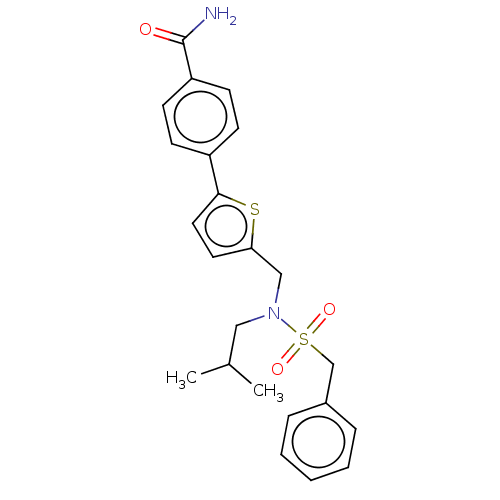
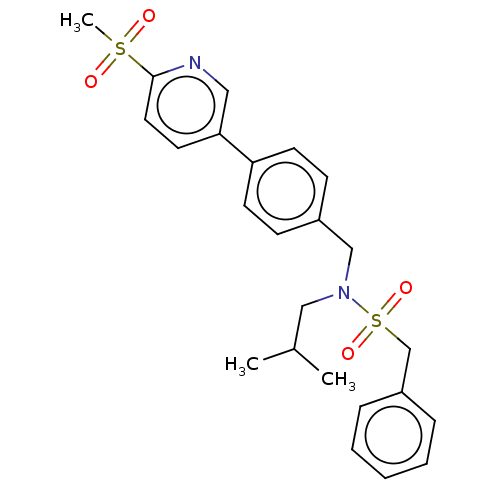
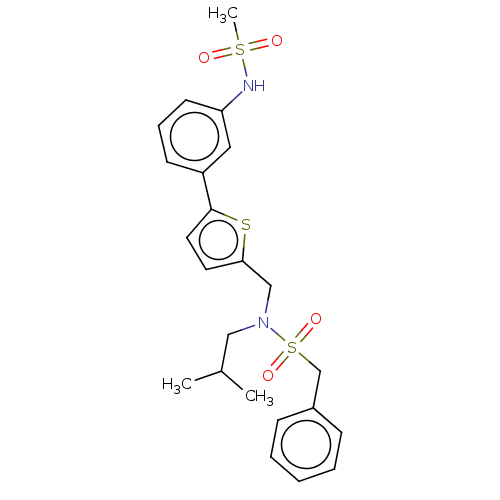
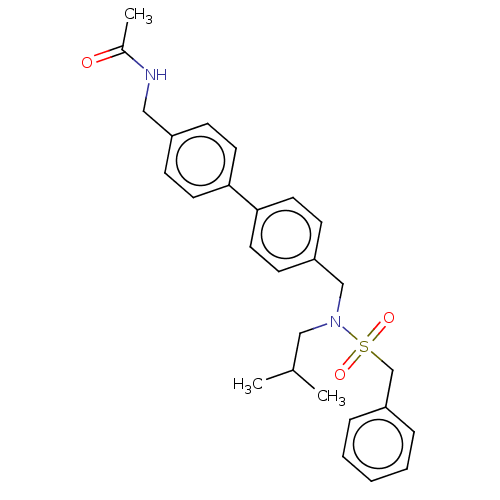
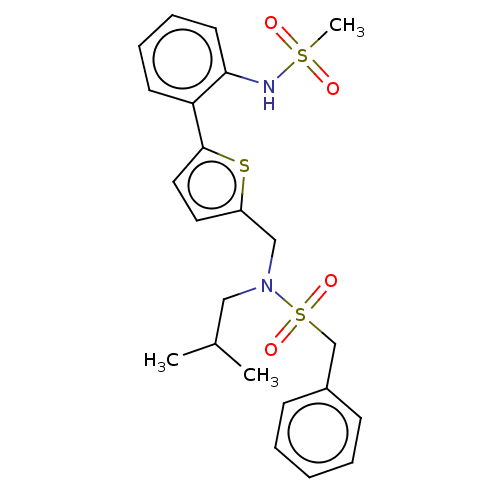
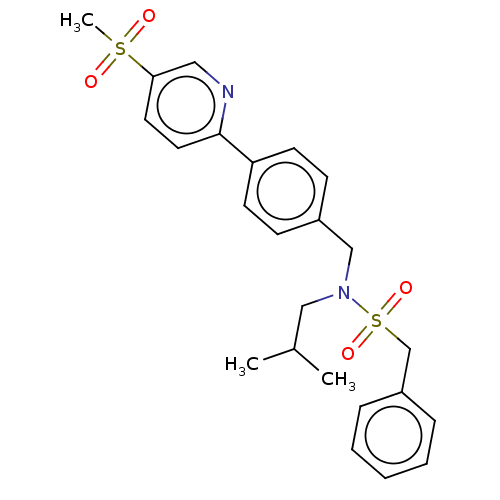
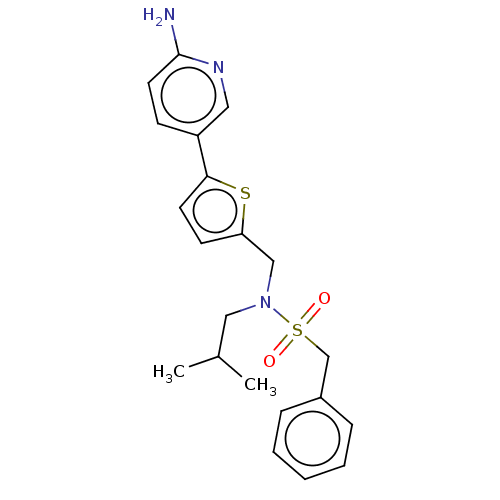
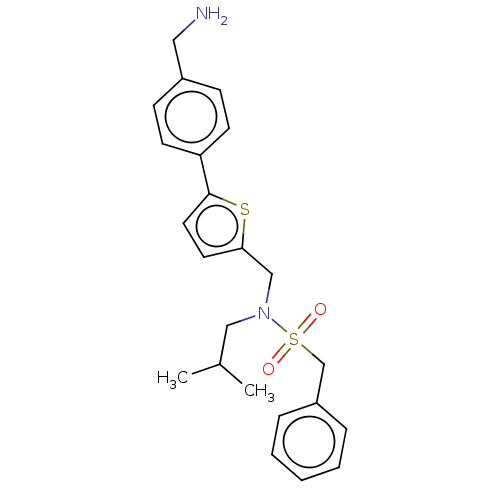
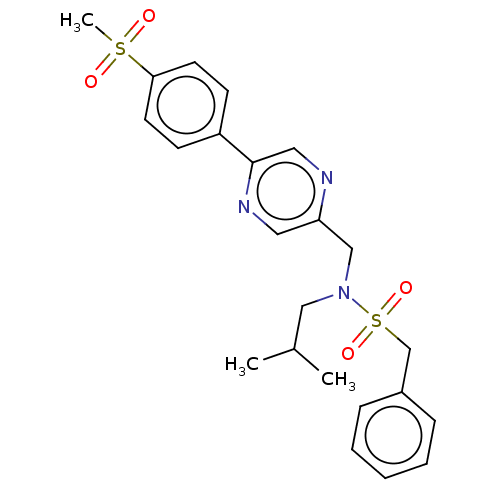
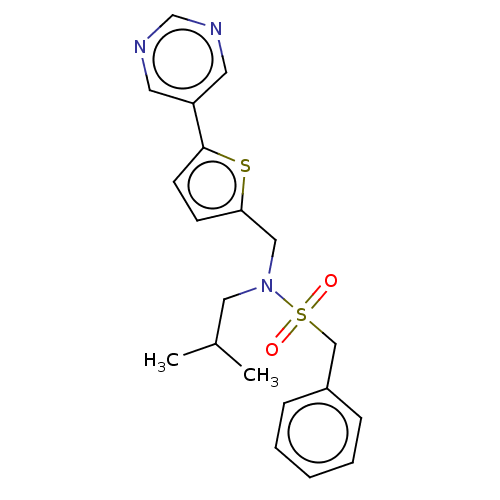
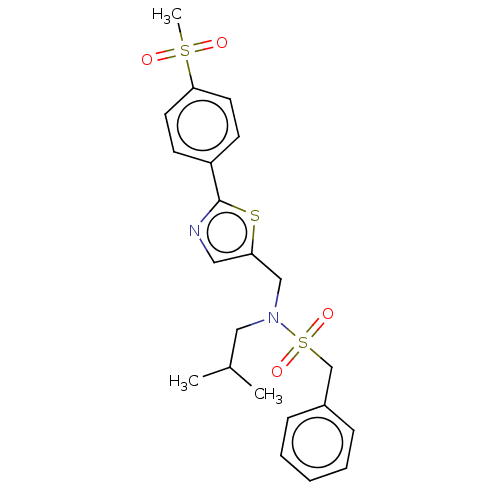
Affinity DataIC50: 32nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 71nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 91nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 370nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:Displacement of [3H]T0901317 from LXRbeta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 610nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 650nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 920nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H2]25-hydroxycholesterol from human RORc-LBD after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+4nMAssay Description:Displacement of [3H]T0901317 from LXRalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inverse agonist activity at human GAL4-fused RORb expressed in HEK293 cells after 5 hrs by luciferase reporter gene based transcriptional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 4.20E+3nMAssay Description:Inverse agonist activity at human GAL4-fused RORa expressed in HEK293 cells after 5 hrs by luciferase reporter gene based transcriptional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.80E+3nMAssay Description:Inverse agonist activity at human GAL4-fused RORa expressed in HEK293 cells after 5 hrs by luciferase reporter gene based transcriptional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.40E+3nMAssay Description:Inverse agonist activity at human GAL4-fused RORa expressed in HEK293 cells after 5 hrs by luciferase reporter gene based transcriptional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.80E+3nMAssay Description:Inverse agonist activity at human GAL4-fused RORa expressed in HEK293 cells after 5 hrs by luciferase reporter gene based transcriptional assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inverse agonist activity at human GAL4-fused RORa expressed in HEK293 cells after 5 hrs by luciferase reporter gene based transcriptional assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.70E+3nMAssay Description:Inverse agonist activity at human GAL4-fused LXRalpha expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity a...More data for this Ligand-Target Pair
Affinity DataEC50: 3.70E+3nMAssay Description:Inverse agonist activity at human GAL4-fused LXRalpha expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity a...More data for this Ligand-Target Pair
Affinity DataEC50: 2.30E+3nMAssay Description:Inverse agonist activity at human GAL4-fused LXRalpha expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity a...More data for this Ligand-Target Pair
Affinity DataEC50: 3.60E+3nMAssay Description:Inverse agonist activity at human GAL4-fused LXRalpha expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity a...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inverse agonist activity at human GAL4-fused LXRalpha expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity a...More data for this Ligand-Target Pair
Affinity DataEC50: 1.90E+3nMAssay Description:Inverse agonist activity at human GAL4-fused LXRbeta expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity af...More data for this Ligand-Target Pair
Affinity DataEC50: 3.90E+3nMAssay Description:Inverse agonist activity at human GAL4-fused LXRbeta expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity af...More data for this Ligand-Target Pair
Affinity DataEC50: 2.50E+3nMAssay Description:Inverse agonist activity at human GAL4-fused LXRbeta expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity af...More data for this Ligand-Target Pair
Affinity DataEC50: 3.80E+3nMAssay Description:Inverse agonist activity at human GAL4-fused LXRbeta expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity af...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inverse agonist activity at human GAL4-fused LXRbeta expressed in HEK293 cells assessed as inhibition of T0901317-induced transcriptional activity af...More data for this Ligand-Target Pair
Affinity DataEC50: 360nMAssay Description:Inverse agonist activity at an N-terminal 6xHis-GST-tag human RORc-LBD assessed as inhibition of recruitment of the SRC1 co-activator peptide after 3...More data for this Ligand-Target Pair
Affinity DataEC50: 460nMAssay Description:Inverse agonist activity at an N-terminal 6xHis-GST-tag human RORc-LBD assessed as inhibition of recruitment of the SRC1 co-activator peptide after 3...More data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+3nMAssay Description:Inverse agonist activity at an N-terminal 6xHis-GST-tag human RORc-LBD assessed as inhibition of recruitment of the SRC1 co-activator peptide after 3...More data for this Ligand-Target Pair
Affinity DataEC50: 4.90E+3nMAssay Description:Inverse agonist activity at an N-terminal 6xHis-GST-tag human RORc-LBD assessed as inhibition of recruitment of the SRC1 co-activator peptide after 3...More data for this Ligand-Target Pair
Affinity DataEC50: 150nMAssay Description:Inverse agonist activity at an N-terminal 6xHis-GST-tag human RORc-LBD assessed as inhibition of recruitment of the SRC1 co-activator peptide after 3...More data for this Ligand-Target Pair
Affinity DataEC50: 130nMAssay Description:Inverse agonist activity at an N-terminal 6xHis-GST-tag human RORc-LBD assessed as inhibition of recruitment of the SRC1 co-activator peptide after 3...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inverse agonist activity at an N-terminal 6xHis-GST-tag human RORc-LBD assessed as inhibition of recruitment of the SRC1 co-activator peptide after 3...More data for this Ligand-Target Pair

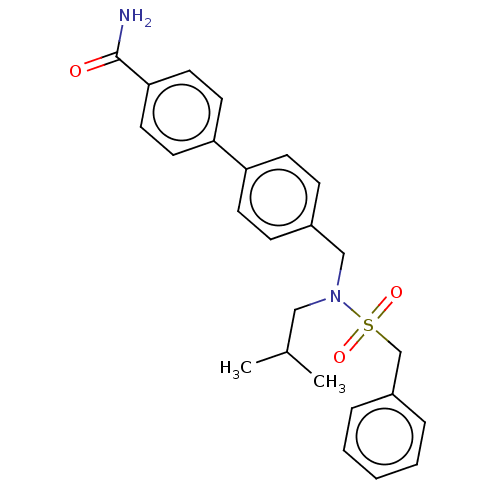
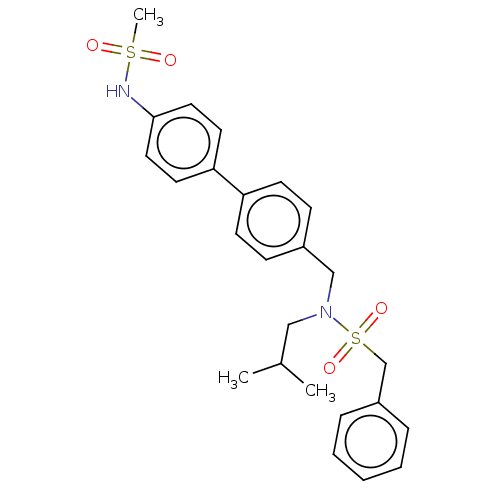

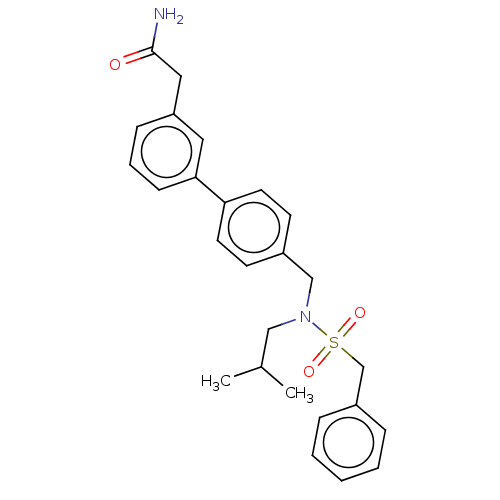
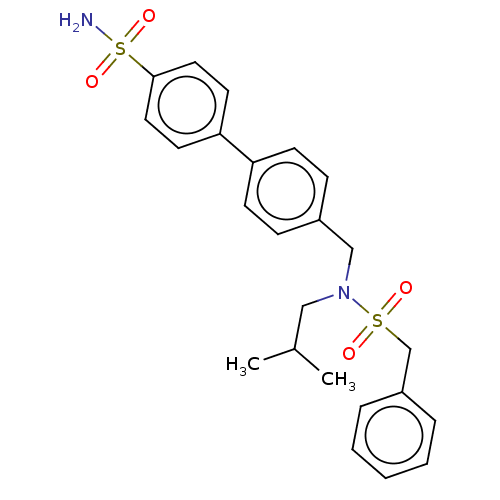
 3D Structure (crystal)
3D Structure (crystal)