- sphingosine 1-phosphate receptor
- sphingosine 1-phosphate receptor 1
- sphingosine-1-phosphate receptor 1
- sphingosine 1-phosphate receptor 2
- sphingosine 1-phosphate receptor 3
- sphingosine 1-phosphate receptor 4
- sphingosine 1-phosphate receptor 5
- sphingosine 1-phosphate receptor gpr6
- sphingosine 1-phosphate receptor 1 (s1p1)
- s1p receptor edg-1
- s1p
- s1p receptor
- decaprenyl-phosphate glcnac-1-phosphate transferase
- udp-glcnac:undecaprenyl-phosphate glcnac-1-phosphate transferase
- sphingosine kinase 2 (sphk2)
- glucose-1-phosphate thymidylyltransferase
- 1-deoxyxylulose-5-phosphate reductoisomerase
- glucose-6-phosphate 1-dehydrogenase
- n-acetylglucosamine-1-phosphate uridyltransferase
- renal sodium-phosphate transport protein 1
- 1-deoxy-d-xylulose 5-phosphate reductoisomerase
- 1-deoxy-d-xylulose-5-phosphate synthase
- glutamine--fructose-6-phosphate aminotransferase [isomerizing] 1
- 1-deoxy-d-xylulose 5-phosphate reductoisomerase, apicoplastic
- phosphatidylinositol 4-phosphate 5-kinase type-1 alpha
- (1->4)-alpha-d-glucan:maltose-1-phosphate alpha-d-maltosyltransferase
- arabinose phosphate isomerase
- dihydroxyacetone phosphate acyltransferase
- lipid-phosphate phosphatase
- orotidine phosphate decarboxylase
- carbamoyl-phosphate synthase (h353n)
- glucosamine-6-phosphate synthase
- glucose-5-phosphate transporter
- glucose-6-phosphate dehydrogenase
- glyceraldehyde-3-phosphate dehydrogenase
- mannose-6-phosphate isomerase
- orotidine 5'-phosphate decarboxylase
- orotidine 5-phosphate decarboxylase
- orotidine-5'-phosphate decarboxylase
- trehalose-6-phosphate phosphatase
- arabinose 5-phosphate isomerase kdsd
- carbamoyl-phosphate synthase [ammonia], mitochondrial
- glucose 6-phosphate dehydrogenase (g6pd)
- glutamine-dependent carbamoyl-phosphate synthase
- glyceraldehyde-3-phosphate dehydrogenase liver
- glyceraldehyde-3-phosphate dehydrogenase, cytosolic
- glyceraldehyde-3-phosphate dehydrogenase, glycosomal
- phosphate regulon sensor protein phor
- sodium-phosphate transport protein 2a
- sodium-phosphate transport protein 2b
- sodium-phosphate transport protein 2c
- 1-phosphatidylinositol 3-phosphate 5-kinase [1-2098,s696n,l932l,q995l,t998s,s1033a,q1183k]
- f420-dependent glucose-6-phosphate dehydrogenase
- glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase
- glutamine--fructose-6-phosphate aminotransferase [isomerizing]
- f420-dependent glucose-6-phosphate dehydrogenase [w44a]
- f420-dependent glucose-6-phosphate dehydrogenase [w44f]
- f420-dependent glucose-6-phosphate dehydrogenase [w44y]
- udp-n-acetylglucosamine--decaprenyl-phosphate n-acetylglucosaminephosphotransferase
- udp-n-acetylglucosamine--dolichyl-phosphate n-acetylglucosaminephosphotransferase
- 2-c-methyl-d-erythritol 4-phosphate synthase
- phosphatidylinositol 4-phosphate 5-kinase type i gamma
- phosphatidylinositol 5-phosphate 4-kinase type-2 beta
- f420-dependent glucose-6-phosphate dehydrogenase mutant (fgd e109q)
- f420-dependent glucose-6-phosphate dehydrogenase mutant (fgd h40a)
- 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase, phe-sensitive
- gag-pol polyprotein [600-1159,y780c]/imidazoleglycerol-phosphate dehydratase [600-1029,y780c]
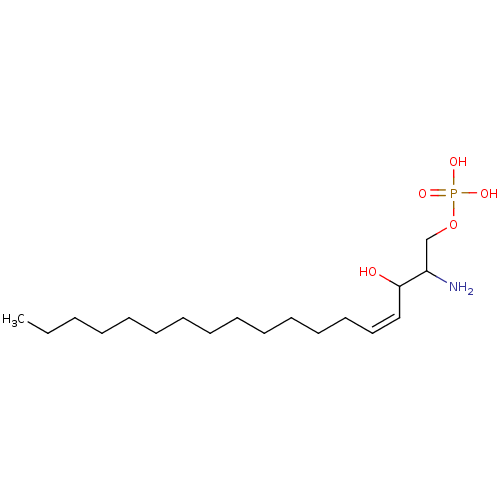 sphingosine-1-phosphate, 33P labeled {[(4Z)-2-amino-3-hydroxyoctadec-4-en-1-yl]oxy}phosphonic acid S1P [32P]S1P [33P]S1P BDBM22202 sphingosine-1-phosphate sphingosine-1-phosphate, 32P labeled
sphingosine-1-phosphate, 33P labeled {[(4Z)-2-amino-3-hydroxyoctadec-4-en-1-yl]oxy}phosphonic acid S1P [32P]S1P [33P]S1P BDBM22202 sphingosine-1-phosphate sphingosine-1-phosphate, 32P labeled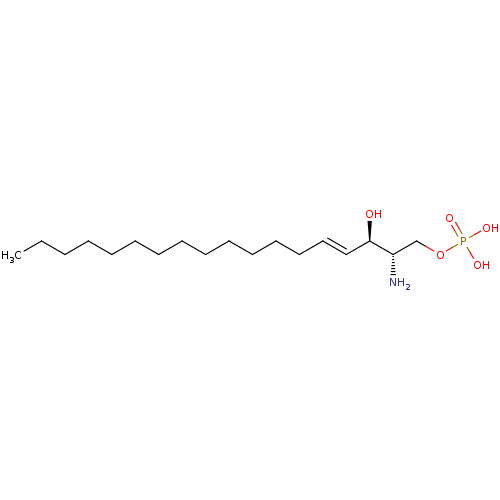 US10676467, Compound S1P (2S,3R,4E)-2-amino-4-octadecene-1,3-diol 1-(dihydrogen phosphate) (2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihydrogen phosphate BDBM50158348 CHEMBL225155 sphingosine-1-phosphate sphingosine 1-phosphate US11584726, Example S1P
US10676467, Compound S1P (2S,3R,4E)-2-amino-4-octadecene-1,3-diol 1-(dihydrogen phosphate) (2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihydrogen phosphate BDBM50158348 CHEMBL225155 sphingosine-1-phosphate sphingosine 1-phosphate US11584726, Example S1P BDBM23161 S1P
BDBM23161 S1P BDBM23162 [32P]S1P
BDBM23162 [32P]S1P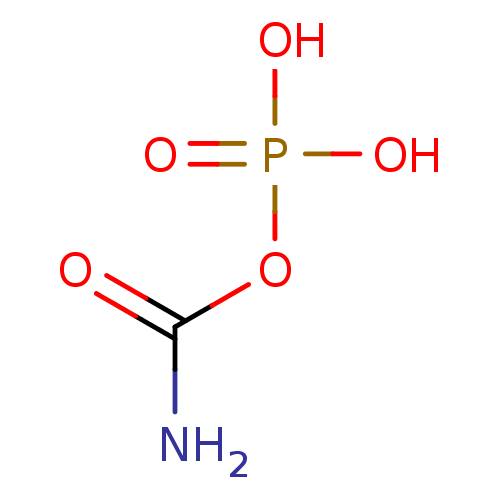 Carbamyl phosphate BDBM50162819 CHEMBL369105 carbamoyl phosphate carbamic phosphoric monoanhydride monocarbamoyl phosphate aminocarbonyl dihydrogen phosphate carbamoyl dihydrogen phosphate
Carbamyl phosphate BDBM50162819 CHEMBL369105 carbamoyl phosphate carbamic phosphoric monoanhydride monocarbamoyl phosphate aminocarbonyl dihydrogen phosphate carbamoyl dihydrogen phosphate (2S,3R)-Sphingosine CHEBI:67106 CHEMBL1835439 BDBM50496601
(2S,3R)-Sphingosine CHEBI:67106 CHEMBL1835439 BDBM50496601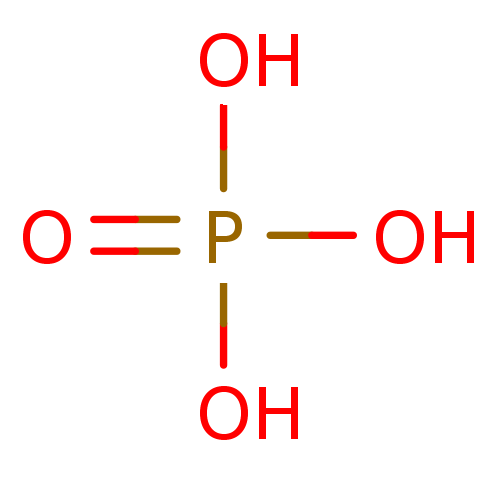 Fragment 1 Phosphate phosphoric acid BDBM14671
Fragment 1 Phosphate phosphoric acid BDBM14671 INCB-18424 PHOSPHATE INCB018424 PHOSPHATE Ruxolitinib (as phosphate) Ruxolitinib phosphate BDBM50649917 INCB-018424 SALT Opzelura Ruxolitinib monophosphate INCB-018424 PHOSPHATE INCB018424 SALT Jakafi Jakavi
INCB-18424 PHOSPHATE INCB018424 PHOSPHATE Ruxolitinib (as phosphate) Ruxolitinib phosphate BDBM50649917 INCB-018424 SALT Opzelura Ruxolitinib monophosphate INCB-018424 PHOSPHATE INCB018424 SALT Jakafi Jakavi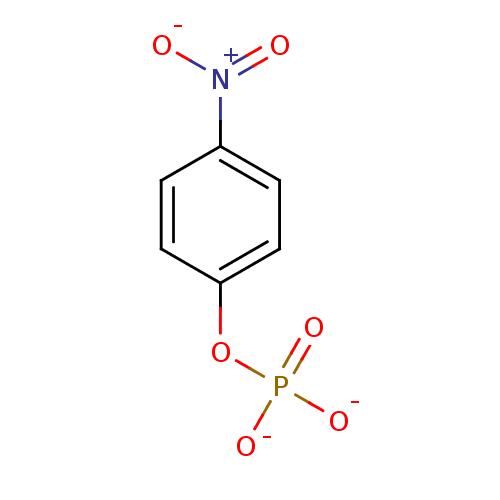 4-Nitrophenyl phosphate disodium salt hexahydrate 4-nitrophenyl phosphate (pNPP) para-nitrophenyl phosphate (pNPP) BDBM13466 disodium (4-nitrophenyl) phosphate
4-Nitrophenyl phosphate disodium salt hexahydrate 4-nitrophenyl phosphate (pNPP) para-nitrophenyl phosphate (pNPP) BDBM13466 disodium (4-nitrophenyl) phosphate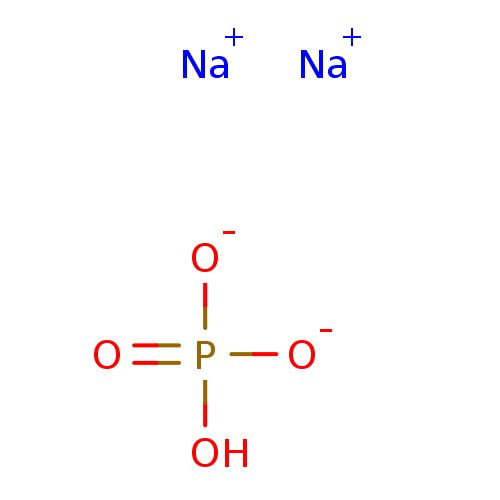 disodium monohydrogen phosphate Na2HPO4 disodium hydrogen phosphate Disodium phosphate disodium acid orthophosphate BDBM50080995 disodium hydrogenphosphate Dibasic sodium phosphate CHEMBL1060
disodium monohydrogen phosphate Na2HPO4 disodium hydrogen phosphate Disodium phosphate disodium acid orthophosphate BDBM50080995 disodium hydrogenphosphate Dibasic sodium phosphate CHEMBL1060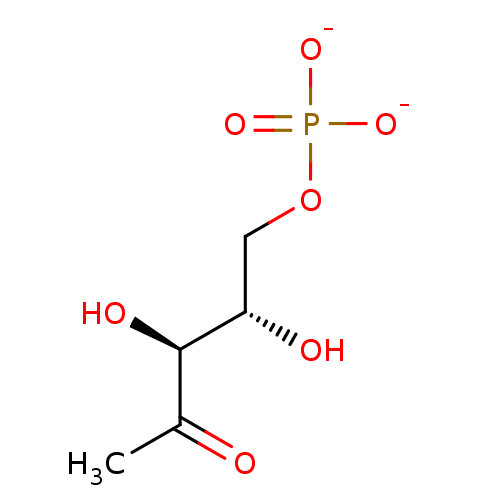 1-deoxy-L-ribulose 5-phosphate BDBM50153712
1-deoxy-L-ribulose 5-phosphate BDBM50153712 Riboflavin 5''-Phosphate BDBM50421345 RIBOFLAVIN 5'-PHOSPHATE E101a
Riboflavin 5''-Phosphate BDBM50421345 RIBOFLAVIN 5'-PHOSPHATE E101a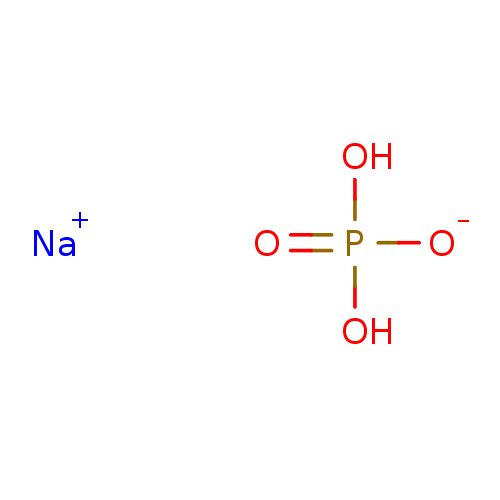 sodium dihydrogen phosphate NaH2PO4 monosodium phosphate CHEMBL1368 BDBM50155534 sodium phosphate, monobasic sodium dihydrogenphosphate sodium phosphate monobasic anhydrous phosphoric acid, monosodium salt
sodium dihydrogen phosphate NaH2PO4 monosodium phosphate CHEMBL1368 BDBM50155534 sodium phosphate, monobasic sodium dihydrogenphosphate sodium phosphate monobasic anhydrous phosphoric acid, monosodium salt Photrexa Riboflavin 5''-phosphate sodium RIBOFLAVIN 5'-PHOSPHATE SODIUM Phosphated riboflavin Riboflavin sodium phosphate BDBM50523758
Photrexa Riboflavin 5''-phosphate sodium RIBOFLAVIN 5'-PHOSPHATE SODIUM Phosphated riboflavin Riboflavin sodium phosphate BDBM50523758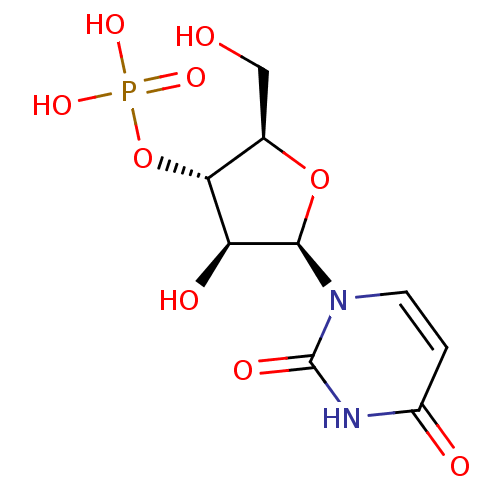 BDBM50292724 arabinouridine 3'-phosphate CHEMBL460901 URACIL ARABINOSE-3'-PHOSPHATE
BDBM50292724 arabinouridine 3'-phosphate CHEMBL460901 URACIL ARABINOSE-3'-PHOSPHATE BDBM50422306 TRICIRIBINE PHOSPHATE Triciribine Phosphate Salt Of Tricyclic Nucleoside
BDBM50422306 TRICIRIBINE PHOSPHATE Triciribine Phosphate Salt Of Tricyclic Nucleoside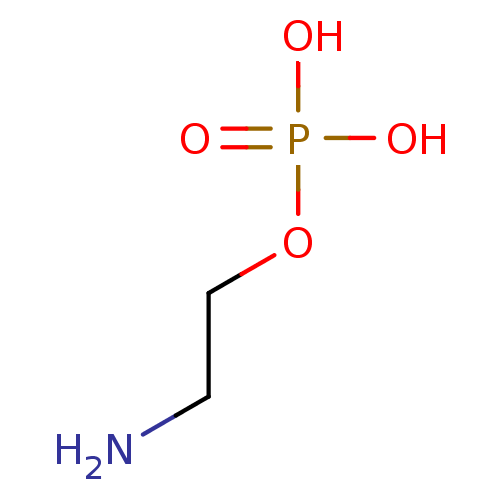 2-amino-ethanol dihydrogen phosphate ethanolamine O-phosphate O-phosphocolamine OPE phosphonoethanolamine monoaminoethyl phosphate colaminphosphoric acid 2-amino-ethanol phosphate colamine phosphate PEA mono(2-aminoethyl) phosphate colamine phosphoric acid 2-aminoethyl dihydrogen phosphate phosphoric acid 2-aminoethyl phenyl ester CHEMBL146972 BDBM50281572 EAP ethanolamine acid phosphate pEtN O-phosphoethanolamine
2-amino-ethanol dihydrogen phosphate ethanolamine O-phosphate O-phosphocolamine OPE phosphonoethanolamine monoaminoethyl phosphate colaminphosphoric acid 2-amino-ethanol phosphate colamine phosphate PEA mono(2-aminoethyl) phosphate colamine phosphoric acid 2-aminoethyl dihydrogen phosphate phosphoric acid 2-aminoethyl phenyl ester CHEMBL146972 BDBM50281572 EAP ethanolamine acid phosphate pEtN O-phosphoethanolamine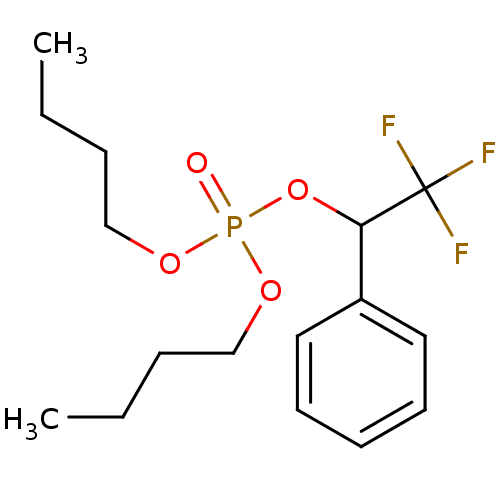 BDBM50300582 CHEMBL573979 Dibutyl 2,2,2-trifluoro-1-phenylethyl phosphate
BDBM50300582 CHEMBL573979 Dibutyl 2,2,2-trifluoro-1-phenylethyl phosphate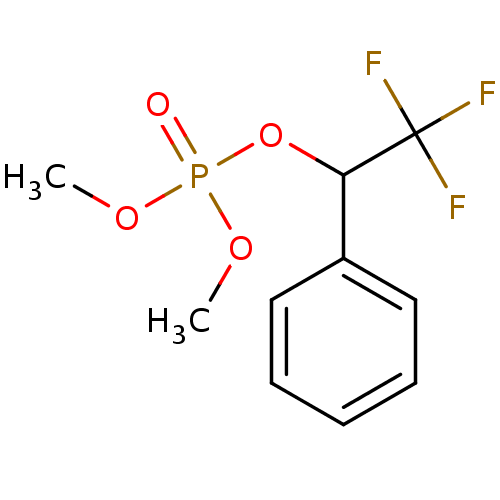 CHEMBL575281 Dimethyl 2,2,2-trifluoro-1-phenylethyl phosphate BDBM50300580
CHEMBL575281 Dimethyl 2,2,2-trifluoro-1-phenylethyl phosphate BDBM50300580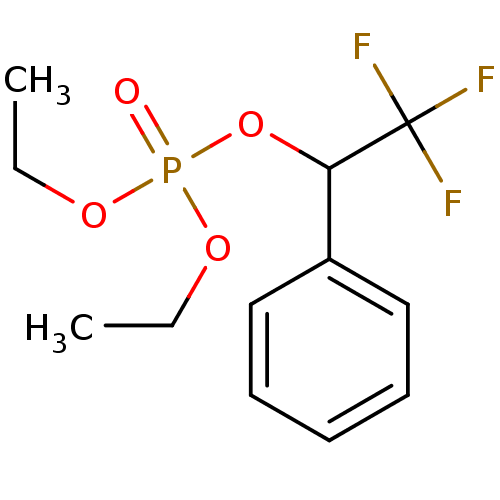 CHEMBL575301 Diethyl 2,2,2-trifluoro-1-phenylethyl phosphate BDBM50300581
CHEMBL575301 Diethyl 2,2,2-trifluoro-1-phenylethyl phosphate BDBM50300581 BDBM241975 Clindamycin phosphate
BDBM241975 Clindamycin phosphate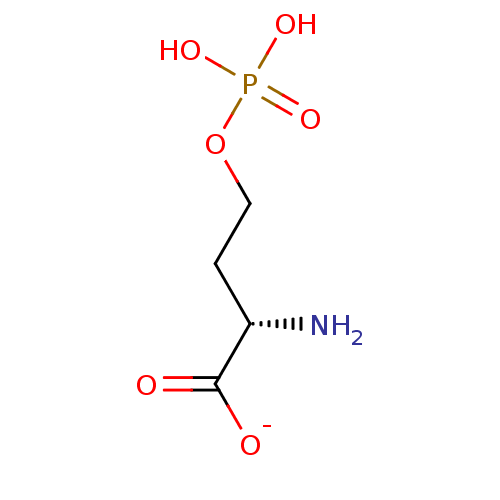 Homoserine phosphate BDBM92999
Homoserine phosphate BDBM92999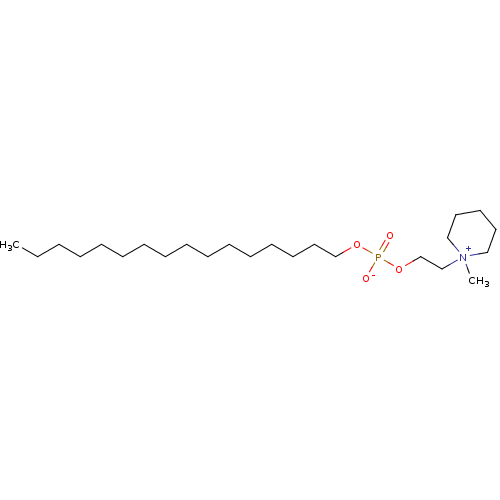 Hexadecyl[2-(N-Methylpiperidinio)ethyl]phosphate hexadecyl 2-(1-methylpiperidinium-1-yl)ethyl phosphate BDBM50051816 (N-methylpiperidino)ethanol hexadecyl phosphonate CHEMBL89618
Hexadecyl[2-(N-Methylpiperidinio)ethyl]phosphate hexadecyl 2-(1-methylpiperidinium-1-yl)ethyl phosphate BDBM50051816 (N-methylpiperidino)ethanol hexadecyl phosphonate CHEMBL89618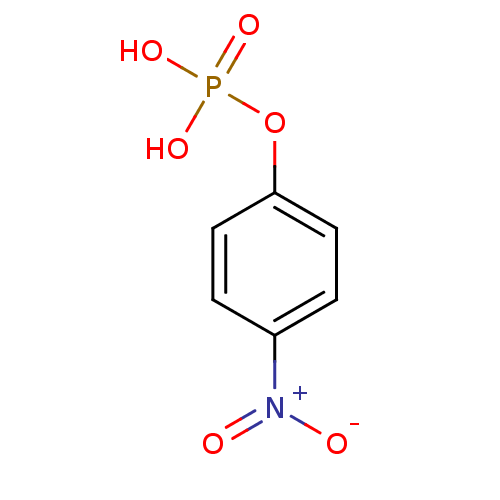 p-nitrophenyl phosphate BDBM24514 CHEMBL24231 4-nitrophenyl phosphate 4-nitrophenoxyphosphonic acid
p-nitrophenyl phosphate BDBM24514 CHEMBL24231 4-nitrophenyl phosphate 4-nitrophenoxyphosphonic acid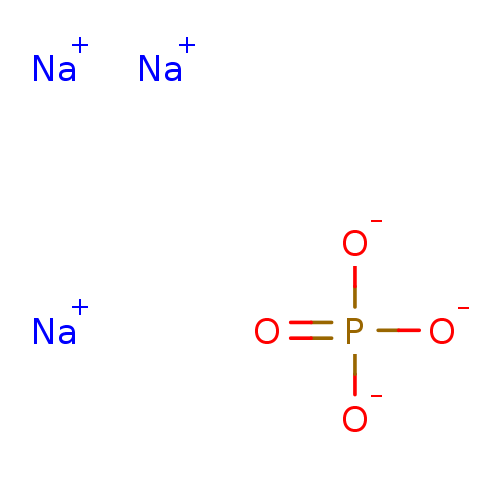 sodium phosphate, tribasic tertiaeres Natriumphosphat(V) tribasic sodium orthophosphate sodium orthophosphate CHEMBL363100 phosphoric acid trisodium salt sodium phosphate tertiary sodium phosphate tribasic sodium phosphate BDBM50155537 Na3PO4 Trinatriumphosphat
sodium phosphate, tribasic tertiaeres Natriumphosphat(V) tribasic sodium orthophosphate sodium orthophosphate CHEMBL363100 phosphoric acid trisodium salt sodium phosphate tertiary sodium phosphate tribasic sodium phosphate BDBM50155537 Na3PO4 Trinatriumphosphat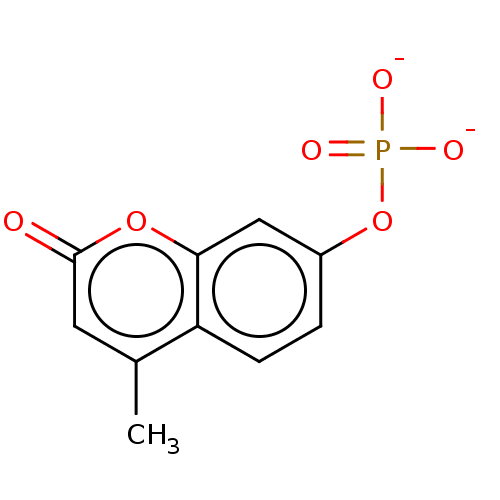 4-Methylumbelliferyl phosphate BDBM213237
4-Methylumbelliferyl phosphate BDBM213237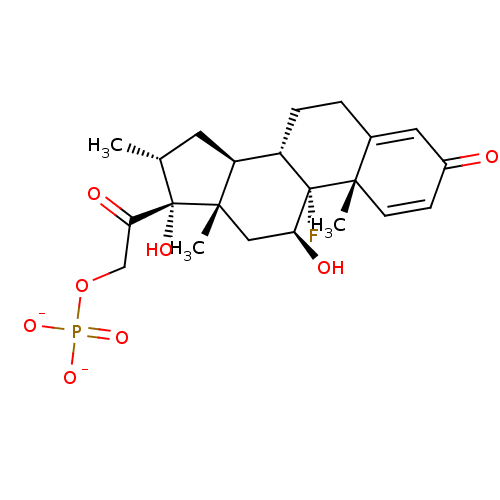 BDBM235670 Dexamethasone sodium phosphate
BDBM235670 Dexamethasone sodium phosphate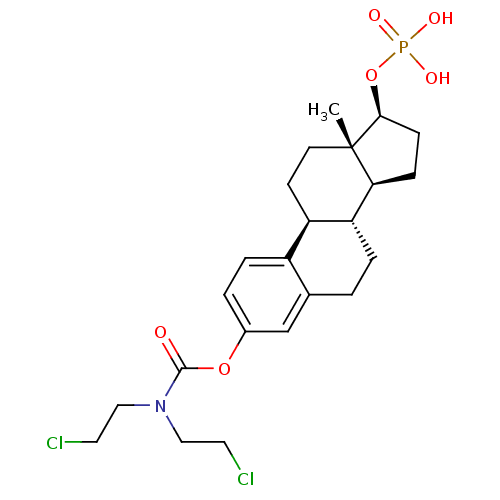 CHEMBL1756 BDBM50333645 Estramustine phosphate
CHEMBL1756 BDBM50333645 Estramustine phosphate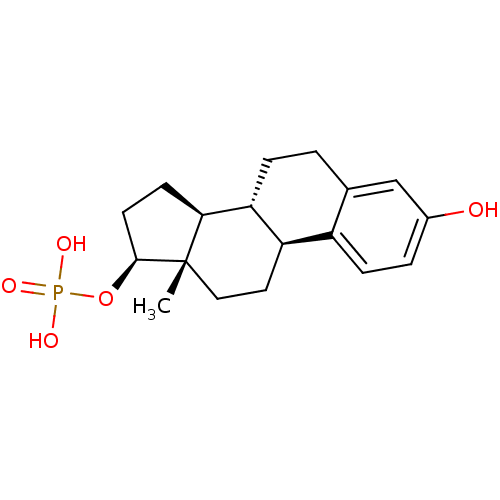 Estradiol phosphate BDBM50333647 CHEMBL1642763
Estradiol phosphate BDBM50333647 CHEMBL1642763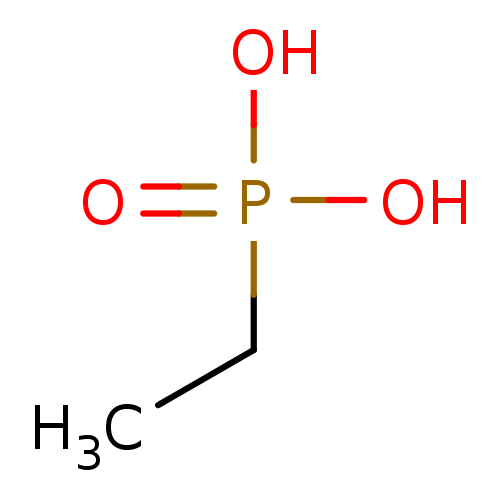 Phosphate analogue, 6 BDBM84557
Phosphate analogue, 6 BDBM84557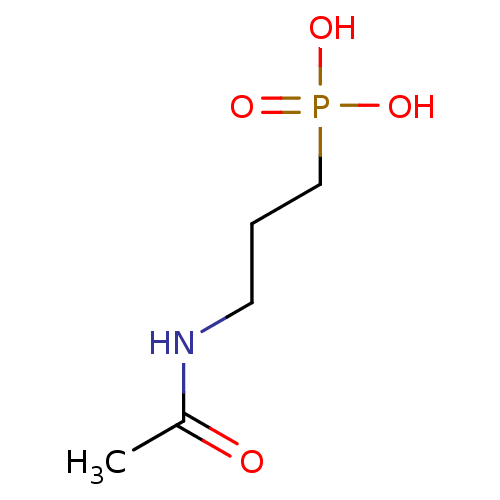 Phosphate analogue, 9 BDBM84558
Phosphate analogue, 9 BDBM84558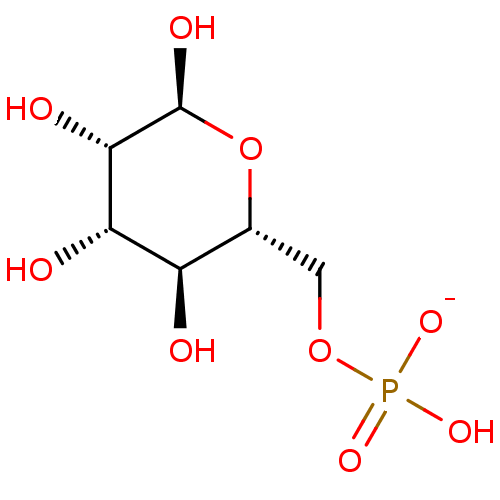 mannose 6-phosphate BDBM50275807
mannose 6-phosphate BDBM50275807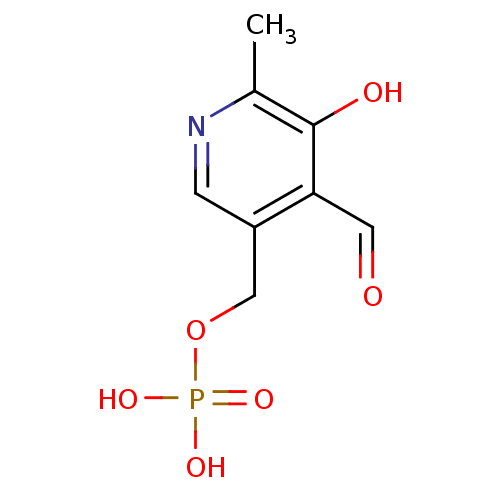 BDBM50118216 pyridoxal 5'-phosphate CHEMBL82202 (4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl dihydrogen phosphate pyridoxal 5'-(dihydrogen phosphate)
BDBM50118216 pyridoxal 5'-phosphate CHEMBL82202 (4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl dihydrogen phosphate pyridoxal 5'-(dihydrogen phosphate)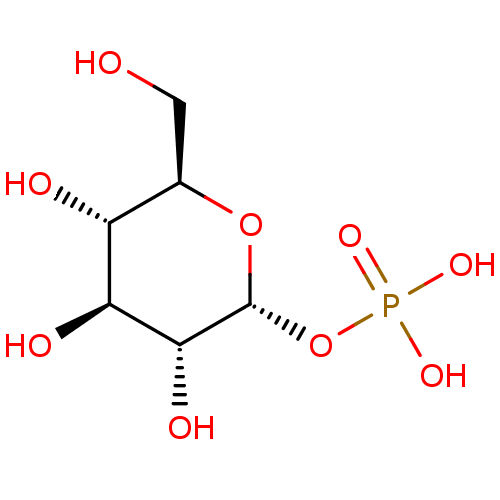 cori ester glucose-1-phosphate alpha-glucose-1-phosphate {[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phosphonic acid BDBM23188
cori ester glucose-1-phosphate alpha-glucose-1-phosphate {[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phosphonic acid BDBM23188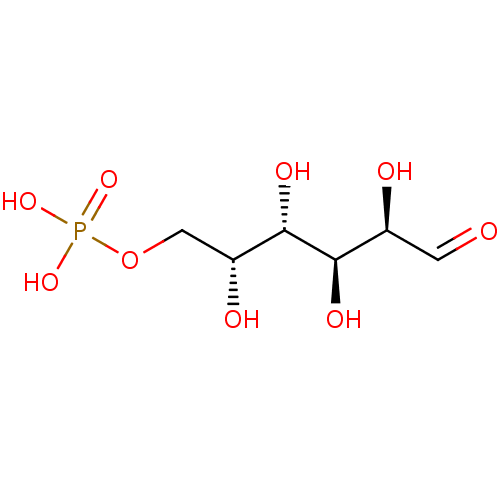 BDBM197174 Glucose-6-phosphate (G6P)
BDBM197174 Glucose-6-phosphate (G6P)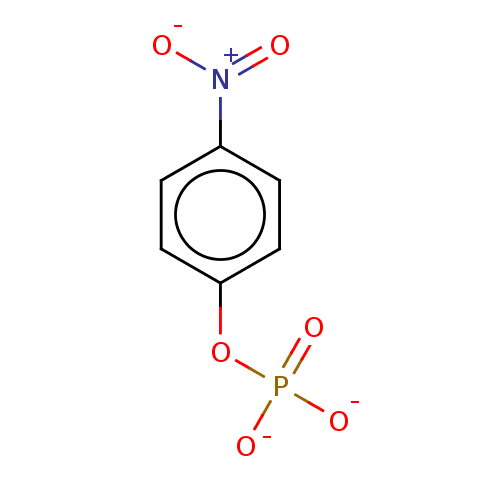 BDBM231698 p-Nitrophenyl phosphate (pNPP)
BDBM231698 p-Nitrophenyl phosphate (pNPP) BDBM84559 Phosphate analogue, (s)-14
BDBM84559 Phosphate analogue, (s)-14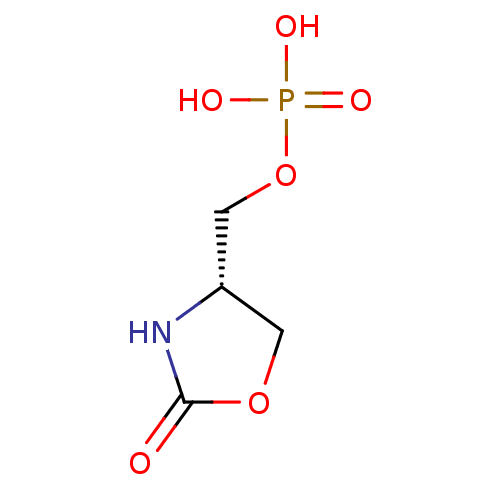 BDBM84560 Phosphate analogue, (S)-21
BDBM84560 Phosphate analogue, (S)-21 para-nitrophenyl phosphate (pNPP) BDBM26100
para-nitrophenyl phosphate (pNPP) BDBM26100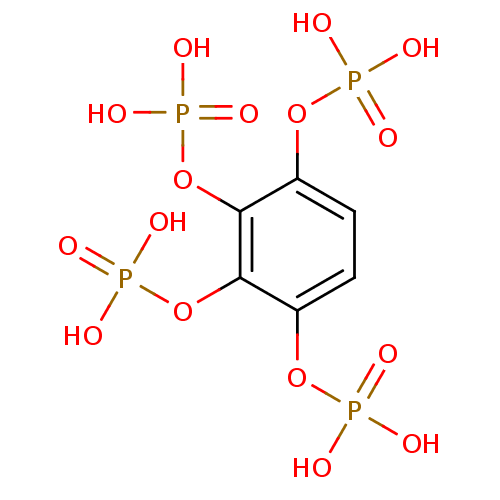 BDBM50304360 BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSPHATE)] benzene-1,2,3,4-tetrayl tetrakis(hydrogen phosphate) CHEMBL595349
BDBM50304360 BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSPHATE)] benzene-1,2,3,4-tetrayl tetrakis(hydrogen phosphate) CHEMBL595349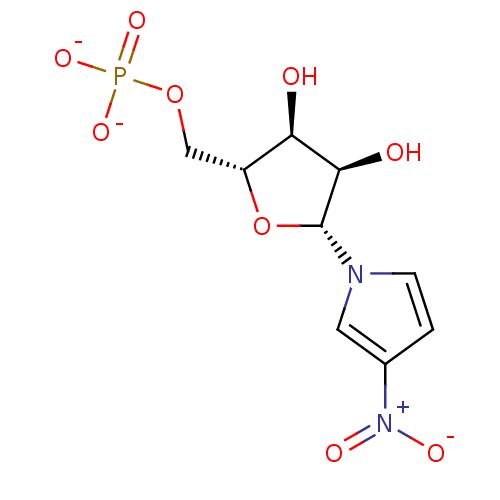 3-Nitro-1-(beta-D-ribofuranosyl)pyrrole 5'-phosphate BDBM50247810 CHEMBL538120
3-Nitro-1-(beta-D-ribofuranosyl)pyrrole 5'-phosphate BDBM50247810 CHEMBL538120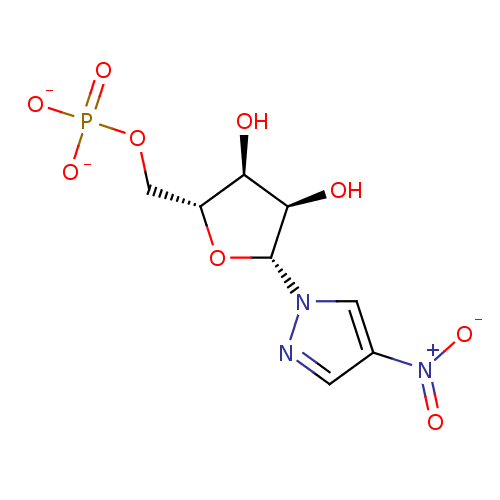 4-Nitro-1-(-beta-D-ribofuranosyl)pyrazole 5'-phosphate BDBM50247822 CHEMBL538121
4-Nitro-1-(-beta-D-ribofuranosyl)pyrazole 5'-phosphate BDBM50247822 CHEMBL538121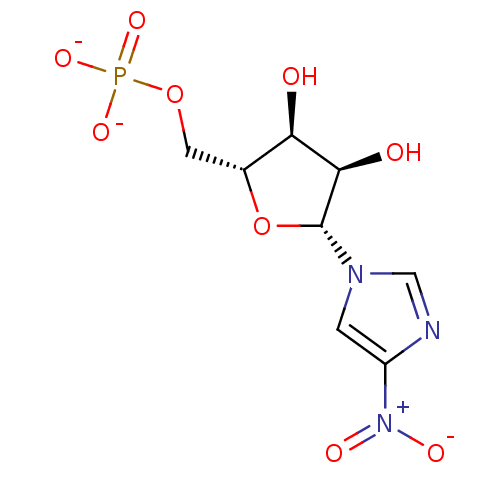 BDBM50247808 CHEMBL538119 3-Nitro-1-(beta-D-ribofuranosyl)imidazole 5'-phosphate
BDBM50247808 CHEMBL538119 3-Nitro-1-(beta-D-ribofuranosyl)imidazole 5'-phosphate phosphacol p-nitrophenyl diethyl phosphate diethyl p-nitrophenyl phosphate O,O-diethyl O-p-nitrophenyl phosphate CHEMBL23838 diethyl 4-nitrophenyl phosphate diethyl paraoxon paraoxon BDBM50240416 ethyl paraoxon phosphoric acid diethyl 4-nitrophenyl ester
phosphacol p-nitrophenyl diethyl phosphate diethyl p-nitrophenyl phosphate O,O-diethyl O-p-nitrophenyl phosphate CHEMBL23838 diethyl 4-nitrophenyl phosphate diethyl paraoxon paraoxon BDBM50240416 ethyl paraoxon phosphoric acid diethyl 4-nitrophenyl ester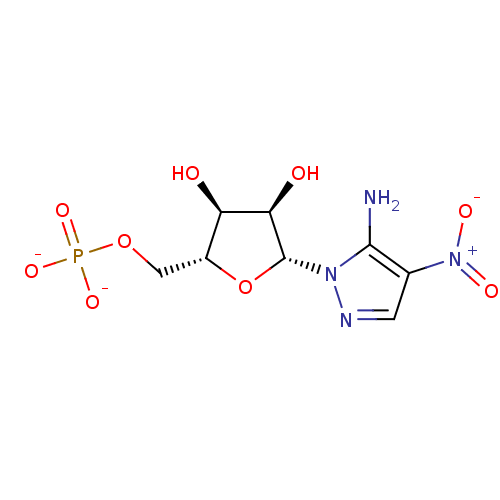 5-Amino-1-(beta-D-ribofuranosyl)-4-nitropyrazole 5'-phosphate CHEMBL554752 BDBM50247824
5-Amino-1-(beta-D-ribofuranosyl)-4-nitropyrazole 5'-phosphate CHEMBL554752 BDBM50247824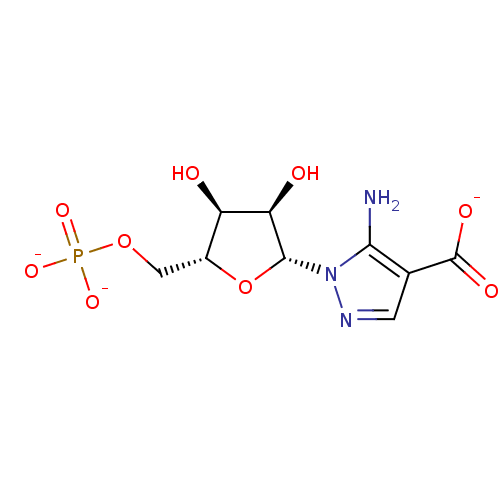 5-Amino-1-(beta-D-ribofuranosyl)pyrazole-4-carboxylate 5'-phosphate BDBM50247823
5-Amino-1-(beta-D-ribofuranosyl)pyrazole-4-carboxylate 5'-phosphate BDBM50247823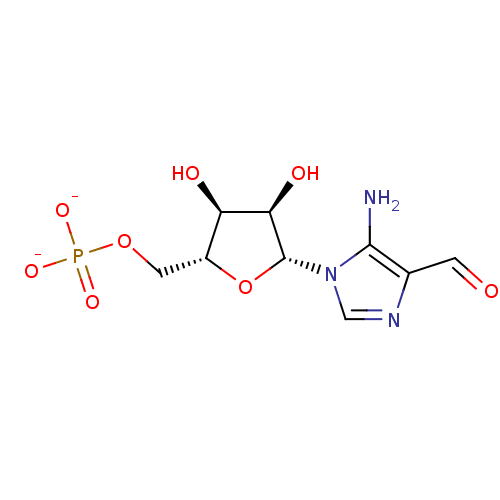 BDBM50247809 5-Amino-1-(beta-D-ribofuranosyl)imidazole-4-carboxaldehyde 5'-phosphate
BDBM50247809 5-Amino-1-(beta-D-ribofuranosyl)imidazole-4-carboxaldehyde 5'-phosphate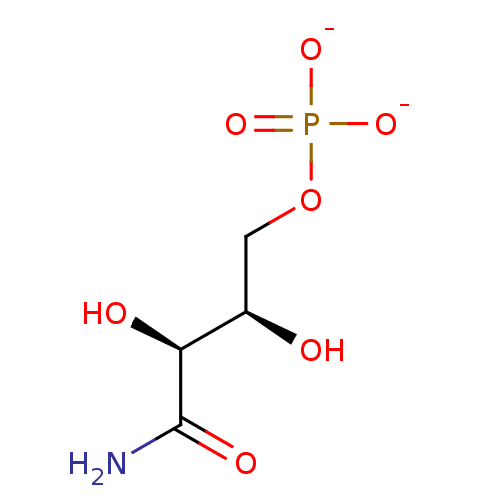 2S,3R-dihydroxybutyramide 4-phosphate BDBM50153711
2S,3R-dihydroxybutyramide 4-phosphate BDBM50153711 CHEBI:63036 BDBM50004328 POTASSIUM PHOSPHATE, MONOBASIC
CHEBI:63036 BDBM50004328 POTASSIUM PHOSPHATE, MONOBASIC CHEMBL186043 BDBM50155535 Carbamoyl phosphate; di sodium
CHEMBL186043 BDBM50155535 Carbamoyl phosphate; di sodium CHLOROQUINE DIPHOSPHATE CHLOROQUINE PHOSPHATE BDBM50411863 ARALEN
CHLOROQUINE DIPHOSPHATE CHLOROQUINE PHOSPHATE BDBM50411863 ARALEN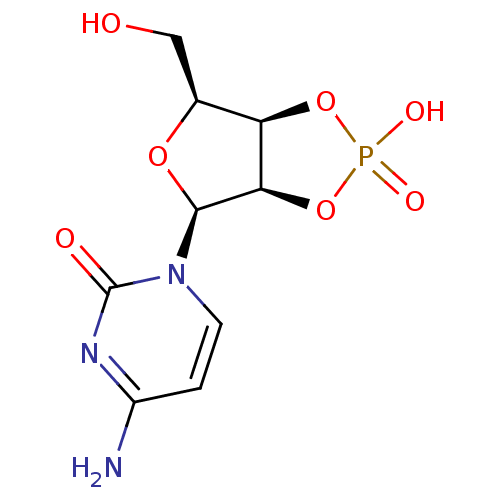 Cytidine 2,3-cyclic phosphate, 6 BDBM31909
Cytidine 2,3-cyclic phosphate, 6 BDBM31909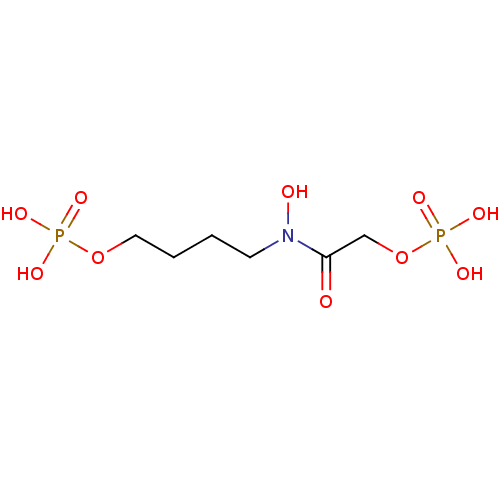 4-{hydroxy[(phosphonooxy)acetyl]amino}butyl dihydrogen phosphate N-(4-hydroxybutyl)-glycolohydroxamicacid bis-phosphate CHEMBL1236228 BDBM50330433
4-{hydroxy[(phosphonooxy)acetyl]amino}butyl dihydrogen phosphate N-(4-hydroxybutyl)-glycolohydroxamicacid bis-phosphate CHEMBL1236228 BDBM50330433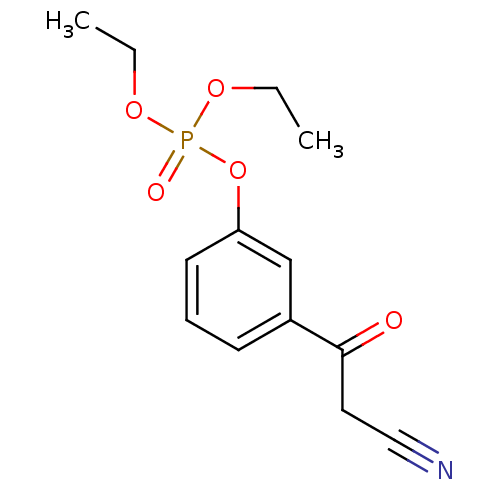 BDBM16190 [3-(2-cyanoacetyl)phenyl] diethyl phosphate 3-(2-Cyanoacetyl)phenyl Diethyl Phosphate beta-ketonitrile 5h
BDBM16190 [3-(2-cyanoacetyl)phenyl] diethyl phosphate 3-(2-Cyanoacetyl)phenyl Diethyl Phosphate beta-ketonitrile 5h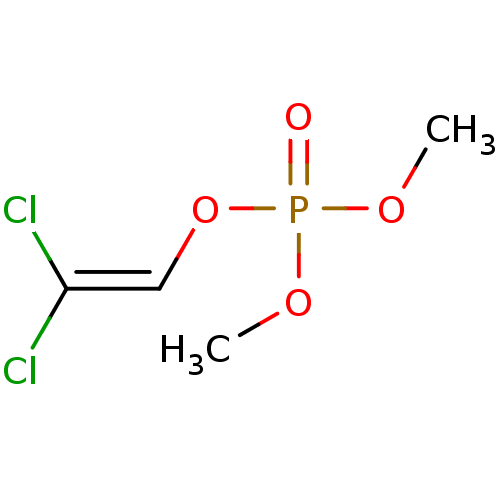 DDVP CHEMBL167911 2,2-Dichloroethenyl dimethyl phosphate Dimethyl-2,2-dichlorovinyl phosphate Phosphoric acid, 2,2-dichloroethenyl dimethyl ester Dimethyl 2,2-dichlorovinyl phosphate BDBM50286926 Phosphoric acid, 2,2-dichlorovinyl dimethyl ester
DDVP CHEMBL167911 2,2-Dichloroethenyl dimethyl phosphate Dimethyl-2,2-dichlorovinyl phosphate Phosphoric acid, 2,2-dichloroethenyl dimethyl ester Dimethyl 2,2-dichlorovinyl phosphate BDBM50286926 Phosphoric acid, 2,2-dichlorovinyl dimethyl ester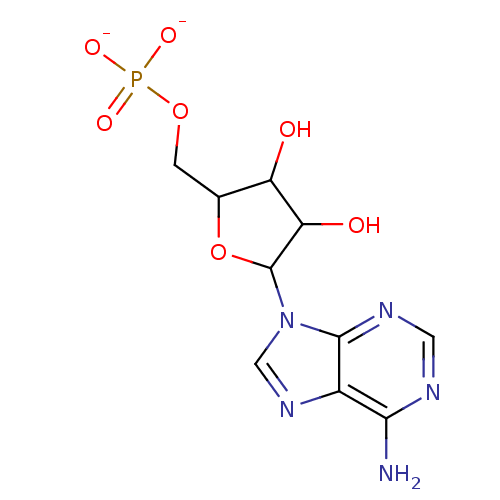 AMP adenosine 5'-phosphate group [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate BDBM92538
AMP adenosine 5'-phosphate group [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate BDBM92538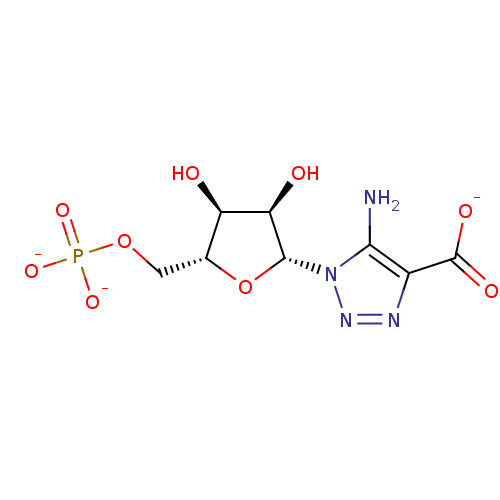 5-Amino-1-(beta-D-ribofuranosyl)-1,2,3-triazole-4-carboxylate 5'-phosphate BDBM50247825
5-Amino-1-(beta-D-ribofuranosyl)-1,2,3-triazole-4-carboxylate 5'-phosphate BDBM50247825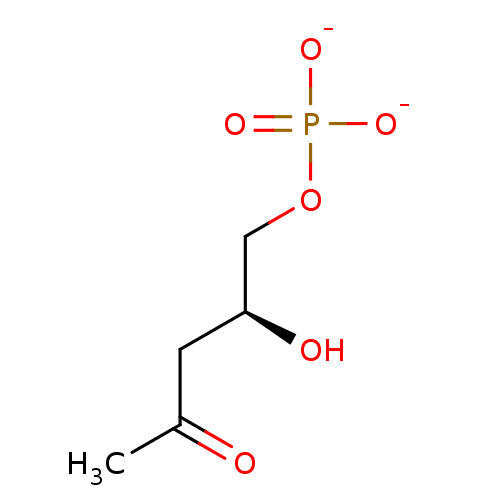 4S-hydroxypentan-2-one 5-phosphate BDBM50153714
4S-hydroxypentan-2-one 5-phosphate BDBM50153714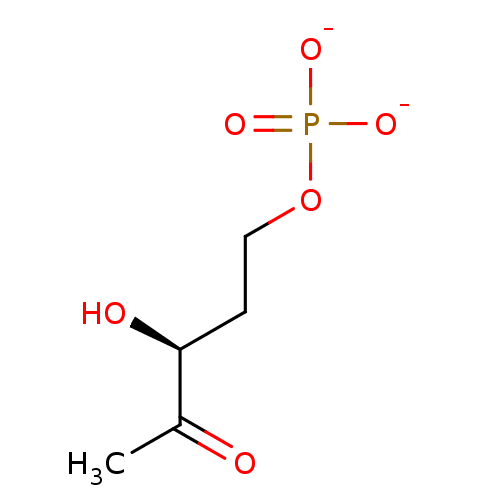 BDBM50153710 3S-hydroxypentan-2-one 5-phosphate
BDBM50153710 3S-hydroxypentan-2-one 5-phosphate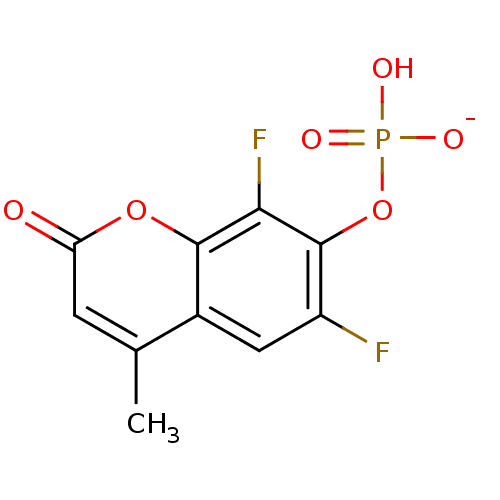 BDBM92531 DiFMUP (6,8-Difluoro-4-methylumbelliferyl phosphate)
BDBM92531 DiFMUP (6,8-Difluoro-4-methylumbelliferyl phosphate)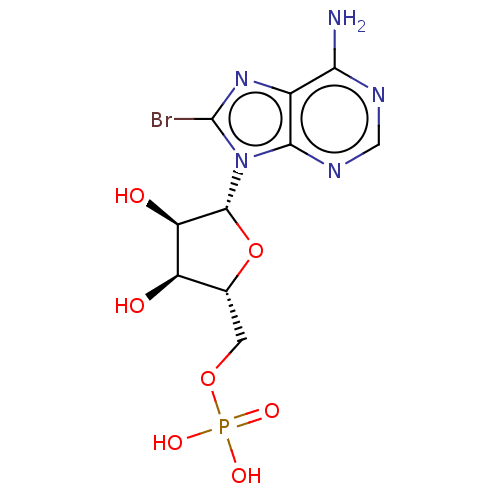 CHEMBL1230617 8-Bromo-Adenosine Mono Phosphate BDBM50222467
CHEMBL1230617 8-Bromo-Adenosine Mono Phosphate BDBM50222467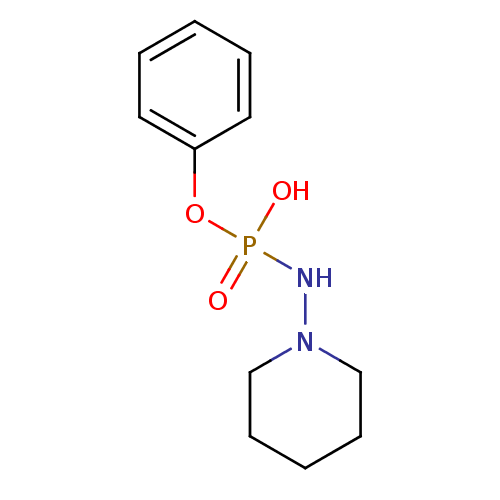 CHEMBL1275924 Phenyl-N-(4-aminopiperidine)phosphate BDBM50330376
CHEMBL1275924 Phenyl-N-(4-aminopiperidine)phosphate BDBM50330376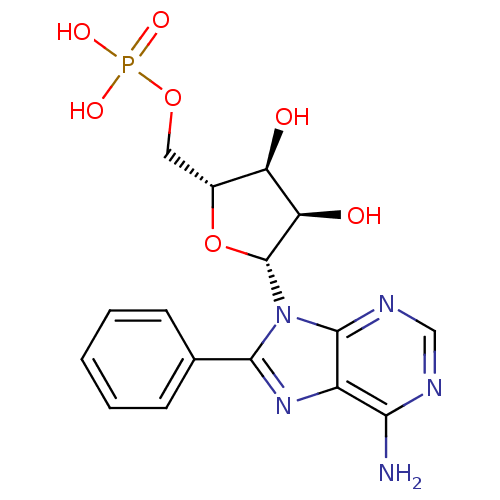 CHEMBL1775011 8-phenyl-Adenosine Mono Phosphate BDBM50343897
CHEMBL1775011 8-phenyl-Adenosine Mono Phosphate BDBM50343897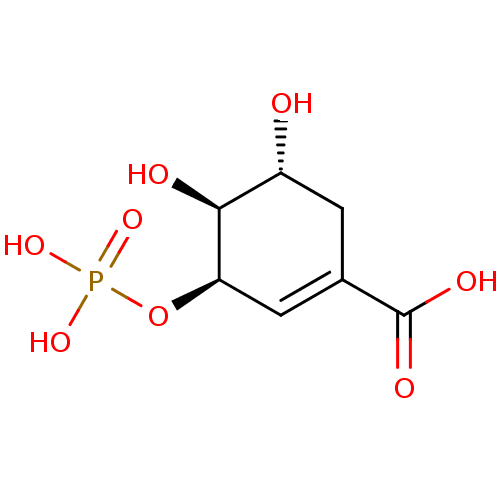 (3R,4S,5R)-4,5-dihydroxy-3-phosphonooxycyclohexene-1-carboxylic acid Shikimate-3-phosphate BDBM100283
(3R,4S,5R)-4,5-dihydroxy-3-phosphonooxycyclohexene-1-carboxylic acid Shikimate-3-phosphate BDBM100283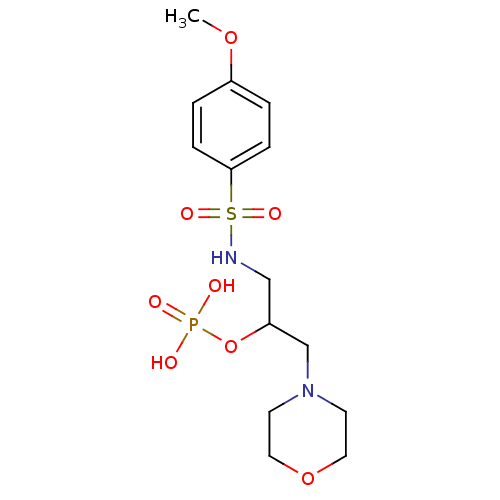 1-(4-Methoxyphenylsulfonamido)-3-morpholinopropan-2-yl dihydrogen phosphate HEA derivative, 7a BDBM50248217 CHEMBL473552
1-(4-Methoxyphenylsulfonamido)-3-morpholinopropan-2-yl dihydrogen phosphate HEA derivative, 7a BDBM50248217 CHEMBL473552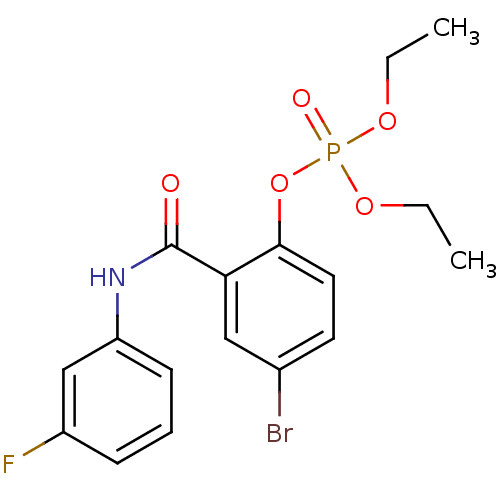 4‐bromo‐2‐[(3‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (1) BDBM150721
4‐bromo‐2‐[(3‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (1) BDBM150721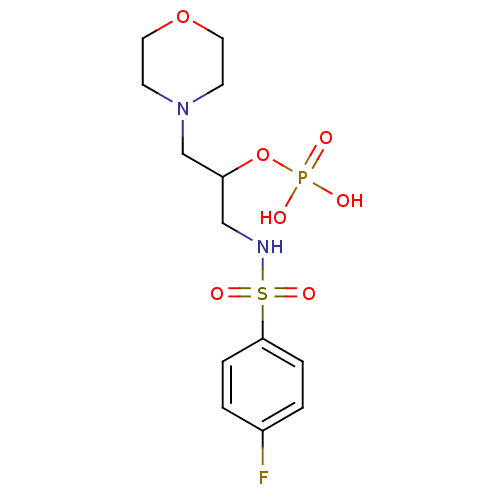 HEA derivative, 7b BDBM50248218 CHEMBL473752 1-(4-Fluorophenylsulfonamido)-3-morpholinopropan-2-yl dihydrogen phosphate
HEA derivative, 7b BDBM50248218 CHEMBL473752 1-(4-Fluorophenylsulfonamido)-3-morpholinopropan-2-yl dihydrogen phosphate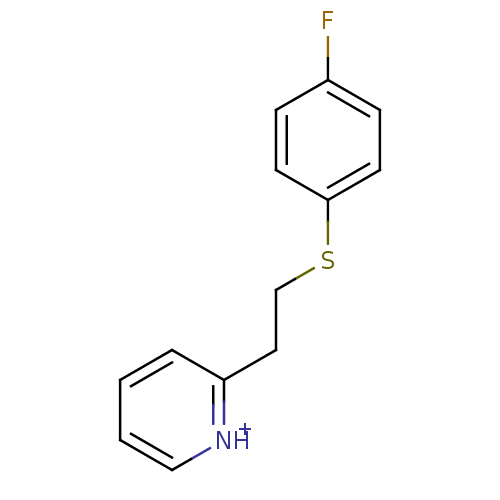 dihydrogen phosphate;2-[2-(4-fluorophenyl)sulfanylethyl]pyridin-1-ium BDBM45710 2-[2-(4-Fluoro-phenylsulfanyl)-ethyl]-pyridinium dihydrogen phosphate;2-[2-[(4-fluorophenyl)thio]ethyl]pyridin-1-ium cid_16682387 MLS000767112 SMR000429527
dihydrogen phosphate;2-[2-(4-fluorophenyl)sulfanylethyl]pyridin-1-ium BDBM45710 2-[2-(4-Fluoro-phenylsulfanyl)-ethyl]-pyridinium dihydrogen phosphate;2-[2-[(4-fluorophenyl)thio]ethyl]pyridin-1-ium cid_16682387 MLS000767112 SMR000429527 CHEMBL1683319 5-O-phosphate-1-N-(4'-(4''-methoxyhenyl))-[1',2',3']-triazol-beta-D-ribofuranoside BDBM50338502
CHEMBL1683319 5-O-phosphate-1-N-(4'-(4''-methoxyhenyl))-[1',2',3']-triazol-beta-D-ribofuranoside BDBM50338502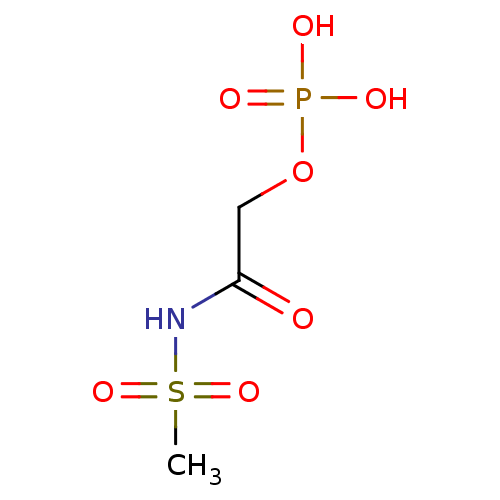 2-(methylsulfonamido)-2-oxoethyl dihydrogen phosphate BDBM50175714 CHEMBL370845
2-(methylsulfonamido)-2-oxoethyl dihydrogen phosphate BDBM50175714 CHEMBL370845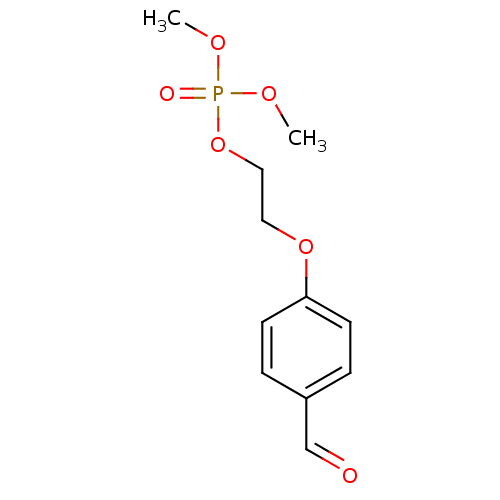 BDBM50300123 2-(4-Formylphenoxy)ethyl dimethyl phosphate CHEMBL574442
BDBM50300123 2-(4-Formylphenoxy)ethyl dimethyl phosphate CHEMBL574442 CHEBI:68603 BETAMETHASONE PHOSPHORIC ACID BDBM50613630 Betamethasone phosphate
CHEBI:68603 BETAMETHASONE PHOSPHORIC ACID BDBM50613630 Betamethasone phosphate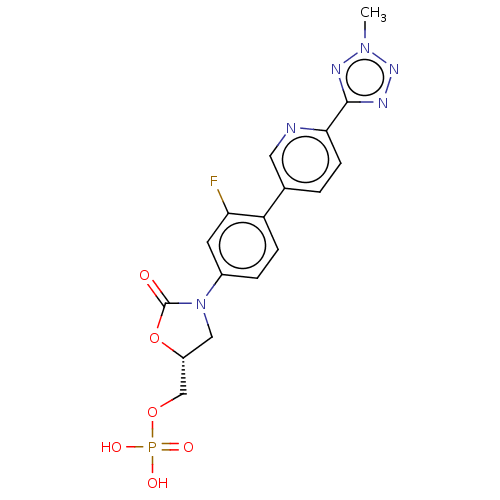 CHEBI:83326 TEDIZOLID PHOSPHATE BDBM50017198 TR-701-FA
CHEBI:83326 TEDIZOLID PHOSPHATE BDBM50017198 TR-701-FA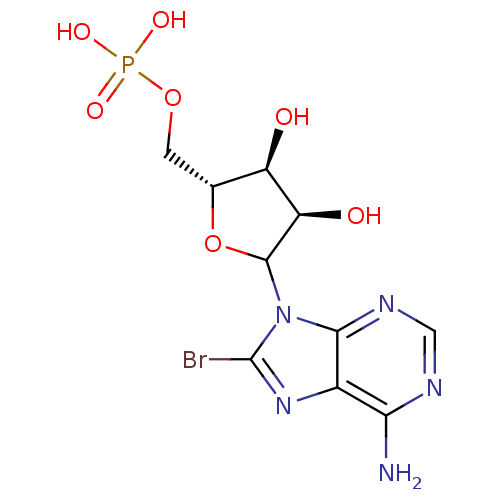 CHEMBL606220 CHEMBL1230617 BDBM50367001 8-Bromo-Adenosine Mono Phosphate
CHEMBL606220 CHEMBL1230617 BDBM50367001 8-Bromo-Adenosine Mono Phosphate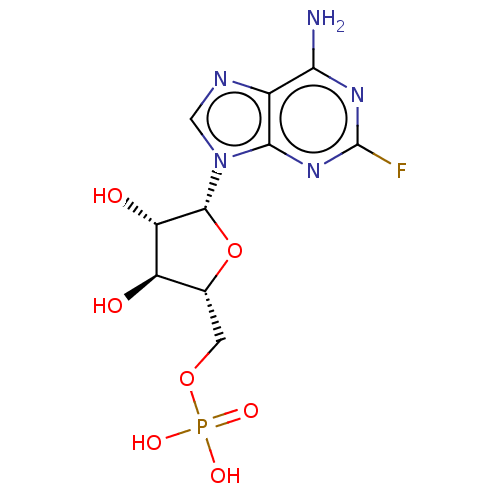 Fludarabine Phosphate Fludarabine Fludara BDBM50248004 CHEBI:63599 Oforta
Fludarabine Phosphate Fludarabine Fludara BDBM50248004 CHEBI:63599 Oforta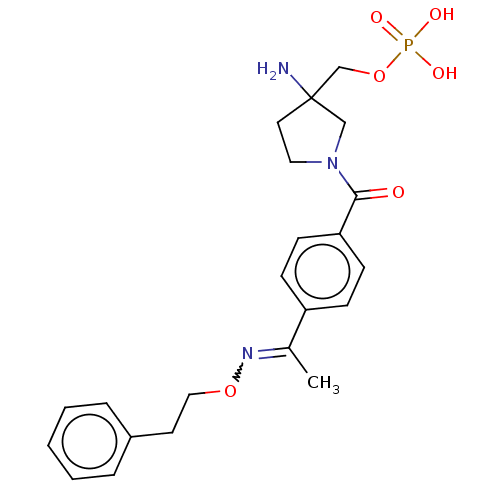 (3-amino-1-(4-(1-(phenethoxyimino)ethyl)benzoyl)pyrrolidin-3-yl)methyl Dihydrogen Phosphate US11059784, Example 55 BDBM511446
(3-amino-1-(4-(1-(phenethoxyimino)ethyl)benzoyl)pyrrolidin-3-yl)methyl Dihydrogen Phosphate US11059784, Example 55 BDBM511446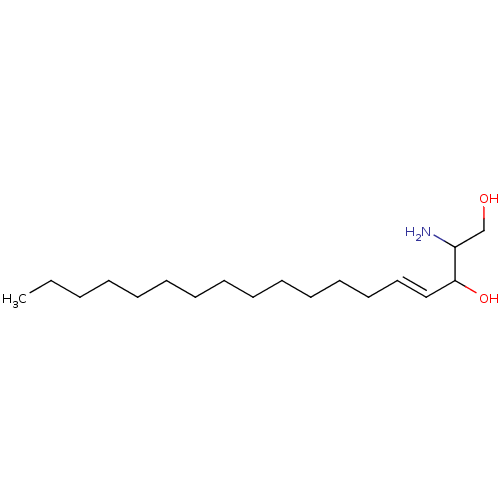 2-Amino-octadec-4-ene-1,3-diol(Sphingosine) BDBM50009730 CHEMBL67166 (E)-2-Amino-octadec-4-ene-1,3-diol
2-Amino-octadec-4-ene-1,3-diol(Sphingosine) BDBM50009730 CHEMBL67166 (E)-2-Amino-octadec-4-ene-1,3-diol ((1R,3R)-1-amino-3-(4-(1-((cyclopropylmethoxy)imino)ethyl)phenyl)cyclopentyl)methyl Dihydrogen Phosphate BDBM511429 US11059784, Example 38
((1R,3R)-1-amino-3-(4-(1-((cyclopropylmethoxy)imino)ethyl)phenyl)cyclopentyl)methyl Dihydrogen Phosphate BDBM511429 US11059784, Example 38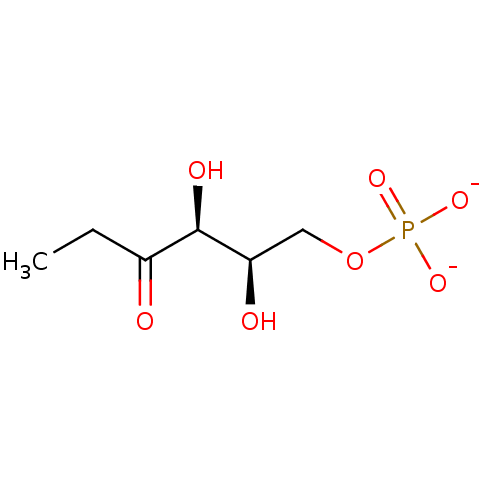 1,2-dideoxy-L-threo-3-hexulose 6-phosphate BDBM50153715
1,2-dideoxy-L-threo-3-hexulose 6-phosphate BDBM50153715 2-Methoxy-4-(nonanamidomethyl)phenyl dihydrogen phosphate (3b) BDBM165191
2-Methoxy-4-(nonanamidomethyl)phenyl dihydrogen phosphate (3b) BDBM165191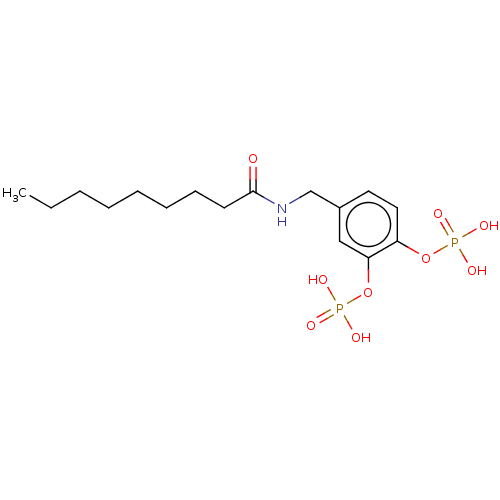 BDBM165193 4-(Nonanamidomethyl)-1,2-phenylene bis(dihydrogen phosphate) (6b)
BDBM165193 4-(Nonanamidomethyl)-1,2-phenylene bis(dihydrogen phosphate) (6b)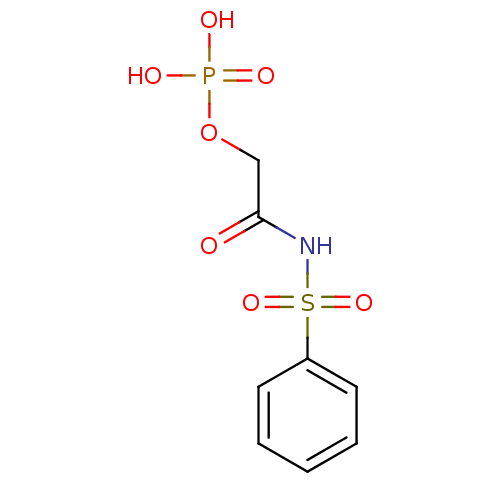 BDBM50175712 CHEMBL199843 2-oxo-2-(phenylsulfonamido)ethyl dihydrogen phosphate
BDBM50175712 CHEMBL199843 2-oxo-2-(phenylsulfonamido)ethyl dihydrogen phosphate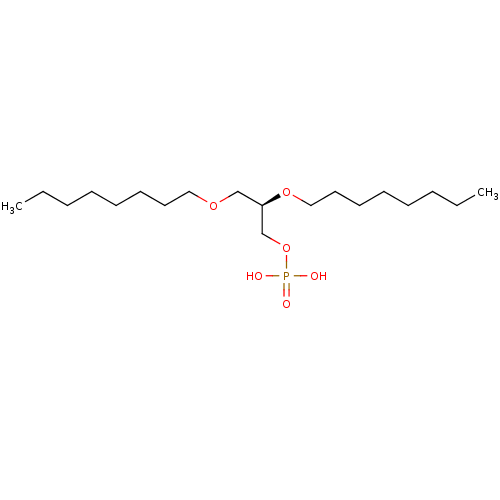 CHEMBL202243 BDBM50176400 (R)-2,3-bis(octyloxy)propyl dihydrogen phosphate
CHEMBL202243 BDBM50176400 (R)-2,3-bis(octyloxy)propyl dihydrogen phosphate Etopophos preservative free Etoposide Phosphate BMY-40481 Etopophos BDBM50247889
Etopophos preservative free Etoposide Phosphate BMY-40481 Etopophos BDBM50247889 RUXOLITINIB PHOSPHATE BDBM50391992 US20250136608, Compound Ruxolitinib INCB018424 SALT Jakafi
RUXOLITINIB PHOSPHATE BDBM50391992 US20250136608, Compound Ruxolitinib INCB018424 SALT Jakafi TCP CAS_78-30-8 BDBM82063 TRI-O-CRESYL PHOSPHATE
TCP CAS_78-30-8 BDBM82063 TRI-O-CRESYL PHOSPHATE beta-nicotinamide adenine dinucleotide phosphate, oxidized form NADP+ BDBM11939
beta-nicotinamide adenine dinucleotide phosphate, oxidized form NADP+ BDBM11939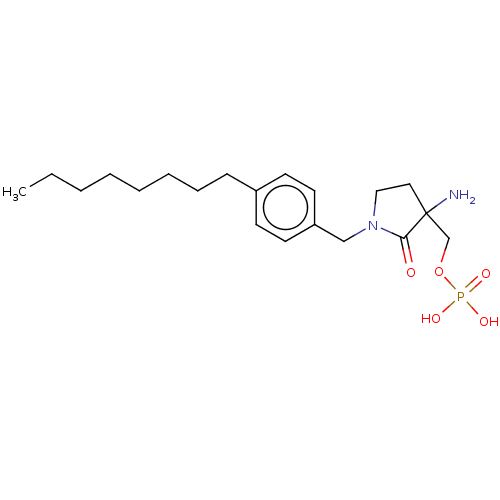 US11046646, Example 14 BDBM506618 (3-amino-1-(4-octylbenzyl)-2-oxopyrrolidin-3-yl)methyl dihydrogen phosphate
US11046646, Example 14 BDBM506618 (3-amino-1-(4-octylbenzyl)-2-oxopyrrolidin-3-yl)methyl dihydrogen phosphate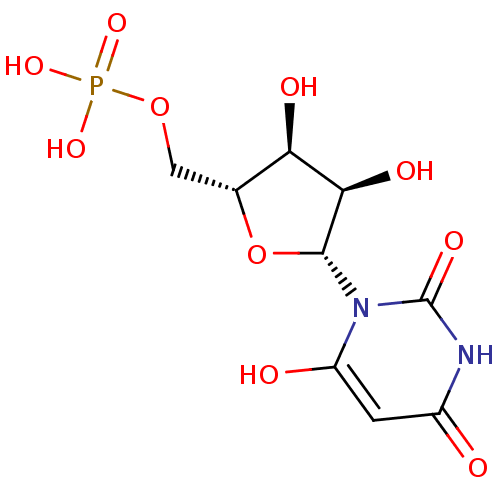 6-hydroxy-UMP 1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione 5'-monophosphate 6-HYDROXYURIDINE-5'-PHOSPHATE 3,4-dihydroxy-5-(6-oxido-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl phosphate BDBM50199178 CHEMBL383923
6-hydroxy-UMP 1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione 5'-monophosphate 6-HYDROXYURIDINE-5'-PHOSPHATE 3,4-dihydroxy-5-(6-oxido-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl phosphate BDBM50199178 CHEMBL383923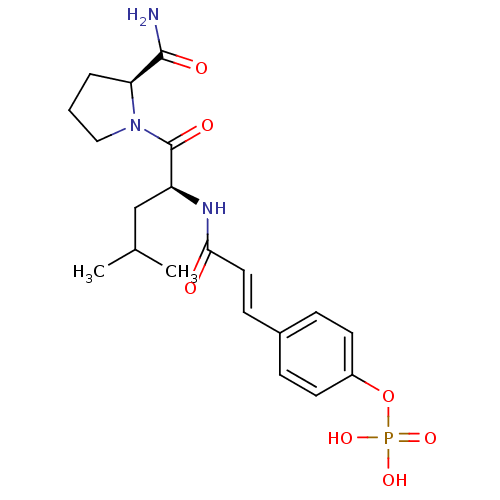 CHEMBL574654 4-(3-((S)-1-((S)-2-carbamoylpyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300616
CHEMBL574654 4-(3-((S)-1-((S)-2-carbamoylpyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300616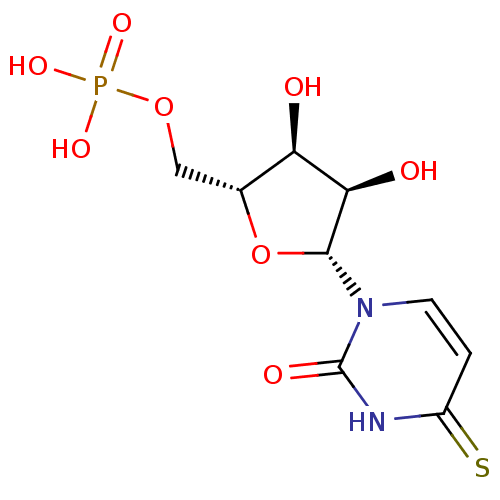 4-THIOURIDINE-5'-MONOPHOSPHATE 4-THIOURIDINE-5'-PHOSPHATE BDBM50341895 ((2R,3S,4R,5R)-3,4-dihydroxy-5-(2-oxo-4-thioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL1230331
4-THIOURIDINE-5'-MONOPHOSPHATE 4-THIOURIDINE-5'-PHOSPHATE BDBM50341895 ((2R,3S,4R,5R)-3,4-dihydroxy-5-(2-oxo-4-thioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL1230331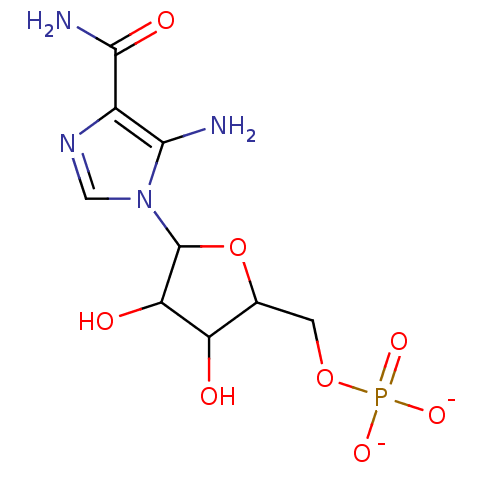 BDBM50080321 ZMP [5-(5-amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate
BDBM50080321 ZMP [5-(5-amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate US10233190, Example 1434 BDBM370515 sodium 1-cyclohexyl-2-(5H-imidazo[5,1- a]isoindol-5-yl)ethyl phosphate
US10233190, Example 1434 BDBM370515 sodium 1-cyclohexyl-2-(5H-imidazo[5,1- a]isoindol-5-yl)ethyl phosphate 2-[(3-bromophenyl)carbamoyl]-5-chlorophenyl diethyl phosphate (20) BDBM150740
2-[(3-bromophenyl)carbamoyl]-5-chlorophenyl diethyl phosphate (20) BDBM150740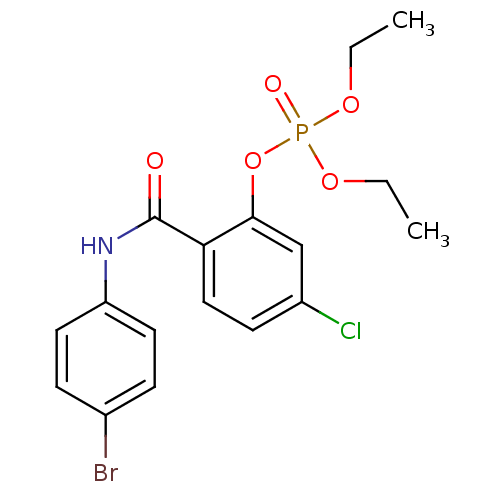 2-[(4-bromophenyl)carbamoyl]-5-chlorophenyl diethyl phosphate (23) BDBM150743
2-[(4-bromophenyl)carbamoyl]-5-chlorophenyl diethyl phosphate (23) BDBM150743 BDBM50032981 Phosphate analogue, 8 (3-Amino-propyl)-phosphonic acid CHEMBL286077
BDBM50032981 Phosphate analogue, 8 (3-Amino-propyl)-phosphonic acid CHEMBL286077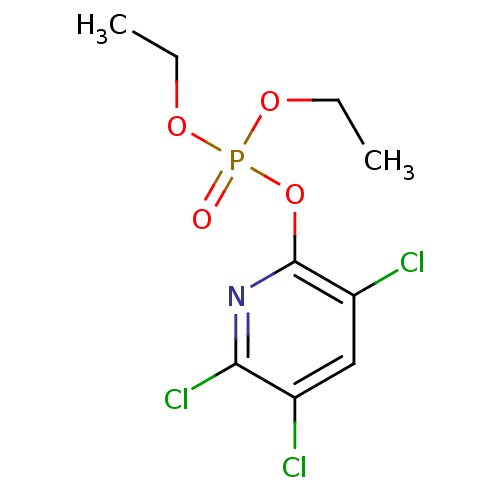 BDBM50247086 Chlorphrifos oxon diethyl 3,5,6-trichloropyridin-2-yl phosphate CHEMBL444970
BDBM50247086 Chlorphrifos oxon diethyl 3,5,6-trichloropyridin-2-yl phosphate CHEMBL444970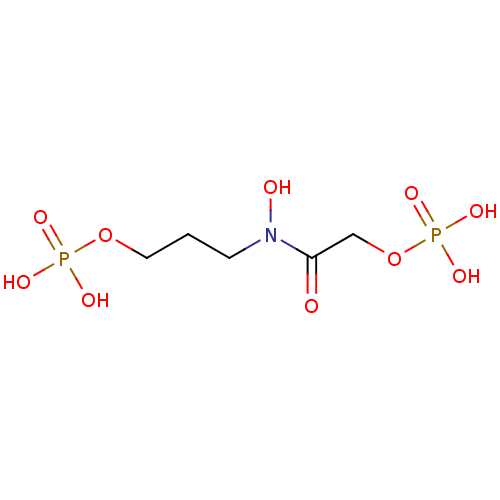 CHEMBL1235276 BDBM50330438 3-{hydroxy[(phosphonooxy)acetyl]amino}propyl dihydrogen phosphate
CHEMBL1235276 BDBM50330438 3-{hydroxy[(phosphonooxy)acetyl]amino}propyl dihydrogen phosphate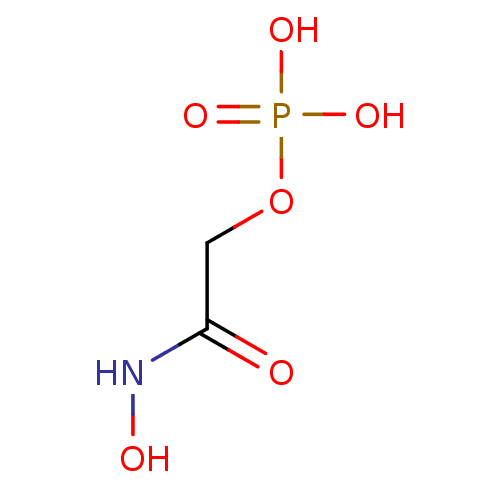 Phosphoglycolohydroxamic Acid BDBM50167777 2-(hydroxyamino)-2-oxoethyl dihydrogen phosphate CHEMBL371668
Phosphoglycolohydroxamic Acid BDBM50167777 2-(hydroxyamino)-2-oxoethyl dihydrogen phosphate CHEMBL371668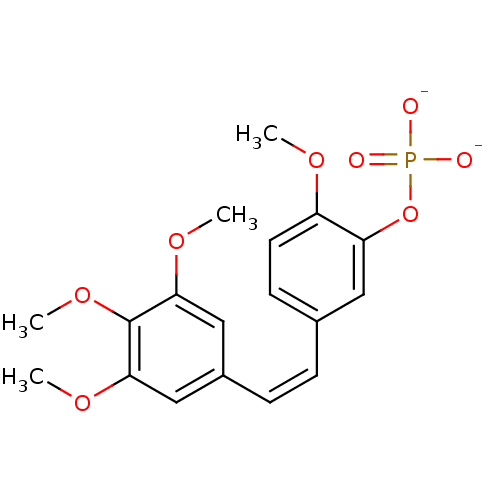 Di-Sodium salt of Phosphoric acid mono-{2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester Disodium Phosphoric acid mono-{2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester combretastatin A-4 sodium phosphate BDBM50064259 Phosphoric acid mono-{2-methoxy-5-[(Z)-2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester; disodium salt sodium(Z)-2-methoxy-5-(3,4,5-trimethoxystyryl)phenyl phosphate combretastatin A-4 phosphate Combretastatin A4 phosphate disodium 2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)vinyl]phenyl phosphate CHEMBL289351 sodium (Z)-5-(3,4,5-trimethoxystyryl)-2-methoxyphenyl phosphate disodium mono(Z)-5-(3,4,5-trimethoxystyryl)-2-methoxyphenyl phosphate
Di-Sodium salt of Phosphoric acid mono-{2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester Disodium Phosphoric acid mono-{2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester combretastatin A-4 sodium phosphate BDBM50064259 Phosphoric acid mono-{2-methoxy-5-[(Z)-2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester; disodium salt sodium(Z)-2-methoxy-5-(3,4,5-trimethoxystyryl)phenyl phosphate combretastatin A-4 phosphate Combretastatin A4 phosphate disodium 2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)vinyl]phenyl phosphate CHEMBL289351 sodium (Z)-5-(3,4,5-trimethoxystyryl)-2-methoxyphenyl phosphate disodium mono(Z)-5-(3,4,5-trimethoxystyryl)-2-methoxyphenyl phosphate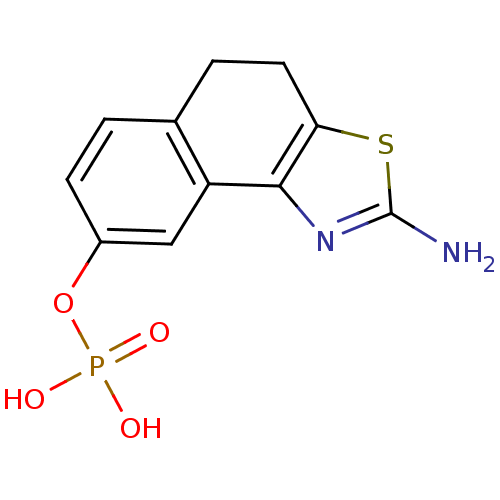 2-amino-4,5-dihydronaphtho[1,2-d][1,3]thiazol-8-yl dihydrogen phosphate 2-amino-4,5-dihydronaphtho[1,2-d]thiazol-8-yl dihydrogen phosphate BDBM50302495 CHEMBL565809
2-amino-4,5-dihydronaphtho[1,2-d][1,3]thiazol-8-yl dihydrogen phosphate 2-amino-4,5-dihydronaphtho[1,2-d]thiazol-8-yl dihydrogen phosphate BDBM50302495 CHEMBL565809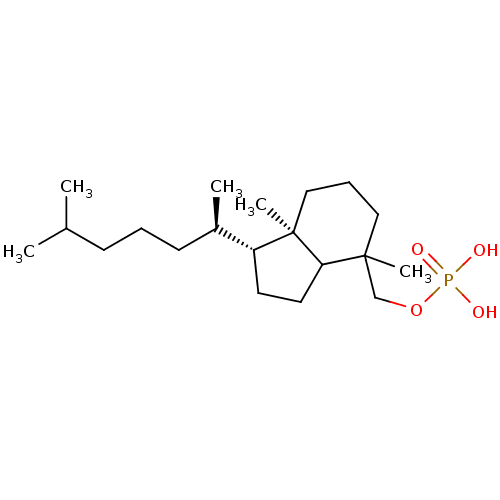 CHEMBL179992 CHEMBL182645 [(1R,4S,7aR)-1-(1,5-dimethylhexyl)-4,7a-dimethyloctahydro-1H-inden-4-yl]methyl dihydrogen phosphate BDBM50150613
CHEMBL179992 CHEMBL182645 [(1R,4S,7aR)-1-(1,5-dimethylhexyl)-4,7a-dimethyloctahydro-1H-inden-4-yl]methyl dihydrogen phosphate BDBM50150613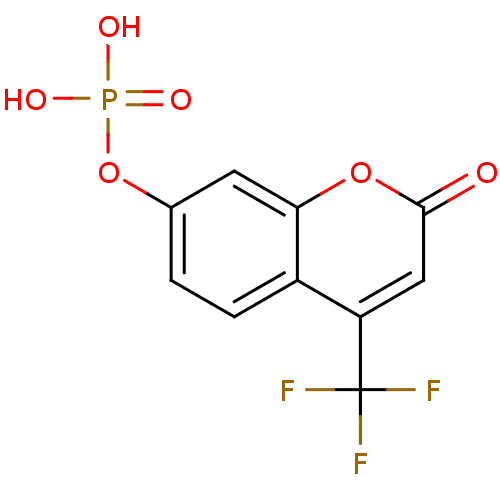 Fragment compound, 1 4-trifluoromethylcoumarin phosphate {[2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl]oxy}phosphonic acid BDBM22598
Fragment compound, 1 4-trifluoromethylcoumarin phosphate {[2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl]oxy}phosphonic acid BDBM22598 US9707205, 19 BDBM255780 4-(4-(3-(3-(2,2,2-trifluoroacetyl)-1H-indol-1-yl)propyl)phenyl)butyl-dihydrogen phosphate
US9707205, 19 BDBM255780 4-(4-(3-(3-(2,2,2-trifluoroacetyl)-1H-indol-1-yl)propyl)phenyl)butyl-dihydrogen phosphate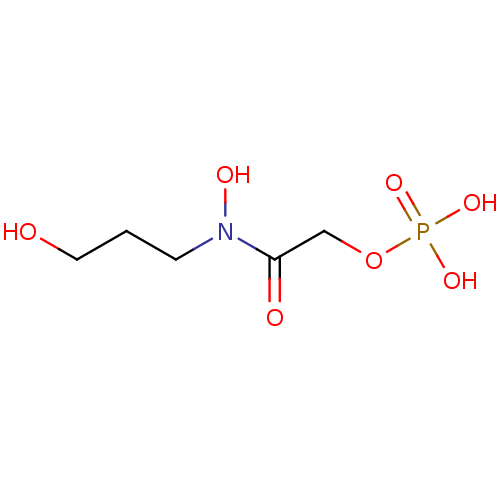 2-(hydroxy(3-hydroxypropyl)amino)-2-oxoethyl hydrogen phosphate BDBM50330436 CHEMBL1276292
2-(hydroxy(3-hydroxypropyl)amino)-2-oxoethyl hydrogen phosphate BDBM50330436 CHEMBL1276292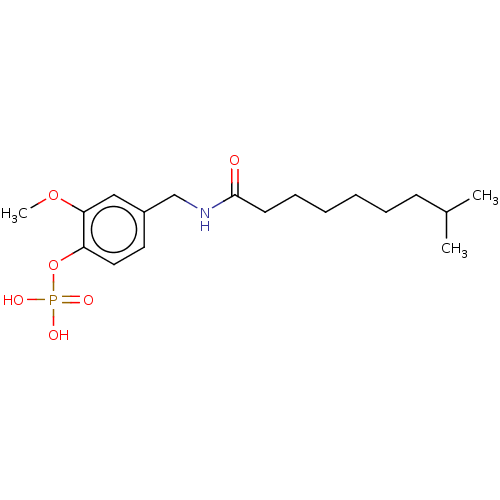 2-Methoxy-4-((8-methylnonanamido)methyl)phenyl dihydrogen phosphate (3a) BDBM165190
2-Methoxy-4-((8-methylnonanamido)methyl)phenyl dihydrogen phosphate (3a) BDBM165190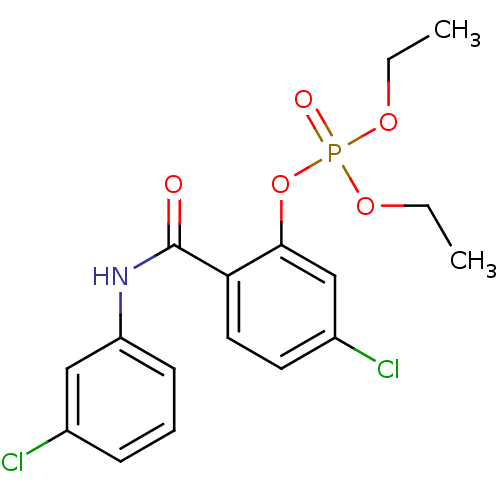 5-chloro-2-[(3-chlorophenyl)carbamoyl]phenyl diethyl phosphate (19) BDBM150739
5-chloro-2-[(3-chlorophenyl)carbamoyl]phenyl diethyl phosphate (19) BDBM150739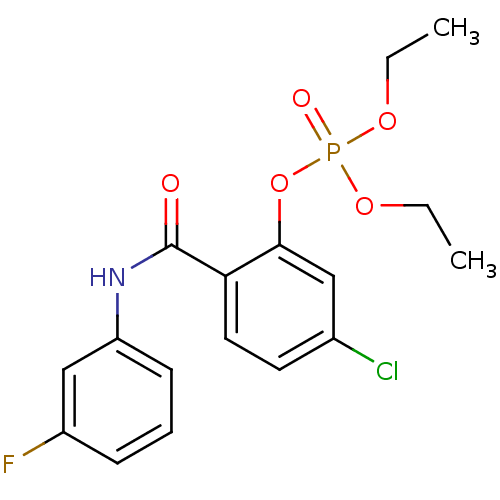 5-chloro-2-[(3-fluorophenyl)carbamoyl]phenyl diethyl phosphate (21) BDBM150741
5-chloro-2-[(3-fluorophenyl)carbamoyl]phenyl diethyl phosphate (21) BDBM150741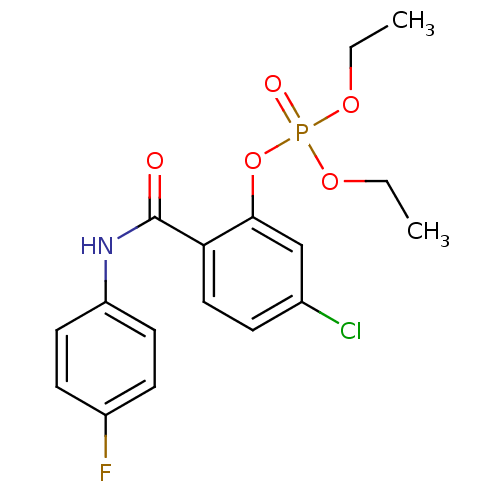 5-chloro-2-[(4-fluorophenyl)carbamoyl]phenyl diethyl phosphate (22) BDBM150742
5-chloro-2-[(4-fluorophenyl)carbamoyl]phenyl diethyl phosphate (22) BDBM150742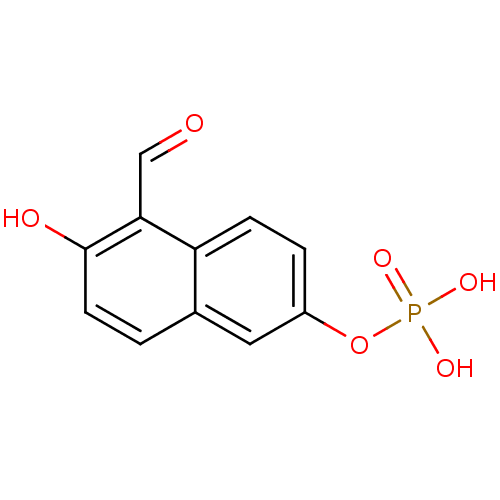 5-formyl-6-hydroxy-2-naphthyl di-sodium phosphate BDBM50183272 CHEMBL382634
5-formyl-6-hydroxy-2-naphthyl di-sodium phosphate BDBM50183272 CHEMBL382634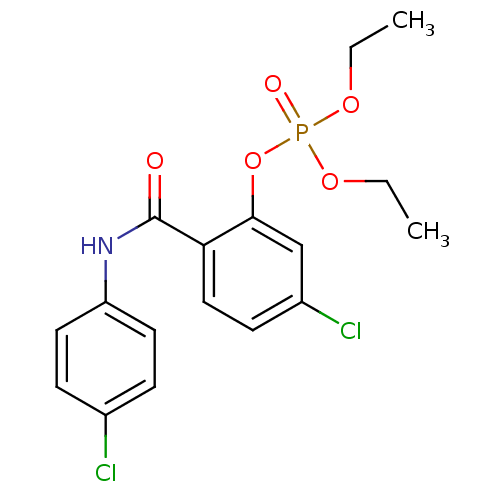 BDBM150744 5-chloro-2-[(4-chlorophenyl)carbamoyl]phenyl diethyl phosphate (24)
BDBM150744 5-chloro-2-[(4-chlorophenyl)carbamoyl]phenyl diethyl phosphate (24)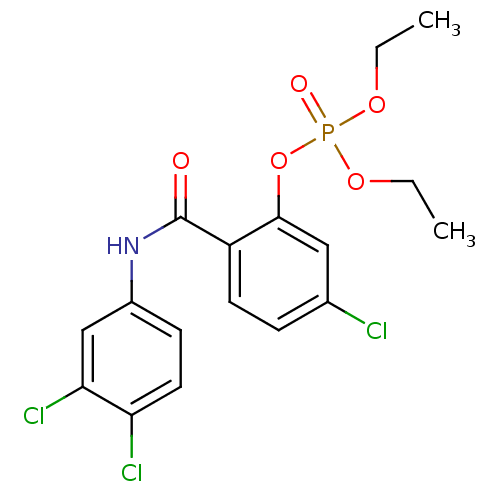 BDBM150745 5-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl diethyl phosphate (25)
BDBM150745 5-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl diethyl phosphate (25)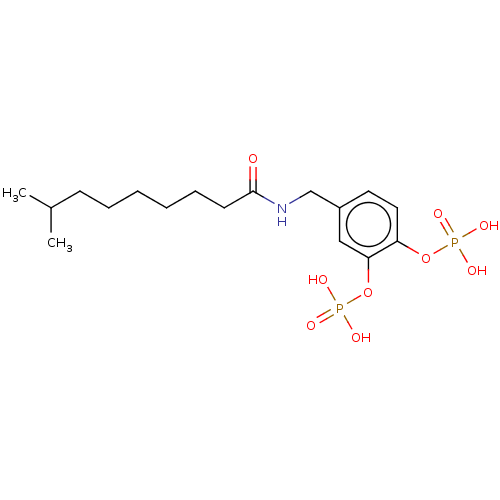 BDBM165192 4-((8-Methylnonanamido)methyl)-1,2-phenylene bis(dihydrogen phosphate) (6a)
BDBM165192 4-((8-Methylnonanamido)methyl)-1,2-phenylene bis(dihydrogen phosphate) (6a)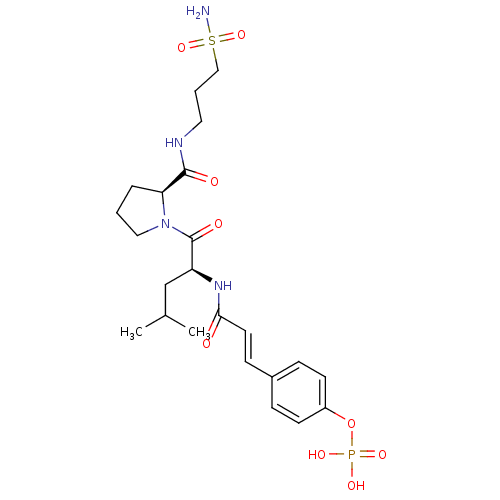 BDBM50300624 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-(3-sulfamoylpropylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL576736
BDBM50300624 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-(3-sulfamoylpropylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL576736 BDBM50300646 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoethylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL574636
BDBM50300646 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoethylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL574636 [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate;triethylazanium SMR000718807 [5-(6-aminopurin-9-yl)-3,4-dihydroxy-2-oxolanyl]methyl hydrogen phosphate;triethylammonium (5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl hydrogen phosphate;triethylammonium BDBM61258 cid_16396156 MLS001306424 [5-(6-aminopurin-9-yl)-3,4-bis(oxidanyl)oxolan-2-yl]methyl hydrogen phosphate;triethylazanium
[5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate;triethylazanium SMR000718807 [5-(6-aminopurin-9-yl)-3,4-dihydroxy-2-oxolanyl]methyl hydrogen phosphate;triethylammonium (5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl hydrogen phosphate;triethylammonium BDBM61258 cid_16396156 MLS001306424 [5-(6-aminopurin-9-yl)-3,4-bis(oxidanyl)oxolan-2-yl]methyl hydrogen phosphate;triethylazanium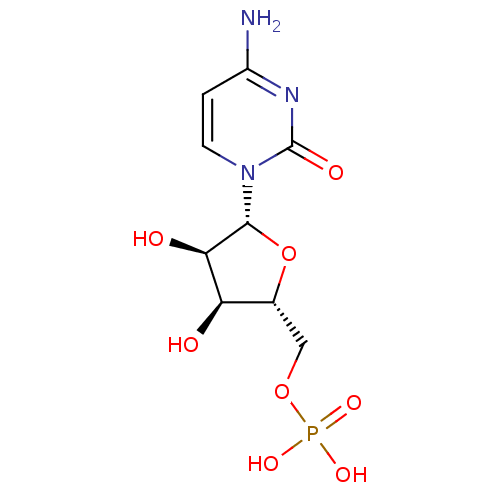 CYTIDINE-5'-MONOPHOSPHATE Phosphoric acid mono-[5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydro-furan-2-ylmethyl] ester CHEMBL307679 cytidine 5'-phosphate ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl dihydrogen phosphate BDBM50310540 ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl dihydrogen phosphate
CYTIDINE-5'-MONOPHOSPHATE Phosphoric acid mono-[5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydro-furan-2-ylmethyl] ester CHEMBL307679 cytidine 5'-phosphate ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl dihydrogen phosphate BDBM50310540 ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl dihydrogen phosphate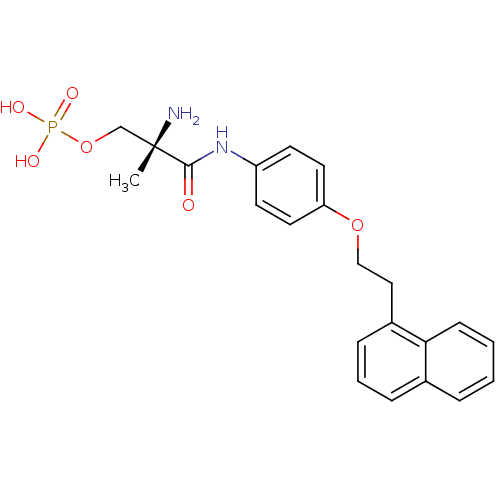 CHEMBL514189 (S)-2-amino-2-methyl-3-(4-(2-(naphthalen-1-yl)ethoxy)phenylamino)-3-oxopropyl dihydrogen phosphate BDBM50249239
CHEMBL514189 (S)-2-amino-2-methyl-3-(4-(2-(naphthalen-1-yl)ethoxy)phenylamino)-3-oxopropyl dihydrogen phosphate BDBM50249239 CHEMBL384771 2-thio-1-beta-D-ribofuranosyl(3H)pyrimidine-2,4-dione 5'-monophosphate BDBM50199180 1-(BETA-D-RIBOFURANOSYL)-2-THIO-URACIL-5'-PHOSPHATE
CHEMBL384771 2-thio-1-beta-D-ribofuranosyl(3H)pyrimidine-2,4-dione 5'-monophosphate BDBM50199180 1-(BETA-D-RIBOFURANOSYL)-2-THIO-URACIL-5'-PHOSPHATE 4-(3-((S)-1-((S)-2-((S)-1-amino-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL578233 BDBM50300617
4-(3-((S)-1-((S)-2-((S)-1-amino-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL578233 BDBM50300617 BDBM50300631 4-(3-((S)-1-((S)-2-((R)-1-amino-1-oxooctan-4-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL576785
BDBM50300631 4-(3-((S)-1-((S)-2-((R)-1-amino-1-oxooctan-4-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL576785 CHEMBL1784780 (S)-2-acetamido-3-((S)-1-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-1-oxopropan-2-ylamino)-1-oxopropan-2-ylamino)-3-oxopropyl dihydrogen phosphate BDBM50345636
CHEMBL1784780 (S)-2-acetamido-3-((S)-1-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-1-oxopropan-2-ylamino)-1-oxopropan-2-ylamino)-3-oxopropyl dihydrogen phosphate BDBM50345636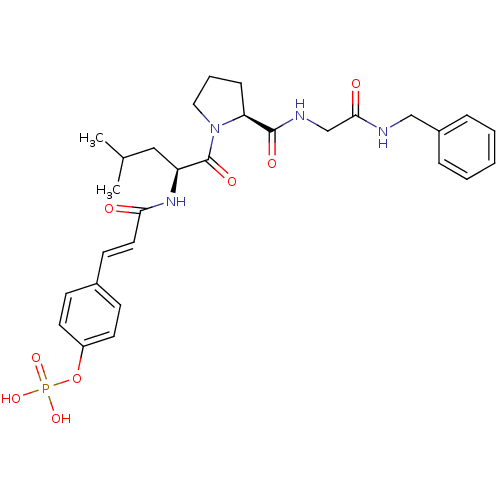 4-(3-((S)-1-((S)-2-(2-(benzylamino)-2-oxoethylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300613 CHEMBL583138
4-(3-((S)-1-((S)-2-(2-(benzylamino)-2-oxoethylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300613 CHEMBL583138 4-(3-((S)-1-((S)-2-(4-(hydroxyamino)-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300625 CHEMBL574875
4-(3-((S)-1-((S)-2-(4-(hydroxyamino)-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300625 CHEMBL574875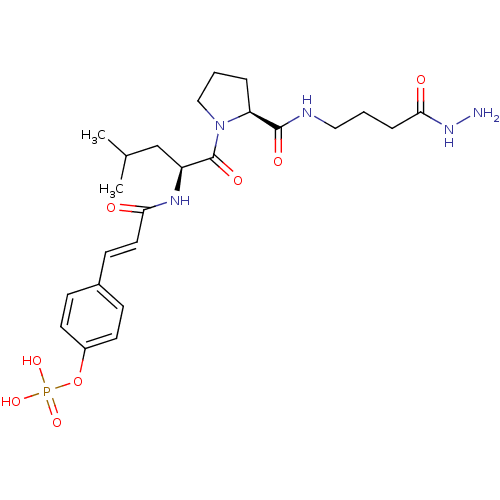 BDBM50300626 CHEMBL575098 4-(3-((S)-1-((S)-2-(4-hydrazinyl-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300626 CHEMBL575098 4-(3-((S)-1-((S)-2-(4-hydrazinyl-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573048 BDBM50300618 4-(3-((S)-1-((S)-2-(4-amino-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL573048 BDBM50300618 4-(3-((S)-1-((S)-2-(4-amino-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL1784770 BDBM50345628 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate
CHEMBL1784770 BDBM50345628 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL573069 BDBM50300647 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-((R)-1-ureidopropan-2-ylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL573069 BDBM50300647 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-((R)-1-ureidopropan-2-ylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate (R)-2-amino-3-(4-octylphenylamino)-3-oxopropyl dihydrogen phosphate CHEMBL227371 BDBM50198840
(R)-2-amino-3-(4-octylphenylamino)-3-oxopropyl dihydrogen phosphate CHEMBL227371 BDBM50198840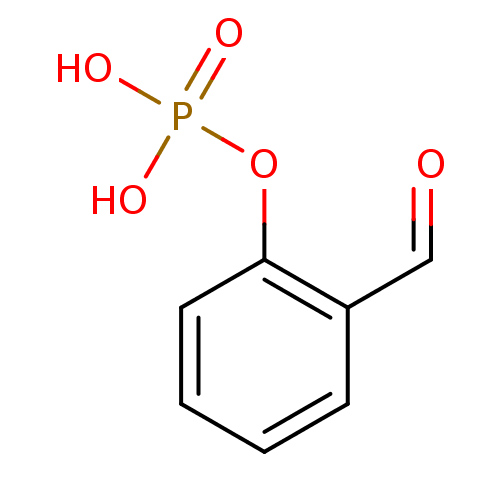 2-formylphenoxyphosphonic acid BDBM14680 2-formylphenyl dihydrogen phosphate RU78262 CHEMBL286678 Fragment 10
2-formylphenoxyphosphonic acid BDBM14680 2-formylphenyl dihydrogen phosphate RU78262 CHEMBL286678 Fragment 10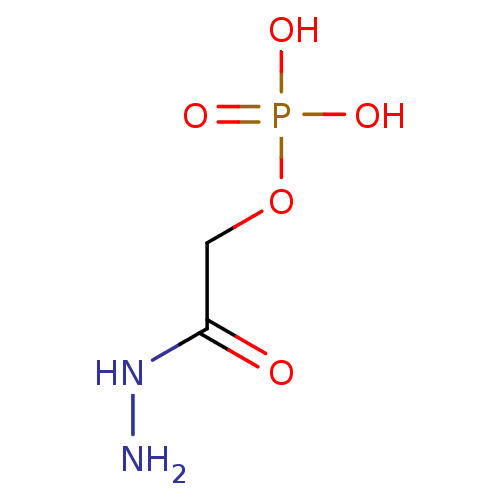 2-hydrazinyl-2-oxoethyl hydrogen phosphate BDBM50167772 Phosphoric acid monohydrazinocarbonylmethyl ester CHEMBL195520
2-hydrazinyl-2-oxoethyl hydrogen phosphate BDBM50167772 Phosphoric acid monohydrazinocarbonylmethyl ester CHEMBL195520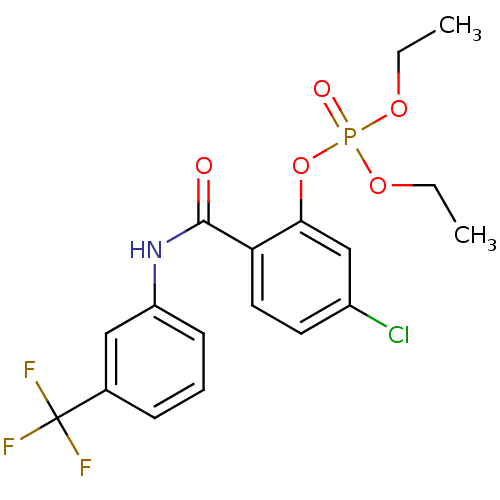 5-chloro-2-{[3-(trifluoromethyl)phenyl]carbamoyl}phenyl diethyl phosphate (26) BDBM150746
5-chloro-2-{[3-(trifluoromethyl)phenyl]carbamoyl}phenyl diethyl phosphate (26) BDBM150746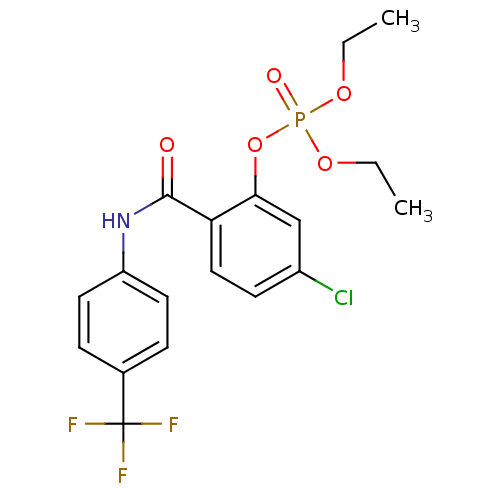 5-chloro-2-{[4-(trifluoromethyl)phenyl]carbamoyl}phenyl diethyl phosphate (27) BDBM150747
5-chloro-2-{[4-(trifluoromethyl)phenyl]carbamoyl}phenyl diethyl phosphate (27) BDBM150747 BDBM50198833 CHEMBL228102 (R)-2-amino-3-(3-octylphenylamino)-3-oxopropyl dihydrogen phosphate
BDBM50198833 CHEMBL228102 (R)-2-amino-3-(3-octylphenylamino)-3-oxopropyl dihydrogen phosphate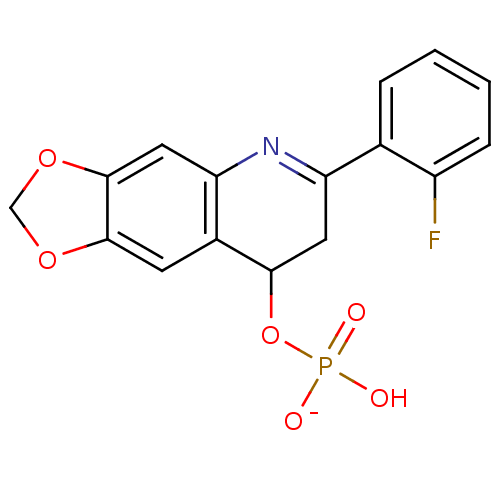 BDBM50312665 Sodium 2-(2-Fluorophenyl)-6,7-methylenedioxyquinolin-4-yl hydrogen phosphate CHEMBL1088572
BDBM50312665 Sodium 2-(2-Fluorophenyl)-6,7-methylenedioxyquinolin-4-yl hydrogen phosphate CHEMBL1088572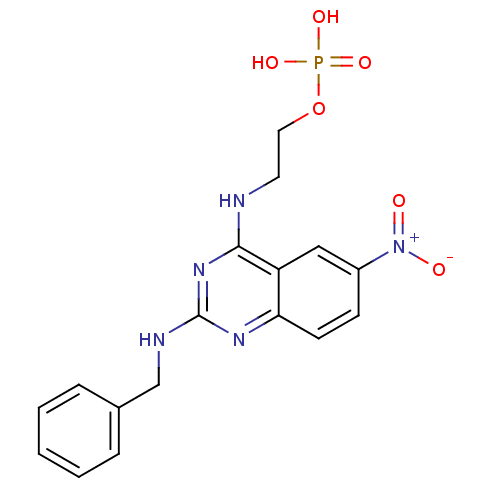 BDBM50343853 2-(2-(Benzylamino)-6-nitroquinazolin-4-ylamino)ethyl dihydrogen phosphate CHEMBL1774786
BDBM50343853 2-(2-(Benzylamino)-6-nitroquinazolin-4-ylamino)ethyl dihydrogen phosphate CHEMBL1774786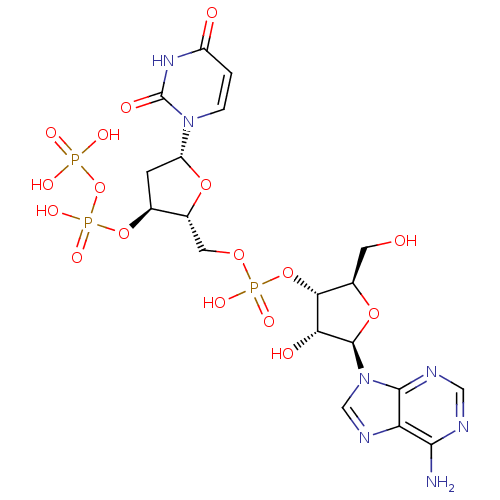 CHEMBL1765479 BDBM50342010 5'-phospho-2'-deoxyuridine-3'-pyrophosphate(P'->5')adenosine3'-phosphate
CHEMBL1765479 BDBM50342010 5'-phospho-2'-deoxyuridine-3'-pyrophosphate(P'->5')adenosine3'-phosphate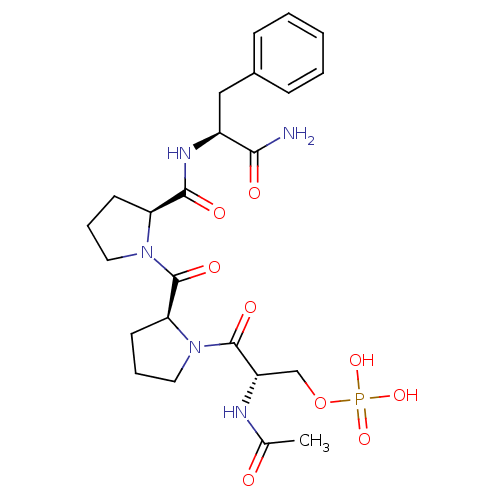 CHEMBL1784781 BDBM50345637 (S)-2-acetamido-3-((S)-2-((S)-2-((S)-1-amino-1-oxo-3-phenylpropan-2-ylcarbamoyl)pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate
CHEMBL1784781 BDBM50345637 (S)-2-acetamido-3-((S)-2-((S)-2-((S)-1-amino-1-oxo-3-phenylpropan-2-ylcarbamoyl)pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate 1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)azetidin-3-yl dihydrogen phosphate BDBM491208 US10975056, Example 152
1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)azetidin-3-yl dihydrogen phosphate BDBM491208 US10975056, Example 152 1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)pyrrolidin-3-yl dihydrogen phosphate BDBM491206 US10975056, Example 150
1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)pyrrolidin-3-yl dihydrogen phosphate BDBM491206 US10975056, Example 150 US10975056, Example 155 BDBM491211 1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)piperidin-4-yl dihydrogen phosphate
US10975056, Example 155 BDBM491211 1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)piperidin-4-yl dihydrogen phosphate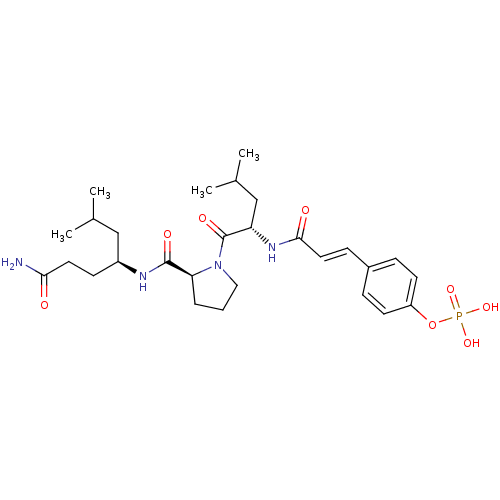 BDBM50300630 CHEMBL574844 4-(3-((S)-1-((S)-2-((S)-1-amino-6-methyl-1-oxoheptan-4-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300630 CHEMBL574844 4-(3-((S)-1-((S)-2-((S)-1-amino-6-methyl-1-oxoheptan-4-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300620 4-(3-((S)-1-((S)-2-(4-amino-4-oxobut-2-ynylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575919
BDBM50300620 4-(3-((S)-1-((S)-2-(4-amino-4-oxobut-2-ynylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575919 BDBM50300622 CHEMBL577593 4-(3-((S)-1-((S)-2-((3S,5S)-5-carbamoylpyrrolidin-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300622 CHEMBL577593 4-(3-((S)-1-((S)-2-((3S,5S)-5-carbamoylpyrrolidin-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate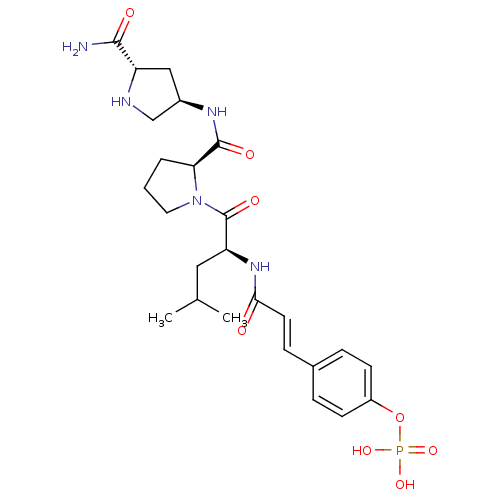 BDBM50300623 CHEMBL573068 4-(3-((S)-1-((S)-2-((3R,5S)-5-carbamoylpyrrolidin-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300623 CHEMBL573068 4-(3-((S)-1-((S)-2-((3R,5S)-5-carbamoylpyrrolidin-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate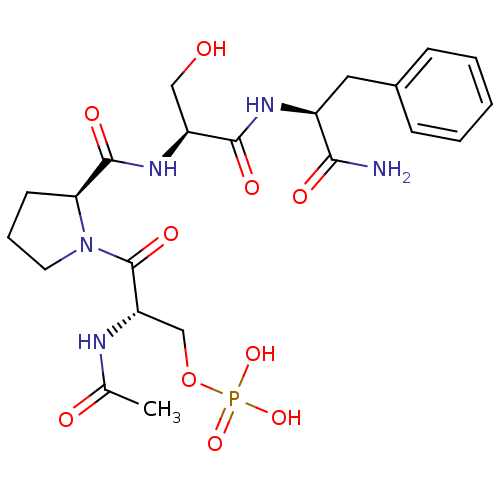 BDBM50345630 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784775
BDBM50345630 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784775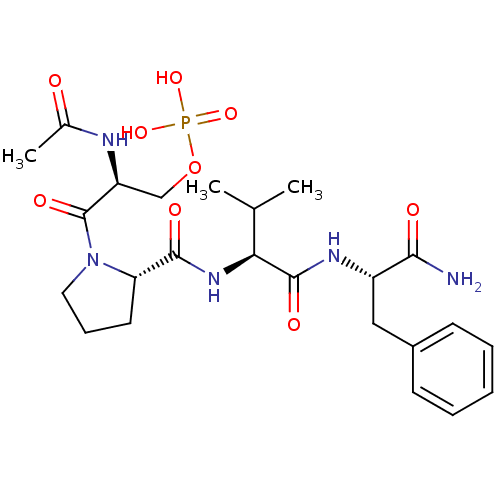 CHEMBL1784703 BDBM50345629 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate
CHEMBL1784703 BDBM50345629 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate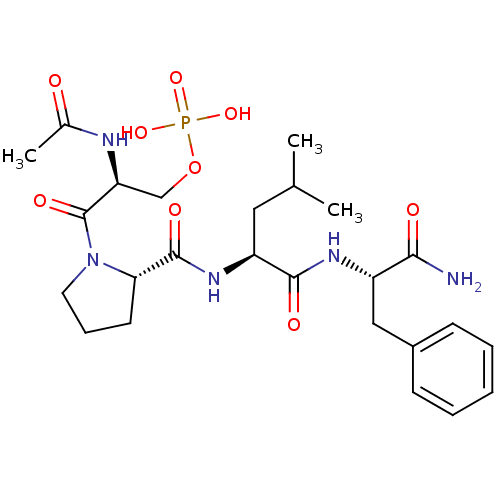 CHEMBL1784777 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-4-methyl-1-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345632
CHEMBL1784777 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-4-methyl-1-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345632 (3R)-1-(6-((2-amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)pyrrolidin-3-yl dihydrogen phosphate BDBM491442 US10975056, Example 416
(3R)-1-(6-((2-amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)pyrrolidin-3-yl dihydrogen phosphate BDBM491442 US10975056, Example 416 [5-[5-fluoranyl-2,4-bis(oxidanylidene)pyrimidin-1-yl]-3-oxidanyl-oxolan-2-yl]methyl [5-[4-(octadecylamino)-2-oxidanylidene-pyrimidin-1-yl]-3,4-bis(oxidanyl)oxolan-2-yl]methyl hydrogen phosphate cid_392613 SMR001565974 [3,4-dihydroxy-5-[2-keto-4-(stearylamino)pyrimidin-1-yl]tetrahydrofuran-2-yl]methyl [5-(5-fluoro-2,4-diketo-pyrimidin-1-yl)-3-hydroxy-tetrahydrofuran-2-yl]methyl hydrogen phosphate MLS002702412 [3,4-dihydroxy-5-[4-(octadecylamino)-2-oxopyrimidin-1-yl]oxolan-2-yl]methyl [5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl hydrogen phosphate [3,4-dihydroxy-5-[4-(octadecylamino)-2-oxo-1-pyrimidinyl]-2-oxolanyl]methyl [5-(5-fluoro-2,4-dioxo-1-pyrimidinyl)-3-hydroxy-2-oxolanyl]methyl hydrogen phosphate BDBM80994
[5-[5-fluoranyl-2,4-bis(oxidanylidene)pyrimidin-1-yl]-3-oxidanyl-oxolan-2-yl]methyl [5-[4-(octadecylamino)-2-oxidanylidene-pyrimidin-1-yl]-3,4-bis(oxidanyl)oxolan-2-yl]methyl hydrogen phosphate cid_392613 SMR001565974 [3,4-dihydroxy-5-[2-keto-4-(stearylamino)pyrimidin-1-yl]tetrahydrofuran-2-yl]methyl [5-(5-fluoro-2,4-diketo-pyrimidin-1-yl)-3-hydroxy-tetrahydrofuran-2-yl]methyl hydrogen phosphate MLS002702412 [3,4-dihydroxy-5-[4-(octadecylamino)-2-oxopyrimidin-1-yl]oxolan-2-yl]methyl [5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl hydrogen phosphate [3,4-dihydroxy-5-[4-(octadecylamino)-2-oxo-1-pyrimidinyl]-2-oxolanyl]methyl [5-(5-fluoro-2,4-dioxo-1-pyrimidinyl)-3-hydroxy-2-oxolanyl]methyl hydrogen phosphate BDBM80994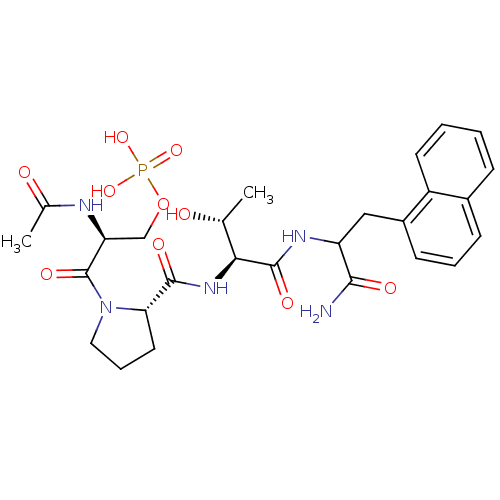 (2S)-2-acetamido-3-((2S)-2-((2S,3R)-1-(1-amino-3-(naphthalen-1-yl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784778 BDBM50345634
(2S)-2-acetamido-3-((2S)-2-((2S,3R)-1-(1-amino-3-(naphthalen-1-yl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784778 BDBM50345634 CHEMBL1784704 (S)-2-acetamido-3-((S)-1-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylamino)-1-oxopropan-2-ylamino)-3-oxopropyl dihydrogen phosphate BDBM50345627
CHEMBL1784704 (S)-2-acetamido-3-((S)-1-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylamino)-1-oxopropan-2-ylamino)-3-oxopropyl dihydrogen phosphate BDBM50345627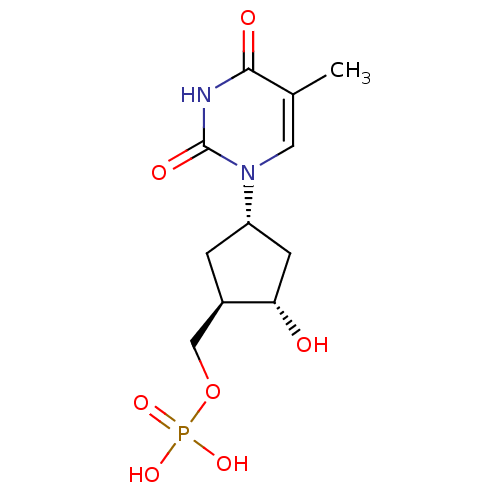 ((1R,2S,4S)-2-hydroxy-4-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)cyclopentyl)methyl dihydrogen phosphate BDBM50223802 CHEMBL393853
((1R,2S,4S)-2-hydroxy-4-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)cyclopentyl)methyl dihydrogen phosphate BDBM50223802 CHEMBL393853 BDBM573689 (R)-3-((1- (methyl- d3)pyrrolidin-2- yl)methyl-d2)- 1H-indol-4-yl dihydrogen phosphate US11453689, Compound I-54
BDBM573689 (R)-3-((1- (methyl- d3)pyrrolidin-2- yl)methyl-d2)- 1H-indol-4-yl dihydrogen phosphate US11453689, Compound I-54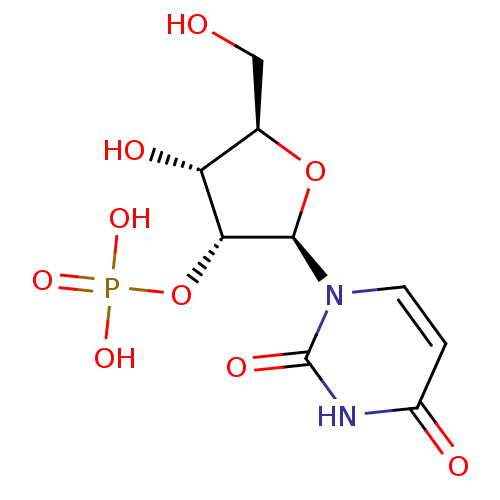 BDBM50292723 uridine 2'-phosphate CHEMBL447360 PHOSPHORIC ACID MONO-[2-(2,4-DIOXO-3,4-DIHYDRO-2H-PYRIMIDIN-1-YL)-4-HYDROXY-5-HYDROXYMETHYL-TETRAHYDRO-FURAN-3-YL] ESTER (2R,3R,4R,5R)-2-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-3-yl dihydrogen phosphate
BDBM50292723 uridine 2'-phosphate CHEMBL447360 PHOSPHORIC ACID MONO-[2-(2,4-DIOXO-3,4-DIHYDRO-2H-PYRIMIDIN-1-YL)-4-HYDROXY-5-HYDROXYMETHYL-TETRAHYDRO-FURAN-3-YL] ESTER (2R,3R,4R,5R)-2-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-3-yl dihydrogen phosphate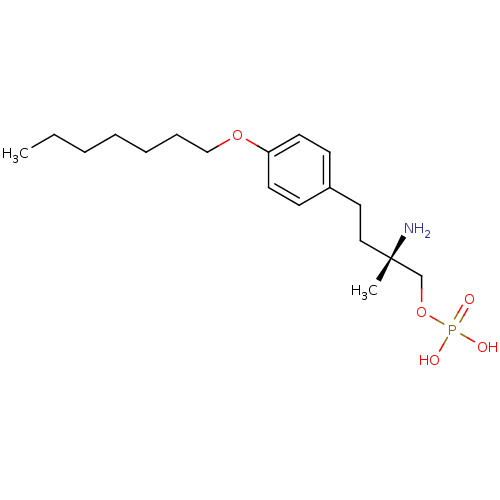 (S)-2-amino-4-(4-(heptyloxy)phenyl)-2-methylbutyl dihydrogen phosphate BDBM50313499 CHEMBL1084929
(S)-2-amino-4-(4-(heptyloxy)phenyl)-2-methylbutyl dihydrogen phosphate BDBM50313499 CHEMBL1084929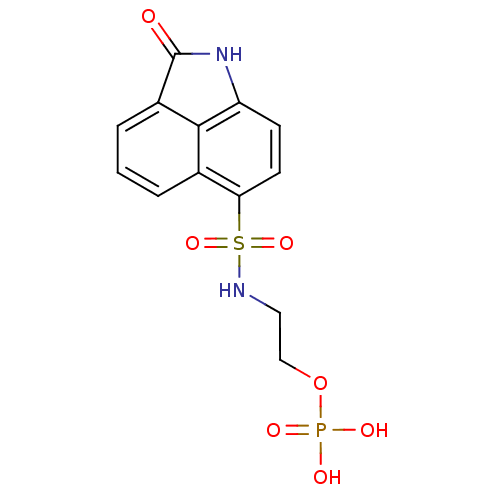 2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)ethyldihydrogen phosphate BDBM50316579 CHEMBL1095991
2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)ethyldihydrogen phosphate BDBM50316579 CHEMBL1095991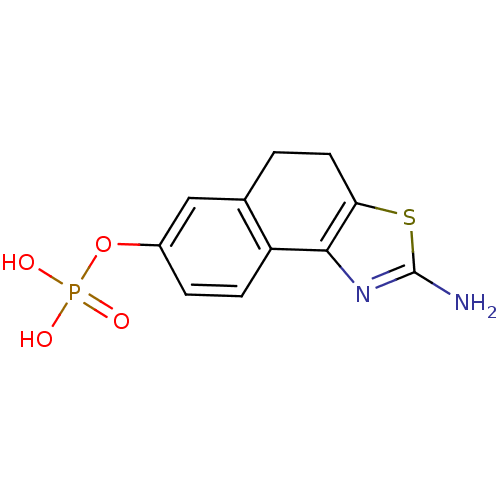 2-amino-4,5-dihydronaphtho[1,2-d]thiazol-7-yl dihydrogen phosphate CHEMBL566049 BDBM50302494
2-amino-4,5-dihydronaphtho[1,2-d]thiazol-7-yl dihydrogen phosphate CHEMBL566049 BDBM50302494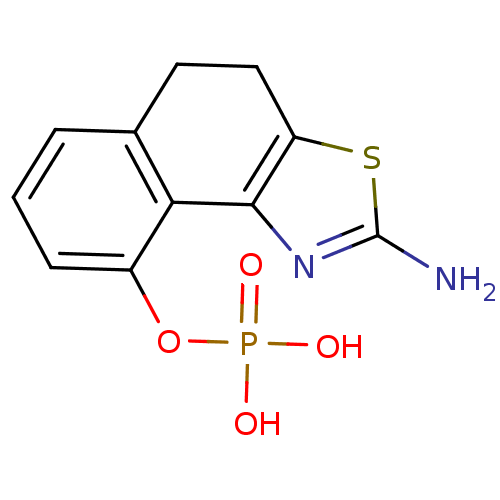 2-amino-4,5-dihydronaphtho[1,2-d]thiazol-9-yl dihydrogen phosphate BDBM50302496 CHEMBL576125
2-amino-4,5-dihydronaphtho[1,2-d]thiazol-9-yl dihydrogen phosphate BDBM50302496 CHEMBL576125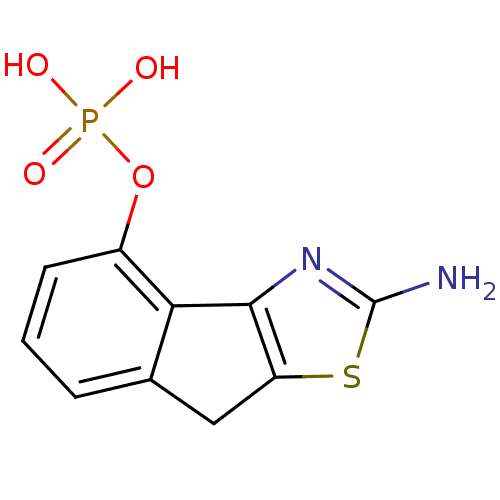 2-amino-8H-indeno[1,2-d]thiazol-4-yl dihydrogen phosphate CHEMBL578134 BDBM50302493
2-amino-8H-indeno[1,2-d]thiazol-4-yl dihydrogen phosphate CHEMBL578134 BDBM50302493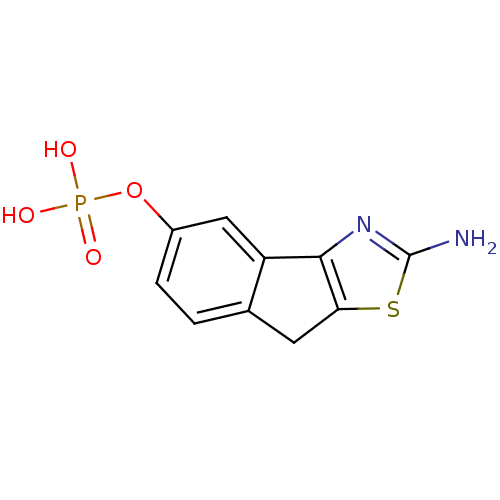 2-amino-8H-indeno[1,2-d]thiazol-5-yl dihydrogen phosphate BDBM50302492 CHEMBL568771
2-amino-8H-indeno[1,2-d]thiazol-5-yl dihydrogen phosphate BDBM50302492 CHEMBL568771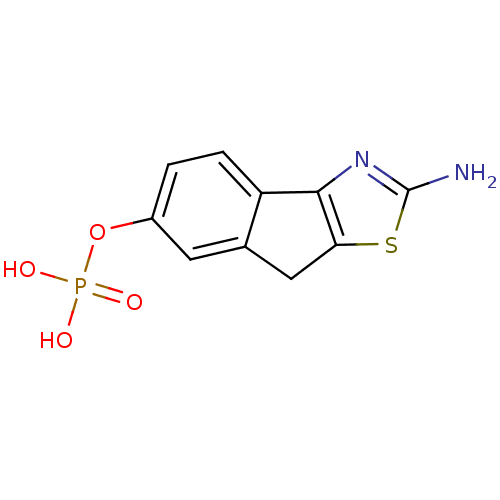 2-amino-8H-indeno[1,2-d]thiazol-6-yl dihydrogen phosphate BDBM50302491 CHEMBL566670
2-amino-8H-indeno[1,2-d]thiazol-6-yl dihydrogen phosphate BDBM50302491 CHEMBL566670 3-(4,5,6,7-Tetrabromo-2H-1,2,3-benzotriazol-2-yl)propyl dihydrogen phosphate BDBM50276680 CHEMBL459343
3-(4,5,6,7-Tetrabromo-2H-1,2,3-benzotriazol-2-yl)propyl dihydrogen phosphate BDBM50276680 CHEMBL459343 BDBM150734 2‐[(4‐bromophenyl)carbamoyl]‐4‐chlorophenyl diethyl phosphate (14)
BDBM150734 2‐[(4‐bromophenyl)carbamoyl]‐4‐chlorophenyl diethyl phosphate (14) BDBM150736 2‐[(3‐bromophenyl)carbamoyl]‐4‐chlorophenyl diethyl phosphate (16)
BDBM150736 2‐[(3‐bromophenyl)carbamoyl]‐4‐chlorophenyl diethyl phosphate (16)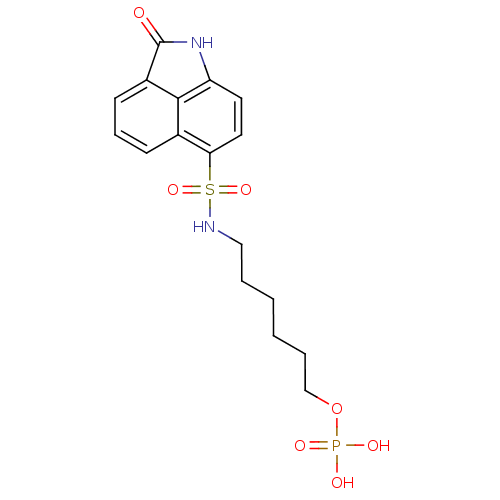 BDBM50316580 6-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)hexyldihydrogen phosphate CHEMBL1095867
BDBM50316580 6-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)hexyldihydrogen phosphate CHEMBL1095867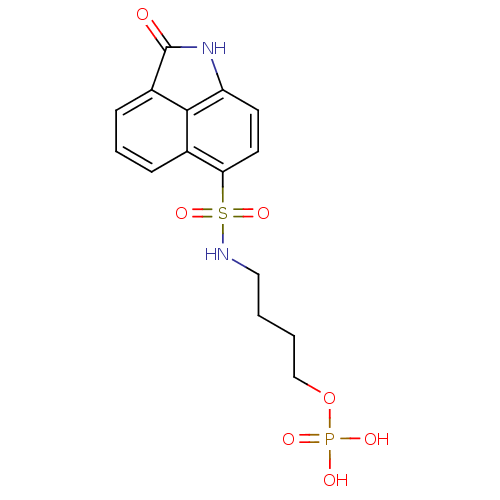 CHEMBL1097477 4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)-butyldihydrogen phosphate BDBM50316577
CHEMBL1097477 4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)-butyldihydrogen phosphate BDBM50316577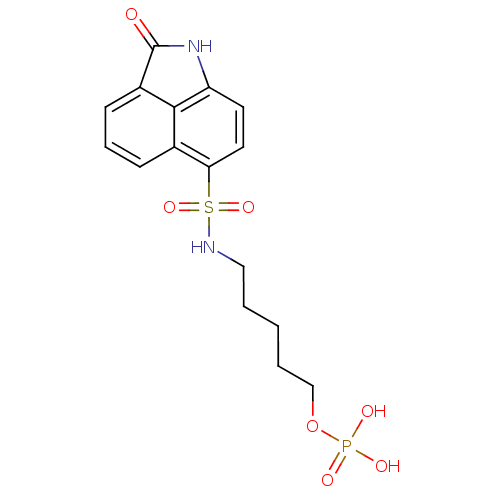 CHEMBL1097478 BDBM50316581 5-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)-pentyldihydrogen phosphate
CHEMBL1097478 BDBM50316581 5-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)-pentyldihydrogen phosphate CHEMBL455775 BDBM50292713 5'-phospho-2'-deoxyuridine 3-pyrophosphate (P'->5') adenosine 3'-phosphate
CHEMBL455775 BDBM50292713 5'-phospho-2'-deoxyuridine 3-pyrophosphate (P'->5') adenosine 3'-phosphate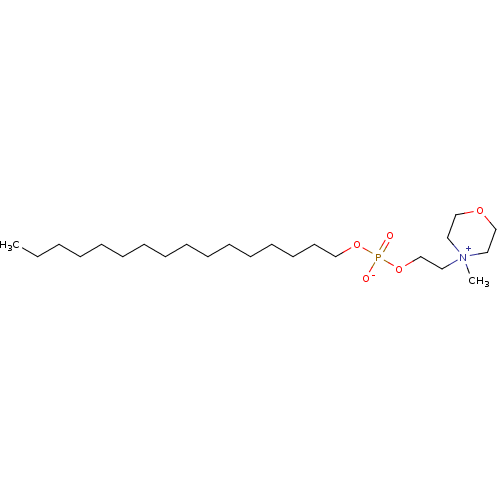 CHEMBL88487 Hexadecyl[2-(N-Methylmorpholinio)ethyl]phosphate BDBM50051809 (N-methylmorpholino)ethanol hexadecyl phosphonate
CHEMBL88487 Hexadecyl[2-(N-Methylmorpholinio)ethyl]phosphate BDBM50051809 (N-methylmorpholino)ethanol hexadecyl phosphonate 4-(3-((S)-1-((S)-2-((R)-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL578635 BDBM50300627
4-(3-((S)-1-((S)-2-((R)-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL578635 BDBM50300627 4-(3-((S)-1-((S)-2-((S)-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573049 BDBM50300628
4-(3-((S)-1-((S)-2-((S)-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573049 BDBM50300628 CHEMBL574202 4-(3-((S)-1-((S)-2-((E)-4-amino-4-oxobut-2-enylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300619
CHEMBL574202 4-(3-((S)-1-((S)-2-((E)-4-amino-4-oxobut-2-enylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300619 (S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784774 BDBM50345623
(S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784774 BDBM50345623 (S)-2-acetamido-3-((S)-2-((2S,3S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-1-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345631 CHEMBL1784776
(S)-2-acetamido-3-((S)-2-((2S,3S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-1-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345631 CHEMBL1784776 4-(3-((S)-1-((S)-2-((S)-5-amino-1-morpholino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL584974 BDBM50300638
4-(3-((S)-1-((S)-2-((S)-5-amino-1-morpholino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL584974 BDBM50300638 4-(3-((S)-1-((S)-2-((S)-6-amino-1-(benzylamino)-1,6-dioxohexan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300615 CHEMBL583754
4-(3-((S)-1-((S)-2-((S)-6-amino-1-(benzylamino)-1,6-dioxohexan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300615 CHEMBL583754 BDBM50300614 4-(3-((S)-1-((S)-2-((S)-4-amino-1-(benzylamino)-1,4-dioxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573901
BDBM50300614 4-(3-((S)-1-((S)-2-((S)-4-amino-1-(benzylamino)-1,4-dioxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573901 BDBM50300621 CHEMBL578430 4-(3-((S)-1-((S)-2-((S)-2-(3-amino-3-oxopropyl)pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300621 CHEMBL578430 4-(3-((S)-1-((S)-2-((S)-2-(3-amino-3-oxopropyl)pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate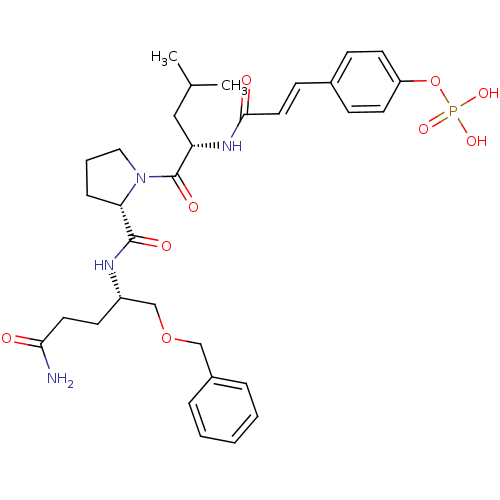 BDBM50300633 CHEMBL573904 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(benzyloxy)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300633 CHEMBL573904 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(benzyloxy)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50345639 CHEMBL1784783 (S)-2-amino-3-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate
BDBM50345639 CHEMBL1784783 (S)-2-amino-3-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate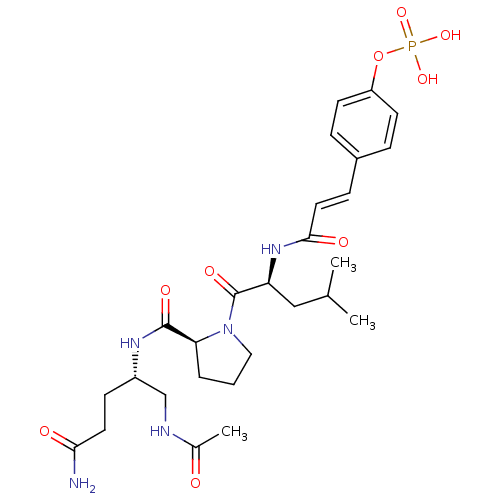 CHEMBL573442 BDBM50300641 4-(3-((S)-1-((S)-2-((S)-1-acetamido-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL573442 BDBM50300641 4-(3-((S)-1-((S)-2-((S)-1-acetamido-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575513 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(dibenzylamino)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300639
CHEMBL575513 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(dibenzylamino)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300639 BDBM491210 2-((1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)piperidin-4-epoxy)ethyl dihydrogen phosphate US10975056, Example 154
BDBM491210 2-((1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)piperidin-4-epoxy)ethyl dihydrogen phosphate US10975056, Example 154 (S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-3-(4-hydroxyphenyl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784779 BDBM50345635
(S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-3-(4-hydroxyphenyl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784779 BDBM50345635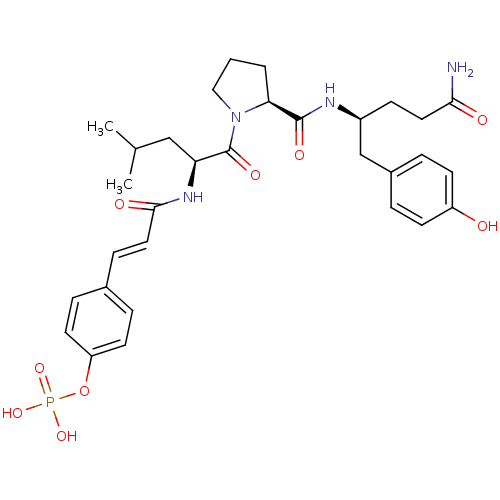 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(4-hydroxyphenyl)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300632 CHEMBL573903
4-(3-((S)-1-((S)-2-((S)-5-amino-1-(4-hydroxyphenyl)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300632 CHEMBL573903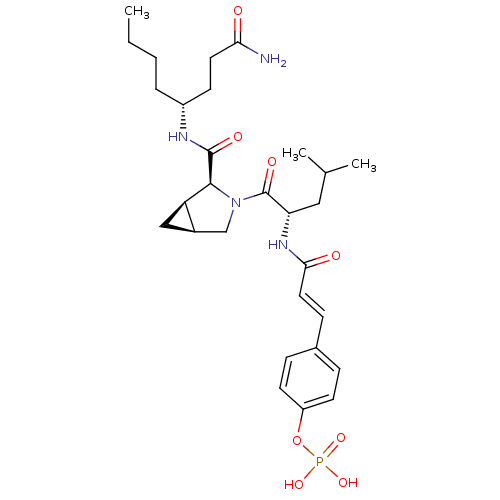 BDBM50300653 4-(3-((S)-1-((cis)-2-((R)-1-amino-1-oxooctan-4-ylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583116
BDBM50300653 4-(3-((S)-1-((cis)-2-((R)-1-amino-1-oxooctan-4-ylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583116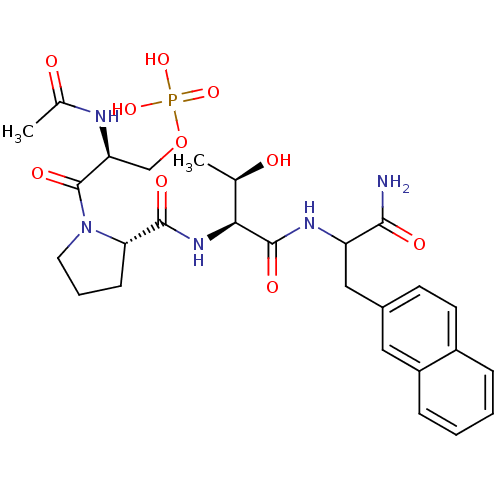 CHEMBL1784769 (2S)-2-acetamido-3-((2S)-2-((2S,3R)-1-(1-amino-3-(naphthalen-2-yl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345633
CHEMBL1784769 (2S)-2-acetamido-3-((2S)-2-((2S,3R)-1-(1-amino-3-(naphthalen-2-yl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345633 (2R,5R,8R,11R)-11-benzyl-2-carbamoyl-8-((S)-1-hydroxyethyl)-5-methyl-4,7,10,13-tetraoxo-3,6,9,12-tetraazatetradecyl dihydrogen phosphate BDBM50345638 CHEMBL1784782
(2R,5R,8R,11R)-11-benzyl-2-carbamoyl-8-((S)-1-hydroxyethyl)-5-methyl-4,7,10,13-tetraoxo-3,6,9,12-tetraazatetradecyl dihydrogen phosphate BDBM50345638 CHEMBL1784782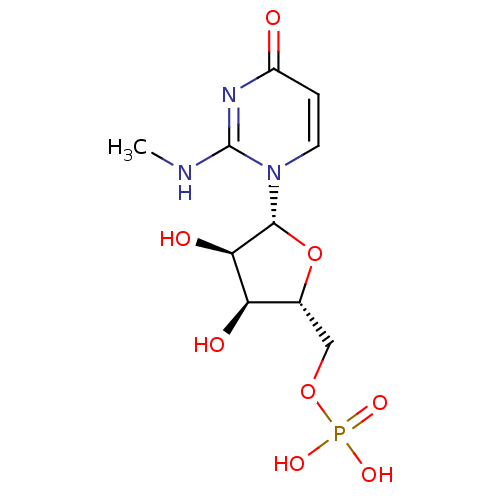 BDBM50341894 ((2R,3S,4R,5R)-3,4-dihydroxy-5-(2-(methylamino)-4-oxopyrimidin-1(4H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL1767423
BDBM50341894 ((2R,3S,4R,5R)-3,4-dihydroxy-5-(2-(methylamino)-4-oxopyrimidin-1(4H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL1767423 BDBM50300636 4-(3-((S)-1-((S)-2-((S)-5-amino-5-oxo-1-(piperidin-1-yl)pentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583963
BDBM50300636 4-(3-((S)-1-((S)-2-((S)-5-amino-5-oxo-1-(piperidin-1-yl)pentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583963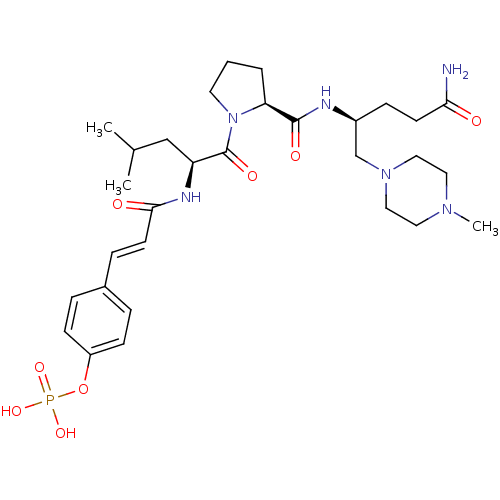 BDBM50300637 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(4-methylpiperazin-1-yl)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573905
BDBM50300637 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(4-methylpiperazin-1-yl)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573905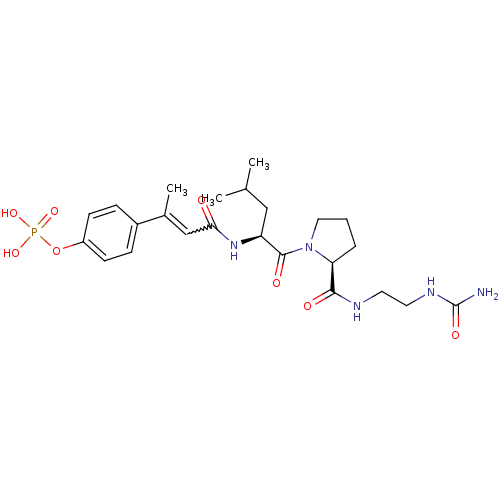 4-((E)-4-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoethylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-4-oxobut-2-en-2-yl)phenyl dihydrogen phosphate BDBM50343638 CHEMBL1774961
4-((E)-4-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoethylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-4-oxobut-2-en-2-yl)phenyl dihydrogen phosphate BDBM50343638 CHEMBL1774961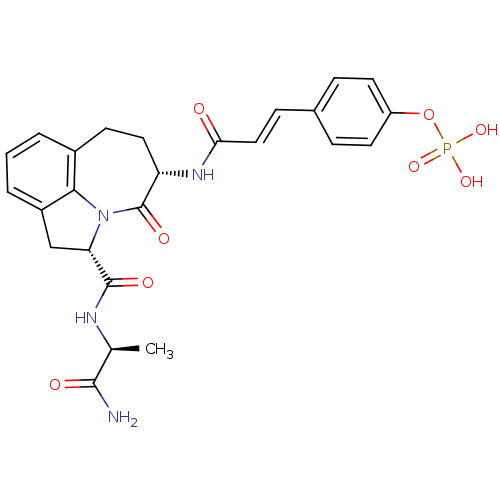 BDBM50267516 CHEMBL486267 4-(3-((3S,6S)-6-((S)-1-amino-1-oxopropan-2-ylcarbamoyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50267516 CHEMBL486267 4-(3-((3S,6S)-6-((S)-1-amino-1-oxopropan-2-ylcarbamoyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate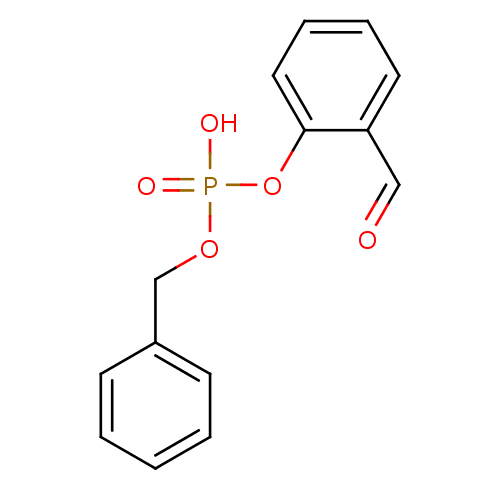 (benzyloxy)(2-formylphenoxy)phosphinic acid PASBN BDBM14688 benzyl 2-formylphenyl hydrogen phosphate Fragment 18
(benzyloxy)(2-formylphenoxy)phosphinic acid PASBN BDBM14688 benzyl 2-formylphenyl hydrogen phosphate Fragment 18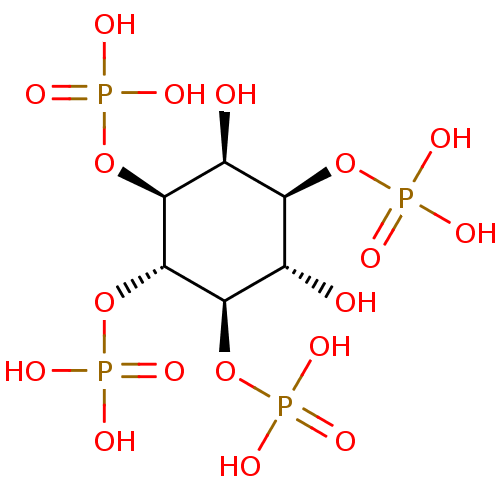 1D-myo-inositol 1,3,4,5-tetrakis(dihydrogen phosphate) CHEMBL23552 BDBM50075651 1D-myo-inositol 1,3,4,5-tetrakisphosphate
1D-myo-inositol 1,3,4,5-tetrakis(dihydrogen phosphate) CHEMBL23552 BDBM50075651 1D-myo-inositol 1,3,4,5-tetrakisphosphate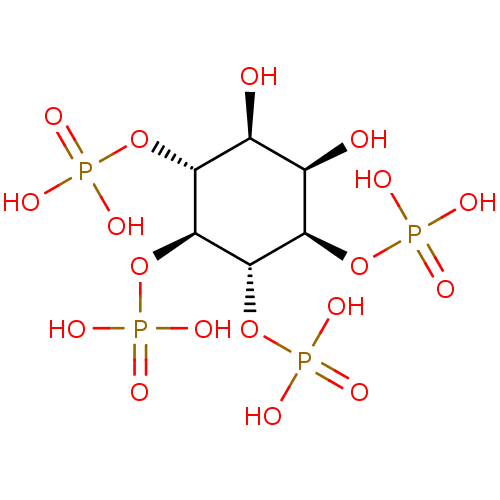 1D-myo-inositol 1,4,5,6-tetrakisphosphate BDBM50075649 CHEMBL282059 1D-myo-inositol 1,4,5,6-tetrakis(dihydrogen phosphate)
1D-myo-inositol 1,4,5,6-tetrakisphosphate BDBM50075649 CHEMBL282059 1D-myo-inositol 1,4,5,6-tetrakis(dihydrogen phosphate)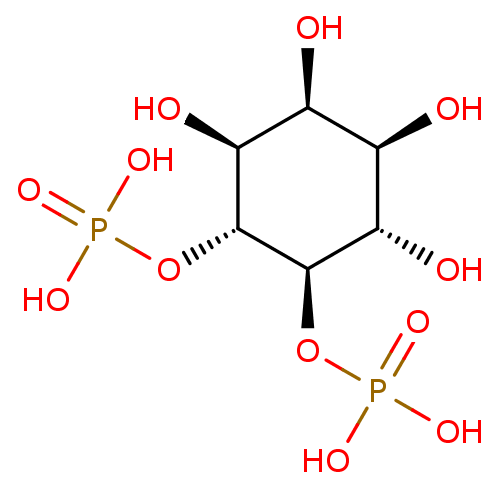 1D-myo-inositol 4,5-bisphosphate BDBM50284584 CHEMBL21825 1D-myo-inositol 4,5-bis(dihydrogen phosphate)
1D-myo-inositol 4,5-bisphosphate BDBM50284584 CHEMBL21825 1D-myo-inositol 4,5-bis(dihydrogen phosphate)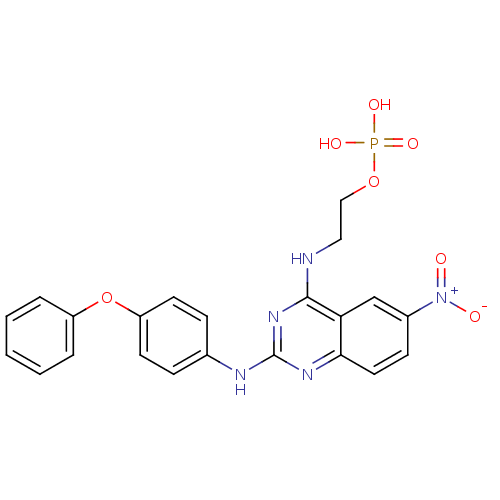 2-(6-Nitro-2-(4-phenoxyphenylamino)quinazolin-4-ylamino)ethyl dihydrogen phosphate BDBM50343852 CHEMBL1774785
2-(6-Nitro-2-(4-phenoxyphenylamino)quinazolin-4-ylamino)ethyl dihydrogen phosphate BDBM50343852 CHEMBL1774785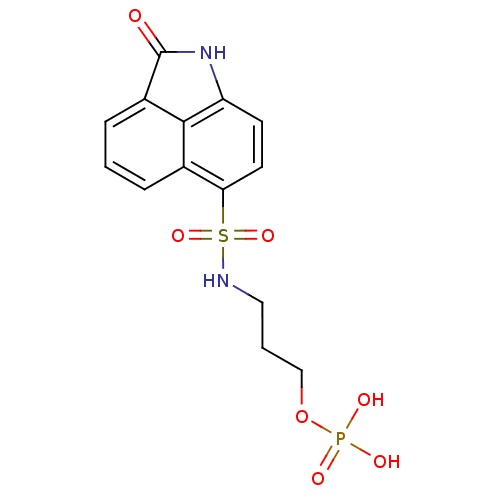 3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)propyl dihydrogen phosphate BDBM50316578 CHEMBL1097476
3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)propyl dihydrogen phosphate BDBM50316578 CHEMBL1097476 4‐bromo‐2‐[(4‐bromophenyl)carbamoyl]phenyl diethyl phosphate (8) BDBM150728
4‐bromo‐2‐[(4‐bromophenyl)carbamoyl]phenyl diethyl phosphate (8) BDBM150728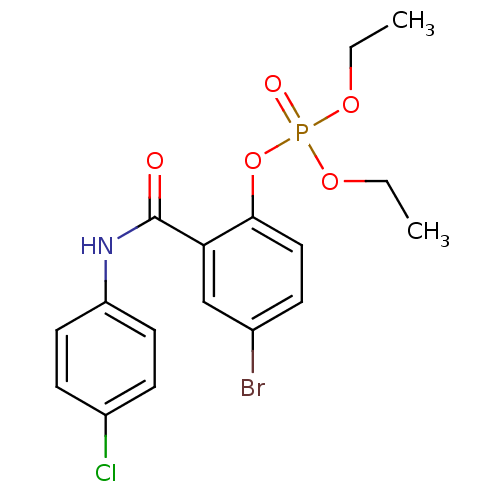 4‐bromo‐2‐[(4‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (3) BDBM150723
4‐bromo‐2‐[(4‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (3) BDBM150723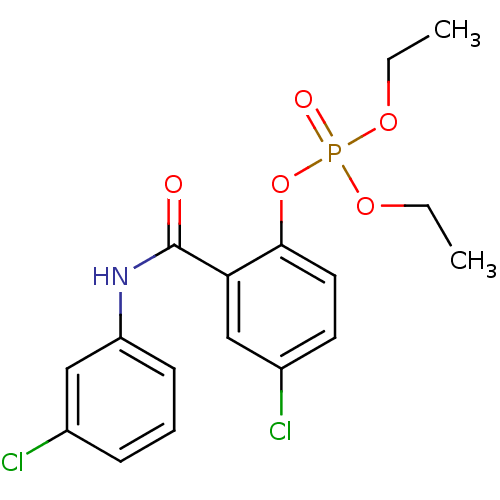 4‐chloro‐2‐[(3‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (10) BDBM150730
4‐chloro‐2‐[(3‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (10) BDBM150730 4‐chloro‐2‐[(3‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (12) BDBM150732
4‐chloro‐2‐[(3‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (12) BDBM150732 4‐chloro‐2‐[(4‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (11) BDBM150731
4‐chloro‐2‐[(4‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (11) BDBM150731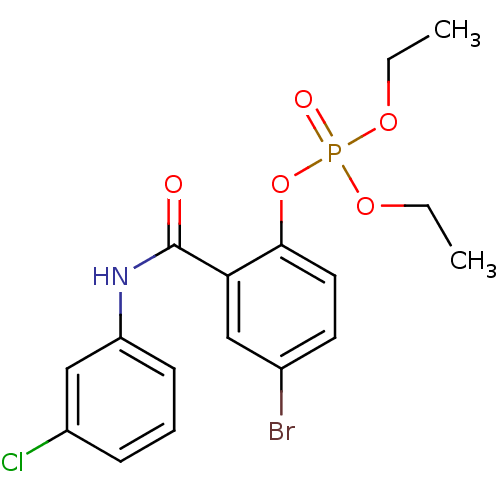 BDBM150722 4‐bromo‐2‐[(3‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (2)
BDBM150722 4‐bromo‐2‐[(3‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (2)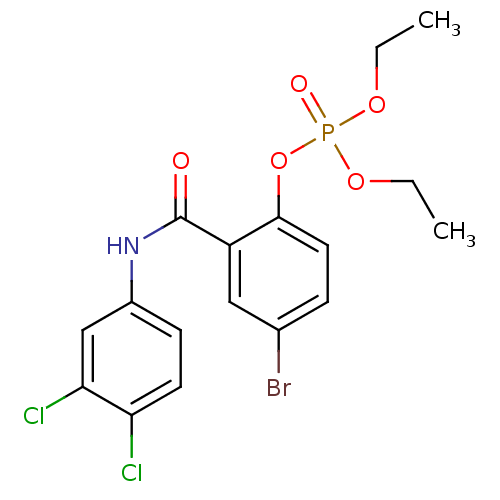 BDBM150724 4‐bromo‐2‐[(3,4‐dichlorophenyl)carbamoyl]phenyl diethyl phosphate (4)
BDBM150724 4‐bromo‐2‐[(3,4‐dichlorophenyl)carbamoyl]phenyl diethyl phosphate (4)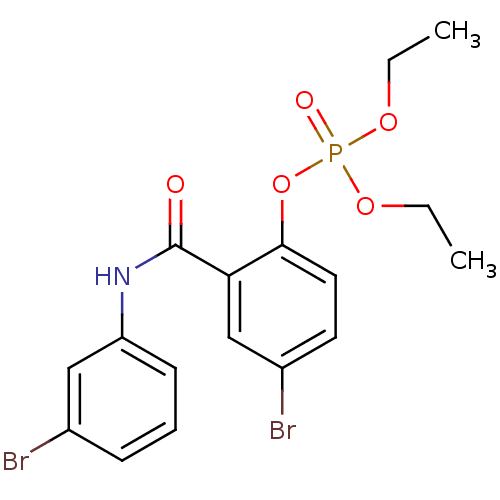 BDBM150727 4‐bromo‐2‐[(3‐bromophenyl)carbamoyl]phenyl diethyl phosphate (7)
BDBM150727 4‐bromo‐2‐[(3‐bromophenyl)carbamoyl]phenyl diethyl phosphate (7)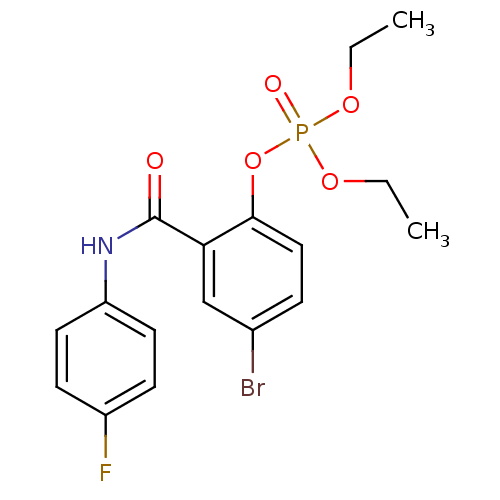 BDBM150729 4‐bromo‐2‐[(4‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (9)
BDBM150729 4‐bromo‐2‐[(4‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (9) BDBM150733 4‐chloro‐2‐[(4‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (13)
BDBM150733 4‐chloro‐2‐[(4‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (13)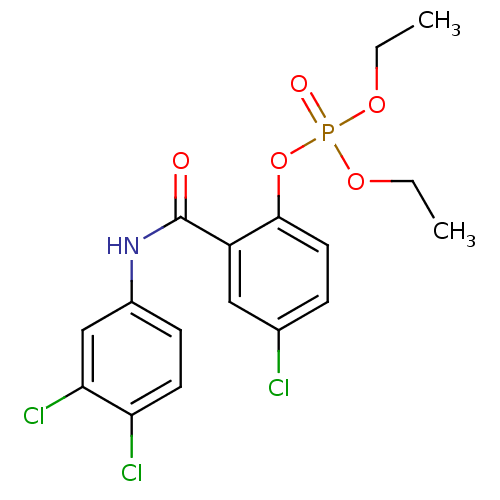 BDBM150735 4‐chloro‐2‐[(3,4‐dichlorophenyl)carbamoyl]phenyl diethyl phosphate (15)
BDBM150735 4‐chloro‐2‐[(3,4‐dichlorophenyl)carbamoyl]phenyl diethyl phosphate (15)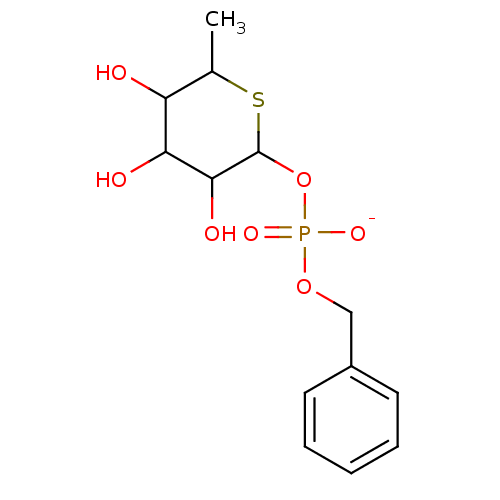 BDBM50287679 CHEMBL61555 Sodium; benzyl 3,4,5-trihydroxy-6-methyltetrahydro-2H-thiopyran-2-yl hydrogen phosphate
BDBM50287679 CHEMBL61555 Sodium; benzyl 3,4,5-trihydroxy-6-methyltetrahydro-2H-thiopyran-2-yl hydrogen phosphate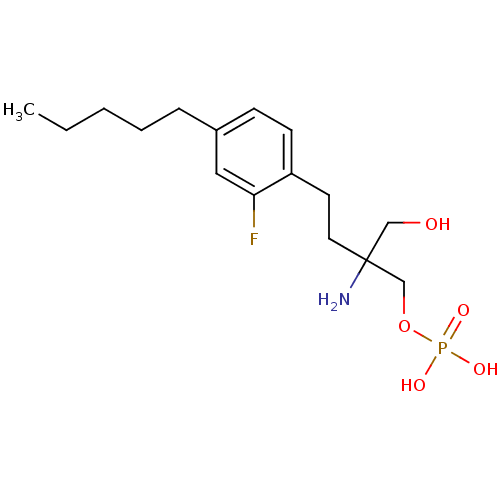 BDBM50315803 2-amino-4-(2-fluoro-4-pentylphenyl)-2-(hydroxymethyl)butyl dihydrogen phosphate CHEMBL1095889
BDBM50315803 2-amino-4-(2-fluoro-4-pentylphenyl)-2-(hydroxymethyl)butyl dihydrogen phosphate CHEMBL1095889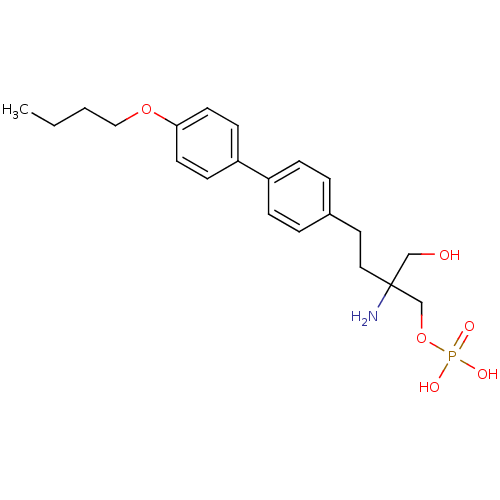 BDBM50315805 2-amino-4-(4'-butoxybiphenyl-4-yl)-2-(hydroxymethyl)butyl dihydrogen phosphate CHEMBL1096211
BDBM50315805 2-amino-4-(4'-butoxybiphenyl-4-yl)-2-(hydroxymethyl)butyl dihydrogen phosphate CHEMBL1096211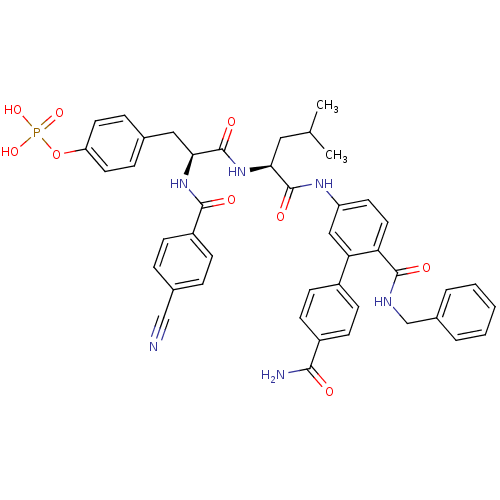 CHEMBL1684872 4-((S)-3-((S)-1-(6-(Benzylcarbamoyl)-4'-carbamoylbiphenyl-3-ylamino)-4-methyl-1-oxopentan-2-ylamino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate BDBM50337199
CHEMBL1684872 4-((S)-3-((S)-1-(6-(Benzylcarbamoyl)-4'-carbamoylbiphenyl-3-ylamino)-4-methyl-1-oxopentan-2-ylamino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate BDBM50337199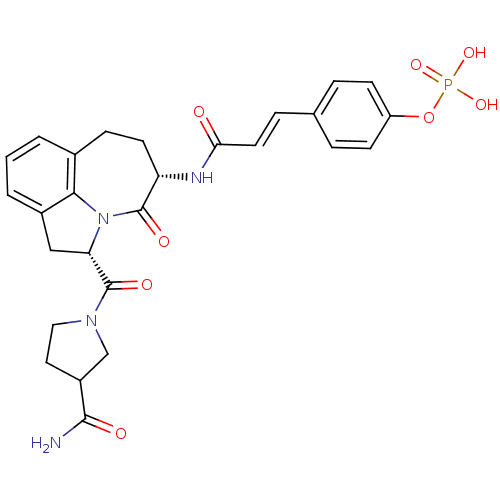 CHEMBL515762 BDBM50267455 4-(3-((3S,6S)-6-(3-carbamoylpyrrolidine-1-carbonyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL515762 BDBM50267455 4-(3-((3S,6S)-6-(3-carbamoylpyrrolidine-1-carbonyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575920 4-(3-((S)-1-((S)-2-((S)-6-amino-2-methyl-6-oxohexan-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300629
CHEMBL575920 4-(3-((S)-1-((S)-2-((S)-6-amino-2-methyl-6-oxohexan-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300629 BDBM699453 (S)-2-(3-(Acrylamidomethyl)-1-(4- (trifluoromethoxy)phenyl)-1H- pyrazolo[3,4-b]pyridin-4-yl)-2- hydroxyethyl dihydrogen phosphate US12110276, Compound 147
BDBM699453 (S)-2-(3-(Acrylamidomethyl)-1-(4- (trifluoromethoxy)phenyl)-1H- pyrazolo[3,4-b]pyridin-4-yl)-2- hydroxyethyl dihydrogen phosphate US12110276, Compound 147 US10988478, Example 145 [1-[2,2-dimethyl-5-(pyrazolo[1,5- a]pyrimidine-3-carbonylamino)-3H- benzofuran-6-yl]-4-piperidyl]methyl diethyl phosphate BDBM494097
US10988478, Example 145 [1-[2,2-dimethyl-5-(pyrazolo[1,5- a]pyrimidine-3-carbonylamino)-3H- benzofuran-6-yl]-4-piperidyl]methyl diethyl phosphate BDBM494097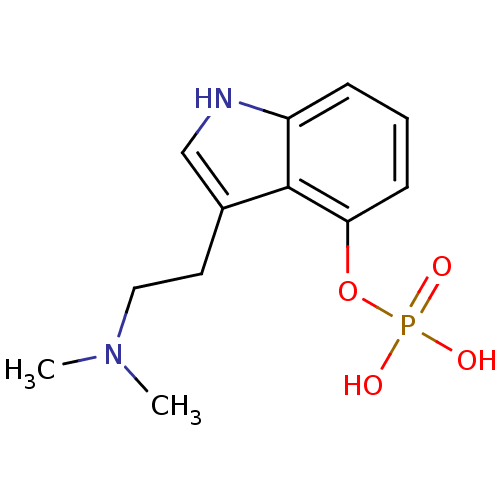 O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine 4-phosphoryloxy-N,N-dimethyltryptamine US11597738, Example 4 BDBM50171269 Indocybin psilocybin 3-[2-(dimethylamino)ethyl]-1H-indol-4-yl dihydrogen phosphate psilocin phosphate ester Psilocybine CHEMBL194378
O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine 4-phosphoryloxy-N,N-dimethyltryptamine US11597738, Example 4 BDBM50171269 Indocybin psilocybin 3-[2-(dimethylamino)ethyl]-1H-indol-4-yl dihydrogen phosphate psilocin phosphate ester Psilocybine CHEMBL194378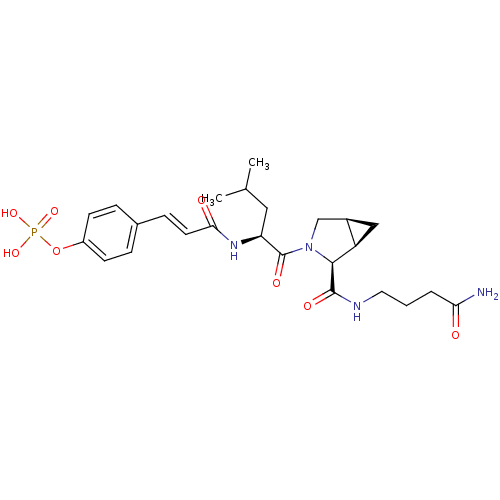 4-(3-((S)-1-((cis)-2-(4-amino-4-oxobutylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL577143 BDBM50300649
4-(3-((S)-1-((cis)-2-(4-amino-4-oxobutylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL577143 BDBM50300649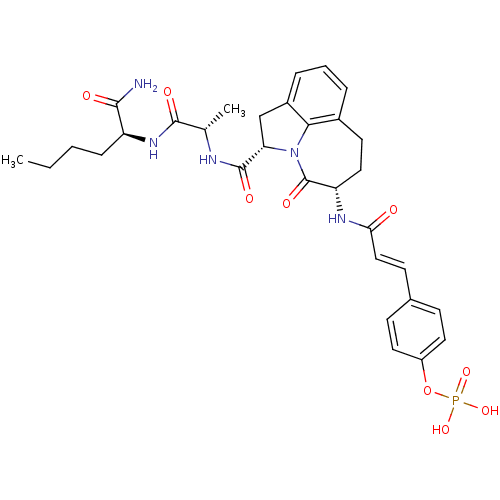 4-(3-((3S,6S)-6-((S)-1-((S)-1-amino-1-oxohexan-2-ylamino)-1-oxopropan-2-ylcarbamoyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL468082 BDBM50267517
4-(3-((3S,6S)-6-((S)-1-((S)-1-amino-1-oxohexan-2-ylamino)-1-oxopropan-2-ylcarbamoyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL468082 BDBM50267517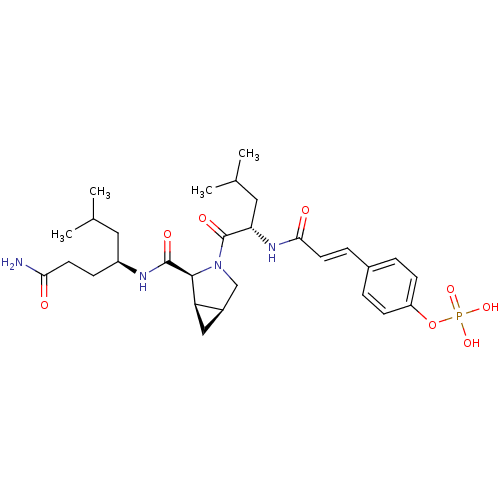 BDBM50300652 4-(3-((S)-1-((cis)-2-((S)-1-amino-6-methyl-1-oxoheptan-4-ylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575484
BDBM50300652 4-(3-((S)-1-((cis)-2-((S)-1-amino-6-methyl-1-oxoheptan-4-ylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575484 BDBM50345626 CHEMBL1784773 (2R,3S)-3-acetamido-4-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-oxobutan-2-yl dihydrogen phosphate
BDBM50345626 CHEMBL1784773 (2R,3S)-3-acetamido-4-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-oxobutan-2-yl dihydrogen phosphate BDBM50300634 4-(3-((S)-1-((S)-2-((2R,3S)-6-amino-2-(benzyloxy)-6-oxohexan-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583758
BDBM50300634 4-(3-((S)-1-((S)-2-((2R,3S)-6-amino-2-(benzyloxy)-6-oxohexan-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583758 BDBM50267448 4-(3-((3S,6S,8aS)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL445970
BDBM50267448 4-(3-((3S,6S,8aS)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL445970 CHEMBL504218 BDBM50267450 4-(3-((3R,6S,8aR)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL504218 BDBM50267450 4-(3-((3R,6S,8aR)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL505207 BDBM50267449 4-(3-((3S,6S,8aR)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL505207 BDBM50267449 4-(3-((3S,6S,8aR)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
- Dyckman, AJ Modulators of Sphingosine-1-phosphate Pathway Biology: Recent Advances of Sphingosine-1-phosphate Receptor 1 (S1P J Med Chem 60: 5267-5289 (2017)
- Xiao, HY; Watterson, SH; Langevine, CM; Srivastava, AS; Ko, SS; Zhang, Y; Cherney, RJ; Guo, WW; Gilmore, JL; Sheppeck, JE; Wu, DR; Li, P; Ramasamy, D; Arunachalam, P; Mathur, A; Taylor, TL; Shuster, DJ; McIntyre, KW; Shen, DR; Yarde, M; Cvijic, ME; Marino, AM; Balimane, PV; Yang, Z; Banas, DM; Cornelius, G; D'Arienzo, CJ; Warrack, BM; Lehman-McKeeman, L; Salter-Cid, LM; Xie, J; Barrish, JC; Carter, PH; Dyckman, AJ; Dhar, TG Identification of Tricyclic Agonists of Sphingosine-1-phosphate Receptor 1 (S1P J Med Chem 59: 9837-9854 (2016)
- Abdel-Magid, AF Sphingosine-1-phosphate (S1P) Receptor Modulators Provide Potential for Diverse Treatments. ACS Med Chem Lett 4: 1014-5 (2013)
- Kurata, H; Kusumi, K; Otsuki, K; Suzuki, R; Kurono, M; Komiya, T; Hagiya, H; Mizuno, H; Shioya, H; Ono, T; Takada, Y; Maeda, T; Matsunaga, N; Kondo, T; Tominaga, S; Nunoya, KI; Kiyoshi, H; Komeno, M; Nakade, S; Habashita, H Discovery of a 1-Methyl-3,4-dihydronaphthalene-Based Sphingosine-1-Phosphate (S1P) Receptor Agonist Ceralifimod (ONO-4641). A S1P J Med Chem 60: 9508-9530 (2017)
- Colandrea, VJ; Legiec, IE; Huo, P; Yan, L; Hale, JJ; Mills, SG; Bergstrom, J; Card, D; Chebret, G; Hajdu, R; Keohane, CA; Milligan, JA; Rosenbach, MJ; Shei, GJ; Mandala, SM 2,5-Disubstituted pyrrolidine carboxylates as potent, orally active sphingosine-1-phosphate (S1P) receptor agonists. Bioorg Med Chem Lett 16: 2905-8 (2006)
- Lanman, BA; Cee, VJ; Cheruku, SR; Frohn, M; Golden, J; Lin, J; Lobera, M; Marantz, Y; Muller, KM; Neira, SC; Pickrell, AJ; Rivenzon-Segal, D; Schutz, N; Sharadendu, A; Yu, X; Zhang, Z; Buys, J; Fiorino, M; Gore, A; Horner, M; Itano, A; McElvain, M; Middleton, S; Schrag, M; Vargas, HM; Xu, H; Xu, Y; Zhang, X; Siu, J Discovery of a Potent, S1P3-Sparing Benzothiazole Agonist of Sphingosine-1-Phosphate Receptor 1 (S1P1) ACS Med Chem Lett 2: 102-106 (2011)
- Yan, L; Budhu, R; Huo, P; Lynch, CL; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Mandala, SM 2-Aryl(pyrrolidin-4-yl)acetic acids are potent agonists of sphingosine-1-phosphate (S1P) receptors. Bioorg Med Chem Lett 16: 3564-8 (2006)
- Swenson, RE Sphingosine 1-phosphate receptor antagonists US Patent US9663511 (2017)
- Zécri, FJ; Albert, R; Landrum, G; Hinterding, K; Cooke, NG; Guerini, D; Streiff, M; Bruns, C; Nuesslein-Hildesheim, B Pyrazole derived from (+)-3-carene; a novel potent, selective scaffold for sphingosine-1-phosphate (S1P(1)) receptor agonists. Bioorg Med Chem Lett 20: 35-7 (2010)
- Beard, RL; Yuan, H; Donello, JE; Liu, X; Duong, T 6-substituted indole-3-carboxylic acid amide compounds having sphingosine-1-phosphate (S1P) receptor antagonist biological activity US Patent US8524917 (2013)
- Clemens, JJ; Davis, MD; Lynch, KR; Macdonald, TL Synthesis of para-alkyl aryl amide analogues of sphingosine-1-phosphate: discovery of potent S1P receptor agonists. Bioorg Med Chem Lett 13: 3401-4 (2003)
- Angst, D; Janser, P; Quancard, J; Buehlmayer, P; Berst, F; Oberer, L; Beerli, C; Streiff, M; Pally, C; Hersperger, R; Bruns, C; Bassilana, F; Bollbuck, B An oral sphingosine 1-phosphate receptor 1 (S1P(1)) antagonist prodrug with efficacy in vivo: discovery, synthesis, and evaluation. J Med Chem 55: 9722-34 (2012)
- Ren, F; Deng, G; Wang, H; Luan, L; Meng, Q; Xu, Q; Xu, H; Xu, X; Zhang, H; Zhao, B; Li, C; Guo, TB; Yang, J; Zhang, W; Zhao, Y; Jia, Q; Lu, H; Xiang, JN; Elliott, JD; Lin, X Discovery of novel 1,2,4-thiadiazole derivatives as potent, orally active agonists of sphingosine 1-phosphate receptor subtype 1 (S1P(1)). J Med Chem 55: 4286-96 (2012)
- Yan, L; Hale, JJ; Lynch, CL; Budhu, R; Gentry, A; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Rosen, H; Mandala, SM Design and synthesis of conformationally constrained 3-(N-alkylamino)propylphosphonic acids as potent agonists of sphingosine-1-phosphate (S1P) receptors. Bioorg Med Chem Lett 14: 4861-6 (2004)
- Urbano, M; Guerrero, M; Rosen, H; Roberts, E Modulators of the Sphingosine 1-phosphate receptor 1. Bioorg Med Chem Lett 23: 6377-89 (2013)
- Roberts, E; Rosen, H; Brown, S; Guerrero, MA; Peng, X; Poddutoori, R Modulators of sphingosine phosphate receptors US Patent US10544136 (2020)
- Hamada, M; Nakamura, M; Kiuchi, M; Marukawa, K; Tomatsu, A; Shimano, K; Sato, N; Sugahara, K; Asayama, M; Takagi, K; Adachi, K Removal of sphingosine 1-phosphate receptor-3 (S1P(3)) agonism is essential, but inadequate to obtain immunomodulating 2-aminopropane-1,3-diol S1P(1) agonists with reduced effect on heart rate. J Med Chem 53: 3154-68 (2010)
- Shrader, CW; Foster, D; Kharel, Y; Huang, T; Lynch, KR; Santos, WL Imidazole-based sphingosine-1-phosphate transporter Spns2 inhibitors. Bioorg Med Chem Lett 96: (2023)
- Marciniak, A; Camp, SM; Garcia, JGN; Polt, R An update on sphingosine-1-phosphate receptor 1 modulators. Bioorg Med Chem Lett 28: 3585-3591 (2018)
- Horan, JC; Sanyal, S; Choi, Y; Hill-Drzewi, M; Patnaude, L; Anderson, S; Fogal, S; Mao, C; Cook, BN; Gueneva-Boucheva, K; Fisher, MB; Hickey, E; Pack, E; Bannen, LC; Chan, DS; Mac, MB; Ng, SM; Wang, Y; Xu, W; Modis, LK; Lemieux, RM Piperazinyl-oxadiazoles as selective sphingosine-1-phosphate receptor agonists. Bioorg Med Chem Lett 24: 4807-11 (2014)
- Yan, L; Huo, P; Doherty, G; Toth, L; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Quackenbush, E; Wickham, A; Mandala, SM Discovery of 3-arylpropionic acids as potent agonists of sphingosine-1-phosphate receptor-1 (S1P1) with high selectivity against all other known S1P receptor subtypes. Bioorg Med Chem Lett 16: 3679-83 (2006)
- Bombrun, A; Schwarz, M; Crosignani, S; Covini, D; Marin, D 6-amino-pyrimidine-4-carboxamide derivatives and related compounds which bind to the sphingosine 1-phosphate (S1P) receptor for the treatment of multiple sclerosis US Patent US9150519 (2015)
- Högenauer, K; Hinterding, K; Nussbaumer, P S1P receptor mediated activity of FTY720 phosphate mimics. Bioorg Med Chem Lett 20: 1485-7 (2010)
- Hennessy, EJ; Grewal, G; Byth, K; Kamhi, VM; Li, D; Lyne, P; Oza, V; Ronco, L; Rooney, MT; Saeh, JC; Su, Q Discovery of heterocyclic sulfonamides as sphingosine 1-phosphate receptor 1 (S1P1) antagonists. Bioorg Med Chem Lett 25: 2041-5 (2015)
- Bell, M; Foley, D; Naylor, C; Robinson, C; Riley, J; Epemolu, O; Scullion, P; Shishikura, Y; Katz, E; McLean, WHI; Wyatt, P; Read, KD; Woodland, A Discovery of super soft-drug modulators of sphingosine-1-phosphate receptor 1. Bioorg Med Chem Lett 28: 3255-3259 (2018)
- Vachal, P; Toth, LM; Hale, JJ; Yan, L; Mills, SG; Chrebet, GL; Koehane, CA; Hajdu, R; Milligan, JA; Rosenbach, MJ; Mandala, S Highly selective and potent agonists of sphingosine-1-phosphate 1 (S1P1) receptor. Bioorg Med Chem Lett 16: 3684-7 (2006)
- Kennedy, AJ; Mathews, TP; Kharel, Y; Field, SD; Moyer, ML; East, JE; Houck, JD; Lynch, KR; Macdonald, TL Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J Med Chem 54: 3524-48 (2011)
- Fritzemeier, R; Foster, D; Peralta, A; Payette, M; Kharel, Y; Huang, T; Lynch, KR; Santos, WL Discovery of In Vivo Active Sphingosine-1-phosphate Transporter (Spns2) Inhibitors. J Med Chem 65: 7656-7681 (2022)
- PubChem, PC Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 1 (S1P1) counterscreen assay PubChem Bioassay (2010)
- Bagdanoff, JT; Donoviel, MS; Nouraldeen, A; Tarver, J; Fu, Q; Carlsen, M; Jessop, TC; Zhang, H; Hazelwood, J; Nguyen, H; Baugh, SD; Gardyan, M; Terranova, KM; Barbosa, J; Yan, J; Bednarz, M; Layek, S; Courtney, LF; Taylor, J; Digeorge-Foushee, AM; Gopinathan, S; Bruce, D; Smith, T; Moran, L; O'Neill, E; Kramer, J; Lai, Z; Kimball, SD; Liu, Q; Sun, W; Yu, S; Swaffield, J; Wilson, A; Main, A; Carson, KG; Oravecz, T; Augeri, DJ Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders. J Med Chem 52: 3941-53 (2009)
- PubChem, PC Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 2 (S1P2) counterscreen assay PubChem Bioassay (2010)
- PubChem, PC Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 3 (S1P3) counterscreen assay PubChem Bioassay (2010)
- PubChem, PC Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 5 (S1P5) counterscreen assay PubChem Bioassay (2010)
- Gilmore, JL; Xiao, HY; Dhar, TGM; Yang, MG; Xiao, Z; Xie, J; Lehman-McKeeman, LD; Gong, L; Sun, H; Lecureux, L; Chen, C; Wu, DR; Dabros, M; Yang, X; Taylor, TL; Zhou, XD; Heimrich, EM; Thomas, R; McIntyre, KW; Borowski, V; Warrack, BM; Li, Y; Shi, H; Levesque, PC; Yang, Z; Marino, AM; Cornelius, G; D'Arienzo, CJ; Mathur, A; Rampulla, R; Gupta, A; Pragalathan, B; Shen, DR; Cvijic, ME; Salter-Cid, LM; Carter, PH; Dyckman, AJ Identification and Preclinical Pharmacology of ((1 R,3 S)-1-Amino-3-(( S)-6-(2-methoxyphenethyl)-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopentyl)methanol (BMS-986166): A Differentiated Sphingosine-1-phosphate Receptor 1 (S1P J Med Chem 62: 2265-2285 (2019)
- Bell, M; Foley, D; Naylor, C; Wood, G; Robinson, C; Riley, J; Epemolu, O; Ellis, L; Scullion, P; Shishikura, Y; Osuna-Cabello, M; Ferguson, L; Pinto, E; Fletcher, D; Katz, E; McLean, WHI; Wyatt, P; Read, KD; Woodland, A Discovery of Soft-Drug Topical Tool Modulators of Sphingosine-1-phosphate Receptor 1 (S1PR1). ACS Med Chem Lett 10: 341-347 (2019)
- Park, SJ; Yeon, SK; Kim, Y; Kim, HJ; Kim, S; Kim, J; Choi, JW; Kim, B; Lee, EH; Kim, R; Seo, SH; Lee, J; Kim, JW; Lee, HY; Hwang, H; Bahn, YS; Cheong, E; Park, JH; Park, KD Discovery of Novel Sphingosine-1-Phosphate-1 Receptor Agonists for the Treatment of Multiple Sclerosis. J Med Chem 65: 3539-3562 (2022)
- Dinges, J; Harris, CM; Wallace, GA; Argiriadi, MA; Queeney, KL; Perron, DC; Dominguez, E; Kebede, T; Desino, KE; Patel, H; Vasudevan, A Hit-to-lead evaluation of a novel class of sphingosine 1-phosphate lyase inhibitors. Bioorg Med Chem Lett 26: 2297-302 (2016)
- Ibrahim, MA; Johnson, HW; Jeong, JW; Lewis, GL; Shi, X; Noguchi, RT; Williams, M; Leahy, JW; Nuss, JM; Woolfrey, J; Banica, M; Bentzien, F; Chou, YC; Gibson, A; Heald, N; Lamb, P; Mattheakis, L; Matthews, D; Shipway, A; Wu, X; Zhang, W; Zhou, S; Shankar, G Discovery of a novel class of potent and orally bioavailable sphingosine 1-phosphate receptor 1 antagonists. J Med Chem 55: 1368-81 (2012)
- Xu, H; Zhang, H; Luan, L; Xu, Y; Li, C; Wang, Y; Han, F; Yang, T; Ren, F; Xiang, JN; Elliott, JD; Zhao, Y; Guo, TB; Lu, H; Zhang, W; Hirst, D; Lindon, M; Lin, X Discovery of thiadiazole amides as potent, S1P3-sparing agonists of sphingosine-1-phosphate 1 (S1P1) receptor. Bioorg Med Chem Lett 22: 2456-9 (2012)
- Sanllehí, P; Abad, JL; Casas, J; Bujons, J; Delgado, A Bacterial versus human sphingosine-1-phosphate lyase (S1PL) in the design of potential S1PL inhibitors. Bioorg Med Chem 24: 4381-4389 (2016)
- Urbano, M; Guerrero, M; Zhao, J; Velaparthi, S; Schaeffer, MT; Brown, S; Rosen, H; Roberts, E SAR analysis of innovative selective small molecule antagonists of sphingosine-1-phosphate 4 (S1P4) receptor. Bioorg Med Chem Lett 21: 5470-4 (2011)
- Tu, Z; Rosenberg, A; Liu, H; Han, J Compositions for binding sphingosine-1-phosphate receptor 1 (S1P1), imaging of S1P1, and methods of use thereof US Patent US10676467 (2020)
- Buzard, DJ; Kim, SH; Lopez, L; Kawasaki, A; Zhu, X; Moody, J; Thoresen, L; Calderon, I; Ullman, B; Han, S; Lehmann, J; Gharbaoui, T; Sengupta, D; Calvano, L; Montalban, AG; Ma, YA; Sage, C; Gao, Y; Semple, G; Edwards, J; Barden, J; Morgan, M; Chen, W; Usmani, K; Chen, C; Sadeque, A; Christopher, RJ; Thatte, J; Fu, L; Solomon, M; Mills, D; Whelan, K; Al-Shamma, H; Gatlin, J; Le, M; Gaidarov, I; Anthony, T; Unett, DJ; Blackburn, A; Rueter, J; Stirn, S; Behan, DP; Jones, RM Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor. ACS Med Chem Lett 5: 1313-7 (2014)
- Evindar, G; Deng, H; Bernier, SG; Doyle, E; Lorusso, J; Morgan, BA; Westlin, WF Exploring amino acids derivatives as potent, selective, and direct agonists of sphingosine-1-phosphate receptor subtype-1. Bioorg Med Chem Lett 23: 472-5 (2012)
- Guo, J; Watterson, SH; Spergel, SH; Kempson, J; Langevine, CM; Shen, DR; Yarde, M; Cvijic, ME; Banas, D; Liu, R; Suchard, SJ; Gillooly, K; Taylor, T; Rex-Rabe, S; Shuster, DJ; McIntyre, KW; Cornelius, G; D'Arienzo, C; Marino, A; Balimane, P; Salter-Cid, L; McKinnon, M; Barrish, JC; Carter, PH; Pitts, WJ; Xie, J; Dyckman, AJ Identification and synthesis of potent and selective pyridyl-isoxazole based agonists of sphingosine-1-phosphate 1 (S1P1). Bioorg Med Chem Lett 26: 2470-4 (2016)
- Burgio, AL; Shrader, CW; Kharel, Y; Huang, T; Salamoun, JM; Lynch, KR; Santos, WL 2-Aminobenzoxazole Derivatives as Potent Inhibitors of the Sphingosine-1-Phosphate Transporter Spinster Homolog 2 (Spns2). J Med Chem 66: 5873-5891 (2023)
- Hanessian, S; Charron, G; Billich, A; Guerini, D Constrained azacyclic analogues of the immunomodulatory agent FTY720 as molecular probes for sphingosine 1-phosphate receptors. Bioorg Med Chem Lett 17: 491-4 (2007)
- Koide, Y; Uemoto, K; Hasegawa, T; Sada, T; Murakami, A; Takasugi, H; Sakurai, A; Mochizuki, N; Takahashi, A; Nishida, A Pharmacophore-based design of sphingosine 1-phosphate-3 receptor antagonists that include a 3,4-dialkoxybenzophenone scaffold. J Med Chem 50: 442-54 (2007)
- Kurata, H; Otsuki, K; Kusumi, K; Kurono, M; Terakado, M; Seko, T; Mizuno, H; Ono, T; Hagiya, H; Minami, M; Nakade, S; Habashita, H Structure-activity relationship studies of sphingosine-1-phosphate receptor agonists with N-cinnamyl-ß-alanine moiety. Bioorg Med Chem Lett 21: 1390-3 (2011)
- Luo, Z; Liu, H; Yu, Y; Gropler, RJ; Klein, RS; Tu, Z Synthesis and evaluation of highly selective quinazoline-2,4-dione ligands for sphingosine-1-phosphate receptor 2. RSC Med Chem 13: 202-207 (2022)
- Xiao, Q; Hu, M; Chen, S; Jin, J; Li, L; Hu, J; Xie, P; Yin, D S1P Bioorg Med Chem Lett 30: (2020)
- Evindar, G; Bernier, SG; Doyle, E; Kavarana, MJ; Satz, AL; Lorusso, J; Blanchette, HS; Saha, AK; Hannig, G; Morgan, BA; Westlin, WF Exploration of amino alcohol derivatives as novel, potent, and highly selective sphingosine-1-phosphate receptor subtype-1 agonists. Bioorg Med Chem Lett 20: 2520-4 (2010)
- Meng, Q; Zhao, B; Xu, Q; Xu, X; Deng, G; Li, C; Luan, L; Ren, F; Wang, H; Xu, H; Xu, Y; Zhang, H; Xiang, JN; Elliott, JD; Guo, TB; Zhao, Y; Zhang, W; Lu, H; Lin, X Indole-propionic acid derivatives as potent, S1P3-sparing and EAE efficacious sphingosine-1-phosphate 1 (S1P1) receptor agonists. Bioorg Med Chem Lett 22: 2794-7 (2012)
- Evindar, G; Bernier, SG; Kavarana, MJ; Doyle, E; Lorusso, J; Kelley, MS; Halley, K; Hutchings, A; Wright, AD; Saha, AK; Hannig, G; Morgan, BA; Westlin, WF Synthesis and evaluation of alkoxy-phenylamides and alkoxy-phenylimidazoles as potent sphingosine-1-phosphate receptor subtype-1 agonists. Bioorg Med Chem Lett 19: 369-72 (2008)
- Luo, Z; Liu, H; Klein, RS; Tu, Z Design, synthesis, and in vitro bioactivity evaluation of fluorine-containing analogues for sphingosine-1-phosphate 2 receptor. Bioorg Med Chem 27: 3619-3631 (2019)
- Guerrero, M; Urbano, M; Zhao, J; Crisp, M; Chase, P; Hodder, P; Schaeffer, MT; Brown, S; Rosen, H; Roberts, E Discovery, design and synthesis of novel potent and selective sphingosine-1-phosphate 4 receptor (S1P4-R) agonists. Bioorg Med Chem Lett 22: 537-42 (2011)
- Sanllehí, P; Abad, JL; Bujons, J; Casas, J; Delgado, A Studies on the inhibition of sphingosine-1-phosphate lyase by stabilized reaction intermediates and stereodefined azido phosphates. Eur J Med Chem 123: 905-915 (2016)
- Clemens, JJ; Davis, MD; Lynch, KR; Macdonald, TL Synthesis of benzimidazole based analogues of sphingosine-1-phosphate: discovery of potent, subtype-selective S1P4 receptor agonists. Bioorg Med Chem Lett 14: 4903-6 (2004)
- Deng, G; Meng, Q; Liu, Q; Xu, X; Xu, Q; Ren, F; Guo, TB; Lu, H; Xiang, JN; Elliott, JD; Lin, X Identification of benzoxazole analogs as novel, S1P(3) sparing S1P(1) agonists. Bioorg Med Chem Lett 22: 3973-7 (2012)
- Hennessy, EJ; Oza, V; Adam, A; Byth, K; Castriotta, L; Grewal, G; Hamilton, GA; Kamhi, VM; Lewis, P; Li, D; Lyne, P; Öster, L; Rooney, MT; Saeh, JC; Sha, L; Su, Q; Wen, S; Xue, Y; Yang, B Identification and Optimization of Benzimidazole Sulfonamides as Orally Bioavailable Sphingosine 1-Phosphate Receptor 1 Antagonists with in Vivo Activity. J Med Chem 58: 7057-75 (2015)
- Watterson, SH; Guo, J; Spergel, SH; Langevine, CM; Moquin, RV; Shen, DR; Yarde, M; Cvijic, ME; Banas, D; Liu, R; Suchard, SJ; Gillooly, K; Taylor, T; Rex-Rabe, S; Shuster, DJ; McIntyre, KW; Cornelius, G; D'Arienzo, C; Marino, A; Balimane, P; Warrack, B; Salter-Cid, L; McKinnon, M; Barrish, JC; Carter, PH; Pitts, WJ; Xie, J; Dyckman, AJ Potent and Selective Agonists of Sphingosine 1-Phosphate 1 (S1P1): Discovery and SAR of a Novel Isoxazole Based Series. J Med Chem 59: 2820-40 (2016)
- Demont, EH; Bailey, JM; Bit, RA; Brown, JA; Campbell, CA; Deeks, N; Dowell, SJ; Eldred, C; Gaskin, P; Gray, JR; Haynes, A; Hirst, DJ; Holmes, DS; Kumar, U; Morse, MA; Osborne, GJ; Renaux, JF; Seal, GA; Smethurst, CA; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of Tetrahydropyrazolopyridine as Sphingosine 1-Phosphate Receptor 3 (S1P3)-Sparing S1P1 Agonists Active at Low Oral Doses. J Med Chem 59: 1003-20 (2016)
- Guerrero, M; Urbano, M; Velaparthi, S; Zhao, J; Schaeffer, MT; Brown, S; Rosen, H; Roberts, E Discovery, design and synthesis of the first reported potent and selective sphingosine-1-phosphate 4 (S1P4) receptor antagonists. Bioorg Med Chem Lett 21: 3632-6 (2011)
- PubChem, PC Dose response cell-based high throughput screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 3 (S1P3) PubChem Bioassay (2009)
- Foss, FW; Snyder, AH; Davis, MD; Rouse, M; Okusa, MD; Lynch, KR; Macdonald, TL Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem 15: 663-77 (2006)
- Hale, JJ; Lynch, CL; Neway, W; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Parent, SA; Chrebet, G; Bergstrom, J; Card, D; Ferrer, M; Hodder, P; Strulovici, B; Rosen, H; Mandala, S A rational utilization of high-throughput screening affords selective, orally bioavailable 1-benzyl-3-carboxyazetidine sphingosine-1-phosphate-1 receptor agonists. J Med Chem 47: 6662-5 (2004)
- Skidmore, J; Heer, J; Johnson, CN; Norton, D; Redshaw, S; Sweeting, J; Hurst, D; Cridland, A; Vesey, D; Wall, I; Ahmed, M; Rivers, D; Myatt, J; Giblin, G; Philpott, K; Kumar, U; Stevens, A; Bit, RA; Haynes, A; Taylor, S; Watson, R; Witherington, J; Demont, E; Heightman, TD Optimization of sphingosine-1-phosphate-1 receptor agonists: effects of acidic, basic, and zwitterionic chemotypes on pharmacokinetic and pharmacodynamic profiles. J Med Chem 57: 10424-42 (2014)
- PubChem, PC Late stage fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) PubChem Bioassay (2010)
- Clemens, JJ; Davis, MD; Lynch, KR; Macdonald, TL Synthesis of 4(5)-phenylimidazole-based analogues of sphingosine-1-phosphate and FTY720: discovery of potent S1P1 receptor agonists. Bioorg Med Chem Lett 15: 3568-72 (2005)
- Li, Z; Chen, W; Hale, JJ; Lynch, CL; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Parent, SA; Bergstrom, J; Card, D; Forrest, M; Quackenbush, EJ; Wickham, LA; Vargas, H; Evans, RM; Rosen, H; Mandala, S Discovery of potent 3,5-diphenyl-1,2,4-oxadiazole sphingosine-1-phosphate-1 (S1P1) receptor agonists with exceptional selectivity against S1P2 and S1P3. J Med Chem 48: 6169-73 (2005)
- Evindar, G; Satz, AL; Bernier, SG; Kavarana, MJ; Doyle, E; Lorusso, J; Taghizadeh, N; Halley, K; Hutchings, A; Kelley, MS; Wright, AD; Saha, AK; Hannig, G; Morgan, BA; Westlin, WF Synthesis and evaluation of arylalkoxy- and biarylalkoxy-phenylamide and phenylimidazoles as potent and selective sphingosine-1-phosphate receptor subtype-1 agonists. Bioorg Med Chem Lett 19: 2315-9 (2009)
- Gilmore, JL; Sheppeck, JE; Watterson, SH; Haque, L; Mukhopadhyay, P; Tebben, AJ; Galella, MA; Shen, DR; Yarde, M; Cvijic, ME; Borowski, V; Gillooly, K; Taylor, T; McIntyre, KW; Warrack, B; Levesque, PC; Li, JP; Cornelius, G; D'Arienzo, C; Marino, A; Balimane, P; Salter-Cid, L; Barrish, JC; Pitts, WJ; Carter, PH; Xie, J; Dyckman, AJ Discovery and Structure-Activity Relationship (SAR) of a Series of Ethanolamine-Based Direct-Acting Agonists of Sphingosine-1-phosphate (S1P1). J Med Chem 59: 6248-64 (2016)
- Yan, L; Huo, P; Hale, JJ; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, MJ; Milligan, JA; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Mandala, SM SAR studies of 3-arylpropionic acids as potent and selective agonists of sphingosine-1-phosphate receptor-1 (S1P1) with enhanced pharmacokinetic properties. Bioorg Med Chem Lett 17: 828-31 (2007)
- Grobelny, DW; Gill, GS S1P receptors modulators US Patent US9181182 (2015)
- PubChem, PC Late stage fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Synthesized analogues PubChem Bioassay (2010)
- PubChem, PC Late-stage fluorescence dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Synthesized compounds PubChem Bioassay (2010)
- Nakamura, T; Asano, M; Sekiguchi, Y; Mizuno, Y; Tamaki, K; Nara, F; Kawase, Y; Yabe, Y; Nakai, D; Kamiyama, E; Urasaki-Kaneno, Y; Shimozato, T; Doi-Komuro, H; Kagari, T; Tomisato, W; Inoue, R; Nagasaki, M; Yuita, H; Oguchi-Oshima, K; Kaneko, R; Nishi, T Synthesis and evaluation of CS-2100, a potent, orally active and S1P(3)- sparing S1P(1) agonist. Eur J Med Chem 51: 92-8 (2012)
- Xiang, Y; Asmussen, G; Booker, M; Hirth, B; Kane, JL; Liao, J; Noson, KD; Yee, C Discovery of novel sphingosine kinase 1 inhibitors. Bioorg Med Chem Lett 19: 6119-21 (2009)
- PubChem, PC Late stage counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 1 (S1P1) PubChem Bioassay (2010)
- James, E; Pertusati, F; Brancale, A; McGuigan, C Kinase-independent phosphoramidate S1P Bioorg Med Chem Lett 27: 1371-1378 (2017)
- Hobson, AD; Harris, CM; van der Kam, EL; Turner, SC; Abibi, A; Aguirre, AL; Bousquet, P; Kebede, T; Konopacki, DB; Gintant, G; Kim, Y; Larson, K; Maull, JW; Moore, NS; Shi, D; Shrestha, A; Tang, X; Zhang, P; Sarris, KK Discovery of A-971432, An Orally Bioavailable Selective Sphingosine-1-Phosphate Receptor 5 (S1P5) Agonist for the Potential Treatment of Neurodegenerative Disorders. J Med Chem 58: 9154-70 (2015)
- PubChem, PC Late-stage fluorescence dose response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): purchased compounds EC50 PubChem Bioassay (2011)
- Wong, L; Tan, SS; Lam, Y; Melendez, AJ Synthesis and evaluation of sphingosine analogues as inhibitors of sphingosine kinases. J Med Chem 52: 3618-26 (2009)
- Lynch, KR; Macdonald, TL; Mathews, TP; Kennedy, A; Kharel, Y Imidamide sphingosine kinase inhibitors US Patent US9421177 (2016)
- PubChem, PC Fluorescence-based counterscreen assay for S1P4 agonists: Cell-based dose response high throughput screening assay to identify agonists of the Sphingosine 1-Phosphate Receptor 1 (S1P1) PubChem Bioassay (2010)
- PubChem, PC Late stage counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 2 (S1P2) PubChem Bioassay (2010)
- PubChem, PC Late stage counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 3 (S1P3) PubChem Bioassay (2010)
- PubChem, PC Late stage counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 5 (S1P5) PubChem Bioassay (2010)
- PubChem, PC Late-stage fluorescence dose response cell-based counterscreening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): inhibition by S1P4-selective antagonist PubChem Bioassay (2010)
- Weiler, S; Braendlin, N; Beerli, C; Bergsdorf, C; Schubart, A; Srinivas, H; Oberhauser, B; Billich, A Orally active 7-substituted (4-benzylphthalazin-1-yl)-2-methylpiperazin-1-yl]nicotinonitriles as active-site inhibitors of sphingosine 1-phosphate lyase for the treatment of multiple sclerosis. J Med Chem 57: 5074-84 (2014)
- Childress, ES; Kharel, Y; Brown, AM; Bevan, DR; Lynch, KR; Santos, WL Transforming Sphingosine Kinase 1 Inhibitors into Dual and Sphingosine Kinase 2 Selective Inhibitors: Design, Synthesis, and in Vivo Activity. J Med Chem 60: 3933-3957 (2017)
- Wang, J; Knapp, S; Pyne, NJ; Pyne, S; Elkins, JM Crystal Structure of Sphingosine Kinase 1 with PF-543. ACS Med Chem Lett 5: 1329-33 (2014)
- Xiang, Y; Hirth, B; Kane, JL; Liao, J; Noson, KD; Yee, C; Asmussen, G; Fitzgerald, M; Klaus, C; Booker, M Discovery of novel sphingosine kinase-1 inhibitors. Part 2. Bioorg Med Chem Lett 20: 4550-4 (2010)
- Luo, D; Zhang, Y; Yang, S; Tian, X; Lv, Y; Guo, Z; Liu, X; Han, G; Liu, S; Wang, W; Cui, S; Qu, X; Wan, S Design, synthesis and biological evaluation of sphingosine-1-phosphate receptor 2 antagonists as potent 5-FU-resistance reversal agents for the treatment of colorectal cancer. Eur J Med Chem 225: (2021)
- Gilmore, JL; Xiao, HY; Dhar, TGM; Yang, M; Xiao, Z; Yang, X; Taylor, TL; McIntyre, KW; Warrack, BM; Shi, H; Levesque, PC; Marino, AM; Cornelius, G; Mathur, A; Shen, DR; Pang, J; Cvijic, ME; Lehman-McKeeman, LD; Sun, H; Xie, J; Salter-Cid, L; Carter, PH; Dyckman, AJ Bicyclic Ligand-Biased Agonists of S1P J Med Chem 64: 1454-1480 (2021)
- Smid, P; Iwema Bakker, WI; Coolen, HK; Sliedregt, LA; van Dongen, MJ; den Hartog, JA; Hobson, A Fused heterocyclic derivatives as S1P modulators US Patent US9670220 (2017)
- Cee, VJ; Frohn, M; Lanman, BA; Golden, J; Muller, K; Neira, S; Pickrell, A; Arnett, H; Buys, J; Gore, A; Fiorino, M; Horner, M; Itano, A; Lee, MR; McElvain, M; Middleton, S; Schrag, M; Rivenzon-Segal, D; Vargas, HM; Xu, H; Xu, Y; Zhang, X; Siu, J; Wong, M Discovery of AMG 369, a Thiazolo[5,4-b]pyridine Agonist of S1P1 and S1P5 ACS Med Chem Lett 2: 107-112 (2011)
- Hengst, JA; Wang, X; Sk, UH; Sharma, AK; Amin, S; Yun, JK Development of a sphingosine kinase 1 specific small-molecule inhibitor. Bioorg Med Chem Lett 20: 7498-502 (2010)
- Bolli, MH; Abele, S; Birker, M; Bravo, R; Bur, D; de Kanter, R; Kohl, C; Grimont, J; Hess, P; Lescop, C; Mathys, B; Müller, C; Nayler, O; Rey, M; Scherz, M; Schmidt, G; Seifert, J; Steiner, B; Velker, J; Weller, T Novel S1P(1) receptor agonists--part 3: from thiophenes to pyridines. J Med Chem 57: 110-30 (2014)
- Santos, WL; Lynch, KR; Macdonald, TL; Kennedy, A; Kharel, Y; Raje, MR; Houck, J Long chain base sphingosine kinase inhibitors US Patent US9688668 (2017)
- Gill, GS; Grobelny, DW S1P receptors modulators and their use thereof US Patent US9707205 (2017)
- Stoit, A; Iwema Bakker, WI; Coolen, HK; van Dongen, MJ; Leflemme, NJ; Hobson, A Spiro-cyclic amine derivatives as S1P modulators US Patent US10179791 (2019)
- Kurata, H; Kusumi, K; Otsuki, K; Suzuki, R; Kurono, M; Takada, Y; Shioya, H; Komiya, T; Mizuno, H; Ono, T; Hagiya, H; Minami, M; Nakade, S; Habashita, H Discovery of S1P agonists with a dihydronaphthalene scaffold. Bioorg Med Chem Lett 21: 3885-9 (2011)
- Vettorazzi, M; Angelina, E; Lima, S; Gonec, T; Otevrel, J; Marvanova, P; Padrtova, T; Mokry, P; Bobal, P; Acosta, LM; Palma, A; Cobo, J; Bobalova, J; Csollei, J; Malik, I; Alvarez, S; Spiegel, S; Jampilek, J; Enriz, RD An integrative study to identify novel scaffolds for sphingosine kinase 1 inhibitors. Eur J Med Chem 139: 461-481 (2017)
- Rosse, G Novel Pyridyloxadiazole Agonists of Sphingosin-1-phosphate. ACS Med Chem Lett 6: 102-3 (2015)
- Butler, KJ; Castro, AA; Dwyer, TS; Hardwick, LM; Iacino, MC; Manore, SG; Mays, KM; McGlade, CA; Hair, LN; Parker, EW; Smith, MR; Turnow, MT; Wilson, MR; Woodson, SR; Cotham, WE; Walla, MD; Hurlbert, JC; Christian Grattan, T Design, synthesis and analysis of novel sphingosine kinase-1 inhibitors to improve oral bioavailability. Bioorg Med Chem Lett 50: (2021)
- Houck, JD; Dawson, TK; Kennedy, AJ; Kharel, Y; Naimon, ND; Field, SD; Lynch, KR; Macdonald, TL Structural Requirements and Docking Analysis of Amidine-Based Sphingosine Kinase 1 Inhibitors Containing Oxadiazoles. ACS Med Chem Lett 7: 487-92 (2016)
- Ma, B; Guckian, KM; Lin, EY; Lee, WC; Scott, D; Kumaravel, G; Macdonald, TL; Lynch, KR; Black, C; Chollate, S; Hahm, K; Hetu, G; Jin, P; Luo, Y; Rohde, E; Rossomando, A; Scannevin, R; Wang, J; Yang, C Stereochemistry-activity relationship of orally active tetralin S1P agonist prodrugs. Bioorg Med Chem Lett 20: 2264-9 (2010)
- Qu, W; Ploessl, K; Truong, H; Kung, MP; Kung, HF Iodophenyl tagged sphingosine derivatives: synthesis and preliminary biological evaluation. Bioorg Med Chem Lett 19: 3382-5 (2009)
- Futerman, AH; Joseph, T; Shaw, WA; Burgess, SW; Li, S Sphingosine analogs and use thereof against bacterial lung infections US Patent US11471430 (2022)
- Hur, W; Rosen, H; Gray, NS A benzo[b]thiophene-based selective type 4 S1P receptor agonist. Bioorg Med Chem Lett 27: 1-5 (2017)
- Guckian, K; Kumaravel, G; Ma, B; Sun, L; Xin, Z; Zhang, L Compounds that are S1P modulating agents and/or ATX modulating agents US Patent US10273234 (2019)
- Pan, S; Gray, NS; Gao, W; Mi, Y; Fan, Y; Wang, X; Tuntland, T; Che, J; Lefebvre, S; Chen, Y; Chu, A; Hinterding, K; Gardin, A; End, P; Heining, P; Bruns, C; Cooke, NG; Nuesslein-Hildesheim, B Discovery of BAF312 (Siponimod), a Potent and Selective S1P Receptor Modulator. ACS Med Chem Lett 4: 333-7 (2013)
- Kurata, H; Kusumi, K; Otsuki, K; Suzuki, R; Kurono, M; Tokuda, N; Takada, Y; Shioya, H; Mizuno, H; Komiya, T; Ono, T; Hagiya, H; Minami, M; Nakade, S; Habashita, H Structure-activity relationship studies of S1P agonists with a dihydronaphthalene scaffold. Bioorg Med Chem Lett 22: 144-8 (2011)
- Knott, K; Kharel, Y; Raje, MR; Lynch, KR; Santos, WL Effect of alkyl chain length on sphingosine kinase 2 selectivity. Bioorg Med Chem Lett 22: 6817-20 (2012)
- Baek, DJ; MacRitchie, N; Anthony, NG; Mackay, SP; Pyne, S; Pyne, NJ; Bittman, R Structure-activity relationships and molecular modeling of sphingosine kinase inhibitors. J Med Chem 56: 9310-27 (2013)
- Noble, NJ; Dubreuil, D; Potter, B Total synthesis of myo-inositol-1-phosphate-4,5-pyrophosphate, a novel second messenger analogue, via myo-inositol-1-phosphate-4,5-bisphosphorothioate Bioorg Med Chem Lett 2: 471-476 (1992)
- Tian, Y; Jin, J; Wang, X; Hu, J; Xiao, Q; Zhou, W; Chen, X; Yin, D Discovery of oxazole and triazole derivatives as potent and selective S1P(1) agonists through pharmacophore-guided design. Eur J Med Chem 85: 1-15 (2014)
- Bolli, MH; Velker, J; Müller, C; Mathys, B; Birker, M; Bravo, R; Bur, D; de Kanter, R; Hess, P; Kohl, C; Lehmann, D; Meyer, S; Nayler, O; Rey, M; Scherz, M; Steiner, B Novel S1P(1) receptor agonists--part 2: from bicyclo[3.1.0]hexane-fused thiophenes to isobutyl substituted thiophenes. J Med Chem 57: 78-97 (2014)
- Byun, HS; Pyne, S; Macritchie, N; Pyne, NJ; Bittman, R Novel sphingosine-containing analogues selectively inhibit sphingosine kinase (SK) isozymes, induce SK1 proteasomal degradation and reduce DNA synthesis in human pulmonary arterial smooth muscle cells. Medchemcomm 4: (2013)
- Li, H; Sibley, CD; Kharel, Y; Huang, T; Brown, AM; Wonilowicz, LG; Bevan, DR; Lynch, KR; Santos, WL Lipophilic tail modifications of 2-(hydroxymethyl)pyrrolidine scaffold reveal dual sphingosine kinase 1 and 2 inhibitors. Bioorg Med Chem 30: (2021)
- Guerrero, M; Poddutoori, R; Urbano, M; Peng, X; Spicer, TP; Chase, PS; Hodder, PS; Schaeffer, MT; Brown, S; Rosen, H; Roberts, E Discovery, design and synthesis of a selective S1P(3) receptor allosteric agonist. Bioorg Med Chem Lett 23: 6346-9 (2013)
- Raje, MR; Knott, K; Kharel, Y; Bissel, P; Lynch, KR; Santos, WL Design, synthesis and biological activity of sphingosine kinase 2 selective inhibitors. Bioorg Med Chem 20: 183-94 (2011)
- Gustin, DJ; Li, Y; Brown, ML; Min, X; Schmitt, MJ; Wanska, M; Wang, X; Connors, R; Johnstone, S; Cardozo, M; Cheng, AC; Jeffries, S; Franks, B; Li, S; Shen, S; Wong, M; Wesche, H; Xu, G; Carlson, TJ; Plant, M; Morgenstern, K; Rex, K; Schmitt, J; Coxon, A; Walker, N; Kayser, F; Wang, Z Structure guided design of a series of sphingosine kinase (SphK) inhibitors. Bioorg Med Chem Lett 23: 4608-16 (2013)
- Saha, AK; Yu, X; Lin, J; Lobera, M; Sharadendu, A; Chereku, S; Schutz, N; Segal, D; Marantz, Y; McCauley, D; Middleton, S; Siu, J; Börli, RW; Buys, J; Horner, M; Salyers, K; Schrag, M; Vargas, HM; Xu, Y; McElvain, M Benzofuran Derivatives as Potent, Orally Active S1P1 Receptor Agonists: A Preclinical Lead Molecule for MS ACS Med Chem Lett 2: 97-101 (2011)
- Adams, DR; Tawati, S; Berretta, G; Rivas, PL; Baiget, J; Jiang, Z; Alsfouk, A; Mackay, SP; Pyne, NJ; Pyne, S Topographical Mapping of Isoform-Selectivity Determinants for J-Channel-Binding Inhibitors of Sphingosine Kinases 1 and 2. J Med Chem 62: 3658-3676 (2019)
- Green, OM; McKenzie, AR; Shapiro, AB; Otterbein, L; Ni, H; Patten, A; Stokes, S; Albert, R; Kawatkar, S; Breed, J Inhibitors of acetyltransferase domain of N-acetylglucosamine-1-phosphate-uridyltransferase/glucosamine-1-phosphate-acetyltransferase (GlmU). Part 1: Hit to lead evaluation of a novel arylsulfonamide series. Bioorg Med Chem Lett 22: 1510-9 (2012)
- Hale, JJ; Doherty, G; Toth, L; Li, Z; Mills, SG; Hajdu, R; Ann Keohane, C; Rosenbach, M; Milligan, J; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Rosen, H; Mandala, S The discovery of 3-(N-alkyl)aminopropylphosphonic acids as potent S1P receptor agonists. Bioorg Med Chem Lett 14: 3495-9 (2004)
- Roberts, E; Rosen, H; Urbano, M; Guerrero, M Sphinogosine-1 -phosphate receptor modulators for treatment of cardiopulmonary disorders US Patent US11034691 (2021)
- Roberts, E; Rosen, H; Urbano, M; Guerrero, MA Sphinogosine-1-phosphate receptor modulators for treatment of cardiopulmonary disorders US Patent US10323029 (2019)
- Vogt, D; Weber, J; Ihlefeld, K; Brüggerhoff, A; Proschak, E; Stark, H Design, synthesis and evaluation of 2-aminothiazole derivatives as sphingosine kinase inhibitors. Bioorg Med Chem 22: 5354-67 (2014)
- Liu, Z; MacRitchie, N; Pyne, S; Pyne, NJ; Bittman, R Synthesis of (S)-FTY720 vinylphosphonate analogues and evaluation of their potential as sphingosine kinase 1 inhibitors and activators. Bioorg Med Chem 21: 2503-10 (2013)
- Hale, JJ; Neway, W; Mills, SG; Hajdu, R; Ann Keohane, C; Rosenbach, M; Milligan, J; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Koo, GC; Koprak, SL; Jackson, JJ; Rosen, H; Mandala, S Potent S1P receptor agonists replicate the pharmacologic actions of the novel immune modulator FTY720. Bioorg Med Chem Lett 14: 3351-5 (2004)
- Schnute, ME; McReynolds, MD; Carroll, J; Chrencik, J; Highkin, MK; Iyanar, K; Jerome, G; Rains, JW; Saabye, M; Scholten, JA; Yates, M; Nagiec, MM Discovery of a Potent and Selective Sphingosine Kinase 1 Inhibitor through the Molecular Combination of Chemotype-Distinct Screening Hits. J Med Chem 60: 2562-2572 (2017)
- Zhang, S; Chen, X; Wu, C; Xu, H; Xie, X; Feng, M; Hu, S; Bai, H; Gao, F; Tong, L; Ding, J; Liu, H; Xie, Z; Wang, J Novel Sphingosine Kinase 1 Inhibitor Suppresses Growth of Solid Tumor and Inhibits the Lung Metastasis of Triple-Negative Breast Cancer. J Med Chem 65: 7697-7716 (2022)
- Deng, L; Endo, K; Kato, M; Cheng, G; Yajima, S; Song, Y Structures of 1-Deoxy-D-Xylulose-5-Phosphate Reductoisomerase/Lipophilic Phosphonate Complexes. ACS Med Chem Lett 2: 165-170 (2011)
- Wang, S; Huo, Y; Zhang, J; Li, L; Cao, F; Song, Y; Zhang, Y; Yang, K Design, synthesis, antitumor activity, and molecular dynamics simulations of novel sphingosine kinase 2 inhibitors. Bioorg Med Chem 93: (2023)
- Hengst, JA; Hegde, S; Paulson, RF; Yun, JK Development of SKI-349, a dual-targeted inhibitor of sphingosine kinase and microtubule polymerization. Bioorg Med Chem Lett 30: (2020)
- Congdon, MD; Kharel, Y; Brown, AM; Lewis, SN; Bevan, DR; Lynch, KR; Santos, WL Structure-Activity Relationship Studies and Molecular Modeling of Naphthalene-Based Sphingosine Kinase 2 Inhibitors. ACS Med Chem Lett 7: 229-34 (2016)
- Congdon, MD; Childress, ES; Patwardhan, NN; Gumkowski, J; Morris, EA; Kharel, Y; Lynch, KR; Santos, WL Structure-activity relationship studies of the lipophilic tail region of sphingosine kinase 2 inhibitors. Bioorg Med Chem Lett 25: 4956-60 (2015)
- Ohno, H; Honda, M; Hamada, N; Miyagaki, J; Iwata, A; Otsuki, K; Maruyama, T; Nakamura, S; Nakanishi, I; Inuki, S; Fujii, N; Oishi, S Identification of selective inhibitors of sphingosine kinases 1 and 2 through a structure-activity relationship study of 4-epi-jaspine B. Bioorg Med Chem 25: 3046-3052 (2017)
- Alphey, MS; Pirrie, L; Torrie, LS; Boulkeroua, WA; Gardiner, M; Sarkar, A; Maringer, M; Oehlmann, W; Brenk, R; Scherman, MS; McNeil, M; Rejzek, M; Field, RA; Singh, M; Gray, D; Westwood, NJ; Naismith, JH Allosteric competitive inhibitors of the glucose-1-phosphate thymidylyltransferase (RmlA) from Pseudomonas aeruginosa. ACS Chem Biol 8: 387-96 (2013)
- Nakanishi, W; Kikuchi, K; Inoue, T; Hirose, K; Iino, M; Nagano, T Hydrophobic modifications at 1-phosphate of inositol 1,4,5-trisphosphate analogues enhance receptor binding. Bioorg Med Chem Lett 12: 911-3 (2002)
- Pettit, GR; Toki, B; Herald, DL; Verdier-Pinard, P; Boyd, MR; Hamel, E; Pettit, RK Antineoplastic agents. 379. Synthesis of phenstatin phosphate. J Med Chem 41: 1688-95 (1998)
- PubChem, PC Human Glucose-6-Phosphate Dehydrogenase Dose Response Selectivity Assay for Inhibitors of Plasmodium falciparum Glucose-6-Phosphate Dehydrogenase PubChem Bioassay (2011)
- Luo, D; Guo, Z; Zhao, X; Wu, L; Liu, X; Zhang, Y; Zhang, Y; Deng, Z; Qu, X; Cui, S; Wan, S Novel 5-fluorouracil sensitizers for colorectal cancer therapy: Design and synthesis of S1P receptor 2 (S1PR2) antagonists. Eur J Med Chem 227: (2022)
- Hale, JJ; Doherty, G; Toth, L; Mills, SG; Hajdu, R; Keohane, CA; Rosenbach, M; Milligan, J; Shei, GJ; Chrebet, G; Bergstrom, J; Card, D; Forrest, M; Sun, SY; West, S; Xie, H; Nomura, N; Rosen, H; Mandala, S Selecting against S1P3 enhances the acute cardiovascular tolerability of 3-(N-benzyl)aminopropylphosphonic acid S1P receptor agonists. Bioorg Med Chem Lett 14: 3501-5 (2004)
- Sibley, CD; Morris, EA; Kharel, Y; Brown, AM; Huang, T; Bevan, DR; Lynch, KR; Santos, WL Discovery of a Small Side Cavity in Sphingosine Kinase 2 that Enhances Inhibitor Potency and Selectivity. J Med Chem 63: 1178-1198 (2020)
- Congdon, M; Fritzemeier, RG; Kharel, Y; Brown, AM; Serbulea, V; Bevan, DR; Lynch, KR; Santos, WL Probing the substitution pattern of indole-based scaffold reveals potent and selective sphingosine kinase 2 inhibitors. Eur J Med Chem 212: (2021)
- Fossetta, J; Deno, G; Gonsiorek, W; Fan, X; Lavey, B; Das, P; Lunn, C; Zavodny, PJ; Lundell, D; Hipkin, RW Pharmacological characterization of human S1P4 using a novel radioligand, [4,5-3H]-dihydrosphingosine-1-phosphate. Br J Pharmacol 142: 851-60 (2004)
- Smith, JM; Vierling, RJ; Meyers, CF Selective inhibition of E. coli 1-deoxy-D-xylulose-5-phosphate synthase by acetylphosphonates(). Medchemcomm 3: 65-67 (2012)
- Fonvielle, M; Mariano, S; Therisod, M New inhibitors of rabbit muscle triose-phosphate isomerase. Bioorg Med Chem Lett 15: 2906-9 (2005)
- Hamilton, NM; Dawson, M; Fairweather, EE; Hamilton, NS; Hitchin, JR; James, DI; Jones, SD; Jordan, AM; Lyons, AJ; Small, HF; Thomson, GJ; Waddell, ID; Ogilvie, DJ Novel steroid inhibitors of glucose 6-phosphate dehydrogenase. J Med Chem 55: 4431-45 (2012)
- Mercklé, L; de Andrés-Gómez, A; Dick, B; Cox, RJ; Godfrey, CR A fragment-based approach to understanding inhibition of 1-deoxy-D-xylulose-5-phosphate reductoisomerase. Chembiochem 6: 1866-74 (2005)
- Qian, Y; Ahmad, M; Chen, S; Gillespie, P; Le, N; Mennona, F; Mischke, S; So, SS; Wang, H; Burghardt, C; Tannu, S; Conde-Knape, K; Kochan, J; Bolin, D Discovery of 1-arylcarbonyl-6,7-dimethoxyisoquinoline derivatives as glutamine fructose-6-phosphate amidotransferase (GFAT) inhibitors. Bioorg Med Chem Lett 21: 6264-9 (2011)
- Mao, J; Eoh, H; He, R; Wang, Y; Wan, B; Franzblau, SG; Crick, DC; Kozikowski, AP Structure-activity relationships of compounds targeting mycobacterium tuberculosis 1-deoxy-D-xylulose 5-phosphate synthase. Bioorg Med Chem Lett 18: 5320-3 (2008)
- Chofor, R; Sooriyaarachchi, S; Risseeuw, MD; Bergfors, T; Pouyez, J; Johny, C; Haymond, A; Everaert, A; Dowd, CS; Maes, L; Coenye, T; Alex, A; Couch, RD; Jones, TA; Wouters, J; Mowbray, SL; Van Calenbergh, S Synthesis and bioactivity ofß-substituted fosmidomycin analogues targeting 1-deoxy-D-xylulose-5-phosphate reductoisomerase. J Med Chem 58: 2988-3001 (2015)
- Miller, MJ; Anderson, KS; Braccolino, DS; Cleary, DG; Gruys, KJ; Han, CY; Lin, KC; Pansegrau, PD; Ream, JE; Sammons, RD; Sikorski, JA EPSP synthase inhibitor design II. The importance of the 3-phosphate group for ligand binding at the shikimate-3-phosphate site & the identification of 3-malonate ethers as novel 3-phosphate mimics. Bioorg Med Chem Lett 3: 1435-1440 (1993)
- Buzard, D; Han, S; Thoresen, L; Moody, J; Lopez, L; Kawasaki, A; Schrader, T; Sage, C; Gao, Y; Edwards, J; Barden, J; Thatte, J; Fu, L; Solomon, M; Liu, L; Al-Shamma, H; Gatlin, J; Le, M; Xing, C; Espinola, S; Jones, RM Discovery and characterization of potent and selective 4-oxo-4-(5-(5-phenyl-1,2,4-oxadiazol-3-yl)indolin-1-yl)butanoic acids as S1P¿? agonists. Bioorg Med Chem Lett 21: 6013-8 (2011)
- Xi, M; Ge, J; Wang, X; Sun, C; Liu, T; Fang, L; Xiao, Q; Yin, D Development of hydroxy-based sphingosine kinase inhibitors and anti-inflammation in dextran sodium sulfate induced colitis in mice. Bioorg Med Chem 24: 3218-30 (2016)
- Zhang, Y; Jumppanen, M; Maksimainen, MM; Auno, S; Awol, Z; Ghemtio, L; Venkannagari, H; Lehtiö, L; Yli-Kauhaluoma, J; Xhaard, H; Boije Af Gennäs, G Adenosine analogs bearing phosphate isosteres as human MDO1 ligands. Bioorg Med Chem 26: 1588-1597 (2018)
- Carreras, C; Charmot, D; Jacobs, JW; Labonte, E; Lewis, JG NHE3-binding compounds and methods for inhibiting phosphate transport US Patent US10272079 (2019)
- Bartee, D; Morris, F; Al-Khouja, A; Freel Meyers, CL Hydroxybenzaldoximes Are D-GAP-Competitive Inhibitors of E. coli 1-Deoxy-D-Xylulose-5-Phosphate Synthase. Chembiochem 16: 1771-81 (2015)
- Kalman, TI; Nie, Z; Kamat, A Mechanism-based inactivation of thymidylate synthase by 5-(3-fluoropropyn-1-yl)-2'-deoxyuridine 5'-phosphate. Bioorg Med Chem Lett 10: 391-4 (2000)
- Henriksson, LM; Unge, T; Carlsson, J; Aqvist, J; Mowbray, SL; Jones, TA Structures of Mycobacterium tuberculosis 1-deoxy-D-xylulose-5-phosphate reductoisomerase provide new insights into catalysis. J Biol Chem 282: 19905-16 (2007)
- Sauer, R; Maurinsh, J; Reith, U; Fülle, F; Klotz, KN; Müller, CE Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A(2A)-selective adenosine receptor antagonists. J Med Chem 43: 440-8 (2000)
- Frohn, M; Cee, VJ; Lanman, BA; Pickrell, AJ; Golden, J; Rivenzon-Segal, D; Middleton, S; Fiorino, M; Xu, H; Schrag, M; Xu, Y; McElvain, M; Muller, K; Siu, J; Bürli, R Novel 5- and 6-subtituted benzothiazoles with improved physicochemical properties: potent S1P¿? agonists with in vivo lymphocyte-depleting activity. Bioorg Med Chem Lett 22: 628-33 (2011)
- Tangadanchu, VKR; Jiang, H; Yu, Y; Graham, TJA; Liu, H; Rogers, BE; Gropler, R; Perlmutter, J; Tu, Z Structure-activity relationship studies and bioactivity evaluation of 1,2,3-triazole containing analogues as a selective sphingosine kinase-2 inhibitors. Eur J Med Chem 206: (2020)
- Yang, MG; Xiao, Z; Dhar, TG; Xiao, HY; Gilmore, JL; Marcoux, D; Xie, JH; McIntyre, KW; Taylor, TL; Borowski, V; Heimrich, E; Li, YW; Feng, J; Fernandes, A; Yang, Z; Balimane, P; Marino, AM; Cornelius, G; Warrack, BM; Mathur, A; Wu, DR; Li, P; Gupta, A; Pragalathan, B; Shen, DR; Cvijic, ME; Lehman-McKeeman, LD; Salter-Cid, L; Barrish, JC; Carter, PH; Dyckman, AJ Asymmetric Hydroboration Approach to the Scalable Synthesis of ((1R,3S)-1-Amino-3-((R)-6-hexyl-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopentyl)methanol (BMS-986104) as a Potent S1P J Med Chem 59: 11138-11147 (2016)
- Deng, L; Diao, J; Chen, P; Pujari, V; Yao, Y; Cheng, G; Crick, DC; Prasad, BV; Song, Y Inhibition of 1-deoxy-D-xylulose-5-phosphate reductoisomerase by lipophilic phosphonates: SAR, QSAR, and crystallographic studies. J Med Chem 54: 4721-34 (2011)
- Veleti, SK; Lindenberger, JJ; Ronning, DR; Sucheck, SJ Synthesis of a C-phosphonate mimic of maltose-1-phosphate and inhibition studies on Mycobacterium tuberculosis GlgE. Bioorg Med Chem 22: 1404-11 (2014)
- Demont, EH; Arpino, S; Bit, RA; Campbell, CA; Deeks, N; Desai, S; Dowell, SJ; Gaskin, P; Gray, JR; Harrison, LA; Haynes, A; Heightman, TD; Holmes, DS; Humphreys, PG; Kumar, U; Morse, MA; Osborne, GJ; Panchal, T; Philpott, KL; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of a brain-penetrant S1P3-sparing direct agonist of the S1P¿? and S1P5 receptors efficacious at low oral dose. J Med Chem 54: 6724-33 (2011)
- Pennington, LD; Croghan, MD; Sham, KK; Pickrell, AJ; Harrington, PE; Frohn, MJ; Lanman, BA; Reed, AB; Lee, MR; Xu, H; McElvain, M; Xu, Y; Zhang, X; Fiorino, M; Horner, M; Morrison, HG; Arnett, HA; Fotsch, C; Tasker, AS; Wong, M; Cee, VJ Quinolinone-based agonists of S1P¿?: use of a N-scan SAR strategy to optimize in vitro and in vivo activity. Bioorg Med Chem Lett 22: 527-31 (2011)
- Pettit, GR; Grealish, MP; Jung, MK; Hamel, E; Pettit, RK; Chapuis, JC; Schmidt, JM Antineoplastic agents. 465. Structural modification of resveratrol: sodium resverastatin phosphate. J Med Chem 45: 2534-42 (2002)
- Freitas, RF; Prokopczyk, IM; Zottis, A; Oliva, G; Andricopulo, AD; Trevisan, MT; Vilegas, W; Silva, MG; Montanari, CA Discovery of novel Trypanosoma cruzi glyceraldehyde-3-phosphate dehydrogenase inhibitors. Bioorg Med Chem 17: 2476-82 (2009)
- Fu, J; Lou, Y; He, Y Fused tricyclic ring derivatives as Src homology-2 phosphate inhibitors US Patent US11034705 (2021)
- Mabiala-Bassiloua, CG; Arthus-Cartier, G; Hannaert, V; Thérisod, H; Sygusch, J; Thérisod, M Mannitol Bis-phosphate Based Inhibitors of Fructose 1,6-Bisphosphate Aldolases. ACS Med Chem Lett 2: 804-808 (2011)
- Chong, KT; Ruwart, MJ; Hinshaw, RR; Wilkinson, KF; Rush, BD; Yancey, MF; Strohbach, JW; Thaisrivongs, S Peptidomimetic HIV protease inhibitors: phosphate prodrugs with improved biological activities. J Med Chem 36: 2575-7 (1993)
- Liu, C; Dunaway-Mariano, D; Mariano, PS Rational design of reversible inhibitors for trehalose 6-phosphate phosphatases. Eur J Med Chem 128: 274-286 (2017)
- Kesharwani, S; Sundriyal, S Non-hydroxamate inhibitors of 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR): A critical review and future perspective. Eur J Med Chem 213: (2021)
- van Steijn, A; Willems, H; de Boer, T; Geurts, J; van Boeckel, C Synthesis of myo-inositol-1-phosphatase inhibitors in which the phosphate group is replaced by less polar groups Bioorg Med Chem Lett 5: 469-474 (1995)
- Kung, PP; Fan, C; Gukasyan, HJ; Huang, B; Kephart, S; Kraus, M; Lee, JH; Sutton, SC; Yamazaki, S; Zehnder, L Design and Characterization of a Pyridone-Containing EZH2 Inhibitor Phosphate Prodrug. J Med Chem 64: 1725-1732 (2021)
- Leitão, A; Andricopulo, AD; Oliva, G; Pupo, MT; de Marchi, AA; Vieira, PC; da Silva, MF; Ferreira, VF; de Souza, MC; Sá, MM; Moraes, VR; Montanari, CA Structure-activity relationships of novel inhibitors of glyceraldehyde-3-phosphate dehydrogenase. Bioorg Med Chem Lett 14: 2199-204 (2004)
- Patwardhan, NN; Morris, EA; Kharel, Y; Raje, MR; Gao, M; Tomsig, JL; Lynch, KR; Santos, WL Structure-activity relationship studies and in vivo activity of guanidine-based sphingosine kinase inhibitors: discovery of SphK1- and SphK2-selective inhibitors. J Med Chem 58: 1879-99 (2015)
- Kwon, YU; Im, J; Choi, G; Kim, YS; Choi, KY; Chung, SK Synthesis of three enantiomeric pairs of scyllo-inositol phosphate and molecular interactions between all possible regioisomers of scyllo-inositol phosphate and inositol 1,4,5-trisphosphate 3-kinase. Bioorg Med Chem Lett 13: 2981-4 (2003)
- Plano, D; Amin, S; Sharma, AK Importance of sphingosine kinase (SphK) as a target in developing cancer therapeutics and recent developments in the synthesis of novel SphK inhibitors. J Med Chem 57: 5509-24 (2014)
- Fredo Naciuk, F; do Nascimento Faria, J; Gonçalves Eufrásio, A; Torres Cordeiro, A; Bruder, M Development of Selective Steroid Inhibitors for the Glucose-6-phosphate Dehydrogenase from ACS Med Chem Lett 11: 1250-1256 (2020)
- Schweitzer, BA; Loida, PJ; CaJacob, CA; Chott, RC; Collantes, EM; Hegde, SG; Mosier, PD; Profeta, S Discovery of imidazole glycerol phosphate dehydratase inhibitors through 3-D database searching. Bioorg Med Chem Lett 12: 1743-6 (2002)
- Kasprzak, AA; Villafranca, JJ Interactive binding between the substrate and allosteric sites of carbamoyl-phosphate synthetase. Biochemistry 27: 8050-6 (1988)
- Rankin, GM; Vullo, D; Supuran, CT; Poulsen, SA Phosphate Chemical Probes Designed for Location Specific Inhibition of Intracellular Carbonic Anhydrases. J Med Chem 58: 7580-90 (2015)
- Yao, ZJ; King, CR; Cao, T; Kelley, J; Milne, GW; Voigt, JH; Burke, TR Potent inhibition of Grb2 SH2 domain binding by non-phosphate-containing ligands. J Med Chem 42: 25-35 (1999)
- Qiao, L; Nan, F; Kunkel, M; Gallegos, A; Powis, G; Kozikowski, AP 3-Deoxy-D-myo-inositol 1-phosphate, 1-phosphonate, and ether lipid analogues as inhibitors of phosphatidylinositol-3-kinase signaling and cancer cell growth. J Med Chem 41: 3303-6 (1998)
- Design, synthesis of ceramide 1-phosphate analogs and their affinity for cytosolic phospholipase A2 as evidenced by surface plasmon resonance.
- Chang, CT; Edwards, MW; Torrence, PF; Mertes, MP 5-Cyano-2'-deoxyuridine 5'-phosphate: a potent competitive inhibitor of thymidylate synthetase. J Med Chem 22: 1137-9 (1979)
- Pereira, JM; Severino, RP; Vieira, PC; Fernandes, JB; da Silva, MF; Zottis, A; Andricopulo, AD; Oliva, G; Corrêa, AG Anacardic acid derivatives as inhibitors of glyceraldehyde-3-phosphate dehydrogenase from Trypanosoma cruzi. Bioorg Med Chem 16: 8889-95 (2008)
- Filipski, KJ; Sammons, MF; Bhattacharya, SK; Panteleev, J; Brown, JA; Loria, PM; Boehm, M; Smith, AC; Shavnya, A; Conn, EL; Song, K; Weng, Y; Facemire, C; Jüppner, H; Clerin, V Discovery of Orally Bioavailable Selective Inhibitors of the Sodium-Phosphate Cotransporter NaPi2a (SLC34A1). ACS Med Chem Lett 9: 440-445 (2018)
- Tritsch, D; Zinglé, C; Rohmer, M; Grosdemange-Billiard, C Flavonoids: true or promiscuous inhibitors of enzyme? The case of deoxyxylulose phosphate reductoisomerase. Bioorg Chem 59: 140-4 (2015)
- Zobel, K; Koehler, MF; Beresini, MH; Caris, LD; Combs, D Phosphate ester serum albumin affinity tags greatly improve peptide half-life in vivo. Bioorg Med Chem Lett 13: 1513-5 (2003)
- Scott, LM; Chen, L; Daniel, KG; Brooks, WH; Guida, WC; Lawrence, HR; Sebti, SM; Lawrence, NJ; Wu, J Shp2 protein tyrosine phosphatase inhibitor activity of estramustine phosphate and its triterpenoid analogs. Bioorg Med Chem Lett 21: 730-3 (2011)
- Manz, TD; Sivakumaren, SC; Yasgar, A; Hall, MD; Davis, MI; Seo, HS; Card, JD; Ficarro, SB; Shim, H; Marto, JA; Dhe-Paganon, S; Sasaki, AT; Boxer, MB; Simeonov, A; Cantley, LC; Shen, M; Zhang, T; Ferguson, FM; Gray, NS Structure-Activity Relationship Study of Covalent Pan-phosphatidylinositol 5-Phosphate 4-Kinase Inhibitors. ACS Med Chem Lett 11: 346-352 (2020)
- Kim, D; Kowalchick, JE; Edmondson, SD; Mastracchio, A; Xu, J; Eiermann, GJ; Leiting, B; Wu, JK; Pryor, KD; Patel, RA; He, H; Lyons, KA; Thornberry, NA; Weber, AE Triazolopiperazine-amides as dipeptidyl peptidase IV inhibitors: close analogs of JANUVIA (sitagliptin phosphate). Bioorg Med Chem Lett 17: 3373-7 (2007)
- Kennedy, KJ; Bressi, JC; Gelb, MH A disubstituted NAD+ analogue is a nanomolar inhibitor of trypanosomal glyceraldehyde-3-phosphate dehydrogenase. Bioorg Med Chem Lett 11: 95-8 (2001)
- Kiuchi, M; Adachi, K; Tomatsu, A; Chino, M; Takeda, S; Tanaka, Y; Maeda, Y; Sato, N; Mitsutomi, N; Sugahara, K; Chiba, K Asymmetric synthesis and biological evaluation of the enantiomeric isomers of the immunosuppressive FTY720-phosphate. Bioorg Med Chem 13: 425-32 (2005)
- Zinglé, C; Tritsch, D; Grosdemange-Billiard, C; Rohmer, M Catechol-rhodanine derivatives: Specific and promiscuous inhibitors of Escherichia coli deoxyxylulose phosphate reductoisomerase (DXR). Bioorg Med Chem 22: 3713-9 (2014)
- Shimazawa, R; Gochomori, M; Shirai, R Design and synthesis of novel Cdc25A-inhibitors having phosphate group as a hydrophilic residue. Bioorg Med Chem Lett 14: 4339-42 (2004)
- Kim, H; Deng, L; Xiong, X; Hunter, WD; Long, MC; Pirrung, MC Glyceraldehyde 3-phosphate dehydrogenase is a cellular target of the insulin mimic demethylasterriquinone B1. J Med Chem 50: 3423-6 (2007)
- Kotoris, CC; Chen, MJ; Taylor, SD Novel phosphate mimetics for the design of non-peptidyl inhibitors of protein tyrosine phosphatases. Bioorg Med Chem Lett 8: 3275-80 (1999)
- Phaosiri, C; Proteau, PJ Substrate analogs for the investigation of deoxyxylulose 5-phosphate reductoisomerase inhibition: synthesis and evaluation. Bioorg Med Chem Lett 14: 5309-12 (2004)
- Evaluation of ketoclomazone and its analogues as inhibitors of 1-deoxy-d-xylulose 5-phosphate synthases and other thiamine diphosphate (ThDP)-dependent enzymes.
- Chou, LC; Chen, CT; Lee, JC; Way, TD; Huang, CH; Huang, SM; Teng, CM; Yamori, T; Wu, TS; Sun, CM; Chien, DS; Qian, K; Morris-Natschke, SL; Lee, KH; Huang, LJ; Kuo, SC Synthesis and preclinical evaluations of 2-(2-fluorophenyl)-6,7-methylenedioxyquinolin-4-one monosodium phosphate (CHM-1-P-Na) as a potent antitumor agent. J Med Chem 53: 1616-26 (2010)
- Maggiora, L; Chang, CT; Hasson, ME; Bigge, CF; Mertes, MP 5-p-benzoquinonyl-2'-deoxyuridine 5'-phosphate: a possible mechanism-based inhibitor of thymidylate synthetase. J Med Chem 26: 1028-36 (1983)
- Schweitzer, BA; Loida, PJ; Thompson-Mize, RL; CaJacob, CA; Hegde, SG Design and synthesis of beta-carboxamido phosphonates as potent inhibitors of imidazole glycerol phosphate dehydratase. Bioorg Med Chem Lett 9: 2053-8 (1999)
- Maemoto, M; Hirata, Y; Hosoe, S; Ouchi, J; Uchii, M; Takada, H; Akizawa, E; Yanagisawa, A; Shuto, S Development of potent non-acylhydrazone inhibitors of intestinal sodium-dependent phosphate transport protein 2b (NaPi2b). Bioorg Med Chem 71: (2022)
- Discovery of Potent and Selective Phosphatidylinositol 3-Phosphate 5-Kinase (PIKfyve) Inhibitors as Methuosis Inducers.
- Akkemik, E; Budak, H; Ciftci, M Effects of some drugs on human erythrocyte glucose 6-phosphate dehydrogenase: an in vitro study. J Enzyme Inhib Med Chem 25: 871-5 (2010)
- Yep, A; Sorenson, RJ; Wilson, MR; Showalter, HD; Larsen, SD; Keller, PR; Woodard, RW Enediol mimics as inhibitors of the D-arabinose 5-phosphate isomerase (KdsD) from Francisella tularensis. Bioorg Med Chem Lett 21: 2679-82 (2011)
- Golojuch, S; Kopcial, M; Strzelecka, D; Kasprzyk, R; Baran, N; Sikorski, PJ; Kowalska, J; Jemielity, J Exploring tryptamine conjugates as pronucleotides of phosphate-modified 7-methylguanine nucleotides targeting cap-dependent translation. Bioorg Med Chem 28: (2020)
- Jedrzejczak, R; Wojciechowski, M; Andruszkiewicz, R; Sowinski, P; Kot-Wasik, A; Milewski, S Inactivation of glucosamine-6-phosphate synthase by N3-oxoacyl derivatives of L-2,3-diaminopropanoic acid. Chembiochem 13: 85-96 (2012)
- Bigham, EC; Gragg, CE; Hall, WR; Kelsey, JE; Mallory, WR; Richardson, DC; Benedict, C; Ray, PH Inhibition of arabinose 5-phosphate isomerase. An approach to the inhibition of bacterial lipopolysaccharide biosynthesis. J Med Chem 27: 717-26 (1984)
- Miran, SG; Chang, SH; Raushel, FM Role of the four conserved histidine residues in the amidotransferase domain of carbamoyl phosphate synthetase. Biochemistry 30: 7901-7 (1991)
- Gosein, V; Miller, GJ Roles of phosphate recognition in inositol 1,3,4,5,6-pentakisphosphate 2-kinase (IPK1) substrate binding and activation. J Biol Chem 288: 26908-13 (2013)
- Aronov, AM; Verlinde, CL; Hol, WG; Gelb, MH Selective tight binding inhibitors of trypanosomal glyceraldehyde-3-phosphate dehydrogenase via structure-based drug design. J Med Chem 41: 4790-9 (1998)
- Kim, YC; Brown, SG; Harden, TK; Boyer, JL; Dubyak, G; King, BF; Burnstock, G; Jacobson, KA Structure-activity relationships of pyridoxal phosphate derivatives as potent and selective antagonists of P2X1 receptors. J Med Chem 44: 340-9 (2001)
- Guida, WC; Elliott, RD; Thomas, HJ; Secrist, JA; Babu, YS; Bugg, CE; Erion, MD; Ealick, SE; Montgomery, JA Structure-based design of inhibitors of purine nucleoside phosphorylase. 4. A study of phosphate mimics. J Med Chem 37: 1109-14 (1994)
- Jeanjean, A; Gary-Bobo, M; Nirdé, P; Leiris, S; Garcia, M; Morère, A Synthesis of new sulfonate and phosphonate derivatives for cation-independent mannose 6-phosphate receptor targeting. Bioorg Med Chem Lett 18: 6240-3 (2008)
- ChEMBL_201317 (CHEMBL806167) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes
- ChEMBL_306053 (CHEMBL833009) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes
- ChEMBL_201334 (CHEMBL806183) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 3 expressed on CHO cell membranes
- ChEMBL_201335 (CHEMBL806184) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 4 expressed on CHO cell membranes
- ChEMBL_201454 (CHEMBL807599) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 5 expressed on CHO cell membranes
- ChEMBL_306054 (CHEMBL833010) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 2 expressed on CHO cell membranes
- ChEMBL_306055 (CHEMBL833011) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 3 expressed on CHO cell membranes
- ChEMBL_306056 (CHEMBL833012) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 4 expressed on CHO cell membranes
- ChEMBL_306057 (CHEMBL833013) Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 5 expressed on CHO cell membranes
- ChEBML_201314 Binding affinity to human Sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEBML_201315 Binding affinity to human sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201314 (CHEMBL800921) Binding affinity to human Sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201316 (CHEMBL806166) Binding affinity towards human Sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEBML_201322 Binding affinity to human sphingosine 1-phosphate receptor 2 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEBML_201324 Binding affinity towards human sphingosine 1-phosphate receptor 2 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEBML_201331 Binding affinity to human sphingosine 1-phosphate receptor 3 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEBML_201333 Binding affinity towards human sphingosine 1-phosphate receptor 3 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEBML_201448 Binding affinity to human sphingosine 1-phosphate receptor 4 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEBML_201449 Binding affinity to human sphingosine 1-phosphate receptor 4 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEBML_201450 Binding affinity towards human sphingosine 1-phosphate receptor 4 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201322 (CHEMBL806172) Binding affinity to human sphingosine 1-phosphate receptor 2 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201323 (CHEMBL806173) Binding affinity to human sphingosine 1-phosphate receptor 2 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201324 (CHEMBL873240) Binding affinity towards human sphingosine 1-phosphate receptor 2 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201331 (CHEMBL806180) Binding affinity to human sphingosine 1-phosphate receptor 3 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201333 (CHEMBL806182) Binding affinity towards human sphingosine 1-phosphate receptor 3 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201448 (CHEMBL873237) Binding affinity to human sphingosine 1-phosphate receptor 4 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201451 (CHEMBL807596) Binding affinity to human sphingosine 1-phosphate receptor 5 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201452 (CHEMBL807597) Binding affinity to human sphingosine 1-phosphate receptor 5 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- ChEMBL_201453 (CHEMBL807598) Binding affinity towards human sphingosine 1-phosphate receptor 5 expressed in CHO cells was determined by using [33P]-S1P as radioligand
- Inhibition Assay Inhibition assay using sphingosine-1-phosphate receptor 3.
- ChEMBL_499713 (CHEMBL980648) Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor
- ChEMBL_499714 (CHEMBL980649) Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor
- ChEMBL_499715 (CHEMBL980650) Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor
- ChEMBL_499716 (CHEMBL980651) Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor
- ChEMBL_540418 (CHEMBL1029035) Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor
- ChEMBL_540419 (CHEMBL1029036) Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor
- ChEMBL_540420 (CHEMBL1029037) Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor
- ChEMBL_540421 (CHEMBL1029038) Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor
- ChEMBL_519984 (CHEMBL951813) Inhibition of sphingosine-1-phosphate lyase in mouse spleen by scintillation counting
- ChEMBL_1575591 (CHEMBL3803908) Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis
- ChEMBL_430293 (CHEMBL913919) Displacement of [33P]sphingosine 1 phosphate from human S1P1 receptor expressed in CHO cells
- ChEMBL_430294 (CHEMBL913920) Displacement of [33P]sphingosine 1 phosphate from human S1P2 receptor expressed in CHO cells
- ChEMBL_430295 (CHEMBL913921) Displacement of [33P]sphingosine 1 phosphate from human S1P3 receptor expressed in CHO cells
- ChEMBL_430296 (CHEMBL913922) Displacement of [33P]sphingosine 1 phosphate from human S1P4 receptor expressed in CHO cells
- ChEMBL_430297 (CHEMBL913923) Displacement of [33P]sphingosine 1 phosphate from human S1P5 receptor expressed in CHO cells
- ChEMBL_1632373 (CHEMBL3875079) Inhibition of PI3Kbeta in HUVEC assessed as reduction in sphingosine-1-phosphate stimulated akt phosphorylation at Ser473 residue preincubated for 15 mins followed by sphingosine-1-phosphate stimulation for 5 mins by HTRF assay
- ChEMBL_304296 (CHEMBL830091) Effective concentration against sphingosine-1-phosphate receptor 1 determined by a [c-35S]-GTP binding assay
- ChEMBL_304306 (CHEMBL830101) Effective concentration against sphingosine 1-phosphate receptor 2 determined by a [c-35S]-GTP binding assay
- ChEMBL_304307 (CHEMBL830102) Effective concentration against sphingosine 1-phosphate receptor 3 determined by a [c-35S]-GTP binding assay
- ChEMBL_304308 (CHEMBL830103) Effective concentration against sphingosine 1-phosphate receptor 4 determined by a [c-35S]-GTP binding assay
- ChEMBL_304309 (CHEMBL830104) Effective concentration against sphingosine 1-phosphate receptor 5 determined by a [c-35S]-GTP binding assay
- Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 1 (S1P1) counterscreen assay Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: U01 AI074564 Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P1_AG_BLA_384_3XEC50 Name: Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 1 (S1P1) counterscreen assay. Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled rece
- ChEMBL_201311 (CHEMBL805799) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 1 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand
- S1P Receptor Binding Assay The assay measures the displacement of [33P]-labeled sphingosine-1-phosphate (S1P) by test compounds from human S1P receptors expressed on CHO cell membranes. Binding was performed for 60 min at room temperature and terminated by collecting the membranes onto GF/B filter plates with a Packard Filtermate Universal Harvester. Filter bound radionuclide was measured on a PerkinElmer 1450 MicroBeta. Specific binding was calculated by subtracting radioactivity that remained in the presence of 1000-fold excess of unlabeled S1P.
- Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 2 (S1P2) counterscreen assay Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: U01 AI074564 Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P2_AG_BLA_384_3XEC50 Name: Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 2 (S1P2) counterscreen assay. Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled rece
- Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 3 (S1P3) counterscreen assay Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: U01 AI074564 Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P3_AG_BLA_384_3XEC50 Name: Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 3 (S1P3) counterscreen assay. Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled rece
- Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 5 (S1P5) counterscreen assay Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: U01 AI074564 Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P5_AG_BLA_384_3XEC50 Name: Late-stage fluorescence-based dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Sphingosine 1-Phosphate Receptor 5 (S1P5) counterscreen assay. Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled rece
- ChEMBL_201312 (CHEMBL800919) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 1 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand (Experiment 1)
- ChEMBL_643861 (CHEMBL1211760) Inhibition of sphingosine kinase 1
- ChEMBL_627320 (CHEMBL1108112) Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting
- ChEMBL_627321 (CHEMBL1108113) Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting
- ChEMBL_627322 (CHEMBL1108114) Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor expressed in HEK293T cells after 60 mins by scintillation counting
- ChEMBL_627323 (CHEMBL1108115) Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor expressed in HEK293T cells after 60 mins by scintillation counting
- ChEMBL_1575592 (CHEMBL3803909) Inhibition of wild-type full-length human sphingosine 1-phosphate lyase transfected in HEK293-H cells preincubated for 30 mins followed by addition of NBD-sphingosine for 3 hrs by fluorescence analysis
- ChEMBL_201313 (CHEMBL800920) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 1 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand. (Experiment 2)
- ChEMBL_201319 (CHEMBL806169) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 2 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand (Experiment 1)
- ChEMBL_201327 (CHEMBL806176) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 3 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand (Experiment 1)
- ChEMBL_201456 (CHEMBL807601) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 5 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand (Experiment 1)
- ChEMBL_304394 (CHEMBL830044) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 1 expressed in HEK293T cells was determined using [gamma-35S]-GTP as radioligand
- ChEMBL_201318 (CHEMBL806168) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 2 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand
- ChEMBL_201326 (CHEMBL806175) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 3 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand
- ChEMBL_201444 (CHEMBL807590) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 4 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand
- ChEMBL_201455 (CHEMBL807600) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 5 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand
- ChEMBL_2161850 (CHEMBL5046711) Displacement of sphingosine D-erythro [3-3H] I- phosphate from human S1P2 receptor expressed in CHO cell membrane
- ChEMBL_304458 (CHEMBL832506) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 1 expressed in HEK293T cells was determined using [gamma-35S]-GTP as radioligand; partial agonist
- ChEMBL_1276448 (CHEMBL3088544) Inhibition of sphingosine kinase 1 (unknown origin)
- ChEMBL_201320 (CHEMBL806170) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 2 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand (Experiment 2)
- ChEMBL_201328 (CHEMBL806177) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 3 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand (Experiment 2)
- ChEMBL_201445 (CHEMBL807591) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 4 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand (Experiment 2)
- ChEMBL_201457 (CHEMBL884095) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 5 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand (Experiment 2)
- ChEMBL_201458 (CHEMBL807602) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 5 expressed in HEK293T cells using [gamma-35S]-GTP as radioligand; Not determined
- ChEMBL_304395 (CHEMBL830045) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 2 expressed in HEK293T cells was determined using [gamma-35S]-GTP as radioligand
- ChEMBL_304396 (CHEMBL830046) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 3 expressed in HEK293T cells was determined using [gamma-35S]-GTP as radioligand
- ChEMBL_304397 (CHEMBL830047) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 4 expressed in HEK293T cells was determined using [gamma-35S]-GTP as radioligand
- ChEMBL_304398 (CHEMBL830048) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 5 expressed in HEK293T cells was determined using [gamma-35S]-GTP as radioligand
- ChEMBL_686925 (CHEMBL1291294) Competitive inhibition of recombinant sphingosine kinase 1 using D-erythro-sphingosine as substrate by Lineweaver-Burke plot analysis
- ChEMBL_1875673 (CHEMBL4377067) Inhibition of human TRPC4 expressed in HEK293 cells assessed as reduction in sphingosine-1-phosphate-induced current at -100 mV by patch clamp assay
- ChEMBL_1875674 (CHEMBL4377068) Inhibition of human TRPC4 expressed in HEK293 cells assessed as reduction in sphingosine-1-phosphate-induced current at +100 mV by patch clamp assay
- ChEMBL_2015690 (CHEMBL4669268) Inhibition of recombinant His6x-tagged human SphK1 expressed in HEK293 cells assessed as reduction in S1P formation using D-erythro-sphingosine as substrate by Western blot analysis
- ChEMBL_304459 (CHEMBL832507) In vitro binding affinity towards human Sphingosine 1-phosphate receptor 5 expressed in HEK293T cells was determined using [gamma-35S]-GTP as radioligand; partial agonist
- [32P] S1P Binding Assay Ki Values were determined by competition of [32P]-S1P binding to stably transfected CHO (S1P1,2,4) or RH7777 (S1P3,5) cells expressing the indicated S1P receptors.
- ChEMBL_1660109 (CHEMBL4009721) Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in S1P formation using sphingosine as substrate after 1 hr by FITC-based caliper assay
- ChEMBL_1365135 (CHEMBL3292342) Competitive inhibition of V5-His-tagged human SphK2 expressed in HEK293 cells assessed as S1P production using sphingosine and [gamma-32P]ATP as substrate by thin layer chromatography
- ChEMBL_1553850 (CHEMBL3767364) Inhibition of recombinant His-tagged human SK1 assessed as production of [32P]-S1P using 10 uM sphingosine as substrate by TLC method in presence of 100 uM [gamma32P]-ATP
- ChEMBL_1553854 (CHEMBL3767368) Inhibition of recombinant SK1 (unknown origin) expressed in Sf9 cells assessed as [33P]S1P formation using D-erythro sphingosine as substrate and gamma[33P]ATP by liquid scintillation counting
- ChEMBL_1553855 (CHEMBL3767369) Inhibition of recombinant SK2 (unknown origin) expressed in Sf9 cells assessed as [33P]S1P formation using D-erythro sphingosine as substrate and gamma[33P]ATP by liquid scintillation counting
- Late stage fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: U01 AI074564 Fast Track Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P4_ANT_BLA_384_3XIC50 Name: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled receptors. Recently, modulation of S1P receptors lo
- Late-stage fluorescence dose response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): purchased compounds EC50 Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: U01 AI074564 Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P4_AG_BLA_384_3XEC50 Name: Late-stage fluorescence dose response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): purchased compounds EC50. Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled receptors. Recently, modulation of S1P receptor
- Late-stage fluorescence dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Synthesized compounds Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: U01 AI074564 Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P4_AG_BLA_384_3XEC50_SYNTHESIZED Name: Late-stage fluorescence dose-response cell-based assay to identify agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Synthesized compounds. Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled receptors. Recently, modulation of S1P
- ChEMBL_1553851 (CHEMBL3767365) Inhibition of recombinant human SK2 assessed as production of [32P] S1P using 10 uM sphingosine as substrate by TLC method in presence of 100 uM [gamma32P]-ATP relative to control
- ChEMBL_627326 (CHEMBL1108118) Antagonist activity at human S1P1 receptor expressed in HEK293T cells assessed as inhibition of sphingosine-1-phosphate-induced gamma-[35S]GTP binding after 30 mins by scintillation counting
- ChEMBL_627327 (CHEMBL1108119) Antagonist activity at human S1P3 receptor expressed in HEK293T cells assessed as inhibition of sphingosine-1-phosphate-induced gamma-[35S]GTP binding after 30 mins by scintillation counting
- ChEMBL_1276446 (CHEMBL3088542) Inhibition of recombinant sphingosine kinase 1 (unknown origin) expressed in HEK293 cells using sphingosine as substrate after 30 mins in presence of [gamma32P]-ATP
- ChEMBL_1578107 (CHEMBL3807062) Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting
- ChEMBL_1660112 (CHEMBL4009724) Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins with substrate measured after 15 mins by LC-MS analysis
- ChEMBL_1276445 (CHEMBL3088541) Inhibition of sphingosine kinase 2 (unknown origin) using sphingosine as substrate after 30 mins in presence of [gamma32P]-ATP
- ChEMBL_590261 (CHEMBL1052796) Inhibition of human sphingosine kinase 1 by off chip mobility shift assay
- ChEMBL_447771 (CHEMBL896780) Inhibition of human S1P expressed in CHOK1 cells
- ChEMBL_1660119 (CHEMBL4009731) Inhibition of SPHK1 in human whole-blood using C17-sphingosine as substrate assessed as reduction in C17-S1P production preincubated for 30 mins followed by substrate addition measured after 10 mins by MS analysis
- Late stage fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Synthesized analogues Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: U01 AI074564 Fast Track Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P4_ANT_BLA_384_3XIC50_Synthesized_Analogues Name: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): Synthesized analogues Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled recept
- Dose response cell-based high throughput screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 3 (S1P3) Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Hugh Rosen, TSRI Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: 1 R03 MH076533-01 Grant Proposal PI: Germana Sanna, TSRI External Assay ID: S1P3_ANT_BLA_384_IC50 Name: Dose response cell-based high throughput screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 3 (S1P3) Description: Sphingosine 1-phosphate (S1P) influences heart rate [1, 2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [1, 4-6] through five related high affinity G-protein coupled receptors [7, 8] which are involved in diverse biology. For example, S1P1 activation is sufficient to control lymphocyte numbers, with no discernable role in sinus rhythm control, whereas S1P3 regulates sinus rhythm but not lymphocyte recirculation.
- Fluorescence-based counterscreen assay for S1P4 agonists: Cell-based dose response high throughput screening assay to identify agonists of the Sphingosine 1-Phosphate Receptor 1 (S1P1) Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Probe Production Center Network (MLPCN) Grant Proposal Number: U01 AI074564 Fast Track Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P1_AG_BLA_1536_3XEC50 Name: Fluorescence-based counterscreen assay for S1P4 agonists: Cell-based dose response high throughput screening assay to identify agonists of the Sphingosine 1-Phosphate Receptor 1 (S1P1) Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-
- Late stage counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 1 (S1P1) Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: U01 AI074564 Fast Track Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P1_ANT_BLA_384_3XIC50 Name: Counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 1 (S1P1) Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled receptors
- ChEMBL_556521 (CHEMBL956516) Inhibition of rabbit muscle glycogen phosphorylase assessed as release of phosphate from glucose 1 phosphate
- ChEMBL_1856910 (CHEMBL4357639) Inhibition of recombinant human SphK2 expressed in baculovirus infected Sf9 cells assessed as decrease in [33P]S1P production using varying level of D-erythro-sphingosine as substrate in presence of [gamma33P]-ATP by scintillation proximity assay
- Late stage counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 2 (S1P2) Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: U01 AI074564 Fast Track Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P2_ANT_BLA_384_3XIC50 Name: Counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 2 (S1P2) Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled receptors
- Late stage counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 3 (S1P3) Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: U01 AI074564 Fast Track Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P3_ANT_BLA_384_3XIC50 Name: Counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 3 (S1P3) Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled receptors
- Late stage counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 5 (S1P5) Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: U01 AI074564 Fast Track Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P5_ANT_BLA_384_3XIC50 Name: Counterscreen assay for S1P4 antagonists: Fluorescence dose response cell-based screening assay for antagonists of the Sphingosine 1-Phosphate Receptor 5 (S1P5) Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity G-protein coupled receptors
- Late-stage fluorescence dose response cell-based counterscreening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): inhibition by S1P4-selective antagonist Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRISMC) Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Michael Oldstone, TSRI Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: U01 AI074564 Fast Track Grant Proposal PI: Michael Oldstone, TSRI External Assay ID: S1P4_AG_BLA_384_3XIC50_Antagonist Name: Late-stage fluorescence dose response cell-based counterscreening assay for antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4): inhibition by S1P4-selective antagonist. Description: Pandemic influenza represents a significant public health threat, due in part to immune cell-mediated lung tissue damage induced during viral infection. Sphingosine 1-phosphate (S1P) is a bioactive phospholipid released by activated blood platelets and serves to influence endothelial integrity, lung epithelial integrity (1), and lymphocyte recirculation (2-5) through five related high affinity
- ChEMBL_447975 (CHEMBL898227) Inhibition of glucose 6 phosphate translocase 1
- ChEMBL_358030 (CHEMBL866347) Displacement of [33P]S1P from S1P1 expressed in CHO cells
- ChEMBL_358031 (CHEMBL853137) Displacement of [33P]S1P from S1P2 expressed in CHO cells
- ChEMBL_358032 (CHEMBL853138) Displacement of [33P]S1P from S1P3 expressed in CHO cells
- ChEMBL_358033 (CHEMBL871182) Displacement of [33P]S1P from S1P4 expressed in CHO cells
- ChEMBL_358034 (CHEMBL871183) Displacement of [33P]S1P from S1P5 expressed in CHO cells
- ChEMBL_620083 (CHEMBL1108551) Inhibition of rabbit muscle glycogen phosphorylase assessed as release of phosphate from glucose-1-phosphate after 25 mins
- Inhibition Assay Inhibition assay using glucose-1-phosphate thymidylyltransferase (RmlA).
- ChEMBL_1749867 (CHEMBL4184627) Displacement of [32P]S1P from recombinant human S1PR4 expressed in cell membranes pretreated for 30 mins followed by [32P]S1P addition measured after 60 mins by scintillation counting method
- ChEMBL_1749868 (CHEMBL4184628) Displacement of [32P]S1P from recombinant human S1PR5 expressed in cell membranes pretreated for 30 mins followed by [32P]S1P addition measured after 60 mins by scintillation counting method
- ChEMBL_1558648 (CHEMBL3771525) Inhibition of rabbit muscle glycogen phosphorylase-a assessed as formation of inorganic phosphate from glucose-1-phosphate by colorimetry
- ChEMBL_595113 (CHEMBL1050600) Inhibition of rabbit muscle glycogen phosphorylase A assessed as release of phosphate from glucose-1-phosphate after 25 mins
- ChEMBL_762684 (CHEMBL1817502) Inhibition of rabbit muscle glycogen phosphorylase 1a assessed as release of phosphate from glucose-1- phosphate after 20 mins
- ChEMBL_762685 (CHEMBL1817503) Inhibition of human liver glycogen phosphorylase 1a assessed as release of phosphate from glucose-1- phosphate after 20 mins
- ChEMBL_374026 (CHEMBL864688) Displacement of [33P]S1P from human S1P1 receptor expressed in CHO cells
- ChEMBL_374027 (CHEMBL864689) Displacement of [33P]S1P from human S1P2 receptor expressed in CHO cells
- ChEMBL_374028 (CHEMBL864690) Displacement of [33P]S1P from human S1P3 receptor expressed in CHO cells
- ChEMBL_374029 (CHEMBL864691) Displacement of [33P]S1P from human S1P4 receptor expressed in CHO cells
- ChEMBL_374030 (CHEMBL864692) Displacement of [33P]S1P from human S1P5 receptor expressed in CHO cells
- ChEMBL_376411 (CHEMBL868516) Inhibition of [33P]S1P binding to S1P1 receptor expressed in CHO cells
- ChEMBL_432845 (CHEMBL915594) Displacement of [32P]S1P from human S1P1 receptor expressed in HEK293T cells
- ChEMBL_432846 (CHEMBL915595) Displacement of [32P]S1P from human S1P3 receptor expressed in HEK293T cells
- ChEMBL_701953 (CHEMBL1657525) Displacement of [33P]S1P from human S1P1R expressed in CHO cell membranes
- ChEMBL_701954 (CHEMBL1657526) Displacement of [33P]S1P from human S1P3R expressed in CHO cell membranes
- ChEMBL_1749865 (CHEMBL4184625) Displacement of [32P]S1P from recombinant human S1PR1 expressed in CHOK1 cell membranes pretreated for 30 mins followed by [32P]S1P addition measured after 60 mins by scintillation counting method
- ChEMBL_99040 (CHEMBL712593) In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphate.
- ChEBML_1553848 Inhibition of SK1 (unknown origin) using 5 uM of sphingosine as substrate
- In Vitro Binding Assay Newly synthesized compounds were first evaluated for binding potency toward S1P1 by a [32P]S1P competitive binding assay following published procedure (J. Rosenberg, H. Liu, Z. Tu, A practical process for the preparation of [32P]S1P and binding assay for S1P receptor ligands, Appl. Radiat. Isot. 102 (2015) 5-9), and using S1P as a reference compound. [32P]S1P was first prepared by incubating sphingosine and [γ-32P]ATP with sphingosine kinase 1 as previously reported (J. Rosenberg, H. Liu, Z. Tu, A practical process for the preparation of [32P]S1P and binding assay for S1P receptor ligands, Appl. Radiat. Isot. 102 (2015) 5-9). [32P]S1P was dissolved in DMSO, and then diluted in the assay buffer (50 mM HEPES-Na with 5 mM MgCl2, 1 mM CaCl2, and 0.5% fatty acid-free bovine serum albumin, pH=7.5). Compounds were dissolved in DMSO and diluted to different concentrations with assay buffer, followed by adding commercial cell membranes expressing recombinant human S1P receptors (1, 2, 3, 4, and 5) in the assay buffer at room temperature in 96-well plate. [32P]S1P solution was then added to give a final volume of 150 μL containing 0.1 nM of [32P]S1P and 1 μg of membrane protein per well. Competitive binding was performed for 60 min at room temperature and terminated by collecting the membranes onto 96-well glass fiber (GF/B) filtration plates (Millipore, Billerica, Mass.). Each filter was washed with 200 μL of assay buffer for five times. The filter bound radionuclide was measured by a Beckman LS3801 scintillation counter using Cherenkov counting. The reported IC50 values were calculated using the 4 parameter equation, least-square non-linear regression curve-fit, with GraphPad Prism software (GraphPad Software, Inc). Each assay was repeated at least three times with duplicate wells for each compound; the reported values (mean±SD, nM) are calculated from the average of all assays. Assays for compounds which showed no activity (IC50>1000 nM) were only repeated twice.
- Dose Response Assay for S1P3 Antagonists Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Assay Provider: The Scripps Research Institute, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: 1 R03 MH076533-01 The biology of S1P receptor subtypes: Sphingosine 1-phosphate (S1P) influences heart rate [1] [2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [1] [4]-[6] through five related high affinity G-protein coupled receptors [7]. Inhibition of lymphocyte recirculation by nonselective S1P receptor agonists produces clinical immunosuppression preventing transplant rejection, but is associated with transient bradycardia. Understanding the contribution of individual receptors has been limited by the unavailability of selective agonists or antagonists for the 5 receptor subtypes. Separation of receptor subtype usage for control of endothelial
- S1P3 Agonist Dose-Response Potency Assay Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Network: Molecular Library Screening Center Network (MLSCN) Proposal number 1 R03 MH076533-01 External Assay ID: (4.3) S1P3_AG_BLA_1536_EC50 Drun1 Name: S1P3 Agonist Dose-Response Potency Assay The biology of S1P receptor subtypes: Sphingosine 1-phosphate (S1P) influences heart rate [1] [2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [1] [4]-[6] through five related high affinity G-protein coupled receptors [7]. Inhibition of lymphocyte recirculation by nonselective S1P receptor agonists produces clinical immunosuppression preventing transplant rejection, but is associated with transient bradycardia. Understanding the contribution of individual receptors has been limited by the unavailability of selective agonists or antagonists for the 5 receptor subtypes. Separation of recept
- ChEMBL_1679175 (CHEMBL4029452) Displacement of [33P]-S1P from human S1P1 receptor expressed in CHO cell membranes
- ChEMBL_1679176 (CHEMBL4029453) Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO cell membranes
- ChEMBL_447602 (CHEMBL896616) Inhibition of human PNP in presence of 1 mM phosphate
- ChEMBL_447604 (CHEMBL896618) Inhibition of calf PNP in presence of 1 mM phosphate
- ChEMBL_1553848 (CHEMBL3767362) Inhibition of SK1 (unknown origin) using 5 uM of sphingosine as substrate
- ChEMBL_1553849 (CHEMBL3767363) Inhibition of SK1 (unknown origin) using 3 uM of sphingosine as substrate
- ChEMBL_1700859 (CHEMBL4051841) Inhibition of recombinant mouse SPHK1 using sphingosine as substrate in presence ATP
- ChEMBL_1700860 (CHEMBL4051842) Inhibition of recombinant mouse SPHK2 using sphingosine as substrate in presence ATP
- ChEMBL_1700865 (CHEMBL4051847) Inhibition of recombinant human SPHK1 using sphingosine as substrate in presence ATP
- ChEMBL_1800086 (CHEMBL4272378) Binding affinity to SK1 (unknown origin) assessed as inhibition of sphingosine phosphorylation
- ChEMBL_1749864 (CHEMBL4184624) Displacement of [32P]S1P from recombinant human S1PR2 expressed in Chem1 cell membranes co-expressing Galpha15 pretreated for 30 mins followed by [32P]S1P addition measured after 60 mins by scintillation counting method
- ChEMBL_1749866 (CHEMBL4184626) Displacement of [32P]S1P from recombinant human S1PR3 expressed in Chem1 cell membranes co-expressing Galpha15 pretreated for 30 mins followed by [32P]S1P addition measured after 60 mins by scintillation counting method
- ChEMBL_1361382 (CHEMBL3293781) Inhibition of human recombinant S1PL (62 to 568) expressed in Sf9 insect cells using S1P as substrate after 1 hr
- ChEMBL_934542 (CHEMBL2317283) Displacement of [33P]S1P from human S1P1 receptor expressed in HEK293T cells after 1 hr by liquid scintillation counting analysis
- ChEMBL_934543 (CHEMBL2317284) Displacement of [33P]S1P from human S1P3 receptor expressed in HEK293T cells after 1 hr by liquid scintillation counting analysis
- S1P Transporter Assay Transporter assays are vectorial and therefore require measurement of the transported analyte in different compartments. The S1P transporter SPNS2 only exports S1P, which obviates measuring uptake of S1P into transporter-expressing cells. Thus, transporter activity was determined by quantifying S1P release from whole cells expressing SPNS2. SPNS2 inhibitor potency was assessed using whole cell assays. HeLa cells expressing mouse SPNS2 were used to determine inhibitor potency (IC50). Cells were plated onto 12 well plates and assayed when the cell monolayers became confluent. Cell growth media (RPMI-1640 containing 10% fetal bovine serum) was replaced with 2 mL of serum-free media (RPMI-1640) containing fatty acid free bovine serum albumin (BSA) (0.2 % w/v) and supplemented with 4-deoxypyridoxine (DOP) (1 mM), NaF (2 mM), Na3VO4 (0.2 mM) to inhibit S1P degradation. Test articles (1 × 10-9 - 1 × 10-4 M) were assayed in duplicate or triplicate. After 18 hours, media was collected, an internal recovery standard (0.005 mL of 5 × 10-7 M deuterated (d7) S 1P in methanol) was added, the BSA was precipitated with trichloroacetic acid and the bound S1P extracted with methanol. S1P and S1P-d7 were measured by liquid chromatography mass spectrometry.
- ChEBML_1682036 Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting method
- ChEMBL_1679172 (CHEMBL4029449) Displacement of [33P]-S1P from S1P3 receptor (unknown origin) expressed in CHO cell membranes
- ChEMBL_1679173 (CHEMBL4029450) Displacement of [33P]-S1P from S1P1 receptor (unknown origin) expressed in CHO cell membranes
- ChEMBL_1681995 (CHEMBL4032272) Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins followed by S1P stimulation measured every 3 secs by fluorescence method
- ChEMBL_1681996 (CHEMBL4032273) Agonist activity at human S1P3 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins followed by S1P stimulation measured every 3 secs by fluorescence method
- ChEMBL_1460187 (CHEMBL3369412) Inhibition of rabbit muscle glycogen phosphorylase a assessed as inhibition of release of phosphate from glucose-1-phosphate after 30 mins by spectrophotometry
- ChEMBL_1630551 (CHEMBL3873257) Inhibition of rabbit muscle glycogen phosphorylase-b assessed as release of inorganic phosphate from glucose-1-phosphate in presence of AMP and glycogen
- ChEMBL_797827 (CHEMBL1942413) Inhibition of rabbit muscle glycogen phosphorylase a assessed as release of phosphate from glucose-1-phosphate after 25 mins using malachite green staining
- ChEMBL_1365136 (CHEMBL3292343) Inhibition of SphK2 (unknown origin) using sphingosine and [gamma-32P]ATP as substrate
- ChEMBL_1582610 (CHEMBL3816951) Inhibition of SphK1 in mouse erythrocytes using sphingosine as substrate by luminescence assay
- ChEMBL_1700862 (CHEMBL4051844) Inhibition of recombinant human SPHK1 using sphingosine as substrate by ADP-Quest assay
- ChEMBL_1700866 (CHEMBL4051848) Inhibition of recombinant human SPHK2 using sphingosine as substrate by ADP-Quest assay
- Dose Response Assays for S1P1 Agonists and Agonism Potentiators - Potentiator Assay 60K MLSMR Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Assay Provider: The Scripps Research Institute, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: 1 R03 MH076533-01 The biology of S1P receptor subtypes Sphingosine 1-phosphate (S1P) influences heart rate (1,2), coronary artery caliber, endothelial integrity, lung epithelial integrity (3) and lymphocyte recirculation (1,4-6) through five related high affinity G-protein coupled receptors (7). Inhibition of lymphocyte recirculation by nonselective S1P receptor agonists produce clinical immunosuppression preventing transplant rejection, but is associated with transient bradycardia. Understanding the contribution of individual receptors has been limited by the unavailability of selective agonists or antagonists for the 5 receptor subtypes. Separation of receptor subtype usage for control of endothelial and
- Kinase Assay Sphingosine kinase activity was assessed as described previously (Kharel, Y., Lee, S., Snyder, A. H., Sheasley-O'Neill, S. L., Morris, M. A., Setiady, Y., Zhu, R., Zigler, M. A., Burcin, T. L., Ley, K., Tung, K. S. K., Engelhard, V. H., Macdonald, T. L. and Lynch, K. R. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biological Chemistry 280: 36865-36872 (2005)). High levels of human sphingosine kinase type 1 (mSK1) and mouse sphingosine kinase type 2 (mSK2) were expressed in Sf9 insect cells by infection with cognate baculoviruses. Crude homogenates were incubated with γ-[32P]ATP and 10 micromolar D-erythro-sphingosine in the presence of 100 micromolar concentrations of test compounds for 20 minutes at 37 C. The product, radiolabeled S1P, was isolated by extraction into organic solvents after acidification and displaced by thin layer chromatography.
- ChEMBL_1619018 (CHEMBL3861187) Inhibition of rabbit skeletal muscle glycogen phosphorylase b assessed as inorganic phosphate release using glucose-1-phosphate as substrate by double-reciprocal plot analysis
- ChEMBL_1619029 (CHEMBL3861198) Inhibition of rabbit skeletal muscle glycogen phosphorylase b assessed as reduction in inorganic phosphate release using glucose-1-phosphate as substrate at pH 6.8
- ChEMBL_753234 (CHEMBL1799389) Inhibition of rabbit muscle glycogen phosphorylase A assessed as release of phosphate from glucose-1-phosphate after 25 mins by microplate reader based method
- Inhibition Activity Assay Human liver glycogen phsphorylase (HLGP) activity was measured in the direction of glycogen synthesis by the release of phosphate from glucose-1-phosphate.
- ChEMBL_1670145 (CHEMBL4020033) Competitive inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using varying levels of glucose-1-phosphate and constant concentration of AMP and glycogen preincubated for 15 mins with AMP and glycogen followed by glucose-1-phosphate addition
- ChEMBL_1518044 (CHEMBL3619045) Displacement of [33P]S1P from human S1P2 expressed in CHO-K1 cells by scintillation counter
- ChEMBL_1518053 (CHEMBL3619054) Displacement of [33P]S1P from human S1P1 expressed in CHO-K1 cells by scintillation counter
- ChEMBL_1518054 (CHEMBL3619055) Displacement of [33P]S1P from human S1P3 expressed in CHO-K1 cells by scintillation counter
- ChEMBL_1518055 (CHEMBL3619056) Displacement of [33P]S1P from human S1P4 expressed in CHO-K1 cells by scintillation counter
- ChEMBL_1518056 (CHEMBL3619057) Displacement of [33P]S1P from human S1P5 expressed in CHO-K1 cells by scintillation counter
- ChEMBL_1682036 (CHEMBL4032313) Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting method
- ChEMBL_2379337 Inhibition of Spns2 in human HeLa cells decrease in extracellular S1P level by LC/MS method
- ChEMBL_1683754 (CHEMBL4034233) Inhibition of human SphK1 using sphingosine as substrate in presence of [gamma-32P]ATP
- ChEMBL_1683755 (CHEMBL4034234) Inhibition of human SphK2 using sphingosine as substrate in presence of [gamma-32P]ATP
- ChEBML_72895 Inhibitory concentration against glyceraldehyde-3-phosphate dehydrogenase was determined as log 1/IC50
- ChEMBL_162032 (CHEMBL770312) Inhibition of calf spleen PNP (purine nucleoside phosphorylase) in 1 mM phosphate
- ChEMBL_162190 (CHEMBL766710) Inhibition of human erythrocytic PNP (purine nucleoside phosphorylase) in 1 mM phosphate
- ChEMBL_463125 (CHEMBL929037) Inhibition of human carbonic anhydrase 1 assessed as 4-nitrophenyl phosphate hydrolysis
- ChEMBL_813023 (CHEMBL2020535) Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for 30 mins prior S1P addition measured after 16 to 18 hrs by luciferase reporter gene assay
- ChEMBL_813024 (CHEMBL2020536) Antagonist activity at S1P2 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for 30 mins prior S1P addition measured after 16 to 18 hrs by luciferase reporter gene assay
- ChEMBL_813025 (CHEMBL2020537) Antagonist activity at S1P3 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for 30 mins prior S1P addition measured after 16 to 18 hrs by luciferase reporter gene assay
- ChEMBL_208010 (CHEMBL816090) Inhibition of HSV-1 thymidine kinase, 1 uM [3H]thymidine and ATP as phosphate donor
- ChEMBL_1733215 (CHEMBL4148751) Inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of glycogen by spectrophotometric method
- ChEMBL_1733216 (CHEMBL4148752) Inhibition of human liver glycogen phosphorylase-a assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of glycogen by spectrophotometric method
- ChEMBL_764140 (CHEMBL1821351) Competitive inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release using alphaD glucose-1-phosphate as substrate at pH 6.8 by spectrophotometric analysis
- ChEMBL_1679185 (CHEMBL4029462) Displacement of [33P]-S1P from human S1P1 receptor expressed in CHO cell membranes after 45 mins
- ChEMBL_632512 (CHEMBL1111160) Displacement of [33P]S1P from human recombinant S1P1 receptor expressed in HEK cells by scintillation counting
- ChEMBL_632513 (CHEMBL1111161) Displacement of [33P]S1P from human recombinant S1P2 receptor expressed in HEK cells by scintillation counting
- ChEMBL_632514 (CHEMBL1111162) Displacement of [33P]S1P from human recombinant S1P3 receptor expressed in HEK cells by scintillation counting
- ChEMBL_632515 (CHEMBL1111163) Displacement of [33P]S1P from human recombinant S1P4 receptor expressed in CHO cells by scintillation counting
- ChEMBL_632516 (CHEMBL1111164) Displacement of [33P]S1P from human recombinant S1P5 receptor expressed in CHO cells by scintillation counting
- Counterscreen for S1P2 Antagonists: Dose Response Cell-Based Screen to Identify Antagonists of CRE-BLA Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Hugh Rosen, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: 1 R03 MH076533-01 Grant Proposal PI: Germana Sanna External Assay ID: CRE _ANT_BLA_384_IC50_Counterscreen_ S1P2_Hits Name: Counterscreen for S1P2 Antagonists: Dose Response Cell-Based Screen to Identify Antagonists of CRE-BLA Description: Sphingosine 1-phosphate (S1P) influences heart rate [1,2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [2,4] through five related high affinity G-protein coupled receptors [5]. Subtype-selective modulators of S1P receptors will be of broad utility in understanding cell functions in vitro and vascular physiology in vivo, as well as de-convoluting the role of individual subtypes in cellular processes. The S1P receptor 2 (S1P2)
- Dose Response Assays for S1P1 Agonists and Agonism Potentiators - Agonist Assay 60K MLSMR Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Assay Provider: The Scripps Research Institute, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: 1 R03 MH076533-01 The biology of S1P receptor subtypes Sphingosine 1-phosphate (S1P) influences heart rate (1,2), coronary artery caliber, endothelial integrity, lung epithelial integrity (3) and lymphocyte recirculation (1,4-6) through five related high affinity G-protein coupled receptors (7). Inhibition of lymphocyte recirculation by nonselective S1P receptor agonists produce clinical immunosuppression preventing transplant rejection, but is associated with transient bradycardia. Understanding the contribution of individual receptors has been limited by the unavailability of selective agonists or antagonists for the 5 receptor subtypes. Separation of receptor subtype usage for control of endothelial and ep
- Dose Response Assays for S1P1 Agonists and Agonism Potentiators - Parental Cell Line Counter Screen 60K MLSMR Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Assay Provider: The Scripps Research Institute, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: 1 R03 MH076533-01 The biology of S1P receptor subtypes Sphingosine 1-phosphate (S1P) influences heart rate (1,2), coronary artery caliber, endothelial integrity, lung epithelial integrity (3) and lymphocyte recirculation (1,4-6) through five related high affinity G-protein coupled receptors (7). Inhibition of lymphocyte recirculation by nonselective S1P receptor agonists produce clinical immunosuppression preventing transplant rejection, but is associated with transient bradycardia. Understanding the contribution of individual receptors has been limited by the unavailability of selective agonists or antagonists for the 5 receptor subtypes. Separation of receptor subtype usage for control of endothelial and ep
- Dose Response Cell Based Assay for Antagonists of the S1P2 Receptor Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Hugh Rosen, TSRI Network: Molecular Library Screening Center Network (MLSCN) Grant Proposal Number: 1 R03 MH076533-01 Grant Proposal PI: Germana Sanna External Assay ID: S1P2_ANT_BLA_384_IC50 Name: Dose Response Cell Based Assay for Antagonists of the S1P2 Receptor Description: Sphingosine 1-phosphate (S1P) influences heart rate [1,2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [2,4] through five related high affinity G-protein coupled receptors [5]. Subtype-selective modulators of S1P receptors will be of broad utility in understanding cell functions in vitro and vascular physiology in vivo, as well as de-convoluting the role of individual subtypes in cellular processes. The S1P receptor 2 (S1P2), also known as endothelial differentiation sphingolipid G-pro
- ChEMBL_304341 (CHEMBL839767) Agonism of human S1P-1 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]GTP-gamma-S as radioligand
- ChEMBL_2055639 (CHEMBL4710640) Inhibition of recombinant SphK2 (unknown origin) using sphingosine as substrate by [gamma32P]-ATP based assay
- Dose Response Cell Based Assay for Agonists of the S1P2 Receptor Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Hugh Rosen, TSRI Grant Proposal Number: 1 R03 MH076533-01 Grant Proposal PI: Germana Sanna External Assay ID: S1P2_AG_BLA_384_EC50 Name: Dose Response Cell Based Assay for Agonists of the S1P2 Receptor Description: Sphingosine 1-phosphate (S1P) influences heart rate [1, 2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [2, 4] through five related high affinity G-protein coupled receptors [5]. Subtype-selective modulators of S1P receptors will be of broad utility in understanding cell functions in vitro and vascular physiology in vivo, as well as de-convoluting the role of individual subtypes in cellular processes. The S1P receptor 2 (S1P2), also known as endothelial differentiation sphingolipid G-protein-coupled receptor 5 (EDG5), signals through Gi, Gq and G1
- Dose Response Cell Based Assay for Agonists of the S1P2 Receptor of Purchased Analogues Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Hugh Rosen, TSRI Grant Proposal Number: 1 R03 MH076533-01 Grant Proposal PI: Germana Sanna External Assay ID: S1P2_AG_BLA_384_EC50_Purchased_Analogues Name: Dose Response Cell Based Assay for Agonists of the S1P2 Receptor of Purchased Analogues Description: Sphingosine 1-phosphate (S1P) influences heart rate [1, 2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [2, 4] through five related high affinity G-protein coupled receptors [5]. Subtype-selective modulators of S1P receptors will be of broad utility in understanding cell functions in vitro and vascular physiology in vivo, as well as de-convoluting the role of individual subtypes in cellular processes. The S1P receptor 2 (S1P2), also known as endothelial differentiation sphingolipid G-protein-coupled recepto
- ChEBML_153319 In vitro inhibition of purine nucleoside phosphorylase from calf spleen in 1 mM phosphate
- ChEMBL_72895 (CHEMBL684062) Inhibitory concentration against glyceraldehyde-3-phosphate dehydrogenase was determined as log 1/IC50
- ChEMBL_2251055 (CHEMBL5165265) Inhibition of SPHK2 (unknown origin) using sphingosine-fluorescein as substrate incubated for 1 hr in presence of ATP by mobility shift assay
- ChEMBL_2251057 (CHEMBL5165267) Inhibition of SPHK1 (unknown origin) using sphingosine-fluorescein as substrate incubated for 1 hr in presence of ATP by mobility shift assay
- ChEMBL_2253406 (CHEMBL5167616) Inhibition of mouse Spns2 transfected in human HeLa cells by measuring S1P release by LC/MS analysis
- ChEMBL_376407 (CHEMBL866633) Activity at S1P2 receptor expressed in CHO cells measured as S1P-induced [35S]GTP-gamma-S uptake
- ChEMBL_376409 (CHEMBL866637) Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTP-gamma-S uptake
- ChEMBL_1733214 (CHEMBL4148750) Inhibition of rabbit muscle glycogen phosphorylase-b assessed as release of inorganic phosphate using glucose-1-phosphate as substrate in presence of AMP and glycogen by spectrophotometric method
- ChEMBL_506139 (CHEMBL937902) Inhibition of Mycobacterium tuberculosis DXR assessed as NADPH-dependent conversion of 1-deoxy-D-xylulose 5-phosphate to 2-C-methyl-D-erythritol 4-phosphate by spectrophotometric method
- ChEMBL_855991 (CHEMBL2161992) Inhibition of human recombinant SphK2 using NBD-sphingosine as substrate after 2 hrs by HPLC analysis
- Counter screen for S1P2 Agonists: Dose Response High Throughput Cell-Based Screen to Identify Activators of CRE-BLA Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Hugh Rosen, TSRI Grant Proposal Number: 1 R03 MH076533-01 Grant Proposal PI: Germana Sanna External Assay ID: Counterscreen for S1P2 Agonists: Dose Response High Throughput Cell-Based Screen to Identify Activators of CRE-BLA Name: CRE_AG_BLA_384_EC50_%ACT Description: Sphingosine 1-phosphate (S1P) influences heart rate [1,2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [2,4] through five related high affinity G-protein coupled receptors [5]. Subtype-selective modulators of S1P receptors will be of broad utility in understanding cell functions in vitro and vascular physiology in vivo, as well as de-convoluting the role of individual subtypes in cellular processes. The S1P receptor 2 (S1P2), also known as endothelial differentiation sphingolipid G-protein-coup
- Counterscreen for S1P2 Agonists: Dose Response High Throughput Cell-Based Screen to Identify Activators of CRE-BLA: S1P2 Purchased Analogues Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay Provider: Hugh Rosen, TSRI Grant Proposal Number: 1 R03 MH076533-01 Grant Proposal PI: Germana Sanna External Assay ID: CRE_AG_BLA_384_EC50_S1P2_Purchased_Analogues Name: Counterscreen for S1P2 Agonists: Dose Response High Throughput Cell-Based Screen to Identify Activators of CRE-BLA: S1P2 Purchased Analogues Description: Sphingosine 1-phosphate (S1P) influences heart rate [1,2], coronary artery caliber, endothelial integrity, lung epithelial integrity [3] and lymphocyte recirculation [2,4] through five related high affinity G-protein coupled receptors [5]. Subtype-selective modulators of S1P receptors will be of broad utility in understanding cell functions in vitro and vascular physiology in vivo, as well as de-convoluting the role of individual subtypes in cellular processes. The S1P receptor 2 (S1P2), also known as endothelial
- ChEMBL_36028 (CHEMBL646429) Inhibitory activity against Arabinose-5-phosphate isomerase
- ChEMBL_153319 (CHEMBL760055) In vitro inhibition of purine nucleoside phosphorylase from calf spleen in 1 mM phosphate
- ChEMBL_1554435 (CHEMBL3767566) Competitive inhibition of rabbit muscle glycogen phosphorylase b in presence of glucose 1-phosphate
- ChEMBL_831739 (CHEMBL2065102) Inhibition of KHKC in human HepG2 cells assessed as level of fructose-1-phosphate
- ChEMBL_1649508 (CHEMBL3998642) Inhibition of rat muscle glycogen phosphorylase A assessed as release of inorganic phosphate from glucose-1- phosphate in presence of glycogen after 30 mins by malachite green based assay
- ChEMBL_376406 (CHEMBL866632) Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTP-gamma-S uptake
- ChEMBL_376408 (CHEMBL866636) Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTP-gamma-S uptake
- ChEMBL_376410 (CHEMBL866638) Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTP-gamma-S uptake
- ChEMBL_1360672 (CHEMBL3282830) Non-competitive inhibition of pyridoxine phosphate oxidase (unknown origin) assessed as oxidation of pyridoxol 5'-phosphate by Lineweaver-Burk plot analysis
- ChEMBL_1300233 (CHEMBL3136697) Inhibition of purified SK2 (unknown origin) using sphingosine and [gamma-32P]ATP as substrate after 30 mins
- ChEMBL_1300234 (CHEMBL3136698) Inhibition of recombinant SK1 (unknown origin) using sphingosine and [gamma-32P]ATP as substrate after 30 mins
- ChEMBL_1365138 (CHEMBL3292345) Competitive inhibition of human recombinant SphK1 using sphingosine and ATP as substrate by ADP-Quest kinase assay
- ChEMBL_1365139 (CHEMBL3292346) Competitive inhibition of human recombinant SphK2 using sphingosine and ATP as substrate by ADP-Quest kinase assay
- ChEMBL_1717750 (CHEMBL4132750) Inhibition of V5-His-tagged human SphK1 using sphingosine as substrate in presence of [gamma-32P]ATP
- ChEMBL_2069852 (CHEMBL4725105) Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by FLIPR assay
- ChEMBL_153321 (CHEMBL760057) Inhibition of purine nucleoside phosphorylase against the enzyme from calf spleen in 1 mM phosphate
- ChEMBL_1619017 (CHEMBL3861186) Inhibition of rabbit muscle glycogen phosphorylase b using alpha-D-glucose-1-phosphate as substrate
- ChEMBL_1630550 (CHEMBL3873256) Competitive inhibition of rabbit muscle glycogen phosphorylase-a assessed as release of inorganic phosphate using varying levels of glucose-1-phosphate and constant concentration of AMP and glycogen measured after 1 min by Dixon plot method
- ChEBML_1682040 Displacement of [33P]-S1P from human S1P2 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method
- ChEBML_1682041 Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method
- ChEBML_1682042 Displacement of [33P]-S1P from human S1P4 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method
- ChEBML_1682043 Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method
- ChEMBL_1365137 (CHEMBL3292344) Competitive inhibition of human recombinant SphK2 assessed as NBD-S1P formation using NBD-Sph as substrate by HPLC analysis
- ChEMBL_1463952 (CHEMBL3405604) Displacement of [33P]-S1P from human S1P2 receptor expressed in CHOK1 cells after 60 mins by scintillation counting analysis
- ChEMBL_1464178 (CHEMBL3407044) Displacement of [33P]-S1P from human S1P1 receptor expressed in CHOK1 cells after 60 mins by scintillation counting analysis
- ChEMBL_1464179 (CHEMBL3407045) Displacement of [33P]-S1P from human S1P3 receptor expressed in CHOK1 cells after 60 mins by scintillation counting analysis
- ChEMBL_1464180 (CHEMBL3407046) Displacement of [33P]-S1P from human S1P4 receptor expressed in CHOK1 cells after 60 mins by scintillation counting analysis
- ChEMBL_1464181 (CHEMBL3407047) Displacement of [33P]-S1P from human S1P5 receptor expressed in CHOK1 cells after 60 mins by scintillation counting analysis
- ChEMBL_1923242 (CHEMBL4426198) Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method
- ChEMBL_2063500 (CHEMBL4718753) Inhibition of mouse liver microsomal S1PL using S1P as substrate incubated for 20 mins by ESI-LC/MS analysis
- ChEMBL_2196310 (CHEMBL5108826) Displacement of [32P]S1P from human recombinant S1PR2 incubated for 60 mins by competitive binding assay based scintillation counter
- ChEMBL_2196311 (CHEMBL5108827) Displacement of [32P]S1P from human recombinant S1PR1 incubated for 60 mins by competitive binding assay based scintillation counter
- ChEMBL_2196312 (CHEMBL5108828) Displacement of [32P]S1P from human recombinant S1PR3 incubated for 60 mins by competitive binding assay based scintillation counter
- ChEMBL_2196313 (CHEMBL5108829) Displacement of [32P]S1P from human recombinant S1PR4 incubated for 60 mins by competitive binding assay based scintillation counter
- ChEMBL_2196314 (CHEMBL5108830) Displacement of [32P]S1P from human recombinant S1PR5 incubated for 60 mins by competitive binding assay based scintillation counter
- ChEMBL_425681 (CHEMBL911997) Antagonist activity against human S1P1 receptor assessed as inhibition of S1P-induced intracellular calcium mobilization in CHO-K1 cells
- ChEMBL_425682 (CHEMBL911998) Antagonist activity against human S1P3 receptor assessed as inhibition of S1P-induced intracellular calcium mobilization in CHO-K1 cells
- ChEMBL_653532 (CHEMBL1226735) Antagonist activity at human S1P1 receptor expressed in CHO cells assessed as inhibition of S1P-induced [35S]GTPgamma binding
- ChEMBL_972985 (CHEMBL2412223) Inhibition of purified human SphK2 assessed as inhibition of formation of [33P]-S1P after 50 mins by scintillation counting
- ChEMBL_972986 (CHEMBL2412224) Inhibition of purified human SphK1 assessed as inhibition of formation of [33P]-S1P after 50 mins by scintillation counting
- ChEMBL_302548 (CHEMBL828250) Binding affinity against rabbit muscle triose-phosphate isomerase
- ChEMBL_305228 (CHEMBL831647) Inhibitory concentration against rabbit muscle triose-phosphate isomerase
- ChEMBL_664344 (CHEMBL1259493) Inhibition of human recombinant renin in phosphate buffer
- ChEMBL_743385 (CHEMBL1767684) Inhibition of mTOR by radiometric phosphate incorporation assay
- ChEMBL_2433318 Inhibition of MAT2A (unknown origin) assessed as release of inorganic phosphate using ATP/L-methionine incubated for 30 mins by colorimetric phosphate assay
- ChEBML_1553858 Inhibition of human SK2 using D-erythro sphingosine as substrate and gamma[33P]ATP by Lineweaver-Burk plot analysis
- ChEBML_1717751 Inhibition of recombinant human GST-tagged SphK1 using [3H]-sphingosine as substrate after 30 mins by scintillation counting method
- ChEMBL_2354974 Inhibition of recombinant SphK2 (unknown origin) using sphingosine as substrate assessed as inhibition constant by Lineweaver-burk plot analysis
- ChEMBL_1670147 (CHEMBL4020035) Competitive inhibition of 6xHis-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorganic phosphate using varying levels of glucose-1-phosphate and constant concentration of AMP and glycogen preincubated for 15 mins with AMP and glycogen followed by glucose-1-phosphate addition by Lineweaver-Burk plot method
- ChEMBL_1546979 (CHEMBL3748082) Binding affinity to Salmonella typhi glucose-1-phosphate cytidylyl-transferase assessed as dissociation constant by spectrophotometry
- ChEMBL_535725 (CHEMBL989742) Inhibition of Mycobacterium tuberculosis cloned 1-deoxy-D-xylulose-5-phosphate synthase expressed in Escherichia coli
- ChEMBL_1630554 (CHEMBL3873260) Competitive inhibition of rabbit muscle glycogen phosphorylase-b assessed as release of inorganic phosphate using varying levels of glucose-1-phosphate and constant concentration of AMP and glycogen measured at 1 min time interval for 1 to 5 mins by Hill plot method
- Fluorescent Coupled Assay The binding affinities to FtsZ were determined by a fluorescent coupled assay for phosphate relase. In this GTPase studies, phosphate liberated during GTP hydrolysis is a substrate of purine nucleoside phosphorylase, which converts 7-methylguanosine to 7-methylguanin and ribose-1-phosphate, resulting in a decrease of fluorescence.
- ChEMBL_1682035 (CHEMBL4032312) Displacement of [33P]-S1P from human S1P1 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method
- ChEMBL_1682040 (CHEMBL4032317) Displacement of [33P]-S1P from human S1P2 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method
- ChEMBL_1682041 (CHEMBL4032318) Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method
- ChEMBL_1682043 (CHEMBL4032320) Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method
- ChEBML_69909 Inhibitory concentration against Trypanosoma brucei glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
- ChEMBL_303122 (CHEMBL829631) Inhibitory activity against Escherichia coli deoxyxylulose 5-phosphate reductoisomerase
- ChEMBL_72881 (CHEMBL684049) TInhibition of glyceraldehyde-3-phosphate dehydrogenase in Leishmania mexicana
- ChEMBL_72891 (CHEMBL684058) Inhibition of glyceraldehyde-3-phosphate dehydrogenase in Trypanosoma brucei
- ChEMBL_72903 (CHEMBL684097) Inhibition of Glyceraldehyde-3-phosphate dehydrogenase in human erythrocytes.
- ChEMBL_765397 (CHEMBL1828245) Inhibition of PTP1B using p-nitrophenyl phosphate as substrate
- ChEMBL_882108 (CHEMBL2214496) Inhibition of TCPTP by para-nitrophenyl phosphate release assay
- ChEMBL_882109 (CHEMBL2214497) Inhibition of PTP1B by para-nitrophenyl phosphate release assay
- ChEBML_1683748 Inhibition of recombinant human SphK1 using NBD-sphingosine as substrate after 2 hrs in presence of ATP by HPLC method
- ChEMBL_1553856 (CHEMBL3767370) Inhibition of recombinant SK2 (unknown origin) using sphingosine as substrate and gamma[32P]ATP by Lineweaver-Burk plot analysis
- ChEMBL_1553857 (CHEMBL3767371) Inhibition of human SK1 using D-erythro sphingosine as substrate and gamma[33P]ATP by Lineweaver-Burk plot analysis
- ChEMBL_1553858 (CHEMBL3767372) Inhibition of human SK2 using D-erythro sphingosine as substrate and gamma[33P]ATP by Lineweaver-Burk plot analysis
- ChEMBL_1717751 (CHEMBL4132751) Inhibition of recombinant human GST-tagged SphK1 using [3H]-sphingosine as substrate after 30 mins by scintillation counting method
- ChEMBL_2267127 Inhibition of recombinant SphK1 (unknown origin) using NBD-sphingosine as substrate in presence of ATP by fluorescence plate reader analysis
- ChEMBL_2267128 Inhibition of recombinant SphK2 (unknown origin) using NBD-sphingosine as substrate in presence of ATP by fluorescence plate reader analysis
- ChEMBL_522923 (CHEMBL995445) Inhibition of human cloned SPHK2 expressed in CHO cells using D-erythro-sphingosine as substrate by Michaelis-Menten plot
- ChEMBL_855992 (CHEMBL2161993) Inhibition of SphK2 using sphingosine as substrate and [32gammaP]ATP after 15 to 20 mins by Cerenkov counting analysis
- ChEMBL_2267133 Inhibition of recombinant GST-tagged SphK1 (unknown origin) (1 to 384 residues) expressed in baculovirus using sphingosine as substrate incubated for 1 hr in presence of ATP by electrophoretic mobility shift assay
- ChEMBL_2267134 Inhibition of recombinant GST-tagged SphK2 (unknown origin) (1 to 618 residues) expressed in baculovirus using sphingosine as substrate incubated for 1 hr in presence of ATP by electrophoretic mobility shift assay
- ChEMBL_767067 (CHEMBL1826116) Inhibition of Mycobacterium tuberculosis DXR assessed as reduction of 1-deoxy-D-xylulose 5-phosphate into 2-C-methyl-D-erythritol-4-phosphate measured for every 5 secs upto 500 secs by spectrophotometry analysis
- ChEMBL_1700861 (CHEMBL4051843) Inhibition of recombinant human C-terminal His-tagged SPHK1 expressed in fall armyworm sf21 cells using sphingosine as substrate after 1 hr by FITC-based caliper assay
- ChEMBL_1550897 (CHEMBL3762852) Displacement of [33P]-S1P from human S1P5 receptor expressed on CHO-K1 cell membranes after 60 mins by scintillation counting method
- ChEMBL_1550913 (CHEMBL3762868) Displacement of [33P]-S1P from human S1P1 receptor expressed on CHO-K1 cell membranes after 60 mins by scintillation counting method
- ChEMBL_1550914 (CHEMBL3762869) Displacement of [33P]-S1P from human S1P2 receptor expressed on CHO-K1 cell membranes after 60 mins by scintillation counting method
- ChEMBL_1550915 (CHEMBL3762870) Displacement of [33P]-S1P from human S1P3 receptor expressed on CHO-K1 cell membranes after 60 mins by scintillation counting method
- ChEMBL_1550916 (CHEMBL3762871) Displacement of [33P]-S1P from human S1P4 receptor expressed on CHO-K1 cell membranes after 60 mins by scintillation counting method
- ChEMBL_1679181 (CHEMBL4029458) Displacement of [33P]-S1P from S1P1 receptor (unknown origin) expressed in CHOK1 cells after 60 mins by microbeta scintillation proximity assay
- ChEMBL_1679182 (CHEMBL4029459) Displacement of [33P]-S1P from S1P3 receptor (unknown origin) expressed in CHOK1 cells after 60 mins by microbeta scintillation proximity assay
- ChEMBL_1885429 (CHEMBL4387011) Displacement of [32P]S1P from recombinant human S1PR2 expressed in commercial cell membranes incubated for 60 mins by scintillation counting method
- ChEMBL_1885430 (CHEMBL4387012) Displacement of [32P]S1P from recombinant human S1PR1 expressed in commercial cell membranes incubated for 60 mins by scintillation counting method
- ChEMBL_1885431 (CHEMBL4387013) Displacement of [32P]S1P from recombinant human S1PR3 expressed in commercial cell membranes incubated for 60 mins by scintillation counting method
- ChEMBL_1885432 (CHEMBL4387014) Displacement of [32P]S1P from recombinant human S1PR4 expressed in commercial cell membranes incubated for 60 mins by scintillation counting method
- ChEMBL_1885433 (CHEMBL4387015) Displacement of [32P]S1P from recombinant human S1PR5 expressed in commercial cell membranes incubated for 60 mins by scintillation counting method
- ChEMBL_1436200 (CHEMBL3386562) Inhibition of Mycobacterium tuberculosis IspC using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric analysis
- ChEMBL_153323 (CHEMBL873308) Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from calf spleen in 1 mM phosphate
- ChEMBL_972706 (CHEMBL2410915) Agonist activity at human GPR142 transfected in HEK293 cells after 1 hr by inositol phosphate accumulation assay
- ChEMBL_1619019 (CHEMBL3861188) Inhibition of human recombinant liver glycogen phosphorylase A expressed in baculovirus infected Sf9 insect cells assessed as release of phosphate from glucose-1- phosphate in presence of 7.5 mM glucose by malachite green based assay
- ChEMBL_1365140 (CHEMBL3292347) Competitive inhibition of C-terminal His6-tagged human recombinant SphK1 expressed in baculovirus-infected Sf9 cells using sphingosine as substrate
- ChEMBL_522922 (CHEMBL995444) Inhibition of EGFP-fused human SPHK1 expressed in CHO cells using D-erythro-sphingosine as substrate by Michaelis-Menten plot
- ChEBML_1970806 Inhibition of recombinant full length GST-tagged human SPHK2 expressed in baculovirus expression system using Sphingosine lipid as substrate in presence of ATP after 1 hr by Adapta assay
- ChEMBL_1583242 (CHEMBL3816960) Inhibition of GST-tagged full length human recombinant SPHK1 expressed in baculovirus using sphingosine as substrate preincubated for 10 mins measured after 1 hr by ADP-quest assay
- ChEMBL_1583243 (CHEMBL3816961) Inhibition of GST-tagged full length human recombinant SPHK2 expressed in baculovirus using sphingosine as substrate preincubated for 10 mins measured after 1 hr by ADP-quest assay
- ChEBML_52541 Inhibition of 2-C-methyl-d-erythritol-4-phosphate cytidyltransferase (YgbP)
- ChEBML_71454 Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]inositol phosphate hydrolysis
- ChEBML_90659 Inhibitory concentration against imidazole glycerol phosphate dehydratase (IGPD) from Cryptococcus neoformans
- ChEMBL_162205 (CHEMBL767214) Binding affinity against phosphate binding site of Purine nucleoside Phosphorylase
- ChEMBL_447603 (CHEMBL896617) Inhibition of human PNP in presence of 0.025 mM phosphate
- ChEMBL_447605 (CHEMBL896619) Inhibition of calf PNP in presence of 0.025 mM phosphate
- ChEMBL_466588 (CHEMBL930238) Displacement of pentamannosyl phosphate bovine serum albumin from M6P/IGF2R
- ChEMBL_555945 (CHEMBL959129) Activity at human GnRH receptor by inositol phosphate functional assay
- ChEMBL_606269 (CHEMBL1069959) Agonist activity against 5HT2B receptor assessed as inositol phosphate accumulation
- ChEMBL_69909 (CHEMBL682259) Inhibitory concentration against Trypanosoma brucei glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
- ChEMBL_69910 (CHEMBL682260) Inhibitory concentration against Trypanosoma brucei glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
- ChEMBL_69911 (CHEMBL682261) Inhibitory concentration against Trypanosoma cruzi glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
- ChEMBL_69912 (CHEMBL682262) Inhibitory concentration against Trypanosoma cruzi glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
- ChEMBL_69913 (CHEMBL682263) Inhibitory concentration against Trypanosoma mexicana glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
- ChEMBL_69915 (CHEMBL682265) Inhibitory concentration against rabbit muscle glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
- ChEMBL_69916 (CHEMBL877826) Inhibitory concentration against rabbit muscle glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
- ChEMBL_775058 (CHEMBL1912253) Noncompetitive inhibition of GFAT in presence of fructose-6-phosphate
- ChEMBL_775079 (CHEMBL1912274) Inhibition of GFAT using N-acetylglucosamine 6-phosphate as substrate
- ChEMBL_835427 (CHEMBL2072133) Agonist activity at mouse GPR142 assessed as inositol phosphate accumulation
- ChEMBL_882103 (CHEMBL2214491) Competitive inhibition of PTP1B by para-nitrophenyl phosphate release assay
- ChEMBL_1511039 (CHEMBL3607180) Antagonist activity against human S1P1 receptor expressed in human U2OS cells co-expressing GFP assessed as inhibition of S1P-induced receptor translocation
- ChEMBL_2372705 Inhibition of mouse Spns2 expressed in Hela cells assessed as S1P release incubated for 18 to 20 hrs by LC-MS/MS analysis
- ChEMBL_813022 (CHEMBL2020534) Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry
- ChEMBL_1363386 (CHEMBL3295521) Antagonist activity at human GPR103 receptor assessed as inhibition of inositol-1-phosphate production by cell-based assay
- ChEMBL_1476391 (CHEMBL3430021) Inhibition of recombinant human PTP1B (1 to 299) expressed in Escherichia coli using p-nitrophenyl phosphate as substrate
- ChEMBL_1630545 (CHEMBL3873251) Competitive inhibition of rabbit muscle glycogen phosphorylase-b in presence of varying glucose-1-phosphate levels and NADP
- ChEMBL_63499 (CHEMBL676490) Ability to block the ET-1-induced hydrolysis of inositol phosphate in Endothelin A receptor of MMQ cells.
- ChEMBL_1670148 (CHEMBL4020036) Competitive inhibition of rabbit muscle glycogen phosphorylase-b in presence of varying levels of glucose-1-phosphate and constant concentration of glycogen and AMP preincubated for 15 mins with AMP and glycogen followed by glucose-1-phosphate addition by Lineweaver-Burk plot method
- ChEMBL_1670149 (CHEMBL4020037) Competitive inhibition of rabbit muscle glycogen phosphorylase-a in presence of varying levels of glucose-1-phosphate and constant concentration of glycogen and AMP preincubated for 15 mins with AMP and glycogen followed by glucose-1-phosphate addition by Lineweaver-Burk plot method
- ChEMBL_1700863 (CHEMBL4051845) Inhibition of recombinant human SPHK1 expressed in Escherichia coli using [3-3H]sphingosine as substrate after 30 mins by scintillation counting
- ChEMBL_1700864 (CHEMBL4051846) Inhibition of recombinant human SPHK2 expressed in Escherichia coli using [3-3H]sphingosine as substrate after 30 mins by scintillation counting
- ChEMBL_2055640 (CHEMBL4710641) Inhibition of recombinant SphK2 (unknown origin) using sphingosine as substrate incubated for 15 to 20 mins by [gamma32P]-ATP based assay
- ChEMBL_2106081 (CHEMBL4814756) Inhibition of Fam20C (unknown origin) assessed as reduction in inorganic phosphate release in presence of ATP measured by Malachite green phosphate staining based micro plate reader assay
- ChEMBL_304342 (CHEMBL839768) Agonism of human S1P-2 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]GTP-gamma-S as radioligand
- ChEMBL_304343 (CHEMBL839769) Agonism of human S1P-3 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]GTP-gamma-S as radioligand
- ChEMBL_304344 (CHEMBL839770) Agonism of human S1P-4 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]GTP-gamma-S as radioligand
- ChEMBL_304345 (CHEMBL839771) Agonism of human S1P-5 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]GTP-gamma-S as radioligand
- Inhibition Assay (piHA-Dm) After kinetic analysis with HPA, piHA-Dm was tested as an inhibitor of ten additional enzymes. The conditions are listed as follows: Agrobacterium faecalis β-glucosidase (2.5 × 10^-4 mg/mL enzyme, 50 mM sodium phosphate pH 7, 4 mM PNP β-gal); Bos taurus β-galactosidase (6.9 × 10^-2 mg/mL enzyme, 50 mM sodium phosphate pH 7, 1 mM PNP β-gal); S. cerevisiae α-glucosidase (5 × 10^-3 mg/mL enzyme, 50 mM sodium phosphate pH 7, 1 mM α-glc); Human maltase-glucoamylase (8.1 × 10^-2 mg/mL enzyme, 50 mM sodium phosphate pH 7, 1 mM α-glc); Mouse maltase-glucoamylase (8.1 × 10^-2 mg/mL enzyme, 50 mM sodium phosphate pH 7, 1 mM α-glc); Butyrivibrio fibrisolvens α-amylase (1.7 × 10^-4 mg/mL enzyme, 50 mM sodium phosphate 100 mM NaCl pH 7, 1 mM CNPG3); Aspergillus oryzae α-amylase (1.9 × 10^-4 mg/mL enzyme, 50 mM sodium phosphate 100 mM NaCl pH 7, 1 mM CNPG3); Porcine pancreatic α-amylase (6.7 × 10^-3 mg/mL enzyme, 50 mM sodium phosphate 100 mM N
- ChEMBL_1436199 (CHEMBL3386561) Inhibition of Escherichia coli M15 recombinant IspC using 1-deoxy-D-xylulose 5-phosphate as substrate by photometric analysis
- ChEMBL_1554437 (CHEMBL3767568) Competitive inhibition of rabbit muscle glycogen phosphorylase b by Lineweaver-Burk plot analysis in presence of glucose 1-phosphate
- ChEMBL_848287 (CHEMBL2149376) Inhibition of human recombinant LAR expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr
- ChEMBL_1630548 (CHEMBL3873254) Competitive inhibition of His6-tagged human liver glycogen phosphorylase-a expressed in Escherichia coli BL21 Gold (DE3) assessed as release of inorganic phosphate using varying levels of glucose-1-phosphate and constant concentration of AMP and glycogen measured after 1 min by Dixon plot method
- ChEMBL_1455461 (CHEMBL3363893) Stimulation of ATPase activity of human MRP2 assessed as inorganic phosphate
- ChEMBL_1455462 (CHEMBL3363894) Inhibition of ATPase activity of human MRP2 assessed as inorganic phosphate
- ChEMBL_1455463 (CHEMBL3363895) Stimulation of ATPase activity of rat Mrp2 assessed as inorganic phosphate
- ChEMBL_1455464 (CHEMBL3363896) Inhibition of ATPase activity of rat Mrp2 assessed as inorganic phosphate
- ChEMBL_1506090 (CHEMBL3595956) Inhibition of human placental alkaline phosphatase using p-nitrophenyl phosphate substrate
- ChEMBL_1990679 (CHEMBL4624414) Inhibition of HSP90 ATPase activity (unknown origin) malachite-green phosphate assay
- ChEMBL_555946 (CHEMBL959130) Activity at rhesus monkey GnRH receptor by inositol phosphate functional assay
- ChEMBL_70744 (CHEMBL681455) Inhibition of rat brain farnesyltransferase in 30 mM potassium phosphate buffer
- ChEMBL_71454 (CHEMBL685983) Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]inositol phosphate hydrolysis
- ChEMBL_72894 (CHEMBL684061) Inhibitory concentration of compound against glyceraldehyde-3-phosphate dehydrogenase was determined
- ChEMBL_741887 (CHEMBL1768448) Inhibition of Itk after 40 mins by radiometric phosphate incorporation assay
- ChEMBL_743384 (CHEMBL1767683) Inhibition of DNAPK after 2 hrs by radiometric phosphate incorporation assay
- ChEMBL_90659 (CHEMBL700890) Inhibitory concentration against imidazole glycerol phosphate dehydratase (IGPD) from Cryptococcus neoformans
- ChEMBL_90660 (CHEMBL700891) Binding affinity imidazole glycerol phosphate dehydratase (IGPD) obtained from Cryptococcus neoformans
- ChEMBL_986855 (CHEMBL2438603) Inhibition of PTP1B (unknown origin) using p-nitrophenyl phosphate as substrate
- ChEMBL_907777 (CHEMBL3066369) Inhibition of Escherichia coli imidazoleglycerol phosphate dehydratase using imidazole glycerol phosphate as substrate incubated for 30 min prior to substrate addition measured for 16 min by spectrophotometric analysis
- ChEMBL_1683746 (CHEMBL4034225) Inhibition of recombinant N-terminal GST-tagged human SphK1 (1 to 384 residues) expressed in baculovirus expression system using sphingosine as substrate after 1 hr in presence of ATP by off-chip mobility shift assay
- ChEMBL_1683747 (CHEMBL4034226) Inhibition of recombinant N-terminal GST-tagged human SphK2 (1 to 618 residues) expressed in baculovirus expression system using sphingosine as substrate after 1 hr in presence of ATP by off-chip mobility shift assay
- ChEMBL_1582612 (CHEMBL3817020) Inhibition of human SphK1 using sphingosine as substrate incubated for 50 mins by scintillation counting analysis in presence of [gamma-33P]ATP
- ChEMBL_1582613 (CHEMBL3817021) Inhibition of human SphK2 using sphingosine as substrate incubated for 50 mins by scintillation counting analysis in presence of [gamma-33P]ATP
- ChEMBL_1700849 (CHEMBL4051831) Inhibition of recombinant human SPHK1 using sphingosine as substrate after 30 mins in presence of gamma-[32P]-ATP by liquid scintillation counting
- ChEMBL_2055635 (CHEMBL4710636) Inhibition of recombinant human Flag-tagged SphK2 transfected in HEK293 cells using d-erythro-sphingosine as substrate by [gamma32P]-ATP based assay
- ChEMBL_2055636 (CHEMBL4710637) Inhibition of recombinant human Flag-tagged SphK1 transfected in HEK293 cells using d-erythro-sphingosine as substrate by [gamma32P]-ATP based assay
- ChEMBL_792465 (CHEMBL1929795) Inhibition of human SphK1 expressed in baculovirus infected Sf9 insect cells using sphingosine and [gamma-(32)P]-ATP by scintillation proximity assay
- ChEMBL_792466 (CHEMBL1929796) Inhibition of mouse SphK2 expressed in baculovirus infected Sf9 insect cells using sphingosine and [gamma-(32)P]-ATP by scintillation proximity assay
- ChEMBL_855989 (CHEMBL2161990) Inhibition of recombinant SphK1 expressed in baculovirus infected Sf9 cells using D-erythro-sphingosine as substrate and [32gammaP]ATP by scintillation counting
- ChEMBL_855990 (CHEMBL2161991) Inhibition of recombinant SphK2 expressed in baculovirus infected Sf9 cells using D-erythro-sphingosine as substrate and [32gammaP]ATP by scintillation counting
- ChEMBL_2126721 (CHEMBL4836066) Antagonist activity at human S1P2 receptor expressed in CHO cells assessed as EC80 S1P-induced calcium flux measured for 3 mins by fluorescence assay
- ChEMBL_972984 (CHEMBL2412222) Inhibition of SphK1 in human WM266-4 cells assessed as inhibition of formation of [17C]-S1P formation after 20 mins by LC-MS analysis
- ChEMBL_105574 (CHEMBL709771) Agonist activity against Metabotropic glutamate receptor 1 expressed in HEK 293 cells was evaluated by measuring total inositol phosphate accumulation
- ChEMBL_1557478 (CHEMBL3771809) Competitive inhibition of rabbit muscle glycogen phosphorylase-b using alpha-D-glucose-1-phosphate as substrate by Dixon plot analysis
- ChEMBL_848283 (CHEMBL2149372) Inhibition of human recombinant PTP-1B expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr
- ChEMBL_848284 (CHEMBL2149373) Inhibition of human recombinant LMW-PTP1 expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr
- ChEMBL_848285 (CHEMBL2149374) Inhibition of human recombinant LMW-PTP2 expressed in Escherichia coli TB1 using p-nitrophenyl phosphate as substrate after 1 hr
- ChEMBL_860250 (CHEMBL2169191) Agonist activity at human GPR142 expressed in HEK293 cells assessed as inositol phosphate accumulation after 1 hr by scintillation counting
- Competitive Inhibition Assay PNPase activity (guanosine to guanine and ribose 1-phosphate) was followed by measuring the decrease in absorbance at 268nm.
- ChEMBL_1582604 (CHEMBL3816945) Inhibition of human recombinant SphK1 using sphingosine as substrate incubated for 20 mins by scintillation counting analysis in presence of [gamma-32P]ATP
- ChEMBL_1582605 (CHEMBL3816946) Inhibition of human recombinant SphK2 using sphingosine as substrate incubated for 20 mins by scintillation counting analysis in presence of [gamma-32P]ATP
- ChEMBL_1683756 (CHEMBL4034235) Inhibition of human SphK1 using sphingosine as substrate after 15 to 20 mins in presence of [gamma-32P]ATP by Cerenkov counting method
- ChEMBL_1683757 (CHEMBL4034236) Inhibition of human SphK2 using sphingosine as substrate after 15 to 20 mins in presence of [gamma-32P]ATP by Cerenkov counting method
- ChEMBL_1700850 (CHEMBL4051832) Inhibition of recombinant human SPHK2 at using sphingosine as substrate after 30 mins in presence of gamma-[32P]-ATP by liquid scintillation counting
- ChEMBL_957868 (CHEMBL2378981) Activation of recombinant GFP-tagged SK1 (unknown origin) expressed in HEK293 cells using sphingosine as substrate after 30 mins by Cerenkov counting analysis
- ChEBML_65103 Competitive inhibitory activity with respect to EPSP (5-enolpyruvyl-shikimate- 3-phosphate) synthase
- ChEMBL_1286129 (CHEMBL3106986) Competitive inhibition of PFKFB3 (unknown origin) using fructose 6-phosphate as substrate
- ChEMBL_1467560 (CHEMBL3411611) Competitive inhibition of PTP1B (unknown origin) using p-nitrophenyl phosphate as substrate
- ChEMBL_147577 (CHEMBL750155) Accumulation of inositol phosphate in 1321N1 astrocytoma cells expressing human P2Y1 purinoceptor
- ChEMBL_1500296 (CHEMBL3588720) Inverse agonist activity at rat ghrelin receptor by inositol phosphate turnover assay
- ChEMBL_153320 (CHEMBL760056) Inhibition of purine nucleoside phosphorylase from calf spleen in 50 mM phosphate
- ChEMBL_1581173 (CHEMBL3810646) Inhibition of recombinant human PTP1B assessed as hydrolysis of p-nitrophenyl phosphate
- ChEMBL_162033 (CHEMBL766673) Inhibition of calf spleen PNP (purine nucleoside phosphorylase) in 50 mM phosphate
- ChEMBL_1888918 (CHEMBL4390595) Inhibition of Mycobacterium tuberculosis H37Ra PTPB using p-nitrophenyl phosphate as substrate
- ChEMBL_2224918 (CHEMBL5138431) Inhibition of recombinant Trypanosoma cruzi TIM using glyceraldehyde 3-phosphate as substrate
- ChEMBL_2475813 Competitive inhibition of Spinacia oleracea RPIA using D-ribose-5-phosphate as substrate
- ChEMBL_2475814 Competitive inhibition of Escherichia coli RPIB using D-ribose-5-phosphate as substrate
- ChEMBL_2475815 Competitive inhibition of Mycobacterium tuberculosis RPIB using D-ribose-5-phosphate as substrate
- ChEMBL_306074 (CHEMBL874554) Inhibition of human GnRH receptor stimulated inositol phosphate accumulation in RBL cells
- ChEMBL_312533 (CHEMBL832576) Inhibition of PGD2-induced inositol phosphate formation at human Prostaglandin D2 receptor
- ChEMBL_322135 (CHEMBL885194) Effective concentration against US28-mediated inositol phosphate production in COS-7 cells
- ChEMBL_463126 (CHEMBL929038) Inhibition of human carbonic anhydrase 2 assessed as 4-nitrophenyl phosphate hydrolysis
- ChEMBL_463127 (CHEMBL929039) Inhibition of mouse carbonic anhydrase 13 assessed as 4-nitrophenyl phosphate hydrolysis
- ChEMBL_504639 (CHEMBL993810) Inhibition of DNA polymerase beta lyase activity by deoxyribose phosphate excision assay
- ChEMBL_598030 (CHEMBL1046299) Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay
- ChEMBL_65087 (CHEMBL673144) Binding affinity against Escherichia coli EPSP(5-enolpyruvyl-shikimate-3-phosphate) synthase
- ChEMBL_72770 (CHEMBL681383) Inhibitory activity measured for Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in Leishmania. mexicana
- ChEMBL_72896 (CHEMBL684063) Inhibitory activity was measured for Glyceraldehyde-3-phosphate dehydrogenase in Trypanosoma brucei
- ChEMBL_72898 (CHEMBL684065) Inhibitory activity was measured for Glyceraldehyde-3-phosphate dehydrogenase in Trypanosoma cruzi.
- ChEMBL_72900 (CHEMBL684215) Inhibitory activity was measured for Glyceraldehyde-3-phosphate dehydrogenase in human(erythrocytes)
- ChEMBL_741888 (CHEMBL1768449) Inhibition of c-Kit after 45 mins by radiometric phosphate incorporation assay
- ChEMBL_1878663 (CHEMBL4380057) Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate in presence of glucose-6-phosphate, glucose-6-phosphate dehydrogenase and NADPH-generating system incubated for 35 mins by fluorometry
- ChEMBL_1878664 (CHEMBL4380058) Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate in presence of glucose-6-phosphate, glucose-6-phosphate dehydrogenase and NADPH-generating system incubated for 15 mins by fluorometry
- ChEMBL_1878665 (CHEMBL4380059) Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate in presence of glucose-6-phosphate, glucose-6-phosphate dehydrogenase and NADPH-generating system incubated for 50 mins by fluorometry
- ChEMBL_604866 (CHEMBL1065975) Inhibition of Escherichia coli type I phosphomannose isomerase assessed as D-mannose 6-phosphate to D-fructose 6-phosphate isomerization at pH 7.1 by PGI/G6PDH coupled enzyme spectrophotometric assay
- ChEMBL_604867 (CHEMBL1065976) Inhibition of Saccharomyces cerevisiae type I phosphomannose isomerase assessed as D-mannose 6-phosphate to D-fructose 6-phosphate isomerization at pH 7.1 by PGI/G6PDH coupled enzyme spectrophotometric assay
- ChEMBL_1276151 (CHEMBL3089317) Antagonist activity at human S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced decrease in cAMP formation by [35S]GTPgammaS binding assay
- ChEMBL_1542579 (CHEMBL3744618) Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB method
- ChEMBL_1542581 (CHEMBL3744620) Displacement of [33P]S1P from S1P3 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB method
- ChEMBL_1542583 (CHEMBL3744622) Displacement of [33P]S1P from S1P1 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB method
- ChEMBL_2161852 (CHEMBL5046713) Antagonist activity at human S1P2 in HFL1 cells assessed as inhibition of EC80 S1P-induced IL8 release incubated for 16 to 24 hrs by ELISA
- ChEMBL_1590325 (CHEMBL3830364) Competitive inhibition of rat S1PL in presence of D(+)-erythro-sphinganine-1-phosphate measured after 15 mins by Dixon plot analysis
- ChEMBL_63519 (CHEMBL873581) Ability to block the ET-1-induced hydrolysis of inositol phosphate in Endothelin B receptor of chinese hamster ovary(CHO) cells.
- ChEMBL_759463 (CHEMBL1810789) Inhibition of KHK-mediated conversion of D-fructose to fructose-1-phosphate after 60 mins by high throughput mass spectrometry analysis
- ChEMBL_804877 (CHEMBL1953925) Inhibition of Streptococcus pneumoniae acetyltransferase activity of GlmU using acetyl-CoA and glucosamine-1-phosphate after 30 mins by Ellman's method
- ChEMBL_861812 (CHEMBL2173405) Inhibition of SYK in rat RBL2H3 cells assessed as reduction in DNP-BSA-stimulated Fcepsilon receptor 1-mediated inositol phosphate release
- ChEBML_1685483 Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins
- ChEBML_64958 Dissociation constant was calculated for Escherichia coli EPSP(5-enolpyruvyl-shikimate-3-phosphate) Synthase
- ChEBML_72905 Compound was tested for the inhibition of Leishmania mexicana GAPDH(glyceraldehyde-3-phosphate dehydrogenase)
- ChEMBL_1449753 (CHEMBL3378176) Inhibition of rabbit skeletal muscle glycogen phosphorylase b assessed as inorganic phosphate release
- ChEMBL_1455848 (CHEMBL3362302) Inhibition of rat DNPH1 assessed as 2-deoxyribose 5-phosphate production by spectrophotometrically
- ChEMBL_1455849 (CHEMBL3362303) Inhibition of human DNPH1 assessed as 2-deoxyribose 5-phosphate production by spectrophotometrically
- ChEMBL_1458320 (CHEMBL3369282) Inhibition of SHP2 (unknown origin) using p-nitrophenyl phosphate substrate by microplate spectrophotometry
- ChEMBL_312848 (CHEMBL874927) Inhibition of U-46,619-induced inositol phosphate accumulation at human Thromboxane A2 receptor
- ChEMBL_48586 (CHEMBL662466) Inhibition of inositol phosphate production induced by Cholecystokinin type B receptor (0.5 nM)
- ChEMBL_516599 (CHEMBL988686) Inhibition of fructose-1,6-bisphosphatase in mouse liver homogenates by colorimetric phosphate assay
- ChEMBL_72772 (CHEMBL681385) Inhibitory activity was measured for Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in Leishmania. mexicana.
 sphingosine-1-phosphate, 33P labeled {[(4Z)-2-amino-3-hydroxyoctadec-4-en-1-yl]oxy}phosphonic acid S1P [32P]S1P [33P]S1P BDBM22202 sphingosine-1-phosphate sphingosine-1-phosphate, 32P labeled
sphingosine-1-phosphate, 33P labeled {[(4Z)-2-amino-3-hydroxyoctadec-4-en-1-yl]oxy}phosphonic acid S1P [32P]S1P [33P]S1P BDBM22202 sphingosine-1-phosphate sphingosine-1-phosphate, 32P labeled US10676467, Compound S1P (2S,3R,4E)-2-amino-4-octadecene-1,3-diol 1-(dihydrogen phosphate) (2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihydrogen phosphate BDBM50158348 CHEMBL225155 sphingosine-1-phosphate sphingosine 1-phosphate US11584726, Example S1P
US10676467, Compound S1P (2S,3R,4E)-2-amino-4-octadecene-1,3-diol 1-(dihydrogen phosphate) (2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihydrogen phosphate BDBM50158348 CHEMBL225155 sphingosine-1-phosphate sphingosine 1-phosphate US11584726, Example S1P BDBM23161 S1P
BDBM23161 S1P BDBM23162 [32P]S1P
BDBM23162 [32P]S1P Carbamyl phosphate BDBM50162819 CHEMBL369105 carbamoyl phosphate carbamic phosphoric monoanhydride monocarbamoyl phosphate aminocarbonyl dihydrogen phosphate carbamoyl dihydrogen phosphate
Carbamyl phosphate BDBM50162819 CHEMBL369105 carbamoyl phosphate carbamic phosphoric monoanhydride monocarbamoyl phosphate aminocarbonyl dihydrogen phosphate carbamoyl dihydrogen phosphate (2S,3R)-Sphingosine CHEBI:67106 CHEMBL1835439 BDBM50496601
(2S,3R)-Sphingosine CHEBI:67106 CHEMBL1835439 BDBM50496601 Fragment 1 Phosphate phosphoric acid BDBM14671
Fragment 1 Phosphate phosphoric acid BDBM14671 INCB-18424 PHOSPHATE INCB018424 PHOSPHATE Ruxolitinib (as phosphate) Ruxolitinib phosphate BDBM50649917 INCB-018424 SALT Opzelura Ruxolitinib monophosphate INCB-018424 PHOSPHATE INCB018424 SALT Jakafi Jakavi
INCB-18424 PHOSPHATE INCB018424 PHOSPHATE Ruxolitinib (as phosphate) Ruxolitinib phosphate BDBM50649917 INCB-018424 SALT Opzelura Ruxolitinib monophosphate INCB-018424 PHOSPHATE INCB018424 SALT Jakafi Jakavi 4-Nitrophenyl phosphate disodium salt hexahydrate 4-nitrophenyl phosphate (pNPP) para-nitrophenyl phosphate (pNPP) BDBM13466 disodium (4-nitrophenyl) phosphate
4-Nitrophenyl phosphate disodium salt hexahydrate 4-nitrophenyl phosphate (pNPP) para-nitrophenyl phosphate (pNPP) BDBM13466 disodium (4-nitrophenyl) phosphate disodium monohydrogen phosphate Na2HPO4 disodium hydrogen phosphate Disodium phosphate disodium acid orthophosphate BDBM50080995 disodium hydrogenphosphate Dibasic sodium phosphate CHEMBL1060
disodium monohydrogen phosphate Na2HPO4 disodium hydrogen phosphate Disodium phosphate disodium acid orthophosphate BDBM50080995 disodium hydrogenphosphate Dibasic sodium phosphate CHEMBL1060 1-deoxy-L-ribulose 5-phosphate BDBM50153712
1-deoxy-L-ribulose 5-phosphate BDBM50153712 Riboflavin 5''-Phosphate BDBM50421345 RIBOFLAVIN 5'-PHOSPHATE E101a
Riboflavin 5''-Phosphate BDBM50421345 RIBOFLAVIN 5'-PHOSPHATE E101a sodium dihydrogen phosphate NaH2PO4 monosodium phosphate CHEMBL1368 BDBM50155534 sodium phosphate, monobasic sodium dihydrogenphosphate sodium phosphate monobasic anhydrous phosphoric acid, monosodium salt
sodium dihydrogen phosphate NaH2PO4 monosodium phosphate CHEMBL1368 BDBM50155534 sodium phosphate, monobasic sodium dihydrogenphosphate sodium phosphate monobasic anhydrous phosphoric acid, monosodium salt Photrexa Riboflavin 5''-phosphate sodium RIBOFLAVIN 5'-PHOSPHATE SODIUM Phosphated riboflavin Riboflavin sodium phosphate BDBM50523758
Photrexa Riboflavin 5''-phosphate sodium RIBOFLAVIN 5'-PHOSPHATE SODIUM Phosphated riboflavin Riboflavin sodium phosphate BDBM50523758 BDBM50292724 arabinouridine 3'-phosphate CHEMBL460901 URACIL ARABINOSE-3'-PHOSPHATE
BDBM50292724 arabinouridine 3'-phosphate CHEMBL460901 URACIL ARABINOSE-3'-PHOSPHATE BDBM50422306 TRICIRIBINE PHOSPHATE Triciribine Phosphate Salt Of Tricyclic Nucleoside
BDBM50422306 TRICIRIBINE PHOSPHATE Triciribine Phosphate Salt Of Tricyclic Nucleoside 2-amino-ethanol dihydrogen phosphate ethanolamine O-phosphate O-phosphocolamine OPE phosphonoethanolamine monoaminoethyl phosphate colaminphosphoric acid 2-amino-ethanol phosphate colamine phosphate PEA mono(2-aminoethyl) phosphate colamine phosphoric acid 2-aminoethyl dihydrogen phosphate phosphoric acid 2-aminoethyl phenyl ester CHEMBL146972 BDBM50281572 EAP ethanolamine acid phosphate pEtN O-phosphoethanolamine
2-amino-ethanol dihydrogen phosphate ethanolamine O-phosphate O-phosphocolamine OPE phosphonoethanolamine monoaminoethyl phosphate colaminphosphoric acid 2-amino-ethanol phosphate colamine phosphate PEA mono(2-aminoethyl) phosphate colamine phosphoric acid 2-aminoethyl dihydrogen phosphate phosphoric acid 2-aminoethyl phenyl ester CHEMBL146972 BDBM50281572 EAP ethanolamine acid phosphate pEtN O-phosphoethanolamine BDBM50300582 CHEMBL573979 Dibutyl 2,2,2-trifluoro-1-phenylethyl phosphate
BDBM50300582 CHEMBL573979 Dibutyl 2,2,2-trifluoro-1-phenylethyl phosphate CHEMBL575281 Dimethyl 2,2,2-trifluoro-1-phenylethyl phosphate BDBM50300580
CHEMBL575281 Dimethyl 2,2,2-trifluoro-1-phenylethyl phosphate BDBM50300580 CHEMBL575301 Diethyl 2,2,2-trifluoro-1-phenylethyl phosphate BDBM50300581
CHEMBL575301 Diethyl 2,2,2-trifluoro-1-phenylethyl phosphate BDBM50300581 BDBM241975 Clindamycin phosphate
BDBM241975 Clindamycin phosphate Homoserine phosphate BDBM92999
Homoserine phosphate BDBM92999 Hexadecyl[2-(N-Methylpiperidinio)ethyl]phosphate hexadecyl 2-(1-methylpiperidinium-1-yl)ethyl phosphate BDBM50051816 (N-methylpiperidino)ethanol hexadecyl phosphonate CHEMBL89618
Hexadecyl[2-(N-Methylpiperidinio)ethyl]phosphate hexadecyl 2-(1-methylpiperidinium-1-yl)ethyl phosphate BDBM50051816 (N-methylpiperidino)ethanol hexadecyl phosphonate CHEMBL89618 p-nitrophenyl phosphate BDBM24514 CHEMBL24231 4-nitrophenyl phosphate 4-nitrophenoxyphosphonic acid
p-nitrophenyl phosphate BDBM24514 CHEMBL24231 4-nitrophenyl phosphate 4-nitrophenoxyphosphonic acid sodium phosphate, tribasic tertiaeres Natriumphosphat(V) tribasic sodium orthophosphate sodium orthophosphate CHEMBL363100 phosphoric acid trisodium salt sodium phosphate tertiary sodium phosphate tribasic sodium phosphate BDBM50155537 Na3PO4 Trinatriumphosphat
sodium phosphate, tribasic tertiaeres Natriumphosphat(V) tribasic sodium orthophosphate sodium orthophosphate CHEMBL363100 phosphoric acid trisodium salt sodium phosphate tertiary sodium phosphate tribasic sodium phosphate BDBM50155537 Na3PO4 Trinatriumphosphat 4-Methylumbelliferyl phosphate BDBM213237
4-Methylumbelliferyl phosphate BDBM213237 BDBM235670 Dexamethasone sodium phosphate
BDBM235670 Dexamethasone sodium phosphate CHEMBL1756 BDBM50333645 Estramustine phosphate
CHEMBL1756 BDBM50333645 Estramustine phosphate Estradiol phosphate BDBM50333647 CHEMBL1642763
Estradiol phosphate BDBM50333647 CHEMBL1642763 Phosphate analogue, 6 BDBM84557
Phosphate analogue, 6 BDBM84557 Phosphate analogue, 9 BDBM84558
Phosphate analogue, 9 BDBM84558 mannose 6-phosphate BDBM50275807
mannose 6-phosphate BDBM50275807 BDBM50118216 pyridoxal 5'-phosphate CHEMBL82202 (4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl dihydrogen phosphate pyridoxal 5'-(dihydrogen phosphate)
BDBM50118216 pyridoxal 5'-phosphate CHEMBL82202 (4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl dihydrogen phosphate pyridoxal 5'-(dihydrogen phosphate) cori ester glucose-1-phosphate alpha-glucose-1-phosphate {[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phosphonic acid BDBM23188
cori ester glucose-1-phosphate alpha-glucose-1-phosphate {[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phosphonic acid BDBM23188 BDBM197174 Glucose-6-phosphate (G6P)
BDBM197174 Glucose-6-phosphate (G6P) BDBM231698 p-Nitrophenyl phosphate (pNPP)
BDBM231698 p-Nitrophenyl phosphate (pNPP) BDBM84559 Phosphate analogue, (s)-14
BDBM84559 Phosphate analogue, (s)-14 BDBM84560 Phosphate analogue, (S)-21
BDBM84560 Phosphate analogue, (S)-21 para-nitrophenyl phosphate (pNPP) BDBM26100
para-nitrophenyl phosphate (pNPP) BDBM26100 BDBM50304360 BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSPHATE)] benzene-1,2,3,4-tetrayl tetrakis(hydrogen phosphate) CHEMBL595349
BDBM50304360 BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSPHATE)] benzene-1,2,3,4-tetrayl tetrakis(hydrogen phosphate) CHEMBL595349 3-Nitro-1-(beta-D-ribofuranosyl)pyrrole 5'-phosphate BDBM50247810 CHEMBL538120
3-Nitro-1-(beta-D-ribofuranosyl)pyrrole 5'-phosphate BDBM50247810 CHEMBL538120 4-Nitro-1-(-beta-D-ribofuranosyl)pyrazole 5'-phosphate BDBM50247822 CHEMBL538121
4-Nitro-1-(-beta-D-ribofuranosyl)pyrazole 5'-phosphate BDBM50247822 CHEMBL538121 BDBM50247808 CHEMBL538119 3-Nitro-1-(beta-D-ribofuranosyl)imidazole 5'-phosphate
BDBM50247808 CHEMBL538119 3-Nitro-1-(beta-D-ribofuranosyl)imidazole 5'-phosphate phosphacol p-nitrophenyl diethyl phosphate diethyl p-nitrophenyl phosphate O,O-diethyl O-p-nitrophenyl phosphate CHEMBL23838 diethyl 4-nitrophenyl phosphate diethyl paraoxon paraoxon BDBM50240416 ethyl paraoxon phosphoric acid diethyl 4-nitrophenyl ester
phosphacol p-nitrophenyl diethyl phosphate diethyl p-nitrophenyl phosphate O,O-diethyl O-p-nitrophenyl phosphate CHEMBL23838 diethyl 4-nitrophenyl phosphate diethyl paraoxon paraoxon BDBM50240416 ethyl paraoxon phosphoric acid diethyl 4-nitrophenyl ester 5-Amino-1-(beta-D-ribofuranosyl)-4-nitropyrazole 5'-phosphate CHEMBL554752 BDBM50247824
5-Amino-1-(beta-D-ribofuranosyl)-4-nitropyrazole 5'-phosphate CHEMBL554752 BDBM50247824 5-Amino-1-(beta-D-ribofuranosyl)pyrazole-4-carboxylate 5'-phosphate BDBM50247823
5-Amino-1-(beta-D-ribofuranosyl)pyrazole-4-carboxylate 5'-phosphate BDBM50247823 BDBM50247809 5-Amino-1-(beta-D-ribofuranosyl)imidazole-4-carboxaldehyde 5'-phosphate
BDBM50247809 5-Amino-1-(beta-D-ribofuranosyl)imidazole-4-carboxaldehyde 5'-phosphate 2S,3R-dihydroxybutyramide 4-phosphate BDBM50153711
2S,3R-dihydroxybutyramide 4-phosphate BDBM50153711 CHEBI:63036 BDBM50004328 POTASSIUM PHOSPHATE, MONOBASIC
CHEBI:63036 BDBM50004328 POTASSIUM PHOSPHATE, MONOBASIC CHEMBL186043 BDBM50155535 Carbamoyl phosphate; di sodium
CHEMBL186043 BDBM50155535 Carbamoyl phosphate; di sodium CHLOROQUINE DIPHOSPHATE CHLOROQUINE PHOSPHATE BDBM50411863 ARALEN
CHLOROQUINE DIPHOSPHATE CHLOROQUINE PHOSPHATE BDBM50411863 ARALEN Cytidine 2,3-cyclic phosphate, 6 BDBM31909
Cytidine 2,3-cyclic phosphate, 6 BDBM31909 4-{hydroxy[(phosphonooxy)acetyl]amino}butyl dihydrogen phosphate N-(4-hydroxybutyl)-glycolohydroxamicacid bis-phosphate CHEMBL1236228 BDBM50330433
4-{hydroxy[(phosphonooxy)acetyl]amino}butyl dihydrogen phosphate N-(4-hydroxybutyl)-glycolohydroxamicacid bis-phosphate CHEMBL1236228 BDBM50330433 BDBM16190 [3-(2-cyanoacetyl)phenyl] diethyl phosphate 3-(2-Cyanoacetyl)phenyl Diethyl Phosphate beta-ketonitrile 5h
BDBM16190 [3-(2-cyanoacetyl)phenyl] diethyl phosphate 3-(2-Cyanoacetyl)phenyl Diethyl Phosphate beta-ketonitrile 5h DDVP CHEMBL167911 2,2-Dichloroethenyl dimethyl phosphate Dimethyl-2,2-dichlorovinyl phosphate Phosphoric acid, 2,2-dichloroethenyl dimethyl ester Dimethyl 2,2-dichlorovinyl phosphate BDBM50286926 Phosphoric acid, 2,2-dichlorovinyl dimethyl ester
DDVP CHEMBL167911 2,2-Dichloroethenyl dimethyl phosphate Dimethyl-2,2-dichlorovinyl phosphate Phosphoric acid, 2,2-dichloroethenyl dimethyl ester Dimethyl 2,2-dichlorovinyl phosphate BDBM50286926 Phosphoric acid, 2,2-dichlorovinyl dimethyl ester AMP adenosine 5'-phosphate group [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate BDBM92538
AMP adenosine 5'-phosphate group [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate BDBM92538 5-Amino-1-(beta-D-ribofuranosyl)-1,2,3-triazole-4-carboxylate 5'-phosphate BDBM50247825
5-Amino-1-(beta-D-ribofuranosyl)-1,2,3-triazole-4-carboxylate 5'-phosphate BDBM50247825 4S-hydroxypentan-2-one 5-phosphate BDBM50153714
4S-hydroxypentan-2-one 5-phosphate BDBM50153714 BDBM50153710 3S-hydroxypentan-2-one 5-phosphate
BDBM50153710 3S-hydroxypentan-2-one 5-phosphate BDBM92531 DiFMUP (6,8-Difluoro-4-methylumbelliferyl phosphate)
BDBM92531 DiFMUP (6,8-Difluoro-4-methylumbelliferyl phosphate) CHEMBL1230617 8-Bromo-Adenosine Mono Phosphate BDBM50222467
CHEMBL1230617 8-Bromo-Adenosine Mono Phosphate BDBM50222467 CHEMBL1275924 Phenyl-N-(4-aminopiperidine)phosphate BDBM50330376
CHEMBL1275924 Phenyl-N-(4-aminopiperidine)phosphate BDBM50330376 CHEMBL1775011 8-phenyl-Adenosine Mono Phosphate BDBM50343897
CHEMBL1775011 8-phenyl-Adenosine Mono Phosphate BDBM50343897 (3R,4S,5R)-4,5-dihydroxy-3-phosphonooxycyclohexene-1-carboxylic acid Shikimate-3-phosphate BDBM100283
(3R,4S,5R)-4,5-dihydroxy-3-phosphonooxycyclohexene-1-carboxylic acid Shikimate-3-phosphate BDBM100283 1-(4-Methoxyphenylsulfonamido)-3-morpholinopropan-2-yl dihydrogen phosphate HEA derivative, 7a BDBM50248217 CHEMBL473552
1-(4-Methoxyphenylsulfonamido)-3-morpholinopropan-2-yl dihydrogen phosphate HEA derivative, 7a BDBM50248217 CHEMBL473552 4‐bromo‐2‐[(3‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (1) BDBM150721
4‐bromo‐2‐[(3‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (1) BDBM150721 HEA derivative, 7b BDBM50248218 CHEMBL473752 1-(4-Fluorophenylsulfonamido)-3-morpholinopropan-2-yl dihydrogen phosphate
HEA derivative, 7b BDBM50248218 CHEMBL473752 1-(4-Fluorophenylsulfonamido)-3-morpholinopropan-2-yl dihydrogen phosphate dihydrogen phosphate;2-[2-(4-fluorophenyl)sulfanylethyl]pyridin-1-ium BDBM45710 2-[2-(4-Fluoro-phenylsulfanyl)-ethyl]-pyridinium dihydrogen phosphate;2-[2-[(4-fluorophenyl)thio]ethyl]pyridin-1-ium cid_16682387 MLS000767112 SMR000429527
dihydrogen phosphate;2-[2-(4-fluorophenyl)sulfanylethyl]pyridin-1-ium BDBM45710 2-[2-(4-Fluoro-phenylsulfanyl)-ethyl]-pyridinium dihydrogen phosphate;2-[2-[(4-fluorophenyl)thio]ethyl]pyridin-1-ium cid_16682387 MLS000767112 SMR000429527 CHEMBL1683319 5-O-phosphate-1-N-(4'-(4''-methoxyhenyl))-[1',2',3']-triazol-beta-D-ribofuranoside BDBM50338502
CHEMBL1683319 5-O-phosphate-1-N-(4'-(4''-methoxyhenyl))-[1',2',3']-triazol-beta-D-ribofuranoside BDBM50338502 2-(methylsulfonamido)-2-oxoethyl dihydrogen phosphate BDBM50175714 CHEMBL370845
2-(methylsulfonamido)-2-oxoethyl dihydrogen phosphate BDBM50175714 CHEMBL370845 BDBM50300123 2-(4-Formylphenoxy)ethyl dimethyl phosphate CHEMBL574442
BDBM50300123 2-(4-Formylphenoxy)ethyl dimethyl phosphate CHEMBL574442 CHEBI:68603 BETAMETHASONE PHOSPHORIC ACID BDBM50613630 Betamethasone phosphate
CHEBI:68603 BETAMETHASONE PHOSPHORIC ACID BDBM50613630 Betamethasone phosphate CHEBI:83326 TEDIZOLID PHOSPHATE BDBM50017198 TR-701-FA
CHEBI:83326 TEDIZOLID PHOSPHATE BDBM50017198 TR-701-FA CHEMBL606220 CHEMBL1230617 BDBM50367001 8-Bromo-Adenosine Mono Phosphate
CHEMBL606220 CHEMBL1230617 BDBM50367001 8-Bromo-Adenosine Mono Phosphate Fludarabine Phosphate Fludarabine Fludara BDBM50248004 CHEBI:63599 Oforta
Fludarabine Phosphate Fludarabine Fludara BDBM50248004 CHEBI:63599 Oforta (3-amino-1-(4-(1-(phenethoxyimino)ethyl)benzoyl)pyrrolidin-3-yl)methyl Dihydrogen Phosphate US11059784, Example 55 BDBM511446
(3-amino-1-(4-(1-(phenethoxyimino)ethyl)benzoyl)pyrrolidin-3-yl)methyl Dihydrogen Phosphate US11059784, Example 55 BDBM511446 2-Amino-octadec-4-ene-1,3-diol(Sphingosine) BDBM50009730 CHEMBL67166 (E)-2-Amino-octadec-4-ene-1,3-diol
2-Amino-octadec-4-ene-1,3-diol(Sphingosine) BDBM50009730 CHEMBL67166 (E)-2-Amino-octadec-4-ene-1,3-diol ((1R,3R)-1-amino-3-(4-(1-((cyclopropylmethoxy)imino)ethyl)phenyl)cyclopentyl)methyl Dihydrogen Phosphate BDBM511429 US11059784, Example 38
((1R,3R)-1-amino-3-(4-(1-((cyclopropylmethoxy)imino)ethyl)phenyl)cyclopentyl)methyl Dihydrogen Phosphate BDBM511429 US11059784, Example 38 1,2-dideoxy-L-threo-3-hexulose 6-phosphate BDBM50153715
1,2-dideoxy-L-threo-3-hexulose 6-phosphate BDBM50153715 2-Methoxy-4-(nonanamidomethyl)phenyl dihydrogen phosphate (3b) BDBM165191
2-Methoxy-4-(nonanamidomethyl)phenyl dihydrogen phosphate (3b) BDBM165191 BDBM165193 4-(Nonanamidomethyl)-1,2-phenylene bis(dihydrogen phosphate) (6b)
BDBM165193 4-(Nonanamidomethyl)-1,2-phenylene bis(dihydrogen phosphate) (6b) BDBM50175712 CHEMBL199843 2-oxo-2-(phenylsulfonamido)ethyl dihydrogen phosphate
BDBM50175712 CHEMBL199843 2-oxo-2-(phenylsulfonamido)ethyl dihydrogen phosphate CHEMBL202243 BDBM50176400 (R)-2,3-bis(octyloxy)propyl dihydrogen phosphate
CHEMBL202243 BDBM50176400 (R)-2,3-bis(octyloxy)propyl dihydrogen phosphate Etopophos preservative free Etoposide Phosphate BMY-40481 Etopophos BDBM50247889
Etopophos preservative free Etoposide Phosphate BMY-40481 Etopophos BDBM50247889 RUXOLITINIB PHOSPHATE BDBM50391992 US20250136608, Compound Ruxolitinib INCB018424 SALT Jakafi
RUXOLITINIB PHOSPHATE BDBM50391992 US20250136608, Compound Ruxolitinib INCB018424 SALT Jakafi TCP CAS_78-30-8 BDBM82063 TRI-O-CRESYL PHOSPHATE
TCP CAS_78-30-8 BDBM82063 TRI-O-CRESYL PHOSPHATE beta-nicotinamide adenine dinucleotide phosphate, oxidized form NADP+ BDBM11939
beta-nicotinamide adenine dinucleotide phosphate, oxidized form NADP+ BDBM11939 US11046646, Example 14 BDBM506618 (3-amino-1-(4-octylbenzyl)-2-oxopyrrolidin-3-yl)methyl dihydrogen phosphate
US11046646, Example 14 BDBM506618 (3-amino-1-(4-octylbenzyl)-2-oxopyrrolidin-3-yl)methyl dihydrogen phosphate 6-hydroxy-UMP 1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione 5'-monophosphate 6-HYDROXYURIDINE-5'-PHOSPHATE 3,4-dihydroxy-5-(6-oxido-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl phosphate BDBM50199178 CHEMBL383923
6-hydroxy-UMP 1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione 5'-monophosphate 6-HYDROXYURIDINE-5'-PHOSPHATE 3,4-dihydroxy-5-(6-oxido-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl phosphate BDBM50199178 CHEMBL383923 CHEMBL574654 4-(3-((S)-1-((S)-2-carbamoylpyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300616
CHEMBL574654 4-(3-((S)-1-((S)-2-carbamoylpyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300616 4-THIOURIDINE-5'-MONOPHOSPHATE 4-THIOURIDINE-5'-PHOSPHATE BDBM50341895 ((2R,3S,4R,5R)-3,4-dihydroxy-5-(2-oxo-4-thioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL1230331
4-THIOURIDINE-5'-MONOPHOSPHATE 4-THIOURIDINE-5'-PHOSPHATE BDBM50341895 ((2R,3S,4R,5R)-3,4-dihydroxy-5-(2-oxo-4-thioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL1230331 BDBM50080321 ZMP [5-(5-amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate
BDBM50080321 ZMP [5-(5-amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate US10233190, Example 1434 BDBM370515 sodium 1-cyclohexyl-2-(5H-imidazo[5,1- a]isoindol-5-yl)ethyl phosphate
US10233190, Example 1434 BDBM370515 sodium 1-cyclohexyl-2-(5H-imidazo[5,1- a]isoindol-5-yl)ethyl phosphate 2-[(3-bromophenyl)carbamoyl]-5-chlorophenyl diethyl phosphate (20) BDBM150740
2-[(3-bromophenyl)carbamoyl]-5-chlorophenyl diethyl phosphate (20) BDBM150740 2-[(4-bromophenyl)carbamoyl]-5-chlorophenyl diethyl phosphate (23) BDBM150743
2-[(4-bromophenyl)carbamoyl]-5-chlorophenyl diethyl phosphate (23) BDBM150743 BDBM50032981 Phosphate analogue, 8 (3-Amino-propyl)-phosphonic acid CHEMBL286077
BDBM50032981 Phosphate analogue, 8 (3-Amino-propyl)-phosphonic acid CHEMBL286077 BDBM50247086 Chlorphrifos oxon diethyl 3,5,6-trichloropyridin-2-yl phosphate CHEMBL444970
BDBM50247086 Chlorphrifos oxon diethyl 3,5,6-trichloropyridin-2-yl phosphate CHEMBL444970 CHEMBL1235276 BDBM50330438 3-{hydroxy[(phosphonooxy)acetyl]amino}propyl dihydrogen phosphate
CHEMBL1235276 BDBM50330438 3-{hydroxy[(phosphonooxy)acetyl]amino}propyl dihydrogen phosphate Phosphoglycolohydroxamic Acid BDBM50167777 2-(hydroxyamino)-2-oxoethyl dihydrogen phosphate CHEMBL371668
Phosphoglycolohydroxamic Acid BDBM50167777 2-(hydroxyamino)-2-oxoethyl dihydrogen phosphate CHEMBL371668 Di-Sodium salt of Phosphoric acid mono-{2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester Disodium Phosphoric acid mono-{2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester combretastatin A-4 sodium phosphate BDBM50064259 Phosphoric acid mono-{2-methoxy-5-[(Z)-2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester; disodium salt sodium(Z)-2-methoxy-5-(3,4,5-trimethoxystyryl)phenyl phosphate combretastatin A-4 phosphate Combretastatin A4 phosphate disodium 2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)vinyl]phenyl phosphate CHEMBL289351 sodium (Z)-5-(3,4,5-trimethoxystyryl)-2-methoxyphenyl phosphate disodium mono(Z)-5-(3,4,5-trimethoxystyryl)-2-methoxyphenyl phosphate
Di-Sodium salt of Phosphoric acid mono-{2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester Disodium Phosphoric acid mono-{2-methoxy-5-[2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester combretastatin A-4 sodium phosphate BDBM50064259 Phosphoric acid mono-{2-methoxy-5-[(Z)-2-(3,4,5-trimethoxy-phenyl)-vinyl]-phenyl} ester; disodium salt sodium(Z)-2-methoxy-5-(3,4,5-trimethoxystyryl)phenyl phosphate combretastatin A-4 phosphate Combretastatin A4 phosphate disodium 2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)vinyl]phenyl phosphate CHEMBL289351 sodium (Z)-5-(3,4,5-trimethoxystyryl)-2-methoxyphenyl phosphate disodium mono(Z)-5-(3,4,5-trimethoxystyryl)-2-methoxyphenyl phosphate 2-amino-4,5-dihydronaphtho[1,2-d][1,3]thiazol-8-yl dihydrogen phosphate 2-amino-4,5-dihydronaphtho[1,2-d]thiazol-8-yl dihydrogen phosphate BDBM50302495 CHEMBL565809
2-amino-4,5-dihydronaphtho[1,2-d][1,3]thiazol-8-yl dihydrogen phosphate 2-amino-4,5-dihydronaphtho[1,2-d]thiazol-8-yl dihydrogen phosphate BDBM50302495 CHEMBL565809 CHEMBL179992 CHEMBL182645 [(1R,4S,7aR)-1-(1,5-dimethylhexyl)-4,7a-dimethyloctahydro-1H-inden-4-yl]methyl dihydrogen phosphate BDBM50150613
CHEMBL179992 CHEMBL182645 [(1R,4S,7aR)-1-(1,5-dimethylhexyl)-4,7a-dimethyloctahydro-1H-inden-4-yl]methyl dihydrogen phosphate BDBM50150613 Fragment compound, 1 4-trifluoromethylcoumarin phosphate {[2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl]oxy}phosphonic acid BDBM22598
Fragment compound, 1 4-trifluoromethylcoumarin phosphate {[2-oxo-4-(trifluoromethyl)-2H-chromen-7-yl]oxy}phosphonic acid BDBM22598 US9707205, 19 BDBM255780 4-(4-(3-(3-(2,2,2-trifluoroacetyl)-1H-indol-1-yl)propyl)phenyl)butyl-dihydrogen phosphate
US9707205, 19 BDBM255780 4-(4-(3-(3-(2,2,2-trifluoroacetyl)-1H-indol-1-yl)propyl)phenyl)butyl-dihydrogen phosphate 2-(hydroxy(3-hydroxypropyl)amino)-2-oxoethyl hydrogen phosphate BDBM50330436 CHEMBL1276292
2-(hydroxy(3-hydroxypropyl)amino)-2-oxoethyl hydrogen phosphate BDBM50330436 CHEMBL1276292 2-Methoxy-4-((8-methylnonanamido)methyl)phenyl dihydrogen phosphate (3a) BDBM165190
2-Methoxy-4-((8-methylnonanamido)methyl)phenyl dihydrogen phosphate (3a) BDBM165190 5-chloro-2-[(3-chlorophenyl)carbamoyl]phenyl diethyl phosphate (19) BDBM150739
5-chloro-2-[(3-chlorophenyl)carbamoyl]phenyl diethyl phosphate (19) BDBM150739 5-chloro-2-[(3-fluorophenyl)carbamoyl]phenyl diethyl phosphate (21) BDBM150741
5-chloro-2-[(3-fluorophenyl)carbamoyl]phenyl diethyl phosphate (21) BDBM150741 5-chloro-2-[(4-fluorophenyl)carbamoyl]phenyl diethyl phosphate (22) BDBM150742
5-chloro-2-[(4-fluorophenyl)carbamoyl]phenyl diethyl phosphate (22) BDBM150742 5-formyl-6-hydroxy-2-naphthyl di-sodium phosphate BDBM50183272 CHEMBL382634
5-formyl-6-hydroxy-2-naphthyl di-sodium phosphate BDBM50183272 CHEMBL382634 BDBM150744 5-chloro-2-[(4-chlorophenyl)carbamoyl]phenyl diethyl phosphate (24)
BDBM150744 5-chloro-2-[(4-chlorophenyl)carbamoyl]phenyl diethyl phosphate (24) BDBM150745 5-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl diethyl phosphate (25)
BDBM150745 5-chloro-2-[(3,4-dichlorophenyl)carbamoyl]phenyl diethyl phosphate (25) BDBM165192 4-((8-Methylnonanamido)methyl)-1,2-phenylene bis(dihydrogen phosphate) (6a)
BDBM165192 4-((8-Methylnonanamido)methyl)-1,2-phenylene bis(dihydrogen phosphate) (6a) BDBM50300624 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-(3-sulfamoylpropylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL576736
BDBM50300624 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-(3-sulfamoylpropylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL576736 BDBM50300646 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoethylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL574636
BDBM50300646 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoethylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL574636 [5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate;triethylazanium SMR000718807 [5-(6-aminopurin-9-yl)-3,4-dihydroxy-2-oxolanyl]methyl hydrogen phosphate;triethylammonium (5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl hydrogen phosphate;triethylammonium BDBM61258 cid_16396156 MLS001306424 [5-(6-aminopurin-9-yl)-3,4-bis(oxidanyl)oxolan-2-yl]methyl hydrogen phosphate;triethylazanium
[5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate;triethylazanium SMR000718807 [5-(6-aminopurin-9-yl)-3,4-dihydroxy-2-oxolanyl]methyl hydrogen phosphate;triethylammonium (5-adenin-9-yl-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl hydrogen phosphate;triethylammonium BDBM61258 cid_16396156 MLS001306424 [5-(6-aminopurin-9-yl)-3,4-bis(oxidanyl)oxolan-2-yl]methyl hydrogen phosphate;triethylazanium CYTIDINE-5'-MONOPHOSPHATE Phosphoric acid mono-[5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydro-furan-2-ylmethyl] ester CHEMBL307679 cytidine 5'-phosphate ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl dihydrogen phosphate BDBM50310540 ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl dihydrogen phosphate
CYTIDINE-5'-MONOPHOSPHATE Phosphoric acid mono-[5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-3,4-dihydroxy-tetrahydro-furan-2-ylmethyl] ester CHEMBL307679 cytidine 5'-phosphate ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl dihydrogen phosphate BDBM50310540 ((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL514189 (S)-2-amino-2-methyl-3-(4-(2-(naphthalen-1-yl)ethoxy)phenylamino)-3-oxopropyl dihydrogen phosphate BDBM50249239
CHEMBL514189 (S)-2-amino-2-methyl-3-(4-(2-(naphthalen-1-yl)ethoxy)phenylamino)-3-oxopropyl dihydrogen phosphate BDBM50249239 CHEMBL384771 2-thio-1-beta-D-ribofuranosyl(3H)pyrimidine-2,4-dione 5'-monophosphate BDBM50199180 1-(BETA-D-RIBOFURANOSYL)-2-THIO-URACIL-5'-PHOSPHATE
CHEMBL384771 2-thio-1-beta-D-ribofuranosyl(3H)pyrimidine-2,4-dione 5'-monophosphate BDBM50199180 1-(BETA-D-RIBOFURANOSYL)-2-THIO-URACIL-5'-PHOSPHATE 4-(3-((S)-1-((S)-2-((S)-1-amino-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL578233 BDBM50300617
4-(3-((S)-1-((S)-2-((S)-1-amino-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL578233 BDBM50300617 BDBM50300631 4-(3-((S)-1-((S)-2-((R)-1-amino-1-oxooctan-4-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL576785
BDBM50300631 4-(3-((S)-1-((S)-2-((R)-1-amino-1-oxooctan-4-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL576785 CHEMBL1784780 (S)-2-acetamido-3-((S)-1-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-1-oxopropan-2-ylamino)-1-oxopropan-2-ylamino)-3-oxopropyl dihydrogen phosphate BDBM50345636
CHEMBL1784780 (S)-2-acetamido-3-((S)-1-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-1-oxopropan-2-ylamino)-1-oxopropan-2-ylamino)-3-oxopropyl dihydrogen phosphate BDBM50345636 4-(3-((S)-1-((S)-2-(2-(benzylamino)-2-oxoethylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300613 CHEMBL583138
4-(3-((S)-1-((S)-2-(2-(benzylamino)-2-oxoethylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300613 CHEMBL583138 4-(3-((S)-1-((S)-2-(4-(hydroxyamino)-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300625 CHEMBL574875
4-(3-((S)-1-((S)-2-(4-(hydroxyamino)-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300625 CHEMBL574875 BDBM50300626 CHEMBL575098 4-(3-((S)-1-((S)-2-(4-hydrazinyl-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300626 CHEMBL575098 4-(3-((S)-1-((S)-2-(4-hydrazinyl-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573048 BDBM50300618 4-(3-((S)-1-((S)-2-(4-amino-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL573048 BDBM50300618 4-(3-((S)-1-((S)-2-(4-amino-4-oxobutylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL1784770 BDBM50345628 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate
CHEMBL1784770 BDBM50345628 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL573069 BDBM50300647 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-((R)-1-ureidopropan-2-ylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL573069 BDBM50300647 4-(3-((S)-4-methyl-1-oxo-1-((S)-2-((R)-1-ureidopropan-2-ylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate (R)-2-amino-3-(4-octylphenylamino)-3-oxopropyl dihydrogen phosphate CHEMBL227371 BDBM50198840
(R)-2-amino-3-(4-octylphenylamino)-3-oxopropyl dihydrogen phosphate CHEMBL227371 BDBM50198840 2-formylphenoxyphosphonic acid BDBM14680 2-formylphenyl dihydrogen phosphate RU78262 CHEMBL286678 Fragment 10
2-formylphenoxyphosphonic acid BDBM14680 2-formylphenyl dihydrogen phosphate RU78262 CHEMBL286678 Fragment 10 2-hydrazinyl-2-oxoethyl hydrogen phosphate BDBM50167772 Phosphoric acid monohydrazinocarbonylmethyl ester CHEMBL195520
2-hydrazinyl-2-oxoethyl hydrogen phosphate BDBM50167772 Phosphoric acid monohydrazinocarbonylmethyl ester CHEMBL195520 5-chloro-2-{[3-(trifluoromethyl)phenyl]carbamoyl}phenyl diethyl phosphate (26) BDBM150746
5-chloro-2-{[3-(trifluoromethyl)phenyl]carbamoyl}phenyl diethyl phosphate (26) BDBM150746 5-chloro-2-{[4-(trifluoromethyl)phenyl]carbamoyl}phenyl diethyl phosphate (27) BDBM150747
5-chloro-2-{[4-(trifluoromethyl)phenyl]carbamoyl}phenyl diethyl phosphate (27) BDBM150747 BDBM50198833 CHEMBL228102 (R)-2-amino-3-(3-octylphenylamino)-3-oxopropyl dihydrogen phosphate
BDBM50198833 CHEMBL228102 (R)-2-amino-3-(3-octylphenylamino)-3-oxopropyl dihydrogen phosphate BDBM50312665 Sodium 2-(2-Fluorophenyl)-6,7-methylenedioxyquinolin-4-yl hydrogen phosphate CHEMBL1088572
BDBM50312665 Sodium 2-(2-Fluorophenyl)-6,7-methylenedioxyquinolin-4-yl hydrogen phosphate CHEMBL1088572 BDBM50343853 2-(2-(Benzylamino)-6-nitroquinazolin-4-ylamino)ethyl dihydrogen phosphate CHEMBL1774786
BDBM50343853 2-(2-(Benzylamino)-6-nitroquinazolin-4-ylamino)ethyl dihydrogen phosphate CHEMBL1774786 CHEMBL1765479 BDBM50342010 5'-phospho-2'-deoxyuridine-3'-pyrophosphate(P'->5')adenosine3'-phosphate
CHEMBL1765479 BDBM50342010 5'-phospho-2'-deoxyuridine-3'-pyrophosphate(P'->5')adenosine3'-phosphate CHEMBL1784781 BDBM50345637 (S)-2-acetamido-3-((S)-2-((S)-2-((S)-1-amino-1-oxo-3-phenylpropan-2-ylcarbamoyl)pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate
CHEMBL1784781 BDBM50345637 (S)-2-acetamido-3-((S)-2-((S)-2-((S)-1-amino-1-oxo-3-phenylpropan-2-ylcarbamoyl)pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate 1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)azetidin-3-yl dihydrogen phosphate BDBM491208 US10975056, Example 152
1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)azetidin-3-yl dihydrogen phosphate BDBM491208 US10975056, Example 152 1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)pyrrolidin-3-yl dihydrogen phosphate BDBM491206 US10975056, Example 150
1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)pyrrolidin-3-yl dihydrogen phosphate BDBM491206 US10975056, Example 150 US10975056, Example 155 BDBM491211 1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)piperidin-4-yl dihydrogen phosphate
US10975056, Example 155 BDBM491211 1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)piperidin-4-yl dihydrogen phosphate BDBM50300630 CHEMBL574844 4-(3-((S)-1-((S)-2-((S)-1-amino-6-methyl-1-oxoheptan-4-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300630 CHEMBL574844 4-(3-((S)-1-((S)-2-((S)-1-amino-6-methyl-1-oxoheptan-4-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300620 4-(3-((S)-1-((S)-2-(4-amino-4-oxobut-2-ynylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575919
BDBM50300620 4-(3-((S)-1-((S)-2-(4-amino-4-oxobut-2-ynylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575919 BDBM50300622 CHEMBL577593 4-(3-((S)-1-((S)-2-((3S,5S)-5-carbamoylpyrrolidin-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300622 CHEMBL577593 4-(3-((S)-1-((S)-2-((3S,5S)-5-carbamoylpyrrolidin-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300623 CHEMBL573068 4-(3-((S)-1-((S)-2-((3R,5S)-5-carbamoylpyrrolidin-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300623 CHEMBL573068 4-(3-((S)-1-((S)-2-((3R,5S)-5-carbamoylpyrrolidin-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50345630 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784775
BDBM50345630 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxopropan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784775 CHEMBL1784703 BDBM50345629 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate
CHEMBL1784703 BDBM50345629 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784777 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-4-methyl-1-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345632
CHEMBL1784777 (S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-4-methyl-1-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345632 (3R)-1-(6-((2-amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)pyrrolidin-3-yl dihydrogen phosphate BDBM491442 US10975056, Example 416
(3R)-1-(6-((2-amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)pyrrolidin-3-yl dihydrogen phosphate BDBM491442 US10975056, Example 416 [5-[5-fluoranyl-2,4-bis(oxidanylidene)pyrimidin-1-yl]-3-oxidanyl-oxolan-2-yl]methyl [5-[4-(octadecylamino)-2-oxidanylidene-pyrimidin-1-yl]-3,4-bis(oxidanyl)oxolan-2-yl]methyl hydrogen phosphate cid_392613 SMR001565974 [3,4-dihydroxy-5-[2-keto-4-(stearylamino)pyrimidin-1-yl]tetrahydrofuran-2-yl]methyl [5-(5-fluoro-2,4-diketo-pyrimidin-1-yl)-3-hydroxy-tetrahydrofuran-2-yl]methyl hydrogen phosphate MLS002702412 [3,4-dihydroxy-5-[4-(octadecylamino)-2-oxopyrimidin-1-yl]oxolan-2-yl]methyl [5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl hydrogen phosphate [3,4-dihydroxy-5-[4-(octadecylamino)-2-oxo-1-pyrimidinyl]-2-oxolanyl]methyl [5-(5-fluoro-2,4-dioxo-1-pyrimidinyl)-3-hydroxy-2-oxolanyl]methyl hydrogen phosphate BDBM80994
[5-[5-fluoranyl-2,4-bis(oxidanylidene)pyrimidin-1-yl]-3-oxidanyl-oxolan-2-yl]methyl [5-[4-(octadecylamino)-2-oxidanylidene-pyrimidin-1-yl]-3,4-bis(oxidanyl)oxolan-2-yl]methyl hydrogen phosphate cid_392613 SMR001565974 [3,4-dihydroxy-5-[2-keto-4-(stearylamino)pyrimidin-1-yl]tetrahydrofuran-2-yl]methyl [5-(5-fluoro-2,4-diketo-pyrimidin-1-yl)-3-hydroxy-tetrahydrofuran-2-yl]methyl hydrogen phosphate MLS002702412 [3,4-dihydroxy-5-[4-(octadecylamino)-2-oxopyrimidin-1-yl]oxolan-2-yl]methyl [5-(5-fluoro-2,4-dioxopyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methyl hydrogen phosphate [3,4-dihydroxy-5-[4-(octadecylamino)-2-oxo-1-pyrimidinyl]-2-oxolanyl]methyl [5-(5-fluoro-2,4-dioxo-1-pyrimidinyl)-3-hydroxy-2-oxolanyl]methyl hydrogen phosphate BDBM80994 (2S)-2-acetamido-3-((2S)-2-((2S,3R)-1-(1-amino-3-(naphthalen-1-yl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784778 BDBM50345634
(2S)-2-acetamido-3-((2S)-2-((2S,3R)-1-(1-amino-3-(naphthalen-1-yl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784778 BDBM50345634 CHEMBL1784704 (S)-2-acetamido-3-((S)-1-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylamino)-1-oxopropan-2-ylamino)-3-oxopropyl dihydrogen phosphate BDBM50345627
CHEMBL1784704 (S)-2-acetamido-3-((S)-1-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylamino)-1-oxopropan-2-ylamino)-3-oxopropyl dihydrogen phosphate BDBM50345627 ((1R,2S,4S)-2-hydroxy-4-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)cyclopentyl)methyl dihydrogen phosphate BDBM50223802 CHEMBL393853
((1R,2S,4S)-2-hydroxy-4-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)cyclopentyl)methyl dihydrogen phosphate BDBM50223802 CHEMBL393853 BDBM573689 (R)-3-((1- (methyl- d3)pyrrolidin-2- yl)methyl-d2)- 1H-indol-4-yl dihydrogen phosphate US11453689, Compound I-54
BDBM573689 (R)-3-((1- (methyl- d3)pyrrolidin-2- yl)methyl-d2)- 1H-indol-4-yl dihydrogen phosphate US11453689, Compound I-54 BDBM50292723 uridine 2'-phosphate CHEMBL447360 PHOSPHORIC ACID MONO-[2-(2,4-DIOXO-3,4-DIHYDRO-2H-PYRIMIDIN-1-YL)-4-HYDROXY-5-HYDROXYMETHYL-TETRAHYDRO-FURAN-3-YL] ESTER (2R,3R,4R,5R)-2-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-3-yl dihydrogen phosphate
BDBM50292723 uridine 2'-phosphate CHEMBL447360 PHOSPHORIC ACID MONO-[2-(2,4-DIOXO-3,4-DIHYDRO-2H-PYRIMIDIN-1-YL)-4-HYDROXY-5-HYDROXYMETHYL-TETRAHYDRO-FURAN-3-YL] ESTER (2R,3R,4R,5R)-2-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-3-yl dihydrogen phosphate (S)-2-amino-4-(4-(heptyloxy)phenyl)-2-methylbutyl dihydrogen phosphate BDBM50313499 CHEMBL1084929
(S)-2-amino-4-(4-(heptyloxy)phenyl)-2-methylbutyl dihydrogen phosphate BDBM50313499 CHEMBL1084929 2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)ethyldihydrogen phosphate BDBM50316579 CHEMBL1095991
2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)ethyldihydrogen phosphate BDBM50316579 CHEMBL1095991 2-amino-4,5-dihydronaphtho[1,2-d]thiazol-7-yl dihydrogen phosphate CHEMBL566049 BDBM50302494
2-amino-4,5-dihydronaphtho[1,2-d]thiazol-7-yl dihydrogen phosphate CHEMBL566049 BDBM50302494 2-amino-4,5-dihydronaphtho[1,2-d]thiazol-9-yl dihydrogen phosphate BDBM50302496 CHEMBL576125
2-amino-4,5-dihydronaphtho[1,2-d]thiazol-9-yl dihydrogen phosphate BDBM50302496 CHEMBL576125 2-amino-8H-indeno[1,2-d]thiazol-4-yl dihydrogen phosphate CHEMBL578134 BDBM50302493
2-amino-8H-indeno[1,2-d]thiazol-4-yl dihydrogen phosphate CHEMBL578134 BDBM50302493 2-amino-8H-indeno[1,2-d]thiazol-5-yl dihydrogen phosphate BDBM50302492 CHEMBL568771
2-amino-8H-indeno[1,2-d]thiazol-5-yl dihydrogen phosphate BDBM50302492 CHEMBL568771 2-amino-8H-indeno[1,2-d]thiazol-6-yl dihydrogen phosphate BDBM50302491 CHEMBL566670
2-amino-8H-indeno[1,2-d]thiazol-6-yl dihydrogen phosphate BDBM50302491 CHEMBL566670 3-(4,5,6,7-Tetrabromo-2H-1,2,3-benzotriazol-2-yl)propyl dihydrogen phosphate BDBM50276680 CHEMBL459343
3-(4,5,6,7-Tetrabromo-2H-1,2,3-benzotriazol-2-yl)propyl dihydrogen phosphate BDBM50276680 CHEMBL459343 BDBM150734 2‐[(4‐bromophenyl)carbamoyl]‐4‐chlorophenyl diethyl phosphate (14)
BDBM150734 2‐[(4‐bromophenyl)carbamoyl]‐4‐chlorophenyl diethyl phosphate (14) BDBM150736 2‐[(3‐bromophenyl)carbamoyl]‐4‐chlorophenyl diethyl phosphate (16)
BDBM150736 2‐[(3‐bromophenyl)carbamoyl]‐4‐chlorophenyl diethyl phosphate (16) BDBM50316580 6-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)hexyldihydrogen phosphate CHEMBL1095867
BDBM50316580 6-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)hexyldihydrogen phosphate CHEMBL1095867 CHEMBL1097477 4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)-butyldihydrogen phosphate BDBM50316577
CHEMBL1097477 4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)-butyldihydrogen phosphate BDBM50316577 CHEMBL1097478 BDBM50316581 5-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)-pentyldihydrogen phosphate
CHEMBL1097478 BDBM50316581 5-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)-pentyldihydrogen phosphate CHEMBL455775 BDBM50292713 5'-phospho-2'-deoxyuridine 3-pyrophosphate (P'->5') adenosine 3'-phosphate
CHEMBL455775 BDBM50292713 5'-phospho-2'-deoxyuridine 3-pyrophosphate (P'->5') adenosine 3'-phosphate CHEMBL88487 Hexadecyl[2-(N-Methylmorpholinio)ethyl]phosphate BDBM50051809 (N-methylmorpholino)ethanol hexadecyl phosphonate
CHEMBL88487 Hexadecyl[2-(N-Methylmorpholinio)ethyl]phosphate BDBM50051809 (N-methylmorpholino)ethanol hexadecyl phosphonate 4-(3-((S)-1-((S)-2-((R)-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL578635 BDBM50300627
4-(3-((S)-1-((S)-2-((R)-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL578635 BDBM50300627 4-(3-((S)-1-((S)-2-((S)-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573049 BDBM50300628
4-(3-((S)-1-((S)-2-((S)-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573049 BDBM50300628 CHEMBL574202 4-(3-((S)-1-((S)-2-((E)-4-amino-4-oxobut-2-enylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300619
CHEMBL574202 4-(3-((S)-1-((S)-2-((E)-4-amino-4-oxobut-2-enylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300619 (S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784774 BDBM50345623
(S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784774 BDBM50345623 (S)-2-acetamido-3-((S)-2-((2S,3S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-1-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345631 CHEMBL1784776
(S)-2-acetamido-3-((S)-2-((2S,3S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-methyl-1-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345631 CHEMBL1784776 4-(3-((S)-1-((S)-2-((S)-5-amino-1-morpholino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL584974 BDBM50300638
4-(3-((S)-1-((S)-2-((S)-5-amino-1-morpholino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL584974 BDBM50300638 4-(3-((S)-1-((S)-2-((S)-6-amino-1-(benzylamino)-1,6-dioxohexan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300615 CHEMBL583754
4-(3-((S)-1-((S)-2-((S)-6-amino-1-(benzylamino)-1,6-dioxohexan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300615 CHEMBL583754 BDBM50300614 4-(3-((S)-1-((S)-2-((S)-4-amino-1-(benzylamino)-1,4-dioxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573901
BDBM50300614 4-(3-((S)-1-((S)-2-((S)-4-amino-1-(benzylamino)-1,4-dioxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573901 BDBM50300621 CHEMBL578430 4-(3-((S)-1-((S)-2-((S)-2-(3-amino-3-oxopropyl)pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300621 CHEMBL578430 4-(3-((S)-1-((S)-2-((S)-2-(3-amino-3-oxopropyl)pyrrolidine-1-carbonyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300633 CHEMBL573904 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(benzyloxy)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50300633 CHEMBL573904 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(benzyloxy)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50345639 CHEMBL1784783 (S)-2-amino-3-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate
BDBM50345639 CHEMBL1784783 (S)-2-amino-3-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL573442 BDBM50300641 4-(3-((S)-1-((S)-2-((S)-1-acetamido-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL573442 BDBM50300641 4-(3-((S)-1-((S)-2-((S)-1-acetamido-5-amino-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575513 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(dibenzylamino)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300639
CHEMBL575513 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(dibenzylamino)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300639 BDBM491210 2-((1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)piperidin-4-epoxy)ethyl dihydrogen phosphate US10975056, Example 154
BDBM491210 2-((1-(6-((2-Amino-2-oxo-1-phenylethyl)thio)-3,5-dicyano-4-ethylpyridin-2-yl)piperidin-4-epoxy)ethyl dihydrogen phosphate US10975056, Example 154 (S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-3-(4-hydroxyphenyl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784779 BDBM50345635
(S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-3-(4-hydroxyphenyl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate CHEMBL1784779 BDBM50345635 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(4-hydroxyphenyl)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300632 CHEMBL573903
4-(3-((S)-1-((S)-2-((S)-5-amino-1-(4-hydroxyphenyl)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300632 CHEMBL573903 BDBM50300653 4-(3-((S)-1-((cis)-2-((R)-1-amino-1-oxooctan-4-ylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583116
BDBM50300653 4-(3-((S)-1-((cis)-2-((R)-1-amino-1-oxooctan-4-ylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583116 CHEMBL1784769 (2S)-2-acetamido-3-((2S)-2-((2S,3R)-1-(1-amino-3-(naphthalen-2-yl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345633
CHEMBL1784769 (2S)-2-acetamido-3-((2S)-2-((2S,3R)-1-(1-amino-3-(naphthalen-2-yl)-1-oxopropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-3-oxopropyl dihydrogen phosphate BDBM50345633 (2R,5R,8R,11R)-11-benzyl-2-carbamoyl-8-((S)-1-hydroxyethyl)-5-methyl-4,7,10,13-tetraoxo-3,6,9,12-tetraazatetradecyl dihydrogen phosphate BDBM50345638 CHEMBL1784782
(2R,5R,8R,11R)-11-benzyl-2-carbamoyl-8-((S)-1-hydroxyethyl)-5-methyl-4,7,10,13-tetraoxo-3,6,9,12-tetraazatetradecyl dihydrogen phosphate BDBM50345638 CHEMBL1784782 BDBM50341894 ((2R,3S,4R,5R)-3,4-dihydroxy-5-(2-(methylamino)-4-oxopyrimidin-1(4H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL1767423
BDBM50341894 ((2R,3S,4R,5R)-3,4-dihydroxy-5-(2-(methylamino)-4-oxopyrimidin-1(4H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate CHEMBL1767423 BDBM50300636 4-(3-((S)-1-((S)-2-((S)-5-amino-5-oxo-1-(piperidin-1-yl)pentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583963
BDBM50300636 4-(3-((S)-1-((S)-2-((S)-5-amino-5-oxo-1-(piperidin-1-yl)pentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583963 BDBM50300637 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(4-methylpiperazin-1-yl)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573905
BDBM50300637 4-(3-((S)-1-((S)-2-((S)-5-amino-1-(4-methylpiperazin-1-yl)-5-oxopentan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL573905 4-((E)-4-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoethylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-4-oxobut-2-en-2-yl)phenyl dihydrogen phosphate BDBM50343638 CHEMBL1774961
4-((E)-4-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoethylcarbamoyl)pyrrolidin-1-yl)pentan-2-ylamino)-4-oxobut-2-en-2-yl)phenyl dihydrogen phosphate BDBM50343638 CHEMBL1774961 BDBM50267516 CHEMBL486267 4-(3-((3S,6S)-6-((S)-1-amino-1-oxopropan-2-ylcarbamoyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
BDBM50267516 CHEMBL486267 4-(3-((3S,6S)-6-((S)-1-amino-1-oxopropan-2-ylcarbamoyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate (benzyloxy)(2-formylphenoxy)phosphinic acid PASBN BDBM14688 benzyl 2-formylphenyl hydrogen phosphate Fragment 18
(benzyloxy)(2-formylphenoxy)phosphinic acid PASBN BDBM14688 benzyl 2-formylphenyl hydrogen phosphate Fragment 18 1D-myo-inositol 1,3,4,5-tetrakis(dihydrogen phosphate) CHEMBL23552 BDBM50075651 1D-myo-inositol 1,3,4,5-tetrakisphosphate
1D-myo-inositol 1,3,4,5-tetrakis(dihydrogen phosphate) CHEMBL23552 BDBM50075651 1D-myo-inositol 1,3,4,5-tetrakisphosphate 1D-myo-inositol 1,4,5,6-tetrakisphosphate BDBM50075649 CHEMBL282059 1D-myo-inositol 1,4,5,6-tetrakis(dihydrogen phosphate)
1D-myo-inositol 1,4,5,6-tetrakisphosphate BDBM50075649 CHEMBL282059 1D-myo-inositol 1,4,5,6-tetrakis(dihydrogen phosphate) 1D-myo-inositol 4,5-bisphosphate BDBM50284584 CHEMBL21825 1D-myo-inositol 4,5-bis(dihydrogen phosphate)
1D-myo-inositol 4,5-bisphosphate BDBM50284584 CHEMBL21825 1D-myo-inositol 4,5-bis(dihydrogen phosphate) 2-(6-Nitro-2-(4-phenoxyphenylamino)quinazolin-4-ylamino)ethyl dihydrogen phosphate BDBM50343852 CHEMBL1774785
2-(6-Nitro-2-(4-phenoxyphenylamino)quinazolin-4-ylamino)ethyl dihydrogen phosphate BDBM50343852 CHEMBL1774785 3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)propyl dihydrogen phosphate BDBM50316578 CHEMBL1097476
3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)propyl dihydrogen phosphate BDBM50316578 CHEMBL1097476 4‐bromo‐2‐[(4‐bromophenyl)carbamoyl]phenyl diethyl phosphate (8) BDBM150728
4‐bromo‐2‐[(4‐bromophenyl)carbamoyl]phenyl diethyl phosphate (8) BDBM150728 4‐bromo‐2‐[(4‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (3) BDBM150723
4‐bromo‐2‐[(4‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (3) BDBM150723 4‐chloro‐2‐[(3‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (10) BDBM150730
4‐chloro‐2‐[(3‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (10) BDBM150730 4‐chloro‐2‐[(3‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (12) BDBM150732
4‐chloro‐2‐[(3‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (12) BDBM150732 4‐chloro‐2‐[(4‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (11) BDBM150731
4‐chloro‐2‐[(4‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (11) BDBM150731 BDBM150722 4‐bromo‐2‐[(3‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (2)
BDBM150722 4‐bromo‐2‐[(3‐chlorophenyl)carbamoyl]phenyl diethyl phosphate (2) BDBM150724 4‐bromo‐2‐[(3,4‐dichlorophenyl)carbamoyl]phenyl diethyl phosphate (4)
BDBM150724 4‐bromo‐2‐[(3,4‐dichlorophenyl)carbamoyl]phenyl diethyl phosphate (4) BDBM150727 4‐bromo‐2‐[(3‐bromophenyl)carbamoyl]phenyl diethyl phosphate (7)
BDBM150727 4‐bromo‐2‐[(3‐bromophenyl)carbamoyl]phenyl diethyl phosphate (7) BDBM150729 4‐bromo‐2‐[(4‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (9)
BDBM150729 4‐bromo‐2‐[(4‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (9) BDBM150733 4‐chloro‐2‐[(4‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (13)
BDBM150733 4‐chloro‐2‐[(4‐fluorophenyl)carbamoyl]phenyl diethyl phosphate (13) BDBM150735 4‐chloro‐2‐[(3,4‐dichlorophenyl)carbamoyl]phenyl diethyl phosphate (15)
BDBM150735 4‐chloro‐2‐[(3,4‐dichlorophenyl)carbamoyl]phenyl diethyl phosphate (15) BDBM50287679 CHEMBL61555 Sodium; benzyl 3,4,5-trihydroxy-6-methyltetrahydro-2H-thiopyran-2-yl hydrogen phosphate
BDBM50287679 CHEMBL61555 Sodium; benzyl 3,4,5-trihydroxy-6-methyltetrahydro-2H-thiopyran-2-yl hydrogen phosphate BDBM50315803 2-amino-4-(2-fluoro-4-pentylphenyl)-2-(hydroxymethyl)butyl dihydrogen phosphate CHEMBL1095889
BDBM50315803 2-amino-4-(2-fluoro-4-pentylphenyl)-2-(hydroxymethyl)butyl dihydrogen phosphate CHEMBL1095889 BDBM50315805 2-amino-4-(4'-butoxybiphenyl-4-yl)-2-(hydroxymethyl)butyl dihydrogen phosphate CHEMBL1096211
BDBM50315805 2-amino-4-(4'-butoxybiphenyl-4-yl)-2-(hydroxymethyl)butyl dihydrogen phosphate CHEMBL1096211 CHEMBL1684872 4-((S)-3-((S)-1-(6-(Benzylcarbamoyl)-4'-carbamoylbiphenyl-3-ylamino)-4-methyl-1-oxopentan-2-ylamino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate BDBM50337199
CHEMBL1684872 4-((S)-3-((S)-1-(6-(Benzylcarbamoyl)-4'-carbamoylbiphenyl-3-ylamino)-4-methyl-1-oxopentan-2-ylamino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate BDBM50337199 CHEMBL515762 BDBM50267455 4-(3-((3S,6S)-6-(3-carbamoylpyrrolidine-1-carbonyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL515762 BDBM50267455 4-(3-((3S,6S)-6-(3-carbamoylpyrrolidine-1-carbonyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575920 4-(3-((S)-1-((S)-2-((S)-6-amino-2-methyl-6-oxohexan-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300629
CHEMBL575920 4-(3-((S)-1-((S)-2-((S)-6-amino-2-methyl-6-oxohexan-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate BDBM50300629 BDBM699453 (S)-2-(3-(Acrylamidomethyl)-1-(4- (trifluoromethoxy)phenyl)-1H- pyrazolo[3,4-b]pyridin-4-yl)-2- hydroxyethyl dihydrogen phosphate US12110276, Compound 147
BDBM699453 (S)-2-(3-(Acrylamidomethyl)-1-(4- (trifluoromethoxy)phenyl)-1H- pyrazolo[3,4-b]pyridin-4-yl)-2- hydroxyethyl dihydrogen phosphate US12110276, Compound 147 US10988478, Example 145 [1-[2,2-dimethyl-5-(pyrazolo[1,5- a]pyrimidine-3-carbonylamino)-3H- benzofuran-6-yl]-4-piperidyl]methyl diethyl phosphate BDBM494097
US10988478, Example 145 [1-[2,2-dimethyl-5-(pyrazolo[1,5- a]pyrimidine-3-carbonylamino)-3H- benzofuran-6-yl]-4-piperidyl]methyl diethyl phosphate BDBM494097 O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine 4-phosphoryloxy-N,N-dimethyltryptamine US11597738, Example 4 BDBM50171269 Indocybin psilocybin 3-[2-(dimethylamino)ethyl]-1H-indol-4-yl dihydrogen phosphate psilocin phosphate ester Psilocybine CHEMBL194378
O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine 4-phosphoryloxy-N,N-dimethyltryptamine US11597738, Example 4 BDBM50171269 Indocybin psilocybin 3-[2-(dimethylamino)ethyl]-1H-indol-4-yl dihydrogen phosphate psilocin phosphate ester Psilocybine CHEMBL194378 4-(3-((S)-1-((cis)-2-(4-amino-4-oxobutylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL577143 BDBM50300649
4-(3-((S)-1-((cis)-2-(4-amino-4-oxobutylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL577143 BDBM50300649 4-(3-((3S,6S)-6-((S)-1-((S)-1-amino-1-oxohexan-2-ylamino)-1-oxopropan-2-ylcarbamoyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL468082 BDBM50267517
4-(3-((3S,6S)-6-((S)-1-((S)-1-amino-1-oxohexan-2-ylamino)-1-oxopropan-2-ylcarbamoyl)-4-oxo-1,2,3,4,6,7-hexahydroazepino[3,2,1-hi]indol-3-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL468082 BDBM50267517 BDBM50300652 4-(3-((S)-1-((cis)-2-((S)-1-amino-6-methyl-1-oxoheptan-4-ylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575484
BDBM50300652 4-(3-((S)-1-((cis)-2-((S)-1-amino-6-methyl-1-oxoheptan-4-ylcarbamoyl)-3-azabicyclo[3.1.0]hexan-3-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL575484 BDBM50345626 CHEMBL1784773 (2R,3S)-3-acetamido-4-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-oxobutan-2-yl dihydrogen phosphate
BDBM50345626 CHEMBL1784773 (2R,3S)-3-acetamido-4-((S)-2-((2S,3R)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidin-1-yl)-4-oxobutan-2-yl dihydrogen phosphate BDBM50300634 4-(3-((S)-1-((S)-2-((2R,3S)-6-amino-2-(benzyloxy)-6-oxohexan-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583758
BDBM50300634 4-(3-((S)-1-((S)-2-((2R,3S)-6-amino-2-(benzyloxy)-6-oxohexan-3-ylcarbamoyl)pyrrolidin-1-yl)-4-methyl-1-oxopentan-2-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL583758 BDBM50267448 4-(3-((3S,6S,8aS)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL445970
BDBM50267448 4-(3-((3S,6S,8aS)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL445970 CHEMBL504218 BDBM50267450 4-(3-((3R,6S,8aR)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL504218 BDBM50267450 4-(3-((3R,6S,8aR)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate CHEMBL505207 BDBM50267449 4-(3-((3S,6S,8aR)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate
CHEMBL505207 BDBM50267449 4-(3-((3S,6S,8aR)-3-((S)-5-amino-1-(benzylamino)-1,5-dioxopentan-2-ylcarbamoyl)-5-oxooctahydroindolizin-6-ylamino)-3-oxoprop-1-enyl)phenyl dihydrogen phosphate