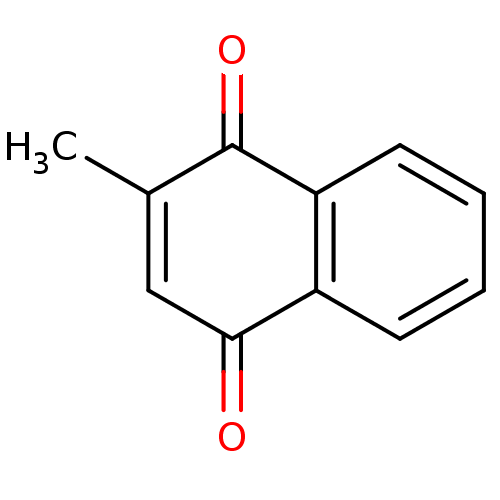
 2-methyl-1,4-naphthoquinone, 5 Menadione (Vitamin K3) 2-methyl-1,4-dihydronaphthalene-1,4-dione CHEMBL590 Menadione BDBM24778 cid_4055 Menadione (5d) Vitamin K3 Menadione, 9
2-methyl-1,4-naphthoquinone, 5 Menadione (Vitamin K3) 2-methyl-1,4-dihydronaphthalene-1,4-dione CHEMBL590 Menadione BDBM24778 cid_4055 Menadione (5d) Vitamin K3 Menadione, 9 US9365563, K3 BDBM236151 US9878991, Compound K3
US9365563, K3 BDBM236151 US9878991, Compound K3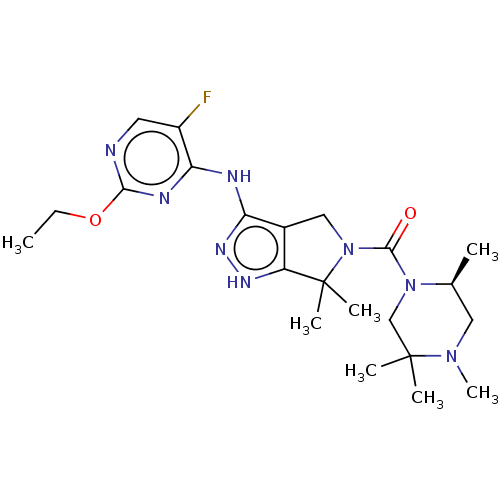 US11220518, Ex. No. K3 BDBM286345 US11780853, Example K3 US9518060, Example K3
US11220518, Ex. No. K3 BDBM286345 US11780853, Example K3 US9518060, Example K3 US9416126, K3 BDBM239345
US9416126, K3 BDBM239345 BDBM365726 US9868748, I-K3
BDBM365726 US9868748, I-K3 US20250188030, Compound K3 BDBM748592
US20250188030, Compound K3 BDBM748592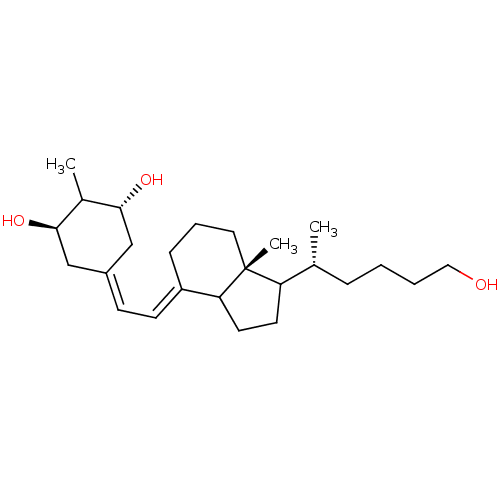 Vitamin D analog, 10 BDBM93065 Vitamin D analog, 14
Vitamin D analog, 10 BDBM93065 Vitamin D analog, 14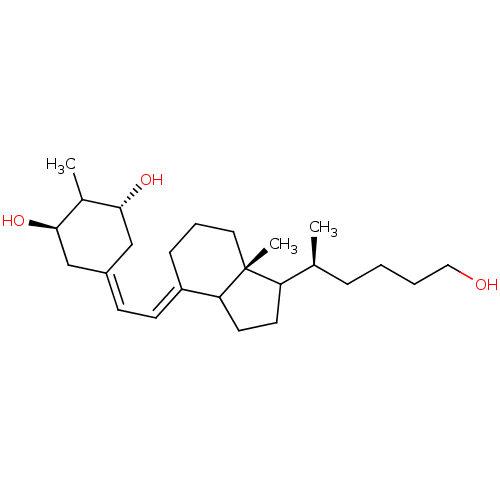 Vitamin D analog, 11 BDBM93062 Vitamin D analog, 15
Vitamin D analog, 11 BDBM93062 Vitamin D analog, 15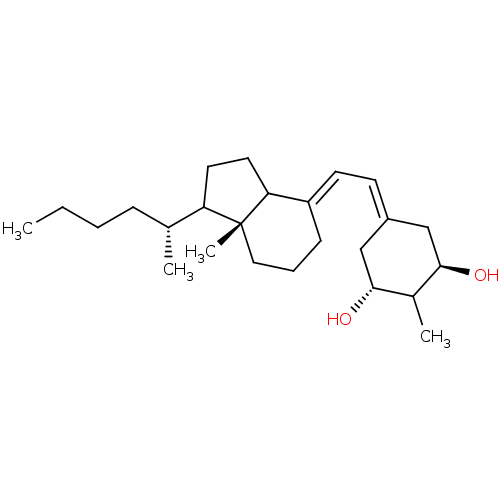 Vitamin D analog, 12 BDBM93066 Vitamin D analog, 16
Vitamin D analog, 12 BDBM93066 Vitamin D analog, 16 Vitamin D analog, 17 BDBM93067 Vitamin D analog, 13
Vitamin D analog, 17 BDBM93067 Vitamin D analog, 13 BDBM218904 US9303033, K3, Table 12A, Compound 6
BDBM218904 US9303033, K3, Table 12A, Compound 6 BDBM512999 Vitamin B12
BDBM512999 Vitamin B12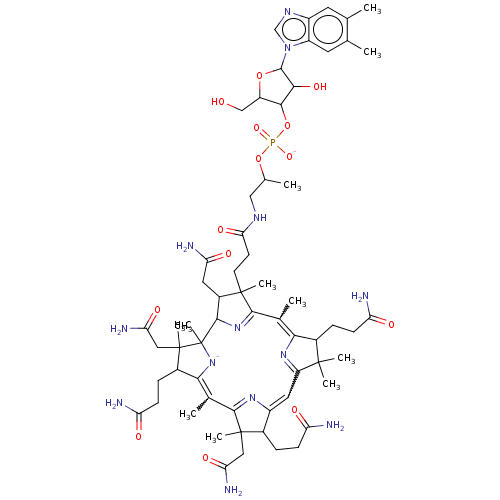 Vitamin B12 BDBM420313
Vitamin B12 BDBM420313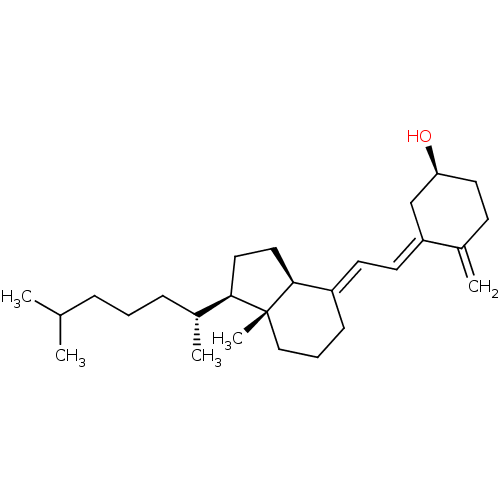 CHEBI:28940 Vitamin D 3 Vitamin D Cholecalciferol 7-Dehydrocholesterol BDBM50030475 Colecalciferol Dihydrocholesterol
CHEBI:28940 Vitamin D 3 Vitamin D Cholecalciferol 7-Dehydrocholesterol BDBM50030475 Colecalciferol Dihydrocholesterol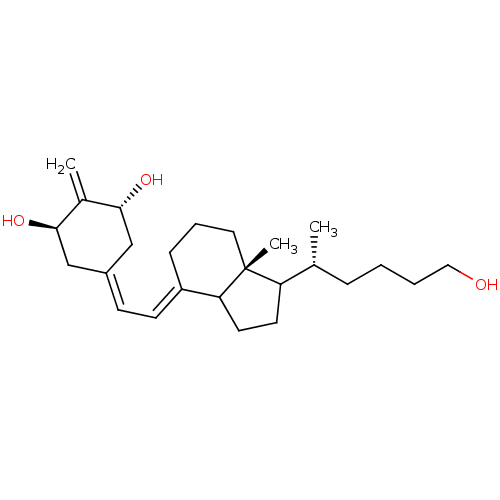 BDBM93061 Vitamin D analog, 6
BDBM93061 Vitamin D analog, 6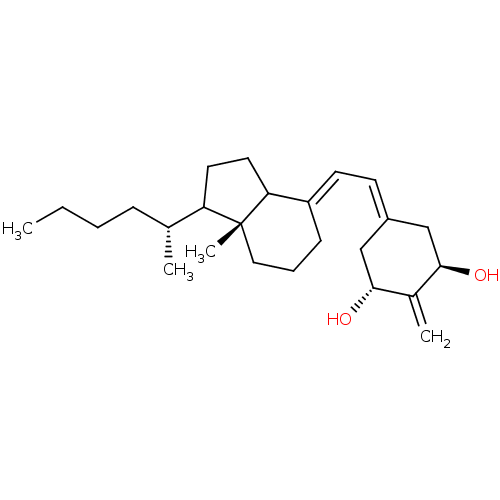 BDBM93063 Vitamin D analog, 8
BDBM93063 Vitamin D analog, 8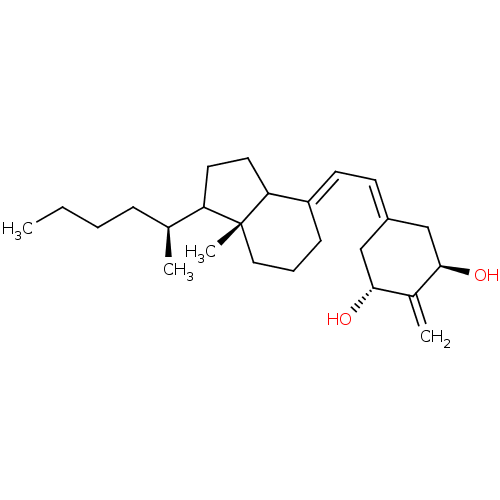 BDBM93064 Vitamin D analog, 9
BDBM93064 Vitamin D analog, 9 BDBM93068 Vitamin D analog, 14
BDBM93068 Vitamin D analog, 14 BDBM93069 Vitamin D analog, 15
BDBM93069 Vitamin D analog, 15 BDBM93070 Vitamin D analog, 16
BDBM93070 Vitamin D analog, 16 BDBM93071 Vitamin D analog, 17
BDBM93071 Vitamin D analog, 17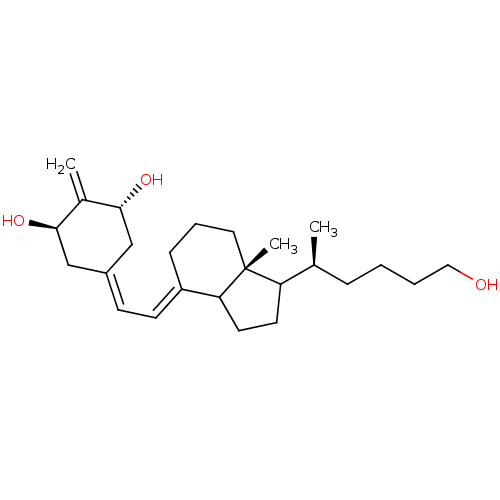 BDBM93072 Vitamin D analog, 7
BDBM93072 Vitamin D analog, 7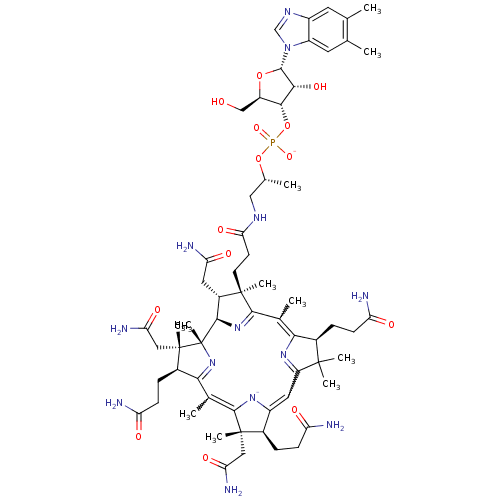 CYANOCOBALAMIN Cobalamin SMR001233181 RUVITE MLS002153809 vitamin B12 cid_25102581 BDBM83973
CYANOCOBALAMIN Cobalamin SMR001233181 RUVITE MLS002153809 vitamin B12 cid_25102581 BDBM83973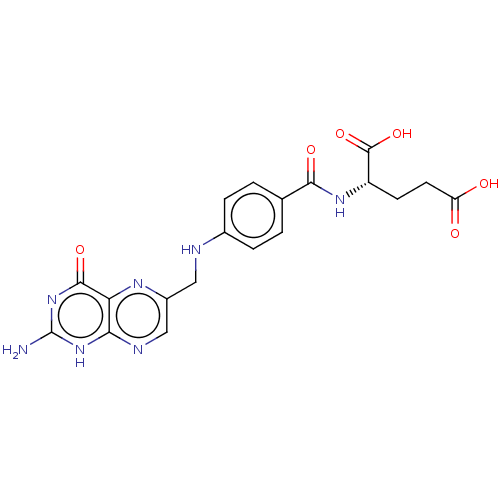 Folic Acid Folvite Pteroylglutamic Acid CHEBI:27470 Natur Flow Folicet Folicare Vitamin Bc Roche Folacin Lexpec BDBM50237629 Preconceive Vitamin M Bio Science Folsan
Folic Acid Folvite Pteroylglutamic Acid CHEBI:27470 Natur Flow Folicet Folicare Vitamin Bc Roche Folacin Lexpec BDBM50237629 Preconceive Vitamin M Bio Science Folsan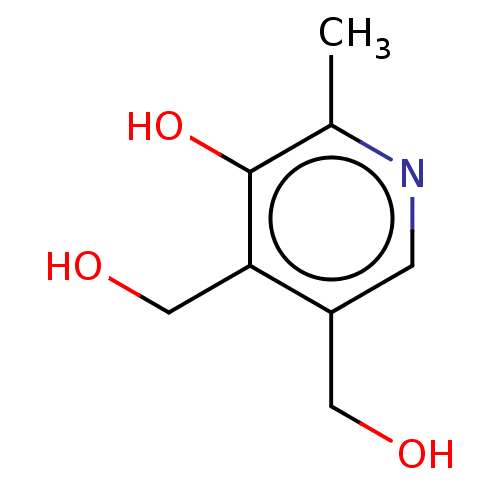 CHEBI:16709 Pyridoxine Hexa-Betalin M.V.I.-12 BDBM50103505 Vitamin B6
CHEBI:16709 Pyridoxine Hexa-Betalin M.V.I.-12 BDBM50103505 Vitamin B6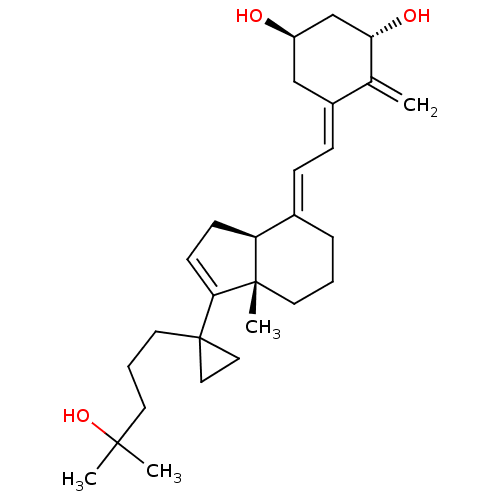 CHEMBL494338 BDBM50257431 1,25-dihydroxy-16-ene-20-cyclopropyl-vitamin D3
CHEMBL494338 BDBM50257431 1,25-dihydroxy-16-ene-20-cyclopropyl-vitamin D3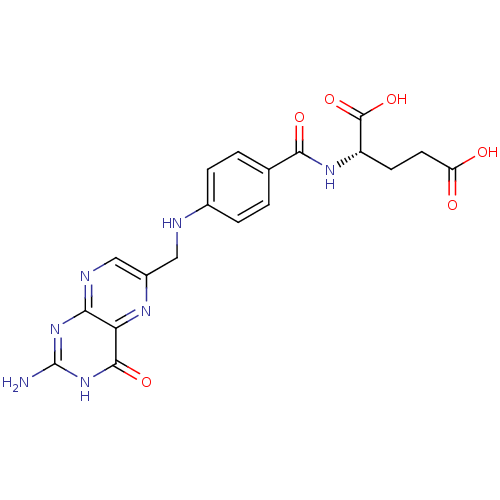 Folvite BDBM50367343 Folacin FOLIC ACID Vitamin B9 (2S)-2-[[4-[(2-amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid Vitamin M
Folvite BDBM50367343 Folacin FOLIC ACID Vitamin B9 (2S)-2-[[4-[(2-amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid Vitamin M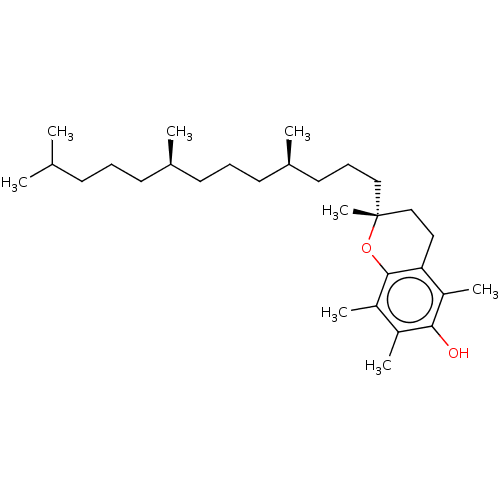 Alpha Tocopherol BDBM50458513 Aquasol E E307 Vitamin E CHEBI:18145 Tocopherol
Alpha Tocopherol BDBM50458513 Aquasol E E307 Vitamin E CHEBI:18145 Tocopherol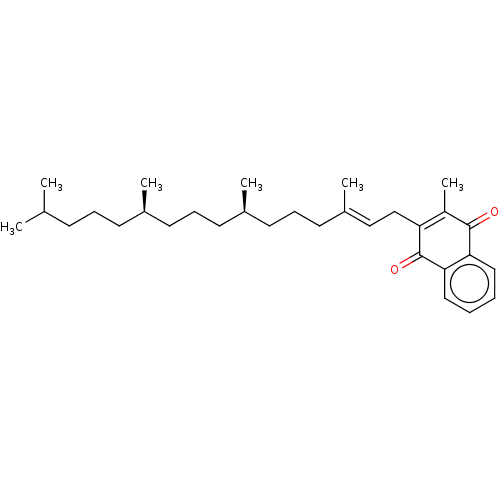 Phytomenadione Konakion mm BDBM50553259 Konakion mm paed Orakay Vitamin k1 Mephyton Kanavit NSC-270681 Aquamephyton Neokay Vitamin K 1 CHEBI:18067 Phylloquinone Phylloquinone e-form Konakion Phytonadione
Phytomenadione Konakion mm BDBM50553259 Konakion mm paed Orakay Vitamin k1 Mephyton Kanavit NSC-270681 Aquamephyton Neokay Vitamin K 1 CHEBI:18067 Phylloquinone Phylloquinone e-form Konakion Phytonadione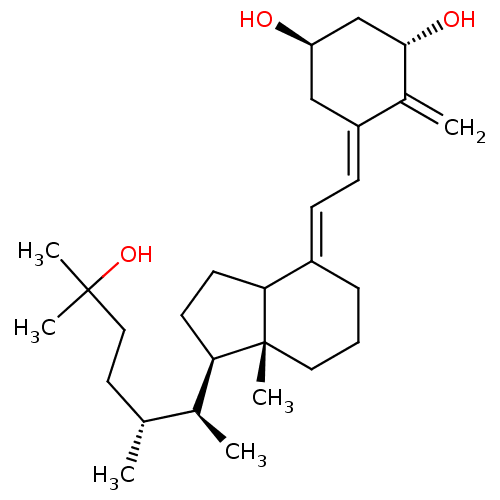 CHEMBL220846 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3 BDBM50205234
CHEMBL220846 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3 BDBM50205234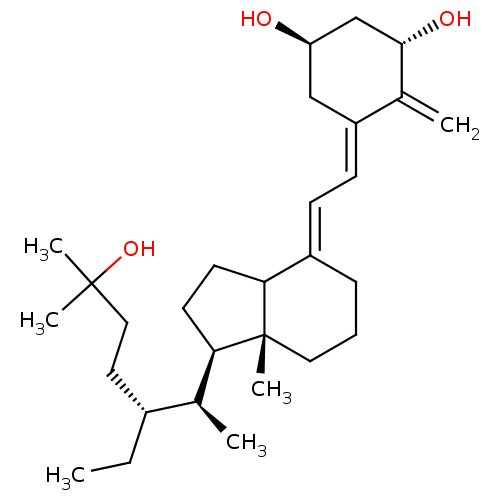 CHEMBL222195 BDBM50205233 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3
CHEMBL222195 BDBM50205233 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3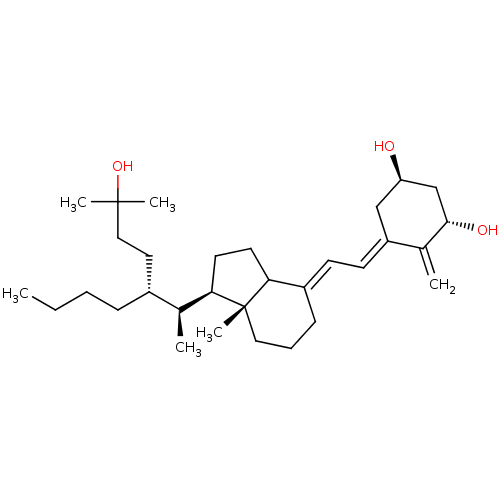 CHEMBL375811 BDBM50205235 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3
CHEMBL375811 BDBM50205235 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3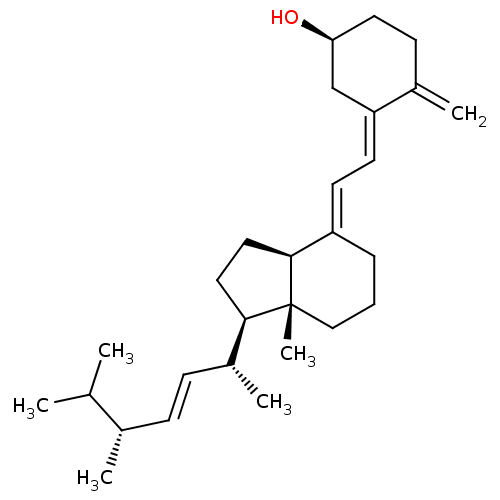 Viosterol in Oil Vitamin D2 Osto-D2 Drisdol Ergoral D2 Uvesterol D BDBM50247883 Caltrate Ergocalciferol Oleovitamin D, Synthetic Oleovitamin D D-Forte Sterogyl Sterogyl 15H Vitamin D 2 Ergo-D2 Calciferol Vitamin D Eciferol D2 Deltalin Calciferol In Arach Oil Lanes Sterogyl-15 CHEBI:28934
Viosterol in Oil Vitamin D2 Osto-D2 Drisdol Ergoral D2 Uvesterol D BDBM50247883 Caltrate Ergocalciferol Oleovitamin D, Synthetic Oleovitamin D D-Forte Sterogyl Sterogyl 15H Vitamin D 2 Ergo-D2 Calciferol Vitamin D Eciferol D2 Deltalin Calciferol In Arach Oil Lanes Sterogyl-15 CHEBI:28934 BDBM50373877 THIAMINE (VIT B1) Betaxin ThOH CA inhibitor, 3 Vitamin B 1 Thiamine
BDBM50373877 THIAMINE (VIT B1) Betaxin ThOH CA inhibitor, 3 Vitamin B 1 Thiamine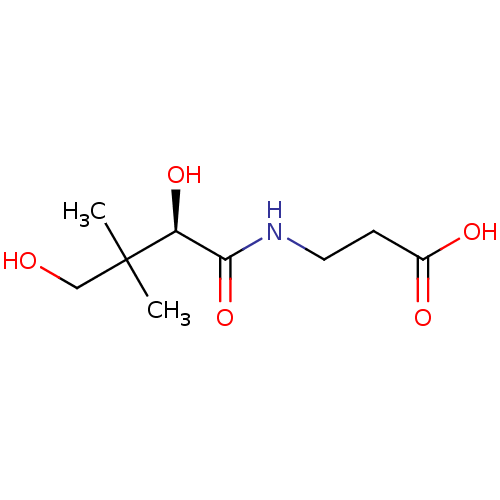 Vitamin B 5 CHEBI:46905 Cantopal BDBM50240040 Calc Pantoth Pantothenic Acid Pantothenic acid
Vitamin B 5 CHEBI:46905 Cantopal BDBM50240040 Calc Pantoth Pantothenic Acid Pantothenic acid 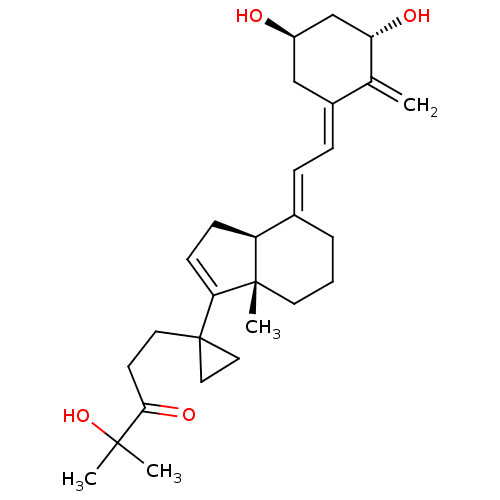 CHEMBL492749 BDBM50293274 1alpha,25(OH)2-24-oxo-16-ene-20-cyclopropyl-vitamin D3
CHEMBL492749 BDBM50293274 1alpha,25(OH)2-24-oxo-16-ene-20-cyclopropyl-vitamin D3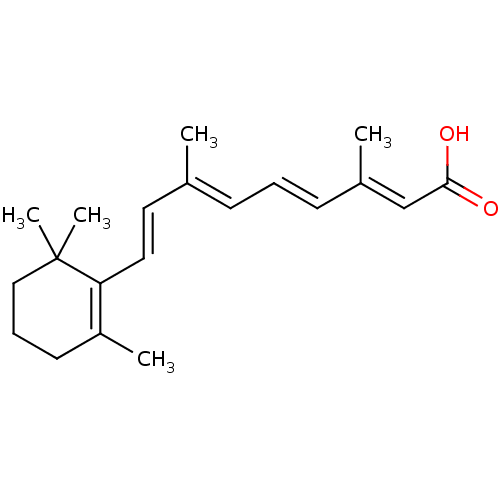 all-trans retinoic acid TRETINOIN MLS000028588 [3H]RA Vitamin A acid ALL-TRANS-RETINOIC ACID [3H]Retinoic acid cid_444795 9-cis-retinoic acid (9cRA) BDBM31883 AT-RA Atralin CHEMBL38 SMR000058245 [3H]tretinoin [3H]Vitamin A acid
all-trans retinoic acid TRETINOIN MLS000028588 [3H]RA Vitamin A acid ALL-TRANS-RETINOIC ACID [3H]Retinoic acid cid_444795 9-cis-retinoic acid (9cRA) BDBM31883 AT-RA Atralin CHEMBL38 SMR000058245 [3H]tretinoin [3H]Vitamin A acid BDBM751371 8-(2-chloro-4-(2- (piperazin-1- yl)ethoxy)phenyl)- 9-((5- chloropyridin-2- yl)methyl)-6-(1- methylcyclopropoxy)- 9H-purine US20250195530, Example K3
BDBM751371 8-(2-chloro-4-(2- (piperazin-1- yl)ethoxy)phenyl)- 9-((5- chloropyridin-2- yl)methyl)-6-(1- methylcyclopropoxy)- 9H-purine US20250195530, Example K3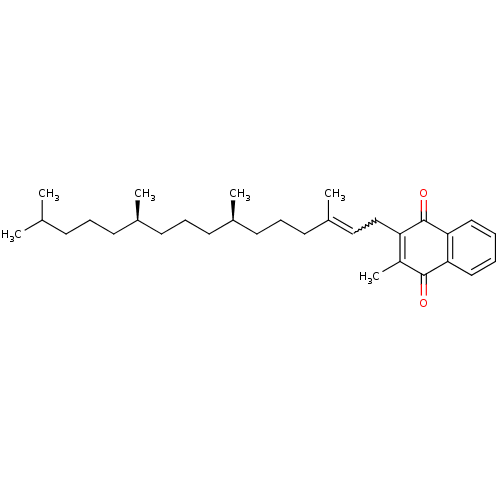 2-methyl-3-[(2E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-yl]-1,4-dihydronaphthalene-1,4-dione vitamin K1, 11 Phylloquinone BDBM24782 Phytonadione
2-methyl-3-[(2E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-yl]-1,4-dihydronaphthalene-1,4-dione vitamin K1, 11 Phylloquinone BDBM24782 Phytonadione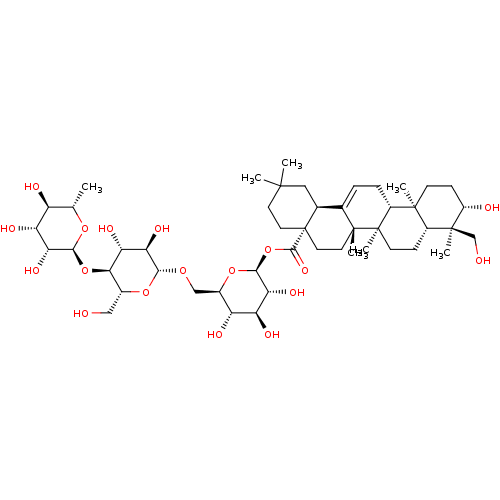 BDBM50322752 CHEMBL540943 kizuta cirensenoside O saponin K3 3beta,23-dihydroxyolean-12-en-28-oic acid O-alpha-L-rhamnopyranosyl-(1->4)-O-beta-D-glucopyranosy1-(1->6)-beta-D-glucopyranosy1 ester
BDBM50322752 CHEMBL540943 kizuta cirensenoside O saponin K3 3beta,23-dihydroxyolean-12-en-28-oic acid O-alpha-L-rhamnopyranosyl-(1->4)-O-beta-D-glucopyranosy1-(1->6)-beta-D-glucopyranosy1 ester Alphalin all-trans-retinyl alcohol all-trans-vitamin A alcohol CHEMBL986 Afaxin all-trans-retinol Chocola A (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol BDBM50092056 Retinol
Alphalin all-trans-retinyl alcohol all-trans-vitamin A alcohol CHEMBL986 Afaxin all-trans-retinol Chocola A (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol BDBM50092056 Retinol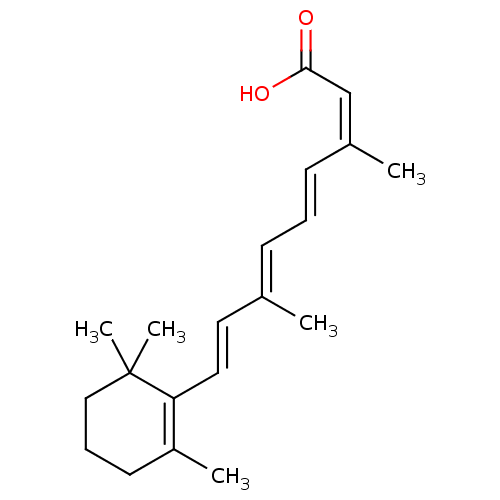 13-RA isotretinoin 13-cis-Vitamin A acid 13-cis-retinoic acid CHEMBL547 (2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid (7E,9E,11E,13Z)-retinoic acid Neovitamin A acid BDBM50031459
13-RA isotretinoin 13-cis-Vitamin A acid 13-cis-retinoic acid CHEMBL547 (2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid (7E,9E,11E,13Z)-retinoic acid Neovitamin A acid BDBM50031459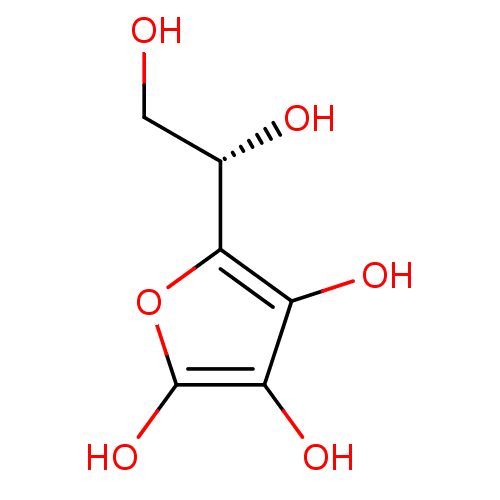 CHEMBL196 (R)-2-((S)-1,2-dihydroxyethyl)-4,5-dihydroxyfuran-3(2H)-one ascorbic acid 5-(1,2-Dihydroxy-ethyl)-3,4-dihydroxy-5H-furan-2-one(AsA) vitamin C (R)-5-((S)-1,2-Dihydroxy-ethyl)-3,4-dihydroxy-5H-furan-2-one (R)-5-((S)-1,2-dihydroxyethyl)-3,4-dihydroxyfuran-2(5H)-one BDBM50090256
CHEMBL196 (R)-2-((S)-1,2-dihydroxyethyl)-4,5-dihydroxyfuran-3(2H)-one ascorbic acid 5-(1,2-Dihydroxy-ethyl)-3,4-dihydroxy-5H-furan-2-one(AsA) vitamin C (R)-5-((S)-1,2-Dihydroxy-ethyl)-3,4-dihydroxy-5H-furan-2-one (R)-5-((S)-1,2-dihydroxyethyl)-3,4-dihydroxyfuran-2(5H)-one BDBM50090256
- Exploring 2-methyl-substituted vitamin K3 derivatives with potent inhibitory activity against the 3CL protease of SARS-CoV-2.
- Coelho Cerqueira, E; Netz, PA; Diniz, C; Petry do Canto, V; Follmer, C Molecular insights into human monoamine oxidase (MAO) inhibition by 1,4-naphthoquinone: evidences for menadione (vitamin K3) acting as a competitive and reversible inhibitor of MAO. Bioorg Med Chem 19: 7416-24 (2011)
- OKAMOTO, S; IBE, K VITAMIN D3-LIKE COMPOUND US Patent US20240317663 (2024)
- Cho, K; Uneuchi, F; Kato-Nakamura, Y; Namekawa, J; Ishizuka, S; Takenouchi, K; Nagasawa, K Structure-activity relationship studies on vitamin D lactam derivatives as vitamin D receptor antagonist. Bioorg Med Chem Lett 18: 4287-90 (2008)
- Nakabayashi, M; Yamada, S; Yoshimoto, N; Tanaka, T; Igarashi, M; Ikura, T; Ito, N; Makishima, M; Tokiwa, H; DeLuca, HF; Shimizu, M Crystal structures of rat vitamin D receptor bound to adamantyl vitamin D analogs: structural basis for vitamin D receptor antagonism and partial agonism. J Med Chem 51: 5320-9 (2008)
- Ryall, RP; Nandi, DL; Silverman, RB Substituted vitamin K epoxide analogues. New competitive inhibitors and substrates of vitamin K1 epoxide reductase. J Med Chem 33: 1790-7 (1990)
- Teske, KA; Bogart, JW; Sanchez, LM; Yu, OB; Preston, JV; Cook, JM; Silvaggi, NR; Bikle, DD; Arnold, LA Synthesis and evaluation of vitamin D receptor-mediated activities of cholesterol and vitamin D metabolites. Eur J Med Chem 109: 238-46 (2016)
- Kawagoe, F; Mendoza, A; Hayata, Y; Asano, L; Kotake, K; Mototani, S; Kawamura, S; Kurosaki, S; Akagi, Y; Takemoto, Y; Nagasawa, K; Nakagawa, H; Uesugi, M; Kittaka, A Discovery of a Vitamin D Receptor-Silent Vitamin D Derivative That Impairs Sterol Regulatory Element-Binding Protein In Vivo. J Med Chem 64: 5689-5709 (2021)
- Sidhu, PS; Nassif, N; McCallum, MM; Teske, K; Feleke, B; Yuan, NY; Nandhikonda, P; Cook, JM; Singh, RK; Bikle, DD; Arnold, LA Development of novel Vitamin D Receptor-Coactivator Inhibitors. ACS Med Chem Lett 5: 199-204 (2014)
- Yamamoto, K; Abe, D; Yoshimoto, N; Choi, M; Yamagishi, K; Tokiwa, H; Shimizu, M; Makishima, M; Yamada, S Vitamin D receptor: ligand recognition and allosteric network. J Med Chem 49: 1313-24 (2006)
- Kudo, T; Ishizawa, M; Maekawa, K; Nakabayashi, M; Watarai, Y; Uchida, H; Tokiwa, H; Ikura, T; Ito, N; Makishima, M; Yamada, S Combination of triple bond and adamantane ring on the vitamin D side chain produced partial agonists for vitamin D receptor. J Med Chem 57: 4073-87 (2014)
- Wang, B; Hao, M; Zhang, C Design, synthesis and biological evaluation of nonsecosteroidal vitamin D Bioorg Med Chem Lett 27: 1428-1436 (2017)
- Sasaki, H; Masuno, H; Kawasaki, H; Yoshihara, A; Numoto, N; Ito, N; Ishida, H; Yamamoto, K; Hirata, N; Kanda, Y; Kawachi, E; Kagechika, H; Tanatani, A Lithocholic Acid Derivatives as Potent Vitamin D Receptor Agonists. J Med Chem 64: 516-526 (2021)
- Lamblin, M; Dabbas, B; Spingarn, R; Mendoza-Sanchez, R; Wang, TT; An, BS; Huang, DC; Kremer, R; White, JH; Gleason, JL Vitamin D receptor agonist/histone deacetylase inhibitor molecular hybrids. Bioorg Med Chem 18: 4119-37 (2010)
- Hosoda, S; Tanatani, A; Wakabayashi, K; Nakano, Y; Miyachi, H; Nagasawa, K; Hashimoto, Y Ligands with dual vitamin D3-agonistic and androgen-antagonistic activities. Bioorg Med Chem Lett 15: 4327-31 (2005)
- Masuno, H; Kazui, Y; Tanatani, A; Fujii, S; Kawachi, E; Ikura, T; Ito, N; Yamamoto, K; Kagechika, H Development of novel lithocholic acid derivatives as vitamin D receptor agonists. Bioorg Med Chem 27: 3674-3681 (2019)
- Dodo, K; Takahashi, M; Yamada, Y; Sugimoto, Y; Hashimoto, Y; Shirai, R Synthesis of a novel class of cdc25A inhibitors from vitamin D3. Bioorg Med Chem Lett 10: 615-7 (2000)
- Gogoi, P; Seoane, S; Sigüeiro, R; Guiberteau, T; Maestro, MA; Pérez-Fernández, R; Rochel, N; Mouriño, A Aromatic-Based Design of Highly Active and Noncalcemic Vitamin D Receptor Agonists. J Med Chem 61: 4928-4937 (2018)
- SMITH, P; ZHANG, Z; PARKER, M; FIDGE, J JAK INHIBITOR WITH A VITAMIN D ANALOG FOR TREATMENT OF SKIN DISEASES US Patent US20250049817 (2025)
- Korytnyk, W; Angelino, N Vitamin B6 antagonists obtained by replacing or modifying the 2-methyl group. J Med Chem 20: 745-9 (1977)
- Anami, Y; Itoh, T; Egawa, D; Yoshimoto, N; Yamamoto, K A mixed population of antagonist and agonist binding conformers in a single crystal explains partial agonism against vitamin D receptor: active vitamin D analogues with 22R-alkyl group. J Med Chem 57: 4351-67 (2014)
- Montagut-Romans, A; Boulven, M; Jacolot, M; Moebs-Sanchez, S; Hascoët, C; Hammed, A; Besse, S; Lemaire, M; Benoit, E; Lattard, V; Popowycz, F Synthesis and biological evaluation of C-3 aliphatic coumarins as vitamin K antagonists. Bioorg Med Chem Lett 27: 1598-1601 (2017)
- Laverny, G; Penna, G; Uskokovic, M; Marczak, S; Maehr, H; Jankowski, P; Ceailles, C; Vouros, P; Smith, B; Robinson, M; Reddy, GS; Adorini, L Synthesis and anti-inflammatory properties of 1alpha,25-dihydroxy-16-ene-20-cyclopropyl-24-oxo-vitamin D3, a hypocalcemic, stable metabolite of 1alpha,25-dihydroxy-16-ene-20-cyclopropyl-vitamin D3. J Med Chem 52: 2204-13 (2009)
- Korytnyk, W; Potti, PG Antagonists of vitamin B6. Simultaneous and stepwise modification of the 2 and 4 positions. J Med Chem 20: 1-5 (1977)
- Shimazawa, R; Suzuki, T; Dodo, K; Shirai, R Design and synthesis of dysidiolide analogs from vitamin D3: novel class of Cdc25A inhibitors. Bioorg Med Chem Lett 14: 3291-4 (2004)
- Demizu, Y; Takahashi, T; Kaneko, F; Sato, Y; Okuda, H; Ochiai, E; Horie, K; Takagi, K; Kakuda, S; Takimoto-Kamimura, M; Kurihara, M Design, synthesis and X-ray crystallographic study of new nonsecosteroidal vitamin D receptor ligands. Bioorg Med Chem Lett 21: 6104-7 (2011)
- Li, X; Himes, RA; Prosser, LC; Christie, CF; Watt, E; Edwards, SF; Metcalf, CS; West, PJ; Wilcox, KS; Chan, SSL; Chou, CJ Discovery of the First Vitamin K Analogue as a Potential Treatment of Pharmacoresistant Seizures. J Med Chem 63: 5865-5878 (2020)
- Islam, I; Ng, KY; Chong, KT; McQuade, TJ; Hui, JO; Wilkinson, KF; Rush, BD; Ruwart, MJ; Borchardt, RT; Fisher, JF Evaluation of a vitamin-cloaking strategy for oligopeptide therapeutics: biotinylated HIV-1 protease inhibitors. J Med Chem 37: 293-304 (1994)
- Mita, Y; Dodo, K; Noguchi-Yachide, T; Miyachi, H; Makishima, M; Hashimoto, Y; Ishikawa, M LXXLL peptide mimetics as inhibitors of the interaction of vitamin D receptor with coactivators. Bioorg Med Chem Lett 20: 1712-7 (2010)
- Misawa, T; Tsuji, G; Takahashi, T; Ochiai, E; Takagi, KI; Horie, K; Kakuda, S; Takimoto-Kamimura, M; Kurihara, M; Demizu, Y Structural development of non-secosteroidal vitamin D receptor (VDR) ligands without any asymmetric carbon. Bioorg Med Chem 26: 6146-6152 (2018)
- Maschinot, CA; Chau, LQ; Wechsler-Reya, RJ; Hadden, MK Synthesis and evaluation of third generation vitamin D3 analogues as inhibitors of Hedgehog signaling. Eur J Med Chem 162: 495-506 (2019)
- Kato, A; Yamao, M; Hashihara, Y; Ishida, H; Itoh, T; Yamamoto, K Vitamin D Analogues with a p-Hydroxyphenyl Group at the C25 Position: Crystal Structure of Vitamin D Receptor Ligand-Binding Domain Complexed with the Ligand Explains the Mechanism Underlying Full Antagonistic Action. J Med Chem 60: 8394-8406 (2017)
- Chen, DU; Kuo, PY; Yang, DY Design and synthesis of novel diphenacoum-derived, conformation-restricted vitamin K 2,3-epoxide reductase inhibitors. Bioorg Med Chem Lett 15: 2665-8 (2005)
- Demizu, Y; Nagoya, S; Shirakawa, M; Kawamura, M; Yamagata, N; Sato, Y; Doi, M; Kurihara, M Development of stapled short helical peptides capable of inhibiting vitamin D receptor (VDR)-coactivator interactions. Bioorg Med Chem Lett 23: 4292-6 (2013)
- Yoshizawa, M; Itoh, T; Hori, T; Kato, A; Anami, Y; Yoshimoto, N; Yamamoto, K Identification of the Histidine Residue in Vitamin D Receptor That Covalently Binds to Electrophilic Ligands. J Med Chem 61: 6339-6349 (2018)
- Misawa, T; Demizu, Y; Kawamura, M; Yamagata, N; Kurihara, M Structural development of stapled short helical peptides as vitamin D receptor (VDR)-coactivator interaction inhibitors. Bioorg Med Chem 23: 1055-61 (2015)
- Belorusova, AY; Martínez, A; Gándara, Z; Gómez, G; Fall, Y; Rochel, N Structure-activity relationship study of vitamin D analogs with oxolane group in their side chain. Eur J Med Chem 134: 86-96 (2017)
- Taban, IM; Zhu, J; DeLuca, HF; Simons, C Analysis of the binding sites of vitamin D 1α-hydroxylase (CYP27B1) and vitamin D 24-hydroxylase (CYP24A1) for the design of selective CYP24A1 inhibitors: Homology modelling, molecular dynamics simulations and identification of key binding requirements. Bioorg Med Chem 25: 5629-5636 (2017)
- Silverman, RB; Oliver, JS 2-(Fluoromethyl)-3-phytyl-1,4-naphthoquinone and its 2,3-epoxide. Inhibition of vitamin K epoxide reductase. J Med Chem 32: 2138-41 (1989)
- Ciesielski, F; Sato, Y; Chebaro, Y; Moras, D; Dejaegere, A; Rochel, N Structural basis for the accommodation of bis- and tris-aromatic derivatives in vitamin D nuclear receptor. J Med Chem 55: 8440-9 (2012)
- Lin, Z; Marepally, SR; Ma, D; Kim, TK; Oak, AS; Myers, LK; Tuckey, RC; Slominski, AT; Miller, DD; Li, W Synthesis and Biological Evaluation of Vitamin D3 Metabolite 20S,23S-Dihydroxyvitamin D3 and Its 23R Epimer. J Med Chem 59: 5102-8 (2016)
- Arichi, N; Fujiwara, S; Ishizawa, M; Makishima, M; Hua, DH; Yamada, KI; Yamaoka, Y; Takasu, K Synthesis and biological evaluation of steroidal derivatives bearing a small ring as vitamin D receptor agonists. Bioorg Med Chem Lett 27: 3408-3411 (2017)
- Chen, B; Kawai, M; Wu-Wong, JR Synthesis of VS-105: A novel and potent vitamin D receptor agonist with reduced hypercalcemic effects. Bioorg Med Chem Lett 23: 5949-52 (2013)
- Sánchez-Abella, L; Fernández, S; Verstuyf, A; Verlinden, L; Gotor, V; Ferrero, M Synthesis, conformational analysis, and biological evaluation of 19-nor-vitamin D3 analogues with A-ring modifications. J Med Chem 52: 6158-62 (2009)
- Yoshimoto, N; Sakamaki, Y; Haeta, M; Kato, A; Inaba, Y; Itoh, T; Nakabayashi, M; Ito, N; Yamamoto, K Butyl pocket formation in the vitamin D receptor strongly affects the agonistic or antagonistic behavior of ligands. J Med Chem 55: 4373-81 (2012)
- Hao, M; Hou, S; Xue, L; Yuan, H; Zhu, L; Wang, C; Wang, B; Tang, C; Zhang, C Further Developments of the Phenyl-Pyrrolyl Pentane Series of Nonsteroidal Vitamin D Receptor Modulators as Anticancer Agents. J Med Chem 61: 3059-3075 (2018)
- Boehm, MF; Fitzgerald, P; Zou, A; Elgort, MG; Bischoff, ED; Mere, L; Mais, DE; Bissonnette, RP; Heyman, RA; Nadzan, AM; Reichman, M; Allegretto, EA Novel nonsecosteroidal vitamin D mimics exert VDR-modulating activities with less calcium mobilization than 1,25-dihydroxyvitamin D3. Chem Biol 6: 265-75 (1999)
- Matsuo, M; Hasegawa, A; Takano, M; Saito, H; Kakuda, S; Chida, T; Takagi, K; Ochiai, E; Horie, K; Harada, Y; Takimoto-Kamimura, M; Takenouchi, K; Sawada, D; Kittaka, A Synthesis of 2α-heteroarylalkyl active vitamin d3 with therapeutic effect on enhancing bone mineral density in vivo. ACS Med Chem Lett 4: 671-4 (2013)
- Korytnyk, W; Potti, PG 4-Halovinyl- and 4-ethynyl-4-deformylpyridoxal derivatives and related analogues as potentially irreversible antagonists of vitamin B6. J Med Chem 20: 567-72 (1977)
- Lamblin, M; Spingarn, R; Wang, TT; Burger, MC; Dabbas, B; Moitessier, N; White, JH; Gleason, JL An o-aminoanilide analogue of 1a,25-dihydroxyvitamin D(3) functions as a strong vitamin D receptor antagonist. J Med Chem 53: 7461-5 (2010)
- Nandhikonda, P; Lynt, WZ; McCallum, MM; Ara, T; Baranowski, AM; Yuan, NY; Pearson, D; Bikle, DD; Guy, RK; Arnold, LA Discovery of the first irreversible small molecule inhibitors of the interaction between the vitamin D receptor and coactivators. J Med Chem 55: 4640-51 (2012)
- Hammed, A; Matagrin, B; Spohn, G; Prouillac, C; Benoit, E; Lattard, V VKORC1L1, an enzyme rescuing the vitamin K 2,3-epoxide reductase activity in some extrahepatic tissues during anticoagulation therapy. J Biol Chem 288: 28733-42 (2013)
- Inaba, Y; Yoshimoto, N; Sakamaki, Y; Nakabayashi, M; Ikura, T; Tamamura, H; Ito, N; Shimizu, M; Yamamoto, K A new class of vitamin D analogues that induce structural rearrangement of the ligand-binding pocket of the receptor. J Med Chem 52: 1438-49 (2010)
- Kang, Z; Wang, C; Tong, Y; Li, Y; Gao, Y; Hou, S; Hao, M; Han, X; Wang, B; Wang, Q; Zhang, C Novel Nonsecosteroidal Vitamin D Receptor Modulator Combined with Gemcitabine Enhances Pancreatic Cancer Therapy through Remodeling of the Tumor Microenvironment. J Med Chem 64: 629-643 (2021)
- Tocchini-Valentini, G; Rochel, N; Wurtz, JM; Moras, D Crystal structures of the vitamin D nuclear receptor liganded with the vitamin D side chain analogues calcipotriol and seocalcitol, receptor agonists of clinical importance. Insights into a structural basis for the switching of calcipotriol to a receptor antagonist by further side chain modificatio J Med Chem 47: 1956-61 (2004)
- Misawa, T; Yorioka, M; Demizu, Y; Noguchi-Yachide, T; Ohoka, N; Kurashima-Kinoshita, M; Motoyoshi, H; Nojiri, H; Kittaka, A; Makishima, M; Naito, M; Kurihara, M Effects of alkyl side chains and terminal hydrophilicity on vitamin D receptor (VDR) agonistic activity based on the diphenylpentane skeleton. Bioorg Med Chem Lett 25: 5362-6 (2015)
- Mita, Y; Dodo, K; Noguchi-Yachide, T; Hashimoto, Y; Ishikawa, M Structure-activity relationship of benzodiazepine derivatives as LXXLL peptide mimetics that inhibit the interaction of vitamin D receptor with coactivators. Bioorg Med Chem 21: 993-1005 (2013)
- Belorusova, AY; Eberhardt, J; Potier, N; Stote, RH; Dejaegere, A; Rochel, N Structural insights into the molecular mechanism of vitamin D receptor activation by lithocholic acid involving a new mode of ligand recognition. J Med Chem 57: 4710-9 (2014)
- Grzywacz, P; Plum, LA; Clagett-Dame, M; Deluca, HF 26- and 27-Methyl groups of 2-substituted, 19-nor-1a,25-dihydroxylated vitamin D compounds are essential for calcium mobilization in vivo. Bioorg Chem 47: 9-16 (2013)
- Zhao, L; Jin, C; Mao, Z; Gopinathan, MB; Rehder, K; Brinton, RD Design, synthesis, and estrogenic activity of a novel estrogen receptor modulator--a hybrid structure of 17beta-estradiol and vitamin E in hippocampal neurons. J Med Chem 50: 4471-81 (2007)
- Kang, ZS; Wang, C; Han, XL; Wang, B; Yuan, HL; Hou, SY; Hao, MX; Du, JJ; Li, YY; Zhou, AW; Zhang, C Sulfonyl-containing phenyl-pyrrolyl pentane analogues: Novel non-secosteroidal vitamin D receptor modulators with favorable physicochemical properties, pharmacokinetic properties and anti-tumor activity. Eur J Med Chem 157: 1174-1191 (2018)
- Anami, Y; Sakamaki, Y; Itoh, T; Inaba, Y; Nakabayashi, M; Ikura, T; Ito, N; Yamamoto, K Fine tuning of agonistic/antagonistic activity for vitamin D receptor by 22-alkyl chain length of ligands: 22S-Hexyl compound unexpectedly restored agonistic activity. Bioorg Med Chem 23: 7274-81 (2015)
- Chatron, N; Boulven, M; Montagut-Romans, A; Ponsot, F; Jacolot, M; Caruel, H; Benoît, E; Popowycz, F; Lattard, V Design of a structure-activity relationship model of vitamin K epoxide reductase (VKORC1) inhibitors combining chemical synthesis of new compounds, enzymatic assays and molecular modelling. Bioorg Med Chem 94: (2023)
- Neukirch, K; Alsabil, K; Dinh, CP; Bilancia, R; Raasch, M; Ville, A; Cerqua, I; Viault, G; Bréard, D; Pace, S; Temml, V; Brunner, E; Jordan, PM; Marques, MC; Loeser, K; Gollowitzer, A; Permann, S; Gerstmeier, J; Lorkowski, S; Stuppner, H; Garscha, U; Rodrigues, T; Bernardes, GJL; Schuster, D; Séraphin, D; Richomme, P; Rossi, A; Mosig, AS; Roviezzo, F; Werz, O; Helesbeux, JJ; Koeberle, A Exploration of Long-Chain Vitamin E Metabolites for the Discovery of a Highly Potent, Orally Effective, and Metabolically Stable 5-LOX Inhibitor that Limits Inflammation. J Med Chem 64: 11496-11526 (2021)
- Saito, N; Matsunaga, T; Saito, H; Anzai, M; Takenouchi, K; Miura, D; Namekawa, J; Ishizuka, S; Kittaka, A Further synthetic and biological studies on vitamin D hormone antagonists based on C24-alkylation and C2alpha-functionalization of 25-dehydro-1alpha-hydroxyvitamin D(3)-26,23-lactones. J Med Chem 49: 7063-75 (2006)
- Calverley, MJ; Binderup, L Synthesis and biological evaluation of MC 1357, a new 20-epi-23-oxa-1α,25-dihydroxy-vitamin D3 analogue with potent non-classical effects Bioorg Med Chem Lett 3: 1845-1848 (1993)
- ChEMBL_305987 (CHEMBL832974) Inhibitory concentration against beef liver Vitamin K epoxide reductase (vitamin K 2,3-epoxide reductase)
- ChEBML_214476 Binding affinity for vitamin K epoxide reductase
- ChEMBL_987815 (CHEMBL2437763) Inhibition of human vitamin D3 receptor
- ChEBML_214470 Apparent kinetic constant for Vitamin K epoxide reductase
- ChEMBL_622880 (CHEMBL1116619) Binding affinity to human vitamin D binding protein
- ChEBML_214154 Displacement from vitamin D receptor in chick intestine: 50% displacement
- ChEMBL_214322 (CHEMBL818350) Effective concentration required for inhibition of Vitamin D3 receptor
- ChEMBL_214155 (CHEMBL818834) Displacement from vitamin D receptor in chick intestine: 50% displacement
- ChEMBL_2260044 (CHEMBL5215055) Agonist activity at Vitamin D receptor (unknown origin) by fluorescence polarization assay
- ChEMBL_514897 (CHEMBL972705) Displacement of [3H]1,25-(OH)2D3 from bovine thymus vitamin D receptor
- ChEMBL_428140 (CHEMBL915778) Displacement of [3H]1,25-(OH)2D3 from vitamin D receptor in bovine thymus
- ChEBML_214183 In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]1
- ChEMBL_214183 (CHEMBL820132) In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]1
- ChEMBL_1657647 (CHEMBL4007117) Inhibition of VKORC1 in rat liver microsomes in presence of 0.003 to 0.2 mM vitamin K
- ChEMBL_412322 (CHEMBL910037) Antagonist activity against 1-alpha,25-dihydroxy vitamin D3-induced HL60 cell differentiation by NBT reduction method
- ChEMBL_1505946 (CHEMBL3595548) Displacement of [3H]-1alpha25-(OH)2D3 from full length recombinant rat vitamin D receptor by scintillation counting analysis
- ChEMBL_2322206 Agonist activity at human Vitamin D receptor assessed as increase in gene transcriptional activity by luciferase reporter gene based assay
- ChEBML_35365 Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equation Ki[k4/(k3 + k4)]
- ChEMBL_1993119 (CHEMBL4626854) Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell voltage clamp method
- ChEMBL_674300 (CHEMBL1274500) Vitamin-D activity at VDR receptor in human HL60 cells assessed as induction of cell differentiation by NBT reduction assay
- ChEMBL_35361 (CHEMBL647943) Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equation Ki[k4/(k3 + k4)]
- ChEMBL_35365 (CHEMBL647946) Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equation Ki[k4/(k3 + k4)]
- ChEMBL_98518 (CHEMBL706539) Effect of inhibitor structure on the slow binding inhibition of Leucine aminopeptidase was determined and Ki* was reported which is obtained by the equation Ki[k4/(k3 + k4)]
- ChEMBL_98522 (CHEMBL706542) Effect of inhibitor structure on the slow binding inhibition of Leucine aminopeptidase was determined and Ki* was reported which is obtained by the equation Ki[k4/(k3 + k4)]
- ChEMBL_1545531 (CHEMBL3749446) Binding affinity to vitamin D3 receptor (unknown origin) after 4 hrs using fluormone VDR red by polar screen VDR competitor assay
- ChEMBL_2322205 Agonist activity at Vitamin D receptor (unknown origin) assessed as increase in fluorescein labeled TRAP220/DRIP-2 coactivator peptide requirement by TR-FRET assay
- ChEMBL_830134 (CHEMBL2061944) Displacement of [3H]1alpha,25-dihydroxyvitamin D3 from human recombinant GST-tagged vitamin D3 receptor LBD expressed in Escherichia coli BL21 after 16 hrs
- ChEMBL_1857759 (CHEMBL4358488) Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HTRF assay
- ChEMBL_2014666 (CHEMBL4668244) Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by immunoblot analysis
- ChEMBL_1545532 (CHEMBL3749447) Agonist activity at vitamin D3 receptor in human HL-60 cells assessed as induction of cell differentiation after 96 hrs by NBT dye-based microscopic analysis
- ChEMBL_1670487 (CHEMBL4020375) Antagonist activity at human vitamin D receptor expressed in HEK293 cells assessed as inhibition of 1,25D3-induced transactivation after 16 hrs by dual luciferase reporter gene assay
- ChEMBL_1670491 (CHEMBL4020379) Displacement of [3H]-1,25-(OH)2D3 from N-terminal GST-tagged human recombinant vitamin D receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) pLys S after 16 hrs
- ChEMBL_1670790 (CHEMBL4020678) Binding affinity to recombinant human GST-tagged VDR LBD (156 to 453 residues) expressed in Echerichia coli BL21 star (DE3) after 40 mins in presence of vitamin D3 by fluorescence polarization assay
- ChEBML_1686154 Agonist activity at vitamin D receptor (unknown origin) expressed in HEK293 cells co-expressing pCMX-RXRalpha and pCMX-beta-galactosidase assessed as induction of receptor transactivation incubated for 16 to 24 hrs by luciferase reporter gene assay
- ChEMBL_2224380 (CHEMBL5137893) Displacement of 25-hydroxy-[26,27-methyl-3H]-vitamin D3 from CYP24 in 1,25(OH)2D3-treated human DU-145 cells using 25-(OH)D3 as substrate pretreated with 1,25(OH)2D3 for 24 hrs followed by compound addition measured after 30 mins by HPLC analysis
- Inhibition Assay Reader: Synergy HT program: tyrosinase 280-490 kinetics: kinetics over 45 minutes, reading at t=10 minutes, Tests in transparent 96-well plates, Phosphate buffer (pH 6.8), Enzyme: mushroom tyrosinase (T-3824, Sigma), Substrate: L-tyrosine (T-3754, Sigma), Positive control: Kojic acid (KA) (60890, Fluka) (reference inhibitor). Reference Molecules for the Test: Kojic acid: 9 μM
- Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Vitamin D Receptor (VDR) Coactivator Assay A VDR ligand binding domain tagged with GST (VDR-LBD(GST)), a fluorescein-TRAP220/DRIP-2-bound coactivator peptide (Fluorescein-peptide), LanthaScreen Tb-anti-GST (Goat) antibody (Tb-anti-GST), TR-FRET co-regulator buffer G, and DTT solution were used from LanthaScreen TR-FRET VDR Coactivator Assay Kit, which was purchased from Invitrogen. Each of the compounds synthesized as described above was dissolved in dimethyl sulfoxide (DMSO for molecular biology, Sigma Aldrich). The solution was diluted to a desired concentration with TR-FRET co-regulator buffer G containing 1 mass % of DMSO. The receptor-tracer-antibody complex solution was added to the compound solution in each well (20 μL) such that each well contained 1.0 nM of VDR-LBD(GST), 2.0 nM of Tb-anti-GST, and 100 nM of Fluorescein-peptide. The resulting mixture was incubated at room temperature for 2 hours. Each well was measured for TR-FRET using a microplate reader (Infinite F200 PRO, Tecan) equipped with an excitation filter at 340 nm (30 nm bandwidth), a terbium emission filter at 495 nm (10 nm bandwidth), and a tracer emission filter at 520 nm (25 nm bandwidth). On the basis of the resulting data, the 50% effective concentration (EC50) of each compound was calculated and evaluated using a graph plotting program (GraphPad Prism ver. 8.2.0) with the saturated activity of natural active vitamin D3 being normalized to 100%.
- Cell-Based Assay The ability of representative disclosed to block influenza infection was assessed in a cell-based assay using A549 human epithelial cells (ATCC CCL-185). Briefly, a spatial infection model for testing drug efficacy and influenza replication was adapted from Lam et al. (Biotechnol Bioeng. (2005) 90(7):793-804). A549 cells were plated on Lab-Tek two well Permanox slides (Nalge Nunc International, Rochester, N.Y.) and allowed to become nearly confluent during an overnight incubation. Culture media used in these experiments was as described above, i.e. in DMEM supplemented with 5% fetal bovine serum, 100 U/ml penicillin, and 100 ug/ml streptomycin. Following overnight incubation, the cells were then washed three times with PBS, and 2 ml of a binary mixture of 2% agar and 2xMEM (with 100 U/ml penicillin, 100 ug/ml streptomycin, 2 mM L-glutamine, and 1xMEM vitamin solution (e.g. Gibco Cat. No. 111120 for 100x solution) was added to the cells.
- Inhibition Assay PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood were used as a source of PDE3 enzyme. 10 mL blood collected in a vacutainer tube (containing K3 EDTA) and centrifuged at 190xg for 15 min at room temperature. Top layer (platelet rich plasma) collected, centrifuged at 2500 g for 5 min at 22° C. (Room temp.) The pellet was washed with 2 ml of 50 mM tris buffer (pH-7.4) containing 1 mM MgCl2 and centrifuged at 2500 g for 5 min. Then 200 μl of assay buffer (from PDE kit, Enzo Life Sciences) was added to the pellet and sonicated at 30 s per ml. Pellet was freeze thawed for three times (-80° C.) in order to rupture the platelet membrane and release the PDE enzyme. Then the cell homogenate was centrifuged at 2500 rpm for 5 min. Supernatant was collected and used as source for PDE3 enzyme. In 96 well plate (Prod. No. BML-KI101), we added supernatant having PDE3 enzyme, PDE3 assay buffer, cAMP substrate, 5' nucleotidase and test or standard compound and incubated for 1 hour at 37° C. The reaction was arrested by the addition 100 μl BIOMOL GREEN reagent incubated in room temp for 20 min.
- PDE3 Inhibition Assay PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood were used as a source of PDE3 enzyme. 10 mL blood collected in a vacutainer tube (containing K3 EDTA) and centrifuged at 190xg for 15 min at room temperature. Top layer (platelet rich plasma) collected, centrifuged at 2500g for 5 min at 22° C. (Room temp.) The pellet was washed with 2 ml of 50 mM tris buffer (pH-7.4) containing 1 mM MgCl2 and centrifuged at 2500 g for 5 min. Then 200 ul of assay buffer (from PDE kit, Enzo Life Sciences) was added to the pellet and sonicated at 30 s per ml. Pellet was freeze thawed for three times (-80° C.) in order to rupture the platelet membrane and release the PDE enzyme. Then the cell homogenate was centrifuged at 2500 rpm for 5 min. Supernatant was collected and used as source for PDE3 enzyme. In 96 well plate (Prod. No. BML-KI101), we added supernatant having PDE3 enzyme, PDE3 assay buffer, cAMP substrate, 5'nucleotidase and test or standard compound and incubated for 1 hour at 37° C. The reaction was arrested by the addition 100 ul BIOMOL GREEN reagent incubated in room temp for 20 min. The green color developed was measured at 620 nm.
- Cellular Assay Alternatively, the present compounds might also be assessed for their capacity to inhibit Btk-dependent FcG receptor-induced IL-8 secretion in human cells. The human myeloid leukemia THP1 cell line (ATCC TIB202) was grown in RPMI 1640 medium supplemented with 10% FCS and 15 nM 1, 25-dihydroxy Vitamin D3 during 4 days before use to induced myeloid differentiation. A sufficient number of tissue-culture grade 384-well plates was coated with human IgG of unknown specificity by incubating overnight at 4° C. with 40 ul/well of a 50 ug/ml IgG solution in PBS. On the day of the experiment, plates were washed 5 times with 80 ul water on a Molecular Devices Aquamax DW4 plate washer. Solutions of the test compounds in 90% DMSO were added to each well on a Hamilton Microlab Star liquid handling station to 40 ul/well tissue culture medium and the total DMSO concentration was adjusted to 0.1%. Differentiated THP1 cells were then added in 40 ul/well to reach a final density of 5'000 cells/well in 80 ul culture medium. After 24 hours, IL-8 secretion was measured in the supernatant by the IL-8 HTRF assay following the protocol of the vendor (CisBio international).
 2-methyl-1,4-naphthoquinone, 5 Menadione (Vitamin K3) 2-methyl-1,4-dihydronaphthalene-1,4-dione CHEMBL590 Menadione BDBM24778 cid_4055 Menadione (5d) Vitamin K3 Menadione, 9
2-methyl-1,4-naphthoquinone, 5 Menadione (Vitamin K3) 2-methyl-1,4-dihydronaphthalene-1,4-dione CHEMBL590 Menadione BDBM24778 cid_4055 Menadione (5d) Vitamin K3 Menadione, 9 US9365563, K3 BDBM236151 US9878991, Compound K3
US9365563, K3 BDBM236151 US9878991, Compound K3 US11220518, Ex. No. K3 BDBM286345 US11780853, Example K3 US9518060, Example K3
US11220518, Ex. No. K3 BDBM286345 US11780853, Example K3 US9518060, Example K3 US9416126, K3 BDBM239345
US9416126, K3 BDBM239345 BDBM365726 US9868748, I-K3
BDBM365726 US9868748, I-K3 US20250188030, Compound K3 BDBM748592
US20250188030, Compound K3 BDBM748592 Vitamin D analog, 10 BDBM93065 Vitamin D analog, 14
Vitamin D analog, 10 BDBM93065 Vitamin D analog, 14 Vitamin D analog, 11 BDBM93062 Vitamin D analog, 15
Vitamin D analog, 11 BDBM93062 Vitamin D analog, 15 Vitamin D analog, 12 BDBM93066 Vitamin D analog, 16
Vitamin D analog, 12 BDBM93066 Vitamin D analog, 16 Vitamin D analog, 17 BDBM93067 Vitamin D analog, 13
Vitamin D analog, 17 BDBM93067 Vitamin D analog, 13 BDBM218904 US9303033, K3, Table 12A, Compound 6
BDBM218904 US9303033, K3, Table 12A, Compound 6 BDBM512999 Vitamin B12
BDBM512999 Vitamin B12 Vitamin B12 BDBM420313
Vitamin B12 BDBM420313 CHEBI:28940 Vitamin D 3 Vitamin D Cholecalciferol 7-Dehydrocholesterol BDBM50030475 Colecalciferol Dihydrocholesterol
CHEBI:28940 Vitamin D 3 Vitamin D Cholecalciferol 7-Dehydrocholesterol BDBM50030475 Colecalciferol Dihydrocholesterol BDBM93061 Vitamin D analog, 6
BDBM93061 Vitamin D analog, 6 BDBM93063 Vitamin D analog, 8
BDBM93063 Vitamin D analog, 8 BDBM93064 Vitamin D analog, 9
BDBM93064 Vitamin D analog, 9 BDBM93068 Vitamin D analog, 14
BDBM93068 Vitamin D analog, 14 BDBM93069 Vitamin D analog, 15
BDBM93069 Vitamin D analog, 15 BDBM93070 Vitamin D analog, 16
BDBM93070 Vitamin D analog, 16 BDBM93071 Vitamin D analog, 17
BDBM93071 Vitamin D analog, 17 BDBM93072 Vitamin D analog, 7
BDBM93072 Vitamin D analog, 7 CYANOCOBALAMIN Cobalamin SMR001233181 RUVITE MLS002153809 vitamin B12 cid_25102581 BDBM83973
CYANOCOBALAMIN Cobalamin SMR001233181 RUVITE MLS002153809 vitamin B12 cid_25102581 BDBM83973 Folic Acid Folvite Pteroylglutamic Acid CHEBI:27470 Natur Flow Folicet Folicare Vitamin Bc Roche Folacin Lexpec BDBM50237629 Preconceive Vitamin M Bio Science Folsan
Folic Acid Folvite Pteroylglutamic Acid CHEBI:27470 Natur Flow Folicet Folicare Vitamin Bc Roche Folacin Lexpec BDBM50237629 Preconceive Vitamin M Bio Science Folsan CHEBI:16709 Pyridoxine Hexa-Betalin M.V.I.-12 BDBM50103505 Vitamin B6
CHEBI:16709 Pyridoxine Hexa-Betalin M.V.I.-12 BDBM50103505 Vitamin B6 CHEMBL494338 BDBM50257431 1,25-dihydroxy-16-ene-20-cyclopropyl-vitamin D3
CHEMBL494338 BDBM50257431 1,25-dihydroxy-16-ene-20-cyclopropyl-vitamin D3 Folvite BDBM50367343 Folacin FOLIC ACID Vitamin B9 (2S)-2-[[4-[(2-amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid Vitamin M
Folvite BDBM50367343 Folacin FOLIC ACID Vitamin B9 (2S)-2-[[4-[(2-amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid Vitamin M Alpha Tocopherol BDBM50458513 Aquasol E E307 Vitamin E CHEBI:18145 Tocopherol
Alpha Tocopherol BDBM50458513 Aquasol E E307 Vitamin E CHEBI:18145 Tocopherol Phytomenadione Konakion mm BDBM50553259 Konakion mm paed Orakay Vitamin k1 Mephyton Kanavit NSC-270681 Aquamephyton Neokay Vitamin K 1 CHEBI:18067 Phylloquinone Phylloquinone e-form Konakion Phytonadione
Phytomenadione Konakion mm BDBM50553259 Konakion mm paed Orakay Vitamin k1 Mephyton Kanavit NSC-270681 Aquamephyton Neokay Vitamin K 1 CHEBI:18067 Phylloquinone Phylloquinone e-form Konakion Phytonadione CHEMBL220846 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3 BDBM50205234
CHEMBL220846 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3 BDBM50205234 CHEMBL222195 BDBM50205233 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3
CHEMBL222195 BDBM50205233 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3 CHEMBL375811 BDBM50205235 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3
CHEMBL375811 BDBM50205235 (20S,22R)-22-methyl-1-alpha-25-dihydroxy-vitamin D3 Viosterol in Oil Vitamin D2 Osto-D2 Drisdol Ergoral D2 Uvesterol D BDBM50247883 Caltrate Ergocalciferol Oleovitamin D, Synthetic Oleovitamin D D-Forte Sterogyl Sterogyl 15H Vitamin D 2 Ergo-D2 Calciferol Vitamin D Eciferol D2 Deltalin Calciferol In Arach Oil Lanes Sterogyl-15 CHEBI:28934
Viosterol in Oil Vitamin D2 Osto-D2 Drisdol Ergoral D2 Uvesterol D BDBM50247883 Caltrate Ergocalciferol Oleovitamin D, Synthetic Oleovitamin D D-Forte Sterogyl Sterogyl 15H Vitamin D 2 Ergo-D2 Calciferol Vitamin D Eciferol D2 Deltalin Calciferol In Arach Oil Lanes Sterogyl-15 CHEBI:28934 BDBM50373877 THIAMINE (VIT B1) Betaxin ThOH CA inhibitor, 3 Vitamin B 1 Thiamine
BDBM50373877 THIAMINE (VIT B1) Betaxin ThOH CA inhibitor, 3 Vitamin B 1 Thiamine Vitamin B 5 CHEBI:46905 Cantopal BDBM50240040 Calc Pantoth Pantothenic Acid Pantothenic acid
Vitamin B 5 CHEBI:46905 Cantopal BDBM50240040 Calc Pantoth Pantothenic Acid Pantothenic acid  CHEMBL492749 BDBM50293274 1alpha,25(OH)2-24-oxo-16-ene-20-cyclopropyl-vitamin D3
CHEMBL492749 BDBM50293274 1alpha,25(OH)2-24-oxo-16-ene-20-cyclopropyl-vitamin D3 all-trans retinoic acid TRETINOIN MLS000028588 [3H]RA Vitamin A acid ALL-TRANS-RETINOIC ACID [3H]Retinoic acid cid_444795 9-cis-retinoic acid (9cRA) BDBM31883 AT-RA Atralin CHEMBL38 SMR000058245 [3H]tretinoin [3H]Vitamin A acid
all-trans retinoic acid TRETINOIN MLS000028588 [3H]RA Vitamin A acid ALL-TRANS-RETINOIC ACID [3H]Retinoic acid cid_444795 9-cis-retinoic acid (9cRA) BDBM31883 AT-RA Atralin CHEMBL38 SMR000058245 [3H]tretinoin [3H]Vitamin A acid BDBM751371 8-(2-chloro-4-(2- (piperazin-1- yl)ethoxy)phenyl)- 9-((5- chloropyridin-2- yl)methyl)-6-(1- methylcyclopropoxy)- 9H-purine US20250195530, Example K3
BDBM751371 8-(2-chloro-4-(2- (piperazin-1- yl)ethoxy)phenyl)- 9-((5- chloropyridin-2- yl)methyl)-6-(1- methylcyclopropoxy)- 9H-purine US20250195530, Example K3 2-methyl-3-[(2E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-yl]-1,4-dihydronaphthalene-1,4-dione vitamin K1, 11 Phylloquinone BDBM24782 Phytonadione
2-methyl-3-[(2E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-yl]-1,4-dihydronaphthalene-1,4-dione vitamin K1, 11 Phylloquinone BDBM24782 Phytonadione BDBM50322752 CHEMBL540943 kizuta cirensenoside O saponin K3 3beta,23-dihydroxyolean-12-en-28-oic acid O-alpha-L-rhamnopyranosyl-(1->4)-O-beta-D-glucopyranosy1-(1->6)-beta-D-glucopyranosy1 ester
BDBM50322752 CHEMBL540943 kizuta cirensenoside O saponin K3 3beta,23-dihydroxyolean-12-en-28-oic acid O-alpha-L-rhamnopyranosyl-(1->4)-O-beta-D-glucopyranosy1-(1->6)-beta-D-glucopyranosy1 ester Alphalin all-trans-retinyl alcohol all-trans-vitamin A alcohol CHEMBL986 Afaxin all-trans-retinol Chocola A (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol BDBM50092056 Retinol
Alphalin all-trans-retinyl alcohol all-trans-vitamin A alcohol CHEMBL986 Afaxin all-trans-retinol Chocola A (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol BDBM50092056 Retinol 13-RA isotretinoin 13-cis-Vitamin A acid 13-cis-retinoic acid CHEMBL547 (2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid (7E,9E,11E,13Z)-retinoic acid Neovitamin A acid BDBM50031459
13-RA isotretinoin 13-cis-Vitamin A acid 13-cis-retinoic acid CHEMBL547 (2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid (7E,9E,11E,13Z)-retinoic acid Neovitamin A acid BDBM50031459 CHEMBL196 (R)-2-((S)-1,2-dihydroxyethyl)-4,5-dihydroxyfuran-3(2H)-one ascorbic acid 5-(1,2-Dihydroxy-ethyl)-3,4-dihydroxy-5H-furan-2-one(AsA) vitamin C (R)-5-((S)-1,2-Dihydroxy-ethyl)-3,4-dihydroxy-5H-furan-2-one (R)-5-((S)-1,2-dihydroxyethyl)-3,4-dihydroxyfuran-2(5H)-one BDBM50090256
CHEMBL196 (R)-2-((S)-1,2-dihydroxyethyl)-4,5-dihydroxyfuran-3(2H)-one ascorbic acid 5-(1,2-Dihydroxy-ethyl)-3,4-dihydroxy-5H-furan-2-one(AsA) vitamin C (R)-5-((S)-1,2-Dihydroxy-ethyl)-3,4-dihydroxy-5H-furan-2-one (R)-5-((S)-1,2-dihydroxyethyl)-3,4-dihydroxyfuran-2(5H)-one BDBM50090256