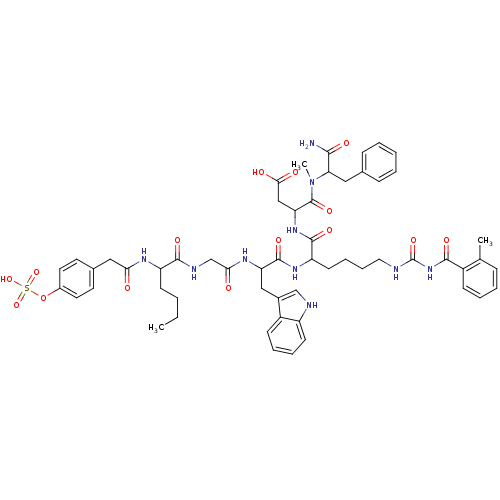
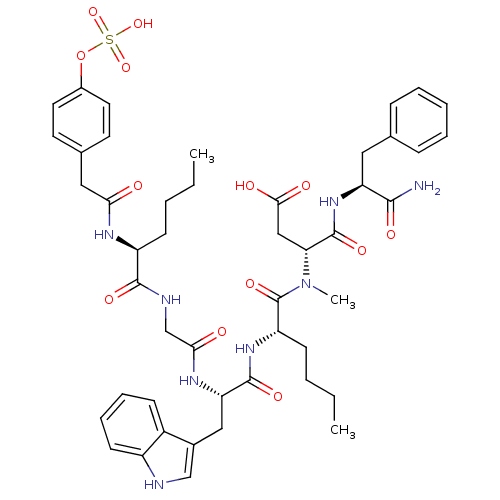
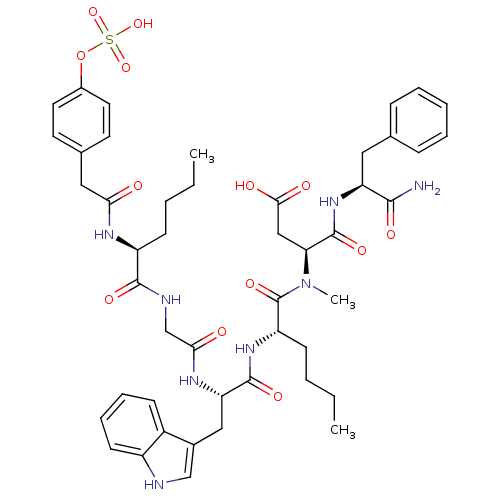
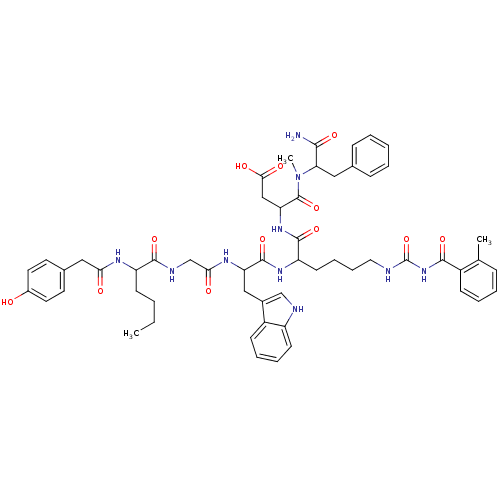
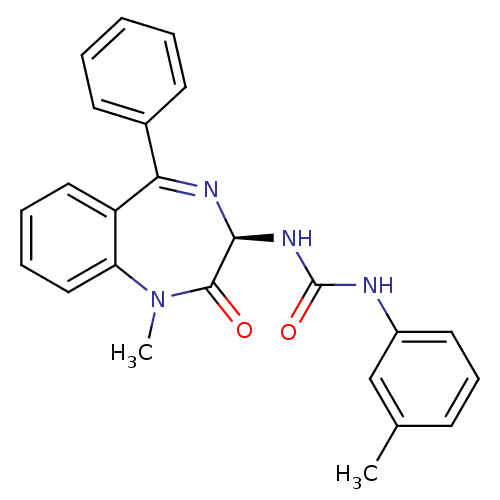
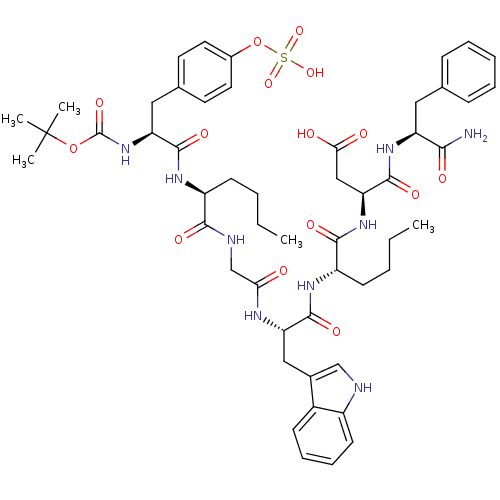
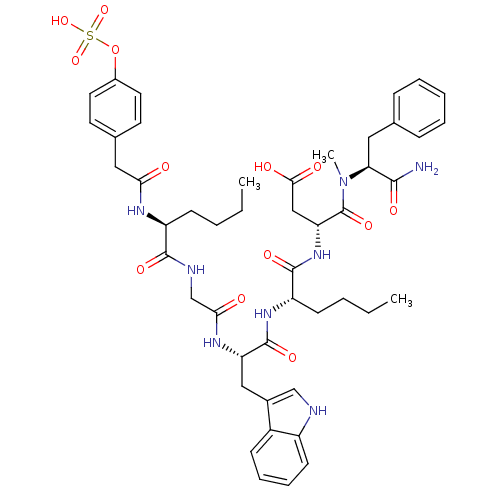
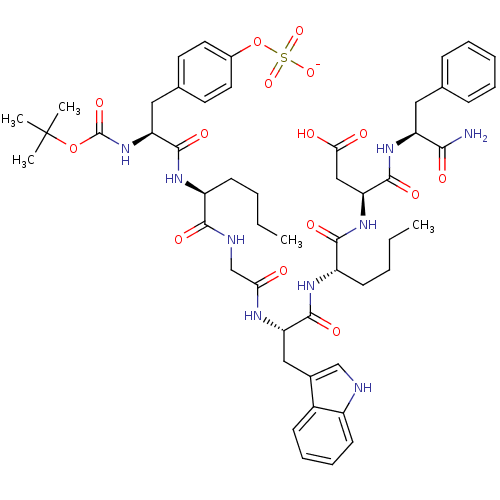
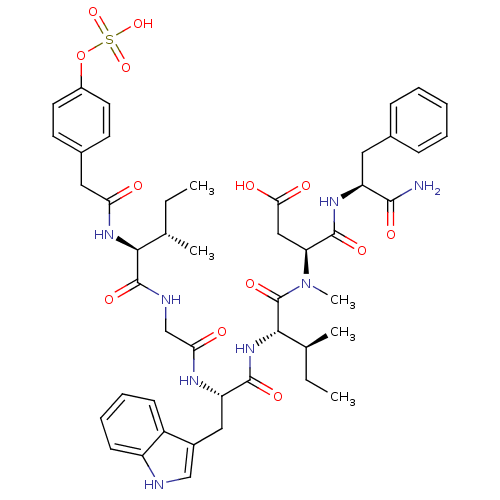
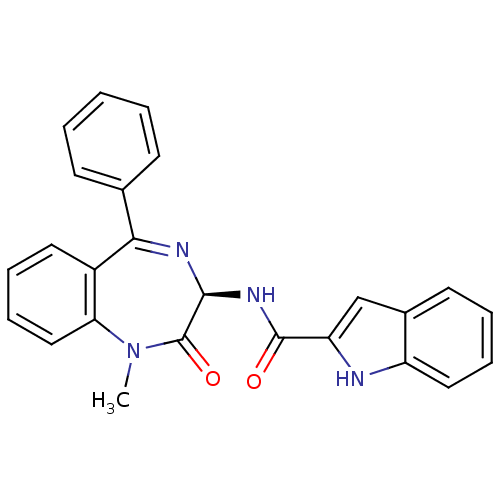
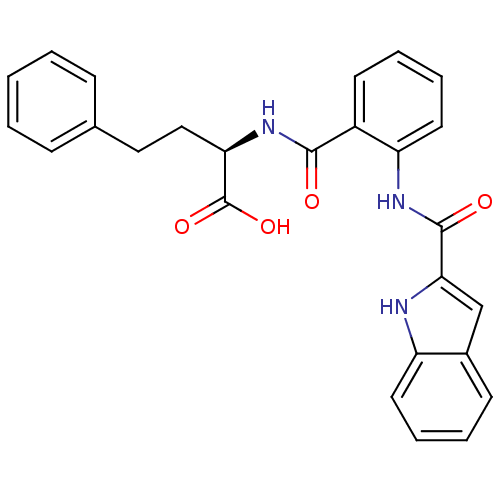
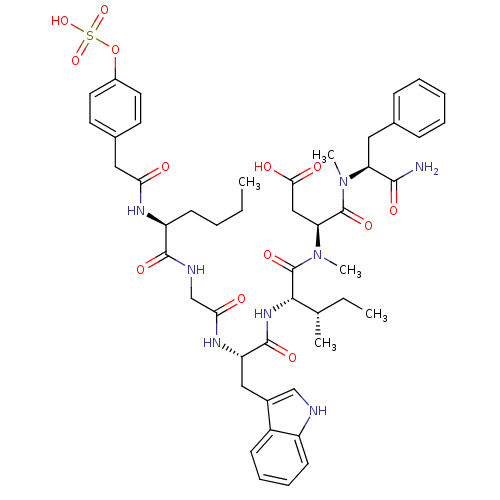
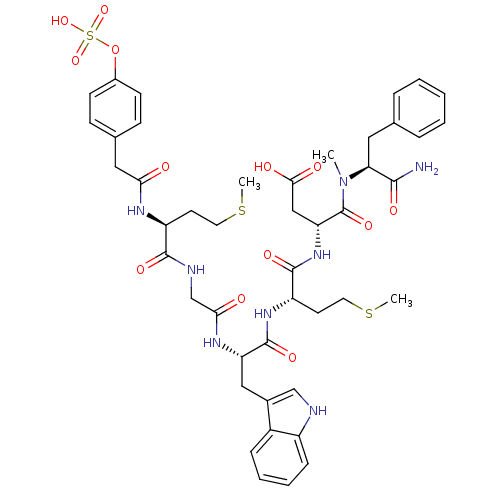
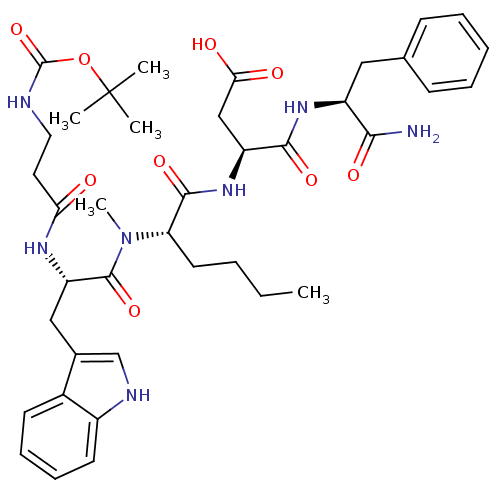
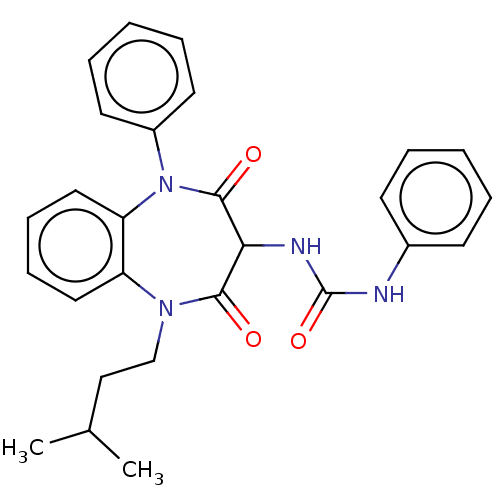
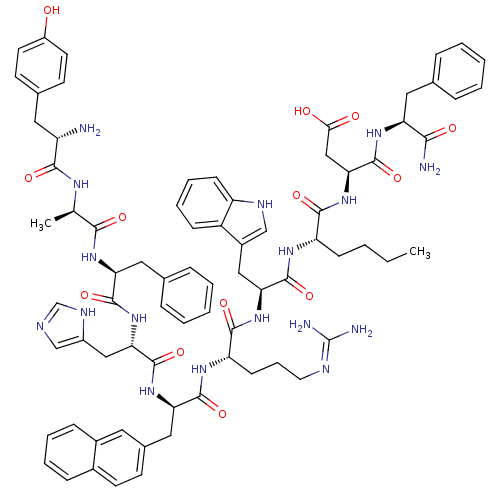
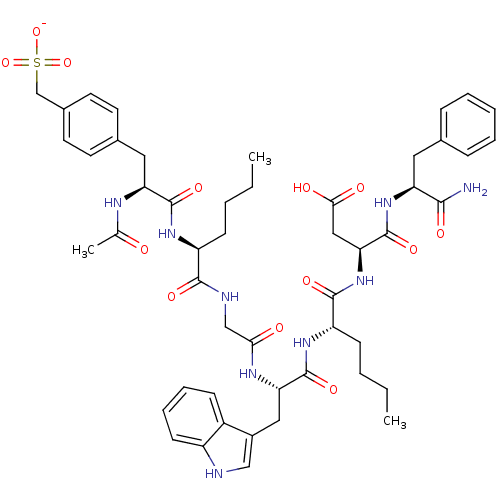
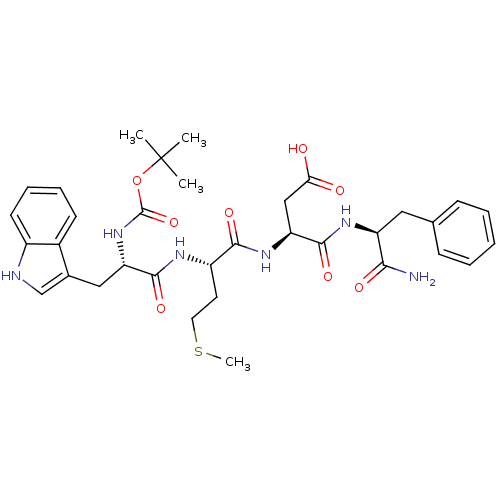
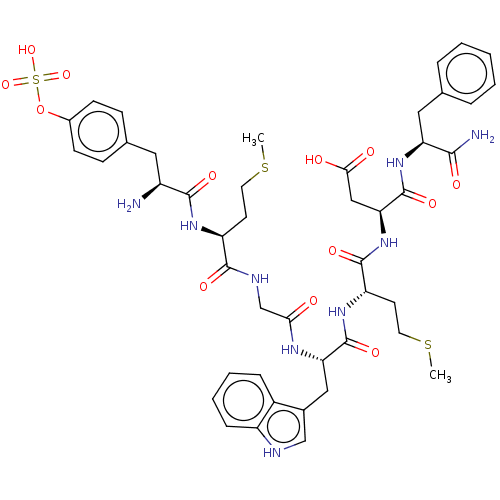
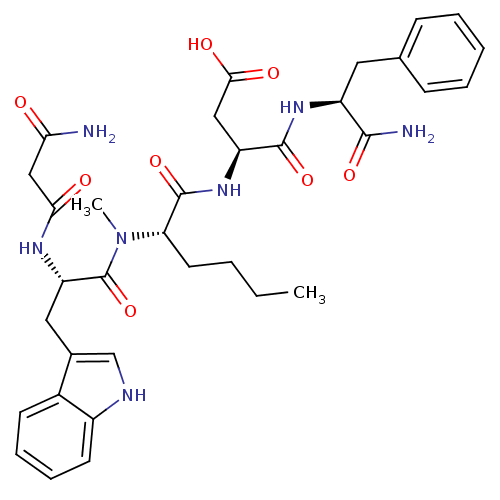
Report error Found 3519 Enz. Inhib. hit(s) with Target = 'Cholecystokinin receptor type A'
Affinity DataKi: 0.0200nMAssay Description:Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0300nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0300nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0300nMAssay Description:Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0700nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.148nMAssay Description:Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.148nMAssay Description:Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.148nMAssay Description:Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranesMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Mus musculus)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 0.191nMAssay Description:Binding affinity measured by inhibiting [3H]Boc[Nle28,31]CCK27-33 specific binding to Cholecystokinin receptor in mouse brain membranes at a KD conce...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.230nMAssay Description:The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig brainMore data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.280nMAssay Description:The compound was tested for the inhibition of [3H]-Propionyl specific binding to CCK-8 receptor of guinea pig brainMore data for this Ligand-Target Pair
Affinity DataKi: 0.280nMAssay Description:Displacement of 0.1 nM [3H]-pCCK-8 from guinea pig pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.280nMAssay Description:Inhibition of [3H]pCCK-8 binding to Cholecystokinin type A receptor of Guinea pig pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreasMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Mus musculus)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Potency in displacing [3H]propionyl-Cholecystokinin from mouse brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.310nMAssay Description:Ability to displace 1 nM [3H]pCCK-8 from Cholecystokinin type A receptor in guinea pig pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.320nMAssay Description:Displacement of [125I]-BH-JMV-179 from wild type human CCK1 receptor expressed in COS7 cells after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Mus musculus)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 0.491nMAssay Description:Binding affinity measured by inhibiting [3H]Boc[Nle28,31]CCK27-33 specific binding to Cholecystokinin receptor in mouse brain membranes at a KD conce...More data for this Ligand-Target Pair
Affinity DataKi: 0.520nMAssay Description:Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreasMore data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Ability to displace 1 nM [3H]pCCK-8 from Cholecystokinin type A receptor in guinea pig pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.620nMAssay Description:Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreasMore data for this Ligand-Target Pair
Affinity DataKi: 0.640nMAssay Description:Tested for the inhibition of [3H]-pCCK-8 binding to Cholecystokinin type A receptor in pancreatic membranes of guinea-pigMore data for this Ligand-Target Pair
Affinity DataKi: 0.640nMAssay Description:The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig brainMore data for this Ligand-Target Pair
Affinity DataKi: >0.700nMAssay Description:Affinity against Cholecystokinin type A receptor on guinea pig pancreatic membranes.More data for this Ligand-Target Pair
Affinity DataKi: >0.700nMAssay Description:Affinity against Cholecystokinin type A receptor on guinea pig pancreatic membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Displacement of [3H]-pCCK-8 from cholecystokinin-A receptor in guinea pig pancreatic membraneMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Compound was tested for binding affinity against CCK1 (cholecystokinin) receptor on guinea pig pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:Ability to displace 1 nM [3H]pCCK-8 from Cholecystokinin type A receptor in guinea pig pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.930nMAssay Description:The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig pancreatic membraneMore data for this Ligand-Target Pair
Affinity DataKi: 0.930nMAssay Description:Displacement of 0.1 nM [3H]-pCCK-8 from guinea pig pancreatic membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.980nMAssay Description:Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.980nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 1.35nMAssay Description:Affinity against Cholecystokinin type A receptor on guinea pig pancreatic membranes.More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Binding affinity was measured by its ability to displace [3H]CCK-8S from CCK-A receptor in rat pancreasMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig pancreatic membraneMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Mus musculus)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]pentagastrin from Cholecystokinin receptor of mouse cerebral cortexMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Mus musculus)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]pentagastrin from Cholecystokinin receptor of mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataKi: 2.05nMAssay Description:Affinity against Cholecystokinin type A receptor on guinea pig pancreatic membranes.More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Displacement of [125I]BH-(Thr,-Nle)-CCK-9 from human CCK1 receptor expressed in COS-7 cells after 60 mins by gamma countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Affinity against Cholecystokinin type A receptor on guinea pig pancreatic membranes.More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Ability to displace 1 nM [3H]pCCK-8 from Cholecystokinin type A receptor in guinea pig pancreatic membranesMore data for this Ligand-Target Pair