Compound (270)
Article Title (109)
Assay (17)
Carboni, JM; Wittman, M; Yang, Z; Lee, F; Greer, A; Hurlburt, W; Hillerman, S; Cao, C; Cantor, GH; Dell-John, J; Chen, C; Discenza, L; Menard, K; Li, A; Trainor, G; Vyas, D; Kramer, R; Attar, RM; Gottardis, MM BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther 8: 3341 -9 Wittman, MD; Carboni, JM; Yang, Z; Lee, FY; Antman, M; Attar, R; Balimane, P; Chang, C; Chen, C; Discenza, L; Frennesson, D; Gottardis, MM; Greer, A; Hurlburt, W; Johnson, W; Langley, DR; Li, A; Li, J; Liu, P; Mastalerz, H; Mathur, A; Menard, K; Patel, K; Sack, J; Sang, X; Saulnier, M; Smith, D; Stefanski, K; Trainor, G; Velaparthi, U; Zhang, G; Zimmermann, K; Vyas, DM Discovery of a 2,4-disubstituted pyrrolo[1,2-f][1,2,4]triazine inhibitor (BMS-754807) of insulin-like growth factor receptor (IGF-1R) kinase in clinical development. J Med Chem 52: 7360 -3 (2009) Veach, DR; Namavari, M; Pillarsetty, N; Santos, EB; Beresten-Kochetkov, T; Lambek, C; Punzalan, BJ; Antczak, C; Smith-Jones, PM; Djaballah, H; Clarkson, B; Larson, SM Synthesis and biological evaluation of a fluorine-18 derivative of dasatinib. J Med Chem 50: 5853 -7 (2007) Kwarcinski, FE; Brandvold, KR; Phadke, S; Beleh, OM; Johnson, TK; Meagher, JL; Seeliger, MA; Stuckey, JA; Soellner, MB Conformation-Selective Analogues of Dasatinib Reveal Insight into Kinase Inhibitor Binding and Selectivity. ACS Chem Biol 11: 1296 -304 (2016) Li, HY; He, DD; Zhao, XJ; Sun, TY; Zhang, Q; Bai, CG; Chen, Y Design and synthesis of novel dasatinib derivatives as inhibitors of leukemia stem cells. Bioorg Med Chem Lett 28: 700 -706 (2018) Hantschel, O; Rix, U; Schmidt, U; Bürckstümmer, T; Kneidinger, M; Schütze, G; Colinge, J; Bennett, KL; Ellmeier, W; Valent, P; Superti-Furga, G The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci U S A 104: 13283 -8 (2007) Liu, L; Hussain, M; Luo, J; Duan, A; Chen, C; Tu, Z; Zhang, J Synthesis and biological evaluation of novel dasatinib analogues as potent DDR1 and DDR2 kinase inhibitors. Chem Biol Drug Des 89: 420 -427 (2017) Washburn, WN; Sun, CQ; Bisacchi, G; Wu, G; Cheng, PT; Sher, PM; Ryono, D; Gavai, AV; Poss, K; Girotra, RN; McCann, PJ; Mikkilineni, AB; Dejneka, TC; Wang, TC; Merchant, Z; Morella, M; Arbeeny, CM; Harper, TW; Slusarchyk, DA; Skwish, S; Russell, AD; Allen, GT; Tesfamariam, B; Frohlich, BH; Abboa-Offei, BE; Cap, M; Waldron, TL; George, RJ; Young, D; Dickinson, KE; Seymour, AA BMS-201620: a selective beta 3 agonist. Bioorg Med Chem Lett 14: 3525 -9 (2004) Priestley, ES; Banville, J; Deon, D; Dubé, L; Gagnon, M; Guy, J; Lapointe, P; Lavallée, JF; Martel, A; Plamondon, S; Rémillard, R; Ruediger, E; Tremblay, F; Posy, SL; Guarino, VR; Richter, JM; Li, J; Gupta, A; Vetrichelvan, M; Balapragalathan, TJ; Mathur, A; Hua, J; Callejo, M; Guay, J; Sum, CS; Cvijic, ME; Watson, C; Wong, P; Yang, J; Bouvier, M; Gordon, DA; Wexler, RR; Marinier, A Discovery of Two Novel Antiplatelet Clinical Candidates (BMS-986120 and BMS-986141) That Antagonize Protease-Activated Receptor 4. J Med Chem 65: 8843 -8854 (2022) Yang, MG; Beaudoin-Bertrand, M; Xiao, Z; Marcoux, D; Weigelt, CA; Yip, S; Wu, DR; Ruzanov, M; Sack, JS; Wang, J; Yarde, M; Li, S; Shuster, DJ; Xie, JH; Sherry, T; Obermeier, MT; Fura, A; Stefanski, K; Cornelius, G; Khandelwal, P; Karmakar, A; Basha, M; Babu, V; Gupta, AK; Mathur, A; Salter-Cid, L; Denton, R; Zhao, Q; Dhar, TGM Tricyclic-Carbocyclic RORγt Inverse Agonists-Discovery of BMS-986313. J Med Chem 64: 2714 -2724 (2021) Das, J; Chen, P; Norris, D; Padmanabha, R; Lin, J; Moquin, RV; Shen, Z; Cook, LS; Doweyko, AM; Pitt, S; Pang, S; Shen, DR; Fang, Q; de Fex, HF; McIntyre, KW; Shuster, DJ; Gillooly, KM; Behnia, K; Schieven, GL; Wityak, J; Barrish, JC 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase i J Med Chem 49: 6819 -32 (2006) Shi, Y; Li, C; Oamppound39Connor, SP; Zhang, J; Shi, M; Bisaha, SN; Wang, Y; Sitkoff, D; Pudzianowski, AT; Huang, C; Klei, HE; Kish, K; Yanchunas, J; Liu, EC; Hartl, KS; Seiler, SM; Steinbacher, TE; Schumacher, WA; Atwal, KS; Stein, PD Aroylguanidine-based factor Xa inhibitors: the discovery of BMS-344577. Bioorg Med Chem Lett 19: 6882 -9 (2009) Misra, RN; Brown, BR; Sher, PM; Patel, MM; Hall, SE; Han, WC; Barrish, JC; Floyd, DM; Sprague, PW; Morrison, RA; Ridgewell, RE; White, RE; DiDonato, GC; Harris, DN; Hedberg, A; Schumacher, WA; Webb, ML; Ogletree, ML Thromboxane receptor antagonist BMS-180291: A new pre-clinical lead Bioorg Med Chem Lett 2: 73 -76 (1992) Washburn, WN; Sher, PM; Poss, KM; Girotra, RN; McCann, PJ; Gavai, AV; Mikkilineni, AB; Mathur, A; Cheng, P; Dejneka, TC; Sun, CQ; Wang, TC; Harper, TW; Russell, AD; Slusarchyk, DA; Skwish, S; Allen, GT; Hillyer, DE; Frohlich, BH; Abboa-Offei, BE; Cap, M; Waldron, TL; George, RJ; Tesfamariam, B; Ciosek, CP; Ryono, D; Young, DA; Dickinson, KE; Seymour, AA; Arbeeny, CM; Gregg, RE Beta 3 agonists. Part 1: evolution from inception to BMS-194449. Bioorg Med Chem Lett 11: 3035 -9 (2001) Gardner, DS; Santella, JB; Duncia, JV; Carter, PH; Dhar, TG; Wu, H; Guo, W; Cavallaro, C; Van Kirk, K; Yarde, M; Briceno, SW; Grafstrom, RR; Liu, R; Patel, SR; Tebben, AJ; Camac, D; Khan, J; Watson, A; Yang, G; Rose, A; Foster, WR; Cvijic, ME; Davies, P; Hynes, J The discovery of BMS-457, a potent and selective CCR1 antagonist. Bioorg Med Chem Lett 23: 3833 -40 (2013) Zhang, W; Ai, J; Shi, D; Peng, X; Ji, Y; Liu, J; Geng, M; Li, Y Discovery of novel type II c-Met inhibitors based on BMS-777607. Eur J Med Chem 80: 254 -66 (2014) Shan, W; Balog, A; Quesnelle, C; Gill, P; Han, WC; Norris, D; Mandal, S; Thiruvenkadam, R; Gona, KB; Thiyagarajan, K; Kandula, S; McGlinchey, K; Menard, K; Wen, ML; Rose, A; White, R; Guarino, V; Shen, DR; Cvijic, ME; Ranasinghe, A; Dai, J; Zhang, Y; Wu, DR; Mathur, A; Rampulla, R; Trainor, G; Hunt, JT; Vite, GD; Westhouse, R; Lee, FY; Gavai, AV BMS-871: a novel orally active pan-Notch inhibitor as an anticancer agent. Bioorg Med Chem Lett 25: 1905 -9 (2015) Shi, Y; Li, J; Kennedy, LJ; Tao, S; Hernández, AS; Lai, Z; Chen, S; Wong, H; Zhu, J; Trehan, A; Lim, NK; Zhang, H; Chen, BC; Locke, KT; O'Malley, KM; Zhang, L; Srivastava, RA; Miao, B; Meyers, DS; Monshizadegan, H; Search, D; Grimm, D; Zhang, R; Harrity, T; Kunselman, LK; Cap, M; Muckelbauer, J; Chang, C; Krystek, SR; Li, YX; Hosagrahara, V; Zhang, L; Kadiyala, P; Xu, C; Blanar, MA; Zahler, R; Mukherjee, R; Cheng, PT; Tino, JA Discovery and Preclinical Evaluation of BMS-711939, an Oxybenzylglycine Based PPARa Selective Agonist. ACS Med Chem Lett 7: 590 -4 (2016) Priestley, ES; De Lucca, I; Zhou, J; Zhou, J; Saiah, E; Stanton, R; Robinson, L; Luettgen, JM; Wei, A; Wen, X; Knabb, RM; Wong, PC; Wexler, RR Discovery and gram-scale synthesis of BMS-593214, a potent, selective FVIIa inhibitor. Bioorg Med Chem Lett 23: 2432 -5 (2013) Liu, C; Lin, J; Langevine, C; Smith, D; Li, J; Tokarski, JS; Khan, J; Ruzanov, M; Strnad, J; Zupa-Fernandez, A; Cheng, L; Gillooly, KM; Shuster, D; Zhang, Y; Thankappan, A; McIntyre, KW; Chaudhry, C; Elzinga, PA; Chiney, M; Chimalakonda, A; Lombardo, LJ; Macor, JE; Carter, PH; Burke, JR; Weinstein, DS Discovery of BMS-986202: A Clinical Tyk2 Inhibitor that Binds to Tyk2 JH2. J Med Chem 64: 677 -694 (2021) Cherney, RJ; Cornelius, LAM; Srivastava, A; Weigelt, CA; Marcoux, D; Duan, JJ; Shi, Q; Batt, DG; Liu, Q; Yip, S; Wu, DR; Ruzanov, M; Sack, J; Khan, J; Wang, J; Yarde, M; Cvijic, ME; Mathur, A; Li, S; Shuster, D; Khandelwal, P; Borowski, V; Xie, J; Obermeier, M; Fura, A; Stefanski, K; Cornelius, G; Tino, JA; Macor, JE; Salter-Cid, L; Denton, R; Zhao, Q; Carter, PH; Dhar, TGM Discovery of BMS-986251: A Clinically Viable, Potent, and Selective RORγt Inverse Agonist. ACS Med Chem Lett 11: 1221 -1227 (2020) Santella, JB; Gardner, DS; Duncia, JV; Wu, H; Dhar, M; Cavallaro, C; Tebben, AJ; Carter, PH; Barrish, JC; Yarde, M; Briceno, SW; Cvijic, ME; Grafstrom, RR; Liu, R; Patel, SR; Watson, AJ; Yang, G; Rose, AV; Vickery, RD; Caceres-Cortes, J; Caporuscio, C; Camac, DM; Khan, JA; An, Y; Foster, WR; Davies, P; Hynes, J Discovery of the CCR1 antagonist, BMS-817399, for the treatment of rheumatoid arthritis. J Med Chem 57: 7550 -64 (2014) Boy, KM; Guernon, JM; Zuev, DS; Xu, L; Zhang, Y; Shi, J; Marcin, LR; Higgins, MA; Wu, YJ; Krishnananthan, S; Li, J; Trehan, A; Smith, D; Toyn, JH; Meredith, JE; Burton, CR; Kimura, SR; Zvyaga, T; Zhuo, X; Lentz, KA; Grace, JE; Denton, R; Morrison, JS; Mathur, A; Albright, CF; Ahlijanian, MK; Olson, RE; Thompson, LA; Macor, JE Identification and Preclinical Evaluation of the Bicyclic Pyrimidine γ-Secretase Modulator BMS-932481. ACS Med Chem Lett 10: 312 -317 (2019) Cherney, RJ; Anjanappa, P; Selvakumar, K; Batt, DG; Brown, GD; Rose, AV; Vuppugalla, R; Chen, J; Pang, J; Xu, S; Yarde, M; Tebben, AJ; Paidi, VR; Cvijic, ME; Mathur, A; Barrish, JC; Mandlekar, S; Zhao, Q; Carter, PH BMS-813160: A Potent CCR2 and CCR5 Dual Antagonist Selected as a Clinical Candidate. ACS Med Chem Lett 12: 1753 -1758 (2021) Yang, F; Snyder, LB; Balakrishnan, A; Brown, JM; Sivarao, DV; Easton, A; Fernandes, A; Gulianello, M; Hanumegowda, UM; Huang, H; Huang, Y; Jones, KM; Li, YW; Matchett, M; Mattson, G; Miller, R; Santone, KS; Senapati, A; Shields, EE; Simutis, FJ; Westphal, R; Whiterock, VJ; Bronson, JJ; Macor, JE; Degnan, AP Discovery and Preclinical Evaluation of BMS-955829, a Potent Positive Allosteric Modulator of mGluR5. ACS Med Chem Lett 7: 289 -93 (2016) Yang, MG; Xiao, Z; Zhao, R; Tebben, AJ; Wang, B; Cherney, RJ; Batt, DG; Brown, GD; Cvijic, ME; Duncia, JV; Gallela, MA; Gardner, DS; Khandelwal, P; Malley, MF; Pang, J; Rose, AV; Santella, JB; Sarjeant, AA; Xu, S; Mathur, A; Mandlekar, S; Vuppugalla, R; Zhao, Q; Carter, PH Discovery of BMS-753426: A Potent Orally Bioavailable Antagonist of CC Chemokine Receptor 2. ACS Med Chem Lett 12: 969 -975 (2021) Zheng, BZ; D'Andrea, SV; Hanumegowda, U; Knipe, JO; Mosure, K; Zhuo, X; Lemm, JA; Liu, M; Rigat, KL; Wang, YK; Fang, H; Poronsky, C; Cutrone, J; Wu, DR; Arunachalam, PN; Balapragalathan, TJ; Arumugam, A; Mathur, A; Meanwell, NA; Gao, M; Roberts, SB; Kadow, JF Discovery of BMS-961955, an allosteric inhibitor of the hepatitis C virus NS5B polymerase. Bioorg Med Chem Lett 27: 3294 -3300 (2017) Pinto, DJP; Orwat, MJ; Smith, LM; Quan, ML; Lam, PYS; Rossi, KA; Apedo, A; Bozarth, JM; Wu, Y; Zheng, JJ; Xin, B; Toussaint, N; Stetsko, P; Gudmundsson, O; Maxwell, B; Crain, EJ; Wong, PC; Lou, Z; Harper, TW; Chacko, SA; Myers, JE; Sheriff, S; Zhang, H; Hou, X; Mathur, A; Seiffert, DA; Wexler, RR; Luettgen, JM; Ewing, WR Discovery of a Parenteral Small Molecule Coagulation Factor XIa Inhibitor Clinical Candidate (BMS-962212). J Med Chem 60: 9703 -9723 (2017) Fisher, LG; Sher, PM; Skwish, S; Michel, IM; Seiler, SM; Dickinson, KE BMS-187257, a potent, selective, and novel heterocyclic β3 adrenergic receptor agonist Bioorg Med Chem Lett 6: 2253 -2258 (1996) Gavai, AV; Sher, PM; Mikkilineni, AB; Poss, KM; McCann, PJ; Girotra, RN; Fisher, LG; Wu, G; Bednarz, MS; Mathur, A; Wang, TC; Sun, CQ; Slusarchyk, DA; Skwish, S; Allen, GT; Hillyer, DE; Frohlich, BH; Abboa-Offei, BE; Cap, M; Waldron, TL; George, RJ; Tesfamariam, B; Harper, TW; Ciosek, CP; Young, DA; Dickinson, KE; Seymour, AA; Arbeeny, CM; Washburn, WN BMS-196085: a potent and selective full agonist of the human beta(3) adrenergic receptor. Bioorg Med Chem Lett 11: 3041 -4 (2001) Marcin, LR; Warrier, J; Thangathirupathy, S; Shi, J; Karageorge, GN; Pearce, BC; Ng, A; Park, H; Kempson, J; Li, J; Zhang, H; Mathur, A; Reddy, AB; Nagaraju, G; Tonukunuru, G; Gupta, GVRKM; Kamble, M; Mannoori, R; Cheruku, S; Jogi, S; Gulia, J; Bastia, T; Sanmathi, C; Aher, J; Kallem, R; Srikumar, BN; Vijaya, KK; Naidu, PS; Paschapur, M; Kalidindi, N; Vikramadithyan, R; Ramarao, M; Denton, R; Molski, T; Shields, E; Subramanian, M; Zhuo, X; Nophsker, M; Simmermacher, J; Sinz, M; Albright, C; Bristow, LJ; Islam, I; Bronson, JJ; Olson, RE; King, D; Thompson, LA; Macor, JE BMS-986163, a Negative Allosteric Modulator of GluN2B with Potential Utility in Major Depressive Disorder. ACS Med Chem Lett 9: 472 -477 (2018) Balog, A; Rampulla, R; Martin, GS; Krystek, SR; Attar, R; Dell-John, J; DiMarco, JD; Fairfax, D; Gougoutas, J; Holst, CL; Nation, A; Rizzo, C; Rossiter, LM; Schweizer, L; Shan, W; Spergel, S; Spires, T; Cornelius, G; Gottardis, M; Trainor, G; Vite, GD; Salvati, ME Discovery of BMS-641988, a Novel Androgen Receptor Antagonist for the Treatment of Prostate Cancer. ACS Med Chem Lett 6: 908 -12 (2015) Wan, H; Schroeder, GM; Hart, AC; Inghrim, J; Grebinski, J; Tokarski, JS; Lorenzi, MV; You, D; Mcdevitt, T; Penhallow, B; Vuppugalla, R; Zhang, Y; Gu, X; Iyer, R; Lombardo, LJ; Trainor, GL; Ruepp, S; Lippy, J; Blat, Y; Sack, JS; Khan, JA; Stefanski, K; Sleczka, B; Mathur, A; Sun, JH; Wong, MK; Wu, DR; Li, P; Gupta, A; Arunachalam, PN; Pragalathan, B; Narayanan, S; K C, N; Kuppusamy, P; Purandare, AV Discovery of a Highly Selective JAK2 Inhibitor, BMS-911543, for the Treatment of Myeloproliferative Neoplasms. ACS Med Chem Lett 6: 850 -5 (2015) Liu, T; Huang, B; Zhan, P; De Clercq, E; Liu, X Discovery of small molecular inhibitors targeting HIV-1 gp120-CD4 interaction drived from BMS-378806. Eur J Med Chem 86: 481 -90 (2014) Huang, A; Jayaraman, L; Fura, A; Vite, GD; Trainor, GL; Gottardis, MM; Spires, TE; Spires, VM; Rizzo, CA; Obermeier, MT; Elzinga, PA; Todderud, G; Fan, Y; Newitt, JA; Beyer, SM; Zhu, Y; Warrack, BM; Goodenough, AK; Tebben, AJ; Doweyko, AM; Gold, DL; Balog, A Discovery of the Selective CYP17A1 Lyase Inhibitor BMS-351 for the Treatment of Prostate Cancer. ACS Med Chem Lett 7: 40 -5 (2016) Dhar, TG; Xiao, HY; Xie, J; Lehman-McKeeman, LD; Wu, DR; Dabros, M; Yang, X; Taylor, TL; Zhou, XD; Heimrich, EM; Thomas, R; McIntyre, KW; Warrack, B; Shi, H; Levesque, PC; Zhu, JL; Hennan, J; Balimane, P; Yang, Z; Marino, AM; Cornelius, G; D'Arienzo, CJ; Mathur, A; Shen, DR; Cvijic, ME; Salter-Cid, L; Barrish, JC; Carter, PH; Dyckman, AJ Identification and Preclinical Pharmacology of BMS-986104: A Differentiated S1P1 Receptor Modulator in Clinical Trials. ACS Med Chem Lett 7: 283 -8 (2016) King, D; Iwuagwu, C; Cook, J; McDonald, IM; Mate, R; Zusi, FC; Hill, MD; Fang, H; Zhao, R; Wang, B; Easton, AE; Miller, R; Post-Munson, D; Knox, RJ; Gallagher, L; Westphal, R; Molski, T; Fan, J; Clarke, W; Benitex, Y; Lentz, KA; Denton, R; Morgan, D; Zaczek, R; Lodge, NJ; Bristow, LJ; Macor, JE; Olson, RE BMS-933043, a Selectivea7 nAChR Partial Agonist for the Treatment of Cognitive Deficits Associated with Schizophrenia. ACS Med Chem Lett 8: 366 -371 (2017) Cherney, EC; Zhang, L; Nara, S; Zhu, X; Gullo-Brown, J; Maley, D; Lin, TA; Hunt, JT; Huang, C; Yang, Z; Darienzo, C; Discenza, L; Ranasinghe, A; Grubb, M; Ziemba, T; Traeger, SC; Li, X; Johnston, K; Kopcho, L; Fereshteh, M; Foster, K; Stefanski, K; Fargnoli, J; Swanson, J; Brown, J; Delpy, D; Seitz, SP; Borzilleri, R; Vite, G; Balog, A Discovery and Preclinical Evaluation of BMS-986242, a Potent, Selective Inhibitor of Indoleamine-2,3-dioxygenase 1. ACS Med Chem Lett 12: 288 -294 (2021) Carpenter, J; Wu, G; Wang, Y; Cook, EM; Wang, T; Sitkoff, D; Rossi, KA; Mosure, K; Zhuo, X; Cao, GG; Ziegler, M; Azzara, AV; Krupinski, J; Soars, MG; Ellsworth, BA; Wacker, DA Discovery of BMS-986318, a Potent Nonbile Acid FXR Agonist for the Treatment of Nonalcoholic Steatohepatitis. ACS Med Chem Lett 12: 1413 -1420 (2021) Elliott, JT; Hoekstra, WJ; Maryanoff, BE; Prestwich, GD Photoactivatable peptides based on BMS-197525: a potent antagonist of the human thrombin receptor (PAR-1). Bioorg Med Chem Lett 9: 279 -84 (1999) Naidu, BN; Walker, MA; Sorenson, ME; Ueda, Y; Matiskella, JD; Connolly, TP; Dicker, IB; Lin, Z; Bollini, S; Terry, BJ; Higley, H; Zheng, M; Parker, DD; Wu, D; Adams, S; Krystal, MR; Meanwell, NA The discovery and preclinical evaluation of BMS-707035, a potent HIV-1 integrase strand transfer inhibitor. Bioorg Med Chem Lett 28: 2124 -2130 (2018) Yang, MG; Xiao, Z; Cherney, RJ; Tebben, AJ; Batt, DG; Brown, GD; Chen, J; Cvijic, ME; Dabros, M; Duncia, JV; Galella, M; Gardner, DS; Khandelwal, P; Ko, SS; Malley, MF; Mo, R; Pang, J; Rose, AV; Santella, JB; Shi, H; Srivastava, A; Traeger, SC; Wang, B; Xu, S; Zhao, R; Barrish, JC; Mandlekar, S; Zhao, Q; Carter, PH Use of a Conformational-Switching Mechanism to Modulate Exposed Polarity: Discovery of CCR2 Antagonist BMS-741672. ACS Med Chem Lett 10: 300 -305 (2019) Velaparthi, U; Darne, CP; Warrier, J; Liu, P; Rahaman, H; Augustine-Rauch, K; Parrish, K; Yang, Z; Swanson, J; Brown, J; Dhar, G; Anandam, A; Holenarsipur, VK; Palanisamy, K; Wautlet, BS; Fereshteh, MP; Lippy, J; Tebben, AJ; Sheriff, S; Ruzanov, M; Yan, C; Gupta, A; Gupta, AK; Vetrichelvan, M; Mathur, A; Gelman, M; Singh, R; Kinsella, T; Murtaza, A; Fargnoli, J; Vite, G; Borzilleri, RM Discovery of BMS-986260, a Potent, Selective, and Orally Bioavailable TGFβR1 Inhibitor as an Immuno-oncology Agent. ACS Med Chem Lett 11: 172 -178 (2020) Richter, JM; Gunaga, P; Yadav, N; Bora, RO; Bhide, R; Rajugowda, N; Govindrajulu, K; Godesi, S; Akuthota, N; Rao, P; Sivaraman, A; Panda, M; Kaspady, M; Gupta, A; Mathur, A; Levesque, PC; Gulia, J; Dokania, M; Ramarao, M; Kole, P; Chacko, S; Lentz, KA; Sivaprasad Lvj, S; Thatipamula, RP; Sridhar, S; Kamble, S; Govindrajan, A; Soleman, SI; Gordon, DA; Wexler, RR; Priestley, ES Discovery of BMS-986308: A Renal Outer Medullary Potassium Channel Inhibitor for the Treatment of Heart Failure. J Med Chem 67: 9731 -9744 Nara, SJ; Jogi, S; Cheruku, S; Kandhasamy, S; Jaipuri, F; Kathi, PK; Reddy, S; Sarodaya, S; Cook, EM; Wang, T; Sitkoff, D; Rossi, KA; Ruzanov, M; Kiefer, SE; Khan, JA; Gao, M; Reddy, S; Sivaprasad Lvj, S; Sane, R; Mosure, K; Zhuo, X; Cao, GG; Ziegler, M; Azzara, A; Krupinski, J; Soars, MG; Ellsworth, BA; Wacker, DA Discovery of BMS-986339, a Pharmacologically Differentiated Farnesoid X Receptor Agonist for the Treatment of Nonalcoholic Steatohepatitis. J Med Chem 65: 8948 -8960 (2022) Sharma, S; Zeng, JY; Zhuang, CM; Zhou, YQ; Yao, HP; Hu, X; Zhang, R; Wang, MH Small-molecule inhibitor BMS-777607 induces breast cancer cell polyploidy with increased resistance to cytotoxic chemotherapy agents. Mol Cancer Ther 12: 725 -36 Zhang, Y; Boy, KM; Wu, YJ; Ramirez, A; Toyn, JH; Ahlijanian, MK; Albright, CF; Zhuo, X; Johnson, BM; Denton, RR; Olson, RE; Thompson, LA; Macor, JE Synthesis of functionalized derivatives of the gamma-secretase modulator BMS-932481 and identification of its major metabolite. Bioorg Med Chem Lett 30: (2020) Luo, G; Chen, L; Conway, CM; Denton, R; Keavy, D; Gulianello, M; Huang, Y; Kostich, W; Lentz, KA; Mercer, SE; Schartman, R; Signor, L; Browning, M; Macor, JE; Dubowchik, GM Discovery of BMS-846372, a Potent and Orally Active Human CGRP Receptor Antagonist for the Treatment of Migraine. ACS Med Chem Lett 3: 337 -341 (2012) Das, J; Lin, J; Moquin, RV; Shen, Z; Spergel, SH; Wityak, J; Doweyko, AM; DeFex, HF; Fang, Q; Pang, S; Pitt, S; Shen, DR; Schieven, GL; Barrish, JC Molecular design, synthesis, and structure-Activity relationships leading to the potent and selective p56(lck) inhibitor BMS-243117. Bioorg Med Chem Lett 13: 2145 -9 (2003) Li, J; Kennedy, LJ; Walker, SJ; Wang, H; Li, JJ; Hong, Z; O'Connor, SP; Ye, XY; Chen, S; Wu, S; Yoon, DS; Nayeem, A; Camac, DM; Ramamurthy, V; Morin, PE; Sheriff, S; Wang, M; Harper, TW; Golla, R; Seethala, R; Harrity, T; Ponticiello, RP; Morgan, NN; Taylor, JR; Zebo, R; Maxwell, B; Moulin, F; Gordon, DA; Robl, JA Discovery of Clinical Candidate BMS-823778 as an Inhibitor of Human 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD-1). ACS Med Chem Lett 9: 1170 -1174 (2018) Gentles, RG; Ding, M; Bender, JA; Bergstrom, CP; Grant-Young, K; Hewawasam, P; Hudyma, T; Martin, S; Nickel, A; Regueiro-Ren, A; Tu, Y; Yang, Z; Yeung, KS; Zheng, X; Chao, S; Sun, JH; Beno, BR; Camac, DM; Chang, CH; Gao, M; Morin, PE; Sheriff, S; Tredup, J; Wan, J; Witmer, MR; Xie, D; Hanumegowda, U; Knipe, J; Mosure, K; Santone, KS; Parker, DD; Zhuo, X; Lemm, J; Liu, M; Pelosi, L; Rigat, K; Voss, S; Wang, Y; Wang, YK; Colonno, RJ; Gao, M; Roberts, SB; Gao, Q; Ng, A; Meanwell, NA; Kadow, JF Discovery and preclinical characterization of the cyclopropylindolobenzazepine BMS-791325, a potent allosteric inhibitor of the hepatitis C virus NS5B polymerase. J Med Chem 57: 1855 -79 (2014) Yeung, KS; Beno, BR; Parcella, K; Bender, JA; Grant-Young, KA; Nickel, A; Gunaga, P; Anjanappa, P; Bora, RO; Selvakumar, K; Rigat, K; Wang, YK; Liu, M; Lemm, J; Mosure, K; Sheriff, S; Wan, C; Witmer, M; Kish, K; Hanumegowda, U; Zhuo, X; Shu, YZ; Parker, D; Haskell, R; Ng, A; Gao, Q; Colston, E; Raybon, J; Grasela, DM; Santone, K; Gao, M; Meanwell, NA; Sinz, M; Soars, MG; Knipe, JO; Roberts, SB; Kadow, JF Discovery of a Hepatitis C Virus NS5B Replicase Palm Site Allosteric Inhibitor (BMS-929075) Advanced to Phase 1 Clinical Studies. J Med Chem 60: 4369 -4385 (2017) O'Hare, T; Walters, DK; Stoffregen, EP; Jia, T; Manley, PW; Mestan, J; Cowan-Jacob, SW; Lee, FY; Heinrich, MC; Deininger, MW; Druker, BJ In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res 65: 4500 -5 Das, J; Kimball, SD; Hall, SE; Han, WC; Iwanowicz, E; Lin, J; Moquin, RV; Reid, JA; Sack, JS; Malley, MF; Chang, CY; Chong, S; Wang-Iverson, DB; Roberts, DG; Seiler, SM; Schumacher, WA; Ogletree, ML Molecular design and structure--activity relationships leading to the potent, selective, and orally active thrombin active site inhibitor BMS-189664. Bioorg Med Chem Lett 12: 45 -9 (2001) Turdi, H; Chao, H; Hangeland, JJ; Ahmad, S; Meng, W; Brigance, R; Zhao, G; Wang, W; Moore, F; Ye, XY; Mathur, A; Hou, X; Kempson, J; Wu, DR; Li, YX; Azzara, AV; Ma, Z; Chu, CH; Chen, L; Cullen, MJ; Rooney, S; Harvey, S; Kopcho, L; Panemangelor, R; Abell, L; O'Malley, K; Keim, WJ; Dierks, E; Chang, S; Foster, K; Apedo, A; Harden, D; Dabros, M; Gao, Q; Pelleymounter, MA; Whaley, JM; Robl, JA; Cheng, D; Lawrence, RM; Devasthale, P Screening Hit to Clinical Candidate: Discovery of BMS-963272, a Potent, Selective MGAT2 Inhibitor for the Treatment of Metabolic Disorders. J Med Chem 64: 14773 -14792 (2021) Padmakar Darne, C; Velaparthi, U; Saulnier, M; Frennesson, D; Liu, P; Huang, A; Tokarski, J; Fura, A; Spires, T; Newitt, J; Spires, VM; Obermeier, MT; Elzinga, PA; Gottardis, MM; Jayaraman, L; Vite, GD; Balog, A The discovery of BMS-737 as a potent, CYP17 lyase-selective inhibitor for the treatment of castration-resistant prostate cancer. Bioorg Med Chem Lett 75: (2022) Sun, LQ; Mull, E; D'Andrea, S; Zheng, B; Hiebert, S; Gillis, E; Bowsher, M; Kandhasamy, S; Baratam, VR; Puttaswamy, S; Pulicharla, N; Vishwakrishnan, S; Reddy, S; Trivedi, R; Sinha, S; Sivaprasad, S; Rao, A; Desai, S; Ghosh, K; Anumula, R; Kumar, A; Rajamani, R; Wang, YK; Fang, H; Mathur, A; Rampulla, R; Zvyaga, TA; Mosure, K; Jenkins, S; Falk, P; Tagore, DM; Chen, C; Rendunchintala, K; Loy, J; Meanwell, NA; McPhee, F; Scola, PM Discovery of BMS-986144, a Third-Generation, Pan-Genotype NS3/4A Protease Inhibitor for the Treatment of Hepatitis C Virus Infection. J Med Chem 63: 14740 -14760 (2020) Asahina, Y; Wurtz, NR; Arakawa, K; Carson, N; Fujii, K; Fukuchi, K; Garcia, R; Hsu, MY; Ishiyama, J; Ito, B; Kick, E; Lupisella, J; Matsushima, S; Ohata, K; Ostrowski, J; Saito, Y; Tsuda, K; Villarreal, F; Yamada, H; Yamaoka, T; Wexler, R; Gordon, D; Kohno, Y Discovery of BMS-986235/LAR-1219: A Potent Formyl Peptide Receptor 2 (FPR2) Selective Agonist for the Prevention of Heart Failure. J Med Chem 63: 9003 -9019 (2020) Wrobleski, ST; Moslin, R; Lin, S; Zhang, Y; Spergel, S; Kempson, J; Tokarski, JS; Strnad, J; Zupa-Fernandez, A; Cheng, L; Shuster, D; Gillooly, K; Yang, X; Heimrich, E; McIntyre, KW; Chaudhry, C; Khan, J; Ruzanov, M; Tredup, J; Mulligan, D; Xie, D; Sun, H; Huang, C; D'Arienzo, C; Aranibar, N; Chiney, M; Chimalakonda, A; Pitts, WJ; Lombardo, L; Carter, PH; Burke, JR; Weinstein, DS Highly Selective Inhibition of Tyrosine Kinase 2 (TYK2) for the Treatment of Autoimmune Diseases: Discovery of the Allosteric Inhibitor BMS-986165. J Med Chem 62: 8973 -8995 (2019) Weinstein, DS; Gong, H; Doweyko, AM; Cunningham, M; Habte, S; Wang, JH; Holloway, DA; Burke, C; Gao, L; Guarino, V; Carman, J; Somerville, JE; Shuster, D; Salter-Cid, L; Dodd, JH; Nadler, SG; Barrish, JC Azaxanthene based selective glucocorticoid receptor modulators: design, synthesis, and pharmacological evaluation of (S)-4-(5-(1-((1,3,4-thiadiazol-2-yl)amino)-2-methyl-1-oxopropan-2-yl)-5H-chromeno[2,3-b]pyridin-2-yl)-2-fluoro-N,N-dimethylbenzamide (BMS-776532) and its methylene homologue (BMS-791 J Med Chem 54: 7318 -33 (2011) Zhou, L; Dockens, RC; Liu-Kreyche, P; Grossman, SJ; Iyer, RA In vitro and in vivo metabolism and pharmacokinetics of BMS-562086, a potent and orally bioavailable corticotropin-releasing factor-1 receptor antagonist. Drug Metab Dispos 40: 1093 -103 (2012) Saulnier, MG; Frennesson, DB; Wittman, MD; Zimmermann, K; Velaparthi, U; Langley, DR; Struzynski, C; Sang, X; Carboni, J; Li, A; Greer, A; Yang, Z; Balimane, P; Gottardis, M; Attar, R; Vyas, D 2-(1H-Imidazol-4-yl)ethanamine and 2-(1H-pyrazol-1-yl)ethanamine side chain variants of the IGF-1R inhibitor BMS-536924. Bioorg Med Chem Lett 18: 1702 -7 (2008) Das, J; Kimball, SD; Reid, JA; Wang, TC; Lau, WF; Roberts, DG; Seiler, SM; Schumacher, WA; Ogletree, ML Thrombin active site inhibitors: chemical synthesis, in vitro and in vivo pharmacological profile of a novel and selective agent BMS-189090 and analogues. Bioorg Med Chem Lett 12: 41 -4 (2001) Naidu, BN; Patel, M; McAuliffe, B; Ding, B; Cianci, C; Simmermacher, J; Jenkins, S; Parker, DD; Sivaprakasam, P; Khan, JA; Kish, K; Lewis, H; Hanumegowda, U; Krystal, M; Meanwell, NA; Kadow, JF Design, Synthesis, and Preclinical Profiling of GSK3739936 (BMS-986180), an Allosteric Inhibitor of HIV-1 Integrase with Broad-Spectrum Activity toward 124/125 Polymorphs. J Med Chem 65: 4949 -4971 (2022) Scola, PM; Wang, AX; Good, AC; Sun, LQ; Combrink, KD; Campbell, JA; Chen, J; Tu, Y; Sin, N; Venables, BL; Sit, SY; Chen, Y; Cocuzza, A; Bilder, DM; D'Andrea, S; Zheng, B; Hewawasam, P; Ding, M; Thuring, J; Li, J; Hernandez, D; Yu, F; Falk, P; Zhai, G; Sheaffer, AK; Chen, C; Lee, MS; Barry, D; Knipe, JO; Li, W; Han, YH; Jenkins, S; Gesenberg, C; Gao, Q; Sinz, MW; Santone, KS; Zvyaga, T; Rajamani, R; Klei, HE; Colonno, RJ; Grasela, DM; Hughes, E; Chien, C; Adams, S; Levesque, PC; Li, D; Zhu, J; Meanwell, NA; McPhee, F Discovery and early clinical evaluation of BMS-605339, a potent and orally efficacious tripeptidic acylsulfonamide NS3 protease inhibitor for the treatment of hepatitis C virus infection. J Med Chem 57: 1708 -29 (2014) Augeri, DJ; Robl, JA; Betebenner, DA; Magnin, DR; Khanna, A; Robertson, JG; Wang, A; Simpkins, LM; Taunk, P; Huang, Q; Han, SP; Abboa-Offei, B; Cap, M; Xin, L; Tao, L; Tozzo, E; Welzel, GE; Egan, DM; Marcinkeviciene, J; Chang, SY; Biller, SA; Kirby, MS; Parker, RA; Hamann, LG Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 48: 5025 -37 (2005) Watterson, SH; Liu, Q; Beaudoin Bertrand, M; Batt, DG; Li, L; Pattoli, MA; Skala, S; Cheng, L; Obermeier, MT; Moore, R; Yang, Z; Vickery, R; Elzinga, PA; Discenza, L; D'Arienzo, C; Gillooly, KM; Taylor, TL; Pulicicchio, C; Zhang, Y; Heimrich, E; McIntyre, KW; Ruan, Q; Westhouse, RA; Catlett, IM; Zheng, N; Chaudhry, C; Dai, J; Galella, MA; Tebben, AJ; Pokross, M; Li, J; Zhao, R; Smith, D; Rampulla, R; Allentoff, A; Wallace, MA; Mathur, A; Salter-Cid, L; Macor, JE; Carter, PH; Fura, A; Burke, JR; Tino, JA Discovery of Branebrutinib (BMS-986195): A Strategy for Identifying a Highly Potent and Selective Covalent Inhibitor Providing Rapid in Vivo Inactivation of Bruton's Tyrosine Kinase (BTK). J Med Chem 62: 3228 -3250 (2019) Hynes, J; Wu, H; Pitt, S; Shen, DR; Zhang, R; Schieven, GL; Gillooly, KM; Shuster, DJ; Taylor, TL; Yang, X; McIntyre, KW; McKinnon, M; Zhang, H; Marathe, PH; Doweyko, AM; Kish, K; Kiefer, SE; Sack, JS; Newitt, JA; Barrish, JC; Dodd, J; Leftheris, K The discovery of (R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbamoyl)phenyl) thiazole-5-carboxamide (BMS-640994)-A potent and efficacious p38alpha MAP kinase inhibitor. Bioorg Med Chem Lett 18: 1762 -7 (2008) Wittman, M; Carboni, J; Attar, R; Balasubramanian, B; Balimane, P; Brassil, P; Beaulieu, F; Chang, C; Clarke, W; Dell, J; Eummer, J; Frennesson, D; Gottardis, M; Greer, A; Hansel, S; Hurlburt, W; Jacobson, B; Krishnananthan, S; Lee, FY; Li, A; Lin, TA; Liu, P; Ouellet, C; Sang, X; Saulnier, MG; Stoffan, K; Sun, Y; Velaparthi, U; Wong, H; Yang, Z; Zimmermann, K; Zoeckler, M; Vyas, D Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem 48: 5639 -43 (2005) Santella, JB; Gardner, DS; Yao, W; Shi, C; Reddy, P; Tebben, AJ; DeLucca, GV; Wacker, DA; Watson, PS; Welch, PK; Wadman, EA; Davies, P; Solomon, KA; Graden, DM; Yeleswaram, S; Mandlekar, S; Kariv, I; Decicco, CP; Ko, SS; Carter, PH; Duncia, JV From rigid cyclic templates to conformationally stabilized acyclic scaffolds. Part I: the discovery of CCR3 antagonist development candidate BMS-639623 with picomolar inhibition potency against eosinophil chemotaxis. Bioorg Med Chem Lett 18: 576 -85 (2008) Yang, MG; Xiao, Z; Dhar, TG; Xiao, HY; Gilmore, JL; Marcoux, D; Xie, JH; McIntyre, KW; Taylor, TL; Borowski, V; Heimrich, E; Li, YW; Feng, J; Fernandes, A; Yang, Z; Balimane, P; Marino, AM; Cornelius, G; Warrack, BM; Mathur, A; Wu, DR; Li, P; Gupta, A; Pragalathan, B; Shen, DR; Cvijic, ME; Lehman-McKeeman, LD; Salter-Cid, L; Barrish, JC; Carter, PH; Dyckman, AJ Asymmetric Hydroboration Approach to the Scalable Synthesis of ((1R,3S)-1-Amino-3-((R)-6-hexyl-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopentyl)methanol (BMS-986104) as a Potent S1P J Med Chem 59: 11138 -11147 (2016) Sher, PM; Mathur, A; Fisher, LG; Wu, G; Skwish, S; Michel, IM; Seiler, SM; Dickinson, KE Carboxyl-promoted enhancement of selectivity for the β3 adrenergic receptor. Negative charge of the sulfonic acid BMS-187413 introduces-β3 binding selectivity Bioorg Med Chem Lett 7: 1583 -1588 (1997) Li, J; Chen, SY; Li, JJ; Wang, H; Hernandez, AS; Tao, S; Musial, CM; Qu, F; Swartz, S; Chao, ST; Flynn, N; Murphy, BJ; Slusarchyk, DA; Seethala, R; Yan, M; Sleph, P; Grover, G; Smith, MA; Beehler, B; Giupponi, L; Dickinson, KE; Zhang, H; Humphreys, WG; Patel, BP; Schwinden, M; Stouch, T; Cheng, PT; Biller, SA; Ewing, WR; Gordon, D; Robl, JA; Tino, JA Discovery of a tetrazole-based growth hormone secretagogue: 4-(hydroxybutyl)carbamic acid 2-{5-[1-(2-amino-2-methylpropionylamino)-2- benzyloxyethyl]tetrazol-1-yl}ethyl ester (BMS-317180). J Med Chem 50: 5890 -3 (2007) Li, J; Kennedy, LJ; Shi, Y; Tao, S; Ye, XY; Chen, SY; Wang, Y; Hernández, AS; Wang, W; Devasthale, PV; Chen, S; Lai, Z; Zhang, H; Wu, S; Smirk, RA; Bolton, SA; Ryono, DE; Zhang, H; Lim, NK; Chen, BC; Locke, KT; O'Malley, KM; Zhang, L; Srivastava, RA; Miao, B; Meyers, DS; Monshizadegan, H; Search, D; Grimm, D; Zhang, R; Harrity, T; Kunselman, LK; Cap, M; Kadiyala, P; Hosagrahara, V; Zhang, L; Xu, C; Li, YX; Muckelbauer, JK; Chang, C; An, Y; Krystek, SR; Blanar, MA; Zahler, R; Mukherjee, R; Cheng, PT; Tino, JA Discovery of an oxybenzylglycine based peroxisome proliferator activated receptor alpha selective agonist 2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)methoxy)benzyl)(methoxycarbonyl)amino)acetic acid (BMS-687453). J Med Chem 53: 2854 -64 (2010) Burke, JR; Pattoli, MA; Gregor, KR; Brassil, PJ; MacMaster, JF; McIntyre, KW; Yang, X; Iotzova, VS; Clarke, W; Strnad, J; Qiu, Y; Zusi, FC BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem 278: 1450 -6 Hunt, JT; Ding, CZ; Batorsky, R; Bednarz, M; Bhide, R; Cho, Y; Chong, S; Chao, S; Gullo-Brown, J; Guo, P; Kim, SH; Lee, FY; Leftheris, K; Miller, A; Mitt, T; Patel, M; Penhallow, BA; Ricca, C; Rose, WC; Schmidt, R; Slusarchyk, WA; Vite, G; Manne, V Discovery of (R)-7-cyano-2,3,4, 5-tetrahydro-1-(1H-imidazol-4-ylmethyl)-3- (phenylmethyl)-4-(2-thienylsulfonyl)-1H-1,4-benzodiazepine (BMS-214662), a farnesyltransferase inhibitor with potent preclinical antitumor activity. J Med Chem 43: 3587 -95 (2000) Liu, C; Lin, J; Wrobleski, ST; Lin, S; Hynes, J; Wu, H; Dyckman, AJ; Li, T; Wityak, J; Gillooly, KM; Pitt, S; Shen, DR; Zhang, RF; McIntyre, KW; Salter-Cid, L; Shuster, DJ; Zhang, H; Marathe, PH; Doweyko, AM; Sack, JS; Kiefer, SE; Kish, KF; Newitt, JA; McKinnon, M; Dodd, JH; Barrish, JC; Schieven, GL; Leftheris, K Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38a MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem 53: 6629 -39 (2010) Wacker, DA; Wang, Y; Broekema, M; Rossi, K; O'Connor, S; Hong, Z; Wu, G; Malmstrom, SE; Hung, CP; LaMarre, L; Chimalakonda, A; Zhang, L; Xin, L; Cai, H; Chu, C; Boehm, S; Zalaznick, J; Ponticiello, R; Sereda, L; Han, SP; Zebo, R; Zinker, B; Luk, CE; Wong, R; Everlof, G; Li, YX; Wu, CK; Lee, M; Griffen, S; Miller, KJ; Krupinski, J; Robl, JA Discovery of 5-chloro-4-((1-(5-chloropyrimidin-2-yl)piperidin-4-yl)oxy)-1-(2-fluoro-4-(methylsulfonyl)phenyl)pyridin-2(1H)-one (BMS-903452), an antidiabetic clinical candidate targeting GPR119. J Med Chem 57: 7499 -508 (2014) Ye, XY; Chen, SY; Wu, S; Yoon, DS; Wang, H; Hong, Z; O'Connor, SP; Li, J; Li, JJ; Kennedy, LJ; Walker, SJ; Nayeem, A; Sheriff, S; Camac, DM; Ramamurthy, V; Morin, PE; Zebo, R; Taylor, JR; Morgan, NN; Ponticiello, RP; Harrity, T; Apedo, A; Golla, R; Seethala, R; Wang, M; Harper, TW; Sleczka, BG; He, B; Kirby, M; Leahy, DK; Li, J; Hanson, RL; Guo, Z; Li, YX; DiMarco, JD; Scaringe, R; Maxwell, B; Moulin, F; Barrish, JC; Gordon, DA; Robl, JA Discovery of Clinical Candidate 2-((2S,6S)-2-Phenyl-6-hydroxyadamantan-2-yl)-1-(3'-hydroxyazetidin-1-yl)ethanone [BMS-816336], an Orally Active Novel Selective 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibitor. J Med Chem 60: 4932 -4948 (2017) Gilmore, JL; Xiao, HY; Dhar, TGM; Yang, MG; Xiao, Z; Xie, J; Lehman-McKeeman, LD; Gong, L; Sun, H; Lecureux, L; Chen, C; Wu, DR; Dabros, M; Yang, X; Taylor, TL; Zhou, XD; Heimrich, EM; Thomas, R; McIntyre, KW; Borowski, V; Warrack, BM; Li, Y; Shi, H; Levesque, PC; Yang, Z; Marino, AM; Cornelius, G; D'Arienzo, CJ; Mathur, A; Rampulla, R; Gupta, A; Pragalathan, B; Shen, DR; Cvijic, ME; Salter-Cid, LM; Carter, PH; Dyckman, AJ Identification and Preclinical Pharmacology of ((1 R,3 S)-1-Amino-3-(( S)-6-(2-methoxyphenethyl)-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopentyl)methanol (BMS-986166): A Differentiated Sphingosine-1-phosphate Receptor 1 (S1P J Med Chem 62: 2265 -2285 (2019) Borzilleri, RM; Bhide, RS; Barrish, JC; D'Arienzo, CJ; Derbin, GM; Fargnoli, J; Hunt, JT; Jeyaseelan, R; Kamath, A; Kukral, DW; Marathe, P; Mortillo, S; Qian, L; Tokarski, JS; Wautlet, BS; Zheng, X; Lombardo, LJ Discovery and evaluation of N-cyclopropyl- 2,4-difluoro-5-((2-(pyridin-2-ylamino)thiazol-5- ylmethyl)amino)benzamide (BMS-605541), a selective and orally efficacious inhibitor of vascular endothelial growth factor receptor-2. J Med Chem 49: 3766 -9 (2006) Misra, RN; Xiao, HY; Kim, KS; Lu, S; Han, WC; Barbosa, SA; Hunt, JT; Rawlins, DB; Shan, W; Ahmed, SZ; Qian, L; Chen, BC; Zhao, R; Bednarz, MS; Kellar, KA; Mulheron, JG; Batorsky, R; Roongta, U; Kamath, A; Marathe, P; Ranadive, SA; Sack, JS; Tokarski, JS; Pavletich, NP; Lee, FY; Webster, KR; Kimball, SD N-(cycloalkylamino)acyl-2-aminothiazole inhibitors of cyclin-dependent kinase 2. N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4- piperidinecarboxamide (BMS-387032), a highly efficacious and selective antitumor agent. J Med Chem 47: 1719 -28 (2004) Schroeder, GM; An, Y; Cai, ZW; Chen, XT; Clark, C; Cornelius, LA; Dai, J; Gullo-Brown, J; Gupta, A; Henley, B; Hunt, JT; Jeyaseelan, R; Kamath, A; Kim, K; Lippy, J; Lombardo, LJ; Manne, V; Oppenheimer, S; Sack, JS; Schmidt, RJ; Shen, G; Stefanski, K; Tokarski, JS; Trainor, GL; Wautlet, BS; Wei, D; Williams, DK; Zhang, Y; Zhang, Y; Fargnoli, J; Borzilleri, RM Discovery of N-(4-(2-Amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a Selective and Orally Efficacious Inhibitor of the Met Kinase Superfamily. J Med Chem 52: 1251 -4 (2009) Ahmad, S; Washburn, WN; Hernandez, AS; Bisaha, S; Ngu, K; Wang, W; Pelleymounter, MA; Longhi, D; Flynn, N; Azzara, AV; Rohrbach, K; Devenny, J; Rooney, S; Thomas, M; Glick, S; Godonis, H; Harvey, S; Zhang, H; Gemzik, B; Janovitz, EB; Huang, C; Zhang, L; Robl, JA; Murphy, BJ Synthesis and Antiobesity Properties of 6-(4-Chlorophenyl)-3-(4-((3,3-difluoro-1-hydroxycyclobutyl)methoxy)-3-methoxyphenyl)thieno[3,2-d]pyrimidin-4(3H)-one (BMS-814580): A Highly Efficacious Melanin Concentrating Hormone Receptor 1 (MCHR1) Inhibitor. J Med Chem 59: 8848 -8858 (2016) Bhide, RS; Cai, ZW; Zhang, YZ; Qian, L; Wei, D; Barbosa, S; Lombardo, LJ; Borzilleri, RM; Zheng, X; Wu, LI; Barrish, JC; Kim, SH; Leavitt, K; Mathur, A; Leith, L; Chao, S; Wautlet, B; Mortillo, S; Jeyaseelan, R; Kukral, D; Hunt, JT; Kamath, A; Fura, A; Vyas, V; Marathe, P; D'Arienzo, C; Derbin, G; Fargnoli, J Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5- methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan- 2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem 49: 2143 -6 (2006) Murugesan, N; Gu, Z; Spergel, S; Young, M; Chen, P; Mathur, A; Leith, L; Hermsmeier, M; Liu, EC; Zhang, R; Bird, E; Waldron, T; Marino, A; Koplowitz, B; Humphreys, WG; Chong, S; Morrison, RA; Webb, ML; Moreland, S; Trippodo, N; Barrish, JC Biphenylsulfonamide endothelin receptor antagonists. 4. Discovery of N-[[2'-[[(4,5-dimethyl-3-isoxazolyl)amino]sulfonyl]-4-(2-oxazolyl)[1,1'-biphenyl]- 2-yl]methyl]-N,3,3-trimethylbutanamide (BMS-207940), a highly potent and orally active ET(A) selective antagonist. J Med Chem 46: 125 -37 (2002) Lombardo, LJ; Lee, FY; Chen, P; Norris, D; Barrish, JC; Behnia, K; Castaneda, S; Cornelius, LA; Das, J; Doweyko, AM; Fairchild, C; Hunt, JT; Inigo, I; Johnston, K; Kamath, A; Kan, D; Klei, H; Marathe, P; Pang, S; Peterson, R; Pitt, S; Schieven, GL; Schmidt, RJ; Tokarski, J; Wen, ML; Wityak, J; Borzilleri, RM Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 47: 6658 -61 (2004) Watterson, SH; Langevine, CM; Van Kirk, K; Kempson, J; Guo, J; Spergel, SH; Das, J; Moquin, RV; Dyckman, AJ; Nirschl, D; Gregor, K; Pattoli, MA; Yang, X; McIntyre, KW; Yang, G; Galella, MA; Booth-Lute, H; Chen, L; Yang, Z; Wang-Iverson, D; McKinnon, M; Dodd, JH; Barrish, JC; Burke, JR; Pitts, WJ Novel tricyclic inhibitors of IKK2: discovery and SAR leading to the identification of 2-methoxy-N-((6-(1-methyl-4-(methylamino)-1,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridin-7-yl)pyridin-2-yl)methyl)acetamide (BMS-066). Bioorg Med Chem Lett 21: 7006 -12 (2011) Watterson, SH; Chen, P; Zhao, Y; Gu, HH; Dhar, TG; Xiao, Z; Ballentine, SK; Shen, Z; Fleener, CA; Rouleau, KA; Obermeier, M; Yang, Z; McIntyre, KW; Shuster, DJ; Witmer, M; Dambach, D; Chao, S; Mathur, A; Chen, BC; Barrish, JC; Robl, JA; Townsend, R; Iwanowicz, EJ Acridone-based inhibitors of inosine 5'-monophosphate dehydrogenase: discovery and SAR leading to the identification of N-(2-(6-(4-ethylpiperazin-1-yl)pyridin-3-yl)propan-2-yl)-2- fluoro-9-oxo-9,10-dihydroacridine-3-carboxamide (BMS-566419). J Med Chem 50: 3730 -42 (2007) Murugesan, N; Gu, Z; Stein, PD; Bisaha, S; Spergel, S; Girotra, R; Lee, VG; Lloyd, J; Misra, RN; Schmidt, J; Mathur, A; Stratton, L; Kelly, YF; Bird, E; Waldron, T; Liu, EC; Zhang, R; Lee, H; Serafino, R; Abboa-Offei, B; Mathers, P; Giancarli, M; Seymour, AA; Webb, ML; Hunt, JT Biphenylsulfonamide endothelin antagonists: structure-activity relationships of a series of mono- and disubstituted analogues and pharmacology of the orally active endothelin antagonist 2'-amino-N- (3,4-dimethyl-5-isoxazolyl)-4'-(2-methylpropyl)[1, 1'-biphenyl]-2-sulfonamide (BMS-187308). J Med Chem 41: 5198 -218 (1999) Potin, D; Launay, M; Monatlik, F; Malabre, P; Fabreguettes, M; Fouquet, A; Maillet, M; Nicolai, E; Dorgeret, L; Chevallier, F; Besse, D; Dufort, M; Caussade, F; Ahmad, SZ; Stetsko, DK; Skala, S; Davis, PM; Balimane, P; Patel, K; Yang, Z; Marathe, P; Postelneck, J; Townsend, RM; Goldfarb, V; Sheriff, S; Einspahr, H; Kish, K; Malley, MF; DiMarco, JD; Gougoutas, JZ; Kadiyala, P; Cheney, DL; Tejwani, RW; Murphy, DK; Mcintyre, KW; Yang, X; Chao, S; Leith, L; Xiao, Z; Mathur, A; Chen, BC; Wu, DR; Traeger, SC; McKinnon, M; Barrish, JC; Robl, JA; Iwanowicz, EJ; Suchard, SJ; Dhar, TG Discovery and development of 5-[(5S,9R)-9-(4-cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-yl-methyl]-3-thiophenecarboxylic acid (BMS-587101)--a small molecule antagonist of leukocyte function associated antigen-1. J Med Chem 49: 6946 -9 (2006) De Lucca, GV; Shi, Q; Liu, Q; Batt, DG; Beaudoin Bertrand, M; Rampulla, R; Mathur, A; Discenza, L; D'Arienzo, C; Dai, J; Obermeier, M; Vickery, R; Zhang, Y; Yang, Z; Marathe, P; Tebben, AJ; Muckelbauer, JK; Chang, CJ; Zhang, H; Gillooly, K; Taylor, T; Pattoli, MA; Skala, S; Kukral, DW; McIntyre, KW; Salter-Cid, L; Fura, A; Burke, JR; Barrish, JC; Carter, PH; Tino, JA Small Molecule Reversible Inhibitors of Bruton's Tyrosine Kinase (BTK): Structure-Activity Relationships Leading to the Identification of 7-(2-Hydroxypropan-2-yl)-4-[2-methyl-3-(4-oxo-3,4-dihydroquinazolin-3-yl)phenyl]-9H-carbazole-1-carboxamide (BMS-935177). J Med Chem 59: 7915 -35 (2016) Devasthale, PV; Chen, S; Jeon, Y; Qu, F; Shao, C; Wang, W; Zhang, H; Cap, M; Farrelly, D; Golla, R; Grover, G; Harrity, T; Ma, Z; Moore, L; Ren, J; Seethala, R; Cheng, L; Sleph, P; Sun, W; Tieman, A; Wetterau, JR; Doweyko, A; Chandrasena, G; Chang, SY; Humphreys, WG; Sasseville, VG; Biller, SA; Ryono, DE; Selan, F; Hariharan, N; Cheng, PT Design and synthesis of N-[(4-methoxyphenoxy)carbonyl]-N-[[4-[2-(5- methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine [Muraglitazar/BMS-298585], a novel peroxisome proliferator-activated receptor alpha/gamma dual agonist with efficacious glucose and lipid-lowering activities. J Med Chem 48: 2248 -50 (2005) Ruel, R; Thibeault, C; L'Heureux, A; Martel, A; Cai, ZW; Wei, D; Qian, L; Barrish, JC; Mathur, A; D'Arienzo, C; Hunt, JT; Kamath, A; Marathe, P; Zhang, Y; Derbin, G; Wautlet, B; Mortillo, S; Jeyaseelan, R; Henley, B; Tejwani, R; Bhide, RS; Trainor, GL; Fargnoli, J; Lombardo, LJ Discovery and preclinical studies of 5-isopropyl-6-(5-methyl-1,3,4-oxadiazol-2-yl)-N-(2-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrrolo[2,1-f][1,2,4]triazin-4-amine (BMS-645737), an in vivo active potent VEGFR-2 inhibitor. Bioorg Med Chem Lett 18: 2985 -9 (2008) Pinto, DJ; Orwat, MJ; Koch, S; Rossi, KA; Alexander, RS; Smallwood, A; Wong, PC; Rendina, AR; Luettgen, JM; Knabb, RM; He, K; Xin, B; Wexler, RR; Lam, PY Discovery of 1-(4-Methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro- 1H-pyrazolo[3,4-c]pyridine-3-carboxamide (Apixaban, BMS-562247), a Highly Potent, Selective, Efficacious, and Orally Bioavailable Inhibitor of Blood Coagulation Factor Xa. J Med Chem 50: 5339 -56 (2007) Devasthale, P; Wang, Y; Wang, W; Fevig, J; Feng, J; Wang, A; Harrity, T; Egan, D; Morgan, N; Cap, M; Fura, A; Klei, HE; Kish, K; Weigelt, C; Sun, L; Levesque, P; Moulin, F; Li, YX; Zahler, R; Kirby, MS; Hamann, LG Optimization of activity, selectivity, and liability profiles in 5-oxopyrrolopyridine DPP4 inhibitors leading to clinical candidate (Sa)-2-(3-(aminomethyl)-4-(2,4-dichlorophenyl)-2-methyl-5-oxo-5H-pyrrolo[3,4-b]pyridin-6(7H)-yl)-N,N-dimethylacetamide (BMS-767778). J Med Chem 56: 7343 -57 (2013) Pinto, DJ; Orwat, MJ; Quan, ML; Han, Q; Galemmo, RA; Amparo, E; Wells, B; Ellis, C; He, MY; Alexander, RS; Rossi, KA; Smallwood, A; Wong, PC; Luettgen, JM; Rendina, AR; Knabb, RM; Mersinger, L; Kettner, C; Bai, S; He, K; Wexler, RR; Lam, PY 1-[3-Aminobenzisoxazol-5'-yl]-3-trifluoromethyl-6-[2'-(3-(R)-hydroxy-N-pyrrolidinyl)methyl-[1,1']-biphen-4-yl]-1,4,5,6-tetrahydropyrazolo-[3,4-c]-pyridin-7-one (BMS-740808) a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. Bioorg Med Chem Lett 16: 4141 -7 (2006) Watterson, SH; Xiao, Z; Dodd, DS; Tortolani, DR; Vaccaro, W; Potin, D; Launay, M; Stetsko, DK; Skala, S; Davis, PM; Lee, D; Yang, X; McIntyre, KW; Balimane, P; Patel, K; Yang, Z; Marathe, P; Kadiyala, P; Tebben, AJ; Sheriff, S; Chang, CY; Ziemba, T; Zhang, H; Chen, BC; DelMonte, AJ; Aranibar, N; McKinnon, M; Barrish, JC; Suchard, SJ; Murali Dhar, TG Small molecule antagonist of leukocyte function associated antigen-1 (LFA-1): structure-activity relationships leading to the identification of 6-((5S,9R)-9-(4-cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]nonan-7-yl)nicotinic acid (BMS-688521). J Med Chem 53: 3814 -30 (2010) Prasad, CV; Zheng, M; Vig, S; Bergstrom, C; Smith, DW; Gao, Q; Yeola, S; Polson, CT; Corsa, JA; Guss, VL; Loo, A; Wang, J; Sleczka, BG; Dangler, C; Robertson, BJ; Hendrick, JP; Roberts, SB; Barten, DM Discovery of (S)-2-((S)-2-(3,5-difluorophenyl)-2-hydroxyacetamido)-N-((S,Z)-3-methyl-4-oxo-4,5-dihydro-3H-benzo[d][1,2]diazepin-5-yl)propanamide (BMS-433796): a gamma-secretase inhibitor with Abeta lowering activity in a transgenic mouse model of Alzheimer's disease. Bioorg Med Chem Lett 17: 4006 -11 (2007) Ahmad, S; Madsen, CS; Stein, PD; Janovitz, E; Huang, C; Ngu, K; Bisaha, S; Kennedy, LJ; Chen, BC; Zhao, R; Sitkoff, D; Monshizadegan, H; Yin, X; Ryan, CS; Zhang, R; Giancarli, M; Bird, E; Chang, M; Chen, X; Setters, R; Search, D; Zhuang, S; Nguyen-Tran, V; Cuff, CA; Harrity, T; Darienzo, CJ; Li, T; Reeves, RA; Blanar, MA; Barrish, JC; Zahler, R; Robl, JA (3R,5S,E)-7-(4-(4-Fluorophenyl)-6-isopropyl-2-(methyl(1-methyl-1H-1,2,4-triazol-5-yl)amino)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoic Acid (BMS-644950): A Rationally Designed Orally Efficacious 3-Hydroxy-3-methylglutaryl Coenzyme-A Reductase Inhibitor with Reduced Myotoxicity Potential. J Med Chem 51: 2722 -33 (2008) Gavai, AV; Fink, BE; Fairfax, DJ; Martin, GS; Rossiter, LM; Holst, CL; Kim, SH; Leavitt, KJ; Mastalerz, H; Han, WC; Norris, D; Goyal, B; Swaminathan, S; Patel, B; Mathur, A; Vyas, DM; Tokarski, JS; Yu, C; Oppenheimer, S; Zhang, H; Marathe, P; Fargnoli, J; Lee, FY; Wong, TW; Vite, GD Discovery and preclinical evaluation of [4-[[1-(3-fluorophenyl)methyl]-1H-indazol-5-ylamino]-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yl]carbamic acid, (3S)-3-morpholinylmethyl ester (BMS-599626), a selective and orally efficacious inhibitor of human epidermal growth factor receptor 1 and 2 kinases. J Med Chem 52: 6527 -30 (2009) Chen, P; Doweyko, AM; Norris, D; Gu, HH; Spergel, SH; Das, J; Moquin, RV; Lin, J; Wityak, J; Iwanowicz, EJ; McIntyre, KW; Shuster, DJ; Behnia, K; Chong, S; de Fex, H; Pang, S; Pitt, S; Shen, DR; Thrall, S; Stanley, P; Kocy, OR; Witmer, MR; Kanner, SB; Schieven, GL; Barrish, JC Imidazoquinoxaline Src-family kinase p56Lck inhibitors: SAR, QSAR, and the discovery of (S)-N-(2-chloro-6-methylphenyl)-2-(3-methyl-1-piperazinyl)imidazo- [1,5-a]pyrido[3,2-e]pyrazin-6-amine (BMS-279700) as a potent and orally active inhibitor with excellent in vivo antiinflammatory activity. J Med Chem 47: 4517 -29 (2004) Luo, G; Chen, L; Conway, CM; Denton, R; Keavy, D; Signor, L; Kostich, W; Lentz, KA; Santone, KS; Schartman, R; Browning, M; Tong, G; Houston, JG; Dubowchik, GM; Macor, JE Discovery of (5S,6S,9R)-5-amino-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-9-yl 4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxylate (BMS-927711): an oral calcitonin gene-related peptide (CGRP) antagonist in clinical trials for treating migraine. J Med Chem 55: 10644 -51 (2012) Watterson, SH; De Lucca, GV; Shi, Q; Langevine, CM; Liu, Q; Batt, DG; Beaudoin Bertrand, M; Gong, H; Dai, J; Yip, S; Li, P; Sun, D; Wu, DR; Wang, C; Zhang, Y; Traeger, SC; Pattoli, MA; Skala, S; Cheng, L; Obermeier, MT; Vickery, R; Discenza, LN; D'Arienzo, CJ; Zhang, Y; Heimrich, E; Gillooly, KM; Taylor, TL; Pulicicchio, C; McIntyre, KW; Galella, MA; Tebben, AJ; Muckelbauer, JK; Chang, C; Rampulla, R; Mathur, A; Salter-Cid, L; Barrish, JC; Carter, PH; Fura, A; Burke, JR; Tino, JA Discovery of 6-Fluoro-5-(R)-(3-(S)-(8-fluoro-1-methyl-2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)-2-methylphenyl)-2-(S)-(2-hydroxypropan-2-yl)-2,3,4,9-tetrahydro-1H-carbazole-8-carboxamide (BMS-986142): A Reversible Inhibitor of Bruton's Tyrosine Kinase (BTK) Conformationally Constrained by Two Locke J Med Chem 59: 9173 -9200 (2016) Wang, T; Yin, Z; Zhang, Z; Bender, JA; Yang, Z; Johnson, G; Yang, Z; Zadjura, LM; D'Arienzo, CJ; DiGiugno Parker, D; Gesenberg, C; Yamanaka, GA; Gong, YF; Ho, HT; Fang, H; Zhou, N; McAuliffe, BV; Eggers, BJ; Fan, L; Nowicka-Sans, B; Dicker, IB; Gao, Q; Colonno, RJ; Lin, PF; Meanwell, NA; Kadow, JF Inhibitors of human immunodeficiency virus type 1 (HIV-1) attachment. 5. An evolution from indole to azaindoles leading to the discovery of 1-(4-benzoylpiperazin-1-yl)-2-(4,7-dimethoxy-1H-pyrrolo[2,3-c]pyridin-3-yl)ethane-1,2-dione (BMS-488043), a drug candidate that demonstrates antiviral activity J Med Chem 52: 7778 -87 (2009) Chaturvedula, PV; Mercer, SE; Pin, SS; Thalody, G; Xu, C; Conway, CM; Keavy, D; Signor, L; Cantor, GH; Mathias, N; Moench, P; Denton, R; Macci, R; Schartman, R; Whiterock, V; Davis, C; Macor, JE; Dubowchik, GM Discovery of (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxamide (BMS-742413): a potent human CGRP antagonist with superior safety profile for the treatment of migraine through intranasal delivery. Bioorg Med Chem Lett 23: 3157 -61 (2013) Cai, ZW; Zhang, Y; Borzilleri, RM; Qian, L; Barbosa, S; Wei, D; Zheng, X; Wu, L; Fan, J; Shi, Z; Wautlet, BS; Mortillo, S; Jeyaseelan, R; Kukral, DW; Kamath, A; Marathe, P; D'Arienzo, C; Derbin, G; Barrish, JC; Robl, JA; Hunt, JT; Lombardo, LJ; Fargnoli, J; Bhide, RS Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215 J Med Chem 51: 1976 -80 (2008) Velaparthi, U; Wittman, M; Liu, P; Carboni, JM; Lee, FY; Attar, R; Balimane, P; Clarke, W; Sinz, MW; Hurlburt, W; Patel, K; Discenza, L; Kim, S; Gottardis, M; Greer, A; Li, A; Saulnier, M; Yang, Z; Zimmermann, K; Trainor, G; Vyas, D Discovery and evaluation of 4-(2-(4-chloro-1H-pyrazol-1-yl)ethylamino)-3-(6-(1-(3-fluoropropyl)piperidin-4-yl)-4-methyl-1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one (BMS-695735), an orally efficacious inhibitor of insulin-like growth factor-1 receptor kinase with broad spectrum in vivo antitumor acti J Med Chem 51: 5897 -900 (2008) Degnan, AP; Chaturvedula, PV; Conway, CM; Cook, DA; Davis, CD; Denton, R; Han, X; Macci, R; Mathias, NR; Moench, P; Pin, SS; Ren, SX; Schartman, R; Signor, LJ; Thalody, G; Widmann, KA; Xu, C; Macor, JE; Dubowchik, GM Discovery of (R)-4-(8-fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-yl)-N-(3-(7-methyl-1H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-yl)propan-2-yl)piperidine-1-carboxamide (BMS-694153): a potent antagonist of the human calcitonin gene-related peptide receptor for migraine with rapid and effic J Med Chem 51: 4858 -61 (2008)
ChEMBL_787405 (CHEMBL1919284) Displacement of [3H]BMS-599240 from recombinant human BACE-1 ChEMBL_575802 (CHEMBL1057726) Binding affinity to BACE assessed as displacement of [3H]BMS-599240 ChEMBL_873597 (CHEMBL2187886) Displacement of [3H]BMS-599240 from BACE1 expressed in HEK293 cells ChEMBL_2362669 Inhibition of wild type human BCR-ABL1 using Tyr2 peptide as substrate incubated for 1 hrs in presence of dasatinib by FRET based Z-LYTE assay ChEMBL_1904780 (CHEMBL4407138) Displacement of [3H]BMS-986120 from His-tagged human PAR4 expressed in HEK293 cell membranes ChEMBL_775085 (CHEMBL1912344) Displacement of [3H]BMS-725519 from rat brain CB1 receptor after 90 mins by scintillation counting ChEMBL_1722333 (CHEMBL4137333) Inhibition of [3H]BMS-488043 binding to HIV-1 JRFL gp120 after 1 hr by scintillation counting method ChEMBL_1904779 (CHEMBL4407137) Displacement of [3H]BMS-986120 from His-tagged human PAR4 expressed in HEK293 cell membranes measured after 2 hrs ChEMBL_775084 (CHEMBL1912343) Displacement of [3H]BMS-725519 from human CB1 receptor expressed in CHO cells after 90 mins by scintillation counting ChEMBL_1523398 (CHEMBL3630425) Displacement of [3H]BMS-599240 from BACE1 (unknown origin) expressed in HEK293 cells at pH 5 after 1.5 hrs by Microbeta liquid scintillation counting analysis ChEMBL_1523399 (CHEMBL3630426) Displacement of [3H]BMS-599240 from BACE1 (unknown origin) expressed in HEK293 cells at pH 6.4 after 1.5 hrs by Microbeta liquid scintillation counting analysis Inhibition of SIK; Abl and Src Kinases by Other Kinase Inhibitor The IC50 for the inhibition of ABL1 by dasatinib, by C7 and by B3 is approximately 1.5 nM, 5.1 nM and 1.6 nM, and of SRC is 1.5 nM, 1.5 nM and 1.5 nM; each, respectively for dasatinib, C7 and by B3 (Table 3A). The compound C12 is also a strong inhibitor of SRC (<100 nM IC50), and is selective to SRC over ABL1. Briefly, a radiometric protein kinase assay (33PanQinase Activity Assay) was used for measuring the kinase activity of the five protein kinases. All kinase assays were performed in 96-well FlashPlates™ from PerkinElmer (Boston, MA, USA) in a 50 uL reaction volume. The reaction cocktail was pipetted in four steps in the following order:25 uL of assay buffer (standard buffer/[gamma-33P]-ATP)10 uL of ATP solution (in water)5 uL of test compound (in 10% DMSO)20 uL enzyme/substrate mix. The assay for all protein kinases contained 70 mM HEPES-NaOH pH7.5, 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, ATP (variable concentrations, corresponding to the apparent ATP-Km of the respective kinase, see Table 2A), [gamma-33P]-ATP (approx. 8 105 cpm per well), protein kinase (variable amount, see Table 2A), and substrate (variable amounts, see Table 2A). hPD-1/hPD-L1 binding assay A negative control, a positive control and drug administration groups were set up, with two duplicate wells in each group. For the positive control group, added 2 μL diluent to a 96-well plate; 4 μL of PD-L1 and 4 uL of PD-1 as diluted according to the instructions; for the negative control group, added 6 μL diluent and 4 μL PD-L1 to a 96-well plate; for the administration group, 2 μL of the compound of the invention (or the positive compound BMS-202), 4 μL of PD-L1 and 4 μL of PD-1 were successively added to a 96-well plate. Sealed the plate with a sealing film, centrifuged at 1000 rpm for 1 minute, and incubated at room temperature for 15 minutes. Then mixed equal volumes of Anti-Tag-Eu3+ and Anti-tag-XL665 as diluted in buffer evenly, then added 10 μL of the mixture to each well, sealed the plate, centrifuged at 1000 rpm for 1 minute, and incubated at room temperature for 2 hours. Removed the sealing film, used EnVision to read the fluorescence intensity at 665 nm and 615 nm, and calculate ratio=Signal 665 nm/Signal 620 nm×104. IC50 of the compounds was calculated using Graphpad. In this experiment, BMS-202 in the patent WO2015034820 of BMS Company was selected as the positive drug. End Point Fluorescence Assay Reaction volumes of 50 μL were used in 96-well plates. Buffer A (1×, 34 μL; 100 mM Tris, pH 8, 10 mM MgCl2) was added to a single row, followed by 15 μL ofenzyme (3.3× concentration) in buffer A with 3-fold dilutions (typically, 125 nM and 42, 14, 4.6, 1.5, 0.5, 0.17, 0.06, 0.02, 0.01, and 0 μM final well concentrations in buffer A). Then, 1 μL of a 500 nM stock of the appropriate dasatinib analogue BODIPY probe in DMSO was added (2% DMSO final). Wells were incubated at rt for 30 min prior to end point read (ex/em 485/535 nm). Reactions had final concentrations of 10 nM BODIPY-probe, 100 mM Tris buffer (pH 8),and 10 mM MgCl2. Cell-Based Assays of c-Kit Mutant Kinase Activity The c-Kit mutant D816V inhibitors were assessed using an engineered BaF3-FL KIT D816V or BaF3-FL KIT V560G/D816V cell line. The BaF3-FL KIT D816V cell lines were created by introduction of KIT mutant (D816V) full length constructs that render the cells dependent on the introduced kinase for growth. Inhibitors of c-Kit mutant D816V kinase reduce or eliminate the mediated c-kit mutant D816V kinase activation, resulting in reduced cell proliferation of the BaF3-FL Kit mutant D816V cells. This inhibition is measured by the effect of compound concentration on cell growth to assess IC50 values. BaF3-FL KIT D816V cells were seeded at 1×104 cells per well of a 96 well cell culture plate in 50 μl of cell culture medium of RPMI Medium 1× (Invitrogen #11875-093) supplemented with 10% FBS (Invitrogen #10438), 1% Non Essential Amino Acids (Invitrogen #11140), 1% Penicillin Streptomycin (Invitrogen #15140), 1% L-Glutamine (Invitrogen #25030-081). Compounds were dissolved in DMSO at a concentration of 5 mM and were serially diluted 1:3 for a total of eight points and added to the cells to a final maximum concentration of 10 μM in 100 μl cell culture medium (final concentration 0.2% DMSO). Cells were also treated with Dasatinib as a positive control. The cells were incubated at 37° C., 5% CO2 for three days. ATPlite Buffer (Perkin Elmer #6016739) and substrate were equilibrated to room temperature, and enzyme/substrate Recombinant Firefly Luciferase/D-Luciferin was reconstituted. The cell plates were equilibrated to room temperature for 30 minutes, then lysed by addition of 25 uL per well of the ATPlite Reagent. The plate was mixed for 5 minutes on a plate shaker to lyse the cells. The plates were read on a Tecan Safire using Luminescence protocol modified to read 0.1s per well. The luminescence reading assesses the ATP content, which correlates directly with cell number such that the reading as a function of compound concentration is used to determine the IC50 value. Automated Whole-Cell Patch Clamp Assay Cell Culture and PreparationFisher rat thyroid (FRT) cells stably expressing human TMEM16A (TMEM16Aabc variant; Dr Luis Galietta, Insituto Giannina, Italy) were cultured in T-75 flasks in Hams F-12 media with Coon's modification (Sigma) supplemented with 10% (v/v) foetal bovine serum, penicillin-streptomycin (10,000 U/mL/10000 pg/mL), G-418 (750 pg/mL), L-glutamine (2 mM) and sodium bicarbonate solution (7.5% v/v). At −90% confluence cells were harvested for experiments by detachment with a 2:1 (v/v) mixture of Detachin (BMS Biotechnology) and 0.25% (w/v) trypsin-EDTA. Cells were diluted to a density of 3.5-4.5×106 cells/mL with media consisting of CHO-S-SFM (Sigma), 25 mM HEPES (Sigma) and Soy bean trypsin inhibitor (Sigma).Whole-Cell Patch Clamp RecordingFRT-TMEM16A cells were whole-cell patch clamped using an automated planar patch clamp system (Qpatch, Sophion). Briefly, once high resistance (GOhm) seals were established between the cells and the planar recording array the patch was ruptured using suction pulses to establish the whole-cell recording configuration of the patch clamp technique. The assay employed the following solutions (all reagents Sigma): Intracellular solution (mM): N-methyl-D-glucamine 130, CaCh 18.2, MgCh 1. HEPES 10, EGTA 10, BAPTA 20, Mg-ATP 2, pH 7.25, 325 mOsm with sucrose.Extracellular solution (mM): N-methyl-D-glucamine 130, CaCl2 2, MgCh 1, HEPES 10, pH 7.3, 320 mOsm with sucrose.The intracellular solution buffers intracellular calcium at levels required to give −20% activation of the maximal TMEM16A mediated current (EC20 for calcium ions). Cells were voltage clamped at a holding potential of −70 mV and a combined voltage step (to +70 mV)/ramp (−90 my to +90 mV) was applied at 0.05 Hz. After a period of current stabilisation test compounds, solubilised in 100% (v/v) DMSO and subsequently diluted into extracellular solution, were applied to generate a cumulative concentration response curve. Each concentration of test compound was incubated for 5 minutes before addition of the next concentration. After the final concentration was tested a supramaximal concentration of either a known active positive modulator or the TMEM16A inhibitor. CaCCinhAOI (Del La Fuente et al, 2008) was added to define the upper and lower limits of the assay.Compound activity was quantified by measuring the increase in current upon compound addition and expressing this as a percentage increase of baseline TMEM16A current level. Percentage increases in current were determined for each concentration and the data plotted as a function of concentration using either the Qpatch software or Graphpad Prism v6.05 providing the concentration which gave 50% of its maximal effect (EC50) and maximum efficacy (percentage of baseline increase). Automated Electrophysiology (Barra) Ion Works Barracuda population patch clamp (PPC). PPC measurements were performed using an IonWorks Barracuda instrument (Molecular Devices Corporation, Union City, Calif.) using either PatchPlate PPC substrates (Molecular Devices Corporation) with 64 apertures per well. The ability to average currents from 64 recordings from each well greatly improves data consistency and recording success rates in the measurement of NaV1.7 mediated ionic currents. Calculated leak current was digitally subtracted from the total cell NaV1.7 current for each sample point acquired.NaV1.7 currents were elicited by a voltage clamp protocol designed to bias the NaV1.7 channels to their inactivated state as follows. From holding potential of −60 mV cells were briefly hyperpolarized to −100 mV for 1.25 sec, then stepped to −20 mV for 20 sec to inactivate the channels. This was followed by a relatively brief hyperpolarization to −100 mv for 300 ms, then a 20 msec test pulse to −20 mV to elicit the NaV1.7 current used to measure the pharmacology of all test compounds. Compounds were incubated for 600 sec between the pre- and post-compound reads. The external recording solution used was (in mM) 137 NaCl, 4 KCl, 1 MgCl2, 1.8 CaCl2, 10 Hepes, 10 glucose, pH to 7.4 with NaOH, and the internal solution used was (in mM) 100 K-gluconate, 40 KCl, 3.2 zMgCl2, 5 EGTA, 10 HEPES pH to 7.2 with KOH. The same solutions were used to record NaV1.5 currents, with the following voltage clamp protocol. NaV1.5 currents were elicited by a voltage clamp protocol designed to bias the NaV1.5 channels to their inactivated state as follows. From holding potential of −40 mV cells were briefly hyperpolarized to −100 mV for 300 ms, then stepped to −10 mV for 20 sec to inactivate the channels. This was followed by a relatively brief hyperpolarization to −100 mv for 30 ms, then a 20 msec test pulse to −10 mV to elicit the NaV1.5 current used to measure the pharmacology of all test compounds. HEK 293 cells expressing NaV1.7 and NaV1.5 channels, were used (Essen Biosciences, Ann Arbor, Mich.). Cells were cultured in T-175 flasks and passaged every 2 to 3 days at 1:3 to 1:6 seeding density dilutions. Cells were grown to 70% to 90% confluence in a flask and removed from the incubator (37° C., 5% CO2) 1 to 3 days after plating. Growth medium was aspirated from the culture flasks. Cells were gently rinsed with 10 ml of PBS (Catalog number: 14190144, Gibco) to remove residual media. Next a total of 2 mL TrypLE (Gibco) solution was added, and the flasks containing cells were sat for 3 min at RT, after which, the cells became visibly rounded and were easily dislodged from the bottom of the flask with a few brief taps on a solid surface. A total of 8 mL of media was added to the flask to inactivate the TrypLE, and the mixture was centrifuged at 910 rpm for 4 min. The cell supernatant was decanted, and the cell pellets were resuspended in 5-6 mL of external solution followed by gentle triturations using a 10 ml pipette, and transferred to a 15 ml conical tube and immediately brought to the IW Barracuda instrument. The cell suspension had a final concentration of 2 to 3 million cells per ml; this corresponds to 10,000 cells added per well.Peak membrane currents were analyzed with IW Barracuda software and exported to Excel for further analysis. Concentration response curve fitting was performed with BMS in-house software. IC50 values were obtained by fits of the Hill equation to the average percent inhibition data plotted versus compound concentration. Concentration-response curves for all test compounds were fitted to a 4-parameter equation: % of control=100 (1+([drug]/IC50)p)−1, where IC50 is the concentration of drug required to inhibit current by 50% and p is the Hill slope.
 DASATINIB BDBM31089
DASATINIB BDBM31089 Dasatinib BDBM82130
Dasatinib BDBM82130 BMS 708163 BMS-70816301 BMS-708163 BMS-708163-01 Avagacestat BDBM50458169
BMS 708163 BMS-70816301 BMS-708163 BMS-708163-01 Avagacestat BDBM50458169 BDBM50578294 Bms 813160 Bms-813160
BDBM50578294 Bms 813160 Bms-813160 med.21724, Compound Dasatinib US20230348453, Compound A8 US10294227, Code Dasatinib BDBM13216 BMS-354825 CHEMBL1421 DASATINIB cid_3062316 N-(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide N-(2-chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide
med.21724, Compound Dasatinib US20230348453, Compound A8 US10294227, Code Dasatinib BDBM13216 BMS-354825 CHEMBL1421 DASATINIB cid_3062316 N-(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide N-(2-chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide BMS 650032 BMS-650032 Asunaprevir BDBM50287594
BMS 650032 BMS-650032 Asunaprevir BDBM50287594 BDBM35316 BMS-344577
BDBM35316 BMS-344577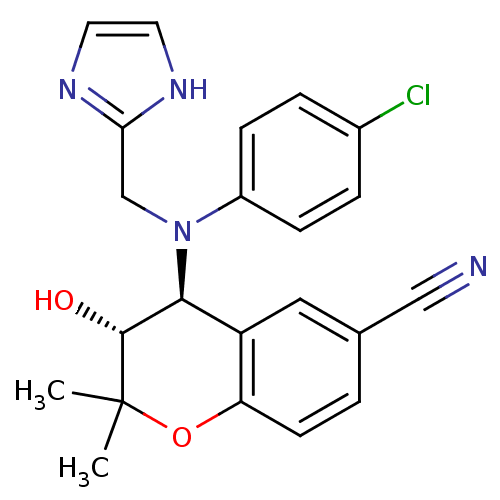 BDBM50409914 BMS-191095
BDBM50409914 BMS-191095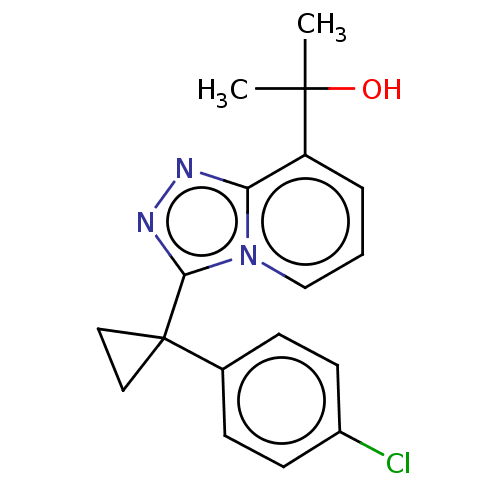 BDBM50507371 BMS-823778
BDBM50507371 BMS-823778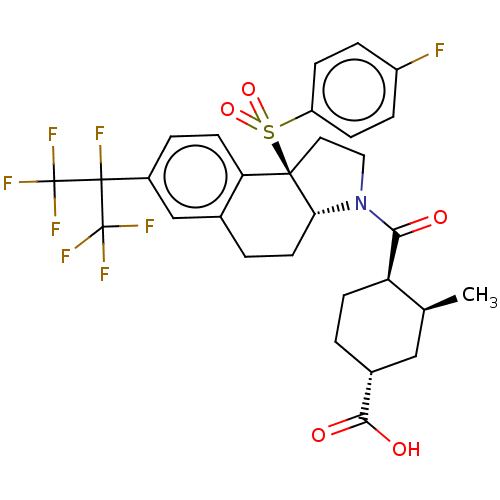 BDBM50541922 Bms-986251
BDBM50541922 Bms-986251 BDBM50558765 Bms-986104
BDBM50558765 Bms-986104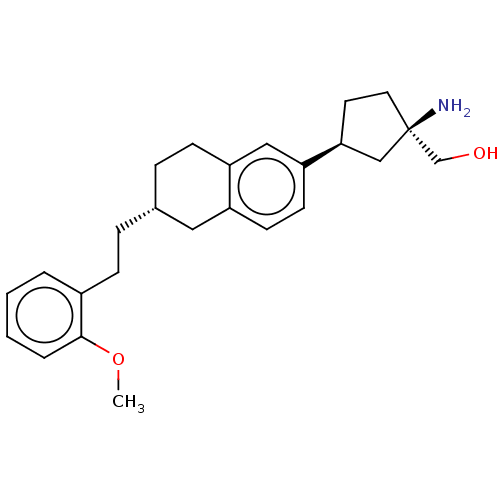 BDBM50563973 Bms-986166
BDBM50563973 Bms-986166 BDBM50633961 Bms-986299
BDBM50633961 Bms-986299 BMS-911543 BDBM50122318
BMS-911543 BDBM50122318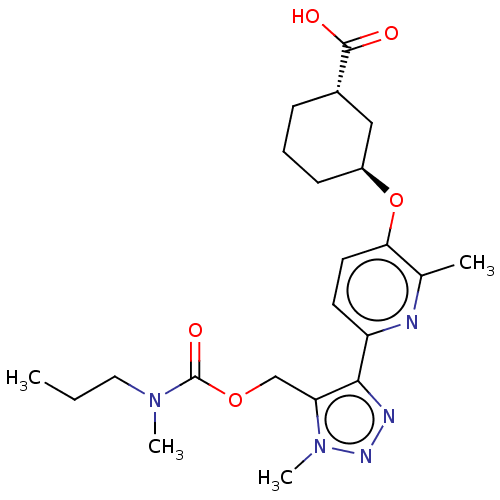 Bms-986278 BDBM50581552
Bms-986278 BDBM50581552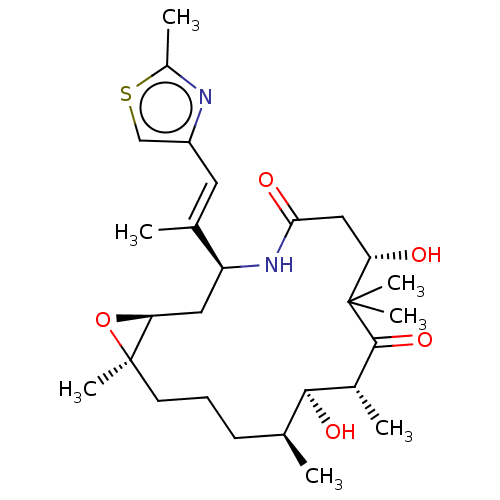 BDBM50564768 NSC-747973 Ixempra kit BMS 247550-01 BMS-247550-01 Ixempra BMS-247550 CHEBI:63605 Azaepothilone b Ixabepilone
BDBM50564768 NSC-747973 Ixempra kit BMS 247550-01 BMS-247550-01 Ixempra BMS-247550 CHEBI:63605 Azaepothilone b Ixabepilone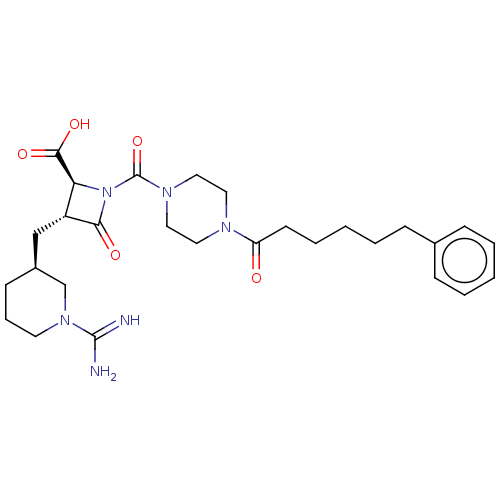 BDBM50217824 CHEMBL70738 BMS-363130
BDBM50217824 CHEMBL70738 BMS-363130 BDBM50353371 BMS-345541 CHEMBL471496
BDBM50353371 BMS-345541 CHEMBL471496 BDBM50387084 BMS-790052 DACLATASVIR
BDBM50387084 BMS-790052 DACLATASVIR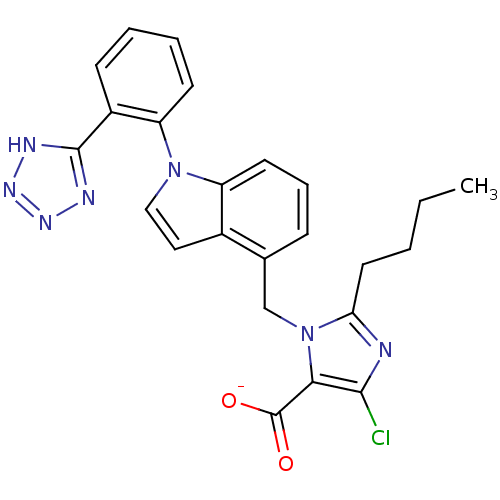 BDBM50449917 CHEMBL2021417 BMS-180560
BDBM50449917 CHEMBL2021417 BMS-180560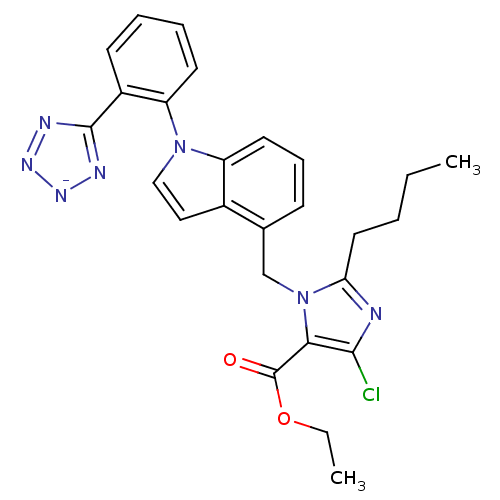 BDBM50449918 CHEMBL2021416 BMS-181688
BDBM50449918 CHEMBL2021416 BMS-181688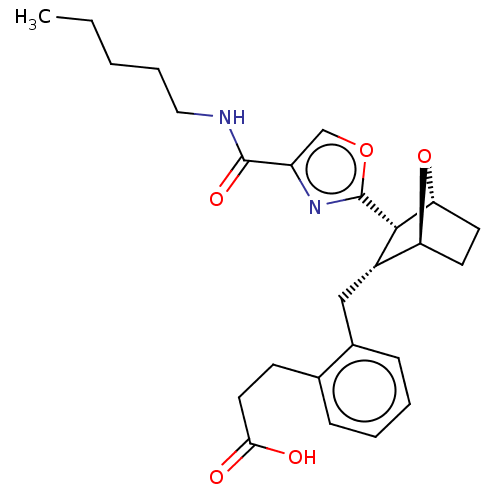 BMS-180291 BDBM50212318 Ifetroban
BMS-180291 BDBM50212318 Ifetroban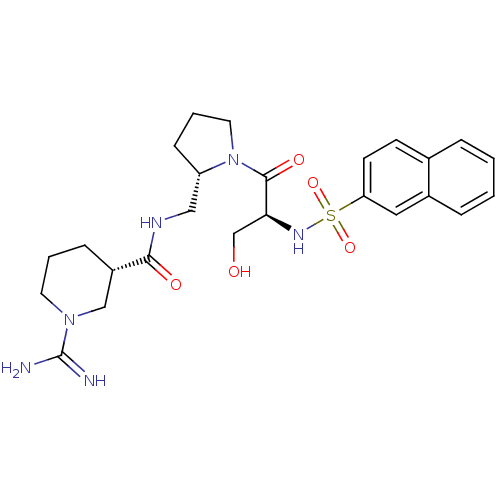 BMS-189090 CHEMBL138877 BDBM50366780
BMS-189090 CHEMBL138877 BDBM50366780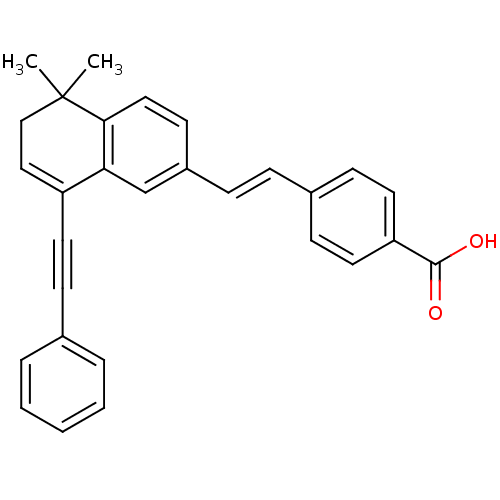 BMS-204493 CHEMBL472172 BDBM50447840
BMS-204493 CHEMBL472172 BDBM50447840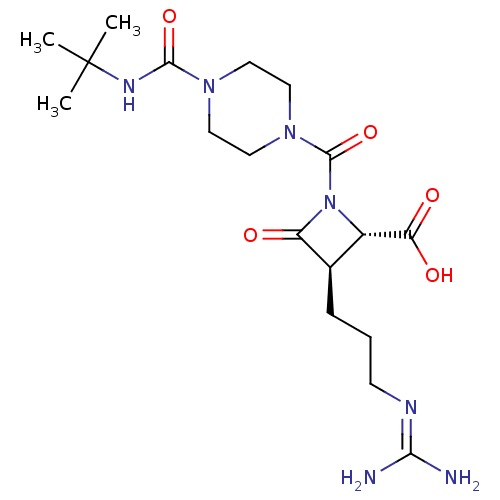 BMS-262084 CHEMBL71037 BDBM50220841
BMS-262084 CHEMBL71037 BDBM50220841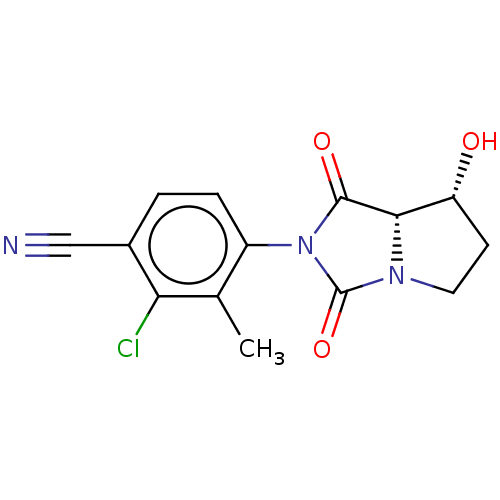 BMS-564929 CHEMBL229264 BDBM50598597
BMS-564929 CHEMBL229264 BDBM50598597 BMS-582664 CHEMBL408444 BDBM50371876
BMS-582664 CHEMBL408444 BDBM50371876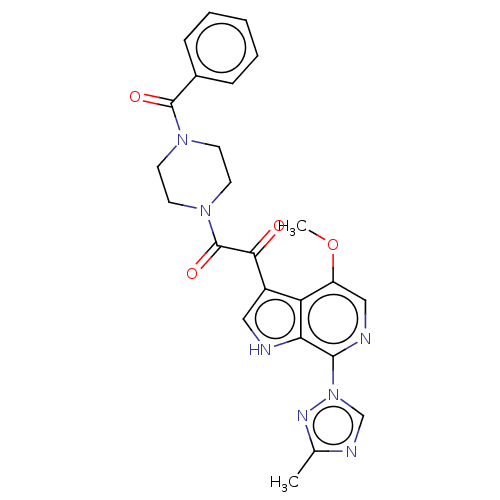 BMS-626529 BDBM50236759 Temsavir
BMS-626529 BDBM50236759 Temsavir BMS-645737 CHEMBL261592 BDBM50377352
BMS-645737 CHEMBL261592 BDBM50377352 BMS-817693 CHEMBL2333637 BDBM50428760
BMS-817693 CHEMBL2333637 BDBM50428760 Beclabuvir BDBM50448498 BMS-791325
Beclabuvir BDBM50448498 BMS-791325 CHEMBL243876 BMS-433771 BDBM50088178
CHEMBL243876 BMS-433771 BDBM50088178 CHEMBL247361 BMS-433796 BDBM50477516
CHEMBL247361 BMS-433796 BDBM50477516 CHEMBL247471 BMS-299897 BDBM50477339
CHEMBL247471 BMS-299897 BDBM50477339 CHEMBL401068 BDBM50487722 BMS-201620
CHEMBL401068 BDBM50487722 BMS-201620 Liafensine BDBM50459930 BMS-820836
Liafensine BDBM50459930 BMS-820836 Hetlioz BMS-214778 BMS-214,778 BDBM50621041 Hetlioz lq CHEBI:79042 TASIMELTEON VEC-162
Hetlioz BMS-214778 BMS-214,778 BDBM50621041 Hetlioz lq CHEBI:79042 TASIMELTEON VEC-162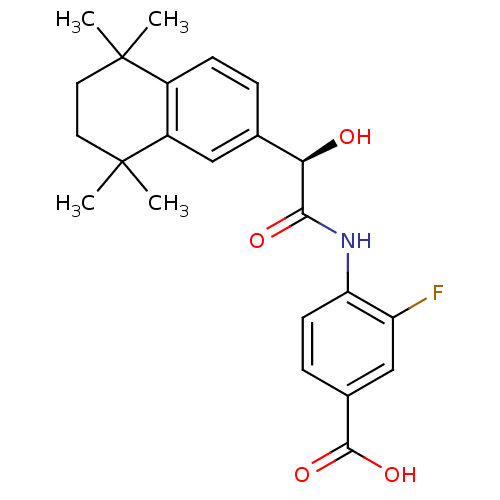 BDBM31889 BMS 961 BMS270394 BMS961
BDBM31889 BMS 961 BMS270394 BMS961 BMS-204352 BDBM50426567 FLINDOKALNER MaxiPost
BMS-204352 BDBM50426567 FLINDOKALNER MaxiPost Bms-955176 GSK-3532795 BDBM50450015
Bms-955176 GSK-3532795 BDBM50450015 Bms-986205 F 001287 BMS 986205 F-001287 BDBM50590772 LINRODOSTAT ONO-7701 ONO 7701
Bms-986205 F 001287 BMS 986205 F-001287 BDBM50590772 LINRODOSTAT ONO-7701 ONO 7701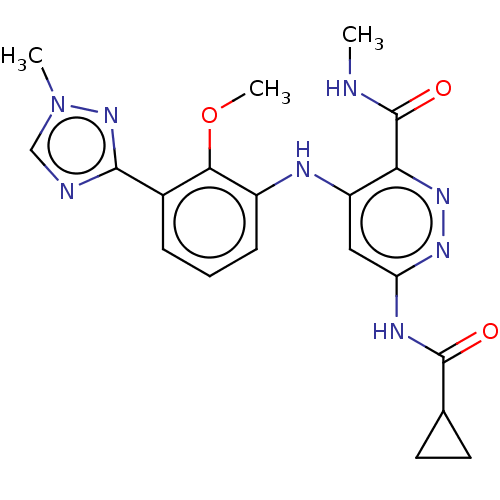 US11578058, Compound Reference (BMS-986165) US11021475, Example 67 US11731956, Example control US20240246944, Control (BMS-986165) BDBM500288 US11021475, Example 52 US20240101548, Compound BMS-986165
US11578058, Compound Reference (BMS-986165) US11021475, Example 67 US11731956, Example control US20240246944, Control (BMS-986165) BDBM500288 US11021475, Example 52 US20240101548, Compound BMS-986165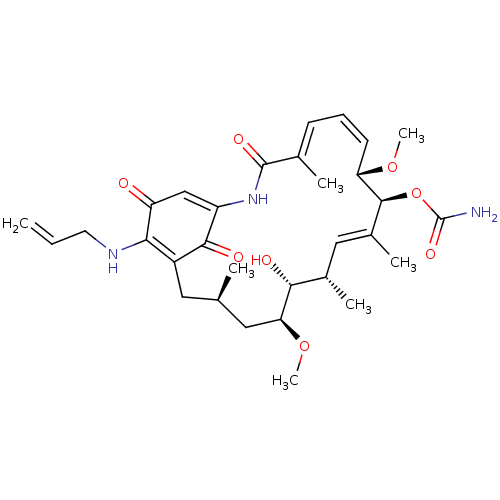 BDBM50008057 CHEBI:64153 BMS-722782 TANESPIMYCIN
BDBM50008057 CHEBI:64153 BMS-722782 TANESPIMYCIN BDBM50011476 ER-30346 RAVUCONAZOLE BMS-207147
BDBM50011476 ER-30346 RAVUCONAZOLE BMS-207147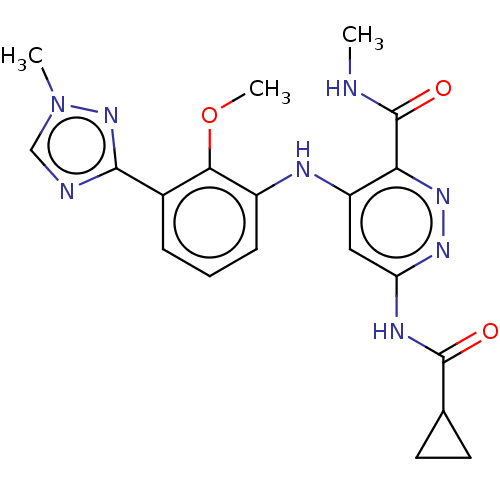 BDBM50507816 Deucravacitinib US12351572, Example control Bms-986165
BDBM50507816 Deucravacitinib US12351572, Example control Bms-986165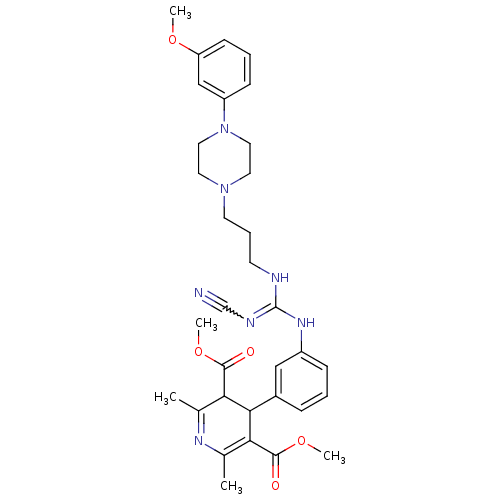 BMS-245782 CHEMBL189548 BDBM50156330 N-arylpiperazine derivative
BMS-245782 CHEMBL189548 BDBM50156330 N-arylpiperazine derivative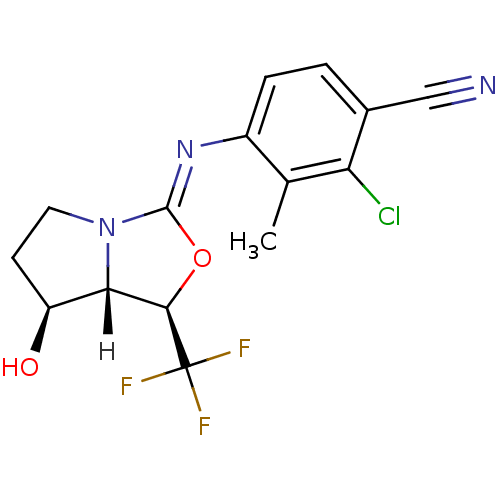 BMS-665139 oxazolidin-2-imine, 6c BDBM29320
BMS-665139 oxazolidin-2-imine, 6c BDBM29320 BMS-908662 BDBM50457451 EXEL-2819 XL-281
BMS-908662 BDBM50457451 EXEL-2819 XL-281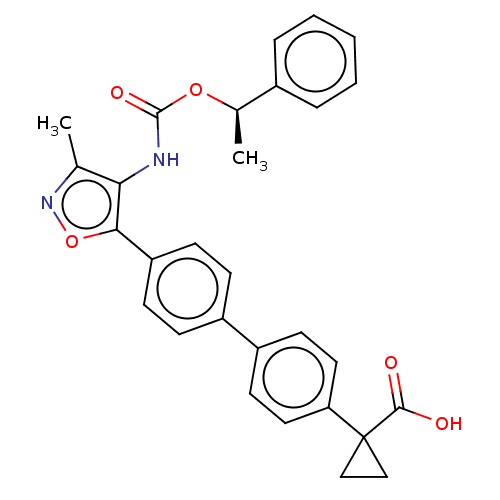 Bms-986020 AP-3152 FREE ACID BDBM50581550
Bms-986020 AP-3152 FREE ACID BDBM50581550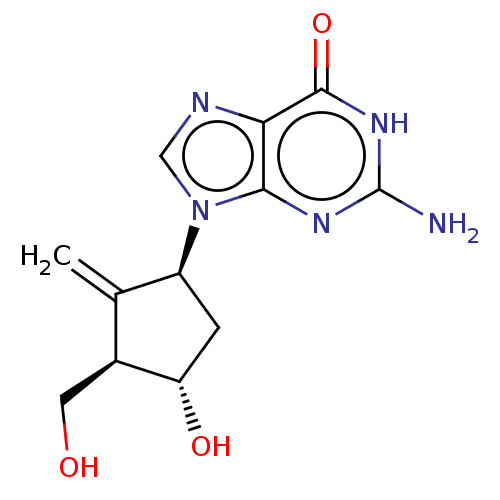 Baraclude Entecavir BDBM50248008 CHEBI:473990 BMS-200475-01 SQ-34676
Baraclude Entecavir BDBM50248008 CHEBI:473990 BMS-200475-01 SQ-34676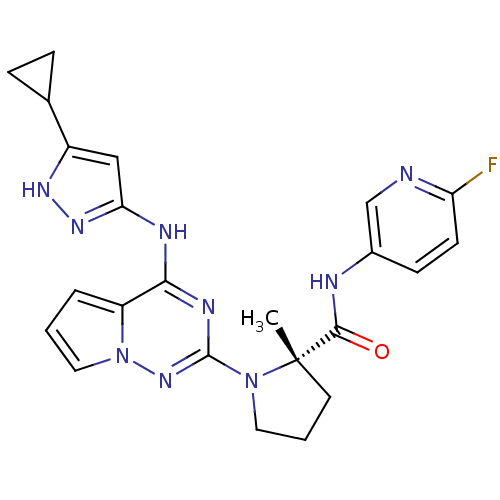 BDBM50299148 BMS-754807 CHEMBL575448 1-{4-[(3-cyclopropyl-1H-pyrazol-5-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl}-N-(6-fluoropyridin-3-yl)-2-methyl-L-prolinamide (S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f][1,2,4]triazin-2-yl)-N-(6-fluoropyridin-3-yl)-2-methylpyrrolidine-2-carboxamide
BDBM50299148 BMS-754807 CHEMBL575448 1-{4-[(3-cyclopropyl-1H-pyrazol-5-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl}-N-(6-fluoropyridin-3-yl)-2-methyl-L-prolinamide (S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f][1,2,4]triazin-2-yl)-N-(6-fluoropyridin-3-yl)-2-methylpyrrolidine-2-carboxamide BDBM50108168 CHEMBL76222 Rebimastat 2-[2-Mercapto-4-(3,4,4-trimethyl-2,5-dioxo-imidazolidin-1-yl)-butyrylamino]-4-methyl-pentanoic acid (2,2-dimethyl-1-methylcarbamoyl-propyl)-amide(BMS-275291) BMS-275291
BDBM50108168 CHEMBL76222 Rebimastat 2-[2-Mercapto-4-(3,4,4-trimethyl-2,5-dioxo-imidazolidin-1-yl)-butyrylamino]-4-methyl-pentanoic acid (2,2-dimethyl-1-methylcarbamoyl-propyl)-amide(BMS-275291) BMS-275291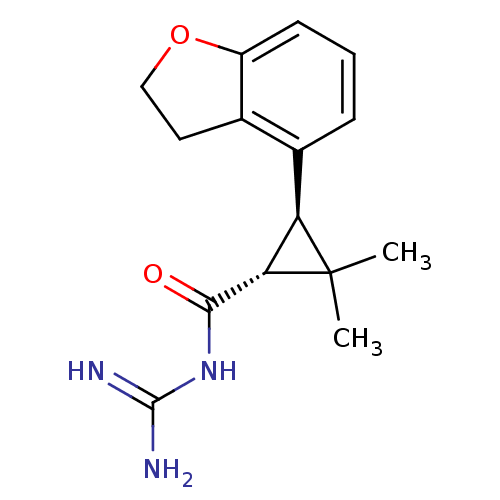 N-[3-(2,3-Dihydro-benzofuran-4-yl)-2,2-dimethyl-cyclopropanecarbonyl]-guanidine (BMS-284640) BMS-284640 CHEMBL51879 BDBM50104844 N-[(1R,3R)-3-(2,3-Dihydro-benzofuran-4-yl)-2,2-dimethyl-cyclopropanecarbonyl]-guanidine
N-[3-(2,3-Dihydro-benzofuran-4-yl)-2,2-dimethyl-cyclopropanecarbonyl]-guanidine (BMS-284640) BMS-284640 CHEMBL51879 BDBM50104844 N-[(1R,3R)-3-(2,3-Dihydro-benzofuran-4-yl)-2,2-dimethyl-cyclopropanecarbonyl]-guanidine US20240327387, Compound BMS202 US20240043392, Compound BMS-202 CHEMBL4089730 US20230303494, Compound BMS202 US9872852, Example 202 BDBM50239947
US20240327387, Compound BMS202 US20240043392, Compound BMS-202 CHEMBL4089730 US20230303494, Compound BMS202 US9872852, Example 202 BDBM50239947 CHEMBL106684 2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide (BMS 187308) 2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide BMS-187308 BDBM50068674
CHEMBL106684 2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide (BMS 187308) 2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide BMS-187308 BDBM50068674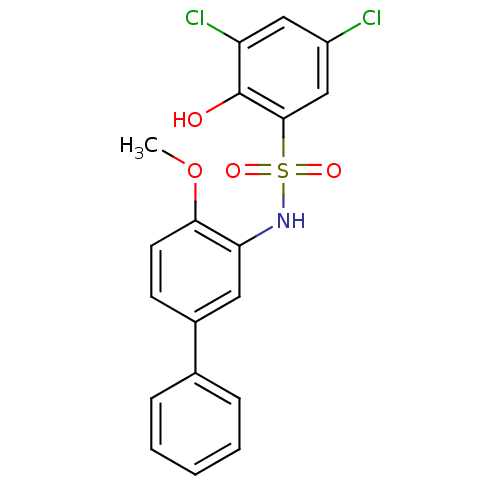 3,5-dichloro-2-hydroxy-N-(4-methoxy-biphenyl-3-yl)-benzenesulfonamide CHEMBL399379 BDBM50209010 US20240398737, Compound BMS-303141
3,5-dichloro-2-hydroxy-N-(4-methoxy-biphenyl-3-yl)-benzenesulfonamide CHEMBL399379 BDBM50209010 US20240398737, Compound BMS-303141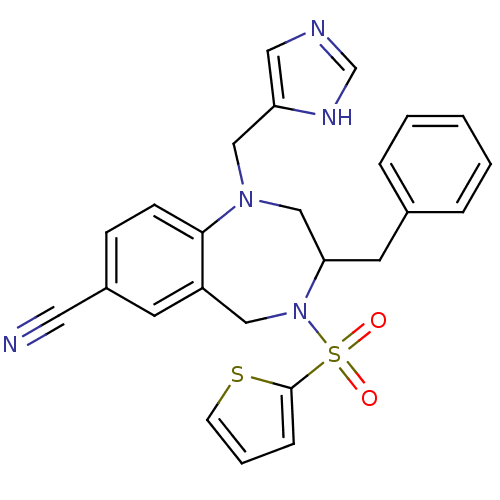 BDBM16178 BMS-214662 3-benzyl-1-(1H-imidazol-5-ylmethyl)-4-(thiophen-2-ylsulfonyl)-2,3,4,5-tetrahydro-1H-1,4-benzodiazepine-7-carbonitrile 3-benzyl-1-(1H-imidazol-5-ylmethyl)-4-(thiophene-2-sulfonyl)-2,3,4,5-tetrahydro-1H-1,4-benzodiazepine-7-carbonitrile BMS 214662
BDBM16178 BMS-214662 3-benzyl-1-(1H-imidazol-5-ylmethyl)-4-(thiophen-2-ylsulfonyl)-2,3,4,5-tetrahydro-1H-1,4-benzodiazepine-7-carbonitrile 3-benzyl-1-(1H-imidazol-5-ylmethyl)-4-(thiophene-2-sulfonyl)-2,3,4,5-tetrahydro-1H-1,4-benzodiazepine-7-carbonitrile BMS 214662 BMS-377806 4-Benzoyl-1-[(4-methoxy-1Hpyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2-(R)-methylpiperazine BMS-378806 1-((R)-4-Benzoyl-2-methyl-piperazin-1-yl)-2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)-ethane-1,2-dione CHEMBL337301 (R)-1-(4-benzoyl-2-methylpiperazin-1-yl)-2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)ethane-1,2-dione BDBM50133282 BMS-806
BMS-377806 4-Benzoyl-1-[(4-methoxy-1Hpyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2-(R)-methylpiperazine BMS-378806 1-((R)-4-Benzoyl-2-methyl-piperazin-1-yl)-2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)-ethane-1,2-dione CHEMBL337301 (R)-1-(4-benzoyl-2-methylpiperazin-1-yl)-2-(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)ethane-1,2-dione BDBM50133282 BMS-806 BDBM13259 N-(2,6-dimethylphenyl)-2-(pyridin-2-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12d
BDBM13259 N-(2,6-dimethylphenyl)-2-(pyridin-2-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12d BDBM13262 BMS-354825 2-Heteroarylamino-thiazole Analog 12g N-(2,6-dimethylphenyl)-2-(pyridazin-3-ylamino)-1,3-thiazole-5-carboxamide
BDBM13262 BMS-354825 2-Heteroarylamino-thiazole Analog 12g N-(2,6-dimethylphenyl)-2-(pyridazin-3-ylamino)-1,3-thiazole-5-carboxamide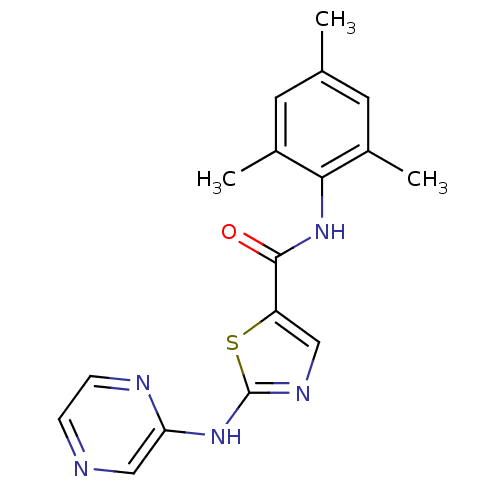 BMS-354825 2-Heteroarylamino-thiazole Analog 12i 2-(pyrazin-2-ylamino)-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13264
BMS-354825 2-Heteroarylamino-thiazole Analog 12i 2-(pyrazin-2-ylamino)-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13264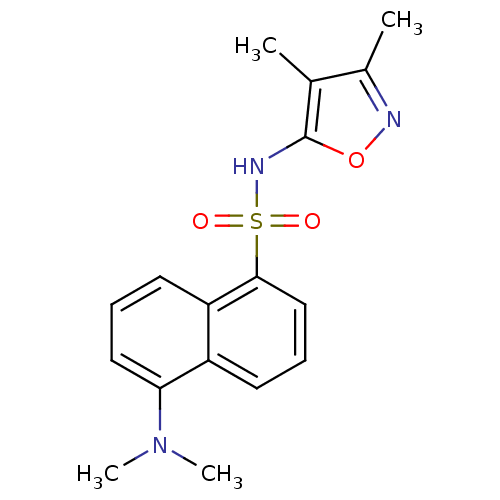 5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide 5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide (BMS 182874) BDBM50034435 CHEMBL267458 BMS-182874 5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-dimethyl-2,3-dihydro-oxazol-5-yl)-amide
5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide 5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide (BMS 182874) BDBM50034435 CHEMBL267458 BMS-182874 5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-dimethyl-2,3-dihydro-oxazol-5-yl)-amide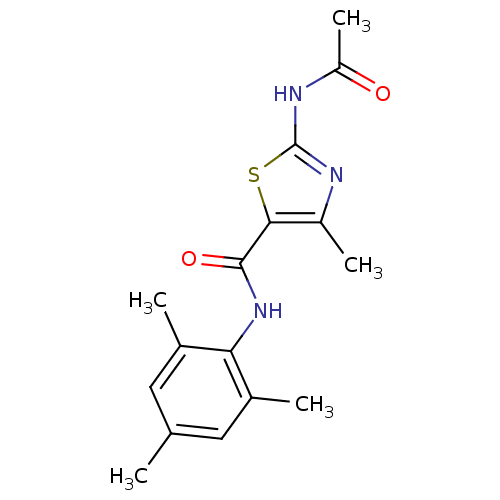 2-acetamido-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 7c BDBM13223
2-acetamido-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 7c BDBM13223 BDBM13261 BMS-354825 2-Heteroarylamino-thiazole Analog 12f N-(2-chloro-6-methylphenyl)-2-(pyridazin-3-ylamino)-1,3-thiazole-5-carboxamide
BDBM13261 BMS-354825 2-Heteroarylamino-thiazole Analog 12f N-(2-chloro-6-methylphenyl)-2-(pyridazin-3-ylamino)-1,3-thiazole-5-carboxamide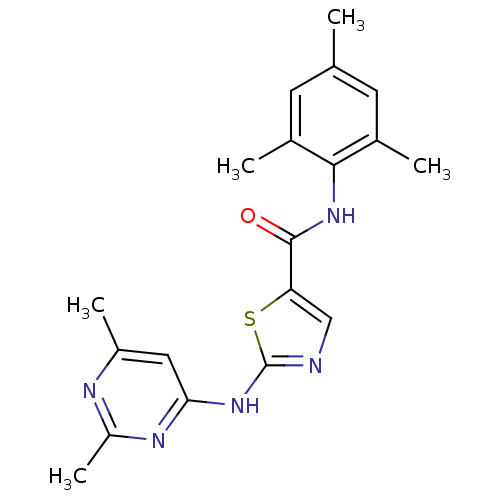 BDBM13270 2-[(2,6-dimethylpyrimidin-4-yl)amino]-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12o
BDBM13270 2-[(2,6-dimethylpyrimidin-4-yl)amino]-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12o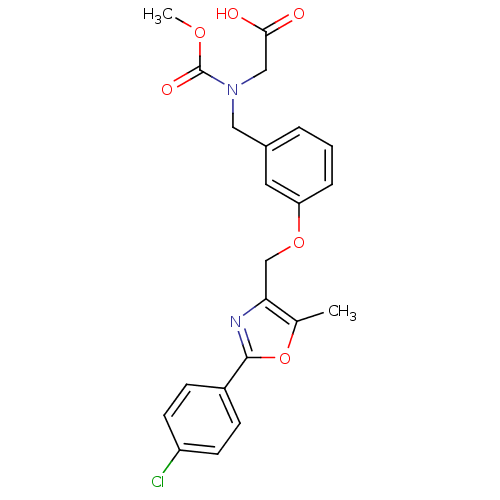 BMS-687453 BDBM28800 2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}phenyl)methyl](methoxycarbonyl)amino}acetic acid
BMS-687453 BDBM28800 2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}phenyl)methyl](methoxycarbonyl)amino}acetic acid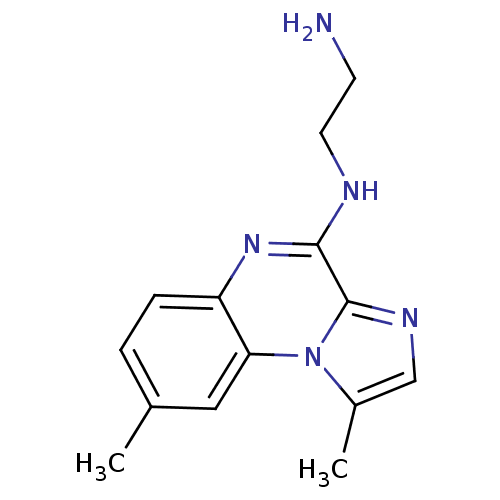 BMS345541 BMS-345541 CHEMBL471496 N-(2-aminoethyl)-3,12-dimethyl-2,5,8-triazatricyclo[7.4.0.0^{2,6}]trideca-1(13),3,5,7,9,11-hexaen-7-amine BDBM25919
BMS345541 BMS-345541 CHEMBL471496 N-(2-aminoethyl)-3,12-dimethyl-2,5,8-triazatricyclo[7.4.0.0^{2,6}]trideca-1(13),3,5,7,9,11-hexaen-7-amine BDBM25919 N-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-4-oxazol-2-yl-biphenyl-2-ylmethyl]-3,3-dimethyl-butyramide CHEMBL277447 BDBM50140757 BMS-207940
N-[2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-4-oxazol-2-yl-biphenyl-2-ylmethyl]-3,3-dimethyl-butyramide CHEMBL277447 BDBM50140757 BMS-207940 pyrazolo[3,4-b]pyridine deriv. BDBM5776 4-butoxy-5-[(2,6-difluoro-4-methylphenyl)carbonyl]-1H-pyrazolo[3,4-b]pyridine BMS-265246
pyrazolo[3,4-b]pyridine deriv. BDBM5776 4-butoxy-5-[(2,6-difluoro-4-methylphenyl)carbonyl]-1H-pyrazolo[3,4-b]pyridine BMS-265246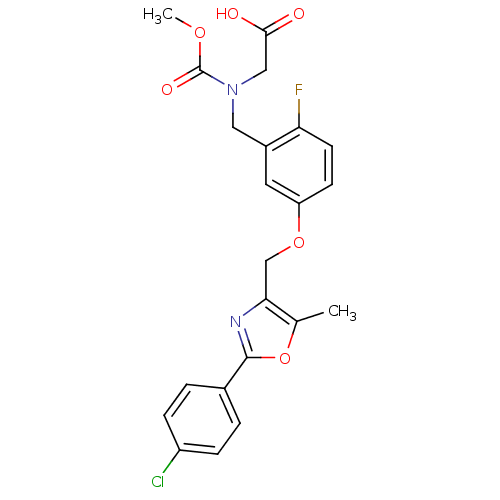 2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}-2-fluorophenyl)methyl](methoxycarbonyl)amino}acetic acid BDBM28802 BMS-711939
2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}-2-fluorophenyl)methyl](methoxycarbonyl)amino}acetic acid BDBM28802 BMS-711939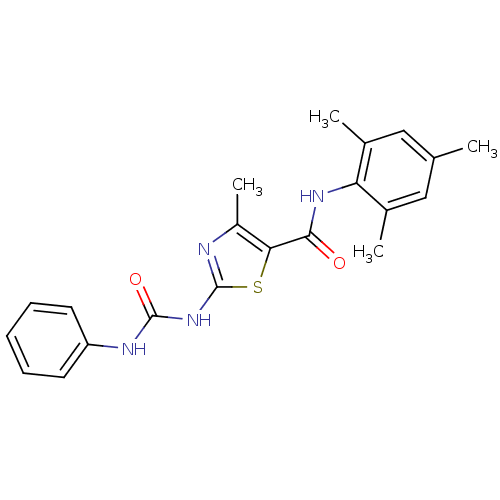 4-methyl-2-[(phenylcarbamoyl)amino]-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 8b BDBM13241
4-methyl-2-[(phenylcarbamoyl)amino]-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 8b BDBM13241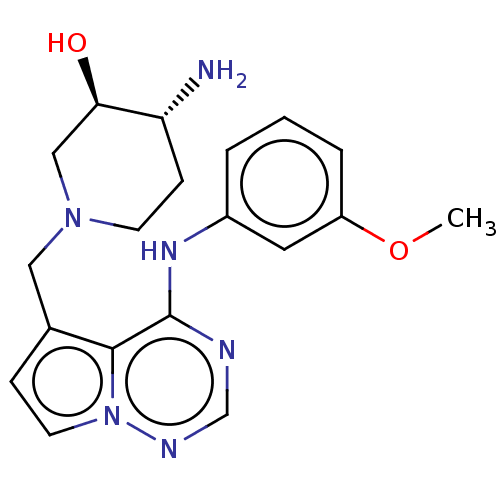 BDBM185148 (3R,4R)-4-amino-1-[[4-(3-methoxyanilino)pyrrolo[2,1-f][1,2,4]triazin-5-yl]methyl]piperidin-3-ol BMS-690514
BDBM185148 (3R,4R)-4-amino-1-[[4-(3-methoxyanilino)pyrrolo[2,1-f][1,2,4]triazin-5-yl]methyl]piperidin-3-ol BMS-690514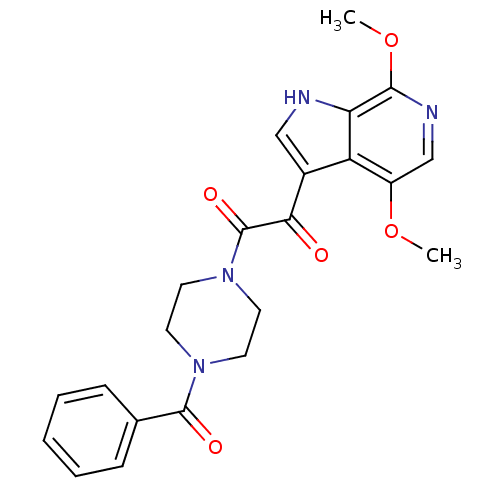 BDBM50228465 CHEMBL238103 1-(4-benzoylpiperazin-1-yl)-2-(4,7-dimethoxy-1H-pyrrolo[2,3-c]pyridin-3-yl)ethane-1,2-dione BMS-488043
BDBM50228465 CHEMBL238103 1-(4-benzoylpiperazin-1-yl)-2-(4,7-dimethoxy-1H-pyrrolo[2,3-c]pyridin-3-yl)ethane-1,2-dione BMS-488043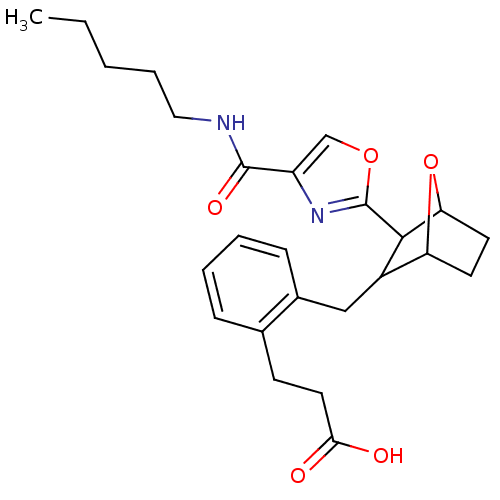 BMS-180291 3-{2-[3-(4-Pentylcarbamoyl-oxazol-2-yl)-7-oxa-bicyclo[2.2.1]hept-2-ylmethyl]-phenyl}-propionic acid CHEMBL283100 BDBM50047274
BMS-180291 3-{2-[3-(4-Pentylcarbamoyl-oxazol-2-yl)-7-oxa-bicyclo[2.2.1]hept-2-ylmethyl]-phenyl}-propionic acid CHEMBL283100 BDBM50047274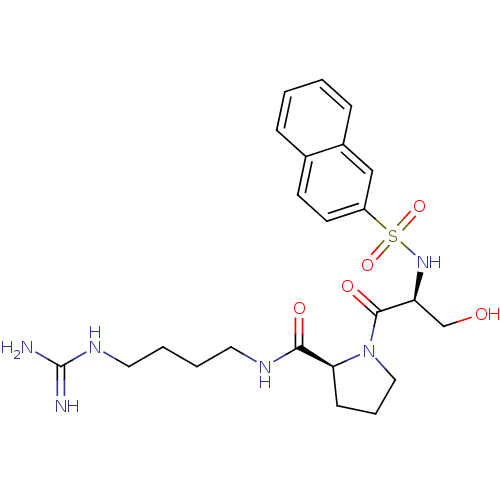 BMS-185367 CHEMBL136082 (S)-1-[(S)-3-Hydroxy-2-(naphthalene-2-sulfonylamino)-propionyl]-pyrrolidine-2-carboxylic acid (4-guanidino-butyl)-amide BDBM50107438
BMS-185367 CHEMBL136082 (S)-1-[(S)-3-Hydroxy-2-(naphthalene-2-sulfonylamino)-propionyl]-pyrrolidine-2-carboxylic acid (4-guanidino-butyl)-amide BDBM50107438 CHEMBL132448 BMS-354825 tert-Butoxycarbamate Analog 5e BDBM13221 tert-butyl N-{5-[(2,6-dichlorophenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate
CHEMBL132448 BMS-354825 tert-Butoxycarbamate Analog 5e BDBM13221 tert-butyl N-{5-[(2,6-dichlorophenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate CHEMBL356065 (S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propionyl)-pyrrolidine-2-carboxylic acid (1-carbamimidoyl-piperidin-4-ylmethyl)-amide BDBM50107463 BMS-189664
CHEMBL356065 (S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propionyl)-pyrrolidine-2-carboxylic acid (1-carbamimidoyl-piperidin-4-ylmethyl)-amide BDBM50107463 BMS-189664 N-(2-chloro-6-methylphenyl)-2-(pyridin-2-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12a BDBM13256 CHEMBL189776
N-(2-chloro-6-methylphenyl)-2-(pyridin-2-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12a BDBM13256 CHEMBL189776 BMS-564929 4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrrolo[1,2-a]imidazolidin-2-yl]-2-chloro-3-methylbenzonitrile BDBM18173 CHEMBL229264
BMS-564929 4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrrolo[1,2-a]imidazolidin-2-yl]-2-chloro-3-methylbenzonitrile BDBM18173 CHEMBL229264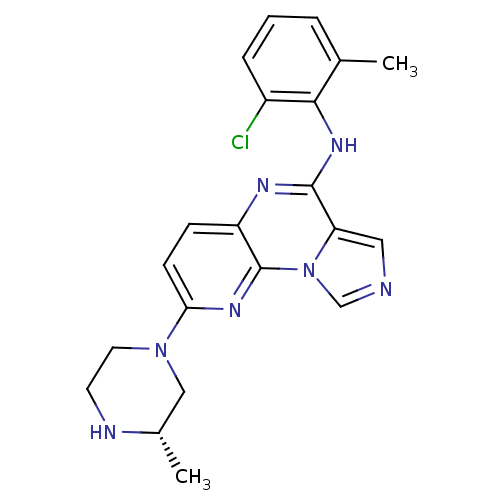 (2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-piperazin-1-yl)-2,5,9,9b-tetraaza-cyclopenta[a]naphthalen-4-yl]-amine BDBM50151366 CHEMBL189338 BMS-279700
(2-Chloro-6-methyl-phenyl)-[8-((S)-3-methyl-piperazin-1-yl)-2,5,9,9b-tetraaza-cyclopenta[a]naphthalen-4-yl]-amine BDBM50151366 CHEMBL189338 BMS-279700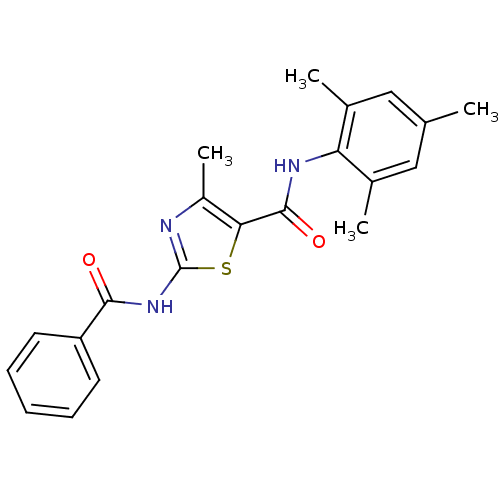 2-N-benzene-4-methyl-5-N-(2,4,6-trimethylphenyl)-1,3-thiazole-2,5-diamido CHEMBL130719 BMS-354825 2-Amino-4-methyl-thiazole Analog 7d BDBM13224
2-N-benzene-4-methyl-5-N-(2,4,6-trimethylphenyl)-1,3-thiazole-2,5-diamido CHEMBL130719 BMS-354825 2-Amino-4-methyl-thiazole Analog 7d BDBM13224 5-N-(2-chloro-6-methylphenyl)-2-N-(2-methylcyclopropane)-1,3-thiazole-2,5-diamido BDBM13249 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7q
5-N-(2-chloro-6-methylphenyl)-2-N-(2-methylcyclopropane)-1,3-thiazole-2,5-diamido BDBM13249 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7q BDBM13248 5-N-(2-chloro-6-methylphenyl)-2-N-(1-methylcyclopropane)-1,3-thiazole-2,5-diamido BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7p
BDBM13248 5-N-(2-chloro-6-methylphenyl)-2-N-(1-methylcyclopropane)-1,3-thiazole-2,5-diamido BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7p N-(2-chloro-6-methylphenyl)-2-[(4,6-dimethylpyrimidin-2-yl)amino]-1,3-thiazole-5-carboxamide BDBM13269 BMS-354825 2-Heteroarylamino-thiazole Analog 12n CHEMBL186564
N-(2-chloro-6-methylphenyl)-2-[(4,6-dimethylpyrimidin-2-yl)amino]-1,3-thiazole-5-carboxamide BDBM13269 BMS-354825 2-Heteroarylamino-thiazole Analog 12n CHEMBL186564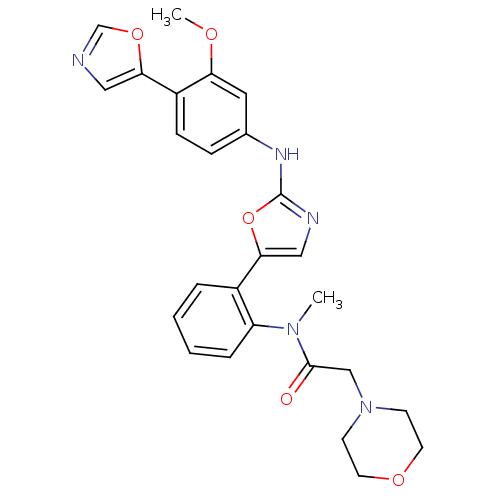 BMS-337197 BDBM50113230 CHEMBL64830 N-{2-[2-(3-Methoxy-4-oxazol-5-yl-phenylamino)-oxazol-5-yl]-phenyl}-N-methyl-2-morpholin-4-yl-acetamide
BMS-337197 BDBM50113230 CHEMBL64830 N-{2-[2-(3-Methoxy-4-oxazol-5-yl-phenylamino)-oxazol-5-yl]-phenyl}-N-methyl-2-morpholin-4-yl-acetamide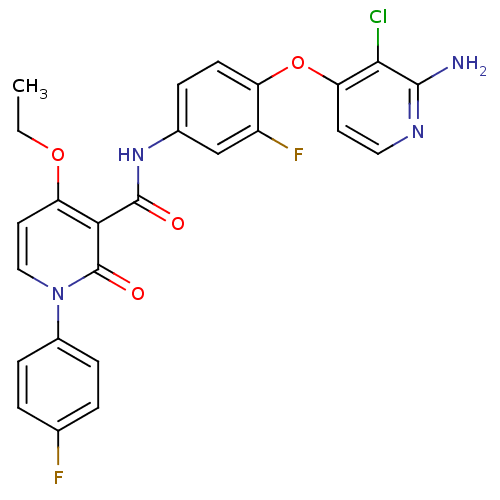 BMS-777607 BDBM28031 N-{4-[(2-amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl}-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide
BMS-777607 BDBM28031 N-{4-[(2-amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl}-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide CHEMBL489100 (R)-2-((R)-3-amino-3-(4-((2-methylquinolin-4-yl)methoxy)phenyl)-2-oxopyrrolidin-1-yl)-N-hydroxy-4-methylpentanamide BDBM50247606 BMS-561392
CHEMBL489100 (R)-2-((R)-3-amino-3-(4-((2-methylquinolin-4-yl)methoxy)phenyl)-2-oxopyrrolidin-1-yl)-N-hydroxy-4-methylpentanamide BDBM50247606 BMS-561392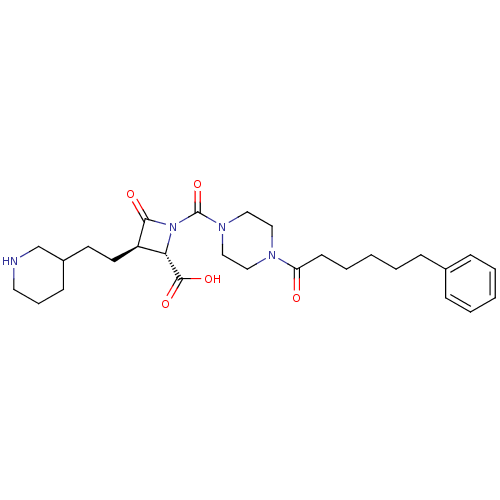 (2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-1-carbonyl]-3-(2-piperidin-3-yl-ethyl)-azetidine-2-carboxylic acid BMS-354326 BDBM50144532 CHEMBL306448
(2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-1-carbonyl]-3-(2-piperidin-3-yl-ethyl)-azetidine-2-carboxylic acid BMS-354326 BDBM50144532 CHEMBL306448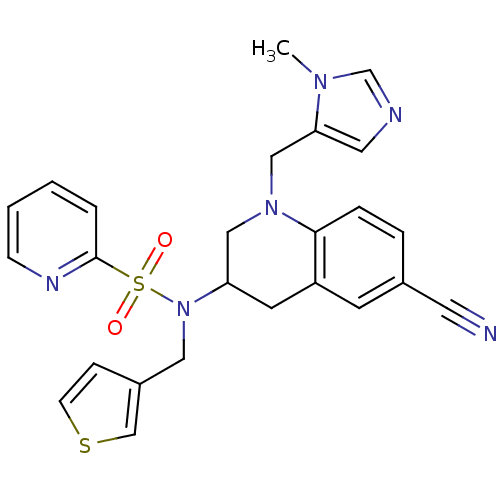 BDBM50164025 BMS-316810 CHEMBL360330 Pyridine-2-sulfonic acid [6-cyano-1-(3-methyl-3H-imidazol-4-ylmethyl)-1,2,3,4-tetrahydro-quinolin-3-yl]-thiophen-3-ylmethyl-amide
BDBM50164025 BMS-316810 CHEMBL360330 Pyridine-2-sulfonic acid [6-cyano-1-(3-methyl-3H-imidazol-4-ylmethyl)-1,2,3,4-tetrahydro-quinolin-3-yl]-thiophen-3-ylmethyl-amide (2S,3S)-7-Amino-3-{[(S)-1-((R)-2-amino-3-phenyl-propionyl)-pyrrolidine-2-carbonyl]-amino}-2-hydroxy-heptanoic acid methyl ester BDBM50280253 CHEMBL41426 BMS-181316
(2S,3S)-7-Amino-3-{[(S)-1-((R)-2-amino-3-phenyl-propionyl)-pyrrolidine-2-carbonyl]-amino}-2-hydroxy-heptanoic acid methyl ester BDBM50280253 CHEMBL41426 BMS-181316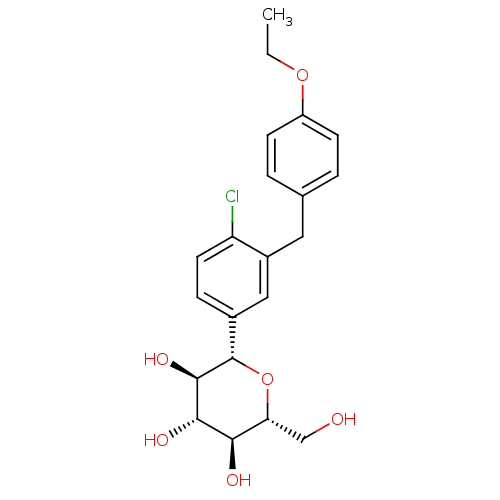 Dapagliflozin BDBM20880 CHEMBL429910 (2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-6-(hydroxymethyl)oxane-3,4,5-triol C-aryl glucoside, 6 BMS-512148
Dapagliflozin BDBM20880 CHEMBL429910 (2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-6-(hydroxymethyl)oxane-3,4,5-triol C-aryl glucoside, 6 BMS-512148 N''-cyano-N-(4-cyanophenyl)-N'-(1,2,2-trimethylpropyl)guanidine (BMS-182264) N''-cyano-N-(4-cyanophenyl)-N'-(1,2,2-trimethylpropyl)guanidine 4-cyanoimino(1,2,2-trimethylpropylamino)methylaminobenzonitrile CHEMBL19169 BDBM50062396
N''-cyano-N-(4-cyanophenyl)-N'-(1,2,2-trimethylpropyl)guanidine (BMS-182264) N''-cyano-N-(4-cyanophenyl)-N'-(1,2,2-trimethylpropyl)guanidine 4-cyanoimino(1,2,2-trimethylpropylamino)methylaminobenzonitrile CHEMBL19169 BDBM50062396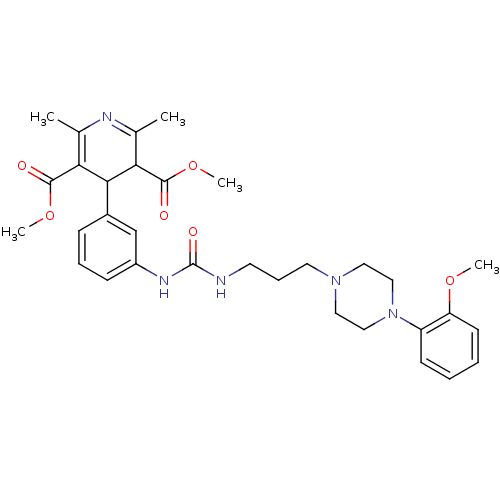 4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propyl}-ureido)-phenyl]-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid dimethyl ester BDBM50109185 BMS-189323 CHEMBL124152
4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-propyl}-ureido)-phenyl]-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid dimethyl ester BDBM50109185 BMS-189323 CHEMBL124152 4-[3-(3-{3-[4-(3-Methoxy-phenyl)-piperidin-1-yl]-propyl}-ureido)-phenyl]-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid dimethyl ester BMS-193885 BDBM50109122 CHEMBL324554
4-[3-(3-{3-[4-(3-Methoxy-phenyl)-piperidin-1-yl]-propyl}-ureido)-phenyl]-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid dimethyl ester BMS-193885 BDBM50109122 CHEMBL324554 Aminobenzisoxazole 2g BMS-740808 Analogue 15 BDBM12688 1-(3-amino-1,2-benzoxazol-5-yl)-N-(4-{2-[(dimethylamino)methyl]phenyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide
Aminobenzisoxazole 2g BMS-740808 Analogue 15 BDBM12688 1-(3-amino-1,2-benzoxazol-5-yl)-N-(4-{2-[(dimethylamino)methyl]phenyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide BDBM50280255 BMS-181412 CHEMBL539241 7-Amino-3-{[(S)-1-((R)-2-amino-3-phenyl-propionyl)-pyrrolidine-2-carbonyl]-amino}-2-hydroxy-2-methoxy-heptanoic acid methyl ester; dihydrochloride
BDBM50280255 BMS-181412 CHEMBL539241 7-Amino-3-{[(S)-1-((R)-2-amino-3-phenyl-propionyl)-pyrrolidine-2-carbonyl]-amino}-2-hydroxy-2-methoxy-heptanoic acid methyl ester; dihydrochloride BMS-181162 3-[(1E,3E,5E)-1-((E)-2-Carboxy-1-methyl-vinyl)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hexa-1,3,5-trienyl]-benzoic acid CHEMBL313583 BDBM50288286
BMS-181162 3-[(1E,3E,5E)-1-((E)-2-Carboxy-1-methyl-vinyl)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hexa-1,3,5-trienyl]-benzoic acid CHEMBL313583 BDBM50288286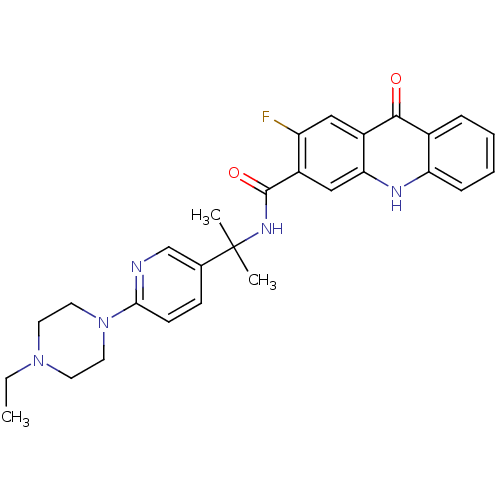 N-{2-[6-(4-ethylpiperazin-1-yl)pyridin-3-yl]propan-2-yl}-2-fluoro-9-oxo-9,10-dihydroacridine-3-carboxamide Acridone-Based Inhibitor, 4m BDBM19289 BMS-566419
N-{2-[6-(4-ethylpiperazin-1-yl)pyridin-3-yl]propan-2-yl}-2-fluoro-9-oxo-9,10-dihydroacridine-3-carboxamide Acridone-Based Inhibitor, 4m BDBM19289 BMS-566419 BDBM50280254 (S)-3-{[(S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine-2-carbonyl]-amino}-7-guanidino-2-hydroxy-heptanoic acid methyl ester with di TFA BMS-182060 CHEMBL290162
BDBM50280254 (S)-3-{[(S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine-2-carbonyl]-amino}-7-guanidino-2-hydroxy-heptanoic acid methyl ester with di TFA BMS-182060 CHEMBL290162 BMS-205603-01 Lamivudine impurity e SQ-7726 NSC-3970 BDBM50549809 CHEBI:17568 Fluorouracil specified compound c Hybar x SQ-8493 Lamivudine impurity e rs SQ-6201 Uracil Pyrod
BMS-205603-01 Lamivudine impurity e SQ-7726 NSC-3970 BDBM50549809 CHEBI:17568 Fluorouracil specified compound c Hybar x SQ-8493 Lamivudine impurity e rs SQ-6201 Uracil Pyrod BMS-317180 BDBM21941 Tetrazole-based compound, 2 2-{5-[(1S)-1-(2-amino-2-methylpropanamido)-2-(benzyloxy)ethyl]-1H-1,2,3,4-tetrazol-1-yl}ethyl N-(4-hydroxybutyl)carbamate CHEMBL398372
BMS-317180 BDBM21941 Tetrazole-based compound, 2 2-{5-[(1S)-1-(2-amino-2-methylpropanamido)-2-(benzyloxy)ethyl]-1H-1,2,3,4-tetrazol-1-yl}ethyl N-(4-hydroxybutyl)carbamate CHEMBL398372 (S)-N2,N2-dimethyl-N5-((2-methylbenzofuran-5-ylamino)(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)azepan-3-ylamino)methylene)pyridine-2,5-dicarboxamide BDBM50328724 CHEMBL570867 BMS-344577
(S)-N2,N2-dimethyl-N5-((2-methylbenzofuran-5-ylamino)(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)azepan-3-ylamino)methylene)pyridine-2,5-dicarboxamide BDBM50328724 CHEMBL570867 BMS-344577 BDBM13260 2-(pyridin-2-ylamino)-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12e N-(2,4,6-Trimethylphenyl)-2-[(2-pyridinyl)amino]-1,3-thiazole-5-carboxamide
BDBM13260 2-(pyridin-2-ylamino)-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12e N-(2,4,6-Trimethylphenyl)-2-[(2-pyridinyl)amino]-1,3-thiazole-5-carboxamide BDBM27879 CHEMBL401930 4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-3-[4-methyl-6-(morpholin-4-yl)-1H-1,3-benzodiazol-2-yl]-1,2-dihydropyridin-2-one US10710978, Compound BMS536924 BMS-536924
BDBM27879 CHEMBL401930 4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-3-[4-methyl-6-(morpholin-4-yl)-1H-1,3-benzodiazol-2-yl]-1,2-dihydropyridin-2-one US10710978, Compound BMS536924 BMS-536924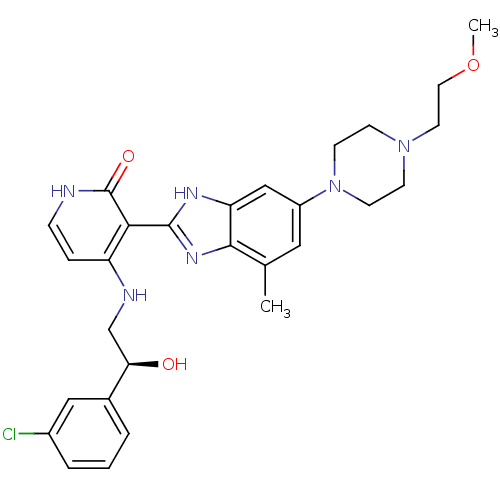 CHEMBL1094165 (S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(6-(4-(2-methoxyethyl)piperazin-1-yl)-4-methyl-1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one BDBM50318112 BMS-577098
CHEMBL1094165 (S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(6-(4-(2-methoxyethyl)piperazin-1-yl)-4-methyl-1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one BDBM50318112 BMS-577098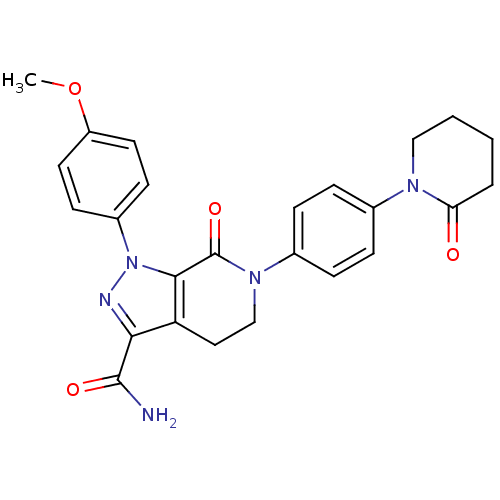 CHEMBL231779 BMS-562247 Apixaban 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridine-3-carboxamide US9938272, Example Apixaban BDBM19023
CHEMBL231779 BMS-562247 Apixaban 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridine-3-carboxamide US9938272, Example Apixaban BDBM19023 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-methyl-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12701 BMS-740808 Analogue 12a
1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-methyl-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12701 BMS-740808 Analogue 12a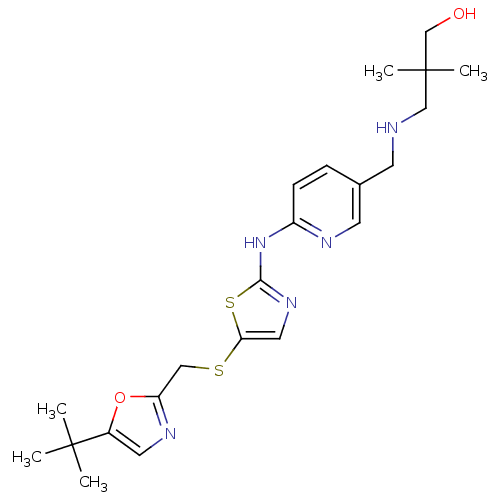 2-aminothiazole deriv. BMS-357075 3-[({6-[(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)amino]pyridin-3-yl}methyl)amino]-2,2-dimethylpropan-1-ol BDBM5726
2-aminothiazole deriv. BMS-357075 3-[({6-[(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)amino]pyridin-3-yl}methyl)amino]-2,2-dimethylpropan-1-ol BDBM5726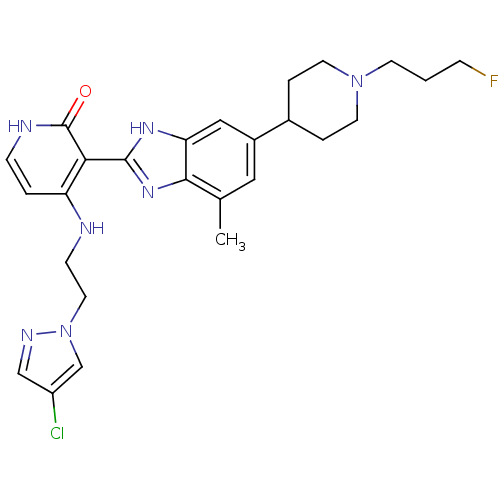 4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-[1-(3-fluoropropyl)piperidin-4-yl]-4-methyl-1H-1,3-benzodiazol-2-yl}-1,2-dihydropyridin-2-one BDBM27888 BMS-695735
4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-[1-(3-fluoropropyl)piperidin-4-yl]-4-methyl-1H-1,3-benzodiazol-2-yl}-1,2-dihydropyridin-2-one BDBM27888 BMS-695735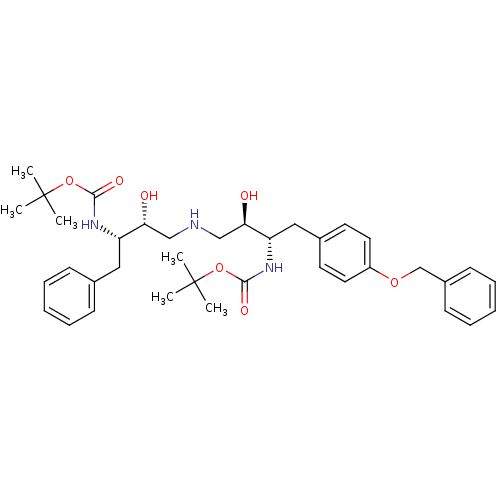 BDBM687 BMS-186318 analog 24 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-4-[4-(benzyloxy)phenyl]-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
BDBM687 BMS-186318 analog 24 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-4-[4-(benzyloxy)phenyl]-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate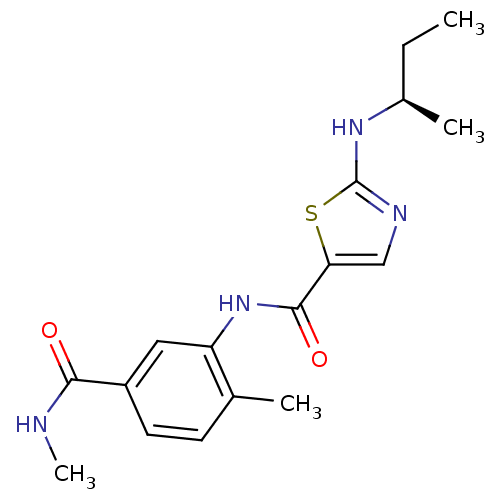 BMS-640994 (R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbamoyl)phenyl)thiazole-5-carboxamide BDBM50236473 N-[2-methyl-5-(methylcarbamoyl)phenyl]-2-{[(1R)-1-methylpropyl]amino}-1,3-thiazole-5-carboxamide CHEMBL258202
BMS-640994 (R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbamoyl)phenyl)thiazole-5-carboxamide BDBM50236473 N-[2-methyl-5-(methylcarbamoyl)phenyl]-2-{[(1R)-1-methylpropyl]amino}-1,3-thiazole-5-carboxamide CHEMBL258202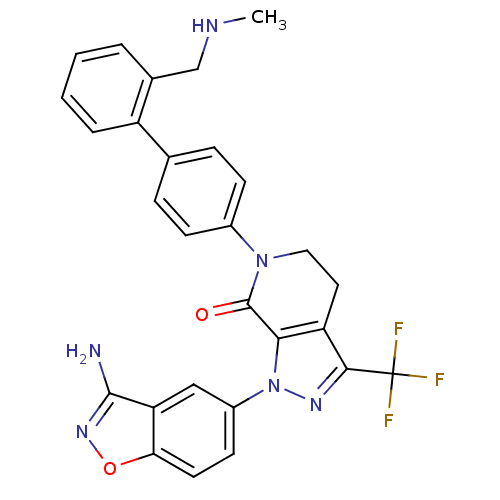 BMS-740808 Analogue 6d 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(methylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12691
BMS-740808 Analogue 6d 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(methylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12691 CHEMBL564417 (S)-3-(4-(2-(4-(2-(2-chlorophenyl)-2-hydroxyethylamino)-2-oxo-1,2-dihydropyridin-3-yl)-7-methyl-1H-benzo[d]imidazol-5-yl)piperazin-1-yl)propanenitrile BMS-554417 BDBM50296350
CHEMBL564417 (S)-3-(4-(2-(4-(2-(2-chlorophenyl)-2-hydroxyethylamino)-2-oxo-1,2-dihydropyridin-3-yl)-7-methyl-1H-benzo[d]imidazol-5-yl)piperazin-1-yl)propanenitrile BMS-554417 BDBM50296350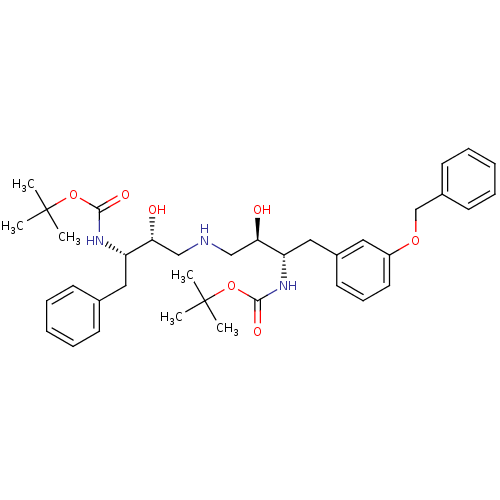 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-4-[3-(benzyloxy)phenyl]-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM688 BMS-186318 analog 25
tert-butyl N-[(2S,3R)-4-{[(2R,3S)-4-[3-(benzyloxy)phenyl]-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM688 BMS-186318 analog 25 1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(piperazin-1-ylmethyl)phenyl]phenyl}-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 6j BDBM12697
1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(piperazin-1-ylmethyl)phenyl]phenyl}-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 6j BDBM12697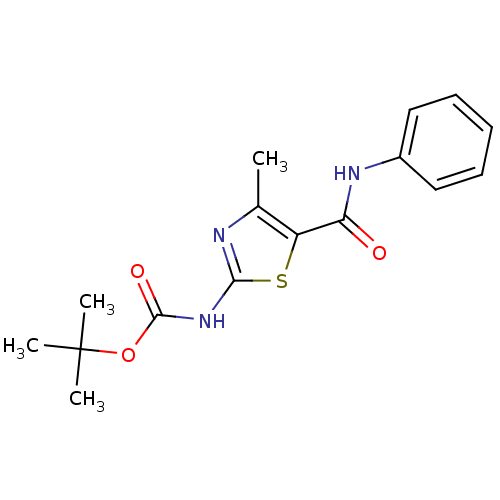 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-phenyl-1,3-thiazole-5-carboxamide tert-butyl N-[4-methyl-5-(phenylcarbamoyl)-1,3-thiazol-2-yl]carbamate BDBM13225 CHEMBL424450 BMS-354825 tert-Butoxycarbamate Analog 5f
2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-phenyl-1,3-thiazole-5-carboxamide tert-butyl N-[4-methyl-5-(phenylcarbamoyl)-1,3-thiazol-2-yl]carbamate BDBM13225 CHEMBL424450 BMS-354825 tert-Butoxycarbamate Analog 5f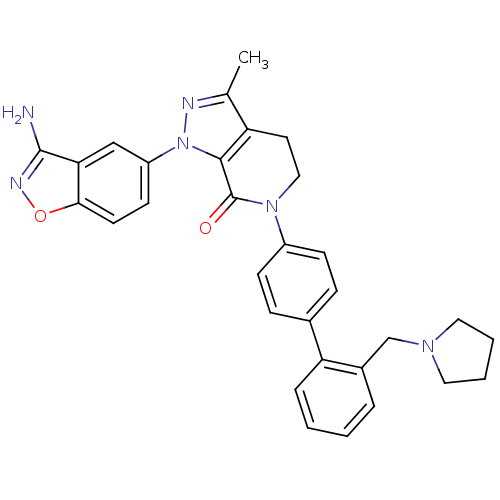 BDBM12702 1-(3-amino-1,2-benzoxazol-5-yl)-3-methyl-6-{4-[2-(pyrrolidin-1-ylmethyl)phenyl]phenyl}-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 12b
BDBM12702 1-(3-amino-1,2-benzoxazol-5-yl)-3-methyl-6-{4-[2-(pyrrolidin-1-ylmethyl)phenyl]phenyl}-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 12b BMS-740808 Analogue 6e 1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(pyrrolidin-1-ylmethyl)phenyl]phenyl}-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12692
BMS-740808 Analogue 6e 1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(pyrrolidin-1-ylmethyl)phenyl]phenyl}-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12692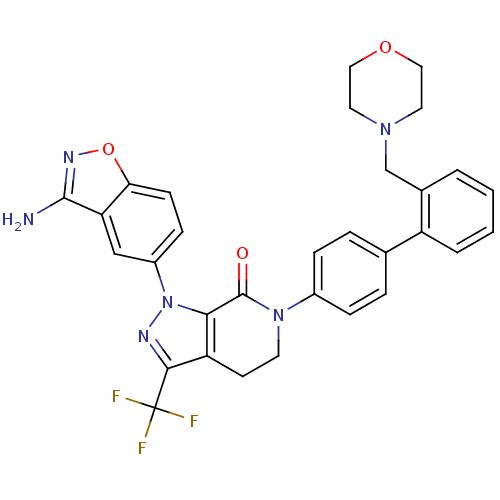 BMS-740808 Analogue 6i BDBM12696 1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(morpholin-4-ylmethyl)phenyl]phenyl}-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one
BMS-740808 Analogue 6i BDBM12696 1-(3-amino-1,2-benzoxazol-5-yl)-6-{4-[2-(morpholin-4-ylmethyl)phenyl]phenyl}-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one N-(2-Chloro-6-methylphenyl)-2-[(ethylcarbonyl)amino]-1,3-thiazole-5-carboxamide N-(2-chloro-6-methylphenyl)-2-propanamido-1,3-thiazole-5-carboxamide BDBM13247 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7o
N-(2-Chloro-6-methylphenyl)-2-[(ethylcarbonyl)amino]-1,3-thiazole-5-carboxamide N-(2-chloro-6-methylphenyl)-2-propanamido-1,3-thiazole-5-carboxamide BDBM13247 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7o N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]cyclohexanecarboxamide BDBM5927 BMS-387032 analog 17 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)cyclohexanecarboxamide
N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]cyclohexanecarboxamide BDBM5927 BMS-387032 analog 17 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)cyclohexanecarboxamide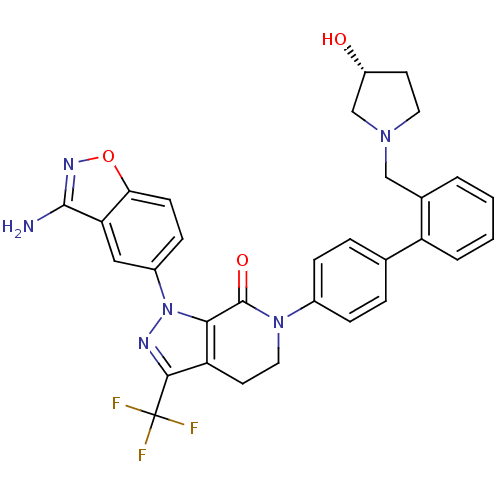 1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12693 BMS-740808
1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12693 BMS-740808 2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethoxy]-quinoline-4-carboxylic acid BMS-183920 BDBM50049201 CHEMBL305365 2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy]-quinoline-4-carboxylic acid
2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethoxy]-quinoline-4-carboxylic acid BMS-183920 BDBM50049201 CHEMBL305365 2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy]-quinoline-4-carboxylic acid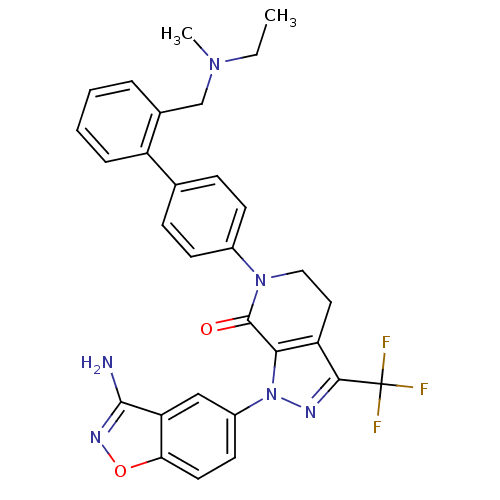 BDBM12690 1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[ethyl(methyl)amino]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 6c
BDBM12690 1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[ethyl(methyl)amino]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 6c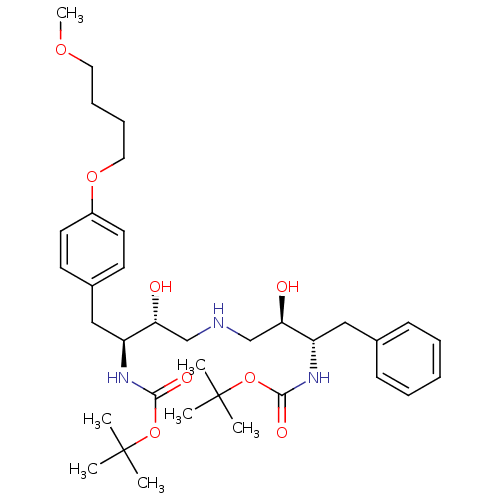 BMS-186318 analog 28 BDBM691 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(4-methoxybutoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
BMS-186318 analog 28 BDBM691 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(4-methoxybutoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-387032 analog 16 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-2-cyclohexylacetamide N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]cyclohexylmethanecarboxamide BDBM5926
BMS-387032 analog 16 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-2-cyclohexylacetamide N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]cyclohexylmethanecarboxamide BDBM5926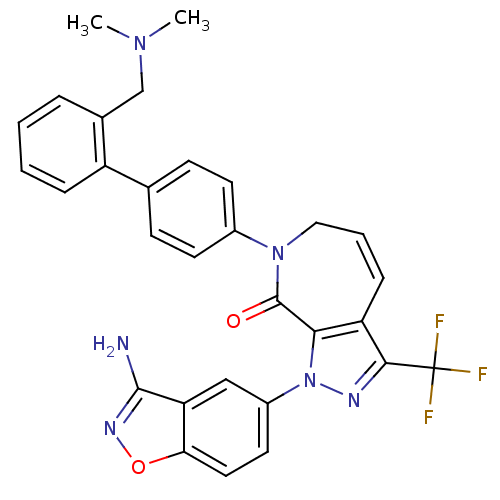 BMS-740808 Analogue 17a 1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,6H,7H,8H-pyrazolo[3,4-c]azepin-8-one BDBM12685 bicyclic pyrazole scaffold
BMS-740808 Analogue 17a 1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,6H,7H,8H-pyrazolo[3,4-c]azepin-8-one BDBM12685 bicyclic pyrazole scaffold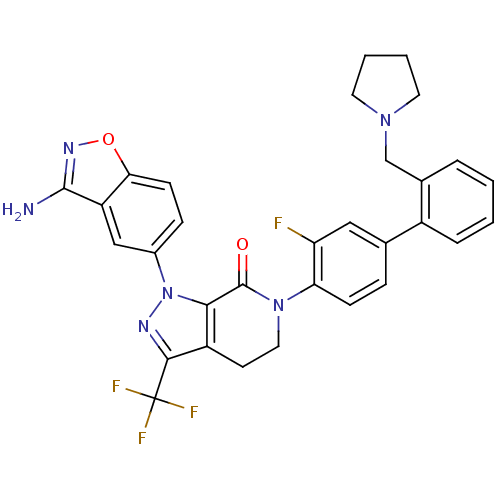 1-(3-amino-1,2-benzoxazol-5-yl)-6-{2-fluoro-4-[2-(pyrrolidin-1-ylmethyl)phenyl]phenyl}-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12699 BMS-740808 Analogue 7b
1-(3-amino-1,2-benzoxazol-5-yl)-6-{2-fluoro-4-[2-(pyrrolidin-1-ylmethyl)phenyl]phenyl}-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12699 BMS-740808 Analogue 7b 2-(3-furoylamino)-N-mesityl-4-methyl-1,3-thiazole-5-carboxamide 2-N-(furan-3-)-4-methyl-5-N-(2,4,6-trimethylphenyl)-1,3-thiazole-2,5-diamido BMS-354825 2-Amino-4-methyl-thiazole Analog 7g BDBM13233
2-(3-furoylamino)-N-mesityl-4-methyl-1,3-thiazole-5-carboxamide 2-N-(furan-3-)-4-methyl-5-N-(2,4,6-trimethylphenyl)-1,3-thiazole-2,5-diamido BMS-354825 2-Amino-4-methyl-thiazole Analog 7g BDBM13233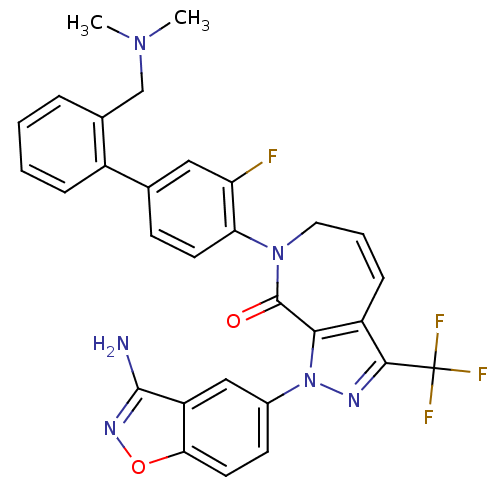 BDBM12686 BMS-740808 Analogue 17b bicyclic pyrazole scaffold 1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethylamino)methyl]phenyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H,6H,7H,8H-pyrazolo[3,4-c]azepin-8-one
BDBM12686 BMS-740808 Analogue 17b bicyclic pyrazole scaffold 1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethylamino)methyl]phenyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H,6H,7H,8H-pyrazolo[3,4-c]azepin-8-one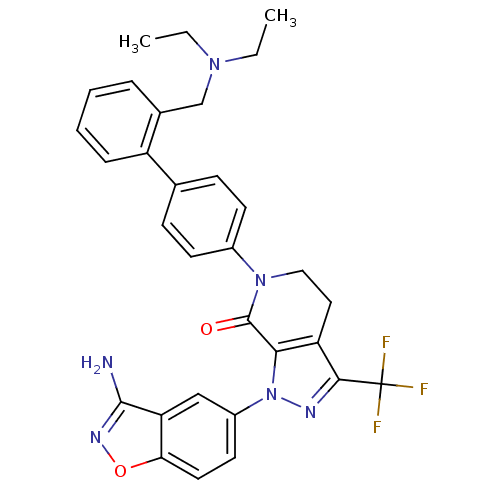 BDBM12689 bicyclic pyrazole scaffold 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(diethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 6b
BDBM12689 bicyclic pyrazole scaffold 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(diethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 6b BDBM13229 tert-butyl N-{5-[(2,4-dimethylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate BMS-354825 tert-Butoxycarbamate Analog 5j 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,4-dimethylphenyl)-1,3-thiazole-5-carboxamide
BDBM13229 tert-butyl N-{5-[(2,4-dimethylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate BMS-354825 tert-Butoxycarbamate Analog 5j 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,4-dimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13258 N-(2-chloro-6-methylphenyl)-2-(pyridin-4-ylamino)-1,3-thiazole-5-carboxamide N-(2-Chloro-6-methylphenyl)-2-[(4-pyridinyl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12c CHEMBL360810
BDBM13258 N-(2-chloro-6-methylphenyl)-2-(pyridin-4-ylamino)-1,3-thiazole-5-carboxamide N-(2-Chloro-6-methylphenyl)-2-[(4-pyridinyl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12c CHEMBL360810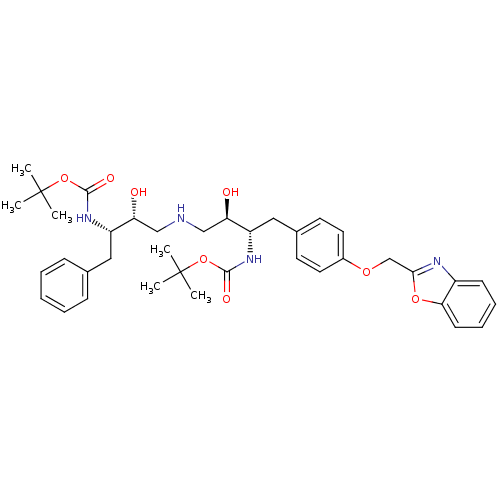 BMS-186318 analog 27 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-4-[4-(1,3-benzoxazol-2-ylmethoxy)phenyl]-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM690
BMS-186318 analog 27 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-4-[4-(1,3-benzoxazol-2-ylmethoxy)phenyl]-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM690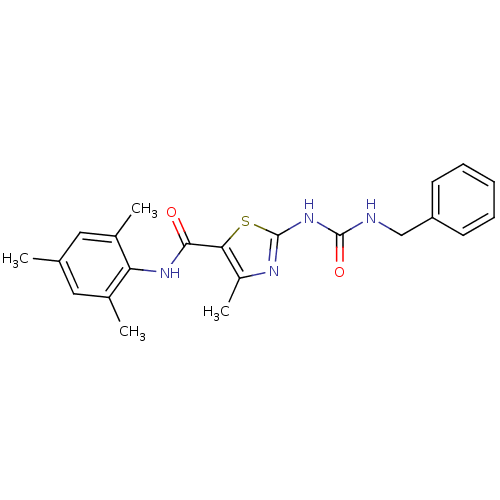 BMS-354825 2-Amino-4-methyl-thiazole Analog 8e 2-[[(Benzylamino)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide 2-[(benzylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13244
BMS-354825 2-Amino-4-methyl-thiazole Analog 8e 2-[[(Benzylamino)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide 2-[(benzylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13244 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7v N-(2-Chloro-6-methylphenyl)-2-[(cyclobutylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-cyclobutane-1,3-thiazole-2,5-diamido BDBM13254
BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7v N-(2-Chloro-6-methylphenyl)-2-[(cyclobutylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-cyclobutane-1,3-thiazole-2,5-diamido BDBM13254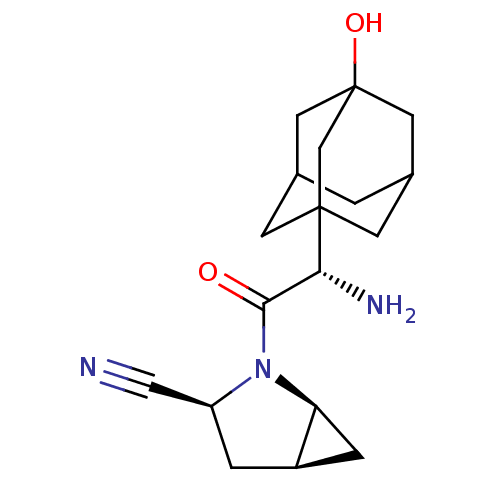 BMS-477118 (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BDBM11542 (S)-3-Hydroxyadamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt Saxagliptin
BMS-477118 (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BDBM11542 (S)-3-Hydroxyadamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt Saxagliptin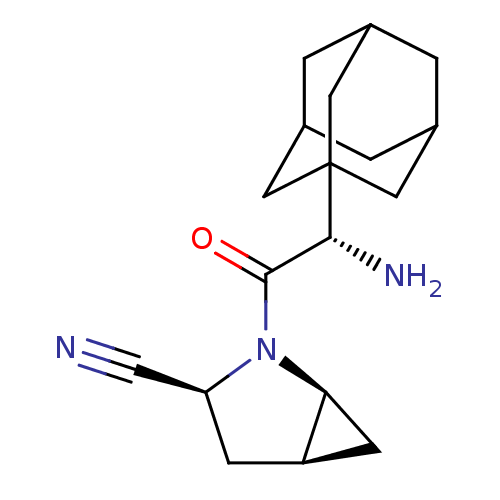 BMS-477118 analogue Saxagliptin Analogue 23 (S)-Adamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt (1S,3S,5S)-2-[(2S)-2-(adamantan-1-yl)-2-aminoacetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BDBM11541
BMS-477118 analogue Saxagliptin Analogue 23 (S)-Adamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt (1S,3S,5S)-2-[(2S)-2-(adamantan-1-yl)-2-aminoacetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BDBM11541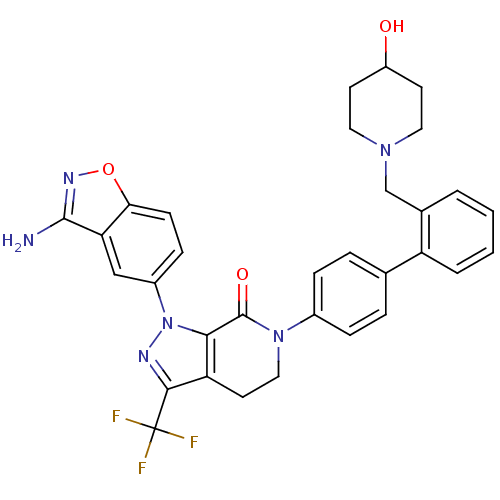 BMS-740808 Analogue 6h 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-hydroxypiperidin-1-yl)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12695
BMS-740808 Analogue 6h 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-hydroxypiperidin-1-yl)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12695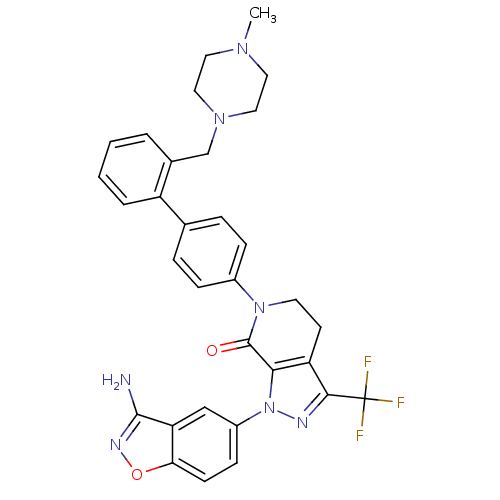 BMS-740808 Analogue 6k 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-methylpiperazin-1-yl)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12698
BMS-740808 Analogue 6k 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(4-methylpiperazin-1-yl)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12698 N-(2-Chloro-6-methylphenyl)-2-[(3-pyridinyl)amino]-1,3-thiazole-5-carboxamide BDBM13257 CHEMBL189675 N-(2-chloro-6-methylphenyl)-2-(pyridin-3-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12b
N-(2-Chloro-6-methylphenyl)-2-[(3-pyridinyl)amino]-1,3-thiazole-5-carboxamide BDBM13257 CHEMBL189675 N-(2-chloro-6-methylphenyl)-2-(pyridin-3-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12b N-(2-Chloro-6-methylphenyl)-2-[(cyclopentylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-cyclopentane-1,3-thiazole-2,5-diamido BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7w BDBM13255
N-(2-Chloro-6-methylphenyl)-2-[(cyclopentylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-cyclopentane-1,3-thiazole-2,5-diamido BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7w BDBM13255 N-[5-((R)-2-{[Bis-(4-difluoromethoxy-phenyl)-methyl]-amino}-1-hydroxy-ethyl)-2-hydroxy-phenyl]-methanesulfonamide (R)-N-(5-(2-(bis(4-(difluoromethoxy)phenyl)methylamino)-1-hydroxyethyl)-2-hydroxyphenyl)methanesulfonamide CHEMBL320915 BMS-194449 BDBM50106794
N-[5-((R)-2-{[Bis-(4-difluoromethoxy-phenyl)-methyl]-amino}-1-hydroxy-ethyl)-2-hydroxy-phenyl]-methanesulfonamide (R)-N-(5-(2-(bis(4-(difluoromethoxy)phenyl)methylamino)-1-hydroxyethyl)-2-hydroxyphenyl)methanesulfonamide CHEMBL320915 BMS-194449 BDBM50106794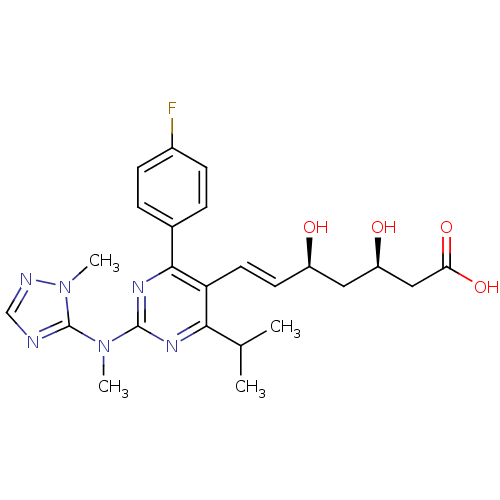 sodium (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1-methyl-1H-1,2,4-triazol-5-yl)amino]-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate BMS-644950 triazole compound, 1b BDBM22163
sodium (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1-methyl-1H-1,2,4-triazol-5-yl)amino]-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate BMS-644950 triazole compound, 1b BDBM22163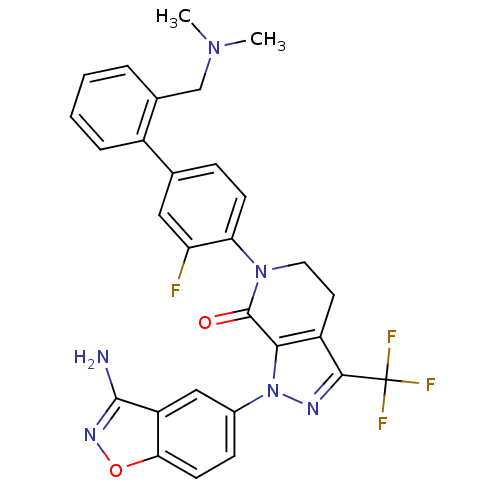 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethylamino)methyl]phenyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one bicyclic pyrazole scaffold BMS-740808 Analogue 7a BDBM12682
1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethylamino)methyl]phenyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one bicyclic pyrazole scaffold BMS-740808 Analogue 7a BDBM12682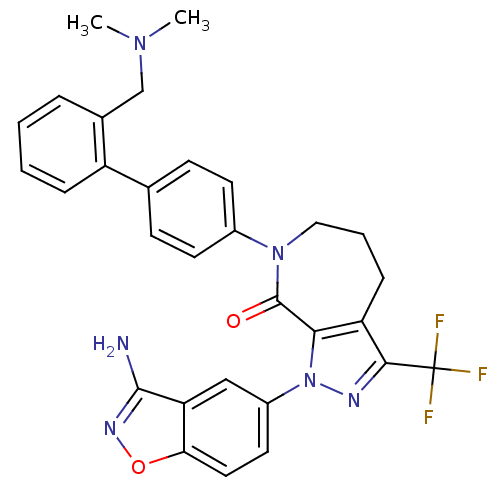 1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H,8H-pyrazolo[3,4-c]azepin-8-one BMS-740808 Analogue 16a BDBM12683 bicyclic pyrazole scaffolds
1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H,8H-pyrazolo[3,4-c]azepin-8-one BMS-740808 Analogue 16a BDBM12683 bicyclic pyrazole scaffolds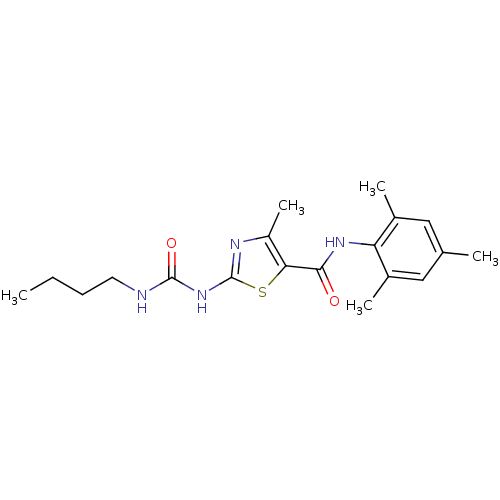 2-[[(Butylamino)-carbonyl]amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide CHEMBL131346 BMS-354825 2-Amino-4-methyl-thiazole Analog 8c 2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13242
2-[[(Butylamino)-carbonyl]amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide CHEMBL131346 BMS-354825 2-Amino-4-methyl-thiazole Analog 8c 2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13242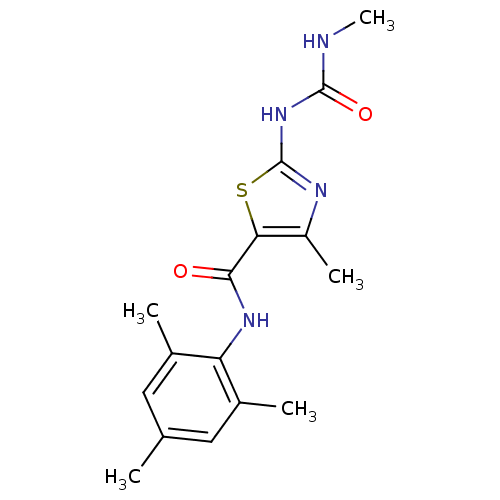 BDBM13240 4-methyl-2-[(methylcarbamoyl)amino]-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide 2-[[(Methylamino)-carbonyl]amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 8a CHEMBL338159
BDBM13240 4-methyl-2-[(methylcarbamoyl)amino]-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide 2-[[(Methylamino)-carbonyl]amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 8a CHEMBL338159 BDBM13250 2-N-benzene-5-N-(2-chloro-6-methylphenyl)-1,3-thiazole-2,5-diamido BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7r N-(2-Chloro-6-methylphenyl)-2-[(phenylcarbonyl)amino]-1,3-thiazole-5-carboxamide CHEMBL131393
BDBM13250 2-N-benzene-5-N-(2-chloro-6-methylphenyl)-1,3-thiazole-2,5-diamido BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7r N-(2-Chloro-6-methylphenyl)-2-[(phenylcarbonyl)amino]-1,3-thiazole-5-carboxamide CHEMBL131393 BMS-354825 2-Amino-4-methyl-thiazole Analog 7e 2-[[(tert-Butyl)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide 2-(2,2-dimethylpropanamido)-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13231
BMS-354825 2-Amino-4-methyl-thiazole Analog 7e 2-[[(tert-Butyl)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide 2-(2,2-dimethylpropanamido)-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13231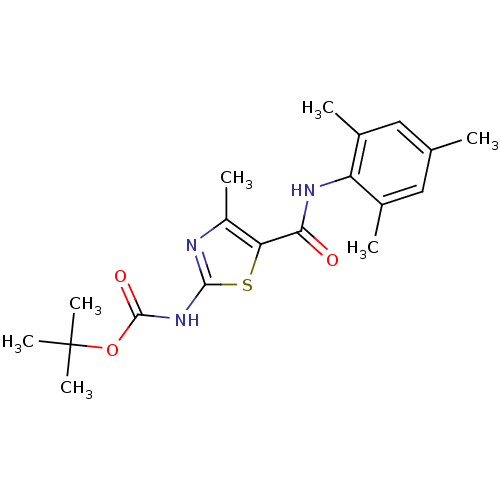 BMS-354825 tert-Butoxycarbamate Analog 5a tert-butyl N-{4-methyl-5-[(2,4,6-trimethylphenyl)carbamoyl]-1,3-thiazol-2-yl}carbamate 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13217 CHEMBL66109
BMS-354825 tert-Butoxycarbamate Analog 5a tert-butyl N-{4-methyl-5-[(2,4,6-trimethylphenyl)carbamoyl]-1,3-thiazol-2-yl}carbamate 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13217 CHEMBL66109 BMS-354825 tert-Butoxycarbamate Analog 5c CHEMBL129454 BDBM13219 tert-butyl N-{5-[(2,6-dimethylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,6-dimethylphenyl)-1,3-thiazole-5-carboxamide
BMS-354825 tert-Butoxycarbamate Analog 5c CHEMBL129454 BDBM13219 tert-butyl N-{5-[(2,6-dimethylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,6-dimethylphenyl)-1,3-thiazole-5-carboxamide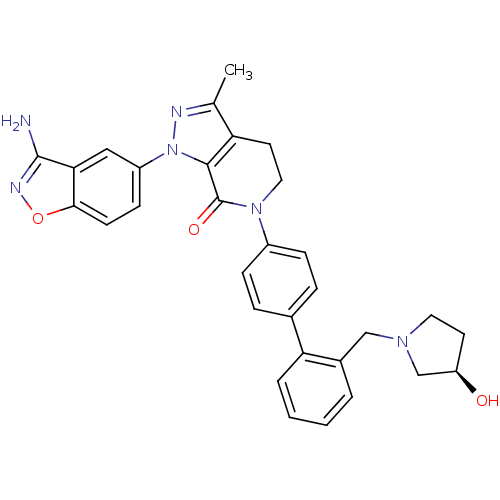 BMS-740808 Analogue 12c BDBM12703 1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-methyl-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one
BMS-740808 Analogue 12c BDBM12703 1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-methyl-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one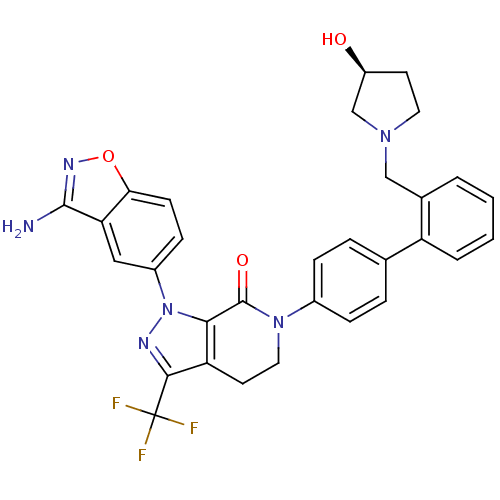 BMS-740808 Analogue 6g 1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3S)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12694
BMS-740808 Analogue 6g 1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3S)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BDBM12694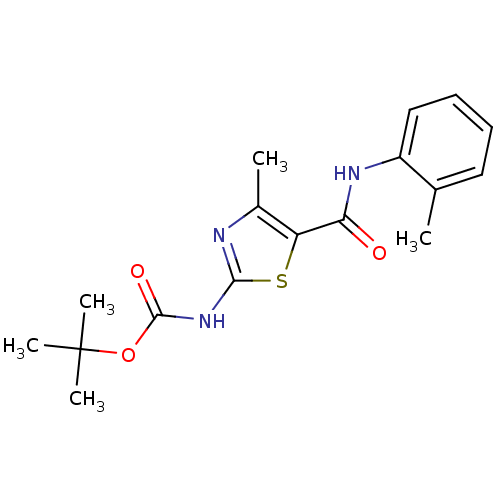 CHEMBL128568 tert-butyl N-{4-methyl-5-[(2-methylphenyl)carbamoyl]-1,3-thiazol-2-yl}carbamate BDBM13226 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-methylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 tert-Butoxycarbamate Analog 5g
CHEMBL128568 tert-butyl N-{4-methyl-5-[(2-methylphenyl)carbamoyl]-1,3-thiazol-2-yl}carbamate BDBM13226 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-methylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 tert-Butoxycarbamate Analog 5g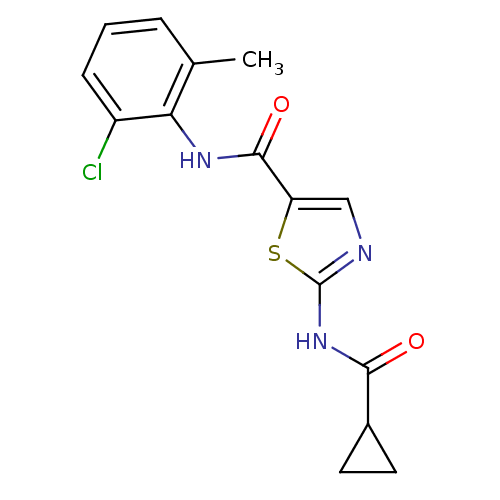 CHEMBL131577 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7n BDBM13246 N-(2-chloro-6-methylphenyl)-2-[(cyclopropylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3-thiazole-2,5-diamido
CHEMBL131577 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7n BDBM13246 N-(2-chloro-6-methylphenyl)-2-[(cyclopropylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3-thiazole-2,5-diamido CHEMBL131661 2-N-(furan-2-)-4-methyl-5-N-(2,4,6-trimethylphenyl)-1,3-thiazole-2,5-diamido BMS-354825 2-Amino-4-methyl-thiazole Analog 7f 2-(2-furoylamino)-N-mesityl-4-methyl-1,3-thiazole-5-carboxamide BDBM13232
CHEMBL131661 2-N-(furan-2-)-4-methyl-5-N-(2,4,6-trimethylphenyl)-1,3-thiazole-2,5-diamido BMS-354825 2-Amino-4-methyl-thiazole Analog 7f 2-(2-furoylamino)-N-mesityl-4-methyl-1,3-thiazole-5-carboxamide BDBM13232 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)pyrrolidine-2-carboxamide (+/-)-N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-2-pyrrolidinecarboxamide BDBM5928 BMS-387032 analog 18
N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)pyrrolidine-2-carboxamide (+/-)-N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-2-pyrrolidinecarboxamide BDBM5928 BMS-387032 analog 18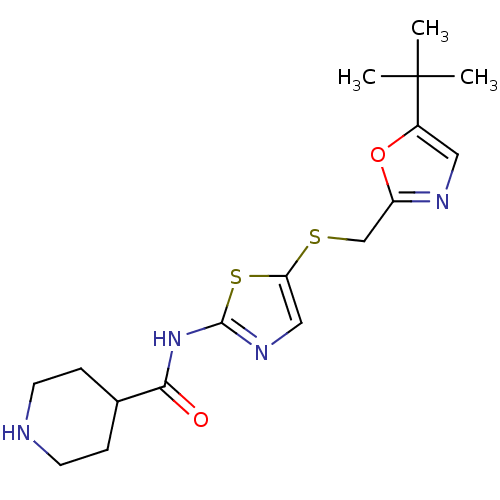 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide BDBM5931 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)piperidine-4-carboxamide CHEMBL296468 BMS-387072 cid_3025986
N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide BDBM5931 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)piperidine-4-carboxamide CHEMBL296468 BMS-387072 cid_3025986 benzyl N-{4-methyl-5-[(2,4,6-trimethylphenyl)carbamoyl]-1,3-thiazol-2-yl}carbamate BMS-354825 2-Amino-4-methyl-thiazole Analog 7b 2-[[(Benzyloxy)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13230
benzyl N-{4-methyl-5-[(2,4,6-trimethylphenyl)carbamoyl]-1,3-thiazol-2-yl}carbamate BMS-354825 2-Amino-4-methyl-thiazole Analog 7b 2-[[(Benzyloxy)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13230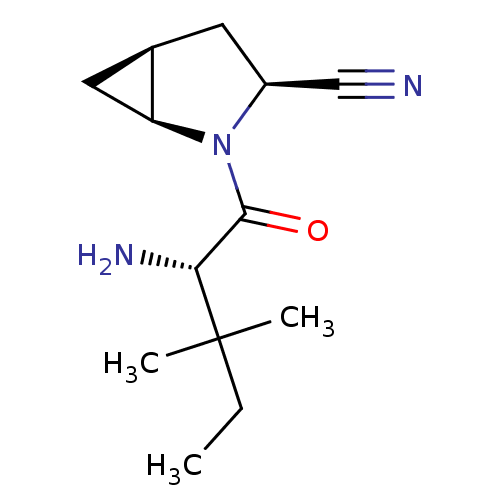 (S)-2-(1,1-Dimethylprop-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt BMS-477118 BDBM11533 (1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpentanoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid Saxagliptin Analogue 10a
(S)-2-(1,1-Dimethylprop-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt BMS-477118 BDBM11533 (1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpentanoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid Saxagliptin Analogue 10a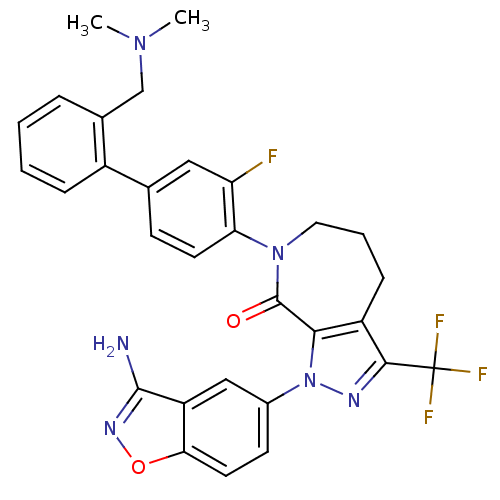 1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethylamino)methyl]phenyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H,8H-pyrazolo[3,4-c]azepin-8-one BMS-740808 Analogue 16b BDBM12684 bicyclic pyrazole scaffold
1-(3-amino-1,2-benzoxazol-5-yl)-7-(4-{2-[(dimethylamino)methyl]phenyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H,8H-pyrazolo[3,4-c]azepin-8-one BMS-740808 Analogue 16b BDBM12684 bicyclic pyrazole scaffold 2-[(tert-butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 8d 2-[[(tert-Butylamino)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13243
2-[(tert-butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 8d 2-[[(tert-Butylamino)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13243 BDBM13251 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7s 5-N-(2-chloro-6-methylphenyl)-2-N-(thiophene-2-)-1,3-thiazole-2,5-diamido N-(2-Chloro-6-methylphenyl)-2-[(2-thienylcarbonyl)amino]-1,3-thiazole-5-carboxamide
BDBM13251 BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7s 5-N-(2-chloro-6-methylphenyl)-2-N-(thiophene-2-)-1,3-thiazole-2,5-diamido N-(2-Chloro-6-methylphenyl)-2-[(2-thienylcarbonyl)amino]-1,3-thiazole-5-carboxamide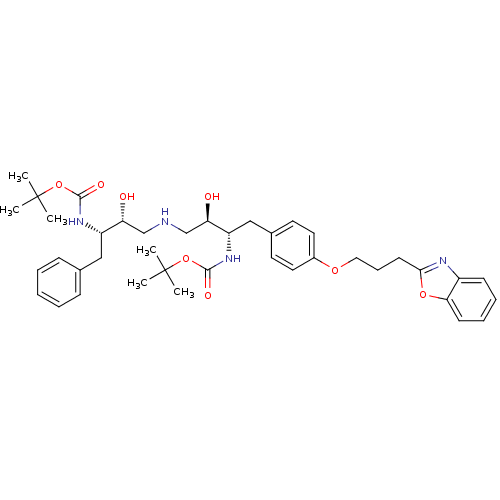 BDBM689 BMS-186318 analog 26 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-4-{4-[3-(1,3-benzoxazol-2-yl)propoxy]phenyl}-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
BDBM689 BMS-186318 analog 26 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-4-{4-[3-(1,3-benzoxazol-2-yl)propoxy]phenyl}-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate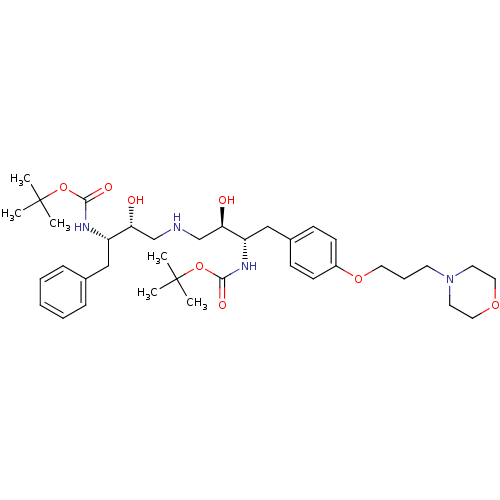 BMS-186318 analog 29 BDBM692 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[3-(morpholin-4-yl)propoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
BMS-186318 analog 29 BDBM692 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[3-(morpholin-4-yl)propoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate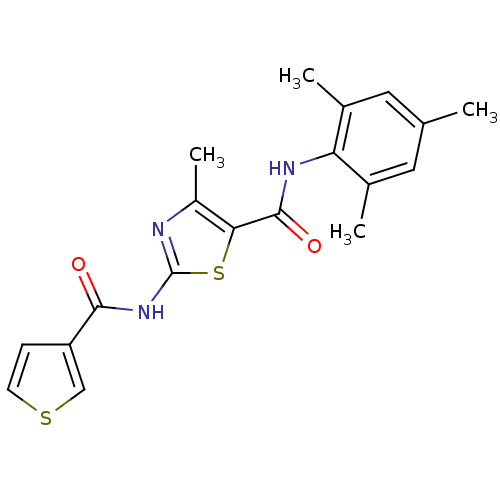 BMS-354825 2-Amino-4-methyl-thiazole Analog 7h 4-methyl-2-N-(thiophene-3-)-5-N-(2,4,6-trimethylphenyl)-1,3-thiazole-2,5-diamido BDBM13234 N-mesityl-4-methyl-2-[(thien-3-ylcarbonyl)amino]-1,3-thiazole-5-carboxamide
BMS-354825 2-Amino-4-methyl-thiazole Analog 7h 4-methyl-2-N-(thiophene-3-)-5-N-(2,4,6-trimethylphenyl)-1,3-thiazole-2,5-diamido BDBM13234 N-mesityl-4-methyl-2-[(thien-3-ylcarbonyl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7t N-(2-Chloro-6-methylphenyl)-2-[(3-thienylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-(thiophene-3-)-1,3-thiazole-2,5-diamido BDBM13252
BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7t N-(2-Chloro-6-methylphenyl)-2-[(3-thienylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-(thiophene-3-)-1,3-thiazole-2,5-diamido BDBM13252 BMS-387032 analog 13 3-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)propanamide BDBM5923 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-2-aminoethanecarboxamide Hydrochloride
BMS-387032 analog 13 3-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)propanamide BDBM5923 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-2-aminoethanecarboxamide Hydrochloride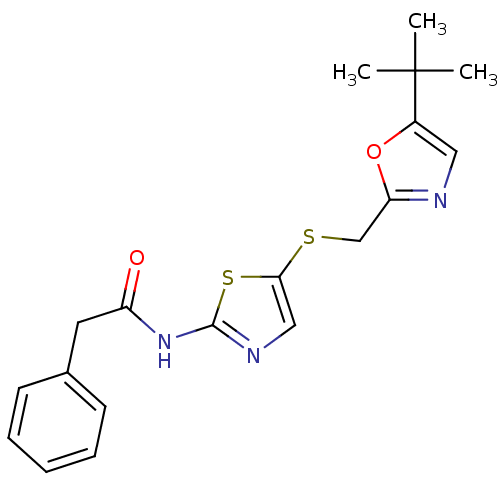 BMS-387032 analog 14 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-2-phenylacetamide BDBM5924 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]phenylmethanecarboxamide Trifluoroacetic Acid Salt
BMS-387032 analog 14 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-2-phenylacetamide BDBM5924 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]phenylmethanecarboxamide Trifluoroacetic Acid Salt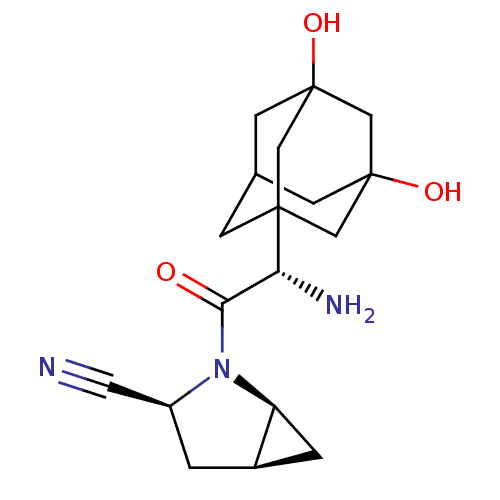 BMS-477118 (S)-3,5-Dihydroxyadamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt Saxagliptin analogue 28 (1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BDBM11543
BMS-477118 (S)-3,5-Dihydroxyadamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt Saxagliptin analogue 28 (1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BDBM11543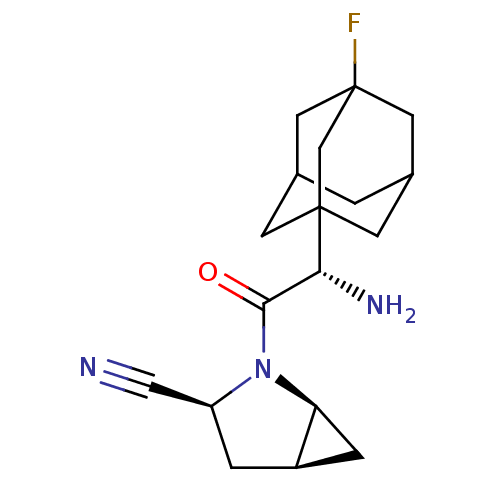 BMS-477118 BDBM11544 (S)-3-Fluoroadamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt Saxagliptin analogue 30 (1S,3S,5S)-2-[(2S)-2-amino-2-(3-fluoroadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid
BMS-477118 BDBM11544 (S)-3-Fluoroadamantylglycine-L-cis-4,5-methanoprolinenitrile TFA salt Saxagliptin analogue 30 (1S,3S,5S)-2-[(2S)-2-amino-2-(3-fluoroadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid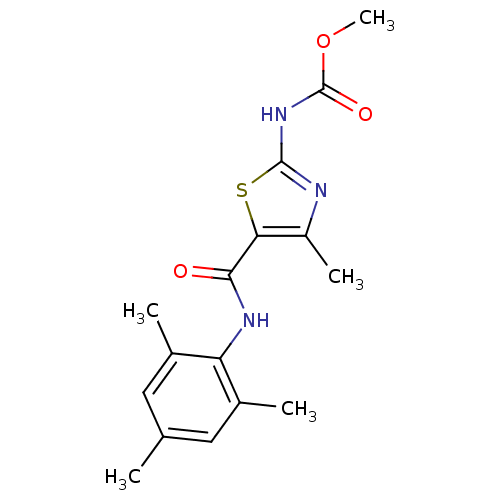 CHEMBL128589 BMS-354825 2-Amino-4-methyl-thiazole Analog 7a methyl N-{4-methyl-5-[(2,4,6-trimethylphenyl)carbamoyl]-1,3-thiazol-2-yl}carbamate 2-[[(Methoxy)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13222
CHEMBL128589 BMS-354825 2-Amino-4-methyl-thiazole Analog 7a methyl N-{4-methyl-5-[(2,4,6-trimethylphenyl)carbamoyl]-1,3-thiazol-2-yl}carbamate 2-[[(Methoxy)carbonyl]-amino]-4-methyl-N-(2,4,6-trimethylphenyl)-1,3-thiazole-5-carboxamide BDBM13222 CHEMBL297570 (+/-)-N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-3-piperidinecarboxamide BDBM5929 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)piperidine-3-carboxamide BMS-387032 analog 19
CHEMBL297570 (+/-)-N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-3-piperidinecarboxamide BDBM5929 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)piperidine-3-carboxamide BMS-387032 analog 19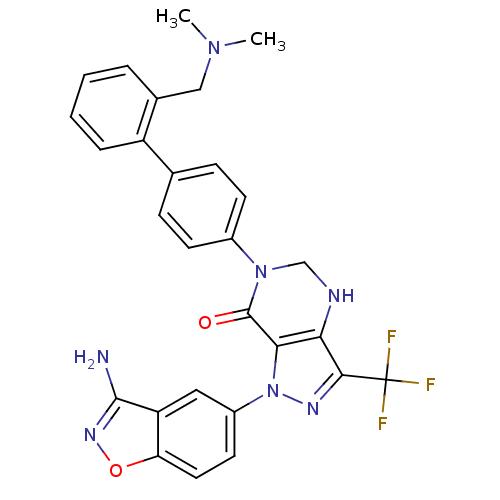 dihydropyrazolo[4,3-d]pyrimidinone 24a BDBM12687 BMS-740808 Analogue 18 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[4,3-d]pyrimidin-7-one
dihydropyrazolo[4,3-d]pyrimidinone 24a BDBM12687 BMS-740808 Analogue 18 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[4,3-d]pyrimidin-7-one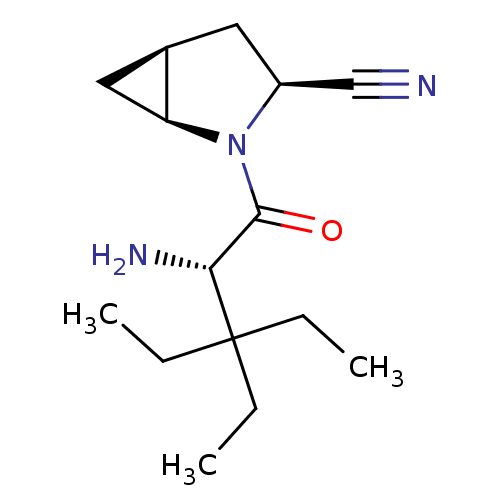 (1S,3S,5S)-2-[(2S)-2-amino-3,3-diethylpentanoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BMS-477118 analogue BDBM11534 Saxagliptin Analogue 10b (S)-2-(1,1-Diethylprop-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt
(1S,3S,5S)-2-[(2S)-2-amino-3,3-diethylpentanoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BMS-477118 analogue BDBM11534 Saxagliptin Analogue 10b (S)-2-(1,1-Diethylprop-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt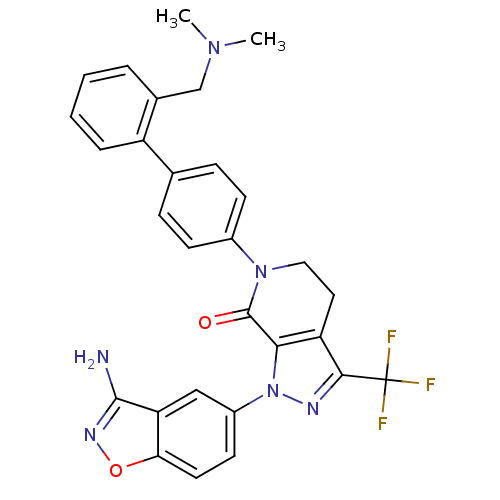 1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one dihydropyrazolopyridinone analogue 9 bicyclic pyrazole scaffold BMS-740808 Analogue 6a BDBM12681
1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one dihydropyrazolopyridinone analogue 9 bicyclic pyrazole scaffold BMS-740808 Analogue 6a BDBM12681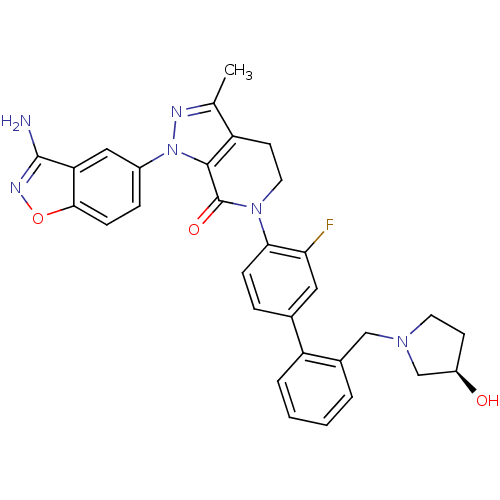 1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-methyl-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 12d BDBM12704
1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-methyl-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 12d BDBM12704 BDBM11529 Saxagliptin Analogue 8d (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclopentyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BMS-477118 analogue (S)-2-(1-Ethenylcyclopent-1-yl)glycine-L-cis-4,5-methanoprolinenitrile
BDBM11529 Saxagliptin Analogue 8d (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclopentyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BMS-477118 analogue (S)-2-(1-Ethenylcyclopent-1-yl)glycine-L-cis-4,5-methanoprolinenitrile BDBM12700 1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 7c
BDBM12700 1-(3-amino-1,2-benzoxazol-5-yl)-6-[2-fluoro-4-(2-{[(3R)-3-hydroxypyrrolidin-1-yl]methyl}phenyl)phenyl]-3-(trifluoromethyl)-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridin-7-one BMS-740808 Analogue 7c BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7u N-(2-Chloro-6-methylphenyl)-2-[(2-furanylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-(furan-2-)-1,3-thiazole-2,5-diamido CHEMBL128070 BDBM13253
BMS-354825 2-Carboxamido-4-hydrido-thiazole Analog 7u N-(2-Chloro-6-methylphenyl)-2-[(2-furanylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-2-N-(furan-2-)-1,3-thiazole-2,5-diamido CHEMBL128070 BDBM13253 N-(2-chloro-6-methylphenyl)-2-[(3,5-dimethylpyrazin-2-yl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12h N-(2-Chloro-6-methylphenyl)-2-[(3,5-dimethyl-2-pyrazinyl)-amino]-1,3-thiazole-5-carboxamide BDBM13263
N-(2-chloro-6-methylphenyl)-2-[(3,5-dimethylpyrazin-2-yl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12h N-(2-Chloro-6-methylphenyl)-2-[(3,5-dimethyl-2-pyrazinyl)-amino]-1,3-thiazole-5-carboxamide BDBM13263 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-methylpiperidinecarboxamide BDBM5930 BMS-387032 analog 20 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-2-(piperidin-4-yl)acetamide
N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-methylpiperidinecarboxamide BDBM5930 BMS-387032 analog 20 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-2-(piperidin-4-yl)acetamide hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BMS-186318 analog 23 BDBM686 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,10-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-[4-[[(4-morpholinyl-carbonyl)oxy]methyl]phenyl]butyl]amino]-2-
hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BMS-186318 analog 23 BDBM686 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,10-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-[4-[[(4-morpholinyl-carbonyl)oxy]methyl]phenyl]butyl]amino]-2- tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[3-(3-oxomorpholin-4-yl)propoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM693 BMS-186318 analog 30
tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[3-(3-oxomorpholin-4-yl)propoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM693 BMS-186318 analog 30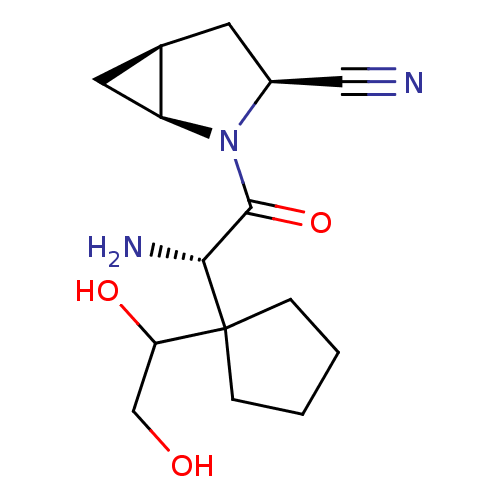 (1S,3S,5S)-2-[(2S)-2-amino-2-[1-(1,2-dihydroxyethyl)cyclopentyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile Saxagliptin Analogue 18d (2S)-2-[1-(1,2-Dihydroxyethyl)cyclopent-1-yl]glycine-L-cis-4,5-methanoprolinenitrile BMS-477118 analogue BDBM11540
(1S,3S,5S)-2-[(2S)-2-amino-2-[1-(1,2-dihydroxyethyl)cyclopentyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile Saxagliptin Analogue 18d (2S)-2-[1-(1,2-Dihydroxyethyl)cyclopent-1-yl]glycine-L-cis-4,5-methanoprolinenitrile BMS-477118 analogue BDBM11540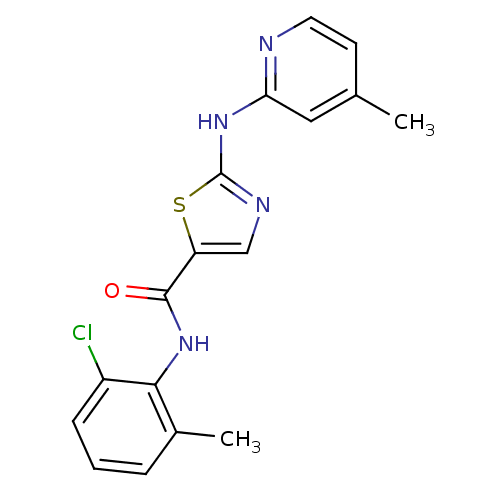 N-(2-Chloro-6-methylphenyl)-2-[(4-methyl-2-pyridinyl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12k CHEMBL186568 BDBM13266 N-(2-chloro-6-methylphenyl)-2-[(4-methylpyridin-2-yl)amino]-1,3-thiazole-5-carboxamide
N-(2-Chloro-6-methylphenyl)-2-[(4-methyl-2-pyridinyl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12k CHEMBL186568 BDBM13266 N-(2-chloro-6-methylphenyl)-2-[(4-methylpyridin-2-yl)amino]-1,3-thiazole-5-carboxamide N-(2-chloro-6-methylphenyl)-2-[(2,6-dimethylpyrimidin-4-yl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12m BDBM13268 CHEMBL364623 N-(2-Chloro-6-methylphenyl)-2-[(2,6-dimethyl-4-pyrimidinyl)-amino]-1,3-thiazole-5-carboxamide
N-(2-chloro-6-methylphenyl)-2-[(2,6-dimethylpyrimidin-4-yl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12m BDBM13268 CHEMBL364623 N-(2-Chloro-6-methylphenyl)-2-[(2,6-dimethyl-4-pyrimidinyl)-amino]-1,3-thiazole-5-carboxamide N-(2-chloro-6-methylphenyl)-2-[(4,6-dimethylpyridin-2-yl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12l N-(2-Chloro-6-methylphenyl)-2-[(4,6-dimethyl-2-pyridinyl)-amino]-1,3-thiazole-5-carboxamide BDBM13267 CHEMBL188478
N-(2-chloro-6-methylphenyl)-2-[(4,6-dimethylpyridin-2-yl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12l N-(2-Chloro-6-methylphenyl)-2-[(4,6-dimethyl-2-pyridinyl)-amino]-1,3-thiazole-5-carboxamide BDBM13267 CHEMBL188478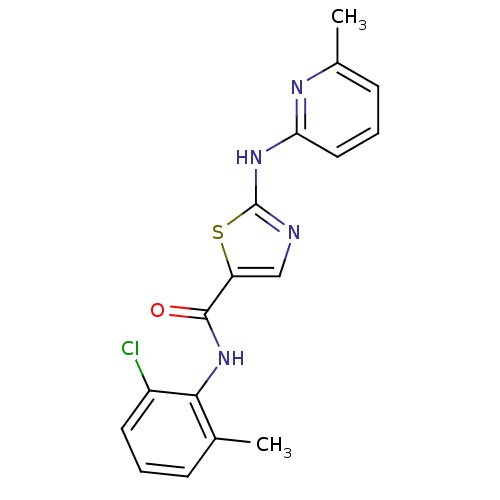 N-(2-chloro-6-methylphenyl)-2-[(6-methylpyridin-2-yl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12j N-(2-Chloro-6-methylphenyl)-2-[(6-methyl-2-pyridinyl)amino]-1,3-thiazole-5-carboxamide BDBM13265 CHEMBL365505
N-(2-chloro-6-methylphenyl)-2-[(6-methylpyridin-2-yl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12j N-(2-Chloro-6-methylphenyl)-2-[(6-methyl-2-pyridinyl)amino]-1,3-thiazole-5-carboxamide BDBM13265 CHEMBL365505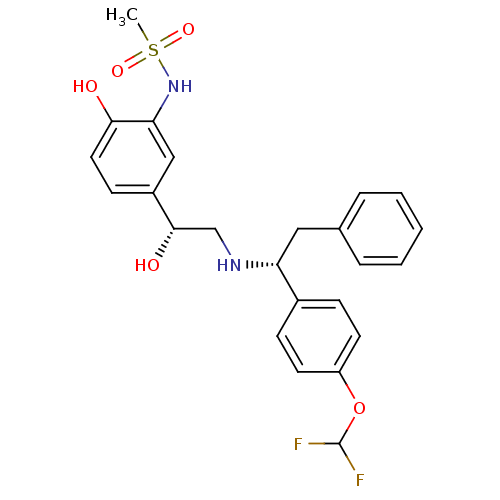 N-(5-{(R)-2-[(R)-1-(4-Difluoromethoxy-phenyl)-2-phenyl-ethylamino]-1-hydroxy-ethyl}-2-hydroxy-phenyl)-methanesulfonamide BDBM50106829 BMS-196085 CHEMBL322862 N-(5-((R)-2-((R)-1-(4-(difluoromethoxy)phenyl)-2-phenylethylamino)-1-hydroxyethyl)-2-hydroxyphenyl)methanesulfonamide
N-(5-{(R)-2-[(R)-1-(4-Difluoromethoxy-phenyl)-2-phenyl-ethylamino]-1-hydroxy-ethyl}-2-hydroxy-phenyl)-methanesulfonamide BDBM50106829 BMS-196085 CHEMBL322862 N-(5-((R)-2-((R)-1-(4-(difluoromethoxy)phenyl)-2-phenylethylamino)-1-hydroxyethyl)-2-hydroxyphenyl)methanesulfonamide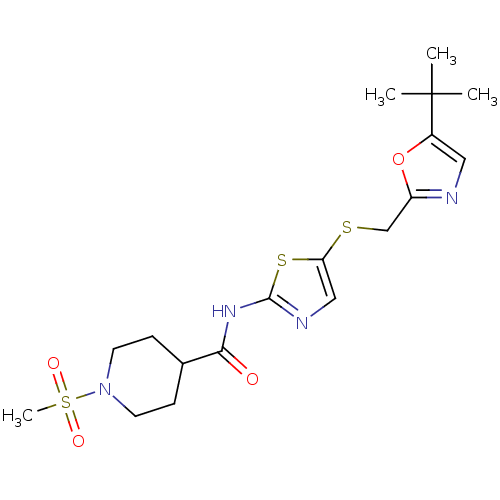 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-1-methanesulfonylpiperidine-4-carboxamide BMS-387032 analog 24 BDBM5934 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-(N-methylsulfonyl)-4-piperidinecarboxamide
N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-1-methanesulfonylpiperidine-4-carboxamide BMS-387032 analog 24 BDBM5934 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-(N-methylsulfonyl)-4-piperidinecarboxamide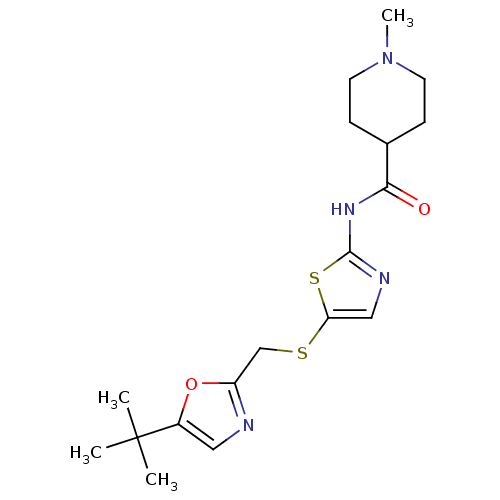 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-1-methylpiperidine-4-carboxamide BMS-387032 analog 22 BDBM5932 1-Methyl-N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide
N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-1-methylpiperidine-4-carboxamide BMS-387032 analog 22 BDBM5932 1-Methyl-N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethylcyclopentyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BMS-477118 analogue BDBM11535 Saxagliptin Analogue 10d (S)-2-(1-Ethylcyclopent-1-yl)-l-glycine-L-cis-4,5-methanoprolinenitrile TFA salt
(1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethylcyclopentyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BMS-477118 analogue BDBM11535 Saxagliptin Analogue 10d (S)-2-(1-Ethylcyclopent-1-yl)-l-glycine-L-cis-4,5-methanoprolinenitrile TFA salt 2-[[(n-Butylamino)carbonyl]-amino]-4-methyl-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide 2-[(butylcarbamoyl)amino]-N-(2-chloro-6-methylphenyl)-4-methyl-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 8f BDBM13245 CHEMBL130160
2-[[(n-Butylamino)carbonyl]-amino]-4-methyl-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide 2-[(butylcarbamoyl)amino]-N-(2-chloro-6-methylphenyl)-4-methyl-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 8f BDBM13245 CHEMBL130160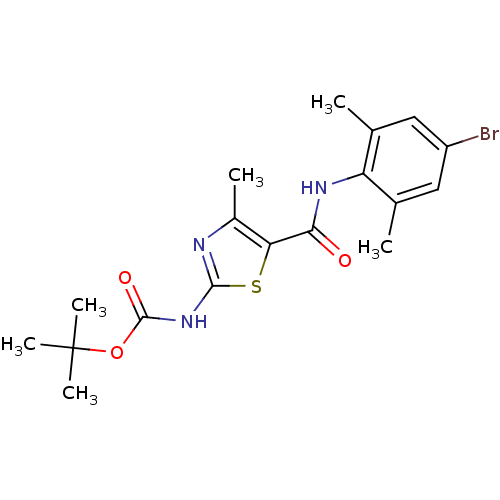 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(4-bromo-2,6-dimethylphenyl)-1,3-thiazole-5-carboxamide CHEMBL340322 tert-butyl N-{5-[(4-bromo-2,6-dimethylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate BMS-354825 tert-Butoxycarbamate Analog 5d BDBM13220
2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(4-bromo-2,6-dimethylphenyl)-1,3-thiazole-5-carboxamide CHEMBL340322 tert-butyl N-{5-[(4-bromo-2,6-dimethylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate BMS-354825 tert-Butoxycarbamate Analog 5d BDBM13220 5-N-(2-chloro-6-methylphenyl)-2-N-(furan-2-)-4-methyl-1,3-thiazole-2,5-diamido BMS-354825 2-Amino-4-methyl-thiazole Analog 7k CHEMBL127500 N-(2-chloro-6-methylphenyl)-2-(2-furoylamino)-4-methyl-1,3-thiazole-5-carboxamide BDBM13237
5-N-(2-chloro-6-methylphenyl)-2-N-(furan-2-)-4-methyl-1,3-thiazole-2,5-diamido BMS-354825 2-Amino-4-methyl-thiazole Analog 7k CHEMBL127500 N-(2-chloro-6-methylphenyl)-2-(2-furoylamino)-4-methyl-1,3-thiazole-5-carboxamide BDBM13237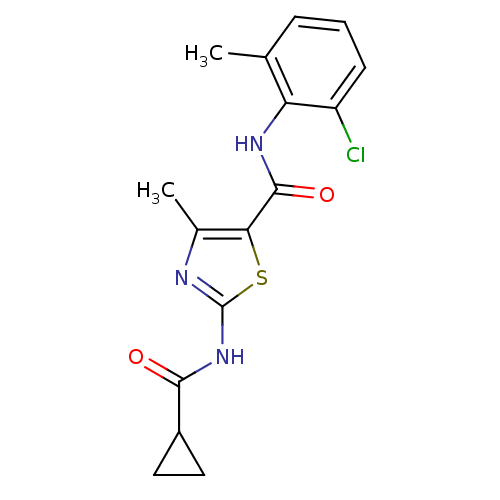 5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-4-methyl-1,3-thiazole-2,5-diamido CHEMBL128377 2-[[(Cyclopropyl)carbonyl]-amino]-4-methyl-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide BDBM13239 BMS-354825 2-Amino-4-methyl-thiazole Analog 7m
5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-4-methyl-1,3-thiazole-2,5-diamido CHEMBL128377 2-[[(Cyclopropyl)carbonyl]-amino]-4-methyl-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide BDBM13239 BMS-354825 2-Amino-4-methyl-thiazole Analog 7m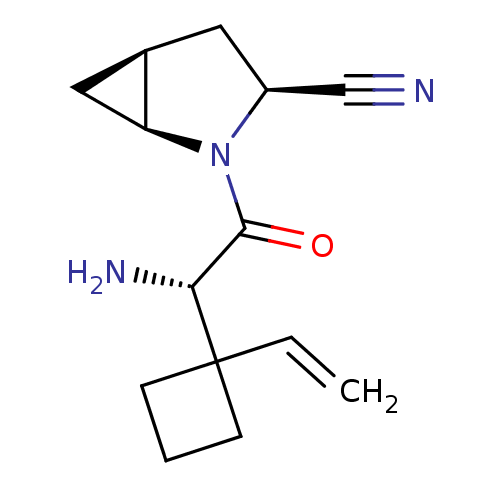 BDBM11528 Saxagliptin Analogue 8c BMS-477118 analogue (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclobutyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (S)-2-(1-Ethenylcyclobut-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt
BDBM11528 Saxagliptin Analogue 8c BMS-477118 analogue (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclobutyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (S)-2-(1-Ethenylcyclobut-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt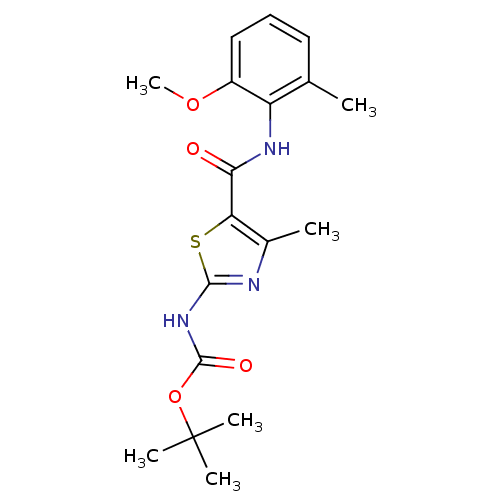 BDBM13228 CHEMBL132399 BMS-354825 tert-Butoxycarbamate Analog 5i tert-butyl N-{5-[(2-methoxy-6-methylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-methoxy-6-methylphenyl)-1,3-thiazole-5-carboxamide
BDBM13228 CHEMBL132399 BMS-354825 tert-Butoxycarbamate Analog 5i tert-butyl N-{5-[(2-methoxy-6-methylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-methoxy-6-methylphenyl)-1,3-thiazole-5-carboxamide BDBM13238 2-N-benzene-5-N-(2-chloro-6-methylphenyl)-4-methyl-1,3-thiazole-2,5-diamido 2-[(Benzoyl)-amino]-4-methyl-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 7l CHEMBL337985
BDBM13238 2-N-benzene-5-N-(2-chloro-6-methylphenyl)-4-methyl-1,3-thiazole-2,5-diamido 2-[(Benzoyl)-amino]-4-methyl-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 7l CHEMBL337985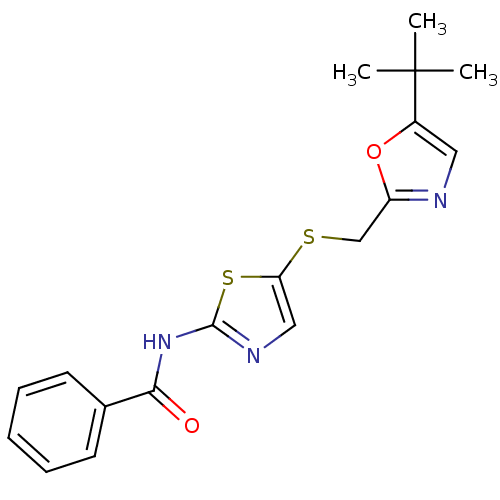 BMS-387032 analog 15 N-[5-[[[5-tert-Butyl-2-oxazolyl]methyl]thio]-2-thiazolyl]benzamide BDBM5683 2-amino-5-thio-substituted thiazole 31 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)benzamide
BMS-387032 analog 15 N-[5-[[[5-tert-Butyl-2-oxazolyl]methyl]thio]-2-thiazolyl]benzamide BDBM5683 2-amino-5-thio-substituted thiazole 31 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)benzamide BMS-387032 analog 26 4-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)cyclohexane-1-carboxamide cis-4-Amino-N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]cyclohexanecarboxamide Hydrochloride BDBM5936
BMS-387032 analog 26 4-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)cyclohexane-1-carboxamide cis-4-Amino-N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]cyclohexanecarboxamide Hydrochloride BDBM5936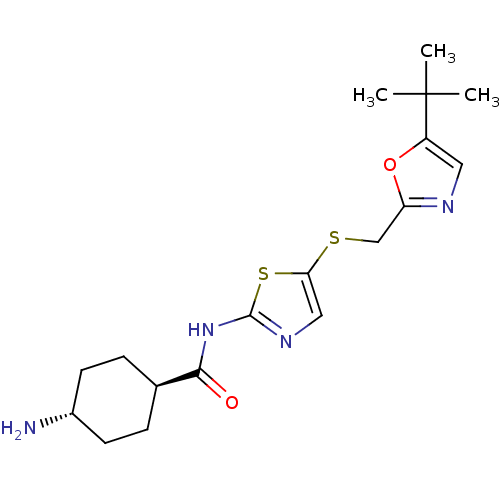 BMS-387032 analog 27 4-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)cyclohexane-1-carboxamide trans-4-Amino-N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]cyclohexanecarboxamide Hydrochloride BDBM5937
BMS-387032 analog 27 4-amino-N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)cyclohexane-1-carboxamide trans-4-Amino-N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]cyclohexanecarboxamide Hydrochloride BDBM5937 BMS-477118 analogue Saxagliptin Analogue 8f BDBM11531 (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcycloheptyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (S)-2-(1-Ethenylcyclohept-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt
BMS-477118 analogue Saxagliptin Analogue 8f BDBM11531 (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcycloheptyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (S)-2-(1-Ethenylcyclohept-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt CABOZANTINIB US12304898, Example Cabozantinib Cometriq US10227329, Compound Cabozantinib US20240174674, Compound Cabozantinib Cabometyx US10202365, Compound Cabozantinib CHEBI:72317 US12338253, Compound Cabozantinib US10464902, Cabozantinib BDBM50021574 BMS-907351 US11279688, Compound Cabozantinib cabozantinib s-malate US10183928, Cabozantinib XL-184 US10273211, Compound Cabozantinib US10774070, Compound Cabozantinib
CABOZANTINIB US12304898, Example Cabozantinib Cometriq US10227329, Compound Cabozantinib US20240174674, Compound Cabozantinib Cabometyx US10202365, Compound Cabozantinib CHEBI:72317 US12338253, Compound Cabozantinib US10464902, Cabozantinib BDBM50021574 BMS-907351 US11279688, Compound Cabozantinib cabozantinib s-malate US10183928, Cabozantinib XL-184 US10273211, Compound Cabozantinib US10774070, Compound Cabozantinib CHEMBL189649 N-(2-Chloro-6-methylphenyl)-2-(6-morpholinopyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide N-(2-chloro-6-methylphenyl)-2-{[6-(morpholin-4-yl)pyrimidin-4-yl]amino}-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12s BDBM13274
CHEMBL189649 N-(2-Chloro-6-methylphenyl)-2-(6-morpholinopyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide N-(2-chloro-6-methylphenyl)-2-{[6-(morpholin-4-yl)pyrimidin-4-yl]amino}-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12s BDBM13274 CHEMBL334333 tert-butyl N-{5-[(2-chloro-6-methylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate BMS-354825 tert-Butoxycarbamate Analog 5b BDBM13218 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide
CHEMBL334333 tert-butyl N-{5-[(2-chloro-6-methylphenyl)carbamoyl]-4-methyl-1,3-thiazol-2-yl}carbamate BMS-354825 tert-Butoxycarbamate Analog 5b BDBM13218 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide Saxagliptin Analogue 8e (S)-2-(1-Ethenylcyclohex-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt BMS-477118 analogue BDBM11530 (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclohexyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid
Saxagliptin Analogue 8e (S)-2-(1-Ethenylcyclohex-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt BMS-477118 analogue BDBM11530 (1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclohexyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpent-4-enoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BMS-477118 analogue BDBM11525 Saxagliptin Analogue 8a (S)-2-(1,1-Dimethylprop-2-en-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt
(1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpent-4-enoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BMS-477118 analogue BDBM11525 Saxagliptin Analogue 8a (S)-2-(1,1-Dimethylprop-2-en-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt (S)-2-(4-Ethenyltetrahydropyran-4-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt BMS-477118 analogue Saxagliptin Analogue 8g (1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethenyloxan-4-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BDBM11532
(S)-2-(4-Ethenyltetrahydropyran-4-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt BMS-477118 analogue Saxagliptin Analogue 8g (1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethenyloxan-4-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid BDBM11532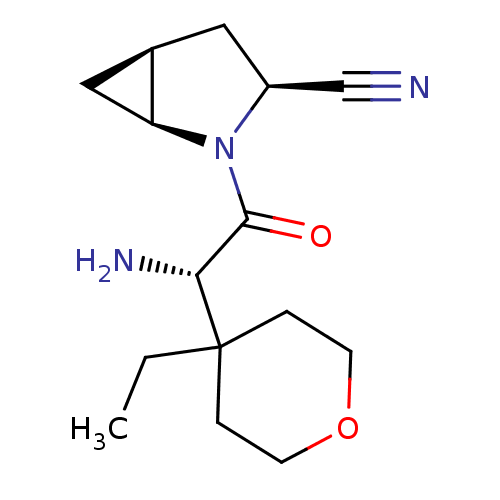 (S)-2-(4-Ethyltetrahydropyran-4-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt BMS-477118 analogue BDBM11536 Saxagliptin Analogue 10g (1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethyloxan-4-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid
(S)-2-(4-Ethyltetrahydropyran-4-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt BMS-477118 analogue BDBM11536 Saxagliptin Analogue 10g (1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethyloxan-4-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid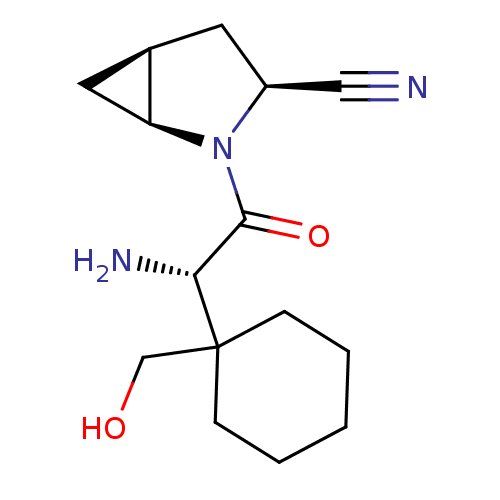 (S)-2-[1-(Hydroxymethyl)cyclohex-1-yl]glycine-L-cis-4,5-methanoprolinenitrile TFA Salt BDBM11539 BMS-477118 analogue (1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyclohexyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid Saxagliptin Analogue 16e
(S)-2-[1-(Hydroxymethyl)cyclohex-1-yl]glycine-L-cis-4,5-methanoprolinenitrile TFA Salt BDBM11539 BMS-477118 analogue (1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyclohexyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid Saxagliptin Analogue 16e 2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-isopropyl6-methylphenyl)-1,3-thiazole-5-carboxamide CHEMBL336330 BDBM13227 BMS-354825 tert-Butoxycarbamate Analog 5h tert-butyl N-(4-methyl-5-{[2-methyl-6-(propan-2-yl)phenyl]carbamoyl}-1,3-thiazol-2-yl)carbamate
2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-isopropyl6-methylphenyl)-1,3-thiazole-5-carboxamide CHEMBL336330 BDBM13227 BMS-354825 tert-Butoxycarbamate Analog 5h tert-butyl N-(4-methyl-5-{[2-methyl-6-(propan-2-yl)phenyl]carbamoyl}-1,3-thiazol-2-yl)carbamate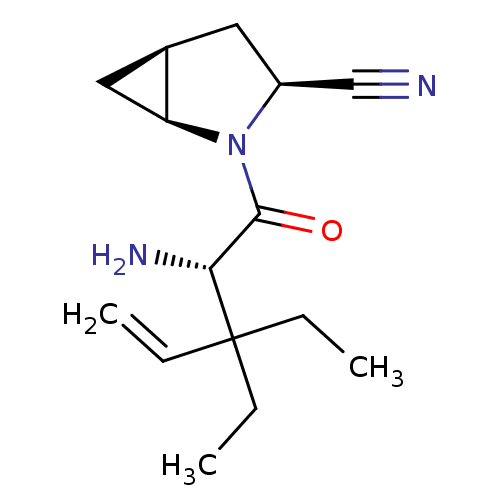 BDBM11527 BMS-477118 analogue Saxagliptin Analogue 8b (1S,3S,5S)-2-[(2S)-2-amino-3,3-diethylpent-4-enoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (S)-2-(1,1-Diethylprop-2-en-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt
BDBM11527 BMS-477118 analogue Saxagliptin Analogue 8b (1S,3S,5S)-2-[(2S)-2-amino-3,3-diethylpent-4-enoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (S)-2-(1,1-Diethylprop-2-en-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt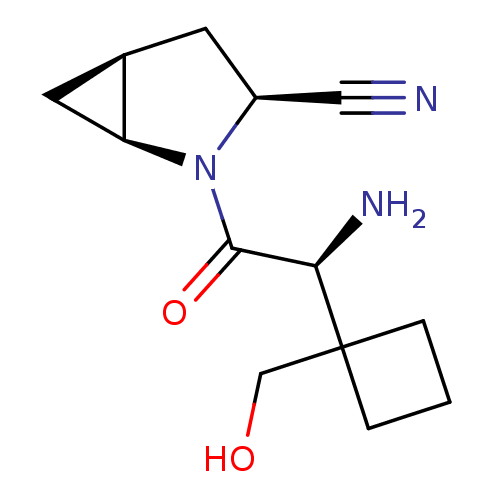 BDBM11537 BMS-477118 analogue (1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyclobutyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (S)-2-[1-(Hydroxymethyl)cyclobut-1-yl]glycine-L-cis-4,5-methanoprolinenitrile TFA Salt Saxagliptin Analogue 16c
BDBM11537 BMS-477118 analogue (1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyclobutyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid (S)-2-[1-(Hydroxymethyl)cyclobut-1-yl]glycine-L-cis-4,5-methanoprolinenitrile TFA Salt Saxagliptin Analogue 16c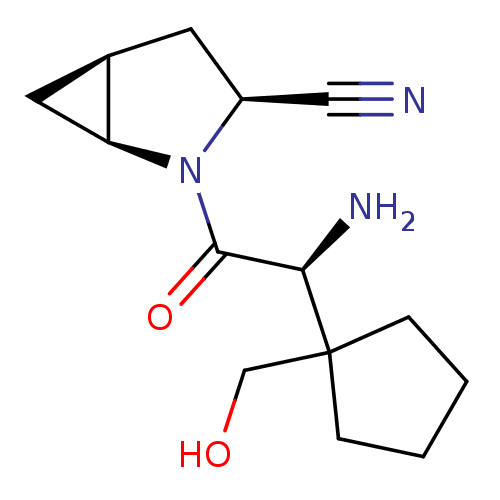 BDBM11538 BMS-477118 analogue (S)-2-[1-(Hydroxymethyl)cyclopent-1-yl]glycine-L-cis-4,5-methanoprolinenitrile TFA Salt (1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyclopentyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid Saxagliptin Analogue 16d
BDBM11538 BMS-477118 analogue (S)-2-[1-(Hydroxymethyl)cyclopent-1-yl]glycine-L-cis-4,5-methanoprolinenitrile TFA Salt (1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyclopentyl]acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid Saxagliptin Analogue 16d BDBM13271 N-(2-Chloro-6-methylphenyl)-2-(6-(2-hydroxyethylamino)pyridin-2-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12p N-(2-chloro-6-methylphenyl)-2-({6-[(2-hydroxyethyl)amino]pyridin-2-yl}amino)-1,3-thiazole-5-carboxamide
BDBM13271 N-(2-Chloro-6-methylphenyl)-2-(6-(2-hydroxyethylamino)pyridin-2-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12p N-(2-chloro-6-methylphenyl)-2-({6-[(2-hydroxyethyl)amino]pyridin-2-yl}amino)-1,3-thiazole-5-carboxamide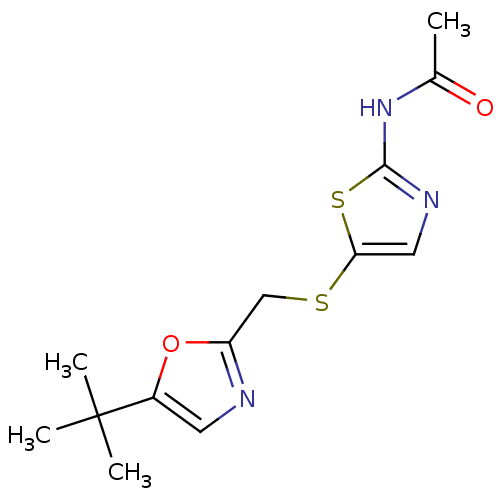 BDBM5660 BMS-387032 analog 12 2-aminothiazole 2 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)acetamide 2-amino-5-thio-substituted thiazole 25 N-[5-[[(5-tert-Butyl-2-oxazolyl)methyl]thio]-2-thiazolyl]acetamide
BDBM5660 BMS-387032 analog 12 2-aminothiazole 2 N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)acetamide 2-amino-5-thio-substituted thiazole 25 N-[5-[[(5-tert-Butyl-2-oxazolyl)methyl]thio]-2-thiazolyl]acetamide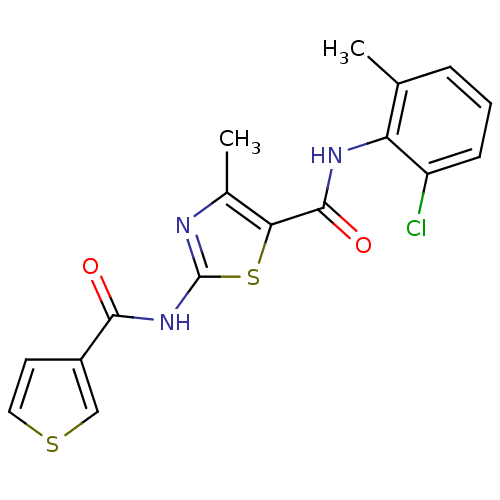 BMS-354825 2-Amino-4-methyl-thiazole Analog 7i BDBM13235 N-(2-chloro-6-methylphenyl)-4-methyl-2-[(thien-3-ylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-4-methyl-2-N-(thiophene-3-)-1,3-thiazole-2,5-diamido
BMS-354825 2-Amino-4-methyl-thiazole Analog 7i BDBM13235 N-(2-chloro-6-methylphenyl)-4-methyl-2-[(thien-3-ylcarbonyl)amino]-1,3-thiazole-5-carboxamide 5-N-(2-chloro-6-methylphenyl)-4-methyl-2-N-(thiophene-3-)-1,3-thiazole-2,5-diamido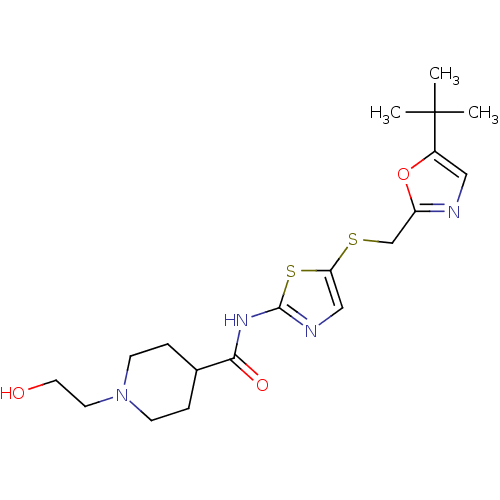 BMS-387032 analog 23 BDBM5933 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-1-(2-hydroxyethyl)-4-piperidinecarboxamide N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-1-(2-hydroxyethyl)piperidine-4-carboxamide
BMS-387032 analog 23 BDBM5933 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-1-(2-hydroxyethyl)-4-piperidinecarboxamide N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-1-(2-hydroxyethyl)piperidine-4-carboxamide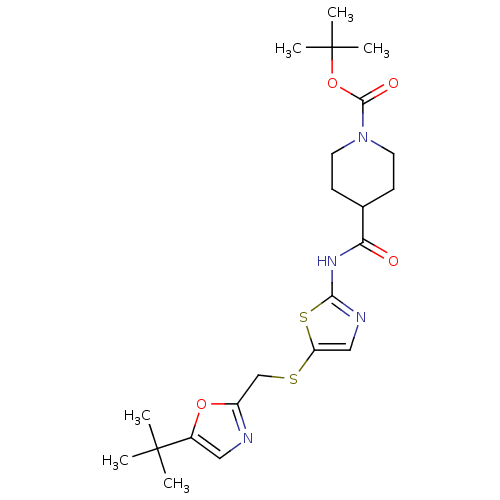 BMS-387032 analog 25 tert-butyl 4-[(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)carbamoyl]piperidine-1-carboxylate BDBM5935 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-(N-tert-butoxycarbonyl)-4-piperidinecarboxamide
BMS-387032 analog 25 tert-butyl 4-[(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)carbamoyl]piperidine-1-carboxylate BDBM5935 N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-(N-tert-butoxycarbonyl)-4-piperidinecarboxamide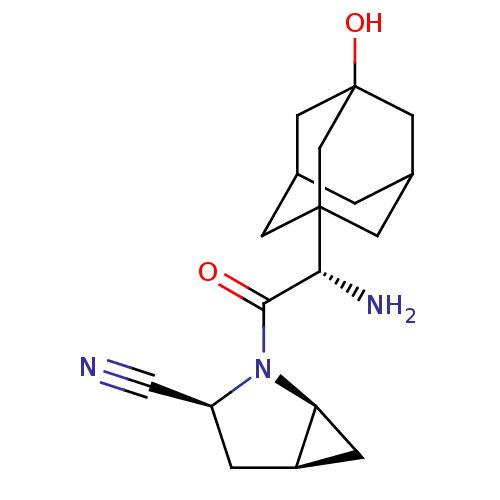 BMS-477118 (1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1-yl)-acetyl]-2-aza-bicyclo[3.1.0]hexane-3-carbonitrile BDBM50225074 SAXAGLIPTIN (1S,6S)-2-(2-adamantan-1-yl-2-amino-acetyl)-2-aza-bicyclo[3.1.0]hexane-3-carbonitrile CHEMBL385517
BMS-477118 (1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1-yl)-acetyl]-2-aza-bicyclo[3.1.0]hexane-3-carbonitrile BDBM50225074 SAXAGLIPTIN (1S,6S)-2-(2-adamantan-1-yl-2-amino-acetyl)-2-aza-bicyclo[3.1.0]hexane-3-carbonitrile CHEMBL385517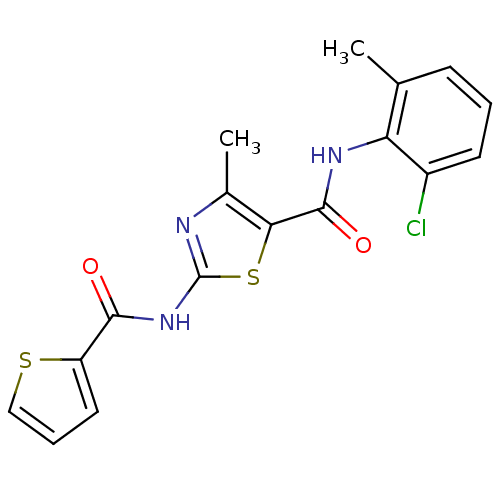 N-(2-chloro-6-methylphenyl)-4-methyl-2-[(thien-2-ylcarbonyl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 7j BDBM13236 5-N-(2-chloro-6-methylphenyl)-4-methyl-2-N-(thiophene-2-)-1,3-thiazole-2,5-diamido
N-(2-chloro-6-methylphenyl)-4-methyl-2-[(thien-2-ylcarbonyl)amino]-1,3-thiazole-5-carboxamide BMS-354825 2-Amino-4-methyl-thiazole Analog 7j BDBM13236 5-N-(2-chloro-6-methylphenyl)-4-methyl-2-N-(thiophene-2-)-1,3-thiazole-2,5-diamido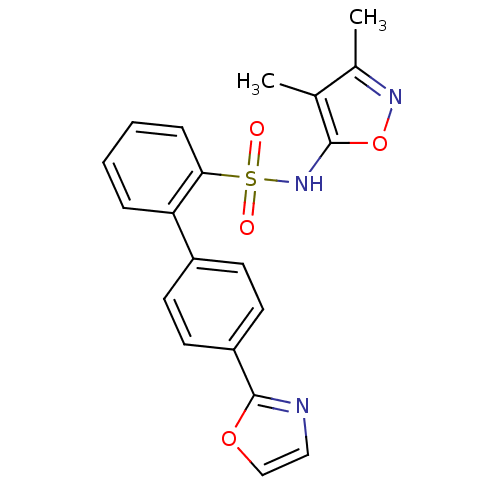 4'-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide CHEMBL24461 BMS-193884 BDBM50091105 4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide 4'-Oxazol-2-yl-biphenyl-2-sulfonicacid(3,4-dimethyl-isoxazol-5-yl)-amide
4'-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide CHEMBL24461 BMS-193884 BDBM50091105 4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide 4'-Oxazol-2-yl-biphenyl-2-sulfonicacid(3,4-dimethyl-isoxazol-5-yl)-amide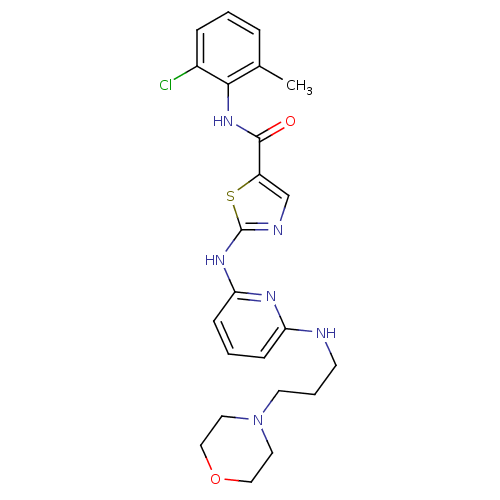 BDBM13272 BMS-354825 2-Heteroarylamino-thiazole Analog 12q N-(2-chloro-6-methylphenyl)-2-[(6-{[3-(morpholin-4-yl)propyl]amino}pyridin-2-yl)amino]-1,3-thiazole-5-carboxamide N-(2-Chloro-6-methylphenyl)-2-(6-(3-morpholinopropylamino)pyridin-2-ylamino)-1,3-thiazole-5-carboxamide
BDBM13272 BMS-354825 2-Heteroarylamino-thiazole Analog 12q N-(2-chloro-6-methylphenyl)-2-[(6-{[3-(morpholin-4-yl)propyl]amino}pyridin-2-yl)amino]-1,3-thiazole-5-carboxamide N-(2-Chloro-6-methylphenyl)-2-(6-(3-morpholinopropylamino)pyridin-2-ylamino)-1,3-thiazole-5-carboxamide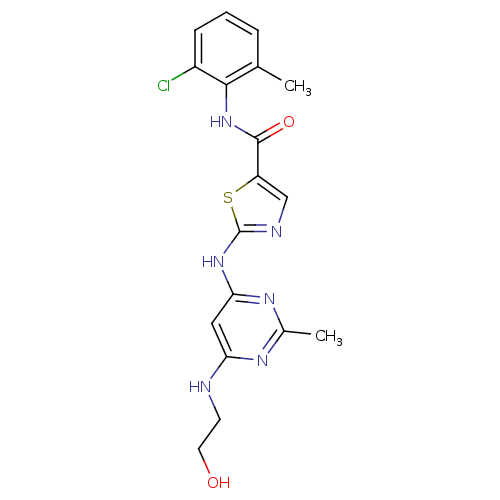 N-(2-Chloro-6-methylphenyl)-2-(6-(2-hydroxyethylamino)-2-methylpyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12v N-(2-chloro-6-methylphenyl)-2-({6-[(2-hydroxyethyl)amino]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide BDBM13277
N-(2-Chloro-6-methylphenyl)-2-(6-(2-hydroxyethylamino)-2-methylpyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12v N-(2-chloro-6-methylphenyl)-2-({6-[(2-hydroxyethyl)amino]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide BDBM13277 N-(2-chloro-6-methylphenyl)-2-{[2-methyl-6-(morpholin-4-yl)pyrimidin-4-yl]amino}-1,3-thiazole-5-carboxamide N-(2-Chloro-6-methylphenyl)-2-(2-methyl-6-morpholinopyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12t BDBM13275
N-(2-chloro-6-methylphenyl)-2-{[2-methyl-6-(morpholin-4-yl)pyrimidin-4-yl]amino}-1,3-thiazole-5-carboxamide N-(2-Chloro-6-methylphenyl)-2-(2-methyl-6-morpholinopyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12t BDBM13275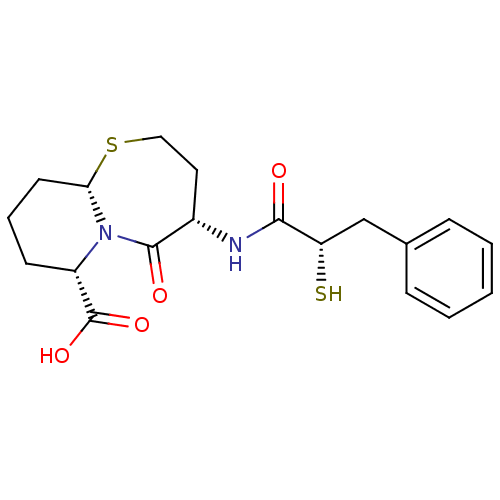 (4S,7S,10aS)-4-((S)-2-mercapto-3-phenylpropanamido)-5-oxo-octahydro-2H-pyrido[2,1-b][1,3]thiazepine-7-carboxylic acid CHEMBL289556 OMAPATRILAT BMS-186716 (4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylamino)-5-oxo-octahydro-9-thia-4a-aza-benzocycloheptene-4-carboxylic acid BDBM50073120
(4S,7S,10aS)-4-((S)-2-mercapto-3-phenylpropanamido)-5-oxo-octahydro-2H-pyrido[2,1-b][1,3]thiazepine-7-carboxylic acid CHEMBL289556 OMAPATRILAT BMS-186716 (4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylamino)-5-oxo-octahydro-9-thia-4a-aza-benzocycloheptene-4-carboxylic acid BDBM50073120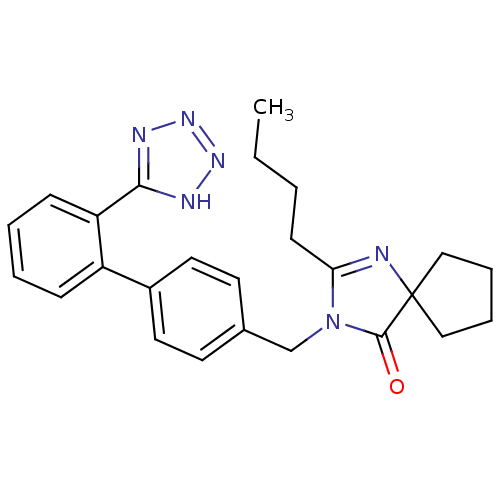 2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one CHEMBL1513 BDBM50042235 IRBESARTAN BMS 186295 2-butyl-3-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one
2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one CHEMBL1513 BDBM50042235 IRBESARTAN BMS 186295 2-butyl-3-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one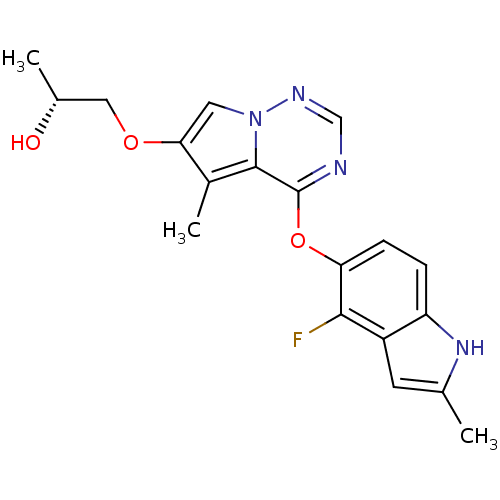 (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-ol CHEMBL377300 (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[1,2-f][1,2,4]triazin-6-yloxy)propan-2-ol BMS-540215 BDBM50184807
(R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-ol CHEMBL377300 (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[1,2-f][1,2,4]triazin-6-yloxy)propan-2-ol BMS-540215 BDBM50184807 9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)-amino]-piperidin-1-yl}-butyl)-9H-fluorene-9-carboxylic acid (2,2,2-trifluoro-ethyl)-amide CHEMBL354541 9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-amino]-piperidin-1-yl}-butyl)-9H-fluorene-9-carboxylic acid (2,2,2-trifluoro-ethyl)-amide BDBM50098320 BMS-201038 LOMITAPIDE
9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)-amino]-piperidin-1-yl}-butyl)-9H-fluorene-9-carboxylic acid (2,2,2-trifluoro-ethyl)-amide CHEMBL354541 9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-amino]-piperidin-1-yl}-butyl)-9H-fluorene-9-carboxylic acid (2,2,2-trifluoro-ethyl)-amide BDBM50098320 BMS-201038 LOMITAPIDE BMS-354825 2-Heteroarylamino-thiazole Analog 12w N-(2-chloro-6-methylphenyl)-2-({6-[4-(hydroxymethyl)piperidin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide N-(2-Chloro-6-methylphenyl)-2-(6-(4-(hydroxymethyl)piperidin-1-yl)-2-methylpyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide BDBM13278
BMS-354825 2-Heteroarylamino-thiazole Analog 12w N-(2-chloro-6-methylphenyl)-2-({6-[4-(hydroxymethyl)piperidin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide N-(2-Chloro-6-methylphenyl)-2-(6-(4-(hydroxymethyl)piperidin-1-yl)-2-methylpyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide BDBM13278 N-(2-Chloro-6-methylphenyl)-2-(2-methyl-6-(3-morpholinopropyl)-pyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12u BDBM13276 N-(2-chloro-6-methylphenyl)-2-[(2-methyl-6-{[3-(morpholin-4-yl)propyl]amino}pyrimidin-4-yl)amino]-1,3-thiazole-5-carboxamide
N-(2-Chloro-6-methylphenyl)-2-(2-methyl-6-(3-morpholinopropyl)-pyrimidin-4-ylamino)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12u BDBM13276 N-(2-chloro-6-methylphenyl)-2-[(2-methyl-6-{[3-(morpholin-4-yl)propyl]amino}pyrimidin-4-yl)amino]-1,3-thiazole-5-carboxamide BDBM13273 N-(2-chloro-6-methylphenyl)-2-[(6-{[3-(1H-imidazol-1-yl)propyl]amino}pyridin-2-yl)amino]-1,3-thiazole-5-carboxamide 2-(6-(3-(1H-Imidazol-1-yl)propylamino)pyridin-2-ylamino)-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12r
BDBM13273 N-(2-chloro-6-methylphenyl)-2-[(6-{[3-(1H-imidazol-1-yl)propyl]amino}pyridin-2-yl)amino]-1,3-thiazole-5-carboxamide 2-(6-(3-(1H-Imidazol-1-yl)propylamino)pyridin-2-ylamino)-N-(2-chloro-6-methylphenyl)-1,3-thiazole-5-carboxamide BMS-354825 2-Heteroarylamino-thiazole Analog 12r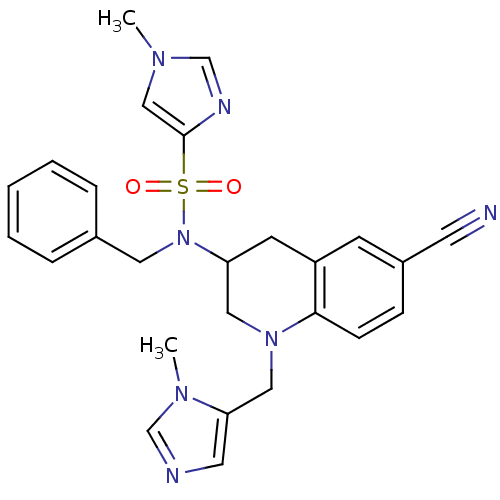 1-Methyl-1H-imidazole-4-sulfonic Acid Benzyl-[6-cyano1-(3-methyl-3H-imidazol-4-ylmethyl)-1,2,3,4-tetrahydroquinolin-3-yl]amide CHEMBL182888 tetrahydroquinoline (THQ)-based inhibitor 4e N-benzyl-N-{6-cyano-1-[(1-methyl-1H-imidazol-5-yl)methyl]-1,2,3,4-tetrahydroquinolin-3-yl}-1-methyl-1H-imidazole-4-sulfonamide BDBM13323 BMS-339941
1-Methyl-1H-imidazole-4-sulfonic Acid Benzyl-[6-cyano1-(3-methyl-3H-imidazol-4-ylmethyl)-1,2,3,4-tetrahydroquinolin-3-yl]amide CHEMBL182888 tetrahydroquinoline (THQ)-based inhibitor 4e N-benzyl-N-{6-cyano-1-[(1-methyl-1H-imidazol-5-yl)methyl]-1,2,3,4-tetrahydroquinolin-3-yl}-1-methyl-1H-imidazole-4-sulfonamide BDBM13323 BMS-339941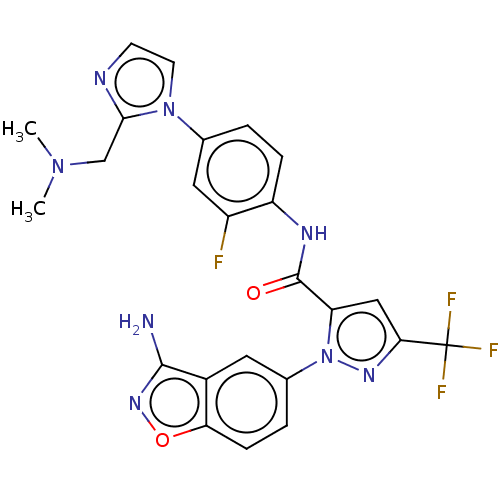 CHEMBL558198 Razaxaban BMS-561389 BDBM12676 1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[2-fluoro-4-[(2-dimethylaminomethyl)imidazol-1-yl]phenyl]-1H-pyrazole-5-carboxyamide Hydrochloride Salt CHEMBL206335 DPC 906 1-(3-amino-1,2-benzoxazol-5-yl)-N-(4-{2-[(dimethylamino)methyl]-1H-imidazol-1-yl}-2-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide
CHEMBL558198 Razaxaban BMS-561389 BDBM12676 1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[2-fluoro-4-[(2-dimethylaminomethyl)imidazol-1-yl]phenyl]-1H-pyrazole-5-carboxyamide Hydrochloride Salt CHEMBL206335 DPC 906 1-(3-amino-1,2-benzoxazol-5-yl)-N-(4-{2-[(dimethylamino)methyl]-1H-imidazol-1-yl}-2-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide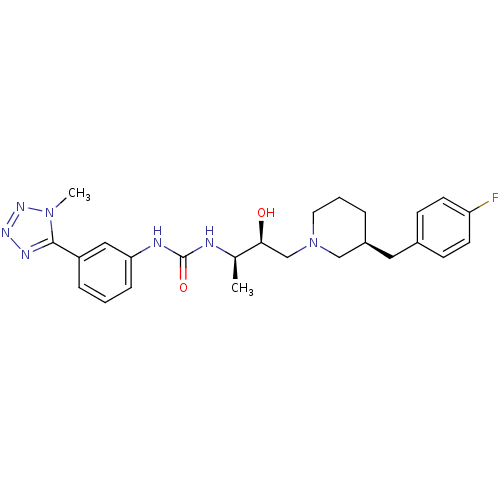 1-((2R,3S)-4-((S)-3-(4-fluorobenzyl)piperidin-1-yl)-3-hydroxybutan-2-yl)-3-(3-(1-methyl-1H-tetrazol-5-yl)phenyl)urea CHEMBL399495 BDBM50231358 BMS-639623 1-{(1R,2S)-3-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-yl]-2-hydroxy-1-methyl-propyl}-3-[3-(1-methyl-1H-tetrazol-5-yl)-phenyl]-urea
1-((2R,3S)-4-((S)-3-(4-fluorobenzyl)piperidin-1-yl)-3-hydroxybutan-2-yl)-3-(3-(1-methyl-1H-tetrazol-5-yl)phenyl)urea CHEMBL399495 BDBM50231358 BMS-639623 1-{(1R,2S)-3-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-yl]-2-hydroxy-1-methyl-propyl}-3-[3-(1-methyl-1H-tetrazol-5-yl)-phenyl]-urea BDBM678 3-{4-[(2S,3R)-2-{[(tert-butoxy)carbonyl]amino}-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-phenylbutyl]amino}-3-hydroxybutyl]phenoxy}propanoic acid BMS-186318 analog 15 [1S-[1R*,2S*(2S*,3R*)]]-[4-[4-(Carboxymethoxy)phenyl]-3-[[3-[[(1,1-dimethylethoxy)carbonyl]amino]-2-hydroxy-1-(phenylmethyl)propyl)carbamic Acid, 1,1-Dimethylethyl Ester
BDBM678 3-{4-[(2S,3R)-2-{[(tert-butoxy)carbonyl]amino}-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-phenylbutyl]amino}-3-hydroxybutyl]phenoxy}propanoic acid BMS-186318 analog 15 [1S-[1R*,2S*(2S*,3R*)]]-[4-[4-(Carboxymethoxy)phenyl]-3-[[3-[[(1,1-dimethylethoxy)carbonyl]amino]-2-hydroxy-1-(phenylmethyl)propyl)carbamic Acid, 1,1-Dimethylethyl Ester BMS-186318 analog 1 [(S,R)-3-[[(R,S)-3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-phenylbutyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-phenylbutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM914 Aminodiol deriv. 9a
BMS-186318 analog 1 [(S,R)-3-[[(R,S)-3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-phenylbutyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-phenylbutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM914 Aminodiol deriv. 9a BMS-387032 analog 2 BDBM5668 N-[5-[[[5-tert-Butyl-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-[[[bis(hydroxymethyl)methyl]amino]methyl]benzeneacetamide Hydrochloride Salt N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-2-(4-{[(1,3-dihydroxypropan-2-yl)amino]methyl}phenyl)acetamide 2-amino-5-thio-substituted thiazole 45
BMS-387032 analog 2 BDBM5668 N-[5-[[[5-tert-Butyl-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-[[[bis(hydroxymethyl)methyl]amino]methyl]benzeneacetamide Hydrochloride Salt N-(5-{[(5-tert-butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)-2-(4-{[(1,3-dihydroxypropan-2-yl)amino]methyl}phenyl)acetamide 2-amino-5-thio-substituted thiazole 45 CHEMBL183536 BDBM13319 BMS-386914 tert-butyl 2-({6-cyano-1-[(1-methyl-1H-imidazol-5-yl)methyl]-1,2,3,4-tetrahydroquinolin-3-yl}(1-methyl-1H-imidazole-4-)sulfonamido)acetate [[6-Cyano-1-(3-methyl-3H-imidazol-4-ylmethyl)-1,2,3,4-tetrahydroquinolin-3-yl]-(1-methyl-1H-imidazole-4-sulfonyl)amino]-acetic Acid tert-Butyl Ester tetrahydroquinoline (THQ)-based inhibitor 4a
CHEMBL183536 BDBM13319 BMS-386914 tert-butyl 2-({6-cyano-1-[(1-methyl-1H-imidazol-5-yl)methyl]-1,2,3,4-tetrahydroquinolin-3-yl}(1-methyl-1H-imidazole-4-)sulfonamido)acetate [[6-Cyano-1-(3-methyl-3H-imidazol-4-ylmethyl)-1,2,3,4-tetrahydroquinolin-3-yl]-(1-methyl-1H-imidazole-4-sulfonyl)amino]-acetic Acid tert-Butyl Ester tetrahydroquinoline (THQ)-based inhibitor 4a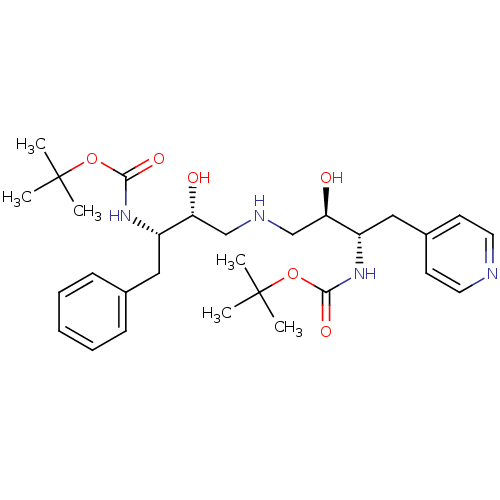 BDBM665 BMS-186318 analog 2 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-phenylbutyl]amino]-2-hydroxy-1-(4-pyridinylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(pyridin-4-yl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
BDBM665 BMS-186318 analog 2 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-phenylbutyl]amino]-2-hydroxy-1-(4-pyridinylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(pyridin-4-yl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate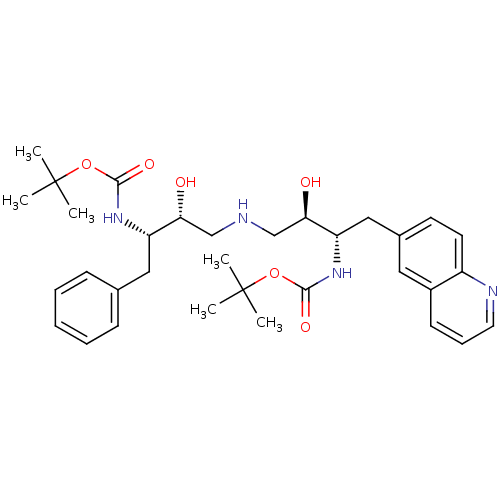 BDBM666 BMS-186318 analog 3 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-phenylbutyl]amino]-2-hydroxy-1-(6-quinolinylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(quinolin-6-yl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
BDBM666 BMS-186318 analog 3 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-phenylbutyl]amino]-2-hydroxy-1-(6-quinolinylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(quinolin-6-yl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate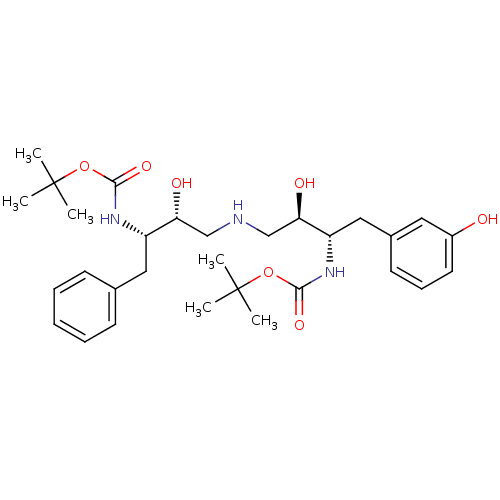 BDBM668 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-(3-hydroxyphenyl)butyl]-amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BMS-186318 analog 5 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(3-hydroxyphenyl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
BDBM668 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-(3-hydroxyphenyl)butyl]-amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BMS-186318 analog 5 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(3-hydroxyphenyl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate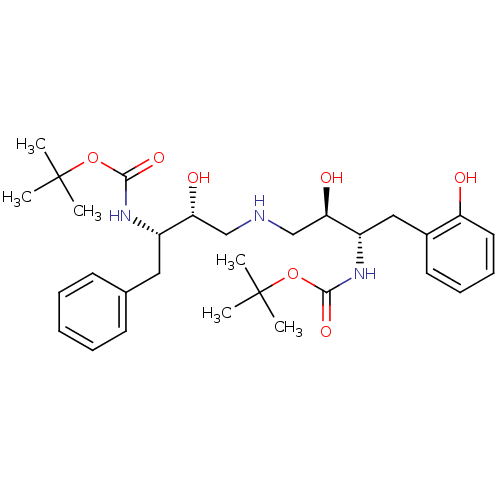 BDBM669 BMS-186318 analog 6 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(2-hydroxyphenyl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-(2-hydroxyphenyl)butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester
BDBM669 BMS-186318 analog 6 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(2-hydroxyphenyl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-(2-hydroxyphenyl)butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester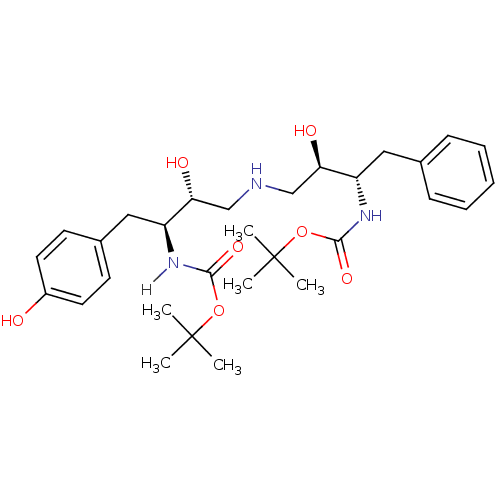 BMS-186318 analog 4 (1S-(1R*,2S*(2S,3R*)))-[3-[[3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-(4-hydroxyphenyl)butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM918 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(4-hydroxyphenyl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate Aminodiol deriv. 15b
BMS-186318 analog 4 (1S-(1R*,2S*(2S,3R*)))-[3-[[3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-(4-hydroxyphenyl)butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM918 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-(4-hydroxyphenyl)butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate Aminodiol deriv. 15b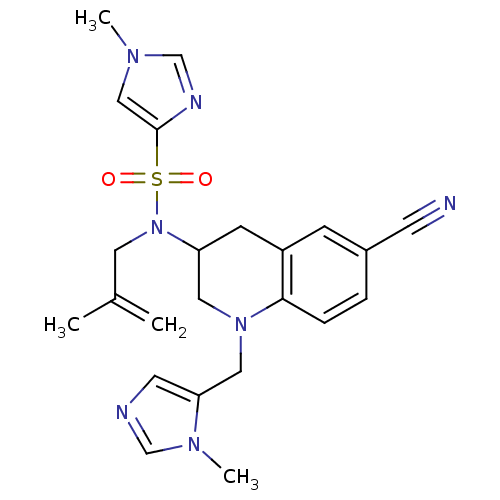 CHEMBL181102 N-{6-cyano-1-[(1-methyl-1H-imidazol-5-yl)methyl]-1,2,3,4-tetrahydroquinolin-3-yl}-1-methyl-N-(2-methylprop-2-en-1-yl)-1H-imidazole-4-sulfonamide BMS-388891 tetrahydroquinoline (THQ)-based inhibitor 4b 1-Methyl-1H-imidazole-4-sulfonic Acid [6-Cyano-1-(3-methyl-3H-imidazol-4-ylmethyl)-1,2,3,4-tetrahydroquinolin-3-yl]-(2-methylallyl)amide BDBM13320
CHEMBL181102 N-{6-cyano-1-[(1-methyl-1H-imidazol-5-yl)methyl]-1,2,3,4-tetrahydroquinolin-3-yl}-1-methyl-N-(2-methylprop-2-en-1-yl)-1H-imidazole-4-sulfonamide BMS-388891 tetrahydroquinoline (THQ)-based inhibitor 4b 1-Methyl-1H-imidazole-4-sulfonic Acid [6-Cyano-1-(3-methyl-3H-imidazol-4-ylmethyl)-1,2,3,4-tetrahydroquinolin-3-yl]-(2-methylallyl)amide BDBM13320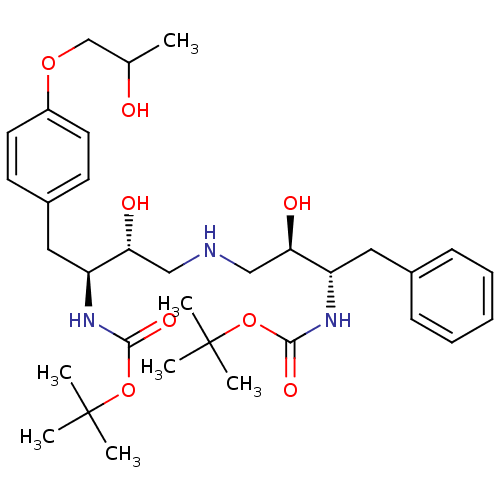 BDBM676 BMS-186318 analog 13 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(2-hydroxypropoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*, 2S*(2S*, 3R*)]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-hydroxypropoxy)phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethyl Ester
BDBM676 BMS-186318 analog 13 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(2-hydroxypropoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*, 2S*(2S*, 3R*)]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-hydroxypropoxy)phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethyl Ester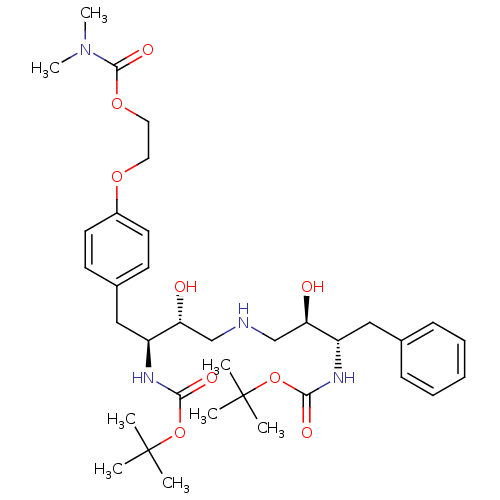 BDBM679 BMS-186318 analog 16 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-4-(4-{2-[(dimethylcarbamoyl)oxy]ethoxy}phenyl)-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[4-[4-[2-[[(Dimethylamino)-carbonyl]oxy]ethoxy]phenyl]-3-[[(1,1-dimethylethoxy)-carbonyl]amino]-2-hydroxybutyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid
BDBM679 BMS-186318 analog 16 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-4-(4-{2-[(dimethylcarbamoyl)oxy]ethoxy}phenyl)-2-hydroxybutyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[4-[4-[2-[[(Dimethylamino)-carbonyl]oxy]ethoxy]phenyl]-3-[[(1,1-dimethylethoxy)-carbonyl]amino]-2-hydroxybutyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid BMS-186318 analog 12 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-methoxyethoxy)phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM675 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(2-methoxyethoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
BMS-186318 analog 12 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-methoxyethoxy)phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM675 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(2-methoxyethoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-hydroxyethoxy)phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BMS-186318 analog 11 BDBM674 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(2-hydroxyethoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
[1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-hydroxyethoxy)phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BMS-186318 analog 11 BDBM674 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(2-hydroxyethoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate 4'-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-ylmethyl)-2'-(3,3-dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide CHEMBL11706 BDBM50117910 BMS-248360 4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-ylmethyl)-2''-(3,3-dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide
4'-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-ylmethyl)-2'-(3,3-dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide CHEMBL11706 BDBM50117910 BMS-248360 4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-ylmethyl)-2''-(3,3-dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-yl)-amide BMS-186318 analog 21 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-(4-morpholinyl)ethoxy]-phenyl]butyl]amino]-2-hydroxy-1- tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(morpholin-4-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM684
BMS-186318 analog 21 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-(4-morpholinyl)ethoxy]-phenyl]butyl]amino]-2-hydroxy-1- tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(morpholin-4-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM684 BMS-186318 analog 8 BDBM671 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(pyridin-2-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-(4-[2-(2-pyridinyl)ethoxy]phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester
BMS-186318 analog 8 BDBM671 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(pyridin-2-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-(4-[2-(2-pyridinyl)ethoxy]phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester CHEMBL450668 (R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-yl)-N-(3-(7-methyl-1H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-yl)propan-2-yl)piperidine-1-carboxamide BDBM50268484 BMS-694153 (R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-indazol-5-yl)-1-oxopropan-2-yl)-4-(8-fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-yl)piperidine-1-carboxamide
CHEMBL450668 (R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-yl)-N-(3-(7-methyl-1H-indazol-5-yl)-1-oxo-1-(4-(piperidin-1-yl)piperidin-1-yl)propan-2-yl)piperidine-1-carboxamide BDBM50268484 BMS-694153 (R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-indazol-5-yl)-1-oxopropan-2-yl)-4-(8-fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-yl)piperidine-1-carboxamide [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-hydroxy-2-methylpropoxy)phenyl]butyl]amino]-2-hydroxy-1- BDBM677 BMS-186318 analog 14 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(2-hydroxy-2-methylpropoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester
[1S-[1R*,2S*(2S*,3R*)]]-[3-[[3[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-hydroxy-2-methylpropoxy)phenyl]butyl]amino]-2-hydroxy-1- BDBM677 BMS-186318 analog 14 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-[4-(2-hydroxy-2-methylpropoxy)phenyl]butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethy Ester [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoamino]-2-hydroxy-4-[4-[3-(4-morpholinyl)-3-oxopropyl]xy)-carbonyl]phenyl]butyl]amino]-2- tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[3-(morpholin-4-yl)-3-oxopropyl]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-186318 analog 20 BDBM683
hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethy Ester [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoamino]-2-hydroxy-4-[4-[3-(4-morpholinyl)-3-oxopropyl]xy)-carbonyl]phenyl]butyl]amino]-2- tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[3-(morpholin-4-yl)-3-oxopropyl]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-186318 analog 20 BDBM683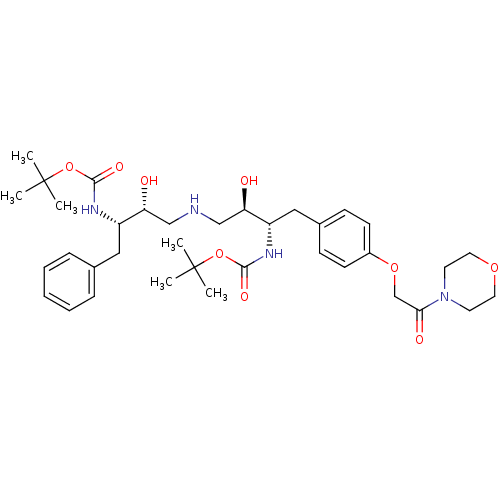 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(morpholin-4-yl)-2-oxoethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM682 hydroxy-1-(phenylmethyl)]propyl]carbamic Acid, 1,1-Dimethylethyl Ester [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-(4-morpholinyl)-2-oxoethoxyphenyl]butyl]amino]-2- BMS-186318 analog 19
tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(morpholin-4-yl)-2-oxoethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM682 hydroxy-1-(phenylmethyl)]propyl]carbamic Acid, 1,1-Dimethylethyl Ester [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-(4-morpholinyl)-2-oxoethoxyphenyl]butyl]amino]-2- BMS-186318 analog 19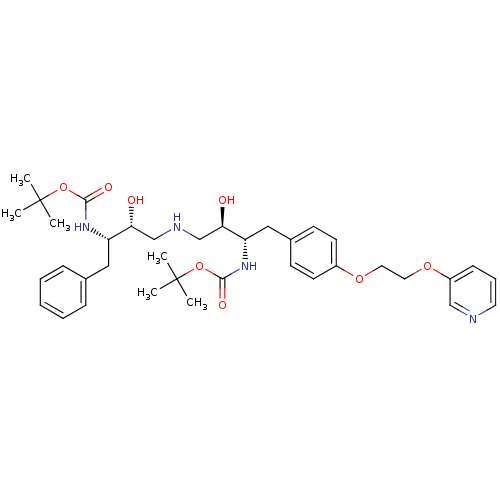 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(pyridin-3-yloxy)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-186318 analog 9 (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM672 [1S-[1R*,2S*(2S*, 3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-[4-[2-(3-pyridinyloxy)ethoxy]phenyl]butyl]amino]-2-hydroxy-1-
tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(pyridin-3-yloxy)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-186318 analog 9 (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM672 [1S-[1R*,2S*(2S*, 3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-[4-[2-(3-pyridinyloxy)ethoxy]phenyl]butyl]amino]-2-hydroxy-1-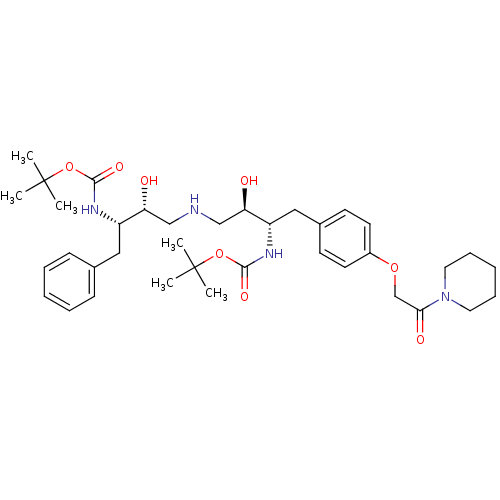 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-oxo-2-(piperidin-1-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-186318 analog 22 BDBM685 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-piperidinylethoxy)-phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester
tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-oxo-2-(piperidin-1-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-186318 analog 22 BDBM685 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-(2-piperidinylethoxy)-phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester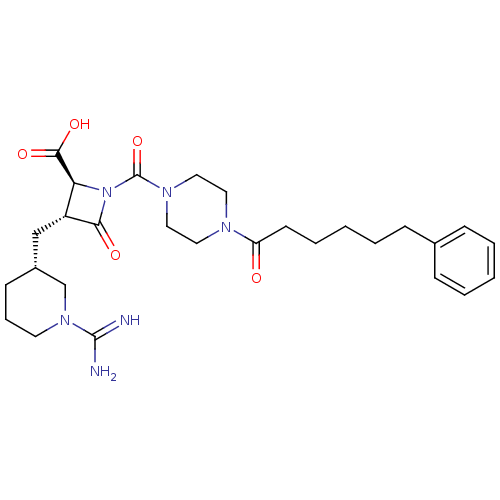 (2S,3R)-3-(1-Carbamimidoyl-piperidin-3-ylmethyl)-4-oxo-1-[4-(5-phenyl-pentanoyl)-piperazine-1-carbonyl]-azetidine-2-carboxylic acid BMS-363131 BDBM50120387 CHEMBL303437 (2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethyl)-4-oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-1-carbonyl]-azetidine-2-carboxylic acid (2S,3R)-3-(1-Carbamimidoyl-piperidin-3-ylmethyl)-4-oxo-1-[4-((R)-6-phenyl-hexanoyl)-piperazine-1-carbonyl]-azetidine-2-carboxylic acid
(2S,3R)-3-(1-Carbamimidoyl-piperidin-3-ylmethyl)-4-oxo-1-[4-(5-phenyl-pentanoyl)-piperazine-1-carbonyl]-azetidine-2-carboxylic acid BMS-363131 BDBM50120387 CHEMBL303437 (2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethyl)-4-oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-1-carbonyl]-azetidine-2-carboxylic acid (2S,3R)-3-(1-Carbamimidoyl-piperidin-3-ylmethyl)-4-oxo-1-[4-((R)-6-phenyl-hexanoyl)-piperazine-1-carbonyl]-azetidine-2-carboxylic acid (S)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethylazepan-3-yl)guanidine BDBM50296258 (S)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)azepan-3-yl)guanidine 1-cyano-2-(2-methyl-1-benzofuran-5-yl)-3-[(3S)-2-oxo-1-(2-oxo-2-pyrrolidin-1-ylethyl)azepan-3-yl]guanidine BMS-269223 CHEMBL551991
(S)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethylazepan-3-yl)guanidine BDBM50296258 (S)-2-cyano-1-(2-methylbenzofuran-5-yl)-3-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)azepan-3-yl)guanidine 1-cyano-2-(2-methyl-1-benzofuran-5-yl)-3-[(3S)-2-oxo-1-(2-oxo-2-pyrrolidin-1-ylethyl)azepan-3-yl]guanidine BMS-269223 CHEMBL551991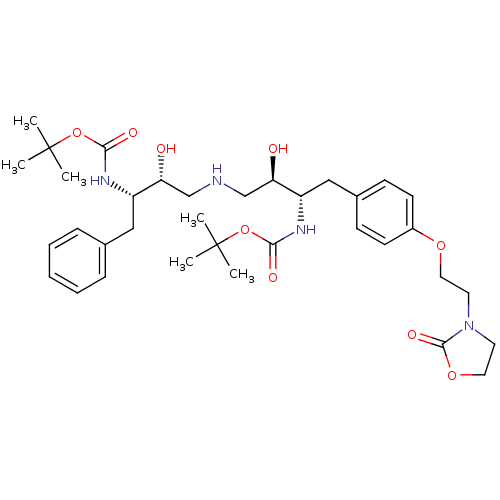 BMS-186318 analog 17 (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethylethyl Ester [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-(2-oxo-3-oxazolidinyl)-ethoxy]phenyl]butyl]amino]-2-hydroxy-1- tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(2-oxo-1,3-oxazolidin-3-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM680
BMS-186318 analog 17 (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethylethyl Ester [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-(2-oxo-3-oxazolidinyl)-ethoxy]phenyl]butyl]amino]-2-hydroxy-1- tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(2-oxo-1,3-oxazolidin-3-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM680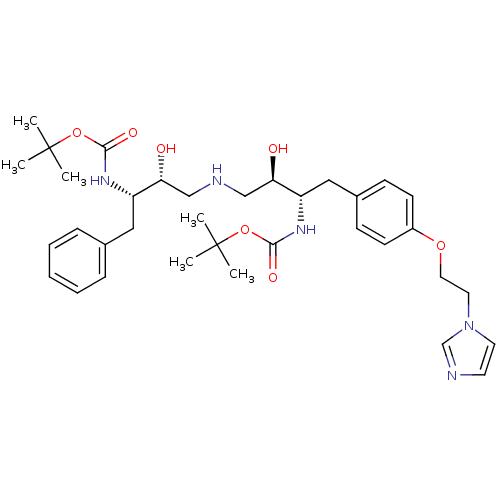 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-[4-[2-(1H-imidazol-1-yl)ethoxy]phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Es tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(1H-imidazol-1-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-186318 analog 7 BDBM670
[1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-hydroxy-4-[4-[2-(1H-imidazol-1-yl)ethoxy]phenyl]butyl]amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Es tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(1H-imidazol-1-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BMS-186318 analog 7 BDBM670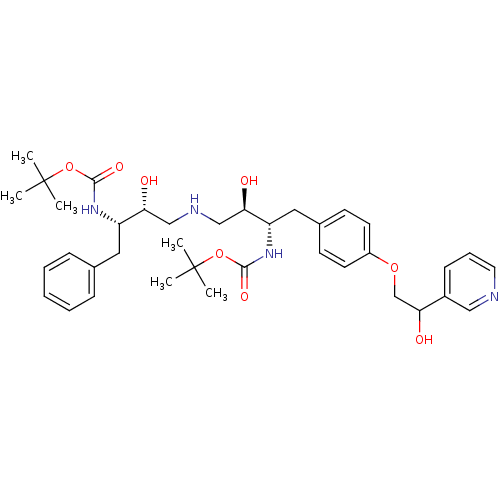 tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-hydroxy-2-(pyridin-3-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-hydroxy-2-(3-pyridinyl)ethoxy]phenyl]butyl]amino]-2-hydroxy-1- BMS-186318 analog 10 (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM673
tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-hydroxy-2-(pyridin-3-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-hydroxy-2-(3-pyridinyl)ethoxy]phenyl]butyl]amino]-2-hydroxy-1- BMS-186318 analog 10 (phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester BDBM673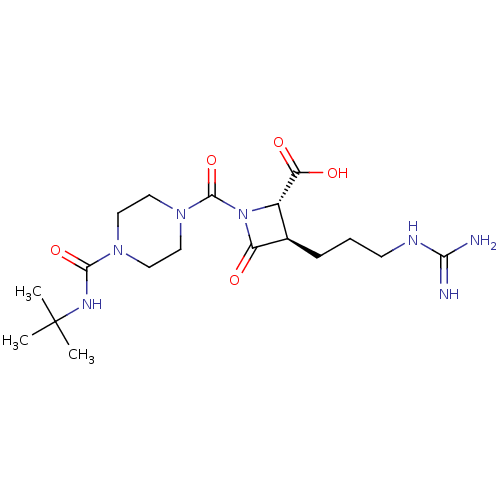 BDBM50120368 BMS-262084 (2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carbonyl)-3-(3-guanidino-propyl)-4-oxo-azetidine-2-carboxylic acid (R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carbonyl)-3-(3-guanidino-propyl)-4-oxo-azetidine-2-carboxylic acid (3S,4R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carbonyl)-3-(3-guanidino-propyl)-4-oxo-azetidine-2-carboxylic acid 1-(4-tert-Butylcarbamoyl-piperazine-1-carbonyl)-3-(3-guanidino-propyl)-4-oxo-azetidine-2-carboxylic acid CHEMBL71037
BDBM50120368 BMS-262084 (2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carbonyl)-3-(3-guanidino-propyl)-4-oxo-azetidine-2-carboxylic acid (R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carbonyl)-3-(3-guanidino-propyl)-4-oxo-azetidine-2-carboxylic acid (3S,4R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carbonyl)-3-(3-guanidino-propyl)-4-oxo-azetidine-2-carboxylic acid 1-(4-tert-Butylcarbamoyl-piperazine-1-carbonyl)-3-(3-guanidino-propyl)-4-oxo-azetidine-2-carboxylic acid CHEMBL71037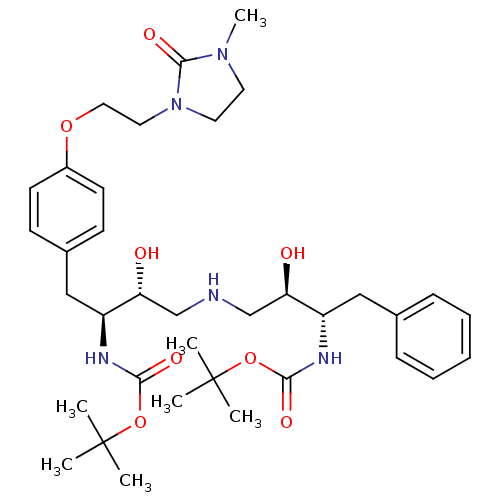 BMS-186318 analog 18 hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(3-methyl-2-oxoimidazolidin-1-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM681 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-(3-methyl-2-oxo-1-imidazolidinyl)ethoxy]phenyl]butyl]amino]-2-
BMS-186318 analog 18 hydroxy-1-(phenylmethyl)propyl]carbamic Acid, 1,1-Dimethylethyl Ester tert-butyl N-[(2S,3R)-4-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-4-{4-[2-(3-methyl-2-oxoimidazolidin-1-yl)ethoxy]phenyl}butyl]amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate BDBM681 [1S-[1R*,2S*(2S*,3R*)]]-[3-[[3-[[(1,1-Dimethylethoxy)-carbonyl]amino]-2-hydroxy-4-[4-[2-(3-methyl-2-oxo-1-imidazolidinyl)ethoxy]phenyl]butyl]amino]-2-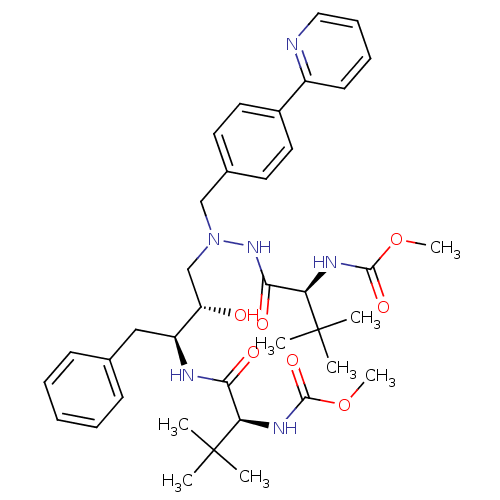 CGP 73547 methyl N-[(1S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)-3,3-dimethyl-butanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenyl-butan-2-yl]carbamoyl]-2,2-dimethyl-propyl]carbamate US10806794, Compound Atazanavir US11938127, Compound atazanavir Atazanavir BDBM13934 Latazanavir BMS 232632 CHEMBL1163 methyl N-[(1S)-1-{[(2S,3S)-3-hydroxy-4-[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-N'-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido]-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate
CGP 73547 methyl N-[(1S)-1-[[(2S,3S)-3-hydroxy-4-[[[(2S)-2-(methoxycarbonylamino)-3,3-dimethyl-butanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenyl-butan-2-yl]carbamoyl]-2,2-dimethyl-propyl]carbamate US10806794, Compound Atazanavir US11938127, Compound atazanavir Atazanavir BDBM13934 Latazanavir BMS 232632 CHEMBL1163 methyl N-[(1S)-1-{[(2S,3S)-3-hydroxy-4-[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-N'-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido]-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate 3-Benzyl-1-(3H-imidazol-4-ylmethyl)-4-(thiophene-2-sulfonyl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-7-carbonitrile (R)-3-Benzyl-1-(3H-imidazol-4-ylmethyl)-4-(thiophene-2-sulfonyl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-7-carbonitrile CHEMBL351706 BMS-214662 (R)-3-Benzyl-1-(1H-imidazol-4-ylmethyl)-4-(thiophene-2-sulfonyl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-7-carbonitrile 3-BENZYL-1-(1H-IMIDAZOL-4-YLMETHYL)-4-(THIEN-2-YLSULFONYL)-2,3,4,5-TETRAHYDRO-1H-1,4-BENZODIAZEPINE-7-CARBONITRILE BDBM50092365 (R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiophen-2-ylsulfonyl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-7-carbonitrile
3-Benzyl-1-(3H-imidazol-4-ylmethyl)-4-(thiophene-2-sulfonyl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-7-carbonitrile (R)-3-Benzyl-1-(3H-imidazol-4-ylmethyl)-4-(thiophene-2-sulfonyl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-7-carbonitrile CHEMBL351706 BMS-214662 (R)-3-Benzyl-1-(1H-imidazol-4-ylmethyl)-4-(thiophene-2-sulfonyl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-7-carbonitrile 3-BENZYL-1-(1H-IMIDAZOL-4-YLMETHYL)-4-(THIEN-2-YLSULFONYL)-2,3,4,5-TETRAHYDRO-1H-1,4-BENZODIAZEPINE-7-CARBONITRILE BDBM50092365 (R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiophen-2-ylsulfonyl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-7-carbonitrile