 BDBM50045333 SB-203580 CHEBI:90705
BDBM50045333 SB-203580 CHEBI:90705 BDBM85859 SB-215505 SB 215505
BDBM85859 SB-215505 SB 215505 SB 234551 SB-234551 BDBM85335
SB 234551 SB-234551 BDBM85335 SB-791016A CHEMBL251572 BDBM50412954 SB-791016
SB-791016A CHEMBL251572 BDBM50412954 SB-791016 BDBM50417257 SB-649868
BDBM50417257 SB-649868 BDBM50419052 SB-399885
BDBM50419052 SB-399885 BDBM50448074 Emindole Sb
BDBM50448074 Emindole Sb SB-258585 BDBM86428
SB-258585 BDBM86428 BDBM50026798 SB-742548 CHEMBL1956072
BDBM50026798 SB-742548 CHEMBL1956072 BDBM50371155 CHEMBL245237 SB-611113
BDBM50371155 CHEMBL245237 SB-611113 BDBM50372467 CHEMBL271102 SB-429201
BDBM50372467 CHEMBL271102 SB-429201 BDBM50400870 CHEMBL559569 SB-612111
BDBM50400870 CHEMBL559569 SB-612111 BDBM50403164 CHEMBL156977 SB-205149
BDBM50403164 CHEMBL156977 SB-205149 BDBM50411414 SB-347804 CHEMBL397570
BDBM50411414 SB-347804 CHEMBL397570 BDBM50412120 SB-714786 CHEMBL425190
BDBM50412120 SB-714786 CHEMBL425190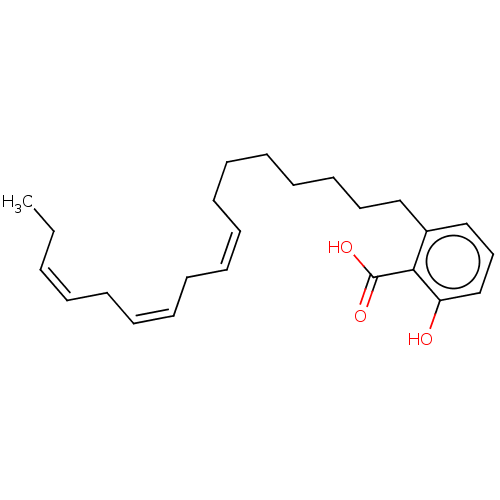 BDBM50469657 SB-202742 CHEMBL33995
BDBM50469657 SB-202742 CHEMBL33995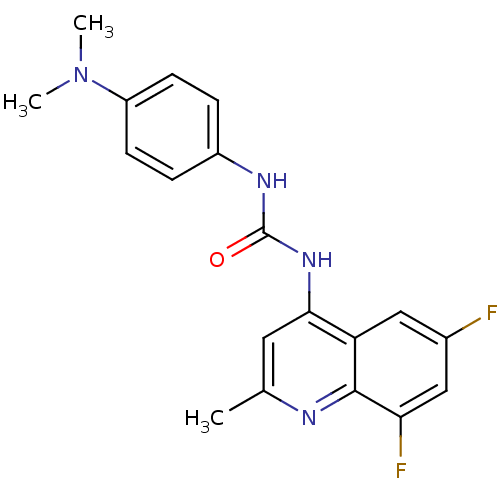 CHEMBL1334465 BDBM50423648 SB-408124
CHEMBL1334465 BDBM50423648 SB-408124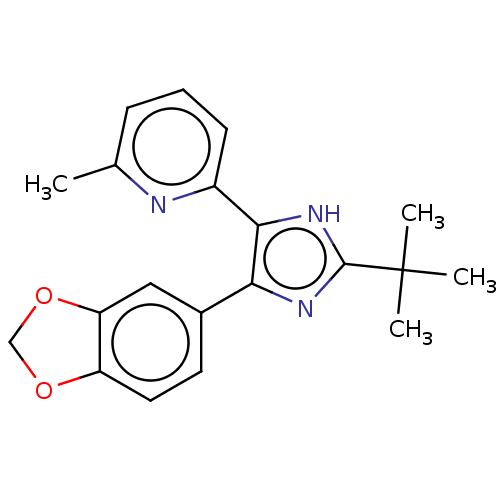 CHEMBL1824446 SB-505124 BDBM50608551
CHEMBL1824446 SB-505124 BDBM50608551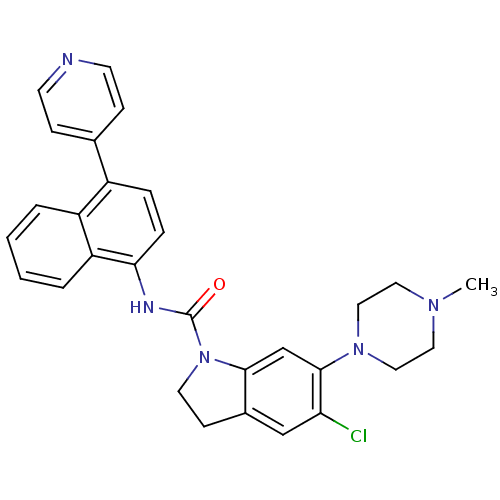 CHEMBL191971 BDBM50410435 SB-272183
CHEMBL191971 BDBM50410435 SB-272183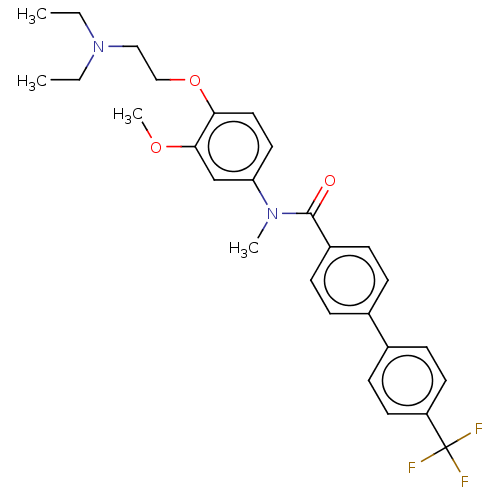 CHEMBL2110360 BDBM50475902 SB-568849
CHEMBL2110360 BDBM50475902 SB-568849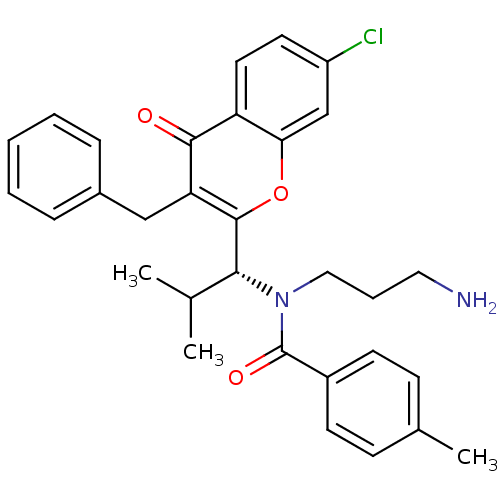 CHEMBL2325429 SB-743921 BDBM50427294
CHEMBL2325429 SB-743921 BDBM50427294 CHEMBL233002 SB-814597 BDBM50411419
CHEMBL233002 SB-814597 BDBM50411419 CHEMBL244083 SB-414796 BDBM50411370
CHEMBL244083 SB-414796 BDBM50411370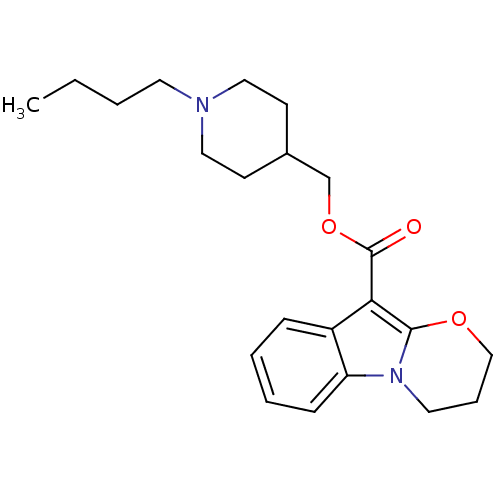 CHEMBL358119 SB-207058 BDBM50421360
CHEMBL358119 SB-207058 BDBM50421360 CHEMBL406800 SB-379278A BDBM50372468
CHEMBL406800 SB-379278A BDBM50372468 CHEMBL565091 BDBM50480755 SB-02066
CHEMBL565091 BDBM50480755 SB-02066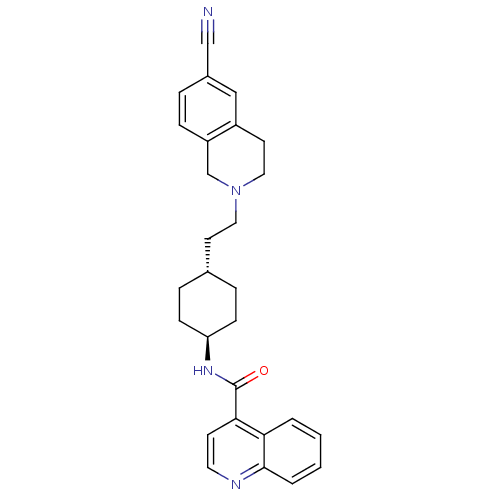 CHEMBL85606 BDBM50370572 SB-277011
CHEMBL85606 BDBM50370572 SB-277011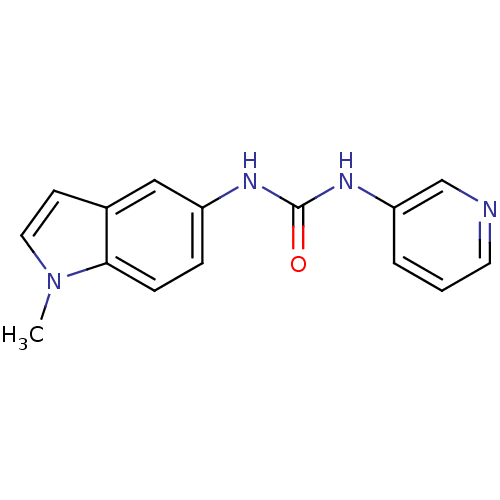 SB 200646 BDBM82272 SB200646a
SB 200646 BDBM82272 SB200646a SB-0304 BDBM50376593 CHEMBL409225
SB-0304 BDBM50376593 CHEMBL409225 SB-203238 CHEMBL506279 BDBM50473786
SB-203238 CHEMBL506279 BDBM50473786 SB-207043 BDBM50366325 CHEMBL606910
SB-207043 BDBM50366325 CHEMBL606910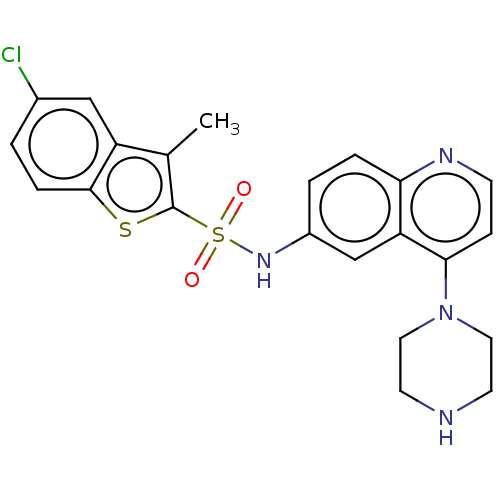 SB-331711 CHEMBL97158 BDBM50001825
SB-331711 CHEMBL97158 BDBM50001825 SB-410220 BDBM50423650 CHEMBL522758
SB-410220 BDBM50423650 CHEMBL522758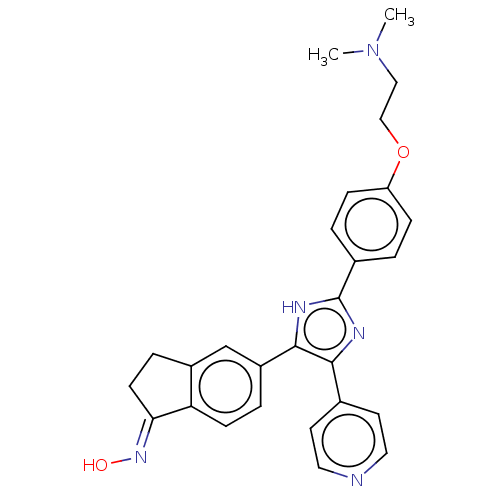 SB-590885 BDBM50457452 SB590885
SB-590885 BDBM50457452 SB590885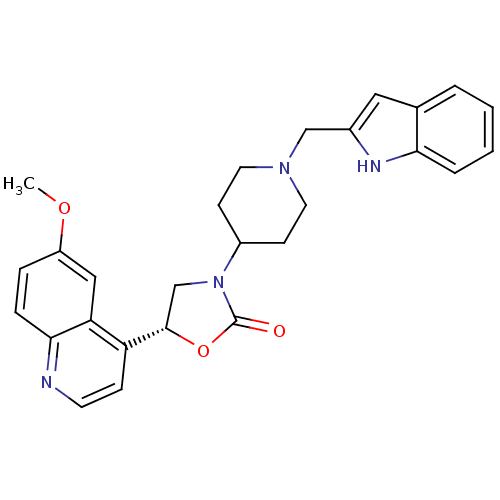 SB-649701 BDBM50423246 CHEMBL245568
SB-649701 BDBM50423246 CHEMBL245568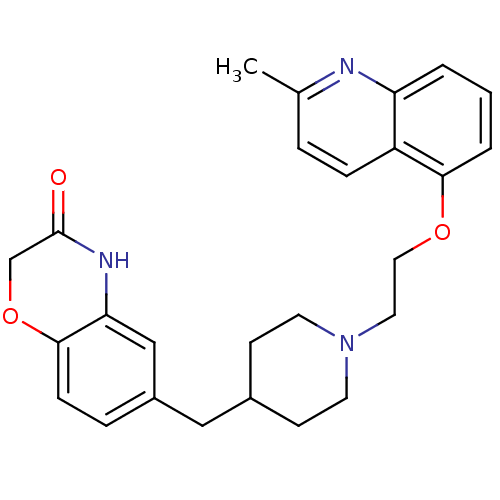 SB-649915 BDBM50412114 CHEMBL183460
SB-649915 BDBM50412114 CHEMBL183460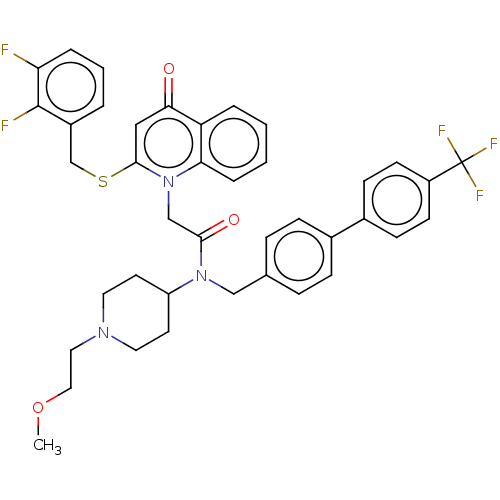 SB-659032 Rilapladib BDBM50205805
SB-659032 Rilapladib BDBM50205805 SB-699551 CHEMBL1181770 BDBM50585908
SB-699551 CHEMBL1181770 BDBM50585908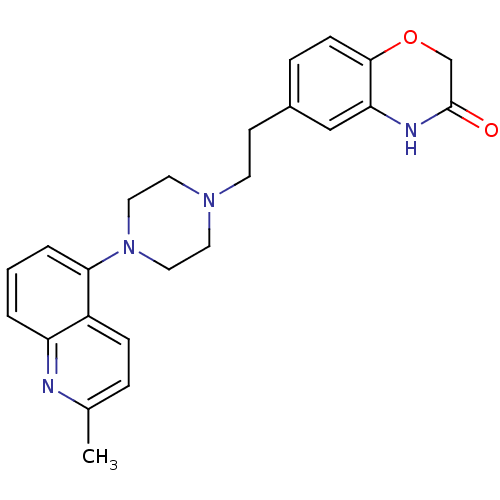 SB-744185 BDBM50412441 CHEMBL490417
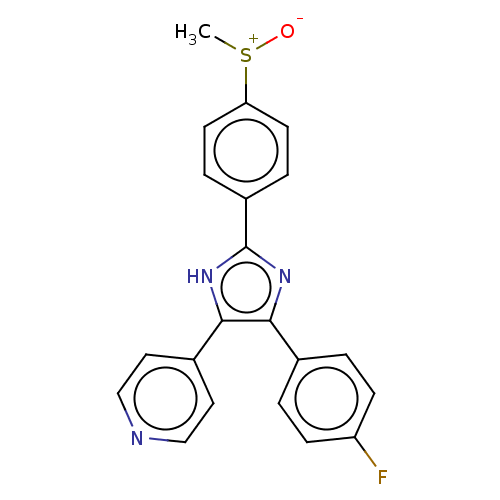
SB-744185 BDBM50412441 CHEMBL490417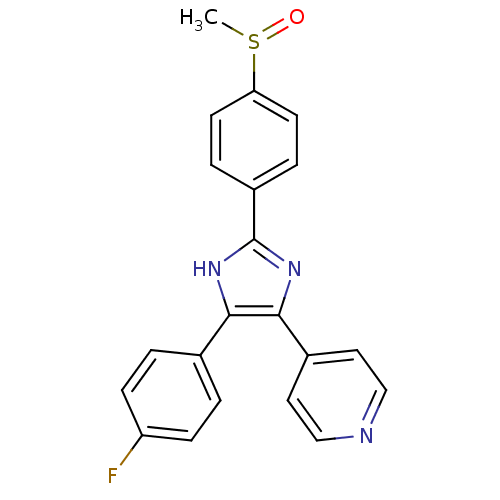 4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine SB-203580 4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine BDBM13336 cid_176155 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine CHEMBL10 SB203580
4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine SB-203580 4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine BDBM13336 cid_176155 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine CHEMBL10 SB203580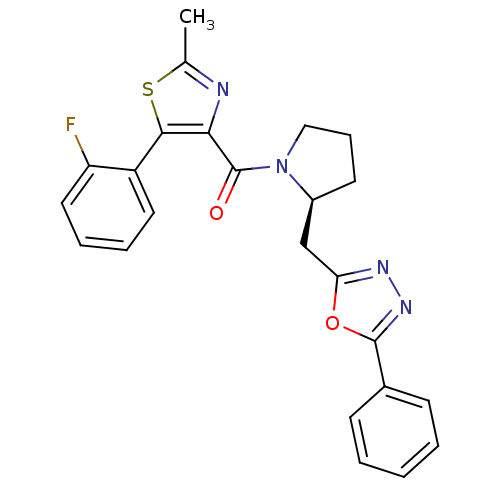 BDBM50423649 CHEMBL2413521 SB-674042 CHEMBL2110363
BDBM50423649 CHEMBL2413521 SB-674042 CHEMBL2110363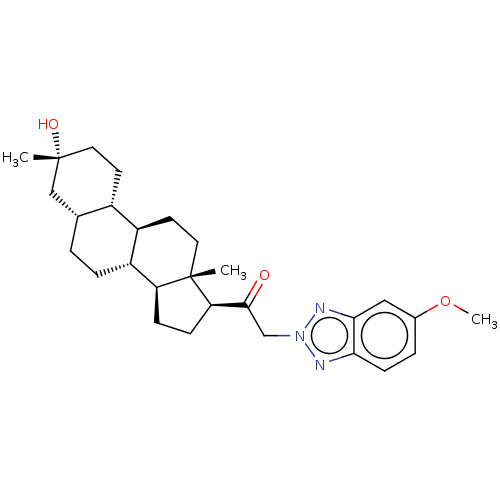 BDBM540760 US11261211, Compound SB-49
BDBM540760 US11261211, Compound SB-49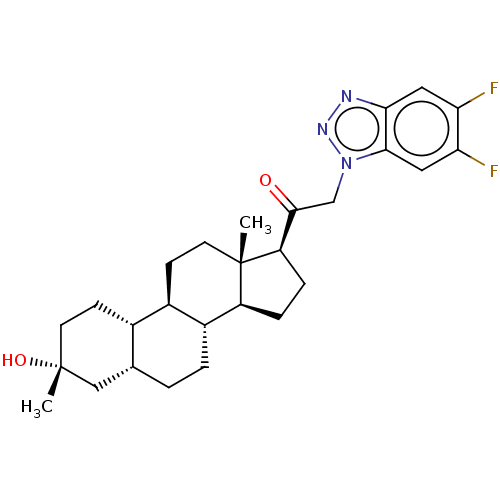 BDBM540761 US11261211, Compound SB-50
BDBM540761 US11261211, Compound SB-50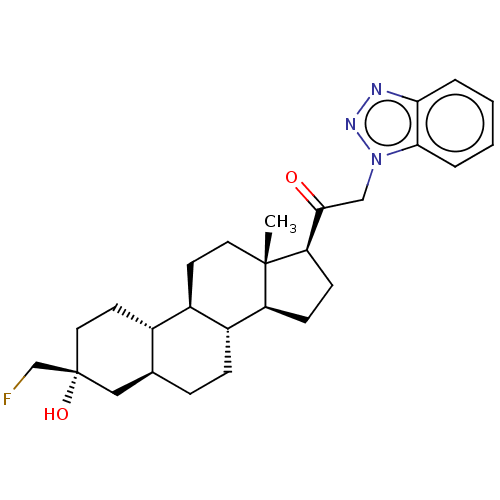 BDBM540770 US11261211, Compound SB-12
BDBM540770 US11261211, Compound SB-12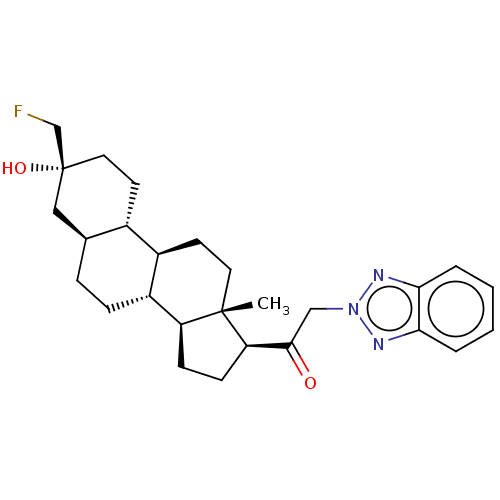 BDBM540771 US11261211, Compound SB-13
BDBM540771 US11261211, Compound SB-13 BDBM540772 US11261211, Compound SB-14
BDBM540772 US11261211, Compound SB-14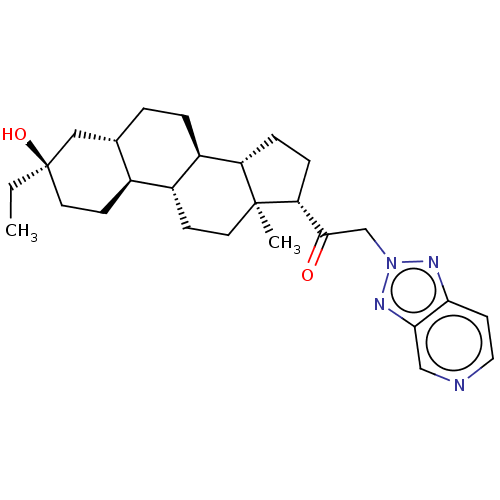 BDBM540773 US11261211, Compound SB-15
BDBM540773 US11261211, Compound SB-15 BDBM540790 US11261211, Compound SB-41
BDBM540790 US11261211, Compound SB-41 BDBM540802 US11261211, Compound SB-40
BDBM540802 US11261211, Compound SB-40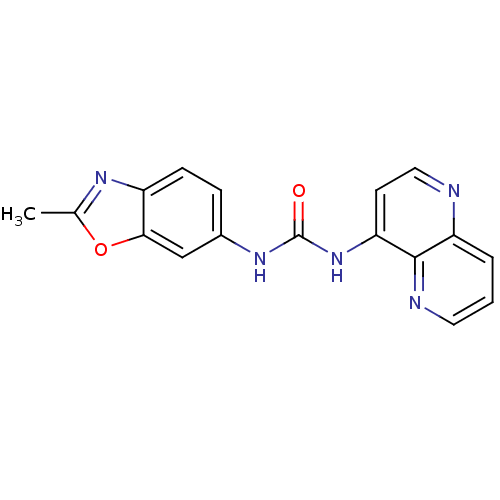 CHEMBL291536 CHEMBL2111553 SB-334867 BDBM50384416
CHEMBL291536 CHEMBL2111553 SB-334867 BDBM50384416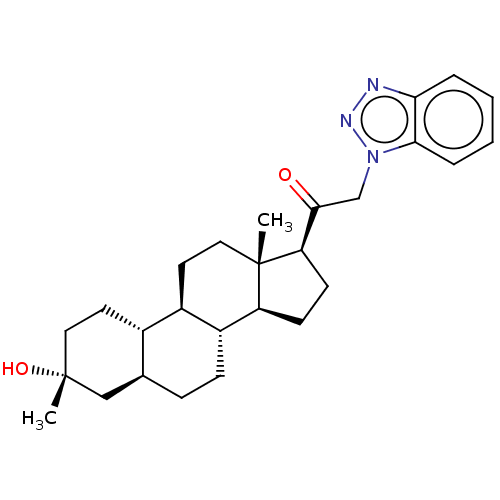 US11261211, Compound SB-1 BDBM540768
US11261211, Compound SB-1 BDBM540768 US11261211, Compound SB-17 BDBM540775
US11261211, Compound SB-17 BDBM540775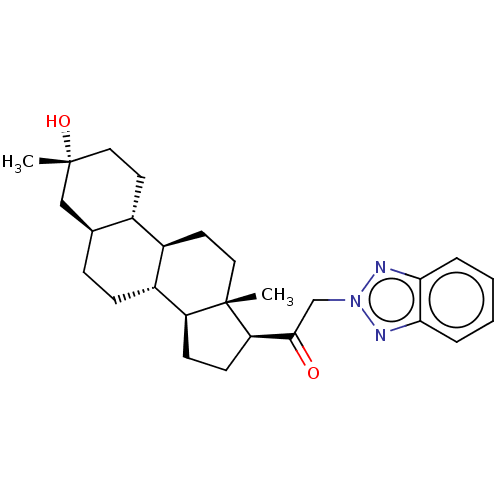 US11261211, Compound SB-2 BDBM540769
US11261211, Compound SB-2 BDBM540769 US11261211, Compound SB-26 BDBM540786
US11261211, Compound SB-26 BDBM540786 US11261211, Compound SB-35 BDBM540780
US11261211, Compound SB-35 BDBM540780 US11261211, Compound SB-37 BDBM540797
US11261211, Compound SB-37 BDBM540797 US11261211, Compound SB-38 BDBM540798
US11261211, Compound SB-38 BDBM540798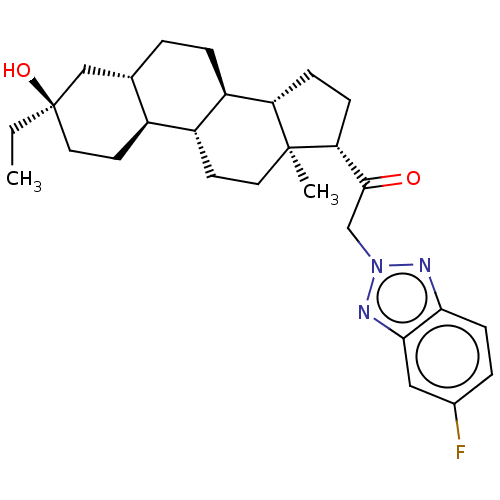 US11261211, Compound SB-39 BDBM540791
US11261211, Compound SB-39 BDBM540791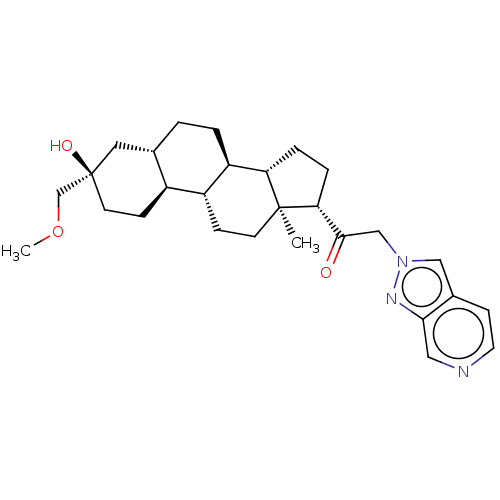 US11261211, Compound SB-45 BDBM540776
US11261211, Compound SB-45 BDBM540776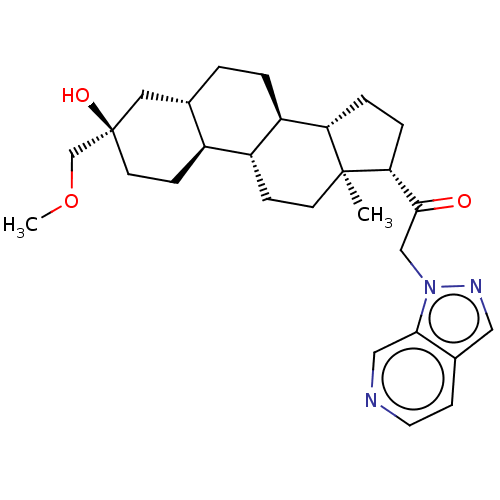 US11261211, Compound SB-46 BDBM540777
US11261211, Compound SB-46 BDBM540777 US11261211, Compound SB-53 BDBM540778
US11261211, Compound SB-53 BDBM540778 US11261211, Compound SB-56 BDBM540779
US11261211, Compound SB-56 BDBM540779 US11261211, Compound SB-60 BDBM540796
US11261211, Compound SB-60 BDBM540796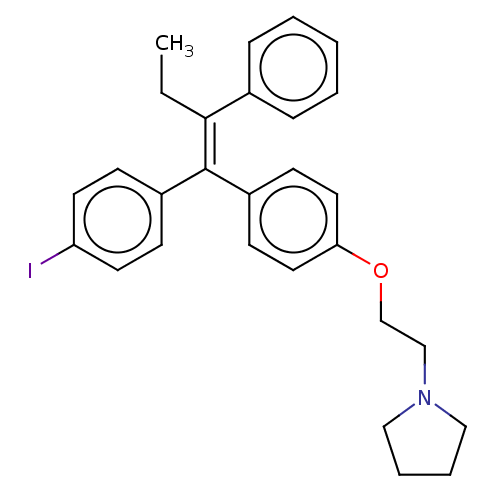 Idoxifene SB-223030 BDBM50219403 CB-7432
Idoxifene SB-223030 BDBM50219403 CB-7432 SB-615575 TCMDC-142445 CHEMBL2098048 BDBM50557135
SB-615575 TCMDC-142445 CHEMBL2098048 BDBM50557135 SB-207266 BDBM85026 N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethyleneoxy)-1H-indole-3-carboxamide SB 207266
SB-207266 BDBM85026 N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethyleneoxy)-1H-indole-3-carboxamide SB 207266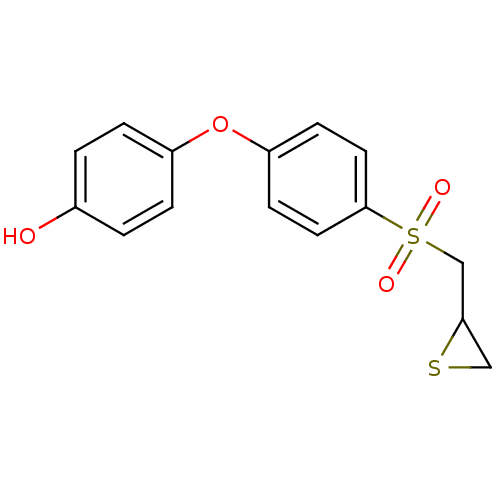 BDBM50388914 US10357546, p-OH SB-3CT CHEMBL2063274
BDBM50388914 US10357546, p-OH SB-3CT CHEMBL2063274 BDBM706133 SB-FAP-11 US20240382629, Example 22
BDBM706133 SB-FAP-11 US20240382629, Example 22 BDBM706134 SB-FAP-09 US20240382629, Example 23
BDBM706134 SB-FAP-09 US20240382629, Example 23 BDBM706135 SB-FAP-01 US20240382629, Example i
BDBM706135 SB-FAP-01 US20240382629, Example i BDBM706136 SB-FAP-02 US20240382629, Example ii
BDBM706136 SB-FAP-02 US20240382629, Example ii BDBM706138 US20240382629, Example iv SB-FAP-06
BDBM706138 US20240382629, Example iv SB-FAP-06 BDBM706139 SB-FAP-07 US20240382629, Example v
BDBM706139 SB-FAP-07 US20240382629, Example v BDBM706143 US20240382629, Example ix SB-FAP-08
BDBM706143 US20240382629, Example ix SB-FAP-08 CAS_181632-25-7 BDBM85097 SB 242084 CHEMBL14563
CAS_181632-25-7 BDBM85097 SB 242084 CHEMBL14563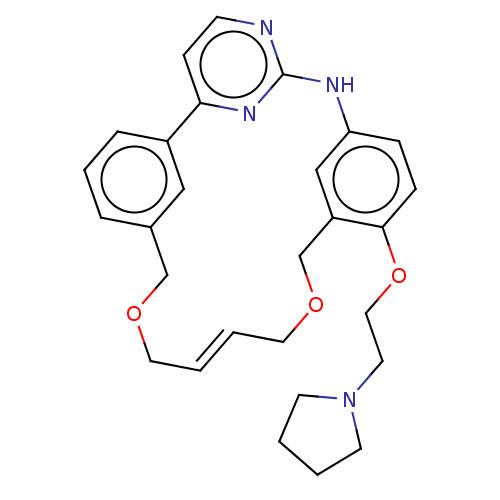 ONX-0803 SB1518 Pacritinib BDBM50210177 SB-1518
ONX-0803 SB1518 Pacritinib BDBM50210177 SB-1518 SB-FAP-04 US20240382629, Example viii BDBM706142
SB-FAP-04 US20240382629, Example viii BDBM706142 SB-FAP-10 US20240382629, Example vi BDBM706140
SB-FAP-10 US20240382629, Example vi BDBM706140 US20240382629, Example iii BDBM706137 SB-FAP-03
US20240382629, Example iii BDBM706137 SB-FAP-03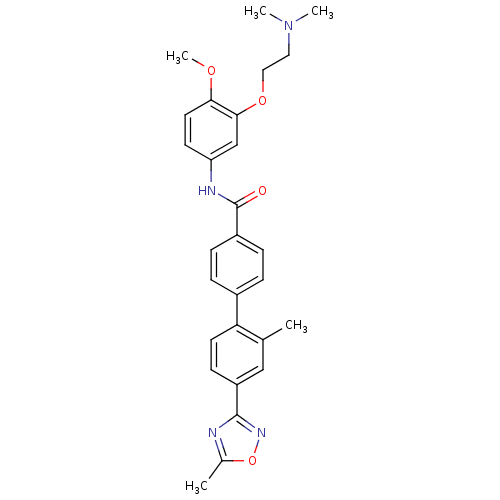 BDBM85166 US20240166639, Example SB41 CAS_3292447 SB 216641 NSC_3292447
BDBM85166 US20240166639, Example SB41 CAS_3292447 SB 216641 NSC_3292447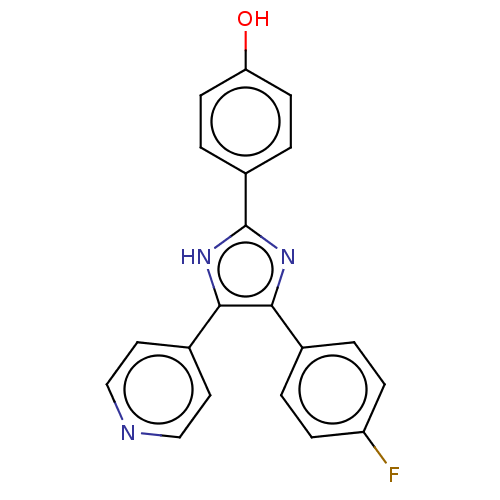 US10865384, Compound SB202190 CHEBI:79090 SB-202190 BDBM50044784
US10865384, Compound SB202190 CHEBI:79090 SB-202190 BDBM50044784 5-Methyl-3,5-dihydro-2H-pyrrolo[2,3-f]indole-1-carboxylic acid pyridin-3-ylamide SB 206553 BDBM50060417 CHEMBL297784 SB-206553
5-Methyl-3,5-dihydro-2H-pyrrolo[2,3-f]indole-1-carboxylic acid pyridin-3-ylamide SB 206553 BDBM50060417 CHEMBL297784 SB-206553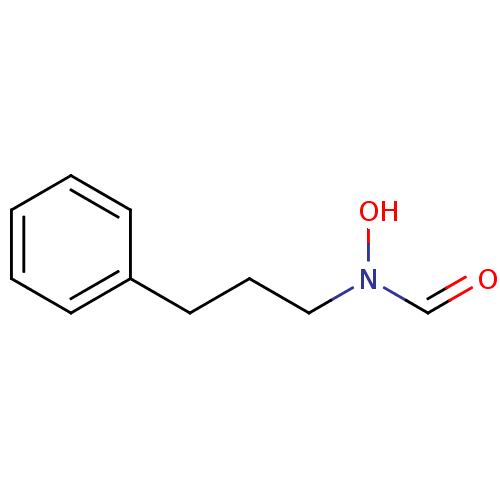 N-hydroxy-N-(3-phenylpropyl)formamide BDBM21683 SB-485345
N-hydroxy-N-(3-phenylpropyl)formamide BDBM21683 SB-485345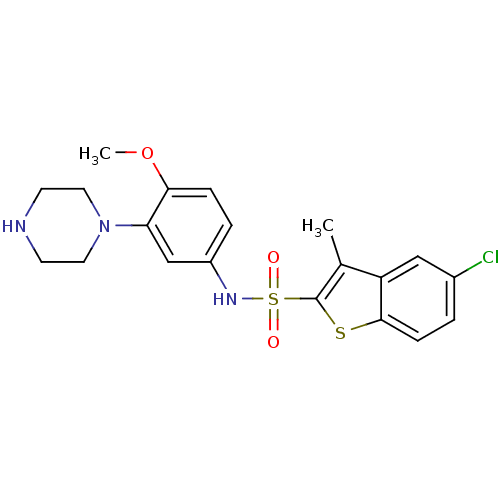 BDBM28583 SB 271046 SB-271046 5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-methyl-1-benzothiophene-2-sulfonamide hydrochloride CHEMBL431298
BDBM28583 SB 271046 SB-271046 5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-methyl-1-benzothiophene-2-sulfonamide hydrochloride CHEMBL431298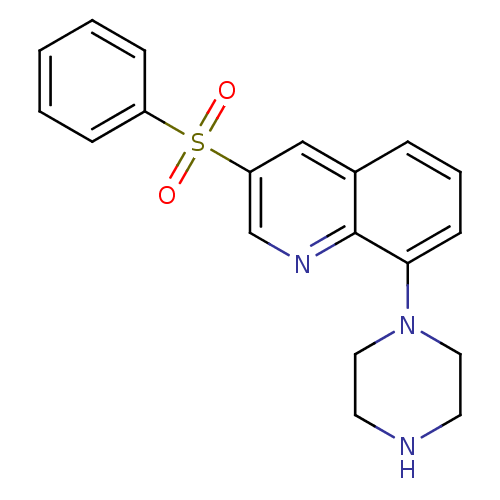 3-benzenesulfonyl-8-piperazin-1-ylquinoline CHEMBL1083390 BDBM50318633 SB-742457
3-benzenesulfonyl-8-piperazin-1-ylquinoline CHEMBL1083390 BDBM50318633 SB-742457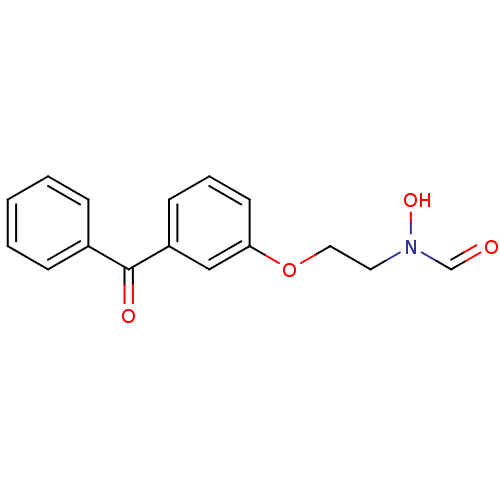 N-[2-(3-benzoylphenoxy)ethyl]-N-hydroxyformamide SB-543668 BDBM21684
N-[2-(3-benzoylphenoxy)ethyl]-N-hydroxyformamide SB-543668 BDBM21684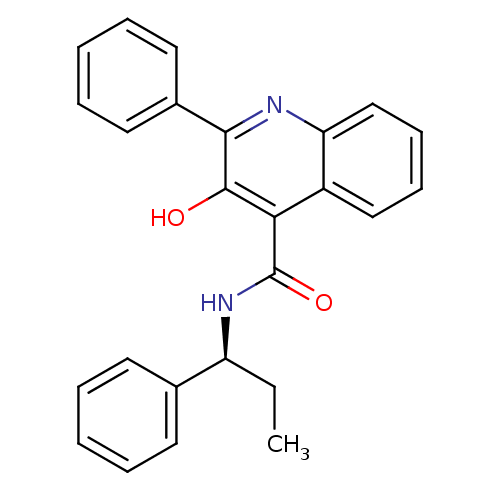 SB-223412 3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide CHEMBL10188 Talnetant SB-2234 BDBM50051293 SB 223412 (S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinoline-4-carboxamide 3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid (1-phenyl-propyl)-amide
SB-223412 3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide CHEMBL10188 Talnetant SB-2234 BDBM50051293 SB 223412 (S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinoline-4-carboxamide 3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid (1-phenyl-propyl)-amide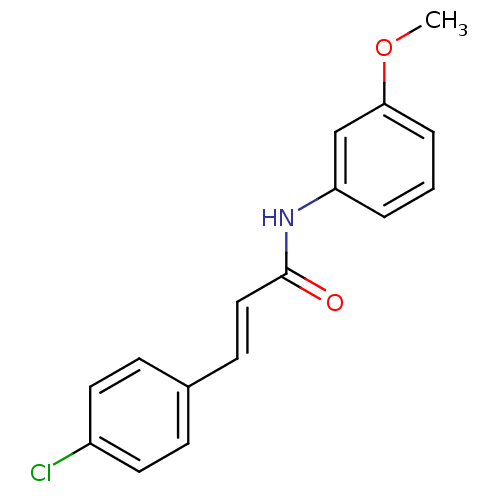 (2E)-3-(4-chlorophenyl)-N-(3-methoxyphenyl)prop-2-enamide BDBM20488 SB-366791
(2E)-3-(4-chlorophenyl)-N-(3-methoxyphenyl)prop-2-enamide BDBM20488 SB-366791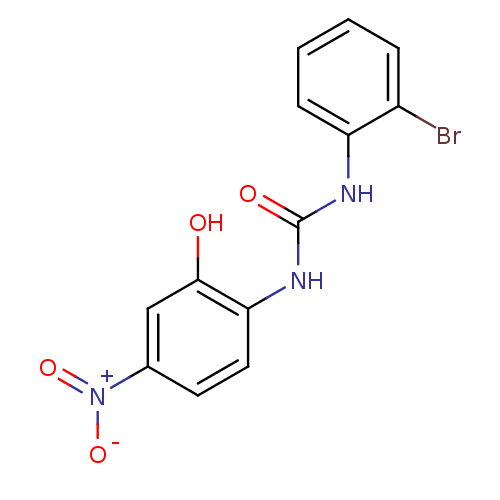 1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea CHEMBL239767 SB-225002 BDBM50203012
1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea CHEMBL239767 SB-225002 BDBM50203012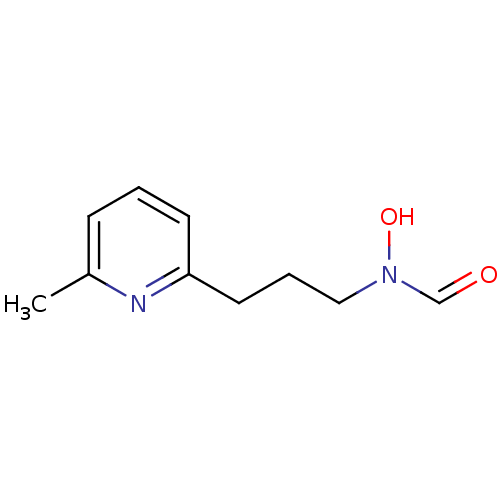 SB-505684 BDBM21685 N-hydroxy-N-[3-(6-methylpyridin-2-yl)propyl]formamide
SB-505684 BDBM21685 N-hydroxy-N-[3-(6-methylpyridin-2-yl)propyl]formamide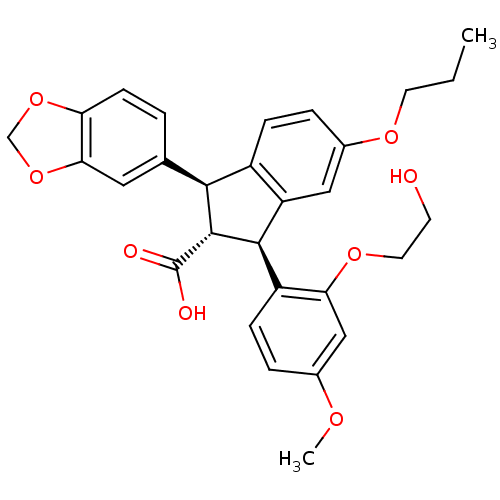 (1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy-ethoxy)-4-methoxy-phenyl]-5-propoxy-indan-2-carboxylic acid SB-217242 Enrasentan SB 217242 CHEMBL431651 BDBM50061077
(1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy-ethoxy)-4-methoxy-phenyl]-5-propoxy-indan-2-carboxylic acid SB-217242 Enrasentan SB 217242 CHEMBL431651 BDBM50061077 1-(1-methylindol-5-yl)-3-pyridin-3-ylurea hydrochloride BDBM84990 SB 200646 SB200646
1-(1-methylindol-5-yl)-3-pyridin-3-ylurea hydrochloride BDBM84990 SB 200646 SB200646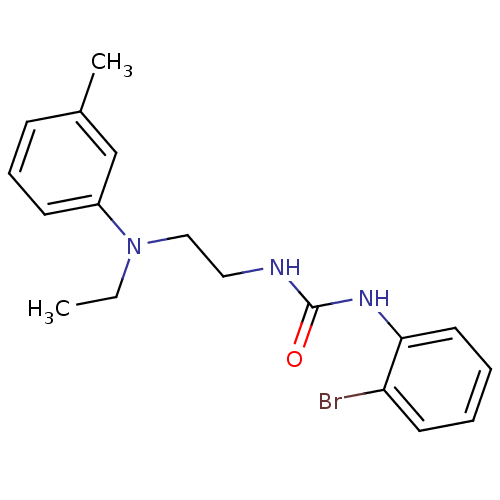 3-(2-bromophenyl)-1-{2-[ethyl(3-methylphenyl)amino]ethyl}urea SB-452533 BDBM20468
3-(2-bromophenyl)-1-{2-[ethyl(3-methylphenyl)amino]ethyl}urea SB-452533 BDBM20468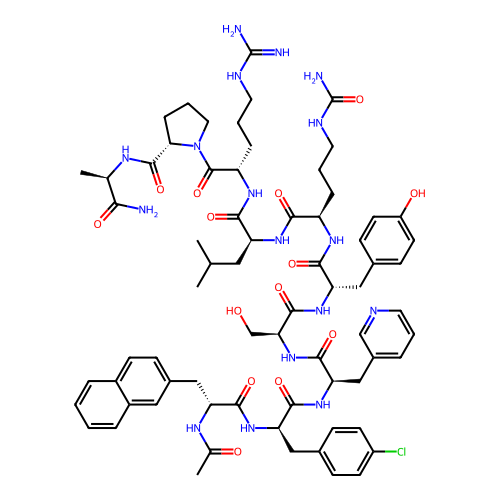 BDBM50248241 Cetrorelix Acetate D-20761 CHEBI:31387 Cetrotide NS-75A SB-075 Acetate Cetrorelix
BDBM50248241 Cetrorelix Acetate D-20761 CHEBI:31387 Cetrotide NS-75A SB-075 Acetate Cetrorelix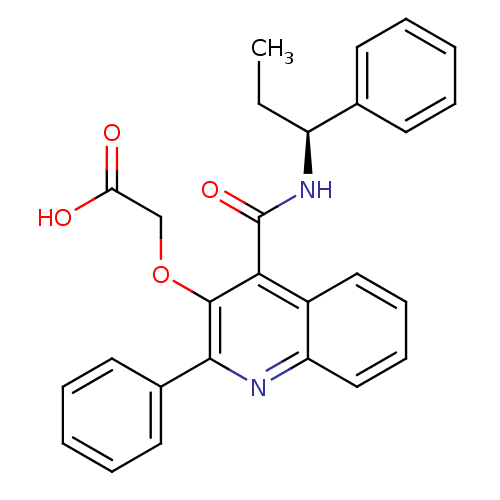 SB 235375 N-[(S)-1-Phenylpropyl]-2-phenyl-3-(carboxymethoxy)quinoline-4-carboxamide BDBM85845
SB 235375 N-[(S)-1-Phenylpropyl]-2-phenyl-3-(carboxymethoxy)quinoline-4-carboxamide BDBM85845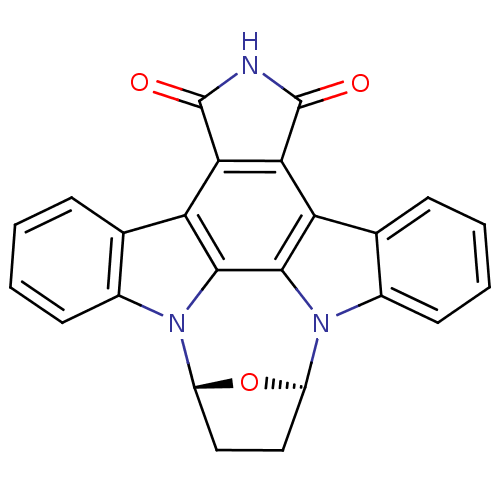 (15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1,6,8(13),9,11,20(25),21,23,26-nonaene-3,5-dione SB-218078 SB 218078 BDBM17140 SB218078
(15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1,6,8(13),9,11,20(25),21,23,26-nonaene-3,5-dione SB-218078 SB 218078 BDBM17140 SB218078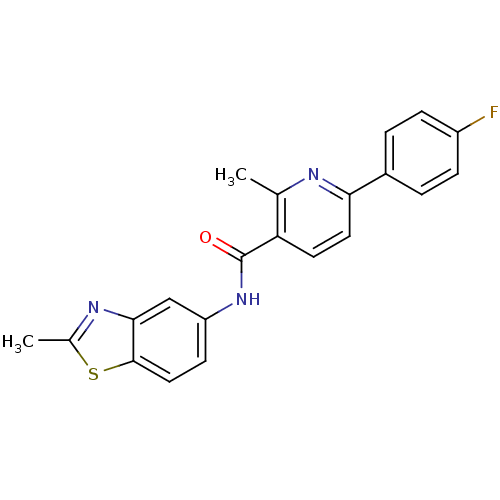 BDBM50263384 CHEMBL514691 6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazol-5-yl)nicotinamide SB-782443
BDBM50263384 CHEMBL514691 6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazol-5-yl)nicotinamide SB-782443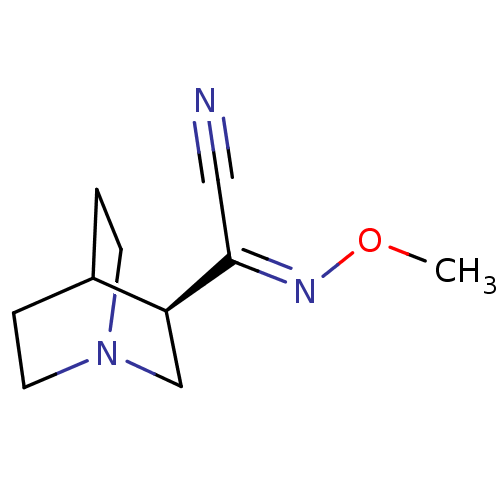 CHEMBL134641 SB-202026 BDBM50061705 (R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino]-acetonitrile
CHEMBL134641 SB-202026 BDBM50061705 (R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino]-acetonitrile N-{3-[2-(3,4-Dimethoxy-phenyl)-ethylamino]-propyl}-4-nitro-benzamide CHEMBL184461 SB-237376 BDBM50151854
N-{3-[2-(3,4-Dimethoxy-phenyl)-ethylamino]-propyl}-4-nitro-benzamide CHEMBL184461 SB-237376 BDBM50151854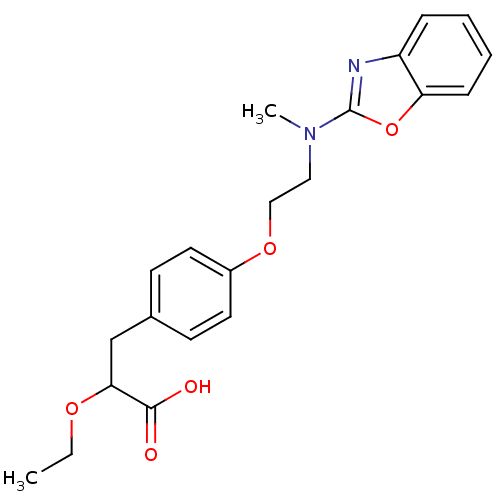 3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid 3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid(SB-213068) BDBM50085043 CHEMBL306229 SB-213068
3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid 3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid(SB-213068) BDBM50085043 CHEMBL306229 SB-213068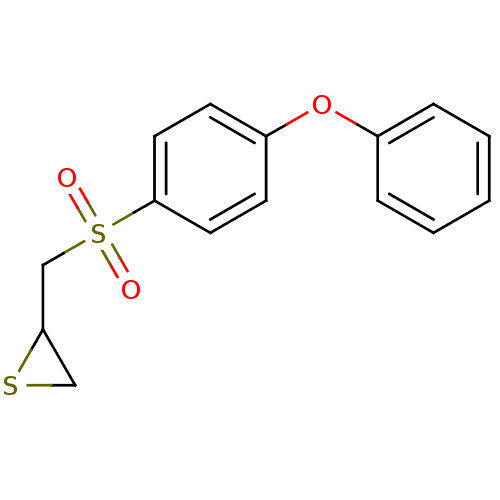 2-((4-phenoxyphenylsulfonyl)methyl)thiirane CHEMBL483857 BDBM50264809 Thiirane (deuterium), 1-d2 Thiirane, 1 US10357546, SB-3CT
2-((4-phenoxyphenylsulfonyl)methyl)thiirane CHEMBL483857 BDBM50264809 Thiirane (deuterium), 1-d2 Thiirane, 1 US10357546, SB-3CT NSC-760125 Rolipram ZK 62 711 ZK-62711 (r,s)-rolipram BDBM50639239 ZK-62771 SB-95952
NSC-760125 Rolipram ZK 62 711 ZK-62711 (r,s)-rolipram BDBM50639239 ZK-62771 SB-95952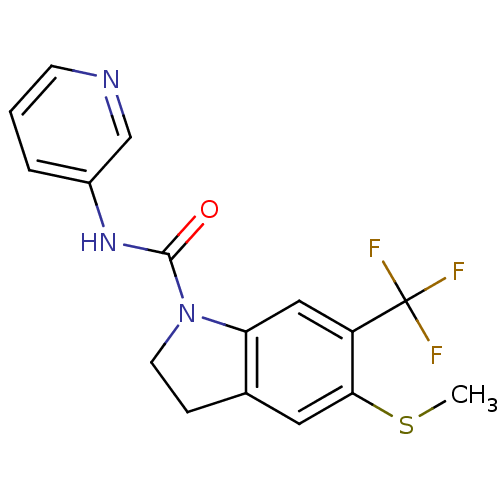 5-Methylsulfanyl-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid pyridin-3-ylamide SB 221284 CHEMBL276140 BDBM50060418
5-Methylsulfanyl-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid pyridin-3-ylamide SB 221284 CHEMBL276140 BDBM50060418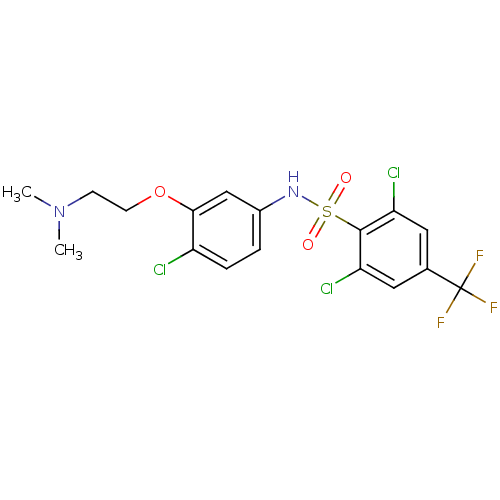 CHEMBL1164033 BDBM50320477 SB-611812 2,6-dichloro-N-(4-chloro-3-(2-(dimethylamino)ethoxy)phenyl)-4-(trifluoromethyl)benzenesulfonamide
CHEMBL1164033 BDBM50320477 SB-611812 2,6-dichloro-N-(4-chloro-3-(2-(dimethylamino)ethoxy)phenyl)-4-(trifluoromethyl)benzenesulfonamide CHEMBL292759 SB-214111 BDBM50073056 4-Bromo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulfonamide
CHEMBL292759 SB-214111 BDBM50073056 4-Bromo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulfonamide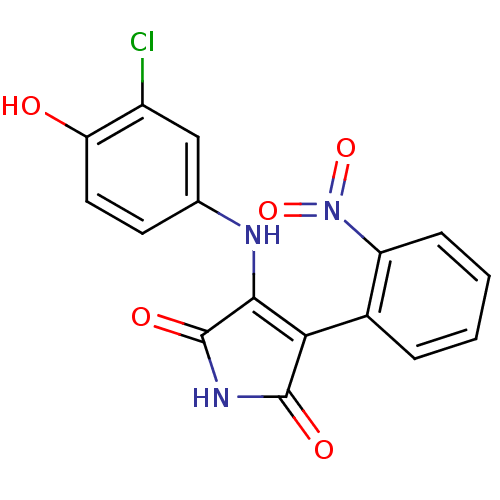 3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-2,5-dihydro-1H-pyrrole-2,5-dione BDBM8297 SB-415286
3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-2,5-dihydro-1H-pyrrole-2,5-dione BDBM8297 SB-415286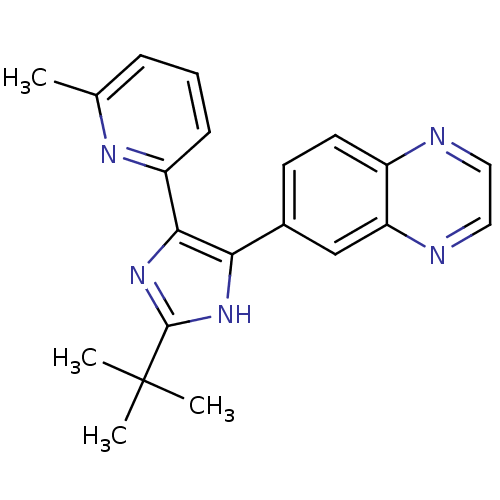 CHEMBL401570 6-(2-tert-butyl-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl)quinoxaline BDBM50320952 SB-525334
CHEMBL401570 6-(2-tert-butyl-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl)quinoxaline BDBM50320952 SB-525334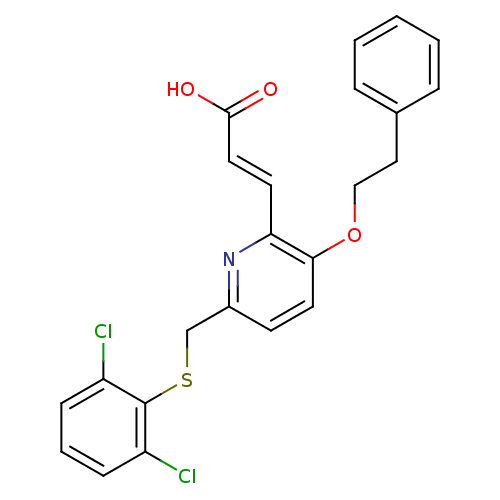 CHEMBL90214 Ticolubant BDBM50052027 (E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phenethyloxy-pyridin-2-yl]-acrylic acid SB-209247
CHEMBL90214 Ticolubant BDBM50052027 (E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phenethyloxy-pyridin-2-yl]-acrylic acid SB-209247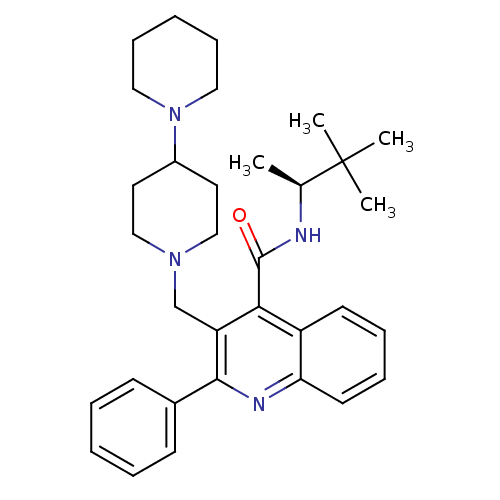 3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoline-4-carboxylic acid (1,2,2-trimethyl-propyl)-amide SB-414240 BDBM50099640 CHEMBL295770
3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoline-4-carboxylic acid (1,2,2-trimethyl-propyl)-amide SB-414240 BDBM50099640 CHEMBL295770 4-{3-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-imidazol-1-yl]-propyl}-morpholine SB-210313 BDBM50053424 CHEMBL14112
4-{3-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-imidazol-1-yl]-propyl}-morpholine SB-210313 BDBM50053424 CHEMBL14112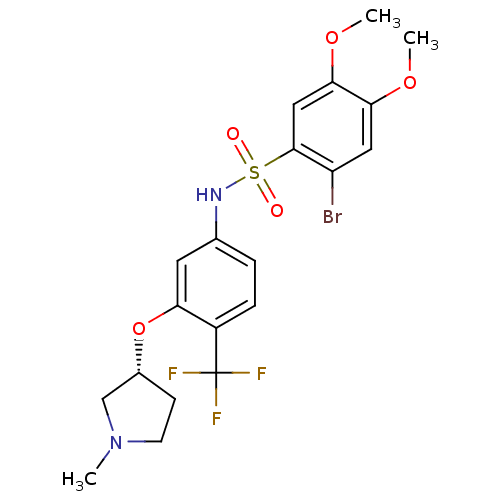 CHEMBL522770 BDBM50249878 (R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin-3-yloxy)-4-(trifluoromethyl)phenyl)benzenesulfonamide SB-706375
CHEMBL522770 BDBM50249878 (R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin-3-yloxy)-4-(trifluoromethyl)phenyl)benzenesulfonamide SB-706375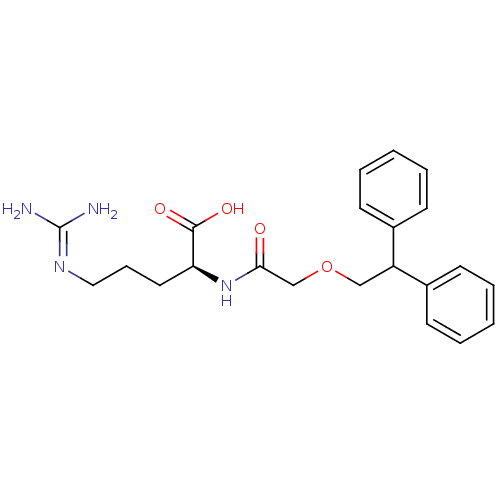 N2-[(2,2-diphenylethoxy)acetyl]-L-arginine (S)-2-(2-(2,2-diphenylethoxy)acetamido)-5-guanidinopentanoic acid CHEMBL389348 SB-290157 BDBM50322650
N2-[(2,2-diphenylethoxy)acetyl]-L-arginine (S)-2-(2-(2,2-diphenylethoxy)acetamido)-5-guanidinopentanoic acid CHEMBL389348 SB-290157 BDBM50322650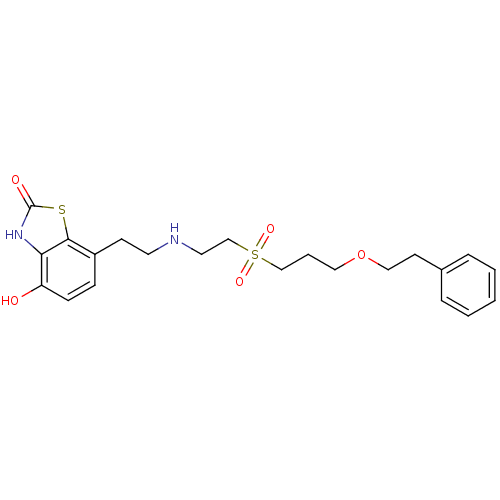 CHEMBL82663 4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfonyl)-ethylamino]-ethyl}-3H-benzothiazol-2-one SB-07499 BDBM50128690
CHEMBL82663 4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfonyl)-ethylamino]-ethyl}-3H-benzothiazol-2-one SB-07499 BDBM50128690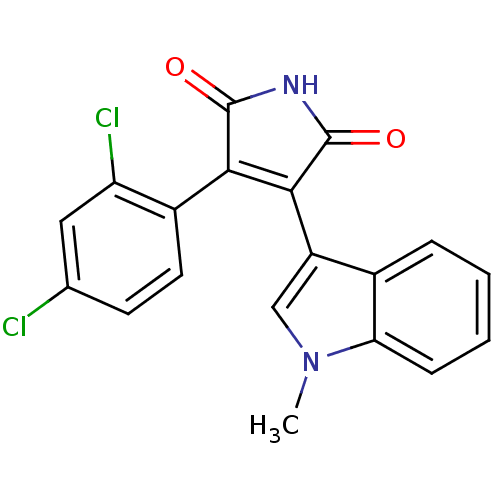 3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione BDBM8296 CHEMBL102714 cid_176158 SB-216763
3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione BDBM8296 CHEMBL102714 cid_176158 SB-216763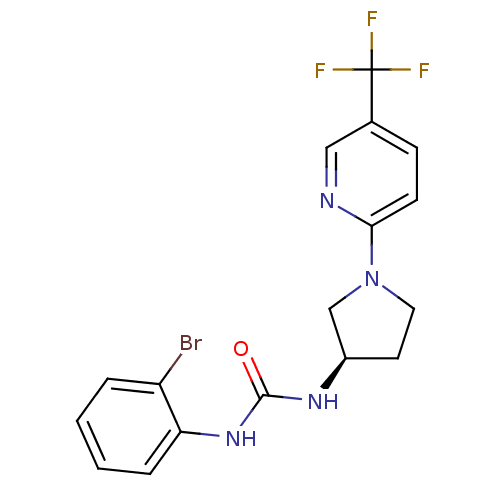 BDBM20504 1-(2-bromophenyl)-3-[(3R)-1-[5-(trifluoromethyl)pyridin-2-yl]pyrrolidin-3-yl]urea BMCL163287 Compound 15 SB-705498
BDBM20504 1-(2-bromophenyl)-3-[(3R)-1-[5-(trifluoromethyl)pyridin-2-yl]pyrrolidin-3-yl]urea BMCL163287 Compound 15 SB-705498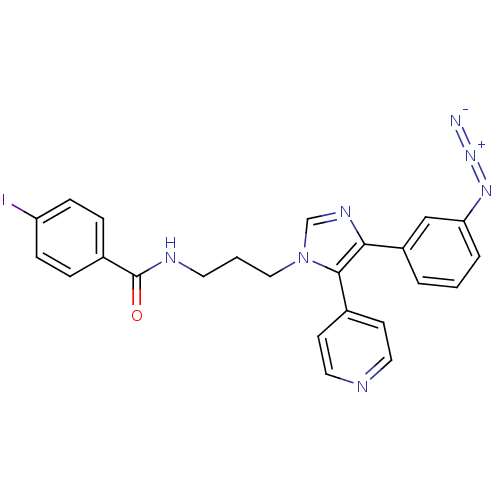 CHEMBL371720 BDBM50173166 SB-227931 N-{3-[4-(3-Azido-phenyl)-5-pyridin-4-yl-imidazol-1-yl]-propyl}-4-iodo-benzamide
CHEMBL371720 BDBM50173166 SB-227931 N-{3-[4-(3-Azido-phenyl)-5-pyridin-4-yl-imidazol-1-yl]-propyl}-4-iodo-benzamide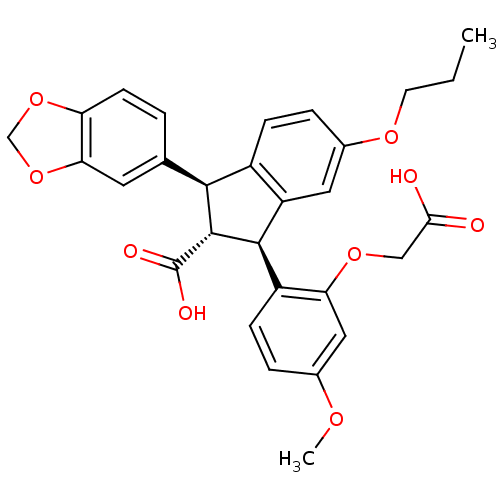 BDBM50041617 SB 209670 SB-209670 (1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymethoxy-4-methoxy-phenyl)-5-propoxy-indan-2-carboxylic acid CHEMBL8823 1-benzo[d][1,3]dioxol-5-ylmethyl-3-(2-carboxymethoxy-4-methoxyphenyl)-5-propoxy-(1R,3S)-2,3-dihydro-1H-2-indenecarboxylic acid
BDBM50041617 SB 209670 SB-209670 (1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymethoxy-4-methoxy-phenyl)-5-propoxy-indan-2-carboxylic acid CHEMBL8823 1-benzo[d][1,3]dioxol-5-ylmethyl-3-(2-carboxymethoxy-4-methoxyphenyl)-5-propoxy-(1R,3S)-2,3-dihydro-1H-2-indenecarboxylic acid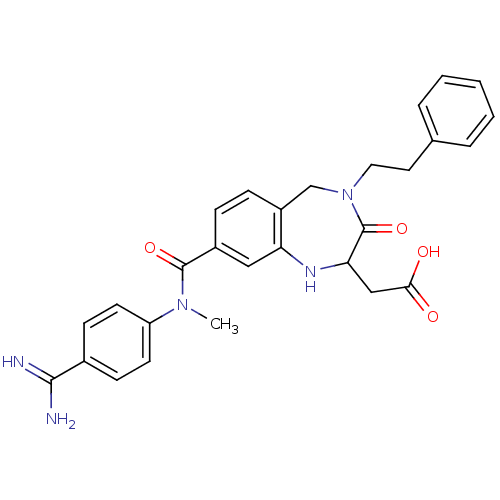 BDBM50054827 SB-208651 {8-[(4-Carbamimidoyl-phenyl)-methyl-carbamoyl]-3-oxo-4-phenethyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid {8-[(4-Carbamimidoyl-phenyl)-methyl-carbamoyl]-3-oxo-4-phenethyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid(SB-208651) CHEMBL85094
BDBM50054827 SB-208651 {8-[(4-Carbamimidoyl-phenyl)-methyl-carbamoyl]-3-oxo-4-phenethyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid {8-[(4-Carbamimidoyl-phenyl)-methyl-carbamoyl]-3-oxo-4-phenethyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid(SB-208651) CHEMBL85094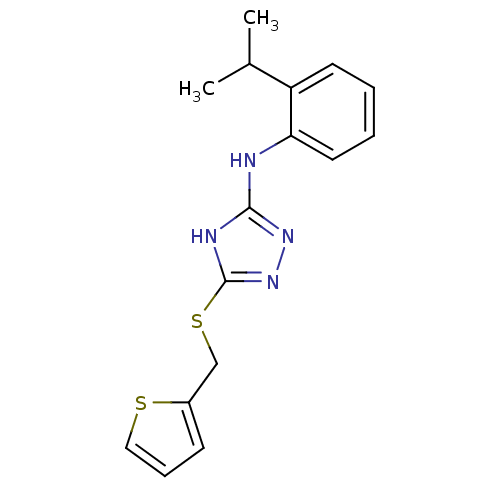 SB-587094 BDBM17443 1,2,4-Triazole Compound, 102 N-[2-(propan-2-yl)phenyl]-5-[(thiophen-2-ylmethyl)sulfanyl]-4H-1,2,4-triazol-3-amine
SB-587094 BDBM17443 1,2,4-Triazole Compound, 102 N-[2-(propan-2-yl)phenyl]-5-[(thiophen-2-ylmethyl)sulfanyl]-4H-1,2,4-triazol-3-amine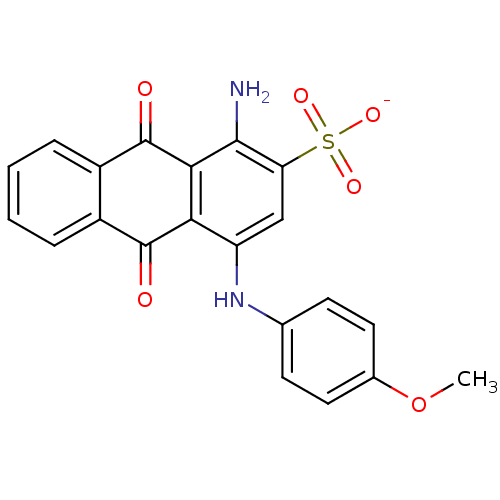 sodium 1-amino-4-(4-methoxyphenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate SB-416 BDBM50227023 1-amino-4-(4-methoxyphenyl)-2-sulfoanthraquinone CHEMBL401735
sodium 1-amino-4-(4-methoxyphenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate SB-416 BDBM50227023 1-amino-4-(4-methoxyphenyl)-2-sulfoanthraquinone CHEMBL401735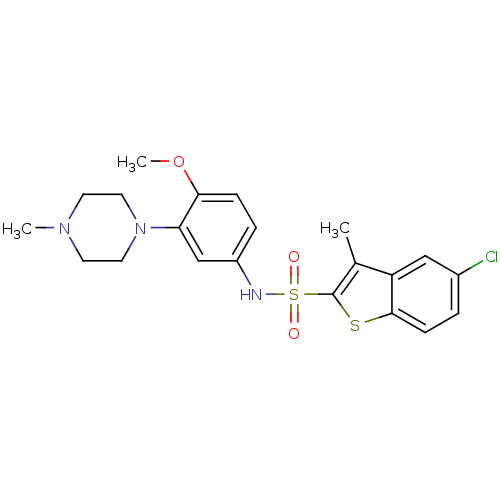 SB-258510 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-amide BDBM50130286 CHEMBL29846
SB-258510 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-amide BDBM50130286 CHEMBL29846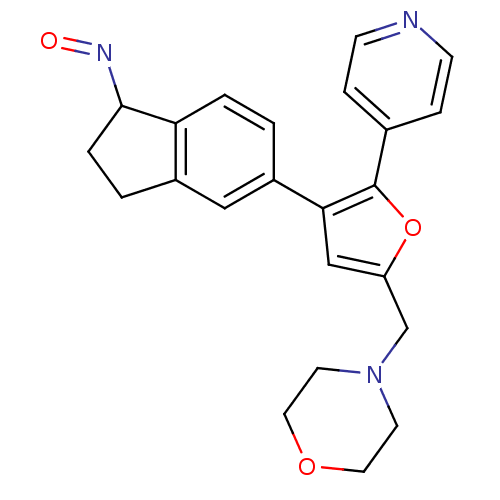 (1E)-5-[5-(morpholin-4-ylmethyl)-2-(pyridin-4-yl)furan-3-yl]-2,3-dihydro-1H-indene-1-hydroxylamine BMCL184373 Compound 17 SB-699393 BDBM26056
(1E)-5-[5-(morpholin-4-ylmethyl)-2-(pyridin-4-yl)furan-3-yl]-2,3-dihydro-1H-indene-1-hydroxylamine BMCL184373 Compound 17 SB-699393 BDBM26056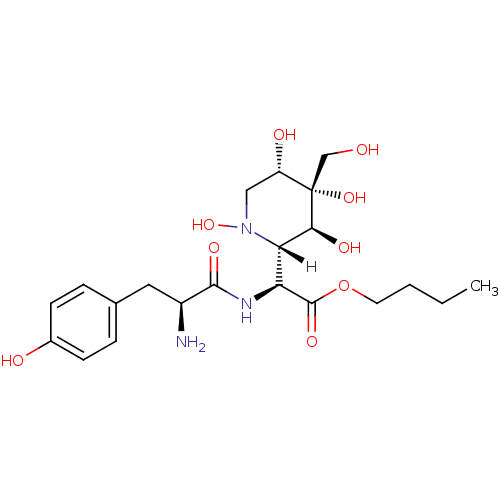 BDBM18131 SB-243545 butyl (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3S,4S,5S)-1,3,4,5-tetrahydroxy-4-(hydroxymethyl)piperidin-2-yl]acetate
BDBM18131 SB-243545 butyl (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3S,4S,5S)-1,3,4,5-tetrahydroxy-4-(hydroxymethyl)piperidin-2-yl]acetate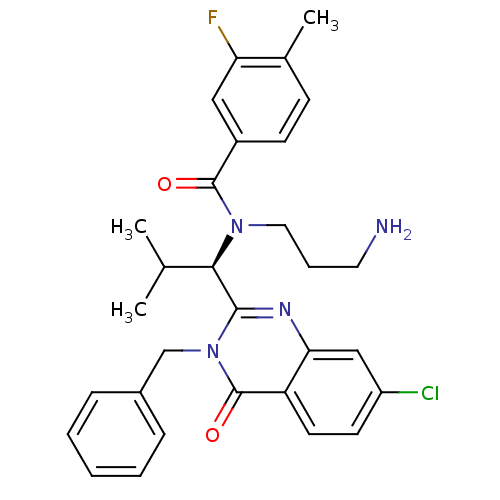 CHEMBL436624 SB-731489 BDBM50220156 (R)-N-(3-aminopropyl)-N-(1-(3-benzyl-7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-2-methylpropyl)-3-fluoro-4-methylbenzamide
CHEMBL436624 SB-731489 BDBM50220156 (R)-N-(3-aminopropyl)-N-(1-(3-benzyl-7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-2-methylpropyl)-3-fluoro-4-methylbenzamide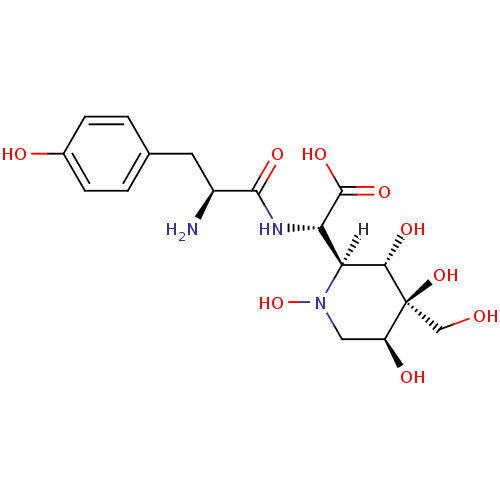 SB-239629 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3S,4S,5S)-1,3,4,5-tetrahydroxy-4-(hydroxymethyl)piperidin-2-yl]acetic acid BDBM18130
SB-239629 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3S,4S,5S)-1,3,4,5-tetrahydroxy-4-(hydroxymethyl)piperidin-2-yl]acetic acid BDBM18130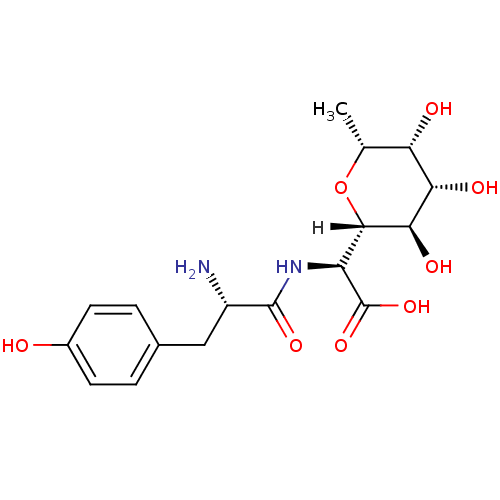 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methyloxan-2-yl]acetic acid SB-284485 BDBM18132 CHEMBL163022
(2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methyloxan-2-yl]acetic acid SB-284485 BDBM18132 CHEMBL163022 CHEMBL490262 N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide SB-298 CHEMBL521280 BDBM50268085
CHEMBL490262 N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide SB-298 CHEMBL521280 BDBM50268085 [3H]-N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide BDBM50268276 SB-298 CHEMBL521280
[3H]-N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide BDBM50268276 SB-298 CHEMBL521280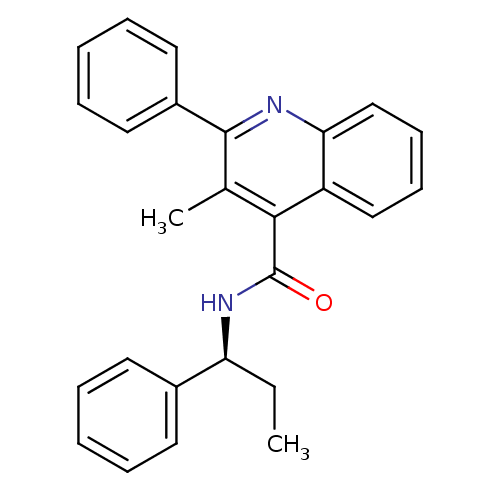 (S)-3-methyl-2-phenyl-N-(1-phenylpropyl)quinoline-4-carboxamide CHEMBL10284 3-Methyl-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide BDBM50051295 SB-222200
(S)-3-methyl-2-phenyl-N-(1-phenylpropyl)quinoline-4-carboxamide CHEMBL10284 3-Methyl-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide BDBM50051295 SB-222200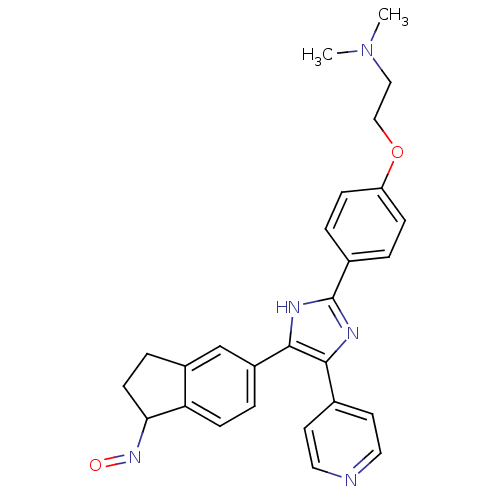 BDBM25391 [2-(4-{4-[(1E)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5-yl]-5-(pyridin-4-yl)-1H-imidazol-2-yl}phenoxy)ethyl]dimethylamine SB-590885 CHEMBL200622 SB590885
BDBM25391 [2-(4-{4-[(1E)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5-yl]-5-(pyridin-4-yl)-1H-imidazol-2-yl}phenoxy)ethyl]dimethylamine SB-590885 CHEMBL200622 SB590885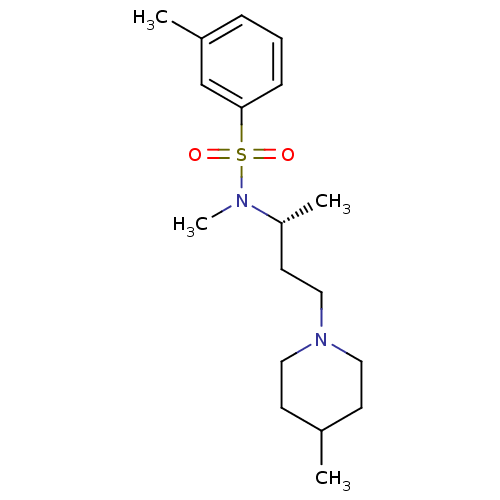 3,N-Dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide 3,N-Dimethyl-N-[(R)-1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide 4-Methyl-1-[(R)-3-methyl-4-(toluene-3-sulfonyl)-pentyl]-piperidine SB-258719 BDBM50098550 CHEMBL12264 3,N-Dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide (SB-258719)
3,N-Dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide 3,N-Dimethyl-N-[(R)-1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide 4-Methyl-1-[(R)-3-methyl-4-(toluene-3-sulfonyl)-pentyl]-piperidine SB-258719 BDBM50098550 CHEMBL12264 3,N-Dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide (SB-258719)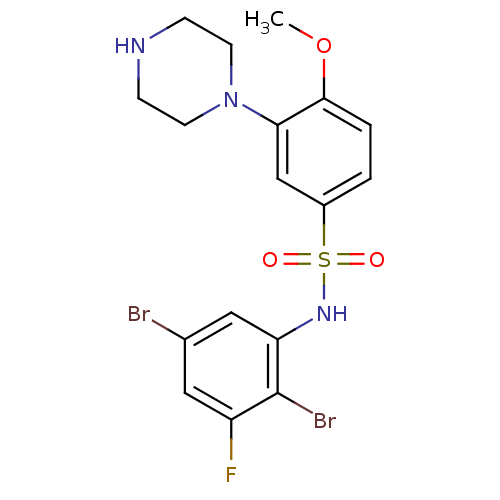 CHEMBL329383 N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide BDBM50130268 N-(2,5-Dibromo-3-fluoro-phenyl)-4-methoxy-3-piperazin-1-yl-benzenesulfonamide SB-357134
CHEMBL329383 N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide BDBM50130268 N-(2,5-Dibromo-3-fluoro-phenyl)-4-methoxy-3-piperazin-1-yl-benzenesulfonamide SB-357134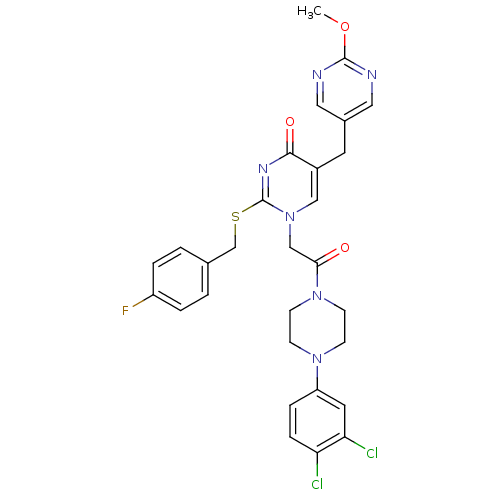 1-{2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-oxo-ethyl}-2-(4-fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimidin-5-ylmethyl)-1H-pyrimidin-4-one BDBM50102161 SB-381320 CHEMBL56954
1-{2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-oxo-ethyl}-2-(4-fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimidin-5-ylmethyl)-1H-pyrimidin-4-one BDBM50102161 SB-381320 CHEMBL56954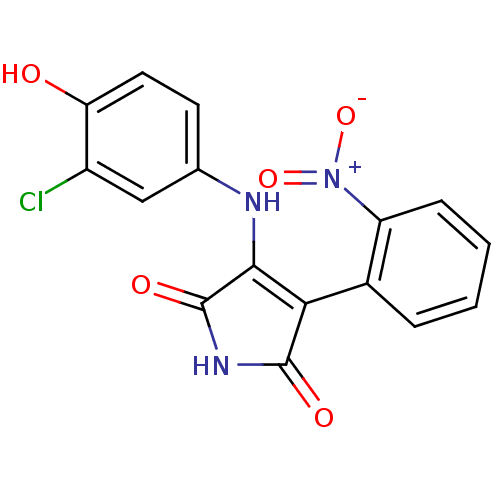 3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phenyl)-pyrrole-2,5-dione CHEMBL322970 SB415286 BDBM50130725 3-(3-chloro-4-hydroxyphenylamino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione SB-415286 cid_4210951
3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phenyl)-pyrrole-2,5-dione CHEMBL322970 SB415286 BDBM50130725 3-(3-chloro-4-hydroxyphenylamino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione SB-415286 cid_4210951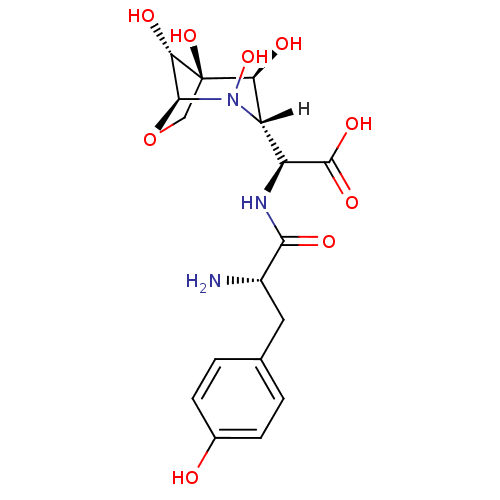 BDBM18128 SB-219383 CHEMBL310012 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(1S,3S,4S,5R,8R)-2,4,5,8-tetrahydroxy-7-oxa-2-azabicyclo[3.2.1]octan-3-yl]acetic acid
BDBM18128 SB-219383 CHEMBL310012 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(1S,3S,4S,5R,8R)-2,4,5,8-tetrahydroxy-7-oxa-2-azabicyclo[3.2.1]octan-3-yl]acetic acid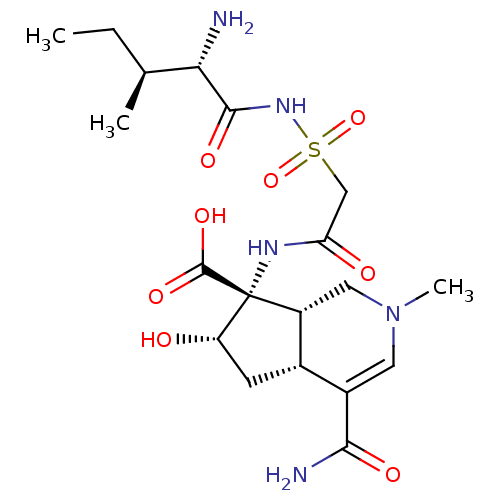 CHEMBL74395 SB-203207 (4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pentanoylsulfamoyl)-acetylamino]-4-carbamoyl-6-hydroxy-2-methyl-2,4a,5,6,7,7a-hexahydro-1H-[2]pyrindine-7-carboxylic acid BDBM50093003
CHEMBL74395 SB-203207 (4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pentanoylsulfamoyl)-acetylamino]-4-carbamoyl-6-hydroxy-2-methyl-2,4a,5,6,7,7a-hexahydro-1H-[2]pyrindine-7-carboxylic acid BDBM50093003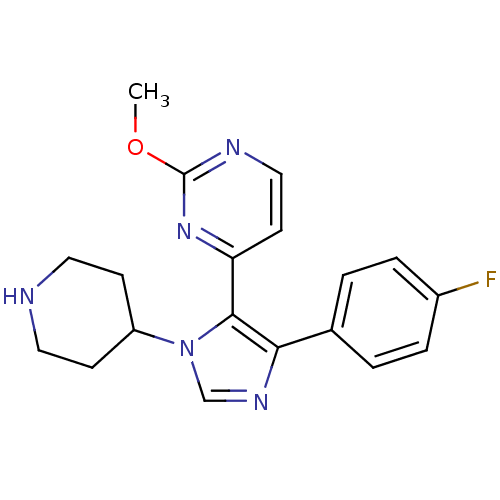 SB242235 BDBM15458 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]-2-methoxypyrimidine 4-[4-(4-fluorophenyl)-1-piperidin-4-yl-1H-imidazol-5-yl]-2-methoxypyrimidine CHEMBL95692 SB-242235
SB242235 BDBM15458 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]-2-methoxypyrimidine 4-[4-(4-fluorophenyl)-1-piperidin-4-yl-1H-imidazol-5-yl]-2-methoxypyrimidine CHEMBL95692 SB-242235 BDBM50343509 SB-203580 4-(4-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-5-yl)pyridine 4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-2,3-dihydro-1H-imidazol-4-yl)pyridine 4-[5-(3-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-3H-imidazol-4-yl]-pyridine 4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-4-yl)pyridine 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-1H-imidazol-4-yl]-pyridine 4-(2-(4-(methylsulfinyl)phenyl)-4-phenyl-1H-imidazol-5-yl)pyridine 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-3H-imidazol-4-yl]-pyridine CHEMBL10 4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfoxide-phenyl)-3H-imidazol-4-yl]-pyridine 4-[5-(4-Fluorophenyl)-2-[4-(methylsulfonyl)phenyl]-1H-imidazol-4-yl]pyridine
BDBM50343509 SB-203580 4-(4-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-5-yl)pyridine 4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-2,3-dihydro-1H-imidazol-4-yl)pyridine 4-[5-(3-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-3H-imidazol-4-yl]-pyridine 4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-4-yl)pyridine 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-1H-imidazol-4-yl]-pyridine 4-(2-(4-(methylsulfinyl)phenyl)-4-phenyl-1H-imidazol-5-yl)pyridine 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-3H-imidazol-4-yl]-pyridine CHEMBL10 4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfoxide-phenyl)-3H-imidazol-4-yl]-pyridine 4-[5-(4-Fluorophenyl)-2-[4-(methylsulfonyl)phenyl]-1H-imidazol-4-yl]pyridine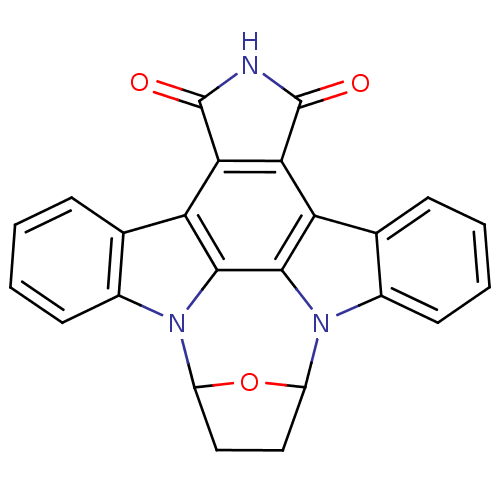 BDBM6763 SB-218078 28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8(13),9,11,20(25),21,23-nonaene-3,5-dione
BDBM6763 SB-218078 28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8(13),9,11,20(25),21,23-nonaene-3,5-dione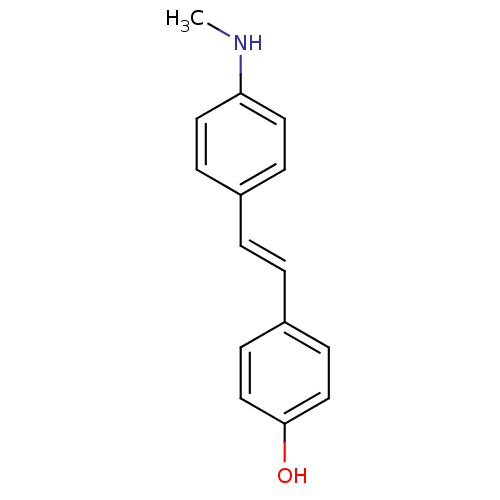 SB-13 BDBM50173647 CHEMBL381642 4-N-methylamino-4'-hydroxystilbene 4-(4-(methylamino)styryl)phenol 4-[(E)-2-(4-Methylamino-phenyl)-vinyl]-phenol [3H]-4-(4-(methylamino)styryl)phenol (E)-4-(4-(methylamino)styryl)phenol
SB-13 BDBM50173647 CHEMBL381642 4-N-methylamino-4'-hydroxystilbene 4-(4-(methylamino)styryl)phenol 4-[(E)-2-(4-Methylamino-phenyl)-vinyl]-phenol [3H]-4-(4-(methylamino)styryl)phenol (E)-4-(4-(methylamino)styryl)phenol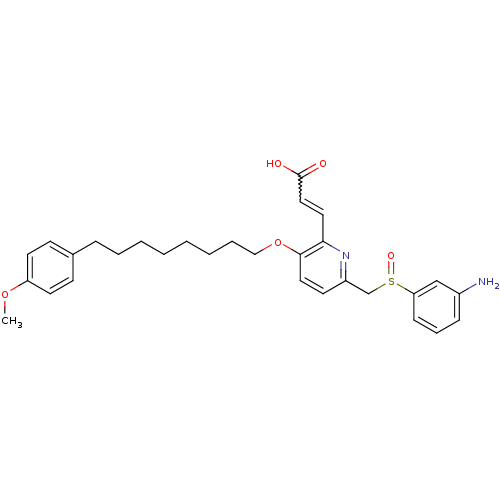 3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-yl}-acrylic acid BDBM50042182 (E)-3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-yl}-acrylic acid CHEMBL112338 SB-201146
3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-yl}-acrylic acid BDBM50042182 (E)-3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-yl}-acrylic acid CHEMBL112338 SB-201146 SB-656104 6-((R)-2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulfonyl)-1H-indole BDBM50130295 CHEMBL95104 6-(2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulfonyl)-1H-indole
SB-656104 6-((R)-2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulfonyl)-1H-indole BDBM50130295 CHEMBL95104 6-(2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulfonyl)-1H-indole 4-(5-ethyl-2-(4-methoxyphenyl)-1H-imidazol-4-yl)pyridine CHEMBL278724 BDBM50284510 4-(4-ethyl-2-(4-methoxyphenyl)-1H-imidazol-5-yl)pyridine 4-[5-Ethyl-2-(4-methoxy-phenyl)-3H-imidazol-4-yl]-pyridine SB-202474
4-(5-ethyl-2-(4-methoxyphenyl)-1H-imidazol-4-yl)pyridine CHEMBL278724 BDBM50284510 4-(4-ethyl-2-(4-methoxyphenyl)-1H-imidazol-5-yl)pyridine 4-[5-Ethyl-2-(4-methoxy-phenyl)-3H-imidazol-4-yl]-pyridine SB-202474 SB-271046 5-chloro-N-(4-methoxy-3-(piperazin-1-yl)phenyl)-3-methylbenzo[b]thiophene-2-sulfonamide 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid (4-methoxy-3-piperazin-1-yl-phenyl)-amide BDBM50090525 CHEMBL431298
SB-271046 5-chloro-N-(4-methoxy-3-(piperazin-1-yl)phenyl)-3-methylbenzo[b]thiophene-2-sulfonamide 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid (4-methoxy-3-piperazin-1-yl-phenyl)-amide BDBM50090525 CHEMBL431298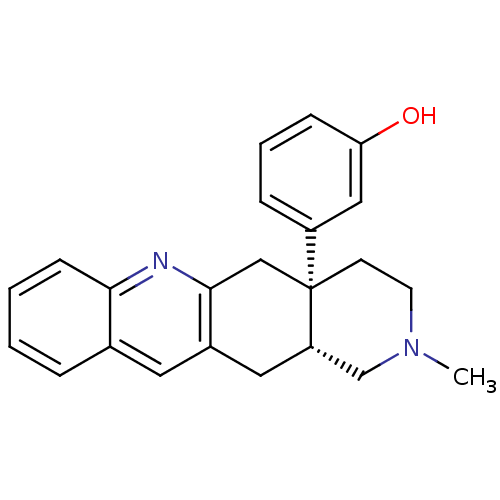 TAN-67 BDBM50290872 Tan-67 (racemic) CHEMBL327745 SB-213698 (-)-rel-3-((4aR,12aS)-2-methyl-1,2,3,4,4a,5,12,12a-octahydropyrido[3,4-b]acridin-4a-yl)phenol 3-((4aS,12aR)-2-Methyl-1,3,4,5,12,12a-hexahydro-2H-2,6-diaza-naphthacen-4a-yl)-phenol
TAN-67 BDBM50290872 Tan-67 (racemic) CHEMBL327745 SB-213698 (-)-rel-3-((4aR,12aS)-2-methyl-1,2,3,4,4a,5,12,12a-octahydropyrido[3,4-b]acridin-4a-yl)phenol 3-((4aS,12aR)-2-Methyl-1,3,4,5,12,12a-hexahydro-2H-2,6-diaza-naphthacen-4a-yl)-phenol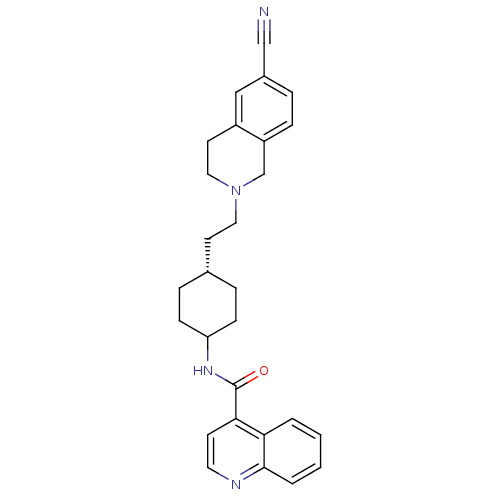 CHEMBL85606 N-(-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide SB-277011-A SB-277011 BDBM50167898 Quinoline-4-carboxylic acid {4-[2-(6-cyano-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-cyclohexyl}-amide N-((1r,4r)-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)ethyl]-cyclohexyl]-4-quinolininecarboxamide trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)-ethyl]cyclo-hexyl]-4-quinolinecarboxamide
CHEMBL85606 N-(-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide SB-277011-A SB-277011 BDBM50167898 Quinoline-4-carboxylic acid {4-[2-(6-cyano-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-cyclohexyl}-amide N-((1r,4r)-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)ethyl]-cyclohexyl]-4-quinolininecarboxamide trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)-ethyl]cyclo-hexyl]-4-quinolinecarboxamide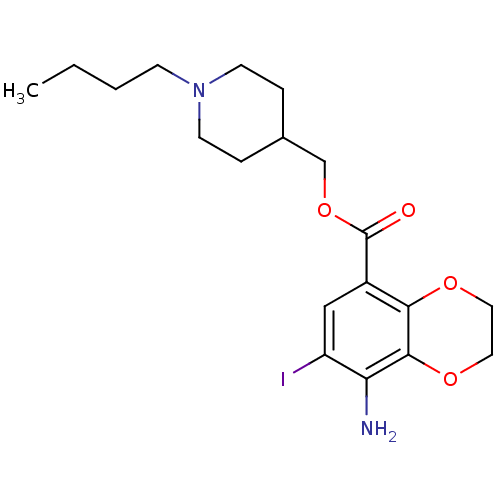 BDBM50327858 SB207710 (1-Butylpiperidin-4-yl)methyl 8-amino-7-iodo-2,3-dihydrobenzo-[b][1,4]dioxine-5-carboxylate CHEMBL114112 8-Amino-7-iodo-2,3-dihydro-benzo[1,4]dioxine-5-carboxylic acid 1-butyl-piperidin-4-ylmethyl ester SB-207710
BDBM50327858 SB207710 (1-Butylpiperidin-4-yl)methyl 8-amino-7-iodo-2,3-dihydrobenzo-[b][1,4]dioxine-5-carboxylate CHEMBL114112 8-Amino-7-iodo-2,3-dihydro-benzo[1,4]dioxine-5-carboxylic acid 1-butyl-piperidin-4-ylmethyl ester SB-207710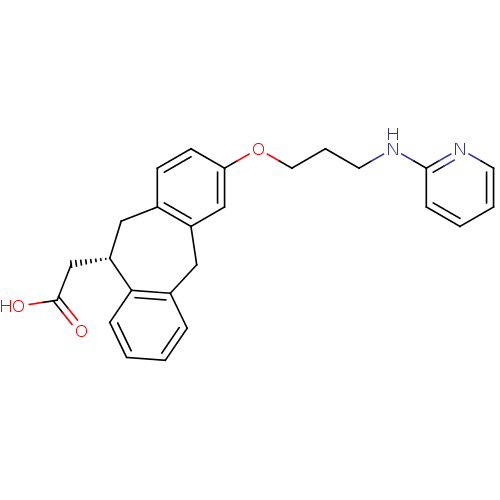 {(S)-3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid {3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid BDBM50078714 SB-265123 CHEMBL288493
{(S)-3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid {3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid BDBM50078714 SB-265123 CHEMBL288493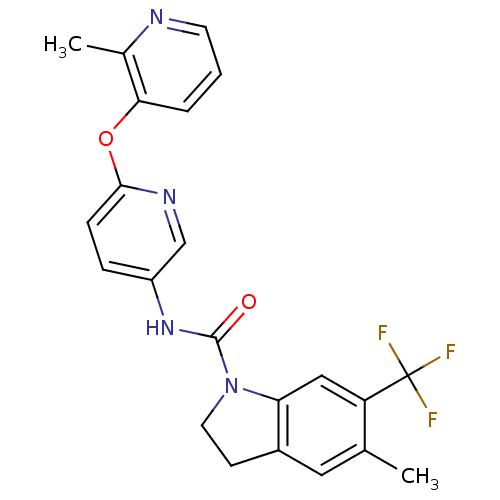 5-Methyl-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid [6-(2-methyl-pyridin-3-yloxy)-pyridin-3-yl]-amide CHEMBL14460 5-methyl-N-(6-(2-methylpyridin-3-yloxy)pyridin-3-yl)-6-(trifluoromethyl)indoline-1-carboxamide BDBM50086065 SB-243213
5-Methyl-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid [6-(2-methyl-pyridin-3-yloxy)-pyridin-3-yl]-amide CHEMBL14460 5-methyl-N-(6-(2-methylpyridin-3-yloxy)pyridin-3-yl)-6-(trifluoromethyl)indoline-1-carboxamide BDBM50086065 SB-243213 BDBM50060416 SB-242084 6-Chloro-5-methyl-2,3-dihydro-indole-1-carboxylic acid [6-(2-methyl-pyridin-3-yloxy)-pyridin-3-yl]-amide 6-chloro-5-methyl-N-(6-(2-methylpyridin-3-yloxy)pyridin-3-yl)indoline-1-carboxamide CHEMBL14563
BDBM50060416 SB-242084 6-Chloro-5-methyl-2,3-dihydro-indole-1-carboxylic acid [6-(2-methyl-pyridin-3-yloxy)-pyridin-3-yl]-amide 6-chloro-5-methyl-N-(6-(2-methylpyridin-3-yloxy)pyridin-3-yl)indoline-1-carboxamide CHEMBL14563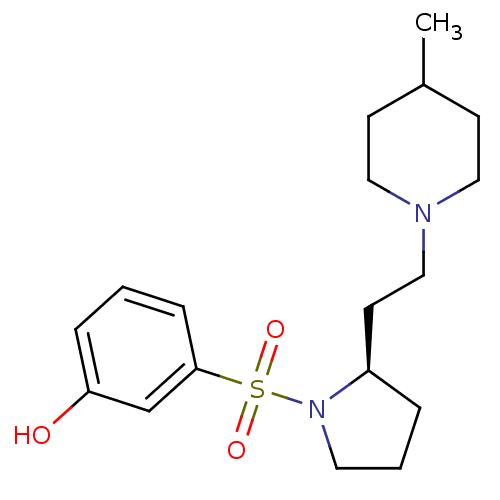 (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl)phenol SB-269970 3-{(R)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol BDBM50098551 CHEMBL282199 3-{2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidin-1-ylsulfonyl)phenol 3-{2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol (SB-269970) 3-{(S)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol
(R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl)phenol SB-269970 3-{(R)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol BDBM50098551 CHEMBL282199 3-{2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidin-1-ylsulfonyl)phenol 3-{2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol (SB-269970) 3-{(S)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol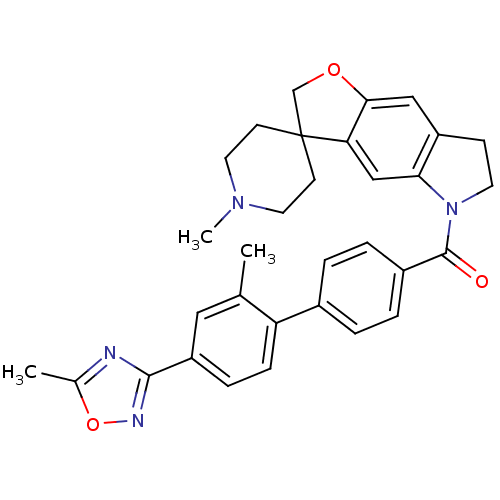 [2'-Methyl-4'-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-yl]-[3-(1-methyl-piperidine)-2,3,6,7-tetrahydro-1-oxa-5-aza-s-indacen-5-yl]-methanone US20240166639, Example SB9 11'-Methyl-5-[[2'-methyl-4'-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydrospiro[furo-[2,3-f]indole-3,4'-piperidine](SB-224289) BDBM50084959 SB-224289 4-[2-methyl-4-(5-methyl-1,2,4-oxadiazol-3-yl)phenyl]phenyl-1'-methylspiro[3,5,6,7-tetrahydro-2H-furo[2,3-f]indole-3,4'-(hexahydropyridine)]-5-ylmethanone CHEMBL281350
[2'-Methyl-4'-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-yl]-[3-(1-methyl-piperidine)-2,3,6,7-tetrahydro-1-oxa-5-aza-s-indacen-5-yl]-methanone US20240166639, Example SB9 11'-Methyl-5-[[2'-methyl-4'-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydrospiro[furo-[2,3-f]indole-3,4'-piperidine](SB-224289) BDBM50084959 SB-224289 4-[2-methyl-4-(5-methyl-1,2,4-oxadiazol-3-yl)phenyl]phenyl-1'-methylspiro[3,5,6,7-tetrahydro-2H-furo[2,3-f]indole-3,4'-(hexahydropyridine)]-5-ylmethanone CHEMBL281350 CHEMBL1824446 SB-505124 US11759530, Compound Table1.2 2-(5-(benzo[d][1,3]dioxol-5-yl)-2-tert-butyl-1H-imidazol-4-yl)-6-methylpyridine CHEMBL226838 2-(4-(benzo[d][1,3]dioxol-5-yl)-2-tert-butyl-1H-imidazol-5-yl)-6-methylpyridine BDBM50298220
CHEMBL1824446 SB-505124 US11759530, Compound Table1.2 2-(5-(benzo[d][1,3]dioxol-5-yl)-2-tert-butyl-1H-imidazol-4-yl)-6-methylpyridine CHEMBL226838 2-(4-(benzo[d][1,3]dioxol-5-yl)-2-tert-butyl-1H-imidazol-5-yl)-6-methylpyridine BDBM50298220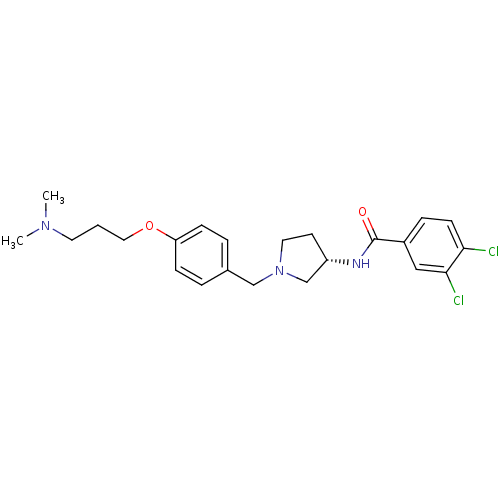 (S)-N-(1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-yl)-3,4-dichlorobenzamide SB-436811 (S)-3,4-dichloro-N-(1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-yl)benzamide CHEMBL366221 3,4-Dichloro-N-{(S)-1-[4-(3-dimethylamino-propoxy)-benzyl]-pyrrolidin-3-yl}-benzamide BDBM50240963
(S)-N-(1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-yl)-3,4-dichlorobenzamide SB-436811 (S)-3,4-dichloro-N-(1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-yl)benzamide CHEMBL366221 3,4-Dichloro-N-{(S)-1-[4-(3-dimethylamino-propoxy)-benzyl]-pyrrolidin-3-yl}-benzamide BDBM50240963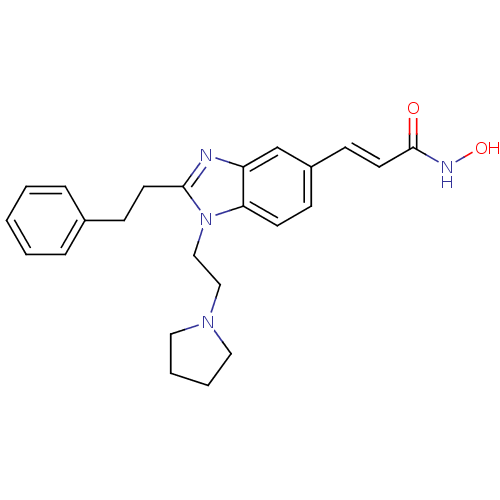 US10736881, Compound 72 BDBM50248476 SB-639 US8551988, 72 N-hydroxy-3-(2-phenethyl-1-(2-(pyrrolidin-1-yl)ethyl)-1H-benzo[d]imidazol-5-yl)acrylamide CHEMBL491316 US10201527, Compound 72 N-hydroxy-3-[2-phenethyl-1-(2-pyrrolidin-1-ylethyl)-1H-benzimidazol-5-yl]acrylamide
US10736881, Compound 72 BDBM50248476 SB-639 US8551988, 72 N-hydroxy-3-(2-phenethyl-1-(2-(pyrrolidin-1-yl)ethyl)-1H-benzo[d]imidazol-5-yl)acrylamide CHEMBL491316 US10201527, Compound 72 N-hydroxy-3-[2-phenethyl-1-(2-pyrrolidin-1-ylethyl)-1H-benzimidazol-5-yl]acrylamide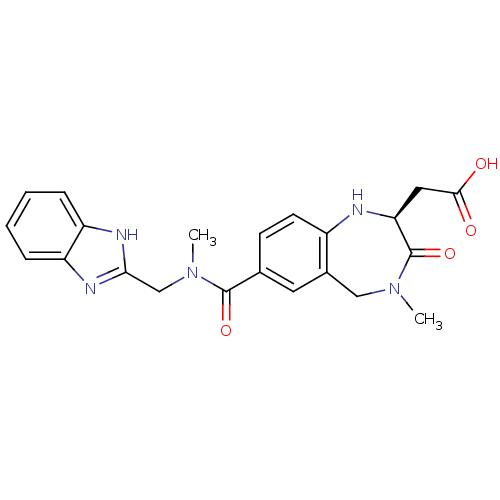 CHEMBL50106 {(S)-7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid {7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid BDBM50059133 SB-223245
CHEMBL50106 {(S)-7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid {7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid BDBM50059133 SB-223245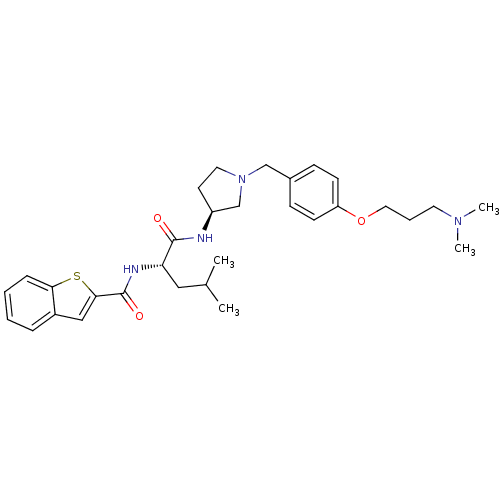 BDBM50240962 Benzo[b]thiophene-2-carboxylic acid ((S)-1-{(R)-1-[4-(3-dimethylamino-propoxy)-benzyl]-pyrrolidin-3-ylcarbamoyl}-3-methyl-butyl)-amide SB-328872 N-((S)-1-((S)-1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-ylamino)-4-methyl-1-oxopentan-2-yl)benzo[b]thiophene-2-carboxamide CHEMBL190533
BDBM50240962 Benzo[b]thiophene-2-carboxylic acid ((S)-1-{(R)-1-[4-(3-dimethylamino-propoxy)-benzyl]-pyrrolidin-3-ylcarbamoyl}-3-methyl-butyl)-amide SB-328872 N-((S)-1-((S)-1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-ylamino)-4-methyl-1-oxopentan-2-yl)benzo[b]thiophene-2-carboxamide CHEMBL190533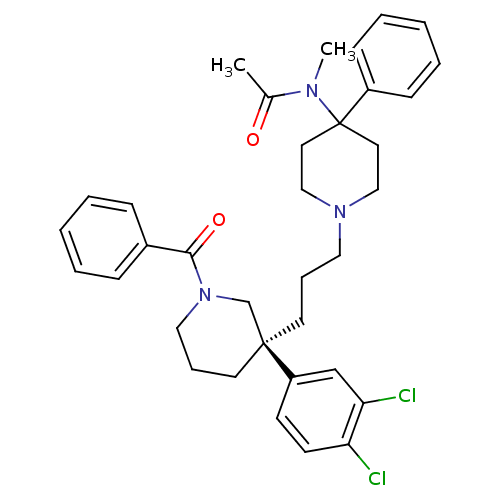 CHEMBL346178 SR-142801 N-(1-{3-[(R)-1-Benzoyl-3-(3,4-dichloro-phenyl)-piperidin-3-yl]-propyl}-4-phenyl-piperidin-4-yl)-N-methyl-acetamide OSANETANT (S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl]prop-1-yl)-4-phenylpiperidin-4-yl)-N-methylacetamine SR-14280 BDBM50291261 SB-236984 CHEMBL2311148
CHEMBL346178 SR-142801 N-(1-{3-[(R)-1-Benzoyl-3-(3,4-dichloro-phenyl)-piperidin-3-yl]-propyl}-4-phenyl-piperidin-4-yl)-N-methyl-acetamide OSANETANT (S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl]prop-1-yl)-4-phenylpiperidin-4-yl)-N-methylacetamine SR-14280 BDBM50291261 SB-236984 CHEMBL2311148 3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)-hexyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid 3-{6-(2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid (SB201993) 3-{6-((E)-2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid CHEMBL422598 SB-201993 BDBM50037385
3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)-hexyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid 3-{6-(2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid (SB201993) 3-{6-((E)-2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid CHEMBL422598 SB-201993 BDBM50037385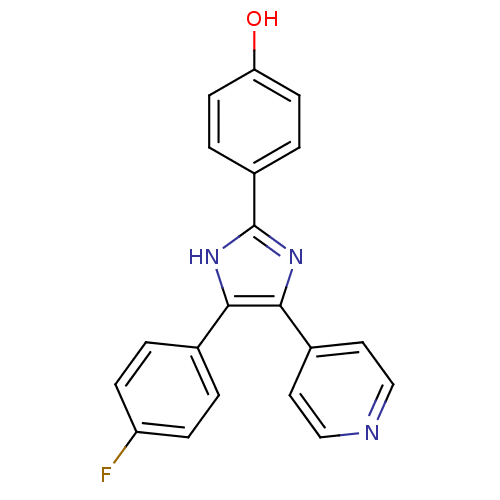 4-[4-(4-fluorophenyl)-5-(4-pyridyl)-4-imidazolin-2-ylidene]cyclohexa-2,5-dien-1-one 4-[4-(4-Fluorophenyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]phenol SB-202190 4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole 4-[5-(4-fluorophenyl)-4-(pyridin-4-yl)-1H-imidazol-2-yl]phenol SB202190 BDBM13531 biotinylated SB202190
4-[4-(4-fluorophenyl)-5-(4-pyridyl)-4-imidazolin-2-ylidene]cyclohexa-2,5-dien-1-one 4-[4-(4-Fluorophenyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]phenol SB-202190 4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole 4-[5-(4-fluorophenyl)-4-(pyridin-4-yl)-1H-imidazol-2-yl]phenol SB202190 BDBM13531 biotinylated SB202190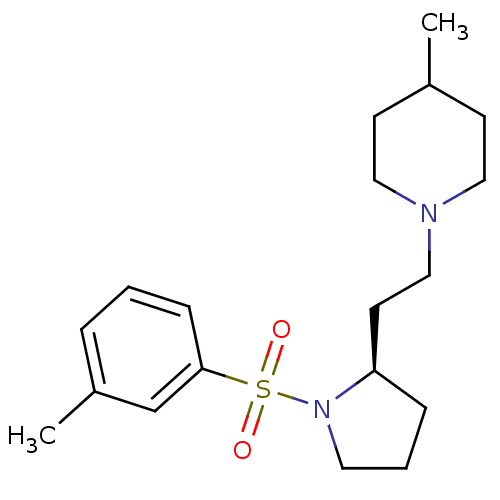 SB-258741 BDBM50130279 CHEMBL12624 (R)-4-Methyl-1-(2-(1-toluene-3-sulfonyl)-pyrrolidin-2-yl)-ethyl)-piperidine (Oxalate salt) 4-Methyl-1-{2-[(R)-1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-Methyl-1-{2-[(S)-1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-Methyl-1-{2-[1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine
SB-258741 BDBM50130279 CHEMBL12624 (R)-4-Methyl-1-(2-(1-toluene-3-sulfonyl)-pyrrolidin-2-yl)-ethyl)-piperidine (Oxalate salt) 4-Methyl-1-{2-[(R)-1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-Methyl-1-{2-[(S)-1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-Methyl-1-{2-[1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-(4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2(3H)-ylidene)cyclohexa-2,5-dienone 4-[5-(4-fluorophenyl)-4-pyridin-4-yl-1H-imidazol-2-yl]phenol BDBM50104383 SB-202190 4-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-1H-imidazol-2-yl]-phenol 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole CHEMBL278041 4-(4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2-yl)phenol
4-(4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2(3H)-ylidene)cyclohexa-2,5-dienone 4-[5-(4-fluorophenyl)-4-pyridin-4-yl-1H-imidazol-2-yl]phenol BDBM50104383 SB-202190 4-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-1H-imidazol-2-yl]-phenol 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole CHEMBL278041 4-(4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2-yl)phenol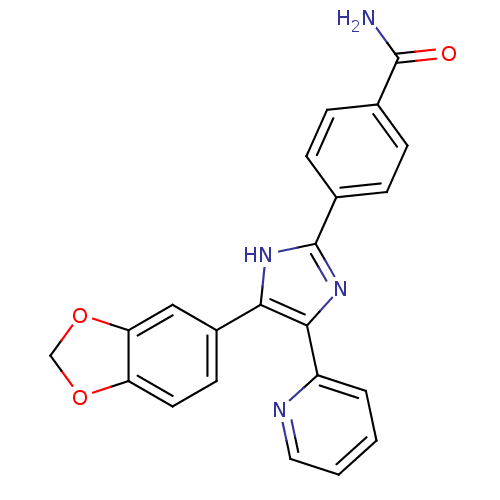 4-(4-Benzo[1,3]dioxol-5-yl-5-pyridin-2-yl-1H-imidazol-2-yl)-benzamide BDBM50110208 cid_4521392 4-(5-(benzo[d][1,3]dioxol-5-yl)-4-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide CHEMBL440084 SB-431542 4-(5-benzo[1,3]dioxol-5-yl-4-pyridin-2-yl-1H-imidazol-2-yl)-benzamide 4-(4-(benzo[d][1,3]dioxol-5-yl)-5-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide
4-(4-Benzo[1,3]dioxol-5-yl-5-pyridin-2-yl-1H-imidazol-2-yl)-benzamide BDBM50110208 cid_4521392 4-(5-(benzo[d][1,3]dioxol-5-yl)-4-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide CHEMBL440084 SB-431542 4-(5-benzo[1,3]dioxol-5-yl-4-pyridin-2-yl-1H-imidazol-2-yl)-benzamide 4-(4-(benzo[d][1,3]dioxol-5-yl)-5-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide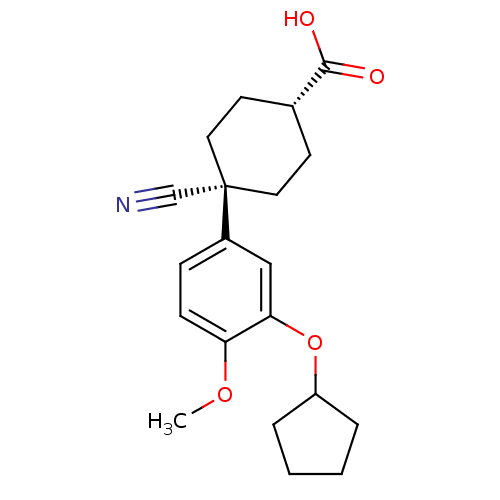 Ariflo 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid (Ariflo) BDBM50346088 CHEMBL511115 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid methyl ester 4-Cyano-3-cyclopentyloxy-4-(4-methoxy-phenyl)-cyclohexanecarboxylic acid 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid(Ariflo) (1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphenyl)cyclohexanecarboxylic acid 6-(3,4-Dimethoxy-phenyl)-5-methyl-4,5-dihydro-2H-pyridazin-3-one 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid SB-207499
Ariflo 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid (Ariflo) BDBM50346088 CHEMBL511115 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid methyl ester 4-Cyano-3-cyclopentyloxy-4-(4-methoxy-phenyl)-cyclohexanecarboxylic acid 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid(Ariflo) (1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphenyl)cyclohexanecarboxylic acid 6-(3,4-Dimethoxy-phenyl)-5-methyl-4,5-dihydro-2H-pyridazin-3-one 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid SB-207499
- Banwell, MG; Crasto, CF; Easton, CJ; Forrest, AK; Karoli, T; March, DR; Mensah, L; Nairn, MR; O'Hanlon, PJ; Oldham, MD; Yue, W Analogues of SB-203207 as inhibitors of tRNA synthetases. Bioorg Med Chem Lett 10: 2263-6 (2001)
- Kennett, GA; Wood, MD; Bright, F; Trail, B; Riley, G; Holland, V; Avenell, KY; Stean, T; Upton, N; Bromidge, S; Forbes, IT; Brown, AM; Middlemiss, DN; Blackburn, TP SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36: 609-20 (1997)
- Coates, NJ; Gilpin, ML; Gwynn, MN; Lewis, DE; Milner, PH; Spear, SR; Tyler, JW SB-202742, a novel beta-lactamase inhibitor isolated from Spondias mombin. J Nat Prod 57: 654-7 (1994)
- Selkirk, JV; Scott, C; Ho, M; Burton, MJ; Watson, J; Gaster, LM; Collin, L; Jones, BJ; Middlemiss, DN; Price, GW SB-224289--a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br J Pharmacol 125: 202-8 (1998)
- Witty, DR; Bateson, J; Hervieu, GJ; Al-Barazanji, K; Jeffrey, P; Hamprecht, D; Haynes, A; Johnson, CN; Muir, AI; O'Hanlon, PJ; Stemp, G; Stevens, AJ; Thewlis, K; Winborn, KY Discovery of potent and stable conformationally constrained analogues of the MCH R1 antagonist SB-568849. Bioorg Med Chem Lett 16: 4872-8 (2006)
- Jarvest, RL; Berge, JM; Brown, P; Hamprecht, DW; McNair, DJ; Mensah, L; O'Hanlon, PJ; Pope, AJ Potent synthetic inhibitors of tyrosyl tRNA synthetase derived from C-pyranosyl analogues of SB-219383. Bioorg Med Chem Lett 11: 715-8 (2001)
- Price, GW; Burton, MJ; Collin, LJ; Duckworth, M; Gaster, L; Göthert, M; Jones, BJ; Roberts, C; Watson, JM; Middlemiss, DN SB-216641 and BRL-15572--compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol 356: 312-20 (1997)
- Forbes, IT; Douglas, S; Gribble, AD; Ife, RJ; Lightfoot, AP; Garner, AE; Riley, GJ; Jeffrey, P; Stevens, AJ; Stean, TO; Thomas, DR SB-656104-A: a novel 5-HT(7) receptor antagonist with improved in vivo properties. Bioorg Med Chem Lett 12: 3341-4 (2002)
- Brown, P; Eggleston, DS; Haltiwanger, RC; Jarvest, RL; Mensah, L; O'Hanlon, PJ; Pope, AJ Synthetic analogues of SB-219383. Novel C-glycosyl peptides as inhibitors of tyrosyl tRNA synthetase. Bioorg Med Chem Lett 11: 711-4 (2001)
- Blackie, JA; Bloomer, JC; Brown, MJ; Cheng, HY; Hammond, B; Hickey, DM; Ife, RJ; Leach, CA; Lewis, VA; Macphee, CH; Milliner, KJ; Moores, KE; Pinto, IL; Smith, SA; Stansfield, IG; Stanway, SJ; Taylor, MA; Theobald, CJ The identification of clinical candidate SB-480848: a potent inhibitor of lipoprotein-associated phospholipase A2. Bioorg Med Chem Lett 13: 1067-70 (2003)
- Rami, HK; Thompson, M; Stemp, G; Fell, S; Jerman, JC; Stevens, AJ; Smart, D; Sargent, B; Sanderson, D; Randall, AD; Gunthorpe, MJ; Davis, JB Discovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med Chem Lett 16: 3287-91 (2006)
- Thomas, DR; Gittins, SA; Collin, LL; Middlemiss, DN; Riley, G; Hagan, J; Gloger, I; Ellis, CE; Forbes, IT; Brown, AM Functional characterisation of the human cloned 5-HT7 receptor (long form); antagonist profile of SB-258719. Br J Pharmacol 124: 1300-6 (1998)
- Jarvest, RL; Berge, JM; Houge-Frydrych, CS; Mensah, LM; O'Hanlon, PJ; Pope, AJ Inhibitors of bacterial tyrosyl tRNA synthetase: synthesis of carbocyclic analogues of the natural product SB-219383. Bioorg Med Chem Lett 11: 2499-502 (2001)
- Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885.
- Berge, JM; Copley, RC; Eggleston, DS; Hamprecht, DW; Jarvest, RL; Mensah, LM; O'Hanlon, PJ; Pope, AJ Inhibitors of bacterial tyrosyl tRNA synthetase: synthesis of four stereoisomeric analogues of the natural product SB-219383. Bioorg Med Chem Lett 10: 1811-4 (2000)
- Reavill, C; Taylor, SG; Wood, MD; Ashmeade, T; Austin, NE; Avenell, KY; Boyfield, I; Branch, CL; Cilia, J; Coldwell, MC; Hadley, MS; Hunter, AJ; Jeffrey, P; Jewitt, F; Johnson, CN; Jones, DN; Medhurst, AD; Middlemiss, DN; Nash, DJ; Riley, GJ; Routledge, C; Stemp, G; Thewlis, KM; Trail, B; Vong, AK; Hagan, JJ Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther 294: 1154-65 (2000)
- Ohlstein, EH; Nambi, P; Hay, DW; Gellai, M; Brooks, DP; Luengo, J; Xiang, JN; Elliott, JD Nonpeptide endothelin receptor antagonists. XI. Pharmacological characterization of SB 234551, a high-affinity and selective nonpeptide ETA receptor antagonist. J Pharmacol Exp Ther 286: 650-6 (1998)
- Wardle, KA; Ellis, ES; Baxter, GS; Kennett, GA; Gaster, LM; Sanger, GJ The effects of SB 204070, a highly potent and selective 5-HT4 receptor antagonist, on guinea-pig distal colon. Br J Pharmacol 112: 789-94 (1994)
- Witty, DR; Bateson, JH; Hervieu, GJ; Jeffrey, P; Johnson, CN; Muir, AI; O'Hanlon, PJ; Stemp, G; Stevens, AJ; Thewlis, KM; Wilson, S; Winborn, KY SAR of biphenyl carboxamide ligands of the human melanin-concentrating hormone receptor 1 (MCH R1): discovery of antagonist SB-568849. Bioorg Med Chem Lett 16: 4865-71 (2006)
- Li, L; Shao, X; Cole, EL; Ohnmacht, SA; Ferrari, V; Hong, YT; Williamson, DJ; Fryer, TD; Quesada, CA; Sherman, P; Riss, PJ; Scott, PJ; Aigbirhio, FI Synthesis and Initial in Vivo Studies with [(11)C]SB-216763: The First Radiolabeled Brain Penetrative Inhibitor of GSK-3. ACS Med Chem Lett 6: 548-52 (2015)
- Blackie, JA; Bloomer, JC; Brown, MJ; Cheng, HY; Elliott, RL; Hammond, B; Hickey, DM; Ife, RJ; Leach, CA; Lewis, VA; Macphee, CH; Milliner, KJ; Moores, KE; Pinto, IL; Smith, SA; Stansfield, IG; Stanway, SJ; Taylor, MA; Theobald, CJ; Whittaker, CM The discovery of SB-435495. A potent, orally active inhibitor of lipoprotein-associated phospholipase A(2) for evaluation in man. Bioorg Med Chem Lett 12: 2603-6 (2002)
- McElhinny, CJ; Lewin, AH; Mascarella, SW; Runyon, S; Brieaddy, L; Carroll, FI Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorg Med Chem Lett 22: 6661-4 (2012)
- Kennett, GA; Wood, MD; Bright, F; Cilia, J; Piper, DC; Gager, T; Thomas, D; Baxter, GS; Forbes, IT; Ham, P; Blackburn, TP In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol 117: 427-434 (1996)
- Sarau, HM; Griswold, DE; Potts, W; Foley, JJ; Schmidt, DB; Webb, EF; Martin, LD; Brawner, ME; Elshourbagy, NA; Medhurst, AD; Giardina, GA; Hay, DW Nonpeptide tachykinin receptor antagonists: I. Pharmacological and pharmacokinetic characterization of SB 223412, a novel, potent and selective neurokinin-3 receptor antagonist. J Pharmacol Exp Ther 281: 1303-11 (1997)
- Bromidge, SM; Clarke, SE; Gager, T; Griffith, K; Jeffrey, P; Jennings, AJ; Joiner, GF; King, FD; Lovell, PJ; Moss, SF; Newman, H; Riley, G; Rogers, D; Routledge, C; Serafinowska, H; Smith, DR Phenyl benzenesulfonamides are novel and selective 5-HT6 antagonists: identification of N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-piperazin-1-ylbenzenesulfonamide (SB-357134). Bioorg Med Chem Lett 11: 55-8 (2001)
- Lovell, PJ; Bromidge, SM; Dabbs, S; Duckworth, DM; Forbes, IT; Jennings, AJ; King, FD; Middlemiss, DN; Rahman, SK; Saunders, DV; Collin, LL; Hagan, JJ; Riley, GJ; Thomas, DR A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phen ol (SB-269970). J Med Chem 43: 342-5 (2000)
- Bromidge, SM; Brown, AM; Clarke, SE; Dodgson, K; Gager, T; Grassam, HL; Jeffrey, PM; Joiner, GF; King, FD; Middlemiss, DN; Moss, SF; Newman, H; Riley, G; Routledge, C; Wyman, P 5-Chloro-N-(4-methoxy-3-piperazin-1-yl- phenyl)-3-methyl-2-benzothiophenesulfon- amide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J Med Chem 42: 202-5 (1999)
- Bromidge, SM; Duckworth, M; Forbes, IT; Ham, P; King, FD; Thewlis, KM; Blaney, FE; Naylor, CB; Blackburn, TP; Kennett, GA; Wood, MD; Clarke, SE 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]- indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem 40: 3494-6 (1997)
- Giardina, GA; Raveglia, LF; Grugni, M; Sarau, HM; Farina, C; Medhurst, AD; Graziani, D; Schmidt, DB; Rigolio, R; Luttmann, M; Cavagnera, S; Foley, JJ; Vecchietti, V; Hay, DW Discovery of a novel class of selective non-peptide antagonists for the human neurokinin-3 receptor. 2. Identification of (S)-N-(1-phenylpropyl)-3-hydroxy-2-phenylquinoline-4-carboxamide (SB 223412). J Med Chem 42: 1053-65 (1999)
- Hay, DW; Giardina, GA; Griswold, DE; Underwood, DC; Kotzer, CJ; Bush, B; Potts, W; Sandhu, P; Lundberg, D; Foley, JJ; Schmidt, DB; Martin, LD; Kilian, D; Legos, JJ; Barone, FC; Luttmann, MA; Grugni, M; Raveglia, LF; Sarau, HM Nonpeptide tachykinin receptor antagonists. III. SB 235375, a low central nervous system-penetrant, potent and selective neurokinin-3 receptor antagonist, inhibits citric acid-induced cough and airways hyper-reactivity in guinea pigs. J Pharmacol Exp Ther 300: 314-23 (2002)
- Bromidge, SM; Brown, F; Cassidy, F; Clark, MS; Dabbs, S; Hadley, MS; Hawkins, J; Loudon, JM; Naylor, CB; Orlek, BS; Riley, GJ Design of [R-(Z)]-(+)-alpha-(methoxyimino)-1-azabicyclo[2.2.2]octane-3-acetonitri le (SB 202026), a functionally selective azabicyclic muscarinic M1 agonist incorporating the N-methoxy imidoyl nitrile group as a novel ester bioisostere. J Med Chem 40: 4265-80 (1998)
- Bromidge, SM; Dabbs, S; Davies, DT; Davies, S; Duckworth, DM; Forbes, IT; Gaster, LM; Ham, P; Jones, GE; King, FD; Mulholland, KR; Saunders, DV; Wyman, PA; Blaney, FE; Clarke, SE; Blackburn, TP; Holland, V; Kennett, GA; Lightowler, S; Middlemiss, DN; Trail, B; Riley, GJ; Wood, MD Biarylcarbamoylindolines are novel and selective 5-HT(2C) receptor inverse agonists: identification of 5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]- 5-pyridyl]carbamoyl]-6-trifluoromethylindoline (SB-243213) as a potential antidepressant/anxiolytic agent. J Med Chem 43: 1123-34 (2000)
- Macdonald, GJ; Branch, CL; Hadley, MS; Johnson, CN; Nash, DJ; Smith, AB; Stemp, G; Thewlis, KM; Vong, AK; Austin, NE; Jeffrey, P; Winborn, KY; Boyfield, I; Hagan, JJ; Middlemiss, DN; Reavill, C; Riley, GJ; Watson, JM; Wood, M; Parker, SG; Ashby, CR Design and synthesis of trans-3-(2-(4-((3-(3-(5-methyl-1,2,4-oxadiazolyl))- phenyl)carboxamido)cyclohexyl)ethyl)-7-methylsulfonyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SB-414796): a potent and selective dopamine D3 receptor antagonist. J Med Chem 46: 4952-64 (2003)
- Stemp, G; Ashmeade, T; Branch, CL; Hadley, MS; Hunter, AJ; Johnson, CN; Nash, DJ; Thewlis, KM; Vong, AK; Austin, NE; Jeffrey, P; Avenell, KY; Boyfield, I; Hagan, JJ; Middlemiss, DN; Reavill, C; Riley, GJ; Routledge, C; Wood, M Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3, 4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): A potent and selective dopamine D(3) receptor antagonist with high oral bioavailability and CNS penetration in the rat. J Med Chem 43: 1878-85 (2000)
- Gaster, LM; Blaney, FE; Davies, S; Duckworth, DM; Ham, P; Jenkins, S; Jennings, AJ; Joiner, GF; King, FD; Mulholland, KR; Wyman, PA; Hagan, JJ; Hatcher, J; Jones, BJ; Middlemiss, DN; Price, GW; Riley, G; Roberts, C; Routledge, C; Selkirk, J; Slade, PD The selective 5-HT1B receptor inverse agonist 1'-methyl-5-[[2'-methyl-4'-(5-methyl-1,2, 4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydro- spiro[furo[2,3-f]indole-3,4'-piperidine] (SB-224289) potently blocks terminal 5-HT autoreceptor function both in vitro and in vivo. J Med Chem 41: 1218-35 (1998)
- Zhou, D; Gross, JL; Adedoyin, AB; Aschmies, SB; Brennan, J; Bowlby, M; Di, L; Kubek, K; Platt, BJ; Wang, Z; Zhang, G; Brandon, N; Comery, TA; Robichaud, AJ J Med Chem 55: 2452-68 (2012)
- Vuorinen, A; Engeli, RT; Leugger, S; Bachmann, F; Akram, M; Atanasov, AG; Waltenberger, B; Temml, V; Stuppner, H; Krenn, L; Ateba, SB; Njamen, D; Davis, RA; Odermatt, A; Schuster, D J Nat Prod 80: 965-974 (2017)
- Patil, PO; Bari, SB; Firke, SD; Deshmukh, PK; Donda, ST; Patil, DA Bioorg Med Chem 21: 2434-50 (2013)
- Berger SB
- Abdullaha, M; Nuthakki, VK; Bharate, SB Eur J Med Chem 207: (2020)
- de Souza, NJ; Gupte, SV; Deshpande, PK; Desai, VN; Bhawsar, SB; Yeole, RD; Shukla, MC; Strahilevitz, J; Hooper, DC; Bozdogan, B; Appelbaum, PC; Jacobs, MR; Shetty, N; Patel, MV; Jha, R; Khorakiwala, HF J Med Chem 48: 5232-42 (2005)
- Bebernitz, GR; Bock, MG; Reddy, DS; Hajare, AK; Vyavahare, V; Bhosale, SB; Kurhade, SE; Salunkhe, V; Shaikh, NS; Bhuniya, D; Palle, PV; Feng, L; Liang, J US Patent USRE49080 (2022)
- McCauley, JA; Theberge, CR; Romano, JJ; Billings, SB; Anderson, KD; Claremon, DA; Freidinger, RM; Bednar, RA; Mosser, SD; Gaul, SL; Connolly, TM; Condra, CL; Xia, M; Cunningham, ME; Bednar, B; Stump, GL; Lynch, JJ; Macaulay, A; Wafford, KA; Koblan, KS; Liverton, NJ J Med Chem 47: 2089-96 (2004)
- Aiello, D; Barnes, MH; Biswas, EE; Biswas, SB; Gu, S; Williams, JD; Bowlin, TL; Moir, DT Bioorg Med Chem 17: 4466-76 (2009)
- Bodendiek, SB; Mahieux, C; Hänsel, W; Wulff, H Eur J Med Chem 44: 1838-52 (2009)
- Liu, J; Kozlowski, JA; Boga, SB; Gao, X; Guiadeen, DG; Cai, J; Liu, S; Wang, D; Wu, H; Yang, C US Patent US10214546 (2019)
- Panchal, RG; Ulrich, RL; Bradfute, SB; Lane, D; Ruthel, G; Kenny, TA; Iversen, PL; Anderson, AO; Gussio, R; Raschke, WC; Bavari, S J Biol Chem 284: 12874-85 (2009)
- Cammerer, SB; Jimenez, C; Jones, S; Gros, L; Lorente, SO; Rodrigues, C; Rodrigues, JC; Caldera, A; Ruiz Perez, LM; da Souza, W; Kaiser, M; Brun, R; Urbina, JA; Gonzalez Pacanowska, D; Gilbert, IH Antimicrob Agents Chemother 51: 4049-61 (2007)
- Cantor SB
- Ekinci, D; Ceyhun, SB; Sentürk, M; Erdem, D; Küfrevioglu, OI; Supuran, CT Bioorg Med Chem 19: 744-8 (2011)
- Park, CE; Jang, YK; Shin, YJ; Kim, JY; Ham, SM; Kim, YG; Min, HK; Cha, SB; Jung, HJ; Lee, JY; Han, SN; Chung, JY; Choi, EJ; Joung, CM; Park, JS; Lee, JW; Cho, NR; Ryu, EJ; Maeng, CY US Patent US11046702 (2021)
- Watanabe, T; Kokubu, N; Charnick, SB; Naito, M; Tsuruo, T; Cohen, D Br J Pharmacol 122: 241-8 (1997)
- Yan, JW; Li, YP; Ye, WJ; Chen, SB; Hou, JQ; Tan, JH; Ou, TM; Li, D; Gu, LQ; Huang, ZS Bioorg Med Chem 20: 2527-34 (2012)
- Cohen, SG; Chishti, SB; Elkind, JL; Reese, H; Cohen, JB J Med Chem 28: 1309-13 (1985)
- Holler, JG; Slotved, HC; Mølgaard, P; Olsen, CE; Christensen, SB Bioorg Med Chem 20: 4514-21 (2012)
- Piotrowski, DW; Futatsugi, K; Casimiro-Garcia, A; Wei, L; Sammons, MF; Herr, M; Jiao, W; Lavergne, SY; Coffey, SB; Wright, SW; Song, K; Loria, PM; Banker, ME; Petersen, DN; Bauman, J J Med Chem 61: 1086-1097 (2018)
- Rottmann, M; McNamara, C; Yeung, BK; Lee, MC; Zou, B; Russell, B; Seitz, P; Plouffe, DM; Dharia, NV; Tan, J; Cohen, SB; Spencer, KR; González-Páez, GE; Lakshminarayana, SB; Goh, A; Suwanarusk, R; Jegla, T; Schmitt, EK; Beck, HP; Brun, R; Nosten, F; Renia, L; Dartois, V; Keller, TH; Fidock, DA; Winzeler, EA; Diagana, TT Science 329: 1175-80 (2010)
- Daval, SB; Valant, C; Bonnet, D; Kellenberger, E; Hibert, M; Galzi, JL; Ilien, B J Med Chem 55: 2125-43 (2012)
- Villalobos, A; Butler, TW; Chapin, DS; Chen, YL; DeMattos, SB; Ives, JL; Jones, SB; Liston, DR; Nagel, AA; Nason, DM J Med Chem 38: 2802-8 (1995)
- Carreras, CW; Siani, MA; Santi, DV; Dillon, SB Anal Biochem 300: 146-51 (2002)
- Nan, X; Zhang, J; Li, HJ; Wu, R; Fang, SB; Zhang, ZZ; Wu, YC Eur J Med Chem 200: (2020)
- Senger, MR; Gomes, Lda C; Ferreira, SB; Kaiser, CR; Ferreira, VF; Silva, FP Chembiochem 13: 1584-93 (2012)
- Coteron, JM; Marco, M; Esquivias, J; Deng, X; White, KL; White, J; Koltun, M; El Mazouni, F; Kokkonda, S; Katneni, K; Bhamidipati, R; Shackleford, DM; Angulo-Barturen, I; Ferrer, SB; Jiménez-Díaz, MB; Gamo, FJ; Goldsmith, EJ; Charman, WN; Bathurst, I; Floyd, D; Matthews, D; Burrows, JN; Rathod, PK; Charman, SA; Phillips, MA J Med Chem 54: 5540-61 (2011)
- Liu, Y; Hao, M; Leggett, AL; Gao, Y; Ficarro, SB; Che, J; He, Z; Olson, CM; Marto, JA; Kwiatkowski, NP; Zhang, T; Gray, NS J Med Chem 63: 6708-6726 (2020)
- Chambers, MS; Hobbs, SC; Fletcher, SR; Matassa, VG; Mitchell, PJ; Watt, AP; Baker, R; Freedman, SB; Patel, S; Smith, AJ Bioorg Med Chem Lett 3: 1919-1924 (1993)
- Miller, MS; Maheshwari, S; McRobb, FM; Kinzler, KW; Amzel, LM; Vogelstein, B; Gabelli, SB Bioorg Med Chem 25: 1481-1486 (2017)
- Gossett, LS; Habeck, LL; Shackelford, KA; Mendelsohn, LG; Gates, SB; Worzalla, JF; Self, TD; Theobald, KS; Andis, SL; Schultz, RM; Shih, C Bioorg Med Chem Lett 9: 75-8 (1999)
- Amaudrut, J; Argiriadi, MA; Barth, M; Breinlinger, EC; Bressac, D; Broqua, P; Calderwood, DJ; Chatar, M; Cusack, KP; Gauld, SB; Jacquet, S; Kamath, RV; Kort, ME; Lepais, V; Luccarini, JM; Masson, P; Montalbetti, C; Mounier, L; Potin, D; Poupardin, O; Rouaud, S; Spitzer, L; Wallace, CD Bioorg Med Chem Lett 29: 1799-1806 (2019)
- Forsyth, T; Kearney, PC; Kim, BG; Johnson, HW; Aay, N; Arcalas, A; Brown, DS; Chan, V; Chen, J; Du, H; Epshteyn, S; Galan, AA; Huynh, TP; Ibrahim, MA; Kane, B; Koltun, ES; Mann, G; Meyr, LE; Lee, MS; Lewis, GL; Noguchi, RT; Pack, M; Ridgway, BH; Shi, X; Takeuchi, CS; Zu, P; Leahy, JW; Nuss, JM; Aoyama, R; Engst, S; Gendreau, SB; Kassees, R; Li, J; Lin, SH; Martini, JF; Stout, T; Tong, P; Woolfrey, J; Zhang, W; Yu, P Bioorg Med Chem Lett 22: 7653-8 (2012)
- Procopiou, PA; Barrett, VJ; Bevan, NJ; Biggadike, K; Butchers, PR; Coe, DM; Conroy, R; Edney, DD; Field, RN; Ford, AJ; Guntrip, SB; Looker, BE; McLay, IM; Monteith, MJ; Morrison, VS; Mutch, PJ; Richards, SA; Sasse, R; Smith, CE J Med Chem 52: 2280-8 (2009)
- HOYT, SB; THOMAS, CJ; FINOCCHIO, CJ; STARCZYNOWSKI, DT; TAWA, GJ; GRACIA MALDONADO, G; ROSEBAUM, JS US Patent US20230303563 (2023)
- Ha, SB; Melman, N; Jacobson, KA; Nair, V Bioorg Med Chem Lett 7: 3085-3090 (1997)
- Anh, DT; Hai, PT; Dung, DTM; Dung, PTP; Huong, LT; Park, EJ; Jun, HW; Kang, JS; Kwon, JH; Tung, TT; Han, SB; Nam, NH Bioorg Med Chem Lett 30: (2020)
- Parker, MF; Barten, DM; Bergstrom, CP; Bronson, JJ; Corsa, JA; Deshpande, MS; Felsenstein, KM; Guss, VL; Hansel, SB; Johnson, G; Keavy, DJ; Lau, WY; Mock, J; Prasad, CV; Polson, CT; Sloan, CP; Smith, DW; Wallace, OB; Wang, HH; Williams, A; Zheng, M Bioorg Med Chem Lett 17: 4432-6 (2007)
- Feldman, HC; Tong, M; Wang, L; Meza-Acevedo, R; Gobillot, TA; Lebedev, I; Gliedt, MJ; Hari, SB; Mitra, AK; Backes, BJ; Papa, FR; Seeliger, MA; Maly, DJ ACS Chem Biol 11: 2195-205 (2016)
- Hahn, KM; Humphreys, W; Helms, AM; Hastie, SB; Macdonald, TL Bioorg Med Chem Lett 1: 471-476 (1991)
- Tumber, A; Nuzzi, A; Hookway, ES; Hatch, SB; Velupillai, S; Johansson, C; Kawamura, A; Savitsky, P; Yapp, C; Szykowska, A; Wu, N; Bountra, C; Strain-Damerell, C; Burgess-Brown, NA; Ruda, GF; Fedorov, O; Munro, S; England, KS; Nowak, RP; Schofield, CJ; La Thangue, NB; Pawlyn, C; Davies, F; Morgan, G; Athanasou, N; Müller, S; Oppermann, U; Brennan, PE Cell Chem Biol 24: 371-380 (2017)
- de Costa, BR; Bowen, WD; Hellewell, SB; George, C; Rothman, RB; Reid, AA; Walker, JM; Jacobson, AE; Rice, KC J Med Chem 32: 1996-2002 (1989)
- Massa, MA; Patt, WC; Ahn, K; Sisneros, AM; Herman, SB; Doherty, A Bioorg Med Chem Lett 8: 2117-22 (1999)
- Colis, LC; Herzon, SB Bioorg Med Chem Lett 26: 3122-6 (2016)
- Hiep, NT; Choi, YH; Kim, N; Hong, SS; Hong, SB; Hwang, BY; Lee, HJ; Lee, SJ; Jang, DS; Lee, D J Nat Prod 75: 784-8 (2012)
- Whitehead, BR; Lo, MM; Ali, A; Park, MK; Hoyt, SB; Xiong, Y; Cai, J; Carswell, E; Cooke, A; MacLean, J; Ratcliffe, P; Robinson, J; Bennett, DJ; Clemas, JA; Wisniewski, T; Struthers, M; Cully, D; MacNeil, DJ Bioorg Med Chem Lett 27: 143-146 (2017)
- Biftu, T; Gamble, NF; Doebber, T; Hwang, SB; Shen, TY; Snyder, J; Springer, JP; Stevenson, R J Med Chem 29: 1917-21 (1986)
- Muthukaman, N; Tambe, M; Deshmukh, S; Pisal, D; Tondlekar, S; Shaikh, M; Sarode, N; Kattige, VG; Pisat, M; Sawant, P; Honnegowda, S; Karande, V; Kulkarni, A; Behera, D; Jadhav, SB; Sangana, RR; Gudi, GS; Khairatkar-Joshi, N; Gharat, LA Bioorg Med Chem Lett 27: 5131-5138 (2017)
- Soth, MJ; Le, K; Di Francesco, ME; Hamilton, MM; Liu, G; Burke, JP; Carroll, CL; Kovacs, JJ; Bardenhagen, JP; Bristow, CA; Cardozo, M; Czako, B; de Stanchina, E; Feng, N; Garvey, JR; Gay, JP; Do, MKG; Greer, J; Han, M; Harris, A; Herrera, Z; Huang, S; Giuliani, V; Jiang, Y; Johnson, SB; Johnson, TA; Kang, Z; Leonard, PG; Liu, Z; McAfoos, T; Miller, M; Morlacchi, P; Mullinax, RA; Palmer, WS; Pang, J; Rogers, N; Rudin, CM; Shepard, HE; Spencer, ND; Theroff, J; Wu, Q; Xu, A; Yau, JA; Draetta, G; Toniatti, C; Heffernan, TP; Jones, P J Med Chem 63: 12957-12977 (2020)
- Villalobos, A; Blake, JF; Biggers, CK; Butler, TW; Chapin, DS; Chen, YL; Ives, JL; Jones, SB; Liston, DR; Nagel, AA J Med Chem 37: 2721-34 (1994)
- Pflimlin, E; Zhou, Z; Amso, Z; Fu, Q; Lee, C; Muppiddi, A; Joseph, SB; Nguyen-Tran, V; Shen, W J Med Chem 63: 382-390 (2020)
- DeCamp, DL; Babé, LM; Salto, R; Lucich, JL; Koo, MS; Kahl, SB; Craik, CS J Med Chem 35: 3426-8 (1992)
- Borthwick, AD; Mills, MT; Brown, JT; Corcoran, JP; De Castro Vasconcelos Goncalves, MB; Kalindjian, SB US Patent US10752616 (2020)
- Lee, YS; Lee, BH; Park, SJ; Kang, SB; Rhim, H; Park, JY; Lee, JH; Jeong, SW; Lee, JY Bioorg Med Chem Lett 14: 3379-84 (2004)
- Chen, P; Doweyko, AM; Norris, D; Gu, HH; Spergel, SH; Das, J; Moquin, RV; Lin, J; Wityak, J; Iwanowicz, EJ; McIntyre, KW; Shuster, DJ; Behnia, K; Chong, S; de Fex, H; Pang, S; Pitt, S; Shen, DR; Thrall, S; Stanley, P; Kocy, OR; Witmer, MR; Kanner, SB; Schieven, GL; Barrish, JC J Med Chem 47: 4517-29 (2004)
- Solomon, VR; Haq, W; Srivastava, K; Puri, SK; Katti, SB J Med Chem 50: 394-8 (2007)
- Charrier, JD; Miller, A; Kay, DP; Brenchley, G; Twin, HC; Collier, PN; Ramaya, S; Keily, SB; Durrant, SJ; Knegtel, RM; Tanner, AJ; Brown, K; Curnock, AP; Jimenez, JM J Med Chem 54: 2341-50 (2011)
- Rich, DH; Green, J; Toth, MV; Marshall, GR; Kent, SB J Med Chem 33: 1285-8 (1990)
- Khan, SB; Hassan Khan, MT; Jang, ES; Akhtar, K; Seo, J; Han, H J Enzyme Inhib Med Chem 25: 812-7 (2010)
- Choi, HG; Ko, E; Cho, J; Son, JB; Ko, YK; Park, J; Kim, SY; Kang, SY; Lee, S; Ryu, HY; Kim, ND; Kim, SB; Lee, S; Kim, D; Lee, SJ; Cho, S; Lee, K; Yu, K; Choi, M; Koo, JW; Hoe, H US Patent US11117892 (2021)
- Gorczynski, MJ; Smitherman, PK; Akiyama, TE; Wood, HB; Berger, JP; King, SB; Morrow, CS J Med Chem 52: 4631-9 (2009)
- Rödl, CB; Vogt, D; Kretschmer, SB; Ihlefeld, K; Barzen, S; Brüggerhoff, A; Achenbach, J; Proschak, E; Steinhilber, D; Stark, H; Hofmann, B Eur J Med Chem 84: 302-11 (2014)
- Cadilla, R; Deaton, DN; Do, Y; Elkins, PA; Ennulat, D; Guss, JH; Holt, J; Jeune, MR; King, AG; Klapwijk, JC; Kramer, HF; Kramer, NJ; Laffan, SB; Masuria, PI; McDougal, AV; Mortenson, PN; Musetti, C; Peckham, GE; Pietrak, BL; Poole, C; Price, DJ; Rendina, AR; Sati, G; Saxty, G; Shearer, BG; Shewchuk, LM; Sneddon, HF; Stewart, EL; Stuart, JD; Thomas, DN; Thomson, SA; Ward, P; Wilson, JW; Xu, T; Youngman, MA Bioorg Med Chem 28: (2020)
- Nzila, A; Rottmann, M; Chitnumsub, P; Kiara, SM; Kamchonwongpaisan, S; Maneeruttanarungroj, C; Taweechai, S; Yeung, BK; Goh, A; Lakshminarayana, SB; Zou, B; Wong, J; Ma, NL; Weaver, M; Keller, TH; Dartois, V; Wittlin, S; Brun, R; Yuthavong, Y; Diagana, TT Antimicrob Agents Chemother 54: 2603-10 (2010)
- Landau, SB; Kagey, MH US Patent US10925881 (2021)
- Jabri, S; Ogawa, AK; Sinz, CJ; Hicks, JD; Cheng, AC; Gao, Y; Yang, S; Bao, J; Hayes, DA; Lang, SB; Taoka, BM; Tian, M; Shearn-Nance, GP; Kuang, R; Lombardo, MJ; Wu, Z; Zhao, Z US Patent US20230286958 (2023)
- Sanghvi, YS; Hanna, NB; Larson, SB; Fujitaki, JM; Willis, RC; Smith, RA; Robins, RK; Revankar, GR J Med Chem 31: 330-5 (1988)
- Gray, WT; Frey, KM; Laskey, SB; Mislak, AC; Spasov, KA; Lee, WG; Bollini, M; Siliciano, RF; Jorgensen, WL; Anderson, KS ACS Med Chem Lett 6: 1075-9 (2015)
- Pratt, LM; Beckett, RP; Davies, SJ; Launchbury, SB; Miller, A; Spavold, ZM; Todd, RS; Whittaker, M Bioorg Med Chem Lett 11: 2585-8 (2001)
- Le Manach, C; Dam, J; Woodland, JG; Kaur, G; Khonde, LP; Brunschwig, C; Njoroge, M; Wicht, KJ; Horatscheck, A; Paquet, T; Boyle, GA; Gibhard, L; Taylor, D; Lawrence, N; Yeo, T; Mok, S; Eastman, RT; Dorjsuren, D; Talley, DC; Guo, H; Simeonov, A; Reader, J; van der Watt, M; Erlank, E; Venter, N; Zawada, JW; Aswat, A; Nardini, L; Coetzer, TL; Lauterbach, SB; Bezuidenhout, BC; Theron, A; Mancama, D; Koekemoer, LL; Birkholtz, LM; Wittlin, S; Delves, M; Ottilie, S; Winzeler, EA; von Geldern, TW; Smith, D; Fidock, DA; Street, LJ; Basarab, GS; Duffy, J; Chibale, K J Med Chem 64: 2291-2309 (2021)
- Kim, J; Song, J; Ji, HD; Yoo, EK; Lee, JE; Lee, SB; Oh, JM; Lee, S; Hwang, JS; Yoon, H; Kim, DS; Lee, SJ; Jeong, M; Lee, S; Kim, KH; Choi, HS; Lee, SW; Park, KG; Lee, IK; Kim, SH; Hwang, H; Jeon, YH; Chin, J; Cho, SJ J Med Chem 62: 1837-1858 (2019)
- Bowser, TE; Bartlett, VJ; Grier, MC; Verma, AK; Warchol, T; Levy, SB; Alekshun, MN Bioorg Med Chem Lett 17: 5652-5 (2007)
- Li, TZ; Hu, J; Sun, JJ; Huang, XY; Geng, CA; Liu, SB; Zhang, XM; Chen, JJ RSC Med Chem 13: 1212-1224 (2022)
- Ghisaidoobe, A; Bikker, P; de Bruijn, AC; Godschalk, FD; Rogaar, E; Guijt, MC; Hagens, P; Halma, JM; Hart, SM; Luitjens, SB; van Rixel, VH; Wijzenbroek, M; Zweegers, T; Donker-Koopman, WE; Strijland, A; Boot, R; Marel, Gv; Overkleeft, HS; Aerts, JM ACS Med Chem Lett 2: 119-123 (2011)
- Christiansen, E; Due-Hansen, ME; Urban, C; Grundmann, M; Schmidt, J; Hansen, SV; Hudson, BD; Zaibi, M; Markussen, SB; Hagesaether, E; Milligan, G; Cawthorne, MA; Kostenis, E; Kassack, MU; Ulven, T J Med Chem 56: 982-92 (2013)
- Boyd, S; Brookfield, JL; Critchlow, SE; Cumming, IA; Curtis, NJ; Debreczeni, J; Degorce, SL; Donald, C; Evans, NJ; Groombridge, S; Hopcroft, P; Jones, NP; Kettle, JG; Lamont, S; Lewis, HJ; MacFaull, P; McLoughlin, SB; Rigoreau, LJ; Smith, JM; St-Gallay, S; Stock, JK; Turnbull, AP; Wheatley, ER; Winter, J; Wingfield, J J Med Chem 58: 3611-25 (2015)
- Jones, MA; Morton, JD; Coxon, JM; McNabb, SB; Lee, HY; Aitken, SG; Mehrtens, JM; Robertson, LJ; Neffe, AT; Miyamoto, S; Bickerstaffe, R; Gately, K; Wood, JM; Abell, AD Bioorg Med Chem 16: 6911-23 (2008)
- Hansch, C; Verma, RP; Kurup, A; Mekapati, SB Bioorg Med Chem Lett 15: 2149-57 (2005)
- Boys, ML; Schretzman, LA; Chandrakumar, NS; Tollefson, MB; Mohler, SB; Downs, VL; Penning, TD; Russell, MA; Wendt, JA; Chen, BB; Stenmark, HG; Wu, H; Spangler, DP; Clare, M; Desai, BN; Khanna, IK; Nguyen, MN; Duffin, T; Engleman, VW; Finn, MB; Freeman, SK; Hanneke, ML; Keene, JL; Klover, JA; Nickols, GA; Nickols, MA; Steininger, CN; Westlin, M; Westlin, W; Yu, YX; Wang, Y; Dalton, CR; Norring, SA Bioorg Med Chem Lett 16: 839-44 (2006)
- Moore, SB; van der Hoek, J; de Capua, A; van Koetsveld, PM; Hofland, LJ; Lamberts, SW; Goodman, M J Med Chem 48: 6643-52 (2005)
- Ebdrup, S; Pettersson, I; Rasmussen, HB; Deussen, HJ; Frost Jensen, A; Mortensen, SB; Fleckner, J; Pridal, L; Nygaard, L; Sauerberg, P J Med Chem 46: 1306-17 (2003)
- Sliskovic, DR; Picard, JA; O'Brien, PM; Liao, P; Roark, WH; Roth, BD; Anderson, MA; Mueller, SB; Bocan, TM; Bousley, RF; Hamelehle, KL; Homan, R; Reindel, JF; Stanfield, RL; Turluck, D; Krause, BR J Med Chem 41: 682-90 (1998)
- Mareddy, J; Nallapati, SB; Anireddy, J; Devi, YP; Mangamoori, LN; Kapavarapu, R; Pal, S Bioorg Med Chem Lett 23: 6721-7 (2013)
- Janssen, CO; Lim, S; Lo, EP; Wan, KF; Yu, VC; Lee, MA; Ng, SB; Everett, MJ; Buss, AD; Lane, DP; Boyce, RS Bioorg Med Chem Lett 18: 5771-3 (2009)
- Cha, MY; Lee, KO; Kim, JW; Lee, CG; Song, JY; Kim, YH; Lee, GS; Park, SB; Kim, MS J Med Chem 52: 6880-8 (2009)
- Gao, YD; Feng, D; Sheridan, RP; Scapin, G; Patel, SB; Wu, JK; Zhang, X; Sinha-Roy, R; Thornberry, NA; Weber, AE; Biftu, T Bioorg Med Chem Lett 17: 3877-9 (2007)
- Pradhan, NS; Patil, NS; Walavalkar, RR; Kulkarni, NS; Pawar, SB; Pawar, TS US Patent US9359341 (2016)
- Wijeratne, A; Xiao, J; Reutter, C; Furness, KW; Leon, R; Zia-Ebrahimi, M; Cavitt, RN; Strelow, JM; Van Horn, RD; Peng, SB; Barda, DA; Engler, TA; Chalmers, MJ ACS Med Chem Lett 9: 557-562 (2018)
- Steadman, VA; Pettit, SB; Poullennec, KG; Lazarides, L; Keats, AJ; Dean, DK; Stanway, SJ; Austin, CA; Sanvoisin, JA; Watt, GM; Fliri, HG; Liclican, AC; Jin, D; Wong, MH; Leavitt, SA; Lee, YJ; Tian, Y; Frey, CR; Appleby, TC; Schmitz, U; Jansa, P; Mackman, RL; Schultz, BE J Med Chem 60: 1000-1017 (2017)
- Dziadulewicz, EK; Ritchie, TJ; Hallett, A; Snell, CR; Davies, JW; Wrigglesworth, R; Dunstan, AR; Bloomfield, GC; Drake, GS; McIntyre, P; Brown, MC; Burgess, GM; Lee, W; Davis, C; Yaqoob, M; Phagoo, SB; Phillips, E; Perkins, MN; Campbell, EA; Davis, AJ; Rang, HP J Med Chem 45: 2160-72 (2002)
- Hopper, AT; Juhl, M; Hornberg, J; Badolo, L; Kilburn, JP; Thougaard, A; Smagin, G; Song, D; Calice, L; Menon, V; Dale, E; Zhang, H; Cajina, M; Nattini, ME; Gandhi, A; Grenon, M; Jones, K; Khayrullina, T; Chandrasena, G; Thomsen, C; Zorn, SH; Brodbeck, R; Poda, SB; Staal, R; Möller, T J Med Chem 64: 4891-4902 (2021)
- Korczynska, M; Le, DD; Younger, N; Gregori-Puigjané, E; Tumber, A; Krojer, T; Velupillai, S; Gileadi, C; Nowak, RP; Iwasa, E; Pollock, SB; Ortiz Torres, I; Oppermann, U; Shoichet, BK; Fujimori, DG J Med Chem 59: 1580-98 (2016)
- Vanover, KE; Weiner, DM; Makhay, M; Veinbergs, I; Gardell, LR; Lameh, J; Del Tredici, AL; Piu, F; Schiffer, HH; Ott, TR; Burstein, ES; Uldam, AK; Thygesen, MB; Schlienger, N; Andersson, CM; Son, TY; Harvey, SC; Powell, SB; Geyer, MA; Tolf, BR; Brann, MR; Davis, RE J Pharmacol Exp Ther 317: 910-8 (2006)
- Kumar, YC; Malviya, M; Chandra, JN; Sadashiva, CT; Kumar, CS; Prasad, SB; Prasanna, DS; Subhash, MN; Rangappa, KS Bioorg Med Chem 16: 5157-63 (2008)
- May, BC; Zorn, JA; Witkop, J; Sherrill, J; Wallace, AC; Legname, G; Prusiner, SB; Cohen, FE J Med Chem 50: 65-73 (2007)
- Munikrishnappa, CS; Puranik, SB; Kumar, GV; Prasad, YR Eur J Med Chem 119: 70-82 (2016)
- Leit, S; Greenwood, J; Carriero, S; Mondal, S; Abel, R; Ashwell, M; Blanchette, H; Boyles, NA; Cartwright, M; Collis, A; Feng, S; Ghanakota, P; Harriman, GC; Hosagrahara, V; Kaila, N; Kapeller, R; Rafi, SB; Romero, DL; Tarantino, PM; Timaniya, J; Toms, AV; Wester, RT; Westlin, W; Srivastava, B; Miao, W; Tummino, P; McElwee, JJ; Edmondson, SD; Masse, CE J Med Chem 66: 10473-10496 (2023)
- Goodfellow, VS; Loweth, CJ; Ravula, SB; Wiemann, T; Nguyen, T; Xu, Y; Todd, DE; Sheppard, D; Pollack, S; Polesskaya, O; Marker, DF; Dewhurst, S; Gelbard, HA J Med Chem 56: 8032-48 (2013)
- Ray, SB; Boinapally, S; Pomper, MG; Horti, A; Das, D; Minn, I; Carroll, L; Cha, H US Patent US20240382629 (2024)
- Koul, S; Ramdas, V; Barawkar, DA; Waman, YB; Prasad, N; Madadi, SK; Shejul, YD; Bonagiri, R; Basu, S; Menon, S; Reddy, SB; Chaturvedi, S; Chennamaneni, SR; Bedse, G; Thakare, R; Gundu, J; Chaudhary, S; De, S; Meru, AV; Palle, V; Chugh, A; Mookhtiar, KA Bioorg Med Chem 25: 1963-1975 (2017)
- Brown, K; Long, JM; Vial, SC; Dedi, N; Dunster, NJ; Renwick, SB; Tanner, AJ; Frantz, JD; Fleming, MA; Cheetham, GM J Biol Chem 279: 18727-32 (2004)
- Lattanzi, R; Spetea, M; Schüllner, F; Rief, SB; Krassnig, R; Negri, L; Schmidhammer, H J Med Chem 48: 3372-8 (2005)
- Ding, M; He, F; Hudyma, TW; Zheng, X; Poss, MA; Kadow, JF; Beno, BR; Rigat, KL; Wang, YK; Fridell, RA; Lemm, JA; Qiu, D; Liu, M; Voss, S; Pelosi, LA; Roberts, SB; Gao, M; Knipe, J; Gentles, RG Bioorg Med Chem Lett 22: 2866-71 (2012)
- Robichaud, J; Bayly, C; Oballa, R; Prasit, P; Mellon, C; Falgueyret, JP; Percival, MD; Wesolowski, G; Rodan, SB Bioorg Med Chem Lett 14: 4291-5 (2004)
- Rong, SB; Enyedy, IJ; Qiao, L; Zhao, L; Ma, D; Pearce, LL; Lorenzo, PS; Stone, JC; Blumberg, PM; Wang, S; Kozikowski, AP J Med Chem 45: 853-60 (2002)
- Zeng, Q; Nair, AG; Rosenblum, SB; Huang, HC; Lesburg, CA; Jiang, Y; Selyutin, O; Chan, TY; Bennett, F; Chen, KX; Venkatraman, S; Sannigrahi, M; Velazquez, F; Duca, JS; Gavalas, S; Huang, Y; Pu, H; Wang, L; Pinto, P; Vibulbhan, B; Agrawal, S; Ferrari, E; Jiang, CK; Li, C; Hesk, D; Gesell, J; Sorota, S; Shih, NY; Njoroge, FG; Kozlowski, JA Bioorg Med Chem Lett 23: 6585-7 (2013)
- Gordon, DE; Jang, GM; Bouhaddou, M; Xu, J; Obernier, K; White, KM; O'Meara, MJ; Rezelj, VV; Guo, JZ; Swaney, DL; Tummino, TA; Hüttenhain, R; Kaake, RM; Richards, AL; Tutuncuoglu, B; Foussard, H; Batra, J; Haas, K; Modak, M; Kim, M; Haas, P; Polacco, BJ; Braberg, H; Fabius, JM; Eckhardt, M; Soucheray, M; Bennett, MJ; Cakir, M; McGregor, MJ; Li, Q; Meyer, B; Roesch, F; Vallet, T; Mac Kain, A; Miorin, L; Moreno, E; Naing, ZZC; Zhou, Y; Peng, S; Shi, Y; Zhang, Z; Shen, W; Kirby, IT; Melnyk, JE; Chorba, JS; Lou, K; Dai, SA; Barrio-Hernandez, I; Memon, D; Hernandez-Armenta, C; Lyu, J; Mathy, CJP; Perica, T; Pilla, KB; Ganesan, SJ; Saltzberg, DJ; Rakesh, R; Liu, X; Rosenthal, SB; Calviello, L; Venkataramanan, S; Liboy-Lugo, J; Lin, Y; Huang, XP; Liu, Y; Wankowicz, SA; Bohn, M; Safari, M; Ugur, FS; Koh, C; Savar, NS; Tran, QD; Shengjuler, D; Fletcher, SJ; O'Neal, MC; Cai, Y; Chang, JCJ; Broadhurst, DJ; Klippsten, S; Sharp, PP; Wenzell, NA; Kuzuoglu-Ozturk, D; Wang, HY; Trenker, R; Young, JM; Cavero, DA; Hiatt, J; Roth, TL; Rathore, U; Subramanian, A; Noack, J; Hubert, M; Stroud, RM; Frankel, AD; Rosenberg, OS; Verba, KA; Agard, DA; Ott, M; Emerman, M; Jura, N; von Zastrow, M; Verdin, E; Ashworth, A; Schwartz, O; d'Enfert, C; Mukherjee, S; Jacobson, M; Malik, HS; Fujimori, DG; Ideker, T; Craik, CS; Floor, SN; Fraser, JS; Gross, JD; Sali, A; Roth, BL; Ruggero, D; Taunton, J; Kortemme, T; Beltrao, P; Vignuzzi, M; García-Sastre, A; Shokat, KM; Shoichet, BK; Krogan, NJ Nature 583: 459-468 (2020)
- Berg, S; Larsson, LG; Rényi, L; Ross, SB; Thorberg, SO; Thorell-Svantesson, G J Med Chem 41: 1934-42 (1998)
- Jalily, PH; Hadfield, JA; Hirst, N; Rossington, SB Bioorg Med Chem Lett 22: 6731-4 (2012)
- Perfetti, MT; Baughman, BM; Dickson, BM; Mu, Y; Cui, G; Mader, P; Dong, A; Norris, JL; Rothbart, SB; Strahl, BD; Brown, PJ; Janzen, WP; Arrowsmith, CH; Mer, G; McBride, KM; James, LI; Frye, SV ACS Chem Biol 10: 1072-81 (2015)
- Pelish, HE; Peterson, JR; Salvarezza, SB; Rodriguez-Boulan, E; Chen, JL; Stamnes, M; Macia, E; Feng, Y; Shair, MD; Kirchhausen, T Nat Chem Biol 2: 39-46 (2006)
- Butler, TW; Blake, JF; Bordner, J; Butler, P; Chenard, BL; Collins, MA; DeCosta, D; Ducat, MJ; Eisenhard, ME; Menniti, FS; Pagnozzi, MJ; Sands, SB; Segelstein, BE; Volberg, W; White, WF; Zhao, D J Med Chem 41: 1172-84 (1998)
- Chang, YT; Wignall, SM; Rosania, GR; Gray, NS; Hanson, SR; Su, AI; Merlie, J; Moon, HS; Sangankar, SB; Perez, O; Heald, R; Schultz, PG J Med Chem 44: 4497-500 (2001)
- Sapkal SB
- Sarikaya, SB; Gülçin, I; Supuran, CT Chem Biol Drug Des 75: 515-20 (2010)
- Kalaba, P; Ilić, M; Aher, NY; Dragačević, V; Wieder, M; Zehl, M; Wackerlig, J; Beyl, S; Sartori, SB; Ebner, K; Roller, A; Lukic, N; Beryozkina, T; Gonzalez, ERP; Neill, P; Khan, JA; Bakulev, V; Leban, JJ; Hering, S; Pifl, C; Singewald, N; Lubec, J; Urban, E; Sitte, HH; Langer, T; Lubec, G J Med Chem 63: 391-417 (2020)
- Pierson, ME; Lyon, RA; Titeler, M; Schulman, SB; Kowalski, P; Glennon, RA J Med Chem 32: 859-63 (1989)
- Sakya, SM; Cheng, H; Lundy Demello, KM; Shavnya, A; Minich, ML; Rast, B; Dutra, J; Li, C; Rafka, RJ; Koss, DA; Li, J; Jaynes, BH; Ziegler, CB; Mann, DW; Petras, CF; Seibel, SB; Silvia, AM; George, DM; Hickman, A; Haven, ML; Lynch, MP Bioorg Med Chem Lett 16: 1202-6 (2006)
- Zhou, Y; Mukherjee, S; Huang, D; Chakraborty, M; Gu, C; Zong, G; Stashko, MA; Pearce, KH; Shears, SB; Chakraborty, A; Wang, H; Wang, X J Med Chem 65: 6869-6887 (2022)
- Hajduk, PJ; Shuker, SB; Nettesheim, DG; Craig, R; Augeri, DJ; Betebenner, D; Albert, DH; Guo, Y; Meadows, RP; Xu, L; Michaelides, M; Davidsen, SK; Fesik, SW J Med Chem 45: 5628-39 (2002)
- Soares de Melo, C; Singh, V; Myrick, A; Simelane, SB; Taylor, D; Brunschwig, C; Lawrence, N; Schnappinger, D; Engelhart, CA; Kumar, A; Parish, T; Su, Q; Myers, TG; Boshoff, HIM; Barry, CE; Sirgel, FA; van Helden, PD; Buchanan, KI; Bayliss, T; Green, SR; Ray, PC; Wyatt, PG; Basarab, GS; Eyermann, CJ; Chibale, K; Ghorpade, SR J Med Chem 64: 719-740 (2021)
- Claremon, DA; Dillard, LW; Dong, C; Fan, Y; Jia, L; Liu, Z; Lotesta, SD; Marcus, A; Singh, SB; Tice, CM; Yuan, J; Zhao, W; Zheng, Y; Zhuang, L US Patent US10399976 (2019)
- Porter, MR; Xiao, H; Wang, J; Smith, SB; Topczewski, JJ ACS Med Chem Lett 10: 1436-1442 (2019)
- Munchhof, MJ; Beebe, JS; Casavant, JM; Cooper, BA; Doty, JL; Higdon, RC; Hillerman, SM; Soderstrom, CI; Knauth, EA; Marx, MA; Rossi, AM; Sobolov, SB; Sun, J Bioorg Med Chem Lett 14: 21-4 (2003)
- Somappa SB
- Li, W; Zhou, W; Song, SB; Shim, SH; Kim, YH J Nat Prod 77: 2611-8 (2014)
- Watts, VJ; Lawler, CP; Gilmore, JH; Southerland, SB; Nichols, DE; Mailman, RB Eur J Pharmacol 242: 165-72 (1993)
- Duan, YT; Sang, YL; Makawana, JA; Teraiya, SB; Yao, YF; Tang, DJ; Tao, XX; Zhu, HL Eur J Med Chem 85: 341-51 (2014)
- Welsch, ME; Zhou, J; Gao, Y; Yan, Y; Porter, G; Agnihotri, G; Li, Y; Lu, H; Chen, Z; Thomas, SB ACS Med Chem Lett 7: 1124-1129 (2016)
- Trattnig, SM; Harpsøe, K; Thygesen, SB; Rahr, LM; Ahring, PK; Balle, T; Jensen, AA J Biol Chem 287: 25241-54 (2012)
- Gong, J; Gan, J; Caceres-Cortes, J; Christopher, LJ; Arora, V; Masson, E; Williams, D; Pursley, J; Allentoff, A; Lago, M; Tran, SB; Iyer, RA Drug Metab Dispos 39: 891-903 (2011)
- Cutshall, NS; Gage, JL; Gruswitz, FA; Khalaf, J; Little, TL; Metz, M; Nguyen, JH; Nollert von Specht, PK; Tsoung, J; Cicirelli, M; Goldstein, SR; Keshipeddy, SK; Kwon, DY; Lemus, RH; Vaddela, SB US Patent US12195427 (2025)
- Vogensen, SB; Jørgensen, L; Madsen, KK; Borkar, N; Wellendorph, P; Skovgaard-Petersen, J; Schousboe, A; White, HS; Krogsgaard-Larsen, P; Clausen, RP J Med Chem 56: 2160-4 (2013)
- Roughley, SD; Browne, H; Macias, AT; Benwell, K; Brooks, T; D'Alessandro, J; Daniels, Z; Dugdale, S; Francis, G; Gibbons, B; Hart, T; Haymes, T; Kennett, G; Lightowler, S; Matassova, N; Mansell, H; Merrett, A; Misra, A; Padfield, A; Parsons, R; Pratt, R; Robertson, A; Simmonite, H; Tan, K; Walls, SB; Wong, M Bioorg Med Chem Lett 22: 901-6 (2012)
- Wang, Z; Wong, IL; Li, FX; Yang, C; Liu, Z; Jiang, T; Jiang, TF; Chow, LM; Wan, SB Bioorg Med Chem 23: 5566-73 (2015)
- Yoon, JS; Kim, G; Jarhad, DB; Kim, HR; Shin, YS; Qu, S; Sahu, PK; Kim, HO; Lee, HW; Wang, SB; Kong, YJ; Chang, TS; Ogando, NS; Kovacikova, K; Snijder, EJ; Posthuma, CC; van Hemert, MJ; Jeong, LS J Med Chem 62: 6346-6362 (2019)
- Poss, MA; Gu, Z; Ryono, DE; Reid, JA; Sieber-McMaster, E; Spitzmiller, ER; Dejneka, T; Dickinson, KE; Williams, SB; Moreland, S; Delaney, CL; Bird, J; Waldron, TL; Schaeffer, TR; Hedberg, S; Petrillo, EW Bioorg Med Chem Lett 4: 145-150 (1994)
- Xu, Y; Rito, CJ; Etgen, GJ; Ardecky, RJ; Bean, JS; Bensch, WR; Bosley, JR; Broderick, CL; Brooks, DA; Dominianni, SJ; Hahn, PJ; Liu, S; Mais, DE; Montrose-Rafizadeh, C; Ogilvie, KM; Oldham, BA; Peters, M; Rungta, DK; Shuker, AJ; Stephenson, GA; Tripp, AE; Wilson, SB; Winneroski, LL; Zink, R; Kauffman, RF; McCarthy, JR J Med Chem 47: 2422-5 (2004)
- Lange, JH; Venhorst, J; van Dongen, MJ; Frankena, J; Bassissi, F; de Bruin, NM; den Besten, C; de Beer, SB; Oostenbrink, C; Markova, N; Kruse, CG Eur J Med Chem 46: 4808-19 (2011)
- Bech, EM; Martos-Maldonado, MC; Wismann, P; Sørensen, KK; van Witteloostuijn, SB; Thygesen, MB; Vrang, N; Jelsing, J; Pedersen, SL; Jensen, KJ J Med Chem 60: 7434-7446 (2017)
- Höfling, SB; Maschauer, S; Hübner, H; Gmeiner, P; Wester, HJ; Prante, O; Heinrich, MR Bioorg Med Chem Lett 20: 6933-7 (2010)
- Oztürk Sarikaya, SB; Topal, F; Sentürk, M; Gülçin, I; Supuran, CT Bioorg Med Chem Lett 21: 4259-62 (2011)
- ChEBML_153639 inhibition of [125I]SB 236636 binding to human PPAR gamma receptor
- ChEBML_153641 Inhibition of [125I]SB 236636 binding to human PPAR gamma receptor
- ChEMBL_153640 (CHEMBL762705) Inhibition of [125I]SB 236636 binding to human PPAR gamma receptor
- ChEMBL_740085 (CHEMBL1763145) Displacement [3H]SB-222200 from of human recombinant NK3 receptor expressed in CHO cells
- ChEMBL_1978 (CHEMBL617583) Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement.
- ChEMBL_1283183 (CHEMBL3101094) Displacement of [3H]SB-674042 from human orexin-1 receptor after 60 mins by scintillation counting analysis
- ChEMBL_1283184 (CHEMBL3101095) Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis
- ChEMBL_1929601 (CHEMBL4432777) Inhibition of PI3Kdelta in human CCRF-SB cells assessed as reduction in AktS473 phosphorylation levels by Western blot analysis
- ChEMBL_1929604 (CHEMBL4432780) Inhibition of PI3Kdelta in human CCRF-SB cells assessed as reduction in S6 phosphorylation levels by Western blot analysis
- ChEMBL_766683 (CHEMBL1827377) Displacement of [125I]SB-258585 from human recombinant 5HT6 receptor using methiothepin after 45 mins by liquid scintillation spectrometry
- ChEMBL_1615912 (CHEMBL3857981) Displacement of [3H]-SB-26997 from human 5-HT7A receptor expressed in HEK293 cell membranes after 60 mins by microbeta counting analysis
- ChEMBL_201063 (CHEMBL805839) Compound was evaluated for its ability to inhibit the binding of 5-HT4 receptor radioligand [125I]SB 207710 to piglet hippocampal membrane
- ChEMBL_2492613 Displacement of [3H]-SB-222200 from recombinant human NK3R expressed in CHO-K1 cells membrane incubated for 2 hrs by microbeta scintillation counting method
- ChEMBL_2067502 (CHEMBL4722755) Inhibition of STAT3 in human SB-590885-sensitive 451Lu cells assessed as reduction in STAT3 transcriptional activity measured after 12 hrs by Dual-luciferase reporter gene assay
- ChEMBL_2067503 (CHEMBL4722756) Inhibition of STAT3 in human SB-590885-resistant 451Lu cells assessed as reduction in STAT3 transcriptional activity measured after 12 hrs by Dual-luciferase reporter gene assay
- ChEMBL_2067504 (CHEMBL4722757) Inhibition of STAT3 in human SB-590885-sensitive MEL1617 cells assessed as reduction in STAT3 transcriptional activity measured after 12 hrs by Dual-luciferase reporter gene assay
- ChEMBL_2067505 (CHEMBL4722758) Inhibition of STAT3 in human SB-590885-resistant MEL1617 cells assessed as reduction in STAT3 transcriptional activity measured after 12 hrs by Dual-luciferase reporter gene assay
- Biological Assay The in vitro affinity of the compounds for the human orexin-1 and orexin-2 receptors was determined by competitive radioligand binding using [3H]SB SB674042 (1-(5-(2-fluoro-phenyl)-2-methyl-thiazol-4-yl)-1-((S)-2-(5-phenyl-(1,3,4)oxadiazol-2-ylmethyl)-pyrrolidin-1-yl)-methanone) (Langmead et al., British Journal of Pharmacology 2004, 141:340-346) and [3H]EMPA (N-ethyl-2[(6-methoxy-pyridin-3-yl)-(toluene-2-sulfonyl)-amino]-N-pyridin-3-ylmethyl acetamide) (Malherbe et al., British Journal of Pharmacology, 2009, 156(8), 1326-1341), respectively. The in vitro functional antagonism of the compounds on the human orexin-1 and orexin-2 receptors was determined using fluorometric imaging plate reader (FLIPR) based calcium assays.
- Binding Activity Assay Human 5-HT2A Receptor, Human 5-HT7 Receptor, and Human D2 Receptor: Binding affinity of the present compound for human 5-HT2A receptor, human 5-HT7 receptor, and human D2 receptor was measured by the following procedures.CHO cell membrane fraction in which human 5-HT2A receptor, human 5-HT7 receptor, or human D2 receptor was expressed was purchased from PerkinElmer, Inc. In a test for evaluating binding affinity, a test compound dissolved in dimethylsulfoxide (DMSO) and each receptor membrane sample diluted in buffer were mixed with [3H]Ketanserin, [3H]SB-269970, or [3H]Spiperone (all purchased from PerkinElmer, Inc.) for 5-HT2A receptor, 5-HT7 receptor, or D2 receptor, respectively. Each mixture was incubated at room temperature for 60 minutes. Non specific binding to receptors was obtained from a competitive binding test in the presence of 10 μmol/L 8-OH-DPAT, 10 μmol/L Mianserin, or 10 μmol/L Spiperone, respectively. Radioactivity caused by binding to receptors was measured with a liquid scintillation counter (PerkinElmer, Inc.), and 50% inhibition concentration was calculated.
- Receptor Binding Assay (1) The prepared membrane was first applied with appropriate amount of homogenized liquid, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container, and appropriate amount of homogenized liquid was added to give 50 ml of membrane suspension, which was reserved for future use.(2) 100 uL of membrane preparation and 100 uL of buffer were added into each reaction tube.(3) 100 uL of homogenized liquid was added into the total binding tube (TB), 100 uL of mianserin (final concentration 10-5 M) was added into the non-specific binding tube (NB), and 100 uL of the test compound (final concentration 10-5 M) was added into the specific binding tube (SB) for each compound.(4) 10 uL of radioactive ligand [3H]-mesulergine was respectively added into each reaction tube (2 parallel tubes were used for each reaction tube, and each of them was placed on ice when adding sample).(5) Each of the reaction tubes was incubated at 37 degree.
- 5-HT2C Binding Assay 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 was transferred to an assay plate according to the plate map for nonspecific binding (Low control: LC). 1 μL of DMSO was transferred to an assay plate according to the plate map for total binding (High control: HC).Unifilter-96 GF/C filter plates were soaked with 50 μL of 0.3% PEI per well for about 0.5 hours at room temperature. When the binding assays were complete, the reaction mixture was filtered through the GF/C filter plates using Perkin Elmer Filtermate Harvester, and each plate was washed 4 times with cold wash buffer. The filter plates were dried for 1 hour at 50° C. After drying, the bottom of the filter plate wells were sealed using Perkin Elmer Unifilter-96 backing seal tape. 50 μL of Perkin Elmer Microscint 20 cocktail was added. The top of the filter plates were sealed with Perkin Elmer TopSeal-A sealing film.
- PDE3A Enzyme Inhibition Assay For the determination of the in vitro effect of example compounds on the PDE3A reactions 2 μl of the respective example compound solution in DMSO (serial dilutions) were placed in wells of microtiter plates (Isoplate-96/200W; Perkin Elmer). 50 μl of a dilution of PDE3A cell extract from Sf9 cells overexpressing human full length PDE3A (SB Drug Discovery, UK) in buffer A (50 mM Tris/HCl pH 7.5, 8.3 mM MgCl2, 1.7 mM EDTA, 0.2% BSA) was added. The dilution of the PDE3A cell extract was chosen such that the reaction kinetics was linear and less than 70% of the substrate was consumed (typical dilution 1:5000). The reaction was started by addition of 50 μl (0.025 μCi) of 1:2000 in buffer A w/o BSA diluted substrate [8-3H]adenosine 3′, 5′-cyclic phosphate (1 μCi/μl; Perkin Elmer). After incubation at room temperature for 60 min, the reaction was stopped by addition of 25 μl of a suspension containing 18 mg/ml yttrium scintillation proximity beads (Perkin Elmer) in water. The microtiter plates were sealed and measured in a Microbeta scintillation counter (PerkinElmer Wallac).
- Receptor Binding Assay 5-HT2C: A solution containing 50 μL of [H]-mesulergine (GE Healthcare) diluted with 50 mmol/L Tris-HCl (pH=7.4) (the final concentration: about 2 nM), 149 μL of 5-HT2C/CHO cell membrane sample (20 μg/well for the amount of protein), and 1 μL of a solution of a test compound dissolved in DMSO or a solvent (DMSO) was reacted at 37° C. for 30 minutes, and then filtered promptly by aspiration under lower pressure with a glass fiber filter coated with a 1% aqueous solution of bovine serum albumin. The filtered substance on the glass fiber filter was washed with 250 μL of 50 mmol/L Tris-HCl (pH=7.4) twice, and then transferred to an ACS-II (Amersham) 4-mL-glass vial. The radioactivity of the filtered substance remained on the filter was measured by liquid scintillation counter. The radioactivity value measured by liquid scintillation counter was deemed to be a receptor binding activity, and the binding inhibition was calculated from the total binding (TB), non-specific binding (NSB), and specific binding (SB) of a test compound according to the following equations.
- Sert Binding Assay Specifically, a solution containing 50 μL of [3H]-citalopram (GE Healthcare) diluted with SERT buffer (50 mmol/L Tris-HCl (pH=7.4) containing 120 mmol/L NaCl and 5 mmol/L KCl) (the final concentration: about 2 nmol/L), 149 μL of h-SERT/CHO cell membrane sample (40 μg/well for the amount of protein), and 1 μL of a solution of a test compound in DMSO or a solvent (DMSO) was reacted at room temperature for 60 minutes, and then filtered promptly by aspiration under lower pressure with a glass fiber filter coated with a 0.05% aqueous polyethyleneimine solution. The filtered substance on the glass fiber filter was washed with 250 μL of SERT buffer twice, and then transferred to an ACS-II (Amersham) 4-mL-glass vial. The radioactivity of the filtered substance remained on the filter was measured by liquid scintillation counter. The radioactivity value measured by liquid scintillation counter was deemed to be a receptor binding activity, and the binding inhibition was calculated from the total binding (TB), non-specific binding (NSB), and specific binding (SB) of a test compound according to the following equations.
- Receptor Binding Assay 5-HT2A: A solution containing 50 μL of [H]-ketanserin (the final concentration: 1 nM), 2 μL of a solution of a test compound in DMSO or a solvent (DMSO), and 148 μL of human 5-HT receptor-expressed CHO cell membrane sample was reacted in a 50 mmol/L Tris-HCl buffer (pH=7.6), and then was let stand at 37° C. for 15 minutes. Then, the resultant was added promptly to a glass fiber filter plate (Multiscreen FB, Millipore) coated with 0.05% Brij 35 and filtered under reduced pressure. The filtered substance on the glass fiber filter was washed with 200 μL of ice-cooled 50 mmol/L Tris-HCl (pH=7.6) twice and repeated to be filtered under reduced pressure, followed by transfer to a vial containing 2 mL of Ecoscint A (National Diagnostics). The radioactivity of the filtered substance remained on the glass fiber filter was measured by liquid scintillation counter. The radioactivity value measured by liquid scintillation counter was deemed to be a receptor binding activity, and the binding inhibition was calculated from the total binding (TB), non-specific binding (NSB), and specific binding (SB) of a test compound according to the following equations.
- Dopamine D2L Receptor Binding Assay The human D2L receptor binding activity of [3H]-spiperone was determined as follows. A solution containing 50 μL of [3H]-spiperone (the final concentration: 0.5 nmol/L), 2 μL of a solution of a test compound in DMSO or a solvent (DMSO), and 148 μL of human D2L receptor-expressed CHO cell membrane sample was reacted in a 50 mmol/L Tris-HCl (pH=7.6) buffer, and then was let stand at room temperature for 60 minutes. Then, the resultant was added promptly to a glass fiber filter plate (Multiscreen FB, Millipore) coated with 0.3% polyethyleneimine (PEI) and filtered under reduced pressure. The filtered substance on the glass fiber filter was washed with 200 μL of ice-cooled 50 mmol/L Tris-HCl (pH=7.6) twice and repeated to be filtered under reduced pressure, followed by transfer to a vial containing 2 mL of Ecoscint A (National Diagnostics). The radioactivity of the filtered substance remained on the glass fiber filter was measured by liquid scintillation counter. The radioactivity value measured by liquid scintillation counter was deemed to be a receptor binding activity, and the binding inhibition was calculated from the total binding (TB), non-specific binding (NSB), and specific binding (SB) of a test drug according to the following equations.
- PDE3B Enzyme Inhibition Assay The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determination of the in vitro effect of example compounds on the PDE3B reactions 2 μl of the respective example compound solution in DMSO (serial dilutions) were placed in wells of microtiter plates (Isoplate-96/200W; Perkin Elmer). 50 μl of a dilution of PDE3B cell extract from Sf9 cells overexpressing human full length PDE3B (SB Drug Discovery, UK) in buffer A (50 mM Tris/HCl pH 7.5, 8.3 mM MgCl2, 1.7 mM EDTA, 0.2% BSA) was added. The dilution of the PDE3B cell extract was chosen such that the reaction kinetics was linear and less than 70% of the substrate was consumed (typical dilution 1:6000). The reaction was started by addition of 50 μl (0.025 μCi) of 1:2000 in buffer A w/o BSA diluted substrate [8-3H]adenosine 3′, 5′-cyclic phosphate (1 μCi/μl; Perkin Elmer). After incubation at room temperature for 60 min, the reaction was stopped by addition of 25 μl of a suspension containing 18 mg/ml yttrium scintillation proximity beads (Perkin Elmer) in water. The microtiter plates were sealed and measured in a Microbeta scintillation counter (PerkinElmer Wallac). IC50 values were determined from sigmoidal curves by plotting percentage PDE3B activity vs log compound concentration.
- Receptor Binding Assay 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container, and appropriate amount of buffer was added to give 50 ml of membrane suspension, which was reserved for future use. (2) 100 μL of membrane preparation and 100 μL of buffer were added into each reaction tube. (3) 100 μL of buffer was added into the total binding tube (TB), 100 μL of 5-HT (final concentration 10-5M) was added into the nonspecific binding tube (NB), 100 μL of the test compound (final concentration 10-5M) was added into the specific binding tube (SB) of each test compound. (4) 10 μL of radioactive ligand 3H-8-OH-DPAT was respectively added into each reaction tube (2 parallel tubes were used for each reaction tube, and each of them was placed on ice when adding sample). (5) Each reaction tube was incubated at 37° C. for 10 min; after the reaction was completed, the bound ligands were rapidly filtered under reduced pressure, and sufficiently washed with ice-chilled assay buffer. The filter was taken out and put into a 3 ml scintillation vial, and 2 ml of toluene scintillation cocktail was added and blended.
- Receptor Binding Assay 5-HT7: (1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container, and appropriate amount of buffer was added to give 50 ml of membrane suspension, which was reserved for future use. (2) 100 μL of membrane preparation and 100 μL of buffer were added into each reaction tube. (3) 100 μL of buffer was added into the total binding tube (TB), 100 μL of ( )-pindolol (final concentration 10-5M) was added into the nonspecific binding tube (NB), 100 μL of the test compound (final concentration 10-5M) was added into the specific binding tube (SB) of each test compound. (4) 10 μL of radioactive ligand 3H-5-CT was respectively added into each reaction tube (2 parallel tubes were used for each reaction tube, and each of them was placed on ice when adding sample). (5) Each of the reaction tubes was incubated at 25° C. for 120 min. After the reaction was completed, the bound ligands were rapidly filtered under reduced pressure, and sufficiently washed with ice-chilled assay buffer. The filter was taken out and put into a 3 ml scintillation vial, and 2 ml of toluene scintillation cocktail was added and blended.
- PDE3A Enzyme Inhibition The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determination of the in vitro effect of example compounds on the PDE3A reactions 2 μl of the respective example compound solution in DMSO (serial dilutions) were placed in wells of microtiter plates (Isoplate-96/200W; Perkin Elmer). 50 μl of a dilution of PDE3A cell extract from Sf9 cells overexpressing human full length PDE3A (SB Drug Discovery, UK) in buffer A (50 mM Tris/HCl pH 7.5, 8.3 mM MgCl2, 1.7 mM EDTA, 0.2% BSA) was added. The dilution of the PDE3A cell extract was chosen such that the reaction kinetics was linear and less than 70% of the substrate was consumed (typical dilution 1:5000). The reaction was started by addition of 50 μl (0.025 μCi) of 1:2000 in buffer A w/o BSA diluted substrate [8-3H]adenosine 3′, 5′-cyclic phosphate (1 μCi/μl; Perkin Elmer). After incubation at room temperature for 60 min, the reaction was stopped by addition of 25 μl of a suspension containing 18 mg/ml yttrium scintillation proximity beads (Perkin Elmer) in water. The microtiter plates were sealed and measured in a Microbeta scintillation counter (PerkinElmer Wallac). IC50 values were determined from sigmoidal curves by plotting percentage PDE3A activity vs log compound concentration.
- PDE3A Enzyme Inhibition The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determination of the in vitro effect of test substances on the PDE3A reactions 2 μl of the respective test compound solution in DMSO (serial dilutions) were is placed in wells of microtiter plates (Isoplate-96/200W; Perkin Elmer). 50 μl of a dilution of PDE3A cell extract from Sf9 cells overexpressing human full length PDE3A (SB Drug Discovery, UK) in buffer A (50 mM Tris/HCl pH 7.5, 8.3 mM MgCl2, 1.7 mM EDTA, 0.2% BSA) was added. The dilution of the PDE3A cell extract was chosen such that the reaction kinetics was linear and less than 70% of the substrate was consumed (typical dilution 1:5000). The reaction was started by addition of 50 μl (0.025 μCi) of 1:2000 in buffer A w/o BSA diluted substrate [8-3H] adenosine 3′,5′-cyclic phosphate (1 μCi/μl; Perkin Elmer). After incubation at room temperature for 60 min, the reaction was stopped by addition of 25 μl of a suspension containing 18 mg/ml yttrium scintillation proximity beads (Perkin Elmer) in water. The microtiter plates were sealed and measured in a Microbeta scintillation counter (PerkinElmer Wallac). IC50 values were determined from sigmoidal curves by plotting percentage PDE3A activity vs log compound concentration.
- PDE3B Enzyme Inhibition The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determination of the in vitro effect of example compounds on the PDE3B reactions 2 μl of the respective example compound solution in DMSO (serial dilutions) were placed in wells of microtiter plates (Isoplate-96/200W; Perkin Elmer). 50 μl of a dilution of PDE3B cell extract from Sf9 cells overexpressing human full length PDE3B (SB Drug Discovery, UK) in buffer A (50 mM Tris/HCl pH 7.5, 8.3 mM MgCl2, 1.7 mM EDTA, 0.2% BSA) was added. The dilution of the PDE3B cell extract was chosen such that the reaction kinetics was linear and less than 70% of the substrate was consumed (typical dilution 1:6000). The reaction was started by addition of 50 μl (0.025 μCi) of 1:2000 in buffer A w/o BSA diluted substrate [8-3H]adenosine 3′, 5′-cyclic phosphate (1 μCi/μl; Perkin Elmer). After incubation at room temperature for 60 min, the reaction was stopped by addition of 25 μl of a suspension containing 18 mg/ml yttrium scintillation proximity beads (Perkin Elmer) in water. The microtiter plates were sealed and measured in a Microbeta scintillation counter (PerkinElmer Wallac). IC50 values were determined from sigmoidal curves by plotting percentage PDE3B activity vs log compound concentration.
- PDE3B Enzyme Inhibition The commercially available 3H-cAMP Scintillation Proximity Assay (SPA, Perkin Elmer) system was used for enzyme inhibition studies. For the determination of the in vitro effect of test substances on the PDE3B reactions 2 μl of the respective test compound solution in DMSO (serial dilutions) were placed in wells of microtiter plates (Isoplate-96/200W; Perkin Elmer). 50 μl of a dilution of PDE3B cell extract from Sf9 cells overexpressing human full length PDE3B (SB Drug Discovery, UK) in buffer A (50 mM Tris/HCl pH 7.5, 8.3 mM MgCl2, 1.7 mM EDTA, 0.2% BSA) was added. The dilution of the PDE3B cell extract was chosen such that the reaction kinetics was linear and less than 70% of the substrate was consumed (typical dilution 1:6000). The reaction was started by addition of 50 μl (0.025 μCi) of 1:2000 in buffer A w/o BSA diluted substrate [8-3H] adenosine 3′,5′-cyclic phosphate (1 μCi/μl; Perkin Elmer). After incubation at room temperature for 60 min, the reaction was stopped by addition of 25 μl of a suspension containing 18 mg/ml yttrium scintillation proximity beads (Perkin Elmer) in water. The microtiter plates were sealed and measured in a Microbeta scintillation counter (PerkinElmer Wallac). IC50 values were determined from sigmoidal curves by plotting percentage PDE3B activity vs log compound concentration.
- Receptor Binding Assay 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container, and appropriate amount of buffer was added to give 50 ml of membrane suspension, which was reserved for future use.(2) 100 μL of membrane preparation and 100 μL of buffer were added into each reaction tube.(3) 100 μL of buffer was added into the total binding tube (TB), 100 μL of Methysergide (final concentration 10−5M) was added into the nonspecific binding tube (NB), 100 μL of the test compound (final concentration 10−5M) was added into the specific binding tube (SB) of each test compound.(4) 10 μL of radioactive ligand 3H-Ketanserin was respectively added into each reaction tube (2 parallel tubes were used for each reaction tube, and each of them was placed on ice when adding sample).(5) Each of the reaction tubes was incubated at 37° C. for 15 min. After the reaction was completed, the bound ligands were rapidly filtered under reduced pressure, and sufficiently washed with ice-chilled assay buffer. The filter was taken out and put into a 3 ml scintillation vial, and 2 ml of toluene scintillation cocktail was added and blended.(6) The scintillation vial were put into Liquid Scintillation Counter for counting.Inhibition rate(I%)=(Total binding tube cpm−compound cpm)/(Total binding tube cpm−nonspecific binding tube cpm)×100% .
- Receptor Binding Assay D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container, and appropriate amount of buffer was added to give 50 ml of membrane suspension, which was reserved for future use.(2) 100 μL of membrane preparation and 100 μL of buffer were added into each reaction tube.(3) 100 μL of buffer was added into the total binding tube (TB), 100 μL of Butaclamol (final concentration 10−5M) was added into the nonspecific binding tube (NB), 100 μL of the test compound (final concentration 10−5M) was added into the specific binding tube (SB) of each test compound.(4) 10 μL of radioactive ligand 3H-Spiperone was respectively added into each reaction tube (2 parallel tubes were used for each reaction tube, and each of them was placed on ice when adding sample).(5) Each of the reaction tubes was incubated at 37° C. for 20 min. After the reaction was completed, the bound ligands were rapidly filtered under reduced pressure, and sufficiently washed with ice-chilled assay buffer. The filter was taken out and put into a 3 ml scintillation vial, and 2 ml of toluene scintillation cocktail was added and blended.(6) The scintillation vials were put into Liquid Scintillation Counter for counting.Inhibition rate(I%)=(Total binding tube cpm−compound cpm)/(Total binding tube cpm−nonspecific binding tube cpm)×100% .
- Receptor Binding Assay Histamine H1: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container, and appropriate amount of buffer (potassium dihydrogen phosphate 1.36 g, 0.1 mol/L sodium hydroxide 79 ml, metered to 200 ml with double-distilled water) was added to give 50 ml of membrane suspension, which was reserved for future use. (2) 100 uL of membrane preparation was added into each reaction tube. (3) 100 uL of buffer was added into the total binding tube (TB), 100 uL of promethazine (final concentration 10-5M) was added into the nonspecific binding tube (NB), 100 uL of the test compound (final concentration 10-5M) was added into the specific binding tube (SB) of each test compound. (4) 10 uL of radioactive ligand 3H-pyrilamine was respectively added into each reaction tube (2 parallel tubes were used for each reaction tube, and each of them was placed on ice when adding sample). (5) Each of the reaction tubes was incubated at 30° C. for 60 min. After the reaction was completed, the bound ligands were rapidly filtered under reduced pressure, and the ice-chilled assay buffer was used for adequate washing. The filter was taken out and put into a 3 ml scintillation vial, and 2 ml of toluene scintillation solution was added and blended. 2* Isotope ligand [3H]-5-CT (85.4 Ci/mmol) was purchased from PerkinElmer Company; (+-)-pindolol was purchased from RBI Company; GF/C glass fiber filter paper was purchased from Whatman Company; Tris was imported and divided into aliquots; PPO and POPOP were purchased from Shanghai No. 1 Reagent Factory; liposoluble scintillation cocktail was purchased from Shanghai Reagent Factory. Beckman LS-6500 Multi-function Liquid Scintillation Counter was used.
- Radioligand Binding Assay Assessments of compound binding to human MC5R (hMC5R)) by displacement of an 125I-labeled NDP-MSH receptor ligand peptide were performed essentially as described in the data sheets produced by Perkin Elmer to accompany their frozen hMC5R membranes (Perkin Elmer catalog number RBXMC5M400UA). [125I] NDP-MSH: Radiolabeled in House and Purified by HPLC: Na125I (0.5 mCi, 17.4 Ci/mg) was added to 50 μL sodium phosphate (50 mM, pH 7.4) in an eppendorf tube precoated with IODOGEN. After incubation for 10 mins the phosphate buffer containing the iodine was added to NDP-MSH (10 μl at 1 mg/mL) in a separate eppendorf tube. This was incubated for a further 10 mins. The iodinated NDP-MSH was purified by HPLC on a Zorbax SB 300 column using solvent A: 0.05% TFA and solvent B: 90% acetonitrile 0.045% TFA with a linear gradient, 0-67% B over 60 mins. The 125I NDP-MSH eluted at 52 mins after the unlabeled starting material (48 min) and was counted and stored in the freezer. It was used within 48 hrs, as radioactive decay and ligand decomposition resulted in greatly reduced specific binding observed after 72 hrs. Incubation buffer: 25 mM HEPES-KOH (pH 7.0), 1.5 mM CaCl2, 1 mM MgSO4, 0.1 M NaCl, 1 mM 1,10-phenanthroline, and 1 Complete™ protease inhibitor tablet/100 mL (Roche, catalog number 1873580). Perkin Elmer frozen hMC5 membranes: catalog number RBXMC5M400UA, 0.4 mL/vial; 400 microassays/vial, 0.78 mg/mL protein concentration. Vials of frozen membranes were thawed rapidly immediately before use, diluted with binding buffer and vortexed. Resuspended membranes were kept on ice until they were added to the wells of the plate. Assays were performed in 96 well polypropylene plates. Membranes (0.78 μg 40 μL of a 1:40 dilution in incubation buffer) were added to [125I] NDP-MSH (0.84 nM; 2200 Ci/mmol) and test compounds in a total volume of 140 μL. This was incubated for 1 hr at 37° C. Non-specific binding was determined with 3 mM NDP-MSH. Plates were filtered using a Tomtec cell harvester with GF/A filters (Wallac) (presoaked in 0.6% polyethylenimine) and washed three times with 1.0 mL ice-cold wash buffer (the above incubation buffer without 1,10-phenanthroline and Complete™ protease inhibitor tablet). The filters were dried in a 37° C. oven, placed in a sample bag and 5 mL Betaplatescint (Wallac) was added. Prepared filters were counted in cassettes in a Microbeta Trilux (Wallac) for 1 min.
 BDBM50045333 SB-203580 CHEBI:90705
BDBM50045333 SB-203580 CHEBI:90705 BDBM85859 SB-215505 SB 215505
BDBM85859 SB-215505 SB 215505 SB 234551 SB-234551 BDBM85335
SB 234551 SB-234551 BDBM85335 SB-791016A CHEMBL251572 BDBM50412954 SB-791016
SB-791016A CHEMBL251572 BDBM50412954 SB-791016 BDBM50417257 SB-649868
BDBM50417257 SB-649868 BDBM50419052 SB-399885
BDBM50419052 SB-399885 BDBM50448074 Emindole Sb
BDBM50448074 Emindole Sb SB-258585 BDBM86428
SB-258585 BDBM86428 BDBM50026798 SB-742548 CHEMBL1956072
BDBM50026798 SB-742548 CHEMBL1956072 BDBM50371155 CHEMBL245237 SB-611113
BDBM50371155 CHEMBL245237 SB-611113 BDBM50372467 CHEMBL271102 SB-429201
BDBM50372467 CHEMBL271102 SB-429201 BDBM50400870 CHEMBL559569 SB-612111
BDBM50400870 CHEMBL559569 SB-612111 BDBM50403164 CHEMBL156977 SB-205149
BDBM50403164 CHEMBL156977 SB-205149 BDBM50411414 SB-347804 CHEMBL397570
BDBM50411414 SB-347804 CHEMBL397570 BDBM50412120 SB-714786 CHEMBL425190
BDBM50412120 SB-714786 CHEMBL425190 BDBM50469657 SB-202742 CHEMBL33995
BDBM50469657 SB-202742 CHEMBL33995 CHEMBL1334465 BDBM50423648 SB-408124
CHEMBL1334465 BDBM50423648 SB-408124 CHEMBL1824446 SB-505124 BDBM50608551
CHEMBL1824446 SB-505124 BDBM50608551 CHEMBL191971 BDBM50410435 SB-272183
CHEMBL191971 BDBM50410435 SB-272183 CHEMBL2110360 BDBM50475902 SB-568849
CHEMBL2110360 BDBM50475902 SB-568849 CHEMBL2325429 SB-743921 BDBM50427294
CHEMBL2325429 SB-743921 BDBM50427294 CHEMBL233002 SB-814597 BDBM50411419
CHEMBL233002 SB-814597 BDBM50411419 CHEMBL244083 SB-414796 BDBM50411370
CHEMBL244083 SB-414796 BDBM50411370 CHEMBL358119 SB-207058 BDBM50421360
CHEMBL358119 SB-207058 BDBM50421360 CHEMBL406800 SB-379278A BDBM50372468
CHEMBL406800 SB-379278A BDBM50372468 CHEMBL565091 BDBM50480755 SB-02066
CHEMBL565091 BDBM50480755 SB-02066 CHEMBL85606 BDBM50370572 SB-277011
CHEMBL85606 BDBM50370572 SB-277011 SB 200646 BDBM82272 SB200646a
SB 200646 BDBM82272 SB200646a SB-0304 BDBM50376593 CHEMBL409225
SB-0304 BDBM50376593 CHEMBL409225 SB-203238 CHEMBL506279 BDBM50473786
SB-203238 CHEMBL506279 BDBM50473786 SB-207043 BDBM50366325 CHEMBL606910
SB-207043 BDBM50366325 CHEMBL606910 SB-331711 CHEMBL97158 BDBM50001825
SB-331711 CHEMBL97158 BDBM50001825 SB-410220 BDBM50423650 CHEMBL522758
SB-410220 BDBM50423650 CHEMBL522758 SB-590885 BDBM50457452 SB590885
SB-590885 BDBM50457452 SB590885 SB-649701 BDBM50423246 CHEMBL245568
SB-649701 BDBM50423246 CHEMBL245568 SB-649915 BDBM50412114 CHEMBL183460
SB-649915 BDBM50412114 CHEMBL183460 SB-659032 Rilapladib BDBM50205805
SB-659032 Rilapladib BDBM50205805 SB-699551 CHEMBL1181770 BDBM50585908
SB-699551 CHEMBL1181770 BDBM50585908 SB-744185 BDBM50412441 CHEMBL490417
SB-744185 BDBM50412441 CHEMBL490417 4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine SB-203580 4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine BDBM13336 cid_176155 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine CHEMBL10 SB203580
4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine SB-203580 4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine BDBM13336 cid_176155 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine CHEMBL10 SB203580 BDBM50423649 CHEMBL2413521 SB-674042 CHEMBL2110363
BDBM50423649 CHEMBL2413521 SB-674042 CHEMBL2110363 BDBM540760 US11261211, Compound SB-49
BDBM540760 US11261211, Compound SB-49 BDBM540761 US11261211, Compound SB-50
BDBM540761 US11261211, Compound SB-50 BDBM540770 US11261211, Compound SB-12
BDBM540770 US11261211, Compound SB-12 BDBM540771 US11261211, Compound SB-13
BDBM540771 US11261211, Compound SB-13 BDBM540772 US11261211, Compound SB-14
BDBM540772 US11261211, Compound SB-14 BDBM540773 US11261211, Compound SB-15
BDBM540773 US11261211, Compound SB-15 BDBM540790 US11261211, Compound SB-41
BDBM540790 US11261211, Compound SB-41 BDBM540802 US11261211, Compound SB-40
BDBM540802 US11261211, Compound SB-40 CHEMBL291536 CHEMBL2111553 SB-334867 BDBM50384416
CHEMBL291536 CHEMBL2111553 SB-334867 BDBM50384416 US11261211, Compound SB-1 BDBM540768
US11261211, Compound SB-1 BDBM540768 US11261211, Compound SB-17 BDBM540775
US11261211, Compound SB-17 BDBM540775 US11261211, Compound SB-2 BDBM540769
US11261211, Compound SB-2 BDBM540769 US11261211, Compound SB-26 BDBM540786
US11261211, Compound SB-26 BDBM540786 US11261211, Compound SB-35 BDBM540780
US11261211, Compound SB-35 BDBM540780 US11261211, Compound SB-37 BDBM540797
US11261211, Compound SB-37 BDBM540797 US11261211, Compound SB-38 BDBM540798
US11261211, Compound SB-38 BDBM540798 US11261211, Compound SB-39 BDBM540791
US11261211, Compound SB-39 BDBM540791 US11261211, Compound SB-45 BDBM540776
US11261211, Compound SB-45 BDBM540776 US11261211, Compound SB-46 BDBM540777
US11261211, Compound SB-46 BDBM540777 US11261211, Compound SB-53 BDBM540778
US11261211, Compound SB-53 BDBM540778 US11261211, Compound SB-56 BDBM540779
US11261211, Compound SB-56 BDBM540779 US11261211, Compound SB-60 BDBM540796
US11261211, Compound SB-60 BDBM540796 Idoxifene SB-223030 BDBM50219403 CB-7432
Idoxifene SB-223030 BDBM50219403 CB-7432 SB-615575 TCMDC-142445 CHEMBL2098048 BDBM50557135
SB-615575 TCMDC-142445 CHEMBL2098048 BDBM50557135 SB-207266 BDBM85026 N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethyleneoxy)-1H-indole-3-carboxamide SB 207266
SB-207266 BDBM85026 N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethyleneoxy)-1H-indole-3-carboxamide SB 207266 BDBM50388914 US10357546, p-OH SB-3CT CHEMBL2063274
BDBM50388914 US10357546, p-OH SB-3CT CHEMBL2063274 BDBM706133 SB-FAP-11 US20240382629, Example 22
BDBM706133 SB-FAP-11 US20240382629, Example 22 BDBM706134 SB-FAP-09 US20240382629, Example 23
BDBM706134 SB-FAP-09 US20240382629, Example 23 BDBM706135 SB-FAP-01 US20240382629, Example i
BDBM706135 SB-FAP-01 US20240382629, Example i BDBM706136 SB-FAP-02 US20240382629, Example ii
BDBM706136 SB-FAP-02 US20240382629, Example ii BDBM706138 US20240382629, Example iv SB-FAP-06
BDBM706138 US20240382629, Example iv SB-FAP-06 BDBM706139 SB-FAP-07 US20240382629, Example v
BDBM706139 SB-FAP-07 US20240382629, Example v BDBM706143 US20240382629, Example ix SB-FAP-08
BDBM706143 US20240382629, Example ix SB-FAP-08 CAS_181632-25-7 BDBM85097 SB 242084 CHEMBL14563
CAS_181632-25-7 BDBM85097 SB 242084 CHEMBL14563 ONX-0803 SB1518 Pacritinib BDBM50210177 SB-1518
ONX-0803 SB1518 Pacritinib BDBM50210177 SB-1518 SB-FAP-04 US20240382629, Example viii BDBM706142
SB-FAP-04 US20240382629, Example viii BDBM706142 SB-FAP-10 US20240382629, Example vi BDBM706140
SB-FAP-10 US20240382629, Example vi BDBM706140 US20240382629, Example iii BDBM706137 SB-FAP-03
US20240382629, Example iii BDBM706137 SB-FAP-03 BDBM85166 US20240166639, Example SB41 CAS_3292447 SB 216641 NSC_3292447
BDBM85166 US20240166639, Example SB41 CAS_3292447 SB 216641 NSC_3292447 US10865384, Compound SB202190 CHEBI:79090 SB-202190 BDBM50044784
US10865384, Compound SB202190 CHEBI:79090 SB-202190 BDBM50044784 5-Methyl-3,5-dihydro-2H-pyrrolo[2,3-f]indole-1-carboxylic acid pyridin-3-ylamide SB 206553 BDBM50060417 CHEMBL297784 SB-206553
5-Methyl-3,5-dihydro-2H-pyrrolo[2,3-f]indole-1-carboxylic acid pyridin-3-ylamide SB 206553 BDBM50060417 CHEMBL297784 SB-206553 N-hydroxy-N-(3-phenylpropyl)formamide BDBM21683 SB-485345
N-hydroxy-N-(3-phenylpropyl)formamide BDBM21683 SB-485345 BDBM28583 SB 271046 SB-271046 5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-methyl-1-benzothiophene-2-sulfonamide hydrochloride CHEMBL431298
BDBM28583 SB 271046 SB-271046 5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-methyl-1-benzothiophene-2-sulfonamide hydrochloride CHEMBL431298 3-benzenesulfonyl-8-piperazin-1-ylquinoline CHEMBL1083390 BDBM50318633 SB-742457
3-benzenesulfonyl-8-piperazin-1-ylquinoline CHEMBL1083390 BDBM50318633 SB-742457 N-[2-(3-benzoylphenoxy)ethyl]-N-hydroxyformamide SB-543668 BDBM21684
N-[2-(3-benzoylphenoxy)ethyl]-N-hydroxyformamide SB-543668 BDBM21684 SB-223412 3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide CHEMBL10188 Talnetant SB-2234 BDBM50051293 SB 223412 (S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinoline-4-carboxamide 3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid (1-phenyl-propyl)-amide
SB-223412 3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide CHEMBL10188 Talnetant SB-2234 BDBM50051293 SB 223412 (S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinoline-4-carboxamide 3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid (1-phenyl-propyl)-amide (2E)-3-(4-chlorophenyl)-N-(3-methoxyphenyl)prop-2-enamide BDBM20488 SB-366791
(2E)-3-(4-chlorophenyl)-N-(3-methoxyphenyl)prop-2-enamide BDBM20488 SB-366791 1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea CHEMBL239767 SB-225002 BDBM50203012
1-(2-bromophenyl)-3-(2-hydroxy-4-nitrophenyl)urea CHEMBL239767 SB-225002 BDBM50203012 SB-505684 BDBM21685 N-hydroxy-N-[3-(6-methylpyridin-2-yl)propyl]formamide
SB-505684 BDBM21685 N-hydroxy-N-[3-(6-methylpyridin-2-yl)propyl]formamide (1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy-ethoxy)-4-methoxy-phenyl]-5-propoxy-indan-2-carboxylic acid SB-217242 Enrasentan SB 217242 CHEMBL431651 BDBM50061077
(1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-[2-(2-hydroxy-ethoxy)-4-methoxy-phenyl]-5-propoxy-indan-2-carboxylic acid SB-217242 Enrasentan SB 217242 CHEMBL431651 BDBM50061077 1-(1-methylindol-5-yl)-3-pyridin-3-ylurea hydrochloride BDBM84990 SB 200646 SB200646
1-(1-methylindol-5-yl)-3-pyridin-3-ylurea hydrochloride BDBM84990 SB 200646 SB200646 3-(2-bromophenyl)-1-{2-[ethyl(3-methylphenyl)amino]ethyl}urea SB-452533 BDBM20468
3-(2-bromophenyl)-1-{2-[ethyl(3-methylphenyl)amino]ethyl}urea SB-452533 BDBM20468 BDBM50248241 Cetrorelix Acetate D-20761 CHEBI:31387 Cetrotide NS-75A SB-075 Acetate Cetrorelix
BDBM50248241 Cetrorelix Acetate D-20761 CHEBI:31387 Cetrotide NS-75A SB-075 Acetate Cetrorelix SB 235375 N-[(S)-1-Phenylpropyl]-2-phenyl-3-(carboxymethoxy)quinoline-4-carboxamide BDBM85845
SB 235375 N-[(S)-1-Phenylpropyl]-2-phenyl-3-(carboxymethoxy)quinoline-4-carboxamide BDBM85845 (15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1,6,8(13),9,11,20(25),21,23,26-nonaene-3,5-dione SB-218078 SB 218078 BDBM17140 SB218078
(15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1,6,8(13),9,11,20(25),21,23,26-nonaene-3,5-dione SB-218078 SB 218078 BDBM17140 SB218078 BDBM50263384 CHEMBL514691 6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazol-5-yl)nicotinamide SB-782443
BDBM50263384 CHEMBL514691 6-(4-fluorophenyl)-2-methyl-N-(2-methylbenzothiazol-5-yl)nicotinamide SB-782443 CHEMBL134641 SB-202026 BDBM50061705 (R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino]-acetonitrile
CHEMBL134641 SB-202026 BDBM50061705 (R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino]-acetonitrile N-{3-[2-(3,4-Dimethoxy-phenyl)-ethylamino]-propyl}-4-nitro-benzamide CHEMBL184461 SB-237376 BDBM50151854
N-{3-[2-(3,4-Dimethoxy-phenyl)-ethylamino]-propyl}-4-nitro-benzamide CHEMBL184461 SB-237376 BDBM50151854 3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid 3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid(SB-213068) BDBM50085043 CHEMBL306229 SB-213068
3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid 3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid(SB-213068) BDBM50085043 CHEMBL306229 SB-213068 2-((4-phenoxyphenylsulfonyl)methyl)thiirane CHEMBL483857 BDBM50264809 Thiirane (deuterium), 1-d2 Thiirane, 1 US10357546, SB-3CT
2-((4-phenoxyphenylsulfonyl)methyl)thiirane CHEMBL483857 BDBM50264809 Thiirane (deuterium), 1-d2 Thiirane, 1 US10357546, SB-3CT NSC-760125 Rolipram ZK 62 711 ZK-62711 (r,s)-rolipram BDBM50639239 ZK-62771 SB-95952
NSC-760125 Rolipram ZK 62 711 ZK-62711 (r,s)-rolipram BDBM50639239 ZK-62771 SB-95952 5-Methylsulfanyl-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid pyridin-3-ylamide SB 221284 CHEMBL276140 BDBM50060418
5-Methylsulfanyl-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid pyridin-3-ylamide SB 221284 CHEMBL276140 BDBM50060418 CHEMBL1164033 BDBM50320477 SB-611812 2,6-dichloro-N-(4-chloro-3-(2-(dimethylamino)ethoxy)phenyl)-4-(trifluoromethyl)benzenesulfonamide
CHEMBL1164033 BDBM50320477 SB-611812 2,6-dichloro-N-(4-chloro-3-(2-(dimethylamino)ethoxy)phenyl)-4-(trifluoromethyl)benzenesulfonamide CHEMBL292759 SB-214111 BDBM50073056 4-Bromo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulfonamide
CHEMBL292759 SB-214111 BDBM50073056 4-Bromo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulfonamide 3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-2,5-dihydro-1H-pyrrole-2,5-dione BDBM8297 SB-415286
3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-2,5-dihydro-1H-pyrrole-2,5-dione BDBM8297 SB-415286 CHEMBL401570 6-(2-tert-butyl-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl)quinoxaline BDBM50320952 SB-525334
CHEMBL401570 6-(2-tert-butyl-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl)quinoxaline BDBM50320952 SB-525334 CHEMBL90214 Ticolubant BDBM50052027 (E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phenethyloxy-pyridin-2-yl]-acrylic acid SB-209247
CHEMBL90214 Ticolubant BDBM50052027 (E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phenethyloxy-pyridin-2-yl]-acrylic acid SB-209247 3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoline-4-carboxylic acid (1,2,2-trimethyl-propyl)-amide SB-414240 BDBM50099640 CHEMBL295770
3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoline-4-carboxylic acid (1,2,2-trimethyl-propyl)-amide SB-414240 BDBM50099640 CHEMBL295770 4-{3-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-imidazol-1-yl]-propyl}-morpholine SB-210313 BDBM50053424 CHEMBL14112
4-{3-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-imidazol-1-yl]-propyl}-morpholine SB-210313 BDBM50053424 CHEMBL14112 CHEMBL522770 BDBM50249878 (R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin-3-yloxy)-4-(trifluoromethyl)phenyl)benzenesulfonamide SB-706375
CHEMBL522770 BDBM50249878 (R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin-3-yloxy)-4-(trifluoromethyl)phenyl)benzenesulfonamide SB-706375 N2-[(2,2-diphenylethoxy)acetyl]-L-arginine (S)-2-(2-(2,2-diphenylethoxy)acetamido)-5-guanidinopentanoic acid CHEMBL389348 SB-290157 BDBM50322650
N2-[(2,2-diphenylethoxy)acetyl]-L-arginine (S)-2-(2-(2,2-diphenylethoxy)acetamido)-5-guanidinopentanoic acid CHEMBL389348 SB-290157 BDBM50322650 CHEMBL82663 4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfonyl)-ethylamino]-ethyl}-3H-benzothiazol-2-one SB-07499 BDBM50128690
CHEMBL82663 4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfonyl)-ethylamino]-ethyl}-3H-benzothiazol-2-one SB-07499 BDBM50128690 3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione BDBM8296 CHEMBL102714 cid_176158 SB-216763
3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione BDBM8296 CHEMBL102714 cid_176158 SB-216763 BDBM20504 1-(2-bromophenyl)-3-[(3R)-1-[5-(trifluoromethyl)pyridin-2-yl]pyrrolidin-3-yl]urea BMCL163287 Compound 15 SB-705498
BDBM20504 1-(2-bromophenyl)-3-[(3R)-1-[5-(trifluoromethyl)pyridin-2-yl]pyrrolidin-3-yl]urea BMCL163287 Compound 15 SB-705498 CHEMBL371720 BDBM50173166 SB-227931 N-{3-[4-(3-Azido-phenyl)-5-pyridin-4-yl-imidazol-1-yl]-propyl}-4-iodo-benzamide
CHEMBL371720 BDBM50173166 SB-227931 N-{3-[4-(3-Azido-phenyl)-5-pyridin-4-yl-imidazol-1-yl]-propyl}-4-iodo-benzamide BDBM50041617 SB 209670 SB-209670 (1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymethoxy-4-methoxy-phenyl)-5-propoxy-indan-2-carboxylic acid CHEMBL8823 1-benzo[d][1,3]dioxol-5-ylmethyl-3-(2-carboxymethoxy-4-methoxyphenyl)-5-propoxy-(1R,3S)-2,3-dihydro-1H-2-indenecarboxylic acid
BDBM50041617 SB 209670 SB-209670 (1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymethoxy-4-methoxy-phenyl)-5-propoxy-indan-2-carboxylic acid CHEMBL8823 1-benzo[d][1,3]dioxol-5-ylmethyl-3-(2-carboxymethoxy-4-methoxyphenyl)-5-propoxy-(1R,3S)-2,3-dihydro-1H-2-indenecarboxylic acid BDBM50054827 SB-208651 {8-[(4-Carbamimidoyl-phenyl)-methyl-carbamoyl]-3-oxo-4-phenethyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid {8-[(4-Carbamimidoyl-phenyl)-methyl-carbamoyl]-3-oxo-4-phenethyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid(SB-208651) CHEMBL85094
BDBM50054827 SB-208651 {8-[(4-Carbamimidoyl-phenyl)-methyl-carbamoyl]-3-oxo-4-phenethyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid {8-[(4-Carbamimidoyl-phenyl)-methyl-carbamoyl]-3-oxo-4-phenethyl-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid(SB-208651) CHEMBL85094 SB-587094 BDBM17443 1,2,4-Triazole Compound, 102 N-[2-(propan-2-yl)phenyl]-5-[(thiophen-2-ylmethyl)sulfanyl]-4H-1,2,4-triazol-3-amine
SB-587094 BDBM17443 1,2,4-Triazole Compound, 102 N-[2-(propan-2-yl)phenyl]-5-[(thiophen-2-ylmethyl)sulfanyl]-4H-1,2,4-triazol-3-amine sodium 1-amino-4-(4-methoxyphenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate SB-416 BDBM50227023 1-amino-4-(4-methoxyphenyl)-2-sulfoanthraquinone CHEMBL401735
sodium 1-amino-4-(4-methoxyphenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate SB-416 BDBM50227023 1-amino-4-(4-methoxyphenyl)-2-sulfoanthraquinone CHEMBL401735 SB-258510 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-amide BDBM50130286 CHEMBL29846
SB-258510 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-amide BDBM50130286 CHEMBL29846 (1E)-5-[5-(morpholin-4-ylmethyl)-2-(pyridin-4-yl)furan-3-yl]-2,3-dihydro-1H-indene-1-hydroxylamine BMCL184373 Compound 17 SB-699393 BDBM26056
(1E)-5-[5-(morpholin-4-ylmethyl)-2-(pyridin-4-yl)furan-3-yl]-2,3-dihydro-1H-indene-1-hydroxylamine BMCL184373 Compound 17 SB-699393 BDBM26056 BDBM18131 SB-243545 butyl (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3S,4S,5S)-1,3,4,5-tetrahydroxy-4-(hydroxymethyl)piperidin-2-yl]acetate
BDBM18131 SB-243545 butyl (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3S,4S,5S)-1,3,4,5-tetrahydroxy-4-(hydroxymethyl)piperidin-2-yl]acetate CHEMBL436624 SB-731489 BDBM50220156 (R)-N-(3-aminopropyl)-N-(1-(3-benzyl-7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-2-methylpropyl)-3-fluoro-4-methylbenzamide
CHEMBL436624 SB-731489 BDBM50220156 (R)-N-(3-aminopropyl)-N-(1-(3-benzyl-7-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-2-methylpropyl)-3-fluoro-4-methylbenzamide SB-239629 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3S,4S,5S)-1,3,4,5-tetrahydroxy-4-(hydroxymethyl)piperidin-2-yl]acetic acid BDBM18130
SB-239629 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3S,4S,5S)-1,3,4,5-tetrahydroxy-4-(hydroxymethyl)piperidin-2-yl]acetic acid BDBM18130 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methyloxan-2-yl]acetic acid SB-284485 BDBM18132 CHEMBL163022
(2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-methyloxan-2-yl]acetic acid SB-284485 BDBM18132 CHEMBL163022 CHEMBL490262 N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide SB-298 CHEMBL521280 BDBM50268085
CHEMBL490262 N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide SB-298 CHEMBL521280 BDBM50268085 [3H]-N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide BDBM50268276 SB-298 CHEMBL521280
[3H]-N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide BDBM50268276 SB-298 CHEMBL521280 (S)-3-methyl-2-phenyl-N-(1-phenylpropyl)quinoline-4-carboxamide CHEMBL10284 3-Methyl-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide BDBM50051295 SB-222200
(S)-3-methyl-2-phenyl-N-(1-phenylpropyl)quinoline-4-carboxamide CHEMBL10284 3-Methyl-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide BDBM50051295 SB-222200 BDBM25391 [2-(4-{4-[(1E)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5-yl]-5-(pyridin-4-yl)-1H-imidazol-2-yl}phenoxy)ethyl]dimethylamine SB-590885 CHEMBL200622 SB590885
BDBM25391 [2-(4-{4-[(1E)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5-yl]-5-(pyridin-4-yl)-1H-imidazol-2-yl}phenoxy)ethyl]dimethylamine SB-590885 CHEMBL200622 SB590885 3,N-Dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide 3,N-Dimethyl-N-[(R)-1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide 4-Methyl-1-[(R)-3-methyl-4-(toluene-3-sulfonyl)-pentyl]-piperidine SB-258719 BDBM50098550 CHEMBL12264 3,N-Dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide (SB-258719)
3,N-Dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide 3,N-Dimethyl-N-[(R)-1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide 4-Methyl-1-[(R)-3-methyl-4-(toluene-3-sulfonyl)-pentyl]-piperidine SB-258719 BDBM50098550 CHEMBL12264 3,N-Dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)-propyl]-benzenesulfonamide (SB-258719) CHEMBL329383 N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide BDBM50130268 N-(2,5-Dibromo-3-fluoro-phenyl)-4-methoxy-3-piperazin-1-yl-benzenesulfonamide SB-357134
CHEMBL329383 N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-(piperazin-1-yl)benzenesulfonamide BDBM50130268 N-(2,5-Dibromo-3-fluoro-phenyl)-4-methoxy-3-piperazin-1-yl-benzenesulfonamide SB-357134 1-{2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-oxo-ethyl}-2-(4-fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimidin-5-ylmethyl)-1H-pyrimidin-4-one BDBM50102161 SB-381320 CHEMBL56954
1-{2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-oxo-ethyl}-2-(4-fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimidin-5-ylmethyl)-1H-pyrimidin-4-one BDBM50102161 SB-381320 CHEMBL56954 3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phenyl)-pyrrole-2,5-dione CHEMBL322970 SB415286 BDBM50130725 3-(3-chloro-4-hydroxyphenylamino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione SB-415286 cid_4210951
3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phenyl)-pyrrole-2,5-dione CHEMBL322970 SB415286 BDBM50130725 3-(3-chloro-4-hydroxyphenylamino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione SB-415286 cid_4210951 BDBM18128 SB-219383 CHEMBL310012 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(1S,3S,4S,5R,8R)-2,4,5,8-tetrahydroxy-7-oxa-2-azabicyclo[3.2.1]octan-3-yl]acetic acid
BDBM18128 SB-219383 CHEMBL310012 (2S)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-2-[(1S,3S,4S,5R,8R)-2,4,5,8-tetrahydroxy-7-oxa-2-azabicyclo[3.2.1]octan-3-yl]acetic acid CHEMBL74395 SB-203207 (4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pentanoylsulfamoyl)-acetylamino]-4-carbamoyl-6-hydroxy-2-methyl-2,4a,5,6,7,7a-hexahydro-1H-[2]pyrindine-7-carboxylic acid BDBM50093003
CHEMBL74395 SB-203207 (4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pentanoylsulfamoyl)-acetylamino]-4-carbamoyl-6-hydroxy-2-methyl-2,4a,5,6,7,7a-hexahydro-1H-[2]pyrindine-7-carboxylic acid BDBM50093003 SB242235 BDBM15458 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]-2-methoxypyrimidine 4-[4-(4-fluorophenyl)-1-piperidin-4-yl-1H-imidazol-5-yl]-2-methoxypyrimidine CHEMBL95692 SB-242235
SB242235 BDBM15458 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]-2-methoxypyrimidine 4-[4-(4-fluorophenyl)-1-piperidin-4-yl-1H-imidazol-5-yl]-2-methoxypyrimidine CHEMBL95692 SB-242235 BDBM50343509 SB-203580 4-(4-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-5-yl)pyridine 4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-2,3-dihydro-1H-imidazol-4-yl)pyridine 4-[5-(3-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-3H-imidazol-4-yl]-pyridine 4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-4-yl)pyridine 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-1H-imidazol-4-yl]-pyridine 4-(2-(4-(methylsulfinyl)phenyl)-4-phenyl-1H-imidazol-5-yl)pyridine 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-3H-imidazol-4-yl]-pyridine CHEMBL10 4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfoxide-phenyl)-3H-imidazol-4-yl]-pyridine 4-[5-(4-Fluorophenyl)-2-[4-(methylsulfonyl)phenyl]-1H-imidazol-4-yl]pyridine
BDBM50343509 SB-203580 4-(4-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-5-yl)pyridine 4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-2,3-dihydro-1H-imidazol-4-yl)pyridine 4-[5-(3-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-3H-imidazol-4-yl]-pyridine 4-(5-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-4-yl)pyridine 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-1H-imidazol-4-yl]-pyridine 4-(2-(4-(methylsulfinyl)phenyl)-4-phenyl-1H-imidazol-5-yl)pyridine 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-phenyl)-3H-imidazol-4-yl]-pyridine CHEMBL10 4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfoxide-phenyl)-3H-imidazol-4-yl]-pyridine 4-[5-(4-Fluorophenyl)-2-[4-(methylsulfonyl)phenyl]-1H-imidazol-4-yl]pyridine BDBM6763 SB-218078 28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8(13),9,11,20(25),21,23-nonaene-3,5-dione
BDBM6763 SB-218078 28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8(13),9,11,20(25),21,23-nonaene-3,5-dione SB-13 BDBM50173647 CHEMBL381642 4-N-methylamino-4'-hydroxystilbene 4-(4-(methylamino)styryl)phenol 4-[(E)-2-(4-Methylamino-phenyl)-vinyl]-phenol [3H]-4-(4-(methylamino)styryl)phenol (E)-4-(4-(methylamino)styryl)phenol
SB-13 BDBM50173647 CHEMBL381642 4-N-methylamino-4'-hydroxystilbene 4-(4-(methylamino)styryl)phenol 4-[(E)-2-(4-Methylamino-phenyl)-vinyl]-phenol [3H]-4-(4-(methylamino)styryl)phenol (E)-4-(4-(methylamino)styryl)phenol 3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-yl}-acrylic acid BDBM50042182 (E)-3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-yl}-acrylic acid CHEMBL112338 SB-201146
3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-yl}-acrylic acid BDBM50042182 (E)-3-{6-(3-Amino-benzenesulfinylmethyl)-3-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-yl}-acrylic acid CHEMBL112338 SB-201146 SB-656104 6-((R)-2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulfonyl)-1H-indole BDBM50130295 CHEMBL95104 6-(2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulfonyl)-1H-indole
SB-656104 6-((R)-2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulfonyl)-1H-indole BDBM50130295 CHEMBL95104 6-(2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulfonyl)-1H-indole 4-(5-ethyl-2-(4-methoxyphenyl)-1H-imidazol-4-yl)pyridine CHEMBL278724 BDBM50284510 4-(4-ethyl-2-(4-methoxyphenyl)-1H-imidazol-5-yl)pyridine 4-[5-Ethyl-2-(4-methoxy-phenyl)-3H-imidazol-4-yl]-pyridine SB-202474
4-(5-ethyl-2-(4-methoxyphenyl)-1H-imidazol-4-yl)pyridine CHEMBL278724 BDBM50284510 4-(4-ethyl-2-(4-methoxyphenyl)-1H-imidazol-5-yl)pyridine 4-[5-Ethyl-2-(4-methoxy-phenyl)-3H-imidazol-4-yl]-pyridine SB-202474 SB-271046 5-chloro-N-(4-methoxy-3-(piperazin-1-yl)phenyl)-3-methylbenzo[b]thiophene-2-sulfonamide 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid (4-methoxy-3-piperazin-1-yl-phenyl)-amide BDBM50090525 CHEMBL431298
SB-271046 5-chloro-N-(4-methoxy-3-(piperazin-1-yl)phenyl)-3-methylbenzo[b]thiophene-2-sulfonamide 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid (4-methoxy-3-piperazin-1-yl-phenyl)-amide BDBM50090525 CHEMBL431298 TAN-67 BDBM50290872 Tan-67 (racemic) CHEMBL327745 SB-213698 (-)-rel-3-((4aR,12aS)-2-methyl-1,2,3,4,4a,5,12,12a-octahydropyrido[3,4-b]acridin-4a-yl)phenol 3-((4aS,12aR)-2-Methyl-1,3,4,5,12,12a-hexahydro-2H-2,6-diaza-naphthacen-4a-yl)-phenol
TAN-67 BDBM50290872 Tan-67 (racemic) CHEMBL327745 SB-213698 (-)-rel-3-((4aR,12aS)-2-methyl-1,2,3,4,4a,5,12,12a-octahydropyrido[3,4-b]acridin-4a-yl)phenol 3-((4aS,12aR)-2-Methyl-1,3,4,5,12,12a-hexahydro-2H-2,6-diaza-naphthacen-4a-yl)-phenol CHEMBL85606 N-(-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide SB-277011-A SB-277011 BDBM50167898 Quinoline-4-carboxylic acid {4-[2-(6-cyano-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-cyclohexyl}-amide N-((1r,4r)-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)ethyl]-cyclohexyl]-4-quinolininecarboxamide trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)-ethyl]cyclo-hexyl]-4-quinolinecarboxamide
CHEMBL85606 N-(-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide SB-277011-A SB-277011 BDBM50167898 Quinoline-4-carboxylic acid {4-[2-(6-cyano-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-cyclohexyl}-amide N-((1r,4r)-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)ethyl]-cyclohexyl]-4-quinolininecarboxamide trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2yl)-ethyl]cyclo-hexyl]-4-quinolinecarboxamide BDBM50327858 SB207710 (1-Butylpiperidin-4-yl)methyl 8-amino-7-iodo-2,3-dihydrobenzo-[b][1,4]dioxine-5-carboxylate CHEMBL114112 8-Amino-7-iodo-2,3-dihydro-benzo[1,4]dioxine-5-carboxylic acid 1-butyl-piperidin-4-ylmethyl ester SB-207710
BDBM50327858 SB207710 (1-Butylpiperidin-4-yl)methyl 8-amino-7-iodo-2,3-dihydrobenzo-[b][1,4]dioxine-5-carboxylate CHEMBL114112 8-Amino-7-iodo-2,3-dihydro-benzo[1,4]dioxine-5-carboxylic acid 1-butyl-piperidin-4-ylmethyl ester SB-207710 {(S)-3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid {3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid BDBM50078714 SB-265123 CHEMBL288493
{(S)-3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid {3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid BDBM50078714 SB-265123 CHEMBL288493 5-Methyl-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid [6-(2-methyl-pyridin-3-yloxy)-pyridin-3-yl]-amide CHEMBL14460 5-methyl-N-(6-(2-methylpyridin-3-yloxy)pyridin-3-yl)-6-(trifluoromethyl)indoline-1-carboxamide BDBM50086065 SB-243213
5-Methyl-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid [6-(2-methyl-pyridin-3-yloxy)-pyridin-3-yl]-amide CHEMBL14460 5-methyl-N-(6-(2-methylpyridin-3-yloxy)pyridin-3-yl)-6-(trifluoromethyl)indoline-1-carboxamide BDBM50086065 SB-243213 BDBM50060416 SB-242084 6-Chloro-5-methyl-2,3-dihydro-indole-1-carboxylic acid [6-(2-methyl-pyridin-3-yloxy)-pyridin-3-yl]-amide 6-chloro-5-methyl-N-(6-(2-methylpyridin-3-yloxy)pyridin-3-yl)indoline-1-carboxamide CHEMBL14563
BDBM50060416 SB-242084 6-Chloro-5-methyl-2,3-dihydro-indole-1-carboxylic acid [6-(2-methyl-pyridin-3-yloxy)-pyridin-3-yl]-amide 6-chloro-5-methyl-N-(6-(2-methylpyridin-3-yloxy)pyridin-3-yl)indoline-1-carboxamide CHEMBL14563 (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl)phenol SB-269970 3-{(R)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol BDBM50098551 CHEMBL282199 3-{2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidin-1-ylsulfonyl)phenol 3-{2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol (SB-269970) 3-{(S)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol
(R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl)phenol SB-269970 3-{(R)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol BDBM50098551 CHEMBL282199 3-{2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidin-1-ylsulfonyl)phenol 3-{2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol (SB-269970) 3-{(S)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrrolidine-1-sulfonyl}-phenol [2'-Methyl-4'-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-yl]-[3-(1-methyl-piperidine)-2,3,6,7-tetrahydro-1-oxa-5-aza-s-indacen-5-yl]-methanone US20240166639, Example SB9 11'-Methyl-5-[[2'-methyl-4'-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydrospiro[furo-[2,3-f]indole-3,4'-piperidine](SB-224289) BDBM50084959 SB-224289 4-[2-methyl-4-(5-methyl-1,2,4-oxadiazol-3-yl)phenyl]phenyl-1'-methylspiro[3,5,6,7-tetrahydro-2H-furo[2,3-f]indole-3,4'-(hexahydropyridine)]-5-ylmethanone CHEMBL281350
[2'-Methyl-4'-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-yl]-[3-(1-methyl-piperidine)-2,3,6,7-tetrahydro-1-oxa-5-aza-s-indacen-5-yl]-methanone US20240166639, Example SB9 11'-Methyl-5-[[2'-methyl-4'-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydrospiro[furo-[2,3-f]indole-3,4'-piperidine](SB-224289) BDBM50084959 SB-224289 4-[2-methyl-4-(5-methyl-1,2,4-oxadiazol-3-yl)phenyl]phenyl-1'-methylspiro[3,5,6,7-tetrahydro-2H-furo[2,3-f]indole-3,4'-(hexahydropyridine)]-5-ylmethanone CHEMBL281350 CHEMBL1824446 SB-505124 US11759530, Compound Table1.2 2-(5-(benzo[d][1,3]dioxol-5-yl)-2-tert-butyl-1H-imidazol-4-yl)-6-methylpyridine CHEMBL226838 2-(4-(benzo[d][1,3]dioxol-5-yl)-2-tert-butyl-1H-imidazol-5-yl)-6-methylpyridine BDBM50298220
CHEMBL1824446 SB-505124 US11759530, Compound Table1.2 2-(5-(benzo[d][1,3]dioxol-5-yl)-2-tert-butyl-1H-imidazol-4-yl)-6-methylpyridine CHEMBL226838 2-(4-(benzo[d][1,3]dioxol-5-yl)-2-tert-butyl-1H-imidazol-5-yl)-6-methylpyridine BDBM50298220 (S)-N-(1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-yl)-3,4-dichlorobenzamide SB-436811 (S)-3,4-dichloro-N-(1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-yl)benzamide CHEMBL366221 3,4-Dichloro-N-{(S)-1-[4-(3-dimethylamino-propoxy)-benzyl]-pyrrolidin-3-yl}-benzamide BDBM50240963
(S)-N-(1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-yl)-3,4-dichlorobenzamide SB-436811 (S)-3,4-dichloro-N-(1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-yl)benzamide CHEMBL366221 3,4-Dichloro-N-{(S)-1-[4-(3-dimethylamino-propoxy)-benzyl]-pyrrolidin-3-yl}-benzamide BDBM50240963 US10736881, Compound 72 BDBM50248476 SB-639 US8551988, 72 N-hydroxy-3-(2-phenethyl-1-(2-(pyrrolidin-1-yl)ethyl)-1H-benzo[d]imidazol-5-yl)acrylamide CHEMBL491316 US10201527, Compound 72 N-hydroxy-3-[2-phenethyl-1-(2-pyrrolidin-1-ylethyl)-1H-benzimidazol-5-yl]acrylamide
US10736881, Compound 72 BDBM50248476 SB-639 US8551988, 72 N-hydroxy-3-(2-phenethyl-1-(2-(pyrrolidin-1-yl)ethyl)-1H-benzo[d]imidazol-5-yl)acrylamide CHEMBL491316 US10201527, Compound 72 N-hydroxy-3-[2-phenethyl-1-(2-pyrrolidin-1-ylethyl)-1H-benzimidazol-5-yl]acrylamide CHEMBL50106 {(S)-7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid {7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid BDBM50059133 SB-223245
CHEMBL50106 {(S)-7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid {7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid BDBM50059133 SB-223245 BDBM50240962 Benzo[b]thiophene-2-carboxylic acid ((S)-1-{(R)-1-[4-(3-dimethylamino-propoxy)-benzyl]-pyrrolidin-3-ylcarbamoyl}-3-methyl-butyl)-amide SB-328872 N-((S)-1-((S)-1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-ylamino)-4-methyl-1-oxopentan-2-yl)benzo[b]thiophene-2-carboxamide CHEMBL190533
BDBM50240962 Benzo[b]thiophene-2-carboxylic acid ((S)-1-{(R)-1-[4-(3-dimethylamino-propoxy)-benzyl]-pyrrolidin-3-ylcarbamoyl}-3-methyl-butyl)-amide SB-328872 N-((S)-1-((S)-1-(4-(3-(dimethylamino)propoxy)benzyl)pyrrolidin-3-ylamino)-4-methyl-1-oxopentan-2-yl)benzo[b]thiophene-2-carboxamide CHEMBL190533 CHEMBL346178 SR-142801 N-(1-{3-[(R)-1-Benzoyl-3-(3,4-dichloro-phenyl)-piperidin-3-yl]-propyl}-4-phenyl-piperidin-4-yl)-N-methyl-acetamide OSANETANT (S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl]prop-1-yl)-4-phenylpiperidin-4-yl)-N-methylacetamine SR-14280 BDBM50291261 SB-236984 CHEMBL2311148
CHEMBL346178 SR-142801 N-(1-{3-[(R)-1-Benzoyl-3-(3,4-dichloro-phenyl)-piperidin-3-yl]-propyl}-4-phenyl-piperidin-4-yl)-N-methyl-acetamide OSANETANT (S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl]prop-1-yl)-4-phenylpiperidin-4-yl)-N-methylacetamine SR-14280 BDBM50291261 SB-236984 CHEMBL2311148 3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)-hexyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid 3-{6-(2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid (SB201993) 3-{6-((E)-2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid CHEMBL422598 SB-201993 BDBM50037385
3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)-hexyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid 3-{6-(2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid (SB201993) 3-{6-((E)-2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid CHEMBL422598 SB-201993 BDBM50037385 4-[4-(4-fluorophenyl)-5-(4-pyridyl)-4-imidazolin-2-ylidene]cyclohexa-2,5-dien-1-one 4-[4-(4-Fluorophenyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]phenol SB-202190 4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole 4-[5-(4-fluorophenyl)-4-(pyridin-4-yl)-1H-imidazol-2-yl]phenol SB202190 BDBM13531 biotinylated SB202190
4-[4-(4-fluorophenyl)-5-(4-pyridyl)-4-imidazolin-2-ylidene]cyclohexa-2,5-dien-1-one 4-[4-(4-Fluorophenyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]phenol SB-202190 4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole 4-[5-(4-fluorophenyl)-4-(pyridin-4-yl)-1H-imidazol-2-yl]phenol SB202190 BDBM13531 biotinylated SB202190 SB-258741 BDBM50130279 CHEMBL12624 (R)-4-Methyl-1-(2-(1-toluene-3-sulfonyl)-pyrrolidin-2-yl)-ethyl)-piperidine (Oxalate salt) 4-Methyl-1-{2-[(R)-1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-Methyl-1-{2-[(S)-1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-Methyl-1-{2-[1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine
SB-258741 BDBM50130279 CHEMBL12624 (R)-4-Methyl-1-(2-(1-toluene-3-sulfonyl)-pyrrolidin-2-yl)-ethyl)-piperidine (Oxalate salt) 4-Methyl-1-{2-[(R)-1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-Methyl-1-{2-[(S)-1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-Methyl-1-{2-[1-(toluene-3-sulfonyl)-pyrrolidin-2-yl]-ethyl}-piperidine 4-(4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2(3H)-ylidene)cyclohexa-2,5-dienone 4-[5-(4-fluorophenyl)-4-pyridin-4-yl-1H-imidazol-2-yl]phenol BDBM50104383 SB-202190 4-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-1H-imidazol-2-yl]-phenol 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole CHEMBL278041 4-(4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2-yl)phenol
4-(4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2(3H)-ylidene)cyclohexa-2,5-dienone 4-[5-(4-fluorophenyl)-4-pyridin-4-yl-1H-imidazol-2-yl]phenol BDBM50104383 SB-202190 4-[4-(4-Fluoro-phenyl)-5-pyridin-4-yl-1H-imidazol-2-yl]-phenol 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole CHEMBL278041 4-(4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2-yl)phenol 4-(4-Benzo[1,3]dioxol-5-yl-5-pyridin-2-yl-1H-imidazol-2-yl)-benzamide BDBM50110208 cid_4521392 4-(5-(benzo[d][1,3]dioxol-5-yl)-4-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide CHEMBL440084 SB-431542 4-(5-benzo[1,3]dioxol-5-yl-4-pyridin-2-yl-1H-imidazol-2-yl)-benzamide 4-(4-(benzo[d][1,3]dioxol-5-yl)-5-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide
4-(4-Benzo[1,3]dioxol-5-yl-5-pyridin-2-yl-1H-imidazol-2-yl)-benzamide BDBM50110208 cid_4521392 4-(5-(benzo[d][1,3]dioxol-5-yl)-4-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide CHEMBL440084 SB-431542 4-(5-benzo[1,3]dioxol-5-yl-4-pyridin-2-yl-1H-imidazol-2-yl)-benzamide 4-(4-(benzo[d][1,3]dioxol-5-yl)-5-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide Ariflo 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid (Ariflo) BDBM50346088 CHEMBL511115 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid methyl ester 4-Cyano-3-cyclopentyloxy-4-(4-methoxy-phenyl)-cyclohexanecarboxylic acid 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid(Ariflo) (1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphenyl)cyclohexanecarboxylic acid 6-(3,4-Dimethoxy-phenyl)-5-methyl-4,5-dihydro-2H-pyridazin-3-one 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid SB-207499
Ariflo 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid (Ariflo) BDBM50346088 CHEMBL511115 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid methyl ester 4-Cyano-3-cyclopentyloxy-4-(4-methoxy-phenyl)-cyclohexanecarboxylic acid 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid(Ariflo) (1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphenyl)cyclohexanecarboxylic acid 6-(3,4-Dimethoxy-phenyl)-5-methyl-4,5-dihydro-2H-pyridazin-3-one 4-Cyano-4-(3-cyclopentyloxy-4-methoxy-phenyl)-cyclohexanecarboxylic acid SB-207499