Target (15)
Compound (888)
Article Title (26)
Article Author (1)
Assay (61)
Byrd, JC; Harrington, B; O'Brien, S; Jones, JA; Schuh, A; Devereux, S; Chaves, J; Wierda, WG; Awan, FT; Brown, JR; Hillmen, P; Stephens, DM; Ghia, P; Barrientos, JC; Pagel, JM; Woyach, J; Johnson, D; Huang, J; Wang, X; Kaptein, A; Lannutti, BJ; Covey, T; Fardis, M; McGreivy, J; Hamdy, A; Rothbaum, W; Izumi, R; Diacovo, TG; Johnson, AJ; Furman, RR Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 374: 323 -32 Miller, WH; Seefeld, MA; Newlander, KA; Uzinskas, IN; Burgess, WJ; Heerding, DA; Yuan, CC; Head, MS; Payne, DJ; Rittenhouse, SF; Moore, TD; Pearson, SC; Berry, V; DeWolf, WE; Keller, PM; Polizzi, BJ; Qiu, X; Janson, CA; Huffman, WF Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI). J Med Chem 45: 3246 -56 (2002) Kitagawa, H; Ozawa, T; Takahata, S; Iida, M Phenylimidazole derivatives as new inhibitors of bacterial enoyl-ACP reductase FabK. Bioorg Med Chem Lett 17: 4982 -6 (2007) Seefeld, MA; Miller, WH; Newlander, KA; Burgess, WJ; DeWolf, WE; Elkins, PA; Head, MS; Jakas, DR; Janson, CA; Keller, PM; Manley, PJ; Moore, TD; Payne, DJ; Pearson, S; Polizzi, BJ; Qiu, X; Rittenhouse, SF; Uzinskas, IN; Wallis, NG; Huffman, WF Indole naphthyridinones as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. J Med Chem 46: 1627 -35 (2003) Bommineni, GR; Kapilashrami, K; Cummings, JE; Lu, Y; Knudson, SE; Gu, C; Walker, SG; Slayden, RA; Tonge, PJ Thiolactomycin-Based Inhibitors of Bacterial β-Ketoacyl-ACP Synthases with in Vivo Activity. J Med Chem 59: 5377 -90 (2016) Takhi, M; Sreenivas, K; Reddy, CK; Munikumar, M; Praveena, K; Sudheer, P; Rao, BN; Ramakanth, G; Sivaranjani, J; Mulik, S; Reddy, YR; Narasimha Rao, K; Pallavi, R; Lakshminarasimhan, A; Panigrahi, SK; Antony, T; Abdullah, I; Lee, YK; Ramachandra, M; Yusof, R; Rahman, NA; Subramanya, H Discovery of azetidine based ene-amides as potent bacterial enoyl ACP reductase (FabI) inhibitors. Eur J Med Chem 84: 382 -94 (2014) Sharma, SK; Parasuraman, P; Kumar, G; Surolia, N; Surolia, A Green tea catechins potentiate triclosan binding to enoyl-ACP reductase from Plasmodium falciparum (PfENR). J Med Chem 50: 765 -75 (2007) Yao, J; Zhang, Q; Min, J; He, J; Yu, Z Novel enoyl-ACP reductase (FabI) potential inhibitors of Escherichia coli from Chinese medicine monomers. Bioorg Med Chem Lett 20: 56 -9 (2010) Wang, H; Lu, Y; Liu, L; Kim, SW; Hooker, JM; Fowler, JS; Tonge, PJ Radiosynthesis and biological evaluation of a novel enoyl-ACP reductase inhibitor for Staphylococcus aureus. Eur J Med Chem 88: 66 -73 (2014) Yogiara, na; Mordukhova, EA; Kim, D; Kim, WG; Hwang, JK; Pan, JG The food-grade antimicrobial xanthorrhizol targets the enoyl-ACP reductase (FabI) in Escherichia coli. Bioorg Med Chem Lett 30: (2020) Herman, SEM; Montraveta, A; Niemann, CU; Mora-Jensen, H; Gulrajani, M; Krantz, F; Mantel, R; Smith, LL; McClanahan, F; Harrington, BK; Colomer, D; Covey, T; Byrd, JC; Izumi, R; Kaptein, A; Ulrich, R; Johnson, AJ; Lannutti, BJ; Wiestner, A; Woyach, JA The Bruton Tyrosine Kinase (BTK) Inhibitor Acalabrutinib Demonstrates Potent On-Target Effects and Efficacy in Two Mouse Models of Chronic Lymphocytic Leukemia. Clin Cancer Res 23: 2831 -2841 Cho, JY; Kwon, YJ; Sohn, MJ; Seok, SJ; Kim, WG Phellinstatin, a new inhibitor of enoyl-ACP reductase produced by the medicinal fungus Phellinus linteus. Bioorg Med Chem Lett 21: 1716 -8 (2011) Neckles, C; Eltschkner, S; Cummings, JE; Hirschbeck, M; Daryaee, F; Bommineni, GR; Zhang, Z; Spagnuolo, L; Yu, W; Davoodi, S; Slayden, RA; Kisker, C; Tonge, PJ Rationalizing the Binding Kinetics for the Inhibition of the Burkholderia pseudomallei FabI1 Enoyl-ACP Reductase. Biochemistry 56: 1865 -1878 (2017) Sampson, PB; Picard, C; Handerson, S; McGrath, TE; Domagala, M; Leeson, A; Romanov, V; Awrey, DE; Thambipillai, D; Bardouniotis, E; Kaplan, N; Berman, JM; Pauls, HW Spiro-naphthyridinone piperidines as inhibitors of S. aureus and E. coli enoyl-ACP reductase (FabI). Bioorg Med Chem Lett 19: 5355 -8 (2009) Tipparaju, SK; Joyasawal, S; Forrester, S; Mulhearn, DC; Pegan, S; Johnson, ME; Mesecar, AD; Kozikowski, AP Design and synthesis of 2-pyridones as novel inhibitors of the Bacillus anthracis enoyl-ACP reductase. Bioorg Med Chem Lett 18: 3565 -9 (2008) Muhammad, A; Anis, I; Ali, Z; Awadelkarim, S; Khan, A; Khalid, A; Shah, MR; Galal, M; Khan, IA; Iqbal Choudhary, M Methylenebissantin: a rare methylene-bridged bisflavonoid from Dodonaea viscosa which inhibits Plasmodium falciparum enoyl-ACP reductase. Bioorg Med Chem Lett 22: 610 -2 (2011) Neckles, C; Pschibul, A; Lai, CT; Hirschbeck, M; Kuper, J; Davoodi, S; Zou, J; Liu, N; Pan, P; Shah, S; Daryaee, F; Bommineni, GR; Lai, C; Simmerling, C; Kisker, C; Tonge, PJ Selectivity of Pyridone- and Diphenyl Ether-Based Inhibitors for the Yersinia pestis FabV Enoyl-ACP Reductase. Biochemistry 55: 2992 -3006 (2016) Belluti, F; Perozzo, R; Lauciello, L; Colizzi, F; Kostrewa, D; Bisi, A; Gobbi, S; Rampa, A; Bolognesi, ML; Recanatini, M; Brun, R; Scapozza, L; Cavalli, A Design, synthesis, and biological and crystallographic evaluation of novel inhibitors of Plasmodium falciparum enoyl-ACP-reductase (PfFabI). J Med Chem 56: 7516 -26 (2013) Ramnauth, J; Surman, MD; Sampson, PB; Forrest, B; Wilson, J; Freeman, E; Manning, DD; Martin, F; Toro, A; Domagala, M; Awrey, DE; Bardouniotis, E; Kaplan, N; Berman, J; Pauls, HW 2,3,4,5-Tetrahydro-1H-pyrido[2,3-b and e][1,4]diazepines as inhibitors of the bacterial enoyl ACP reductase, FabI. Bioorg Med Chem Lett 19: 5359 -62 (2009) Schiebel, J; Chang, A; Shah, S; Lu, Y; Liu, L; Pan, P; Hirschbeck, MW; Tareilus, M; Eltschkner, S; Yu, W; Cummings, JE; Knudson, SE; Bommineni, GR; Walker, SG; Slayden, RA; Sotriffer, CA; Tonge, PJ; Kisker, C Rational design of broad spectrum antibacterial activity based on a clinically relevant enoyl-acyl carrier protein (ACP) reductase inhibitor. J Biol Chem 289: 15987 -6005 (2014) Bonnac, L; Gao, GY; Chen, L; Felczak, K; Bennett, EM; Xu, H; Kim, T; Liu, N; Oh, H; Tonge, PJ; Pankiewicz, KW Synthesis of 4-phenoxybenzamide adenine dinucleotide as NAD analogue with inhibitory activity against enoyl-ACP reductase (InhA) of Mycobacterium tuberculosis. Bioorg Med Chem Lett 17: 4588 -91 (2007) Alhamadsheh, MM; Waters, NC; Huddler, DP; Kreishman-Deitrick, M; Florova, G; Reynolds, KA Synthesis and biological evaluation of thiazolidine-2-one 1,1-dioxide as inhibitors of Escherichia coli beta-ketoacyl-ACP-synthase III (FabH). Bioorg Med Chem Lett 17: 879 -83 (2007) Bromberg, KD; Mitchell, TR; Upadhyay, AK; Jakob, CG; Jhala, MA; Comess, KM; Lasko, LM; Li, C; Tuzon, CT; Dai, Y; Li, F; Eram, MS; Nuber, A; Soni, NB; Manaves, V; Algire, MA; Sweis, RF; Torrent, M; Schotta, G; Sun, C; Michaelides, MR; Shoemaker, AR; Arrowsmith, CH; Brown, PJ; Santhakumar, V; Martin, A; Rice, JC; Chiang, GG; Vedadi, M; Barsyte-Lovejoy, D; Pappano, WN The SUV4-20 inhibitor A-196 verifies a role for epigenetics in genomic integrity. Nat Chem Biol 13: 317 -324 (2017) Protasevich, II; Brouillette, CG; Snow, ME; Dunham, S; Rubin, JR; Gogliotti, R; Siegel, K Role of inhibitor aliphatic chain in the thermodynamics of inhibitor binding to Escherichia coli enoyl-ACP reductase and the Phe203Leu mutant: a proposed mechanism for drug resistance. Biochemistry 43: 13380 -9 (2004) Zhang, SX; Bastow, KF; Tachibana, Y; Kuo, SC; Hamel, E; Mauger, A; Narayanan, VL; Lee, KH Antitumor agents. 196. Substituted 2-thienyl-1,8-naphthyridin-4-ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem 42: 4081 -7 (1999) Vanover, KE; Weiner, DM; Makhay, M; Veinbergs, I; Gardell, LR; Lameh, J; Del Tredici, AL; Piu, F; Schiffer, HH; Ott, TR; Burstein, ES; Uldam, AK; Thygesen, MB; Schlienger, N; Andersson, CM; Son, TY; Harvey, SC; Powell, SB; Geyer, MA; Tolf, BR; Brann, MR; Davis, RE Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317: 910 -8 (2006)
ChEMBL_953658 (CHEMBL2352562) Inhibition of Toxoplasma gondii enoyl reductase assessed as conversion of trans-2-acyl-ACP to acyl-ACP ChEMBL_1431241 (CHEMBL3385666) Inhibition of Staphylococcus aureus enoyl ACP reductase ChEMBL_479301 (CHEMBL933673) Inhibition of Bacillus anthracis Enoyl ACP reductase ChEMBL_450119 (CHEMBL900394) Inhibition of Streptococcus pneumoniae enoyl-ACP reductase FabK ChEMBL_450121 (CHEMBL900393) Inhibition of Escherichia coli enoyl-ACP reductase FabI ChEMBL_1290812 (CHEMBL3118190) Inhibition of Toxoplasma gondii enoyl-ACP reductase-NADH complex ChEMBL_436468 (CHEMBL904777) Inhibition of Plasmodium falciparum recombinant enoyl ACP reductase expressed in BL21 (DE3) cells ChEMBL_728028 (CHEMBL1686899) Inhibition of Staphylococcus aureus enoyl-ACP reductase assessed as increase of NADPH level ChEBML_37595 In vitro inhibitory activity against the recombinant Mycobacterium tuberculosis Beta-ketoacyl-ACP synthase III mtFabH condensing enzyme. ChEMBL_88041 (CHEMBL695903) In vitro inhibitory activity against acyl carrier protein synthase (AcpS) in Bacillus subtilis GST-Acp-HTRFassay ChEBML_88040 In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassay ChEMBL_2019646 (CHEMBL4673459) Inhibition of Plasmodium falciparum enoyl-ACP reductase using crotonoyl-CoA as substrate by UV-Vis spectrophotometric method ChEMBL_436470 (CHEMBL904775) Inhibition of Plasmodium falciparum recombinant enoyl ACP reductase expressed in BL21 (DE3) cells with respect to NADH FabH Enzyme Assay Escherichia coli FabH assays were carried out using standard coupled trichloroacetic acid precipitation assay to determine the rate formation of radiolabeled 3-ketoacyl-ACP from malonyl-ACP. Mycobacterium tuberculosis FabH assay was done by sustituting malonyl-CoA for MACP. ChEMBL_436469 (CHEMBL904776) Inhibition of Plasmodium falciparum recombinant enoyl ACP reductase expressed in BL21 (DE3) cells with respect to crotonyl CoA ChEMBL_604715 (CHEMBL1070535) Inhibition of Escherichia coli Beta-ketoacyl-ACP synthase III expressed in Escherichia coli BL21 (DE3) by liquid scintillation counting ChEMBL_1455655 (CHEMBL3365684) Inhibition of Staphylococcus aureus FabI-mediated reduction of enoyl-ACP preincubated for 30 mins measured after 2 hrs by spectrophotometry ChEMBL_795989 (CHEMBL1936261) Inhibition of Plasmodium falciparum enoyl-ACP reductase assessed as oxidation of NADH to NAD+ after 10 mins by spectrophotometric analysis ChEMBL_603807 (CHEMBL1042945) Inhibition of Escherichia coli MG1655 enoyl-ACP reductase overexpressed in Escherichia coli M15 assessed as oxidation of NADH to NAD+ after 5 mins ChEMBL_761713 (CHEMBL1816807) Inhibition of Plasmodium falciparum Enoyl-ACP reductase using crotonyl-CoA as substrate peincubated for 5 mins measured after 10 mins of substrate addition by UV-vis spectrophotometry ChEMBL_1788620 (CHEMBL4260354) Agonist activity at human LXRbeta-LBD (196 to 461 amino acids) expressed in HEK293T cells by luciferase reporter gene assay ChEMBL_2157259 (CHEMBL5041919) Displacement of 5-FITC-(Acp)-Bth-D-pThr-Pip-2-NaI-Q from N-terminal-GST-fused human Pin1 expressed in Escherichia coli BL21(DE3) incubated for 12 hrs by fluorescence polarization assay ChEMBL_1673639 (CHEMBL4023668) Transactivation of recombinant GST-tagged PPARgamma (unknown origin) expressed in Escherichia coli assessed as N-terminal biotin-labeled PGC1alpha (196 to 221 residues) co-activator recruitment by TR-FRET assay ChEMBL_2184290 (CHEMBL5096372) Inhibition of recombinant His6-tagged human Bcl-xL (1 to 196 residues) expressed in Escherichia coli using biotinylated Bim BH3 peptide as substrate incubated for 60 mins by TR-FRET analysis ChEMBL_1855917 (CHEMBL4356646) Inhibition of recombinant C-terminal His6x-tagged human Bcl-xL (1 to 196 residues) expressed in Escherichia coli cells interaction with biotinylated human Bim (51 to 76 residues) incubated for 60 mins by HTRF assay ChEMBL_1973165 (CHEMBL4605983) Inhibition of human GST-tagged USP21 CD (196 to 565 residues) expressed in Escherichia coli assessed as cleavage of Ubiquitin-Rhodamine110-glycine to Ubiquitin and Rhodamine110-glycine using Ubiquitin-Rhodamine110-glycine as substrate by fluorescence based analysis ChEMBL_2089955 (CHEMBL4771218) Inhibition of human full-length ABCB1 expressed in FLp-In-293 cells assessed as potentiation of doxorubicin-induced cytotoxicity by measuring doxorubicin IC50 at 2.5 uM measured after 72 hrs by SRB assay (Rvb = 2264 +/- 196 nM) ChEMBL_2089956 (CHEMBL4771219) Inhibition of human full-length ABCB1 expressed in FLp-In-293 cells assessed as potentiation of doxorubicin-induced cytotoxicity by measuring doxorubicin IC50 at 5 uM measured after 72 hrs by SRB assay (Rvb = 2264 +/- 196 nM) FabD/FabH Coupled Assay The potency of FabH inhibition (IC50) was determined using [3H]- or [14C]-radiolabeled substrates. This was accomplished at fixed concentrations of acetyl-CoA in a coupled FabD/FabH assay system that generates the substrate malonyl-ACP in situ. Subsequently, the FabH acetylation reaction was performed to exclude the possible contribution of undesired FabD inhibitory activity. The incorporation of the 3H (or [14C]) signal in the final product was read by liquid scintillation. Radioligand Binding Assay Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2A) min incubation at 21 deg C, assays were terminated by rapid filtration with GF/B using a Canberra Packard filtermate 196. The radioactivity was assessed using a Canberra Packard TopCount microplate scintillation counter. FAAH Activity Assay In this assay, 0.05 Units of hFAAH was pre-incubated for 30 min at 37 C with 2 uL of 11a, 11b, 11f at different concentrations (0.01 - 100 uM) or vehicin 196 uL of assay buffer (10 mM Tris-HCl, 1 mM EDTA (pH 7.6) and 0.1% w/v fatty acid-free BSA). 2 uL of AEA/[3H]-AEA mixture was added to the solution (final concenttion in assay was 10 nM) and incubated for 15 min at 37 C. Mycobacterium tuberculosis Pantothenate Synthetase Assay Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening Centers Network (MLSCN) Award: 1R03MH076412-01 Multi-drug resistant Mycobacterium tuberculosis is becoming an increased health problem, especially in immunocompromised individuals with HIV. This form of TB is more difficult to treat and as a result has a higher mortality rate. Because of this, the discovery of drugs targeting novel pathways such as the synthesis of pantothenate has become increasingly important. Pantothenate synthetase (PS; EC 6.3.2.1), encoded by the panC gene, catalyzes the essential ATP-dependent condensation of D-pantoate and alpha-alanine to form pantothenate in bacteria, yeast and plants; pantothenate is a key precursor for the biosynthesis of coenzyme A (CoA) and acyl carrier protein (ACP). The activity of PS was measured spectrophotometrically by coupling the formation of AMP to the reactions of myokinase, pyr Mycobacterium tuberculosis Pantothenate Synthetase Secondary Assay Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening Centers Network (MLSCN) Award: 1R03MH076412-01 Multi-drug resistant Mycobacterium tuberculosis is becoming an increased health problem, especially in immunocompromised individuals with HIV. This form of TB is more difficult to treat and as a result has a higher mortality rate. Because of this, the discovery of drugs targeting novel pathways such as the synthesis of pantothenate has become increasingly important. Pantothenate synthetase (PS; EC 6.3.2.1), encoded by the panC gene, catalyzes the essential ATP-dependent condensation of D-pantoate and alpha-alanine to form pantothenate in bacteria, yeast and plants; pantothenate is a key precursor for the biosynthesis of coenzyme A (CoA) and acyl carrier protein (ACP). The activity of PS was measured spectrophotometrically by coupling the formation of AMP to the reactions of myokinase, pyruva Inhibition of Fabl Protein Inhibition of Fabl enzyme from Acinetobacter baumannii and Escherichia coli was tested by measuring the rate of NADH consumption (Aabsorbance at 340 nm/min) at 30° C. in 96-well plate format using an automated plate reader in the presence or absence of the test compounds. The assay mixture contained 100 mM Tris-HCl, pH 7.25 (A. baumannii) or 7.5 (E. coli), 100 mM ammonium acetate, 0.02% (A. baumannii) or 0.05% (E. coli) Pluronic F-68, 25 μM crotonyl ACP, 50 μM NADH, 25 μM (A. baumannii) or 50 μM (E. coli) recombinant Fabl protein, and 7.5% DMSO. Test compounds were added at concentrations ranging from 0.17 to 10,000 nM in a final well volume of 100 μl. This dose-response inhibitory assay was performed using a 10-point, serial dilution series for each test compound. IC50 values for each test compound were assigned from logistical sigmoid curve-fitting of the inhibition dose response curves. BTK Target Occupancy Ramos B cells (ATCC, cat no. CRL-1923) were plated in 24-wells culture plates at 2×106 cells per well in a total volume of 900 μL DMEMF12+10% FBS+2 mM L-Glutamine+Pen/Strep. Allow the cells to rest 1 h at 5-7% CO2 and 37° C.Serial dilutions log 10 from 10 mM to 316 nM of test compounds are made in 100% DMSO, followed by a 100-fold dilution into culture medium.For each well, 100 μL was then transferred to well plate containing 900 μL of Ramos B cells. Final compound concentration range in the assay varied from 10 μM to 0.316 nM, with a final DMSO concentration of 0.1% and incubated at 5-7% CO2 and 37° C. for 2h. Afterwards, cells are collected for the measurement of the BTK target occupancy using the BTK target occupancy ELISA as outlined below.The percent of drug-bound BTK in Ramos B cell samples was determined by an ELISA based method as follows: OptiPlate 96-well plates (Perkin Elmer) were coated with 125 ng/well anti-BTK Ab (BD Biosciences) and blocked with BSA (Sigma-Aldrich). Samples containing Ramos B cells were lysed in ice cold lysis buffer containing 50 mM Tris-HCl pH 7.5, 250 mM sucrose, 5 mM MgCl2, 1 mM dithiothreitol (DTT), 0.05% digitonin, and protease inhibitor cocktail (Sigma-Aldrich). Cell lysates were then incubated for 1 h in the absence or presence of 1 μM acalabrutinib, a saturating concentration that results in complete BTK occupancy. Final amount of cell lysate used per well in BTK target occupancy ELISA is representative of 2×105 Ramos B cells. The difference with the signal of the cell lysates not incubated with an excess acalabrutinib represents free BTK (not occupied by a BTK inhibitor). Samples were incubated for 1 h with biotin tag compound of Formula (II) (100 nM). This probe will bind covalently to Cys481 in the ATP pocket in BTK when the ATP pocket is not occupied by a covalent BTK inhibitor. Each sample was then added in duplicate to the prepared Optiplate and incubated for 2h at ambient temperature. Plates were washed with PBS+0.05% Tween20 four times. Streptavidin-HRP (Invitrogen; ELISA grade) was added at 100 μL/well (120 ng/mL) and incubated for 1 hour at room temperature. Plates were washed with PBS+0.05% Tween20 three times and then washed with PBS (without Tween 20) two times. One hundred L/well of SuperSignal ELISA Femto Substrate (ThermoFisher Scientific) was added and then chemiluminescence was measured after 1 minute (EnVision plate reader; PerkinElmer). FLIPR Assay Assay Buffer: The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10× HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). FLIPR Assay Assay Buffer: The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10×HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). FLIPR Assay Assay Buffer: The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10×HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4. 5.33 mM KC, 0.441 mM KH2PO4. 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). FLIPR Assay The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10xHBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (flanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). FLIPR Assay The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10×HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). FLIPR Assays The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10× HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). FLIPR Assays The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10×HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). In Vitro Assay The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10×HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). FP Assay (In Vitro) The inhibitory activity of test compounds on the binding between Nrf2 and Keap1 was determined by fluorescence polarization. A solution of 50 mM Tris-HCl pH. 8.0 (NACALAI, REF: 06938-15), and 5 mM DTT (SIGMA-ALDRICH, REF: 646563-10×0.5 mL) was used as a buffer solution. 8 μL of a buffer solution containing 40 nM GST-fused human Keap1 (amino acid residues: 325-642) protein (Protein tech, REF: Ag0779) was taken in a black 384-well plate (final concentration 20 nM), and 4 μL of the test compound solution prepared to each concentration in buffer solution was added thereto. A well containing a portion of the buffer solution without Keap1 was placed as a negative control. A well with no compound was used as a positive control. 40 nM of FITC-labeled Nrf2 peptide (FITC-X-LQLDEETGEFLPIQ (X=ε-Acp), Peptide Institute Inc.) was added thereto (10 nM final concentration). The wells were incubated at room temperature for 2 hr, and then fluorescence polarization was measured with a plate reader SPECTRAMAX Paradigm (Molecular devices) at excitation wavelength of 485 nm and fluorescence wavelength of 535 nm. The fluorescence polarization of the negative control was set to 100% inhibition and that of the positive control to 0% inhibition, and the inhibitory rate upon addition of the test compound was calculated using the following formula. Binding Assay for gamma1-Containing GABAA Subtypes Radioligand binding assays were carried out in a volume of 200 μL (96-well plates) which contained 100 μL of cell membranes, [3H]RO7239181 at a concentration of 1.5 nM (α5β2γ1) or 20-30 nM (α1β2γ1, α2β2γ1) and the test compound in the range of [0.3-10000]×10−9 M. Nonspecific binding was defined by 10×10−6 (α5β2γ1) and 30×10−6 M RO7239181 and typically represented less than 5% (α5β2γ1) and less than 20% (α1β2β1, α2β2γ1) of the total binding. Assays were incubated to equilibrium for 1 hour at 4° C. and then, membranes were filtered onto unifilter (96-well white microplate with bonded GF/C filters preincubated 20-50 minutes in 0.3% Polyethylenimine) with a Filtermate 196 harvester (Packard BioScience) and washed 4 times with cold Potassium Phosphate 10 mM pH 7.4, KCl 100 mM binding buffer. After anhydrousing, filter-retained radioactivity was detected by liquid scintillation counting. Ki values were calculated using Excel-Fit (Microsoft) and are the means of two determinations. Enzyme Inhibition Assay Human SCD-1 enzyme activity using HepG2 cell microsomes after treating with inhibitory compounds (% inhibition):Human hepatocarcinoma HepG2 cells (ATCC, HB-8065) are cultured to confluence and trypsinised. The cell pellet is taken up with 10 mM Tris (pH 7.4) sucrose (250 mM) DTT (1 mM) buffer and the cells are lysed by sonication. The microsomes are obtained after centrifugation at 10,000 g for 20 minutes at 4 C. followed by centrifugation of the supernatant at 100,000 g for 60 minutes at 4 C. The pellet is taken up with 10 mM Tris (pH 7.4) sucrose (250 mM) buffer at 4 C. and the microsomal proteins are assayed and stored at -196 C. (liquid nitrogen).The enzyme reaction measures the conversion of stearic acid (C18:0 fatty acid) to oleic acid (C18:1 fatty acid) by SCD-1. The enzyme reaction is started by adding 125 ug of HepG2 cell microsomal fraction to tubes (total reaction volume of 500 ul) containing 62 uM of stearic acid. Radioligand binding assays Radioligand binding assays were carried out in a volume of 200 μL (96-well plates) which contained 100 μL of cell membranes, [3H]RO7239181 at a concentration of 1.5 nM (α5β2γ1) or 20-30 nM (α1β2γ1, α2β2γ1) and the test compound in the range of [0.3-10000]×10−9 M. Nonspecific binding was defined by 10×10−6 (α5β2γ1) and 30×10−6 M RO7239181 and typically represented less than 5% (α5β2γ1) and less than 20% (α1β2γ1, α2β2γ1) of the total binding. Assays were incubated to equilibrium for 1 hour at 4° C. and then, membranes were filtered onto unifilter (96-well white microplate with bonded GF/C filters preincubated 20-50 minutes in 0.3% Polyethylenimine) with a Filtermate 196 harvester (Packard BioScience) and washed 4 times with cold Potassium Phosphate 10 mM pH 7.4, KCl 100 mM binding buffer. After anhydrousing, filter-retained radioactivity was detected by liquid scintillation counting. Ki values were calculated using Excel-Fit (Microsoft) and are the means of two determinations. Assay of FatA Acyl-ACP Thioesterase To determine the level of FatA thioesterase activity in the presence of test compounds, an assay buffer consisting of 50 mM Tris-HCl pH 8.0, 0.15M NaCl, 5 mM EDTA, 10 μM oleoyl-CoA is combined with enzyme (1-80 nM Arabidopsis FatA thioesterase) to a final volume of 100 μl. The assay is carried out in black 96-well microtitre plates into which test compounds dissolved in DMSO (1% v/v final assay concentration) have been dispensed prior to the addition of other assay components. Once all assay components have been added, the plates are incubated at room temperature for 5 min during which time the reaction progresses at a linear rate. Enzymatic activity is simultaneously stopped, and detection reagent added, by the addition of 10 μl solution of 220 μM 7-diethylamino-3-(4′-maleimidylphenyl)-4-methylcoumarin (“CPM”) dissolved in 50% v/v ethanol. After an appropriate time to allow for near complete reaction of the CoASH formed by the enzyme with CPM, the fluorescence is read in a plate reader using excitation and emission wavelengths of 390 nm and 470 nm respectively. The assay signal associated with maximal (uninhibited) enzyme activity is determined with DMSO present in the reaction at 1% v/v final concentration and the assay signal associated with full enzyme inhibition is determined from wells to which no enzyme has been added. Electrophysiology Assay In vitro assays were performed in a recombinant cell line expressing cDNA encoding the alpha subunit (Nav1.7, SCN9a, PN1, NE) of human Nav1.7 (Accession No. NM_002977). The cell line was provided by investigators at Yale University (Cummins et al, J. Neurosci. 18(23): 9607-9619 (1998)). For dominant selection of the Nav1.7-expressing clones, the expression plasmid co-expressed the neomycin resistance gene. The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10×HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). FLIPR Assay Assay Buffer: The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10xHBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). At the start of the assay, the media was flicked from the cells and the wells were washed once with 50 ul/well assay buffer (1x Hank's balanced salt solution without sodium bicarbonate or phenol red, 20 mM Hepes, pH 7.3) and then pre-incubated with the test articles, CoroNa Green AM sodium dye (for cell loading) and KCl for re FLIPR Assay In vitro assays were performed in a recombinant cell line expressing cDNA encoding the alpha subunit (Nav1.7, SCN9a, PN1, NE) of human Nav1.7 (Accession No. NM_002977). The cell line was provided by investigators at Yale University (Cummins et al, J. Neurosci. 18(23): 9607-9619 (1998)). For dominant selection of the Nav1.7-expressing clones, the expression plasmid co-expressed the neomycin resistance gene. The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10×HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). Radioactivity-Based Assay In this assay [3H]AdoMet (PerkinElmer Life Sciences; catalog number NET155V250UC)was used as a methyl donor to methylate biotinylated histone peptide substrates. Reaction mixtures contained 10 μM biotinylated peptide and 200 μM AdoMet (4 μM [3H]AdoMet plus 196 μM cold AdoMet) in 10mM Tris-HCl buffer, pH 8.5, with 0.01%Triton X-100 and 10 mM DTT in a final volume of 20 μl. The reaction was started by adding PRDM9(final concentration of 1 nM). Samples were incubated for 30 min at 23 °C and the reaction was quenched by addition of 20 μl of 7.5 M guanidinium hydrochloride followed by addition of 160 μl of 20 mM Tris-HCl buffer, pH 8.0. Samples were added to wells of a streptavidin/scintillant-coated microplate (FlashPlate® PLUS; Perkin-Elmer Life Sciences). Biotinylated peptides would be captured in each well through their interaction with streptavidin, thereby bringing any incorporated [3H]methyl in proximity of scintillant. The amount of methylated peptide was quantified by tracing the radioactivity (cpm) as measured by the TopCount NXTTM Microplate Scintillation and Luminescence Counter (PerkinElmer Life Sciences). Radioligand Binding Assay Radioligand binding assays were carried out in a volume of 200 μL (96-well plates) which contained 100 μL of cell membranes, [3H]RO7239181 at a concentration of 1.5 nM (α5β2γ1) or 20-30 nM (α1β2γ1, α2β2γ1) and the test compound in the range of [0.3-1000]×10−9 M. Nonspecific binding was defined by 10×10−6 (α5β2γ1) and 30×10−6 M RO7239181 and typically represented less than 5% (α5β2γ1) and less than 20% (α1β2γ1, α2β2γ1) of the total binding. Assays were incubated to equilibrium for 1 hour at 4° C. and then, membranes were filtered onto unifilter (96-well white microplate with bonded GF/C filters preincubated 20-50 minutes in 0.3% Polyethylenimine) with a Filtermate 196 harvester (Packard BioScience) and washed 4 times with cold Potassium Phosphate 10 mM pH 7.4, KCl 100 mM binding buffer. After anhydrousing, filter-retained radioactivity was detected by liquid scintillation counting. Ki values were calculated using Excel-Fit (Microsoft) and are the means of two determinations. RadioligandBinding Assay Radioligand binding assays were carried out in a volume of 200 μL (96-well plates) which contained 100 μL of cell membranes, [3H]RO7239181 at a concentration of 1.5 nM (α5β2γ1) or 20-30 nM (α1β2γ1, α2β2γ1) and the test compound in the range of [0.3-10000]=10−9 M. Nonspecific binding was defined by 10×10−6 (α5β2γ1) and 30×10−6 M RO7239181 and typically represented less than 5% (α5β2γ1) and less than 20% (β1β2γ1, α2β2γ1) of the total binding. Assays were incubated to equilibrium for 1 hour at 4° C. and then, membranes were filtered onto unifilter (96-well white microplate with bonded GF/C filters preincubated 20-50 minutes in 0.3% Polyethylenimine) with a Filtermate 196 harvester (Packard BioScience) and washed 4 times with cold Potassium Phosphate 10 mM pH 7.4, KCl 100 mM binding buffer. After anhydrousing, filter-retained radioactivity was detected by liquid scintillation counting. Ki values were calculated using Excel-Fit (Microsoft) and are the means of two determinations. HEK-Blue-hTLR7 assay HEK-Blue-hTLR7 cells were incubated at a density of 250,000˜450,000 cells/mL in a volume of 180 μL in a 96-well plate in Dulbecco's Modified Eagle's medium (DMEM) containing 4.5 g/L glucose, 50 U/mL penicillin, 50 mg/mL streptomycin, 100 mg/mL Normocin, 2 mM L-glutamine, 10% (V/V) heat-inactivated fetal bovine serum for 24 hrs. Then the HEK-Blue-hTLR-7 cells were incubated with addition of 20 μL test compound in a serial dilution in the presence of final DMSO at 1% and perform incubation under 37° C. in a CO2 incubator for 20 hrs. Then 20 μL of the supernatant from each well was incubated with 180 μL Quanti-blue substrate solution at 37° C. for 2 hrs and the absorbance was read at 620˜655 nm using a spectrophotometer. The signalling pathway that TLR7 activation leads to downstream NF-κB activation has been widely accepted, and therefore similar reporter assay was also widely used for evaluating TLR7 agonist (Tsuneyasu Kaisho and Takashi Tanaka, Trends in Immunology, Volume 29, Issue 7, July 2008, Pages 329.sci; Hiroaki Hemmi et al, Nature Immunology 3, 196-200 (2002)). fluorescence polarization assay The fluorescent labeled small molecule used in the present invention was VER-00051001 (synthesized by reference to the synthetic method described in JMC, 2008, 51, 196-218). The reaction was carried out in a 384-well black plate, using the reaction hydrophobic protein HFB buffer: 100 mM Tris.Cl pH 7.4, 20 mM KCl, 6 mM MgCl2, 5 μg/mL BSA, 25 nM full-length Hsp90U, 10 nM VER-51001. The volume of the reaction system was 50 mL, containing 5 nM GM-BODIPY (geldanamycin), 30 nM HSP90 and the test small molecular compound or DMSO (dimethyl sulfoxide), wherein the DMSO content was 2% o. Additional two group wells were established, wherein the wells only added with HFB buffer were used as blank controls, and the wells further added with 5 nM GM-BODIPY were used as negative controls. The reaction was carried out at 4° C. for 12-16 hours. The mP value was measured by Biotek microplate reader at 485 nm of excitation wavelength and 535 nm of emission wavelength. The inhibition rate was calculated using the following equation:(Compound-free mP−Compound mP)/(Compound-free mP−negative control mP)×100%After the inhibitory rates of the compound at different concentrations were calculated, the IC50 of the compound can be calculated. Surface Plasmon Resonance (SPR) Assay Recombinant full-length hnRNPA1 (aa 1-320) and truncated versions of hnRNPA1, including the N-terminal RNA binding domain (aa 1-196), the middle region (aa 182-268), and the C-terminal region (aa 268-320), were individually covalently coupled to a dextran matrix (CM5 chip) using a Biacore T200 system (GE Healthcare), following the manufacturer's protocols. Immobilized proteins were activated with N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide to form a carbodiamide linkage between carboxyl moieties on the dextran and primary amide groups on the protein. hnRNPA1 variants were dissolved in 10 mM acetate buffer (full-length, pH 4; N-terminal RNA binding domain and the C-terminal region, pH 4.5; middle region, pH 5) to a concentration of 20 μg/ml and flowed over the activated surface for 5 min with PBS. Unreacted groups were blocked with ethanolamine. PBS containing 0.05% (v/v) P20 (surfactant) and 5% DMSO was used for priming and conditioning the surface of the chips. Quercetin was serially diluted (from 200 to 3 μM) in PBS buffer and maintained in DMSO (5% (v/v)) (final concentrations from 10 to 0.15 μM). The baseline response was determined for 120 s (30 μl/min) before perfusing the immobilized proteins with the compounds to allow association to occur. Dissociation was then monitored over a period of an additional 120 s. The immobilized proteins were regenerated with 50 mM NaOH. In Vitro Assay In vitro assays were performed in a recombinant cell line expressing cDNA encoding the alpha subunit (Nav1.7, SCN9a, PN1, NE) of human Nav1.7 (Accession No. NM_002977). The cell line was provided by investigators at Yale University (Cummins et al, J. Neurosci. 18(23): 9607-9619 (1998)). For dominant selection of the Nav1.7-expressing clones, the expression plasmid co-expressed the neomycin resistance gene. The cell line was constructed in the human embryonic kidney cell line, HEK293, under the influence of the CMV major late promoter, and stable clones were selected using limiting dilution cloning and antibiotic selection using the neomycin analogue, G418. Recombinant beta and gamma subunits were not introduced into this cell line. Additional cell lines expressing recombinant Nav1.7 cloned from other species can also be used, alone or in combination with various beta subunits, gamma subunits or chaperones.The assay buffer was formulated by removing 120 mL from a 1 L bottle of fresh, sterile dH2O (Mediatech, Herndon, Va.) and adding 100 mL of 10×HBSS that does not contain Ca++ or Mg++ (Gibco, Invitrogen, Grand Island, N.Y.) followed by 20 mL of 1.0 M Hepes, pH 7.3 (Fisher Scientific, BP299-100). The final buffer consisted of 20 mM Hepes, pH 7.3, 1.261 mM CaCl2, 0.493 mM MgCl2, 0.407 mM Mg(SO)4, 5.33 mM KCl, 0.441 mM KH2PO4, 137 mM NaCl, 0.336 mM Na2HPO4 and 0.556 mM D-glucose (Hanks et al., Proc. Soc. Exp. Biol. Med. 71:196 (1949)), and the simple formulation was typically the basic buffer throughout the assay (i.e., all wash and addition steps). [3H]SR142801 Competition Binding Assay hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK limited, Buckinghamshire, UK) and membrane isolated from HEK293 cells transiently expressing recombinant human NK3 receptor. After thawing, the membrane homogenates were centrifuged at 48,000×g for 10 mM at 4° C., the pellets were resuspended in the 50 mM Tris-HCl, 4 mM MnCl2, 1 μM phosphoramidon, 0.1% BSA binding buffer at pH 7.4 to a final assay concentration of 5 μg protein/well. For inhibition experiments, membranes were incubated with [3H]SR142801 at a concentration equal to KD value of radioligand and 10 concentrations of the inhibitory compound (0.0003-10 μM) (in a total reaction volume of 500 μl) for 75 min at room temperature (RT). At the end of the incubation, membranes were filtered onto unitfilter (96-well white microplate with bonded GF/C filter preincubated 1 h in 0.3% PEI+0.3% BSA, Packard BioScience, Meriden, Conn.) with a Filtermate 196 harvester (Packard BioScience) and washed 4 times with ice-cold 50 mM Tris-HCl, pH 7.4 buffer. Nonspecific binding was measured in the presence of 10 μM SB222200 for both radioligands. The radioactivity on the filter was counted (5 min) on a Packard Top-count microplate scintillation counter with quenching correction after addition of 45 μl of microscint 40 (Canberra Packard S.A., Zürich, Switzerland) and shaking for 1 h. HEK293-hTLR-7 assay HEK293-Blue-hTLR7 cells were incubated at a density of 250,000-450,000 cells/mL in a volume of 180 μL in a 96-well plate in Dulbecco's Modified Eagle's medium (DMEM) containing 4.5 g/l glucose, 50 U/ml penicillin, 50 mg/ml streptomycin, 100 mg/ml Normocin, 2 mM L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum for 24 h. Then the HEK293-Blue-hTLR-7 cells were incubated with addition of 20 μL test compound in a serial dilution in the presence of final DMSO at 1% and perform incubation under 37° C. in a CO2 incubator for 20 hours. Then 20 μL of the supernatant from each well was incubated with 180 μL Quanti-blue substrate solution at 37° C. for 1-3 hours and the absorbance was read at 620-655 nm using a spectrophotometer. The signalling pathway that TLR7 activation leads to downstream NF-κB activation has been widely accepted, and therefore similar reporter assay was also widely used for evaluating TLR7 agonist (Tsuneyasu Kaisho and Takashi Tanaka, Trends in Immunology, Volume 29, Issue 7, July 2008, Pages 329.sci; Hiroaki Hemmi et al, Nature Immunology 3, 196-200 (2002).The compounds of the present invention were tested in the above assay for their TLR7 agonism activity as described herein and results are listed in Table 1. The Examples were found to have EC50 of about 3 μM to about 500 μM. Particular compounds of formula (I) or (Ia) were found to have EC50 of about 3 μM to about 200 μM. Radioligand Binding Assay In this assay, COS-7 cells are transiently transfected with the hFP recombinant plasmid using LipofectAMINE Reagent. Forty-eight hours later, the transfected cells are washed with Hank's Balanced Salt Solution (HBSS, without CaCl2, MgCl2, MgSO4, or phenol red). The cells are detached with versene, and HBSS is added. The mixture is centrifuged at 200 g for 10 minutes, at 4° C. to pellet the cells. The pellet is resuspended in Phosphate-Buffered Saline-EDTA buffer (PBS; 1 mM EDTA; pH 7.4; 4° C.). The cells are disrupted by nitrogen cavitation (Parr model 4639), at 800 psi, for 15 minutes at 4° C. The mixture is centrifuged at 1000 g for 10 minutes at 4° C. The supernatant is centrifuged at 100,000 g for 60 minutes at 4° C. The pellet is resuspended to 1 mg protein/mL TME buffer (50 mM Tris; 10 mM MgCl2; 0.1 mM EDTA; pH 6.0; 4° C.) based on protein levels measured using the Pierce BCA Protein Assay kit. The homogenate is mixed for 10 seconds using a Kinematica POLYTRON (available from KINEMATICA AG, Luzernerstrasse147A CH-6014 Littau, Switzerland). The membrane preparations are then stored at −80° C., until thawed for assay use. The receptor competition binding assays are developed in a 96 well format. Each well contains 100 g of hFP membrane, 5 nM (3 H) PGF2α, and the various competing compounds in a total volume of 200 L. The plates are incubated at 23° C. for 1 hour. The incubation is terminated by rapid filtration using the Packard Filtermate 196 harvester through Packard UNIFILTER GF/B filters (available from Packard Instrument Co., Inc. of Downers Grove Ill.) pre-wetted with TME buffer. The filter is washed four times with TME buffer. Packard Microscint 20, a high efficiency liquid scintillation cocktail, is added to the filter plate wells and the plates remain at room temperature for three hours prior to counting. The plates are read on a Packard TOPCOUNT Microplate Scintillation Counter (also available from Packard Instrument Co., Inc.)
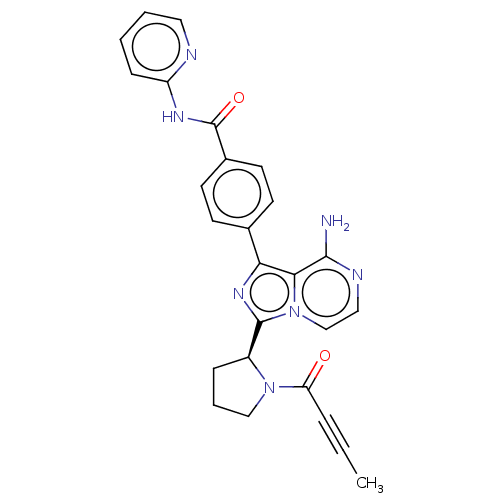 US10919899, ACP-196 US10934296, Example 6 US10239883, Example 6 US9758524, Example 6 ACP-196 US11420975, Acalabrutinlb Acalabrutinib BDBM50175583 US10793576, Example ACP-196
US10919899, ACP-196 US10934296, Example 6 US10239883, Example 6 US9758524, Example 6 ACP-196 US11420975, Acalabrutinlb Acalabrutinib BDBM50175583 US10793576, Example ACP-196 BDBM737806 US12291534, Compound Acalabrutinib
BDBM737806 US12291534, Compound Acalabrutinib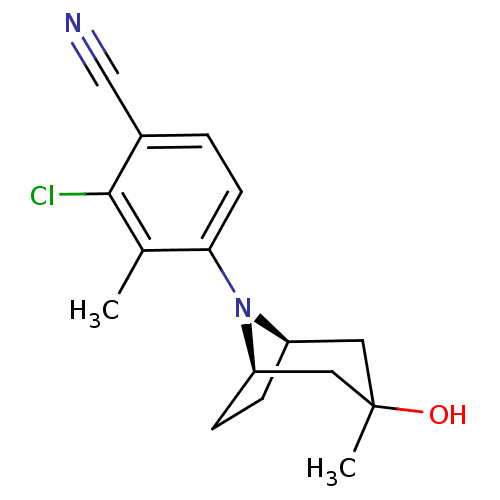 ACP-105 CHEMBL570435 BDBM50415086
ACP-105 CHEMBL570435 BDBM50415086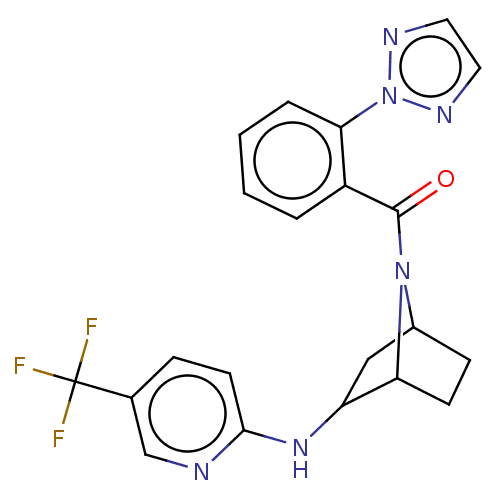 BDBM164066 US9475819, 196 US9062078, 196 US9637496, 196 US9695183, 196
BDBM164066 US9475819, 196 US9062078, 196 US9637496, 196 US9695183, 196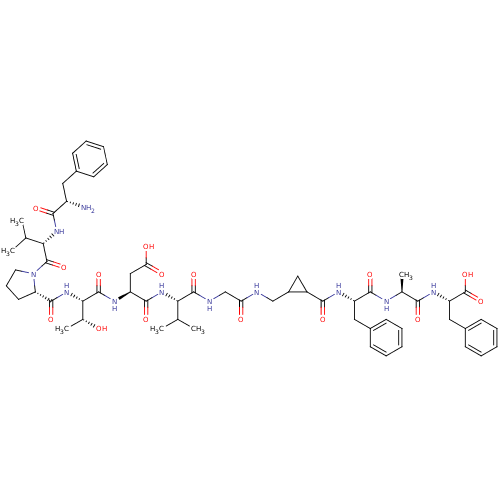 BDBM50062170 CHEMBL428655 FVPTDVG-Acp-FAF
BDBM50062170 CHEMBL428655 FVPTDVG-Acp-FAF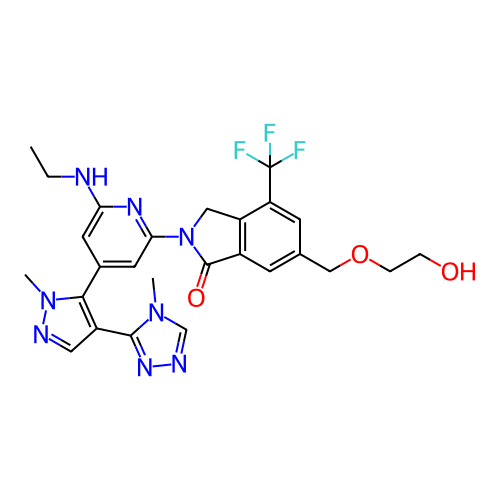 BDBM747473 US12325697, Compound ACP-3
BDBM747473 US12325697, Compound ACP-3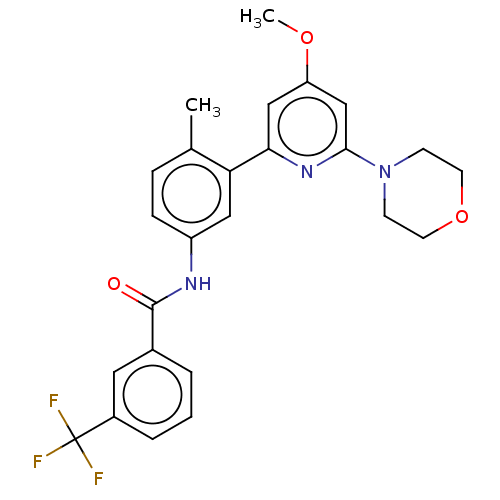 US9242969, 196 US10709712, Example 196 BDBM202843 US10245267, Example 196 US9694016, 196
US9242969, 196 US10709712, Example 196 BDBM202843 US10245267, Example 196 US9694016, 196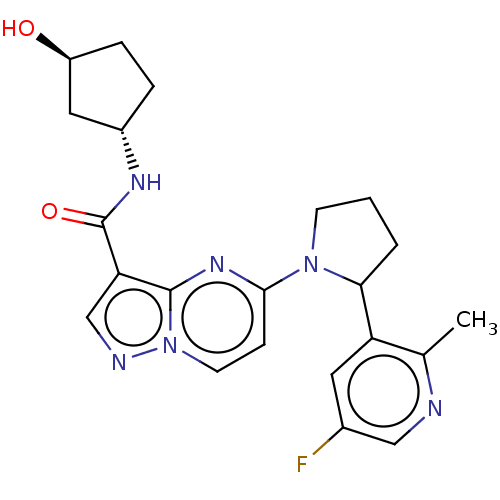 BDBM127823 US10758542, Example 196 US9782415, Example 196 US8791123, 196 US9796724, Example 196 US10251889, Example 196
BDBM127823 US10758542, Example 196 US9782415, Example 196 US8791123, 196 US9796724, Example 196 US10251889, Example 196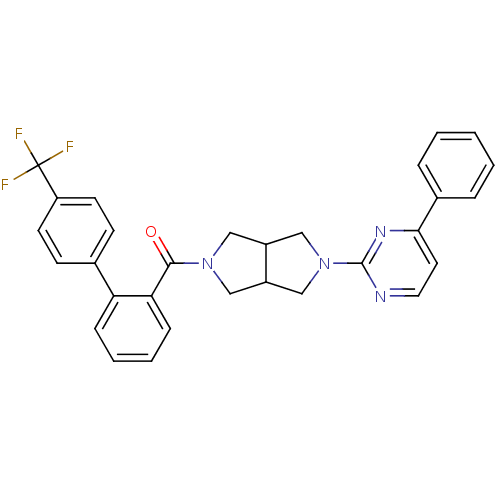 USRE48841, Example 196 US8653263, 196 US9586962, Example 196 BDBM118543 US11059828, Example 196 US11667644, Example 196
USRE48841, Example 196 US8653263, 196 US9586962, Example 196 BDBM118543 US11059828, Example 196 US11667644, Example 196 US10238633, Example 196 US9713606, 196 US8987249, 196 BDBM150522
US10238633, Example 196 US9713606, 196 US8987249, 196 BDBM150522 US10112937, Example 196 BDBM254299 US10703749, Example 196 US9464084, 196 US10150765, Example 196
US10112937, Example 196 BDBM254299 US10703749, Example 196 US9464084, 196 US10150765, Example 196 BDBM305180 US10881652, Example 196 US11648243, Example 196 US10144734, Example 196 US10172845, Example 196 US10441581, Example 196
BDBM305180 US10881652, Example 196 US11648243, Example 196 US10144734, Example 196 US10172845, Example 196 US10441581, Example 196 US10112942, Example 196 US10172851, Example 196 BDBM296462 US10137124, Example 196 US10953005, Example 196 US10555944, Example 196
US10112942, Example 196 US10172851, Example 196 BDBM296462 US10137124, Example 196 US10953005, Example 196 US10555944, Example 196 US11021515, Compound 196 BDBM442003 US11667673, Compound 196 US10689414, Compound 196 US10640534, Compound 196 US10717764, Compound 196
US11021515, Compound 196 BDBM442003 US11667673, Compound 196 US10689414, Compound 196 US10640534, Compound 196 US10717764, Compound 196 US10391089, Compound 196 US10307413, Compound 196 BDBM178150 US10980794, Cmpd No 196 US9675614, 196
US10391089, Compound 196 US10307413, Compound 196 BDBM178150 US10980794, Cmpd No 196 US9675614, 196 US11220509, Example 196 BDBM513835 US11091495, Example 196 US11485738, Example 196 US20240398811, Example 196
US11220509, Example 196 BDBM513835 US11091495, Example 196 US11485738, Example 196 US20240398811, Example 196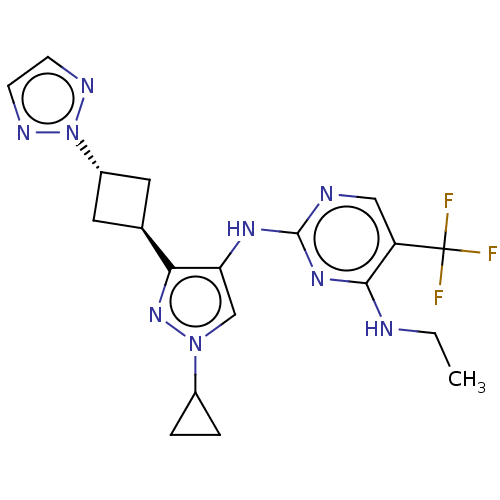 US11591316, Compound 196 BDBM384510 US10590114, No. 196 US11111235, No. 196 US9932325, Example 196
US11591316, Compound 196 BDBM384510 US10590114, No. 196 US11111235, No. 196 US9932325, Example 196 BDBM224518 US9321760, 196 US9662330, Compound 196 US10080750, Compound 196
BDBM224518 US9321760, 196 US9662330, Compound 196 US10080750, Compound 196 BDBM246290 US10183932, Example 196 US9434690, 196 US9822102, Example 196
BDBM246290 US10183932, Example 196 US9434690, 196 US9822102, Example 196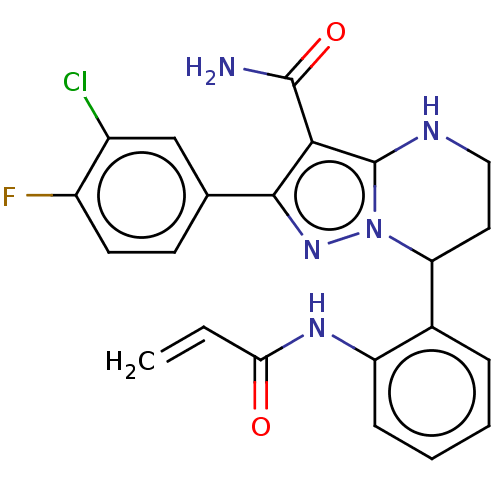 BDBM250071 US10005782, Compound 196 US9447106, 196 US9556188, Compound 196
BDBM250071 US10005782, Compound 196 US9447106, 196 US9556188, Compound 196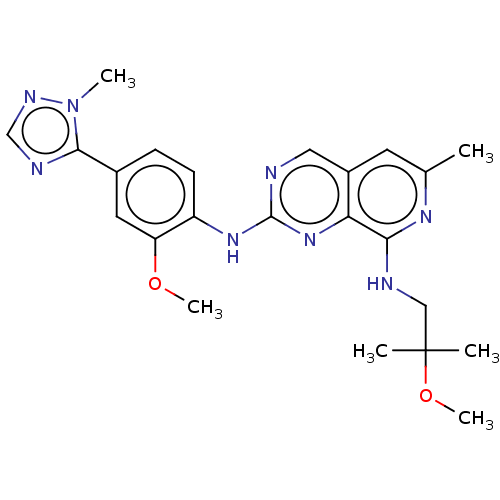 US11046688, Example 196 US10479788, Example 196 US9409907, 196 BDBM241352
US11046688, Example 196 US10479788, Example 196 US9409907, 196 BDBM241352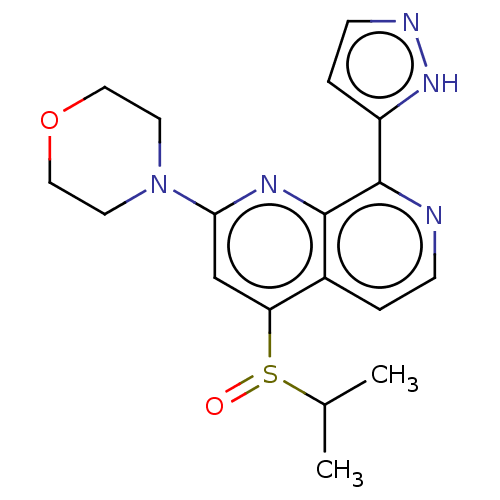 US11529356, Example 196 US10772893, Example 196 BDBM268164 US9549932, 196
US11529356, Example 196 US10772893, Example 196 BDBM268164 US9549932, 196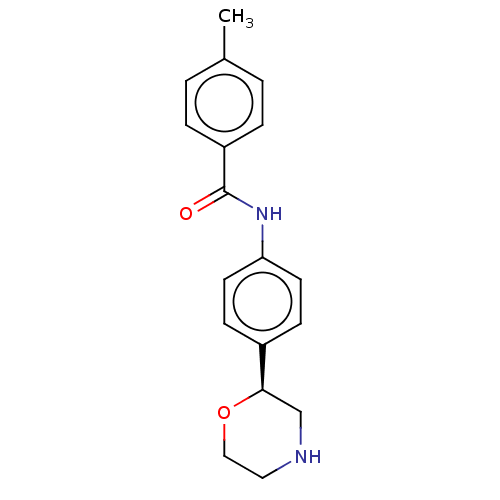 US9452980, 196 BDBM250286 US10501411, Example 196 US11697636, Example 196
US9452980, 196 BDBM250286 US10501411, Example 196 US11697636, Example 196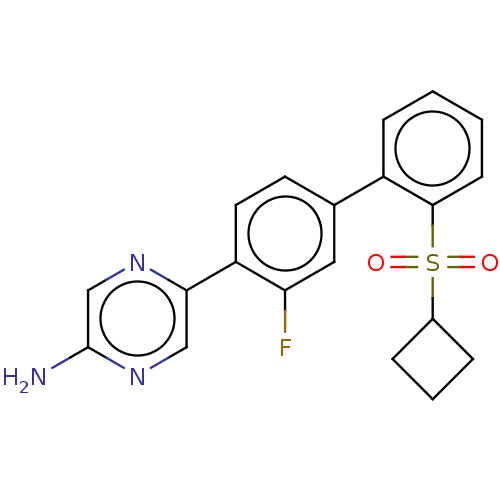 US9745328, Compound 196 US9884878, Compound 196 BDBM168797 US9079866, 196
US9745328, Compound 196 US9884878, Compound 196 BDBM168797 US9079866, 196 BDBM110422 US9206232, 196 US8613914, 196
BDBM110422 US9206232, 196 US8613914, 196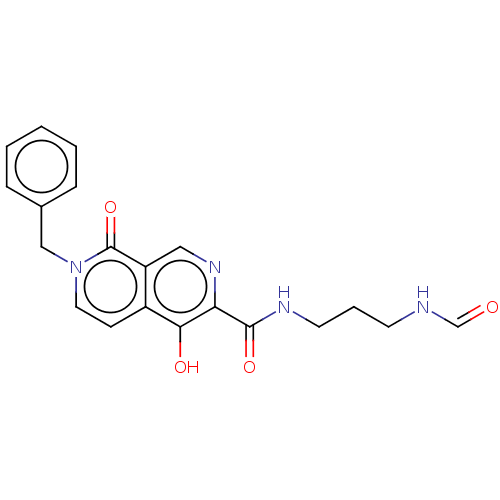 BDBM141737 US8921389, 196 US9695170 196
BDBM141737 US8921389, 196 US9695170 196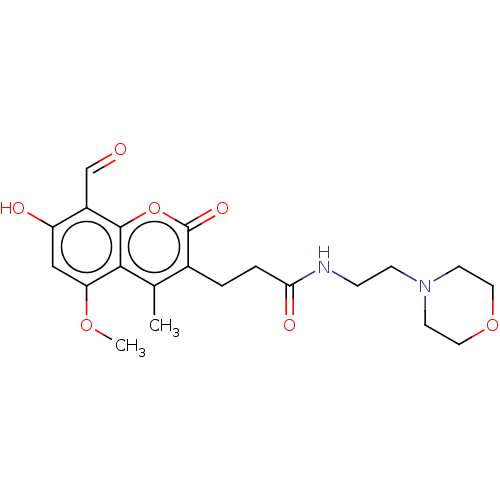 BDBM160466 US9040714, 196 US9693992, 196
BDBM160466 US9040714, 196 US9693992, 196 BDBM165161 US9682971, 196 US9067917, 196
BDBM165161 US9682971, 196 US9067917, 196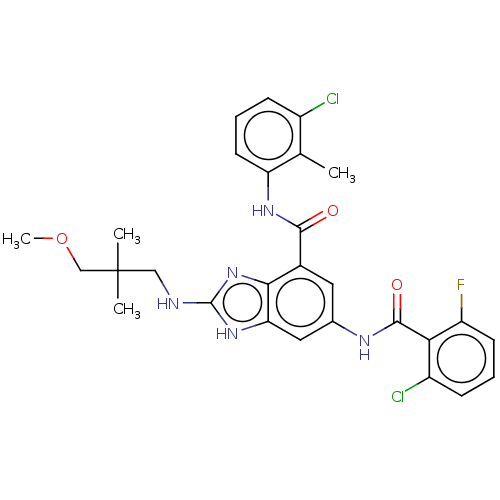 BDBM197596 US9732066, 196 US9216968, 196
BDBM197596 US9732066, 196 US9216968, 196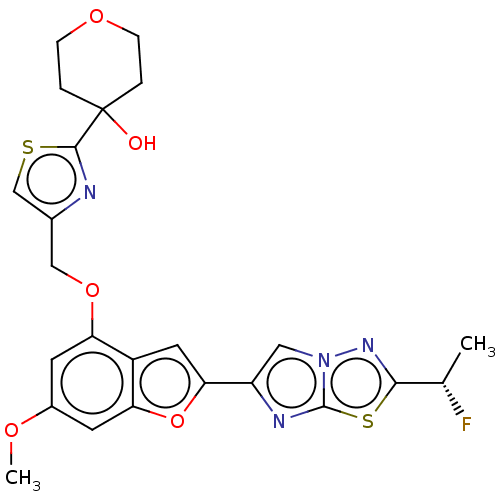 US10047103, 196 US9688695, 196 BDBM176163
US10047103, 196 US9688695, 196 BDBM176163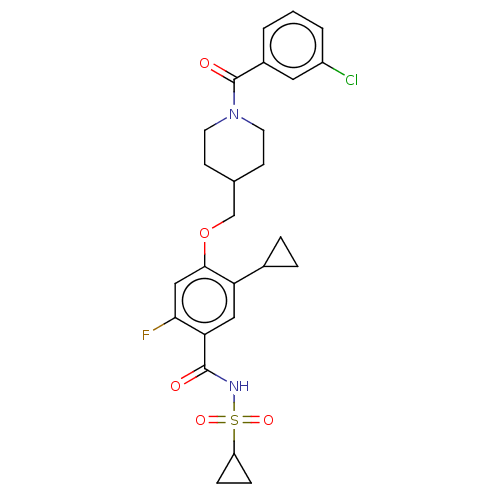 US9694002, 196 US9546164, 196 BDBM71049
US9694002, 196 US9546164, 196 BDBM71049 US9688629, 196 US9802915, Example 196 BDBM165583 US11623921, Example 196 US10604504, Example 196 US9688629, 194 US9688629, 195 US9920031, Example 196
US9688629, 196 US9802915, Example 196 BDBM165583 US11623921, Example 196 US10604504, Example 196 US9688629, 194 US9688629, 195 US9920031, Example 196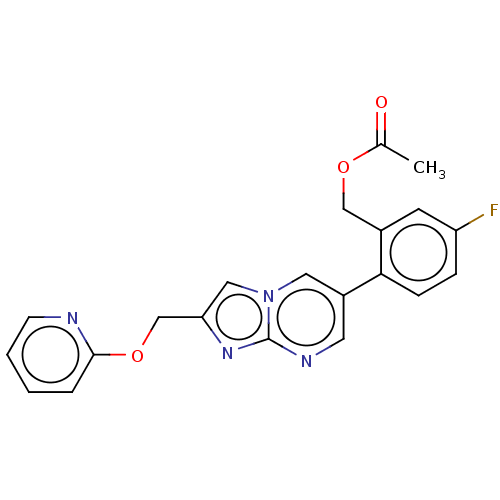 BDBM292341 US11046702, Example 196 US10100057, Example 196 US9745310, Example 196
BDBM292341 US11046702, Example 196 US10100057, Example 196 US9745310, Example 196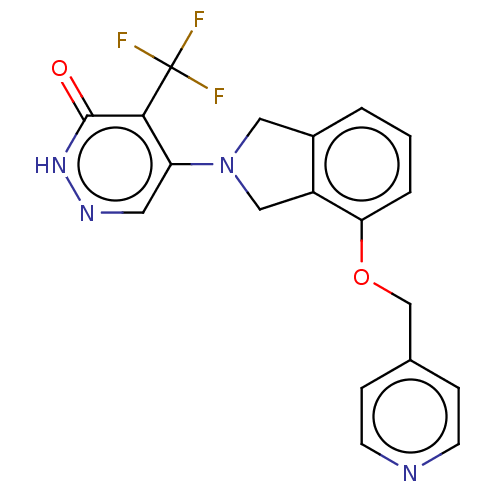 BDBM431251 US11014913, Example 196 US10870641, Example 196 US10550105, Example 196
BDBM431251 US11014913, Example 196 US10870641, Example 196 US10550105, Example 196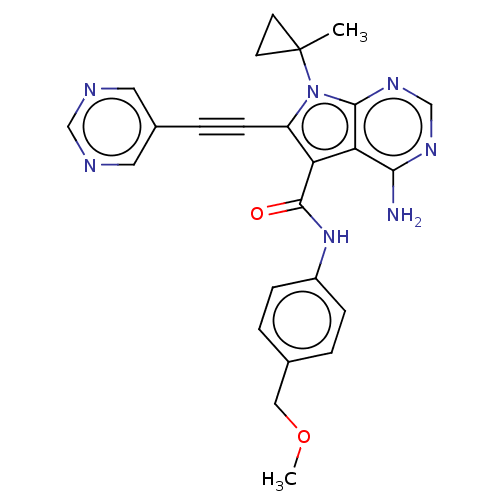 Ex. Cpd. 196 US11046696, Compound 196 BDBM311744 US10807986, Example 196
Ex. Cpd. 196 US11046696, Compound 196 BDBM311744 US10807986, Example 196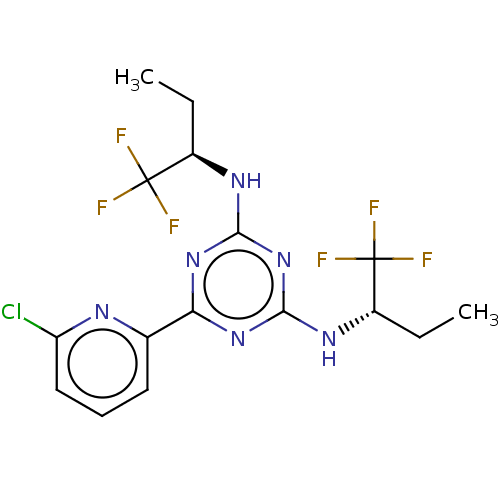 US10172864, Compound 196 US10028961, Compound 196 US10946023, Compound 196 BDBM280043
US10172864, Compound 196 US10028961, Compound 196 US10946023, Compound 196 BDBM280043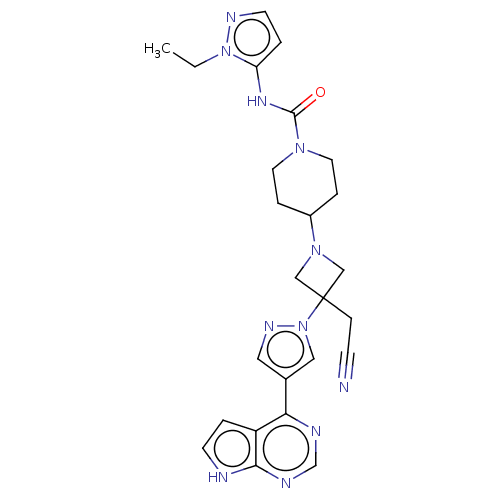 US10695337, Example 196 US9999619, Example 196 US11285140, Example 196 BDBM400441
US10695337, Example 196 US9999619, Example 196 US11285140, Example 196 BDBM400441 US11091484, Example 196 US10442803, Example 196 BDBM343597 US9777003, Example 196
US11091484, Example 196 US10442803, Example 196 BDBM343597 US9777003, Example 196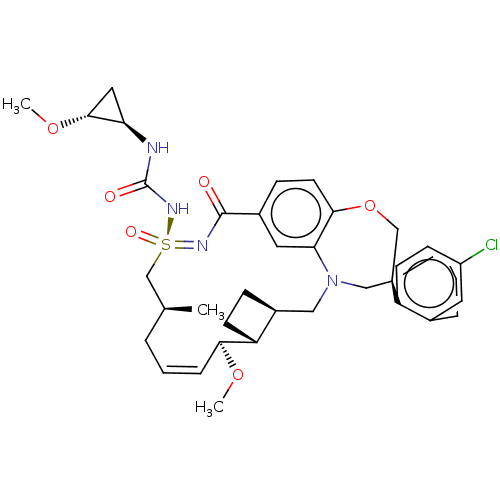 US11643400, Example 196 US10703733, Example 196 US10988451, Example 196 BDBM449583
US11643400, Example 196 US10703733, Example 196 US10988451, Example 196 BDBM449583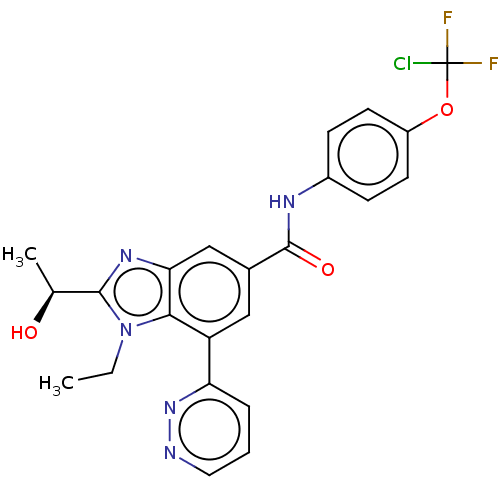 US20240101536, Example 196 BDBM477848 US12240835, Example 196 US10889571, Example 196
US20240101536, Example 196 BDBM477848 US12240835, Example 196 US10889571, Example 196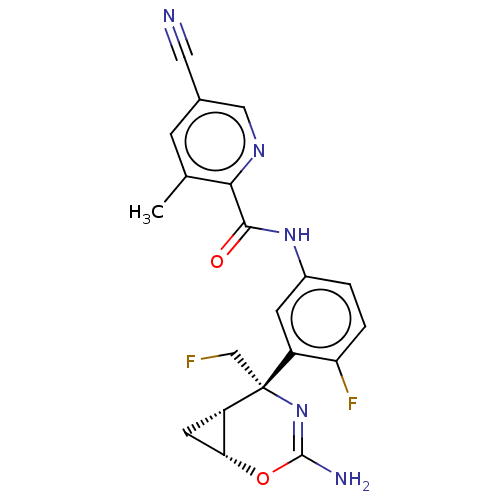 BDBM171562 US9085576, 196 US9611261, Example 196
BDBM171562 US9085576, 196 US9611261, Example 196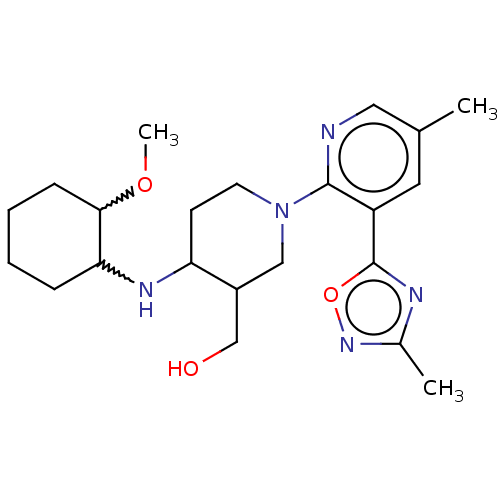 US10118915, Compound 196 BDBM156203 US9682966, 196
US10118915, Compound 196 BDBM156203 US9682966, 196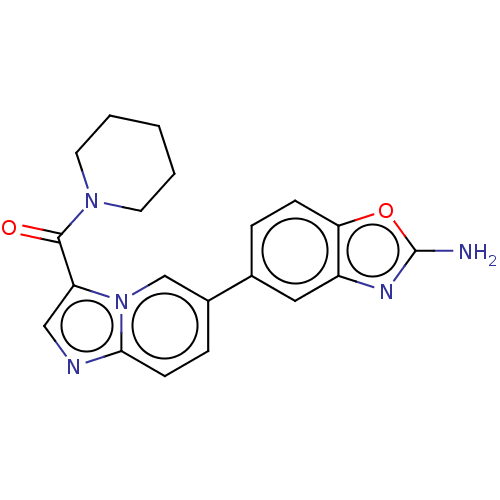 US11547697, Compound 196 BDBM127349 US9682141, 196
US11547697, Compound 196 BDBM127349 US9682141, 196 US9212139, 196 BDBM196496 US10202344, Compound 196
US9212139, 196 BDBM196496 US10202344, Compound 196 US9296736, 196 US9593129, Example 196 BDBM215236
US9296736, 196 US9593129, Example 196 BDBM215236 US9409908, 196 BDBM241564 US9951071, Example 196
US9409908, 196 BDBM241564 US9951071, Example 196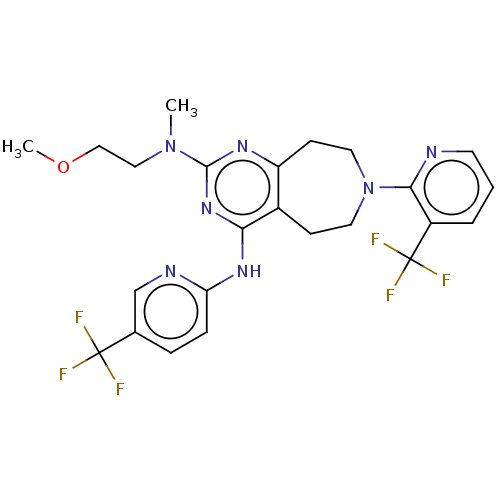 US9422293, 196 US9738649, Example 196 BDBM244961
US9422293, 196 US9738649, Example 196 BDBM244961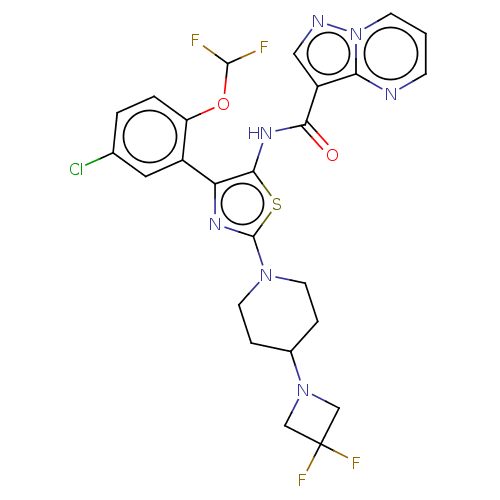 US9604984, Example 196 US9346815, 196 BDBM232650
US9604984, Example 196 US9346815, 196 BDBM232650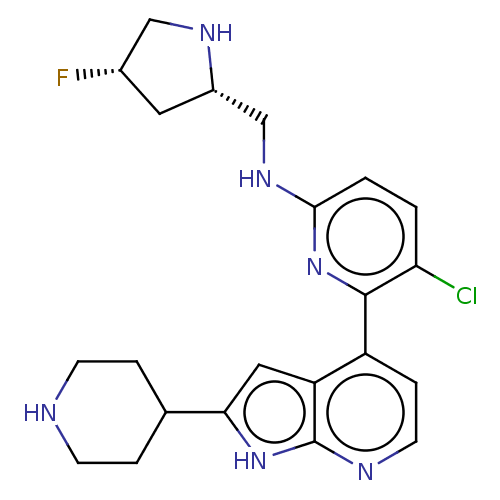 US9796708, Example 196 BDBM167709 US9073922, 196
US9796708, Example 196 BDBM167709 US9073922, 196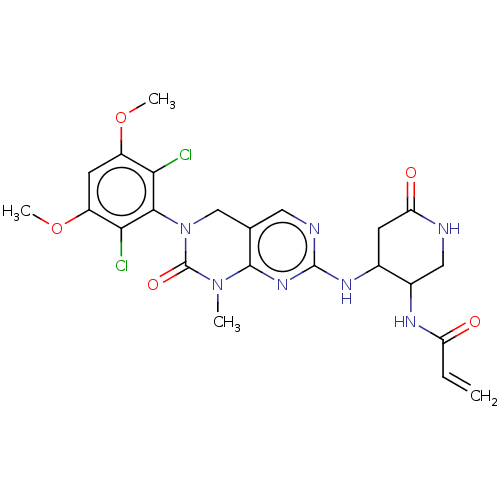 US10618902, Compound I-196 BDBM99850 US10774052, Compound I-196 US10189794, Compound I-196 US9695132, I-196
US10618902, Compound I-196 BDBM99850 US10774052, Compound I-196 US10189794, Compound I-196 US9695132, I-196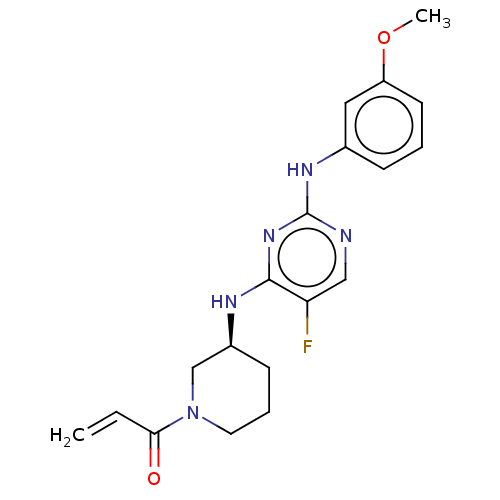 BDBM397343 US10828300, Compound I-196 US9987276, Compound I-196 US11351168, Compound I-196 US10596172, Compound I-196
BDBM397343 US10828300, Compound I-196 US9987276, Compound I-196 US11351168, Compound I-196 US10596172, Compound I-196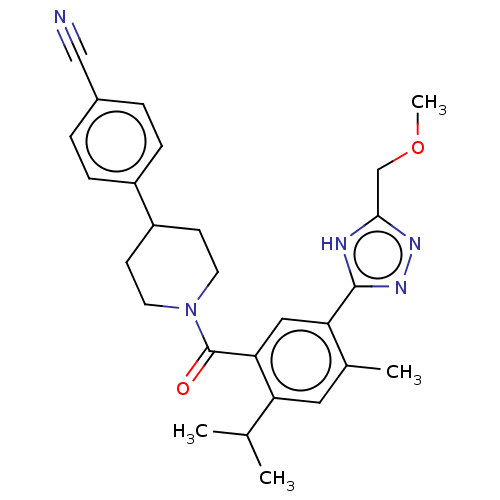 BDBM137128 US20250017938, Compound 001-196 US8871790, 196
BDBM137128 US20250017938, Compound 001-196 US8871790, 196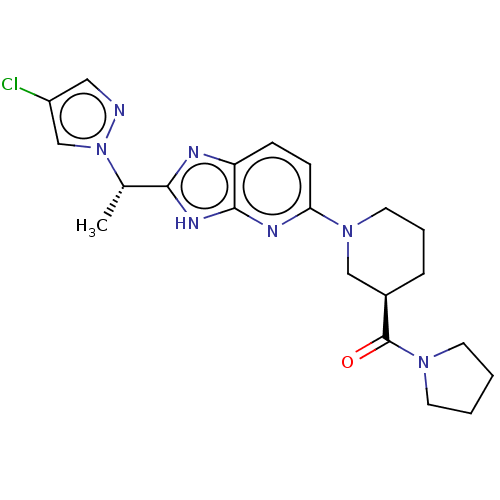 BDBM215629 US9296745, 196-B US9296745, 196-A
BDBM215629 US9296745, 196-B US9296745, 196-A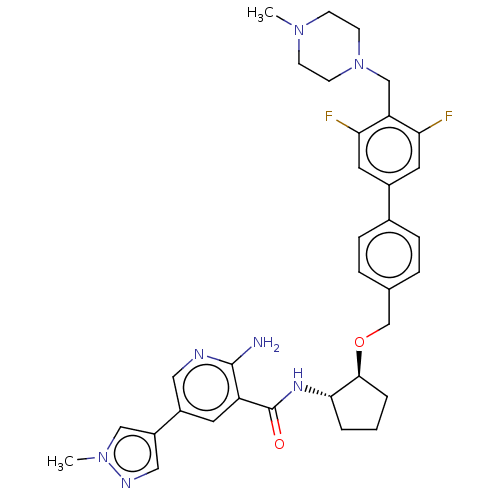 BDBM298790 US10125118, Example 196 US10947215, Example 196
BDBM298790 US10125118, Example 196 US10947215, Example 196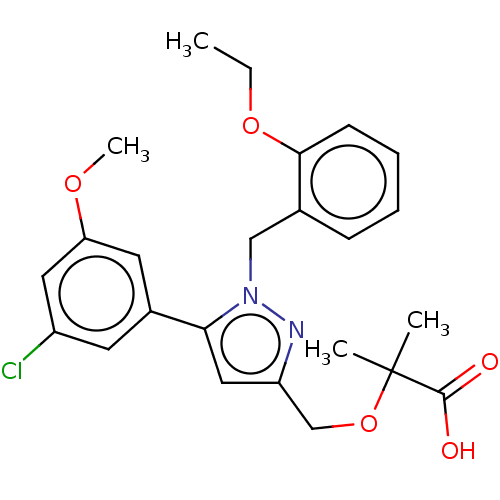 BDBM356846 US10214492, Example 196 US11292767, Example 196
BDBM356846 US10214492, Example 196 US11292767, Example 196 BDBM393899 US10597367, Example 196 US9969726, Example 196
BDBM393899 US10597367, Example 196 US9969726, Example 196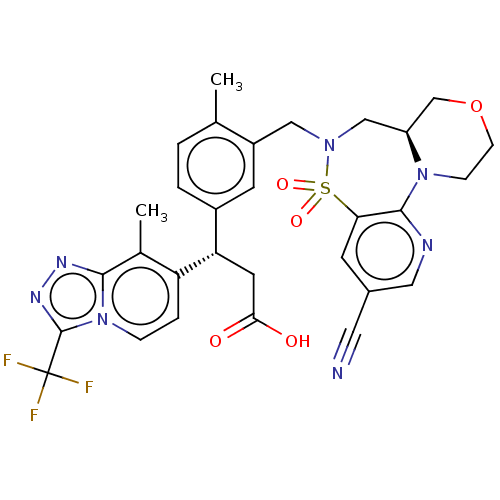 BDBM486749 US11427601, Example 196 US10947252, Example 196
BDBM486749 US11427601, Example 196 US10947252, Example 196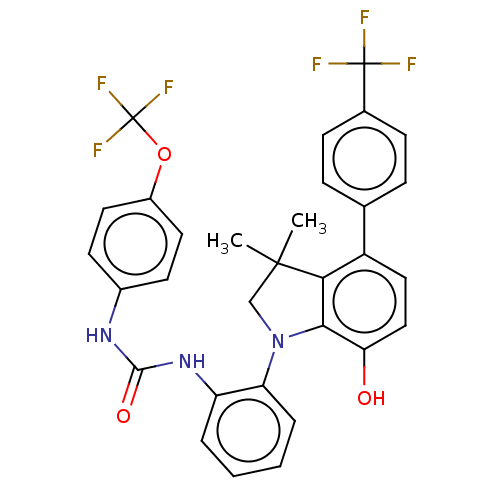 BDBM50045620 CHEMBL3314300 US9540323, example 196 US9540323, 196
BDBM50045620 CHEMBL3314300 US9540323, example 196 US9540323, 196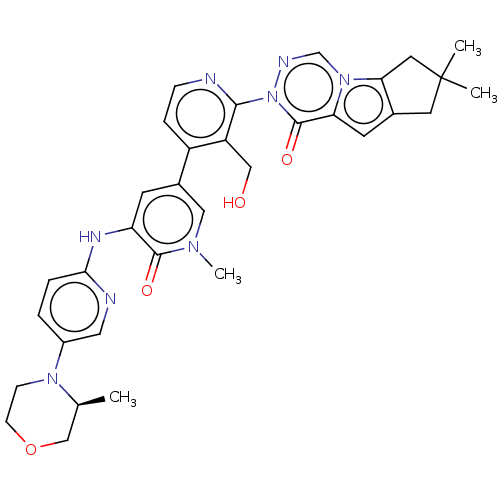 BDBM578348 US11478474, Compound 196 US12070457, Compound 196
BDBM578348 US11478474, Compound 196 US12070457, Compound 196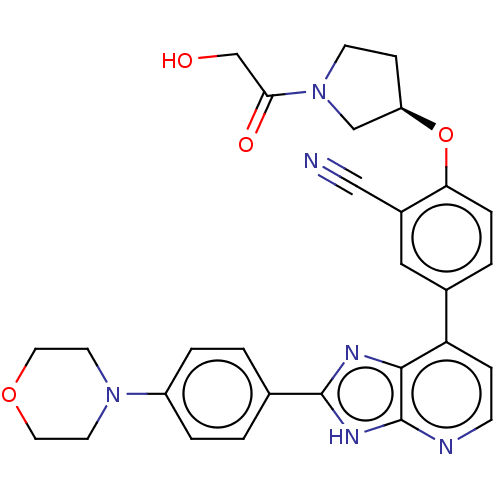 US10072001, Example 196 US10259811, Example 196 BDBM277019
US10072001, Example 196 US10259811, Example 196 BDBM277019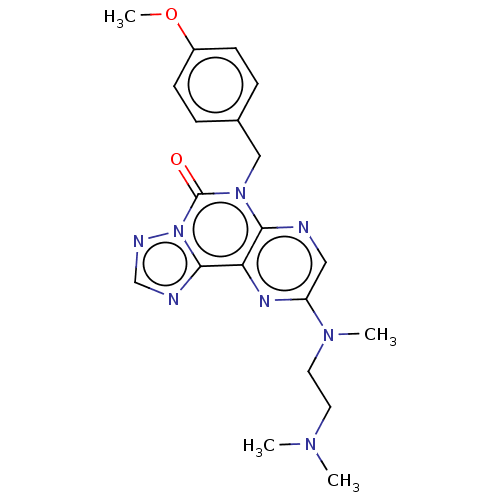 US10105367, Example 196 BDBM292910 US10376514, Example 196
US10105367, Example 196 BDBM292910 US10376514, Example 196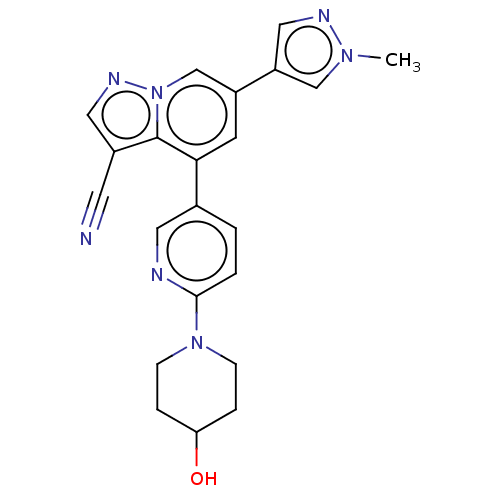 US10174028, Example 196 BDBM284186 US10023570, Example 196
US10174028, Example 196 BDBM284186 US10023570, Example 196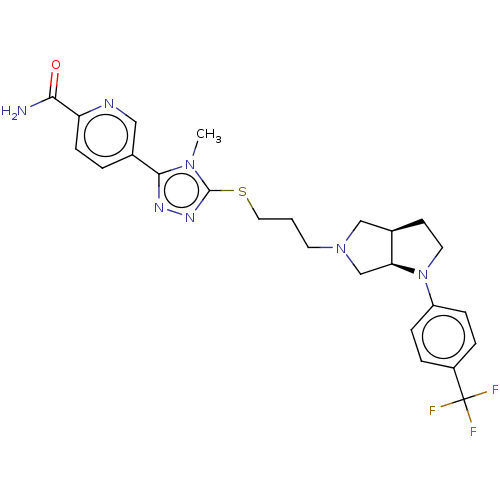 US10273244, Example 196 US10584135, Example 196 BDBM382315
US10273244, Example 196 US10584135, Example 196 BDBM382315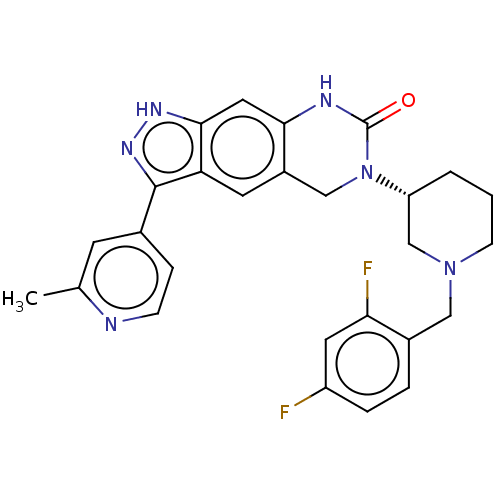 US10301317, Example 196 US9624228, Example 196 BDBM318240
US10301317, Example 196 US9624228, Example 196 BDBM318240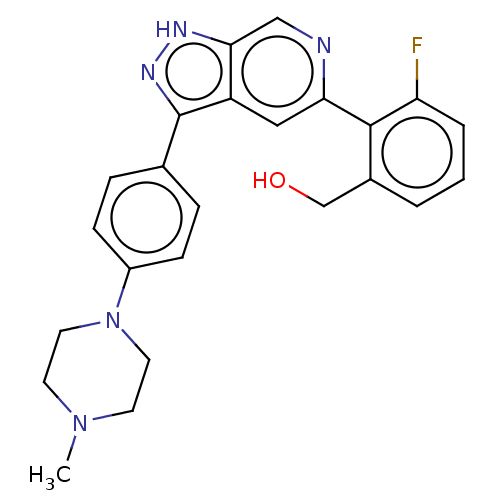 US10435405, Example 196 BDBM414779 US10934288, Example 196
US10435405, Example 196 BDBM414779 US10934288, Example 196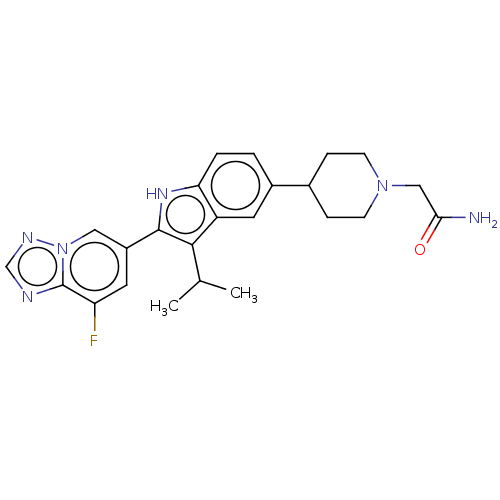 US10478424, Example 196 US10071079, Example 196 BDBM273413
US10478424, Example 196 US10071079, Example 196 BDBM273413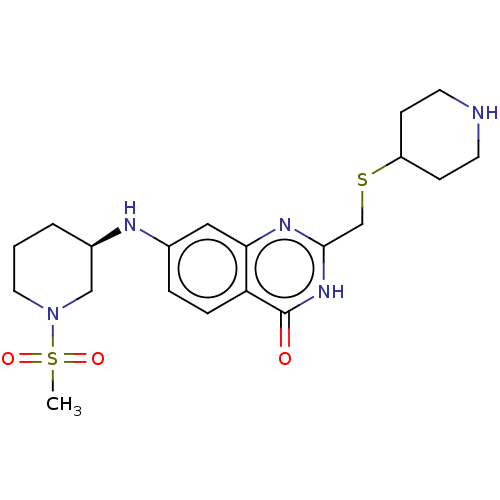 US10562891, Example 196 BDBM434274 US11008308, Example 196
US10562891, Example 196 BDBM434274 US11008308, Example 196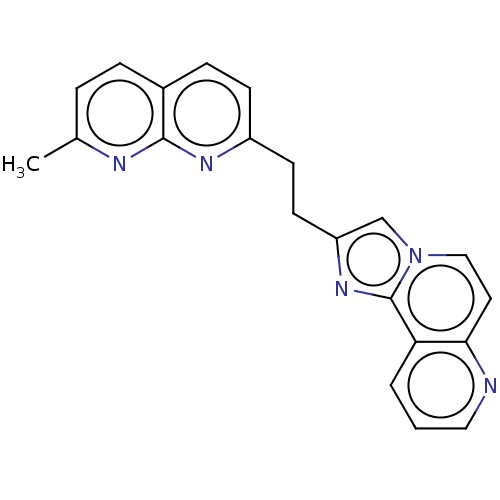 US10562916, Compound 196 BDBM362634 US9834564, Compound 196
US10562916, Compound 196 BDBM362634 US9834564, Compound 196 US10577374, Compound 196 US9790229, Compound 196 BDBM348473
US10577374, Compound 196 US9790229, Compound 196 BDBM348473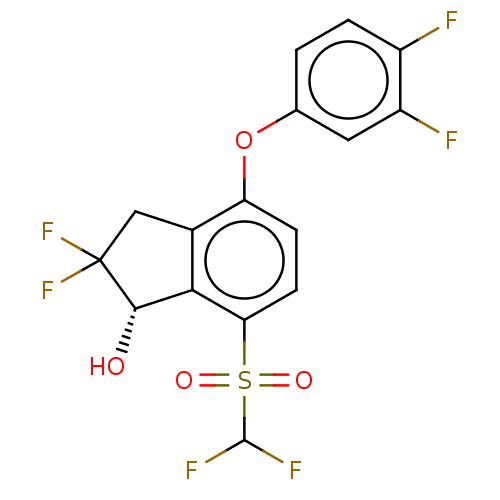 US10597366, Compound 196 US9896418, Compound 196 BDBM373021
US10597366, Compound 196 US9896418, Compound 196 BDBM373021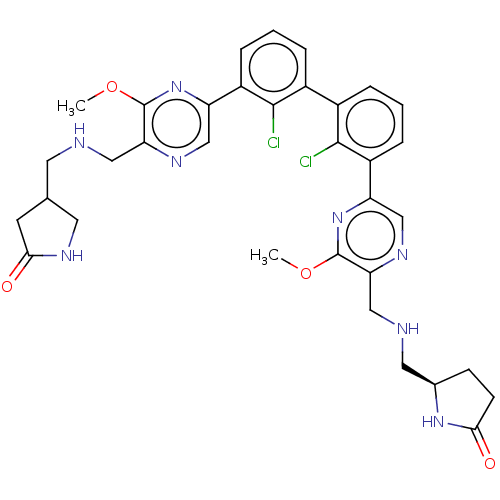 US10710986, Example 196 BDBM452056 US11555029, No. 196
US10710986, Example 196 BDBM452056 US11555029, No. 196 US10711020, Example 196 BDBM382487 US10273259, Example 196
US10711020, Example 196 BDBM382487 US10273259, Example 196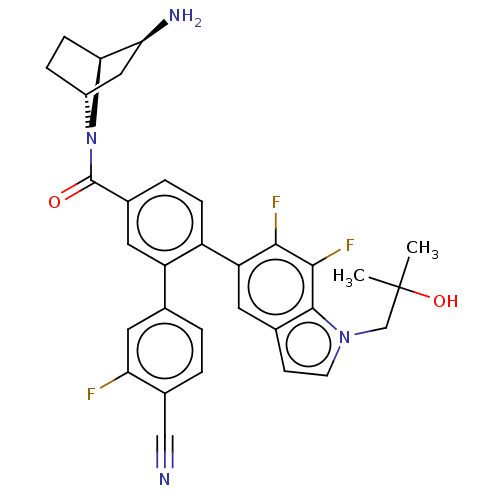 US10723742, Example 196 BDBM456470 US11510915, Example 196
US10723742, Example 196 BDBM456470 US11510915, Example 196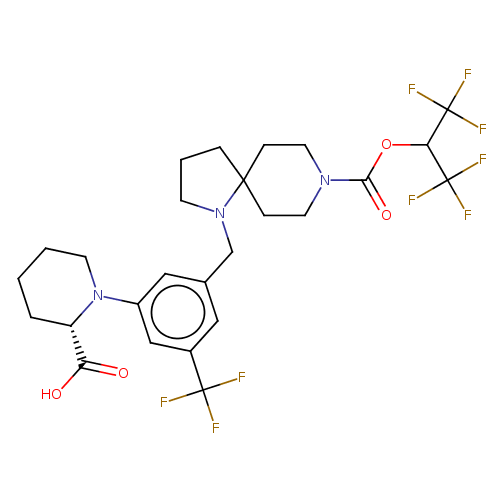 US10781211, Example 196 US10030020, Example 196 BDBM280651
US10781211, Example 196 US10030020, Example 196 BDBM280651 US10800783, Example 196 BDBM467204 US11396512, Example 196
US10800783, Example 196 BDBM467204 US11396512, Example 196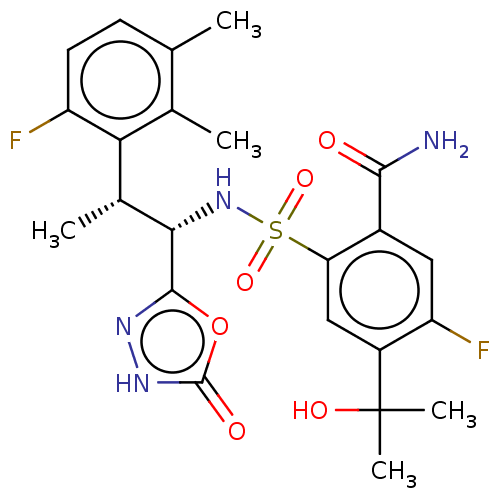 US10889555, Example 196 BDBM477413 US11634395, Example 196
US10889555, Example 196 BDBM477413 US11634395, Example 196 US11001575, Example 196 BDBM417579 US10457669, Example 196
US11001575, Example 196 BDBM417579 US10457669, Example 196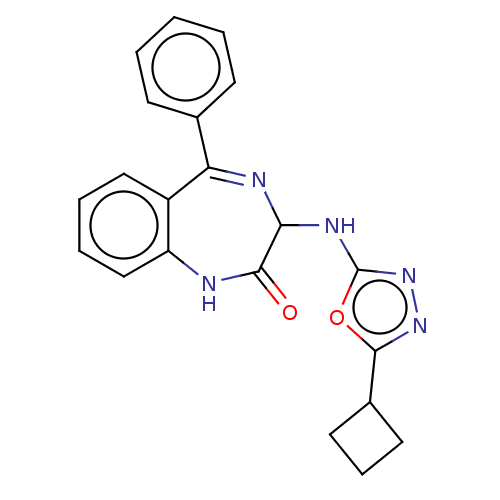 US11390631, Example 196 BDBM561659 US12268694, Example 196
US11390631, Example 196 BDBM561659 US12268694, Example 196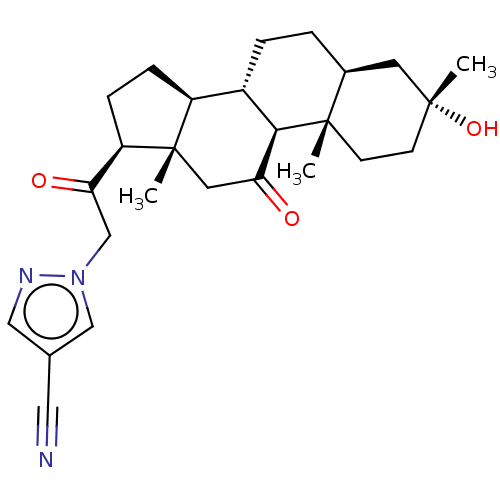 US11542297, Compound 196 BDBM586019 US11530237, Compound 196
US11542297, Compound 196 BDBM586019 US11530237, Compound 196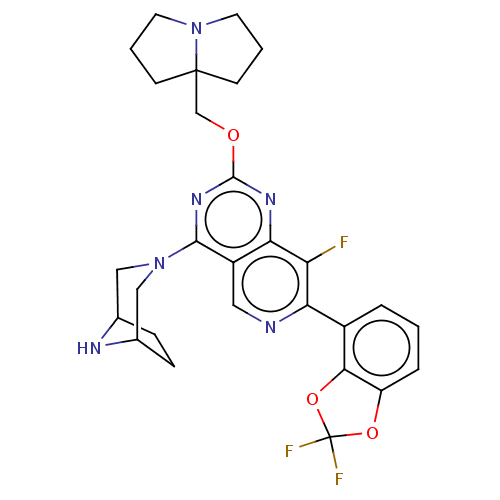 US20230279025, Example 196 BDBM573611 US11453683, Example 196
US20230279025, Example 196 BDBM573611 US11453683, Example 196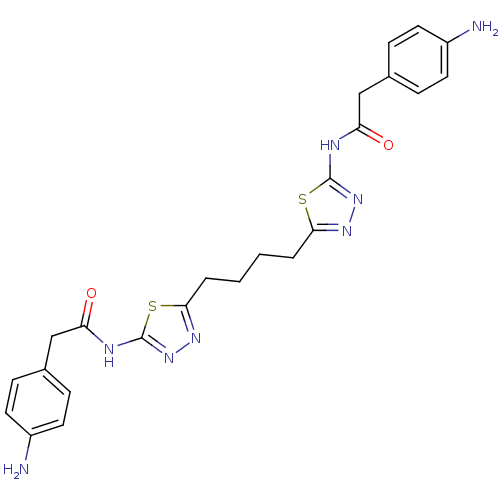 US9938267, Cmpd ID 196 US8604016, 196 BDBM108621
US9938267, Cmpd ID 196 US8604016, 196 BDBM108621 BDBM423741 US11046698, Compound I-196 US10508120, Compound I-196 US10577373, Compound I-196
BDBM423741 US11046698, Compound I-196 US10508120, Compound I-196 US10577373, Compound I-196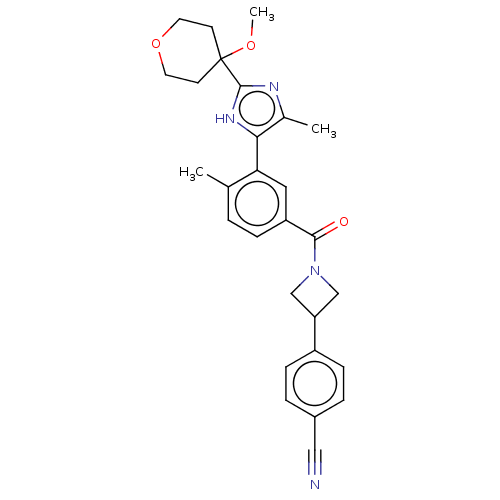 US10226449, cpd 196 US20250017938, Compound 002-196 BDBM367378
US10226449, cpd 196 US20250017938, Compound 002-196 BDBM367378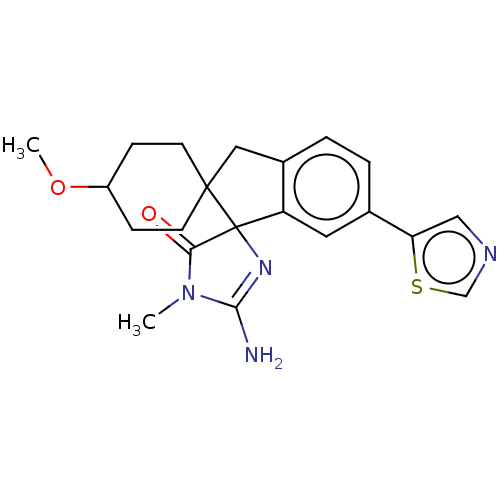 US10336717, Compound 196 BDBM195136 US9212153, 196,Ex. 157
US10336717, Compound 196 BDBM195136 US9212153, 196,Ex. 157 US10442772, Example 2-196 BDBM390564 US9957235, 2-196
US10442772, Example 2-196 BDBM390564 US9957235, 2-196 US10662186, Compound 196 US10988476, Compound I-196 BDBM444615
US10662186, Compound 196 US10988476, Compound I-196 BDBM444615 US11124504, Cpd. No. 196 BDBM450902 US10676469, Compound 196
US11124504, Cpd. No. 196 BDBM450902 US10676469, Compound 196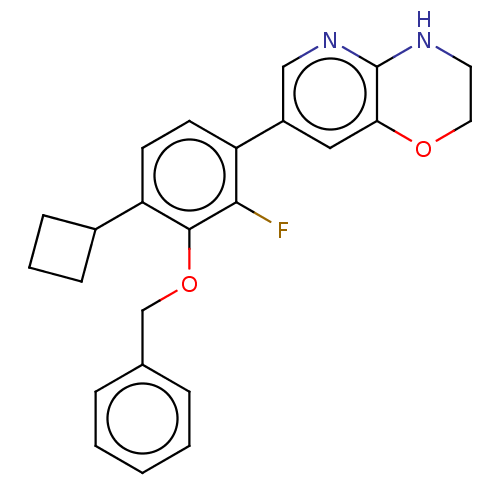 US9732093, Compound 196 BDBM167102 US9073876, 186 US9073876, 196
US9732093, Compound 196 BDBM167102 US9073876, 186 US9073876, 196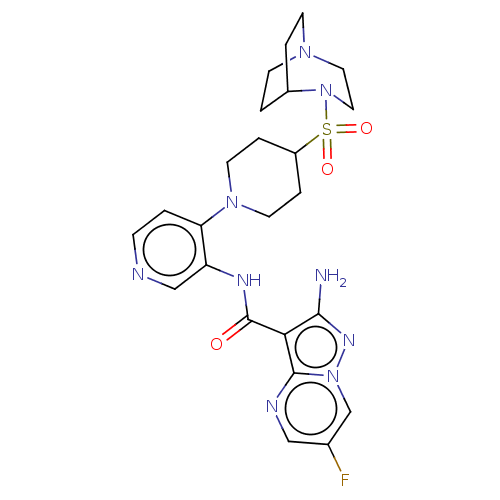 BDBM411763 US20230271963, Compound I-N-196 US10392391, Compound I-N-196 US10787452, Compound I-N-196 US11117900, Compound I-N-196 US11370798, Cmpd. # I-N-196
BDBM411763 US20230271963, Compound I-N-196 US10392391, Compound I-N-196 US10787452, Compound I-N-196 US11117900, Compound I-N-196 US11370798, Cmpd. # I-N-196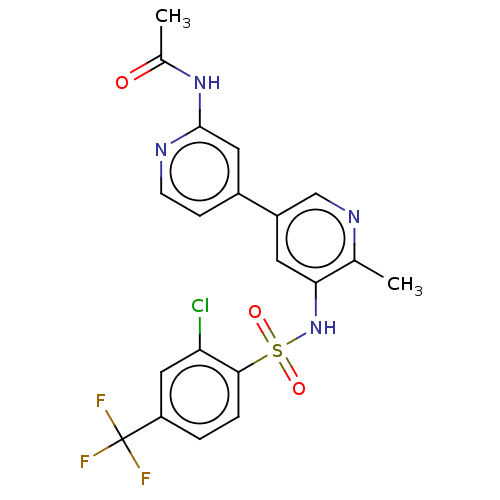 BDBM338020 US9751854, Compound I-196 US9802960, Compound I-196
BDBM338020 US9751854, Compound I-196 US9802960, Compound I-196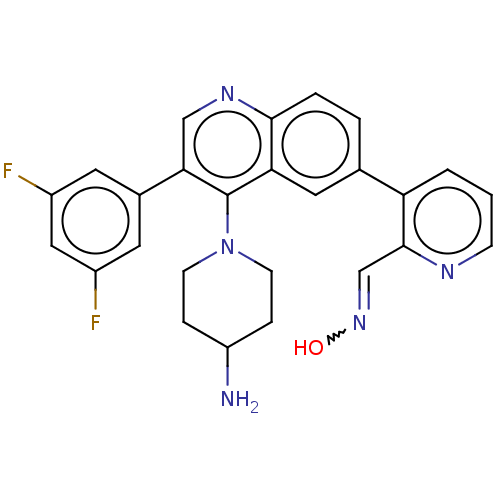 BDBM405727 US10597377, Compound 2-196 US10351547, Compound 2-196
BDBM405727 US10597377, Compound 2-196 US10351547, Compound 2-196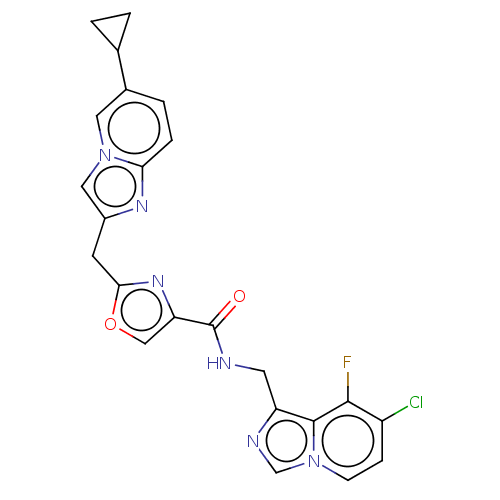 BDBM455178 US10730874, Compound I-196 US11352356, Compound I-196
BDBM455178 US10730874, Compound I-196 US11352356, Compound I-196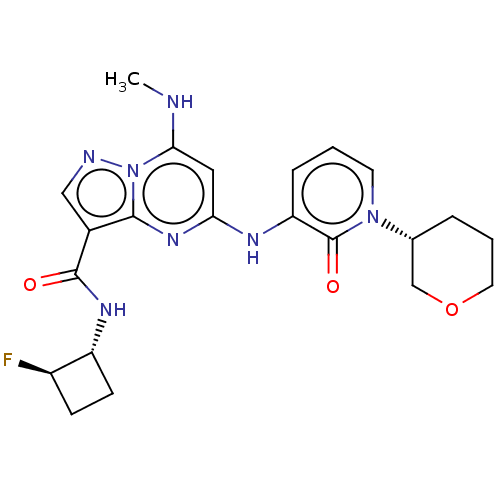 BDBM565630 US12221453, Compound I-196 US11414431, Compound I-196
BDBM565630 US12221453, Compound I-196 US11414431, Compound I-196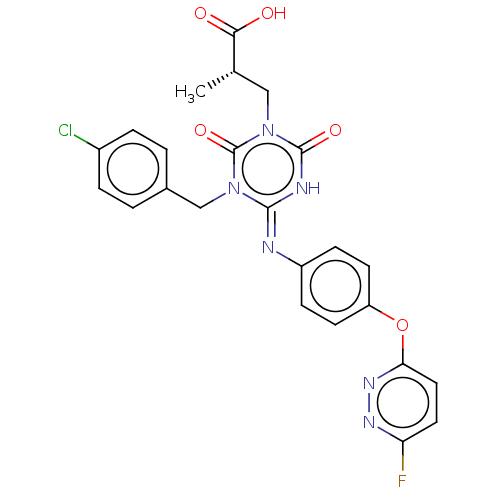 US10065941, Compound R-196 US9732060, Compound R-196 BDBM271881
US10065941, Compound R-196 US9732060, Compound R-196 BDBM271881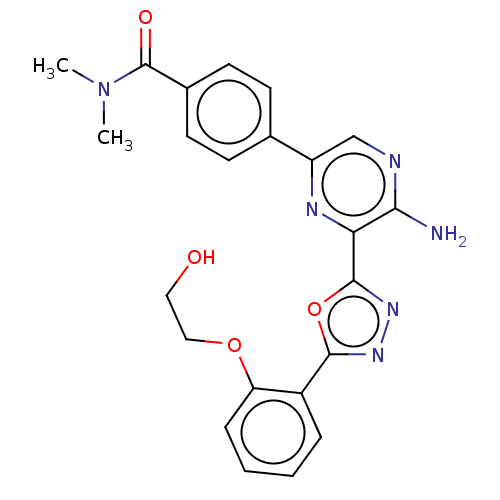 US10479784, Compound IA-196 BDBM420692 US10961232, Compound IA-196
US10479784, Compound IA-196 BDBM420692 US10961232, Compound IA-196 US10647713, Compound I-196 BDBM442235 US11396508, Compound I-196
US10647713, Compound I-196 BDBM442235 US11396508, Compound I-196 US11208415, Example I-196 BDBM429097 US10533010, Example I-196
US11208415, Example I-196 BDBM429097 US10533010, Example I-196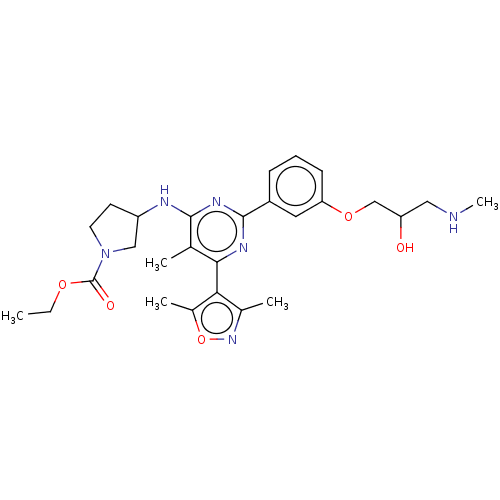 US20230279020, Example 196-1a BDBM439887 US10633389, Example 196-1a
US20230279020, Example 196-1a BDBM439887 US10633389, Example 196-1a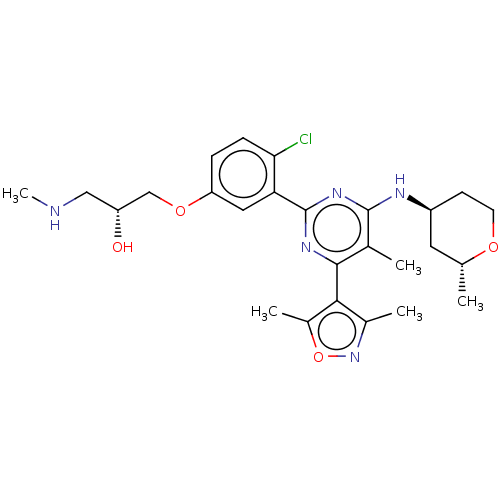 US20230279020, Example 196-3 BDBM440530 US10633389, Example 196-3
US20230279020, Example 196-3 BDBM440530 US10633389, Example 196-3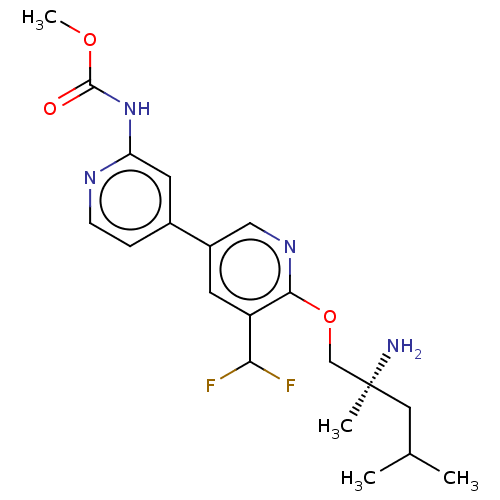 (S)-methyl (6-((2-amino-2,4-dimethylpentyl)oxy)-5-(difluoromethyl)-[3,4′-bipyridin]-2′-yl)carbamate US9902722, Example 196 US10351563, Example 196 US10155760, Example 196 BDBM311339 US10544120, Example 196 US10981910, Example 196 US10723734, Example 196
(S)-methyl (6-((2-amino-2,4-dimethylpentyl)oxy)-5-(difluoromethyl)-[3,4′-bipyridin]-2′-yl)carbamate US9902722, Example 196 US10351563, Example 196 US10155760, Example 196 BDBM311339 US10544120, Example 196 US10981910, Example 196 US10723734, Example 196 BDBM103219 US8541407, 196
BDBM103219 US8541407, 196 BDBM104417 US8575186, 196
BDBM104417 US8575186, 196 BDBM104776 US8569308, 196
BDBM104776 US8569308, 196 BDBM112198 US8623889, 196
BDBM112198 US8623889, 196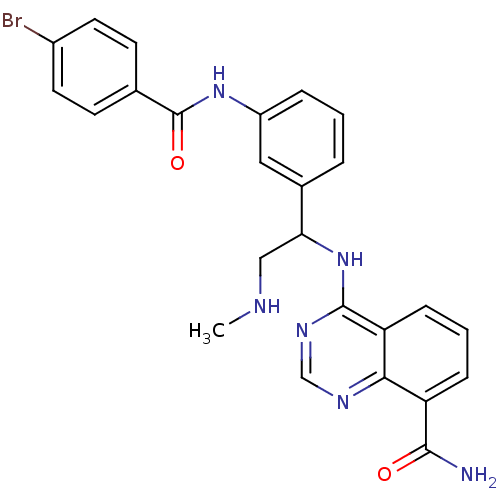 BDBM116513 US8637532, 196
BDBM116513 US8637532, 196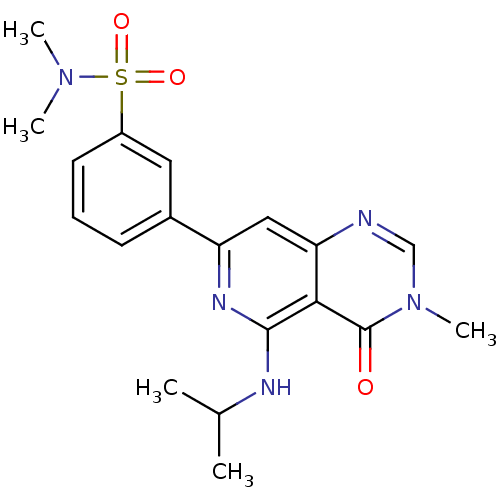 BDBM121757 US8722692, 196
BDBM121757 US8722692, 196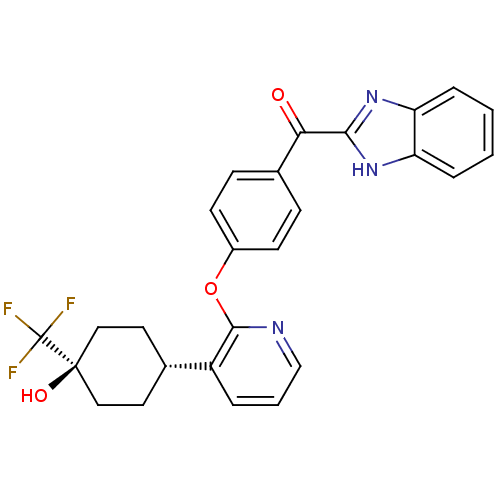 BDBM124519 US8759532, 196
BDBM124519 US8759532, 196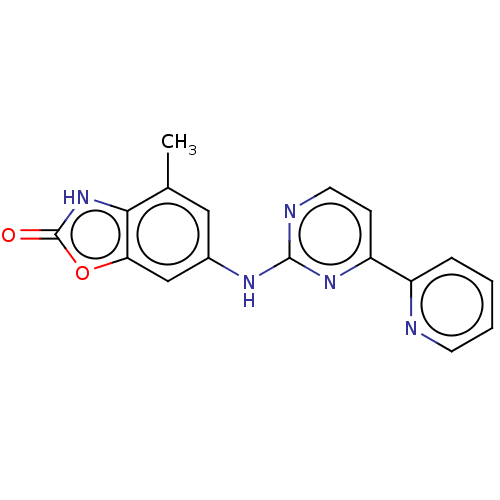 BDBM125801 US8772305, 196
BDBM125801 US8772305, 196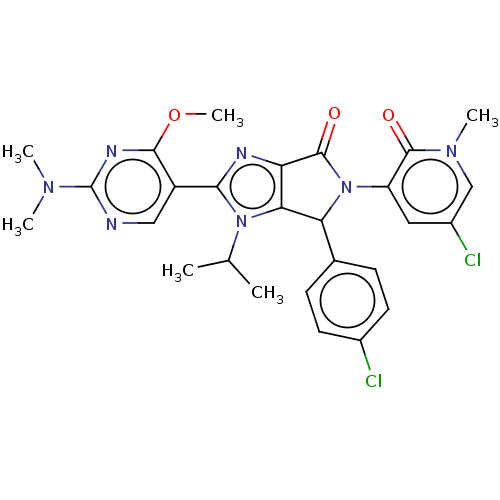 BDBM129915 US8815926, 196
BDBM129915 US8815926, 196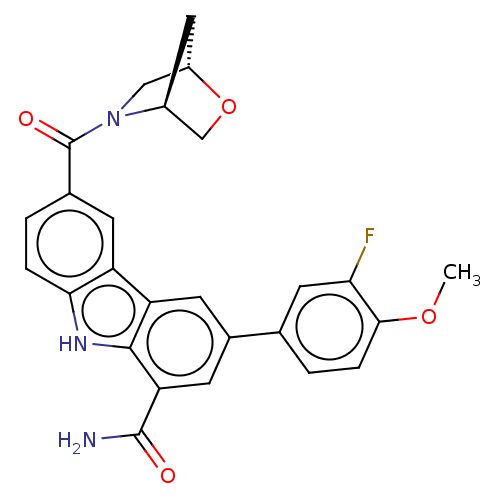 BDBM130290 US8815840, 196
BDBM130290 US8815840, 196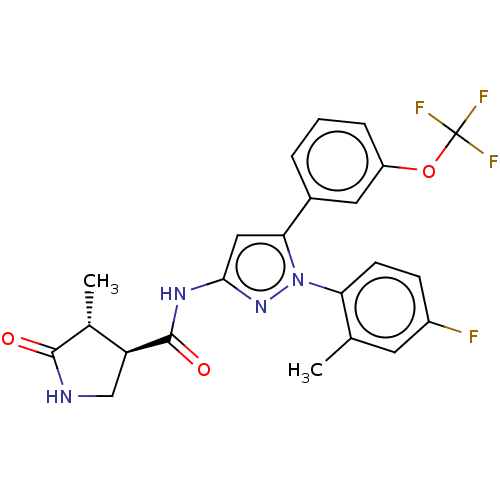 BDBM132918 US8846746, 196
BDBM132918 US8846746, 196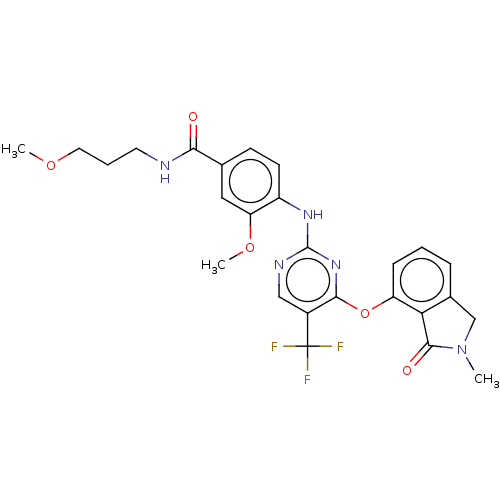 BDBM134194 US8846689, 196
BDBM134194 US8846689, 196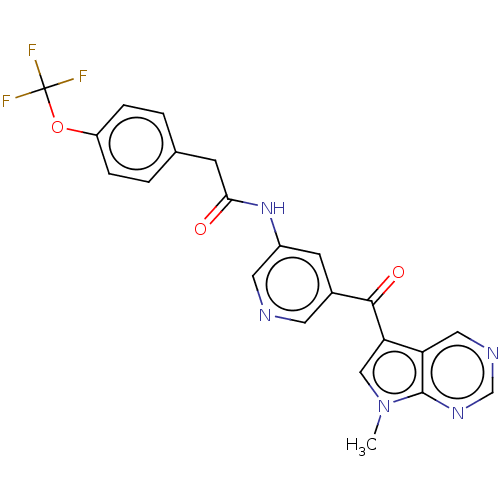 BDBM134733 US8846698, 196
BDBM134733 US8846698, 196 BDBM135544 US8853258, 196
BDBM135544 US8853258, 196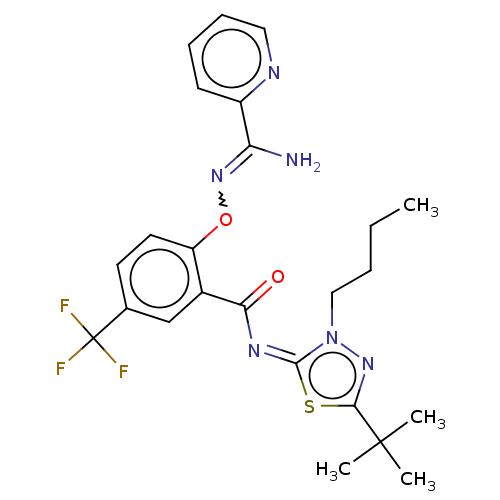 BDBM135907 US8859596, 196
BDBM135907 US8859596, 196 BDBM13736 US8846929, 196
BDBM13736 US8846929, 196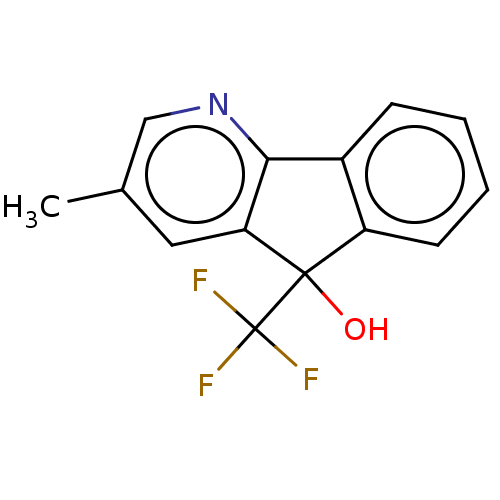 BDBM137603 US8871934, 196
BDBM137603 US8871934, 196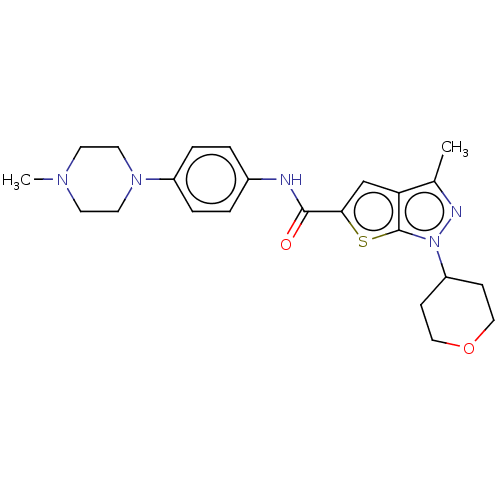 BDBM140102 US8901315, 196
BDBM140102 US8901315, 196 BDBM146485 US8957068, 196
BDBM146485 US8957068, 196 BDBM148470 US8962637, 196
BDBM148470 US8962637, 196 BDBM149017 US8962648, 196
BDBM149017 US8962648, 196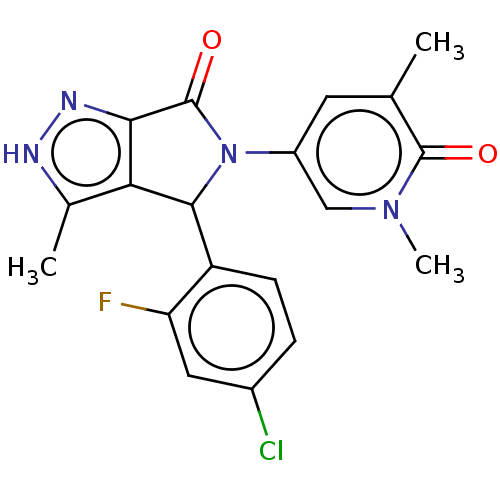 BDBM150128 US8975417, 196
BDBM150128 US8975417, 196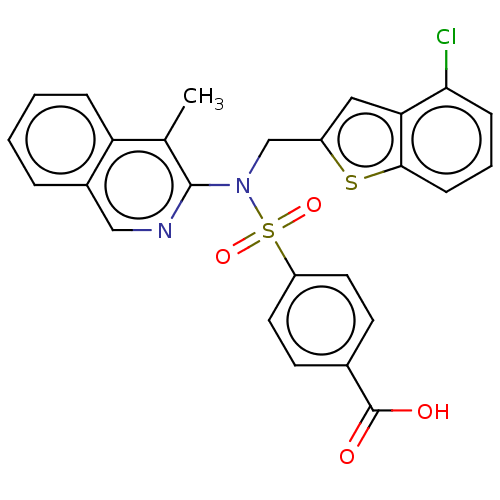 BDBM151760 US8987445, 196
BDBM151760 US8987445, 196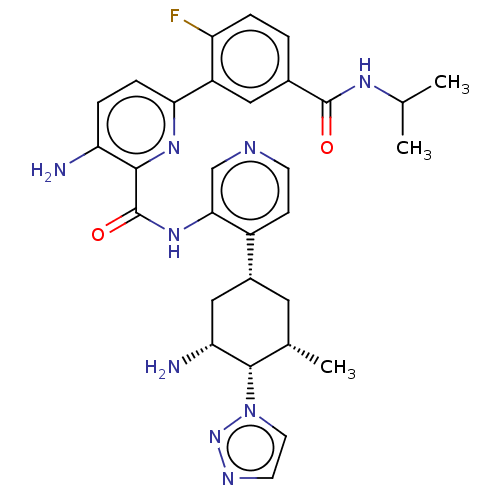 BDBM152002 US8987457, 196
BDBM152002 US8987457, 196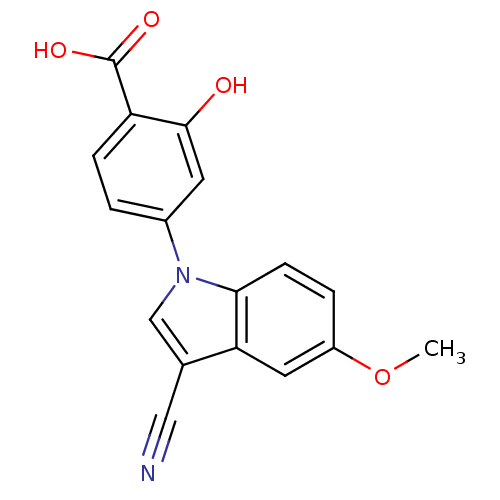 BDBM153050 US8993616, 196
BDBM153050 US8993616, 196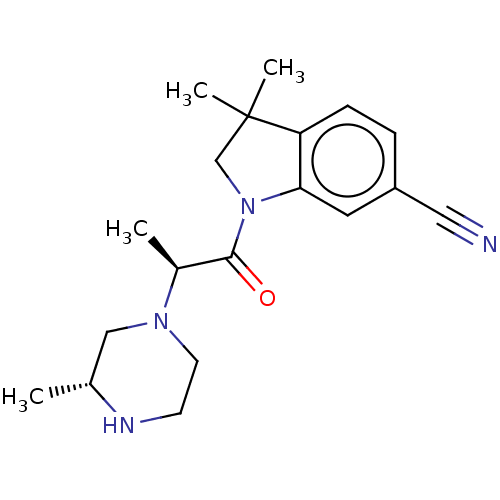 BDBM156145 US9018214, 196
BDBM156145 US9018214, 196 BDBM157355 US9023865, 196
BDBM157355 US9023865, 196 BDBM162238 US9051280, 196
BDBM162238 US9051280, 196 BDBM164719 US9067871, 196
BDBM164719 US9067871, 196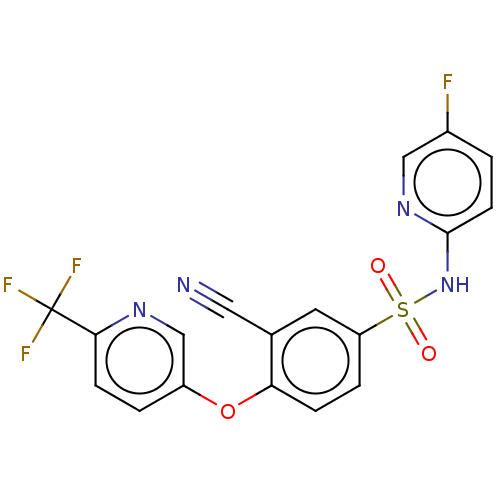 BDBM165857 US9067922, 196
BDBM165857 US9067922, 196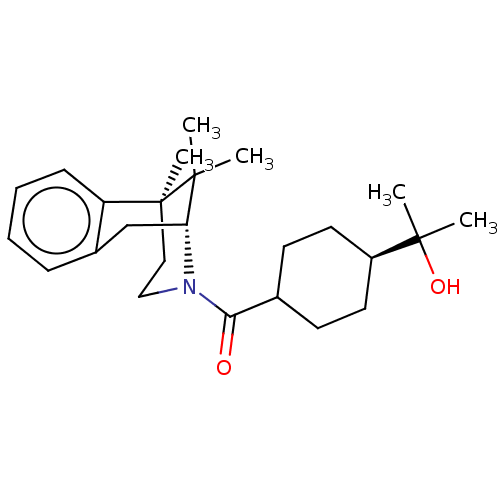 BDBM166900 US9073870, 196
BDBM166900 US9073870, 196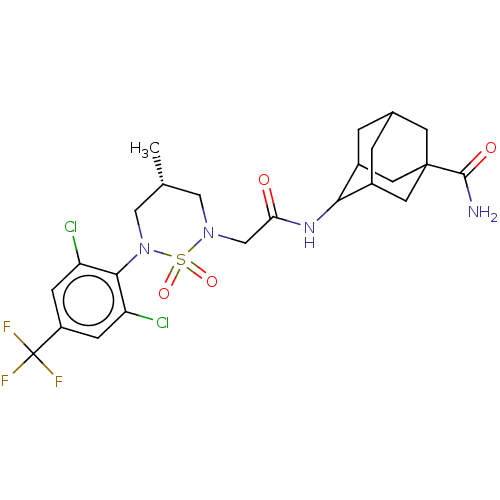 BDBM167451 US9073906, 196
BDBM167451 US9073906, 196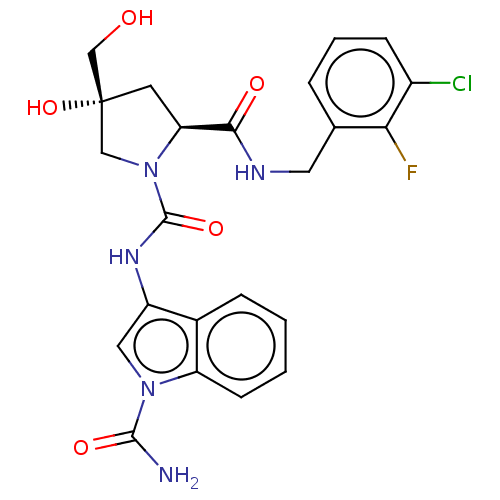 BDBM170768 US9085555, 196
BDBM170768 US9085555, 196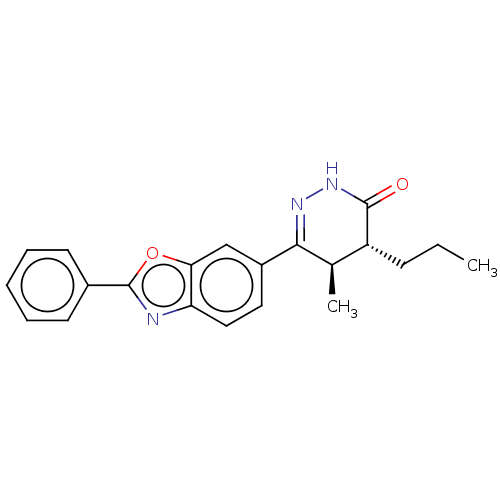 BDBM180630 US8772323, 196
BDBM180630 US8772323, 196 BDBM187646 US9169252, 196
BDBM187646 US9169252, 196 BDBM188930 US9169260, 196
BDBM188930 US9169260, 196 BDBM196091 US9206199, 196
BDBM196091 US9206199, 196 BDBM200205 US9234000, 196
BDBM200205 US9234000, 196 BDBM204486 US9242996, 196
BDBM204486 US9242996, 196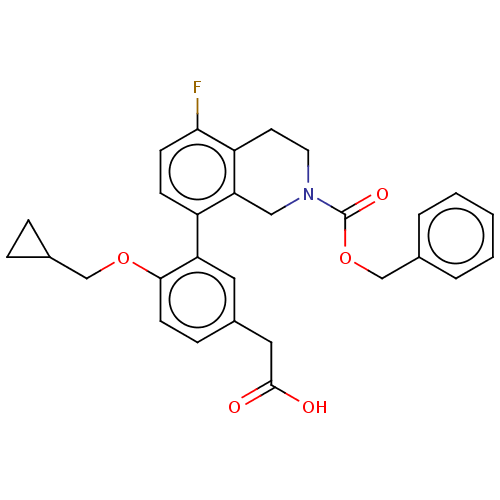 BDBM205727 US9255090, 196
BDBM205727 US9255090, 196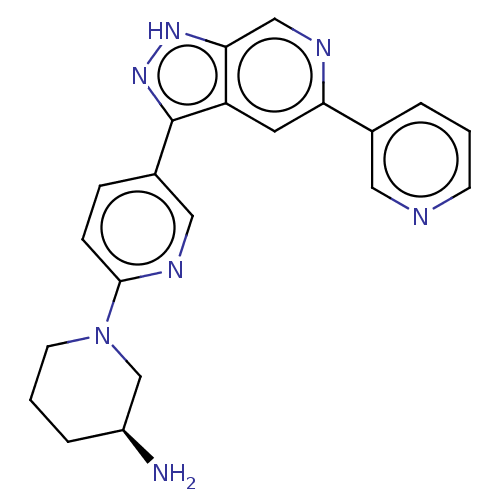 BDBM206450 US9260425, 196
BDBM206450 US9260425, 196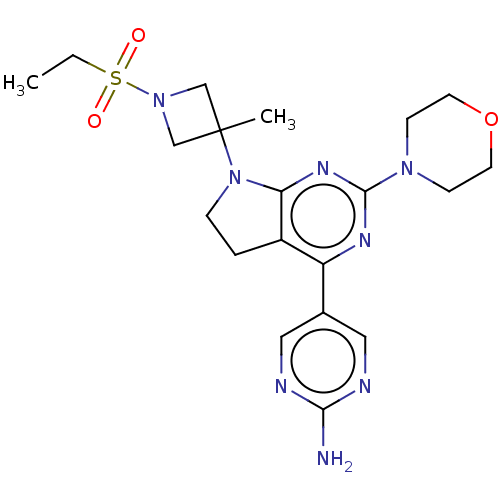 BDBM207219 US9260439, 196
BDBM207219 US9260439, 196 BDBM210484 US9290451, 196
BDBM210484 US9290451, 196 BDBM224936 US9321756, 196
BDBM224936 US9321756, 196 BDBM228474 US9556135, 196
BDBM228474 US9556135, 196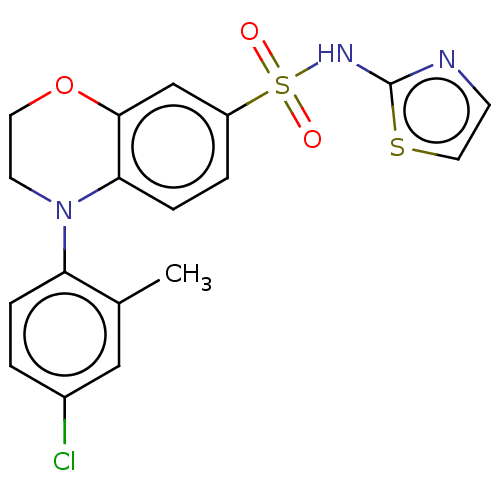 BDBM231449 US9346798, 196
BDBM231449 US9346798, 196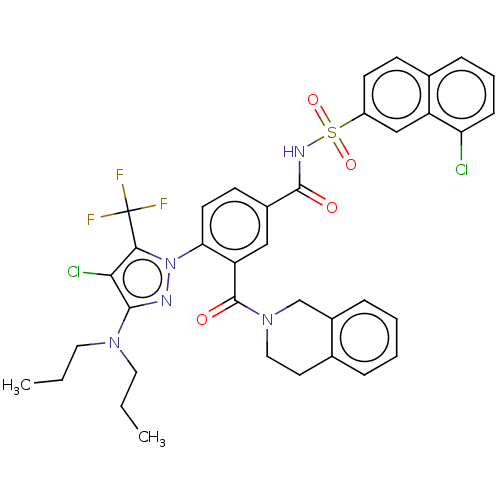 BDBM232429 US9346795, 196
BDBM232429 US9346795, 196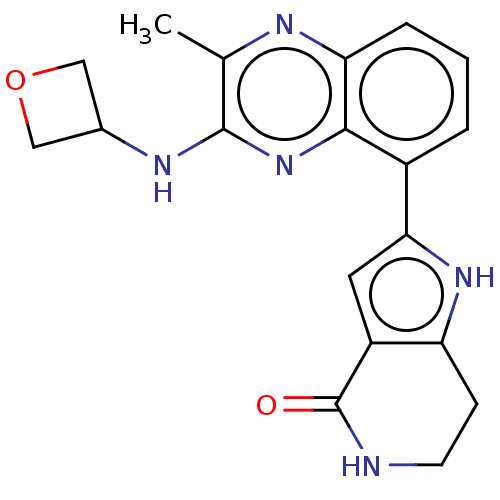 BDBM238550 US9394297, 196
BDBM238550 US9394297, 196 BDBM247935 US9434719, 196
BDBM247935 US9434719, 196 BDBM250892 US9452986, 196
BDBM250892 US9452986, 196 BDBM253236 US9458171, 196
BDBM253236 US9458171, 196 BDBM254871 US9493412, 196
BDBM254871 US9493412, 196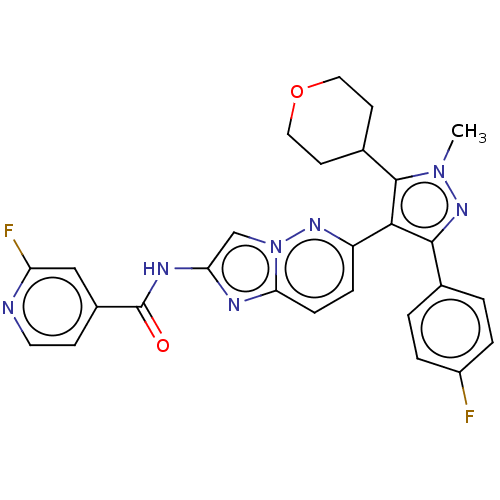 BDBM255237 US9475817, 196
BDBM255237 US9475817, 196 BDBM255445 US9481682, 196
BDBM255445 US9481682, 196 BDBM256018 US9481672, 196
BDBM256018 US9481672, 196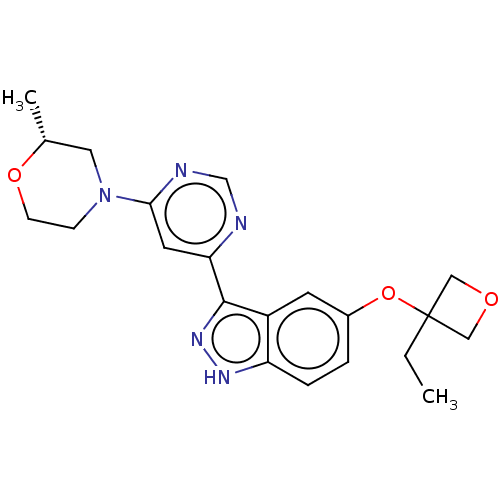 BDBM257352 US9493440, 196
BDBM257352 US9493440, 196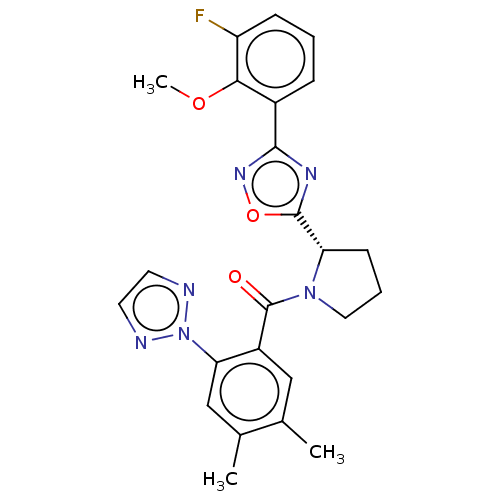 BDBM257727 US9493446, 196
BDBM257727 US9493446, 196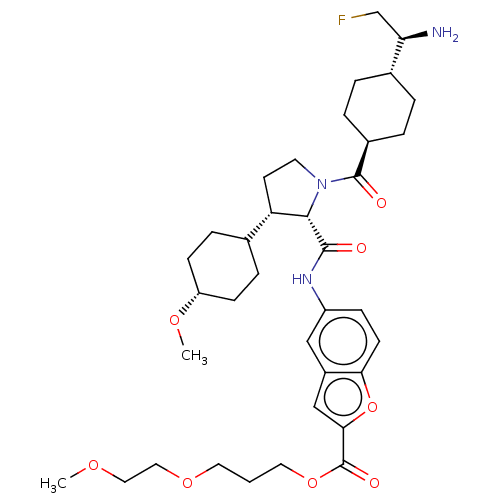 BDBM339923 US9758480, 196
BDBM339923 US9758480, 196 BDBM393616 US09969700, 196
BDBM393616 US09969700, 196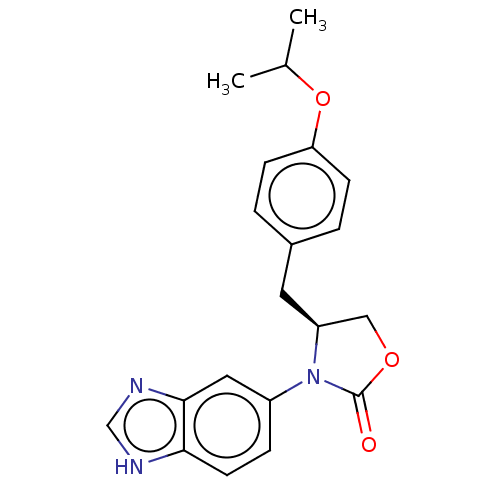 BDBM64992 US8486940, 196
BDBM64992 US8486940, 196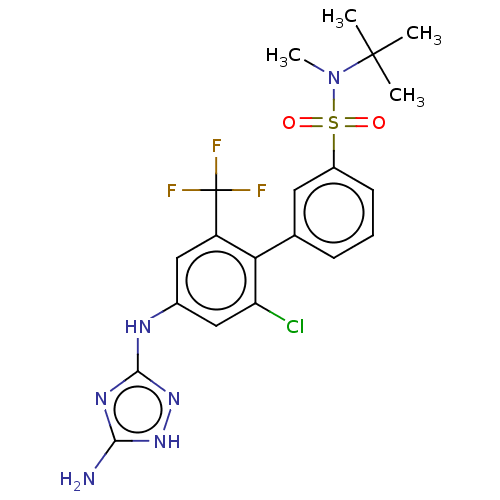 BDBM70672 US9693997, 196
BDBM70672 US9693997, 196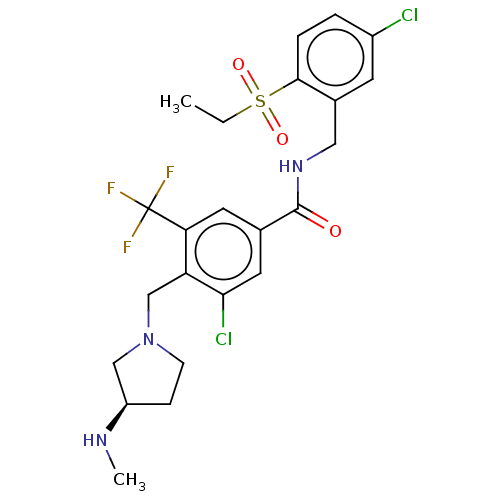 BDBM88308 US9695118, 196
BDBM88308 US9695118, 196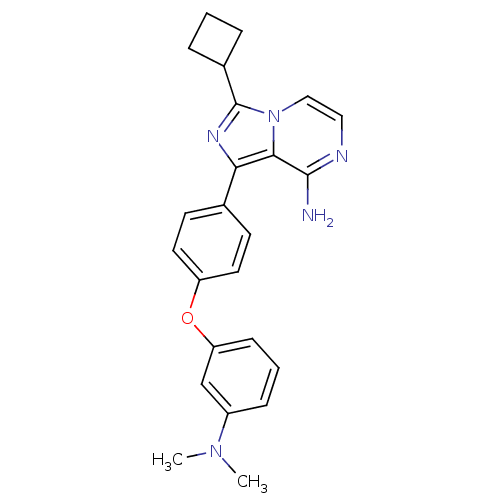 BDBM98384 US8481733, 196
BDBM98384 US8481733, 196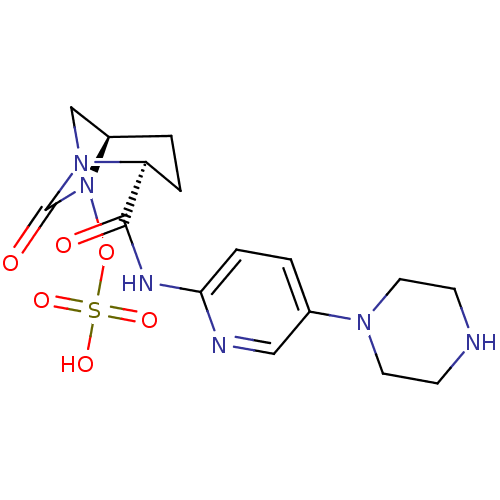 BDBM98806 US8487093, 196
BDBM98806 US8487093, 196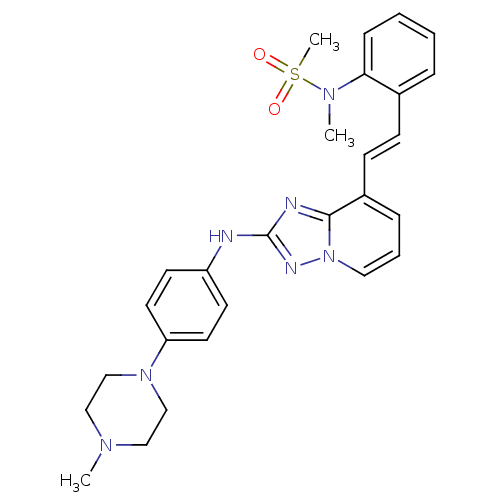 BDBM99695 US8501936, 196
BDBM99695 US8501936, 196 US8497265, 196 BDBM98870
US8497265, 196 BDBM98870 US8530648, 196 BDBM102259
US8530648, 196 BDBM102259 US8604061, 196 BDBM109459
US8604061, 196 BDBM109459 US8653100, 196 BDBM141302
US8653100, 196 BDBM141302 US8669266, 196 BDBM119417
US8669266, 196 BDBM119417 US8680275, 196 BDBM119639
US8680275, 196 BDBM119639 US8772480, 196 BDBM125366
US8772480, 196 BDBM125366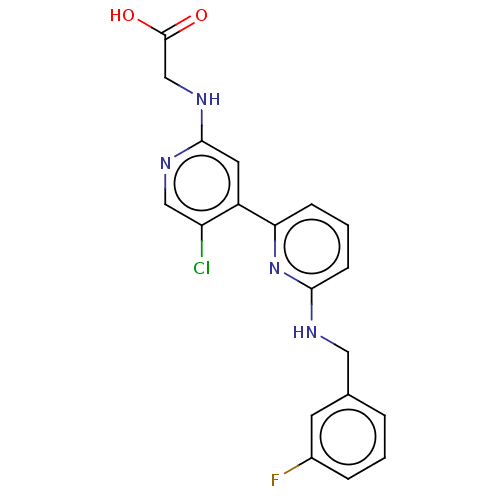 US8778951, 196 BDBM126386
US8778951, 196 BDBM126386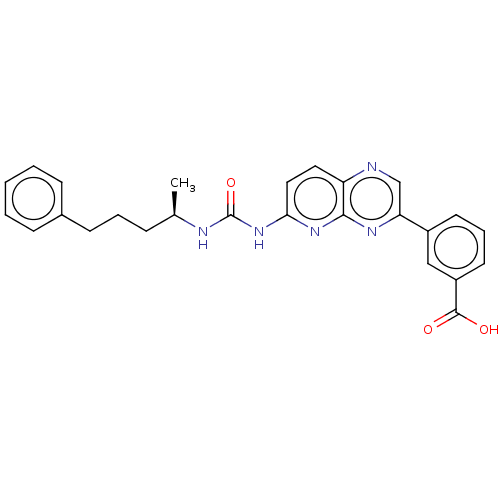 US8791118, 196 BDBM127622
US8791118, 196 BDBM127622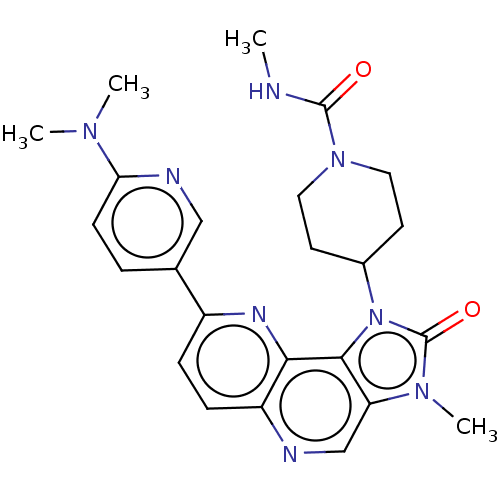 US8791131, 196 BDBM127422
US8791131, 196 BDBM127422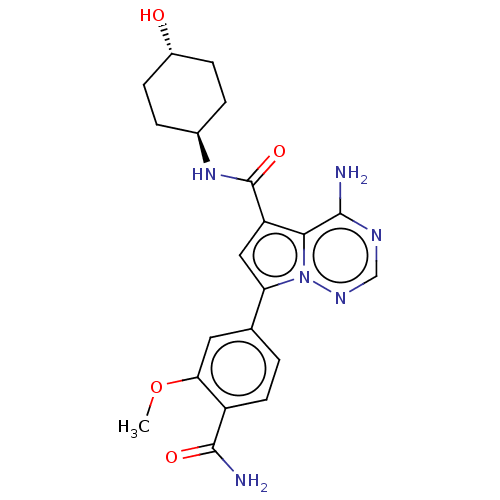 US8791257, 196 BDBM127341
US8791257, 196 BDBM127341 US8802673, 196 BDBM129554
US8802673, 196 BDBM129554 US8841312, 196 BDBM132449
US8841312, 196 BDBM132449 US8846719, 196 BDBM133507
US8846719, 196 BDBM133507 US8912224, 196 BDBM140764
US8912224, 196 BDBM140764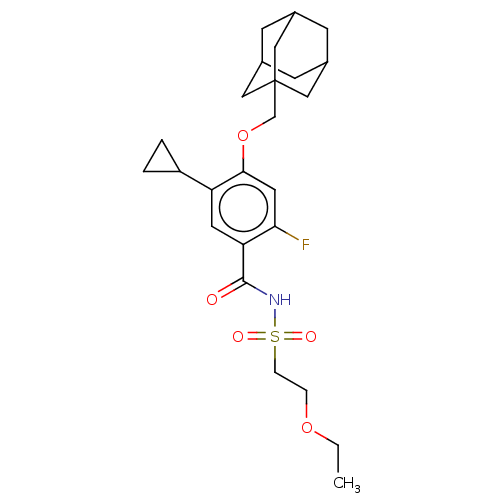 US8952169, 196 BDBM145417
US8952169, 196 BDBM145417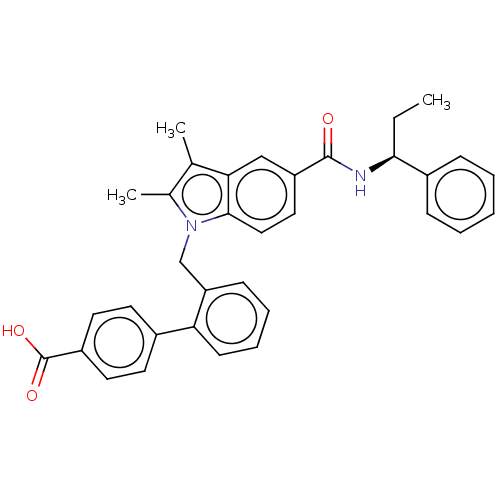 US8957093, 196 BDBM147359
US8957093, 196 BDBM147359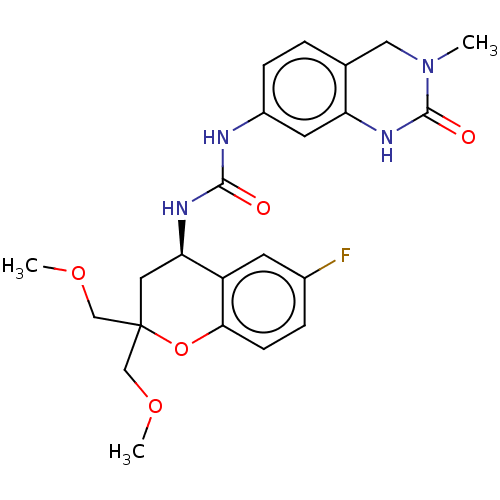 US8969325, 196 BDBM143995
US8969325, 196 BDBM143995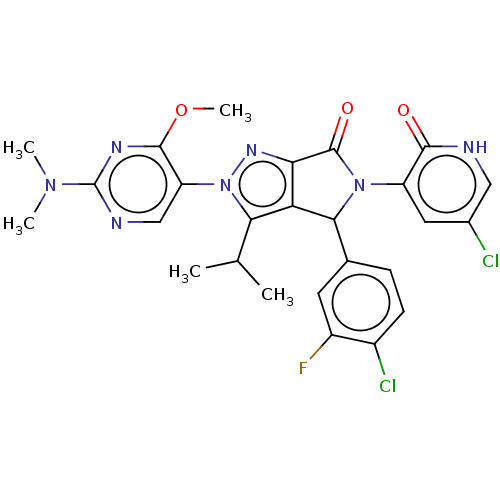 US8969341, 196 BDBM143723
US8969341, 196 BDBM143723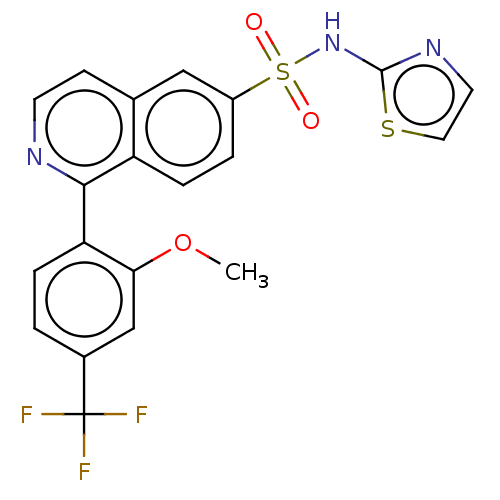 US9012443, 196 BDBM154187
US9012443, 196 BDBM154187 US9012651, 196 BDBM155759
US9012651, 196 BDBM155759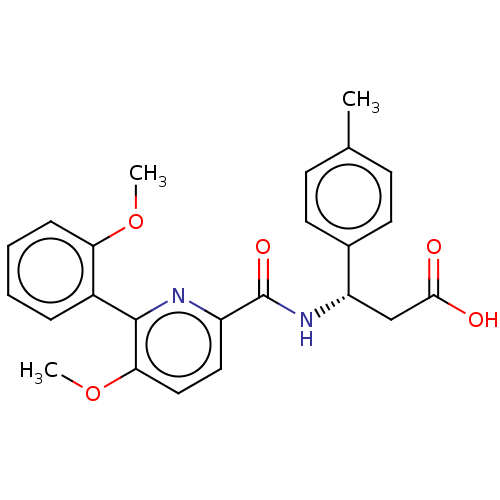 US9029559, 196 BDBM156769
US9029559, 196 BDBM156769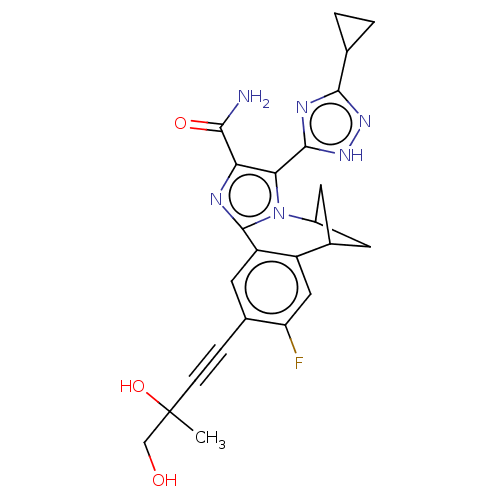 US9034866, 196 BDBM158721
US9034866, 196 BDBM158721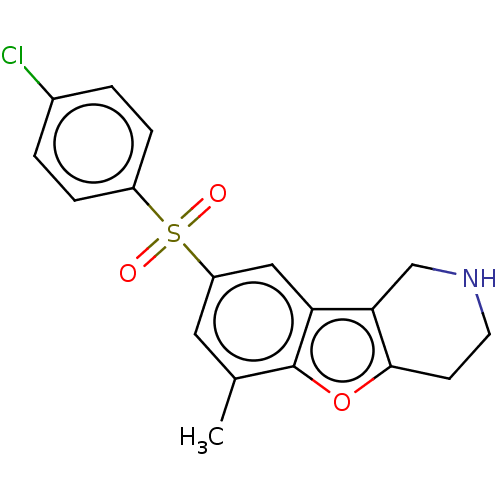 US9067949, 196 BDBM166339
US9067949, 196 BDBM166339 US9073876, 196 BDBM167112
US9073876, 196 BDBM167112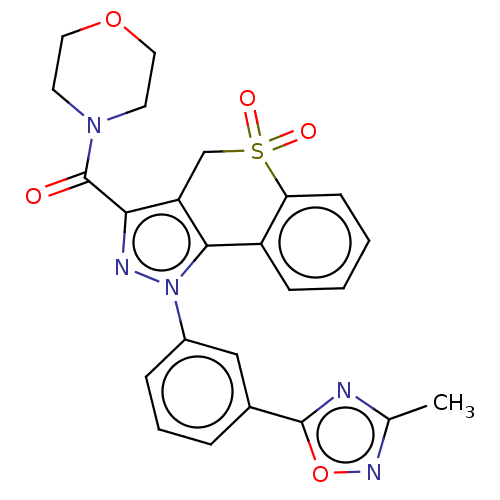 US9073940, 196 BDBM169847
US9073940, 196 BDBM169847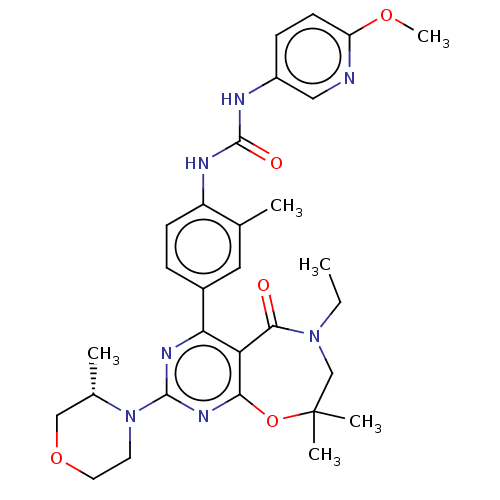 US9120812, 196 BDBM178411
US9120812, 196 BDBM178411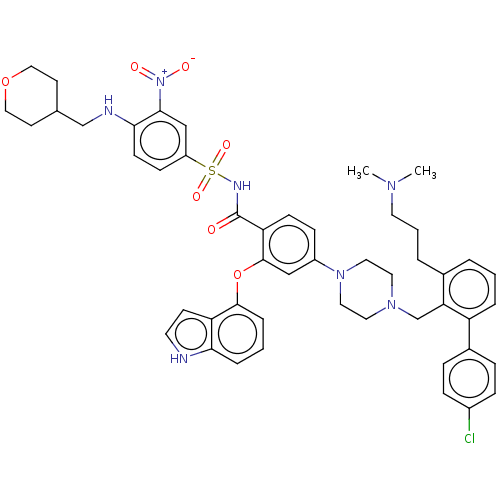 US9125913, 196 BDBM178631
US9125913, 196 BDBM178631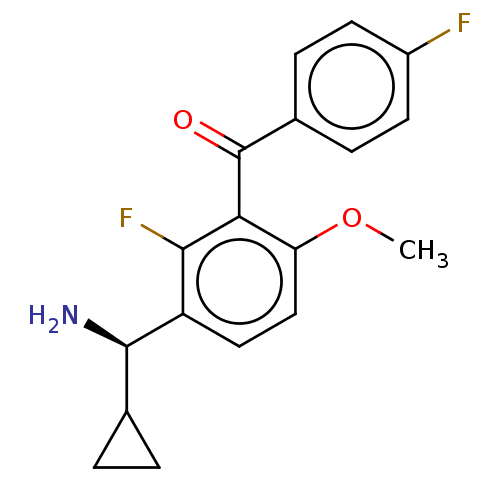 US9145354, 196 BDBM183558
US9145354, 196 BDBM183558 US9145380, 196 BDBM181993
US9145380, 196 BDBM181993 US9145392, 196 BDBM182495
US9145392, 196 BDBM182495 US9163021, 196 BDBM186529
US9163021, 196 BDBM186529 US9169246, 196 BDBM188656
US9169246, 196 BDBM188656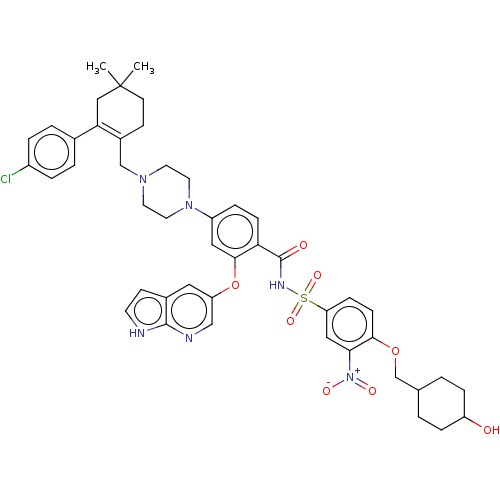 US9174982, 196 BDBM189633
US9174982, 196 BDBM189633 US9175003, 196 BDBM190033
US9175003, 196 BDBM190033 US9181272, 196 BDBM191426
US9181272, 196 BDBM191426 US9187424, 196 BDBM201430
US9187424, 196 BDBM201430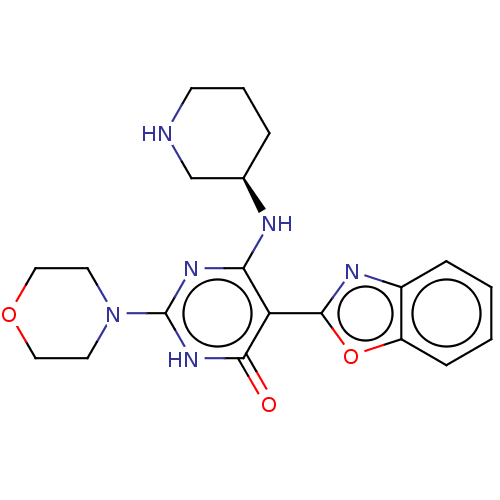 US9221809, 196 BDBM203638
US9221809, 196 BDBM203638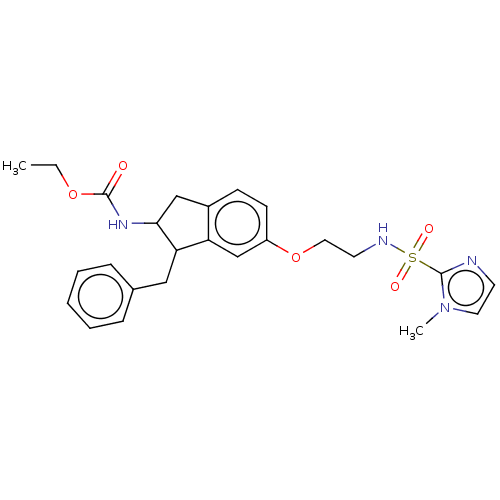 US9227930, 196 BDBM199949
US9227930, 196 BDBM199949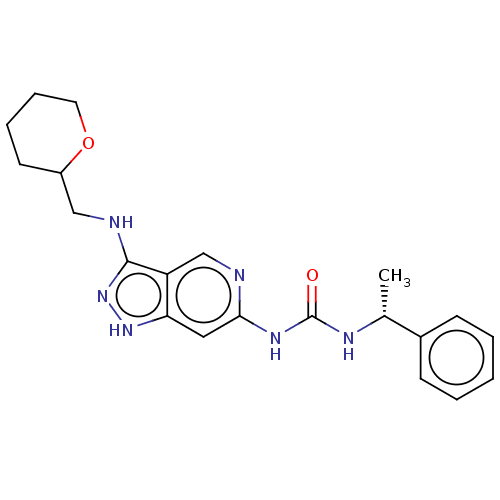 US9233979, 196 BDBM201765
US9233979, 196 BDBM201765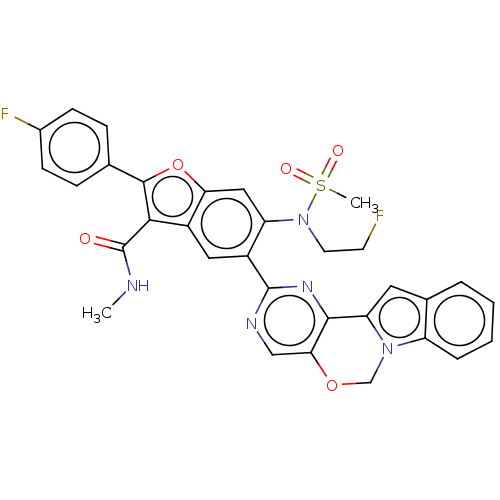 US9265773, 196 BDBM208251
US9265773, 196 BDBM208251 US9266876, 196 BDBM208729
US9266876, 196 BDBM208729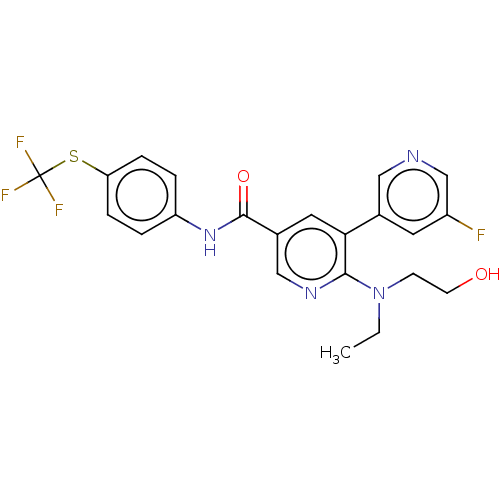 US9278981, 196 BDBM213604
US9278981, 196 BDBM213604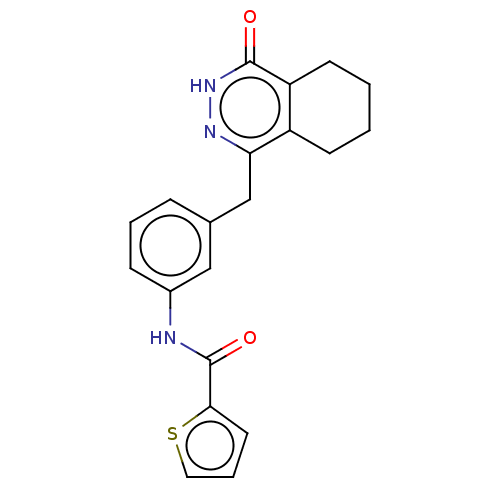 US9283222, 196 BDBM214134
US9283222, 196 BDBM214134 US9296734, 196 BDBM215003
US9296734, 196 BDBM215003 US9296741, 196 BDBM220607
US9296741, 196 BDBM220607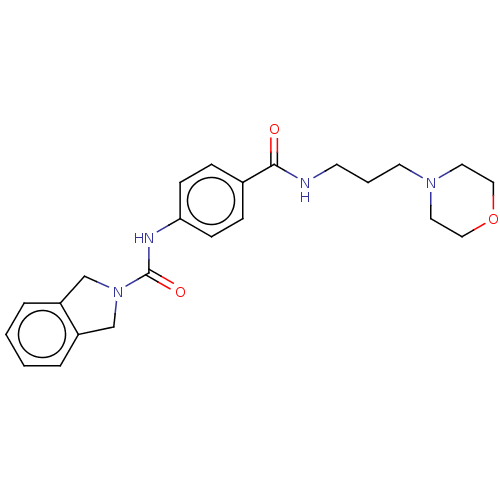 US9302989, 196 BDBM215996
US9302989, 196 BDBM215996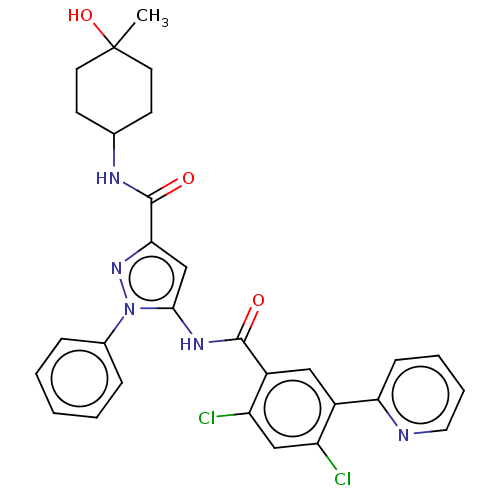 US9328096, 196 BDBM226503
US9328096, 196 BDBM226503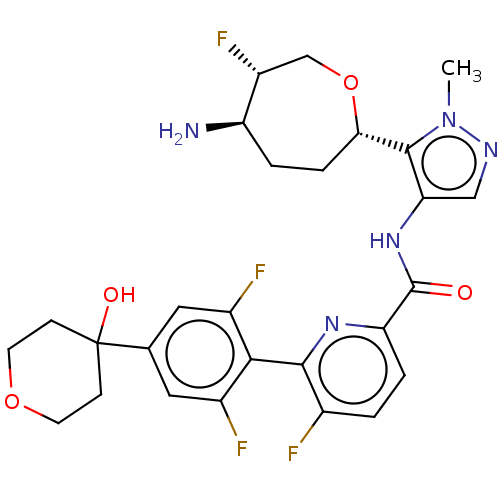 US9328106, 196 BDBM227245
US9328106, 196 BDBM227245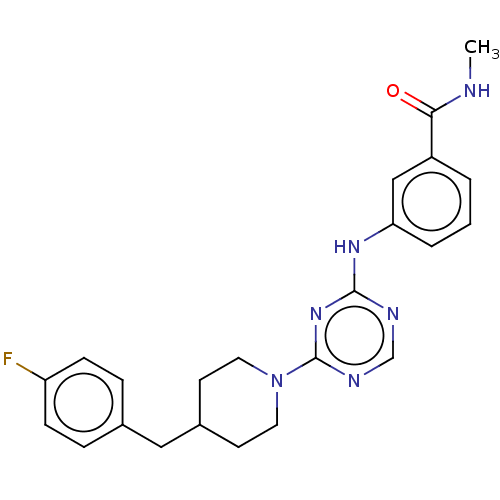 US9334269, 196 BDBM229869
US9334269, 196 BDBM229869 US9340517, 196 BDBM231809
US9340517, 196 BDBM231809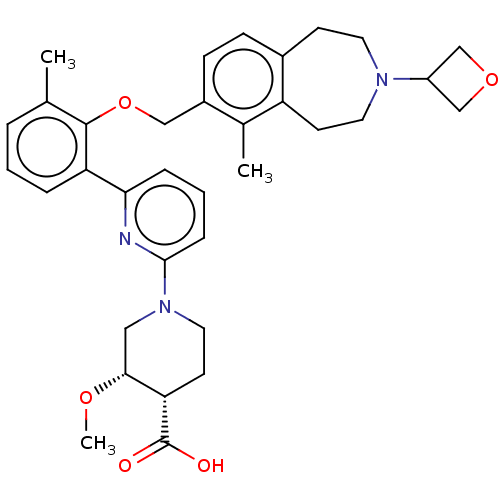 US9353090, 196 BDBM234068
US9353090, 196 BDBM234068 US9365576, 196 BDBM236443
US9365576, 196 BDBM236443 US9409866, 196 BDBM240481
US9409866, 196 BDBM240481 US9428502, 196 BDBM244587
US9428502, 196 BDBM244587 US9434711, 196 BDBM248342
US9434711, 196 BDBM248342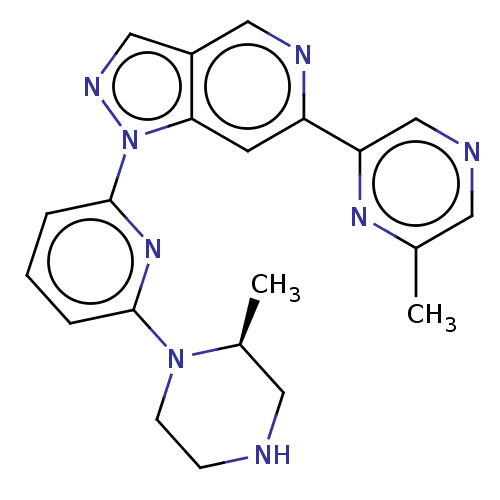 US9434725, 196 BDBM248965
US9434725, 196 BDBM248965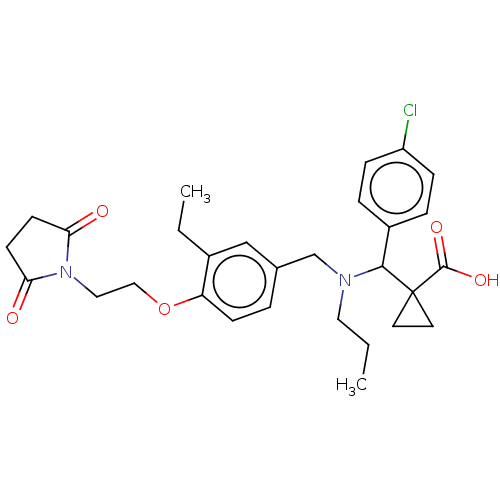 US9447038, 196 BDBM249768
US9447038, 196 BDBM249768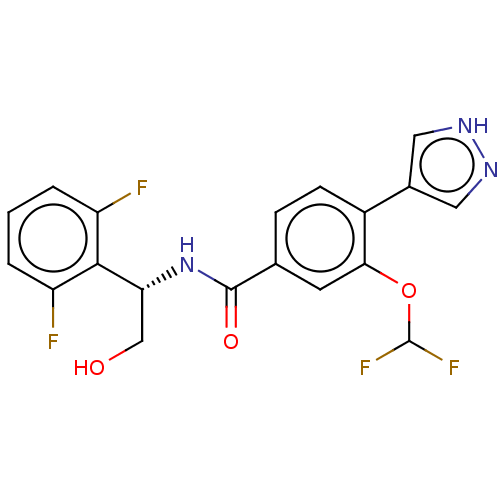 US9458110, 196 BDBM252124
US9458110, 196 BDBM252124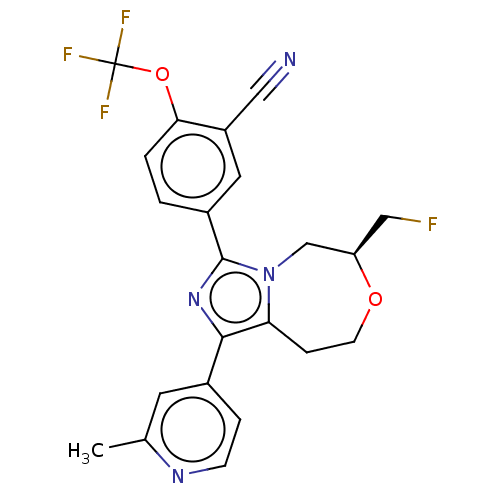 US9458176, 196 BDBM252799
US9458176, 196 BDBM252799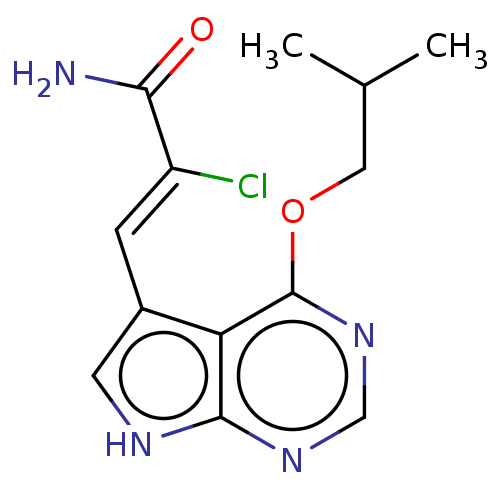 US9505765, 196 BDBM254972
US9505765, 196 BDBM254972 US9598415, 196 BDBM302284
US9598415, 196 BDBM302284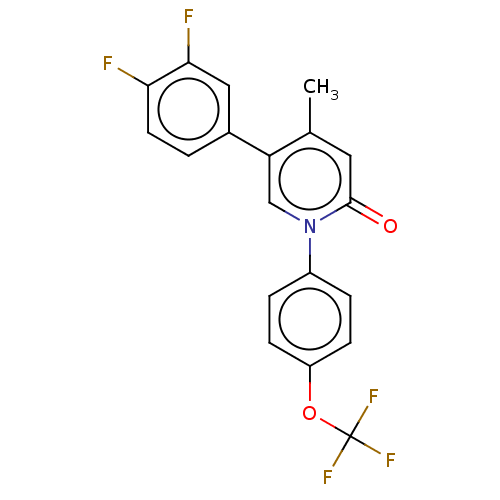 US9675593, 196 BDBM169539
US9675593, 196 BDBM169539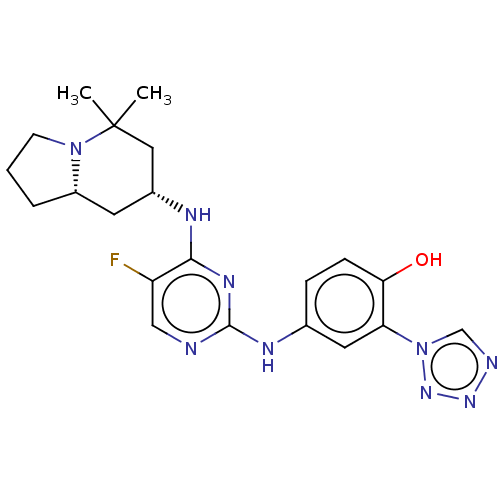 US9682976, 196 BDBM161758
US9682976, 196 BDBM161758 US9688629, 196 BDBM165585
US9688629, 196 BDBM165585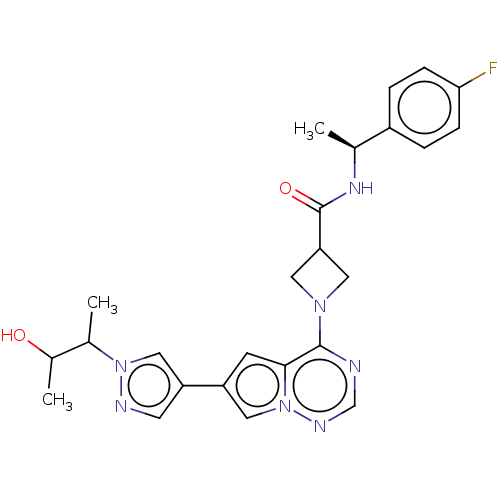 US9688680, 196 BDBM175649
US9688680, 196 BDBM175649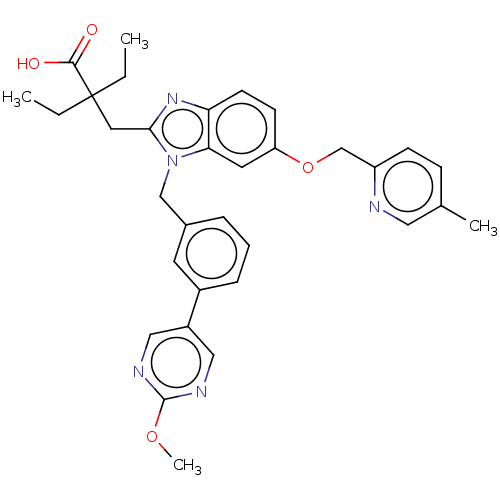 US9695149, 196 BDBM65679
US9695149, 196 BDBM65679 US10189845, Example 196A BDBM325534 US10730880, Example 196 US11028093, Example 196
US10189845, Example 196A BDBM325534 US10730880, Example 196 US11028093, Example 196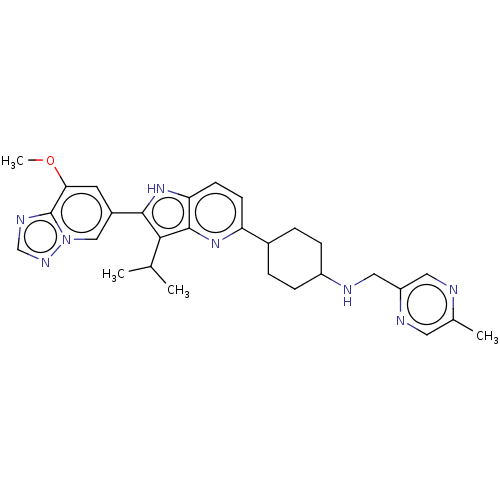 US10544143, Example 190 US10730877, Example 196 US11053244, Example 196 BDBM427757
US10544143, Example 190 US10730877, Example 196 US11053244, Example 196 BDBM427757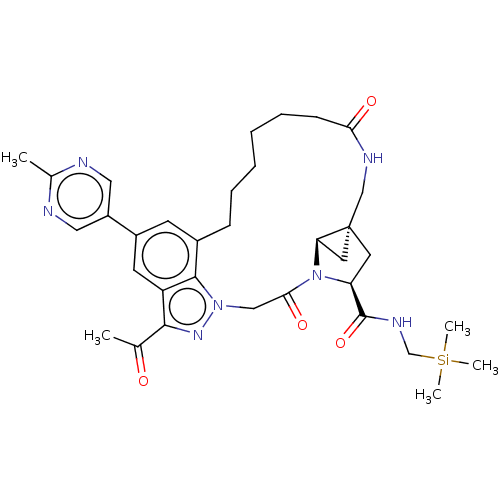 US11718626, Compound T-196 US11053253, Cmp No. T-196 BDBM516775
US11718626, Compound T-196 US11053253, Cmp No. T-196 BDBM516775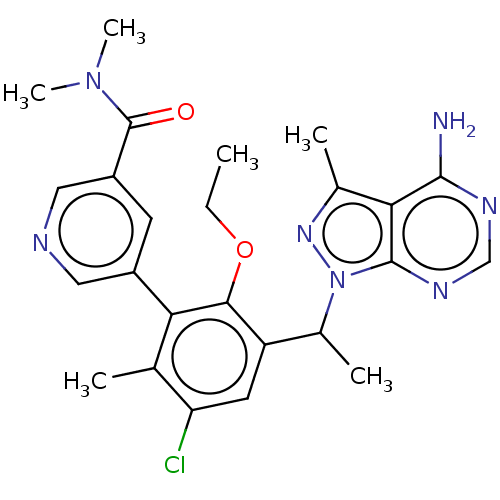 BDBM261173 US10092570, Example 196 US11433071, Example 196 US9730939, Example 196 US10646492, Example 196 US9707233, 196 US10376513, Example 196 5-(3-(1-(4-amino-3-methyl-1H- pyrazolo[3,4-d]pyrimidin-1- yl)ethyl)-5-chloro-2-ethoxy-6- methylphenyl)-N,N- dimethylnicotinamide3
BDBM261173 US10092570, Example 196 US11433071, Example 196 US9730939, Example 196 US10646492, Example 196 US9707233, 196 US10376513, Example 196 5-(3-(1-(4-amino-3-methyl-1H- pyrazolo[3,4-d]pyrimidin-1- yl)ethyl)-5-chloro-2-ethoxy-6- methylphenyl)-N,N- dimethylnicotinamide3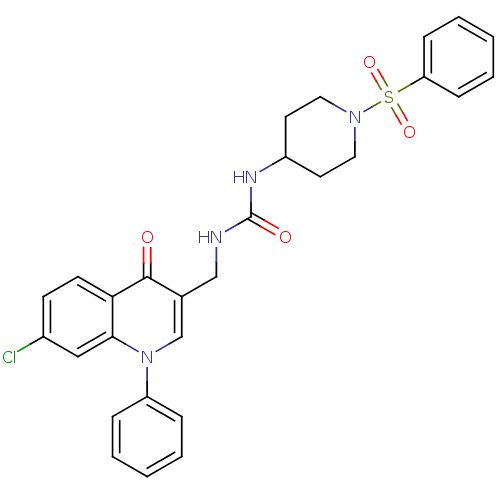 BDBM100636 US8501732, I-196
BDBM100636 US8501732, I-196 BDBM106283 US8575197, II-196
BDBM106283 US8575197, II-196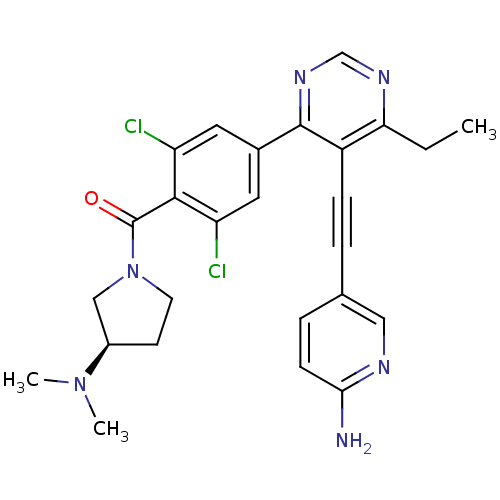 BDBM116047 US8633183, E-196
BDBM116047 US8633183, E-196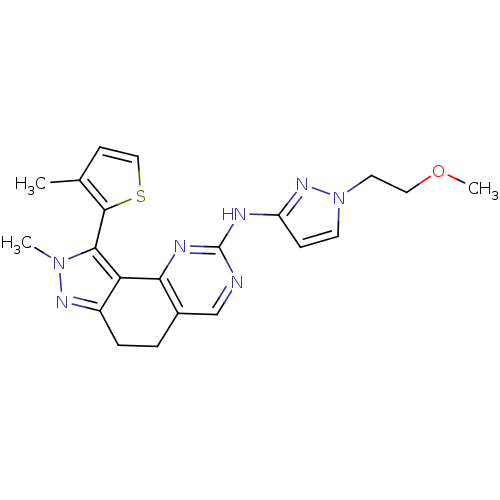 BDBM122726 US8735386, I-196
BDBM122726 US8735386, I-196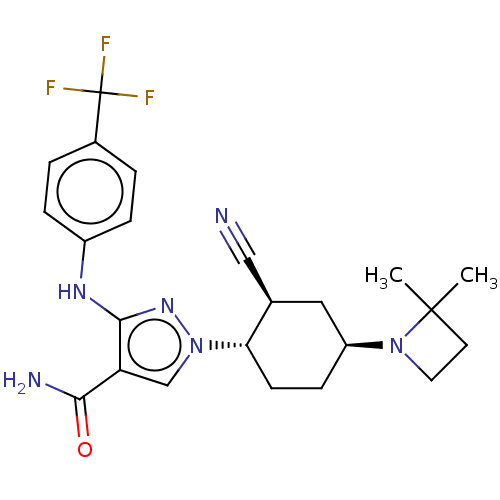 BDBM147872 US8962608, 28-196
BDBM147872 US8962608, 28-196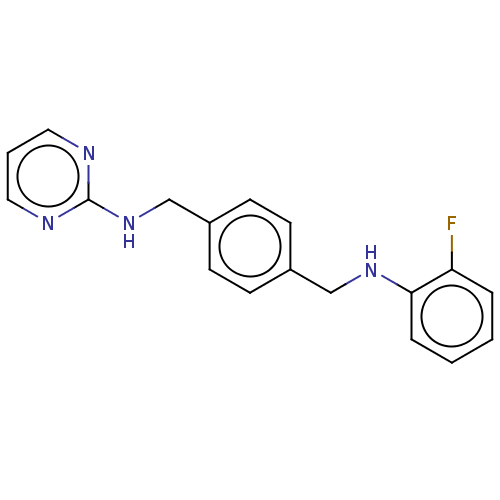 BDBM194564 US9205085, MSX- 196
BDBM194564 US9205085, MSX- 196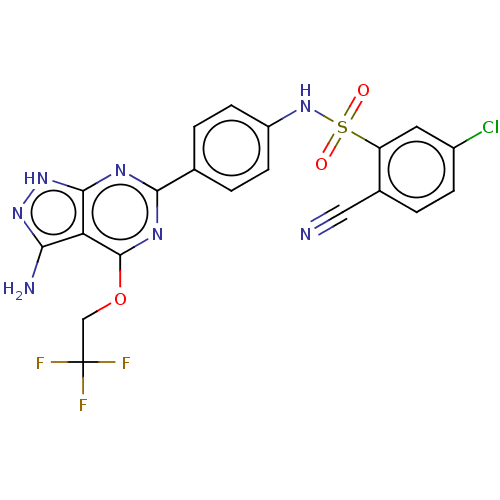 BDBM268792 US9718825, Example 196
BDBM268792 US9718825, Example 196 BDBM270801 US10059705, II-196
BDBM270801 US10059705, II-196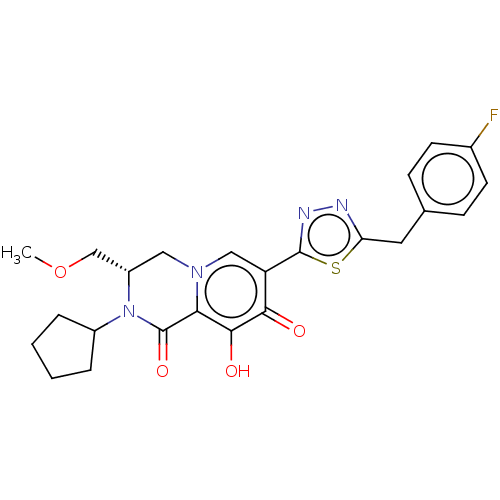 BDBM272354 US10065950, Example 196
BDBM272354 US10065950, Example 196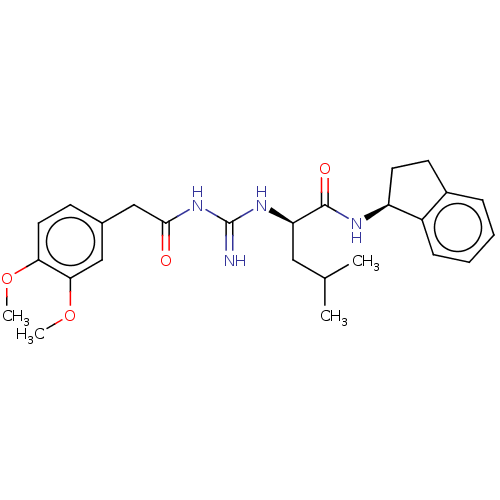 BDBM275594 US9884814, Compound 196
BDBM275594 US9884814, Compound 196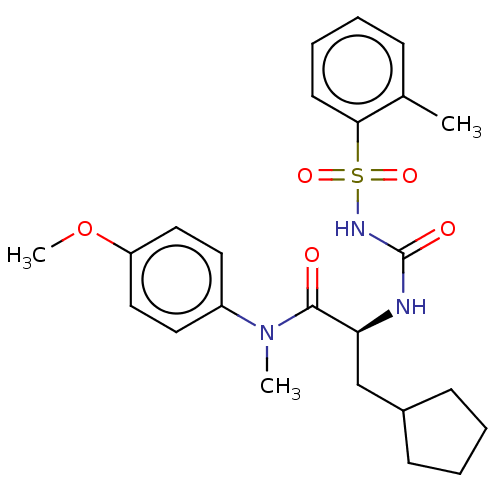 BDBM279371 US10035760, Example 196
BDBM279371 US10035760, Example 196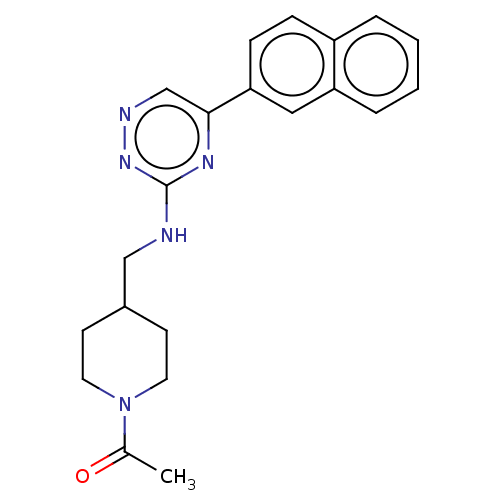 BDBM280319 US10029993, Example 196
BDBM280319 US10029993, Example 196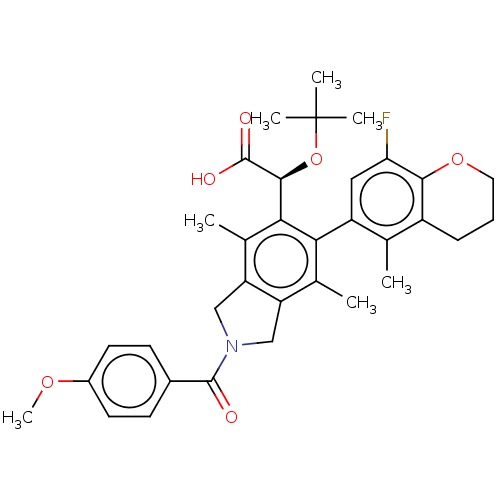 BDBM294719 US10112899, Example 196
BDBM294719 US10112899, Example 196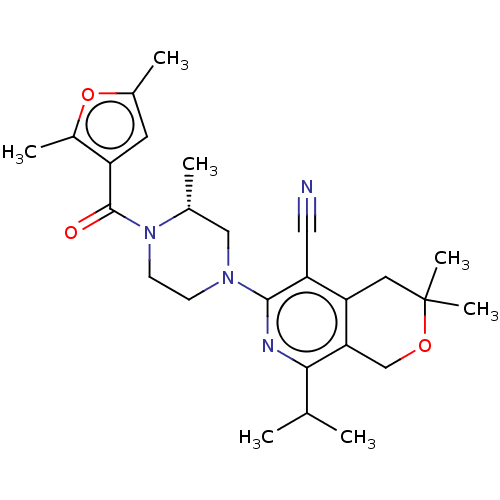 BDBM326499 US9662327, Compound 196
BDBM326499 US9662327, Compound 196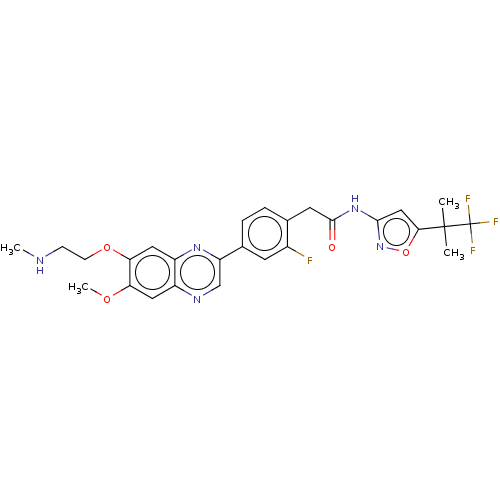 BDBM331775 US9725465, Example 196
BDBM331775 US9725465, Example 196 BDBM343902 US9777008, Compound 196
BDBM343902 US9777008, Compound 196 BDBM347990 US9790221, Compound 196
BDBM347990 US9790221, Compound 196 BDBM348277 US9790228, Compound 196
BDBM348277 US9790228, Compound 196 BDBM356196 US9815846, Compound 196
BDBM356196 US9815846, Compound 196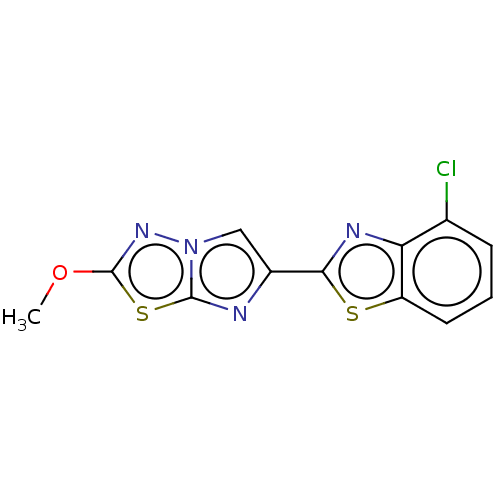 BDBM364737 US9862730, Example 196
BDBM364737 US9862730, Example 196 BDBM379672 US9926281, Compound 196
BDBM379672 US9926281, Compound 196 BDBM390309 US9951086, Example 196
BDBM390309 US9951086, Example 196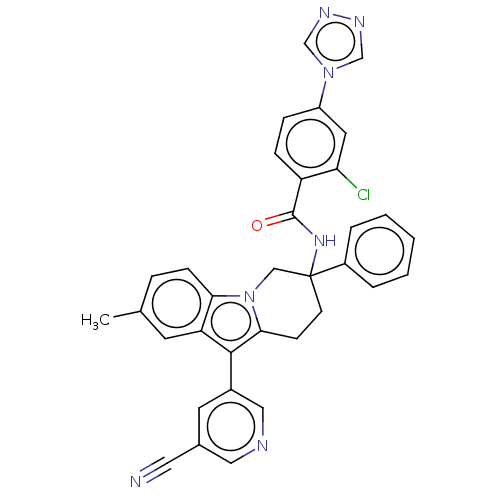 BDBM406021 US10351558, Example 196
BDBM406021 US10351558, Example 196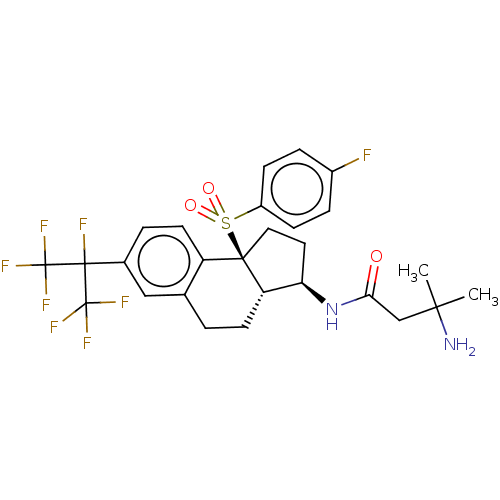 BDBM414309 US10435369, Example 196
BDBM414309 US10435369, Example 196 BDBM419534 US10457703, Example 196
BDBM419534 US10457703, Example 196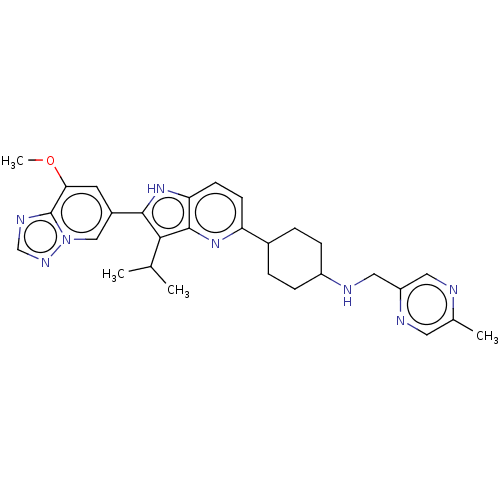 BDBM427763 US10544143, Example 196
BDBM427763 US10544143, Example 196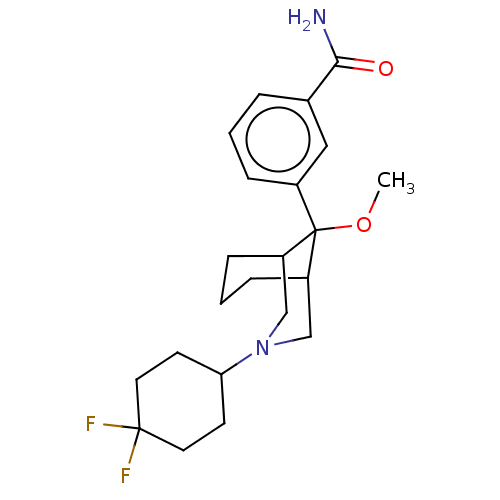 BDBM439006 US10604489, Compound 196
BDBM439006 US10604489, Compound 196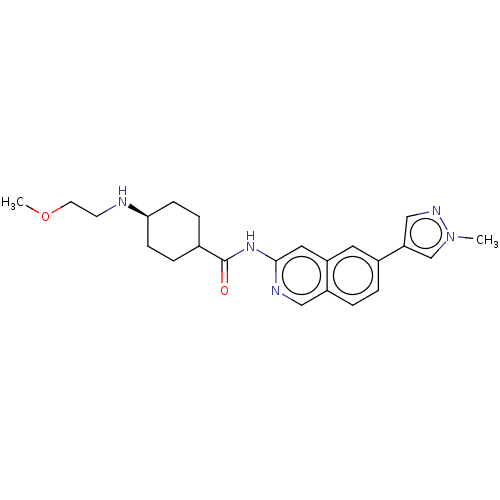 BDBM439282 US10604514, Compound 196
BDBM439282 US10604514, Compound 196 BDBM443612 US10660877, Example 196
BDBM443612 US10660877, Example 196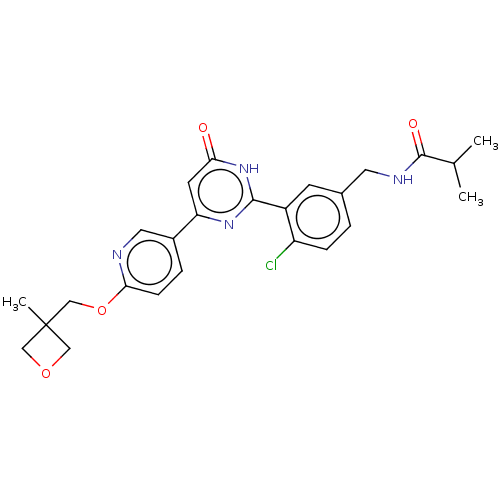 BDBM451596 US10710967, Example 196
BDBM451596 US10710967, Example 196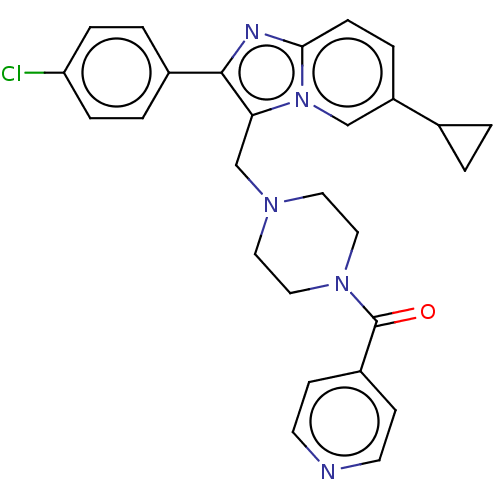 BDBM459038 US10759794, Example 196
BDBM459038 US10759794, Example 196 BDBM473245 US10844044, Example 196
BDBM473245 US10844044, Example 196 BDBM484945 US10934302, Example 196
BDBM484945 US10934302, Example 196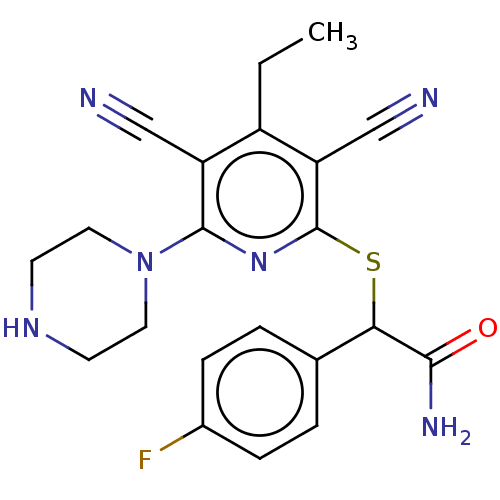 BDBM491249 US10975056, Example 196
BDBM491249 US10975056, Example 196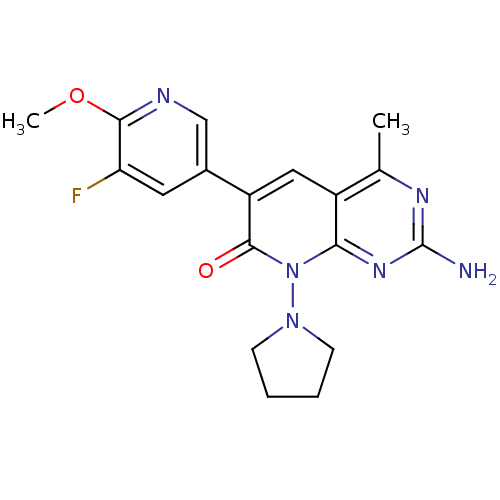 BDBM50387582 US8633204, 196 CHEMBL2057736
BDBM50387582 US8633204, 196 CHEMBL2057736 BDBM515891 US11053225, Compound 196
BDBM515891 US11053225, Compound 196 BDBM520915 US11149018, Example 196
BDBM520915 US11149018, Example 196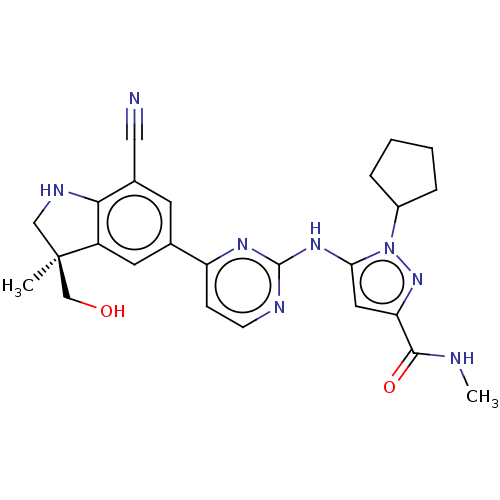 BDBM523705 US11136311, Compound 196
BDBM523705 US11136311, Compound 196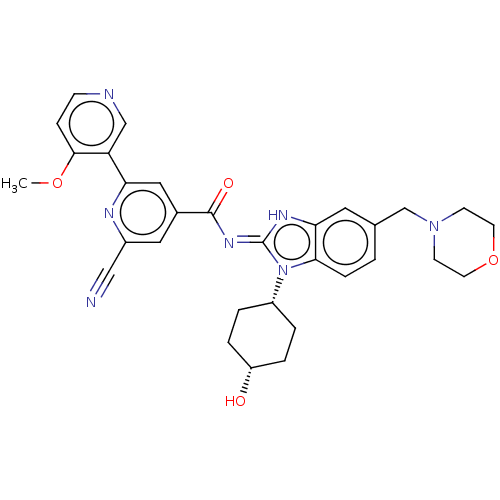 BDBM525518 US11174245, # I-196
BDBM525518 US11174245, # I-196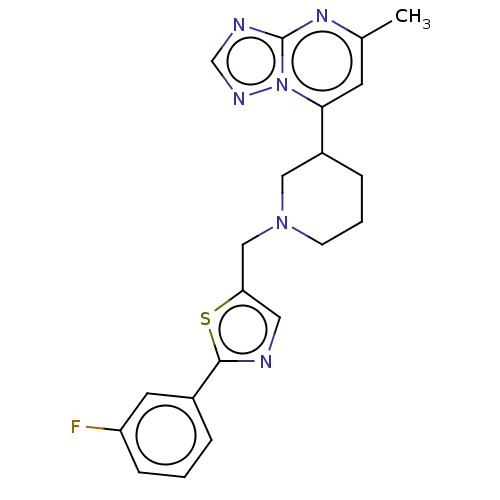 BDBM526993 US11186582, Example 196
BDBM526993 US11186582, Example 196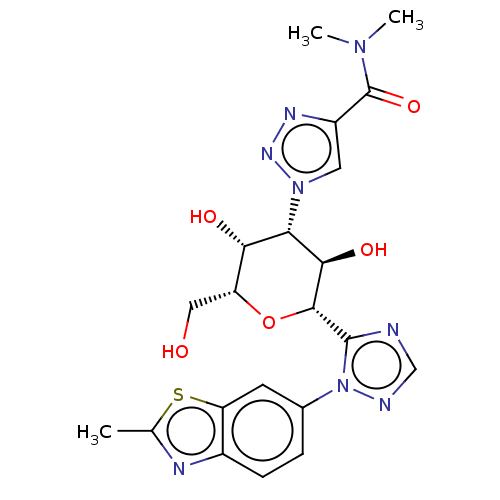 BDBM541593 US11267811, Example 196
BDBM541593 US11267811, Example 196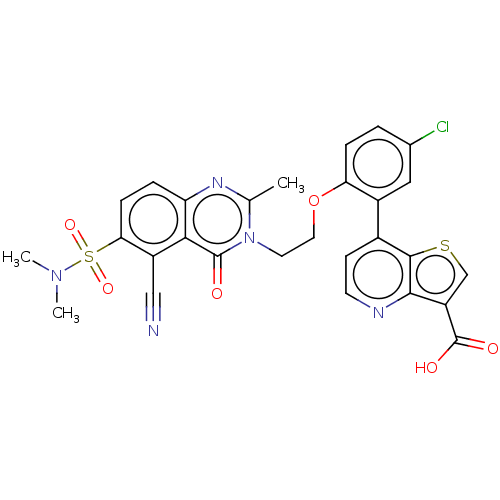 BDBM544712 US11286268, Compound 196
BDBM544712 US11286268, Compound 196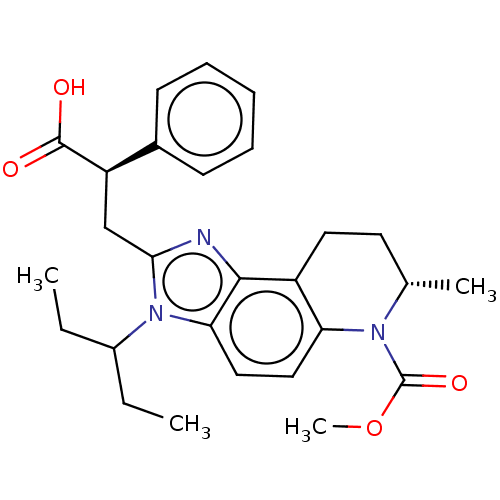 BDBM546923 US11292791, Example 196
BDBM546923 US11292791, Example 196 BDBM556315 US11897851, Compound 196
BDBM556315 US11897851, Compound 196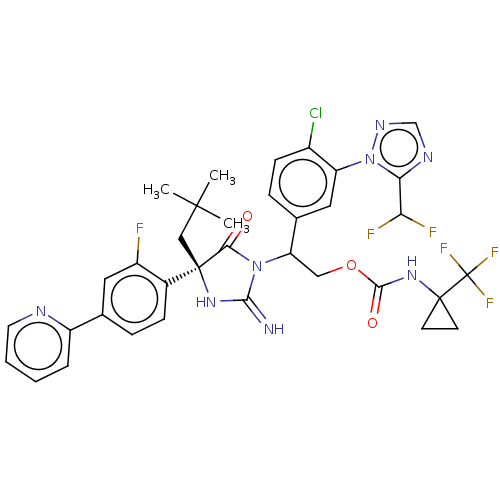 BDBM556698 US11352329, COMPD # 196
BDBM556698 US11352329, COMPD # 196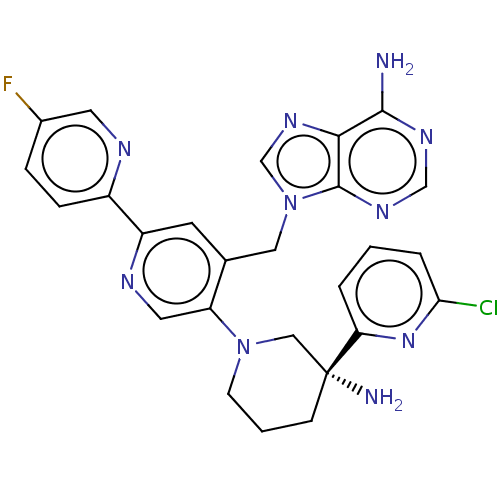 BDBM567995 US11420970, Example 196
BDBM567995 US11420970, Example 196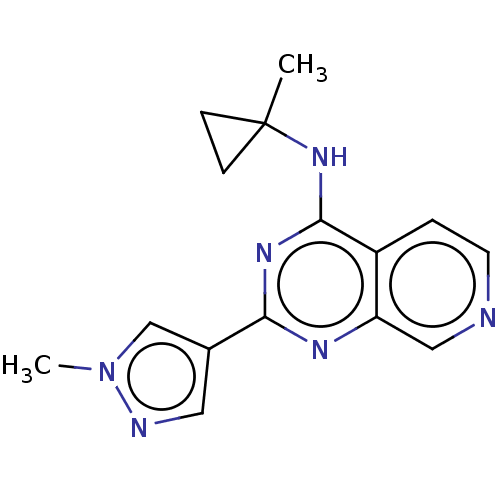 BDBM574367 US11458138, Example 196
BDBM574367 US11458138, Example 196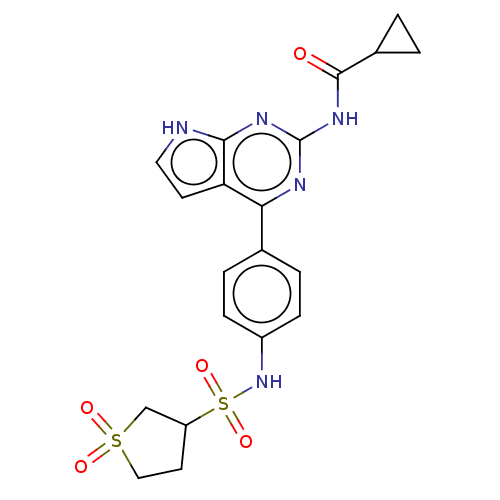 BDBM584448 US11524968, Example 196
BDBM584448 US11524968, Example 196 BDBM585251 US11530197, Compound 196
BDBM585251 US11530197, Compound 196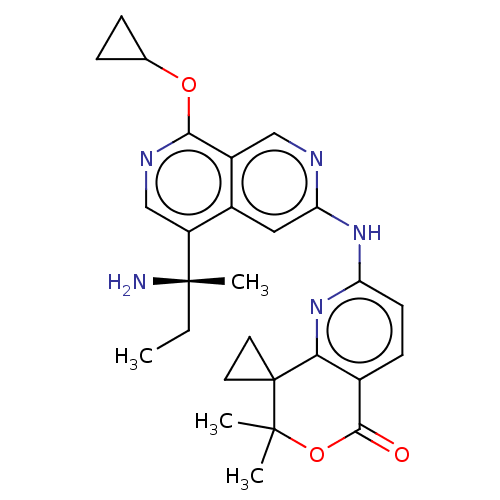 BDBM587486 US11534441, Compound 196
BDBM587486 US11534441, Compound 196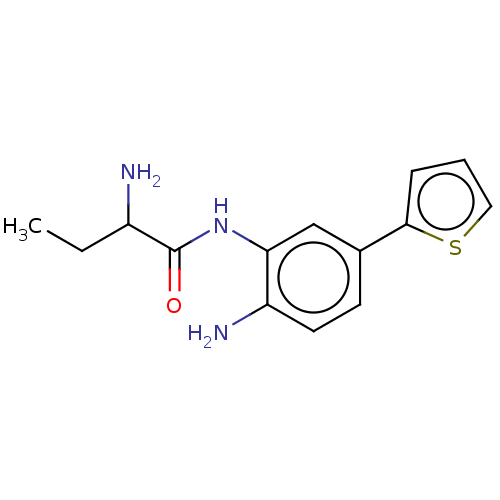 BDBM592501 US11572368, Compound 196
BDBM592501 US11572368, Compound 196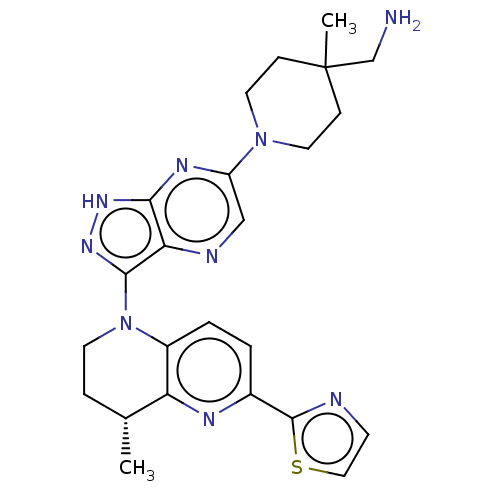 BDBM596081 US11591336, Compound 196
BDBM596081 US11591336, Compound 196 BDBM604081 US11660293, Example 196
BDBM604081 US11660293, Example 196 BDBM607258 US11685745, Example 196
BDBM607258 US11685745, Example 196 BDBM608627 US11697648, Example 196
BDBM608627 US11697648, Example 196 BDBM612934 US11725000, Compound 196
BDBM612934 US11725000, Compound 196 BDBM615405 US20230271949, Example 196
BDBM615405 US20230271949, Example 196 BDBM622236 US20230312550, Example 196
BDBM622236 US20230312550, Example 196 BDBM622484 US20230312576, Compound 196
BDBM622484 US20230312576, Compound 196 BDBM623178 US11780845, Example 196
BDBM623178 US11780845, Example 196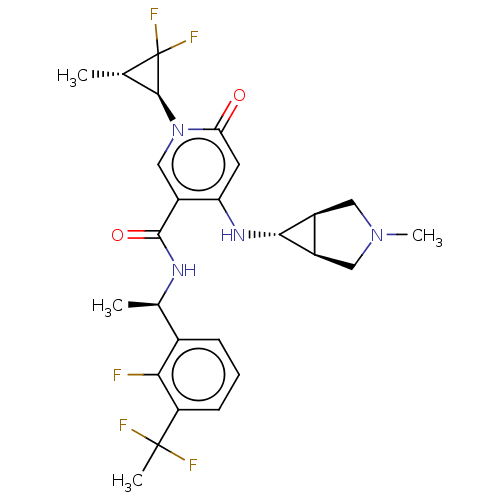 BDBM631613 US20230348424, Example 196
BDBM631613 US20230348424, Example 196 BDBM631926 US20230348426, Example 196
BDBM631926 US20230348426, Example 196 BDBM634296 US20230357253, Compound 196
BDBM634296 US20230357253, Compound 196 BDBM634747 US11814367, Compound 196
BDBM634747 US11814367, Compound 196 BDBM637281 US20230382904, Compound 196
BDBM637281 US20230382904, Compound 196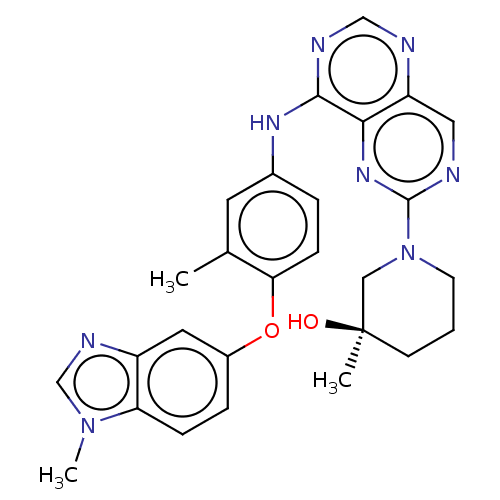 BDBM638093 US11834453, Example 196
BDBM638093 US11834453, Example 196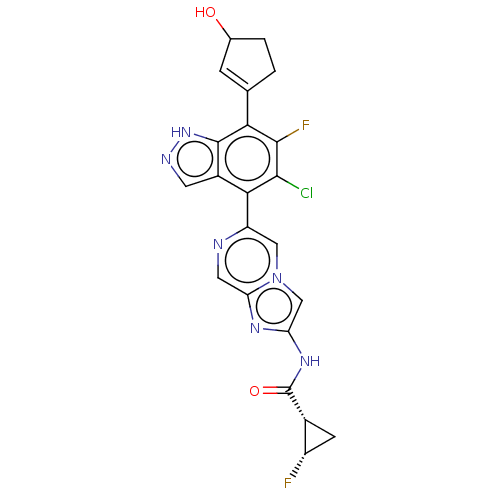 BDBM638601 US11834467, Example 196
BDBM638601 US11834467, Example 196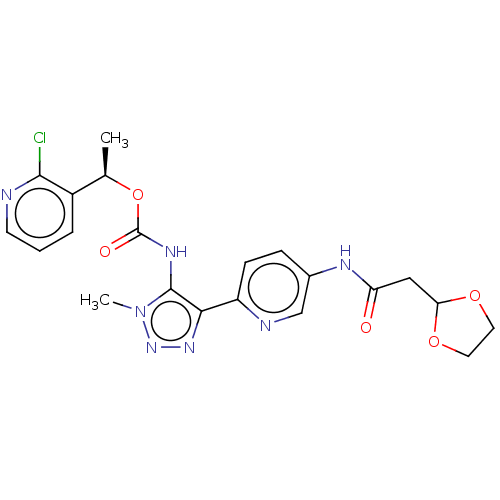 BDBM654251 US11912686, Compound 196
BDBM654251 US11912686, Compound 196 BDBM658317 US20240083900, Example 196
BDBM658317 US20240083900, Example 196 BDBM659079 US20240092758, Example 196
BDBM659079 US20240092758, Example 196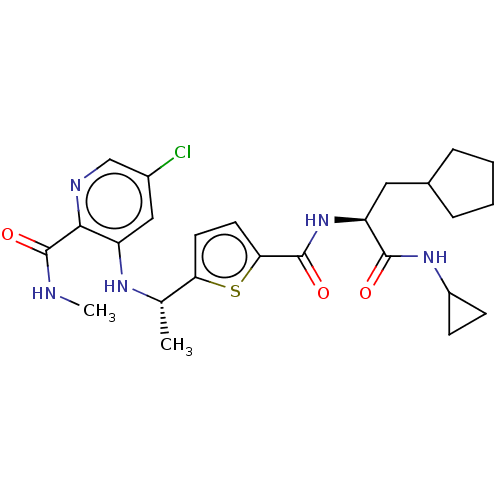 BDBM665512 US20240116910, Compound 196
BDBM665512 US20240116910, Compound 196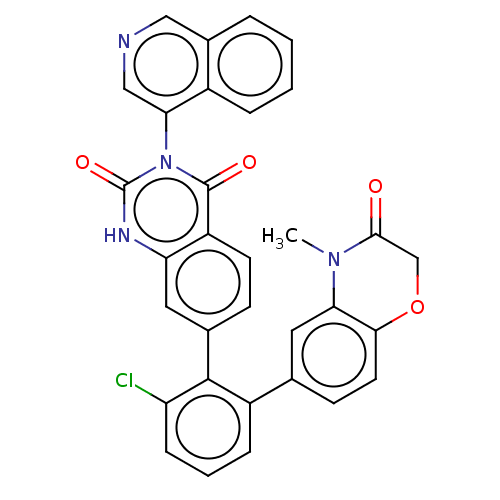 BDBM666163 US20240116946, Example 196
BDBM666163 US20240116946, Example 196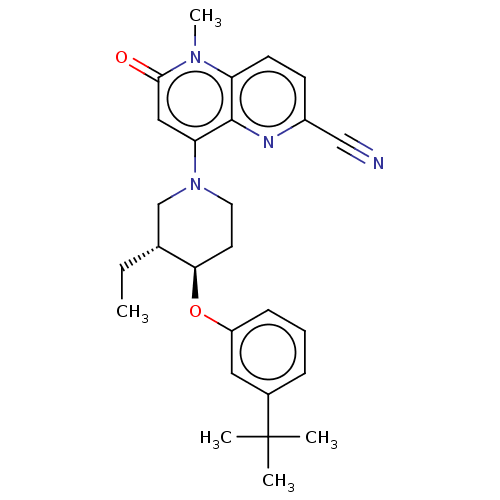 BDBM669209 US11964973, Example 196
BDBM669209 US11964973, Example 196 BDBM669794 US20240132480, Example 196
BDBM669794 US20240132480, Example 196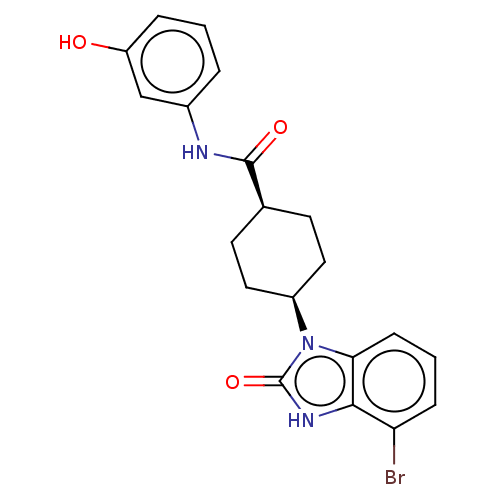 BDBM670252 US11970474, Example 196
BDBM670252 US11970474, Example 196 BDBM677532 US20240174662, Example 196
BDBM677532 US20240174662, Example 196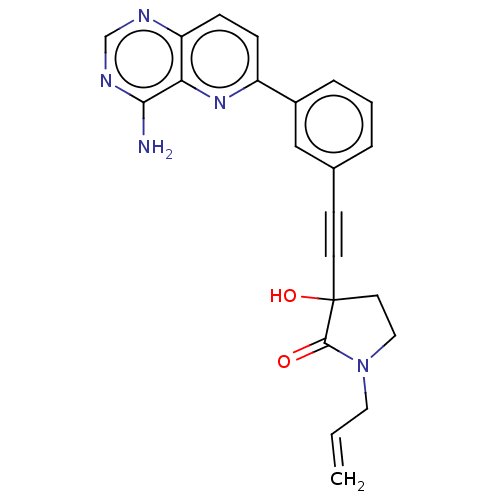 BDBM681637 US20240199605, Example 196
BDBM681637 US20240199605, Example 196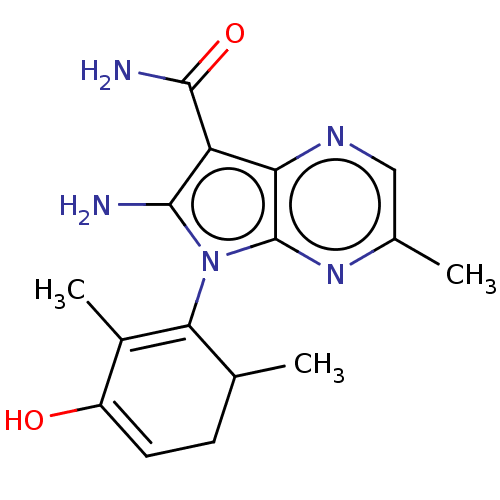 BDBM682450 US20240207300, Compound 196
BDBM682450 US20240207300, Compound 196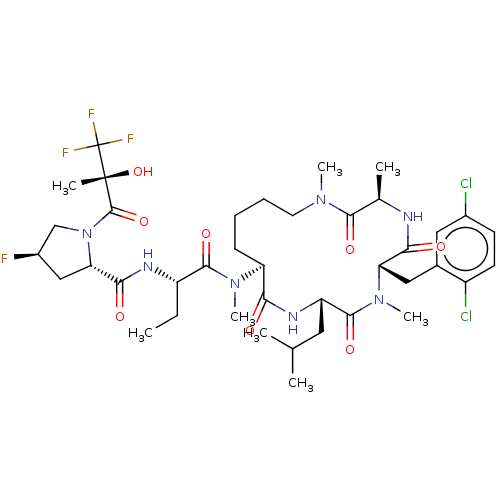 BDBM684200 US20240218021, Example 196
BDBM684200 US20240218021, Example 196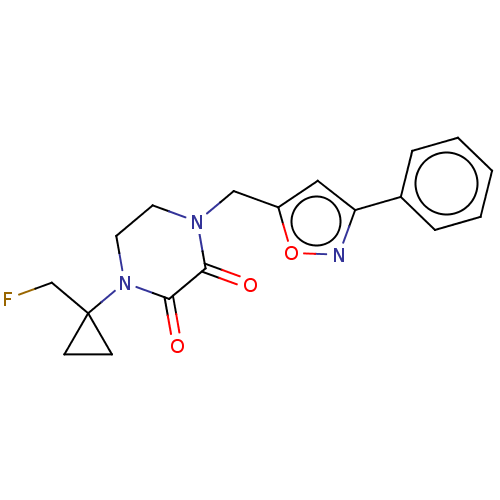 BDBM686751 US20240246937, Example 196
BDBM686751 US20240246937, Example 196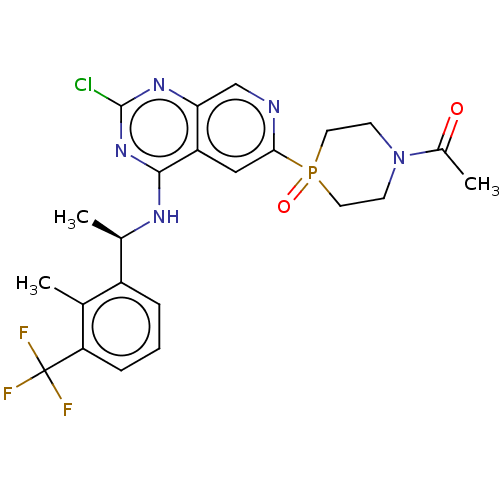 BDBM688082 US20240247015, Example 196
BDBM688082 US20240247015, Example 196 BDBM695030 US20240294551, Example 196
BDBM695030 US20240294551, Example 196 BDBM702445 US20240360148, Example 196
BDBM702445 US20240360148, Example 196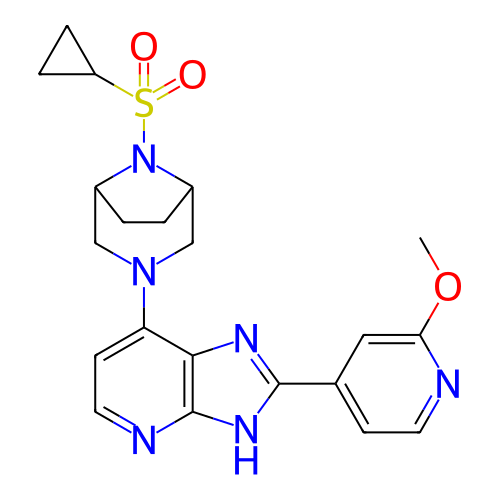 BDBM702842 US20240360157, Example 196
BDBM702842 US20240360157, Example 196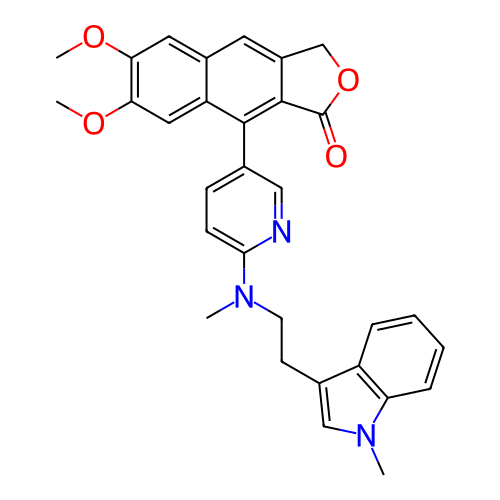 BDBM703249 US20240368104, Example 196
BDBM703249 US20240368104, Example 196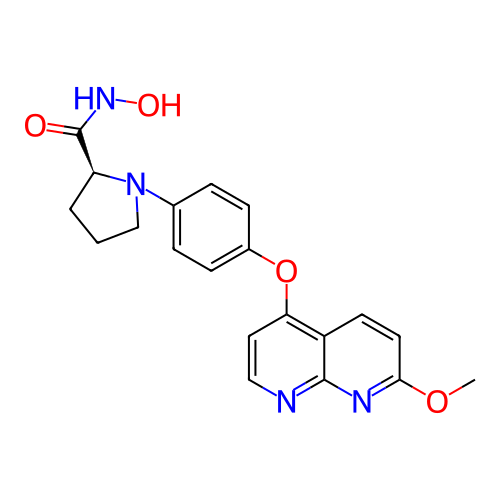 BDBM706320 US20240383893, Compound 196
BDBM706320 US20240383893, Compound 196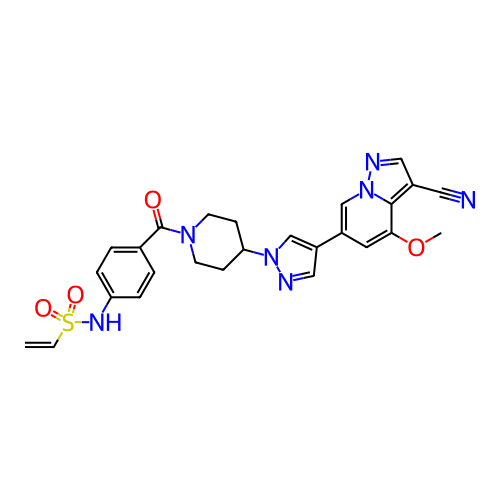 BDBM709664 US12180207, Example 196
BDBM709664 US12180207, Example 196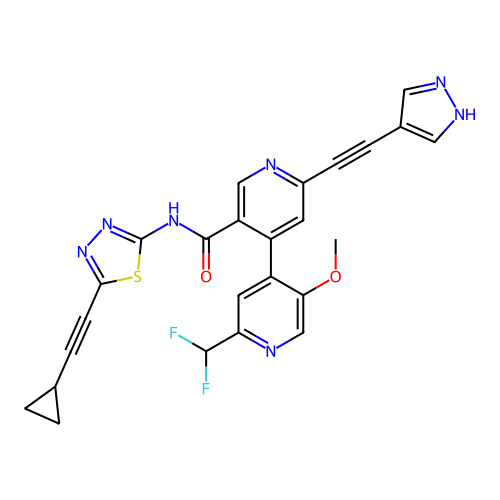 BDBM715032 US20250026748, Compound 196
BDBM715032 US20250026748, Compound 196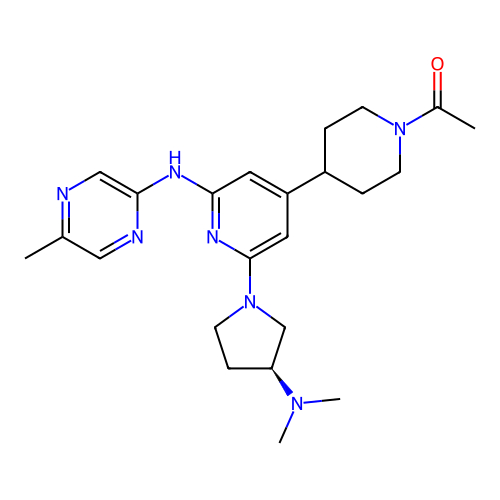 BDBM716297 US20250032489, Compound 196
BDBM716297 US20250032489, Compound 196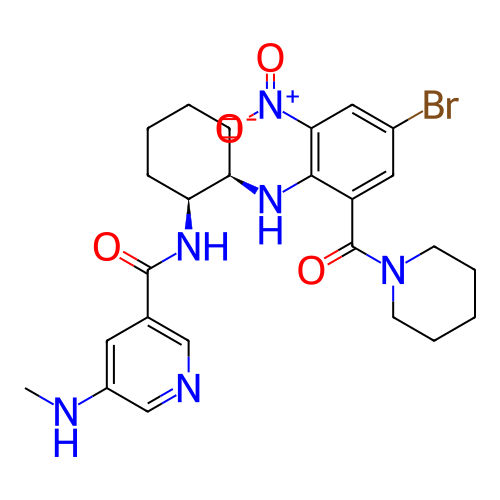 BDBM718341 US20250042889, Example 196
BDBM718341 US20250042889, Example 196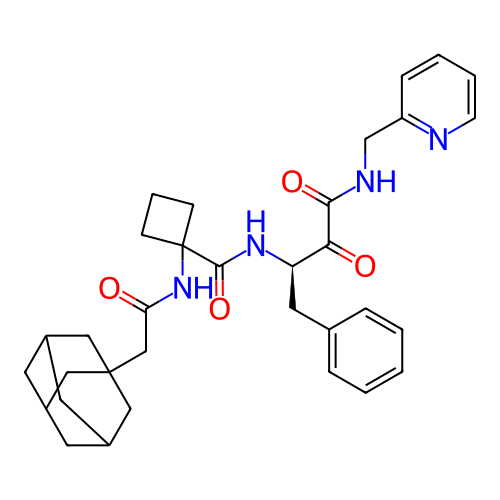 BDBM723622 US20250064789, Compound 196
BDBM723622 US20250064789, Compound 196 BDBM730983 US20250122183, Example 196
BDBM730983 US20250122183, Example 196 BDBM734653 US20250129067, Compound 196
BDBM734653 US20250129067, Compound 196 BDBM735609 US20250129103, Compound 196
BDBM735609 US20250129103, Compound 196 BDBM735768 US20250129104, Compound 196
BDBM735768 US20250129104, Compound 196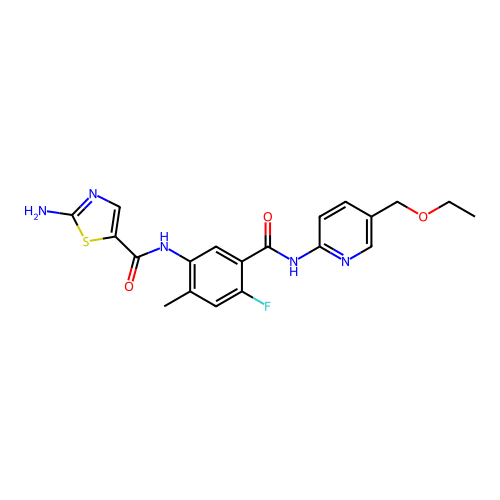 BDBM738637 US20250145606, Example 196
BDBM738637 US20250145606, Example 196 BDBM751930 US20250197382, Compound 196
BDBM751930 US20250197382, Compound 196 BDBM756664 US20250215013, Example 196
BDBM756664 US20250215013, Example 196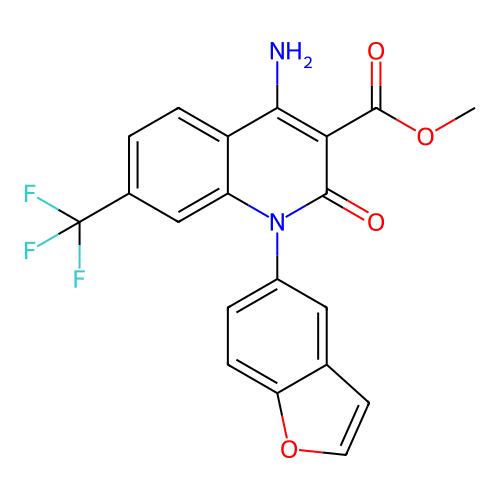 BDBM756915 US20250221979, Example 196
BDBM756915 US20250221979, Example 196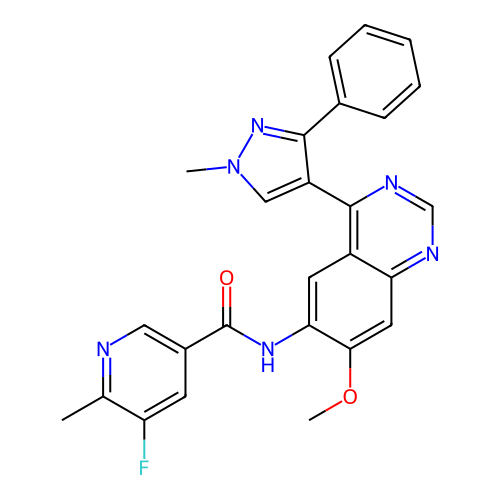 BDBM759604 US20250236608, Example 196
BDBM759604 US20250236608, Example 196 BDBM761205 US20250243180, Compound 196
BDBM761205 US20250243180, Compound 196 BDBM763173 US12384753, Example 196
BDBM763173 US12384753, Example 196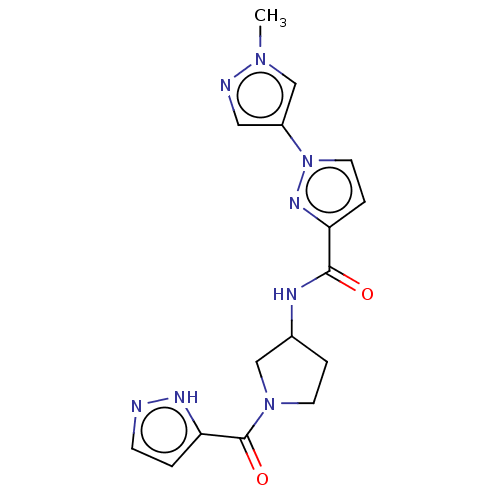 US10022354, Example 196 BDBM281256
US10022354, Example 196 BDBM281256 US10112929, Example 196 BDBM295469
US10112929, Example 196 BDBM295469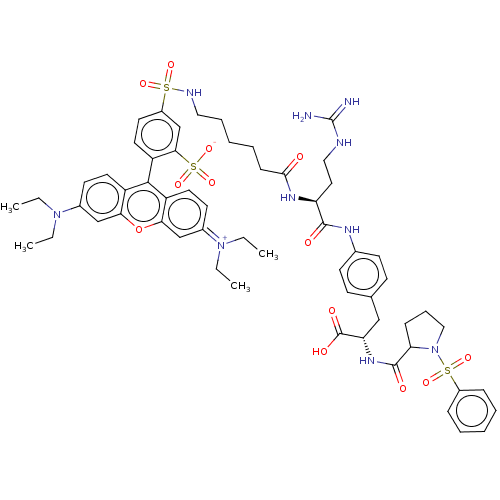 US10131658, Compound 196 BDBM300926
US10131658, Compound 196 BDBM300926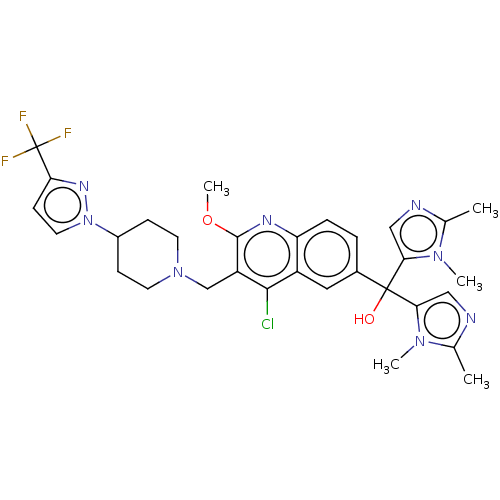 US10201546, Example 196 BDBM339335
US10201546, Example 196 BDBM339335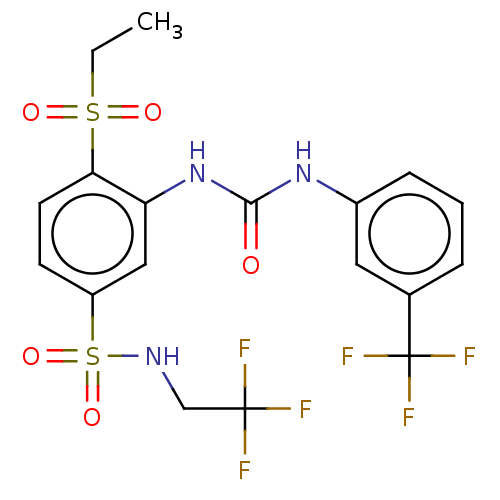 US10202339, Compound 196 BDBM339751
US10202339, Compound 196 BDBM339751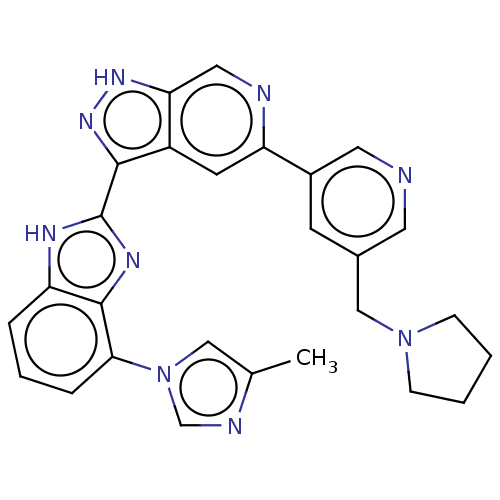 US10202377, Compound 196 BDBM346301
US10202377, Compound 196 BDBM346301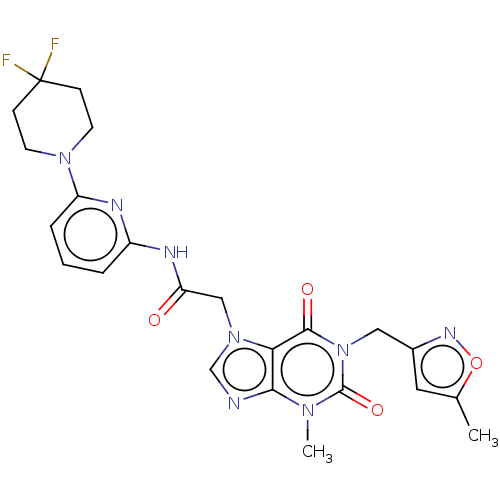 US10221177, Compound 196 BDBM361829
US10221177, Compound 196 BDBM361829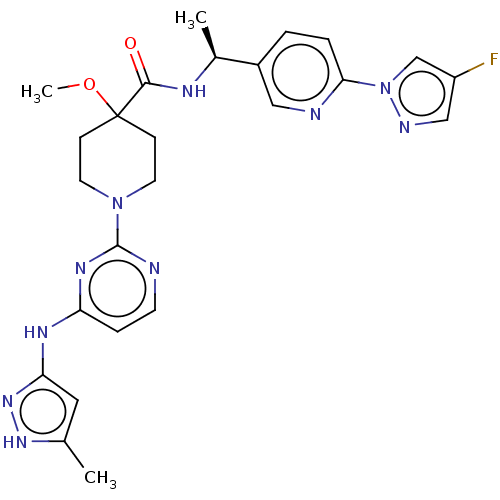 US10227329, Compound 196 BDBM367784
US10227329, Compound 196 BDBM367784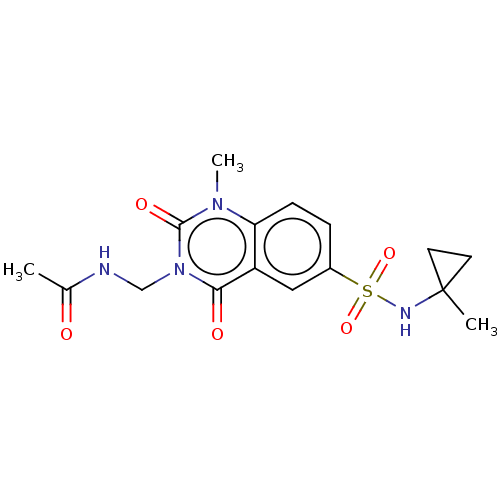 US10239843, Example 196 BDBM371089
US10239843, Example 196 BDBM371089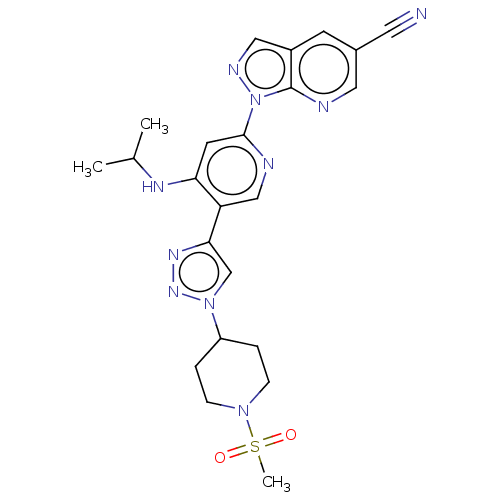 US10294229, Example 196 BDBM388048
US10294229, Example 196 BDBM388048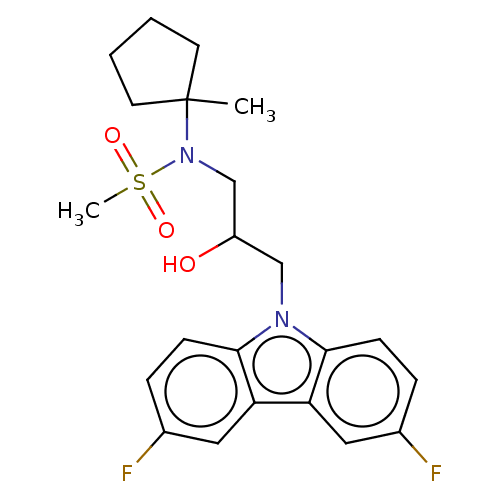 US10383880, Example 196 BDBM410986
US10383880, Example 196 BDBM410986 US10457654, Example 196 BDBM417336
US10457654, Example 196 BDBM417336 US10570121, Example 196 BDBM435690
US10570121, Example 196 BDBM435690 US10618903, Example 196 BDBM438629
US10618903, Example 196 BDBM438629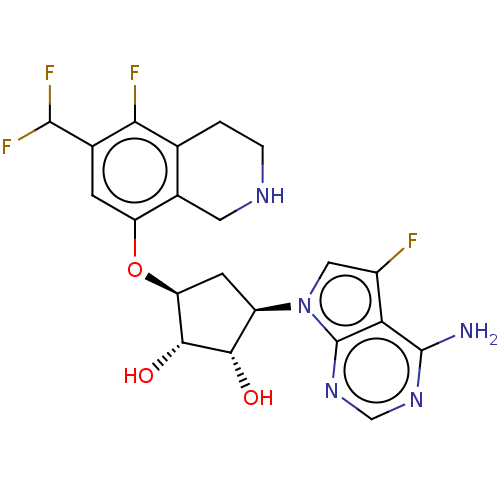 US10709709, Example 196 BDBM451366
US10709709, Example 196 BDBM451366 US10730863, Example 196 BDBM453840
US10730863, Example 196 BDBM453840 US10766903, Example 196 BDBM462584
US10766903, Example 196 BDBM462584 US10774053, Compound 196 BDBM461063
US10774053, Compound 196 BDBM461063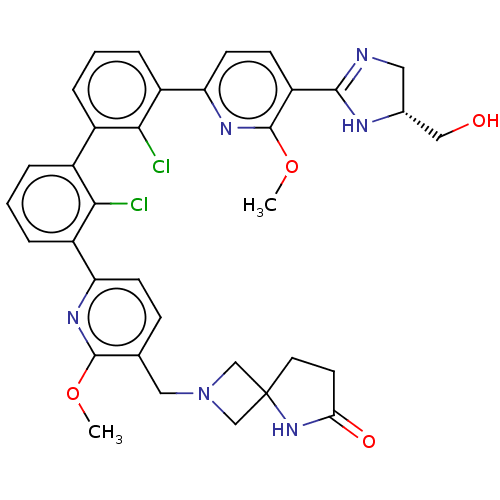 US10899735, No. 196 BDBM479737
US10899735, No. 196 BDBM479737 US10906888, Example 196 BDBM480979
US10906888, Example 196 BDBM480979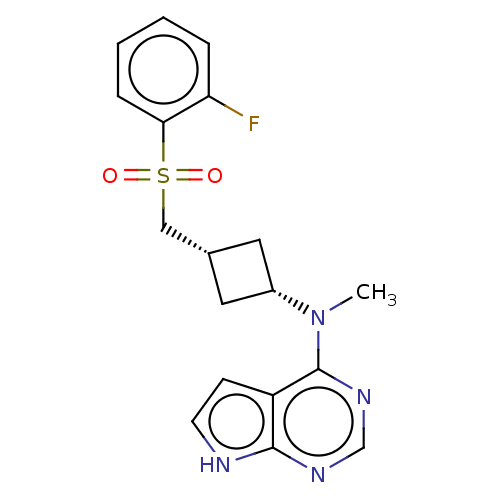 US10966980, Example 196 BDBM489942
US10966980, Example 196 BDBM489942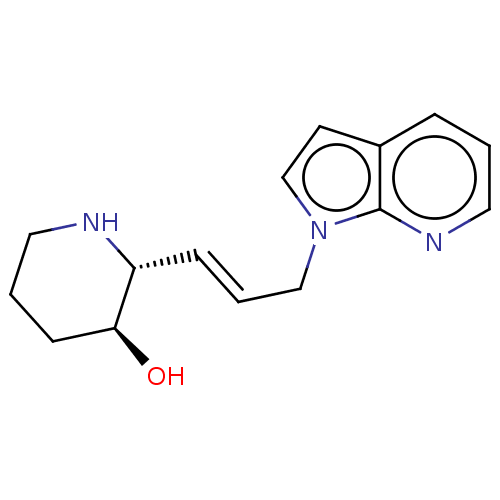 US10981917, Example 196 BDBM493495
US10981917, Example 196 BDBM493495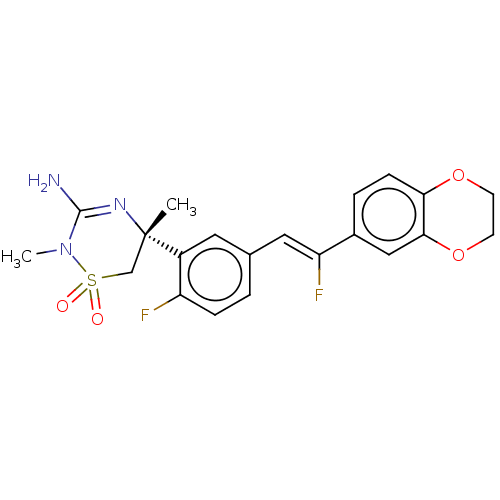 US11021493, Example 196 BDBM501277
US11021493, Example 196 BDBM501277 US11028090, Example 196 BDBM502309
US11028090, Example 196 BDBM502309 US11045457, Example 196 BDBM506595
US11045457, Example 196 BDBM506595 US11053226, Example 196 BDBM516106
US11053226, Example 196 BDBM516106 US11136296, Example 196 BDBM523465
US11136296, Example 196 BDBM523465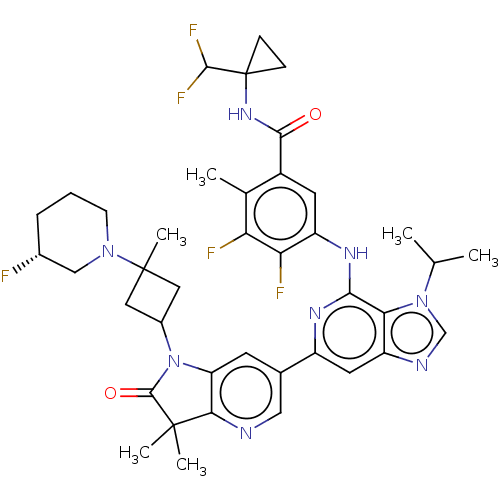 US11203591, Example 196 BDBM529789
US11203591, Example 196 BDBM529789 US11208404, Compound 196 BDBM532135
US11208404, Compound 196 BDBM532135 US11225469, Compound 196 BDBM533696
US11225469, Compound 196 BDBM533696 US11242361, Compound 196 BDBM536589
US11242361, Compound 196 BDBM536589 US11254668, Example 196 BDBM538855
US11254668, Example 196 BDBM538855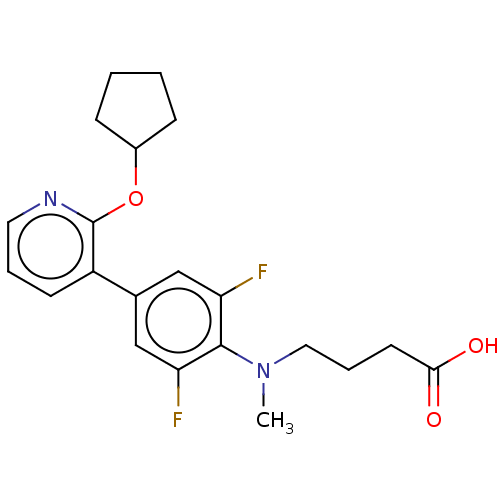 US11261186, Example 196 BDBM540269
US11261186, Example 196 BDBM540269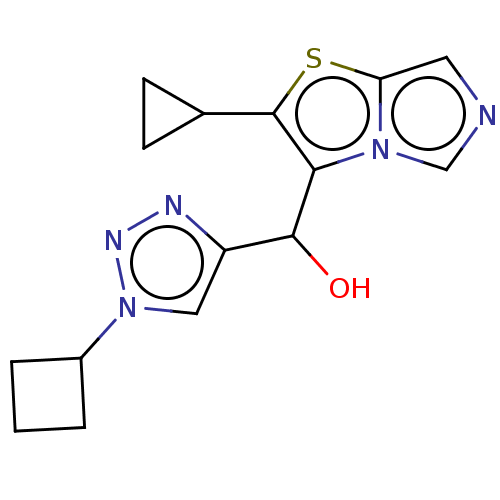 US11267824, Example 196 BDBM541990
US11267824, Example 196 BDBM541990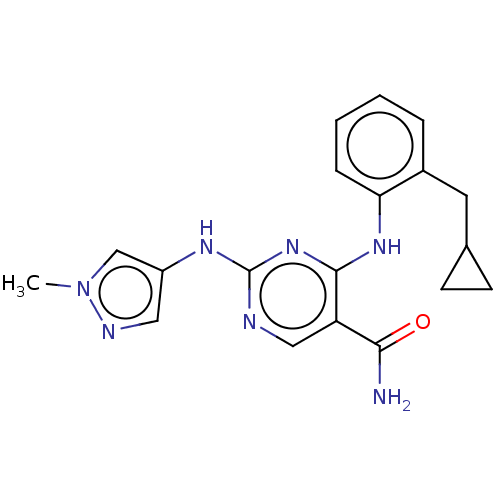 US11344549, Example 196 BDBM555520
US11344549, Example 196 BDBM555520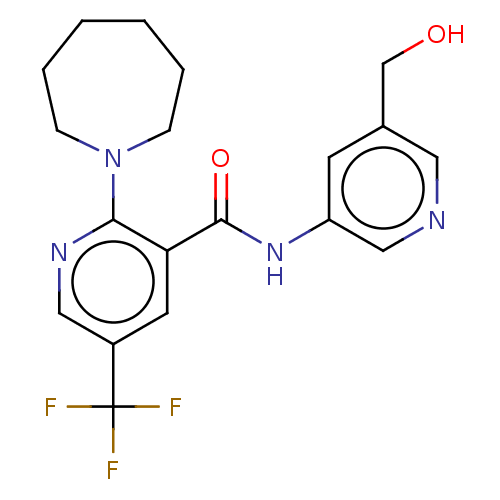 US11377438, Example 196 BDBM560156
US11377438, Example 196 BDBM560156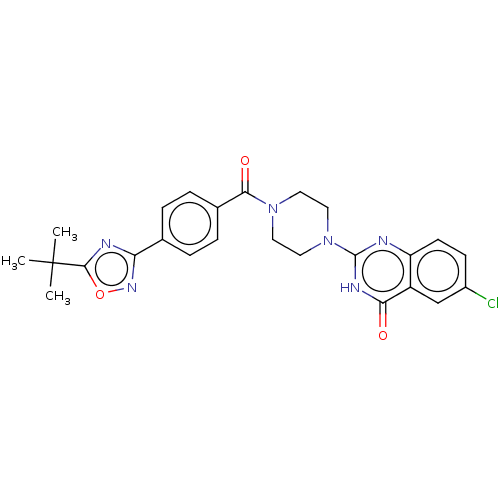 US11390610, Example 196 BDBM561367
US11390610, Example 196 BDBM561367 US11420973, Example 196 BDBM568318
US11420973, Example 196 BDBM568318 US11548892, Compound 196 BDBM583073
US11548892, Compound 196 BDBM583073 US11559538, Example 196 BDBM589949
US11559538, Example 196 BDBM589949 US11596639, Example 196 BDBM596498
US11596639, Example 196 BDBM596498 US11649255, Example 196 BDBM603223
US11649255, Example 196 BDBM603223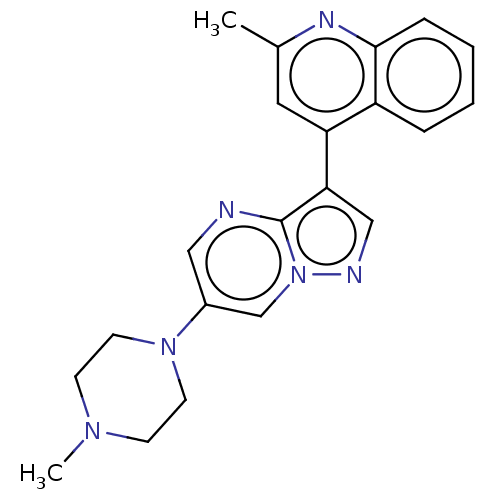 US11654147, Compound 196 BDBM603759
US11654147, Compound 196 BDBM603759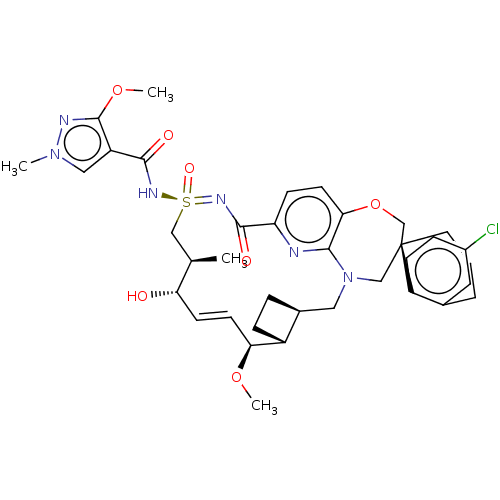 US11667652, Example 196 BDBM605402
US11667652, Example 196 BDBM605402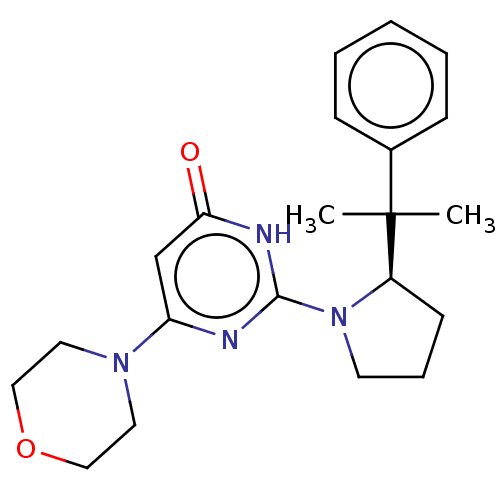 US11685734, Example 196 BDBM606909
US11685734, Example 196 BDBM606909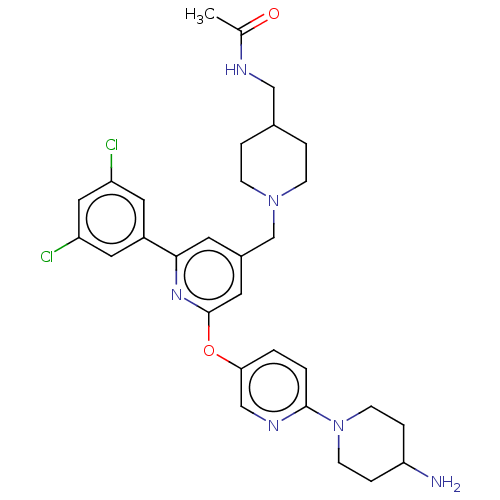 US11773078, Example 196 BDBM621444
US11773078, Example 196 BDBM621444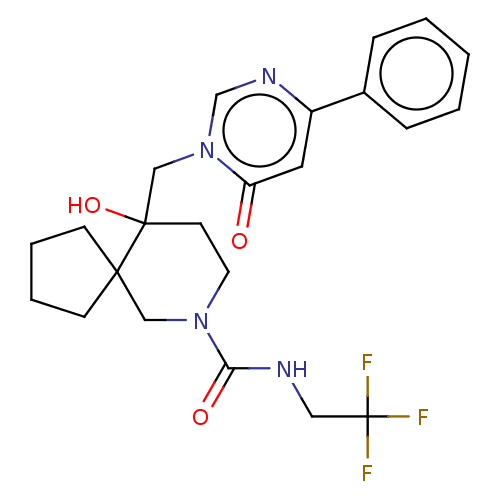 US11807646, Example 196 BDBM633782
US11807646, Example 196 BDBM633782 US11845723, Example 196 BDBM641302
US11845723, Example 196 BDBM641302 US11912703, Example 196 BDBM655424
US11912703, Example 196 BDBM655424 US11964953, Example 196 BDBM668932
US11964953, Example 196 BDBM668932 US12128028, Example 196 BDBM701800
US12128028, Example 196 BDBM701800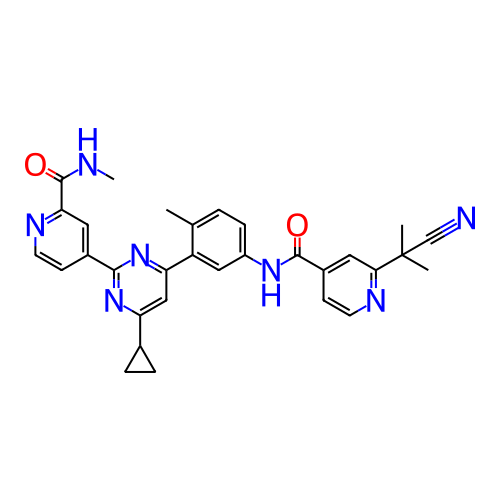 US12319655, Example 196 BDBM745716
US12319655, Example 196 BDBM745716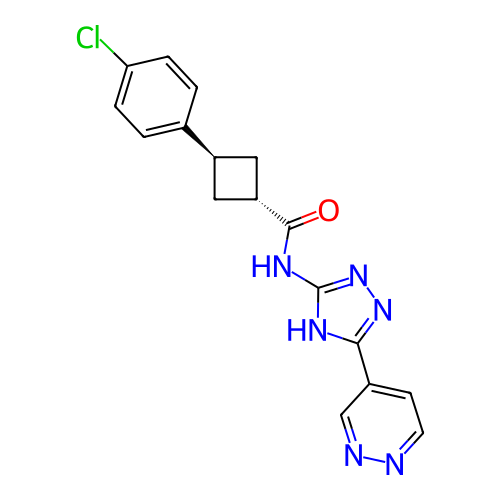 US12331033, Example 196 BDBM749574
US12331033, Example 196 BDBM749574 US12338220, Example 196 BDBM753199
US12338220, Example 196 BDBM753199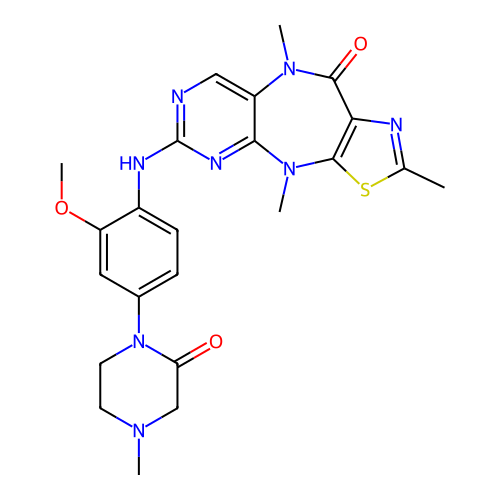 US12365696, Compound 196 BDBM759382
US12365696, Compound 196 BDBM759382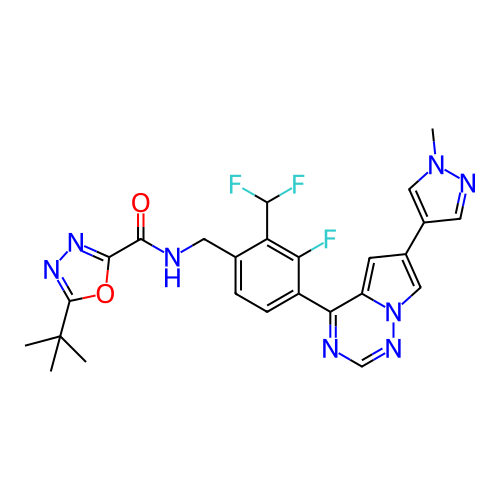 US12391697, Example 196 BDBM765567
US12391697, Example 196 BDBM765567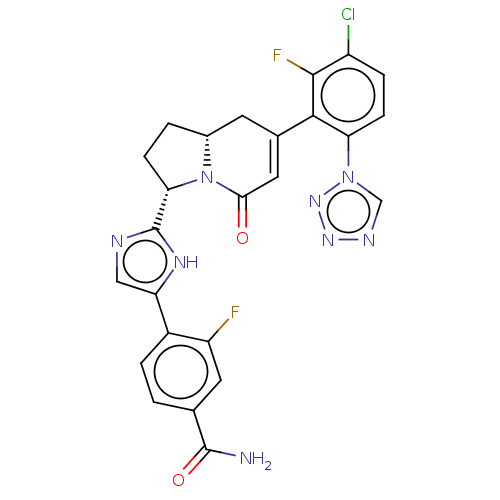 US20230295157, Example 196 BDBM619872
US20230295157, Example 196 BDBM619872 US20230322747, Compound 196 BDBM625151
US20230322747, Compound 196 BDBM625151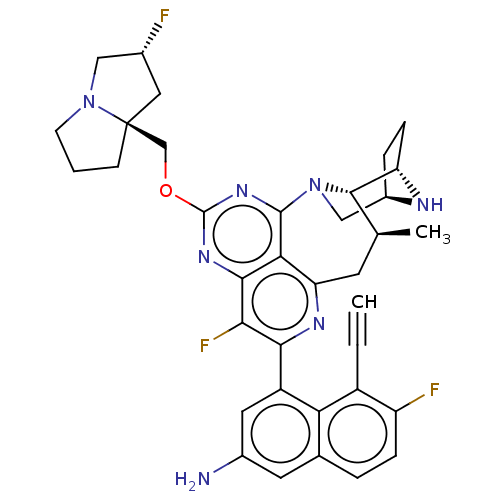 US20230339981, Example 196 BDBM629508
US20230339981, Example 196 BDBM629508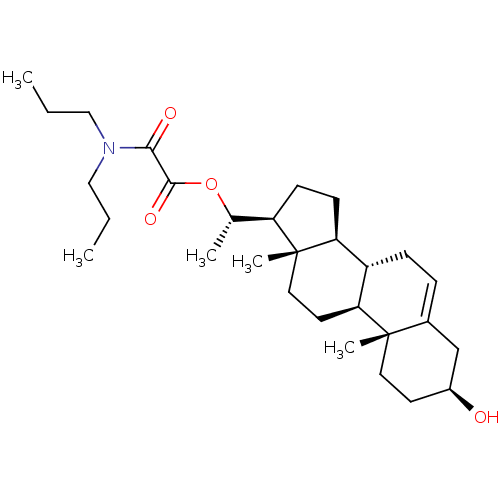 US20230340011, Example 196. BDBM629898
US20230340011, Example 196. BDBM629898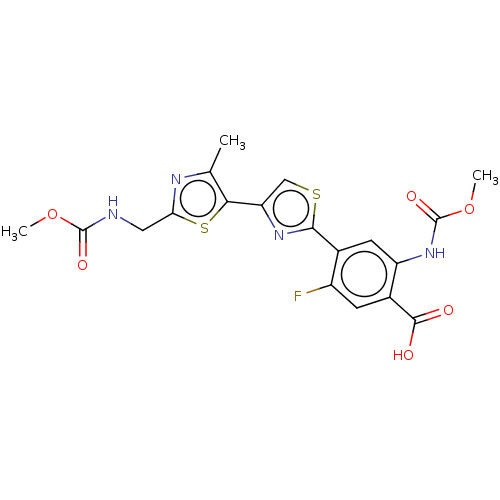 US20230348450, Example 196 BDBM632811
US20230348450, Example 196 BDBM632811 US20230365546, Compound 196 BDBM636413
US20230365546, Compound 196 BDBM636413 US20240002391, Compound 196 BDBM643384
US20240002391, Compound 196 BDBM643384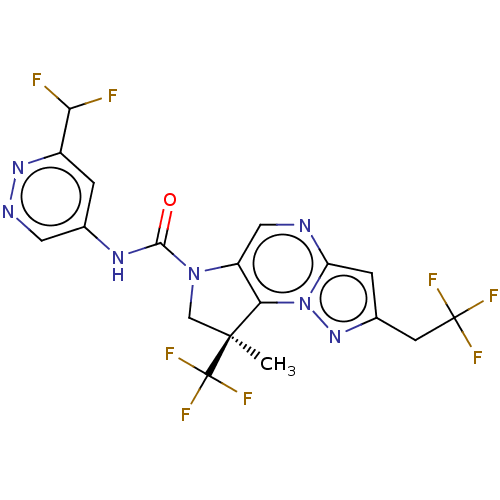 US20240018157, Example 196 BDBM645994
US20240018157, Example 196 BDBM645994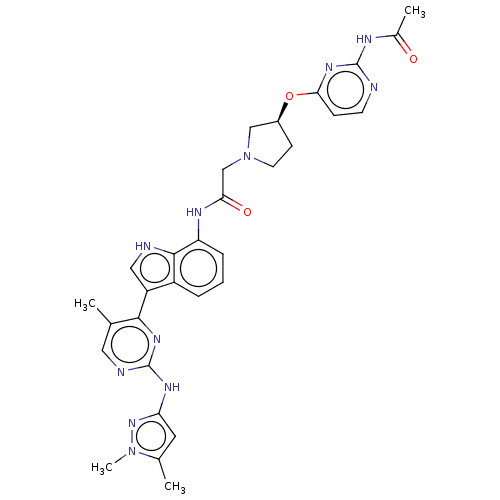 US20240025884, Example 196 BDBM646985
US20240025884, Example 196 BDBM646985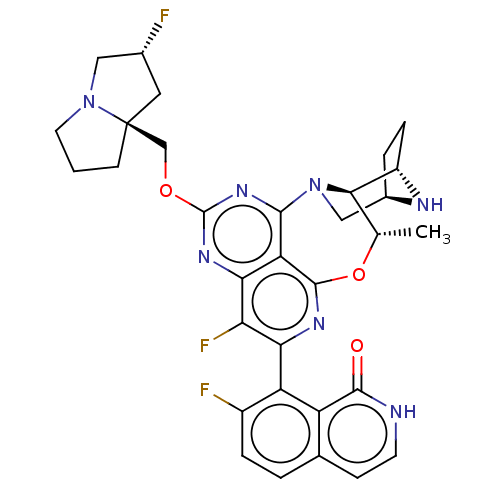 US20240025919, Compound 196 BDBM647959
US20240025919, Compound 196 BDBM647959 US20240043427, Example 196 BDBM650110
US20240043427, Example 196 BDBM650110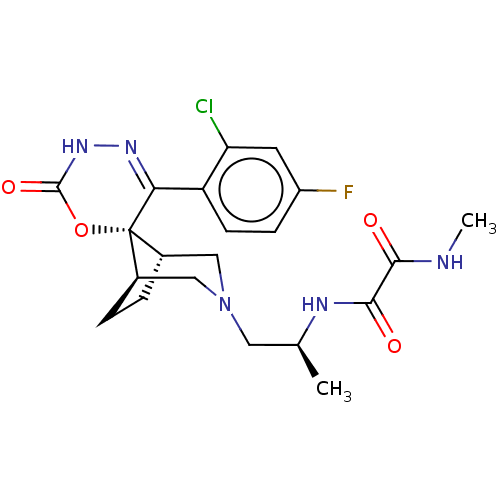 US20240101572, Example 196 BDBM661587
US20240101572, Example 196 BDBM661587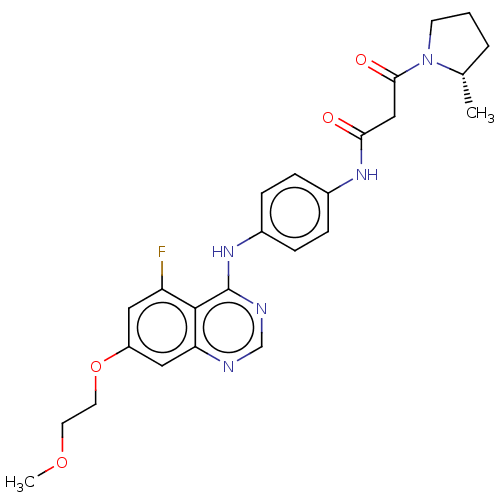 US20240116877, Example 196 BDBM664925
US20240116877, Example 196 BDBM664925 US20240140931, Compound 196 BDBM670575
US20240140931, Compound 196 BDBM670575 US20240158414, Example 196 BDBM675437
US20240158414, Example 196 BDBM675437 US20240199649, Compound 196 BDBM681897
US20240199649, Compound 196 BDBM681897 US20240262804, Example 196 BDBM689722
US20240262804, Example 196 BDBM689722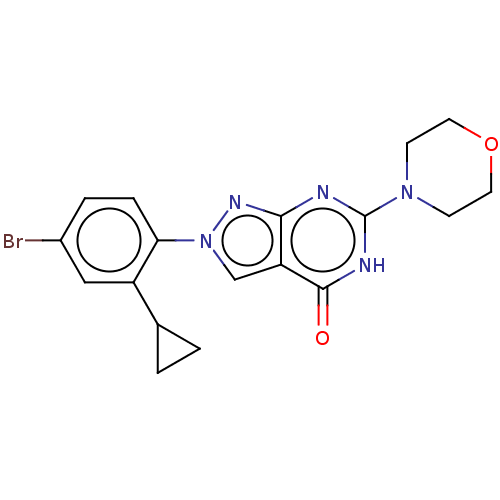 US20240287079, Example 196 BDBM694025
US20240287079, Example 196 BDBM694025 US20240316047, Example 196 BDBM697414
US20240316047, Example 196 BDBM697414 US20250034135, Compound 196 BDBM716758
US20250034135, Compound 196 BDBM716758 US20250059174, Example 196 BDBM721933
US20250059174, Example 196 BDBM721933 US20250059220, Compound 196 BDBM723083
US20250059220, Compound 196 BDBM723083 US20250074911, Example 196 BDBM725990
US20250074911, Example 196 BDBM725990 US20250084074, Example 196 BDBM726882
US20250084074, Example 196 BDBM726882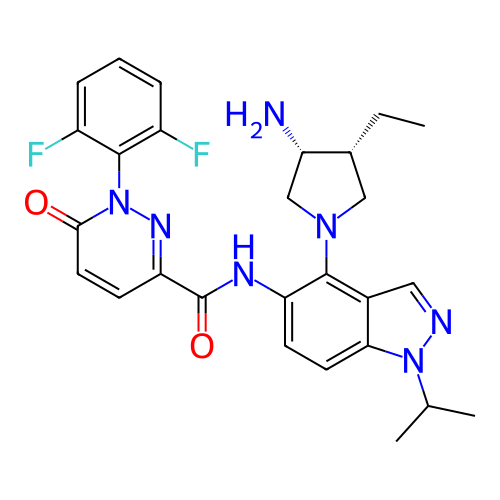 US20250122174, Example 196 BDBM730614
US20250122174, Example 196 BDBM730614 US20250129078, Compound 196 BDBM735178
US20250129078, Compound 196 BDBM735178 US20250145633, Example 196 BDBM738982
US20250145633, Example 196 BDBM738982 US20250154139, Compound 196 BDBM740569
US20250154139, Compound 196 BDBM740569 US20250170122, Compound 196 BDBM744272
US20250170122, Compound 196 BDBM744272 US20250179047, Example 196 BDBM747026
US20250179047, Example 196 BDBM747026 US20250188079, Compound 196 BDBM749143
US20250188079, Compound 196 BDBM749143 US20250195475, Example 196 BDBM750544
US20250195475, Example 196 BDBM750544 US20250195518, Example 196 BDBM750602
US20250195518, Example 196 BDBM750602 US20250206717, Example 196 BDBM753783
US20250206717, Example 196 BDBM753783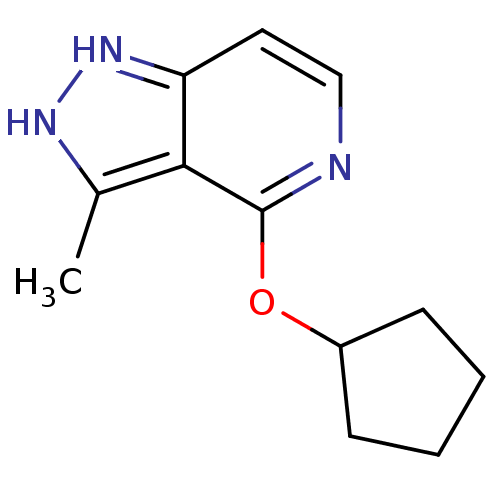 US8569281, 196 BDBM105159 CHEMBL2152709
US8569281, 196 BDBM105159 CHEMBL2152709 US8633183, D-196 BDBM115812
US8633183, D-196 BDBM115812 US8653087, III-196 BDBM117516
US8653087, III-196 BDBM117516 US9150577, 196 CHEMBL1933145 BDBM50360285
US9150577, 196 CHEMBL1933145 BDBM50360285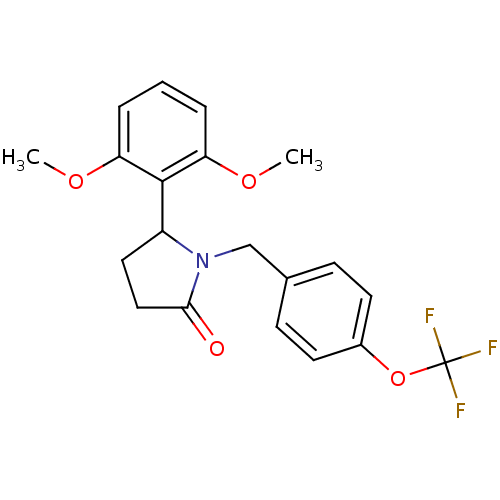 US9242970, 196 CHEMBL3113773 BDBM50447783
US9242970, 196 CHEMBL3113773 BDBM50447783 US9296745, 196-B BDBM215630
US9296745, 196-B BDBM215630 US9359338, Exe. 196 BDBM235586
US9359338, Exe. 196 BDBM235586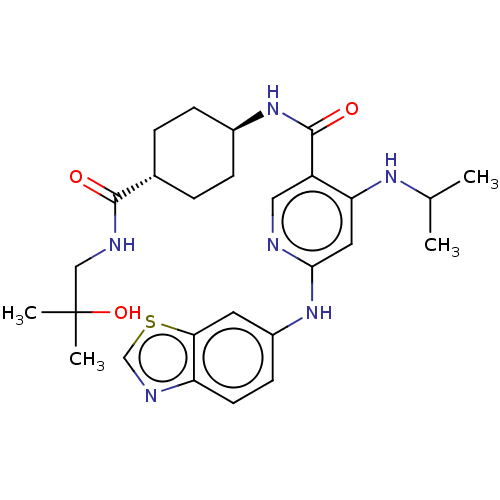 US9546153, ex. 196 BDBM209849
US9546153, ex. 196 BDBM209849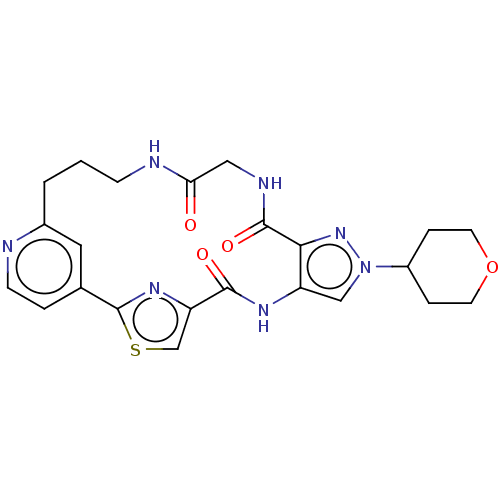 US9617282, Example 196 BDBM317421
US9617282, Example 196 BDBM317421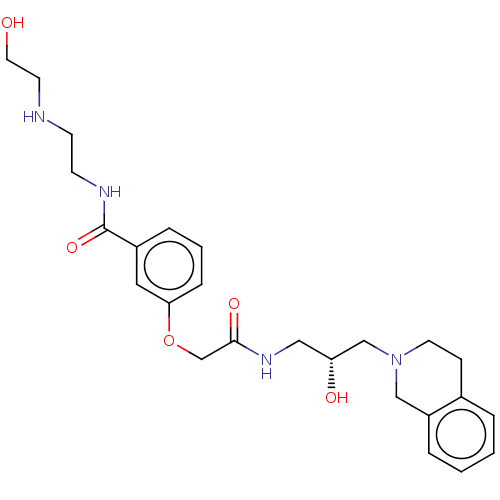 US9745291, Compound 196 BDBM337150
US9745291, Compound 196 BDBM337150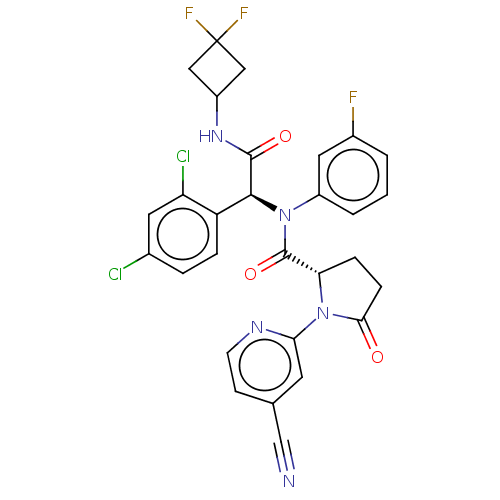 US9850277, Compound 196 BDBM363709
US9850277, Compound 196 BDBM363709 US9873693, Compound 196 BDBM366273
US9873693, Compound 196 BDBM366273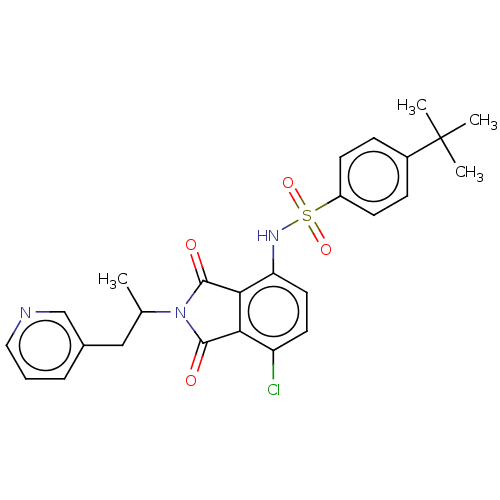 US9969687, Compound 196 BDBM393532
US9969687, Compound 196 BDBM393532 WO2022091056, Example 196 BDBM548205
WO2022091056, Example 196 BDBM548205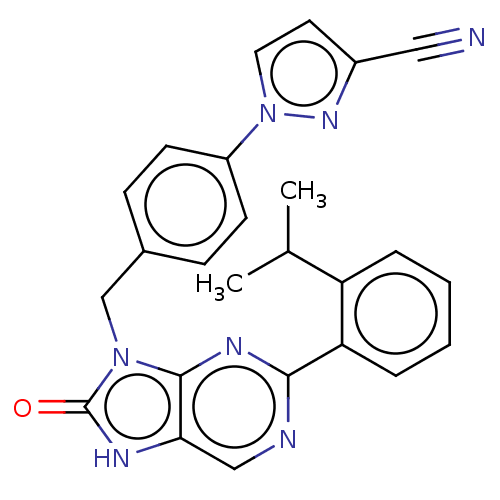 BDBM324990 US10189841, Compound I-196 1-(4-((2-(2- US10399980, Compound I-196
BDBM324990 US10189841, Compound I-196 1-(4-((2-(2- US10399980, Compound I-196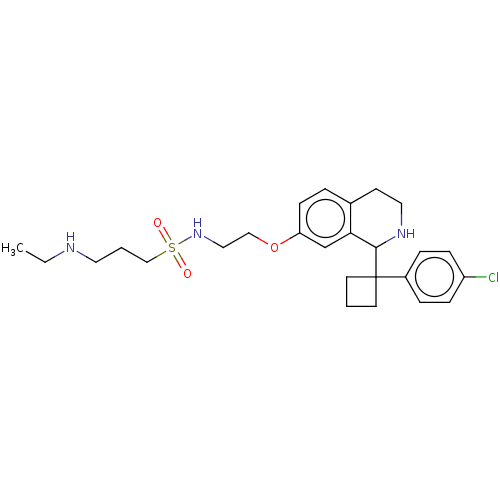 BDBM141293 US8653100, 196 US8653100, 187
BDBM141293 US8653100, 196 US8653100, 187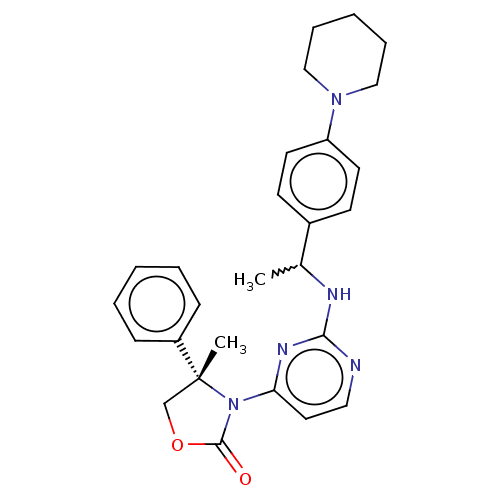 BDBM146148 US8957068, 195 US8957068, 196
BDBM146148 US8957068, 195 US8957068, 196 BDBM179490 US9675697, Cpd. No. 196
BDBM179490 US9675697, Cpd. No. 196 BDBM195177 US9212153, 239,Ex. 196
BDBM195177 US9212153, 239,Ex. 196 BDBM201429 US9187424, 196 US9187424, 195
BDBM201429 US9187424, 196 US9187424, 195 BDBM244778 US9550763, Compound I-196
BDBM244778 US9550763, Compound I-196 BDBM332907 US10196390, Compound I-196
BDBM332907 US10196390, Compound I-196 BDBM351101 US9796700, Compound I-196
BDBM351101 US9796700, Compound I-196 BDBM370309 US10233188, Example 196 BDBM467204
BDBM370309 US10233188, Example 196 BDBM467204 BDBM441272 US10640495, Example I-196
BDBM441272 US10640495, Example I-196 BDBM461729 US10774071, Example B-196
BDBM461729 US10774071, Example B-196 BDBM465546 US10793568, Compound I-196
BDBM465546 US10793568, Compound I-196 BDBM482129 US10913753, Compound I-196
BDBM482129 US10913753, Compound I-196 BDBM513406 US11091472, Compound S-196
BDBM513406 US11091472, Compound S-196 BDBM526852 US11186577, Cmpd. No. 196
BDBM526852 US11186577, Cmpd. No. 196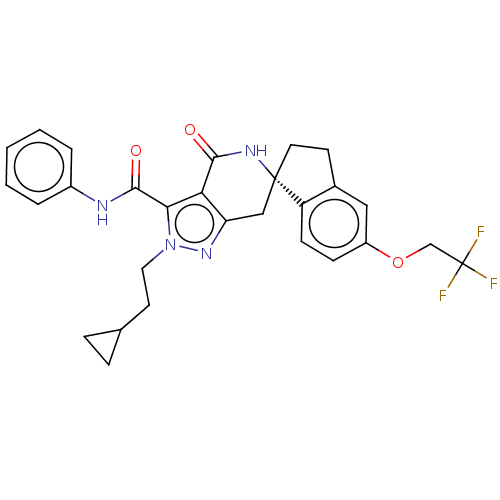 BDBM528985 US11198695, Example I-196
BDBM528985 US11198695, Example I-196 BDBM532937 US11214565, Example D-196
BDBM532937 US11214565, Example D-196 BDBM554747 US11339144, Compound I-196
BDBM554747 US11339144, Compound I-196 BDBM566827 US11420958, Ex. No. 196
BDBM566827 US11420958, Ex. No. 196 BDBM582187 US11518764, Compound I-196
BDBM582187 US11518764, Compound I-196 BDBM589455 US11555012, Compound I-196
BDBM589455 US11555012, Compound I-196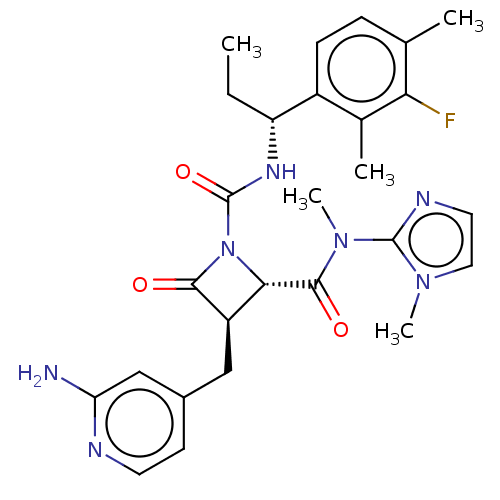 BDBM607798 US11691962, Compound 1-196
BDBM607798 US11691962, Compound 1-196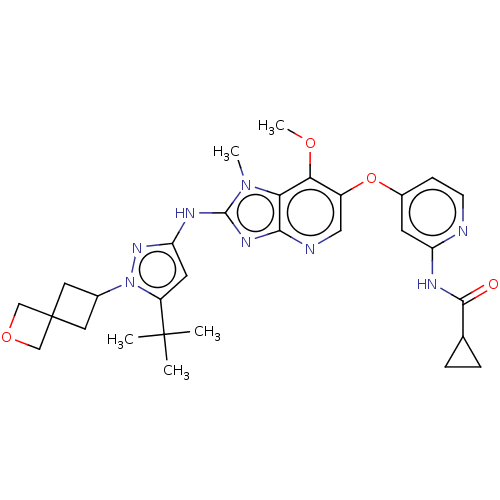 BDBM608047 US11691963, Example I-196
BDBM608047 US11691963, Example I-196 BDBM638979 US20230390274, Compound A-196
BDBM638979 US20230390274, Compound A-196 BDBM713517 US20250019387, Table 1a.196
BDBM713517 US20250019387, Table 1a.196 BDBM727335 US20250090540, Example I-196
BDBM727335 US20250090540, Example I-196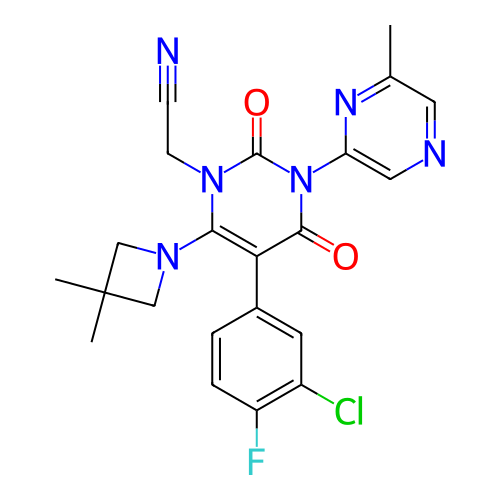 BDBM728582 US20250092056, Compound I-196
BDBM728582 US20250092056, Compound I-196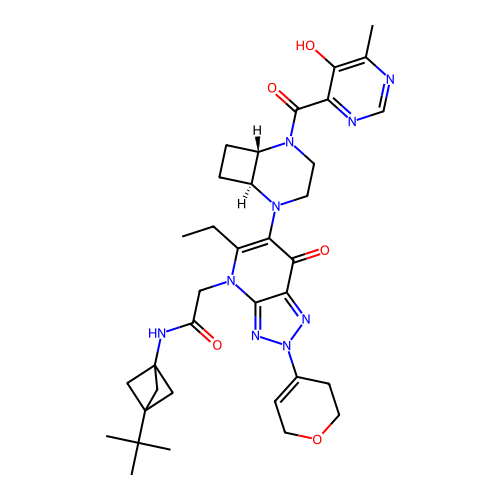 BDBM734959 US20250129069, Compound I-196
BDBM734959 US20250129069, Compound I-196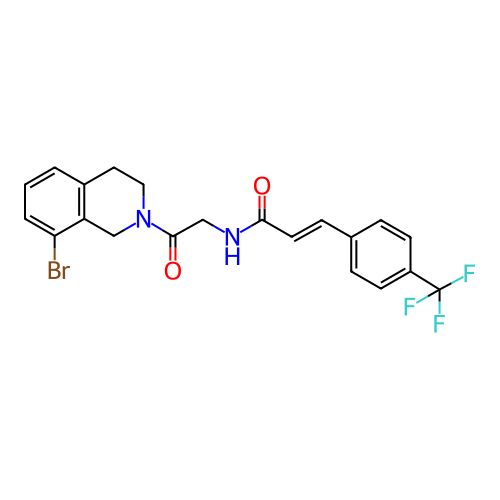 BDBM744596 US20250170126, Compound I-196
BDBM744596 US20250170126, Compound I-196 CHEMBL2417788 US11279687, Compound 196 BDBM50439675
CHEMBL2417788 US11279687, Compound 196 BDBM50439675 CHEMBL4452794 US11274105, Example 196 BDBM50514217
CHEMBL4452794 US11274105, Example 196 BDBM50514217 CHEMBL4641728 BDBM50541705 US11926614, Example 196
CHEMBL4641728 BDBM50541705 US11926614, Example 196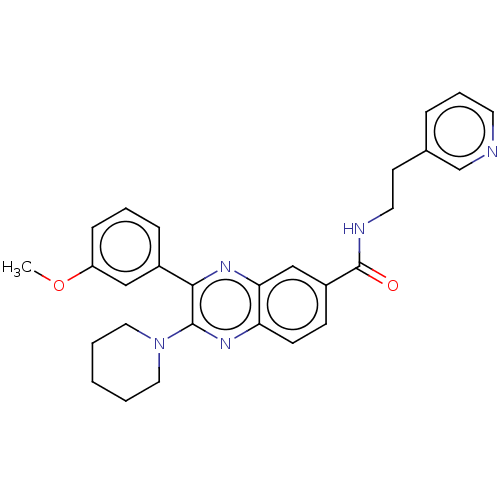 US10144742, Compound I-196 BDBM306034
US10144742, Compound I-196 BDBM306034 US10150728, Example I-196 BDBM306983
US10150728, Example I-196 BDBM306983 US10189809, Compound I-196 BDBM323943
US10189809, Compound I-196 BDBM323943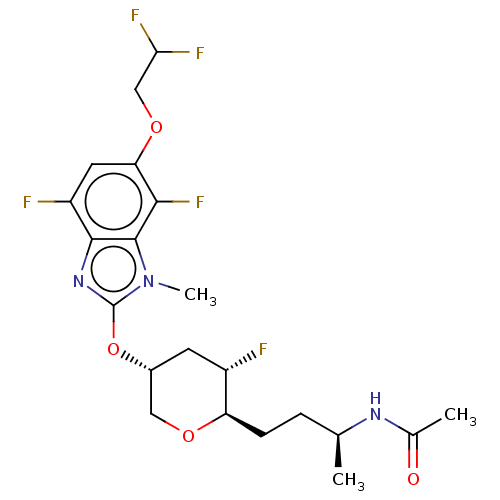 US10233156, Example I-196 BDBM369657
US10233156, Example I-196 BDBM369657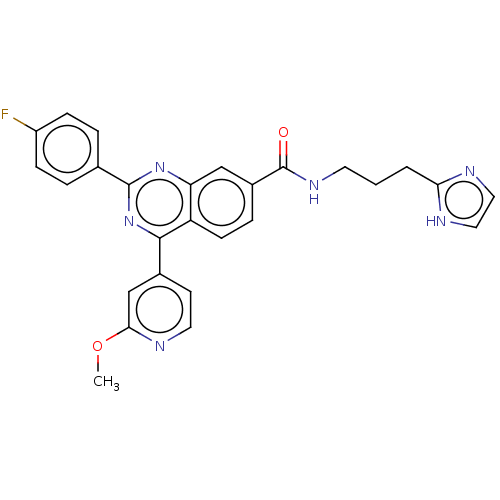 US10323018, Compound I-196 BDBM398467
US10323018, Compound I-196 BDBM398467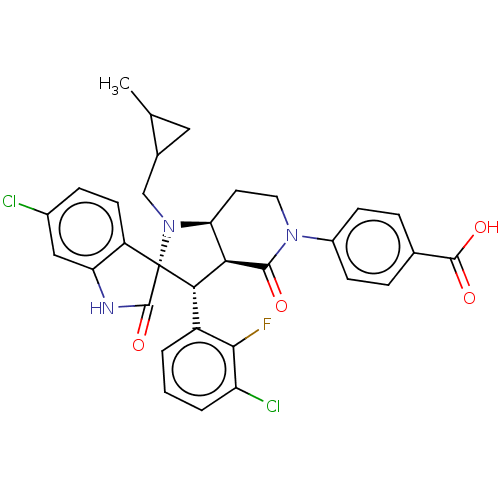 US10576064, Example I-196 BDBM431991
US10576064, Example I-196 BDBM431991 US10781204, Compound I-196 BDBM463719
US10781204, Compound I-196 BDBM463719 US10793563, Compound I-196 BDBM464864
US10793563, Compound I-196 BDBM464864 US10919885, Compound No. 196 BDBM483047
US10919885, Compound No. 196 BDBM483047 US11198695, Example II-196 BDBM529260
US11198695, Example II-196 BDBM529260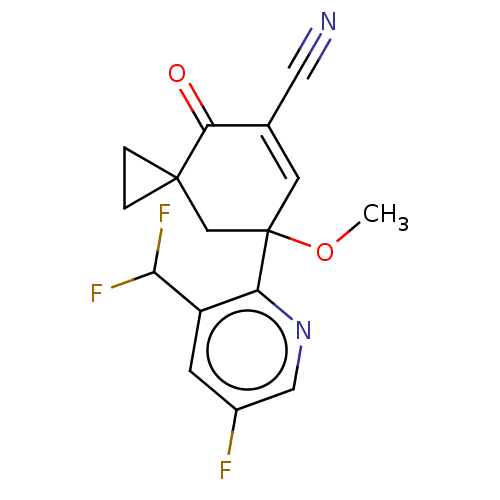 US11479539, Compound 196 CHEMBL4462699 BDBM50502723
US11479539, Compound 196 CHEMBL4462699 BDBM50502723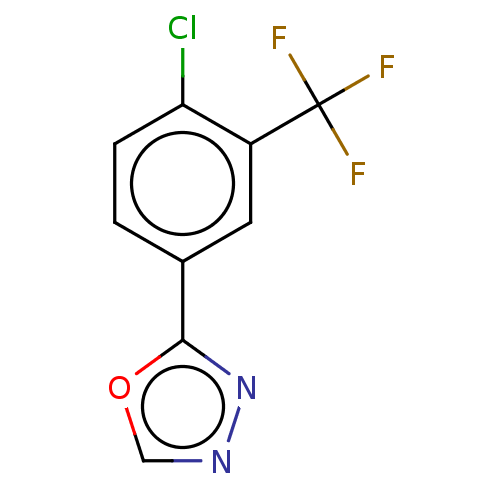 US11634391, Compound 196 CHEMBL4760228 BDBM50554450
US11634391, Compound 196 CHEMBL4760228 BDBM50554450 US11685732, Compound I-196 BDBM606552
US11685732, Compound I-196 BDBM606552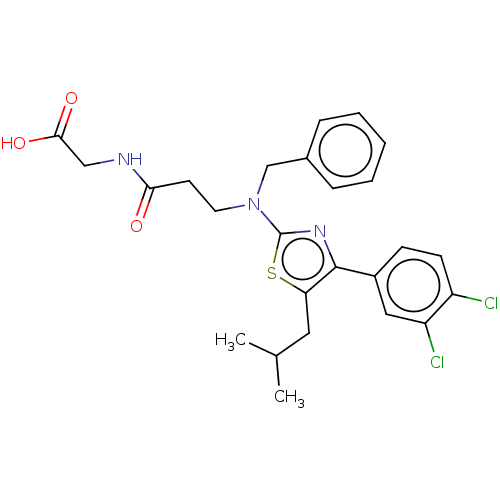 US11753403, Compound I-196 BDBM617056
US11753403, Compound I-196 BDBM617056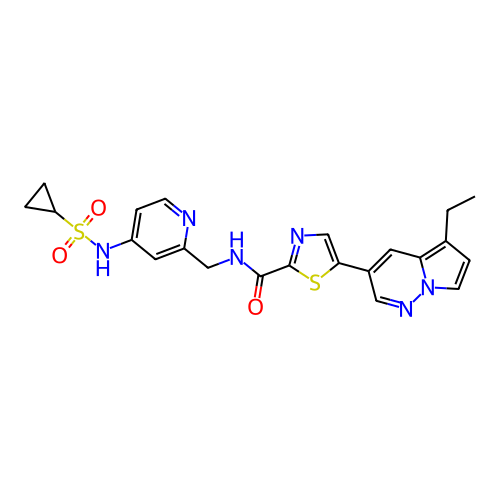 US12331046, Compound I-196 BDBM750057
US12331046, Compound I-196 BDBM750057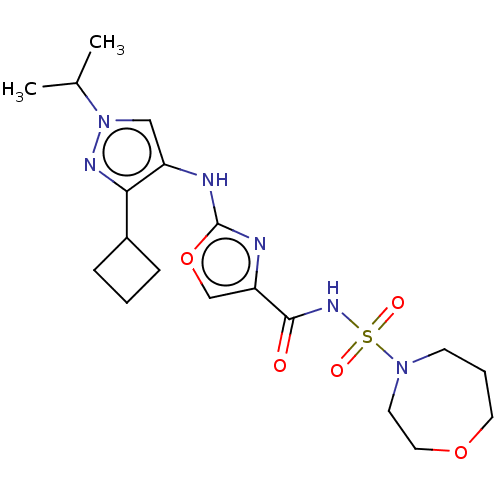 US20230399319, Example 2-196 BDBM640642
US20230399319, Example 2-196 BDBM640642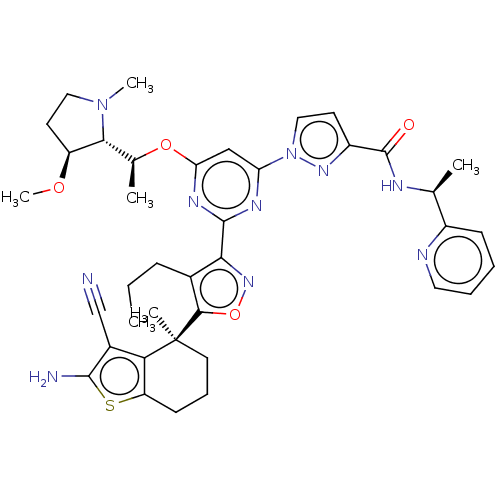 US20240174690, Example II-196 BDBM678347
US20240174690, Example II-196 BDBM678347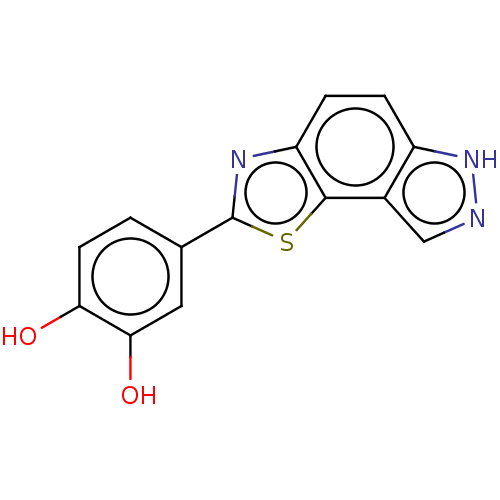 US20240199561, Compound FC-196 BDBM681344
US20240199561, Compound FC-196 BDBM681344 US20240294545, Compound A-196 BDBM694774
US20240294545, Compound A-196 BDBM694774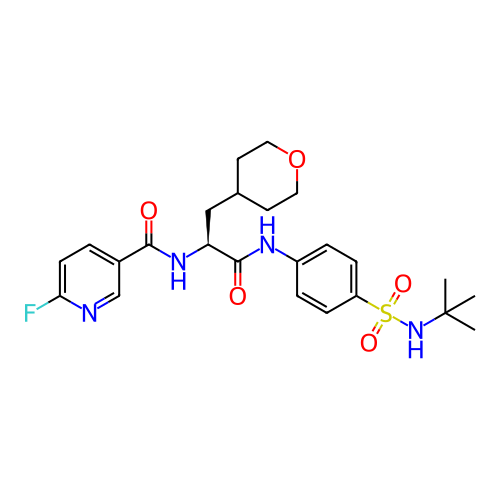 US20250162989, Compound I-196 BDBM741394
US20250162989, Compound I-196 BDBM741394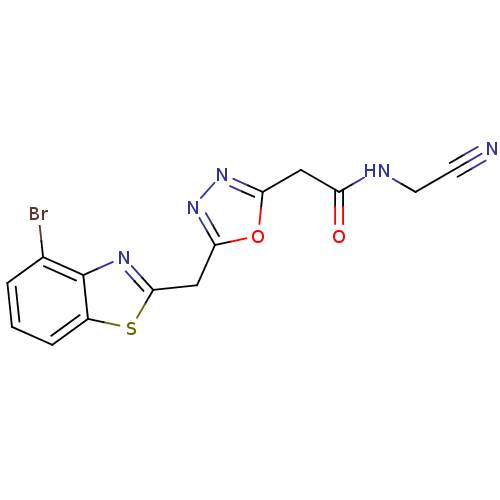 US8754113, I-2-196 BDBM123892
US8754113, I-2-196 BDBM123892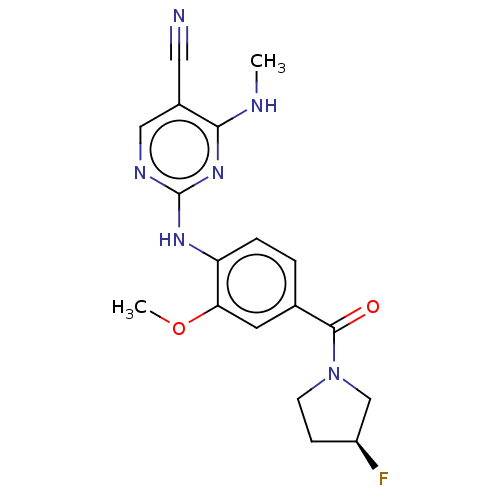 US8802674, 196 US8802674, 210 BDBM129108
US8802674, 196 US8802674, 210 BDBM129108 US9682940, 197 US9682940, 196 BDBM118934
US9682940, 197 US9682940, 196 BDBM118934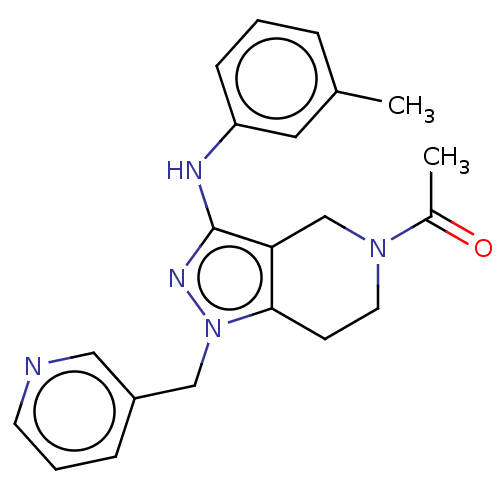 US9763922, Example 196 CHEMBL3986361 BDBM50200434
US9763922, Example 196 CHEMBL3986361 BDBM50200434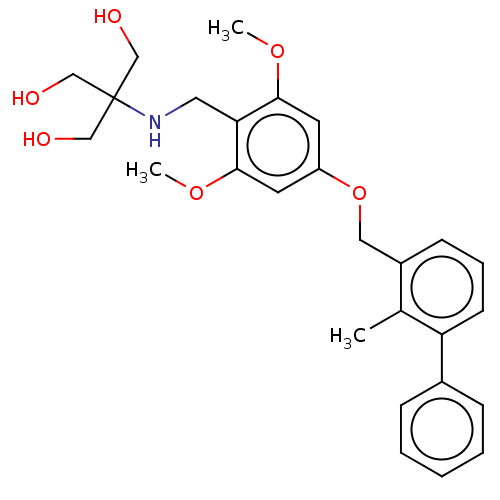 US9872852, Example 196 CHEMBL3582257 BDBM50091329
US9872852, Example 196 CHEMBL3582257 BDBM50091329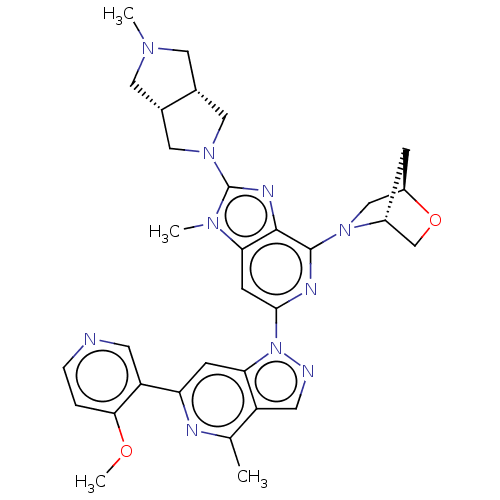 WO2022090481, Example I-196 BDBM547975
WO2022090481, Example I-196 BDBM547975 US10034861, Example 196 US10512632, Example 196 BDBM279165 US11634416, Example 196 1-isopropyl-3-methyl-5-(2-propoxy-3-pyridyl)-N-(1H-pyrazol-4-ylmethyl)pyrazolo[4,3-b]pyridin-7-amine US11491140, Example 196
US10034861, Example 196 US10512632, Example 196 BDBM279165 US11634416, Example 196 1-isopropyl-3-methyl-5-(2-propoxy-3-pyridyl)-N-(1H-pyrazol-4-ylmethyl)pyrazolo[4,3-b]pyridin-7-amine US11491140, Example 196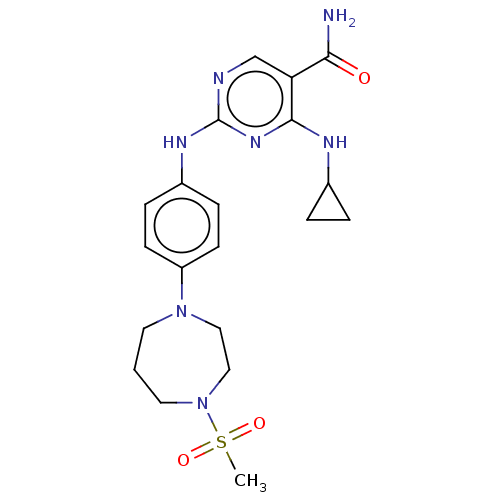 4-(cyclopropylamino)-2-(4-(4-(methylsulfonyl)-1,4-diazepan-1-yl)phenylamino)pyrimidine-5-carboxamide BDBM365480 US10533001, Example 196 US11414410, Example 196 US9868729, Example 196
4-(cyclopropylamino)-2-(4-(4-(methylsulfonyl)-1,4-diazepan-1-yl)phenylamino)pyrimidine-5-carboxamide BDBM365480 US10533001, Example 196 US11414410, Example 196 US9868729, Example 196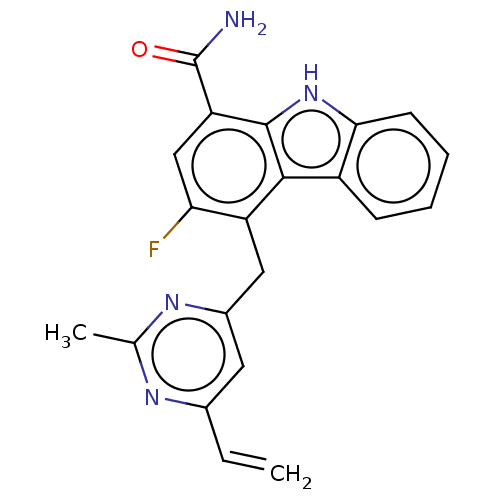 BDBM377503 3-fluoro-4-((2-methyl-6- vinylpyrimidin-4- yl)methyl)-9H-carbazole-1- carboxamide US10676434, Example 196 US10266491, Example 196 US11053197, Example 196
BDBM377503 3-fluoro-4-((2-methyl-6- vinylpyrimidin-4- yl)methyl)-9H-carbazole-1- carboxamide US10676434, Example 196 US10266491, Example 196 US11053197, Example 196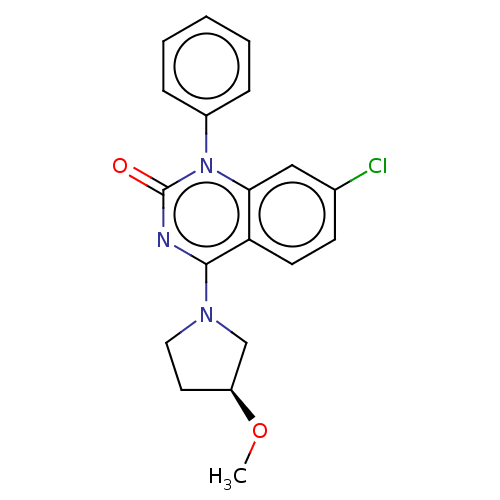 US11130759, Cpd. No. 196 (S)-7-chloro-4-(3-methoxypyrrolidin-1- yl)-1-phenylquinazolin-2(1H)-one US11084798, Cpd No 196 US11046691, Compound 196 BDBM507783
US11130759, Cpd. No. 196 (S)-7-chloro-4-(3-methoxypyrrolidin-1- yl)-1-phenylquinazolin-2(1H)-one US11084798, Cpd No 196 US11046691, Compound 196 BDBM507783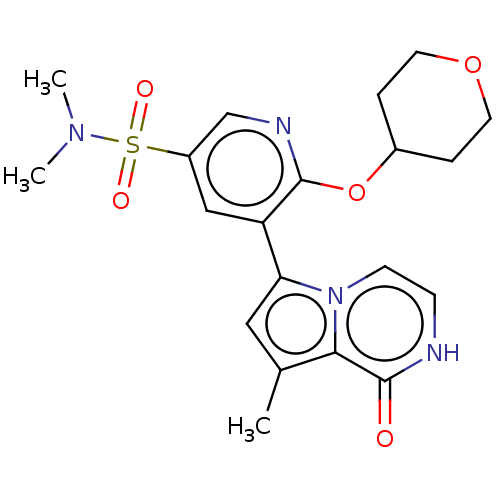 BDBM570181 US11427593, Compound ZB-BD-196
BDBM570181 US11427593, Compound ZB-BD-196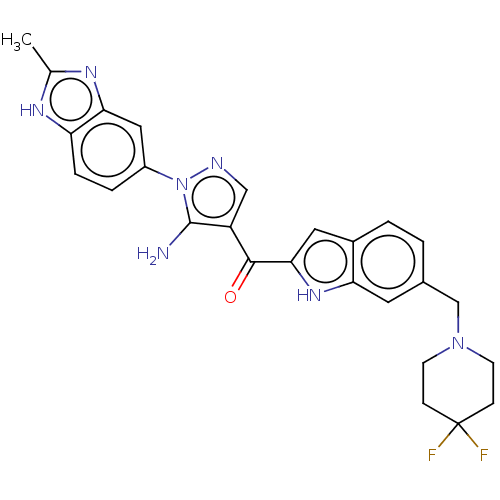 US10689705, Compound 7 BDBM130903 US8829199, 196
US10689705, Compound 7 BDBM130903 US8829199, 196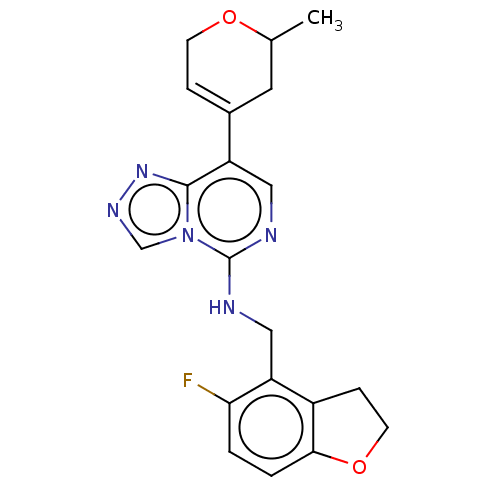 US11013745, Compound SL-ZYE-196 BDBM498168
US11013745, Compound SL-ZYE-196 BDBM498168 US11591319, ID lqr-8-196 BDBM595509
US11591319, ID lqr-8-196 BDBM595509 US8575186, 195/196 US8575186, 212 BDBM104432
US8575186, 195/196 US8575186, 212 BDBM104432 BDBM323584 US10188615, Example 196 US10639286, Example 196 4-(3-(Cyclohexylmethoxy)Phenyl)but-3-Yn-1-Amine
BDBM323584 US10188615, Example 196 US10639286, Example 196 4-(3-(Cyclohexylmethoxy)Phenyl)but-3-Yn-1-Amine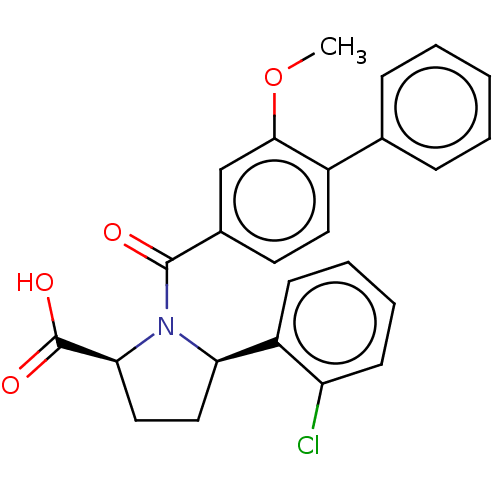 US10017468, Compound 196 US11072582, Compound 196 US10781171, Compound 196 (2S,5R)-5-(2-chlorophenyl)-1-(2-methoxy-[1,1'-biphenyl]-4-carbonyl)pyrrolidine-2-carboxylic acid BDBM403931
US10017468, Compound 196 US11072582, Compound 196 US10781171, Compound 196 (2S,5R)-5-(2-chlorophenyl)-1-(2-methoxy-[1,1'-biphenyl]-4-carbonyl)pyrrolidine-2-carboxylic acid BDBM403931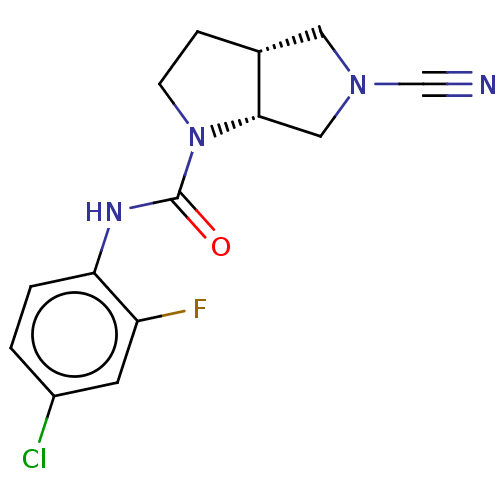 US10343992, Example 196 BDBM404544 US11390584, Example 196 (3aR,6aR)-N-(4-chloro-2-fluorophenyl)-5-cyanohexahydropyrrolo[3, 4-b]pyrrole-1(2H)-carboxamide US10689345, Example 196
US10343992, Example 196 BDBM404544 US11390584, Example 196 (3aR,6aR)-N-(4-chloro-2-fluorophenyl)-5-cyanohexahydropyrrolo[3, 4-b]pyrrole-1(2H)-carboxamide US10689345, Example 196 US11261191, Example 196 BDBM388866 2-Fluoro-N-methyl-4-[3-(1-quinolin-6-ylcyclopropyl)imidazo[1,2-a]pyrimidin-6-yl]benzenesulfonamide US9944645, 196 US10738052, Example 196
US11261191, Example 196 BDBM388866 2-Fluoro-N-methyl-4-[3-(1-quinolin-6-ylcyclopropyl)imidazo[1,2-a]pyrimidin-6-yl]benzenesulfonamide US9944645, 196 US10738052, Example 196 3-Cyclohexylmethyl-6,6- dimethyl-3,5,6,7-tetrahydro- benzoimidazol-4-one BDBM473929 US10858342, Compound C-ACP
3-Cyclohexylmethyl-6,6- dimethyl-3,5,6,7-tetrahydro- benzoimidazol-4-one BDBM473929 US10858342, Compound C-ACP 1-{4-[(2S)-2,3-dihydro-1,4- benzodioxin-2- yl]benzyl]pyrrolidine BDBM326981 US9662339, 196 US11957671, Compound 196
1-{4-[(2S)-2,3-dihydro-1,4- benzodioxin-2- yl]benzyl]pyrrolidine BDBM326981 US9662339, 196 US11957671, Compound 196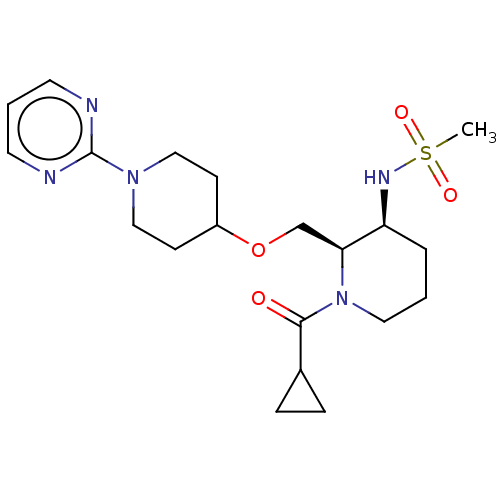 BDBM386222 US11292766, Example 196 US10508083, Example 196 N-(cis-1-(cyclopropylcarbonyl)-2- (((1-(pyrimidin-2-yl)piperidin-4- yl)oxy)methyl)piperidin-3- yl)methanesulfonamide US10287305, Example 196
BDBM386222 US11292766, Example 196 US10508083, Example 196 N-(cis-1-(cyclopropylcarbonyl)-2- (((1-(pyrimidin-2-yl)piperidin-4- yl)oxy)methyl)piperidin-3- yl)methanesulfonamide US10287305, Example 196 US11352360, Example 196' US9884868, Example 196' (6-(1-(2,2-difluoroethyl)-4-(4-fluorophenyl)-1H- imidazol-5-yl)imidazo[1,2-b]pyridazin-3- yl)methanamine US10287295, Example 196' BDBM282993
US11352360, Example 196' US9884868, Example 196' (6-(1-(2,2-difluoroethyl)-4-(4-fluorophenyl)-1H- imidazol-5-yl)imidazo[1,2-b]pyridazin-3- yl)methanamine US10287295, Example 196' BDBM282993 BDBM219522 US9303033, O27, Table 37A, Compound 196
BDBM219522 US9303033, O27, Table 37A, Compound 196 BDBM270800 US10059705, II-196 US10059705, II-195
BDBM270800 US10059705, II-196 US10059705, II-195 BDBM433721 US10562878, Compound 200 US10562878, Compound 196
BDBM433721 US10562878, Compound 200 US10562878, Compound 196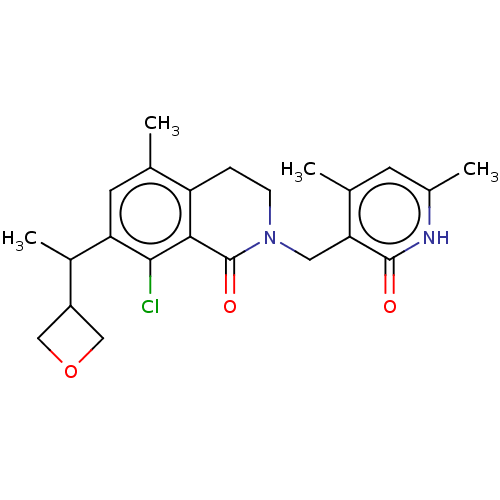 BDBM435689 US10570121, Example 195 US10570121, Example 196
BDBM435689 US10570121, Example 195 US10570121, Example 196 BDBM490319 US10968215, Compound 196 US10968215, Compound 207
BDBM490319 US10968215, Compound 196 US10968215, Compound 207 BDBM520914 US11149018, Example 196 US11149018, Example 195
BDBM520914 US11149018, Example 196 US11149018, Example 195 BDBM548188 WO2022091056, Example 196 WO2022091056, Example 179
BDBM548188 WO2022091056, Example 196 WO2022091056, Example 179 BDBM563882 Roche-Dataset for PDE10A, Compound 196
BDBM563882 Roche-Dataset for PDE10A, Compound 196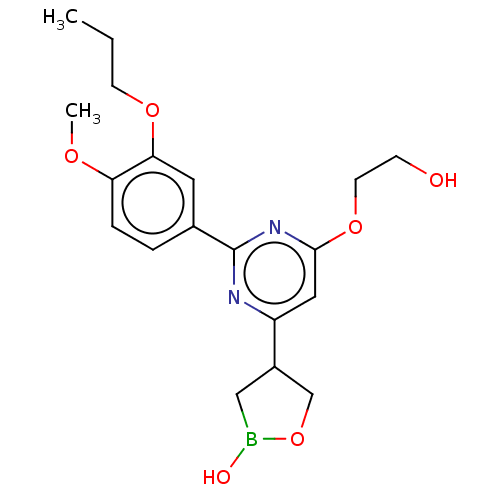 BDBM589948 US11559538, Example 196 US11559538, Example 195
BDBM589948 US11559538, Example 196 US11559538, Example 195 BDBM594396 US11584747, Example 196 US11584747, Example 197
BDBM594396 US11584747, Example 196 US11584747, Example 197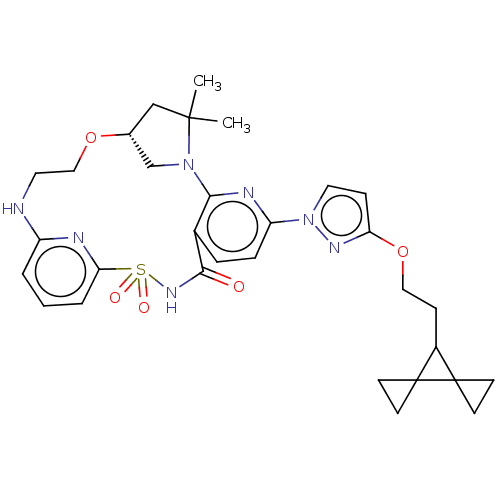 BDBM644783 US11866450, Compound 196 US11866450, Compound 197
BDBM644783 US11866450, Compound 196 US11866450, Compound 197 BDBM710558 US12187701, Compound FMF-03-196-1
BDBM710558 US12187701, Compound FMF-03-196-1 BDBM710559 US12187701, Compound FMF-03-196-2
BDBM710559 US12187701, Compound FMF-03-196-2 BDBM710589 US12187701, Compound FMF-04-196-1
BDBM710589 US12187701, Compound FMF-04-196-1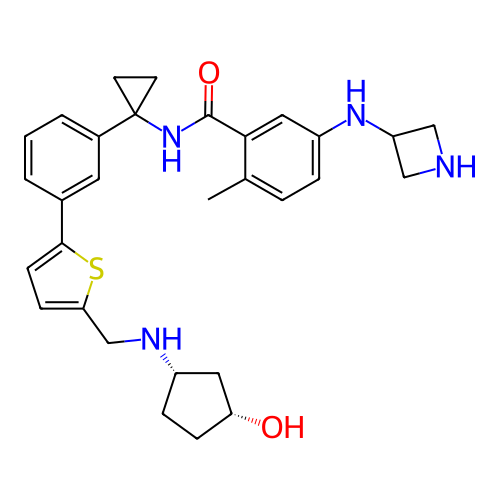 BDBM714169 US20250025443, Example 203 US20250025443, Compound 196
BDBM714169 US20250025443, Example 203 US20250025443, Compound 196 US10323022, Example 199 BDBM398719 US10323022, Example 196
US10323022, Example 199 BDBM398719 US10323022, Example 196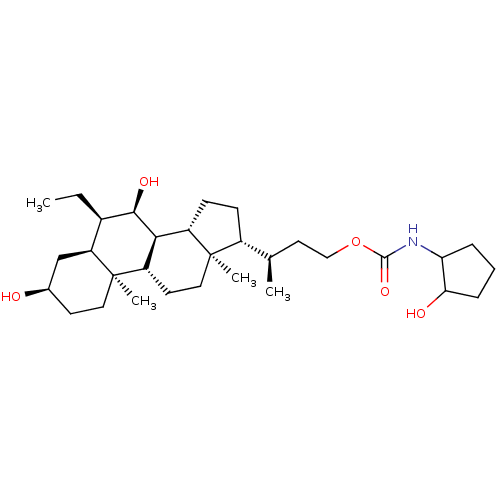 US10457703, Example 196 BDBM419533 US10457703, Example 195
US10457703, Example 196 BDBM419533 US10457703, Example 195 US11053225, Compound 101 BDBM515820 US11053225, Compound 196
US11053225, Compound 101 BDBM515820 US11053225, Compound 196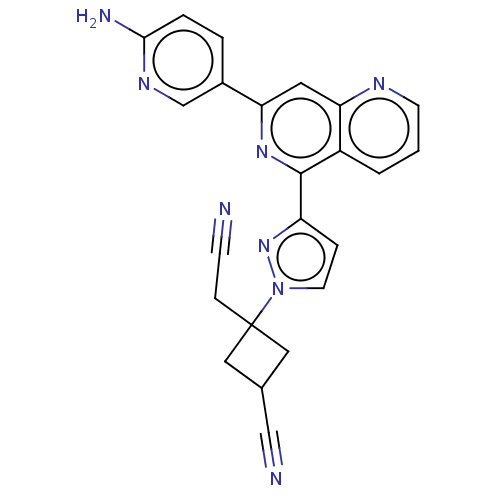 US11254668, Example 195 US11254668, Example 196 BDBM538854
US11254668, Example 195 US11254668, Example 196 BDBM538854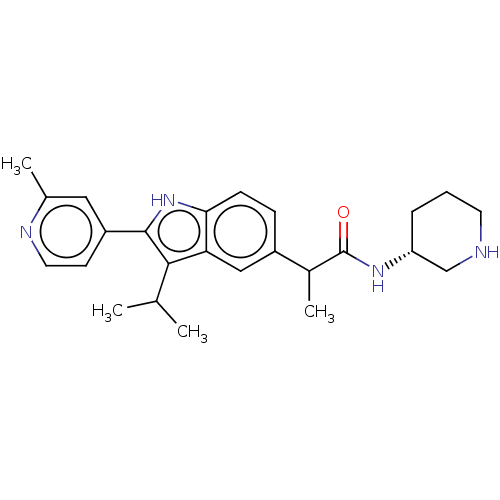 US11420973, Example 195 BDBM568317 US11420973, Example 196
US11420973, Example 195 BDBM568317 US11420973, Example 196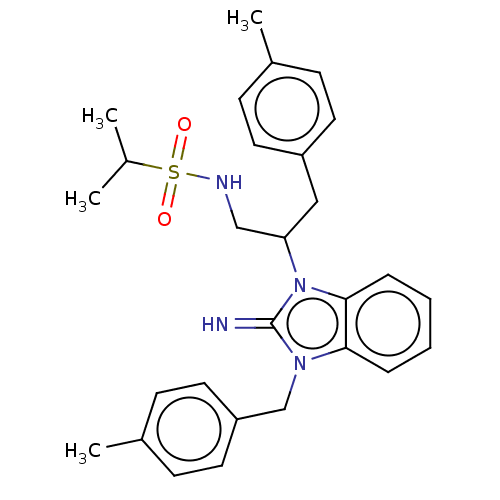 US11479560, Title KM-4-196-1 BDBM579294
US11479560, Title KM-4-196-1 BDBM579294 US11999676, Compound 197 BDBM679333 US11999676, Compound 196
US11999676, Compound 197 BDBM679333 US11999676, Compound 196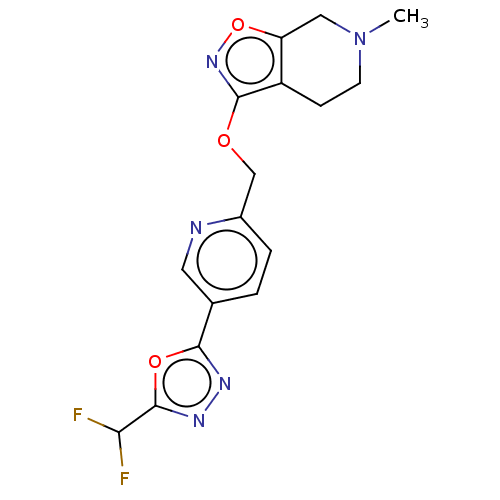 US20230322747, Compound 191 US20230322747, Compound 196 BDBM625150
US20230322747, Compound 191 US20230322747, Compound 196 BDBM625150 US20230322789, Example 163 US20230322789, Compound 196 BDBM625562
US20230322789, Example 163 US20230322789, Compound 196 BDBM625562 US20250025443, Example 196 US20250025443, Compound 338 BDBM714162
US20250025443, Example 196 US20250025443, Compound 338 BDBM714162 US8497281, 196 BDBM99418 US9663470, 129 US9120769, 129
US8497281, 196 BDBM99418 US9663470, 129 US9120769, 129 US9181272, 196 US9181272, 184 US9181272, 195 BDBM191414
US9181272, 196 US9181272, 184 US9181272, 195 BDBM191414 US9771320, Example 197 US9771320, Example 196 BDBM341962
US9771320, Example 197 US9771320, Example 196 BDBM341962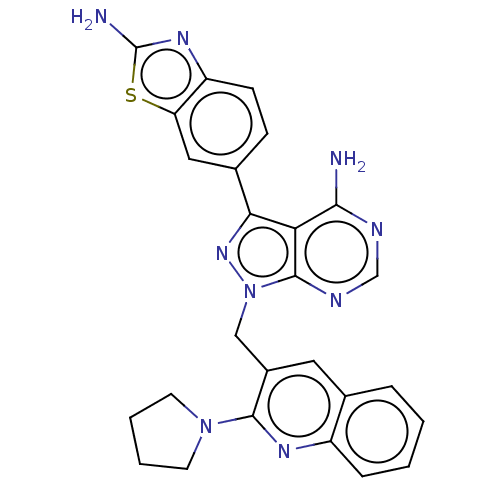 US9790228, Compound 79 BDBM348160 US9790228, Compound 196
US9790228, Compound 79 BDBM348160 US9790228, Compound 196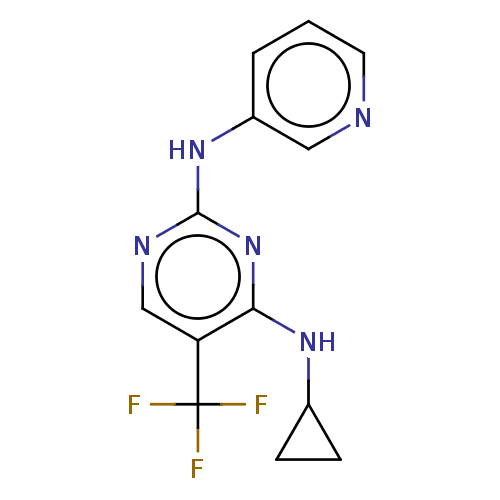 N4-cyclopropyl-N2-(pyridin-3-yl)-5-(trifluoromethyl) pyrimidine-2,4-diamine US10266549, Example 196 US10774092, Example 196 BDBM379312
N4-cyclopropyl-N2-(pyridin-3-yl)-5-(trifluoromethyl) pyrimidine-2,4-diamine US10266549, Example 196 US10774092, Example 196 BDBM379312 BDBM316701 US20250134869, Example 196 US11111242, Example 196 2-{[(3R,4R)-1-acryloyl-3-hydroxypiperidin-4-yl]amino}-N-(propan-2-yl)-5H-pyrrolo[2,3-b]pyrazine-7-carboxamide US9617258, Example 196
BDBM316701 US20250134869, Example 196 US11111242, Example 196 2-{[(3R,4R)-1-acryloyl-3-hydroxypiperidin-4-yl]amino}-N-(propan-2-yl)-5H-pyrrolo[2,3-b]pyrazine-7-carboxamide US9617258, Example 196 Intermediate 1-2-29 BDBM415096 US10428044, Example 196
Intermediate 1-2-29 BDBM415096 US10428044, Example 196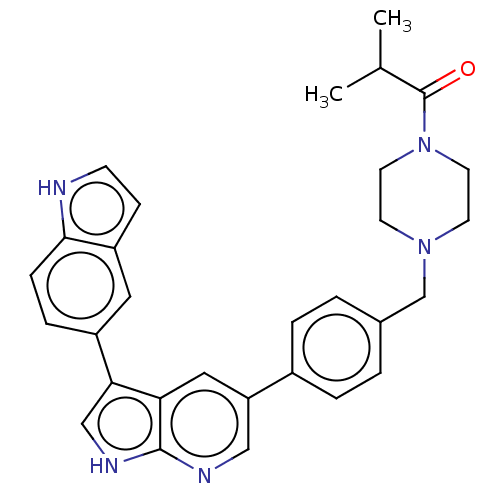 US10485800, Example 196 BDBM420229 US10485800, Cmpd ID GS
US10485800, Example 196 BDBM420229 US10485800, Cmpd ID GS 2-[[1-[4-Chloro-3-(difluoromethoxy)phenyl]triazol-4-yl]methoxy]-5-(3- US10233173, Example 196 US10071988, Example 196 BDBM276258
2-[[1-[4-Chloro-3-(difluoromethoxy)phenyl]triazol-4-yl]methoxy]-5-(3- US10233173, Example 196 US10071988, Example 196 BDBM276258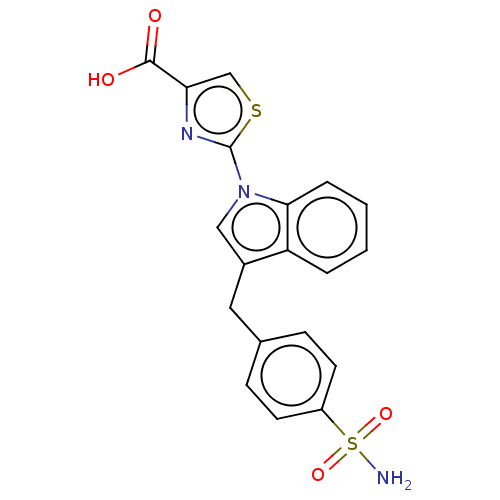 US10961200, Compound 196 2-(3-(4- sulfamoylbenzyl)- 1H-indol-1- yl)thiazole-4- carboxylic acid BDBM488818 US11247971, Cmpd ID 196
US10961200, Compound 196 2-(3-(4- sulfamoylbenzyl)- 1H-indol-1- yl)thiazole-4- carboxylic acid BDBM488818 US11247971, Cmpd ID 196 US11319329, Ex # 196 N-((1R,3S)-3-Acrylamidocyclopentyl)-5-(*S)-(2-methyl-4- phenoxyphenyl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide US10934310, Ex # 196 BDBM485362 US12065446, Example 196
US11319329, Ex # 196 N-((1R,3S)-3-Acrylamidocyclopentyl)-5-(*S)-(2-methyl-4- phenoxyphenyl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide US10934310, Ex # 196 BDBM485362 US12065446, Example 196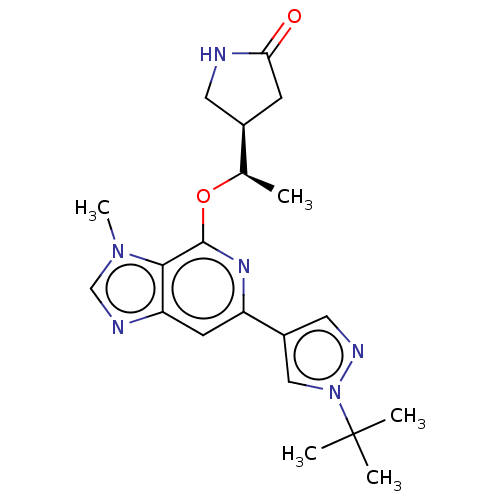 BDBM311137 example 3A.02, on page 196 of WO15017610
BDBM311137 example 3A.02, on page 196 of WO15017610 BDBM522638 US11161848, Compound I-196 1-(4-((2-(2-
BDBM522638 US11161848, Compound I-196 1-(4-((2-(2- BDBM680977 US12012467, Example 196 US12012467, Compound ZBB-02-113
BDBM680977 US12012467, Example 196 US12012467, Compound ZBB-02-113 CHEMBL1829759 US8575157, 196 BDBM50353405 US8592410, 93 US8592410, Comparator 8
CHEMBL1829759 US8575157, 196 BDBM50353405 US8592410, 93 US8592410, Comparator 8 US8575186, 130/179/180 BDBM104367 US8575186, 196 US8575186, 195
US8575186, 130/179/180 BDBM104367 US8575186, 196 US8575186, 195 US8703768, 196 Roche-Dataset for PDE10A, Compound 870 BDBM120596
US8703768, 196 Roche-Dataset for PDE10A, Compound 870 BDBM120596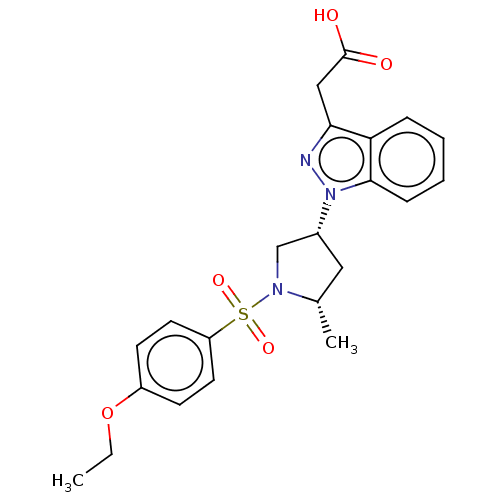 US9725442, Compound I-196 BDBM330644 US9725442, Compound I-211
US9725442, Compound I-196 BDBM330644 US9725442, Compound I-211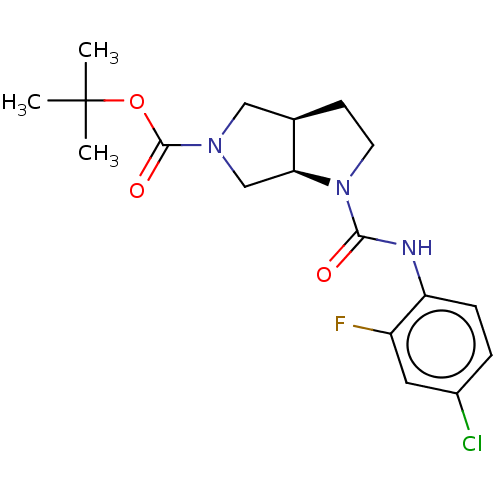 BDBM505524 US11066365, Example 196 US11053198, Example 196 (3aR,6aR)-N-(4-chloro-2-fluorophenyl)-5-cyanohexahydropyrrolo[3,4-b]pyrrole-1 (2H)-carboxamide
BDBM505524 US11066365, Example 196 US11053198, Example 196 (3aR,6aR)-N-(4-chloro-2-fluorophenyl)-5-cyanohexahydropyrrolo[3,4-b]pyrrole-1 (2H)-carboxamide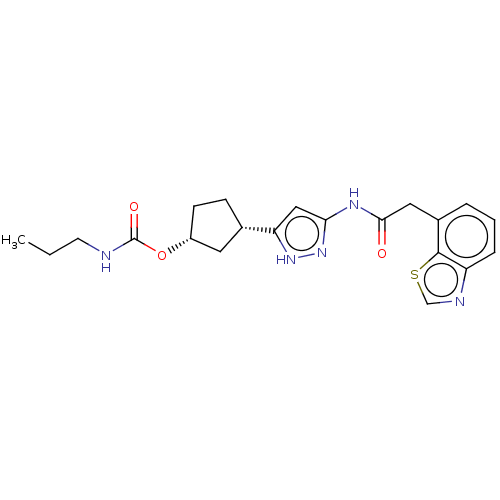 US11718603, Example 196 BDBM498371 (1R,3S)-3-{3-[(1,3- benzothiazol-7- ylacetyl)amino]-1H-pyrazol-5- yl}cyclopentyl propylcarbamate US11014911, Example 196
US11718603, Example 196 BDBM498371 (1R,3S)-3-{3-[(1,3- benzothiazol-7- ylacetyl)amino]-1H-pyrazol-5- yl}cyclopentyl propylcarbamate US11014911, Example 196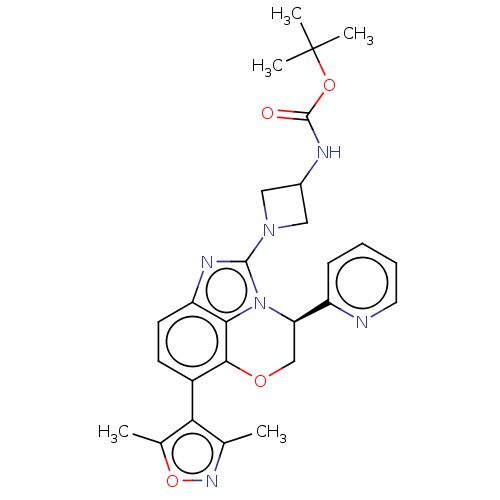 US11498926, Example 196 US9624241, Example 196 tert-butyl {1-[(4S)-7-(3,5- dimethylisoxazol-4-yl)-4-pyridin-2-yl- 4,5-dihydroimidazo[1,5,4- de][1,4]benzoxazin-2-yl]azetidin-3- yl}carbamate BDBM318668 US10464947, Example 196
US11498926, Example 196 US9624241, Example 196 tert-butyl {1-[(4S)-7-(3,5- dimethylisoxazol-4-yl)-4-pyridin-2-yl- 4,5-dihydroimidazo[1,5,4- de][1,4]benzoxazin-2-yl]azetidin-3- yl}carbamate BDBM318668 US10464947, Example 196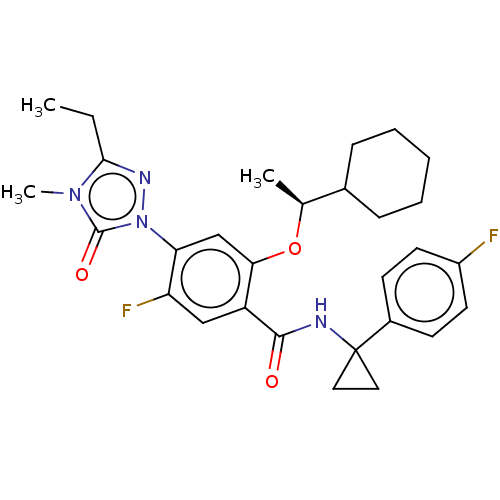 2-[(1S)-1-cyclohexylethoxy]-4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl)-5-fluoro-N-[1-(4-fluorophenyl)cyclopropyl]benzamide US11130745, Example 196 US11713304, Example 196 BDBM470536 US10815215, Example 196
2-[(1S)-1-cyclohexylethoxy]-4-(3-ethyl-4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl)-5-fluoro-N-[1-(4-fluorophenyl)cyclopropyl]benzamide US11130745, Example 196 US11713304, Example 196 BDBM470536 US10815215, Example 196 BDBM395584 (R)-1-((7-cyano-2- (3'-(3-(((R)-1-hydroxy propan-2-ylamino)- methyl)-1,7- naphthyridin-8- ylamino)-2,2'- dimethyl biphenyl- 3-yl)benzo[d]oxazol- US10800768, Example 196 US10308644, Example 196 US11339149, Example 196
BDBM395584 (R)-1-((7-cyano-2- (3'-(3-(((R)-1-hydroxy propan-2-ylamino)- methyl)-1,7- naphthyridin-8- ylamino)-2,2'- dimethyl biphenyl- 3-yl)benzo[d]oxazol- US10800768, Example 196 US10308644, Example 196 US11339149, Example 196 US10526329, Compound 196 US11072611, Compound 196 US9670204, 196 (R)-5-((4-(2-ethyl-3-((4-(4-fluorophenyl)thiazol-2-yl)(methyl)amino)imidazo[1,2-a]pyridin-6-yl)piperidin-1-yl)methyl)oxazolidin-2-one BDBM193292
US10526329, Compound 196 US11072611, Compound 196 US9670204, 196 (R)-5-((4-(2-ethyl-3-((4-(4-fluorophenyl)thiazol-2-yl)(methyl)amino)imidazo[1,2-a]pyridin-6-yl)piperidin-1-yl)methyl)oxazolidin-2-one BDBM193292 US11053246, Example 196 BDBM301380 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-(2-fluorophenyl)-8-(2-morpholin-4-ylethyl)-1,3,4,7-tetrahydro-2H-pyrrolo[3',2':5,6]pyrido[4,3-d]pyrimidin-2-one US10131667, Example 196 US9611267, Example 196
US11053246, Example 196 BDBM301380 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-(2-fluorophenyl)-8-(2-morpholin-4-ylethyl)-1,3,4,7-tetrahydro-2H-pyrrolo[3',2':5,6]pyrido[4,3-d]pyrimidin-2-one US10131667, Example 196 US9611267, Example 196 US10221163, Example 196 BDBM361039 US10544130, Example 196 N1-(2-aminoethyl)-4-(6-aminopyridin-3-yl)-3-(2H-tetrazol-5-yl)benzene-1,2-disulfonamide
US10221163, Example 196 BDBM361039 US10544130, Example 196 N1-(2-aminoethyl)-4-(6-aminopyridin-3-yl)-3-(2H-tetrazol-5-yl)benzene-1,2-disulfonamide US10336775, Example 196 (S)-2-(4-(1-(benzo[d][1,3]dioxol-5-yl)ethyl)piperazin-1-yl)-5-methoxypyrimidine BDBM408272 US11046712, No 196
US10336775, Example 196 (S)-2-(4-(1-(benzo[d][1,3]dioxol-5-yl)ethyl)piperazin-1-yl)-5-methoxypyrimidine BDBM408272 US11046712, No 196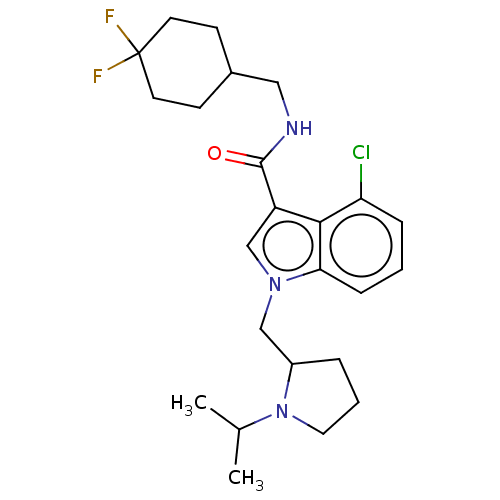 US10676433, Compound 196 BDBM398255 4-chloro-N-((4,4-difluorocyclohexyl)methyl)-1-((1-isopropylpyrrolidin-2-yl)methyl)-1H-indole-3-carboxamide US10323000, Compound 196
US10676433, Compound 196 BDBM398255 4-chloro-N-((4,4-difluorocyclohexyl)methyl)-1-((1-isopropylpyrrolidin-2-yl)methyl)-1H-indole-3-carboxamide US10323000, Compound 196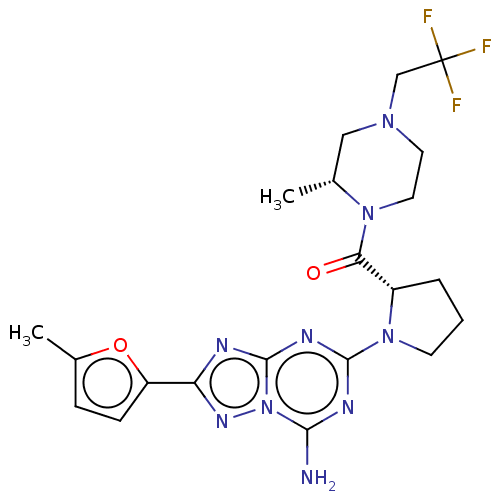 BDBM683541 ((S)-1-(7-amino-2-(5- US20240217978, Example 196
BDBM683541 ((S)-1-(7-amino-2-(5- US20240217978, Example 196 US10202369, Example 195 US10174016, Example 196 US10472354, Example 195 BDBM320029
US10202369, Example 195 US10174016, Example 196 US10472354, Example 195 BDBM320029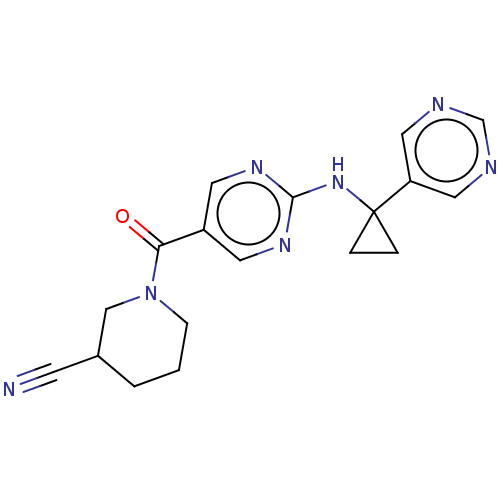 US10906888, Example 196 US10906888, Example 197 US10906888, Example 195 BDBM480978
US10906888, Example 196 US10906888, Example 197 US10906888, Example 195 BDBM480978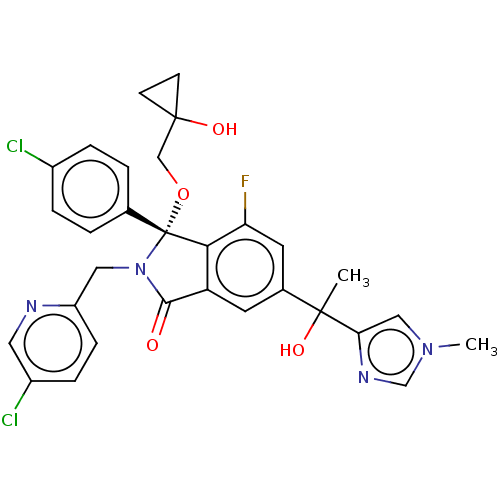 US20230338337, Compound 195 US11236047, Example 196 US11236047, Example 195 BDBM535420
US20230338337, Compound 195 US11236047, Example 196 US11236047, Example 195 BDBM535420 1-(2-(3-chloro-5- (trifluoromethyl)- benzyl)-2,8- diazaspiro[4.5]- decane-8-carbonyl)-1H- pyrazole-3- carboxamide US11655217, Example 196 US10927105, Ex 196 BDBM483714
1-(2-(3-chloro-5- (trifluoromethyl)- benzyl)-2,8- diazaspiro[4.5]- decane-8-carbonyl)-1H- pyrazole-3- carboxamide US11655217, Example 196 US10927105, Ex 196 BDBM483714 US10617680, Example 196 US11020380, Example 196 3-Dimethylamino-1-methyl-5-[1-(1-phenyl-ethyl)-1H- pyrazol-4-yl]-1H-pyridin-2-one BDBM437462
US10617680, Example 196 US11020380, Example 196 3-Dimethylamino-1-methyl-5-[1-(1-phenyl-ethyl)-1H- pyrazol-4-yl]-1H-pyridin-2-one BDBM437462 3-[5-(Difluoromethyl)-1,3,4-oxadiazol-2-yl]-N-[1-(fluoromethyl)cyclopropyl]-1-methyl-2-oxo-benzimidazole-5-sulfonamide US10995073, Example 196 BDBM421606 US10508086, Example 196
3-[5-(Difluoromethyl)-1,3,4-oxadiazol-2-yl]-N-[1-(fluoromethyl)cyclopropyl]-1-methyl-2-oxo-benzimidazole-5-sulfonamide US10995073, Example 196 BDBM421606 US10508086, Example 196 US11439633, Example 196 (5S,8S)-N-((R)-2,3- dihydro-1H- inden-1-yl)-5- fluoro-8- hydroxy-5,6,7,8- tetrahydroquinoline- 5-carboxamide US11077100, Example 196 BDBM510336
US11439633, Example 196 (5S,8S)-N-((R)-2,3- dihydro-1H- inden-1-yl)-5- fluoro-8- hydroxy-5,6,7,8- tetrahydroquinoline- 5-carboxamide US11077100, Example 196 BDBM510336 US9663496, Compound 196 US10377742, Compound 196 5-(4-(4-fluorophenoxy) piperidine-1-carbonyl)- N-(1-(4-(pyrrolidin-1- yl)benzyl)piperidin-4- yl)picolinamide BDBM328058
US9663496, Compound 196 US10377742, Compound 196 5-(4-(4-fluorophenoxy) piperidine-1-carbonyl)- N-(1-(4-(pyrrolidin-1- yl)benzyl)piperidin-4- yl)picolinamide BDBM328058 US10246453, Example 196 (S)-5-chloro-2-fluoro-4-(methyl(1-(3-phenylpropyl)pyrrolidin-3-yl)amino)-N-(thiazol-4-yl)benzenesulfonamide US10815229, Example 196 BDBM373929
US10246453, Example 196 (S)-5-chloro-2-fluoro-4-(methyl(1-(3-phenylpropyl)pyrrolidin-3-yl)amino)-N-(thiazol-4-yl)benzenesulfonamide US10815229, Example 196 BDBM373929 BDBM189558 US20240043404, Example 120 US9174982, 120 US10213433, Compound 196 US11369599, Compound 120
BDBM189558 US20240043404, Example 120 US9174982, 120 US10213433, Compound 196 US11369599, Compound 120 3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-((2-(1-methyl-1H-indole-5-carboxamido)ethyl)sulfonyl)-1H-indole-2-carboxamide BDBM282791 US10844032, Example 196 US10093640, Example 196
3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-((2-(1-methyl-1H-indole-5-carboxamido)ethyl)sulfonyl)-1H-indole-2-carboxamide BDBM282791 US10844032, Example 196 US10093640, Example 196 US10617676, Example 196 1-[2-(3,3-Difluoropyrrolidin-1-yl)-2-oxo-ethyl]-3-methyl-6-[3- (trifluoromethyl)phenyl]imidazo[4,5-b]pyridin-2-one US11207298, Example 196 BDBM436861
US10617676, Example 196 1-[2-(3,3-Difluoropyrrolidin-1-yl)-2-oxo-ethyl]-3-methyl-6-[3- (trifluoromethyl)phenyl]imidazo[4,5-b]pyridin-2-one US11207298, Example 196 BDBM436861 US11479551, Example 4-196 US11492351, Example 4-196 BDBM579012 6-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin-6-yl)-1-methyl-1H- pyrazol-5-yl)chromane-5-carbonitrile
US11479551, Example 4-196 US11492351, Example 4-196 BDBM579012 6-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin-6-yl)-1-methyl-1H- pyrazol-5-yl)chromane-5-carbonitrile US11548890, Compound I-196 4-[7-(hydroxymeth- yl)imidazo[1,2- a]pyridin-3- yl]-7-[(5- morpholino-2- pyridyl)amino] isoindolin-1-one BDBM500537 US11021481, Compound I-196
US11548890, Compound I-196 4-[7-(hydroxymeth- yl)imidazo[1,2- a]pyridin-3- yl]-7-[(5- morpholino-2- pyridyl)amino] isoindolin-1-one BDBM500537 US11021481, Compound I-196 BDBM593504 US11578066, Compound I-196 US20230381148, Compound I-196 N-({5-[5-(difluoromethyl)-1,3,4-oxadiazol-2- yl]-1,3-thiazol-2-yl)methyl)-2-[(1R,4R)-2- oxa-5-azabicyclo[2.2.1]heptan-5-yl]-N- (pyridin-3-yl)ethane-1-sulfonamide US20240269137, Compound I-196
BDBM593504 US11578066, Compound I-196 US20230381148, Compound I-196 N-({5-[5-(difluoromethyl)-1,3,4-oxadiazol-2- yl]-1,3-thiazol-2-yl)methyl)-2-[(1R,4R)-2- oxa-5-azabicyclo[2.2.1]heptan-5-yl]-N- (pyridin-3-yl)ethane-1-sulfonamide US20240269137, Compound I-196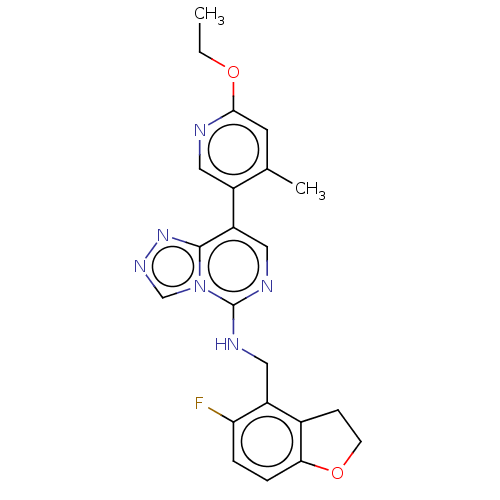 8-(6-ethoxy-4-methylpyridin-3-yl)-N-((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)-[1,2,4]triazolo[4,3-c]pyrimidin-5-amine BDBM291881 US9580437, Example 196 US11207325, Example 196
8-(6-ethoxy-4-methylpyridin-3-yl)-N-((5-fluoro-2,3-dihydrobenzofuran-4-yl)methyl)-[1,2,4]triazolo[4,3-c]pyrimidin-5-amine BDBM291881 US9580437, Example 196 US11207325, Example 196 BDBM297693 US10118902, Example 196 US10584103, Example 196 1-{2-(1-Fluoro-cyclopropyl)-4-[4- (2-methoxy-phenyl)-piperidin-1- yl]-pyrido[3,4-d]pyrimidin-6-yl}- pyrrolidin-3-ol
BDBM297693 US10118902, Example 196 US10584103, Example 196 1-{2-(1-Fluoro-cyclopropyl)-4-[4- (2-methoxy-phenyl)-piperidin-1- yl]-pyrido[3,4-d]pyrimidin-6-yl}- pyrrolidin-3-ol US10053462, 196 US9540388, 196 (6r,10s)-11-{[2-chloro-3-(trifluoromethyl)phenyl]carbonyl}-3-[4-(trifluoromethyl)pyridin-2-yl]-5,6,7,8,9,10-hexahydro-6,10-epimino[1,2,4]triazolo[4,3-a]azocine BDBM203318
US10053462, 196 US9540388, 196 (6r,10s)-11-{[2-chloro-3-(trifluoromethyl)phenyl]carbonyl}-3-[4-(trifluoromethyl)pyridin-2-yl]-5,6,7,8,9,10-hexahydro-6,10-epimino[1,2,4]triazolo[4,3-a]azocine BDBM203318 BDBM396571 3-(Azetidin-3-yloxy)-2-benzyloxy-5-chloro-pyridine US9981909, Example 196
BDBM396571 3-(Azetidin-3-yloxy)-2-benzyloxy-5-chloro-pyridine US9981909, Example 196 BDBM4719 Benzoic Acid deriv. 196 3-carbamimidamido-5-[(1Z)-(hydroxyimino)methyl]benzoic acid
BDBM4719 Benzoic Acid deriv. 196 3-carbamimidamido-5-[(1Z)-(hydroxyimino)methyl]benzoic acid BDBM691735 4-(difluoromethyl)benzyl (4-(pyridin-4- ylmethyl)phenyl)carbamate US20240279215, Compound 196
BDBM691735 4-(difluoromethyl)benzyl (4-(pyridin-4- ylmethyl)phenyl)carbamate US20240279215, Compound 196 US10112929, Example 196 US10112929, Example 197 US10112929, Example 170 BDBM295444 US10112929, Example 171
US10112929, Example 196 US10112929, Example 197 US10112929, Example 170 BDBM295444 US10112929, Example 171 US11078201, Compound I-196 US11028085, Compound I-177 BDBM502324 US11548890, Compound I-177
US11078201, Compound I-196 US11028085, Compound I-177 BDBM502324 US11548890, Compound I-177 BDBM518057 8-(4,4-difluoropiperidine-1-carbonyl)-4-[(2R)-3-(3,4-dihydro-1H-isoquinolin-2-yl)-2-hydroxy- propyl]-2,3-dihydro-1,4-benzoxazepin-5-one US11111237, Example 196 US11725001, Example 196
BDBM518057 8-(4,4-difluoropiperidine-1-carbonyl)-4-[(2R)-3-(3,4-dihydro-1H-isoquinolin-2-yl)-2-hydroxy- propyl]-2,3-dihydro-1,4-benzoxazepin-5-one US11111237, Example 196 US11725001, Example 196 US10344025, Example 196 BDBM404852 tert-butyl 3-((5-(3-fluoro-4-(4- (methylcarbamoyl)-1H-1,2,3- triazol-1-yl)butyl)-1,3,4- thiadiazole-2- carboxamido)methyl)piperidine-1- carboxylate US11370786, Example 196
US10344025, Example 196 BDBM404852 tert-butyl 3-((5-(3-fluoro-4-(4- (methylcarbamoyl)-1H-1,2,3- triazol-1-yl)butyl)-1,3,4- thiadiazole-2- carboxamido)methyl)piperidine-1- carboxylate US11370786, Example 196 BDBM327389 N-{2-[(3-nitrobenzyl)sulfinyl]phenyl}-1-benzofuran-2-sulfonamide US9663460, Example 196
BDBM327389 N-{2-[(3-nitrobenzyl)sulfinyl]phenyl}-1-benzofuran-2-sulfonamide US9663460, Example 196 US10266526, Compound 196 BDBM378068 N-((1r,4r)-4-(3-aminopropanamido)cyclohexyl)isoquinoline-1-carboxamide
US10266526, Compound 196 BDBM378068 N-((1r,4r)-4-(3-aminopropanamido)cyclohexyl)isoquinoline-1-carboxamide US20230159469, Example 196 BDBM633485 N -(4-(4-oxo-3,4-dihydrophthalazin-1-yl)benzyl)cyclopropanesulfonamide
US20230159469, Example 196 BDBM633485 N -(4-(4-oxo-3,4-dihydrophthalazin-1-yl)benzyl)cyclopropanesulfonamide sodium 6-chloro-7-(4-(2- ethylphenethylcarbamoyl) phenoxy)chroman-4- carboxylate US9556139, 196 BDBM234893
sodium 6-chloro-7-(4-(2- ethylphenethylcarbamoyl) phenoxy)chroman-4- carboxylate US9556139, 196 BDBM234893 2-(1-(4-((4-(4-(2-hydroxyethyl)piperidin- 1-yl)phenyl)amino)-5-oxo-5,6- dihydropyrimido[4,5-d]pyridazin-2-yl) piperidin-4-yl)acetonitrile US10647720, Ex. # 196 BDBM322826 US10183944, Example 196
2-(1-(4-((4-(4-(2-hydroxyethyl)piperidin- 1-yl)phenyl)amino)-5-oxo-5,6- dihydropyrimido[4,5-d]pyridazin-2-yl) piperidin-4-yl)acetonitrile US10647720, Ex. # 196 BDBM322826 US10183944, Example 196 BDBM386266 1-(2-((2S,4R)-4- fluoro-2-(2- hydroxypyridin-3- ylcarbamoyl) pyrrolidin-1-yl)-2- oxoethyl)-5- (pyridazin-4-yl)- 1H-indazole-3- carboxamide US10822352, Comp No. 196 US10287301, Compound 196
BDBM386266 1-(2-((2S,4R)-4- fluoro-2-(2- hydroxypyridin-3- ylcarbamoyl) pyrrolidin-1-yl)-2- oxoethyl)-5- (pyridazin-4-yl)- 1H-indazole-3- carboxamide US10822352, Comp No. 196 US10287301, Compound 196 BDBM413803 US10421742, Example 196 US11180471, Example 196 2-[4-{5-Chloro-2-[5-(difluoromethyl)-1,3,4-thiadiazol-2-yl]phenyl}-5-methoxy-2-oxopyridin-1(2H)-yl]-N-(quinoxalin-6-yl)butanamide (Racemate)
BDBM413803 US10421742, Example 196 US11180471, Example 196 2-[4-{5-Chloro-2-[5-(difluoromethyl)-1,3,4-thiadiazol-2-yl]phenyl}-5-methoxy-2-oxopyridin-1(2H)-yl]-N-(quinoxalin-6-yl)butanamide (Racemate) N-((6-amino-2,4-dimethylpyridin-3-yl)methyl)- 2-((3-chloroquinolin-6-yl)methyl)-6-(5-oxo-4,5- dihydro-1H-1,2,4-triazol-3-yl)isonicotinamide BDBM283547 US10023557, Example 196 US10308637, Example 196
N-((6-amino-2,4-dimethylpyridin-3-yl)methyl)- 2-((3-chloroquinolin-6-yl)methyl)-6-(5-oxo-4,5- dihydro-1H-1,2,4-triazol-3-yl)isonicotinamide BDBM283547 US10023557, Example 196 US10308637, Example 196 US9669031, 196 8′-chloro-6′-((5-ethoxypyrimidin-4-yl)amino)-2′H-spiro[cyclohexane-1,3′-imidazo[1,5-a]pyridine]-1′,5′-dione (Cpd. No. 196) BDBM168347
US9669031, 196 8′-chloro-6′-((5-ethoxypyrimidin-4-yl)amino)-2′H-spiro[cyclohexane-1,3′-imidazo[1,5-a]pyridine]-1′,5′-dione (Cpd. No. 196) BDBM168347 BDBM272891 US10065970, Compound 196 US10435414, Compound 196 (S)-2-cyclopropyl-2- ((1-(2-oxo-2,3- dihydrobenzo[d]oxazol- 5-yl)-8,9-dihydro- 7H-6-oxa-2,9a- diazabenzo[cd]azulen-4- yl)amino)acetamide
BDBM272891 US10065970, Compound 196 US10435414, Compound 196 (S)-2-cyclopropyl-2- ((1-(2-oxo-2,3- dihydrobenzo[d]oxazol- 5-yl)-8,9-dihydro- 7H-6-oxa-2,9a- diazabenzo[cd]azulen-4- yl)amino)acetamide US10202369, Example 196 3-(5-methyl-1,3-thiazol-2-yl)-5-(tetrahydro-2H-pyran-4-yloxy)-N-{1-[5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl]ethyl}benzamide US10174016, Example 195 BDBM320028 US10472354, Example 196
US10202369, Example 196 3-(5-methyl-1,3-thiazol-2-yl)-5-(tetrahydro-2H-pyran-4-yloxy)-N-{1-[5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl]ethyl}benzamide US10174016, Example 195 BDBM320028 US10472354, Example 196 US11702414, Example 196 BDBM609236 N-(4-(5-(6-(3-cyanopyrrolo[1,2-b]pyridazin-7-yl)-4-(oxetan-3-ylamino)pyridin-3-yl)-1,3,4-thiadiazol-2-yl)-4-fluorocyclohexyl)acetamide (Example 195 and 196)
US11702414, Example 196 BDBM609236 N-(4-(5-(6-(3-cyanopyrrolo[1,2-b]pyridazin-7-yl)-4-(oxetan-3-ylamino)pyridin-3-yl)-1,3,4-thiadiazol-2-yl)-4-fluorocyclohexyl)acetamide (Example 195 and 196) US9611277, Example 196 (3-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)((1S,4S,6R)-6-((5-(trifluoromethyl)pyrazin-2-yl)amino)-2-azabicyclo[2.2.1]heptan-2-yl)methanone BDBM315015 US10183953, Example 196
US9611277, Example 196 (3-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)((1S,4S,6R)-6-((5-(trifluoromethyl)pyrazin-2-yl)amino)-2-azabicyclo[2.2.1]heptan-2-yl)methanone BDBM315015 US10183953, Example 196 3-({[(4R)-7-{[4-(3,3-difluoroazetidin-1-yl)phenyl](methyl)amino}- US10106534, Example 196 BDBM293854
3-({[(4R)-7-{[4-(3,3-difluoroazetidin-1-yl)phenyl](methyl)amino}- US10106534, Example 196 BDBM293854 4-[2-(cyclopropylmethylamino)-5- methylsulfonylphenyl]-7-fluoro-2- methylisoquinolin-1-one BDBM485995 US10941160, Example 196
4-[2-(cyclopropylmethylamino)-5- methylsulfonylphenyl]-7-fluoro-2- methylisoquinolin-1-one BDBM485995 US10941160, Example 196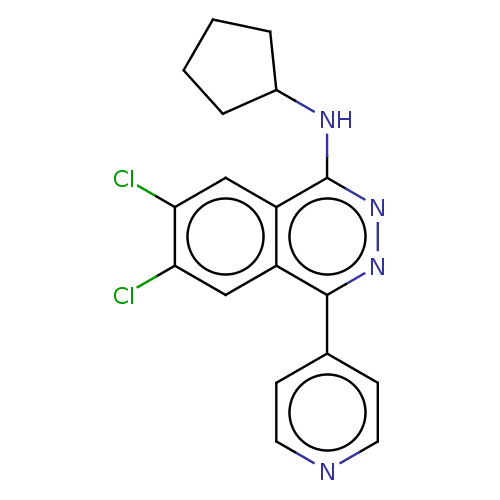 A-196 (3) BDBM223981 6,7-Dichloro-N-cyclopentyl-4-(pyridin-4-yl)phthalazin-1-amine
A-196 (3) BDBM223981 6,7-Dichloro-N-cyclopentyl-4-(pyridin-4-yl)phthalazin-1-amine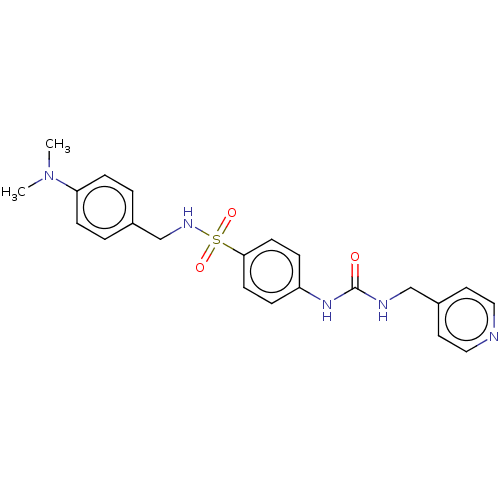 US11452717, Example 196 BDBM572734 N-(4-Dimethylamino-benzyl)-4-(3-pyridin-4-ylmethyl-ureido)-benzenesulfonamide
US11452717, Example 196 BDBM572734 N-(4-Dimethylamino-benzyl)-4-(3-pyridin-4-ylmethyl-ureido)-benzenesulfonamide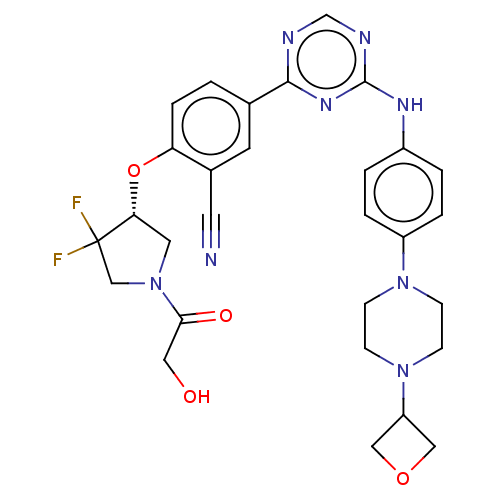 US10253019, Example 196 US10040781, Example 196 (R)-2-((4,4-difluoro-1-(2- hydroxyacetyl)pyrrolidin-3- yl)oxy)-5-(4-((4-(4-(oxetan- 3-yl)piperazin-1- yl)phenyl)amino)-1,3,5- triazin-2-yl)benzonitrile BDBM277974
US10253019, Example 196 US10040781, Example 196 (R)-2-((4,4-difluoro-1-(2- hydroxyacetyl)pyrrolidin-3- yl)oxy)-5-(4-((4-(4-(oxetan- 3-yl)piperazin-1- yl)phenyl)amino)-1,3,5- triazin-2-yl)benzonitrile BDBM277974 US10800792, Example 196 BDBM467544 (R,E)-N-(1-(3-(1-Aminocyclopropyl)-2-cyanoacryloyl)piperidin-3- yl)-5-(2-methyl-4-phenoxyphenyl)-4-oxo-4,5-dihydro-3H-1-thia- 3,5,8-triazaacenaphthylene-2-carboxamide; US10822348, Example 196
US10800792, Example 196 BDBM467544 (R,E)-N-(1-(3-(1-Aminocyclopropyl)-2-cyanoacryloyl)piperidin-3- yl)-5-(2-methyl-4-phenoxyphenyl)-4-oxo-4,5-dihydro-3H-1-thia- 3,5,8-triazaacenaphthylene-2-carboxamide; US10822348, Example 196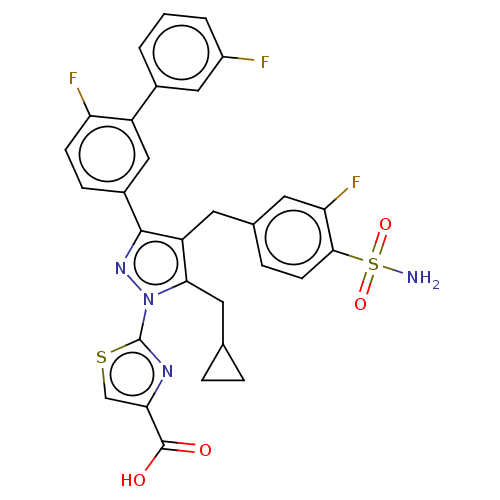 US10954228, Compound 196 BDBM488241 2-(5-(cyclopropylmethyl)-3-(3',6- difluoro-[1,1'-biphenyl]-3-yl)- 4-(3-fluoro-4-sulfamoylbenzyl)- 1H-pyrazol-1-yl)thiazole-4- carboxylic acid US11247971, Cmpd ID 371 US11752138, Compound 196
US10954228, Compound 196 BDBM488241 2-(5-(cyclopropylmethyl)-3-(3',6- difluoro-[1,1'-biphenyl]-3-yl)- 4-(3-fluoro-4-sulfamoylbenzyl)- 1H-pyrazol-1-yl)thiazole-4- carboxylic acid US11247971, Cmpd ID 371 US11752138, Compound 196 4-(1-oxoisoindolin-5-yl)-3-(1H- tetrazol-5-yl)pyridine-2- sulfonamide BDBM262344 US9708336, 196
4-(1-oxoisoindolin-5-yl)-3-(1H- tetrazol-5-yl)pyridine-2- sulfonamide BDBM262344 US9708336, 196 4-[2-(cyclopropylmethylamino)-5- methylsulfonylphenyl]-7-fluoro-2- methylisoquinolin-1-one BDBM285151 BDBM485995 US10023592, Example 196
4-[2-(cyclopropylmethylamino)-5- methylsulfonylphenyl]-7-fluoro-2- methylisoquinolin-1-one BDBM285151 BDBM485995 US10023592, Example 196 5-[2-Chloro-3-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)phenyl]-1,3- BDBM436024 US10611730, Example 196
5-[2-Chloro-3-(5-cyclopropyl-1,3,4-oxadiazol-2-yl)phenyl]-1,3- BDBM436024 US10611730, Example 196 US20250129027, Example 196 3-(5-(1,3,4-oxadiazol-2-yl)pyridin-3-yl)-5-fluorophenyl benzylcarbamate BDBM734220
US20250129027, Example 196 3-(5-(1,3,4-oxadiazol-2-yl)pyridin-3-yl)-5-fluorophenyl benzylcarbamate BDBM734220 US11702414, Example 195 US11702414, Example 196 BDBM609235 N-(4-(5-(6-(3-cyanopyrrolo[1,2-b]pyridazin-7-yl)-4-(oxetan-3-ylamino)pyridin-3-yl)-1,3,4-thiadiazol-2-yl)-4-fluorocyclohexyl)acetamide (Example 195 and 196)
US11702414, Example 195 US11702414, Example 196 BDBM609235 N-(4-(5-(6-(3-cyanopyrrolo[1,2-b]pyridazin-7-yl)-4-(oxetan-3-ylamino)pyridin-3-yl)-1,3,4-thiadiazol-2-yl)-4-fluorocyclohexyl)acetamide (Example 195 and 196) (4R)-4-methyl-2-oxo-N-phenyl-1,3,4,5-tetrahydro-1,5-benzodiazepine-6-carboxamide BDBM349388 US10206931, Example 196
(4R)-4-methyl-2-oxo-N-phenyl-1,3,4,5-tetrahydro-1,5-benzodiazepine-6-carboxamide BDBM349388 US10206931, Example 196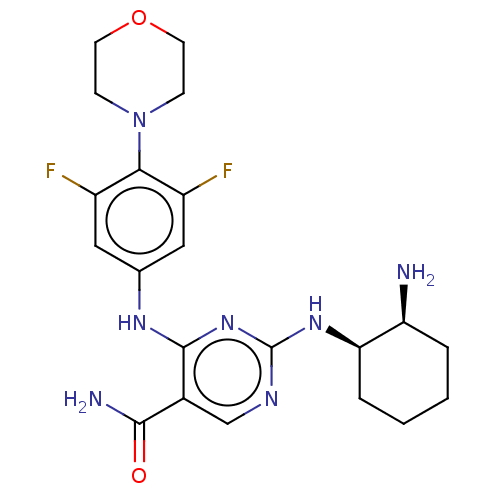 2-((1R,2S)-2-aminocyclohexylamino)-4-(3,5-difluoro-4-morpholinophenylamino)pyrimidine-5-carboxamide US9579320, Example 196 BDBM290797
2-((1R,2S)-2-aminocyclohexylamino)-4-(3,5-difluoro-4-morpholinophenylamino)pyrimidine-5-carboxamide US9579320, Example 196 BDBM290797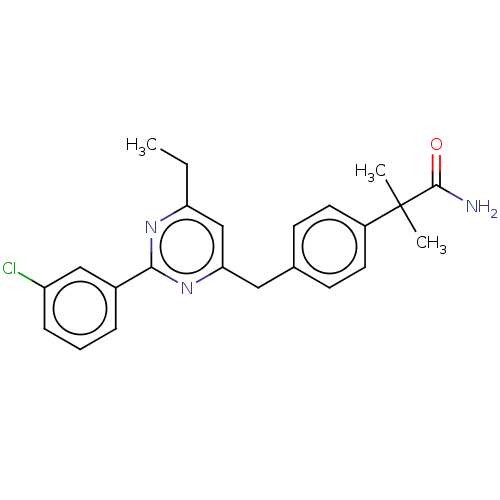 2-(4-((2-(3-Chlorophenyl)-6-ethylpyrimidin-4-yl)methyl)phenyl)-2-methylpropanamide US11401286, Example 196 BDBM562624
2-(4-((2-(3-Chlorophenyl)-6-ethylpyrimidin-4-yl)methyl)phenyl)-2-methylpropanamide US11401286, Example 196 BDBM562624 5-[2-Cyclopentyl-7-(3-fluoroazetidin-1-yl)pyrazolo[1,5-c]pyrimidin-3- BDBM426256 US10513523, Example 196
5-[2-Cyclopentyl-7-(3-fluoroazetidin-1-yl)pyrazolo[1,5-c]pyrimidin-3- BDBM426256 US10513523, Example 196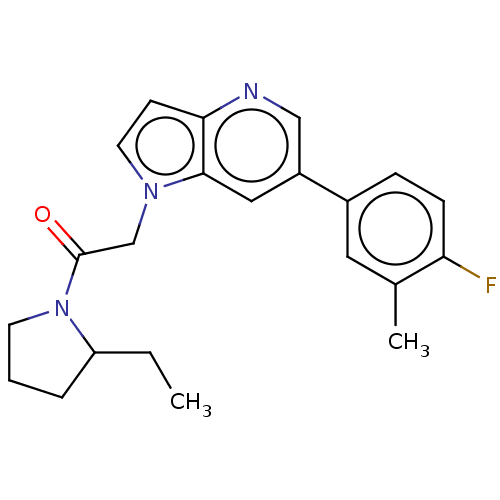 BDBM409304 (R/S)-1-(2-Ethylpyrrolidin-1-yl)-2-[6-(4-fluoro-3-methyl- US10377753, Example 196
BDBM409304 (R/S)-1-(2-Ethylpyrrolidin-1-yl)-2-[6-(4-fluoro-3-methyl- US10377753, Example 196 BDBM612508 (S)-or (R)- N'-((4-cyano-2,6- diisopropylphenyl)carbamoyl)-4- (methylsulfonyl)benzene- sulfonimidamide US11724992, Example 196
BDBM612508 (S)-or (R)- N'-((4-cyano-2,6- diisopropylphenyl)carbamoyl)-4- (methylsulfonyl)benzene- sulfonimidamide US11724992, Example 196 BDBM757557 6-(Dimethylamino)-N-((2-methylquinolin-8-yl)sulfonyl)-4-(trifluoromethyl)benzofuran-2-carboxamide US12357603, Example 196
BDBM757557 6-(Dimethylamino)-N-((2-methylquinolin-8-yl)sulfonyl)-4-(trifluoromethyl)benzofuran-2-carboxamide US12357603, Example 196 US10654814, Example 196 2-(2'-fluoro-3-methoxybiphenyl-4- yl)-N-hydroxy-1,3-benzoxazole-5- carboxamide BDBM443070
US10654814, Example 196 2-(2'-fluoro-3-methoxybiphenyl-4- yl)-N-hydroxy-1,3-benzoxazole-5- carboxamide BDBM443070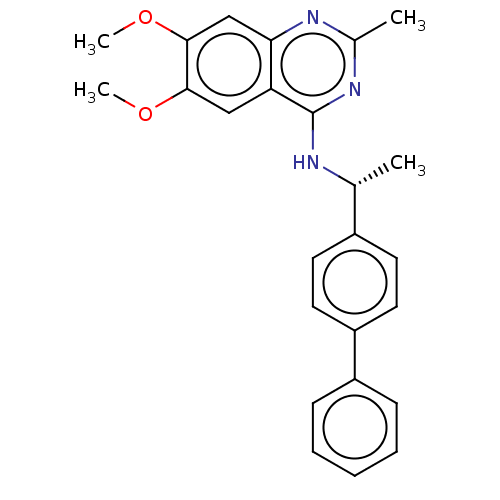 US20240083857, Example 196 BDBM657517 N-[(1R)-1-(biphenyl-4-yl)ethyl]-6,7-dimethoxy-2-methylquinazolin-4-amine
US20240083857, Example 196 BDBM657517 N-[(1R)-1-(biphenyl-4-yl)ethyl]-6,7-dimethoxy-2-methylquinazolin-4-amine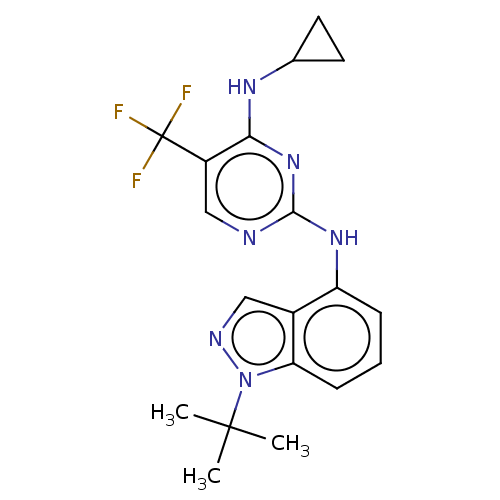 US20240174647, Example 196 BDBM677275 N2-(1-tert-butylindazol-4-yl)-N4-cyclopropyl-5-(trifluoromethyl)pyrimidine-2,4-diamine
US20240174647, Example 196 BDBM677275 N2-(1-tert-butylindazol-4-yl)-N4-cyclopropyl-5-(trifluoromethyl)pyrimidine-2,4-diamine US20250171431, Compound 196 4-(6-chloro-3- quinolylamino)-2-[3- methoxy-4-(3- piperidinopropoxy) phenylamino]pyrimidine BDBM744901
US20250171431, Compound 196 4-(6-chloro-3- quinolylamino)-2-[3- methoxy-4-(3- piperidinopropoxy) phenylamino]pyrimidine BDBM744901 [4-cyclobutyl-1-(p-tolylmethyl)piperazin-2-yl]-(6-methoxy-2-naphthyl)methanone US10280149, Example 196 BDBM383714
[4-cyclobutyl-1-(p-tolylmethyl)piperazin-2-yl]-(6-methoxy-2-naphthyl)methanone US10280149, Example 196 BDBM383714 7-(benzenesulfonyl)-4-(2-ethylthiomorpholin-4-yl)-5H-pyrrolo[3,2-d]pyrimidin-6-amine BDBM271077 US10059713, Example 196
7-(benzenesulfonyl)-4-(2-ethylthiomorpholin-4-yl)-5H-pyrrolo[3,2-d]pyrimidin-6-amine BDBM271077 US10059713, Example 196 BDBM262340 US9708336, 192 4-(1-oxoisoindolin-5-yl)-3-(1H- tetrazol-5-yl)pyridine-2- sulfonamide US9708336, 196
BDBM262340 US9708336, 192 4-(1-oxoisoindolin-5-yl)-3-(1H- tetrazol-5-yl)pyridine-2- sulfonamide US9708336, 196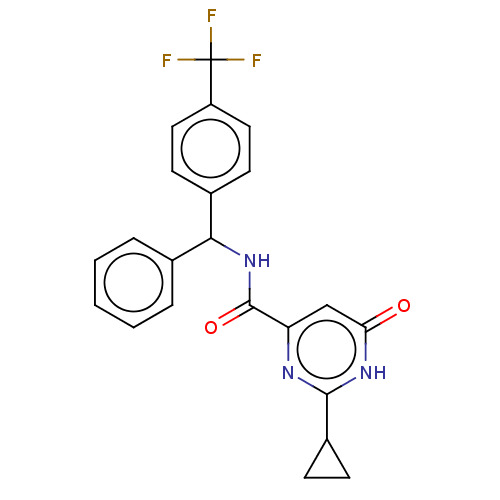 BDBM355105 US9815796, Example 196 2-cyclopropyl-6-oxo-N-{phenyl[4- (trifluoromethyl)phenyl]methyl}-1,6- dihydropyrimidine-4-carboxamide
BDBM355105 US9815796, Example 196 2-cyclopropyl-6-oxo-N-{phenyl[4- (trifluoromethyl)phenyl]methyl}-1,6- dihydropyrimidine-4-carboxamide BDBM387612 US10294212, No. 196 N3-(3,4- Difluoro- phenyl)-7-(2- methoxy- phenyl)furo [2,3-c]pyridine- 2,3-diamine
BDBM387612 US10294212, No. 196 N3-(3,4- Difluoro- phenyl)-7-(2- methoxy- phenyl)furo [2,3-c]pyridine- 2,3-diamine BDBM399872 N-(1-(4-(3,4-dichlorobenzyl)piperazine- 1-carbonyl)-1H-pyrazol-4-yl)-N- methylacetamide US10323038, Example 196
BDBM399872 N-(1-(4-(3,4-dichlorobenzyl)piperazine- 1-carbonyl)-1H-pyrazol-4-yl)-N- methylacetamide US10323038, Example 196 BDBM411200 US10385022, Example 196 Methyl 2-(N-(4-(2-fluorophenyl)pyridin-3-yl)-2,6-bis(trifluoromethyl)isonicotinamido)acetate
BDBM411200 US10385022, Example 196 Methyl 2-(N-(4-(2-fluorophenyl)pyridin-3-yl)-2,6-bis(trifluoromethyl)isonicotinamido)acetate BDBM426738 US10544106, Compound C194 US11208388, Example 196 2-(4-(8-(3-acrylamidophenyl)quinazolin-6- yl)-3-fluorophenoxy)isonicotinamide
BDBM426738 US10544106, Compound C194 US11208388, Example 196 2-(4-(8-(3-acrylamidophenyl)quinazolin-6- yl)-3-fluorophenoxy)isonicotinamide BDBM432497 US10577363, Compound 196 N-((3S,4S)-1-(4-aminobutanoyl)-3- methylpiperidin-4-yl)-5- cyclopropylisoxazole-3-carboxamide
BDBM432497 US10577363, Compound 196 N-((3S,4S)-1-(4-aminobutanoyl)-3- methylpiperidin-4-yl)-5- cyclopropylisoxazole-3-carboxamide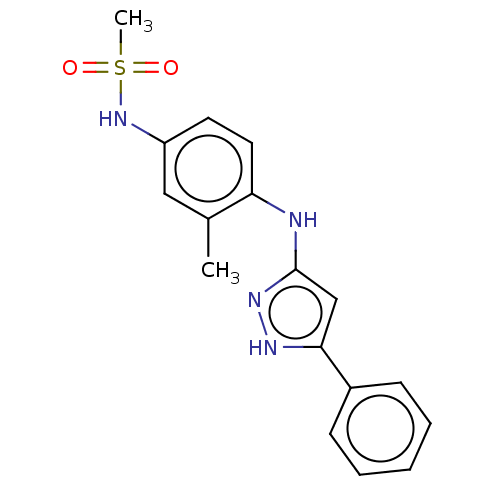 BDBM579752 US11485711, Compound 196 N-(3-methyl-4-((5-phenyl-1H- pyrazol-3- yl)amino)phenyl)methanesulfon- amide
BDBM579752 US11485711, Compound 196 N-(3-methyl-4-((5-phenyl-1H- pyrazol-3- yl)amino)phenyl)methanesulfon- amide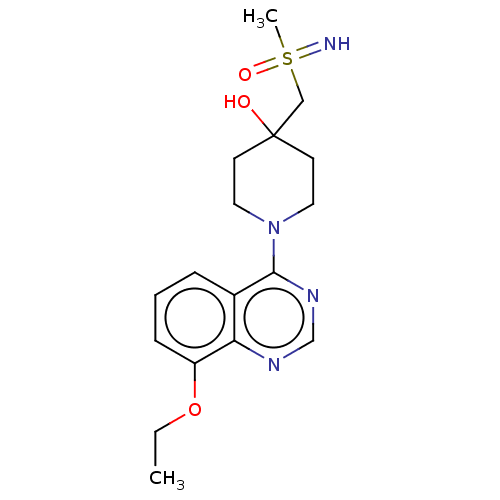 BDBM624657 US11780849, Compound 196 {[1-(8-ethoxyquinazolin-4-yl)-4- hydroxypiperidin-4-yl]methyl}(imino)methyl- lambda6-sulfanone
BDBM624657 US11780849, Compound 196 {[1-(8-ethoxyquinazolin-4-yl)-4- hydroxypiperidin-4-yl]methyl}(imino)methyl- lambda6-sulfanone (2R)-2-(5-fluoro-2-methoxypyridin-4-yl)-1-{(2S)-7-methyl-6-[2-(morpholin-4-yl)pyrimidin-4-yl]-3,4-dihydro-1H-spiro[1,8-naphthyridine-2,3'-pyrrolidin]-1'-yl}propan-1-one US12187727, Example 196 BDBM711659 US20250084084, Example 196
(2R)-2-(5-fluoro-2-methoxypyridin-4-yl)-1-{(2S)-7-methyl-6-[2-(morpholin-4-yl)pyrimidin-4-yl]-3,4-dihydro-1H-spiro[1,8-naphthyridine-2,3'-pyrrolidin]-1'-yl}propan-1-one US12187727, Example 196 BDBM711659 US20250084084, Example 196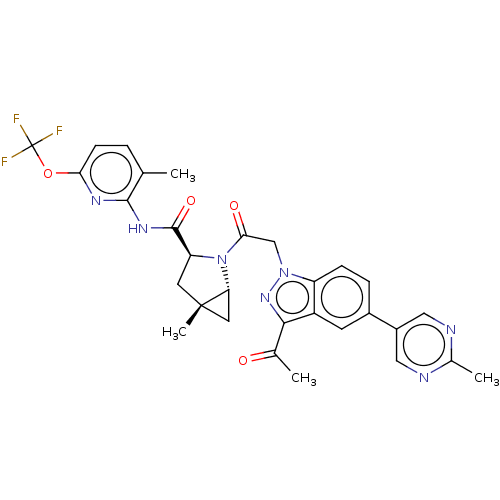 US11084800, Cpd No. 196 (1R,3S,5R)-2-(2-(3-acetyl-5-(2- methylpyrimidin-5-yl)-1H-indazol-1- yl)acetyl)-5-methyl-N-(3-methyl-6- (trifluoromethoxy)pyridin-2-yl)-2- azabicyclo[3.1.0]hexane-3-carboxamide US11708351, Compound 196 BDBM511881
US11084800, Cpd No. 196 (1R,3S,5R)-2-(2-(3-acetyl-5-(2- methylpyrimidin-5-yl)-1H-indazol-1- yl)acetyl)-5-methyl-N-(3-methyl-6- (trifluoromethoxy)pyridin-2-yl)-2- azabicyclo[3.1.0]hexane-3-carboxamide US11708351, Compound 196 BDBM511881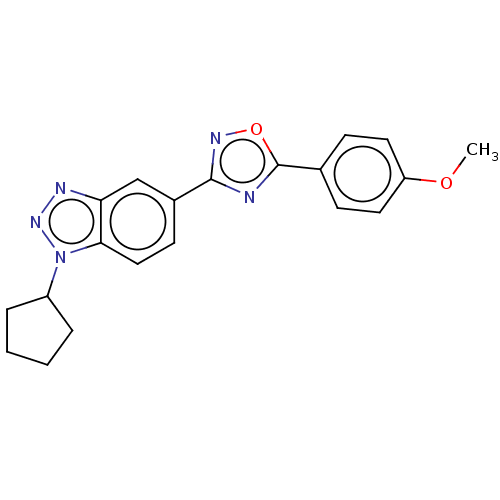 3-(1-cyclopentyl-1H- benzo[d][1,2,3]triazol-5-yl)-5-(4- methoxyphenyl)-1,2,4-oxadiazole BDBM654586 US11912693, Compound 196
3-(1-cyclopentyl-1H- benzo[d][1,2,3]triazol-5-yl)-5-(4- methoxyphenyl)-1,2,4-oxadiazole BDBM654586 US11912693, Compound 196 BDBM557146 4-Benzyl-1- {[2'-(4,5- dimethyl-1H- imidazol-2-yl)- 3,4'-bipyridin- 5- yl]carbonyl} US11352340, Example 196
BDBM557146 4-Benzyl-1- {[2'-(4,5- dimethyl-1H- imidazol-2-yl)- 3,4'-bipyridin- 5- yl]carbonyl} US11352340, Example 196 BDBM612214 N-[3-methyl-8-(5- methylfuran-2- yl)imidazo[1,2-a]pyrazin-6- yl]cyclopropanecarboxamide US11718622, Compound 196
BDBM612214 N-[3-methyl-8-(5- methylfuran-2- yl)imidazo[1,2-a]pyrazin-6- yl]cyclopropanecarboxamide US11718622, Compound 196 BDBM616381 5-(4,6-dichloro-5- hydroxypicolinamido)-N-((3- (trifluoromethyl)pyridin-2- yl)methyl)thiazole-4- carboxamide US20230278981, Example 196
BDBM616381 5-(4,6-dichloro-5- hydroxypicolinamido)-N-((3- (trifluoromethyl)pyridin-2- yl)methyl)thiazole-4- carboxamide US20230278981, Example 196 BDBM644250 US11866430, Example 196 4-(4-benzhydrylpiperazin-1-yl)-1-methyl-2-oxo-1,2-dihydro-1,5-naphthyridine-3-carbonitrile
BDBM644250 US11866430, Example 196 4-(4-benzhydrylpiperazin-1-yl)-1-methyl-2-oxo-1,2-dihydro-1,5-naphthyridine-3-carbonitrile N-benzyl-6-(4-(2-chloro-4-cyanophenyl)-5-hydroxy-1H-pyrazol-1-yl)nicotinamide BDBM413243 US10407409, Example 196
N-benzyl-6-(4-(2-chloro-4-cyanophenyl)-5-hydroxy-1H-pyrazol-1-yl)nicotinamide BDBM413243 US10407409, Example 196 US11312712, Example 196 BDBM551084 2-Ethoxy-3-(3-fluoro-1H-indazol-5-yl)-5-(trifluoromethyl)imidazo[4,5-b]pyridine;
US11312712, Example 196 BDBM551084 2-Ethoxy-3-(3-fluoro-1H-indazol-5-yl)-5-(trifluoromethyl)imidazo[4,5-b]pyridine;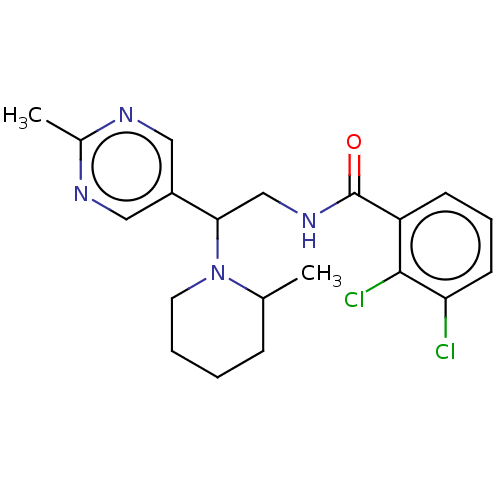 US9415055, 2,3-dichloro-N-(2-(2-methylpiperidin-1-yl)-2-(2-methylpyrimidin-5-yl)ethyl)benzamide BDBM242195 US9593105, 196
US9415055, 2,3-dichloro-N-(2-(2-methylpiperidin-1-yl)-2-(2-methylpyrimidin-5-yl)ethyl)benzamide BDBM242195 US9593105, 196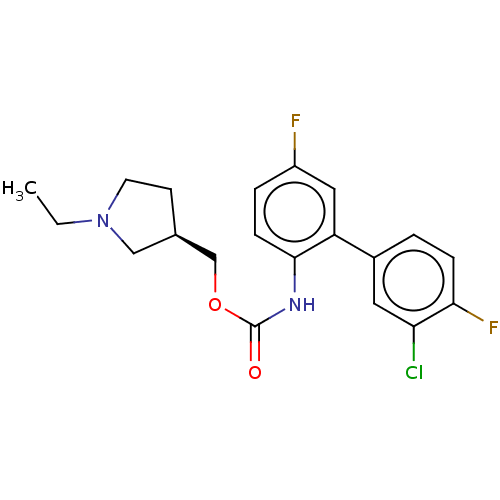 (R)-(1-ethylpyrrolidin-3-yl)methyl (3'-chloro-4',5-difluoro-[1,1'-biphenyl]-2-yl)carbamate US9828339, Example 196 BDBM360291
(R)-(1-ethylpyrrolidin-3-yl)methyl (3'-chloro-4',5-difluoro-[1,1'-biphenyl]-2-yl)carbamate US9828339, Example 196 BDBM360291 (R)-N-(3-(3,5-dimethylisoxazol-4-yl)-4-(piperidin-2- ylmethoxy)phenyl)-4-methyloxazole-5-carboxamide US20240376090, Compound 196 BDBM704826
(R)-N-(3-(3,5-dimethylisoxazol-4-yl)-4-(piperidin-2- ylmethoxy)phenyl)-4-methyloxazole-5-carboxamide US20240376090, Compound 196 BDBM704826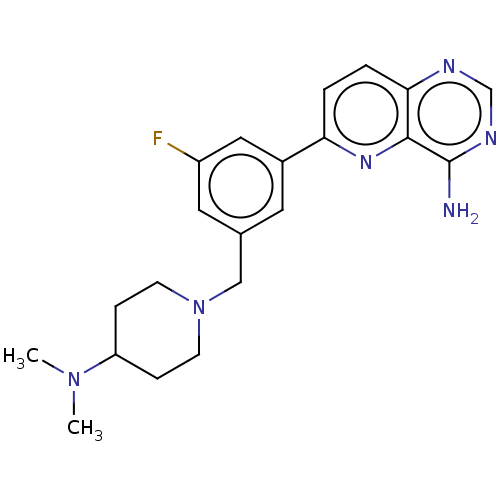 6-[3-[[4-(dimethylamino)-1-piperidyl]methyl]-5-fluoro-phenyl]pyrido[3,2-d]pyrimidin-4-amine BDBM363953 US9855269, Compound 196
6-[3-[[4-(dimethylamino)-1-piperidyl]methyl]-5-fluoro-phenyl]pyrido[3,2-d]pyrimidin-4-amine BDBM363953 US9855269, Compound 196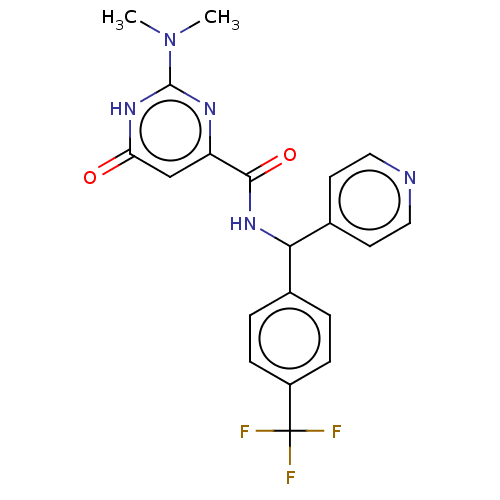 BDBM384976 US10285989, Example 196 2-(dimethylamino)-6-oxo-N- {pyridin-4-yl[4- (trifluoromethyl)phenyl]methyl}- 1,6-dihydropyrimidine-4- carboxamide
BDBM384976 US10285989, Example 196 2-(dimethylamino)-6-oxo-N- {pyridin-4-yl[4- (trifluoromethyl)phenyl]methyl}- 1,6-dihydropyrimidine-4- carboxamide BDBM385793 2-methoxyethyl N-[5-(4- cyano-3-fluorophenyl)- [1,2,4]triazolo[1,5-a]pyridin- 7-yl]carbamate US10287286, Example 196
BDBM385793 2-methoxyethyl N-[5-(4- cyano-3-fluorophenyl)- [1,2,4]triazolo[1,5-a]pyridin- 7-yl]carbamate US10287286, Example 196 BDBM585581 US11530210, Example 196 6-(3-Chloro-4-fluoro-phenyl)-1-[(5-methoxy-3- pyridyl)methyl]pyrazolo[4,3-b]pyridine
BDBM585581 US11530210, Example 196 6-(3-Chloro-4-fluoro-phenyl)-1-[(5-methoxy-3- pyridyl)methyl]pyrazolo[4,3-b]pyridine BDBM732652 (R)-N-methyl-N-(2,2,2-trifluoro-1-(4- methoxyphenyl)ethyl)imidazo[1,2- a]pyrazine-2-sulfonamide US20250115569, Example 196
BDBM732652 (R)-N-methyl-N-(2,2,2-trifluoro-1-(4- methoxyphenyl)ethyl)imidazo[1,2- a]pyrazine-2-sulfonamide US20250115569, Example 196 N-[3-(4-amino- quinazolin-6- yl)-5-fluoro- phenyl]- 3-methoxy- propanamide US9592235, Example 196 US9855269, Compound 43 BDBM299403
N-[3-(4-amino- quinazolin-6- yl)-5-fluoro- phenyl]- 3-methoxy- propanamide US9592235, Example 196 US9855269, Compound 43 BDBM299403 Trans-4-(4-(((1S,2R)-2- (5-ethylpyridin-2- yl)cyclopropyl)methoxy)- 2-methylpyrimidin-5- yl)cyclohexanol US9663513, 196 BDBM329583
Trans-4-(4-(((1S,2R)-2- (5-ethylpyridin-2- yl)cyclopropyl)methoxy)- 2-methylpyrimidin-5- yl)cyclohexanol US9663513, 196 BDBM329583 US11440922, Example 196 3-{(3E)-3-[1-(3- methoxybenzoyl)-3,3- dimethylpiperidin-4- ylidene]prop-1-yn-1- yl}benzonitrile BDBM571436
US11440922, Example 196 3-{(3E)-3-[1-(3- methoxybenzoyl)-3,3- dimethylpiperidin-4- ylidene]prop-1-yn-1- yl}benzonitrile BDBM571436 acs.jmedchem.1c00409_ST.196 CHEMBL23453 3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylamino)-5-fluoro-4-oxo-pentanoic acid methyl ester BDBM50140548
acs.jmedchem.1c00409_ST.196 CHEMBL23453 3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylamino)-5-fluoro-4-oxo-pentanoic acid methyl ester BDBM50140548 (R)-5-(3-(3,3-dimethylureido)piperidin-1-yl)-3-(4-(pyridin-2-ylcarbamoyl)phenylamino)pyrazine-2-carboxamide US9656988, Example 196 BDBM309727
(R)-5-(3-(3,3-dimethylureido)piperidin-1-yl)-3-(4-(pyridin-2-ylcarbamoyl)phenylamino)pyrazine-2-carboxamide US9656988, Example 196 BDBM309727 (S)-quinuclidin-3-yl((R)-7-fluoro-6-(3-isopropoxyphenyl)-2,2-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)carbamate BDBM690052 US20240262819, Example 196
(S)-quinuclidin-3-yl((R)-7-fluoro-6-(3-isopropoxyphenyl)-2,2-dimethyl-1,2,3,4-tetrahydronaphthalen-1-yl)carbamate BDBM690052 US20240262819, Example 196 1-((3-(4-Aminoimidazo[2,1-f][1,2,4]triazin-7-yl)-4- methylphenyl)sulfonyl)-4,4-difluoropiperidin-3-ol BDBM421153 US10479795, Example 196
1-((3-(4-Aminoimidazo[2,1-f][1,2,4]triazin-7-yl)-4- methylphenyl)sulfonyl)-4,4-difluoropiperidin-3-ol BDBM421153 US10479795, Example 196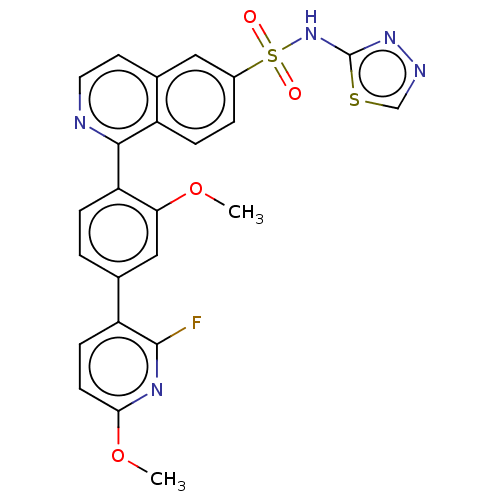 1-(4-(2-fluoro-6-methoxy-3-pyridinyl)-2-methoxyphenyl)-N-1,3,4-thiadiazol-2-yl-6-isoquinolinesulfonamide US9776995, Example 196 BDBM343003
1-(4-(2-fluoro-6-methoxy-3-pyridinyl)-2-methoxyphenyl)-N-1,3,4-thiadiazol-2-yl-6-isoquinolinesulfonamide US9776995, Example 196 BDBM343003 2-(1-methyl-3-(2-((4- (trifluoromethyl)phenyl)amino)phenyl)-1H- pyrazol-5-yl)ethan-1-ol US11186554, Compound 196 BDBM526414
2-(1-methyl-3-(2-((4- (trifluoromethyl)phenyl)amino)phenyl)-1H- pyrazol-5-yl)ethan-1-ol US11186554, Compound 196 BDBM526414 2-[4-(3-Methoxy-pyridine-2-carbonyl)-piperazin-1-ylmethyl]-thiazole-5-carboxylic acid 2,4-dichloro-benzylamide US9776991, Example 196 BDBM342826
2-[4-(3-Methoxy-pyridine-2-carbonyl)-piperazin-1-ylmethyl]-thiazole-5-carboxylic acid 2,4-dichloro-benzylamide US9776991, Example 196 BDBM342826 3-cyano-N-(3-(isoxazol-4- yl)-1H-indazol-5-yl)-1,4- dimethyl-1H-pyrazole-5- carboxamide BDBM569049 US11427558, Example 196
3-cyano-N-(3-(isoxazol-4- yl)-1H-indazol-5-yl)-1,4- dimethyl-1H-pyrazole-5- carboxamide BDBM569049 US11427558, Example 196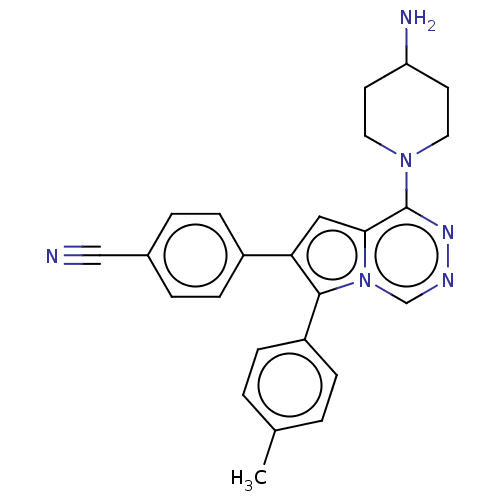 4-(1-(4-aminopiperidin-1-yl)-6-(p-tolyl)pyrrolo[1,2-d][1,2,4]triazin-7-yl)benzonitrile BDBM683239 US20240216357, Example 196
4-(1-(4-aminopiperidin-1-yl)-6-(p-tolyl)pyrrolo[1,2-d][1,2,4]triazin-7-yl)benzonitrile BDBM683239 US20240216357, Example 196 5-Amino-N-(5-chloropyridin-2-yl)-1-(2-(pyridin-3-yl)phenyl)-1H-pyrazole-4-carboxamide US10214509, Example 196 BDBM357008
5-Amino-N-(5-chloropyridin-2-yl)-1-(2-(pyridin-3-yl)phenyl)-1H-pyrazole-4-carboxamide US10214509, Example 196 BDBM357008 BDBM299188 US11311541, Example 196 US10125144, Example 16 N-(2-(5-Methoxyisoindolin-2-yl)-5,6-dimethylpyrimidin-4-yl)-1H-indazol-5-amine
BDBM299188 US11311541, Example 196 US10125144, Example 16 N-(2-(5-Methoxyisoindolin-2-yl)-5,6-dimethylpyrimidin-4-yl)-1H-indazol-5-amine BDBM630606 US11802111, Example 196 ((1s,3s)-3-Hydroxy-3-methylcyclobutyl)(6-(2-(trifluoromethoxy)benzyl)-2-azaspiro[3.3]heptan-2-yl)methanone
BDBM630606 US11802111, Example 196 ((1s,3s)-3-Hydroxy-3-methylcyclobutyl)(6-(2-(trifluoromethoxy)benzyl)-2-azaspiro[3.3]heptan-2-yl)methanone BDBM646691 US20240025883, Example 196 6-bromo-N-[5-(2,2- difluoroethyl)-4-methoxy- pyrimidin-2-yl]-7-methoxy-1H- indole-3-sulfonamide
BDBM646691 US20240025883, Example 196 6-bromo-N-[5-(2,2- difluoroethyl)-4-methoxy- pyrimidin-2-yl]-7-methoxy-1H- indole-3-sulfonamide BDBM667289 US11957687, Compound 196 N-(3-chloro-9H-xanthen-9- yl)-4-methyl-2-oxo-6- (trifluoromethyl)-1,2- dihydropyridine-3- carboxamide
BDBM667289 US11957687, Compound 196 N-(3-chloro-9H-xanthen-9- yl)-4-methyl-2-oxo-6- (trifluoromethyl)-1,2- dihydropyridine-3- carboxamide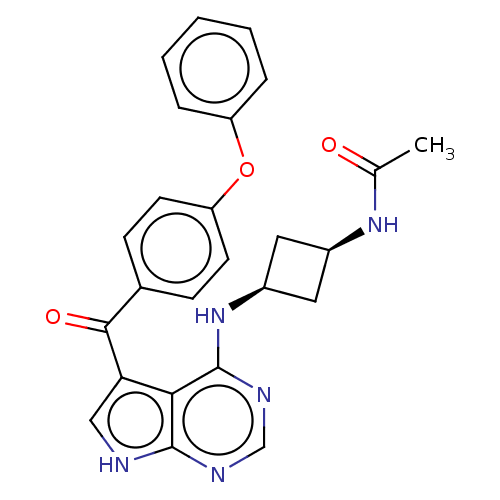 N-(cis-3-((5-(4-phenoxybenzoyl)-7H- pyrrolo[2,3-d]pyrimidin-4- yl)amino)cyclobutyl)acetamide US11020398, Compound I-196 BDBM499884
N-(cis-3-((5-(4-phenoxybenzoyl)-7H- pyrrolo[2,3-d]pyrimidin-4- yl)amino)cyclobutyl)acetamide US11020398, Compound I-196 BDBM499884 N-[4-(4-oxo-3,4- dihydrophthalazin-1- yl)phenyl]-3-phenyl-4,5- dihydro-1,2-oxazole-5- carboxamide BDBM380201 US9926282, Example 196
N-[4-(4-oxo-3,4- dihydrophthalazin-1- yl)phenyl]-3-phenyl-4,5- dihydro-1,2-oxazole-5- carboxamide BDBM380201 US9926282, Example 196 US10246426, Example 196 N-[2-(4-Cyano-benzyl)-2H-[1,2,3]triazol-4-yl]-2-[4-(3,3-difluoro-cyclobutoxy)-phenyl]-acetamide BDBM372642
US10246426, Example 196 N-[2-(4-Cyano-benzyl)-2H-[1,2,3]triazol-4-yl]-2-[4-(3,3-difluoro-cyclobutoxy)-phenyl]-acetamide BDBM372642 US10251893, Cpd 196 N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(4-(5-fluoropyrimidin-4-yl)phenyl)acetamide BDBM375001
US10251893, Cpd 196 N-(5-(4-acetylpiperazin-1-yl)pyridin-2-yl)-2-(4-(5-fluoropyrimidin-4-yl)phenyl)acetamide BDBM375001 US10626112, Example 196 (2S)-1-(benzofuran-2- ylsulfonyl)-N-[[3-[5- (trifluoromethyl)pyrazin-2- yl]phenyl]methyl]- pyrrolidine-2-carboxamide BDBM611303
US10626112, Example 196 (2S)-1-(benzofuran-2- ylsulfonyl)-N-[[3-[5- (trifluoromethyl)pyrazin-2- yl]phenyl]methyl]- pyrrolidine-2-carboxamide BDBM611303 US10781204, Compound I-170 US10781204, Compound I-196 US10781204, Compound I-87 US11434240, Compound I-200 US10781204, Compound I-200 BDBM463608
US10781204, Compound I-170 US10781204, Compound I-196 US10781204, Compound I-87 US11434240, Compound I-200 US10781204, Compound I-200 BDBM463608 US10793582, Example 196 1-[(R)-2-(6-methyl[1,3]thiazolo[4,5-b]pyridin-2-yl)pyrrolidin-1-yl]-2-phenylethanone BDBM466307
US10793582, Example 196 1-[(R)-2-(6-methyl[1,3]thiazolo[4,5-b]pyridin-2-yl)pyrrolidin-1-yl]-2-phenylethanone BDBM466307 US11174268, Example 196 BDBM526089 4-((2-(azetidin-1-ylmethyl)-3-fluorobenzyl)amino)-2,6-difluoro-N-(thiazol-4-yl)benzenesulfonamide 2,2,2-trifluoroacetate
US11174268, Example 196 BDBM526089 4-((2-(azetidin-1-ylmethyl)-3-fluorobenzyl)amino)-2,6-difluoro-N-(thiazol-4-yl)benzenesulfonamide 2,2,2-trifluoroacetate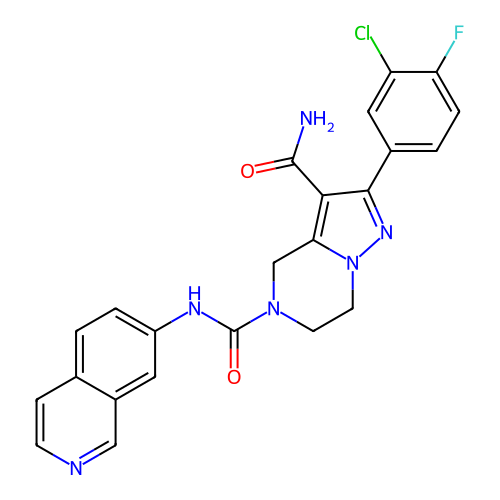 US9598423, Example 196 BDBM302629 2-(3-Chloro-4- fluorophenyl)-N5- (isoquinolin-7-yl)-6,7- dihydropyrazolo[1,5-a] pyrazine-3,5(4H)- dicarboxamide
US9598423, Example 196 BDBM302629 2-(3-Chloro-4- fluorophenyl)-N5- (isoquinolin-7-yl)-6,7- dihydropyrazolo[1,5-a] pyrazine-3,5(4H)- dicarboxamide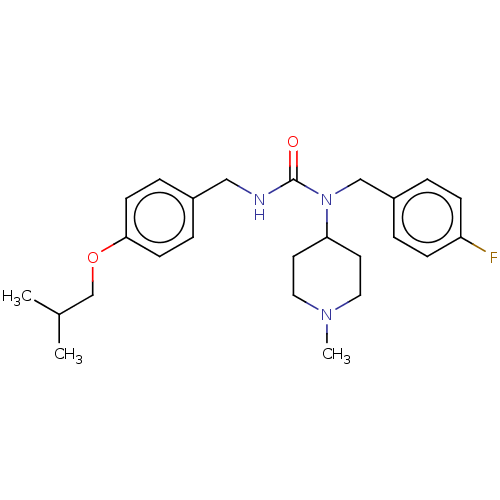 bis(1-(4-fluorobenzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(2-methylpropoxy)benzyl)urea) (2R,3R)-2,3-dihydroxybutanedioate BDBM139370 ACP-103 Nuplazid US20230348421, Compound Pimavanserin Pimavanserin tartrate WO2023288027, Cmpd PIMA Pimavanserin hydrochloride Pimavanserin
bis(1-(4-fluorobenzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(2-methylpropoxy)benzyl)urea) (2R,3R)-2,3-dihydroxybutanedioate BDBM139370 ACP-103 Nuplazid US20230348421, Compound Pimavanserin Pimavanserin tartrate WO2023288027, Cmpd PIMA Pimavanserin hydrochloride Pimavanserin (8-(2-(pyridin-4-yl)pyrido[3,4-d]pyrimidin-4-yl)-2,8-diazaspiro[4.5]decan-3-yl)methanol BDBM678471 US20240174696, Compound 196
(8-(2-(pyridin-4-yl)pyrido[3,4-d]pyrimidin-4-yl)-2,8-diazaspiro[4.5]decan-3-yl)methanol BDBM678471 US20240174696, Compound 196 (R)-4-(2-(5-fluoropyridin-2-yl)-4-(trifluoromethyl)phenyl)-N-(5-fluoropyrimidin-2-yl)chroman-7-sulfonamide US10239869, Example 196 BDBM371770
(R)-4-(2-(5-fluoropyridin-2-yl)-4-(trifluoromethyl)phenyl)-N-(5-fluoropyrimidin-2-yl)chroman-7-sulfonamide US10239869, Example 196 BDBM371770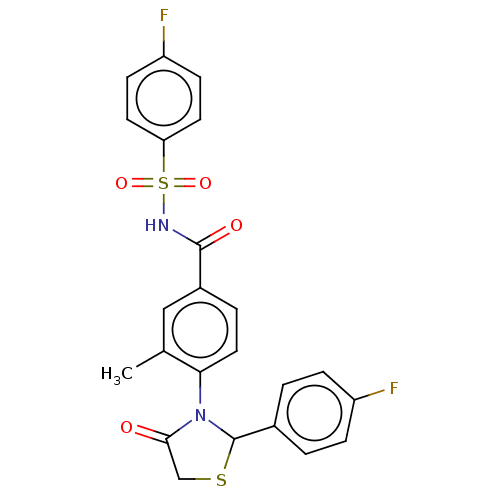 2,3-Dihydro-1,4-benzodioxin-2-ylmethyl 4-[2-(4-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-methylbenzoate BDBM426891 US10544113, No. 196
2,3-Dihydro-1,4-benzodioxin-2-ylmethyl 4-[2-(4-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-methylbenzoate BDBM426891 US10544113, No. 196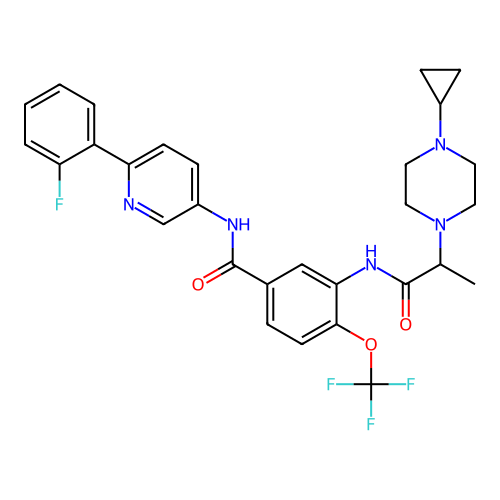 3-{[2-(4-cyclopropylpiperazin-1- yl)propanoyl]amino}-N-[6-(2- fluorophenyl)pyridin-3-yl]-4- (trifluoromethoxy)benzamide BDBM300626 US10130633, Example 196
3-{[2-(4-cyclopropylpiperazin-1- yl)propanoyl]amino}-N-[6-(2- fluorophenyl)pyridin-3-yl]-4- (trifluoromethoxy)benzamide BDBM300626 US10130633, Example 196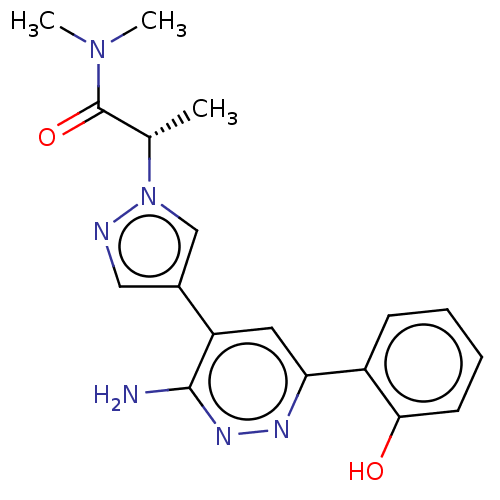 BDBM394593 (2S)-2-[4-[3-amino- 6-(2- hydroxyphenyl) pyridazin-4-yl]pyrazol- 1-yl]-N,N-dimethyl- propanamide US10308614, Example 196
BDBM394593 (2S)-2-[4-[3-amino- 6-(2- hydroxyphenyl) pyridazin-4-yl]pyrazol- 1-yl]-N,N-dimethyl- propanamide US10308614, Example 196 BDBM556961 US11352339, Example 196 3-(4-Fluoro-3-(6-(piperidin-1-yl)pyridin-2-yl)-1H-pyrazol-5-yl)pyrrolidine-1-carbonitrile
BDBM556961 US11352339, Example 196 3-(4-Fluoro-3-(6-(piperidin-1-yl)pyridin-2-yl)-1H-pyrazol-5-yl)pyrrolidine-1-carbonitrile N-(3-((5-amino-6-(2,3-dichlorophenyl)-1,2,4-triazin-3-yl)amino)-2-hydroxypropyl)-2-(2-methoxyethoxy)acetamide BDBM331951 US10189859, Compound 196
N-(3-((5-amino-6-(2,3-dichlorophenyl)-1,2,4-triazin-3-yl)amino)-2-hydroxypropyl)-2-(2-methoxyethoxy)acetamide BDBM331951 US10189859, Compound 196 N-[[6-(2-cyclohexylacetyl)-6- azaspiro[2.5]octan-2-yl]methyl]- 1,3-dihydropyrrolo[3,4- c]pyridine-2-carboxamide BDBM455608 US10730889, Example 196
N-[[6-(2-cyclohexylacetyl)-6- azaspiro[2.5]octan-2-yl]methyl]- 1,3-dihydropyrrolo[3,4- c]pyridine-2-carboxamide BDBM455608 US10730889, Example 196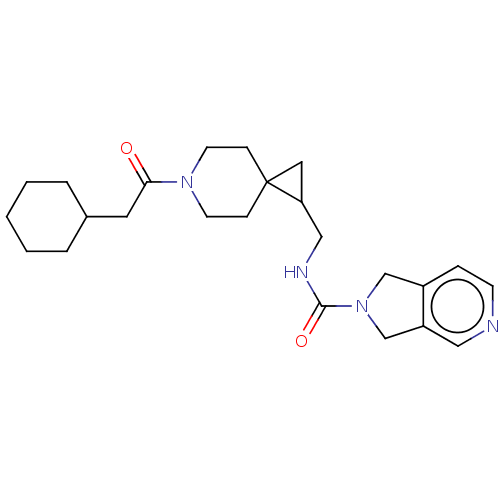 N-[[6-(2-cyclohexylacetyl)-6- azaspiro[2.5]octan-2-yl]methyl]- 1,3-dihydropyrrolo[3,4- c]pyridine-2-carboxamide US11485745, Example 196 BDBM580307
N-[[6-(2-cyclohexylacetyl)-6- azaspiro[2.5]octan-2-yl]methyl]- 1,3-dihydropyrrolo[3,4- c]pyridine-2-carboxamide US11485745, Example 196 BDBM580307 US10206907, Compound 196 Cyclopropanecarboxylic acid {5-[4-(1-hydroxy-pyridin-3- ylmethoxy)-phenyl]- [1,2,4]triazolo[1,5-a]pyridin-2- yl}-amide BDBM349077
US10206907, Compound 196 Cyclopropanecarboxylic acid {5-[4-(1-hydroxy-pyridin-3- ylmethoxy)-phenyl]- [1,2,4]triazolo[1,5-a]pyridin-2- yl}-amide BDBM349077 US10329294, Example 196 N-(2-bromothiophen-3- yl)-5-{[(1R,2S)-2- hydroxycyclohexyl]amino} pyrazolo[1,5- a]pyrimidine-3- carboxamide trifluoroacetate BDBM402622
US10329294, Example 196 N-(2-bromothiophen-3- yl)-5-{[(1R,2S)-2- hydroxycyclohexyl]amino} pyrazolo[1,5- a]pyrimidine-3- carboxamide trifluoroacetate BDBM402622 US12065436, Compound 196 BDBM690807 (S)-N-(6-(5-(difluoromethyl)-1,2,4- oxadiazol-3-yl)-2,3-dihydrofuro[3,2- b]pyridin-3-yl)-2- methylisonicotinamide
US12065436, Compound 196 BDBM690807 (S)-N-(6-(5-(difluoromethyl)-1,2,4- oxadiazol-3-yl)-2,3-dihydrofuro[3,2- b]pyridin-3-yl)-2- methylisonicotinamide 2-hydroxy-N-((5-(2-((4- (trifluoromethyl)benzo[d]oxazol-2- yl)thio)acetyl)thiophen-2- yl)methyl)acetamide US20250051319, Compound 196 BDBM719980
2-hydroxy-N-((5-(2-((4- (trifluoromethyl)benzo[d]oxazol-2- yl)thio)acetyl)thiophen-2- yl)methyl)acetamide US20250051319, Compound 196 BDBM719980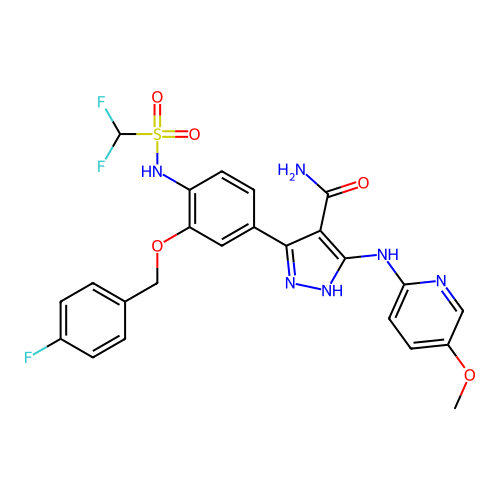 3-(4-((difluoromethyl)sulfonamido)-3- ((4-fluorobenzyl)oxy)phenyl)-5-((6- methoxypyridin-3-yl)amino)-1H- pyrazole-4-carboxamide BDBM725446 US20250074891, Compound 196
3-(4-((difluoromethyl)sulfonamido)-3- ((4-fluorobenzyl)oxy)phenyl)-5-((6- methoxypyridin-3-yl)amino)-1H- pyrazole-4-carboxamide BDBM725446 US20250074891, Compound 196 6-[(4,4-difluoro-2- methylcyclohexyl)carbonyl]- 8-(4,4-difluoropiperidin-1-yl)- 6,11-dihydro-5H-pyrido[2,3- b][1,5]benzodiazepine US10442819, Example 196 BDBM416050
6-[(4,4-difluoro-2- methylcyclohexyl)carbonyl]- 8-(4,4-difluoropiperidin-1-yl)- 6,11-dihydro-5H-pyrido[2,3- b][1,5]benzodiazepine US10442819, Example 196 BDBM416050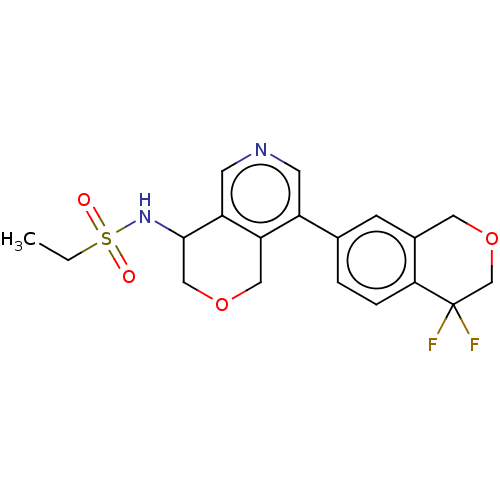 BDBM380992 Ethanesulfonic acid [8-(4,4-difluoro- isochroman-7-yl)-3,4-dihydro-1H- pyrano[4,3-c]pyridin-4-yl]-amide, Enantiomer II US9890171, 196
BDBM380992 Ethanesulfonic acid [8-(4,4-difluoro- isochroman-7-yl)-3,4-dihydro-1H- pyrano[4,3-c]pyridin-4-yl]-amide, Enantiomer II US9890171, 196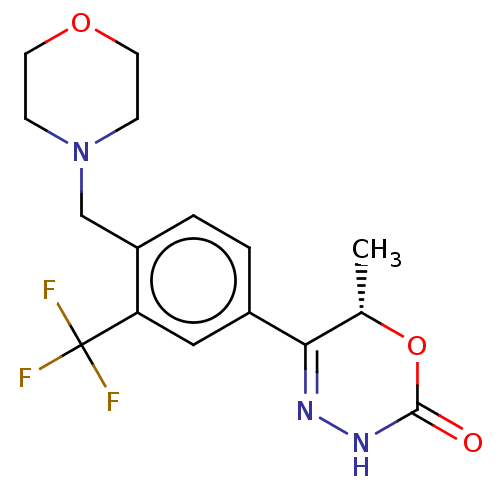 BDBM568733 (6S)-6-Methyl-5-{4-[(morpholin-4-yl)methyl]-3-(trifluoromethyl)phenyl}-3,6-dihydro-2H-1,3,4-oxadiazin-2-one US11427553, Example 196
BDBM568733 (6S)-6-Methyl-5-{4-[(morpholin-4-yl)methyl]-3-(trifluoromethyl)phenyl}-3,6-dihydro-2H-1,3,4-oxadiazin-2-one US11427553, Example 196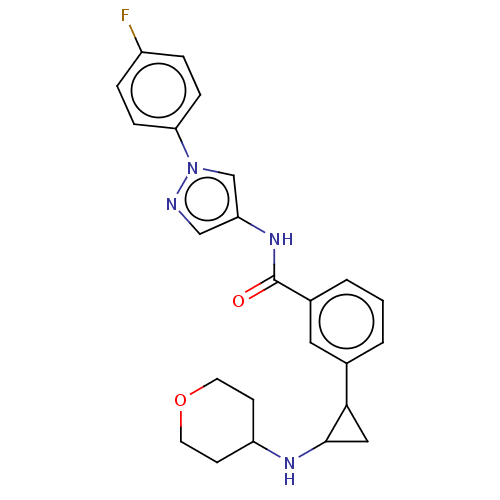 N-(1-(4-fluorophenyl)- 1H-pyrazol-4-yl)-3- (trans-2-(tetrahydro-2H- pyran-4-ylamino)cyclo- propyl)benzamide dihydrochloride US9751885, 196 BDBM338761
N-(1-(4-fluorophenyl)- 1H-pyrazol-4-yl)-3- (trans-2-(tetrahydro-2H- pyran-4-ylamino)cyclo- propyl)benzamide dihydrochloride US9751885, 196 BDBM338761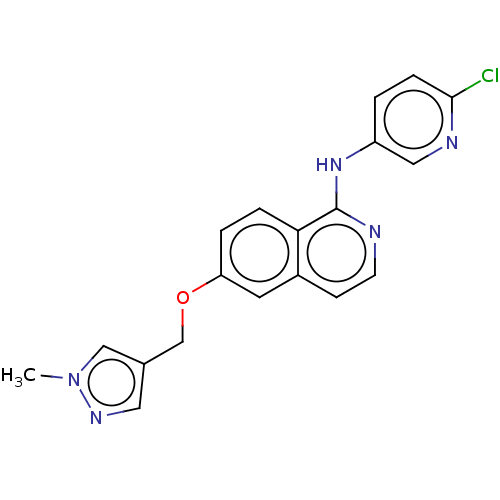 N-(6-chloropyridin-3-yl)-6-((1- methyl-1H-pyrazol-4- yl)methoxy)isoquinolin-1- amine US20240101531, Example 196 BDBM660810 US20240101531, Example 386
N-(6-chloropyridin-3-yl)-6-((1- methyl-1H-pyrazol-4- yl)methoxy)isoquinolin-1- amine US20240101531, Example 196 BDBM660810 US20240101531, Example 386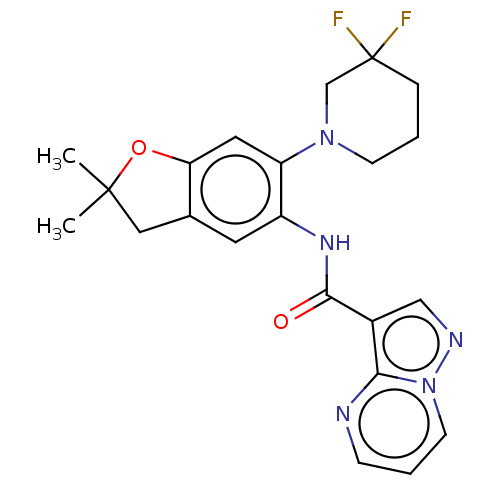 US10988478, Example 196 BDBM494148 N-(6-(3,3- difluoropiperidin- 1-yl)-2,2- dimethyl-2,3- dihydrobenzo- furan-5- yl)pyrazolo[1,5- a]pyrimidine-3- carboxamide
US10988478, Example 196 BDBM494148 N-(6-(3,3- difluoropiperidin- 1-yl)-2,2- dimethyl-2,3- dihydrobenzo- furan-5- yl)pyrazolo[1,5- a]pyrimidine-3- carboxamide US11998539, Example 196 1,6-dimethyl-4-[4-(6-methyl-1,3-benzoxazol-2-yl)piperidin-1-yl]-2-oxo-1,2-dihydroquinoline-3-carboxamide BDBM678754
US11998539, Example 196 1,6-dimethyl-4-[4-(6-methyl-1,3-benzoxazol-2-yl)piperidin-1-yl]-2-oxo-1,2-dihydroquinoline-3-carboxamide BDBM678754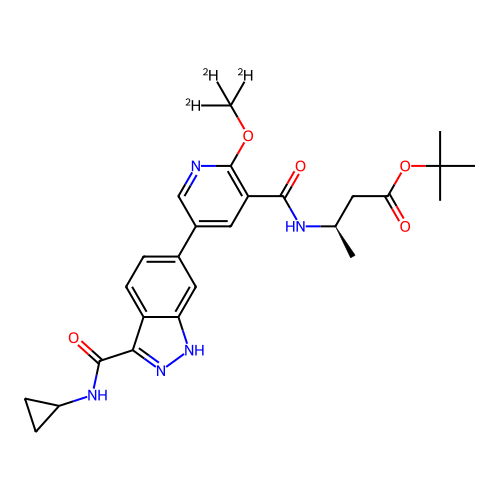 tert-butyl (3R)-3-({5-[3-(cyclo-propylcarbamoyl)-1H-indazol-6-yl]-2-(deutero)methoxypyridin-3-yl}formamido)butanoate US12172975, Example 196 BDBM709220
tert-butyl (3R)-3-({5-[3-(cyclo-propylcarbamoyl)-1H-indazol-6-yl]-2-(deutero)methoxypyridin-3-yl}formamido)butanoate US12172975, Example 196 BDBM709220 (R)-2,2-difluoro-64,4-dimethyl-67-morpholino-7-oxa- 5-aza-6(1,5)-phthalazina-1(4,1)-piperidina-3(1,3)- benzenacyclotridecaphane US20230339952, Compound 196 BDBM629672
(R)-2,2-difluoro-64,4-dimethyl-67-morpholino-7-oxa- 5-aza-6(1,5)-phthalazina-1(4,1)-piperidina-3(1,3)- benzenacyclotridecaphane US20230339952, Compound 196 BDBM629672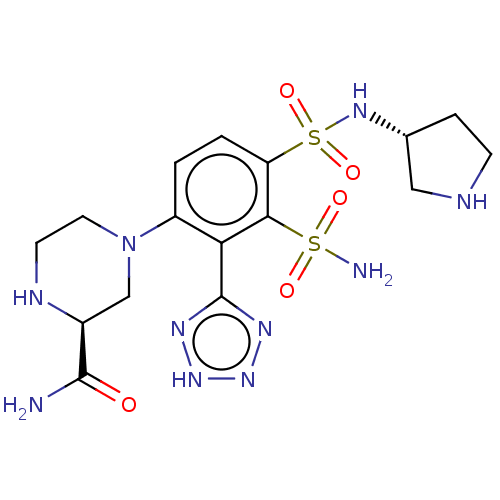 (S)-4-(4-(N-((R)-pyrrolidin-3-yl)sulfamoyl)-3-sulfamoyl-2-(2H-tetrazol-5-yl)phenyl)piperazine-2-carboxamide BDBM531064 US11207312, Example 196
(S)-4-(4-(N-((R)-pyrrolidin-3-yl)sulfamoyl)-3-sulfamoyl-2-(2H-tetrazol-5-yl)phenyl)piperazine-2-carboxamide BDBM531064 US11207312, Example 196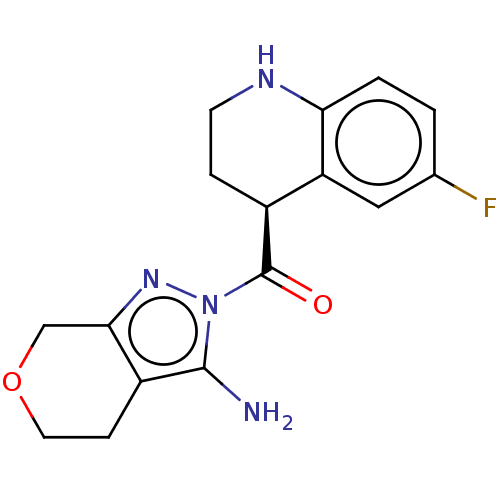 (S*)-(3-amino- 4,5- dihydropyrano[3, 4-c]pyrazol- 2(7H)-yl)(6- fluoro-1,2,3,4- tetrahydro- quinolin-4- yl)methanone BDBM552021 US11312723, Example 196
(S*)-(3-amino- 4,5- dihydropyrano[3, 4-c]pyrazol- 2(7H)-yl)(6- fluoro-1,2,3,4- tetrahydro- quinolin-4- yl)methanone BDBM552021 US11312723, Example 196 1-isopropyl-3-methyl-5-(2-propoxy-3-pyridyl)- N-(1H-pyrazol-4-ylmethyl)pyrazolo[4,3- b]pyridin-7-amine BDBM501567 US11026923, Example 196
1-isopropyl-3-methyl-5-(2-propoxy-3-pyridyl)- N-(1H-pyrazol-4-ylmethyl)pyrazolo[4,3- b]pyridin-7-amine BDBM501567 US11026923, Example 196 1-isopropyl-3-methyl-5-(2-propoxy-3-pyridyl)- N-(1H-pyrazol-4-ylmethyl)pyrazolo[4,3- b]pyridin-7-amine US10806718, Example 196 BDBM468508
1-isopropyl-3-methyl-5-(2-propoxy-3-pyridyl)- N-(1H-pyrazol-4-ylmethyl)pyrazolo[4,3- b]pyridin-7-amine US10806718, Example 196 BDBM468508 2-Ethyl-5-(1-methyl-1H-indazol-5-yl)-3-(pyridin-2-yl)pyrazolo[1,5-a]pyrimidin-7(4H)-one BDBM487388 US10953012, Example 196
2-Ethyl-5-(1-methyl-1H-indazol-5-yl)-3-(pyridin-2-yl)pyrazolo[1,5-a]pyrimidin-7(4H)-one BDBM487388 US10953012, Example 196 BDBM438844 3-((1R,5S,9r)-3-(4,4-difluorocyclohexyl)-9-methoxy-3-azabicyclo[3.3.1]nonan-9-yl)benzamide hydrochloride US11180455, Compound 196 US10604489, Compound 9
BDBM438844 3-((1R,5S,9r)-3-(4,4-difluorocyclohexyl)-9-methoxy-3-azabicyclo[3.3.1]nonan-9-yl)benzamide hydrochloride US11180455, Compound 196 US10604489, Compound 9 BDBM588266 US11542259, Compound 196 1-N'-(4-fluorophenyl)-1-N- [4-[6-(3-hydroxyoxetan- 3-yl)-7- methoxyquinolin-4- yl]oxyphenyl]cyclopropane- 1,1-dicarboxamide
BDBM588266 US11542259, Compound 196 1-N'-(4-fluorophenyl)-1-N- [4-[6-(3-hydroxyoxetan- 3-yl)-7- methoxyquinolin-4- yl]oxyphenyl]cyclopropane- 1,1-dicarboxamide BDBM703719 1-[3-fluoro-5- isobutyl-2-(2H- tetrazol-5-yl)phen- yl]-4-[(3-fluoro-2- pyridyl)methyl]- piperazine US20240368133, Compound A-196
BDBM703719 1-[3-fluoro-5- isobutyl-2-(2H- tetrazol-5-yl)phen- yl]-4-[(3-fluoro-2- pyridyl)methyl]- piperazine US20240368133, Compound A-196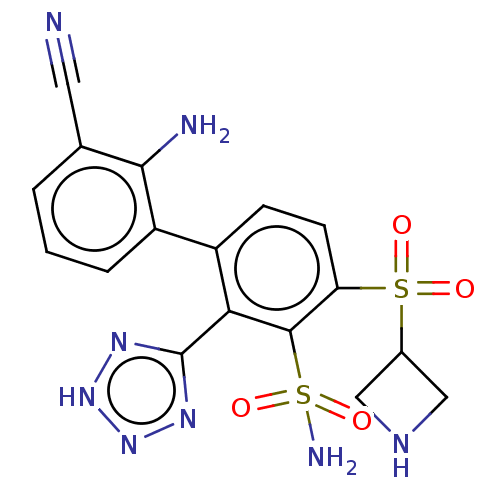 US10227331, Example 196 BDBM368009 2′-Amino-4-(azetidin-3-ylsulfonyl)-3′-cyano-2-(2H-tetrazol-5-yl)-[1,1′-biphenyl]-3-sulfonamide
US10227331, Example 196 BDBM368009 2′-Amino-4-(azetidin-3-ylsulfonyl)-3′-cyano-2-(2H-tetrazol-5-yl)-[1,1′-biphenyl]-3-sulfonamide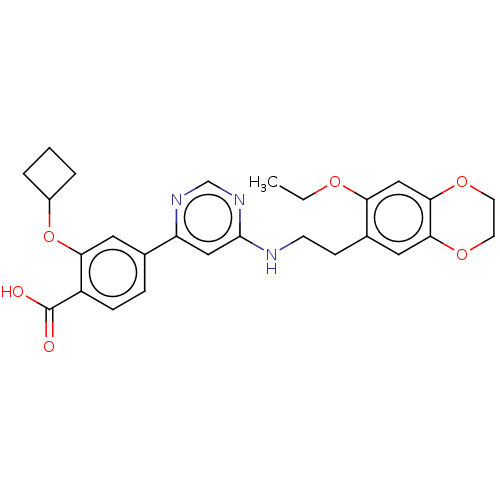 US11839613, Example 196 2-Cyclobutoxy-4-{6-[2-(7-ethoxy-2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethylamino]- pyrimidin-4-yl}-benzoic acid BDBM640075
US11839613, Example 196 2-Cyclobutoxy-4-{6-[2-(7-ethoxy-2,3-dihydro-benzo[1,4]dioxin-6-yl)-ethylamino]- pyrimidin-4-yl}-benzoic acid BDBM640075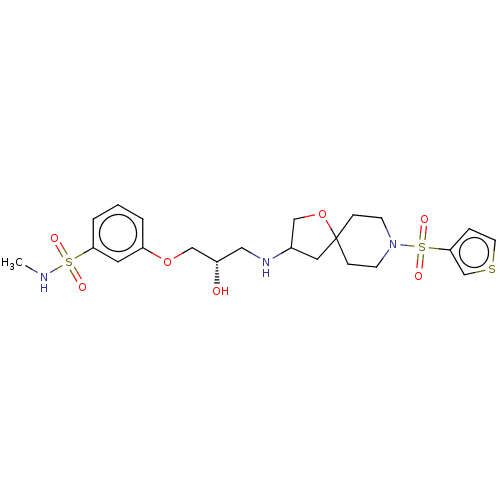 US11987588, Compound 196 BDBM675727 3-((2S)-2-hydroxy-3-(8-(thiophen-3-ylsulfonyl)-1-oxa-8-azaspiro[4.5]decan-3-ylamino)propoxy)-N-methylbenzenesulfonamide
US11987588, Compound 196 BDBM675727 3-((2S)-2-hydroxy-3-(8-(thiophen-3-ylsulfonyl)-1-oxa-8-azaspiro[4.5]decan-3-ylamino)propoxy)-N-methylbenzenesulfonamide US20250163063, Example 196 (3R,4R)-1-(cyclopropylsulfonyl)-4- ((7-(3,3-dimethylcyclobutyl)-5- fluoropyrrolo[2,1-f][1,2,4]triazin-2- yl)amino)piperidin-3-ol BDBM742677
US20250163063, Example 196 (3R,4R)-1-(cyclopropylsulfonyl)-4- ((7-(3,3-dimethylcyclobutyl)-5- fluoropyrrolo[2,1-f][1,2,4]triazin-2- yl)amino)piperidin-3-ol BDBM742677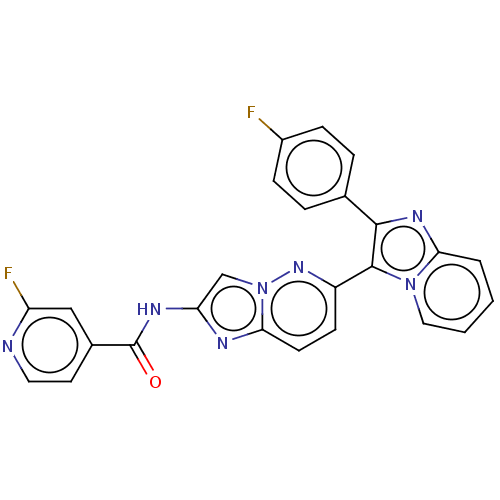 US9556179, Compound 196 2-fluoro-N-(6-(2-(4- fluorophenyl)imidazo[1,2-a] pyridin-3-yl)imidazo[1,2-b] pyridazin-2-yl)isonicotinamide BDBM274622
US9556179, Compound 196 2-fluoro-N-(6-(2-(4- fluorophenyl)imidazo[1,2-a] pyridin-3-yl)imidazo[1,2-b] pyridazin-2-yl)isonicotinamide BDBM274622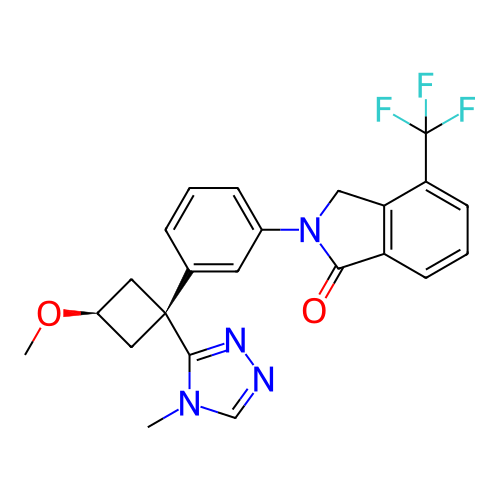 2-(3-((1r,3r)-3-methoxy-1-(4-methyl-4H- 1,2,4-triazol-3-yl)cyclobutyl)phenyl)-4- (trifluoromethyl)isoindolin-1-one US12187709, Compound 196 BDBM710838
2-(3-((1r,3r)-3-methoxy-1-(4-methyl-4H- 1,2,4-triazol-3-yl)cyclobutyl)phenyl)-4- (trifluoromethyl)isoindolin-1-one US12187709, Compound 196 BDBM710838 BDBM297980 US10118930, Example 196 3-[3-(4-tert-butylphenyl)-1,2,4-oxadiazol-5-yl]-7-(piperidin-4-yl)pyrazolo[1,5-a]pyrimidin-5(4H)-one hydrochloride
BDBM297980 US10118930, Example 196 3-[3-(4-tert-butylphenyl)-1,2,4-oxadiazol-5-yl]-7-(piperidin-4-yl)pyrazolo[1,5-a]pyrimidin-5(4H)-one hydrochloride BDBM312450 2-[6,6-Difluoro-3-(3-methyl-[1,2,4]thiadiazol-5-yl)-4,5,6,7-tetrahydro-benzo[b]thiophen-2-ylcarbamoyl]-cyclopent-1-enecarboxylic acid US9604977, Example 196
BDBM312450 2-[6,6-Difluoro-3-(3-methyl-[1,2,4]thiadiazol-5-yl)-4,5,6,7-tetrahydro-benzo[b]thiophen-2-ylcarbamoyl]-cyclopent-1-enecarboxylic acid US9604977, Example 196 BDBM517588 USRE48711, Example 196 3-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(4-cyclopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)-2-methylbenzamide
BDBM517588 USRE48711, Example 196 3-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(4-cyclopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)-2-methylbenzamide BDBM530119 4-[4-fluoro-1-(5- fluoro-4-methoxy- 6-methyl- pyrimidin-2- yl)piperidine-4- carbonyl]-3,5- dihydro-2H- pyrido[3,4- US11203600, Example 196
BDBM530119 4-[4-fluoro-1-(5- fluoro-4-methoxy- 6-methyl- pyrimidin-2- yl)piperidine-4- carbonyl]-3,5- dihydro-2H- pyrido[3,4- US11203600, Example 196 BDBM610388 N-(2-chloro-5-(2,3-dihydrobenzo[b][1,4]dioxine-6-carboxamido)phenyl)-2-((4-ethylpiperazin-1-yl)methyl)quinoline-6-carboxamide US9701664, Example 196
BDBM610388 N-(2-chloro-5-(2,3-dihydrobenzo[b][1,4]dioxine-6-carboxamido)phenyl)-2-((4-ethylpiperazin-1-yl)methyl)quinoline-6-carboxamide US9701664, Example 196 BDBM613455 methyl N-[4-carbamoyl-1-[4- (cyanomethyl)-3-fluoro-1-[(6- phenyl-3-pyridyl)methyl]-4- piperidyl]pyrazol-3-yl]carbamate US11731943, Example 196
BDBM613455 methyl N-[4-carbamoyl-1-[4- (cyanomethyl)-3-fluoro-1-[(6- phenyl-3-pyridyl)methyl]-4- piperidyl]pyrazol-3-yl]carbamate US11731943, Example 196 BDBM760517 N-[4-[(6,7-dimethoxy-1,5- naphthyridin-4-yl)oxy]phenyl]-5-(4- fluoro-2-methylphenyl)-1,6-dimethyl- 4-oxopyridine-3-carboxamide US12371428, Compound 196
BDBM760517 N-[4-[(6,7-dimethoxy-1,5- naphthyridin-4-yl)oxy]phenyl]-5-(4- fluoro-2-methylphenyl)-1,6-dimethyl- 4-oxopyridine-3-carboxamide US12371428, Compound 196 N-{trans-4-(3-fluorophenyl)-1-[(1-methyl-1H-imidazol-4-yl)sulfonyl]pyrrolidin-3-yl}-5-(trifluoromethyl)pyridin-3-amine US9656955, Example 196 BDBM308682
N-{trans-4-(3-fluorophenyl)-1-[(1-methyl-1H-imidazol-4-yl)sulfonyl]pyrrolidin-3-yl}-5-(trifluoromethyl)pyridin-3-amine US9656955, Example 196 BDBM308682 US10173991, Example 196 N-[(cyclopropyl- carbamoyl) methyl]-2-[5- fluoro-6-(1- methyl-1H- indazol-6-yl)-1,3- benzothiazol- 2-yl]-2- methanesulfonyl- acetamide BDBM319153
US10173991, Example 196 N-[(cyclopropyl- carbamoyl) methyl]-2-[5- fluoro-6-(1- methyl-1H- indazol-6-yl)-1,3- benzothiazol- 2-yl]-2- methanesulfonyl- acetamide BDBM319153 US10183938, Compound (R)-196 BDBM322443 (R)-7-cyclopropyl-6-methyl-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[2.2.2]octan]-3'-yl)benzo[b]thiophene-2-carboxamide
US10183938, Compound (R)-196 BDBM322443 (R)-7-cyclopropyl-6-methyl-N-(1'-azaspiro[cyclopropane-1,2'-bicyclo[2.2.2]octan]-3'-yl)benzo[b]thiophene-2-carboxamide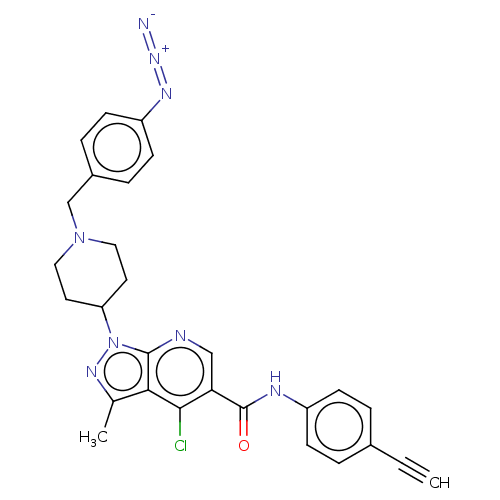 US10272074, Example 196 BDBM381363 1-(1-(4-azidobenzyl)piperidin-4- yl)-4-chloro-N-(4-ethynylphenyl)- 3-methyl-1H-pyrazolo[3,4- b]pyridine-5-carboxamide
US10272074, Example 196 BDBM381363 1-(1-(4-azidobenzyl)piperidin-4- yl)-4-chloro-N-(4-ethynylphenyl)- 3-methyl-1H-pyrazolo[3,4- b]pyridine-5-carboxamide US10508109, Example 196 4-(2-(5-chloro-2-(3- (dimethylamino)propoxy)-4- methoxyphenyl)imidazo[1,2- a]pyridine-7-yl)-N,N- dimethylpiperazine-1- sulfonamide BDBM423010
US10508109, Example 196 4-(2-(5-chloro-2-(3- (dimethylamino)propoxy)-4- methoxyphenyl)imidazo[1,2- a]pyridine-7-yl)-N,N- dimethylpiperazine-1- sulfonamide BDBM423010 US10543207, Example 196 BDBM426518 N1,N3-bis(2-(2-(2-(3-(6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenylsulfonamido)ethoxy)ethoxy)ethyl)-2,2-dimethylmalonamide
US10543207, Example 196 BDBM426518 N1,N3-bis(2-(2-(2-(3-(6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenylsulfonamido)ethoxy)ethoxy)ethyl)-2,2-dimethylmalonamide US11648254, Compound 196 (R)-6-(4-ethylpiperazin-1-yl)-N-(1-(2- fluoro-3-(trifluoromethyl)phenyl) ethyl)-7-methoxypyrido[2,3-d]pyrimidin- 4-amine BDBM602297
US11648254, Compound 196 (R)-6-(4-ethylpiperazin-1-yl)-N-(1-(2- fluoro-3-(trifluoromethyl)phenyl) ethyl)-7-methoxypyrido[2,3-d]pyrimidin- 4-amine BDBM602297 US12338237, Example 196 (S)-quinuclidin-3-yl (6'-(2-(2-methoxyethoxy)phenyl)-3',4'-dihydro-1'H-spiro[cyclopropane-1,2'-naphthalen]-1'-yl)carbamate BDBM753438
US12338237, Example 196 (S)-quinuclidin-3-yl (6'-(2-(2-methoxyethoxy)phenyl)-3',4'-dihydro-1'H-spiro[cyclopropane-1,2'-naphthalen]-1'-yl)carbamate BDBM753438 (R)-N-((S)-2-(dimethylamino)-3-(4-methyl-1H-indazol-5-yl)propyl)-3-(pyridin-4-yl)-3-(1-(trifluoromethyl)cyclopropyl)propanamide BDBM601257 US11634396, Compound 196
(R)-N-((S)-2-(dimethylamino)-3-(4-methyl-1H-indazol-5-yl)propyl)-3-(pyridin-4-yl)-3-(1-(trifluoromethyl)cyclopropyl)propanamide BDBM601257 US11634396, Compound 196 1,1-dimethylethyl 2-[3-({[4-[(3,4- dichlorophenyl)amino]-6- (methyloxy)quinazolin-7-yl]oxy}methyl)-1,2,4- oxadiazol-5-yl]piperidine-1-carboxylate BDBM351476 US9796704, Entry 196
1,1-dimethylethyl 2-[3-({[4-[(3,4- dichlorophenyl)amino]-6- (methyloxy)quinazolin-7-yl]oxy}methyl)-1,2,4- oxadiazol-5-yl]piperidine-1-carboxylate BDBM351476 US9796704, Entry 196 2-{4-[5-Amino-6-((S)-(3- amino-piperidin-1-yl)- pyrazin-2-yl]- benzylamino}-N-(4- fluoro-phenyl)-5- trifluoromethyl-nicotinamide BDBM282316 US10030016, Example 196
2-{4-[5-Amino-6-((S)-(3- amino-piperidin-1-yl)- pyrazin-2-yl]- benzylamino}-N-(4- fluoro-phenyl)-5- trifluoromethyl-nicotinamide BDBM282316 US10030016, Example 196 4-(3-{[6-(4-chloro-3,5-dimethyl-1H-pyrazol-1-yl)pyrimidin-4-yl]amino}-1,4-dimethyl-1H-pyrazol-5-yl)benzonitrile US11208400, Example 196 BDBM531563
4-(3-{[6-(4-chloro-3,5-dimethyl-1H-pyrazol-1-yl)pyrimidin-4-yl]amino}-1,4-dimethyl-1H-pyrazol-5-yl)benzonitrile US11208400, Example 196 BDBM531563 5-(1H-imidazol-1-yl)-N-((1r,4r)-4-(2-methoxyethoxy)cyclohexyl)-2-methyl-2H-pyrazolo[3,4-c]pyridine-7-carboxamide and BDBM587947 US11535621, Example 196
5-(1H-imidazol-1-yl)-N-((1r,4r)-4-(2-methoxyethoxy)cyclohexyl)-2-methyl-2H-pyrazolo[3,4-c]pyridine-7-carboxamide and BDBM587947 US11535621, Example 196 6-(azetidin-1-yl)-N-[5-(3,6-dihydro-2H-pyran-4-yl)-2-methoxybenzene-1-sulfonyl]-4-fluoro-1-benzofuran-2-carboxamide BDBM534089 WO2022081807, Example 196
6-(azetidin-1-yl)-N-[5-(3,6-dihydro-2H-pyran-4-yl)-2-methoxybenzene-1-sulfonyl]-4-fluoro-1-benzofuran-2-carboxamide BDBM534089 WO2022081807, Example 196 BDBM315246 (−) 3-{3-[(cyclopentyl{3-fluoro-4-[5-(2,2,2-trifluoroethyl)pyridin-3-yl]phenyl}acetyl)amino]-2-methylphenyl}propanoic acid, Single Enantiomer US10172814, Example 196
BDBM315246 (−) 3-{3-[(cyclopentyl{3-fluoro-4-[5-(2,2,2-trifluoroethyl)pyridin-3-yl]phenyl}acetyl)amino]-2-methylphenyl}propanoic acid, Single Enantiomer US10172814, Example 196 BDBM406598 US10336739, Example 196 2-{5-[6-butyl-5- (2,6- dimethoxyphenyl)- 2,4- dihydroxypyridin- 3-yl]-1,3,4- oxadiazol-2-yl}-1- (morpholin-4- yl)ethan-1-one
BDBM406598 US10336739, Example 196 2-{5-[6-butyl-5- (2,6- dimethoxyphenyl)- 2,4- dihydroxypyridin- 3-yl]-1,3,4- oxadiazol-2-yl}-1- (morpholin-4- yl)ethan-1-one BDBM464314 US10787450, Example 196 3-(5-(1H-pyrazol-4- yl)pyridin-2-yl)-1-(3- methoxybenzyl)-8- (oxetane-3-carbonyl)- 1,3,8- triazaspiro[4.5]decan- 2-one
BDBM464314 US10787450, Example 196 3-(5-(1H-pyrazol-4- yl)pyridin-2-yl)-1-(3- methoxybenzyl)-8- (oxetane-3-carbonyl)- 1,3,8- triazaspiro[4.5]decan- 2-one BDBM593218 US11578061, Example 196 N-((1R,4R)-4-(((2-((1- cyclopropyl-3-methyl-1H-pyrazol- 4-yl)amino)-5-fluoropyrimidin-4- yl)oxy)methyl)cyclohexyl) acetamide
BDBM593218 US11578061, Example 196 N-((1R,4R)-4-(((2-((1- cyclopropyl-3-methyl-1H-pyrazol- 4-yl)amino)-5-fluoropyrimidin-4- yl)oxy)methyl)cyclohexyl) acetamide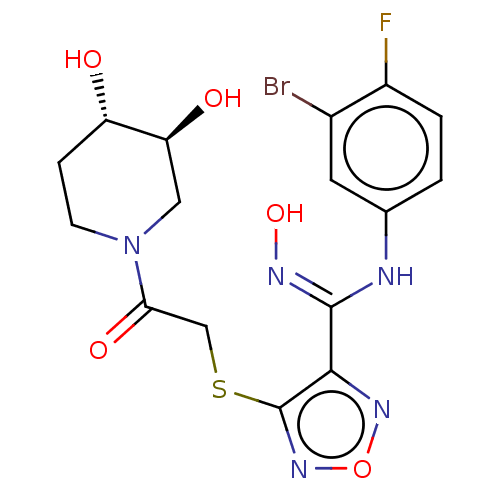 N-(3-bromo-4-fluorophenyl)-4- ((2-((3S,4S)-3,4- dihydroxypiperidin-1-yl)-2- oxoethyl)thio)-N'-hydroxy-1,2,5- oxadiazole-3-carboximidamide BDBM430006 US10538497, Example 196
N-(3-bromo-4-fluorophenyl)-4- ((2-((3S,4S)-3,4- dihydroxypiperidin-1-yl)-2- oxoethyl)thio)-N'-hydroxy-1,2,5- oxadiazole-3-carboximidamide BDBM430006 US10538497, Example 196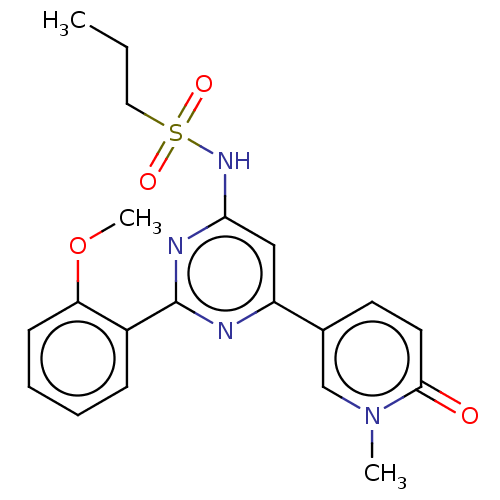 Propane-1-sulfonic acid [2-(2- methoxy-phenyl)-6-(1-methyl-6- oxo-1,6-dihydro-pyridin-3-yl)- pyrimidin-4-yl]-amide US10202360, Example 196 BDBM345651
Propane-1-sulfonic acid [2-(2- methoxy-phenyl)-6-(1-methyl-6- oxo-1,6-dihydro-pyridin-3-yl)- pyrimidin-4-yl]-amide US10202360, Example 196 BDBM345651 US10052306, 196 BDBM237380 rac-((3aR,r,6aS)-2-(2- hydroxy-2-(5- hydroxypyridin-2- yl)ethyl)-5-(3-meth- oxybenzyl)octahydro- cyclopenta[c]pyrrol-5-ol
US10052306, 196 BDBM237380 rac-((3aR,r,6aS)-2-(2- hydroxy-2-(5- hydroxypyridin-2- yl)ethyl)-5-(3-meth- oxybenzyl)octahydro- cyclopenta[c]pyrrol-5-ol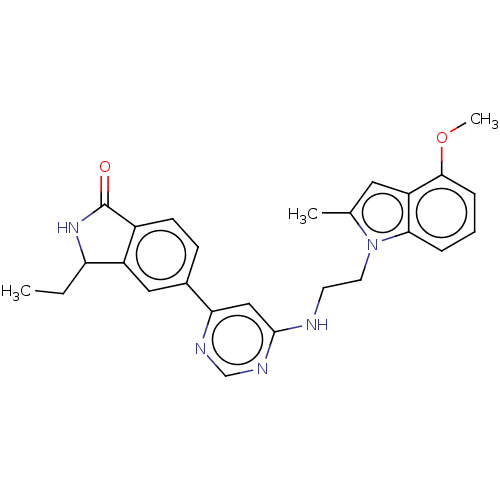 US12011444, Example 196 BDBM679981 rac-3-Ethyl-5-{6-[2-(4-methoxy-2-methyl-indol-1-yl)-ethylamino]-pyrimidin-4-yl}-2,3- dihydro-isoindol-1-one
US12011444, Example 196 BDBM679981 rac-3-Ethyl-5-{6-[2-(4-methoxy-2-methyl-indol-1-yl)-ethylamino]-pyrimidin-4-yl}-2,3- dihydro-isoindol-1-one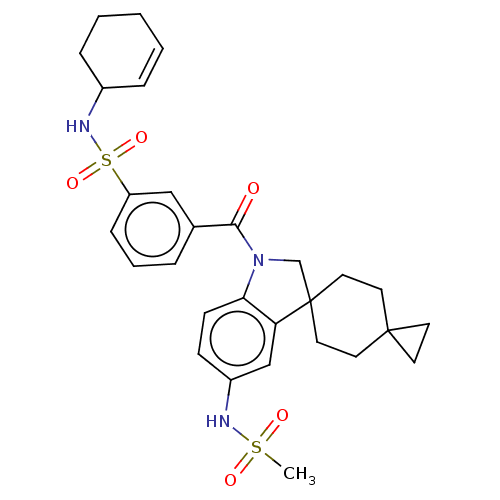 US12084420, Compound 196 N-(cyclohex-2-en-1-yl)-3-(5$#8243;- (methylsulfonamido)dispiro[cyclopropane- 1,1'-cyclohexane-4',3$#8243;-indoline]- 1$#8243;-carbonyl)benzenesulfonamide BDBM695625
US12084420, Compound 196 N-(cyclohex-2-en-1-yl)-3-(5$#8243;- (methylsulfonamido)dispiro[cyclopropane- 1,1'-cyclohexane-4',3$#8243;-indoline]- 1$#8243;-carbonyl)benzenesulfonamide BDBM695625 US20240262826, Compound 196 N-(2-methyl-5-(2-(1-(2,2,2-trifluoroethyl)azetidin-3-yl)-2H-tetrazol-5-yl)phenyl)pyrazolo[1,5-a]pyridine-3-carboxamide BDBM690251
US20240262826, Compound 196 N-(2-methyl-5-(2-(1-(2,2,2-trifluoroethyl)azetidin-3-yl)-2H-tetrazol-5-yl)phenyl)pyrazolo[1,5-a]pyridine-3-carboxamide BDBM690251 [1-[5-(6-Methyl-pyridazin-3-yl)-pyrimidin-2-yl]-spiro[1,2-dihydro-indole-3,1'-cyclopropane]-6-yl]-morpholin-4-yl-methanone BDBM332235 US10189854, Compound 196
[1-[5-(6-Methyl-pyridazin-3-yl)-pyrimidin-2-yl]-spiro[1,2-dihydro-indole-3,1'-cyclopropane]-6-yl]-morpholin-4-yl-methanone BDBM332235 US10189854, Compound 196 [3-(1-ethyl-8-oxo- spiro[6,7-dihydro-4H- pyrazolo[3,4-c]azepine- 5,4'-tetrahydropyran]-3- yl)-2,2-dimethyl-propyl] 3- methoxybenzoate BDBM466095 US10793580, Example 196
[3-(1-ethyl-8-oxo- spiro[6,7-dihydro-4H- pyrazolo[3,4-c]azepine- 5,4'-tetrahydropyran]-3- yl)-2,2-dimethyl-propyl] 3- methoxybenzoate BDBM466095 US10793580, Example 196 BDBM470809 US10821115, Example 196 US11224601, Example 196 (1S,3'R,6'R,7'S,8'E,11'S,12'R)- 6-chloro-7'-methoxy-11',12'- dimethyl-7'-((4-methyl-1- piperazinyl) methyl)-3,4- dihydro-2H,15'H- spiro[naphthalene-1,22'- [20]oxa[13]thia[1,14] diazatetracyclo [14.7.2.0~3,6~.0~19,24~] pentacosa[8,16,18,24]tetraen]- 15'-one 13',13'-dioxide
BDBM470809 US10821115, Example 196 US11224601, Example 196 (1S,3'R,6'R,7'S,8'E,11'S,12'R)- 6-chloro-7'-methoxy-11',12'- dimethyl-7'-((4-methyl-1- piperazinyl) methyl)-3,4- dihydro-2H,15'H- spiro[naphthalene-1,22'- [20]oxa[13]thia[1,14] diazatetracyclo [14.7.2.0~3,6~.0~19,24~] pentacosa[8,16,18,24]tetraen]- 15'-one 13',13'-dioxide 1-[(3S,4R)-1-cyclobutyl-4- phenylpyrrolidin-3-yl]-3-[1- (2-methylpyridin-4-yl)-1H- pyrazolo[3,4-c]pyridin-5- yl]urea US9884048, Example 196 BDBM275302
1-[(3S,4R)-1-cyclobutyl-4- phenylpyrrolidin-3-yl]-3-[1- (2-methylpyridin-4-yl)-1H- pyrazolo[3,4-c]pyridin-5- yl]urea US9884048, Example 196 BDBM275302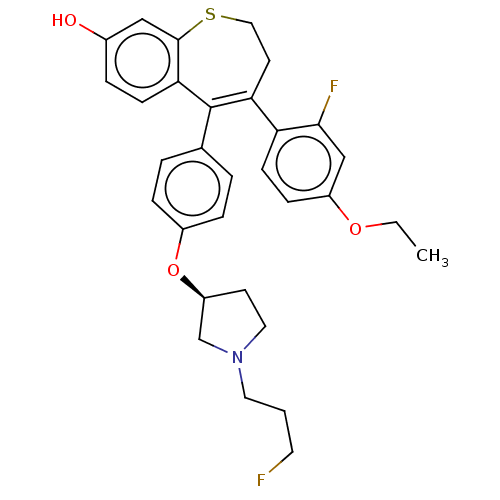 4-(4-ethoxy-2- fluoro-phenyl)- 5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]oxyphenyl]- 2,3-dihydro-1- benzothiepin-8- ol BDBM521372 US11149031, Example 196
4-(4-ethoxy-2- fluoro-phenyl)- 5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]oxyphenyl]- 2,3-dihydro-1- benzothiepin-8- ol BDBM521372 US11149031, Example 196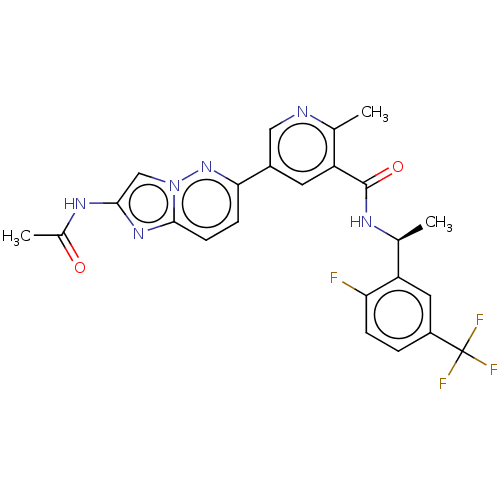 5-{2-acetamidoimidazo[1,2-b] pyridazin-6-yl}-N-[(1S)- 1-[2-fluoro-5-(trifluoromethyl) phenyl](2,2,2-deutero)ethyl]-2- methylpyridine-3-carboxamide US11440913, Example 196 BDBM571129
5-{2-acetamidoimidazo[1,2-b] pyridazin-6-yl}-N-[(1S)- 1-[2-fluoro-5-(trifluoromethyl) phenyl](2,2,2-deutero)ethyl]-2- methylpyridine-3-carboxamide US11440913, Example 196 BDBM571129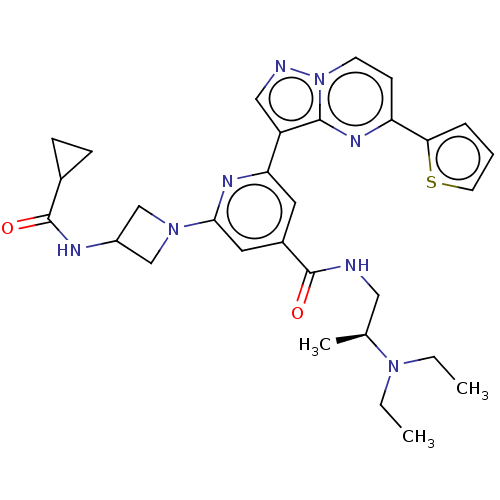 BDBM292540 (S)-2-(3-(cyclopropanecarboxamido)azetidin-1-yl)-N-(2-(diethylamino)propyl)-6-(5-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidin-3-yl)isonicotinamide US10100058, Example 196
BDBM292540 (S)-2-(3-(cyclopropanecarboxamido)azetidin-1-yl)-N-(2-(diethylamino)propyl)-6-(5-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidin-3-yl)isonicotinamide US10100058, Example 196 BDBM299826 US9593097, Example 196 4-(4-{[(4- aminopyrimidin-5- yl)oxy]methyl} piperidin-1-yl)-6- {[(1R,2S)-2- cyanocyclopropyl] methoxy}-N- cyclopentyl-1,3,5- triazine-2- carboxamide
BDBM299826 US9593097, Example 196 4-(4-{[(4- aminopyrimidin-5- yl)oxy]methyl} piperidin-1-yl)-6- {[(1R,2S)-2- cyanocyclopropyl] methoxy}-N- cyclopentyl-1,3,5- triazine-2- carboxamide BDBM306553 (1S,8R)-N12-[(6-amino-2,4- dimethylypyridin-3-yl)methyl]- N4-(4-fluorophenyl)-N4-methyl- 15-oxatetracyclo[6.6.1.02,7.09,14] pentadeca-2,4,6,9,11,13- hexaene-4,12-dicarboxamide US10144746, Compound 196
BDBM306553 (1S,8R)-N12-[(6-amino-2,4- dimethylypyridin-3-yl)methyl]- N4-(4-fluorophenyl)-N4-methyl- 15-oxatetracyclo[6.6.1.02,7.09,14] pentadeca-2,4,6,9,11,13- hexaene-4,12-dicarboxamide US10144746, Compound 196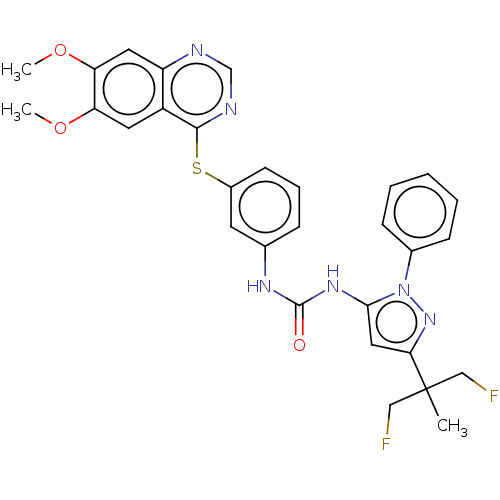 BDBM333647 1-[3-(1,3- difluoro-2- methylpropan- 2-yl)-1- phenyl-1H- pyrazol-5- yl]-3-[3-(6,7- dimethoxy- quinazolin- 4- ylthio)phenyl] urea US9730937, Example 196
BDBM333647 1-[3-(1,3- difluoro-2- methylpropan- 2-yl)-1- phenyl-1H- pyrazol-5- yl]-3-[3-(6,7- dimethoxy- quinazolin- 4- ylthio)phenyl] urea US9730937, Example 196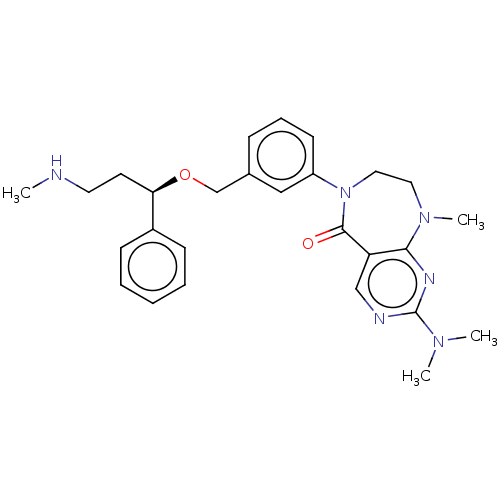 BDBM436421 (R)-2-(dimethylamino)- 9-methyl-6-(3-((3- (methylamino)-1- phenylpropoxy)methyl) phenyl)-6,7,8,9- tetrahydro-5H- pyrimido[4,5- e][1,4]diazepin-5-one US10590140, Example 196
BDBM436421 (R)-2-(dimethylamino)- 9-methyl-6-(3-((3- (methylamino)-1- phenylpropoxy)methyl) phenyl)-6,7,8,9- tetrahydro-5H- pyrimido[4,5- e][1,4]diazepin-5-one US10590140, Example 196 BDBM493267 (1-((1r,4r)-4-(Cyanomethyl)cyclohexyl)-1,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridin-2-yl)methyl (3S,5S,7S)-adamantan-1-ylcarbamate US10981911, Example 196
BDBM493267 (1-((1r,4r)-4-(Cyanomethyl)cyclohexyl)-1,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridin-2-yl)methyl (3S,5S,7S)-adamantan-1-ylcarbamate US10981911, Example 196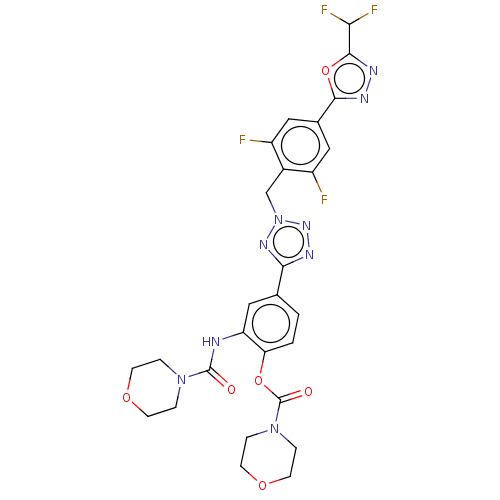 BDBM618426 US20230286970, Compound 196 4-(2-(4-(5-(difluoromethyl)-1,3,4-oxadiazol-2-yl)-2,6-difluorobenzyl)-2H-tetrazol-5-yl)-2-(morpholine-4-carboxamido)phenyl morpholine-4-carboxylate
BDBM618426 US20230286970, Compound 196 4-(2-(4-(5-(difluoromethyl)-1,3,4-oxadiazol-2-yl)-2,6-difluorobenzyl)-2H-tetrazol-5-yl)-2-(morpholine-4-carboxamido)phenyl morpholine-4-carboxylate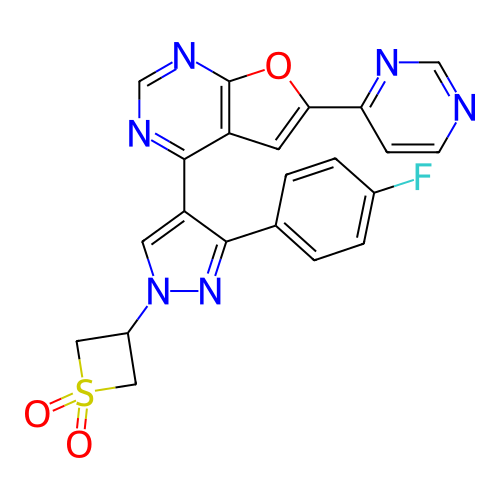 BDBM717375 US20250034159, Example 196 3-{3-(4-fluorophenyl)-4-[6- (pyrimidin-4-yl)furo[2,3- d]pyrimidin-4-y1]-1H-pyrazol-1- yl}-126-thietane-1,1-dione
BDBM717375 US20250034159, Example 196 3-{3-(4-fluorophenyl)-4-[6- (pyrimidin-4-yl)furo[2,3- d]pyrimidin-4-y1]-1H-pyrazol-1- yl}-126-thietane-1,1-dione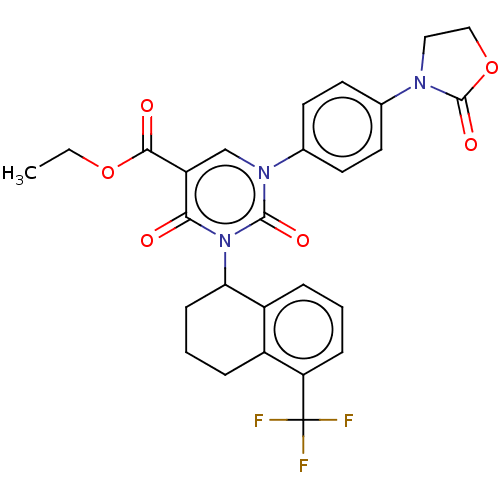 Ethyl 2,4-dioxo-1-[4-(2-oxo-1,3-oxazolidin-3-yl)phenyl]-3-[5-(trifluoromethyl)-1,2,3,4-tetrahydronaphthalen-1-yl]-1,2,3,4-tetrahydropyrimidine-5-carboxylate (racemate) BDBM337802 US9751843, 196
Ethyl 2,4-dioxo-1-[4-(2-oxo-1,3-oxazolidin-3-yl)phenyl]-3-[5-(trifluoromethyl)-1,2,3,4-tetrahydronaphthalen-1-yl]-1,2,3,4-tetrahydropyrimidine-5-carboxylate (racemate) BDBM337802 US9751843, 196 US10988487, Example 196 BDBM494800 N-(4-fluorobicyclo[4.2.0]octa- 1,3,5-trien-7-yl)-N'-hydroxy-4- [(3-oxocyclopent-1-en-1- yl)amino]-1,2,5-oxadiazole-3- carboximidamide
US10988487, Example 196 BDBM494800 N-(4-fluorobicyclo[4.2.0]octa- 1,3,5-trien-7-yl)-N'-hydroxy-4- [(3-oxocyclopent-1-en-1- yl)amino]-1,2,5-oxadiazole-3- carboximidamide rac-(1R,2S)-2- (cyano(phenyl)(1-((1-(4- (pyridin-4- ylsulfonyl)phenyl)azetidin-3- yl)methyl)piperidin-4- yl)methylcyclopentyl propionate BDBM480136 US10899738, Cpd. No 196
rac-(1R,2S)-2- (cyano(phenyl)(1-((1-(4- (pyridin-4- ylsulfonyl)phenyl)azetidin-3- yl)methyl)piperidin-4- yl)methylcyclopentyl propionate BDBM480136 US10899738, Cpd. No 196 tert-butyl 2-{[4-({2-[6-(difluoromethyl)pyridin-2-yl]pyrrolo[2,1-f][1,2,4]triazin-4-yl}amino)pyridin-3-yl]formamido}acetate US10336761, Example 196 BDBM406913
tert-butyl 2-{[4-({2-[6-(difluoromethyl)pyridin-2-yl]pyrrolo[2,1-f][1,2,4]triazin-4-yl}amino)pyridin-3-yl]formamido}acetate US10336761, Example 196 BDBM406913 (1R,2R)-N-(8-amino-5-fluoro-6-(4- methylpyridin-3-yl)-2,7-naphthyridin-3- yl)-2-(1H-pyrazol-4-yl)cyclopropane-1- carboxamide BDBM503915 US11034692, Compound 196
(1R,2R)-N-(8-amino-5-fluoro-6-(4- methylpyridin-3-yl)-2,7-naphthyridin-3- yl)-2-(1H-pyrazol-4-yl)cyclopropane-1- carboxamide BDBM503915 US11034692, Compound 196 (R)-4-(3-(1-(but-2-ynoyl) pyrrolidin-3-yl)-5- ethoxyimidazo[1,5-a]pyrazin- 1-yl)-N-(4- (trifluoromethyl)pyridin- 2-yl)benzamide US10933063, Compound 196 BDBM484397
(R)-4-(3-(1-(but-2-ynoyl) pyrrolidin-3-yl)-5- ethoxyimidazo[1,5-a]pyrazin- 1-yl)-N-(4- (trifluoromethyl)pyridin- 2-yl)benzamide US10933063, Compound 196 BDBM484397 (S)-N-(1-(4-chloro-5-(4- fluorophenyl)-1H-imidazol-2-yl)-7- (isoxazol-3-yl)-7-oxoheptyl)-1- methylazetidine-3-carboxamide 2,3-dihydroxysuccinate BDBM749904 US12331044, Example 196
(S)-N-(1-(4-chloro-5-(4- fluorophenyl)-1H-imidazol-2-yl)-7- (isoxazol-3-yl)-7-oxoheptyl)-1- methylazetidine-3-carboxamide 2,3-dihydroxysuccinate BDBM749904 US12331044, Example 196 5-[[(1R)-1-[3-(1,1-difluoro-2- hydroxy- ethyl)phenyl]ethyl]amino]-3-ethyl- 3-methoxy-1,8-dimethyl- pyrrolo[2,3-g]phthalazin-2-one BDBM752766 US20250197407, Compound 196
5-[[(1R)-1-[3-(1,1-difluoro-2- hydroxy- ethyl)phenyl]ethyl]amino]-3-ethyl- 3-methoxy-1,8-dimethyl- pyrrolo[2,3-g]phthalazin-2-one BDBM752766 US20250197407, Compound 196 BDBM304891 5-Cyclopentyl-3-(4-methyl-3-(((R)-4-methyl-1,1-dioxido-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepin-2-yl)methyl)phenyl)pentanoic acid US10144731, Example 196
BDBM304891 5-Cyclopentyl-3-(4-methyl-3-(((R)-4-methyl-1,1-dioxido-3,4-dihydro-2H-benzo[b][1,4,5]oxathiazepin-2-yl)methyl)phenyl)pentanoic acid US10144731, Example 196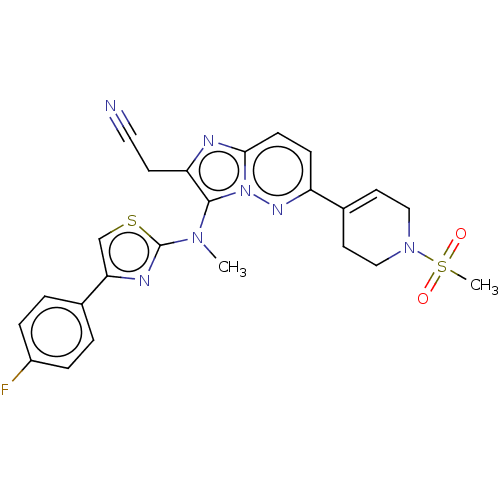 BDBM354527 2-(3-((4-(4- fluorophenyl) thiazol-2- yl)(methyl)amino)- 6-(1- (methylsulfonyl)- 1,2,3,6- tetrahydropyridin- 4-yl)imidazo[1,2- b]pyridazin-2- yl)acetonitrile US9796719, Compound 196
BDBM354527 2-(3-((4-(4- fluorophenyl) thiazol-2- yl)(methyl)amino)- 6-(1- (methylsulfonyl)- 1,2,3,6- tetrahydropyridin- 4-yl)imidazo[1,2- b]pyridazin-2- yl)acetonitrile US9796719, Compound 196 BDBM383481 N-[[(1S,8R)-5-(2,6- difluorophenyl)-11,11-dimethyl- 3,4- diazatricyclo[6.2.1.02,7]undeca- 2,4,6-trien-1-yl]methyl]-N-ethyl- 6-(hydroxymethyl)pyrazine-2- carboxamide US10280144, Compound 196
BDBM383481 N-[[(1S,8R)-5-(2,6- difluorophenyl)-11,11-dimethyl- 3,4- diazatricyclo[6.2.1.02,7]undeca- 2,4,6-trien-1-yl]methyl]-N-ethyl- 6-(hydroxymethyl)pyrazine-2- carboxamide US10280144, Compound 196 BDBM422289 5-[4-(6-fluoro-2- methylpyridin-3- yl)benzenesulfonyl]-6- hydroxy-1-[(1R)-2- methoxy-1- phenylethyl]-2-(3- methylbutyl)-1,4- dihydropyrimidin-4-one US10508104, Example 196
BDBM422289 5-[4-(6-fluoro-2- methylpyridin-3- yl)benzenesulfonyl]-6- hydroxy-1-[(1R)-2- methoxy-1- phenylethyl]-2-(3- methylbutyl)-1,4- dihydropyrimidin-4-one US10508104, Example 196 BDBM503891 US11034692, Compound 196 trans-N-(8-amino-5-fluoro-6-(4- methylpyridin-3-yl)-2,7-naphthyridin-3- yl)-2-(1H-pyrazol-4- yl)cyclopropanecarboxamide US11034692, Compound 172
BDBM503891 US11034692, Compound 196 trans-N-(8-amino-5-fluoro-6-(4- methylpyridin-3-yl)-2,7-naphthyridin-3- yl)-2-(1H-pyrazol-4- yl)cyclopropanecarboxamide US11034692, Compound 172 BDBM504781 US11066396, Example 196 (3S)-N- tert-butyl- 4-{3- fluoro-4- [5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3- yl]phenyl}- N-methyl- 5-oxo- morpholine- 3- carboxamide
BDBM504781 US11066396, Example 196 (3S)-N- tert-butyl- 4-{3- fluoro-4- [5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3- yl]phenyl}- N-methyl- 5-oxo- morpholine- 3- carboxamide BDBM553147 US11319299, Example 196 N-[5-(6- acetamidopyridin-3-yl)-4- fluoro-2-[rac-(3R)-3,4- dimethylpiperazin-1- yl]phenyl]-6-oxo-4- (trifluoromethyl)-1H- pyridine-3-carboxamide
BDBM553147 US11319299, Example 196 N-[5-(6- acetamidopyridin-3-yl)-4- fluoro-2-[rac-(3R)-3,4- dimethylpiperazin-1- yl]phenyl]-6-oxo-4- (trifluoromethyl)-1H- pyridine-3-carboxamide BDBM755942 US20250214991, Compound 196 3-(5-(3-amino-7-((3- hydroxypyrrolidin-1-yl)methyl)- 1H-pyrazolo[4,3-b]pyridin-5-yl)-1- oxoisoindolin-2-yl)piperidine-2,6- dione
BDBM755942 US20250214991, Compound 196 3-(5-(3-amino-7-((3- hydroxypyrrolidin-1-yl)methyl)- 1H-pyrazolo[4,3-b]pyridin-5-yl)-1- oxoisoindolin-2-yl)piperidine-2,6- dione US10092575, Example 196 BDBM289707 8#(2,2-Dimethyltetrahydro-2H-pyran-4-yl)(ethyl)amino)methyl)-6-(4-methoxybenzyl)thieno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidin-5(6H)-one
US10092575, Example 196 BDBM289707 8#(2,2-Dimethyltetrahydro-2H-pyran-4-yl)(ethyl)amino)methyl)-6-(4-methoxybenzyl)thieno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidin-5(6H)-one US10227299, Example 196 BDBM367508 (6RS)-2-2-[(4-Fluorobenzoyl)amino]pyridin-4-yl-N-(2-hydroxyethyl)-N-methyl-4-oxo-3-(phenylamino)-4,5,6,7-tetrahydro-1H-indol-6-carboxamide
US10227299, Example 196 BDBM367508 (6RS)-2-2-[(4-Fluorobenzoyl)amino]pyridin-4-yl-N-(2-hydroxyethyl)-N-methyl-4-oxo-3-(phenylamino)-4,5,6,7-tetrahydro-1H-indol-6-carboxamide US10780090, Compound I-196 {(1R,2S,4R)-4-[(5-{[4-(3,6-dihydro-2H-pyran-4-ylmethyl)-2- thienyl]carbonyl}pyrimidin-4-yl)amino]-2-hydroxycyclopentyl}methyl sulfamate BDBM462873
US10780090, Compound I-196 {(1R,2S,4R)-4-[(5-{[4-(3,6-dihydro-2H-pyran-4-ylmethyl)-2- thienyl]carbonyl}pyrimidin-4-yl)amino]-2-hydroxycyclopentyl}methyl sulfamate BDBM462873 US11554118, Compound 196 BDBM589423 (R)-4-(3-(1-(but-2-ynoyl)pyrrolidin-3- yl)-5-ethoxyimidazo[1,5-a]pyrazin-1- yl)-N-(4-(trifluoromethyl)pyridin-2- yl)benzamide
US11554118, Compound 196 BDBM589423 (R)-4-(3-(1-(but-2-ynoyl)pyrrolidin-3- yl)-5-ethoxyimidazo[1,5-a]pyrazin-1- yl)-N-(4-(trifluoromethyl)pyridin-2- yl)benzamide US20230365537, Example 196 BDBM636067 (1R,2S)-2-(3-{[6-(methanesulfonyl)-3-methoxypyridin-2-yl]amino}-1H-indazol-6-yl)-5'-methoxyspiro[cyclopropane-1,3'-indol]-2'(1'H)-one
US20230365537, Example 196 BDBM636067 (1R,2S)-2-(3-{[6-(methanesulfonyl)-3-methoxypyridin-2-yl]amino}-1H-indazol-6-yl)-5'-methoxyspiro[cyclopropane-1,3'-indol]-2'(1'H)-one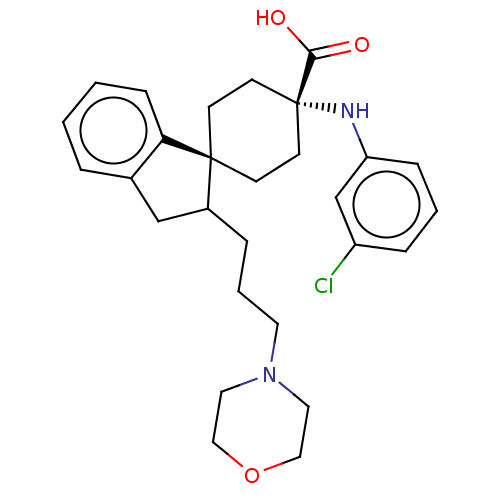 US20240150293, Example 196 BDBM673061 (1r,4r)-4-(3-chloroanilino)-2′-[3-(morpholin-4-yl)propyl]-2′,3′-dihydrospiro[cyclohexane-1,1′-indene]-4-carboxylic acid
US20240150293, Example 196 BDBM673061 (1r,4r)-4-(3-chloroanilino)-2′-[3-(morpholin-4-yl)propyl]-2′,3′-dihydrospiro[cyclohexane-1,1′-indene]-4-carboxylic acid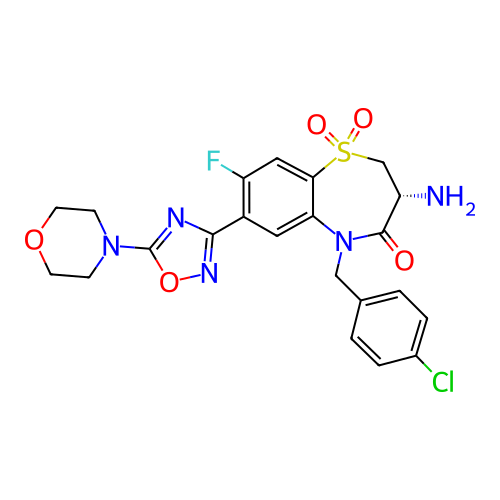 US20240368150, Example 196 BDBM703787 (3R)-3-amino-5-[(4- chlorophenyl)methyl]-8-fluoro- 7-(5-morpholino- 1,2,4-oxadiazol- 3-yl)-1,1-dioxo-2,3-dihydro- 1lambda6,5-benzothiazepin-4-one (*)
US20240368150, Example 196 BDBM703787 (3R)-3-amino-5-[(4- chlorophenyl)methyl]-8-fluoro- 7-(5-morpholino- 1,2,4-oxadiazol- 3-yl)-1,1-dioxo-2,3-dihydro- 1lambda6,5-benzothiazepin-4-one (*)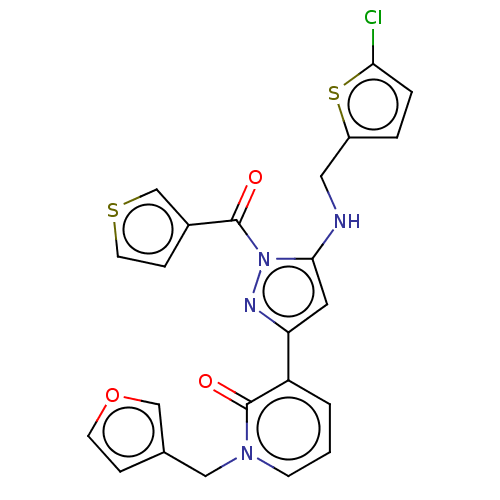 US9687479, 196 3-(5-[(5-chlorothiophen-2-yl)methyl]amino-1-[(thiophen-3-yl)carbonyl]-1H-pyrazol-3-yl)-1-(furan-3-ylmethyl)-1,2-dihydropyridin-2-one BDBM163274
US9687479, 196 3-(5-[(5-chlorothiophen-2-yl)methyl]amino-1-[(thiophen-3-yl)carbonyl]-1H-pyrazol-3-yl)-1-(furan-3-ylmethyl)-1,2-dihydropyridin-2-one BDBM163274 [(2R,3S,5R)-5-{4-amino-3-[(3,4-dimethylphenyl)sulfanyl]-1H-pyrazolo[3,4- d]pyrimidin-1-yl}-3-hydroxytetrahydrofuran-2-yl]methyl sulfamate US10865208, Compound I-196 BDBM476141
[(2R,3S,5R)-5-{4-amino-3-[(3,4-dimethylphenyl)sulfanyl]-1H-pyrazolo[3,4- d]pyrimidin-1-yl}-3-hydroxytetrahydrofuran-2-yl]methyl sulfamate US10865208, Compound I-196 BDBM476141 (4'-fluoro-[1,1'-biphenyl]-4-yl)(3-(2-(thiophen-2-yl)thiazol-4-yl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone US10544150, Compound 196 BDBM430775
(4'-fluoro-[1,1'-biphenyl]-4-yl)(3-(2-(thiophen-2-yl)thiazol-4-yl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone US10544150, Compound 196 BDBM430775 1-[1-(2-fluorophenyl)-1H-indazol-4-yl]-3-[(3R,5S)-5-(3-fluorophenyl)tetrahydrofuran-3-yl]imidazolidin-2-one 1-[1-(2-fluorophenyl)-1H- US9783527, Example 196 BDBM344894
1-[1-(2-fluorophenyl)-1H-indazol-4-yl]-3-[(3R,5S)-5-(3-fluorophenyl)tetrahydrofuran-3-yl]imidazolidin-2-one 1-[1-(2-fluorophenyl)-1H- US9783527, Example 196 BDBM344894 3-(2-amino-[1,2,4]triazolo[1,5-α]pyridin-7-yl)-6-(difluoromethyl)-2-fluoro-N-(2-fluoro-3-(4-fluorophenyl)-3-hydroxybutyl-4,4,4-d3)benzamide BDBM534460 WO2022086828, Example 196
3-(2-amino-[1,2,4]triazolo[1,5-α]pyridin-7-yl)-6-(difluoromethyl)-2-fluoro-N-(2-fluoro-3-(4-fluorophenyl)-3-hydroxybutyl-4,4,4-d3)benzamide BDBM534460 WO2022086828, Example 196 BDBM312906 1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin-3- yl]ethynyl]phenyl]-5-(meth- ylamino)pyrazolo[3,4-d]thia- zole-3-carboxamide US9605005, Example 196
BDBM312906 1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin-3- yl]ethynyl]phenyl]-5-(meth- ylamino)pyrazolo[3,4-d]thia- zole-3-carboxamide US9605005, Example 196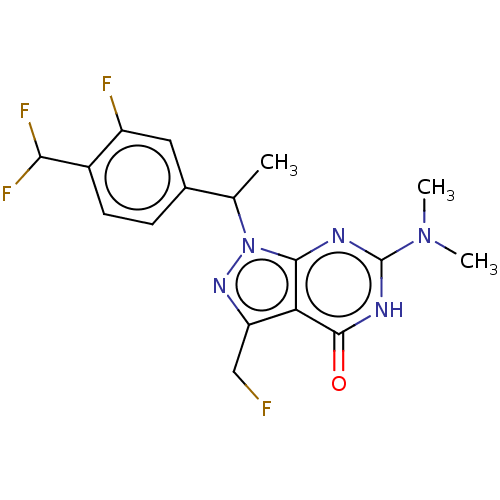 BDBM320791 (R)- or (S)-1-(1-(4-(Difluoromethyl)-3- fluorophenyl)ethyl)-6-(dimethylamino)-3- (fluoromethyl)-1H-pyrazolo[3,4- d]pyrimidin-4(5H)-one US10174037, Example 197 US10174037, Example 196
BDBM320791 (R)- or (S)-1-(1-(4-(Difluoromethyl)-3- fluorophenyl)ethyl)-6-(dimethylamino)-3- (fluoromethyl)-1H-pyrazolo[3,4- d]pyrimidin-4(5H)-one US10174037, Example 197 US10174037, Example 196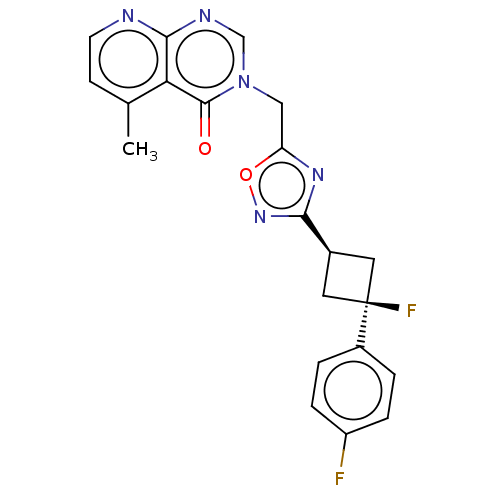 BDBM452874 3-((3-((1s,3s)-3- fluoro-3-(4-fluoro- phenyl)cyclobutyl)- 1,2,4-oxadiazol-5-yl) methyl)-5-methyl- pyrido[2,3-d]pyrimidin- 4(3H)-one US10711004, Example 196
BDBM452874 3-((3-((1s,3s)-3- fluoro-3-(4-fluoro- phenyl)cyclobutyl)- 1,2,4-oxadiazol-5-yl) methyl)-5-methyl- pyrido[2,3-d]pyrimidin- 4(3H)-one US10711004, Example 196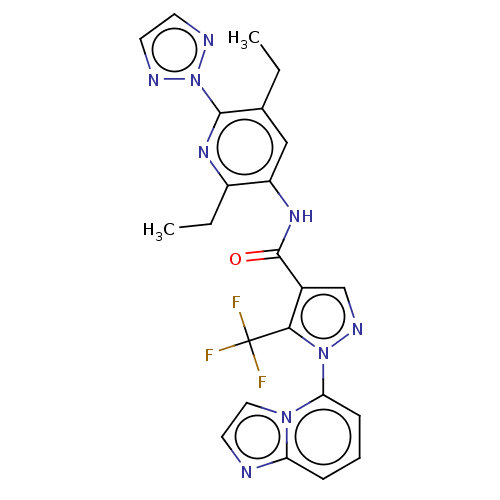 BDBM487774 N-(2,5-diethyl-6-(2H-1,2,3- triazol-2-yl)pyridin-3-yl)-1- (imidazo[1,2-a]pyridin-5-yl)-5- (trifluoromethyl)-1H-pyrazole-4- carboxamide US10954214, Compound 196
BDBM487774 N-(2,5-diethyl-6-(2H-1,2,3- triazol-2-yl)pyridin-3-yl)-1- (imidazo[1,2-a]pyridin-5-yl)-5- (trifluoromethyl)-1H-pyrazole-4- carboxamide US10954214, Compound 196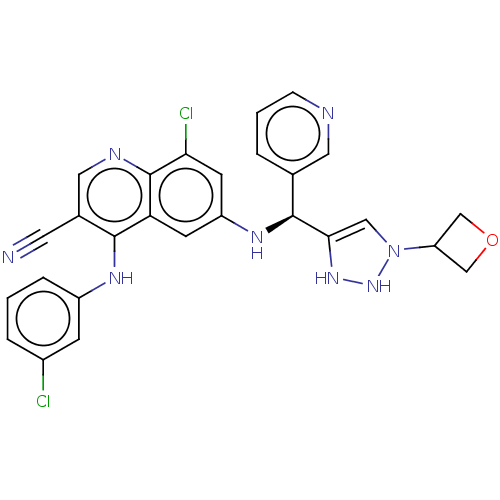 BDBM505545 US11066414, Compound 196 (S)-8-chloro-4-((3- chlorophenyl)amino)-6-(((1-(oxetan- 3-yl)-1H-1,2,3-triazol-4-yl)(pyridin- 3-yl)methyl)amino)quinoline-3- carbonitrile
BDBM505545 US11066414, Compound 196 (S)-8-chloro-4-((3- chlorophenyl)amino)-6-(((1-(oxetan- 3-yl)-1H-1,2,3-triazol-4-yl)(pyridin- 3-yl)methyl)amino)quinoline-3- carbonitrile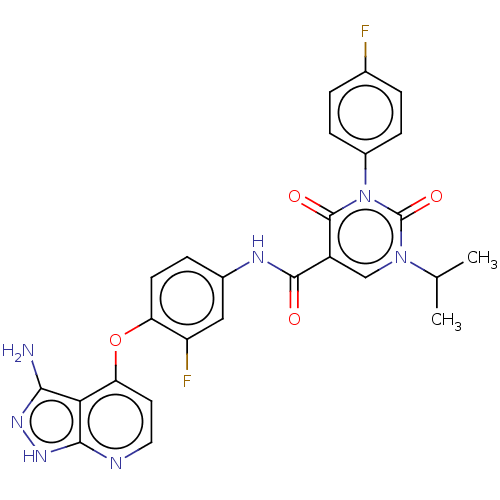 BDBM517126 N-(4-((3-amino-1H-pyrazolo[3,4-b]pyridin-4-yl)oxy)-3-fluorophenyl)-3-(4-fluorophenyl)-1-isopropyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide US11104676, Example 196
BDBM517126 N-(4-((3-amino-1H-pyrazolo[3,4-b]pyridin-4-yl)oxy)-3-fluorophenyl)-3-(4-fluorophenyl)-1-isopropyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide US11104676, Example 196 BDBM537371 3-(((1s,4s)-4-(6-(ethylamino)-3-(1- methyl-1H-pyrazol-4-yl)-1H- pyrazolo[4,3-c]pyridin-1- yl)cyclohexyl)oxy)pyridazine-4- carbonitrile US11247990, Example 196
BDBM537371 3-(((1s,4s)-4-(6-(ethylamino)-3-(1- methyl-1H-pyrazol-4-yl)-1H- pyrazolo[4,3-c]pyridin-1- yl)cyclohexyl)oxy)pyridazine-4- carbonitrile US11247990, Example 196 BDBM597309 US11597728, Example 196 (1-(3-Fluorophenyl)-1H-1,2,4-triazol-3-yl)((5R,9S)-2-methyl-3- (3,4,5-trifluorophenyl)-4,5,6,7,8,9-hexahydro-2H-5,9- epiminocycloocta[c]pyrazol-10-yl)methanone;
BDBM597309 US11597728, Example 196 (1-(3-Fluorophenyl)-1H-1,2,4-triazol-3-yl)((5R,9S)-2-methyl-3- (3,4,5-trifluorophenyl)-4,5,6,7,8,9-hexahydro-2H-5,9- epiminocycloocta[c]pyrazol-10-yl)methanone; BDBM599058 US11618753, Example 196 5-[4-amino-5- (trifluoromethyl) pyrrolo[2,1- f][1,2,4]triazin-7- yl]-N-{1-[(2,2- difluorocyclopropyl) methyl]-4- fluoropyrrolidin-3- yl}-2- methoxypyridine-3- carboxamide
BDBM599058 US11618753, Example 196 5-[4-amino-5- (trifluoromethyl) pyrrolo[2,1- f][1,2,4]triazin-7- yl]-N-{1-[(2,2- difluorocyclopropyl) methyl]-4- fluoropyrrolidin-3- yl}-2- methoxypyridine-3- carboxamide BDBM688975 US20240190890, Compound I-196 (R)-3-(5-cyclopropyl-2-vinylpyridin-4-yl)-10-methyl-9,10,11,12-tetrahydro-8H-[1,4]diazepino[5',6':4,5]thieno[3,2-f]quinolin-8-one
BDBM688975 US20240190890, Compound I-196 (R)-3-(5-cyclopropyl-2-vinylpyridin-4-yl)-10-methyl-9,10,11,12-tetrahydro-8H-[1,4]diazepino[5',6':4,5]thieno[3,2-f]quinolin-8-one US10233182, Example 196 (R)—N-(1-(1-(2-Chlorophenyl)-3-methyl-4-oxo-1,3,8-triazaspiro[4.5]decan-8-yl)-3-methyl-1-oxobutan-2-yl)-3-methylbenzamide BDBM370025
US10233182, Example 196 (R)—N-(1-(1-(2-Chlorophenyl)-3-methyl-4-oxo-1,3,8-triazaspiro[4.5]decan-8-yl)-3-methyl-1-oxobutan-2-yl)-3-methylbenzamide BDBM370025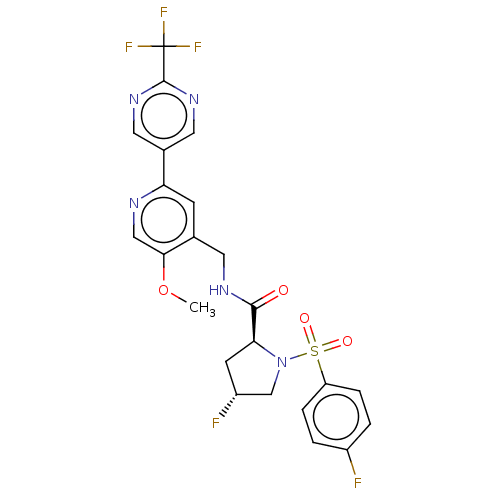 US11236046, Example 196 Preparation of (2S,4R)-4-fluoro-1-(4-fluorophenylsulfonyl)-N-((5-methoxy-2-(2-(trifluoromethyl)pyrimidin-5-yl)pyridin-4-yl)methyl)pyrrolidine-2-carboxamide BDBM535117
US11236046, Example 196 Preparation of (2S,4R)-4-fluoro-1-(4-fluorophenylsulfonyl)-N-((5-methoxy-2-(2-(trifluoromethyl)pyrimidin-5-yl)pyridin-4-yl)methyl)pyrrolidine-2-carboxamide BDBM535117 US12071425, Compound 196 (S)-N-(2,3,6-trifluoro-4-((3-(2-(piperidin- 3-ylamino)pyrimidin-4-yl)pyridin-2- yl)oxy)phenyl)-1-(6- (trifluoromethyl)pyridin-3- yl)methanesulfonamide BDBM693286
US12071425, Compound 196 (S)-N-(2,3,6-trifluoro-4-((3-(2-(piperidin- 3-ylamino)pyrimidin-4-yl)pyridin-2- yl)oxy)phenyl)-1-(6- (trifluoromethyl)pyridin-3- yl)methanesulfonamide BDBM693286 US20240109865, Example 196. ethyl 6-[5-[5-[(1R)-1-(3,5- dichloro-2-fluoro-4- pyridyl)ethoxy]-1H-indazol-3- yl]-2-pyridyl]-2,6- diazaspiro[3.3]heptane-2- carboxylate BDBM662773
US20240109865, Example 196. ethyl 6-[5-[5-[(1R)-1-(3,5- dichloro-2-fluoro-4- pyridyl)ethoxy]-1H-indazol-3- yl]-2-pyridyl]-2,6- diazaspiro[3.3]heptane-2- carboxylate BDBM662773 US20250136570, Compound 196 BDBM736711 rac-N-(4-amino-4'-fluoro-[1,1'-biphenyl]-3-yl)-4-((1S,3R)-1-oxido-3-phenyl-4,5-dihydro-3H-1λ6-isothiazol-1-yl)benzamide
US20250136570, Compound 196 BDBM736711 rac-N-(4-amino-4'-fluoro-[1,1'-biphenyl]-3-yl)-4-((1S,3R)-1-oxido-3-phenyl-4,5-dihydro-3H-1λ6-isothiazol-1-yl)benzamide  US9725449, Example 196 5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-4-yl(phenyl)methyl]-11-(pyrrolidine-1-sulfonyl)-3,8,10-triazatricyclo[7.4.0.02,7]trideca-1(9),2 BDBM331362
US9725449, Example 196 5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-4-yl(phenyl)methyl]-11-(pyrrolidine-1-sulfonyl)-3,8,10-triazatricyclo[7.4.0.02,7]trideca-1(9),2 BDBM331362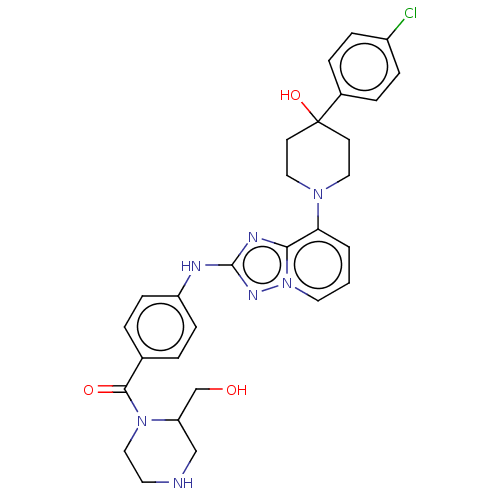 US9873709, Example 1-196 BDBM366599 [4-[[8-[4-(4- chlorophenyl)-4- hydroxy-1-piperidyl]- [1,2,4]triazolo[1,5- a]pyridin-2- yl]amino]phenyl]-[2- (hydroxymethyl)piperazin- 1-yl]methanone
US9873709, Example 1-196 BDBM366599 [4-[[8-[4-(4- chlorophenyl)-4- hydroxy-1-piperidyl]- [1,2,4]triazolo[1,5- a]pyridin-2- yl]amino]phenyl]-[2- (hydroxymethyl)piperazin- 1-yl]methanone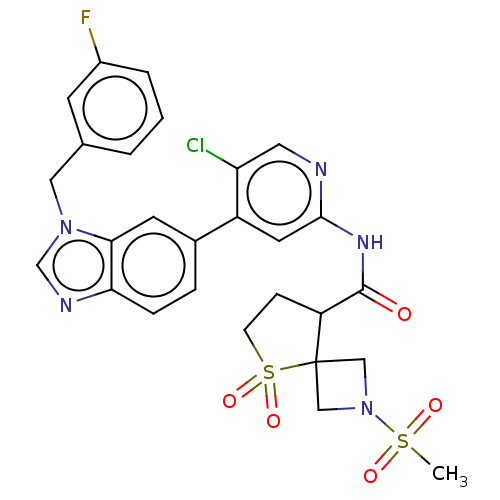 BDBM307900 US9650358, Example 196 N-{5-chloro-4-[1-(3-fluorobenzyl)-1H-benzimidazol-6-yl]pyridin-2-yl}-2-(methylsulfonyl)-5-thia-2-azaspiro[3.4]octane-8-carboxamide 5,5-dioxide
BDBM307900 US9650358, Example 196 N-{5-chloro-4-[1-(3-fluorobenzyl)-1H-benzimidazol-6-yl]pyridin-2-yl}-2-(methylsulfonyl)-5-thia-2-azaspiro[3.4]octane-8-carboxamide 5,5-dioxide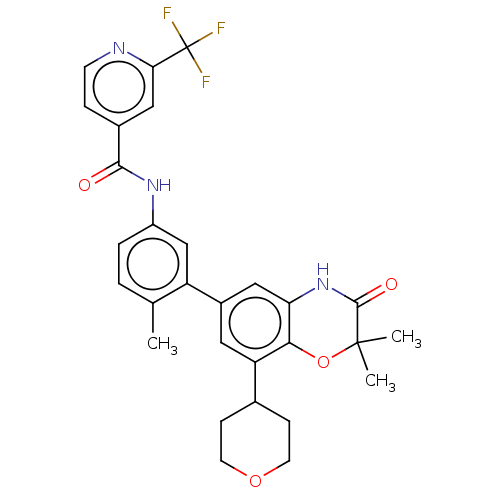 BDBM313572 US10167279, Example 196 N-(3-(2,2-dimethyl-3- oxo-8-(tetrahydro-2H- pyran-4-yl)-3,4-dihydro- 2H-benzo[b][1,4]oxazin- 6-yl)-4-methylphenyl)-2- (trifluoromethyl) isonicotinamide
BDBM313572 US10167279, Example 196 N-(3-(2,2-dimethyl-3- oxo-8-(tetrahydro-2H- pyran-4-yl)-3,4-dihydro- 2H-benzo[b][1,4]oxazin- 6-yl)-4-methylphenyl)-2- (trifluoromethyl) isonicotinamide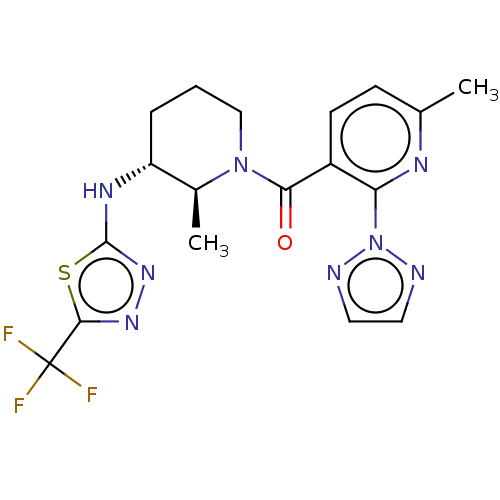 BDBM314578 US9611251, 196 (6-methyl-2-(2H-1,2,3-triazol-2-yl)pyridin-3-yl)((2S*,3R*)-2-methyl-3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)piperidin-1-yl)methanone
BDBM314578 US9611251, 196 (6-methyl-2-(2H-1,2,3-triazol-2-yl)pyridin-3-yl)((2S*,3R*)-2-methyl-3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)piperidin-1-yl)methanone BDBM407255 (R)-methyl 5-((2-(3- cyanopyrrolo[1,2- b]pyridazin-7-yl)-5- ((2-fluoro-3-hydroxy-3- methylbutyl)carbamoyl) pyridin-4- yl)amino)isoindoline-2- carboxylate US10336762, Compound 196
BDBM407255 (R)-methyl 5-((2-(3- cyanopyrrolo[1,2- b]pyridazin-7-yl)-5- ((2-fluoro-3-hydroxy-3- methylbutyl)carbamoyl) pyridin-4- yl)amino)isoindoline-2- carboxylate US10336762, Compound 196 BDBM570023 (+/−)-[2-(4-{[3-(2,3-difluorophenyl)-1H-pyrrolo[2,3-b]pyridin-4-yl]oxy}-3,5-difluoroanilino)-5-fluoro-5,6-dihydro-4H-1,3-oxazin-5-yl]methanol US11427578, Example 196
BDBM570023 (+/−)-[2-(4-{[3-(2,3-difluorophenyl)-1H-pyrrolo[2,3-b]pyridin-4-yl]oxy}-3,5-difluoroanilino)-5-fluoro-5,6-dihydro-4H-1,3-oxazin-5-yl]methanol US11427578, Example 196 BDBM591104 US11566003, Compound 196 (1S,2R,3S)-N-(8-amino-7-fluoro-6-(4-methylpyridin-3-yl)isoquinolin-3-yl)-2-methyl-3-(1-methyl-1H-pyrazol-4-yl)cyclopropanecarboxamide
BDBM591104 US11566003, Compound 196 (1S,2R,3S)-N-(8-amino-7-fluoro-6-(4-methylpyridin-3-yl)isoquinolin-3-yl)-2-methyl-3-(1-methyl-1H-pyrazol-4-yl)cyclopropanecarboxamide BDBM738391 US20250145589, Example 196 Preparation of (E)-2,4-difluoro-N-(5-(8-fluoro-4-(4-(4-oxopent-2-enoyl)piperazin-1-yl)quinazoline-6-(yl)-2-methoxypyridin-3-yl)benzenesulfonamide
BDBM738391 US20250145589, Example 196 Preparation of (E)-2,4-difluoro-N-(5-(8-fluoro-4-(4-(4-oxopent-2-enoyl)piperazin-1-yl)quinazoline-6-(yl)-2-methoxypyridin-3-yl)benzenesulfonamide N-(4-(8-amino-3-isopropyl-5-(4-(methylamino)cyclohex-1-en-1-yl)imidazo[1,5-a]pyrazin-1-yl)-2,5-difluorophenyl)-1-(2-chlorophenyl)methanesulfonamide BDBM602975 US11649237, Example 196
N-(4-(8-amino-3-isopropyl-5-(4-(methylamino)cyclohex-1-en-1-yl)imidazo[1,5-a]pyrazin-1-yl)-2,5-difluorophenyl)-1-(2-chlorophenyl)methanesulfonamide BDBM602975 US11649237, Example 196 US10906915, Ex. 196 BDBM481401 [(2R)-3-(3-ethyl-4-oxo- spiro[6,8-dihydro-5H- pyrazolo[4,3-c]azepine- 7,4'-tetrahydropyran]-1-yl)- 2-methyl-propyl] 4-chloro- 2-methyl-benzoate
US10906915, Ex. 196 BDBM481401 [(2R)-3-(3-ethyl-4-oxo- spiro[6,8-dihydro-5H- pyrazolo[4,3-c]azepine- 7,4'-tetrahydropyran]-1-yl)- 2-methyl-propyl] 4-chloro- 2-methyl-benzoate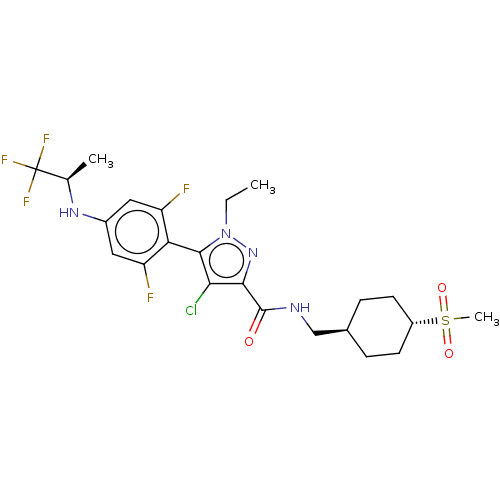 US10975037, Example 196 BDBM491002 4-Chloro-5-(2,6-difluoro-4-(((R)-1,1,1-trifluoropropan-2-yl)amino)phenyl)-1-ethyl-N-(((1r,4R)-4-(methylsulfonyl)cyclohexyl)methyl)-1H-pyrazole-3-carboxamide
US10975037, Example 196 BDBM491002 4-Chloro-5-(2,6-difluoro-4-(((R)-1,1,1-trifluoropropan-2-yl)amino)phenyl)-1-ethyl-N-(((1r,4R)-4-(methylsulfonyl)cyclohexyl)methyl)-1H-pyrazole-3-carboxamide US20230391786, Example 196 BDBM639727 6-Amino-N-[2-[[3,4-dichloro-10-(1H-pyrazol-4-yl)-6,7,8,9-tetrahydropyrido[1,2-a]indol-7-yl]amino]-2-oxo-ethyl]hexanamide formic acid salt
US20230391786, Example 196 BDBM639727 6-Amino-N-[2-[[3,4-dichloro-10-(1H-pyrazol-4-yl)-6,7,8,9-tetrahydropyrido[1,2-a]indol-7-yl]amino]-2-oxo-ethyl]hexanamide formic acid salt 1-((10-Hydroxy-7-(3- (trifluoromethyl)cyclopentane-1-carbonyl)- 7-azaspiro[4.5]decan-10-yl)methyl)-N,N- dimethyl-6-oxo-4-phenyl-1,6- dihydropyridine-3-carboxamide US11053213, Example 196 BDBM514454
1-((10-Hydroxy-7-(3- (trifluoromethyl)cyclopentane-1-carbonyl)- 7-azaspiro[4.5]decan-10-yl)methyl)-N,N- dimethyl-6-oxo-4-phenyl-1,6- dihydropyridine-3-carboxamide US11053213, Example 196 BDBM514454 BDBM663358 4-(6-((1S,6R,7R)-7-(aminomethyl)-7-(2- fluorophenyl)-3-azabicyclo[4.1.0]heptan-3-yl)-1H- pyrazolo[3,4-b]pyrazin-3-yl)-5-chloropyridin-2- amine US20240109900, Example 196
BDBM663358 4-(6-((1S,6R,7R)-7-(aminomethyl)-7-(2- fluorophenyl)-3-azabicyclo[4.1.0]heptan-3-yl)-1H- pyrazolo[3,4-b]pyrazin-3-yl)-5-chloropyridin-2- amine US20240109900, Example 196 BDBM728150 US20250091994, Example 196 (Z)-1-acetyl-2-((4- (2-(1-methyl-1H- pyrazol-4-yl)phen- yl)-6-(morpholine- 4-carbonyl)quin- olin-2-yl)meth- ylene)-indolin-3- one
BDBM728150 US20250091994, Example 196 (Z)-1-acetyl-2-((4- (2-(1-methyl-1H- pyrazol-4-yl)phen- yl)-6-(morpholine- 4-carbonyl)quin- olin-2-yl)meth- ylene)-indolin-3- one US10047096, 196 BDBM227935 4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-[(4-isopropyl-3,3-dimethyl-5-oxopiperazin-1-yl)methyl]-6,11-dihydro-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one
US10047096, 196 BDBM227935 4-[(2-Fluoro-5-hydroxy-4-methylphenyl)amino]-8-[(4-isopropyl-3,3-dimethyl-5-oxopiperazin-1-yl)methyl]-6,11-dihydro-5H-pyrido[2,3-b][1,5]benzodiazepin-5-one US20230270751, Example 196 BDBM614636 2-(2,6-Difluorophenyl)-N-[(3S)-9-fluoro-2-oxo-5-phenyl-1,3-dihydro-1,4-benzodiazepin-3-yl]-6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxamide
US20230270751, Example 196 BDBM614636 2-(2,6-Difluorophenyl)-N-[(3S)-9-fluoro-2-oxo-5-phenyl-1,3-dihydro-1,4-benzodiazepin-3-yl]-6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxamide US20250114338, Example 196 BDBM731781 (1R,3R)-1-[2,6-difluoro-4-[1- (3-fluoropropyl)azetidin-3- yl]sulfanyl-phenyl]-2-(2- fluoro-2-methyl-propyl)-3- methyl-1,3,4,9- tetrahydropyrido[3,4-b]indole
US20250114338, Example 196 BDBM731781 (1R,3R)-1-[2,6-difluoro-4-[1- (3-fluoropropyl)azetidin-3- yl]sulfanyl-phenyl]-2-(2- fluoro-2-methyl-propyl)-3- methyl-1,3,4,9- tetrahydropyrido[3,4-b]indole US9718828, Example, 196 BDBM267603 4-(8-amino-3-((cis)-6-methyl-1-(3-(methylthio)propanoyl) piperidin-3-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide
US9718828, Example, 196 BDBM267603 4-(8-amino-3-((cis)-6-methyl-1-(3-(methylthio)propanoyl) piperidin-3-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide 2-((2-methoxy-4-(7-methyl-2,7-diazaspiro[3.5]nonan-2-yl)phenyl)amino)-4-((2'-methyl-3'-oxospiro[cyclopropane-1,1'-isoindolin]-4'-yl)oxy)pyrimidine-5-carbonitrile BDBM740101 US20250154134, Compound 196
2-((2-methoxy-4-(7-methyl-2,7-diazaspiro[3.5]nonan-2-yl)phenyl)amino)-4-((2'-methyl-3'-oxospiro[cyclopropane-1,1'-isoindolin]-4'-yl)oxy)pyrimidine-5-carbonitrile BDBM740101 US20250154134, Compound 196 4-(2-(1-(3-Chloro-2-fluorophenyl)-1H-1,2,3-triazole-4-carbonyl)-5-((S)-3-hydroxy-2-oxopyrrolidin-1-yl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamido)benzoic acid, TFA salt BDBM335968 US9738655, Example 196
4-(2-(1-(3-Chloro-2-fluorophenyl)-1H-1,2,3-triazole-4-carbonyl)-5-((S)-3-hydroxy-2-oxopyrrolidin-1-yl)-1,2,3,4-tetrahydroisoquinoline-1-carboxamido)benzoic acid, TFA salt BDBM335968 US9738655, Example 196 5-[4-[(3S)- 1-(3- fluoropropyl)- pyrrolidin-3- yl]oxy- phenyl]-6- (2,2,3,3- tetra- fluoro-1,4- benzo- dioxon-6-yl)-8,9- dihydro- 7H- benzo[7]- annulen-2-ol BDBM263898 US9714221, Example 196
5-[4-[(3S)- 1-(3- fluoropropyl)- pyrrolidin-3- yl]oxy- phenyl]-6- (2,2,3,3- tetra- fluoro-1,4- benzo- dioxon-6-yl)-8,9- dihydro- 7H- benzo[7]- annulen-2-ol BDBM263898 US9714221, Example 196 5-{9-Fluoro-5-[(S)-(2-fluorophenyl)(oxan-4-yl)methyl]-7-methanesulfonyl-5H-pyrido[3,2-b]indol-3-yl}-4-(2H3)methyl-1-methyl-1H-1,2,3-triazole US10112941, Example 196 BDBM297089
5-{9-Fluoro-5-[(S)-(2-fluorophenyl)(oxan-4-yl)methyl]-7-methanesulfonyl-5H-pyrido[3,2-b]indol-3-yl}-4-(2H3)methyl-1-methyl-1H-1,2,3-triazole US10112941, Example 196 BDBM297089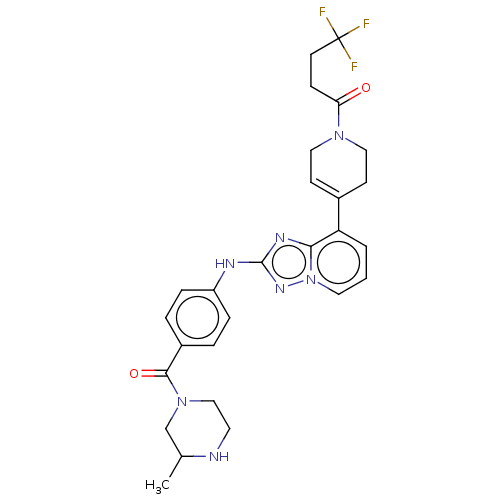 BDBM366902 4,4,4-trifluoro-1-[4-[2- [4-(3- methylpiperazine-1- carbonyl)anilino]- [1,2,4]triazolo[1,5- a]pyridin-8-yl]-3,6- dihydro-2H-pyridin-1- yl]butan-1-one US9873709, Example 2-196
BDBM366902 4,4,4-trifluoro-1-[4-[2- [4-(3- methylpiperazine-1- carbonyl)anilino]- [1,2,4]triazolo[1,5- a]pyridin-8-yl]-3,6- dihydro-2H-pyridin-1- yl]butan-1-one US9873709, Example 2-196 BDBM556024 US11345681, Example 196 5-Amino-3-[2-(6-chloro-1-ethyl-1,3-benzodiazol-5-yl)ethynyl]-1-[(3S,5R)-5-(methoxymethyl)-1-(prop-2-enoyl)pyrrolidin-3-yl]pyrazole-4-carboxamide
BDBM556024 US11345681, Example 196 5-Amino-3-[2-(6-chloro-1-ethyl-1,3-benzodiazol-5-yl)ethynyl]-1-[(3S,5R)-5-(methoxymethyl)-1-(prop-2-enoyl)pyrrolidin-3-yl]pyrazole-4-carboxamide US10214537, Example 196 BDBM358127 4-(3-(4-Amino-5-(1-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-5-yl)pyrrolo[2,1-f][1,2,4]triazin-7-yl)phenoxy)tetrahydro-2H-thiopyran 1,1-dioxide
US10214537, Example 196 BDBM358127 4-(3-(4-Amino-5-(1-(tetrahydro-2H-pyran-4-yl)-1H-pyrazol-5-yl)pyrrolo[2,1-f][1,2,4]triazin-7-yl)phenoxy)tetrahydro-2H-thiopyran 1,1-dioxide US11130769, Ex. No. 196 BDBM520497 (3R,6R,12S,22S)-6′-Chloro-11,12-dimethyl-15,15-dioxo-spiro[8,20-dioxa-15-thia-1,11,14-triazatetracyclo[14.7.2.03,6.019,24]pentacosa-16,18,24-triene-22,1′-tetralin]-10,13-dione
US11130769, Ex. No. 196 BDBM520497 (3R,6R,12S,22S)-6′-Chloro-11,12-dimethyl-15,15-dioxo-spiro[8,20-dioxa-15-thia-1,11,14-triazatetracyclo[14.7.2.03,6.019,24]pentacosa-16,18,24-triene-22,1′-tetralin]-10,13-dione US11472791, Example 196 (S)-4-(1-(2-Chloro-4- (((tetrahydro-2H-pyran-3- yl)amino)methyl)phenyl)- 1H-pyrazol-4-yl)-2-((1- (methylsulfonyl)piperidin- 4-yl)amino)pyrimidine- 5-carbonitrile BDBM576874
US11472791, Example 196 (S)-4-(1-(2-Chloro-4- (((tetrahydro-2H-pyran-3- yl)amino)methyl)phenyl)- 1H-pyrazol-4-yl)-2-((1- (methylsulfonyl)piperidin- 4-yl)amino)pyrimidine- 5-carbonitrile BDBM576874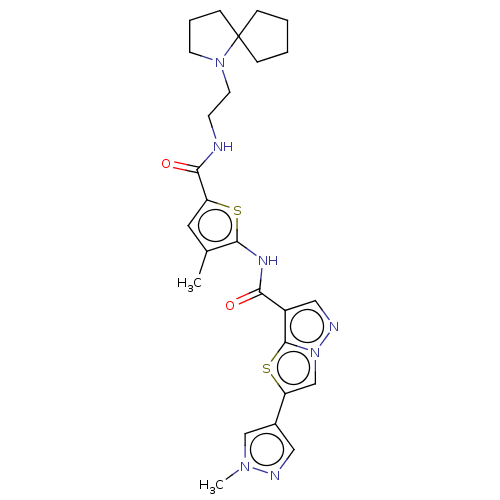 US20240109917, Example 196 N-(5-((2-(1-azaspiro[4.4]nonan- 1-yl)ethyl)carbamoyl)-3- methylthiophen-2-yl)-2-(1- methyl-1H-pyrazol-4- yl)pyrazolo[5,1-b]thiazole-7- carboxamide BDBM664115
US20240109917, Example 196 N-(5-((2-(1-azaspiro[4.4]nonan- 1-yl)ethyl)carbamoyl)-3- methylthiophen-2-yl)-2-(1- methyl-1H-pyrazol-4- yl)pyrazolo[5,1-b]thiazole-7- carboxamide BDBM664115 BDBM288585 trans-4-{(2R)-2-[8-amino-5- chloro-1-(4-{[4- (difluoromethyl)pyridin-2- yl]carbamoyl}phenyl)imidazo [1,5-a]pyrazin-3-yl]morpholin- 4-yl}-1- methylcyclohexanecarboxylic acid US10087188, Example 196
BDBM288585 trans-4-{(2R)-2-[8-amino-5- chloro-1-(4-{[4- (difluoromethyl)pyridin-2- yl]carbamoyl}phenyl)imidazo [1,5-a]pyrazin-3-yl]morpholin- 4-yl}-1- methylcyclohexanecarboxylic acid US10087188, Example 196 BDBM314664 [(1R,3R,4S,6R)-3-[(5-chloro-2-pyridyl)oxy]-2,2-dideuterio-6-methyl-5-azabicyclo[2.2.1]heptan-5-yl]-[6-methyl-3-(triazol-2-yl)-2-pyridyl]methanone US9611262, Example 196
BDBM314664 [(1R,3R,4S,6R)-3-[(5-chloro-2-pyridyl)oxy]-2,2-dideuterio-6-methyl-5-azabicyclo[2.2.1]heptan-5-yl]-[6-methyl-3-(triazol-2-yl)-2-pyridyl]methanone US9611262, Example 196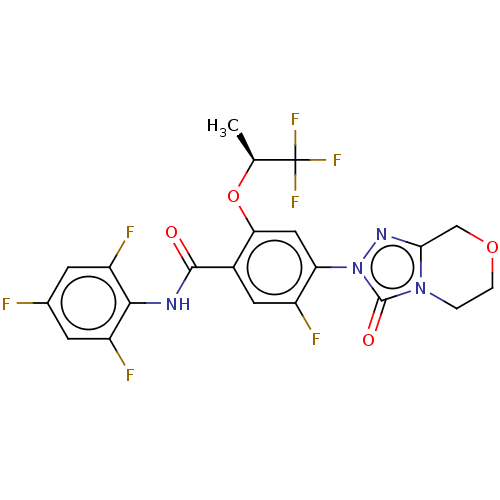 BDBM490528 US10968216, Example 196 5-fluoro-4-(3-oxo-5,6-dihydro-3H-[1,2,4]triazolo[3,4-c][1,4]oxazin-2(8H)-yl)-N-(2,4,6-trifluoro-phenyl)-2-{[(2S)-1,1,1-trifluoropropan-2-yl]oxy}benzamide
BDBM490528 US10968216, Example 196 5-fluoro-4-(3-oxo-5,6-dihydro-3H-[1,2,4]triazolo[3,4-c][1,4]oxazin-2(8H)-yl)-N-(2,4,6-trifluoro-phenyl)-2-{[(2S)-1,1,1-trifluoropropan-2-yl]oxy}benzamide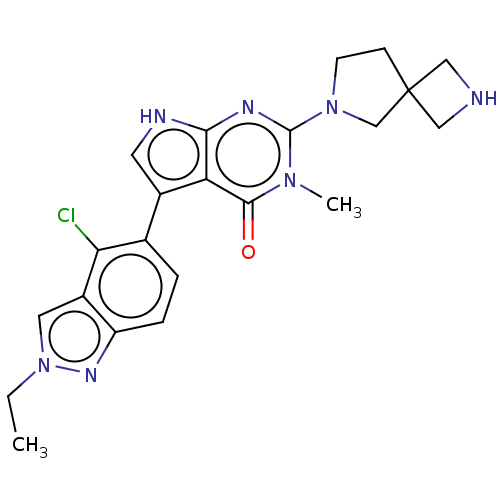 BDBM685115 rac-5-(4- chloro-2- ethyl-2H- indazol-5-yl)- 2-{2,6-diaza- spiro[3.4] octan-6-yl}- 3-methyl- 3H,4H,7H- pyrrolo[2,3-d] pyrimidin- 4-one US12037345, Example 196
BDBM685115 rac-5-(4- chloro-2- ethyl-2H- indazol-5-yl)- 2-{2,6-diaza- spiro[3.4] octan-6-yl}- 3-methyl- 3H,4H,7H- pyrrolo[2,3-d] pyrimidin- 4-one US12037345, Example 196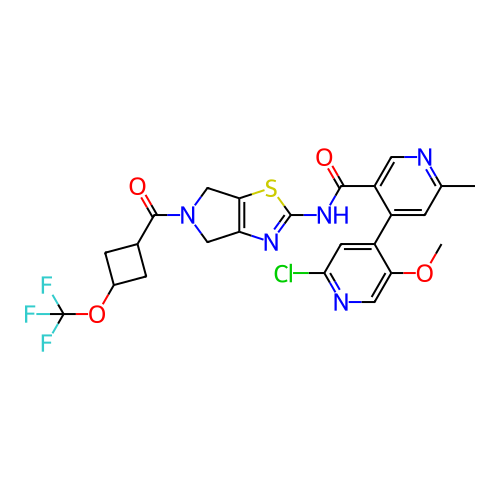 BDBM758566 (Cis and Trans)-2'-chloro-5'-methoxy-6- methyl-N-(5-(3- (trifluoromethoxy)cyclobutane-1- carbonyl)-5,6-dihydro-4H-pyrrolo[3,4- d]thiazol-2-yl)-[4,4'-bipyridine]-3- carboxamide US20250230171, Compound 196
BDBM758566 (Cis and Trans)-2'-chloro-5'-methoxy-6- methyl-N-(5-(3- (trifluoromethoxy)cyclobutane-1- carbonyl)-5,6-dihydro-4H-pyrrolo[3,4- d]thiazol-2-yl)-[4,4'-bipyridine]-3- carboxamide US20250230171, Compound 196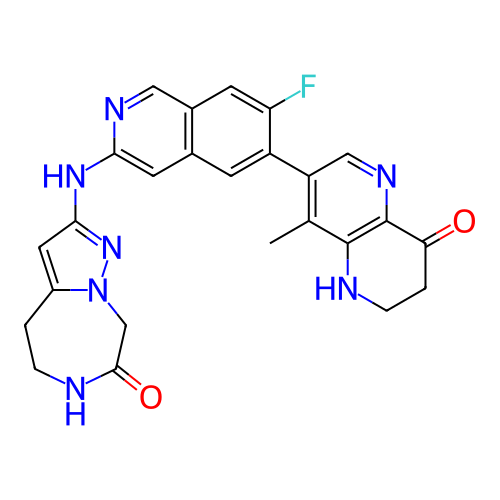 BDBM762638 US12378249, Compound 196 2-((7-fluoro-6-(4-methyl-8-oxo-5,6,7,8- tetrahydro-1,5-naphthyridin-3-yl)isoquinolin-3- yl)amino)-5,6-dihydro-4H-pyrazolo[1,5- d][1,4]diazepin-7(8H)-one
BDBM762638 US12378249, Compound 196 2-((7-fluoro-6-(4-methyl-8-oxo-5,6,7,8- tetrahydro-1,5-naphthyridin-3-yl)isoquinolin-3- yl)amino)-5,6-dihydro-4H-pyrazolo[1,5- d][1,4]diazepin-7(8H)-one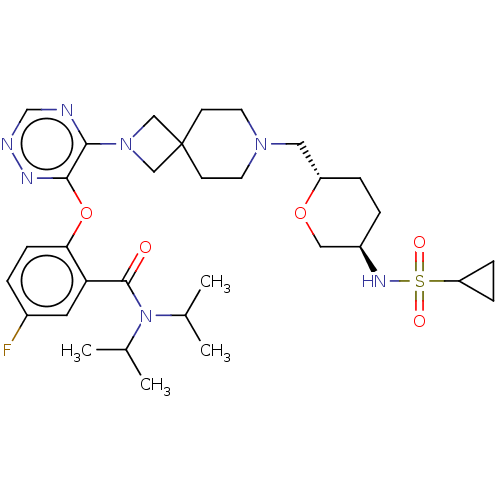 US11919901, Compound 196 BDBM656801 2-((5-(7-(((2S,5R)-5- (Cyclopropanesulfonamido)tetrahydro-2H-pyran-2- yl)methyl)-2,7-diazaspiro[3.5]nonan-2-yl)-1,2,4- triazin-6-yl)oxy)-5-fluoro-N,N- diisopropylbenzamide
US11919901, Compound 196 BDBM656801 2-((5-(7-(((2S,5R)-5- (Cyclopropanesulfonamido)tetrahydro-2H-pyran-2- yl)methyl)-2,7-diazaspiro[3.5]nonan-2-yl)-1,2,4- triazin-6-yl)oxy)-5-fluoro-N,N- diisopropylbenzamide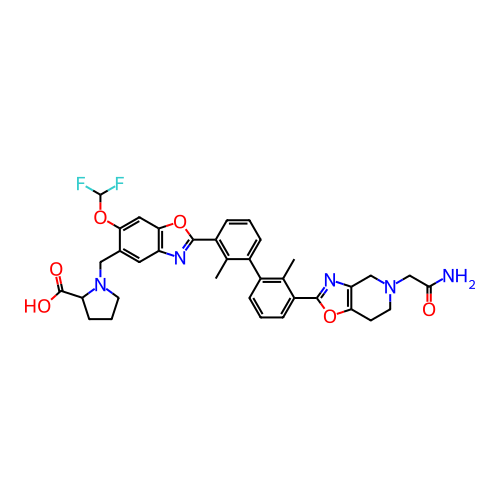 US12187713, Example 196 ((2-(3'-(5-(2-amino-2-oxoethyl)- 4,5,6,7-tetrahydrooxazolo[4,5- c]pyridin-2-yl)-2,2'-dimethyl-[1,1'- biphenyl]-3-yl)-6- (difluoromethoxy)benzo[d]oxazol- 5-yl)methyl)proline BDBM711340
US12187713, Example 196 ((2-(3'-(5-(2-amino-2-oxoethyl)- 4,5,6,7-tetrahydrooxazolo[4,5- c]pyridin-2-yl)-2,2'-dimethyl-[1,1'- biphenyl]-3-yl)-6- (difluoromethoxy)benzo[d]oxazol- 5-yl)methyl)proline BDBM711340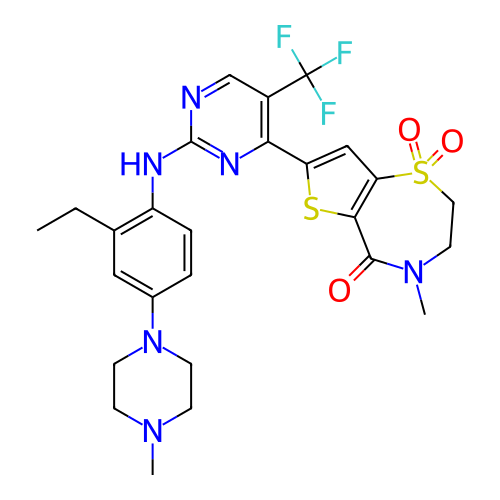 US20250144094, Example 196 BDBM737964 7-(2-((2-ethyl-4-(4- methylpiperazin-1- yl)phenyl)amino)-5- (trifluoromethyl)pyrimidin-4-yl)-4- methyl-3,4-dihydrothieno[2,3- f][1,4]thiazepin-5(2H)-one 1,1- dioxide
US20250144094, Example 196 BDBM737964 7-(2-((2-ethyl-4-(4- methylpiperazin-1- yl)phenyl)amino)-5- (trifluoromethyl)pyrimidin-4-yl)-4- methyl-3,4-dihydrothieno[2,3- f][1,4]thiazepin-5(2H)-one 1,1- dioxide 2-[1-[6-[(1S,8R)-5-(2,6-difluorophenyl)- 11,11-dimethyl-3,4- diazatricyclo[6.2.1.02,7]undeca-2(7),3,5- trien-1-yl]pyrazin-2-yl]-1,2,4-triazol-3- yl]propane-1,3-diol BDBM497702 US11008312, Example 196
2-[1-[6-[(1S,8R)-5-(2,6-difluorophenyl)- 11,11-dimethyl-3,4- diazatricyclo[6.2.1.02,7]undeca-2(7),3,5- trien-1-yl]pyrazin-2-yl]-1,2,4-triazol-3- yl]propane-1,3-diol BDBM497702 US11008312, Example 196 4-(1-(((2R,3S,4R,5R)-5-(6-amino-2-chloro-9H-purin-9-yl)-3-ethynyl-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)-1-carboxy-2-phenylethyl)thiazole-2-carboxylic acid BDBM511270 US11078228, Example 196
4-(1-(((2R,3S,4R,5R)-5-(6-amino-2-chloro-9H-purin-9-yl)-3-ethynyl-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)-1-carboxy-2-phenylethyl)thiazole-2-carboxylic acid BDBM511270 US11078228, Example 196 4-(1-(2-Chloro-4- ((3-fluoro-3-methyl- azetidin-1-yl)methyl)- phenyl)-1H-imidazol- 4-yl)-N-(1-(methyl- sulfonyl)piperidin-4- yl)-5-(trifluoromethyl)- pyrimidin-2-amine US11427567, Example 196 BDBM569551
4-(1-(2-Chloro-4- ((3-fluoro-3-methyl- azetidin-1-yl)methyl)- phenyl)-1H-imidazol- 4-yl)-N-(1-(methyl- sulfonyl)piperidin-4- yl)-5-(trifluoromethyl)- pyrimidin-2-amine US11427567, Example 196 BDBM569551 BDBM492055 US10975068, Example 196 4-((S)-4,4-Difluoro-2-methylpyrrolidine-1-carbonyl)-N—((R)-3-hydroxy-3-methylbutan-2-yl)-5-(6-((1-methhylcyclobutyl)amino)-4-(trifluoromethyl)pyridine-3-yl)thiazole-2-carboxamide
BDBM492055 US10975068, Example 196 4-((S)-4,4-Difluoro-2-methylpyrrolidine-1-carbonyl)-N—((R)-3-hydroxy-3-methylbutan-2-yl)-5-(6-((1-methhylcyclobutyl)amino)-4-(trifluoromethyl)pyridine-3-yl)thiazole-2-carboxamide BDBM499291 US11014963, Example 196 (2S)-2-[[2-(2,5-Dichlorophenyl)-2,2-difluoroacetyl]amino]-3-hydroxy-N-[(1S)-1-(4-methoxyphenyl)-2-oxo-2-[[(3S)-1,1,1-trifluoro-4-methyl-2-oxopentan-3-yl]amino]ethyl]propanamide
BDBM499291 US11014963, Example 196 (2S)-2-[[2-(2,5-Dichlorophenyl)-2,2-difluoroacetyl]amino]-3-hydroxy-N-[(1S)-1-(4-methoxyphenyl)-2-oxo-2-[[(3S)-1,1,1-trifluoro-4-methyl-2-oxopentan-3-yl]amino]ethyl]propanamide US11311520, Example 196 8-methyl-6-(7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-2′,3′,5′,6′-tetrahydro-2H-spiro[imidazo[1,5-a]pyridine-3,4′-thiopyran]-1,5-dione BDBM550702
US11311520, Example 196 8-methyl-6-(7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-2′,3′,5′,6′-tetrahydro-2H-spiro[imidazo[1,5-a]pyridine-3,4′-thiopyran]-1,5-dione BDBM550702 [(1R,2R,4S,5R)-5-[(5-chloro-2-pyridyl)oxy]-2-methyl-3-azabicyclo[2.2.1]heptan-3-yl]-[6-methyl-3-(triazol-2-yl)-2-pyridyl]methanone BDBM314618 US9611262, Example 196 US9611262, Example 122
[(1R,2R,4S,5R)-5-[(5-chloro-2-pyridyl)oxy]-2-methyl-3-azabicyclo[2.2.1]heptan-3-yl]-[6-methyl-3-(triazol-2-yl)-2-pyridyl]methanone BDBM314618 US9611262, Example 196 US9611262, Example 122 {(3S)-3-[3-({4-[(1,1- dimethylpropyl)sulfonyl] phenyl}amino)-4-oxo-4,5- dihydro-1H-pyrazolo[4,3- c]pyridin-1-yl]tetrahydro- 2H-pyran-3-yl}acetonitrile (from I-17B) BDBM391125 US9957264, Example 5-196
{(3S)-3-[3-({4-[(1,1- dimethylpropyl)sulfonyl] phenyl}amino)-4-oxo-4,5- dihydro-1H-pyrazolo[4,3- c]pyridin-1-yl]tetrahydro- 2H-pyran-3-yl}acetonitrile (from I-17B) BDBM391125 US9957264, Example 5-196 (6R,8aS)-6-[8-amino-1- (2-fluoro-4-{1- hydroxy-1-[3- (trifluoromethyl)pheyl] ethyl}phenyl)imidazo [1,5-a)pyrazin-3-yl]-2,2- dimethylhexahydroindolizin- 3(2H)-one BDBM359064 US10214546, Example 196 US10214546, Example 197
(6R,8aS)-6-[8-amino-1- (2-fluoro-4-{1- hydroxy-1-[3- (trifluoromethyl)pheyl] ethyl}phenyl)imidazo [1,5-a)pyrazin-3-yl]-2,2- dimethylhexahydroindolizin- 3(2H)-one BDBM359064 US10214546, Example 196 US10214546, Example 197 BDBM615897 US20230271954, Compound 196 cis-8-((-4-((((R or S)-1,4-dioxan-2-yl)methyl) (benzo[d][1,3]dioxol-4-yl)amino)cyclohexyl)(methyl) amino)-5-methyl-6-oxo-5,6-dihydro-1,5- naphthyridine-2-carbonitrile
BDBM615897 US20230271954, Compound 196 cis-8-((-4-((((R or S)-1,4-dioxan-2-yl)methyl) (benzo[d][1,3]dioxol-4-yl)amino)cyclohexyl)(methyl) amino)-5-methyl-6-oxo-5,6-dihydro-1,5- naphthyridine-2-carbonitrile Methyl (5-(((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro[benzo[d][1,3]oxazine-4,3′-pyrrolidin]-1′-yl)-1-oxo-3-phenylpropan-2-yl)carbamoyl)thiophen-2-yl)carbamate US10093683, Example 196 BDBM289843
Methyl (5-(((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro[benzo[d][1,3]oxazine-4,3′-pyrrolidin]-1′-yl)-1-oxo-3-phenylpropan-2-yl)carbamoyl)thiophen-2-yl)carbamate US10093683, Example 196 BDBM289843 2-(3-(3-((S)-fluoro(4-methyl-4H-1,2,4- triazol-3-yl)methyl)oxetan-3-yl)phenyl)- 6-(((S)-2-isopropyl-4- (methylsulfonyl)piperazin-1-yl)methyl)- 4-(trifluoromethyl)isoindolin-1-one BDBM758018 US20250230147, Compound 196
2-(3-(3-((S)-fluoro(4-methyl-4H-1,2,4- triazol-3-yl)methyl)oxetan-3-yl)phenyl)- 6-(((S)-2-isopropyl-4- (methylsulfonyl)piperazin-1-yl)methyl)- 4-(trifluoromethyl)isoindolin-1-one BDBM758018 US20250230147, Compound 196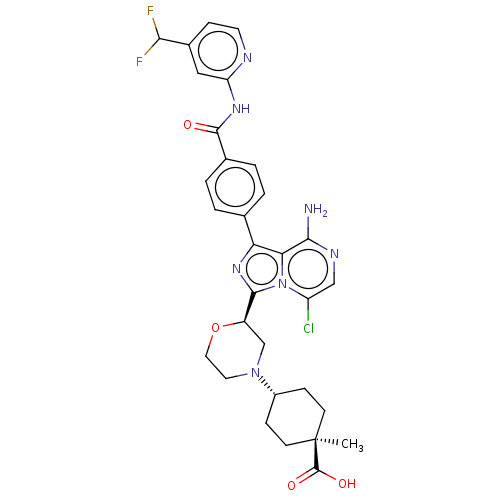 BDBM288584 trans-4-{(2R)-2-[8-amino-5- chloro-1-(4-{[4- (difluoromethyl)pyridin-2- yl]carbamoyl}phenyl)imidazo [1,5-a]pyrazin-3-yl]morpholin- 4-yl}-1- methylcyclohexanecarboxylic acid US10087188, Example 195 US10087188, Example 196
BDBM288584 trans-4-{(2R)-2-[8-amino-5- chloro-1-(4-{[4- (difluoromethyl)pyridin-2- yl]carbamoyl}phenyl)imidazo [1,5-a]pyrazin-3-yl]morpholin- 4-yl}-1- methylcyclohexanecarboxylic acid US10087188, Example 195 US10087188, Example 196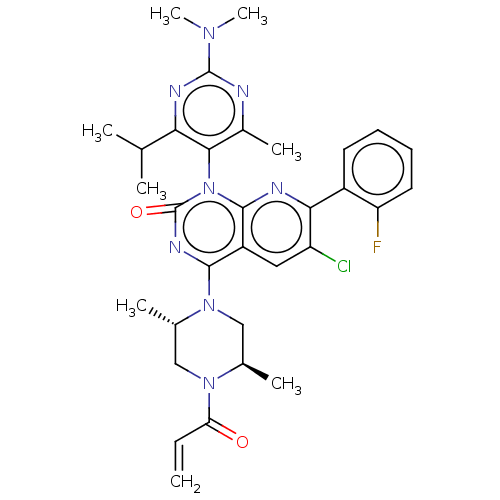 BDBM544381 US11285156, Ex.# 196 4-((2S,5R)-4-Acryloyl-2,5-dimethylpiperazin-1-yl)-6-chloro-1-(2-(dimethylamino)-4-isopropyl-6-methylpyrimidin-5-yl)-7-(2-fluorophenyl)pyrido[2,3-d]pyrimidin-2(1H)-one, Single Isomer
BDBM544381 US11285156, Ex.# 196 4-((2S,5R)-4-Acryloyl-2,5-dimethylpiperazin-1-yl)-6-chloro-1-(2-(dimethylamino)-4-isopropyl-6-methylpyrimidin-5-yl)-7-(2-fluorophenyl)pyrido[2,3-d]pyrimidin-2(1H)-one, Single Isomer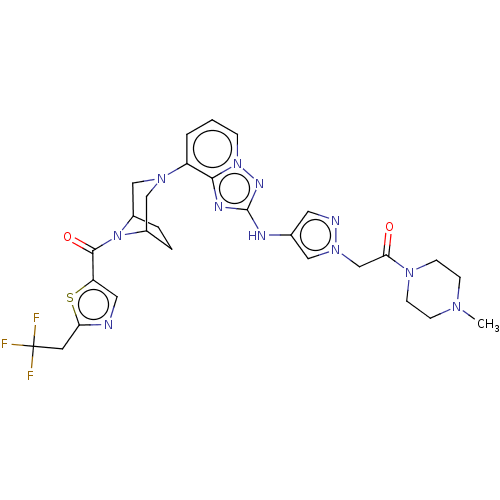 US10189836, Example 196 1-(4-methylpiperazin-1- yl)-2-[4-[[8-[8-(2-(2,2,2- trifluoroethypthiazole-5- carbonyl]-3,8- diazabicyclo[3.2.1]octan- 3-yl]-[1,2,4]triazolo[1,5-a] pyridin-2- yl]amino]pyrazol-1- yl]ethanone BDBM324633
US10189836, Example 196 1-(4-methylpiperazin-1- yl)-2-[4-[[8-[8-(2-(2,2,2- trifluoroethypthiazole-5- carbonyl]-3,8- diazabicyclo[3.2.1]octan- 3-yl]-[1,2,4]triazolo[1,5-a] pyridin-2- yl]amino]pyrazol-1- yl]ethanone BDBM324633 US12187742, Example 196 3-Chloro-5-fluoro-N-(5-(3'-methyl-2'-oxo- 2',3'-dihydrospiro[cyclobulane-1,1'- pyrrolo[2,3-c]quinolin]-8'-yl)-2-(3- (piperidin-1-yl)propoxy)pyridin-3- yl)benzenesulfonamide hydrochloride BDBM711847
US12187742, Example 196 3-Chloro-5-fluoro-N-(5-(3'-methyl-2'-oxo- 2',3'-dihydrospiro[cyclobulane-1,1'- pyrrolo[2,3-c]quinolin]-8'-yl)-2-(3- (piperidin-1-yl)propoxy)pyridin-3- yl)benzenesulfonamide hydrochloride BDBM711847 BDBM737754 (2R,3R,5R,6R)—N-(3,5-dichlorophenyl)-5-hydroxy-N-(1S,2S)-2-hydroxycyclohexyl-3-(2-hydroxyethoxy)-6-(hydroxymethyl)-4-(4-(3,4,5-trifluorophenyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-2-carboxamide US12291519, Example 196
BDBM737754 (2R,3R,5R,6R)—N-(3,5-dichlorophenyl)-5-hydroxy-N-(1S,2S)-2-hydroxycyclohexyl-3-(2-hydroxyethoxy)-6-(hydroxymethyl)-4-(4-(3,4,5-trifluorophenyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-2-carboxamide US12291519, Example 196 BDBM551650 (2R,3R or 2S,3S)-3-(4-((R)-3-(5-amino-9-fluoro-8-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)piperidin- 1-yl)-5-methyl-1H-pyrazol-1-yl)butan-2-ol US11312719, Example 196
BDBM551650 (2R,3R or 2S,3S)-3-(4-((R)-3-(5-amino-9-fluoro-8-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)piperidin- 1-yl)-5-methyl-1H-pyrazol-1-yl)butan-2-ol US11312719, Example 196 BDBM740218 N-(1-(7-chloro-1,2,3,4- tetrahydronaphthalen-2- yl)-2-((2-((R)-4- isopropyl-2- oxoimidazolidin-1-yl)- 2-(methylcarbamoyl)- 2,3-dihydro-1H-inden- 5-yl)amino)-2- oxoethyl)-1-methyl-1H- pyrazole-5-carboxamide US20250154138, Example 196
BDBM740218 N-(1-(7-chloro-1,2,3,4- tetrahydronaphthalen-2- yl)-2-((2-((R)-4- isopropyl-2- oxoimidazolidin-1-yl)- 2-(methylcarbamoyl)- 2,3-dihydro-1H-inden- 5-yl)amino)-2- oxoethyl)-1-methyl-1H- pyrazole-5-carboxamide US20250154138, Example 196 (8S,11S,14S)-8-((1H-Indol-3-yl)methyl)-11-(4-aminobutyl)-14-(3-aminopropyl)-4-chloro-1-(2-methoxypyridin-4-yl)-9-methyl-5,6,8,9,11,12,15,16-octahydrobenzo[b]pyrido[4,3-p][1,5,8,11,14]thiatetraazacycloheptadecine-7,10,13(14H)-trione US10030047, Example 196 BDBM281014
(8S,11S,14S)-8-((1H-Indol-3-yl)methyl)-11-(4-aminobutyl)-14-(3-aminopropyl)-4-chloro-1-(2-methoxypyridin-4-yl)-9-methyl-5,6,8,9,11,12,15,16-octahydrobenzo[b]pyrido[4,3-p][1,5,8,11,14]thiatetraazacycloheptadecine-7,10,13(14H)-trione US10030047, Example 196 BDBM281014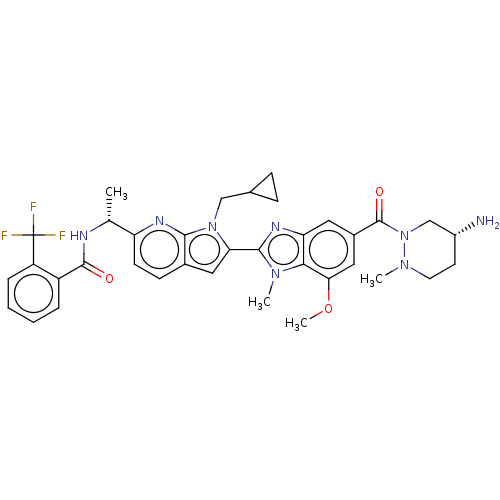 BDBM646211 US11878965, Example 196 N-((R)-1-(2-(5-((R)-5-amino-2- methylhexahydropyridazine-1- carbonyl)-7-methoxy-1-methyl-1H- benzo[d]imidazol-2-yl)-1- (cyclopropylmethyl)-1H-pyrrolo[2,3- b]pyridin-6-yl)ethyl)-2- (trifluoromethyl)benzamide
BDBM646211 US11878965, Example 196 N-((R)-1-(2-(5-((R)-5-amino-2- methylhexahydropyridazine-1- carbonyl)-7-methoxy-1-methyl-1H- benzo[d]imidazol-2-yl)-1- (cyclopropylmethyl)-1H-pyrrolo[2,3- b]pyridin-6-yl)ethyl)-2- (trifluoromethyl)benzamide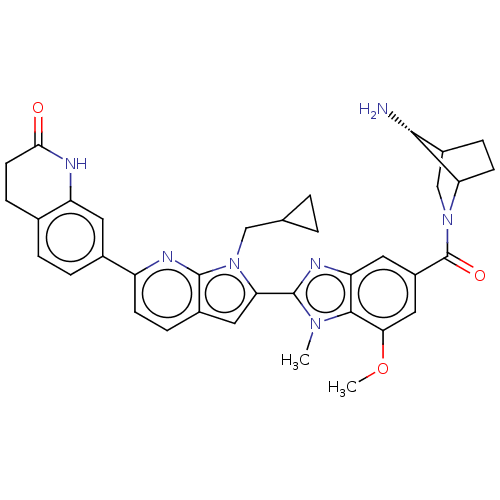 7-(2-{5-[(1R,4R,7R)-7-amino-2- azabicyclo[2.2.1]heptane-2- carbonyl]-7-methoxy-1-methyl-1H- 1,3-benzodiazol-2-yl}-1- (cyclopropylmethyl)-1H-pyrrolo[2,3- b]pyridin-6-yl)-1,2,3,4- tetrahydroquinolin-2-one BDBM583304 US11524959, Compound 196.
7-(2-{5-[(1R,4R,7R)-7-amino-2- azabicyclo[2.2.1]heptane-2- carbonyl]-7-methoxy-1-methyl-1H- 1,3-benzodiazol-2-yl}-1- (cyclopropylmethyl)-1H-pyrrolo[2,3- b]pyridin-6-yl)-1,2,3,4- tetrahydroquinolin-2-one BDBM583304 US11524959, Compound 196. BDBM551649 (2S,3S or 2R,3R)-3-(4-((R)-3-(5-amino-9-fluoro-8-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)piperidin- 1-yl)-5-methyl-1H-pyrazol-1-yl)butan-2-ol US11312719, Example 195 US11312719, Example 196
BDBM551649 (2S,3S or 2R,3R)-3-(4-((R)-3-(5-amino-9-fluoro-8-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)piperidin- 1-yl)-5-methyl-1H-pyrazol-1-yl)butan-2-ol US11312719, Example 195 US11312719, Example 196 BDBM554334 rac-((R)-2-(((2S,3R,4S,5S)-5-(6-chloro-4- ((cyclopropylmethyl)amino)-1H-pyrazolo[3,4- d]pyrimidin-1-yl)-3,4- dihydroxytetrahydrofuran-2-yl)methoxy)-1- hydroxy-3-(2H-tetrazol-5-yl)propan-2- yl)phosphonic acid US11325938, Ex 196
BDBM554334 rac-((R)-2-(((2S,3R,4S,5S)-5-(6-chloro-4- ((cyclopropylmethyl)amino)-1H-pyrazolo[3,4- d]pyrimidin-1-yl)-3,4- dihydroxytetrahydrofuran-2-yl)methoxy)-1- hydroxy-3-(2H-tetrazol-5-yl)propan-2- yl)phosphonic acid US11325938, Ex 196 US20240148821, Compound 196 (3S,9S,18S,21S,25R,28S,34S)-3-[2-[3-chloro-4-(trifluoromethyl)phenyl]ethyl]-9-(cyclohexylmethyl)-22-ethyl-28-isopropyl-7,10,13,16,21,25,29-heptamethyl-18-[(1S)-1-methylpropyl]spiro[1,4,7,10,13,16,19,22,26,29,32-undecazabicyclo[32.3 0]heptatriacontane-31,1'-cyclopentane]-2,5,8,11,14,17,20,23,27,30,33-undecone BDBM671202
US20240148821, Compound 196 (3S,9S,18S,21S,25R,28S,34S)-3-[2-[3-chloro-4-(trifluoromethyl)phenyl]ethyl]-9-(cyclohexylmethyl)-22-ethyl-28-isopropyl-7,10,13,16,21,25,29-heptamethyl-18-[(1S)-1-methylpropyl]spiro[1,4,7,10,13,16,19,22,26,29,32-undecazabicyclo[32.3 0]heptatriacontane-31,1'-cyclopentane]-2,5,8,11,14,17,20,23,27,30,33-undecone BDBM671202 BDBM743661 US12310975, Example 196 2-((6-((5-chloro-2-(4-((4- (2-(2,6-dioxopiperidin-3- yl)-1,3-dioxoisoindolin-5- yl)-2-oxopiperazin-1- yl)methyl)piperidin-1- yl)pyrimidin-4- yl)amino)-1-methyl-2- oxo-1,2-dihydroquinolin- 3-yl)oxy)-N- methylacetamide
BDBM743661 US12310975, Example 196 2-((6-((5-chloro-2-(4-((4- (2-(2,6-dioxopiperidin-3- yl)-1,3-dioxoisoindolin-5- yl)-2-oxopiperazin-1- yl)methyl)piperidin-1- yl)pyrimidin-4- yl)amino)-1-methyl-2- oxo-1,2-dihydroquinolin- 3-yl)oxy)-N- methylacetamide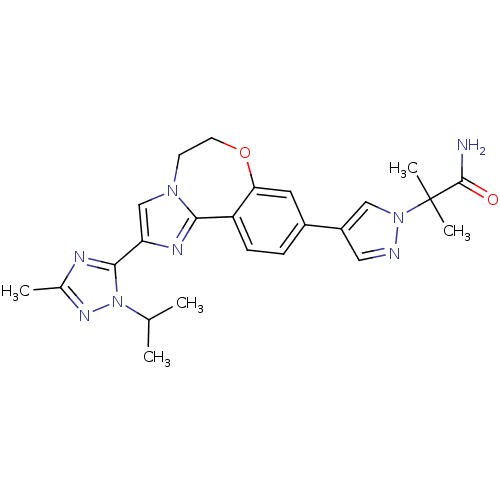 GDC 0032 US10851091, Compound taselisib CHEMBL2387080 GDC-0032 Roche RG7604 US8242104, Compound 196 2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)-1H-pyrazol-1-yl)-2-methylpropanamide BDBM50434806 Taselisib
GDC 0032 US10851091, Compound taselisib CHEMBL2387080 GDC-0032 Roche RG7604 US8242104, Compound 196 2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)-1H-pyrazol-1-yl)-2-methylpropanamide BDBM50434806 Taselisib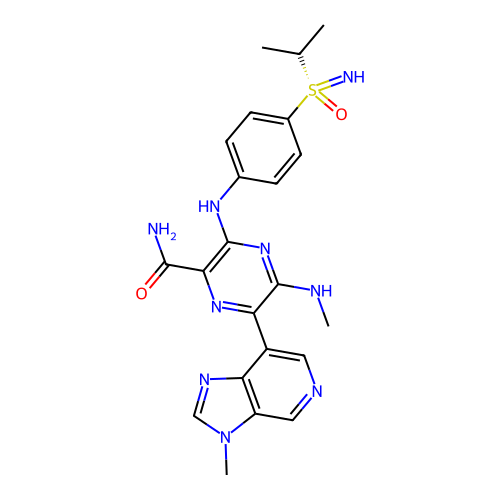 rel-(R)-3-[4-(Isopropylsulfonimidoyl)anilino]-5-(methylamino)-6-(3-methylimidazo[4,5-c]pyridin-7-yl)pyrazine-2-carboxamide and rel-(S)-3-[4-(isopropylsulfonimidoyl)anilino]-5-(methylamino)-6-(3-methylimidazo[4,5-c]pyridin-7-yl)pyrazine-2-carboxamide BDBM704643 US20240374606, Example 196
rel-(R)-3-[4-(Isopropylsulfonimidoyl)anilino]-5-(methylamino)-6-(3-methylimidazo[4,5-c]pyridin-7-yl)pyrazine-2-carboxamide and rel-(S)-3-[4-(isopropylsulfonimidoyl)anilino]-5-(methylamino)-6-(3-methylimidazo[4,5-c]pyridin-7-yl)pyrazine-2-carboxamide BDBM704643 US20240374606, Example 196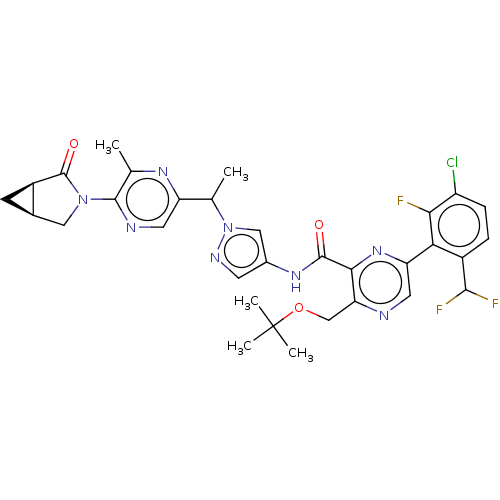 3-(tert-Butoxymethyl)-6-(3-chloro-6-(difluoromethyl)-2-fluorophenyl)-N-(1-((S and R)-1-(6-methyl-5-((1R,5S)-2-oxo-3-azabicyclo[3.1.0]hexan-3-yl)pyrazin-2-yl)ethyl)-1H-pyrazol-4-yl)pyrazine-2-carboxamide BDBM617984 US20230286958, Example 196
3-(tert-Butoxymethyl)-6-(3-chloro-6-(difluoromethyl)-2-fluorophenyl)-N-(1-((S and R)-1-(6-methyl-5-((1R,5S)-2-oxo-3-azabicyclo[3.1.0]hexan-3-yl)pyrazin-2-yl)ethyl)-1H-pyrazol-4-yl)pyrazine-2-carboxamide BDBM617984 US20230286958, Example 196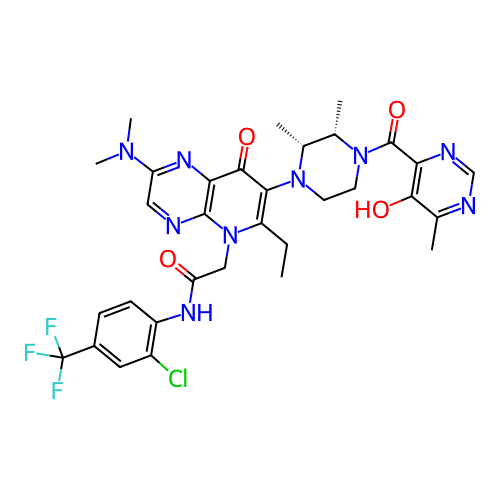 US20250034136, Compound I-196 BDBM717025 N-(2-chloro-4-(trifluoromethyl)phenyl)-2-(2-(dimethylamino)-6-ethyl- 7-(4-(5-hydroxy-6-methylpyrimidine-4-carbonyl)-2,3- dimethylpiperazin-1-yl)-8-oxopyrido[2,3-b]pyrazin-5(8H)-yl)acetamide (single stereoisomer, first eluting compound as stereoisomer 1, cis)
US20250034136, Compound I-196 BDBM717025 N-(2-chloro-4-(trifluoromethyl)phenyl)-2-(2-(dimethylamino)-6-ethyl- 7-(4-(5-hydroxy-6-methylpyrimidine-4-carbonyl)-2,3- dimethylpiperazin-1-yl)-8-oxopyrido[2,3-b]pyrazin-5(8H)-yl)acetamide (single stereoisomer, first eluting compound as stereoisomer 1, cis)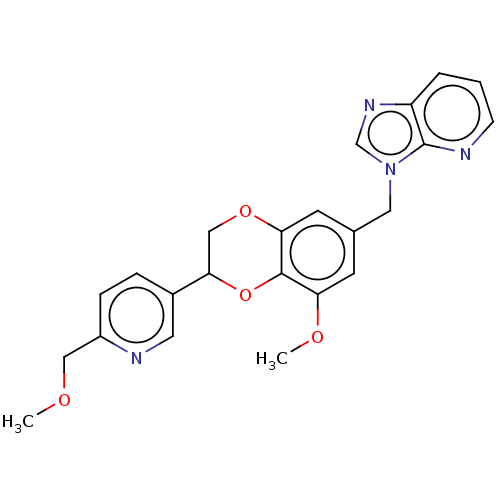 3-((8-methoxy-2-(6-(methoxymethyl)pyridin-3-yl)-2,3-dihydrobenzo[b][1,4]dioxin-6-yl)methyl)-3H-imidazo[4,5-b]pyridine BDBM542917 3-((8-methoxy-2-(6-(methoxymethyl)pyridin-3-yl)-2,3- dihydrobenzo[b][1,4]dioxin-6-yl)methyl)-3H-imidazo[4,5- b]pyridine US11274108, Example 1-196
3-((8-methoxy-2-(6-(methoxymethyl)pyridin-3-yl)-2,3-dihydrobenzo[b][1,4]dioxin-6-yl)methyl)-3H-imidazo[4,5-b]pyridine BDBM542917 3-((8-methoxy-2-(6-(methoxymethyl)pyridin-3-yl)-2,3- dihydrobenzo[b][1,4]dioxin-6-yl)methyl)-3H-imidazo[4,5- b]pyridine US11274108, Example 1-196 5-amino-3-[(5S,7R,8R,12aR,14R,15S,15aR)-14- (4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-15- fluoro-2,10-dihydroxy-2,10-disulfidooctahydro- 12H-5,8-methanofuro[3,2- l][1,3,6,9,11,2,10]pentaoxadiphosphacyclotetra- decin-7-yl]-3,6-dihydro-7H-[1,2,3]triazolo[4,5- d]pyrimidin-7-one (Diastereomer 3) BDBM574068 US11453697, Example 196
5-amino-3-[(5S,7R,8R,12aR,14R,15S,15aR)-14- (4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-15- fluoro-2,10-dihydroxy-2,10-disulfidooctahydro- 12H-5,8-methanofuro[3,2- l][1,3,6,9,11,2,10]pentaoxadiphosphacyclotetra- decin-7-yl]-3,6-dihydro-7H-[1,2,3]triazolo[4,5- d]pyrimidin-7-one (Diastereomer 3) BDBM574068 US11453697, Example 196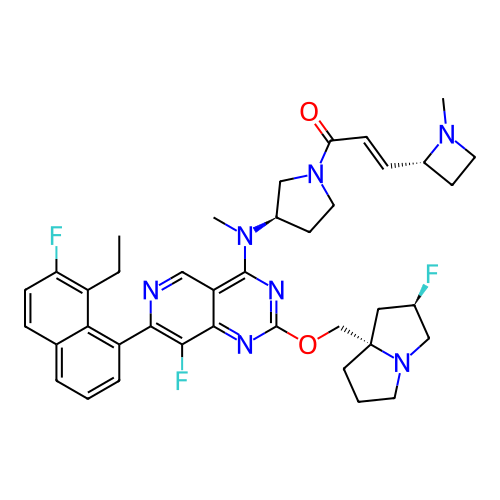 US20250019387, Example 196 BDBM713284 (Method 11):(E)-1-((R)-3-((7-(8-ethyl-7-fluoronaphthalen-1-yl)-8-fluoro-2-(((2R,7aS)-2-fluorotetrahydro-1H-pyrrolizin-7a(5H)-yl)methoxy)pyrido[4,3-d]pyrimidin-4-yl)(methyl)amino)pyrrolidin-1-yl)-3-((R)-1-methylazetidin-2-yl)prop-2-en-1-one
US20250019387, Example 196 BDBM713284 (Method 11):(E)-1-((R)-3-((7-(8-ethyl-7-fluoronaphthalen-1-yl)-8-fluoro-2-(((2R,7aS)-2-fluorotetrahydro-1H-pyrrolizin-7a(5H)-yl)methoxy)pyrido[4,3-d]pyrimidin-4-yl)(methyl)amino)pyrrolidin-1-yl)-3-((R)-1-methylazetidin-2-yl)prop-2-en-1-one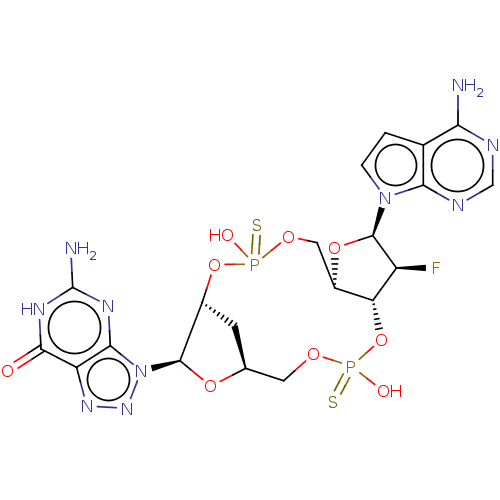 5-amino-3-[(5S,7R,8R,12aR,14R,15S,15aR)-14- (4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-15- fluoro-2,10-dihydroxy-2,10-disulfidooctahydro- 12H-5,8-methanofuro[3,2- l][1,3,6,9,11,2,10]pentaoxadiphosphacyclotetra- decin-7-yl]-3,6-dihydro-7H-[1,2,3]triazolo[4,5- d]pyrimidin-7-one (Diastereomer 1) BDBM574066 US11453697, Example 196 US11453697, Example 194 US11453697, Example 195
5-amino-3-[(5S,7R,8R,12aR,14R,15S,15aR)-14- (4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-15- fluoro-2,10-dihydroxy-2,10-disulfidooctahydro- 12H-5,8-methanofuro[3,2- l][1,3,6,9,11,2,10]pentaoxadiphosphacyclotetra- decin-7-yl]-3,6-dihydro-7H-[1,2,3]triazolo[4,5- d]pyrimidin-7-one (Diastereomer 1) BDBM574066 US11453697, Example 196 US11453697, Example 194 US11453697, Example 195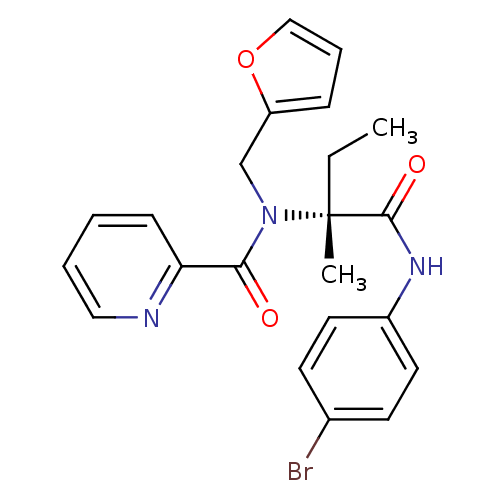 N-[(1R)-1-[(4-bromophenyl)carbamoyl]-1-methyl-propyl]-N-(2-furfuryl)picolinamide N-[(2R)-1-(4-bromoanilino)-2-methyl-1-oxobutan-2-yl]-N-(2-furanylmethyl)-2-pyridinecarboxamide BDBM65816 KUC103880N cid_44543531 N-[(2R)-1-(4-bromoanilino)-2-methyl-1-oxobutan-2-yl]-N-(furan-2-ylmethyl)pyridine-2-carboxamide KSC-5-196 N-[(2R)-1-[(4-bromophenyl)amino]-2-methyl-1-oxidanylidene-butan-2-yl]-N-(furan-2-ylmethyl)pyridine-2-carboxamide
N-[(1R)-1-[(4-bromophenyl)carbamoyl]-1-methyl-propyl]-N-(2-furfuryl)picolinamide N-[(2R)-1-(4-bromoanilino)-2-methyl-1-oxobutan-2-yl]-N-(2-furanylmethyl)-2-pyridinecarboxamide BDBM65816 KUC103880N cid_44543531 N-[(2R)-1-(4-bromoanilino)-2-methyl-1-oxobutan-2-yl]-N-(furan-2-ylmethyl)pyridine-2-carboxamide KSC-5-196 N-[(2R)-1-[(4-bromophenyl)amino]-2-methyl-1-oxidanylidene-butan-2-yl]-N-(furan-2-ylmethyl)pyridine-2-carboxamide (4,5-Dimethyl- thiazol-2-yl)-[6- fluoro-5-(7- morpholin-4-yl- pyrido[4,3-d}- pyrimidin-4-yl)- pyridin-3-yl]- methanol1H NMR (500 MHz, DMSO-d6) ppm = 9.16 (s,1H), 8.90 (d, J = 2.4, 1H), 8.53 (d, J = 2.3, 1H),8.27 (dd, J = 9.1, 2.4, 1H), 7.06 (d, J = 4.9, 1H),6.99 (s, 1H), 6.08 (d, J = 4.9, 1H), 3.76-3.69(m, 8H), 2.30 (s, 3H), 2.21 (s, 3H) US11065253, Example 204 US9732094, Example 204 BDBM315774 US10172859, Example 203 US10172859, Example 204 US10172859, Example 196
(4,5-Dimethyl- thiazol-2-yl)-[6- fluoro-5-(7- morpholin-4-yl- pyrido[4,3-d}- pyrimidin-4-yl)- pyridin-3-yl]- methanol1H NMR (500 MHz, DMSO-d6) ppm = 9.16 (s,1H), 8.90 (d, J = 2.4, 1H), 8.53 (d, J = 2.3, 1H),8.27 (dd, J = 9.1, 2.4, 1H), 7.06 (d, J = 4.9, 1H),6.99 (s, 1H), 6.08 (d, J = 4.9, 1H), 3.76-3.69(m, 8H), 2.30 (s, 3H), 2.21 (s, 3H) US11065253, Example 204 US9732094, Example 204 BDBM315774 US10172859, Example 203 US10172859, Example 204 US10172859, Example 196