fry, DW; Kraker, AJ; Denny, WA Tyrosine kinase inhibitors. 1. Structure-activity relationships for inhibition of epidermal growth factor receptor tyrosine kinase activity by 2,3-dihydro-2-thioxo-1H-indole-3-alkanoic acids and 2,2'-dithiobis(1H-indole-3-alkanoic acids). J Med Chem 36: 2459-69 (1993)
- Ishizuka, H; Konno, K; Naganuma, H; Sasahara, K; Kawahara, Y; Niinuma, K; Suzuki, H; Sugiyama, Y Temocaprilat, a novel angiotensin-converting enzyme inhibitor, is excreted in bile via an ATP-dependent active transporter (cMOAT) that is deficient in Eisai hyperbilirubinemic mutant rats (EHBR). J Pharmacol Exp Ther 280: 1304-11 (1997)
- Vo-Quang, Y; Carniato, D; Vo-Quang, L; Lacoste, AM; Neuzil, E; Le Goffic, F (beta-Chloro-alpha-aminoethyl)phosphonic acids as inhibitors of alanine racemase and D-alanine:D-alanine ligase. J Med Chem 29: 148-51 (1986)
- Gazvoda, M; Beranic, N; Turk, S; Burja, B; Kocevar, M; Rižner, TL; Gobec, S; Polanc, S 2,3-diarylpropenoic acids as selective non-steroidal inhibitors of type-5 17ß-hydroxysteroid dehydrogenase (AKR1C3). Eur J Med Chem 62: 89-97 (2013)
- Winn, M; von Geldern, TW; Opgenorth, TJ; Jae, HS; Tasker, AS; Boyd, SA; Kester, JA; Mantei, RA; Bal, R; Sorensen, BK; Wu-Wong, JR; Chiou, WJ; Dixon, DB; Novosad, EI; Hernandez, L; Marsh, KC 2,4-Diarylpyrrolidine-3-carboxylic acids--potent ETA selective endothelin receptor antagonists. 1. Discovery of A-127722. J Med Chem 39: 1039-48 (1996)
- Horvath, A; Nussbaumer, P; Wolff, B; Billich, A 2-(1-adamantyl)-4-(thio)chromenone-6-carboxylic acids: potent reversible inhibitors of human steroid sulfatase. J Med Chem 47: 4268-76 (2004)
- Yan, L; Budhu, R; Huo, P; Lynch, CL; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Mandala, SM 2-Aryl(pyrrolidin-4-yl)acetic acids are potent agonists of sphingosine-1-phosphate (S1P) receptors. Bioorg Med Chem Lett 16: 3564-8 (2006)
- Adams, DR; Abraham, A; Asano, J; Breslin, C; Dick, CA; Ixkes, U; Johnston, BF; Johnston, D; Kewnay, J; Mackay, SP; MacKenzie, SJ; McFarlane, M; Mitchell, L; Spinks, D; Takano, Y 2-Aryl-3,3,3-trifluoro-2-hydroxypropionic acids: a new class of protein tyrosine phosphatase 1B inhibitors. Bioorg Med Chem Lett 17: 6579-83 (2007)
- Flipo, M; Beghyn, T; Charton, J; Leroux, VA; Deprez, BP; Deprez-Poulain, RF A library of novel hydroxamic acids targeting the metallo-protease family: design, parallel synthesis and screening. Bioorg Med Chem 15: 63-76 (2006)
- Rajaram, S; Ramulu, U; Ramesh, D; Srikanth, D; Bhattacharya, P; Prabhakar, P; Kalivendi, SV; Babu, KS; Venkateswarlu, Y; Navath, S Anti-cancer evaluation of carboxamides of furano-sesquiterpene carboxylic acids from the soft coral Sinularia kavarattiensis. Bioorg Med Chem Lett 23: 6234-8 (2013)
- Marastoni, E; Bartoli, S; Berettoni, M; Cipollone, A; Ettorre, A; Fincham, CI; Mauro, S; Paris, M; Porcelloni, M; Bigioni, M; Binaschi, M; Nardelli, F; Parlani, M; Maggi, CA; Fattori, D Benzofused hydroxamic acids: useful fragments for the preparation of histone deacetylase inhibitors. Part 1: hit identification. Bioorg Med Chem Lett 23: 4091-5 (2013)
- Valgeirsson, J; Nielsen, EO; Peters, D; Mathiesen, C; Kristensen, AS; Madsen, U Bioisosteric modifications of 2-arylureidobenzoic acids: selective noncompetitive antagonists for the homomeric kainate receptor subtype GluR5. J Med Chem 47: 6948-57 (2004)
- Cincinelli, R; Zwick, V; Musso, L; Zuco, V; De Cesare, M; Zunino, F; Simoes-Pires, C; Nurisso, A; Giannini, G; Cuendet, M; Dallavalle, S Biphenyl-4-yl-acrylohydroxamic acids: Identification of a novel indolyl-substituted HDAC inhibitor with antitumor activity. Eur J Med Chem 112: 99-105 (2016)
- Breuer, E; Salomon, CJ; Katz, Y; Chen, W; Lu, S; Röschenthaler, GV; Hadar, R; Reich, R Carbamoylphosphonates, a new class of in vivo active matrix metalloproteinase inhibitors. 1. Alkyl- and cycloalkylcarbamoylphosphonic acids. J Med Chem 47: 2826-32 (2004)
- Oon Han, H; Kim, SH; Kim, KH; Hur, GC; Joo Yim, H; Chung, HK; Ho Woo, S; Dong Koo, K; Lee, CS; Sung Koh, J; Kim, GT Design and synthesis of oxime ethers of alpha-acyl-beta-phenylpropanoic acids as PPAR dual agonists. Bioorg Med Chem Lett 17: 937-41 (2007)
- Hanson, GJ; Vuletich, JL; Bedell, LJ; Bono, CP; Howard, SC; Welply, JK; Woulfe, SL; Zacheis, ML Design of MHC class II (DR4) ligands using conformationally restricted imino acids at p3 and p5 Bioorg Med Chem Lett 6: 1931-1936 (1996)
- Szymanska, E; Chalupnik, P; Szczepanska, K; Cuñado Moral, AM; Pickering, DS; Nielsen, B; Johansen, TN; Kiec-Kononowicz, K Design, synthesis and structure-activity relationships of novel phenylalanine-based amino acids as kainate receptors ligands. Bioorg Med Chem Lett 26: 5568-5572 (2016)
- Richter, HG; Angehrn, P; Hubschwerlen, C; Kania, M; Page, MG; Specklin, JL; Winkler, FK Design, synthesis, and evaluation of 2 beta-alkenyl penam sulfone acids as inhibitors of beta-lactamases. J Med Chem 39: 3712-22 (1996)
- Koukoulitsa, C; Bailly, F; Pegklidou, K; Demopoulos, VJ; Cotelle, P Evaluation of aldose reductase inhibition and docking studies of 6'-nitro and 6',6''-dinitrorosmarinic acids. Eur J Med Chem 45: 1663-6 (2010)
- Kotani, T; Nagaki, Y; Ishii, A; Konishi, Y; Yago, H; Suehiro, S; Okukado, N; Okamoto, K Highly selective aldose reductase inhibitors. 3. Structural diversity of 3-(arylmethyl)-2,4,5-trioxoimidazolidine-1-acetic acids. J Med Chem 40: 684-94 (1997)
- Angibaud, P; Van Emelen, K; Decrane, L; van Brandt, S; Ten Holte, P; Pilatte, I; Roux, B; Poncelet, V; Speybrouck, D; Queguiner, L; Gaurrand, S; Mariën, A; Floren, W; Janssen, L; Verdonck, M; van Dun, J; van Gompel, J; Gilissen, R; Mackie, C; Du Jardin, M; Peeters, J; Noppe, M; Van Hijfte, L; Freyne, E; Page, M; Janicot, M; Arts, J Identification of a series of substituted 2-piperazinyl-5-pyrimidylhydroxamic acids as potent histone deacetylase inhibitors. Bioorg Med Chem Lett 20: 294-8 (2010)
- Liu, M; Huang, J; Chen, DX; Jiang, C Identification of indole-3-carboxylic acids as non-ATP-competitive Polo-like kinase 1 (Plk1) inhibitors. Bioorg Med Chem Lett 25: 431-4 (2015)
- Stachel, SJ; Egbertson, MS; Wai, J; Machacek, M; Toolan, DM; Swestock, J; Eddins, DM; Puri, V; McGaughey, G; Su, HP; Perlow, D; Wang, D; Ma, L; Parthasarathy, G; Reid, JC; Abeywickrema, PD; Smith, SM; Uslaner, JM Indole acids as a novel PDE2 inhibitor chemotype that demonstrate pro-cognitive activity in multiple species. Bioorg Med Chem Lett 28: 1122-1126 (2018)
- Ashton, WT; Cantone, CL; Tolman, RL; Greenlee, WJ; Lynch, RJ; Schorn, TW; Strouse, JF; Siegl, PK Inhibitors of human renin with C-termini derived from amides and esters of alpha-mercaptoalkanoic acids. J Med Chem 35: 2772-81 (1992)
- Petrović, MM; Roschger, C; Chaudary, S; Zierer, A; Mladenović, M; Marković, V; Trifunović, S; Joksović, MD Low cytotoxic quinoline-4-carboxylic acids derived from vanillin precursors as potential human dihydroorotate dehydrogenase inhibitors. Bioorg Med Chem Lett 46: (2021)
- Pombrio, JM; Giangreco, A; Li, L; Wempe, MF; Anders, MW; Sweet, DH; Pritchard, JB; Ballatori, N Mercapturic acids (N-acetylcysteine S-conjugates) as endogenous substrates for the renal organic anion transporter-1. Mol Pharmacol 60: 1091-9 (2001)
- Salomé, C; Salomé-Grosjean, E; Stables, JP; Kohn, H Merging the structural motifs of functionalized amino acids and alpha-aminoamides: compounds with significant anticonvulsant activities. J Med Chem 53: 3756-71 (2010)
- Zhou, X; Born, EJ; Allen, C; Holstein, SA; Wiemer, DF N-Oxide derivatives of 3-(3-pyridyl)-2-phosphonopropanoic acids as potential inhibitors of Rab geranylgeranylation. Bioorg Med Chem Lett 25: 2331-4 (2015)
- Zhang, J; Didierlaurent, S; Fortin, M; Lefrançois, D; Uridat, E; Vevert, JP Nonpeptide endothelin antagonists: from lower affinity pyrazol-5-ols to higher affinity pyrazole-5-carboxylic acids. Bioorg Med Chem Lett 10: 1351-5 (2000)
- Gavande, N; Yamamoto, I; Salam, NK; Ai, TH; Burden, PM; Johnston, GA; Hanrahan, JR Novel Cyclic Phosphinic Acids as GABAC ρ Receptor Antagonists: Design, Synthesis, and Pharmacology ACS Med Chem Lett 2: 11-16 (2011)
- Takano, R; Yoshida, M; Inoue, M; Honda, T; Nakashima, R; Matsumoto, K; Yano, T; Ogata, T; Watanabe, N; Hirouchi, M; Kimura, T; Toda, N Optimization of 3-aryl-3-ethoxypropanoic acids and discovery of the potent GPR40 agonist DS-1558. Bioorg Med Chem 23: 5546-65 (2015)
- Walsh, TF; Fitch, KJ; Williams, DL; Murphy, KL; Nolan, NA; Pettibone, DJ; Chang, RS; O'Malley, SS; Clineschmidt, BV; Veber, DF; Greenlee, WJ Potent dual antagonists of endothelin and angiotensin II receptors derived from α-phenoxyphenylacetic acids (Part III) Bioorg Med Chem Lett 5: 1155-1158 (1995)
- Halab, L; Becker, JA; Darula, Z; Tourwé, D; Kieffer, BL; Simonin, F; Lubell, WD Probing opioid receptor interactions with azacycloalkane amino acids. Synthesis of a potent and selective ORL1 antagonist. J Med Chem 45: 5353-7 (2002)
- Van der Poorten, O; Knuhtsen, A; Sejer Pedersen, D; Ballet, S; Tourwé, D Side Chain Cyclized Aromatic Amino Acids: Great Tools as Local Constraints in Peptide and Peptidomimetic Design. J Med Chem 59: 10865-10890 (2016)
- Christiansen, E; Due-Hansen, ME; Urban, C; Merten, N; Pfleiderer, M; Karlsen, KK; Rasmussen, SS; Steensgaard, M; Hamacher, A; Schmidt, J; Drewke, C; Petersen, RK; Kristiansen, K; Ullrich, S; Kostenis, E; Kassack, MU; Ulven, T Structure-Activity Study of Dihydrocinnamic Acids and Discovery of the Potent FFA1 (GPR40) Agonist TUG-469. ACS Med Chem Lett 1: 345-349 (2010)
- Andrés, M; Buil, MA; Calbet, M; Casado, O; Castro, J; Eastwood, PR; Eichhorn, P; Ferrer, M; Forns, P; Moreno, I; Petit, S; Roberts, RS Structure-activity relationships (SAR) and structure-kinetic relationships (SKR) of pyrrolopiperidinone acetic acids as CRTh2 antagonists. Bioorg Med Chem Lett 24: 5111-7 (2014)
- Jin, H; Cianchetta, G; Devasagayaraj, A; Gu, K; Marinelli, B; Samala, L; Scott, S; Stouch, T; Tunoori, A; Wang, Y; Zang, Y; Zhang, C; David Kimball, S; Main, AJ; Ding, ZM; Sun, W; Yang, Q; Yu, XQ; Powell, DR; Wilson, A; Liu, Q; Shi, ZC Substituted 3-(4-(1,3,5-triazin-2-yl)-phenyl)-2-aminopropanoic acids as novel tryptophan hydroxylase inhibitors. Bioorg Med Chem Lett 19: 5229-32 (2009)
- Galaka, T; Falcone, BN; Li, C; Szajnman, SH; Moreno, SNJ; Docampo, R; Rodriguez, JB Synthesis and biological evaluation of 1-alkylaminomethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii. Bioorg Med Chem 27: 3663-3673 (2019)
- Donkor, IO; Korukonda, R Synthesis and calpain inhibitory activity of peptidomimetic compounds with constrained amino acids at the P2 position. Bioorg Med Chem Lett 18: 4806-8 (2008)
- Faridoon, na; Mnkandhla, D; Isaacs, M; Hoppe, HC; Kaye, PT Synthesis and evaluation of substituted 4-arylimino-3-hydroxybutanoic acids as potential HIV-1 integrase inhibitors. Bioorg Med Chem Lett 28: 1067-1070 (2018)
- Bar-Tana, J; Ben-Shoshan, S; Blum, J; Migron, Y; Hertz, R; Pill, J; Rose-Khan, G; Witte, EC Synthesis and hypolipidemic and antidiabetogenic activities of beta,beta,beta',beta'-tetrasubstituted, long-chain dioic acids. J Med Chem 32: 2072-84 (1989)
- Lazarova, TI; Jin, L; Rynkiewicz, M; Gorga, JC; Bibbins, F; Meyers, HV; Babine, R; Strickler, J Synthesis and in vitro biological evaluation of aryl boronic acids as potential inhibitors of factor XIa. Bioorg Med Chem Lett 16: 5022-7 (2006)
- Acher, FC; Tellier, FJ; Azerad, R; Brabet, IN; Fagni, L; Pin, JP Synthesis and pharmacological characterization of aminocyclopentanetricarboxylic acids: new tools to discriminate between metabotropic glutamate receptor subtypes. J Med Chem 40: 3119-29 (1997)
- Sharma, H; Sanchez, TW; Neamati, N; Detorio, M; Schinazi, RF; Cheng, X; Buolamwini, JK Synthesis, docking, and biological studies of phenanthrene β-diketo acids as novel HIV-1 integrase inhibitors. Bioorg Med Chem Lett 23: 6146-51 (2013)
- Sparey, T; Abeywickrema, P; Almond, S; Brandon, N; Byrne, N; Campbell, A; Hutson, PH; Jacobson, M; Jones, B; Munshi, S; Pascarella, D; Pike, A; Prasad, GS; Sachs, N; Sakatis, M; Sardana, V; Venkatraman, S; Young, MB The discovery of fused pyrrole carboxylic acids as novel, potent D-amino acid oxidase (DAO) inhibitors. Bioorg Med Chem Lett 18: 3386-91 (2008)
- Metcalf, MD; Aceto, MD; Harris, LS; Woods, JH; Traynor, JR; Coop, A; May, EL The influence of esters and carboxylic acids as the N-substituent of opioids. Part 1: Benzomorphans. Bioorg Med Chem 16: 869-73 (2008)
- Main, AJ; Bhagwat, SS; Boswell, C; Goldstein, R; Gude, C; Cohen, DS; Furness, P; Lee, W; Louzan, M Thromboxane receptor antagonism combined with thromboxane synthase inhibition. 3. Pyridinylalkyl-substituted 8-[(arylsulfonyl)amino]octanoic acids. J Med Chem 35: 4366-72 (1992)
- Kato, K; Ohkawa, S; Terao, S; Terashita, Z; Nishikawa, K Thromboxane synthetase inhibitors (TXSI). Design, synthesis, and evaluation of a novel series of omega-pyridylalkenoic acids. J Med Chem 28: 287-94 (1985)
- Lu, Q; Yang, YT; Chen, CS; Davis, M; Byrd, JC; Etherton, MR; Umar, A; Chen, CS Zn2+-chelating motif-tethered short-chain fatty acids as a novel class of histone deacetylase inhibitors. J Med Chem 47: 467-74 (2004)
- Nocentini, A; Lucidi, A; Perut, F; Massa, A; Tomaselli, D; Gratteri, P; Baldini, N; Rotili, D; Mai, A; Supuran, CT α,γ-Diketocarboxylic Acids and Their Esters Act as Carbonic Anhydrase IX and XII Selective Inhibitors. ACS Med Chem Lett 10: 661-665 (2019)
- Drysdale, MJ; Hind, SL; Jansen, M; Reinhard, JF Synthesis and SAR of 4-aryl-2-hydroxy-4-oxobut-2-enoic acids and esters and 2-amino-4-aryl-4-oxobut-2-enoic acids and esters: potent inhibitors of kynurenine-3-hydroxylase as potential neuroprotective agents. J Med Chem 43: 123-7 (2000)
- Buzard, DJ; Lopez, L; Moody, J; Kawasaki, A; Schrader, TO; Kasem, M; Johnson, B; Zhu, X; Thoresen, L; Kim, SH; Gharbaoui, T; Sengupta, D; Calvano, L; Krishnan, A; Gao, Y; Semple, G; Edwards, J; Barden, J; Morgan, M; Usmani, K; Chen, C; Sadeque, A; Chen, W; Christopher, RJ; Thatte, J; Fu, L; Solomon, M; Whelan, K; Al-Shamma, H; Gatlin, J; Gaidarov, I; Anthony, T; Le, M; Unett, DJ; Stirn, S; Blackburn, A; Behan, DP; Jones, RM (7-Benzyloxy-2,3-dihydro-1H-pyrrolo[1,2-a]indol-1-yl)acetic Acids as S1P1 Functional Antagonists. ACS Med Chem Lett 5: 1334-9 (2014)
- Kim, DH; Guinosso, CJ; Buzby, GC; Herbst, DR; McCaully, RJ; Wicks, TC; Wendt, RL (Mercaptopropanoyl)indoline-2-carboxylic acids and related compounds as potent angiotensin converting enzyme inhibitors and antihypertensive agents. J Med Chem 26: 394-403 (1983)
- Mehndiratta, S; Qian, B; Chuang, JY; Liou, JP; Shih, JC -Methylpropargylamine-Conjugated Hydroxamic Acids as Dual Inhibitors of Monoamine Oxidase A and Histone Deacetylase for Glioma Treatment. J Med Chem 65: 2208-2224 (2022)
- Schulte, ML; Khodadadi, AB; Cuthbertson, ML; Smith, JA; Manning, HC 2-Amino-4-bis(aryloxybenzyl)aminobutanoic acids: A novel scaffold for inhibition of ASCT2-mediated glutamine transport. Bioorg Med Chem Lett 26: 1044-7 (2016)
- Silverman, RB; Durkee, SC; Invergo, BJ 4-Amino-2-(substituted methyl)-2-butenoic acids: substrates and potent inhibitors of gamma-aminobutyric acid aminotransferase. J Med Chem 29: 764-70 (1986)
- Fröhner, W; Lopez-Garcia, LA; Neimanis, S; Weber, N; Navratil, J; Maurer, F; Stroba, A; Zhang, H; Biondi, RM; Engel, M 4-benzimidazolyl-3-phenylbutanoic acids as novel PIF-pocket-targeting allosteric inhibitors of protein kinase PKC¿. J Med Chem 54: 6714-23 (2011)
- Cvijetic, IN; Tanç, M; Juranic, IO; Verbic, TŽ; Supuran, CT; Drakulic, BJ 5-Aryl-1H-pyrazole-3-carboxylic acids as selective inhibitors of human carbonic anhydrases IX and XII. Bioorg Med Chem 23: 4649-59 (2015)
- Wang, Y; Xing, Y; Liu, X; Ji, H; Kai, M; Chen, Z; Yu, J; Zhao, D; Ren, H; Wang, R A new class of highly potent and selective endomorphin-1 analogues containinga-methylene-ß-aminopropanoic acids (map). J Med Chem 55: 6224-36 (2012)
- Burkholder, TP; Le, TB; Giroux, EL; Flynn, GA Acid-catalyzed O-allylation of β-hydroxy-α-amino acids: an entry into conformationally constrained dipeptide surrogates Bioorg Med Chem Lett 2: 579-582 (1992)
- Xia, Y; Yang, ZY; Xia, P; Bastow, KF; Nakanishi, Y; Nampoothiri, P; Hamel, E; Brossi, A; Lee, KH Antitumor agents. Part 226: synthesis and cytotoxicity of 2-phenyl-4-quinolone acetic acids and their esters. Bioorg Med Chem Lett 13: 2891-3 (2003)
- Aotsuka, T; Abe, N; Fukushima, K; Ashizawa, N; Yoshida, M Benzothiazol-2-ylcarboxylic acids with diverse spacers: A novel class of potent, orally active aldose reductase inhibitors Bioorg Med Chem Lett 7: 1677-1682 (1997)
- Aicher, TD; VanHuis, CA; MacLean, J; Andresen, BM; Barr, KJ; Bienstock, C; Daniels, M; Liu, K; Liu, Y; White, C; Sciammetta, N; Simov, V Carbamate benzoxazine propionic acids and acid derivatives for modulation of RORgamma activity and the treatment of disease US Patent US9783511 (2017)
- Sarikaya, SB; Gülçin, I; Supuran, CT Carbonic anhydrase inhibitors: Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 75: 515-20 (2010)
- Sodji, QH; Kornacki, JR; McDonald, JF; Mrksich, M; Oyelere, AK Design and structure activity relationship of tumor-homing histone deacetylase inhibitors conjugated to folic and pteroic acids. Eur J Med Chem 96: 340-59 (2015)
- Shimazawa, R; Kuriyama, M; Shirai, R Design and synthesis of N-alkyl oxindolylidene acetic acids as a new class of potent Cdc25A inhibitors. Bioorg Med Chem Lett 18: 3350-3 (2008)
- Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; McEachern, E; Metz, M; Harwig, C; Li, TS; Yang, W; Bogucki, D; Zhu, Y; Langille, J; Veale, D; Ba, T; Bey, M; Baird, I; Kaller, A; Krumpak, M; Leitch, D; Satori, M; Vocadlo, K; Guay, D; Nan, S; Yee, H; Crawford, J; Chen, G; Wilson, T; Carpenter, B; Gauthier, D; Macfarland, R; Mosi, R; Bodart, V; Wong, R; Fricker, S; Schols, D Design of substituted imidazolidinylpiperidinylbenzoic acids as chemokine receptor 5 antagonists: potent inhibitors of R5 HIV-1 replication. J Med Chem 56: 8049-65 (2013)
- Thakur, A; Tawa, GJ; Henderson, MJ; Danchik, C; Liu, S; Shah, P; Wang, AQ; Dunn, G; Kabir, M; Padilha, EC; Xu, X; Simeonov, A; Kharbanda, S; Stone, R; Grewal, G Design, Synthesis, and Biological Evaluation of Quinazolin-4-one-Based Hydroxamic Acids as Dual PI3K/HDAC Inhibitors. J Med Chem 63: 4256-4292 (2020)
- Kankanala, J; Kirby, KA; Liu, F; Miller, L; Nagy, E; Wilson, DJ; Parniak, MA; Sarafianos, SG; Wang, Z Design, Synthesis, and Biological Evaluations of Hydroxypyridonecarboxylic Acids as Inhibitors of HIV Reverse Transcriptase Associated RNase H. J Med Chem 59: 5051-62 (2016)
- Shi, J; Lei, M; Wu, W; Feng, H; Wang, J; Chen, S; Zhu, Y; Hu, S; Liu, Z; Jiang, C Design, synthesis and docking studies of novel dipeptidyl boronic acid proteasome inhibitors constructed fromaa- andaß-amino acids. Bioorg Med Chem Lett 26: 1958-62 (2016)
- Zhang, H; Ryono, DE; Devasthale, P; Wang, W; O'Malley, K; Farrelly, D; Gu, L; Harrity, T; Cap, M; Chu, C; Locke, K; Zhang, L; Lippy, J; Kunselman, L; Morgan, N; Flynn, N; Moore, L; Hosagrahara, V; Zhang, L; Kadiyala, P; Xu, C; Doweyko, AM; Bell, A; Chang, C; Muckelbauer, J; Zahler, R; Hariharan, N; Cheng, PT Design, synthesis and structure-activity relationships of azole acids as novel, potent dual PPAR alpha/gamma agonists. Bioorg Med Chem Lett 19: 1451-6 (2009)
- Hamer, RR; Tegeler, JJ; Kurtz, ES; Allen, RC; Bailey, SC; Elliott, ME; Hellyer, L; Helsley, GC; Przekop, P; Freed, BS; White, J; Martin, LL Dibenzoxepinone hydroxylamines and hydroxamic acids: dual inhibitors of cyclooxygenase and 5-lipoxygenase with potent topical antiinflammatory activity. J Med Chem 39: 246-52 (1996)
- Barawkar, DA; Bandyopadhyay, A; Deshpande, A; Koul, S; Kandalkar, S; Patil, P; Khose, G; Vyas, S; Mone, M; Bhosale, S; Singh, U; De, S; Meru, A; Gundu, J; Chugh, A; Palle, VP; Mookhtiar, KA; Vacca, JP; Chakravarty, PK; Nargund, RP; Wright, SD; Roy, S; Graziano, MP; Cully, D; Cai, TQ; Singh, SB Discovery of pyrazole carboxylic acids as potent inhibitors of rat long chain L-2-hydroxy acid oxidase. Bioorg Med Chem Lett 22: 4341-7 (2012)
- Evindar, G; Deng, H; Bernier, SG; Doyle, E; Lorusso, J; Morgan, BA; Westlin, WF Exploring amino acids derivatives as potent, selective, and direct agonists of sphingosine-1-phosphate receptor subtype-1. Bioorg Med Chem Lett 23: 472-5 (2012)
- Black, PN; Ahowesso, C; Montefusco, D; Saini, N; DiRusso, CC Fatty Acid Transport Proteins: Targeting FATP2 as a Gatekeeper Involved in the Transport of Exogenous Fatty Acids. Medchemcomm 7: 612-622 (2016)
- Imbriglio, JE; Chang, S; Liang, R; Raghavan, S; Schmidt, D; Smenton, A; Tria, S; Schrader, TO; Jung, JK; Esser, C; Holt, TG; Wolff, MS; Taggart, AK; Cheng, K; Carballo-Jane, E; Waters, MG; Tata, JR; Colletti, SL GPR109a agonists. Part 2: pyrazole-acids as agonists of the human orphan G-protein coupled receptor GPR109a. Bioorg Med Chem Lett 20: 4472-4 (2010)
- Casiraghi, G; Rassu, G; Auzzas, L; Burreddu, P; Gaetani, E; Battistini, L; Zanardi, F; Curti, C; Nicastro, G; Belvisi, L; Motto, I; Castorina, M; Giannini, G; Pisano, C Grafting aminocyclopentane carboxylic acids onto the RGD tripeptide sequence generates low nanomolar alphaVbeta3/alphaVbeta5 integrin dual binders. J Med Chem 48: 7675-87 (2005)
- Magesh, S; Savita, V; Moriya, S; Suzuki, T; Miyagi, T; Ishida, H; Kiso, M Human sialidase inhibitors: design, synthesis, and biological evaluation of 4-acetamido-5-acylamido-2-fluoro benzoic acids. Bioorg Med Chem 17: 4595-603 (2009)
- Greiner, C; Zettl, H; Koeberle, A; Pergola, C; Northoff, H; Schubert-Zsilavecz, M; Werz, O Identification of 2-mercaptohexanoic acids as dual inhibitors of 5-lipoxygenase and microsomal prostaglandin E2 synthase-1. Bioorg Med Chem 19: 3394-401 (2011)
- Keenan, RM; Weinstock, J; Finkelstein, JA; Franz, RG; Gaitanopoulos, DE; Girard, GR; Hill, DT; Morgan, TM; Samanen, JM; Hempel, J Imidazole-5-acrylic acids: potent nonpeptide angiotensin II receptor antagonists designed using a novel peptide pharmacophore model. J Med Chem 35: 3858-72 (1992)
- Reiter, LA; Rizzi, JP; Pandit, J; Lasut, MJ; McGahee, SM; Parikh, VD; Blake, JF; Danley, DE; Laird, ER; Lopez-Anaya, A; Lopresti-Morrow, LL; Mansour, MN; Martinelli, GJ; Mitchell, PG; Owens, BS; Pauly, TA; Reeves, LM; Schulte, GK; Yocum, SA Inhibition of MMP-1 and MMP-13 with phosphinic acids that exploit binding in the S2 pocket. Bioorg Med Chem Lett 9: 127-32 (1999)
- Rossello, A; Nuti, E; Carelli, P; Orlandini, E; Macchia, M; Nencetti, S; Zandomeneghi, M; Balzano, F; Uccello Barretta, G; Albini, A; Benelli, R; Cercignani, G; Murphy, G; Balsamo, A N-i-Propoxy-N-biphenylsulfonylaminobutylhydroxamic acids as potent and selective inhibitors of MMP-2 and MT1-MMP. Bioorg Med Chem Lett 15: 1321-6 (2005)
- Elimam, DM; Elgazar, AA; Bonardi, A; Abdelfadil, M; Nocentini, A; El-Domany, RA; Abdel-Aziz, HA; Badria, FA; Supuran, CT; Eldehna, WM Natural inspired piperine-based sulfonamides and carboxylic acids as carbonic anhydrase inhibitors: Design, synthesis and biological evaluation. Eur J Med Chem 225: (2021)
- Shi, GQ; Dropinski, JF; Zhang, Y; Santini, C; Sahoo, SP; Berger, JP; Macnaul, KL; Zhou, G; Agrawal, A; Alvaro, R; Cai, TQ; Hernandez, M; Wright, SD; Moller, DE; Heck, JV; Meinke, PT Novel 2,3-dihydrobenzofuran-2-carboxylic acids: highly potent and subtype-selective PPARalpha agonists with potent hypolipidemic activity. J Med Chem 48: 5589-99 (2005)
- Miyamae, Y; Kurisu, M; Murakami, K; Han, J; Isoda, H; Irie, K; Shigemori, H Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid β-protein. Bioorg Med Chem 20: 5844-9 (2012)
- von Geldern, TW; Tasker, AS; Sorensen, BK; Winn, M; Szczepankiewicz, BG; Dixon, DB; Chiou, WJ; Wang, L; Wessale, JL; Adler, A; Marsh, KC; Nguyen, B; Opgenorth, TJ Pyrrolidine-3-carboxylic acids as endothelin antagonists. 4. Side chain conformational restriction leads to ET(B) selectivity. J Med Chem 42: 3668-78 (1999)
- Jae, HS; Winn, M; von Geldern, TW; Sorensen, BK; Chiou, WJ; Nguyen, B; Marsh, KC; Opgenorth, TJ Pyrrolidine-3-carboxylic acids as endothelin antagonists. 5. Highly selective, potent, and orally active ET(A) antagonists. J Med Chem 44: 3978-84 (2001)
- Sato, M; Kawakami, H; Motomura, T; Aramaki, H; Matsuda, T; Yamashita, M; Ito, Y; Matsuzaki, Y; Yamataka, K; Ikeda, S; Shinkai, H Quinolone carboxylic acids as a novel monoketo acid class of human immunodeficiency virus type 1 integrase inhibitors. J Med Chem 52: 4869-82 (2009)
- Hayashi, T; Sugimoto, T; Takewaki, N; Takeguchi, N; Tran, VD; O'Connor, SJ; Rucker, PV; Overman, LE Silyl ethers of cycloheptene, novel proton pump inhibitors obtained during the total synthesis of the scopadulcic acids Bioorg Med Chem Lett 5: 2943-2946 (1995)
- Zhao, ZY; Tong, YP; Jiang, W; Zang, Y; Xiong, J; Li, J; Hu, JF Structurally Diverse Triterpene-26-oic Acids as Potential Dual ACL and ACC1 Inhibitors from the Vulnerable Conifer J Nat Prod 86: 1487-1499 (2023)
- Belley, M; Gallant, M; Roy, B; Houde, K; Lachance, N; Labelle, M; Trimble, LA; Chauret, N; Li, C; Sawyer, N; Tremblay, N; Lamontagne, S; Carrière, MC; Denis, D; Greig, GM; Slipetz, D; Metters, KM; Gordon, R; Chan, CC; Zamboni, RJ Structure-activity relationship studies on ortho-substituted cinnamic acids, a new class of selective EP(3) antagonists. Bioorg Med Chem Lett 15: 527-30 (2005)
- Wei, J; Shao, X; Gong, M; Zhu, B; Cui, Y; Gao, Y; Wang, R. Structure-activity relationships of novel endomorphin-2 analogues with N-O turns induced by alpha-aminoxy acids. Bioorg Med Chem Lett 15: 2986-2989 (2005)
- Sibille, P; Lopez, S; Brabet, I; Valenti, O; Oueslati, N; Gaven, F; Goudet, C; Bertrand, HO; Neyton, J; Marino, MJ; Amalric, M; Pin, JP; Acher, FC Synthesis and biological evaluation of 1-amino-2-phosphonomethylcyclopropanecarboxylic acids, new group III metabotropic glutamate receptor agonists. J Med Chem 50: 3585-95 (2007)
- Hsu, KC; Chu, JC; Tseng, HJ; Liu, CI; Wang, HC; Lin, TE; Lee, HS; Hsin, LW; Wang, AH; Lin, CH; Huang, WJ Synthesis and biological evaluation of phenothiazine derivative-containing hydroxamic acids as potent class II histone deacetylase inhibitors. Eur J Med Chem 219: (2021)
- Lakshminarayana, N; Prasad, YR; Gharat, L; Thomas, A; Narayanan, S; Raghuram, A; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel dibenzo[b,d]furan carboxylic acids as potential anti-diabetic agents. Eur J Med Chem 45: 3709-18 (2010)
- Carrigan, CN; Bartlett, RD; Esslinger, CS; Cybulski, KA; Tongcharoensirikul, P; Bridges, RJ; Thompson, CM Synthesis and in vitro pharmacology of substituted quinoline-2,4-dicarboxylic acids as inhibitors of vesicular glutamate transport. J Med Chem 45: 2260-76 (2002)
- Blankley, CJ; Kaltenbronn, JS; DeJohn, DE; Werner, A; Bennett, LR; Bobowski, G; Krolls, U; Johnson, DR; Pearlman, WM; Hoefle, ML Synthesis and structure-activity relationships of potent new angiotensin converting enzyme inhibitors containing saturated bicyclic amino acids. J Med Chem 30: 992-8 (1987)
- Khudina, OG; Makhaeva, GF; Elkina, NA; Boltneva, NP; Serebryakova, OG; Shchegolkov, EV; Rudakova, EV; Lushchekina, SV; Burgart, YV; Bachurin, SO; Richardson, RJ; Saloutin, VI Synthesis of 2-arylhydrazinylidene-3-oxo-4,4,4-trifluorobutanoic acids as new selective carboxylesterase inhibitors and radical scavengers. Bioorg Med Chem Lett 29: (2019)
- Penning, TD; Russell, MA; Chen, BB; Chen, HY; Desai, BN; Docter, SH; Edwards, DJ; Gesicki, GJ; Liang, CD; Malecha, JW; Yu, SS; Engleman, VW; Freeman, SK; Hanneke, ML; Shannon, KE; Westlin, MM; Nickols, GA Synthesis of cinnamic acids and related isosteres as potent and selective alpha v beta 3 receptor antagonists. Bioorg Med Chem Lett 14: 1471-6 (2004)
- Seki, H; Pellett, S; Silhár, P; Stowe, GN; Blanco, B; Lardy, MA; Johnson, EA; Janda, KD Synthesis/biological evaluation of hydroxamic acids and their prodrugs as inhibitors for Botulinum neurotoxin A light chain. Bioorg Med Chem 22: 1208-17 (2014)
- Danelon, GO; Mata, EG; Mascaretti, OA; Girardini, J; Marro, M; Roveri, OA Synthesis and β-lactamase inhibitory evaluation of novel 6α-halo-2β-chloromethyl-2α-methylpenam-3α-carboxylic acids and their sulfones and 6α-halo-2β-mercaptobenzothiazolylmethyl-2α-methylpenam-3α-carboxylic acids Bioorg Med Chem Lett 5: 2037-2040 (1995)
- Pellicciari, R; Costantino, G; Camaioni, E; Sadeghpour, BM; Entrena, A; Willson, TM; Fiorucci, S; Clerici, C; Gioiello, A Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem 47: 4559-69 (2004)
- Pellicciari, R; Passeri, D; De Franco, F; Mostarda, S; Filipponi, P; Colliva, C; Gadaleta, RM; Franco, P; Carotti, A; Macchiarulo, A; Roda, A; Moschetta, A; Gioiello, A Discovery of 3α,7α,11β-Trihydroxy-6α-ethyl-5β-cholan-24-oic Acid (TC-100), a Novel Bile Acid as Potent and Highly Selective FXR Agonist for Enterohepatic Disorders. J Med Chem 59: 9201-9214 (2016)
- Koyama, H; Miller, DJ; Boueres, JK; Desai, RC; Jones, AB; Berger, JP; MacNaul, KL; Kelly, LJ; Doebber, TW; Wu, MS; Zhou, G; Wang, PR; Ippolito, MC; Chao, YS; Agrawal, AK; Franklin, R; Heck, JV; Wright, SD; Moller, DE; Sahoo, SP (2R)-2-ethylchromane-2-carboxylic acids: discovery of novel PPARalpha/gamma dual agonists as antihyperglycemic and hypolipidemic agents. J Med Chem 47: 3255-63 (2004)
- Biava, M; Porretta, GC; Cappelli, A; Vomero, S; Manetti, F; Botta, M; Sautebin, L; Rossi, A; Makovec, F; Anzini, M 1,5-Diarylpyrrole-3-acetic acids and esters as novel classes of potent and highly selective cyclooxygenase-2 inhibitors. J Med Chem 48: 3428-32 (2005)
- Subramanyam, C; Bell, MR; Carabateas, P; Court, JJ; Dority, JA; Ferguson, E; Gordon, R; Hlasta, DJ; Kumar, V; Saindane, M 2,6-Disubstituted aryl carboxylic acids, leaving groups"par excellence" for benzisothiazolone inhibitors of human leukocyte elastase. J Med Chem 37: 2623-6 (1994)
- Andrés, M; Bravo, M; Buil, MA; Calbet, M; Castillo, M; Castro, J; Eichhorn, P; Ferrer, M; Lehner, MD; Moreno, I; Roberts, RS; Sevilla, S 2-(1H-Pyrazol-1-yl)acetic acids as chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes (CRTh2) antagonists. Eur J Med Chem 71: 168-84 (2014)
- Biot, C; Bauer, H; Schirmer, RH; Davioud-Charvet, E 5-substituted tetrazoles as bioisosteres of carboxylic acids. Bioisosterism and mechanistic studies on glutathione reductase inhibitors as antimalarials. J Med Chem 47: 5972-83 (2004)
- Weglarz-Tomczak, E; Berlicki, L; Pawelczak, M; Nocek, B; Joachimiak, A; Mucha, A A structural insight into the P1S1 binding mode of diaminoethylphosphonic and phosphinic acids, selective inhibitors of alanine aminopeptidases. Eur J Med Chem 117: 187-96 (2016)
- Nair, V; Uchil, V; Neamati, N Beta-diketo acids with purine nucleobase scaffolds: novel, selective inhibitors of the strand transfer step of HIV integrase. Bioorg Med Chem Lett 16: 1920-3 (2006)
- Bochen, A; Marelli, UK; Otto, E; Pallarola, D; Mas-Moruno, C; Di Leva, FS; Boehm, H; Spatz, JP; Novellino, E; Kessler, H; Marinelli, L Biselectivity of isoDGR peptides for fibronectin binding integrin subtypesa5ß1 andavß6: conformational control through flanking amino acids. J Med Chem 56: 1509-19 (2013)
- Gurrapu, S; Jonnalagadda, SK; Alam, MA; Ronayne, CT; Nelson, GL; Solano, LN; Lueth, EA; Drewes, LR; Mereddy, VR Coumarin carboxylic acids as monocarboxylate transporter 1 inhibitors: In vitro and in vivo studies as potential anticancer agents. Bioorg Med Chem Lett 26: 3282-3286 (2016)
- Schann, S; Menet, C; Arvault, P; Mercier, G; Frauli, M; Mayer, S; Hubert, N; Triballeau, N; Bertrand, HO; Acher, F; Neuville, P Design and synthesis of APTCs (aminopyrrolidinetricarboxylic acids): identification of a new group III metabotropic glutamate receptor selective agonist. Bioorg Med Chem Lett 16: 4856-60 (2006)
- Steffan, T; Renukappa-Gutke, T; Höfner, G; Wanner, KT Design, synthesis and SAR studies of GABA uptake inhibitors derived from 2-substituted pyrrolidine-2-yl-acetic acids. Bioorg Med Chem 23: 1284-306 (2015)
- Fu, H; Hou, X; Wang, L; Dun, Y; Yang, X; Fang, H Design, synthesis and biological evaluation of 3-aryl-rhodanine benzoic acids as anti-apoptotic protein Bcl-2 inhibitors. Bioorg Med Chem Lett 25: 5265-9 (2015)
- Kim, NJ; Lee, KO; Koo, BW; Li, F; Yoo, JK; Park, HJ; Min, KH; Lim, JI; Kim, MK; Kim, JK; Suh, YG Design, synthesis, and structure-activity relationship of carbamate-tethered aryl propanoic acids as novel PPARalpha/gamma dual agonists. Bioorg Med Chem Lett 17: 3595-8 (2007)
- Nieto, CT; Gonzalez-Nunez, V; Rodríguez, RE; Diez, D; Garrido, NM Design, synthesis, pharmacological evaluation and molecular dynamics ofß-amino acids morphan-derivatives as novel ligands for opioid receptors. Eur J Med Chem 101: 150-62 (2015)
- Vaillancourt, M; Vanasse, B; Cohen, E; Sauv, G Difunctional enols of N-protected amino acids as low molecular weight and novel inhibitors of HIV-1 protease. Bioorg Med Chem Lett 3: 1169-1174 (1993)
- Zhang, H; Lapointe, BT; Anthony, N; Azevedo, R; Cals, J; Correll, CC; Daniels, M; Deshmukh, S; van Eenenaam, H; Ferguson, H; Hegde, LG; Karstens, WJ; Maclean, J; Miller, JR; Moy, LY; Simov, V; Nagpal, S; Oubrie, A; Palte, RL; Parthasarathy, G; Sciammetta, N; van der Stelt, M; Woodhouse, JD; Trotter, BW; Barr, K Discovery of N-(Indazol-3-yl)piperidine-4-carboxylic Acids as ROR��t Allosteric Inhibitors for Autoimmune Diseases ACS Med Chem Lett 11: 114-119 (2020)
- Asada, M; Obitsu, T; Nagase, T; Sugimoto, I; Yamaura, Y; Sato, K; Narita, M; Ohuchida, S; Nakai, H; Toda, M Discovery of a series of acrylic acids and their derivatives as chemical leads for selective EP3 receptor antagonists. Bioorg Med Chem 17: 6567-82 (2009)
- Identification of 3-(9H-carbazol-9-yl)-2-(1,3-dioxoisoindolin-2-yl)propanoic acids as promising DNMT1 inhibitors.
- Liu, KG; Smith, JS; Ayscue, AH; Henke, BR; Lambert, MH; Leesnitzer, LM; Plunket, KD; Willson, TM; Sternbach, DD Identification of a series of oxadiazole-substituted alpha-isopropoxy phenylpropanoic acids with activity on PPARalpha, PPARgamma, and PPARdelta. Bioorg Med Chem Lett 11: 2385-8 (2001)
- Oh, J; Hwang, IH; Hong, CE; Lyu, SY; Na, M Inhibition of fatty acid synthase by ginkgolic acids from the leaves of Ginkgo biloba and their cytotoxic activity. J Enzyme Inhib Med Chem 28: 565-8 (2013)
- Graham, DW; Ashton, WT; Barash, L; Brown, JE; Brown, RD; Canning, LF; Chen, A; Springer, JP; Rogers, EF Inhibition of the mammalian beta-lactamase renal dipeptidase (dehydropeptidase-I) by (Z)-2-(acylamino)-3-substituted-propenoic acids. J Med Chem 30: 1074-90 (1987)
- Tan, MC; Matsuoka, S; Ano, H; Ishida, H; Hirose, M; Sato, F; Sugiyama, S; Murata, M Interaction kinetics of liposome-incorporated unsaturated fatty acids with fatty acid-binding protein 3 by surface plasmon resonance. Bioorg Med Chem 22: 1804-8 (2014)
- Rosowsky, A; Bader, H; Kohler, W; Freisheim, JH; Moran, RG Methotrexate analogues. 34. Replacement of the glutamate moiety in methotrexate and aminopterin by long-chain 2-aminoalkanedioic acids. J Med Chem 31: 1338-44 (1988)
- Burdick, DJ; Marsters, JC; Aliagas-Martin, I; Stanley, M; Beresini, M; Clark, K; McDowell, RS; Gadek, TR N-Benzoyl amino acids as ICAM/LFA-1 inhibitors. Part 2: structure-activity relationship of the benzoyl moiety. Bioorg Med Chem Lett 14: 2055-9 (2004)
- de Vicente, J; Hendricks, RT; Smith, DB; Fell, JB; Fischer, J; Spencer, SR; Stengel, PJ; Mohr, P; Robinson, JE; Blake, JF; Hilgenkamp, RK; Yee, C; Zhao, J; Elworthy, TR; Tracy, J; Chin, E; Li, J; Lui, A; Wang, B; Oshiro, C; Harris, SF; Ghate, M; Leveque, VJ; Najera, I; Le Pogam, S; Rajyaguru, S; Ao-Ieong, G; Alexandrova, L; Fitch, B; Brandl, M; Masjedizadeh, M; Wu, SY; de Keczer, S; Voronin, T Non-nucleoside inhibitors of HCV polymerase NS5B. Part 3: synthesis and optimization studies of benzothiazine-substituted tetramic acids. Bioorg Med Chem Lett 19: 5648-51 (2009)
- Hudkins, RL; Mailman, RB; DeHaven-Hudkins, DL Novel (4-phenylpiperidinyl)- and (4-phenylpiperazinyl)alkyl-spaced esters of 1-phenylcyclopentanecarboxylic acids as potent sigma-selective compounds. J Med Chem 37: 1964-70 (1994)
- Hone, AJ; Fisher, F; Christensen, S; Gajewiak, J; Larkin, D; Whiteaker, P; McIntosh, JM PeIA-5466: A Novel Peptide Antagonist Containing Non-natural Amino Acids That Selectively Targets α3β2 Nicotinic Acetylcholine Receptors. J Med Chem 62: 6262-6275 (2019)
- Jungheim, LN; Shepherd, TA; Baxter, AJ; Burgess, J; Hatch, SD; Lubbehusen, P; Wiskerchen, M; Muesing, MA Potent human immunodeficiency virus type 1 protease inhibitors that utilize noncoded D-amino acids as P2/P3 ligands. J Med Chem 39: 96-108 (1996)
- Kogan, TP; Dupré, B; Keller, KM; Scott, IL; Bui, H; Market, RV; Beck, PJ; Voytus, JA; Revelle, BM; Scott, D Rational design and synthesis of small molecule, non-oligosaccharide selectin inhibitors: (alpha-D-mannopyranosyloxy)biphenyl-substituted carboxylic acids. J Med Chem 38: 4976-84 (1996)
- Ma, D; Jiang, Y; Chen, F; Gong, LK; Ding, K; Xu, Y; Wang, R; Ge, A; Ren, J; Li, J; Li, J; Ye, Q Selective inhibition of matrix metalloproteinase isozymes and in vivo protection against emphysema by substituted gamma-keto carboxylic acids. J Med Chem 49: 456-8 (2006)
- Luker, T; Bonnert, R; Schmidt, J; Sargent, C; Paine, SW; Thom, S; Pairaudeau, G; Patel, A; Mohammed, R; Akam, E; Dougall, I; Davis, AM; Abbott, P; Brough, S; Millichip, I; McInally, T Switching between agonists and antagonists at CRTh2 in a series of highly potent and selective biaryl phenoxyacetic acids. Bioorg Med Chem Lett 21: 3616-21 (2011)
- Guan, Q; Cheng, Z; Ma, X; Wang, L; Feng, D; Cui, Y; Bao, K; Wu, L; Zhang, W Synthesis and bioevaluation of 2-phenyl-4-methyl-1,3-selenazole-5-carboxylic acids as potent xanthine oxidase inhibitors. Eur J Med Chem 85: 508-16 (2014)
- Jendralla, H; Granzer, E; von Kerekjarto, B; Krause, R; Schacht, U; Baader, E; Bartmann, W; Beck, G; Bergmann, A; Kesseler, K Synthesis and biological activity of new HMG-CoA reductase inhibitors. 3. Lactones of 6-phenoxy-3,5-dihydroxyhexanoic acids. J Med Chem 34: 2962-83 (1991)
- Sæther, T; Paulsen, SM; Tungen, JE; Vik, A; Aursnes, M; Holen, T; Hansen, TV; Nebb, HI Synthesis and biological evaluations of marine oxohexadecenoic acids: PPARα/γ dual agonism and anti-diabetic target gene effects. Eur J Med Chem 155: 736-753 (2018)
- Zervosen, A; Bouillez, A; Herman, A; Amoroso, A; Joris, B; Sauvage, E; Charlier, P; Luxen, A Synthesis and evaluation of boronic acids as inhibitors of Penicillin Binding Proteins of classes A, B and C. Bioorg Med Chem 20: 3915-24 (2012)
- Wang, S; Ying, Z; Huang, Y; Li, Y; Hu, M; Kang, K; Wang, H; Shao, J; Wu, G; Yu, Y; Du, Y; Chen, W Synthesis and structure-activity optimization of 7-azaindoles containing aza-β-amino acids targeting the influenza PB2 subunit. Eur J Med Chem 250: (2023)
- Kubota, K; Kurebayashi, H; Miyachi, H; Tobe, M; Onishi, M; Isobe, Y Synthesis and structure-activity relationships of phenothiazine carboxylic acids having pyrimidine-dione as novel histamine H(1) antagonists. Bioorg Med Chem Lett 19: 2766-71 (2009)
- Conti, P; De Amici, M; Grazioso, G; Roda, G; Pinto, A; Hansen, KB; Nielsen, B; Madsen, U; Bräuner-Osborne, H; Egebjerg, J; Vestri, V; Pellegrini-Giampietro, DE; Sibille, P; Acher, FC; De Micheli, C Synthesis, binding affinity at glutamic acid receptors, neuroprotective effects, and molecular modeling investigation of novel dihydroisoxazole amino acids. J Med Chem 48: 6315-25 (2005)
- Wright, SW; Harris, RR; Kerr, JS; Green, AM; Pinto, DJ; Bruin, EM; Collins, RJ; Dorow, RL; Mantegna, LR; Sherk, SR Synthesis, chemical, and biological properties of vinylogous hydroxamic acids: dual inhibitors of 5-lipoxygenase and IL-1 biosynthesis. J Med Chem 35: 4061-8 (1992)
- Song, WQ; Liu, ML; Yuan, LC; Li, SY; Wang, YN; Xiao, ZP; Zhu, HL Synthesis, evaluation and mechanism exploration of 2-(N-(3-nitrophenyl)-N-phenylsulfonyl)aminoacetohydroxamic acids as novel urease inhibitors. Bioorg Med Chem Lett 78: (2022)
- Xiao, ZP; Peng, ZY; Dong, JJ; Deng, RC; Wang, XD; Ouyang, H; Yang, P; He, J; Wang, YF; Zhu, M; Peng, XC; Peng, WX; Zhu, HL Synthesis, molecular docking and kinetic properties of β-hydroxy-β-phenylpropionyl-hydroxamic acids as Helicobacter pylori urease inhibitors. Eur J Med Chem 68: 212-21 (2013)
- Laufer, SA; Augustin, J; Dannhardt, G; Kiefer, W (6,7-Diaryldihydropyrrolizin-5-yl)acetic acids, a novel class of potent dual inhibitors of both cyclooxygenase and 5-lipoxygenase. J Med Chem 37: 1894-7 (1994)
- Semple, G; Skinner, PJ; Cherrier, MC; Webb, PJ; Sage, CR; Tamura, SY; Chen, R; Richman, JG; Connolly, DT 1-Alkyl-benzotriazole-5-carboxylic acids are highly selective agonists of the human orphan G-protein-coupled receptor GPR109b. J Med Chem 49: 1227-30 (2006)
- Williams, TM; Ciccarone, TM; MacTough, SC; Bock, RL; Conner, MW; Davide, JP; Hamilton, K; Koblan, KS; Kohl, NE; Kral, AM; Mosser, SD; Omer, CA; Pompliano, DL; Rands, E; Schaber, MD; Shah, D; Wilson, FR; Gibbs, JB; Graham, SL; Hartman, GD; Oliff, AI; Smith, RL 2-substituted piperazines as constrained amino acids. Application to the synthesis of potent, non carboxylic acid inhibitors of farnesyltransferase. J Med Chem 39: 1345-8 (1996)
- Banoglu, E; Çelikoglu, E; Völker, S; Olgaç, A; Gerstmeier, J; Garscha, U; Çaliskan, B; Schubert, US; Carotti, A; Macchiarulo, A; Werz, O 4,5-Diarylisoxazol-3-carboxylic acids: A new class of leukotriene biosynthesis inhibitors potentially targeting 5-lipoxygenase-activating protein (FLAP). Eur J Med Chem 113: 1-10 (2016)
- Costi, R; Di Santo, R; Artico, M; Roux, A; Ragno, R; Massa, S; Tramontano, E; La Colla, M; Loddo, R; Marongiu, ME; Pani, A; La Colla, P 6-aryl-2,4-dioxo-5-hexenoic acids, novel integrase inhibitors active against HIV-1 multiplication in cell-based assays. Bioorg Med Chem Lett 14: 1745-9 (2004)
- Funke, M; Thimm, D; Schiedel, AC; Müller, CE 8-Benzamidochromen-4-one-2-carboxylic acids: potent and selective agonists for the orphan G protein-coupled receptor GPR35. J Med Chem 56: 5182-97 (2013)
- Cozzi, P; Giordani, A; Menichincheri, M; Pillan, A; Pinciroli, V; Rossi, A; Tonani, R; Volpi, D; Tamburin, M; Ferrario, R Agents combining thromboxane receptor antagonism with thromboxane synthase inhibition: [[[2-(1H-imidazol-1-yl)ethylidene]amino]oxy]alkanoic acids. J Med Chem 37: 3588-604 (1994)
- Innocenti, A; Beyza Oztürk Sarikaya, S; Gülçin, I; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 18: 2159-64 (2010)
- Innocenti, A; Winum, JY; Hall, RA; Mühlschlegel, FA; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of the fungal beta-carbonic anhydrases from Candida albicans and Cryptococcus neoformans with boronic acids. Bioorg Med Chem Lett 19: 2642-5 (2009)
- Ito, K; Suzuki, H; Sugiyama, Y Charged amino acids in the transmembrane domains are involved in the determination of the substrate specificity of rat Mrp2. Mol Pharmacol 59: 1077-85 (2001)
- Hill, DT; Girard, GR; Weinstock, J; Edwards, RM; Weidley, EF; Ohlstein, E; Peishoff, CE; Baker, E; Aiyar, N D and L-N-[(1-benzyl-1H-imidazol-5-yl)-alkyl]-amino acids as angiotensin II AT-1 antagonists Bioorg Med Chem Lett 5: 19-24 (1995)
- Koul, S; Kurhade, S; Bhosale, S; Naik, K; Salunkhe, V; Ravula, S; Punde, P; Velayutham, R; Tiwari, A; Ahl, D; Malkapuram, S; Mudagala, V; Raje, A; Umrani, D; Tambe, S; Patil, P; Singh, U; Bhuniya, D; Hariharan, N; Mookhtiar, K Design and synthesis of novel spirocyclic carboxylic acids as potent and orally bioavailable DGAT1 inhibitors and their biological evaluation. Bioorg Med Chem Lett 62: (2022)
- McKittrick, BA; Stamford, AW; Xiaoyu, Weng; Ke, Ma; Chackalamannil, S; Czarniecki, M; Cleven, R; Fawzi, AB Design and synthesis of phosphinic acids that triply inhibit endothelin converting enzyme, angiotensin converting enzyme and neutral endopeptidase 24.11 Bioorg Med Chem Lett 6: 1629-1634 (1996)
- Filosa, R; Carmela Fulco, M; Marinozzi, M; Giacchè, N; Macchiarulo, A; Peduto, A; Massa, A; de Caprariis, P; Thomsen, C; Christoffersen, CT; Pellicciari, R Design, synthesis and biological evaluation of novel bicyclo[1.1.1]pentane-based omega-acidic amino acids as glutamate receptors ligands. Bioorg Med Chem 17: 242-50 (2008)
- Ye, XY; Li, YX; Farrelly, D; Flynn, N; Gu, L; Locke, KT; Lippy, J; O'Malley, K; Twamley, C; Zhang, L; Ryono, DE; Zahler, R; Hariharan, N; Cheng, PT Design, synthesis, and structure-activity relationships of piperidine and dehydropiperidine carboxylic acids as novel, potent dual PPARalpha/gamma agonists. Bioorg Med Chem Lett 18: 3545-50 (2008)
- De Filippis, B; Giancristofaro, A; Ammazzalorso, A; D'Angelo, A; Fantacuzzi, M; Giampietro, L; Maccallini, C; Petruzzelli, M; Amoroso, R Discovery of gemfibrozil analogues that activate PPARa and enhance the expression of gene CPT1A involved in fatty acids catabolism. Eur J Med Chem 46: 5218-24 (2011)
- Black, WC; Bayly, C; Belley, M; Chan, CC; Charleson, S; Denis, D; Gauthier, JY; Gordon, R; Guay, D; Kargman, S; Lau, CK; Leblanc, Y; Mancini, J; Ouellet, M; Percival, D; Roy, P; Skorey, K; Tagari, P; Vickers, P; Wong, E From indomethacin to a selective COX-2 inhibitor: Development of indolalkanoic acids as potent and selective cyclooxygenase-2 inhibitors Bioorg Med Chem Lett 6: 725-730 (1996)
- Nikolaou, A; Ninou, I; Kokotou, MG; Kaffe, E; Afantitis, A; Aidinis, V; Kokotos, G Hydroxamic Acids Constitute a Novel Class of Autotaxin Inhibitors that Exhibit in Vivo Efficacy in a Pulmonary Fibrosis Model. J Med Chem 61: 3697-3711 (2018)
- Charton, J; Gauriot, M; Guo, Q; Hennuyer, N; Marechal, X; Dumont, J; Hamdane, M; Pottiez, V; Landry, V; Sperandio, O; Flipo, M; Buee, L; Staels, B; Leroux, F; Tang, WJ; Deprez, B; Deprez-Poulain, R Imidazole-derived 2-[N-carbamoylmethyl-alkylamino]acetic acids, substrate-dependent modulators of insulin-degrading enzyme in amyloid-ß hydrolysis. Eur J Med Chem 79: 184-93 (2014)
- Geurink, PP; van der Linden, WA; Mirabella, AC; Gallastegui, N; de Bruin, G; Blom, AE; Voges, MJ; Mock, ED; Florea, BI; van der Marel, GA; Driessen, C; van der Stelt, M; Groll, M; Overkleeft, HS; Kisselev, AF Incorporation of non-natural amino acids improves cell permeability and potency of specific inhibitors of proteasome trypsin-like sites. J Med Chem 56: 1262-75 (2013)
- Mahindroo, N; Wang, CC; Liao, CC; Huang, CF; Lu, IL; Lien, TW; Peng, YH; Huang, WJ; Lin, YT; Hsu, MC; Lin, CH; Tsai, CH; Hsu, JT; Chen, X; Lyu, PC; Chao, YS; Wu, SY; Hsieh, HP Indol-1-yl acetic acids as peroxisome proliferator-activated receptor agonists: design, synthesis, structural biology, and molecular docking studies. J Med Chem 49: 1212-6 (2006)
- García-Echeverría, C; Gay, B; Rahuel, J; Furet, P Mapping the X(+1) binding site of the Grb2-SH2 domain with alpha,alpha-disubstituted cyclic alpha-amino acids. Bioorg Med Chem Lett 9: 2915-20 (1999)
- Ekanayake, KB; Mahawaththa, MC; Qianzhu, H; Abdelkader, EH; George, J; Ullrich, S; Murphy, RB; Fry, SE; Johansen-Leete, J; Payne, RJ; Nitsche, C; Huber, T; Otting, G Probing Ligand Binding Sites on Large Proteins by Nuclear Magnetic Resonance Spectroscopy of Genetically Encoded Non-Canonical Amino Acids. J Med Chem 66: 5289-5304 (2023)
- Liang, C; Tian, D; Liu, Y; Li, H; Zhu, J; Li, M; Xin, M; Xia, J Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur J Med Chem 174: 130-141 (2019)
- Kromann, H; Sløk, FA; Stensbøl, TB; Bräuner-Osborne, H; Madsen, U; Krogsgaard-Larsen, P Selective antagonists at group I metabotropic glutamate receptors: synthesis and molecular pharmacology of 4-aryl-3-isoxazolol amino acids. J Med Chem 45: 988-91 (2002)
- Takashiro, E; Hayakawa, I; Nitta, T; Kasuya, A; Miyamoto, S; Ozawa, Y; Yagi, R; Yamamoto, I; Shibayama, T; Nakagawa, A; Yabe, Y Structure-activity relationship of HIV-1 protease inhibitors containing alpha-hydroxy-beta-amino acids. Detailed study of P1 site. Bioorg Med Chem 7: 2063-72 (1999)
- Arnsmann, M; Hanekamp, W; Elfringhoff, AS; Lehr, M Structure-activity relationship studies on 1-(2-oxopropyl)indole-5-carboxylic acids acting as inhibitors of cytosolic phospholipase A Eur J Med Chem 125: 1107-1114 (2017)
- Alonso, JA; Andrés, M; Bravo, M; Buil, MA; Calbet, M; Castro, J; Eastwood, PR; Eichhorn, P; Esteve, C; Gómez, E; González, J; Mir, M; Petit, S; Roberts, RS; Vidal, B; Vidal, L; Vilaseca, P; Zanuy, M Structure-activity relationships (SAR) and structure-kinetic relationships (SKR) of bicyclic heteroaromatic acetic acids as potent CRTh2 antagonists I. Bioorg Med Chem Lett 24: 5118-22 (2014)
- Faghih, R; Dwight, W; Black, L; Liu, H; Gentles, R; Phelan, K; Esbenshade, TA; Ireland, L; Miller, TR; Kang, CH; Krueger, KM; Fox, GB; Hancock, AA; Bennani, YL Structure-activity relationships of non-imidazole H(3) receptor ligands. Part 2: binding preference for D-amino acids motifs. Bioorg Med Chem Lett 12: 2035-7 (2002)
- Shuman, RT; Rothenberger, RB; Campbell, CS; Smith, GF; Gifford-Moore, DS; Paschal, JW; Gesellchen, PD Structure-activity study of tripeptide thrombin inhibitors using alpha-alkyl amino acids and other conformationally constrained amino acid substitutions. J Med Chem 38: 4446-53 (1995)
- Hibi, S; Tagami, K; Kikuchi, K; Yoshimura, H; Tai, K; Hida, T; Tokuhara, N; Yamauchi, T; Nagai, M Syntheses and evaluation of naphthalenyl- and chromenyl-pyrrolyl-benzoic acids as potent and selective retinoic acid receptor alpha agonists. Bioorg Med Chem Lett 10: 623-5 (2000)
- Kerscher-Hack, S; Renukappa-Gutke, T; Höfner, G; Wanner, KT Synthesis and biological evaluation of a series of N-alkylated imidazole alkanoic acids as mGAT3 selective GABA uptake inhibitors. Eur J Med Chem 124: 852-880 (2016)
- Patel, HJ; Olgun, N; Lengyel, I; Reznik, S; Stephani, RA Synthesis and pharmacological activity of 1,3,6-trisubstituted-4-oxo-1,4-dihydroquinoline-2-carboxylic acids as selective ET(A) antagonists. Bioorg Med Chem Lett 20: 6840-4 (2010)
- Hum, G; Lee, J; Taylor, SD Synthesis of [difluoro-(3-alkenylphenyl)-methyl]-phosphonic acids on non-crosslinked polystyrene and their evaluation as inhibitors of PTP1B. Bioorg Med Chem Lett 12: 3471-4 (2002)
- Ekodo Voundi, M; Hanekamp, W; Lehr, M Synthesis, activity and metabolic stability of propan-2-one substituted tetrazolylalkanoic acids as dual inhibitors of cytosolic phospholipase A RSC Med Chem 14: 2079-2088 (2023)
- Dufresne, C; Wilson, KE; Singh, SB; Zink, DL; Bergstrom, JD; Rew, D; Polishook, JD; Meinz, M; Huang, L; Silverman, KC; Lingham, RB; Mojena, M; Cascales, C; Pelaéz, F; Gibbs, JB Zaragozic Acids D and D2: Potent Inhibitors of Squalene Synthase and of Ras Farnesyl-Protein Transferase J Nat Prod 56: 1923-1929 (1993)
- Qiu, J; Silverman, RB A new class of conformationally rigid analogues of 4-amino-5-halopentanoic acids, potent inactivators of gamma-aminobutyric acid aminotransferase. J Med Chem 43: 706-20 (2000)
- Gorczynski, MJ; Smitherman, PK; Akiyama, TE; Wood, HB; Berger, JP; King, SB; Morrow, CS Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by nitroalkene fatty acids: importance of nitration position and degree of unsaturation. J Med Chem 52: 4631-9 (2009)
- Dubois, L; Pietrancosta, N; Cabaye, A; Fanget, I; Debacker, C; Gilormini, PA; Dansette, PM; Dairou, J; Biot, C; Froissart, R; Goupil-Lamy, A; Bertrand, HO; Acher, FC; McCort-Tranchepain, I; Gasnier, B; Anne, C Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin. J Med Chem 63: 8231-8249 (2020)
- Tanis, SP; Plewe, MB; Johnson, TW; Butler, SL; Dalvie, D; DeLisle, D; Dress, KR; Hu, Q; Huang, B; Kuehler, JE; Kuki, A; Liu, W; Peng, Q; Smith, GL; Solowiej, J; Tran, KT; Wang, H; Yang, A; Yin, C; Yu, X; Zhang, J; Zhu, H Azaindole N-methyl hydroxamic acids as HIV-1 integrase inhibitors-II. The impact of physicochemical properties on ADME and PK. Bioorg Med Chem Lett 20: 7429-34 (2010)
- Shepherd, TA; Jungheim, LN; Baxter, AJ D-amino acids as novel P2/P3 ligands for inhibitors of HIV-1 protease Bioorg Med Chem Lett 4: 1391-1396 (1994)
- Yan, L; Hale, JJ; Lynch, CL; Budhu, R; Gentry, A; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Rosen, H; Mandala, SM Design and synthesis of conformationally constrained 3-(N-alkylamino)propylphosphonic acids as potent agonists of sphingosine-1-phosphate (S1P) receptors. Bioorg Med Chem Lett 14: 4861-6 (2004)
- Ning, C; Bi, Y; He, Y; Huang, W; Liu, L; Li, Y; Zhang, S; Liu, X; Yu, N Design, synthesis and biological evaluation of di-substituted cinnamic hydroxamic acids bearing urea/thiourea unit as potent histone deacetylase inhibitors. Bioorg Med Chem Lett 23: 6432-5 (2013)
- Hauguel, C; Ducellier, S; Provot, O; Ibrahim, N; Lamaa, D; Balcerowiak, C; Letribot, B; Nascimento, M; Blanchard, V; Askenatzis, L; Levaique, H; Bignon, J; Baschieri, F; Bauvais, C; Bollot, G; Renko, D; Deroussent, A; Prost, B; Laisne, MC; Michallet, S; Lafanechère, L; Papot, S; Montagnac, G; Tran, C; Alami, M; Apcher, S; Hamze, A Design, synthesis and biological evaluation of quinoline-2-carbonitrile-based hydroxamic acids as dual tubulin polymerization and histone deacetylases inhibitors. Eur J Med Chem 240: (2022)
- Design, synthesis, and biological evaluation of 5-(1H-indol-5-yl)isoxazole-3-carboxylic acids as novel xanthine oxidase inhibitors.
- Gim, HJ; Li, H; Jeong, JH; Lee, SJ; Sung, MK; Song, MY; Park, BH; Oh, SJ; Ryu, JH; Jeon, R Design, synthesis, and biological evaluation of a series of alkoxy-3-indolylacetic acids as peroxisome proliferator-activated receptor¿/d agonists. Bioorg Med Chem 23: 3322-36 (2015)
- Merlino, F; Zhou, Y; Cai, M; Carotenuto, A; Yousif, AM; Brancaccio, D; Di Maro, S; Zappavigna, S; Limatola, A; Novellino, E; Grieco, P; Hruby, VJ Development of Macrocyclic Peptidomimetics Containing Constrained α,α-Dialkylated Amino Acids with Potent and Selective Activity at Human Melanocortin Receptors. J Med Chem 61: 4263-4269 (2018)
- Weinhart, CG; Wifling, D; Schmidt, MF; Neu, E; Höring, C; Clark, T; Gmeiner, P; Keller, M Dibenzodiazepinone-type muscarinic receptor antagonists conjugated to basic peptides: Impact of the linker moiety and unnatural amino acids on M Eur J Med Chem 213: (2021)
- Balboni, G; Salvadori, S; Guerrini, R; Negri, L; Giannini, E; Bryant, SD; Jinsmaa, Y; Lazarus, LH Direct influence of C-terminally substituted amino acids in the Dmt-Tic pharmacophore on delta-opioid receptor selectivity and antagonism. J Med Chem 47: 4066-71 (2004)
- Adeniji, AO; Twenter, BM; Byrns, MC; Jin, Y; Winkler, JD; Penning, TM Discovery of substituted 3-(phenylamino)benzoic acids as potent and selective inhibitors of type 5 17ß-hydroxysteroid dehydrogenase (AKR1C3). Bioorg Med Chem Lett 21: 1464-8 (2011)
- Nair, RN; Mishra, JK; Li, F; Tortosa, M; Yang, C; Doherty, JR; Cameron, M; Cleveland, JL; Roush, WR; Bannister, TD Exploiting the co-reliance of tumours upon transport of amino acids and lactate: Gln and Tyr conjugates of MCT1 inhibitors. Medchemcomm 7: 900-905 (2016)
- Sahner, JH; Empting, M; Kamal, A; Weidel, E; Groh, M; Börger, C; Hartmann, RW Exploring the chemical space of ureidothiophene-2-carboxylic acids as inhibitors of the quorum sensing enzyme PqsD from Pseudomonas aeruginosa. Eur J Med Chem 96: 14-21 (2015)
- Williams, DE; Steinø, A; de Voogd, NJ; Mauk, AG; Andersen, RJ Halicloic acids A and B isolated from the marine sponge Haliclona sp. collected in the Philippines inhibit indoleamine 2,3-dioxygenase. J Nat Prod 75: 1451-8 (2012)
- Flossdorf, J; Pratorius, HJ; Kula, MR Influence of side-chain structure of aliphatic amino acids on binding to isoleucyl-tRNA synthetase from Escherichia coli MRE 600. Eur J Biochem 66: 147-55 (1976)
- Mouchlis, VD; Magrioti, V; Barbayianni, E; Cermak, N; Oslund, RC; Mavromoustakos, TM; Gelb, MH; Kokotos, G Inhibition of secreted phospholipases A2 by 2-oxoamides based ona-amino acids: Synthesis, in vitro evaluation and molecular docking calculations. Bioorg Med Chem 19: 735-43 (2011)
- Barf, T; Lehmann, F; Hammer, K; Haile, S; Axen, E; Medina, C; Uppenberg, J; Svensson, S; Rondahl, L; Lundbäck, T N-Benzyl-indolo carboxylic acids: Design and synthesis of potent and selective adipocyte fatty-acid binding protein (A-FABP) inhibitors. Bioorg Med Chem Lett 19: 1745-8 (2009)
- Baston, E; Hartmann, RW N-substituted 4-(5-indolyl)benzoic acids. Synthesis and evaluation of steroid 5alpha-reductase type I and II inhibitory activity. Bioorg Med Chem Lett 9: 1601-6 (1999)
- Gorham, RD; Forest, DL; Khoury, GA; Smadbeck, J; Beecher, CN; Healy, ED; Tamamis, P; Archontis, G; Larive, CK; Floudas, CA; Radeke, MJ; Johnson, LV; Morikis, D New compstatin peptides containing N-terminal extensions and non-natural amino acids exhibit potent complement inhibition and improved solubility characteristics. J Med Chem 58: 814-26 (2015)
- Otake, K; Azukizawa, S; Fukui, M; Kunishiro, K; Kamemoto, H; Kanda, M; Miike, T; Kasai, M; Shirahase, H Novel (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids: peroxisome proliferator-activated receptor¿ selective agonists with protein-tyrosine phosphatase 1B inhibition. Bioorg Med Chem 20: 1060-75 (2012)
- Patil, AD; Chan, JA; Lois-Flamberg, P; Mayer, RJ; Westley, JW Novel Acetylenic Acids from the Root Bark of Paramacrolobium caeruleum: Inhibitors of 3-Hydroxy-3-methyl-glutaryl Coenzyme A Reductase J Nat Prod 52: 153-161 (1989)
- Trujillo, JI; Meyers, MJ; Anderson, DR; Hegde, S; Mahoney, MW; Vernier, WF; Buchler, IP; Wu, KK; Yang, S; Yang, S; Hartmann, SJ; Reitz, DB Novel tetrahydro-beta-carboline-1-carboxylic acids as inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2). Bioorg Med Chem Lett 17: 4657-63 (2007)
- Heyl, DL; Dandabathula, M; Kurtz, KR; Mousigian, C Opioid receptor binding requirements for the delta-selective peptide deltorphin. I: Phe3 replacement with ring-substituted and heterocyclic amino acids. J Med Chem 38: 1242-6 (1995)
- Liu, G; Henry, KJ; Szczepankiewicz, BG; Winn, M; Kozmina, NS; Boyd, SA; Wasicak, J; von Geldern, TW; Wu-Wong, JR; Chiou, WJ; Dixon, DB; Nguyen, B; Marsh, KC; Opgenorth, TJ Pyrrolidine-3-carboxylic acids as endothelin antagonists. 3. Discovery of a potent, 2-nonaryl, highly selective ETA antagonist (A-216546). J Med Chem 41: 3261-75 (1998)
- Yu, CW; Hung, PY; Yang, HT; Ho, YH; Lai, HY; Cheng, YS; Chern, JW Quinazolin-2,4-dione-Based Hydroxamic Acids as Selective Histone Deacetylase-6 Inhibitors for Treatment of Non-Small Cell Lung Cancer. J Med Chem 62: 857-874 (2019)
- Zhu, YF; Wang, XC; Connors, P; Wilcoxen, K; Gao, Y; Gross, R; Strack, N; Gross, T; McCarthy, JR; Xie, Q; Ling, N; Chen, C Quinoline-carboxylic acids are potent inhibitors that inhibit the binding of insulin-like growth factor (IGF) to IGF-binding proteins. Bioorg Med Chem Lett 13: 1931-4 (2003)
- Sagi, K; Nakagawa, T; Yamanashi, M; Makino, S; Takahashi, M; Takayanagi, M; Takenaka, K; Suzuki, N; Oono, S; Kataoka, N; Ishikawa, K; Shima, S; Fukuda, Y; Kayahara, T; Takehana, S; Shima, Y; Tashiro, K; Yamamoto, H; Yoshimoto, R; Iwata, S; Tsuji, T; Sakurai, K; Shoji, M Rational design, synthesis, and structure-activity relationships of novel factor Xa inhibitors: (2-substituted-4-amidinophenyl)pyruvic and -propionic acids. J Med Chem 46: 1845-57 (2003)
- Xu, LL; Li, CC; An, LY; Dai, Z; Chen, XY; You, QD; Hu, C; Di, B Selective apoptosis-inducing activity of synthetic hydrocarbon-stapled SOS1 helix with d-amino acids in H358 cancer cells expressing KRAS Eur J Med Chem 185: (2020)
- Szajnman, SH; Ravaschino, EL; Docampo, R; Rodriguez, JB Synthesis and biological evaluation of 1-amino-1,1-bisphosphonates derived from fatty acids against Trypanosoma cruzi targeting farnesyl pyrophosphate synthase. Bioorg Med Chem Lett 15: 4685-90 (2005)
- Szajnman, SH; García Liñares, GE; Li, ZH; Jiang, C; Galizzi, M; Bontempi, EJ; Ferella, M; Moreno, SN; Docampo, R; Rodriguez, JB Synthesis and biological evaluation of 2-alkylaminoethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase. Bioorg Med Chem 16: 3283-90 (2008)
- Lehr, M Synthesis, biological evaluation, and structure-activity relationships of 3-acylindole-2-carboxylic acids as inhibitors of the cytosolic phospholipase A2. J Med Chem 40: 2694-705 (1997)
- van de Plassche, MAT; O'Neill, TJ; Seeholzer, T; Turk, B; Krappmann, D; Verhelst, SHL Use of Non-Natural Amino Acids for the Design and Synthesis of a Selective, Cell-Permeable MALT1 Activity-Based Probe. J Med Chem 63: 3996-4004 (2020)
- Stokker, GE; Hoffman, WF; Alberts, AW; Cragoe, EJ; Deana, AA; Gilfillan, JL; Huff, JW; Novello, FC; Prugh, JD; Smith, RL 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 1. Structural modification of 5-substituted 3,5-dihydroxypentanoic acids and their lactone derivatives. J Med Chem 28: 347-58 (1985)
- Loddo, R; Novelli, F; Sparatore, A; Tasso, B; Tonelli, M; Boido, V; Sparatore, F; Collu, G; Delogu, I; Giliberti, G; La Colla, P Antiviral activity of benzotriazole derivatives. 5-[4-(Benzotriazol-2-yl)phenoxy]-2,2-dimethylpentanoic acids potently and selectively inhibit Coxsackie Virus B5. Bioorg Med Chem 23: 7024-34 (2015)
- Goodfellow, VS; Marathe, MV; Kuhlman, KG; Fitzpatrick, TD; Cuadrado, D; Hanson, W; Zuzack, JS; Ross, SE; Wieczorek, M; Burkard, M; Whalley, ET Bradykinin receptor antagonists containing N-substituted amino acids: in vitro and in vivo B(2) and B(1) receptor antagonist activity. J Med Chem 39: 1472-84 (1996)
- Arvanitis, AG; Arnold, CR; Fitzgerald, LW; Frietze, WE; Olson, RE; Gilligan, PJ; Robertson, DW CRF ligands via Suzuki and Negishi couplings of 3-pyridyl boronic acids or halides with 2-benzyloxy-4-chloro-3-nitropyridine. Bioorg Med Chem Lett 13: 289-91 (2003)
- Rekharsky , M; Yamamura, H; Kawai, M; Inoue, Y Critical Difference in Chiral Recognition of N-Cbz-d/l-aspartic and -glutamic Acids by Mono- and Bis(Trimethylammonio)-β-cyclodextrins J Am Chem Soc 123: 5360-5361 (2000)
- Guo, B; Guo, S; Huang, J; Li, J; Li, J; Chen, Q; Zhou, X; Xie, X; Yang, Y Design and optimization of 2,3-dihydrobenzo[b][1,4]dioxine propanoic acids as novel GPR40 agonists with improved pharmacokinetic and safety profiles. Bioorg Med Chem 26: 5780-5791 (2018)
- Xu, Y; Rito, CJ; Etgen, GJ; Ardecky, RJ; Bean, JS; Bensch, WR; Bosley, JR; Broderick, CL; Brooks, DA; Dominianni, SJ; Hahn, PJ; Liu, S; Mais, DE; Montrose-Rafizadeh, C; Ogilvie, KM; Oldham, BA; Peters, M; Rungta, DK; Shuker, AJ; Stephenson, GA; Tripp, AE; Wilson, SB; Winneroski, LL; Zink, R; Kauffman, RF; McCarthy, JR Design and synthesis of alpha-aryloxy-alpha-methylhydrocinnamic acids: a novel class of dual peroxisome proliferator-activated receptor alpha/gamma agonists. J Med Chem 47: 2422-5 (2004)
- Michellys, PY; Ardecky, RJ; Chen, JH; D'Arrigo, J; Grese, TA; Karanewsky, DS; Leibowitz, MD; Liu, S; Mais, DA; Mapes, CM; Montrose-Rafizadeh, C; Ogilvie, KM; Reifel-Miller, A; Rungta, D; Thompson, AW; Tyhonas, JS; Boehm, MF Design, synthesis, and structure-activity relationship studies of novel 6,7-locked-[7-(2-alkoxy-3,5-dialkylbenzene)-3-methylocta]-2,4,6-trienoic acids. J Med Chem 46: 4087-103 (2003)
- Elgaher, WA; Sharma, KK; Haupenthal, J; Saladini, F; Pires, M; Real, E; Mély, Y; Hartmann, RW Discovery and Structure-Based Optimization of 2-Ureidothiophene-3-carboxylic Acids as Dual Bacterial RNA Polymerase and Viral Reverse Transcriptase Inhibitors. J Med Chem 59: 7212-22 (2016)
- Koppitz, M; Bräuer, N; Ter Laak, A; Irlbacher, H; Rotgeri, A; Coelho, AM; Walter, D; Steinmeyer, A; Zollner, TM; Peters, M; Nagel, J Discovery and optimization of pyridyl-cycloalkyl-carboxylic acids as inhibitors of microsomal prostaglandin E synthase-1 for the treatment of endometriosis. Bioorg Med Chem Lett 29: 2700-2705 (2019)
- Summa, V; Petrocchi, A; Pace, P; Matassa, VG; De Francesco, R; Altamura, S; Tomei, L; Koch, U; Neuner, P Discovery of alpha,gamma-diketo acids as potent selective and reversible inhibitors of hepatitis C virus NS5b RNA-dependent RNA polymerase. J Med Chem 47: 14-7 (2003)
- Chan, L; Das, SK; Reddy, TJ; Poisson, C; Proulx, M; Pereira, O; Courchesne, M; Roy, C; Wang, W; Siddiqui, A; Yannopoulos, CG; Nguyen-Ba, N; Labrecque, D; Bethell, R; Hamel, M; Courtemanche-Asselin, P; L'Heureux, L; David, M; Nicolas, O; Brunette, S; Bilimoria, D; Bédard, J Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 1: Sulfonamides. Bioorg Med Chem Lett 14: 793-6 (2004)
- Fass, DM; Shah, R; Ghosh, B; Hennig, K; Norton, S; Zhao, WN; Reis, SA; Klein, PS; Mazitschek, R; Maglathlin, RL; Lewis, TA; Haggarty, SJ Effect of Inhibiting Histone Deacetylase with Short-Chain Carboxylic Acids and Their Hydroxamic Acid Analogs on Vertebrate Development and Neuronal Chromatin. ACS Med Chem Lett 2: 39-42 (2011)
- Ruenitz, PC; Bourne, CS; Sullivan, KJ; Moore, SA Estrogenic triarylethylene acetic acids: effect of structural variation on estrogen receptor affinity and estrogenic potency and efficacy in MCF-7 cells. J Med Chem 39: 4853-9 (1996)
- Sparks, SM; Aquino, C; Banker, P; Collins, JL; Cowan, D; Diaz, C; Dock, ST; Hertzog, DL; Liang, X; Swiger, ED; Yuen, J; Chen, G; Jayawickreme, C; Moncol, D; Nystrom, C; Rash, V; Rimele, T; Roller, S; Ross, S Exploration of phenylpropanoic acids as agonists of the free fatty acid receptor 4 (FFA4): Identification of an orally efficacious FFA4 agonist. Bioorg Med Chem Lett 27: 1278-1283 (2017)
- Jansen, M; Potschka, H; Brandt, C; Löscher, W; Dannhardt, G Hydantoin-substituted 4,6-dichloroindole-2-carboxylic acids as ligands with high affinity for the glycine binding site of the NMDA receptor. J Med Chem 46: 64-73 (2002)
- Dhanoa, DS; Bagley, SW; Chang, RS; Lotti, VJ; Chen, TB; Kivlighn, SD; Zingaro, GJ; Siegl, PK; Patchett, AA; Greenlee, WJ Non-peptide angiotensin II receptor antagonists. 2. Design, synthesis, and biological activity of N-substituted (phenylamino)phenylacetic acids and acyl sulfonamides. J Med Chem 36: 4239-49 (1994)
- Abed, DA; Lee, S; Wen, X; Ali, AR; Mangipudy, V; Aleksunes, LM; Hu, L Optimization of 1,4-bis(arylsulfonamido)naphthalene-N,N'-diacetic acids as inhibitors of Keap1-Nrf2 protein-protein interaction to suppress neuroinflammation. Bioorg Med Chem 44: (2021)
- Alonso, JA; Andrés, M; Bravo, M; Calbet, M; Eastwood, PR; Eichhorn, P; Esteve, C; Ferrer, M; Gómez, E; González, J; Mir, M; Moreno, I; Petit, S; Roberts, RS; Sevilla, S; Vidal, B; Vidal, L; Vilaseca, P; Zanuy, M Structure-activity relationships (SAR) and structure-kinetic relationships (SKR) of bicyclic heteroaromatic acetic acids as potent CRTh2 antagonists II: lead optimization. Bioorg Med Chem Lett 24: 5123-6 (2014)
- Arduin, M; Spagnolo, B; Calò, G; Guerrini, R; Carrà, G; Fischetti, C; Trapella, C; Marzola, E; McDonald, J; Lambert, DG; Regoli, D; Salvadori, S Synthesis and biological activity of nociceptin/orphanin FQ analogues substituted in position 7 or 11 with Calpha,alpha-dialkylated amino acids. Bioorg Med Chem 15: 4434-43 (2007)
- Rosso, VS; Szajnman, SH; Malayil, L; Galizzi, M; Moreno, SN; Docampo, R; Rodriguez, JB Synthesis and biological evaluation of new 2-alkylaminoethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase. Bioorg Med Chem 19: 2211-7 (2011)
- Aranapakam, V; Grosu, GT; Davis, JM; Hu, B; Ellingboe, J; Baker, JL; Skotnicki, JS; Zask, A; DiJoseph, JF; Sung, A; Sharr, MA; Killar, LM; Walter, T; Jin, G; Cowling, R Synthesis and structure-activity relationship of alpha-sulfonylhydroxamic acids as novel, orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis. J Med Chem 46: 2361-75 (2003)
- Wendt, JA; Wu, H; Stenmark, HG; Boys, ML; Downs, VL; Penning, TD; Chen, BB; Wang, Y; Duffin, T; Finn, MB; Keene, JL; Engleman, VW; Freeman, SK; Hanneke, ML; Shannon, KE; Nickols, MA; Steininger, CN; Westlin, M; Klover, JA; Westlin, W; Nickols, GA; Russell, MA Synthesis of 2,5-thiazole butanoic acids as potent and selective alpha(v)beta3 integrin receptor antagonists with improved oral pharmacokinetic properties. Bioorg Med Chem Lett 16: 845-9 (2006)
- Yoshimura, Y; Ohara, C; Imahori, T; Saito, Y; Kato, A; Miyauchi, S; Adachi, I; Takahata, H Synthesis of both enantiomers of hydroxypipecolic acid derivatives equivalent to 5-azapyranuronic acids and evaluation of their inhibitory activities against glycosidases. Bioorg Med Chem 16: 8273-86 (2008)
- Verhoff, M; Seitz, S; Paul, M; Noha, SM; Jauch, J; Schuster, D; Werz, O Tetra- and pentacyclic triterpene acids from the ancient anti-inflammatory remedy frankincense as inhibitors of microsomal prostaglandin E(2) synthase-1. J Nat Prod 77: 1445-51 (2014)
- Rekharsky, MV; Mayhew, MP; Goldberg, RN; Ross, PD; Yamashoji, Y; Inoue, Y Thermodynamic and Nuclear Magnetic Resonance Study of the Reactions of α- and β-Cyclodextrin with Acids, Aliphatic Amines, and Cyclic Alcohols J Phys Chem B 101: 87-100 (1997)
- Hedfors, A; Appelqvist, T; Carlsson, B; Bladh, LG; Litten, C; Agback, P; Grynfarb, M; Koehler, KF; Malm, J Thyroid receptor ligands. 3. Design and synthesis of 3,5-dihalo-4-alkoxyphenylalkanoic acids as indirect antagonists of the thyroid hormone receptor. J Med Chem 48: 3114-7 (2005)
- Katayama, S; Ae, N; Kodo, T; Masumoto, S; Hourai, S; Tamamura, C; Tanaka, H; Nagata, R Tricyclic indole-2-carboxylic acids: highly in vivo active and selective antagonists for the glycine binding site of the NMDA receptor. J Med Chem 46: 691-701 (2003)
- Niinuma, K; Takenaka, O; Horie, T; Kobayashi, K; Kato, Y; Suzuki, H; Sugiyama, Y Kinetic analysis of the primary active transport of conjugated metabolites across the bile canalicular membrane: comparative study of S-(2,4-dinitrophenyl)-glutathione and 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole glucuronide. J Pharmacol Exp Ther 282: 866-72 (1997)
- Jamieson, SM; Brooke, DG; Heinrich, D; Atwell, GJ; Silva, S; Hamilton, EJ; Turnbull, AP; Rigoreau, LJ; Trivier, E; Soudy, C; Samlal, SS; Owen, PJ; Schroeder, E; Raynham, T; Flanagan, JU; Denny, WA 3-(3,4-Dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic Acids: highly potent and selective inhibitors of the type 5 17-ß-hydroxysteroid dehydrogenase AKR1C3. J Med Chem 55: 7746-58 (2012)
- Angelastro, MR; Mehdi, S; Burkhart, JP; Peet, NP; Bey, P Alpha-diketone and alpha-keto ester derivatives of N-protected amino acids and peptides as novel inhibitors of cysteine and serine proteinases. J Med Chem 33: 11-3 (1990)
- Evans, KA; Chai, D; Graybill, TL; Burton, G; Sarisky, RT; Lin-Goerke, J; Johnston, VK; Rivero, RA An efficient, asymmetric solid-phase synthesis of benzothiadiazine-substituted tetramic acids: potent inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. Bioorg Med Chem Lett 16: 2205-8 (2006)
- Trân, K; Van Den Hauwe, R; Sainsily, X; Couvineau, P; Côté, J; Simard, L; Echevarria, M; Murza, A; Serre, A; Théroux, L; Saibi, S; Haroune, L; Longpré, JM; Lesur, O; Auger-Messier, M; Spino, C; Bouvier, M; Sarret, P; Ballet, S; Marsault, É Constraining the Side Chain of C-Terminal Amino Acids in Apelin-13 Greatly Increases Affinity, Modulates Signaling, and Improves the Pharmacokinetic Profile. J Med Chem 64: 5345-5364 (2021)
- Boys, ML; Schretzman, LA; Chandrakumar, NS; Tollefson, MB; Mohler, SB; Downs, VL; Penning, TD; Russell, MA; Wendt, JA; Chen, BB; Stenmark, HG; Wu, H; Spangler, DP; Clare, M; Desai, BN; Khanna, IK; Nguyen, MN; Duffin, T; Engleman, VW; Finn, MB; Freeman, SK; Hanneke, ML; Keene, JL; Klover, JA; Nickols, GA; Nickols, MA; Steininger, CN; Westlin, M; Westlin, W; Yu, YX; Wang, Y; Dalton, CR; Norring, SA Convergent, parallel synthesis of a series of beta-substituted 1,2,4-oxadiazole butanoic acids as potent and selective alpha(v)beta3 receptor antagonists. Bioorg Med Chem Lett 16: 839-44 (2006)
- Ambo, A; Niizuma, H; Sasaki, A; Kohara, H; Sasaki, Y Dermorphin tetrapeptide analogues with 2',6'-dimethylphenylalanine (Dmp) substituted for aromatic amino acids have high mu opioid receptor binding and biological activities. Bioorg Med Chem Lett 13: 1269-72 (2003)
- Peukert, S; Sun, Y; Zhang, R; Hurley, B; Sabio, M; Shen, X; Gray, C; Dzink-Fox, J; Tao, J; Cebula, R; Wattanasin, S Design and structure-activity relationships of potent and selective inhibitors of undecaprenyl pyrophosphate synthase (UPPS): tetramic, tetronic acids and dihydropyridin-2-ones. Bioorg Med Chem Lett 18: 1840-4 (2008)
- Minehira, D; Takeda, D; Urata, H; Kato, A; Adachi, I; Wang, X; Matsuya, Y; Sugimoto, K; Takemura, M; Endo, S; Matsunaga, T; Hara, A; Koseki, J; Narukawa, K; Hirono, S; Toyooka, N Design, synthesis, and biological evaluation of novel (1-thioxo-1,2,3,4-tetrahydro-ß-carbolin-9-yl)acetic acids as selective inhibitors for AKR1B1. Bioorg Med Chem 20: 356-67 (2011)
- Xue, CB; Voss, ME; Nelson, DJ; Duan, JJ; Cherney, RJ; Jacobson, IC; He, X; Roderick, J; Chen, L; Corbett, RL; Wang, L; Meyer, DT; Kennedy, K; DeGradodagger, WF; Hardman, KD; Teleha, CA; Jaffee, BD; Liu, RQ; Copeland, RA; Covington, MB; Christ, DD; Trzaskos, JM; Newton, RC; Magolda, RL; Wexler, RR; Decicco, CP Design, synthesis, and structure-activity relationships of macrocyclic hydroxamic acids that inhibit tumor necrosis factor alpha release in vitro and in vivo. J Med Chem 44: 2636-60 (2001)
- Duan, JJ; Chen, L; Wasserman, ZR; Lu, Z; Liu, RQ; Covington, MB; Qian, M; Hardman, KD; Magolda, RL; Newton, RC; Christ, DD; Wexler, RR; Decicco, CP Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: design, synthesis, and structure-activity relationships. J Med Chem 45: 4954-7 (2002)
- Chan, L; Pereira, O; Reddy, TJ; Das, SK; Poisson, C; Courchesne, M; Proulx, M; Siddiqui, A; Yannopoulos, CG; Nguyen-Ba, N; Roy, C; Nasturica, D; Moinet, C; Bethell, R; Hamel, M; L'Heureux, L; David, M; Nicolas, O; Courtemanche-Asselin, P; Brunette, S; Bilimoria, D; Bédard, J Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 2: tertiary amides. Bioorg Med Chem Lett 14: 797-800 (2004)
- ONICIU, DC; STEINBERG, GR; HEATON, S; NEWTON, RS; LALLY, JS; GAUTAM, J FUNCTIONALIZED LONG-CHAIN HYDROCARBON MONO- AND DI-CARBOXYLIC ACIDS AND DERIVATIVES THEREOF, AND THEIR USE FOR THE PREVENTION OR TREATMENT OF DISEASE US Patent US20240398737 (2024)
- Price, S; Bordogna, W; Braganza, R; Bull, RJ; Dyke, HJ; Gardan, S; Gill, M; Harris, NV; Heald, RA; van den Heuvel, M; Lockey, PM; Lloyd, J; Molina, AG; Roach, AG; Roussel, F; Sutton, JM; White, AB Identification and optimisation of a series of substituted 5-pyridin-2-yl-thiophene-2-hydroxamic acids as potent histone deacetylase (HDAC) inhibitors. Bioorg Med Chem Lett 17: 363-9 (2007)
- Degorce, SL; Bailey, A; Callis, R; De Savi, C; Ducray, R; Lamont, G; MacFaul, P; Maudet, M; Martin, S; Morgentin, R; Norman, RA; Peru, A; Pink, JH; Plé, PA; Roberts, B; Scott, JS Investigation of (E)-3-[4-(2-Oxo-3-aryl-chromen-4-yl)oxyphenyl]acrylic Acids as Oral Selective Estrogen Receptor Down-Regulators. J Med Chem 58: 3522-33 (2015)
- Harris, GH; Dufresne, C; Joshua, H; Koch, LA; Zink, DL; Salmon, PM; Göklen, KE; Kurtz, MM; Rew, DJ; Bergstrom, JD; Wilson, KE Isolation, structure determination and squalene synthase activity of L-731,120 and L-731,128, alkyl citrate analogs of zaragozic acids A and B Bioorg Med Chem Lett 5: 2403-2408 (1995)
- Zajdel, P; Subra, G; Bojarski, AJ; Duszynska, B; Tatarczynska, E; Nikiforuk, A; Chojnacka-Wójcik, E; Pawlowski, M; Martinez, J Novel class of arylpiperazines containing N-acylated amino acids: their synthesis, 5-HT1A, 5-HT2A receptor affinity, and in vivo pharmacological evaluation. Bioorg Med Chem 15: 2907-19 (2007)
- Dubois, M; Bailly, F; Mbemba, G; Mouscadet, JF; Debyser, Z; Witvrouw, M; Cotelle, P Reaction of rosmarinic acid with nitrite ions in acidic conditions: discovery of nitro- and dinitrorosmarinic acids as new anti-HIV-1 agents. J Med Chem 51: 2575-9 (2008)
- Yan, L; Huo, P; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Mandala, SM SAR studies of 3-arylpropionic acids as potent and selective agonists of sphingosine-1-phosphate receptor-1 (S1P1) with enhanced pharmacokinetic properties. Bioorg Med Chem Lett 17: 828-31 (2007)
- Cross, PE; Dickinson, RP; Parry, MJ; Randall, MJ Selective thromboxane synthetase inhibitors. 4. 2-(1H-imidazol-1-ylmethyl) carboxylic acids of benzo[b]furan, benzo[b]thiophene, indole, and naphthalene. J Med Chem 29: 1643-50 (1986)
- Niiyama, K; Takahashi, H; Nagase, T; Kojima, H; Amano, Y; Katsuki, K; Yamakawa, T; Ozaki, S; Ihara, M; Yano, M; Fukuroda, T; Nishikibe, M; Ishikawa, K Structure-Activity relationships of 2-substituted 5,7-Diarylcyclopenteno[1,2-b]pyridine-6-carboxylic acids as a novel class of endothelin receptor antagonists. Bioorg Med Chem Lett 12: 3041-5 (2002)
- King, PJ; Ma, G; Miao, W; Jia, Q; McDougall, BR; Reinecke, MG; Cornell, C; Kuan, J; Kim, TR; Robinson, WE Structure-activity relationships: analogues of the dicaffeoylquinic and dicaffeoyltartaric acids as potent inhibitors of human immunodeficiency virus type 1 integrase and replication. J Med Chem 42: 497-509 (1999)
- Cincinelli, R; Dallavalle, S; Nannei, R; Carella, S; De Zani, D; Merlini, L; Penco, S; Garattini, E; Giannini, G; Pisano, C; Vesci, L; Carminati, P; Zuco, V; Zanchi, C; Zunino, F Synthesis and structure-activity relationships of a new series of retinoid-related biphenyl-4-ylacrylic acids endowed with antiproliferative and proapoptotic activity. J Med Chem 48: 4931-46 (2005)
- Duplantier, AJ; Biggers, MS; Chambers, RJ; Cheng, JB; Cooper, K; Damon, DB; Eggler, JF; Kraus, KG; Marfat, A; Masamune, H; Pillar, JS; Shirley, JT; Umland, JP; Watson, JW Biarylcarboxylic acids and -amides: inhibition of phosphodiesterase type IV versus [3H]rolipram binding activity and their relationship to emetic behavior in the ferret. J Med Chem 39: 120-5 (1996)
- Tomoo, T; Nakatsuka, T; Katayama, T; Hayashi, Y; Fujieda, Y; Terakawa, M; Nagahira, K Design, synthesis, and biological evaluation of 3-(1-Aryl-1H-indol-5-yl)propanoic acids as new indole-based cytosolic phospholipase A2a inhibitors. J Med Chem 57: 7244-62 (2014)
- Suh, YG; Kim, NJ; Koo, BW; Lee, KO; Moon, SH; Shin, DH; Jung, JW; Paek, SM; Chang, DJ; Li, F; Kang, HJ; Le, TV; Chae, YN; Shin, CY; Kim, MK; Lim, JI; Ryu, JS; Park, HJ Design, synthesis, and biological evaluation of novel constrained meta-substituted phenyl propanoic acids as peroxisome proliferator-activated receptor alpha and gamma dual agonists. J Med Chem 51: 6318-33 (2008)
- Faull, AW; Brewster, AG; Brown, GR; Smithers, MJ; Jackson, R Dual-acting thromboxane receptor antagonist/synthase inhibitors: synthesis and biological properties of [2-substituted-4-(3-pyridyl)-1,3-dioxan-5-yl] alkenoic acids. J Med Chem 38: 686-94 (1995)
- Summa, V; Petrocchi, A; Matassa, VG; Taliani, M; Laufer, R; De Francesco, R; Altamura, S; Pace, P HCV NS5b RNA-dependent RNA polymerase inhibitors: from alpha,gamma-diketoacids to 4,5-dihydroxypyrimidine- or 3-methyl-5-hydroxypyrimidinonecarboxylic acids. Design and synthesis. J Med Chem 47: 5336-9 (2004)
- Hoffman, HE; Jirásková, J; Cígler, P; Sanda, M; Schraml, J; Konvalinka, J Hydroxamic acids as a novel family of serine racemase inhibitors: mechanistic analysis reveals different modes of interaction with the pyridoxal-5'-phosphate cofactor. J Med Chem 52: 6032-41 (2009)
- Price, S; Bordogna, W; Bull, RJ; Clark, DE; Crackett, PH; Dyke, HJ; Gill, M; Harris, NV; Gorski, J; Lloyd, J; Lockey, PM; Mullett, J; Roach, AG; Roussel, F; White, AB Identification and optimisation of a series of substituted 5-(1H-pyrazol-3-yl)-thiophene-2-hydroxamic acids as potent histone deacetylase (HDAC) inhibitors. Bioorg Med Chem Lett 17: 370-5 (2007)
- Identification of 4-(6-((2-methoxyphenyl)amino)pyrazin-2-yl)benzoic acids as CSNK2A inhibitors with antiviral activity and improved selectivity over PIM3.
- Carroll, FI; Abraham, P; Lewin, AH; Parham, KA; Boja, JW; Kuhar, M Isopropyl and phenyl esters of 3 beta-(4-substituted phenyl)tropan-2 beta-carboxylic acids. Potent and selective compounds for the dopamine transporter. J Med Chem 35: 2497-500 (1992)
- Laghezza, A; Pochetti, G; Lavecchia, A; Fracchiolla, G; Faliti, S; Piemontese, L; Di Giovanni, C; Iacobazzi, V; Infantino, V; Montanari, R; Capelli, D; Tortorella, P; Loiodice, F New 2-(aryloxy)-3-phenylpropanoic acids as peroxisome proliferator-activated receptora/¿ dual agonists able to upregulate mitochondrial carnitine shuttle system gene expression. J Med Chem 56: 60-72 (2013)
- Coleman, PJ; Brashear, KM; Hunt, CA; Hoffman, WF; Hutchinson, JH; Breslin, MJ; McVean, CA; Askew, BC; Hartman, GD; Rodan, SB; Rodan, GA; Leu, CT; Prueksaritanont, T; Fernandez-Metzler, C; Ma, B; Libby, LA; Merkle, KM; Stump, GL; Wallace, AA; Lynch, JJ; Lynch, R; Duggan, ME Non-peptide alpha(v)beta(3) antagonists. Part 3: identification of potent RGD mimetics incorporating novel beta-amino acids as aspartic acid replacements. Bioorg Med Chem Lett 12: 31-4 (2001)
- Bedford, CD; Miura, M; Bottaro, JC; Howd, RA; Nolen, HW Nonquaternary cholinesterase reactivators. 4. Dialkylaminoalkyl thioesters of alpha-keto thiohydroximic acids as reactivators of ethyl methylphosphonyl- and 1,2,2-trimethylpropyl methylphosphonyl-acetylcholinesterase in vitro. J Med Chem 29: 1689-96 (1986)
- Saha, AK; End, DW Novel beta-(imidazol-4-yl)-beta-amino acids: solid-phase synthesis and study of their inhibitory activity against geranylgeranyl protein transferase type I. Bioorg Med Chem Lett 15: 1713-9 (2005)
- Thorarensen, A; Li, J; Wakefield, BD; Romero, DL; Marotti, KR; Sweeney, MT; Zurenko, GE; Sarver, RW Preparation of novel anthranilic acids as antibacterial agents: extensive evaluation of structural and physical properties on antibacterial activity and human serum albumin affinity. Bioorg Med Chem Lett 17: 3113-6 (2007)
- Cross, PE; Dickinson, RP; Parry, MJ; Randall, MJ Selective thromboxane synthetase inhibitors. 3. 1H-imidazol-1-yl-substituted benzo[b]furan-, benzo[b]thiophene-, and indole-2- and -3-carboxylic acids. J Med Chem 29: 1637-43 (1986)
- Kocalka, P; Rejman, D; Vanek, V; Rinnová, M; Tomecková, I; Králíková, S; Petrová, M; Páv, O; Pohl, R; Budesínský, M; Liboska, R; Tocík, Z; Panova, N; Votruba, I; Rosenberg, I Structural diversity of nucleoside phosphonic acids as a key factor in the discovery of potent inhibitors of rat T-cell lymphoma thymidine phosphorylase. Bioorg Med Chem Lett 20: 862-5 (2010)
- Jacobson, IC; Reddy, PG; Wasserman, ZR; Hardman, KD; Covington, MB; Arner, EC; Copeland, RA; Decicco, CP; Magolda, RL Structure-based design and synthesis of a series of hydroxamic acids with a quaternary-hydroxy group in P1 as inhibitors of matrix metalloproteinases. Bioorg Med Chem Lett 8: 837-42 (1999)
- Beck, G; Kesseler, K; Baader, E; Bartmann, W; Bergmann, A; Granzer, E; Jendralla, H; von Kerekjarto, B; Krause, R; Paulus, E Synthesis and biological activity of new HMG-CoA reductase inhibitors. 1. Lactones of pyridine- and pyrimidine-substituted 3,5-dihydroxy-6-heptenoic (-heptanoic) acids. J Med Chem 33: 52-60 (1990)
- Wilhelm, A; Lopez-Garcia, LA; Busschots, K; Fröhner, W; Maurer, F; Boettcher, S; Zhang, H; Schulze, JO; Biondi, RM; Engel, M 2-(3-Oxo-1,3-diphenylpropyl)malonic acids as potent allosteric ligands of the PIF pocket of phosphoinositide-dependent kinase-1: development and prodrug concept. J Med Chem 55: 9817-30 (2012)
- Hoffman, WF; Alberts, AW; Cragoe, EJ; Deana, AA; Evans, BE; Gilfillan, JL; Gould, NP; Huff, JW; Novello, FC; Prugh, JD 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 2. Structural modification of 7-(substituted aryl)-3,5-dihydroxy-6-heptenoic acids and their lactone derivatives. J Med Chem 29: 159-69 (1986)
- Zheng, X; Wu, Y; Wu, D; Wang, X; Zhang, C; Guo, X; Luo, HB 3D-QSAR studies of 3-(3,4-dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic acids as AKR1C3 inhibitors: Highlight the importance of molecular docking in conformation generation. Bioorg Med Chem Lett 26: 5631-5638 (2016)
- Coats, EA; Shah, KJ; Milstein, SR; Genther, CS; Nene, DM; Roesener, J; Schmidt, J; Pleiss, M; Wagner, E; Baker, JK 4-hydroxyquinoline-3-carboxylic acids as inhibitors of cell respiration. 2. Quantitative structure-activity relationship of dehydrogenase enzyme and Ehrlich ascites tumor cell inhibitions. J Med Chem 25: 57-63 (1982)
- Gribble, AD; Dolle, RE; Shaw, A; McNair, D; Novelli, R; Novelli, CE; Slingsby, BP; Shah, VP; Tew, D; Saxty, BA; Allen, M; Groot, PH; Pearce, N; Yates, J ATP-citrate lyase as a target for hypolipidemic intervention. Design and synthesis of 2-substituted butanedioic acids as novel, potent inhibitors of the enzyme. J Med Chem 39: 3569-84 (1996)
- Ott, GR; Asakawa, N; Lu, Z; Liu, RQ; Covington, MB; Vaddi, K; Qian, M; Newton, RC; Christ, DD; Traskos, JM; Decicco, CP; Duan, JJ Alpha,beta-cyclic-beta-benzamido hydroxamic acids: novel templates for the design, synthesis, and evaluation of selective inhibitors of TNF-alpha converting enzyme (TACE). Bioorg Med Chem Lett 18: 694-9 (2008)
- Müller, K; Prinz, H Antipsoriatic anthrones with modulated redox properties. 4. Synthesis and biological activity of novel 9,10-dihydro-1,8-dihydroxy-9-oxo-2-anthracenecarboxylic and -hydroxamic acids. J Med Chem 40: 2780-7 (1997)
- Häcker, HG; Leyers, S; Wiendlocha, J; Gütschow, M; Wiese, M Aromatic 2-(thio)ureidocarboxylic acids as a new family of modulators of multidrug resistance-associated protein 1: synthesis, biological evaluation, and structure-activity relationships. J Med Chem 52: 4586-95 (2009)
- Zeng, LF; Zhang, HS; Wang, YH; Sanchez, T; Zheng, YT; Neamati, N; Long, YQ Efficient synthesis and utilization of phenyl-substituted heteroaromatic carboxylic acids as aryl diketo acid isosteres in the design of novel HIV-1 integrase inhibitors. Bioorg Med Chem Lett 18: 4521-4 (2008)
- Negoro, N; Sasaki, S; Ito, M; Kitamura, S; Tsujihata, Y; Ito, R; Suzuki, M; Takeuchi, K; Suzuki, N; Miyazaki, J; Santou, T; Odani, T; Kanzaki, N; Funami, M; Tanaka, T; Yasuma, T; Momose, Y Identification of fused-ring alkanoic acids with improved pharmacokinetic profiles that act as G protein-coupled receptor 40/free fatty acid receptor 1 agonists. J Med Chem 55: 1538-52 (2012)
- Brashear, KM; Hunt, CA; Kucer, BT; Duggan, ME; Hartman, GD; Rodan, GA; Rodan, SB; Leu, CT; Prueksaritanont, T; Fernandez-Metzler, C; Barrish, A; Homnick, CF; Hutchinson, JH; Coleman, PJ Non-peptide alpha(v)beta(3) antagonists. Part 5: identification of potent RGD mimetics incorporating 2-aryl beta-amino acids as aspartic acid replacements. Bioorg Med Chem Lett 12: 3483-6 (2002)
- Silverman, RB; Nanavati, SM Selective inhibition of gamma-aminobutyric acid aminotransferase by (3R,4R),(3S,4S)- and (3R,4S),(3S,4R)-4-amino-5-fluoro-3-phenylpentanoic acids. J Med Chem 33: 931-6 (1990)
- Nicolaou, I; Demopoulos, VJ Substituted pyrrol-1-ylacetic acids that combine aldose reductase enzyme inhibitory activity and ability to prevent the nonenzymatic irreversible modification of proteins from monosaccharides. J Med Chem 46: 417-26 (2003)
- Perchonock, CD; Uzinskas, I; McCarthy, ME; Erhard, KF; Gleason, JG; Wasserman, MA; Muccitelli, RM; DeVan, JF; Tucker, SS; Vickery, LM Synthesis and structure-activity relationship studies of a series of 5-aryl-4,6-dithianonanedioic acids and related compounds: a novel class of leukotriene antagonists. J Med Chem 29: 1442-52 (1986)
- Uprety, R; Váradi, A; Allaoa, A; Redel-Traub, GN; Palmer, TC; Feinberg, EN; Ferris, AC; Pande, VS; Pasternak, GW; Majumdar, S Synthesis of spiro-2,6-dioxopiperazine and spiro-2,6-dioxopyrazine scaffolds using amino acids in a three-component reaction to generate potential Sigma-1 (σ Eur J Med Chem 164: 241-251 (2019)
- Fresneau, P; Cussac, M; Morand, JM; Szymonski, B; Tranqui, D; Leclerc, G Synthesis, activity, and molecular modeling of new 2, 4-dioxo-5-(naphthylmethylene)-3-thiazolidineacetic acids and 2-thioxo analogues as potent aldose reductase inhibitors. J Med Chem 41: 4706-15 (1998)
- Lemonnier, G; Lion, C; Quirion, JC; Pin, JP; Goudet, C; Jubault, P ¿-amino-ß-fluorocyclopropanecarboxylic acids as a new tool for drug development: synthesis of glutamic acid analogs and agonist activity towards metabotropic glutamate receptor 4. Bioorg Med Chem 20: 4716-26 (2012)
- Stokker, GE; Alberts, AW; Anderson, PS; Cragoe, EJ; Deana, AA; Gilfillan, JL; Hirshfield, J; Holtz, WJ; Hoffman, WF; Huff, JW 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 3. 7-(3,5-Disubstituted [1,1'-biphenyl]-2-yl)-3,5-dihydroxy-6-heptenoic acids and their lactone derivatives. J Med Chem 29: 170-81 (1986)
- Stokker, GE; Alberts, AW; Gilfillan, JL; Huff, JW; Smith, RL 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 5. 6-(Fluoren-9-yl)- and 6-(fluoren-9-ylidenyl)-3,5-dihydroxyhexanoic acids and their lactone derivatives. J Med Chem 29: 852-5 (1986)
- Yan, L; Huo, P; Doherty, G; Toth, L; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Quackenbush, E; Wickham, A; Mandala, SM Discovery of 3-arylpropionic acids as potent agonists of sphingosine-1-phosphate receptor-1 (S1P1) with high selectivity against all other known S1P receptor subtypes. Bioorg Med Chem Lett 16: 3679-83 (2006)
- Kaila, N; Follows, B; Leung, L; Thomason, J; Huang, A; Moretto, A; Janz, K; Lowe, M; Mansour, TS; Hubeau, C; Page, K; Morgan, P; Fish, S; Xu, X; Williams, C; Saiah, E Discovery of isoquinolinone indole acetic acids as antagonists of chemoattractant receptor homologous molecule expressed on Th2 cells (CRTH2) for the treatment of allergic inflammatory diseases. J Med Chem 57: 1299-322 (2014)
- Lü, MH; Wang, ZP; Xing, LZ; Zhang, W; Han, F; Huang, GL; Liu, W; Zhang, YX; Xu, J; Cui, J Hybrids of polyphenolic/quinone acids, the potential preventive and therapeutic drugs for PD: Disaggregate α-Syn fibrils, inhibit inclusions, and repair damaged neurons in mice. Eur J Med Chem 249: (2023)
- Dal Pozzo, A; Ni, M; Muzi, L; de Castiglione, R; Mondelli, R; Mazzini, S; Penco, S; Pisano, C; Castorina, M; Giannini, G Incorporation of the unusual C(alpha)-fluoroalkylamino acids into cyclopeptides: synthesis of arginine-glycine-aspartate (RGD) analogues and study of their conformational and biological behavior. J Med Chem 49: 1808-17 (2006)
- Pothier, J; Riederer, MA; Peter, O; Leroy, X; Valdenaire, A; Gnerre, C; Fretz, H Novel 2-(2-(benzylthio)-1H-benzo[d]imidazol-1-yl)acetic acids: discovery and hit-to-lead evolution of a selective CRTh2 receptor antagonist chemotype. Bioorg Med Chem Lett 22: 4660-4 (2012)
- Optimization of α-amido boronic acids via cryo-electron microscopy analysis: Discovery of a novel highly selective immunoproteasome subunit LMP7 (β5i)/LMP2 (β1i) dual inhibitor.
- Molinski, TF; Reynolds, KA; Morinaka, BI Symplocin A, a linear peptide from the Bahamian cyanobacterium Symploca sp. Configurational analysis of N,N-dimethylamino acids by chiral-phase HPLC of naphthacyl esters. J Nat Prod 75: 425-31 (2012)
- Senthilkumar, P; Dinakaran, M; Banerjee, D; Devakaram, RV; Yogeeswari, P; China, A; Nagaraja, V; Sriram, D Synthesis and antimycobacterial evaluation of newer 1-cyclopropyl-1,4-dihydro-6-fluoro-7-(substituted secondary amino)-8-methoxy-5-(sub)-4-oxoquinoline-3-carboxylic acids. Bioorg Med Chem 16: 2558-69 (2008)
- Aranapakam, V; Davis, JM; Grosu, GT; Baker, J; Ellingboe, J; Zask, A; Levin, JI; Sandanayaka, VP; Du, M; Skotnicki, JS; DiJoseph, JF; Sung, A; Sharr, MA; Killar, LM; Walter, T; Jin, G; Cowling, R; Tillett, J; Zhao, W; McDevitt, J; Xu, ZB Synthesis and structure-activity relationship of N-substituted 4-arylsulfonylpiperidine-4-hydroxamic acids as novel, orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis. J Med Chem 46: 2376-96 (2003)
- Domagala, JM; Hagen, SE; Heifetz, CL; Hutt, MP; Mich, TF; Sanchez, JP; Trehan, AK 7-substituted 5-amino-1-cyclopropyl-6,8-difluoro-1,4-dihydro-4-oxo-3- quinolinecarboxylic acids: synthesis and biological activity of a new class of quinolone antibacterials. J Med Chem 31: 503-6 (1988)
- Ballet, S; Feytens, D; Buysse, K; Chung, NN; Lemieux, C; Tumati, S; Keresztes, A; Van Duppen, J; Lai, J; Varga, E; Porreca, F; Schiller, PW; Vanden Broeck, J; Tourwé, D Design of novel neurokinin 1 receptor antagonists based on conformationally constrained aromatic amino acids and discovery of a potent chimeric opioid agonist-neurokinin 1 receptor antagonist. J Med Chem 54: 2467-76 (2011)
- Ye, XY; Chen, SY; Nayeem, A; Golla, R; Seethala, R; Wang, M; Harper, T; Sleczka, BG; Li, YX; He, B; Kirby, M; Gordon, DA; Robl, JA Design, synthesis, and SAR studies of novel polycyclic acids as potent and selective inhibitors of human 11ß-hydroxysteroid dehydrogenase type 1 (11ß-HSD-1). Bioorg Med Chem Lett 21: 6699-704 (2011)
- Khan, M; Yates, SL; Tedford, CE; Kirschbaum, K; Phillips, JG Diastereoselective synthesis of trans-2-(1-triphenylmethyl-1H-imidazol-4-yl)cyclopropanecarboxylic acids: key intermediates for the preparation of potent and chiral histamine H3 receptor agents Bioorg Med Chem Lett 7: 3017-3022 (1997)
- Buzard, D; Han, S; Thoresen, L; Moody, J; Lopez, L; Kawasaki, A; Schrader, T; Sage, C; Gao, Y; Edwards, J; Barden, J; Thatte, J; Fu, L; Solomon, M; Liu, L; Al-Shamma, H; Gatlin, J; Le, M; Xing, C; Espinola, S; Jones, RM Discovery and characterization of potent and selective 4-oxo-4-(5-(5-phenyl-1,2,4-oxadiazol-3-yl)indolin-1-yl)butanoic acids as S1P¿? agonists. Bioorg Med Chem Lett 21: 6013-8 (2011)
- Lee, S; Abed, DA; Nguyen, MU; Verzi, MP; Hu, L Structure-activity relationships of 1,4-bis(arylsulfonamido)-benzene or naphthalene-N,N'-diacetic acids with varying C2-substituents as inhibitors of Keap1-Nrf2 protein-protein interaction. Eur J Med Chem 237: (2022)
- Jendralla, H; Baader, E; Bartmann, W; Beck, G; Bergmann, A; Granzer, E; von Kerekjarto, B; Kesseler, K; Krause, R; Schubert, W Synthesis and biological activity of new HMG-CoA reductase inhibitors. 2. Derivatives of 7-(1H-pyrrol-3-yl)-substituted-3,5-dihydroxyhept-6(E)-enoic (-heptanoic) acids. J Med Chem 33: 61-70 (1990)
- Tomita, K; Tsuzuki, Y; Shibamori, K; Tashima, M; Kajikawa, F; Sato, Y; Kashimoto, S; Chiba, K; Hino, K Synthesis and structure-activity relationships of novel 7-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acids as antitumor agents. Part 1. J Med Chem 45: 5564-75 (2002)
- Boatman, PD; Lauring, B; Schrader, TO; Kasem, M; Johnson, BR; Skinner, P; Jung, JK; Xu, J; Cherrier, MC; Webb, PJ; Semple, G; Sage, CR; Knudsen, J; Chen, R; Luo, WL; Caro, L; Cote, J; Lai, E; Wagner, J; Taggart, AK; Carballo-Jane, E; Hammond, M; Colletti, SL; Tata, JR; Connolly, DT; Waters, MG; Richman, JG (1aR,5aR)1a,3,5,5a-Tetrahydro-1H-2,3-diaza-cyclopropa[a]pentalene-4-carboxylic acid (MK-1903): a potent GPR109a agonist that lowers free fatty acids in humans. J Med Chem 55: 3644-66 (2012)
- Brooks, DA; Etgen, GJ; Rito, CJ; Shuker, AJ; Dominianni, SJ; Warshawsky, AM; Ardecky, R; Paterniti, JR; Tyhonas, J; Karanewsky, DS; Kauffman, RF; Broderick, CL; Oldham, BA; Montrose-Rafizadeh, C; Winneroski, LL; Faul, MM; McCarthy, JR Design and synthesis of 2-methyl-2-[4-(2-[5-methyl-2-aryloxazol-4-yl]ethoxy)phenoxy]propionic acids: a new class of dual PPARalpha/gamma agonists. J Med Chem 44: 2061-4 (2001)
- Shiohara, H; Nakamura, T; Kikuchi, N; Ozawa, T; Matsuzawa, A; Nagano, R; Ohnota, H; Miyamoto, T; Ichikawa, K; Hashizume, K Design, synthesis, and structure-activity relationship (SAR) of N-[7-(4-hydroxyphenoxy)-6-methylindan-4-yl]malonamic acids as thyroid hormone receptorß (TRß) selective agonists. Bioorg Med Chem 21: 592-607 (2013)
- Inguimbert, N; Poras, H; Teffo, F; Beslot, F; Selkti, M; Tomas, A; Scalbert, E; Bennejean, C; Renard, P; Fournié-Zaluski, MC; Roques, BP N-[2-(Indan-1-yl)-3-mercapto-propionyl] amino acids as highly potent inhibitors of the three vasopeptidases (NEP, ACE, ECE): In vitro and In vivo activities. Bioorg Med Chem Lett 12: 2001-5 (2002)
- Fracchiolla, G; Laghezza, A; Piemontese, L; Tortorella, P; Mazza, F; Montanari, R; Pochetti, G; Lavecchia, A; Novellino, E; Pierno, S; Conte Camerino, D; Loiodice, F New 2-aryloxy-3-phenyl-propanoic acids as peroxisome proliferator-activated receptors alpha/gamma dual agonists with improved potency and reduced adverse effects on skeletal muscle function. J Med Chem 52: 6382-93 (2009)
- Lehr, M Structure-activity relationships of (4-acylpyrrol-2-yl)alkanoic acids as inhibitors of the cytosolic phospholipase A2: variation of the substituents in positions 1, 3, and 5. J Med Chem 40: 3381-92 (1997)
- Long, YQ; Xue, T; Song, YL; Liu, ZL; Huang, SX; Yu, Q Synthesis and utilization of chiral alpha-methylated alpha-amino acids with a carboxyalkyl side chain in the design of novel Grb2-SH2 peptide inhibitors free of phosphotyrosine. J Med Chem 51: 6371-80 (2008)
- Manley, PW; Tuffin, DP; Allanson, NM; Buckle, PE; Lad, N; Lai, SM; Lunt, DO; Porter, RA; Wade, PJ Thromboxane synthase inhibitors. Synthesis and pharmacological activity of (R)-, (S)-, and (+/-)-2,2-dimethyl-6-[2-(1H-imidazol-1-yl)-1-[[(4-methoxyphenyl)- methoxy]methyl]ethoxy]hexanoic acids. J Med Chem 30: 1812-8 (1987)
- Kaila, N; Huang, A; Moretto, A; Follows, B; Janz, K; Lowe, M; Thomason, J; Mansour, TS; Hubeau, C; Page, K; Morgan, P; Fish, S; Xu, X; Williams, C; Saiah, E Diazine indole acetic acids as potent, selective, and orally bioavailable antagonists of chemoattractant receptor homologous molecule expressed on Th2 cells (CRTH2) for the treatment of allergic inflammatory diseases. J Med Chem 55: 5088-109 (2012)
- Piscitelli, F; Coluccia, A; Brancale, A; La Regina, G; Sansone, A; Giordano, C; Balzarini, J; Maga, G; Zanoli, S; Samuele, A; Cirilli, R; La Torre, F; Lavecchia, A; Novellino, E; Silvestri, R Indolylarylsulfones bearing natural and unnatural amino acids. Discovery of potent inhibitors of HIV-1 non-nucleoside wild type and resistant mutant strains reverse transcriptase and coxsackie B4 virus. J Med Chem 52: 1922-34 (2009)
- Chłoń-Rzepa, G; Ślusarczyk, M; Jankowska, A; Gawalska, A; Bucki, A; Kołaczkowski, M; Świerczek, A; Pociecha, K; Wyska, E; Zygmunt, M; Kazek, G; Sałat, K; Pawłowski, M Novel amide derivatives of 1,3-dimethyl-2,6-dioxopurin-7-yl-alkylcarboxylic acids as multifunctional TRPA1 antagonists and PDE4/7 inhibitors: A new approach for the treatment of pain. Eur J Med Chem 158: 517-533 (2018)
- Stroba, A; Schaeffer, F; Hindie, V; Lopez-Garcia, L; Adrian, I; Fröhner, W; Hartmann, RW; Biondi, RM; Engel, M 3,5-Diphenylpent-2-enoic acids as allosteric activators of the protein kinase PDK1: structure-activity relationships and thermodynamic characterization of binding as paradigms for PIF-binding pocket-targeting compounds. J Med Chem 52: 4683-93 (2009)
- Zhu, YF; Wilcoxen, K; Gross, T; Connors, P; Strack, N; Gross, R; Huang, CQ; McCarthy, JR; Xie, Q; Ling, N; Chen, C 6,7-dihydroxyisoquinoline-3-carboxylic acids are potent inhibitors on the binding of insulin-like growth factor (IGF) to IGF-binding proteins: optimization of the 1-position benzoyl side chain. Bioorg Med Chem Lett 13: 1927-30 (2003)
- Costi, R; Métifiot, M; Esposito, F; Cuzzucoli Crucitti, G; Pescatori, L; Messore, A; Scipione, L; Tortorella, S; Zinzula, L; Novellino, E; Pommier, Y; Tramontano, E; Marchand, C; Di Santo, R 6-(1-Benzyl-1H-pyrrol-2-yl)-2,4-dioxo-5-hexenoic acids as dual inhibitors of recombinant HIV-1 integrase and ribonuclease H, synthesized by a parallel synthesis approach. J Med Chem 56: 8588-98 (2013)
- Ott, GR; Asakawa, N; Liu, RQ; Covington, MB; Qian, M; Vaddi, K; Newton, RC; Trzaskos, JM; Christ, DD; Galya, L; Scholz, T; Marshall, W; Duan, JJ Alpha,Beta-cyclic-beta-benzamido hydroxamic acids: Novel oxaspiro[4.4]nonane templates for the discovery of potent, selective, orally bioavailable inhibitors of tumor necrosis factor-alpha converting enzyme (TACE). Bioorg Med Chem Lett 18: 1288-92 (2008)
- Seto, S; Okada, K; Kiyota, K; Isogai, S; Iwago, M; Shinozaki, T; Kitamura, Y; Kohno, Y; Murakami, K Design, synthesis, and structure-activity relationship studies of novel 2,4,6-trisubstituted-5-pyrimidinecarboxylic acids as peroxisome proliferator-activated receptor gamma (PPARgamma) partial agonists with comparable antidiabetic efficacy to rosiglitazone. J Med Chem 53: 5012-24 (2010)
- Sechi, M; Innocenti, A; Pala, N; Rogolino, D; Carcelli, M; Scozzafava, A; Supuran, CT Inhibition ofa-class cytosolic human carbonic anhydrases I, II, IX and XII, andß-class fungal enzymes by carboxylic acids and their derivatives: new isoform-I selective nanomolar inhibitors. Bioorg Med Chem Lett 22: 5801-6 (2012)
- Ottanà, R; Maccari, R; Amuso, S; Wolber, G; Schuster, D; Herdlinger, S; Manao, G; Camici, G; Paoli, P New 4-[(5-arylidene-2-arylimino-4-oxo-3-thiazolidinyl)methyl]benzoic acids active as protein tyrosine phosphatase inhibitors endowed with insulinomimetic effect on mouse C2C12 skeletal muscle cells. Eur J Med Chem 50: 332-43 (2012)
- Xue, CB; He, X; Corbett, RL; Roderick, J; Wasserman, ZR; Liu, RQ; Jaffee, BD; Covington, MB; Qian, M; Trzaskos, JM; Newton, RC; Magolda, RL; Wexler, RR; Decicco, CP Discovery of macrocyclic hydroxamic acids containing biphenylmethyl derivatives at P1', a series of selective TNF-alpha converting enzyme inhibitors with potent cellular activity in the inhibition of TNF-alpha release. J Med Chem 44: 3351-4 (2001)
- Qian, WJ; Park, JE; Lee, KS; Burke, TR Non-proteinogenic amino acids in the pThr-2 position of a pentamer peptide that confer high binding affinity for the polo box domain (PBD) of polo-like kinase 1 (Plk1). Bioorg Med Chem Lett 22: 7306-8 (2012)
- Berlicki, L; Kaske, M; Gutièrrez-Abad, R; Bernhardt, G; Illa, O; Ortuno, RM; Cabrele, C; Buschauer, A; Reiser, O Replacement of Thr32 and Gln34 in the C-terminal neuropeptide Y fragment 25-36 by cis-cyclobutane and cis-cyclopentane β-amino acids shifts selectivity toward the Y(4) receptor. J Med Chem 56: 8422-31 (2013)
- Golani, LK; George, C; Zhao, S; Raghavan, S; Orr, S; Wallace, A; Wilson, MR; Hou, Z; Matherly, LH; Gangjee, A Structure-activity profiles of novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolates with modified amino acids for cellular uptake by folate receptorsa andß and the proton-coupled folate transporter. J Med Chem 57: 8152-66 (2014)
- Kotake, Y; Iijima, A; Yoshimatsu, K; Tamai, N; Ozawa, Y; Koyanagi, N; Kitoh, K; Nomura, H Synthesis and antitumor activities of novel 6-5 fused ring heterocycle antifolates: N-[4-[omega-(2-amino-4-substituted-6,7-dihydrocyclopenta [d]pyrimidin-5-yl)alkyl]benzoyl]-L-glutamic acids. J Med Chem 37: 1616-24 (1994)
- Abdellatif, KR; Chowdhury, MA; Dong, Y; Chen, QH; Knaus, EE Diazen-1-ium-1,2-diolated and nitrooxyethyl nitric oxide donor ester prodrugs of anti-inflammatory (E)-2-(aryl)-3-(4-methanesulfonylphenyl)acrylic acids: synthesis, cyclooxygenase inhibition, and nitric oxide release studies. Bioorg Med Chem 16: 3302-8 (2008)
- Van Zandt, MC; Doan, B; Sawicki, DR; Sredy, J; Podjarny, AD Discovery of [3-(4,5,7-trifluoro-benzothiazol-2-ylmethyl)-pyrrolo[2,3-b]pyridin-1-yl]acetic acids as highly potent and selective inhibitors of aldose reductase for treatment of chronic diabetic complications. Bioorg Med Chem Lett 19: 2006-8 (2009)
- Yanagisawa, H; Amemiya, Y; Kanazaki, T; Shimoji, Y; Fujimoto, K; Kitahara, Y; Sada, T; Mizuno, M; Ikeda, M; Miyamoto, S; Furukawa, Y; Koike, H Nonpeptide angiotensin II receptor antagonists: synthesis, biological activities, and structure-activity relationships of imidazole-5-carboxylic acids bearing alkyl, alkenyl, and hydroxyalkyl substituents at the 4-position and their related compounds. J Med Chem 39: 323-38 (1996)
- Alonso, JA; Andrés, M; Bravo, M; Buil, MA; Calbet, M; Castro, J; Eastwood, PR; Esteve, C; Ferrer, M; Forns, P; Gómez, E; González, J; Lozoya, E; Mir, M; Moreno, I; Petit, S; Roberts, RS; Sevilla, S; Vidal, B; Vidal, L; Vilaseca, P Structure-activity relationships (SAR) and structure-kinetic relationships (SKR) of bicyclic heteroaromatic acetic acids as potent CRTh2 antagonists III: the role of a hydrogen-bond acceptor in long receptor residence times. Bioorg Med Chem Lett 24: 5127-33 (2014)
- Yokomatsu, T; Hayakawa, Y; Suemune, K; Kihara, T; Soeda, S; Shimeno, H; Shibuya, S Synthesis and biological evaluation of 1,1-difluoro-2-(tetrahydro-3-furanyl)ethylphosphonic acids possessing a N9-purinylmethyl functional group at the ring. a new class of inhibitors for purine nucleoside phosphorylases. Bioorg Med Chem Lett 9: 2833-6 (1999)
- Han, F; Jiang, B; Lü, MH; Wang, ZP; Liu, W; Zhang, YX; Xu, J Hybrids of polyphenolic acids and xanthone, the potential preventive and therapeutic effects on PD: Design, synthesis, in vitro anti-aggregation of α-synuclein, and disaggregation against the existed α-synuclein oligomer and fibril. Bioorg Med Chem 66: (2022)
- Maccari, R; Wolber, G; Genovese, M; Sardelli, G; Talagayev, V; Balestri, F; Luti, S; Santi, A; Moschini, R; Del Corso, A; Paoli, P; Ottanà, R Designed multiple ligands for the treatment of type 2 diabetes mellitus and its complications: Discovery of (5-arylidene-4-oxo-2-thioxothiazolidin-3-yl)alkanoic acids active as novel dual-targeted PTP1B/AKR1B1 inhibitors. Eur J Med Chem 252: (2023)
- Zajdel, P; Subra, G; Verdie, P; Bojarski, AJ; Duszynska, B; Basista, K; Obniska, J; Martinez, J; Pawlowski, M The influence of an ethylene spacer on the 5-HT(1A) and 5-HT(2A) receptor affinity of arylpiperazine derivatives of amides with N-acylated amino acids and 3-differently substituted pyrrolidine-2,5-diones. Eur J Med Chem 44: 800-8 (2009)
- Monn, JA; Valli, MJ; Massey, SM; Hansen, MM; Kress, TJ; Wepsiec, JP; Harkness, AR; Grutsch, JL; Wright, RA; Johnson, BG; Andis, SL; Kingston, A; Tomlinson, R; Lewis, R; Griffey, KR; Tizzano, JP; Schoepp, DD Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors J Med Chem 42: 1027-40 (1999)
- Negoro, N; Sasaki, S; Mikami, S; Ito, M; Tsujihata, Y; Ito, R; Suzuki, M; Takeuchi, K; Suzuki, N; Miyazaki, J; Santou, T; Odani, T; Kanzaki, N; Funami, M; Morohashi, A; Nonaka, M; Matsunaga, S; Yasuma, T; Momose, Y Optimization of (2,3-dihydro-1-benzofuran-3-yl)acetic acids: discovery of a non-free fatty acid-like, highly bioavailable G protein-coupled receptor 40/free fatty acid receptor 1 agonist as a glucose-dependent insulinotropic agent. J Med Chem 55: 3960-74 (2012)
- Inagaki, H; Takahashi, H; Takemura, M Synthesis and antibacterial activity of novel 6-fluoro-1-[(1R,2S)-2-fluorocyclopropan-1-yl]-4-oxoquinoline-3-carboxylic acids bearing cyclopropane-fused 2-amino-8-azabicyclo[4.3.0]nonan-8-yl substituents at the C-7 position. Bioorg Med Chem Lett 14: 5193-8 (2004)
- Hruby, VJ; Lu, D; Sharma, SD; Castrucci, AL; Kesterson, RA; al-Obeidi, FA; Hadley, ME; Cone, RD Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem 38: 3454-61 (1995)
- Gribble, AD; Ife, RJ; Shaw, A; McNair, D; Novelli, CE; Bakewell, S; Shah, VP; Dolle, RE; Groot, PH; Pearce, N; Yates, J; Tew, D; Boyd, H; Ashman, S; Eggleston, DS; Haltiwanger, RC; Okafo, G ATP-Citrate lyase as a target for hypolipidemic intervention. 2. Synthesis and evaluation of (3R,5S)-omega-substituted-3-carboxy-3, 5-dihydroxyalkanoic acids and their gamma-lactone prodrugs as inhibitors of the enzyme in vitro and in vivo. J Med Chem 41: 3582-95 (1998)
- Zhao, J; Mao, Q; Lin, F; Zhang, B; Sun, M; Zhang, T; Wang, S Intramolecular hydrogen bond interruption and scaffold hopping of TMC-5 led to 2-(4-alkoxy-3-cyanophenyl)pyrimidine-4/5-carboxylic acids and 6-(4-alkoxy-3-cyanophenyl)-1,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-ones as potent pyrimidine-based xanthine oxidase inhibitors. Eur J Med Chem 229: (2022)
- ChEMBL_534427 (CHEMBL971353) Inhibition of bile acid beta-glucosidase activity of human liver GBA2
- ChEMBL_837935 (CHEMBL2075104) TP_TRANSPORTER: inhibition of SN-38 uptake in bile canalicular membrane vesicles
- ChEMBL_1520854 (CHEMBL3626239) Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytes
- ChEMBL_836158 (CHEMBL2075057) TP_TRANSPORTER: inhibition of Temocaprilat uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836172 (CHEMBL2075071) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836173 (CHEMBL2075072) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836174 (CHEMBL2075073) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836175 (CHEMBL2075074) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836176 (CHEMBL2075075) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836182 (CHEMBL2075081) TP_TRANSPORTER: inhibition of BQ-485 uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836184 (CHEMBL2075083) TP_TRANSPORTER: inhibition of 5-methyltetrahydrofolate uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836450 (CHEMBL2076890) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836945 (CHEMBL2075785) TP_TRANSPORTER: inhibition of SN-38 uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836948 (CHEMBL2075788) TP_TRANSPORTER: inhibition of L-MTX uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_836949 (CHEMBL2075789) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_838268 (CHEMBL2076216) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_838314 (CHEMBL2076812) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_839174 (CHEMBL2077845) TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_839289 (CHEMBL2077960) TP_TRANSPORTER: inhibition of BQ-123 uptake in bile canalicular membrane vesicles from SD rat
- ChEMBL_1895086 (CHEMBL4397121) Inhibition of C-terminal GST-tagged human SET1B (1629 to 1923 amino acids) expressed in Escherichia coli using core histone as substrate in presence of WDR5 (22 to 334 amino acids),RbBP5 (1 to 538 amino acids),Ash2L (2 to 534 amino acids),DPY-30 (1 to 99 amino acids) by fluorescence method
- ChEMBL_306878 (CHEMBL828684) In vitro inhibition of taurocholate binding to liver bile acid transporter expressed in CHO cells
- ChEMBL_836947 (CHEMBL2075787) TP_TRANSPORTER: inhibition of LTC4 uptake (LTC4: 0.05 uM) in bile canalicular membranes from Wistar rat
- ChEMBL_306883 (CHEMBL828689) In vitro inhibition of taurocholate binding to human ileal bile acid transporter expressed in CHO cells
- ChEMBL_836946 (CHEMBL2075786) TP_TRANSPORTER: inhibition of Naf-GlcA uptake (Naf-GlcA: 0.1 uM) in bile canalicular membrane vesicles of Wistar rat
- ChEBML_28895 In vitro Inhibition of apical sodium-dependent bile acid transporter by measuring the uptake of [3H]taurocholate in hamster ileal ring
- ChEMBL_753132 (CHEMBL1799132) Inhibition of human truncated LSD1 lacking N-terminal 184 amino acids
- ChEMBL_628491 (CHEMBL1104613) Inhibition of human mTOR (1360-2549 amino acids) expressed in HEK293 cells
- ChEBML_41854 In vitro for inhibition of [14C]taurocholate uptake in baby hamster kidney cells transfected with the cDNA of human Bile acid transporter (H14 cells)
- Inhibition Assay Inhibition of HIV-protease activity for selected acids at P3-P3' positions.
- ChEBML_35875 In vitro inhibitory activity against uptake of [14C]taurocholate in baby hamster kidney cells transfected with cDNA from human Apical Sodium-Codependent Bile Acid Transporter
- ChEMBL_1933900 (CHEMBL4479552) Inhibition of human recombinant DOT1L (1 to 420 amino acids) expressed in Escherichia coli
- ChEMBL_559411 (CHEMBL1017265) Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) expressed in SF9 cells
- ChEMBL_790766 (CHEMBL1926219) Inhibition of recombinant human AKT1 using GSK3 (amino acids 14 to 27) as substrate
- ChEMBL_820572 (CHEMBL2040309) Inhibition of human N-myristoyltransferase 1 using human pp60Src92-16 amino acids) as substrate
- ChEMBL_820573 (CHEMBL2040310) Inhibition of human N-myristoyltransferase 2 using human pp60Src92-16 amino acids) as substrate
- ChEMBL_967551 (CHEMBL2398945) Inhibition of wild type GST-tagged LRRK2 ((1326 to 2517 amino acids) (unknown origin)
- ChEMBL_1910044 (CHEMBL4412490) Inhibition of N-terminal His-tagged human TAK1 (1 to 303 amino acids)/human TAB1 (437 to 504 amino acids) expressed in baculovirus expression system using fluorescence-labeled substrate by off-chip mobility shift assay
- ChEMBL_1285739 (CHEMBL3107728) Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method
- ChEMBL_2170548 (CHEMBL5055607) Inhibition of C-terminal His-tagged WNV NSB2 (52 to 96 amino acids)-NS3 (1 to 184 amino acids) protease expressed in Escherichia coli BL21(DE3) using Boc-GRK-AMC as substrate measured by flourescence based plate reader assay
- Glycocholic acid Uptake Radiometric Assay ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric SPA Inhibitor IC50 Mean IC50 (nM) was determined.
- ChEMBL_1285740 (CHEMBL3107729) Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by LC-MS analysis
- ChEMBL_787631 (CHEMBL1918084) Binding affinity to recombinant rat brain PLCdelta1 PH domain (amino acids 11 to 140) by SPR analysis
- ChEMBL_787632 (CHEMBL1918085) Binding affinity to recombinant rat brain Grp1 PH domain (amino acids 1 to 120) by SPR analysis
- ChEMBL_2170549 (CHEMBL5055608) Competitive inhibition of C-terminal His-tagged WNV NSB2 (52 to 96 amino acids)-NS3 (1 to 184 amino acids) protease expressed in Escherichia coli BL21(DE3) using Boc-GRK-AMC as substrate measured by flourescence based plate reader assay
- ChEMBL_863095 (CHEMBL2175491) Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry
- ChEMBL_966836 (CHEMBL2400837) Binding affinity to substance P receptor (1 to 7 amino acids) binding site in rat spinal cord membranes
- ChEMBL_2238135 (CHEMBL5152031) Inhibition of human recombinant PARP5a (E1023 to T1327 amino acids) incubated for 4 hrs by fluorescence anisotropy binding assay
- ChEMBL_2333315 Inhibition of GST-fused G9a (amino acids 685 to 1000) (unknown origin) expressed in Escherichia coli BL21 by DELFIA assay
- ChEMBL_2333316 Inhibition of GST-fused GLP (amino acids 610 to 917) (unknown origin) expressed in Escherichia coli BL21 by DELFIA assay
- ChEMBL_968095 (CHEMBL2401547) Binding affinity to MDM2 (25 to 109 amino acids) (unknown origin) after 30 mins by surface plasmon resosnance analysis
- ChEMBL_1578159 (CHEMBL3807172) Inhibition of recombinant LSD1 (157 to 852 amino acids) (unknown origin) expressed in Escherichia coli BL21(DE) using H3K4me2 substrate
- ChEMBL_1788619 (CHEMBL4260353) Agonist activity at human LXRalpha-LBD (182 to 447 amino acids) expressed in HEK293T cells by luciferase reporter gene assay
- ChEMBL_1788620 (CHEMBL4260354) Agonist activity at human LXRbeta-LBD (196 to 461 amino acids) expressed in HEK293T cells by luciferase reporter gene assay
- ChEMBL_864591 (CHEMBL2176640) Inhibition of human BACE1 (43-454 amino acids) expressed in Escherichia coli BL21 using SEVNLDAEFRHDSGYEK-biotinafter 3 hrs by ELISA
- ChEMBL_935054 (CHEMBL2320919) Inhibition of human HER2 cytoplasmic domain (amino acids 676 to 1245) expressed in Sf9 cells by DELFIA time resolved fluorometry
- ChEMBL_968766 (CHEMBL2399319) Inhibition of full-length human KRas4B (amino acids 1 to 188)-SOS interaction assessed as inhibition of nucleotide exchange activity
- ChEMBL_2170550 (CHEMBL5055609) Inhibition of C-terminal His-tagged ZIKV NSB2 (52 to 96 amino acids)-NS3 (1 to 184 amino acids) protease expressed in Escherichia coli BL21(DE3) preincubated for 10 mins followed by addition of Boc-GKR-AMC substrate and measured by flourescence based plate reader assay
- ChEMBL_2170551 (CHEMBL5055610) Competitive inhibition of C-terminal His-tagged ZIKV NSB2 (52 to 96 amino acids)-NS3 (1 to 184 amino acids) protease expressed in Escherichia coli BL21(DE3) preincubated for 10 mins followed by addition of Boc-GKR-AMC substrate and measured by flourescence based plate reader assay
- ChEMBL_1331153 (CHEMBL3226853) Reversible inhibition of recombinant LSD1 catalytic domain (178 to 831 amino acids) (unknown origin) expressed in baculovirus infected insect Sf9 cells
- ChEMBL_771323 (CHEMBL1839595) Inhibition of human FAS assessed as synthesis of long chain fatty acids from malonyl CoA after 60 mins by fluorescence assay
- ChEMBL_787633 (CHEMBL1918086) Binding affinity to recombinant rat brain Grp1 PH domain (amino acids 1 to 120) after 10 mins by pull-down assay
- ChEMBL_961840 (CHEMBL2390132) Inhibition of human recombinant PDE4B (152 to 564 amino acids) using cAMP as substrate after 30 mins by plate reader analysis
- ChEMBL_968096 (CHEMBL2398954) Inhibition of TMRA-p53 binding to MDM2 (25 to 109 amino acids) (unknown origin) after 30 mins by fluorescence polarization assay
- Determination of Compound's ROCK Inhibitory Activity In Vitro (Z'Lyte Assay) Recombinant ROCK1 (amino acids 1-535) and ROCK2 (amino acids 1-552) proteins were purchased from ThermoFisher Scientific. Compound's activities were measured by Z′-lyte kinase kit (ThermoFisher Scientific) and IC50 was calculated
- ChEMBL_1296166 (CHEMBL3132000) Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluorimetric analysis
- ChEMBL_1331154 (CHEMBL3226854) Time-dependent inhibition of recombinant LSD1 catalytic domain (178 to 831 amino acids) (unknown origin) expressed in baculovirus infected insect Sf9 cells
- ChEMBL_1456579 (CHEMBL3369961) Inhibition of human PDE10A (amino acids 14 to 779) using [3H]-labelled cyclic nucleotide as substrate after 1 hr b beta counting
- ChEMBL_1931157 (CHEMBL4434408) Inhibition of human recombinant GST tagged JAK3 kinase domain (781 to 1124 amino acids) expressed in insect cells in presence of ATP
- ChEMBL_2057037 (CHEMBL4712038) Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 20 uM DiFMUP by DiFMUP assay
- ChEMBL_2057038 (CHEMBL4712039) Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay
- ChEMBL_944555 (CHEMBL2339071) Binding affinity to recombinant His-tagged Grb10 interacting GYF protein 2 (745-1030 amino acids) (unknown origin) by surface plasmon resonance analysis
- ChEMBL_953654 (CHEMBL2352558) Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay
- ChEMBL_968765 (CHEMBL2399318) Inhibition of full-length human KRas4B (amino acids 1 to 188)-SOS interaction assessed as inhibition of SOS-mediated nucleotide release activity
- ChEMBL_985355 (CHEMBL2432164) Inhibition of recombinant GST-tagged FAK catalytic domain region (amino acids 411-686) (unknown origin) expressed in Sf9 cells after 5 mins
- Pharmaceutical Effect Mean Inhibitory Effect ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric SPA Inhibitor IC50 Mean IC50 (nM) was determined for the compounds of examples 1-14.
- ChEMBL_1879629 (CHEMBL4381023) Inhibition GST-tagged recombinant HRAS G12V mutant (1 to 166 amino acids) (unknown origin) expressed in Escherichia coli C41(DE3) by SPR assay
- ChEMBL_1898487 (CHEMBL4400602) Inhibition of N-terminal GST-fused human FGFR1 cytoplasmic domain (398 to 822(end) amino acids) using CSKtide substrate incubated for 90 mins
- ChEMBL_1898488 (CHEMBL4400603) Inhibition of N-terminal GST-fused human FGFR2 cytoplasmic domain (399 to 821(end) amino acids) using CSKtide substrate incubated for 90 mins
- ChEMBL_1898489 (CHEMBL4400604) Inhibition of N-terminal GST-fused human FGFR3 cytoplasmic domain (436 to 806(end) amino acids) using CSKtide substrate incubated for 90 mins
- ChEMBL_1898490 (CHEMBL4400605) Inhibition of N-terminal GST-fused human FGFR4 cytoplasmic domain (460 to 802(end) amino acids) using CSKtide substrate incubated for 90 mins
- ChEMBL_2122326 (CHEMBL4831473) Inhibition of ACSS2 in human HCT-15 cells assessed as inhibition of 14C acetate incorporation into fatty acids by microbeta scintillation counting analysis
- ChEMBL_2585389 Binding affinity to human recombinant PYK2 (amino acids 420-691) expressed in Escherichia coli, BL21 (DE3) assessed as equilibrium dissociation constant by SPR assay
- ChEMBL_773166 (CHEMBL1838642) Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis
- ChEMBL_973989 (CHEMBL2410314) Competitive inhibition of N-terminal GST-tagged human PLK4 (1 to 391 amino acids) expressed in Escherichia coli in the presence of ATP
- ChEMBL_1336210 (CHEMBL3241458) Binding affinity to recombinant human RPA70 A/B region (181 to 422 amino acids) expressed in Escherichia coli Rosetta by fluorescence polarization anisotropy assay
- ChEMBL_1444239 (CHEMBL3380178) Inhibition of N-terminal GST-fused full-length human IRAK4 (1 to 460 amino acids) assessed as reduction in phosphorylated substrates by Caliper assay
- ChEMBL_1557232 (CHEMBL3773607) Inhibition of GST-tagged human SIRT1 (133 to 747 amino acids) using ZMAL as substrate after 4 hrs by fluorescence-based microplate reader method
- ChEMBL_1855685 (CHEMBL4356414) Inhibition of human full-length USP7 (1 to 1102 amino acids) expressed in Escherichia coli BL21(DE3) incubated 30 mins by Ubiquitin-AMC assay
- ChEMBL_1898483 (CHEMBL4400598) Inhibition of N-terminal GST-tagged recombinant human FGFR1 (456 to 765 amino acids) incubated for 60 mins by time-resolved fluorescence kinase assay
- ChEMBL_2585379 Inhibition of purified-activated FAK kinase domain (amino acids 410-689) (unknown origin) using Glu and Tyr, p(Glu/Tyr) peptide in presence of ATP
- ChEMBL_1885551 (CHEMBL4387133) Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression system assessed as inhibition constant using Ulight-4E-BP1 as substrate preincubated for 30 mins followed by substrate addition and incubated for 90 mins by TR-FRET assay
- ChEMBL_1885552 (CHEMBL4387134) Inhibition of human full length GST-tagged CDK6 (1 to 326(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression system assessed as inhibition constant using Ulight-4E-BP1 as substrate preincubated for 30 mins followed by substrate addition and incubated for 90 mins by TR-FRET assay
- ChEMBL_1885555 (CHEMBL4387137) Inhibition of human full length GST-tagged CDK2 (1 to 298(end) amino acids)/Cyclin E1 (1 to 410(end) amino acids) expressed in baculovirus expression system assessed as inhibition constant using Ulight-4E-BP1 as substrate preincubated for 30 mins followed by substrate addition and incubated for 90 mins by TR-FRET assay
- ChEMBL_1336033 (CHEMBL3240326) Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay
- ChEMBL_1336209 (CHEMBL3241316) Binding affinity to recombinant human RPA70 N-terminal domain (1 to 120 amino acids) expressed in Escherichia coli BL21-DE3 by fluorescence polarization anisotropy assay
- ChEMBL_1337212 (CHEMBL3240620) Inhibition of autophosphorylation of EGFR cytoplasmic domain (amino acids 645 to 1186) (unknown origin) expressed in Sf9 cells after 1 hr by time-resolved fluorometry
- ChEMBL_1459715 (CHEMBL3370433) Binding affinity to N-terminal His6-tagged pVHL (54 to 213 amino acids) (unknown origin) expressed in Escherichia coli BL21 (DE3) by isothermal titration calorimetry
- ChEMBL_1619578 (CHEMBL3861747) Inhibition of N-terminal GST tagged human TrkC catalytic domain (456 to 825 amino acids) using fluorescence-labeled substrate by off-chip mobility shift assay
- ChEMBL_876373 (CHEMBL2186306) Inhibition of human recombinant mTOR (1360 to 2549 amino acids) assessed as reduction in phosphorylation of (GFP)-4-EBP1 after 30 mins by FRET assay
- ChEMBL_1337213 (CHEMBL3240621) Inhibition of autophosphorylation of human HER2 cytoplasmic domain (amino acids 676 to 1245) (unknown origin) expressed in Sf9 cells after 1 hr by time-resolved fluorometry
- ChEMBL_1499595 (CHEMBL3584541) Inhibition of N-terminal His6-tagged human recombinant PAK4 (300 to 591 amino acids) using peptide-7 substrate by pyruvate kinase and lactate dehydrogenase coupled assay
- ChEMBL_1557218 (CHEMBL3773441) Inhibition of N-terminal his6-tagged human SIRT2 (25 to 389 amino acids) using ZMAL as substrate after 4 hrs by fluorescence-based microplate reader method
- ChEMBL_2020129 (CHEMBL4673942) Inhibition of N-terminal Flag tagged- human HDAC10 (2 to 631 amino acids) expressed in baculovirus infected Sf9 cells using Fluor deLys substrate by fluorescence method
- ChEMBL_967970 (CHEMBL2401074) Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 mins by competition assay
- ChEMBL_1281650 (CHEMBL3102587) Inhibition of JNK3 (39 to 402 amino acids) (unknown origin) expressed in Escherichia coli BL21(DE3) using biotinylated ATF2 as substrate after 15 mins by HTRF assay
- ChEMBL_1453489 (CHEMBL3362176) Inhibition of human PDE4D2 catalytic domain (86 to 413 amino acids) expressed in Escherichia coli strain BL21 after 15 mins using [3H]-cAMP by liquid scintillation counting
- ChEMBL_1453676 (CHEMBL3363782) Inhibition of human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using pteroyl-di-L-glutamate substrate by HPLC method
- ChEMBL_1499749 (CHEMBL3585198) Inhibition of PKAalpha (1 to 351 amino acids) (unknown origin) using Leu-Arg-Arg-Ala-Ser-Leu-Gly as substrate by pyruvate kinase/lactate dehydrogenase coupled assay
- ChEMBL_1672604 (CHEMBL4022633) Inhibition of N-terminal GST fused human recombinant JAK1 (866 to 1154 amino acids) expressed in insect cells using ATP and peptide substrate (FITC-C6-KKHTDDGYMPMSPGVA-NH2
- ChEMBL_1672605 (CHEMBL4022634) Inhibition of N-terminal GST fused recombinant JAK2 (831 to 1132 amino acids) (unknown origin) expressed in insect cells using ATP and peptide substrate (5FAM-GEEPLYWSFPAKKK-NH2
- ChEMBL_1672607 (CHEMBL4022636) Inhibition of N-terminal GST fused recombinant JAK3 (781 to 1124 amino acids) (unknown origin) expressed in insect cells using ATP and peptide substrate (5FAM-GEEPLYWSFPAKKK-NH2
- ChEMBL_1759898 (CHEMBL4194906) Binding affinity to BRD4 bromodomain 1 (K57 to E168 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromodomain TR-FRET binding assay
- ChEMBL_1759899 (CHEMBL4194907) Binding affinity to BRD4 bromodomain 2 (E352 to M457 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromodomain TR-FRET binding assay
- ChEMBL_934773 (CHEMBL2318907) Inhibition of EGFR cytoplasmic domain (amino acids 645 to 1186) (unknown origin) expressed in Sf9 cells assessed as reduction in enzyme autophosphorylation by DELFIA time resolved fluorometry
- ChEMBL_965919 (CHEMBL2394350) Inhibition of human recombinant CLK3 (275 to 632 amino acids) expressed in Escherichia coli BL21(DE3) preincubated for 10 mins prior to substrate addition by LDH assay
- ChEMBL_1280886 (CHEMBL3095184) Inhibition of human endothelial lipase using HDL as substrate assessed as release of free fatty acids preincubated for 30 mins followed by substrate addition measured after 2 hrs
- ChEMBL_1759900 (CHEMBL4194908) Binding affinity to BRDT bromodomain 1 to 2 (N21to P380 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromodomain TR-FRET binding assay
- ChEMBL_1812686 (CHEMBL4312260) Inhibition of recombinant human N-terminal FLAG-tagged mTOR (1362 to end amino acids) using ULight-4E-BP1 as substrate incubated for 1 hr by LANCE Ultra assay
- ChEMBL_2081810 (CHEMBL4737601) Inhibition of recombinant human N-terminal FLAG-tagged mTOR (1362 to end amino acids) using ULight-4E-BP1 as substrate incubated for 1 hr by LANCE Ultra assay
- ChEMBL_794139 (CHEMBL1931877) Inhibition of human recombinant BACE1 ectodomain (1 to 460 amino acids) assessed as inhibition of proteolytic cleavage of Rhodamine-EVNLDAEFK-Quencher substrate after 35 mins by FRET assay
- ChEMBL_973993 (CHEMBL2410318) Inhibition of N-terminal GST-tagged human PLK4 (1 to 391 amino acids) expressed in Escherichia coli using TMB as substrate after 30 mins by indirect ELISA assay
- Competitive Ligand Binding Assay Utilizing an analytical ultracentrifuge, the binding constants of various amino acids and isoleucine analogues to IleRS were estimated from their competition with radioactively labeled L-isoleucine.
- ChEMBL_1448250 (CHEMBL3375023) Inhibition of histidine-tagged recombinant EGFR cytoplasmic domain (amino acids 645-1186) (unknown origin) expressed in Sf9 cells assessed as reduction in enzyme autophosphorylation by DELFIA/time-resolved fluorometry
- ChEMBL_1520320 (CHEMBL3626370) Displacement of [3H]-DHT from human GST fused AR-LBD (627 to 919 amino acids) transfected in Escherichia coli HB 101 after 15 hrs by liquid scintillation counting assay
- ChEMBL_1719680 (CHEMBL4134680) Inhibition of recombinant LSD1 (157 to 852 amino acids) (unknown origin) expressed in Escherichia coli BL21(DE) using H3K4me2 substrate by amplex red and horseradish peroxidase based fluorescence assay
- ChEMBL_1759896 (CHEMBL4194904) Binding affinity to BRD2 bromodomain 1 to 2 (G73 to A560 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromodomain TR-FRET binding assay
- ChEMBL_1759897 (CHEMBL4194905) Binding affinity to BRD3 bromodomain 1 to 2 (P24 to P416 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromodomain TR-FRET binding assay
- ChEMBL_2132786 (CHEMBL4842301) Inhibition of ACSS2 in human HCT-15 cells assessed as inhibition of 14C acetate incorporation into fatty acids measured after 24 hrs by scintillation counting based cellular lipids assay
- ChEMBL_967971 (CHEMBL2401075) Binding affinity to recombinant human His-tagged MDM2 (1 to 118 amino acids) using p53-based PMDM6-F as probe after 15 to 30 mins by fluorescence polarization assay
- ChEMBL_1290541 (CHEMBL3119990) Displacement of [3H]-raclopride from high-affinity state of human dopamine D2L receptor (443 amino acids) expressed in LTK deficient mouse fibroblasts after 60 mins by liquid scintillation counting analysis
- ChEMBL_1438398 (CHEMBL3383627) Inhibition of catalytically active human SIRT3 (102 to 399 amino acids) expressed in Escherichia coli BL21 (DE3) cells using fluorogenic 7-amino-4-methylcoumarin (AMC)-labeled peptide by fluorescence assay
- ChEMBL_1826717 (CHEMBL4326591) Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ATP by ADP-Glo luminescence assay
- ChEMBL_1926625 (CHEMBL4429697) Inhibition of recombinant human C-terminal 6His-tagged JAK2 (808 to end amino acids) expressed in Sf21 cells measured after 1 hr in presence of ATP by TR-FRET assay
- ChEMBL_2253497 (CHEMBL5167707) Agonist activity at human CLpP (57 to 277 amino acids) expressed in Escherichia coli using fluorogenic peptide AC-WLA-AMC as substrate incubated for 10 mins by fluorescence based assay
- ChEMBL_2263838 Agonist activity at human recombinant CLpP (57 to 277 amino acids) expressed in Escherichia coli using fluorogenic peptide AC-WLA-AMC as substrate incubated for 10 mins by fluorescence based assay
- ChEMBL_1438404 (CHEMBL3383633) Inhibition of catalytically active human SIRT3 (102 to 399 amino acids) expressed in Escherichia coli BL21 (DE3) cells using (Ac)RHKK(Ac)-AMC substrate by fluorescence assay based enzyme kinetic analysis
- ChEMBL_1457123 (CHEMBL3369467) Inhibition of c-terminal 6xHis-tagged Bcl-2 (amino acids 1 to 204) (unknown origin) preincubated for 1 hr prior to substrate addition measured after 20 mins by fluorescence polarization assay
- ChEMBL_1457124 (CHEMBL3369468) Inhibition of c-terminal 6xHis-tagged Bcl-xL (amino acids 1 to 209) (unknown origin) preincubated for 1 hr prior to substrate addition measured after 20 mins by fluorescence polarization assay
- ChEMBL_1619576 (CHEMBL3861745) Inhibition of N-terminal GST tagged human TrkA cytoplasmic domain (436 to 790 amino acids) pre-incubated for 15 mins before peptide substrate addition for 180 mins by fluorescence based assay
- ChEMBL_1619577 (CHEMBL3861746) Inhibition of N-terminal GST tagged human TrkB cytoplasmic domain (456 to 822 amino acids) pre-incubated for 15 mins before peptide substrate addition for 180 mins by fluorescence based assay
- ChEMBL_1759876 (CHEMBL4194884) Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by bromodomain TR-FRET binding assay
- ChEMBL_1799155 (CHEMBL4271447) Inhibition of human recombinant CDK9/cyclin T expressed in insect cells using pRb fragment (773 to 928 amino acids) and [gamma-33P]ATP incubated fro 30 mins by scintillation counting analysis
- ChEMBL_965921 (CHEMBL2394352) Inhibition of human recombinant CLK1 (148 to 484 amino acids) expressed in Escherichia coli BL21(DE3) using AFRREWSPGKEAKK as substrate preincubated for 10 mins prior to substrate addition by LDH assay
- ChEMBL_966838 (CHEMBL2400839) Displacement of [3H]SP1-7 from substance P receptor (1 to 7 amino acids) binding site in Sprague-Dawley rat spinal cord membranes after 60 mins by liquid scintillation counting analysis
- ChEMBL_1453678 (CHEMBL3363784) Binding affinity to human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using biotin-tagged compound immobilized on neutravidin after 9 mins by SPR assay
- ChEMBL_1523511 (CHEMBL3631015) Inhibition of N-terminally GST- tagged human DLK catalytic domain (1 to 520 amino acids) using N-terminally HIS-tagged MKK4 K131M as substrate incubated for 60 mins by TR-FRET assay
- ChEMBL_1905311 (CHEMBL4407669) Inhibition of GST-tagged recombinant human DDR1 (440 to 876 amino acids) expressed in baculovirus using fluorescein-poly GAT as substrate after 1 hr by FRET-based LanthaScreen Eu kinase binding assay
- ChEBML_1691957 Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for 20 followed by substrate addition measured for 25 mins by FRET analysis
- ChEMBL_1434484 (CHEMBL3388870) Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli assessed as reduction in H2O2 release using synthetic mono-methylated H3-K4 peptide (21 amino acids) substrate by horseradish peroxidase coupled enzyme assay
- ChEMBL_863264 (CHEMBL2176207) Inhibition of human recombinant SIRT2 expressed in Escherichia coli cells using acetylated Lys side chain amino acids 379-382 (Arg-His-Lys-Lys(Ac)) p53 conjugated with aminomethylcoumarin as substrate by fluorescence assay
- ChEMBL_863265 (CHEMBL2176208) Inhibition of human recombinant SIRT1 expressed in Escherichia coli cells using acetylated Lys side chain amino acids 379-382 (Arg-His-Lys-Lys(Ac)) p53 conjugated with aminomethylcoumarin as substrate by fluorescence assay
- ChEMBL_1438405 (CHEMBL3383634) Inhibition of catalytically active human SIRT3 (102 to 399 amino acids) expressed in Escherichia coli BL21 (DE3) cells using (Ac)RHKK(Ac)-AMC substrate by fluorescence assay based Morrison's equation based enzyme kinetic analysis
- ChEMBL_1691957 (CHEMBL4042606) Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for 20 followed by substrate addition measured for 25 mins by FRET analysis
- ChEMBL_1691958 (CHEMBL4042607) Uncompetitive inhibition of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for 20 followed by substrate addition measured for 25 mins by Lineweaver-Burk plot analysis
- ChEMBL_1798921 (CHEMBL4271213) Inhibition of 6x-His-tagged human EGFR cytoplasmic domain (645 to 1186 amino acids) assessed as reduction in autophosphorylation pre-incubated for 10 mins and measured after 1 hr by DELFIA/time-resolved fluorometry
- ChEMBL_772464 (CHEMBL1839255) Agonist activity at human amino-terminal polyhistidine-tagged FXR alpha LBD (amino acids 237 to 472) assessed as cofactor peptide SRC-1 interaction with receptor ligand binding domain after 2 hrs by FRET assay
- ChEMBL_1335833 (CHEMBL3239942) Inhibition of human coronavirus NL63 PLP2 (amino acids 1565 to 1894) expressed in Escherichia coli BL21 (DE3) cells assessed as reduction of AMC release using Z-RLRGG-AMC as substrate by multimode plate reader analysis
- ChEMBL_1476207 (CHEMBL3429393) Inhibition of PTP1B catalytic domain (1 to 321 amino acids) (unknown origin) expressed in Escherichia coli Rosetta (DE3) incubated for 30 mins followed by UV irradiation with 365 nm for 45 mins by pNPP assay
- ChEMBL_1798716 (CHEMBL4271008) Inhibition of wild type N-terminal GST tagged human recombinant EGFR (695 to end amino acids) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Glu, Tyr) Peptide substrate by ADP-Glo assay
- ChEMBL_1860111 (CHEMBL4360967) Displacement of Alexafluor labelled kinase tracer314 from recombinant human C-terminal GST-tagged mTOR (1360 to 2549 amino acids) expressed in insect cells incubated for 1 hr by FRET-based LanthaScreen Eu kinase binding assay
- ChEMBL_816345 (CHEMBL2024743) Inhibition of N-terminal hexa-histidine tagged human cloned Eg5 (1 to 368 amino acids) expressed in Escherichia coli BL21 (DE3) assessed as reduction in basal ATPase activity by pyruvate kinase/lactate dehydrogenase-linked assay
- ChEMBL_1438406 (CHEMBL3383635) Competitive inhibition of catalytically active human SIRT3 (102 to 399 amino acids) expressed in Escherichia coli BL21 (DE3) cells using varying (Ac)RHKK(Ac)-AMC substrate by fluorescence assay based Morrison's equation based enzyme kinetic analysis
- ChEMBL_1465147 (CHEMBL3405660) Inhibition of human recombinant SIRT5 isoform 1 (34 to 302 amino acids) expressed in Escherichia coli BL21 (DE3) codon plus RIL cells assessed as reduction in deacetylase activity using acetylated chicken histone substrate and [3H]-NAD+
- ChEMBL_1476206 (CHEMBL3429392) Inhibition of PTP1B catalytic domain (1 to 321 amino acids) (unknown origin) expressed in Escherichia coli Rosetta (DE3) incubated for 30 mins in absence of UV irradiation with 365 nm for 45 mins by pNPP assay
- ChEMBL_1476965 (CHEMBL3430220) Inhibition of GST-tagged human recombinant EGFR catalytic domain (668 to 1210 amino acids) L858R mutant expressed in Sf9 cells pre-incubated for 30 mins before ATP and substrate addition by homogeneous time-resolved FRET assay
- ChEMBL_1798715 (CHEMBL4271007) Inhibition of N-terminal GST tagged human recombinant EGFR L858R/T790M mutant (695 to end amino acids) expressed in baculovirus infected Sf9 insect cells using Poly (4:1 Glu, Tyr) Peptide substrate by ADP-Glo assay
- ChEMBL_1806397 (CHEMBL4305756) Displacement of tracer 222 from GST-tagged recombinant human AAK1 catalytic domain (1 to 510 amino acids) expressed in insect cells shaken for 30 secs and incubated for 1 hr by LanthaScreen Eu kinase binding assay
- ChEMBL_1970699 (CHEMBL4603517) Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 amino acids) expressed in baculovirus expression system using ERM (LRRKtide) peptide as substrate incubated for 60 mins in presence of ATP by Adapta assay
- ChEMBL_816346 (CHEMBL2024744) Inhibition of N-terminal hexa-histidine tagged human cloned Eg5 (1 to 368 amino acids) expressed in Escherichia coli BL21 (DE3) assessed as reduction of MT-stimulated ATPase activity by pyruvate kinase/lactate dehydrogenase-linked assay
- ChEMBL_1476966 (CHEMBL3430221) Inhibition of GST-tagged human recombinant EGFR catalytic domain (668 to 1210 amino acids) T790M/L858R mutant expressed in Sf9 cells pre-incubated for 30 mins before ATP and substrate addition by homogeneous time-resolved FRET assay
- ChEMBL_1499748 (CHEMBL3585197) Inhibition of ROCK1 (6 to 553 amino acids) (unknown origin) using Lys-Lys-Arg-Asn-Arg-Thr-Leu-Ser-Val as substrate preincubated for 15 mins followed by ATP addition by pyruvate kinase/lactate dehydrogenase coupled assay
- ChEMBL_1579231 (CHEMBL3812311) Inhibition of N-terminal GST-tagged recombinant human PDGFRbeta (amino acids 557-end) expressed in baculovirus infected insect Sf9 cells using Poly(4:1 Glu, Tyr) peptide as substrate after 1 hr by promega ADP-glo assay
- ChEMBL_1806441 (CHEMBL4305800) Inhibition of C-terminal recombinant human ClpP (57 to 277 amino acids) expressed in Escherichia coli Rosetta2 cells using Suc-Leu-Tyr-AMC as substrate incubated for 1 hr and measured for 90 mins by fluorescence assay
- ChEMBL_1873666 (CHEMBL4374955) Inhibition of bacterial N-terminal His-tagged TEV protease site linked TEM-1 (24 to 286 amino acids) expressed in Escherichia coli Transetta (DE3) preincubated for 10 mins followed by FC5 fluorescent substrate addition by fluorescence assay
- ChEMBL_1873903 (CHEMBL4375192) Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate incubated for 90 mins by microfluidic off-Chip Mobility Shift Assay
- ChEMBL_1874103 (CHEMBL4375392) Inhibition of integrin alphavbeta1 (unknown origin) expressed in human A549 cells assessed as reduction in cell adhesion to GST-LAP1 (242 to 252 amino acids) incubated for 30 mins by fluorescent probe BCECF-AM based fluorescence assay
- ChEMBL_1874104 (CHEMBL4375393) Inhibition of integrin alphavbeta3 (unknown origin) expressed in human K562 cells assessed as reduction in cell adhesion to GST-LAP1 (242 to 252 amino acids) incubated for 30 mins by fluorescent probe BCECF-AM based fluorescence assay
- ChEMBL_1874106 (CHEMBL4375395) Inhibition of integrin alphavbeta8 (unknown origin) expressed in human K562 cells assessed as reduction in cell adhesion to GST-LAP1 (242 to 252 amino acids) incubated for 30 mins by fluorescent probe BCECF-AM based fluorescence assay
- ChEMBL_1874107 (CHEMBL4375396) Inhibition of integrin alphavbeta6 (unknown origin) expressed in human K562 cells assessed as reduction in cell adhesion to GST-LAP1 (242 to 252 amino acids) incubated for 30 mins by fluorescent probe BCECF-AM based fluorescence assay
- ChEMBL_1467488 (CHEMBL3411111) Inhibition of human recombinant KDM1A/CoREST complex expressed in Escherichia coli using mono-methylated H3-K4 peptide containing 21 amino acids as substrate preincubated for 15 mins followed by substrate addition measured for 12 mins by fluorescence assay
- ChEMBL_1472393 (CHEMBL3420114) Inhibition of hexahistidine-tagged human recombinant ARTD8 catalytic domain (1611 to 1801 amino acids) expressed in Escherichia coli BL21(DE3) assessed as inhibition of ADP-ribosyltransferase activity incubated for 15 mins using biotin-NAD+ by chemiluminescence detection based assay
- ChEMBL_1472394 (CHEMBL3420115) Inhibition of hexahistidine-tagged human recombinant ARTD7 catalytic domain (459 to 656 amino acids) expressed in Escherichia coli BL21(DE3) assessed as inhibition of ADP-ribosyltransferase activity incubated for 15 mins using biotin-NAD+ by chemiluminescence detection based assay
- ChEMBL_1496877 (CHEMBL3580357) Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity using NAD+ by chemiluminescence detection based assay
- ChEMBL_2050770 (CHEMBL4705469) Inhibition of N-terminal GST tagged wild-type human ALK cytoplasmic domain (1058-1620 amino acids) expressed Sf9 cells pre-incubated for 30 mins before addition of Ulight-CKKSRGDYMTMQIG substrate and measured after 90 mins by fluorescence based assay
- ChEMBL_785410 (CHEMBL1919789) Inhibition of C-terminal His6-tagged human DNA polymerase eta (amino acids 1 to 511) using poly(dA)/oligo(dT)18 (A/T = 2/1) and dTTP as the DNA template-primer and nucleotide substrate after 60 mins
- ChEMBL_785412 (CHEMBL1919791) Inhibition of C-terminal His6-tagged human DNA polymerase kappa (amino acids 1 to 560) using poly(dA)/oligo(dT)18 (A/T = 2/1) and dTTP as the DNA template-primer and nucleotide substrate after 60 mins
- ChEMBL_787970 (CHEMBL1918904) Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated for 1 hrs before substrate addition measured after 15 mins by fluorimetry
- ChEMBL_787971 (CHEMBL1918905) Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated for 1 hrs before substrate addition measured after 15 mins by fluorimetry
- ChEMBL_787972 (CHEMBL1918906) Inhibition of human MMP1 catalytic domain (amino acids 100 to 262) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated for 1 hrs before substrate addition measured after 15 mins by fluorimetry
- ChEMBL_787973 (CHEMBL1918907) Inhibition of human MMP3 catalytic domain (amino acids 100 to 265) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated for 1 hrs before substrate addition measured after 15 mins by fluorimetry
- ChEMBL_787974 (CHEMBL1918908) Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated for 1 hrs before substrate addition measured after 15 mins by fluorimetry
- ChEMBL_1438407 (CHEMBL3383636) Non-competitive inhibition of catalytically active human SIRT3 (102 to 399 amino acids) expressed in Escherichia coli BL21 (DE3) cells using (Ac)RHKK(Ac)-AMC substrate and varying NAD+ levels by fluorescence assay based Morrison's equation based enzyme kinetic analysis
- ChEMBL_1476209 (CHEMBL3429395) Inhibition of PTP1B catalytic domain (1 to 321 amino acids) (unknown origin) expressed in Escherichia coli Rosetta (DE3) incubated for 30 mins followed by UV irradiation with 365 nm for 45 mins in presence of 1 mM DTT by pNPP assay
- ChEMBL_938233 (CHEMBL2328536) Inhibition of C-terminal His-tagged human recombinant HDAC9 (amino acids 604- 1066) expressed in baculovirus expression system using fluorogenic acetylated peptide as substrate preincubated with enzyme for 10 mins prior to substrate addition measured after 60 mins by fluorescence analysis
- ChEMBL_966729 (CHEMBL2400289) Inhibition of GST-tagged Aurora kinase A catalytic domain (123 to 401 amino acids) (unknown origin) expressed in sf9 cells using tetra(LRRWSLG) as substrate preincubated for 15 mins prior to substrate addition measured after 90 mins by luminescence assay
- Ligand-Induced Uptake of [35S]-GTP-gamma-S EC50s were determined by measuring the binding of [35S]-GTP-gamma-S to S1P receptors expressed in transfected Chinese hamster ovary (CHO) cell membranes. It is found that these modified 3-arylpropionic acids appeared to be more potent when evaluated in the functional assay than in the competitive binding assay, and their EC50 values served as a better guide for interpreting in vivo activity. All 3-arylpropionic acids and their modified analogs are found to be inactive against S1P2,4 receptors (IC50 > 10 uM).
- ChEMBL_1363643 (CHEMBL3293161) Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH2 as substrate incubated for 15 mins prior to ATP addition measured after 1 hr by microfluidic mobility shift assay
- ChEMBL_1363644 (CHEMBL3293162) Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH2 as substrate incubated for 15 mins prior to ATP addition measured after 1 hr by microfluidic mobility shift assay
- ChEMBL_1476208 (CHEMBL3429394) Inhibition of PTP1B catalytic domain (1 to 321 amino acids) (unknown origin) expressed in Escherichia coli Rosetta (DE3) incubated for 30 mins in absence of UV irradiation with 365 nm for 45 mins and in presence of 1 mM DTT by pNPP assay
- ChEMBL_1843891 (CHEMBL4344318) Inhibition of N-terminal His6-tagged human Bcl-xL (with internal deletion of 45 to 85 amino acids and C-terminal truncation for 212 to 233 residues) expressed in Escherichia coli Rosetta2 DE3 by fluorescent labeled FAM-Bid peptide based fluorescence polarization assay
- ChEMBL_1873760 (CHEMBL4375049) Inhibition of human C-terminal His-tagged and N-terminal GST-tagged EGFR L858R/T790M/C797S triple mutant ( 668 to 1210 amino acids) expressed in baculovirus infected Sf9 insect cells using Poly(Glu,Tyr) 4:1 as substrate after 60 mins by ELISA
- ChEMBL_1995343 (CHEMBL4629238) Inhibition of N-terminal GST-tagged human FGFR3 cytoplasmic domain (436-806 end amino acids residues) expressed in baculovirus expression system preincubated for 30 to 120 mins followed by incubation with substrate and ATP for 30 mins by off-chip mobility shift assay
- ChEMBL_1995346 (CHEMBL4629241) Inhibition of N-terminal GST-tagged human FGFR1 cytoplasmic domain (398-822 end amino acids residues) expressed in baculovirus expression system preincubated for 30 to 120 mins followed by incubation with substrate and ATP for 30 mins by off-chip mobility shift assay
- ChEMBL_1995347 (CHEMBL4629242) Inhibition of N-terminal GST-tagged human FGFR2 cytoplasmic domain (399-821 end amino acids residues) expressed in baculovirus expression system preincubated for 30 to 120 mins followed by incubation with substrate and ATP for 30 mins by off-chip mobility shift assay
- ChEMBL_1995348 (CHEMBL4629243) Inhibition of N-terminal GST-tagged human FGFR4 cytoplasmic domain (460-802 end amino acids residues) expressed in baculovirus expression system preincubated for 30 to 120 mins followed by incubation with substrate and ATP for 30 mins by off-chip mobility shift assay
- ChEMBL_2105715 (CHEMBL4814390) Inhibition of N-terminal hexahistidine-tagged human LSD1 (1 to 852 amino acids) expressed in Escherichia coli BL21 (DE3) cells using H3K4me2 peptide as substrate preincubated for 5 mins followed by substrate addition and measured for 30 mins by peroxidase-coupled method
- Enzyme Assay ATR for use in the in vitro enzyme assay was obtained from HeLa nuclear extract (CIL Biotech, Mons, Belgium) by immunoprecipitation with rabbit polycolonal antiserum raised to amino acids 400-480 of ATR (Tibbetts R S et, al, 1999, GenesDev.13:152-157).
- ChEMBL_1576591 (CHEMBL3807386) Inhibition of wild type His-tagged c-KIT (544 to 976 amino acids) (unknown origin) expressed in insect cells using poly[Glu:Tyr] (4:1) as substrate preincubated for 20 mins followed by [33P-gamma]ATP addition and subsequent inhibition for 2 hrs by radiometric assay
- ChEMBL_1576592 (CHEMBL3807387) Inhibition of N-terminal GST-His-tagged c-KIT (544 to 976 amino acids) D816V mutant (unknown origin) expressed in Sf9 insect cells using poly[Glu:Tyr] (4:1) as substrate preincubated for 20 mins followed by [33P-gamma]ATP addition and subsequent inhibition for 2 hrs by radiometric assay
- LRRK2 Enzymatic Assay LRRKtide substrate (peptide sequence RLGRDKYKTLRQIRQ, derived from human ezrin [amino acids 561-573], moesin [amino acids 539-553] and radixin [amino acids 558-570], obtained from SignalChem, catalogue #L10-58, reconstituted in 20 mM Tris-HCl at pH 7.5 to a final concentration of 1 mg/mL, assay concentration 20 μM) and recombinant human LRRK2 (catalytic domain only [amino acids 970-2527], GST-tagged, expressed in insect cells, obtained from ThermoFisher Scientific, catalogue #PV4874, 0.35 mg/mL, assay concentration 30 nM) were mixed in assay buffer (20 mM Hepes pH 7.5, 10 mM MgCl, 1 mM EGTA, 0.02% Brij35, 0.02 mg/ml BSA, 0.1 mM NaVO, 2 mM DTT, 1% DMSO). Compounds of interest (in DMSO, serial 3-fold dilution from 10 μM to 0.5 nM) or control (1% DMSO) were dispensed into the kinase reaction mixture by Acoustic technology (Echo550; nanoliter range). After incubation at room temperature for 20 minutes, the kinase reaction was initiated by addition of [P]-ATP (Specific activity 10 μCi/μl) and the mixture was incubated at room temperature for 2 hours. The reaction was then stopped by spotting the reaction mixture on strips of phosphocellulose P81 paper. Following washing, the radioactivity of the P81 paper was measured and kinase activity data were expressed as the percent remaining kinase activity in test samples compared to vehicle (dimethyl sulfoxide) reactions.
- TR-FRET Assay Ten nanomolar of 6× Histidine-tagged BIR2 domain, corresponding to amino acids 124-240 of XIAP, or BIR3 domain, corresponding to amino acids 241-356 of XIAP, was mixed with 20 nM of the peptide AVPIAQKSEK-(ε-biotin)-OH 1:2 TFA, in the presence of 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 1 mM dithiothreitol (DTT) and 0.1 mg/mL bovine serum albumin (BSA). Following a 45 min. incubation at 37° C., Europium-Streptavidin and Allophycocyanin conjugated anti-Histidine antibody were added to a final concentration of 1.5 nM and 15 nM, respectively. Time-resolved fluorescence resonance energy transfer (TR-FRET) signals were measured 1 hour later at room temperature.
- FAS Inhibitory Assay To each microtube (final volume: 100 μL), FAS was added (20-30 μg protein) in a buffer containing 100 mM potassium phosphate (pH 7.0), 2.5 mM dithiothreitol and 2.0 mM EDTA with or without test compounds. The mixtures were preincubated at 37°C for 60 min and the reaction was started by the addition of a substrate mixture containing 0.25 mM NADPH, 0.4 nmol of malonyl CoA and 0.02 μCi of [3H] acetyl CoA. Following incubation at 37°C for 10 min, the reaction was terminated with 60% HClO4. Fatty acids were extracted with 1 mL of hexane and incorporation of radioactivity into the fatty acids was assessed by scintillation counting [Na et al., Bioorg. Med. Chem. Lett., 16:4738-4742].
- Pseudotyped HCV particle (HCVpp) reporter assay Plasmids expressing HCV E1 and E2 envelope proteins of GT1a H77 strain (Proc Natl Acad Sci USA 1997 94:8738-43) or GT1b Con1 strain (Science 1999 285:110-3) were constructed by cloning the nucleic acids encoding the last 60 amino acids of HCV core protein and all of the HCV E1 and E2 proteins into pcDNA3.1(+) vector. Plasmid pVSV-G expressing the glycoprotein G of the vesicular stomatitis virus (VSV G) is from Clontech (cat #631530). The HIV packaging construct expressing the firefly luciferase reporter gene was modified based on the envelope defective pNL.4.3.Luc-R .E vector (Virology 1995 206:935-44) by further deleting part of the HIV envelope protein.
- TR-FRET Assay Ten nanomolar of 6x Histidine-tagged BIR2 domain, corresponding to amino acids 124-240 of XIAP, or BIR3 domain, corresponding to amino acids 241-356 of XIAP, was mixed with 20 nM of the peptide AVPIAQKSEK-(8-biotin)-OH 1:2 TFA, in the presence of 50 mM Tris-C1, pH 7.5, 100 mM NaCl, 1 mM dithiothreitol (DTT) and 0.1 mg/mL bovine serum albumin (BSA). Following a 45 min. incubation at 37° C., Europium-Streptavidin and Allophycocyanin conjugated anti-Histidine antibody were added to a final concentration of 1.5 nM and 15 nM, respectively. Time-resolved fluorescence resonance energy transfer (TR-FRET) signals were measured 1 hour later at room temperature. Test compound potency was assessed at 10 serially diluted concentrations.
- Release Assay SH-SY5Y cells were cultured in DMEM/F-12 with Glutamax, 10% FCS and 1% non-essential amino acids and cryopreserved and stored at -140 C. at a concentration of 7.5-9.5x106 cells per vial. Thaw cells and seed at a conc. of around 10000 cells/well in DMEM/F-12 with Glutamax, 10% FCS and 1% non-essential amino acids to a 384-well tissue culture treated plate, 100 uL cell susp/well. The cell plates were then incubated for 7-24 h at 37 C., 5% CO2. The cell medium was removed, followed by addition of 30 uL compound diluted in DMEM/F-12 with Glutamax, 10% FCS, 1% non-essential amino acids and 1% PeSt to a final conc. of 1% DMSO. The compounds were incubated with the cells for 17 h (overnight) at 37 C., 5% CO2. Meso Scale Discovery (MSD) plates were used for the detection of sAPPbeta release. MSD sAPPbeta plates were blocked in 1% BSA in Tris wash buffer (40 uL/well) for 1 h on shake at r.t. and washed 1 time in Tris wash buffer (40 uL/well).
- Release Assay SH-SY5Y cells were cultured in DMEM/F-12 with Glutamax, 10% FCS and 1% non-essential amino acids and cryopreserved and stored at -140 C. at a concentration of 7.5-9.5x106 cells per vial. Thaw cells and seed at a conc. of around 10000 cells/well in DMEM/F-12 with Glutamax, 10% FCS and 1% non-essential amino acids to a 384-well tissue culture treated plate, 1004, cell susp/well. The cell plates were then incubated for 7-24 h at 37 C., 5% CO2. The cell medium was removed, followed by addition of 30 uL compound diluted in DMEM/F-12 with Glutamax, 10% FCS, 1% non-essential amino acids and 1% PeSt to a final conc. of 1% DMSO. The compounds were incubated with the cells for 17 h (overnight) at 37 C., 5% CO2. Meso Scale Discovery (MSD) plates were used for the detection of sAPPbeta release. MSD sAPPbeta plates were blocked in 1% BSA in Tris wash buffer (40 uL/well) for 1 h on shake at r.t. and washed 1 time in Tris wash buffer (40 uL/well).
- SOS-Catalyzed Nucleotide Exchange Assay (version 1) Recombinant KRAS G12C (amino acids 1-169, SEQ ID NO:1), KRAS G12D (amino acids 1-169, SEQ ID NO:2), KRAS G12V (amino acids 1-169, SEQ ID NO:3) and SOS1 (amino acids 564-1049, SEQ ID NO:4) proteins were expressed in E. coli and purified by affinity chromatography. To prepare each BODIPY FL GDP-bound KRAS mutant protein, 50 uM KRAS mutant proteins were incubated with 0.5 mM BODIPY FL GDP (Invitrogen, G22360) in a loading buffer (20 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT and 2.5 mM EDTA) for 1 hour on ice. After the incubation, MgCl2 was added to a final concentration of 10 mM, followed by incubation at room temperature for 30 minutes. The mixtures were allowed to pass through a NAP-5 column to remove free nucleotides and purified BODIPY FL GDP-bound KRAS G12C, G12D and G12V proteins were used for compound evaluation. For the measurement of the inhibitory activity of compounds on GDP-GTP exchange rate of recombinant KRAS mutants, each BODIPY FL GDP-bound KRAS mutant protein whose concentration is 25 nM was incubated with various concentrations of compound in a reaction buffer (20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM MgCl2, 2 mM DTT, 0.1% Tween 20) at 25 C. for 1 hour. After the incubation, recombinant SOS1 and GMPPNP (Jena Bioscience GmbH, NU-401) were added and incubated at room temperature for 30 minutes to proceed SOS1-dependent GDP-GTP exchange reaction on KRAS mutants.
- SOS-Catalyzed Nucleotide Exchange Assay (version 2) Recombinant KRAS G12C (amino acids 1-169, SEQ ID NO:1), KRAS G12D (amino acids 1-169, SEQ ID NO:2), KRAS G12V (amino acids 1-169, SEQ ID NO:3) and SOS1 (amino acids 564-1049, SEQ ID NO:4) proteins were expressed in E. coli and purified by affinity chromatography. To prepare each BODIPY FL GDP-bound KRAS mutant protein, 50 uM KRAS mutant proteins were incubated with 0.5 mM BODIPY FL GDP (Invitrogen, G22360) in a loading buffer (20 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT and 2.5 mM EDTA) for 1 hour on ice. After the incubation, MgCl2 was added to a final concentration of 10 mM, followed by incubation at room temperature for 30 minutes. The mixtures were allowed to pass through a NAP-5 column to remove free nucleotides and purified BODIPY FL GDP-bound KRAS G12C, G12D and G12V proteins were used for compound evaluation. For the measurement of the inhibitory activity of compounds on GDP-GTP exchange rate of recombinant KRAS mutants, each BODIPY FL GDP-bound KRAS mutant protein whose concentration is 2.5 nM was incubated with various concentrations of compound in a reaction buffer (20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM MgCl2, 2 mM DTT, 0.1% Tween 20) at 25 C. for 1 hour. After the incubation, recombinant SOS1 and GMPPNP (Jena Bioscience GmbH, NU-401) were added and incubated at room temperature for 30 minutes to proceed SOS1-dependent GDP-GTP exchange reaction on KRAS mutants.
- ChEMBL_1570257 (CHEMBL3795199) Binding affinity to FKBP51 FK506-binding domain (1 to 140 amino acids) (unknown origin) incubated for 30 mins using fluorescein-conjugated 2-(5-((2-(3-((R)-3-(3,4-dimethoxyphenyl)-1-((S)-1-(3,3-dimethyl-2-oxopentanoyl)piperidine-2-carbonyloxy)propyl)phenoxy)acetamido)methyl)-6-hydroxy-3-oxo-3H-xanthen-9-yl)benzoic acid by competitive fluorescence polarization assay
- TR-FRET Assay Ten nanomolar of 6× Histidine-tagged BIR2 domain, corresponding to amino acids 124-240 of XIAP, or BIR3 domain, corresponding to amino acids 241-356 of XIAP, was mixed with 20 nM of the peptide AVPIAQKSEK-(ε-biotin)-OH 1:2 TFA, in the presence of 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 1 mM dithiothreitol (DTT) and 0.1 mg/mL bovine serum albumin (BSA). Following a 45 min. incubation at 37° C., Europium-Streptavidin and Allophycocyanin conjugated anti-Histidine antibody were added to a final concentration of 1.5 nM and 15 nM, respectively. Time-resolved fluorescence resonance energy transfer (TR-FRET) signals were measured 1 hour later at room temperature. Test compound potency was assessed at 10 serially diluted concentrations.
- Binding Assay The murine STING [3H] 2′,3′-cGAMP scintillation proximity competition binding assay was performed in the same manner as the human STING competition binding assay (described above) with the exception that murine STING protein (amino acids 154-378, SEQ ID NO: 3) was utilized. 2′,2′-cGAMP was used as a reference compound and had an IC50 of 0.055 μM.
- Inhibition Assay Jak2: Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 10 μM Omnia Y7 peptide (Catalog # IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 4 nM Jak2 in a total volume of 20 μL. Human Jak2 kinase domain comprising amino acids 808-1132.
- Enzyme Inhibition Assay Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino acids 831-1132, AstraZeneca R&D Boston), or JAK3 (amino acids 781-1124, AstraZeneca R&D Boston) under buffer conditions of 50 mM HEPES pH 7.3, 1 mM DTT, 0.01% Tween-20, 50 μg/ml BSA, and 10 mM MgCl2. JAK enzyme was expressed as N-terminal GST fusion in insect cells and purified by glutathione-affinity and size-exclusion chromatographies. Enzymes were assayed at their approximated high end of physiological ATP concentration of 5 mM, in the presence of inhibitor dosed at 30, 3, 0.3, 0.03, 0.003 and 0 μM final test concentrations.For JAK1, 4 nM of enzyme was incubated with 1.5 μM peptide substrate (FITC-C6-KKHTDDGYMPMSPGVA-NH2 (SEQ ID NO:1), Intonation, Boston, Mass.). For JAK2, 0.3 nM enzyme was incubated with 1.5 μM peptide substrate (5FAM-GEEPLYWSFPAKKK-NH2 (SEQ ID NO:2), Intonation, Boston, Mass.), For JAK3, 0.1 nM enzyme was incubated with 1.5 μM peptide substrate (5FAM-GEEPLYWSFPAKKK-NII2 (SEQ ID NO:2), Intonation, Boston, Mass.). Phosphorylated and unphosphotylated peptides were separated and quantified by a Caliper LC3000 system (Caliper Life Sciences, MA) for calculating percent inhibition.
- Time Resolved Fluorescence Energy Transfer (TR-FRET) Assay The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfer (or Foerster resonance energy transfer) describes an energy transfer between donor and acceptor fluorescent molecules. For this assay, human MDM2 protein (amino acids 2-188) and human MDM4 protein (amino acids 2-185), tagged with a C-terminal biotin moiety, are used in combination with a Europium labeled streptavidin (Perkin Elmer, Inc., Waltham, MA, USA) serving as the donor fluorophore. The p53 derived, Cy5 labeled peptide Cy5-TFSDLWKLL (p53 aa18-26) is the energy acceptor. Upon excitation of the donor molecule at 340nm, binding interaction between MDM2 or MDM4 and the p53 peptide induces energy transfer and enhanced response at the acceptor emission wavelength at 665 nm.
- Time Resolved Fluorescence Energy Transfer (TR-FRET) Assay The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfer (or Foerster resonance energy transfer) describes an energy transfer between donor and acceptor fluorescent molecules. For this assay, human MDM2 protein (amino acids 2-188) and human MDM4 protein (amino acids 2-185), tagged with a C-terminal biotin moiety, are used in combination with a Europium labeled streptavidin (Perkin Elmer, Inc., Waltham, Mass., USA) serving as the donor fluorophore. The p53 derived, Cy5 labeled peptide Cy5-TFSDLWKLL (p53 aa18-26) is the energy acceptor. Upon excitation of the donor molecule at 340 nm, binding interaction between MDM2 or MDM4 and the p53 peptide induces energy transfer and enhanced response at the acceptor emission wavelength at 665 nm.
- Inhibition Assay Jak1: Compounds of Formula I were screened for their ability to inhibit Jak1 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 8 μM Omnia Y12 peptide (Catalog # IVGN KPZ3121C; Invitrogen Corporation, Carlsbad, Calif.) and 5 nM Jak1 in a total volume of 20 μL. GST-tagged human Jak1 kinase domain comprising amino acids 866-1154.
- Inhibition Assay Jak3: Compounds of Formula I were screened for their ability to inhibit Jak3 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 10 μM Omnia Y7 peptide (Catalog # IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 1.5 nM Jak3 in a total volume of 20 μL. GST-tagged human Jak3 kinase domain comprising amino acids 781-1124
- SOS1 GDP TR-FRET Assay This assay was used to identify compounds which competitively interact with the binding of KRAS protein to SOS1 in the presence of GDP. GST-tagged WT KRAS (amino acids 1-188), GST-tagged KRAS (amino acids 1-188) G12D and His- tagged SOS1 protein (amino acids 564-1049) was expressed in E. coli and purified. All protein and reaction solutions were prepared in assay buffer containing DPBS pH7.5, 0.1% BSA, and 0.05% Tween 20. Purified GST-tagged WT KRAS or KRAS G12D protein (37.5 nM final assay concentration) and GDP (Sigma, 10 μM final assay concentration) mixture, a serially diluted compound (top final concentration is 50 uM or 10 uM, 3-fold serially diluted, 10 points) and His-tagged SOS1 protein (18 nM final assay concentration) were added into the assay plates (384 well microplate, black, Corning) sequentially. Plates are incubated at 24° C. for 1 hr. Following the incubation, Mab Anti-6His-Tb cryptate (Cisbio) and Mab Anti GST-D2 (Cisbio) were added and further incubated at 24° C. for another 1 hr. The TR-FRET signals (ex337nm, em665nm/620nm) were read on BMG PHERAstar FSX instrument. The inhibition percentage of KRAS protein binding with SOS1 in presence of increasing concentrations of compounds was calculated based on the ratio of fluorescence at 665 nm to that at 620 nm.
- TR-FRET Assay for BIR2 and BIR3 Ten nanomolar of 6× Histidine-tagged BIR2 domain, corresponding to amino acids 124-240 of XIAP, or BIR3 domain, corresponding to amino acids 241-356 of XIAP, was mixed with 20 nM of the peptide AVPIAQKSEK-(c-biotin)-OH 1:2 TFA, in the presence of 50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 1 mM dithiothreitol (DTT) and 0.1 mg/mL bovine serum albumin (BSA). Following a 45 min. incubation at 37° C., Europium-Streptavidin and Allophycocyanin conjugated anti-Histidine antibody were added to a final concentration of 1.5 nM and 15 nM, respectively. Time-resolved fluorescence resonance energy transfer (TR-FRET) signals were measured 1 hour later at room temperature. Test compound potency was assessed at 10 serially diluted concentrations. Percentage of inhibition at each concentration was determined to generate an IC50 value for each test compound.
- Tet-Inducible cAMP Assay A human-mouse chimeric GPR119 expression construct encoding 3 copies of the FLAG epitope tag, the first 198 amino acids of human GPR119 and the C-terminal 137 amino acids of the mouse receptor was cloned into a tetracycline inducible vector pcDNA5/FRT/TO (Invitrogen #V6520-20), which includes a hygromycin-resistance marker. Tightly controlled receptor expression was achieved by stable integration of this construct into the genome of a specific host cell line, Flp-In-T-Rex-HEK293, expressing the tetracycline repressor (Invitrogen). Once a stable hygromycin-resistant cell line was generated, the cells were maintained at 37° C. in a humidified 5% CO2 atmosphere in culture medium consisting of Dulbecco's modified Eagle's medium (DMEM; Invitrogen #11960) supplemented with 2 mM L-glutamine, 10% fetal bovine serum, 200 μg/ml hygromycin B, and 15 μg/ml blasticidin.
- Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 10 μM Omnia Y7 peptide (Catalog # IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 4 nM Jak2 in a total volume of 20 μL. Human Jak2 kinase domain comprising amino acids 808-1132 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV4210).
- Jak1 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak1 using the general enzyme inhibition assay method, in which the assay mixture contained 500 uM ATP, 8 uM Omnia Y12 peptide (Catalog # IVGN KPZ3121C; Invitrogen Corporation, Carlsbad, Calif.) and 5 nM Jak1 in a total volume of 20 uL. GST-tagged human Jak1 kinase domain comprising amino acids 866-1154 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV4774).
- Jak2 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture contained 500 uM ATP, 10 uM Omnia Y7 peptide (Catalog # IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 4 nM Jak2 in a total volume of 20 uL. Human Jak2 kinase domain comprising amino acids 808-1132 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV4210).
- Jak3 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak3 using the general enzyme inhibition assay method, in which the assay mixture contained 500 M ATP, 10 uM Omnia Y7 peptide (Catalog # IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 1.5 nM Jak3 in a total volume of 20 uL. GST-tagged human Jak3 kinase domain comprising amino acids 781-1124 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV3855).
- Fluorescence Resonance Energy Transfer (FRET) Assay Potency of test compounds were determined by measurement of their inhibition of BACE1 activity toward a fluorescent substrate. Experiments were performed by reference to the procedure as described in Ermolieff, et al. (Biochemistry 39:12450-12456 (2000), the teachings of which are incorporated hereby in their entirety). Briefly, the recombinant protease unit of BACE1 was prepared from E. coli expression as inclusion bodies, refolded, and purified as described in Lin, et al., (Proc. Nat. Acad. Sci. 97:1456-1460 (2000)). Fluorogenic substrate, MCA-SEVNLDAEFK(DNP)-NH2 (SEQ ID NO:1) was purchased. (M-2485, Bachem Americas, Torrance, Calif.). The substrate was derived from 10 amino acids of the human amyloid precursor protein (APP), with the Swedish variant amino acids at the beta-secretase cleavage site. The terminal amino acid was modified from arginine to lysine to facilitate derivatization with a functional group for detection by autofluorescence.
- Kat6a Activity Assay Recombinant human His-tagged Kat6a protein (amino acids 194-810), was purified in-house from Baculovirus-infected insect cells (Sf9). Histone H4 peptide (amino acids 1-24, SGRGKGGKGLGKGGAKRHRK-VLRD-K(Btn)-amide), which was used as substrate, was synthesized by Biosyntan GmbH, Berlin, Germany. Acetyl Coenzyme A was purchased from Sigma-Aldrich (#A-2056).Kat6a was incubated for 60 mins at 22° C. in the presence of different concentrations of test substances (0 μM, and within the range 0.01-20 μM) in assay buffer [25 mM Tris/HCl pH 8, 1 mM EGTA, 2.5 mM Glutathion, 0.02% Chicken Albumin, 0.05% Pluronic F127, 25 mM NaCl, 220 nM H4 peptide and 600 nM Acetyl Coenzym A].The reaction was stopped by addition of Detection Solution (25 mM HEPES pH 7.5, 0.1% BSA, 22 nM SAXL665 (Cisbio #610SAXLE), 100 μM Anacardic Acid (Enzo #ALX-270-381), 1 nM Anti-Histone H4 (ACETYL K8) Antibody (ABCAM #AB15823) and 0.5 nM Anti-Rabbit IgG Eu (Perkin Elmer #AD0083).
- Enzyme Inhibition Assay Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, PV4774, Carlsbad, Calif.), JAK2 (amino acids 831-1132), or JAK3 (amino acids 781-1124) under buffer conditions of 50 mM HEPES pH 7.3, 1 mM DTT, 0.01% Tween 20, 50 μg/mL BSA, and 10 mM MgCl2. JAK enzyme was expressed as an N-terminal GST fusion in insect cells and purified by glutathione-affinity and size-exclusion chromatographies. Enzymes were assayed both at their respective ATP Km (JAK1: 55 μM, JAK2: 15 μM, JAK3: 3 μM) and the approximated high end of physiological ATP concentration of 5 mM, in the presence of inhibitor dosed at 30, 3, 0.3, 0.03, 0.003 and 0 μM final test concentrations. For JAK1, 6 nM of enzyme (for Km ATP assay) or 4 nM enzyme (for high ATP assay) was incubated with 1.5 μM peptide substrate (FITC-C6-KKHTDDGYMPMSPGVA-NH2 (SEQ ID NO:1)). For JAK2, 0.8 nM of enzyme (for Km ATP assay) or 0.3 nM enzyme (for high ATP assay) was incubated with 1.5 μM peptide substrate (5FAM-GEEPLYWSFPAKKK-NH2 (SEQ ID NO:2)). For JAK3, 0.2 nM of enzyme (for Km ATP assay) or 0.1 nM enzyme (for high ATP assay) was incubated with 1.5 μM peptide substrate (5FAM-GEEPLYWSFPAKKK-NH2 (SEQ ID NO:2), Intonation, Boston, Mass.).
- Human FXR (NR1H4) Assay The potency and efficacy of the compounds of the invention on TGR5 receptor was evaluated using in vitro assays which carried out using the express kit from DiscoverX (cAMP HUNTER eXpress GPBAR1 CHO-K1 GPCR Assay; Cataloguer number: 95-0049E2CP2S)GPBAR1 (G protein-coupled bile acid receptor 1) encodes a member of the G protein-coupled receptor (GPCR) superfamily. GPBAR1 activation following ligand binding initiates a series of second messenger cascades that result in a cellular response. Treatment of CHO cells expressing GPBAR1 with bile acids induces the production of intracellular cAMP and internalization of the receptor. The potency and efficacy of compound for GPBAR1 activation by measuring cyclic adenosine monophosphate (cyclic AMP or cAMP) levels in live cells using a competitive immunoassay based on Enzyme Fragment Complementation (EFC).In briefly, following seeding the cells into the white, 96 well microplate, place it in a 37° C., 5% CO2 in a humidified incubator for 18-24 hours prior to testing. On second day, proceed to the appropriate cAMP HUNTER eXpress Protocol according to the manufacturer's instructions. Dissolve agonist compound in DMSO at the desired stock concentration, and prepare 3-fold serial dilutions of agonist compound in Cell Assay Buffer. The concentration of each dilution should be prepared at 4× of the final screening concentration (i.e. 15 μL compound+45 μL Cell Assay Buffer/cAMP Antibody Reagent). For each dilution, the final concentration of solvent should remain constant. Transfer 15 μL diluted compound the assay plate and incubate the plate for 30 minutes at 37° C. Following agonist incubation, add 60 μL of working cAMP detection reagents/cAMP Solution mixture (cAMP Lysis Buffer, Substrate Reagent 1, cAMP Solution D) to the appropriate wells. Incubate for 1 hour at room temperature (23° C.), protected from light. Add 60 μl of cAMP Solution A to the appropriate wells. Incubate for 3 hours at room temperature (23° C.), protected from light. Read samples on Envision standard luminescence plate reader. Calculate of average EC50 after logarithm transformation.
- Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak1 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 8 μM Omnia Y12 peptide (Catalog # IVGN KPZ3121C; Invitrogen Corporation, Carlsbad, Calif.) and 5 nM Jak1 in a total volume of 20 μL. GST-tagged human Jak1 kinase domain comprising amino acids 866-1154 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV4774).
- Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak3 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 10 μM Omnia Y7 peptide (Catalog # IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 1.5 nM Jak3 in a total volume of 20 μL. GST-tagged human Jak3 kinase domain comprising amino acids 781-1124 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV3855).
- Jak1 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak1 using the general enzyme inhibition assay method, in which the assay mixture contained 50 μM ATP, 8 μM Omnia Y12 peptide (Catalog #IVGN KPZ3121C; Invitrogen Corporation, Carlsbad, Calif.) and 5 nM Jak1 in a total volume of 20 μL. GST-tagged human Jak1 kinase domain comprising amino acids 866-1154 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog #IVGN PV4774).
- Jak1 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak1 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 8 μM Omnia Y12 peptide (Catalog # IVGN KPZ3121C; Invitrogen Corporation, Carlsbad, Calif.) and 5 nM Jak1 in a total volume of 20 μL. GST-tagged human Jak1 kinase domain comprising amino acids 866-1154 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV4774).
- Jak2 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture contained 50 μM ATP, 10 μM Omnia Y7 peptide (Catalog #IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 4 nM Jak2 in a total volume of 20 μL. Human Jak2 kinase domain comprising amino acids 808-1132 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog #IVGN PV4210).
- Jak2 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 10 μM Omnia Y7 peptide (Catalog # IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 4 nM Jak2 in a total volume of 20 μL. Human Jak2 kinase domain comprising amino acids 808-1132 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV4210).
- Jak3 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak3 using the general enzyme inhibition assay method, in which the assay mixture contained 50 μM ATP, 10 μM Omnia Y7 peptide (Catalog #IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 1.5 nM Jak3 in a total volume of 20 μL. GST-tagged human Jak3 kinase domain comprising amino acids 781-1124 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog #IVGN PV3855).
- Jak3 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Jak3 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 10 μM Omnia Y7 peptide (Catalog # IVGN KNZ3071C, Invitrogen Corporation, Carlsbad, Calif.) and 1.5 nM Jak3 in a total volume of 20 μL. GST-tagged human Jak3 kinase domain comprising amino acids 781-1124 was purchased from Invitrogen Corporation, Carlsbad, Calif. (catalog # IVGN PV3855).
- Enzyme Inhibition Assay Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino acids 831-1132), or JAK3 (amino acids 781-1124) under buffer conditions of 50 mM HEPES pH 7.3, 1 mM DTT, 0.01% Tween 20, 50 μg/mL BSA, and 10 mM MgCl2. JAK enzyme was expressed as an N-terminal GST fusion in insect cells and purified by glutathione-affinity and size-exclusion chromatographies. Enzymes were assayed both at their respective ATP Km (JAK1: 55 μM, JAK2: 15 μM, JAK3: 3 μM) and the approximated high end of physiological ATP concentration of 5 mM, in the presence of inhibitor dosed at 30, 3, 0.3, 0.03, 0.003 and 0 μM final test concentrations. For JAK1, 6 nM of enzyme (for Km ATP assay) or 4 nM enzyme (for high ATP assay) was incubated with 1.5 μM peptide substrate (FITC-C6-KKHTDDGYMPMSPGVA-NH2 (SEQ ID NO:1), Intonation, Boston, Mass.). For JAK2, 0.8 nM of enzyme (for Km ATP assay) or 0.3 nM enzyme (for high ATP assay) was incubated with 1.5 μM peptide substrate (5FAM-GEEPLYWSFPAKKK-NH2 (SEQ ID NO:2), Intonation, Boston, Mass.). For JAK3, 0.2 nM of enzyme (for Km ATP assay) or 0.1 nM enzyme (for high ATP assay) was incubated with 1.5 μM peptide substrate (5FAM-GEEPLYWSFPAKKK-NH2 (SEQ ID NO:2), Intonation, Boston, Mass.). Phosphorylated and unphosphorylated peptides were separated and quantified by a Caliper LC3000 system (Caliper Life Sciences, MA) for calculating percent inhibition.
- PERK In Vitro Activity Assay In vitro Inhibition of PERK Enzyme Activity (isolated) Recombinant human EIF2AK2 (PKR) catalytic domain (amino acids 252-551), EIF2AK3 (PERK) catalytic domain (amino acids 536-1116), GFP-eIF2a substrate, and Terbium-labelled phospho-eIF2a antibody is obtained (Invitrogen, Carlsbad, CA).Express and purify HIS-SUMO-GCN2 catalytic domain (amino acids 584-1019) from E. coli. Perform TR-FRET kinase assays in the absence or presence of inhibitors in a reaction buffer consisting of 50 mM HEPES, pH 7.5, 10 mM MgCb, 1.0 mM EGTA, and 0.01% Brij-35, and 100-200 nM GFP-eIF2a substrate. PKR assays contain 14 ng/mL enzyme and 2.5 μM ATP (Km, −2.5 μM), PERK assays contain 62.5 ng/mL enzyme and 1.5 μM ATP (Km. app −1.5 uM), and GCN2 assays contain 3 nM enzyme and 90 μM ATP (Km, −200 uM). Add test compound, initiate the reaction by addition of enzyme, and incubate at room temperature for 45 minutes. Stop the reaction by addition of EDTA to a final concentration of 10 mM, add Terbium-labelled phospho-eIF2a antibody at a final concentration of 2 nM, and incubate for 90 minutes. Monitor the resulting fluorescence in an EnVison® Multilabel reader (PerkinElmer, Waltham, MA). Determine TR-FRET ratios and the resulting IC50 values using a 4-parameter nonlinear logistic equation as shown: Y=(A+((B−A)/(1+(C/x)AD)))) where, Y=% specific inhibition, A=Bottom of the curve, B=Top of the curve, C=absolute IC50 (concentration causing 50% inhibition), and D=hill slope.
- Alpha Screen Assay A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2) SEQ ID NO: 1). FAK enzyme was expressed in insect cells as catalytic domain (amino acids 411-686) N-terminally tagged with six histidine amino acids and a Tobacco Etch Virus (TeV) cleavage sequence. After lysing the cells by sonication, the kinase was purified by Ni-Immobilised Metal Affinity Chromatography, TeV cleavage leaving a N-terminal glycine, and gel filtration. The 15 ul assay reactions are run in Greiner brand white 384-well low volume plates. All reactions contained 10 mM HEPES pH 7.4, 25 mM NaCI, 10 mM MgCl2, 0.01% (v/v) Tween-20, 50 uM Na3V04, 0.01% (w/v) albumin from chicken egg white, 111 nM peptide substrate, 80 pM ATP, and 4 ng/reaction FAK enzyme, with the enzyme being omitted from negative control reactions. Compounds were added in a volume of 100 nl from dilution series made up in DMSO.
- Biochemical Alpha Screen Assay A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1SEQ ID NO: 1). FAK enzyme was expressed in insect cells as catalytic domain (amino acids 411-686)N-terminally tagged with six histidine amino acids and a Tobacco Etch Virus (TeV) cleavage sequence. After lysing the cells by sonication, the kinase was purified by Ni-Immobilised Metal Affinity Chromatography chromatography, TeV cleavage leaving a N-terminal glycine, and gel filtration. The 15 ul assay reactions are run in Greiner brand white 384-well low volume plates. All reactions contained 10 mM HEPES pH 7.4, 25 mM NaCl, 10 mM MgCl2, 0.01% (v/v) Tween-20, 50 uM Na3V04, 0.01% (w/v) albumin from chicken egg white, 111 nM peptide substrate, 80 uM ATP, and 4 ng/reaction FAK enzyme, with the enzyme being omitted from negative control reactions. Compounds were added in a volume of 100 nl from dilution series made up in DMSO.
- Time Resolved Fluorescence Energy Transfer (TR-FRET) Assay The inhibition of p53-Hdm2 and p53-Hdm4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfer (or Foerster resonance energy transfer) describes an energy transfer between donor and acceptor fluorescent molecules. For this assay, MDM2 protein (amino acids 2-188) and MDM4 protein (amino acids 2-185), tagged with a C-terminal Biotin moiety, are used in combination with a Europium labeled streptavidin (Perkin Elmer, Inc., Waltham, Mass., USA) serving as the donor fluorophore. The p53 derived, Cy5 labeled peptide Cy5-TFSDLWKLL (p53 aa 18-26) is the energy acceptor. Upon excitation of the donor molecule at 340 nm, binding interaction between MDM2 or MDM4 and the p53 peptide induces energy transfer and enhanced response at the acceptor emission wavelength at 665 nm. Disruption of the formation of the p53-MDM2 or p53-MDM4 complex due to an inhibitor molecule binding to the p53 binding site of MDM2 or MDM4 results in increased donor emission.
- AlphaScreen Assay 20 nM ofGST-tagged HCNlc40 protein (corresponding to the C terminal 40 amino acids of HCN1) was incubated with 200 nM of His-tagged TRIP8b(241-602) and varying concentrations of the test compound as described in our prior report (Han et al., 2015). After a 3 h incubation, anti-GST AlphaScreen acceptor beads were added for 1.5 h followed by AlphaScreen nickel chelate donor beads for 1.5 h. AlphaScreen signal was quantified using a PerkinElmer (Waltham, MA) EnSpire multimode plate reader
- Inhibition Assay Tyk2: Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture contained 500 μM ATP, 8 μM Omnia Y12 peptide (Catalog # IVGN KPZ3121C; Invitrogen Corporation, Carlsbad, Calif.) and 1 nM Tyk2 in a total volume of 20 μL. Human Tyk2 kinase domain, comprising amino acids 886 to 1187 with 10 additional histidine residues (histidine tag) on the carboxy terminus, was expressed and purified from bacculovirus.
- Fluorimetric Assay Inhibitory constants (Ki) and IC50 values were determined under saturating substrate conditions by using a fluorimetric assay (components were purchased separately). This assay system allows detection of a fluorescent signal upon SIRT6 deacetylation of the fluorescent substrate peptide (AMC-p53) with amino acids 379-382 (Arg-His-Lys-LysAc). Sirtuin deacetylation of the substrate renders the fluorophore-substrate bond susceptible to treatment with lysyl endopeptidase. Trypsin cleavage releases the fluorophore, resulting in an increase in fluorescence. Fluorescence intensity was measured on a Synergy HT Multi-Mode microplate fluorescence reader.
- Spectrophotometric 384 Well Assay Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked kinetic method measuring ADP generated during the ACC reaction using a coupled lactate dehydrogenase/pyruvate kinase reaction. For biological testing, a human ACC2 construct which lacks the 128 amino acids at the N-terminus for increased solubility (nt 385-6966 in Genbank entry AJ575592) is cloned. The protein is then expressed in insect cells using a baculoviral expression system. Protein purification is performed by anion exchange.
- Time Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Assay Test Example 2: Potency of compounds were also measured using another fluorogenic substrate, TruPoint BACE1 Substrate Eu-CEVNLDAEFK-QSY 7 (SEQ ID NO:3) (AD0258, PerkinElmer, Boston Mass.). This substrate also has Swedish variant amino acids at the β-secretase cleavage site, with a fluorescent europium (Eu) chelate coupled to one end and a quencher of europium fluorescence (QSY7) coupled to the other end via lysine. If the sample contains BACE1 activity, the Eu chelate and the quencher will be separated as the substrate is cleaved.
- Tyk2 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture contained 50 μM ATP, 8 μM Omnia Y12 peptide (Catalog #IVGN KPZ3121C; Invitrogen Corporation, Carlsbad, Calif.) and 1 nM Tyk2 in a total volume of 20 μL. Human Tyk2 kinase domain, comprising amino acids 886 to 1187 with 10 additional histidine residues (histidine tag) on the carboxy terminus, was expressed and purified from bacculovirus in-house at Array BioPharma Inc. (Boulder, Colo.).
- Tyk2 Inhibition Assay Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture contained 500 uM ATP, 8 uM Omnia Y12 peptide (Catalog # IVGN KPZ3121C; Invitrogen Corporation, Carlsbad, Calif.) and 1 nM Tyk2 in a total volume of 20 uL. Human Tyk2 kinase domain, comprising amino acids 886 to 1187 with 10 additional histidine residues (histidine tag) on the carboxy terminus, was expressed and purified from baculovirus in-house at Array BioPharma Inc. (Boulder, Colo.).
- Urease Inhibition Assay The inhibition studies of soybean urease were initiated with boric acid and boronic acids (butylboronic acid, 4-bromophenylboronic acid, and phenylboronic acid). Also, heavy metal ions (HgCl2, AgCl, and Cu(CH3COO)2) and sodium salts of mineral acids (NaF, NaCl, NaNO3, and Na2SO4) were investigated for their inhibitory effects. Stock solutions of inhibitors, except for 4-bromophenylboronicacid, were prepared in 0.05 M Tris-acetate buffer, pH 7.0, and were suitably diluted for experiments, whereas a stock solution of 4-bromophenylboronic acid was prepared in absolute ethanol and subsequently diluted in respective buffer. The activity assay was carried out at standard conditions as described earlier in the presence of varying concentrations of inhibitors. The yellow-orange colored solution was measured at 405 nm on a Unicam UV-2 spectrophotometer. The amount of NH3 liberated in the reaction mixture was estimated by calibrating Nessler's reagent with standard NH4Cl solution. One enzyme unit is defined as the amount of urease required to liberate 1 μmol of ammonia per minute under our test conditions (0.1 M urea, 0.05 M Trisacetatebuffer, pH 7.0, 37°C).
 carbocysteine (R)-S-(carboxymethyl)cysteine (2R)-2-amino-3-[(carboxymethyl)sulfanyl]propanoic acidS-(carboxymethyl)-L-cysteine BDBM50213735 CHEMBL396416 S-(carboxymethyl)-(R)-cysteine S-carboxymethyl-L-cysteine (L)-2-Amino-3-(carboxymethylthio)propionic acid L-3-((carboxymethyl)thio)alanine
carbocysteine (R)-S-(carboxymethyl)cysteine (2R)-2-amino-3-[(carboxymethyl)sulfanyl]propanoic acidS-(carboxymethyl)-L-cysteine BDBM50213735 CHEMBL396416 S-(carboxymethyl)-(R)-cysteine S-carboxymethyl-L-cysteine (L)-2-Amino-3-(carboxymethylthio)propionic acid L-3-((carboxymethyl)thio)alanine leukotriene E4 (5S-(5R*,6S*(S*),7E,9E,11Z,14Z))-6-((2-Amino-2-carboxyethyl)thio)-5-hydroxy-7,9,11,14-eicosatetraenoic acid BDBM50297387 LTE4 CHEMBL509456 5S-hydroxy,6R-(S-cysteinyl),7E,9E,11Z,14Z-eicosatetraenoic acid (5S,6R,7E,9E,11Z,14Z)-6-(cystein-S-yl)-5-hydroxyicosa-7,9,11,14-tetraenoic acidS-{(1R,2E,4E,6Z,9Z)-1-[(1S)-4-carboxy-1-hydroxybutyl]pentadeca-2,4,6,9-tetraen-1-yl}-L-cysteine
leukotriene E4 (5S-(5R*,6S*(S*),7E,9E,11Z,14Z))-6-((2-Amino-2-carboxyethyl)thio)-5-hydroxy-7,9,11,14-eicosatetraenoic acid BDBM50297387 LTE4 CHEMBL509456 5S-hydroxy,6R-(S-cysteinyl),7E,9E,11Z,14Z-eicosatetraenoic acid (5S,6R,7E,9E,11Z,14Z)-6-(cystein-S-yl)-5-hydroxyicosa-7,9,11,14-tetraenoic acidS-{(1R,2E,4E,6Z,9Z)-1-[(1S)-4-carboxy-1-hydroxybutyl]pentadeca-2,4,6,9-tetraen-1-yl}-L-cysteine