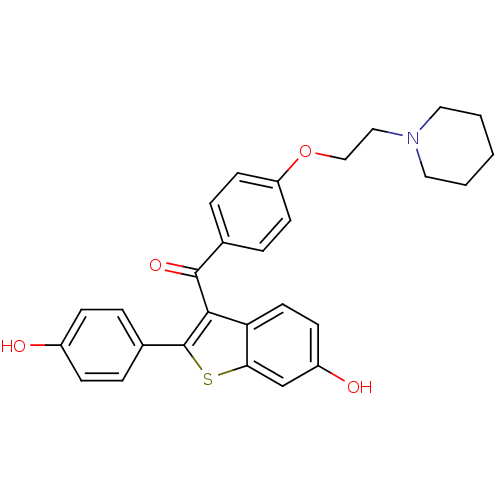
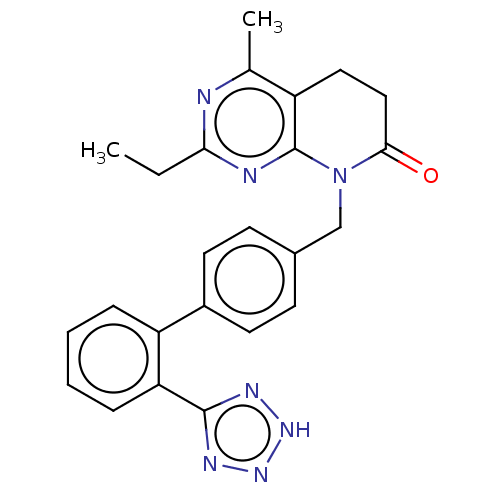
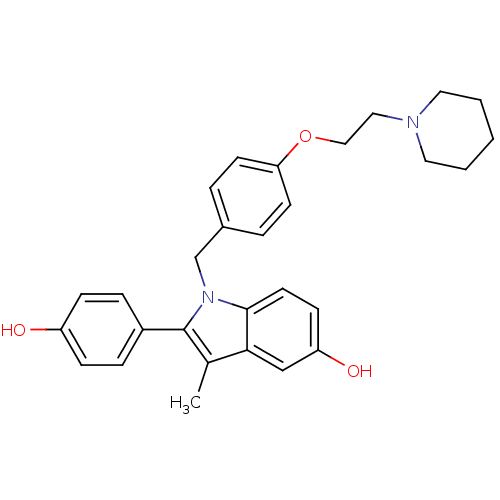
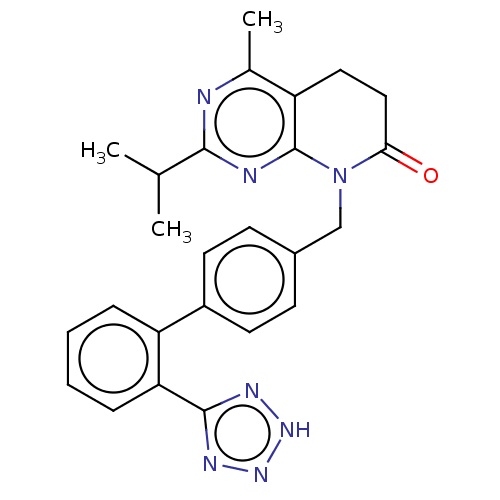
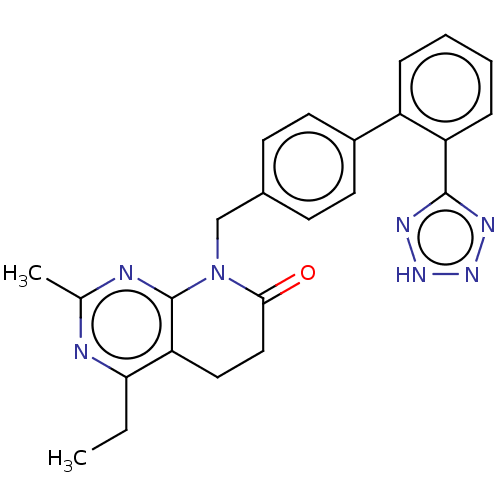
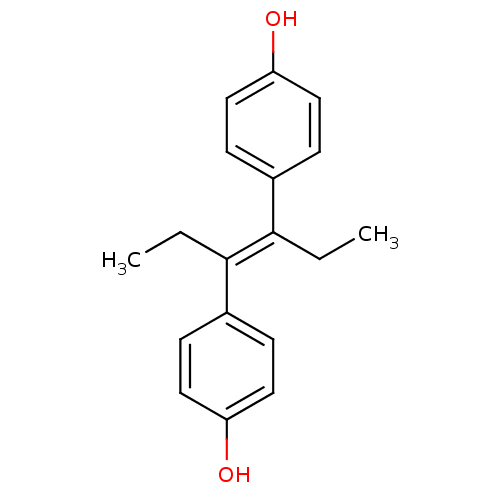
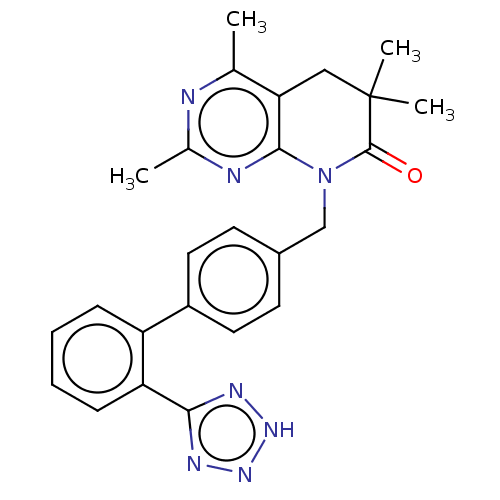
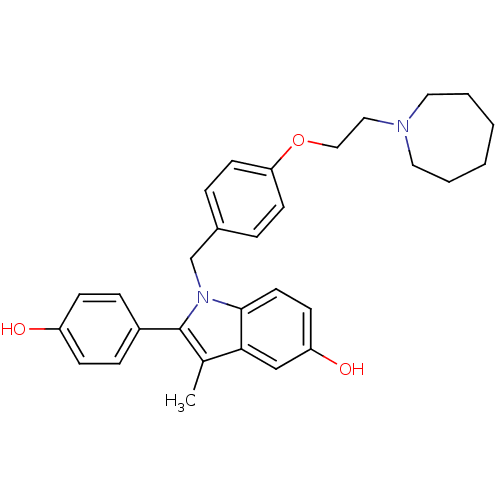
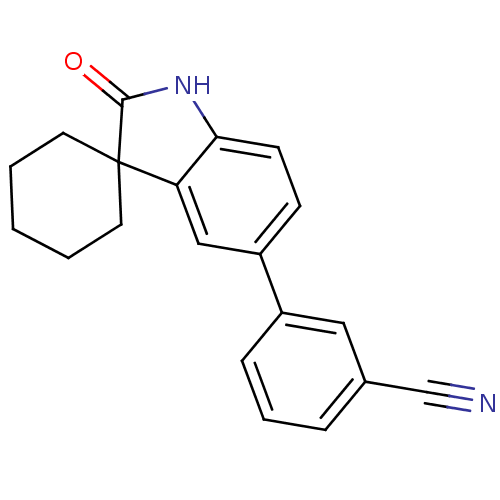
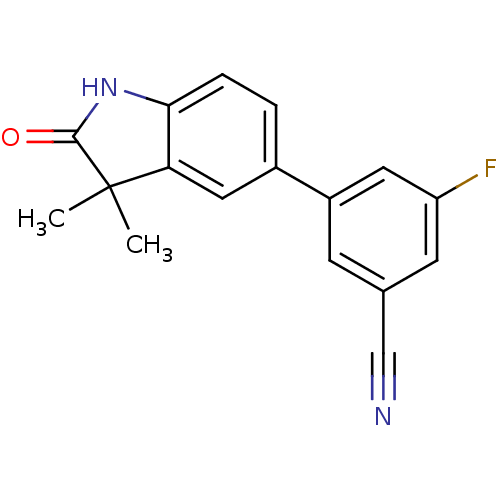
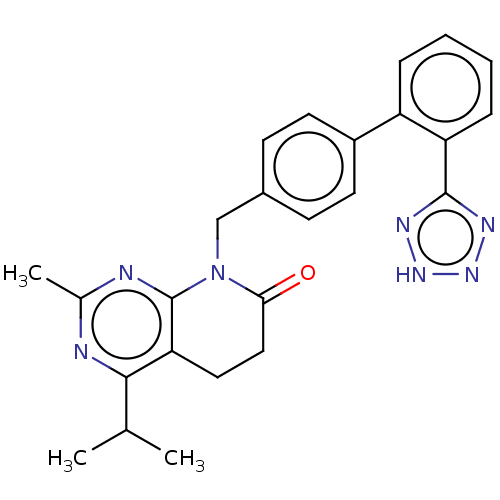
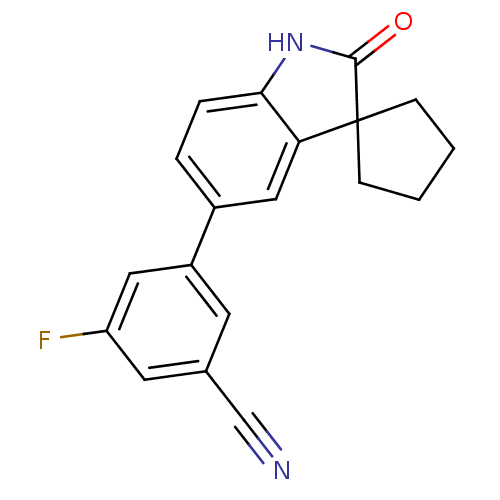
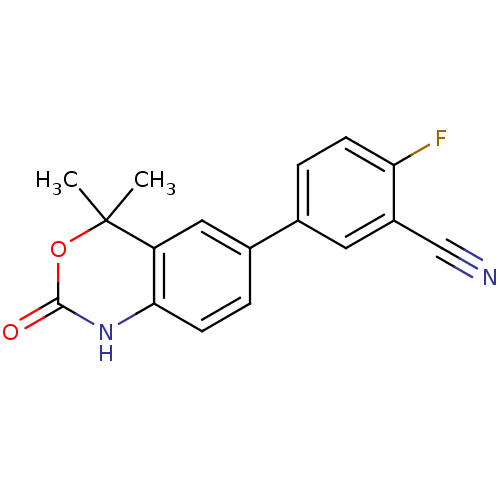
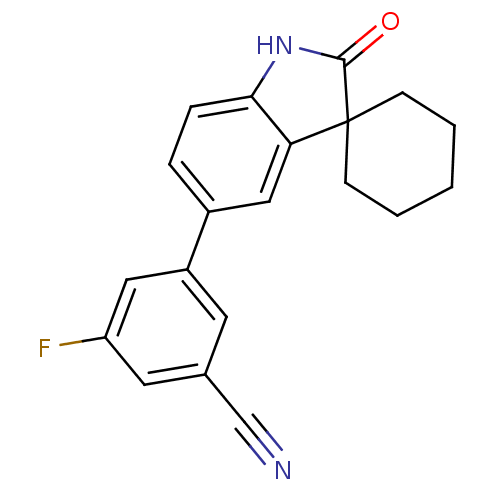
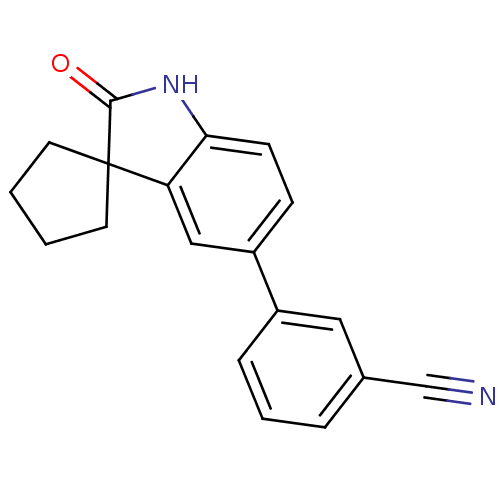
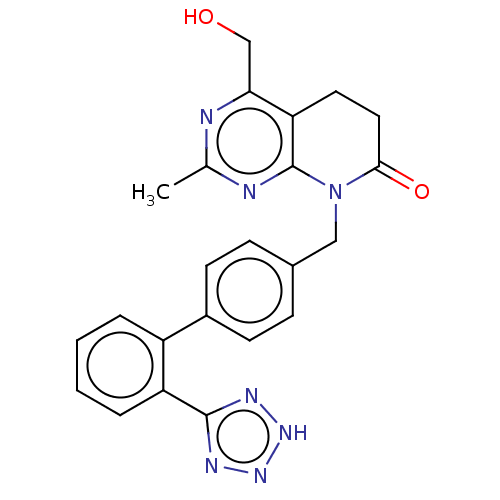
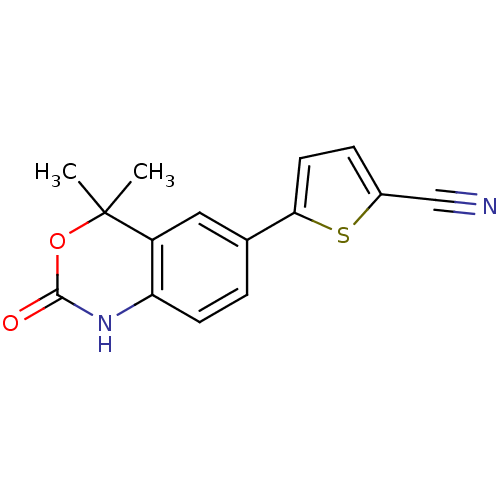
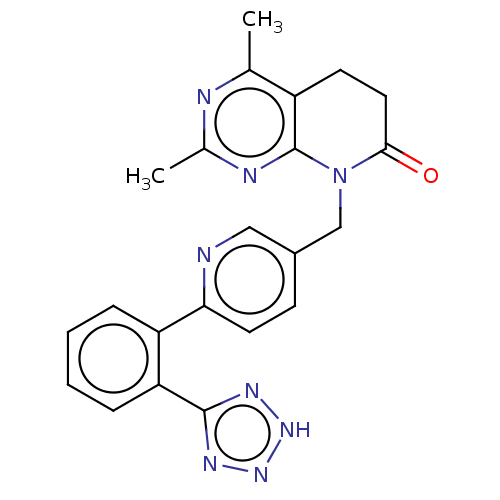
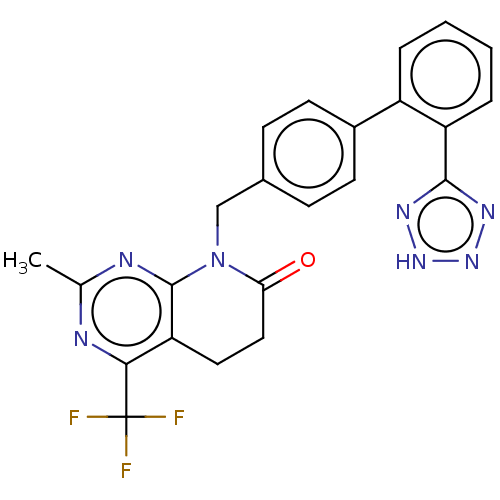
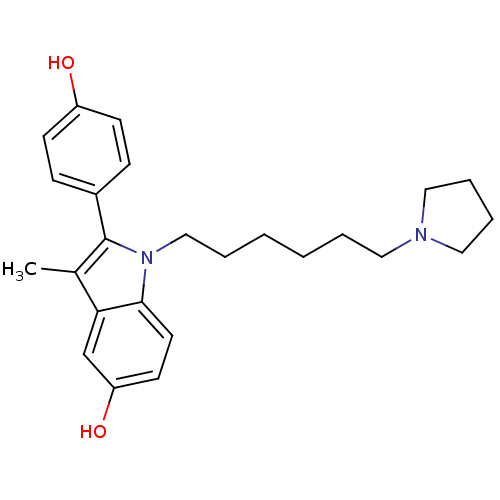
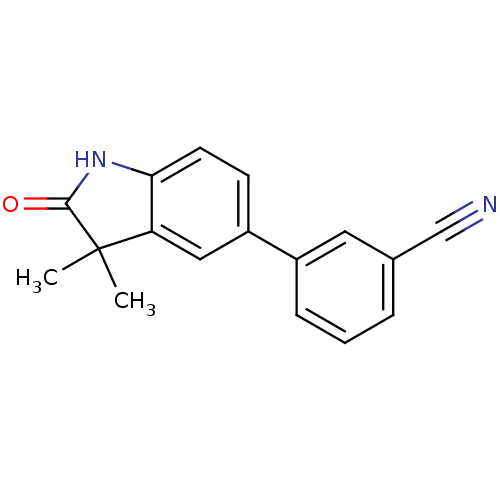
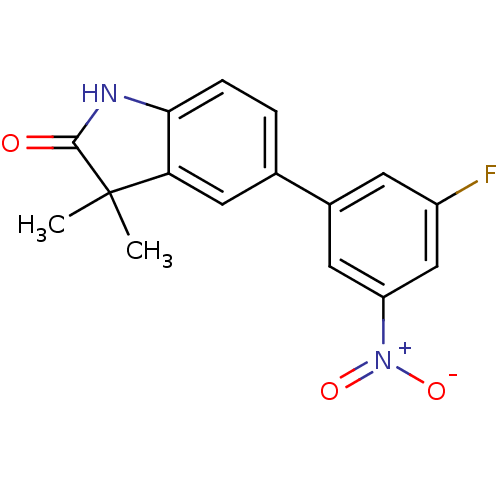
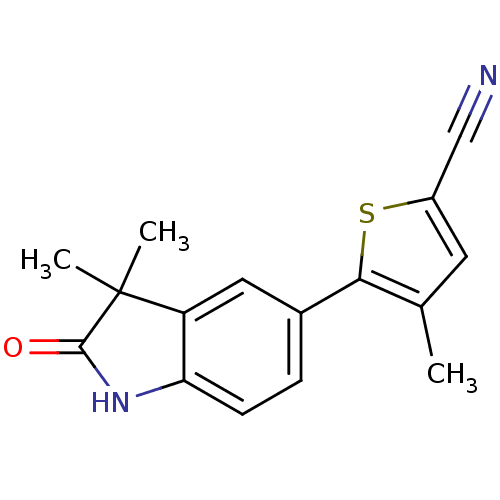
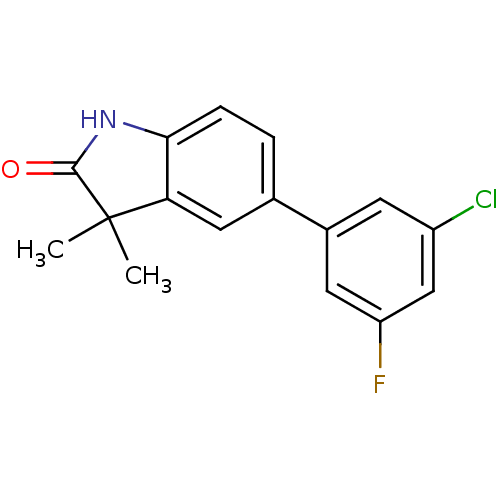
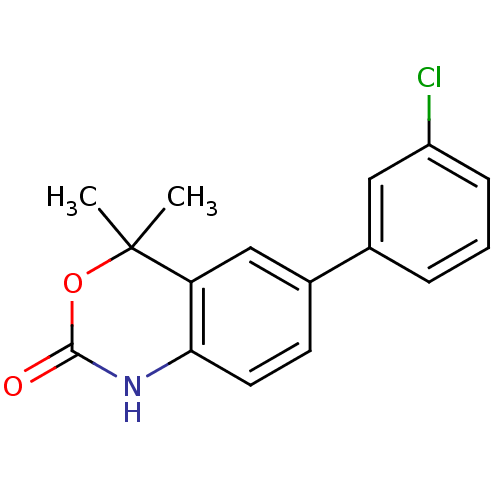
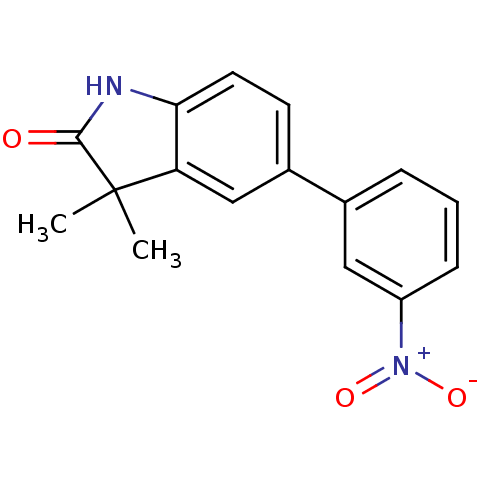
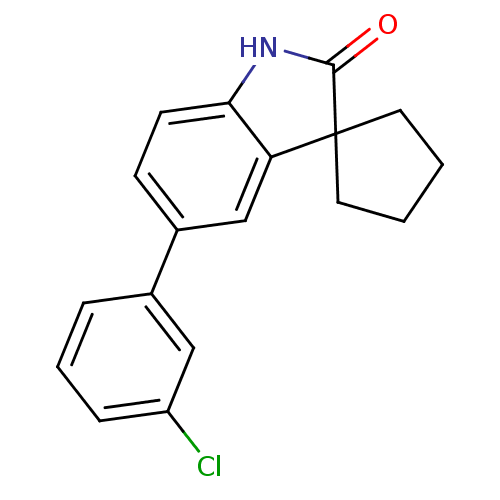
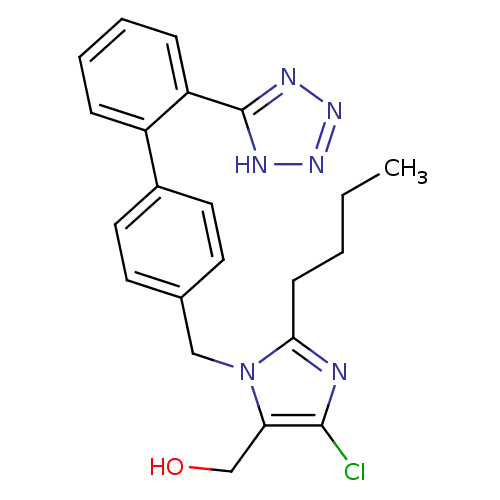
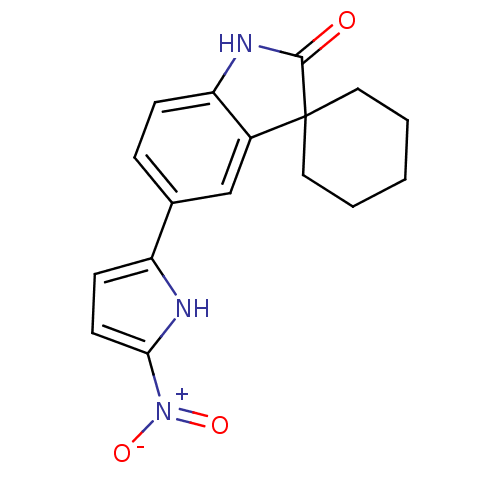
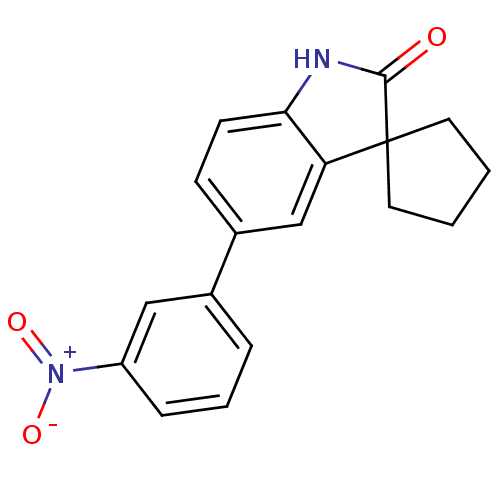
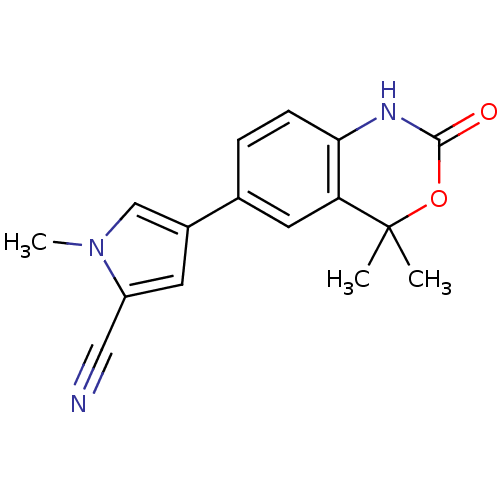
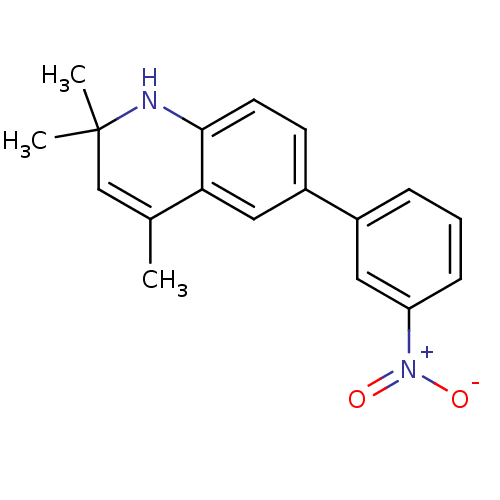
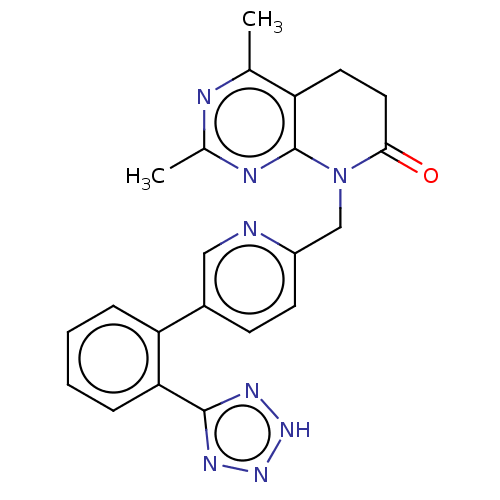
Affinity DataIC50: 0.100nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.720nMAssay Description:In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiolMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiolMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of [3H]-17-beta-estradiol binding to human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiolMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In vitro inhibition of [3H]-17-beta-estradiol binding to human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In vitro inhibitory concentration against [3H]-17-beta-estradiol binding to human estrogen receptor 2More data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 6.60nMAssay Description:Ability to block progesterone induced alkaline phosphatase activity in T47D human breast cancer cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Ability to block progesterone induced alkaline phosphatase activity in T47D human breast cancer cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:In vitro inhibition of [3H]-17-beta-estradiol binding to human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:In vitro inhibition of [3H]-17-beta-estradiol binding to human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Ability to block progesterone induced alkaline phosphatase activity in T47D human breast cancer cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:In vitro inhibition of [3H]-17-beta-estradiol binding to human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Ability to block progesterone induced alkaline phosphatase activity in T47D human breast cancer cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:In vitro inhibitory concentration against [3H]-17-beta-estradiol binding to human estrogen receptor 2More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Ability to block progesterone induced alkaline phosphatase activity in T47D human breast cancer cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:In vitro inhibitory concentration against [3H]-17-beta-estradiol binding to human estrogen receptor 2More data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:In vitro inhibition of specific binding of [125 I] A II to rat liver membrane.More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)