Target (4)
Compound (503)
Article Title (2)
Assay (78)
Wang, G; Goyal, N; Hopkinson, B Preparation of L-proline based aeruginosin 298-A analogs: optimization of the P1-moiety. Bioorg Med Chem Lett 19: 3798 -803 (2009) Wortmann, L; Lindenthal, B; Muhn, P; Walter, A; Nubbemeyer, R; Heldmann, D; Sobek, L; Morandi, F; Schrey, AK; Moosmayer, D; Günther, J; Kuhnke, J; Koppitz, M; Lücking, U; Röhn, U; Schäfer, M; Nowak-Reppel, K; Kühne, R; Weinmann, H; Langer, G Discovery of BAY-298 and BAY-899: Tetrahydro-1,6-naphthyridine-Based, Potent, and Selective Antagonists of the Luteinizing Hormone Receptor Which Reduce Sex Hormone Levels in Vivo. J Med Chem 62: 10321 -10341 (2019)
ChEMBL_303339 (CHEMBL840155) Inhibition of [3H]PSB-298 binding to adenosine A2b receptor ChEBML_62882 Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue ChEMBL_302166 (CHEMBL829234) Dissociation constant for [3H]ORG2058 at human progesterone receptor at 298 K ChEMBL_1788587 (CHEMBL4260321) Binding affinity to recombinant Pseudomonas aeruginosa PqsD at 298 K by ITC assay ChEMBL_302155 (CHEMBL830056) Dissociation constant for [3H]R-1881 binding to human androgen receptor at 298 K ChEMBL_30310 (CHEMBL638564) Binding affinity for human adenosine A2B receptor using [3H]-PSB-298 in CHO cell membranes ChEMBL_1734653 (CHEMBL4150189) Inhibition of PTP1B (1 to 298 residues) (unknown origin) using p-nitrophenylphosphate as substrate after 30 mins ChEMBL_2476329 Inhibition of N-terminal GST-tagged human CDK2 (1 to 298 residues) extracted from Escherichia coli BL21 (DE3) ChEMBL_30420 (CHEMBL645974) Displacement of [3H]PSB-298 from human adenosine A2B receptor expressed in HEK293 cells at 10 uM ChEMBL_2285993 Inhibition of Flag-tagged human PTP1B (1 to 298 residues) expressed in Escherichia coli BL21 using FDP as substrate ChEMBL_2286054 Inhibition of human PTP1B (1 to 298 residues) expressed in Escherichia coli using FDP as substrate by microplate reader ChEMBL_303584 (CHEMBL828125) Ability to displace [3H]spiperone from human Dopamine receptor D4.2 stably transfected in human embryonic kidney 298 cells ChEMBL_1473380 (CHEMBL3419340) Activation of tumorigenic wild type B-Raf in human IPC-298 cells with Ras mutation assessed as phosphorylation of ERK ChEMBL_1614459 (CHEMBL3856528) Binding affinity to full length human CDK2 (1 to 298 residues) expressed in bacterial expression system by KINOMEscan competition assay NMR (OBClip) The experimental studies were carried out in 20 mM sodium phosphate buffer at pH7.4, at a temperature of 298 K. UV/VIS The experimental studies were carried out in 20 mM sodium phosphate buffer at pH7.4, at a temperature of 298 K. ChEBML_61346 In vitro binding affinity on D3 receptor is inhibition of binding of [125I]- NCQ 298 to Sf9 cells infected with recombinant baculovirus ChEMBL_30419 (CHEMBL645973) Displacement of [3H]PSB-298 from human adenosine A2B receptor expressed in HEK293 cells at 10 uM; Less than 10% inhibition ChEMBL_2232553 (CHEMBL5146325) Binding affinity to human CDK2 (1 to 298 residues) expressed in Escherichia coli Tuner(DE3) assessed as dissociation constant by fluorescence spectroscopy ChEMBL_2476328 Binding affinity to N-terminal GST-tagged human CDK2 (1 to 298 residues) extracted from Escherichia coli BL21 (DE3) assessed as dissociation constant ChEMBL_1879019 (CHEMBL4380413) Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs ChEMBL_1879023 (CHEMBL4380417) Inhibition of human thrombin using Boc-VPR-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs ChEMBL_2459548 Binding affinity to recombinant human RXR beta LBD (298 to 533 residues) expressed in Escherichia coli Rosetta assessed as dissociation constant by ITC method ChEMBL_1879018 (CHEMBL4380412) Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs ChEMBL_1879021 (CHEMBL4380415) Inhibition of human neutrophil elastase using MeOSuc-AAPV-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs ChEMBL_1930989 (CHEMBL4434240) Inhibition of human FLAG-tagged PTP1B catalytic domain (1 to 298) expressed in Escherichia coli using fluorescein diphosphate as substrate by fluorescence based method ChEMBL_1879024 (CHEMBL4380418) Inhibition of human coagulation factor 12a using Ac-QRFR-pNA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs ChEMBL_2210433 (CHEMBL5123382) Inhibition of human full-length recombinant CDK2 (1 to 298 residues) expressed in baculovirus infected Sf21 insect cells in presence of ATP by enzymatic assay ChEMBL_1708203 (CHEMBL4059436) Binding affinity to human N-terminal 6His-tagged HSP90alpha (1 to 236 residues) expressed in Escherichia coli BL21star/Rosetta2 (DE3) at 298 K by ITC assay ChEMBL_1586220 (CHEMBL3819935) Inhibition of flag-tagged human PTP1B (1 to 298 residues) catalytic domain expressed in Escherichia coli BL21 cells using fluorescein diphosphate as substrate by microplate reader method ChEMBL_2125832 (CHEMBL4835177) Inhibition of human recombinant MMP14 (AA112 to 298) assessed as cleavage of fluorescent substrate using 390 MMP FRET as substrate incubated for 30 mins by biochemical assay ChEMBL_1765701 (CHEMBL4200948) Binding affinity to IDE E111Q mutant (unknown origin) using N-terminal FITC-labeled compound after 2 hrs in absence of light at 298 K by fluorescence polarization assay ChEMBL_1871266 (CHEMBL4372433) Binding affinity to human N-terminal His6-tagged PTP1B catalytic domain (1 to 298 residues) expressed in Escherichia coli strain Rosetta2 (DE3) by switch sense technology based assay ChEMBL_1879020 (CHEMBL4380414) Inhibition of recombinant human KLK7 expressed in Pichia pastoris X-33 using KHLY-pNA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs ChEMBL_1879017 (CHEMBL4380411) Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 and 298 K by spectrophotometry analysis ChEMBL_2459534 Partial agonist activity at recombinant human RXR beta LBD (298 to 533 residues) expressed in Escherichia coli Rosetta incubated for 16 hrs by Gal4-hybrid reporter gene based Dual-glo luciferase assay ChEMBL_1879030 (CHEMBL4380424) Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells assessed as decrease in angiotensin 1 cleavage to angiotensin 2 incubated for 120 mins at 298 K by UPLC-MS analysis ChEMBL_1614390 (CHEMBL3856459) Inhibition of human recombinant full length N-terminal GST-tagged CDK2 (1 to 298 residues)/cyclin E1 (1 to 395 residues) expressed in Sf9 insect cells using histone 3 as substrate by 33P-gamma ATP based assay ChEMBL_1721239 (CHEMBL4136239) Antagonist activity at recombinant human His6-tagged ERalpha LBD (298 to 554 residues) expressed in Escherichia coli assessed as inhibition of estradiol-induced fluorescein-labeled PGC1alpha coactivator peptide binding after overnight incubation by Lantascreen TR-FRET assay ChEMBL_2473699 Inhibition of human full length CDK2 (1 to 298 residues)/N-terminal GST-tagged Cyclin E1 (1 to 410 residues) expressed in baculovirus expression system using fluorescence-based kinase probe as substrate by stopped flow fluorescence spectrometric analysis ChEMBL_1685777 (CHEMBL4036256) Inhibition of full length human CDC2 (1 to 298 residues)/human N-terminal GST-tagged CyclinE1 (1 to 410 residues) expressed in baculovirus expression system using histone H1 as substrate measured after 90 mins by Kinase-Glo luminescence assay ChEMBL_1721240 (CHEMBL4136240) Antagonist activity at recombinant human His6-tagged ERalpha LBD (298 to 554 residues) D538G mutant expressed in Escherichia coli assessed as inhibition of estradiol-induced fluorescein-labeled PGC1alpha coactivator peptide binding after overnight incubation by Lantascreen TR-FRET assay ChEMBL_1721241 (CHEMBL4136241) Antagonist activity at recombinant human His6-tagged ERalpha LBD (298 to 554 residues) Y537S mutant expressed in Escherichia coli assessed as inhibition of estradiol-induced fluorescein-labeled PGC1alpha coactivator peptide binding after overnight incubation by Lantascreen TR-FRET assay ChEMBL_1825562 (CHEMBL4325326) Inhibition of N-terminal GST-tagged full-length human CDK2 (1 to 298 residues)/Cyclin E (1 to 410 residues) expressed in baculovirus expression system using histone H1 as susbtrate after 90 mins by Kinase-Glo luminescence assay ChEMBL_2135502 (CHEMBL4845112) Inhibition of full length human CDK2 (1 to 298 end residues)/N-terminal GST-tagged CyclinA2 (1 to 432 residues) expressed in baculovirus expression system preincubated for 10 mins followed by substrate and ATP addition by mobility shift assay ChEMBL_2449499 Inhibition of N-terminal GST-tagged full-length human CDK2(1 to 298 residues) /FLAG-tagged human Cyclin E1 (1 to 410 residues) expressed in baculovirus-infected Sf21 cells incubated for 60 mins in presence of ATP by HTRF assay ChEMBL_1743830 (CHEMBL4178340) Inhibition of recombinant human Cdk2 (1 to 298 residues)/human N-terminal GST-tagged cyclinE (1 to 410 residues) expressed in baculovirus expression system using histone H1 as substrate after 90 mins in presence of ATP by kinase-glo assay ChEMBL_1871257 (CHEMBL4372424) Inhibition of human N-terminal His6-tagged PTP1B catalytic domain (1 to 298 residues) expressed in Escherichia coli strain Rosetta2 (DE3) using DiFMUP as substrate preincubated for 10 mins followed by substrate addition and measured after 10 mins by fluorescence assay ChEMBL_2081650 (CHEMBL4737441) Inhibition of human N-terminal GST-tagged CDK2 (1 to 298 residues)/Cyclin A (174 to 432 residues) expressed in Escherichia coli Turner (DE3) cells assessed as reduction in ADP formation using PKTPKKAKKL as substrate by resorufin dye based fluorescence assay ChEMBL_2473708 Reversible inhibition of human full length CDK2 (1 to 298 residues)/N-terminal GST-tagged Cyclin E1 (1 to 410 residues) expressed in baculovirus expression system using fluorescence-based kinase probe as substrate measured for 60 mins by fluorescence based analysis ChEMBL_1806447 (CHEMBL4305806) Inhibition of recombinant human full length N-terminal GST-tagged CDK2 (1 to 298 residues)/cyclin A2 (1 to 432 residues) expressed in baculovirus expression system using eIF4E-binding protein 1 peptide and ATP as substrate incubated for 30 mins by LANCE ultra kinase assay ChEMBL_1871267 (CHEMBL4372434) Binding affinity to human N-terminal His6-tagged PTP1B catalytic domain (1 to 298 residues) expressed in Escherichia coli strain Rosetta2 (DE3) assessed as association rate constant using DiFMUP as substrate preincubated for 10 mins followed by substrate addition measured for 10 mins fluorescence assay ChEMBL_1871268 (CHEMBL4372435) Binding affinity to human N-terminal His6-tagged PTP1B catalytic domain (1 to 298 residues) expressed in Escherichia coli strain Rosetta2 (DE3) assessed as dissociation rate constant using DiFMUP as substrate preincubated for 10 mins followed by substrate addition measured for 10 mins fluorescence assay ChEMBL_1516442 (CHEMBL3619460) Binding affinity to N-terminal His-tagged recombinant human CDK2 (1 to 298 amino acid residues) expressed in Escherichia coli BL21 assessed as inhibition of interaction with cyclin A3 by measuring 250 uM HHASPRK phosphorylation by ADP-Glo Max assay in presence of 75 uM ATP ChEMBL_1516443 (CHEMBL3619461) Binding affinity to N-terminal His-tagged recombinant human CDK2 (1 to 298 amino acid residues) expressed in Escherichia coli BL21 assessed as inhibition of interaction with cyclin A3 by measuring 250 uM HHASPRK phosphorylation by ADP-Glo Max assay in presence of 375 uM ATP ChEMBL_1516444 (CHEMBL3619462) Binding affinity to N-terminal His-tagged recombinant human CDK2 (1 to 298 amino acid residues) expressed in Escherichia coli BL21 assessed as inhibition of interaction with cyclin A3 by measuring 250 uM HHASPRK phosphorylation by ADP-Glo Max assay in presence of 750 uM ATP ChEMBL_1516445 (CHEMBL3619463) Binding affinity to N-terminal His-tagged recombinant human CDK2 (1 to 298 amino acid residues) expressed in Escherichia coli BL21 assessed as inhibition of interaction with cyclin A3 by measuring 1.25 mM HHASPRK phosphorylation by ADP-Glo Max assay in presence of 75 uM ATP ChEMBL_1516446 (CHEMBL3619464) Binding affinity to N-terminal His-tagged recombinant human CDK2 (1 to 298 amino acid residues) expressed in Escherichia coli BL21 assessed as inhibition of interaction with cyclin A3 by measuring 2.5 mM HHASPRK phosphorylation by ADP-Glo Max assay in presence of 75 uM ATP ChEMBL_2163802 (CHEMBL5048663) Inhibition of full length N-terminal GST tagged human CDK2(1 to 298 residues)/CyclinA2 (1 to 432 residues) expressed in baculovirus expression system using FL peptide as substrate preincubated for 30 mins followed by substrate addition further incubated for 120 mins by EZ reader method ChEMBL_2373797 Inhibition of human recombinant N-terminal IgG tagged and C-terminal His tagged MASP2 (298 to 686 aa residues) expressed in Expi293 cells using Z-Gly-Arg-SBzl peptide and BES-Thio as substrate pretreated with compound followed by substrate addition incubated for 60 mins by multilabel plate reader method ChEMBL_2467950 Inhibition of full length N-terminal GST-tagged human CDK2 (1 to 298 residues)/cyclin E1 (1 to 410 residues) expressed in Sf21 cells using histone H1 as substrate preincubated for 10 mins followed by substrate and ATP addition and measured after 60 mins by ADP-Glo reagent based microplate reader assay Nuclear Magnetic Resonance (NMR) Assay NMR spectra were acquired on Bruker Avance 600 MHz spectrometers at 298 K using a 5 mm triple-resonance HCN cryoprobe. Ligand binding was detected using WaterLOGSY and T1p-filtered 1D experiments in combination with 2D 1H-15N TROSY-HSQC experiments to provide binding-site and kd information. ChEMBL_2464448 Inhibition of full length human CDK2 (1 to 298 residues)/N-terminal GST-tagged human Cyclin E1 (1 to 410 residues) expressed in baculovirus expression system preincubated for 10 mins followed by substrate addition and measured after 60 mins in presence of ATP by ADP-Glo reagent based luminescence microtiter plate reader analysis ChEMBL_2470787 Inhibition of full-length N-terminal GST-tagged human CDK2 (1 to 298 residues) / GST-tagged CyclinA2 (1 to 432 residues) expressed in baculovirus expression system preincubated for 30 mins followed by ATP and ULight-histone H3 peptide/ULight-eIF4E binding protein addition and measured after 2 hrs by LANCE Ultra TR-FRET assay ChEMBL_1885555 (CHEMBL4387137) Inhibition of human full length GST-tagged CDK2 (1 to 298(end) amino acids)/Cyclin E1 (1 to 410(end) amino acids) expressed in baculovirus expression system assessed as inhibition constant using Ulight-4E-BP1 as substrate preincubated for 30 mins followed by substrate addition and incubated for 90 mins by TR-FRET assay NMR (OAH or OAMe) The NMR experiments were carried out in 10 mM sodium phosphate buffer at a pH of 11.3, while the ITC experiments were performed in 50 mM sodium phosphate buffer at pH 11.5. Both sets of experiments were conducted at 298 K, except that the NMR results for OAMe-G4 were obtained at 278 K. AICAR Tfase Inhibition Assay Recombinant human AICAR Tfase was used in the inhibition assay. The reaction was monitored at 298 nm by measuring the increase in absorbance corresponding to the formation of THF. The THF was generated in the transfer reaction of the formyl group from cofactor to AICAR to produce 5-formyl-AICAR. Double reciprocal plots were used to determine the Ki values. Fluorescence Polarization Assay The fluorescence polarization assays were performed at 298 K following a protocol modified from previously reported protocol [Zhuo et al., Mol. Biosyst., 12:2532-2540]. Synthetic peptides were labeled with fluorescein. Titrations were conducted by monitoring polarization as a function of increasing amounts of the proteins of TnC N-terminal domain added to 20 nM fluorescein-peptides in a buffer 50 mM HEPES, pH 7.0, 0.1% Tween 20, 200 mM NaCl and 15 mM DTT. Assay of Enzyme Activity The activity was assayed by measuring the rate of change in NADPH fluorescence (at 455 nm with an excitation wavelength of 340 nm) at 298 K. When the fluorescence due to the high concentration of inhibitor interfered with the fluorometric assay, the enzyme activity was determined by measuring the rate of change in NADPH absorbance at 340 nm. The inhibitor constant, Ki, was determined from a Lineweaver-Burk plot using five concentrations of substrate, and is expressed as the mean +/- standard error of at least three determinations. AICAR Tfase Inhibition Assay The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The reaction was initiated by adding the substrate AICAR to reaction mixtures containing ATIC, test compounds, and different concentrations of cofactor. Using a SpectraMax Plus384 microplate reader, the reaction was monitored at 298 nm by measuring the increase in absorbance corresponding to the formation of THF. The THF was generated in the transfer reaction of the formyl group from cofactor to AICAR to produce 5-formyl-AICAR. IC50 values were measured from dose-response curves. Isothermal Titration Calorimetry (ITC) ITC titrations were performed using an ITC200 instrument (Microcal Inc.,Malvern). DMSO stock solutions (20 mM) of fragments were diluted 1:20 (v/v) into ITC titration buffer (50 mM tricine, 2 mM MnCl2, pH = 8.5) to give a 1 mM final compound concentration at 5% (v/v) DMSO. Aliquots of the same buffer batch (stored at −253 K) were used as for protein storage to avoid a buffer mismatch. H6PqsE was diluted in the same buffer to 100 μM, and DMSO concentration was adjusted to 5% (v/v). Titrations were carried out at 298 K using 19 injections of 2 μL every 180 s. Areas under the peaks were integrated. Baseline peaks at the endof a titration accounting for the heat of dilution and mixing were subtracted from the measurement. Data were fitted to a 1:1 binding model (MicroCal Origin 7 software). NMR Assay All NMR spectra were acquired at 298 K on a Bruker DRX 600 MHz spectrometer equipped with a cryoprobe. Typically, NMR samples contained 0.1-0.2 mM protein in 50 mM KH2PO4 and 50 mM Na2HPO4, pH 7.4, containing 150 mM NaCl and 5 mM β-Mercaptoethanol. Water suppression was carried out using the WATERGATE sequence. NMR data were processed using the Bruker program Xwin-NMR version 3.5. NMR ligand binding experiments were carried out in an analogous way to those previously described. See D'Silva L., et. al., J. Am. Chem. Soc. 2005, 127, 13220-13226 and Popowicz G. M., et. al., Cell Cycle. 2007, 6, 2386-2392. The maximum concentration of DMSO at the end of titration experiments was less than 1%. The pH was maintained constant during the entire titration. The 1H-15N-HSQC spectra were recorded using fast HSQC pulse sequence as described by Mori et. al., J. Magn. Reson. B 1995, 108, 94-98. Binding Assay hERG: The purpose of the hERG binding assay is to evaluate the effects of test compounds on the voltage-dependent potassium channel encoded by the human ether go go-related gene (hERG) using a constitutively expressing CHO cell line on the Nanion Syncropatch 384PE automated patch clamp system.The assay was conducted as follows with all reagents used at room temperature unless otherwise stated.Reagent Preparations Include:1. Internal "IC700" solution used to perfuse the underside of chip (in mM), KF 130, KCl 20, MgCl2 1, EGTA 10 and HEPES 10, (all Sigma-Aldrich; pH 7.2-7.3 using 10 M KOH, 320 mOsm) and supplemented with 25 μM escin.2. External and cell buffer (in mM), NaCl 137, KCl 4, HEPES 10, D-glucose 10, CaCl2 2, MgCl2 1 (pH7.4, NaOH)3. NMDG "reference" buffer used to establish a stable baseline prior to the addition of test compounds, NaCl 80, KCl 4, CaCl2 2, MgCl2 1, NMDG Cl 60, D-Glucose monohydrate 5, HEPES 10 (pH7.4 NaOH 298 mOsm)4. Seal enhancer used to improve seal quality of cells, NaCl 80, KCl 3, CaCl2 10, HEPES 10, MgCl2 1 (pH7.4 NaOH) HCMV antiviral assay Antiviral assays for HCMV DNA were carried out by DNA hybridization as reported by Dankner, W. M., Scholl, D., Stanat, S. C., Martin, M., Souke, R. L. and Spector, S. A., J. Virol. Methods 21:293-298, 1990. Briefly, subconfluent MRC-5 cells in 24-well culture dishes were pretreated for 24 h with various concentrations of drug in Eagle s minimum essential medium (E-MEM) containing 2% FBS and antibiotics. The medium was removed and HCMV strains added aba dilution that will result in a 3-4+ cytopathic effect (CPE) in the no-drug wells in 5 days. The virus was absorbed for 1′ h at 37° C., aspirated and replaced with the drug dilutions. After 5 days of incubation HCMV DNA was quantified in triplicate by nucleic acid hybridization using a CMV Antiviral Susceptibility Test Kit from Diagnostic Hybrids, Inc. (Athens, Ohio). The medium was removed and cells lysed according to the manufacturer s instructions. After absorption of the lysate, the Hybriwix filters were hybridized overnight at 60° C. The Hybriwix were washed for 30 min at 73° C. and counted in a gamma counter. The results are expressed as EC50 (the 50% inhibitory concentration). hERG Electrophysiological Assay Electrophysiological recordings (all performed at RT) from stably transfected CHO hKv11.1 cells were obtained using the Nanion Syncropatch 768PE. Test compounds, vehicle or positive controls were added with 6 compound plates each at a different concentration to allow cumulative dosing onto cells (10 mM, 3.167 mM, 1 mM, 0.3167 mM, 0.1 mM, 0.03167 mM). 600 Figure US11325906-20220510-P00001of compound is resuspended into 90 μl of reference buffer (in mM, NaCl 80, KCL 4, CaCl 5, MgCl 1, NMDG Cl 60, D-Glucose monohydrate 5, HEPES 10 (pH7.4 HCL, 298 mOsm) for a final compound concentration of 39.6 μM, 13.2 μM, 4.4 μM, 1.46 μM, 0.48 μM, 0.16 μM. For each Nanion Syncropatch 768PE run, the current amplitude in each cell in the presence of extracellular solution (in mM, NaCl 80, KCL 4, CaCl 5, MgCl 1, NMDG Cl 60, D-Glucose monohydrate 5, HEPES 10 (pH7.4 HCL, 298 mOsm) is measured with all liquid additions performed using the Syncropatch liquid handling system. Add 40 μL external solution (in mM, HBPS, CaCl2 2, MgCl2 1 (pH7.4, NaOH) to 384 well multihole medium resistance recording chip and perfuse internal buffer (in mM, KF 130, KCl 20, MgCl2 1, EGTA 10, HEPES 10, Escin 25 (all Sigma-Aldrich; pH 7.2-7.30 using 10 M KOH, 320 mOsm) to the underside of plate. Dispense 20 μL of cells at a density of 1e6 cells/ml maintained at 9° C. into each well of the chip followed by 20 μL of seal enhancer (in mM, NaCl 80, KCl 3, CaCl 10, HEPES 10, MgCl 1 (pH7.4 NaOH). Perform wash step leaving a residual volume of 40 μL. Dispense 40 μL of reference buffer to establish a stable baseline prior to the addition of test compounds, with a removal step of 40 μL after 3 min, repeat this step. Dispense 40 μL of compound concentration 1 (0.16 μM), real time recordings for 3 min exposure prior to removal of 40 μL. This step is repeated for 5 further subsequent compound plates to generate cumulative curve analysis. All data is leak subtracted, 2 pulses to −80 mV 100 ms with 100 ms delay. Outward K+ currents are then evoked by voltage step to +60 mV from a holding potential of −90 mV, Each pulse is delivered at a frequency of 2 Hz with a 15 s pulse interval. Binding Assay hERG (human ether go go-related gene) potassium channels are essential for normal electrical activity in the heart. Arrhythmia can be induced by a blockage of hERG channels by a diverse group of drugs. This side effect is a common reason for drug failure in preclinical safety trials and therefore minimisation of hERG channel blocking activity may be a desirable property for drug candidates.The purpose of the hERG binding assay is to evaluate the effects of test compounds on the voltage-dependent potassium channel encoded by the human ether go go-related gene (hERG) using a constitutively expressing CHO cell line on the Nanion Syncropatch 384PE automated patch clamp system.The assay was conducted as follows with all reagents used at room temperature unless otherwise stated.Reagent Preparations Include:1. Internal IC700 solution used to perfuse the underside of chip (in mM), KF 130, KCl 20, MgCl2 1, EGTA 10 and HEPES 10, (all Sigma-Aldrich; pH 7.2-7.3 using 10 M KOH, 320 mOsm) and supplemented with 25 □M escin.2. External and cell buffer (in mM), NaCl 137, KCl 4, HEPES 10, D-glucose 10, CaCl2 2, MgCl2 1 (pH7.4, NaOH)3. NMDG reference buffer used to establish a stable baseline prior to the addition of test compounds, NaCl 80, KCl 4, CaCl2 2, MgCl2 1, NMDG Cl 60, D-Glucose monohydrate 5, HEPES 10 (pH7.4 NaOH 298 mOsm)4. Seal enhancer used to improve seal quality of cells, NaCl 80, KCl 3, CaCl2 10, HEPES 10, MgCl2 1 (pH7.4 NaOH) Inhibitory Assay An inhibitory assay was conducted using the previously described p-nitrophenylphosphate assay (Rayapureddi, J. P. et al. Nature 426, 295-298 (2003)). Briefly, ED was incubated in a reaction mixture containing 20 mM MES pH 6, 2 mM MgCl2, 125 μM inhibitor, 3.4 mM para-nitrophenol phosphate (pNPP) and 0.01 μg/μL enzyme. The amount of 4-nitrophenol (pNP) produced was monitored at 405 nM on a BioTek EL808 plate reader. A similar protocol was used to screen for inhibitor activity using hEYA3 and hEYA2(ED).The compounds were then tested using full-length human recombinant, purified EYA3 and pNPP as a substrate. Compounds were dissolved in DMSO and diluted as needed. IC50 values were determined by adding varying amounts of inhibitor (0-400 μM) to reaction mixtures containing 20 mM MES pH 6, 2 mM MgCl2, 2% DMSO, 3.4 mM pNPP, and 0.01 μg/L enzyme. Reactions were incubated at 30° C. for 30 minutes and quenched with 100 mM EDTA pH 10. IC50 values were then calculated directly from regression curves using PRISM software. All reported values are the mean of two independent experiments.These results were mirrored when an alternate substrate, a 10 amino acid phosphopeptide representing the C-terminus of the known EYA substrate γ-H2AX (Cook, P. J. et al. Nature 458, 591-596, (2009); Krishnan, N. et al. J. Biol. Chem. (2009), the contents of which are incorporated by reference in their entireties). The phospho-peptide KKATQASQEpY (SEQ. ID 5) was obtained from Genscript. Peptide assays were conducted in 20 mM MES pH 6, 2 mM MgCl2, and a range of peptide concentrations from 0 to 300 μM as previously described in the incorporated materials of Rayapureddi, J. P. (2003). IC50 values were then calculated using PRISM software. Calcein Quenching Fluostar Assay A calcein quenching fluostar assay was performed in order to investigate the biological activity of the newly synthesized examples 1 to 57. This type of assay is disclosed in J. Biol. Chem., 2011, 286, 44319-44325 and Am. J. Physiol. Renal Physiol. (2010), 298, F224-230.The buffers used in the assay were prepared with the following compounds and quantities.500 ml of 4× buffer:3.2 mM MgSO4.7H2O (0.395 g)20 mM KCl (0.746 g)7.2 mM CaCl.2H2O (0.530 g)100 mM NaHepes (13.02 g)pH 7.4 w. HClTetracyclin Stock: Wash buffer (μl) Sucrose buffer (μl) 4x buffer 80000 35000 NaCl (1M) 34080 14910 H2O 199520 18970 Probenecid 6400 2800 Sucrose (1M) 0 68320 Total 320000 140000The total probenecid required to prepare the wash buffer and sucrose buffer is 6400+2800=9200 μl. An additional 500 μl of probenecid (5 plates at 100 μl each) is also required. Therefore, the total probenecid required is 9200 μl+500 μl=9700 μl. Sufficient probenecid is prepared using:690 mg probenecid;4850 μl NaOH 1M;1213 μl 4× buffer; and3638 μl H2O.Assay Experimental Protocol:1) Two days prior to commencement of the assay, seed 10,000 cells/well of 96 well black clear bottom plate (Greiner Poly-lysin plate). A 1:1 mix of Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12 (DMEM: F12) was obtained from Gibco. Tetracycline stock of 5 mg/ml in 96% ethanol is used. Medium: DMEM/F12/10% Donor Bovine Serum, Human AQP9 cell line+1:270,000 tetracyclin, mouse AQP9 cell line+1:2,700,000 tetracycline.2) Day of assay: Flick/slam off the medium and add 50 μl/well of loading solution: 5 ml DMEM/F12/10% Donor Bovine Serum, 25 μl Calcein AM from freshly dissolved aliquot in 50 μl DMSO (VWR #734-1434), and 100 μl Probenecid.3) Incubate the well for 90 minutes at 37° C.4) Perform one wash with 75 μl wash buffer.5) Add 75 μl of an example compound prepared in wash buffer per well.Example compounds are prepared in 500 μl U bottom PP plates (NUNC). 2.7 μl Substance in DMSO are added to row A; 180 μl of wash buffer+1% DMSO are added to rows B H. 90 μl from row A are transferred and mixed with all other wells (up to row G) to make a 3-fold dilution series.6) Assay in FLUOstar Optima at 25° C. Settings buffer addition at 135 μl/seconds, add 75 μl/well, record time course for 30 seconds, add sucrose buffer 3.6 seconds into recording.7) Normalization to initial in Excel.8) Fit to exponential decay function in GraphPad Prism 5.0, then arrange half live shrinking values according to wells and fit dose-response curves.
 USRE48841, Example 298 US11667644, Example 298 US8653263, 298 US9586962, Example 298 BDBM118645 US11059828, Example 298
USRE48841, Example 298 US11667644, Example 298 US8653263, 298 US9586962, Example 298 BDBM118645 US11059828, Example 298 BDBM150624 US10238633, Example 298 US9713606, 298 US8987249, 298
BDBM150624 US10238633, Example 298 US9713606, 298 US8987249, 298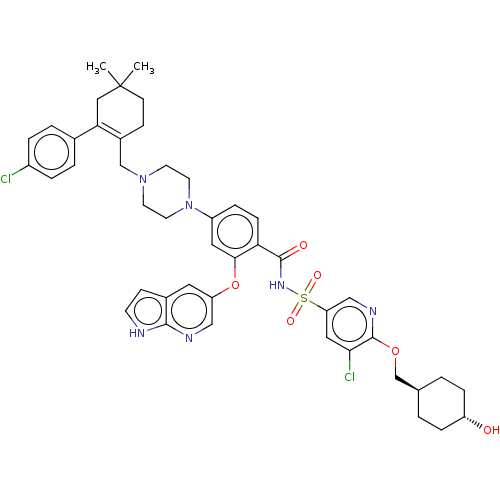 US10213433, Compound 298 US20240043404, Example 298 US9174982, 298 US11369599, Compound 298 BDBM189728
US10213433, Compound 298 US20240043404, Example 298 US9174982, 298 US11369599, Compound 298 BDBM189728 BDBM385142 US10287267, Compound 298 US10556885, Compound 298 US11673881, Compound 298 US10508099, Compound 298 US11174244, Compound 298
BDBM385142 US10287267, Compound 298 US10556885, Compound 298 US11673881, Compound 298 US10508099, Compound 298 US11174244, Compound 298 US10137124, Example 298 US10112942, Example 298 US10172851, Example 298 BDBM296564 US10555944, Example 298 US10953005, Example 298
US10137124, Example 298 US10112942, Example 298 US10172851, Example 298 BDBM296564 US10555944, Example 298 US10953005, Example 298 US10307413, Compound 298 US10980794, Cmpd No 298 US10391089, Compound 298 US9675614, 298 BDBM179260
US10307413, Compound 298 US10980794, Cmpd No 298 US10391089, Compound 298 US9675614, 298 BDBM179260 US10646492, Example 298 US9932341, Example 298 US10092570, Example 298 BDBM289297 US9730939, Example 298
US10646492, Example 298 US9932341, Example 298 US10092570, Example 298 BDBM289297 US9730939, Example 298 US10245267, Example 298 US9694016, 298 US10709712, Example 298 BDBM77854
US10245267, Example 298 US9694016, 298 US10709712, Example 298 BDBM77854 US9452980, 298 US10501411, Example 298 US11697636, Example 298 BDBM250388
US9452980, 298 US10501411, Example 298 US11697636, Example 298 BDBM250388 US9688672, Example 298 US9434719, 298 BDBM247696 US10112931, Example 298
US9688672, Example 298 US9434719, 298 BDBM247696 US10112931, Example 298 US9745328, Compound 298 US9079866, 298 BDBM168897 US9884878, Compound 298
US9745328, Compound 298 US9079866, 298 BDBM168897 US9884878, Compound 298 BDBM71191 US9546164, 298 US9694002, 298
BDBM71191 US9546164, 298 US9694002, 298 US10047103, 298 US9688695, 298 BDBM176265
US10047103, 298 US9688695, 298 BDBM176265 US8952157, 298 US9303025, 298 BDBM145107
US8952157, 298 US9303025, 298 BDBM145107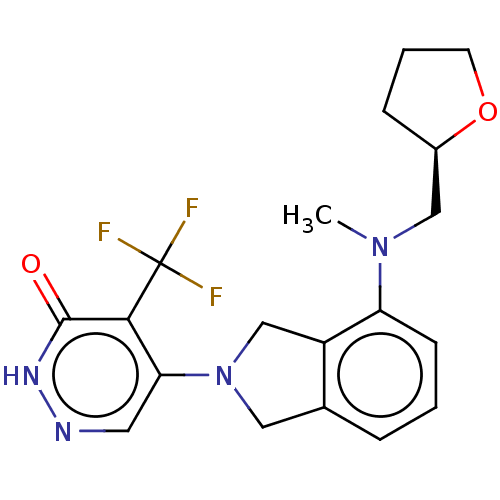 BDBM431353 US10870641, Example 298 US10550105, Example 298 US11014913, Example 298
BDBM431353 US10870641, Example 298 US10550105, Example 298 US11014913, Example 298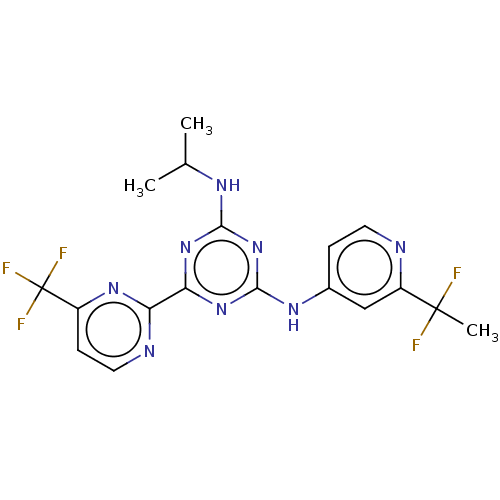 US10028961, Compound 298 US10946023, Compound 298 US10172864, Compound 298 BDBM280145
US10028961, Compound 298 US10946023, Compound 298 US10172864, Compound 298 BDBM280145 US11702424, Example 298 US10793579, Example 298 BDBM403144 US10329302, Example 298
US11702424, Example 298 US10793579, Example 298 BDBM403144 US10329302, Example 298 US9999619, Example 298 US11285140, Example 298 US10695337, Example 298 BDBM400542
US9999619, Example 298 US11285140, Example 298 US10695337, Example 298 BDBM400542 BDBM135661 US11547697, Compound 298 US9682141, 298
BDBM135661 US11547697, Compound 298 US9682141, 298 BDBM167807 US9073922, 298 US9796708, Example 298
BDBM167807 US9073922, 298 US9796708, Example 298 BDBM171664 US9085576, 298 US9611261, Example 298
BDBM171664 US9085576, 298 US9611261, Example 298 BDBM232752 US9604984, Example 298 US9346815, 298
BDBM232752 US9604984, Example 298 US9346815, 298 US9556187, Example 298 BDBM198501 US9216999, 298
US9556187, Example 298 BDBM198501 US9216999, 298 US9593129, Example 298 US9296736, 298 BDBM215335
US9593129, Example 298 US9296736, 298 BDBM215335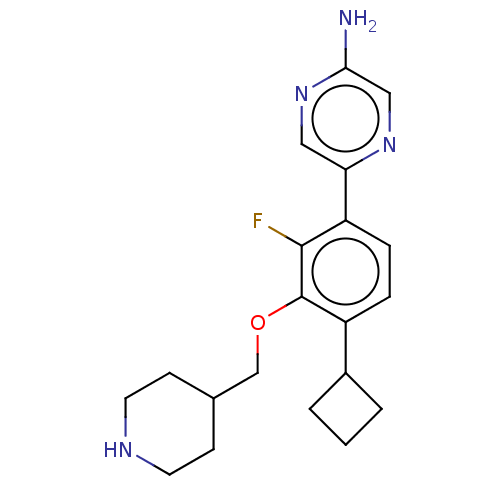 US9732093, Compound 298 US9073876, 298 BDBM167217
US9732093, Compound 298 US9073876, 298 BDBM167217 BDBM108718 US8604016, 298 US9938267, Cmpd ID 298
BDBM108718 US8604016, 298 US9938267, Cmpd ID 298 BDBM273515 US10478424, Example 298 US10071079, Example 298
BDBM273515 US10478424, Example 298 US10071079, Example 298 BDBM284288 US10174027, Example 298 US10023570, Example 298
BDBM284288 US10174027, Example 298 US10023570, Example 298 BDBM348575 US9790229, Compound 298 US10577374, Compound 298
BDBM348575 US9790229, Compound 298 US10577374, Compound 298 BDBM434379 US10562891, Example 298 US11008308, Example 298
BDBM434379 US10562891, Example 298 US11008308, Example 298 BDBM477526 US10889555, Example 298 US11634395, Example 298
BDBM477526 US10889555, Example 298 US11634395, Example 298 BDBM486850 US10947252, Example 298 US11427601, Example 298
BDBM486850 US10947252, Example 298 US11427601, Example 298 US10105367, Example 298 BDBM293012 US10376514, Example 298
US10105367, Example 298 BDBM293012 US10376514, Example 298 US10125118, Example 298 BDBM298892 US10947215, Example 298
US10125118, Example 298 BDBM298892 US10947215, Example 298 US10273259, Example 298 US10711020, Example 298 BDBM382589
US10273259, Example 298 US10711020, Example 298 BDBM382589 US10301317, Example 298 US9624228, Example 298 BDBM318341
US10301317, Example 298 US9624228, Example 298 BDBM318341 US10577367, Example 298 US10246456, Example 298 BDBM374486
US10577367, Example 298 US10246456, Example 298 BDBM374486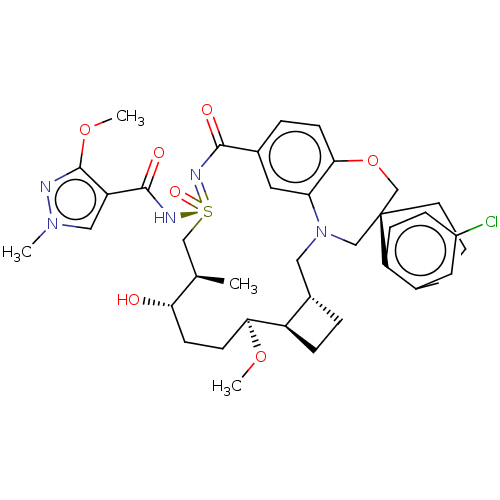 US10703733, Example 298 BDBM449685 US11643400, Example 298
US10703733, Example 298 BDBM449685 US11643400, Example 298 US10774053, Compound 298 US11352329, COMPD # 298 BDBM461130
US10774053, Compound 298 US11352329, COMPD # 298 BDBM461130 US11453683, Example 298 BDBM573445 US20230279025, Example 298
US11453683, Example 298 BDBM573445 US20230279025, Example 298 US11555029, No. 298 US10710986, Example 298 BDBM452160
US11555029, No. 298 US10710986, Example 298 BDBM452160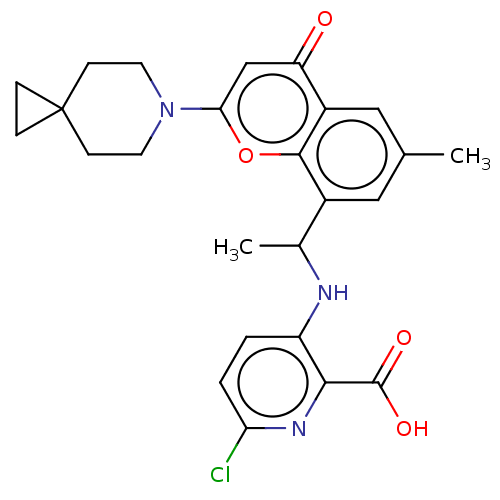 US20230286960, Example 298 US11649227, Example 298 BDBM602693
US20230286960, Example 298 US11649227, Example 298 BDBM602693 BDBM423843 US11046698, Compound I-298 US10577373, Compound I-298 US10508120, Compound I-298
BDBM423843 US11046698, Compound I-298 US10577373, Compound I-298 US10508120, Compound I-298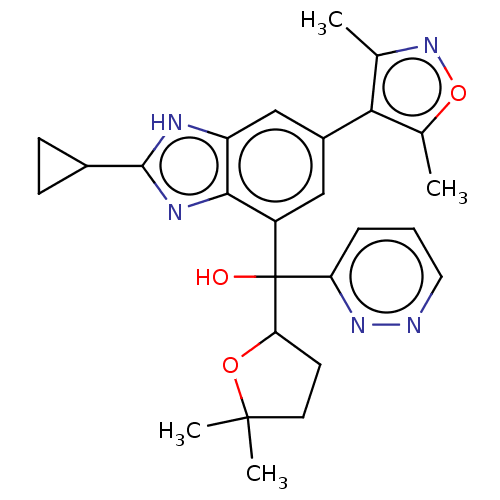 US10017501, Compound 1020-298 BDBM249314 US9458145, 1020-298
US10017501, Compound 1020-298 BDBM249314 US9458145, 1020-298 BDBM271987 US9732060, Compound R-298 US10065941, Compound R-298
BDBM271987 US9732060, Compound R-298 US10065941, Compound R-298 BDBM442337 US11396508, Compound I-298 US10647713, Compound I-298
BDBM442337 US11396508, Compound I-298 US10647713, Compound I-298 BDBM565730 US12221453, Compound I-298 US11414431, Compound I-298
BDBM565730 US12221453, Compound I-298 US11414431, Compound I-298 US10633389, Example 298-1a BDBM439988 US20230279020, Example 298-1a
US10633389, Example 298-1a BDBM439988 US20230279020, Example 298-1a US11208415, Example I-298 US10533010, Example I-298 BDBM429171
US11208415, Example I-298 US10533010, Example I-298 BDBM429171 US11433071, Example 298-1 US9707233, 298 (1st peak) BDBM261275
US11433071, Example 298-1 US9707233, 298 (1st peak) BDBM261275 BDBM115429 US8637500, 298
BDBM115429 US8637500, 298 BDBM121859 US8722692, 298
BDBM121859 US8722692, 298 BDBM124612 US8759532, 298
BDBM124612 US8759532, 298 BDBM128704 US8796244, 298
BDBM128704 US8796244, 298 BDBM144097 US8969325, 298
BDBM144097 US8969325, 298 BDBM146246 US8957068, 298
BDBM146246 US8957068, 298 BDBM147444 US8957093, 298
BDBM147444 US8957093, 298 BDBM149048 US8962648, 298
BDBM149048 US8962648, 298 BDBM153098 US8993616, 298
BDBM153098 US8993616, 298 BDBM154280 US9012443, 298
BDBM154280 US9012443, 298 BDBM156862 US9029559, 298
BDBM156862 US9029559, 298 BDBM158005 US9023882, 298
BDBM158005 US9023882, 298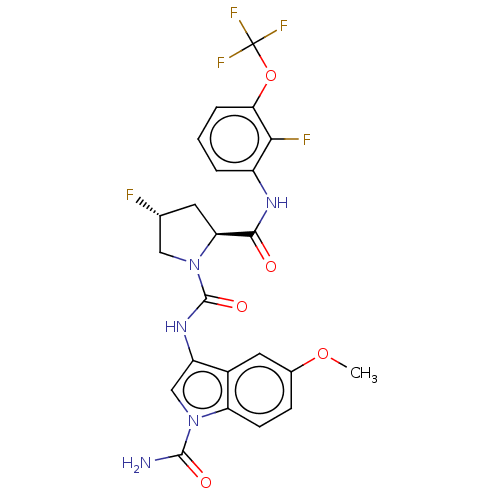 BDBM170870 US9085555, 298
BDBM170870 US9085555, 298 BDBM175753 US9688680, 298
BDBM175753 US9688680, 298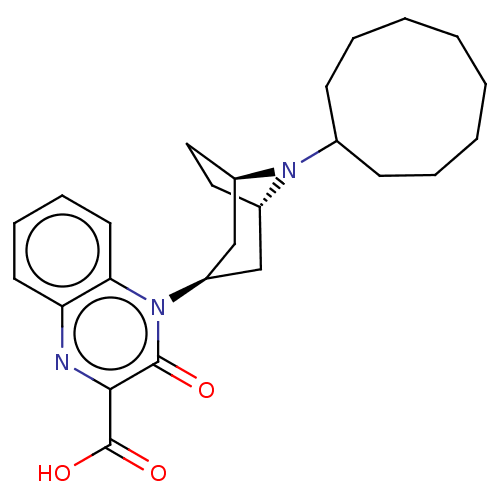 BDBM17605 US8846929, 298
BDBM17605 US8846929, 298 BDBM178729 US9125913, 298
BDBM178729 US9125913, 298 BDBM182597 US9145392, 298
BDBM182597 US9145392, 298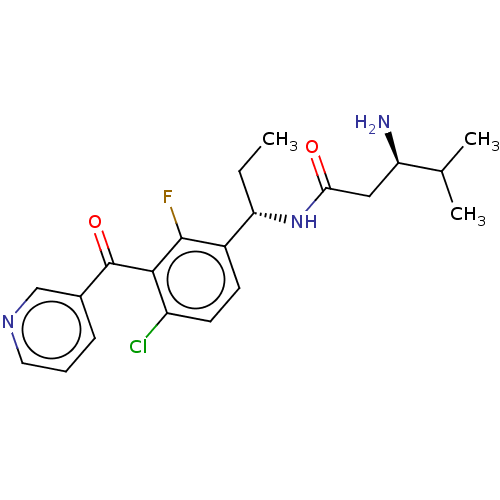 BDBM183631 US9145354, 298
BDBM183631 US9145354, 298 BDBM187747 US9169252, 298
BDBM187747 US9169252, 298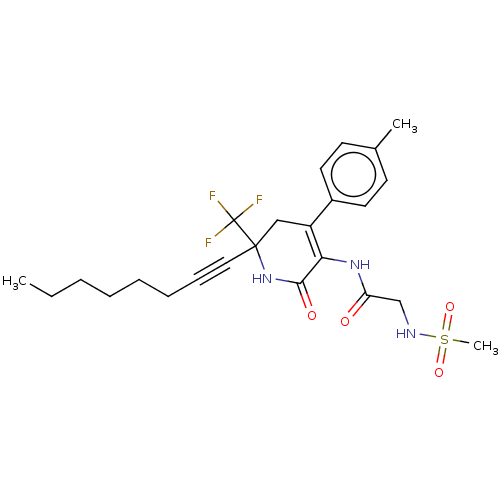 BDBM201531 US9187424, 298
BDBM201531 US9187424, 298 BDBM201867 US9233979, 298
BDBM201867 US9233979, 298 BDBM203119 US9242970, 298
BDBM203119 US9242970, 298 BDBM204224 US9242996, 298
BDBM204224 US9242996, 298 BDBM206545 US9260425, 298
BDBM206545 US9260425, 298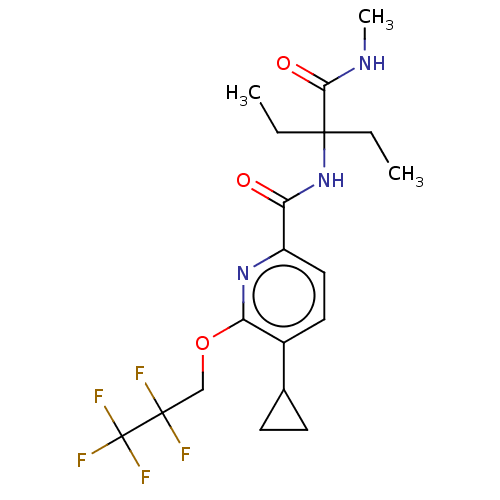 BDBM210611 US9290451, 298
BDBM210611 US9290451, 298 BDBM213706 US9278981, 298
BDBM213706 US9278981, 298 BDBM216533 US9302989, 298
BDBM216533 US9302989, 298 BDBM225031 US9321756, 298
BDBM225031 US9321756, 298 BDBM226605 US9328096, 298
BDBM226605 US9328096, 298 BDBM229971 US9334269, 298
BDBM229971 US9334269, 298 BDBM231911 US9340517, 298
BDBM231911 US9340517, 298 BDBM234165 US9353090, 298
BDBM234165 US9353090, 298 BDBM240583 US9409866, 298
BDBM240583 US9409866, 298 BDBM258847 US9499482, 298
BDBM258847 US9499482, 298 BDBM30610 US8853258, 298
BDBM30610 US8853258, 298 BDBM327751 US9663469, 298
BDBM327751 US9663469, 298 BDBM77729 US9695118, 298
BDBM77729 US9695118, 298 BDBM99781 US8501936, 298
BDBM99781 US8501936, 298 BDBM99782 US8501936, 298
BDBM99782 US8501936, 298 US8481733, 298 BDBM98486
US8481733, 298 BDBM98486 US8604061, 298 BDBM109528
US8604061, 298 BDBM109528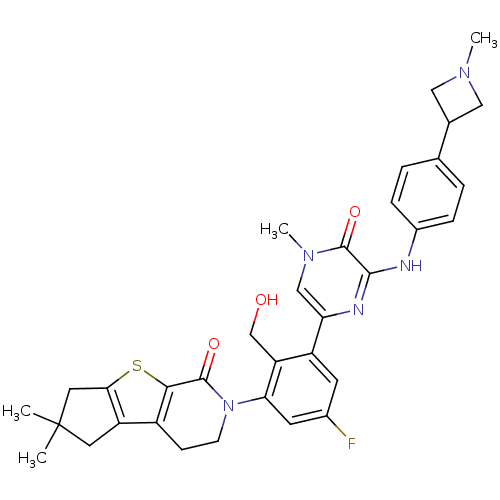 US8618107, 298 BDBM111985
US8618107, 298 BDBM111985 US8623889, 298 BDBM112300
US8623889, 298 BDBM112300 US8637532, 298 BDBM116604
US8637532, 298 BDBM116604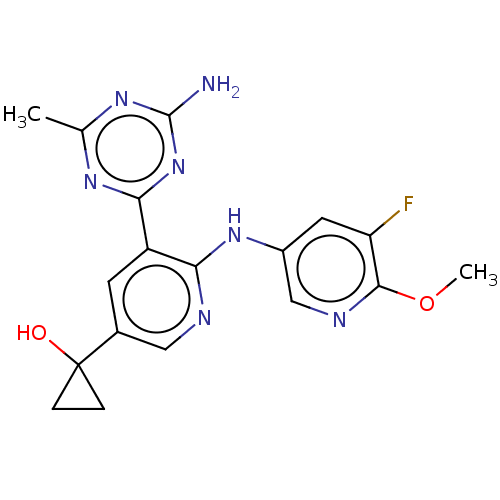 US8772480, 298 BDBM125443
US8772480, 298 BDBM125443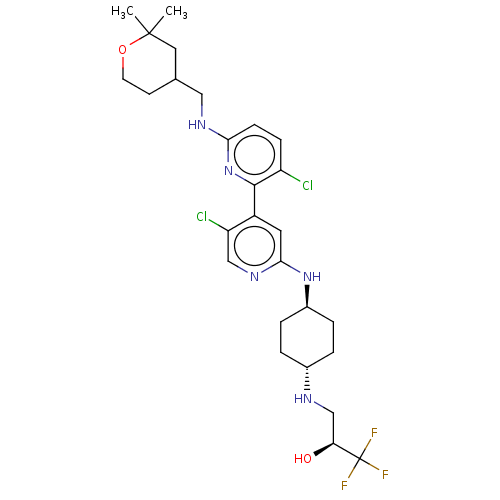 US8778951, 298 BDBM126488
US8778951, 298 BDBM126488 US8802674, 298 BDBM129191
US8802674, 298 BDBM129191 US8815926, 298 BDBM130016
US8815926, 298 BDBM130016 US8841312, 298 BDBM132549
US8841312, 298 BDBM132549 US8846698, 298 BDBM134830
US8846698, 298 BDBM134830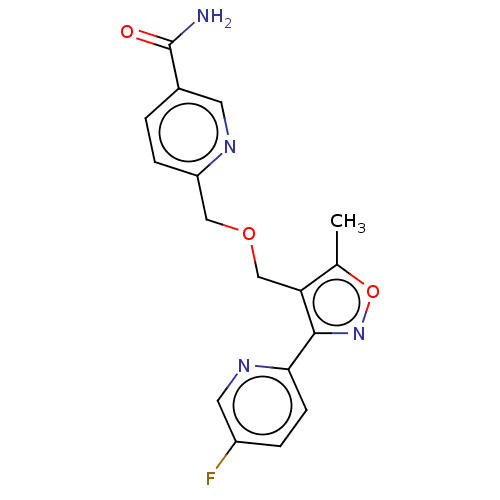 US8846719, 298 BDBM133595
US8846719, 298 BDBM133595 US8871934, 298 BDBM137705
US8871934, 298 BDBM137705 US8912224, 298 BDBM140861
US8912224, 298 BDBM140861 US8921368, 298 BDBM141486
US8921368, 298 BDBM141486 US8952169, 298 BDBM145502
US8952169, 298 BDBM145502 US9023865, 298 BDBM157457
US9023865, 298 BDBM157457 US9034866, 298 BDBM158823
US9034866, 298 BDBM158823 US9067871, 298 BDBM164842
US9067871, 298 BDBM164842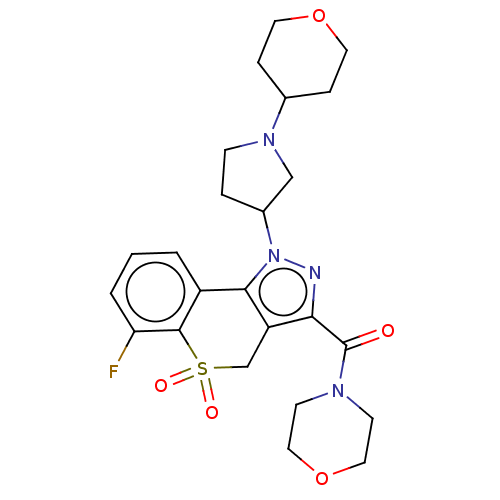 US9073940, 298 BDBM169948
US9073940, 298 BDBM169948 US9090628, 298 BDBM172638
US9090628, 298 BDBM172638 US9126931, 298 BDBM178983
US9126931, 298 BDBM178983 US9169260, 298 BDBM189032
US9169260, 298 BDBM189032 US9181272, 298 BDBM191528
US9181272, 298 BDBM191528 US9221809, 298 BDBM203747
US9221809, 298 BDBM203747 US9226922, 298 BDBM199662
US9226922, 298 BDBM199662 US9255090, 298 BDBM205829
US9255090, 298 BDBM205829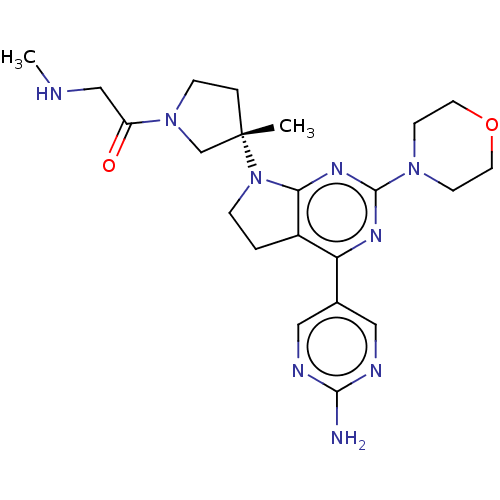 US9260439, 298 BDBM207414
US9260439, 298 BDBM207414 US9283222, 298 BDBM214235
US9283222, 298 BDBM214235 US9296741, 298 BDBM220709
US9296741, 298 BDBM220709 US9328106, 298 BDBM227347
US9328106, 298 BDBM227347 US9394297, 298 BDBM238638
US9394297, 298 BDBM238638 US9434725, 298 BDBM249067
US9434725, 298 BDBM249067 US9452986, 298 BDBM250994
US9452986, 298 BDBM250994 US9458110, 298 BDBM252227
US9458110, 298 BDBM252227 US9458171, 298 BDBM253338
US9458171, 298 BDBM253338 US9481672, 298 BDBM256114
US9481672, 298 BDBM256114 US9493446, 298 BDBM257829
US9493446, 298 BDBM257829 US9675593, 298 BDBM170478
US9675593, 298 BDBM170478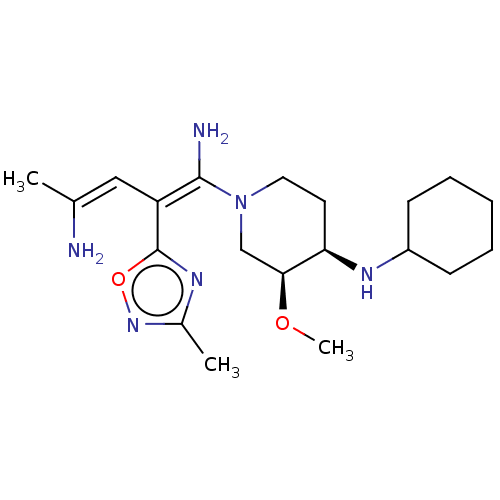 US9682966, 298 BDBM156511
US9682966, 298 BDBM156511 US9682976, 298 BDBM161869
US9682976, 298 BDBM161869 US9150546, I-298 US9718790, I-0298 BDBM183145 US9688643, I-298
US9150546, I-298 US9718790, I-0298 BDBM183145 US9688643, I-298 BDBM105788 US8575197, I-298
BDBM105788 US8575197, I-298 BDBM116144 US8633183, E-298
BDBM116144 US8633183, E-298 BDBM117618 US8653087, III-298
BDBM117618 US8653087, III-298 BDBM242556 US9422240, 1-298
BDBM242556 US9422240, 1-298 BDBM268873 US9718825, Example 298
BDBM268873 US9718825, Example 298 BDBM272454 US10065950, Example 298
BDBM272454 US10065950, Example 298 BDBM294827 US10112899, Example 298
BDBM294827 US10112899, Example 298 BDBM297182 US10112941, Example 298
BDBM297182 US10112941, Example 298 BDBM298035 US10118915, Compound 298
BDBM298035 US10118915, Compound 298 BDBM326601 US9662327, Compound 298
BDBM326601 US9662327, Compound 298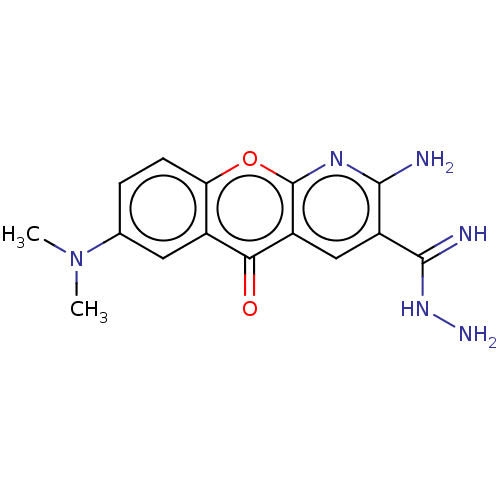 BDBM357931 US10214536, Compound 298
BDBM357931 US10214536, Compound 298 BDBM364842 US9862730, Example 298
BDBM364842 US9862730, Example 298 BDBM367603 US10227299, Example 298
BDBM367603 US10227299, Example 298 BDBM371191 US10239843, Example 298
BDBM371191 US10239843, Example 298 BDBM380303 US9926282, Example 298
BDBM380303 US9926282, Example 298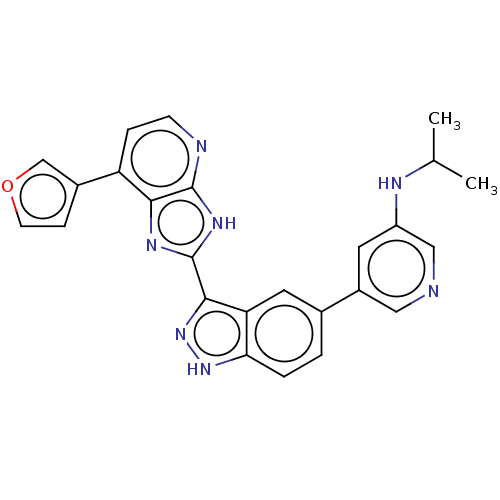 BDBM384016 US10280166, Compound 298
BDBM384016 US10280166, Compound 298 BDBM388149 US10294229, Example 298
BDBM388149 US10294229, Example 298 BDBM390411 US9951086, Example 298
BDBM390411 US9951086, Example 298 BDBM410476 US10377770, Example 298
BDBM410476 US10377770, Example 298 BDBM451703 US10710967, Example 298
BDBM451703 US10710967, Example 298 BDBM479839 US10899735, No. 298
BDBM479839 US10899735, No. 298 BDBM516208 US11053226, Example 298
BDBM516208 US11053226, Example 298 BDBM527265 US11186582, Example 298
BDBM527265 US11186582, Example 298 BDBM536606 US11242361, Compound 298
BDBM536606 US11242361, Compound 298 BDBM538390 US11254663, Example 298
BDBM538390 US11254663, Example 298 BDBM544814 US11286268, Compound 298
BDBM544814 US11286268, Compound 298 BDBM563297 US11407740, Compound 298
BDBM563297 US11407740, Compound 298 BDBM583406 US11524959, Compound 298.
BDBM583406 US11524959, Compound 298. BDBM584550 US11524968, Example 298
BDBM584550 US11524968, Example 298 BDBM588671 US11548892, Compound 298
BDBM588671 US11548892, Compound 298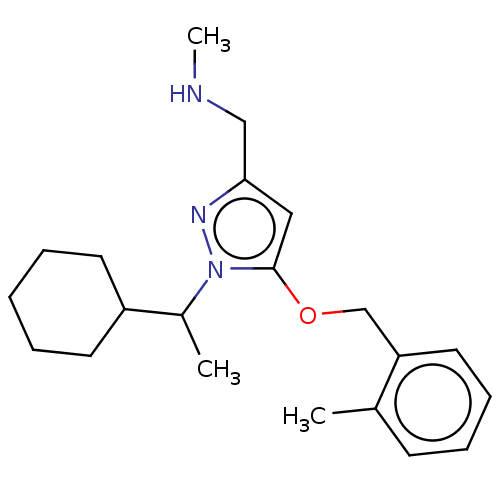 BDBM593013 US11576897, Example 298
BDBM593013 US11576897, Example 298 BDBM596798 US11596639, Example 298
BDBM596798 US11596639, Example 298 BDBM603325 US11649255, Example 298
BDBM603325 US11649255, Example 298 BDBM610850 US10626095, Example 298
BDBM610850 US10626095, Example 298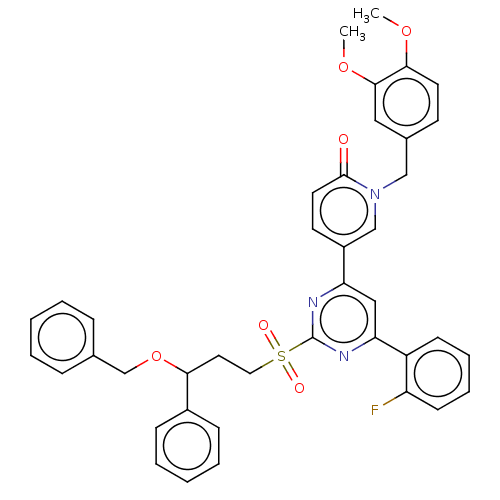 BDBM613036 US11725000, Compound 298
BDBM613036 US11725000, Compound 298 BDBM615507 US20230271949, Example 298
BDBM615507 US20230271949, Example 298 BDBM623279 US11780845, Example 298
BDBM623279 US11780845, Example 298 BDBM629999 US20230340011, Example 298.
BDBM629999 US20230340011, Example 298. BDBM631715 US20230348424, Example 298
BDBM631715 US20230348424, Example 298 BDBM633884 US11807646, Example 298
BDBM633884 US11807646, Example 298 BDBM634849 US11814367, Compound 298
BDBM634849 US11814367, Compound 298 BDBM637383 US20230382904, Compound 298
BDBM637383 US20230382904, Compound 298 BDBM638703 US11834467, Example 298
BDBM638703 US11834467, Example 298 BDBM639829 US20230391786, Example 298
BDBM639829 US20230391786, Example 298 BDBM641404 US11845723, Example 298
BDBM641404 US11845723, Example 298 BDBM647087 US20240025884, Example 298
BDBM647087 US20240025884, Example 298 BDBM655165 US11912703, Example 298
BDBM655165 US11912703, Example 298 BDBM669309 US11964973, Example 298
BDBM669309 US11964973, Example 298 BDBM670354 US11970474, Example 298
BDBM670354 US11970474, Example 298 BDBM670681 US20240140931, Compound 298
BDBM670681 US20240140931, Compound 298 BDBM681738 US20240199605, Example 298
BDBM681738 US20240199605, Example 298 BDBM686146 US20240245673, Example 298
BDBM686146 US20240245673, Example 298 BDBM689811 US20240262804, Example 298
BDBM689811 US20240262804, Example 298 BDBM695132 US20240294551, Example 298
BDBM695132 US20240294551, Example 298 BDBM697516 US20240316047, Example 298
BDBM697516 US20240316047, Example 298 BDBM718443 US20250042889, Example 298
BDBM718443 US20250042889, Example 298 BDBM723722 US20250064789, Compound 298
BDBM723722 US20250064789, Compound 298 BDBM734747 US20250129067, Compound 298
BDBM734747 US20250129067, Compound 298 BDBM735280 US20250129078, Compound 298
BDBM735280 US20250129078, Compound 298 BDBM735710 US20250129103, Compound 298
BDBM735710 US20250129103, Compound 298 BDBM739049 US20250145633, Example 298
BDBM739049 US20250145633, Example 298 BDBM745816 US12319655, Example 298
BDBM745816 US12319655, Example 298 BDBM749675 US12331033, Example 298
BDBM749675 US12331033, Example 298 BDBM752032 US20250197382, Compound 298
BDBM752032 US20250197382, Compound 298 BDBM756975 US20250221979, Example 298
BDBM756975 US20250221979, Example 298 BDBM759704 US20250236608, Example 298
BDBM759704 US20250236608, Example 298 US10112929, Example 298 BDBM295568
US10112929, Example 298 BDBM295568 US10189854, Compound 298 BDBM332334
US10189854, Compound 298 BDBM332334 US10202339, Compound 298 BDBM339519
US10202339, Compound 298 BDBM339519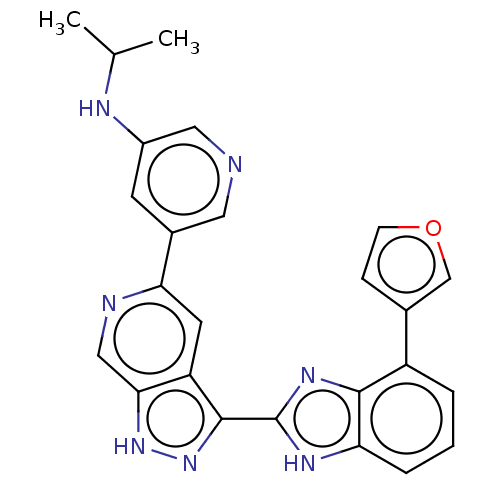 US10202377, Compound 298 BDBM346324
US10202377, Compound 298 BDBM346324 US10214519, Example 298 BDBM357542
US10214519, Example 298 BDBM357542 US10597366, Compound 298 BDBM438043
US10597366, Compound 298 BDBM438043 US10660877, Example 298 BDBM443711
US10660877, Example 298 BDBM443711 US10844044, Example 298 BDBM473287
US10844044, Example 298 BDBM473287 US10961200, Compound 298 BDBM488919
US10961200, Compound 298 BDBM488919 US10994015, Example 298 BDBM495002
US10994015, Example 298 BDBM495002 US11034692, Compound 298 BDBM504029
US11034692, Compound 298 BDBM504029 US11254668, Example 298 BDBM538955
US11254668, Example 298 BDBM538955 US11261186, Example 298 BDBM540367
US11261186, Example 298 BDBM540367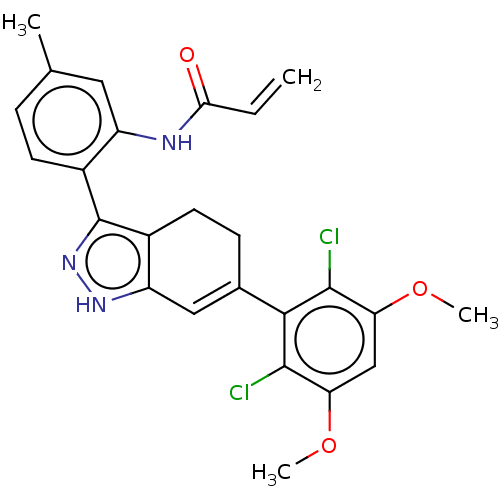 US11279697, Compound 298 BDBM543437
US11279697, Compound 298 BDBM543437 US11292791, Example 298 BDBM547025
US11292791, Example 298 BDBM547025 US11352340, Example 298 BDBM557245
US11352340, Example 298 BDBM557245 US11427558, Example 298 BDBM569151
US11427558, Example 298 BDBM569151 US11458138, Example 298 BDBM574418
US11458138, Example 298 BDBM574418 US11591336, Compound 298 BDBM596010
US11591336, Compound 298 BDBM596010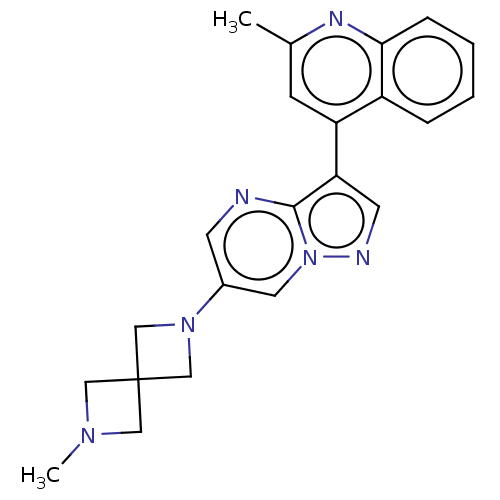 US11654147, Compound 298 BDBM603850
US11654147, Compound 298 BDBM603850 US11787780, Example 298 BDBM626590
US11787780, Example 298 BDBM626590 US11834453, Example 298 BDBM638195
US11834453, Example 298 BDBM638195 US11912686, Compound 298 BDBM654352
US11912686, Compound 298 BDBM654352 US12378224, Example 298 BDBM762056
US12378224, Example 298 BDBM762056 US12384753, Example 298 BDBM763275
US12384753, Example 298 BDBM763275 US20230286970, Compound 298 BDBM618201
US20230286970, Compound 298 BDBM618201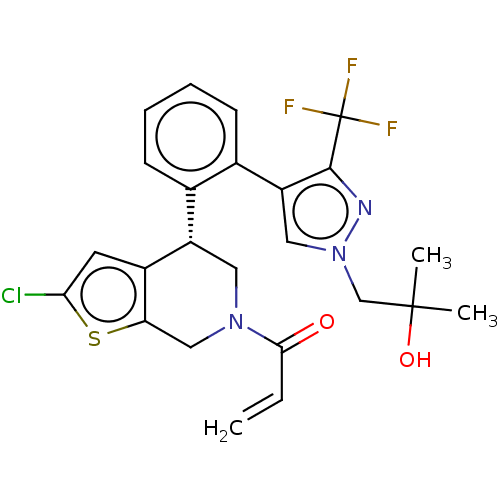 US20230303584, Compound 298 BDBM621206
US20230303584, Compound 298 BDBM621206 US20230322846, Example 298 BDBM625804
US20230322846, Example 298 BDBM625804 US20230348426, Example 298 BDBM632028
US20230348426, Example 298 BDBM632028 US20230348450, Example 298 BDBM632910
US20230348450, Example 298 BDBM632910 US20240043427, Example 298 BDBM650210
US20240043427, Example 298 BDBM650210 US20240092758, Example 298 BDBM659181
US20240092758, Example 298 BDBM659181 US20240101572, Example 298 BDBM661689
US20240101572, Example 298 BDBM661689 US20240116946, Example 298 BDBM666265
US20240116946, Example 298 BDBM666265 US20240174662, Example 298 BDBM677634
US20240174662, Example 298 BDBM677634 US20240207300, Compound 298 BDBM682542
US20240207300, Compound 298 BDBM682542 US20240218021, Example 298 BDBM684402
US20240218021, Example 298 BDBM684402 US20240246937, Example 298 BDBM686854
US20240246937, Example 298 BDBM686854 US20240246964, Compound 298 BDBM687277
US20240246964, Compound 298 BDBM687277 US20240287079, Example 298 BDBM694121
US20240287079, Example 298 BDBM694121 US20250026748, Compound 298 BDBM715134
US20250026748, Compound 298 BDBM715134 US20250059174, Example 298 BDBM722037
US20250059174, Example 298 BDBM722037 US20250059220, Compound 298 BDBM723186
US20250059220, Compound 298 BDBM723186 US20250122174, Example 298 BDBM730715
US20250122174, Example 298 BDBM730715 US20250170122, Compound 298 BDBM744370
US20250170122, Compound 298 BDBM744370 US9546153, ex. 298 BDBM210175
US9546153, ex. 298 BDBM210175 US9763922, Example 298 BDBM340774
US9763922, Example 298 BDBM340774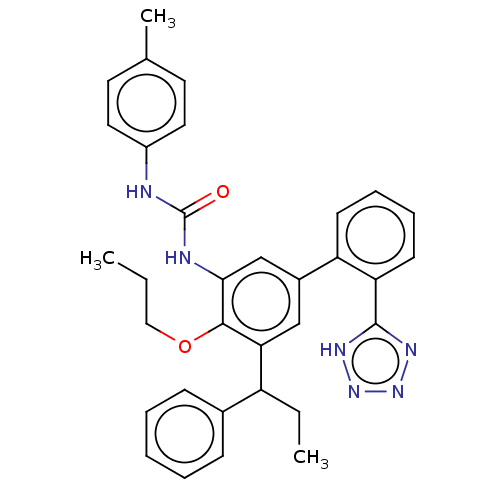 US9765018, Example 298 BDBM340838
US9765018, Example 298 BDBM340838 US9771320, Example 298 BDBM342064
US9771320, Example 298 BDBM342064 US9777008, Compound 298 BDBM344004
US9777008, Compound 298 BDBM344004 US9873693, Compound 298 BDBM366368
US9873693, Compound 298 BDBM366368 US9884814, Compound 298 BDBM275685
US9884814, Compound 298 BDBM275685 US9926281, Compound 298 BDBM379807
US9926281, Compound 298 BDBM379807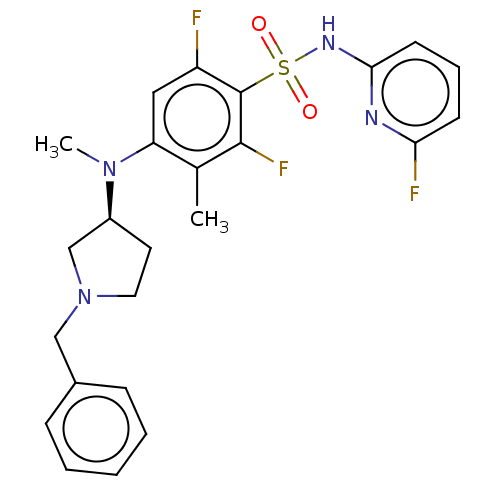 US10662184, Example 298 US10246453, Example 298 US11299490, Example 298 BDBM374031 US10815229, Example 298 (S)-4-((1-benzylpyrrolidin-3-yl)(methyl)amino)-2,6-difluoro-N-(6-fluoropyridin-2-yl)-3-methylbenzenesulfonamide formate
US10662184, Example 298 US10246453, Example 298 US11299490, Example 298 BDBM374031 US10815229, Example 298 (S)-4-((1-benzylpyrrolidin-3-yl)(methyl)amino)-2,6-difluoro-N-(6-fluoropyridin-2-yl)-3-methylbenzenesulfonamide formate BDBM129161 US8802674, 298 US8802674, 334
BDBM129161 US8802674, 298 US8802674, 334 BDBM196434 US9212130, I-298 BDBM196374
BDBM196434 US9212130, I-298 BDBM196374 BDBM2013 US8501936, 298 US8501936, 300
BDBM2013 US8501936, 298 US8501936, 300 BDBM246646 US9550763, Compound I-298
BDBM246646 US9550763, Compound I-298 BDBM346697 US10202379, Reference Example 298
BDBM346697 US10202379, Reference Example 298 BDBM441104 US10640495, Example I-298
BDBM441104 US10640495, Example I-298 BDBM454167 US10716791, Code KP-298
BDBM454167 US10716791, Code KP-298 BDBM455290 US10730874, Compound I-298
BDBM455290 US10730874, Compound I-298 BDBM464966 US10793563, Compound I-298
BDBM464966 US10793563, Compound I-298 BDBM465647 US10793568, Compound I-298
BDBM465647 US10793568, Compound I-298 BDBM557652 US11352356, Compound I-298
BDBM557652 US11352356, Compound I-298 BDBM566929 US11420958, Ex. No. 298
BDBM566929 US11420958, Ex. No. 298 BDBM582289 US11518764, Compound I-298
BDBM582289 US11518764, Compound I-298 BDBM606654 US11685732, Compound I-298
BDBM606654 US11685732, Compound I-298 BDBM617726 US20230286948, Compound HYBI-298
BDBM617726 US20230286948, Compound HYBI-298 BDBM640739 US20230399319, Example 2-298
BDBM640739 US20230399319, Example 2-298 BDBM728667 US20250092056, Compound I-298
BDBM728667 US20250092056, Compound I-298 BDBM742028 US20250163057, Compound I-298
BDBM742028 US20250163057, Compound I-298 CHEMBL4128967 BDBM50271428 US11225469, Compound 298
CHEMBL4128967 BDBM50271428 US11225469, Compound 298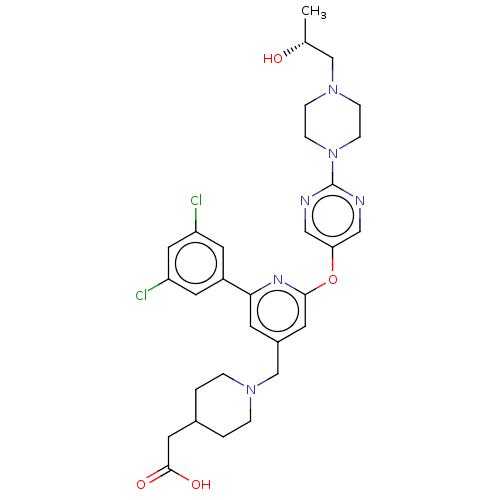 CHEMBL5175809 BDBM50601759 US11773078, Example 298
CHEMBL5175809 BDBM50601759 US11773078, Example 298 US10144742, Compound I-298 BDBM306136
US10144742, Compound I-298 BDBM306136 US10150728, Example I-298 BDBM307085
US10150728, Example I-298 BDBM307085 US10576064, Example I-298 BDBM432051
US10576064, Example I-298 BDBM432051 US10618902, Compound I-298 BDBM438617
US10618902, Compound I-298 BDBM438617 US10919885, Compound No. 298 BDBM483149
US10919885, Compound No. 298 BDBM483149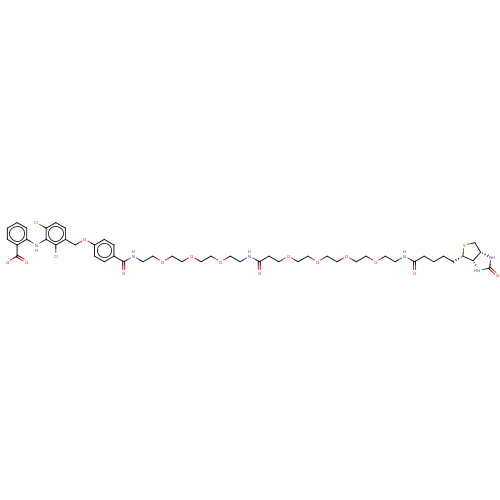 US11173137, Compound AK3-298 BDBM525306
US11173137, Compound AK3-298 BDBM525306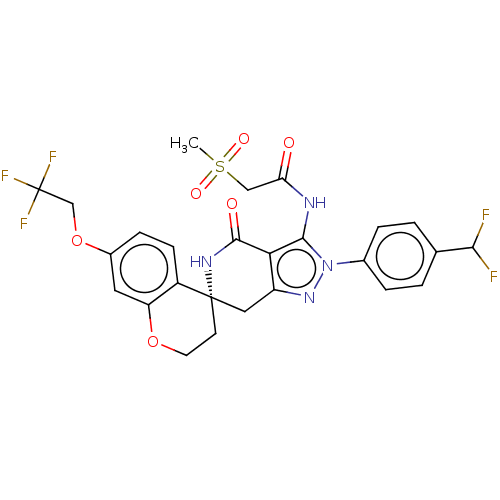 US11198695, Example II-298 BDBM529360
US11198695, Example II-298 BDBM529360 US11339144, Compound I-298 BDBM554849
US11339144, Compound I-298 BDBM554849 US11555012, Compound I-298 BDBM589557
US11555012, Compound I-298 BDBM589557 US12053473, Example I-298 BDBM689452
US12053473, Example I-298 BDBM689452 US20230390274, Compound A-298 BDBM638981
US20230390274, Compound A-298 BDBM638981 US20240150321, Compound I-298 BDBM673987
US20240150321, Compound I-298 BDBM673987 US20250019387, Table 1a.298 BDBM713573
US20250019387, Table 1a.298 BDBM713573 US20250090540, Example I-298 BDBM727437
US20250090540, Example I-298 BDBM727437 US9212153, 354,Ex. 298 BDBM195279
US9212153, 354,Ex. 298 BDBM195279 US9725442, Compound I-298 BDBM330730
US9725442, Compound I-298 BDBM330730 US9802960, Compound I-298 BDBM352719
US9802960, Compound I-298 BDBM352719 BDBM268265 4-(3-fluorophenyl)-2-(morpholin- 4-yl)-8-(1H-pyrazol-5-yl)-1,7- naphthyridine US9549932, 298 US10772893, Example 298 US11529356, Example 298
BDBM268265 4-(3-fluorophenyl)-2-(morpholin- 4-yl)-8-(1H-pyrazol-5-yl)-1,7- naphthyridine US9549932, 298 US10772893, Example 298 US11529356, Example 298 BDBM561761 Step a US11390631, Example 298
BDBM561761 Step a US11390631, Example 298 BDBM731857 Step b US12268694, Example 298
BDBM731857 Step b US12268694, Example 298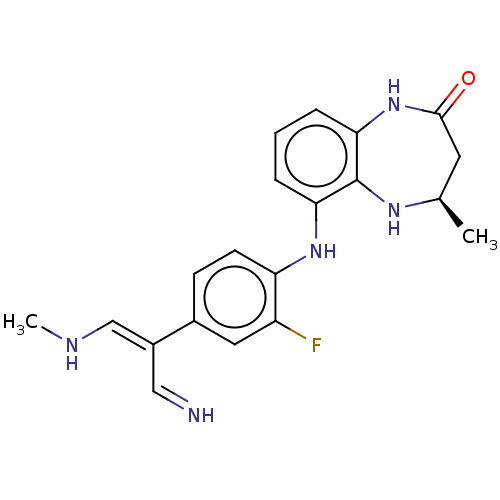 US10206931, Example 298 methylpyrazol-4- BDBM349490
US10206931, Example 298 methylpyrazol-4- BDBM349490 US11479560, Title WZ-I-298 BDBM579342
US11479560, Title WZ-I-298 BDBM579342 1-(5-(3-cyano-6-(2- hydroxy-2- methylpropoxy)pyrazolo [1,5-a]pyridin-4-yl)pyridin- 2-yl)-4-methyl-N- ((tetrahydro-2H-pyran-3- yl)methyl)piperidine-4- carboxamide US11648243, Example 298 US10144734, Example 298 US10172845, Example 298 US10441581, Example 298 US10881652, Example 298 BDBM305282
1-(5-(3-cyano-6-(2- hydroxy-2- methylpropoxy)pyrazolo [1,5-a]pyridin-4-yl)pyridin- 2-yl)-4-methyl-N- ((tetrahydro-2H-pyran-3- yl)methyl)piperidine-4- carboxamide US11648243, Example 298 US10144734, Example 298 US10172845, Example 298 US10441581, Example 298 US10881652, Example 298 BDBM305282 methyl cis-3- ((methylsulfonyl)amino)-2-(((cis-4- (3-(trifluoromethoxy)phenyl)- cyclohexyl)oxy)methyl)piperidine-1- carboxylate US11292766, Example 298 US10508083, Example 298 BDBM386853 US10287305, Example 298
methyl cis-3- ((methylsulfonyl)amino)-2-(((cis-4- (3-(trifluoromethoxy)phenyl)- cyclohexyl)oxy)methyl)piperidine-1- carboxylate US11292766, Example 298 US10508083, Example 298 BDBM386853 US10287305, Example 298 US10017468, Compound 298 US11072582, Compound 298 US10781171, Compound 298 BDBM404102 (2S,5R)-5-(2-chlorophenyl)-1-(2'-fluoro-4'-methoxy-[1,1'-biphenyl]-4-carbonyl)pyrrolidine-2-carboxylic acid
US10017468, Compound 298 US11072582, Compound 298 US10781171, Compound 298 BDBM404102 (2S,5R)-5-(2-chlorophenyl)-1-(2'-fluoro-4'-methoxy-[1,1'-biphenyl]-4-carbonyl)pyrrolidine-2-carboxylic acid BDBM219624 US9303033, M31, Table 37A, Compound 298
BDBM219624 US9303033, M31, Table 37A, Compound 298 BDBM410474 US10377770, Example 298 US10377770, Example 296
BDBM410474 US10377770, Example 298 US10377770, Example 296 BDBM417679 US10457669, Example 298 US11001575, Example 299
BDBM417679 US10457669, Example 298 US11001575, Example 299 BDBM563984 Roche-Dataset for PDE10A, Compound 298
BDBM563984 Roche-Dataset for PDE10A, Compound 298 BDBM613557 (General Procedure T) US11731943, Example 298
BDBM613557 (General Procedure T) US11731943, Example 298 BDBM684399 US20240218021, Example 296 US20240218021, Example 298
BDBM684399 US20240218021, Example 296 US20240218021, Example 298 US10112929, Example 298 US10112929, Example 297 BDBM295567
US10112929, Example 298 US10112929, Example 297 BDBM295567 US11420970, Example 298 US11420970, Example 299 BDBM568096
US11420970, Example 298 US11420970, Example 299 BDBM568096 US11584747, Example 298 US11584747, Example 299 BDBM594491
US11584747, Example 298 US11584747, Example 299 BDBM594491 US20250025443, Compound 298 US20250025443, Example 187 BDBM714153
US20250025443, Compound 298 US20250025443, Example 187 BDBM714153 US20250025443, Example 298 US20250025443, Compound 431 BDBM714264
US20250025443, Example 298 US20250025443, Compound 431 BDBM714264 US9884868, Example 298' 6-(2-amino-1-(2,2-difluoroethyl)-4-(4-fluorophenyl)- 1H-imidazol-5-yl)imidazo[1,2-a]pyridine-3-carbonitrile US11352360, Example 298' BDBM283098 US10287295, Example 298'
US9884868, Example 298' 6-(2-amino-1-(2,2-difluoroethyl)-4-(4-fluorophenyl)- 1H-imidazol-5-yl)imidazo[1,2-a]pyridine-3-carbonitrile US11352360, Example 298' BDBM283098 US10287295, Example 298' BDBM316801 US11111242, Example 298 US20250134869, Example 298 2-[(2S,5R)-4-acryloyl-2,5-dimethylpiperazin-1-yl]-N-(propan-2-yl)-5H-pyrrolo[2,3-b]pyrazine-7-carboxamide US9617258, Example 298
BDBM316801 US11111242, Example 298 US20250134869, Example 298 2-[(2S,5R)-4-acryloyl-2,5-dimethylpiperazin-1-yl]-N-(propan-2-yl)-5H-pyrrolo[2,3-b]pyrazine-7-carboxamide US9617258, Example 298 US10071988, Example 298 US10233173, Example 298 [2-[[1-[3-(1,1-Difluoroethyl)phenyl]triazol-4-yl]methoxy]pyrimidin-5- BDBM276359
US10071988, Example 298 US10233173, Example 298 [2-[[1-[3-(1,1-Difluoroethyl)phenyl]triazol-4-yl]methoxy]pyrimidin-5- BDBM276359 US10112941, Example 297 US10112941, Example 298 BDBM297181 BDBM297328
US10112941, Example 297 US10112941, Example 298 BDBM297181 BDBM297328 US10023557, Example 298 BDBM283652 N-((1-aminoisoquinolin-6-yl)methyl)-2-((3- aminoquinolin-6-yl)methyl)isonicotinamide US10308637, Example 298
US10023557, Example 298 BDBM283652 N-((1-aminoisoquinolin-6-yl)methyl)-2-((3- aminoquinolin-6-yl)methyl)isonicotinamide US10308637, Example 298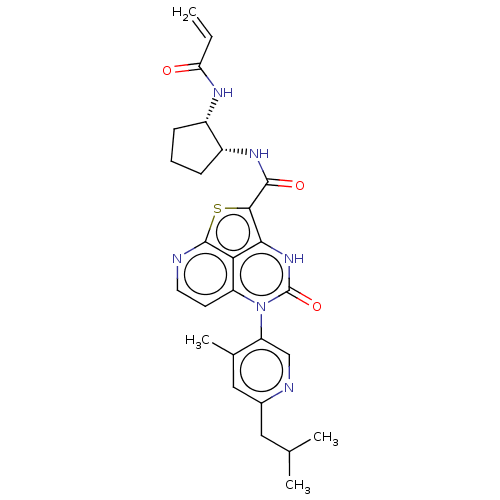 N-((1R,2S)-2-Acrylamidocyclopentyl)-5-(S)-(6-isobutyl-4- methylpyridin-3-yl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide US12065446, Example 298 BDBM485462 US10934310, Ex # 298 US11319329, Ex # 298
N-((1R,2S)-2-Acrylamidocyclopentyl)-5-(S)-(6-isobutyl-4- methylpyridin-3-yl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide US12065446, Example 298 BDBM485462 US10934310, Ex # 298 US11319329, Ex # 298 Roche-Dataset for PDE10A, Compound 689 US8703768, 298 BDBM120697
Roche-Dataset for PDE10A, Compound 689 US8703768, 298 BDBM120697 US9409866, 297 US9409866, 298 US9409866, 295 BDBM240580 US9409866, 296
US9409866, 297 US9409866, 298 US9409866, 295 BDBM240580 US9409866, 296 5-(4-(3,4- difluorobenzoyl) piperidine-1-carbonyl)- N-(1-(3,5- difluorobenzyl)piperidin- 4-yl)picolinamide BDBM328160 US10377742, Compound 298 US9663496, Compound 298
5-(4-(3,4- difluorobenzoyl) piperidine-1-carbonyl)- N-(1-(3,5- difluorobenzyl)piperidin- 4-yl)picolinamide BDBM328160 US10377742, Compound 298 US9663496, Compound 298 BDBM436963 US11207298, Example 298 US10617676, Example 298 6-(3,4-Difluorophenyl)-1-[(5-fluoro-3-pyridyl)methyl]-3H-imidazo[4,5- b]pyridin-2-one
BDBM436963 US11207298, Example 298 US10617676, Example 298 6-(3,4-Difluorophenyl)-1-[(5-fluoro-3-pyridyl)methyl]-3H-imidazo[4,5- b]pyridin-2-one BDBM510438 US11439633, Example 298 US11077100, Example 298 (5S,8S)-N-(2,4- dichlorobenzyl)-5- fluoro-8-hydroxy-8- ((methylsulfonyl) methyl)-5,6,7,8- tetrahydroquinoline- 5-carboxamide
BDBM510438 US11439633, Example 298 US11077100, Example 298 (5S,8S)-N-(2,4- dichlorobenzyl)-5- fluoro-8-hydroxy-8- ((methylsulfonyl) methyl)-5,6,7,8- tetrahydroquinoline- 5-carboxamide US10988451, Example 298 BDBM449686 US10703733, Example 299 US11643400, Example 299
US10988451, Example 298 BDBM449686 US10703733, Example 299 US11643400, Example 299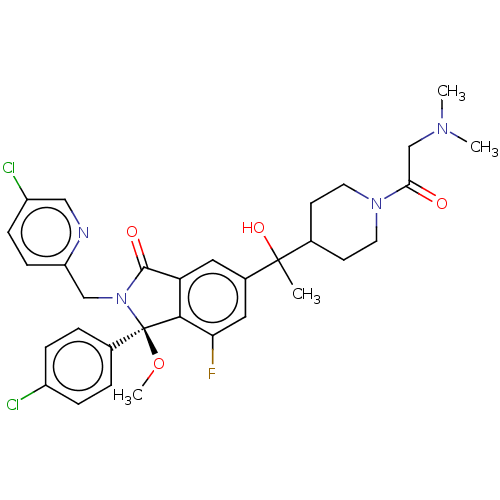 US11236047, Example 299 BDBM535385 US11236047, Example 298 US20230338337, Compound 299
US11236047, Example 299 BDBM535385 US11236047, Example 298 US20230338337, Compound 299 US20240217978, Example 298 BDBM683642 (S)-(1-(7-amino-2-(5-
US20240217978, Example 298 BDBM683642 (S)-(1-(7-amino-2-(5- US11078201, Compound I-298 BDBM500638 7-((5-(4- hydroxy-4- (methoxymeth- yl)piperidin-1- yl)pyridin-2- yl)amino)-4- (1-methyl-1H- pyrrolo[2,3- b]pyridin-4- yl)isoindolin- 1-one US11021481, Compound I-298 US11548890, Compound I-298
US11078201, Compound I-298 BDBM500638 7-((5-(4- hydroxy-4- (methoxymeth- yl)piperidin-1- yl)pyridin-2- yl)amino)-4- (1-methyl-1H- pyrrolo[2,3- b]pyridin-4- yl)isoindolin- 1-one US11021481, Compound I-298 US11548890, Compound I-298 (1R,3S)-3-(3-{[(5-methyl-1,3,4- oxadiazol-2-yl)acetyl]amino}- 1H-pyrazol-5-yl)cyclopentyl tert-butylcarbamate US11718603, Example 298 BDBM498473 US11014911, Example 298
(1R,3S)-3-(3-{[(5-methyl-1,3,4- oxadiazol-2-yl)acetyl]amino}- 1H-pyrazol-5-yl)cyclopentyl tert-butylcarbamate US11718603, Example 298 BDBM498473 US11014911, Example 298 N-{3-[({2-[(4-aminopyrimidin-2-yl)amino]- 5-(trifluoromethyl)pyrimidin-4- yl}amino)methyl]pyridin-2-yl}-N- methylmethane-sulfonamide (298) BDBM418798 US10450297, Example 298
N-{3-[({2-[(4-aminopyrimidin-2-yl)amino]- 5-(trifluoromethyl)pyrimidin-4- yl}amino)methyl]pyridin-2-yl}-N- methylmethane-sulfonamide (298) BDBM418798 US10450297, Example 298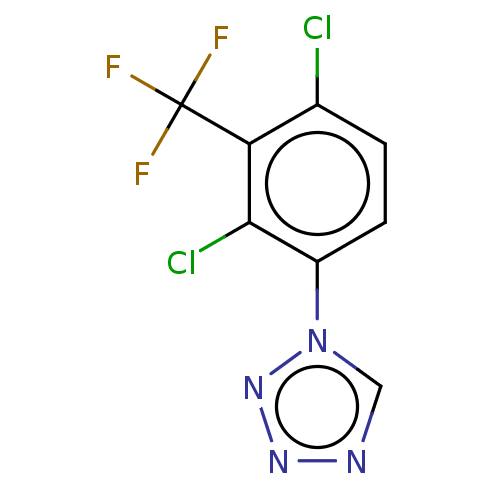 1-(2,4-dichloro-3-(trifluoromethyl)phenyl)-1H-tetrazole BDBM601035 US11634391, Compound 298
1-(2,4-dichloro-3-(trifluoromethyl)phenyl)-1H-tetrazole BDBM601035 US11634391, Compound 298 N-((1r,4r)-4-aminocyclohexyl)pyrazine-2-carboxamide US10266526, Compound 298 BDBM378168
N-((1r,4r)-4-aminocyclohexyl)pyrazine-2-carboxamide US10266526, Compound 298 BDBM378168 BDBM322928 2-(azepan-1-yl)-4-((4-(4-(2-fluoroethyl) piperidin-1-yl)phenyl)amino) pyrimido[4,5-d]pyridazin-5-(6H)-one US10647720, Ex. # 298 US10183944, Example 298
BDBM322928 2-(azepan-1-yl)-4-((4-(4-(2-fluoroethyl) piperidin-1-yl)phenyl)amino) pyrimido[4,5-d]pyridazin-5-(6H)-one US10647720, Ex. # 298 US10183944, Example 298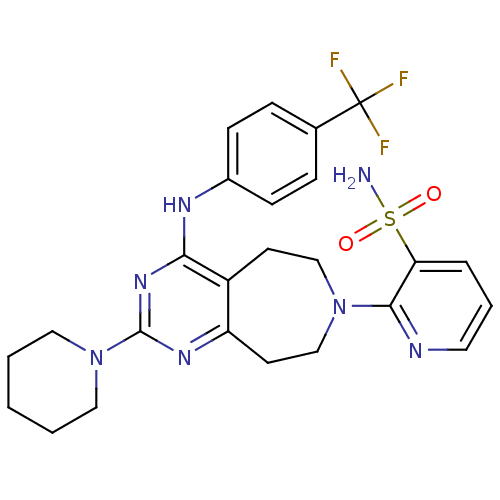 CHEMBL1290373 BDBM50331146 2-(2-(piperidin-1-yl)-4-(4-(trifluoromethyl)phenylamino)-8,9-dihydro-5H-pyrimido[5,4-d]azepin-7(6H)-yl)pyridine-3-sulfonamide US9422293, 298 US9738649, Example 298
CHEMBL1290373 BDBM50331146 2-(2-(piperidin-1-yl)-4-(4-(trifluoromethyl)phenylamino)-8,9-dihydro-5H-pyrimido[5,4-d]azepin-7(6H)-yl)pyridine-3-sulfonamide US9422293, 298 US9738649, Example 298 US11020380, Example 298 BDBM437564 US10617680, Example 298 4-(4-Methoxy-phenyl)-1-methyl-5-{1-[2-(1H-tetrazol-5- yl)-phenyl]-1H-pyrazol-4-yl}-1H-pyridin-2-one
US11020380, Example 298 BDBM437564 US10617680, Example 298 4-(4-Methoxy-phenyl)-1-methyl-5-{1-[2-(1H-tetrazol-5- yl)-phenyl]-1H-pyrazol-4-yl}-1H-pyridin-2-one US10377753, Example 298 1-(Azetidin-1-yl)-2-[3-chloro-6-[3- BDBM409429
US10377753, Example 298 1-(Azetidin-1-yl)-2-[3-chloro-6-[3- BDBM409429 US11420958, Ex. No. 292 US11420958, Ex. No. 312 US11420958, Ex. No. 298 BDBM566923
US11420958, Ex. No. 292 US11420958, Ex. No. 312 US11420958, Ex. No. 298 BDBM566923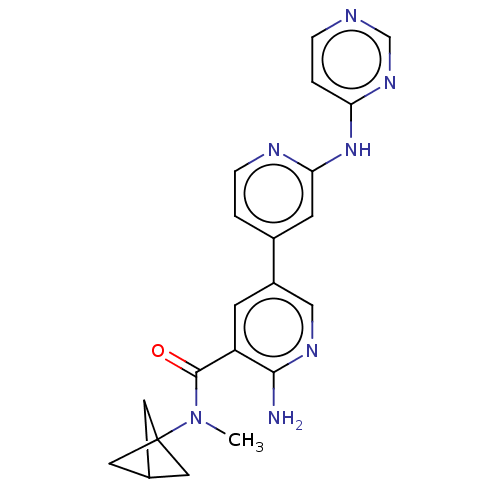 US9751854, Compound I-303 US9802960, Compound I-303 US9751854, Compound I-298 BDBM338122
US9751854, Compound I-303 US9802960, Compound I-303 US9751854, Compound I-298 BDBM338122 BDBM467645 US10800792, Example 298 5-(2-Methyl-4-phenoxyphenyl)-N-(2-(1-methylpyrrolidin-2-yl)ethyl)- 4-oxo-4,5-dihydro-3H-1-thia-3,5,8-triazaacenaphthylene-2- carboxamide; US10822348, Example 298
BDBM467645 US10800792, Example 298 5-(2-Methyl-4-phenoxyphenyl)-N-(2-(1-methylpyrrolidin-2-yl)ethyl)- 4-oxo-4,5-dihydro-3H-1-thia-3,5,8-triazaacenaphthylene-2- carboxamide; US10822348, Example 298 N-(pyridin-2-ylmethyl)-1-(4-{4-[2- (pyridin-3-yl)acetamido]-1H-1,2,3- triazol-1-yl}butyl)-1H-1,2,3-triazole- 4-carboxamide US11370786, Example 298 US10344025, Example 298 BDBM404954
N-(pyridin-2-ylmethyl)-1-(4-{4-[2- (pyridin-3-yl)acetamido]-1H-1,2,3- triazol-1-yl}butyl)-1H-1,2,3-triazole- 4-carboxamide US11370786, Example 298 US10344025, Example 298 BDBM404954 US10544130, Example 298 (R)-4-(2-amino-1H-benzo[d]imidazol-4-yl)-N1-(3-amino-2-hydroxypropyl)-3-(2H-tetrazol-5-yl)-benzene-1,2-disulfonamide US10221163, Example 298 BDBM361141
US10544130, Example 298 (R)-4-(2-amino-1H-benzo[d]imidazol-4-yl)-N1-(3-amino-2-hydroxypropyl)-3-(2H-tetrazol-5-yl)-benzene-1,2-disulfonamide US10221163, Example 298 BDBM361141 US11485745, Example 298 US10730889, Example 298 N-[[6-(2-tert-butyl-4-methyl- pyrazole-3-carbonyl)-6- azaspiro[2.5]octan-2- yl]methyl]furo[2,3-c]pyridine-2- carboxamide BDBM455705
US11485745, Example 298 US10730889, Example 298 N-[[6-(2-tert-butyl-4-methyl- pyrazole-3-carbonyl)-6- azaspiro[2.5]octan-2- yl]methyl]furo[2,3-c]pyridine-2- carboxamide BDBM455705 2-(2-isopropylphenyl)-9-(4-(1-methyl-1H-1,2,3- BDBM522740 US11161848, Compound I-298
2-(2-isopropylphenyl)-9-(4-(1-methyl-1H-1,2,3- BDBM522740 US11161848, Compound I-298 BDBM315117 (5-methyl-3-(pyrimidin-2-yl)pyridin-2-yl)((1S,4R,6R)-6-((5-(trifluoromethyl)pyridin-2-yl)oxy)-2-azabicyclo[2.2.1]heptan-2-yl)methanone US10183953, Example 298 US9611277, Example 298
BDBM315117 (5-methyl-3-(pyrimidin-2-yl)pyridin-2-yl)((1S,4R,6R)-6-((5-(trifluoromethyl)pyridin-2-yl)oxy)-2-azabicyclo[2.2.1]heptan-2-yl)methanone US10183953, Example 298 US9611277, Example 298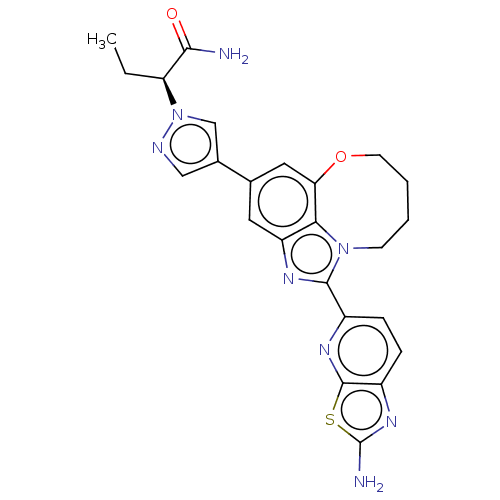 US10065970, Example 298 (S)-2-(4-(1-(2- aminothiazolo[5,4- b]pyridin-5-yl)- 7,8,9,10-tetrahydro-6- oxa-2,10a- diazacycloocta[cd]inden- 4-yl)-1H-pyrazol-1- yl)butanamide BDBM272993 US10435414, Example 298
US10065970, Example 298 (S)-2-(4-(1-(2- aminothiazolo[5,4- b]pyridin-5-yl)- 7,8,9,10-tetrahydro-6- oxa-2,10a- diazacycloocta[cd]inden- 4-yl)-1H-pyrazol-1- yl)butanamide BDBM272993 US10435414, Example 298 5-(4-ethylphenoxy)- 1H-1,2,3-triazole-4- carboxylic acid BDBM560927 US11389456, Ex. No. I-298
5-(4-ethylphenoxy)- 1H-1,2,3-triazole-4- carboxylic acid BDBM560927 US11389456, Ex. No. I-298 BDBM396671 [2-(Azetidin-3-yloxy)-4-chloro-phenyl]-(3-chloro-phenyl)-methanone US9981909, Example 298
BDBM396671 [2-(Azetidin-3-yloxy)-4-chloro-phenyl]-(3-chloro-phenyl)-methanone US9981909, Example 298 BDBM691837 US20240279215, Compound 298 4-chlorobenzyl (4-((6- (difluoromethyl)-N- methylnicotinamido)meth- yl)phenyl)carbamate
BDBM691837 US20240279215, Compound 298 4-chlorobenzyl (4-((6- (difluoromethyl)-N- methylnicotinamido)meth- yl)phenyl)carbamate US10189841, Compound I-298 2-(2-isopropylphenyl)-9-(4-(1-methyl-1H-1,2,3-triazol- BDBM325092
US10189841, Compound I-298 2-(2-isopropylphenyl)-9-(4-(1-methyl-1H-1,2,3-triazol- BDBM325092 BDBM320131 US10174016, Example 298 3-[5-(propan-2-yl)- 1,3-thiazol-2-yl]-5- [(3S)- tetrahydrofuran-3- ylmethoxy]-N- {(1R)-1-[6- (trifluoromethyl) pyridazin-3- yl]ethyl}benzamide US10202369, Example 299 US10472354, Example 298
BDBM320131 US10174016, Example 298 3-[5-(propan-2-yl)- 1,3-thiazol-2-yl]-5- [(3S)- tetrahydrofuran-3- ylmethoxy]-N- {(1R)-1-[6- (trifluoromethyl) pyridazin-3- yl]ethyl}benzamide US10202369, Example 299 US10472354, Example 298 US10434101, Compound I-8 US11096942, Compound I-8 US9796700, Compound I-298 BDBM149408 US8975249, I-8
US10434101, Compound I-8 US11096942, Compound I-8 US9796700, Compound I-298 BDBM149408 US8975249, I-8 3-(3-Bromo-1H-indazol-5-yl)-2-isopropyl-5-(trifluoromethyl)imidazo[4,5- US11312712, Example 298 BDBM551181
3-(3-Bromo-1H-indazol-5-yl)-2-isopropyl-5-(trifluoromethyl)imidazo[4,5- US11312712, Example 298 BDBM551181 3-(6-Aminopyridin-3-yl)-2-(1H-tetrazol-5-yl)benzene sulfonamide, TFA salt BDBM262446 US9708336, 298
3-(6-Aminopyridin-3-yl)-2-(1H-tetrazol-5-yl)benzene sulfonamide, TFA salt BDBM262446 US9708336, 298 8-Bromo-3-[2-ethylsulfanyl-5-(trifluoromethoxy)anilino]-6-(trifluoromethyl)quinazolin-4-one BDBM401061 US10005739, Example 298
8-Bromo-3-[2-ethylsulfanyl-5-(trifluoromethoxy)anilino]-6-(trifluoromethyl)quinazolin-4-one BDBM401061 US10005739, Example 298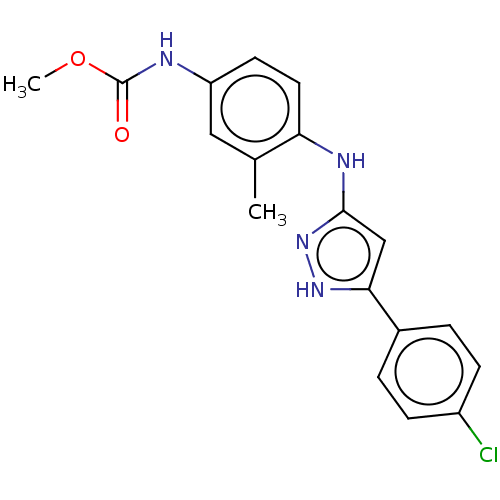 BDBM579821 methyl (4-((5-(4-chlorophenyl)-1H- pyrazol-3-yl)amino)-3- methylphenyl)carbamate US11485711, Compound 298
BDBM579821 methyl (4-((5-(4-chlorophenyl)-1H- pyrazol-3-yl)amino)-3- methylphenyl)carbamate US11485711, Compound 298 US11845767, Example 298 BDBM642253 (5-amino-5-(1-(4-bromobenzyl)-1H- tetrazol-5-yl)pentyl)boronic acid
US11845767, Example 298 BDBM642253 (5-amino-5-(1-(4-bromobenzyl)-1H- tetrazol-5-yl)pentyl)boronic acid US9855269, Compound 298 6-[3-[(cyclopropylmethylamino)methyl]-5-fluoro-phenyl]pyrido[3,2-d]pyrimidin-4-amine BDBM364055
US9855269, Compound 298 6-[3-[(cyclopropylmethylamino)methyl]-5-fluoro-phenyl]pyrido[3,2-d]pyrimidin-4-amine BDBM364055 BDBM261240 US10092570, Example 263 US11433071, Example 263 US9730939, Example 263 US10646492, Example 263 US10376513, Example 298 US9707233, 263
BDBM261240 US10092570, Example 263 US11433071, Example 263 US9730939, Example 263 US10646492, Example 263 US10376513, Example 298 US9707233, 263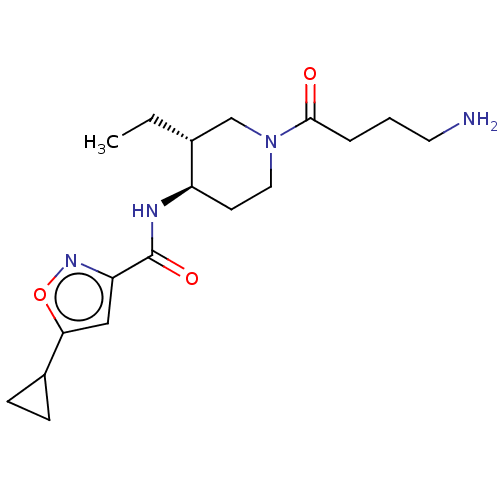 BDBM432598 US10577363, Compound 298 N-((3R,4R)-1-(4-aminobutanoyl)-3- ethylpiperidin-4-yl)-5- cyclopropylisoxazole-3-carboxamide
BDBM432598 US10577363, Compound 298 N-((3R,4R)-1-(4-aminobutanoyl)-3- ethylpiperidin-4-yl)-5- cyclopropylisoxazole-3-carboxamide BDBM543287 US11279687, Compound 298 1-(4-{[4-(morpholin-4- yl)benzene]sulfonyl}phenyl)-3-(pyridin-3- ylmethyl)urea
BDBM543287 US11279687, Compound 298 1-(4-{[4-(morpholin-4- yl)benzene]sulfonyl}phenyl)-3-(pyridin-3- ylmethyl)urea BDBM660912 (S)-N-(6-chloropyridin-3-yl)-6- ((tetrahydrofuran-3- yl)oxy)isoquinolin-1-amine US20240101531, Example 298
BDBM660912 (S)-N-(6-chloropyridin-3-yl)-6- ((tetrahydrofuran-3- yl)oxy)isoquinolin-1-amine US20240101531, Example 298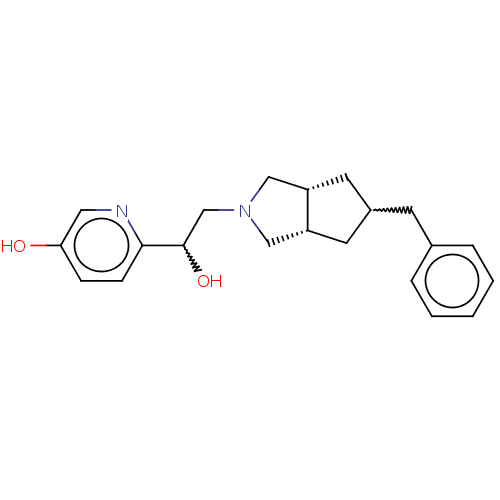 rac-6-(2-{5-benzyl- octahydrocyclopenta[c] pyrrol-2-yl}-1- hydroxyethyl)pyridin-3- ol US10052306, 298 BDBM238859
rac-6-(2-{5-benzyl- octahydrocyclopenta[c] pyrrol-2-yl}-1- hydroxyethyl)pyridin-3- ol US10052306, 298 BDBM238859 1-({5-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3- yl]pyridin- 2-yl} methoxy) phthalazine BDBM504890 US11066396, Example 298
1-({5-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3- yl]pyridin- 2-yl} methoxy) phthalazine BDBM504890 US11066396, Example 298 5-(4-(2-(2-methylpyridin-4- yl)ethynyl)phenoxy)-1H-1,2,3- triazole-4-carboxylic acid BDBM581202 US11504367, Example 298
5-(4-(2-(2-methylpyridin-4- yl)ethynyl)phenoxy)-1H-1,2,3- triazole-4-carboxylic acid BDBM581202 US11504367, Example 298 6-((2-aminoethyl)sulfonyl)-3- (1H-indazol-7-yl)-2-(1H- tetrazol-5-yl) benzenesulfonamide US10227331, Example 298 BDBM368111
6-((2-aminoethyl)sulfonyl)-3- (1H-indazol-7-yl)-2-(1H- tetrazol-5-yl) benzenesulfonamide US10227331, Example 298 BDBM368111 BDBM480237 US10899738, Cpd. No 298 4-(3-(4-(1-cyclohexyl- 1,2,3,4- tetrahydroisoquinolin-1- yl)piperidin-1- yl)propoxy)benzonitrile
BDBM480237 US10899738, Cpd. No 298 4-(3-(4-(1-cyclohexyl- 1,2,3,4- tetrahydroisoquinolin-1- yl)piperidin-1- yl)propoxy)benzonitrile BDBM494249 US10988478, Example 298 N-(6- (Cyclopropylmethoxy)- 2,2- dimethyl-2,3- dihydrobenzofuran-5- yl)pyrazolo[1,5- alpha]pyrimidine-3- carboxamide
BDBM494249 US10988478, Example 298 N-(6- (Cyclopropylmethoxy)- 2,2- dimethyl-2,3- dihydrobenzofuran-5- yl)pyrazolo[1,5- alpha]pyrimidine-3- carboxamide (2S)-1-(benzofuran-2-ylsulfonyl)-N- [[3-[[4-(trifluoromethyl)phenoxy]- methyl]phenyl]methyl]pyrrolidine-2- carboxamide BDBM611405 US10626112, Example 298
(2S)-1-(benzofuran-2-ylsulfonyl)-N- [[3-[[4-(trifluoromethyl)phenoxy]- methyl]phenyl]methyl]pyrrolidine-2- carboxamide BDBM611405 US10626112, Example 298 1-(2,3'-difluoro-5-methoxy-4-biphenylyl)-N-3-isoxazolyl-2-oxo-1,2-dihydro-6-quinolinesulfonamide US9776995, Example 298 BDBM343128
1-(2,3'-difluoro-5-methoxy-4-biphenylyl)-N-3-isoxazolyl-2-oxo-1,2-dihydro-6-quinolinesulfonamide US9776995, Example 298 BDBM343128 4-(7,8-difluoro-3- quinolylamino)-2-{3- methoxy-4-[(1s,3s)-3- (dimethylamino)cyclobutoxy] phenylamino}pyrimidine BDBM745002 US20250171431, Compound 298
4-(7,8-difluoro-3- quinolylamino)-2-{3- methoxy-4-[(1s,3s)-3- (dimethylamino)cyclobutoxy] phenylamino}pyrimidine BDBM745002 US20250171431, Compound 298 BDBM267697 US9718828, Example, 298 5-chloro-1-iodo-3-(tetrahydro-2H-pyran-4-yl)imidazo[1,5-a]pyrazin-8-amine
BDBM267697 US9718828, Example, 298 5-chloro-1-iodo-3-(tetrahydro-2H-pyran-4-yl)imidazo[1,5-a]pyrazin-8-amine BDBM355207 2-cyclopropyl-N-{(1R)-1-[4-(1- methylethyl)phenyl]ethyl}-6-oxo-1,6- dihydropyrimidine-4-carboxamide US9815796, Example 298
BDBM355207 2-cyclopropyl-N-{(1R)-1-[4-(1- methylethyl)phenyl]ethyl}-6-oxo-1,6- dihydropyrimidine-4-carboxamide US9815796, Example 298 BDBM363875 US9856220, Example 298 N-(2-sec-Butoxy-4-methylbenzyl)-2-ethyl-6,7-dimethoxy-1-oxo-1,2-dihydroisoquinoline-4-carboxamide
BDBM363875 US9856220, Example 298 N-(2-sec-Butoxy-4-methylbenzyl)-2-ethyl-6,7-dimethoxy-1-oxo-1,2-dihydroisoquinoline-4-carboxamide BDBM449312 N-[[4-[4-(2- methoxyethyl)piperazin-1- yl]sulfonylphenyl]methyl]furo [2,3-c]pyridine-2-carboxamide US10696692, Example 298
BDBM449312 N-[[4-[4-(2- methoxyethyl)piperazin-1- yl]sulfonylphenyl]methyl]furo [2,3-c]pyridine-2-carboxamide US10696692, Example 298 US11634396, Compound 298 BDBM601359 (S)-4-cyclopropyl-N-((S)-2-(dimethylamino)-3-(4-hydroxyphenyl)propyl)-3-(pyridin-4-yl)butanamide
US11634396, Compound 298 BDBM601359 (S)-4-cyclopropyl-N-((S)-2-(dimethylamino)-3-(4-hydroxyphenyl)propyl)-3-(pyridin-4-yl)butanamide  BDBM511959 methyl (((1R,3S,5R)- 2-(2-(3-acetyl-7- methyl-5-(2- methylpyrimidin-5- yl)-1H-indazol-1- yl)acetyl)-3-((6- bromo-3- methylpyridin-2- yl)carbamoyl)-2- azabicyclo[3.1.0]hexan- 5-yl)methyl)-L- prolinate US11084800, Cpd No. 298 US11708351, Compound 298
BDBM511959 methyl (((1R,3S,5R)- 2-(2-(3-acetyl-7- methyl-5-(2- methylpyrimidin-5- yl)-1H-indazol-1- yl)acetyl)-3-((6- bromo-3- methylpyridin-2- yl)carbamoyl)-2- azabicyclo[3.1.0]hexan- 5-yl)methyl)-L- prolinate US11084800, Cpd No. 298 US11708351, Compound 298 (R)-1-(7-(Hydroxymethyl)-1-methyl-1H-pyrazolo[3,4-c]pyridin-5-yl)-3-(1-phenylethyl)urea BDBM275419 US9884048, Example 298
(R)-1-(7-(Hydroxymethyl)-1-methyl-1H-pyrazolo[3,4-c]pyridin-5-yl)-3-(1-phenylethyl)urea BDBM275419 US9884048, Example 298 2-(5-Chloro-2-thienyl)-6-cyclopropyl-N-[4-(1H-tetrazol-5-ylmethyl)phenyl]pyrimidin-4-amine BDBM562726 US11401286, Example 298
2-(5-Chloro-2-thienyl)-6-cyclopropyl-N-[4-(1H-tetrazol-5-ylmethyl)phenyl]pyrimidin-4-amine BDBM562726 US11401286, Example 298 3-Methanesulfonyl-N-(2,2,2-trifluoro-ethyl)-N-[4-(2-trifluoromethoxy-phenyl)-pyridin-3-yl]-5-trifluoromethyl-benzamide BDBM411302 US10385022, Example 298
3-Methanesulfonyl-N-(2,2,2-trifluoro-ethyl)-N-[4-(2-trifluoromethoxy-phenyl)-pyridin-3-yl]-5-trifluoromethyl-benzamide BDBM411302 US10385022, Example 298 6-[3-(pyridin-3-yl)-1,2,4-oxadiazol-5- yl]-3,4-dihydrospiro[1-benzopyran- 2,1'-cyclopentane]-4-one US11912693, Compound 298 BDBM654688
6-[3-(pyridin-3-yl)-1,2,4-oxadiazol-5- yl]-3,4-dihydrospiro[1-benzopyran- 2,1'-cyclopentane]-4-one US11912693, Compound 298 BDBM654688 7-(2-fluorobenzenesulfonyl)-N2-methyl-4-(2-methylmorpholin-4-yl)-5H-pyrrolo[3,2-d]pyrimidine-2,6-diamine US10059713, Example 298 BDBM271179
7-(2-fluorobenzenesulfonyl)-N2-methyl-4-(2-methylmorpholin-4-yl)-5H-pyrrolo[3,2-d]pyrimidine-2,6-diamine US10059713, Example 298 BDBM271179 BDBM285253 US10023592, Example 298 N-[4-[1-(cyclobutylmethyl)-5-methyl-6- oxopyridin-3-yl]-5-(2,4- difluorophenoxy)pyrimidin-2-yl] methanesulfonamide
BDBM285253 US10023592, Example 298 N-[4-[1-(cyclobutylmethyl)-5-methyl-6- oxopyridin-3-yl]-5-(2,4- difluorophenoxy)pyrimidin-2-yl] methanesulfonamide Ethyl 2-methyl-5-oxo-7-(piperidin-4-yl)-4,5-dihydropyrazolo[1,5-a]pyrimidine-3-carboxylate hydrochloride BDBM298088 US10118930, Example 298
Ethyl 2-methyl-5-oxo-7-(piperidin-4-yl)-4,5-dihydropyrazolo[1,5-a]pyrimidine-3-carboxylate hydrochloride BDBM298088 US10118930, Example 298 N-(2-(3,8-diazabicyclo[3.2.1]octan-8-yl)-5-(trifluoromethyl)pyrimidin-4-yl)-1H-indazol-5-amine BDBM598627 US11613531, Example 298
N-(2-(3,8-diazabicyclo[3.2.1]octan-8-yl)-5-(trifluoromethyl)pyrimidin-4-yl)-1H-indazol-5-amine BDBM598627 US11613531, Example 298 US10544113, No. 298 N-[2-(2-Fluorophenyl)ethyl]-4-[2-(4-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-methylbenzamide BDBM426944
US10544113, No. 298 N-[2-(2-Fluorophenyl)ethyl]-4-[2-(4-fluorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-3-methylbenzamide BDBM426944 US10941160, Example 298 BDBM486097 N-[4-[1-(cyclobutylmethyl)-5-methyl-6- oxopyridin-3-yl]-5-(2,4- difluorophenoxy)pyrimidin-2-yl] methanesulfonamide
US10941160, Example 298 BDBM486097 N-[4-[1-(cyclobutylmethyl)-5-methyl-6- oxopyridin-3-yl]-5-(2,4- difluorophenoxy)pyrimidin-2-yl] methanesulfonamide US20240150293, Example 298 BDBM673163 (1s,4s)-4-(3-chloroanilino)-2′-(4-chlorophenyl)spiro[cyclohexane-1,1′-indene]-4-carboxylic acid
US20240150293, Example 298 BDBM673163 (1s,4s)-4-(3-chloroanilino)-2′-(4-chlorophenyl)spiro[cyclohexane-1,1′-indene]-4-carboxylic acid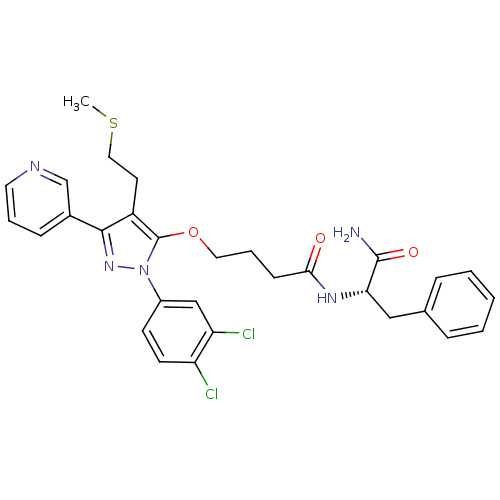 GGTI-DU40 BDBM14713 N-[(S)-1-carbamoyl-2-phenylethyl]-4-[2-(3,4-di-chlorophenyl)-4-(2-methylsulfanylethyl)-5-pyridin-3-yl-2-H-pyrazol-3-yloxy]butyramide N-[(1S)-1-carbamoyl-2-phenylethyl]-4-{[1-(3,4-dichlorophenyl)-4-[2-(methylsulfanyl)ethyl]-3-(pyridin-3-yl)-1H-pyrazol-5-yl]oxy}butanamide
GGTI-DU40 BDBM14713 N-[(S)-1-carbamoyl-2-phenylethyl]-4-[2-(3,4-di-chlorophenyl)-4-(2-methylsulfanylethyl)-5-pyridin-3-yl-2-H-pyrazol-3-yloxy]butyramide N-[(1S)-1-carbamoyl-2-phenylethyl]-4-{[1-(3,4-dichlorophenyl)-4-[2-(methylsulfanyl)ethyl]-3-(pyridin-3-yl)-1H-pyrazol-5-yl]oxy}butanamide BDBM309829 US9656988, Example 298 (R)-ethyl 1-(5-carbamoyl-6-(4-(1-cyclopentylpiperidin-4-yl)phenylamino)pyrazin-2-yl)piperidin-3-ylcarbamate
BDBM309829 US9656988, Example 298 (R)-ethyl 1-(5-carbamoyl-6-(4-(1-cyclopentylpiperidin-4-yl)phenylamino)pyrazin-2-yl)piperidin-3-ylcarbamate Cyclopropanecarboxylic acid {5-[4-(3- dimethylaminomethyl- azetidin-1-ylmethyl)-phenyl]- [1,2,4]triazolo[1,5-a]pyridin-2- yl}-amide US10206907, Compound 298 BDBM349163
Cyclopropanecarboxylic acid {5-[4-(3- dimethylaminomethyl- azetidin-1-ylmethyl)-phenyl]- [1,2,4]triazolo[1,5-a]pyridin-2- yl}-amide US10206907, Compound 298 BDBM349163 US11174268, Example 298 5-chloro-2-fluoro-4-(((6-fluoro-1H-indol-7-yl)methyl)amino)-N-(thiazol-4-yl)benzenesulfonamide BDBM526191
US11174268, Example 298 5-chloro-2-fluoro-4-(((6-fluoro-1H-indol-7-yl)methyl)amino)-N-(thiazol-4-yl)benzenesulfonamide BDBM526191 US12065436, Compound 298 (R)-1-cyclopentyl-3-(5-(5- (difluoromethyl)-1,2,4-oxadiazol-3- yl)-2,3-dihydro-1H-inden-1-yl)urea BDBM690909
US12065436, Compound 298 (R)-1-cyclopentyl-3-(5-(5- (difluoromethyl)-1,2,4-oxadiazol-3- yl)-2,3-dihydro-1H-inden-1-yl)urea BDBM690909 2-(4-(3-amino-1H-pyrazolo [3,4-b]pyridin-5-yl) benzylamino)-N-(oxetan- 3-yl)-5-(trifluoromethyl) nicotinamide BDBM282418 US10030016, Example 298
2-(4-(3-amino-1H-pyrazolo [3,4-b]pyridin-5-yl) benzylamino)-N-(oxetan- 3-yl)-5-(trifluoromethyl) nicotinamide BDBM282418 US10030016, Example 298 4-((3-(8-amino-6-(trifluoromethyl)imidazo[1,2-a]pyrazin-3- yl)-4-methylphenyl)sulfonyl)-1-methylpiperazin-2-one US10479795, Example 298 BDBM421229
4-((3-(8-amino-6-(trifluoromethyl)imidazo[1,2-a]pyrazin-3- yl)-4-methylphenyl)sulfonyl)-1-methylpiperazin-2-one US10479795, Example 298 BDBM421229 6-[3-(4-methyl- 1,2,4-triazol-3-yl)- 1-piperidyl]pyrido [3,2-d]pyrimidin- 4-amine US9855269, Compound 145 US9592235, Example 298 BDBM299525
6-[3-(4-methyl- 1,2,4-triazol-3-yl)- 1-piperidyl]pyrido [3,2-d]pyrimidin- 4-amine US9855269, Compound 145 US9592235, Example 298 BDBM299525 BDBM422395 US10508104, Example 298 5-(4-bromo-3- fluorobenzenesulfonyl)- 2-(ethoxymethyl)-1-[1- (3-fluorophenyl)-2- methoxyethyl]-6- hydroxy-1,4- dihydropyrimidin-4-one
BDBM422395 US10508104, Example 298 5-(4-bromo-3- fluorobenzenesulfonyl)- 2-(ethoxymethyl)-1-[1- (3-fluorophenyl)-2- methoxyethyl]-6- hydroxy-1,4- dihydropyrimidin-4-one BDBM502392 2-(R)- hydroxy-N- [4-(7- methoxy-1- methyl- [1,2,4]tri- azolo[4,3- a]quinoxaline- 4-ylamino)- butyl]-3- US11028090, Example 298
BDBM502392 2-(R)- hydroxy-N- [4-(7- methoxy-1- methyl- [1,2,4]tri- azolo[4,3- a]quinoxaline- 4-ylamino)- butyl]-3- US11028090, Example 298 N-(2-ethyl-5-methyl-2,3- dihydro-1H-inden-1-yl)-2- oxo-6-(trifluoromethyl)-1,2- dihydropyridine-3- carboxamide US11957687, Compound 298 BDBM667642
N-(2-ethyl-5-methyl-2,3- dihydro-1H-inden-1-yl)-2- oxo-6-(trifluoromethyl)-1,2- dihydropyridine-3- carboxamide US11957687, Compound 298 BDBM667642 US20240083857, Example 298 2-{[2-(5-{1-[(6,7-dimethoxy-2-methylquinazolin-4-yl)amino]ethyl}thiophen-2-yl)benzyl](methyl)amino}ethanol BDBM657619
US20240083857, Example 298 2-{[2-(5-{1-[(6,7-dimethoxy-2-methylquinazolin-4-yl)amino]ethyl}thiophen-2-yl)benzyl](methyl)amino}ethanol BDBM657619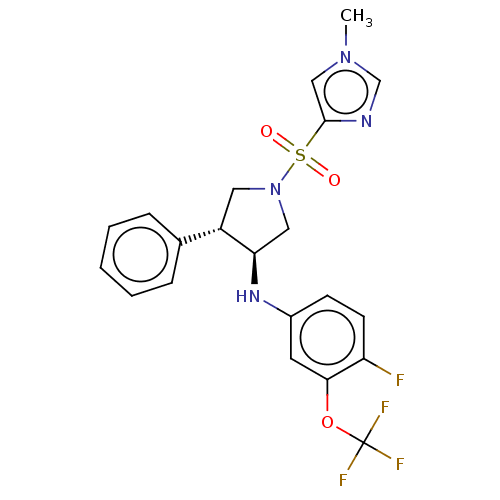 US9656955, Example 298 BDBM308784 trans-N-[4-fluoro-3-(trifluoromethoxy)phenyl]-1-[(1-methyl-1H-imidazol-4-yl)sulfonyl]-4-phenylpyrrolidin-3-amine
US9656955, Example 298 BDBM308784 trans-N-[4-fluoro-3-(trifluoromethoxy)phenyl]-1-[(1-methyl-1H-imidazol-4-yl)sulfonyl]-4-phenylpyrrolidin-3-amine 1-amino-N-{5-chloro-4-[1-(tetrahydro-2H-pyran-4-ylmethyl)-1H-benzimidazol-6-yl]pyridin-2-yl}cyclopentanecarboxamide US9650358, Example 298 BDBM308002
1-amino-N-{5-chloro-4-[1-(tetrahydro-2H-pyran-4-ylmethyl)-1H-benzimidazol-6-yl]pyridin-2-yl}cyclopentanecarboxamide US9650358, Example 298 BDBM308002 (S)-2,4-dimethyl-1-((5-(2-methylpyrimidin-4-yl)-3-(oxazol-5-yl)pyridin-2-yl)oxy)pentan-2-amine US10544120, Example 298 BDBM427292
(S)-2,4-dimethyl-1-((5-(2-methylpyrimidin-4-yl)-3-(oxazol-5-yl)pyridin-2-yl)oxy)pentan-2-amine US10544120, Example 298 BDBM427292 4-(3-(2H-1,2,3-triazol-2-yl)phenylamino)-2-((3R,4R)-3-aminotetrahydro-2H-pyran-4-ylamino)pyrimidine-5-carboxamide US9579320, Example 298 BDBM290885
4-(3-(2H-1,2,3-triazol-2-yl)phenylamino)-2-((3R,4R)-3-aminotetrahydro-2H-pyran-4-ylamino)pyrimidine-5-carboxamide US9579320, Example 298 BDBM290885 5-[2-(2-Fluoro-6-methyl-phenoxy)- 6-(1-phenyl-ethylamino)-pyrimidin- 4-yl]-1-methyl-1H-pyridin-2-one BDBM345753 US10202360, Example 298
5-[2-(2-Fluoro-6-methyl-phenoxy)- 6-(1-phenyl-ethylamino)-pyrimidin- 4-yl]-1-methyl-1H-pyridin-2-one BDBM345753 US10202360, Example 298 BDBM417420 2-(2-isopropylphenyl)-9-(4-(1-methyl-1H-1,2,3-triazol-5-yl)benzyl)-7,9-dihydro-8H-purin-8-one US10399980, Compound I-298
BDBM417420 2-(2-isopropylphenyl)-9-(4-(1-methyl-1H-1,2,3-triazol-5-yl)benzyl)-7,9-dihydro-8H-purin-8-one US10399980, Compound I-298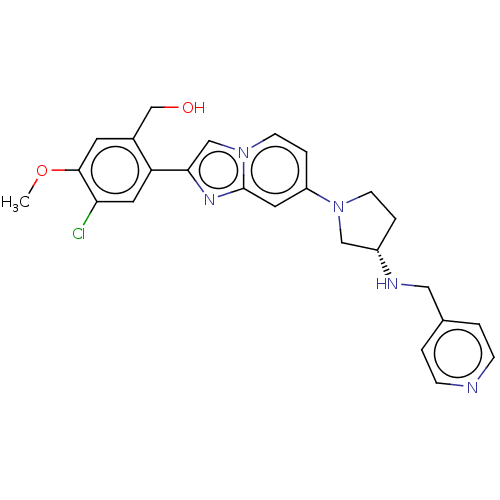 BDBM423112 US10508109, Example 298 (S)-1-(2-(5-chloro-2,4- dimethoxyphenyl)imidazo[1,2- a]pyridin-7-yl)-N-(pyridin-4- ylmethyl)pyrrolidin-3-amine
BDBM423112 US10508109, Example 298 (S)-1-(2-(5-chloro-2,4- dimethoxyphenyl)imidazo[1,2- a]pyridin-7-yl)-N-(pyridin-4- ylmethyl)pyrrolidin-3-amine N-{[2-(cyclopropylmethoxy)-4,6- difluorophenyl]methyl}-5-{2- acetamidoimidazo[1,2-b]pyridazin-6-yl}- 2-methoxy-6-methylpyridine-3- carboxamide BDBM571231 US11440913, Example 298
N-{[2-(cyclopropylmethoxy)-4,6- difluorophenyl]methyl}-5-{2- acetamidoimidazo[1,2-b]pyridazin-6-yl}- 2-methoxy-6-methylpyridine-3- carboxamide BDBM571231 US11440913, Example 298 US10118930, Example 9 Ethyl 2-methyl-5-oxo-7-(piperidin-4-yl)-4,5-dihydropyrazolo[1,5-a]pyrimidine-3-carboxylate trifluoroacetate BDBM297784 US10118930, Example 298
US10118930, Example 9 Ethyl 2-methyl-5-oxo-7-(piperidin-4-yl)-4,5-dihydropyrazolo[1,5-a]pyrimidine-3-carboxylate trifluoroacetate BDBM297784 US10118930, Example 298 US10329294, Example 298 BDBM402714 N-[1-(1-methylethyl)- 3-(trifluoromethyl)- 1H-pyrazol-4-yl]-5- piperazin-1- ylpyrazolo[1,5- a]pyrimidine-3- carboxamide trifluoroacetate
US10329294, Example 298 BDBM402714 N-[1-(1-methylethyl)- 3-(trifluoromethyl)- 1H-pyrazol-4-yl]-5- piperazin-1- ylpyrazolo[1,5- a]pyrimidine-3- carboxamide trifluoroacetate US10975056, Example 298 BDBM491343 N-(4-(((3,5-Dicyano-4-ethyl-6-(4-methyl-1,4-diazepan-1-yl)pyridin-2-yl)thio)methyl)phenyl)acetamide
US10975056, Example 298 BDBM491343 N-(4-(((3,5-Dicyano-4-ethyl-6-(4-methyl-1,4-diazepan-1-yl)pyridin-2-yl)thio)methyl)phenyl)acetamide US11839613, Example 298 (2-Ethoxy-4-{6-[2-(5-methoxy-1-methyl-1H-indazol-6-yl)-ethylamino]-pyrimidin-4-yl}- phenoxy)-acetic acid BDBM640177
US11839613, Example 298 (2-Ethoxy-4-{6-[2-(5-methoxy-1-methyl-1H-indazol-6-yl)-ethylamino]-pyrimidin-4-yl}- phenoxy)-acetic acid BDBM640177 (1R,8S)-N-[(4-carbamimidoyl- 2,6-difluoro-phenyl)methyl]- 12-(propan-2-yl)-15- oxatetracyclo[6.6.1.02,7.09,14] pentadeca-2,4,6,9,11,13-hexaene- 4-carboxamide BDBM306654 US10144746, Compound 298
(1R,8S)-N-[(4-carbamimidoyl- 2,6-difluoro-phenyl)methyl]- 12-(propan-2-yl)-15- oxatetracyclo[6.6.1.02,7.09,14] pentadeca-2,4,6,9,11,13-hexaene- 4-carboxamide BDBM306654 US10144746, Compound 298 (5,6-Dimethoxypyridin-2-yl)((5R,9S)-2-methyl-3-(3,4,5- trifluorophenyl)-4,5,6,7,8,9-hexahydro-2H-5,9- epiminocycloocta[c]pyrazol-10-yl)methanone; US11597728, Example 298 BDBM597411
(5,6-Dimethoxypyridin-2-yl)((5R,9S)-2-methyl-3-(3,4,5- trifluorophenyl)-4,5,6,7,8,9-hexahydro-2H-5,9- epiminocycloocta[c]pyrazol-10-yl)methanone; US11597728, Example 298 BDBM597411 2-((4-(1H-indazol-7-yl)- 2-sulfamoyl-3-(1H- tetrazol-5- yl)phenyl)sulfonyl) ethanaminium 2,2,2- trifluoroacetate US10227331, Example 173 BDBM367986 US10227331, Example 298
2-((4-(1H-indazol-7-yl)- 2-sulfamoyl-3-(1H- tetrazol-5- yl)phenyl)sulfonyl) ethanaminium 2,2,2- trifluoroacetate US10227331, Example 173 BDBM367986 US10227331, Example 298 5-[5-[(1R)-1-(3,5-dichloro-4- pyridyl)ethoxy]-1H-indazol-3- yl]-2-(4-hydroxy-1- piperidyl)pyridine-3- carbonitrile BDBM662865 US20240109865, Example 298.
5-[5-[(1R)-1-(3,5-dichloro-4- pyridyl)ethoxy]-1H-indazol-3- yl]-2-(4-hydroxy-1- piperidyl)pyridine-3- carbonitrile BDBM662865 US20240109865, Example 298. 6-fluoro-1-methyl-4-[4-(5-methyl-1,3-benzoxazol-2-yl)piperidin-1-yl]-2-oxo-1,2-dihydroquinoline-3-carboxamide US11998539, Example 298 BDBM678856
6-fluoro-1-methyl-4-[4-(5-methyl-1,3-benzoxazol-2-yl)piperidin-1-yl]-2-oxo-1,2-dihydroquinoline-3-carboxamide US11998539, Example 298 BDBM678856 BDBM313672 N-(6'-ethoxy-5'-(3- fluorotetrahydro- furan-3-yl)-2- methyl-[3,3'- bipyridin]-5-yl)-2- (2-fluoropropan-2- yl)isonicotinamide US10167279, Example 298
BDBM313672 N-(6'-ethoxy-5'-(3- fluorotetrahydro- furan-3-yl)-2- methyl-[3,3'- bipyridin]-5-yl)-2- (2-fluoropropan-2- yl)isonicotinamide US10167279, Example 298 BDBM412989 US10407409, Example 298 4-(1-(5-(3-(cyclopropyl(methyl)amino)azetidine-1-carbonyl)pyridin-2-yl)-5-hydroxy-1H-pyrazol-4-yl)-3-methylbenzonitrile
BDBM412989 US10407409, Example 298 4-(1-(5-(3-(cyclopropyl(methyl)amino)azetidine-1-carbonyl)pyridin-2-yl)-5-hydroxy-1H-pyrazol-4-yl)-3-methylbenzonitrile BDBM695693 US12084420, Compound 298 N-(tert-butyl)-2-hydroxy-5-(5$#8243;- (methylsulfonamido)dispiro[cyclopropane- 1,1'-cyclohexane-4',3$#8243;-indoline]- 1$#8243;-carbonyl)benzenesulfonamide
BDBM695693 US12084420, Compound 298 N-(tert-butyl)-2-hydroxy-5-(5$#8243;- (methylsulfonamido)dispiro[cyclopropane- 1,1'-cyclohexane-4',3$#8243;-indoline]- 1$#8243;-carbonyl)benzenesulfonamide BDBM716062 US12208088, Compound 298 3-(4'-Chloro-1',2'-dihydrospiro[cyclopropane-1,3'- pyrrolo[2,3-b]pyridin]-5'-yl)-N,N-dimethyl-1H- indole-7-carboxamide
BDBM716062 US12208088, Compound 298 3-(4'-Chloro-1',2'-dihydrospiro[cyclopropane-1,3'- pyrrolo[2,3-b]pyridin]-5'-yl)-N,N-dimethyl-1H- indole-7-carboxamide US11724992, Example 298 (R) or (S)-N'- ((3-fluoro-2,6- diisopropyl- phenyl)carba- moyl)-2-(2- hydroxy- propan-2-yl) thiazole-5- sulfonimi- damide BDBM612608
US11724992, Example 298 (R) or (S)-N'- ((3-fluoro-2,6- diisopropyl- phenyl)carba- moyl)-2-(2- hydroxy- propan-2-yl) thiazole-5- sulfonimi- damide BDBM612608 US9687479, 298 tert-butyl 4-(5-[(5-chlorothiophen-2-yl)methyl]amino-1-[(thiophen-3-yl)carbonyl]-1H-pyrazol-3-yl)piperidine-1-carboxylateIUPACname BDBM163432
US9687479, 298 tert-butyl 4-(5-[(5-chlorothiophen-2-yl)methyl]amino-1-[(thiophen-3-yl)carbonyl]-1H-pyrazol-3-yl)piperidine-1-carboxylateIUPACname BDBM163432 (S)-2-(6-Hydroxy-4-oxo-1-(1-(4- (trifluoromethyl)phenyl)ethyl)-4,5- dihydro-1H-pyrazolo[3,4- d]pyrimidin-3-yl)acetonitrile BDBM320892 US10174037, Example 298
(S)-2-(6-Hydroxy-4-oxo-1-(1-(4- (trifluoromethyl)phenyl)ethyl)-4,5- dihydro-1H-pyrazolo[3,4- d]pyrimidin-3-yl)acetonitrile BDBM320892 US10174037, Example 298 2-[2-Chloro-4-fluoro- 5-(7-morpholin-4-yl- quinazolin-4-yl)- phenyl]-2-[1,2,4]- triazolo[4,3-a]pyridin- 5-ylacetamide US11065253, Example 298 BDBM504810
2-[2-Chloro-4-fluoro- 5-(7-morpholin-4-yl- quinazolin-4-yl)- phenyl]-2-[1,2,4]- triazolo[4,3-a]pyridin- 5-ylacetamide US11065253, Example 298 BDBM504810 2-fluoro-N-(6-(4-(4-fluoro-3- methylphenyl)-1,2-dimethyl-1H- imidazol-5-yl)imidazo[1,2-b] pyridazin-2-yl)isonicotinamide US9556179, Compound 298 BDBM274723
2-fluoro-N-(6-(4-(4-fluoro-3- methylphenyl)-1,2-dimethyl-1H- imidazol-5-yl)imidazo[1,2-b] pyridazin-2-yl)isonicotinamide US9556179, Compound 298 BDBM274723 3-((S)-3-((R)-8-(4'-(cyanomethoxy)biphenyl-3-ylsulfonyl)-1-oxa-8-azaspiro[4.5]decan-3-ylamino)-2-hydroxypropoxy)-N-methylbenzenesulfonamide US11987588, Compound 298 BDBM675829
3-((S)-3-((R)-8-(4'-(cyanomethoxy)biphenyl-3-ylsulfonyl)-1-oxa-8-azaspiro[4.5]decan-3-ylamino)-2-hydroxypropoxy)-N-methylbenzenesulfonamide US11987588, Compound 298 BDBM675829 6-chloro-4-{4-[(3,5-difluoro-2-hydroxyphenyl)methyl]piperazin-1-yl}-1-methyl-2-oxo-1,2-dihydro-1,5-naphthyridine-3-carbonitrile BDBM644320 US11866430, Example 298
6-chloro-4-{4-[(3,5-difluoro-2-hydroxyphenyl)methyl]piperazin-1-yl}-1-methyl-2-oxo-1,2-dihydro-1,5-naphthyridine-3-carbonitrile BDBM644320 US11866430, Example 298 BDBM333748 1-(3-tert- butyl-1-p- tolyl-1H- pyrazol-5- yl)-3-(2- chloro-5- (6,7- dimethoxy- quinazolin- 4- yloxy)phenyl) urea US9730937, Example 298
BDBM333748 1-(3-tert- butyl-1-p- tolyl-1H- pyrazol-5- yl)-3-(2- chloro-5- (6,7- dimethoxy- quinazolin- 4- yloxy)phenyl) urea US9730937, Example 298 BDBM710940 US12187709, Compound 298 2-(3-(1-(4-methyl-4H-1,2,4-triazol-3- yl)cyclohexyl)phenyl)-6-(((1- methylcyclobutyl)amino)methyl)-4- (trifluoromethyl)isoindolin-1-one
BDBM710940 US12187709, Compound 298 2-(3-(1-(4-methyl-4H-1,2,4-triazol-3- yl)cyclohexyl)phenyl)-6-(((1- methylcyclobutyl)amino)methyl)-4- (trifluoromethyl)isoindolin-1-one BDBM760610 N-[4-[(6,7-dimethoxy-1,5- naphthyridin-4-yl)oxy]-3- fluorophenyl]-1-(4-fluoro-2- methylphenyl)-4,6-dimethyl-2- oxopyridine-3-carboxamide US12371428, Compound 298
BDBM760610 N-[4-[(6,7-dimethoxy-1,5- naphthyridin-4-yl)oxy]-3- fluorophenyl]-1-(4-fluoro-2- methylphenyl)-4,6-dimethyl-2- oxopyridine-3-carboxamide US12371428, Compound 298 N-Cyclopropyl-7-hydroxy-4-neopentyl-5-oxo-2-(4-(trifluoromethyl)-1H-pyrazol-3-yl)-4,5-dihydropyrazolo[1,5-a]pyrimidine-6-carboxamide BDBM659861 US20240092784, Example 298
N-Cyclopropyl-7-hydroxy-4-neopentyl-5-oxo-2-(4-(trifluoromethyl)-1H-pyrazol-3-yl)-4,5-dihydropyrazolo[1,5-a]pyrimidine-6-carboxamide BDBM659861 US20240092784, Example 298 US10173991, Example 298 N-[(cyclopropyl- carbamoyl) methyl]-2-(2- methoxyethane- sulfonyl)-2-{6-[6- (pyaolidin-1- yl)pyridin-3-yl]- 1,3-benzothiazol- 2-yl}acetamide BDBM319254
US10173991, Example 298 N-[(cyclopropyl- carbamoyl) methyl]-2-(2- methoxyethane- sulfonyl)-2-{6-[6- (pyaolidin-1- yl)pyridin-3-yl]- 1,3-benzothiazol- 2-yl}acetamide BDBM319254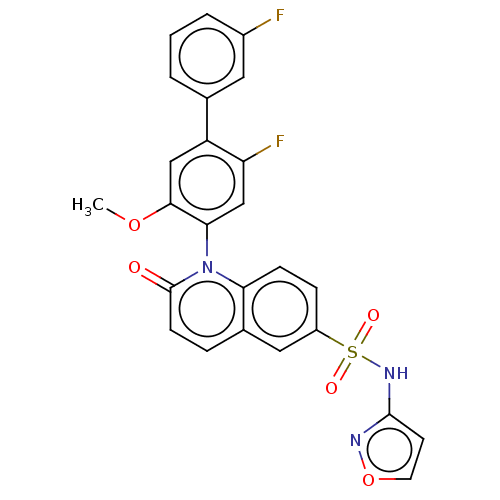 US9776995, Example 42 BDBM342945 US9776995, Example 299 US9776995, Example 298 1-(2,3''-difluoro-5-methoxy-4-biphenylyl)-N-3-isoxazolyl-2-oxo-1,2-dihydro-6-quinolinesulfonamide
US9776995, Example 42 BDBM342945 US9776995, Example 299 US9776995, Example 298 1-(2,3''-difluoro-5-methoxy-4-biphenylyl)-N-3-isoxazolyl-2-oxo-1,2-dihydro-6-quinolinesulfonamide (3-fluoro-2-(oxazol-2-yl)phenyl)((1S,2R,4R)-2-((5-(trifluoromethyl)pyridin-2-yl)amino)-7-azabicyclo[2.2.1]heptan-7-yl)methanone BDBM326633 US9637496, 298
(3-fluoro-2-(oxazol-2-yl)phenyl)((1S,2R,4R)-2-((5-(trifluoromethyl)pyridin-2-yl)amino)-7-azabicyclo[2.2.1]heptan-7-yl)methanone BDBM326633 US9637496, 298 BDBM407356 6-(3-cyanopyrrolo[1,2-b] pyridazin-7-yl)-N-((R)-2- fluoro-3-hydroxy-3- methylbutyl)-4-(((1r,4R)-4- isobutyramidocyclohexyl) amino)nicotinamide US10336762, Compound 298
BDBM407356 6-(3-cyanopyrrolo[1,2-b] pyridazin-7-yl)-N-((R)-2- fluoro-3-hydroxy-3- methylbutyl)-4-(((1r,4R)-4- isobutyramidocyclohexyl) amino)nicotinamide US10336762, Compound 298 BDBM680083 US12011444, Example 298 {6-[4-(2-Amino-5-methyl-thiazol-4-yl)-phenyl]-pyrimidin-4-yl}-[2-(7-fluoro-2,4-dimethyl- indol-1-yl)-ethyl]-amine
BDBM680083 US12011444, Example 298 {6-[4-(2-Amino-5-methyl-thiazol-4-yl)-phenyl]-pyrimidin-4-yl}-[2-(7-fluoro-2,4-dimethyl- indol-1-yl)-ethyl]-amine BDBM758869 US20250230171, Compound 298 4-(2-methoxyphenyl)-6-methyl-N-(5- (pyrazolo[1,5-a]pyridine-2-carbonyl)- 5,6-dihydro-4H-pyrrolo[3,4-d]thiazol-2- yl)nicotinamide
BDBM758869 US20250230171, Compound 298 4-(2-methoxyphenyl)-6-methyl-N-(5- (pyrazolo[1,5-a]pyridine-2-carbonyl)- 5,6-dihydro-4H-pyrrolo[3,4-d]thiazol-2- yl)nicotinamide CHEMBL490262 N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide SB-298 CHEMBL521280 BDBM50268085
CHEMBL490262 N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide SB-298 CHEMBL521280 BDBM50268085 US10189841, Compound I-298 BDBM325079 US10189841, Compound I-285 2-(2-isopropylphenyl)-7-methyl-9-(4-(1-methyl-1H- US11161848, Compound I-285 US10399980, Compound I-285
US10189841, Compound I-298 BDBM325079 US10189841, Compound I-285 2-(2-isopropylphenyl)-7-methyl-9-(4-(1-methyl-1H- US11161848, Compound I-285 US10399980, Compound I-285 [3H]-N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide BDBM50268276 SB-298 CHEMBL521280
[3H]-N-(2-hydroxyethyl)-2-(4-(1-methyl-2,4-dioxo-3-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)phenoxy)acetamide BDBM50268276 SB-298 CHEMBL521280 ((1s,3s)-3-Hydroxy-3-methylcyclobutyl)(6-((1-methyl-1H-pyrrolo[3,2-c]pyridin-6-yl)methyl)-2-azaspiro[3.3]heptan-2-yl)methanone BDBM630708 US11802111, Example 298
((1s,3s)-3-Hydroxy-3-methylcyclobutyl)(6-((1-methyl-1H-pyrrolo[3,2-c]pyridin-6-yl)methyl)-2-azaspiro[3.3]heptan-2-yl)methanone BDBM630708 US11802111, Example 298 5-[[(5-chloro-3-pyridyl)-methyl-amino]methyl]-N-[(1S)-1-(cyclohexyl methyl)-2-[[4-(dimethylamino)cyclohexyl]amino]-2-oxo-ethyl]thiophene-2-carboxamide BDBM665608 US20240116910, Compound 298
5-[[(5-chloro-3-pyridyl)-methyl-amino]methyl]-N-[(1S)-1-(cyclohexyl methyl)-2-[[4-(dimethylamino)cyclohexyl]amino]-2-oxo-ethyl]thiophene-2-carboxamide BDBM665608 US20240116910, Compound 298 BDBM299928 US9593097, Example 298 4-{[(1S,2R)-2- cyanocyclopropyl] methoxy}-N- (cyclopropylmethyl)- 6-[4-(1H- pyrazolo[3,4- b]pyridin-3- yl)piperidin-1-yl]- 1,3,5-triazine-2- carboxamide
BDBM299928 US9593097, Example 298 4-{[(1S,2R)-2- cyanocyclopropyl] methoxy}-N- (cyclopropylmethyl)- 6-[4-(1H- pyrazolo[3,4- b]pyridin-3- yl)piperidin-1-yl]- 1,3,5-triazine-2- carboxamide N-(1-(5-(6-(3-cyanopyrrolo[1,2-b]pyridazin-7-yl)-4-(isopropylamino)pyridin-3-yl)-1,3,4-thiadiazol-2-yl)azepan-4-yl)acetamide US11702414, Example 298 BDBM609332
N-(1-(5-(6-(3-cyanopyrrolo[1,2-b]pyridazin-7-yl)-4-(isopropylamino)pyridin-3-yl)-1,3,4-thiadiazol-2-yl)azepan-4-yl)acetamide US11702414, Example 298 BDBM609332 US10954214, Compound 298 BDBM487876 1-(benzo[d]thiazol-4-yl)-N-(5- cyano-2-methyl-4-(2H-1,2,3- triazol-2-yl)phenyl)-5- (trifluoromethyl)-1H-pyrazole-4- carboxamide
US10954214, Compound 298 BDBM487876 1-(benzo[d]thiazol-4-yl)-N-(5- cyano-2-methyl-4-(2H-1,2,3- triazol-2-yl)phenyl)-5- (trifluoromethyl)-1H-pyrazole-4- carboxamide 5-(4-((3-fluoro-4-(piperazin-1-yl)phenyl)amino)-1,3,5-triazin-2-yl)-2-(((3R,4S)-3-fluorotetrahydro-2H-pyran-4-yl)oxy)benzonitrile BDBM375573 US10253019, Example 298
5-(4-((3-fluoro-4-(piperazin-1-yl)phenyl)amino)-1,3,5-triazin-2-yl)-2-(((3R,4S)-3-fluorotetrahydro-2H-pyran-4-yl)oxy)benzonitrile BDBM375573 US10253019, Example 298 6-{[trans-4-(1- methylethoxy)cyclohexyl]car- bonyl}-8-(6-oxa-3- azabicyclo[3.1.1]hept-3-yl)- 6,11-dihydro-5H-pyrido[2,3- b][1,5]benzodiazepine BDBM416153 US10442819, Example 298
6-{[trans-4-(1- methylethoxy)cyclohexyl]car- bonyl}-8-(6-oxa-3- azabicyclo[3.1.1]hept-3-yl)- 6,11-dihydro-5H-pyrido[2,3- b][1,5]benzodiazepine BDBM416153 US10442819, Example 298 BDBM273099 US10065972, Example 298 (R)-N-benzyl-1-[5-(3-fluoropyridin-2-yl)-6-methyl-7-oxo-6,7-dihydro-[1,3]thiazolo[5,4-d]pyrimidin-2-yl]pyrrolidine-2-carboxamide
BDBM273099 US10065972, Example 298 (R)-N-benzyl-1-[5-(3-fluoropyridin-2-yl)-6-methyl-7-oxo-6,7-dihydro-[1,3]thiazolo[5,4-d]pyrimidin-2-yl]pyrrolidine-2-carboxamide BDBM497814 US11008312, Example 298 (1S,8R)-5-(2,6-difluorophenyl)-11,11- dimethyl-1-[2-[1- (methylsulfonylmethyl)imidazol-4- yl]pyrimidin-4-yl]-3,4- diazatricyclo[6.2.1.02,7]undeca-2(7),3,5- triene
BDBM497814 US11008312, Example 298 (1S,8R)-5-(2,6-difluorophenyl)-11,11- dimethyl-1-[2-[1- (methylsulfonylmethyl)imidazol-4- yl]pyrimidin-4-yl]-3,4- diazatricyclo[6.2.1.02,7]undeca-2(7),3,5- triene BDBM725548 US20250074891, Compound 298 3-(4- ((difluoro- methyl)sulfon- amido)-3-(1-(4- fluorophenyl)-2- methoxy- ethoxy)phenyl)- 5-((5-methyl- isoxazol- 3-yl)amino)-1H- pyrazole-4- carboxamide
BDBM725548 US20250074891, Compound 298 3-(4- ((difluoro- methyl)sulfon- amido)-3-(1-(4- fluorophenyl)-2- methoxy- ethoxy)phenyl)- 5-((5-methyl- isoxazol- 3-yl)amino)-1H- pyrazole-4- carboxamide US10040781, Example 298 BDBM278076 5-(4-((3-fluoro-4-(piperazin-1-yl)phenyl)amino)-1,3,5-triazin-2-yl)-2-(((3R,4S)-3-fluorotetrahydro-2H-pyran-4-yl)oxy)benzonitrile
US10040781, Example 298 BDBM278076 5-(4-((3-fluoro-4-(piperazin-1-yl)phenyl)amino)-1,3,5-triazin-2-yl)-2-(((3R,4S)-3-fluorotetrahydro-2H-pyran-4-yl)oxy)benzonitrile US11208400, Example 298 4-[5-({6-[4-(2-hydroxypropan-2-yl)-3,5-dimethyl-1H-pyrazol-1-yl]pyrimidin-4-yl}amino)-1,4-dimethyl-1H-pyrazol-3-yl]benzonitrile BDBM531665
US11208400, Example 298 4-[5-({6-[4-(2-hydroxypropan-2-yl)-3,5-dimethyl-1H-pyrazol-1-yl]pyrimidin-4-yl}amino)-1,4-dimethyl-1H-pyrazol-3-yl]benzonitrile BDBM531665 US9783527, Example 298 1-[2-(3-fluoroazetidin-1-yl)- 1-[2-(3-fluoroazetidin-1-yl)-2-oxoethyl]-3-[1-(2-fluorophenyl)-1H-indazol-4-yl]imidazolidin-2-one BDBM344996
US9783527, Example 298 1-[2-(3-fluoroazetidin-1-yl)- 1-[2-(3-fluoroazetidin-1-yl)-2-oxoethyl]-3-[1-(2-fluorophenyl)-1H-indazol-4-yl]imidazolidin-2-one BDBM344996 [3-(1-ethyl-8-oxo-spiro[6,7- dihydro-4H-pyrazolo[3,4- c]azepine-5,4'-tetrahydro- pyran]-3-yl)-2,2-dimethyl- propyl] 1-methylpyrazole-3- carboxylate BDBM466204 US10793580, Example 298
[3-(1-ethyl-8-oxo-spiro[6,7- dihydro-4H-pyrazolo[3,4- c]azepine-5,4'-tetrahydro- pyran]-3-yl)-2,2-dimethyl- propyl] 1-methylpyrazole-3- carboxylate BDBM466204 US10793580, Example 298 [3-(3-ethyl-4-oxo-spiro[6,8- dihydro-5H-pyrazolo[4,3- c]azepine-7,4'- tetrahydropyran]-1-yl)-2,2- dimethyl-propyl] 4-cyano-2- fluoro-benzoate BDBM481502 US10906915, Ex. 298
[3-(3-ethyl-4-oxo-spiro[6,8- dihydro-5H-pyrazolo[4,3- c]azepine-7,4'- tetrahydropyran]-1-yl)-2,2- dimethyl-propyl] 4-cyano-2- fluoro-benzoate BDBM481502 US10906915, Ex. 298 (1R,3R)-3-[8- (methoxycarbonyl)- 3-[(2R)-1- phenylpropan- 2-yl]- 3H,6H,7H,8H, 9H- imidazo[4,5- h]isoquinolin- 2- yl]cyclohexane- 1-carboxylic acid BDBM749245 US20250188079, Compound 298
(1R,3R)-3-[8- (methoxycarbonyl)- 3-[(2R)-1- phenylpropan- 2-yl]- 3H,6H,7H,8H, 9H- imidazo[4,5- h]isoquinolin- 2- yl]cyclohexane- 1-carboxylic acid BDBM749245 US20250188079, Compound 298 BDBM407014 US10336761, Example 298 3-fluoro-N-(5-{[(5-phenyl-1H-pyrazol-3-yl)amino]methyl}-2-(pyridin-2-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)pyridin-4-amine
BDBM407014 US10336761, Example 298 3-fluoro-N-(5-{[(5-phenyl-1H-pyrazol-3-yl)amino]methyl}-2-(pyridin-2-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)pyridin-4-amine BDBM505649 US11066414, Compound 298 (R)-8-chloro-4-((3-chloro-4-fluoro- phenyl)amino)-6-(((1-isopropyl-1H- 1,2,3-triazol-4- yl)(oxazol-5-yl)methyl)- amino)quinoline-3-carbonitrile
BDBM505649 US11066414, Compound 298 (R)-8-chloro-4-((3-chloro-4-fluoro- phenyl)amino)-6-(((1-isopropyl-1H- 1,2,3-triazol-4- yl)(oxazol-5-yl)methyl)- amino)quinoline-3-carbonitrile BDBM553242 4-fluoro-N-[4-fluoro-5- (2-morpholin-4-ylpyrimidin- 5-yl)-2-[rac-(3R)-3,4- dimethylpiperazin-1- yl]phenyl]-2- (trifluoromethyl)benzamide US11319299, Example 299 US11319299, Example 298
BDBM553242 4-fluoro-N-[4-fluoro-5- (2-morpholin-4-ylpyrimidin- 5-yl)-2-[rac-(3R)-3,4- dimethylpiperazin-1- yl]phenyl]-2- (trifluoromethyl)benzamide US11319299, Example 299 US11319299, Example 298 N-((S)-2-(diethylamino)propyl)-2-((S)-2-(hydroxy(methyl)carbamoyl)azetidin-1-yl)-6-(5-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidin-3-yl)isonicotinamide BDBM292642 US10100058, Example 298
N-((S)-2-(diethylamino)propyl)-2-((S)-2-(hydroxy(methyl)carbamoyl)azetidin-1-yl)-6-(5-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidin-3-yl)isonicotinamide BDBM292642 US10100058, Example 298 US9725449, Example 298 2-{5-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl]-8-[(S)-oxan-4-yl(phenyl)methyl]-3,8,11-triazatricyclo[7.4.0.02,7]trideca-1(9),2 BDBM331461
US9725449, Example 298 2-{5-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl]-8-[(S)-oxan-4-yl(phenyl)methyl]-3,8,11-triazatricyclo[7.4.0.02,7]trideca-1(9),2 BDBM331461 US10821115, Example 298 US11224601, Example 298 (1S,3'R,6'R,7'R,8'E,11'S,12'R)- 6-chloro-7'-(((9aR)-8,8- difluorooctahydro-2H- pyrido[1,2-a]pyrazin-2- yl)methyl)-7'-methoxy-11',12'- dimethyl-3,4-dihydro-2H,15'H- spiro[naphthalene-1,22'- [20]oxa[13]thia[1,14] diazatetracyclo [14.7.2.0~3,6~.0~19,24~] pentacosa[8,16,18,24]tetraen]- 15'-one 13',13'-dioxide BDBM470915
US10821115, Example 298 US11224601, Example 298 (1S,3'R,6'R,7'R,8'E,11'S,12'R)- 6-chloro-7'-(((9aR)-8,8- difluorooctahydro-2H- pyrido[1,2-a]pyrazin-2- yl)methyl)-7'-methoxy-11',12'- dimethyl-3,4-dihydro-2H,15'H- spiro[naphthalene-1,22'- [20]oxa[13]thia[1,14] diazatetracyclo [14.7.2.0~3,6~.0~19,24~] pentacosa[8,16,18,24]tetraen]- 15'-one 13',13'-dioxide BDBM470915 1-(4-(((R)-1-(3-(difluoromethyl)-2- fluorophenyl)ethyl)amino)-2-methyl- 7-(((R)- tetrahydrofuran-3-yl)oxy)pyrido[2,3-d] pyrimidin-6-yl)cyclopropane-1- carbonitrile BDBM602406 US11648254, Compound 298
1-(4-(((R)-1-(3-(difluoromethyl)-2- fluorophenyl)ethyl)amino)-2-methyl- 7-(((R)- tetrahydrofuran-3-yl)oxy)pyrido[2,3-d] pyrimidin-6-yl)cyclopropane-1- carbonitrile BDBM602406 US11648254, Compound 298 3-(2-amino-[1,2,4]triazolo[1,5-α]pyri din-7-yl)-6-chl oro-2 -fluoro-N-((2R,3S)-2-fluoro-3-(4-fluorophenyl)-3-hydroxypropyl)benzamide WO2022086828, Example 298 BDBM534562
3-(2-amino-[1,2,4]triazolo[1,5-α]pyri din-7-yl)-6-chl oro-2 -fluoro-N-((2R,3S)-2-fluoro-3-(4-fluorophenyl)-3-hydroxypropyl)benzamide WO2022086828, Example 298 BDBM534562 BDBM492157 US10975068, Example 298 5-(4-(Difluoromethyl)-6-(((R)-3,3-dimethylbutan-2-yl)amino)pyridin-3-yl)-N-(2-hydroxy-2-methylpropyl)-4-((S)-2-methylpyrrolidine-1-carbonyl)thiazole-2-carboxamide
BDBM492157 US10975068, Example 298 5-(4-(Difluoromethyl)-6-(((R)-3,3-dimethylbutan-2-yl)amino)pyridin-3-yl)-N-(2-hydroxy-2-methylpropyl)-4-((S)-2-methylpyrrolidine-1-carbonyl)thiazole-2-carboxamide N-((3R,4S)-3-Fluoro-1- (methylsulfonyl)piperidin- 4-yl)-4-(1-(3- fluoropyridin-4-yl)-2- methyl-1H-imidazol-4- yl)-5- (trifluoromethyl)pyrimidin- 2-amine BDBM569653 US11427567, Example 298
N-((3R,4S)-3-Fluoro-1- (methylsulfonyl)piperidin- 4-yl)-4-(1-(3- fluoropyridin-4-yl)-2- methyl-1H-imidazol-4- yl)-5- (trifluoromethyl)pyrimidin- 2-amine BDBM569653 US11427567, Example 298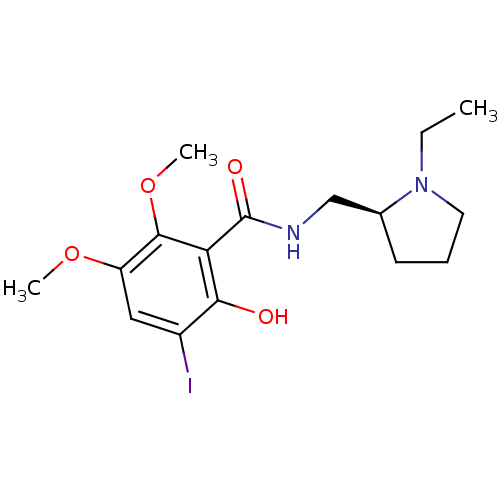 N-(1-Ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-3-iodo-5,6-dimethoxy-benzamide (ioxipride) BDBM50040079 N-(1-Ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-3-iodo-5,6-dimethoxy-benzamide( NCQ 298) CHEMBL308524
N-(1-Ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-3-iodo-5,6-dimethoxy-benzamide (ioxipride) BDBM50040079 N-(1-Ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-3-iodo-5,6-dimethoxy-benzamide( NCQ 298) CHEMBL308524 US20250163063, Example 298 BDBM742776 (3S,4R)-4-((7-(5-(1,1,1-trifluoro-2- methylpropan-2-yl)pyridin-2- yl)pyrrolo[2,1-f][1,2,4]triazin-2- yl)amino)tetrahydro-2H-pyran-3-ol
US20250163063, Example 298 BDBM742776 (3S,4R)-4-((7-(5-(1,1,1-trifluoro-2- methylpropan-2-yl)pyridin-2- yl)pyrrolo[2,1-f][1,2,4]triazin-2- yl)amino)tetrahydro-2H-pyran-3-ol US9738655, Example 298 4-(2-(3-(3-chloro-2-fluoro-6- (trifluoromethyl)phenyl)-4,5- dihydroisoxazole-5-carbonyl)-5- (4-methoxypiperidin-1-yl)- 1,2,3,4-tetrahydroisoquinoline-1- carboxamido)benzoic acid, TFA salt BDBM336075
US9738655, Example 298 4-(2-(3-(3-chloro-2-fluoro-6- (trifluoromethyl)phenyl)-4,5- dihydroisoxazole-5-carbonyl)-5- (4-methoxypiperidin-1-yl)- 1,2,3,4-tetrahydroisoquinoline-1- carboxamido)benzoic acid, TFA salt BDBM336075 ethyl 4-[2-[4-[4-(4- methylpiperazin-1- yl)piperidine-1- carbonyl]anilino]- [1,2,4]triazolo[1,5- a]pyridin-8-yl]-3,6- dihydro-2H-pyridine- 1-carboxylate BDBM367002 US9873709, Example 2-298
ethyl 4-[2-[4-[4-(4- methylpiperazin-1- yl)piperidine-1- carbonyl]anilino]- [1,2,4]triazolo[1,5- a]pyridin-8-yl]-3,6- dihydro-2H-pyridine- 1-carboxylate BDBM367002 US9873709, Example 2-298 2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fluoroisoquinolin- 3-yl)amino)-6-ethyl-5,6-dihydro-4H- pyrazolo [1,5-d][1,4]diazepin-7(8H)-one BDBM591232 US11566003, Compound 298
2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fluoroisoquinolin- 3-yl)amino)-6-ethyl-5,6-dihydro-4H- pyrazolo [1,5-d][1,4]diazepin-7(8H)-one BDBM591232 US11566003, Compound 298 5-(6-((1S,6R,7R)-7-(aminomethyl)-7-(2- fluorophenyl)-3-azabicyclo[4.1.0]heptan-3-yl)-1H- pyrazolo[3,4-b]pyrazin-3-yl)-3,4-dihydroisoquinolin- 1(2H)-one BDBM663460 US20240109900, Example 298
5-(6-((1S,6R,7R)-7-(aminomethyl)-7-(2- fluorophenyl)-3-azabicyclo[4.1.0]heptan-3-yl)-1H- pyrazolo[3,4-b]pyrazin-3-yl)-3,4-dihydroisoquinolin- 1(2H)-one BDBM663460 US20240109900, Example 298 BDBM537473 5-(6-(ethylamino)-1- ((1s,4s)-4-((3-fluoro-4- (trifluoromethyl)pyridin-2- yl)oxy)cyclohexyl)-1H- pyrazolo[4,3-c]pyridin-3- yl)-1-methylpyridin-2(1H)- one US11247990, Example 298
BDBM537473 5-(6-(ethylamino)-1- ((1s,4s)-4-((3-fluoro-4- (trifluoromethyl)pyridin-2- yl)oxy)cyclohexyl)-1H- pyrazolo[4,3-c]pyridin-3- yl)-1-methylpyridin-2(1H)- one US11247990, Example 298 US10214537, Example 298 BDBM358229 1-(4-(3-(4-amino-5-(1-(tetrahydro- 2H-pyran-4-yl)-1H-1,2,3-triazol-5- yl)pyrrolo[2,1-f][1,2,4]triazin-7-yl) phenyl)piperazin-1-yl)ethanone
US10214537, Example 298 BDBM358229 1-(4-(3-(4-amino-5-(1-(tetrahydro- 2H-pyran-4-yl)-1H-1,2,3-triazol-5- yl)pyrrolo[2,1-f][1,2,4]triazin-7-yl) phenyl)piperazin-1-yl)ethanone US10323022, Example 298 1-((3S,4R)-4-(3,4- difluorophenyl)-1-(2- methoxyethyl)pyrrolidin-3-yl)- 3-(4-methyl-3-(3-methyl-1,2,4- oxadiazol-5-yl)-1-phenyl-1H- pyrazol-5-yl)urea BDBM398821
US10323022, Example 298 1-((3S,4R)-4-(3,4- difluorophenyl)-1-(2- methoxyethyl)pyrrolidin-3-yl)- 3-(4-methyl-3-(3-methyl-1,2,4- oxadiazol-5-yl)-1-phenyl-1H- pyrazol-5-yl)urea BDBM398821 US11618753, Example 298 5-[4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][1,2,4]triazin-7-yl]- N-[(3S,4S)-1-(3,3-difluorocyclo- butanecarbonyl)-4-methyl- pyrrolidin-3-yl]-2-methoxy- pyridine-3-carboxamide BDBM599160
US11618753, Example 298 5-[4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][1,2,4]triazin-7-yl]- N-[(3S,4S)-1-(3,3-difluorocyclo- butanecarbonyl)-4-methyl- pyrrolidin-3-yl]-2-methoxy- pyridine-3-carboxamide BDBM599160 US20250230147, Compound 298 BDBM758117 2-(6-(cyclopentylamino)-4-(3-((4- methyl-4H-1,2,4-triazol-3- yl)methyl)oxetan-3-yl)pyridin-2-yl)-6- (((1-methylcyclobutyl)amino)methyl)-4- (trifluoromethyl)isoindolin-1-one
US20250230147, Compound 298 BDBM758117 2-(6-(cyclopentylamino)-4-(3-((4- methyl-4H-1,2,4-triazol-3- yl)methyl)oxetan-3-yl)pyridin-2-yl)-6- (((1-methylcyclobutyl)amino)methyl)-4- (trifluoromethyl)isoindolin-1-one 4-[[8-[4-(2-amino-2- oxo-ethyl)-4-(4- chlorophenyl)-1- piperidyl]- [1,2,4]triazolo[1,5- a]pyridin-2-yl]amino]- N-methyl-N-(1- methyl-4- piperidyl)benzamide BDBM366701 US9873709, Example 1-298
4-[[8-[4-(2-amino-2- oxo-ethyl)-4-(4- chlorophenyl)-1- piperidyl]- [1,2,4]triazolo[1,5- a]pyridin-2-yl]amino]- N-methyl-N-(1- methyl-4- piperidyl)benzamide BDBM366701 US9873709, Example 1-298 BDBM619974 N-(2-(2-((3S,8aR*)-7-(3-Chloro-2-fluoro-6-(1H-tetrazol-1-yl)phenyl)-5-oxo-1,2,3,5,8,8a-hexahydroindolizin-3-yl)-1-methyl-1H-imidazol-4-yl)phenyl)cyclopropanecarboxamide US20230295157, Example 298
BDBM619974 N-(2-(2-((3S,8aR*)-7-(3-Chloro-2-fluoro-6-(1H-tetrazol-1-yl)phenyl)-5-oxo-1,2,3,5,8,8a-hexahydroindolizin-3-yl)-1-methyl-1H-imidazol-4-yl)phenyl)cyclopropanecarboxamide US20230295157, Example 298 N-(2-methyl-5-(2- (methyl(tetrahydro-2H-pyran-4- yl)amino)acetamido)pyridin-3- yl)-2-(1- ((methylsulfonyl)methyl)-1H- pyrazol-4-yl)pyrazolo[5,1- b]thiazole-7-carboxamide US20240109917, Example 298 BDBM664215
N-(2-methyl-5-(2- (methyl(tetrahydro-2H-pyran-4- yl)amino)acetamido)pyridin-3- yl)-2-(1- ((methylsulfonyl)methyl)-1H- pyrazol-4-yl)pyrazolo[5,1- b]thiazole-7-carboxamide US20240109917, Example 298 BDBM664215 US10590140, Example 298 BDBM436522 2-(trimethylsilyl)ethyl (S)-(3-(3-(8-chloro-1- methyl-5-oxo-1,2,3,5- tetrahydro-4H- pyrido[4,3-e] [1,4]diazepin-4- yl)phenoxy)-3- (thiophen-2- yl)propyl)(methyl) carbamate
US10590140, Example 298 BDBM436522 2-(trimethylsilyl)ethyl (S)-(3-(3-(8-chloro-1- methyl-5-oxo-1,2,3,5- tetrahydro-4H- pyrido[4,3-e] [1,4]diazepin-4- yl)phenoxy)-3- (thiophen-2- yl)propyl)(methyl) carbamate US9738655, Example 297 US9738655, Example 298 4-(2-(3-(3-chloro-2-fluoro-6- (trifluoromethyl)phenyl)-4,5- dihydroisoxazole-5-carbonyl)-5- (4-methoxypiperidin-1-yl)- 1,2,3,4-tetrahydroisoquinoline-1- carboxamido)benzoic acid, TFA salt BDBM336074
US9738655, Example 297 US9738655, Example 298 4-(2-(3-(3-chloro-2-fluoro-6- (trifluoromethyl)phenyl)-4,5- dihydroisoxazole-5-carbonyl)-5- (4-methoxypiperidin-1-yl)- 1,2,3,4-tetrahydroisoquinoline-1- carboxamido)benzoic acid, TFA salt BDBM336074 BDBM731910 (R)-3-((1R,3R)-1-(2,6- difluoro-4-(2-(3- (fluoromethyl)azetidin-1- yl)ethoxy)phenyl)-3-methyl- 1,3,4,9-tetrahydro-2H- pyrido[3,4-b]indol-2-yl)- N,N,2-trimethylpropanamide US20250114338, Example 298
BDBM731910 (R)-3-((1R,3R)-1-(2,6- difluoro-4-(2-(3- (fluoromethyl)azetidin-1- yl)ethoxy)phenyl)-3-methyl- 1,3,4,9-tetrahydro-2H- pyrido[3,4-b]indol-2-yl)- N,N,2-trimethylpropanamide US20250114338, Example 298 BDBM753884 US20250206717, Example 298 Preparation of trans-1-(4-((6-(4-(3,4-dihydroisoquinoline-2(1H)-yl)-3-hydroxypiperidine-1-carbonyl)-2-(1H-imidazole-1-yl)pyrimidine-4-yl)amino)piperidine-1-yl)ethane-1-one
BDBM753884 US20250206717, Example 298 Preparation of trans-1-(4-((6-(4-(3,4-dihydroisoquinoline-2(1H)-yl)-3-hydroxypiperidine-1-carbonyl)-2-(1H-imidazole-1-yl)pyrimidine-4-yl)amino)piperidine-1-yl)ethane-1-one N-(6-methoxy-8-quinolyl)-2,4-dinitro-benzenesulfonamide BDBM40733 N-(6-methoxyquinolin-8-yl)-2,4-dinitro-benzenesulfonamide TG1-298-3 cid_16072224 N-(6-methoxyquinolin-8-yl)-2,4-dinitrobenzenesulfonamide N-(6-methoxy-8-quinolinyl)-2,4-dinitrobenzenesulfonamide
N-(6-methoxy-8-quinolyl)-2,4-dinitro-benzenesulfonamide BDBM40733 N-(6-methoxyquinolin-8-yl)-2,4-dinitro-benzenesulfonamide TG1-298-3 cid_16072224 N-(6-methoxyquinolin-8-yl)-2,4-dinitrobenzenesulfonamide N-(6-methoxy-8-quinolinyl)-2,4-dinitrobenzenesulfonamide US10287301, Compound 298 BDBM386369 (1R,3S,5R)-2-(2-(3- acetyl-5-(2- methylpyrazolo[1,5- a]pyrimidin-6-yl)- 1H-indol-1- yl)acetyl)-N-(6- bromopyridin-2- yl)-2- azabicyclo[3.1.0] hexane-3- carboxamide
US10287301, Compound 298 BDBM386369 (1R,3S,5R)-2-(2-(3- acetyl-5-(2- methylpyrazolo[1,5- a]pyrimidin-6-yl)- 1H-indol-1- yl)acetyl)-N-(6- bromopyridin-2- yl)-2- azabicyclo[3.1.0] hexane-3- carboxamide ((2-(3'-(6,7-difluoro-1-methyl-5- (pyrrolidin-1-ylmethyl)-1H- benzo[d]imidazol-2-yl)-2,2'-dimethyl-[1,1'- biphenyl]-3-yl)-6- (difluoromethoxy)benzo[d]oxazol-5- yl)methyl)-L-proline US12187713, Example 298 BDBM711442
((2-(3'-(6,7-difluoro-1-methyl-5- (pyrrolidin-1-ylmethyl)-1H- benzo[d]imidazol-2-yl)-2,2'-dimethyl-[1,1'- biphenyl]-3-yl)-6- (difluoromethoxy)benzo[d]oxazol-5- yl)methyl)-L-proline US12187713, Example 298 BDBM711442 rac-2-{[1-aminopropan-2-yl]oxy}-N-(2-chloro-6-fluorophenyl)-5-fluoro-4-(3-oxo-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyridin-2(3H)-yl)benzamide, salt with hydrochloric acid BDBM490630 US10968216, Example 298
rac-2-{[1-aminopropan-2-yl]oxy}-N-(2-chloro-6-fluorophenyl)-5-fluoro-4-(3-oxo-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyridin-2(3H)-yl)benzamide, salt with hydrochloric acid BDBM490630 US10968216, Example 298 BDBM693380 US12071425, Compound 300 US12071425, Compound 301 US12071425, Compound 298 N-(4-((3-(2-((1,1-difluoro-5- azaspiro[2.5]octan-7- yl)amino)pyrimidin-4-yl)pyridin-2- yl)oxy)-2,3,6-trifluorophenyl)-1- phenylmethanesulfonamide US12071425, Compound 299
BDBM693380 US12071425, Compound 300 US12071425, Compound 301 US12071425, Compound 298 N-(4-((3-(2-((1,1-difluoro-5- azaspiro[2.5]octan-7- yl)amino)pyrimidin-4-yl)pyridin-2- yl)oxy)-2,3,6-trifluorophenyl)-1- phenylmethanesulfonamide US12071425, Compound 299 US20250144094, Example 298 7-(2-((2-Ethyl-4-(1-(2,2,2- trifluoroethyl)piperidin-4- yl)phenyl)amino)-5- (trifluoromethyl)pyrimidin-4-yl)-4- (oxetan-3-yl)-3,4- dihydrothieno[2,3-f][1,4]thiazepin- 5(2H)-one 1,1-dioxide BDBM738066
US20250144094, Example 298 7-(2-((2-Ethyl-4-(1-(2,2,2- trifluoroethyl)piperidin-4- yl)phenyl)amino)-5- (trifluoromethyl)pyrimidin-4-yl)-4- (oxetan-3-yl)-3,4- dihydrothieno[2,3-f][1,4]thiazepin- 5(2H)-one 1,1-dioxide BDBM738066 Preparation of (14S)-8-[3-(2-{dispiro[2.0.2.1]heptan-7-yl}-2,2-dideuterio-ethoxy)-1H-pyrazol-1-yl]-12,12-dimethyl(20-deuterio)-2λ6-thia-3,9,11,18,23-pentaazatetracyclo[17.3.1.111,14.05,10]tetracosa-1(23),5,7,9,19,21-hexaene-2,2,4-trione US11866450, Compound 298 BDBM644883
Preparation of (14S)-8-[3-(2-{dispiro[2.0.2.1]heptan-7-yl}-2,2-dideuterio-ethoxy)-1H-pyrazol-1-yl]-12,12-dimethyl(20-deuterio)-2λ6-thia-3,9,11,18,23-pentaazatetracyclo[17.3.1.111,14.05,10]tetracosa-1(23),5,7,9,19,21-hexaene-2,2,4-trione US11866450, Compound 298 BDBM644883 US10287301, Compound 257 (1R,3S,5R)-2-(2-(3- acetyl-5-(2- methylpyrazolo[1,5- a]pyrimidin-6-yl)- 1H-indazol-1- yl)acetyl)-N-(6- bromopyridin-2- yl)-2- azabicyclo[3.1.0] hexane-3- carboxamide BDBM386328 US10822352, Comp No. 298
US10287301, Compound 257 (1R,3S,5R)-2-(2-(3- acetyl-5-(2- methylpyrazolo[1,5- a]pyrimidin-6-yl)- 1H-indazol-1- yl)acetyl)-N-(6- bromopyridin-2- yl)-2- azabicyclo[3.1.0] hexane-3- carboxamide BDBM386328 US10822352, Comp No. 298 [(3R,6R,7S,8E,22R)-6′-Chloro-12,12-dimethyl-13,15,15-trioxo-spiro[11,20-dioxa-15-thia-1,14-diazatetracyclo[14.7.2.03,6.019,24]pentacosa-8,16,18,24-tetraene-22,1′-isochromane]-7-yl] N-methyl-N-(oxetan-3-yl)carbamate BDBM520601 US11130769, Ex. No. 298
[(3R,6R,7S,8E,22R)-6′-Chloro-12,12-dimethyl-13,15,15-trioxo-spiro[11,20-dioxa-15-thia-1,14-diazatetracyclo[14.7.2.03,6.019,24]pentacosa-8,16,18,24-tetraene-22,1′-isochromane]-7-yl] N-methyl-N-(oxetan-3-yl)carbamate BDBM520601 US11130769, Ex. No. 298 6-((Z)-2-(5-((1S,5S,6S)-3-amino-1-((1R,5R)-3,7-dioxa-9-azabicyclo[3.3.1]nonane-9-carbonyl)-5-methyl-2-thia-4-azabicyclo[4.1.0]hept-3-en-5-yl)-6-fluoropyridin-3-yl)-1-fluorovinyl)nicotinonitrile US10246429, Example 298 BDBM372892
6-((Z)-2-(5-((1S,5S,6S)-3-amino-1-((1R,5R)-3,7-dioxa-9-azabicyclo[3.3.1]nonane-9-carbonyl)-5-methyl-2-thia-4-azabicyclo[4.1.0]hept-3-en-5-yl)-6-fluoropyridin-3-yl)-1-fluorovinyl)nicotinonitrile US10246429, Example 298 BDBM372892 BDBM646310 N-((R)-1-(2-(5-((1S,4R,5R)-4- amino-2-azabicyclo[3.2.1]octane- 2-carbonyl)-7-methoxy-1-methyl- 1H-benzo[d]imidazol-2-yl)-1- (cyclopropylmethyl)-1H- pyrrolo[2,3-b]pyridin-6-yl)ethyl)- 2-chloroisonicotinamide US11878965, Example 298
BDBM646310 N-((R)-1-(2-(5-((1S,4R,5R)-4- amino-2-azabicyclo[3.2.1]octane- 2-carbonyl)-7-methoxy-1-methyl- 1H-benzo[d]imidazol-2-yl)-1- (cyclopropylmethyl)-1H- pyrrolo[2,3-b]pyridin-6-yl)ethyl)- 2-chloroisonicotinamide US11878965, Example 298 BDBM671286 US20240148821, Compound 298 (3S,9S,18S,21S,25S,28S,34S)-9-(cyclohexylmethyl)-3-[2-[3-fluoro-4-(trifluoromethyl)phenyl]ethyl]-21,28-diisobutyl-7,10,13,16,22,29-hexamethyl-18-[(1S)-1-methylpropyl]-25-(piperidine-1-carbonyl)spiro[1,4,7,10,13,16,19,22,26,29,32-undecazabicyclo[32.3.0]heptatriacontane-31,1'-cyclopentane]-2,5,8,11,14,17,20,23,27,30,33-undecone
BDBM671286 US20240148821, Compound 298 (3S,9S,18S,21S,25S,28S,34S)-9-(cyclohexylmethyl)-3-[2-[3-fluoro-4-(trifluoromethyl)phenyl]ethyl]-21,28-diisobutyl-7,10,13,16,22,29-hexamethyl-18-[(1S)-1-methylpropyl]-25-(piperidine-1-carbonyl)spiro[1,4,7,10,13,16,19,22,26,29,32-undecazabicyclo[32.3.0]heptatriacontane-31,1'-cyclopentane]-2,5,8,11,14,17,20,23,27,30,33-undecone US10172859, Example 298 2-[2-Chloro-4-fluoro- 5-(7-morpholin-4-yl- quinazolin-4-yl)- phenyl]-2-[1,2,4]- triazolo[4,3-a]pyridin- 5-ylacetamide1H NMR (500 MHz, DMSO-d6) ppm = 9.21 (d,J = 0.8, 1H), 9.08 (s, 1H), 8.13 (s, 1H), 7.88 (d,J = 9.5, 1H), 7.79 (d, J = 9.2, 1H), 7.73 (s, 1H), 7.57-7.48 (m, 3H), 7.38 (dd, J = 9.2, 6.9, 1H), 7.19 (d,J = 2.3, 1H), 6.57 (d, J = 6.9, 1H), 5.84 (s, 1H), 3.80-3.75 (m, 4H), 3.48-3.42 (m, 4H). BDBM315871 US9732094, Example 298
US10172859, Example 298 2-[2-Chloro-4-fluoro- 5-(7-morpholin-4-yl- quinazolin-4-yl)- phenyl]-2-[1,2,4]- triazolo[4,3-a]pyridin- 5-ylacetamide1H NMR (500 MHz, DMSO-d6) ppm = 9.21 (d,J = 0.8, 1H), 9.08 (s, 1H), 8.13 (s, 1H), 7.88 (d,J = 9.5, 1H), 7.79 (d, J = 9.2, 1H), 7.73 (s, 1H), 7.57-7.48 (m, 3H), 7.38 (dd, J = 9.2, 6.9, 1H), 7.19 (d,J = 2.3, 1H), 6.57 (d, J = 6.9, 1H), 5.84 (s, 1H), 3.80-3.75 (m, 4H), 3.48-3.42 (m, 4H). BDBM315871 US9732094, Example 298 3-cyano-4-fluoranyl-N-(6-methoxyquinolin-8-yl)benzenesulfonamide cid_16072228 3-cyano-4-fluoro-N-(6-methoxy-8-quinolinyl)benzenesulfonamide 3-cyano-4-fluoro-N-(6-methoxy-8-quinolyl)benzenesulfonamide 3-cyano-4-fluoro-N-(6-methoxyquinolin-8-yl)benzenesulfonamide BDBM32141 TG1-298-2
3-cyano-4-fluoranyl-N-(6-methoxyquinolin-8-yl)benzenesulfonamide cid_16072228 3-cyano-4-fluoro-N-(6-methoxy-8-quinolinyl)benzenesulfonamide 3-cyano-4-fluoro-N-(6-methoxy-8-quinolyl)benzenesulfonamide 3-cyano-4-fluoro-N-(6-methoxyquinolin-8-yl)benzenesulfonamide BDBM32141 TG1-298-2 BDBM463046 [(1R,2S,4R)-4-{[5-({4-[(4R)-2-chloro-6,7-dihydro-4H-furo[3,2-c]pyran-4-yl]-5-methyl- 2-thienyl}carbonyl)pyrimidin-4-yl]amino}-2-hydroxycyclopentyl]methyl sulfamate US10780090, Compound I-298 US10780090, Compound I-298a US10780090, Compound I-298b
BDBM463046 [(1R,2S,4R)-4-{[5-({4-[(4R)-2-chloro-6,7-dihydro-4H-furo[3,2-c]pyran-4-yl]-5-methyl- 2-thienyl}carbonyl)pyrimidin-4-yl]amino}-2-hydroxycyclopentyl]methyl sulfamate US10780090, Compound I-298 US10780090, Compound I-298a US10780090, Compound I-298b BDBM573980 US11453697, Example 298 2-amino-9- [(5R,7R,8R,12aR,14R,15S,15aR,16R)-15- fluoro-2,10,16-trihydroxy-14-(6-oxo-1,6- dihydro-9H-purin-9-yl)-2,10- disulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]- 1,9-dihydro-6H-purin-6-one (Diastereomer 3)
BDBM573980 US11453697, Example 298 2-amino-9- [(5R,7R,8R,12aR,14R,15S,15aR,16R)-15- fluoro-2,10,16-trihydroxy-14-(6-oxo-1,6- dihydro-9H-purin-9-yl)-2,10- disulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]- 1,9-dihydro-6H-purin-6-one (Diastereomer 3) (Method 8): 1-((R)-3-((7-(8-ethynyl-7-fluoronaphthalen-1-yl)-8-fluoro-2-(((2R,7aS)-2-fluorotetrahydro-1H-pyrrolizin-7a(5H)-yl)methoxy)pyrido[4,3-d]pyrimidin-4-yl)(methyl)amino)pyrrolidin-1-yl)-2-(1-methylazetidin-3-ylidene)ethan-1-one BDBM713388 US20250019387, Example 298
(Method 8): 1-((R)-3-((7-(8-ethynyl-7-fluoronaphthalen-1-yl)-8-fluoro-2-(((2R,7aS)-2-fluorotetrahydro-1H-pyrrolizin-7a(5H)-yl)methoxy)pyrido[4,3-d]pyrimidin-4-yl)(methyl)amino)pyrrolidin-1-yl)-2-(1-methylazetidin-3-ylidene)ethan-1-one BDBM713388 US20250019387, Example 298 BDBM705134 2-((1H-pyrrolo[2,3-b]pyridin-5-yl) oxy)-4-(6-((R)-4-((2,3- dihydrobenzofuran-6-yl)methyl)-2- (2-isopropylphenyl)piperazin-1-yl)- 2-azaspiro[3.3]heptan-2-yl)-N-((4- ((((1r,4r)-4-hydroxy-4- methylcyclohexyl)methyl)amino)- 3-nitrophenyl)sulfonyl)benzamide US20240376097, Example 298
BDBM705134 2-((1H-pyrrolo[2,3-b]pyridin-5-yl) oxy)-4-(6-((R)-4-((2,3- dihydrobenzofuran-6-yl)methyl)-2- (2-isopropylphenyl)piperazin-1-yl)- 2-azaspiro[3.3]heptan-2-yl)-N-((4- ((((1r,4r)-4-hydroxy-4- methylcyclohexyl)methyl)amino)- 3-nitrophenyl)sulfonyl)benzamide US20240376097, Example 298 US12310975, Example 298 2-{[6-({5-chloro-2-[4- ({6-[2-(2,6- dioxopiperidin-3-yl)-1,3- dioxo-2,3-dihydro-1H- isoindol-5-yl]-2,6- diazaspiro[3.3]heptan-2- yl}methyl)piperidin-1- yl]pyrimidin-4- yl}amino)-1-ethyl-2-oxo- 1,2-dihydroquinolin-3- yl]oxy}-N- methylacetamide BDBM743759
US12310975, Example 298 2-{[6-({5-chloro-2-[4- ({6-[2-(2,6- dioxopiperidin-3-yl)-1,3- dioxo-2,3-dihydro-1H- isoindol-5-yl]-2,6- diazaspiro[3.3]heptan-2- yl}methyl)piperidin-1- yl]pyrimidin-4- yl}amino)-1-ethyl-2-oxo- 1,2-dihydroquinolin-3- yl]oxy}-N- methylacetamide BDBM743759 US11034692, Compound 299 BDBM504030 (1R,2R)—N-[8-amino-6-(2-methylphenyl)-2,7-naphthyridin-3-yl]-2-(1-methyl-1H-pyrazol-4-yl)cyclopropane-1-carboxamide (Compound 298) and (1S,2S)—N-[8-amino-6-(2-methylphenyl)-2,7-naphthyridin-3-yl]-2-(1-methyl-1H-pyrazol-4-yl)cyclopropane-1-carboxamide
US11034692, Compound 299 BDBM504030 (1R,2R)—N-[8-amino-6-(2-methylphenyl)-2,7-naphthyridin-3-yl]-2-(1-methyl-1H-pyrazol-4-yl)cyclopropane-1-carboxamide (Compound 298) and (1S,2S)—N-[8-amino-6-(2-methylphenyl)-2,7-naphthyridin-3-yl]-2-(1-methyl-1H-pyrazol-4-yl)cyclopropane-1-carboxamide  US11203600, Example 298 BDBM530217 Preparation of 4-[(4R)-3,3-difluoro-4-methyl-1-pyrimidin-2-yl-piperidine-4-carbonyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile and 4-[(4S)-3,3-difluoro-4-methyl-1-pyrimidin-2-yl-piperidine-4-carbonyl]-3,5-dihydro-2H-pyrido [3,4-f][1,4]oxazepine-9-carbonitrile
US11203600, Example 298 BDBM530217 Preparation of 4-[(4R)-3,3-difluoro-4-methyl-1-pyrimidin-2-yl-piperidine-4-carbonyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile and 4-[(4S)-3,3-difluoro-4-methyl-1-pyrimidin-2-yl-piperidine-4-carbonyl]-3,5-dihydro-2H-pyrido [3,4-f][1,4]oxazepine-9-carbonitrile US11453697, Example 299 US11453697, Example 298 US11453697, Example 296 2-amino-9- [(5R,7R,8R,12aR,14R,15S,15aR,16R)-15- fluoro-2,10,16-trihydroxy-14-(6-oxo-1,6- dihydro-9H-purin-9-yl)-2,10- disulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]- 1,9-dihydro-6H-purin-6-one (Diastereomer 1) BDBM573978
US11453697, Example 299 US11453697, Example 298 US11453697, Example 296 2-amino-9- [(5R,7R,8R,12aR,14R,15S,15aR,16R)-15- fluoro-2,10,16-trihydroxy-14-(6-oxo-1,6- dihydro-9H-purin-9-yl)-2,10- disulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]- 1,9-dihydro-6H-purin-6-one (Diastereomer 1) BDBM573978 US11866450, Compound 301 US11866450, Compound 253 US11866450, Compound 298 Preparation of (14S)-8-[3-(2-{dispiro[2.0.2.1]heptan-7-yl}ethoxy)-1H-pyrazol-1-yl]-12,12-dimethyl-2λ6-thia-3,9,11,18,23-pentaazatetracyclo[17.3.1.111,14.05,10]tetracosa-1(22),5,7,9,19(23),20-hexaene-2,2,4-trione US11866450, Compound 300 BDBM644782 US11866450, Compound 195 US11866450, Compound 252 US11866450, Compound 274
US11866450, Compound 301 US11866450, Compound 253 US11866450, Compound 298 Preparation of (14S)-8-[3-(2-{dispiro[2.0.2.1]heptan-7-yl}ethoxy)-1H-pyrazol-1-yl]-12,12-dimethyl-2λ6-thia-3,9,11,18,23-pentaazatetracyclo[17.3.1.111,14.05,10]tetracosa-1(22),5,7,9,19(23),20-hexaene-2,2,4-trione US11866450, Compound 300 BDBM644782 US11866450, Compound 195 US11866450, Compound 252 US11866450, Compound 274 US11203600, Example 298 BDBM530216 Preparation of 4-[(4R)-3,3-difluoro-4-methyl-1-pyrimidin-2-yl-piperidine-4-carbonyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile and 4-[(4S)-3,3-difluoro-4-methyl-1-pyrimidin-2-yl-piperidine-4-carbonyl]-3,5-dihydro-2H-pyrido [3,4-f][1,4]oxazepine-9-carbonitrile US11203600, Example 297
US11203600, Example 298 BDBM530216 Preparation of 4-[(4R)-3,3-difluoro-4-methyl-1-pyrimidin-2-yl-piperidine-4-carbonyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile and 4-[(4S)-3,3-difluoro-4-methyl-1-pyrimidin-2-yl-piperidine-4-carbonyl]-3,5-dihydro-2H-pyrido [3,4-f][1,4]oxazepine-9-carbonitrile US11203600, Example 297 US10435369, Example 299 US10435369, Example 298 BDBM414411 4-fluoro-N-((3R,3aS,9bS)-9b-((4-fluorophenyl)sulfonyl)-7-(perfluoropropan-2-yl)-2,3,3a,4,5,9b-hexahydro-1H-cyclopenta[a]naphthalen-3-yl)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide and N-((3R,3aS,9bS)-9b-((4-fluorophenyl)sulfonyl)-7-(perfluoropropan-2-yl)-2,3,3a4,5,9b-hexahydro-1H-cyclopenta[a]naphthalen-3-yl)-3,6-dihydro-2H-thiopyran-4-carboxamide 1,1-dioxide
US10435369, Example 299 US10435369, Example 298 BDBM414411 4-fluoro-N-((3R,3aS,9bS)-9b-((4-fluorophenyl)sulfonyl)-7-(perfluoropropan-2-yl)-2,3,3a,4,5,9b-hexahydro-1H-cyclopenta[a]naphthalen-3-yl)tetrahydro-2H-thiopyran-4-carboxamide 1,1-dioxide and N-((3R,3aS,9bS)-9b-((4-fluorophenyl)sulfonyl)-7-(perfluoropropan-2-yl)-2,3,3a4,5,9b-hexahydro-1H-cyclopenta[a]naphthalen-3-yl)-3,6-dihydro-2H-thiopyran-4-carboxamide 1,1-dioxide