Compound (576)
Article Title (3)
Assay (26)
Sridhar, J; Sfondouris, ME; Bratton, MR; Nguyen, TL; Townley, I; Klein Stevens, CL; Jones, FE Identification of quinones as HER2 inhibitors for the treatment of trastuzumab resistant breast cancer. Bioorg Med Chem Lett 24: 126 -31 (2014) Identification of a novel potent CDK inhibitor degrading cyclinK with a superb activity to reverse trastuzumab-resistance in HER2-positive breast cancer in vivo. Singh, A; Chang, TY; Kaur, N; Hsu, KC; Yen, Y; Lin, TE; Lai, MJ; Lee, SB; Liou, JP CAP rigidification of MS-275 and chidamide leads to enhanced antiproliferative effects mediated through HDAC1, 2 and tubulin polymerization inhibition. Eur J Med Chem 215: (2021)
ChEMBL_481422 (CHEMBL1008680) Inhibition of GST-fused human recombinant Cdc25B2 (275-539) catalytic domain ChEMBL_42939 (CHEMBL654581) Inhibition of [3H]cAMP (NEN NET-275) binding to Calcium-Independent Phosphodiesterase from rat brain. ChEMBL_27738 (CHEMBL644205) Displacement of [3H]DPCPX from human Adenosine A1 receptor expressed in CHO cells; range 275-386 ChEMBL_462156 (CHEMBL944984) Inhibition of HDAC1 in human T24 cells assessed as induction of histone H4 acetylation after 16 hrs relative to MS-275 ChEMBL_1458488 (CHEMBL3370374) Inhibition of SUMO-tagged human TTK (1-275 residues) compound pre-incubated for 15 mins prior ATP addition by MBP-based assay ChEMBL_965919 (CHEMBL2394350) Inhibition of human recombinant CLK3 (275 to 632 amino acids) expressed in Escherichia coli BL21(DE3) preincubated for 10 mins prior to substrate addition by LDH assay ChEBML_1654001 Inhibition of human CLK3 (275 to 632 residues) expressed in Escherichia coli BL21(DE3) preincubated for 10 mins followed by substrate addition by pyruvate kinase and lactate dehydrogenase coupled enzyme assay ChEMBL_1654001 (CHEMBL4003367) Inhibition of human CLK3 (275 to 632 residues) expressed in Escherichia coli BL21(DE3) preincubated for 10 mins followed by substrate addition by pyruvate kinase and lactate dehydrogenase coupled enzyme assay ChEMBL_1924421 (CHEMBL4427377) Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated for 5 mins before ATP addition by indirect ELISA ChEMBL_2193632 (CHEMBL5105992) Inhibition of human CLK3-4 (275 to 632 residues) expressed in Escherichia coli BL21 (DE3) Turner using S6-peptide as substrate incubated for 60 mins in presence of [gamma-32P]ATP by radiometric kinase assay ChEMBL_1628974 (CHEMBL3871600) Inhibition of human recombinant full-length N-terminal GST-tagged Aurora C (1 to 275 end residues) expressed in baculovirus expression system using GLRRASLG-NH2 as substrate after 20 mins in presence of [gamma-33P]-ATP by liquid scintillation counting Radioligand Binding Assay The compounds were evaluated using well established radioligand binding assays protocols (Galli, A. et al., J. Exp. Biol. 1995, 198, 2197-2212; Giros, B. et al., Trends Pharmcol. Sci. 1993, 14, 43-49; Gu, H. et al., J. Biol. Chem. 1994, 269(10), 7124-7130; Shearman, L. P. et al, Am. J. Physiol., 1998, 275(6 Pt 1), C1621-1629; Wolf, W. A. et al., J. Biol. Chem. 1992, 267(29), 20820-20825). The human recombinant transporter proteins dopamine (DAT), norepinephrine (NET) and serotonin (SERT) were selected for the in vitro assays. CYP19 Inhibition Assay The enzyme was obtained from the microsome fraction of fresh human placenta (St. Josephs Krankenhaus, Saarbrucken-Dudweiler, Germany) according to the method of Thompson and Siiteri (Thompson, E. A. & Siiteri, P. K., J. Biol. Chem. 249: 5364-5372 (1974)). The isolated microsomes were suspended in a mini mum volume of phosphate buffer (0.05 M; pH 7.4; 20% glycerol). In addition, DTT (10 mM) and EDTA (1 mM) were added to protect the enzyme from degradation reactions. The protein concentration was determined according to Lowry et al. (Lowry, O. H. et al., J. Biol. Chem. 193: 265-275 (1951)) and should be about 35 mg/ml after the processing. Radioligand Binding Assay The compounds were evaluated using well established radioligand binding assays protocols (Galli, A. et al., J. Exp. Biol. 1995, 198, 2197-2212; Giros, B. et al., Trends Pharmcol. Sci. 1993, 14, 43-49; Gu, H. et al., J. Biol. Chem. 1994, 269(10), 7124-7130; Shearman, L. P. et al, Am. J. Physiol., 1998, 275 (6 Pt 1), C1621-1629; Wolf, W. A. et al., J. Biol. Chem. 1992, 267(29), 20820-20825). The human recombinant transporter proteins dopamine (DAT), norepinephrine (NET) and serotonin (SERT) were selected for the in vitro assays. The radioligand binding assays were carried out at 11 different test concentrations 0.1 nm to 1 μM. In Vitro Pharmacology Assay The monoamine transporters inhibitory activities of selected cycloalkylmethylamines of Formula (I) are reported herein. The compounds were evaluated using well established radioligand binding assays protocols (Galli, A. et al., J. Exp. Biol. 1995, 198, 2197-2212; Giros, B. et al., Trends Pharmcol. Sci. 1993, 14, 43-49; Gu, H. et al., J. Biol. Chem. 1994, 269(10), 7124-7130; Shearman, L. P. et al, Am. J. Physiol., 1998, 275(6 Pt 1), C1621-1629; Wolf, W. A. et al., J. Biol. Chem. 1992, 267(29), 20820-20825). The human recombinant transporter proteins dopamine (DAT), norepinephrine (NET) and serotonin (SERT) were selected for the in vitro assays. The radioligand binding assays were carried out at 11 different test concentrations 0.1 nM to 1 μM. Radioligand Binding Assay The monoamine transporters inhibitory activities of selected compounds cycloalkylmethylamine derivatives comprising Formula (I) are reported herein. The compounds were evaluated using well established radioligand binding assays protocols (Galli, A. et al., J. Exp. Biol. 1995, 198, 2197-2212; Giros, B. et al., Trends Pharmcol. Sci. 1993, 14, 43-49; Gu, H. et al., J. Biol. Chem. 1994, 269(10), 7124-7130; Shearman, L. P. et al, Am. J. Physiol., 1998, 275(6 Pt 1), C1621-1629; Wolf, W. A. et al., J. Biol. Chem. 1992, 267(29), 20820-20825). The human recombinant transporter proteins dopamine (DAT), norepinephrine (NET) and serotonin (SERT) were selected for the in vitro assays. The radioligand binding assays were carried out at 11 different test concentrations 0.1 nm to 1 μM. Biological Assay To determine the effectiveness of representative compounds of this invention as histamine-3 receptor ligands (H3 receptor ligands), the following tests were conducted according to previously described methods (see Arrang J M, et al. European Journal of Pharmacology 1990; 188: 219-227; Tedford C E, et al. Journal of Pharmacology and Experimental Therapeutics 1995; 275: 598-604; Leurs R, et al. Journal of Pharmacology and Experimental Therapeutics 1996; 276: 1009-1015; and Cheng Y-C, et al. Biochemical Pharmacology 1973; 22: 3099-3108). Membrane preparations were incubated with [3H]-N-alpha-methylhistamine (0.5-1.0 nM) in the presence or absence of increasing concentrations of ligands for H3 receptor competition binding. The binding incubations were conducted in a final volume of 0.5 mL TE buffer at 25° C. and were terminated after 30 minutes. Thioperamide (30 uM) was used to define non-specific binding. All binding reactions were terminated by filtration under vacuum. RIPK2 Inhibition Assay Active RIPK2 was purchased from Life Technologies as His-tagged of catalytic domain (amin acids 1-299) of human RIPK2 kinase expressed in insect cells Amino terminal 6 histidine, sumo tagged human TTK (residues 1-275) was expressed in E. coli, and purified to >95% homogeneity by Ni2+ agarose, gel filtration, and ion exchange chromatography.RIPK2 activity was measured using an indirect ELISA detection system. His-RIPK2 (0.6 nM) was incubated in the presence of 6 μM ATP (Sigma cat #A7699), 20 mM Hepes, pH 7.5, 1 mM EGTA, 2.5 mM MgCl2, 2.5 mM MnCl2 and 0.01% Triton X-100 in a 96 well microtitre plate pre-coated with amino terminal 6 histidine, sumo tagged TTK (amino acid residues 1-275). The reaction was allowed to proceed for 30 minutes, followed by 5 washes of the plate with Wash Buffer (phosphate buffered saline supplemented with 0.2% Tween 20), and incubation for 30 minutes with a 1:3000 dilution of primary antibody (Cell Signaling cat #9381). The plate was washed 5 times with Wash Buffer, incubated for 30 minutes in the presence of secondary antibody coupled to horse radish peroxidase (BioRad cat #1721019, 1:3000 concentration), washed an additional 5 times with Wash Buffer, and incubated in the presence of TMB substrate (Sigma cat #T0440). The colorimetric reaction was allowed to continue for 5 minutes, followed by addition of stop solution (0.5 N H2SO4), and quantified by detection at 450 nm with either a monoChromatic or filter based plate reader (Molecular Devices M5 or Beckman DTX880, respectively).Compound inhibition was determined at either a fixed concentration (10 μM) or at a variable inhibitor concentration (typically 50 μM to 0.1 μM in a 10 point dose response titration). Compounds were pre-incubated in the presence of enzyme for 15 minutes prior to addition of ATP and the activity remaining quantified using the above described activity assay. The % Inhibition of a compound was determined using the following formula; % Inhibition=100×(1−(experimental value−background value)/(high activity control−background value)). The IC50 value was determined using a non-linear 4 point logistic curve fit (XLfit4, IDBS) with the formula; (A+(B/(1+((x/C){circumflex over ( )} D)))), where A=background value, B=range, C=inflection point, D=curve fit parameter. RIPK2 Inhibition Assay RIPK2 activity was measured using an indirect ELISA detection system. His RIPK2 (0.6 nM) was incubated in the presence of 6 μM ATP (Sigma cat # A7699), 20 mM Hepes, pH 7.5, 1 mM EGTA, 2.5 mM MgCl2, 2.5 mM MnCl2 and 0.01% Triton X-100 in a 96 well microtitre plate pre-coated with amino terminal 6 histidine, sumo tagged TTK (amino acid residues 1-275). The reaction was allowed to proceed for 30 minutes, followed by 5 washes of the plate with Wash Buffer (phosphate buffered saline supplemented with 0.2% Tween 20), and incubation for 30 minutes with a 1:3000 dilution of primary antibody (Cell Signaling cat #9381). The plate was washed 5 times with Wash Buffer, incubated for 30 minutes in the presence of secondary antibody coupled to horse radish peroxidase (BioRad cat #1721019, 1:3000 concentration), washed an additional 5 times with Wash Buffer, and incubated in the presence of TMB substrate (Sigma cat # T0440). The colorimetric reaction was allowed to continue for 5 minutes, followed by addition of stop solution (0.5 N H2SO4), and quantified by detection at 450 nm with either a monoChromatic or filter based plate reader (Molecular Devices M5 or Beckman DTX880, respectively). CYP3A4 Inhibitory Activity Assay Test compounds, DMSO (negative control), and ketoconazole (positive control) were diluted to 4× final concentrations in water. The standard final Compound concentrations were 37, 111, 333, 1000, and 3000 nM. 12.5 μL of the Compound dilutions were transferred to a white 96-well plate. 1450 μL (enough for a whole plate) of 4× assay buffer (400 mM potassium phosphate buffer (10 mL 1M potassium phosphate buffer: 8.02 mL 1M K2HPO4 +1.98 mL 1M KH2PO4 (1.4 g K2HPO4 +0.27 g KH2PO4 in 10 mL H2O), 32 μM Luciferin-IPA (Promega V9002)) 580 μl of 1 M K3PO4 buffer, 870 μL H2O, 14 μL of 3 mM Luciferin-IPA, and 18 μL of human liver microsome (Sigma M0317-1VL) was made. 12.5 μL of 4× assay buffer was added to each well. For the well of blank control, 12.5 μL of 4× assay buffer without liver microsome was added. The plate was incubated at room temperature for 15 minutes. 2.75 mL NADPH buffer was made as follows: 2.42 mL H2O, 275 μL solution A and 55 μL solution B (NADPH regeneration system, Promega V9510). 25 μL of the buffer was added to each well. The plates were incubated at 37° C. for 11 minutes. 50 μL of luciferin detection reagent (Promega V9002) was added and the plates were incubated at room temperature for 5 minutes. The plate was read with a luminometer. 3H]-Spiperone Binding Assay CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were re-suspended in 20 mM HEPES, 2 mM EDTA (pH 7.4), homogenised and centrifuged at 40,000 g (20 min, 4° C.). After re-suspension, homogenization and centrifugation as above, the final pellet was re-suspended in 20 mM HEPES, 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA (pH 7.4) and aliquots were kept at −80° C. [3H]-Spiperone Binding experiments were performed in 96 deep-well polypropylene plates in 50 mM Tris/HCl, 120 mM NaCl, 5 mM KCl, 5 mM MgCl2 (pH 7.4). Compounds of invention were serially diluted in DMSO at 100 fold final concentrations in the assay (1% DMSO final in the assay). Displacement was performed in the presence of 0.08 nM [3H]-Spiperone. The reaction was initiated by the addition of membrane suspension (2 μg of protein for CHO-hD2 membranes) and lasted for 120 min at 23° C. in a final volume of 1000 μl. Non specific binding (NSB) was determined in the presence of 0.1 μM Spiperone. The binding reaction was stopped by rapid filtration through GF/B filterplates pre-soaked in 0.5% polyetylenimmine (PEI) using a Packard cell harvester. After washing with ice-cold 0.9% NaCl, the plate was left to dry before the addition of Microscint 20 (50 μl/well, PerkinElmer). Radioactivity was counted with a TopCount (PerkinElmer). Data were analysed by non-linear regression analysis using GraphPad Prism 5.0 (GraphPad Software) or XLfit Version 5.2.0.0 (Copyright 2006-2009 ID Business Solutions Ltd). Saturation binding experiments were performed similar to the competition binding experiments using a radioligand concentrations ranging from 0.011 to 3.0 nM. Ref: Durcan M. J. et al. (1995). Is Clozapine selective for the dopamine D4 receptor? Life Sciences, 57: 275-283. Petrus J. et al. (2001). Caliper Endpoint Assay for HDAC Enzymatic Activity Assay HDAC reactions were assembled in 384 well plates (Greiner) in a total volume of 20 μL as follows: HDAC proteins (and their regulatory subunit, if applicable) were pre-diluted in the assay buffer comprising: 100 mM HEPES, pH 7.5, 0.1% BSA, 0.01% Triton X-100, 25 mM KCl and dispensed into a 384 well plate (10 μL per well). Test compounds were serially pre-diluted in 100% DMSO using 3-fold dilution steps and added to the protein samples by acoustic dispensing (Labcyte Echo). Concentration of DMSO was equalized to 1% in all samples. Final compound concentration in assays typically ranged from 100 μM to 0.00056 μM for a 12-point concentration-response format. Reference compounds such as TSA (trichostatin A) and MS-275, were tested in an identical manner.Control samples (0%-inhibition in the absence of inhibitor, DMSO only) and 100%-inhibition (in the absence of enzyme) were assembled in replicates of four (for each caliper sipper) and used to calculate the %-inhibition in the presence of compounds. At this step compounds were pre-incubated with enzyme for 30 minutes at room temperature (20-23° C.). The reactions were initiated by addition of 10 μL of the FAM-labeled substrate peptide (see table above) pre-diluted in the same assay buffer. Final concentration of substrate peptide was 1 μM. The reactions were allowed to proceed at room temperature (20-23° C.). Typical incubation times for each HDAC, based on pre-determined enzyme progress curves, vary and are listed in table above.Following incubation, the reactions were quenched by addition of 50 μL of termination buffer (100 mM HEPES, pH7.5, 0.01% Triton X-100, 0.05% SDS). Terminated plates were analyzed on a microfluidic electrophoresis instrument (Caliper LabChip® 3000, Caliper Life Sciences/Perkin Elmer) which enables electrophoretic separation of deacetylated product from acetylated substrate. A change in the relative intensity of the peptide substrate and product is the parameter measured. [3H]-Spiperone Binding Assay at hD2 Recombinant Receptor CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were re-suspended in 20 mM HEPES, 2 mM EDTA (pH 7.4), homogenised and centrifuged at 40,000 g (20 min, 4° C.). After re-suspension, homogenization and centrifugation as above, the final pellet was re-suspended in 20 mM HEPES, 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA (pH 7.4) and aliquots were kept at −80° C. [3H]-Spiperone Binding experiments were performed in 96 deep-well polypropylene plates in 50 mM Tris/HCl, 120 mM NaCl, 5 mM KCl, 5 mM MgCl2 (pH 7.4). Compounds of invention were serially diluted in DMSO at 100 fold final concentrations in the assay (1% DMSO final in the assay). Displacement was performed in the presence of 0.08 nM [3H]-Spiperone. The reaction was initiated by the addition of membrane suspension (2 μg of protein for CHO-hD2 membranes) and lasted for 120 min at 23° C. in a final volume of 1000 μl. Non specific binding (NSB) was determined in the presence of 0.1 μM Spiperone. The binding reaction was stopped by rapid filtration through GF/B filterplates pre-soaked in 0.5% polyetylenimmine (PEI) using a Packard cell harvester. After washing with ice-cold 0.9% NaCl, the plate was left to dry before the addition of Microscint 20 (50 μl/well, PerkinElmer). Radioactivity was counted with a TopCount (PerkinElmer). Data were analysed by non-linear regression analysis using GraphPad Prism 5.0 (GraphPad Software) or XLfit Version 5.2.0.0 (Copyright 2006-2009 ID Business Solutions Ltd). Saturation binding experiments were performed similar to the competition binding experiments using a radioligand concentrations ranging from 0.011 to 3.0 nM. Ref: Durcan M. J. et al. (1995). Is Clozapine selective for the dopamine D4 receptor? Life Sciences, 57: 275-283. Petrus J. et al. (2001). Real-time analysis of dopamine: antagonist interactions at recombinant human D2long receptor upon modulation of its activation state. Brit. J. Pharmacol. 134, 88±97. [3H]-Spiperone Binding Assay at hD2 recombinant receptor CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were re-suspended in 20 mM HEPES, 2 mM EDTA (pH 7.4), homogenised and centrifuged at 40,000 g (20 min, 4° C.). After re-suspension, homogenization and centrifugation as above, the final pellet was re-suspended in 20 mM HEPES, 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA (pH 7.4) and aliquots were kept at −80° C. [3H]-Spiperone Binding experiments were performed in 96 deep-well polypropylene plates in 50 mM Tris/HCl, 120 mM NaCl, 5 mM KCl, 5 mM MgCl2 (pH 7.4). Compounds of invention were serially diluted in DMSO at 100 fold final concentrations in the assay (1% DMSO final in the assay). Displacement was performed in the presence of 0.08 nM [3H]-Spiperone. The reaction was initiated by the addition of membrane suspension (2 μg of protein for CHO-hD2 membranes) and lasted for 120 min at 23° C. in a final volume of 1000 μl. Non specific binding (NSB) was determined in the presence of 0.1 μM Spiperone. The binding reaction was stopped by rapid filtration through GF/B filterplates pre-soaked in 0.5% polyetylenimmine (PEI) using a Packard cell harvester. After washing with ice-cold 0.9% NaCl, the plate was left to dry before the addition of Microscint 20 (50 μl/well, PerkinElmer). Radioactivity was counted with a TopCount (PerkinElmer). Data were analysed by non-linear regression analysis using GraphPad Prism 5.0 (GraphPad Software) or XLfit Version 5.2.0.0 (Copyright 2006-2009 ID Business Solutions Ltd). Saturation binding experiments were performed similar to the competition binding experiments using a radioligand concentrations ranging from 0.011 to 3.0 nM. Ref: Durcan M. J. et al. (1995). Is Clozapine selective for the dopamine D4 receptor? Life Sciences, 57: 275-283. Petrus J. et al. (2001). Real-time analysis of dopamine: antagonist interactions at recombinant human D2long receptor upon modulation of its activation state. Brit. J. Pharmacol. 134, 88±97. [3H]BRL 43694 Competition Binding (h-5HT3 Assay [3H]BRL 43694 competition binding assay was performed under contract by Cerep Poitiers, France following the methods described in Hope, A. G et al., Characterization of a human 5-hydroxytryptamine3 receptor type A (h5-HT3R-AS) subunit stably expressed in HEK 293 cells, Brit. J. Pharmacol., (1996) 118: 1237-1245.In brief, Chinese Hamster Ovary (CHO) cells stably expressing human 5-HT3 serotonin receptors, grown to confluence in 175 cm2 flasks. Following aspiration of the culture medium, cells were harvested by mechanical agitation in ice cold PBS containing (in mM): (150 NaCl, 8 K2HPO4, 2 KH2PO4, pH 7.4, 37° C.), centrifuged at 4,000 g for 10 min and subsequently stored as a cell pellet at −80 C. When required, the pellet was thawed and resuspended in ice cold homogenization buffer (Tris 50 mM, EGTA 5.0 mM, phenylmethylsulphonylfluoride 0.1 mM, pH 7.6) and homogenized. The homogenate was centrifuged at 48,000 g for 10 minutes at 40° C. The resulting pellet was resuspended in ice cold binding buffer comprising (in mM): NaCl 140, KCl 2.8, CaCl2 1.0; MgCl2, 2.0; HEPES 10 (pH 7.4) and centrifuged as above. The pellet was resuspended in ice cold binding buffer and the protein concentration was determined by the method of Lowry et al., Protein measurement with the Folin phenol reagent, J. Biol. Chem., (1953) 193, 265-275). The membrane homogenate was adjusted to a protein concentration of approximately 600 mg/mL in binding buffer. Assay tubes were loaded with equal volumes of binding buffer containing [3H]BRL 43694 and test compound and 0.5 mL of membrane homogenate in a total reaction volume of 1 ml. Binding was initiated by the addition of the membrane homogenate and allowed to proceed for 120 min. at room temperature. Bound and free radioligand were separated by the addition of 3 ml of ice-cold binding buffer and immediate vacuum filtration through pre-soaked (0.1% (v/v) polyethyleneimine) Whatman GF/B filters. Filters were washed with a further 2×3 mL applications of binding buffer and counted for radioactivity using a scintillation counter.The results were expressed as a percent inhibition of control specific binding obtained in the presence of the test compounds where Inhibition (%)=100−[(measured specific binding/control specific binding)×100]. Cell-Based Assay The assay involves using a cell line that expresses the NR1 subunit together with either NR2C or NR2D. These cell lines can be prepared by transfecting a cell line with an appropriate vector that includes the DNA encoding the NR2C or NR2D receptors. One suitable cell line is BHK-1 (Syrian hamster kidney BHK-21 is a subclone (clone 13) of the parental line established from the kidneys of five unsexed, one-day-old hamsters in 1961).The NR2D receptor cDNA has also been cloned, for example, in 293T cells (Glover et al., Interaction of the N-Methyl-D-Aspartic Acid Receptor NR2D Subunit with the c-Abl Tyrosine Kinase*, J. Biol. Chem., Vol. 275, Issue 17, 12725-12729, Apr. 28, 2000). The cDNA for NR2D is also described in this reference.An NR2D cDNA (clone designation pNR2D422) is also disclosed in Arvanian, et al., Viral Delivery of NR2D Subunits Reduces Mg2+ Block of NMDA Receptor and Restores NT-3-Induced Potentiation of AMPA-Kainate Responses in Maturing Rat Motoneurons, J Neurophysiol 92: 2394-2404, 2004.The cDNA for the NR2C is described, for example, in Lin, Y. J., Bovetto, S, Carver, J. M., and Giordano, T., Cloning of the cDNA for the human NMDA receptor NR2C subunit and its expression in the central nervous system and periphery, Molecular Brain Research, 1996, vol. 43, no 1-2, pp. 57-64 (41 ref.). Lin et al. describe several overlapping cDNA clones containing 3995 nucleotides of the human 2C NMDA receptor subunit (NR2C) that were isolated from human hippocampal and cerebellar cDNA libraries. The predicted protein sequence is 1233 amino acids long. Lin et al. noted that readily detectable levels of NR2C are present in the hippocampus, amygdala, caudate nucleus, corpus callosum, subthalamic nuclei and thalamus, as well as the heart, skeletal muscle and pancreas, demonstrating a widespread expression pattern of the NR2C gene, both in the CNS and in the periphery.In one embodiment, the high throughput bioassay uses commercially-available BHK-21 cell lines expressing NR1 under control of the Tet-On system (Clontech) (Hansen et al (2008), and which constitutively express either NR2C or NR2D. FIG. 4A illustrates vector design for the NR2D cell line. A similar strategy can be used for the NR2C cell line, except that the NR2C cDNA is used in place of NR2D cDNA.Stable expression of NMDA receptor subunits is cytotoxic. To avoid this toxicity, the culture media can be supplemented with NMDA receptor antagonists, for example, DL-APV and 7Cl-kynurenate. Functional NR1 expression can be induced by doxycyclin before the assay.
 US9475819, 275 BDBM164145 US9637496, 275 US9062078, 275 US9695183, 275
US9475819, 275 BDBM164145 US9637496, 275 US9062078, 275 US9695183, 275 US11667644, Example 275 USRE48841, Example 275 US9586962, Example 275 US8653263, 275 BDBM118622 US11059828, Example 275
US11667644, Example 275 USRE48841, Example 275 US9586962, Example 275 US8653263, 275 BDBM118622 US11059828, Example 275 BDBM150601 US10238633, Example 275 US9713606, 275 US8987249, 275
BDBM150601 US10238633, Example 275 US9713606, 275 US8987249, 275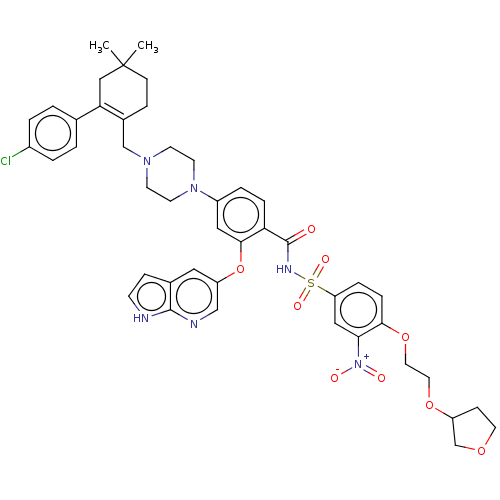 BDBM189705 US10213433, Compound 275 US9174982, 275 US20240043404, Example 275 US11369599, Compound 275
BDBM189705 US10213433, Compound 275 US9174982, 275 US20240043404, Example 275 US11369599, Compound 275 US10703749, Example 275 US10112937, Example 275 US9464084, 275 US10150765, Example 275 BDBM254368
US10703749, Example 275 US10112937, Example 275 US9464084, 275 US10150765, Example 275 BDBM254368 US10137124, Example 275 US10112942, Example 275 BDBM296541 US10555944, Example 275 US10953005, Example 275 US10172851, Example 275
US10137124, Example 275 US10112942, Example 275 BDBM296541 US10555944, Example 275 US10953005, Example 275 US10172851, Example 275 US10287267, Compound 275 US10556885, Compound 275 US10508099, Compound 275 BDBM385119 US11673881, Compound 275 US11174244, Compound 275
US10287267, Compound 275 US10556885, Compound 275 US10508099, Compound 275 BDBM385119 US11673881, Compound 275 US11174244, Compound 275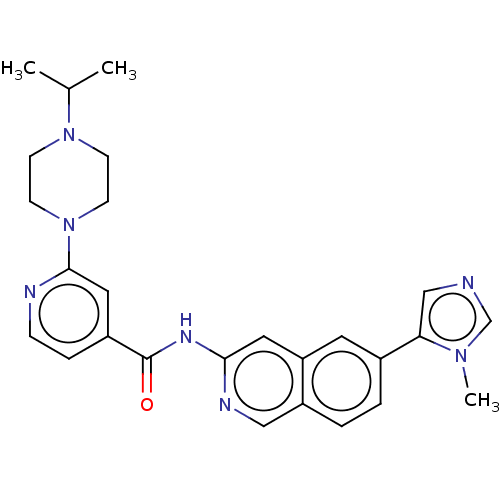 US11548872, Compound 275 BDBM293564 US10106527, Compound 275 US10544128, Compound 275 US9951048, Compound 275 US20230286945, Compound 275
US11548872, Compound 275 BDBM293564 US10106527, Compound 275 US10544128, Compound 275 US9951048, Compound 275 US20230286945, Compound 275 US10307413, Compound 275 US10980794, Cmpd No 275 US10391089, Compound 275 US9675614, 275 BDBM179229
US10307413, Compound 275 US10980794, Cmpd No 275 US10391089, Compound 275 US9675614, 275 BDBM179229 BDBM268242 US10772893, Example 275 US11529356, Example 275 US9549932, 275
BDBM268242 US10772893, Example 275 US11529356, Example 275 US9549932, 275 BDBM77828 US10245267, Example 275 US9694016, 275 US10709712, Example 275
BDBM77828 US10245267, Example 275 US9694016, 275 US10709712, Example 275 US9452980, 275 US10501411, Example 275 US11697636, Example 275 BDBM250365
US9452980, 275 US10501411, Example 275 US11697636, Example 275 BDBM250365 US9745328, Compound 275 US9884878, Compound 275 BDBM168876 US9079866, 275
US9745328, Compound 275 US9884878, Compound 275 BDBM168876 US9079866, 275 BDBM145085 US8952157, 275 US9303025, 275
BDBM145085 US8952157, 275 US9303025, 275 US10047103, 275 US9688695, 275 BDBM176242
US10047103, 275 US9688695, 275 BDBM176242 US9546164, 275 BDBM71154 US9694002, 275
US9546164, 275 BDBM71154 US9694002, 275 BDBM244665 US20250017938, Compound 002-275 US10226449, cpd 275 US9428502, 275
BDBM244665 US20250017938, Compound 002-275 US10226449, cpd 275 US9428502, 275 BDBM431330 US10870641, Example 275 US11014913, Example 275 US10550105, Example 275
BDBM431330 US10870641, Example 275 US11014913, Example 275 US10550105, Example 275 US10172864, Compound 275 US10028961, Compound 275 US10946023, Compound 275 BDBM280122
US10172864, Compound 275 US10028961, Compound 275 US10946023, Compound 275 BDBM280122 US10881652, Example 275 US10441581, Example 275 US11648243, Example 275 BDBM415965
US10881652, Example 275 US10441581, Example 275 US11648243, Example 275 BDBM415965 US10988451, Example 275 US10703733, Example 275 BDBM449662 US11643400, Example 275
US10988451, Example 275 US10703733, Example 275 BDBM449662 US11643400, Example 275 US9999619, Example 275 US11285140, Example 275 US10695337, Example 275 BDBM400519
US9999619, Example 275 US11285140, Example 275 US10695337, Example 275 BDBM400519 BDBM167784 US9073922, 275 US9796708, Example 275
BDBM167784 US9073922, 275 US9796708, Example 275 BDBM171641 US9085576, 275 US9611261, Example 275
BDBM171641 US9085576, 275 US9611261, Example 275 BDBM245036 US9738649, Example 275 US9422293, 275
BDBM245036 US9738649, Example 275 US9422293, 275 US11547697, Compound 275 BDBM132455 US9682141, 275
US11547697, Compound 275 BDBM132455 US9682141, 275 US9216999, 275 US9556187, Example 275 BDBM198478
US9216999, 275 US9556187, Example 275 BDBM198478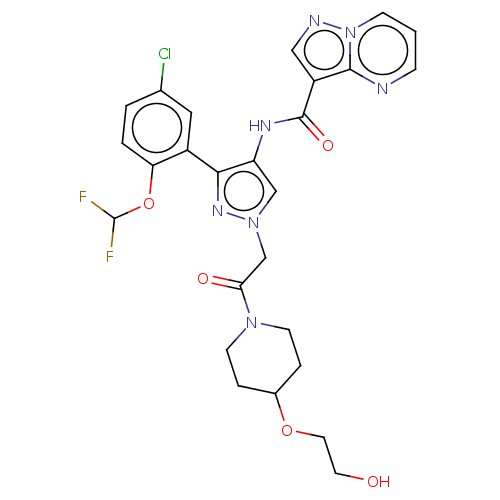 US9604984, Example 275 BDBM232729 US9346815, 275
US9604984, Example 275 BDBM232729 US9346815, 275 US9682966, 275 BDBM156485 US10118915, Compound 275
US9682966, 275 BDBM156485 US10118915, Compound 275 BDBM137205 US8871790, 275 US20250017938, Compound 001-275
BDBM137205 US8871790, 275 US20250017938, Compound 001-275 BDBM273492 US10478424, Example 275 US10071079, Example 275
BDBM273492 US10478424, Example 275 US10071079, Example 275 BDBM284265 US10174027, Example 275 US10023570, Example 275
BDBM284265 US10174027, Example 275 US10023570, Example 275 BDBM305259 US10172845, Example 275 US10144734, Example 275
BDBM305259 US10172845, Example 275 US10144734, Example 275 BDBM318318 US10301317, Example 275 US9624228, Example 275
BDBM318318 US10301317, Example 275 US9624228, Example 275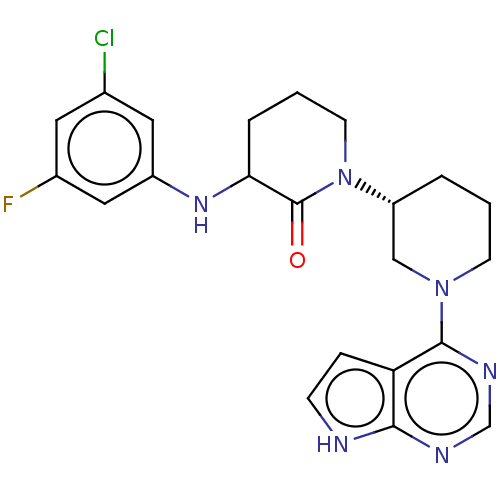 BDBM348552 US9790229, Compound 275 US10577374, Compound 275
BDBM348552 US9790229, Compound 275 US10577374, Compound 275 BDBM434356 US10562891, Example 275 US11008308, Example 275
BDBM434356 US10562891, Example 275 US11008308, Example 275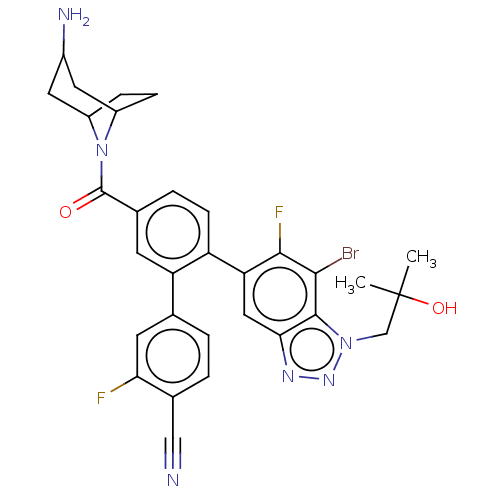 BDBM456546 US10723742, Example 275 US11510915, Example 275
BDBM456546 US10723742, Example 275 US11510915, Example 275 BDBM477506 US10889555, Example 275 US11634395, Example 275
BDBM477506 US10889555, Example 275 US11634395, Example 275 BDBM486827 US11427601, Example 275 US10947252, Example 275
BDBM486827 US11427601, Example 275 US10947252, Example 275 BDBM561738 US11390631, Example 275 US12268694, Example 275
BDBM561738 US11390631, Example 275 US12268694, Example 275 US10105367, Example 275 BDBM292989 US10376514, Example 275
US10105367, Example 275 BDBM292989 US10376514, Example 275 US10125118, Example 275 US10947215, Example 275 BDBM298869
US10125118, Example 275 US10947215, Example 275 BDBM298869 US10273259, Example 275 US10711020, Example 275 BDBM382566
US10273259, Example 275 US10711020, Example 275 BDBM382566 US10774053, Compound 275 US11352329, COMPD # 275 BDBM461082
US10774053, Compound 275 US11352329, COMPD # 275 BDBM461082 US10793579, Example 275 US11702424, Example 275 BDBM466161
US10793579, Example 275 US11702424, Example 275 BDBM466161 US11453683, Example 275 US20230279025, Example 275 BDBM573417
US11453683, Example 275 US20230279025, Example 275 BDBM573417 US11555029, No. 275 BDBM452136 US10710986, Example 275
US11555029, No. 275 BDBM452136 US10710986, Example 275 BDBM423820 US11046698, Compound I-275 US10508120, Compound I-275 US10577373, Compound I-275
BDBM423820 US11046698, Compound I-275 US10508120, Compound I-275 US10577373, Compound I-275 BDBM261252 US11433071, Example 275 US9707233, 276 US9707233, 275
BDBM261252 US11433071, Example 275 US9707233, 276 US9707233, 275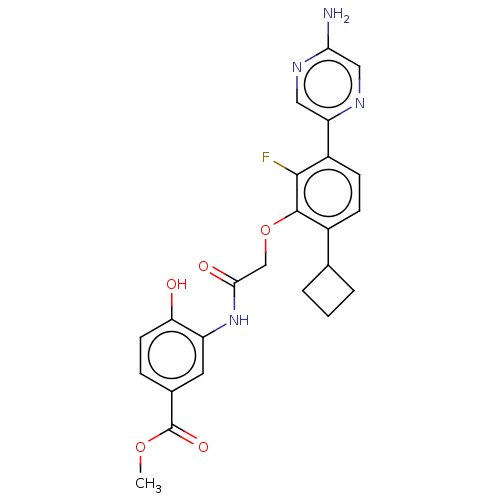 US9732093, Compound 275 US9073876, 263 US9073876, 275 BDBM167182
US9732093, Compound 275 US9073876, 263 US9073876, 275 BDBM167182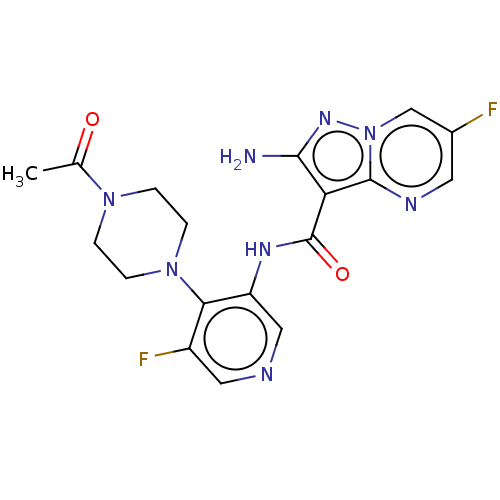 BDBM411840 US10392391, Compound I-N-275 US11117900, Compound I-N-275 US20230271963, Compound I-N-275 US11370798, Cmpd. # I-N-275 US10787452, Compound I-N-275
BDBM411840 US10392391, Compound I-N-275 US11117900, Compound I-N-275 US20230271963, Compound I-N-275 US11370798, Cmpd. # I-N-275 US10787452, Compound I-N-275 BDBM247673 US10112931, Example 275 US9434719, 275 as TFA salt
BDBM247673 US10112931, Example 275 US9434719, 275 as TFA salt BDBM405805 US10597377, Compound 2-275 US10351547, Compound 2-275
BDBM405805 US10597377, Compound 2-275 US10351547, Compound 2-275 BDBM439965 US10633389, Example 275-1a US20230279020, Example 275-1a
BDBM439965 US10633389, Example 275-1a US20230279020, Example 275-1a BDBM440608 US20230279020, Example 275-3 US10633389, Example 275-3
BDBM440608 US20230279020, Example 275-3 US10633389, Example 275-3 BDBM455267 US10730874, Compound I-275 US11352356, Compound I-275
BDBM455267 US10730874, Compound I-275 US11352356, Compound I-275 US10479784, Compound IA-275 BDBM420776 US10961232, Compound IA-275
US10479784, Compound IA-275 BDBM420776 US10961232, Compound IA-275 US10533010, Example I-275 US11208415, Example I-275 BDBM428889
US10533010, Example I-275 US11208415, Example I-275 BDBM428889 US11396508, Compound I-275 BDBM442314 US10647713, Compound I-275
US11396508, Compound I-275 BDBM442314 US10647713, Compound I-275 US12221453, Compound I-275 US11414431, Compound I-275 BDBM565707
US12221453, Compound I-275 US11414431, Compound I-275 BDBM565707 US20240374587, Example I-275 BDBM529063 US11198695, Example I-275
US20240374587, Example I-275 BDBM529063 US11198695, Example I-275 US9732060, Compound I-275 BDBM272054 US10065941, Compound I-275
US9732060, Compound I-275 BDBM272054 US10065941, Compound I-275 US9732060, Compound R-275 BDBM271941 US10065941, Compound R-275
US9732060, Compound R-275 BDBM271941 US10065941, Compound R-275 BDBM108695 US8604016, 275
BDBM108695 US8604016, 275 BDBM111963 US8618107, 275
BDBM111963 US8618107, 275 BDBM118135 US9682940, 275
BDBM118135 US9682940, 275 BDBM121836 US8722692, 275
BDBM121836 US8722692, 275 BDBM125880 US8772305, 275
BDBM125880 US8772305, 275 BDBM129172 US8802674, 275
BDBM129172 US8802674, 275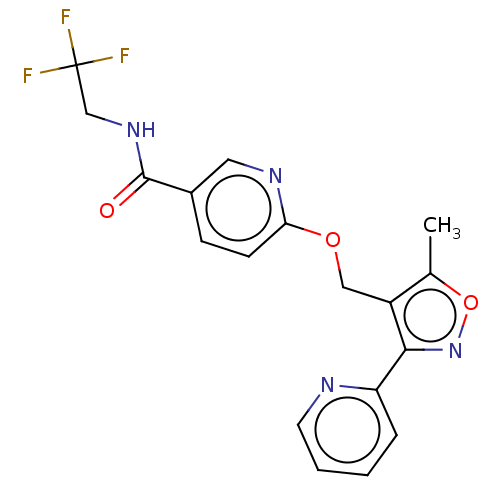 BDBM133583 US8846719, 275
BDBM133583 US8846719, 275 BDBM134809 US8846698, 275
BDBM134809 US8846698, 275 BDBM140142 US8901315, 275
BDBM140142 US8901315, 275 BDBM140839 US8912224, 275
BDBM140839 US8912224, 275 BDBM144074 US8969325, 275
BDBM144074 US8969325, 275 BDBM146223 US8957068, 275
BDBM146223 US8957068, 275 BDBM149041 US8962648, 275
BDBM149041 US8962648, 275 BDBM151086 US8987268, 275
BDBM151086 US8987268, 275 BDBM155828 US9012651, 275
BDBM155828 US9012651, 275 BDBM157434 US9023865, 275
BDBM157434 US9023865, 275 BDBM157982 US9023882, 275
BDBM157982 US9023882, 275 BDBM161839 US9682976, 275
BDBM161839 US9682976, 275 BDBM175730 US9688680, 275
BDBM175730 US9688680, 275 BDBM178707 US9125913, 275
BDBM178707 US9125913, 275 BDBM182574 US9145392, 275
BDBM182574 US9145392, 275 BDBM183611 US9145354, 275
BDBM183611 US9145354, 275 BDBM187724 US9169252, 275
BDBM187724 US9169252, 275 BDBM188733 US9169246, 275
BDBM188733 US9169246, 275 BDBM189009 US9169260, 275
BDBM189009 US9169260, 275 BDBM190079 US9175003, 275
BDBM190079 US9175003, 275 BDBM201508 US9187424, 275
BDBM201508 US9187424, 275 BDBM201844 US9233979, 275
BDBM201844 US9233979, 275 BDBM204201 US9242996, 275
BDBM204201 US9242996, 275 BDBM206522 US9260425, 275
BDBM206522 US9260425, 275 BDBM207391 US9260439, 275
BDBM207391 US9260439, 275 BDBM210577 US9290451, 275
BDBM210577 US9290451, 275 BDBM220686 US9296741, 275
BDBM220686 US9296741, 275 BDBM226582 US9328096, 275
BDBM226582 US9328096, 275 BDBM229948 US9334269, 275
BDBM229948 US9334269, 275 BDBM234144 US9353090, 275
BDBM234144 US9353090, 275 BDBM248411 US9434711, 275
BDBM248411 US9434711, 275 BDBM250971 US9452986, 275
BDBM250971 US9452986, 275 BDBM257806 US9493446, 275
BDBM257806 US9493446, 275 BDBM30588 US8853258, 275
BDBM30588 US8853258, 275 BDBM327728 US9663469, 275
BDBM327728 US9663469, 275 BDBM389084 USRE46792, 275
BDBM389084 USRE46792, 275 US8481733, 275 BDBM98463
US8481733, 275 BDBM98463 US8501936, 275 BDBM99759
US8501936, 275 BDBM99759 US8586617, 275 BDBM106648
US8586617, 275 BDBM106648 US8592431, 275 BDBM107406
US8592431, 275 BDBM107406 US8623889, 275 BDBM112277
US8623889, 275 BDBM112277 US8637532, 275 BDBM116583
US8637532, 275 BDBM116583 US8759532, 275 BDBM124591
US8759532, 275 BDBM124591 US8772480, 275 BDBM125423
US8772480, 275 BDBM125423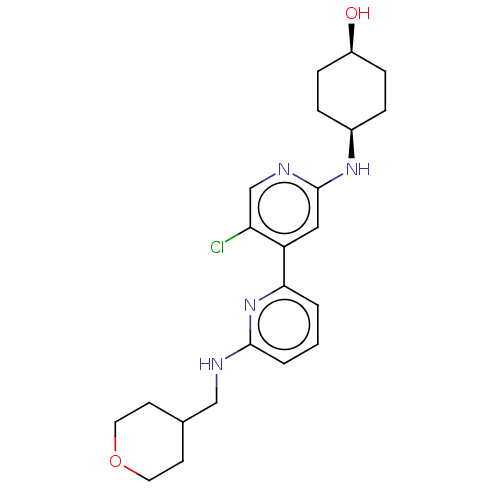 US8778951, 275 BDBM126465
US8778951, 275 BDBM126465 US8791131, 275 BDBM127497
US8791131, 275 BDBM127497 US8815926, 275 BDBM129994
US8815926, 275 BDBM129994 US8841312, 275 BDBM132526
US8841312, 275 BDBM132526 US8871934, 275 BDBM137682
US8871934, 275 BDBM137682 US8957093, 275 BDBM147437
US8957093, 275 BDBM147437 US9012443, 275 BDBM154259
US9012443, 275 BDBM154259 US9029559, 275 BDBM156843
US9029559, 275 BDBM156843 US9034866, 275 BDBM158800
US9034866, 275 BDBM158800 US9067871, 275 BDBM164810
US9067871, 275 BDBM164810 US9073876, 275 BDBM167194
US9073876, 275 BDBM167194 US9073940, 275 BDBM169925
US9073940, 275 BDBM169925 US9085555, 275 BDBM170847
US9085555, 275 BDBM170847 US9090628, 275 BDBM172615
US9090628, 275 BDBM172615 US9181272, 275 BDBM191505
US9181272, 275 BDBM191505 US9221809, 275 BDBM203724
US9221809, 275 BDBM203724 US9226922, 275 BDBM199639
US9226922, 275 BDBM199639 US9242970, 275 BDBM203096
US9242970, 275 BDBM203096 US9255090, 275 BDBM205806
US9255090, 275 BDBM205806 US9278981, 275 BDBM213683
US9278981, 275 BDBM213683 US9283222, 275 BDBM214212
US9283222, 275 BDBM214212 US9296736, 275 BDBM215313
US9296736, 275 BDBM215313 US9302989, 275 BDBM217011
US9302989, 275 BDBM217011 US9321756, 275 BDBM225009
US9321756, 275 BDBM225009 US9328106, 275 BDBM227324
US9328106, 275 BDBM227324 US9340517, 275 BDBM231888
US9340517, 275 BDBM231888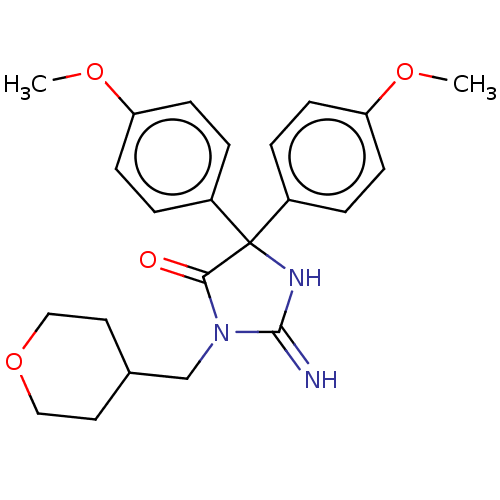 US9353089, 275 BDBM234541
US9353089, 275 BDBM234541 US9434725, 275 BDBM249044
US9434725, 275 BDBM249044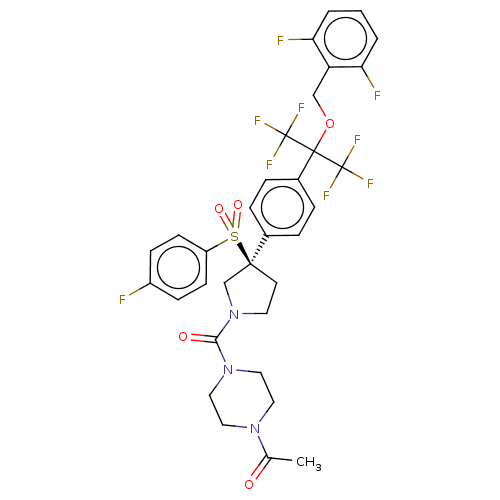 US9458171, 275 BDBM253315
US9458171, 275 BDBM253315 US9481672, 275 BDBM256093
US9481672, 275 BDBM256093 US9499482, 275 BDBM258840
US9499482, 275 BDBM258840 US9675593, 275 BDBM170403
US9675593, 275 BDBM170403 US9693992, 275 BDBM70583
US9693992, 275 BDBM70583 US9693997, 275 BDBM70780
US9693997, 275 BDBM70780 US10730877, Example 275 BDBM427840 US10544143, Example 273 US11053244, Example 275
US10730877, Example 275 BDBM427840 US10544143, Example 273 US11053244, Example 275 US9150546, I-275 US9718790, I-0275 BDBM183123 US9688643, I-275
US9150546, I-275 US9718790, I-0275 BDBM183123 US9688643, I-275 BDBM105765 US8575197, I-275
BDBM105765 US8575197, I-275 BDBM106362 US8575197, II-275
BDBM106362 US8575197, II-275 BDBM116125 US8633183, E-275
BDBM116125 US8633183, E-275 BDBM122805 US8735386, I-275
BDBM122805 US8735386, I-275 BDBM210136 US9546153, ex. 275
BDBM210136 US9546153, ex. 275 BDBM272431 US10065950, Example 275
BDBM272431 US10065950, Example 275 BDBM280716 US10030020, Example 275
BDBM280716 US10030020, Example 275 BDBM294804 US10112899, Example 275
BDBM294804 US10112899, Example 275 BDBM326578 US9662327, Compound 275
BDBM326578 US9662327, Compound 275 BDBM337228 US9745291, Compound 275
BDBM337228 US9745291, Compound 275 BDBM357519 US10214519, Example 275
BDBM357519 US10214519, Example 275 BDBM372868 US10246429, Example 275
BDBM372868 US10246429, Example 275 BDBM390388 US9951086, Example 275
BDBM390388 US9951086, Example 275 BDBM403121 US10329302, Example 275
BDBM403121 US10329302, Example 275 BDBM410454 US10377770, Example 275
BDBM410454 US10377770, Example 275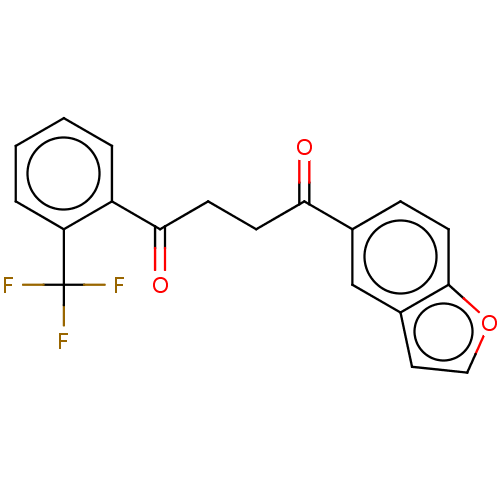 BDBM418351 US10457672, Compound 275
BDBM418351 US10457672, Compound 275 BDBM427842 US10544143, Example 275
BDBM427842 US10544143, Example 275 BDBM451678 US10710967, Example 275
BDBM451678 US10710967, Example 275 BDBM453881 US10730863, Example 275
BDBM453881 US10730863, Example 275 BDBM479816 US10899735, No. 275
BDBM479816 US10899735, No. 275 BDBM485024 US10934302, Example 275
BDBM485024 US10934302, Example 275 BDBM491321 US10975056, Example 275
BDBM491321 US10975056, Example 275 BDBM494226 US10988478, Example 275
BDBM494226 US10988478, Example 275 BDBM502373 US11028090, Example 275
BDBM502373 US11028090, Example 275 BDBM512681 acs.jmedchem.1c00409_ST.275
BDBM512681 acs.jmedchem.1c00409_ST.275 BDBM516185 US11053226, Example 275
BDBM516185 US11053226, Example 275 BDBM527021 US11186582, Example 275
BDBM527021 US11186582, Example 275 BDBM529869 US11203591, Example 275
BDBM529869 US11203591, Example 275 BDBM534539 WO2022086828, Example 275
BDBM534539 WO2022086828, Example 275 BDBM538367 US11254663, Example 275
BDBM538367 US11254663, Example 275 BDBM538932 US11254668, Example 275
BDBM538932 US11254668, Example 275 BDBM540344 US11261186, Example 275
BDBM540344 US11261186, Example 275 BDBM569128 US11427558, Example 275
BDBM569128 US11427558, Example 275 BDBM574395 US11458138, Example 275
BDBM574395 US11458138, Example 275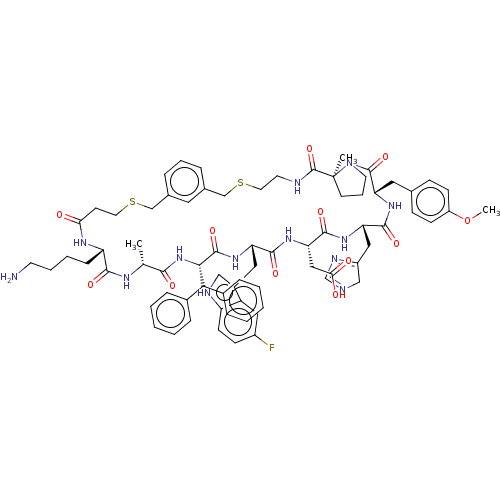 BDBM586214 US11530244, Compound 275
BDBM586214 US11530244, Compound 275 BDBM588028 US11535621, Example 275
BDBM588028 US11535621, Example 275 BDBM588650 US11548892, Compound 275
BDBM588650 US11548892, Compound 275 BDBM593002 US11576897, Example 275
BDBM593002 US11576897, Example 275 BDBM599137 US11618753, Example 275
BDBM599137 US11618753, Example 275 BDBM603302 US11649255, Example 275
BDBM603302 US11649255, Example 275 BDBM603835 US11654147, Compound 275
BDBM603835 US11654147, Compound 275 BDBM607313 US11685745, Example 275
BDBM607313 US11685745, Example 275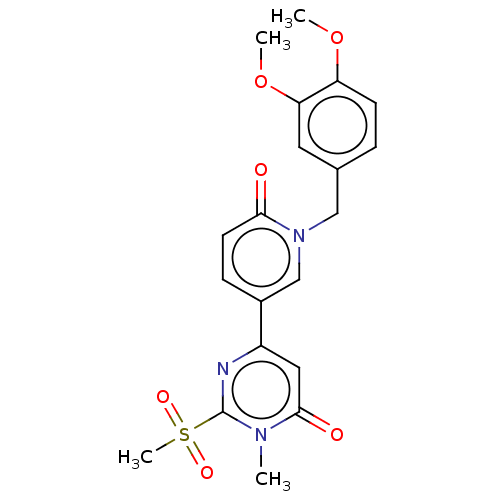 BDBM613013 US11725000, Compound 275
BDBM613013 US11725000, Compound 275 BDBM615484 US20230271949, Example 275
BDBM615484 US20230271949, Example 275 BDBM623256 US11780845, Example 275
BDBM623256 US11780845, Example 275 BDBM625786 US20230322846, Example 275
BDBM625786 US20230322846, Example 275 BDBM629976 US20230340011, Example 275.
BDBM629976 US20230340011, Example 275. BDBM631338 US11802133, Example 275
BDBM631338 US11802133, Example 275 BDBM634826 US11814367, Compound 275
BDBM634826 US11814367, Compound 275 BDBM637360 US20230382904, Compound 275
BDBM637360 US20230382904, Compound 275 BDBM638172 US11834453, Example 275
BDBM638172 US11834453, Example 275 BDBM638680 US11834467, Example 275
BDBM638680 US11834467, Example 275 BDBM641381 US11845723, Example 275
BDBM641381 US11845723, Example 275 BDBM646770 US20240025883, Example 275
BDBM646770 US20240025883, Example 275 BDBM648034 US20240025919, Compound 275
BDBM648034 US20240025919, Compound 275 BDBM654329 US11912686, Compound 275
BDBM654329 US11912686, Compound 275 BDBM659158 US20240092758, Example 275
BDBM659158 US20240092758, Example 275 BDBM665591 US20240116910, Compound 275
BDBM665591 US20240116910, Compound 275 BDBM666242 US20240116946, Example 275
BDBM666242 US20240116946, Example 275 BDBM670331 US11970474, Example 275
BDBM670331 US11970474, Example 275 BDBM670589 US20240140931, Compound 275
BDBM670589 US20240140931, Compound 275 BDBM677935 US20240174674, Compound 275
BDBM677935 US20240174674, Compound 275 BDBM681716 US20240199605, Example 275
BDBM681716 US20240199605, Example 275 BDBM686123 US20240245673, Example 275
BDBM686123 US20240245673, Example 275 BDBM686831 US20240246937, Example 275
BDBM686831 US20240246937, Example 275 BDBM689788 US20240262804, Example 275
BDBM689788 US20240262804, Example 275 BDBM694098 US20240287079, Example 275
BDBM694098 US20240287079, Example 275 BDBM697493 US20240316047, Example 275
BDBM697493 US20240316047, Example 275 BDBM715111 US20250026748, Compound 275
BDBM715111 US20250026748, Compound 275 BDBM716219 US12209085, Example 275
BDBM716219 US12209085, Example 275 BDBM718420 US20250042889, Example 275
BDBM718420 US20250042889, Example 275 BDBM722013 US20250059174, Example 275
BDBM722013 US20250059174, Example 275 BDBM723699 US20250064789, Compound 275
BDBM723699 US20250064789, Compound 275 BDBM730692 US20250122174, Example 275
BDBM730692 US20250122174, Example 275 BDBM734728 US20250129067, Compound 275
BDBM734728 US20250129067, Compound 275 BDBM735257 US20250129078, Compound 275
BDBM735257 US20250129078, Compound 275 BDBM735687 US20250129103, Compound 275
BDBM735687 US20250129103, Compound 275 BDBM739028 US20250145633, Example 275
BDBM739028 US20250145633, Example 275 BDBM744348 US20250170122, Compound 275
BDBM744348 US20250170122, Compound 275 BDBM745793 US12319655, Example 275
BDBM745793 US12319655, Example 275 BDBM749652 US12331033, Example 275
BDBM749652 US12331033, Example 275 BDBM752009 US20250197382, Compound 275
BDBM752009 US20250197382, Compound 275 BDBM753862 US20250206717, Example 275
BDBM753862 US20250206717, Example 275 BDBM756962 US20250221979, Example 275
BDBM756962 US20250221979, Example 275 BDBM762016 US12378224, Example 275
BDBM762016 US12378224, Example 275 US10112929, Example 275 BDBM295545
US10112929, Example 275 BDBM295545 US10112941, Example 275 BDBM297159
US10112941, Example 275 BDBM297159 US10189854, Compound 275 BDBM332311
US10189854, Compound 275 BDBM332311 US10202339, Compound 275 BDBM339497
US10202339, Compound 275 BDBM339497 US10227299, Example 275 BDBM367585
US10227299, Example 275 BDBM367585 US10239843, Example 275 BDBM371168
US10239843, Example 275 BDBM371168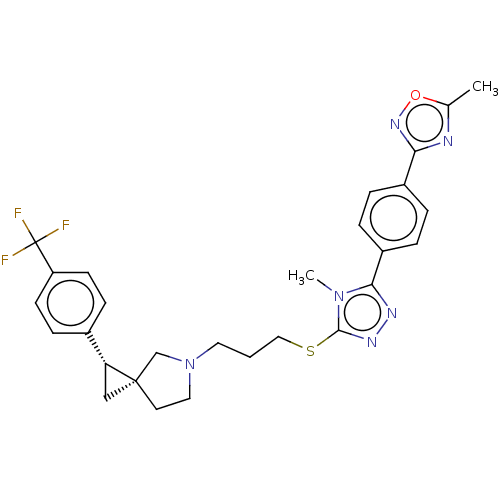 US10239870, Example 275 BDBM372015
US10239870, Example 275 BDBM372015 US10246456, Example 275 BDBM374154
US10246456, Example 275 BDBM374154 US10285982, Compound 275 BDBM384700
US10285982, Compound 275 BDBM384700 US10285983, Compound 275 BDBM384773
US10285983, Compound 275 BDBM384773 US10294229, Example 275 BDBM388126
US10294229, Example 275 BDBM388126 US10323022, Example 275 BDBM398798
US10323022, Example 275 BDBM398798 US10336717, Compound 275 BDBM403990
US10336717, Compound 275 BDBM403990 US10435369, Example 275 BDBM414388
US10435369, Example 275 BDBM414388 US10544141, Compound 275 BDBM427486
US10544141, Compound 275 BDBM427486 US10562878, Compound 275 BDBM433766
US10562878, Compound 275 BDBM433766 US10618903, Example 275 BDBM438708
US10618903, Example 275 BDBM438708 US10626095, Example 275 BDBM610848
US10626095, Example 275 BDBM610848 US10660877, Example 275 BDBM443689
US10660877, Example 275 BDBM443689 US10703755, Example 275 BDBM450094
US10703755, Example 275 BDBM450094 US10781211, Example 275 BDBM464037
US10781211, Example 275 BDBM464037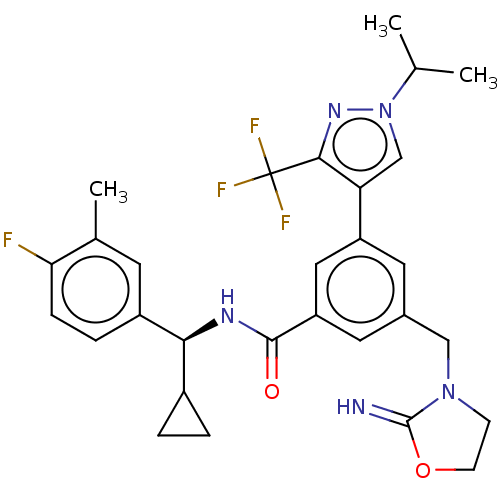 US10844044, Example 275 BDBM473193
US10844044, Example 275 BDBM473193 US10961200, Compound 275 BDBM488896
US10961200, Compound 275 BDBM488896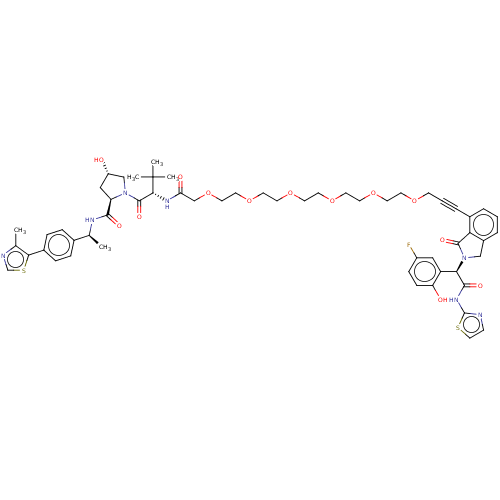 US10994015, Example 275 BDBM494979
US10994015, Example 275 BDBM494979 US11021493, Example 275 BDBM501356
US11021493, Example 275 BDBM501356 US11208404, Compound 275 BDBM532147
US11208404, Compound 275 BDBM532147 US11267811, Example 275 BDBM541656
US11267811, Example 275 BDBM541656 US11292791, Example 275 BDBM547002
US11292791, Example 275 BDBM547002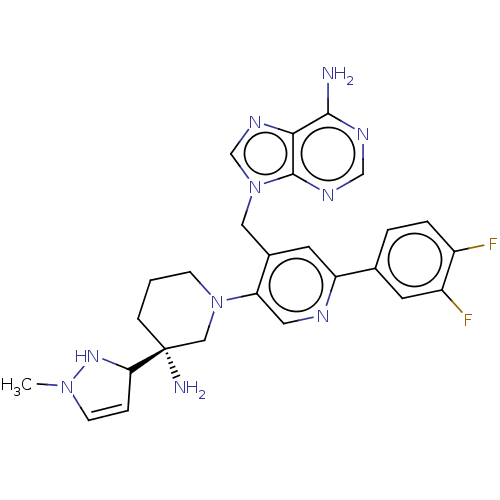 US11420970, Example 275 BDBM568073
US11420970, Example 275 BDBM568073 US11524959, Compound 275. BDBM583383
US11524959, Compound 275. BDBM583383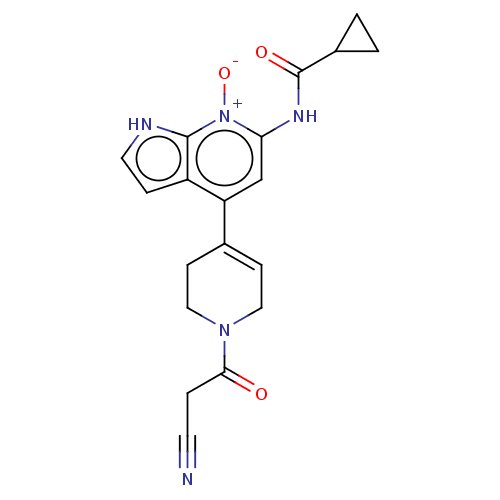 US11524968, Example 275 BDBM584527
US11524968, Example 275 BDBM584527 US11591336, Compound 275 BDBM595918
US11591336, Compound 275 BDBM595918 US11596639, Example 275 BDBM596729
US11596639, Example 275 BDBM596729 US11773078, Example 275 BDBM621523
US11773078, Example 275 BDBM621523 US12128028, Example 275 BDBM701879
US12128028, Example 275 BDBM701879 US12384753, Example 275 BDBM763252
US12384753, Example 275 BDBM763252 US20230286970, Compound 275 BDBM618155
US20230286970, Compound 275 BDBM618155 US20230348450, Example 275 BDBM632887
US20230348450, Example 275 BDBM632887 US20240002391, Compound 275 BDBM643439
US20240002391, Compound 275 BDBM643439 US20240025884, Example 275 BDBM647064
US20240025884, Example 275 BDBM647064 US20240043427, Example 275 BDBM650187
US20240043427, Example 275 BDBM650187 US20240101572, Example 275 BDBM661666
US20240101572, Example 275 BDBM661666 US20240174662, Example 275 BDBM677611
US20240174662, Example 275 BDBM677611 US20240216357, Example 275 BDBM683313
US20240216357, Example 275 BDBM683313 US20240218021, Example 275 BDBM684357
US20240218021, Example 275 BDBM684357 US20240246964, Compound 275 BDBM687254
US20240246964, Compound 275 BDBM687254 US20240294551, Example 275 BDBM695109
US20240294551, Example 275 BDBM695109 US20240368104, Example 275 BDBM703328
US20240368104, Example 275 BDBM703328 US20250059220, Compound 275 BDBM723163
US20250059220, Compound 275 BDBM723163 US20250084074, Example 275 BDBM726961
US20250084074, Example 275 BDBM726961 US20250188079, Compound 275 BDBM749222
US20250188079, Compound 275 BDBM749222 US20250243180, Compound 275 BDBM761283
US20250243180, Compound 275 BDBM761283 US8653087, III-275 BDBM117595
US8653087, III-275 BDBM117595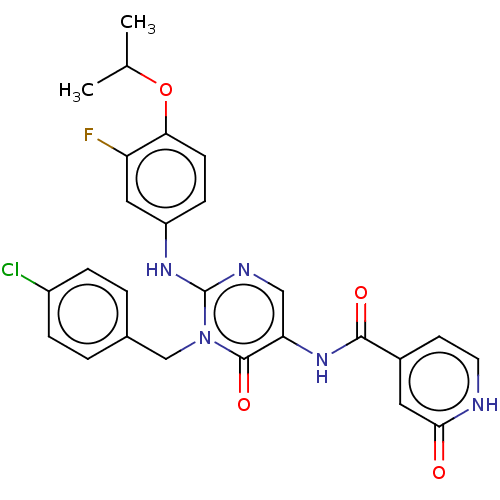 US9212130, I-275 BDBM196355
US9212130, I-275 BDBM196355 US9422240, 1-275 BDBM242545
US9422240, 1-275 BDBM242545 US9771320, Example 275 BDBM342041
US9771320, Example 275 BDBM342041 US9777008, Compound 275 BDBM343981
US9777008, Compound 275 BDBM343981 US9790221, Compound 275 BDBM348067
US9790221, Compound 275 BDBM348067 US9862730, Example 275 BDBM364819
US9862730, Example 275 BDBM364819 US9873693, Compound 275 BDBM366347
US9873693, Compound 275 BDBM366347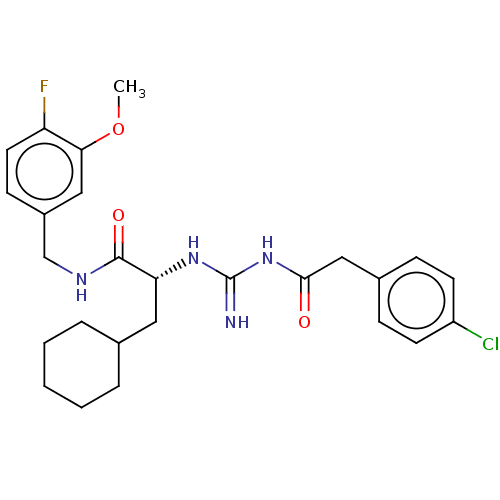 US9884814, Compound 275 BDBM275664
US9884814, Compound 275 BDBM275664 US9926281, Compound 275 BDBM379851
US9926281, Compound 275 BDBM379851 US9969687, Compound 275 BDBM393595
US9969687, Compound 275 BDBM393595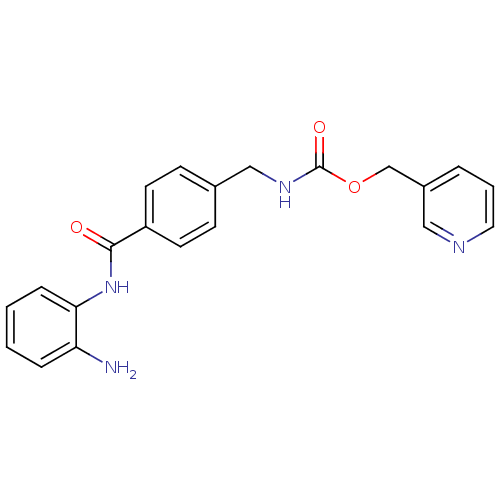 MS-275 US20240327418, Example MS275 US20240150300, Compound MS-275 US9265734, MS-275 benzamide-type inhibitor, 3 pyridin-3-ylmethyl N-({4-[(2-aminophenyl)carbamoyl]phenyl}methyl)carbamate US11672788, Compound Entinostat CHEMBL27759 US9796664, Compound MS-275 BDBM19410 US11377423, MS-275
MS-275 US20240327418, Example MS275 US20240150300, Compound MS-275 US9265734, MS-275 benzamide-type inhibitor, 3 pyridin-3-ylmethyl N-({4-[(2-aminophenyl)carbamoyl]phenyl}methyl)carbamate US11672788, Compound Entinostat CHEMBL27759 US9796664, Compound MS-275 BDBM19410 US11377423, MS-275 BDBM195209 US9212153, 275,Ex. 226
BDBM195209 US9212153, 275,Ex. 226 BDBM307062 US10150728, Example I-275
BDBM307062 US10150728, Example I-275 BDBM330713 US9725442, Compound I-275
BDBM330713 US9725442, Compound I-275 BDBM387052 US9938267, Cmpd ID 275
BDBM387052 US9938267, Cmpd ID 275 BDBM464942 US10793563, Compound I-275
BDBM464942 US10793563, Compound I-275 BDBM483126 US10919885, Compound No. 275
BDBM483126 US10919885, Compound No. 275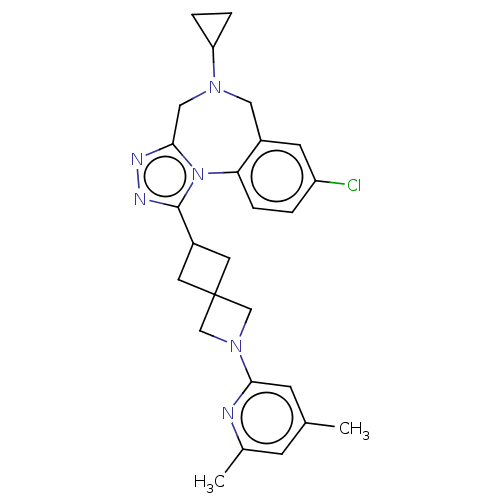 BDBM526927 US11186577, Cmpd. No. 275
BDBM526927 US11186577, Cmpd. No. 275 BDBM566906 US11420958, Ex. No. 275
BDBM566906 US11420958, Ex. No. 275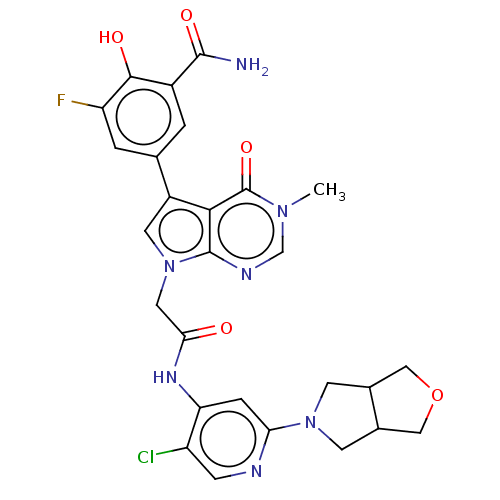 BDBM582266 US11518764, Compound I-275
BDBM582266 US11518764, Compound I-275 BDBM606631 US11685732, Compound I-275
BDBM606631 US11685732, Compound I-275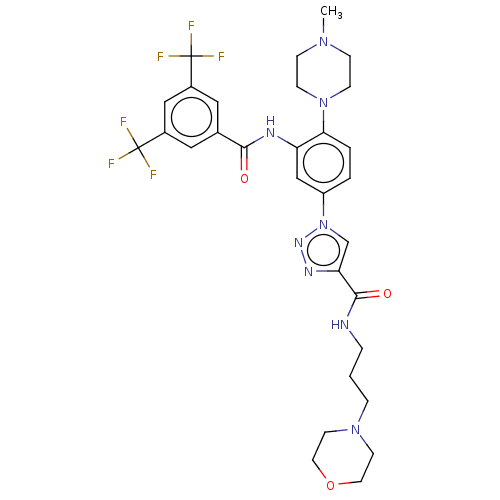 BDBM617715 US20230286948, Compound HYBI-275
BDBM617715 US20230286948, Compound HYBI-275 BDBM639064 US20230390274, Compound A-275
BDBM639064 US20230390274, Compound A-275 BDBM640718 US20230399319, Example 2-275
BDBM640718 US20230399319, Example 2-275 BDBM673964 US20240150321, Compound I-275
BDBM673964 US20240150321, Compound I-275 BDBM689434 US12053473, Example I-275
BDBM689434 US12053473, Example I-275 BDBM727414 US20250090540, Example I-275
BDBM727414 US20250090540, Example I-275 BDBM728648 US20250092056, Compound I-275
BDBM728648 US20250092056, Compound I-275 BDBM741601 US20250162989, Compound I-275
BDBM741601 US20250162989, Compound I-275 BDBM742005 US20250163057, Compound I-275
BDBM742005 US20250163057, Compound I-275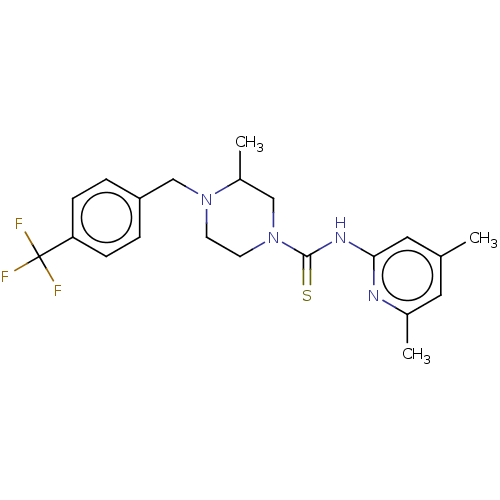 CHEMBL4127154 US11225469, Compound 275 BDBM50271427
CHEMBL4127154 US11225469, Compound 275 BDBM50271427 US10144742, Compound I-275 BDBM306113
US10144742, Compound I-275 BDBM306113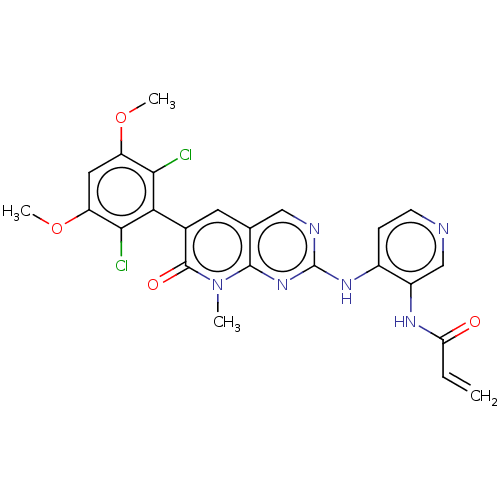 US10618902, Compound I-275 BDBM438594
US10618902, Compound I-275 BDBM438594 US10640495, Example I-275 BDBM441099
US10640495, Example I-275 BDBM441099 US11339144, Compound I-275 BDBM554826
US11339144, Compound I-275 BDBM554826 US11458149, Compound I-275 BDBM574661
US11458149, Compound I-275 BDBM574661 US11555012, Compound I-275 BDBM589534
US11555012, Compound I-275 BDBM589534 US11560370, Compound AIA-275 BDBM590019
US11560370, Compound AIA-275 BDBM590019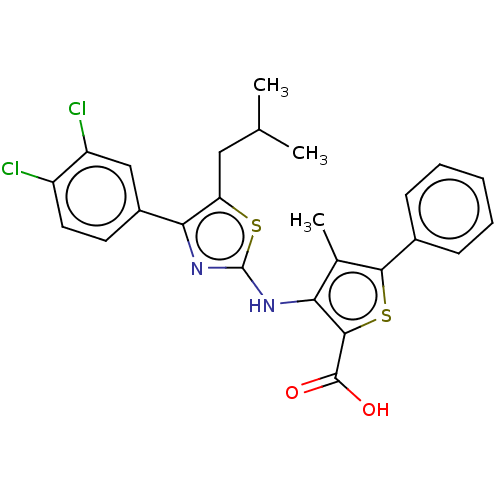 US11753403, Compound I-275 BDBM617358
US11753403, Compound I-275 BDBM617358 US12331046, Compound I-275 BDBM750154
US12331046, Compound I-275 BDBM750154 US8815926, 245 US8815926, 275 BDBM129964
US8815926, 245 US8815926, 275 BDBM129964 US9212153, 326,Ex. 275 BDBM195261
US9212153, 326,Ex. 275 BDBM195261 US9409866, 275 BDBM240560 US9409866, 276
US9409866, 275 BDBM240560 US9409866, 276 US9458110, 275 US9458110, 276 BDBM252203
US9458110, 275 US9458110, 276 BDBM252203 US9550763, Compound I-275 BDBM246630
US9550763, Compound I-275 BDBM246630 US9682940, 105 BDBM115786 US9682940, 275
US9682940, 105 BDBM115786 US9682940, 275 US11591319, ID lqr-8-275 BDBM595518
US11591319, ID lqr-8-275 BDBM595518 (2S,5R)-5-(3-fluorophenyl)-1-(2'-methoxy-[1,1'-biphenyl]-4-carbonyl)pyrrolidine-2-carboxylic acid US10017468, Compound 275 US11072582, Compound 275 US10781171, Compound 275 BDBM403997
(2S,5R)-5-(3-fluorophenyl)-1-(2'-methoxy-[1,1'-biphenyl]-4-carbonyl)pyrrolidine-2-carboxylic acid US10017468, Compound 275 US11072582, Compound 275 US10781171, Compound 275 BDBM403997 BDBM316779 US11111242, Example 275 US20250134869, Example 275 2-[(1-acryloylpiperidin-4-yl)amino]-N-(2-methoxyethyl)-5H-pyrrolo[2,3-b]pyrazine-7-carboxamide US9617258, Example 275
BDBM316779 US11111242, Example 275 US20250134869, Example 275 2-[(1-acryloylpiperidin-4-yl)amino]-N-(2-methoxyethyl)-5H-pyrrolo[2,3-b]pyrazine-7-carboxamide US9617258, Example 275 US10071988, Example 275 US10233173, Example 275 2-[[1-[3-(Difluoromethyl)phenyl]triazol-4-yl]methoxy]-5- BDBM276336
US10071988, Example 275 US10233173, Example 275 2-[[1-[3-(Difluoromethyl)phenyl]triazol-4-yl]methoxy]-5- BDBM276336 BDBM219601 US9303033, P30, Table 37A, Compound 275
BDBM219601 US9303033, P30, Table 37A, Compound 275 BDBM433763 US10562878, Compound 275 US10562878, Compound 272
BDBM433763 US10562878, Compound 275 US10562878, Compound 272 BDBM544791 US11286268, Compound 776 US11286268, Compound 275
BDBM544791 US11286268, Compound 776 US11286268, Compound 275 BDBM563961 Roche-Dataset for PDE10A, Compound 275
BDBM563961 Roche-Dataset for PDE10A, Compound 275 US10206931, Example 275 BDBM349467 (4R)-6-(1-
US10206931, Example 275 BDBM349467 (4R)-6-(1- US10294229, Example 275 US10294229, Example 274 BDBM388125
US10294229, Example 275 US10294229, Example 274 BDBM388125 US10435369, Example 275 US10435369, Example 274 BDBM414387
US10435369, Example 275 US10435369, Example 274 BDBM414387 US10781211, Example 275 BDBM464007 US10781211, Example 238
US10781211, Example 275 BDBM464007 US10781211, Example 238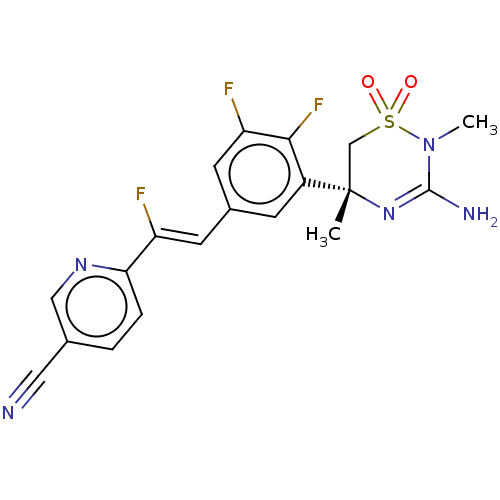 US11021493, Example 107 US11021493, Example 275 BDBM501188
US11021493, Example 107 US11021493, Example 275 BDBM501188 US11427567, Example 275 BDBM569630 US11427567, Example 276
US11427567, Example 275 BDBM569630 US11427567, Example 276 US11584747, Example 276 BDBM594469 US11584747, Example 275
US11584747, Example 276 BDBM594469 US11584747, Example 275 US20230348426, Example 290 BDBM632005 US20230348426, Example 275
US20230348426, Example 290 BDBM632005 US20230348426, Example 275 US20250025443, Compound 275 BDBM714081 US20250025443, Example 115
US20250025443, Compound 275 BDBM714081 US20250025443, Example 115 US20250025443, Example 275 US20250025443, Compound 420 BDBM714241
US20250025443, Example 275 US20250025443, Compound 420 BDBM714241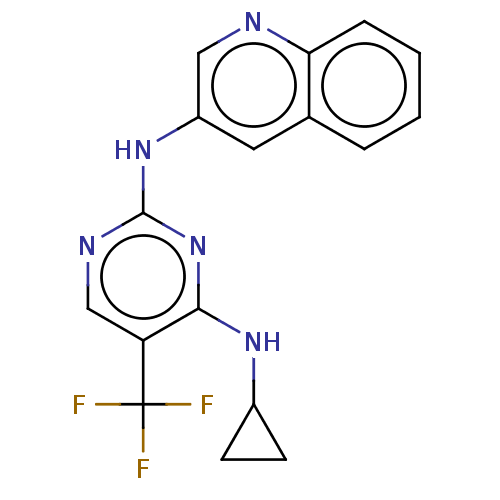 US10266549, Example 275 N4-cyclopropyl-N2-(quinolin-3-yl)-5-(trifluoromethyl) pyrimidine-2,4-diamine BDBM379387 US10774092, Example 275
US10266549, Example 275 N4-cyclopropyl-N2-(quinolin-3-yl)-5-(trifluoromethyl) pyrimidine-2,4-diamine BDBM379387 US10774092, Example 275 US11352360, Example 275' US9884868, Example 275' BDBM283074 US10287295, Example 275' N-((6-(1-(2,2-difluoroethyl)-4-(4-fluorophenyl)-1H- imidazol-5-yl)imidazo[1,2-a]pyridin-3-yl)methyl)-2,2- difluoroethanamine
US11352360, Example 275' US9884868, Example 275' BDBM283074 US10287295, Example 275' N-((6-(1-(2,2-difluoroethyl)-4-(4-fluorophenyl)-1H- imidazol-5-yl)imidazo[1,2-a]pyridin-3-yl)methyl)-2,2- difluoroethanamine methyl cis-3- ((methylsulfonyl)amino)-2- (((1s,4's)-3H-spiro[2-benzofuran- 1,1'-cyclohexan]-4'- yloxy)methyl)piperidine-1- carboxylate US11292766, Example 275 BDBM386837 US10508083, Example 275 US10287305, Example 275
methyl cis-3- ((methylsulfonyl)amino)-2- (((1s,4's)-3H-spiro[2-benzofuran- 1,1'-cyclohexan]-4'- yloxy)methyl)piperidine-1- carboxylate US11292766, Example 275 BDBM386837 US10508083, Example 275 US10287305, Example 275 US12012467, Compound DI-808 US12012467, Example 275 BDBM681128
US12012467, Compound DI-808 US12012467, Example 275 BDBM681128 US9303033, Example 275, Table 52, Compound 1 BDBM220007
US9303033, Example 275, Table 52, Compound 1 BDBM220007 BDBM325069 US10189841, Compound I-275 9-(4-(1-isopropyl-1H-imidazol-4-yl)benzyl)-2-(2- US10399980, Compound I-275
BDBM325069 US10189841, Compound I-275 9-(4-(1-isopropyl-1H-imidazol-4-yl)benzyl)-2-(2- US10399980, Compound I-275 N-((1R,3S)-3-Acrylamidocyclohexyl)-5-(*R)-(4-isopropoxy-2- methylphenyl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide US12065446, Example 275 US11319329, Ex # 275 US10934310, Ex # 275 BDBM485441
N-((1R,3S)-3-Acrylamidocyclohexyl)-5-(*R)-(4-isopropoxy-2- methylphenyl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide US12065446, Example 275 US11319329, Ex # 275 US10934310, Ex # 275 BDBM485441 US11130759, Cpd. No. 275 US11084798, Cpd No 275 US11046691, Compound 275 BDBM508035 4-{[(cis)-3-hydroxy-3-(trifluoromethyl)- cyclobutyl]amino}-1-(2-chlorophenyl)- 7-(trifluoromethyl)pyrido[2,3-d]- pyrimidin-2(1H)-one
US11130759, Cpd. No. 275 US11084798, Cpd No 275 US11046691, Compound 275 BDBM508035 4-{[(cis)-3-hydroxy-3-(trifluoromethyl)- cyclobutyl]amino}-1-(2-chlorophenyl)- 7-(trifluoromethyl)pyrido[2,3-d]- pyrimidin-2(1H)-one BDBM465624 US10793568, Compound I-301 US10793568, Compound I-275
BDBM465624 US10793568, Compound I-301 US10793568, Compound I-275 US11198695, Example II-275 US11198695, Example II-276 BDBM529338
US11198695, Example II-275 US11198695, Example II-276 BDBM529338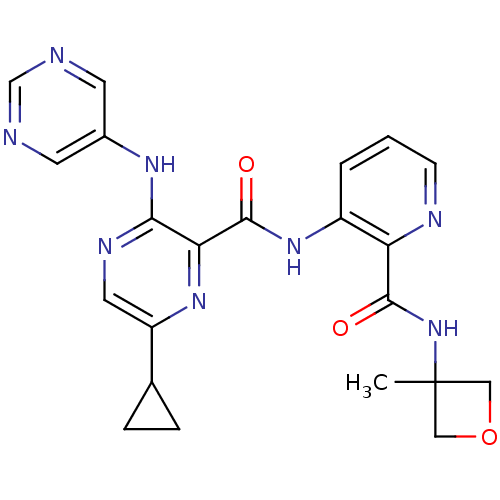 US8703768, 275 Roche-Dataset for PDE10A, Compound 770 BDBM120674
US8703768, 275 Roche-Dataset for PDE10A, Compound 770 BDBM120674 US9802960, Compound I-277 US9751854, Compound I-275 BDBM338099
US9802960, Compound I-277 US9751854, Compound I-275 BDBM338099 BDBM328137 US10377742, Compound 275 N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(1-(4- methoxyphenyl) piperidin-4- ylamino)picolinamide US9663496, Compound 275
BDBM328137 US10377742, Compound 275 N-(1-(4- cyanobenzyl)piperidin-4- yl)-5-(1-(4- methoxyphenyl) piperidin-4- ylamino)picolinamide US9663496, Compound 275 1-(4-((3-chloro-5- (trifluoromethyl)- benzyl)oxy)- piperidine- 1-carbonyl)-1H- pyrazole-3- carboxylic acid US11655217, Example 275 BDBM483776 US10927105, Ex 275
1-(4-((3-chloro-5- (trifluoromethyl)- benzyl)oxy)- piperidine- 1-carbonyl)-1H- pyrazole-3- carboxylic acid US11655217, Example 275 BDBM483776 US10927105, Ex 275 6-(3,4-Difluorophenyl)-1-[(4-methyl-3-pyridyl)methyl]-3H-imidazo[4,5- b]pyridin-2-one BDBM436940 US10617676, Example 275 US11207298, Example 275
6-(3,4-Difluorophenyl)-1-[(4-methyl-3-pyridyl)methyl]-3H-imidazo[4,5- b]pyridin-2-one BDBM436940 US10617676, Example 275 US11207298, Example 275 BDBM283628 N-((6-amino-2,4-dimethylpyridin-3-yl)methyl)- 2-((3-chloroquinolin-6- yl)(hydroxy)methyl)isonicotinamide US10023557, Example 275 US10308637, Example 275
BDBM283628 N-((6-amino-2,4-dimethylpyridin-3-yl)methyl)- 2-((3-chloroquinolin-6- yl)(hydroxy)methyl)isonicotinamide US10023557, Example 275 US10308637, Example 275 BDBM510415 US11439633, Example 275 US11077100, Example 275 (5S,8R)-5-fluoro-8- hydroxy-8- methyl-N-((1- morpholinocyclohexyl) methyl)-5,6,7,8- tetrahydroquinoline- 5-carboxamide
BDBM510415 US11439633, Example 275 US11077100, Example 275 (5S,8R)-5-fluoro-8- hydroxy-8- methyl-N-((1- morpholinocyclohexyl) methyl)-5,6,7,8- tetrahydroquinoline- 5-carboxamide US11718603, Example 275 BDBM498450 US11014911, Example 275 (1R,3S)-3-(3-{[(4- methoxyphenyl)acetyl]amino}- 1H-pyrazol-5-yl)cyclopentyl propan-2-ylcarbamate
US11718603, Example 275 BDBM498450 US11014911, Example 275 (1R,3S)-3-(3-{[(4- methoxyphenyl)acetyl]amino}- 1H-pyrazol-5-yl)cyclopentyl propan-2-ylcarbamate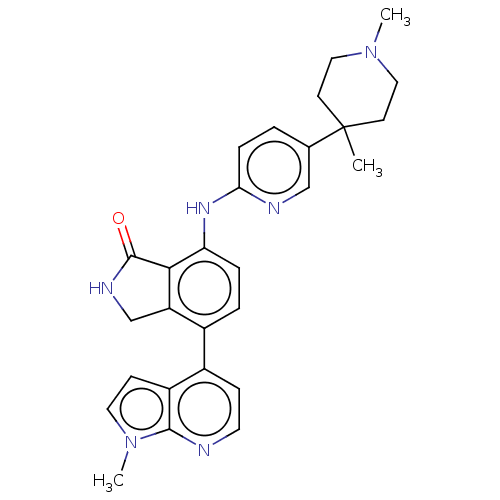 US11548890, Compound I-275 US11078201, Compound I-275 US11021481, Compound I-275 BDBM500616 7-((5-(1,4- dimethyl-piperidin-4- yl)pyridin-2- yl)amino)-4- (1-methyl-1H- pyrrolo[2,3- b]pyridin-4- yl)isoindolin- 1-one
US11548890, Compound I-275 US11078201, Compound I-275 US11021481, Compound I-275 BDBM500616 7-((5-(1,4- dimethyl-piperidin-4- yl)pyridin-2- yl)amino)-4- (1-methyl-1H- pyrrolo[2,3- b]pyridin-4- yl)isoindolin- 1-one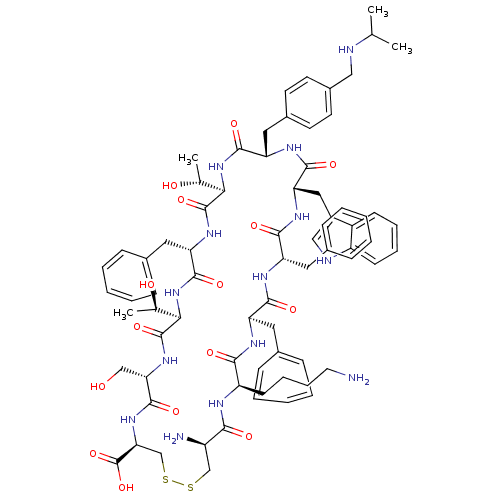 CHEMBL386023 BDBM50159394 Des-AA1,2,5-[D-Trp8,IAmp9]SRIF CH-275
CHEMBL386023 BDBM50159394 Des-AA1,2,5-[D-Trp8,IAmp9]SRIF CH-275 US11912703, Example 59 BDBM655121 US11912703, Example 275 US11912703, Example 276
US11912703, Example 59 BDBM655121 US11912703, Example 275 US11912703, Example 276 US10246453, Example 275 (S)-4-((1-benzylpyrrolidin-3-yl)oxy)-2,6-difluoro-N-(thiazol-4-yl)-3-vinylbenzenesulfonamide 2,2,2-trifluoroacetate BDBM374008 US10815229, Example 275
US10246453, Example 275 (S)-4-((1-benzylpyrrolidin-3-yl)oxy)-2,6-difluoro-N-(thiazol-4-yl)-3-vinylbenzenesulfonamide 2,2,2-trifluoroacetate BDBM374008 US10815229, Example 275 BDBM322098 US11312688, Example 275 1-{5-[(5-chloro-2-fluorobenzyl)oxy]-1-(3-methoxy-3-methylbutyl)-1H-pyrazol-3-yl}-N-methylmethanamine US10183913, Example 275
BDBM322098 US11312688, Example 275 1-{5-[(5-chloro-2-fluorobenzyl)oxy]-1-(3-methoxy-3-methylbutyl)-1H-pyrazol-3-yl}-N-methylmethanamine US10183913, Example 275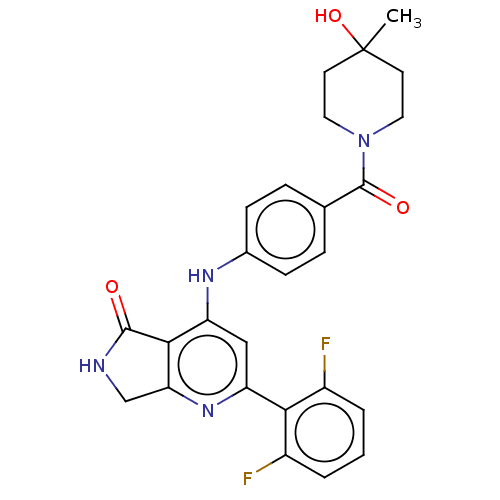 US11040967, Compound 12 BDBM332986 US10196390, Compound I-275 US10336752, Compound 12
US11040967, Compound 12 BDBM332986 US10196390, Compound I-275 US10336752, Compound 12 BDBM467622 US10800792, Example 275 5-(2-Methyl-4-phenoxyphenyl)-N-(2-morpholinoethyl)-4-oxo-4,5- dihydro-3H-1-thia-3,5,8-triazaacenaphthylene-2-carboxamide; US10822348, Example 275
BDBM467622 US10800792, Example 275 5-(2-Methyl-4-phenoxyphenyl)-N-(2-morpholinoethyl)-4-oxo-4,5- dihydro-3H-1-thia-3,5,8-triazaacenaphthylene-2-carboxamide; US10822348, Example 275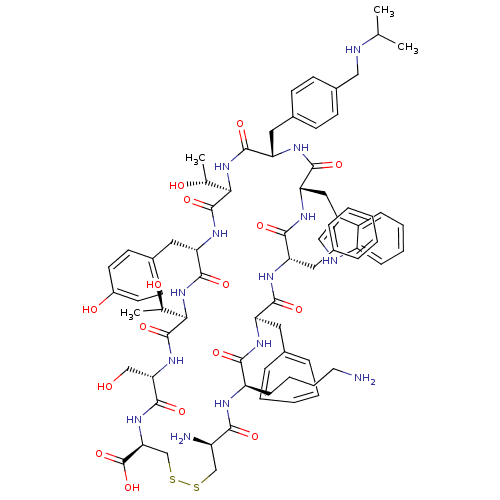 CHEMBL268205 Des-AA1,2,5-[D-Trp8,IAmp9,Tyr11]SRIF [Tyr11]CH-275 BDBM50159420
CHEMBL268205 Des-AA1,2,5-[D-Trp8,IAmp9,Tyr11]SRIF [Tyr11]CH-275 BDBM50159420 (R)-2-((1-(2- aminobenzo[d]thiazol- 5-yl)-7,8,9,10- tetrahydro-6-oxa-2,10a- diazacycloocta[cd]inden-4- yl)oxy)propanamide US10065970, Example 275 BDBM272970 US10435414, Example 275
(R)-2-((1-(2- aminobenzo[d]thiazol- 5-yl)-7,8,9,10- tetrahydro-6-oxa-2,10a- diazacycloocta[cd]inden-4- yl)oxy)propanamide US10065970, Example 275 BDBM272970 US10435414, Example 275 US10544130, Example 275 (R)-4-(2-(aminomethyl)benzo[d]thiazol-5-yl)-N1-(pyrrolidin-3-yl)-3-(2H-tetrazol-5-yl)benzene-1,2-disulfonamide BDBM361118 US10221163, Example 275
US10544130, Example 275 (R)-4-(2-(aminomethyl)benzo[d]thiazol-5-yl)-N1-(pyrrolidin-3-yl)-3-(2H-tetrazol-5-yl)benzene-1,2-disulfonamide BDBM361118 US10221163, Example 275 US10617680, Example 275 2-{4-[4-(4-Chloro-phenyl)-1-methyl-6-oxo-1,6-dihydro- pyridin-3-yl]-pyrazol-1-yl}-6-fluoro-benzonitrile US11020380, Example 275 BDBM437541
US10617680, Example 275 2-{4-[4-(4-Chloro-phenyl)-1-methyl-6-oxo-1,6-dihydro- pyridin-3-yl]-pyrazol-1-yl}-6-fluoro-benzonitrile US11020380, Example 275 BDBM437541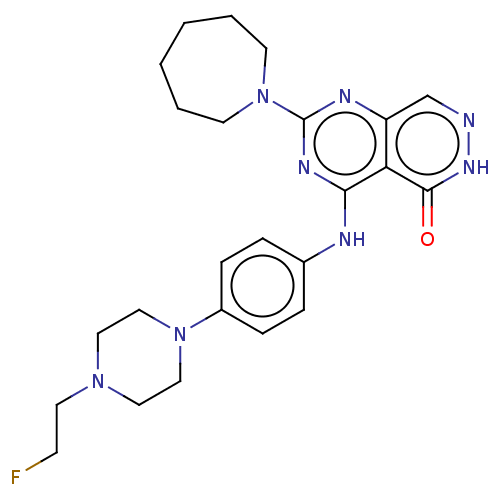 US10647720, Ex. # 275 BDBM322905 US10183944, Example 275 2-(azepan-1-yl)-4-((4-(4-(2-fluoroethyl) piperazin-1-yl)phenyl)amino) pyrimido[4,5-d]pyridazin-5(6H)-one
US10647720, Ex. # 275 BDBM322905 US10183944, Example 275 2-(azepan-1-yl)-4-((4-(4-(2-fluoroethyl) piperazin-1-yl)phenyl)amino) pyrimido[4,5-d]pyridazin-5(6H)-one (R)-1-(4-(6-(2-(4-cyclopropylpyridin- 2-yl)acetamido)pyridazin-3-yl)-2- fluorobutyl)-N-methyl-1H-1,2,3- triazole-4-carboxamide US10344025, Example 275 US11370786, Example 275 BDBM404931
(R)-1-(4-(6-(2-(4-cyclopropylpyridin- 2-yl)acetamido)pyridazin-3-yl)-2- fluorobutyl)-N-methyl-1H-1,2,3- triazole-4-carboxamide US10344025, Example 275 US11370786, Example 275 BDBM404931 5-(4-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)amino)-1,3,5-triazin-2-yl)-2-((tetrahydro-2H-pyran-4-yl)oxy)benzonitrile US10040781, Example 275 BDBM278053 US10253019, Example 275
5-(4-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)amino)-1,3,5-triazin-2-yl)-2-((tetrahydro-2H-pyran-4-yl)oxy)benzonitrile US10040781, Example 275 BDBM278053 US10253019, Example 275 US20240217978, Example 275 BDBM683619 (S)-1-(4-(1-(7-amino-2-(furan-2-
US20240217978, Example 275 BDBM683619 (S)-1-(4-(1-(7-amino-2-(furan-2- N-{3-[({2-[(4-{[(3R)-3-hydroxypiperidin-1- yl]carbonyl}phenyl)amino]-5- (trifluoromethyl)pyrimidin-4- yl}amino)methyl]pyridin-2-yl}-N- methylmethane-sulfonamide (275) BDBM418777 US10450297, Example 275
N-{3-[({2-[(4-{[(3R)-3-hydroxypiperidin-1- yl]carbonyl}phenyl)amino]-5- (trifluoromethyl)pyrimidin-4- yl}amino)methyl]pyridin-2-yl}-N- methylmethane-sulfonamide (275) BDBM418777 US10450297, Example 275 (S)-3-[4-Chloro-2-(4-chloro-phenoxy)-phenoxy]-pyrrolidine BDBM396648 US9981909, Example 275
(S)-3-[4-Chloro-2-(4-chloro-phenoxy)-phenoxy]-pyrrolidine BDBM396648 US9981909, Example 275 BDBM601013 (1-(3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methanol US11634391, Compound 275
BDBM601013 (1-(3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methanol US11634391, Compound 275 BDBM691814 4- (difluoromethoxy)benzyl (4-((6- methylnicotinamido)meth- yl)phenyl)carbamate US20240279215, Compound 275
BDBM691814 4- (difluoromethoxy)benzyl (4-((6- methylnicotinamido)meth- yl)phenyl)carbamate US20240279215, Compound 275 (R/S)-(2-(2H-1,2,3-triazol-2-yl)phenyl)(6-((5-(trifluoromethyl)pyrazin-2-yl)amino)-2-azabicyclo[2.2.2]octan-2-yl)methanone US9611277, Example 275 US9611277, Example 254 US10183953, Example 275 BDBM315073
(R/S)-(2-(2H-1,2,3-triazol-2-yl)phenyl)(6-((5-(trifluoromethyl)pyrazin-2-yl)amino)-2-azabicyclo[2.2.2]octan-2-yl)methanone US9611277, Example 275 US9611277, Example 254 US10183953, Example 275 BDBM315073 US11001575, Example 275 2-(6-{5-chloro-2-[(oxan- 4-yl)amino]pyrimidin-4- yl}-1-oxo-2,3-dihydro- 1H-isoindol-2-yl)-N- [(1S,2R)-2- hydroxycyclopentyl] acetamide US10457669, Example 275 BDBM417658
US11001575, Example 275 2-(6-{5-chloro-2-[(oxan- 4-yl)amino]pyrimidin-4- yl}-1-oxo-2,3-dihydro- 1H-isoindol-2-yl)-N- [(1S,2R)-2- hydroxycyclopentyl] acetamide US10457669, Example 275 BDBM417658 BDBM262423 6-cyclopropyl-3-(quinolin-4-yl)-2-(2H-tetrazol-5-yl)benzenesulfonamide US9708336, 275
BDBM262423 6-cyclopropyl-3-(quinolin-4-yl)-2-(2H-tetrazol-5-yl)benzenesulfonamide US9708336, 275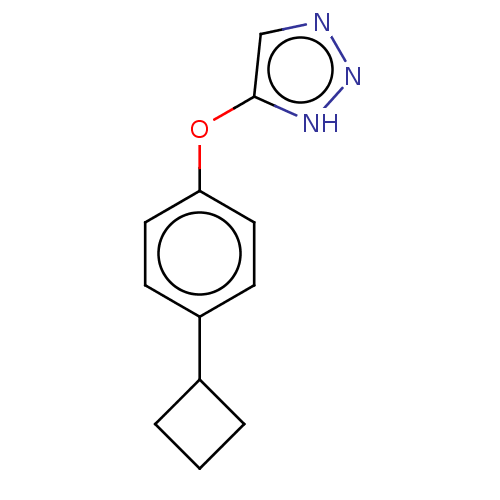 BDBM560904 5-(4- cyclobutylphenoxy)- 1H-1,2,3-triazole-4- carboxylic acid US11389456, Ex. No. I-275
BDBM560904 5-(4- cyclobutylphenoxy)- 1H-1,2,3-triazole-4- carboxylic acid US11389456, Ex. No. I-275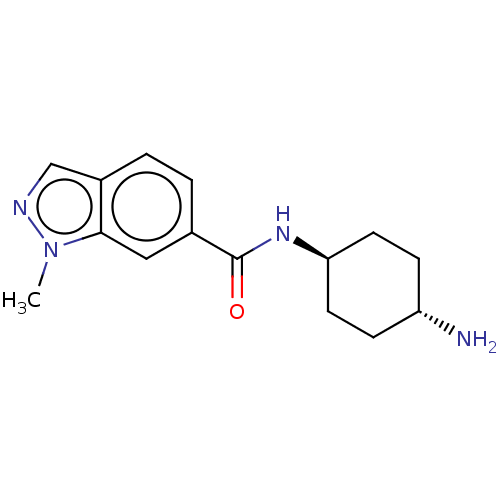 US10266526, Compound 275 N-((1r,4r)-4-aminocyclohexyl)-1-methyl-1H-indazole-6-carboxamide BDBM378146
US10266526, Compound 275 N-((1r,4r)-4-aminocyclohexyl)-1-methyl-1H-indazole-6-carboxamide BDBM378146 US11279687, Compound 275 BDBM543264 3-{4-[(3-chlorobenzene)sulfonyl]phenyl}-1- (pyridin-3-ylmethyl)urea
US11279687, Compound 275 BDBM543264 3-{4-[(3-chlorobenzene)sulfonyl]phenyl}-1- (pyridin-3-ylmethyl)urea 4-((4-ethoxycyclohexyl)methoxy)- 5-fluoro-N-(4- morpholinophenyl)pyrimidin-2- amine BDBM593296 US11578061, Example 275
4-((4-ethoxycyclohexyl)methoxy)- 5-fluoro-N-(4- morpholinophenyl)pyrimidin-2- amine BDBM593296 US11578061, Example 275 BDBM285230 US10023592, Example 275 3-chloro-5-[2-(cyclopropylmethylamino)-5- ethylsulfonylphenyl]-1-methyl-pyridine-2-one
BDBM285230 US10023592, Example 275 3-chloro-5-[2-(cyclopropylmethylamino)-5- ethylsulfonylphenyl]-1-methyl-pyridine-2-one BDBM522717 US11161848, Compound I-275 9-(4-(1-isopropyl-1H-imidazol-4-yl)benzyl)-2-(2-
BDBM522717 US11161848, Compound I-275 9-(4-(1-isopropyl-1H-imidazol-4-yl)benzyl)-2-(2- N-[(4-tetrahydropyran-3- ylsulfonylphenyl)methyl]furo [2,3-c]pyridine-2-carboxamide BDBM449291 US10696692, Example 275
N-[(4-tetrahydropyran-3- ylsulfonylphenyl)methyl]furo [2,3-c]pyridine-2-carboxamide BDBM449291 US10696692, Example 275 US10941160, Example 275 BDBM486074 3-chloro-5-[2-(cyclopropylmethylamino)-5- ethylsulfonylphenyl]-1-methyl-pyridine-2-one
US10941160, Example 275 BDBM486074 3-chloro-5-[2-(cyclopropylmethylamino)-5- ethylsulfonylphenyl]-1-methyl-pyridine-2-one (2S,4R)-1-(2-(3- acetyl-5-(2- methylpyrazolo[1,5- a]pyrimidin-6-yl)- 1H-indazol-1- yl)acetyl)-4-fluoro- N-(2-fluoro-3- methoxyphenyl) pyrrolidine-2- carboxamide US10287301, Compound 275 BDBM386346 US10822352, Comp No. 275
(2S,4R)-1-(2-(3- acetyl-5-(2- methylpyrazolo[1,5- a]pyrimidin-6-yl)- 1H-indazol-1- yl)acetyl)-4-fluoro- N-(2-fluoro-3- methoxyphenyl) pyrrolidine-2- carboxamide US10287301, Compound 275 BDBM386346 US10822352, Comp No. 275 (R/S)-2-Cyclopropyl-1-[6-(4-fluoro-3-methyl-phenyl)pyrrolo[3,2- BDBM409402 US10377753, Example 275
(R/S)-2-Cyclopropyl-1-[6-(4-fluoro-3-methyl-phenyl)pyrrolo[3,2- BDBM409402 US10377753, Example 275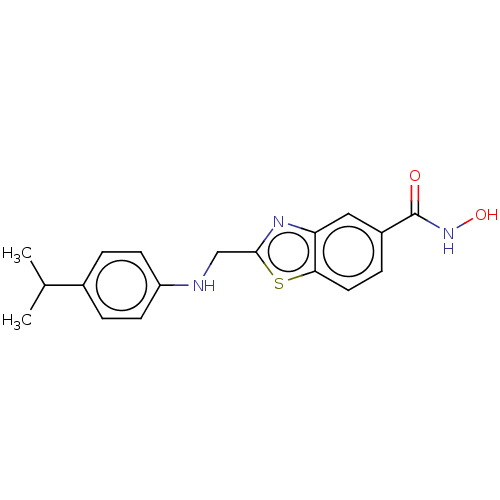 BDBM443092 N-hydroxy-2-({[4-(1- methylethyl)phenyl]amino}methyl)- 1,3-benzothiazole-5-carboxamide US10654814, Example 275
BDBM443092 N-hydroxy-2-({[4-(1- methylethyl)phenyl]amino}methyl)- 1,3-benzothiazole-5-carboxamide US10654814, Example 275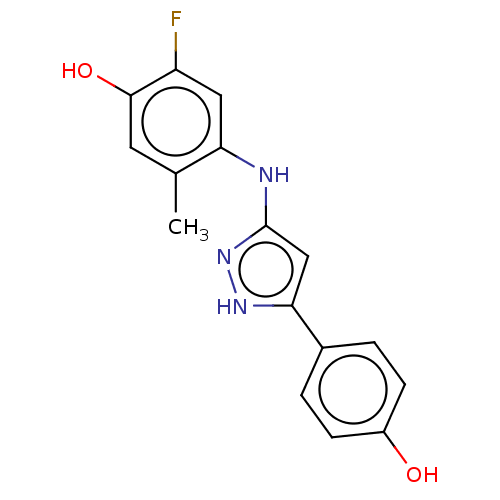 BDBM579798 2-fluoro-4-((5-(4-hydroxyphenyl)- 1H-pyrazol-3-yl)amino)-5- methylphenol US11485711, Compound 275
BDBM579798 2-fluoro-4-((5-(4-hydroxyphenyl)- 1H-pyrazol-3-yl)amino)-5- methylphenol US11485711, Compound 275 BDBM612586 BDBM612724 BDBM612725 N'-(1,2,3,5,6,7-hexahydro- s-indacen-4-ylcarbamoyl)- 6-isobutylpyridine-3- sulfonimidamide US11724992, Example 275
BDBM612586 BDBM612724 BDBM612725 N'-(1,2,3,5,6,7-hexahydro- s-indacen-4-ylcarbamoyl)- 6-isobutylpyridine-3- sulfonimidamide US11724992, Example 275 US11634396, Compound 275 2-chloro-4-((S)-2-(dimethylamino)-3-((S)-3-phenylbutanamido)propyl)phenyl acetate BDBM601336
US11634396, Compound 275 2-chloro-4-((S)-2-(dimethylamino)-3-((S)-3-phenylbutanamido)propyl)phenyl acetate BDBM601336 3-(4-aminopyrido[3,2-d]pyrimidin-6-yl)-N-(2-pyrrolidin-1-ylethyl)benzamide BDBM364032 US9855269, Compound 275
3-(4-aminopyrido[3,2-d]pyrimidin-6-yl)-N-(2-pyrrolidin-1-ylethyl)benzamide BDBM364032 US9855269, Compound 275 BDBM413280 6-(4-(4-cyanophenyl)-5-hydroxy-1H-pyrazol-1-yl)-N-cyclopropyl-4-methylnicotinamide US10407409, Example 275
BDBM413280 6-(4-(4-cyanophenyl)-5-hydroxy-1H-pyrazol-1-yl)-N-cyclopropyl-4-methylnicotinamide US10407409, Example 275 BDBM585660 US11530210, Example 275 6-[3-(Difluoromethyl)-4-fluoro-phenyl]-1-(pyrimidin-5- ylmethyl)pyrazolo[4,3-b]pyridine
BDBM585660 US11530210, Example 275 6-[3-(Difluoromethyl)-4-fluoro-phenyl]-1-(pyrimidin-5- ylmethyl)pyrazolo[4,3-b]pyridine N-3-isoxazolyl-1-(5-methoxy-2,2',4',5'-tetramethyl-4-biphenylyl)-6-isoquinolinesulfonamide BDBM343104 US9776995, Example 275
N-3-isoxazolyl-1-(5-methoxy-2,2',4',5'-tetramethyl-4-biphenylyl)-6-isoquinolinesulfonamide BDBM343104 US9776995, Example 275 US10544113, No. 275 N-(Adamantan-1-ylmethyl)-4-(2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-3-methylbenzamide BDBM426930
US10544113, No. 275 N-(Adamantan-1-ylmethyl)-4-(2-(4-fluorophenyl)-4-oxothiazolidin-3-yl)-3-methylbenzamide BDBM426930 US20250136553, Compound 275 5-((1H-pyrazol-1-yl)methyl)-N-((2,6-dimethoxy-4- methylphenyl)sulfonyl)-6-methoxypicolinamide BDBM736550
US20250136553, Compound 275 5-((1H-pyrazol-1-yl)methyl)-N-((2,6-dimethoxy-4- methylphenyl)sulfonyl)-6-methoxypicolinamide BDBM736550 US9598423, Example 275 N5-(4-Cyanophenyl)-2-(3,5- dimethylphenyl)-6,7- dihydropyrazolo[1,5-a] pyrazine-3,5(4H)- dicarboxamide BDBM302707
US9598423, Example 275 N5-(4-Cyanophenyl)-2-(3,5- dimethylphenyl)-6,7- dihydropyrazolo[1,5-a] pyrazine-3,5(4H)- dicarboxamide BDBM302707 BDBM334120 US10646492, Example 275 US9730939, Example 274 4-[1-(4-Amino-3-methyl-1H- pyrazolo[3,4-d]pyrimidin-1- yl)ethyl]-6-chloro-3-methoxy-2-[1- (tetrahydrofuran-3-yl)azetidin-3- yl]benzonitrile US10376513, Example 276 US9730939, Example 275
BDBM334120 US10646492, Example 275 US9730939, Example 274 4-[1-(4-Amino-3-methyl-1H- pyrazolo[3,4-d]pyrimidin-1- yl)ethyl]-6-chloro-3-methoxy-2-[1- (tetrahydrofuran-3-yl)azetidin-3- yl]benzonitrile US10376513, Example 276 US9730939, Example 275 US20230348424, Example 276 US20230348424, Example 275 BDBM631692 N-((S)-1-(3-(difluoromethyl)-2-fluorophenyl)-2,2,2- trifluoroethyl)-1-(1-(difluoromethyl)cyclopropyl)-4-(((1R,5S,6s)-3-methyl-3- azabicyclo[3.1.0]hexan-6-yl)amino)-6-oxo-1,6-dihydropyridine-3-carboxamide (Example 275)
US20230348424, Example 276 US20230348424, Example 275 BDBM631692 N-((S)-1-(3-(difluoromethyl)-2-fluorophenyl)-2,2,2- trifluoroethyl)-1-(1-(difluoromethyl)cyclopropyl)-4-(((1R,5S,6s)-3-methyl-3- azabicyclo[3.1.0]hexan-6-yl)amino)-6-oxo-1,6-dihydropyridine-3-carboxamide (Example 275) 3-(1-cyclopentyl-1H- benzo[d][1,2,3]triazol-5-yl)-5-(3,4- dimethoxyphenyl)-1,2,4-oxadiazole US11912693, Compound 275 BDBM654665
3-(1-cyclopentyl-1H- benzo[d][1,2,3]triazol-5-yl)-5-(3,4- dimethoxyphenyl)-1,2,4-oxadiazole US11912693, Compound 275 BDBM654665 5-[2,5-Bis(trifluoromethyl)imidazo[4,5-b]pyridin-3-yl]-7-methyl-indolin-2-one; US11312712, Example 275 BDBM551158
5-[2,5-Bis(trifluoromethyl)imidazo[4,5-b]pyridin-3-yl]-7-methyl-indolin-2-one; US11312712, Example 275 BDBM551158 6-(difluoromethyl)-N-(3- methyl-9H-thioxanthen-9- yl)-2-oxo-1,2- dihydropyridine-3- carboxamide BDBM667544 US11957687, Compound 275
6-(difluoromethyl)-N-(3- methyl-9H-thioxanthen-9- yl)-2-oxo-1,2- dihydropyridine-3- carboxamide BDBM667544 US11957687, Compound 275 6-oxo-2-phenyl-N-{(1R)-1-[4- (trifluoromethoxy)phenyl]ethyl}-1,6- dihydropyrimidine-4-carboxamide BDBM355184 US9815796, Example 275
6-oxo-2-phenyl-N-{(1R)-1-[4- (trifluoromethoxy)phenyl]ethyl}-1,6- dihydropyrimidine-4-carboxamide BDBM355184 US9815796, Example 275 BDBM613534 1-[1-(cyanomethyl)-4-[4- (cyclopenten-1- yl)anilino]cyclohexyl]-3- (cyclopropanecarbonylamino) pyrazole-4-carboxamide US11731943, Example 275
BDBM613534 1-[1-(cyanomethyl)-4-[4- (cyclopenten-1- yl)anilino]cyclohexyl]-3- (cyclopropanecarbonylamino) pyrazole-4-carboxamide US11731943, Example 275 5-(3-fluoro-5-(2-(3- (methylsulfonyl)phenyl)ethynyl) phenoxy)-1H-1,2,3-triazole- 4-carboxylic acid BDBM581177 US11504367, Example 275
5-(3-fluoro-5-(2-(3- (methylsulfonyl)phenyl)ethynyl) phenoxy)-1H-1,2,3-triazole- 4-carboxylic acid BDBM581177 US11504367, Example 275 5-(6-(benzyl(methyl)amino)-2-(2,4- difluorophenoxy)pyrimidin-4-yl)-1- methylpyridin-2(1H)-one BDBM345730 US10202360, Example 275
5-(6-(benzyl(methyl)amino)-2-(2,4- difluorophenoxy)pyrimidin-4-yl)-1- methylpyridin-2(1H)-one BDBM345730 US10202360, Example 275 BDBM660889 US20240101531, Example 275 ((1-((6-chloropyridin-3- yl)amino)isoquinolin-6- yl)amino)(methyl)(oxetan-3-yl)- lambda6-sulfanone
BDBM660889 US20240101531, Example 275 ((1-((6-chloropyridin-3- yl)amino)isoquinolin-6- yl)amino)(methyl)(oxetan-3-yl)- lambda6-sulfanone US11066396, Example 275 BDBM504867 3-({3- fluoro-4-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3- yl]phenyl} methoxy)- 7-methoxy-
US11066396, Example 275 BDBM504867 3-({3- fluoro-4-[5- (trifluoro- methyl)- 1,2,4-oxa- diazol-3- yl]phenyl} methoxy)- 7-methoxy- US11839613, Example 275 2-Ethylsulfanyl-4-{6-[2-(6-fluoro-benzofuran-5-yl)-ethylamino]-pyrimidin-4- yl}-benzoic acid BDBM640154
US11839613, Example 275 2-Ethylsulfanyl-4-{6-[2-(6-fluoro-benzofuran-5-yl)-ethylamino]-pyrimidin-4- yl}-benzoic acid BDBM640154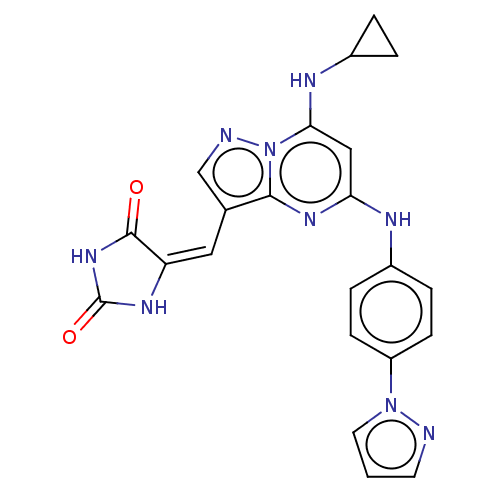 US9303033, Example 275, Table 52, Compound 1 US9303033, C39, Table 43A, Compound 6 US9303033, R1, Table 4A, Compound 12 BDBM218860
US9303033, Example 275, Table 52, Compound 1 US9303033, C39, Table 43A, Compound 6 US9303033, R1, Table 4A, Compound 12 BDBM218860 BDBM511938 US11084800, Cpd No. 275 (1R,3S,5R)-2-(2-(3- acetyl-5-(2- methoxypyrimidin-5- yl)-7-methyl-1H- pyrazolo[3,4- c]pyridin-1-yl)acetyl)- N-(6-bromo-3- methylpyridin-2-yl)-5- methyl-2- azabicyclo[3.1.0] hexane-3-carboxamide US11708351, Compound 275
BDBM511938 US11084800, Cpd No. 275 (1R,3S,5R)-2-(2-(3- acetyl-5-(2- methoxypyrimidin-5- yl)-7-methyl-1H- pyrazolo[3,4- c]pyridin-1-yl)acetyl)- N-(6-bromo-3- methylpyridin-2-yl)-5- methyl-2- azabicyclo[3.1.0] hexane-3-carboxamide US11708351, Compound 275 (1R,2R)-2-[({2,6-dimethoxy-4-[(2- methyl-3- phenylphenyl)methoxy]phenyl} methyl)amino]cyclohexan-1-ol BDBM366023 US9872852, Example 275
(1R,2R)-2-[({2,6-dimethoxy-4-[(2- methyl-3- phenylphenyl)methoxy]phenyl} methyl)amino]cyclohexan-1-ol BDBM366023 US9872852, Example 275 6-chloro-3-(2-{3-methoxy-4- [(1s,3s)-3- (dimethylamino)cyclobutoxy] phenylamino}-4- pyrimidinylamino)-2- pyridinecarboxamide US20250171431, Compound 275 BDBM744980
6-chloro-3-(2-{3-methoxy-4- [(1s,3s)-3- (dimethylamino)cyclobutoxy] phenylamino}-4- pyrimidinylamino)-2- pyridinecarboxamide US20250171431, Compound 275 BDBM744980 BDBM327192 US9662339, 275 (S)-3-[4-(1,1-Dioxo-1lambda6- [1,4]thiazepan-4-ylmethyl)- phenyl]-2,3-dihydro- [1,4]dioxino[2,3-b]pyridine
BDBM327192 US9662339, 275 (S)-3-[4-(1,1-Dioxo-1lambda6- [1,4]thiazepan-4-ylmethyl)- phenyl]-2,3-dihydro- [1,4]dioxino[2,3-b]pyridine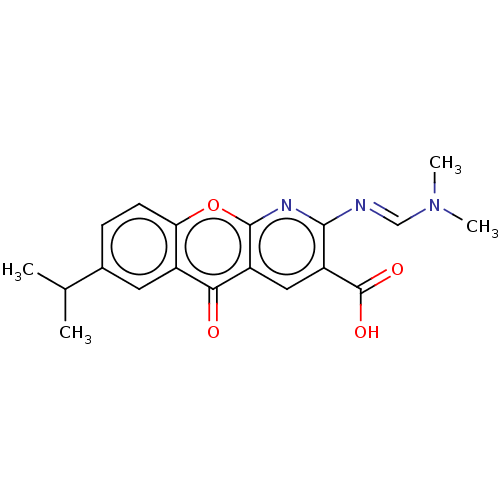 BDBM357915 US10214536, Compound 275 (E)-2-(((Dimethylamino)methylene)amino)-7-isopropyl-5-oxo-5H-chromeno[2,3-b]pyridine-3-carboxylic acid
BDBM357915 US10214536, Compound 275 (E)-2-(((Dimethylamino)methylene)amino)-7-isopropyl-5-oxo-5H-chromeno[2,3-b]pyridine-3-carboxylic acid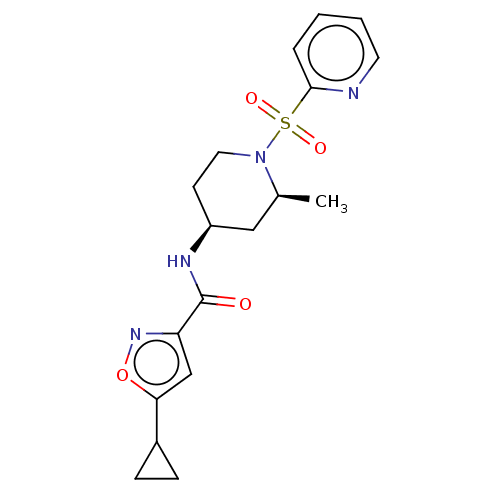 BDBM432575 US10577363, Compound 275 5-cyclopropyl-N-((2S,4S)-2-methyl-1- (pyridin-2-ylsulfonyl)piperidin-4- yl)isoxazole-3-carboxamide
BDBM432575 US10577363, Compound 275 5-cyclopropyl-N-((2S,4S)-2-methyl-1- (pyridin-2-ylsulfonyl)piperidin-4- yl)isoxazole-3-carboxamide US10696692, Example 168 Furo[2,3-c]pyridine-2- carboxylic acid 4- (tetrahydro-pyran-3- sulfonyl)-benzylamide (racemic) BDBM449189 US10696692, Example 275
US10696692, Example 168 Furo[2,3-c]pyridine-2- carboxylic acid 4- (tetrahydro-pyran-3- sulfonyl)-benzylamide (racemic) BDBM449189 US10696692, Example 275 US11998539, Example 275 4-[4-(1,3- benzoxazol-2- yl)piperidin-1-yl]- 1-methyl-2-oxo- 1,2- dihydroquinoline- 3,7-dicarbonitrile BDBM678833
US11998539, Example 275 4-[4-(1,3- benzoxazol-2- yl)piperidin-1-yl]- 1-methyl-2-oxo- 1,2- dihydroquinoline- 3,7-dicarbonitrile BDBM678833 (6-(4-(Difluoromethyl)-2-methoxybenzyl)-2-azaspiro[3.3]heptan-2-yl)((1s,3s)-3-hydroxy-3-methylcyclobutyl)methanone BDBM630685 US11802111, Example 275
(6-(4-(Difluoromethyl)-2-methoxybenzyl)-2-azaspiro[3.3]heptan-2-yl)((1s,3s)-3-hydroxy-3-methylcyclobutyl)methanone BDBM630685 US11802111, Example 275 BDBM290870 US9579320, Example 275 2-((1R,2S)-2-aminocyclohexylamino)-4-(3-methyl-4-(2H-1,2,3-triazol-2-yl)phenylamino)pyrimidine-5-carboxamide
BDBM290870 US9579320, Example 275 2-((1R,2S)-2-aminocyclohexylamino)-4-(3-methyl-4-(2H-1,2,3-triazol-2-yl)phenylamino)pyrimidine-5-carboxamide N-[1-{5-[5-(aminomethyl)furan-2-yl]thiophen-2-yl}ethyl]-6,7-dimethoxy-2-methylquinazolin-4-amine BDBM657596 US20240083857, Example 275
N-[1-{5-[5-(aminomethyl)furan-2-yl]thiophen-2-yl}ethyl]-6,7-dimethoxy-2-methylquinazolin-4-amine BDBM657596 US20240083857, Example 275 N6-Cyclopropyl-7-hydroxy-4-(2-methoxy-2-methylpropyl)-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidine-2,6-dicarboxamide BDBM659838 US20240092784, Example 275
N6-Cyclopropyl-7-hydroxy-4-(2-methoxy-2-methylpropyl)-5-oxo-4,5-dihydropyrazolo[1,5-a]pyrimidine-2,6-dicarboxamide BDBM659838 US20240092784, Example 275 US10206907, Compound 275 1-{4-[2- (Cyclopropanecarbonyl- amino)-[1,2,4]triazolo[1,5- a]pyridin-5-yl]-benzoyl}- piperidine-2-carboxylic acid amide BDBM349141
US10206907, Compound 275 1-{4-[2- (Cyclopropanecarbonyl- amino)-[1,2,4]triazolo[1,5- a]pyridin-5-yl]-benzoyl}- piperidine-2-carboxylic acid amide BDBM349141 US10294212, No. 275 4-(2-Amino-3- ((3-chloro-4- fluorophenyl) amino)furo[2,3- c]pyridin-7-yl)- N-pentyl- picolinamide BDBM387695
US10294212, No. 275 4-(2-Amino-3- ((3-chloro-4- fluorophenyl) amino)furo[2,3- c]pyridin-7-yl)- N-pentyl- picolinamide BDBM387695 US10508109, Example 275 BDBM423089 tert-butyl 4-(2-(5-chloro-2,4- dimethoxyphenyl)imidazo[1,2- a]pyridin-7-yl)-2- methylpiperazine-1-carboxylate
US10508109, Example 275 BDBM423089 tert-butyl 4-(2-(5-chloro-2,4- dimethoxyphenyl)imidazo[1,2- a]pyridin-7-yl)-2- methylpiperazine-1-carboxylate US11174268, Example 275 BDBM526168 (S)-4-((1-(5-chloro-2-fluorophenyl)ethyl)amino)-2-fluoro-5-methyl-N-(pyrimidin-4-yl)benzenesulfonamide
US11174268, Example 275 BDBM526168 (S)-4-((1-(5-chloro-2-fluorophenyl)ethyl)amino)-2-fluoro-5-methyl-N-(pyrimidin-4-yl)benzenesulfonamide US11845767, Example 275 (5-amino-5-(1-(2-oxo-2-(pyrrolidin-1- yl)ethyl)-1H-tetrazol-5-yl)pentyl)boronic acid BDBM642230
US11845767, Example 275 (5-amino-5-(1-(2-oxo-2-(pyrrolidin-1- yl)ethyl)-1H-tetrazol-5-yl)pentyl)boronic acid BDBM642230 US9556179, Compound 275 N-(6-(1-benzyl-4-(4-fluorophenyl)-1H- imidazol-5-yl)imidazo[1,2-b]pyridazin-2- yl)pivalamide BDBM274700
US9556179, Compound 275 N-(6-(1-benzyl-4-(4-fluorophenyl)-1H- imidazol-5-yl)imidazo[1,2-b]pyridazin-2- yl)pivalamide BDBM274700 4-[(RS,SR)-2,3-dimethylmorpholin-4-yl]-7-(2-fluorobenzenesulfonyl)-2-methyl-5H-pyrrolo[3,2-d]pyrimidin-6-amine US10059713, Example 275 BDBM271154
4-[(RS,SR)-2,3-dimethylmorpholin-4-yl]-7-(2-fluorobenzenesulfonyl)-2-methyl-5H-pyrrolo[3,2-d]pyrimidin-6-amine US10059713, Example 275 BDBM271154 6-[3-[(5-methyl-2- pyridyl)methyl] pyrrolidin-1-yl] pyrido[3,2-d] pyrimidin-4- amine US9592235, Example 275 US9855269, Compound 122 BDBM299502
6-[3-[(5-methyl-2- pyridyl)methyl] pyrrolidin-1-yl] pyrido[3,2-d] pyrimidin-4- amine US9592235, Example 275 US9855269, Compound 122 BDBM299502 BDBM407333 US10336762, Compound 275 6-(3-cyanopyrrolo[1,2- b]pyridazin-7-yl)-N- methyl-4-(((1r,4r)-4- (2,2,2-trifluoroacetamido) cyclohexyl)amino) nicotinamide
BDBM407333 US10336762, Compound 275 6-(3-cyanopyrrolo[1,2- b]pyridazin-7-yl)-N- methyl-4-(((1r,4r)-4- (2,2,2-trifluoroacetamido) cyclohexyl)amino) nicotinamide BDBM725525 3-(4- ((difluoro- methyl)sulfon- amido)-3- (thiazol-2- ylmethoxy) phenyl)-5- (pyrazin-2- ylamino)- 1H-pyrazole-4- carboxamide US20250074891, Compound 275
BDBM725525 3-(4- ((difluoro- methyl)sulfon- amido)-3- (thiazol-2- ylmethoxy) phenyl)-5- (pyrazin-2- ylamino)- 1H-pyrazole-4- carboxamide US20250074891, Compound 275 US10173991, Example 275 2-[5-(4- cyanophenyl)-6- fluoro-1,3- benzothiazol-2- yl]-N- [(cyclopropyl- carbamoyl) methyl]-2- (propane-2- sulfonyl)acetamide BDBM319232
US10173991, Example 275 2-[5-(4- cyanophenyl)-6- fluoro-1,3- benzothiazol-2- yl]-N- [(cyclopropyl- carbamoyl) methyl]-2- (propane-2- sulfonyl)acetamide BDBM319232 US10323038, Example 275 N-(1-(5-(5-chloro-2- (trifluoromethyl)benzyl)octahydropyrrolo [3,4-c]pyrrole-2-carbonyl)-1H-pyrazol-4- yl)methanesulfonamide BDBM399914
US10323038, Example 275 N-(1-(5-(5-chloro-2- (trifluoromethyl)benzyl)octahydropyrrolo [3,4-c]pyrrole-2-carbonyl)-1H-pyrazol-4- yl)methanesulfonamide BDBM399914 US10577367, Example 275 1-(6-(3-(6-methylpyridin-2- yl)imidazo[1,5-a]pyridin-6- yl)pyridin-2-yl)propan-1- amine BDBM433380
US10577367, Example 275 1-(6-(3-(6-methylpyridin-2- yl)imidazo[1,5-a]pyridin-6- yl)pyridin-2-yl)propan-1- amine BDBM433380 (1R,2R)-2-[({2,6-dimethoxy-4-[(2- methyl-3-phenylphenyl) methoxy]phenyl}methyl)amino] cyclohexan-1-ol US9872852, Example 25 US9872852, Example 275 BDBM365773
(1R,2R)-2-[({2,6-dimethoxy-4-[(2- methyl-3-phenylphenyl) methoxy]phenyl}methyl)amino] cyclohexan-1-ol US9872852, Example 25 US9872852, Example 275 BDBM365773 (1r,4r)-4-(3-chloroanilino)-2′-(cyclohex-1-en-1-yl)spiro[cyclohexane-1,1′-indene]-4-carboxylic acid BDBM673140 US20240150293, Example 275
(1r,4r)-4-(3-chloroanilino)-2′-(cyclohex-1-en-1-yl)spiro[cyclohexane-1,1′-indene]-4-carboxylic acid BDBM673140 US20240150293, Example 275 (2S)-2-[(5-fluorobenzofuran- 2-yl)sulfonyl-2- propynylamino]-N-[[2-[2- hydroxy-4- (trifluoromethyl)phenyl]-4- pyridyl]methyl]propanamide BDBM611382 US10626112, Example 275
(2S)-2-[(5-fluorobenzofuran- 2-yl)sulfonyl-2- propynylamino]-N-[[2-[2- hydroxy-4- (trifluoromethyl)phenyl]-4- pyridyl]methyl]propanamide BDBM611382 US10626112, Example 275 (7S)-4,7,8-trimethyl-2-((cis-3-((5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl)cyclobutyl)amino)-7,8-dihydropteridin-6(5H)-one US20230312587, Compound 275 BDBM622567
(7S)-4,7,8-trimethyl-2-((cis-3-((5-(trifluoromethyl)-1H-pyrazol-1-yl)methyl)cyclobutyl)amino)-7,8-dihydropteridin-6(5H)-one US20230312587, Compound 275 BDBM622567 1-(3-tert- Butyl-1-p- tolyl-1H- pyrazol-5- yl)-3-(3-(6,7- dimethoxy- quinazolin- 4- ylthio)phenyl) urea BDBM333725 US9730937, Example 275
1-(3-tert- Butyl-1-p- tolyl-1H- pyrazol-5- yl)-3-(3-(6,7- dimethoxy- quinazolin- 4- ylthio)phenyl) urea BDBM333725 US9730937, Example 275 2-(4-((2-(3-Chlorophenyl)-5,5-dioxido-7,8-dihydro-6H-thiopyrano[3,2-d]pyrimidin-4-yl)amino)phenyl)acetic acid BDBM562703 US11401286, Example 275
2-(4-((2-(3-Chlorophenyl)-5,5-dioxido-7,8-dihydro-6H-thiopyrano[3,2-d]pyrimidin-4-yl)amino)phenyl)acetic acid BDBM562703 US11401286, Example 275 BDBM351554 US9796704, Entry 275 2-({2-[2-({[4-[(3,4-dichlorophenyl)amino]-6- (methyloxy)quinazolin-7- yl]oxy}methyl)morpholin-4- yl]ethyl}oxy)ethanol
BDBM351554 US9796704, Entry 275 2-({2-[2-({[4-[(3,4-dichlorophenyl)amino]-6- (methyloxy)quinazolin-7- yl]oxy}methyl)morpholin-4- yl]ethyl}oxy)ethanol BDBM411279 US10385022, Example 275 3-(Azetidin-3-ylsu lfanyl)-N-[4-(4-fluoro-2-methoxy-phenyl)-pyridin-3-yl]-N-methyl-5-trifluoromethyl-benzamide
BDBM411279 US10385022, Example 275 3-(Azetidin-3-ylsu lfanyl)-N-[4-(4-fluoro-2-methoxy-phenyl)-pyridin-3-yl]-N-methyl-5-trifluoromethyl-benzamide BDBM571208 2-chloro-5-{2-acetamidoimidazo[1,2- b]pyridazin-6-yl}-N-{[2-fluoro-5- (trifluoromethyl)phenyl]methyl}pyridine- 3-carboxamide US11440913, Example 275
BDBM571208 2-chloro-5-{2-acetamidoimidazo[1,2- b]pyridazin-6-yl}-N-{[2-fluoro-5- (trifluoromethyl)phenyl]methyl}pyridine- 3-carboxamide US11440913, Example 275 BDBM680060 US12011444, Example 275 3-Ethoxy-5-{6-[2-(4,5,7-trifluoro-2-methyl-indol-1-yl)-ethylamino]-pyrimidin-4-yl}- thiophene-2-carboxylic acid
BDBM680060 US12011444, Example 275 3-Ethoxy-5-{6-[2-(4,5,7-trifluoro-2-methyl-indol-1-yl)-ethylamino]-pyrimidin-4-yl}- thiophene-2-carboxylic acid US11807646, Example 275 BDBM633861 3-((7-(2- Cyclopropylpiperazine-1- carbonyl)-10-hydroxy-7- azaspiro[4.5]decan-10- yl)methyl)-6-phenylpyrimidin- 4(3H)-one
US11807646, Example 275 BDBM633861 3-((7-(2- Cyclopropylpiperazine-1- carbonyl)-10-hydroxy-7- azaspiro[4.5]decan-10- yl)methyl)-6-phenylpyrimidin- 4(3H)-one US9656955, Example 275 BDBM308761 trans-N-(3-chloro-4-fluorophenyl)-4-(4-fluorophenyl)-1-[(1-methyl-1H-imidazol-4-yl)sulfonyl]pyrrolidine-3-carboxamide
US9656955, Example 275 BDBM308761 trans-N-(3-chloro-4-fluorophenyl)-4-(4-fluorophenyl)-1-[(1-methyl-1H-imidazol-4-yl)sulfonyl]pyrrolidine-3-carboxamide trans-N-[8-amino-5-ethenyl-6-(4- methylpyridin-3-yl)-2,7-naphthyridin-3- yl]-2-(cyanomethyl)cyclopropane-1- carboxamide BDBM504011 US11034692, Compound 275
trans-N-[8-amino-5-ethenyl-6-(4- methylpyridin-3-yl)-2,7-naphthyridin-3- yl]-2-(cyanomethyl)cyclopropane-1- carboxamide BDBM504011 US11034692, Compound 275 1-[(1S,2S)-2- US9783527, Example 275 BDBM344973 1-[(1S,2S)-2-(benzyloxy)cyclohexyl]-3-[1-(2-fluorophenyl)-1H-indazol-4-yl]imidazolidin-2-one
1-[(1S,2S)-2- US9783527, Example 275 BDBM344973 1-[(1S,2S)-2-(benzyloxy)cyclohexyl]-3-[1-(2-fluorophenyl)-1H-indazol-4-yl]imidazolidin-2-one 2-(1-ethyl-8-oxo-spiro[6,7- dihydro-4H-pyrazolo[3,4- c]azepine-5,4'- tetrahydropyran]-3-yl)ethyl isothiazole-4-carboxylate BDBM466178 US10793580, Example 275
2-(1-ethyl-8-oxo-spiro[6,7- dihydro-4H-pyrazolo[3,4- c]azepine-5,4'- tetrahydropyran]-3-yl)ethyl isothiazole-4-carboxylate BDBM466178 US10793580, Example 275 2-(3,8-diazabicyclo[3.2.1]octan-8-yl)-N-(1H-indazol-5-yl)-6,7-dihydro-5H-pyrrolo[3,4-d]pyrimidin-4-amine BDBM598604 US11613531, Example 275
2-(3,8-diazabicyclo[3.2.1]octan-8-yl)-N-(1H-indazol-5-yl)-6,7-dihydro-5H-pyrrolo[3,4-d]pyrimidin-4-amine BDBM598604 US11613531, Example 275 3-{5-[(5- chloropyridin-2- yl)methyl]- 1,3,4-oxadiazol-2- yl}-6- (ethoxymethyl)- 5-(2-hydroxy-6- methoxyphenyl) pyridine-2,4-diol US10336739, Example 275 BDBM406661
3-{5-[(5- chloropyridin-2- yl)methyl]- 1,3,4-oxadiazol-2- yl}-6- (ethoxymethyl)- 5-(2-hydroxy-6- methoxyphenyl) pyridine-2,4-diol US10336739, Example 275 BDBM406661 8-chloro-5-methoxy-4-(2-methyl-2,8-diazaspiro[4.5]decan-8-yl)-2-(pyridin-4-yl)pyrido[3,4-d]pyrimidine BDBM678549 US20240174696, Compound 275
8-chloro-5-methoxy-4-(2-methyl-2,8-diazaspiro[4.5]decan-8-yl)-2-(pyridin-4-yl)pyrido[3,4-d]pyrimidine BDBM678549 US20240174696, Compound 275 BDBM282395 (R)-2-(4-(3-amino-1H- pyrazolo[3,4-b]pyridin- 5-yl)benzylamino)-N- (1-(4-bromophenyl) ethyl)-5- (trifluoromethyl) nicotinamide US10030016, Example 275
BDBM282395 (R)-2-(4-(3-amino-1H- pyrazolo[3,4-b]pyridin- 5-yl)benzylamino)-N- (1-(4-bromophenyl) ethyl)-5- (trifluoromethyl) nicotinamide US10030016, Example 275 BDBM455682 N-([6-[2-(1,4-dimethylpiperidin-4-yl)acetyl]-6-azaspiro[2.5]octan-1-yl]methyl)furo[2,3-c]pyridine-2-carboxamide US10730889, Example 275
BDBM455682 N-([6-[2-(1,4-dimethylpiperidin-4-yl)acetyl]-6-azaspiro[2.5]octan-1-yl]methyl)furo[2,3-c]pyridine-2-carboxamide US10730889, Example 275 BDBM669010 US11964953, Example 275 rac-2-(N-[4-amino-5-[6-[4-(trifluoromethyl)-1-piperidyl]pyridine-3-carbonyl]thiazol-2-yl]-4-fluoro-anilino)propanamide
BDBM669010 US11964953, Example 275 rac-2-(N-[4-amino-5-[6-[4-(trifluoromethyl)-1-piperidyl]pyridine-3-carbonyl]thiazol-2-yl]-4-fluoro-anilino)propanamide BDBM760587 US12371428, Compound 275 1-(4-fluorophenyl)-N-[3-fluoro-4-[(7- phenylmethoxy-1,5-naphthyridin-4- yl)oxy]phenyl]-6-methyl-2- oxopyridine-3-carboxamide
BDBM760587 US12371428, Compound 275 1-(4-fluorophenyl)-N-[3-fluoro-4-[(7- phenylmethoxy-1,5-naphthyridin-4- yl)oxy]phenyl]-6-methyl-2- oxopyridine-3-carboxamide Preparation of N-{4-[4-oxo-3-(phenylamino)-4,5,6,7-tetrahydro-1H-indol-2-yl]pyridin-2-yl}-1H-pyrazole-5-carboxamide US10428044, Example 275 BDBM415170
Preparation of N-{4-[4-oxo-3-(phenylamino)-4,5,6,7-tetrahydro-1H-indol-2-yl]pyridin-2-yl}-1H-pyrazole-5-carboxamide US10428044, Example 275 BDBM415170 US10227331, Example 275 3-(2-amino-1-methyl-1H-benzo[d]imidazol-4-yl)-6-((R)-2-aminopropylsulfonyl)-2-(2H-tetrazol-5-yl)benzenesulfonamide BDBM368088
US10227331, Example 275 3-(2-amino-1-methyl-1H-benzo[d]imidazol-4-yl)-6-((R)-2-aminopropylsulfonyl)-2-(2H-tetrazol-5-yl)benzenesulfonamide BDBM368088 US10544150, Compound 275 (3-(5-methylthiazol-4-yl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)(4-(thiophen-2-yl)phenyl)methanone BDBM430846
US10544150, Compound 275 (3-(5-methylthiazol-4-yl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)(4-(thiophen-2-yl)phenyl)methanone BDBM430846 US11352340, Example 275 BDBM557223 N-[(1R)-2- Methoxy-1- phenylethyl]-5- methyl-2-(5- morpholin-4-yl-3, 4'-bipyridin-2'-yl) 1H-imidazole-4-
US11352340, Example 275 BDBM557223 N-[(1R)-2- Methoxy-1- phenylethyl]-5- methyl-2-(5- morpholin-4-yl-3, 4'-bipyridin-2'-yl) 1H-imidazole-4- US11485745, Example 275 BDBM580384 N-[[6-[2-(1,4-dimethyl-4- piperidyl)acetyl]-6- azaspiro[2.5]octan-2- yl]methyl]furo[2,3-c]pyridine-2- carboxamide
US11485745, Example 275 BDBM580384 N-[[6-[2-(1,4-dimethyl-4- piperidyl)acetyl]-6- azaspiro[2.5]octan-2- yl]methyl]furo[2,3-c]pyridine-2- carboxamide US12065436, Compound 275 (R)-4-methyl-N-(5-(3-(methyl-d3)- 1,2,4-oxadiazol-5-yl)-2,3-dihydro-1H- inden-1-yl)isoxazole-5-carboxamide BDBM690886
US12065436, Compound 275 (R)-4-methyl-N-(5-(3-(methyl-d3)- 1,2,4-oxadiazol-5-yl)-2,3-dihydro-1H- inden-1-yl)isoxazole-5-carboxamide BDBM690886 US12084420, Compound 275 BDBM695677 N-(tert-butyl)-4-(5$#8243;- (ethylsulfonamido)dispiro[cyclopropane- 1,1'-cyclohexane-4',3$#8243;-indoline]-1$#8243;- carbonyl)thiophene-2-sulfonamide
US12084420, Compound 275 BDBM695677 N-(tert-butyl)-4-(5$#8243;- (ethylsulfonamido)dispiro[cyclopropane- 1,1'-cyclohexane-4',3$#8243;-indoline]-1$#8243;- carbonyl)thiophene-2-sulfonamide US9687479, 275 1-(5-[(5-chlorothiophen-2-yl)methyl]amino-4-fluoro-3-phenyl-1H-pyrazol-1-yl)-3-hydroxy-2,2-dimethylpropan-1-one BDBM163400
US9687479, 275 1-(5-[(5-chlorothiophen-2-yl)methyl]amino-4-fluoro-3-phenyl-1H-pyrazol-1-yl)-3-hydroxy-2,2-dimethylpropan-1-one BDBM163400 US11203600, Example 276 BDBM530194 Preparation of 4-[2-methyl-2-[3-(triazol-2-yl)cyclobutyl]propanoyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile (Example 275) and 4-[2-methyl-2-[3-(triazol-1-yl)cyclobutyl]propanoyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile (Example 276) US11203600, Example 275
US11203600, Example 276 BDBM530194 Preparation of 4-[2-methyl-2-[3-(triazol-2-yl)cyclobutyl]propanoyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile (Example 275) and 4-[2-methyl-2-[3-(triazol-1-yl)cyclobutyl]propanoyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile (Example 276) US11203600, Example 275 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-((2-(cyclohexanecarboxamido)ethyl)sulfonyl)-1-(pyridin-3-ylmethyl)-1H-indole-2-carboxamide US10093640, Example 275 BDBM289067
6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-((2-(cyclohexanecarboxamido)ethyl)sulfonyl)-1-(pyridin-3-ylmethyl)-1H-indole-2-carboxamide US10093640, Example 275 BDBM289067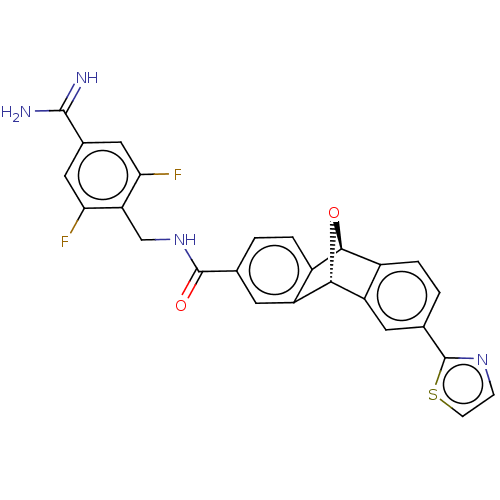 BDBM306632 (1S,8R)-N-[(4-carbamimidoyl- 2,6-difluoro-phenyl)methyl]- 12-(1,3-thiazol-2-yl)-15- oxatetracyclo[6.6.1.02,7.09,14] pentadeca-2,4,6,9,11,13- hexaene-4-carboxamide US10144746, Compound 275
BDBM306632 (1S,8R)-N-[(4-carbamimidoyl- 2,6-difluoro-phenyl)methyl]- 12-(1,3-thiazol-2-yl)-15- oxatetracyclo[6.6.1.02,7.09,14] pentadeca-2,4,6,9,11,13- hexaene-4-carboxamide US10144746, Compound 275 BDBM380280 US9926282, Example 275 4-[4-(2-oxo-2-(6- [(3S)-oxolan-3-yloxy]- 2,3-dihydro-1H-indol- 1-yl}ethyl)phenyl]- 1,2-dihydrophthalazin- 1-one
BDBM380280 US9926282, Example 275 4-[4-(2-oxo-2-(6- [(3S)-oxolan-3-yloxy]- 2,3-dihydro-1H-indol- 1-yl}ethyl)phenyl]- 1,2-dihydrophthalazin- 1-one BDBM755006 N-[4-[2-[[4-(dimethyl- amino)cyclohexyl]- amino]-8-isopropyl-7- oxo-pteridin-6-yl]-2- fluoro-phenyl]nor- carane-7-sulfonamide US12344603, Compound 275
BDBM755006 N-[4-[2-[[4-(dimethyl- amino)cyclohexyl]- amino]-8-isopropyl-7- oxo-pteridin-6-yl]-2- fluoro-phenyl]nor- carane-7-sulfonamide US12344603, Compound 275 BDBM758699 US20250230171, Compound 275 N-(5-(N-(tert-butyl)sulfamoyl)-5,6- dihydro-4H-pyrrolo[3,4-d]thiazol-2-yl)- 4-(5-cyano-2-methoxyphenyl)-6- methylnicotinamide
BDBM758699 US20250230171, Compound 275 N-(5-(N-(tert-butyl)sulfamoyl)-5,6- dihydro-4H-pyrrolo[3,4-d]thiazol-2-yl)- 4-(5-cyano-2-methoxyphenyl)-6- methylnicotinamide US10167279, Example 275 BDBM313649 (R)-N-(3-(2-(2,3- dihydroxypropoxy)-6- (tetrahydro-2H-pyran-4- yl)pyridin-4-yl)-4- methylphenyl)-2-(1- (trifluoromethyl) cyclopropyl) isonicotinamide
US10167279, Example 275 BDBM313649 (R)-N-(3-(2-(2,3- dihydroxypropoxy)-6- (tetrahydro-2H-pyran-4- yl)pyridin-4-yl)-4- methylphenyl)-2-(1- (trifluoromethyl) cyclopropyl) isonicotinamide US11149018, Example 275 (+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]quinolin-4-yl}carbonyl)amino]-4-(2-chlorophenyl)pentanoic acid (Enantiomer 1) BDBM520995
US11149018, Example 275 (+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]quinolin-4-yl}carbonyl)amino]-4-(2-chlorophenyl)pentanoic acid (Enantiomer 1) BDBM520995 US11866430, Example 275 BDBM644297 4-{4-[(5-bromo-2-hydroxyphenyl)methyl]piperazin-1-yl}-6-chloro-1-methyl-2-oxo-1,2-dihydro-1,5-naphthyridine-3-carbonitrile
US11866430, Example 275 BDBM644297 4-{4-[(5-bromo-2-hydroxyphenyl)methyl]piperazin-1-yl}-6-chloro-1-methyl-2-oxo-1,2-dihydro-1,5-naphthyridine-3-carbonitrile US9656988, Example 275 (R)-3-(4-(1-cyclopentylpiperidin-4-yl)phenylamino)-5-(3-(3-ethyl-2-oxoimidazolidin-1-yl)piperidin-1-yl)pyrazine-2-carboxamide BDBM309806
US9656988, Example 275 (R)-3-(4-(1-cyclopentylpiperidin-4-yl)phenylamino)-5-(3-(3-ethyl-2-oxoimidazolidin-1-yl)piperidin-1-yl)pyrazine-2-carboxamide BDBM309806 US9738655, Example 275 4-(2-(1-(3-chloro-2- fluoroplienyl)-1H-1,2,3-triazole- 4-carbonyl)-5-methoxy-1,2,3,4- tetrahydroisoquinoline-1- carboxamido)benzoic acid, TFA salt BDBM336053
US9738655, Example 275 4-(2-(1-(3-chloro-2- fluoroplienyl)-1H-1,2,3-triazole- 4-carbonyl)-5-methoxy-1,2,3,4- tetrahydroisoquinoline-1- carboxamido)benzoic acid, TFA salt BDBM336053 2-((3aR,5r,6aS)-5-benzyl- 5- hydroxyhexahydrocyclo- penta[c]pyrrol-2(1H)-yl)- 1-(4'-hydroxy-[1,1'- biphenyl]-3-yl)ethanone US10052306, 275 BDBM238283
2-((3aR,5r,6aS)-5-benzyl- 5- hydroxyhexahydrocyclo- penta[c]pyrrol-2(1H)-yl)- 1-(4'-hydroxy-[1,1'- biphenyl]-3-yl)ethanone US10052306, 275 BDBM238283 3-((2S)-3-(8-(3-(6-aminopyridin-3-yl)phenylsulfonyl)-1-oxa-8-azaspiro[4.5]decan-3-ylamino)-2-hydroxypropoxy)-N,N-dimethylbenzenesulfonamide US11987588, Compound 275 BDBM675806
3-((2S)-3-(8-(3-(6-aminopyridin-3-yl)phenylsulfonyl)-1-oxa-8-azaspiro[4.5]decan-3-ylamino)-2-hydroxypropoxy)-N,N-dimethylbenzenesulfonamide US11987588, Compound 275 BDBM675806 4-chloro-1-((1-isopropyl-1H- imidazol-2-yl)methyl)-3-methyl-N- (4-(trifluoromethyl)phenyl)-1H- pyrazolo[3,4-b]pyridine-5- carboxamide BDBM381442 US10272074, Example 275
4-chloro-1-((1-isopropyl-1H- imidazol-2-yl)methyl)-3-methyl-N- (4-(trifluoromethyl)phenyl)-1H- pyrazolo[3,4-b]pyridine-5- carboxamide BDBM381442 US10272074, Example 275 BDBM307979 US9650358, Example 275 N-{5-chloro-4-[1-(3-fluorobenzyl)-1H-benzimidazol-6-yl]pyridin-2-yl}-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyridine-6-carboxamide
BDBM307979 US9650358, Example 275 N-{5-chloro-4-[1-(3-fluorobenzyl)-1H-benzimidazol-6-yl]pyridin-2-yl}-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyridine-6-carboxamide BDBM320870 US10174037, Example 275 (R)- or (S)-1-(1-(4- Cyclopropylphenyl)ethyl)-3- (difluoromethyl)-6-hydroxy- 1H-pyrazolo[3,4-d]pyrimidin- 4(5H)-one US10174037, Example 276
BDBM320870 US10174037, Example 275 (R)- or (S)-1-(1-(4- Cyclopropylphenyl)ethyl)-3- (difluoromethyl)-6-hydroxy- 1H-pyrazolo[3,4-d]pyrimidin- 4(5H)-one US10174037, Example 276 BDBM599035 US11618753, Example 171 US11618753, Example 275 5-[4-amino-5- (trifluoromethyl) pyrrolo[2,1- f][1,2,4]triazin- 7-yl]-2-methyl- N-(3- phenylbutyl) pyridine-3- carboxamide
BDBM599035 US11618753, Example 171 US11618753, Example 275 5-[4-amino-5- (trifluoromethyl) pyrrolo[2,1- f][1,2,4]triazin- 7-yl]-2-methyl- N-(3- phenylbutyl) pyridine-3- carboxamide N-{6-[(3-cyclopropyl-1 H-pyrazol-5-yl)amino]-5-methoxy-1,2-benzoxazol-3-yl}-4-(1,2-dimethoxyethyl)-2,6-dimethoxybenzene-1-sulfonamide US20250122183, Example 275 BDBM731062
N-{6-[(3-cyclopropyl-1 H-pyrazol-5-yl)amino]-5-methoxy-1,2-benzoxazol-3-yl}-4-(1,2-dimethoxyethyl)-2,6-dimethoxybenzene-1-sulfonamide US20250122183, Example 275 BDBM731062 US9725449, Example 275 BDBM331438 N-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-(2-fluorophenyl)(oxan-4-yl)methyl]-3,8,10-triazatricyclo[7.4.0.02,7]trideca-1(13),2
US9725449, Example 275 BDBM331438 N-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-(2-fluorophenyl)(oxan-4-yl)methyl]-3,8,10-triazatricyclo[7.4.0.02,7]trideca-1(13),2 [(2S)-3-(3-ethyl-4-oxo- spiro[6,8-dihydro-5H- pyrazolo[4,3-c]azepine- 7,4'-tetrahydropyran]-1- yl)-2-methyl-propyl] 4- methylbenzoate US10906915, Ex. 275 BDBM481480
[(2S)-3-(3-ethyl-4-oxo- spiro[6,8-dihydro-5H- pyrazolo[4,3-c]azepine- 7,4'-tetrahydropyran]-1- yl)-2-methyl-propyl] 4- methylbenzoate US10906915, Ex. 275 BDBM481480 2-[[3-[(1R,5R,6S)-3- phenyl-6-bicyclo [3.1.0]hex-2-enyl]- 1,2,4-oxadiazol-5-yl] methyl]pyrido[1,2-a] pyrazine-1,6-dione US10711004, Example 275 BDBM452953
2-[[3-[(1R,5R,6S)-3- phenyl-6-bicyclo [3.1.0]hex-2-enyl]- 1,2,4-oxadiazol-5-yl] methyl]pyrido[1,2-a] pyrazine-1,6-dione US10711004, Example 275 BDBM452953 3-[4-({4-[(3-fluoropyridin-4-yl)amino]-2-(pyridin-2-yl)pyrrolo[2,1-f][1,2,4]triazin-5-yl}methyl)piperazin-1-yl]phenol US10336761, Example 275 BDBM406991
3-[4-({4-[(3-fluoropyridin-4-yl)amino]-2-(pyridin-2-yl)pyrrolo[2,1-f][1,2,4]triazin-5-yl}methyl)piperazin-1-yl]phenol US10336761, Example 275 BDBM406991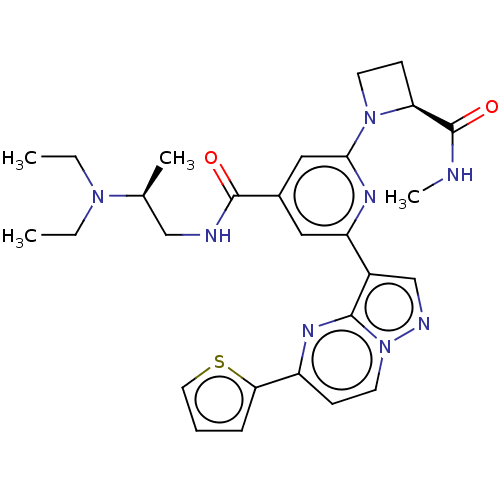 BDBM292619 US10100058, Example 275 N-((S)-2-(diethylamino)propyl)-2-((S)-2-(methylcarbamoyl)azetidin-1-yl)-6-(5-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidin-3-yl)isonicotinamide
BDBM292619 US10100058, Example 275 N-((S)-2-(diethylamino)propyl)-2-((S)-2-(methylcarbamoyl)azetidin-1-yl)-6-(5-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidin-3-yl)isonicotinamide BDBM406660 3-{5-[(5- chloropyridin- 2-yl)methyl]- 1,3,4-oxadiazol- 2-yl}-6- (ethoxymethyl)- 5-(2-hydroxy-6- methoxyphenyl) pyridine-2,4-diol US10336739, Example 274 US10336739, Example 275
BDBM406660 3-{5-[(5- chloropyridin- 2-yl)methyl]- 1,3,4-oxadiazol- 2-yl}-6- (ethoxymethyl)- 5-(2-hydroxy-6- methoxyphenyl) pyridine-2,4-diol US10336739, Example 274 US10336739, Example 275 BDBM556101 1-((3S,5R)-1-Acryloyl-5-(methoxymethyl)pyrrolidin-3-yl)-3-((1-methyl-1H-indazol-4-yl)ethynyl)-5-(methylamino)-1H-pyrazole-4-carboxamide US11345681, Example 275
BDBM556101 1-((3S,5R)-1-Acryloyl-5-(methoxymethyl)pyrrolidin-3-yl)-3-((1-methyl-1H-indazol-4-yl)ethynyl)-5-(methylamino)-1H-pyrazole-4-carboxamide US11345681, Example 275 BDBM635450 1-(tert-Butyl)-5-fluoro-N-(3-(3-fluoro-2-methyl- 8-morpholinoimidazo[1,2-a]pyridin-6-yl)-4- methylphenyl)-1H-pyrazole-4-carboxamide US11814384, Example 275
BDBM635450 1-(tert-Butyl)-5-fluoro-N-(3-(3-fluoro-2-methyl- 8-morpholinoimidazo[1,2-a]pyridin-6-yl)-4- methylphenyl)-1H-pyrazole-4-carboxamide US11814384, Example 275 Preparation of 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-((2-(cyclohexanecarboxamido)ethyl)sulfonyl)-1-(pyridin-3-ylmethyl)-1H-indole-2-carboxamide US10844032, Example 275 BDBM473391
Preparation of 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-N-((2-(cyclohexanecarboxamido)ethyl)sulfonyl)-1-(pyridin-3-ylmethyl)-1H-indole-2-carboxamide US10844032, Example 275 BDBM473391 US11485745, Example 149 US11485745, Example 275 N-[[6-[2-(1-methyl-4- piperidyl)acetyl]-6- azaspiro[2.5]octan-2- yl]methyl]furo[2,3-c]pyridine-2- carboxamide BDBM580261
US11485745, Example 149 US11485745, Example 275 N-[[6-[2-(1-methyl-4- piperidyl)acetyl]-6- azaspiro[2.5]octan-2- yl]methyl]furo[2,3-c]pyridine-2- carboxamide BDBM580261 US9611277, Example 275 (2-(2H-1,2,3-triazol-2-yl)phenyl)((1S,4R,6R)-6-((5-(trifluoromethyl)pyrazin-2-yl)amino)-2-azabicyclo[2.2.2]octan-2-yl)methanone BDBM315094
US9611277, Example 275 (2-(2H-1,2,3-triazol-2-yl)phenyl)((1S,4R,6R)-6-((5-(trifluoromethyl)pyrazin-2-yl)amino)-2-azabicyclo[2.2.2]octan-2-yl)methanone BDBM315094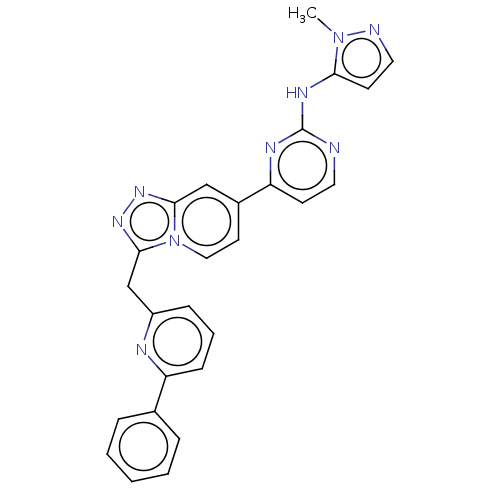 US9670208, Example 275 N-(1-methyl-1H-pyrazol-5-yl)-4-(3-((6-phenylpyridin-2-yl)methyl)-[1,2,4]triazolo[4,3-a]pyridin-7-yl)pyrimidin-2-amine BDBM193411
US9670208, Example 275 N-(1-methyl-1H-pyrazol-5-yl)-4-(3-((6-phenylpyridin-2-yl)methyl)-[1,2,4]triazolo[4,3-a]pyridin-7-yl)pyrimidin-2-amine BDBM193411 (3S,4R)-4-((7-(5-(1,1-difluoropropan- 2-yl)pyridin-2-yl)pyrrolo[2,1- f][1,2,4]triazin-2-yl)amino)tetrahydro- 2H-pyran-3-ol US20250163063, Example 275 BDBM742753
(3S,4R)-4-((7-(5-(1,1-difluoropropan- 2-yl)pyridin-2-yl)pyrrolo[2,1- f][1,2,4]triazin-2-yl)amino)tetrahydro- 2H-pyran-3-ol US20250163063, Example 275 BDBM742753 BDBM267676 US9718828, Example, 275 (R)-4-(4-amino-1-(1-(2,2,2-trifluoroacetyl)piperidin-3-yl)-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide
BDBM267676 US9718828, Example, 275 (R)-4-(4-amino-1-(1-(2,2,2-trifluoroacetyl)piperidin-3-yl)-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-N-(4-(trifluoromethyl)pyridin-2-yl)benzamide BDBM416130 6-[(trans-4- methoxycyclohexyl)carbonyl]- 8-(2-methyl-2,4,6,7- tetrahydro-5H-pyrazolo[4,3- c]pyridin-5-yl)-6,11-dihydro- 5H-pyrido[2,3- b][1,5]benzodiazepine US10442819, Example 275
BDBM416130 6-[(trans-4- methoxycyclohexyl)carbonyl]- 8-(2-methyl-2,4,6,7- tetrahydro-5H-pyrazolo[4,3- c]pyridin-5-yl)-6,11-dihydro- 5H-pyrido[2,3- b][1,5]benzodiazepine US10442819, Example 275 BDBM436500 US10590140, Example 275 2-(ethylamino)-6-(3-(2-(4- (3-hydroxyphenyl) piperidin-1-yl)ethyl) phenyl)-9-methyl- 6,7,8,9-tetrahydro-5H- pyrimido[4,5- e][1,4]diazepin-5-one
BDBM436500 US10590140, Example 275 2-(ethylamino)-6-(3-(2-(4- (3-hydroxyphenyl) piperidin-1-yl)ethyl) phenyl)-9-methyl- 6,7,8,9-tetrahydro-5H- pyrimido[4,5- e][1,4]diazepin-5-one BDBM568812 (rac)-5-{4-[1-amino-3-azabicyclo[3.1.0]hexan-3-yl]-3-(trifluoromethyl)phenyl}-3,6-dihydro-2H-1,3,4-oxadiazin-2-one-salt with hydrochloric acid US11427553, Example 275
BDBM568812 (rac)-5-{4-[1-amino-3-azabicyclo[3.1.0]hexan-3-yl]-3-(trifluoromethyl)phenyl}-3,6-dihydro-2H-1,3,4-oxadiazin-2-one-salt with hydrochloric acid US11427553, Example 275 BDBM664194 US20240109917, Example 275 N-(5-(2-(3,3-dimethylazetidin-1- yl)acetamido)-2-methylpyridin- 3-yl)-2-(1-(methylsulfonyl)-1H- pyrrol-3-yl)pyrazolo[5,1- b]thiazole-7-carboxamide
BDBM664194 US20240109917, Example 275 N-(5-(2-(3,3-dimethylazetidin-1- yl)acetamido)-2-methylpyridin- 3-yl)-2-(1-(methylsulfonyl)-1H- pyrrol-3-yl)pyrazolo[5,1- b]thiazole-7-carboxamide US10954214, Compound 275 N-(5-cyano-6-(2H-1,2,3-triazol- 2-yl)pyridin-3-yl)-1- (imidazo[1,2-a]pyrazin-5-yl)-5- (trifluoromethyl)-1H-pyrazole-4- carboxamide BDBM487853
US10954214, Compound 275 N-(5-cyano-6-(2H-1,2,3-triazol- 2-yl)pyridin-3-yl)-1- (imidazo[1,2-a]pyrazin-5-yl)-5- (trifluoromethyl)-1H-pyrazole-4- carboxamide BDBM487853 BDBM275396 US9884048, Example 275 1-{(3S,4S)-4-[(5- fluoropyridin-3- yl)oxy]pyrrolidin-3-yl}-3-[1- (2-methylpyridin-4-yl)-1H- pyrazolo[3,4-c]pyridin-5- yl]urea
BDBM275396 US9884048, Example 275 1-{(3S,4S)-4-[(5- fluoropyridin-3- yl)oxy]pyrrolidin-3-yl}-3-[1- (2-methylpyridin-4-yl)-1H- pyrazolo[3,4-c]pyridin-5- yl]urea BDBM299597 US9593129, Example 275 2-((3R,5R,6S)-5-(3-chlorophenyl)-6-(4-chlorophenyl)-1-((S)-1-cyclopropyl-2-(N-phenylethylsulfonamido)ethyl)-3-methyl-2-oxopiperidin-3-yl)acetic acid
BDBM299597 US9593129, Example 275 2-((3R,5R,6S)-5-(3-chlorophenyl)-6-(4-chlorophenyl)-1-((S)-1-cyclopropyl-2-(N-phenylethylsulfonamido)ethyl)-3-methyl-2-oxopiperidin-3-yl)acetic acid BDBM480214 rac-3-((1S,2R)-2- (cyano(phenyl)(1-((1-(4- (pyridin-4- ylsulfonyl)phenyl)azetidin-3- yl)methyl)piperidin-4- yl)methyl)cyclopentyl)-1,1- dimethylurea US10899738, Cpd. No 275
BDBM480214 rac-3-((1S,2R)-2- (cyano(phenyl)(1-((1-(4- (pyridin-4- ylsulfonyl)phenyl)azetidin-3- yl)methyl)piperidin-4- yl)methyl)cyclopentyl)-1,1- dimethylurea US10899738, Cpd. No 275 BDBM497789 (1S,8R)-5-(2,6-difluorophenyl)-11,11- dimethyl-1-[2-[2-(methylsulfonylmethyl)- 1,2,4-triazol-3-yl]pyrimidin-4-yl]-3,4- diazatricyclo[6.2.1.02,7]undeca-2(7),3,5- triene US11008312, Example 275
BDBM497789 (1S,8R)-5-(2,6-difluorophenyl)-11,11- dimethyl-1-[2-[2-(methylsulfonylmethyl)- 1,2,4-triazol-3-yl]pyrimidin-4-yl]-3,4- diazatricyclo[6.2.1.02,7]undeca-2(7),3,5- triene US11008312, Example 275 BDBM717454 US20250034159, Example 275 1-{3-(4-fluorophenyl)-4-[6-(1- methyl-1H-pyrazol-3- yl)furo[2,3-d]pyrimidin-4-yl]- 1H-pyrazol-1-yl}-2- methylpropan-2-ol
BDBM717454 US20250034159, Example 275 1-{3-(4-fluorophenyl)-4-[6-(1- methyl-1H-pyrazol-3- yl)furo[2,3-d]pyrimidin-4-yl]- 1H-pyrazol-1-yl}-2- methylpropan-2-ol US11597728, Example 275 BDBM597388 (3-Fluoro-5-(4H-1,2,4-triazol-4-yl)phenyl)((5R,9S)-2-methyl-3- (3,4,5-trifluorophenyl)-4,5,6,7,8,9-hexahydro-2H-5,9- epiminocycloocta[c]pyrazol-10-yl)methanone;
US11597728, Example 275 BDBM597388 (3-Fluoro-5-(4H-1,2,4-triazol-4-yl)phenyl)((5R,9S)-2-methyl-3- (3,4,5-trifluorophenyl)-4,5,6,7,8,9-hexahydro-2H-5,9- epiminocycloocta[c]pyrazol-10-yl)methanone; US9763922, Example 275 1-[3-[3-fluoro-4-(1-methylpyrazol-4-yl)anilino]-1-(2-hydroxy-1-methyl-ethyl)-6,7-dihydro-4H-pyrazolo[4,3-c]pyridin-5-yl]ethanone BDBM340749
US9763922, Example 275 1-[3-[3-fluoro-4-(1-methylpyrazol-4-yl)anilino]-1-(2-hydroxy-1-methyl-ethyl)-6,7-dihydro-4H-pyrazolo[4,3-c]pyridin-5-yl]ethanone BDBM340749 5-(5-(3-benzyl-1-((1-methyl-1H-1,2,3-triazol-4-yl)sulfonyl)pyrrolidin-3-yl)-6-methyl-1H-indazol-1-yl)-1-methylpyridin-2(1H)-one BDBM626576 US11787780, Example 275
5-(5-(3-benzyl-1-((1-methyl-1H-1,2,3-triazol-4-yl)sulfonyl)pyrrolidin-3-yl)-6-methyl-1H-indazol-1-yl)-1-methylpyridin-2(1H)-one BDBM626576 US11787780, Example 275 BDBM298064 7-(piperidin-4-yl)-3-{5-[2-(1H-1,2,4-triazol-1-yl)propan-2-yl]-1,3,4-oxadiazol-2-yl}pyrazolo[1,5-a]pyrimidin-5(4H)-one hydrochloride US10118930, Example 275
BDBM298064 7-(piperidin-4-yl)-3-{5-[2-(1H-1,2,4-triazol-1-yl)propan-2-yl]-1,3,4-oxadiazol-2-yl}pyrazolo[1,5-a]pyrimidin-5(4H)-one hydrochloride US10118930, Example 275 BDBM299905 (3,3- difluoroazetidin-1- yl)(2-{[(2R)-1- methoxypropan-2- yl]oxy}-6-[4-(1H- pyrazolo[3,4- b]pyridin-3- yl)piperidin-1- yl]pyrimidin-4- yl)methanone US9593097, Example 275
BDBM299905 (3,3- difluoroazetidin-1- yl)(2-{[(2R)-1- methoxypropan-2- yl]oxy}-6-[4-(1H- pyrazolo[3,4- b]pyridin-3- yl)piperidin-1- yl]pyrimidin-4- yl)methanone US9593097, Example 275 BDBM334121 4-[1-(4-Amino-3-methyl-1H- pyrazolo[3,4-d]pyrimidin-1- yl)ethyl]-6-chloro-3-methoxy-2-[1- (tetrahydrofuran-3-yl)azetidin-3- yl]benzonitrile US9730939, Example 275
BDBM334121 4-[1-(4-Amino-3-methyl-1H- pyrazolo[3,4-d]pyrimidin-1- yl)ethyl]-6-chloro-3-methoxy-2-[1- (tetrahydrofuran-3-yl)azetidin-3- yl]benzonitrile US9730939, Example 275 BDBM505627 US11066414, Compound 275 (S)-8-chloro-4-((3-chloro-4- fluorophenyl)amino)-6-(((4,5,6,7- tetrahydrothieno[2,3-c]pyridin-3- yl)(1H-1,2,3-triazol-4-yl)methyl)- amino)quinoline-3-carbonitrile
BDBM505627 US11066414, Compound 275 (S)-8-chloro-4-((3-chloro-4- fluorophenyl)amino)-6-(((4,5,6,7- tetrahydrothieno[2,3-c]pyridin-3- yl)(1H-1,2,3-triazol-4-yl)methyl)- amino)quinoline-3-carbonitrile BDBM537450 US11247990, Example 275 (3-(((1 s,4s)-4-(3-(1-methyl-1H-pyrazol-4-yl)-6-(methylamino)-1H-pyrazolo[4,3-c]pyridin-1-yl)cyclohexyl)oxy)pyridin-2-yl)methanol
BDBM537450 US11247990, Example 275 (3-(((1 s,4s)-4-(3-(1-methyl-1H-pyrazol-4-yl)-6-(methylamino)-1H-pyrazolo[4,3-c]pyridin-1-yl)cyclohexyl)oxy)pyridin-2-yl)methanol BDBM636146 5-ethoxy-6-({6-[(1R,2S)-5'-methoxy-2'-oxo-1',2'-dihydrospiro[cyclopropane-1,3'-indol]-2-yl]-1H-indazol-3-yl}amino)-N-methylpyridine-3-sulfonamide US20230365537, Example 275
BDBM636146 5-ethoxy-6-({6-[(1R,2S)-5'-methoxy-2'-oxo-1',2'-dihydrospiro[cyclopropane-1,3'-indol]-2-yl]-1H-indazol-3-yl}amino)-N-methylpyridine-3-sulfonamide US20230365537, Example 275 BDBM639806 US20230391786, Example 275 4-[6,7-Dichloro-9-[(2,2-difluoroacetyl)amino]-10-(1H-pyrazol-4-yl)-3,4-dihydro-1H-pyrazino[1,2-a]indol-2-yl]-N,N-dimethyl-4-oxo-butanamide
BDBM639806 US20230391786, Example 275 4-[6,7-Dichloro-9-[(2,2-difluoroacetyl)amino]-10-(1H-pyrazol-4-yl)-3,4-dihydro-1H-pyrazino[1,2-a]indol-2-yl]-N,N-dimethyl-4-oxo-butanamide KSC-6-275 4-chloro-2-[[(4-chlorophenyl)-oxomethyl]amino]benzoic acid 4-chloranyl-2-[(4-chlorophenyl)carbonylamino]benzoic acid 4-chloro-2-[(4-chlorobenzoyl)amino]benzoic acid BDBM61994 cid_587114 KUC105797N
KSC-6-275 4-chloro-2-[[(4-chlorophenyl)-oxomethyl]amino]benzoic acid 4-chloranyl-2-[(4-chlorophenyl)carbonylamino]benzoic acid 4-chloro-2-[(4-chlorobenzoyl)amino]benzoic acid BDBM61994 cid_587114 KUC105797N US10174016, Example 275 3-[5-(propan-2-yl)- 1,3-thiazol-2-yl]-5- [(2R)- tetrahydrofuran-2- ylmethoxy]-N- {(1R)-1-[2- (trifluoromethyl) pyrimidin-5- yl]ethyl} benzamide US10202369, Example 281 BDBM320108
US10174016, Example 275 3-[5-(propan-2-yl)- 1,3-thiazol-2-yl]-5- [(2R)- tetrahydrofuran-2- ylmethoxy]-N- {(1R)-1-[2- (trifluoromethyl) pyrimidin-5- yl]ethyl} benzamide US10202369, Example 281 BDBM320108 US11208400, Example 275 6-(3,5-dimethyl-1H-pyrazol-1-yl)-N-[5-(4-fluorophenyl)-1-methyl-4-{[3-(trifluoromethyl)azetidin-1-yl]methyl}-1H-pyrazol-3-yl]pyrimidin-4-amine BDBM531642
US11208400, Example 275 6-(3,5-dimethyl-1H-pyrazol-1-yl)-N-[5-(4-fluorophenyl)-1-methyl-4-{[3-(trifluoromethyl)azetidin-1-yl]methyl}-1H-pyrazol-3-yl]pyrimidin-4-amine BDBM531642 US12071425, Compound 275 1-(2-fluorophenyl)-N-(2,3,6-trifluoro-4- ((3-(2-(((3S,5S)-5-fluoro-5- methylpiperidin-3-yl)amino)pyrimidin-4- yl)pyridin-2- yl)oxy)phenyl)methanesulfonamide BDBM693356
US12071425, Compound 275 1-(2-fluorophenyl)-N-(2,3,6-trifluoro-4- ((3-(2-(((3S,5S)-5-fluoro-5- methylpiperidin-3-yl)amino)pyrimidin-4- yl)pyridin-2- yl)oxy)phenyl)methanesulfonamide BDBM693356 (R)-1-(4-((1-(3-(difluoromethyl)-2- fluorophenyl)ethyl)amino)-7-methoxy- 2-((tetrahydro-2H-pyran-4- yl)methoxy)pyrido[2,3-d] pyrimidin-6-yl)cyclopropane-1- carbonitrile US11648254, Compound 275 BDBM602383
(R)-1-(4-((1-(3-(difluoromethyl)-2- fluorophenyl)ethyl)amino)-7-methoxy- 2-((tetrahydro-2H-pyran-4- yl)methoxy)pyrido[2,3-d] pyrimidin-6-yl)cyclopropane-1- carbonitrile US11648254, Compound 275 BDBM602383 1-[5-[6-chloro-5-[(1R)-1-(3,5- dichloro-2-methyl-4- pyridyl)ethoxy]-1H-indazol-3- yl]-3-fluoro-2-pyridyl]-N,3- dimethyl-azetidin-3-amine BDBM662851 US20240109865, Example 275.
1-[5-[6-chloro-5-[(1R)-1-(3,5- dichloro-2-methyl-4- pyridyl)ethoxy]-1H-indazol-3- yl]-3-fluoro-2-pyridyl]-N,3- dimethyl-azetidin-3-amine BDBM662851 US20240109865, Example 275. 2-(3-((R)-1-(4-methyl-4H-1,2,4-triazol-3- yl)-2-((R)-tetrahydrofuran-3- yl)ethyl)phenyl)-6-(((1- methylcyclobutyl)amino)methyl)-4- (trifluoromethyl)isoindolin-1-one US12187709, Compound 275 BDBM710917
2-(3-((R)-1-(4-methyl-4H-1,2,4-triazol-3- yl)-2-((R)-tetrahydrofuran-3- yl)ethyl)phenyl)-6-(((1- methylcyclobutyl)amino)methyl)-4- (trifluoromethyl)isoindolin-1-one US12187709, Compound 275 BDBM710917 BDBM492134 US10975068, Example 275 4-[(2S)-4,4-Difluoro-2-methyl-pyrrolidine-1-carbonyl]-5-[4-(difluoromethyl)-6-[[1-(trifluoromethyl)cyclopropyl]methylamino]-3-pyridyl]-N-(2-hydroxy-2-methyl-propyl)thiazole-2-carboxamide
BDBM492134 US10975068, Example 275 4-[(2S)-4,4-Difluoro-2-methyl-pyrrolidine-1-carbonyl]-5-[4-(difluoromethyl)-6-[[1-(trifluoromethyl)cyclopropyl]methylamino]-3-pyridyl]-N-(2-hydroxy-2-methyl-propyl)thiazole-2-carboxamide BDBM690329 US20240262826, Compound 275 (S)-N-(2-chloro-5-(2-cyclopropyl-2H-tetrazol-5-yl)phenyl)-5-(1-(2,3-dihydroxypropyl)-3,5-dimethyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carboxamide
BDBM690329 US20240262826, Compound 275 (S)-N-(2-chloro-5-(2-cyclopropyl-2H-tetrazol-5-yl)phenyl)-5-(1-(2,3-dihydroxypropyl)-3,5-dimethyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyridine-3-carboxamide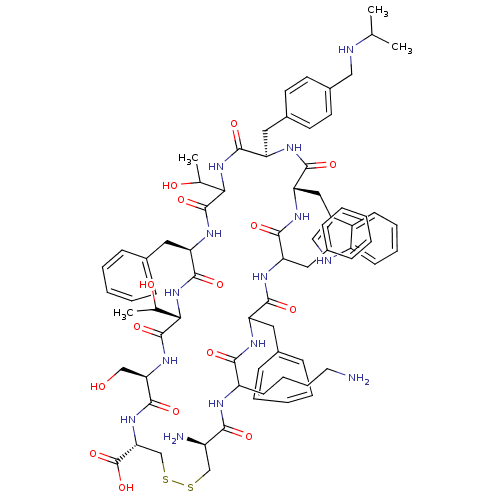 CH-275 CH275 BDBM85080 L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-4-[(Isopropylamino)methyl]-L-Phe-L-Thr-L-Phe-L-Thr-L-Ser-L-Cys(1)-OH
CH-275 CH275 BDBM85080 L-Cys(1)-L-Lys-L-Phe-L-Phe-D-Trp-4-[(Isopropylamino)methyl]-L-Phe-L-Thr-L-Phe-L-Thr-L-Ser-L-Cys(1)-OH N,1-dimethyl-4-[4-[[8- [1-(4,4,4- trifluorobutanoyl)-3,6- dihydro-2H-pyridin-4- yl]-[1,2,4]triazolo[1,5- a]pyridin-2- yl]amino]benzoyl] piperazine-2-carboxamide BDBM366979 US9873709, Example 2-275
N,1-dimethyl-4-[4-[[8- [1-(4,4,4- trifluorobutanoyl)-3,6- dihydro-2H-pyridin-4- yl]-[1,2,4]triazolo[1,5- a]pyridin-2- yl]amino]benzoyl] piperazine-2-carboxamide BDBM366979 US9873709, Example 2-275 US11866450, Compound 275 BDBM644860 Preparation of 21,21-dimethyl-4-(3-{3-[1-(trifluoromethyl)cyclopropyl]propoxy}-1H-pyrazol-1-yl)-10λ6-thia-1,3,9,14,23-pentaazatetracyclo[17.2.1.111,14.02,7]tricosa-2,4,6,11(23),12-pentaene-8,10,10-trione
US11866450, Compound 275 BDBM644860 Preparation of 21,21-dimethyl-4-(3-{3-[1-(trifluoromethyl)cyclopropyl]propoxy}-1H-pyrazol-1-yl)-10λ6-thia-1,3,9,14,23-pentaazatetracyclo[17.2.1.111,14.02,7]tricosa-2,4,6,11(23),12-pentaene-8,10,10-trione  7-(5-(5-(3-(2-hydroxy-2-methylpropanoyl)-3,6-diazabicyclo[3.1.1]heptan-6-yl)-1,3,4-thiadiazol-2-yl)-4-(isopropylamino)pyridin-2-yl)pyrrolo[1,2-b]pyridazine-3-carbonitrile US11702414, Example 275 BDBM609310
7-(5-(5-(3-(2-hydroxy-2-methylpropanoyl)-3,6-diazabicyclo[3.1.1]heptan-6-yl)-1,3,4-thiadiazol-2-yl)-4-(isopropylamino)pyridin-2-yl)pyrrolo[1,2-b]pyridazine-3-carbonitrile US11702414, Example 275 BDBM609310 US12187713, Example 275 BDBM711419 ((6-(difluoromethoxy)-2-(3'-(5-((3- (difluoromethyl)pyrrolidin-1-yl)methyl)-6- methoxypyridin-2-yl)-2,2'-dimethyl-[1,1'- biphenyl]-3-yl)benzo[d]oxazol-5-yl)methyl)-L- proline
US12187713, Example 275 BDBM711419 ((6-(difluoromethoxy)-2-(3'-(5-((3- (difluoromethyl)pyrrolidin-1-yl)methyl)-6- methoxypyridin-2-yl)-2,2'-dimethyl-[1,1'- biphenyl]-3-yl)benzo[d]oxazol-5-yl)methyl)-L- proline 1-(4-(3-(4-amino-5-(1-(tetrahydro-2H-pyran-4-yl)- 1H-1,2,3-triazol-5-yl)pyrrolo[2,1-f][1,2,4]triazin- 7-yl)phenyl)-3,3-dimethylpiperazin-1-yl)ethanone US10214537, Example 275 BDBM358206
1-(4-(3-(4-amino-5-(1-(tetrahydro-2H-pyran-4-yl)- 1H-1,2,3-triazol-5-yl)pyrrolo[2,1-f][1,2,4]triazin- 7-yl)phenyl)-3,3-dimethylpiperazin-1-yl)ethanone US10214537, Example 275 BDBM358206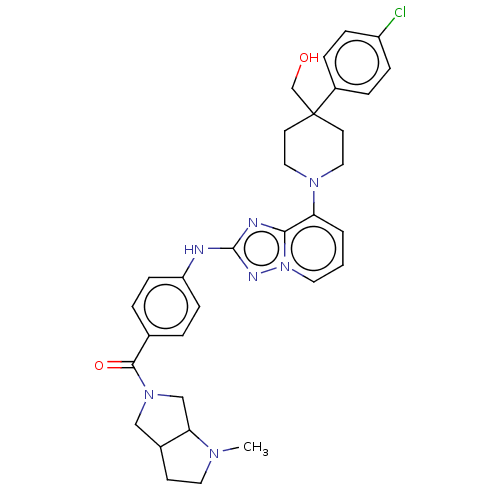 BDBM366678 US9873709, Example 1-275 [4-[[8-[4-(4- chlorophenyl)-4- (hydroxymethyl)-1- piperidyl]- [1,2,4]triazolo[1,5- a]pyridin-2- yl]amino]phenyl]-(1- methyl-2,3,3a,4,6,6a- hexahydropyrrolo[2,3- c]pyrrol-5- yl)methanone
BDBM366678 US9873709, Example 1-275 [4-[[8-[4-(4- chlorophenyl)-4- (hydroxymethyl)-1- piperidyl]- [1,2,4]triazolo[1,5- a]pyridin-2- yl]amino]phenyl]-(1- methyl-2,3,3a,4,6,6a- hexahydropyrrolo[2,3- c]pyrrol-5- yl)methanone BDBM462988 US10780090, Compound I-275 [(1R,2S,4R)-4-{[5-({4-[(4S)-6,7-dihydro-4H-thieno[3,2-c]pyran-4-yl]-5-methyl-2- thienyl}carbonyl)pyrimidin-4-yl]amino}-2-hydroxycyclopentyl]methyl sulfamate
BDBM462988 US10780090, Compound I-275 [(1R,2S,4R)-4-{[5-({4-[(4S)-6,7-dihydro-4H-thieno[3,2-c]pyran-4-yl]-5-methyl-2- thienyl}carbonyl)pyrimidin-4-yl]amino}-2-hydroxycyclopentyl]methyl sulfamate BDBM576953 4-(1-(6- ((Isopropylamino)methyl)- 2-methylpyridin-3-yl)- 1H-pyrazol-4-yl)-N-(1- ((1-methyl-1H-imidazol- 4-yl)sulfonyl)piperidin-4- yl)-5- (trifluoromethyl)pyrimidin- 2-amine US11472791, Example 275
BDBM576953 4-(1-(6- ((Isopropylamino)methyl)- 2-methylpyridin-3-yl)- 1H-pyrazol-4-yl)-N-(1- ((1-methyl-1H-imidazol- 4-yl)sulfonyl)piperidin-4- yl)-5- (trifluoromethyl)pyrimidin- 2-amine US11472791, Example 275 US10093683, Example 275 BDBM289854 Methyl (4-(3-((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro[benzo[d][1,3]oxazine-4,3′-pyrrolidin]-1′-yl)-1-oxo-3-phenylpropan-2-yl)ureido)phenyl)carbamate
US10093683, Example 275 BDBM289854 Methyl (4-(3-((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro[benzo[d][1,3]oxazine-4,3′-pyrrolidin]-1′-yl)-1-oxo-3-phenylpropan-2-yl)ureido)phenyl)carbamate 4-[1-(4-Amino-3-methyl-1H- pyrazolo[3,4-d]pyrimidin-1- yl)ethyl]-6-chloro-3-methoxy-2-[1- (tetrahydrofuran-3-yl)azetidin-3- yl]benzonitrile (from peak 1)5 US10092570, Example 275 BDBM289274
4-[1-(4-Amino-3-methyl-1H- pyrazolo[3,4-d]pyrimidin-1- yl)ethyl]-6-chloro-3-methoxy-2-[1- (tetrahydrofuran-3-yl)azetidin-3- yl]benzonitrile (from peak 1)5 US10092570, Example 275 BDBM289274 BDBM490607 2-{[(2S)-1-(dimethylamino)propan-2-yl]oxy}-5-fluoro-N-(2-methoxy-4-methylpyridin-3-yl)-4-(3-oxo-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyridin-2(3H)-yl)benzamide US10968216, Example 275
BDBM490607 2-{[(2S)-1-(dimethylamino)propan-2-yl]oxy}-5-fluoro-N-(2-methoxy-4-methylpyridin-3-yl)-4-(3-oxo-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyridin-2(3H)-yl)benzamide US10968216, Example 275 BDBM731880 (R)-2-fluoro-3-((1R,3R)-1-(4- (2-(3-(fluoromethyl)azetidin- 1-yl)ethoxy)phenyl)-3- methyl-1,3,4,9-tetrahydro- 2H-pyrido[3,4-b]indol-2-yl)- 2-methylpropan-1-ol US20250114338, Example 275
BDBM731880 (R)-2-fluoro-3-((1R,3R)-1-(4- (2-(3-(fluoromethyl)azetidin- 1-yl)ethoxy)phenyl)-3- methyl-1,3,4,9-tetrahydro- 2H-pyrido[3,4-b]indol-2-yl)- 2-methylpropan-1-ol US20250114338, Example 275 US20250230147, Compound 275 (S)-2-(6-(allylamino)-4-(1-((4-methyl- 4H-1,2,4-triazol-3- yl)methyl)cyclobutyl)pyridin-2-yl)-6-((2- isopropyl-4-methylpiperazin-1- yl)methyl)-4-(trifluoromethyl)isoindolin- 1-one BDBM758094
US20250230147, Compound 275 (S)-2-(6-(allylamino)-4-(1-((4-methyl- 4H-1,2,4-triazol-3- yl)methyl)cyclobutyl)pyridin-2-yl)-6-((2- isopropyl-4-methylpiperazin-1- yl)methyl)-4-(trifluoromethyl)isoindolin- 1-one BDBM758094 BDBM663437 ((1R,5S,6r)-3-(3-(4-chloro-2-methyl-2H-indazol-5- yl)-1H-pyrazolo[3,4-b]pyrazin-6-yl)-6-(5- methylisoxazol-3-yl)-3-azabicyclo[3.1.0]hexan-6- yl)methanamine US20240109900, Example 275
BDBM663437 ((1R,5S,6r)-3-(3-(4-chloro-2-methyl-2H-indazol-5- yl)-1H-pyrazolo[3,4-b]pyrazin-6-yl)-6-(5- methylisoxazol-3-yl)-3-azabicyclo[3.1.0]hexan-6- yl)methanamine US20240109900, Example 275 N-[5-[1-(5-chloropyrimidin- 2-yl)-3,6-dihydro-2H- pyridin-4-yl]-4-fluoro-2- [rac-(3R,5S)-3,4,5- trimethylpiperazin-1- yl]phenyl]-6-oxo-4- (trifluoromethyl)-1H- pyridine-3-carboxamide BDBM553220 US11319299, Example 275
N-[5-[1-(5-chloropyrimidin- 2-yl)-3,6-dihydro-2H- pyridin-4-yl]-4-fluoro-2- [rac-(3R,5S)-3,4,5- trimethylpiperazin-1- yl]phenyl]-6-oxo-4- (trifluoromethyl)-1H- pyridine-3-carboxamide BDBM553220 US11319299, Example 275 BDBM619951 US20230295157, Example 275 N-(3-(2-((3S,8aS)-7-(3-Chloro-2-fluoro-6-(1H-tetrazol-1-yl)phenyl)-5-oxo-1,2,3,5,8,8a-hexahydroindolizin-3-yl)-1H-imidazol-4-yl)-1-methyl-1H-pyrazol-4-yl)cyclopropanecarboxamide
BDBM619951 US20230295157, Example 275 N-(3-(2-((3S,8aS)-7-(3-Chloro-2-fluoro-6-(1H-tetrazol-1-yl)phenyl)-5-oxo-1,2,3,5,8,8a-hexahydroindolizin-3-yl)-1H-imidazol-4-yl)-1-methyl-1H-pyrazol-4-yl)cyclopropanecarboxamide US20250144094, Example 275 7-(2-((7-ethyl-2-(oxetan-3- ylmethyl)-1,2,3,4- tetrahydroisoquinolin-6-yl)amino)- 5-(trifluoromethyl)pyrimidin-4-yl)- 4-(oxetan-3-y1)-3,4- dihydrothieno[2,3-f][1,4]thiazepin- 5(2H)-one 1,1-dioxide BDBM738043
US20250144094, Example 275 7-(2-((7-ethyl-2-(oxetan-3- ylmethyl)-1,2,3,4- tetrahydroisoquinolin-6-yl)amino)- 5-(trifluoromethyl)pyrimidin-4-yl)- 4-(oxetan-3-y1)-3,4- dihydrothieno[2,3-f][1,4]thiazepin- 5(2H)-one 1,1-dioxide BDBM738043 US20230348424, Example 276 BDBM631693 N-((S)-1-(3-(difluoromethyl)-2-fluorophenyl)-2,2,2- trifluoroethyl)-1-(1-(difluoromethyl)cyclopropyl)-4-(((1R,5S,6s)-3-methyl-3- azabicyclo[3.1.0]hexan-6-yl)amino)-6-oxo-1,6-dihydropyridine-3-carboxamide (Example 275)
US20230348424, Example 276 BDBM631693 N-((S)-1-(3-(difluoromethyl)-2-fluorophenyl)-2,2,2- trifluoroethyl)-1-(1-(difluoromethyl)cyclopropyl)-4-(((1R,5S,6s)-3-methyl-3- azabicyclo[3.1.0]hexan-6-yl)amino)-6-oxo-1,6-dihydropyridine-3-carboxamide (Example 275) 1-((4-((3S,5R or 3R,5S)-5-(5-amino-9-fluoro-8-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)-5-methylpiperidin- 1-yl)-5-methyl-1H-pyrazol-1-yl)-2-methylpropan-2-ol BDBM551729 US11312719, Example 275
1-((4-((3S,5R or 3R,5S)-5-(5-amino-9-fluoro-8-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)-5-methylpiperidin- 1-yl)-5-methyl-1H-pyrazol-1-yl)-2-methylpropan-2-ol BDBM551729 US11312719, Example 275 US12310975, Example 275 BDBM743736 2-[4-({4-[5-chloro-4-({1- methyl-3- [(methylcarbamoyl) methoxy]-2-oxo-1,2- dihydroquinolin-6- yl}amino)pyrimidin-2- yl]piperazin-1- yl}methyl)piperidin-1- yl]-N-(2,6- dioxopiperidin-3-yl)-6- fluorobenzamide
US12310975, Example 275 BDBM743736 2-[4-({4-[5-chloro-4-({1- methyl-3- [(methylcarbamoyl) methoxy]-2-oxo-1,2- dihydroquinolin-6- yl}amino)pyrimidin-2- yl]piperazin-1- yl}methyl)piperidin-1- yl]-N-(2,6- dioxopiperidin-3-yl)-6- fluorobenzamide (3R,6R,7S,8E,22S)-6′-Chloro-7-[2-(3-fluoroazetidin-1-yl)ethoxy]-11,12,12-trimethyl-15,15-dioxo-spiro[20-oxa-15-thia-1,11,14-triazatetracyclo[14.7.2.03,6.019,24]pentacosa-8,16,18,24-tetraene-22,1′-tetralin]-13-one BDBM520578 US11130769, Ex. No. 275
(3R,6R,7S,8E,22S)-6′-Chloro-7-[2-(3-fluoroazetidin-1-yl)ethoxy]-11,12,12-trimethyl-15,15-dioxo-spiro[20-oxa-15-thia-1,11,14-triazatetracyclo[14.7.2.03,6.019,24]pentacosa-8,16,18,24-tetraene-22,1′-tetralin]-13-one BDBM520578 US11130769, Ex. No. 275 BDBM646284 US11878965, Example 275 ((2S,5R)-5-amino-2- methylpiperidin-1-yl)(2-(1- (cyclopropylmethyl)-6-(5- methyltetrazolo[1,5-a]pyridin-6-yl)- 1H-pyrrolo[2,3-b]pyridin-2-yl)-7- methoxy-1-methyl-1H- benzo[d]imidazol-5-yl)methanone
BDBM646284 US11878965, Example 275 ((2S,5R)-5-amino-2- methylpiperidin-1-yl)(2-(1- (cyclopropylmethyl)-6-(5- methyltetrazolo[1,5-a]pyridin-6-yl)- 1H-pyrrolo[2,3-b]pyridin-2-yl)-7- methoxy-1-methyl-1H- benzo[d]imidazol-5-yl)methanone BDBM671266 (3S,9S,18S,21S,25R,28S,31R,34S)-3-[2-[3-chloro-4-(trifluoromethyl)phenyl]ethyl]-9-(cyclohexylmethyl)-31-ethyl-21-isobutyl-28-isopropyl-7,10,13,16,22,25,29,31-octamethyl-18-[(1S)-1-methylpropyl]-1,4,7,10,13,16,19,22,26,29,32-undecazabicyclo[32.3.0]heptatriacontane-2,5,8,11,14,17,20,23,27,30,33-undecone US20240148821, Compound 275
BDBM671266 (3S,9S,18S,21S,25R,28S,31R,34S)-3-[2-[3-chloro-4-(trifluoromethyl)phenyl]ethyl]-9-(cyclohexylmethyl)-31-ethyl-21-isobutyl-28-isopropyl-7,10,13,16,22,25,29,31-octamethyl-18-[(1S)-1-methylpropyl]-1,4,7,10,13,16,19,22,26,29,32-undecazabicyclo[32.3.0]heptatriacontane-2,5,8,11,14,17,20,23,27,30,33-undecone US20240148821, Compound 275 BDBM740378 N-((2S)-1-((2-((2- chloro-6- fluorophenyl)carbamoyl)- 2-((R)-4-isopropyl-2- oxoimidazolidin-1-yl)- 2,3-dihydro-1H-inden- 5-yl)amino)-3,3- dicyclopropyl-1- oxopropan-2-yl)-4- methyl-1,2,5- oxadiazole-3- carboxamide US20250154138, Example 275
BDBM740378 N-((2S)-1-((2-((2- chloro-6- fluorophenyl)carbamoyl)- 2-((R)-4-isopropyl-2- oxoimidazolidin-1-yl)- 2,3-dihydro-1H-inden- 5-yl)amino)-3,3- dicyclopropyl-1- oxopropan-2-yl)-4- methyl-1,2,5- oxadiazole-3- carboxamide US20250154138, Example 275 1-((4-((3R,5S or 3S,5R)-5-(5-amino-9-fluoro-8-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)-5-methylpiperidin- 1-yl)-5-methyl-1H-pyrazol-1-yl)-2-methylpropan-2-ol BDBM551728 US11312719, Example 274 US11312719, Example 275
1-((4-((3R,5S or 3S,5R)-5-(5-amino-9-fluoro-8-methoxy- [1,2,4]triazolo[1,5-c]quinazolin-2-yl)-5-methylpiperidin- 1-yl)-5-methyl-1H-pyrazol-1-yl)-2-methylpropan-2-ol BDBM551728 US11312719, Example 274 US11312719, Example 275 US11566003, Compound 275 (S)-2-((8-amino-7-fluoro-6-(7-hydroxy- 6,7-dihydro-5H-cyclopenta[c]pyridin-4- yl)isoquinolin-3-yl)amino)-6-isopropyl- 5,6-dihydro-4H-pyrazolo[1,5- d][1,4]diazepin-7(8H)-one (Absolute stereochemistry arbitrarily assigned) BDBM591209
US11566003, Compound 275 (S)-2-((8-amino-7-fluoro-6-(7-hydroxy- 6,7-dihydro-5H-cyclopenta[c]pyridin-4- yl)isoquinolin-3-yl)amino)-6-isopropyl- 5,6-dihydro-4H-pyrazolo[1,5- d][1,4]diazepin-7(8H)-one (Absolute stereochemistry arbitrarily assigned) BDBM591209 BDBM391204 tert-butyl (3R,4R)-4- (cyanomethyl)-3-fluoro-4-(3- {[4-(1-methoxyethyl)phenyl] amino}-4-oxo-4,5-dihydro- 1H-pyrazolo[4,3-c]pyridin-1- yl)piperidine-1-carboxylate (from I-7-2B, as a mixture of ether diastereomers) US9957264, Example 5-275
BDBM391204 tert-butyl (3R,4R)-4- (cyanomethyl)-3-fluoro-4-(3- {[4-(1-methoxyethyl)phenyl] amino}-4-oxo-4,5-dihydro- 1H-pyrazolo[4,3-c]pyridin-1- yl)piperidine-1-carboxylate (from I-7-2B, as a mixture of ether diastereomers) US9957264, Example 5-275 US20250034136, Compound I-275 BDBM717102 N-[2-chloro-4-(trifluoromethyl)phenyl]-2-[2-(dimethylamino)-7- [(1S,6S)-5-(2-ethoxy-4-hydroxy-5-methylpyridine-3-carbonyl)-2,5- diazabicyclo[4.2.0]octan-2-yl]-6-ethyl-8-oxo-5H,8H-pyrido[2,3- b]pyrazin-5-yl]acetamide
US20250034136, Compound I-275 BDBM717102 N-[2-chloro-4-(trifluoromethyl)phenyl]-2-[2-(dimethylamino)-7- [(1S,6S)-5-(2-ethoxy-4-hydroxy-5-methylpyridine-3-carbonyl)-2,5- diazabicyclo[4.2.0]octan-2-yl]-6-ethyl-8-oxo-5H,8H-pyrido[2,3- b]pyrazin-5-yl]acetamide 2-((1H-pyrrolo[2,3-b]pyridin-5-yl) oxy)-N-((4-((((1r,4r)-4-hydroxy-4- methylcyclohexyl)methyl)amino)- 3-nitrophenyl)sulfonyl)-4-(6-((R)- 2-(2-isopropylphenyl)-4-(3- methoxy-4-methylbenzyl) piperazin-1-yl)-2-azaspiro[3.3] heptan-2-yl)benzamide US20240376097, Example 275 BDBM705115
2-((1H-pyrrolo[2,3-b]pyridin-5-yl) oxy)-N-((4-((((1r,4r)-4-hydroxy-4- methylcyclohexyl)methyl)amino)- 3-nitrophenyl)sulfonyl)-4-(6-((R)- 2-(2-isopropylphenyl)-4-(3- methoxy-4-methylbenzyl) piperazin-1-yl)-2-azaspiro[3.3] heptan-2-yl)benzamide US20240376097, Example 275 BDBM705115 (Method 1):(E)-1-(3-(((7-(8-chloronaphthalen-1-yl)-8-fluoro-2-(((2R,7aS)-2-fluorohexahydro-1H-pyrrolizin-7a-yl)methoxy)pyrido[4,3-d]pyrimidin-4-yl)(methyl)amino)methyl)azetidin-1-yl)-3-(2,6-dimethylpyrimidin-4-yl)prop-2-en-1-one BDBM713364 US20250019387, Example 275
(Method 1):(E)-1-(3-(((7-(8-chloronaphthalen-1-yl)-8-fluoro-2-(((2R,7aS)-2-fluorohexahydro-1H-pyrrolizin-7a-yl)methoxy)pyrido[4,3-d]pyrimidin-4-yl)(methyl)amino)methyl)azetidin-1-yl)-3-(2,6-dimethylpyrimidin-4-yl)prop-2-en-1-one BDBM713364 US20250019387, Example 275 BDBM574143 US11453697, Example 275 US11453697, Example 276 2-amino-9-[(5S,7R,8R,12aR,14R,15aS)-14-(4- amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2,10- dihydroxy-2-oxido-10-sulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]-1,9- dihydro-6H-purin-6-one (Diastereomer 1)
BDBM574143 US11453697, Example 275 US11453697, Example 276 2-amino-9-[(5S,7R,8R,12aR,14R,15aS)-14-(4- amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2,10- dihydroxy-2-oxido-10-sulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]-1,9- dihydro-6H-purin-6-one (Diastereomer 1) (1S,5S)—N-(4-(1-(2,2-difluoroethyl)-3-phenyl-1H-pyrazol-4-yl)-7-methoxyquinazolin-6-yl)-3-oxabicyclo[3.1.0]hexane-1-carboxamide and (1R,5R)—N-(4-(1-(2,2-difluoroethyl)-3-phenyl-1H-pyrazol-4-yl)-7-methoxyquinazolin-6-yl)-3-oxabicyclo[3.1.0]hexane-1-carboxamide BDBM759681 US20250236608, Example 275
(1S,5S)—N-(4-(1-(2,2-difluoroethyl)-3-phenyl-1H-pyrazol-4-yl)-7-methoxyquinazolin-6-yl)-3-oxabicyclo[3.1.0]hexane-1-carboxamide and (1R,5R)—N-(4-(1-(2,2-difluoroethyl)-3-phenyl-1H-pyrazol-4-yl)-7-methoxyquinazolin-6-yl)-3-oxabicyclo[3.1.0]hexane-1-carboxamide BDBM759681 US20250236608, Example 275 US11203600, Example 276 BDBM530195 Preparation of 4-[2-methyl-2-[3-(triazol-2-yl)cyclobutyl]propanoyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile (Example 275) and 4-[2-methyl-2-[3-(triazol-1-yl)cyclobutyl]propanoyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile (Example 276)
US11203600, Example 276 BDBM530195 Preparation of 4-[2-methyl-2-[3-(triazol-2-yl)cyclobutyl]propanoyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile (Example 275) and 4-[2-methyl-2-[3-(triazol-1-yl)cyclobutyl]propanoyl]-3,5-dihydro-2H-pyrido[3,4-f][1,4]oxazepine-9-carbonitrile (Example 276) US10821115, Example 275 BDBM470890 (1S,3'R,6'R,7'S,8'E,11'S,12'R)- 6-chloro-7'-methoxy-11',12'- dimethyl-7'-((4-((3R)- tetrahydro-3-furanyl)-1- piperazinyl)methyl)-3,4- dihydro-2H,15'H- spiro[naphthalene-1,22'- [20]oxa[13]thia[1,14] diazatetracyclo [14.7.2.0~3,6~.0~19,24~] pentacosa[8,16,18,24]tetraen]- 15'-one 13',13'-dioxide
US10821115, Example 275 BDBM470890 (1S,3'R,6'R,7'S,8'E,11'S,12'R)- 6-chloro-7'-methoxy-11',12'- dimethyl-7'-((4-((3R)- tetrahydro-3-furanyl)-1- piperazinyl)methyl)-3,4- dihydro-2H,15'H- spiro[naphthalene-1,22'- [20]oxa[13]thia[1,14] diazatetracyclo [14.7.2.0~3,6~.0~19,24~] pentacosa[8,16,18,24]tetraen]- 15'-one 13',13'-dioxide