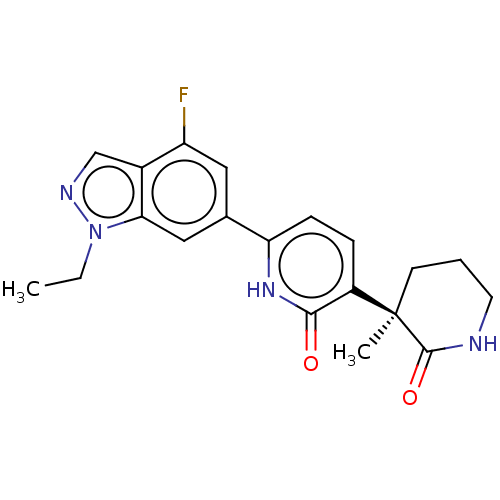
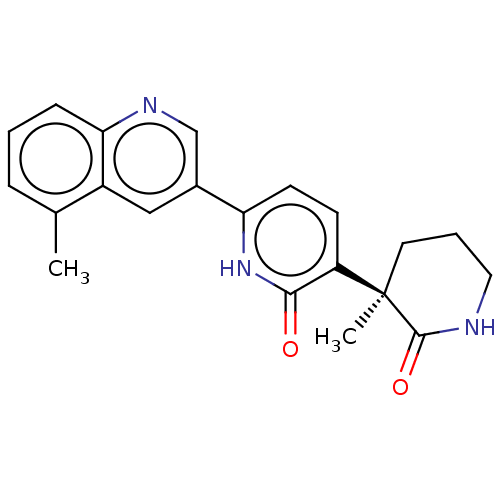
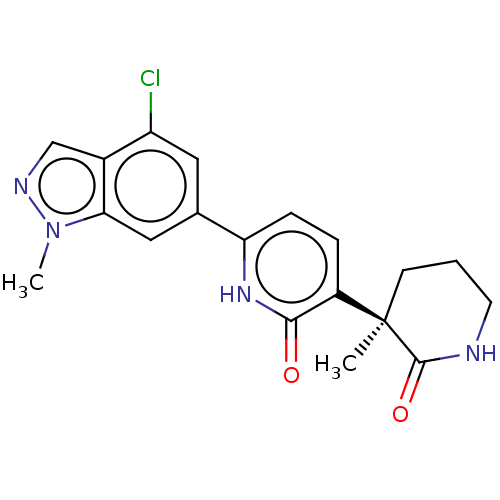
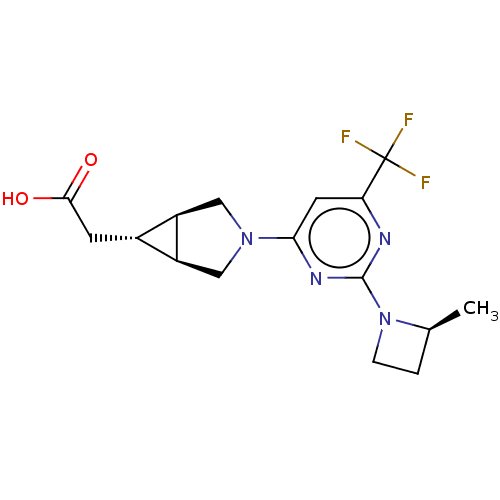
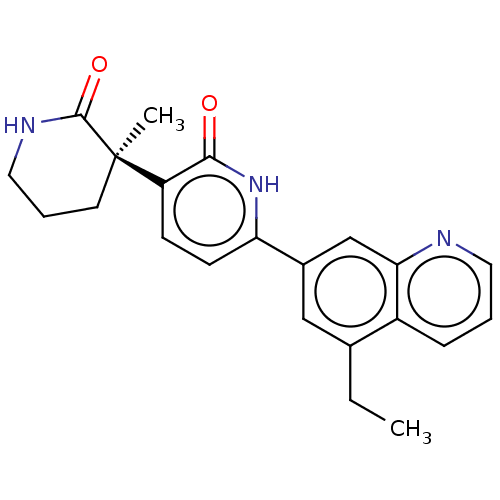
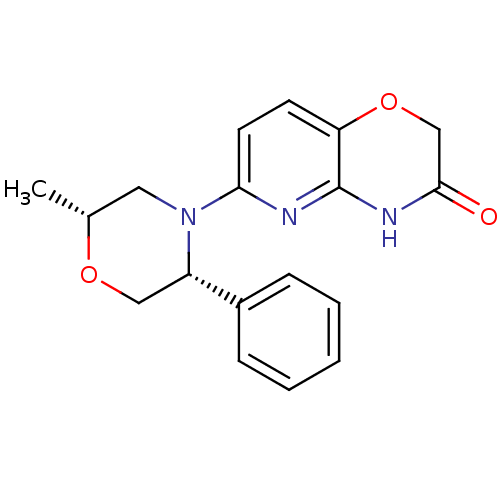
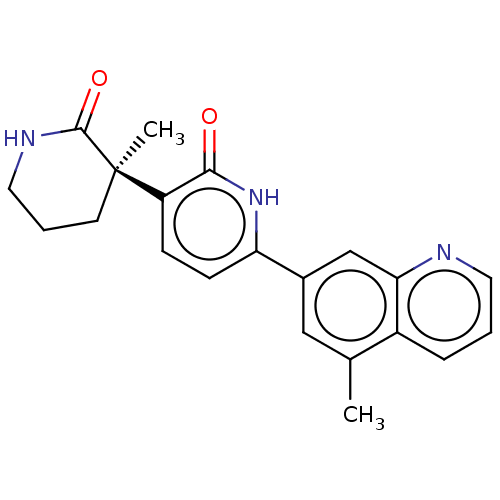
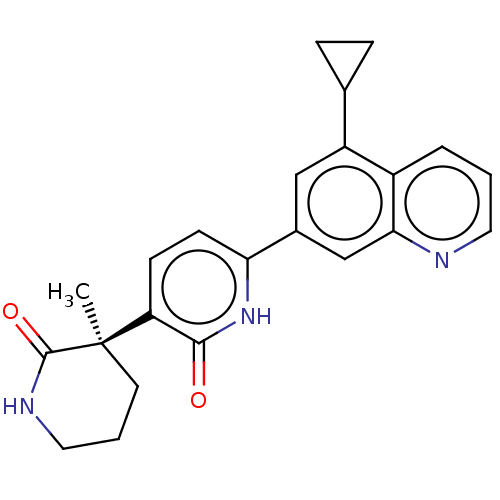
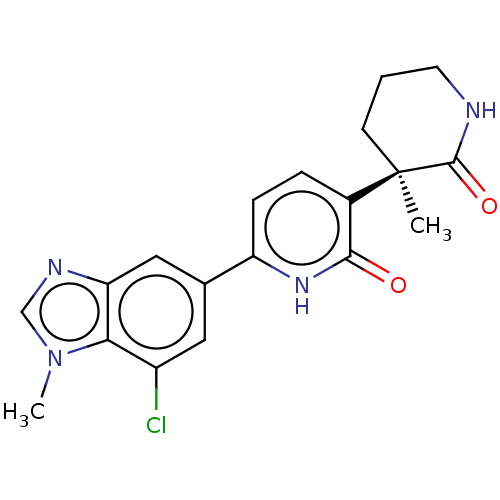
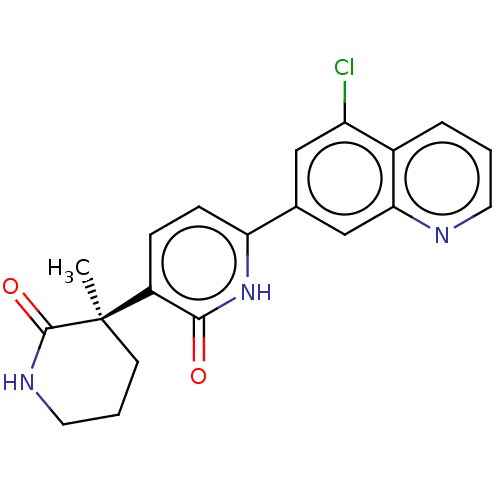
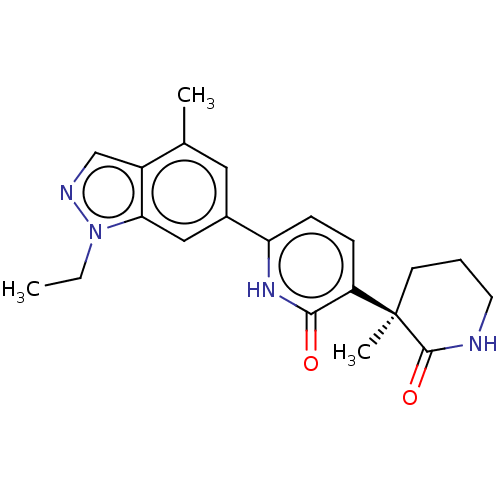
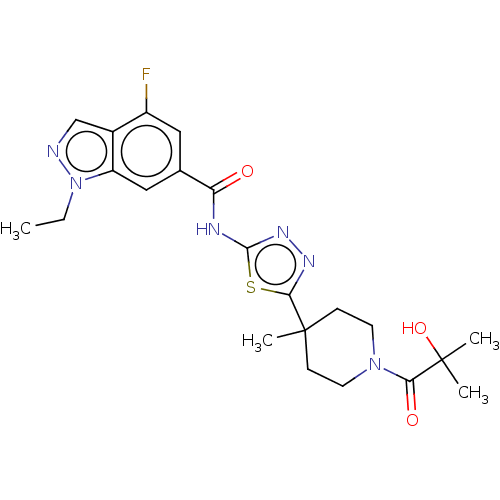
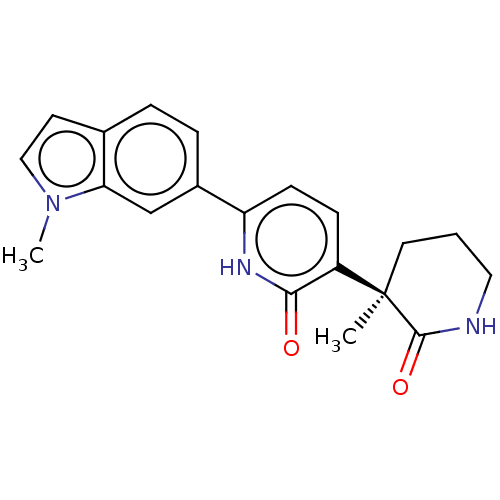
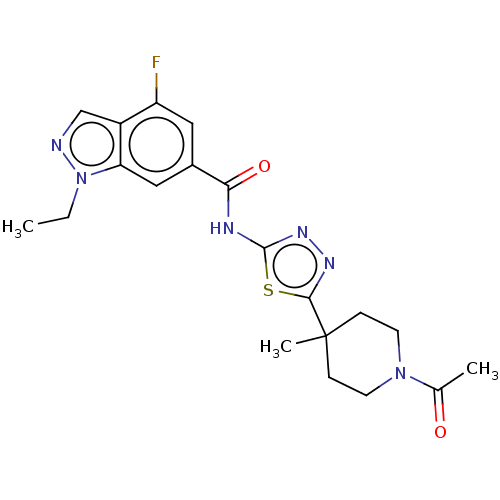
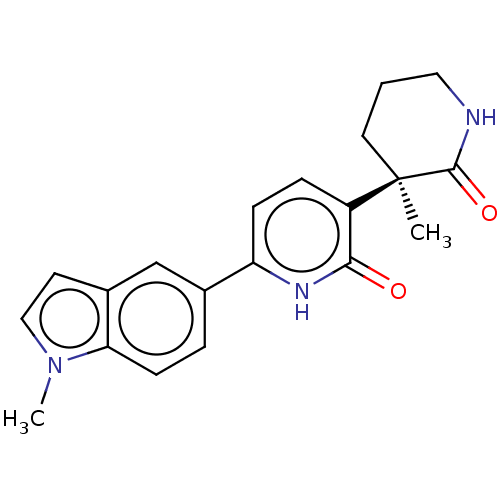
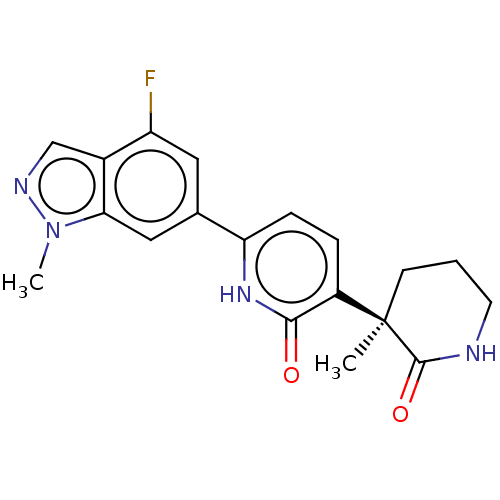
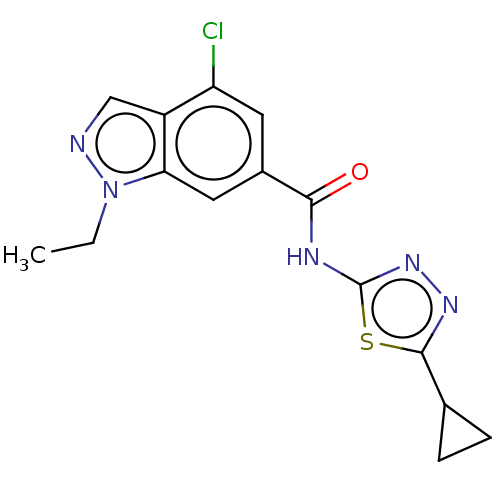
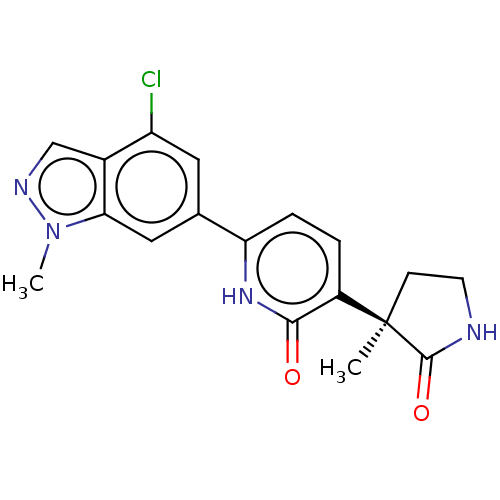
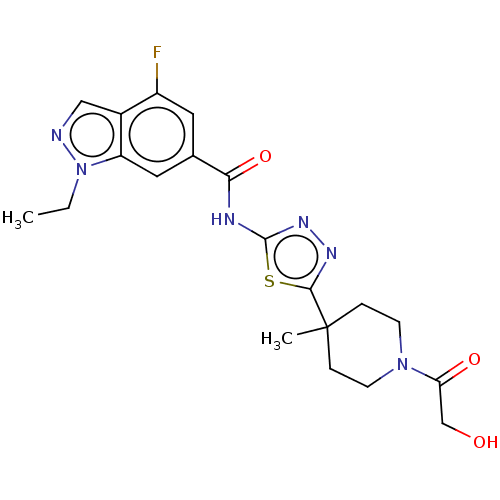
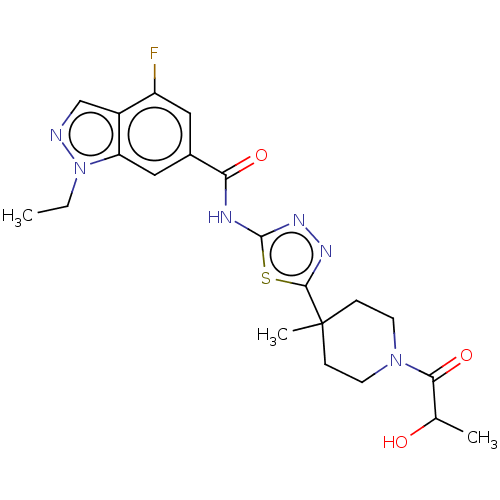
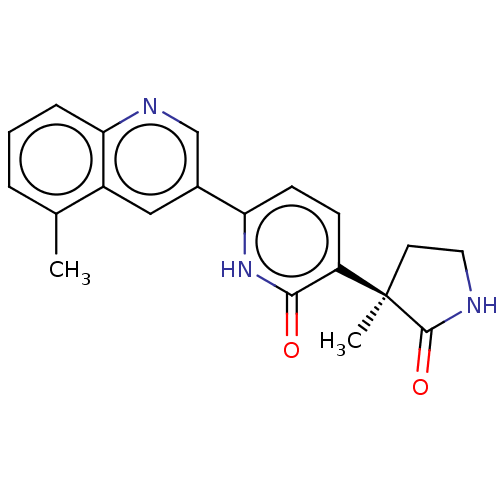
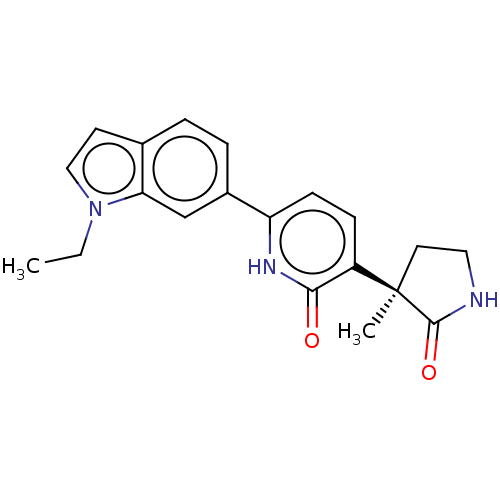
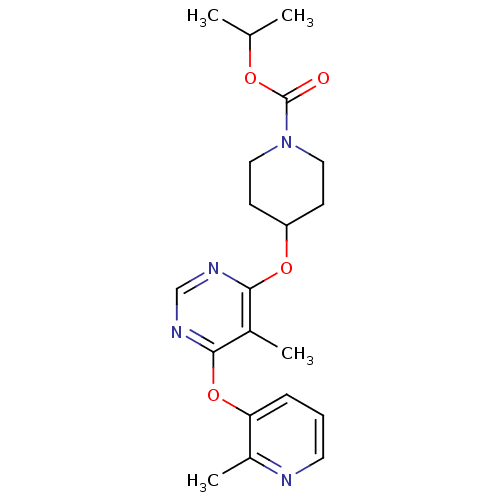
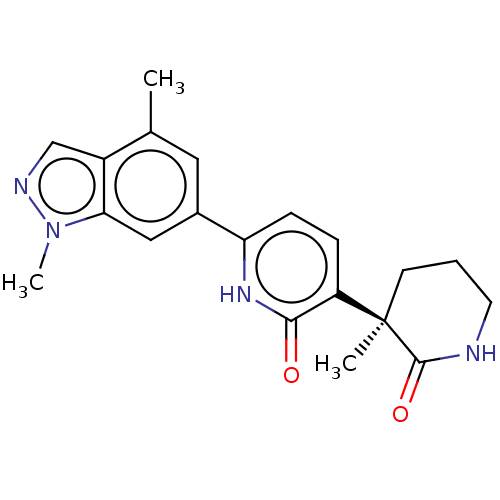
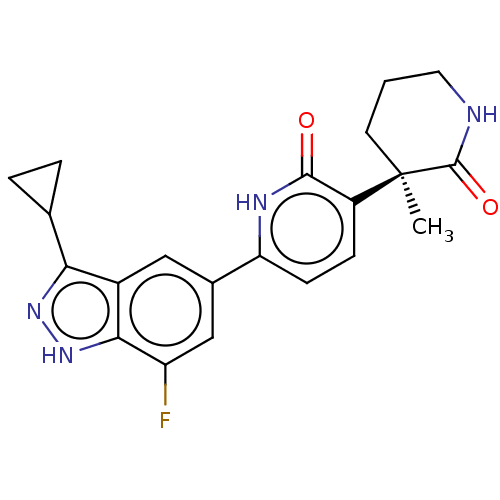
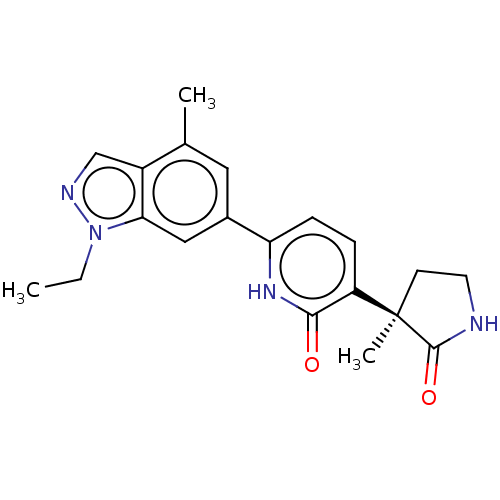
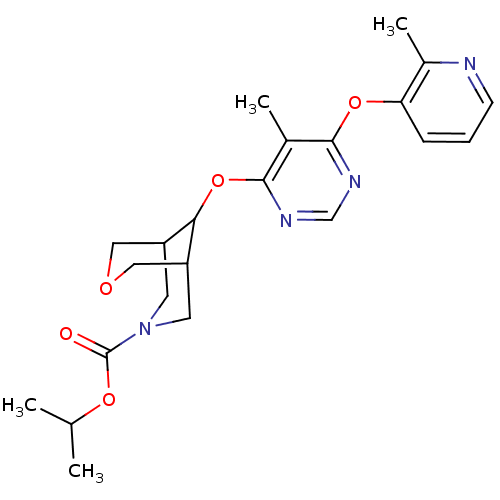
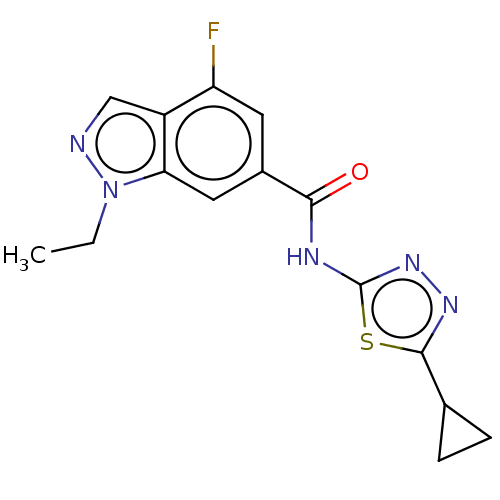
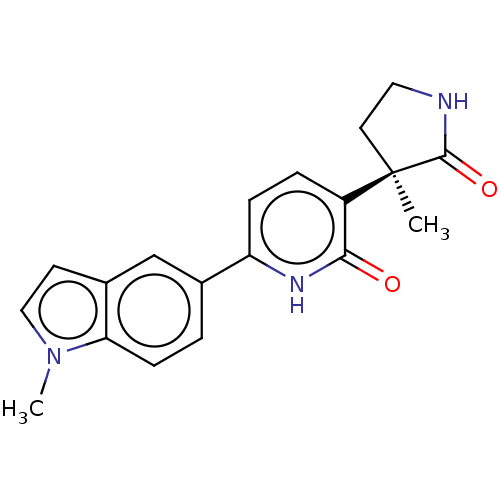
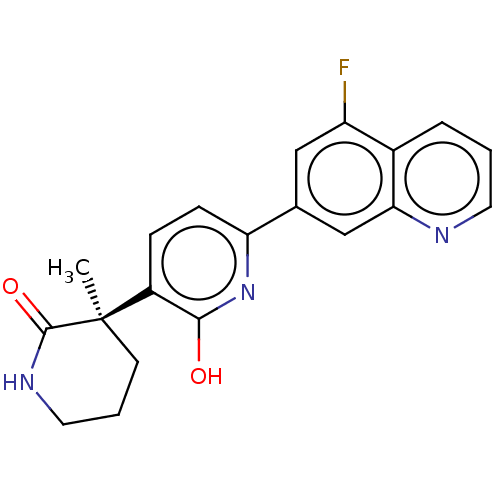
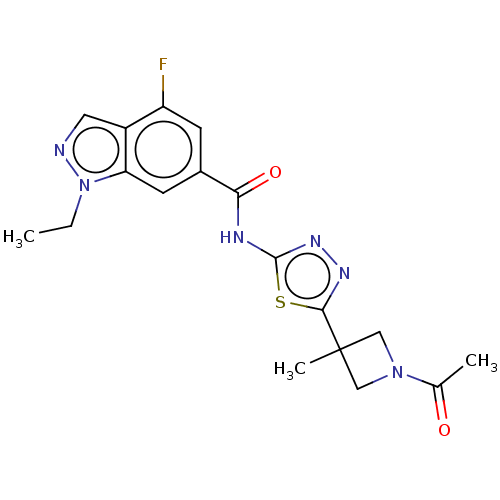
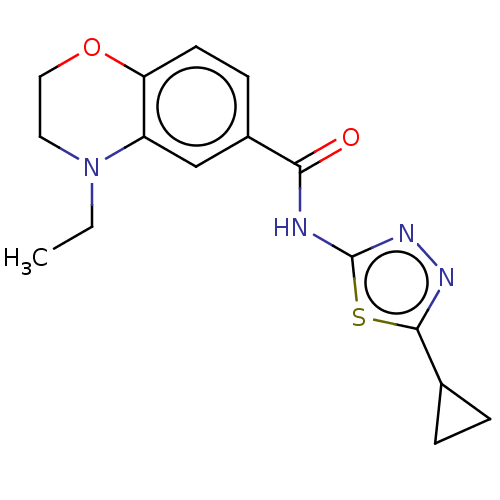
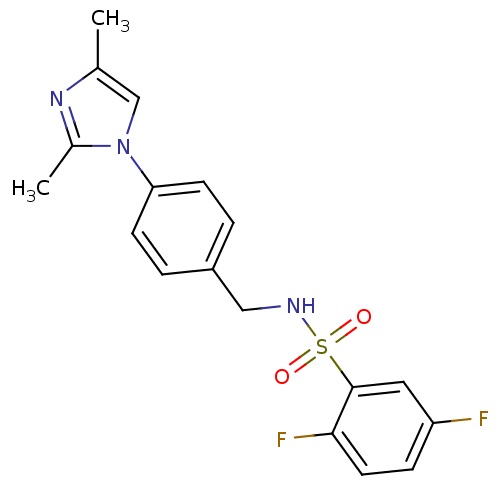
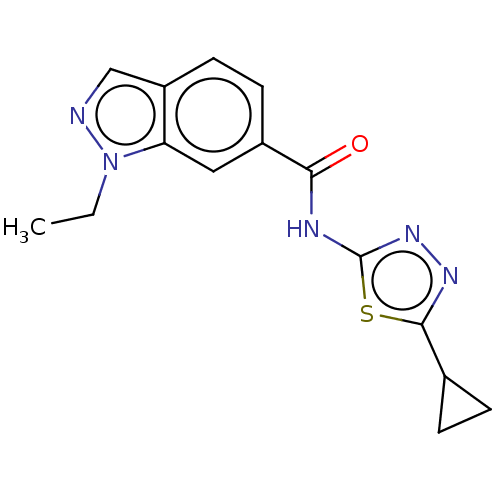
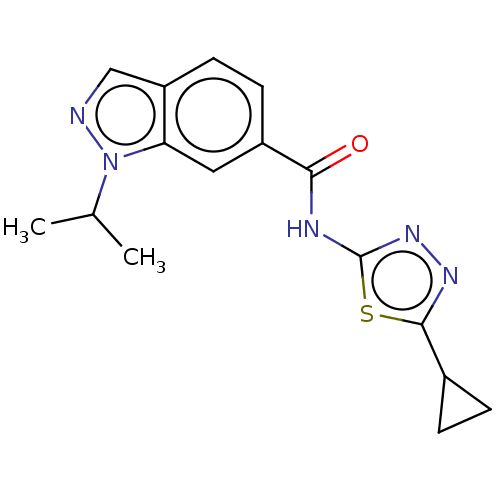
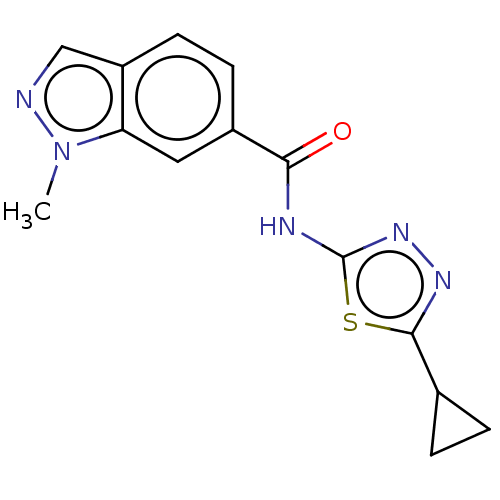
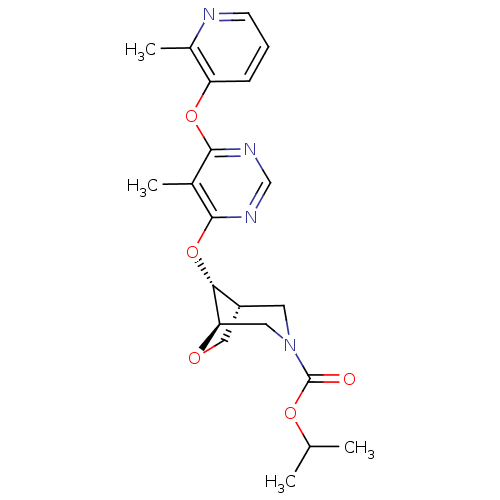
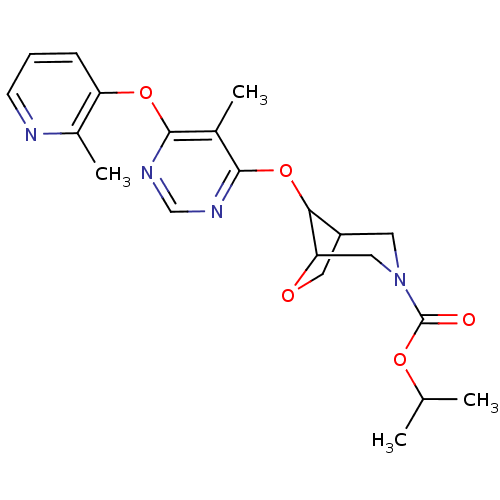
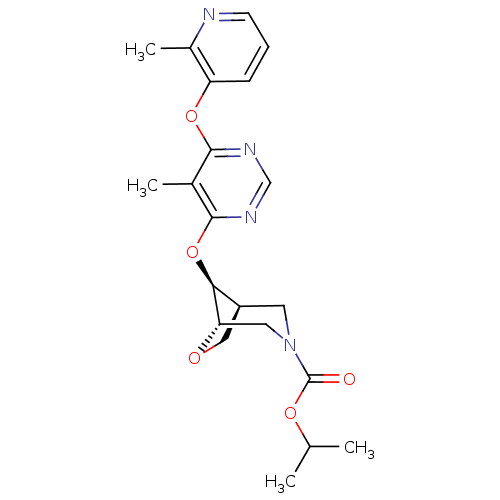
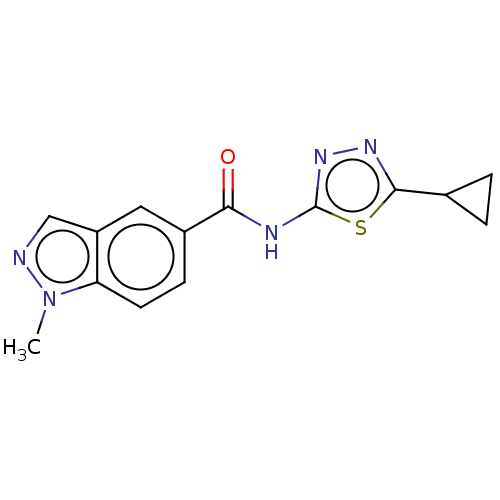
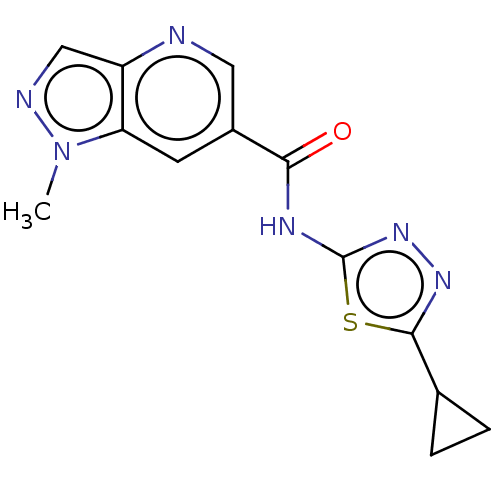
Affinity DataKi: 2nM ΔG°: -49.7kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P...More data for this Ligand-Target Pair
Affinity DataKi: 3.60nM ΔG°: -48.2kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 4.40nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ...More data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P...More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Displacement of [3H]-aldosterone from human GST-tagged MR ligand binding domain after 4 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 7.20nMAssay Description:To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P...More data for this Ligand-Target Pair
Affinity DataKi: 7.30nMAssay Description:To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P...More data for this Ligand-Target Pair
Affinity DataKi: 7.80nM ΔG°: -46.3kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P...More data for this Ligand-Target Pair
Affinity DataKi: 9.5nM ΔG°: -45.8kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 11.2nM ΔG°: -45.4kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 11.9nM ΔG°: -45.2kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 12.2nM ΔG°: -45.2kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 13.9nM ΔG°: -44.8kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 14.2nM ΔG°: -44.8kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 18.6nMAssay Description:To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P...More data for this Ligand-Target Pair
Affinity DataKi: 19.9nM ΔG°: -44.0kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -43.4kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 29.2nM ΔG°: -43.0kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 31.6nM ΔG°: -42.8kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 46nM ΔG°: -41.9kJ/molepH: 7.4 T: 2°CAssay Description:Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ...More data for this Ligand-Target Pair
Affinity DataKi: 48.6nMAssay Description:To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P...More data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 65nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 77nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 113nMAssay Description:Displacement of [3H]aldosterone from GST-tagged human mineralocorticoid receptor ligand binding domain after 4 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 584nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.07E+3nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.18E+3nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 2.27E+3nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 5.89E+3nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: >9.65E+3nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in human Chem1 cell membrane by scintillation proximity assayMore data for this Ligand-Target Pair