 BDBM50227275 PD-117558
BDBM50227275 PD-117558 BDBM50366632 PD-170292
BDBM50366632 PD-170292 BDBM50230684 CHEMBL353157 PD-135118
BDBM50230684 CHEMBL353157 PD-135118 BDBM50565138 CHEMBL595227 PD-404182
BDBM50565138 CHEMBL595227 PD-404182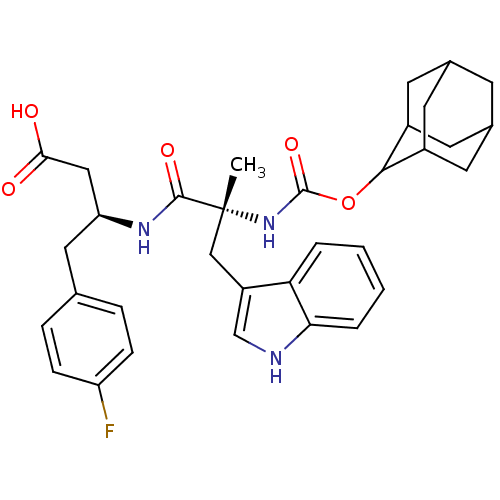 CHEMBL2062146 BDBM50451028 PD-149164
CHEMBL2062146 BDBM50451028 PD-149164 CHEMBL2062154 BDBM50449787 PD-134308
CHEMBL2062154 BDBM50449787 PD-134308 CHEMBL535139 PD-123177 BDBM50370405
CHEMBL535139 PD-123177 BDBM50370405 PD-0184264 BDBM50595935 CHEMBL481949
PD-0184264 BDBM50595935 CHEMBL481949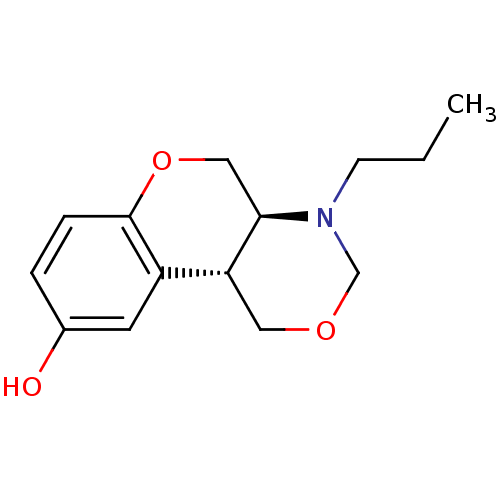 PD-128907 BDBM50369174 CHEMBL94015
PD-128907 BDBM50369174 CHEMBL94015 PD-150606 CHEMBL366254 BDBM50466172
PD-150606 CHEMBL366254 BDBM50466172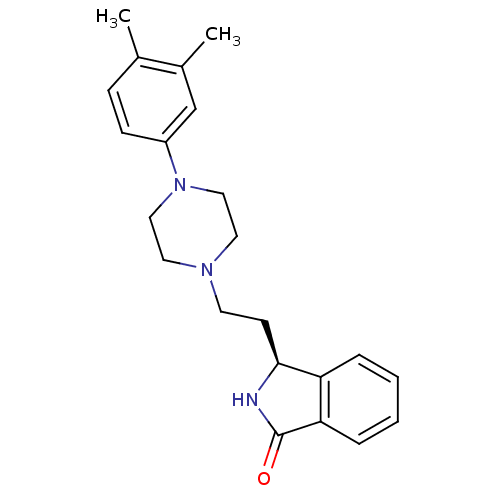 (S)-3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one BDBM50070514 PD-18126 CHEMBL37170 PD-172938 3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one (PD-18126)
(S)-3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one BDBM50070514 PD-18126 CHEMBL37170 PD-172938 3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one (PD-18126)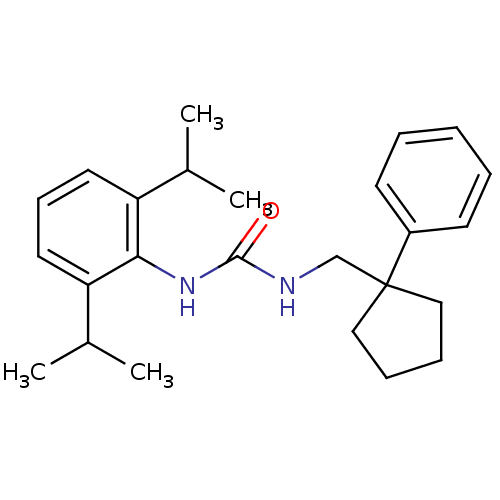 1-(2,6-Diisopropyl-phenyl)-3-(1-phenyl-cyclopentylmethyl)-urea BDBM50038526 PD-129337 CHEMBL296055
1-(2,6-Diisopropyl-phenyl)-3-(1-phenyl-cyclopentylmethyl)-urea BDBM50038526 PD-129337 CHEMBL296055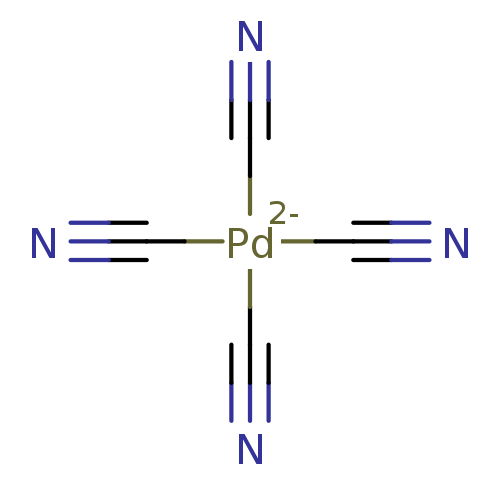 BDBM50164088 [Pd(CN)4]2- CHEMBL362447
BDBM50164088 [Pd(CN)4]2- CHEMBL362447 BDBM689140 US12049474, Compound NP-PD-042
BDBM689140 US12049474, Compound NP-PD-042 BDBM689141 US12049474, Compound NP-PD-051
BDBM689141 US12049474, Compound NP-PD-051 BDBM689142 US12049474, Compound NP-PD-102
BDBM689142 US12049474, Compound NP-PD-102 BDBM689145 US12049474, Compound NP-PD-149
BDBM689145 US12049474, Compound NP-PD-149 BDBM689147 US12049474, Compound NP-PD-130
BDBM689147 US12049474, Compound NP-PD-130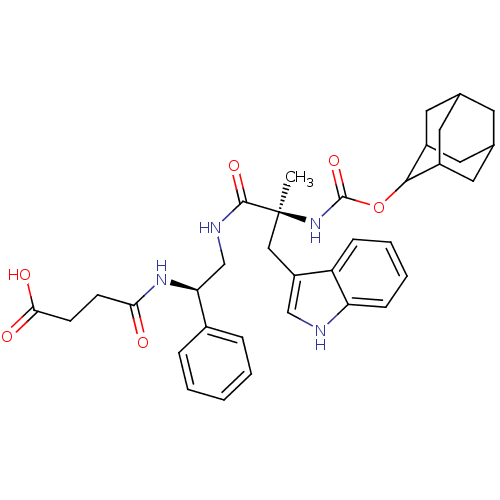 PD-137342 CHEMBL2062144 CI-988 BDBM50422043
PD-137342 CHEMBL2062144 CI-988 BDBM50422043 US12049474, Compound NP-PD-105 BDBM689143
US12049474, Compound NP-PD-105 BDBM689143 US12049474, Compound NP-PD-158 BDBM689146
US12049474, Compound NP-PD-158 BDBM689146 BDBM50080296 PD-32577 2-(1-azepanylmethyl)-4-{1-[3-(1-azepanylmethyl)-4-hydroxyphenyl]-1-methylethyl}phenol CHEMBL421719
BDBM50080296 PD-32577 2-(1-azepanylmethyl)-4-{1-[3-(1-azepanylmethyl)-4-hydroxyphenyl]-1-methylethyl}phenol CHEMBL421719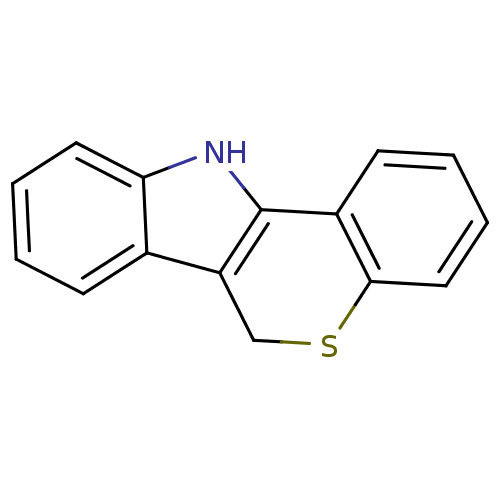 6,11-dihydrothiochromeno[4,3-b]indole PD-146176 BDBM50208823 CHEMBL180917
6,11-dihydrothiochromeno[4,3-b]indole PD-146176 BDBM50208823 CHEMBL180917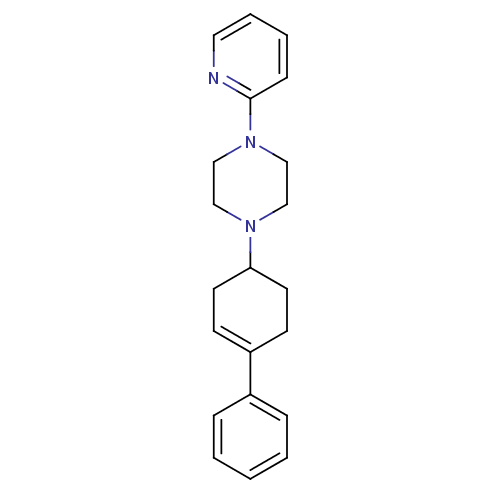 CHEMBL157422 BDBM50281610 PD-135478 1-(4-Phenyl-cyclohex-3-enyl)-4-pyridin-2-yl-piperazine
CHEMBL157422 BDBM50281610 PD-135478 1-(4-Phenyl-cyclohex-3-enyl)-4-pyridin-2-yl-piperazine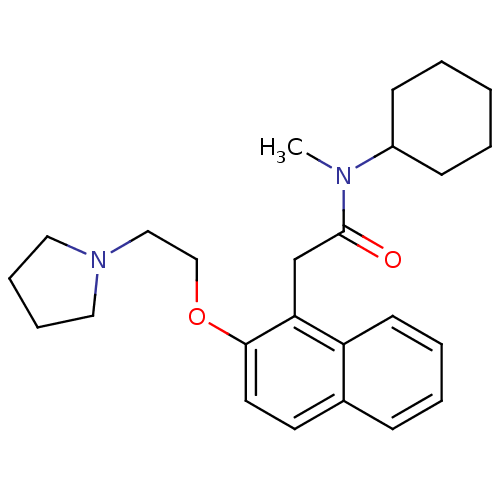 N-Cyclohexyl-N-methyl-2-[2-(2-pyrrolidin-1-yl-ethoxy)-naphthalen-1-yl]-acetamide BDBM50281660 CHEMBL353654 PD-148282
N-Cyclohexyl-N-methyl-2-[2-(2-pyrrolidin-1-yl-ethoxy)-naphthalen-1-yl]-acetamide BDBM50281660 CHEMBL353654 PD-148282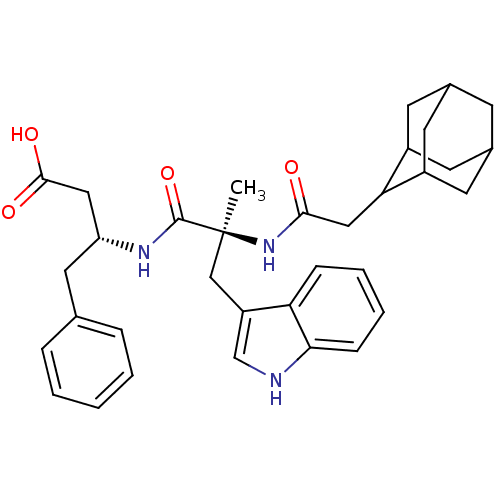 BDBM50092154 3-[2-(2-Adamantan-2-yl-acetylamino)-3-(1H-indol-3-yl)-2-methyl-propionylamino]-4-phenyl-butyric acid(PD 140458) PD-140458 CHEMBL420320
BDBM50092154 3-[2-(2-Adamantan-2-yl-acetylamino)-3-(1H-indol-3-yl)-2-methyl-propionylamino]-4-phenyl-butyric acid(PD 140458) PD-140458 CHEMBL420320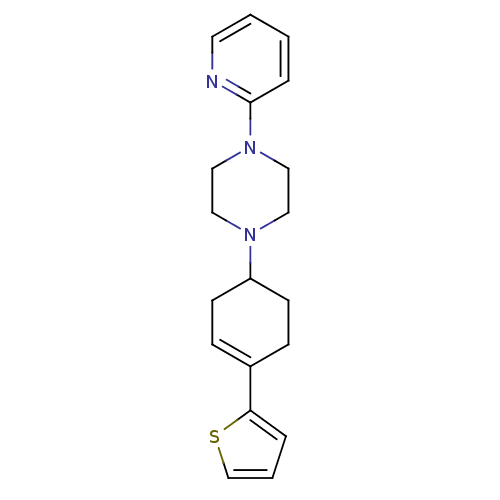 BDBM50281605 1-Pyridin-2-yl-4-(4-thiophen-2-yl-cyclohex-3-enyl)-piperazine PD-135188 CHEMBL157087
BDBM50281605 1-Pyridin-2-yl-4-(4-thiophen-2-yl-cyclohex-3-enyl)-piperazine PD-135188 CHEMBL157087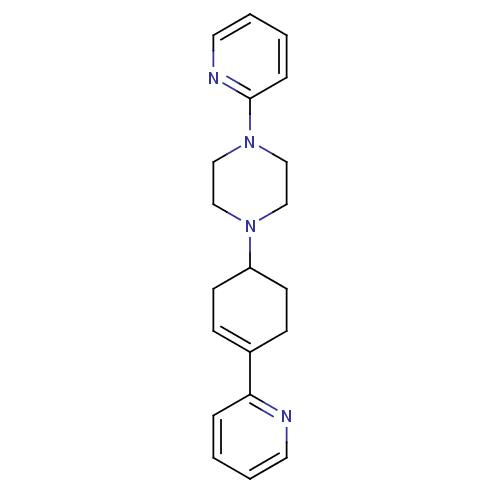 BDBM50281607 PD-135222 1-Pyridin-2-yl-4-(4-pyridin-2-yl-cyclohex-3-enyl)-piperazine CHEMBL422187
BDBM50281607 PD-135222 1-Pyridin-2-yl-4-(4-pyridin-2-yl-cyclohex-3-enyl)-piperazine CHEMBL422187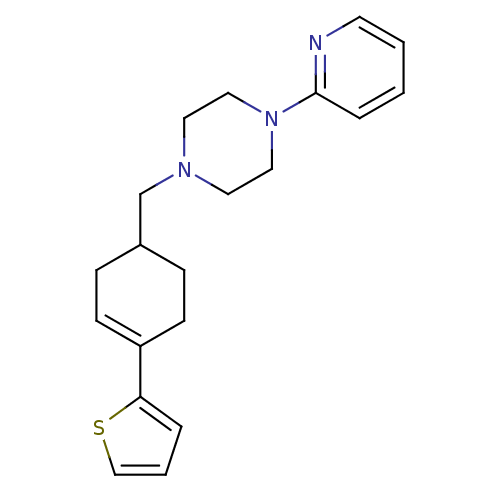 BDBM50281608 CHEMBL349994 1-Pyridin-2-yl-4-(4-thiophen-2-yl-cyclohex-3-enylmethyl)-piperazine PD-135146
BDBM50281608 CHEMBL349994 1-Pyridin-2-yl-4-(4-thiophen-2-yl-cyclohex-3-enylmethyl)-piperazine PD-135146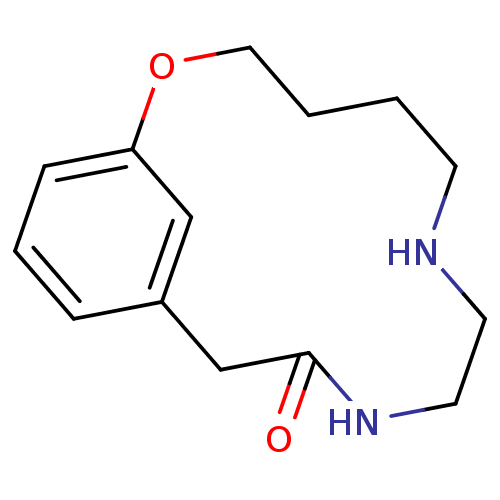 CHEMBL353264 PD-146884 2-Oxa-7,10-diaza-bicyclo[11.3.1]heptadeca-1(17),13,15-trien-11-one BDBM50281661
CHEMBL353264 PD-146884 2-Oxa-7,10-diaza-bicyclo[11.3.1]heptadeca-1(17),13,15-trien-11-one BDBM50281661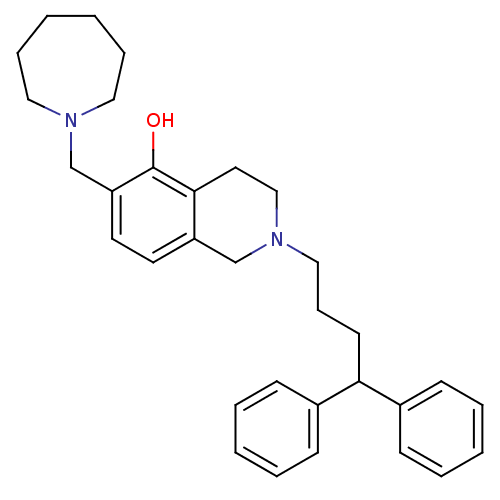 PD-157667 BDBM50071611 CHEMBL78017 6-Azepan-1-ylmethyl-2-(4,4-diphenyl-butyl)-1,2,3,4-tetrahydro-isoquinolin-5-ol
PD-157667 BDBM50071611 CHEMBL78017 6-Azepan-1-ylmethyl-2-(4,4-diphenyl-butyl)-1,2,3,4-tetrahydro-isoquinolin-5-ol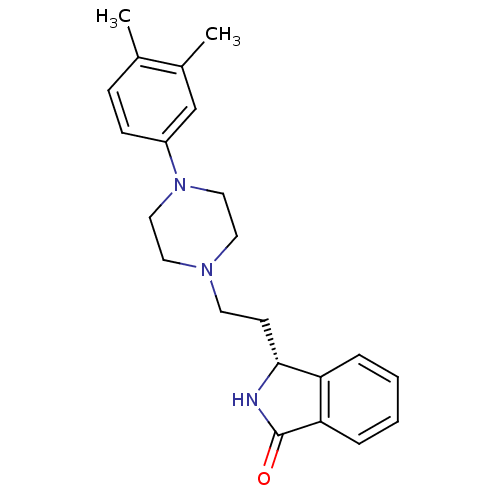 CHEMBL35093 (R)-3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one BDBM50070518 PD-172939
CHEMBL35093 (R)-3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one BDBM50070518 PD-172939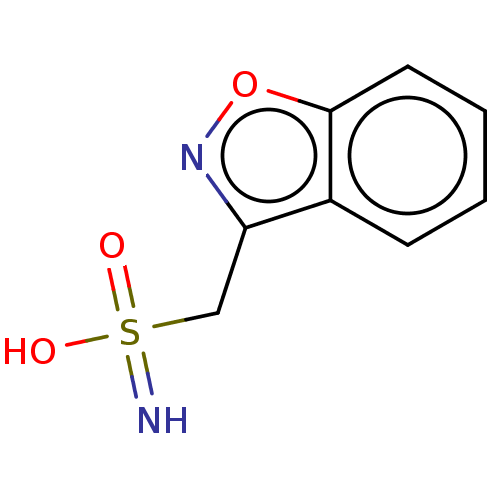 PD-110843 AD-810 Zonegran BDBM50028010 CI-912 CHEBI:10127 Zonisamide
PD-110843 AD-810 Zonegran BDBM50028010 CI-912 CHEBI:10127 Zonisamide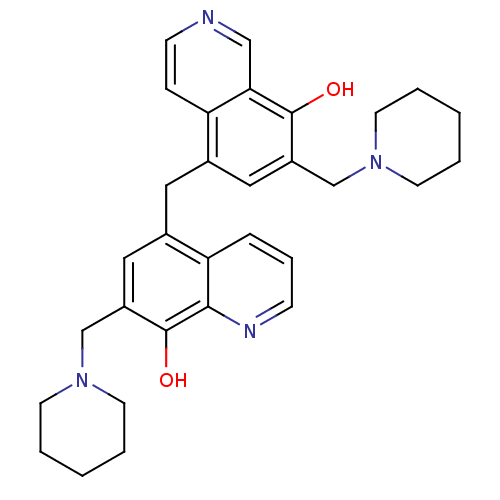 5-(8-Hydroxy-7-piperidin-1-ylmethyl-isoquinolin-5-ylmethyl)-7-piperidin-1-ylmethyl-quinolin-8-ol BDBM50071612 PD-29361 CHEMBL312381
5-(8-Hydroxy-7-piperidin-1-ylmethyl-isoquinolin-5-ylmethyl)-7-piperidin-1-ylmethyl-quinolin-8-ol BDBM50071612 PD-29361 CHEMBL312381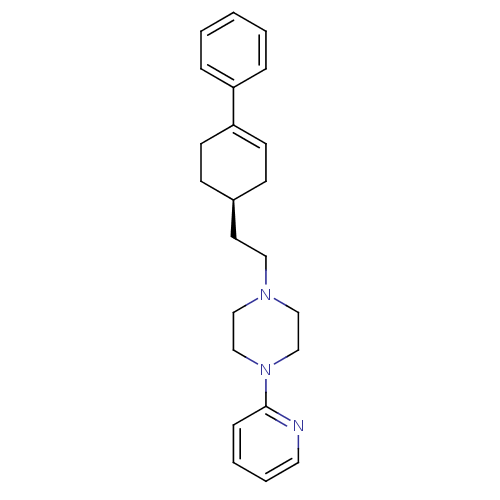 BDBM50055724 CHEMBL418937 1-[2-((S)-4-Phenyl-cyclohex-3-enyl)-ethyl]-4-pyridin-2-yl-piperazine PD-137789
BDBM50055724 CHEMBL418937 1-[2-((S)-4-Phenyl-cyclohex-3-enyl)-ethyl]-4-pyridin-2-yl-piperazine PD-137789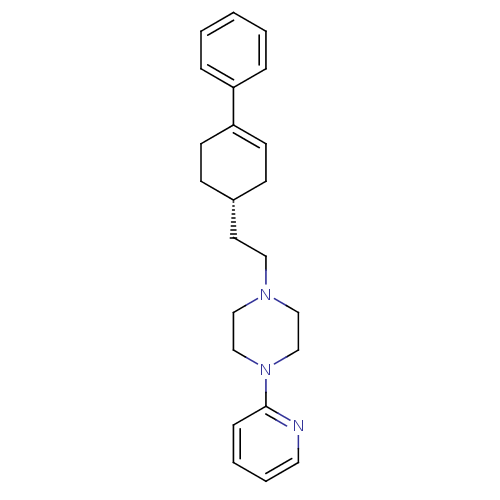 BDBM50281606 1-[2-((R)-4-Phenyl-cyclohex-3-enyl)-ethyl]-4-pyridin-2-yl-piperazine PD-137821 CHEMBL348547
BDBM50281606 1-[2-((R)-4-Phenyl-cyclohex-3-enyl)-ethyl]-4-pyridin-2-yl-piperazine PD-137821 CHEMBL348547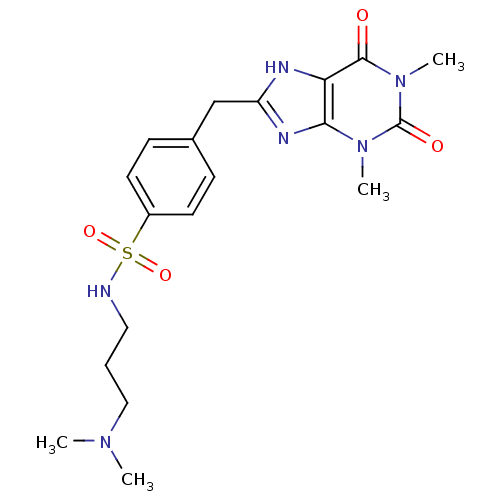 BDBM82039 8-{4-[N-(3-Dimethylaminopropyl)-sulfonamido]phenyl}-1,3-Dipropylxanthine (PD 113,297)
BDBM82039 8-{4-[N-(3-Dimethylaminopropyl)-sulfonamido]phenyl}-1,3-Dipropylxanthine (PD 113,297)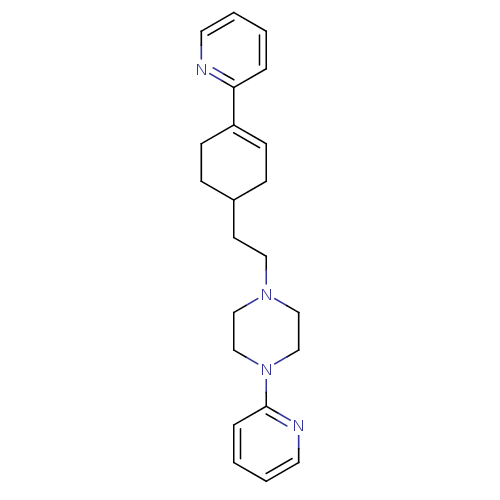 BDBM50281609 CHEMBL346075 1-Pyridin-2-yl-4-[2-(4-pyridin-2-yl-cyclohex-3-enyl)-ethyl]-piperazine PD-135540
BDBM50281609 CHEMBL346075 1-Pyridin-2-yl-4-[2-(4-pyridin-2-yl-cyclohex-3-enyl)-ethyl]-piperazine PD-135540 CHEMBL346940 PD-135111 BDBM50281611 1-Pyridin-2-yl-4-[2-(4-thiophen-2-yl-cyclohex-3-enyl)-ethyl]-piperazine
CHEMBL346940 PD-135111 BDBM50281611 1-Pyridin-2-yl-4-[2-(4-thiophen-2-yl-cyclohex-3-enyl)-ethyl]-piperazine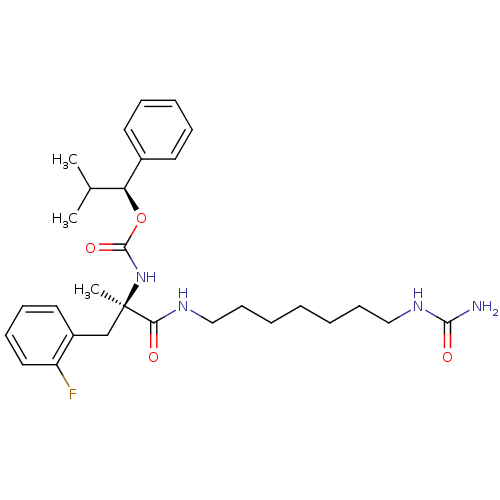 PD-160946 [(R)-2-(2-Fluoro-phenyl)-1-methyl-1-(7-ureido-heptylcarbamoyl)-ethyl]-carbamic acid (S)-2-methyl-1-phenyl-propyl ester BDBM50050652 CHEMBL47146
PD-160946 [(R)-2-(2-Fluoro-phenyl)-1-methyl-1-(7-ureido-heptylcarbamoyl)-ethyl]-carbamic acid (S)-2-methyl-1-phenyl-propyl ester BDBM50050652 CHEMBL47146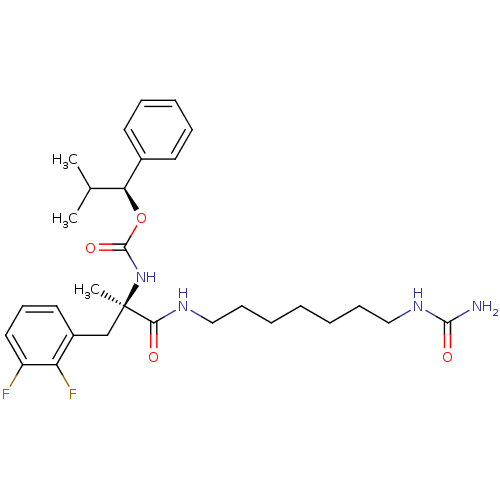 PD-161182 [(R)-2-(2,3-Difluoro-phenyl)-1-methyl-1-(7-ureido-heptylcarbamoyl)-ethyl]-carbamic acid (S)-2-methyl-1-phenyl-propyl ester BDBM50050641 CHEMBL45340
PD-161182 [(R)-2-(2,3-Difluoro-phenyl)-1-methyl-1-(7-ureido-heptylcarbamoyl)-ethyl]-carbamic acid (S)-2-methyl-1-phenyl-propyl ester BDBM50050641 CHEMBL45340 6-(4-Amino-benzenesulfonyl)-5-nitro-quinolin-8-ylamine BDBM50287526 CHEMBL53016 PD-9262
6-(4-Amino-benzenesulfonyl)-5-nitro-quinolin-8-ylamine BDBM50287526 CHEMBL53016 PD-9262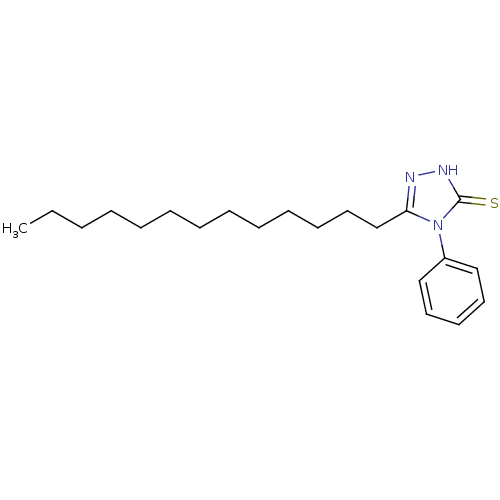 PD-140195 4-Phenyl-5-tridecyl-4H-[1,2,4]triazole-3-thiol CHEMBL61135 BDBM50287670
PD-140195 4-Phenyl-5-tridecyl-4H-[1,2,4]triazole-3-thiol CHEMBL61135 BDBM50287670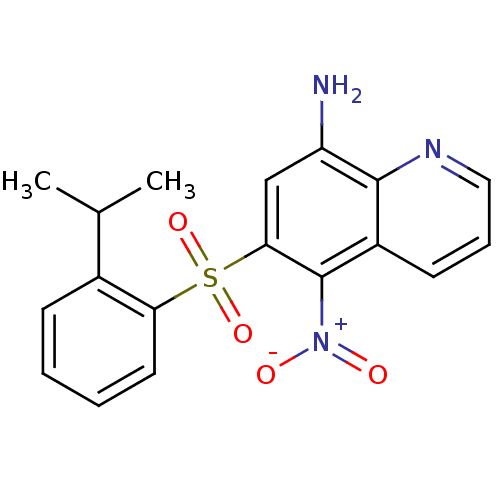 PD-160170 CHEMBL422942 6-(2-Isopropyl-benzenesulfonyl)-5-nitro-quinolin-8-ylamine BDBM50287527
PD-160170 CHEMBL422942 6-(2-Isopropyl-benzenesulfonyl)-5-nitro-quinolin-8-ylamine BDBM50287527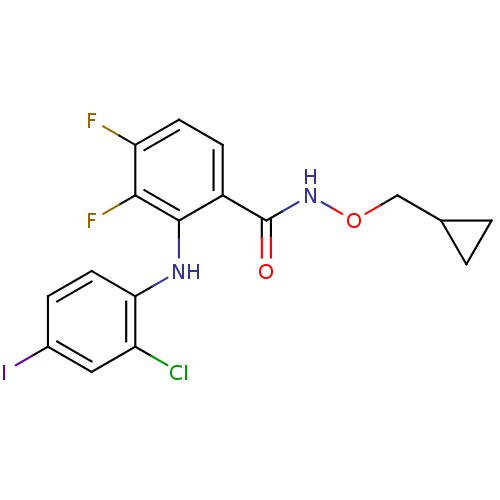 CHEMBL105442 US8575391, 341 CI-1040 BDBM50132260 PD-18435 PD-184352 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-benzamide 2-(2-chloro-4-iodophenylamino)-N-(cyclopropylmethoxy)-3,4-difluorobenzamide
CHEMBL105442 US8575391, 341 CI-1040 BDBM50132260 PD-18435 PD-184352 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-benzamide 2-(2-chloro-4-iodophenylamino)-N-(cyclopropylmethoxy)-3,4-difluorobenzamide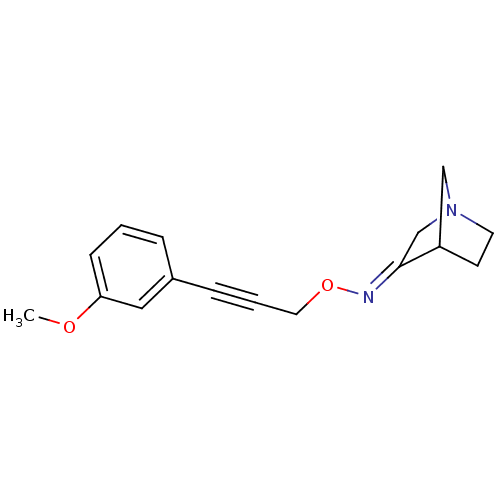 1-Aza-bicyclo[2.2.1]heptan-3-one O-[3-(3-methoxy-phenyl)-prop-2-ynyl]-oxime BDBM50065223 PD-151832 CHEMBL81878
1-Aza-bicyclo[2.2.1]heptan-3-one O-[3-(3-methoxy-phenyl)-prop-2-ynyl]-oxime BDBM50065223 PD-151832 CHEMBL81878 CHEMBL78406 Thiophene-2-carboxylic acid {4-[2-(4-pyridin-2-yl-piperazin-1-yl)-ethyl]-cyclohexyl}-amide PD-137557 BDBM50290229
CHEMBL78406 Thiophene-2-carboxylic acid {4-[2-(4-pyridin-2-yl-piperazin-1-yl)-ethyl]-cyclohexyl}-amide PD-137557 BDBM50290229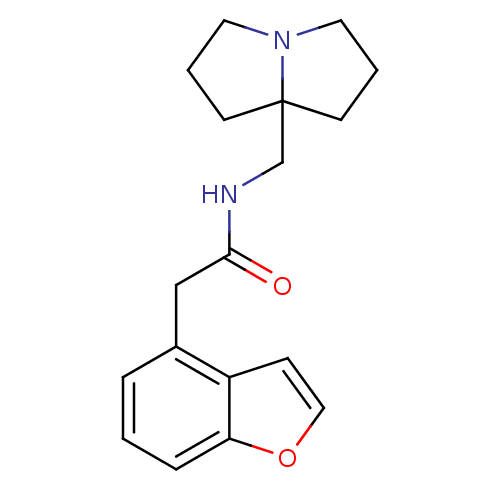 CHEMBL170603 BDBM50281662 PD-146795 2-Benzofuran-4-yl-N-(tetrahydro-pyrrolizin-7a-ylmethyl)-acetamide
CHEMBL170603 BDBM50281662 PD-146795 2-Benzofuran-4-yl-N-(tetrahydro-pyrrolizin-7a-ylmethyl)-acetamide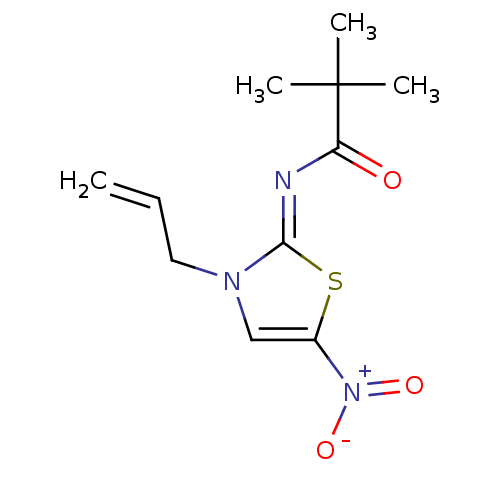 CHEMBL455856 BDBM50266362 PD-068235 (Z)-N-(3-allyl-5-nitrothiazol-2(3H)-ylidene)pivalamide
CHEMBL455856 BDBM50266362 PD-068235 (Z)-N-(3-allyl-5-nitrothiazol-2(3H)-ylidene)pivalamide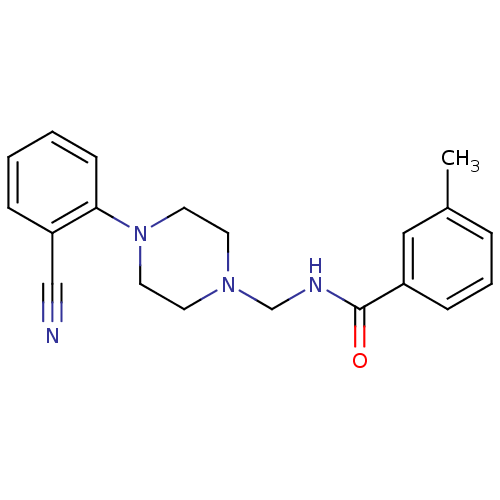 CHEMBL45244 BDBM50058225 N-((4-(2-cyanophenyl)piperazin-1-yl)methyl)-3-methylbenzamide PD-168077 N-[4-(2-Cyano-phenyl)-piperazin-1-ylmethyl]-3-methyl-benzamide
CHEMBL45244 BDBM50058225 N-((4-(2-cyanophenyl)piperazin-1-yl)methyl)-3-methylbenzamide PD-168077 N-[4-(2-Cyano-phenyl)-piperazin-1-ylmethyl]-3-methyl-benzamide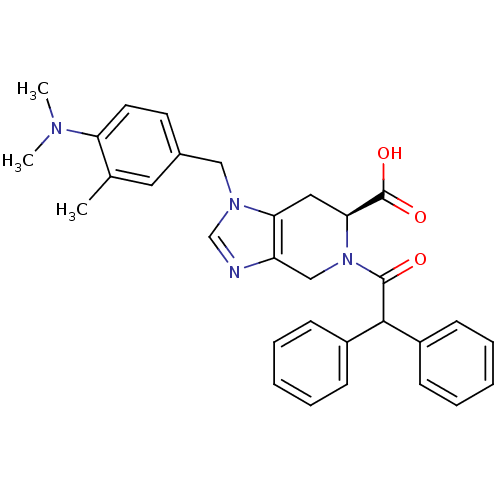 PD-123319 BDBM50282396 CHEMBL157946 (S)-1-(4-Dimethylamino-3-methyl-benzyl)-5-diphenylacetyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid
PD-123319 BDBM50282396 CHEMBL157946 (S)-1-(4-Dimethylamino-3-methyl-benzyl)-5-diphenylacetyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid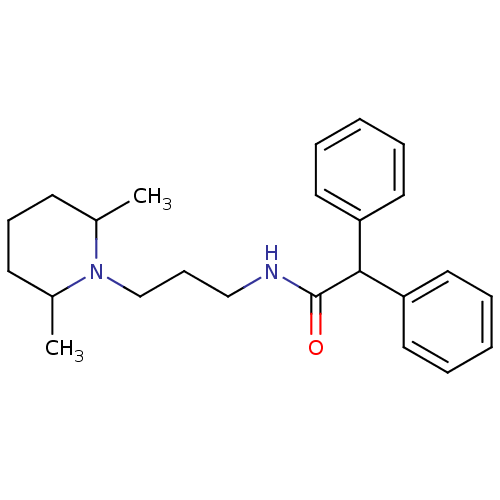 CHEMBL282833 N-[3-(2,6-Dimethyl-piperidin-1-yl)-propyl]-2,2-diphenyl-acetamide(PD85639) BDBM50043993 PD-85639 N-[3-(2,6-Dimethyl-piperidin-1-yl)-propyl]-2,2-diphenyl-acetamide
CHEMBL282833 N-[3-(2,6-Dimethyl-piperidin-1-yl)-propyl]-2,2-diphenyl-acetamide(PD85639) BDBM50043993 PD-85639 N-[3-(2,6-Dimethyl-piperidin-1-yl)-propyl]-2,2-diphenyl-acetamide
- Ren, X; Hess, R; Hummel, J; Lacharity, JJ; Li, X; Manns, S; Qian, D; Wang, X; Wei, B COMBINATION THERAPY COMPRISING DGK INHIBITORS and PD-1/PD-L1 INHIBITORS US Patent US20250186450 (2025)
- Wang, H; Carlsen, PN; Huang, T; Li, Y; Lin, L; Qi, C; Thekkat, PU; Wang, X; Wu, L; Yao, W; Zhu, W Combination Therapy Comprising A2A/A2B and PD-1/PD-L1 Inhibitors US Patent US20250041310 (2025)
- Sun, JG; Gao, Y; Gao, YS; Dai, XJ; Chen, P Identification of the exosomal PD-L1 inhibitor to promote the PD-1 targeting therapy of gastric cancer. Eur J Med Chem 268:
- Hu, J; Zhang, D; Tian, K; Ren, C; Li, H; Lin, C; Huang, X; Liu, J; Mao, W; Zhang, J Small-molecule LRRK2 inhibitors for PD therapy: Current achievements and future perspectives. Eur J Med Chem 256: (2023)
- Abdel-Magid, AF Inhibitors of the PD-1/PD-L1 Pathway Can Mobilize the Immune System: An Innovative Potential Therapy for Cancer and Chronic Infections. ACS Med Chem Lett 6: 489-90 (2015)
- Aktoudianakis, E; Cho, A; Du, Z; Graupe, M; Lad, LT; Machicao Tello, PA; Medley, JW; Metobo, SE; Mukherjee, PK; Naduthambi, D; Parkhill, EQ; Phillips, BW; Simonovich, SP; Squires, NH; Wang, P; Watkins, WJ; Xu, J; Yang, KS; Ziebenhaus, CA PD-1/PD-L1 inhibitors US Patent US10710986 (2020)
- Boisgerault, N; Bertrand, P Inside PD-1/PD-L1,2 with their inhibitors. Eur J Med Chem 256: (2023)
- van der Straat, R; Draijer, R; Surmiak, E; Butera, R; Land, L; Magiera-Mularz, K; Musielak, B; Plewka, J; Holak, TA; Dömling, A 1,5-Disubstituted tetrazoles as PD-1/PD-L1 antagonists. RSC Med Chem 15: 1210-1215 (2024)
- DANN, SG; MILLER, NL; VANARSDALE, TL COMBINATION THERAPY US Patent US20230321042 (2023)
- SATCHI-FAINARO, R; FLORINDO, H; GUEDES, R; ACÚRCIO, R MODULATORS OF PD-L1/PD-1 INTERACTION AND USES THEREOF US Patent US20230390287 (2023)
- Guo, J; Yu, F; Zhang, K; Jiang, S; Zhang, X; Wang, T Beyond inhibition against the PD-1/PD-L1 pathway: development of PD-L1 inhibitors targeting internalization and degradation of PD-L1. RSC Med Chem 15: 1096-1108 (2024)
- Wu, M; Wu, Y; Jin, Y; Mao, X; Zeng, S; Yu, H; Zhang, J; Jin, Y; Wu, Y; Xu, T; Chen, Y; Wang, Y; Yao, X; Che, J; Huang, W; Dong, X Discovery of an Exceptionally Orally Bioavailable and Potent HPK1 PROTAC with Enhancement of Antitumor Efficacy of Anti-PD-L1 Therapy. J Med Chem 67: 13852-13878
- Wang, T; Cai, S; Cheng, Y; Zhang, W; Wang, M; Sun, H; Guo, B; Li, Z; Xiao, Y; Jiang, S Discovery of Small-Molecule Inhibitors of the PD-1/PD-L1 Axis That Promote PD-L1 Internalization and Degradation. J Med Chem 65: 3879-3893 (2022)
- Negi, A; Murphy, PV Development of Mcl-1 inhibitors for cancer therapy. Eur J Med Chem 210: (2021)
- Butera, R; Ważyńska, M; Magiera-Mularz, K; Plewka, J; Musielak, B; Surmiak, E; Sala, D; Kitel, R; de Bruyn, M; Nijman, HW; Elsinga, PH; Holak, TA; Dömling, A Design, Synthesis, and Biological Evaluation of Imidazopyridines as PD-1/PD-L1 Antagonists. ACS Med Chem Lett 12: 768-773 (2021)
- Lin, X; Lu, X; Luo, G; Xiang, H Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur J Med Chem 186: (2020)
- Le Biannic, R; Magnez, R; Klupsch, F; Leleu-Chavain, N; Thiroux, B; Tardy, M; El Bouazzati, H; Dezitter, X; Renault, N; Vergoten, G; Bailly, C; Quesnel, B; Thuru, X; Millet, R Pyrazolones as inhibitors of immune checkpoint blocking the PD-1/PD-L1 interaction. Eur J Med Chem 236: (2022)
- Lu, L; Qi, Z; Wang, T; Zhang, X; Zhang, K; Wang, K; Cheng, Y; Xiao, Y; Li, Z; Jiang, S Design, Synthesis, and Evaluation of PD-1/PD-L1 Antagonists Bearing a Benzamide Scaffold. ACS Med Chem Lett 13: 586-592 (2022)
- Konieczny, M; Musielak, B; Kocik, J; Skalniak, L; Sala, D; Czub, M; Magiera-Mularz, K; Rodriguez, I; Myrcha, M; Stec, M; Siedlar, M; Holak, TA; Plewka, J Di-bromo-Based Small-Molecule Inhibitors of the PD-1/PD-L1 Immune Checkpoint. J Med Chem 63: 11271-11285 (2020)
- Guzik, K; Zak, KM; Grudnik, P; Magiera, K; Musielak, B; Törner, R; Skalniak, L; Dömling, A; Dubin, G; Holak, TA Small-Molecule Inhibitors of the Programmed Cell Death-1/Programmed Death-Ligand 1 (PD-1/PD-L1) Interaction via Transiently Induced Protein States and Dimerization of PD-L1. J Med Chem 60: 5857-5867 (2017)
- Sasmal, P; Kumar Babasahib, S; Prashantha Kumar, BR; Manjunathaiah Raghavendra, N Biphenyl-based small molecule inhibitors: Novel cancer immunotherapeutic agents targeting PD-1/PD-L1 interaction. Bioorg Med Chem 73: (2022)
- Hec-Gałązka, A; Tyrcha, U; Barczyński, J; Bielski, P; Mikitiuk, M; Gudz, GP; Kitel, R; Musielak, B; Plewka, J; Sitar, T; Holak, TA Nonsymmetrically Substituted 1,1'-Biphenyl-Based Small Molecule Inhibitors of the PD-1/PD-L1 Interaction. ACS Med Chem Lett 15: 828-836
- Klaus, C; Raimondi, MA; Daigle, SR; Pollock, RM Combination therapy for treating cancer US Patent US9446064 (2016)
- Parlati, F; Orford, K; Whiting, SH Combination therapy with glutaminase inhibitors US Patent US10278968 (2019)
- Dunham, RM; Margolis, D; Tai, VW; Tang, J Compounds useful in HIV therapy US Patent US11492361 (2022)
- Iqbal, M; Messina McLaughlin, PA; Dunn, D; Mallya, S; Husten, J; Ator, MA; Chatterjee, S Proteasome inhibitors for cancer therapy. Bioorg Med Chem 20: 2362-8 (2012)
- Cooper, CB; Huang, H; Zhang, D; Fotouhi, N; Kaneko, T Substituted phenyloxazolidinones for antimicrobial therapy US Patent US11964949 (2024)
- Pan, C; Yang, H; Lu, Y; Hu, S; Wu, Y; He, Q; Dong, X Recent advance of peptide-based molecules and nonpeptidic small-molecules modulating PD-1/PD-L1 protein-protein interaction or targeting PD-L1 protein degradation. Eur J Med Chem 213: (2021)
- Deng, H; Han, Y; Liu, L; Zhang, H; Liu, D; Wen, J; Huang, M; Zhao, L Targeting Myeloid Leukemia-1 in Cancer Therapy: Advances and Directions. J Med Chem 67: 5963-5998
- Shen, L; Wang, B; Wang, SP; Ji, SK; Fu, MJ; Wang, SW; Hou, WQ; Dai, XJ; Liu, HM Combination Therapy and Dual-Target Inhibitors Based on LSD1: New Emerging Tools in Cancer Therapy. J Med Chem 67: 922-951
- Cai, S; Wang, K; Qi, Z; Ye, K; Zhou, X; Jiang, S; Zhang, K; Zhang, X; Wang, T Design, synthesis, and evaluation of PD-1/PD-L1 small-molecule inhibitors bearing a rigid indane scaffold. Eur J Med Chem 256: (2023)
- Wu, X; Meng, Y; Liu, L; Gong, G; Zhang, H; Hou, Y; Liu, C; Wu, D; Qin, M Insights into non-peptide small-molecule inhibitors of the PD-1/PD-L1 interaction: Development and perspective. Bioorg Med Chem 33: (2021)
- Cui, JJ; Zhai, D Combination therapy involving diaryl macrocyclic compounds US Patent US11291667 (2022)
- Williams, M; Kowaluk, EA; Arneric, SP Emerging molecular approaches to pain therapy. J Med Chem 42: 1481-500 (1999)
- Bilcer, GM; Kelly, TA Granzyme B directed imaging and therapy US Patent US11667645 (2023)
- Manning, HC; Schulte, M Metabolism probes for therapy and diagnosis US Patent US10189805 (2019)
- Chiamvimonvat, N; Hammock, BD; Sirish, P Methods of improving cell-based therapy US Patent US11723929 (2023)
- Mederski, W; Fuchss, T; Emde, U; Buchstaller, H Morpholinylbenzotriazines for use in cancer therapy US Patent US9187469 (2015)
- An, Q; Li, C; Chen, Y; Deng, Y; Yang, T; Luo, Y Repurposed drug candidates for antituberculosis therapy. Eur J Med Chem 192: (2020)
- Li, X; Dou, J; You, Q; Jiang, Z Inhibitors of BCL2A1/Bfl-1 protein: Potential stock in cancer therapy. Eur J Med Chem 220: (2021)
- Huang, X; Chen, H; Dai, X; Xu, M; Wang, K; Feng, Z Design, synthesis, and structure-activity relationship of PD-1/PD-L1 inhibitors with a benzo[d]isoxazole scaffold. Bioorg Med Chem Lett 52: (2021)
- Xu, Y; Du, H; Guo, W; Liu, B; Yan, W; Zhang, C; Qin, L; Huang, J; Wang, H; Wu, S; Ren, W; Zou, Y; Wang, J; Zhu, Q; Xu, Y; Gu, H Discovery of Highly Potent Small-Molecule PD-1/PD-L1 Inhibitors with a Novel Scaffold for Cancer Immunotherapy. J Med Chem 67: 4083-4099
- Zhang, Y; Yin, F; Luo, Z; Li, S; Li, X; Wan, S; Chen, Y; Kong, L; Wang, X Improving tumor sensitivity by the introduction of an ester chain to triaryl derivatives targeting PD-1/PD-L1. Eur J Med Chem 271:
- Chen, T; Li, Q; Liu, Z; Chen, Y; Feng, F; Sun, H Peptide-based and small synthetic molecule inhibitors on PD-1/PD-L1 pathway: A new choice for immunotherapy? Eur J Med Chem 161: 378-398 (2019)
- Abdel-Magid, AF Adenosine Receptor Antagonists as Potential Cancer Therapy. ACS Med Chem Lett 12: 1892-1893 (2021)
- FERRARI, N; SAINI, HK; AHN, JS BIOMARKERS FOR CANCER THERAPY USING MDM2 ANTAGONISTS US Patent US20230338337 (2023)
- Bylund, J; Ek, ME; Gravenfors, Y; Nordvall, G; Minidis, A; Vallin, KS; Viklund, J; Holenz, J; Von Berg, S; Sohn, D Bis-(sulfonylamino) derivatives for use in therapy US Patent US9145380 (2015)
- Conway, SJ Bromodomains: are readers right for epigenetic therapy? ACS Med Chem Lett 3: 691-694 (2012)
- Assad, A Combination therapy for treatment of hematological diseases US Patent US11324749 (2022)
- Errico, JP; Mugrage, B; Turchi, I; Sills, M; Ong, J; Allocco, J; Wines, P; Bastos, M Combination therapy with MDM2 and EFGR inhibitors US Patent US9023354 (2015)
- Abdel-Magid, AF Inhibitors of BRD4 as Potential Cancer Therapy. ACS Med Chem Lett 7: 728-9 (2016)
- Kargbo, RB Ligand Design for Cereblon Based Immunomodulatory Therapy. ACS Med Chem Lett 11: 1088-1089 (2020)
- Bi, Y; Carson, KG; Harriman, GC; Thangapandian, S; Kuper, CJ; Degorce, SL; Whittaker, BP; Lewis, A; Palomero-Vazquez, MA PYRAZOLYLSULFONAMIDE COMPOUNDS AND THEIR USE IN THERAPY US Patent US20240150321 (2024)
- Sattler, M; Popowicz, G; Dawidowski, M; Emmanouilidis, L; Erdmann, R; Schliebs, W; Kalel, V Pyrazolopyridine derivatives and their use in therapy US Patent US10138242 (2018)
- BJÖRK, A; HEDLUND, G SUBSTITUTED BENZAMIDES AND THEIR USE IN THERAPY US Patent US20250064761 (2025)
- Shuttleworth, SJ; Tomassi, CD Scriptaid isosteres and their use in therapy US Patent US8748458 (2014)
- Cázares-Körner, A; Helleday, T; Visnes, T; Wallner, O; Koolmeister, T Substituted benzodiazoles and use thereof in therapy US Patent US11970474 (2024)
- Yang, G; Li, Y; Zhao, Y; Ouyang, L; Chen, Y; Liu, B; Liu, J Targeting Atg4B for cancer therapy: Chemical mediators. Eur J Med Chem 209: (2021)
- Lodola, A; Giorgio, C; Incerti, M; Zanotti, I; Tognolini, M Targeting Eph/ephrin system in cancer therapy. Eur J Med Chem 142: 152-162 (2017)
- Miao, Q; Ma, K; Chen, D; Wu, X; Jiang, S Targeting tropomyosin receptor kinase for cancer therapy. Eur J Med Chem 175: 129-148 (2019)
- Song, Z; Liu, B; Peng, X; Gu, W; Sun, Y; Xing, L; Xu, Y; Geng, M; Ai, J; Zhang, A Design, Synthesis, and Pharmacological Evaluation of Biaryl-Containing PD-1/PD-L1 Interaction Inhibitors Bearing a Unique Difluoromethyleneoxy Linkage. J Med Chem 64: 16687-16702 (2021)
- Wang, K; Cai, S; Cheng, Y; Qi, Z; Ni, X; Zhang, K; Xiao, Y; Zhang, X; Wang, T Discovery of Benzo[d]oxazoles as Novel Dual Small-Molecule Inhibitors Targeting PD-1/PD-L1 and VISTA Pathway. J Med Chem 67: 18526-18548
- Zaber, J; Skalniak, L; Gudz, GP; Hec-Gałązka, A; Zarnik, M; Tyrcha, U; Stec, M; Siedlar, M; Holak, TA; Sitar, T; Muszak, D N-methylmorpholine incorporation into the structure of biphenyl leads to the bioactive inhibitor of PD-1/PD-L1 interaction. Bioorg Med Chem Lett 110:
- Wang, Y; Kun Huang, na; Gao, Y; Yuan, D; Ling, L; Liu, J; Wu, S; Chen, R; Li, H; Xiong, Y; Liu, H; Ma, J Discovery of quinazoline derivatives as novel small-molecule inhibitors targeting the programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) interaction. Eur J Med Chem 229: (2022)
- Malathong, V; Mali, VR; McMurtrie, DJ; Punna, S; Roth, HS; Singh, R; Yang, J; Zhang, P Indanes as PD-L1 inhibitors US Patent US11872217 (2024)
- Perry, E; Mills, JJ; Zhao, B; Wang, F; Sun, Q; Christov, PP; Tarr, JC; Rietz, TA; Olejniczak, ET; Lee, T; Fesik, S Fragment-based screening of programmed death ligand 1 (PD-L1). Bioorg Med Chem Lett 29: 786-790 (2019)
- Bylock, LA Benzodioxane inhibitors of leukotriene production for combination therapy US Patent US9662339 (2017)
- Woolford, AJ; Howard, S; Buck, IM; Chessari, G; Johnson, CN; Tamanini, E; Day, JE; Chiarparin, E; Heightman, TD; Frederickson, M; Griffiths-Jones, CM Bicyclic heterocycle compounds and their uses in therapy US Patent US9018214 (2015)
- Cantley, LC; Hopkins, B; Goncalves, M; Mukherjee, S Combination therapy for PI3K-associated disease or disorder US Patent US12324807 (2025)
- Sonawane, YA; Taylor, MA; Napoleon, JV; Rana, S; Contreras, JI; Natarajan, A Cyclin Dependent Kinase 9 Inhibitors for Cancer Therapy. J Med Chem 59: 8667-8684 (2016)
- Shuttleworth, SJ; Tomassi, CD; Cecil, AR; MacCormick, S; Nodes, WJ; Silva, FA Histone deacetylase inhibitors and their use in therapy US Patent US10150763 (2018)
- Rauh, D; Gontla, R; Weisner, J Kinase inhibitors and their use in cancer therapy US Patent US10550114 (2020)
- Fu, S; Zheng, Q; Zhang, D; Lin, C; Ouyang, L; Zhang, J; Chen, L Medicinal chemistry strategies targeting PRMT5 for cancer therapy. Eur J Med Chem 244: (2022)
- Liang, Q; Liu, M; Li, J; Tong, R; Hu, Y; Bai, L; Shi, J NAE modulators: A potential therapy for gastric carcinoma. Eur J Med Chem 231: (2022)
- de Los Ríos, C; Marco-Contelles, J Tacrines for Alzheimer's disease therapy. III. The PyridoTacrines. Eur J Med Chem 166: 381-389 (2019)
- Velnati, S; Ruffo, E; Massarotti, A; Talmon, M; Varma, KSS; Gesu, A; Fresu, LG; Snow, AL; Bertoni, A; Capello, D; Tron, GC; Graziani, A; Baldanzi, G Identification of a novel DGKα inhibitor for XLP-1 therapy by virtual screening. Eur J Med Chem 164: 378-390 (2019)
- Tang, K; Wang, B; Yu, B; Liu, HM Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors and PROTAC-based degraders for cancer therapy. Eur J Med Chem 227: (2022)
- Zhang, J; Zhang, L; Wang, J; Ouyang, L; Wang, Y Polo-like Kinase 1 Inhibitors in Human Cancer Therapy: Development and Therapeutic Potential. J Med Chem 65: 10133-10160 (2022)
- Wang, H; Shen, L; Chen, L; Gao, Y; Ma, L; Lian, W; Zhang, Z; Liu, H; Yang, H; Wang, J; Zhao, D; Cheng, M Design, synthesis, pharmacological evaluation, and computational study of benzo[d] isothiazol-based small molecule inhibitors targeting PD-1/PD-l1 interaction. Eur J Med Chem 275:
- Yang, X; Cheng, B; Xiao, Y; Xue, M; Liu, T; Cao, H; Chen, J Discovery of novel CA-4 analogs as dual inhibitors of tubulin polymerization and PD-1/PD-L1 interaction for cancer treatment. Eur J Med Chem 213: (2021)
- Sun, C; Yin, M; Cheng, Y; Kuang, Z; Liu, X; Wang, G; Wang, X; Yuan, K; Min, W; Dong, J; Hou, Y; Hu, L; Zhang, G; Pei, W; Wang, L; Sun, Y; Yu, X; Xiao, Y; Deng, H; Yang, P Novel Small-Molecule PD-L1 Inhibitor Induces PD-L1 Internalization and Optimizes the Immune Microenvironment. J Med Chem 66: 2064-2083 (2023)
- Meng, Y; Chu, C; Niu, X; Cheng, L; Wu, D; Liu, L; Zhang, S; Li, T; Hou, Y; Liu, Y; Qin, M Discovery of 4-phenylindolines containing a (5-cyanopyridin-3-yl)methoxy moiety as potent inhibitors of the PD-1/PD-L1 interaction. Bioorg Med Chem Lett 63: (2022)
- Lange, C; Punna, S; Singh, R; Yang, J; Zhang, P Indane-amines as PD-L1 antagonists US Patent US10568874 (2020)
- Peel, M; Smith, P Combination therapy comprising JAK pathway inhibitor and rock inhibitor US Patent US11918581 (2024)
- Liu, T; Wang, Y; Wang, J; Ren, C; Chen, H; Zhang, J DYRK1A inhibitors for disease therapy: Current status and perspectives. Eur J Med Chem 229: (2022)
- Wei, C; Bajpai, R; Sharma, H; Heitmeier, M; Jain, AD; Matulis, SM; Nooka, AK; Mishra, RK; Hruz, PW; Schiltz, GE; Shanmugam, M Development of GLUT4-selective antagonists for multiple myeloma therapy. Eur J Med Chem 139: 573-586 (2017)
- Webb, RL; Schiering, N; Sedrani, R; Maibaum, J Direct renin inhibitors as a new therapy for hypertension. J Med Chem 53: 7490-520 (2010)
- Elwaie, TA; Abbas, SE; Aly, EI; George, RF; Ali, H; Kraiouchkine, N; Abdelwahed, KS; Fandy, TE; El Sayed, KA; Abd Elmageed, ZY; Ali, HI HER2 Kinase-Targeted Breast Cancer Therapy: Design, Synthesis, and J Med Chem 63: 15906-15945 (2020)
- Luan, Y; Li, J; Bernatchez, JA; Li, R Kinase and Histone Deacetylase Hybrid Inhibitors for Cancer Therapy. J Med Chem 62: 3171-3183 (2019)
- Pillaiyar, T; Laufer, S Kinases as Potential Therapeutic Targets for Anti-coronaviral Therapy. J Med Chem 65: 955-982 (2022)
- Tautz, L; Senis, YA; Oury, C; Rahmouni, S Perspective: Tyrosine phosphatases as novel targets for antiplatelet therapy. Bioorg Med Chem 23: 2786-97 (2015)
- Dar, A; Godara, P; Prusty, D; Bashir, M Plasmodium falciparum topoisomerases: Emerging targets for anti-malarial therapy. Eur J Med Chem 265:
- Abdel-Magid, AF Potential of Cyclin-Dependent Kinase Inhibitors as Cancer Therapy. ACS Med Chem Lett 12: 182-184 (2021)
- Halder, P; Rai, A; Talukdar, V; Das, P; Lakkaniga, NR Pyrazolopyridine-based kinase inhibitors for anti-cancer targeted therapy. RSC Med Chem 15: 1452-1470
- Ayati, A; Moghimi, S; Toolabi, M; Foroumadi, A Pyrimidine-based EGFR TK inhibitors in targeted cancer therapy. Eur J Med Chem 221: (2021)
- Tian, E; Zhou, C; Quan, S; Su, C; Zhang, G; Yu, Q; Li, J; Zhang, J RIPK2 inhibitors for disease therapy: Current status and perspectives. Eur J Med Chem 259:
- Song, Y; Wang, S; Zhao, M; Yang, X; Yu, B Strategies Targeting Protein Tyrosine Phosphatase SHP2 for Cancer Therapy. J Med Chem 65: 3066-3079 (2022)
- Velu, SE; Wu, H; Zhang, Q; Nijampatnam, B Streptococcus mutans glucosyl transferase inhibitors for dental caries therapy US Patent US11866416 (2024)
- Sharma, PC; Bansal, KK; Sharma, A; Sharma, D; Deep, A Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur J Med Chem 188: (2020)
- Pietsch, M; Wodtke, R; Pietzsch, J; Löser, R Tissue transglutaminase: an emerging target for therapy and imaging. Bioorg Med Chem Lett 23: 6528-43 (2014)
- Wang, YQ; Wang, PY; Wang, YT; Yang, GF; Zhang, A; Miao, ZH An Update on Poly(ADP-ribose)polymerase-1 (PARP-1) Inhibitors: Opportunities and Challenges in Cancer Therapy. J Med Chem 59: 9575-9598 (2016)
- Zhang, F; Zhang, H; Zhou, S; Plewka, J; Wang, M; Sun, S; Wu, C; Yu, Q; Zhu, M; Awadasseid, A; Wu, Y; Magiera-Mularz, K; Zhang, W Design, synthesis, and evaluation of antitumor activity of 2-arylmethoxy-4-(2-fluoromethyl-biphenyl-3-ylmethoxy) benzylamine derivatives as PD-1/PD-l1 inhibitors. Eur J Med Chem 276:
- Liu, J; Cheng, Y; Yuan, L; Liu, T; Ruan, Y; Ren, Y; Li, L; Jiang, S; Xiao, Y; Chen, J Discovery and Crystallography Study of Novel Biphenyl Ether and Oxadiazole Thioether (Non-Arylmethylamine)-Based Small-Molecule PD-1/PD-L1 Inhibitors as Immunotherapeutic Agents. J Med Chem 66: 13172-13188 (2023)
- Li, J; Xi, W; Li, X; Sun, H; Li, Y Advances in inhibition of protein-protein interactions targeting hypoxia-inducible factor-1 for cancer therapy. Bioorg Med Chem 27: 1145-1158 (2019)
- Adhikari, A; Chauhan, K; Adhikari, M; Tiwari, AK Colony Stimulating Factor-1 Receptor: An emerging target for neuroinflammation PET imaging and AD therapy. Bioorg Med Chem 100:
- Feng, X; Liao, D; Liu, D; Ping, A; Li, Z; Bian, J Development of Indoleamine 2,3-Dioxygenase 1 Inhibitors for Cancer Therapy and Beyond: A Recent Perspective. J Med Chem 63: 15115-15139 (2020)
- Zhou, XL; Zhao, F; Xu, YT; Guan, YY; Yu, T; Zhang, YZ; Duan, YC; Zhao, Y A comprehensive review of BET-targeting PROTACs for cancer therapy. Bioorg Med Chem 73: (2022)
- Singh, M; Kaur, M; Kukreja, H; Chugh, R; Silakari, O; Singh, D Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur J Med Chem 70: 165-88 (2013)
- Diab, S; Yu, M; Wang, S CDK7 Inhibitors in Cancer Therapy: The Sweet Smell of Success? J Med Chem 63: 7458-7474 (2020)
- KUGLER, DG; QIN, J; MAIER, A; OLSON, R; WICHROSKI, M; VELAPARTHI, U; DARNE, CP; DASGUPTA, B; GRUNENFELDER, DC; WARRIER, JS; RAHAMAN, H; JALAGAM, PR; ROY, S; SIVAKUMAR, P COMBINATION OF A T CELL THERAPY AND A DGK INHIBITOR US Patent US20240108654 (2024)
- de Almeida, SMV; Santos Soares, JC; Dos Santos, KL; Alves, JEF; Ribeiro, AG; Jacob, ÍTT; da Silva Ferreira, CJ; Dos Santos, JC; de Oliveira, JF; de Carvalho Junior, LB; de Lima, MDCA COVID-19 therapy: What weapons do we bring into battle? Bioorg Med Chem 28: (2020)
- Tadesse, S; Caldon, EC; Tilley, W; Wang, S Cyclin-Dependent Kinase 2 Inhibitors in Cancer Therapy: An Update. J Med Chem 62: 4233-4251 (2019)
- Yang, J; Tian, E; Chen, L; Liu, Z; Ren, Y; Mao, W; Zhang, Y; Zhang, J Development and therapeutic perspectives of CXCR4 antagonists for disease therapy. Eur J Med Chem 275:
- Xie, Z; Meng, Z; Yang, X; Duan, Y; Wang, Q; Liao, C Factor XIa Inhibitors in Anticoagulation Therapy: Recent Advances and Perspectives. J Med Chem 66: 5332-5363 (2023)
- Slusher, BS; Le, A; Tsukamoto, T Glutaminase inhibitor discovery and nanoparticle-enhanced delivery for cancer therapy US Patent US11191732 (2021)
- TATE, EW; BELL, AS; BONNERT, R; CARR, R; RITCHIE, TJ IMIDAZO[1,2-A]PYRIDINE COMPOUNDS AND THEIR USE IN THERAPY US Patent US20240166643 (2024)
- Lynch, KR; Kharel, Y; Santos, WL; Fritzemeier, RG; Burgio, AL; Shrader, C; Foster, D INHIBITORS OF SPINSTER HOMOLOG 2 (SPNS2) FOR USE IN THERAPY US Patent US20230331683 (2023)
- Papadimitropoulou, A; Makri, M; Zoidis, G MYC the oncogene from hell: Novel opportunities for cancer therapy. Eur J Med Chem 267:
- Lee, MJ; Bhattarai, D; Jang, H; Baek, A; Yeo, IJ; Lee, S; Miller, Z; Lee, S; Hong, JT; Kim, DE; Lee, W; Kim, KB Macrocyclic Immunoproteasome Inhibitors as a Potential Therapy for Alzheimer's Disease. J Med Chem 64: 10934-10950 (2021)
- Abdel-Magid, AF P21-Activated Kinase 4 (PAK4) Inhibitors as Potential Cancer Therapy. ACS Med Chem Lett 6: 17-8 (2015)
- Kargbo, RB PARP7 Inhibition: A Promising Pathway to Advancements in Cancer Therapy. ACS Med Chem Lett 14: 1141-1143 (2023)
- Petrov, SA; Zyk, NY; Machulkin, AE; Beloglazkina, EK; Majouga, AG PSMA-targeted low-molecular double conjugates for diagnostics and therapy. Eur J Med Chem 225: (2021)
- Coelingh Bennink, HJ; Bunschoten, EJ Pharmaceutical composition comprising estetrol derivatives for use in cancer therapy US Patent US9034854 (2015)
- Coelingh Bennink, HJ; Bunschoten, EJ Pharmaceutical compositions comprising estetrol derivatives for use in cancer therapy US Patent US9561238 (2017)
- Estrela, JM; Mena, S; Obrador, E; Benlloch, M; Castellano, G; Salvador, R; Dellinger, RW Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J Med Chem 60: 9413-9436 (2017)
- do Carmo Carreiras, M; Ismaili, L; Marco-Contelles, J Propargylamine-derived multi-target directed ligands for Alzheimer's disease therapy. Bioorg Med Chem Lett 30: (2020)
- Song, R; Qiao, W; He, J; Huang, J; Luo, Y; Yang, T Proteases and Their Modulators in Cancer Therapy: Challenges and Opportunities. J Med Chem 64: 2851-2877 (2021)
- Mehta, N; Kaur, M; Singh, M; Chand, S; Vyas, B; Silakari, P; Bahia, MS; Silakari, O Purinergic receptor P2X₇: a novel target for anti-inflammatory therapy. Bioorg Med Chem 22: 54-88 (2014)
- Guo, K; Ma, X; Li, J; Zhang, C; Wu, L Recent advances in combretastatin A-4 codrugs for cancer therapy. Eur J Med Chem 241: (2022)
- Yang, X; Xu, L; Yang, L; Xu, S Research progress of STAT3-based dual inhibitors for cancer therapy. Bioorg Med Chem 91: (2023)
- Fantacuzzi, M; Amoroso, R; Carradori, S; De Filippis, B Resveratrol-based compounds and neurodegeneration: Recent insight in multitarget therapy. Eur J Med Chem 233: (2022)
- Pomper, MG; Mease, RC; Ray, S; Chen, Y; Yang, X Scaffolds and multifunctional intermediates for imaging PSMA and cancer therapy US Patent US10736974 (2020)
- Chu, T; Karmakar, J; Haggie, PM; Tan, JA; Master, R; Ramaswamy, K; Verkman, AS; Anderson, MO; Cil, O Selective isoxazolopyrimidine PAT1 (SLC26A6) inhibitors for therapy of intestinal disorders. RSC Med Chem 14: 2342-2347 (2023)
- Liang, X; Chen, R; Wang, C; Wang, Y; Zhang, J Targeting HSP90 for Cancer Therapy: Current Progress and Emerging Prospects. J Med Chem 67: 15968-15995
- Kargbo, RB Targeting the KRAS G12D Mutant as Potential Therapy in Cancer. ACS Med Chem Lett 12: 1212-1213 (2021)
- Ochse, M; Lange, U; Braje, W; Behl, B; Hornberger, W; Mezler, M; Amberg, W; Hutchins, CW Tetrahydroisoquinolines, pharmaceutical compositions containing them, and their use in therapy US Patent US8653100 (2014)
- Kargbo, RB Tumor-Targeted Bivalent Protein Degradation for Application in Cancer Therapy. ACS Med Chem Lett 12: 326-327 (2021)
- Josa-Culleré, L; Llebaria, A Visible-Light-Controlled Histone Deacetylase Inhibitors for Targeted Cancer Therapy. J Med Chem 66: 1909-1927 (2023)
- ACKERMAN, SE; ALONSO, MN; DORNAN, D; KOWANETZ, M; KUDIRKA, R; LEE, A; MALLET, W; SAFINA, B; ZHOU, M ANTI-PD-L1 IMMUNOCONJUGATES, AND USES THEREOF US Patent US20240033370 (2024)
- Malathong, V; Fan, P; Lange, C; Mali, VR; McMurtrie, DJ; Punna, S; Roth, HS; Singh, R; Yang, J; Zhang, P Compounds for treatment of PD-L1 diseases US Patent US11485708 (2022)
- Wu, T; Raboisson, PJ; Gonzalvez, F; Stoycheva, AD; Deval, J; Liu, C; Zhang, Q Methods and compositions for targeting PD-L1 US Patent US11760761 (2023)
- Zhang, H; Zhou, S; Plewka, J; Wu, C; Zhu, M; Yu, Q; Musielak, B; Wang, X; Awadasseid, A; Magiera-Mularz, K; Wu, Y; Zhang, W Design, Synthesis, and Antitumor Activity Evaluation of 2-Arylmethoxy-4-(2,2'-dihalogen-substituted biphenyl-3-ylmethoxy) Benzylamine Derivatives as Potent PD-1/PD-L1 Inhibitors. J Med Chem 66: 10579-10603 (2023)
- Cheng, B; Wang, W; Niu, X; Ren, Y; Liu, T; Cao, H; Wang, S; Tu, Y; Chen, J; Liu, S; Yang, X; Chen, J Discovery of Novel and Highly Potent Resorcinol Dibenzyl Ether-Based PD-1/PD-L1 Inhibitors with Improved Drug-like and Pharmacokinetic Properties for Cancer Treatment. J Med Chem 63: 15946-15959 (2020)
- Yang, Y; Wang, K; Chen, H; Feng, Z Design, synthesis, evaluation, and SAR of 4-phenylindoline derivatives, a novel class of small-molecule inhibitors of the programmed cell death-1/ programmed cell death-ligand 1 (PD-1/PD-L1) interaction. Eur J Med Chem 211: (2021)
- Hu, X; Zhang, J; Zhang, Y; Jiao, F; Wang, J; Chen, H; Ouyang, L; Wang, Y Dual-target inhibitors of poly (ADP-ribose) polymerase-1 for cancer therapy: Advances, challenges, and opportunities. Eur J Med Chem 230: (2022)
- ECKMANN, J; FRIESS, T; HERTING, F; PETTAZZONI, PF; WICHMANN, J METHODS AND COMPOSITIONS COMPRISING A BRAF INHIBITOR AND A PD-1 BINDING ANTAGONIST US Patent US20240197735 (2024)
- Hersperger, R; Buchheit, KH; Cammisuli, S; Enz, A; Lohse, O; Ponelle, M; Schuler, W; Schweitzer, A; Walker, C; Zehender, H; Zenke, G; Zimmerlin, AG; Zollinger, M; Mazzoni, L; Fozard, JR A locally active antiinflammatory macrolide (MLD987) for inhalation therapy of asthma. J Med Chem 47: 4950-7 (2004)
- Pohlki, F; Lange, U; Amberg, W; Ochse, M; Behl, B; Hutchins, CW Aminoindane derivatives, pharmaceutical compositions containing them, and their use in therapy US Patent US9227930 (2016)
- Amberg, W; Ochse, M; Lange, U; Kling, A; Behl, B; Hornberger, W; Mezler, M; Hutchins, CW Aminotetraline derivatives, pharmaceutical compositions containing them, and their use in therapy US Patent US9067871 (2015)
- May, PC; Mergott, DJ Combination Alzheimer therapy using anti-N3pGlu Abeta antibodies + a BACE inhibitor US Patent US9999624 (2018)
- Chen, L; Mao, W; Ren, C; Li, J; Zhang, J Comprehensive Insights that Targeting PIM for Cancer Therapy: Prospects and Obstacles. J Med Chem 67: 38-64
- Philip, S; Kumarasiri, M; Teo, T; Yu, M; Wang, S Cyclin-Dependent Kinase 8: A New Hope in Targeted Cancer Therapy? J Med Chem 61: 5073-5092 (2018)
- Liu, J; Shikhman, AR; Lotz, MK; Wong, CH Hexosaminidase inhibitors as new drug candidates for the therapy of osteoarthritis. Chem Biol 8: 701-11 (2001)
- Sakamoto, H; Okamoto, K; Aoki, M; Kato, H; Katsume, A; Ohta, A; Tsukuda, T; Shimma, N; Aoki, Y; Arisawa, M; Kohara, M; Sudoh, M Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat Chem Biol 1: 333-7 (2006)
- Abdel-Magid, AF Inhibitors of Tropomyosin-Receptor Kinases (Trk's): Potential Pain Therapy and More. ACS Med Chem Lett 5: 8-9 (2014)
- Abdel-Magid, AF Nav1.7 Inhibitors: Potential Effective Therapy for the Treatment of Chronic Pain. ACS Med Chem Lett 6: 956-7 (2015)
- Malek, R; Arribas, RL; Palomino-Antolin, A; Totoson, P; Demougeot, C; Kobrlova, T; Soukup, O; Iriepa, I; Moraleda, I; Diez-Iriepa, D; Godyń, J; Panek, D; Malawska, B; Głuch-Lutwin, M; Mordyl, B; Siwek, A; Chabchoub, F; Marco-Contelles, J; Kiec-Kononowicz, K; Egea, J; de Los Ríos, C; Ismaili, L New Dual Small Molecules for Alzheimer's Disease Therapy Combining Histamine H J Med Chem 62: 11416-11422 (2019)
- Zhu, HY; Desai, J; Cooper, AB; Wang, J; Rane, DF; Kirschmeier, P; Strickland, C; Liu, M; Nomeir, AA; Girijavallabhan, VM New class of azaheptapyridine FPT inhibitors as potential cancer therapy agents. Bioorg Med Chem Lett 24: 1228-31 (2014)
- Wexler, RR; Greenlee, WJ; Irvin, JD; Goldberg, MR; Prendergast, K; Smith, RD; Timmermans, PB Nonpeptide angiotensin II receptor antagonists: the next generation in antihypertensive therapy. J Med Chem 39: 625-56 (1996)
- Pohlki, F; Lange, U; Amberg, W; Ochse, M; Behl, B; Hornberger, W; Mezler, M; Hutchins, CW; Turner, S Phenalkylamine derivatives, pharmaceutical compositions containing them, and their use in therapy US Patent US9238619 (2016)
- Amberg, W; Pohlki, F; Lange, U; Wang, YX; Zhao, HH; Li, H; Brewer, JT; Zanze, I; Dietrich, J; Vasudevan, A; Djuric, SW; Lao, Y; Hutchins, CW Pyrrolidine derivatives, pharmaceutical compositions containing them, and their use in therapy US Patent US9656955 (2017)
- Cheng, B; Pan, W; Xing, Y; Xiao, Y; Chen, J; Xu, Z Recent advances in DDR (DNA damage response) inhibitors for cancer therapy. Eur J Med Chem 230: (2022)
- Yang, X Research progress of LSD1-based dual-target agents for cancer therapy. Bioorg Med Chem 101: (2024)
- Kargbo, RB Selective Cyclin-Dependent Kinase Inhibitors and Their Application in Cancer Therapy. ACS Med Chem Lett 13: 1561-1563 (2022)
- Hill, JE; Linder, MK; Davies, KS; Sawada, GA; Morgan, J; Ohulchanskyy, TY; Detty, MR Selenorhodamine photosensitizers for photodynamic therapy of P-glycoprotein-expressing cancer cells. J Med Chem 57: 8622-34 (2014)
- Matsubara, T; Onishi, A; Saito, T; Shimada, A; Inoue, H; Taki, T; Nagata, K; Okahata, Y; Sato, T Sialic acid-mimic peptides as hemagglutinin inhibitors for anti-influenza therapy. J Med Chem 53: 4441-9 (2010)
- Silverman, RB; Meyskens, FL; Yang, S; Ji, H; Xue, F; Poulos, TL Specific nNOS inhibitors for the therapy and prevention of human melanoma US Patent US9090589 (2015)
- Liu, C; Li, Y; Liu, Z; Cao, C; Lin, M; Chen, X; Yuan, M; Fan, Y; Gu, X; Wang, L; Yang, F; Ye, F; Jin, J Structure-based discovery of potent CARM1 inhibitors for colorectal cancer therapy. Eur J Med Chem 269:
- OGIWARA, H; SASAKI, M THERAPY BASED ON SYNTHETIC LETHALITY IN SWI/SNF COMPLEX-DYSFUNCTION CANCER US Patent US20240122941 (2024)
- Cuffaro, D; Ciccone, L; Rossello, A; Nuti, E; Santamaria, S Targeting Aggrecanases for Osteoarthritis Therapy: From Zinc Chelation to Exosite Inhibition. J Med Chem 65: 13505-13532 (2022)
- Zeng, J; Zhang, J; Sun, Y; Wang, J; Ren, C; Banerjee, S; Ouyang, L; Wang, Y Targeting EZH2 for cancer therapy: From current progress to novel strategies. Eur J Med Chem 238: (2022)
- Pache, L; Chanda, SK; Vamos, MD; Cosford, ND; Teriete, P; Marlett, J; Diaz, A; Young, JA Use of inhibitor of apoptosis protein (IAP) antagonists in HIV therapy US Patent US10300074 (2019)
- Wu, L; Li, Z; Yao, W PD-L1/STING conjugates and methods of use US Patent US11596692 (2023)
- Xu, J; Kong, Y; Zhu, P; Du, M; Liang, X; Tong, Y; Li, X; Dong, C Progress in small-molecule inhibitors targeting PD-L1. RSC Med Chem 15: 1161-1175 (2024)
- Fan, P; Lange, C; Mali, VR; McMurtrie, DJ; Malathong, V; Punna, S; Singh, R; Yang, J; Zeng, Y; Zhang, P Triaryl compounds for treatment of PD-L1 diseases US Patent US11266643 (2022)
- Bengtsson, C; Borhade, S; Haraldsson, M; Helleday, T; Henriksson, M; Homan, E; Paulin, C; Sandberg, L; Scobie, M; Stenmark, P; Vallin, K 2,6-diamino-3,4-dihydropyrimidin-4-one derivatives and use thereof in therapy US Patent US11504368 (2022)
- Xi, M; Zhu, J; Zhang, F; Shen, H; Chen, J; Xiao, Z; Huangfu, Y; Wu, C; Sun, H; Xia, G Antibody-drug conjugates for targeted cancer therapy: Recent advances in potential payloads. Eur J Med Chem 276:
- Wang, ZC; Shen, FQ; Yang, MR; You, LX; Chen, LZ; Zhu, HL; Lu, YD; Kong, FL; Wang, MH Dihydropyrazothiazole derivatives as potential MMP-2/MMP-8 inhibitors for cancer therapy. Bioorg Med Chem Lett 28: 3816-3821 (2018)
- Chen, J; Zhao, T; He, F; Zhong, Y; Wang, S; Tang, Z; Qiu, Y; Wu, Z; Fang, M Discovery of bipyridine amide derivatives targeting pRXRα-PLK1 interaction for anticancer therapy. Eur J Med Chem 254: (2023)
- Fu, J; Wang, Y; Sun, Y; Wu, G; Lu, A; Zhang, S; Goodnow, R; Gilmer, T; Kastan, M; Kirsch, D Dual ATM and DNA-PK inhibitors for use in anti-tumor therapy US Patent US12187742 (2025)
- Liu, T; Wan, Y; Xiao, Y; Xia, C; Duan, G Dual-Target Inhibitors Based on HDACs: Novel Antitumor Agents for Cancer Therapy. J Med Chem 63: 8977-9002 (2020)
- Kargbo, RB Histone Deacetylase Inhibitors as Treatment for Targeting Multiple Components in Cancer Therapy. ACS Med Chem Lett 9: 167-168 (2018)
- Palmer, BD; Ching, L Inhibitors of tryptophan dioxygenases (IDO1 and TDO) and their use in therapy US Patent US11414428 (2022)
- Chatzisideri, T; Leonidis, G; Karampelas, T; Skavatsou, E; Velentza-Almpani, A; Bianchini, F; Tamvakopoulos, C; Sarli, V Integrin-Mediated Targeted Cancer Therapy Using c(RGDyK)-Based Conjugates of Gemcitabine. J Med Chem 65: 271-284 (2022)
- Ge, Z; Hao, M; Xu, M; Su, Z; Kang, Z; Xue, L; Zhang, C Novel nonsecosteroidal VDR ligands with phenyl-pyrrolyl pentane skeleton for cancer therapy. Eur J Med Chem 107: 48-62 (2016)
- Drygin, D; Haddach, M; Pierre, F; Ryckman, DM Potential use of selective and nonselective Pim kinase inhibitors for cancer therapy. J Med Chem 55: 8199-208 (2012)
- Zheng, Q; Zhang, W; Rao, GW Protein Lysine Methyltransferase SMYD2: A Promising Small Molecule Target for Cancer Therapy. J Med Chem 65: 10119-10132 (2022)
- Wang, Z; Wu, D; Zhao, X; Liu, C; Jia, S; He, Q; Huang, F; Cheng, Z; Lu, T; Chen, Y; Chen, Y; Yang, P; Lu, S Rational discovery of dual FLT3/HDAC inhibitors as a potential AML therapy. Eur J Med Chem 260:
- Shuai, W; Wang, G; Zhang, Y; Bu, F; Zhang, S; Miller, DD; Li, W; Ouyang, L; Wang, Y Recent Progress on Tubulin Inhibitors with Dual Targeting Capabilities for Cancer Therapy. J Med Chem 64: 7963-7990 (2021)
- Ma, S; Long, G; Jiang, Z; Zhang, Y; Sun, L; Pan, Y; You, Q; Guo, X Recent advances in targeting histone H3 lysine 36 methyltransferases for cancer therapy. Eur J Med Chem 274:
- Liang, Q; Wang, J; Zhao, L; Hou, J; Hu, Y; Shi, J Recent advances of dual FGFR inhibitors as a novel therapy for cancer. Eur J Med Chem 214: (2021)
- Yu, Z; Liang, YC; Berton, S; Liu, L; Zou, J; Chen, L; Xu, Z; Luo, C; Sun, J; Yang, W Small Molecule Targeting PPM1A Activates Autophagy for Mycobacterium tuberculosis Host-Directed Therapy. J Med Chem 67: 11917-11936
- Tang, W; Zhao, G Small molecules targeting HIF-1α pathway for cancer therapy in recent years. Bioorg Med Chem 28: (2020)
- Zhang, R; Wang, Y; Wu, A; Wang, J; Zhang, J Strategies of targeting CYP51 for IFIs therapy: Emerging prospects, opportunities and challenges. Eur J Med Chem 259:
- Jiang, Y; Li, X; Hou, J; Huang, Y; Wang, X; Jia, Y; Wang, Q; Xu, W; Zhang, J; Zhang, Y Synthesis and biological characterization of ubenimex-fluorouracil conjugates for anti-cancer therapy. Eur J Med Chem 143: 334-347 (2018)
- Zheng, J; Li, B; Wu, Y; Wu, X; Wang, Y Targeting Arginine Methyltransferase PRMT5 for Cancer Therapy: Updated Progress and Novel Strategies. J Med Chem 66: 8407-8427 (2023)
- Wang, ZH; Li, DD; Chen, WL; You, QD; Guo, XK Targeting protein-protein interaction between MLL1 and reciprocal proteins for leukemia therapy. Bioorg Med Chem 26: 356-365 (2018)
- Lacivita, E; Perrone, R; Margari, L; Leopoldo, M Targets for Drug Therapy for Autism Spectrum Disorder: Challenges and Future Directions. J Med Chem 60: 9114-9141 (2017)
- Li, R; Pourpak, A; Morris, SW Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J Med Chem 52: 4981-5004 (2010)
- Xu, Y; Wu, H; Huang, L; Zhai, B; Li, X; Xu, S; Wu, X; Zhu, Q; Xu, Q Rational design, synthesis and biological evaluation of dual PARP-1/2 and TNKS1/2 inhibitors for cancer therapy. Eur J Med Chem 237: (2022)
- Nozal, V; Martínez-González, L; Gomez-Almeria, M; Gonzalo-Consuegra, C; Santana, P; Chaikuad, A; Pérez-Cuevas, E; Knapp, S; Lietha, D; Ramírez, D; Petralla, S; Monti, B; Gil, C; Martín-Requero, A; Palomo, V; de Lago, E; Martinez, A TDP-43 Modulation by Tau-Tubulin Kinase 1 Inhibitors: A New Avenue for Future Amyotrophic Lateral Sclerosis Therapy. J Med Chem 65: 1585-1607 (2022)
- Ruengsatra, T; Soponpong, J; Nalinratana, N; Jirapongwattana, N; Dunkoksung, W; Rattanangkool, E; Deesiri, S; Srisa, J; Songthammanuphap, S; Udomnilobol, U; Prueksaritanont, T Design, synthesis, and optimization of novel PD-L1 inhibitors and the identification of a highly potent and orally bioavailable PD-L1 inhibitor. Eur J Med Chem 277:
- Wang, K; Zhang, X; Cheng, Y; Qi, Z; Ye, K; Zhang, K; Jiang, S; Liu, Y; Xiao, Y; Wang, T Discovery of Novel PD-L1 Inhibitors That Induce the Dimerization, Internalization, and Degradation of PD-L1 Based on the Fragment Coupling Strategy. J Med Chem 66: 16807-16827 (2023)
- Amberg, W; Pohlki, F; Lange, U; Wang, Y; Brewer, J; Vasudevan, A; Lao, Y; Hutchins, C; Zhao, H; Li, H Aminotetraline and aminoindane derivatives, pharmaceutical compositions containing them, and their use in therapy US Patent US9586942 (2017)
- Li, H; Cai, X; Yang, X; Zhang, X An overview of PROTACs targeting MDM2 as a novel approach for cancer therapy. Eur J Med Chem 272:
- Becker, DP; Lutz, JR., MR CARBORANE HYDROXAMIC ACID MATRIX METALLOPROTEINASE INHIBITORS AND AGENTS FOR BORON NEUTRON CAPTURE THERAPY US Patent US20240050571 (2024)
- Feng, Z; Zhu, S; Li, W; Yao, M; Song, H; Wang, RB Current approaches and strategies to identify Hedgehog signaling pathway inhibitors for cancer therapy. Eur J Med Chem 244: (2022)
- BICKERDIKE, MJ; WANGPAICHITR, M DUAL INHIBITORS OF TRYPTOPHAN DIOXYGENASES (IDO1 AND TDO) AND THEIR USE IN THERAPY US Patent US20250186410 (2025)
- Chen, X; Wang, C; Lu, D; Luo, H; Li, S; Yin, F; Luo, Z; Cui, N; Kong, L; Wang, X Design, synthesis and mechanism studies of dual EZH2/BRD4 inhibitors for cancer therapy. Bioorg Med Chem 91: (2023)
- Huang, W; Hulverson, MA; Choi, R; Arnold, SLM; Zhang, Z; McCloskey, MC; Whitman, GR; Hackman, RC; Rivas, KL; Barrett, LK; Ojo, KK; Van Voorhis, WC; Fan, E Development of 5-Aminopyrazole-4-carboxamide-based Bumped-Kinase Inhibitors for Cryptosporidiosis Therapy. J Med Chem 62: 3135-3146 (2019)
- Sun, D; Zhao, Y; Zhang, S; Zhang, L; Liu, B; Ouyang, L Dual-target kinase drug design: Current strategies and future directions in cancer therapy. Eur J Med Chem 188: (2020)
- Wang, S; Yuan, XH; Wang, SQ; Zhao, W; Chen, XB; Yu, B FDA-approved pyrimidine-fused bicyclic heterocycles for cancer therapy: Synthesis and clinical application. Eur J Med Chem 214: (2021)
- Abdel-Magid, AF LRRK2 Kinase Inhibitors as Possible Therapy for Parkinson's Disease and Other Neurodegenerative Disorders. ACS Med Chem Lett 10: 846-847 (2019)
- Yuan, Z; Chen, S; Sun, Q; Wang, N; Li, D; Miao, S; Gao, C; Chen, Y; Tan, C; Jiang, Y Olaparib hydroxamic acid derivatives as dual PARP and HDAC inhibitors for cancer therapy. Bioorg Med Chem 25: 4100-4109 (2017)
- Yuan, X; Bu, H; Zhou, J; Yang, CY; Zhang, H Recent Advances of SHP2 Inhibitors in Cancer Therapy: Current Development and Clinical Application. J Med Chem 63: 11368-11396 (2020)
- Xu, P; Ge, R Roles and drug development of METTL3 (methyltransferase-like 3) in anti-tumor therapy. Eur J Med Chem 230: (2022)
- Zheng, H; Youdim, MB; Fridkin, M Site-activated chelators targeting acetylcholinesterase and monoamine oxidase for Alzheimer's therapy. ACS Chem Biol 5: 603-10 (2010)
- Zheng, H; Youdim, MB; Fridkin, M Site-activated multifunctional chelator with acetylcholinesterase and neuroprotective-neurorestorative moieties for Alzheimer's therapy. J Med Chem 52: 4095-8 (2009)
- Xi, M; Chen, Y; Yang, H; Xu, H; Du, K; Wu, C; Xu, Y; Deng, L; Luo, X; Yu, L; Wu, Y; Gao, X; Cai, T; Chen, B; Shen, R; Sun, H Small molecule PROTACs in targeted therapy: An emerging strategy to induce protein degradation. Eur J Med Chem 174: 159-180 (2019)
- Rana, S; Sonawane, YA; Taylor, MA; Kizhake, S; Zahid, M; Natarajan, A Synthesis of aminopyrazole analogs and their evaluation as CDK inhibitors for cancer therapy. Bioorg Med Chem Lett 28: 3736-3740 (2018)
- Chen, S; Yang, Y; Yuan, Y; Bo Liu, na Targeting PIM kinases in cancer therapy: An update on pharmacological small-molecule inhibitors. Eur J Med Chem 264:
- Lange, U; Amberg, W; Ochse, M; Behl, B; Pohlki, F; Hutchins, CW Tetraline and indane derivatives, pharmaceutical compositions containing them, and their use in therapy US Patent US9051280 (2015)
- Beato, A; Gori, A; Boucherle, B; Peuchmaur, M; Haudecoeur, R β-Carboline as a Privileged Scaffold for Multitarget Strategies in Alzheimer's Disease Therapy. J Med Chem 64: 1392-1422 (2021)
- Sun, H; Chen, D; Zhan, S; Wu, W; Xu, H; Luo, C; Su, H; Feng, Y; Shao, W; Wan, A; Zhou, B; Wan, G; Bu, X Design and Discovery of Natural Cyclopeptide Skeleton Based Programmed Death Ligand 1 Inhibitor as Immune Modulator for Cancer Therapy. J Med Chem 63: 11286-11301 (2020)
- Zhang, H; Zhang, Y; Feng, Z; Shuai, M; Ma, X; Wang, S; Yu, S; Deng, R; Luo, D; Shi, J; Pu, C; Li, R Discovery of Novel Proteolysis-Targeting Chimera Molecules as Degraders of Programmed Cell Death-Ligand 1 for Breast Cancer Therapy. J Med Chem 67: 10589-10600
- Zhao, Y; Zhang, LX; Jiang, T; Long, J; Ma, ZY; Lu, AP; Cheng, Y; Cao, DS The ups and downs of Poly(ADP-ribose) Polymerase-1 inhibitors in cancer therapy-Current progress and future direction. Eur J Med Chem 203: (2020)
- Fan, P; Lange, CW; Lui, RM; McMurtrie, DJ; Scamp, RJ; Yang, J; Zeng, Y; Zhang, P Heteroaryl-biphenyl amides for the treatment of PD-L1 diseases US Patent US11713307 (2023)
- Fan, P; Lange, CW; Lui, RM; McMurtrie, DJ; Scamp, RJ; Yang, J; Zeng, Y; Zhang, P Heteroaryl-biphenyl amines for the treatment of PD-L1 diseases US Patent US11866429 (2024)
- Hangeland, JJ; Abell, LM; Adam, LP; Jiang, J; Friends, TJ; Haque, LE; Neels, J; Onorato, JM; Chen, AYA; Taylor, DS; Yin, X; Harrity, TW; Basso, MD; Yang, R; Sleph, PG; Gordon, DA; Huang, CS; Wexler, RR; Finlay, HJ; Lawrence, RM PK/PD Disconnect Observed with a Reversible Endothelial Lipase Inhibitor. ACS Med Chem Lett 9: 673-678 (2018)
- Surmiak, E; Ząber, J; Plewka, J; Wojtanowicz, G; Kocik-Krol, J; Kruc, O; Muszak, D; Rodríguez, I; Musielak, B; Viviano, M; Castellano, S; Skalniak, L; Magiera-Mularz, K; Holak, TA; Kalinowska-Tłuścik, J Solubilizer Tag Effect on PD-L1/Inhibitor Binding Properties for ACS Med Chem Lett 15: 36-44 (2024)
- Ravez, S; Spillier, Q; Marteau, R; Feron, O; Frédérick, R Challenges and Opportunities in the Development of Serine Synthetic Pathway Inhibitors for Cancer Therapy. J Med Chem 60: 1227-1237 (2017)
- Xu, Q; Li, T; Chen, H; Kong, J; Zhang, L; Yin, H Design and optimisation of a small-molecule TLR2/4 antagonist for anti-tumour therapy. RSC Med Chem 12: 1771-1779 (2021)
- Reßing, N; Schliehe-Diecks, J; Watson, PR; Sönnichsen, M; Cragin, AD; Schöler, A; Yang, J; Schäker-Hübner, L; Borkhardt, A; Christianson, DW; Bhatia, S; Hansen, FK Development of Fluorinated Peptoid-Based Histone Deacetylase (HDAC) Inhibitors for Therapy-Resistant Acute Leukemia. J Med Chem 65: 15457-15472 (2022)
- Gao, D; Tang, S; Cen, Y; Yuan, L; Lan, X; Li, QH; Lin, GQ; Huang, M; Tian, P Discovery of Novel Drug-like PHGDH Inhibitors to Disrupt Serine Biosynthesis for Cancer Therapy. J Med Chem 66: 285-305 (2023)
- Wang, C; Wang, R; Chen, Y; Wang, L; Zhou, S; Wang, H Discovery of an EGFR tyrosine kinase inhibitor from Ilex latifolia in breast cancer therapy. Bioorg Med Chem Lett 29: 1282-1290 (2019)
- Hu, X; Li, J; Zhang, H; Yu, Q; Wang, Y; Li, X; Long, L; Jiang, W; Wang, Z Discovery of dual inhibitors of topoisomerase I and Cyclooxygenase-2 for colon cancer therapy. Eur J Med Chem 240: (2022)
- Dai, Q; Yuan, Z; Sun, Q; Ao, Z; He, B; Jiang, Y Discovery of novel nucleoside derivatives as selective lysine acetyltransferase p300 inhibitors for cancer therapy. Bioorg Med Chem Lett 104:
- Nocentini, A; Ceruso, M; Bua, S; Lomelino, CL; Andring, JT; McKenna, R; Lanzi, C; Sgambellone, S; Pecori, R; Matucci, R; Filippi, L; Gratteri, P; Carta, F; Masini, E; Selleri, S; Supuran, CT Discovery of β-Adrenergic Receptors Blocker-Carbonic Anhydrase Inhibitor Hybrids for Multitargeted Antiglaucoma Therapy. J Med Chem 61: 5380-5394 (2018)
- Siebenbuerger, L; Hernandez-Olmos, V; Abdelsamie, AS; Frotscher, M; van Koppen, CJ; Marchais-Oberwinkler, S; Scheuer, C; Laschke, MW; Menger, MD; Boerger, C; Hartmann, RW Highly Potent 17β-HSD2 Inhibitors with a Promising Pharmacokinetic Profile for Targeted Osteoporosis Therapy. J Med Chem 61: 10724-10738 (2018)
- Arshad, JZ; Hanif, M Hydroxypyrone derivatives in drug discovery: from chelation therapy to rational design of metalloenzyme inhibitors. RSC Med Chem 13: 1127-1149 (2022)
- Ondetti, MA; Cushman, DW Inhibition of the renin-angiotensin system. A new approach to the therapy of hypertension. J Med Chem 24: 355-61 (1981)
- Lawandi, J; Gerber-Lemaire, S; Juillerat-Jeanneret, L; Moitessier, N Inhibitors of prolyl oligopeptidases for the therapy of human diseases: defining diseases and inhibitors. J Med Chem 53: 3423-38 (2010)
- Chen, X; Huang, Y; Chen, B; Liu, H; Cai, Y; Yang, Y Insight into the design of FGFR4 selective inhibitors in cancer therapy: Prospects and challenges. Eur J Med Chem 263:
- Friedlos, F; Denny, WA; Palmer, BD; Springer, CJ Mustard prodrugs for activation by Escherichia coli nitroreductase in gene-directed enzyme prodrug therapy. J Med Chem 40: 1270-5 (1997)
- Khan, TH; Eno-Amooquaye, EA; Searle, F; Browne, PJ; Osborn, HM; Burke, PJ Novel inhibitors of carboxypeptidase G2 (CPG2): potential use in antibody-directed enzyme prodrug therapy. J Med Chem 42: 951-6 (1999)
- Wang, Y; Qin, L; Chen, W; Chen, Q; Sun, J; Wang, G Novel strategies to improve tumour therapy by targeting the proteins MCT1, MCT4 and LAT1. Eur J Med Chem 226: (2021)
- Han, B; Wang, M; Li, J; Chen, Q; Sun, N; Yang, X; Zhang, Q Perspectives and new aspects of histone deacetylase inhibitors in the therapy of CNS diseases. Eur J Med Chem 258:
- Joharapurkar, AA; Pandya, VB; Patel, VJ; Desai, RC; Jain, MR Prolyl Hydroxylase Inhibitors: A Breakthrough in the Therapy of Anemia Associated with Chronic Diseases. J Med Chem 61: 6964-6982 (2018)
- Qin, W; Xie, M; Qin, X; Fang, Q; Yin, F; Li, Z Recent advances in peptidomimetics antagonists targeting estrogen receptor α-coactivator interaction in cancer therapy. Bioorg Med Chem Lett 28: 2827-2836 (2018)
- Duan, YC; Zhang, SJ; Shi, XJ; Jin, LF; Yu, T; Song, Y; Guan, YY Research progress of dual inhibitors targeting crosstalk between histone epigenetic modulators for cancer therapy. Eur J Med Chem 222: (2021)
- Abdel-Magid, AF Rho kinase inhibitors: potentially versatile therapy for the treatment of cardiovascular diseases and more. ACS Med Chem Lett 6: 371-2 (2015)
- Zhang, Z; Guo, Z; Xu, X; Cao, D; Yang, H; Li, Y; Shi, Q; Du, Z; Guo, X; Wang, X; Chen, D; Zhang, Y; Chen, L; Zhou, K; Li, J; Geng, M; Huang, X; Xiong, B Structure-Based Discovery of Potent CARM1 Inhibitors for Solid Tumor and Cancer Immunology Therapy. J Med Chem 64: 16650-16674 (2021)
- Wang, C; Lu, X Targeting MET: Discovery of Small Molecule Inhibitors as Non-Small Cell Lung Cancer Therapy. J Med Chem 66: 7670-7697 (2023)
- Clement, OO; Freeman, CM; Hartmann, RW; Handratta, VD; Vasaitis, TS; Brodie, AM; Njar, VC Three dimensional pharmacophore modeling of human CYP17 inhibitors. Potential agents for prostate cancer therapy. J Med Chem 46: 2345-51 (2003)
- Wang, ZX; Li, QQ; Cai, J; Wu, JZ; Wang, JJ; Zhang, MY; Wang, QX; Tong, ZJ; Yang, J; Wei, TH; Zhou, Y; Dai, WC; Ding, N; Leng, XJ; Sun, SL; Xue, X; Yu, YC; Yang, Y; Li, NG; Shi, ZH Unraveling the Promise of RET Inhibitors in Precision Cancer Therapy by Targeting RET Mutations. J Med Chem 67: 4346-4375
- Dong, Q; Tong, M; Yu, X; Wang, L; Ao, J; Guan, D; Tang, Y; Liu, J; Long, L; Tong, Y; Fang, S; Zhou, H; Huang, Y; Gong, L; Lou, L; Huang, W Carbohydrate Strengthens the Immunotherapeutic Effect of Small-Molecule PD-L1 Inhibitors. J Med Chem 66: 7179-7204 (2023)
- Fetse, J; Zhao, Z; Liu, H; Mamani, UF; Mustafa, B; Adhikary, P; Ibrahim, M; Liu, Y; Patel, P; Nakhjiri, M; Alahmari, M; Li, G; Cheng, K Discovery of Cyclic Peptide Inhibitors Targeting PD-L1 for Cancer Immunotherapy. J Med Chem 65: 12002-12013 (2022)
- Ważyńska, MA; Butera, R; Requesens, M; Plat, A; Zarganes-Tzitzikas, T; Neochoritis, CG; Plewka, J; Skalniak, L; Kocik-Krol, J; Musielak, B; Magiera-Mularz, K; Rodriguez, I; Blok, SN; de Bruyn, M; Nijman, HW; Elsinga, PH; Holak, TA; Dömling, A Design, Synthesis, and Biological Evaluation of 2-Hydroxy-4-phenylthiophene-3-carbonitrile as PD-L1 Antagonist and Its Comparison to Available Small Molecular PD-L1 Inhibitors. J Med Chem 66: 9577-9591 (2023)
- Carroll, FI 2002 Medicinal Chemistry Division Award address: monoamine transporters and opioid receptors. Targets for addiction therapy. J Med Chem 46: 1775-94 (2003)
- Hu, J; Fu, S; Zhan, Z; Zhang, J Advancements in dual-target inhibitors of PI3K for tumor therapy: Clinical progress, development strategies, prospects. Eur J Med Chem 265:
- Kozikowski, AP; Fauq, AH; Miller, JH; McKinney, M Alzheimer's therapy: an approach to novel muscarinic ligands based upon the naturally occurring alkaloid himbacine. Bioorg Med Chem Lett 2: 797-802 (1992)
- Wei, Y; Zhong, S; Yang, H; Wang, X; Lv, B; Bian, Y; Pei, Y; Xu, C; Zhao, Q; Wu, Y; Luo, D; Wang, F; Sun, H; Chen, Y Current therapy in amyotrophic lateral sclerosis (ALS): A review on past and future therapeutic strategies. Eur J Med Chem 272:
- Lu, Y; Gutgesell, LM; Xiong, R; Zhao, J; Li, Y; Rosales, CI; Hollas, M; Shen, Z; Gordon-Blake, J; Dye, K; Wang, Y; Lee, S; Chen, H; He, D; Dubrovyskyii, O; Zhao, H; Huang, F; Lasek, AW; Tonetti, DA; Thatcher, GRJ Design and Synthesis of Basic Selective Estrogen Receptor Degraders for Endocrine Therapy Resistant Breast Cancer. J Med Chem 62: 11301-11323 (2019)
- Deng, J; Feng, E; Ma, S; Zhang, Y; Liu, X; Li, H; Huang, H; Zhu, J; Zhu, W; Shen, X; Miao, L; Liu, H; Jiang, H; Li, J Design and synthesis of small molecule RhoA inhibitors: a new promising therapy for cardiovascular diseases? J Med Chem 54: 4508-22 (2011)
- Shen, Z; Ratia, K; Cooper, L; Kong, D; Lee, H; Kwon, Y; Li, Y; Alqarni, S; Huang, F; Dubrovskyi, O; Rong, L; Thatcher, GRJ; Xiong, R Design of SARS-CoV-2 PLpro Inhibitors for COVID-19 Antiviral Therapy Leveraging Binding Cooperativity. J Med Chem (2021)
- Jiao, P; Jin, P; Li, C; Cui, L; Dong, L; Pan, B; Song, W; Ma, L; Dong, J; Song, L; Jin, X; Li, F; Wan, M; Lv, Z; Geng, Q Design, synthesis and in vitro evaluation of amidoximes as histone deacetylase inhibitors for cancer therapy. Bioorg Med Chem Lett 26: 4679-4683 (2016)
- Kargbo, RB Dual Inhibition of KRAS G12C and G12D Mutants as a Potential Treatment in Cancer Therapy. ACS Med Chem Lett 12: 1512-1513 (2021)
- Wu, S; Huang, Y; Wang, T; Li, K; Lu, J; Huang, M; Dong, G; Sheng, C Evodiamine-Inspired Topoisomerase-Histone Deacetylase Dual Inhibitors: Novel Orally Active Antitumor Agents for Leukemia Therapy. J Med Chem 65: 4818-4831 (2022)
- Lu, X; Chen, H; Patterson, AV; Smaill, JB; Ding, K Fibroblast Growth Factor Receptor 4 (FGFR4) Selective Inhibitors as Hepatocellular Carcinoma Therapy: Advances and Prospects. J Med Chem 62: 2905-2915 (2019)
- Picaud, S; Fedorov, O; Thanasopoulou, A; Leonards, K; Jones, K; Meier, J; Olzscha, H; Monteiro, O; Martin, S; Philpott, M; Tumber, A; Filippakopoulos, P; Yapp, C; Wells, C; Che, KH; Bannister, A; Robson, S; Kumar, U; Parr, N; Lee, K; Lugo, D; Jeffrey, P; Taylor, S; Vecellio, ML; Bountra, C; Brennan, PE; O'Mahony, A; Velichko, S; Müller, S; Hay, D; Daniels, DL; Urh, M; La Thangue, NB; Kouzarides, T; Prinjha, R; Schwaller, J; Knapp, S Generation of a Selective Small Molecule Inhibitor of the CBP/p300 Bromodomain for Leukemia Therapy. Cancer Res 75: 5106-5119 (2015)
- Carrión, MD; Rubio-Ruiz, B; Franco-Montalban, F; Amoia, P; Zuccarini, MC; De Simone, C; Camacho, ME; Amoroso, R; Maccallini, C New amidine-benzenesulfonamides as iNOS inhibitors for the therapy of the triple negative breast cancer. Eur J Med Chem 248: (2023)
- Jiang, ZY; Lu, MC; You, QD Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Inhibition: An Emerging Strategy in Cancer Therapy. J Med Chem 62: 3840-3856 (2019)
- Blum, E; Zhang, J; Zaluski, J; Einstein, DE; Korshin, EE; Kubas, A; Gruzman, A; Tochtrop, GP; Kiser, PD; Palczewski, K Rational Alteration of Pharmacokinetics of Chiral Fluorinated and Deuterated Derivatives of Emixustat for Retinal Therapy. J Med Chem 64: 8287-8302 (2021)
- Zhang, L; Zhang, J; Wang, J; Ren, C; Tang, P; Ouyang, L; Wang, Y Recent advances of human dihydroorotate dehydrogenase inhibitors for cancer therapy: Current development and future perspectives. Eur J Med Chem 232: (2022)
- Zhou, Y; Zou, J; Xu, J; Zhou, Y; Cen, X; Zhao, Y Recent advances of mitochondrial complex I inhibitors for cancer therapy: Current status and future perspectives. Eur J Med Chem 251: (2023)
- Fang, Y; Ma, H; Zhang, X; Zhang, P; Li, Y; He, S; Sheng, C; Dong, G Smart glypican-3-targeting peptide-chlorin e6 conjugates for targeted photodynamic therapy of hepatocellular carcinoma. Eur J Med Chem 264:
- Colombo, R; Caldarelli, M; Mennecozzi, M; Giorgini, ML; Sola, F; Cappella, P; Perrera, C; Depaolini, SR; Rusconi, L; Cucchi, U; Avanzi, N; Bertrand, JA; Bossi, RT; Pesenti, E; Galvani, A; Isacchi, A; Colotta, F; Donati, D; Moll, J Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res 70: 10255-64
- Dgachi, Y; Sokolov, O; Luzet, V; Godyn, J; Panek, D; Bonet, A; Martin, H; Iriepa, I; Moraleda, I; García-Iriepa, C; Janockova, J; Richert, L; Soukup, O; Malawska, B; Chabchoub, F; Marco-Contelles, J; Ismaili, L Tetrahydropyranodiquinolin-8-amines as new, non hepatotoxic, antioxidant, and acetylcholinesterase inhibitors for Alzheimer's disease therapy. Eur J Med Chem 126: 576-589 (2017)
- Poulter, S; Austin, N; Armstrong, R; Barnes, M; Bucknell, SJ; Higueruelo, A; Banerjee, J; Mead, A; Mould, R; MacSweeney, C; O'Brien, MA; Stott, LA; Watson, SP The Identification of GPR52 Agonist HTL0041178, a Potential Therapy for Schizophrenia and Related Psychiatric Disorders. ACS Med Chem Lett 14: 499-505 (2023)
- Liao, G; Yang, D; Ma, L; Li, W; Hu, L; Zeng, L; Wu, P; Duan, L; Liu, Z The development of piperidinones as potent MDM2-P53 protein-protein interaction inhibitors for cancer therapy. Eur J Med Chem 159: 1-9 (2018)
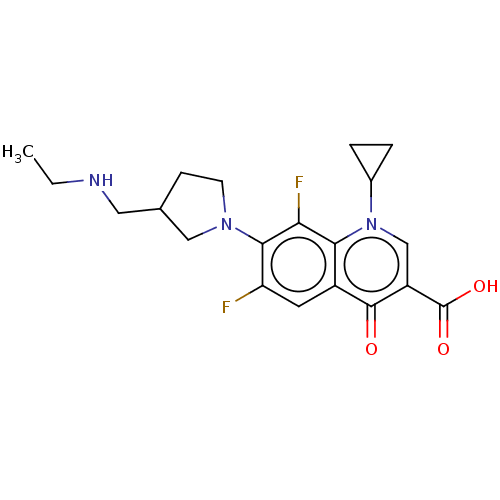
- Martín-Martínez, M; Latorre, M; García-López, MT; Cenarruzabeitia, E; Del Río, J; González-Muñiz, R Effects of the incorporation of IBTM beta-turn mimetics into the dipeptoid CCK(1) receptor agonist PD 170292. Bioorg Med Chem Lett 12: 109-12 (2001)
- Fotsch, C; Wang, M Blockade of glucocorticoid excess at the tissue level: inhibitors of 11beta-hydroxysteroid dehydrogenase type 1 as a therapy for type 2 diabetes. J Med Chem 51: 4851-7 (2008)
- Jeffries, DE; Borza, CM; Blobaum, AL; Pozzi, A; Lindsley, CW Discovery of VU6015929: A Selective Discoidin Domain Receptor 1/2 (DDR1/2) Inhibitor to Explore the Role of DDR1 in Antifibrotic Therapy. ACS Med Chem Lett 11: 29-33 (2020)
- Jiang, L; Deng, J; Lu, X; Shang, K; Shou, J; Wang, B; Wu, D; Xu, X; Xu, Y; Zhang, Y; Zheng, M Compound having PD-L1 inhibitory activity, preparation method therefor and use thereof US Patent US11787766 (2023)
- Thoma, G; Veenstra, S; Strang, R; Blanz, J; Vangrevelinghe, E; Berghausen, J; Lee, CC; Zerwes, HG Orally bioavailable Syk inhibitors with activity in a rat PK/PD model. Bioorg Med Chem Lett 25: 4642-7 (2015)
- Amberg, W; Pohlki, F; Lange, U; Wang, Y; Brewer, J; Vasudevan, A; Lao, Y; Hutchins, C; Zhao, H; Li, H Aminochromane, aminothiochromane and amino-1,2,3,4-tetrahydroquinoline derivatives, pharmaceutical compositions containing them, and their use in therapy US Patent US9586945 (2017)
- Golonko, A; Pienkowski, T; Swislocka, R; Lazny, R; Roszko, M; Lewandowski, W Another look at phenolic compounds in cancer therapy the effect of polyphenols on ubiquitin-proteasome system. Eur J Med Chem 167: 291-311 (2019)
- Wacker, DA; Santella, JB; Gardner, DS; Varnes, JG; Estrella, M; DeLucca, GV; Ko, SS; Tanabe, K; Watson, PS; Welch, PK; Covington, M; Stowell, NC; Wadman, EA; Davies, P; Solomon, KA; Newton, RC; Trainor, GL; Friedman, SM; Decicco, CP; Duncia, JV CCR3 antagonists: a potential new therapy for the treatment of asthma. Discovery and structure-activity relationships. Bioorg Med Chem Lett 12: 1785-9 (2002)
- Feng, KR; Wang, F; Shi, XW; Tan, YX; Zhao, JY; Zhang, JW; Li, QH; Lin, GQ; Gao, D; Tian, P Design, synthesis and biological evaluation of novel potent STAT3 inhibitors based on BBI608 for cancer therapy. Eur J Med Chem 201: (2020)
- Zha, C; Deng, W; Fu, Y; Tang, S; Lan, X; Ye, Y; Su, Y; Jiang, L; Chen, Y; Huang, Y; Ding, J; Geng, M; Huang, M; Wan, H Design, synthesis and biological evaluation of tetrahydronaphthyridine derivatives as bioavailable CDK4/6 inhibitors for cancer therapy. Eur J Med Chem 148: 140-153 (2018)
- Duan, Y; Qin, W; Suo, F; Zhai, X; Guan, Y; Wang, X; Zheng, Y; Liu, H Design, synthesis and in vitro evaluation of stilbene derivatives as novel LSD1 inhibitors for AML therapy. Bioorg Med Chem 26: 6000-6014 (2018)
- Yang, C; Gong, Y; Deng, M; Ling, Y; Wang, J; Zhou, Y Discovery of a photosensitizing PI3K inhibitor for tumor therapy: Design, synthesis and in vitro biological evaluation. Bioorg Med Chem Lett 94: (2023)
- Guo, W; Wang, M; Yang, Z; Liu, D; Ma, B; Zhao, Y; Chen, Y; Hu, Y Recent advances in small molecule and peptide inhibitors of glucose-regulated protein 78 for cancer therapy. Eur J Med Chem 261:
- Xu, L; Xu, B; Wang, J; Gao, Y; He, X; Xie, T; Ye, XY Recent advances of novel fourth generation EGFR inhibitors in overcoming C797S mutation of lung cancer therapy. Eur J Med Chem 245:
- Yang, P; Zhu, Y; Zheng, Q; Meng, S; Wu, Y; Shuai, W; Sun, Q; Wang, G Recent advances of β-catenin small molecule inhibitors for cancer therapy: Current development and future perspectives. Eur J Med Chem 243: (2022)
- Zhao, Y; Guan, YY; Zhao, F; Yu, T; Zhang, SJ; Zhang, YZ; Duan, YC; Zhou, XL Recent strategies targeting Embryonic Ectoderm Development (EED) for cancer therapy: Allosteric inhibitors, PPI inhibitors, and PROTACs. Eur J Med Chem 231: (2022)
- Liu, C; Miao, R; Raza, F; Qian, H; Tian, X Research progress and challenges of TRPV1 channel modulators as a prospective therapy for diabetic neuropathic pain. Eur J Med Chem 245: (2023)
- Liu, T; Wu, Z; He, Y; Xiao, Y; Xia, C Single and dual target inhibitors based on Bcl-2: Promising anti-tumor agents for cancer therapy. Eur J Med Chem 201: (2020)
- Pismataro, MC; Astolfi, A; Barreca, ML; Pacetti, M; Schenone, S; Bandiera, T; Carbone, A; Massari, S Small Molecules Targeting DNA Polymerase Theta (POLθ) as Promising Synthetic Lethal Agents for Precision Cancer Therapy. J Med Chem 66: 6498-6522 (2023)
- Lepesheva, GI; Ott, RD; Hargrove, TY; Kleshchenko, YY; Schuster, I; Nes, WD; Hill, GC; Villalta, F; Waterman, MR Sterol 14alpha-demethylase as a potential target for antitrypanosomal therapy: enzyme inhibition and parasite cell growth. Chem Biol 14: 1283-93 (2007)
- Okamura, T; Kurogi, Y; Hashimoto, K; Sato, S; Nishikawa, H; Kiryu, K; Nagao, Y Structure-activity relationships of adenosine A3 receptor ligands: new potential therapy for the treatment of glaucoma. Bioorg Med Chem Lett 14: 3775-9 (2004)
- Xiong, Y; Wiltsie, J; Woods, A; Guo, J; Pivnichny, JV; Tang, W; Bansal, A; Cummings, RT; Cunningham, BR; Friedlander, AM; Douglas, CM; Salowe, SP; Zaller, DM; Scolnick, EM; Schmatz, DM; Bartizal, K; Hermes, JD; MacCoss, M; Chapman, KT The discovery of a potent and selective lethal factor inhibitor for adjunct therapy of anthrax infection. Bioorg Med Chem Lett 16: 964-8 (2006)
- Wakeling, AE; Guy, SP; Woodburn, JR; Ashton, SE; Curry, BJ; Barker, AJ; Gibson, KH ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 62: 5749-54
- BUHRLAGE, S; GRIFFIN, J; WEISBERG, E; YANG, J; LIU, X; MAGIN, R; HU, B; CHAN, WC SMALL MOLECULE INHIBITION OF DEUBIQUITINATING ENZYME JOSEPHIN DOMAIN CONTAINING 1 (JOSD1) AS A TARGETED THERAPY FOR LEUKEMIAS WITH MUTANT JANUS KINASE 2 (JAK2) US Patent US20240335427 (2024)
- Wang, X; Xu, W; Wang, Z; Yu, Q; Yuan, L; Liu, Y; Sang, J; Li, W; Zhu, S; Jiang, W; Li, Z; Zhang, W; Dang, Y Sokotrasterol Sulfate Suppresses IFN-γ-Induced PD-L1 Expression by Inhibiting JAK Activity. J Nat Prod 87: 713-721 (2024)
- Lee, S; Cil, O; Haggie, PM; Verkman, AS 4,8-Dimethylcoumarin Inhibitors of Intestinal Anion Exchanger slc26a3 (Downregulated in Adenoma) for Anti-Absorptive Therapy of Constipation. J Med Chem 62: 8330-8337 (2019)
- Yin, L; Li, H; Liu, W; Yao, Z; Cheng, Z; Zhang, H; Zou, H A highly potent CDK4/6 inhibitor was rationally designed to overcome blood brain barrier in gliobastoma therapy. Eur J Med Chem 144: 1-28 (2018)
- Zhang, WX; Huang, J; Tian, XY; Liu, YH; Jia, MQ; Wang, W; Jin, CY; Song, J; Zhang, SY A review of progress in o-aminobenzamide-based HDAC inhibitors with dual targeting capabilities for cancer therapy. Eur J Med Chem 259:
- Wong, JC; Guo, L; Peng, Z; Zhang, W; Zhang, N; Lai, W; Zhang, Z; Zhang, C; Zhang, X; Song, S; Pan, D; Xie, C; Li, J; Ning, Z; Lu, X; He, Y; Chen, L Application of p21 and klf2 reporter gene assays to identify selective histone deacetylase inhibitors for cancer therapy. Bioorg Med Chem Lett 21: 110-6 (2010)
- Liu, L; Zhang, L; Chen, X; Yang, K; Cui, H; Qian, R; Zhao, S; Wang, L; Su, X; Zhao, M; Wang, M; Hu, Z; Lu, T; Zhu, Y; Zhou, QQ; Yao, Y Design and synthesis of 1H-benzo[d]imidazole selective HDAC6 inhibitors with potential therapy for multiple myeloma. Eur J Med Chem 261:
- Li, C; Zhu, M; Liu, S; Zhang, J; Ye, H; Zhang, C; Ji, D; Tang, H; Zhang, Y; Wu, J; Huang, Z Development of Nitric Oxide-Donating Netarsudil Derivatives as a Synergistic Therapy for Glaucoma with Reduced Ocular Irritation. J Med Chem 67: 16311-16327
- Yang, Z; Shen, M; Tang, M; Zhang, W; Cui, X; Zhang, Z; Pei, H; Li, Y; Hu, M; Bai, P; Chen, L Discovery of 1,2,4-oxadiazole-Containing hydroxamic acid derivatives as histone deacetylase inhibitors potential application in cancer therapy. Eur J Med Chem 178: 116-130 (2019)
- Lee, SY; Namasivayam, V; Boshta, NM; Perotti, A; Mirza, S; Bua, S; Supuran, CT; Müller, CE Discovery of potent nucleotide pyrophosphatase/phosphodiesterase3 (NPP3) inhibitors with ancillary carbonic anhydrase inhibition for cancer (immuno)therapy. RSC Med Chem 12: 1187-1206 (2021)
- Tremblay, ML; Pike, K; Perez Quintero, LA Enhancing CD8+ T cells for adoptive T cell therapy by inhibiting PTPN1 (PTP1B) and PTPN2 (TC-PTP) US Patent US11597739 (2023)
- Gu, X; Zhang, H; Jiao, M; Han, B; Zhang, Z; Li, J; Zhang, Q Histone deacetylase 6 inhibitors with blood-brain barrier penetration as a potential strategy for CNS-Disorders therapy. Eur J Med Chem 229: (2022)
- Amberg, W; Lange, U; Pohlki, F; Santandrea, E; Hutchins, C N-substituted aminobenzocycloheptene, aminotetraline, aminoindane and phenalkylamine derivatives, pharmaceutical compositions containing them, and their use in therapy US Patent US8846741 (2014)
- Luo, D; Guo, Z; Zhao, X; Wu, L; Liu, X; Zhang, Y; Zhang, Y; Deng, Z; Qu, X; Cui, S; Wan, S Novel 5-fluorouracil sensitizers for colorectal cancer therapy: Design and synthesis of S1P receptor 2 (S1PR2) antagonists. Eur J Med Chem 227: (2022)
- Angeli, A; Chelli, I; Lucarini, L; Sgambellone, S; Marri, S; Villano, S; Ferraroni, M; De Luca, V; Capasso, C; Carta, F; Supuran, CT Novel Carbonic Anhydrase Inhibitors with Dual-Tail Core Sulfonamide Show Potent and Lasting Effects for Glaucoma Therapy. J Med Chem 67: 3066-3089
- Li, Y; Lv, Y; Zhang, C; Fu, B; Liu, Y; Hu, J Recent advances in the development of dual ALK/ROS1 inhibitors for non-small cell lung cancer therapy. Eur J Med Chem 257:
- James, DA; Swamy, N; Paz, N; Hanson, RN; Ray, R Synthesis and estrogen receptor binding affinity of a porphyrin-estradiol conjugate for targeted photodynamic therapy of cancer. Bioorg Med Chem Lett 9: 2379-84 (1999)
- Dubuc, C; Langlois, R; Bénard, F; Cauchon, N; Klarskov, K; Tone, P; van Lier, JE Targeting gastrin-releasing peptide receptors of prostate cancer cells for photodynamic therapy with a phthalocyanine-bombesin conjugate. Bioorg Med Chem Lett 18: 2424-7 (2008)
- Liu, Y; Wang, X; Wang, G; Yang, Y; Yuan, Y; Ouyang, L The past, present and future of potential small-molecule drugs targeting p53-MDM2/MDMX for cancer therapy. Eur J Med Chem 176: 92-104 (2019)
- Panek, RL; Lu, GH; Dahring, TK; Batley, BL; Connolly, C; Hamby, JM; Brown, KJ In vitro biological characterization and antiangiogenic effects of PD 166866, a selective inhibitor of the FGF-1 receptor tyrosine kinase. J Pharmacol Exp Ther 286: 569-77 (1998)
- Sun, Z; Xu, C; Cheng, J; Yang, Z; Liu, T; Deng, B; Zhang, X; Peng, X; Chen, J Discovery of Novel HDAC3 Inhibitors with PD-L1 Downregulating/Degrading and Antitumor Immune Effects. J Med Chem 67: 13067-13088
- Wang, S; Kong, Z; Shi, Y; Shao, C; Wang, W; Su, Z; Liu, J; Zhou, Y; Fei, X; Cheng, B; Chen, J; Lu, Y; Xiao, J Discovery of Small and Bifunctional Molecules Targeting PD-L1/CD73 for Cancer Dual Immunotherapy. J Med Chem 67: 9447-9464
- Eden, J; Hall, M; Higginbottom, M; Horwell, D; Howson, W; Hughes, J; Jordan, R; Lewthwaite, R; Martin, K; McKnight, A; Pinnock, R; Pritchard, M; Suman-Chauhan, N; Williams, S PD 165929 the first high affinity non-peptide neuromedin-B (NMB) receptor selective antagonist Bioorg Med Chem Lett 6: 2617-2622 (1996)
- Ashwood, V; Brownhill, V; Higginbottom, M; Horwell, DC; Hughes, J; Lewthwaite, RA; McKnight, AT; Pinnock, RD; Pritchard, MC; Suman-Chauhan, N; Webb, C; Williams, SC PD 176252--the first high affinity non-peptide gastrin-releasing peptide (BB2) receptor antagonist. Bioorg Med Chem Lett 8: 2589-94 (1999)
- Cheng, B; Xiao, Y; Xue, M; Cao, H; Chen, J Recent Advances in the Development of PD-L1 Modulators: Degraders, Downregulators, and Covalent Inhibitors. J Med Chem 63: 15389-15398 (2020)
- Jaen, JC; Caprathe, BW; Wise, LD; Meltzer, LT; Pugsley, TA; Huffner, TG Synthesis and pharmacological evaluation of the enantiomers of the dopamine autoreceptor agonist PD 135385 Bioorg Med Chem Lett 3: 639-644 (1993)
- Volgraf, M; Chan, L; Huestis, MP; Purkey, HE; Burkard, M; Geck Do, M; Harris, J; Hunt, KW; Liu, X; Lyssikatos, JP; Rana, S; Thomas, AA; Vigers, GP; Siu, M Synthesis, characterization, and PK/PD studies of a series of spirocyclic pyranochromene BACE1 inhibitors. Bioorg Med Chem Lett 24: 2477-80 (2014)
- Lu, T; Schubert, C; Cummings, MD; Bignan, G; Connolly, PJ; Smans, K; Ludovici, D; Parker, MH; Meyer, C; Rocaboy, C; Alexander, R; Grasberger, B; De Breucker, S; Esser, N; Fraiponts, E; Gilissen, R; Janssens, B; Peeters, D; Van Nuffel, L; Vermeulen, P; Bischoff, J; Meerpoel, L Design and synthesis of a series of bioavailable fatty acid synthase (FASN) KR domain inhibitors for cancer therapy. Bioorg Med Chem Lett 28: 2159-2164 (2018)
- Dilger, AK; Pabbisetty, KB; Corte, JR; De Lucca, I; Fang, T; Yang, W; Pinto, DJP; Wang, Y; Zhu, Y; Mathur, A; Li, J; Hou, X; Smith, D; Sun, D; Zhang, H; Krishnananthan, S; Wu, DR; Myers, JE; Sheriff, S; Rossi, KA; Chacko, S; Zheng, JJ; Galella, MA; Ziemba, T; Dierks, EA; Bozarth, JM; Wu, Y; Crain, E; Wong, PC; Luettgen, JM; Wexler, RR; Ewing, WR Discovery of Milvexian, a High-Affinity, Orally Bioavailable Inhibitor of Factor XIa in Clinical Studies for Antithrombotic Therapy. J Med Chem 65: 1770-1785 (2022)
- Zhuang, Z; Miao, YL; Song, SS; Leng, GT; Zhang, XF; He, Q; Ding, J; He, JX; Yang, CH Discovery of pyrrolo[2,3-d]pyrimidin-4-one derivative YCH3124 as a potent USP7 inhibitor for cancer therapy. Eur J Med Chem 277:
- Li, X; Wang, C; Li, S; Yin, F; Luo, H; Zhang, Y; Luo, Z; Chen, Y; Wan, S; Kong, L; Wang, X Dual target PARP1/EZH2 inhibitors inducing excessive autophagy and producing synthetic lethality for triple-negative breast cancer therapy. Eur J Med Chem 265:
- Leverson, JD; Phillips, DC; Mitten, MJ; Boghaert, ER; Diaz, D; Tahir, SK; Belmont, LD; Nimmer, P; Xiao, Y; Ma, XM; Lowes, KN; Kovar, P; Chen, J; Jin, S; Smith, M; Xue, J; Zhang, H; Oleksijew, A; Magoc, TJ; Vaidya, KS; Albert, DH; Tarrant, JM; La, N; Wang, L; Tao, ZF; Wendt, MD; Sampath, D; Rosenberg, SH; Tse, C; Huang, DC; Fairbrother, WJ; Elmore, SW; Souers, AJ Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 7:
- Zhao, C; Tang, C; Li, C; Ning, W; Hu, Z; Xin, L; Zhou, HB; Huang, J Novel hybrid conjugates with dual estrogen receptor α degradation and histone deacetylase inhibitory activities for breast cancer therapy. Bioorg Med Chem 40: (2021)
- Dong, J; Cheng, XD; Zhang, WD; Qin, JJ Recent Update on Development of Small-Molecule STAT3 Inhibitors for Cancer Therapy: From Phosphorylation Inhibition to Protein Degradation. J Med Chem 64: 8884-8915 (2021)
- Sagar, S; Singh, S; Mallareddy, JR; Sonawane, YA; Napoleon, JV; Rana, S; Contreras, JI; Rajesh, C; Ezell, EL; Kizhake, S; Garrison, JC; Radhakrishnan, P; Natarajan, A Structure activity relationship (SAR) study identifies a quinoxaline urea analog that modulates IKKβ phosphorylation for pancreatic cancer therapy. Eur J Med Chem 222: (2021)
- Li, G; Shao, Y; Pan, Y; Li, Y; Wang, Y; Wang, L; Wang, X; Shao, K; Wang, S; Liu, N; Zhang, J; Zhao, W; Nakamura, H Total synthesis and biological evaluation of 7-hydroxyneolamellarin A as hypoxia-inducible factor-1α inhibitor for cancer therapy. Bioorg Med Chem Lett 50: (2021)
- Hammed, A; Matagrin, B; Spohn, G; Prouillac, C; Benoit, E; Lattard, V VKORC1L1, an enzyme rescuing the vitamin K 2,3-epoxide reductase activity in some extrahepatic tissues during anticoagulation therapy. J Biol Chem 288: 28733-42 (2013)
- Dong, Y; Wang, S; Wang, C; Li, Z; Ma, Y; Liu, G Antagonizing NOD2 Signaling with Conjugates of Paclitaxel and Muramyl Dipeptide Derivatives Sensitizes Paclitaxel Therapy and Significantly Prevents Tumor Metastasis. J Med Chem 60: 1219-1224 (2017)
- Yan, YY; Zhang, XX; Xiao, Y; Shen, XB; Jian, YJ; Wang, YM; She, ZH; Liu, MM; Liu, XH Design and Synthesis of a 2-Amino-pyridine Derivative as a Potent CDK8 Inhibitor for Anti-colorectal Cancer Therapy. J Med Chem 65: 13216-13239 (2022)
- Hattori, K; Kohchi, Y; Oikawa, N; Suda, H; Ura, M; Ishikawa, T; Miwa, M; Endoh, M; Eda, H; Tanimura, H; Kawashima, A; Horii, I; Ishitsuka, H; Shimma, N Design and synthesis of the tumor-activated prodrug of dihydropyrimidine dehydrogenase (DPD) inhibitor, RO0094889 for combination therapy with capecitabine. Bioorg Med Chem Lett 13: 867-72 (2003)
- Yuan, Z; Chen, S; Chen, C; Chen, J; Chen, C; Dai, Q; Gao, C; Jiang, Y Design, synthesis and biological evaluation of 4-amidobenzimidazole acridine derivatives as dual PARP and Topo inhibitors for cancer therapy. Eur J Med Chem 138: 1135-1146 (2017)
- Fan, X; Li, J; Long, L; Shi, T; Liu, D; Tan, W; Zhang, H; Wu, X; Lei, X; Wang, Z Design, synthesis and biological evaluation of N-anthraniloyl tryptamine derivatives as pleiotropic molecules for the therapy of malignant glioma. Eur J Med Chem 222: (2021)
- Qiu, G; Xie, J; Li, F; Han, K; Long, Q; Kowah, JAH; Gao, R; Wang, L; Liu, X Design, synthesis and biological evaluation of matrine contains benzimidazole derivatives as dual TOPOI and PARP inhibitors for cancer therapy. Eur J Med Chem 270:
- Li, Y; Peng, P; Tang, L; Hu, Y; Hu, Y; Sheng, R Design, synthesis and evaluation of rivastigmine and curcumin hybrids as site-activated multitarget-directed ligands for Alzheimer's disease therapy. Bioorg Med Chem 22: 4717-25 (2014)
- Lill, AP; Rödl, CB; Steinhilber, D; Stark, H; Hofmann, B Development and evaluation of ST-1829 based on 5-benzylidene-2-phenylthiazolones as promising agent for anti-leukotriene therapy. Eur J Med Chem 89: 503-23 (2014)
- Baeriswyl, V; Calzavarini, S; Gerschheimer, C; Diderich, P; Angelillo-Scherrer, A; Heinis, C Development of a selective peptide macrocycle inhibitor of coagulation factor XII toward the generation of a safe antithrombotic therapy. J Med Chem 56: 3742-6 (2013)
- Sestito, S; Bacci, A; Chiarugi, S; Runfola, M; Gado, F; Margheritis, E; Gul, S; Riveiro, ME; Vazquez, R; Huguet, S; Manera, C; Rezai, K; Garau, G; Rapposelli, S Development of potent dual PDK1/AurA kinase inhibitors for cancer therapy: Lead-optimization, structural insights, and ADME-Tox profile. Eur J Med Chem 226: (2021)
- Adhikari, N; Baidya, SK; Jha, T Effective anti-aromatase therapy to battle against estrogen-mediated breast cancer: Comparative SAR/QSAR assessment on steroidal aromatase inhibitors. Eur J Med Chem 208: (2020)
- Badran, MM; Abbas, SH; Tateishi, H; Maemoto, Y; Toma, T; Ito, A; Fujita, M; Otsuka, M; Abdel-Aziz, M; Radwan, MO Ligand-based design and synthesis of new trityl histamine and trityl cysteamine derivatives as SIRT2 inhibitors for cancer therapy. Eur J Med Chem 269:
- Desphande, A; Xia, G; Boerma, LJ; Vines, KK; Atigadda, VR; Lobo-Ruppert, S; Grubbs, CJ; Moeinpour, FL; Smith, CD; Christov, K; Brouillette, WJ; Muccio, DD Methyl-substituted conformationally constrained rexinoid agonists for the retinoid X receptors demonstrate improved efficacy for cancer therapy and prevention. Bioorg Med Chem 22: 178-85 (2013)
- Soltan, OM; Shoman, ME; Abdel-Aziz, SA; Narumi, A; Konno, H; Abdel-Aziz, M Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur J Med Chem 225: (2021)
- Brunetti, L; Loiodice, F; Piemontese, L; Tortorella, P; Laghezza, A New Approaches to Cancer Therapy: Combining Fatty Acid Amide Hydrolase (FAAH) Inhibition with Peroxisome Proliferator-Activated Receptors (PPARs) Activation. J Med Chem 62: 10995-11003 (2019)
- Kang, Z; Wang, C; Tong, Y; Li, Y; Gao, Y; Hou, S; Hao, M; Han, X; Wang, B; Wang, Q; Zhang, C Novel Nonsecosteroidal Vitamin D Receptor Modulator Combined with Gemcitabine Enhances Pancreatic Cancer Therapy through Remodeling of the Tumor Microenvironment. J Med Chem 64: 629-643 (2021)
- Zhang, XJ; Liu, MH; Luo, YS; Han, GY; Ma, ZQ; Huang, F; Wang, Y; Miao, ZY; Zhang, WN; Sheng, CQ; Yao, JZ Novel dual-mode antitumor chlorin-based derivatives as potent photosensitizers and histone deacetylase inhibitors for photodynamic therapy and chemotherapy. Eur J Med Chem 217: (2021)
- Liu, XJ; Xu-Liu, na; Pang, XJ; -Ying Yuan, X; Yu, GX; Li, YR; Guan, YF; Zhang, YB; Song, J; Zhang, QR; Zhang, SY Progress in the development of small molecular inhibitors of the Bruton's tyrosine kinase (BTK) as a promising cancer therapy. Bioorg Med Chem 47: (2021)
- Seneci, P; Bianchi, A; Battaglia, C; Belvisi, L; Bolognesi, M; Caprini, A; Cossu, F; Franco, E; Matteo, M; Delia, D; Drago, C; Khaled, A; Lecis, D; Manzoni, L; Marizzoni, M; Mastrangelo, E; Milani, M; Motto, I; Moroni, E; Potenza, D; Rizzo, V; Servida, F; Turlizzi, E; Varrone, M; Vasile, F; Scolastico, C Rational design, synthesis and characterization of potent, non-peptidic Smac mimics/XIAP inhibitors as proapoptotic agents for cancer therapy. Bioorg Med Chem 17: 5834-56 (2009)
- She, W; Liu, T; Li, H; Wang, Z; Guo, Z; Liu, Y; Liu, Y Reprogramming Energy Metabolism with Synthesized PDK Inhibitors Based on Dichloroacetate Derivatives and Targeted Delivery Systems for Enhanced Cancer Therapy. J Med Chem 66: 14683-14699 (2023)
- Abdel-Magid, AF Selective Estrogen Receptor Degraders (SERDs): A Promising Treatment to Overcome Resistance to Endocrine Therapy in ERα-Positive Breast Cancer. ACS Med Chem Lett 8: 1129-1131 (2017)
- Brunetti, L; Leuci, R; Carrieri, A; Catto, M; Occhineri, S; Vinci, G; Gambacorta, L; Baltrukevich, H; Chaves, S; Laghezza, A; Altomare, CD; Tortorella, P; Santos, MA; Loiodice, F; Piemontese, L Structure-based design of novel donepezil-like hybrids for a multi-target approach to the therapy of Alzheimer's disease. Eur J Med Chem 237: (2022)
- Zhao, L; Brinton, RD Structure-based virtual screening for plant-based ERbeta-selective ligands as potential preventative therapy against age-related neurodegenerative diseases. J Med Chem 48: 3463-6 (2005)
- Xiang, S; Wang, J; Huang, H; Wang, Z; Song, X; Zhou, Y; Jin, F; He, X; Zhang, ZM; Tu, Z; Ding, K; Zhang, Z; Lu, X Switch type I to type II TRK inhibitors for combating clinical resistance induced by xDFG mutation for cancer therapy. Eur J Med Chem 245: (2023)
- Sivaraman, A; Kim, DG; Bhattarai, D; Kim, M; Lee, HY; Lim, S; Kong, J; Goo, JI; Shim, S; Lee, S; Suh, YG; Choi, Y; Kim, S; Lee, K Synthesis and Structure-Activity Relationships of Arylsulfonamides as AIMP2-DX2 Inhibitors for the Development of a Novel Anticancer Therapy. J Med Chem 63: 5139-5158 (2020)
- Yeon, SK; Choi, JW; Park, JH; Lee, YR; Kim, HJ; Shin, SJ; Jang, BK; Kim, S; Bahn, YS; Han, G; Lee, YS; Pae, AN; Park, KD Synthesis and evaluation of biaryl derivatives for structural characterization of selective monoamine oxidase B inhibitors toward Parkinson's disease therapy. Bioorg Med Chem 26: 232-244 (2018)
- Li, R; Wu, Y; Li, Y; Shuai, W; Wang, A; Zhu, Y; Hu, X; Xia, Y; Ouyang, L; Wang, G Targeted regulated cell death with small molecule compounds in colorectal cancer: Current perspectives of targeted therapy and molecular mechanisms. Eur J Med Chem 265:
- Orafaie, A; Sadeghian, H; Bahrami, AR; Rafatpanah, H; Matin, MM Design, synthesis and evaluation of PD-L1 peptide antagonists as new anticancer agents for immunotherapy. Bioorg Med Chem 30: (2021)
- Miao, SX; Wan, LX; He, ZX; Zhou, XL; Li, X; Gao, F Pd-Catalyzed Direct Diversification of Natural Anti-Alzheimer's Disease Drug: Synthesis and Biological Evaluation of J Nat Prod 84: 2374-2379 (2021)
- Vandromme, L; Piguel, S; Lozach, O; Meijer, L; Legraverend, M; Grierson, DS Suzuki-type Pd(0) coupling reactions in the synthesis of 2-arylpurines as Cdk inhibitors. Bioorg Med Chem Lett 16: 3144-6 (2006)
- Stachel, SJ; Egbertson, MS; Wai, J; Machacek, M; Toolan, DM; Swestock, J; Eddins, DM; Puri, V; McGaughey, G; Su, HP; Perlow, D; Wang, D; Ma, L; Parthasarathy, G; Reid, JC; Abeywickrema, PD; Smith, SM; Uslaner, JM Bioorg Med Chem Lett 28: 1122-1126 (2018)
- Kung, HF; Newman, S; Choi, SR; Oya, S; Hou, C; Zhuang, ZP; Acton, PD; Plössl, K; Winkler, J; Kung, MP J Med Chem 47: 5258-64 (2004)
- O'Neill, DJ; Adedoyin, A; Alfinito, PD; Bray, JA; Cosmi, S; Deecher, DC; Fensome, A; Harrison, J; Leventhal, L; Mann, C; McComas, CC; Sullivan, NR; Spangler, TB; Uveges, AJ; Trybulski, EJ; Whiteside, GT; Zhang, P J Med Chem 53: 4511-21 (2010)
- Foley, DW; Pathak, RB; Phillips, TR; Wilson, GL; Bailey, PD; Pieri, M; Senan, A; Meredith, D Eur J Med Chem 156: 180-189 (2018)
- Doucet-Personeni, C; Bentley, PD; Fletcher, RJ; Kinkaid, A; Kryger, G; Pirard, B; Taylor, A; Taylor, R; Taylor, J; Viner, R; Silman, I; Sussman, JL; Greenblatt, HM; Lewis, T J Med Chem 44: 3203-15 (2001)
- Boatman, PD; Richman, JG; Semple, G J Med Chem 51: 7653-62 (2008)
- Humphries, PS; Benbow, JW; Bonin, PD; Boyer, D; Doran, SD; Frisbie, RK; Piotrowski, DW; Balan, G; Bechle, BM; Conn, EL; Dirico, KJ; Oliver, RM; Soeller, WC; Southers, JA; Yang, X Bioorg Med Chem Lett 19: 2400-3 (2009)
- Boudreau, PD; Miller, BW; McCall, LI; Almaliti, J; Reher, R; Hirata, K; Le, T; Siqueira-Neto, JL; Hook, V; Gerwick, WH J Med Chem 62: 9026-9044 (2019)
- Krause-Heuer, AM; Fraser-Spears, R; Dobrowolski, JC; Ashford, ME; Wyatt, NA; Roberts, MP; Gould, GG; Cheah, WC; Ng, CKL; Bhadbhade, M; Zhang, B; Greguric, I; Wheate, NJ; Kumar, N; Koek, W; Callaghan, PD; Daws, LC; Fraser, BH Eur J Med Chem 137: 476-487 (2017)
- DeCarr, LB; Buckholz, TM; Coish, PD; Fathi, Z; Fisk, SE; Mays, MR; O'Connor, SJ; Lumb, KJ Bioorg Med Chem Lett 17: 538-41 (2007)
- Berry, DA; Gilbertsen, RB; Cook, PD J Med Chem 29: 2034-7 (1986)
- Bamborough, P; Barnett, HA; Becher, I; Bird, MJ; Chung, CW; Craggs, PD; Demont, EH; Diallo, H; Fallon, DJ; Gordon, LJ; Grandi, P; Hobbs, CI; Hooper-Greenhill, E; Jones, EJ; Law, RP; Le Gall, A; Lugo, D; Michon, AM; Mitchell, DJ; Prinjha, RK; Sheppard, RJ; Watson, AJ; Watson, RJ ACS Med Chem Lett 7: 552-7 (2016)
- Huang, C; Moree, WJ; Zamani-Kord, S; Li, BF; Tucci, FC; Malany, S; Wen, J; Wang, H; Hoare, SR; Yang, C; Madan, A; Crowe, PD; Beaton, G Bioorg Med Chem Lett 21: 947-51 (2011)
- Perez, DI; Palomo, V; Pérez, C; Gil, C; Dans, PD; Luque, FJ; Conde, S; Martínez, A J Med Chem 54: 4042-56 (2011)
- Bit, RA; Davis, PD; Elliott, LH; Harris, W; Hill, CH; Keech, E; Kumar, H; Lawton, G; Maw, A; Nixon, JS J Med Chem 36: 21-9 (1993)
- Devanesan, PD; Bobst, AM J Med Chem 29: 1237-42 (1987)
- Tecle, H; Barrett, SD; Lauffer, DJ; Augelli-Szafran, C; Brann, MR; Callahan, MJ; Caprathe, BW; Davis, RE; Doyle, PD; Eubanks, D; Lipiniski, W; Mirzadegan, T; Moos, WH; Moreland, DW; Nelson, CB; Pavia, MR; Raby, C; Schwarz, RD; Spencer, CJ; Thomas, AJ; Jaen, JC J Med Chem 41: 2524-36 (1998)
- Marques, EF; Bueno, MA; Duarte, PD; Silva, LR; Martinelli, AM; dos Santos, CY; Severino, RP; Brömme, D; Vieira, PC; Corrêa, AG Eur J Med Chem 54: 10-21 (2012)
- Edwards, PD; Andisik, DW; Strimpler, AM; Gomes, B; Tuthill, PA J Med Chem 39: 1112-24 (1996)
- Muccio, DD; Brouillette, WJ; Breitman, TR; Taimi, M; Emanuel, PD; Zhang, X; Chen, G; Sani, BP; Venepally, P; Reddy, L; Alam, M; Simpson-Herren, L; Hill, DL J Med Chem 41: 1679-87 (1998)
- Osborne, JD; Matthews, TP; McHardy, T; Proisy, N; Cheung, KM; Lainchbury, M; Brown, N; Walton, MI; Eve, PD; Boxall, KJ; Hayes, A; Henley, AT; Valenti, MR; De Haven Brandon, AK; Box, G; Jamin, Y; Robinson, SP; Westwood, IM; van Montfort, RL; Leonard, PM; Lamers, MB; Reader, JC; Aherne, GW; Raynaud, FI; Eccles, SA; Garrett, MD; Collins, I J Med Chem 59: 5221-37 (2016)
- Marques, GVL; Marques, DPA; Clarindo, FA; Avendaño-Villarreal, JA; Guerra, FS; Fernandes, PD; Dos Santos, EN; Gusevskaya, EV; Kohlhoff, M; Moreira, FA; Andrade, LAF; Fonseca, FGD; Dos-Reis, JGAC; Oliveira, RB Eur J Med Chem 260:
- Chen, T; Reich, NW; Bell, N; Finn, PD; Rodriguez, D; Kohler, J; Kozuka, K; He, L; Spencer, AG; Charmot, D; Navre, M; Carreras, CW; Koo-McCoy, S; Tabora, J; Caldwell, JS; Jacobs, JW; Lewis, JG J Med Chem 61: 7589-7613 (2018)
- Fathalla, RK; Fröhner, W; Bader, CD; Fischer, PD; Dahlem, C; Chatterjee, D; Mathea, S; Kiemer, AK; Arthanari, H; Müller, R; Abdel-Halim, M; Ducho, C; Engel, M J Med Chem 65: 14740-14763 (2022)
- LaMarche, MJ; Acker, M; Argintaru, A; Bauer, D; Boisclair, J; Chan, H; Chen, CH; Chen, YN; Chen, Z; Deng, Z; Dore, M; Dunstan, D; Fan, J; Fekkes, P; Firestone, B; Fodor, M; Garcia-Fortanet, J; Fortin, PD; Fridrich, C; Giraldes, J; Glick, M; Grunenfelder, D; Hao, HX; Hentemann, M; Ho, S; Jouk, A; Kang, ZB; Karki, R; Kato, M; Keen, N; Koenig, R; LaBonte, LR; Larrow, J; Liu, G; Liu, S; Majumdar, D; Mathieu, S; Meyer, MJ; Mohseni, M; Ntaganda, R; Palermo, M; Perez, L; Pu, M; Ramsey, T; Reilly, J; Sarver, P; Sellers, WR; Sendzik, M; Shultz, MD; Slisz, J; Slocum, K; Smith, T; Spence, S; Stams, T; Straub, C; Tamez, V; Toure, BB; Towler, C; Wang, P; Wang, H; Williams, SL; Yang, F; Yu, B; Zhang, JH; Zhu, S J Med Chem 63: 13578-13594 (2020)
- YOSHIKAWA, M; SAITOH, M; KATO, T; NAKAYAMA, Y; SEKI, T; NAKAGAWA, Y; TOMINARI, Y; SETO, M; SASAKI, Y; OKANIWA, M; ODA, T; SHIBUYA, A; HIDAKA, K; SHIOKAWA, Z; MURATA, S; OKABE, A; NAKADA, Y; MOCHIZUKI, M; FREEZE, BS; TAWARAISHI, T; WADA, Y; GREENSPAN, PD US Patent US20250205270 (2025)
- Nishiguchi, GA; Atallah, G; Bellamacina, C; Burger, MT; Ding, Y; Feucht, PH; Garcia, PD; Han, W; Klivansky, L; Lindvall, M Bioorg Med Chem Lett 21: 6366-9 (2011)
- Shuman, RT; Rothenberger, RB; Campbell, CS; Smith, GF; Gifford-Moore, DS; Gesellchen, PD J Med Chem 36: 314-9 (1993)
- Hong, WD; Gibbons, PD; Leung, SC; Amewu, R; Stocks, PA; Stachulski, A; Horta, P; Cristiano, MLS; Shone, AE; Moss, D; Ardrey, A; Sharma, R; Warman, AJ; Bedingfield, PTP; Fisher, NE; Aljayyoussi, G; Mead, S; Caws, M; Berry, NG; Ward, SA; Biagini, GA; O'Neill, PM; Nixon, GL J Med Chem 60: 3703-3726 (2017)
- McCleland, BW; Davis, RS; Palovich, MR; Widdowson, KL; Werner, ML; Burman, M; Foley, JJ; Schmidt, DB; Sarau, HM; Rogers, M; Salyers, KL; Gorycki, PD; Roethke, TJ; Stelman, GJ; Azzarano, LM; Ward, KW; Busch-Petersen, J Bioorg Med Chem Lett 17: 1713-7 (2007)
- Greenspan, PD; Fujimoto, RA; Marshall, PJ; Raychaudhuri, A; Lipson, KE; Zhou, H; Doti, RA; Coppa, DE; Zhu, L; Pelletier, R; Uziel-Fusi, S; Jackson, RH; Chin, MH; Kotyuk, BL; Fitt, JJ J Med Chem 42: 164-72 (1999)
- Hadida, S; Van Goor, F; Zhou, J; Arumugam, V; McCartney, J; Hazlewood, A; Decker, C; Negulescu, P; Grootenhuis, PD J Med Chem 57: 9776-95 (2014)
- Dawson, MI; Xia, Z; Liu, G; Ye, M; Fontana, JA; Farhana, L; Patel, BB; Arumugarajah, S; Bhuiyan, M; Zhang, XK; Han, YH; Stallcup, WB; Fukushi, J; Mustelin, T; Tautz, L; Su, Y; Harris, DL; Waleh, N; Hobbs, PD; Jong, L; Chao, WR; Schiff, LJ; Sani, BP J Med Chem 50: 2622-39 (2007)
- Taylor, NR; Cleasby, A; Singh, O; Skarzynski, T; Wonacott, AJ; Smith, PW; Sollis, SL; Howes, PD; Cherry, PC; Bethell, R; Colman, P; Varghese, J J Med Chem 41: 798-807 (1998)
- Cameron, KO; Lefker, BA; Chu-Moyer, MY; Crawford, DT; Jardine, PD; DeNinno, SL; Gilbert, S; Grasser, WA; Ke, H; Lu, B; Owen, TA; Paralkar, VM; Qi, H; Scott, DO; Thompson, DD; Tjoa, CM; Zawistoski, MP Bioorg Med Chem Lett 16: 1799-802 (2006)
- Semple, G; Ashworth, DM; Baker, GR; Batt, AR; Baxter, AJ; Benzies, DW; Elliot, LH; Evans, D; Franklin, RJ; Hudson, P; Jenkins, PD; Pitt, GR; Rooker, DP; Sheppard, A; Szelke, M; Yamamoto, S; Isomura, Y Bioorg Med Chem Lett 7: 1337-1342 (1997)
- Wang, HY; Cao, ZX; Li, LL; Jiang, PD; Zhao, YL; Luo, SD; Yang, L; Wei, YQ; Yang, SY Bioorg Med Chem Lett 18: 4972-7 (2008)
- Singer, JM; Wilson, MW; Johnson, PD; Graham, SR; Cooke, LW; Roof, RL; Boxer, PA; Gold, LH; Meltzer, LT; Janssen, A; Roush, N; Campbell, JE; Su, TZ; Hurst, SI; Stoner, CL; Schwarz, JB Bioorg Med Chem Lett 19: 2409-12 (2009)
- Anandan, SK; Webb, HK; Chen, D; Wang, YX; Aavula, BR; Cases, S; Cheng, Y; Do, ZN; Mehra, U; Tran, V; Vincelette, J; Waszczuk, J; White, K; Wong, KR; Zhang, LN; Jones, PD; Hammock, BD; Patel, DV; Whitcomb, R; MacIntyre, DE; Sabry, J; Gless, R Bioorg Med Chem Lett 21: 983-8 (2011)
- Bandgar, BP; Totre, JV; Gawande, SS; Khobragade, CN; Warangkar, SC; Kadam, PD Bioorg Med Chem 18: 6149-55 (2010)
- Baettig, U; Brown, L; Brundish, D; Dell, C; Furzer, A; Garman, S; Janus, D; Kane, PD; Smith, G; Walker, CV; Cockcroft, X; Ambler, J; Mitchelson, A; Talbot, MD; Tweed, M; Wills, N Bioorg Med Chem Lett 10: 1563-6 (2000)
- Leger, PR; Hu, DX; Biannic, B; Bui, M; Han, X; Karbarz, E; Maung, J; Okano, A; Osipov, M; Shibuya, GM; Young, K; Higgs, C; Abraham, B; Bradford, D; Cho, C; Colas, C; Jacobson, S; Ohol, YM; Pookot, D; Rana, P; Sanchez, J; Shah, N; Sun, M; Wong, S; Brockstedt, DG; Kassner, PD; Schwarz, JB; Wustrow, DJ J Med Chem 63: 5398-5420 (2020)
- Lopes da Rosa, J; Bajaj, V; Spoonamore, J; Kaufman, PD Bioorg Med Chem Lett 23: 2853-9 (2013)
- McCoull, W; Hennessy, EJ; Blades, K; Chuaqui, C; Dowling, JE; Ferguson, AD; Goldberg, FW; Howe, N; Jones, CR; Kemmitt, PD; Lamont, G; Varnes, JG; Ward, RA; Yang, B ACS Med Chem Lett 7: 1118-1123 (2016)
- Waldschmidt, HV; Homan, KT; Cruz-Rodríguez, O; Cato, MC; Waninger-Saroni, J; Larimore, KM; Cannavo, A; Song, J; Cheung, JY; Kirchhoff, PD; Koch, WJ; Tesmer, JJ; Larsen, SD J Med Chem 59: 3793-807 (2016)
- Blum, E; Zhang, J; Zaluski, J; Einstein, DE; Korshin, EE; Kubas, A; Gruzman, A; Tochtrop, GP; Kiser, PD; Palczewski, K J Med Chem 64: 8287-8302 (2021)
- Morellato-Castillo, L; Acharya, P; Combes, O; Michiels, J; Descours, A; Ramos, OH; Yang, Y; Vanham, G; Ariën, KK; Kwong, PD; Martin, L; Kessler, P J Med Chem 56: 5033-47 (2013)
- Milne, JC; Lambert, PD; Schenk, S; Carney, DP; Smith, JJ; Gagne, DJ; Jin, L; Boss, O; Perni, RB; Vu, CB; Bemis, JE; Xie, R; Disch, JS; Ng, PY; Nunes, JJ; Lynch, AV; Yang, H; Galonek, H; Israelian, K; Choy, W; Iffland, A; Lavu, S; Medvedik, O; Sinclair, DA; Olefsky, JM; Jirousek, MR; Elliott, PJ; Westphal, CH Nature 450: 712-716 (2007)
- Drilon, A; Nagasubramanian, R; Blake, JF; Ku, N; Tuch, BB; Ebata, K; Smith, S; Lauriault, V; Kolakowski, GR; Brandhuber, BJ; Larsen, PD; Bouhana, KS; Winski, SL; Hamor, R; Wu, WI; Parker, A; Morales, TH; Sullivan, FX; DeWolf, WE; Wollenberg, LA; Gordon, PR; Douglas-Lindsay, DN; Scaltriti, M; Benayed, R; Raj, S; Hanusch, B; Schram, AM; Jonsson, P; Berger, MF; Hechtman, JF; Taylor, BS; Andrews, S; Rothenberg, SM; Hyman, DM Cancer Discov 7: 963-972
- Rowley, M; Broughton, HB; Collins, I; Baker, R; Emms, F; Marwood, R; Patel, S; Patel, S; Ragan, CI; Freedman, SB; Leeson, PD J Med Chem 39: 1943-5 (1996)
- Brown, MF; Bahnck, KB; Blumberg, LC; Brissette, WH; Burrell, SA; Driscoll, JP; Fedeles, F; Fisher, MB; Foti, RS; Gladue, RP; Guzman-Martinez, A; Hayward, MM; Lira, PD; Lillie, BM; Lu, Y; Lundquist, GD; McElroy, EB; McGlynn, MA; Paradis, TJ; Poss, CS; Roache, JH; Shavnya, A; Shepard, RM; Trevena, KA; Tylaska, LA Bioorg Med Chem Lett 17: 3109-12 (2007)
- Johannes, JW; Almeida, L; Daly, K; Ferguson, AD; Grosskurth, SE; Guan, H; Howard, T; Ioannidis, S; Kazmirski, S; Lamb, ML; Larsen, NA; Lyne, PD; Mikule, K; Ogoe, C; Peng, B; Petteruti, P; Read, JA; Su, N; Sylvester, M; Throner, S; Wang, W; Wang, X; Wu, J; Ye, Q; Yu, Y; Zheng, X; Scott, DA Bioorg Med Chem Lett 25: 5743-7 (2015)
- González-López, M; Welsh, K; Finlay, D; Ardecky, RJ; Ganji, SR; Su, Y; Yuan, H; Teriete, P; Mace, PD; Riedl, SJ; Vuori, K; Reed, JC; Cosford, ND Bioorg Med Chem Lett 21: 4332-6 (2011)
- Hartman, GD; Egbertson, MS; Halczenko, W; Laswell, WL; Duggan, ME; Smith, RL; Naylor, AM; Manno, PD; Lynch, RJ; Zhang, G J Med Chem 35: 4640-2 (1993)
- Sheppard, GS; Pireh, D; Carrera, GM; Bures, MG; Heyman, HR; Steinman, DH; Davidsen, SK; Phillips, JG; Guinn, DE; May, PD J Med Chem 37: 2011-32 (1994)
- Mehta, PD; Sengar, NP; Pathak, AK Eur J Med Chem 45: 5541-60 (2010)
- Durant, GJ; Ganellin, CR; Hills, DW; Miles, PD; Parsons, ME; Pepper, ES; White, GR J Med Chem 28: 1414-22 (1985)
- Harfenist, M; Joyner, CT; Mize, PD; White, HL J Med Chem 37: 2085-9 (1994)
- Dewkar, GK; Peddi, S; Mosier, PD; Roth, BL; Westkaemper, RB Bioorg Med Chem Lett 18: 5268-71 (2008)
- Meegalla, SK; Plössl, K; Kung, MP; Chumpradit, S; Stevenson, DA; Kushner, SA; McElgin, WT; Mozley, PD; Kung, HF J Med Chem 40: 9-17 (1997)
- Rynearson, KD; Buckle, RN; Barnes, KD; Herr, RJ; Mayhew, NJ; Paquette, WD; Sakwa, SA; Nguyen, PD; Johnson, G; Tanzi, RE; Wagner, SL Bioorg Med Chem Lett 26: 3928-37 (2016)
- Horwell, DC; Nichols, PD; Roberts, E Bioorg Med Chem Lett 4: 2263-2266 (1994)
- Kendall, JD; O'Connor, PD; Marshall, AJ; Frédérick, R; Marshall, ES; Lill, CL; Lee, WJ; Kolekar, S; Chao, M; Malik, A; Yu, S; Chaussade, C; Buchanan, C; Rewcastle, GW; Baguley, BC; Flanagan, JU; Jamieson, SM; Denny, WA; Shepherd, PR Bioorg Med Chem 20: 69-85 (2011)
- Corey, SD; Pansegrau, PD; Walker, MC; Sikorski, JA Bioorg Med Chem Lett 3: 2857-2862 (1993)
- Patel, HJ; Patel, PD; Ochiana, SO; Yan, P; Sun, W; Patel, MR; Shah, SK; Tramentozzi, E; Brooks, J; Bolaender, A; Shrestha, L; Stephani, R; Finotti, P; Leifer, C; Li, Z; Gewirth, DT; Taldone, T; Chiosis, G J Med Chem 58: 3922-43 (2015)
- Lagu, B; Pio, B; Lebedev, R; Yang, M; Pelton, PD Bioorg Med Chem Lett 17: 3497-503 (2007)
- Pierson, PD; Fettes, A; Freichel, C; Gatti-McArthur, S; Hertel, C; Huwyler, J; Mohr, P; Nakagawa, T; Nettekoven, M; Plancher, JM; Raab, S; Richter, H; Roche, O; Rodríguez Sarmiento, RM; Schmitt, M; Schuler, F; Takahashi, T; Taylor, S; Ullmer, C; Wiegand, R J Med Chem 52: 3855-68 (2009)
- Ganapathy, ME; Prasad, PD; Mackenzie, B; Ganapathy, V; Leibach, FH Biochim Biophys Acta 1324: 296-308 (1997)
- Vargas, R; Perola, E; Ramsden, PD; Wenglowsky, SM; Wilson, D US Patent US20250074895 (2025)
- Palin, R; Clark, JK; Evans, L; Feilden, H; Fletcher, D; Hamilton, NM; Houghton, AK; Jones, PS; McArthur, D; Montgomery, B; Ratcliffe, PD; Smith, AR; Sutherland, A; Weston, MA; Wishart, G Bioorg Med Chem Lett 19: 6441-6 (2009)
- Groziak, MP; Huan, ZW; Ding, H; Meng, Z; Stevens, WC; Robinson, PD J Med Chem 40: 3336-45 (1997)
- Hassett, MR; Sternberg, AR; Roepe, PD Biochemistry 56: 4326-4334
- Ross, PD; Rekharsky, MV Biophys J 71: 2144-54 (1996)
- Lee, J; Choi, J; Hwang, H; Song, H; Kim, J; Kim, S; Koh, JS; Lee, J; Lee, T; Choi, Y; Park, S; Lee, IY; Suh, B; Salgaonkar, PD; Jung, D US Patent US9212178 (2015)
- Sant' Anna, Gda S; Machado, P; Sauzem, PD; Rosa, FA; Rubin, MA; Ferreira, J; Bonacorso, HG; Zanatta, N; Martins, MA Bioorg Med Chem Lett 19: 546-9 (2008)
- Jordan, JB; Poppe, L; Xia, X; Cheng, AC; Sun, Y; Michelsen, K; Eastwood, H; Schnier, PD; Nixey, T; Zhong, W J Med Chem 55: 678-87 (2012)
- Dios, A; Mitchell, RA; Aljabari, B; Lubetsky, J; O'Connor, K; Liao, H; Senter, PD; Manogue, KR; Lolis, E; Metz, C; Bucala, R; Callaway, DJ; Al-Abed, Y J Med Chem 45: 2410-6 (2002)
- Gaster, LM; Blaney, FE; Davies, S; Duckworth, DM; Ham, P; Jenkins, S; Jennings, AJ; Joiner, GF; King, FD; Mulholland, KR; Wyman, PA; Hagan, JJ; Hatcher, J; Jones, BJ; Middlemiss, DN; Price, GW; Riley, G; Roberts, C; Routledge, C; Selkirk, J; Slade, PD J Med Chem 41: 1218-35 (1998)
- De Savi, C; Pape, A; Sawyer, Y; Milne, D; Davies, C; Cumming, JG; Ting, A; Lamont, S; Smith, PD; Tart, J; Page, K; Moore, P Bioorg Med Chem Lett 21: 3301-6 (2011)
- Letourneau, JJ; Stroke, IL; Hilbert, DW; Cole, AG; Sturzenbecker, LJ; Marinelli, BA; Quintero, JG; Sabalski, J; Li, Y; Ma, L; Pechik, I; Stein, PD; Webb, ML Bioorg Med Chem Lett 28: 3601-3605 (2018)
- Andersen, KE; Sørensen, JL; Huusfeldt, PO; Knutsen, LJ; Lau, J; Lundt, BF; Petersen, H; Suzdak, PD; Swedberg, MD J Med Chem 42: 4281-91 (1999)
- Agoston, GE; Vaughan, R; Lever, JR; Izenwasser, S; Terry, PD; Newman, AH Bioorg Med Chem Lett 7: 3027-3032 (1997)
- Heathcock, CH; Hadley, CR; Rosen, T; Theisen, PD; Hecker, SJ J Med Chem 30: 1858-73 (1987)
- Powell, JW; Mann, CA; Toth, PD; Nolan, S; Steinert, A; Ove, C; Seffernick, JT; Wozniak, DJ; Kebriaei, R; Lindert, S; Osheroff, N; Yalowich, JC; Mitton-Fry, MJ ACS Med Chem Lett 15: 1287-1297
- Rodgers, TL; Townsend, PD; Burnell, D; Jones, ML; Richards, SA; McLeish, TC; Pohl, E; Wilson, MR; Cann, MJ PLoS Biol 11: 1-15 (2013)
- Bondinell, WE; Chapin, FW; Girard, GR; Kaiser, C; Krog, AJ; Pavloff, AM; Schwartz, MS; Silvestri, JS; Vaidya, PD; Lam, BL; Wellman, GR; Pendleton, RG J Med Chem 23: 506-11 (1980)
- Wise, JG; Nanayakkara, AK; Aljowni, M; Chen, G; De Oliveira, MC; Ammerman, L; Olengue, K; Lippert, AR; Vogel, PD J Med Chem 62: 10645-10663 (2019)
- Shawn, C; Paul, CD; Lee, DR; Andrew, F; Magdalena, K; Yvette, LS; Timothy, LA; Vincent, M; Walter, PD; Andre, S; Mike, TM; Tao, W; Maria, WH WIPO WO2022112919 (2022)
- Bungard, CJ; Williams, PD; Schulz, J; Wiscount, CM; Holloway, MK; Loughran, HM; Manikowski, JJ; Su, HP; Bennett, DJ; Chang, L; Chu, XJ; Crespo, A; Dwyer, MP; Keertikar, K; Morriello, GJ; Stamford, AW; Waddell, ST; Zhong, B; Hu, B; Ji, T; Diamond, TL; Bahnck-Teets, C; Carroll, SS; Fay, JF; Min, X; Morris, W; Ballard, JE; Miller, MD; McCauley, JA ACS Med Chem Lett 8: 1292-1297 (2017)
- St-Denis, Y; Lévesque, S; Bachand, B; Edmunds, JJ; Leblond, L; Préville, P; Tarazi, M; Winocour, PD; Siddiqui, MA Bioorg Med Chem Lett 12: 1181-4 (2002)
- Vo, CD; Shebert, HL; Zikovich, S; Dryer, RA; Huang, TP; Moran, LJ; Cho, J; Wassarman, DR; Falahee, BE; Young, PD; Gu, GH; Heinl, JF; Hammond, JW; Jackvony, TN; Frederick, TE; Blair, JA Bioorg Med Chem Lett 27: 5235-5244 (2017)
- Converso, A; Hartingh, T; Garbaccio, RM; Tasber, E; Rickert, K; Fraley, ME; Yan, Y; Kreatsoulas, C; Stirdivant, S; Drakas, B; Walsh, ES; Hamilton, K; Buser, CA; Mao, X; Abrams, MT; Beck, SC; Tao, W; Lobell, R; Sepp-Lorenzino, L; Zugay-Murphy, J; Sardana, V; Munshi, SK; Jezequel-Sur, SM; Zuck, PD; Hartman, GD Bioorg Med Chem Lett 19: 1240-4 (2009)
- Soares de Melo, C; Singh, V; Myrick, A; Simelane, SB; Taylor, D; Brunschwig, C; Lawrence, N; Schnappinger, D; Engelhart, CA; Kumar, A; Parish, T; Su, Q; Myers, TG; Boshoff, HIM; Barry, CE; Sirgel, FA; van Helden, PD; Buchanan, KI; Bayliss, T; Green, SR; Ray, PC; Wyatt, PG; Basarab, GS; Eyermann, CJ; Chibale, K; Ghorpade, SR J Med Chem 64: 719-740 (2021)
- Dang, Q; Brown, BS; Liu, Y; Rydzewski, RM; Robinson, ED; van Poelje, PD; Reddy, MR; Erion, MD J Med Chem 52: 2880-98 (2009)
- Chi, G; Manos-Turvey, A; O'Connor, PD; Johnston, JM; Evans, GL; Baker, EN; Payne, RJ; Lott, JS; Bulloch, EM Biochemistry 51: 4868-79 (2012)
- ChEMBL_2273446 Inhibition of human PD-1/PD-L1 interaction
- ChEMBL_2489301 Inhibition of mouse PD-1/PD-L1 interaction
- ChEMBL_2378267 Inhibition of PD-1/PD-L1 (unknown origin) interaction
- ChEMBL_2450079 Inhibition of PD-1/PD-L1 interaction (unknown origin)
- ChEMBL_2184690 (CHEMBL5096772) Inhibition of PD-1/PD-L1 interaction (unknown origin)
- ChEMBL_2241734 (CHEMBL5155944) Inhibition of PD-1/PD-L1 (unknown origin) interaction
- ChEMBL_2378134 Antagonist activity at PD-1/PD-L1 interaction (unknown origin)
- ChEMBL_2378276 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction
- ChEMBL_2450080 Inhibition of human PD-1/PD-L1 interaction by HTRF analysis
- ChEMBL_2454243 Inhibition of PD-1/PD-L1 (unknown origin) protein protein interaction
- ChEMBL_2477236 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction
- ChEMBL_2327515 Inhibition of PD-1 binding to human PD-L1 by HTRF assay
- ChEMBL_2360361 Inhibition of PD-1/PD-L1 interaction (unknown origin) by HTRF assay
- ChEMBL_2432495 Inhibition of PD-1/PD-L1 (unknown origin) interaction by HTRF assay
- ChEMBL_2152646 (CHEMBL5037193) Inhibition of PD-1/PD-L1 interaction (unknown origin) preincubated with PD-L1 for 15 mins followed by PD-1 addition measured after 90 mins by TR-FRET assay
- ChEMBL_2480579 Inhibition of human PD-1/PD-L1 interaction incubated for 1 hr by HTRF assay
- ChEMBL_2149677 (CHEMBL5034139) Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based HTRF assay
- ChEMBL_2132481 (CHEMBL4841996) Inhibition of PD-1/PD-L1 interaction (unknown origin) by HTRF assay
- ChEMBL_2184692 (CHEMBL5096774) Inhibition of human PD-1/PD-L1 interaction by HTRF binding assay
- ChEMBL_2378262 Inhibition of PD-1/PD-L1 interaction (unknown origin) by TR-FRET assay
- ChEMBL_2352901 Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 1 hr by HTRF assay
- ChEMBL_2378135 Antagonist activity at human PD-1/PD-L1 interaction measured after 1 hr by HTRF assay
- ChEMBL_2378264 Inhibition of PD-1/PD-L1 (unknown origin) interaction incubated for 1 hr by HTRF assay
- ChEMBL_2184691 (CHEMBL5096773) Inhibition of PD-1/PD-L1 interaction (unknown origin) by HTRF binding assay
- ChEMBL_2206840 (CHEMBL5119548) Inhibition of human PD-1/PD-L1 interaction by surface plasmon resonance analysis
- ChEMBL_2356354 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction by HTRF assay
- ChEMBL_2480900 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction by HTRF assay
- ChEMBL_2241733 (CHEMBL5155943) Inhibition of PD-1-Ig/His-PD-L1 (unknown origin) protein-protein interaction incubated with PD-L1 for 15 mins followed by addition of PD-1 for 15 mins by HTRF assay
- ChEMBL_2307870 Inhibition of europium-labeled PD-1/biotinylated PD-L1 interaction (unknown origin) preincubated for 15 mins with PD-L1 followed by PD-1 addition and measured after 90 mins by TR-FRET assay
- ChEMBL_2281189 Inhibition of PD-1 (unknown origin)
- ChEMBL_2325350 Inhibition of human PD-1/PD-L1 interaction assessed as induction of PD-L1 dimerization incubated for 2 hrs by HTRF analysis
- ChEMBL_2250882 (CHEMBL5165092) Inhibition of human PD-1/PD-L1 interaction in CHO-K1 cells transfected with PD-1-YFP/SHP-2-CFP and measured by FRET assay
- ChEMBL_2083840 (CHEMBL4739631) Antagonist activity at PD1/PD-L1 (unknown origin) assessed as disruption of PD-1/PD-L1 complex by measuring PD-L1 dimerization measured after 2 hrs by HTRF assay
- ChEMBL_2341374 Inhibition of PD-1 (unknown origin)/PD-L1 (unknown origin) interaction by HTRF binding assay
- ChEMBL_2357139 Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 15 mins by ELISA
- ChEMBL_2378269 Inhibition of human PD-1/PD-L1 interaction incubated for 24 hrs by HTRF assay
- ChEMBL_2378273 Inhibition of PD-1 (unknown origin)/PD-L1 (unknown origin) interaction by HTRF binding assay
- ChEMBL_2378274 Inhibition of PD-1/PD-L1 interaction (unknown origin) by AlphaLISA assay relative to control
- ChEMBL_2480581 Inhibition of human PD-1/PD-L1 interaction incubated for 2 hr by HTRF assay
- ChEMBL_1750186 (CHEMBL4184946) Inhibition of His-tagged PD-L1 binding to PD-1-Ig (unknown origin) preincubated with PD-L1 for 15 mins followed by PD-1-Ig addition measured after 15 mins by HTRF binding assay
- ChEMBL_2132495 (CHEMBL4842010) Inhibition of PD-1/PD-L1 interaction in human Jurkat T cells expressing PD-1 cocultured with CHO cells expressing PD-Ll/TCR assessed as activation of Jurkat cells by luciferase reporter gene assay
- ChEMBL_2360353 Inhibition of PD-I/PD-L1 interaction (unknown origin)
- ChEMBL_2460173 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction incubated for 1 hr by HTRF assay
- ChEMBL_2327504 Inhibition of Ig-tagged PD-1 (unknown origin)/His-tagged PD-L1 (unknown origin) protein-protein interaction preincubated with PD-L1 for 15 mins followed by PD-1 addition measured after 15 mins by HTRF assay
- ChEMBL_2445171 Inhibition of Eu-tagged PD-1(unknown origin) /biotin-tagged PD-L1 (unknown origin) interaction preincubated with PD-L1 for 15 mins followed by PD-1 addition and measured after 90 mins by TR-FRET assay
- ChEMBL_2303331 Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 24 hrs by HTRF assay
- ChEMBL_2328179 Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 60 min by HTRF assay
- ChEMBL_2332832 Inhibition of PD-1/PD-L1 (unknown origin) interaction incubated for 15 mins by HTRF assay
- ChEMBL_2378121 Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 24 hrs by HTRF assay
- ChEMBL_2378263 Inhibition of PD-1/PD-L1 (unknown origin) interaction incubated for 15 mins by HTRF assay
- ChEMBL_2378396 Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 24 hrs by HTRF assay
- ChEMBL_2423595 Inhibition of PD-1/PD-L1 (unknown origin) interaction incubated for 15 mins by HTRF assay
- ChEMBL_2441564 Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 15 min by HTRF assay
- ChEMBL_2184689 (CHEMBL5096771) Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 1 to 24 hrs by HTRF assay
- ChEMBL_2184686 (CHEMBL5096768) Inhibition of PD-1/PD-L1 interaction (unknown origin) incubated for 15 mins by HTRF assay
- ChEMBL_2332862 Inhibition of human PD-1/PD-L1 interaction overexpressed in human HEK293T cells by flow cytometry analysis
- ChEMBL_2378265 Inhibition of PD-1/PD-L1 (unknown origin) interaction incubated for 2 hr by TR-FRET assay
- ChEMBL_2378272 Inhibition of human PD-1/PD-L1 interaction overexpressed in human HEK293T cells by flow cytometry analysis
- ChEMBL_2460174 Inhibition of PD-1/PD-L1 (unknown origin) interaction expressed in human HEK293T cells by HTRF analysis
- ChEMBL_2485930 Inhibition of human PD-1 to PD-L1 protein-protein interaction by homogeneous-time resolved fluorescence assay
- ChEMBL_2110972 (CHEMBL4819822) Inhibition of PD-1/PD-L1 interaction in CHO cells expressing PD-Ll/TCR cocultured with human Jurkat cells expressing PD-1 assessed as activation of effector Jurkat T cells incubated for 6 hrs by luciferase reporter gene assay
- ChEMBL_2360356 Inhibition of PD-I/PD-L1 interaction (unknown origin) by ELISA
- ChEMBL_2184695 (CHEMBL5096777) Inhibition of recombinant human PD-1/PD-L1 interaction incubated for 40 mins by HTRF binding assay
- ChEMBL_2314396 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction incubated for 105 mins by HTRF assay
- ChEMBL_2432493 Inhibition of His-tagged PD-1/PD-L1 (unknown origin) interaction incubated for 15 mins by HTRF assay
- ChEMBL_2460167 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction expressed in HEK293 cells by HTRF method
- ChEMBL_2489288 Inhibition of Fc-tagged recombinant human PD-1/His-tagged recombinant human PD-L1 interaction by ALPHA assay
- ChEMBL_2489290 Inhibition of biotinylated PD-1/His-tagged PD-L1 (unknown origin) incubated for 60 mins by ALPHA assay
- ChEMBL_2156092 (CHEMBL5040752) Inhibition of PD-1/PDL-1 (unknown origin) binding
- ChEMBL_2360369 Inhibition of PD-I/PD-L1 interaction (unknown origin) by HTRF assay
- ChEMBL_2184693 (CHEMBL5096775) Inhibition of His-tagged PD-1/PD-L1 interaction (unknown origin) incubated for 1 to 4 hrs by HTRF binding assay
- ChEMBL_2332855 Inhibition of PD-1/His-tagged PD-L1 (unknown origin) protein-protein interaction incubated for 90 mins by AlphaLISA
- ChEMBL_2356351 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction incubated for 15 mins by TR-FRET assay
- ChEMBL_2360490 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction incubated for 15 mins by TR-FRET assay
- ChEMBL_2378275 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction incubated for 15 mins by TR-FRET assay
- ChEMBL_2477238 Inhibition of PD-1/PD-L1 (unknown origin) protein-protein interaction incubated for 15 mins by TR-FRET assay
- ChEMBL_2148569 (CHEMBL5032967) Inhibition of human PD-1/PD-L1 interaction in human Jurkat cells expressing PD-1/NFAT/Luc cocultured with CHO-K1 cells expressing PD-L1/aAPC assessed as Jurkat T-lymphocyte activation measured after 6 hrs by luciferase reporter gene based luminescence assay
- ChEMBL_2360350 Inhibition of PD-I/PD-L2 interaction (unknown origin) expressed in HEK293 cells
- ChEMBL_2265318 Inhibition of human PD-1/PDL1 protein-protein interaction
- ChEMBL_2450078 Inhibition of human recombinant PD-1 by HTRF analysis
- ChEMBL_2432196 Inhibition of PD-1/PD-L1 (unknown origin) interaction assessed as inhibition rate incubated for 1 hr by HTRF assay relative to control
- ChEMBL_2307873 Inhibition of PD-1 Fc IgG fusion protein binding to PD-L1 in human NCI-H460 cells incubated for 6 hrs by DAPI/PD-1 anti-human IgG DyLight 488 staining based fluorescence microscopy
- ChEMBL_2148560 (CHEMBL5032958) Inhibition of human PD-1/PD-L1 interaction assessed as blockade activity incubated for 15 mins by HTRF assay
- ChEMBL_2206838 (CHEMBL5119546) Inhibition of human recombinant PD-1/PD-L1 interaction preincubated with compound for 2 hrs followed by PD-1 protein addition and measured after 1.5 hrs by inhibitor screening ELISA assay pair kit method
- ChEMBL_2241722 (CHEMBL5155932) Inhibition of Tag2-PD-1/Tag1-PD-L1 (unknown origin) protein-protein interaction incubated for 15 mins by HTRF assay
- ChEMBL_2206839 (CHEMBL5119547) Inhibition of mouse recombinant PD-1/PD-L1 interaction preincubated with compound for 2 hrs followed by PD-1 protein addition and measured after 1.5 hrs by by inhibitor screening ELISA assay pair kit method
- ChEMBL_2069507 (CHEMBL4724760) Inhibition of human PD-1/PDL1 by HTRF assay
- ChEMBL_2083843 (CHEMBL4739634) Antagonist activity at human PD1/PD-L1 preincubated with PD-L1 for 1 hr followed by incubation with PD1 for 1 hr by ELISA
- ChEMBL_2341287 Inhibition of GST-tagged PD-1 (unknown origin)/His-tagged PD-L1 (unknown origin) interaction by HTRF binding assay relative to control
- ChEMBL_2360351 Inhibition of PD-I/PD-L2 interaction (unknown origin) expressed in human MDA-MB-23 cells
- ChEMBL_2378268 Inhibition of human PD-L1/PD-L1 interaction incubated for 15 mins by TR-FRET assay
- ChEMBL_2069506 (CHEMBL4724759) Inhibition of PD-1/PDL1 (unknown origin) by HTRF assay
- ChEMBL_2169505 (CHEMBL5054564) Inhibition of PD-1/PDL1 protein-protein interaction (unknown origin)
- PD-1/PD-L1 NFA T Reporter Assay Compounds were tested in a functional co-culture reporter assay in which TCR-mediated NFAT activity is inhibited by the engagement of PD-1 with PD-L1. Blocking the PD-1/PD-L1 interaction impairs PD-1 mediated blunting of TCR signaling and significantly increases NFAT-mediated transcription of luciferase. CHO cells expressing surface-bound anti-CD3 antibodies and PD-L1 (artificial antigen presenting cells, aAPC-PD-L1) were first seeded overnight. Jurkat cells overexpressing PD-1 and expressing a luciferase construct under NFAT control are diluted in RPMI assay medium (RPMI 1640 with 2% FBS), mixed with compounds, and immediately seeded on the monolayer of aAPC-PD-L1. The co-culture is then incubated for 6 hrs at 37° C. Luciferase activity is assessed by adding the ONE-Glo reagent and measuring luminescence with a plate reader. EC50 values are calculated from the fit of the dose-response curves to a four-parameter equation.
- ChEMBL_2281190 Inhibition of PD-L1 (unknown origin)
- ChEMBL_2164099 (CHEMBL5048960) Inhibition of PD-1/PD-L1 (unknown origin) interaction pre-incubated for 15 mins and measured after 90 mins by TR-FRET assay
- ChEMBL_2327513 Inhibition of His-tagged PD-1 (unknown origin)/hFc-tagged PD-L1 (unknown origin) protein-protein interaction incubated for 15 mins by HTRF assay
- ChEMBL_2327512 Inhibition of C-terminal 3S-IG-tagged recombinant human PD-1 (25 to 167 residues)/C-terminal 6xHis-tagged recombinant human PD-L1 (18 to 239 residues) expressed in HEK293T cells protein-protein interaction preincubated with PD-L1 for 15 mins followed by PD-1 addition measured after 15 mins by HTRF assay
- ChEMBL_2360352 Inhibition of PD-I/PD-L1 interaction (unknown origin) incubated for 15 mins by TR-FRET assay
- ChEMBL_2156093 (CHEMBL5040753) Inhibition of human recombinant PD-1/PDL-1 interaction measured by HTRF assay
- ChEMBL_2320908 Inhibition of human PD-1/PDL1 interaction incubated for 1 hr by HTRF assay
- ChEMBL_2028153 (CHEMBL4682311) Inhibition of PD-1/PDL1 interaction (unknown origin) by HTRF assay
- ChEMBL_2265317 Inhibition of human PD-1/PDL1 protein-protein interaction by HTRF assay
- ChEMBL_2434121 Inhibition of PD-1/PDL1 interaction (unknown origin) in by HTRF assay
- Binding of PD-1 to PD-L1 Using Homogenous Time-Resolved Fluorescence (HTRF) Binding Assay Homogenous Time-Resolved Fluorescence (HTRF) Binding Assay. The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and PD-L1 protein extracellular domains were expressed as fusion proteins with detection tags, for PD-1, the tag was the Fc portion of Immunoglobulin (PD-1-Ig) and for PD-L1 it was the 6 histidine motif (PD-L1-His). All binding studies were performed in an HTRF assay buffer consisting of dPBS supplemented with 0.1% (with) bovine serum albumin and 0.05% (v/v) Tween-20. For the h/PD-L1-His binding assay, inhibitors were pre-incubated with PD-L1-His (10 nM final) for 15 m in 4 μl of assay buffer, followed by addition of PD-1-Ig (20 nM final) in 1 μl of assay buffer and further incubation for 15 m. HTRF detection was achieved using europium cryptate-labeled anti-Ig (1 nM final) and allophycocyanin (APC) labeled anti-His (20 nM final). Antibodies were diluted in HTRF detection buffer and 5 μl was dispensed on top of the binding reaction. The reaction mixture was allowed to equilibrate for 30 minutes and the resulting signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer. Additional binding assays were established between the human proteins PD-1-Ig/PD-L2-His (20 & 5 nM, respectively) and CD80-His/PD-L1-Ig (100 & 10 nM, respectively).
- ChEMBL_1791472 (CHEMBL4263391) Inhibition of PD-L1 (unknown origin)
- ChEMBL_2184684 (CHEMBL5096766) Inhibition of PD-L1 (unknown origin)
- ChEMBL_2265320 Binding affinity to human PD-1 assessed as dissociation constant by SPR assay
- ChEMBL_2265321 Inhibition of human PD-1/PDL1 protein-protein interaction by TR-FRET assay
- ChEMBL_2273447 Inhibition of human PD-1 expressed in rat PBMCs/human PD-L1 expressed in MDA-MB-231 cells protein-protein interaction assessed as increase in PBMCs proliferation
- ChEMBL_2360371 Inhibition of recombinant human PD-1 (33 to 150 residues)/recombinant human PD-L1 (18 to 134 residues) expressed Escherichia coli BL21 (DE3) cells by HTFR assay
- ChEMBL_2360354 Inhibition of Fc-fused recombinant human PD-I/His-tagged recombinant human PD-L1 interaction incubated overnight by HTRF assay
- ChEMBL_2127477 (CHEMBL4836822) Inhibition of PD-1/PD-L1 interaction in human Jurkat cells cocultured with CHO cells expressing PD-Ll/TCRAct assessed as activation of effector Jurkat cells incubated for 6 hrs by luciferase reporter gene based immune checkpoint blockade cell-based assay
- Homogenous Time-Resolved Fluorescence (HTRF) Binding Assay The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and PD-L1 protein extracellular domains were expressed as fusion proteins with detection tags, for PD-1, the tag was the Fc portion of Immunoglobulin (PD-1-Ig) and for PD-L1 it was the 6 histidine motif (PD-L1-His). All binding studies were performed in an HTRF assay buffer consisting of dPBS supplemented with 0.1% (with) bovine serum albumin and 0.05% (v/v) Tween-20. For the h/PD-L1-His binding assay, inhibitors were pre-incubated with PD-L1-His (10 nM final) for 15 m in 4 μl of assay buffer, followed by addition of PD-1-Ig (20 nM final) in 1 μl of assay buffer and further incubation for 15 m. HTRF detection was achieved using europium crypate-labeled anti-Ig (1 nM final) and allophycocyanin (APC) labeled anti-His (20 nM final). Antibodies were diluted in HTRF detection buffer and 5 μl was dispensed on top of the binding reaction. The reaction mixture was allowed to equilibrate for 30 minutes and the resulting signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer. Additional binding assays were established between the human proteins PD-1-Ig/PD-L2-His (20 & 5 nM, respectively) and CD80-His/PD-L1-Ig (100 & 10 nM, respectively).
- Homogenous Time-Resolved Fluorescence (HTRF) binding assay The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and PD-L1 protein extracellular domains were expressed as fusion proteins with detection tags, for PD-1, the tag was the Fc portion of Immunoglobulin (PD-1-Ig) and for PD-L1 it was the 6 histidine motif (PD-L1-His). All binding studies were performed in an HTRF assay buffer consisting of dPBS supplemented with 0.1% (with) bovine serum albumin and 0.05% (v/v) Tween-20. For the h/PD-L1-His binding assay, inhibitors were pre-incubated with PD-L1-His (10 nM final) for 15 m in 4 μl of assay buffer, followed by addition of PD-1-Ig (20 nM final) in 1 μl of assay buffer and further incubation for 15 m. HTRF detection was achieved using europium crypate-labeled anti-Ig (1 nM final) and allophycocyanin (APC) labeled anti-His (20 nM final). Antibodies were diluted in HTRF detection buffer and 5 μl was dispensed on top of the binding reaction. The reaction mixture was allowed to equilibrate for 30 minutes and the resulting signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer. Additional binding assays were established between the human proteins PD-1-Ig/PD-L2-His (20 & 5 nM, respectively) and CD8O-His/PD-L1-Ig (100 & 10 nM, respectively).
- ChEMBL_2102732 (CHEMBL4811128) Inhibition of PD1/PD-L1 (unknown origin)
- Homogeneous time resolved fluorescence (HTRF) The identified compounds were tested for their capacity to inhibit the PD-1/PD-L1 interaction using in vitro functional assays. Homogeneous time resolved fluorescence (HTRF) was used to evaluate those 94 hits identified in silico. The BMS202 inhibitor, known as a small-molecule inhibitor of PD-1/PD-L1 interaction, was used as a reference. Compounds were tested at 100 μM and the determination of the inhibition levels was based on the HTRF signal reduction. Hits were defined as compounds that inhibited at least 50% of the PD-1/PD-L1 interaction.
- ChEMBL_2088599 (CHEMBL4769862) Inhibition of PD-1/PDL1 protein-protein interaction (unknown origin) by HTRF assay
- ChEMBL_2169504 (CHEMBL5054563) Inhibition of PD-1/PDL1 protein-protein interaction (unknown origin) by HTRF assay
- ChEMBL_2265319 Inhibition of human PD-1/PDL1 protein-protein interaction by luminescence proximity homogeneous assay
- ChEMBL_2249588 (CHEMBL5163798) Inhibition of biotinylated tagged PD-1 (unknown origin)/His tagged PD-L1 (unknown origin) protein-protein interaction assessed as inhibition constant incubated for 90 mins by AlphaLISA assay
- ChEMBL_2487935 Inhibition of Eu-tagged PD-1 (unknown origin)/C-terminal Fc fusion (human IgG1)/Avi-tagged biotinylated human PDL1 (19 to 239 residues) extracted from HEK293 cells interaction preincubated for 15 mins with PD-L1 followed by PD-1 addition and measured after 90 mins by TR-FRET assay
- ChEMBL_2051342 (CHEMBL4706041) Inhibition of PD-1/PDL1 (unknown origin) measured after 2 hrs by HTRF assay
- ChEMBL_2083844 (CHEMBL4739635) Antagonist activity at PD1/PD-L1 (unknown origin)
- ChEMBL_2378261 Inhibition of PD-L1 (unknown origin) by HTRF assay
- ChEMBL_2432487 Inhibition of PD-L1 (unknown origin) by HTRF assay
- ChEMBL_2360357 Inhibition of biotinylated human PD-I /His-tagged PD-L1 (unknown origin) interaction incubated for 90 mins in dark condition by AlphaLISA assay
- ChEMBL_2157805 (CHEMBL5042465) Inhibition of PD-1/PDL-1 (unknown origin) interaction incubated for 15 mins by TR-FRET assay
- ChEMBL_2455069 Inhibition of interaction of PD-1 (unknown origin) expressed in Jurkat T cells co-transfected with NFAT response element/PD-L1 (unknown origin) expressed in CHO-K1 cells by reporter bioassay
- Homogenous Time-Resolved Fluorescence (HTRF) Binding Assay The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and PD-L1 protein extracellular domains were expressed as fusion proteins with detection tags, for PD-1, the tag was the Fc portion of Immunoglobulin (PD-1-Ig) and for PD-L1 it was the 6 histidine motif (PD-L1-His). All binding studies were performed in an HTRF assay buffer consisting of dPBS supplemented with 0.1% (with) bovine serum albumin and 0.05% (v/v) Tween-20. For the h/PD-L1-His binding assay, inhibitors were pre-incubated with PD-L1-His (10 nM final) for 15m in 4 μl of assay buffer, followed by addition of PD-1-Ig (20 nM final) in 1 μ1 of assay buffer and further incubation for 15m. HTRF detection was achieved using europium crypate-labeled anti-Ig (1 nM final) and allophycocyanin (APC) labeled anti-His (20 nM final). Antibodies were diluted in HTRF detection buffer and 5 μl was dispensed on top of the binding reaction. The reaction mixture was allowed to equilibrate for 30 minutes and the resulting signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer. Additional binding assays were established between the human proteins PD-1-Ig/PD-L2-His (20 & 5 nM, respectively) and CD8O-His/PD-L1-Ig (100 & 10 nM, respectively). Recombinant Proteins: Human PD-1 (25-167) with a C-terminal human Fc domain of immunoglobulin G (Ig) epitope tag [hPD-1 (25-167)-3S-IG] and human PD-L1 (18-239) with a C-terminal His epitope tag [hPD-L1(18-239)-TVMV-His] were expressed in HEK293T cells and purified sequentially by ProteinA affinity chromatography and size exclusion chromatography. Human PD-L2-His and CD8O-His was obtained through commercial sources.
- Homogenous Time-Resolved Fluorescence (HTRF) Binding Assay The interaction of PD-1 and PD-L1 can be assessed using soluble, purified preparations of the extracellular domains of the two proteins. The PD-1 and PD-L1 protein extracellular domains were expressed as fusion proteins with detection tags, for PD-1, the tag was the Fc portion of Immunoglobulin (PD-1-Ig) and for PD-L1 it was the 6 histidine motif (PD-L1-His). All binding studies were performed in an HTRF assay buffer consisting of dPBS supplemented with 0.1% (with) bovine serum albumin and 0.05% (v/v) Tween-20. For the h/PD-L1-His binding assay, inhibitors were pre-incubated with PD-L1-His (10 nM final) for 15m in 4 μl of assay buffer, followed by addition of PD-1-Ig (20 nM final) in 1 μl of assay buffer and further incubation for 15 m. HTRF detection was achieved using europium crypate-labeled anti-Ig (1 nM final) and allophycocyanin (APC) labeled anti-His (20 nM final). Antibodies were diluted in HTRF detection buffer and 5 μl was dispensed on top of the binding reaction. The reaction mixture was allowed to equilibrate for 60 minutes and the resulting signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer. Additional binding assays were established between the human proteins PD-1-Ig/PD-L2-His (20 & 2 nM, respectively) and CD80-His/PD-L1-Ig (100 & 10 nM, respectively).Recombinant Proteins: Human PD-1 (25-167) with a C-terminal human Fc domain of immunoglobulin G (Ig) epitope tag [hPD-1 (25-167)-3S-IG] and human PD-L1 (18-239) with a C-terminal His epitope tag [hPD-L1(18-239)-TVMV-His] were expressed in HEK293T cells and purified sequentially by ProteinA affinity chromatography and size exclusion chromatography. Human PD-L2-His and CD80-His was obtained through commercial sources.
- ChEMBL_1667595 (CHEMBL4017391) Binding affinity to His-tagged PD-L1 (unknown origin) assessed as reduction in PD-L1/PD1-Ig interaction preincubated with PD-L1 for 15 mins followed by addition of PD1-Ig measured after 15 mins by HTRF assay
- ChEMBL_2184680 (CHEMBL5096762) Inhibition of PD-L1 (unknown origin) by HTRF assay
- ChEMBL_2109284 (CHEMBL4817959) Inhibition of Tag2-PD-1/Tag1-PDL-1 (unknown origin) interaction incubated for 15 mins by TR-FRET assay
- ChEMBL_2169506 (CHEMBL5054565) Inhibition of human PD-1/PDL1 protein-protein interaction measured after 30 mins by HTRF assay
- ChEMBL_2360367 Inhibition of human recombinant extracellular domain PD-L1 in human DU-145 cells incubated for 1 hr
- Inhibitory Activity Assay 1. In vitro activity evaluation: Cisbio PD-1/PD-L1 binding assay kit was applied for the detection method of in vitro enzymology level.Screening Principles and Methods of PD-1/PD-L1 Small Molecule Inhibitors1) Principle: PD-1 protein is with HIS tag, and PD-1 ligand PD-L1 is with hFc tag. Eu labeled anti-hFc antibody and XL665 labeled anti-HIS antibody are combined with the above two label proteins respectively. After laser excitation, energy can be transferred from donor Eu to receptor XL665, allowing XL665 to glow. After adding inhibitors (compounds or antibodies), blocking the binding of PD-1 and PD-L1 makes the distance between Eu and XL665 far away, the energy can not be transferred, and XL665 does not glow.2) Experimental method: The specific method can be referred to Cisbio's PD-1/PD-L1 Kit (item 64CUS000C-2). Reagents should be dispensed in the following order. For 384-well white ELISA plate, 2 μl of diluent or target compound diluted with diluent was added to each well, and then 4 μl of PD-1 protein and 4 μl of PD-L1 protein were added per well, incubated for 15 min at room temperature; and 10 μl of a mixture of anti-Tag1-Eu3+ and anti-Tag2-XL665 was added per well and incubated for 1 h to 4 h at room temperature and the fluorescence signals at 665 nm and 620 nm were measured with an Envison instrument. HTRF rate=(665 nm/620 nm)*104. 8-10 concentrations were detected for each compound and IC50 was calculated by Graphpad software.
- ChEMBL_2051372 (CHEMBL4706071) Binding affinity to human PD-L1 by surface plasmon resonance
- ChEMBL_2051373 (CHEMBL4706072) Binding affinity to mouse PD-L1 by surface plasmon resonance
- In Vitro Activity Assay In vitro activity evaluation: Cisbio PD-1/PD-L1 binding assay kit was applied for the detection method of in vitro enzymology level.Screening Principles and Methods of PD-1/PD-L1 Small Molecule Inhibitors1) Principle: PD-1 protein is with HIS tag, and PD-1 ligand PD-L1 is with hFc tag. Eu labeled anti-hFc antibody and XL665 labeled anti-HIS antibody are combined with the above two label proteins respectively. After laser excitation, energy can be transferred from donor Eu to receptor XL665, allowing XL665 to glow. After adding inhibitors (compounds or antibodies), blocking the binding of PD-1 and PD-L1 makes the distance between Eu and XL665 far away, the energy can not be transferred, and XL665 does not glow.2) Experimental method: The specific method can be referred to Cisbio's PD-1/PD-L1 Kit (item 64CUS000C-2). Reagents should be dispensed in the following order. For 384-well white ELISA plate, 2 μl of diluent or target compound diluted with diluent was added to each well, and then 4 μl of PD-1 protein and 4 μl of PD-L1 protein were added per well, incubated for 15 min at room temperature; and 10 μl of a mixture of anti-Tag1-Eu3+ and anti-Tag2-XL665 was added per well and incubated for 1 h to 4 h at room temperature and the fluorescence signals at 665 nm and 620 nm were measured with an Envison instrument. HTRF rate=(665 nm/620 nm)*104. 8-10 concentrations were detected for each compound and IC50 was calculated by Graphpad software.
- Homogenous Time-Resolved Fluorescence (HTRF) Assays Assays of Binding of Soluble PD-1 to Soluble PD-L1. Soluble PD-1 and soluble PD-L1 refers to proteins with carboxyl-end truncations that remove the transmembrane-spanning regions and are fused to heterologous sequences, specifically the Fc portion of the human immunoglobuling G sequence (Ig) or the hexahistidine epitope tag (His). All binding studies were performed in an HTRF assay buffer consisting of dPBS supplemented with 0.1% (w/v) bovine serum albumin and 0.05% (v/v) Tween-20. For the PD-1-Ig/PD-L1-His binding assay, inhibitors were pre-incubated with PD-L1-His (10 nM final) for 15m in 4 μl of assay buffer, followed by addition of PD-1-Ig (20 nM final) in 1 μl of assay buffer and further incubation for 15 m. PD-L1 fusion proteins from either human, cynomologous macaques, mouse, or other species were used. HTRF detection was achieved using europium crypate-labeled anti-Ig monoclonal antibody (1 nM final) and allophycocyanin (APC) labeled anti-His monoclonal antibody (20 nM final). Antibodies were diluted in HTRF detection buffer and 5 μl was dispensed on top of binding reaction. The reaction was allowed to equilibrate for 30 minutes and signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer. Additional binding assays were established between PD-1-Ig/PD-L2-His (20 and 5 nM, respectively), CD80-His/PD-L1-Ig (100 and 10 nM, respectively) and CD80-His/CTLA4-Ig (10 and 5 nM, respectively).
- ChEMBL_2489302 Induction of internalization of PD-L1 (unknown origin) expressed in CHO-K1 cells assessed as reduction in surface PD-L1 expression incubated for 16 hrs by flow cytometry
- ChEMBL_2480574 Inhibition of PD-1/PD-L1 interaction in human Jurkat T cells co-cultured with CHO/TCRAct/PDL1 cells assessed as restoration of T cell activation measured for 6 hrs by luminescence based immune checkpoint blockade assay
- ChEMBL_2051370 (CHEMBL4706069) Binding affinity to human PD-L1 by isothermal titration calorimetric analysis
- ChEMBL_2051371 (CHEMBL4706070) Binding affinity to mouse PD-L1 by isothermal titration calorimetric analysis
- ChEMBL_2358445 Inhibition of human PD-L1 incubated for 15 mins by ELISA method
- ChEMBL_2378271 Inhibition of human PD-L1 expressed in Escherichia coli BL21 (DE3) incubated for 1 hr by TR-FRET assay
- Homogenous Time-Resolved Fluorescence (HTRF) Binding Assays The ability of the macrocyclic peptides of the present disclosure to bind to PD-L1 was investigated using a PD-1/PD-L1 Homogenous Time-Resolved Fluorescence (HTRF) binding assay.MethodsHomogenous Time-Resolved Fluorescence (HTRF) Assays of Binding of Soluble PD-1 to Soluble PD-L1. Soluble PD-1 and soluble PD-L1 refers to proteins with carboxyl-end truncations that remove the transmembrane-spanning regions and are fused to heterologous sequences, specifically the Fc portion of the human immunoglobuling G sequence (Ig) or the hexahistidine epitope tag (His). All binding studies were performed in an HTRF assay buffer consisting of dPBS supplemented with 0.1% (w/v) bovine serum albumin and 0.05% (v/v) Tween-20. For the PD-1-Ig/PD-L1-His binding assay, inhibitors were pre-incubated with PD-L1-His (10 nM final) for 15m in 4 μl of assay buffer, followed by addition of PD-1-Ig (20 nM final) in 1 μl of assay buffer and further incubation for 15 m. PD-L1 fusion proteins from either human, cynomologous macaques, mouse, or other species were used. HTRF detection was achieved using europium crypate-labeled anti-Ig monoclonal antibody (1 nM final) and allophycocyanin (APC) labeled anti-His monoclonal antibody (20 nM final). Antibodies were diluted in HTRF detection buffer and 5 μl was dispensed on top of binding reaction. The reaction was allowed to equilibrate for 30 minutes and signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer. Additional binding assays were established between PD-1-Ig/PD-L2-His (20 and 5 nM, respectively), CD8O-His/PD-L1-Ig (100 and 10 nM, respectively) and CD80-His/CTLA4-Ig (10 and 5 nM, respectively). Binding/competition studies between biotinylated Compound No. 71 and human PD-L1-His were performed as follows. Macrocyclic peptide inhibitors were pre-incubated with PD-L1-His (10 nM final) for 60 minutes in 4 μl of assay buffer followed by addition of biotinylated Compound No. 71 (0.5 nM final) in 1 μl of assay buffer. Binding was allowed to equilibrate for 30 minutes followed by addition of europium crypated labeled Streptavidin (2.5 pM final) and APC-labeled anti-His (20 nM final) in 5 μl of HTRF buffer. The reaction was allowed to equilibrate for 30 m and signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer.
- ChEMBL_2184681 (CHEMBL5096763) Binding affinity to human recombinant PD-L1 by surface plasmon resonance assay
- ChEMBL_2273453 Inhibition of PD-L1 in mouse splenocytes assessed as increase in INFgamma release
- ChEMBL_2273454 Inhibition of PD-L1 in human PBMCs assessed as increase in INFgamma production
- ChEMBL_2273455 Inhibition of PD-L2 in human PBMCs assessed as increase in INFgamma production
- ChEMBL_2273457 Inhibition of PD-L2 in mouse splenocytes assessed as increase in INFgamma production
- ChEMBL_2307871 Binding affinity to human PD-L1 assessed as dissociation constant by MST analysis
- ChEMBL_2341288 Binding affinity to human PD-L1 assessed as dissociation constant by SPR analysis
- ChEMBL_2341289 Binding affinity to mouse PD-L1 assessed as dissociation constant by SPR analysis
- ChEMBL_2341290 Binding affinity to mouse PD-L1 assessed as dissociation constant by MST analysis
- ChEMBL_2432491 Inhibition of PD-L1 (unknown origin) incubated for 15 mins by HTRF assay
- ChEMBL_2017415 (CHEMBL4670993) Inhibition of hPD-1/hPD-L1 immune checkpoint using PD-L1 expressing human aAPCs and PD-1 expressing human Jurkat cells assessed as activation of effector Jurkat T cells incubated for 6 hrs by luciferase reporter gene based immune checkpoint blockade cell-based assay
- Homogeneous Time-Resolved Fluorescence (HTRF) PD1/PD-L1 binding assay kit (Cisbio, Cat #63ADK000CPDEC) was used, which includes two proteins, Tag 1-PD-L1 and Tag 2-PD-1, and two antibodies, Anti-Tag1-Eu3+ and Anti-Tag2-XL 665. Assay principle: Anti-tag1-Eu3+ is the HTRF donor, and Anti-Tag2-XL 665 is the HTRF acceptor. When Tag 1-PD-L1 and Tag 2-PD-1 interact, the added HTRF donor and the acceptor are close to each other. After the donor receives the excitation energy, it transfers part of the energy to the acceptor, thus producing 665 nm emission signal. When the compound is added to block the PD1/PD-L1 interaction, only 620 nm emission light was detected. The inhibitory effect of the compound was determined by analyzing the ratio of 665 nm/620 nm. Tag 1-PD-L1 was diluted with Diluent buffer (cat #62DLBDDF) to a working concentration of 10 nM, Tag 2-PD-1 was diluted with Diluent buffer to a working concentration of 500 nM, and Anti-Tag1-Eu3+ was diluted with detection buffer (cat #62DB1FDG) by 1:100, Anti-Tag2-XL 665 was diluted with detection buffer by 1:20, and the test compound was diluted with Diluent buffer to a final concentration of 2×. In a 384-well plate, 2 μL of compound was added to each well, and then 4 μL of Tag 1-PD-L1, 4 μL of Tag 2-PD-1 were added and incubated at room temperature for 15 minutes.
- ChEMBL_2368518 Inhibition of interaction of Fc(IgG1)-Avi)-(Biotin)-fused human PDL1 (19 to 239 residues)/Eu-labeled human PD-1 (25 to 167 residues) expressed in HEK293 cells pretreated with PDL1 for 15 mins followed by PD-1 addition and measured after 90 mins by TR-FRET assay
- ChEMBL_2330952 Inhibition of POLQ PD (unknown origin) (1819 to 2590 residues) by fluorescence based analysis
- ChEMBL_2357141 Binding affinity to PD-L1 (unknown origin) assessed as dissociation constant by SPR analysis
- ChEMBL_2432212 Binding affinity to recombinant human PD-L1 expressed in HEK293 cells by SPR analysis
- ChEMBL_2445173 Binding affinity to PD-L1 (unknown origin) assessed as dissociation constant by SPR analysis
- ChEMBL_2485932 Binding affinity to recombinant human PD-L1 expressed in HEK293 cells by MST assay
- ChEMBL_40191 (CHEMBL652238) Inhibition of binding of [125I]-PD 142251 to CCK-B receptor was determined
- ChEMBL_40192 (CHEMBL652239) Inhibition of binding of [125I]-PD 142308 to CCK-B receptor was determined
- ChEMBL_40217 (CHEMBL653063) Inhibition of binding of [125I]-PD 142308 to CCK-B receptor was determined
- ChEMBL_2294203 Inhibition of C-terminal his tagged human PD-1/human Fc tagged PDL-1 interaction expressed in HEK293 cells incubated for 2 hrs AlphaScreen assay
- PD-1/PD-L1 Homogenous Time-Resolved Fluorescence (HTRF) binding assay Homogenous Time-Resolved Fluorescence (HTRF) Assays of Binding of Soluble PD-1 to Soluble PD-L1. Soluble PD-1 and soluble PD-L1 refers to proteins with carboxyl-end truncations that remove the transmembrane-spanning regions and are fused to heterologous sequences, specifically the Fc portion of the human immunoglobuling G sequence (Ig) or the hexahistidine epitope tag (His). All binding studies were performed in an HTRF assay buffer consisting of dPBS supplemented with 0.1% (w/v) bovine serum albumin and 0.05% (v/v) Tween-20. For the PD-1-Ig/PD-L1-His binding assay, inhibitors were pre-incubated with PD-L1-His (10 nM final) for 15 m in 4 μl of assay buffer, followed by addition of PD-1-Ig (20 nM final) in 1 μl of assay buffer and further incubation for 15 m. PD-L1 fusion proteins from either human, cynomologous macaques, mouse, or other species were used. HTRF detection was achieved using europium crypate-labeled anti-Ig monoclonal antibody (1 nM final) and allophycocyanin (APC) labeled anti-His monoclonal antibody (20 nM final). Antibodies were diluted in HTRF detection buffer and 5 μl was dispensed on top of binding reaction. The reaction was allowed to equilibrate for 30 minutes and signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer. Additional binding assays were established between PD-1-Ig/PD-L2-His (20 and 5 nM, respectively), CD80-His/PD-L1-Ig (100 and 10 nM, respectively) and CD80-His/CTLA4-Ig (10 and 5 nM, respectively). Binding/competition studies between biotinylated Compound No. 71 and human PD-L1-His were performed as follows. Macrocyclic peptide inhibitors were pre-incubated with PD-L1-His (10 nM final) for 60 minutes in 4 μl of assay buffer followed by addition of biotinylated Compound No. 71 (0.5 nM final) in 1 μl of assay buffer. Binding was allowed to equilibrate for 30 minutes followed by addition of europium crypated labeled Streptavidin (2.5 pM final) and APC-labeled anti-His (20 nM final) in 5 μl of HTRF buffer. The reaction was allowed to equilibrate for 30 m and signal (665 nm/620 nm ratio) was obtained using an EnVision fluorometer.
- ChEMBL_2356356 Binding affinity to recombinant human PD-L1 (1 to 238 residues) expressed in HEK293 cells assessed as dissociation constant by SPR analysis
- ChEMBL_2378260 Inhibition of human PD-L1 (18 to 134 residues) expressed in Escherichia coli BL21 (DE3) incubated for 1 hr by spectrophometric analysis
 BDBM50227275 PD-117558
BDBM50227275 PD-117558 BDBM50366632 PD-170292
BDBM50366632 PD-170292 BDBM50230684 CHEMBL353157 PD-135118
BDBM50230684 CHEMBL353157 PD-135118 BDBM50565138 CHEMBL595227 PD-404182
BDBM50565138 CHEMBL595227 PD-404182 CHEMBL2062146 BDBM50451028 PD-149164
CHEMBL2062146 BDBM50451028 PD-149164 CHEMBL2062154 BDBM50449787 PD-134308
CHEMBL2062154 BDBM50449787 PD-134308 CHEMBL535139 PD-123177 BDBM50370405
CHEMBL535139 PD-123177 BDBM50370405 PD-0184264 BDBM50595935 CHEMBL481949
PD-0184264 BDBM50595935 CHEMBL481949 PD-128907 BDBM50369174 CHEMBL94015
PD-128907 BDBM50369174 CHEMBL94015 PD-150606 CHEMBL366254 BDBM50466172
PD-150606 CHEMBL366254 BDBM50466172 (S)-3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one BDBM50070514 PD-18126 CHEMBL37170 PD-172938 3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one (PD-18126)
(S)-3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one BDBM50070514 PD-18126 CHEMBL37170 PD-172938 3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one (PD-18126) 1-(2,6-Diisopropyl-phenyl)-3-(1-phenyl-cyclopentylmethyl)-urea BDBM50038526 PD-129337 CHEMBL296055
1-(2,6-Diisopropyl-phenyl)-3-(1-phenyl-cyclopentylmethyl)-urea BDBM50038526 PD-129337 CHEMBL296055 BDBM50164088 [Pd(CN)4]2- CHEMBL362447
BDBM50164088 [Pd(CN)4]2- CHEMBL362447 BDBM689140 US12049474, Compound NP-PD-042
BDBM689140 US12049474, Compound NP-PD-042 BDBM689141 US12049474, Compound NP-PD-051
BDBM689141 US12049474, Compound NP-PD-051 BDBM689142 US12049474, Compound NP-PD-102
BDBM689142 US12049474, Compound NP-PD-102 BDBM689145 US12049474, Compound NP-PD-149
BDBM689145 US12049474, Compound NP-PD-149 BDBM689147 US12049474, Compound NP-PD-130
BDBM689147 US12049474, Compound NP-PD-130 PD-137342 CHEMBL2062144 CI-988 BDBM50422043
PD-137342 CHEMBL2062144 CI-988 BDBM50422043 US12049474, Compound NP-PD-105 BDBM689143
US12049474, Compound NP-PD-105 BDBM689143 US12049474, Compound NP-PD-158 BDBM689146
US12049474, Compound NP-PD-158 BDBM689146 BDBM50080296 PD-32577 2-(1-azepanylmethyl)-4-{1-[3-(1-azepanylmethyl)-4-hydroxyphenyl]-1-methylethyl}phenol CHEMBL421719
BDBM50080296 PD-32577 2-(1-azepanylmethyl)-4-{1-[3-(1-azepanylmethyl)-4-hydroxyphenyl]-1-methylethyl}phenol CHEMBL421719 6,11-dihydrothiochromeno[4,3-b]indole PD-146176 BDBM50208823 CHEMBL180917
6,11-dihydrothiochromeno[4,3-b]indole PD-146176 BDBM50208823 CHEMBL180917 CHEMBL157422 BDBM50281610 PD-135478 1-(4-Phenyl-cyclohex-3-enyl)-4-pyridin-2-yl-piperazine
CHEMBL157422 BDBM50281610 PD-135478 1-(4-Phenyl-cyclohex-3-enyl)-4-pyridin-2-yl-piperazine N-Cyclohexyl-N-methyl-2-[2-(2-pyrrolidin-1-yl-ethoxy)-naphthalen-1-yl]-acetamide BDBM50281660 CHEMBL353654 PD-148282
N-Cyclohexyl-N-methyl-2-[2-(2-pyrrolidin-1-yl-ethoxy)-naphthalen-1-yl]-acetamide BDBM50281660 CHEMBL353654 PD-148282 BDBM50092154 3-[2-(2-Adamantan-2-yl-acetylamino)-3-(1H-indol-3-yl)-2-methyl-propionylamino]-4-phenyl-butyric acid(PD 140458) PD-140458 CHEMBL420320
BDBM50092154 3-[2-(2-Adamantan-2-yl-acetylamino)-3-(1H-indol-3-yl)-2-methyl-propionylamino]-4-phenyl-butyric acid(PD 140458) PD-140458 CHEMBL420320 BDBM50281605 1-Pyridin-2-yl-4-(4-thiophen-2-yl-cyclohex-3-enyl)-piperazine PD-135188 CHEMBL157087
BDBM50281605 1-Pyridin-2-yl-4-(4-thiophen-2-yl-cyclohex-3-enyl)-piperazine PD-135188 CHEMBL157087 BDBM50281607 PD-135222 1-Pyridin-2-yl-4-(4-pyridin-2-yl-cyclohex-3-enyl)-piperazine CHEMBL422187
BDBM50281607 PD-135222 1-Pyridin-2-yl-4-(4-pyridin-2-yl-cyclohex-3-enyl)-piperazine CHEMBL422187 BDBM50281608 CHEMBL349994 1-Pyridin-2-yl-4-(4-thiophen-2-yl-cyclohex-3-enylmethyl)-piperazine PD-135146
BDBM50281608 CHEMBL349994 1-Pyridin-2-yl-4-(4-thiophen-2-yl-cyclohex-3-enylmethyl)-piperazine PD-135146 CHEMBL353264 PD-146884 2-Oxa-7,10-diaza-bicyclo[11.3.1]heptadeca-1(17),13,15-trien-11-one BDBM50281661
CHEMBL353264 PD-146884 2-Oxa-7,10-diaza-bicyclo[11.3.1]heptadeca-1(17),13,15-trien-11-one BDBM50281661 PD-157667 BDBM50071611 CHEMBL78017 6-Azepan-1-ylmethyl-2-(4,4-diphenyl-butyl)-1,2,3,4-tetrahydro-isoquinolin-5-ol
PD-157667 BDBM50071611 CHEMBL78017 6-Azepan-1-ylmethyl-2-(4,4-diphenyl-butyl)-1,2,3,4-tetrahydro-isoquinolin-5-ol CHEMBL35093 (R)-3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one BDBM50070518 PD-172939
CHEMBL35093 (R)-3-{2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-ethyl}-2,3-dihydro-isoindol-1-one BDBM50070518 PD-172939 PD-110843 AD-810 Zonegran BDBM50028010 CI-912 CHEBI:10127 Zonisamide
PD-110843 AD-810 Zonegran BDBM50028010 CI-912 CHEBI:10127 Zonisamide 5-(8-Hydroxy-7-piperidin-1-ylmethyl-isoquinolin-5-ylmethyl)-7-piperidin-1-ylmethyl-quinolin-8-ol BDBM50071612 PD-29361 CHEMBL312381
5-(8-Hydroxy-7-piperidin-1-ylmethyl-isoquinolin-5-ylmethyl)-7-piperidin-1-ylmethyl-quinolin-8-ol BDBM50071612 PD-29361 CHEMBL312381 BDBM50055724 CHEMBL418937 1-[2-((S)-4-Phenyl-cyclohex-3-enyl)-ethyl]-4-pyridin-2-yl-piperazine PD-137789
BDBM50055724 CHEMBL418937 1-[2-((S)-4-Phenyl-cyclohex-3-enyl)-ethyl]-4-pyridin-2-yl-piperazine PD-137789 BDBM50281606 1-[2-((R)-4-Phenyl-cyclohex-3-enyl)-ethyl]-4-pyridin-2-yl-piperazine PD-137821 CHEMBL348547
BDBM50281606 1-[2-((R)-4-Phenyl-cyclohex-3-enyl)-ethyl]-4-pyridin-2-yl-piperazine PD-137821 CHEMBL348547 BDBM82039 8-{4-[N-(3-Dimethylaminopropyl)-sulfonamido]phenyl}-1,3-Dipropylxanthine (PD 113,297)
BDBM82039 8-{4-[N-(3-Dimethylaminopropyl)-sulfonamido]phenyl}-1,3-Dipropylxanthine (PD 113,297) BDBM50281609 CHEMBL346075 1-Pyridin-2-yl-4-[2-(4-pyridin-2-yl-cyclohex-3-enyl)-ethyl]-piperazine PD-135540
BDBM50281609 CHEMBL346075 1-Pyridin-2-yl-4-[2-(4-pyridin-2-yl-cyclohex-3-enyl)-ethyl]-piperazine PD-135540 CHEMBL346940 PD-135111 BDBM50281611 1-Pyridin-2-yl-4-[2-(4-thiophen-2-yl-cyclohex-3-enyl)-ethyl]-piperazine
CHEMBL346940 PD-135111 BDBM50281611 1-Pyridin-2-yl-4-[2-(4-thiophen-2-yl-cyclohex-3-enyl)-ethyl]-piperazine PD-160946 [(R)-2-(2-Fluoro-phenyl)-1-methyl-1-(7-ureido-heptylcarbamoyl)-ethyl]-carbamic acid (S)-2-methyl-1-phenyl-propyl ester BDBM50050652 CHEMBL47146
PD-160946 [(R)-2-(2-Fluoro-phenyl)-1-methyl-1-(7-ureido-heptylcarbamoyl)-ethyl]-carbamic acid (S)-2-methyl-1-phenyl-propyl ester BDBM50050652 CHEMBL47146 PD-161182 [(R)-2-(2,3-Difluoro-phenyl)-1-methyl-1-(7-ureido-heptylcarbamoyl)-ethyl]-carbamic acid (S)-2-methyl-1-phenyl-propyl ester BDBM50050641 CHEMBL45340
PD-161182 [(R)-2-(2,3-Difluoro-phenyl)-1-methyl-1-(7-ureido-heptylcarbamoyl)-ethyl]-carbamic acid (S)-2-methyl-1-phenyl-propyl ester BDBM50050641 CHEMBL45340 6-(4-Amino-benzenesulfonyl)-5-nitro-quinolin-8-ylamine BDBM50287526 CHEMBL53016 PD-9262
6-(4-Amino-benzenesulfonyl)-5-nitro-quinolin-8-ylamine BDBM50287526 CHEMBL53016 PD-9262 PD-140195 4-Phenyl-5-tridecyl-4H-[1,2,4]triazole-3-thiol CHEMBL61135 BDBM50287670
PD-140195 4-Phenyl-5-tridecyl-4H-[1,2,4]triazole-3-thiol CHEMBL61135 BDBM50287670 PD-160170 CHEMBL422942 6-(2-Isopropyl-benzenesulfonyl)-5-nitro-quinolin-8-ylamine BDBM50287527
PD-160170 CHEMBL422942 6-(2-Isopropyl-benzenesulfonyl)-5-nitro-quinolin-8-ylamine BDBM50287527 CHEMBL105442 US8575391, 341 CI-1040 BDBM50132260 PD-18435 PD-184352 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-benzamide 2-(2-chloro-4-iodophenylamino)-N-(cyclopropylmethoxy)-3,4-difluorobenzamide
CHEMBL105442 US8575391, 341 CI-1040 BDBM50132260 PD-18435 PD-184352 2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmethoxy-3,4-difluoro-benzamide 2-(2-chloro-4-iodophenylamino)-N-(cyclopropylmethoxy)-3,4-difluorobenzamide 1-Aza-bicyclo[2.2.1]heptan-3-one O-[3-(3-methoxy-phenyl)-prop-2-ynyl]-oxime BDBM50065223 PD-151832 CHEMBL81878
1-Aza-bicyclo[2.2.1]heptan-3-one O-[3-(3-methoxy-phenyl)-prop-2-ynyl]-oxime BDBM50065223 PD-151832 CHEMBL81878 CHEMBL78406 Thiophene-2-carboxylic acid {4-[2-(4-pyridin-2-yl-piperazin-1-yl)-ethyl]-cyclohexyl}-amide PD-137557 BDBM50290229
CHEMBL78406 Thiophene-2-carboxylic acid {4-[2-(4-pyridin-2-yl-piperazin-1-yl)-ethyl]-cyclohexyl}-amide PD-137557 BDBM50290229 CHEMBL170603 BDBM50281662 PD-146795 2-Benzofuran-4-yl-N-(tetrahydro-pyrrolizin-7a-ylmethyl)-acetamide
CHEMBL170603 BDBM50281662 PD-146795 2-Benzofuran-4-yl-N-(tetrahydro-pyrrolizin-7a-ylmethyl)-acetamide CHEMBL455856 BDBM50266362 PD-068235 (Z)-N-(3-allyl-5-nitrothiazol-2(3H)-ylidene)pivalamide
CHEMBL455856 BDBM50266362 PD-068235 (Z)-N-(3-allyl-5-nitrothiazol-2(3H)-ylidene)pivalamide CHEMBL45244 BDBM50058225 N-((4-(2-cyanophenyl)piperazin-1-yl)methyl)-3-methylbenzamide PD-168077 N-[4-(2-Cyano-phenyl)-piperazin-1-ylmethyl]-3-methyl-benzamide
CHEMBL45244 BDBM50058225 N-((4-(2-cyanophenyl)piperazin-1-yl)methyl)-3-methylbenzamide PD-168077 N-[4-(2-Cyano-phenyl)-piperazin-1-ylmethyl]-3-methyl-benzamide PD-123319 BDBM50282396 CHEMBL157946 (S)-1-(4-Dimethylamino-3-methyl-benzyl)-5-diphenylacetyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid
PD-123319 BDBM50282396 CHEMBL157946 (S)-1-(4-Dimethylamino-3-methyl-benzyl)-5-diphenylacetyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid CHEMBL282833 N-[3-(2,6-Dimethyl-piperidin-1-yl)-propyl]-2,2-diphenyl-acetamide(PD85639) BDBM50043993 PD-85639 N-[3-(2,6-Dimethyl-piperidin-1-yl)-propyl]-2,2-diphenyl-acetamide
CHEMBL282833 N-[3-(2,6-Dimethyl-piperidin-1-yl)-propyl]-2,2-diphenyl-acetamide(PD85639) BDBM50043993 PD-85639 N-[3-(2,6-Dimethyl-piperidin-1-yl)-propyl]-2,2-diphenyl-acetamide