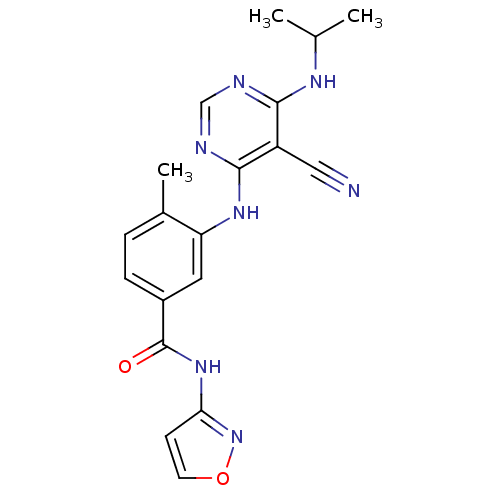
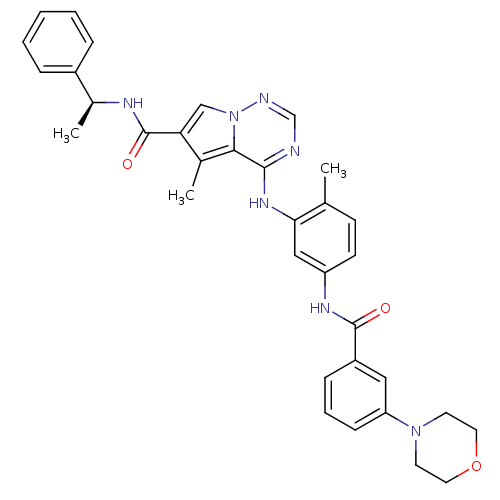
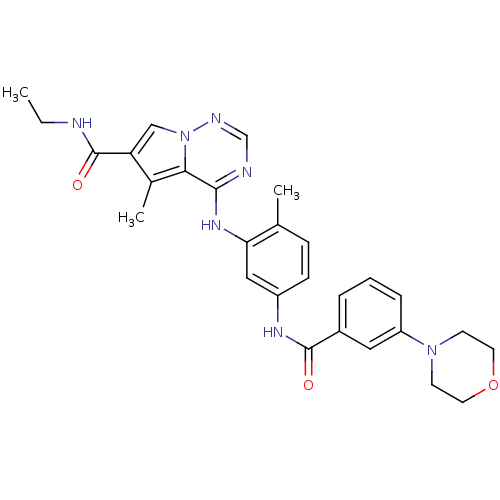
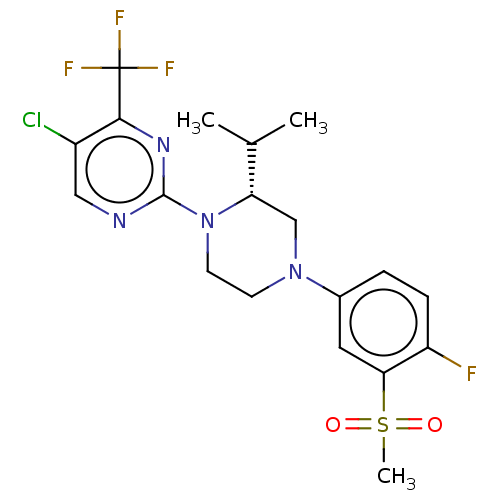
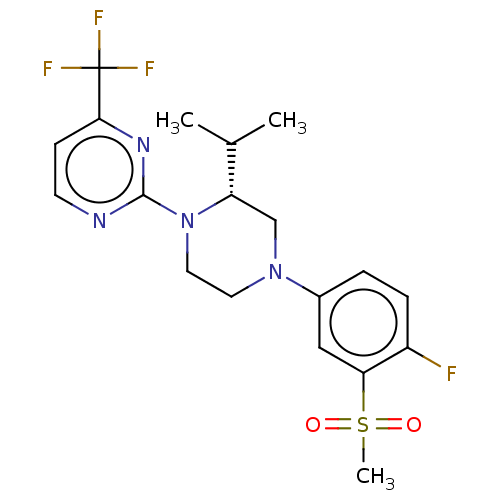
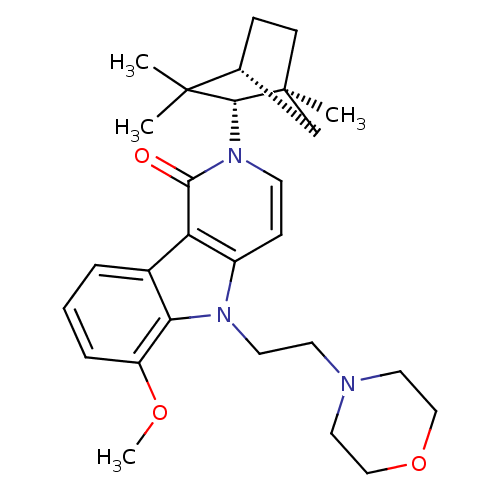
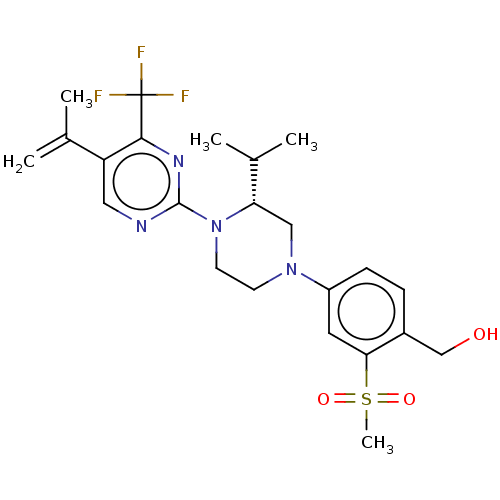
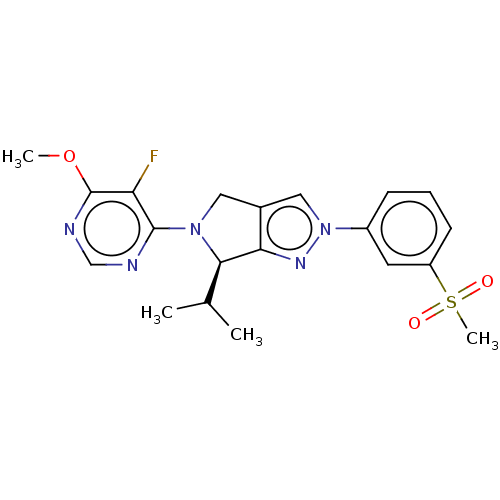
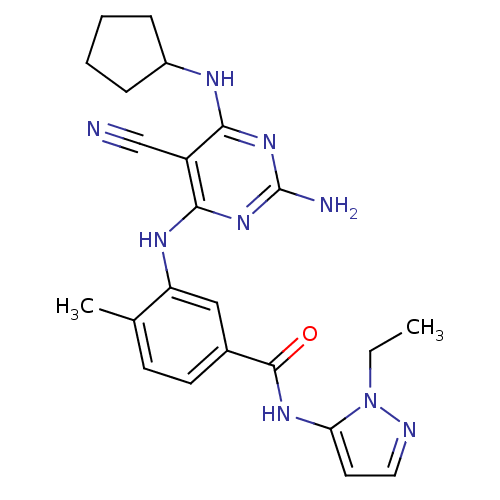
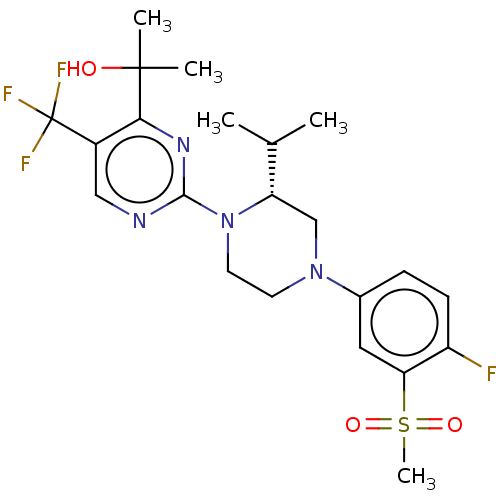
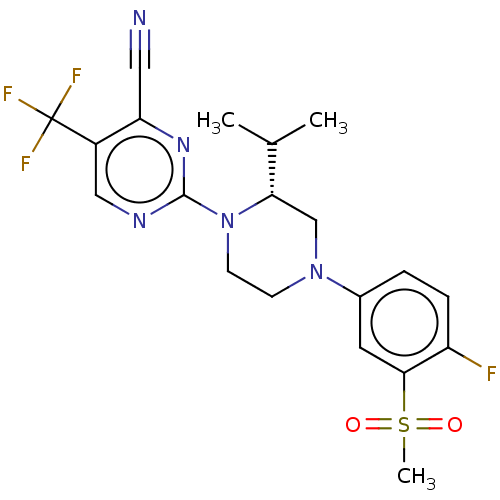
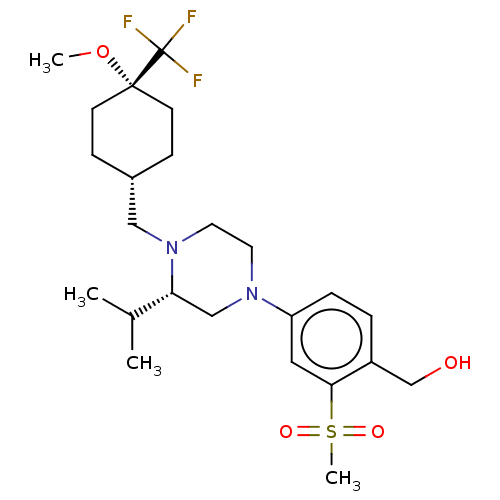
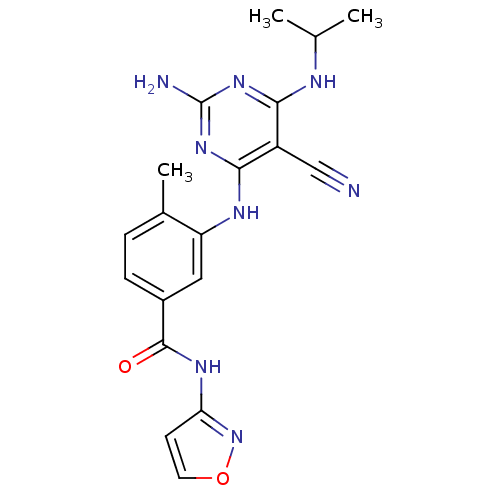
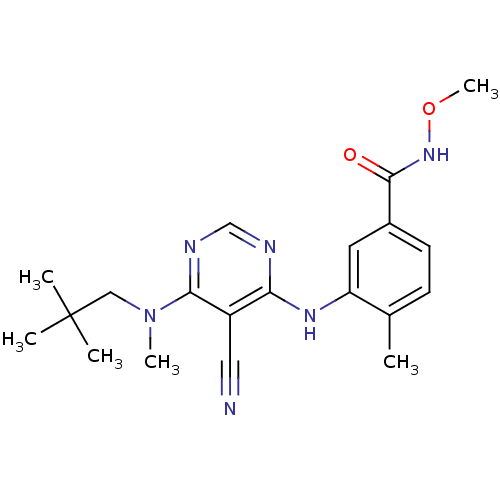
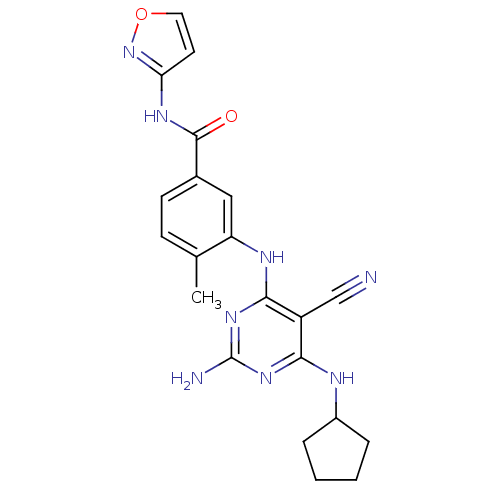
Affinity DataKi: 0.0470nM ΔG°: -58.4kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
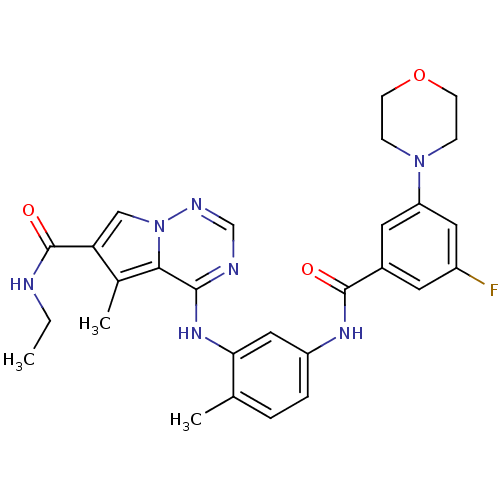
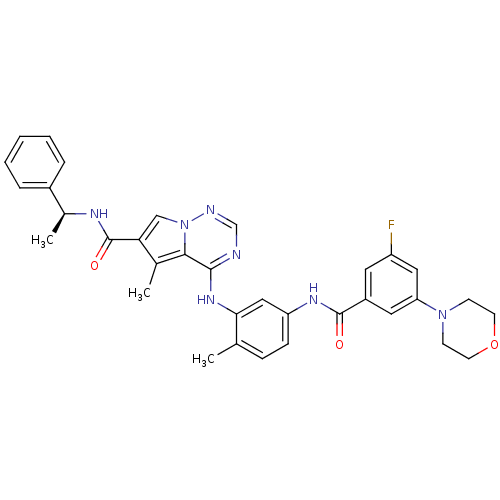
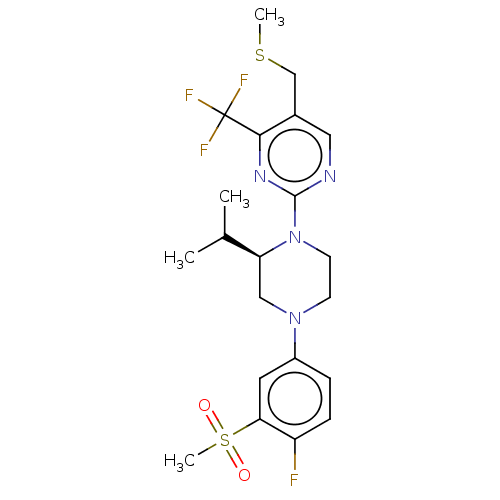
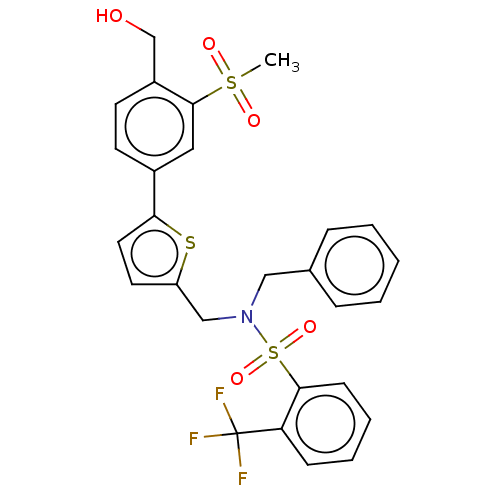
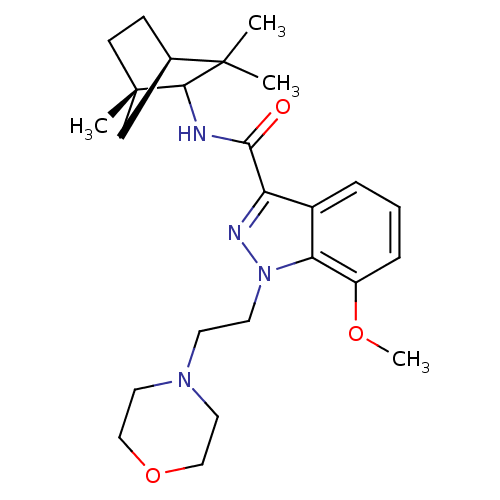
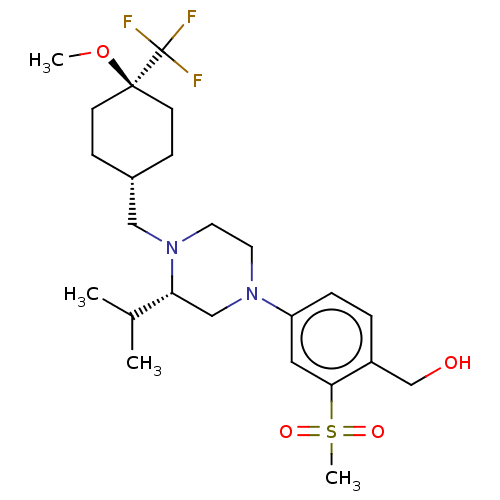
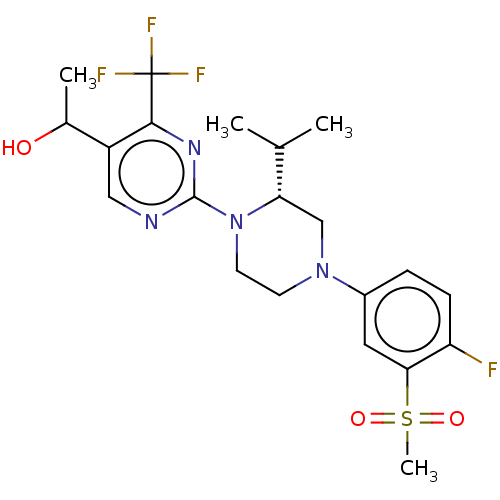
Affinity DataKi: 0.0500nM ΔG°: -58.2kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
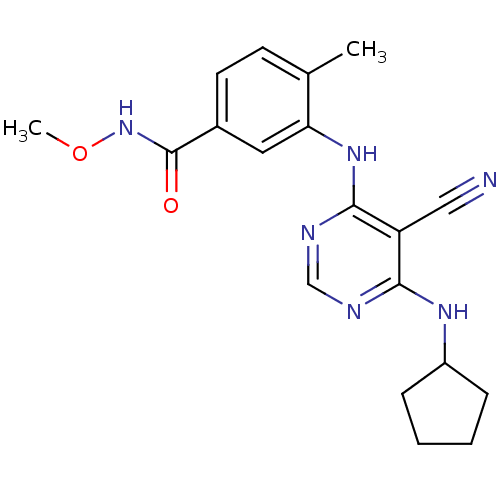
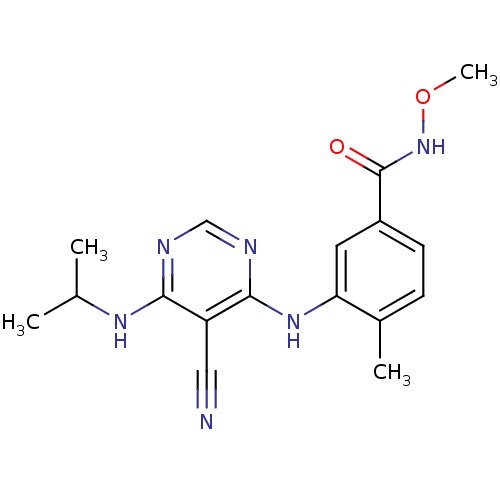
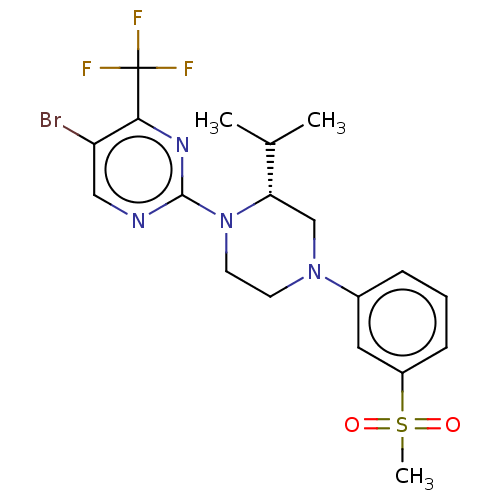
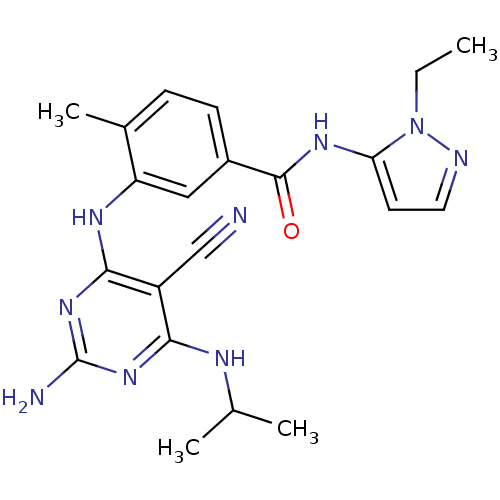
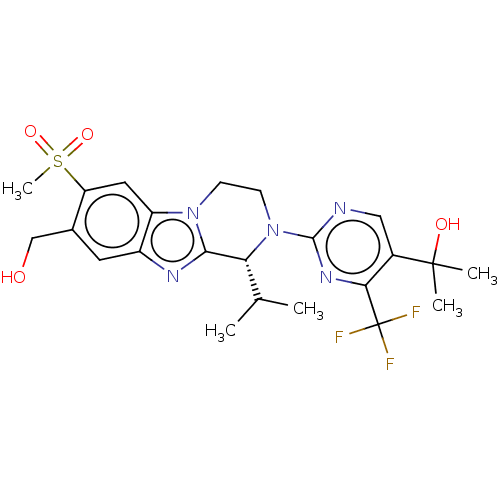
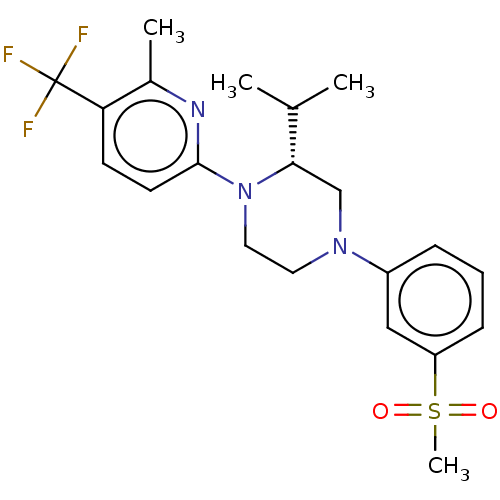
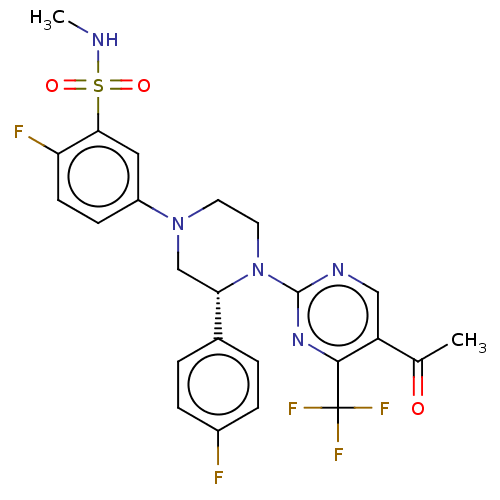
Affinity DataKi: 0.0570nM ΔG°: -57.9kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
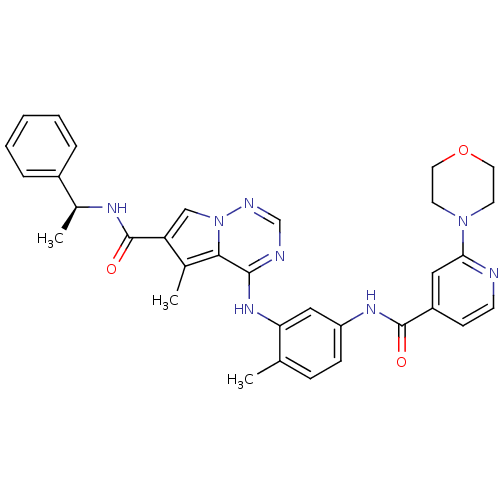
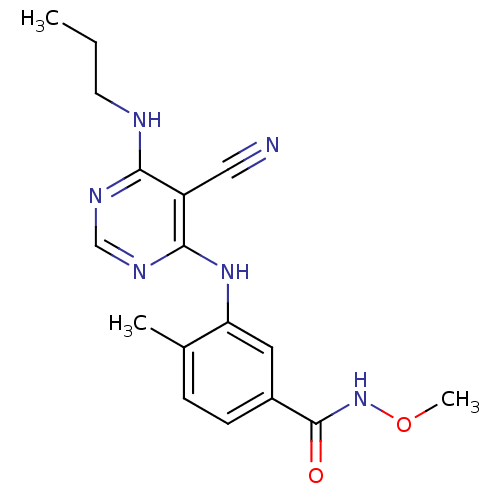
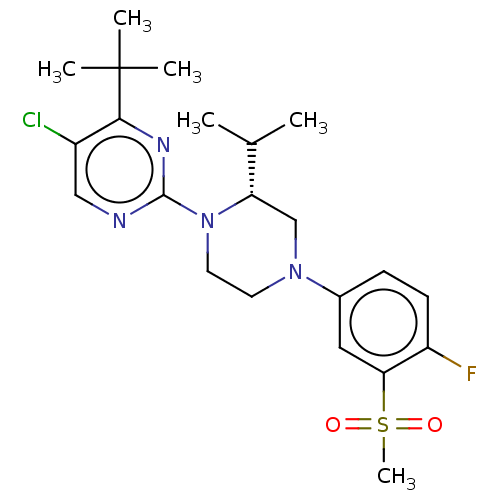
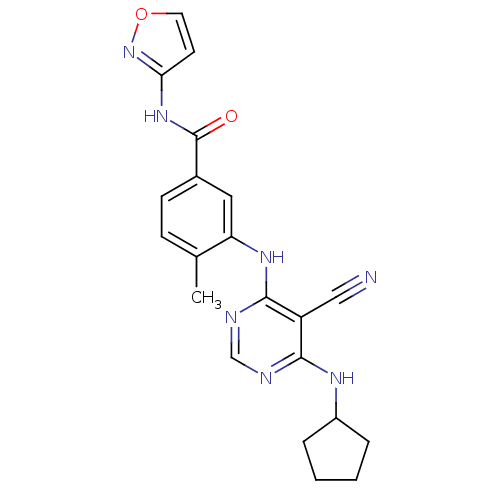
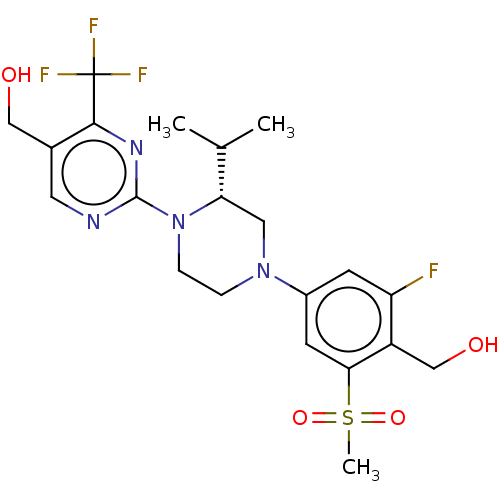
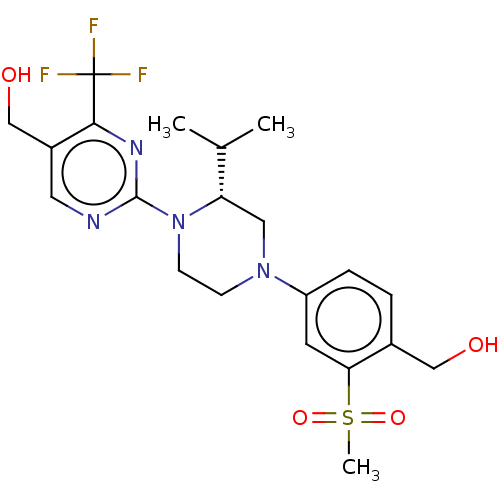
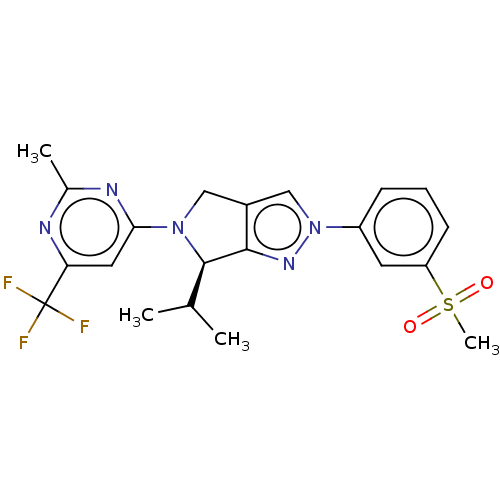
Affinity DataKi: 0.0570nM ΔG°: -57.9kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nM ΔG°: -55.5kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nM ΔG°: -55.3kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
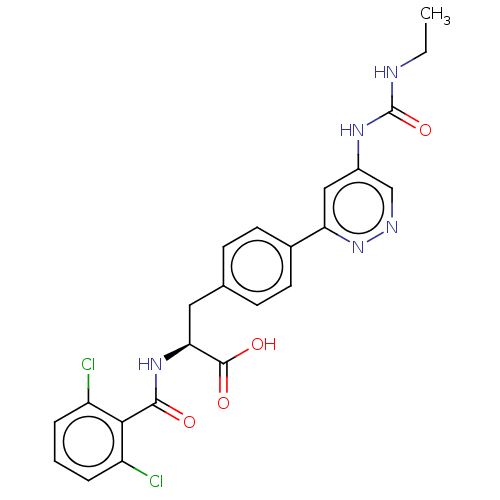
Affinity DataKi: 0.166nMAssay Description:Displacement of [3H] C8 from human integrin alphavbeta1 incubated for 6 hrs by competition binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylationMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylationMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinity of compound was determined against to human cannabinoid receptor 2 in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.410nM ΔG°: -53.0kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Inhibition of p38alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.420nM ΔG°: -53.0kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Inhibition of p38alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.460nMAssay Description:Inhibition of p38alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.540nMAssay Description:Inhibition of p38alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.610nM ΔG°: -52.1kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 0.970nM ΔG°: -50.9kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 0.980nMAssay Description:Inhibition of p38alphaMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H]More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nM ΔG°: -49.9kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nM ΔG°: -49.7kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Binding affinity of compound was determined against to human cannabinoid receptor 1 in chinese hamster ovary cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nM ΔG°: -49.3kJ/molepH: 7.4 T: 2°CAssay Description:The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity against human cannabinoid receptor 2 in chinese hamster ovary cells using WIN-55212-2 mesylate[57-3H]More data for this Ligand-Target Pair
Affinity DataKi: 2nM ΔG°: -49.7kJ/moleT: 2°CAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair

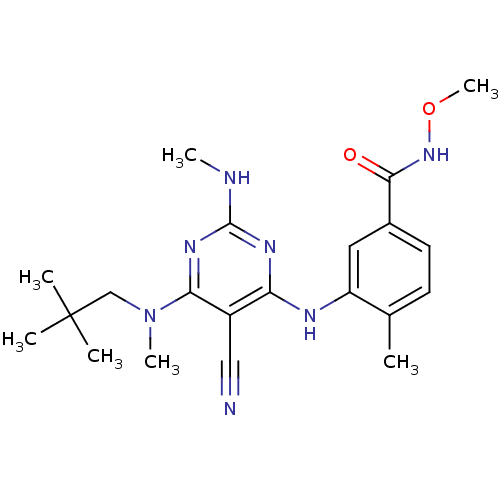
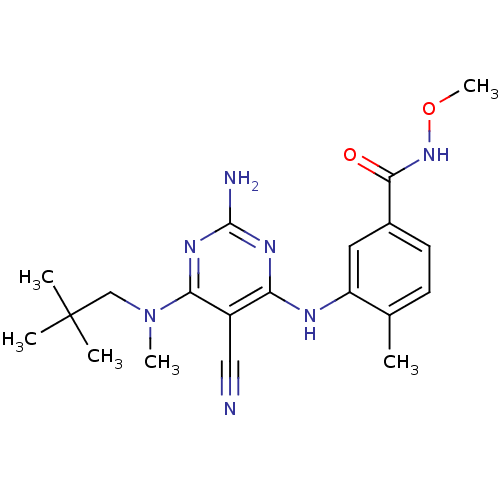
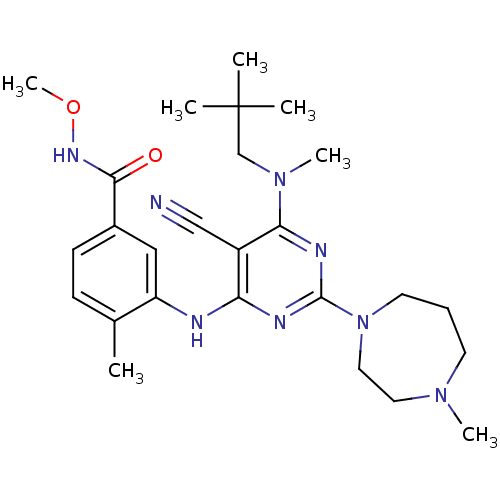
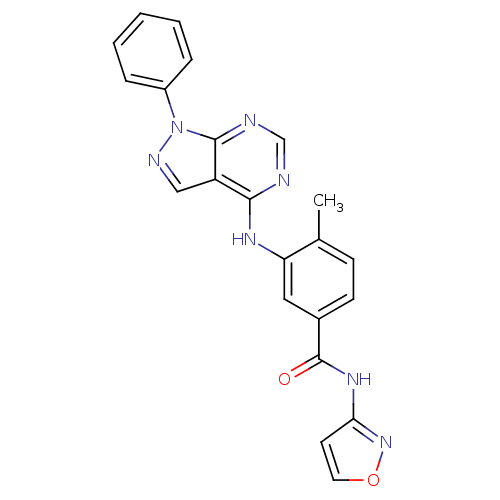
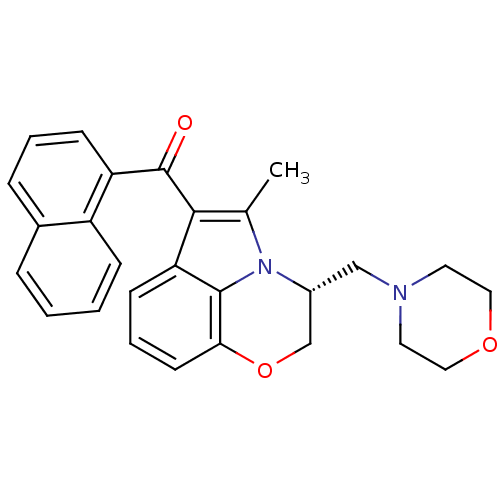
 3D Structure (crystal)
3D Structure (crystal)