Target (1)
Compound (146)
Article Title (27)
Article Author (2)
Assay (44)
Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: in vitro studies. Brown, BS; Keddy, R; Perner, RJ; DiDomenico, S; Koenig, JR; Jinkerson, TK; Hannick, SM; McDonald, HA; Bianchi, BR; Honore, P; Puttfarcken, PS; Moreland, RB; Marsh, KC; Faltynek, CR; Lee, CH Discovery of TRPV1 antagonist ABT-116. Bioorg Med Chem Lett 20: 3291 -4 (2010) Curtin, ML; Florjancic, AS; Heyman, HR; Michaelides, MR; Garland, RB; Holms, JH; Steinman, DH; Dellaria, JF; Gong, J; Wada, CK; Guo, Y; Elmore, IB; Tapang, P; Albert, DH; Magoc, TJ; Marcotte, PA; Bouska, JJ; Goodfellow, CL; Bauch, JL; Marsh, KC; Morgan, DW; Davidsen, SK Discovery and characterization of the potent, selective and orally bioavailable MMP inhibitor ABT-770. Bioorg Med Chem Lett 11: 1557 -60 (2001) In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). Clark, MJ; Wright, T; Bertrand, PP; Bornstein, JC; Jenkinson, KM; Verlinden, M; Furness, JB Erythromycin derivatives ABT 229 and GM 611 act on motilin receptors in the rabbit duodenum. Clin Exp Pharmacol Physiol 26: 242 -5 (1999) Sham, HL; Betebenner, DA; Herrin, T; Kumar, G; Saldivar, A; Vasavanonda, S; Molla, A; Kempf, DJ; Plattner, JJ; Norbeck, DW Synthesis and antiviral activities of the major metabolites of the HIV protease inhibitor ABT-378 (Lopinavir). Bioorg Med Chem Lett 11: 1351 -3 (2001) Zhang, CX; Ge, ZM; Cheng, TM; Li, RT Synthesis and analgesic activity of secondary amine analogues of pyridylmethylamine and positional isomeric analogues of ABT-594. Bioorg Med Chem Lett 16: 2013 -6 (2006) Jantos, K; Kling, A; Mack, H; Hornberger, W; Moeller, A; Nimmrich, V; Lao, Y; Nijsen, M Discovery of ABT-957: 1-Benzyl-5-oxopyrrolidine-2-carboxamides as selective calpain inhibitors with enhanced metabolic stability. Bioorg Med Chem Lett 29: 1968 -1973 (2019) Zhang, Q; Xia, Z; Joshi, S; Scott, VE; Jarvis, MF Optimization of ADME Properties for Sulfonamides Leading to the Discovery of a T-Type Calcium Channel Blocker, ABT-639. ACS Med Chem Lett 6: 641 -4 (2015) Dallanoce, C; Magrone, P; Matera, C; Lo Presti, L; De Amici, M; Riganti, L; Clementi, F; Gotti, C; De Micheli, C Synthesis of novel chiral¿2-isoxazoline derivatives related to ABT-418 and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes. Eur J Med Chem 45: 5594 -601 (2010) Brooks, CD; Stewart, AO; Basha, A; Bhatia, P; Ratajczyk, JD; Martin, JG; Craig, RA; Kolasa, T; Bouska, JB; Lanni, C (R)-(+)-N-[3-[5-[(4-fluorophenyl)methyl]-2-thienyl]-1-methyl- 2-propynyl]-N-hydroxyurea (ABT-761), a second-generation 5-lipoxygenase inhibitor. J Med Chem 38: 4768 -75 (1996) Wagner, R; Randolph, JT; Patel, SV; Nelson, L; Matulenko, MA; Keddy, R; Pratt, JK; Liu, D; Krueger, AC; Donner, PL; Hutchinson, DK; Flentge, C; Betebenner, D; Rockway, T; Maring, CJ; Ng, TI; Krishnan, P; Pilot-Matias, T; Collins, C; Panchal, N; Reisch, T; Dekhtyar, T; Mondal, R; Stolarik, DF; Gao, Y; Gao, W; Beno, DA; Kati, WM Highlights of the Structure-Activity Relationships of Benzimidazole Linked Pyrrolidines Leading to the Discovery of the Hepatitis C Virus NS5A Inhibitor Pibrentasvir (ABT-530). J Med Chem 61: 4052 -4066 (2018) Arneric, SP; Sullivan, JP; Briggs, CA; Donnelly-Roberts, D; Anderson, DJ; Raszkiewicz, JL; Hughes, ML; Cadman, ED; Adams, P; Garvey, DS (S)-3-methyl-5-(1-methyl-2-pyrrolidinyl) isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: I. In vitro characterization. J Pharmacol Exp Ther 270: 310 -8 (1994) Penning, TD; Zhu, GD; Gandhi, VB; Gong, J; Liu, X; Shi, Y; Klinghofer, V; Johnson, EF; Donawho, CK; Frost, DJ; Bontcheva-Diaz, V; Bouska, JJ; Osterling, DJ; Olson, AM; Marsh, KC; Luo, Y; Giranda, VL Discovery of the Poly(ADP-ribose) polymerase (PARP) inhibitor 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide (ABT-888) for the treatment of cancer. J Med Chem 52: 514 -23 (2009) Lin, NH; Gunn, DE; Ryther, KB; Garvey, DS; Donnelly-Roberts, DL; Decker, MW; Brioni, JD; Buckley, MJ; Rodrigues, AD; Marsh, KG; Anderson, DJ; Buccafusco, JJ; Prendergast, MA; Sullivan, JP; Williams, M; Arneric, SP; Holladay, MW Structure-activity studies on 2-methyl-3-(2(S)-pyrrolidinylmethoxy) pyridine (ABT-089): an orally bioavailable 3-pyridyl ether nicotinic acetylcholine receptor ligand with cognition-enhancing properties. J Med Chem 40: 385 -90 (1997) Esbenshade, TA; Fox, GB; Krueger, KM; Miller, TR; Kang, CH; Denny, LI; Witte, DG; Yao, BB; Pan, L; Wetter, J; Marsh, K; Bennani, YL; Cowart, MD; Sullivan, JP; Hancock, AA Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: I. Potent and selective histamine H3 receptor antagonist with drug-like properties. J Pharmacol Exp Ther 313: 165 -75 (2005) Cowart, M; Latshaw, SP; Bhatia, P; Daanen, JF; Rohde, J; Nelson, SL; Patel, M; Kolasa, T; Nakane, M; Uchic, ME; Miller, LN; Terranova, MA; Chang, R; Donnelly-Roberts, DL; Namovic, MT; Hollingsworth, PR; Martino, BR; Lynch, JJ; Sullivan, JP; Hsieh, GC; Moreland, RB; Brioni, JD; Stewart, AO Discovery of 2-(4-pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole (ABT-724), a dopaminergic agent with a novel mode of action for the potential treatment of erectile dysfunction. J Med Chem 47: 3853 -64 (2004) Holladay, MW; Wasicak, JT; Lin, NH; He, Y; Ryther, KB; Bannon, AW; Buckley, MJ; Kim, DJ; Decker, MW; Anderson, DJ; Campbell, JE; Kuntzweiler, TA; Donnelly-Roberts, DL; Piattoni-Kaplan, M; Briggs, CA; Williams, M; Arneric, SP Identification and initial structure-activity relationships of (R)-5-(2-azetidinylmethoxy)-2-chloropyridine (ABT-594), a potent, orally active, non-opiate analgesic agent acting via neuronal nicotinic acetylcholine receptors. J Med Chem 41: 407 -12 (1998) Gomtsyan, A; Bayburt, EK; Schmidt, RG; Surowy, CS; Honore, P; Marsh, KC; Hannick, SM; McDonald, HA; Wetter, JM; Sullivan, JP; Jarvis, MF; Faltynek, CR; Lee, CH Identification of (R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)urea (ABT-102) as a potent TRPV1 antagonist for pain management. J Med Chem 51: 392 -5 (2008) Dai, Y; Hartandi, K; Ji, Z; Ahmed, AA; Albert, DH; Bauch, JL; Bouska, JJ; Bousquet, PF; Cunha, GA; Glaser, KB; Harris, CM; Hickman, D; Guo, J; Li, J; Marcotte, PA; Marsh, KC; Moskey, MD; Martin, RL; Olson, AM; Osterling, DJ; Pease, LJ; Soni, NB; Stewart, KD; Stoll, VS; Tapang, P; Reuter, DR; Davidsen, SK; Michaelides, MR Discovery of N-(4-(3-amino-1H-indazol-4-yl)phenyl)-N'-(2-fluoro-5-methylphenyl)urea (ABT-869), a 3-aminoindazole-based orally active multitargeted receptor tyrosine kinase inhibitor. J Med Chem 50: 1584 -97 (2007) Holladay, MW; Bai, H; Li, Y; Lin, NH; Daanen, JF; Ryther, KB; Wasicak, JT; Kincaid, JF; He, Y; Hettinger, AM; Huang, P; Anderson, DJ; Bannon, AW; Buckley, MJ; Campbell, JE; Donnelly-Roberts, DL; Gunther, KL; Kim, DJ; Kuntzweiler, TA; Sullivan, JP; Decker, MW; Arneric, SP Structure-activity studies related to ABT-594, a potent nonopioid analgesic agent: effect of pyridine and azetidine ring substitutions on nicotinic acetylcholine receptor binding affinity and analgesic activity in mice. Bioorg Med Chem Lett 8: 2797 -802 (1999) Altenbach, RJ; Khilevich, A; Meyer, MD; Buckner, SA; Milicic, I; Daza, AV; Brune, ME; O'Neill, AB; Gauvin, DM; Cain, JC; Nakane, M; Holladay, MW; Williams, M; Brioni, JD; Sullivan, JP N-[3-(1H-imidazol-4-ylmethyl)phenyl]ethanesulfonamide (ABT-866, 1),(1) a novel alpha(1)-adrenoceptor ligand with an enhanced in vitro and in vivo profile relative to phenylpropanolamine and midodrine. J Med Chem 45: 4395 -7 (2002) Patel, MV; Kolasa, T; Mortell, K; Matulenko, MA; Hakeem, AA; Rohde, JJ; Nelson, SL; Cowart, MD; Nakane, M; Miller, LN; Uchic, ME; Terranova, MA; El-Kouhen, OF; Donnelly-Roberts, DL; Namovic, MT; Hollingsworth, PR; Chang, R; Martino, BR; Wetter, JM; Marsh, KC; Martin, R; Darbyshire, JF; Gintant, G; Hsieh, GC; Moreland, RB; Sullivan, JP; Brioni, JD; Stewart, AO Discovery of 3-methyl-N-(1-oxy-3',4',5',6'-tetrahydro-2'H-[2,4'-bipyridine]-1'-ylmethyl)benzamide (ABT-670), an orally bioavailable dopamine D4 agonist for the treatment of erectile dysfunction. J Med Chem 49: 7450 -65 (2006) Michaelides, MR; Hong, Y; DiDomenico, S; Asin, KE; Britton, DR; Lin, CW; Williams, M; Shiosaki, K (5aR,11bS)-4,5,5a,6,7,11b-hexahydro-2-propyl-3-thia-5-azacyclopent-1- ena[c]-phenanthrene-9,10-diol (A-86929): a potent and selective dopamine D1 agonist that maintains behavioral efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431). J Med Chem 38: 3445 -7 (1995) Jarvis, MF; Yu, H; Kohlhaas, K; Alexander, K; Lee, CH; Jiang, M; Bhagwat, SS; Williams, M; Kowaluk, EA ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2, 3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse. J Pharmacol Exp Ther 295: 1156 -64 (2000) Madar, DJ; Kopecka, H; Pireh, D; Yong, H; Pei, Z; Li, X; Wiedeman, PE; Djuric, SW; Von Geldern, TW; Fickes, MG; Bhagavatula, L; McDermott, T; Wittenberger, S; Richards, SJ; Longenecker, KL; Stewart, KD; Lubben, TH; Ballaron, SJ; Stashko, MA; Long, MA; Wells, H; Zinker, BA; Mika, AK; Beno, DW; Kempf-Grote, AJ; Polakowski, J; Segreti, J; Reinhart, GA; Fryer, RM; Sham, HL; Trevillyan, JM Discovery of 2-[4-{{2-(2S,5R)-2-cyano-5-ethynyl-1-pyrrolidinyl]-2-oxoethyl]amino]-4-methyl-1-piperidinyl]-4-pyridinecarboxylic acid (ABT-279): a very potent, selective, effective, and well-tolerated inhibitor of dipeptidyl peptidase-IV, useful for the treatment of diabetes. J Med Chem 49: 6416 -20 (2006) Pei, Z; Li, X; von Geldern, TW; Madar, DJ; Longenecker, K; Yong, H; Lubben, TH; Stewart, KD; Zinker, BA; Backes, BJ; Judd, AS; Mulhern, M; Ballaron, SJ; Stashko, MA; Mika, AK; Beno, DW; Reinhart, GA; Fryer, RM; Preusser, LC; Kempf-Grote, AJ; Sham, HL; Trevillyan, JM Discovery of ((4R,5S)-5-amino-4-(2,4,5- trifluorophenyl)cyclohex-1-enyl)-(3- (trifluoromethyl)-5,6-dihydro- [1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone (ABT-341), a highly potent, selective, orally efficacious, and safe dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 49: 6439 -42 (2006)
Clemens, JJ; Coon, T; Busch, BB; Asgian, JL; Hudson, S; Termin, A; Flores, TB; Tran, D; Chiang, P; Sperry, S; Gross, R; Abt, J; Heim, R; Lechner, S; Twin, H; Studley, J; Brenchley, G; Collier, PN; Pierard, F; Miller, A; Mak, C; Dvornikovs, V; Jimenez, JM; Stamos, D Bioorg Med Chem Lett 24: 3398 -402 (2014) Biller, SA; Abt, JW; Pudzianowski, AT; Rich, LC; Slusarchyk, DA; Ciosek, C Bioorg Med Chem Lett 3: 595 -600 (1993)
ChEMBL_306020 (CHEMBL833535) Inhibition of Farnesyl transferase inhibitor (ABT-839) ChEMBL_2187516 (CHEMBL5099598) Inhibition of full length N-terminal GST/C-terminal his-tagged human recombinant HDAC4 (627 to 1084 residues) expressed in Sf9 insect cells ChEBML_1686577 Inhibition of human N-terminal GST-tagged/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected Sf9 insect cells by fluorescence assay ChEMBL_1431998 (CHEMBL3388103) Inhibition of N-terminal GST-tagged human HDAC4 (627 to 1085 residues) using fluorogenic acetyl-Lys(trifluoroacetyl)-AMC substrate incubated for 2 hrs by fluorescence assay ChEMBL_1686577 (CHEMBL4037056) Inhibition of human N-terminal GST-tagged/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected Sf9 insect cells by fluorescence assay ChEBML_1684750 Inhibition of N-terminal GST-tagged human HDAC4 (627 to 1085 residues) expressed in baculovirus expression system using AMC-labeled RHKKAc as substrate after 2 hrs by fluorescence assay ChEMBL_1927617 (CHEMBL4430689) Inhibition of recombinant C-terminal His-tagged/N-terminal GST-tagged human HDAC4 (627 to 1084 residues) expressed in baculovirus expression system using fluorogenic substrate by fluorescence assay ChEMBL_993098 (CHEMBL2446673) Displacement of [3H]-DHT from human GST-tagged androgen receptor LBD (627 to 919) expressed in Escherichia coli HB-101 after 15 hrs by liquid scintillation counting analysis ChEMBL_1520320 (CHEMBL3626370) Displacement of [3H]-DHT from human GST fused AR-LBD (627 to 919 amino acids) transfected in Escherichia coli HB 101 after 15 hrs by liquid scintillation counting assay ChEMBL_1684750 (CHEMBL4035229) Inhibition of N-terminal GST-tagged human HDAC4 (627 to 1085 residues) expressed in baculovirus expression system using AMC-labeled RHKKAc as substrate after 2 hrs by fluorescence assay ChEMBL_2135626 (CHEMBL4845236) Inhibition of N-terminal GST-tagged full length human HDAC5 (627 to 1084 residues) expressed in baculovirus expression system using Ac-peptide-AMC as substrate incubated for 1 hr by fluorescence method ChEMBL_1822315 (CHEMBL4322079) Inhibition of recombinant human full length C-terminal GST-tagged HDAC4 (627 to end residues) expressed in baculovirus Sf9 insect cells using HDAC class 2a substrate incubated for 30 mins by fluorescence assay ChEMBL_1829483 (CHEMBL4329357) Inhibition of recombinant N-terminal GST-tagged /C-terminal His-tagged human HDAC4 (627 to 1084 residues) expressed in Baculovirus infected insect cells using Boc-Lys(trifluoroacetyl)-AMC as substrate by fluorimetric assay ChEBML_1572331 Inhibition of human recombinant C-terminal His-tagged, N-terminal GST-tagged HDAC4 (627 to 1084 residues) expressed in insect cells using RHK-K(Ac)-AMC as substrate incubated for 60 mins by fluorescence assay ChEMBL_1678051 (CHEMBL4028194) Inhibition of recombinant human C-terminal His-tagged/N-terminal GST-tagged HDAC4 (627 to 1084 residues) expressed in insect cells using Boc-K(Ac)-AMC as substrate after 60 mins by fluorescence assay ChEMBL_1728627 (CHEMBL4143905) Inhibition of recombinant human N-terminal GST-tagged/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected Sf21 insect cells using (Boc-Lys(trifluoroacetyl)-AMC) as substrate by fluorescence assay ChEMBL_2135625 (CHEMBL4845235) Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC4 (627 to 1084 residues) expressed in baculovirus expression system using Ac-peptide-AMC as substrate incubated for 1 hr by fluorescence method ChEMBL_2146502 (CHEMBL5030848) Inhibition of recombinant human N-terminal GST-tagged/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected Sf9 insect cells using fluorogenic substrate measured after 60 mins by FRET assay ChEMBL_1571079 (CHEMBL3794865) Inhibition of human recombinant N-terminal GST-tagged C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in insect cells using Boc-K(TFA)-AMC as substrate incubated for 60 mins by fluorescence assay ChEMBL_2092318 (CHEMBL4773581) Inhibition of recombinant human N-terminal GST-tagged HDAC4 (627 to end residues) expressed in baculovirus infected Sf9 insect cells using AcLeu-Gly-Lys(Tfa)-AMC as substrate measured after 30 mins by fluorescence assay ChEMBL_2119616 (CHEMBL4828682) Inhibition of recombinant human N-terminal GST-tagged and C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in Sf9 insect cells using Nepsilon-Trifluoroacetyl L-lysine as substrate by fluorescent microplate reader assay ChEMBL_2199365 (CHEMBL5111881) Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC4 (627 to 1084 residues) expressed in baculovirus expression system using trifluoroacetyl lysine as substrate incubated for 2 hrs by Multimode microplate reader analysis ChEMBL_2243517 (CHEMBL5157727) Inhibition of recombinant human N-terminal GST-fused/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected Sf9 insect cells using fluorogenic HDAC class 2a substrate measured after 30 mins by fluorimetry ChEMBL_2456854 Inhibition of N-terminal GST-tagged and C-terminal His-tagged human HDAC4 (627 to 1084 end residues) expressed in baculovirus infected Sf9 cells using Ac-peptide as substrate incubated for 1 hr by plate reader analysis ChEMBL_1919910 (CHEMBL4422755) Inhibition of recombinant human N-terminal GST-tagged/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected Sf21 insect cells using 7-AMC-labelled HDAC substrate measured after 30 mins by fluorescence assay ChEMBL_2052186 (CHEMBL4707187) Inhibition of recombinant human N-terminal GST-tagged and C-terminal His-tagged HDAC4 (627 to 1084 end residues) expressed in baculovirus infected Sf9 cells using fluorogenic HDAC class2a as substrate measured after 30 mins by fluorescence based assay ChEMBL_2188091 (CHEMBL5100173) Inhibition of recombinant human N-terminal GST-tagged/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected Sf9 insect cells using Boc-Lys (trifluoroacetyl)-AMC as substrate incubated for 90 mins by microplate reader analysis ChEMBL_2104902 (CHEMBL4813405) Inhibition of N-terminal GST-tagged/C-terminal His-tagged human recombinant HDAC4 (627 to 1084 residues) expressed in baculovirus-infected Sf9 cells assessed as reduction in 7-amino-4-methylcoumarin release measured every 5 mins by fluorescence based analysis ChEMBL_1591058 (CHEMBL3831143) Inhibition of N-terminal GST-tagged and C-terminal His-tagged human recombinant HDAC4 (627 to 1084 residues ) expressed in baculovirus coexpressed in fall armyworm Sf9 cells using carboxyfluorescein (FAM)-labeled acetylated/ trifluoroacetylated peptide as substrate after 60 mins by fluorescence assay ChEMBL_1674061 (CHEMBL4024090) Inhibition of recombinant human N-terminal GST-tagged/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus expression system using Boc-Lys(trifluoroacetyl)-AMC as substrate preincubated for 1 hr followed by substrate addition measured after 2 hrs by fluorescence assay ChEMBL_2031687 (CHEMBL4685845) Inhibition of recombinant human N-terminal GST-tagged/C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus expression system using Boc-Lys(TFA)-AMC as substrate preincubated for 5 mins followed by substrate addition and measured after 30 mins by fluorescence assay ChEMBL_2114400 (CHEMBL4823341) Inhibition of recombinant human N-terminal GST-fusion tagged/C-terminal GST-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected insect cells using Boc-Lys(TFA)-AMC as substrate preincubated for 5 mins followed by substrate addition measured after 90 mins by fluorimetry ChEMBL_2359062 Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC4 (627 to 1084 residues) expressed in Sf9 insect cells using Boc-Lys(Tfa)-AMC as fluorogenic substrate preincubated for 60 mins followed by substrate addition and measured after 90 mins by microplate reader analysis ChEMBL_1838324 (CHEMBL4338457) Inhibition of recombinant human N-terminal GST-fused and C-terminal His-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected insect cells using Boc-Lys(TFa)-AMC as substrate preincubated for 5 mins followed by substrate addition and measured after 90 mins by fluorescence assay ChEMBL_2112637 (CHEMBL4821487) Inhibition of N-terminal GST-tagged and C-terminal His-tagged human HDAC4 (627 to 1084 residues) expressed in baculovirus infected Sf9 cells using Boc Lys(TFA)-AMC as substrate preincubated for 5 mins followed by substrate addition and further incubated for 30 mins by fluorogenic assay ChEMBL_2340973 Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC4 (627 to 1084 residues) expressed in baculovirus-infected Sf9 cells using Boc-Lys(trifluoroacety1)-AMC as substrate preincubated with enzyme for 1 hr followed by substrate addition and measured after 30 mins by fluorescence based analysis ChEMBL_2427793 Inhibition of C-terminal His-tagged/N-terminal GST-tagged human HDAC4 (627 to 1084 residues) expressed in baculovirus-infected Sf9 cells using Boc-Lys-(trifluoroacetyl)-AMC as substrate preincubated with enzyme for 1 hr followed by substrate addition and measured after 2 hrs by fluorescence analysis ChEMBL_1590566 (CHEMBL3829035) Inhibition of N-terminal GST/C-terminal His-tagged human recombinant HDAC4 (627 to 1084 residues) expressed in baculovirus infected insect Sf9 cells using Ac-peptide-AMC as substrate assessed as release of AMC preincubated for 15 mins followed by substrate addition measured after 1 hr by fluorescence assay ChEMBL_2361634 Inhibition of N-terminal GST-tagged/C-terminal His tagged human recombinant HDAC4 (627 to 1084 residues) expressed in baculovirus-infected Sf9 cells using Ac-Leu-Gly-Lys(trifluoroAc)-AMC as substrate preincubated with enzyme for 15 mins followed by substrate addition and measured after 60 mins by fluorescence based analysis ChEMBL_1897697 (CHEMBL4399732) Inhibition of C-terminal His-tagged/N-terminal GST-tagged recombinant human HDAC4 (627 to 1084 residues) expressed in Baculovirus infected insect cells using Boc-Lys(TFa)-AMC as substrate preincubated for 5 mins followed by substrate addition and further incubation for 90 mins measured after 15 mins by microplate reader based fluorescence assay Filter-Binding-Assay PI3Kalpha assay described herein provides IC50 values indicating the activity of the compounds inhibiting PI3 kinase alpha activity Inhibition of PI3 kinase is expected to be indicative of activity in treating conditions of excessive or anomalous cell proliferation, such as cancers. See also J. A. Engelman, Nature Reviews Cancer, 2009, 9, 550-562; A. Carnero, Expert Opin. Investig. Drugs, 2009, 18, 1265-1277 and P. Liu et al., Nature Reviews Drug Discovery, 2009, 8, 627-64. Filter-Binding-Assay This PI3Kalpha assay provides an IC50 value indicative of the activity of the compounds to inhibit PI3 kinase alpha activity. Inhibition of PI3 kinase would be expected to be indicative of activity in treating conditions of excessive or anomalous cell proliferation, such as cancers. See also J. A. Engelman, Nature Reviews Cancer, 2009, 9, 550-562; A. Carnero, Expert Opin. Investig. Drugs, 2009, 18, 1265-1277 and P. Liu et al., Nature Reviews Drug Discovery, 2009, 8, 627-64. BCL-2 Competition Binding (Fluorescence Polarization) Assay The fluorescence-labeled 23 amino acid peptide BH3 was purchased from CalBiochem (NLWAAQRYGRELRRMSDKFVD). An unbound Fluorescein labeled BH3 peptide emits random light with respect to the plane of polarization plane of excited light, resulting in a lower polarization degree (mP) value. When the peptide is bound to BCL-2, the complex tumble slower and the emitted light can have a higher level of polarization, resulting in a higher mP value. This binding assay was performed in 96-well plate and with each assay contained 15 and 30 nM of labeled peptide and purified BCL-2 protein (purchased from R&D Systems, Inc). The assay buffer contained 20 mM Hepes (pH 7.0), 50 mM KCl, 5 mM MgCl2, 20 mM Na2MoO4, 0.1 mg/ml Bovine Gamma Globulin and 0.01% NP40. Compounds were diluted in DMSO and added to the final assay with concentration range from 20 μM to 2 nM. The polarization degree (mP) value was determined by BioTek Synergy II with background subtraction after 3 hours of incubation at room temperature. IC50 was calculated using Prism software with sigmoidal dose-response curve fitting. ABT-737 was used as reference compound. Such assays, carried out with a range of doses of test compounds, allowed the determination of an approximate IC50 value. Bcl-2 Competition Binding (Fluorescence Polarization) Assay The fluorescence-labeled 23 amino acid peptide BH3 was purchased from CalBiochem (NLWAAQRYGRELRRMSDKFVD, SEQ ID NO: 1). An unbound Fluorescein labeled BH3 peptide emits random light with respect to the plane of polarization plane of excited light, resulting in a lower polarization degree (mP) value. When the peptide is bound to Bcl-2, the complex tumble slower and the emitted light can have a higher level of polarization, resulting in a higher mP value. This binding assay was performed in 96-well plate and with each assay contained 15 and 30 nM of labeled peptide and purified Bcl-2 protein (purchased from R&D Systems, Inc). The assay buffer contained 20 mM Hepes (pH 7.0), 50 mM KCl, 5 mM MgCl2, 20 mM Na2MoO4, 0.1 mg/ml Bovine Gamma Globulin and 0.01% NP40. Compounds were diluted in DMSO and added to the final assay with concentration range from 20 uM to 2 nM. The polarization degree (mP) value was determined by BioTek Synergy II with background subtraction after 3 hours of incubation at room temperature. IC50 was calculated using Prism software with sigmoidal dose-response curve fitting. ABT-737 was used as reference compound. Such assays, carried out with a range of doses of test compounds, allow the determination of an approximate IC50 value. Although the inhibitory properties of the compounds of the present invention vary with structural change as expected, the activity generally exhibited by these agents is in the range of IC50=0.1-1000 nM.
 US10172845, Example 627 US10441581, Example 627 US10144734, Example 627 US11648243, Example 627 BDBM305609
US10172845, Example 627 US10441581, Example 627 US10144734, Example 627 US11648243, Example 627 BDBM305609 US10709712, Example 627 BDBM84355 US10245267, Example 627 US9694016, 627
US10709712, Example 627 BDBM84355 US10245267, Example 627 US9694016, 627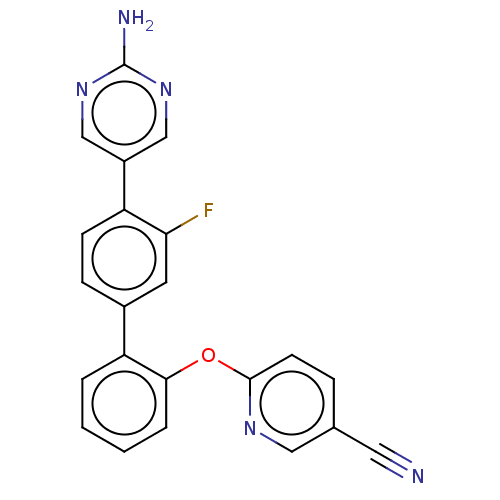 US9079866, 627 BDBM169226 US9884878, Compound 627 US9745328, Compound 627
US9079866, 627 BDBM169226 US9884878, Compound 627 US9745328, Compound 627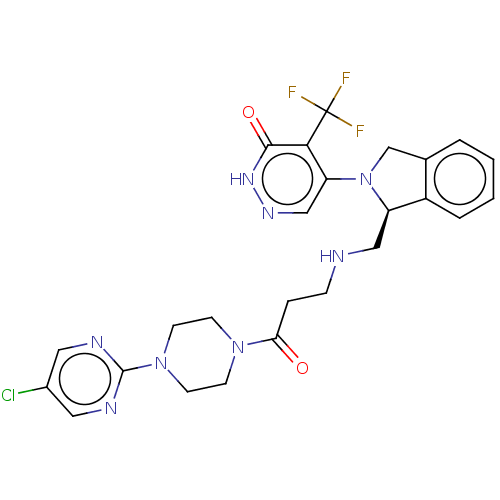 US11014913, Example 627 US10870641, Example 627 US10550105, Example 627 BDBM431761
US11014913, Example 627 US10870641, Example 627 US10550105, Example 627 BDBM431761 US11053244, Example 627 US10730877, Example 627 BDBM428191 US10544143, Example 627
US11053244, Example 627 US10730877, Example 627 BDBM428191 US10544143, Example 627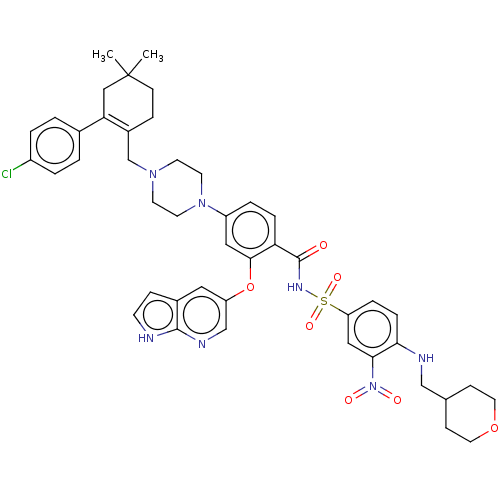 BDBM50162774 ABT-199 US11420968, Example ABT-199 Venetoclax
BDBM50162774 ABT-199 US11420968, Example ABT-199 Venetoclax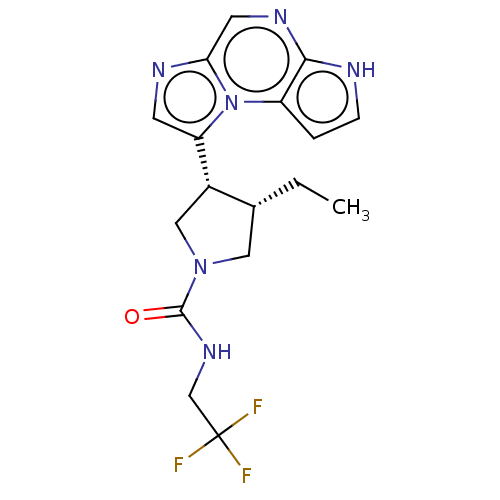 US10961228, Example ABT-494 BDBM50503287 Rinvoq ABT-494 Upadacitinib
US10961228, Example ABT-494 BDBM50503287 Rinvoq ABT-494 Upadacitinib BDBM109045 US9938267, Cmpd ID 627 US8604016, 627
BDBM109045 US9938267, Cmpd ID 627 US8604016, 627 US10071079, Example 627 US10478424, Example 627 BDBM273841
US10071079, Example 627 US10478424, Example 627 BDBM273841 US10710986, Example 627 BDBM452486 US11555029, No. 627
US10710986, Example 627 BDBM452486 US11555029, No. 627 US11427601, Example 627 BDBM487114 US10947252, Example 627
US11427601, Example 627 BDBM487114 US10947252, Example 627 ABT-229 BDBM86685
ABT-229 BDBM86685 US10377755, Example ABT-199 US20240166646, Example ABT-199 US11369599, Compound 369 BDBM60828 US10213433, Compound 5 US11590126, Example ABT-199 BDBM189459 US11318134, Example ABT-199 US11718611, Example T-7 US9174982, 369 ABT-199 US20240043404, Example 369
US10377755, Example ABT-199 US20240166646, Example ABT-199 US11369599, Compound 369 BDBM60828 US10213433, Compound 5 US11590126, Example ABT-199 BDBM189459 US11318134, Example ABT-199 US11718611, Example T-7 US9174982, 369 ABT-199 US20240043404, Example 369 BDBM566059 US11414431, Compound I-627 US12221453, Compound I-627
BDBM566059 US11414431, Compound I-627 US12221453, Compound I-627 US10647713, Compound I-627 BDBM442665 US11396508, Compound I-627
US10647713, Compound I-627 BDBM442665 US11396508, Compound I-627 US20230279020, Example 627-1a US10633389, Example 627-1a BDBM440277
US20230279020, Example 627-1a US10633389, Example 627-1a BDBM440277 BDBM116901 US8637532, 627
BDBM116901 US8637532, 627 BDBM122187 US8722692, 627
BDBM122187 US8722692, 627 BDBM138034 US8871934, 627
BDBM138034 US8871934, 627 BDBM159150 US9034866, 627
BDBM159150 US9034866, 627 BDBM171197 US9085555, 627
BDBM171197 US9085555, 627 BDBM177261 US9120749, 627
BDBM177261 US9120749, 627 BDBM214515 US9283222, 627
BDBM214515 US9283222, 627 BDBM216366 US9302989, 627
BDBM216366 US9302989, 627 BDBM217785 US9212182, 627
BDBM217785 US9212182, 627 BDBM226939 US9328096, 627
BDBM226939 US9328096, 627 BDBM248692 US9434711, 627
BDBM248692 US9434711, 627 US9023865, 627 BDBM157786
US9023865, 627 BDBM157786 US9169252, 627 BDBM188096
US9169252, 627 BDBM188096 BDBM747490 US12325697, Compound ABT-2
BDBM747490 US12325697, Compound ABT-2 US10377755, Example ABT-263 BDBM417035
US10377755, Example ABT-263 BDBM417035 BDBM394968 US10307413, Compound 627
BDBM394968 US10307413, Compound 627 BDBM444038 US10660877, Example 627
BDBM444038 US10660877, Example 627 BDBM454157 US10730863, Example 627
BDBM454157 US10730863, Example 627 BDBM491644 US10975056, Example 627
BDBM491644 US10975056, Example 627 BDBM597028 US11596639, Example 627
BDBM597028 US11596639, Example 627 BDBM603654 US11649255, Example 627
BDBM603654 US11649255, Example 627 BDBM623608 US11780845, Example 627
BDBM623608 US11780845, Example 627 BDBM630327 US20230340011, Example 627.
BDBM630327 US20230340011, Example 627. BDBM635178 US11814367, Compound 627
BDBM635178 US11814367, Compound 627 BDBM643561 US20240002391, Compound 627
BDBM643561 US20240002391, Compound 627 BDBM650526 US20240043427, Example 627
BDBM650526 US20240043427, Example 627 BDBM666594 US20240116946, Example 627
BDBM666594 US20240116946, Example 627 BDBM702915 US20240366536, Compound 627
BDBM702915 US20240366536, Compound 627 BDBM705501 US20160046618, Example 627
BDBM705501 US20160046618, Example 627 BDBM739294 US20250145633, Example 627
BDBM739294 US20250145633, Example 627 BDBM763582 US12384753, Example 627
BDBM763582 US12384753, Example 627 US10323022, Example 627 BDBM399150
US10323022, Example 627 BDBM399150 US10626095, Example 627 BDBM610860
US10626095, Example 627 BDBM610860 US10881652, Example 627 BDBM477541
US10881652, Example 627 BDBM477541 US11286268, Compound 627 BDBM545141
US11286268, Compound 627 BDBM545141 US11524959, Compound 627. BDBM583735
US11524959, Compound 627. BDBM583735 US11524968, Example 627 BDBM584878
US11524968, Example 627 BDBM584878 US11845723, Example 627 BDBM641725
US11845723, Example 627 BDBM641725 US20230348426, Example 627 BDBM632357
US20230348426, Example 627 BDBM632357 US20240218021, Example 627 BDBM684368
US20240218021, Example 627 BDBM684368 US20240246964, Compound 627 BDBM687606
US20240246964, Compound 627 BDBM687606 US20240316047, Example 627 BDBM697846
US20240316047, Example 627 BDBM697846 US20250129104, Compound 627 BDBM735817
US20250129104, Compound 627 BDBM735817 US9718825, Example 627 BDBM269181
US9718825, Example 627 BDBM269181 ABT-267 BDBM50453112 Ombitasvir CHEBI:85183
ABT-267 BDBM50453112 Ombitasvir CHEBI:85183 CHEBI:62880 BDBM50445857 ABT-888 Veliparib
CHEBI:62880 BDBM50445857 ABT-888 Veliparib Pibrentasvir ABT-530 A-1325912.0 BDBM50453100
Pibrentasvir ABT-530 A-1325912.0 BDBM50453100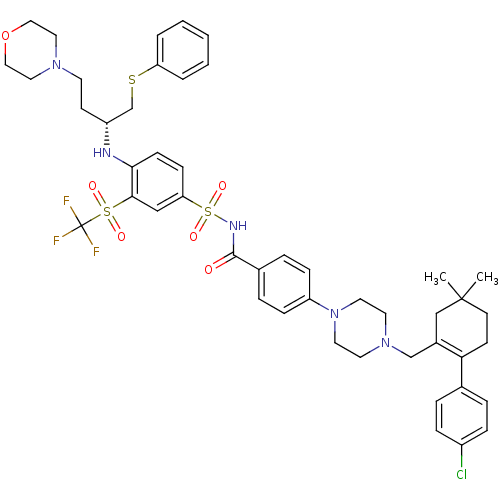 US11590126, Example ABT-263 US11318134, Example ABT-263 ABT-263 US11344546, Example ABT-263 US11420968, Example ABT-263 (R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-1-enyl)methyl)piperazin-1-yl)-N-(4-(4-morpholino-1-(phenylthio)butan-2-ylamino)-3-(trifluoromethylsulfonyl)phenylsulfonyl)benzamide BDBM50270877 CHEMBL443684
US11590126, Example ABT-263 US11318134, Example ABT-263 ABT-263 US11344546, Example ABT-263 US11420968, Example ABT-263 (R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex-1-enyl)methyl)piperazin-1-yl)-N-(4-(4-morpholino-1-(phenylthio)butan-2-ylamino)-3-(trifluoromethylsulfonyl)phenylsulfonyl)benzamide BDBM50270877 CHEMBL443684 BDBM307399 US10150728, Example I-627
BDBM307399 US10150728, Example I-627 BDBM321831 US10183021, Compound I-627
BDBM321831 US10183021, Compound I-627 BDBM441131 US10640495, Example I-627
BDBM441131 US10640495, Example I-627 BDBM727765 US20250090540, Example I-627
BDBM727765 US20250090540, Example I-627 US20250163057, Compound I-627 BDBM742339
US20250163057, Compound I-627 BDBM742339 BDBM508316 US11084798, Cpd No 627 US11046691, Compound 627 7-ethyl-4-(methylamino)-1-phenyl- pyrido[2,3-d]pyrimidin-2(1H)-one US11130759, Cpd. No. 627
BDBM508316 US11084798, Cpd No 627 US11046691, Compound 627 7-ethyl-4-(methylamino)-1-phenyl- pyrido[2,3-d]pyrimidin-2(1H)-one US11130759, Cpd. No. 627 ABT-348 BDBM50381716 ILORASERTIB US8722890, 1 US8722890, 2
ABT-348 BDBM50381716 ILORASERTIB US8722890, 1 US8722890, 2 Delafloxacin BDBM50560872 WQ-3034 ABT-492 RX-3341
Delafloxacin BDBM50560872 WQ-3034 ABT-492 RX-3341 BDBM564313 Roche-Dataset for PDE10A, Compound 627
BDBM564313 Roche-Dataset for PDE10A, Compound 627 BDBM714357 US20250025443, Example 391 US20250025443, Compound 627
BDBM714357 US20250025443, Example 391 US20250025443, Compound 627 US11292791, Example 917 BDBM547354 US11292791, Example 627
US11292791, Example 917 BDBM547354 US11292791, Example 627 US20250025443, Example 627 US20250025443, Compound 816 BDBM714591
US20250025443, Example 627 US20250025443, Compound 816 BDBM714591 US9169252, 626 BDBM188094 US9169252, 627 US9169252, 625
US9169252, 626 BDBM188094 US9169252, 627 US9169252, 625 US10233173, Example 627 2-[[1-[4-Chloro-3-(difluoromethyl)phenyl]triazol-4-yl]methoxy]-4- BDBM276687 US10071988, Example 627
US10233173, Example 627 2-[[1-[4-Chloro-3-(difluoromethyl)phenyl]triazol-4-yl]methoxy]-4- BDBM276687 US10071988, Example 627 US9604926, Compound CM-627 BDBM312214 US9724435, Compound CM627
US9604926, Compound CM-627 BDBM312214 US9724435, Compound CM627 A-12825760 Glecaprevir A-1282576 A-1282576.0 ABT-493 BDBM50573891
A-12825760 Glecaprevir A-1282576 A-1282576.0 ABT-493 BDBM50573891 ABT-333 Dasabuvir sodium monohydrate BDBM50549803 CHEBI:85182 Exviera Dasabuvir
ABT-333 Dasabuvir sodium monohydrate BDBM50549803 CHEBI:85182 Exviera Dasabuvir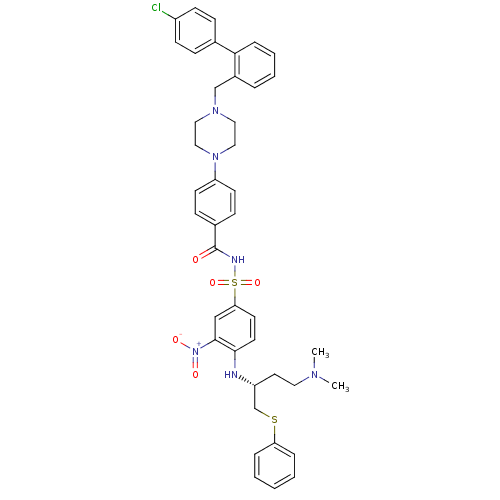 CHEMBL376408 4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1-yl)-N-[(4-{[(2R)-4-(dimethylamino)-1-(phenylsulfanyl)butan-2-yl]amino}-3-nitrobenzene)sulfonyl]benzamide US11760752, Compound ABT-737 BDBM21447 ABT-737 US9125913, ABT-737 N-Benylpiperazine derivative, 2
CHEMBL376408 4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1-yl)-N-[(4-{[(2R)-4-(dimethylamino)-1-(phenylsulfanyl)butan-2-yl]amino}-3-nitrobenzene)sulfonyl]benzamide US11760752, Compound ABT-737 BDBM21447 ABT-737 US9125913, ABT-737 N-Benylpiperazine derivative, 2 USRE48547, Compound 7 BDBM420280 USRE47740, Compound 7 SX-627
USRE48547, Compound 7 BDBM420280 USRE47740, Compound 7 SX-627 BDBM382916 US10711020, Example 628 US10273259, Example 628 US10273259, Example 627
BDBM382916 US10711020, Example 628 US10273259, Example 628 US10273259, Example 627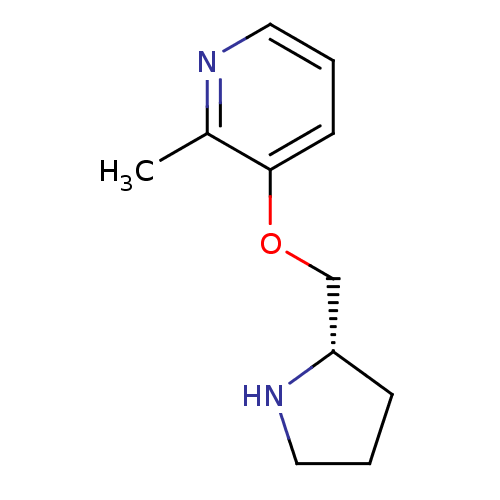 2-Methyl-3-((S)-1-pyrrolidin-2-ylmethoxy)-pyridine CHEMBL127071 BDBM50056147 ABT-089
2-Methyl-3-((S)-1-pyrrolidin-2-ylmethoxy)-pyridine CHEMBL127071 BDBM50056147 ABT-089 3-Methyl-5-(1-methyl-pyrrolidin-2-yl)-isoxazole ABT-418 BDBM50171341 CHEMBL188981
3-Methyl-5-(1-methyl-pyrrolidin-2-yl)-isoxazole ABT-418 BDBM50171341 CHEMBL188981 US11021481, Compound I-627 US11548890, Compound I-627 (S)-4-(7- fluoroimidazo [1,2-a]pyridin- 3-yl)-7-((5-(6- (2-hydroxypropa n-2-yl)-1,4- oxazepan-4- yl)pyridin-2- yl)amino)isoin- dolin-1-one BDBM500962 US11078201, Compound I-627
US11021481, Compound I-627 US11548890, Compound I-627 (S)-4-(7- fluoroimidazo [1,2-a]pyridin- 3-yl)-7-((5-(6- (2-hydroxypropa n-2-yl)-1,4- oxazepan-4- yl)pyridin-2- yl)amino)isoin- dolin-1-one BDBM500962 US11078201, Compound I-627 US10822348, Example 627 US10800792, Example 627 BDBM467966 tert-Butyl 4-(4-oxo-5-(4-phenoxyphenyl)-4,5-dihydro-3H-1-thia- 3,5,8-triazaacenaphthylene-2-carboxamido)piperidine-1-carboxylate;
US10822348, Example 627 US10800792, Example 627 BDBM467966 tert-Butyl 4-(4-oxo-5-(4-phenoxyphenyl)-4,5-dihydro-3H-1-thia- 3,5,8-triazaacenaphthylene-2-carboxamido)piperidine-1-carboxylate; US10174027, Example 627 US10023570, Example 627 BDBM284597 6-(1,5-dimethyl-1H- pyrazol-3-yl)-4-(6- (piperazin-1-yl)pyridin-3- yl)pyrazolo[1,5-a]pyridine- 3-carbonitrile dihydrochloride
US10174027, Example 627 US10023570, Example 627 BDBM284597 6-(1,5-dimethyl-1H- pyrazol-3-yl)-4-(6- (piperazin-1-yl)pyridin-3- yl)pyrazolo[1,5-a]pyridine- 3-carbonitrile dihydrochloride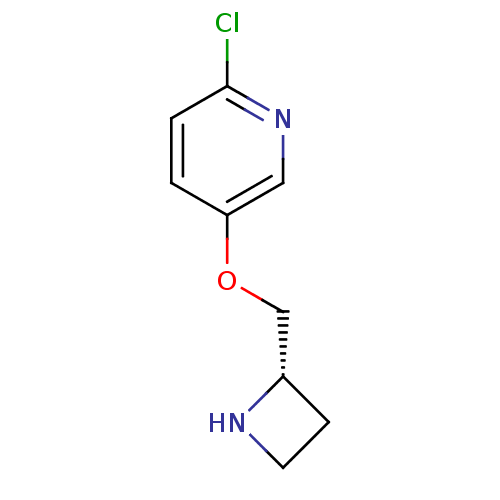 5-((S)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine A-98593 CHEMBL439766 BDBM50062639 ABT-594
5-((S)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine A-98593 CHEMBL439766 BDBM50062639 ABT-594 US11718603, Example 627 US11014911, Example 627 BDBM498802 (1R,3S)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]amino}-1H-pyrazol-5- yl)cyclopentyl (2S,3R)-3- hydroxy-2,3-dimethylazetidine- 1-carboxylate
US11718603, Example 627 US11014911, Example 627 BDBM498802 (1R,3S)-3-(3-{[(6- methoxypyridin-3- yl)acetyl]amino}-1H-pyrazol-5- yl)cyclopentyl (2S,3R)-3- hydroxy-2,3-dimethylazetidine- 1-carboxylate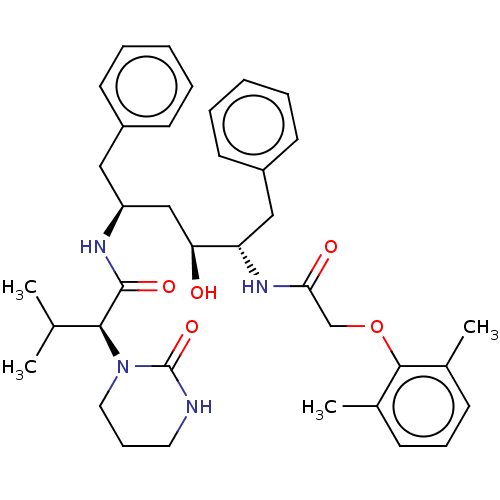 med.21724, Compound 185 ABT-378 A-157378-0 BDBM50180655 Lopinavir CHEBI:31781 A-157378.0 Kaletra
med.21724, Compound 185 ABT-378 A-157378-0 BDBM50180655 Lopinavir CHEBI:31781 A-157378.0 Kaletra Example 627A tert-butyl thieno[3,2-b]pyridin-7-ylcarbamate BDBM673480 US20240150293, Example 627
Example 627A tert-butyl thieno[3,2-b]pyridin-7-ylcarbamate BDBM673480 US20240150293, Example 627 Tiagabine Abbott-70569 NO-05-0328 Abbott-70569.1 ABT-569 Gabitril TIAGABINE HYDROCHLORIDE BDBM50368628 NNC-05-0328
Tiagabine Abbott-70569 NO-05-0328 Abbott-70569.1 ABT-569 Gabitril TIAGABINE HYDROCHLORIDE BDBM50368628 NNC-05-0328 US10858342, Compound C-ABT BDBM473907 3-(6-Chloro-pyridin-3-yl)- 6,6-dimethyl-3,5,6,7- tetrahydro-benzoimidazol-4- one
US10858342, Compound C-ABT BDBM473907 3-(6-Chloro-pyridin-3-yl)- 6,6-dimethyl-3,5,6,7- tetrahydro-benzoimidazol-4- one US11001575, Example 627 US10457669, Example 796 (2R)-2-{6-[5-chloro- 2- (methylamino) pyrimidin-4-yl]-1- oxo-2,3-dihydro-1H- isoindol-2-yl}- N-[(1S)-2-hydroxy- 1-(3-methoxyphenyl) ethyl]propanamide BDBM417962 US10457669, Example 627
US11001575, Example 627 US10457669, Example 796 (2R)-2-{6-[5-chloro- 2- (methylamino) pyrimidin-4-yl]-1- oxo-2,3-dihydro-1H- isoindol-2-yl}- N-[(1S)-2-hydroxy- 1-(3-methoxyphenyl) ethyl]propanamide BDBM417962 US10457669, Example 627 1-(Azetidin-1-yl)-2-[6-(2,4-difluoro-3-methyl-phenyl)-3-methyl- US10377753, Example 627 BDBM409759
1-(Azetidin-1-yl)-2-[6-(2,4-difluoro-3-methyl-phenyl)-3-methyl- US10377753, Example 627 BDBM409759 US10508120, Compound I-627 US11046698, Compound I-687 US10577373, Compound I-687 US10508120, Compound I-687 BDBM424172
US10508120, Compound I-627 US11046698, Compound I-687 US10577373, Compound I-687 US10508120, Compound I-687 BDBM424172 (1R,3S,5R)-2-(2- (3-acetyl-5-(2- methylpyrimidin- 5-yl)-1H-indazol- 1-yl)acetyl)-N-(3- chloro-2- fluorobenzyl)-5- methyl-2- azabicyclo[3.1.0] hexane-3- carboxamide US10822352, Comp No. 627 BDBM386678 US10287301, Compound 627
(1R,3S,5R)-2-(2- (3-acetyl-5-(2- methylpyrimidin- 5-yl)-1H-indazol- 1-yl)acetyl)-N-(3- chloro-2- fluorobenzyl)-5- methyl-2- azabicyclo[3.1.0] hexane-3- carboxamide US10822352, Comp No. 627 BDBM386678 US10287301, Compound 627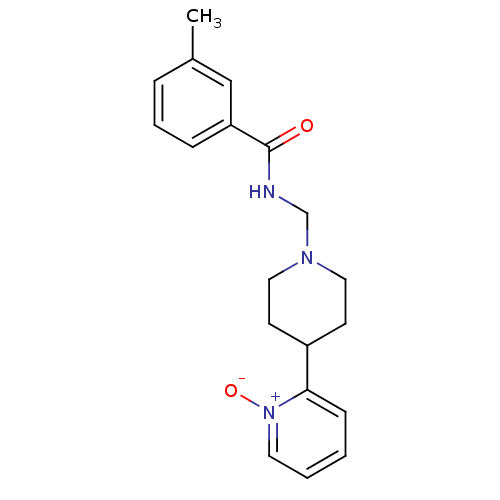 CHEMBL219182 methyl-N-(1-oxy-3',4',5',6'-tetrahydro-2'H-[2,4'-bipyridine]-1'ylmethyl)benzamide BDBM50200050 ABT-670
CHEMBL219182 methyl-N-(1-oxy-3',4',5',6'-tetrahydro-2'H-[2,4'-bipyridine]-1'ylmethyl)benzamide BDBM50200050 ABT-670 US9708336, 627 3-[6-[3- (dimethylamino)pyrrolidin-1- yl]-3-pyridyl]-2-(1H-tetrazol- 5-yl)benzenesulfonamide BDBM262774
US9708336, 627 3-[6-[3- (dimethylamino)pyrrolidin-1- yl]-3-pyridyl]-2-(1H-tetrazol- 5-yl)benzenesulfonamide BDBM262774 (1S,6R)-3-[(3-carbamoylphenyl)carbamoyl]-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate ABT-341 analogue 22 BDBM12655
(1S,6R)-3-[(3-carbamoylphenyl)carbamoyl]-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate ABT-341 analogue 22 BDBM12655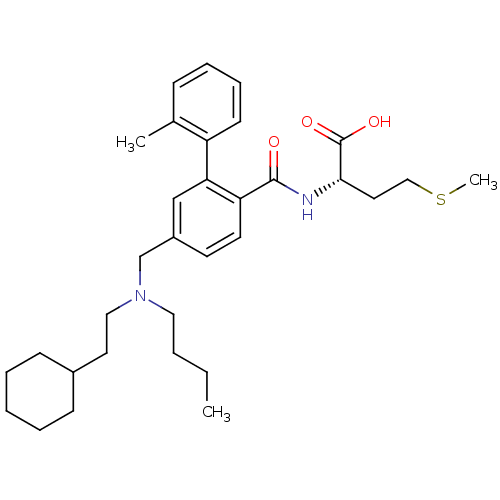 CHEMBL29982 BDBM17325 ABT-839 (2S)-2-[(4-{[butyl(2-cyclohexylethyl)amino]methyl}-2-(2-methylphenyl)phenyl)formamido]-4-(methylsulfanyl)butanoic acid
CHEMBL29982 BDBM17325 ABT-839 (2S)-2-[(4-{[butyl(2-cyclohexylethyl)amino]methyl}-2-(2-methylphenyl)phenyl)formamido]-4-(methylsulfanyl)butanoic acid 2-methyl-6-oxo-N-{1-[4-(2,2,2- trifluoroethyl)phenyl]cyclopropyl}- 1,6-dihydropyrimidine-4- carboxamide US9815796, Example 627 BDBM355536
2-methyl-6-oxo-N-{1-[4-(2,2,2- trifluoroethyl)phenyl]cyclopropyl}- 1,6-dihydropyrimidine-4- carboxamide US9815796, Example 627 BDBM355536 US9796708, Example 627 4-(4,5-difluoro-2-methoxyphenyl)-2-(1,2,3,6-tetrahydropyridin-4-yl)-1H-pyrrolo[2,3-b]pyridine BDBM353395
US9796708, Example 627 4-(4,5-difluoro-2-methoxyphenyl)-2-(1,2,3,6-tetrahydropyridin-4-yl)-1H-pyrrolo[2,3-b]pyridine BDBM353395 (1S,6R)-3-{[(3-carbamoylphenyl)amino]methyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate BDBM12653 ABT-341 analogue 19
(1S,6R)-3-{[(3-carbamoylphenyl)amino]methyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate BDBM12653 ABT-341 analogue 19 BDBM12652 ABT-341 analogue 14 (1S,6R)-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethyl)-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate
BDBM12652 ABT-341 analogue 14 (1S,6R)-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethyl)-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate Trans-4-(4-fluorophenyl)-N-hexyl-1-[(1-methyl-1H-imidazol-4-yl)sulfonyl]pyrrolidine-3-carboxamide BDBM309105 US9656955, Example 627
Trans-4-(4-fluorophenyl)-N-hexyl-1-[(1-methyl-1H-imidazol-4-yl)sulfonyl]pyrrolidine-3-carboxamide BDBM309105 US9656955, Example 627 US11912693, Compound 627 5-(1-isopropyl-1H- benzo[d][1,2,3]triazol-5-yl)-3-(3- methoxypyridin-2-yl)-1,2,4- oxadiazole BDBM655014
US11912693, Compound 627 5-(1-isopropyl-1H- benzo[d][1,2,3]triazol-5-yl)-3-(3- methoxypyridin-2-yl)-1,2,4- oxadiazole BDBM655014 US12065436, Compound 627 (R)-N-(5-(5-ethyl-1,2,4-oxadiazol-3- yl)-2,3-dihydro-1H-inden-1-yl)-4- methylnicotinamide BDBM691238
US12065436, Compound 627 (R)-N-(5-(5-ethyl-1,2,4-oxadiazol-3- yl)-2,3-dihydro-1H-inden-1-yl)-4- methylnicotinamide BDBM691238 3-[(5-Chloro-2-ethylsulfonylphenyl)methyl]-8-fluoro-7-[(4-methylpiperazin-1-yl)methyl]-6-(trifluoromethyl)quinazolin-4-one BDBM401208 US10005739, Example 627
3-[(5-Chloro-2-ethylsulfonylphenyl)methyl]-8-fluoro-7-[(4-methylpiperazin-1-yl)methyl]-6-(trifluoromethyl)quinazolin-4-one BDBM401208 US10005739, Example 627 N-((3S,4R)-1-((4-(2-aminopropan-2- yl)phenyl)sulfonyl)-3-methylpiperidin-4- yl)-5-cyclopropylisoxazole-3-carboxamide US10577363, Compound 627 BDBM432924
N-((3S,4R)-1-((4-(2-aminopropan-2- yl)phenyl)sulfonyl)-3-methylpiperidin-4- yl)-5-cyclopropylisoxazole-3-carboxamide US10577363, Compound 627 BDBM432924 4-{6-[2-(6,7-Difluoro-2,4-dimethyl-indol-1-yl)-ethylamino]-pyrimidin-4-yl}-2-fluoro-6- propoxy-benzoic acid US12011444, Example 627 BDBM680412
4-{6-[2-(6,7-Difluoro-2,4-dimethyl-indol-1-yl)-ethylamino]-pyrimidin-4-yl}-2-fluoro-6- propoxy-benzoic acid US12011444, Example 627 BDBM680412 BDBM371516 N-[1-(Fluoromethyl)cyclopropyl]-3-[(3-methylisoxazol-5-yl)methyl]-2,4-dioxo-1-(1,3,4-thiadiazol-2-ylmethyl)quinazoline-6-sulfonamide US10239843, Example 627
BDBM371516 N-[1-(Fluoromethyl)cyclopropyl]-3-[(3-methylisoxazol-5-yl)methyl]-2,4-dioxo-1-(1,3,4-thiadiazol-2-ylmethyl)quinazoline-6-sulfonamide US10239843, Example 627 BDBM502659 US11028090, Example 627 US11028090, Example 628 [4-(1- isopropyl-7- methoxy- [1,2,4]tria- zolo[4,3- a]quinoxaline- 4- ylamino)- butyl]-3- methyl- butyramide
BDBM502659 US11028090, Example 627 US11028090, Example 628 [4-(1- isopropyl-7- methoxy- [1,2,4]tria- zolo[4,3- a]quinoxaline- 4- ylamino)- butyl]-3- methyl- butyramide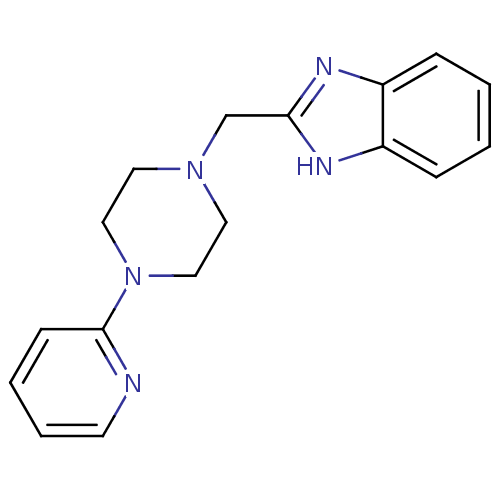 2-(4-Pyridin-2-yl-piperazin-1-ylmethyl)-1H-benzoimidazole CHEMBL440687 ABT-724 BDBM50145075 2-((4-(pyridin-2-yl)piperazin-1-yl)methyl)-1H-benzo[d]imidazole
2-(4-Pyridin-2-yl-piperazin-1-ylmethyl)-1H-benzoimidazole CHEMBL440687 ABT-724 BDBM50145075 2-((4-(pyridin-2-yl)piperazin-1-yl)methyl)-1H-benzo[d]imidazole BDBM358550 US10214537, Example 627 methyl 2-(4-acetyl-3,3-dimethyl-2- oxopiperazin-1-yl)-4-(4-amino-5- chloropyrrolo[2,1-f][1,2,4]triazin-7- yl)benzoate
BDBM358550 US10214537, Example 627 methyl 2-(4-acetyl-3,3-dimethyl-2- oxopiperazin-1-yl)-4-(4-amino-5- chloropyrrolo[2,1-f][1,2,4]triazin-7- yl)benzoate US10174028, Example 627 BDBM321067 6-(1,5-dimethyl-1H- pyrazol-3-yl)-4-(6- (piperazin-1-yl)pyridin-3- yl)pyrazolo[1,5-a]pyridine- 3-carbonitrile dihydrochloride
US10174028, Example 627 BDBM321067 6-(1,5-dimethyl-1H- pyrazol-3-yl)-4-(6- (piperazin-1-yl)pyridin-3- yl)pyrazolo[1,5-a]pyridine- 3-carbonitrile dihydrochloride (3S,4R)-4-((5,6-dichloro-7-(1- (difluoromethyl)cyclobutyl)pyrrolo[2, 1-f][1,2,4]triazin-2- yl)amino)tetrahydro-2H-pyran-3-ol US20250163063, Example 627 BDBM743104
(3S,4R)-4-((5,6-dichloro-7-(1- (difluoromethyl)cyclobutyl)pyrrolo[2, 1-f][1,2,4]triazin-2- yl)amino)tetrahydro-2H-pyran-3-ol US20250163063, Example 627 BDBM743104 BDBM505978 (S)-6-(((8-fluoroquinolin-5-yl)(1-(1-(trifluoro- methyl)cyclopropyl)-1H-1,2,3-triazol-4- yl)methyl)amino)-4- (neopentylamino)quinoline- 3,8-dicarbonitrile US11066414, Compound 627
BDBM505978 (S)-6-(((8-fluoroquinolin-5-yl)(1-(1-(trifluoro- methyl)cyclopropyl)-1H-1,2,3-triazol-4- yl)methyl)amino)-4- (neopentylamino)quinoline- 3,8-dicarbonitrile US11066414, Compound 627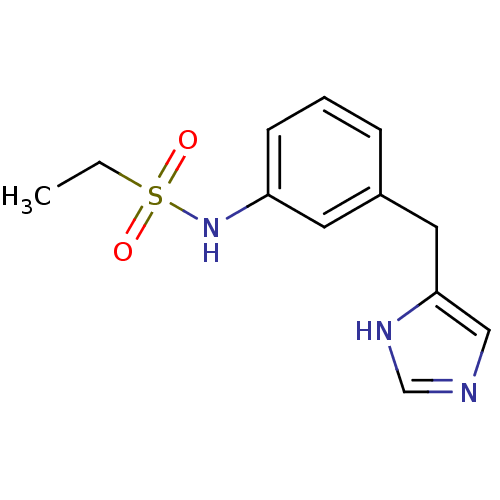 CHEMBL326702 N-[3-(1H-imidazol-4-ylmethyl)-phenyl]ethanesulfonamide N-(3-((1H-imidazol-4-yl)methyl)phenyl)ethanesulfonamide ABT-866 BDBM50118705 Ethanesulfonic acid [3-(1H-imidazol-4-ylmethyl)-phenyl]-amide
CHEMBL326702 N-[3-(1H-imidazol-4-ylmethyl)-phenyl]ethanesulfonamide N-(3-((1H-imidazol-4-yl)methyl)phenyl)ethanesulfonamide ABT-866 BDBM50118705 Ethanesulfonic acid [3-(1H-imidazol-4-ylmethyl)-phenyl]-amide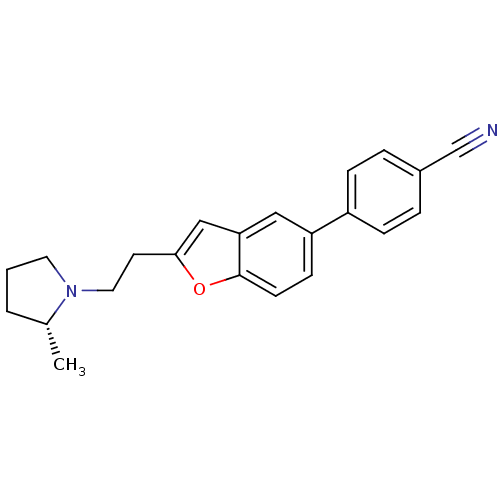 (R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofuran-5-yl)benzonitrile 4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-benzofuran-5-yl}-benzonitrile ABT-239 CHEMBL351231 BDBM50139391
(R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofuran-5-yl)benzonitrile 4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-benzofuran-5-yl}-benzonitrile ABT-239 CHEMBL351231 BDBM50139391 N-((1R,3R,5S)-8-(((1r,4R)-4-(benzylamino)cyclohexyl)sulfonyl)-8-azabicyclo[3.2.1]octan-3-yl)-2,2-difluorobenzo[d][1,3]dioxole-5-carboxamide BDBM378494 US10266526, Compound 627
N-((1R,3R,5S)-8-(((1r,4R)-4-(benzylamino)cyclohexyl)sulfonyl)-8-azabicyclo[3.2.1]octan-3-yl)-2,2-difluorobenzo[d][1,3]dioxole-5-carboxamide BDBM378494 US10266526, Compound 627 BDBM407685 US10336762, Compound 627 (R)-4-((4-(1,3,4-oxadiazol-2- yl)phenyl)amino)-6-(3- cyanopyrrolo[1,2-b] pyridazin-7-yl)-N- (2-fluoro-3-hydroxy-3- methylbutyl)nicotinamide
BDBM407685 US10336762, Compound 627 (R)-4-((4-(1,3,4-oxadiazol-2- yl)phenyl)amino)-6-(3- cyanopyrrolo[1,2-b] pyridazin-7-yl)-N- (2-fluoro-3-hydroxy-3- methylbutyl)nicotinamide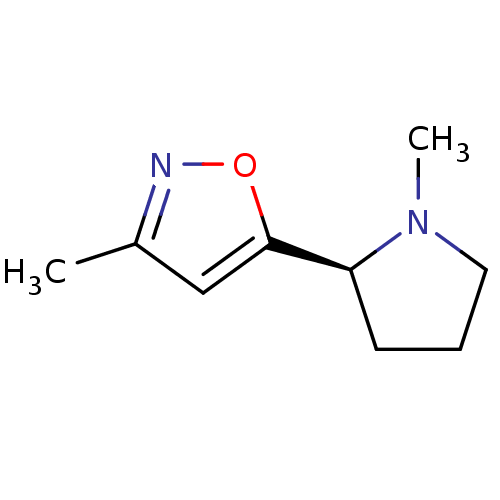 (S)-3-methyl-5-(1-methylpyrrolidin-2-yl)isoxazole BDBM50035398 3-Methyl-5-((S)-1-methyl-pyrrolidin-2-yl)-isoxazole ABT-418 (S)-1-Methyl-2-(3-methyl-isoxazol-5-yl)-pyrrolidinium CHEMBL274525
(S)-3-methyl-5-(1-methylpyrrolidin-2-yl)isoxazole BDBM50035398 3-Methyl-5-((S)-1-methyl-pyrrolidin-2-yl)-isoxazole ABT-418 (S)-1-Methyl-2-(3-methyl-isoxazol-5-yl)-pyrrolidinium CHEMBL274525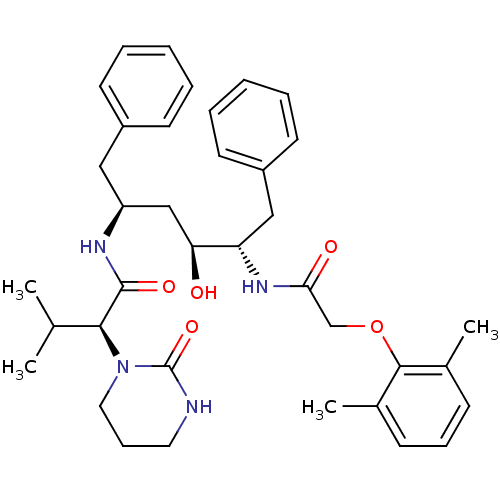 CHEMBL729 ABT-378 BDBM578 LPV Aluviran Lopinavir (2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
CHEMBL729 ABT-378 BDBM578 LPV Aluviran Lopinavir (2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide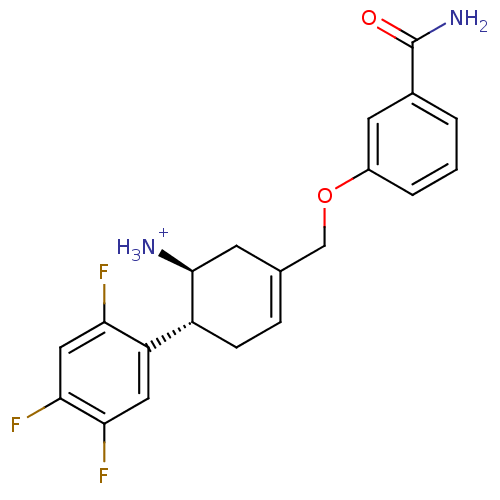 BDBM12654 ABT-341 analogue 17 3-{[trans-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex-1-en-1-yl]methoxy}benzamide (1S,6R)-3-(3-carbamoylphenoxymethyl)-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate
BDBM12654 ABT-341 analogue 17 3-{[trans-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex-1-en-1-yl]methoxy}benzamide (1S,6R)-3-(3-carbamoylphenoxymethyl)-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate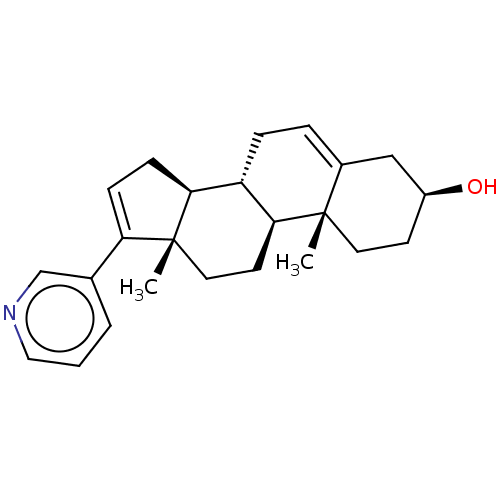 CB 7598 CHEMBL254328 (1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3-yl)tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-7,13-dien-5-ol ABT Abiraterone US9487554, Abiraterone BDBM25458 US9611270, Example 5, abiraterone
CB 7598 CHEMBL254328 (1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3-yl)tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-7,13-dien-5-ol ABT Abiraterone US9487554, Abiraterone BDBM25458 US9611270, Example 5, abiraterone BDBM553541 US11319299, Example 627 4-(difluoromethyl)-N-[5- [2,4-difluoro-5-(2,4,4- trimethylpentan-2- ylcarbamoyl)phenyl]-4- fluoro-2-[rac-(3R,5S)- 3,4,5-trimethylpiperazin- 1-yl]phenyl]-6-oxo-1H- pyridine-3-carboxamide
BDBM553541 US11319299, Example 627 4-(difluoromethyl)-N-[5- [2,4-difluoro-5-(2,4,4- trimethylpentan-2- ylcarbamoyl)phenyl]-4- fluoro-2-[rac-(3R,5S)- 3,4,5-trimethylpiperazin- 1-yl]phenyl]-6-oxo-1H- pyridine-3-carboxamide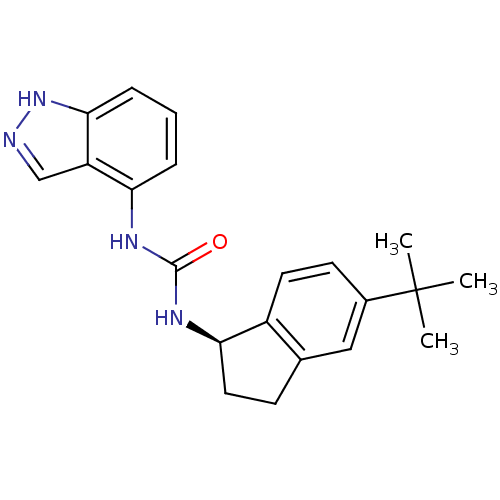 ABT-102 CHEMBL398338 N-[(1R)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-N'-1H-indazol-4-ylurea (R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)urea BDBM50232114
ABT-102 CHEMBL398338 N-[(1R)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-N'-1H-indazol-4-ylurea (R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)urea BDBM50232114 (E)-N-Cyclopropyl-3-(3-(3,3-difluoropyrrolidin-1-yl)-3-oxoprop-1-en-1-yl)-7-hydroxy-2-methyl-4-neopentyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidine-6-carboxamide BDBM660190 US20240092784, Example 627
(E)-N-Cyclopropyl-3-(3-(3,3-difluoropyrrolidin-1-yl)-3-oxoprop-1-en-1-yl)-7-hydroxy-2-methyl-4-neopentyl-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidine-6-carboxamide BDBM660190 US20240092784, Example 627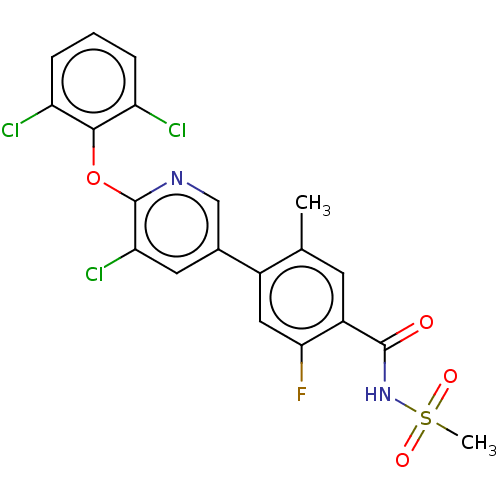 4-(5-chloro-6-(2,6-dichlorophenoxy)-3-pyridinyl)-2-fluoro-5-methyl-N-(methylsulfonyl)benzamide US9663508, Example 627 BDBM329126 4-(5-chloro-6-(2,6- dichlorophenoxy)-3-pyridinyl)- 2-fluoro-5-methyl-N- (methylsulfonyl)benzamide
4-(5-chloro-6-(2,6-dichlorophenoxy)-3-pyridinyl)-2-fluoro-5-methyl-N-(methylsulfonyl)benzamide US9663508, Example 627 BDBM329126 4-(5-chloro-6-(2,6- dichlorophenoxy)-3-pyridinyl)- 2-fluoro-5-methyl-N- (methylsulfonyl)benzamide US11618753, Example 627 BDBM599466 5-[4-amino-5- (trifluoromethyl)pyrrolo[2,1- f][1,2,4]triazin-7-yl]-3-fluoro-N- [(3R,4S)-4-fluoro-1-(3,3,3- trifluoro-2- methylpropanoyl)pyrrolidin-3- yl]-2-(fluoromethyl)benzamide
US11618753, Example 627 BDBM599466 5-[4-amino-5- (trifluoromethyl)pyrrolo[2,1- f][1,2,4]triazin-7-yl]-3-fluoro-N- [(3R,4S)-4-fluoro-1-(3,3,3- trifluoro-2- methylpropanoyl)pyrrolidin-3- yl]-2-(fluoromethyl)benzamide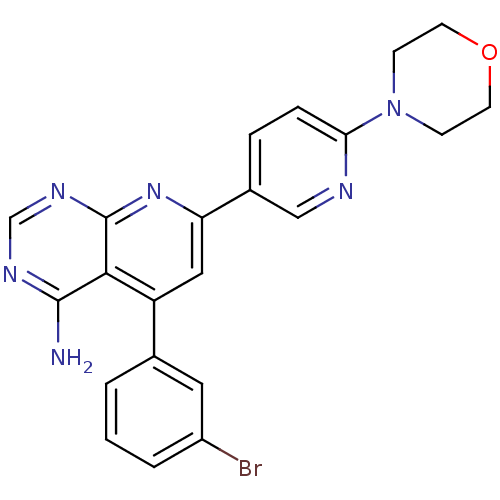 5-(3-bromophenyl)-7-(6-morpholin-4-ylpyridin-3-yl)pyrido[2,3-d]pyrimidin-4-ylamine 5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-yl)-pyrido[2,3-d]pyrimidin-4-ylamine ABT-702 CHEMBL66089 BDBM50094703
5-(3-bromophenyl)-7-(6-morpholin-4-ylpyridin-3-yl)pyrido[2,3-d]pyrimidin-4-ylamine 5-(3-Bromo-phenyl)-7-(6-morpholin-4-yl-pyridin-3-yl)-pyrido[2,3-d]pyrimidin-4-ylamine ABT-702 CHEMBL66089 BDBM50094703 BDBM499947 US11020398, Compound I-627 (4-chloro-3-(4-(((3R,6S)-6- (hydroxymethyl)tetrahydro-2H-pyran-3- yl)amino)-7H-pyrrolo[2,3-d]pyrimidine- 5-carbonyl)phenyl)(4-(pyridin-2- yl)piperazin-1-yl)methanone
BDBM499947 US11020398, Compound I-627 (4-chloro-3-(4-(((3R,6S)-6- (hydroxymethyl)tetrahydro-2H-pyran-3- yl)amino)-7H-pyrrolo[2,3-d]pyrimidine- 5-carbonyl)phenyl)(4-(pyridin-2- yl)piperazin-1-yl)methanone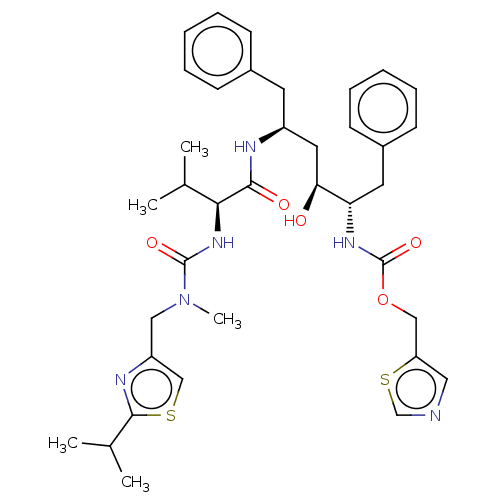 CHEMBL163 Norvir Ritonavir RTV 1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[(2S)-3-methyl-2-{[methyl({[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl})carbamoyl]amino}butanamido]-1,6-diphenylhexan-2-yl]carbamate ABT-538 BDBM520
CHEMBL163 Norvir Ritonavir RTV 1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[(2S)-3-methyl-2-{[methyl({[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl})carbamoyl]amino}butanamido]-1,6-diphenylhexan-2-yl]carbamate ABT-538 BDBM520 BDBM684860 US20240228512, Example 627 4-(10-((R)-1-(2-aminopyridin-3-yl)ethyl)-4-fluoro-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-9,10-dihydro-8H-7-oxa-1,3,6,10-tetraazacyclohepta[de]naphthalen-5-yl)-6-methyl-5-(trifluoromethyl)pyridin-2-amine
BDBM684860 US20240228512, Example 627 4-(10-((R)-1-(2-aminopyridin-3-yl)ethyl)-4-fluoro-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-9,10-dihydro-8H-7-oxa-1,3,6,10-tetraazacyclohepta[de]naphthalen-5-yl)-6-methyl-5-(trifluoromethyl)pyridin-2-amine BDBM671558 (3S,9S,18S,21S,25S,28S,34S)-3-[2-[3-chloro-4-(trifluoromethyl)phenyl]ethyl]-9-(cyclohexylmethyl)-18-(cyclopentylmethyl)-21-isobutyl-28-isopropyl-7,10,13,16,22,26,29-heptamethyl-25-(piperidine-1-carbonyl)spiro[1,4,7,10,13,16,19,22,26,29,32-undecazabicyclo[32.3.0]heptatriacontane-31,1'-cyclopentane]-2,5,8,11,14,17,20,23,27,30,33-undecone US20240148821, Compound 627
BDBM671558 (3S,9S,18S,21S,25S,28S,34S)-3-[2-[3-chloro-4-(trifluoromethyl)phenyl]ethyl]-9-(cyclohexylmethyl)-18-(cyclopentylmethyl)-21-isobutyl-28-isopropyl-7,10,13,16,22,26,29-heptamethyl-25-(piperidine-1-carbonyl)spiro[1,4,7,10,13,16,19,22,26,29,32-undecazabicyclo[32.3.0]heptatriacontane-31,1'-cyclopentane]-2,5,8,11,14,17,20,23,27,30,33-undecone US20240148821, Compound 627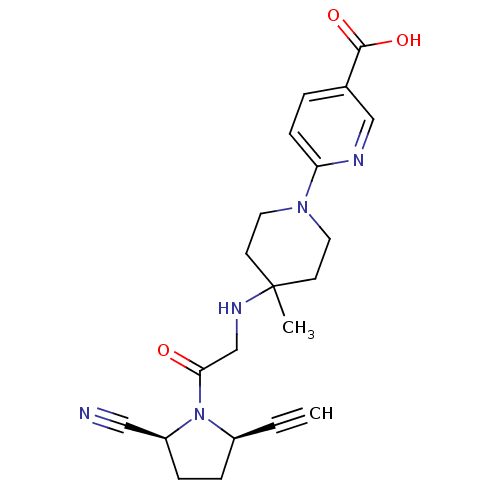 (2S,5R)-5-ethynyl-1-(N-(4-methyl-1-(5-carboxy-pyridin-2-yl)piperidin-4-yl)glycyl)pyrrolidine-2-carbonitrile BDBM12647 ABT-279 analogue C5-substituted pyrrolidine 41 6-[4-({2-[(2S,5R)-2-cyano-5-ethynylpyrrolidin-1-yl]-2-oxoethyl}amino)-4-methylpiperidin-1-yl]pyridine-3-carboxylic acid
(2S,5R)-5-ethynyl-1-(N-(4-methyl-1-(5-carboxy-pyridin-2-yl)piperidin-4-yl)glycyl)pyrrolidine-2-carbonitrile BDBM12647 ABT-279 analogue C5-substituted pyrrolidine 41 6-[4-({2-[(2S,5R)-2-cyano-5-ethynylpyrrolidin-1-yl]-2-oxoethyl}amino)-4-methylpiperidin-1-yl]pyridine-3-carboxylic acid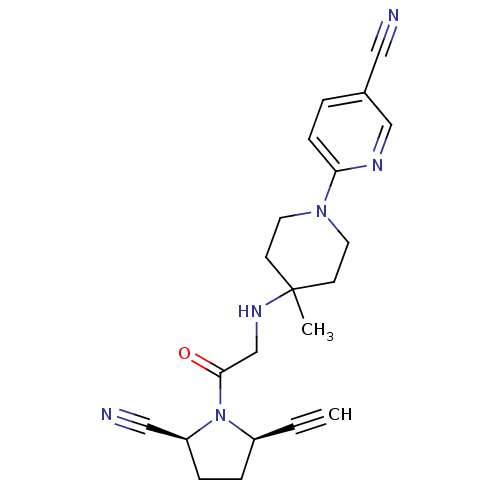 BDBM12646 ABT-279 analogue C5-substituted pyrrolidine 40 (2S,5R)-5-ethynyl-1-(N-(4-methyl-1-(5-cyano-pyridin-2-yl)piperidin-4-yl)glycyl)pyrrolidine-2-carbonitrile 6-[4-({2-[(2S,5R)-2-cyano-5-ethynylpyrrolidin-1-yl]-2-oxoethyl}amino)-4-methylpiperidin-1-yl]pyridine-3-carbonitrile
BDBM12646 ABT-279 analogue C5-substituted pyrrolidine 40 (2S,5R)-5-ethynyl-1-(N-(4-methyl-1-(5-cyano-pyridin-2-yl)piperidin-4-yl)glycyl)pyrrolidine-2-carbonitrile 6-[4-({2-[(2S,5R)-2-cyano-5-ethynylpyrrolidin-1-yl]-2-oxoethyl}amino)-4-methylpiperidin-1-yl]pyridine-3-carbonitrile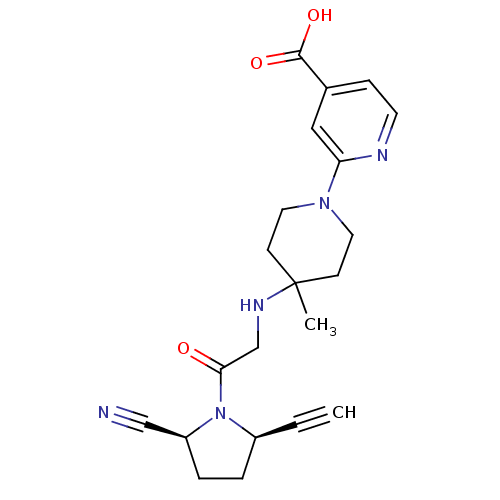 2-[4-{{2-(2S,5R)-2-Cyano-5-ethynyl-1-pyrrolidinyl]-2-oxoethyl]amino]-4-methyl-1-piperidinyl]-4-pyridinecarboxylic Acid 2-[4-({2-[(2S,5R)-2-cyano-5-ethynylpyrrolidin-1-yl]-2-oxoethyl}amino)-4-methylpiperidin-1-yl]pyridine-4-carboxylic acid ABT-279 BDBM12648 (2S,5R)-5-Ethynyl-1-{N-(4-methyl-1-(4-carboxy-pyridin-2-yl)-piperidin-4-yl)glycyl}pyrrolidine-2-carbonitrile
2-[4-{{2-(2S,5R)-2-Cyano-5-ethynyl-1-pyrrolidinyl]-2-oxoethyl]amino]-4-methyl-1-piperidinyl]-4-pyridinecarboxylic Acid 2-[4-({2-[(2S,5R)-2-cyano-5-ethynylpyrrolidin-1-yl]-2-oxoethyl}amino)-4-methylpiperidin-1-yl]pyridine-4-carboxylic acid ABT-279 BDBM12648 (2S,5R)-5-Ethynyl-1-{N-(4-methyl-1-(4-carboxy-pyridin-2-yl)-piperidin-4-yl)glycyl}pyrrolidine-2-carbonitrile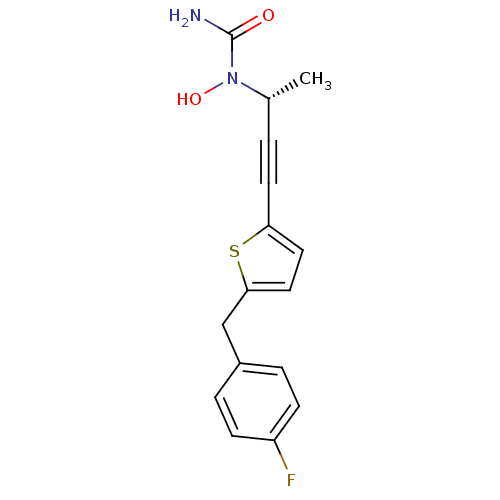 A-85761 (R)-(+)-N-{3-[5-(4-fluorobenzyl)thien-2-yl]-1-methylprop-2-ynyl}-N-hydroxyurea N-{(1R)-3-[5-(4-fluorobenzyl)thien-2-yl]-1-methylprop-2-ynyl}-N-hydroxyurea Atreleuton CHEMBL59356 N-{(1R)-3-[5-(4-fluorobenzyl)thien-2-yl]-1-methylprop-2-ynyl}-N-hydroxyurea (ABT-761) {3-[5-(4-Fluoro-benzyl)-thiophen-2-yl]-1-methyl-prop-2-ynyl}-hydroxy urea BDBM50029781
A-85761 (R)-(+)-N-{3-[5-(4-fluorobenzyl)thien-2-yl]-1-methylprop-2-ynyl}-N-hydroxyurea N-{(1R)-3-[5-(4-fluorobenzyl)thien-2-yl]-1-methylprop-2-ynyl}-N-hydroxyurea Atreleuton CHEMBL59356 N-{(1R)-3-[5-(4-fluorobenzyl)thien-2-yl]-1-methylprop-2-ynyl}-N-hydroxyurea (ABT-761) {3-[5-(4-Fluoro-benzyl)-thiophen-2-yl]-1-methyl-prop-2-ynyl}-hydroxy urea BDBM50029781 1-[4-(3-azanyl-1H-indazol-4-yl)phenyl]-3-(2-fluoranyl-5-methyl-phenyl)urea 1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-5-methyl-phenyl)urea CHEMBL223360 1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-5-methylphenyl)urea 3-[4-(3-amino-1H-indazol-4-yl)phenyl]-1-(2-fluoro-5-methylphenyl)urea ABT-869 US20250129067, Compound Linifanib BDBM21079 Aminoindazole, 3 cid_11485656
1-[4-(3-azanyl-1H-indazol-4-yl)phenyl]-3-(2-fluoranyl-5-methyl-phenyl)urea 1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-5-methyl-phenyl)urea CHEMBL223360 1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-5-methylphenyl)urea 3-[4-(3-amino-1H-indazol-4-yl)phenyl]-1-(2-fluoro-5-methylphenyl)urea ABT-869 US20250129067, Compound Linifanib BDBM21079 Aminoindazole, 3 cid_11485656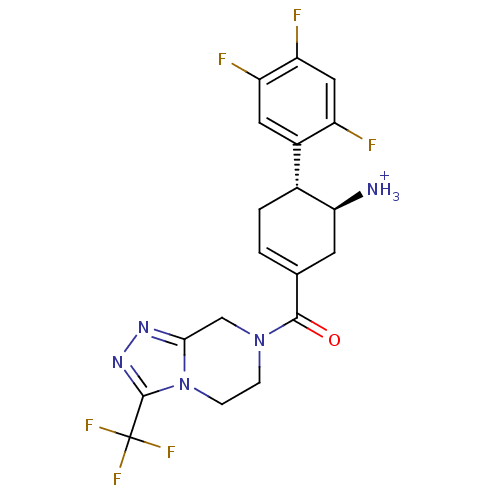 (1S,6R)-3-{[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]carbonyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium trifluoroacetate BDBM12656 (1S,6R)-3-{[3-(trifluoromethyl)-5H,6H,7H,8H-[1,2,4]triazolo[3,4-a]pyrazin-7-yl]carbonyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate ((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex-1-enyl)-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone ABT-341
(1S,6R)-3-{[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]carbonyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium trifluoroacetate BDBM12656 (1S,6R)-3-{[3-(trifluoromethyl)-5H,6H,7H,8H-[1,2,4]triazolo[3,4-a]pyrazin-7-yl]carbonyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate ((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex-1-enyl)-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone ABT-341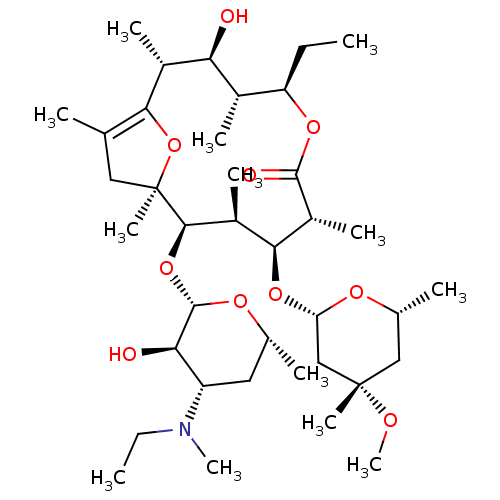 ABT-229 5-Ethyl-11-[4-(ethyl-methyl-amino)-3-hydroxy-6-methyl-tetrahydro-pyran-2-yloxy]-3-hydroxy-9-(4-methoxy-4,6-dimethyl-tetrahydro-pyran-2-yloxy)-2,4,8,10,12,14-hexamethyl-6,15-dioxa-bicyclo[10.2.1]pentadec-1(14)-en-7-one (2R,3S,4R,5R,8R,9S,10S,11R,12S)-5-ethyl-11-((2S,3R,4S,6R)-4-(ethyl(methyl)amino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-3-hydroxy-9-((2S,4S,6S)-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yloxy)-2,4,8,10,12,14-hexamethyl-6,15-dioxabicyclo[10.2.1]pentadec-1(14)-en-7-one CHEMBL1628277 BDBM50344952
ABT-229 5-Ethyl-11-[4-(ethyl-methyl-amino)-3-hydroxy-6-methyl-tetrahydro-pyran-2-yloxy]-3-hydroxy-9-(4-methoxy-4,6-dimethyl-tetrahydro-pyran-2-yloxy)-2,4,8,10,12,14-hexamethyl-6,15-dioxa-bicyclo[10.2.1]pentadec-1(14)-en-7-one (2R,3S,4R,5R,8R,9S,10S,11R,12S)-5-ethyl-11-((2S,3R,4S,6R)-4-(ethyl(methyl)amino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-3-hydroxy-9-((2S,4S,6S)-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yloxy)-2,4,8,10,12,14-hexamethyl-6,15-dioxabicyclo[10.2.1]pentadec-1(14)-en-7-one CHEMBL1628277 BDBM50344952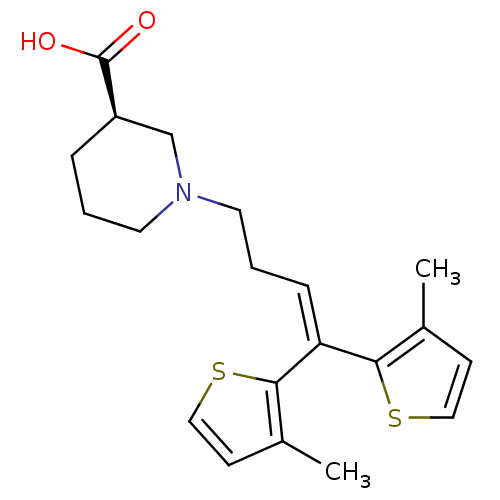 1-[4,4-Bis-(3-methyl-4,5-dihydro-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid (R)-1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid NO-05-0328 Gabitril Tiagabine hydrochloride BDBM50039251 (R)-1-(4,4-bis(3-methylthiophen-2-yl)but-3-enyl)piperidine-3-carboxylic acid hydrochloride A-70569-1 (S)-1-(4,4-bis(3-methylthiophen-2-yl)but-3-enyl)piperidine-3-carboxylic acid ABBOTT-70569 1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid; hydrochloride CHEMBL1027 ABT-569 ABBOTT-70569-1 NNC-05-0328 TIAGABINE (R)-1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid; hydrochloride
1-[4,4-Bis-(3-methyl-4,5-dihydro-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid (R)-1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid NO-05-0328 Gabitril Tiagabine hydrochloride BDBM50039251 (R)-1-(4,4-bis(3-methylthiophen-2-yl)but-3-enyl)piperidine-3-carboxylic acid hydrochloride A-70569-1 (S)-1-(4,4-bis(3-methylthiophen-2-yl)but-3-enyl)piperidine-3-carboxylic acid ABBOTT-70569 1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid; hydrochloride CHEMBL1027 ABT-569 ABBOTT-70569-1 NNC-05-0328 TIAGABINE (R)-1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid; hydrochloride