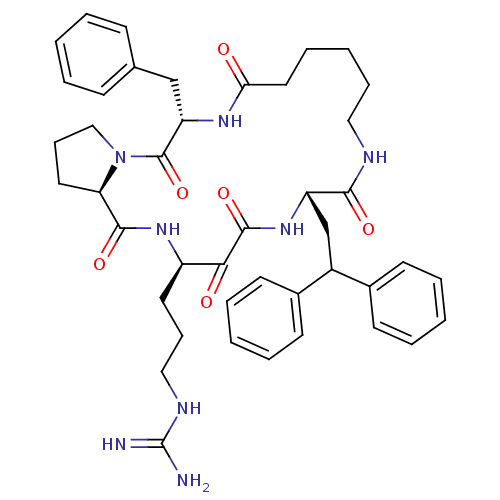
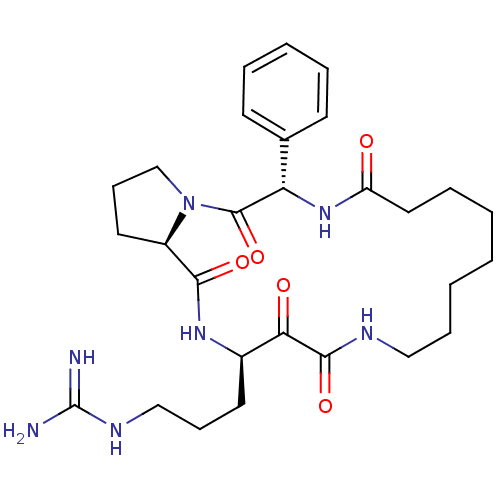
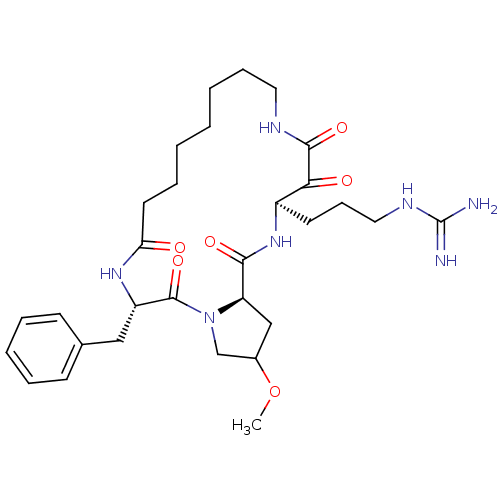
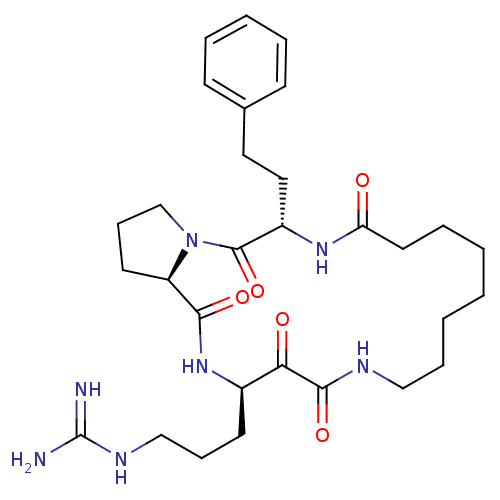
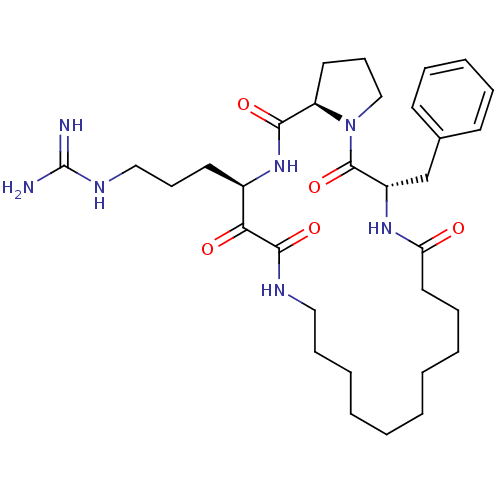
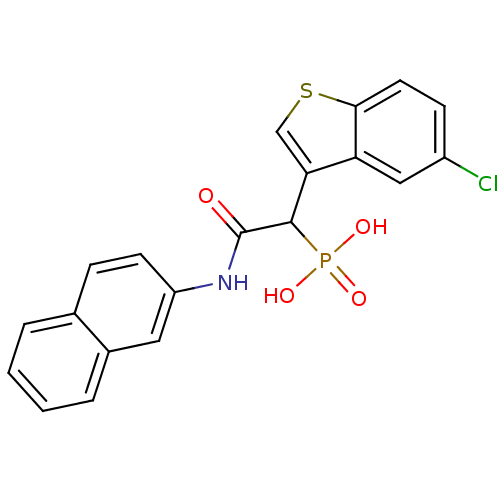
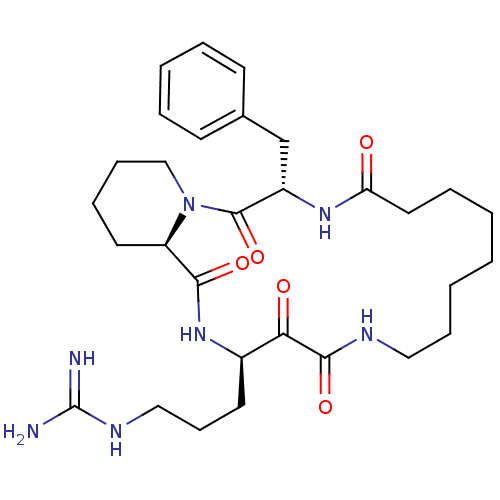
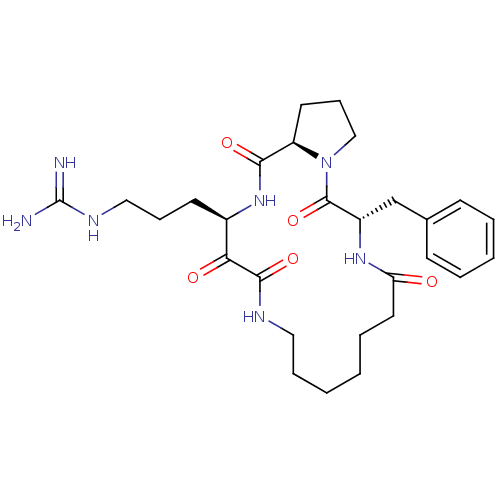
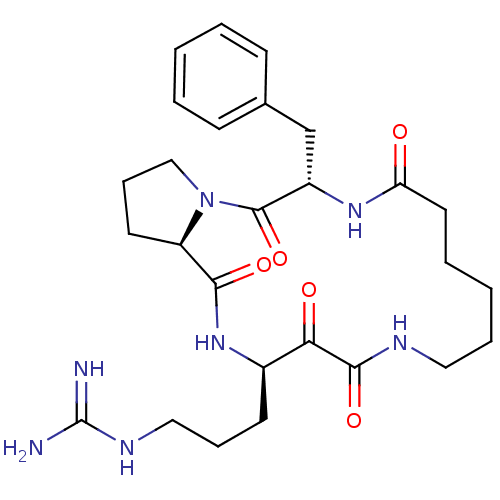
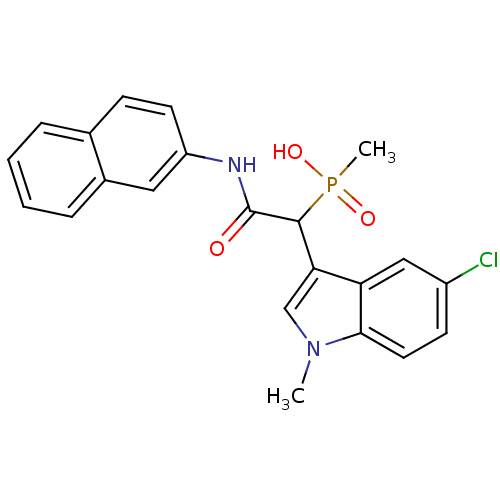
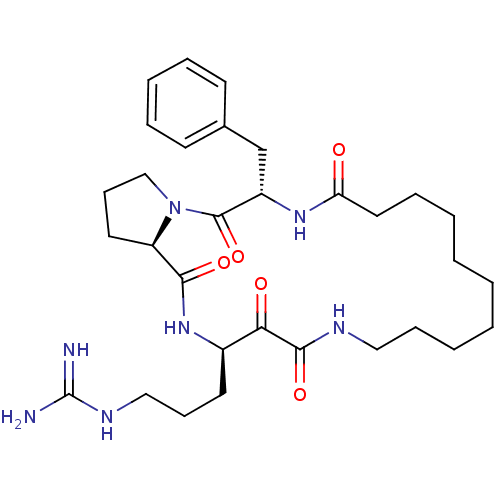
Affinity DataKi: 1nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 4.10nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 5.30nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
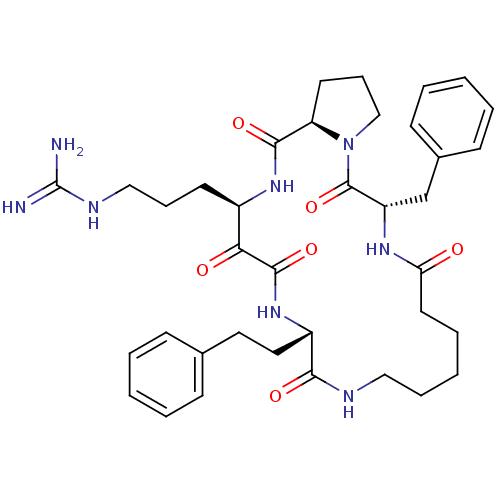
Affinity DataKi: 11nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
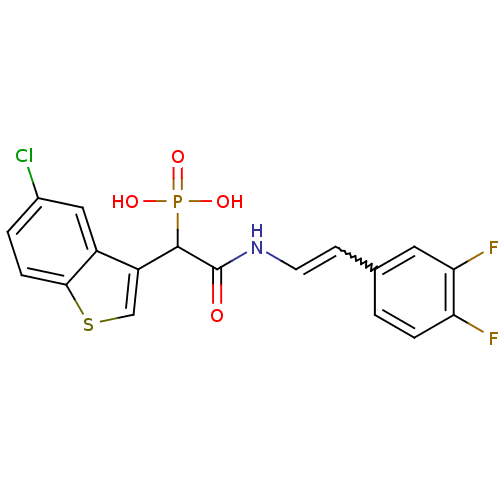
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 36nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
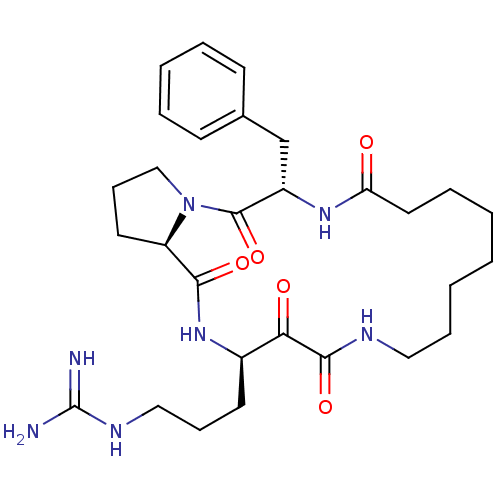
Affinity DataKi: 38nMAssay Description:Inhibition of human neutrophil Cat GMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 86nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 159nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 163nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 280nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 473nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 710nMAssay Description:Inhibition of bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human thrombinMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 9.50E+3nMAssay Description:Inhibition of human neutrophil Cat GMore data for this Ligand-Target Pair
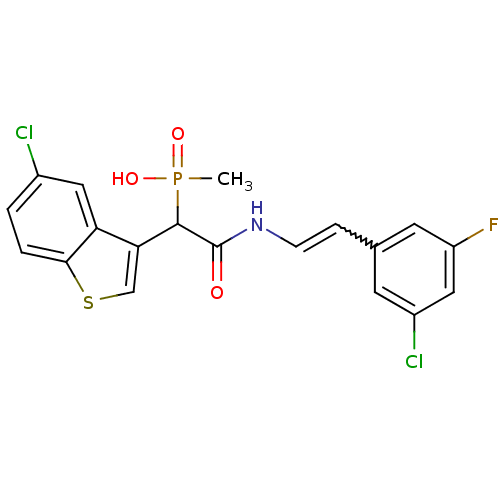
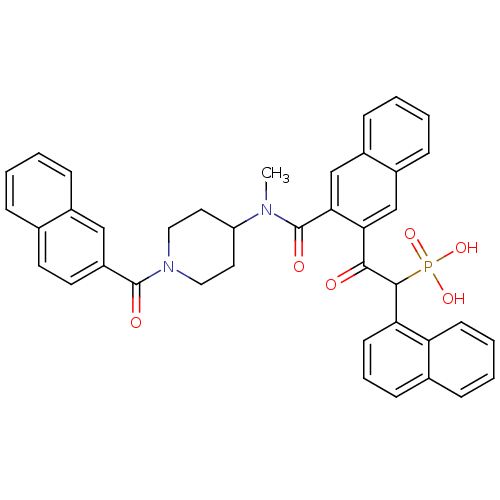
Affinity DataIC50: 0.800nMAssay Description:The reaction procedure was conducted as follows:1) Prepare substrate in freshly prepared Reaction Buffer.2) MnCl2 (2 mM) was added as a co-factor lis...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Binding affinity towards V2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO-K1 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]-rimonabant from human CB1 receptor expressed in CHO cell membranes after 60 mins by TopCount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:The reaction procedure was conducted as follows:1) Prepare substrate in freshly prepared Reaction Buffer.2) MnCl2 (2 mM) was added as a co-factor lis...More data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:The reaction procedure was conducted as follows:1) Prepare substrate in freshly prepared Reaction Buffer.2) MnCl2 (2 mM) was added as a co-factor lis...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO-K1 cell membranesMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Displacement of [3H]-rimonabant from human CB1 receptor expressed in CHO cell membranes after 60 mins by TopCount methodMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of [3H]-AVP from human V2 receptors expressed in HEK-293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:Displacement of [3H]-CP-55940 from human CB1 receptor expressed in CHO-K1 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 7.30nMAssay Description:The reaction procedure was conducted as follows:1) Prepare substrate in freshly prepared Reaction Buffer.2) MnCl2 (2 mM) was added as a co-factor lis...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMAssay Description:The reaction procedure was conducted as follows:1) Prepare substrate in freshly prepared Reaction Buffer.2) MnCl2 (2 mM) was added as a co-factor lis...More data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Displacement of [3H]-AVP from human V2 receptors expressed in HEK-293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:The reaction procedure was conducted as follows:1) Prepare substrate in freshly prepared Reaction Buffer.2) MnCl2 (2 mM) was added as a co-factor lis...More data for this Ligand-Target Pair
Affinity DataIC50: 9.5nMAssay Description:The reaction procedure was conducted as follows:1) Prepare substrate in freshly prepared Reaction Buffer.2) MnCl2 (2 mM) was added as a co-factor lis...More data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Displacement of [3H]-AVP from human V2 receptors expressed in HEK-293 cellsMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The reaction procedure was conducted as follows:1) Prepare substrate in freshly prepared Reaction Buffer.2) MnCl2 (2 mM) was added as a co-factor lis...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)