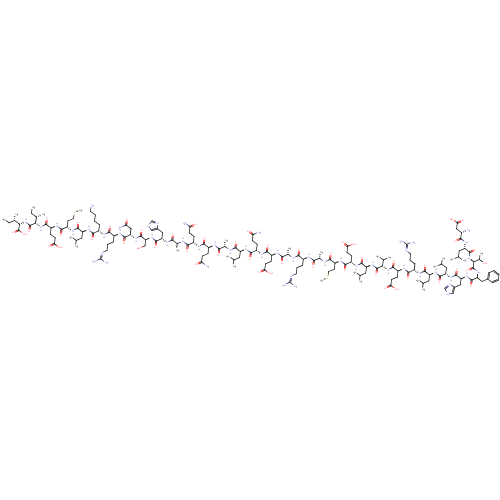
 BDBM86193 Alpha-Helical CRF CAS_0 NSC_0
BDBM86193 Alpha-Helical CRF CAS_0 NSC_0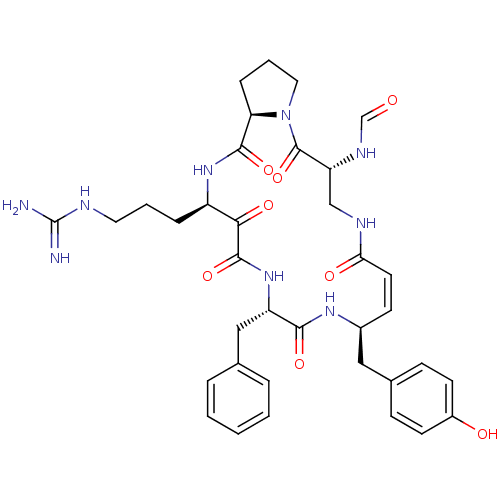 BDBM50288457 CHEMBL317974 macrocyclic tripeptide motif
BDBM50288457 CHEMBL317974 macrocyclic tripeptide motif
- Yin, H; Hamilton, AD Terephthalamide derivatives as mimetics of the helical region of Bak peptide target Bcl-xL protein. Bioorg Med Chem Lett 14: 1375-9 (2004)
- Mita, Y; Dodo, K; Noguchi-Yachide, T; Miyachi, H; Makishima, M; Hashimoto, Y; Ishikawa, M LXXLL peptide mimetics as inhibitors of the interaction of vitamin D receptor with coactivators. Bioorg Med Chem Lett 20: 1712-7 (2010)
- Murage, EN; Schroeder, JC; Beinborn, M; Ahn, JM Search for alpha-helical propensity in the receptor-bound conformation of glucagon-like peptide-1. Bioorg Med Chem 16: 10106-12 (2008)
- Luo, Q; Ma, Y; Liang, H; Feng, Y; Liu, N; Lian, C; Zhu, L; Ye, Y; Liu, Z; Hou, Z; Chen, S; Wang, Y; Dai, C; Song, C; Zhang, M; He, Z; Xing, Y; Zhong, W; Li, S; Wu, J; Lu, F; Yin, F; Li, Z Covalent Peptide LSD1 Inhibitor Specifically Recognizes Cys360 in the Enzyme-Active Region. J Med Chem 66: 15409-15423 (2023)
- Guerlavais, V; Sawyer, TK; Carvajal, L; Chang, YS; Graves, B; Ren, JG; Sutton, D; Olson, KA; Packman, K; Darlak, K; Elkin, C; Feyfant, E; Kesavan, K; Gangurde, P; Vassilev, LT; Nash, HM; Vukovic, V; Aivado, M; Annis, DA Discovery of Sulanemadlin (ALRN-6924), the First Cell-Permeating, Stabilized α-Helical Peptide in Clinical Development. J Med Chem 66: 9401-9417 (2023)
- Gao, Y; Voigt, J; Zhao, H; Pais, GC; Zhang, X; Wu, L; Zhang, ZY; Burke, TR Utilization of a peptide lead for the discovery of a novel PTP1B-binding motif. J Med Chem 44: 2869-78 (2001)
- Anananuchatkul, T; Tsutsumi, H; Miki, T; Mihara, H hDM2 protein-binding peptides screened from stapled α-helical peptide phage display libraries with different types of staple linkers. Bioorg Med Chem Lett 30: (2020)
- Liu, D; Yang, B; Cao, R; Huang, Z A chemical strategy to promote helical peptide-protein interactions involved in apoptosis. Bioorg Med Chem Lett 15: 4467-9 (2005)
- Khoo, KK; Wilson, MJ; Smith, BJ; Zhang, MM; Gulyas, J; Yoshikami, D; Rivier, JE; Bulaj, G; Norton, RS Lactam-stabilized helical analogues of the analgesicµ-conotoxin KIIIA. J Med Chem 54: 7558-66 (2011)
- Martinez, J; Magous, R; Lignon, MF; Laur, J; Castro, B; Bali, JP Synthesis and biological activity of new peptide segments of gastrin exhibiting gastrin antagonist property. J Med Chem 27: 1597-601 (1985)
- Mita, Y; Dodo, K; Noguchi-Yachide, T; Hashimoto, Y; Ishikawa, M Structure-activity relationship of benzodiazepine derivatives as LXXLL peptide mimetics that inhibit the interaction of vitamin D receptor with coactivators. Bioorg Med Chem 21: 993-1005 (2013)
- Liu, G; Liu, G; Hou, R; Hou, R; Xu, L; Xu, L; Zhang, X; Zhang, X; Yan, J; Yan, J; Xing, C; Xing, C; Xu, K; Xu, K; Zhuang, C; Zhuang, C Crystallography-Guided Optimizations of the Keap1-Nrf2 Inhibitors on the Solvent Exposed Region: From Symmetric to Asymmetric Naphthalenesulfonamides. J Med Chem 65: 8289-8302 (2022)
- Horwell, DC; Howson, W; Ratcliffe, G; Willems, H The design of dipeptide helical mimetics: the synthesis and biological activity of trisubstituted indanes Bioorg Med Chem Lett 4: 2825-2830 (1994)
- M'Kadmi, C; Cabral, A; Barrile, F; Giribaldi, J; Cantel, S; Damian, M; Mary, S; Denoyelle, S; Dutertre, S; Péraldi-Roux, S; Neasta, J; Oiry, C; Banères, JL; Marie, J; Perello, M; Fehrentz, JA N-Terminal Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Region Exhibits Inverse Agonist Activity toward the Ghrelin Receptor. J Med Chem 62: 965-973 (2019)
- Peng, YH; Shiao, HY; Tu, CH; Liu, PM; Hsu, JT; Amancha, PK; Wu, JS; Coumar, MS; Chen, CH; Wang, SY; Lin, WH; Sun, HY; Chao, YS; Lyu, PC; Hsieh, HP; Wu, SY Protein kinase inhibitor design by targeting the Asp-Phe-Gly (DFG) motif: the role of the DFG motif in the design of epidermal growth factor receptor inhibitors. J Med Chem 56: 3889-903 (2013)
- Xu, X; Weitzberg, M; Keyes, RF; Li, Q; Wang, R; Wang, X; Zhang, X; Frevert, EU; Camp, HS; Beutel, BA; Sham, HL; Gu, YG The synthesis and structure-activity relationship studies of selective acetyl-CoA carboxylase inhibitors containing 4-(thiazol-5-yl)but-3-yn-2-amino motif: polar region modifications. Bioorg Med Chem Lett 17: 1803-7 (2007)
- Zawahir, Z; Neamati, N Inhibition of HIV-1 integrase activity by synthetic peptides derived from the HIV-1 HXB2 Pol region of the viral genome. Bioorg Med Chem Lett 16: 5199-202 (2006)
- Kreutter, KD; Lu, T; Lee, L; Giardino, EC; Patel, S; Huang, H; Xu, G; Fitzgerald, M; Haertlein, BJ; Mohan, V; Crysler, C; Eisennagel, S; Dasgupta, M; McMillan, M; Spurlino, JC; Huebert, ND; Maryanoff, BE; Tomczuk, BE; Damiano, BP; Player, MR Orally efficacious thrombin inhibitors with cyanofluorophenylacetamide as the P2 motif. Bioorg Med Chem Lett 18: 2865-70 (2008)
- Novick, PA; Lopes, DH; Branson, KM; Esteras-Chopo, A; Graef, IA; Bitan, G; Pande, VS Design of β-amyloid aggregation inhibitors from a predicted structural motif. J Med Chem 55: 3002-10 (2012)
- Lima, LM; Silva, Vde A; Palmieri, Lde C; Oliveira, MC; Foguel, D; Polikarpov, I Identification of a novel ligand binding motif in the transthyretin channel. Bioorg Med Chem 18: 100-10 (2010)
- Williams, LK; Zhang, X; Caner, S; Tysoe, C; Nguyen, NT; Wicki, J; Williams, DE; Coleman, J; McNeill, JH; Yuen, V; Andersen, RJ; Withers, SG; Brayer, GD The amylase inhibitor montbretin A reveals a new glycosidase inhibition motif. Nat Chem Biol 11: 691-6 (2015)
- Erdeljac, N; Thiehoff, C; Jumde, RP; Daniliuc, CG; Höppner, S; Faust, A; Hirsch, AKH; Gilmour, R Validating the 1,2-Difluoro Motif As a Hybrid Bioisostere of CF J Med Chem 63: 6225-6237 (2020)
- Rutledge, LD; Perlman, JH; Gershengorn, MC; Marshall, GR; Moeller, KD Conformationally restricted TRH analogs: a probe for the pyroglutamate region. J Med Chem 39: 1571-4 (1996)
- Szczepańska, K; Pockes, S; Podlewska, S; Höring, C; Mika, K; Latacz, G; Bednarski, M; Siwek, A; Karcz, T; Nagl, M; Bresinsky, M; Mönnich, D; Seibel, U; Kuder, KJ; Kotańska, M; Stark, H; Elz, S; Kieć-Kononowicz, K Structural modifications in the distal, regulatory region of histamine H Eur J Med Chem 213: (2021)
- Zhao, X; Liu, H; Zhang, JC; Cai, J Helical sulfonyl-γ-AApeptides for the inhibition of HIV-1 fusion and HIF-1α signaling. RSC Med Chem 15: 1418-1423
- Yin, H; Lee, GI; Sedey, KA; Rodriguez, JM; Wang, HG; Sebti, SM; Hamilton, AD Terephthalamide derivatives as mimetics of helical peptides: disruption of the Bcl-x(L)/Bak interaction. J Am Chem Soc 127: 5463-8 (2005)
- Carro, L; Raviña, E; Domínguez, E; Brea, J; Loza, MI; Masaguer, CF Synthesis and binding affinity of potential atypical antipsychotics with the tetrahydroquinazolinone motif. Bioorg Med Chem Lett 19: 6059-62 (2009)
- Di Maro, S; Trotta, AM; Brancaccio, D; Di Leva, FS; La Pietra, V; Ieranò, C; Napolitano, M; Portella, L; D'Alterio, C; Siciliano, RA; Sementa, D; Tomassi, S; Carotenuto, A; Novellino, E; Scala, S; Marinelli, L Exploring the N-Terminal Region of C-X-C Motif Chemokine 12 (CXCL12): Identification of Plasma-Stable Cyclic Peptides As Novel, Potent C-X-C Chemokine Receptor Type 4 (CXCR4) Antagonists. J Med Chem 59: 8369-80 (2016)
- Cho, Y; Kim, MS; Kim, HS; Ann, J; Lee, J; Pearce, LV; Pavlyukovets, VA; Morgan, MA; Blumberg, PM; Lee, J The SAR analysis of TRPV1 agonists with thea-methylated B-region. Bioorg Med Chem Lett 22: 5227-31 (2012)
- Furuya, N; Momose, T; Katsuno, K; Fushimi, N; Muranaka, H; Handa, C; Ozawa, T; Kinoshita, T The juxtamembrane region of TrkA kinase is critical for inhibitor selectivity. Bioorg Med Chem Lett 27: 1233-1236 (2017)
- Leftheris, K; Kline, T; Natarajan, S; DeVirgilio, MK; Cho, YH; Pluscec, J; Ricca, C; Robinson, S; Seizinger, BR; Manne, V; Meyers, CA Peptide based P21RAS farnesyl transferase inhibitors: systematic modification of the tetrapeptide CA1A2X motif Bioorg Med Chem Lett 4: 887-892 (1994)
- Hughes, RO; Maddux, T; Joseph Rogier, D; Lu, S; Walker, JK; Jon Jacobsen, E; Rumsey, JM; Zheng, Y; Macinnes, A; Bond, BR; Han, S Investigation of the pyrazinones as PDE5 inhibitors: evaluation of regioisomeric projections into the solvent region. Bioorg Med Chem Lett 21: 6348-52 (2011)
- Kaczor, AA; Kijkowska-Murak, UA; Matosiuk, D Theoretical studies on the structure and symmetry of the transmembrane region of glutamatergic GluR5 receptor. J Med Chem 51: 3765-76 (2008)
- Wang, H; Fei, YJ; Ganapathy, V; Leibach, FH Electrophysiological characteristics of the proton-coupled peptide transporter PEPT2 cloned from rat brain. Am J Physiol 275: 967-75 (1998)
- Takayama, K; Noguchi, Y; Aoki, S; Takayama, S; Yoshida, M; Asari, T; Yakushiji, F; Nishimatsu, S; Ohsawa, Y; Itoh, F; Negishi, Y; Sunada, Y; Hayashi, Y Identification of the minimum peptide from mouse myostatin prodomain for human myostatin inhibition. J Med Chem 58: 1544-9 (2015)
- Klee, N; Wong, PE; Baragaña, B; Mazouni, FE; Phillips, MA; Barrett, MP; Gilbert, IH Selective delivery of 2-hydroxy APA to Trypanosoma brucei using the melamine motif. Bioorg Med Chem Lett 20: 4364-6 (2010)
- Hoekstra, WJ; Hulshizer, BL; McComsey, DF; Andrade-Gordon, P; Kauffman, JA; Addo, MF; Oksenberg, D; Scarborough, RM; Maryanoff, BE Thrombin receptor (PAR-1) antagonists. Heterocycle-based peptidomimetics of the SFLLR agonist motif. Bioorg Med Chem Lett 8: 1649-54 (1999)
- Blackburn, BK; Lee, A; Baier, M; Kohl, B; Olivero, AG; Matamoros, R; Robarge, KD; McDowell, RS From peptide to non-peptide. 3. Atropisomeric GPIIbIIIa antagonists containing the 3,4-dihydro-1H-1,4-benzodiazepine-2,5-dione nucleus. J Med Chem 40: 717-29 (1997)
- Chowdhury, MG; Kalmegh, V; Kapoor, S; Kamble, V; Shard, A Imidazopyrimidine: from a relatively exotic scaffold to an evolving structural motif in drug discovery. RSC Med Chem 15: 1488-1507
- Luo, JG; Wang, XB; Ma, L; Kong, LY Gypsophin: a novel alpha-glucosidase inhibitory cyclic peptide from the roots of Gypsophila oldhamiana. Bioorg Med Chem Lett 17: 4460-3 (2007)
- Wildemann, D; Erdmann, F; Alvarez, BH; Stoller, G; Zhou, XZ; Fanghänel, J; Schutkowski, M; Lu, KP; Fischer, G Nanomolar inhibitors of the peptidyl prolyl cis/trans isomerase Pin1 from combinatorial peptide libraries. J Med Chem 49: 2147-50 (2006)
- Kath, JC; DiRico, AP; Gladue, RP; Martin, WH; McElroy, EB; Stock, IA; Tylaska, LA; Zheng, D The discovery of structurally novel CCR1 antagonists derived from a hydroxyethylene peptide isostere template. Bioorg Med Chem Lett 14: 2163-7 (2004)
- Imamura, Y; Umezawa, N; Osawa, S; Shimada, N; Higo, T; Yokoshima, S; Fukuyama, T; Iwatsubo, T; Kato, N; Tomita, T; Higuchi, T Effect of helical conformation and side chain structure on γ-secretase inhibition by β-peptide foldamers: insight into substrate recognition. J Med Chem 56: 1443-54 (2013)
- Perreault, S; Arjmand, F; Chandrasekhar, J; Hao, J; Keegan, KS; Koditek, D; Lepist, EI; Matson, CK; McGrath, ME; Patel, L; Sedillo, K; Therrien, J; Till, NA; Tomkinson, A; Treiberg, J; Zherebina, Y; Phillips, G Discovery of an Atropisomeric PI3Kβ Selective Inhibitor through Optimization of the Hinge Binding Motif. ACS Med Chem Lett 11: 1236-1243 (2020)
- Lu, X; Wu, L; Liu, X; Wang, S; Zhang, C BH3 mimetics derived from Bim-BH3 domain core region show PTP1B inhibitory activity. Bioorg Med Chem Lett 29: 244-247 (2019)
- Jagtap, PK; Garg, D; Kapp, TG; Will, CL; Demmer, O; Lührmann, R; Kessler, H; Sattler, M Rational Design of Cyclic Peptide Inhibitors of U2AF Homology Motif (UHM) Domains To Modulate Pre-mRNA Splicing. J Med Chem 59: 10190-10197 (2016)
- Malamas, MS; Erdei, J; Gunawan, I; Barnes, K; Hui, Y; Johnson, M; Robichaud, A; Zhou, P; Yan, Y; Solvibile, W; Turner, J; Fan, KY; Chopra, R; Bard, J; Pangalos, MN New pyrazolyl and thienyl aminohydantoins as potent BACE1 inhibitors: exploring the S2' region. Bioorg Med Chem Lett 21: 5164-70 (2011)
- Irie, K; Yanai, Y; Ohigashi, H; Wender, PA; Miller, BL Synthesis and characterization of the second cysteine-rich region of mouse skin PKCGh Bioorg Med Chem Lett 6: 353-356 (1996)
- Castanedo, G; Clark, K; Wang, S; Tsui, V; Wong, M; Nicholas, J; Wickramasinghe, D; Marsters, JC; Sutherlin, D CDK2/cyclinA inhibitors: targeting the cyclinA recruitment site with small molecules derived from peptide leads. Bioorg Med Chem Lett 16: 1716-20 (2006)
- Codony, S; Calvó-Tusell, C; Valverde, E; Osuna, S; Morisseau, C; Loza, MI; Brea, J; Pérez, C; Rodríguez-Franco, MI; Pizarro-Delgado, J; Corpas, R; Griñán-Ferré, C; Pallàs, M; Sanfeliu, C; Vázquez-Carrera, M; Hammock, BD; Feixas, F; Vázquez, S From the Design to the J Med Chem 64: 5429-5446 (2021)
- Montalban, AG; Boman, E; Chang, CD; Ceide, SC; Dahl, R; Dalesandro, D; Delaet, NG; Erb, E; Ernst, JT; Gibbs, A; Kahl, J; Kessler, L; Kucharski, J; Lum, C; Lundström, J; Miller, S; Nakanishi, H; Roberts, E; Saiah, E; Sullivan, R; Urban, J; Wang, Z; Larson, CJ Optimization of alpha-ketoamide based p38 inhibitors through modifications to the region that binds to the allosteric site. Bioorg Med Chem Lett 20: 4819-24 (2010)
- Knaup, FH; Meyners, C; Sugiarto, WO; Wedel, S; Springer, M; Walz, C; Geiger, TM; Schmidt, M; Sisignano, M; Hausch, F Structure-Based Discovery of a New Selectivity-Enabling Motif for the FK506-Binding Protein 51. J Med Chem 66: 5965-5980 (2023)
- Bassetto, M; Zaluski, J; Li, B; Zhang, J; Badiee, M; Kiser, PD; Tochtrop, GP Tuning the Metabolic Stability of Visual Cycle Modulators through Modification of an RPE65 Recognition Motif. J Med Chem 66: 8140-8158 (2023)
- Alboreggia, G; Udompholkul, P; Baggio, C; Muzzarelli, K; Assar, Z; Pellecchia, M Histidine-Covalent Stapled Alpha-Helical Peptides Targeting hMcl-1. J Med Chem 67: 8172-8185
- Levy, DE; Wang, DX; Lu, Q; Chen, Z; Perumattam, J; Xu, YJ; Liclican, A; Higaki, J; Dong, H; Laney, M; Mavunkel, B; Dugar, S Aryl-indolyl maleimides as inhibitors of CaMKIIdelta. Part 1: SAR of the aryl region. Bioorg Med Chem Lett 18: 2390-4 (2008)
- Lampa, AK; Bergman, SM; Gustafsson, SS; Alogheli, H; Akerblom, EB; Lindeberg, GG; Svensson, RM; Artursson, P; Danielson, UH; Karlén, A; Sandström, A Novel Peptidomimetic Hepatitis C Virus NS3/4A Protease Inhibitors Spanning the P2-P1' Region. ACS Med Chem Lett 5: 249-54 (2014)
- Congdon, MD; Childress, ES; Patwardhan, NN; Gumkowski, J; Morris, EA; Kharel, Y; Lynch, KR; Santos, WL Structure-activity relationship studies of the lipophilic tail region of sphingosine kinase 2 inhibitors. Bioorg Med Chem Lett 25: 4956-60 (2015)
- Wang, GT; Lane, B; Fesik, SW; Petros, A; Luly, J; Krafft, GA Synthesis and FKBP binding of small molecule mimics of the tricarbonyl region of FK506 Bioorg Med Chem Lett 4: 1161-1166 (1994)
- Lee, T; Seo, YH Targeting the hydrophobic region of Hsp90's ATP binding pocket with novel 1,3,5-triazines. Bioorg Med Chem Lett 23: 6427-31 (2013)
- Chan, CB; Poulie, CBM; Wismann, SS; Soelberg, J; Kristensen, JL The Alkaloids from J Nat Prod 84: 2398-2407 (2021)
- Gazdik, M; Jarman, KE; O'Neill, MT; Hodder, AN; Lowes, KN; Jousset Sabroux, H; Cowman, AF; Boddey, JA; Sleebs, BE Exploration of the P3 region of PEXEL peptidomimetics leads to a potent inhibitor of the Plasmodium protease, plasmepsin V. Bioorg Med Chem 24: 1993-2010 (2016)
- Salerno, L; Amata, E; Romeo, G; Marrazzo, A; Prezzavento, O; Floresta, G; Sorrenti, V; Barbagallo, I; Rescifina, A; Pittalà, V Potholing of the hydrophobic heme oxygenase-1 western region for the search of potent and selective imidazole-based inhibitors. Eur J Med Chem 148: 54-62 (2018)
- Zhou, HB; Collins, ML; Gunther, JR; Comninos, JS; Katzenellenbogen, JA Bicyclo[2.2.2]octanes: close structural mimics of the nuclear receptor-binding motif of steroid receptor coactivators. Bioorg Med Chem Lett 17: 4118-22 (2007)
- Wang, BB; Maghami, N; Goodlin, VL; Smith, PJ Critical structural motif for the catalytic inhibition of human topoisomerase II by UK-1 and analogs. Bioorg Med Chem Lett 14: 3221-6 (2004)
- Ujjainwalla, F; Warner, D; Snedden, C; Grisson, RD; Walsh, TF; Wyvratt, MJ; Kalyani, RN; Macneil, T; Tang, R; Weinberg, DH; Van der Ploeg, L; Goulet, MT Design and syntheses of melanocortin subtype-4 receptor agonists. Part 2: discovery of the dihydropyridazinone motif. Bioorg Med Chem Lett 15: 4023-8 (2005)
- Summer, SL; Kell, SA; Zhu, Z; Moore, R; Liotta, DC; Myers, SJ; Koszalka, GW; Traynelis, SF; Menaldino, DS Di-aryl Sulfonamide Motif Adds π-Stacking Bulk in Negative Allosteric Modulators of the NMDA Receptor. ACS Med Chem Lett 10: 248-254 (2019)
- Frankowski, KJ; Slauson, SR; Lovell, KM; Phillips, AM; Streicher, JM; Zhou, L; Whipple, DA; Schoenen, FJ; Prisinzano, TE; Bohn, LM; Aubé, J Potency enhancement of the¿-opioid receptor antagonist probe ML140 through sulfonamide constraint utilizing a tetrahydroisoquinoline motif. Bioorg Med Chem 23: 3948-56 (2015)
- Lo, HY; Nemoto, PA; Kim, JM; Hao, MH; Qian, KC; Farrow, NA; Albaugh, DR; Fowler, DM; Schneiderman, RD; Michael August, E; Martin, L; Hill-Drzewi, M; Pullen, SS; Takahashi, H; De Lombaert, S Benzimidazolone as potent chymase inhibitor: modulation of reactive metabolite formation in the hydrophobic (P1) region. Bioorg Med Chem Lett 21: 4533-9 (2011)
- Dax, SL; Cook, SC Cyclic urea HIV protease inhibitors containing alkynyl- and alkenyl-tethered heterocycles in the P2 region Bioorg Med Chem Lett 6: 797-802 (1996)
- Aki, C; Chao, J; Ferreira, JA; Dwyer, MP; Yu, Y; Chao, J; Merritt, RJ; Lai, G; Wu, M; Hipkin, RW; Fan, X; Gonsiorek, W; Fosseta, J; Rindgen, D; Fine, J; Lundell, D; Taveras, AG; Biju, P Diaminocyclobutenediones as potent and orally bioavailable CXCR2 receptor antagonists: SAR in the phenolic amide region. Bioorg Med Chem Lett 19: 4446-9 (2009)
- Krstenansky, JL; Owen, TJ; Yates, MT; Mao, SJ Anticoagulant peptides: nature of the interaction of the C-terminal region of hirudin with a noncatalytic binding site on thrombin. J Med Chem 30: 1688-91 (1987)
- Furet, P; Salem, B; Mesrouze, Y; Schmelzle, T; Lewis, I; Kallen, J; Chène, P Structure-based design of potent linear peptide inhibitors of the YAP-TEAD protein-protein interaction derived from the YAP omega-loop sequence. Bioorg Med Chem Lett 29: 2316-2319 (2019)
- Yamaguchi, Y; Menear, K; Cohen, NC; Mah, R; Cumin, F; Schnell, C; Wood, JM; Maibaum, J The P1N-isopropyl motif bearing hydroxyethylene dipeptide isostere analogues of aliskiren are in vitro potent inhibitors of the human aspartyl protease renin. Bioorg Med Chem Lett 19: 4863-7 (2009)
- Wångsell, F; Russo, F; Sävmarker, J; Rosenquist, A; Samuelsson, B; Larhed, M Design and synthesis of BACE-1 inhibitors utilizing a tertiary hydroxyl motif as the transition state mimic. Bioorg Med Chem Lett 19: 4711-4 (2009)
- Li, T; Shiotani, K; Miyazaki, A; Fujita, Y; Tsuda, Y; Ambo, A; Sasaki, Y; Jinsmaa, Y; Marczak, E; Bryant, SD; Lazarus, LH; Okada, Y New series of potent delta-opioid antagonists containing the H-Dmt-Tic-NH-hexyl-NH-R motif. Bioorg Med Chem Lett 15: 5517-20 (2005)
- Robello, M; Barresi, E; Baglini, E; Salerno, S; Taliani, S; Settimo, FD The Alpha Keto Amide Moiety as a Privileged Motif in Medicinal Chemistry: Current Insights and Emerging Opportunities. J Med Chem 64: 3508-3545 (2021)
- Williams, TM; Stump, CA; Nguyen, DN; Quigley, AG; Bell, IM; Gallicchio, SN; Zartman, CB; Wan, BL; Penna, KD; Kunapuli, P; Kane, SA; Koblan, KS; Mosser, SD; Rutledge, RZ; Salvatore, C; Fay, JF; Vacca, JP; Graham, SL Non-peptide calcitonin gene-related peptide receptor antagonists from a benzodiazepinone lead. Bioorg Med Chem Lett 16: 2595-8 (2006)
- Hansen, KØ; Andersen, JH; Bayer, A; Pandey, SK; Lorentzen, M; Jørgensen, KB; Sydnes, MO; Guttormsen, Y; Baumann, M; Koch, U; Klebl, B; Eickhoff, J; Haug, BE; Isaksson, J; Hansen, EH Kinase Chemodiversity from the Arctic: The Breitfussins. J Med Chem 62: 10167-10181 (2019)
- McAllister, SD; Rizvi, G; Anavi-Goffer, S; Hurst, DP; Barnett-Norris, J; Lynch, DL; Reggio, PH; Abood, ME An aromatic microdomain at the cannabinoid CB(1) receptor constitutes an agonist/inverse agonist binding region. J Med Chem 46: 5139-52 (2003)
- Els, S; Schild, E; Petersen, PS; Kilian, TM; Mokrosinski, J; Frimurer, TM; Chollet, C; Schwartz, TW; Holst, B; Beck-Sickinger, AG An aromatic region to induce a switch between agonism and inverse agonism at the ghrelin receptor. J Med Chem 55: 7437-49 (2012)
- Probst, GD; Bowers, S; Sealy, JM; Stupi, B; Dressen, D; Jagodzinska, BM; Aquino, J; Gailunas, A; Truong, AP; Tso, L; Xu, YZ; Hom, RK; John, V; Tung, JS; Pleiss, MA; Tucker, JA; Konradi, AW; Sham, HL; Jagodzinski, J; Toth, G; Brecht, E; Yao, N; Pan, H; Lin, M; Artis, DR; Ruslim, L; Bova, MP; Sinha, S; Yednock, TA; Gauby, S; Zmolek, W; Quinn, KP; Sauer, JM Design and synthesis of hydroxyethylamine (HEA) BACE-1 inhibitors: structure-activity relationship of the aryl region. Bioorg Med Chem Lett 20: 6034-9 (2010)
- Hong, S; Kim, J; Yun, SM; Lee, H; Park, Y; Hong, SS; Hong, S Discovery of new benzothiazole-based inhibitors of breakpoint cluster region-Abelson kinase including the T315I mutant. J Med Chem 56: 3531-45 (2013)
- Kang, JS; Lee, J; Nam, LB; Yoo, OK; Pham, KT; Duong, TH; Keum, YS Homoharringtonine stabilizes secondary structure of guanine-rich sequence existing in the 5'-untranslated region of Nrf2. Bioorg Med Chem Lett 29: 2189-2196 (2019)
- Gao, B; Wenzel, A; Grimm, C; Vavricka, SR; Benke, D; Meier, PJ; Remè, CE Localization of organic anion transport protein 2 in the apical region of rat retinal pigment epithelium. Invest Ophthalmol Vis Sci 43: 510-4 (2002)
- Pagé, D; Naismith, A; Schmidt, R; Coupal, M; Labarre, M; Gosselin, M; Bellemare, D; Payza, K; Brown, W Novel C-terminus modifications of the Dmt-Tic motif: a new class of dipeptide analogues showing altered pharmacological profiles toward the opioid receptors. J Med Chem 44: 2387-90 (2001)
- Bihel, F; Das, C; Bowman, MJ; Wolfe, MS Discovery of a Subnanomolar helical D-tridecapeptide inhibitor of gamma-secretase. J Med Chem 47: 3931-3 (2004)
- Han, X; Civiello, RL; Conway, CM; Cook, DA; Davis, CD; Macci, R; Pin, SS; Ren, SX; Schartman, R; Signor, LJ; Thalody, G; Widmann, KA; Xu, C; Chaturvedula, PV; Macor, JE; Dubowchik, GM The synthesis and SAR of calcitonin gene-related peptide (CGRP) receptor antagonists derived from tyrosine surrogates. Part 1. Bioorg Med Chem Lett 22: 4723-7 (2012)
- Han, X; Civiello, RL; Conway, CM; Cook, DA; Davis, CD; Degnan, AP; Jiang, XJ; Macci, R; Mathias, NR; Moench, P; Pin, SS; Schartman, R; Signor, LJ; Thalody, G; Tora, G; Whiterock, V; Xu, C; Macor, JE; Dubowchik, GM The synthesis and SAR of calcitonin gene-related peptide (CGRP) receptor antagonists derived from tyrosine surrogates. Part 2. Bioorg Med Chem Lett 23: 1870-3 (2013)
- Katz, BA; Elrod, K; Luong, C; Rice, MJ; Mackman, RL; Sprengeler, PA; Spencer, J; Hataye, J; Janc, J; Link, J; Litvak, J; Rai, R; Rice, K; Sideris, S; Verner, E; Young, W A novel serine protease inhibition motif involving a multi-centered short hydrogen bonding network at the active site. J Mol Biol 307: 1451-86 (2001)
- Geng, K; Xia, Z; Ji, Y; Zhang, RR; Sun, D; Ai, J; Song, Z; Geng, M; Zhang, A Discovery of 2,4-diarylaminopyrimidines bearing a resorcinol motif as novel ALK inhibitors to overcome the G1202R resistant mutation. Eur J Med Chem 144: 386-397 (2018)
- Stepniewski, TM; Filipek, S Non-peptide ligand binding to the formyl peptide receptor FPR2--A comparison to peptide ligand binding modes. Bioorg Med Chem 23: 4072-81 (2015)
- Wu, J; Tao, Y; Zhang, M; Howard, MH; Gutteridge, S; Ding, J Crystal structures of Saccharomyces cerevisiae N-myristoyltransferase with bound myristoyl-CoA and inhibitors reveal the functional roles of the N-terminal region. J Biol Chem 282: 22185-94 (2007)
- Tapadar, S; He, R; Luchini, DN; Billadeau, DD; Kozikowski, AP Isoxazole moiety in the linker region of HDAC inhibitors adjacent to the Zn-chelating group: effects on HDAC biology and antiproliferative activity. Bioorg Med Chem Lett 19: 3023-6 (2009)
- Nishimura, N; Norman, MH; Liu, L; Yang, KC; Ashton, KS; Bartberger, MD; Chmait, S; Chen, J; Cupples, R; Fotsch, C; Helmering, J; Jordan, SR; Kunz, RK; Pennington, LD; Poon, SF; Siegmund, A; Sivits, G; Lloyd, DJ; Hale, C; St Jean, DJ Small molecule disruptors of the glucokinase-glucokinase regulatory protein interaction: 3. Structure-activity relationships within the aryl carbinol region of the N-arylsulfonamido-N'-arylpiperazine series. J Med Chem 57: 3094-116 (2014)
- Houghten, RA; Dooley, CT The use of synthetic peptide combinatorial libraries for the determination of peptide ligands in radio-receptor assays: Opioid peptides Bioorg Med Chem Lett 3: 405-412 (1993)
- Muratspahić, E; Tomašević, N; Koehbach, J; Duerrauer, L; Hadžić, S; Castro, J; Schober, G; Sideromenos, S; Clark, RJ; Brierley, SM; Craik, DJ; Gruber, CW Design of a Stable Cyclic Peptide Analgesic Derived from Sunflower Seeds that Targets the κ-Opioid Receptor for the Treatment of Chronic Abdominal Pain. J Med Chem 64: 9042-9055 (2021)
- Rist, B; Entzeroth, M; Beck-Sickinger, AG From micromolar to nanomolar affinity: a systematic approach to identify the binding site of CGRP at the human calcitonin gene-related peptide 1 receptor. J Med Chem 41: 117-23 (1998)
- Dean, TT; Jelú-Reyes, J; Allen, AC; Moore, TW Peptide-Drug Conjugates: An Emerging Direction for the Next Generation of Peptide Therapeutics. J Med Chem 67: 1641-1661
- Nair, MG; Chen, SY; Kisliuk, RL; Gaumont, Y; Strumpf, D Folate analogues altered in the C9-N10 bridge region. 16. Synthesis and antifolate activity of 11-thiohomoaminopterin. J Med Chem 23: 899-903 (1980)
- Kim, YS; Kil, MJ; Kang, SU; Ryu, H; Kim, MS; Cho, Y; Bhondwe, RS; Thorat, SA; Sun, W; Liu, K; Lee, JH; Choi, S; Pearce, LV; Pavlyukovets, VA; Morgan, MA; Tran, R; Lazar, J; Blumberg, PM; Lee, J N-4-t-Butylbenzyl 2-(4-methylsulfonylaminophenyl) propanamide TRPV1 antagonists: Structure-activity relationships in the A-region. Bioorg Med Chem 20: 215-24 (2011)
- Pennington, MW; Czerwinski, A; Norton, RS Peptide therapeutics from venom: Current status and potential. Bioorg Med Chem 26: 2738-2758 (2018)
- Van den Eynde, I; Laus, G; Schiller, PW; Kosson, P; Chung, NN; Lipkowski, AW; Tourwé, D A new structural motif for mu-opioid antagonists. J Med Chem 48: 3644-8 (2005)
- Juettner, NE; Bogen, JP; Bauer, TA; Knapp, S; Pfeifer, F; Huettenhain, SH; Meusinger, R; Kraemer, A; Fuchsbauer, HL Decoding the Papain Inhibitor from J Nat Prod 83: 2983-2995 (2020)
- Guan, P; Wang, L; Hou, X; Wan, Y; Xu, W; Tang, W; Fang, H Improved antiproliferative activity of 1,3,4-thiadiazole-containing histone deacetylase (HDAC) inhibitors by introduction of the heteroaromatic surface recognition motif. Bioorg Med Chem 22: 5766-75 (2014)
- Lavecchia, A; Cosconati, S; Novellino, E Architecture of the human urotensin II receptor: comparison of the binding domains of peptide and non-peptide urotensin II agonists. J Med Chem 48: 2480-92 (2005)
- Stepan, AF; Subramanyam, C; Efremov, IV; Dutra, JK; O'Sullivan, TJ; DiRico, KJ; McDonald, WS; Won, A; Dorff, PH; Nolan, CE; Becker, SL; Pustilnik, LR; Riddell, DR; Kauffman, GW; Kormos, BL; Zhang, L; Lu, Y; Capetta, SH; Green, ME; Karki, K; Sibley, E; Atchison, KP; Hallgren, AJ; Oborski, CE; Robshaw, AE; Sneed, B; O'Donnell, CJ Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J Med Chem 55: 3414-24 (2012)
- Ashwood, V; Brownhill, V; Higginbottom, M; Horwell, DC; Hughes, J; Lewthwaite, RA; McKnight, AT; Pinnock, RD; Pritchard, MC; Suman-Chauhan, N; Webb, C; Williams, SC PD 176252--the first high affinity non-peptide gastrin-releasing peptide (BB2) receptor antagonist. Bioorg Med Chem Lett 8: 2589-94 (1999)
- Simoni, D; Roberti, M; Rondanin, R; Baruchello, R; Rossi, M; Invidiata, FP; Merighi, S; Varani, K; Gessi, S; Borea, PA; Marino, S; Cavallini, S; Bianchi, C; Siniscalchi, A Effects of two-carbon bridge region methoxylation of benztropine: discovery of novel chiral ligands for the dopamine transporter. Bioorg Med Chem Lett 11: 823-7 (2001)
- Crawford, TD; Audia, JE; Bellon, S; Burdick, DJ; Bommi-Reddy, A; Côté, A; Cummings, RT; Duplessis, M; Flynn, EM; Hewitt, M; Huang, HR; Jayaram, H; Jiang, Y; Joshi, S; Kiefer, JR; Murray, J; Nasveschuk, CG; Neiss, A; Pardo, E; Romero, FA; Sandy, P; Sims, RJ; Tang, Y; Taylor, AM; Tsui, V; Wang, J; Wang, S; Wang, Y; Xu, Z; Zawadzke, L; Zhu, X; Albrecht, BK; Magnuson, SR; Cochran, AG GNE-886: A Potent and Selective Inhibitor of the Cat Eye Syndrome Chromosome Region Candidate 2 Bromodomain (CECR2). ACS Med Chem Lett 8: 737-741 (2017)
- Holst, B; Mokrosinski, J; Lang, M; Brandt, E; Nygaard, R; Frimurer, TM; Beck-Sickinger, AG; Schwartz, TW Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J Biol Chem 282: 15799-811 (2007)
- Schmid, CR; Sluka, JP; Duke, KM; Glasebrook, AW Novel nonsteroidal selective estrogen receptor modulators. Carbon and heteroatom replacement of oxygen in the ethoxypiperidine region of raloxifene. Bioorg Med Chem Lett 9: 523-8 (1999)
- Sall, DJ; Grunewald, GL Inhibition of phenylethanolamine N-methyltransferase (PNMT) by aromatic hydroxy-substituted 1,2,3,4,-tetrahydroisoquinolines: further studies on the hydrophilic pocket of the aromatic ring binding region of the active site. J Med Chem 30: 2208-16 (1988)
- Brandsch, M; Thunecke, F; Küllertz, G; Schutkowski, M; Fischer, G; Neubert, K Evidence for the absolute conformational specificity of the intestinal H+/peptide symporter, PEPT1. J Biol Chem 273: 3861-4 (1998)
- Emonds-Alt, X; Bichon, D; Ducoux, JP; Heaulme, M; Miloux, B; Poncelet, M; Proietto, V; Van Broeck, D; Vilain, P; Neliat, G SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci 56: -32 (1995)
- Tu, Y; Wu, C; Kang, Y; Li, Q; Zhu, C; Li, Y Bioactivity-guided identification of flavonoids with cholinesterase and β-amyloid peptide aggregation inhibitory effects from the seeds of Millettia pachycarpa. Bioorg Med Chem Lett 29: 1194-1198 (2019)
- Kumaran, D; Adler, M; Levit, M; Krebs, M; Sweeney, R; Swaminathan, S Interactions of a potent cyclic peptide inhibitor with the light chain of botulinum neurotoxin A: Insights from X-ray crystallography. Bioorg Med Chem 23: 7264-73 (2015)
- Windley, MJ; Escoubas, P; Valenzuela, SM; Nicholson, GM A novel family of insect-selective peptide neurotoxins targeting insect large-conductance calcium-activated K+ channels isolated from the venom of the theraphosid spider Eucratoscelus constrictus. Mol Pharmacol 80: 1-13 (2011)
- Coombs, GS; Hazzard, J; Corey, DR Kinetic characterization of a peptide inhibitor of trypsin isolated from a synthetic peptide combinatorial library Bioorg Med Chem Lett 5: 611-614 (1995)
- Weiser, PT; Chang, CY; McDonnell, DP; Hanson, RN 4,4'-Unsymmetrically substituted 3,3'-biphenyl alpha helical proteomimetics as potential coactivator binding inhibitors. Bioorg Med Chem 22: 917-26 (2014)
- Wang, Z; Song, T; Feng, Y; Guo, Z; Fan, Y; Xu, W; Liu, L; Wang, A; Zhang, Z Bcl-2/MDM2 Dual Inhibitors Based on Universal Pyramid-Like α-Helical Mimetics. J Med Chem 59: 3152-62 (2016)
- D de Araujo, A; Lim, J; Wu, KC; Xiang, Y; Good, AC; Skerlj, R; Fairlie, DP Bicyclic Helical Peptides as Dual Inhibitors Selective for Bcl2A1 and Mcl-1 Proteins. J Med Chem 61: 2962-2972 (2018)
- Zhao, Z; Dai, X; Li, C; Wang, X; Tian, J; Feng, Y; Xie, J; Ma, C; Nie, Z; Fan, P; Qian, M; He, X; Wu, S; Zhang, Y; Zheng, X Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect. Eur J Med Chem 186: (2020)
- Day, HA; Pavlou, P; Waller, ZA i-Motif DNA: structure, stability and targeting with ligands. Bioorg Med Chem 22: 4407-18 (2014)
- Knütter, I; Theis, S; Hartrodt, B; Born, I; Brandsch, M; Daniel, H; Neubert, K A novel inhibitor of the mammalian peptide transporter PEPT1. Biochemistry 40: 4454-8 (2001)
- Moinet, C; Contour-Galcéra, MO; Poitout, L; Morgan, B; Gordon, T; Rouber, P; Thurieau, C Novel non-peptide ligands for the somatostatin sst3 receptor. Bioorg Med Chem Lett 11: 991-5 (2001)
- Liu, J; Gao, S; Zhou, W; Chen, Y; Wang, Z; Zeng, Z; Zhou, H; Lin, T Dihydrotrichodimerol Purified from the Marine Fungus J Nat Prod 86: 1189-1201 (2023)
- Fu, Y; Ding, X; Zhang, X; Shao, X; Zhao, J; Xu, Y; Luo, X; Zhao, W Diterpenoids from the Root Bark of J Nat Prod 83: 1229-1237 (2020)
- Roth, GJ; Binder, R; Colbatzky, F; Dallinger, C; Schlenker-Herceg, R; Hilberg, F; Wollin, SL; Kaiser, R Nintedanib: from discovery to the clinic. J Med Chem 58: 1053-63 (2015)
- Wang, N; Song, J; Jang, KH; Lee, HS; Li, X; Oh, KB; Shin, J Sesterterpenoids from the sponge Sarcotragus sp. J Nat Prod 71: 551-7 (2008)
- Gaudreau, P; Paradis, H; Langelier, Y; Brazeau, P Synthesis and inhibitory potency of peptides corresponding to the subunit 2 C-terminal region of herpes virus ribonucleotide reductases. J Med Chem 33: 723-30 (1990)
- Stierle, AA; Stierle, DB; Patacini, B The berkeleyamides, amides from the acid lake fungus Penicillum rubrum. J Nat Prod 71: 856-60 (2008)
- Fehrenbach, T; Cui, Y; Faulstich, H; Keppler, D Characterization of the transport of the bicyclic peptide phalloidin by human hepatic transport proteins. Naunyn Schmiedebergs Arch Pharmacol 368: 415-20 (2003)
- Bernad, N; Burgaud, BG; Horwell, DC; Lewthwaite, RA; Martinez, J; Pritchard, MC The design and synthesis of the high efficacy, non-peptide CCK1 receptor agonist PD170292. Bioorg Med Chem Lett 10: 1245-8 (2000)
- Mogil, JS; Pasternak, GW The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev 53: 381-415 (2001)
- Sakakibara, R; Sasaki, W; Onda, Y; Yamaguchi, M; Ushirogochi, H; Hiraga, Y; Sato, K; Nishio, M; Egi, Y; Takedomi, K; Shimizu, H; Ohbora, T; Akahoshi, F Discovery of Novel Pyrazole-Based Selective Aldosterone Synthase (CYP11B2) Inhibitors: A New Template to Coordinate the Heme-Iron Motif of CYP11B2. J Med Chem 61: 5594-5608 (2018)
- Valentine, JJ; Nakanishi, S; Hageman, DL; Snider, R; Spencer, RW; Vinick, FJ CP-70,030 and CP-75,998: The first non-peptide antagonists of bombesin and gastrin releasing peptide Bioorg Med Chem Lett 2: 333-338 (1992)
- Butini, S; Brindisi, M; Cosconati, S; Marinelli, L; Borrelli, G; Coccone, SS; Ramunno, A; Campiani, G; Novellino, E; Zanoli, S; Samuele, A; Giorgi, G; Bergamini, A; Mattia, MD; Lalli, S; Galletti, B; Gemma, S; Maga, G Specific Targeting of Highly Conserved Residues in the HIV-1 Reverse Transcriptase Primer Grip Region. 2. Stereoselective Interaction to Overcome the Effects of Drug Resistant Mutations. J Med Chem 52: 1224-8 (2009)
- Innocenti, A; Pastorekova, S; Pastorek, J; Scozzafava, A; De Simone, G; Supuran, CT The proteoglycan region of the tumor-associated carbonic anhydrase isoform IX acts as anintrinsic buffer optimizing CO2 hydration at acidic pH values characteristic of solid tumors. Bioorg Med Chem Lett 19: 5825-8 (2009)
- Demizu, Y; Misawa, T; Nagakubo, T; Kanda, Y; Okuhira, K; Sekino, Y; Naito, M; Kurihara, M Structural development of stabilized helical peptides as inhibitors of estrogen receptor (ER)-mediated transcription. Bioorg Med Chem 23: 4132-8 (2015)
- Suzuki, S; Urano, E; Hashimoto, C; Tsutsumi, H; Nakahara, T; Tanaka, T; Nakanishi, Y; Maddali, K; Han, Y; Hamatake, M; Miyauchi, K; Pommier, Y; Beutler, JA; Sugiura, W; Fuji, H; Hoshino, T; Itotani, K; Nomura, W; Narumi, T; Yamamoto, N; Komano, JA; Tamamura, H Peptide HIV-1 integrase inhibitors from HIV-1 gene products. J Med Chem 53: 5356-60 (2010)
- Thorat, SA; Kang, DW; Ryu, H; Kim, MS; Kim, HS; Ann, J; Ha, T; Kim, SE; Son, K; Choi, S; Blumberg, PM; Frank, R; Bahrenberg, G; Schiene, K; Christoph, T; Lee, J 2-(3-Fluoro-4-methylsulfonylaminophenyl)propanamides as potent TRPV1 antagonists: structure activity relationships of the 2-oxy pyridine C-region. Eur J Med Chem 64: 589-602 (2013)
- Nair, MG; Bridges, TW; Henkel, TJ; Kisliuk, RL; Gaumont, Y; Sirotnak, FM Folate analogues altered in the C9-N10 bridge region. 18. Synthesis and antitumor evaluation of 11-oxahomoaminopterin and related compounds. J Med Chem 24: 1068-73 (1981)
- Hallén, S; Björquist, A; Ostlund-Lindqvist, AM; Sachs, G Identification of a region of the ileal-type sodium/bile acid cotransporter interacting with a competitive bile acid transport inhibitor. Biochemistry 41: 14916-14924 (2002)
- Pastor, M; Cruciani, G; Clementi, S Smart region definition: a new way to improve the predictive ability and interpretability of three-dimensional quantitative structure-activity relationships. J Med Chem 40: 1455-64 (1997)
- Rampa, A; Mancini, F; De Simone, A; Falchi, F; Belluti, F; Di Martino, RM; Gobbi, S; Andrisano, V; Tarozzi, A; Bartolini, M; Cavalli, A; Bisi, A From AChE to BACE1 inhibitors: The role of the amine on the indanone scaffold. Bioorg Med Chem Lett 25: 2804-8 (2015)
- Graham, JM; Coughenour, LL; Barr, BM; Rock, DL; Nikam, SS 1-Aminoindanes as novel motif with potential atypical antipsychotic properties. Bioorg Med Chem Lett 18: 489-93 (2008)
- Chakraborty, S; Mallick, D; Goswami, M; Guengerich, FP; Chakrabarty, A; Chowdhury, G The Natural Products Withaferin A and Withanone from the Medicinal Herb J Nat Prod 85: 2340-2350 (2022)
- Roll, DM; Barbieri, LR; Bigelis, R; McDonald, LA; Arias, DA; Chang, LP; Singh, MP; Luckman, SW; Berrodin, TJ; Yudt, MR The lecanindoles, nonsteroidal progestins from the terrestrial fungus Verticillium lecanii 6144. J Nat Prod 72: 1944-8 (2009)
- Wang, H; Wang, J; Zhao, X; Ye, R; Sun, L; Wang, J; Li, L; Liang, H; Wang, S; Lu, Y Discovery of an Anti-TNF-α 9-mer Peptide from a T7 Phage Display Library for the Treatment of Inflammatory Bowel Disease. J Med Chem 66: 6981-6993 (2023)
- Jacobsson, E; Peigneur, S; Andersson, HS; Laborde, Q; Strand, M; Tytgat, J; Göransson, U Functional Characterization of the Nemertide α Family of Peptide Toxins. J Nat Prod 84: 2121-2128 (2021)
- Lynagh, T; Kiontke, S; Meyhoff-Madsen, M; Gless, BH; Johannesen, J; Kattelmann, S; Christiansen, A; Dufva, M; Laustsen, AH; Devkota, K; Olsen, CA; Kümmel, D; Pless, SA; Lohse, B Peptide Inhibitors of the α-Cobratoxin-Nicotinic Acetylcholine Receptor Interaction. J Med Chem 63: 13709-13718 (2020)
- Einsiedel, J; Hübner, H; Hervet, M; Härterich, S; Koschatzky, S; Gmeiner, P Peptide backbone modifications on the C-terminal hexapeptide of neurotensin. Bioorg Med Chem Lett 18: 2013-8 (2008)
- Llinàs-Brunet, M; Bailey, M; Fazal, G; Goulet, S; Halmos, T; Laplante, S; Maurice, R; Poirier, M; Poupart, MA; Thibeault, D; Wernic, D; Lamarre, D Peptide-based inhibitors of the hepatitis C virus serine protease. Bioorg Med Chem Lett 8: 1713-8 (1999)
- Steel, RJ; O'Connell, MA; Searcey, M Perfluoroarene-based peptide macrocycles that inhibit the Nrf2/Keap1 interaction. Bioorg Med Chem Lett 28: 2728-2731 (2018)
- Knudsen, LB; Kiel, D; Teng, M; Behrens, C; Bhumralkar, D; Kodra, JT; Holst, JJ; Jeppesen, CB; Johnson, MD; de Jong, JC; Jorgensen, AS; Kercher, T; Kostrowicki, J; Madsen, P; Olesen, PH; Petersen, JS; Poulsen, F; Sidelmann, UG; Sturis, J; Truesdale, L; May, J; Lau, J Small-molecule agonists for the glucagon-like peptide 1 receptor. Proc Natl Acad Sci U S A 104: 937-42 (2007)
- Sgrignani, J; Cecchinato, V; Fassi, EMA; D'Agostino, G; Garofalo, M; Danelon, G; Pedotti, M; Simonelli, L; Varani, L; Grazioso, G; Uguccioni, M; Cavalli, A Systematic Development of Peptide Inhibitors Targeting the CXCL12/HMGB1 Interaction. J Med Chem 64: 13439-13450 (2021)
- Lumangtad, LA; Bell, TW The signal peptide as a new target for drug design. Bioorg Med Chem Lett 30: (2020)
- Asari, T; Takayama, K; Nakamura, A; Shimada, T; Taguchi, A; Hayashi, Y Structural Basis for the Effective Myostatin Inhibition of the Mouse Myostatin Prodomain-Derived Minimum Peptide. ACS Med Chem Lett 8: 113-117 (2017)
- Wang, L; Coric, P; Broussy, S; Di Stasi, R; Zhou, L; D'Andrea, LD; Ji, L; Vidal, M; Bouaziz, S; Liu, WQ Structural studies of the binding of an antagonistic cyclic peptide to the VEGFR1 domain 2. Eur J Med Chem 169: 65-75 (2019)
- Dziadulewicz, EK; Brown, MC; Dunstan, AR; Lee, W; Said, NB; Garratt, PJ The design of non-peptide human bradykinin B2 receptor antagonists employing the benzodiazepine peptidomimetic scaffold. Bioorg Med Chem Lett 9: 463-8 (1999)
- Song, YL; Peach, ML; Roller, PP; Qiu, S; Wang, S; Long, YQ Discovery of a novel nonphosphorylated pentapeptide motif displaying high affinity for Grb2-SH2 domain by the utilization of 3'-substituted tyrosine derivatives. J Med Chem 49: 1585-96 (2006)
- Fischer, C; Lamer, T; Fernandez, K; Gheblawi, M; Wang, W; Pascoe, C; Lambkin, G; Iturrioz, X; Llorens-Cortes, C; Oudit, GY; Vederas, JC Optimizing PEG-Extended Apelin Analogues as Cardioprotective Drug Leads: Importance of the KFRR Motif and Aromatic Head Group for Improved Physiological Activity. J Med Chem 63: 12073-12082 (2020)
- Miranda, LP; Holder, JR; Shi, L; Bennett, B; Aral, J; Gegg, CV; Wright, M; Walker, K; Doellgast, G; Rogers, R; Li, H; Valladares, V; Salyers, K; Johnson, E; Wild, K Identification of potent, selective, and metabolically stable peptide antagonists to the calcitonin gene-related peptide (CGRP) receptor. J Med Chem 51: 7889-97 (2008)
- Xia, M; Sreedharan, SP; Bolin, DR; Gaufo, GO; Goetzl, EJ Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. J Pharmacol Exp Ther 281: 629-33 (1997)
- Gebauer, S; Knütter, I; Hartrodt, B; Brandsch, M; Neubert, K; Thondorf, I Three-dimensional quantitative structure-activity relationship analyses of peptide substrates of the mammalian H+/peptide cotransporter PEPT1. J Med Chem 46: 5725-34 (2003)
- Geissler, T; Brandt, W; Porzel, A; Schlenzig, D; Kehlen, A; Wessjohann, L; Arnold, N Acetylcholinesterase inhibitors from the toadstool Cortinarius infractus. Bioorg Med Chem 18: 2173-7 (2010)
- Hong, W; Zhang, Y; Yang, J; Xia, MY; Luo, JF; Li, XN; Wang, YH; Wang, JS Alkaloids from the Branches and Leaves of J Nat Prod 82: 3221-3226 (2019)
- Yang, JH; Kondratyuk, TP; Jermihov, KC; Marler, LE; Qiu, X; Choi, Y; Cao, H; Yu, R; Sturdy, M; Huang, R; Liu, Y; Wang, LQ; Mesecar, AD; van Breemen, RB; Pezzuto, JM; Fong, HH; Chen, YG; Zhang, HJ Bioactive compounds from the fern Lepisorus contortus. J Nat Prod 74: 129-36 (2011)
- Shi, YN; Tu, ZC; Wang, XL; Yan, YM; Fang, P; Zuo, ZL; Hou, B; Yang, TH; Cheng, YX Bioactive compounds from the insect Aspongopus chinensis. Bioorg Med Chem Lett 24: 5164-9 (2014)
- Figueroa, M; González, Mdel C; Rodríguez-Sotres, R; Sosa-Peinado, A; González-Andrade, M; Cerda-García-Rojas, CM; Mata, R Calmodulin inhibitors from the fungus Emericella sp. Bioorg Med Chem 17: 2167-74 (2009)
- Jiao, WH; Li, J; Wang, D; Zhang, MM; Liu, LY; Sun, F; Li, JY; Capon, RJ; Lin, HW Cinerols, Nitrogenous Meroterpenoids from the Marine Sponge J Nat Prod 82: 2586-2593 (2019)
- Chear, NJ; León, F; Sharma, A; Kanumuri, SRR; Zwolinski, G; Abboud, KA; Singh, D; Restrepo, LF; Patel, A; Hiranita, T; Ramanathan, S; Hampson, AJ; McMahon, LR; McCurdy, CR Exploring the Chemistry of Alkaloids from Malaysian J Nat Prod 84: 1034-1043 (2021)
- Jung, M; Jang, KH; Kim, B; Lee, BH; Choi, BW; Oh, KB; Shin, J Meroditerpenoids from the brown alga Sargassum siliquastrum. J Nat Prod 71: 1714-9 (2008)
- Cho, Y; Bawkar, C; Hyun, JM; Song, MJ; Jeong, K; Lee, YJ Norterpene Cyclic Peroxides from the Marine Sponge J Nat Prod 87: 358-364 (2024)
- Song, J; Jeong, W; Wang, N; Lee, HS; Sim, CJ; Oh, KB; Shin, J Scalarane sesterterpenes from the sponge Smenospongia sp. J Nat Prod 71: 1866-71 (2008)
- Huang, XC; Li, J; Li, ZY; Shi, L; Guo, YW Sesquiterpenes from the Hainan Sponge Dysidea septosa. J Nat Prod 71: 1399-403 (2008)
- Saifudin, A; Tanaka, K; Kadota, S; Tezuka, Y Sesquiterpenes from the rhizomes of Curcuma heyneana. J Nat Prod 76: 223-9 (2013)
- Bae, J; Jeon, JE; Lee, YJ; Lee, HS; Sim, CJ; Oh, KB; Shin, J Sesterterpenes from the tropical sponge Coscinoderma sp. J Nat Prod 74: 1805-11 (2011)
- Stierle, AA; Stierle, DB; Decato, D; Priestley, ND; Alverson, JB; Hoody, J; McGrath, K; Klepacki, D The Berkeleylactones, Antibiotic Macrolides from Fungal Coculture. J Nat Prod 80: 1150-1160 (2017)
- Arai, MA; Akamine, R; Hayashi, N; Koyano, T; Kowithayakorn, T; Ishibashi, M The Notch Inhibitors Isolated from Nerium indicum. J Nat Prod 81: 1235-1240 (2018)
- Williams, DE; Cassel, J; Zhu, JL; Yang, JX; de Voogd, NJ; Matainaho, T; Salvino, JM; Wang, YA; Montaner, LJ; Tietjen, I; Andersen, RJ Thorectidiol A Isolated from the Marine Sponge J Nat Prod 86: 582-588 (2023)
- Walpole, CS; Wrigglesworth, R; Bevan, S; Campbell, EA; Dray, A; James, IF; Masdin, KJ; Perkins, MN; Winter, J Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 2. The amide bond"B-region". J Med Chem 36: 2373-80 (1993)
- Pochetti, G; Montanari, R; Gege, C; Chevrier, C; Taveras, AG; Mazza, F Extra Binding Region Induced by Non-Zinc Chelating Inhibitors into the S(1)' Subsite of Matrix Metalloproteinase 8 (MMP-8) (dagger). J Med Chem 52: 1040-9 (2009)
- Demizu, Y; Nagoya, S; Shirakawa, M; Kawamura, M; Yamagata, N; Sato, Y; Doi, M; Kurihara, M Development of stapled short helical peptides capable of inhibiting vitamin D receptor (VDR)-coactivator interactions. Bioorg Med Chem Lett 23: 4292-6 (2013)
- de Araujo, AD; Lim, J; Good, AC; Skerlj, RT; Fairlie, DP Electrophilic Helical Peptides That Bond Covalently, Irreversibly, and Selectively in a Protein-Protein Interaction Site. ACS Med Chem Lett 8: 22-26 (2017)
- Misawa, T; Demizu, Y; Kawamura, M; Yamagata, N; Kurihara, M Structural development of stapled short helical peptides as vitamin D receptor (VDR)-coactivator interaction inhibitors. Bioorg Med Chem 23: 1055-61 (2015)
- Yin, H; Lee, GI; Sedey, KA; Kutzki, O; Park, HS; Orner, BP; Ernst, JT; Wang, HG; Sebti, SM; Hamilton, AD Terphenyl-Based Bak BH3 alpha-helical proteomimetics as low-molecular-weight antagonists of Bcl-xL. J Am Chem Soc 127: 10191-6 (2005)
- Debbab, A; Aly, AH; Edrada-Ebel, R; Wray, V; Müller, WE; Totzke, F; Zirrgiebel, U; Schächtele, C; Kubbutat, MH; Lin, WH; Mosaddak, M; Hakiki, A; Proksch, P; Ebel, R Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J Nat Prod 72: 626-31 (2009)
- Mwakwari, SC; Guerrant, W; Patil, V; Khan, SI; Tekwani, BL; Gurard-Levin, ZA; Mrksich, M; Oyelere, AK Non-peptide macrocyclic histone deacetylase inhibitors derived from tricyclic ketolide skeleton. J Med Chem 53: 6100-11 (2010)
- Smith, HK; Beckett, RP; Clements, JM; Doel, S; East, SP; Launchbury, SB; Pratt, LM; Spavold, ZM; Thomas, W; Todd, RS; Whittaker, M Structure-activity relationships of the peptide deformylase inhibitor BB-3497: modification of the metal binding group. Bioorg Med Chem Lett 12: 3595-9 (2002)
- Katzman, BM; Cox, BD; Prosser, AR; Alcaraz, AA; Murat, B; Héroux, M; Tebben, A; Zhang, Y; Schroeder, GM; Snyder, JP; Wilson, LJ; Liotta, DC Tetrahydroisoquinoline CXCR4 Antagonists Adopt a Hybrid Binding Mode within the Peptide Subpocket of the CXCR4 Receptor. ACS Med Chem Lett 10: 67-73 (2019)
- Chong, H; Qiu, Z; Su, Y; He, Y The N-Terminal T-T Motif of a Third-Generation HIV-1 Fusion Inhibitor Is Not Required for Binding Affinity and Antiviral Activity. J Med Chem 58: 6378-88 (2015)
- Davies, SJ; Ayscough, AP; Beckett, RP; Bragg, RA; Clements, JM; Doel, S; Grew, C; Launchbury, SB; Perkins, GM; Pratt, LM; Smith, HK; Spavold, ZM; Thomas, SW; Todd, RS; Whittaker, M Structure-activity relationships of the peptide deformylase inhibitor BB-3497: modification of the methylene spacer and the P1' side chain. Bioorg Med Chem Lett 13: 2709-13 (2003)
- Huang, H; Feng, X; Xiao, Z; Liu, L; Li, H; Ma, L; Lu, Y; Ju, J; She, Z; Lin, Y Azaphilones and p-terphenyls from the mangrove endophytic fungus Penicillium chermesinum (ZH4-E2) isolated from the South China Sea. J Nat Prod 74: 997-1002 (2011)
- Wang, Jx; Bray, AM; DiPasquale, AJ; Maeji, N; Geysen, H Application of the multipin peptide synthesis technique for peptide receptor binding studies: Substance P as A model system Bioorg Med Chem Lett 3: 447-450 (1993)
- Greco, MN; Powell, ET; Hecker, LR; Andrade-Gordon, P; Kauffman, JA; Lewis, JM; Ganesh, V; Tulinsky, A; Maryanoff, BE Novel thrombin inhibitors that are based on a macrocyclic tripeptide motif Bioorg Med Chem Lett 6: 2947-2952 (1996)
- Filipski, KJ; Guzman-Perez, A; Bian, J; Perreault, C; Aspnes, GE; Didiuk, MT; Dow, RL; Hank, RF; Jones, CS; Maguire, RJ; Tu, M; Zeng, D; Liu, S; Knafels, JD; Litchfield, J; Atkinson, K; Derksen, DR; Bourbonais, F; Gajiwala, KS; Hickey, M; Johnson, TO; Humphries, PS; Pfefferkorn, JA Pyrimidone-based series of glucokinase activators with alternative donor-acceptor motif. Bioorg Med Chem Lett 23: 4571-8 (2013)
- Ahmadi, R; Emami, S Recent applications of vinyl sulfone motif in drug design and discovery. Eur J Med Chem 234: (2022)
- Mucha, A; Kafarski, P; Berlicki, L Remarkable potential of thea-aminophosphonate/phosphinate structural motif in medicinal chemistry. J Med Chem 54: 5955-80 (2011)
- Pepanian, A; Binbay, FA; Roy, S; Nubbemeyer, B; Koley, A; Rhodes, CA; Ammer, H; Pei, D; Ghosh, P; Imhof, D Bicyclic Peptide Library Screening for the Identification of Gαi Protein Modulators. J Med Chem 66: 12396-12406 (2023)
- Aeed, PA; Young, CL; Nagiec, MM; Elhammer, AP Inhibition of inositol phosphorylceramide synthase by the cyclic peptide aureobasidin A. Antimicrob Agents Chemother 53: 496-504 (2009)
- Giuliani, G; Cappelli, A; Matarrese, M; Masiello, V; Turolla, EA; Monterisi, C; Fazio, F; Anzini, M; Pericot Mohr, G; Riitano, D; Finetti, F; Morbidelli, L; Ziche, M; Giorgi, G; Vomero, S Non-peptide NK1 receptor ligands based on the 4-phenylpyridine moiety. Bioorg Med Chem 19: 2242-51 (2011)
- Kok, ZY; Stoddart, LA; Mistry, SJ; Mocking, TAM; Vischer, HF; Leurs, R; Hill, SJ; Mistry, SN; Kellam, B Optimization of Peptide Linker-Based Fluorescent Ligands for the Histamine H J Med Chem 65: 8258-8288 (2022)
- Talma, M; Maślanka, M; Mucha, A Recent developments in the synthesis and applications of phosphinic peptide analogs. Bioorg Med Chem Lett 29: 1031-1042 (2019)
- Joshi, AA; Murray, TF; Aldrich, JV Structure-Activity Relationships of the Peptide Kappa Opioid Receptor Antagonist Zyklophin. J Med Chem 58: 8783-95 (2015)
- Boteju, LW; Zalewska, T; Yamamura, HI; Hruby, VJ Tryptophan-norleucine 1,5-disubstituted tetrazoles as cis peptide bond mimics: Investigation of the bioactive conformation of a potent and selective peptide for the cholecystokinin-B receptor Bioorg Med Chem Lett 3: 2011-2016 (1993)
- Walpole, CS; Wrigglesworth, R; Bevan, S; Campbell, EA; Dray, A; James, IF; Masdin, KJ; Perkins, MN; Winter, J Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 3. The hydrophobic side-chain"C-region". J Med Chem 36: 2381-9 (1993)
- Pfefferkorn, JA; Nugent, R; Gross, RJ; Greene, M; Mitchell, MA; Reding, MT; Funk, LA; Anderson, R; Wells, PA; Shelly, JA; Anstadt, R; Finzel, BC; Harris, MS; Kilkuskie, RE; Kopta, LA; Schwende, FJ Inhibitors of HCV NS5B polymerase. Part 2: Evaluation of the northern region of (2Z)-2-benzoylamino-3-(4-phenoxy-phenyl)-acrylic acid. Bioorg Med Chem Lett 15: 2812-8 (2005)
- McKinnie, SMK; Wang, W; Fischer, C; McDonald, T; Kalin, KR; Iturrioz, X; Llorens-Cortes, C; Oudit, GY; Vederas, JC Synthetic Modification within the"RPRL" Region of Apelin Peptides: Impact on Cardiovascular Activity and Stability to Neprilysin and Plasma Degradation. J Med Chem 60: 6408-6427 (2017)
- Jeong, JH; Oh, YJ; Lho, Y; Park, SY; Liu, KH; Ha, E; Seo, YH Targeting the entry region of Hsp90's ATP binding pocket with a novel 6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl amide. Eur J Med Chem 124: 1069-1080 (2016)
- Wang, C; Wang, X; Wang, H; Pu, J; Li, Q; Li, J; Liu, Y; Lu, L; Jiang, S A "Two-Birds-One-Stone" Approach toward the Design of Bifunctional Human Immunodeficiency Virus Type 1 Entry Inhibitors Targeting the CCR5 Coreceptor and gp41 N-Terminal Heptad Repeat Region. J Med Chem 64: 11460-11471 (2021)
- Di Fiore, A; Pedone, C; D'Ambrosio, K; Scozzafava, A; De Simone, G; Supuran, CT Carbonic anhydrase inhibitors: Valdecoxib binds to a different active site region of the human isoform II as compared to the structurally related cyclooxygenase II"selective" inhibitor celecoxib. Bioorg Med Chem Lett 16: 437-42 (2005)
- Jiang, X; Huang, B; Olotu, FA; Li, J; Kang, D; Wang, Z; De Clercq, E; Soliman, MES; Pannecouque, C; Liu, X; Zhan, P Exploiting the tolerant region I of the non-nucleoside reverse transcriptase inhibitor (NNRTI) binding pocket. Part 2: Discovery of diarylpyrimidine derivatives as potent HIV-1 NNRTIs with high Fsp Eur J Med Chem 213: (2021)
- Carney, JR; Scheuer, PJ; Kelly-Borges, M A new bastadin from the sponge Psammaplysilla purpurea. J Nat Prod 56: 153-7 (1993)
- Trinh Thi Thanh, V; Doan Thi Mai, H; Pham, VC; Litaudon, M; Dumontet, V; Guéritte, F; Nguyen, VH; Chau, VM Acetylcholinesterase inhibitors from the leaves of Macaranga kurzii. J Nat Prod 75: 2012-2015 (2012)
- Kawagishi, H; Miyazawa, T; Kume, H; Arimoto, Y; Inakuma, T Aldehyde dehydrogenase inhibitors from the mushroom Clitocybe clavipes. J Nat Prod 65: 1712-4 (2002)
- Carroll, AR; Addepalli, R; Fechner, G; Smith, J; Guymer, GP; Forster, PI; Quinn, RJ Alkaloids from the Australian rainforest tree Ochrosia moorei. J Nat Prod 71: 1063-5 (2008)
- Ren, J; Ding, SS; Zhu, A; Cao, F; Zhu, HJ Bioactive Azaphilone Derivatives from the Fungus Talaromyces aculeatus. J Nat Prod 80: 2199-2203 (2017)
- Wang, LY; Chen, MH; Wu, J; Sun, H; Liu, W; Qu, YH; Li, YC; Wu, YZ; Li, R; Zhang, D; Wang, SJ; Lin, S Bioactive Glycosides from the Twigs of Litsea cubeba. J Nat Prod 80: 1808-1818 (2017)
- Yang, P; Liu, DQ; Liang, TJ; Li, J; Zhang, HY; Liu, AH; Guo, YW; Mao, SC Bioactive constituents from the green alga Caulerpa racemosa. Bioorg Med Chem 23: 38-45 (2014)
- Dai, Y; Zhou, GX; Kurihara, H; Ye, WC; Yao, XS Biphenyl glycosides from the fruit of Pyracantha fortuneana. J Nat Prod 69: 1022-4 (2006)
- Lerch, ML; Harper, MK; Faulkner, DJ Brominated polyacetylenes from the Philippines sponge Diplastrella sp. J Nat Prod 66: 667-70 (2003)
- Gan, M; Liu, M; Liu, B; Lin, S; Zhang, Y; Zi, J; Song, W; Ye, F; Chen, X; Shi, J Cucurbitane glucosides from the root of Machilus yaoshansis. J Nat Prod 74: 2431-7 (2011)
- Jeon, JE; Na, Z; Jung, M; Lee, HS; Sim, CJ; Nahm, K; Oh, KB; Shin, J Discorhabdins from the Korean marine sponge Sceptrella sp. J Nat Prod 73: 258-62 (2010)
- Cui, L; Thuong, PT; Lee, HS; Ndinteh, DT; Mbafor, JT; Fomum, ZT; Oh, WK Flavanones from the stem bark of Erythrina abyssinica. Bioorg Med Chem 16: 10356-62 (2008)
- Bracegirdle, J; Gordon, DP; Harvey, JE; Keyzers, RA Kinase-Inhibitory Nucleoside Derivatives from the Pacific Bryozoan J Nat Prod 83: 547-551 (2020)
- Phan, CS; Mehjabin, JJ; Anas, ARJ; Hayasaka, M; Onoki, R; Wang, J; Umezawa, T; Washio, K; Morikawa, M; Okino, T Nostosin G and Spiroidesin B from the Cyanobacterium J Nat Prod 85: 2000-2005 (2022)
- Cai, R; Jiang, H; Mo, Y; Guo, H; Li, C; Long, Y; Zang, Z; She, Z Ophiobolin-Type Sesterterpenoids from the Mangrove Endophytic Fungus J Nat Prod 82: 2268-2278 (2019)
- Raksat, A; Phukhatmuen, P; Yang, J; Maneerat, W; Charoensup, R; Andersen, RJ; Wang, YA; Pyne, SG; Laphookhieo, S Phloroglucinol Benzophenones and Xanthones from the Leaves of J Nat Prod 83: 164-168 (2020)
- Fan, JR; Kuang, Y; Dong, ZY; Yi, Y; Zhou, YX; Li, B; Qiao, X; Ye, M Prenylated Phenolic Compounds from the Aerial Parts of J Nat Prod 83: 814-824 (2020)
- Chen, QC; Youn, U; Min, BS; Bae, K Pyronane monoterpenoids from the fruit of Gardenia jasminoides. J Nat Prod 71: 995-9 (2008)
- Willson, TM; Brown, PJ; Sternbach, DD; Henke, BR The PPARs: from orphan receptors to drug discovery. J Med Chem 43: 527-50 (2000)
- Arai, MA; Masada, A; Ohtsuka, T; Kageyama, R; Ishibashi, M The first Hes1 dimer inhibitors from natural products. Bioorg Med Chem Lett 19: 5778-81 (2009)
- Anas, AR; Kisugi, T; Umezawa, T; Matsuda, F; Campitelli, MR; Quinn, RJ; Okino, T Thrombin inhibitors from the freshwater cyanobacterium Anabaena compacta. J Nat Prod 75: 1546-52 (2012)
- Dai, J; Sorribas, A; Yoshida, WY; Kelly, M; Williams, PG Xestosaprols from the Indonesian marine sponge Xestospongia sp. J Nat Prod 73: 1188-91 (2010)
- Rodriguez, M; Lignon, MF; Galas, MC; Fulcrand, P; Mendre, C; Aumelas, A; Laur, J; Martinez, J Synthesis and biological activities of pseudopeptide analogues of the C-terminal heptapeptide of cholecystokinin. On the importance of the peptide bonds. J Med Chem 30: 1366-73 (1987)
- Adiv, S; Carmeli, S Protease inhibitors from Microcystis aeruginosa bloom material collected from the Dalton Reservoir, Israel. J Nat Prod 76: 2307-15 (2013)
- Lauer-Fields, JL; Minond, D; Chase, PS; Baillargeon, PE; Saldanha, SA; Stawikowska, R; Hodder, P; Fields, GB High throughput screening of potentially selective MMP-13 exosite inhibitors utilizing a triple-helical FRET substrate. Bioorg Med Chem 17: 990-1005 (2009)
- Vig, BS; Zheng, MQ; Murray, TF; Aldrich, JV Effects of the substitution of Phe4 in the opioid peptide [D-Ala8]dynorphin A-(1-11)NH2. J Med Chem 46: 4002-8 (2003)
- Xie, X; He, J Multivalent peptide dendrimers inhibit the fusion of viral-cellular membranes and the cellular NF-κB signaling pathway. Eur J Med Chem 230: (2022)
- Pilla, E; Kilisch, M; Lenz, C; Urlaub, H; Geiss-Friedlander, R The SUMO1-E67 interacting loop peptide is an allosteric inhibitor of the dipeptidyl peptidases 8 and 9. J Biol Chem 288: 32787-96 (2013)
- Bell, IM; Gallicchio, SN; Stump, CA; Bruno, JG; Fan, H; Gantert, LT; Hostetler, ED; Kemmerer, AL; McWherter, M; Moore, EL; Mosser, SD; Purcell, ML; Riffel, K; Salvatore, CA; Sanabria-Bohórquez, S; Staas, DD; White, RB; Williams, M; Zartman, CB; Cook, JJ; Hargreaves, RJ; Kane, SA; Graham, SL; Selnick, HG [(11)C]MK-4232: The First Positron Emission Tomography Tracer for the Calcitonin Gene-Related Peptide Receptor. ACS Med Chem Lett 4: 863-8 (2013)
- Su, DS; Lim, JJ; Tinney, E; Tucker, TJ; Saggar, S; Sisko, JT; Wan, BL; Young, MB; Anderson, KD; Rudd, D; Munshi, V; Bahnck, C; Felock, PJ; Lu, M; Lai, MT; Touch, S; Moyer, G; Distefano, DJ; Flynn, JA; Liang, Y; Sanchez, R; Perlow-Poehnelt, R; Miller, M; Vacca, JP; Williams, TM; Anthony, NJ Biaryl ethers as potent allosteric inhibitors of reverse transcriptase and its key mutant viruses: aryl substituted pyrazole as a surrogate for the pyrazolopyridine motif. Bioorg Med Chem Lett 20: 4328-32 (2010)
- Wang, K; Xu, CH; Tong, L; Wang, B; Liu, AH; Li, SW; Mao, SC Coriaceumins A-D, the First Nitrogen-Containing Crenulide Diterpenoids from the Brown Alga J Nat Prod 87: 121-131 (2024)
- Olivon, F; Nothias, LF; Dumontet, V; Retailleau, P; Berger, S; Ferry, G; Cohen, W; Pfeiffer, B; Boutin, JA; Scalbert, E; Roussi, F; Litaudon, M Natural Inhibitors of the RhoA-p115 Complex from the Bark of Meiogyne baillonii. J Nat Prod 81: 1610-1618 (2018)
- Ben Salem, S; Jabrane, A; Harzallah-Skhiri, F; Ben Jannet, H New bioactive dihydrofuranocoumarins from the roots of the Tunisian Ferula lutea (Poir.) Maire. Bioorg Med Chem Lett 23: 4248-52 (2013)
- Vullo, D; De Luca, V; Del Prete, S; Carginale, V; Scozzafava, A; Osman, SM; AlOthman, Z; Capasso, C; Supuran, CT Sulfonamide inhibition studies of the¿-carbonic anhydrase from the Antarctic bacterium Colwellia psychrerythraea. Bioorg Med Chem Lett 26: 1253-9 (2016)
- Vullo, D; De Luca, V; Del Prete, S; Carginale, V; Scozzafava, A; Capasso, C; Supuran, CT Sulfonamide inhibition studies of the¿-carbonic anhydrase from the Antarctic bacterium Pseudoalteromonas haloplanktis. Bioorg Med Chem Lett 25: 3550-5 (2015)
- Vullo, D; De Luca, V; Del Prete, S; Carginale, V; Scozzafava, A; Capasso, C; Supuran, CT Sulfonamide inhibition studies of the¿-carbonic anhydrase from the Antarctic cyanobacterium Nostoc commune. Bioorg Med Chem 23: 1728-34 (2015)
- Wang, C; McFadyen, IJ; Traynor, JR; Mosberg, HI Design of a high affinity peptidomimetic opioid agonist from peptide pharmacophore models. Bioorg Med Chem Lett 8: 2685-8 (1999)
- Odagami, T; Tsuda, Y; Kogami, Y; Kouji, H; Okada, Y Identification of new agonists of urotensin-II from a cyclic peptide library. Bioorg Med Chem 17: 6742-7 (2009)
- Camps, P; Gómez, E; Muñoz-Torrero, D; Badia, A; Clos, MV; Curutchet, C; Muñoz-Muriedas, J; Luque, FJ Binding of 13-amidohuprines to acetylcholinesterase: exploring the ligand-induced conformational change of the gly117-gly118 peptide bond in the oxyanion hole. J Med Chem 49: 6833-40 (2006)
- Pasquini, S; De Rosa, M; Pedani, V; Mugnaini, C; Guida, F; Luongo, L; De Chiaro, M; Maione, S; Dragoni, S; Frosini, M; Ligresti, A; Di Marzo, V; Corelli, F Investigations on the 4-quinolone-3-carboxylic acid motif. 4. Identification of new potent and selective ligands for the cannabinoid type 2 receptor with diverse substitution patterns and antihyperalgesic effects in mice. J Med Chem 54: 5444-53 (2011)
- Lee, J; Kang, SU; Kil, MJ; Shin, M; Lim, JO; Choi, HK; Jin, MK; Kim, SY; Kim, SE; Lee, YS; Min, KH; Kim, YH; Ha, HJ; Tran, R; Welter, JD; Wang, Y; Szabo, T; Pearce, LV; Lundberg, DJ; Toth, A; Pavlyukovets, VA; Morgan, MA; Blumberg, PM Analysis of structure-activity relationships for the 'A-region' of N-(4-t-butylbenzyl)-N'-[4-(methylsulfonylamino)benzyl]thiourea analogues as TRPV1 antagonists. Bioorg Med Chem Lett 15: 4136-42 (2005)
- Lee, J; Jin, MK; Kang, SU; Kim, SY; Lee, J; Shin, M; Hwang, J; Cho, S; Choi, YS; Choi, HK; Kim, SE; Suh, YG; Lee, YS; Kim, YH; Ha, HJ; Toth, A; Pearce, LV; Tran, R; Szabo, T; Welter, JD; Lundberg, DJ; Wang, Y; Lazar, J; Pavlyukovets, VA; Morgan, MA; Blumberg, PM Analysis of structure-activity relationships for the 'B-region' of N-(4-t-butylbenzyl)-N'-[4-(methylsulfonylamino)benzyl]-thiourea analogues as TRPV1 antagonists. Bioorg Med Chem Lett 15: 4143-50 (2005)
- Pfefferkorn, JA; Greene, ML; Nugent, RA; Gross, RJ; Mitchell, MA; Finzel, BC; Harris, MS; Wells, PA; Shelly, JA; Anstadt, RA; Kilkuskie, RE; Kopta, LA; Schwende, FJ Inhibitors of HCV NS5B polymerase. Part 1: Evaluation of the southern region of (2Z)-2-(benzoylamino)-3-(5-phenyl-2-furyl)acrylic acid. Bioorg Med Chem Lett 15: 2481-6 (2005)
- Ahn, YM; Clare, M; Ensinger, CL; Hood, MM; Lord, JW; Lu, WP; Miller, DF; Patt, WC; Smith, BD; Vogeti, L; Kaufman, MD; Petillo, PA; Wise, SC; Abendroth, J; Chun, L; Clark, R; Feese, M; Kim, H; Stewart, L; Flynn, DL Switch control pocket inhibitors of p38-MAP kinase. Durable type II inhibitors that do not require binding into the canonical ATP hinge region. Bioorg Med Chem Lett 20: 5793-8 (2010)
- Porter, RA; Chan, WN; Coulton, S; Johns, A; Hadley, MS; Widdowson, K; Jerman, JC; Brough, SJ; Coldwell, M; Smart, D; Jewitt, F; Jeffrey, P; Austin, N 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett 11: 1907-10 (2001)
- Mollica, A; Pinnen, F; Costante, R; Locatelli, M; Stefanucci, A; Pieretti, S; Davis, P; Lai, J; Rankin, D; Porreca, F; Hruby, VJ Biological active analogues of the opioid peptide biphalin: mixeda/ß(3)-peptides. J Med Chem 56: 3419-23 (2013)
- Hossain, MA; Bathgate, RAD Challenges in the design of insulin and relaxin/insulin-like peptide mimetics. Bioorg Med Chem 26: 2827-2841 (2018)
- Thakkar, R; Upreti, D; Ishiguro, S; Tamura, M; Comer, J Computational design of a cyclic peptide that inhibits the CTLA4 immune checkpoint. RSC Med Chem 14: 658-670 (2023)
- Duggineni, S; Mitra, S; Lamberto, I; Han, X; Xu, Y; An, J; Pasquale, EB; Huang, Z Design and Synthesis of Potent Bivalent Peptide Agonists Targeting the EphA2 Receptor. ACS Med Chem Lett 4: 344-8 (2013)
- Evans, BE; Bock, MG; Rittle, KE; DiPardo, RM; Whitter, WL; Veber, DF; Anderson, PS; Freidinger, RM Design of potent, orally effective, nonpeptidal antagonists of the peptide hormone cholecystokinin. Proc Natl Acad Sci U S A 83: 4918-22 (1986)
- Bursavich, MG; Rich, DH Designing non-peptide peptidomimetics in the 21st century: inhibitors targeting conformational ensembles. J Med Chem 45: 541-58 (2002)
- Harrison, RES; Zewde, NT; Narkhede, YB; Hsu, RV; Morikis, D; Vullev, VI; Palermo, G Factor H-Inspired Design of Peptide Biomarkers of the Complement C3d Protein. ACS Med Chem Lett 11: 1054-1059 (2020)
- Röver, S; Adam, G; Cesura, AM; Galley, G; Jenck, F; Monsma, FJ; Wichmann, J; Dautzenberg, FM High-affinity, non-peptide agonists for the ORL1 (orphanin FQ/nociceptin) receptor. J Med Chem 43: 1329-38 (2001)
- Herrero, S; Suarez-Gea, M; Gonzalez-Muniz, R; Garcia-Lopez, M; Herranz, R; Ballaz, S; Barber, A; Fortuno, A; Del Rio, J Pseudopeptide CCK-4 analogues incorporating the [CH(CN)NH] peptide bond surrogate Bioorg Med Chem Lett 7: 855-860 (1997)
- Ulysse, LG; Chmielewski, J Restricting the flexibility of crosslinked, interfacial peptide inhibitors of HIV-1 protease. Bioorg Med Chem Lett 8: 3281-6 (1999)
- Zhou, J; Li, Y; Huang, W; Shi, W; Qian, H Source and exploration of the peptides used to construct peptide-drug conjugates. Eur J Med Chem 224: (2021)
- Horwell, DC; Howson, W; Ratcliffe, GS; Rees, DC The design of a dipeptide library for screening at peptide receptor sites Bioorg Med Chem Lett 3: 799-802 (1993)
- Molinski, TF; Reynolds, KA; Morinaka, BI Symplocin A, a linear peptide from the Bahamian cyanobacterium Symploca sp. Configurational analysis of N,N-dimethylamino acids by chiral-phase HPLC of naphthacyl esters. J Nat Prod 75: 425-31 (2012)
- Gademann, K; Kimmerlin, T; Hoyer, D; Seebach, D Peptide folding induces high and selective affinity of a linear and small beta-peptide to the human somatostatin receptor 4. J Med Chem 44: 2460-8 (2001)
- Zhang, C; Wang, X; Liu, H; Zhang, M; Geng, M; Sun, L; Shen, A; Zhang, A Design, synthesis and pharmacological evaluation of 4,5-diarylisoxazols bearing amino acid residues within the 3-amido motif as potent heat shock protein 90 (Hsp90) inhibitors. Eur J Med Chem 125: 315-326 (2017)
- Georgsson, J; Bergström, F; Nordqvist, A; Watson, MJ; Blundell, CD; Johansson, MJ; Petersson, AU; Yuan, ZQ; Zhou, Y; Kristensson, L; Kakol-Palm, D; Tyrchan, C; Wellner, E; Bauer, U; Brodin, P; Svensson Henriksson, A GPR103 antagonists demonstrating anorexigenic activity in vivo: design and development of pyrrolo[2,3-c]pyridines that mimic the C-terminal Arg-Phe motif of QRFP26. J Med Chem 57: 5935-48 (2014)
- Borner, T; Tinsley, IC; Milliken, BT; Doebley, SA; Najjar, NR; Kerwood, DJ; De Jonghe, BC; Hayes, MR; Doyle, RP Creation of a Peptide Antagonist of the GFRAL-RET Receptor Complex for the Treatment of GDF15-Induced Malaise. J Med Chem 66: 11237-11249 (2023)
- de Veer, SJ; Wang, CK; Harris, JM; Craik, DJ; Swedberg, JE Improving the Selectivity of Engineered Protease Inhibitors: Optimizing the P2 Prime Residue Using a Versatile Cyclic Peptide Library. J Med Chem 58: 8257-68 (2015)
- Davies, SJ; Ayscough, AP; Beckett, RP; Clements, JM; Doel, S; Pratt, LM; Spavold, ZM; Thomas, SW; Whittaker, M Structure--activity relationships of the peptide deformylase inhibitor BB-3497: modification of the P2' and P3' side chains. Bioorg Med Chem Lett 13: 2715-8 (2003)
- Ovadia, O; Linde, Y; Haskell-Luevano, C; Dirain, ML; Sheynis, T; Jelinek, R; Gilon, C; Hoffman, A The effect of backbone cyclization on PK/PD properties of bioactive peptide-peptoid hybrids: the melanocortin agonist paradigm. Bioorg Med Chem 18: 580-9 (2010)
- Vullo, D; Del Prete, S; Fisher, GM; Andrews, KT; Poulsen, SA; Capasso, C; Supuran, CT Sulfonamide inhibition studies of the¿-class carbonic anhydrase from the malaria pathogen Plasmodium falciparum. Bioorg Med Chem 23: 526-31 (2015)
- Vullo, D; De Luca, V; Scozzafava, A; Carginale, V; Rossi, M; Supuran, CT; Capasso, C Anion inhibition studies of the fastest carbonic anhydrase (CA) known, the extremo-CA from the bacterium Sulfurihydrogenibium azorense. Bioorg Med Chem Lett 22: 7142-5 (2012)
- Wu, Y; Shi, C; Sun, X; Wu, X; Sun, H Synthesis, biological evaluation and docking studies of octane-carboxamide based renin inhibitors with extended segments toward S3' site of renin. Bioorg Med Chem 19: 4238-49 (2011)
- Han, X; Gao, H; Lai, H; Zhu, W; Wang, Y Anti-Aβ42 Aggregative Polyketides from the Antarctic Psychrophilic Fungus J Nat Prod 86: 882-890 (2023)
- Nguyen, CN; Trinh, BTD; Tran, TB; Nguyen, LT; Jäger, AK; Nguyen, LD Anti-diabetic xanthones from the bark of Garcinia xanthochymus. Bioorg Med Chem Lett 27: 3301-3304 (2017)
- Alilou, M; Dibwe, DF; Schwaiger, S; Khodami, M; Troppmair, J; Awale, S; Stuppner, H Antiausterity Activity of Secondary Metabolites from the Roots of J Nat Prod 83: 1099-1106 (2020)
- Li, YT; Yang, C; Wu, Y; Lv, JJ; Feng, X; Tian, X; Zhou, Z; Pan, X; Liu, S; Tian, LW Axial Chiral Binaphthoquinone and Perylenequinones from the Stromata of J Nat Prod 84: 436-443 (2021)
- Won, TH; Jeon, JE; Lee, SH; Rho, BJ; Oh, KB; Shin, J Beta-carboline alkaloids derived from the ascidian Synoicum sp. Bioorg Med Chem 20: 4082-7 (2012)
- Arvizu-Espinosa, MG; von Poser, GL; Henriques, AT; Mendoza-Ruiz, A; Cardador-Martínez, A; Gesto-Borroto, R; Núñez-Aragón, PN; Villarreal-Ortega, ML; Sharma, A; Cardoso-Taketa, A Bioactive Dimeric Acylphloroglucinols from the Mexican Fern Elaphoglossum paleaceum. J Nat Prod 82: 785-791 (2019)
- Homhual, S; Bunyapraphatsara, N; Kondratyuk, T; Herunsalee, A; Chaukul, W; Pezzuto, JM; Fong, HH; Zhang, HJ Bioactive dammarane triterpenes from the mangrove plant Bruguiera gymnorrhiza. J Nat Prod 69: 421-4 (2006)
- Gualtieri, MJ; Malafronte, N; Vassallo, A; Braca, A; Cotugno, R; Vasaturo, M; De Tommasi, N; Dal Piaz, F Bioactive limonoids from the leaves of Azaridachta indica (Neem). J Nat Prod 77: 596-602 (2014)
- Ge, HM; Huang, B; Tan, SH; Shi, da H; Song, YC; Tan, RX Bioactive oligostilbenoids from the stem bark of Hopea exalata. J Nat Prod 69: 1800-2 (2006)
- Eder, C; Schupp, P; Proksch, P; Wray, V; Steube, K; Müller, CE; Frobenius, W; Herderich, M; van Soest, RW Bioactive pyridoacridine alkaloids from the micronesian sponge Oceanapia sp. J Nat Prod 61: 301-5 (1998)
- He, ZZ; Yan, JF; Song, ZJ; Ye, F; Liao, X; Peng, SL; Ding, LS Chemical constituents from the aerial parts of Artemisia minor. J Nat Prod 72: 1198-201 (2009)
- Stierle, AA; Stierle, DB; Decato, D; Alverson, J; Apedaile, L Cryptic Biosynthesis of the Berkeleypenostatins from Coculture of Extremophilic J Nat Prod 84: 1656-1665 (2021)
- Chen, C; Qiang, S; Lou, L; Zhao, W Cucurbitane-type triterpenoids from the stems of Cucumis melo. J Nat Prod 72: 824-9 (2010)
- Kodani, S; Ishida, K; Murakami, M Dehydroradiosumin, a trypsin inhibitor from the cyanobacterium Anabaena cylindrica. J Nat Prod 61: 854-6 (1998)
- Mu, H; Tang, S; Zuo, Q; Huang, M; Zhao, W Dihydro-β-agarofuran-Type Sesquiterpenoids from the Seeds of J Nat Prod 84: 588-600 (2021)
- Chawengrum, P; Boonsombat, J; Mahidol, C; Eurtivong, C; Kittakoop, P; Thongnest, S; Ruchirawat, S Diterpenoids with Aromatase Inhibitory Activity from the Rhizomes of J Nat Prod 84: 1738-1747 (2021)
- Fiene, A; Baqi, Y; Malik, EM; Newton, P; Li, W; Lee, SY; Hartland, EL; Müller, CE Inhibitors for the bacterial ectonucleotidase Lp1NTPDase from Legionella pneumophila. Bioorg Med Chem 24: 4363-4371 (2016)
- Cui, L; Ndinteh, DT; Na, M; Thuong, PT; Silike-Muruumu, J; Njamen, D; Mbafor, JT; Fomum, ZT; Ahn, JS; Oh, WK Isoprenylated flavonoids from the stem bark of Erythrina abyssinica. J Nat Prod 70: 1039-42 (2007)
- Kaneko, A; Tsukada, M; Fukai, M; Suzuki, T; Nishio, K; Miki, K; Kinoshita, K; Takahashi, K; Koyama, K KDR Kinase Inhibitor Isolated from the Mushroom Boletopsis leucomelas. J Nat Prod 73: 1002-4 (2010)
- Yao, FH; Liang, X; Shen, WB; Lu, XH; Li, GC; Qi, SH Microascones, Decahydrofluorene-Class Alkaloids from the Marine-Derived Fungus J Nat Prod 87: 810-819 (2024)
- Turroni, S; Brigidi, P; Cavalli, A; Candela, M Microbiota-Host Transgenomic Metabolism, Bioactive Molecules from the Inside. J Med Chem 61: 47-61 (2018)
- Horton, PA; Longley, RE; Kelly-Borges, M; McConnell, OJ; Ballas, LM New cytotoxic peroxylactones from the marine sponge, Plakinastrella onkodes. J Nat Prod 57: 1374-81 (1994)
- Cui, Y; Yim, JH; Lee, DS; Kim, YC; Oh, H New diterpene furanoids from the Antarctic lichen Huea sp. Bioorg Med Chem Lett 22: 7393-6 (2012)
- Hwang, JH; Hong, SS; Han, XH; Hwang, JS; Lee, D; Lee, H; Yun, YP; Kim, Y; Ro, JS; Hwang, BY Prenylated xanthones from the root bark of Cudrania tricuspidata. J Nat Prod 70: 1207-9 (2007)
- Zaman, KAU; Park, JH; DeVine, L; Hu, Z; Wu, X; Kim, HS; Cao, S Secondary Metabolites from the Leather Coral-Derived Fungal Strain J Nat Prod 84: 466-473 (2021)
- Li, J; Tang, H; Kurtán, T; Mándi, A; Zhuang, CL; Su, L; Zheng, GL; Zhang, W Swinhoeisterols from the South China Sea Sponge Theonella swinhoei. J Nat Prod 81: 1645-1650 (2018)
- Niemann, H; Lin, W; Müller, WE; Kubbutat, M; Lai, D; Proksch, P Trimeric hemibastadin congener from the marine sponge Ianthella basta. J Nat Prod 76: 121-5 (2013)
- Wada, S; Iida, A; Tanaka, R Triterpenoid constituents isolated from the bark of Abies sachalinensis. J Nat Prod 65: 1657-9 (2002)
- Ngoc, TM; Lee, I; Ha, do T; Kim, H; Min, B; Bae, K Tyrosinase-inhibitory constituents from the twigs of Cinnamomum cassia. J Nat Prod 72: 1205-8 (2009)
- Lacivita, E; Patarnello, D; Stroth, N; Caroli, A; Niso, M; Contino, M; De Giorgio, P; Di Pilato, P; Colabufo, NA; Berardi, F; Perrone, R; Svenningsson, P; Hedlund, PB; Leopoldo, M Investigations on the 1-(2-biphenyl)piperazine motif: identification of new potent and selective ligands for the serotonin(7) (5-HT(7)) receptor with agonist or antagonist action in vitro or ex vivo. J Med Chem 55: 6375-80 (2012)
- Tasdemir, D; Topaloglu, B; Perozzo, R; Brun, R; O'Neill, R; Carballeira, NM; Zhang, X; Tonge, PJ; Linden, A; Rüedi, P Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg Med Chem 15: 6834-45 (2007)
- Chakravarty, S; Wilkins, D; Kyle, DJ Design of potent, cyclic peptide bradykinin receptor antagonists from conformationally constrained linear peptides. J Med Chem 36: 2569-71 (1993)
- Maes, M; Levin, A; Hayouka, Z; Shalev, DE; Loyter, A; Friedler, A Peptide inhibitors of HIV-1 integrase: from mechanistic studies to improved lead compounds. Bioorg Med Chem 17: 7635-42 (2009)
- Ramamoorthy, S; Liu, W; Ma, YY; Yang-Feng, TL; Ganapathy, V; Leibach, FH Proton/peptide cotransporter (PEPT 2) from human kidney: functional characterization and chromosomal localization. Biochim Biophys Acta 1240: 1-4 (1996)
- Šimelis, K; Saraç, H; Salah, E; Nishio, K; McAllister, TE; Corner, TP; Tumber, A; Belle, R; Schofield, CJ; Suga, H; Kawamura, A Selective targeting of human TET1 by cyclic peptide inhibitors: Insights from biochemical profiling. Bioorg Med Chem 99:
- Bech, EM; Martos-Maldonado, MC; Wismann, P; Sørensen, KK; van Witteloostuijn, SB; Thygesen, MB; Vrang, N; Jelsing, J; Pedersen, SL; Jensen, KJ Peptide Half-Life Extension: Divalent, Small-Molecule Albumin Interactions Direct the Systemic Properties of Glucagon-Like Peptide 1 (GLP-1) Analogues. J Med Chem 60: 7434-7446 (2017)
- Pasquini, S; Mugnaini, C; Tintori, C; Botta, M; Trejos, A; Arvela, RK; Larhed, M; Witvrouw, M; Michiels, M; Christ, F; Debyser, Z; Corelli, F Investigations on the 4-quinolone-3-carboxylic acid motif. 1. Synthesis and structure-activity relationship of a class of human immunodeficiency virus type 1 integrase inhibitors. J Med Chem 51: 5125-9 (2008)
- Jehle, K; Cato, L; Neeb, A; Muhle-Goll, C; Jung, N; Smith, EW; Buzon, V; Carbó, LR; Estébanez-Perpiñá, E; Schmitz, K; Fruk, L; Luy, B; Chen, Y; Cox, MB; Bräse, S; Brown, M; Cato, AC Coregulator control of androgen receptor action by a novel nuclear receptor-binding motif. J Biol Chem 289: 8839-51 (2014)
- Cominetti, MM; Goffin, SA; Raffel, E; Turner, KD; Ramoutar, JC; O'Connell, MA; Howell, LA; Searcey, M Identification of a new p53/MDM2 inhibitor motif inspired by studies of chlorofusin. Bioorg Med Chem Lett 25: 4878-80 (2015)
- Atkinson, GE; Cowan, A; McInnes, C; Zheleva, DI; Fischer, PM; Chan, WC Peptide inhibitors of CDK2-cyclin A that target the cyclin recruitment-site: structural variants of the C-terminal Phe. Bioorg Med Chem Lett 12: 2501-5 (2002)
- Luca, VD; Vullo, D; Scozzafava, A; Carginale, V; Rossi, M; Supuran, CT; Capasso, C Ana-carbonic anhydrase from the thermophilic bacterium Sulphurihydrogenibium azorense is the fastest enzyme known for the CO2 hydration reaction. Bioorg Med Chem 21: 1465-9 (2013)
- Sugiyama, D; Kusuhara, H; Shitara, Y; Abe, T; Meier, PJ; Sekine, T; Endou, H; Suzuki, H; Sugiyama, Y Characterization of the efflux transport of 17beta-estradiol-D-17beta-glucuronide from the brain across the blood-brain barrier. J Pharmacol Exp Ther 298: 316-22 (2001)
- Surup, F; Shojaei, H; von Zezschwitz, P; Kunze, B; Grond, S Iromycins from Streptomyces sp. and from synthesis: new inhibitors of the mitochondrial electron transport chain. Bioorg Med Chem 16: 1738-46 (2008)
- Griffith, DA; Edmonds, DJ; Fortin, JP; Kalgutkar, AS; Kuzmiski, JB; Loria, PM; Saxena, AR; Bagley, SW; Buckeridge, C; Curto, JM; Derksen, DR; Dias, JM; Griffor, MC; Han, S; Jackson, VM; Landis, MS; Lettiere, D; Limberakis, C; Liu, Y; Mathiowetz, AM; Patel, JC; Piotrowski, DW; Price, DA; Ruggeri, RB; Tess, DA A Small-Molecule Oral Agonist of the Human Glucagon-like Peptide-1 Receptor. J Med Chem 65: 8208-8226 (2022)
- Nakayama, J; Uemura, Y; Nishiguchi, K; Yoshimura, N; Igarashi, Y; Sonomoto, K Ambuic acid inhibits the biosynthesis of cyclic peptide quormones in gram-positive bacteria. Antimicrob Agents Chemother 53: 580-6 (2009)
- Lipkowski, AW; Misicka, A; Davis, P; Stropova, D; Janders, J; Lachwa, M; Porreca, F; Yamamura, HI; Hruby, VJ Biological activity of fragments and analogues of the potent dimeric opioid peptide, biphalin. Bioorg Med Chem Lett 9: 2763-6 (1999)
- Salvino, JM; Seoane, PR; Douty, BD; Awad, MM; Dolle, RE; Houck, WT; Faunce, DM; Sawutz, DG Design of potent non-peptide competitive antagonists of the human bradykinin B2 receptor. J Med Chem 36: 2583-4 (1993)
- Lau, J; Bloch, P; Schäffer, L; Pettersson, I; Spetzler, J; Kofoed, J; Madsen, K; Knudsen, LB; McGuire, J; Steensgaard, DB; Strauss, HM; Gram, DX; Knudsen, SM; Nielsen, FS; Thygesen, P; Reedtz-Runge, S; Kruse, T Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J Med Chem 58: 7370-80 (2015)
- Dougherty, PG; Wen, J; Pan, X; Koley, A; Ren, JG; Sahni, A; Basu, R; Salim, H; Appiah Kubi, G; Qian, Z; Pei, D Enhancing the Cell Permeability of Stapled Peptides with a Cyclic Cell-Penetrating Peptide. J Med Chem 62: 10098-10107 (2019)
- McComsey, DF; Hawkins, MJ; Andrade-Gordon, P; Addo, MF; Oksenberg, D; Maryanoff, BE Heterocycle-peptide hybrid compounds. Aminotriazole-containing agonists of the thrombin receptor (PAR-1). Bioorg Med Chem Lett 9: 1423-8 (1999)
- Bendzunas, NG; Dörfler, S; Autenrieth, K; Bertinetti, D; Machal, EMF; Kennedy, EJ; Herberg, FW Investigating PKA-RII specificity using analogs of the PKA:AKAP peptide inhibitor STAD-2. Bioorg Med Chem 26: 1174-1178 (2018)
- Duprez, W; Premkumar, L; Halili, MA; Lindahl, F; Reid, RC; Fairlie, DP; Martin, JL Peptide inhibitors of the Escherichia coli DsbA oxidative machinery essential for bacterial virulence. J Med Chem 58: 577-87 (2015)
- Lowe, JA; Drozda, SE; Snider, RM; Longo, KP; Bordner, J Preparation and radiolabelling of CP-96,345, the first non-peptide substance P antagonist Bioorg Med Chem Lett 1: 129-132 (1991)
- Plut, E; Calderón, JC; Stanojlović, V; Gattor, AO; Höring, C; Humphrys, LJ; Konieczny, A; Kerres, S; Schubert, M; Keller, M; Cabrele, C; Clark, T; Reiser, O Stereochemistry-Driven Interactions of α,γ-Peptide Ligands with the Neuropeptide Y Y J Med Chem 66: 9642-9657 (2023)
- Terada, T; Sawada, K; Irie, M; Saito, H; Hashimoto, Y; Inui, K Structural requirements for determining the substrate affinity of peptide transporters PEPT1 and PEPT2. Pflugers Arch 440: 679-84 (2000)
- Mezo, AR; McDonnell, KA; Castro, A; Fraley, C Structure-activity relationships of a peptide inhibitor of the human FcRn:human IgG interaction. Bioorg Med Chem 16: 6394-405 (2008)
- Veale, CA; Alford, VC; Aharony, D; Banville, DL; Bialecki, RA; Brown, FJ; Damewood, JR; Dantzman, CL; Edwards, PD; Jacobs, RT; Mauger, RC; Murphy, MM; Palmer, W; Pine, KK; Rumsey, WL; Garcia-Davenport, LE; Shaw, A; Steelman, GB; Surian, JM; Vacek, EP The discovery of non-basic atrial natriuretic peptide clearance receptor antagonists. Part 1. Bioorg Med Chem Lett 10: 1949-52 (2001)
- Judge, RA; Zhu, H; Upadhyay, AK; Bodelle, PM; Hutchins, CW; Torrent, M; Marin, VL; Yu, W; Vedadi, M; Li, F; Brown, PJ; Pappano, WN; Sun, C; Petros, AM Turning a Substrate Peptide into a Potent Inhibitor for the Histone Methyltransferase SETD8. ACS Med Chem Lett 7: 1102-1106 (2016)
- Ganapathy, ME; Huang, W; Wang, H; Ganapathy, V; Leibach, FH Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun 246: 470-5 (1998)
- Montanari, R; Saccoccia, F; Scotti, E; Crestani, M; Godio, C; Gilardi, F; Loiodice, F; Fracchiolla, G; Laghezza, A; Tortorella, P; Lavecchia, A; Novellino, E; Mazza, F; Aschi, M; Pochetti, G Crystal structure of the peroxisome proliferator-activated receptor gamma (PPARgamma) ligand binding domain complexed with a novel partial agonist: a new region of the hydrophobic pocket could be exploited for drug design. J Med Chem 51: 7768-76 (2008)
- Greve, H; Meis, S; Kassack, MU; Kehraus, S; Krick, A; Wright, AD; König, GM New iantherans from the marine sponge Ianthella quadrangulata: novel agonists of the P2Y(11) receptor. J Med Chem 50: 5600-7 (2007)
- Hegde, VR; Borges, S; Patel, M; Das, PR; Wu, B; Gullo, VP; Chan, TM New potential antitumor compounds from the plant Aristolochia manshuriensis as inhibitors of the CDK2 enzyme. Bioorg Med Chem Lett 20: 1344-6 (2010)
- Chu, BC; DeWolf, T; Vogel, HJ Role of the two structural domains from the periplasmic Escherichia coli histidine-binding protein HisJ. J Biol Chem 288: 31409-22 (2013)
- Xie, M; Zhao, H; Liu, Q; Zhu, Y; Yin, F; Liang, Y; Jiang, Y; Wang, D; Hu, K; Qin, X; Wang, Z; Wu, Y; Xu, N; Ye, X; Wang, T; Li, Z Structural Basis of Inhibition of ERα-Coactivator Interaction by High-Affinity N-Terminus Isoaspartic Acid Tethered Helical Peptides. J Med Chem 60: 8731-8740 (2017)
- Ruiz-Santaquiteria, M; de Castro, S; Toro, MA; de Lucio, H; Gutiérrez, KJ; Sánchez-Murcia, PA; Jiménez, MÁ; Gago, F; Jiménez-Ruiz, A; Camarasa, MJ; Velázquez, S Trypanothione reductase inhibition and anti-leishmanial activity of all-hydrocarbon stapled α-helical peptides with improved proteolytic stability. Eur J Med Chem 149: 238-247 (2018)
- Summerfield, RL; Daigle, DM; Mayer, S; Mallik, D; Hughes, DW; Jackson, SG; Sulek, M; Organ, MG; Brown, ED; Junop, MS A 2.13 A structure of E. coli dihydrofolate reductase bound to a novel competitive inhibitor reveals a new binding surface involving the M20 loop region. J Med Chem 49: 6977-86 (2006)
- Sun, Y; Feng, D; Zhou, Z; Zhang, T; De Clercq, E; Pannecouque, C; Kang, D; Zhan, P; Liu, X In situ click chemistry-based discovery of 1,2,3-triazole-derived diarylpyrimidines as novel HIV-1 NNRTIs by exploiting the tolerant region I in binding pocket. Bioorg Med Chem 96: (2023)
- Pan, P; Vermelho, AB; Scozzafava, A; Parkkila, S; Capasso, C; Supuran, CT Anion inhibition studies of the α-carbonic anhydrase from the protozoan pathogen Trypanosoma cruzi, the causative agent of Chagas disease. Bioorg Med Chem 21: 4472-6 (2013)
- Smith, LJ; Rought Whitta, G; Dolenc, J; Wang, D; van Gunsteren, WF A molecular dynamics simulation investigation of the relative stability of the cyclic peptide octreotide and its deprotonated and its (CF Bioorg Med Chem 24: 4936-4948 (2016)
- Billard, E; Hébert, TE; Chatenet, D Discovery of New Allosteric Modulators of the Urotensinergic System through Substitution of the Urotensin II-Related Peptide (URP) Phenylalanine Residue. J Med Chem 61: 8707-8716 (2018)
- Vincenzi, M; Mercurio, FA; Palumbo, R; La Manna, S; Pirone, L; Marasco, D; Pedone, EM; Leone, M Inhibition of the EphA2-Sam/Ship2-Sam Association through Peptide Ligands: Studying the Combined Effect of Charge and Aromatic Character. J Med Chem 67: 16649-16663
- Li, HY; Zawahir, Z; Song, LD; Long, YQ; Neamati, N Sequence-based design and discovery of peptide inhibitors of HIV-1 integrase: insight into the binding mode of the enzyme. J Med Chem 49: 4477-86 (2006)
- Biegel, A; Gebauer, S; Brandsch, M; Neubert, K; Thondorf, I Structural requirements for the substrates of the H+/peptide cotransporter PEPT2 determined by three-dimensional quantitative structure-activity relationship analysis. J Med Chem 49: 4286-96 (2006)
- Furet, P; Chène, P; De Pover, A; Valat, TS; Lisztwan, JH; Kallen, J; Masuya, K The central valine concept provides an entry in a new class of non peptide inhibitors of the p53-MDM2 interaction. Bioorg Med Chem Lett 22: 3498-502 (2012)
- Han, SY; Chin, YW A FLT3-inhibitory constituent from the rhizomes of Anemarrhena asphodeloides. J Enzyme Inhib Med Chem 26: 445-8 (2011)
- Zhan, G; Zhou, J; Liu, J; Huang, J; Zhang, H; Liu, R; Yao, G Acetylcholinesterase Inhibitory Alkaloids from the Whole Plants of Zephyranthes carinata. J Nat Prod 80: 2462-2471 (2017)
- Ahn, S; Ma, CT; Choi, JM; An, S; Lee, M; Le, THV; Pyo, JJ; Lee, J; Choi, MS; Kwon, SW; Park, JH; Noh, M Adiponectin-Secretion-Promoting Phenylethylchromones from the Agarwood of Aquilaria malaccensis. J Nat Prod 82: 259-264 (2019)
- Wang, JN; Zhang, HJ; Li, JQ; Ding, WJ; Ma, ZJ Bioactive Indolocarbazoles from the Marine-Derived Streptomyces sp. DT-A61. J Nat Prod 81: 949-956 (2018)
- Shi, LS; Chao, CH; Shen, DY; Chan, HH; Chen, CH; Liao, YR; Wu, SJ; Leu, YL; Shen, YC; Kuo, YH; Lee, EJ; Qian, K; Wu, TS; Lee, KH Biologically active constituents from the fruiting body of Taiwanofungus camphoratus. Bioorg Med Chem 19: 677-83 (2011)
- Gardner, CR; Davies, KA; Zhang, Y; Brzozowski, M; Czabotar, PE; Murphy, JM; Lessene, G From (Tool)Bench to Bedside: The Potential of Necroptosis Inhibitors. J Med Chem 66: 2361-2385 (2023)
- Shin, J; Seo, Y; Rho, JR; Cho, KW Isolation of Polyhydroxysteroids from the Gorgonian Acabaria undulata J Nat Prod 59: 679-682 (1996)
- Papadimitropoulou, A; Makri, M; Zoidis, G MYC the oncogene from hell: Novel opportunities for cancer therapy. Eur J Med Chem 267:
- Jiang, YJ; Zhang, DS; Zhang, HJ; Li, JQ; Ding, WJ; Xu, CD; Ma, ZJ Medermycin-Type Naphthoquinones from the Marine-Derived Streptomyces sp. XMA39. J Nat Prod 81: 2120-2124 (2018)
- Yatsu, G; Kino, Y; Sasaki, H; Satoh, JI; Kinoshita, K; Koyama, K Meroterpenoids with BACE1 Inhibitory Activity from the Fruiting Body of J Nat Prod 82: 1797-1801 (2019)
- Parrish, SM; Neupane, RP; Harper, MK; Head, J; Williams, PG Myrmenaphthol A, Isolated from a Hawaiian Sponge of the Genus J Nat Prod 82: 2668-2671 (2019)
- Folmer, F; Harrison, WT; Tabudravu, JN; Jaspars, M; Aalbersberg, W; Feussner, K; Wright, AD; Dicato, M; Diederich, M NF-kappaB-inhibiting naphthopyrones from the Fijian echinoderm Comanthus parvicirrus. J Nat Prod 71: 106-11 (2008)
- Iwagawa, T; Kaneko, M; Okamura, H; Nakatani, M; van Soest RWM, na New alkaloids from the papua new guinean sponge agelas nakamurai J Nat Prod 61: 1310-2 (1998)
- Yasuda, K; Kizu, H; Yamashita, T; Kameda, Y; Kato, A; Nash, RJ; Fleet, GW; Molyneux, RJ; Asano, N New sugar-mimic alkaloids from the pods of Angylocalyx pynaertii. J Nat Prod 65: 198-202 (2002)
- Zhang, AL; Ye, Q; Li, BG; Qi, HY; Zhang, GL Phenolic and triterpene glycosides from the stems of Ilex litseaefolia. J Nat Prod 68: 1531-5 (2005)
- Lee, JS; Cho, YS; Park, EJ; Kim, J; Oh, WK; Lee, HS; Ahn, JS Phospholipase Cgamma1 inhibitory principles from the sarcotestas of Ginkgo biloba. J Nat Prod 61: 867-71 (1998)
- Davis, RA; Simpson, MM; Nugent, RB; Carroll, AR; Avery, VM; Rali, T; Chen, H; Qurallo, B; Quinn, RJ Pim2 inhibitors from the Papua New Guinean plant Cupaniopsis macropetala. J Nat Prod 71: 451-2 (2008)
- Bao, J; Miao, S; Kayser, F; Kotliar, AJ; Baker, RK; Doss, GA; Felix, JP; Bugianesi, RM; Slaughter, RS; Kaczorowski, GJ; Garcia, ML; Ha, SN; Castonguay, L; Koo, GC; Shah, K; Springer, MS; Staruch, MJ; Parsons, WH; Rupprecht, KM Potent Kv1.3 inhibitors from correolide-modification of the C18 position. Bioorg Med Chem Lett 15: 447-51 (2004)
- Freyer, AJ; Patil, AD; Killmer, L; Zuber, G; Myers, C; Johnson, RK Rigidone, a sesquiterpene o-quinone from the gorgonian Pseudopterogorgia rigida. J Nat Prod 60: 309-11 (1997)
- Li, J; Gu, BB; Sun, F; Xu, JR; Jiao, WH; Yu, HB; Han, BN; Yang, F; Zhang, XC; Lin, HW Sesquiterpene Quinones/Hydroquinones from the Marine Sponge Spongia pertusa Esper. J Nat Prod 80: 1436-1445 (2017)
- Xiong, J; Wang, LJ; Qian, J; Wang, PP; Wang, XJ; Ma, GL; Zeng, H; Li, J; Hu, JF Structurally Diverse Sesquiterpenoids from the Endangered Ornamental Plant Michelia shiluensis. J Nat Prod 81: 2195-2204 (2018)
- Vasiljevik, T; Groer, CE; Lehner, K; Navarro, H; Prisinzano, TE Studies toward the Development of Antiproliferative Neoclerodanes from Salvinorin A. J Nat Prod 77: 1817-24 (2014)
- Burton, J; Wood, SG; Lynch, M; Plaut, AG Substrate analogue inhibitors of the IgA1 proteinases from Neisseria gonorrhoeae. J Med Chem 31: 1647-51 (1988)
- Qiao, Y; Zheng, H; Li, L; Zhang, J; Li, Y; Li, S; Zhu, R; Zhou, J; Zhao, S; Jiang, Y; Lou, H Terpenoids with vasorelaxant effects from the Chinese liverwort Scapania carinthiaca. Bioorg Med Chem 26: 4320-4328 (2018)
- Liu, J; Li, Z; Yan, H; Wang, L; Chen, J The design and synthesis of ALS inhibitors from pharmacophore models. Bioorg Med Chem Lett 9: 1927-32 (1999)
- Dai, J; Sorribas, A; Yoshida, WY; Kelly, M; Williams, PG Topsentinols, 24-isopropyl steroids from the marine sponge Topsentia sp. J Nat Prod 73: 1597-600 (2010)
- Letschert, K; Komatsu, M; Hummel-Eisenbeiss, J; Keppler, D Vectorial transport of the peptide CCK-8 by double-transfected MDCKII cells stably expressing the organic anion transporter OATP1B3 (OATP8) and the export pump ABCC2. J Pharmacol Exp Ther 313: 549-56 (2005)
- O'Rourke, MF; Iversen, LJ; Lomasney, JW; Bylund, DB Species orthologs of the alpha-2A adrenergic receptor: the pharmacological properties of the bovine and rat receptors differ from the human and porcine receptors. J Pharmacol Exp Ther 271: 735-40 (1994)
- Wiener, JJ; Wickboldt, AT; Nguyen, S; Sun, S; Rynberg, R; Rizzolio, M; Karlsson, L; Edwards, JP; Grice, CA Pyrazole-based arylalkyne cathepsin S inhibitors. Part III: modification of P4 region. Bioorg Med Chem Lett 23: 1070-4 (2013)
- Li, X; Staszewski, L; Xu, H Use of T1R3 venus flytrap region polypeptide to screen for taste modulators US Patent US9459250 (2016)
- Sweeney, ZK; Fu, J; Wiedmann, B From chemical tools to clinical medicines: nonimmunosuppressive cyclophilin inhibitors derived from the cyclosporin and sanglifehrin scaffolds. J Med Chem 57: 7145-59 (2014)
- Inoue, M; Tanabe, H; Nakashima, K; Ishida, Y; Kotani, H Rexinoids isolated from Sophora tonkinensis with a gene expression profile distinct from the synthetic rexinoid bexarotene. J Nat Prod 77: 1670-7 (2014)
- Canzoneri, JC; Chen, PC; Oyelere, AK Design and synthesis of novel histone deacetylase inhibitor derived from nuclear localization signal peptide. Bioorg Med Chem Lett 19: 6588-90 (2009)
- Xu, W; Chen, G; Liew, OW; Zuo, Z; Jiang, H; Zhu, W Novel non-peptide beta-secretase inhibitors derived from structure-based virtual screening and bioassay. Bioorg Med Chem Lett 19: 3188-92 (2009)
- Qin, X; Chen, H; Tu, L; Ma, Y; Liu, N; Zhang, H; Li, D; Riedl, B; Bierer, D; Yin, F; Li, Z Potent Inhibition of HIF1α and p300 Interaction by a Constrained Peptide Derived from CITED2. J Med Chem 64: 13693-13703 (2021)
- Samaradivakara, SP; Samarasekera, R; Handunnetti, SM; Weerasena, OVDSJ; Al-Hamashi, AA; Slama, JT; Taylor, WR; Alhadidi, Q; Shah, ZA; Perera, L; Tillekeratne, LMV A Bioactive Resveratrol Trimer from the Stem Bark of the Sri Lankan Endemic Plant Vateria copallifera. J Nat Prod 81: 1693-1700 (2018)
- Hieble, JP; Bondinell, WE; Ruffolo, RR Alpha- and beta-adrenoceptors: from the gene to the clinic. 1. Molecular biology and adrenoceptor subclassification. J Med Chem 38: 3415-44 (1995)
- Isik, S; Kockar, F; Arslan, O; Guler, OO; Innocenti, A; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the yeast Saccharomyces cerevisiae with anions. Bioorg Med Chem Lett 18: 6327-31 (2008)
- Segala, E; Guo, D; Cheng, RK; Bortolato, A; Deflorian, F; Doré, AS; Errey, JC; Heitman, LH; IJzerman, AP; Marshall, FH; Cooke, RM Controlling the Dissociation of Ligands from the Adenosine A2A Receptor through Modulation of Salt Bridge Strength. J Med Chem 59: 6470-9 (2016)
- Escalante, A; Gattuso, M; Pérez, P; Zacchino, S Evidence for the mechanism of action of the antifungal phytolaccoside B isolated from Phytolacca tetramera Hauman. J Nat Prod 71: 1720-5 (2008)
- Lefas, G; Chaconas, G High-throughput screening identifies three inhibitor classes of the telomere resolvase from the lyme disease spirochete. Antimicrob Agents Chemother 53: 4441-9 (2009)
- Yamanokuchi, R; Imada, K; Miyazaki, M; Kato, H; Watanabe, T; Fujimuro, M; Saeki, Y; Yoshinaga, S; Terasawa, H; Iwasaki, N; Rotinsulu, H; Losung, F; Mangindaan, RE; Namikoshi, M; de Voogd, NJ; Yokosawa, H; Tsukamoto, S Hyrtioreticulins A-E, indole alkaloids inhibiting the ubiquitin-activating enzyme, from the marine sponge Hyrtios reticulatus. Bioorg Med Chem 20: 4437-42 (2012)
- Maresca, A; Vullo, D; Scozzafava, A; Supuran, CT Inhibition of the alpha- and beta-carbonic anhydrases from the gastric pathogen Helycobacter pylori with anions. J Enzyme Inhib Med Chem 28: 388-91 (2013)
- Melchiorre, C; Bolognesi, ML; Minarini, A; Rosini, M; Tumiatti, V Polyamines in drug discovery: from the universal template approach to the multitarget-directed ligand design strategy. J Med Chem 53: 5906-14 (2010)
- Ghosh, D; Lo, J; Egbuta, C Recent Progress in the Discovery of Next Generation Inhibitors of Aromatase from the Structure-Function Perspective. J Med Chem 59: 5131-48 (2016)
- Wen, J; Liao, H; Stachowski, K; Hempfling, JP; Qian, Z; Yuan, C; Foster, MP; Pei, D Rational design of cell-permeable cyclic peptides containing a d-Pro-l-Pro motif. Bioorg Med Chem 28: (2020)
- Smith, RJ; Perez-Ternero, C; Conole, D; Martin, C; Myers, SH; Hobbs, AJ; Selwood, DL A Series of Substituted Bis-Aminotriazines Are Activators of the Natriuretic Peptide Receptor C. J Med Chem 65: 5495-5513 (2022)
- Lavrador, K; Murphy, B; Saunders, J; Struthers, S; Wang, X; Williams, J A screening library for peptide activated G-protein coupled receptors. 1. The test set. J Med Chem 47: 6864-74 (2004)
- Howson, W; Hodgson, J; Richardson, R; Walton, L; Guard, S; Watling, K An SAR study for the non-peptide substance P receptor (NK1) antagonist, CP-96,345. Bioorg Med Chem Lett 2: 559-564 (1992)
- Lewis, CA; Longcore, KE; Miller, SJ; Wender, PA An approach to the site-selective diversification of apoptolidin A with peptide-based catalysts. J Nat Prod 72: 1864-9 (2009)
- Burgey, CS; Stump, CA; Nguyen, DN; Deng, JZ; Quigley, AG; Norton, BR; Bell, IM; Mosser, SD; Salvatore, CA; Rutledge, RZ; Kane, SA; Koblan, KS; Vacca, JP; Graham, SL; Williams, TM Benzodiazepine calcitonin gene-related peptide (CGRP) receptor antagonists: optimization of the 4-substituted piperidine. Bioorg Med Chem Lett 16: 5052-6 (2006)
- Silvestri, AP; Zhang, Q; Ping, Y; Muir, EW; Zhao, J; Chakka, SK; Wang, G; Bray, WM; Chen, W; Fribourgh, JL; Tripathi, S; He, Y; Rubin, SM; Satz, AL; Pye, CR; Kuai, L; Su, W; Schwochert, JA DNA-Encoded Macrocyclic Peptide Libraries Enable the Discovery of a Neutral MDM2-p53 Inhibitor. ACS Med Chem Lett 14: 820-826 (2023)
- Duncan, KE; Dempsey, BR; Killip, LE; Adams, J; Bailey, ML; Lajoie, GA; Litchfield, DW; Brandl, CJ; Shaw, GS; Shilton, BH Discovery and characterization of a nonphosphorylated cyclic peptide inhibitor of the peptidylprolyl isomerase, Pin1. J Med Chem 54: 3854-65 (2011)
- Sawada, K; Terada, T; Saito, H; Hashimoto, Y; Inui, K Effects of glibenclamide on glycylsarcosine transport by the rat peptide transporters PEPT1 and PEPT2. Br J Pharmacol 128: 1159-64 (1999)
- Twum, K; Bhattacharjee, A; Laryea, ET; Esposto, J; Omolloh, G; Mortensen, S; Jaradi, M; Stock, NL; Schileru, N; Elias, B; Pszenica, E; McCormick, TM; Martic, S; Beyeh, NK Functionalized resorcinarenes effectively disrupt the aggregation of αA66-80 crystallin peptide related to cataracts. RSC Med Chem 12: 2022-2030 (2021)
- DeVita, RJ; Goulet, MT; Wyvratt, MJ; Fisher, MH; Lo, JL; Yang, YT; Cheng, K; Smith, RG Investigation of the 4-O-alkylamine substituent of non-peptide quinolone GnRH receptor antagonists. Bioorg Med Chem Lett 9: 2621-4 (1999)
- Götz, MG; Godwin, K; Price, R; Dorn, R; Merrill-Steskal, G; Klemmer, W; Hansen, H; Produturi, G; Rocha, M; Palmer, M; Molacek, L; Strater, Z; Groll, M Macrocyclic Oxindole Peptide Epoxyketones-A Comparative Study of Macrocyclic Inhibitors of the 20S Proteasome. ACS Med Chem Lett 15: 533-539 (2024)
- Hu, X; Nguyen, KT; Jiang, VC; Lofland, D; Moser, HE; Pei, D Macrocyclic inhibitors for peptide deformylase: a structure-activity relationship study of the ring size. J Med Chem 47: 4941-9 (2004)
- Eden, J; Hall, M; Higginbottom, M; Horwell, D; Howson, W; Hughes, J; Jordan, R; Lewthwaite, R; Martin, K; McKnight, A; Pinnock, R; Pritchard, M; Suman-Chauhan, N; Williams, S PD 165929 the first high affinity non-peptide neuromedin-B (NMB) receptor selective antagonist Bioorg Med Chem Lett 6: 2617-2622 (1996)
- Liu, F; Park, JE; Qian, WJ; Lim, D; Scharow, A; Berg, T; Yaffe, MB; Lee, KS; Burke, TR Peptoid-Peptide hybrid ligands targeting the polo box domain of polo-like kinase 1. Chembiochem 13: 1291-6 (2012)
- Suzuki, R; Brown, GA; Christopher, JA; Scully, CCG; Congreve, M Recent Developments in Therapeutic Peptides for the Glucagon-like Peptide 1 and 2 Receptors. J Med Chem 63: 905-927 (2020)
- Lavey, NP; Coker, JA; Ruben, EA; Duerfeldt, AS Sclerotiamide: The First Non-Peptide-Based Natural Product Activator of Bacterial Caseinolytic Protease P. J Nat Prod 79: 1193-7 (2016)
- Jenkins, RJ; Heslip, KA; Meagher, JL; Stuckey, JA; Dotson, GD Structural basis for the recognition of peptide RJPXD33 by acyltransferases in lipid A biosynthesis. J Biol Chem 289: 15527-35 (2014)
- Le Marec, O; Neveu, C; Lefranc, B; Dubessy, C; Boutin, JA; Do-Régo, JC; Costentin, J; Tonon, MC; Tena-Sempere, M; Vaudry, H; Leprince, J Structure-activity relationships of a series of analogues of the RFamide-related peptide 26RFa. J Med Chem 54: 4806-14 (2011)
- Boden, P; Eden, J; Hodgson, J; Horwell, D; Pritchard, M; Raphy, J; Suman-Chauhan, N The development of a novel series of non-peptide tachykinin NK3 receptor selective antagonists Bioorg Med Chem Lett 5: 1773-1778 (1995)
- Luthin, DR; Hong, Y; Pathak, VP; Paderes, G; Nared-Hood, KD; Castro, MA; Vazir, H; Li, H; Tompkins, E; Christie, L; May, JM; Anderson, MB The discovery of novel small molecule non-peptide gonadotropin releasing hormone (GnRH) receptor antagonists. Bioorg Med Chem Lett 12: 3467-70 (2002)
- Cao, R; Li, L; Xu, Z; Li, J; Wu, D; Wang, Y; Zhu, H The lipidation and glycosylation enabling bioactivity enhancement and structural change of antibacterial peptide G3. Bioorg Med Chem Lett 90: (2023)
- Prete, SD; Vullo, D; Osman, SM; Scozzafava, A; AlOthman, Z; Capasso, C; Supuran, CT Sulfonamide inhibition study of the carbonic anhydrases from the bacterial pathogen Porphyromonas gingivalis: theß-class (PgiCAb) versus the¿-class (PgiCA) enzymes. Bioorg Med Chem 22: 4537-43 (2014)
- DeNinno, MP; Schoenleber, R; Perner, RJ; Lijewski, L; Asin, KE; Britton, DR; MacKenzie, R; Kebabian, JW Synthesis and dopaminergic activity of 3-substituted 1-(aminomethyl)-3,4-dihydro-5,6-dihydroxy-1H-2-benzopyrans: characterization of an auxiliary binding region in the D1 receptor. J Med Chem 34: 2561-9 (1991)
- Velagapudi, UK; Langelier, MF; Delgado-Martin, C; Diolaiti, ME; Bakker, S; Ashworth, A; Patel, BA; Shao, X; Pascal, JM; Talele, TT Design and Synthesis of Poly(ADP-ribose) Polymerase Inhibitors: Impact of Adenosine Pocket-Binding Motif Appendage to the 3-Oxo-2,3-dihydrobenzofuran-7-carboxamide on Potency and Selectivity. J Med Chem 62: 5330-5357 (2019)
- Pasquini, S; De Rosa, M; Ligresti, A; Mugnaini, C; Brizzi, A; Caradonna, NP; Cascio, MG; Bolognini, D; Pertwee, RG; Di Marzo, V; Corelli, F Investigations on the 4-quinolone-3-carboxylic acid motif. 6. Synthesis and pharmacological evaluation of 7-substituted quinolone-3-carboxamide derivatives as high affinity ligands for cannabinoid receptors. Eur J Med Chem 58: 30-43 (2012)
- Yi, X; Tran, E; Odiba, JO; Qin, CX; Ritchie, RH; Baell, JB The formyl peptide receptors FPR1 and FPR2 as targets for inflammatory disorders: recent advances in the development of small-molecule agonists. Eur J Med Chem 265:
- Yadav, S; Pandey, A; Mali, SN From lab to nature: Recent advancements in the journey of gastroprotective agents from medicinal chemistry to phytotherapy. Eur J Med Chem 272:
- Ruffolo, RR; Bondinell, W; Hieble, JP Alpha- and beta-adrenoceptors: from the gene to the clinic. 2. Structure-activity relationships and therapeutic applications. J Med Chem 38: 3681-716 (1995)
- Innocenti, A; Zimmerman, S; Ferry, JG; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the methanoarchaeon Methanobacterium thermoautotrophicum (Cab) with anions. Bioorg Med Chem Lett 14: 4563-7 (2004)
- Innocenti, A; Leewattanapasuk, W; Mühlschlegel, FA; Mastrolorenzo, A; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the pathogenic yeast Candida glabrata with anions. Bioorg Med Chem Lett 19: 4802-5 (2009)
- Del Prete, S; Vullo, D; di Fonzo, P; Carginale, V; Supuran, CT; Capasso, C Comparison of the anion inhibition profiles of theß- and¿-carbonic anhydrases from the pathogenic bacterium Burkholderia pseudomallei. Bioorg Med Chem 25: 2010-2015 (2017)
- Nakao, Y; Oku, N; Matsunaga, S; Fusetani, N Cyclotheonamides E2 and E3, new potent serine protease inhibitors from the marine sponge of the genus Theonella. J Nat Prod 61: 667-70 (1998)
- Gerusz, V; Denis, A; Faivre, F; Bonvin, Y; Oxoby, M; Briet, S; LeFralliec, G; Oliveira, C; Desroy, N; Raymond, C; Peltier, L; Moreau, F; Escaich, S; Vongsouthi, V; Floquet, S; Drocourt, E; Walton, A; Prouvensier, L; Saccomani, M; Durant, L; Genevard, JM; Sam-Sambo, V; Soulama-Mouze, C From triclosan toward the clinic: discovery of nonbiocidal, potent FabI inhibitors for the treatment of resistant bacteria. J Med Chem 55: 9914-28 (2012)
- Sutton, J; Clark, DE; Higgs, C; de Groot, MJ; Harris, NV; Taylor, A; Lockey, PM; Maubach, K; Woodrooffe, A; Davis, RJ; Coleman, RA; Clark, KL From virtual to clinical: The discovery of PGN-1531, a novel antagonist of the prostanoid EP4 receptor. Bioorg Med Chem Lett 24: 2212-21 (2014)
- Cui, Q; Huang, C; Liu, JY; Zhang, JT Small Molecule Inhibitors Targeting the "Undruggable" Survivin: The Past, Present, and Future from a Medicinal Chemist's Perspective. J Med Chem 66: 16515-16545 (2023)
- Lin, L; Lu, L; Yuan, C; Wang, A; Zhu, M; Fu, X; Xing, S The dual inhibition against the activity and expression of tyrosine phosphatase PRL-3 from a rhodanine derivative. Bioorg Med Chem Lett 41: (2021)
- Patil, AD; Freyer, AJ; Eggleston, DS; Haltiwanger, RC; Bean, MF; Taylor, PB; Caranfa, MJ; Breen, AL; Bartus, HR; Johnson, RK The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J Med Chem 36: 4131-8 (1994)
- Potterat, O; Wagner, K; Gemmecker, G; Mack, J; Puder, C; Vettermann, R; Streicher, R BI-32169, a bicyclic 19-peptide with strong glucagon receptor antagonist activity from Streptomyces sp. J Nat Prod 67: 1528-31 (2004)
- Skovbakke, SL; Skovbakke, SL; Skovbakke, SL; Holdfeldt, A; Holdfeldt, A; Holdfeldt, A; Nielsen, C; Nielsen, C; Nielsen, C; Hansen, AM; Hansen, AM; Hansen, AM; Perez-Gassol, I; Perez-Gassol, I; Perez-Gassol, I; Dahlgren, C; Dahlgren, C; Dahlgren, C; Forsman, H; Forsman, H; Forsman, H; Franzyk, H; Franzyk, H; Franzyk, H Combining Elements from Two Antagonists of Formyl Peptide Receptor 2 Generates More Potent Peptidomimetic Antagonists. J Med Chem 60: 6991-6997 (2017)
- Kwon, YJ; Sohn, MJ; Kim, CJ; Koshino, H; Kim, WG Flavimycins A and B, dimeric 1,3-dihydroisobenzofurans with peptide deformylase inhibitory activity from Aspergillus flavipes. J Nat Prod 75: 271-4 (2012)
- da Silva-Júnior, EF; de Araújo-Júnior, JX Peptide derivatives as inhibitors of NS2B-NS3 protease from Dengue, West Nile, and Zika flaviviruses. Bioorg Med Chem 27: 3963-3978 (2019)
- Lai, D; Yu, S; van Ofwegen, L; Totzke, F; Proksch, P; Lin, W 9,10-secosteroids, protein kinase inhibitors from the Chinese gorgonian Astrogorgia sp. Bioorg Med Chem 19: 6873-80 (2011)
- Jang, KH; Chung, SC; Shin, J; Lee, SH; Kim, TI; Lee, HS; Oh, KB Aaptamines as sortase A inhibitors from the tropical sponge Aaptos aaptos. Bioorg Med Chem Lett 17: 5366-9 (2007)
- Kodani, S; Ishida, K; Murakami, M Aeruginosin 103-A, a thrombin inhibitor from the cyanobacterium Microcystis viridis. J Nat Prod 61: 1046-8 (1998)
- Liu, XL; Li, S; Meng, DL Anti-gout nor-oleanane triterpenoids from the leaves of Stauntonia brachyanthera. Bioorg Med Chem Lett 26: 2874-2879 (2016)
- Huang, YT; Chang, HS; Wang, GJ; Cheng, MJ; Chen, CH; Yang, YJ; Chen, IS Anti-inflammatory endiandric acid analogues from the roots of Beilschmiedia tsangii. J Nat Prod 74: 1875-80 (2011)
- Hwang, BS; Lee, IK; Choi, HJ; Yun, BS Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg Med Chem Lett 25: 3256-60 (2015)
- Gao, E; Zhou, ZQ; Zou, J; Yu, Y; Feng, XL; Chen, GD; He, RR; Yao, XS; Gao, H Bioactive Asarone-Derived Phenylpropanoids from the Rhizome of Acorus tatarinowii Schott. J Nat Prod 80: 2923-2929 (2017)
- Liu, Q; Yang, QM; Hu, HJ; Yang, L; Yang, YB; Chou, GX; Wang, ZT Bioactive diterpenoids and flavonoids from the aerial parts of Scoparia dulcis. J Nat Prod 77: 1594-600 (2014)
- Zhang, JF; Zhong, WC; Li, YC; Song, YQ; Xia, GY; Tian, GH; Ge, GB; Lin, S Bioactivity-Guided Discovery of Human Carboxylesterase Inhibitors from the Roots of J Nat Prod 83: 2940-2949 (2020)
- Won, TH; Jeon, JE; Kim, SH; Lee, SH; Rho, BJ; Oh, DC; Oh, KB; Shin, J Brominated aromatic furanones and related esters from the ascidian Synoicum sp. J Nat Prod 75: 2055-61 (2012)
- Jeong, T; Ahn, J; Kim, Y; Bok, S; Kwon, B; Shin, J; Seo, Y CETP inhibitory activity of ceramides, isolated from the gorgonian Acabaria Undulata Bioorg Med Chem Lett 7: 1481-1482 (1997)
- Horsfall, LE; Garau, G; Liénard, BM; Dideberg, O; Schofield, CJ; Frère, JM; Galleni, M Competitive inhibitors of the CphA metallo-beta-lactamase from Aeromonas hydrophila. Antimicrob Agents Chemother 51: 2136-42 (2007)
- Pan, Y; Weng, J; Kabaleeswaran, V; Li, H; Cao, Y; Bhosle, RC; Zhou, M Cortisone dissociates the Shaker family K+ channels from their beta subunits. Nat Chem Biol 4: 708-14 (2008)
- Badal, S; Gallimore, W; Huang, G; Tzeng, TR; Delgoda, R Cytotoxic and potent CYP1 inhibitors from the marine algae Cymopolia barbata. Org Med Chem Lett 2: 21 (2012)
- Hegde, VR; Dai, P; Ladislaw, C; Patel, MG; Puar, MS; Pachter, JA D4 dopamine receptor-selective compounds from the Chinese plant Phoebe chekiangensis Bioorg Med Chem Lett 7: 1207-1212 (1997)
- Wang, J; Liang, Z; Li, K; Yang, B; Liu, Y; Fang, W; Tang, L; Zhou, X Ene-yne Hydroquinones from a Marine-derived Strain of the Fungus J Nat Prod 83: 1258-1264 (2020)
- Henley, ZA; Bax, BD; Inglesby, LM; Champigny, A; Gaines, S; Faulder, P; Le, J; Thomas, DA; Washio, Y; Baldwin, IR From PIM1 to PI3Kδ via GSK3β: Target Hopping through the Kinome. ACS Med Chem Lett 8: 1093-1098 (2017)
- Jautelat, R; Brumby, T; Schäfer, M; Briem, H; Eisenbrand, G; Schwahn, S; Krüger, M; Lücking, U; Prien, O; Siemeister, G From the insoluble dye indirubin towards highly active, soluble CDK2-inhibitors. Chembiochem 6: 531-40 (2005)
- Wu, T; Salim, AA; Cui, H; Khalil, ZG; Bernhardt, PV; Capon, RJ Glenthenamines A-F: Enamine Pyranonaphthoquinones from the Australian Pasture Plant Derived J Nat Prod 85: 337-344 (2022)
- Huang, CY; Stauffer, TM; Strickland, CL; Reader, JC; Huang, H; Li, G; Cooper, AB; Doll, RJ; Ganguly, AK; Baldwin, JJ; Rokosz, LL Guiding farnesyltransferase inhibitors from an ECLiPS library to the catalytic zinc. Bioorg Med Chem Lett 16: 507-11 (2005)
- Devkota, KP; Ratnayake, R; Colburn, NH; Wilson, JA; Henrich, CJ; McMahon, JB; Beutler, JA Inhibitors of the oncogenic transcription factor AP-1 from Podocarpus latifolius. J Nat Prod 74: 374-7 (2011)
- Phainuphong, P; Rukachaisirikul, V; Saithong, S; Phongpaichit, S; Bowornwiriyapan, K; Muanprasat, C; Srimaroeng, C; Duangjai, A; Sakayaroj, J Lovastatin Analogues from the Soil-Derived Fungus Aspergillus sclerotiorum PSU-RSPG178. J Nat Prod 79: 1500-7 (2016)
- Poirel, L; Rodriguez-Martinez, JM; Plésiat, P; Nordmann, P Naturally occurring Class A ss-lactamases from the Burkholderia cepacia complex. Antimicrob Agents Chemother 53: 876-82 (2009)
- Hsiao, CC; Rombouts, F; Gijsen, HJM New evolutions in the BACE1 inhibitor field from 2014 to 2018. Bioorg Med Chem Lett 29: 761-777 (2019)
- Yu, S; Deng, Z; Proksch, P; Lin, W Oculatol, oculatolide, and A-nor sterols from the sponge Haliclona oculata. J Nat Prod 69: 1330-4 (2006)
- Balboni, G; Salvadori, S; Marczak, ED; Knapp, BI; Bidlack, JM; Lazarus, LH; Peng, X; Si, YG; Neumeyer, JL Opioid bifunctional ligands from morphine and the opioid pharmacophore Dmt-Tic. Eur J Med Chem 46: 799-803 (2011)
- Le, DH; Takenaka, Y; Hamada, N; Mizushina, Y; Tanahashi, T Polyketides from the cultured lichen mycobiont of a Vietnamese Pyrenula sp. J Nat Prod 77: 1404-12 (2014)
- Son, IH; Chung, IM; Lee, SI; Yang, HD; Moon, HI Pomiferin, histone deacetylase inhibitor isolated from the fruits of Maclura pomifera. Bioorg Med Chem Lett 17: 4753-5 (2007)
- El Amrani, M; Lai, D; Debbab, A; Aly, AH; Siems, K; Seidel, C; Schnekenburger, M; Gaigneaux, A; Diederich, M; Feger, D; Lin, W; Proksch, P Protein kinase and HDAC inhibitors from the endophytic fungus Epicoccum nigrum. J Nat Prod 77: 49-56 (2014)
- Yao, FH; Liang, X; Lu, XH; Cheng, X; Luo, LX; Qi, SH Pyrrospirones K-Q, Decahydrofluorene-Class Alkaloids from the Marine-Derived Fungus J Nat Prod 85: 2071-2081 (2022)
- Kalauni, SK; Choudhary, MI; Shaheen, F; Manandhar, MD; Atta-ur-Rahman, A; Gewali, MB; Khalid, A Steroidal alkaloids from the leaves of Sarcococca coriacea of Nepalese origin. J Nat Prod 64: 842-4 (2001)
- Huang, ZH; Liang, X; Li, CJ; Gu, Q; Ma, X; Qi, SH Talaromynoids A-I, Highly Oxygenated Meroterpenoids from the Marine-Derived Fungus J Nat Prod 84: 2727-2737 (2021)
- Puder, C; Wagner, K; Vettermann, R; Hauptmann, R; Potterat, O Terphenylquinone inhibitors of the src protein tyrosine kinase from Stilbella sp. J Nat Prod 68: 323-6 (2005)
- Wood, SG; Lynch, M; Plaut, AG; Burton, J Tetrapeptide inhibitors of the IgA1 proteinases from type I Neisseria gonorrhoeae. J Med Chem 32: 2407-11 (1989)
- Bertron, JL; Cho, HP; Garcia-Barrantes, PM; Panarese, JD; Salovich, JM; Nance, KD; Engers, DW; Rook, JM; Blobaum, AL; Niswender, CM; Stauffer, SR; Conn, PJ; Lindsley, CW The discovery of VU0486846: steep SAR from a series of M Bioorg Med Chem Lett 28: 2175-2179 (2018)
- Look, GC; Schullek, JR; Holmes, CP; Chinn, JP; Gordon, EM; Gallop, MA The identification of cyclooxygenase-1 inhibitors from 4-thiazolidinone combinatorial libraries Bioorg Med Chem Lett 6: 707-712 (1996)
- Xi, FM; Li, CT; Han, J; Yu, SS; Wu, ZJ; Chen, WS Thiophenes, polyacetylenes and terpenes from the aerial parts of Eclipata prostrate. Bioorg Med Chem 22: 6515-22 (2015)
- Avalon, NE; Nafie, J; De Marco Verissimo, C; Warrensford, LC; Dietrick, SG; Pittman, AR; Young, RM; Kearns, FL; Smalley, T; Binning, JM; Dalton, JP; Johnson, MP; Woodcock, HL; Allcock, AL; Baker, BJ Tuaimenal A, a Meroterpene from the Irish Deep-Sea Soft Coral J Nat Prod 85: 1315-1323 (2022)
- Choi, H; Hwang, H; Chin, J; Kim, E; Lee, J; Nam, SJ; Lee, BC; Rho, BJ; Kang, H Tuberatolides, potent FXR antagonists from the Korean marine tunicate Botryllus tuberatus. J Nat Prod 74: 90-4 (2011)
- Tian, LW; Feng, Y; Tran, TD; Shimizu, Y; Pfeifer, T; Forster, PI; Quinn, RJ Tyrosyl-DNA Phosphodiesterase I Inhibitors from the Australian Plant Macropteranthes leichhardtii. J Nat Prod 78: 1756-60 (2015)
- Ryu, HW; Curtis-Long, MJ; Jung, S; Jin, YM; Cho, JK; Ryu, YB; Lee, WS; Park, KH Xanthones with neuraminidase inhibitory activity from the seedcases of Garcinia mangostana. Bioorg Med Chem 18: 6258-64 (2010)
- Willson, M; Alric, I; Perie, J; Sanejouand, YH Yeast hexokinase inhibitors designed from the 3-D enzyme structure rebuilding. J Enzym Inhib 12: 101-21 (1997)
- Arif Lodhi, M; Iqbal Choudhary, M; Malik, A; Ahmad, S alpha-Chymotrypsin inhibition studies on the lignans from Vitex negundo Linn. J Enzyme Inhib Med Chem 23: 400-5 (2008)
- Barrow, CJ; Doleman, MS; Bobko, MA; Cooper, R Structure determination, pharmacological evaluation, and structure-activity studies of a new cyclic peptide substance P antagonist containing the new amino acid 3-prenyl-beta-hydroxytyrosine, isolated from Aspergillus flavipes. J Med Chem 37: 356-63 (1994)
- Kovar, SE; Fourman, C; Kinstedt, C; Williams, B; Morris, C; Cho, KJ; Ketcha, DM Chalcones bearing a 3,4,5-trimethoxyphenyl motif are capable of selectively inhibiting oncogenic K-Ras signaling. Bioorg Med Chem Lett 30: (2020)
- Gadhiya, S; Cordone, P; Pal, RK; Gallicchio, E; Wickstrom, L; Kurtzman, T; Ramsey, S; Harding, WW New Dopamine D3-Selective Receptor Ligands Containing a 6-Methoxy-1,2,3,4-tetrahydroisoquinolin-7-ol Motif. ACS Med Chem Lett 9: 990-995 (2018)
- Wang, CX; Zhang, ZL; Yin, QK; Tu, JL; Wang, JE; Xu, YH; Rao, Y; Ou, TM; Huang, SL; Li, D; Wang, HG; Li, QJ; Tan, JH; Chen, SB; Huang, ZS Design, Synthesis, and Evaluation of New Quinazolinone Derivatives that Inhibit Bloom Syndrome Protein (BLM) Helicase, Trigger DNA Damage at the Telomere Region, and Synergize with PARP Inhibitors. J Med Chem 63: 9752-9772 (2020)
- Pasquini, S; Botta, L; Semeraro, T; Mugnaini, C; Ligresti, A; Palazzo, E; Maione, S; Di Marzo, V; Corelli, F Investigations on the 4-quinolone-3-carboxylic acid motif. 2. Synthesis and structure-activity relationship of potent and selective cannabinoid-2 receptor agonists endowed with analgesic activity in vivo. J Med Chem 51: 5075-84 (2008)
- Li, CY; Yap, K; Swedberg, JE; Craik, DJ; de Veer, SJ Binding Loop Substitutions in the Cyclic Peptide SFTI-1 Generate Potent and Selective Chymase Inhibitors. J Med Chem 63: 816-826 (2020)
- Ontoria, JM; Biancofiore, I; Fezzardi, P; Ferrigno, F; Torrente, E; Colarusso, S; Bianchi, E; Andreini, M; Patsilinakos, A; Kempf, G; Augustin, M; Steinbacher, S; Summa, V; Pacifici, R; Muñoz-Sanjuan, I; Park, L; Bresciani, A; Dominguez, C; Sherman, LT; Harper, S Combined Peptide and Small-Molecule Approach toward Nonacidic THIQ Inhibitors of the KEAP1/NRF2 Interaction. ACS Med Chem Lett 11: 740-746 (2020)
- Zhang, Z; Pan, H; Guo, L; Cai, C; Chen, T; Zhang, Z; Yang, X; Zheng, H; Jiang, C; Wang, Z; Yang, Y; Wang, Z; Zhang, X; Zhang, Y; Liu, D Design and Evaluation of 3-Phenyloxetane Derivative Agonists of the Glucagon-Like Peptide-1 Receptor. J Med Chem 67: 14820-14839
- Hojo, K; Hossain, MA; Tailhades, J; Shabanpoor, F; Wong, LL; Ong-Pålsson, EE; Kastman, HE; Ma, S; Gundlach, AL; Rosengren, KJ; Wade, JD; Bathgate, RA Development of a Single-Chain Peptide Agonist of the Relaxin-3 Receptor Using Hydrocarbon Stapling. J Med Chem 59: 7445-56 (2016)
- Fleming, MC; Chiou, LF; Tumbale, PP; Droby, GN; Lim, J; Norris-Drouin, JL; Williams, JG; Pearce, KH; Williams, RS; Vaziri, C; Bowers, AA Discovery and Structural Basis of the Selectivity of Potent Cyclic Peptide Inhibitors of MAGE-A4. J Med Chem 65: 7231-7245 (2022)
- Madsen, P; Knudsen, LB; Wiberg, FC; Carr, RD Discovery and structure-activity relationship of the first non-peptide competitive human glucagon receptor antagonists. J Med Chem 41: 5150-7 (1999)
- Willard, FS; Wainscott, DB; Showalter, AD; Stutsman, C; Ma, W; Cardona, GR; Zink, RW; Corkins, CM; Chen, Q; Yumibe, N; Agejas, J; Cumming, GR; Minguez, JM; Jiménez, A; Mateo, AI; Castaño, AM; Briere, DA; Sloop, KW; Bueno, AB Discovery of an Orally Efficacious Positive Allosteric Modulator of the Glucagon-like Peptide-1 Receptor. J Med Chem 64: 3439-3448 (2021)
- Stilz, HU; Guba, W; Jablonka, B; Just, M; Klingler, O; König, W; Wehner, V; Zoller, G Discovery of an orally active non-peptide fibrinogen receptor antagonist based on the hydantoin scaffold. J Med Chem 44: 1158-76 (2001)
- Haviv, F; Fitzpatrick, TD; Swenson, RE; Nichols, CJ; Mort, NA; Bush, EN; Diaz, G; Bammert, G; Nguyen, A; Rhutasel, NS Effect of N-methyl substitution of the peptide bonds in luteinizing hormone-releasing hormone agonists. J Med Chem 36: 363-9 (1993)
- de Veer, SJ; Li, CY; Swedberg, JE; Schroeder, CI; Craik, DJ Engineering potent mesotrypsin inhibitors based on the plant-derived cyclic peptide, sunflower trypsin inhibitor-1. Eur J Med Chem 155: 695-704 (2018)
- Ahn, DR; Kim, SY; Lee, MJ; Yang, EG HIF-1alpha peptide derivatives with modifications at the hydroxyproline residue as activators of HIF-1alpha. Bioorg Med Chem Lett 19: 4403-5 (2009)
- Tian, S; Swedberg, JE; Li, CY; Craik, DJ; de Veer, SJ Iterative Optimization of the Cyclic Peptide SFTI-1 Yields Potent Inhibitors of Neutrophil Proteinase 3. ACS Med Chem Lett 10: 1234-1239 (2019)
- Ettari, R; Previti, S; Maiorana, S; Amendola, G; Wagner, A; Cosconati, S; Schirmeister, T; Hellmich, UA; Zappalà, M Optimization Strategy of Novel Peptide-Based Michael Acceptors for the Treatment of Human African Trypanosomiasis. J Med Chem 62: 10617-10629 (2019)
- Hruby, VJ Peptide science: exploring the use of chemical principles and interdisciplinary collaboration for understanding life processes. J Med Chem 46: 4215-31 (2003)
- Teng, M; Johnson, MD; Thomas, C; Kiel, D; Lakis, JN; Kercher, T; Aytes, S; Kostrowicki, J; Bhumralkar, D; Truesdale, L; May, J; Sidelman, U; Kodra, JT; Jørgensen, AS; Olesen, PH; de Jong, JC; Madsen, P; Behrens, C; Pettersson, I; Knudsen, LB; Holst, JJ; Lau, J Small molecule ago-allosteric modulators of the human glucagon-like peptide-1 (hGLP-1) receptor. Bioorg Med Chem Lett 17: 5472-8 (2007)
- Horwell, DC; Morrell, AI; Roberts, E The design and synthesis of non-peptide ligands with affinity and selectivity for tachykinin receptors Bioorg Med Chem Lett 6: 165-166 (1996)
- Pickett, JE; Nagakura, K; Pasternak, AR; Grinnell, SG; Majumdar, S; Lewis, JS; Pasternak, GW Sandmeyer reaction repurposed for the site-selective, non-oxidizing radioiodination of fully-deprotected peptides: studies on the endogenous opioid peptide α-neoendorphin. Bioorg Med Chem Lett 23: 4347-50 (2013)
- Long, F; Rouquette-Loughlin, C; Shafer, WM; Yu, EW Functional cloning and characterization of the multidrug efflux pumps NorM from Neisseria gonorrhoeae and YdhE from Escherichia coli. Antimicrob Agents Chemother 52: 3052-60 (2008)
- Loya, S; Reshef, V; Mizrachi, E; Silberstein, C; Rachamim, Y; Carmeli, S; Hizi, A The inhibition of the reverse transcriptase of HIV-1 by the natural sulfoglycolipids from cyanobacteria: contribution of different moieties to their high potency. J Nat Prod 61: 891-5 (1998)
- Li, CH; Zhang, JY; Zhang, XY; Li, SH; Gao, JM An overview of grayanane diterpenoids and their biological activities from the Ericaceae family in the last seven years. Eur J Med Chem 166: 400-416 (2019)
- Isik, S; Kockar, F; Aydin, M; Arslan, O; Guler, OO; Innocenti, A; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors: inhibition of the beta-class enzyme from the yeast Saccharomyces cerevisiae with sulfonamides and sulfamates. Bioorg Med Chem 17: 1158-63 (2009)
- Del Prete, S; Vullo, D; De Luca, V; Carginale, V; Osman, SM; AlOthman, Z; Supuran, CT; Capasso, C Comparison of the sulfonamide inhibition profiles of thea-,ß- and¿-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem Lett 26: 1941-6 (2016)
- Shaw, SJ; Goff, DA; Lin, N; Singh, R; Li, W; McLaughlin, J; Baltgalvis, KA; Payan, DG; Kinsella, TM Developing DYRK inhibitors derived from the meridianins as a means of increasing levels of NFAT in the nucleus. Bioorg Med Chem Lett 27: 2617-2621 (2017)
- Melancon, BJ; Lamers, AP; Bridges, TM; Sulikowski, GA; Utley, TJ; Sheffler, DJ; Noetzel, MJ; Morrison, RD; Scott Daniels, J; Niswender, CM; Jones, CK; Conn, PJ; Lindsley, CW; Wood, MR Development of a more highly selective M(1) antagonist from the continued optimization of the MLPCN Probe ML012. Bioorg Med Chem Lett 22: 1044-8 (2012)
- Seo, C; Sohn, JH; Oh, H; Kim, BY; Ahn, JS Isolation of the protein tyrosine phosphatase 1B inhibitory metabolite from the marine-derived fungus Cosmospora sp. SF-5060. Bioorg Med Chem Lett 19: 6095-7 (2009)
- Carroll, AR; Ngo, A; Quinn, RJ; Redburn, J; Hooper, JN Petrosamine B, an inhibitor of the Helicobacter pylori enzyme aspartyl semialdehyde dehydrogenase from the Australian sponge Oceanapia sp. J Nat Prod 68: 804-6 (2005)
- Vullo, D; Luca, VD; Scozzafava, A; Carginale, V; Rossi, M; Supuran, CT; Capasso, C The alpha-carbonic anhydrase from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1 is highly susceptible to inhibition by sulfonamides. Bioorg Med Chem 21: 1534-8 (2013)
- Thomas, JB; Navarro, H; Warner, KR; Gilmour, B The identification of nonpeptide neurotensin receptor partial agonists from the potent antagonist SR48692 using a calcium mobilization assay. Bioorg Med Chem Lett 19: 1438-41 (2009)
- Giardina, GA; Sarau, HM; Farina, C; Medhurst, AD; Grugni, M; Raveglia, LF; Schmidt, DB; Rigolio, R; Luttmann, M; Vecchietti, V; Hay, DW Discovery of a novel class of selective non-peptide antagonists for the human neurokinin-3 receptor. 1. Identification of the 4-quinolinecarboxamide framework. J Med Chem 40: 1794-807 (1997)
- Lang, M; De Pol, S; Baldauf, C; Hofmann, HJ; Reiser, O; Beck-Sickinger, AG Identification of the key residue of calcitonin gene related peptide (CGRP) 27-37 to obtain antagonists with picomolar affinity at the CGRP receptor. J Med Chem 49: 616-24 (2006)
- Luca, S; Verdoliva, V; Saviano, M Peptide Ligands Specifically Targeting HER2 Receptor and the Role Played by a Synthetic Model System of the Receptor Extracellular Domain: Hypothesized Future Perspectives. J Med Chem 63: 15333-15343 (2020)
- Gourlet, P; Woussen-Colle, MC; Robberecht, P; de Neef, P; Cauvin, A; Vandermeers-Piret, MC; Vandermeers, A; Christophe, J Structural requirements for the binding of the pituitary adenylate-cyclase-activating peptide to receptors and adenylate-cyclase activation in pancreatic and neuronal membranes. Eur J Biochem 195: 535-41 (1991)
- Reymond, MT; Delmas, L; Koerber, SC; Brown, MR; Rivier, JE Truncated, branched, and/or cyclic analogues of neuropeptide Y: importance of the pancreatic peptide fold in the design of specific Y2 receptor ligands. J Med Chem 35: 3653-9 (1992)
- Wang, C; Zhao, L; Xia, S; Zhang, T; Cao, R; Liang, G; Li, Y; Meng, G; Wang, W; Shi, W; Zhong, W; Jiang, S; Liu, K De Novo Design of α-Helical Lipopeptides Targeting Viral Fusion Proteins: A Promising Strategy for Relatively Broad-Spectrum Antiviral Drug Discovery. J Med Chem 61: 8734-8745 (2018)
- Qiu, Z; Yan, M; Li, Q; Liu, D; Van den Steen, PE; Wang, M; Opdenakker, G; Hu, J Definition of peptide inhibitors from a synthetic peptide library by targeting gelatinase B/matrix metalloproteinase-9 (MMP-9) and TNF-a converting enzyme (TACE/ADAM-17). J Enzyme Inhib Med Chem 27: 533-40 (2012)
- Glennon, RA; Chaurasia, C; Titeler, M Binding of indolylalkylamines at 5-HT2 serotonin receptors: examination of a hydrophobic binding region. J Med Chem 33: 2777-84 (1990)
- Ngo, VTH; Hoang, VH; Tran, PT; Van Manh, N; Ann, J; Kim, E; Cui, M; Choi, S; Lee, J; Kim, H; Ha, HJ; Choi, K; Kim, YH; Lee, J Structure-activity relationship investigation of Phe-Arg mimetic region of human glutaminyl cyclase inhibitors. Bioorg Med Chem 26: 3133-3144 (2018)
- Sun, Y; Zhou, Z; Feng, D; Jing, L; Zhao, F; Wang, Z; Zhang, T; Lin, H; Song, H; De Clercq, E; Pannecouque, C; Zhan, P; Liu, X; Kang, D Lead Optimization and Avoidance of Metabolic-perturbing Motif Developing Novel Diarylpyrimidines as Potent HIV-1 NNRTIs. J Med Chem 65: 15608-15626 (2022)
- Hanessian, S; Therrien, E; van Otterlo, WA; Bayrakdarian, M; Nilsson, I; Fjellström, O; Xue, Y Phenolic P2/P3 core motif as thrombin inhibitors--design, synthesis, and X-ray co-crystal structure. Bioorg Med Chem Lett 16: 1032-6 (2006)
- Wagner, FF; Olson, DE; Gale, JP; Kaya, T; Weïwer, M; Aidoud, N; Thomas, M; Davoine, EL; Lemercier, BC; Zhang, YL; Holson, EB Potent and selective inhibition of histone deacetylase 6 (HDAC6) does not require a surface-binding motif. J Med Chem 56: 1772-6 (2013)
- Halland, N; Czech, J; Czechtizky, W; Evers, A; Follmann, M; Kohlmann, M; Schreuder, HA; Kallus, C Sulfamide as Zinc Binding Motif in Small Molecule Inhibitors of Activated Thrombin Activatable Fibrinolysis Inhibitor (TAFIa). J Med Chem 59: 9567-9573 (2016)
- Lu, Q; Yang, YT; Chen, CS; Davis, M; Byrd, JC; Etherton, MR; Umar, A; Chen, CS Zn2+-chelating motif-tethered short-chain fatty acids as a novel class of histone deacetylase inhibitors. J Med Chem 47: 467-74 (2004)
- Nemecz, Akos; Taylor, Palmer Creating an a7 Nicotinic Acetylcholine Recognition Domain from the Acetylcholine-binding Protein Taylor Research Group (2014)
- Wessels, M; König, GM; Wright, AD A new tyrosine kinase inhibitor from the marine brown alga Stypopodium zonale. J Nat Prod 62: 927-30 (1999)
- Nhoek, P; Ahn, S; Pel, P; Kim, YM; Huh, J; Kim, HW; Noh, M; Chin, YW Alkaloids and Coumarins with Adiponectin-Secretion-Promoting Activities from the Leaves of J Nat Prod 86: 138-148 (2023)
- Ramli, RA; Lie, W; Pyne, SG Alkaloids from the roots of Stichoneuron caudatum and their acetylcholinesterase inhibitory activities. J Nat Prod 77: 894-901 (2014)
- Jaidee, W; Andersen, RJ; Chavez, MAG; Wang, YA; Patrick, BO; Pyne, SG; Muanprasat, C; Borwornpinyo, S; Laphookhieo, S Amides and Flavonoids from the Fruit and Leaf Extracts of Melodorum siamensis. J Nat Prod 82: 283-292 (2019)
- Guile, SD; Alcaraz, L; Birkinshaw, TN; Bowers, KC; Ebden, MR; Furber, M; Stocks, MJ Antagonists of the P2X(7) receptor. From lead identification to drug development. J Med Chem 52: 3123-41 (2009)
- Yang, XW; Zhao, J; Cui, YX; Liu, XH; Ma, CM; Hattori, M; Zhang, LH Anti-HIV-1 protease triterpenoid saponins from the seeds of Aesculus chinensis. J Nat Prod 62: 1510-3 (1999)
- Wibowo, M; Levrier, C; Sadowski, MC; Nelson, CC; Wang, Q; Holst, J; Healy, PC; Hofmann, A; Davis, RA Bioactive Dihydro-ß-agarofuran Sesquiterpenoids from the Australian Rainforest Plant Maytenus bilocularis. J Nat Prod 79: 1445-53 (2016)
- Liang, LF; Wang, XJ; Zhang, HY; Liu, HL; Li, J; Lan, LF; Zhang, W; Guo, YW Bioactive polyhydroxylated steroids from the Hainan soft coral Sinularia depressa Tixier-Durivault. Bioorg Med Chem Lett 23: 1334-7 (2013)
- Oh, KB; Mar, W; Kim, S; Kim, JY; Oh, MN; Kim, JG; Shin, D; Sim, CJ; Shin, J Bis(indole) alkaloids as sortase A inhibitors from the sponge Spongosorites sp. Bioorg Med Chem Lett 15: 4927-31 (2005)
- Wu, B; Chen, J; Qu, H; Cheng, Y Complex sesquiterpenoids with tyrosinase inhibitory activity from the leaves of Chloranthus tianmushanensis. J Nat Prod 71: 877-80 (2008)
- Wu, TS; Hsu, MY; Kuo, PC; Sreenivasulu, B; Damu, AG; Su, CR; Li, CY; Chang, HC Constituents from the leaves of Phellodendron amurense var. wilsonii and their bioactivity. J Nat Prod 66: 1207-11 (2003)
- Hajduk, PJ; Gomtsyan, A; Didomenico, S; Cowart, M; Bayburt, EK; Solomon, L; Severin, J; Smith, R; Walter, K; Holzman, TF; Stewart, A; McGaraughty, S; Jarvis, MF; Kowaluk, EA; Fesik, SW Design of adenosine kinase inhibitors from the NMR-based screening of fragments. J Med Chem 43: 4781-6 (2000)
- Fredo Naciuk, F; do Nascimento Faria, J; Gonçalves Eufrásio, A; Torres Cordeiro, A; Bruder, M Development of Selective Steroid Inhibitors for the Glucose-6-phosphate Dehydrogenase from ACS Med Chem Lett 11: 1250-1256 (2020)
- Pereira, GAN; da Silva, EB; Braga, SFP; Leite, PG; Martins, LC; Vieira, RP; Soh, WT; Villela, FS; Costa, FMR; Ray, D; de Andrade, SF; Brandstetter, H; Oliveira, RB; Caffrey, CR; Machado, FS; Ferreira, RS Discovery and characterization of trypanocidal cysteine protease inhibitors from the 'malaria box'. Eur J Med Chem 179: 765-778 (2019)
- Li, H; Zhang, M; Li, H; Yu, H; Chen, S; Wu, W; Sun, P Discovery of Venturicidin Congeners and Identification of the Biosynthetic Gene Cluster from J Nat Prod 84: 110-119 (2021)
- Tapiolas, DM; Bowden, BF; Abou-Mansour, E; Willis, RH; Doyle, JR; Muirhead, AN; Liptrot, C; Llewellyn, LE; Wolff, CW; Wright, AD; Motti, CA Eusynstyelamides A, B, and C, nNOS inhibitors, from the ascidian Eusynstyela latericius. J Nat Prod 72: 1115-20 (2009)
- Jacobson, KA; Salmaso, V; Suresh, RR; Tosh, DK Expanding the repertoire of methanocarba nucleosides from purinergic signaling to diverse targets. RSC Med Chem 12: 1808-1825 (2021)
- Jiang, W; Jiang, Y; Luo, Y; Qiao, W; Yang, T Facilitating the development of molecular glues: Opportunities from serendipity and rational design. Eur J Med Chem 263:
- Saario, SM; Poso, A; Juvonen, RO; Järvinen, T; Salo-Ahen, OM Fatty acid amide hydrolase inhibitors from virtual screening of the endocannabinoid system. J Med Chem 49: 4650-6 (2006)
- Boss, C; Bolli, MH; Gatfield, J From bosentan (Tracleer®) to macitentan (Opsumit®): The medicinal chemistry perspective. Bioorg Med Chem Lett 26: 3381-94 (2016)
- Katavic, PL; Venables, DA; Forster, PI; Guymer, G; Carroll, AR Grandisines C-G, indolizidine alkaloids from the Australian rainforest tree Elaeocarpus grandis. J Nat Prod 69: 1295-9 (2006)
- Piao, SJ; Zhang, HJ; Lu, HY; Yang, F; Jiao, WH; Yi, YH; Chen, WS; Lin, HW Hippolides A-H, acyclic manoalide derivatives from the marine sponge Hippospongia lachne. J Nat Prod 74: 1248-54 (2011)
- Pereira, A; Vottero, E; Roberge, M; Mauk, AG; Andersen, RJ Indoleamine 2,3-dioxygenase inhibitors from the Northeastern Pacific Marine Hydroid Garveia annulata. J Nat Prod 69: 1496-9 (2006)
- Masuda, Y; Fujihara, K; Hayashi, S; Sasaki, H; Kino, Y; Kamauchi, H; Noji, M; Satoh, JI; Takanami, T; Kinoshita, K; Koyama, K Inhibition of BACE1 and Amyloid-β Aggregation by Meroterpenoids from the Mushroom J Nat Prod 84: 1748-1754 (2021)
- Ung, PM; Song, W; Cheng, L; Zhao, X; Hu, H; Chen, L; Schlessinger, A Inhibitor Discovery for the Human GLUT1 from Homology Modeling and Virtual Screening. ACS Chem Biol 11: 1908-16 (2016)
- Bankeu, JJ; Khayala, R; Lenta, BN; Noungoué, DT; Ngouela, SA; Mustafa, SA; Asaad, K; Choudhary, MI; Prigge, ST; Hasanov, R; Nkengfack, AE; Tsamo, E; Ali, MS Isoflavone dimers and other bioactive constituents from the figs of Ficus mucuso. J Nat Prod 74: 1370-8 (2011)
- Kochanowska-Karamyan, AJ; Araujo, HC; Zhang, X; El-Alfy, A; Carvalho, P; Avery, MA; Holmbo, SD; Magolan, J; Hamann, MT Isolation and Synthesis of Veranamine, an Antidepressant Lead from the Marine Sponge J Nat Prod 83: 1092-1098 (2020)
- Musso-Buendía, JA; Vidal, AE; Kasinthan, G; Nguyen, C; Carrero-Lérida, J; Ruiz-Pérez, LM; Wilson, K; Johansson, NG; Gilbert, IH; González-Pacanowska, D Kinetic properties and inhibition of the dimeric dUTPase-dUDPase from Campylobacter jejuni. J Enzyme Inhib Med Chem 24: 111-6 (2009)
- Feng, L; Zhang, Y; Liu, YC; Liu, Y; Luo, SH; Huang, CS; Li, SH Leucoflavonine, a new bioactive racemic flavoalkaloid from the leaves of Leucosceptrum canum. Bioorg Med Chem 27: 442-446 (2019)
- Yan, P; Ritt, DA; Zlotkowski, K; Bokesch, HR; Reinhold, WC; Schneekloth, JS; Morrison, DK; Gustafson, KR Macrophilones from the Marine Hydroid Macrorhynchia philippina Can Inhibit ERK Cascade Signaling. J Nat Prod 81: 1666-1672 (2018)
- Qiu, S; Yang, WZ; Yao, CL; Shi, XJ; Li, JY; Lou, Y; Duan, YN; Wu, WY; Guo, DA Malonylginsenosides with Potential Antidiabetic Activities from the Flower Buds of Panax ginseng. J Nat Prod 80: 899-908 (2017)
- Nakashima, S; Matsuda, H; Oda, Y; Nakamura, S; Xu, F; Yoshikawa, M Melanogenesis inhibitors from the desert plant Anastatica hierochuntica in B16 melanoma cells. Bioorg Med Chem 18: 2337-45 (2010)
- Matsuda, H; Nakashima, S; Oda, Y; Nakamura, S; Yoshikawa, M Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg Med Chem 17: 6048-53 (2009)
- Williams, DE; Telliez, JB; Liu, J; Tahir, A; van Soest, R; Andersen, RJ Meroterpenoid MAPKAP (MK2) inhibitors isolated from the indonesian marine sponge Acanthodendrilla sp. J Nat Prod 67: 2127-9 (2004)
- Rosazza, JP; Duffel, MW; El-Marakby, S; Ahn, SH Metabolism of the Catharanthus Alkaloids: from Streptomyces griseus to Monoamine Oxidase B J Nat Prod 55: 269-284 (1992)
- Zafrir, E; Carmeli, S Micropeptins from an Israeli fishpond water bloom of the cyanobacterium Microcystis sp. J Nat Prod 73: 352-8 (2010)
- Gunasekera, SP; Miller, MW; Kwan, JC; Luesch, H; Paul, VJ Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium Dichothrix utahensis. J Nat Prod 73: 459-62 (2010)
- Sato, S; Takeo, J; Aoyama, C; Kawahara, H Na+-glucose cotransporter (SGLT) inhibitory flavonoids from the roots of Sophora flavescens. Bioorg Med Chem 15: 3445-9 (2007)
- Murakami, M; Suzuki, S; Itou, Y; Kodani, S; Ishida, K New anabaenopeptins, potent carboxypeptidase-A inhibitors from the cyanobacterium Aphanizomenon flos-aquae. J Nat Prod 63: 1280-2 (2000)
- Liu, L; Jokela, J; Wahlsten, M; Nowruzi, B; Permi, P; Zhang, YZ; Xhaard, H; Fewer, DP; Sivonen, K Nostosins, Trypsin Inhibitors Isolated from the Terrestrial Cyanobacterium Nostoc sp. Strain FSN. J Nat Prod 77: 1784-90 (2014)
- Huang, XF; Dong, YH; Wang, JH; Ke, HM; Song, GQ; Xu, DF Novel PDE5 inhibitors derived from rutaecarpine for the treatment of Alzheimer's disease. Bioorg Med Chem Lett 30: (2020)
- Wu, CC; Yen, MH; Yang, SC; Lin, CN Phloroglucinols with antioxidant activity and xanthonolignoids from the heartwood of Hypericum geminiflorum. J Nat Prod 71: 1027-31 (2008)
- Gunasekera, SP; Isbrucker, RA; Longley, RE; Wright, AE; Pomponi, SA; Reed, JK Plakolide A, a new gamma-lactone from the marine sponge Plakortis sp. J Nat Prod 67: 110-1 (2004)
- Prevatt-Smith, KM; Lovell, KM; Simpson, DS; Day, VW; Douglas, JT; Bosch, P; Dersch, CM; Rothman, RB; Kivell, B; Prisinzano, TE Potential Drug Abuse Therapeutics Derived from the Hallucinogenic Natural Product Salvinorin A. Medchemcomm 2: 1217-1222 (2011)
- Dai, J; Yoshida, WY; Kelly, M; Williams, P Pregnane-10,2-carbolactones from a Hawaiian Marine Sponge in the Genus Myrmekioderma. J Nat Prod 79: 1464-7 (2016)
- Cheng, ZB; Deng, YL; Fan, CQ; Han, QH; Lin, SL; Tang, GH; Luo, HB; Yin, S Prostaglandin Derivatives: Nonaromatic Phosphodiesterase-4 Inhibitors from the Soft Coral Sarcophyton ehrenbergi. J Nat Prod 77: 1928-36 (2014)
- Li, X; Li, X; Liu, F; Li, S; Shi, D Rational Multitargeted Drug Design Strategy from the Perspective of a Medicinal Chemist. J Med Chem 64: 10581-10605 (2021)
- Williams, PG; Asolkar, RN; Kondratyuk, T; Pezzuto, JM; Jensen, PR; Fenical, W Saliniketals A and B, bicyclic polyketides from the marine actinomycete Salinispora arenicola. J Nat Prod 70: 83-8 (2007)
- Tran, TD; Wilson, BAP; Henrich, CJ; Staudt, LM; Krumpe, LRH; Smith, EA; King, J; Wendt, KL; Stchigel, AM; Miller, AN; Cichewicz, RH; O'Keefe, BR; Gustafson, KR Secondary Metabolites from the Fungus Dictyosporium sp. and Their MALT1 Inhibitory Activities. J Nat Prod 82: 154-162 (2019)
- Murayama, S; Imae, Y; Takada, K; Kikuchi, J; Nakao, Y; van Soest, RW; Okada, S; Matsunaga, S Shishicrellastatins, inhibitors of cathepsin B, from the marine sponge Crella (Yvesia) spinulata. Bioorg Med Chem 19: 6594-8 (2011)
- Sai, M; Vietor, J; Kornmayer, M; Egner, M; López-García, Ú; Höfner, G; Pabel, J; Marschner, JA; Wein, T; Merk, D Structure-Guided Design of Nurr1 Agonists Derived from the Natural Ligand Dihydroxyindole. J Med Chem 66: 13556-13567 (2023)
- Vullo, D; Del Prete, S; Osman, SM; De Luca, V; Scozzafava, A; Alothman, Z; Supuran, CT; Capasso, C Sulfonamide inhibition studies of thed-carbonic anhydrase from the diatom Thalassiosira weissflogii. Bioorg Med Chem Lett 24: 275-9 (2013)
- Kato, H; El-Desoky, AH; Takeishi, Y; Nehira, T; Angkouw, ED; Mangindaan, REP; de Voogd, NJ; Tsukamoto, S Tetradehydrohalicyclamine B, a new proteasome inhibitor from the marine sponge Acanthostrongylophora ingens. Bioorg Med Chem Lett 29: 8-10 (2019)
- Geurs, S; Clarisse, D; De Bosscher, K; D'hooghe, M The Zinc-Binding Group Effect: Lessons from Non-Hydroxamic Acid Vorinostat Analogs. J Med Chem 66: 7698-7729 (2023)
- Fletcher, SR; McIver, E; Lewis, S; Burkamp, F; Leech, C; Mason, G; Boyce, S; Morrison, D; Richards, G; Sutton, K; Jones, AB The search for novel TRPV1-antagonists: from carboxamides to benzimidazoles and indazolones. Bioorg Med Chem Lett 16: 2872-6 (2006)
- Jiao, WH; Salim, AA; Khalil, ZG; Dewapriya, P; Lin, HW; Butler, MS; Capon, RJ Trivirensols: Selectively Bacteriostatic Sesquiterpene Trimers from the Australian Termite Nest-Derived Fungus J Nat Prod 82: 3165-3175 (2019)
- Kim, SS; Hyun, CG; Choi, YH; Lee, NH Tyrosinase inhibitory activities of the compounds isolated from Neolitsea aciculata (Blume) Koidz. J Enzyme Inhib Med Chem 28: 685-9 (2013)
- Aspnes, GE; Bagley, SW; Coffey, SB; Conn, EL; Curto, JM; Edmonds, DJ; Genovino, J; Griffith, DA; Ingle, G; Jiao, W; Limberakis, C; Mathiowetz, AM; Piotrowski, DW; Rose, CR; Ruggeri, RB; Wei, L 6-Azaspiro[2.5]octanes as small molecule agonists of the human glucagon-like peptide-1 receptor. Bioorg Med Chem Lett 94: (2023)
- Grieco, P; Carotenuto, A; Campiglia, P; Zampelli, E; Patacchini, R; Maggi, CA; Novellino, E; Rovero, P A new, potent urotensin II receptor peptide agonist containing a Pen residue at the disulfide bridge. J Med Chem 45: 4391-4 (2002)
- VanAtten, MK; Ensinger, CL; Chiu, AT; McCall, DE; Nguyen, TT; Wexler, RR; Timmermans, PB A novel series of selective, non-peptide inhibitors of angiotensin II binding to the AT2 site. J Med Chem 36: 3985-92 (1994)
- Sigal, GB; Whitesides, GM Benzenesulfonamide-peptide conjugates as probes for secondary binding sites near the active site of carbonic anhydrase Bioorg Med Chem Lett 6: 559-564 (1996)
- Neal, CR; Owens, CE; Taylor, LP; Hoversten, MT; Akil, H; Watson, SJ Binding and GTPgammaS autoradiographic analysis of preproorphanin precursor peptide products at the ORL1 and opioid receptors. J Chem Neuroanat 25: 233-47 (2003)
- Tombling, BJ; Lammi, C; Lawrence, N; Gilding, EK; Grazioso, G; Craik, DJ; Wang, CK Bioactive Cyclization Optimizes the Affinity of a Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Peptide Inhibitor. J Med Chem 64: 2523-2533 (2021)
- Haskell-Luevano, C; Holder, JR; Monck, EK; Bauzo, RM Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors. J Med Chem 44: 2247-52 (2001)
- Szedlacsek, HS; Bajusz, D; Badea, RA; Pop, A; Bică, CC; Ravasz, L; Mittli, D; Mátyás, D; Necula-Petrăreanu, G; Munteanu, CVA; Papp, I; Juhász, G; Hritcu, L; Keserű, GM; Szedlacsek, SE Designed Peptide Inhibitors of STEP Phosphatase-GluA2 AMPA Receptor Interaction Enhance the Cognitive Performance in Rats. J Med Chem 65: 217-233 (2022)
- Algar, S; Martín-Martínez, M; González-Muñiz, R Evolution in non-peptide α-helix mimetics on the road to effective protein-protein interaction modulators. Eur J Med Chem 211: (2021)
- Zhang, Y; Eigenbrot, C; Zhou, L; Shia, S; Li, W; Quan, C; Tom, J; Moran, P; Di Lello, P; Skelton, NJ; Kong-Beltran, M; Peterson, A; Kirchhofer, D Identification of a small peptide that inhibits PCSK9 protein binding to the low density lipoprotein receptor. J Biol Chem 289: 942-55 (2014)
- Meng, H; Krishnaji, ST; Beinborn, M; Kumar, K Influence of selective fluorination on the biological activity and proteolytic stability of glucagon-like peptide-1. J Med Chem 51: 7303-7 (2009)
- Ganapathy, ME; Prasad, PD; Mackenzie, B; Ganapathy, V; Leibach, FH Interaction of anionic cephalosporins with the intestinal and renal peptide transporters PEPT 1 and PEPT 2. Biochim Biophys Acta 1324: 296-308 (1997)
- Olson, EJ; Lechtenberg, BC; Zhao, C; Rubio de la Torre, E; Lamberto, I; Riedl, SJ; Dawson, PE; Pasquale, EB Modifications of a Nanomolar Cyclic Peptide Antagonist for the EphA4 Receptor To Achieve High Plasma Stability. ACS Med Chem Lett 7: 841-6 (2016)
- Rosenkilde, MM; Gerlach, LO; Hatse, S; Skerlj, RT; Schols, D; Bridger, GJ; Schwartz, TW Molecular mechanism of action of monocyclam versus bicyclam non-peptide antagonists in the CXCR4 chemokine receptor. J Biol Chem 282: 27354-65 (2007)
- Shahripour, A; Plummer, MS; Lunney, EA; Para, KS; Stankovic, CJ; Rubin, JR; Humblet, C; Fergus, JH; Marks, JS; Herrera, R; Hubbell, SE; Saltiel, AR; Sawyer, TK Novel phosphotyrosine mimetics in the design of peptide ligands for pp60src SH2 domain Bioorg Med Chem Lett 6: 1209-1214 (1996)
- Schiller, PW; Nguyen, TM; Maziak, LA; Wilkes, BC; Lemieux, C Structure-activity relationships of cyclic opioid peptide analogues containing a phenylalanine residue in the 3-position. J Med Chem 30: 2094-9 (1987)
- Wilson, DL; Meininger, I; Strater, Z; Steiner, S; Tomlin, F; Wu, J; Jamali, H; Krappmann, D; Götz, MG Synthesis and Evaluation of Macrocyclic Peptide Aldehydes as Potent and Selective Inhibitors of the 20S Proteasome. ACS Med Chem Lett 7: 250-5 (2016)
- Burden, JE; Davis, P; Porreca, F; Spatola, AF Synthesis and biological activities of position one and three transposed analogs of the opioid peptide YKFA. Bioorg Med Chem Lett 9: 3441-6 (2000)
- Jones, AB; Acton, JJ; Rivetna, MN; Cummings, RT; Cubbon, RM; Nichols, EA; Schwartz, CD; Wicker, LS; Hermes, JD Tetrapeptide derived inhibitors of complexation of a class II MHC: the peptide backbone is not inviolate. Bioorg Med Chem Lett 9: 2109-14 (1999)
- Peng, D; Cao, B; Zhou, YJ; Long, YQ The chemical diversity and structure-based evolution of non-peptide CXCR4 antagonists with diverse therapeutic potential. Eur J Med Chem 149: 148-169 (2018)
- Girlich, D; Poirel, L; Nordmann, P Novel ambler class A carbapenem-hydrolyzing beta-lactamase from a Pseudomonas fluorescens isolate from the Seine River, Paris, France. Antimicrob Agents Chemother 54: 328-32 (2009)
- Del Prete, S; Vullo, D; De Luca, V; Carginale, V; Osman, SM; AlOthman, Z; Supuran, CT; Capasso, C Cloning, expression, purification and sulfonamide inhibition profile of the complete domain of the η-carbonic anhydrase from Plasmodium falciparum. Bioorg Med Chem Lett 26: 4184-90 (2016)
- Springthorpe, B; Bailey, A; Barton, P; Birkinshaw, TN; Bonnert, RV; Brown, RC; Chapman, D; Dixon, J; Guile, SD; Humphries, RG; Hunt, SF; Ince, F; Ingall, AH; Kirk, IP; Leeson, PD; Leff, P; Lewis, RJ; Martin, BP; McGinnity, DF; Mortimore, MP; Paine, SW; Pairaudeau, G; Patel, A; Rigby, AJ; Riley, RJ; Teobald, BJ; Tomlinson, W; Webborn, PJ; Willis, PA From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett 17: 6013-8 (2007)
- Nishimori, I; Minakuchi, T; Vullo, D; Scozzafava, A; Supuran, CT Inhibition studies of theß-carbonic anhydrases from the bacterial pathogen Salmonella enterica serovar Typhimurium with sulfonamides and sulfamates. Bioorg Med Chem 19: 5023-30 (2011)
- Hazra, S; Kurz, SG; Wolff, K; Nguyen, L; Bonomo, RA; Blanchard, JS Kinetic and Structural Characterization of the Interaction of 6-Methylidene Penem 2 with the ß-Lactamase from Mycobacterium tuberculosis. Biochemistry 54: 5657-64 (2015)
- Kossack, R; Breinlinger, S; Nguyen, T; Moschny, J; Straetener, J; Berscheid, A; Brötz-Oesterhelt, H; Enke, H; Schirmeister, T; Niedermeyer, THJ Nostotrebin 6 Related Cyclopentenediones and δ-Lactones with Broad Activity Spectrum Isolated from the Cultivation Medium of the Cyanobacterium J Nat Prod 83: 392-400 (2020)
- Wilson, DP; Wan, ZK; Xu, WX; Kirincich, SJ; Follows, BC; Joseph-McCarthy, D; Foreman, K; Moretto, A; Wu, J; Zhu, M; Binnun, E; Zhang, YL; Tam, M; Erbe, DV; Tobin, J; Xu, X; Leung, L; Shilling, A; Tam, SY; Mansour, TS; Lee, J Structure-based optimization of protein tyrosine phosphatase 1B inhibitors: from the active site to the second phosphotyrosine binding site. J Med Chem 50: 4681-98 (2007)
- Del Prete, S; Vullo, D; Osman, SM; AlOthman, Z; Supuran, CT; Capasso, C Sulfonamide inhibition profiles of theβ-carbonic anhydrase from the pathogenic bacterium Francisella tularensis responsible of the febrile illness tularemia. Bioorg Med Chem 25: 3555-3561 (2017)
- Allam, HA; Fahim, SH; F Abo-Ashour, M; Nocentini, A; Elbakry, ME; Abdelrahman, MA; Eldehna, WM; Ibrahim, HS; Supuran, CT Application of hydrazino and hydrazido linkers to connect benzenesulfonamides with hydrophilic/phobic tails for targeting the middle region of human carbonic anhydrases active site: Selective inhibitors of hCA IX. Eur J Med Chem 179: 547-556 (2019)
- Pasquini, S; Ligresti, A; Mugnaini, C; Semeraro, T; Cicione, L; De Rosa, M; Guida, F; Luongo, L; De Chiaro, M; Cascio, MG; Bolognini, D; Marini, P; Pertwee, R; Maione, S; Di Marzo, V; Corelli, F Investigations on the 4-quinolone-3-carboxylic acid motif. 3. Synthesis, structure-affinity relationships, and pharmacological characterization of 6-substituted 4-quinolone-3-carboxamides as highly selective cannabinoid-2 receptor ligands. J Med Chem 53: 5915-28 (2010)
- Fotsch, C; Smith, DM; Adams, JA; Cheetham, J; Croghan, M; Doherty, EM; Hale, C; Jarosinski, MA; Kelly, MG; Norman, MH; Tamayo, NA; Xi, N; Baumgartner, JW Design of a new peptidomimetic agonist for the melanocortin receptors based on the solution structure of the peptide ligand, Ac-Nle-cyclo[Asp-Pro-DPhe-Arg-Trp-Lys]-NH(2). Bioorg Med Chem Lett 13: 2337-40 (2003)
- Coleman, DR; Ren, Z; Mandal, PK; Cameron, AG; Dyer, GA; Muranjan, S; Campbell, M; Chen, X; McMurray, JS Investigation of the binding determinants of phosphopeptides targeted to the SRC homology 2 domain of the signal transducer and activator of transcription 3. Development of a high-affinity peptide inhibitor. J Med Chem 48: 6661-70 (2005)
- PubChem, PC Dose-response biochemical assay for antagonists of the interaction between the Eph receptor B4 (EphB4) and its ligand ephrin-B2 via TNYL-RAW peptide PubChem Bioassay (2007)
- Igarashi, H; Ito, T; Pradhan, TK; Mantey, SA; Hou, W; Coy, DH; Jensen, RT Elucidation of the vasoactive intestinal peptide pharmacophore for VPAC(2) receptors in human and rat and comparison to the pharmacophore for VPAC(1) receptors. J Pharmacol Exp Ther 303: 445-60 (2002)
- Carney, DW; Leffler, AE; Bell, JA; Chandrasinghe, AS; Cheng, C; Chang, E; Dornford, A; Dougan, DR; Frye, LL; Grimes, ME; Knehans, T; Knight, JL; Komandla, M; Lane, W; Li, H; Newman, SR; Phimister, K; Saikatendu, KS; Silverstein, H; Vafaei, S Exploiting high-energy hydration sites for the discovery of potent peptide aldehyde inhibitors of the SARS-CoV-2 main protease with cellular antiviral activity. Bioorg Med Chem 103:
- Yamamoto, T; Nair, P; Jacobsen, NE; Davis, P; Ma, SW; Navratilova, E; Moye, S; Lai, J; Yamamura, HI; Vanderah, TW; Porreca, F; Hruby, VJ The importance of micelle-bound states for the bioactivities of bifunctional peptide derivatives for delta/mu opioid receptor agonists and neurokinin 1 receptor antagonists. J Med Chem 51: 6334-47 (2008)
- Randolph, JT; Sauer, DR; Haviv, F; Nilius, AM; Greer, J Elimination of antibacterial activities of non-peptide luteinizing hormone-releasing hormone (LHRH) antagonists derived from erythromycin A. Bioorg Med Chem Lett 14: 1599-602 (2004)
- Bower, KE; Lam, SN; Oates, BD; Del Rosario, JR; Corner, E; Osothprarop, TF; Kinhikar, AG; Hoye, JA; Preston, RR; Murphy, RE; Campbell, LA; Huang, H; Jimenez, J; Cao, X; Chen, G; Ainekulu, ZW; Datt, AB; Levin, NJ; Doppalapudi, VR; Pirie-Shepherd, SR; Bradshaw, C; Woodnutt, G; Lappe, RW Evolution of potent and stable placental-growth-factor-1-targeting CovX-bodies from phage display peptide discovery. J Med Chem 54: 1256-65 (2011)
- Yoshida, S; Uehara, S; Kondo, N; Takahashi, Y; Yamamoto, S; Kameda, A; Kawagoe, S; Inoue, N; Yamada, M; Yoshimura, N; Tachibana, Y Peptide-to-Small Molecule: A Pharmacophore-Guided Small Molecule Lead Generation Strategy from High-Affinity Macrocyclic Peptides. J Med Chem 65: 10655-10673 (2022)
- Gavenonis, J; Jonas, NE; Kritzer, JA Potential C-terminal-domain inhibitors of heat shock protein 90 derived from a C-terminal peptide helix. Bioorg Med Chem 22: 3989-93 (2014)
- Boeglin, D; Hamdan, FF; Melendez, RE; Cluzeau, J; Laperriere, A; Héroux, M; Bouvier, M; Lubell, WD Calcitonin gene-related peptide analogues with aza and indolizidinone amino acid residues reveal conformational requirements for antagonist activity at the human calcitonin gene-related peptide 1 receptor. J Med Chem 50: 1401-8 (2007)
- Orritt, KM; Feng, L; Newell, JF; Sutton, JN; Grossman, S; Germe, T; Abbott, LR; Jackson, HL; Bury, BKL; Maxwell, A; McPhillie, MJ; Fishwick, CWG design of type II topoisomerase inhibitors as potential antimicrobial agents targeting a novel binding region. RSC Med Chem 13: 831-839 (2022)
- Lee, S; Kim, C; Ann, J; Thorat, SA; Kim, E; Park, J; Choi, S; Blumberg, PM; Frank-Foltyn, R; Bahrenberg, G; Stockhausen, H; Christoph, T; Lee, J Pyrazole C-region analogues of 2-(3-fluoro-4-methylsulfonylaminophenyl)propanamides as potent TRPV1 antagonists. Bioorg Med Chem Lett 27: 4383-4388 (2017)
- Ryu, H; Seo, S; Lee, JY; Ha, TH; Lee, S; Jung, A; Ann, J; Kim, SE; Yoon, S; Hong, M; Blumberg, PM; Frank-Foltyn, R; Bahrenberg, G; Schiene, K; Stockhausen, H; Christoph, T; Frormann, S; Lee, J Pyridine C-region analogs of 2-(3-fluoro-4-methylsulfonylaminophenyl)propanamides as potent TRPV1 antagonists. Eur J Med Chem 93: 101-8 (2015)
- Huang, B; Chen, W; Zhao, T; Li, Z; Jiang, X; Ginex, T; Vílchez, D; Luque, FJ; Kang, D; Gao, P; Zhang, J; Tian, Y; Daelemans, D; De Clercq, E; Pannecouque, C; Zhan, P; Liu, X Exploiting the Tolerant Region I of the Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) Binding Pocket: Discovery of Potent Diarylpyrimidine-Typed HIV-1 NNRTIs against Wild-Type and E138K Mutant Virus with Significantly Improved Water Solubility and Favorable Safety Profiles. J Med Chem 62: 2083-2098 (2019)
- Udugamasooriya, DG; Ritchie, C; Brekken, RA; Kodadek, T A peptoid antagonist of VEGF receptor 2 recognizes a 'hotspot' in the extracellular domain distinct from the hormone-binding site. Bioorg Med Chem 16: 6338-43 (2008)
- Gouda, H; Yanai, Y; Sugawara, A; Sunazuka, T; Omura, S; Hirono, S Computational analysis of the binding affinities of the natural-product cyclopentapeptides argifin and argadin to chitinase B from Serratia marcescens. Bioorg Med Chem 16: 3565-79 (2008)
- Choung, W; Jung, HJ; Nam, EH; Yang, D; Yoo, B; Choi, H; Lee, BR; Park, M; Jang, SM; Lim, JS; Kim, KH; Chin, J; Jung, K; Lee, G; Kim, SH Discovery of the bifunctional modulator of angiotensin II type 1 receptor (AT1R) and PPARγ derived from the AT1R antagonist, Fimasartan. Bioorg Med Chem Lett 28: 3155-3160 (2018)
- Sahner, JH; Empting, M; Kamal, A; Weidel, E; Groh, M; Börger, C; Hartmann, RW Exploring the chemical space of ureidothiophene-2-carboxylic acids as inhibitors of the quorum sensing enzyme PqsD from Pseudomonas aeruginosa. Eur J Med Chem 96: 14-21 (2015)
- Williams, DE; Steinø, A; de Voogd, NJ; Mauk, AG; Andersen, RJ Halicloic acids A and B isolated from the marine sponge Haliclona sp. collected in the Philippines inhibit indoleamine 2,3-dioxygenase. J Nat Prod 75: 1451-8 (2012)
- El Dine, RS; El Halawany, AM; Ma, CM; Hattori, M Inhibition of the dimerization and active site of HIV-1 protease by secondary metabolites from the Vietnamese mushroom Ganoderma colossum. J Nat Prod 72: 2019-23 (2009)
- Yang, SP; Zhang, XW; Ai, J; Gan, LS; Xu, JB; Wang, Y; Su, ZS; Wang, L; Ding, J; Geng, MY; Yue, JM Potent HGF/c-Met axis inhibitors from Eucalyptus globulus: the coupling of phloroglucinol and sesquiterpenoid is essential for the activity. J Med Chem 55: 8183-7 (2012)
- Day, CL; Smits, C; Fan, FC; Lee, EF; Fairlie, WD; Hinds, MG Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol 380: 958-71 (2008)
- Reggio, PH; Basu-Dutt, S; Barnett-Norris, J; Castro, MT; Hurst, DP; Seltzman, HH; Roche, MJ; Gilliam, AF; Thomas, BF; Stevenson, LA; Pertwee, RG; Abood, ME The bioactive conformation of aminoalkylindoles at the cannabinoid CB1 and CB2 receptors: insights gained from (E)- and (Z)-naphthylidene indenes. J Med Chem 41: 5177-87 (1999)
- Wijtmans, M; Scholten, DJ; Roumen, L; Canals, M; Custers, H; Glas, M; Vreeker, MC; de Kanter, FJ; de Graaf, C; Smit, MJ; de Esch, IJ; Leurs, R Chemical subtleties in small-molecule modulation of peptide receptor function: the case of CXCR3 biaryl-type ligands. J Med Chem 55: 10572-83 (2012)
- Nam, NH; Ye, G; Sun, G; Parang, K Conformationally constrained peptide analogues of pTyr-Glu-Glu-Ile as inhibitors of the Src SH2 domain binding. J Med Chem 47: 3131-41 (2004)
- Holder, JR; Bauzo, RM; Xiang, Z; Scott, J; Haskell-Luevano, C Design and pharmacology of peptoids and peptide-peptoid hybrids based on the melanocortin agonists core tetrapeptide sequence. Bioorg Med Chem Lett 13: 4505-9 (2003)
- Llinàs-Brunet, M; Bailey, M; Fazal, G; Ghiro, E; Gorys, V; Goulet, S; Halmos, T; Maurice, R; Poirier, M; Poupart, MA; Rancourt, J; Thibeault, D; Wernic, D; Lamarre, D Highly potent and selective peptide-based inhibitors of the hepatitis C virus serine protease: towards smaller inhibitors. Bioorg Med Chem Lett 10: 2267-70 (2001)
- Wu, X; Meng, Y; Liu, L; Gong, G; Zhang, H; Hou, Y; Liu, C; Wu, D; Qin, M Insights into non-peptide small-molecule inhibitors of the PD-1/PD-L1 interaction: Development and perspective. Bioorg Med Chem 33: (2021)
- Xiong, X; Wang, N; Zhang, Y; Zhao, W; Pang, N; Fu, K; Zhou, N; Zhou, X; Guo, D Long-Residence Time Peptide Antagonist for the Vasopressin V2 Receptor to Treat Autosomal Dominant Polycystic Kidney Disease. J Med Chem 67: 5935-5944
- Li, G; Haq, W; Xiang, L; Lou, BS; Hughes, R; De Leon, IA; Davis, P; Gillespie, TJ; Romanowski, M; Zhu, X; Misicka, A; Lipkowski, AW; Porreca, F; Davis, TP; Yamamura, HI; O'Brien, DF; Hruby, VJ Modifications of the 4,4'-residues and SAR studies of Biphalin, a highly potent opioid receptor active peptide. Bioorg Med Chem Lett 8: 555-60 (1999)
- Teufel, DP; Bennett, G; Harrison, H; van Rietschoten, K; Pavan, S; Stace, C; Le Floch, F; Van Bergen, T; Vermassen, E; Barbeaux, P; Hu, TT; Feyen, JHM; Vanhove, M Stable and Long-Lasting, Novel Bicyclic Peptide Plasma Kallikrein Inhibitors for the Treatment of Diabetic Macular Edema. J Med Chem 61: 2823-2836 (2018)
- Madden, MM; Muppidi, A; Li, Z; Li, X; Chen, J; Lin, Q Synthesis of cell-permeable stapled peptide dual inhibitors of the p53-Mdm2/Mdmx interactions via photoinduced cycloaddition. Bioorg Med Chem Lett 21: 1472-5 (2011)
- Boden, P; Eden, JM; Hodgson, J; Horwell, DC; Hughes, J; McKnight, AT; Lewthwaite, RA; Pritchard, MC; Raphy, J; Meecham, K; Ratcliffe, GS; Suman-Chauhan, N; Woodruff, GN Use of a dipeptide chemical library in the development of non-peptide tachykinin NK3 receptor selective antagonists. J Med Chem 39: 1664-75 (1996)
- Shi, ZD; Karki, RG; Oishi, S; Worthy, KM; Bindu, LK; Dharmawardana, PG; Nicklaus, MC; Bottaro, DP; Fisher, RJ; Burke, TR Utilization of a nitrobenzoxadiazole (NBD) fluorophore in the design of a Grb2 SH2 domain-binding peptide mimetic. Bioorg Med Chem Lett 15: 1385-8 (2005)
- AlphaScreen Assay AlphaScreen was used with the aim of identifying novel modulators by taking advantage of the bimolecular interaction prevailing between FXR and the LXXLL motif present in the NR box of the steroid receptor coactivator 1 (SRC-1).Human FXR-LBD-GST was incubated with increasing concentrations of the indicated ligands in the presence of biotinylated LXXLL SRC-1 peptide. The AlphaScreen signal increases when the complex receptor-coactivator is formed.
- ChEMBL_48921 (CHEMBL660751) Binding affinity towards 23-nt RNA oligonucleotide from the 5'-untranslated region of the cholestery ester transfer protein (CETP)
- ChEMBL_2469668 Antagonist activity at C-terminal RLucII-fused human ER-alpha expressed in HEK293T cells co-expressing CoA-YFP-fused LXXLL coactivator motif from NCOA2 in presence of E2 by BRET assay
- ChEMBL_304408 (CHEMBL831813) Effective concentration for recruitment of SRC-1 LxxLL-containing peptide to human Farnesoid X receptor
- ChEBML_34991 Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor.
- ChEBML_34995 Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A.
- ChEMBL_35453 (CHEMBL643724) Inhibitory concentration against B-Cell Lymphoma xL (Bcl-xL) Bak-peptide binding region
- ChEMBL_815757 (CHEMBL2025841) Displacement of fluorescein-labeled phosphotyrosine-peptide derived from the interleukin-6 receptor subunit gp130 from the STAT3 after 15 mins by fluorescent polarization assay
- MAO-A and MAO-B Assay The MAO-A and MAO-B is purchased from Active Motif (Cat#31502, Cat#31503). Biochemical Kit is purchased from Promega (MAO-Glo Assay, V1402). The inhibition of selectivity assay was preformed in light of the manufacturer's protocol.
- ChEMBL_2071588 (CHEMBL4727122) Displacement of propidium iodide from PAS region of electric eel AChE by fluorescence assay
- IMAP Assay Cdk9/cyclinT1 is purchased from Millipore, cat #14-685. The final total protein concentration in the assay 4 nM. The 5TAMRA-cdk7tide peptide substrate, 5TAMRA-YSPTSPSYSPTSPSYSTPSPS--COOH, is purchased from Molecular Devices, cat#R7352. The final concentration of peptide substrate is 100 nM. The ATP substrate (Adenosine-5'-triphosphate) is purchased from Roche Diagnostics, cat#1140965. The final concentration of ATP substrate is 6 uM. IMAP (Immobilized Metal Assay for Phosphochemicals) Progressive Binding reagent is purchased from Molecular Devices, cat#R8139. Fluorescence polarization (FP) is used for detection. The 5TAMRA-cdk7tide peptide is phosphorylated by Cdk9/cyclinT1 kinase using the ATP substrate. The Phospho-5TAMRA-cdk7tide peptide substrate is bound to the IMAP Progressive Binding Reagent.
- ChEMBL_461 (CHEMBL615691) The ability of the peptide to inhibit the binding of 50 pM [125I]gastrin releasing peptide to intact S-3T3 cells
- ChEMBL_2112634 (CHEMBL4821484) Displacement of [3H]-ketanserin from Wistar rat frontal region 5-HT2AR incubated for 30 mins
- ChEMBL_851510 (CHEMBL2156749) Displacement of [3H]ABP688 from human mGluR5a transmembrane region expressed in mouse L(tk-) cells
- Fluorescence Polarization Assay Assays effective in monitoring the inhibition of the MLL binding to menin were developed during experiments performed during the development of embodiments of the present invention. A fluorescein-labeled 12-amino acid peptide derived from MLL containing the high affinity menin binding motif was produced (Yokoyama et al., Cell., 2005. 123(2): p. 207-18., herein incorporated by reference in its entirety). Upon binding of the peptide (1.7 kDa) to the much larger menin (-67 kDa), the rotational correlation time of the fluorophore (peptide labeled with fluorescein at N-terminus) changes significantly, resulting in a substantial increase in the measured fluorescence polarization and fluorescence anisotropy (excitation at 500 nm, emission at 525 nm). The fluorescence polarization (FP) assay was utilized to determine the Kd for the binding of menin and the MLL peptide using a serial dilution of menin and 50 nM fluorescein-labeled MLL peptide.
- ChEMBL_2112633 (CHEMBL4821483) Displacement of [3H]-5-OH-DPAT from Wistar rat frontal region 5-HT1AR incubated for 30 mins
- Inhibition Assay NSC-87877 ranked among top 10% (175th) of the compounds with the best GLIDE scores for the docking to the human Shp2 PTP domain in our virtual screening of 2368 3D structures derived from the NCI Diversity Set. Computer docking of NSC-87877 (FIG. 2) suggested that the B-ring sulfonic acid group forms hydrogen bond with the backbone NH group of Arg-465. Arg-465 is a conserved residue in the PTP signature motif (motif 9) VHCSXGXGR[T/S]G located at the base of the PTP catalytic cleft (Andersen et al., 2001). The A-ring sulfonic acid forms hydrogen bonds with the side-chain NH3 group of Lys-280 and the side-chain NH2 group of Asn-281. Lys-280/Asn-281 are non-conserved PTP residues located adjacent to the phosphotyrosine recognition loop (motif 1) (Andersen et al., 2001). The interaction between aromatic rings of the compound and the protein contributes to the binding through hydrophobic stabilization.
- PKA assay IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a protein or peptide substrate.
- PKC assay IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a protein or peptide substrate.
- ChEMBL_770205 (CHEMBL1832995) Displacement of H4Ac4 peptide from first bromodomain of human BRD2 by peptide displacement assay
- ChEMBL_770206 (CHEMBL1832996) Displacement of H4Ac4 peptide from first bromodomain of human BRD4 by peptide displacement assay
- ChEMBL_2057667 (CHEMBL4712668) Displacement of propidium iodide from PAS-region of electric eel AChE assessed as dissociation constant by spectrofluorimetric analysis
- ChEBML_146550 The ability to displace [3H]-naloxone from the Opioid receptor mu isolated from rat brain membrane.
- ChEMBL_36341 (CHEMBL650168) Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats
- ChEMBL_303746 (CHEMBL829789) Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continuous assay
- PKA Inhibition Assay IC50 is the inhibitor concentration, which inhibits 50% of PKA activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a synthetic kemptide peptide substrate.
- Phosphorylase Kinasse Assay IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a protein or peptide substrate.
- ChEMBL_1517687 (CHEMBL3620075) Inhibition of kelch domain of Keap1 (unknown origin) interaction to high affinity ETGE motif from Nrf2 by fluorescence polarization assay
- Tyrosine Kinase Inhibition Assay IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a protein or peptide substrate.
- ChEMBL_303773 (CHEMBL830130) Ability to inhibit the hydrolysis of chromogenic 4-chlorophenylbutyric acid ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continuous assay
- TACE Inhibition Assay The compounds were tested for TACE inhibition using fluorescence resonance energy transfer (FRET) assay. TACE catalyzed cleavage of the substrate peptide liberates the fluoropore from the proximity of the adjacent quenching moiety, and an increase in fluorescence signal results.
- ChEBML_148701 The ability to displace [3H]naloxone from the Opioid receptor mu 1 isolated from rat brain membrane.
- ChEBML_148837 Ability to displace [3H]naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.
- ChEMBL_146550 (CHEMBL754969) The ability to displace [3H]-naloxone from the Opioid receptor mu isolated from rat brain membrane.
- ChEMBL_146552 (CHEMBL754970) The ability to displace [3H]naloxone from the opioid receptor mu isolated from rat brain membrane.
- Fluorescence Polarization Assay In the fluorescence polarization assay, the Bcl-XL protein is incubated with a fluorescein-tagged Bak-BH3 peptide. The Bcl-XL:Bak-BH3 peptide complex is then titrated with the test compounds, and the displacement of the Bak-BH3 peptide is measured as a function of decreasing polarized fluorescence. The fluorescence polarization values were determined using a Tecan GeniosPro plate reader using the excitation/emission wavelengths 485/535 nm. The binding affinities are reported as the inhibitory concentration of the titrant required to displace 50% of the Bak-BH3 peptide (IC50).
- Protease Inhibition Assay The Ki values were obtained from the IC50 values estimated from an inhibitor dose-response curve with the spectroscopic assay and the chromogenic substrate.
- SARS-CoV 3CL Protease Inhibition Assay The effects of compound on enzyme activity were measured by using a fluorogenic peptide cleavage assay. Enhanced fluorescence caused by cleavage of the substrate peptide was monitored. IC50 values were obtained from dose response curves.
- MK-2 IC50 Value Determination The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in reaction buffer containing enzyme and test compound in the presence of the HSP-peptide with [gamma-33P]ATP /ATP. After the reaction was terminated and [gamma-33P]ATP was removed from solution by the addition of AG 1X8 ion exchange resin. An aliquot was removed from the quenched reaction mixture and added to a 96-well plate. Microscint-40 (Packard) was added and the amount of phosphorylated-peptide was determined.
- Src Inhibition Assay IC50 is the inhibitor concentration, which inhibits 50% of pp60c-src activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a synthetic gastrin-based peptide substrate.
- ChEMBL_1721968 (CHEMBL4136968) Agonist activity at His tagged RORgammat LBD (unknown origin) expressed in baculovirus infected sf9 cells assessed as biotinylated-LXXLL peptide coactivator recruitment incubated for overnight by TR-FRET Assay
- ChEMBL_2272602 Displacement of biotinylated Bim peptide from Bcl-2 (unknown origin)
- ChEMBL_2432156 Displacement of tetra-acetylated H4 peptide from ATAD2B (unknown origin)
- ChEMBL_811654 (CHEMBL2014167) Displacement of [125I]peptide YY from human NPY5 receptor
- ChEMBL_148701 (CHEMBL751073) The ability to displace [3H]naloxone from the Opioid receptor mu 1 isolated from rat brain membrane.
- ChEMBL_148837 (CHEMBL753955) Ability to displace [3H]naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.
- ULK1 inhibition assay ULK1 inhibition assay is a screening assay to identify compounds that inhibit kinase activity of ULK1 using the ULKtide peptide. In some embodiments, the method contacting a candidate compound, ULK1 and a recombinant ULKtide peptide (or variant thereof); detecting phosphorylation of the recombinant peptide in the presence and absence of the candidate compound; and identifying a compound that inhibits kinase activity of ULK1 if phosphorylation of the recombinant peptide is decreased in the presence of the candidate compound compared to in the absence of the candidate compound.
- ChEMBL_146553 (CHEMBL754971) The ability to displace [3H]naloxone from the opioid receptor mu isolated from rat brain membrane; >= no displacement
- ChEMBL_2440286 Displacement of BRC4-biotinylated peptide from human RAD51 by competitive ELISA
- ChEMBL_770203 (CHEMBL1832993) Displacement of H3K56Ac from human CREBBP by peptide displacement assay
- LANCE Ultra Assay The LANCE Ultra assay kit was purchased from PerkinElmer. The ULight-MBP peptide, Europium labeled Antibody and LANCE Detection buffer were purchased from PerkinElmer. The APT and dimethylsulfoxide were purchased from Sigma-Aldrich. The 5× Kinase buffer were purchased from Invitrogen. The MAP kinase 1 (Mek1), inactive Erk1 were purchased from Millipore. A Mek1 kinase assay (LANCE, PerkinElmer) was developed for supporting compound profiling and lead optimization. In this assay, un-phosphorylated/inactive Erk1 (Millipore) was used as the substrate for Mek1 (Millipore). Then the phosphorylated Erk1 was able to phosphorylate ULight-MBP peptide (PerkinElmer). The phosphorylated peptide was detected by Europium-anti-phospho-MBP (PerkinElmer). In a reaction, the activity of Mek1 (0.5 nM) was measured in a buffer containing 50 μM ATP, 2 nM inactive Erk1, 2 nM ULight-MBP peptide, and a compound for 90 min at 23° C. After quenching the reaction with xxx, 2 nM Europium-anti-phospho-MBP was added to the reaction mixture and incubated for 60 min, followed by a detection using EnVision Multilabel Plate Reader (PerkinElmer).
- PPIase Assay The promising ligand from the results obtained from ESI-MS assay are subjected to PPIase in vitro assay. The PPIase assay assesses the abil9ity of ligands to inhibit the PPIase activity of CypA.
- ChEMBL_149149 (CHEMBL759240) The ability to displace [3H]naloxone from the Opioid receptor mu 1 isolated from rat brain membrane; >= no displacement
- ChEBML_164495 Inhibitory activity against peptide binding to the RRE RNA was determined
- ChEMBL_154313 (CHEMBL762798) Inhibition of the isolated native E. coli peptide deformylase (PDF)
- ChEBML_212946 Binding affinity to uridine kinase from L1210. was determined from the dixon plot
- ChEBML_212947 Binding affinity to uridine kinase from L1210. was determined from the dixon plot
- ChEBML_217700 The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei
- ChEBML_30523 The compound was tested for the inhibition of alanine racemase from Pseudomonas aeruginosa
- ChEMBL_1884179 (CHEMBL4385761) Inhibition of tau-tau binding (unknown origin) assessed as increase in dissolved paired helical filaments
- ChEMBL_1736592 (CHEMBL4152128) Inhibition of Keap1 Kelch domain (unknown origin) /Nrf2 (unknown origin) interaction using fluorescein-labeled 9-mer peptide containing NRF2 Neh2 domain ETGE motif by fluorescence anisotropy assay
- Biochemical Assay A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen assay can be used to evaluate Btk activity through quantification of its phosphorylated peptide product. The FRET (Fluorescence Resonance Energy Transfer) that occurs between the fluorescein on the peptide product and the terbium on the detection antibody decreases with the addition of inhibitors of Btk that reduce the phosphorylation of the peptide.
- In Vitro Kinase Inhibition Assay IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled ATP to the peptide substrate. Use of radiolabeled ATP allows this transfer to be monitored by scintillation counting.
- Mobility-Shift kinase assay Compounds of the examples provided herein were assessed for their ability to inhibit Syk kinase by utilizing Caliper Life Sciences' proprietary LabChip technology. The off-chip incubation mobility-shift kinase assay uses a microfluidic chip to measure the conversion of a fluorescent peptide substrate to a phosphorylated product. The reaction mixture, from a microtiter plate well, is introduced through a capillary sipper onto the chip, where the nonphosphorylated substrate and phosphorylated product are separated by electrophoresis and detected via laser induced fluorescence. The signature of the fluorescence signal over time reveals the extent of the reaction. The phosphorylated product migrates through the chip faster than the non-phosphorylated substrate, and signals from the two forms of the peptide appear as distinct peaks.
- ChEMBL_149148 (CHEMBL759239) The ability to displace [3H]naloxone from the Opioid receptor mu 1 isolated from rat brain membrane; >= absolutely no % change
- ChEBML_155034 Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in Sf9 cells.
- ChEMBL_744058 (CHEMBL1768208) Displacement of biotin-labeled JIP1 peptide from JNK1 by DELFIA assay
- ChEMBL_807449 (CHEMBL1958571) Displacement of acetylated histone peptide from BRD2-BD1,2 by FRET assay
- ChEMBL_807450 (CHEMBL1958572) Displacement of acetylated histone peptide from BRD3-BD1,2 by FRET assay
- ChEMBL_807451 (CHEMBL1958573) Displacement of acetylated histone peptide from BRD4-BD1,2 by FRET assay
- Pin1 Inhibition Assay Assay was carried out measuring the MCA fluorescence using Suc-Ala-Glu-Pro-Phe-MCA as substrate purchased from Japan Peptide Institute Co.
- ChEBML_104575 Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate.
- ChEBML_106803 Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate.
- Fluorescent Peptide Displacement Assay Test compound was incubated for 15 minutes with 30 nM peptide fluorescein-FMDYWEGLN (Fl 9mer) and 120 nM (glycine-HDM2 17-125) in assay buffer. The polarization of the fluorescein label was measured by excitation at 485 nm and emission at 530 nm. Percent inhibition was calculated from the decrease in the fluorescence polarization of the labeled peptide upon displacement from HDM2 by the compounds, using buffer with Fl 9mer but without HDM2 as background. Data were analyzed by non-linear least squares fitting to a hyperbolic binding function. The measured IC50s of the p53 wild-type (QETFSDLWKLLP) and optimized (MPRFMDYWEGLN) peptides are 2000 and 250 nM, respectively. Under these conditions, the IC50 is 2.5 fold higher than the dissociation binding constant (Kd).
- In Vitro Kinase Assay The IC50 for the different compounds was determined with Invitrogen Z'-LYTE kinase assay kit Ser/Thr 1 peptide PV3174 by following the manufacturer's instructions. To determine the IC50 of receptor tyrosine kinase, an assay based on the ELISA principle with kinases from Biomol (N-terminally fused to GST), POD-coupled antibody from Calbiochem (PY20)) and BM chem chemiluminescence ELISA substrate from Roche was performed.
- ChEMBL_164495 (CHEMBL771472) Inhibitory activity against peptide binding to the RRE RNA was determined
- Protease Inhibition Assay The inhibition assays were performed in microtiter plate wells by mixing enzyme and fluorescent peptide substrate in the presence of inhibitor compounds. The increase of fluorescence was detected using fluorimeter-luminometer. Ki values were calculated from the IC50 values estimated from a dose-response curve with the fluorescent assay using the equation Ki = (IC50 - [E]/2)/ (1 +[S]/Km), where [E] and [S] are the protease and substrate concentrations.
- ChEBML_139952 Displacement of [3H]-Pz (pirenzepine) from the muscarinic receptor M1 of the rat hippocampus
- ChEMBL_217700 (CHEMBL821239) The compound was evaluated for the inhibition of dTMP synthetase from Lactobacillus casei
- ChEMBL_28150 (CHEMBL644105) Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes
- In Vitro Assay The IC50 of the compounds for inhibiting the interaction of a fluorescent NRF2 peptide and the Kelch domain of KEAP1 was determined using fluorescence anisotropy.
- Kinase Assay A coupled-enzyme assay was used to quantify the ADP generated in the kinase reaction with S6 peptide (RRRLSSLRA) as the phosphoacceptorsubstrate.
- EGFR IC50 Determination The kinase reaction for EGFR consisted of BSA-supplemented kinase buffer, kinase, peptide, and ATP. For IC50 determinations, 10 different concentrations (ranging from 0.1 nM to 10 uM) of inhibitor were used in duplicate. Each experiment was repeated at least twice. Following the kinase reaction, Development Solution (included with the kit) was added to cleave the remaining unphosphorylated peptide. Following a 1 h incubation time with Development Solution, Stop Solution (included with the kit) was added to the reaction mixture. Fluorescence was then measured with a Spectramax M5 plate reader from Molecular Devices or a Safire2 from Tecan. Upon excitation of coumarin at 400 nm, fluorescence emission was measured at 445 nm (coumarin) and 520 nm (fluorescein). The ratio of coumarin to fluorescein emission was used to calculate the percentage of phosphorylation of the peptide by the kinase.
- PKA In Vitro Enzyme Assay The recombinant alpha catalytic subunit of bovine PKA was assayed with a peptide substrate and test compound in the presence of 40 uM ATP/ [gamma-33P]ATP in 96-well plates. IC50 is the concentration of inhibitor that inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] labeled ATP to substrate peptide. IC50 values were determined from an eight-point concentration range run in duplicate and calculated in GraphPad Prism 4.00.
- PKB In Vitro Enzyme Assay The purified PKB beta enzyme was assayed with a peptide substrate and test compound in the presence of 30 uM ATP/ [gamma-33P]ATP in 96-well plates. IC50 is the concentration of inhibitor that inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] labeled ATP to substrate peptide. IC50 values were determined from an eight-point concentration range run in duplicate and calculated in GraphPad Prism 4.00.
- ChEMBL_155034 (CHEMBL765476) Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in Sf9 cells.
- ChEMBL_155036 (CHEMBL765478) Inhibition of human Phosphodiesterase 4A isoform using construct representing the common region of spliced variants expressed as GST-fusion proteins in Sf9 cells.
- ROCK 1 Kinase Activity Assay The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptide was quantified by Scintillation Proximity Assay (SPA - Amersham Pharmacia).
- ChEBML_208276 Evaluated for the inhibition of trehalase from porcine kidneys
- ChEMBL_1804490 (CHEMBL4303093) Displacement of [3H]-DTG from the Sigma2 receptor
- ChEMBL_1804491 (CHEMBL4303094) Displacement of [3H]-pentazocin from the Sigma1 receptor
- ChEMBL_438659 (CHEMBL888990) Displacement of [125I] ITAC from the CXCR3 receptor
- ChEMBL_48915 (CHEMBL660745) Inhibition of the Pancreatic Cholesterol Esterase from porcine
- FRET-Based Assay The protease activity of the full-length NS3-NS4a and the protease domain were measured using a FRET-based assay using a peptide substrate derived from the NS4A/B cleavage site and labeled at one end with a quencher (QXL520) and at the other with a fluorophore (5-FAMsp; Anaspec).
- ChEMBL_1287023 (CHEMBL3111334) Displacement of H4Ac4 peptide from CECR2 bromodomain (unknown origin) by AlphaScreen assay
- ChEMBL_217807 (CHEMBL823263) Displacement of a non-peptide radioligand from human recombinant alphaV-beta3 integrin
- ChEMBL_439337 (CHEMBL888453) Displacement of FAM-Bim peptide from human Bcl2 by fluorescence polarization assay
- ChEMBL_439339 (CHEMBL888455) Displacement of FAM-Bim peptide from human Mcl1 by fluorescence polarization assay
- ChEMBL_537290 (CHEMBL985386) Displacement of fluorescein labeled Bax peptide from Bcl2 by fluorescence polarization assay
- ChEMBL_639438 (CHEMBL1168411) Displacement of fluorescently-tagged BH3 peptide from Bcl-XL by fluorimetric assay
- ChEMBL_807448 (CHEMBL1958570) Displacement of acetylated histone peptide from CREBBP by luminescence proximity homogenous assay
- ChEMBL_811269 (CHEMBL2015221) Displacement of fluorescein-labeled p53 peptide from HDM2 by fluorescence polarization assay
- ChEMBL_971499 (CHEMBL2406561) Displacement of [125I]MCH peptide from human MCHR1 expressed in CHOK1 cells
- Src IC50 Determination IC50 values were determined with the Z lyte assay system (Invitrogen). The reactions were performed in 384-well small volume plates from Greiner (#784076). The kinase reaction for cSrc consisted of kinase buffer, kinase, peptide and ATP. For each IC50 determination, 16 different concentrations of inhibitor (range from 250,000 to 10 nM) were used in triplicate and at least three independent experiments were performed. Before starting the kinase reaction, enzyme and inhibitor were incubated for 40 min. Following the kinase reaction, Development Solution (included with the kit) was added to cleave the remaining unphosphorylated peptide. Following a 1 h incubation time with Development Solution, Stop Solution (included with the kit) was added to the reaction mixture. Fluorescence was then measured with a Spectramax M5 plate reader from Molecular Devices or a Safire2 from Tecan. Upon excitation of coumarin at 400 nm, fluorescence emission was measured at 445 nm (coumarin) and 520 nm (fluorescein). The ratio of coumarin to fluorescein emission was used to calculate the percentage of phosphorylation of the peptide by the kinase.
- Enzyme Assay The Aurora assays described here are performed on two Caliper Life Sciences systems: the LC3000 and the Desktop Profiler. These provide data on enzyme activity via measurement of the relative amounts of phosphorylated or unphosphorylated fluorescently labelled substrate peptide at the end of an enzymatic reaction. These different states of peptide are resolved by applying a potential difference across the sample. The presence of the charged phosphate group on the product (as opposed to the substrate) causes a different peptide mobility between the two peptides. This is visualized by excitation of the fluorescent label on the substrate and product peptides and represented as peaks within the analysis software.
- ChEBML_3868 The compound was tested for the inhibition of Arachidonate 5-lipoxygenase purified from porcine leukocytes.
- ChEMBL_41588 (CHEMBL654882) Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum
- Enzyme Inhbition Assay Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore whose emission is quenched in the intact peptide.
- Competitive Binding Assay Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference in polarization values of fluorescein-conjugated peptide reflects the bound and unbound state of the probe. IC50s were calculated based on the binding of the fluorescein-conjugated peptide to the protein with compound addition relative to vehicle-alone control samples.
- Inhibitory Effect on HER2-Phosphorylating Activity (In Vitro) For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, ProfilerPro Peptide 22 from PerkinElmer Inc. was used as a substrate on the basis of the report (PLoS One, 6 (7), e21487, 2011) on HER2 kinase reaction using, as a substrate, a peptide having the same sequence (5-FAM-EEPLYWSFPAKKK-CONH2) as that of ProfilerPro Peptide 22. The purified recombinant human HER2 protein used in the test was purchased from Carna Biosciences, Inc.For the inhibitory activity measurement of each compound, the synthesis example compound was first serially diluted with dimethyl sulfoxide (DMSO). Next, the HER2 protein, the substrate peptide (final concentration: 0.5 μM), manganese chloride (final concentration: 10 mM), ATP (final concentration: 6 μM), and the solution of the synthesis example compound in DMSO (final concentration of DMSO: 5%) were added into a buffer solution for kinase reaction (15 mM Tris (pH 7.5), 2 mM dithiothreitol, and 0.01% Tween 20), and the mixture was incubated at 25° C. for 40 minutes for kinase reaction. The reaction was terminated by adding EDTA (final concentration: 30 mM) thereto. Finally, the unphosphorylated substrate peptide (S) and the phosphorylated peptide (P) were separated and detected by microcapillary electrophoresis using LabChip EZ Reader II (PerkinElmer Inc.).
- Enzymatic Assay Compounds were tested for their inhibition of the addition of L-Ala, D-Glu, L-Lys or D-Ala-D-Ala to nucleotide precursors catalyzed by MurC from Escherichia coli, MurD from E. coli, MurE from Staphylococcus aureus, and MurF from E coli. The detection of the orthophosphate generated during the reaction was based on the colorimetric Malachite green method.
- GW0385 Assay The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The k1 is calculated from spectrofluorometric data in the presence of saquinavir. The k-1 is calculated from the time course for displacement of [3H]GW0385 from E[3H]GW0385 by nonlabeled GW0385.
- HER2-Phosphorylating Activity For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, ProfilerPro Peptide 22 from PerkinElmer Inc. was used as a substrate on the basis of the report (PLoS One, 6 (7), e21487, 2011) on HER2 kinase reaction using, as a substrate, a peptide having the same sequence (5-FAM-EEPLYWSFPAKKK-CONH2) as that of ProfilerPro Peptide 22. The purified recombinant human HER2 protein used in the test was purchased from Carna Biosciences, Inc. Also, staurosporine (Eur. J. Biochem., 234, p. 317-322, 1995; and Nat. Biotechnol., 26 (1), p. 127-132, 2008), which is a multikinase inhibitor having Her2 inhibitory activity, was purchased from Enzo Life Sciences, Inc. (item No.: ALX-380-014) and used as a positive control in this test.For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dimethyl sulfoxide (DMSO). Next, the HER2 protein, the substrate peptide (final concentration: 0.5 uM), manganese chloride (final concentration: 10 mM), ATP (final concentration: 6 uM), and the solution of the compound of the present invention in DMSO (final concentration of DMSO: 5%) were added into a buffer solution for kinase reaction (15 mM Tris (pH 7.5), 2 mM dithiothreitol, and 0.01% Tween 20), and the mixture was incubated at 25° C. for 40 minutes for kinase reaction. The reaction was terminated by adding EDTA (final concentration: 30 mM) thereto. Finally, the unphosphorylated substrate peptide (S) and the phosphorylated peptide (P) were separated and detected by microcapillary electrophoresis using LabChip EZ Reader II (PerkinElmer Inc.). The amount of phosphorylation reaction was determined from the respective peak heights of S and P. The compound concentration which can suppress the phosphorylation reaction by 50% was defined as an IC50 value (nM).
- Measurement of Biochemical Activity of Compounds In order to assess the activity of chemical compounds against the relevant kinase of interest, the Caliper LifeSciences electrophoretic mobility shift technology platform is used. Fluorescently labeled substrate peptide is incubated in the presence of kinase and ATP so that a reflective proportion of the peptide is phosphorylated. At the end of the reaction, the mix of phosphorylated (product) and non-phosphorylated (substrate) peptides are passed through the microfluidic system of the Caliper EZ Reader 2, under an applied potential difference. The presence of the phosphate group on the product peptide provides a difference in mass and charge between those of the substrate peptide, resulting in a separation of the substrate and product pools in the sample. As the pools pass a LEDS within the instrument, these pools are detected and resolved as separate peaks. The ratio between these peaks therefore reflects the activity of the chemical matter at that concentration in that well, under those conditions.
- Measurement of Biochemical Activity of Compounds In order to assess the activity of chemical compounds against the relevant kinase of interest, the Caliper LifeSciences electrophoretic mobility shift technology platform was used. Fluorescently labeled substrate peptide was incubated in the presence of kinase and ATP so that a reflective proportion of the peptide was phosphorylated. At the end of the reaction, the mix of phosphorylated (product) and non-phosphorylated (substrate) peptides were passed through the microfluidic system of the Caliper EZ Reader 2, under an applied potential difference. The presence of the phosphate group on the product peptide provides a difference in mass and charge between those of the substrate peptide, resulting in a separation of the substrate and product pools in the sample. As the pools pass a LEDS within the instrument, these pools are detected and resolved as separate peaks. The ratio between these peaks therefore reflects the activity of the chemical matter at that concentration in that well, under those conditions.
- ChEBML_35004 Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriuretic peptide receptor C).
- ChEMBL_155035 (CHEMBL765477) Inhibitory concentration against human PDE4A isoform using a construct representing the common region of spliced variants expressed as GST-fusion protein in Sf9 cells
- Fluorescence Polarization Cell-Free Homogeneous Dose Retest to Confirm Inhibitors of the LANA Histone H2A/H2B Interaction Primary Collaborators: Kenneth Kaye,Brigham & Womens,Boston MA,kkaye@rics.bwh.harvard.edu,617-525-4256 Chantal Beauchemin,Brigham & Womens,Boston MA,cbeauchemin@rics.bwh.harvard.edu,617-525-4256 Keywords: KSHV, LANA, fluorescence polarization, nucleosomes, viral persistence Assay Overview: Screening for inhibitors that reduce or prevent the binding of LANA to the acidic region of the H2A/H2B histone dimer interface. Synthetic LANA peptide is labeled with an amino terminal FITC fluorophore linked via a beta alanine residue. The peptide contains the first 23 amino acids of the LANA protein that is essential for binding to the H2A/H2B interface. Nucleosomes were purifed from chicken Erythrocytes as a source of intact H2A/H2B dimers. Fluorescence is measured in the S and P planes with a Perkin Elmer Viewlux after 1 hour incubation. Expected Outcome: Compounds will be identified that inhibit the interaction of LANA 1-23 peptide with purified nucleosomes (containing H2A/H2B histone
- ChEMBL_2266265 Inverse agonist activity at 6His-tagged RORA LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of biotinylated SRC1-2 derived CPSSHSSLTERHKILHRLLQEGSPS-NH2 containing LXXLL coactivator peptide incubated for 3 hrs by TR-FRET assay
- ChEMBL_2266266 Inverse agonist activity at 6His-tagged RORB LBD (unknown origin) expressed in Escherichia coli assessed as inhibition of biotinylated SRC1-2 derived CPSSHSSLTERHKILHRLLQEGSPS-NH2 containing LXXLL coactivator peptide incubated for 3 hrs by TR-FRET assay
- Kinase Inhibition Assay A coupled-enzyme assay was used to quantify the ADP generated in the kinase reaction with S6 peptide (RRRLSSLRA) as the phosphoacceptor substrate.
- Enzymatic Activity Assay The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed to measure the ability of the isolated enzyme to catalyze the transfer of phosphate from ATP to serine/threonine residues in a fluorescein-labeled substrate peptide, using recombinant human SGK-1 enzyme produced in a baculovirus system (Biomol, Hamburg, Germany, Cat. No. 4-331). The synthesized fluorescent labeled peptide substrate contained (5(6)-Carboxyfluorescein)-RPRAATF-NH2. The phosphorylated substrate peptide and non-phosphorylated substrate peptide were separated with caliper life science's lab-chip technology based on a micro fluidics method. All fluid flow was established on the chip by applying a vacuum of a few psi to the waste well transporting fluid from various sources through interconnecting channels. Because the phosphoryl group is doubly negatively charged, under the pressure-driven hydrodynamic flow.
- Fluorescence Assay The promising ligand from the results obtained from ESI-MS assay are subjected to fluorescence in vitro assay. The fluorescence assay takes advantage of the presence of a single tryptophan residue (Trp121) located close to the active site of CypA.
- Inhibition Assay Crithidia fasciculata trypanothione reductase (cfTR) was purifed from E.coli using the expression system. The activity of the cfTR was observed from the rate of oxidation of the co-substrate NADPH to NADP+ as measured by a decrease in absorbance at 340nm.
- ChEBML_157702 Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLC
- ChEMBL_28151 (CHEMBL644106) Compound was evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes,
- ChEMBL_65474 (CHEMBL677304) Binding ability determined by the displacement of [125I]ET1 from the human endothelin A receptor
- Pim Kinase Assay The primary screen and evaluation of the compounds was conducted using an ATP-depletion assay. Recombinant human Pim kinase was incubated with S6 kinase/Rsk-2 peptide 2 in the presence 100 uM of compounds from the screening library (DIVERSet collection of the ChemBridge Corporation). The Kinase-Glo luciferase kit (Promega) was used to measure residual ATP levels after the kinase reaction.
- Determination of kinetic parameters and the Ki of effective compounds using the FRET peptide Kinetic parameters were obtained using various concentrations of FRET peptide in the fluorescent assay. The maximal velocity (Vmax) and Michaelis Menten constant (Km) were calculated from the Eadie Hofstee plot. If the type of inhibitionwas found to be competitive using a Lineweaver Burk double reciprocal plot, then the inhibitory constant (Ki) for 3CLpro was estimated using the equation:Ki = IC50/(1+[substrate]/Km).Plots were performed, and kinetic parameters were calculated, using Prism software (Graphpad Software, San Diego, CA).
- ChEMBL_1287019 (CHEMBL3111330) Displacement of H3K14(Ac) peptide from TIF1alpha bromodomain (unknown origin) by AlphaScreen assay
- ChEMBL_1287021 (CHEMBL3111332) Displacement of H3K14(Ac) peptide from BAZ2B bromodomain (unknown origin) by AlphaScreen assay
- ChEMBL_1287022 (CHEMBL3111333) Displacement of H3K56(Ac) peptide from CREBBP bromodomain (unknown origin) by AlphaScreen assay
- ChEMBL_1287025 (CHEMBL3111336) Displacement of H4Ac4 peptide from BRD4 bromodomain-1 (unknown origin) by AlphaScreen assay
- ChEMBL_1350281 (CHEMBL3267619) Displacement of fluorescent P4 peptide from MDM2 (unknown origin) by fluorescence polarization assay
- ChEMBL_2281552 Displacement of fluorescent-labeled BID-BH3 peptide from His-tagged BFL-1 (unknown origin)
- ChEMBL_434380 (CHEMBL919858) Displacement of [15I]IMPY from Abeta peptide plaques in Alzheimer's disease patient brain
- ChEMBL_439338 (CHEMBL888454) Displacement of FAM-Bim peptide from human Bcl-XL by fluorescence polarization assay
- ChEMBL_537289 (CHEMBL985385) Displacement of fluorescein labeled BAD peptide from Bcl-XL by fluorescence polarization assay
- ChEMBL_684920 (CHEMBL1286335) Displacement of [125I]peptide YY from NPY2 receptor in human KAN-TS cells
- ChEMBL_807446 (CHEMBL1958568) Displacement of acetylated histone peptide from BRD4-BD1 by luminescence proximity homogenous assay
- ChEMBL_807447 (CHEMBL1958569) Displacement of acetylated histone peptide from BRD4-BD2 by luminescence proximity homogenous assay
- Activity Assay The primary assay for compound inhibitory activity was the ATP depletion assay using the PDKtide substrate purchase from Millipore Corporation.
- ChEMBL_63064 (CHEMBL673631) Displacement of the radioligand [3H]spiperone from D2 receptor
- Literature assay The assays are from references cited in this article.
- Spectrophotometic Assay The IC50 values were obtained from a spectrophotometric assay.
- ChEMBL_501393 (CHEMBL1010576) Inhibition of human recombinant Arg235 region of BACE1 by FRET assay
- ChEMBL_143828 (CHEMBL748713) Compound was tested for the displacement of [125I]-PYY (SK-N-MC) from Neuropeptide Y receptor type 1 in the membranes prepared from cells
- ChEBML_35003 Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes (Atrionatriuretic peptide receptor B)
- ChEMBL_35004 (CHEMBL649027) Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriuretic peptide receptor C).
- ChEMBL_1776554 (CHEMBL4233546) Binding affinity to human GST-tagged 14-3-3zeta expressed in Escherichia coli assessed as Kd for 14-3-3zeta binding to H+-ATPase peptide motif by isothermal titration calorimetric method
- Inhibition Assay Purified recombinant human FGFR3 protein was purchased from Carna Biosciences, Inc. When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR3 kinase activity, a biotinylated peptide (biotin-EEPLYWSFPAKKK) was synthesized for use as a substrate by utilizing the amino acid sequence of FL-Peptide 22 (Caliper Life Sciences, Inc.) with biotin. The purified recombinant human FGFR3 protein used in the test was purchased from Carna Biosciences, Inc. In the measurement of the inhibitory effect of the compounds, first, a test compound was gradually diluted with dimethylsulfoxide (DMSO) to a concentration 20 times higher than the final concentration. Next, the purified human FGFR3 protein, substrate peptide (final concentration: 250 nM), magnesium chloride (final concentration: 5 mM), ATP (final concentration: 190 .mu.M), and the test compound DMSO solution (final concentration of DMSO: 5%) were added to a reaction buffer.
- Inhibition Assay Purified recombinant human FGFR4 protein was purchased from Carna Biosciences, Inc. When setting conditions for the measurement of the inhibitory effect of the compounds on FGFR4 kinase activity, a biotinylated peptide (biotin-EEPLYWSFPAKKK) was synthesized for use as a substrate by utilizing the amino acid sequence of FL-Peptide 22 (Caliper Life Sciences, Inc.) with biotin. The purified recombinant human FGFR4 protein used in the test was purchased from Carna Biosciences, Inc. In the measurement of the inhibitory effect of the compounds, first, a test compound was gradually diluted with dimethylsulfoxide (DMSO) to a concentration 20 times higher than the final concentration. Next, the purified human FGFR4 protein, substrate peptide (final concentration: 250 nM), magnesium chloride (final concentration: 5 mM), ATP (final concentration: 190 .mu.M), and the test compound DMSO solution (final concentration of DMSO: 5%) were added to a reaction buffer.
- Inhibition of Cellular Release of Amyloid Peptide 1-40 Chinese hamster ovary cells are transfected with the human gene for amyloid precursor protein. The cells are plated at a density of 8000 cells/well into 96-well microtiter plates and cultivated for 24 hours in DMEM cell culture medium containing 10% FCS. The test compound is added to the cells at various concentrations, and the cells are cultivated for 24 hours in the presence of the test compound. The supernatants are collected, and the concentration of amyloid peptide 1-40 is determined using state of the art immunoassay techniques, for example sandwich ELISA, homogenous time-resolved fluorescence (HTRF) immunoassay, or electro-chemiluminescence immunoassay. The potency of the compound is calculated from the percentage of inhibition of amyloid peptide release as a function of the test compound concentration.
- ChEMBL_38359 (CHEMBL651291) In vitro inhibitory activity against Bak-Bh3 peptide binding to the Bcl-2
- Kinase Inhibition Assay For kinase inhibition assays, the final concentrations of the enzymes were 15, 14.5, 14.5, and 8.8 nM for CLK1, CLK2, CLK3, and Dyrk1A (Invitrogen, catalog no. PV3785), respectively. The compound concentrations ranged from 15503 to 121 nM and from 1550 to 12 nM. The RS domainderived peptide (GRSRSRSRSR) (Anaspec, catalog no. 61722) for CLK1 and CLK3, Dyrktide (RRRFRPASPLRGPPK) (Anaspec, catalog no. 62698) for Dyrk1A, and S6K peptide for CLK2 were used as substrates at a concentration of 5 μM.
- Surface Plasmon Resonance (SPR) Assay Recombinant full-length hnRNPA1 (aa 1-320) and truncated versions of hnRNPA1, including the N-terminal RNA binding domain (aa 1-196), the middle region (aa 182-268), and the C-terminal region (aa 268-320), were individually covalently coupled to a dextran matrix (CM5 chip) using a Biacore T200 system (GE Healthcare), following the manufacturer's protocols. Immobilized proteins were activated with N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide to form a carbodiamide linkage between carboxyl moieties on the dextran and primary amide groups on the protein. hnRNPA1 variants were dissolved in 10 mM acetate buffer (full-length, pH 4; N-terminal RNA binding domain and the C-terminal region, pH 4.5; middle region, pH 5) to a concentration of 20 μg/ml and flowed over the activated surface for 5 min with PBS. Unreacted groups were blocked with ethanolamine. PBS containing 0.05% (v/v) P20 (surfactant) and 5% DMSO was used for priming and conditioning the surface of the chips. Quercetin was serially diluted (from 200 to 3 μM) in PBS buffer and maintained in DMSO (5% (v/v)) (final concentrations from 10 to 0.15 μM). The baseline response was determined for 120 s (30 μl/min) before perfusing the immobilized proteins with the compounds to allow association to occur. Dissociation was then monitored over a period of an additional 120 s. The immobilized proteins were regenerated with 50 mM NaOH.
- ICMT Biochemical Assay An ICMT enzymatic assay was developed using Promega's Methyltransferase-GloTMreagents. In the assay, ICMT catalyzes the methylation of the N-Acetyl-S-farnesyl-L-cysteine by transferring a methyl group from SAM to the farnesylated cysteine and further converts the SAM to SAH. The methyltransferase activity is measured based on the amount of SAH produced from the reaction through the use of coupling enzymes that convert the SAH to ATP. The MTase-Glo detection solution then catalyzes the formation of light from ATP.For the IC50 determination, the compounds were incubated for 30 minutes with 0.04 ug/well ICMT membrane extract from Sf-9 cells. A final concentration of 2.0 μM and 20 μM of SAM and peptide were added and further incubated for 90 minutes at room temperature before adding the MTase Glo and detection reagent. Reaction signals were detected using microplate readers on luminescent mode (Satire Tecan). The IC50 was determined by nonlinear regression, using GraphPad Prism version, 5.03.
- ChEBML_58717 Compound was tested for the displacement of [3H]spiperone from Dopamine receptor D2 from rat striatal membranes.
- Enzyme Inhibition Assay Stromelysin inhibitory activity is based on the hydrolysis of substance P by recombinant human stromelysin to generate a fragment, substance P 7-11, which can be quantitated by HPLC. The IC50 is calculated from control reaction without the inhibitor. The mean IC50 was obtained from two independent measurements, and the standard deviation of the mean was less than 10%. The mean Ki values reported have been derived from the mean IC50.
- ChEMBL_4198 (CHEMBL620000) The compound was tested for the in vitro inhibition of 5-lipoxygenase from the 20000 g supernatant of RBI-1 cells
- ChEBML_60687 Displacement of the radioligand [3H]spiperone from the cloned human dopamine receptor D4 expressed in CHO cells
- ChEMBL_201273 (CHEMBL804962) Binding affinity against the sigma receptor from guinea pig brain using the radioligand [3H]SKF-10047
- ChEMBL_32319 (CHEMBL643512) The compound was evaluated for the binding affinity towards alpha-2 adrenergic receptor from rat cortex
- ChEMBL_70660 (CHEMBL678650) The compound was tested for the inhibition of Gamma-amino-N-butyrate transaminase from pig brain
- Sigma 1 Assay The test was performed with the radioligand [3H]-(+)-pentazocine (42.5 Ci/mmol) from Perkin Elmer.
- ChEMBL_35003 (CHEMBL649026) Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes (Atrionatriuretic peptide receptor B)
- Kinase Inhibition Assay IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a protein or peptide substrate. Use of 32P-labeled ATP allows this transfer to be monitored by scintillation counting or by autoradiography of the substrate
- Kinase Inhibition Assay IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a protein or peptide substrate. Use of 32P-labeled ATP allows this transfer to be monitored by scintillation counting or by autoradiography of the substrate
- Kinase Inhibition Assay IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labeled ATP to a protein or peptide substrate. Use of 32P-labeled ATP allows this transfer to be monitored by scintillation counting or by autoradiography of the substrate.
- Kinase Inhibition Assay IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] labeled ATP to a protein or peptide substrate. Use of 33P-labeled ATP allows this transfer to be monitored by scintillation counting or by autoradiography of the substrate.
- Biochemical Assay Biochemical Assay: The biochemical assay is in a AlphaScreen format. The kinase reaction is based on the IRAK-4 phosphorylation of a biotin labeled peptide. The phosphopeptide is incubated with anti-phosphothreonine antibody as well as streptavidin- and protein A-coated beads. Binding of the protein-A coated beads to the antibody and the streptavidin beads to the peptide, leads to an energy transfer from one bead to the other, ultimately producing a luminescent/fluorescent signal.Generally, the kinase reaction is carried out at 1 nM IRAK4, 1.6 μM peptide, 1 mM ATP in reaction buffer 50 mM Hepes, 60 mM NaCl, 5 mM MgCl2, 0.25 mM MnCl2, 2 mM DTT, 0.01% BSA, 0.01% Tween-20) for 3.5 h at RT.
- Biochemical Assay The biochemical assay is in a AlphaScreen format. The kinase reaction is based on the IRAK-4 phosphorylation of a biotin labeled peptide. The phosphopeptide is incubated with anti-phosphothreonine antibody as well as streptavidin- and protein A-coated beads. Binding of the protein-A coated beads to the antibody and the streptavidin beads to the peptide, leads to an energy transfer from one bead to the other, ultimately producing a luminescent/fluorescent signal.Generally, the kinase reaction is carried out at 1 nM IRAK4, 1.6 μM peptide, 1 mM ATP in reaction buffer 50 mM Hepes, 60 mM NaCl, 5 mM MgCl2, 0.25 mM MnCl2, 2 mM DTT, 0.01% BSA, 0.01% Tween-20) for 3.5 h at RT.
- Biochemical Assay The biochemical assay is in a AlphaScreen format. The kinase reaction is based on the IRAK-4 phosphorylation of a biotin labeled peptide. The phosphopeptide is incubated with anti-phosphothreonine antibody as well as streptavidin- and protein A-coated beads. Binding of the protein-A coated beads to the antibody and the streptavidin beads to the peptide, leads to an energy transfer from one bead to the other, ultimately producing a luminescent/fluorescent signal.Generally, the kinase reaction is carried out at 1 nM IRAK4, 1.6 μM peptide, 10 μM ATP in reaction buffer 50 mM Hepes, 60 mM NaCl, 5 mM MgCl2, 0.25 mM MnCl2, 2 mM DTT, 0.01% BSA, 0.01% Tween-20) for 3.5 h at RT.
- Scintillation Proximity Assay (SPA) The compound inhibitory activity was determined by incubation with purified PLK1 and biotinylated peptide substrate in the presence ATP/[gamma-33P]ATP. After incubation, the phosphorylated substrate was separated from nonincorporated radioactive ATP using SPA beads. Incorporated radioactivity was measured using Packard TopCount.
- ChEMBL_1287020 (CHEMBL3111331) Displacement of H3K14(Ac) peptide from PB1-5 bromodomain (unknown origin) by AlphaScreen assay
- ChEMBL_1472592 (CHEMBL3417957) Displacement of fluorescent labeled NOXA peptide from recombinant human MCL1 by fluorescence polarization assay
- ChEMBL_1748314 (CHEMBL4182824) Displacement of Bax-derived peptide from Bcl-2 (unknown origin) by fluorescence polarization assay
- ChEMBL_1748315 (CHEMBL4182825) Displacement of Bad-derived peptide from Bcl-XL (unknown origin) by fluorescence polarization assay
- ChEMBL_1813645 (CHEMBL4313219) Displacement of biotinylated H4 peptide from ATAD2B bromodomain (unknown origin) by TR-FRET assay
- ChEMBL_2112707 (CHEMBL4821557) Displacement of Bak BH3 domain peptide from human Bax by competitive fluorescence polarization assay
- ChEMBL_302256 (CHEMBL829490) Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization
- ChEMBL_304472 (CHEMBL832688) Displacement of PRP-1 peptide from human Nck kinase SH3 domain by fluorescence polarization
- ChEMBL_304473 (CHEMBL832689) Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization
- ChEMBL_304474 (CHEMBL832690) Displacement of PRP-2 peptide from human Hck kinase SH3 domain by fluorescence polarization
- ChEMBL_429498 (CHEMBL918621) Displacement of FAM-Bid BH3 peptide from human Mcl1 by FP-based binding assay
- ChEMBL_429499 (CHEMBL918622) Displacement of biotinylated Bim BH3 peptide from recombinant His-tagged Mcl1 by ELISA assay
- ChEMBL_429500 (CHEMBL918623) Displacement of biotinylated Bim BH3 peptide from recombinant His-tagged Bcl2 by ELISA assay
- ChEMBL_441637 (CHEMBL891868) Displacement of Flu-Bak peptide from recombinant antiapoptotic Bcl2 protein by fluorescence polarization assay
- ChEMBL_453427 (CHEMBL885426) Displacement of [125]peptide YY from human recombinant Y1 receptor expressed in CHO cells
- ChEMBL_500250 (CHEMBL969585) Displacement of fluorescently-tagged BH3 peptide from Bcl-2 by chemiluminescent nitrogen detection method
- ChEMBL_500251 (CHEMBL969586) Displacement of fluorescently-tagged BH3 peptide from Bcl-XL by chemiluminescent nitrogen detection method
- ChEMBL_593415 (CHEMBL1040543) Displacement of biotin-Ahx-PSpYVNVQN peptide from GST fused Grb2 SH2 domain by ELISA
- ChEMBL_610766 (CHEMBL1064401) Displacement of fluorescein-labeled Bak-BH3 peptide from human Mcl1 by fluorescence polarization assay
- ChEMBL_613509 (CHEMBL1072275) Displacement of FAM-Bid peptide from human recombinant Bcl-xL by fluorescence polarization assay
- ChEMBL_684919 (CHEMBL1286334) Displacement of [125I]peptide YY from NPY1 receptor in human SK-N-MC cells
- ChEMBL_306303 (CHEMBL828595) Inhibitory concentration against hepatitis C virus NS4A binding region of NS3 protease
- Biochemical Assay In brief, this Rpn11 bioassay employs a fluorescent polarization readout based on the ability of the 26S proteasome to cleave the protein substrate including four tandem ubiquitin proteins fused to a peptide having a unique cysteine labeled with a fluorophore. Cleavage of this substrate by Rpn11 at the junction between the fourth ubiquitin and the peptide, releases the low molecular weight fluorescent peptide. Accordingly, inhibition of fluorescence correlates with inhibition of Rpn11. Inhibition is reported as the half maximal inhibitory concentration (IC50) for the candidate compound.The catalytic JAB1/MPN/Mov34 metalloenzyme (JAMM) motif of Rpn11 is found in 7 different human proteins including the Csn5 subunit of the COP9 signalosome, AMSH, AMSH-LP, the BRCC36 subunit of BRISC, MPND, and MYSM1. All of these enzymes cleave the isopeptide linkage that joins ubiquitin (or the ubiquitin-like protein Nedd8 in the case of Csn5) to a second molecule of ubiquitin or to a substrate. The conserved JAMM domain has the consensus sequence EXnHS/THX7SXXD, in which the histidine (His) and aspartic acid (Asp) residues bind the Zn2+ ion and the fourth coordination site is occupied by a water molecule that is engaged in hydrogen bonding with a conserved glutamic acid (Glu). The Zn2+ acts as a Lewis acid and increases the nucleophilic character of the bound water enough to allow hydrolytic cleavage of the isopeptide bond.
- ChEBML_101760 Inhibition of the Collagenase enzyme was determined from purified NSO cells.
- ChEBML_102089 Inhibition of the Stromelysin enzyme was determined from purified NSO cells.
- ChEBML_140542 Displacement of [3H]glycine from glycine site on the NMDA receptor.
- ChEBML_27804 Tested for the ability to inhibit Acetylcholinesterase (AChE) from Torpedo californica
- ChEMBL_152918 (CHEMBL758788) Inhibition of phosphodiesterase 3 (PDE3) isolated from the dog aorta
- ChEMBL_158991 (CHEMBL763757) Evaluated for inhibition of Protease from the crude plate mixture
- ChEMBL_208540 (CHEMBL813634) The binding affinity towards thrombin obtained from human purified enzymes.
- ChEMBL_48913 (CHEMBL660743) Concentration required for the inhibition of ACAT from rat gut
- ChEMBL_51850 (CHEMBL664073) Inhibition of cruzain, the major cysteine protease from Trypanosoma cruzi
- ChEMBL_143850 (CHEMBL747207) Compound was tested for the displacement of [125I]-PYY(3-36) (SK-N-BE2) from Neuropeptide Y receptor type 2 in the membranes prepared from cells
- Radiometric Filtration Assay The mTOR activity was determined by measuring the amount of [gamma-33 P] phosphae from ATP incorporated in the substrate protein.
- Heme Binding Assay Heme binding was tracked by difference spectroscopy in the Soret region of the UV−visible spectrum. Successive aliquots of 0.5 mM hemin in 0.1 M NaOH were added to both the sample cuvette, which contained 10 μM apo-HutZ, and the referencecuvette. Spectra were recorded 3 min after the addition of each heme aliquot.
- Chk1 Enzymatic Assay Chk1 kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor in the presence of 100uM ATP/[gamma-33P] ATP. 33P incorporation was measured using a Perkin-Elmer Trilux scintillation counter. The IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from 33P labeled ATP to the substrate.
- Kinase assays for IC50 determinations Kinase activity was assayed in reaction buffer containing substrate peptide, enzyme, and inhibitor compound in the presence ATP/[gamma-33P] ATP. 33P incorporation was measured using a Perkin-Elmer Trilux scintillation counter. The IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from 33P labeled ATP to the substrate.
- Cardiac Myofibril ATPase Assay Following example 15, the counter screen was done using frozen myofibril pellets obtained from cardiac tissue. The assay was done in the same manner as above, with the following notable exceptions: the final well concentration of myofibrils was 1.0 mg/mL and KCl was omitted from the recipe.
- ChEMBL_196788 (CHEMBL799731) Inhibitory activity against peptide binding to the Rev Response Element RNA IIB was determined
- Protease Inhibition Assay Inhibition constants were determined by a spectrophotometric assay with the chromogenic peptide substrate.
- Kinase Assay Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The extend of peptide was determined by Homogeneous Time Resolved Fluorescence (HTRF) using a lanthanide chelate (Lance)-coupled monoclonal antibody specific for the phosphopeptide in combination with a streptavidin-linked allophycocyanin (SA-APC) fluorophore which will bind to the biotin moiety on the peptide.
- ChEMBL_58484 (CHEMBL670415) Ability to displace [3H]spiperone from D2 dopamine receptor isolated from the striata of male Wistar rats
- ChEMBL_58717 (CHEMBL671110) Compound was tested for the displacement of [3H]spiperone from Dopamine receptor D2 from rat striatal membranes.
- ChEMBL_61408 (CHEMBL673349) Compound was tested for the displacement of [3H]spiperone from dopamine receptor D2 from rat striatal membranes.
- ChEBML_140302 In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptors
- ChEBML_60221 Displacement of the radioligand [3H]spiperone from the cloned human Dopamine receptor D2 long expressed in CHO cells
- ChEMBL_152935 (CHEMBL757135) Concentration at which 50% of the activity of the Phosphodiesterase 4 from Human U937 cells is inhibited
- ChEMBL_63674 (CHEMBL670477) Binding ability determined by the displacement of [125 I]ET-3 from the human Endothelin B receptor
- Enzymatic Assay The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an amino acid side chain of a commercially available peptide (amino acid sequence EVPRRKSLVGTPYWM).
- Radioligand Binding Assay IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition constant (Ki) was calculated from IC50 value by the Cheng and Prusoff equation.
- Inhibition Assay The PPlase activity of recombinant CypA or D, produced by thrombin cleavage of GST-CypA or D, was determined by following the rate of hydrolysis of N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide by chymotrypsin. Chymotrypsin only hydrolyzes the trans form of the peptide, and hydrolysis of the cis form, the concentration of which is maximized by using a stock dissolved in trifluoroethanol containing 470 mM LiCl, is limited by the rate of cis-trans isomerization. CypA or D was equilibrated for 1 h at 5° C. with selected test article using a drug concentration range from 0.1 to 20 nM. The reaction was started by addition of the peptide, and the change in absorbance was monitored spectrophotometrically at 10 data points per second. The blank rates of hydrolysis (in the absence of CypA or D) were subtracted from the rates in the presence of CypA or D.
- Fluorimetric Assay The MMP assay were performed evaluating the ability of compounds 1a-6a and 1b-6b to prevent the hydrolysis of the fluorescence-quenched peptide substrate Mca-Pro-Gly-Leu-Dpa-ala-Arg-NH2.
- FRET Assay for LRH-1 The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-derived peptide. Detection of the associated complex was measured by time resolved fluorescence energy transfer (TR-FRET). The EC50s of the test compound was estimated from a plot using the ratio of fluorescence values collected at 671 nm to fluorescence values collected 618 nm versus concentration of test compound added. Test compounds (agonists) that increased the affinity of the receptors for the peptide yielded an increase in fluorescent signal.
- TR-FRET Assay Protein arginine methyltransferase 5 (PRMT5) is a type II arginine methyltransferase that catalyze mono- and symmetric demethylation on arginine residues of histone or non-histone proteins in presence of S-adenosylmethionine (AdoMet or SAM) a cofactor responsible for donating the methyl group. PRMT5 is reported to be overexpressed in several human cancers. To identify compounds that inhibit the PRMT5 and decrease its activity, a TR-FRET based assay has been established. Time-resolved fluorescence resonance energy transfer (TR-FRET) HTS assays are homogeneous proximity assays where the interaction of two dye-labeled binding partners is detected by the energy transfer between a donor and an acceptor dye, and the subsequent light emission by the acceptor dye. PRMT5 catalyzes Histone H4 peptide [1-16] which is biotin tagged to the Lysine amino acid at carboxyl end, in presence of S-adenosyl-1-methionine (SAM) to methylate the peptide. The antibody specific to mono methylated H4 peptide (H4R3) with Ig conjugate binds to the methylated peptide, indirectly binding to the Europium lanthanide. SureLight Allophycocyanin-Streptavidin binds to the biotin tag of the peptide, therefore accepting the energy transferred from the Europium lanthanide. This energy transfer between Europium to SureLight Allophycocyanin is a direct measure of the activity/inhibition of the PRMT5 enzyme.
- Scintillation Proximity Assay (SPA) The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-33P]ATP. After incubation, the phosphorylated substrate was separated from nonincorporated radioactive ATP using SPA beads. Incorporated radioactivity was measured using Packard TopCount.
- ChEBML_49899 Tested for the 50% displacement of [125I]CCK-8 from membrane preparation isolated from CHO-K1 cells stably transfected with the cDNA of human Cholecystokinin type A receptor
- Peptidyl Prolyl Isomerase (PPIase) Inibition Assay PPIase(Rotamase) activity of FKBP12 was assayed using the peptide N-succinyl Ala-Leu-Pro-Phe p-nitroanilide as substrate. It is based on the observation that chymotrypsin cleaves the C-terminal amide bond only in the trans conformer of the chromogenic substrate. The rapid hydrolysis perturbs the cis-trans conformational equilibrium, which allows one to monitor the PPIase-catalyzed cis-to-trans isomerization. The absorbance at 390 nM versus time was monitored for up to 400 s. Rate constants were generated from the absorbance versus time plots at 8-12 compound concentrations. The Kiapp was determined from the corrected rate constants using an IC50 equation or a tight-binding equation by Morrison.
- Ray2010 Assay 74 Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.
- ChEMBL_799260 (CHEMBL1941810) Noncompetitive inhibition of human full length recombinant MMP13 using triple-helical fTHP-15 as substrate by Lineweaver-Burk plot analysis
- ChEMBL_799327 (CHEMBL1941964) Inhibition of MMP1 expressed in Escherichia coli using triple-helical fTHP-15 as substrate after 4 hrs by FRET assay
- Enzymatic Activity Assay The compounds were tested for serum and glucocorticoid-regulated kinase 1 (SGK-1) inhibitory activity in a substrate phosphorylation assay designed to measure the ability of the isolated enzyme to catalyze the transfer of phosphate from ATP to serine/threonine residues in a fluorescein-labeled substrate peptide, using recombinant human SGK-1 enzyme produced in a baculovirus system (Biomol, Hamburg, Germany, Cat. No. 4-331). The synthesized fluorescent labeled peptide substrate contained (5(6)-Carboxyfluorescein)-RPRAATF-NH2. The phosphorylated substrate peptide and non-phosphorylated substrate peptide were separated with caliper life science's lab-chip technology based on a micro fluidics method. All fluid flow was established on the chip by applying a vacuum of a few psi to the waste well transporting fluid from various sources through interconnecting channels. Because the phosphoryl group is doubly negatively charged, under the pressure-driven hydrodynamic flow and the voltage-driven flow within the electric field, the fluorescent labeled peptide substrate and its phosphorylation product appear at different times in the detection window to the detection point. Substrate turnover can thus be determined as the ratio of the product peak area and the sum of substrate peak area and product peak area.The enzyme reaction was carried out in a buffer containing 25 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 2 mM MnCl2, 2 mM DTT, and 0.03% bovine serum albumine. The enzyme was pre-incubated with the test compound for 30 min at 24° C. The kinase reaction was initiated by addition of the substrate mixture containing the peptide substrate (final concentration 1 μM) and ATP (final concentration 10 μM). After 60 min incubation at 37° C., the enzyme reaction was terminated by adding a buffer containing 100 mM Hepes (pH 7.4) and 35 mM EDTA.
- Enzyme Assay The inhibitory activity of compounds against AURKA, AURKB, JAK1, JAK2 and JAK3 was determined in Z′-LYTE assays run by ThermoFisher Scientific as part of their SelectScreen Biochemical Kinase Profiling Service. The Z′-LYTE biochemical assay format employs a fluorescence-based, coupled-enzyme format and is based on the differential sensitivity of phosphorylated and non-phosphorylated peptides to proteolytic cleavage. The peptide substrate is labeled with two fluorophores one at each end that make up a FRET pair. In the primary reaction, the kinase transfers the gamma-phosphate of ATP to a single tyrosine, serine or threonine residue in a synthetic FRET-peptide. In the secondary reaction, a site-specific protease recognises and cleaves non-phosphorylated FRET-peptides. Phosphorylation of FRET-peptides suppresses cleavage by the Development Reagent. Cleavage disrupts FRET between the donor (i.e., coumarin) and acceptor (i.e., fluorescein) fluorophore on the FRET-peptide, whereas uncleaved, phosphorylated FRET-peptides maintain FRET. A ratiometric method, which calculates the ratio (the Emission Ratio) of donor emission to acceptor emission after excitation of the donor fluorophore at 400 nm, is used to quantitate reaction progress. Both cleaved and uncleaved FRET-peptides contribute to the fluorescence signals and therefore to the Emission Ratio. The extent of phosphorylation of the FRET-peptide can be calculated from the Emission Ratio. The Emission Ratio will remain low if the FRET-peptide is phosphorylated (i.e., no kinase inhibition) and will be high if the FRET-peptide is non-phosphorylated (i.e., kinase inhibition).
- Isothermal Titration Calorimetry Studies of STAT3 Binding The binding isotherm from the integrated thermogram fit using the one-site model in the PEAQ-ITC software generated from the titration of Compound 1 into purified STAT3 show KD of 880 nM. The signature plot show the thermodynamics parameters for the titration reveals ΔH=−21.1 kJ/mol, ΔG=−34.6 kJ/mol, and −TΔS=−13.2 kJ/mol.
- ChEBML_1711442 Displacement of [125I]peptide YY from human Y1 receptor after 120 mins by scintillation counting analysis
- ChEBML_1711443 Displacement of [125I]peptide YY from human Y2 receptor after 120 mins by scintillation counting analysis
- ChEMBL_1460643 (CHEMBL3395884) Inhibition of DNA-PK purified from HeLa nuclear extracts assessed as EPPLSQEAFADLWKKR peptide substrate phosphorylation
- ChEMBL_1767769 (CHEMBL4219881) Displacement of FAM-labeled HIF-1alpha peptide from VBC (unknown origin) by fluorescence polarization assay
- ChEMBL_1833236 (CHEMBL4333244) Displacement of fluorescence-labelled Ac-ARA peptide from WDR5 (unknown origin) by fluorescence polarization assay
- ChEMBL_2074780 (CHEMBL4730314) Displacement of FAM-labeled HIF-1alpha peptide from VCB (unknown origin) by fluorescence polarization assay
- ChEMBL_2230035 (CHEMBL5143807) Displacement of CPSF6 peptide from HIV1 capsid protein by surface plasmon resonance-based competition assay
- ChEMBL_2230036 (CHEMBL5143808) Displacement of NUP153 peptide from HIV1 capsid protein by surface plasmon resonance-based competition assay
- ChEMBL_429496 (CHEMBL918619) Displacement of FAM-Bid BH3 peptide from human recombinant Bcl2 by FP-based binding assay
- ChEMBL_429497 (CHEMBL918620) Displacement of FAM-Bak BH3 peptide from human Bcl-xL by FP-based binding assay
- ChEMBL_441639 (CHEMBL891870) Displacement of Flu-Bak peptide from recombinant antiapoptotic Bcl-w protein by fluorescence polarization assay
- ChEMBL_610765 (CHEMBL1064400) Displacement of fluorescein-labeled Bak-BH3 peptide from human Bcl-XL by fluorescence polarization assay
- ChEMBL_643624 (CHEMBL1212488) Displacement of [125I]peptide YY from human recombinant Y1 receptor expressed in SK-NM- cell
- ChEMBL_643625 (CHEMBL1212489) Displacement of [125I]peptide YY from human recombinant Y2 receptor expressed in KAN-TS cells
- BTK Inhibitory Activity With regard to the setting of the conditions for a method for measuring the inhibitory activity of a compound against BTK kinase activity in vitro, it is described in the consumable reagent supplies price list for LabChip (registered trademark) series of PerkinElmer, Inc. that FL-PEPTIDE 2 corresponds to a substrate peptide for the measurement of BTK kinase activity. Therefore, FL-PEPTIDE 2 was used as a substrate. The purified recombinant human BTK protein used in the test was purchased from Carna Biosciences, Inc.With regard to the measurement of the inhibitory activity of the compounds, firstly, the compounds of the present invention were diluted stepwise with dimethyl sulfoxide (DMSO). Subsequently, BTK protein, a substrate peptide (final concentration was 1 μM), magnesium chloride (final concentration was 10 mM), ATP (final concentration was 45 μM), and a DMSO solution of the compounds of the present invention (final concentration of DMSO was 5%) were added to a buffer solution for kinase reaction (20 mM HEPES (pH 7.5), 2 mM dithiotheitol, 0.01% Triton X-100), and after the solution was incubated for 40 minutes at 25° C., a kinase reaction was carried out. The reaction was terminated by adding EDTA thereto in order to obtain a final concentration of 30 mM. Finally, a substrate peptide that was not phosphorylated (S) and a phosphorylated peptide (P) were separated and detected by microchannel capillary electrophoresis using a LabChip EZ Reader II (PerkinElmer, Inc.). The amounts of phosphorylation reaction were determined from the respective peak heights of S and P, and the compound concentration at which the phosphorylation reaction could be suppressed in 50% was defined as the IC50 value (nM).
- CB Receptor Binding Assay IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition constant (Ki) was calculated from IC50 value by the Cheng and Prusoff equation.
- Functional Peptidyl Prolyl Isomerase (PPlase) Spectrophotometric Assay Cyclophilins are enzymes that catalyse the cis-trans isomerisation of peptide bonds to the amino acid proline. This event is important in many biological processes, including protein folding and signal transduction. Cyclosporins and the compounds of the present invention inhibit this catalytic activity. The method to measure this activity and its inhibition is similar to that described by Jankowski (Analytical Biochemistry, 252, 2, 299-307 (1997)). The assay measures the cis to trans isomerisation of a peptide substrate catalysed by a PPlase enzyme using a UV/Vis spectrophotometer. This assay is a manual single cuvette-based assay. A first order rate equation is fitted to the absorbance data to obtain a rate constant. The catalytic rate is calculated from the enzymatic rate minus the background rate. The Ki (the concentration required to produce half maximum inhibition) for an inhibitor is obtained from the rate constant plotted against the inhibitor concentration.
- Biochemical Activity Assay for BRD4 1) using BRD4-BD1 protein and BRD4-BD2 protein from BPS company for the experiments; as well as polypeptides from ANASPEC company; detection reagents from Perkinelmer company;2) screening compounds by applying the experimental principle of TR-FRET;3) testing the compounds.
- Radioligand Displacement Assay The receptor-binding assay was conducted using [3H]4-hydroxytamoxifen (4-OHT) as the radioligand. To estimate the binding affinity, the IC50 values (the concentrations for the half-maximal inhibition) were calculated from the dose-response curves by using the nonlinear analysis program ALLFIT.
- Inhibition Assay Chinese hamster ovary cells are transfected with the human gene for amyloid precursor protein. The cells are plated at a density of 8000 cells/well into 96-well microtiter plates and cultivated for 24 hours in DMEM cell culture medium containing 10% FCS. The test compound is added to the cells at various concentrations, and the cells are cultivated for 24 hours in the presence of the test compound. The supernatants are collected, and the concentration of amyloid peptide 1-40 is determined using state of the art immunoassay techniques, for example sandwich ELISA, homogenous time-resolved fluorescence (HTRF) immunoassay, or electro-chemiluminescence immunoassay. The potency of the compound is calculated from the percentage of inhibition of amyloid peptide release as a function of the test compound concentration.
- ChEMBL_142637 (CHEMBL746905) Binding affinity for Norepinephrine transporter is assessed from the ability to displace [3H]nisoxetine from rat frontal cortex
- ChEMBL_200909 (CHEMBL880920) Inhibitory concentration bound to tryptophan containing region of Serum albumin in fluorescence quenching
- Fluorescence Polarization Assay A fluorescence polarization (FP) assay monitor the fluorescein-labeled C-terminal Hsp90 peptide-TPRA interaction. FP assay will also confirm binding interaction between hit compounds identified from AlphaScreen assay.
- ChEBML_75879 The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600.
- ChEMBL_139910 (CHEMBL746846) Allosteric potency against the dissociation of radioligand [3H]N-methylscopolamine from the porcine cardiac Muscarinic acetylcholine receptor M2
- ChEMBL_140072 (CHEMBL747450) Displacement of [3H]-OXO-M (oxotremorine-M) from the central muscarinic receptor sites of the rat brain membranes
- ChEMBL_32315 (CHEMBL646332) The ability to displace [3H]clonidine from the Alpha-2 adrenergic receptor was determined in rat brain membrane
- ChEMBL_33508 (CHEMBL647005) The compound's binding affinity against Alpha-2B adrenergic receptor from rat kidney homogenate in the presence of phentolamine
- BACE Assay For each compound being tested, the BACE activity was monitored in a fluorescence quenching assay (FRET) using the ectodomain of BACE (aa 1-454) fused to a myc-his tag and secreted from HEK293/BACEect. cells into OptiMEM (Invitrogen) as enzyme source and a substrate peptide derived from the APP-Swedish mutation which possesses a Cy3-fluorophore at the N-terminus and a Cy5Q-quencher at the C-terminus (Cy3-SEVNLDAEFK-Cy5Q-NH2; Amersham).
- Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Using human recombinant KRAS G12D, SOS and c-Raf proteins, the inhibitory activity of subject compounds on the complex formation of the proteins was examined by a time-resolved fluorescence resonance energy transfer (TR-FRET) method.Biotinylated AviTag-KRAS G12D (amino acid region of 1-185, GDP) (2.5 μL; 400 nM) and subject compounds dissolved in an assay buffer (50 mM HEPES, 150 mM NaCl, 5 mM MgCl2, 0.05% Tween 20, pH 7.0) were added to a 384-well plate (from Corning) in a liquid volume of 2.5 μL at 40,000 nM to 40 nM. Son of Sevenless (SOS) (amino acid region of 564-1049, 2.5 μL; 1.3 μM) and c-Raf (amino acid region of 51-131) GST (2.5 μL; 130 nM) containing GTP (from Sigma-Aldrich; 2 μM) were added to the plate, and the plate was allowed to stand for 1 hour at room temperature. Then, a mixture liquid (10 μL) of LANCE Ulight-anti-GST (from PerkinElmer; 120 nM) and LANCE Eu-W1024 labeled Streptoavidin (from PerkinElmer; 100 ng/mL) was added, and the 620-nm and 665-nm fluorescence intensities were measured using EnVision 2104 (from PerkinElmer) under the conditions of an excitation wavelength of 337 nm. After standardizing the values with the fluorescence intensity at a reference wavelength of 620 nm, the 50% inhibitory concentrations (IC50) were calculated by Sigmoid-Emax model nonlinear regression analysis with the signaling value in treatment with the solvent taken as 0% inhibition and with the signaling value without the addition of GTP taken as 100% inhibition.
- Immunofluorescence Resonance Energy Transfer (FRET) Assay The FRET assay was performed essentially as described in Gruninger-Leitch et al., Journal of Biological Chemistry (2002) 277(7) 4687-93 (Substrate and inhibitor profile of BACE (beta-secretase) and comparison with other mammalian aspartic proteases). In summary, a peptide is designed that is cleaved by the protease. The peptide is labelled with dabcyl at the N terminus and Lucifer Yellow at the C-terminus, such that for an intact peptide the Lucifer Yellow fluorescence is quenched by the dabcyl. When the peptide is cut by BACE2, the quenching is removed and a fluorescent signal is generated. The assay was performed as described in Grueninger et al. 2002 at pH 4.5 using a substrate concentration of 5 μM. A FRET peptide based on the TMEM27 sequence was devised. dabcyl-QTLEFLKIPS-LucY (SEQ ID NO: 1). BACE2 had a high activity against this sequence, which is unrelated to the known APP-based substrates. Conversely, BACE1 had insignificant activity against this peptide.

